User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Estimating insulin resistance may help predict stroke, death in T2D
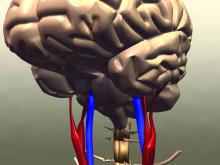
Calculating the estimated glucose disposal rate (eGDR) as a proxy for the level of insulin resistance may be useful way to determine if someone with type 2 diabetes (T2D) is at risk for having a first stroke, Swedish researchers have found.
In a large population-based study, the lower the eGDR score went, the higher the risk for having a first stroke became.
The eGDR score was also predictive of the chance of dying from any or a cardiovascular cause, Alexander Zabala, MD, reported at the annual meeting of the European Association for the Study of Diabetes (Abstract OP 01-4).
The link between insulin resistance and an increased risk for stroke has been known for some time, and not just in people with T2D. However, the current way of determining insulin resistance is not suitable for widespread practice.
“The goal standard technique for measuring insulin resistance is the euglycemic clamp method,” said Dr. Zabala, an internal medical resident at Södersjukhuset hospital and researcher at the Karolinska Institutet in Stockholm.
“For that reason, [the eGDR], a method based on readily available clinical factors – waist circumference, hypertension, and glycosylated hemoglobin was developed,” he explained. Body mass index can also be used in place of waist circumference, he qualified.
The eGDR has already been proven to be very precise in people with type 1 diabetes, said Dr. Zabala, and could be an “excellent tool to measure insulin resistance in a large patient population.”
Investigating the link between eGDR and first stroke risk
The aim of the study he presented was to see if changes in the eGDR were associated with changes in the risk of someone with T2D experiencing a first stroke, or dying from a cardiovascular or other cause.
An observational cohort was formed by first considering data on all adult patients with T2D who were logged in the Swedish National Diabetes Registry (NDR) during 2004-2016. Then anyone with a history of stroke, or with any missing data on the clinical variables needed to calculate the eGDR, were excluded.
This resulted in an overall population of 104,697 individuals, aged a mean of 63 years, who had developed T2D at around the age of 59 years. About 44% of the study population were women. The mean eGDR for the whole population was 5.6 mg/kg per min.
The study subjects were grouped according to four eGDR levels: 24,706 were in the lowest quartile of eGDR (less than 4 mg/kg per min), signifying the highest level of insulin resistance, and 18,762 were in the upper quartile of eGDR (greater than 8 mg/kg per min), signifying the lowest level of insulin resistance. The middle two groups had an eGDR between 4 and 6 mg/kg per min (40,187), and 6 and 8 mg/kg/min (21,042).
Data from the NDR were then combined with the Swedish Cause of Death register, the Swedish In-patient Care Diagnoses registry, and the Longitudinal Database for Health Insurance and Labour Market Studies (LISA) to determine the rates of stroke, ischemic stroke, hemorrhagic stroke, all-cause mortality, and cardiovascular mortality.
Increasing insulin resistance ups risk for stroke, death
After a median follow-up of 5.6 years, 4% (4,201) of the study population had had a stroke.
“We clearly see an increased occurrence of first-time stroke in the group with the lowest eGDR, indicating worst insulin resistance, in comparison with the group with the highest eGDR, indicating less insulin resistance,” Dr. Zabala reported.
After adjustment for potential confounding factors, including age at baseline, gender, diabetes duration, among other variables, the risk for stroke was lowest in those with a high eGDR value and highest for those with a low eGDR value.
Using individuals with the lowest eGDR (less than 4 mg/kg per min) and thus greatest risk of stroke as the reference, adjusted hazard ratios (aHR) for first-time stroke were: 0.60, 0.68, and 0.77 for those with an eGDR of greater than 8, 6-8, and 4-6 mg/kg per min, respectively.
The corresponding values for risk of ischemic stroke were 0.55, 0.68, and 0.75. Regarding hemorrhagic stroke, there was no statistically significant correlation between eGDR levels and stroke occurrence. This was due to the small number of cases recorded.
As for all-cause and cardiovascular mortality, a similar pattern was seen, with higher rates of death linked to increasing insulin resistance. Adjusted hazard ratios according to increasing insulin resistance (decreasing eGDR scores) for all-cause death were 0.68, 0.75, and 0.82 and for cardiovascular mortality were 0.65, 0.75, and 0.82.
A sensitivity analysis, using BMI instead of waist circumference to calculate the eGDR, showed a similar pattern, and “interestingly, a correlation between eGDR levels and risk of hemorrhagic stroke.” Dr. Zabala said.
Limitations and take-homes
Of course, this is an observational cohort study, so no conclusions on causality can be made and there are no data on the use of anti-diabetic treatments specifically. But there are strengths such as covering almost all adults with T2D in Sweden and a relatively long-follow-up time.
The findings suggest that “eGDR, which may reflect insulin resistance may be a useful risk marker for stroke and death in people with type 2 diabetes,” said Dr. Zabala.
“You had a very large cohort, and that certainly makes your results very valid,” observed Peter Novodvorsky, MUDr. (Hons), PhD, MRCP, a consultant diabetologist in Trenčín, Slovakia.
Dr. Novodvorsky, who chaired the session, picked up on the lack of information about how many people were taking newer diabetes drugs, such as the glucagon-like peptide 1 receptor antagonists and sodium glucose-lowering transport 2 inhibitors.
“As we all know, these might have protective effects which are not necessarily related to the glucose lowering or insulin resistance-lowering” effects, so could have influenced the results. In terms of how practical the eGDR is for clinical practice, Dr. Zabala observed in a press release: “eGDR could be used to help T2D patients better understand and manage their risk of stroke and death.
“It could also be of importance in research. In this era of personalized medicine, better stratification of type 2 diabetes patients will help optimize clinical trials and further vital research into treatment, diagnosis, care and prevention.”
The research was a collaboration between the Karolinska Institutet, Gothenburg University and the Swedish National Diabetes Registry. Dr. Zabala and coauthors reported having no conflicts of interest.
Calculating the estimated glucose disposal rate (eGDR) as a proxy for the level of insulin resistance may be useful way to determine if someone with type 2 diabetes (T2D) is at risk for having a first stroke, Swedish researchers have found.
In a large population-based study, the lower the eGDR score went, the higher the risk for having a first stroke became.
The eGDR score was also predictive of the chance of dying from any or a cardiovascular cause, Alexander Zabala, MD, reported at the annual meeting of the European Association for the Study of Diabetes (Abstract OP 01-4).
The link between insulin resistance and an increased risk for stroke has been known for some time, and not just in people with T2D. However, the current way of determining insulin resistance is not suitable for widespread practice.
“The goal standard technique for measuring insulin resistance is the euglycemic clamp method,” said Dr. Zabala, an internal medical resident at Södersjukhuset hospital and researcher at the Karolinska Institutet in Stockholm.
“For that reason, [the eGDR], a method based on readily available clinical factors – waist circumference, hypertension, and glycosylated hemoglobin was developed,” he explained. Body mass index can also be used in place of waist circumference, he qualified.
The eGDR has already been proven to be very precise in people with type 1 diabetes, said Dr. Zabala, and could be an “excellent tool to measure insulin resistance in a large patient population.”
Investigating the link between eGDR and first stroke risk
The aim of the study he presented was to see if changes in the eGDR were associated with changes in the risk of someone with T2D experiencing a first stroke, or dying from a cardiovascular or other cause.
An observational cohort was formed by first considering data on all adult patients with T2D who were logged in the Swedish National Diabetes Registry (NDR) during 2004-2016. Then anyone with a history of stroke, or with any missing data on the clinical variables needed to calculate the eGDR, were excluded.
This resulted in an overall population of 104,697 individuals, aged a mean of 63 years, who had developed T2D at around the age of 59 years. About 44% of the study population were women. The mean eGDR for the whole population was 5.6 mg/kg per min.
The study subjects were grouped according to four eGDR levels: 24,706 were in the lowest quartile of eGDR (less than 4 mg/kg per min), signifying the highest level of insulin resistance, and 18,762 were in the upper quartile of eGDR (greater than 8 mg/kg per min), signifying the lowest level of insulin resistance. The middle two groups had an eGDR between 4 and 6 mg/kg per min (40,187), and 6 and 8 mg/kg/min (21,042).
Data from the NDR were then combined with the Swedish Cause of Death register, the Swedish In-patient Care Diagnoses registry, and the Longitudinal Database for Health Insurance and Labour Market Studies (LISA) to determine the rates of stroke, ischemic stroke, hemorrhagic stroke, all-cause mortality, and cardiovascular mortality.
Increasing insulin resistance ups risk for stroke, death
After a median follow-up of 5.6 years, 4% (4,201) of the study population had had a stroke.
“We clearly see an increased occurrence of first-time stroke in the group with the lowest eGDR, indicating worst insulin resistance, in comparison with the group with the highest eGDR, indicating less insulin resistance,” Dr. Zabala reported.
After adjustment for potential confounding factors, including age at baseline, gender, diabetes duration, among other variables, the risk for stroke was lowest in those with a high eGDR value and highest for those with a low eGDR value.
Using individuals with the lowest eGDR (less than 4 mg/kg per min) and thus greatest risk of stroke as the reference, adjusted hazard ratios (aHR) for first-time stroke were: 0.60, 0.68, and 0.77 for those with an eGDR of greater than 8, 6-8, and 4-6 mg/kg per min, respectively.
The corresponding values for risk of ischemic stroke were 0.55, 0.68, and 0.75. Regarding hemorrhagic stroke, there was no statistically significant correlation between eGDR levels and stroke occurrence. This was due to the small number of cases recorded.
As for all-cause and cardiovascular mortality, a similar pattern was seen, with higher rates of death linked to increasing insulin resistance. Adjusted hazard ratios according to increasing insulin resistance (decreasing eGDR scores) for all-cause death were 0.68, 0.75, and 0.82 and for cardiovascular mortality were 0.65, 0.75, and 0.82.
A sensitivity analysis, using BMI instead of waist circumference to calculate the eGDR, showed a similar pattern, and “interestingly, a correlation between eGDR levels and risk of hemorrhagic stroke.” Dr. Zabala said.
Limitations and take-homes
Of course, this is an observational cohort study, so no conclusions on causality can be made and there are no data on the use of anti-diabetic treatments specifically. But there are strengths such as covering almost all adults with T2D in Sweden and a relatively long-follow-up time.
The findings suggest that “eGDR, which may reflect insulin resistance may be a useful risk marker for stroke and death in people with type 2 diabetes,” said Dr. Zabala.
“You had a very large cohort, and that certainly makes your results very valid,” observed Peter Novodvorsky, MUDr. (Hons), PhD, MRCP, a consultant diabetologist in Trenčín, Slovakia.
Dr. Novodvorsky, who chaired the session, picked up on the lack of information about how many people were taking newer diabetes drugs, such as the glucagon-like peptide 1 receptor antagonists and sodium glucose-lowering transport 2 inhibitors.
“As we all know, these might have protective effects which are not necessarily related to the glucose lowering or insulin resistance-lowering” effects, so could have influenced the results. In terms of how practical the eGDR is for clinical practice, Dr. Zabala observed in a press release: “eGDR could be used to help T2D patients better understand and manage their risk of stroke and death.
“It could also be of importance in research. In this era of personalized medicine, better stratification of type 2 diabetes patients will help optimize clinical trials and further vital research into treatment, diagnosis, care and prevention.”
The research was a collaboration between the Karolinska Institutet, Gothenburg University and the Swedish National Diabetes Registry. Dr. Zabala and coauthors reported having no conflicts of interest.
Calculating the estimated glucose disposal rate (eGDR) as a proxy for the level of insulin resistance may be useful way to determine if someone with type 2 diabetes (T2D) is at risk for having a first stroke, Swedish researchers have found.
In a large population-based study, the lower the eGDR score went, the higher the risk for having a first stroke became.
The eGDR score was also predictive of the chance of dying from any or a cardiovascular cause, Alexander Zabala, MD, reported at the annual meeting of the European Association for the Study of Diabetes (Abstract OP 01-4).
The link between insulin resistance and an increased risk for stroke has been known for some time, and not just in people with T2D. However, the current way of determining insulin resistance is not suitable for widespread practice.
“The goal standard technique for measuring insulin resistance is the euglycemic clamp method,” said Dr. Zabala, an internal medical resident at Södersjukhuset hospital and researcher at the Karolinska Institutet in Stockholm.
“For that reason, [the eGDR], a method based on readily available clinical factors – waist circumference, hypertension, and glycosylated hemoglobin was developed,” he explained. Body mass index can also be used in place of waist circumference, he qualified.
The eGDR has already been proven to be very precise in people with type 1 diabetes, said Dr. Zabala, and could be an “excellent tool to measure insulin resistance in a large patient population.”
Investigating the link between eGDR and first stroke risk
The aim of the study he presented was to see if changes in the eGDR were associated with changes in the risk of someone with T2D experiencing a first stroke, or dying from a cardiovascular or other cause.
An observational cohort was formed by first considering data on all adult patients with T2D who were logged in the Swedish National Diabetes Registry (NDR) during 2004-2016. Then anyone with a history of stroke, or with any missing data on the clinical variables needed to calculate the eGDR, were excluded.
This resulted in an overall population of 104,697 individuals, aged a mean of 63 years, who had developed T2D at around the age of 59 years. About 44% of the study population were women. The mean eGDR for the whole population was 5.6 mg/kg per min.
The study subjects were grouped according to four eGDR levels: 24,706 were in the lowest quartile of eGDR (less than 4 mg/kg per min), signifying the highest level of insulin resistance, and 18,762 were in the upper quartile of eGDR (greater than 8 mg/kg per min), signifying the lowest level of insulin resistance. The middle two groups had an eGDR between 4 and 6 mg/kg per min (40,187), and 6 and 8 mg/kg/min (21,042).
Data from the NDR were then combined with the Swedish Cause of Death register, the Swedish In-patient Care Diagnoses registry, and the Longitudinal Database for Health Insurance and Labour Market Studies (LISA) to determine the rates of stroke, ischemic stroke, hemorrhagic stroke, all-cause mortality, and cardiovascular mortality.
Increasing insulin resistance ups risk for stroke, death
After a median follow-up of 5.6 years, 4% (4,201) of the study population had had a stroke.
“We clearly see an increased occurrence of first-time stroke in the group with the lowest eGDR, indicating worst insulin resistance, in comparison with the group with the highest eGDR, indicating less insulin resistance,” Dr. Zabala reported.
After adjustment for potential confounding factors, including age at baseline, gender, diabetes duration, among other variables, the risk for stroke was lowest in those with a high eGDR value and highest for those with a low eGDR value.
Using individuals with the lowest eGDR (less than 4 mg/kg per min) and thus greatest risk of stroke as the reference, adjusted hazard ratios (aHR) for first-time stroke were: 0.60, 0.68, and 0.77 for those with an eGDR of greater than 8, 6-8, and 4-6 mg/kg per min, respectively.
The corresponding values for risk of ischemic stroke were 0.55, 0.68, and 0.75. Regarding hemorrhagic stroke, there was no statistically significant correlation between eGDR levels and stroke occurrence. This was due to the small number of cases recorded.
