User login
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
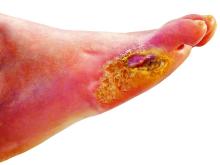
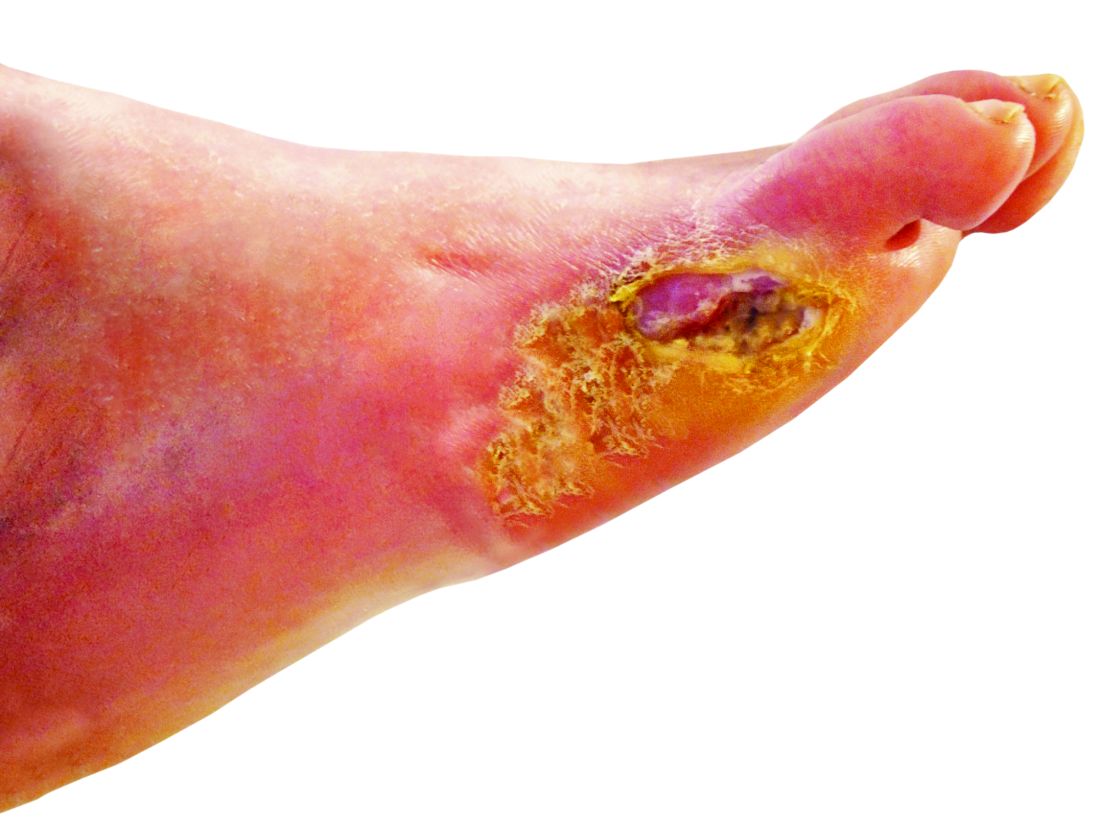
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
‘Amazing’ data for cheap beta-blocker gel for diabetic foot ulcers
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2022
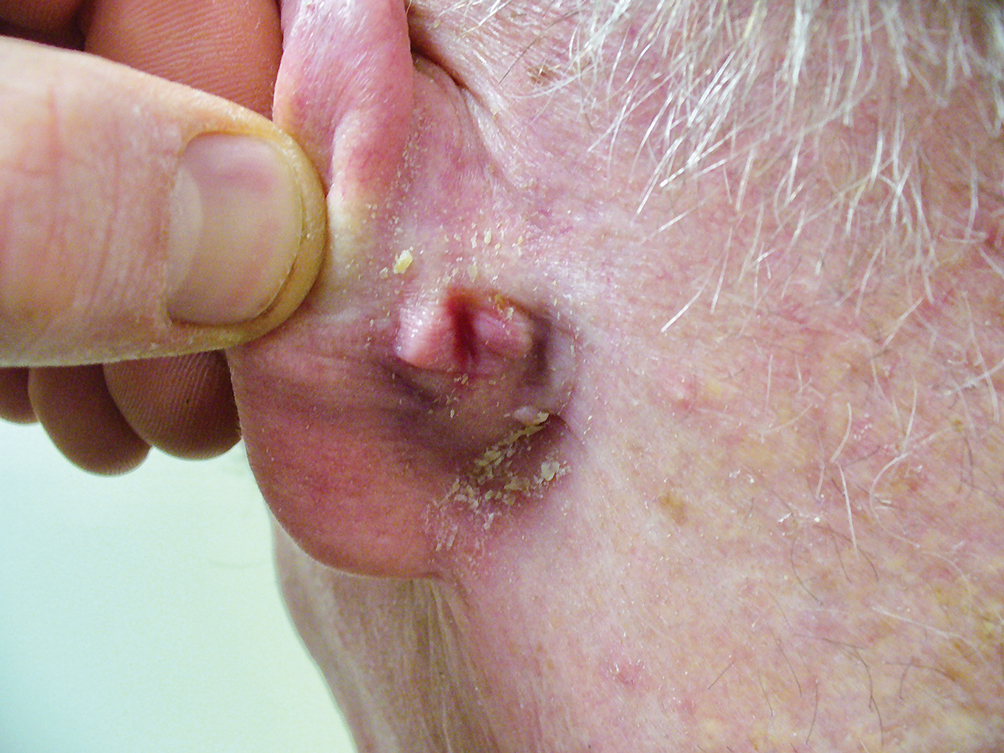
Pink Nodule Behind the Ear
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
A 62-year-old man presented to the dermatology office with a 1.5-cm, pink, rubbery nodule behind the left ear that sometimes was tender. He stated that the lesion gradually grew in size over the last 2 years, and it developed after he was fitted for new glasses.
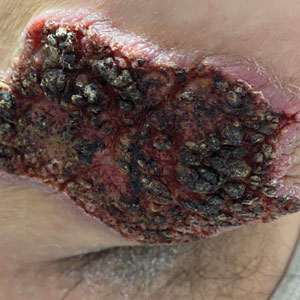
Multiple Fingerlike Projections on the Leg
The Diagnosis: Elephantiasis Nostras Verrucosa
Histopathology revealed a benign fibroepithelial polyp demonstrating areas of hyperkeratosis, acanthosis, and focal papillomatosis (Figure, A). Increased superficial vessels with dilated lymphatics, stellate fibroblasts, edematous stroma, and plasmolymphocytosis also were noted (Figure, B). Clinical and histopathological findings led to a diagnosis of lymphedema papules in the setting of elephantiasis nostra verrucosa (ENV).
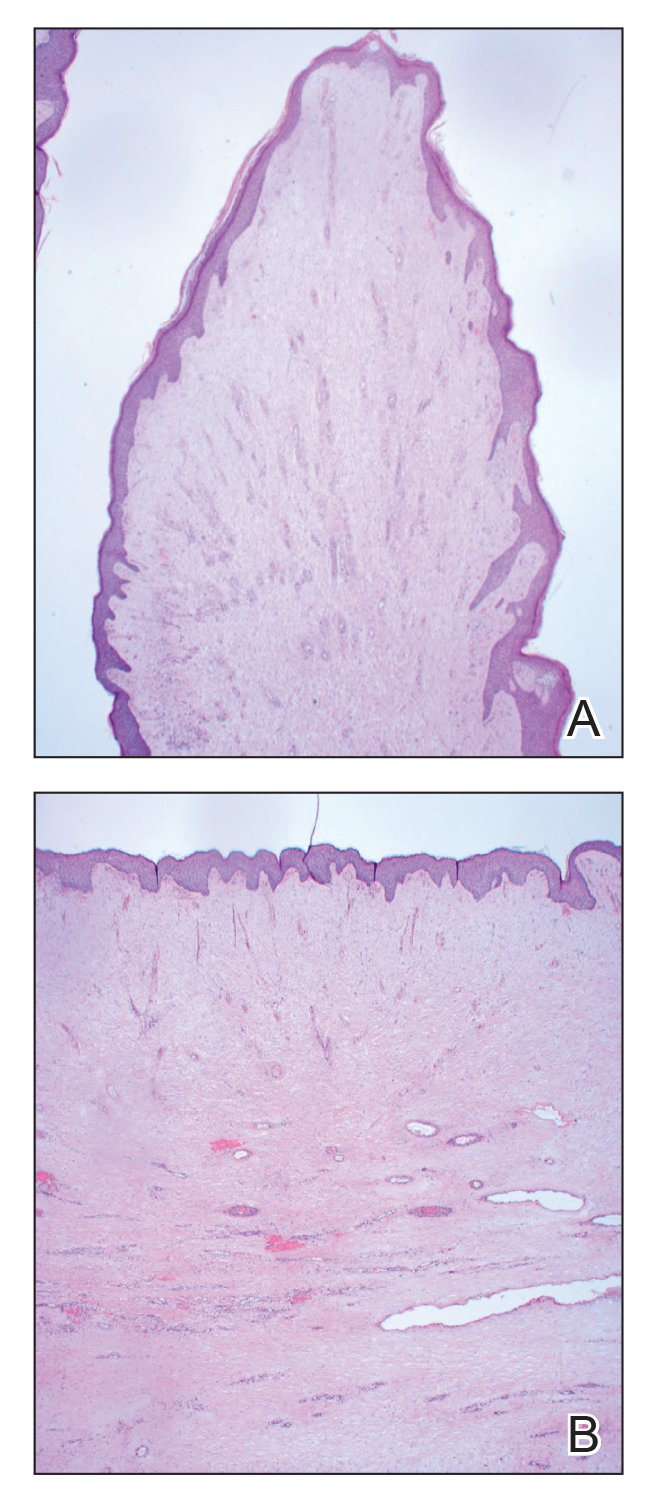
Elephantiasis nostras verrucosa is a complication of long-standing nonfilarial obstruction of lymphatic drainage leading to grotesque enlargement of the affected areas. Common cutaneous manifestations of ENV include nonpitting edema, dermal fibrosis, and extensive hyperkeratosis with verrucous and papillomatous lesions.1 In the beginning stages of ENV, the skin has a cobblestonelike appearance. As the disease progresses, the verrucous lesions continue to enlarge, giving the affected area a mossy appearance. Although less common, groupings of large papillomas similar to our patient’s presentation also