User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
A Joint Effort to Save the Joints: What Dermatologists Need to Know About Psoriatic Arthritis
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
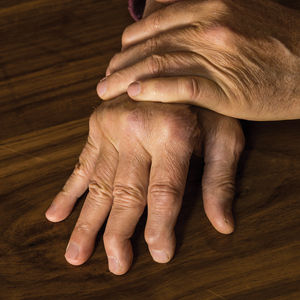
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Interacting With Dermatology Patients Online: Private Practice vs Academic Institute Website Content
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.
Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).
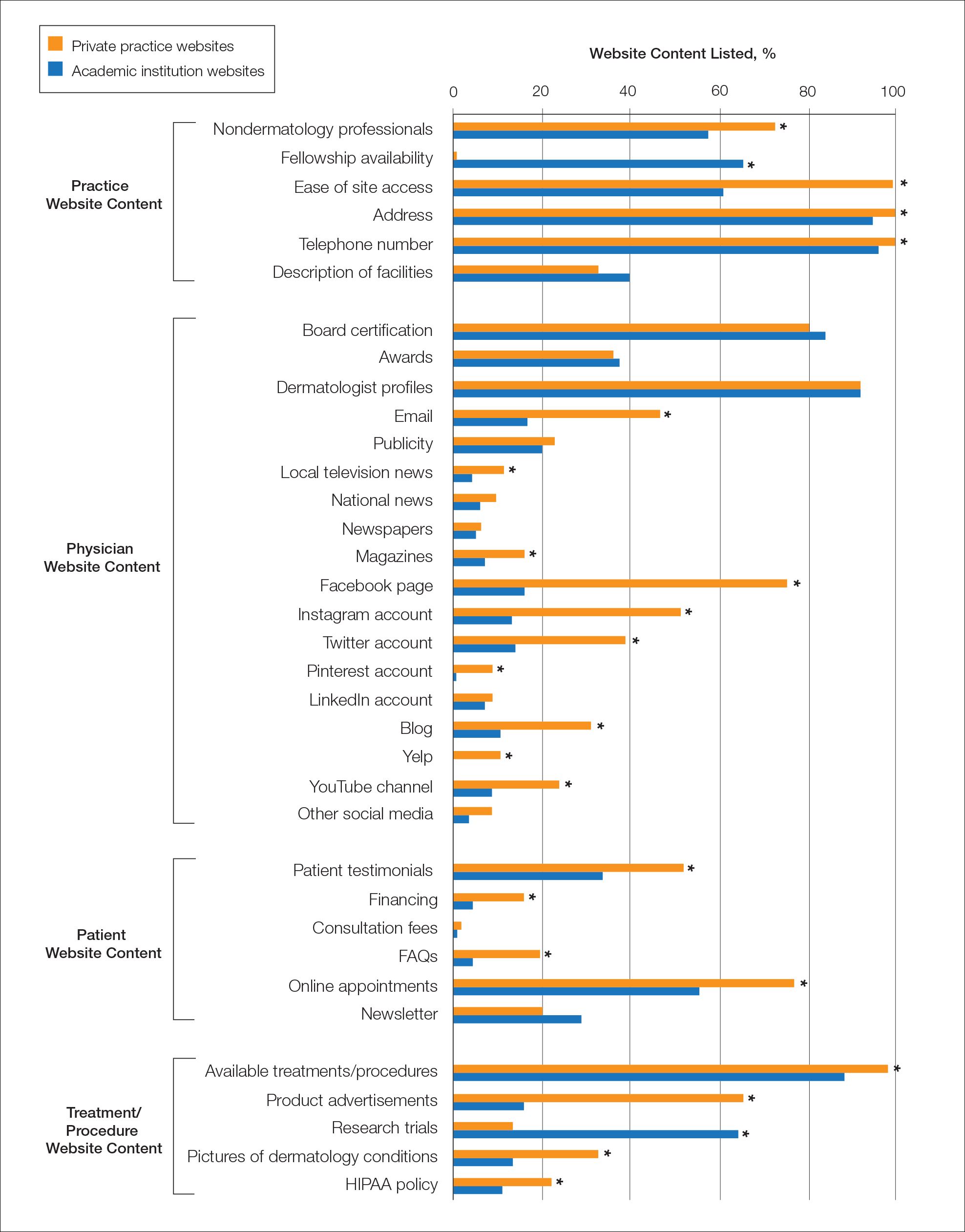
When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
Patients are finding it easier to use online resources to discover health care providers who fit their personalized needs. In the United States, approximately 70% of individuals use the internet to find health care information, and 80% are influenced by the information presented to them on health care websites.1 Patients utilize the internet to better understand treatments offered by providers and their prices as well as how other patients have rated their experience. Providers in private practice also have noticed that many patients are referring themselves vs obtaining a referral from another provider.2 As a result, it is critical for practice websites to have information that is of value to their patients, including the unique qualities and treatments offered. The purpose of this study was to analyze the differences between the content presented on dermatology private practice websites and academic institutional websites.