As for all-cause and cardiovascular mortality, a similar pattern was seen, with higher rates of death linked to increasing insulin resistance. Adjusted hazard ratios according to increasing insulin resistance (decreasing eGDR scores) for all-cause death were 0.68, 0.75, and 0.82 and for cardiovascular mortality were 0.65, 0.75, and 0.82.
A sensitivity analysis, using BMI instead of waist circumference to calculate the eGDR, showed a similar pattern, and “interestingly, a correlation between eGDR levels and risk of hemorrhagic stroke.” Dr. Zabala said.
Limitations and take-homes
Of course, this is an observational cohort study, so no conclusions on causality can be made and there are no data on the use of anti-diabetic treatments specifically. But there are strengths such as covering almost all adults with T2D in Sweden and a relatively long-follow-up time.
The findings suggest that “eGDR, which may reflect insulin resistance may be a useful risk marker for stroke and death in people with type 2 diabetes,” said Dr. Zabala.
“You had a very large cohort, and that certainly makes your results very valid,” observed Peter Novodvorsky, MUDr. (Hons), PhD, MRCP, a consultant diabetologist in Trenčín, Slovakia.
Dr. Novodvorsky, who chaired the session, picked up on the lack of information about how many people were taking newer diabetes drugs, such as the glucagon-like peptide 1 receptor antagonists and sodium glucose-lowering transport 2 inhibitors.
“As we all know, these might have protective effects which are not necessarily related to the glucose lowering or insulin resistance-lowering” effects, so could have influenced the results. In terms of how practical the eGDR is for clinical practice, Dr. Zabala observed in a press release: “eGDR could be used to help T2D patients better understand and manage their risk of stroke and death.
“It could also be of importance in research. In this era of personalized medicine, better stratification of type 2 diabetes patients will help optimize clinical trials and further vital research into treatment, diagnosis, care and prevention.”
The research was a collaboration between the Karolinska Institutet, Gothenburg University and the Swedish National Diabetes Registry. Dr. Zabala and coauthors reported having no conflicts of interest.
FROM EASD 2021
Comorbidities larger factor than race in COVID ICU deaths?
Racial/ethnic disparities in COVID-19 mortality rates may be related more to comorbidities than to demographics, suggest authors of a new study.
Researchers compared the length of stay in intensive care units in two suburban hospitals for patients with severe SARS-CoV-2 infections. Their study shows that although the incidence of comorbidities and rates of use of mechanical ventilation and death were higher among Black patients than among patients of other races, length of stay in the ICU was generally similar for patients of all races. The study was conducted by Tripti Kumar, DO, from Lankenau Medical Center, Wynnewood, Pennsylvania, and colleagues.
“Racial disparities are observed in the United States concerning COVID-19, and studies have discovered that minority populations are at ongoing risk for health inequity,” Dr. Kumar said in a narrated e-poster presented during the American College of Chest Physicians (CHEST) 2021 Annual Meeting.
“Primary prevention initiatives should take precedence in mitigating the effect that comorbidities have on these vulnerable populations to help reduce necessity for mechanical ventilation, hospital length of stay, and overall mortality,” she said.
Higher death rates for Black patients
At the time the study was conducted, the COVID-19 death rate in the United States had topped 500,000 (as of this writing, it stands at 726,000). Of those who died, 22.4% were Black, 18.1% were Hispanic, and 3.6% were of Asian descent. The numbers of COVID-19 diagnoses and deaths were significantly higher in U.S. counties where the proportions of Black residents were higher, the authors note.
To see whether differences in COVID-19 outcomes were reflected in ICU length of stay, the researchers conducted a retrospective chart review of data on 162 patients admitted to ICUs at Paoli Hospital and Lankenau Medical Center, both in the suburban Philadelphia town of Wynnewood.
All patients were diagnosed with COVID-19 from March through June 2020.
In all, 60% of the study population were Black, 35% were White, 3% were Asian, and 2% were Hispanic. Women composed 46% of the sample.
The average length of ICU stay, which was the primary endpoint, was similar among Black patients (15.4 days), White patients (15.5 days), and Asians (16 days). The shortest average hospital stay was among Hispanic patients, at 11.3 days.
The investigators determined that among all races, the prevalence of type 2 diabetes, obesity, hypertension, and smoking was highest among Black patients.
Overall, nearly 85% of patients required mechanical ventilation. Among the patients who required it, 86% were Black, 84% were White, 66% were Hispanic, and 75% were Asian.
Overall mortality was 62%. It was higher among Black patients, at 60%, than among White patients, at 33%. The investigators did not report mortality rates for Hispanic or Asian patients.
Missing data
Demondes Haynes, MD, FCCP, professor of medicine in the Division of Pulmonary and Critical Care and associate dean for admissions at the University of Mississippi Medical Center and School of Medicine, Jackson, who was not involved in the study, told this news organization that there are some gaps in the study that make it difficult to draw strong conclusions about the findings.
“For sure, comorbidities contribute a great deal to mortality, but is there something else going on? I think this poster is incomplete in that it cannot answer that question,” he said in an interview.
He noted that the use of retrospective rather than prospective data makes it hard to account for potential confounders.
“I agree that these findings show the potential contribution of comorbidities, but to me, this is a little incomplete to make that a definitive statement,” he said.
“I can’t argue with their recommendation for primary prevention – we definitely want to do primary prevention to decrease comorbidities. Would it decrease overall mortality? It might, it sure might, for just COVID-19 I’d say no, we need more information.”
No funding source for the study was reported. Dr. Kumar and colleagues and Dr. Haynes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Racial/ethnic disparities in COVID-19 mortality rates may be related more to comorbidities than to demographics, suggest authors of a new study.
Researchers compared the length of stay in intensive care units in two suburban hospitals for patients with severe SARS-CoV-2 infections. Their study shows that although the incidence of comorbidities and rates of use of mechanical ventilation and death were higher among Black patients than among patients of other races, length of stay in the ICU was generally similar for patients of all races. The study was conducted by Tripti Kumar, DO, from Lankenau Medical Center, Wynnewood, Pennsylvania, and colleagues.
“Racial disparities are observed in the United States concerning COVID-19, and studies have discovered that minority populations are at ongoing risk for health inequity,” Dr. Kumar said in a narrated e-poster presented during the American College of Chest Physicians (CHEST) 2021 Annual Meeting.
“Primary prevention initiatives should take precedence in mitigating the effect that comorbidities have on these vulnerable populations to help reduce necessity for mechanical ventilation, hospital length of stay, and overall mortality,” she said.
Higher death rates for Black patients
At the time the study was conducted, the COVID-19 death rate in the United States had topped 500,000 (as of this writing, it stands at 726,000). Of those who died, 22.4% were Black, 18.1% were Hispanic, and 3.6% were of Asian descent. The numbers of COVID-19 diagnoses and deaths were significantly higher in U.S. counties where the proportions of Black residents were higher, the authors note.
To see whether differences in COVID-19 outcomes were reflected in ICU length of stay, the researchers conducted a retrospective chart review of data on 162 patients admitted to ICUs at Paoli Hospital and Lankenau Medical Center, both in the suburban Philadelphia town of Wynnewood.
All patients were diagnosed with COVID-19 from March through June 2020.
In all, 60% of the study population were Black, 35% were White, 3% were Asian, and 2% were Hispanic. Women composed 46% of the sample.
The average length of ICU stay, which was the primary endpoint, was similar among Black patients (15.4 days), White patients (15.5 days), and Asians (16 days). The shortest average hospital stay was among Hispanic patients, at 11.3 days.
The investigators determined that among all races, the prevalence of type 2 diabetes, obesity, hypertension, and smoking was highest among Black patients.
Overall, nearly 85% of patients required mechanical ventilation. Among the patients who required it, 86% were Black, 84% were White, 66% were Hispanic, and 75% were Asian.
Overall mortality was 62%. It was higher among Black patients, at 60%, than among White patients, at 33%. The investigators did not report mortality rates for Hispanic or Asian patients.
Missing data
Demondes Haynes, MD, FCCP, professor of medicine in the Division of Pulmonary and Critical Care and associate dean for admissions at the University of Mississippi Medical Center and School of Medicine, Jackson, who was not involved in the study, told this news organization that there are some gaps in the study that make it difficult to draw strong conclusions about the findings.
“For sure, comorbidities contribute a great deal to mortality, but is there something else going on? I think this poster is incomplete in that it cannot answer that question,” he said in an interview.
He noted that the use of retrospective rather than prospective data makes it hard to account for potential confounders.
“I agree that these findings show the potential contribution of comorbidities, but to me, this is a little incomplete to make that a definitive statement,” he said.
“I can’t argue with their recommendation for primary prevention – we definitely want to do primary prevention to decrease comorbidities. Would it decrease overall mortality? It might, it sure might, for just COVID-19 I’d say no, we need more information.”
No funding source for the study was reported. Dr. Kumar and colleagues and Dr. Haynes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Racial/ethnic disparities in COVID-19 mortality rates may be related more to comorbidities than to demographics, suggest authors of a new study.
Researchers compared the length of stay in intensive care units in two suburban hospitals for patients with severe SARS-CoV-2 infections. Their study shows that although the incidence of comorbidities and rates of use of mechanical ventilation and death were higher among Black patients than among patients of other races, length of stay in the ICU was generally similar for patients of all races. The study was conducted by Tripti Kumar, DO, from Lankenau Medical Center, Wynnewood, Pennsylvania, and colleagues.
“Racial disparities are observed in the United States concerning COVID-19, and studies have discovered that minority populations are at ongoing risk for health inequity,” Dr. Kumar said in a narrated e-poster presented during the American College of Chest Physicians (CHEST) 2021 Annual Meeting.
“Primary prevention initiatives should take precedence in mitigating the effect that comorbidities have on these vulnerable populations to help reduce necessity for mechanical ventilation, hospital length of stay, and overall mortality,” she said.
Higher death rates for Black patients
At the time the study was conducted, the COVID-19 death rate in the United States had topped 500,000 (as of this writing, it stands at 726,000). Of those who died, 22.4% were Black, 18.1% were Hispanic, and 3.6% were of Asian descent. The numbers of COVID-19 diagnoses and deaths were significantly higher in U.S. counties where the proportions of Black residents were higher, the authors note.
To see whether differences in COVID-19 outcomes were reflected in ICU length of stay, the researchers conducted a retrospective chart review of data on 162 patients admitted to ICUs at Paoli Hospital and Lankenau Medical Center, both in the suburban Philadelphia town of Wynnewood.
All patients were diagnosed with COVID-19 from March through June 2020.
In all, 60% of the study population were Black, 35% were White, 3% were Asian, and 2% were Hispanic. Women composed 46% of the sample.
The average length of ICU stay, which was the primary endpoint, was similar among Black patients (15.4 days), White patients (15.5 days), and Asians (16 days). The shortest average hospital stay was among Hispanic patients, at 11.3 days.
The investigators determined that among all races, the prevalence of type 2 diabetes, obesity, hypertension, and smoking was highest among Black patients.
Overall, nearly 85% of patients required mechanical ventilation. Among the patients who required it, 86% were Black, 84% were White, 66% were Hispanic, and 75% were Asian.
Overall mortality was 62%. It was higher among Black patients, at 60%, than among White patients, at 33%. The investigators did not report mortality rates for Hispanic or Asian patients.
Missing data
Demondes Haynes, MD, FCCP, professor of medicine in the Division of Pulmonary and Critical Care and associate dean for admissions at the University of Mississippi Medical Center and School of Medicine, Jackson, who was not involved in the study, told this news organization that there are some gaps in the study that make it difficult to draw strong conclusions about the findings.
“For sure, comorbidities contribute a great deal to mortality, but is there something else going on? I think this poster is incomplete in that it cannot answer that question,” he said in an interview.
He noted that the use of retrospective rather than prospective data makes it hard to account for potential confounders.
“I agree that these findings show the potential contribution of comorbidities, but to me, this is a little incomplete to make that a definitive statement,” he said.
“I can’t argue with their recommendation for primary prevention – we definitely want to do primary prevention to decrease comorbidities. Would it decrease overall mortality? It might, it sure might, for just COVID-19 I’d say no, we need more information.”
