User login
Dapper homolog 2 shows promise for pulmonary fibrosis
Dapper homolog 2 attenuated pulmonary fibrosis development and suppressed glycosis in myofibroblasts, suggesting potential as a therapeutic target for idiopathic pulmonary fibrosis, based on data from mouse models.
Idiopathic pulmonary fibrosis (IPF) remains a challenge with poor prognosis, and current therapeutic options are limited, wrote Xiaofan Lai, of Sun Yat-sen University, Guangzhou, China, and colleagues. Previous studies suggest that myofibroblasts are key contributors to fibrosis in IPF, they said.
Dishevelled-associated antagonist of beta-catenin 2 (DACT2) is an antagonist in the DACT gene family and associated with tissue development and injury, but its function and potential therapeutic role in IPF has not been explored; specifically, “whether DACT2 participates in the dysregulated glycolysis of myofibroblasts remains unknown,” they said.
In a study published in the International Journal of Biological Macromolecules, the researchers examined adeno-associated virus serotype 6 (AAV6)-mediated DACT2 overexpression in experimental pulmonary fibrosis using mouse models.
The researchers injected AAV6 vectors into the lungs of mice to overexpress DACT2. The DACT2 overexpression “effectively attenuated both bleomycin-induced and AdTGF-beta-1-induced pulmonary fibrosis murine models in vivo, as evidenced by the alleviation of myofibroblast differentiation and collagen accumulation,” they said.
They found that overexpression of DACT2 was associated with glucose uptake, extracellular acidification rate, intracellular adenosine-triphosphate (ATP) level, and lactate levels of myofibroblasts.
The researchers also conducted in vitro experiments in which they treated lung fibroblasts with cycloheximide (CHX), a protein synthesis inhibitor. These experiments showed that DACT2 inhibited differentiation of lung myofibroblasts by downregulating lactate dehydrogenase A (LDHA), which caused suppression of glycolysis in myofibroblasts.
“Aerobic glycolysis is an important method of energy generation, and several studies have shown that enhanced glycolysis facilitates the progression of pulmonary fibrosis,” the researchers wrote in their discussion.
More research is needed outside of mouse models and in vitro studies, but the current study is the first known to explore the relationship between DACT2 and LDHA in pulmonary fibrosis, and the results provide evidence of the potential benefits of DACT2 in treating lung disorders, the researchers wrote.
“We hope this research will lay the theoretical foundation for finding novel therapeutics to alleviate or reverse the development of pulmonary fibrosis and other chronic lung disorders,” they concluded.
The study was supported by the National Natural Science Foundation of China and the Regional Joint Fund-Youth Fund projects of Guangdong Province. The researchers had no financial conflicts to disclose.
Dapper homolog 2 attenuated pulmonary fibrosis development and suppressed glycosis in myofibroblasts, suggesting potential as a therapeutic target for idiopathic pulmonary fibrosis, based on data from mouse models.
Idiopathic pulmonary fibrosis (IPF) remains a challenge with poor prognosis, and current therapeutic options are limited, wrote Xiaofan Lai, of Sun Yat-sen University, Guangzhou, China, and colleagues. Previous studies suggest that myofibroblasts are key contributors to fibrosis in IPF, they said.
Dishevelled-associated antagonist of beta-catenin 2 (DACT2) is an antagonist in the DACT gene family and associated with tissue development and injury, but its function and potential therapeutic role in IPF has not been explored; specifically, “whether DACT2 participates in the dysregulated glycolysis of myofibroblasts remains unknown,” they said.
In a study published in the International Journal of Biological Macromolecules, the researchers examined adeno-associated virus serotype 6 (AAV6)-mediated DACT2 overexpression in experimental pulmonary fibrosis using mouse models.
The researchers injected AAV6 vectors into the lungs of mice to overexpress DACT2. The DACT2 overexpression “effectively attenuated both bleomycin-induced and AdTGF-beta-1-induced pulmonary fibrosis murine models in vivo, as evidenced by the alleviation of myofibroblast differentiation and collagen accumulation,” they said.
They found that overexpression of DACT2 was associated with glucose uptake, extracellular acidification rate, intracellular adenosine-triphosphate (ATP) level, and lactate levels of myofibroblasts.
The researchers also conducted in vitro experiments in which they treated lung fibroblasts with cycloheximide (CHX), a protein synthesis inhibitor. These experiments showed that DACT2 inhibited differentiation of lung myofibroblasts by downregulating lactate dehydrogenase A (LDHA), which caused suppression of glycolysis in myofibroblasts.
“Aerobic glycolysis is an important method of energy generation, and several studies have shown that enhanced glycolysis facilitates the progression of pulmonary fibrosis,” the researchers wrote in their discussion.
More research is needed outside of mouse models and in vitro studies, but the current study is the first known to explore the relationship between DACT2 and LDHA in pulmonary fibrosis, and the results provide evidence of the potential benefits of DACT2 in treating lung disorders, the researchers wrote.
“We hope this research will lay the theoretical foundation for finding novel therapeutics to alleviate or reverse the development of pulmonary fibrosis and other chronic lung disorders,” they concluded.
The study was supported by the National Natural Science Foundation of China and the Regional Joint Fund-Youth Fund projects of Guangdong Province. The researchers had no financial conflicts to disclose.
Dapper homolog 2 attenuated pulmonary fibrosis development and suppressed glycosis in myofibroblasts, suggesting potential as a therapeutic target for idiopathic pulmonary fibrosis, based on data from mouse models.
Idiopathic pulmonary fibrosis (IPF) remains a challenge with poor prognosis, and current therapeutic options are limited, wrote Xiaofan Lai, of Sun Yat-sen University, Guangzhou, China, and colleagues. Previous studies suggest that myofibroblasts are key contributors to fibrosis in IPF, they said.
Dishevelled-associated antagonist of beta-catenin 2 (DACT2) is an antagonist in the DACT gene family and associated with tissue development and injury, but its function and potential therapeutic role in IPF has not been explored; specifically, “whether DACT2 participates in the dysregulated glycolysis of myofibroblasts remains unknown,” they said.