Methods
Websites Searched —All 140 academic dermatology programs, including both allopathic and osteopathic programs, were queried from the Association of American Medical Colleges (AAMC) database in March 2022. 3 First, the dermatology departmental websites for each program were analyzed to see if they contained information pertinent to patients. Any website that lacked this information or only had information relevant to the dermatology residency program was excluded from the study. After exclusion, a total of 113 websites were used in the academic website cohort. The private practices were found through an incognito Google search with the search term dermatologist and matched to be within 5 miles of each academic institution. The private practices that included at least one board-certified dermatologist and received the highest number of reviews on Google compared to other practices in the same region—a measure of online reputation—were selected to be in the private practice cohort (N = 113). Any duplicate practices, practices belonging to the same conglomerate company, or multispecialty clinics were excluded from the study. Board-certified dermatologists were confirmed using the Find a Dermatologist tool on the American Academy of Dermatology (AAD) website. 4
Website Assessments —Each website was assessed using 23 criteria divided into 4 categories: practice, physician(s), patient, and treatment/procedure (Table). Criteria for social media and publicity were further assessed. Criteria for social media included links on the website to a Facebook page, an Instagram account, a Twitter account, a Pinterest account, a LinkedIn account, a blog, a Yelp page, a YouTube channel, and/or any other social media. Criteria for publicity included links on the website to local television news, national news, newspapers, and/or magazines. 5-8 Ease of site access was determined if the website was the first search result found on Google when searching for each website. Nondermatology professionals included listing of mid-level providers or researchers.

Four individuals (V.S.J., A.C.B., M.E.O., and M.B.B.) independently assessed each of the websites using the established criteria. Each criterion was defined and discussed prior to data collection to maintain consistency. The criteria were determined as being present if the website clearly displayed, stated, explained, or linked to the relevant content. If the website did not directly contain the content, it was determined that the criteria were absent. One other individual (J.P.) independently cross-examined the data for consistency and evaluated for any discrepancies. 8
A raw analysis was done between each cohort. Another analysis was done that controlled for population density and the proportionate population age in each city 9 in which an academic institution/private practice was located. We proposed that more densely populated cities naturally may have more competition between practices, which may result in more optimized websites. 10 We also anticipated similar findings in cities with younger populations, as the younger demographic may be more likely to utilize and value online information when compared to older populations. 11 The websites for each cohort were equally divided into 3 tiers of population density (not shown) and population age (not shown).
Statistical Analysis —Statistical analysis was completed using descriptive statistics, χ 2 testing, and Fisher exact tests where appropriate with a predetermined level of significance of P < .05 in Microsoft Excel.
Results
Demographics —A total of 226 websites from both private practices and academic institutions were evaluated. Of them, only 108 private practices and 108 academic institutions listed practicing dermatologists on their site. Of 108 private practices, 76 (70.4%) had more than one practicing board-certified dermatologist. Of 108 academic institutions, all 108 (100%) institutions had more than one practicing board-certified dermatologist.
Of the dermatologists who practiced at academic institutions (n=2014) and private practices (n=817), 1157 (57.4%) and 419 (51.2%) were females, respectively. The population density of the cities with each of these practices/institutions ranged from 137 individuals per square kilometer to 11,232 individuals per square kilometer (mean [SD] population density, 2579 [2485] individuals per square kilometer). Densely populated, moderately populated, and sparsely populated cities had a median population density of 4618, 1708, and 760 individuals per square kilometer, respectively. The data also were divided into 3 age groups. In the older population tier, the median percentage of individuals older than 64 years was 14.2%, the median percentage of individuals aged 18 to 64 years was 63.8%, and the median percentage of individuals aged 5 to 17 years was 14.9%. In the moderately aged population tier, the median percentage of individuals older than 64 years was 10.2%, the median percentage of individuals aged 18 to 64 years was 70.3%, and the median percentage of individuals aged 5 to 17 years was 13.6%. In the younger population tier, the median percentage of individuals older than 64 years was 12%, the median percentage of individuals aged 18 to 64 years was 66.8%, and the median percentage of individuals aged 5 to 17 years was 15%.