can form.2 Ulcer formation is more likely to occur in advanced disease states, increasing the risk for bacterial and fungal colonization. Elephantiasis nostras verrucosa classically affects the legs; however, this condition can develop in any area with chronic lymphedema. Cases of ENV involving the arms, abdomen, scrotum, and ear have been documented.3-5
The pathogenesis of ENV involves the proliferation of fibroblasts and fibrosis secondary to lymphostasis and inflammation.6 When interstitial fluid builds up in the affected region, the protein-rich fluid is believed to trigger fibrogenesis and increase macrophage, keratinocyte, and adipocyte activity.7 Because of this inflammatory process, dilation and fibrosis of the lymphatic channels develop. Lymphatic obstruction can have several etiologies, most notably infection and malignancy. Staphylococcal lymphangitis and erysipelas create fibrosis of the lymphatic system and are the main infectious causes of ENV.6 Large tumors or lymphomas are insidious causes of lymphatic obstruction and should be ruled out when investigating for ENV. Other risk factors include obesity, chronic venous insufficiency, surgery, trauma, radiation, and uncontrolled congestive heart failure.1,6,8
An ENV diagnosis is clinicopathologic, involving a comprehensive metabolic panel and complete blood cell count with differential. A biopsy is needed for pathologic confirmation and to rule out malignancy. Histologically, ENV is characterized by pseudoepitheliomatous hyperplasia, dermal fibrosis, hyperkeratosis of the epidermis, and dilated lymphatic vessels.6,8 Additional studies for diagnosis include wound and lymph node culture, Wood lamp examination, and lymphoscintigraphy.