No funding source for the study was reported. Dr. Kumar and colleagues and Dr. Haynes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
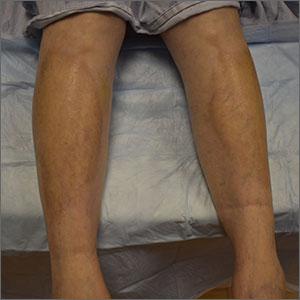
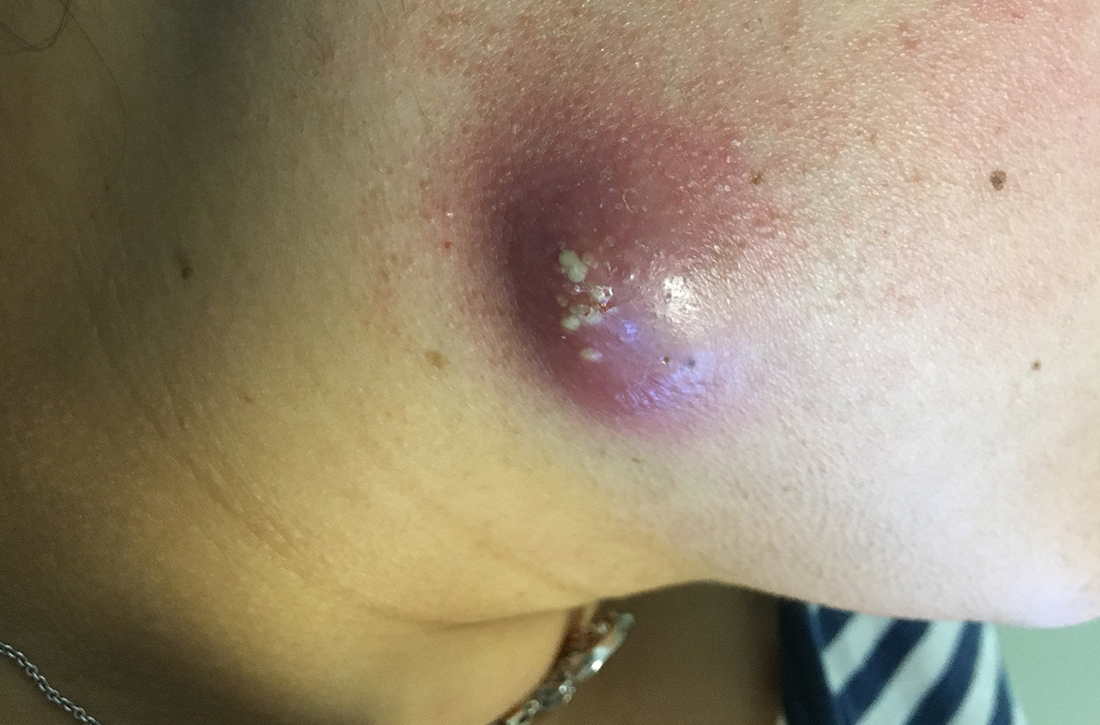
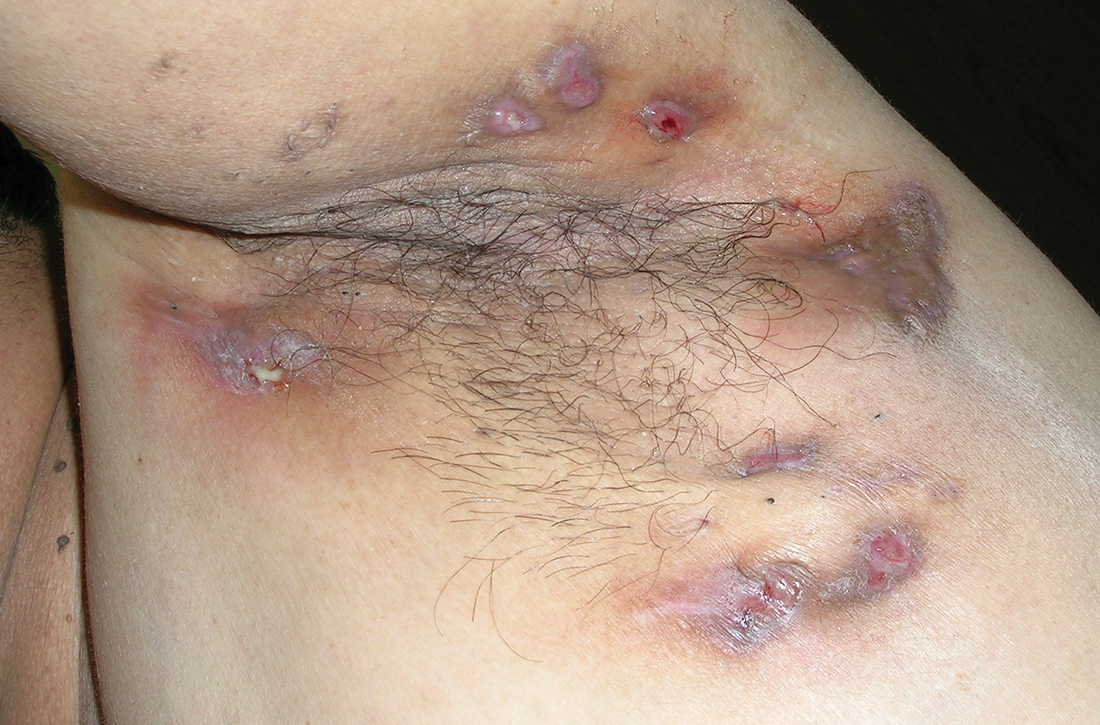
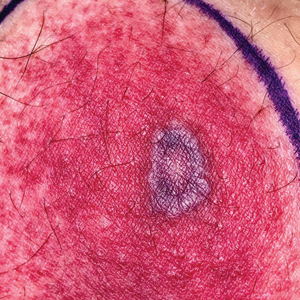
Yellow pruritic eruption
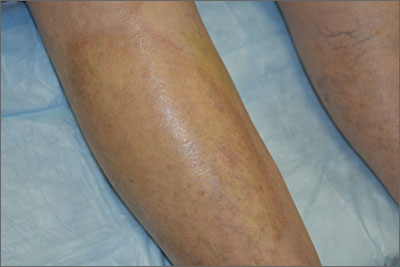
This pruritic, eruption with yellowing atrophy and telangiectasias on the lower extremities is a classic presentation for necrobiosis lipoidica (NL).
NL is a chronic granulomatous disorder with a predilection for the lower extremities. Patients with NL present with progressive, yellow-brown atrophic plaques on the pretibial aspect of the legs. The plaques have underlying telangiectasias, revealed by the atrophy, and may ulcerate. While these lesions are primarily asymptomatic, associated symptoms may include pruritus, pain, or altered sensation on the affected skin. The classic pathology of NL is notable for altered collagen bundles layered with palisading granulomas extending deep into the dermis. Other notable findings may include mixed inflammatory cells, multinucleated giant cells, and plasma cells; mucin is notably absent.
There is an established relationship between NL and diabetes. When these 2 entities are present, the skin eruption may be referred to as “necrobiosis lipoidica diabeticorum” or “NLD.” Only a small percentage of patients with diabetes will develop NL. Furthermore, there is growing evidence to suggest that NL may be associated with other comorbidities, such as obesity, hypertension, dyslipidemia, and thyroid disease.1 Additionally, squamous cell carcinoma has been reported as arising within skin affected by NL.2
The etiology of NL is not completely understood. Current theories suggest that blood vessel inflammation related to autoimmune factors may be at work.2 The differential diagnosis of NL includes granuloma annulare, pretibial myxedema, stasis dermatitis, panniculitis, morphea, and lichen sclerosis.
NL can be refractory to therapy. Paramount to management is the avoidance of trauma to the affected skin. Topical therapies include corticosteroids, tretinoin, and tacrolimus. Systemic immunomodulation with infliximab, etanercept, thalidomide, and cyclosporine has also been trialed. There is evidence for the utility of pentoxifylline (400 mg po tid), a xanthine derivative often used for peripheral artery disease, to reverse ulceration that can arise in NL.
The patient in this case opted for topical therapy with clobetasol 0.05% ointment and tacrolimus 0.1% ointment. She was subsequently lost to follow-up.
Image courtesy of Cyrelle Fermin, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Cyrelle Fermin, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155:455-459. doi: 10.1001/jamadermatol.2018.5635
2. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi: 10.1016/j.det.2015.03.003


This pruritic, eruption with yellowing atrophy and telangiectasias on the lower extremities is a classic presentation for necrobiosis lipoidica (NL).
NL is a chronic granulomatous disorder with a predilection for the lower extremities. Patients with NL present with progressive, yellow-brown atrophic plaques on the pretibial aspect of the legs. The plaques have underlying telangiectasias, revealed by the atrophy, and may ulcerate. While these lesions are primarily asymptomatic, associated symptoms may include pruritus, pain, or altered sensation on the affected skin. The classic pathology of NL is notable for altered collagen bundles layered with palisading granulomas extending deep into the dermis. Other notable findings may include mixed inflammatory cells, multinucleated giant cells, and plasma cells; mucin is notably absent.
There is an established relationship between NL and diabetes. When these 2 entities are present, the skin eruption may be referred to as “necrobiosis lipoidica diabeticorum” or “NLD.” Only a small percentage of patients with diabetes will develop NL. Furthermore, there is growing evidence to suggest that NL may be associated with other comorbidities, such as obesity, hypertension, dyslipidemia, and thyroid disease.1 Additionally, squamous cell carcinoma has been reported as arising within skin affected by NL.2
The etiology of NL is not completely understood. Current theories suggest that blood vessel inflammation related to autoimmune factors may be at work.2 The differential diagnosis of NL includes granuloma annulare, pretibial myxedema, stasis dermatitis, panniculitis, morphea, and lichen sclerosis.
NL can be refractory to therapy. Paramount to management is the avoidance of trauma to the affected skin. Topical therapies include corticosteroids, tretinoin, and tacrolimus. Systemic immunomodulation with infliximab, etanercept, thalidomide, and cyclosporine has also been trialed. There is evidence for the utility of pentoxifylline (400 mg po tid), a xanthine derivative often used for peripheral artery disease, to reverse ulceration that can arise in NL.
The patient in this case opted for topical therapy with clobetasol 0.05% ointment and tacrolimus 0.1% ointment. She was subsequently lost to follow-up.
Image courtesy of Cyrelle Fermin, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Cyrelle Fermin, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.


This pruritic, eruption with yellowing atrophy and telangiectasias on the lower extremities is a classic presentation for necrobiosis lipoidica (NL).
NL is a chronic granulomatous disorder with a predilection for the lower extremities. Patients with NL present with progressive, yellow-brown atrophic plaques on the pretibial aspect of the legs. The plaques have underlying telangiectasias, revealed by the atrophy, and may ulcerate. While these lesions are primarily asymptomatic, associated symptoms may include pruritus, pain, or altered sensation on the affected skin. The classic pathology of NL is notable for altered collagen bundles layered with palisading granulomas extending deep into the dermis. Other notable findings may include mixed inflammatory cells, multinucleated giant cells, and plasma cells; mucin is notably absent.
There is an established relationship between NL and diabetes. When these 2 entities are present, the skin eruption may be referred to as “necrobiosis lipoidica diabeticorum” or “NLD.” Only a small percentage of patients with diabetes will develop NL. Furthermore, there is growing evidence to suggest that NL may be associated with other comorbidities, such as obesity, hypertension, dyslipidemia, and thyroid disease.1 Additionally, squamous cell carcinoma has been reported as arising within skin affected by NL.2
The etiology of NL is not completely understood. Current theories suggest that blood vessel inflammation related to autoimmune factors may be at work.2 The differential diagnosis of NL includes granuloma annulare, pretibial myxedema, stasis dermatitis, panniculitis, morphea, and lichen sclerosis.
NL can be refractory to therapy. Paramount to management is the avoidance of trauma to the affected skin. Topical therapies include corticosteroids, tretinoin, and tacrolimus. Systemic immunomodulation with infliximab, etanercept, thalidomide, and cyclosporine has also been trialed. There is evidence for the utility of pentoxifylline (400 mg po tid), a xanthine derivative often used for peripheral artery disease, to reverse ulceration that can arise in NL.
The patient in this case opted for topical therapy with clobetasol 0.05% ointment and tacrolimus 0.1% ointment. She was subsequently lost to follow-up.
Image courtesy of Cyrelle Fermin, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Cyrelle Fermin, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155:455-459. doi: 10.1001/jamadermatol.2018.5635
2. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi: 10.1016/j.det.2015.03.003
1. Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155:455-459. doi: 10.1001/jamadermatol.2018.5635
2. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi: 10.1016/j.det.2015.03.003
FDA authorizes boosters for Moderna, J&J, allows mix-and-match
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
in people who are eligible to get them.
The move to amend the Emergency Use Authorization for these vaccines gives the vaccine experts on the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices latitude to recommend a mix-and-match strategy if they feel the science supports it.
The committee convenes Oct. 21 for a day-long meeting to make its recommendations for additional doses.
People who’ve previously received two doses of the Moderna mRNA vaccine, which is now called Spikevax, are eligible for a third dose of any COVID-19 vaccine if they are 6 months past their second dose and are:
- 65 years of age or older
- 18 to 64 years of age, but at high risk for severe COVID-19 because of an underlying health condition
- 18 to 64 years of age and at high risk for exposure to the SARS-CoV-2 virus because they live in a group setting, such as a prison or care home, or work in a risky occupation, such as healthcare
People who’ve previously received a dose of the Johnson & Johnson vaccine are eligible for a second dose of any COVID-19 vaccine if they are over the age of 18 and at least 2 months past their vaccination.
“Today’s actions demonstrate our commitment to public health in proactively fighting against the COVID-19 pandemic,” said Acting FDA Commissioner Janet Woodcock, MD, in a news release. “As the pandemic continues to impact the country, science has shown that vaccination continues to be the safest and most effective way to prevent COVID-19, including the most serious consequences of the disease, such as hospitalization and death.
“The available data suggest waning immunity in some populations who are fully vaccinated. The availability of these authorized boosters is important for continued protection against COVID-19 disease.”
A version of this article was first published on Medscape.com.
Bone risk: Is time since menopause a better predictor than age?
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
White House announces vaccination plans for younger children
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
No increased risk of relugolix side effects in fibroid, endometriosis patients
Side effects from relugolix combination therapy (Myfembree) in premenopausal women treated for uterine fibroids and endometriosis are minimal, according to research presented at the American Society for Reproductive Medicine’s 2021 meeting.
The Food and Drug Administration approved relugolix, a daily oral gonadotropin-releasing hormone antagonist medication, earlier this year to treat heavy menstrual bleeding associated with uterine fibroids. It has not received Food and Drug Administration approval to treat endometriosis yet.
“It was a good kind of vindication about the safety of relugolix combination therapy,” Ayman Al-Hendy, MD, PhD, gynecologist and endoscopic surgeon at the University of Chicago, said in an interview.
Researchers led by Dr. Al-Hendy analyzed the results from two 24-week clinical trials that examined the effects of relugolix on premenopausal women between the ages of 18 and 50 suffering from uterine fibroids and endometriosis, both of which found that the treatment was well tolerated. With 1,344 patients in total, researchers found that the most common side effects of the treatment were headache, which occurred in 24.3% of participants, and hot flush, which affected 10.6%.
However, the prevalence of adverse reactions was similar to that of the placebo group in which 21.4% of participants experienced headaches and 6.4% experienced hot flushes, which, according to Dr. Al-Hendy, means that there is “really no increased risk” of experiencing an adverse event while taking relugolix.
“If we follow a large number of patients [with uterine fibroids or endometriosis], they will have some of these symptoms like headache or hot flushes or fatigue and so on. Either because it just happens in women for no known reason or because maybe the disease itself is causing some of these symptoms. The question is does the treatment in this case increase the frequency of these events?” Dr. Al-Hendy said.
“As long as it’s similar, fairly similar, or close between the [treatment and placebo group], then we know it’s not because of the medication,” Dr. Al-Hendy added.
Other adverse reactions that occurred while taking relugolix were “relatively rare” Dr. Al-Hendy said during his presentation. About 5.5% of those who took relugolix had uterine bleeding, 3.4% had decreased libido, 1.9% suffered from hyperhidrosis, 1.2% experienced night sweats, and 1.3% suffered from vaginal dryness.
The study shows that the risk profile of relugolix combination therapy is favorable and the side effects are relatively mild compared with past treatment options used to treat fibroids or endometriosis, said J. Ricardo Loret de Mola, MD, FACOG, FACS, who was not involved in the study.
However, Dr. Loret de Mola emphasized that this treatment isn’t for women who are seeking fertility or to get pregnant so it’s important for physicians to ask patients about their goals for treatment. Relugolix treatment could be a way for fibroid patients in their reproductive age to buy time and reduce the number of surgeries needed to get them to “the point where they would be ready to become mothers.”
He said surgery could be the right option for endometriosis patients who want to have children in the near future.
“This is an additional tool that we have available now that’s effective,” Dr. Loret de Mola said. “It is not going to cure either one of the two conditions, but could buy enough time for patients to be able to reach their goals, which is not having symptoms of endometriosis and fibroids after menopause or for people who just want to buy time.”
Dr. Al-Hendy said he hopes his findings reassure and encourage health care providers to discuss with patients different options for treating fibroids, and not just counsel them about surgery.
“So more awareness of these nonsurgical options hopefully will offer our patients a wide range of options when they seek help with fibroids and then against endometriosis [if or when] it’s [FDA]-approved,” Dr. Al-Hendy said.
None of the experts interviewed had conflicts of interest.
Side effects from relugolix combination therapy (Myfembree) in premenopausal women treated for uterine fibroids and endometriosis are minimal, according to research presented at the American Society for Reproductive Medicine’s 2021 meeting.
The Food and Drug Administration approved relugolix, a daily oral gonadotropin-releasing hormone antagonist medication, earlier this year to treat heavy menstrual bleeding associated with uterine fibroids. It has not received Food and Drug Administration approval to treat endometriosis yet.