Practice and Physician Content—In the raw analysis (Figure), the most commonly listed types of content (>90% of websites) in both private practice and academic sites was address (range, 95% to 100%), telephone number (range, 97% to 100%), and dermatologist profiles (both 92%). The least commonly listed types of content in both cohorts was publicity (range, 20% to 23%). Private practices were more likely to list profiles of nondermatology professionals (73% vs 56%; P<.02), email (47% vs 17%; P<.0001), and social media (29% vs 8%; P<.0001) compared with academic institution websites. Although Facebook was the most-linked social media account for both groups, 75% of private practice sites included the link compared with 16% of academic institutions. Academic institutions were more likely to list fellowship availability (66% vs 1%; P<.0001). Accessing each website was significantly easier in the private practice cohort (99% vs 61%; P<.0001).

When controlling for population density, private practices were only more likely to list nondermatology professionals’ profiles in densely populated cities when compared with academic institutions (73% vs 41%; P<.01). Academic institutions continued to list fellowship availability more often than private practices regardless of population density. The same trend was observed for private practices with ease of site access and listing of social media.
When controlling for population age, similar trends were seen as when controlling for population density. However, private practices listing nondermatology professionals’ profiles was only more likely in the cities with a proportionately younger population when compared with academic institutions (74% vs 47%; P<.04).
Patient and Treatment/Procedure—The most commonly listed content types on both private practice websites and academic institution websites were available treatments/procedures (range, 89% to 98%). The least commonly listed content included financing for elective procedures (range, 4% to 16%), consultation fees (range, 1% to 2%), FAQs (frequently asked questions)(range, 4% to 20%), and HIPAA (Health Insurance Portability and Accountability Act) policy (range, 12% to 22%). Private practices were more likely to list patient testimonials (52% vs 35%; P<.005), financing (16% vs 4%; P<.005), FAQs (20% vs 4%; P<.001), online appointments (77% vs 56%; P<.001), available treatments/procedures (98% vs 86%; P<.004), product advertisements (66% vs 16%; P<.0001), pictures of dermatology conditions (33% vs 13%; P<.001), and HIPAA policy (22% vs 12%; P<.04). Academic institutions were more likely to list research trials (65% vs 13%; P<.0001).
When controlling for population density, private practices were only more likely to list patient testimonials in densely populated (P=.035) and moderately populated cities (P=.019). The same trend was observed for online appointments in densely populated (P=.0023) and moderately populated cities (P=.037). Private practices continued to list product availability more often than academic institutions regardless of population density or population age. Academic institutions also continued to list research trials more often than private practices regardless of population density or population age.
Comment
Our study uniquely analyzed the differences in website content between private practices and academic institutions in dermatology. Of the 140 academic institutions accredited by the Accreditation Council for Graduate Medical Education (ACGME), only 113 had patient-pertinent websites.
Access to Websites —There was a significant difference in many website content criteria between the 2 groups. Private practice sites were easier to access via a Google search when compared with academic sites, which likely is influenced by the Google search algorithm that ranks websites higher based on several criteria including but not limited to keyword use in the title tag, link popularity of the site, and historic ranking. 12,13 Academic sites often were only accessible through portals found on their main institutional site or institution’s residency site.
Role of Social Media —Social media has been found to assist in educating patients on medical practices as well as selecting a physician. 14,15 Our study found that private practice websites listed links to social media more often than their academic counterparts. Social media consumption is increasing, in part due to the COVID-19 pandemic, and it may be optimal for patients and practices alike to include links on their websites. 16 Facebook and Instagram were listed more often on private practice sites when compared with academic institution sites, which was similar to a recent study analyzing the websites of plastic surgery private practices (N = 310) in which 90% of private practices included some type of social media, with Instagram and Facebook being the most used. 8 Social networking accounts can act as convenient platforms for marketing, providing patient education, and generating referrals, which suggests that the prominence of their usage in private practice poses benefits in patient decision-making when seeking care. 17-19 A study analyzing the impact of Facebook in medicine concluded that a Facebook page can serve as an effective vehicle for medical education, particularly in younger generations that favor technology-oriented teaching methods. 20 A survey on trends in cosmetic facial procedures in plastic surgery found that the most influential online methods patients used for choosing their providers were social media platforms and practice websites. Front-page placement on Google also was commonly associated with the number of social media followers. 21,22 A lack of social media prominence could hinder a website’s potential to reach patients.