Given the chronic and progressive nature of the disease, ENV is difficult to treat. There currently is no standard of treatment, but the mainstay of management involves reducing peripheral edema. Lifestyle changes including weight loss, extremity elevation, and increased ambulation are helpful first-line therapies.3 Compression of the affected extremity using stockings or intermittent pneumatic compression devices has proven to be beneficial with long-term use.7 Patients should be followed for wound care to prevent the infection of ulcers.2 Pharmacologic treatments include systemic retinoids, which have been shown to reduce the appearance of hyperkeratosis, verrucous lesions, and papillomatous nodules.6 Prophylactic antibiotics are reserved for advanced stages of disease or in patients with recurrent infections.2,7 In severe cases of ENV that are unresponsive to medical management, surgical intervention such as lymphatic anastomosis and debulking may be considered.9,10
Other diagnoses to consider for ENV include pretibial myxedema, lymphatic filariasis, Stewart-Treves syndrome, and papillomatosis cutis carcinoides. Pretibial myxedema is an uncommon dermatologic manifestation of Graves disease. It is a local autoimmune reaction in the cutaneous tissue characterized by hyperpigmentation, nonpitting edema, and nodules on the anterior leg. Histopathology shows increased hyaluronic acid and chondroitin as well as compression of dermal lymphatics.11
Filariasis is a parasitic infection caused by Wuchereria bancrofti, Brugia malayi or Brugia timori, and Onchocerca volvulus.6 This condition presents with elephantiasis of the affected extremities but should be considered in areas endemic for filarial parasites such as tropical and subtropical countries.12 Eosinophilia and identification of microfilaria in a peripheral blood smear would indicate parasitic infection. Stewart-Treves syndrome is a rare angiosarcoma that arises in areas of chronic lymphedema. This condition classically is seen on the upper extremities following a mastectomy with lymphadenectomy, lymph node irradiation, or both.