In a study published in the International Journal of Biological Macromolecules, the researchers examined adeno-associated virus serotype 6 (AAV6)-mediated DACT2 overexpression in experimental pulmonary fibrosis using mouse models.
The researchers injected AAV6 vectors into the lungs of mice to overexpress DACT2. The DACT2 overexpression “effectively attenuated both bleomycin-induced and AdTGF-beta-1-induced pulmonary fibrosis murine models in vivo, as evidenced by the alleviation of myofibroblast differentiation and collagen accumulation,” they said.
They found that overexpression of DACT2 was associated with glucose uptake, extracellular acidification rate, intracellular adenosine-triphosphate (ATP) level, and lactate levels of myofibroblasts.
The researchers also conducted in vitro experiments in which they treated lung fibroblasts with cycloheximide (CHX), a protein synthesis inhibitor. These experiments showed that DACT2 inhibited differentiation of lung myofibroblasts by downregulating lactate dehydrogenase A (LDHA), which caused suppression of glycolysis in myofibroblasts.
“Aerobic glycolysis is an important method of energy generation, and several studies have shown that enhanced glycolysis facilitates the progression of pulmonary fibrosis,” the researchers wrote in their discussion.
More research is needed outside of mouse models and in vitro studies, but the current study is the first known to explore the relationship between DACT2 and LDHA in pulmonary fibrosis, and the results provide evidence of the potential benefits of DACT2 in treating lung disorders, the researchers wrote.
“We hope this research will lay the theoretical foundation for finding novel therapeutics to alleviate or reverse the development of pulmonary fibrosis and other chronic lung disorders,” they concluded.
The study was supported by the National Natural Science Foundation of China and the Regional Joint Fund-Youth Fund projects of Guangdong Province. The researchers had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES
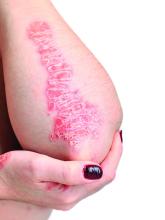
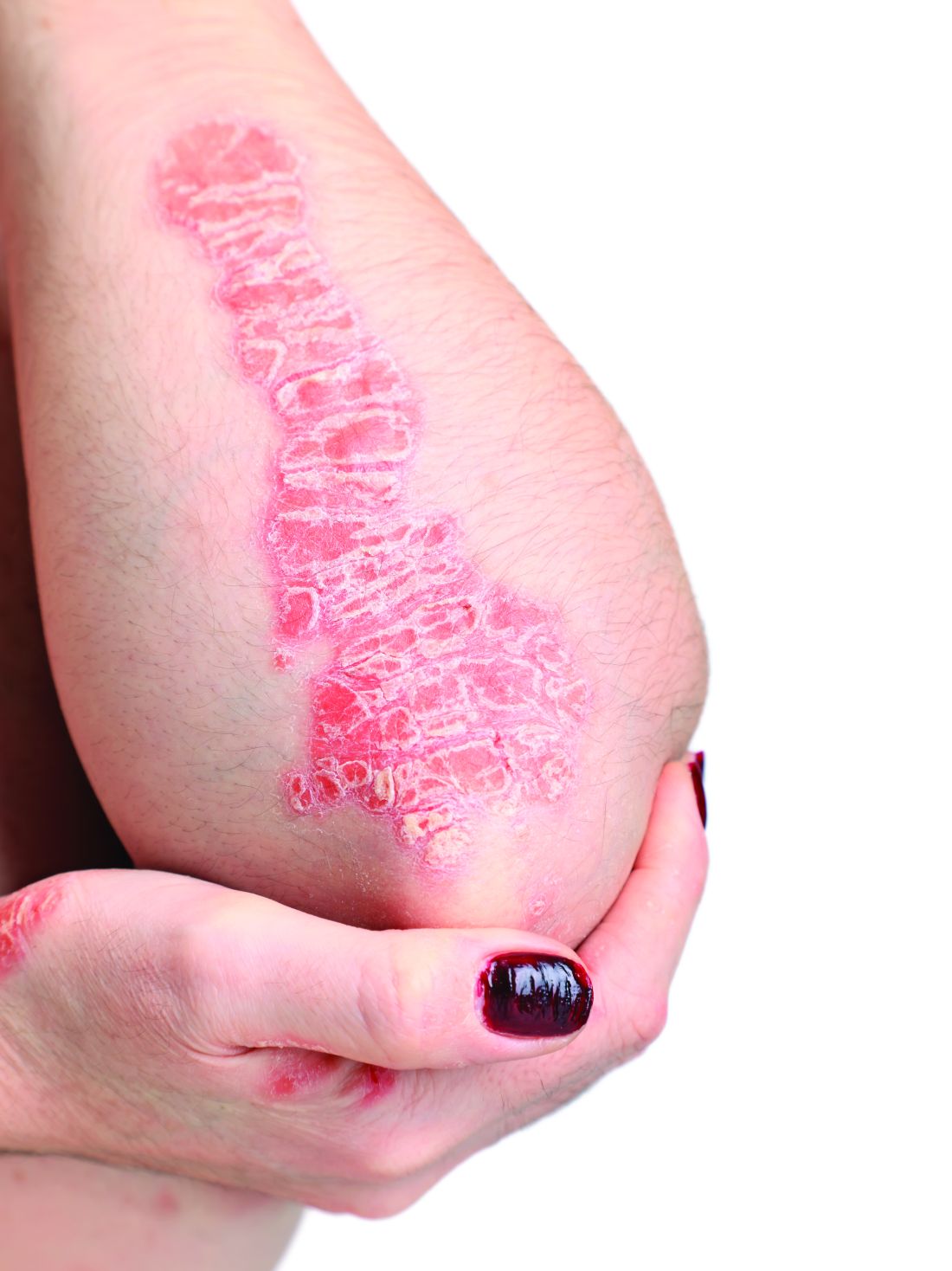
Topical psoriasis treatments
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Fat-free mass index tied to outcomes in underweight COPD patients
Higher fat-free mass was tied to exercise outcomes in patients with chronic obstructive pulmonary disease who were underweight but not in those who were obese or nearly obese, based on data from more than 2,000 individuals.
Change in body composition, including a lower fat-free mass index (FFMI), often occurs in patients with COPD irrespective of body weight, write Felipe V.C. Machado, MSc, of Maastricht University Medical Center, the Netherlands, and colleagues.