Communication With Practices —Our study also found significant differences in other metrics related to a patient’s ability to directly communicate with a practice, such as physical addresses, telephone numbers, products available for direct purchase, and online appointment booking, all of which were listed more often on private practice websites compared with academic institution websites. Online appointment booking also was found more frequently on private practice websites. Although physical addresses and telephone numbers were listed significantly more often on private practice sites, this information was ubiquitous and easily accessible elsewhere. Academic institution websites listed research trials and fellowship training significantly more often than private practices. These differences imply a divergence in focus between private practices and academic institutions, likely because academic institutions are funded in large part from research grants, begetting a cycle of academic contribution. 23 In contrast, private practices may not rely as heavily on academic revenue and may be more likely to prioritize other revenue streams such as product sales. 24
HIPAA Policy —Surprisingly, HIPAA policy rarely was listed on any private (22%) or academic site (12%). Conversely, in the plastic surgery study, HIPAA policy was listed much more often, with more than half of private practices with board-certified plastic surgeons accredited in the year 2015 including it on their website, 8 which may suggest that surgically oriented specialties, particularly cosmetic subspecialties, aim to more noticeably display their privacy policies for patient reassurance.
Study Limitations —There are several limitations of our study. First, it is common for a conglomerate company to own multiple private practices in different specialties. As with academic sites, private practice sites may be limited by the hosting platforms, which often are tedious to navigate. Also noteworthy is the emergence of designated social media management positions—both by practice employees and by third-party firms 25 —but the impact of these positions in private practices and academic institutions has not been fully explored. Finally, inclusion criteria and standardized criteria definitions were chosen based on the precedent established by the authors of similar analyses in plastic surgery and radiology. 5-8 Further investigation into the most valued aspects of care by patients within the context of the type of practice chosen would be valuable in refining inclusion criteria. Additionally, this study did not stratify the data collected based on factors such as gender, race, and geographical location; studies conducted on website traffic analysis patterns that focus on these aspects likely would further explain the significance of these findings. Differences in the length of time to the next available appointment between private practices and academic institutions also may help support our findings. Finally, there is a need for further investigation into the preferences of patients themselves garnered from website traffic alone.
Conclusion
Our study examined a diverse compilation of private practice and academic institution websites and uncovered numerous differences in content. As technology and health care continuously evolve, it is imperative that both private practices and academic institutions are actively adapting to optimize their online presence. In doing so, patients will be better equipped at accessing provider information, gaining familiarity with the practice, and understanding treatment options.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
- Gentry ZL, Ananthasekar S, Yeatts M, et al. Can patients find an endocrine surgeon? how hospital websites hide the expertise of these medical professionals. Am J Surg . 2021;221:101-105.
- Pollack CE, Rastegar A, Keating NL, et al. Is self-referral associated with higher quality care? Health Serv Res . 2015;50:1472-1490.
- Association of American Medical Colleges. Residency Explorer TM tool. Accessed May 15, 2023. https://students-residents.aamc.org/apply-smart-residency/residency-explorer-tool
- Find a dermatologist. American Academy of Dermatology website. Accessed May 15, 2023. https://find-a-derm.aad.org/
- Johnson EJ, Doshi AM, Rosenkrantz AB. Strengths and deficiencies in the content of US radiology private practices’ websites. J Am Coll Radiol. 2017;14:431-435.
- Brunk D. Medical website expert shares design tips. Dermatology News . February 9, 2012. Accessed May 15, 2023. https://www.mdedge.com/dermatology/article/47413/health-policy/medical-website-expert-shares-design-tips
- Kuhnigk O, Ramuschkat M, Schreiner J, et al. Internet presence of neurologists, psychiatrists and medical psychotherapists in private practice [in German]. Psychiatr Prax . 2013;41:142-147.
- Ananthasekar S, Patel JJ, Patel NJ, et al. The content of US plastic surgery private practices’ websites. Ann Plast Surg . 2021;86(6S suppl 5):S578-S584.
- US Census Bureau. Age and Sex: 2021. Updated December 2, 2021. Accessed March 15, 2023. https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059
- Porter ME. The competitive advantage of the inner city. Harvard Business Review . Published August 1, 2014. https://hbr.org/1995/05/the-competitive-advantage-of-the-inner-city
- Clark PG. The social allocation of health care resources: ethical dilemmas in age-group competition. Gerontologist. 1985;25:119-125.
- Su A-J, Hu YC, Kuzmanovic A, et al. How to improve your Google ranking: myths and reality. ACM Transactions on the Web . 2014;8. https://dl.acm.org/doi/abs/10.1145/2579990
- McCormick K. 39 ways to increase traffic to your website. WordStream website. Published March 28, 2023. Accessed May 22, 2023. https://www.wordstream.com/blog/ws/2014/08/14/increase-traffic-to-my-website
- Montemurro P, Porcnik A, Hedén P, et al. The influence of social media and easily accessible online information on the aesthetic plastic surgery practice: literature review and our own experience. Aesthetic Plast Surg . 2015;39:270-277.