Stewart-Treves syndrome presents with coalescing purpuric macules and nodules that eventually coalesce into cutaneous masses. Histopathology reveals proliferating vascular channels that split apart dermal collagen with hyperchromatism and pleomorphism in the tumor endothelial cells that line these channels.13
Papillomatosis cutis carcinoides is a low-grade squamous cell carcinoma that occurs secondary to human papillomavirus commonly affecting the mouth, anogenital area, and the plantar surfaces of the feet. It presents with exophytic growths and ulcerated tumors that are unilateral and asymmetrical. The presence of blunt-shaped tumor projections extending deep into the dermis to form sinuses and keratin-filled cysts is characteristic of papillomatosis cutis carcinoides.14
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64: 1104-1110. doi:10.1016/j.jaad.2010.04.047
- Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care. 2017;30:305-318. doi:10.1097/01.ASW.0000520501.23702.82
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004; 3:446-448.
- Nakai K, Taoka R, Sugimoto M, et al. Genital elephantiasis possibly caused by chronic inguinal eczema with streptococcal infection. J Dermatol. 2019;46:E196-E198. doi:10.1111/1346-8138.14746
- Carlson JA, Mazza J, Kircher K, et al. Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. Am J Dermatopathol. 2008;30:67-72. doi:10.1097/DAD.0b013e31815cd937
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146. doi:10.2165/00128071-200809030-00001
- Yoho RM, Budny AM, Pea AS. Elephantiasis nostras verrucosa. J Am Podiatr Med Assoc. 2006;96:442-444. doi:10.7547/0960442
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-920. doi:10.1016/j.jaad.2006.12.004
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941. doi:10.1111/j.1524-4725.2004.30267.x
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138:152-161. doi:10.1001/archsurg.138.2.152
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309. doi:10.2165 /00128071-200506050-00003
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi:10.1186/1475-2883-6-2
- Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves syndrome. JAMA Dermatol. 2021;157:721. doi:10.1001/jamadermatol.2021.0341
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-24. doi:10.1016/0190-9622(95)90177-9
The Diagnosis: Elephantiasis Nostras Verrucosa
Histopathology revealed a benign fibroepithelial polyp demonstrating areas of hyperkeratosis, acanthosis, and focal papillomatosis (Figure, A). Increased superficial vessels with dilated lymphatics, stellate fibroblasts, edematous stroma, and plasmolymphocytosis also were noted (Figure, B). Clinical and histopathological findings led to a diagnosis of lymphedema papules in the setting of elephantiasis nostra verrucosa (ENV).

Elephantiasis nostras verrucosa is a complication of long-standing nonfilarial obstruction of lymphatic drainage leading to grotesque enlargement of the affected areas. Common cutaneous manifestations of ENV include nonpitting edema, dermal fibrosis, and extensive hyperkeratosis with verrucous and papillomatous lesions.1 In the beginning stages of ENV, the skin has a cobblestonelike appearance. As the disease progresses, the verrucous lesions continue to enlarge, giving the affected area a mossy appearance. Although less common, groupings of large papillomas similar to our patient’s presentation also can form.2 Ulcer formation is more likely to occur in advanced disease states, increasing the risk for bacterial and fungal colonization. Elephantiasis nostras verrucosa classically affects the legs; however, this condition can develop in any area with chronic lymphedema. Cases of ENV involving the arms, abdomen, scrotum, and ear have been documented.3-5
The pathogenesis of ENV involves the proliferation of fibroblasts and fibrosis secondary to lymphostasis and inflammation.6 When interstitial fluid builds up in the affected region, the protein-rich fluid is believed to trigger fibrogenesis and increase macrophage, keratinocyte, and adipocyte activity.7 Because of this inflammatory process, dilation and fibrosis of the lymphatic channels develop. Lymphatic obstruction can have several etiologies, most notably infection and malignancy. Staphylococcal lymphangitis and erysipelas create fibrosis of the lymphatic system and are the main infectious causes of ENV.6 Large tumors or lymphomas are insidious causes of lymphatic obstruction and should be ruled out when investigating for ENV. Other risk factors include obesity, chronic venous insufficiency, surgery, trauma, radiation, and uncontrolled congestive heart failure.1,6,8
An ENV diagnosis is clinicopathologic, involving a comprehensive metabolic panel and complete blood cell count with differential. A biopsy is needed for pathologic confirmation and to rule out malignancy. Histologically, ENV is characterized by pseudoepitheliomatous hyperplasia, dermal fibrosis, hyperkeratosis of the epidermis, and dilated lymphatic vessels.6,8 Additional studies for diagnosis include wound and lymph node culture, Wood lamp examination, and lymphoscintigraphy.