However, the impact of changes in FFMI on outcomes including exercise capacity, health-related quality of life (HRQL), and systemic inflammation in patients with COPD stratified by BMI has not been well studied, they said.
In a study published in the journal CHEST, the researchers reviewed data from the COPD and Systemic Consequences – Comorbidities Network (COSYCONET) cohort. The study population included 2,137 adults with COPD (mean age 65 years; 61% men). Patients were divided into four groups based on weight: underweight (UW), normal weight (NW), pre-obese (PO), and obese (OB). These groups accounted for 12.3%, 31.3%, 39.6%, and 16.8%, respectively, of the study population.
Exercise capacity was assessed using the 6-minute walk distance test (6MWD), health-related quality of life was assessed using the Saint George’s Respiratory Questionnaire for COPD, and systemic inflammation was assessed using blood markers including white blood cell (WBC) count and C-reactive protein (CRP). Body composition was assessed using bioelectrical impedance analysis (BIA).
Overall, the frequency of low FFMI decreased from lower to higher BMI groups, occurring in 81% of UW patients, 53% of NW patients, 42% of PO patients, and 39% of OB patients.
Notably, after adjusting for multiple variables, after controlling for lung function (forced expiratory volume in 1 second – FEV1), the researchers wrote.
However, compared with the other BMI groups, NW patients with high FFMI showed the greatest exercise capacity and health-related quality of life on average, with the lowest degree of airflow limitation (FEV1, 59.5), lowest proportion of patients with mMRC greater than 2 (27%), highest levels of physical activity (International Physical Activity Questionnaire score), best exercise capacity (6MWD, 77) and highest HRQL (SGRQ total score 37).
Body composition was associated differently with exercise capacity, HRQL, and systemic inflammation according to BMI group, the researchers write in their discussion. “We found that stratification using BMI allowed discrimination of groups of patients with COPD who showed slight but significant differences in lung function, exercise capacity, HRQL and systemic inflammation,” they say.
The findings were limited by several factors, including the use of BIA for body composition, which may be subject to hydration and fed conditions, the researchers noted. Other limitations included the use of reference values from a general population sample younger than much of the study population and the cross-sectional design that does not prove causality, the researchers noted.
However, the results support those of previous studies and suggest that normal weight and high FFMI is the most favorable combination to promote positive outcomes in COPD, they conclude. “Clinicians and researchers should consider screening patients with COPD for body composition abnormalities through a combination of BMI and FFMI classifications rather than each of the two indexes alone,” they say.
The COSYCONET study is supported by the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET) in collaboration with the German Center for Lung Research (DZL). The study also was funded by AstraZeneca, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma, Chiesi, GlaxoSmithKline, and multiple other pharmaceutical companies.
Mr. Machado disclosed financial support from ZonMW.
A version of this article first appeared on Medscape.com.
Higher fat-free mass was tied to exercise outcomes in patients with chronic obstructive pulmonary disease who were underweight but not in those who were obese or nearly obese, based on data from more than 2,000 individuals.
Change in body composition, including a lower fat-free mass index (FFMI), often occurs in patients with COPD irrespective of body weight, write Felipe V.C. Machado, MSc, of Maastricht University Medical Center, the Netherlands, and colleagues.
However, the impact of changes in FFMI on outcomes including exercise capacity, health-related quality of life (HRQL), and systemic inflammation in patients with COPD stratified by BMI has not been well studied, they said.
In a study published in the journal CHEST, the researchers reviewed data from the COPD and Systemic Consequences – Comorbidities Network (COSYCONET) cohort. The study population included 2,137 adults with COPD (mean age 65 years; 61% men). Patients were divided into four groups based on weight: underweight (UW), normal weight (NW), pre-obese (PO), and obese (OB). These groups accounted for 12.3%, 31.3%, 39.6%, and 16.8%, respectively, of the study population.
Exercise capacity was assessed using the 6-minute walk distance test (6MWD), health-related quality of life was assessed using the Saint George’s Respiratory Questionnaire for COPD, and systemic inflammation was assessed using blood markers including white blood cell (WBC) count and C-reactive protein (CRP). Body composition was assessed using bioelectrical impedance analysis (BIA).
Overall, the frequency of low FFMI decreased from lower to higher BMI groups, occurring in 81% of UW patients, 53% of NW patients, 42% of PO patients, and 39% of OB patients.
Notably, after adjusting for multiple variables, after controlling for lung function (forced expiratory volume in 1 second – FEV1), the researchers wrote.
However, compared with the other BMI groups, NW patients with high FFMI showed the greatest exercise capacity and health-related quality of life on average, with the lowest degree of airflow limitation (FEV1, 59.5), lowest proportion of patients with mMRC greater than 2 (27%), highest levels of physical activity (International Physical Activity Questionnaire score), best exercise capacity (6MWD, 77) and highest HRQL (SGRQ total score 37).
Body composition was associated differently with exercise capacity, HRQL, and systemic inflammation according to BMI group, the researchers write in their discussion. “We found that stratification using BMI allowed discrimination of groups of patients with COPD who showed slight but significant differences in lung function, exercise capacity, HRQL and systemic inflammation,” they say.
The findings were limited by several factors, including the use of BIA for body composition, which may be subject to hydration and fed conditions, the researchers noted. Other limitations included the use of reference values from a general population sample younger than much of the study population and the cross-sectional design that does not prove causality, the researchers noted.
However, the results support those of previous studies and suggest that normal weight and high FFMI is the most favorable combination to promote positive outcomes in COPD, they conclude. “Clinicians and researchers should consider screening patients with COPD for body composition abnormalities through a combination of BMI and FFMI classifications rather than each of the two indexes alone,” they say.
The COSYCONET study is supported by the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET) in collaboration with the German Center for Lung Research (DZL). The study also was funded by AstraZeneca, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma, Chiesi, GlaxoSmithKline, and multiple other pharmaceutical companies.
Mr. Machado disclosed financial support from ZonMW.
A version of this article first appeared on Medscape.com.
Higher fat-free mass was tied to exercise outcomes in patients with chronic obstructive pulmonary disease who were underweight but not in those who were obese or nearly obese, based on data from more than 2,000 individuals.