- Steehler KR, Steehler MK, Pierce ML, et al. Social media’s role in otolaryngology–head and neck surgery. Otolaryngol Head Neck Surg . 2013;149:521-524.
- Tsao S-F, Chen H, Tisseverasinghe T, et al. What social media told us in the time of COVID-19: a scoping review. Lancet Digit Health . 2021;3:E175-E194.
- Geist R, Militello M, Albrecht JM, et al. Social media and clinical research in dermatology. Curr Dermatol Rep . 2021;10:105-111.
- McLawhorn AS, De Martino I, Fehring KA, et al. Social media and your practice: navigating the surgeon-patient relationship. Curr Rev Musculoskelet Med . 2016;9:487-495.
- Thomas RB, Johnson PT, Fishman EK. Social media for global education: pearls and pitfalls of using Facebook, Twitter, and Instagram. J Am Coll Radiol . 2018;15:1513-1516.
- Lugo-Fagundo C, Johnson MB, Thomas RB, et al. New frontiers in education: Facebook as a vehicle for medical information delivery. J Am Coll Radiol . 2016;13:316-319.
- Ho T-VT, Dayan SH. How to leverage social media in private practice. Facial Plast Surg Clin North Am . 2020;28:515-522.
- Fan KL, Graziano F, Economides JM, et al. The public’s preferences on plastic surgery social media engagement and professionalism. Plast Reconstr Surg . 2019;143:619-630.
- Jacob BA, Lefgren L. The impact of research grant funding on scientific productivity. J Public Econ. 2011;95:1168-1177.
- Baumann L. Ethics in cosmetic dermatology. Clin Dermatol. 2012;30:522-527.
- Miller AR, Tucker C. Active social media management: the case of health care. Info Sys Res . 2013;24:52-70.
Practice Points
- Dermatologists at both private practices and academic institutions should understand that website content often may be the most accessible source of information about the practice available to patients and should be as specific and detailed as possible.
- When compared to private practices, academic institutions largely fail to have a social media presence, which may limit patient interaction with their websites.
Glitter Effects of Nail Art on Optical Coherence Tomography
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).
We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.
Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Lower racial disparity in melanoma diagnoses in vets than U.S. men overall, study finds
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
a new analysis shows.
“The trend of a lower racial disparity in the VA in the proportion of melanomas with local disease and in the proportion of distant metastasis at presentation was observed across age groups,” wrote Martin A. Weinstock MD, PhD, and Rachel K. Lim, of the department of dermatology at Brown University, Providence, R.I., and the Center for Dermatoepidemiology at the VA Providence Healthcare System. The study was published online in the Journal of the American Academy of Dermatology.
“Melanoma was the fourth-most common cancer [diagnosed] in male VA patients in 2010,” wrote the authors, who also pointed out that “prior surveys found that 11%-13% of U.S. active-duty personnel routinely use sunscreen despite significant occupational sun exposure. Racial disparities are important concerns in the VA and elsewhere.”
To compare the stage of melanoma at presentation among White and non-Whites patients in the VA and in the general U.S. population, the researchers identified invasive cutaneous melanoma cases from 2000 to 2019 in the VA Corporate Data Warehouse and the Surveillance, Epidemiology and End Results Program (SEER).
They restricted the analysis to men because of the small proportion of women in the at-risk veteran population and excluded cases with an age younger than 20, those with unknown histology, and melanoma in situ. The researchers performed two-tailed z-tests to evaluate the difference in proportions of melanoma stages between the veteran population and the general population.
The analysis included 44,077 cases of invasive melanoma in the VA and 217,030 in SEER. Racial disparities in melanoma staging were substantially less pronounced in the VA than in SEER.
In the VA, localized disease represented 77.9% of melanomas among Whites versus 71.0% among non-Whites. But in SEER, localized disease represented 80.7% of melanomas among Whites versus 61.5% in non-Whites – over double the VA disparity (P < .0001).
Likewise, the disparity between Whites and nonwhites observed for regional or distant metastatic disease at presentation in the VA was lower than the disparity observed in SEER. For example, in the VA, distant metastatic disease at presentation represented 6.1% of melanomas among Whites versus 8.6% among non-Whites, while in SEER it represented 4.8% of melanomas among Whites versus 11.3% in non-Whites – again, more than double the VA disparity (P < .0001).
“These differences between the VA and SEER were less marked” among those older than 65 years, the researchers wrote. “Notably, the differences between VA and SEER in racial disparities among those greater than 65 in age were still significant for localized disease and for distant metastasis.”