Given the chronic and progressive nature of the disease, ENV is difficult to treat. There currently is no standard of treatment, but the mainstay of management involves reducing peripheral edema. Lifestyle changes including weight loss, extremity elevation, and increased ambulation are helpful first-line therapies.3 Compression of the affected extremity using stockings or intermittent pneumatic compression devices has proven to be beneficial with long-term use.7 Patients should be followed for wound care to prevent the infection of ulcers.2 Pharmacologic treatments include systemic retinoids, which have been shown to reduce the appearance of hyperkeratosis, verrucous lesions, and papillomatous nodules.6 Prophylactic antibiotics are reserved for advanced stages of disease or in patients with recurrent infections.2,7 In severe cases of ENV that are unresponsive to medical management, surgical intervention such as lymphatic anastomosis and debulking may be considered.9,10
Other diagnoses to consider for ENV include pretibial myxedema, lymphatic filariasis, Stewart-Treves syndrome, and papillomatosis cutis carcinoides. Pretibial myxedema is an uncommon dermatologic manifestation of Graves disease. It is a local autoimmune reaction in the cutaneous tissue characterized by hyperpigmentation, nonpitting edema, and nodules on the anterior leg. Histopathology shows increased hyaluronic acid and chondroitin as well as compression of dermal lymphatics.11
Filariasis is a parasitic infection caused by Wuchereria bancrofti, Brugia malayi or Brugia timori, and Onchocerca volvulus.6 This condition presents with elephantiasis of the affected extremities but should be considered in areas endemic for filarial parasites such as tropical and subtropical countries.12 Eosinophilia and identification of microfilaria in a peripheral blood smear would indicate parasitic infection. Stewart-Treves syndrome is a rare angiosarcoma that arises in areas of chronic lymphedema. This condition classically is seen on the upper extremities following a mastectomy with lymphadenectomy, lymph node irradiation, or both.
Stewart-Treves syndrome presents with coalescing purpuric macules and nodules that eventually coalesce into cutaneous masses. Histopathology reveals proliferating vascular channels that split apart dermal collagen with hyperchromatism and pleomorphism in the tumor endothelial cells that line these channels.13
Papillomatosis cutis carcinoides is a low-grade squamous cell carcinoma that occurs secondary to human papillomavirus commonly affecting the mouth, anogenital area, and the plantar surfaces of the feet. It presents with exophytic growths and ulcerated tumors that are unilateral and asymmetrical. The presence of blunt-shaped tumor projections extending deep into the dermis to form sinuses and keratin-filled cysts is characteristic of papillomatosis cutis carcinoides.14
The Diagnosis: Elephantiasis Nostras Verrucosa
Histopathology revealed a benign fibroepithelial polyp demonstrating areas of hyperkeratosis, acanthosis, and focal papillomatosis (Figure, A). Increased superficial vessels with dilated lymphatics, stellate fibroblasts, edematous stroma, and plasmolymphocytosis also were noted (Figure, B). Clinical and histopathological findings led to a diagnosis of lymphedema papules in the setting of elephantiasis nostra verrucosa (ENV).

Elephantiasis nostras verrucosa is a complication of long-standing nonfilarial obstruction of lymphatic drainage leading to grotesque enlargement of the affected areas. Common cutaneous manifestations of ENV include nonpitting edema, dermal fibrosis, and extensive hyperkeratosis with verrucous and papillomatous lesions.1 In the beginning stages of ENV, the skin has a cobblestonelike appearance. As the disease progresses, the verrucous lesions continue to enlarge, giving the affected area a mossy appearance. Although less common, groupings of large papillomas similar to our patient’s presentation also can form.2 Ulcer formation is more likely to occur in advanced disease states, increasing the risk for bacterial and fungal colonization. Elephantiasis nostras verrucosa classically affects the legs; however, this condition can develop in any area with chronic lymphedema. Cases of ENV involving the arms, abdomen, scrotum, and ear have been documented.3-5
The pathogenesis of ENV involves the proliferation of fibroblasts and fibrosis secondary to lymphostasis and inflammation.6 When interstitial fluid builds up in the affected region, the protein-rich fluid is believed to trigger fibrogenesis and increase macrophage, keratinocyte, and adipocyte activity.7 Because of this inflammatory process, dilation and fibrosis of the lymphatic channels develop. Lymphatic obstruction can have several etiologies, most notably infection and malignancy. Staphylococcal lymphangitis and erysipelas create fibrosis of the lymphatic system and are the main infectious causes of ENV.6 Large tumors or lymphomas are insidious causes of lymphatic obstruction and should be ruled out when investigating for ENV. Other risk factors include obesity, chronic venous insufficiency, surgery, trauma, radiation, and uncontrolled congestive heart failure.1,6,8
An ENV diagnosis is clinicopathologic, involving a comprehensive metabolic panel and complete blood cell count with differential. A biopsy is needed for pathologic confirmation and to rule out malignancy. Histologically, ENV is characterized by pseudoepitheliomatous hyperplasia, dermal fibrosis, hyperkeratosis of the epidermis, and dilated lymphatic vessels.6,8 Additional studies for diagnosis include wound and lymph node culture, Wood lamp examination, and lymphoscintigraphy.