Change in body composition, including a lower fat-free mass index (FFMI), often occurs in patients with COPD irrespective of body weight, write Felipe V.C. Machado, MSc, of Maastricht University Medical Center, the Netherlands, and colleagues.
However, the impact of changes in FFMI on outcomes including exercise capacity, health-related quality of life (HRQL), and systemic inflammation in patients with COPD stratified by BMI has not been well studied, they said.
In a study published in the journal CHEST, the researchers reviewed data from the COPD and Systemic Consequences – Comorbidities Network (COSYCONET) cohort. The study population included 2,137 adults with COPD (mean age 65 years; 61% men). Patients were divided into four groups based on weight: underweight (UW), normal weight (NW), pre-obese (PO), and obese (OB). These groups accounted for 12.3%, 31.3%, 39.6%, and 16.8%, respectively, of the study population.
Exercise capacity was assessed using the 6-minute walk distance test (6MWD), health-related quality of life was assessed using the Saint George’s Respiratory Questionnaire for COPD, and systemic inflammation was assessed using blood markers including white blood cell (WBC) count and C-reactive protein (CRP). Body composition was assessed using bioelectrical impedance analysis (BIA).
Overall, the frequency of low FFMI decreased from lower to higher BMI groups, occurring in 81% of UW patients, 53% of NW patients, 42% of PO patients, and 39% of OB patients.
Notably, after adjusting for multiple variables, after controlling for lung function (forced expiratory volume in 1 second – FEV1), the researchers wrote.
However, compared with the other BMI groups, NW patients with high FFMI showed the greatest exercise capacity and health-related quality of life on average, with the lowest degree of airflow limitation (FEV1, 59.5), lowest proportion of patients with mMRC greater than 2 (27%), highest levels of physical activity (International Physical Activity Questionnaire score), best exercise capacity (6MWD, 77) and highest HRQL (SGRQ total score 37).
Body composition was associated differently with exercise capacity, HRQL, and systemic inflammation according to BMI group, the researchers write in their discussion. “We found that stratification using BMI allowed discrimination of groups of patients with COPD who showed slight but significant differences in lung function, exercise capacity, HRQL and systemic inflammation,” they say.
The findings were limited by several factors, including the use of BIA for body composition, which may be subject to hydration and fed conditions, the researchers noted. Other limitations included the use of reference values from a general population sample younger than much of the study population and the cross-sectional design that does not prove causality, the researchers noted.
However, the results support those of previous studies and suggest that normal weight and high FFMI is the most favorable combination to promote positive outcomes in COPD, they conclude. “Clinicians and researchers should consider screening patients with COPD for body composition abnormalities through a combination of BMI and FFMI classifications rather than each of the two indexes alone,” they say.
The COSYCONET study is supported by the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET) in collaboration with the German Center for Lung Research (DZL). The study also was funded by AstraZeneca, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma, Chiesi, GlaxoSmithKline, and multiple other pharmaceutical companies.
Mr. Machado disclosed financial support from ZonMW.
A version of this article first appeared on Medscape.com.
From the Journal CHEST
Serum trace metals relate to lower risk of sleep disorders
, based on data from 3,660 individuals.
Previous research has shown an association between trace metals and sleep and sleep patterns, but data on the impact of serum trace metals on sleep disorders have been limited, wrote Ming-Gang Deng, MD, of Wuhan (China) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) 2011-2016 to calculate the odds ratios of sleep disorders and serum zinc (Zn), copper (Cu), and selenium (Se). The study population included adults aged 18 years and older, with an average age of 47.6 years. Approximately half of the participants were men, and the majority was non-Hispanic white. Serum Zn, Cu, and Se were identified at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health. The lower limits of detection for Zn, Cu, and Se were 2.9 mcg/dL, 2.5 mcg/dL, and 4.5 mcg/L, respectively. Sleep disorders were assessed based on self-reports of discussions with health professionals about sleep disorders, and via the Sleep Disorder Questionnaire.
After adjusting for sociodemographic, behavioral characteristics, and health characteristics, adults in the highest tertiles of serum Zn had a 30% reduced risk of sleep disorders, compared with those in the lowest tertiles of serum Zn (odds ratio, 0.70; P = .035). In measures of trace metals ratios, serum Zn/Cu and Zn/Se also were significantly associated with reduced risk of sleep disorders for individuals in the highest tertiles, compared with those in the lowest tertiles (OR, 0.62 and OR, 0.68, respectively).
However, serum Cu, Se and Cu/Se were not associated with sleep disorder risk.
Sociodemographic factors included age, sex, race, education level, family income level; behavioral characteristics included smoking, alcohol consumption, physical activity, and caffeine intake.
The researchers also used a restricted cubic spline model to examine the dose-response relationships between serum trace metals, serum trace metals ratios, and sleep disorders. In this analysis, higher levels of serum Zn, Zn/Cu, and Zn/Se were related to reduced risk of sleep disorders, while no significant association appeared between serum Cu, Se, or Cu/Se and sleep disorders risk.
The findings showing a lack of association between Se and sleep disorders were not consistent with previous studies, the researchers wrote in their discussion. Previous research has shown that a higher Se was less likely to be associated with trouble falling asleep, and has shown a potential treatment effect of Se on obstructive sleep apnea, they said.
“Although serum Cu and Se levels were not correlated to sleep disorders in our study, the Zn/Cu and Zn/Se may provide some novel insights,” they wrote. For example, Zn/Cu has been used as a predictor of several clinical complications related to an increased risk of sleep disorders including cardiovascular disease, cancer, and major depressive disorder, they noted.
The findings were limited by several factors including the cross-sectional design, use of self-reports, and the inability to examine relationships between trace metals and specific sleep disorder symptoms, such as restless legs syndrome, insomnia, and obstructive sleep apnea, the researchers noted.
However, the results were strengthened by the large national sample, and support data from previous studies, they said.
“The inverse associations of serum Zn, and Zn/Cu, Zn/Se with sleep disorders enlightened us that increasing Zn intake may be an excellent approach to prevent sleep disorders due to its benefits from these three aspects,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, based on data from 3,660 individuals.