The findings suggest that the VA “may be more effective in reducing racial disparities in melanoma stage at diagnosis, potentially due to all patients in the VA dataset having insured access to health care, regardless of socioeconomic status,” the researchers concluded. Similarly, the decreased difference in racial disparities observed in patients older than 65 across systems “may be related to the availability of Medicare to the older general populations. The authors acknowledged several study limitations, such as the predominantly elderly and male VA population, potentially underreported utilization of non-VA dermatologic care, and variation in geographic regions covered by each database.
Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the work, said in an interview he would have liked to see a more detailed breakdown of the younger patients, “for those in their 30s and 40s, to see if this trend held up.”
He would have also liked to see how the data trended over time, adding, “while this, broadly, may be good news for our veterans, attributing this finding to a reduction in access disparity or some other organizational intervention seems a little premature. Regardless, Dr. Weinstock has given us, once again, information from our veterans to probe for the betterment of all patients.”
The researchers reported having no relevant disclosures and the study had no funding. Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Oval Brown Plaque on the Palm
The Diagnosis: Poroma
Histopathology showed an endophytic expansion of the epidermis by bland, uniform, basaloid epithelial cells with focal ductal differentiation and an abrupt transition with surrounding epidermal keratinocytes (Figure), consistent with a diagnosis of poroma. The patient elected to monitor the lesion rather than to have it excised.
Eccrine poroma, used interchangeably with the term poroma, is a rare benign adnexal tumor of the eccrine sweat glands resulting from proliferation of the acrosyringium.1,2 It often occurs on the palms or soles, though it also can arise anywhere sweat glands are present.1 Eccrine poromas often appear in middle-aged individuals as singular, well-circumscribed, red-brown papules or nodules.3 A characteristic feature is a shallow, cup-shaped depression within the larger papule or nodule.1
Because the condition is benign and often asymptomatic, it can be safely monitored for progression.1 However, if the lesion is symptomatic or located in a sensitive area, complete excision is curative.4 Eccrine poromas can recur, making close monitoring following excision important.5 The development of bleeding, itching, or pain in a previously asymptomatic lesion may indicate possible malignant transformation, which occurs in only 18% of cases.6
The differential diagnosis includes basal cell carcinoma, circumscribed acral hypokeratosis, Kaposi sarcoma, and pyogenic granuloma. Basal cell carcinoma is the most common type of skin cancer.7 In rare cases it has been shown to present on the palms or soles as a slowgrowing, reddish-pink papule or plaque with central ulceration. It typically is asymptomatic. Histopathology shows dermal nests of basaloid cells with peripheral palisading, stromal mucin, and peritumoral clefts. Treatment is surgical excision.7
Circumscribed acral hypokeratosis presents on the palms or soles as a solitary, shallow, well-defined lesion with a flat base and raised border.8 It often is red-pink in color and most frequently occurs in middle-aged women. Although the cause of the condition is unknown, it is thought to be the result of trauma or human papillomavirus infection.8 Biopsy results characteristically show hypokeratosis demarcated by a sharp and frayed cutoff from uninvolved acral skin with discrete hypogranulosis, dilated blood vessels in the papillary dermis, and slightly thickened collagen fibers in the reticular dermis.9 Surgical excision is a potential treatment option, as topical corticosteroids, retinoids, and calcipotriene have not been shown to be effective; spontaneous resolution has been reported.8
Kaposi sarcoma is a vascular neoplasm that is associated with human herpesvirus 8 infection.10 It typically presents on mucocutaneous sites and the lower extremities. Palmar involvement has been reported in rare cases, occurring as a solitary, well-demarcated, violaceous macule or patch that may be painful.10-12 Characteristic histopathologic features include a proliferation in the dermis of slitlike vascular spaces and spindle cell proliferation.13 Treatment options include cryosurgery; pulsed dye laser; and topical, intralesional, or systemic chemotherapy agents, depending on the stage of the patient’s disease. Antiretroviral therapy is indicated for patients with Kaposi sarcoma secondary to AIDS.14
Pyogenic granuloma presents as a solitary red-brown or bluish-black papule or nodule that bleeds easily when manipulated.15 It commonly occurs following trauma, typically on the fingers, feet, and lips.6 Although benign, potential complications include ulceration and blood loss. Pyogenic granulomas can be treated via curettage and cautery, excision, cryosurgery, or pulsed dye laser.15
- Wankhade V, Singh R, Sadhwani V, et al. Eccrine poroma. Indian Dermatol Online J. 2015;6:304-305.
- Yorulmaz A, Aksoy GG, Ozhamam EU. A growing mass under the nail: subungual eccrine poroma. Skin Appendage Disord. 2020;6:254-257.