“It was a good kind of vindication about the safety of relugolix combination therapy,” Ayman Al-Hendy, MD, PhD, gynecologist and endoscopic surgeon at the University of Chicago, said in an interview.
Researchers led by Dr. Al-Hendy analyzed the results from two 24-week clinical trials that examined the effects of relugolix on premenopausal women between the ages of 18 and 50 suffering from uterine fibroids and endometriosis, both of which found that the treatment was well tolerated. With 1,344 patients in total, researchers found that the most common side effects of the treatment were headache, which occurred in 24.3% of participants, and hot flush, which affected 10.6%.
However, the prevalence of adverse reactions was similar to that of the placebo group in which 21.4% of participants experienced headaches and 6.4% experienced hot flushes, which, according to Dr. Al-Hendy, means that there is “really no increased risk” of experiencing an adverse event while taking relugolix.
“If we follow a large number of patients [with uterine fibroids or endometriosis], they will have some of these symptoms like headache or hot flushes or fatigue and so on. Either because it just happens in women for no known reason or because maybe the disease itself is causing some of these symptoms. The question is does the treatment in this case increase the frequency of these events?” Dr. Al-Hendy said.
“As long as it’s similar, fairly similar, or close between the [treatment and placebo group], then we know it’s not because of the medication,” Dr. Al-Hendy added.
Other adverse reactions that occurred while taking relugolix were “relatively rare” Dr. Al-Hendy said during his presentation. About 5.5% of those who took relugolix had uterine bleeding, 3.4% had decreased libido, 1.9% suffered from hyperhidrosis, 1.2% experienced night sweats, and 1.3% suffered from vaginal dryness.
The study shows that the risk profile of relugolix combination therapy is favorable and the side effects are relatively mild compared with past treatment options used to treat fibroids or endometriosis, said J. Ricardo Loret de Mola, MD, FACOG, FACS, who was not involved in the study.
However, Dr. Loret de Mola emphasized that this treatment isn’t for women who are seeking fertility or to get pregnant so it’s important for physicians to ask patients about their goals for treatment. Relugolix treatment could be a way for fibroid patients in their reproductive age to buy time and reduce the number of surgeries needed to get them to “the point where they would be ready to become mothers.”
He said surgery could be the right option for endometriosis patients who want to have children in the near future.
“This is an additional tool that we have available now that’s effective,” Dr. Loret de Mola said. “It is not going to cure either one of the two conditions, but could buy enough time for patients to be able to reach their goals, which is not having symptoms of endometriosis and fibroids after menopause or for people who just want to buy time.”
Dr. Al-Hendy said he hopes his findings reassure and encourage health care providers to discuss with patients different options for treating fibroids, and not just counsel them about surgery.
“So more awareness of these nonsurgical options hopefully will offer our patients a wide range of options when they seek help with fibroids and then against endometriosis [if or when] it’s [FDA]-approved,” Dr. Al-Hendy said.
None of the experts interviewed had conflicts of interest.
Side effects from relugolix combination therapy (Myfembree) in premenopausal women treated for uterine fibroids and endometriosis are minimal, according to research presented at the American Society for Reproductive Medicine’s 2021 meeting.
The Food and Drug Administration approved relugolix, a daily oral gonadotropin-releasing hormone antagonist medication, earlier this year to treat heavy menstrual bleeding associated with uterine fibroids. It has not received Food and Drug Administration approval to treat endometriosis yet.
“It was a good kind of vindication about the safety of relugolix combination therapy,” Ayman Al-Hendy, MD, PhD, gynecologist and endoscopic surgeon at the University of Chicago, said in an interview.
Researchers led by Dr. Al-Hendy analyzed the results from two 24-week clinical trials that examined the effects of relugolix on premenopausal women between the ages of 18 and 50 suffering from uterine fibroids and endometriosis, both of which found that the treatment was well tolerated. With 1,344 patients in total, researchers found that the most common side effects of the treatment were headache, which occurred in 24.3% of participants, and hot flush, which affected 10.6%.
However, the prevalence of adverse reactions was similar to that of the placebo group in which 21.4% of participants experienced headaches and 6.4% experienced hot flushes, which, according to Dr. Al-Hendy, means that there is “really no increased risk” of experiencing an adverse event while taking relugolix.
“If we follow a large number of patients [with uterine fibroids or endometriosis], they will have some of these symptoms like headache or hot flushes or fatigue and so on. Either because it just happens in women for no known reason or because maybe the disease itself is causing some of these symptoms. The question is does the treatment in this case increase the frequency of these events?” Dr. Al-Hendy said.
“As long as it’s similar, fairly similar, or close between the [treatment and placebo group], then we know it’s not because of the medication,” Dr. Al-Hendy added.
Other adverse reactions that occurred while taking relugolix were “relatively rare” Dr. Al-Hendy said during his presentation. About 5.5% of those who took relugolix had uterine bleeding, 3.4% had decreased libido, 1.9% suffered from hyperhidrosis, 1.2% experienced night sweats, and 1.3% suffered from vaginal dryness.
The study shows that the risk profile of relugolix combination therapy is favorable and the side effects are relatively mild compared with past treatment options used to treat fibroids or endometriosis, said J. Ricardo Loret de Mola, MD, FACOG, FACS, who was not involved in the study.
However, Dr. Loret de Mola emphasized that this treatment isn’t for women who are seeking fertility or to get pregnant so it’s important for physicians to ask patients about their goals for treatment. Relugolix treatment could be a way for fibroid patients in their reproductive age to buy time and reduce the number of surgeries needed to get them to “the point where they would be ready to become mothers.”
He said surgery could be the right option for endometriosis patients who want to have children in the near future.
“This is an additional tool that we have available now that’s effective,” Dr. Loret de Mola said. “It is not going to cure either one of the two conditions, but could buy enough time for patients to be able to reach their goals, which is not having symptoms of endometriosis and fibroids after menopause or for people who just want to buy time.”
Dr. Al-Hendy said he hopes his findings reassure and encourage health care providers to discuss with patients different options for treating fibroids, and not just counsel them about surgery.
“So more awareness of these nonsurgical options hopefully will offer our patients a wide range of options when they seek help with fibroids and then against endometriosis [if or when] it’s [FDA]-approved,” Dr. Al-Hendy said.
None of the experts interviewed had conflicts of interest.
FROM ASRM 2021
Painful facial abscess
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.
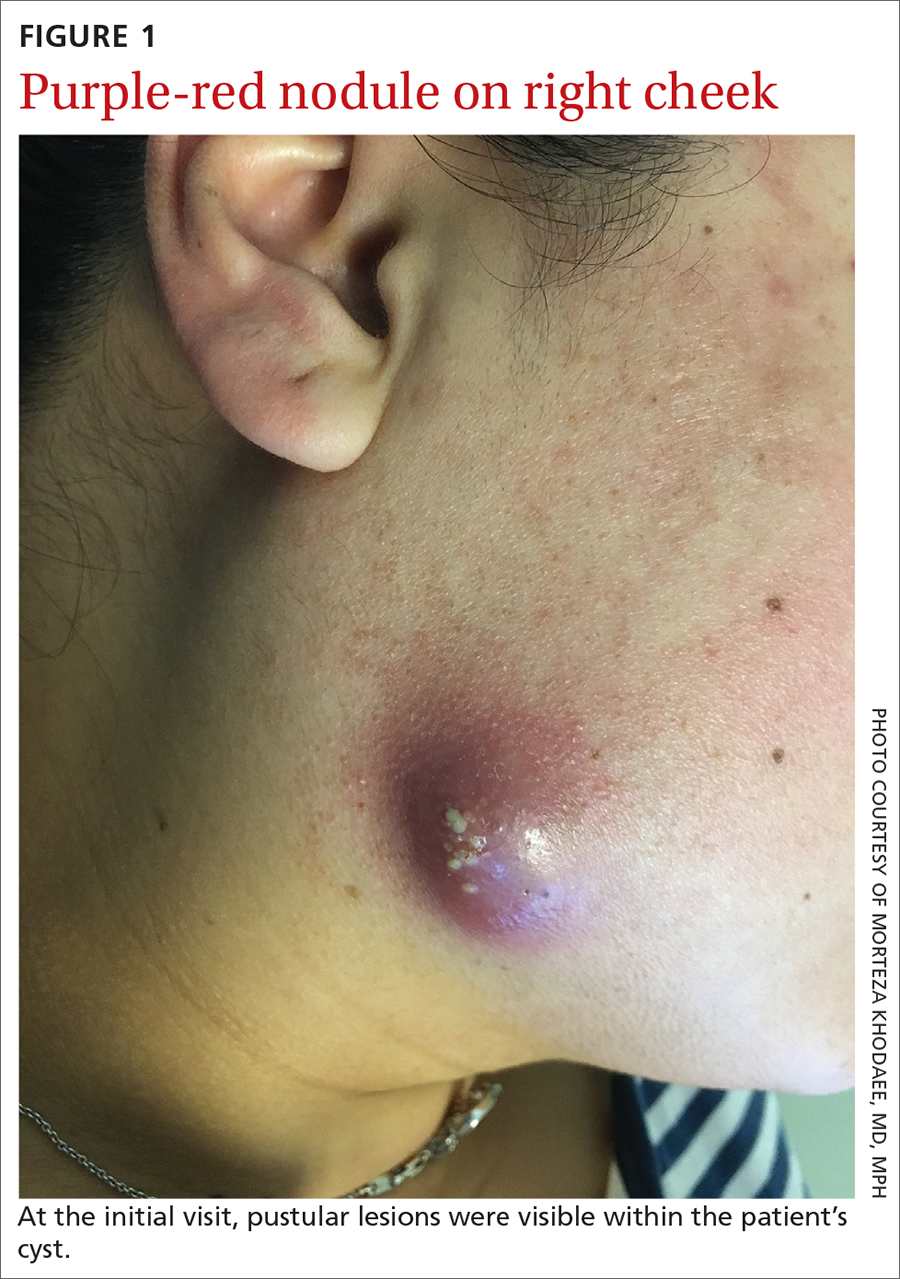
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).
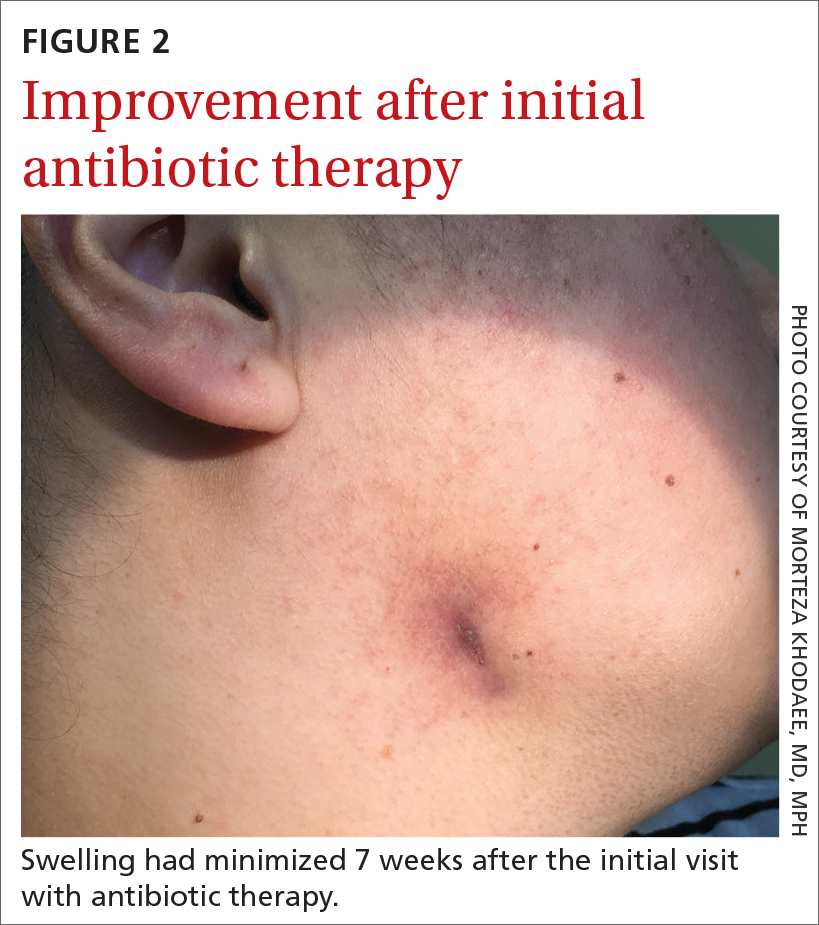
The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.
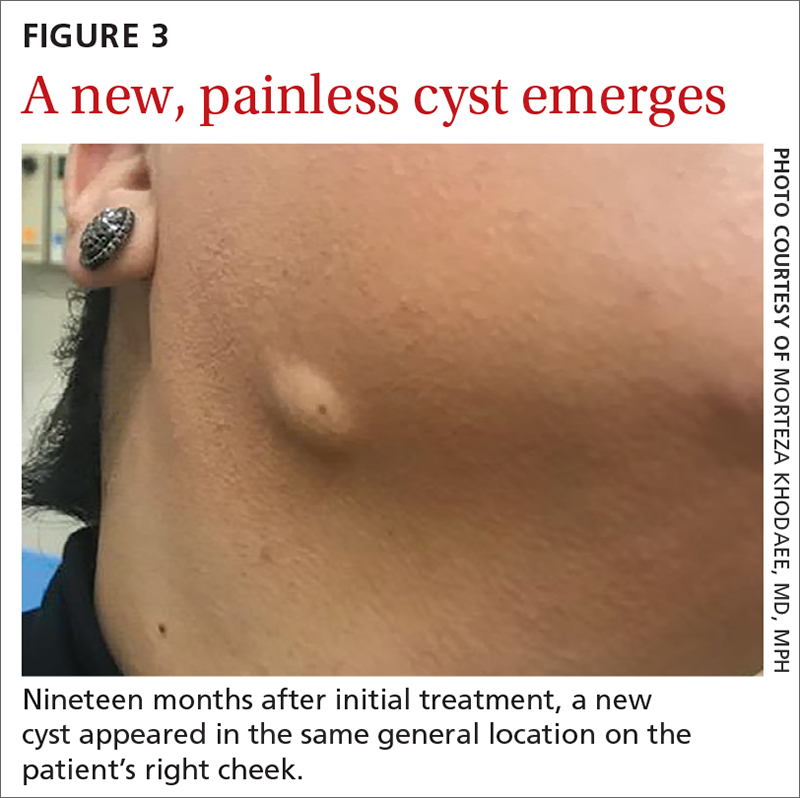
Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.