Previous research has shown an association between trace metals and sleep and sleep patterns, but data on the impact of serum trace metals on sleep disorders have been limited, wrote Ming-Gang Deng, MD, of Wuhan (China) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) 2011-2016 to calculate the odds ratios of sleep disorders and serum zinc (Zn), copper (Cu), and selenium (Se). The study population included adults aged 18 years and older, with an average age of 47.6 years. Approximately half of the participants were men, and the majority was non-Hispanic white. Serum Zn, Cu, and Se were identified at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health. The lower limits of detection for Zn, Cu, and Se were 2.9 mcg/dL, 2.5 mcg/dL, and 4.5 mcg/L, respectively. Sleep disorders were assessed based on self-reports of discussions with health professionals about sleep disorders, and via the Sleep Disorder Questionnaire.
After adjusting for sociodemographic, behavioral characteristics, and health characteristics, adults in the highest tertiles of serum Zn had a 30% reduced risk of sleep disorders, compared with those in the lowest tertiles of serum Zn (odds ratio, 0.70; P = .035). In measures of trace metals ratios, serum Zn/Cu and Zn/Se also were significantly associated with reduced risk of sleep disorders for individuals in the highest tertiles, compared with those in the lowest tertiles (OR, 0.62 and OR, 0.68, respectively).
However, serum Cu, Se and Cu/Se were not associated with sleep disorder risk.
Sociodemographic factors included age, sex, race, education level, family income level; behavioral characteristics included smoking, alcohol consumption, physical activity, and caffeine intake.
The researchers also used a restricted cubic spline model to examine the dose-response relationships between serum trace metals, serum trace metals ratios, and sleep disorders. In this analysis, higher levels of serum Zn, Zn/Cu, and Zn/Se were related to reduced risk of sleep disorders, while no significant association appeared between serum Cu, Se, or Cu/Se and sleep disorders risk.
The findings showing a lack of association between Se and sleep disorders were not consistent with previous studies, the researchers wrote in their discussion. Previous research has shown that a higher Se was less likely to be associated with trouble falling asleep, and has shown a potential treatment effect of Se on obstructive sleep apnea, they said.
“Although serum Cu and Se levels were not correlated to sleep disorders in our study, the Zn/Cu and Zn/Se may provide some novel insights,” they wrote. For example, Zn/Cu has been used as a predictor of several clinical complications related to an increased risk of sleep disorders including cardiovascular disease, cancer, and major depressive disorder, they noted.
The findings were limited by several factors including the cross-sectional design, use of self-reports, and the inability to examine relationships between trace metals and specific sleep disorder symptoms, such as restless legs syndrome, insomnia, and obstructive sleep apnea, the researchers noted.
However, the results were strengthened by the large national sample, and support data from previous studies, they said.
“The inverse associations of serum Zn, and Zn/Cu, Zn/Se with sleep disorders enlightened us that increasing Zn intake may be an excellent approach to prevent sleep disorders due to its benefits from these three aspects,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, based on data from 3,660 individuals.
Previous research has shown an association between trace metals and sleep and sleep patterns, but data on the impact of serum trace metals on sleep disorders have been limited, wrote Ming-Gang Deng, MD, of Wuhan (China) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) 2011-2016 to calculate the odds ratios of sleep disorders and serum zinc (Zn), copper (Cu), and selenium (Se). The study population included adults aged 18 years and older, with an average age of 47.6 years. Approximately half of the participants were men, and the majority was non-Hispanic white. Serum Zn, Cu, and Se were identified at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health. The lower limits of detection for Zn, Cu, and Se were 2.9 mcg/dL, 2.5 mcg/dL, and 4.5 mcg/L, respectively. Sleep disorders were assessed based on self-reports of discussions with health professionals about sleep disorders, and via the Sleep Disorder Questionnaire.
After adjusting for sociodemographic, behavioral characteristics, and health characteristics, adults in the highest tertiles of serum Zn had a 30% reduced risk of sleep disorders, compared with those in the lowest tertiles of serum Zn (odds ratio, 0.70; P = .035). In measures of trace metals ratios, serum Zn/Cu and Zn/Se also were significantly associated with reduced risk of sleep disorders for individuals in the highest tertiles, compared with those in the lowest tertiles (OR, 0.62 and OR, 0.68, respectively).
However, serum Cu, Se and Cu/Se were not associated with sleep disorder risk.
Sociodemographic factors included age, sex, race, education level, family income level; behavioral characteristics included smoking, alcohol consumption, physical activity, and caffeine intake.
The researchers also used a restricted cubic spline model to examine the dose-response relationships between serum trace metals, serum trace metals ratios, and sleep disorders. In this analysis, higher levels of serum Zn, Zn/Cu, and Zn/Se were related to reduced risk of sleep disorders, while no significant association appeared between serum Cu, Se, or Cu/Se and sleep disorders risk.
The findings showing a lack of association between Se and sleep disorders were not consistent with previous studies, the researchers wrote in their discussion. Previous research has shown that a higher Se was less likely to be associated with trouble falling asleep, and has shown a potential treatment effect of Se on obstructive sleep apnea, they said.
“Although serum Cu and Se levels were not correlated to sleep disorders in our study, the Zn/Cu and Zn/Se may provide some novel insights,” they wrote. For example, Zn/Cu has been used as a predictor of several clinical complications related to an increased risk of sleep disorders including cardiovascular disease, cancer, and major depressive disorder, they noted.
The findings were limited by several factors including the cross-sectional design, use of self-reports, and the inability to examine relationships between trace metals and specific sleep disorder symptoms, such as restless legs syndrome, insomnia, and obstructive sleep apnea, the researchers noted.
However, the results were strengthened by the large national sample, and support data from previous studies, they said.
“The inverse associations of serum Zn, and Zn/Cu, Zn/Se with sleep disorders enlightened us that increasing Zn intake may be an excellent approach to prevent sleep disorders due to its benefits from these three aspects,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Sleep-disordered breathing promotes elevated arterial stiffness and preeclampsia
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
FDA will review pediatric indication for roflumilast cream
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
Severe OSA tied to poor prognoses in stroke patients
Patients with acute ischemic stroke had a worse prognosis if they had also experienced severe obstructive sleep apnea (OSA), based on data from 125 individuals.