- Wang Y, Liu M, Zheng Y, et al. Eccrine poroma presented as spindleshaped plaque: a case report. Medicine (Baltimore). 2021;100:E25971. doi:10.1097/MD.0000000000025971
- Sharma M, Singh M, Gupta K, et al. Eccrine poroma of the eyelid. Indian J Ophthalmol. 2020;68:2522.
- Rasool MN, Hawary MB. Benign eccrine poroma in the palm of the hand. Ann Saudi Med. 2004;24:46-47.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms [published online April 2, 2014]. Int J Dermatol. 2014;53:1053-1061. doi:10.1111/ijd.12448
- López-Sánchez C, Ferguson P, Collgros H. Basal cell carcinoma of the palm: an unusual presentation of a common tumour [published online August 6, 2019]. Australas J Dermatol. 2020;61:69-70. doi:10.1111/ajd.13129
- Berk DR, Böer A, Bauschard FD, et al. Circumscribed acral hypokeratosis [published online April 6, 2007]. J Am Acad Dermatol. 2007;57:292-296. doi:10.1016/j.jaad.2007.02.022
- Majluf-Cáceres P, Vera-Kellet C, González-Bombardiere S. New dermoscopic keys for circumscribed acral hypokeratosis: report of four cases. Dermatol Pract Concept. 2021;11:E2021010. doi:10.5826/dpc.1102a10
- Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: role of iron filings in its development? Br J Dermatol. 2003;148:1061-1063. doi:10.1046/j.1365-2133.2003.05331.x
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Al Zolibani AA, Al Robaee AA. Primary palmoplantar Kaposi’s sarcoma: an unusual presentation. Skinmed. 2006;5:248-249. doi:10.1111/j.1540-9740.2006.04662.x
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Etemad SA, Dewan AK. Kaposi sarcoma updates [published online July 10, 2019]. Dermatol Clin. 2019;37:505-517. doi:10.1016/j. det.2019.05.008
- Murthy SC, Nagaraj A. Pyogenic granuloma. Indian Pediatr. 2012;49:855. doi:10.1007/s13312-012-0184-4
The Diagnosis: Poroma
Histopathology showed an endophytic expansion of the epidermis by bland, uniform, basaloid epithelial cells with focal ductal differentiation and an abrupt transition with surrounding epidermal keratinocytes (Figure), consistent with a diagnosis of poroma. The patient elected to monitor the lesion rather than to have it excised.

Eccrine poroma, used interchangeably with the term poroma, is a rare benign adnexal tumor of the eccrine sweat glands resulting from proliferation of the acrosyringium.1,2 It often occurs on the palms or soles, though it also can arise anywhere sweat glands are present.1 Eccrine poromas often appear in middle-aged individuals as singular, well-circumscribed, red-brown papules or nodules.3 A characteristic feature is a shallow, cup-shaped depression within the larger papule or nodule.1
Because the condition is benign and often asymptomatic, it can be safely monitored for progression.1 However, if the lesion is symptomatic or located in a sensitive area, complete excision is curative.4 Eccrine poromas can recur, making close monitoring following excision important.5 The development of bleeding, itching, or pain in a previously asymptomatic lesion may indicate possible malignant transformation, which occurs in only 18% of cases.6
The differential diagnosis includes basal cell carcinoma, circumscribed acral hypokeratosis, Kaposi sarcoma, and pyogenic granuloma. Basal cell carcinoma is the most common type of skin cancer.7 In rare cases it has been shown to present on the palms or soles as a slowgrowing, reddish-pink papule or plaque with central ulceration. It typically is asymptomatic. Histopathology shows dermal nests of basaloid cells with peripheral palisading, stromal mucin, and peritumoral clefts. Treatment is surgical excision.7
Circumscribed acral hypokeratosis presents on the palms or soles as a solitary, shallow, well-defined lesion with a flat base and raised border.8 It often is red-pink in color and most frequently occurs in middle-aged women. Although the cause of the condition is unknown, it is thought to be the result of trauma or human papillomavirus infection.8 Biopsy results characteristically show hypokeratosis demarcated by a sharp and frayed cutoff from uninvolved acral skin with discrete hypogranulosis, dilated blood vessels in the papillary dermis, and slightly thickened collagen fibers in the reticular dermis.9 Surgical excision is a potential treatment option, as topical corticosteroids, retinoids, and calcipotriene have not been shown to be effective; spontaneous resolution has been reported.8
Kaposi sarcoma is a vascular neoplasm that is associated with human herpesvirus 8 infection.10 It typically presents on mucocutaneous sites and the lower extremities. Palmar involvement has been reported in rare cases, occurring as a solitary, well-demarcated, violaceous macule or patch that may be painful.10-12 Characteristic histopathologic features include a proliferation in the dermis of slitlike vascular spaces and spindle cell proliferation.13 Treatment options include cryosurgery; pulsed dye laser; and topical, intralesional, or systemic chemotherapy agents, depending on the stage of the patient’s disease. Antiretroviral therapy is indicated for patients with Kaposi sarcoma secondary to AIDS.14
Pyogenic granuloma presents as a solitary red-brown or bluish-black papule or nodule that bleeds easily when manipulated.15 It commonly occurs following trauma, typically on the fingers, feet, and lips.6 Although benign, potential complications include ulceration and blood loss. Pyogenic granulomas can be treated via curettage and cautery, excision, cryosurgery, or pulsed dye laser.15
The Diagnosis: Poroma
Histopathology showed an endophytic expansion of the epidermis by bland, uniform, basaloid epithelial cells with focal ductal differentiation and an abrupt transition with surrounding epidermal keratinocytes (Figure), consistent with a diagnosis of poroma. The patient elected to monitor the lesion rather than to have it excised.