Given the chronic and progressive nature of the disease, ENV is difficult to treat. There currently is no standard of treatment, but the mainstay of management involves reducing peripheral edema. Lifestyle changes including weight loss, extremity elevation, and increased ambulation are helpful first-line therapies.3 Compression of the affected extremity using stockings or intermittent pneumatic compression devices has proven to be beneficial with long-term use.7 Patients should be followed for wound care to prevent the infection of ulcers.2 Pharmacologic treatments include systemic retinoids, which have been shown to reduce the appearance of hyperkeratosis, verrucous lesions, and papillomatous nodules.6 Prophylactic antibiotics are reserved for advanced stages of disease or in patients with recurrent infections.2,7 In severe cases of ENV that are unresponsive to medical management, surgical intervention such as lymphatic anastomosis and debulking may be considered.9,10
Other diagnoses to consider for ENV include pretibial myxedema, lymphatic filariasis, Stewart-Treves syndrome, and papillomatosis cutis carcinoides. Pretibial myxedema is an uncommon dermatologic manifestation of Graves disease. It is a local autoimmune reaction in the cutaneous tissue characterized by hyperpigmentation, nonpitting edema, and nodules on the anterior leg. Histopathology shows increased hyaluronic acid and chondroitin as well as compression of dermal lymphatics.11
Filariasis is a parasitic infection caused by Wuchereria bancrofti, Brugia malayi or Brugia timori, and Onchocerca volvulus.6 This condition presents with elephantiasis of the affected extremities but should be considered in areas endemic for filarial parasites such as tropical and subtropical countries.12 Eosinophilia and identification of microfilaria in a peripheral blood smear would indicate parasitic infection. Stewart-Treves syndrome is a rare angiosarcoma that arises in areas of chronic lymphedema. This condition classically is seen on the upper extremities following a mastectomy with lymphadenectomy, lymph node irradiation, or both.
Stewart-Treves syndrome presents with coalescing purpuric macules and nodules that eventually coalesce into cutaneous masses. Histopathology reveals proliferating vascular channels that split apart dermal collagen with hyperchromatism and pleomorphism in the tumor endothelial cells that line these channels.13
Papillomatosis cutis carcinoides is a low-grade squamous cell carcinoma that occurs secondary to human papillomavirus commonly affecting the mouth, anogenital area, and the plantar surfaces of the feet. It presents with exophytic growths and ulcerated tumors that are unilateral and asymmetrical. The presence of blunt-shaped tumor projections extending deep into the dermis to form sinuses and keratin-filled cysts is characteristic of papillomatosis cutis carcinoides.14
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64: 1104-1110. doi:10.1016/j.jaad.2010.04.047
- Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care. 2017;30:305-318. doi:10.1097/01.ASW.0000520501.23702.82
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004; 3:446-448.
- Nakai K, Taoka R, Sugimoto M, et al. Genital elephantiasis possibly caused by chronic inguinal eczema with streptococcal infection. J Dermatol. 2019;46:E196-E198. doi:10.1111/1346-8138.14746
- Carlson JA, Mazza J, Kircher K, et al. Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. Am J Dermatopathol. 2008;30:67-72. doi:10.1097/DAD.0b013e31815cd937
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146. doi:10.2165/00128071-200809030-00001
- Yoho RM, Budny AM, Pea AS. Elephantiasis nostras verrucosa. J Am Podiatr Med Assoc. 2006;96:442-444. doi:10.7547/0960442
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-920. doi:10.1016/j.jaad.2006.12.004
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941. doi:10.1111/j.1524-4725.2004.30267.x
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138:152-161. doi:10.1001/archsurg.138.2.152
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309. doi:10.2165 /00128071-200506050-00003
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi:10.1186/1475-2883-6-2
- Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves syndrome. JAMA Dermatol. 2021;157:721. doi:10.1001/jamadermatol.2021.0341
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-24. doi:10.1016/0190-9622(95)90177-9
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64: 1104-1110. doi:10.1016/j.jaad.2010.04.047
- Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care. 2017;30:305-318. doi:10.1097/01.ASW.0000520501.23702.82
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004; 3:446-448.