OSA is on the rise, and is associated with pathophysiological changes, and data from previous studies suggest that severe OSA doubles the risk of stroke and increases risk of stroke recurrence, according to Juan Xu, PhD, of Soochow University, Suzhou, China, and colleagues.
“There is a high comorbidity between stroke and OSA,” and effective sleep is important to cerebral function recovery, the researchers wrote. Early prediction of stroke prognosis may inform treatment in stroke patients, but the value of OSA as a predictor of functional prognosis has not been explored.
In a study published in Sleep Medicine, the researchers analyzed data from 125 adults with mild to moderate ischemic stroke and OSA. The participants underwent polysomnography within a week of stroke onset between January 2015 and June 2020 and were grouped by severity according to apnea-hypopnea index (AHI) of either less than 30/h (not severe) or 30/h or higher (severe). The mean age of the patients was 58 years, and 87% were men. Approximately one-third of the participants met the criteria for severe OSA.
The researchers assessed the impact of OSA on functional prognosis in the acute phase of stroke, and reviewed quantitative electroencephalography (EEG) markers in stroke patients during sleep.
Overall, individuals with severe OSA were significantly more likely than those with less severe OSA to have comorbid hypertension (85.4% vs. 56%; P = .002) and a higher body mass index (28 vs. 24; P < .001). Other factors including blood pressure, smoking history, alcohol use, and comorbid diabetes were similar between the groups.
Quantitative EEG among patients with severe OSA showed lower relative power of high-frequency bands (alpha, beta, and sigma). The EEG also showed higher delta/alpha power ratio and slowing ratio, and higher delta relative power (delta RP) in severe OSA (P < .05 for all).
In addition, severe OSA was associated with more than triple the risk (3.6-fold increase) of poor prognosis, defined as a Modified Rankin Scale score of 3 or higher (24.4% for severe OSA vs. 8.3% for nonsevere OSA; P = .03).
the researchers wrote. “Integrating the alteration of quantitative EEG parameters may improve the accuracy of early predictions of functional prognosis in patients with stroke.”
The findings were limited by several factors including the retrospective design and the lack of a sizable non-OSA control group, the researchers noted. Other limitations included the use of an AHI of 30/h or higher to define severity and the use of data from medical histories, with the potential for information bias, and the use of only 30-second continuous polysomnography segments.
However, the results suggest that increased delta RP and TSR, and decreased alpha, beta, and sigma RP, may be independent predictors of a poor functional prognosis in stroke patients with OSA, and that the prognosis could be improved by treating the OSA, they concluded.
The study was supported by the Natural Science Foundation of China and the Discipline Construction Program of the Second Affiliated Hospital of Soochow University. The researchers reported no financial conflicts.
A version of this article first appeared on Medscape.com.
Patients with acute ischemic stroke had a worse prognosis if they had also experienced severe obstructive sleep apnea (OSA), based on data from 125 individuals.
OSA is on the rise, and is associated with pathophysiological changes, and data from previous studies suggest that severe OSA doubles the risk of stroke and increases risk of stroke recurrence, according to Juan Xu, PhD, of Soochow University, Suzhou, China, and colleagues.
“There is a high comorbidity between stroke and OSA,” and effective sleep is important to cerebral function recovery, the researchers wrote. Early prediction of stroke prognosis may inform treatment in stroke patients, but the value of OSA as a predictor of functional prognosis has not been explored.
In a study published in Sleep Medicine, the researchers analyzed data from 125 adults with mild to moderate ischemic stroke and OSA. The participants underwent polysomnography within a week of stroke onset between January 2015 and June 2020 and were grouped by severity according to apnea-hypopnea index (AHI) of either less than 30/h (not severe) or 30/h or higher (severe). The mean age of the patients was 58 years, and 87% were men. Approximately one-third of the participants met the criteria for severe OSA.
The researchers assessed the impact of OSA on functional prognosis in the acute phase of stroke, and reviewed quantitative electroencephalography (EEG) markers in stroke patients during sleep.
Overall, individuals with severe OSA were significantly more likely than those with less severe OSA to have comorbid hypertension (85.4% vs. 56%; P = .002) and a higher body mass index (28 vs. 24; P < .001). Other factors including blood pressure, smoking history, alcohol use, and comorbid diabetes were similar between the groups.
Quantitative EEG among patients with severe OSA showed lower relative power of high-frequency bands (alpha, beta, and sigma). The EEG also showed higher delta/alpha power ratio and slowing ratio, and higher delta relative power (delta RP) in severe OSA (P < .05 for all).
In addition, severe OSA was associated with more than triple the risk (3.6-fold increase) of poor prognosis, defined as a Modified Rankin Scale score of 3 or higher (24.4% for severe OSA vs. 8.3% for nonsevere OSA; P = .03).
the researchers wrote. “Integrating the alteration of quantitative EEG parameters may improve the accuracy of early predictions of functional prognosis in patients with stroke.”
The findings were limited by several factors including the retrospective design and the lack of a sizable non-OSA control group, the researchers noted. Other limitations included the use of an AHI of 30/h or higher to define severity and the use of data from medical histories, with the potential for information bias, and the use of only 30-second continuous polysomnography segments.
However, the results suggest that increased delta RP and TSR, and decreased alpha, beta, and sigma RP, may be independent predictors of a poor functional prognosis in stroke patients with OSA, and that the prognosis could be improved by treating the OSA, they concluded.
The study was supported by the Natural Science Foundation of China and the Discipline Construction Program of the Second Affiliated Hospital of Soochow University. The researchers reported no financial conflicts.
A version of this article first appeared on Medscape.com.
Patients with acute ischemic stroke had a worse prognosis if they had also experienced severe obstructive sleep apnea (OSA), based on data from 125 individuals.
OSA is on the rise, and is associated with pathophysiological changes, and data from previous studies suggest that severe OSA doubles the risk of stroke and increases risk of stroke recurrence, according to Juan Xu, PhD, of Soochow University, Suzhou, China, and colleagues.