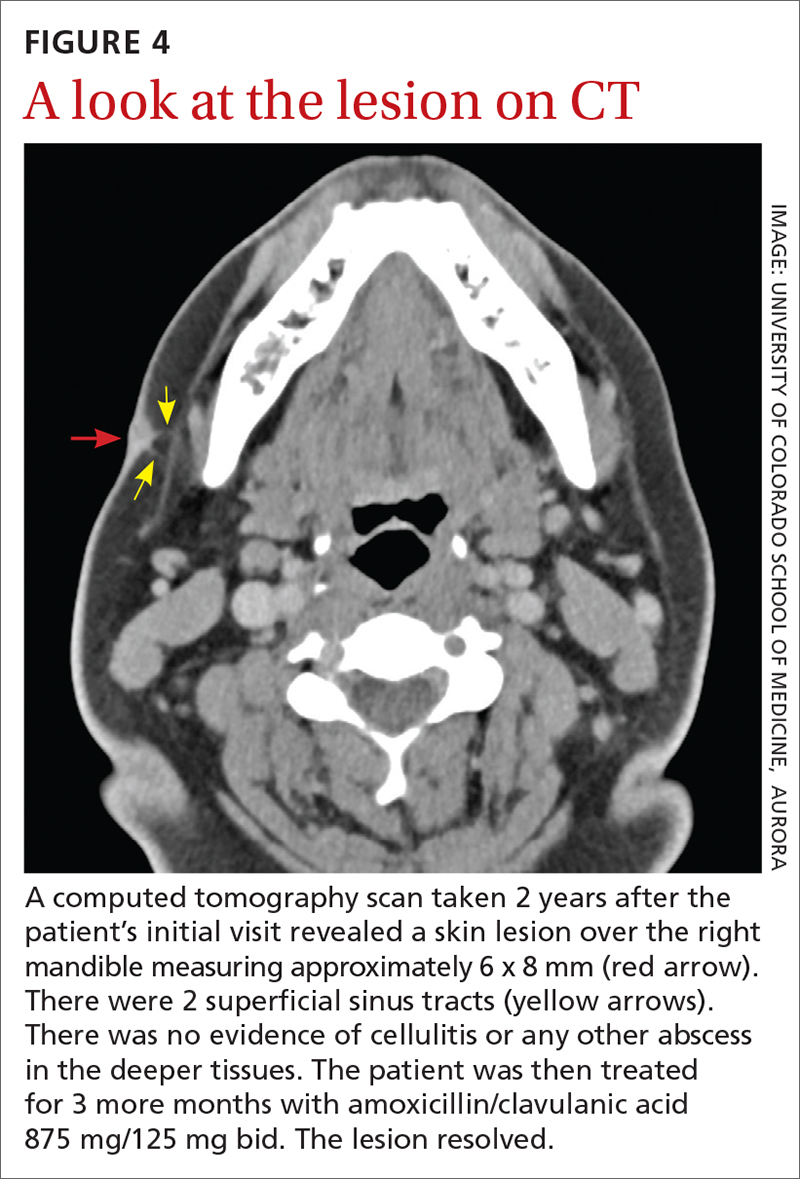
We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).

The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.

Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
A 35-year-old woman presented to our clinic with a purple-red cyst on her right cheek that had been present for about 4 years but had worsened over the prior 2 weeks (FIGURE 1). She said she was experiencing excruciating pain and that the cyst had purulent drainage. She denied any history of diabetes, dental problems, recent trauma, or an inciting event.
On physical examination, there was no cervical lymphadenopathy, and her vital signs were normal. An incision and drainage procedure was performed. About 2 mL of purulent fluid was extracted and sent for aerobic and anaerobic cultures.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cervicofacial actinomycosis
Direct Gram stain showed gram-positive cocci, so the patient was started on a 7-day course of cephalexin 500 mg tid. Five days later, the anaerobic culture grew Actinomyces neuii, revealing the diagnosis as cervicofacial actinomycosis; the patient stopped taking cephalexin. The patient was then switched to a 3-month course of amoxicillin 875 mg bid.
Actinomyces are natural inhabitants of the human oropharynx and gastrointestinal and genitourinary tracts.1-4 They are filamentous, gram-positive rods with characteristic sulfur granules (although these are not always present).1-4 It is believed that actinomycosis is endogenously acquired from deep tissue either through dental trauma, penetrating wounds, or compound fractures.2,4
The most common presentations of actinomycosis include cervicofacial (sometimes referred to as “lumpy jaw syndrome”), followed by abdominopelvic and thoracic/pulmonary, manifestations.2-4 Primary cutaneous actinomycosis is rare.5-9 Actinomycosis infection often manifests with indolent constitutional symptoms such as fatigue and anorexia.1 Most cases occur in men ages 20 to 60 years, although cases in women are increasingly being reported.2-4
Risk factors include poor dental hygiene or dental procedures, alcoholism, intrauterine device use, immunosuppression, appendicitis, and diverticulitis.2-4 The exact cause of this patient’s actinomycosis was unknown, as she did not have any known risk factors.
Furunculosis and sporotrichosis are part of the differential
Actinomycosis is often called a “great mimicker” due to its ability to masquerade as infection, malignancy, or fungus.1 The differential diagnosis for this patient’s presentation included bacterial soft-tissue infection (eg, furunculosis), infected epidermoid cyst, cutaneous tuberculosis, sporotrichosis, deep fungal infection, and nocardiosis.
Continue to: Furunculosis was initially suspected
Furunculosis was initially suspected, but the original wound culture demonstrated actinomycoses instead of traditional gram-positive bacteria.
A clinical diagnosis
The diagnosis of actinomycosis is usually made clinically, but definitive confirmation requires culture, which can be challenging with a slow-growing facultative or strict anaerobe that may take up to 14 days to appear.2-4 A Gram stain can aid in the diagnosis, but overall, there is a high false-negative rate in identifying actinomycosis.1,3,4
Treatment time can be lengthy, but prognosis is favorable
Unfortunately, there are no randomized controlled studies for treatment of actinomycosis. The majority of evidence for treatment comes from in vitro and clinical case studies.2-4,10 In general, prognosis of actinomycosis is favorable with low mortality, but chronic infection without complete resolution of symptoms can occur.1-4,7,8,10
First-line therapy for actinomycosis is a beta-lactam antibiotic, typically penicillin G or amoxicillin.2-4,10 High doses of prolonged intravenous (IV) and oral antibiotic therapy (2 to 12 months) based on location and complexity are standard.3,11 However, if there is minimal bone involvement and the patient shows rapid improvement, treatment could be shortened to a 4 to 6–week oral regimen.1,11 Surgical intervention can also shorten the required length of antibiotic duration.1,10
Cutaneous actinomycosis Tx. Amoxicillin/clavulanic acid has been shown to be an effective treatment for cutaneous actinomycosis, especially if polymicrobial infection is suspected.5,6 Individualized regimens for cutaneous actinomycosis—based on severity, location, and treatment response—are acceptable with close monitoring.1,2,11
Continue to: A lengthy recovery for our patient
A lengthy recovery for our patient
Seven weeks after the initial visit, the patient reported that she had taken only 20 days’ worth of the recommended 3-month course of amoxicillin. Fortunately, the lesion appeared to be healing well with no apparent fluid collection (FIGURE 2).

The patient was then prescribed, and completed, a 3-month course of amoxicillin/clavulanic acid
Nineteen months after initial treatment, the lesion reappeared as a painless cyst in a similar location (FIGURE 3). Plastic Surgery incised and drained the lesion and Infectious Diseases continued her on 3 months of amoxicillin/clavulanic acid 875 mg/125 mg bid, which she did complete.

Due to the continued presence of the lesion, a computed tomography scan of the face was ordered 2 years after the initial visit and demonstrated a superficial skin lesion with no mandibular involvement (FIGURE 4). She was then treated with 3 more months of amoxicillin/clavulanic acid 875 mg/125 mg bid, with the possibility of deep debridement if not improved. However, debridement was unnecessary as the cyst did not recur.

We believe that the course of this patient’s treatment was protracted because she never took oral antibiotics for more than 3 months at a time, and thus, her infection never completely resolved. In retrospect, we would have treated her more aggressively from the outset.
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
1. Najmi AH, Najmi IH, Tawhari MMH, et al. Cutaneous actinomycosis and long-term management through using oral and topical antibiotics: a case report. Clin Pract. 2018;8:1102. doi: 10.4081/ cp.2018.1102
2. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. StatPearls Publishing; 2021.
3. Valour F, Sénécha A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-97. doi: 10.2147/IDR.S39601
4. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099
5. Akhtar M, Zade MP, Shahane PL, et al. Scalp actinomycosis presenting as soft tissue tumour: a case report with literature review. Int J Surg Case Rep. 2015;16:99-101. doi: 10.1016/ j.ijscr.2015.09.030
6. Bose M, Ghosh R, Mukherjee K, et al. Primary cutaneous actinomycosis:a case report. J Clin Diagn Res. 2014;8:YD03-5. doi: 10.7860/JCDR/2014/8286.4591
7. Cataño JC, Gómez Villegas SI. Images in clinical medicine. Cutaneous actinomycosis. N Engl J Med. 2016;374:1773. doi: 10.1056/ NEJMicm1511213
8. Mehta V, Balachandran C. Primary cutaneous actinomycosis on the chest wall. Dermatol Online J. 2008;14:13.
9. Piggott SA, Khodaee M. A bump in the groin: cutaneous actinomycosis. J Family Community Med. 2017;24:203. doi: 10.4103/jfcm.JFCM_79_17
10. Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J Dermatolog Treat. 2017;28:59-64. doi: 10.1080/09546634.2016.1178373
11. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;;7:183-197. doi: 10.2147/IDR.S39601
Painful lumps in the axilla
A 30-year-old man presented to the clinic with a complaint of small painful lumps in his armpit. He stated that he initially experienced some itching and discomfort, but after a while he noticed some red, tender, swollen areas. He also mentioned an odorous yellow fluid that would sometimes drain from the lumps. Since first noticing them 2 years earlier, he reported that the nodules had disappeared and reappeared on their own several times.
On physical exam, several small red subcutaneous nodules were present in the axilla and tender to palpation (FIGURE 1A). The patient also had comedonal acne on his back (FIGURE 1B). The patient’s body mass index was 31, and he was a nonsmoker.
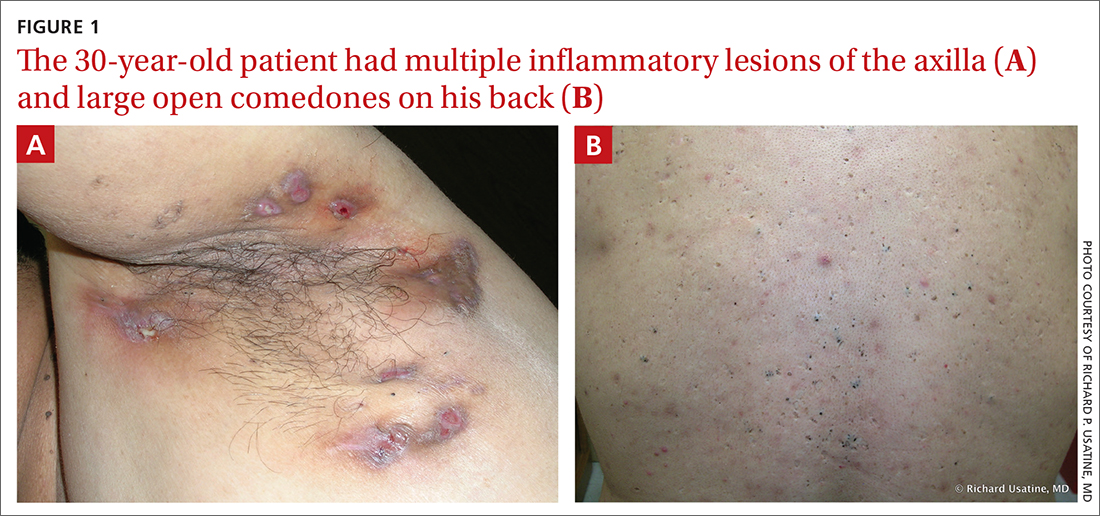
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Hidradenitis suppurativa
The characteristic location and morphology of the lesions, along with the chronicity and odor, were critical in arriving at a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic, inflammatory skin condition that normally manifests in areas of apocrine sweat glands, including the axilla, groin, and perianal, perineal, and inframammary locations.1 It begins when an abnormal hair follicle gets occluded and ruptures, spilling keratin and bacteria into the dermis. An inflammatory response can ensue with surrounding neutrophils and lymphocytes, which leads to abscess formation and destruction of the pilosebaceous unit. Sinus tracts form between the lesions, and a cycle of scarring, fistulas, and contractures can occur.
In this case, the comedones from acne conglobata on the patient’s back indicated a more global follicular occlusion disorder. The characteristic triad is hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp—of which the patient had 2.
Other potential causes of the pathology include abnormal secretion of apocrine glands, abnormal antimicrobial peptides, deficient numbers of sebaceous glands, and abnormal invaginations of the epidermis.2 Increased levels of tumor necrosis factor alpha and other cytokines have been detected in HS lesions and are a potential target for therapy.
The prevalence of HS in the United States is approximately 0.1%.3 The condition typically begins between the ages of 18 and 39 years. The ratio of women to men affected by the condition is 3:1.2 There is no evident racial or ethnic predilection. There is an association with diabetes and Crohn disease.3 Obesity and smoking are risk factors.1
Continue to: The differential includes an array of common skin conditions
The differential includes an array of common skin conditions
The differential diagnosis in this case included carbuncles, cysts, acne, and abscesses.
A furuncle or carbuncle can result from an infection of hair follicles that can manifest as individual (furuncle) or clusters of (carbuncle) red, painful boils. They form on parts of the skin where hair grows, including the face, neck, armpits, shoulders, and buttocks. They respond well to treatment with antibiotics and incision and drainage. They can be recurrent but usually don’t cluster together in apocrine-rich areas, as seen with HS.
Epidermal inclusion cysts are keratin-filled inclusion cysts with epithelial-lined cyst walls. The cysts are subcutaneous and occasionally more superficial. They can occur almost anywhere but are most often found on the back, scalp, neck, face, and chest. They are usually solitary; however, when there are multiple cysts, they are not linked by sinus tracts as found in HS.
Inflammatory acne lesions tend to form on the face, neck, back, chest, and shoulders, while HS lesions appear most often in apocrine-rich intertriginous areas.
Skin abscesses are local deep infections of the skin caused by bacterial pathogens. The most common agent is Staphylococcus aureus (frequently methicillin resistant). Injection drug use and immunosuppression are risk factors. Although bacteria do not cause HS lesions, bacteria can exacerbate HS through colonization.
Continue to: No lab test needed to diagnosis hidradenitis suppurativa
No lab test needed to diagnose hidradenitis suppurativa
Diagnosis of HS is largely clinical and based on a patient’s history and physical exam findings.2 No specific laboratory test is needed.
Although the patient in this case did have comedonal acne on his back, the lesions that prompted his visit were in an apocrine-rich area, were recurrent, and broke open on their own to release foul-smelling contents—all typical characteristics of HS.
Treatment depends on the severity of the condition
There are 3 stages of HS: Hurley stage I involves abscess formation without tracts or scars. Hurley stage II involves recurrent abscesses with sinus tracts and scarring. Hurley stage III has diffuse involvement with multiple interconnected sinus tracts and abscesses across an entire area.2 Our patient fits into Hurley stage III.