- Nakai K, Taoka R, Sugimoto M, et al. Genital elephantiasis possibly caused by chronic inguinal eczema with streptococcal infection. J Dermatol. 2019;46:E196-E198. doi:10.1111/1346-8138.14746
- Carlson JA, Mazza J, Kircher K, et al. Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. Am J Dermatopathol. 2008;30:67-72. doi:10.1097/DAD.0b013e31815cd937
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146. doi:10.2165/00128071-200809030-00001
- Yoho RM, Budny AM, Pea AS. Elephantiasis nostras verrucosa. J Am Podiatr Med Assoc. 2006;96:442-444. doi:10.7547/0960442
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-920. doi:10.1016/j.jaad.2006.12.004
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941. doi:10.1111/j.1524-4725.2004.30267.x
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138:152-161. doi:10.1001/archsurg.138.2.152
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309. doi:10.2165 /00128071-200506050-00003
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi:10.1186/1475-2883-6-2
- Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves syndrome. JAMA Dermatol. 2021;157:721. doi:10.1001/jamadermatol.2021.0341
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-24. doi:10.1016/0190-9622(95)90177-9
A 61-year-old man presented with painful skin growths on the right pretibial region of several months’ duration. The patient reported pain due to friction between the lesions and underlying skin, leading to erosions. His medical history was remarkable for morbid obesity (body mass index of 62), chronic venous stasis, and chronic lymphedema. The patient was followed for wound care of venous stasis ulcers. Dermatologic examination revealed multiple 5- to 30-mm, flesh-colored, fingerlike projections on the right tibial region. A biopsy was obtained and submitted for histopathologic analysis.
Topical gene therapy for dystrophic epidermolysis bullosa shows promise
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
INDIANAPOLIS – An investigational compared with placebo, according to results from a small phase 3 study.
DEB is a serious, ultra-rare genetic blistering disease caused by mutations in the COL7A1 gene, encoding for type VII collagen and leading to skin fragility and wounds. No approved therapies are currently available. In the study, treatment was generally well tolerated.
“B-VEC is the first treatment that has not only been shown to be effective, but the first to directly target the defect through topical application,” the study’s principal investigator, Shireen V. Guide, MD, said in an interview during a poster session at the annual meeting of the Society for Pediatric Dermatology. “It delivers type VII collagen gene therapy to these patients, which allows healing in areas that they may have had open since birth. It’s been life-changing for them.”
B-VEC is a herpes simplex virus (HSV-1)-based topical, redosable gene therapy being developed by Krystal Biotech that is designed to restore functional COL7 protein by delivering the COL7A1 gene. For the phase 3, multicenter, double-blind, placebo-controlled study known GEM-3, Dr. Guide, who practices dermatology in Rancho Santa Margarita, Calif., and her colleagues, including Peter Marinkovich, MD, from Stanford (Calif.) University, and Mercedes Gonzalez, MD, from the University of Miami, enrolled 31 patients aged 6 months and older with genetically confirmed DEB. Each patient had one wound treated randomized 1:1 to treatment with B-VEC once a week or placebo for 6 months. The mean age of the 31 study participants was 17 years, 65% were male, 65% were White, and 19% were Asian.
The primary endpoint was complete wound healing (defined as 100% wound closure from exact wound area at baseline, specified as skin re-epithelialization without drainage) at 6 months. Additional endpoints included complete wound healing at 3 months and change in pain associated with wound dressing changes.
At 3 months, 70% of wounds treated with B-VEC met the endpoint of complete wound healing, compared with 20% of wounds treated with placebo (P < .005). At 6 months, 67% of wounds treated with B-VEC met the endpoint of complete wound healing compared with 22% of those treated with placebo (P < .005).
Of the total wounds that closed at 3 months, 67% of wounds treated with B-VEC were also closed at 6 months, compared with 33% of those treated with placebo (P = .02). In other findings, a trend toward decreased pain was observed in wounds treated with B-VEC vs. those treated with placebo.
B-VEC was well tolerated with no treatment-related serious adverse events or discontinuations. Three patients experienced a total of five serious adverse events during the study: anemia (two events), and cellulitis, diarrhea, and positive blood culture (one event each). None were considered related to the study drug.