“There is a high comorbidity between stroke and OSA,” and effective sleep is important to cerebral function recovery, the researchers wrote. Early prediction of stroke prognosis may inform treatment in stroke patients, but the value of OSA as a predictor of functional prognosis has not been explored.
In a study published in Sleep Medicine, the researchers analyzed data from 125 adults with mild to moderate ischemic stroke and OSA. The participants underwent polysomnography within a week of stroke onset between January 2015 and June 2020 and were grouped by severity according to apnea-hypopnea index (AHI) of either less than 30/h (not severe) or 30/h or higher (severe). The mean age of the patients was 58 years, and 87% were men. Approximately one-third of the participants met the criteria for severe OSA.
The researchers assessed the impact of OSA on functional prognosis in the acute phase of stroke, and reviewed quantitative electroencephalography (EEG) markers in stroke patients during sleep.
Overall, individuals with severe OSA were significantly more likely than those with less severe OSA to have comorbid hypertension (85.4% vs. 56%; P = .002) and a higher body mass index (28 vs. 24; P < .001). Other factors including blood pressure, smoking history, alcohol use, and comorbid diabetes were similar between the groups.
Quantitative EEG among patients with severe OSA showed lower relative power of high-frequency bands (alpha, beta, and sigma). The EEG also showed higher delta/alpha power ratio and slowing ratio, and higher delta relative power (delta RP) in severe OSA (P < .05 for all).
In addition, severe OSA was associated with more than triple the risk (3.6-fold increase) of poor prognosis, defined as a Modified Rankin Scale score of 3 or higher (24.4% for severe OSA vs. 8.3% for nonsevere OSA; P = .03).
the researchers wrote. “Integrating the alteration of quantitative EEG parameters may improve the accuracy of early predictions of functional prognosis in patients with stroke.”
The findings were limited by several factors including the retrospective design and the lack of a sizable non-OSA control group, the researchers noted. Other limitations included the use of an AHI of 30/h or higher to define severity and the use of data from medical histories, with the potential for information bias, and the use of only 30-second continuous polysomnography segments.
However, the results suggest that increased delta RP and TSR, and decreased alpha, beta, and sigma RP, may be independent predictors of a poor functional prognosis in stroke patients with OSA, and that the prognosis could be improved by treating the OSA, they concluded.
The study was supported by the Natural Science Foundation of China and the Discipline Construction Program of the Second Affiliated Hospital of Soochow University. The researchers reported no financial conflicts.
A version of this article first appeared on Medscape.com.
FROM SLEEP MEDICINE
Bite-sized bouts of exercise: Why they are valuable and what they are missing
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Mothers’ sleep issues promote poor outcomes for infants
or insomnia, based on data from approximately 5,000 infants.
Sleep disturbance is common during pregnancy, and “sleep disorders during pregnancy can have significant consequences for both the pregnant person and their infant,” write Jennifer N. Felder, PhD, of the University of California, San Francisco, and colleagues.
However, data on the impact of maternal insomnia on specific infant outcomes are limited, they said.
In a study published recently in the journal Sleep Health, the researchers reviewed data from 3,371 pregnant women diagnosed with sleep apnea and 3,213 with insomnia. Of these, 2,357 and 2,212 were matched with controls in a propensity-score analysis. The referent controls were matched for maternal characteristics, obstetric factors, and infant factors among individuals without a sleep disorder. All were singleton pregnancies.
Adverse infant outcomes included the following:
- One- and 5-minute Apgar scores less than 7.
- Respiratory distress syndrome.
- Neonatal intensive care unit admission.
- Hypoglycemia.
- Infant death.
- Hospital stay of longer than 2 days for vaginal delivery or longer than 4 days for cesarean delivery.
- Emergency department visit before 3 months of age.
- Emergency department visit in the first year of life.
- Composite measure of adverse infant outcomes.
Compared with matched controls, the infants born to mothers with sleep apnea had a significantly increased risk for any adverse outcome (50.1% vs. 53.5%) and of the specific outcomes of low 1-minute Apgar scores (6.3% vs. 9.6%), neonatal ICU stays (6.3% vs. 8.4%), and an emergency department visit in the first year of life (33.6% vs. 36.9%).
For infants born to mothers with insomnia, the only significant difference in outcomes compared with controls was an increased likelihood of an emergency department visit (37.2% vs. 32.3%).
“Research on possible mechanisms of the relation between maternal prenatal sleep apnea and poorer birth and infant outcomes associations is small but growing, implicating systemic inflammation and late or prolonged fetal heart rate decelerations,” the researchers write in their discussion.
Research on insomnia during pregnancy and adverse infant outcomes is limited, and the largest studies have been complicated by the effects of insomnia medication; therefore, “our finding that infants born to mothers with an insomnia diagnosis were at increased risk of only emergency room visit, but no other analyzed infant outcomes, is important and novel,” they note.
The findings were limited by several factors, including the reliance on medical records, which may lack details on how routinely health care professionals assessed sleep disorders, the researchers noted. “Consequently, the findings presented here may reflect more severe cases of insomnia and sleep apnea, and may not represent the population of individuals with diagnosed sleep apnea or insomnia during pregnancy generally,” the authors say. Other limitations included a lack of information on treatment of sleep disorders and on the timing of diagnosis (before pregnancy or during pregnancy).
However, the results were strengthened by the large, population-based sample and use of codes to highlight research questions, the researchers said.
In light of the health consequences of sleep disorders in pregnancy, the data suggest that sleep apnea and insomnia in pregnant women may serve as targets for risk assessment of adverse infant outcomes, and more research is needed to determine whether addressing sleep issues reduces these outcomes, they concluded.
The study was supported by the University of California, San Francisco, Preterm Birth Initiative and by grants to lead author Dr. Felder from the National Center for Complementary and Integrative Health and to a coauthor from the National Heart, Lung, and Blood Institute. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or insomnia, based on data from approximately 5,000 infants.
Sleep disturbance is common during pregnancy, and “sleep disorders during pregnancy can have significant consequences for both the pregnant person and their infant,” write Jennifer N. Felder, PhD, of the University of California, San Francisco, and colleagues.
However, data on the impact of maternal insomnia on specific infant outcomes are limited, they said.