Evidence-based treatment of mild disease (Hurley stage I) includes topical clindamycin 1% solution/gel bid or doxycycline 100 mg bid for widespread disease (Hurley stage II or resistant stage I).2 Chlorhexidine and benzoyl peroxide washes are also often recommended.3 If a patient does not respond to this treatment or the condition is moderate to severe, then clindamycin 300 po bid (with or without rifampin 600/d po) for 10 weeks should be considered.4,5 In a randomized placebo-controlled trial that compared the efficacy of oral clindamycin vs clindamycin plus rifampin in patients with HS, both therapeutic options were statistically equivalent.5 One small, randomized controlled study of patients with mild-to-moderate HS showed that tetracycline 500 mg bid for 3 months resulted in fewer abscesses and nodules but was not superior to topical clindamycin.3
If the patient doesn’t show improvement (Hurley stage III), then adalimumab is an option, as follows: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly, if needed.4 Adalimumab is currently the only FDA-approved treatment for HS. Infliximab by IV infusion can be effective in improving pain, disease severity, and quality of life in patients with moderate-to-severe HS.3 This patient was also a candidate for treatment with systemic retinoids (isotretinoin or acitretin), which could have helped both the HS and the acne conglobata.
Continue to: Intralesional steroid injectiosn with triamcinolone
Intralesional steroid injections with triamcinolone 10 mg/mL can reduce local pain and inflammation rapidly. Pain management is also critical, as HS is painful. First-line therapy includes nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants, and serotonin and norepinephrine reuptake inhibitors.2 Opiate analgesics may be needed for breakthrough pain in patients with severe disease. Avoiding tight clothing, harsh products, and adhesive dressings, as well as using clear petroleum jelly, can prevent skin trauma and help with healing. Weight loss and smoking cessation are also associated with better outcomes.6,7
If medical management fails …
If there is no improvement with medical management, it may be time to consider local procedures such as unroofing/deroofing, punch debridement, skin-tissue-sparing excision with electrosurgical peeling, and laser excision. Incision and drainage may be necessary for acutely inflamed, painful abscesses but should not be routinely performed because lesions can recur.3
Referral to a plastic surgeon is necessary when patients are considering wide excisions of largely affected areas. Even when surgical excisions are performed, medical treatment is needed to prevent new lesions and recurrences.
Our patient was treated initially with oral doxycycline 100 mg bid and intralesional triamcinolone (10 mg/mL) in the most tender lesions. He was also provided with a prescription for ibuprofen 800 mg tid to be taken with meals. The family physician encouraged the patient to lose weight. The patient derived some benefit from treatment but continued to experience new painful lesions.
The physician prescribed oral clindamycin 300 mg bid at a follow-up visit to replace the oral doxycycline. When this failed, the patient was sent for labs to determine if he would be a candidate for adalimumab. When the screening labs were normal, a prescription for adalimumab was provided: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly.4
1. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi: 10.2147/CCID.S111019
2. Ballard K, Shuman VL. Hidradenitis Suppurativa. StatPearls Publishing; 2021.
3. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
4. Alikhan A, Lymch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. doi:10.10126/j.jaad.2008.11.911
5. Caro RDC, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
6. Hendricks AJ, Hirt PA, Sekhon S, et al. Non-pharmacologic approaches for hidradenitis suppurativa - a systematic review. J Dermatolog Treat. 2021;32:11-18. doi: 10.1080/09546634.2019.1621981
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
A 30-year-old man presented to the clinic with a complaint of small painful lumps in his armpit. He stated that he initially experienced some itching and discomfort, but after a while he noticed some red, tender, swollen areas. He also mentioned an odorous yellow fluid that would sometimes drain from the lumps. Since first noticing them 2 years earlier, he reported that the nodules had disappeared and reappeared on their own several times.
On physical exam, several small red subcutaneous nodules were present in the axilla and tender to palpation (FIGURE 1A). The patient also had comedonal acne on his back (FIGURE 1B). The patient’s body mass index was 31, and he was a nonsmoker.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Hidradenitis suppurativa
The characteristic location and morphology of the lesions, along with the chronicity and odor, were critical in arriving at a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic, inflammatory skin condition that normally manifests in areas of apocrine sweat glands, including the axilla, groin, and perianal, perineal, and inframammary locations.1 It begins when an abnormal hair follicle gets occluded and ruptures, spilling keratin and bacteria into the dermis. An inflammatory response can ensue with surrounding neutrophils and lymphocytes, which leads to abscess formation and destruction of the pilosebaceous unit. Sinus tracts form between the lesions, and a cycle of scarring, fistulas, and contractures can occur.
In this case, the comedones from acne conglobata on the patient’s back indicated a more global follicular occlusion disorder. The characteristic triad is hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp—of which the patient had 2.
Other potential causes of the pathology include abnormal secretion of apocrine glands, abnormal antimicrobial peptides, deficient numbers of sebaceous glands, and abnormal invaginations of the epidermis.2 Increased levels of tumor necrosis factor alpha and other cytokines have been detected in HS lesions and are a potential target for therapy.
The prevalence of HS in the United States is approximately 0.1%.3 The condition typically begins between the ages of 18 and 39 years. The ratio of women to men affected by the condition is 3:1.2 There is no evident racial or ethnic predilection. There is an association with diabetes and Crohn disease.3 Obesity and smoking are risk factors.1
Continue to: The differential includes an array of common skin conditions
The differential includes an array of common skin conditions
The differential diagnosis in this case included carbuncles, cysts, acne, and abscesses.
A furuncle or carbuncle can result from an infection of hair follicles that can manifest as individual (furuncle) or clusters of (carbuncle) red, painful boils. They form on parts of the skin where hair grows, including the face, neck, armpits, shoulders, and buttocks. They respond well to treatment with antibiotics and incision and drainage. They can be recurrent but usually don’t cluster together in apocrine-rich areas, as seen with HS.
Epidermal inclusion cysts are keratin-filled inclusion cysts with epithelial-lined cyst walls. The cysts are subcutaneous and occasionally more superficial. They can occur almost anywhere but are most often found on the back, scalp, neck, face, and chest. They are usually solitary; however, when there are multiple cysts, they are not linked by sinus tracts as found in HS.
Inflammatory acne lesions tend to form on the face, neck, back, chest, and shoulders, while HS lesions appear most often in apocrine-rich intertriginous areas.
Skin abscesses are local deep infections of the skin caused by bacterial pathogens. The most common agent is Staphylococcus aureus (frequently methicillin resistant). Injection drug use and immunosuppression are risk factors. Although bacteria do not cause HS lesions, bacteria can exacerbate HS through colonization.
Continue to: No lab test needed to diagnosis hidradenitis suppurativa
No lab test needed to diagnose hidradenitis suppurativa
Diagnosis of HS is largely clinical and based on a patient’s history and physical exam findings.2 No specific laboratory test is needed.
Although the patient in this case did have comedonal acne on his back, the lesions that prompted his visit were in an apocrine-rich area, were recurrent, and broke open on their own to release foul-smelling contents—all typical characteristics of HS.
Treatment depends on the severity of the condition
There are 3 stages of HS: Hurley stage I involves abscess formation without tracts or scars. Hurley stage II involves recurrent abscesses with sinus tracts and scarring. Hurley stage III has diffuse involvement with multiple interconnected sinus tracts and abscesses across an entire area.2 Our patient fits into Hurley stage III.
Evidence-based treatment of mild disease (Hurley stage I) includes topical clindamycin 1% solution/gel bid or doxycycline 100 mg bid for widespread disease (Hurley stage II or resistant stage I).2 Chlorhexidine and benzoyl peroxide washes are also often recommended.3 If a patient does not respond to this treatment or the condition is moderate to severe, then clindamycin 300 po bid (with or without rifampin 600/d po) for 10 weeks should be considered.4,5 In a randomized placebo-controlled trial that compared the efficacy of oral clindamycin vs clindamycin plus rifampin in patients with HS, both therapeutic options were statistically equivalent.5 One small, randomized controlled study of patients with mild-to-moderate HS showed that tetracycline 500 mg bid for 3 months resulted in fewer abscesses and nodules but was not superior to topical clindamycin.3
If the patient doesn’t show improvement (Hurley stage III), then adalimumab is an option, as follows: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly, if needed.4 Adalimumab is currently the only FDA-approved treatment for HS. Infliximab by IV infusion can be effective in improving pain, disease severity, and quality of life in patients with moderate-to-severe HS.3 This patient was also a candidate for treatment with systemic retinoids (isotretinoin or acitretin), which could have helped both the HS and the acne conglobata.
Continue to: Intralesional steroid injectiosn with triamcinolone
Intralesional steroid injections with triamcinolone 10 mg/mL can reduce local pain and inflammation rapidly. Pain management is also critical, as HS is painful. First-line therapy includes nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants, and serotonin and norepinephrine reuptake inhibitors.2 Opiate analgesics may be needed for breakthrough pain in patients with severe disease. Avoiding tight clothing, harsh products, and adhesive dressings, as well as using clear petroleum jelly, can prevent skin trauma and help with healing. Weight loss and smoking cessation are also associated with better outcomes.6,7
If medical management fails …
If there is no improvement with medical management, it may be time to consider local procedures such as unroofing/deroofing, punch debridement, skin-tissue-sparing excision with electrosurgical peeling, and laser excision. Incision and drainage may be necessary for acutely inflamed, painful abscesses but should not be routinely performed because lesions can recur.3
Referral to a plastic surgeon is necessary when patients are considering wide excisions of largely affected areas. Even when surgical excisions are performed, medical treatment is needed to prevent new lesions and recurrences.
Our patient was treated initially with oral doxycycline 100 mg bid and intralesional triamcinolone (10 mg/mL) in the most tender lesions. He was also provided with a prescription for ibuprofen 800 mg tid to be taken with meals. The family physician encouraged the patient to lose weight. The patient derived some benefit from treatment but continued to experience new painful lesions.
The physician prescribed oral clindamycin 300 mg bid at a follow-up visit to replace the oral doxycycline. When this failed, the patient was sent for labs to determine if he would be a candidate for adalimumab. When the screening labs were normal, a prescription for adalimumab was provided: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly.4
A 30-year-old man presented to the clinic with a complaint of small painful lumps in his armpit. He stated that he initially experienced some itching and discomfort, but after a while he noticed some red, tender, swollen areas. He also mentioned an odorous yellow fluid that would sometimes drain from the lumps. Since first noticing them 2 years earlier, he reported that the nodules had disappeared and reappeared on their own several times.
On physical exam, several small red subcutaneous nodules were present in the axilla and tender to palpation (FIGURE 1A). The patient also had comedonal acne on his back (FIGURE 1B). The patient’s body mass index was 31, and he was a nonsmoker.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Hidradenitis suppurativa
The characteristic location and morphology of the lesions, along with the chronicity and odor, were critical in arriving at a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic, inflammatory skin condition that normally manifests in areas of apocrine sweat glands, including the axilla, groin, and perianal, perineal, and inframammary locations.1 It begins when an abnormal hair follicle gets occluded and ruptures, spilling keratin and bacteria into the dermis. An inflammatory response can ensue with surrounding neutrophils and lymphocytes, which leads to abscess formation and destruction of the pilosebaceous unit. Sinus tracts form between the lesions, and a cycle of scarring, fistulas, and contractures can occur.
In this case, the comedones from acne conglobata on the patient’s back indicated a more global follicular occlusion disorder. The characteristic triad is hidradenitis suppurativa, acne conglobata, and dissecting cellulitis of the scalp—of which the patient had 2.
Other potential causes of the pathology include abnormal secretion of apocrine glands, abnormal antimicrobial peptides, deficient numbers of sebaceous glands, and abnormal invaginations of the epidermis.2 Increased levels of tumor necrosis factor alpha and other cytokines have been detected in HS lesions and are a potential target for therapy.
The prevalence of HS in the United States is approximately 0.1%.3 The condition typically begins between the ages of 18 and 39 years. The ratio of women to men affected by the condition is 3:1.2 There is no evident racial or ethnic predilection. There is an association with diabetes and Crohn disease.3 Obesity and smoking are risk factors.1
Continue to: The differential includes an array of common skin conditions
The differential includes an array of common skin conditions
The differential diagnosis in this case included carbuncles, cysts, acne, and abscesses.
A furuncle or carbuncle can result from an infection of hair follicles that can manifest as individual (furuncle) or clusters of (carbuncle) red, painful boils. They form on parts of the skin where hair grows, including the face, neck, armpits, shoulders, and buttocks. They respond well to treatment with antibiotics and incision and drainage. They can be recurrent but usually don’t cluster together in apocrine-rich areas, as seen with HS.
Epidermal inclusion cysts are keratin-filled inclusion cysts with epithelial-lined cyst walls. The cysts are subcutaneous and occasionally more superficial. They can occur almost anywhere but are most often found on the back, scalp, neck, face, and chest. They are usually solitary; however, when there are multiple cysts, they are not linked by sinus tracts as found in HS.
Inflammatory acne lesions tend to form on the face, neck, back, chest, and shoulders, while HS lesions appear most often in apocrine-rich intertriginous areas.
Skin abscesses are local deep infections of the skin caused by bacterial pathogens. The most common agent is Staphylococcus aureus (frequently methicillin resistant). Injection drug use and immunosuppression are risk factors. Although bacteria do not cause HS lesions, bacteria can exacerbate HS through colonization.
Continue to: No lab test needed to diagnosis hidradenitis suppurativa
No lab test needed to diagnose hidradenitis suppurativa
Diagnosis of HS is largely clinical and based on a patient’s history and physical exam findings.2 No specific laboratory test is needed.
Although the patient in this case did have comedonal acne on his back, the lesions that prompted his visit were in an apocrine-rich area, were recurrent, and broke open on their own to release foul-smelling contents—all typical characteristics of HS.
Treatment depends on the severity of the condition
There are 3 stages of HS: Hurley stage I involves abscess formation without tracts or scars. Hurley stage II involves recurrent abscesses with sinus tracts and scarring. Hurley stage III has diffuse involvement with multiple interconnected sinus tracts and abscesses across an entire area.2 Our patient fits into Hurley stage III.
Evidence-based treatment of mild disease (Hurley stage I) includes topical clindamycin 1% solution/gel bid or doxycycline 100 mg bid for widespread disease (Hurley stage II or resistant stage I).2 Chlorhexidine and benzoyl peroxide washes are also often recommended.3 If a patient does not respond to this treatment or the condition is moderate to severe, then clindamycin 300 po bid (with or without rifampin 600/d po) for 10 weeks should be considered.4,5 In a randomized placebo-controlled trial that compared the efficacy of oral clindamycin vs clindamycin plus rifampin in patients with HS, both therapeutic options were statistically equivalent.5 One small, randomized controlled study of patients with mild-to-moderate HS showed that tetracycline 500 mg bid for 3 months resulted in fewer abscesses and nodules but was not superior to topical clindamycin.3
If the patient doesn’t show improvement (Hurley stage III), then adalimumab is an option, as follows: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly, if needed.4 Adalimumab is currently the only FDA-approved treatment for HS. Infliximab by IV infusion can be effective in improving pain, disease severity, and quality of life in patients with moderate-to-severe HS.3 This patient was also a candidate for treatment with systemic retinoids (isotretinoin or acitretin), which could have helped both the HS and the acne conglobata.