Dr. Guide, who is on staff at Children’s Health of Orange County, Orange, Calif., characterized B-VEC as “very novel because it’s very practical.”
To date, all treatments for DEB “have been extremely labor intensive, including skin grafting and hospitalizations. It’s a topical application that can be done in the office and potentially applied at home in the future. It’s also durable. Not only are the [treated] areas closing, but they are staying closed.”
Kalyani S. Marathe, MD, MPH, director of the dermatology division at Cincinnati Children’s Hospital, who was asked to comment on the study, said that topical application of B-VEC “allows the side effect profile to be very favorable. The results are remarkable in the amount of wound healing and reduction in pain.”
The tolerability of this medication “is crucial,” she added. “EB patients have a lot of pain from their wounds and so any treatment needs to be as painless as possible for it to be usable. I’m very excited about the next phase of studies for this medication and hopeful that it heralds new treatments for our EB patients.”
In June 2022, the manufacturer announced that it had submitted a biologics license application to the Food and Drug Administration for approval of B-VEC for the treatment of DEB, and that it anticipates submitting an application for marketing authorization with the European Medical Agency (EMA) in the second half of 2022.
Dr. Guide disclosed that she has served as an investigator for Krystal Biotech, Innovaderm Research, Arcutis, Premier Research, Paidion, and Castle Biosciences. Dr. Marathe disclosed that she has served as an adviser for Verrica, and that Cincinnati Children’s Hospital is a site for the next phase studies for B-VEC.
*This story was updated on July 25.
AT SPD 2022
Fungated Eroded Plaque on the Arm
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.
Topical gel for epidermolysis bullosa shows ongoing benefit
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
Surgical Specimens and Margins
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.
Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
Practice Points
- Margin analysis in simple excisions can provide useful information by proxy about more than the 1% of the margin often quoted in the literature.
- Simple excisions of uncomplicated keratinocytic carcinomas are associated with high cure rates.
Simple Intraoperative Technique to Improve Wound Edge Approximation for Residents
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.
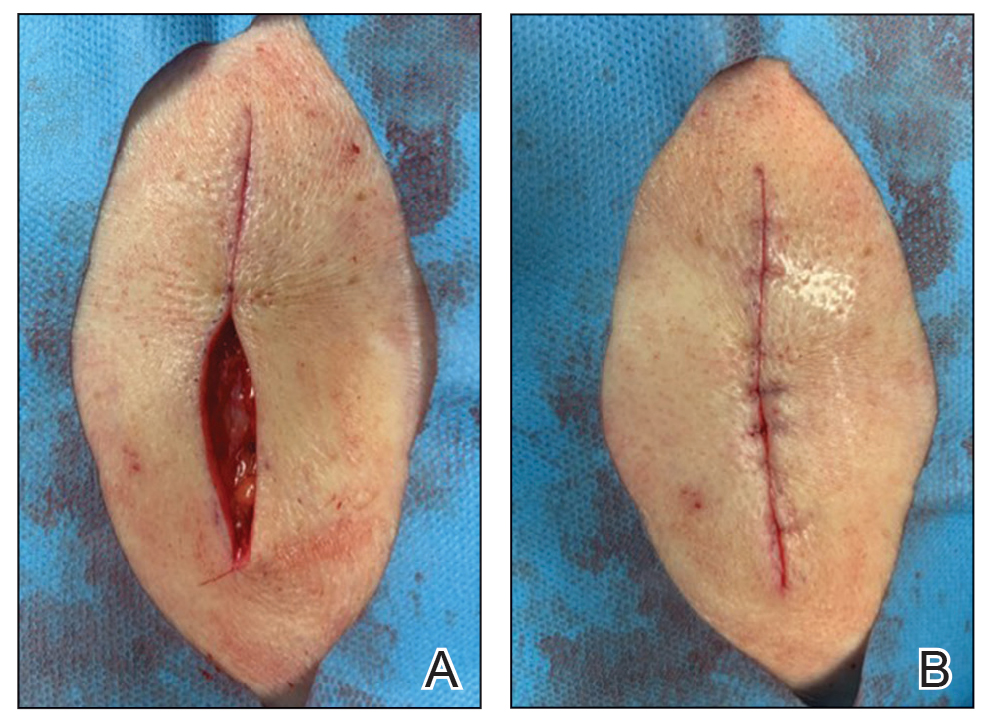
Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.

Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.

Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Combo of excision, cryosurgery found to benefit keloid scar outcomes
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
FROM JAMA DERMATOLOGY