In a study published recently in the journal Sleep Health, the researchers reviewed data from 3,371 pregnant women diagnosed with sleep apnea and 3,213 with insomnia. Of these, 2,357 and 2,212 were matched with controls in a propensity-score analysis. The referent controls were matched for maternal characteristics, obstetric factors, and infant factors among individuals without a sleep disorder. All were singleton pregnancies.
Adverse infant outcomes included the following:
- One- and 5-minute Apgar scores less than 7.
- Respiratory distress syndrome.
- Neonatal intensive care unit admission.
- Hypoglycemia.
- Infant death.
- Hospital stay of longer than 2 days for vaginal delivery or longer than 4 days for cesarean delivery.
- Emergency department visit before 3 months of age.
- Emergency department visit in the first year of life.
- Composite measure of adverse infant outcomes.
Compared with matched controls, the infants born to mothers with sleep apnea had a significantly increased risk for any adverse outcome (50.1% vs. 53.5%) and of the specific outcomes of low 1-minute Apgar scores (6.3% vs. 9.6%), neonatal ICU stays (6.3% vs. 8.4%), and an emergency department visit in the first year of life (33.6% vs. 36.9%).
For infants born to mothers with insomnia, the only significant difference in outcomes compared with controls was an increased likelihood of an emergency department visit (37.2% vs. 32.3%).
“Research on possible mechanisms of the relation between maternal prenatal sleep apnea and poorer birth and infant outcomes associations is small but growing, implicating systemic inflammation and late or prolonged fetal heart rate decelerations,” the researchers write in their discussion.
Research on insomnia during pregnancy and adverse infant outcomes is limited, and the largest studies have been complicated by the effects of insomnia medication; therefore, “our finding that infants born to mothers with an insomnia diagnosis were at increased risk of only emergency room visit, but no other analyzed infant outcomes, is important and novel,” they note.
The findings were limited by several factors, including the reliance on medical records, which may lack details on how routinely health care professionals assessed sleep disorders, the researchers noted. “Consequently, the findings presented here may reflect more severe cases of insomnia and sleep apnea, and may not represent the population of individuals with diagnosed sleep apnea or insomnia during pregnancy generally,” the authors say. Other limitations included a lack of information on treatment of sleep disorders and on the timing of diagnosis (before pregnancy or during pregnancy).
However, the results were strengthened by the large, population-based sample and use of codes to highlight research questions, the researchers said.
In light of the health consequences of sleep disorders in pregnancy, the data suggest that sleep apnea and insomnia in pregnant women may serve as targets for risk assessment of adverse infant outcomes, and more research is needed to determine whether addressing sleep issues reduces these outcomes, they concluded.
The study was supported by the University of California, San Francisco, Preterm Birth Initiative and by grants to lead author Dr. Felder from the National Center for Complementary and Integrative Health and to a coauthor from the National Heart, Lung, and Blood Institute. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or insomnia, based on data from approximately 5,000 infants.
Sleep disturbance is common during pregnancy, and “sleep disorders during pregnancy can have significant consequences for both the pregnant person and their infant,” write Jennifer N. Felder, PhD, of the University of California, San Francisco, and colleagues.
However, data on the impact of maternal insomnia on specific infant outcomes are limited, they said.
In a study published recently in the journal Sleep Health, the researchers reviewed data from 3,371 pregnant women diagnosed with sleep apnea and 3,213 with insomnia. Of these, 2,357 and 2,212 were matched with controls in a propensity-score analysis. The referent controls were matched for maternal characteristics, obstetric factors, and infant factors among individuals without a sleep disorder. All were singleton pregnancies.
Adverse infant outcomes included the following:
- One- and 5-minute Apgar scores less than 7.
- Respiratory distress syndrome.
- Neonatal intensive care unit admission.
- Hypoglycemia.
- Infant death.
- Hospital stay of longer than 2 days for vaginal delivery or longer than 4 days for cesarean delivery.
- Emergency department visit before 3 months of age.
- Emergency department visit in the first year of life.
- Composite measure of adverse infant outcomes.
Compared with matched controls, the infants born to mothers with sleep apnea had a significantly increased risk for any adverse outcome (50.1% vs. 53.5%) and of the specific outcomes of low 1-minute Apgar scores (6.3% vs. 9.6%), neonatal ICU stays (6.3% vs. 8.4%), and an emergency department visit in the first year of life (33.6% vs. 36.9%).
For infants born to mothers with insomnia, the only significant difference in outcomes compared with controls was an increased likelihood of an emergency department visit (37.2% vs. 32.3%).
“Research on possible mechanisms of the relation between maternal prenatal sleep apnea and poorer birth and infant outcomes associations is small but growing, implicating systemic inflammation and late or prolonged fetal heart rate decelerations,” the researchers write in their discussion.
Research on insomnia during pregnancy and adverse infant outcomes is limited, and the largest studies have been complicated by the effects of insomnia medication; therefore, “our finding that infants born to mothers with an insomnia diagnosis were at increased risk of only emergency room visit, but no other analyzed infant outcomes, is important and novel,” they note.
The findings were limited by several factors, including the reliance on medical records, which may lack details on how routinely health care professionals assessed sleep disorders, the researchers noted. “Consequently, the findings presented here may reflect more severe cases of insomnia and sleep apnea, and may not represent the population of individuals with diagnosed sleep apnea or insomnia during pregnancy generally,” the authors say. Other limitations included a lack of information on treatment of sleep disorders and on the timing of diagnosis (before pregnancy or during pregnancy).
However, the results were strengthened by the large, population-based sample and use of codes to highlight research questions, the researchers said.
In light of the health consequences of sleep disorders in pregnancy, the data suggest that sleep apnea and insomnia in pregnant women may serve as targets for risk assessment of adverse infant outcomes, and more research is needed to determine whether addressing sleep issues reduces these outcomes, they concluded.
The study was supported by the University of California, San Francisco, Preterm Birth Initiative and by grants to lead author Dr. Felder from the National Center for Complementary and Integrative Health and to a coauthor from the National Heart, Lung, and Blood Institute. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP HEALTH
FDA approves Idacio as eighth adalimumab biosimilar in U.S.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.