Continue to: Intralesional steroid injectiosn with triamcinolone
Intralesional steroid injections with triamcinolone 10 mg/mL can reduce local pain and inflammation rapidly. Pain management is also critical, as HS is painful. First-line therapy includes nonsteroidal anti-inflammatory drugs, acetaminophen, atypical anticonvulsants, and serotonin and norepinephrine reuptake inhibitors.2 Opiate analgesics may be needed for breakthrough pain in patients with severe disease. Avoiding tight clothing, harsh products, and adhesive dressings, as well as using clear petroleum jelly, can prevent skin trauma and help with healing. Weight loss and smoking cessation are also associated with better outcomes.6,7
If medical management fails …
If there is no improvement with medical management, it may be time to consider local procedures such as unroofing/deroofing, punch debridement, skin-tissue-sparing excision with electrosurgical peeling, and laser excision. Incision and drainage may be necessary for acutely inflamed, painful abscesses but should not be routinely performed because lesions can recur.3
Referral to a plastic surgeon is necessary when patients are considering wide excisions of largely affected areas. Even when surgical excisions are performed, medical treatment is needed to prevent new lesions and recurrences.
Our patient was treated initially with oral doxycycline 100 mg bid and intralesional triamcinolone (10 mg/mL) in the most tender lesions. He was also provided with a prescription for ibuprofen 800 mg tid to be taken with meals. The family physician encouraged the patient to lose weight. The patient derived some benefit from treatment but continued to experience new painful lesions.
The physician prescribed oral clindamycin 300 mg bid at a follow-up visit to replace the oral doxycycline. When this failed, the patient was sent for labs to determine if he would be a candidate for adalimumab. When the screening labs were normal, a prescription for adalimumab was provided: 160 mg subcutaneously at Week 0, 80 mg at Week 2, and then 40 mg weekly.4
1. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi: 10.2147/CCID.S111019
2. Ballard K, Shuman VL. Hidradenitis Suppurativa. StatPearls Publishing; 2021.
3. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
4. Alikhan A, Lymch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. doi:10.10126/j.jaad.2008.11.911
5. Caro RDC, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
6. Hendricks AJ, Hirt PA, Sekhon S, et al. Non-pharmacologic approaches for hidradenitis suppurativa - a systematic review. J Dermatolog Treat. 2021;32:11-18. doi: 10.1080/09546634.2019.1621981
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
1. Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi: 10.2147/CCID.S111019
2. Ballard K, Shuman VL. Hidradenitis Suppurativa. StatPearls Publishing; 2021.
3. Wipperman J, Bragg DA, Litzner B. Hidradenitis suppurativa: rapid evidence review. Am Fam Physician. 2019;100:562-569.
4. Alikhan A, Lymch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561. doi:10.10126/j.jaad.2008.11.911
5. Caro RDC, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
6. Hendricks AJ, Hirt PA, Sekhon S, et al. Non-pharmacologic approaches for hidradenitis suppurativa - a systematic review. J Dermatolog Treat. 2021;32:11-18. doi: 10.1080/09546634.2019.1621981
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi: 10.1016/j.jaad.2019.02.067
Tender Annular Plaque on the Thigh
The Diagnosis: Ecthyma Gangrenosum
Histopathology revealed basophilic bacterial rods around necrotic vessels with thrombosis and edema (Figure). Blood and tissue cultures grew Pseudomonas aeruginosa. Based on the histopathology and clinical presentation, a diagnosis of P aeruginosa–associated ecthyma gangrenosum (EG) was made. The patient’s symptoms resolved with intravenous cefepime, and he later was transitioned to oral levofloxacin for outpatient treatment.

Ecthyma gangrenosum is an uncommon cutaneous manifestation of bacteremia that most commonly occurs secondary to P aeruginosa in immunocompromised patients, particularly patients with severe neutropenia in the setting of recent chemotherapy.1,2 Ecthyma gangrenosum can occur anywhere on the body, predominantly in moist areas such as the axillae and groin; the arms and legs, such as in our patient, as well as the trunk and face also may be involved.3 Other causes of EG skin lesions include methicillin-resistant Staphylococcus aureus, Citrobacter freundii, Escherichia coli, fungi such as Candida, and viruses such as herpes simplex virus.2,4-6 Common predisposing conditions associated with EG include neutropenia, leukemia, HIV, diabetes mellitus, extensive burn wounds, and a history of immunosuppressive medications. It also has been known to occur in otherwise healthy, immunocompetent individuals with no difference in clinical manifestation.2
The diagnosis is clinicopathologic, with initial evaluation including blood and wound cultures as well as a complete blood cell count once EG is suspected. An excisional or punch biopsy is performed for confirmation, showing many gram-negative, rod-shaped bacteria in cases of pseudomonal EG.7 Histopathology is characterized by bacterial perivascular invasion that then leads to secondary arteriole thrombosis, tissue edema, and separation of the epidermis.7,8 Resultant ischemic necrosis results in the classic macroscopic appearance of an erythematous macule that rapidly progresses into a central necrotic lesion surrounded by an erythematous or violaceous halo after undergoing a hemorrhagic bullous stage.1,9 A Wood lamp can be used to expedite the diagnosis, as Pseudomonas bacteria excretes a pigment (pyoverdine) that fluoresces yellowish green.10
Ecthyma gangrenosum can be classified as a primary skin lesion that may or may not be followed by bacteremia or as a lesion secondary to pseudomonal bacteremia.11 Bacteremia has been reported in half of cases, with hematogenous metastasis of the infection, likely in manifestations with multiple bilateral lesions.2 Our patient’s presentation of a single lesion revealed a positive blood culture result. Lesions also can develop by direct inoculation of the epidermis causing local destruction of the surrounding tissue. The nonbacteremic form of EG has been associated with a lower mortality rate of around 15% compared to patients with bacteremia ranging from 38% to 96%.12 The presence of neutropenia is the most important prognostic factor for mortality at the time of diagnosis.13
Prompt empiric therapy should be initiated after obtaining wound and blood cultures in those with infection until the causative organism and its susceptibility are identified. Pseudomonal infections account for 4% of all cases of hospital-acquired bacteremia and are the third leading cause of gram-negative bloodstream infection.7 Initial broad-spectrum antibiotics include antipseudomonal β-lactams (piperacillin-tazobactam), cephalosporins (cefepime), fluoroquinolones (levofloxacin), and carbapenems (imipenem).1,7 Medical therapy alone may be sufficient without requiring extensive surgical debridement to remove necrotic tissue in some patients. Surgical debridement usually is warranted for lesions larger than 10 cm in diameter.3 Our patient was treated with intravenous cefepime with resolution and was followed with outpatient oral levofloxacin as appropriate. A high index of suspicion should be maintained for relapsing pseudomonal EG infection among patients with AIDS, as the reported recurrence rate is 57%.14
Clinically, the differential diagnosis of EG presenting in immunocompromised patients or individuals with underlying malignancy includes pyoderma gangrenosum, papulonecrotic tuberculid, and leukemia cutis. An erythematous rash with central necrosis presenting in a patient with systemic symptoms is pathognomonic for erythema migrans and should be considered as a diagnostic possibility in areas endemic for Lyme disease in the United States, including the northeastern, mid-Atlantic, and north-central regions.15 A thorough history, physical examination, basic laboratory studies, and histopathology are critical to differentiate between these entities with similar macroscopic features. Pyoderma gangrenosum histologically manifests as a noninfectious, deep, suppurative folliculitis with leukocytoclastic vasculitis in 40% of cases.16 Although papulonecrotic tuberculid can present with dermal necrosis resulting from a hypersensitivity reaction to antigenic components of mycobacteria, there typically are granulomatous infiltrates present and a lack of observed organisms on histopathology.17 Although leukemia cutis infrequently occurs in patients diagnosed with leukemia, its salient features on pathology are nodular or diffuse infiltrates of leukemic cells in the dermis and subcutis with a high nuclear-to-cytoplasmic ratio, often with prominent nucleoli.18 Lyme disease can present in various ways; however, cutaneous involvement in the primary lesion is histologically characterized by a perivascular lymphohistiocytic infiltrate containing plasma cells at the periphery of the expanding annular lesion and eosinophils present at the center.19
- Abdou A, Hassam B. Ecthyma gangrenosum [in French]. Pan Afr Med J. 2018;30:95. doi:10.11604/pamj.2018.30.95.6244
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis. 2015;34:633-639. doi:10.1007/s10096-014-2277-6
- Vaiman M, Lasarovitch T, Heller L, et al. Ecthyma gangrenosum versus ecthyma-like lesions: should we separate these conditions? Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:69-72. doi:10.15570 /actaapa.2015.18
- Reich HL, Williams Fadeyi D, Naik NS, et al. Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol. 2004;50(5 suppl): S114-S117. doi:10.1016/j.jaad.2003.09.019
- Hawkley T, Chang D, Pollard W, et al. Ecthyma gangrenosum caused by Citrobacter freundii [published online July 27, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220996
- Santhaseelan RG, Muralidhar V. Non-pseudomonal ecthyma gangrenosum caused by methicillin-resistant Staphylococcus aureus (MRSA) in a chronic alcoholic patient [published online August 3, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220983m
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections [published online May 29, 2018]. Drugs Context. 2018;7:212527. doi:10.7573/dic.212527
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662. doi:10.1097/DAD.0000000000000766
- Sarkar S, Patra AK, Mondal M. Ecthyma gangrenosum in the periorbital region in a previously healthy immunocompetent woman without bacteremia. Indian Dermatol Online J. 2016;7:36-39. doi:10.4103/2229-5178.174326
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Van den Broek PJ, Van der Meer JWM, Kunst MW. The pathogenesis of ecthyma gangrenosum. J Infect. 1979;1:263-267. doi:10.1016 /S0163-4453(79)91329-X
- Downey DM, O’Bryan MC, Burdette SD, et al. Ecthyma gangrenosum in a patient with toxic epidermal necrolysis. J Burn Care Res. 2007;28:198-202. doi:10.1097/BCR.0B013E31802CA481
- Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, et al. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92:698-700. doi:10.1590/abd1806-4841.20175580
- Khan MO, Montecalvo MA, Davis I, et al. Ecthyma gangrenosum in patients with acquired immunodeficiency syndrome. Cutis. 2000;66:121-123.
- Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998; 352:557-565.
- Su WP, Schroeter AL, Perry HO, et al. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13:323-330. doi:10.1111/j.1600-0560.1986.tb00466.x
- Tirumalae R, Yeliur IK, Antony M, et al. Papulonecrotic tuberculidclinicopathologic and molecular features of 12 Indian patients. Dermatol Pract Concept. 2014;4:17-22. doi:10.5826/dpc.0402a03
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis [published online August 15, 2015]. Dermatol Online J. 2015;21:13030/qt6m18g35f
- Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167-174. doi:10.4103/0019-5154.110822
The Diagnosis: Ecthyma Gangrenosum
Histopathology revealed basophilic bacterial rods around necrotic vessels with thrombosis and edema (Figure). Blood and tissue cultures grew Pseudomonas aeruginosa. Based on the histopathology and clinical presentation, a diagnosis of P aeruginosa–associated ecthyma gangrenosum (EG) was made. The patient’s symptoms resolved with intravenous cefepime, and he later was transitioned to oral levofloxacin for outpatient treatment.

Ecthyma gangrenosum is an uncommon cutaneous manifestation of bacteremia that most commonly occurs secondary to P aeruginosa in immunocompromised patients, particularly patients with severe neutropenia in the setting of recent chemotherapy.1,2 Ecthyma gangrenosum can occur anywhere on the body, predominantly in moist areas such as the axillae and groin; the arms and legs, such as in our patient, as well as the trunk and face also may be involved.3 Other causes of EG skin lesions include methicillin-resistant Staphylococcus aureus, Citrobacter freundii, Escherichia coli, fungi such as Candida, and viruses such as herpes simplex virus.2,4-6 Common predisposing conditions associated with EG include neutropenia, leukemia, HIV, diabetes mellitus, extensive burn wounds, and a history of immunosuppressive medications. It also has been known to occur in otherwise healthy, immunocompetent individuals with no difference in clinical manifestation.2
The diagnosis is clinicopathologic, with initial evaluation including blood and wound cultures as well as a complete blood cell count once EG is suspected. An excisional or punch biopsy is performed for confirmation, showing many gram-negative, rod-shaped bacteria in cases of pseudomonal EG.7 Histopathology is characterized by bacterial perivascular invasion that then leads to secondary arteriole thrombosis, tissue edema, and separation of the epidermis.7,8 Resultant ischemic necrosis results in the classic macroscopic appearance of an erythematous macule that rapidly progresses into a central necrotic lesion surrounded by an erythematous or violaceous halo after undergoing a hemorrhagic bullous stage.1,9 A Wood lamp can be used to expedite the diagnosis, as Pseudomonas bacteria excretes a pigment (pyoverdine) that fluoresces yellowish green.10
Ecthyma gangrenosum can be classified as a primary skin lesion that may or may not be followed by bacteremia or as a lesion secondary to pseudomonal bacteremia.11 Bacteremia has been reported in half of cases, with hematogenous metastasis of the infection, likely in manifestations with multiple bilateral lesions.2 Our patient’s presentation of a single lesion revealed a positive blood culture result. Lesions also can develop by direct inoculation of the epidermis causing local destruction of the surrounding tissue. The nonbacteremic form of EG has been associated with a lower mortality rate of around 15% compared to patients with bacteremia ranging from 38% to 96%.12 The presence of neutropenia is the most important prognostic factor for mortality at the time of diagnosis.13
Prompt empiric therapy should be initiated after obtaining wound and blood cultures in those with infection until the causative organism and its susceptibility are identified. Pseudomonal infections account for 4% of all cases of hospital-acquired bacteremia and are the third leading cause of gram-negative bloodstream infection.7 Initial broad-spectrum antibiotics include antipseudomonal β-lactams (piperacillin-tazobactam), cephalosporins (cefepime), fluoroquinolones (levofloxacin), and carbapenems (imipenem).1,7 Medical therapy alone may be sufficient without requiring extensive surgical debridement to remove necrotic tissue in some patients. Surgical debridement usually is warranted for lesions larger than 10 cm in diameter.3 Our patient was treated with intravenous cefepime with resolution and was followed with outpatient oral levofloxacin as appropriate. A high index of suspicion should be maintained for relapsing pseudomonal EG infection among patients with AIDS, as the reported recurrence rate is 57%.14
Clinically, the differential diagnosis of EG presenting in immunocompromised patients or individuals with underlying malignancy includes pyoderma gangrenosum, papulonecrotic tuberculid, and leukemia cutis. An erythematous rash with central necrosis presenting in a patient with systemic symptoms is pathognomonic for erythema migrans and should be considered as a diagnostic possibility in areas endemic for Lyme disease in the United States, including the northeastern, mid-Atlantic, and north-central regions.15 A thorough history, physical examination, basic laboratory studies, and histopathology are critical to differentiate between these entities with similar macroscopic features. Pyoderma gangrenosum histologically manifests as a noninfectious, deep, suppurative folliculitis with leukocytoclastic vasculitis in 40% of cases.16 Although papulonecrotic tuberculid can present with dermal necrosis resulting from a hypersensitivity reaction to antigenic components of mycobacteria, there typically are granulomatous infiltrates present and a lack of observed organisms on histopathology.17 Although leukemia cutis infrequently occurs in patients diagnosed with leukemia, its salient features on pathology are nodular or diffuse infiltrates of leukemic cells in the dermis and subcutis with a high nuclear-to-cytoplasmic ratio, often with prominent nucleoli.18 Lyme disease can present in various ways; however, cutaneous involvement in the primary lesion is histologically characterized by a perivascular lymphohistiocytic infiltrate containing plasma cells at the periphery of the expanding annular lesion and eosinophils present at the center.19
The Diagnosis: Ecthyma Gangrenosum
Histopathology revealed basophilic bacterial rods around necrotic vessels with thrombosis and edema (Figure). Blood and tissue cultures grew Pseudomonas aeruginosa. Based on the histopathology and clinical presentation, a diagnosis of P aeruginosa–associated ecthyma gangrenosum (EG) was made. The patient’s symptoms resolved with intravenous cefepime, and he later was transitioned to oral levofloxacin for outpatient treatment.

Ecthyma gangrenosum is an uncommon cutaneous manifestation of bacteremia that most commonly occurs secondary to P aeruginosa in immunocompromised patients, particularly patients with severe neutropenia in the setting of recent chemotherapy.1,2 Ecthyma gangrenosum can occur anywhere on the body, predominantly in moist areas such as the axillae and groin; the arms and legs, such as in our patient, as well as the trunk and face also may be involved.3 Other causes of EG skin lesions include methicillin-resistant Staphylococcus aureus, Citrobacter freundii, Escherichia coli, fungi such as Candida, and viruses such as herpes simplex virus.2,4-6 Common predisposing conditions associated with EG include neutropenia, leukemia, HIV, diabetes mellitus, extensive burn wounds, and a history of immunosuppressive medications. It also has been known to occur in otherwise healthy, immunocompetent individuals with no difference in clinical manifestation.2
The diagnosis is clinicopathologic, with initial evaluation including blood and wound cultures as well as a complete blood cell count once EG is suspected. An excisional or punch biopsy is performed for confirmation, showing many gram-negative, rod-shaped bacteria in cases of pseudomonal EG.7 Histopathology is characterized by bacterial perivascular invasion that then leads to secondary arteriole thrombosis, tissue edema, and separation of the epidermis.7,8 Resultant ischemic necrosis results in the classic macroscopic appearance of an erythematous macule that rapidly progresses into a central necrotic lesion surrounded by an erythematous or violaceous halo after undergoing a hemorrhagic bullous stage.1,9 A Wood lamp can be used to expedite the diagnosis, as Pseudomonas bacteria excretes a pigment (pyoverdine) that fluoresces yellowish green.10
Ecthyma gangrenosum can be classified as a primary skin lesion that may or may not be followed by bacteremia or as a lesion secondary to pseudomonal bacteremia.11 Bacteremia has been reported in half of cases, with hematogenous metastasis of the infection, likely in manifestations with multiple bilateral lesions.2 Our patient’s presentation of a single lesion revealed a positive blood culture result. Lesions also can develop by direct inoculation of the epidermis causing local destruction of the surrounding tissue. The nonbacteremic form of EG has been associated with a lower mortality rate of around 15% compared to patients with bacteremia ranging from 38% to 96%.12 The presence of neutropenia is the most important prognostic factor for mortality at the time of diagnosis.13
Prompt empiric therapy should be initiated after obtaining wound and blood cultures in those with infection until the causative organism and its susceptibility are identified. Pseudomonal infections account for 4% of all cases of hospital-acquired bacteremia and are the third leading cause of gram-negative bloodstream infection.7 Initial broad-spectrum antibiotics include antipseudomonal β-lactams (piperacillin-tazobactam), cephalosporins (cefepime), fluoroquinolones (levofloxacin), and carbapenems (imipenem).1,7 Medical therapy alone may be sufficient without requiring extensive surgical debridement to remove necrotic tissue in some patients. Surgical debridement usually is warranted for lesions larger than 10 cm in diameter.3 Our patient was treated with intravenous cefepime with resolution and was followed with outpatient oral levofloxacin as appropriate. A high index of suspicion should be maintained for relapsing pseudomonal EG infection among patients with AIDS, as the reported recurrence rate is 57%.14
Clinically, the differential diagnosis of EG presenting in immunocompromised patients or individuals with underlying malignancy includes pyoderma gangrenosum, papulonecrotic tuberculid, and leukemia cutis. An erythematous rash with central necrosis presenting in a patient with systemic symptoms is pathognomonic for erythema migrans and should be considered as a diagnostic possibility in areas endemic for Lyme disease in the United States, including the northeastern, mid-Atlantic, and north-central regions.15 A thorough history, physical examination, basic laboratory studies, and histopathology are critical to differentiate between these entities with similar macroscopic features. Pyoderma gangrenosum histologically manifests as a noninfectious, deep, suppurative folliculitis with leukocytoclastic vasculitis in 40% of cases.16 Although papulonecrotic tuberculid can present with dermal necrosis resulting from a hypersensitivity reaction to antigenic components of mycobacteria, there typically are granulomatous infiltrates present and a lack of observed organisms on histopathology.17 Although leukemia cutis infrequently occurs in patients diagnosed with leukemia, its salient features on pathology are nodular or diffuse infiltrates of leukemic cells in the dermis and subcutis with a high nuclear-to-cytoplasmic ratio, often with prominent nucleoli.18 Lyme disease can present in various ways; however, cutaneous involvement in the primary lesion is histologically characterized by a perivascular lymphohistiocytic infiltrate containing plasma cells at the periphery of the expanding annular lesion and eosinophils present at the center.19
- Abdou A, Hassam B. Ecthyma gangrenosum [in French]. Pan Afr Med J. 2018;30:95. doi:10.11604/pamj.2018.30.95.6244
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis. 2015;34:633-639. doi:10.1007/s10096-014-2277-6
- Vaiman M, Lasarovitch T, Heller L, et al. Ecthyma gangrenosum versus ecthyma-like lesions: should we separate these conditions? Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:69-72. doi:10.15570 /actaapa.2015.18
- Reich HL, Williams Fadeyi D, Naik NS, et al. Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol. 2004;50(5 suppl): S114-S117. doi:10.1016/j.jaad.2003.09.019
- Hawkley T, Chang D, Pollard W, et al. Ecthyma gangrenosum caused by Citrobacter freundii [published online July 27, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220996
- Santhaseelan RG, Muralidhar V. Non-pseudomonal ecthyma gangrenosum caused by methicillin-resistant Staphylococcus aureus (MRSA) in a chronic alcoholic patient [published online August 3, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220983m
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections [published online May 29, 2018]. Drugs Context. 2018;7:212527. doi:10.7573/dic.212527
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662. doi:10.1097/DAD.0000000000000766
- Sarkar S, Patra AK, Mondal M. Ecthyma gangrenosum in the periorbital region in a previously healthy immunocompetent woman without bacteremia. Indian Dermatol Online J. 2016;7:36-39. doi:10.4103/2229-5178.174326
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Van den Broek PJ, Van der Meer JWM, Kunst MW. The pathogenesis of ecthyma gangrenosum. J Infect. 1979;1:263-267. doi:10.1016 /S0163-4453(79)91329-X
- Downey DM, O’Bryan MC, Burdette SD, et al. Ecthyma gangrenosum in a patient with toxic epidermal necrolysis. J Burn Care Res. 2007;28:198-202. doi:10.1097/BCR.0B013E31802CA481
- Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, et al. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92:698-700. doi:10.1590/abd1806-4841.20175580
- Khan MO, Montecalvo MA, Davis I, et al. Ecthyma gangrenosum in patients with acquired immunodeficiency syndrome. Cutis. 2000;66:121-123.
- Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998; 352:557-565.
- Su WP, Schroeter AL, Perry HO, et al. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13:323-330. doi:10.1111/j.1600-0560.1986.tb00466.x
- Tirumalae R, Yeliur IK, Antony M, et al. Papulonecrotic tuberculidclinicopathologic and molecular features of 12 Indian patients. Dermatol Pract Concept. 2014;4:17-22. doi:10.5826/dpc.0402a03
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis [published online August 15, 2015]. Dermatol Online J. 2015;21:13030/qt6m18g35f
- Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167-174. doi:10.4103/0019-5154.110822
- Abdou A, Hassam B. Ecthyma gangrenosum [in French]. Pan Afr Med J. 2018;30:95. doi:10.11604/pamj.2018.30.95.6244
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis. 2015;34:633-639. doi:10.1007/s10096-014-2277-6
- Vaiman M, Lasarovitch T, Heller L, et al. Ecthyma gangrenosum versus ecthyma-like lesions: should we separate these conditions? Acta Dermatovenerol Alp Pannonica Adriat. 2015;24:69-72. doi:10.15570 /actaapa.2015.18
- Reich HL, Williams Fadeyi D, Naik NS, et al. Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol. 2004;50(5 suppl): S114-S117. doi:10.1016/j.jaad.2003.09.019
- Hawkley T, Chang D, Pollard W, et al. Ecthyma gangrenosum caused by Citrobacter freundii [published online July 27, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220996
- Santhaseelan RG, Muralidhar V. Non-pseudomonal ecthyma gangrenosum caused by methicillin-resistant Staphylococcus aureus (MRSA) in a chronic alcoholic patient [published online August 3, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-220983m
- Bassetti M, Vena A, Croxatto A, et al. How to manage Pseudomonas aeruginosa infections [published online May 29, 2018]. Drugs Context. 2018;7:212527. doi:10.7573/dic.212527
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662. doi:10.1097/DAD.0000000000000766
- Sarkar S, Patra AK, Mondal M. Ecthyma gangrenosum in the periorbital region in a previously healthy immunocompetent woman without bacteremia. Indian Dermatol Online J. 2016;7:36-39. doi:10.4103/2229-5178.174326
- Ponka D, Baddar F. Wood lamp examination. Can Fam Physician. 2012;58:976.
- Van den Broek PJ, Van der Meer JWM, Kunst MW. The pathogenesis of ecthyma gangrenosum. J Infect. 1979;1:263-267. doi:10.1016 /S0163-4453(79)91329-X
- Downey DM, O’Bryan MC, Burdette SD, et al. Ecthyma gangrenosum in a patient with toxic epidermal necrolysis. J Burn Care Res. 2007;28:198-202. doi:10.1097/BCR.0B013E31802CA481
- Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, et al. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92:698-700. doi:10.1590/abd1806-4841.20175580
- Khan MO, Montecalvo MA, Davis I, et al. Ecthyma gangrenosum in patients with acquired immunodeficiency syndrome. Cutis. 2000;66:121-123.
- Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998; 352:557-565.
- Su WP, Schroeter AL, Perry HO, et al. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13:323-330. doi:10.1111/j.1600-0560.1986.tb00466.x
- Tirumalae R, Yeliur IK, Antony M, et al. Papulonecrotic tuberculidclinicopathologic and molecular features of 12 Indian patients. Dermatol Pract Concept. 2014;4:17-22. doi:10.5826/dpc.0402a03
- Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lymphoglandular bodies: a clue to acute lymphoblastic leukemia cutis [published online August 15, 2015]. Dermatol Online J. 2015;21:13030/qt6m18g35f
- Vasudevan B, Chatterjee M. Lyme borreliosis and skin. Indian J Dermatol. 2013;58:167-174. doi:10.4103/0019-5154.110822
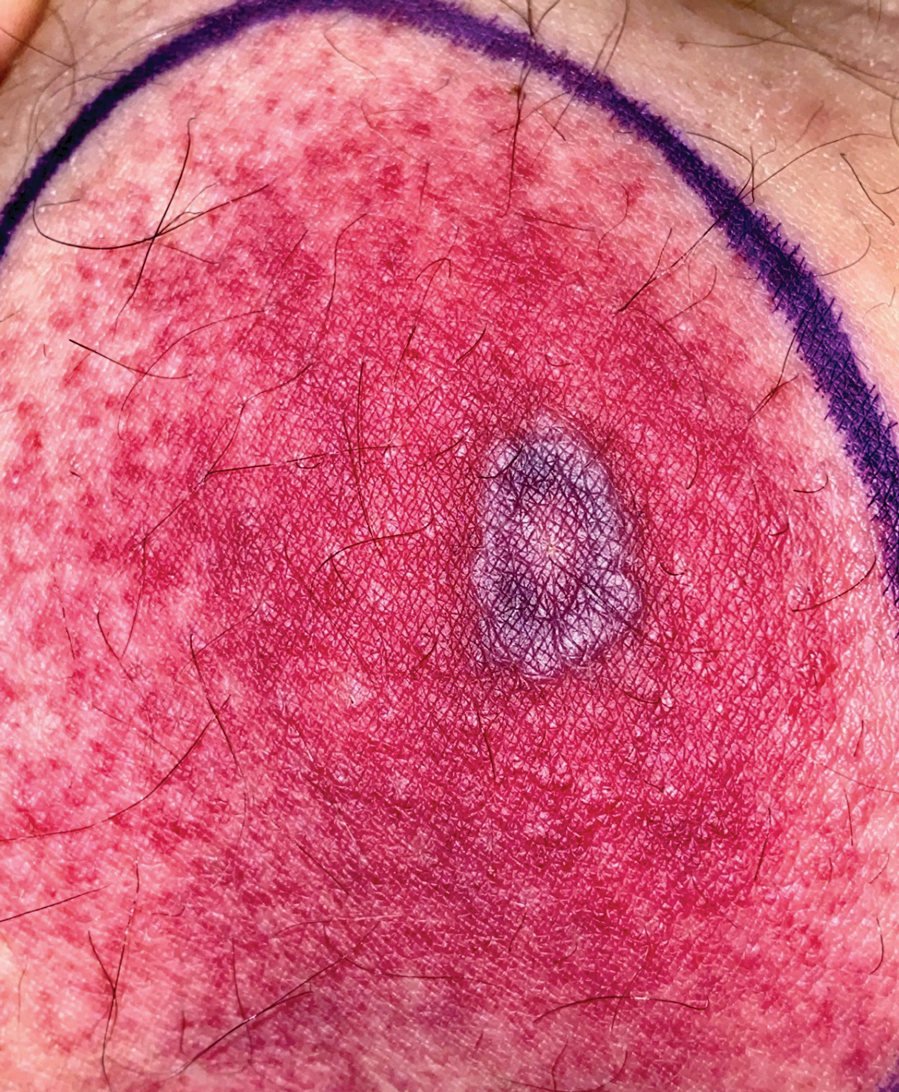
A 58-year-old man who was receiving gilteritinib therapy for relapsed acute myeloid leukemia presented to the emergency department with a painful, rapidly enlarging lesion on the right medial thigh of 2 days’ duration that was accompanied by fever (temperature, 39.2 °C) and body aches. Physical examination revealed a tender annular plaque with a dark violaceous halo overlying a larger area of erythema and induration. Laboratory evaluation revealed a white blood cell count of 600/μL (reference range, 4500–11,000/μL) and an absolute neutrophil count of 200/μL (reference range, 1800–7000/μL). A biopsy was performed.