Eccrine poroma, used interchangeably with the term poroma, is a rare benign adnexal tumor of the eccrine sweat glands resulting from proliferation of the acrosyringium.1,2 It often occurs on the palms or soles, though it also can arise anywhere sweat glands are present.1 Eccrine poromas often appear in middle-aged individuals as singular, well-circumscribed, red-brown papules or nodules.3 A characteristic feature is a shallow, cup-shaped depression within the larger papule or nodule.1
Because the condition is benign and often asymptomatic, it can be safely monitored for progression.1 However, if the lesion is symptomatic or located in a sensitive area, complete excision is curative.4 Eccrine poromas can recur, making close monitoring following excision important.5 The development of bleeding, itching, or pain in a previously asymptomatic lesion may indicate possible malignant transformation, which occurs in only 18% of cases.6
The differential diagnosis includes basal cell carcinoma, circumscribed acral hypokeratosis, Kaposi sarcoma, and pyogenic granuloma. Basal cell carcinoma is the most common type of skin cancer.7 In rare cases it has been shown to present on the palms or soles as a slowgrowing, reddish-pink papule or plaque with central ulceration. It typically is asymptomatic. Histopathology shows dermal nests of basaloid cells with peripheral palisading, stromal mucin, and peritumoral clefts. Treatment is surgical excision.7
Circumscribed acral hypokeratosis presents on the palms or soles as a solitary, shallow, well-defined lesion with a flat base and raised border.8 It often is red-pink in color and most frequently occurs in middle-aged women. Although the cause of the condition is unknown, it is thought to be the result of trauma or human papillomavirus infection.8 Biopsy results characteristically show hypokeratosis demarcated by a sharp and frayed cutoff from uninvolved acral skin with discrete hypogranulosis, dilated blood vessels in the papillary dermis, and slightly thickened collagen fibers in the reticular dermis.9 Surgical excision is a potential treatment option, as topical corticosteroids, retinoids, and calcipotriene have not been shown to be effective; spontaneous resolution has been reported.8
Kaposi sarcoma is a vascular neoplasm that is associated with human herpesvirus 8 infection.10 It typically presents on mucocutaneous sites and the lower extremities. Palmar involvement has been reported in rare cases, occurring as a solitary, well-demarcated, violaceous macule or patch that may be painful.10-12 Characteristic histopathologic features include a proliferation in the dermis of slitlike vascular spaces and spindle cell proliferation.13 Treatment options include cryosurgery; pulsed dye laser; and topical, intralesional, or systemic chemotherapy agents, depending on the stage of the patient’s disease. Antiretroviral therapy is indicated for patients with Kaposi sarcoma secondary to AIDS.14
Pyogenic granuloma presents as a solitary red-brown or bluish-black papule or nodule that bleeds easily when manipulated.15 It commonly occurs following trauma, typically on the fingers, feet, and lips.6 Although benign, potential complications include ulceration and blood loss. Pyogenic granulomas can be treated via curettage and cautery, excision, cryosurgery, or pulsed dye laser.15
- Wankhade V, Singh R, Sadhwani V, et al. Eccrine poroma. Indian Dermatol Online J. 2015;6:304-305.
- Yorulmaz A, Aksoy GG, Ozhamam EU. A growing mass under the nail: subungual eccrine poroma. Skin Appendage Disord. 2020;6:254-257.
- Wang Y, Liu M, Zheng Y, et al. Eccrine poroma presented as spindleshaped plaque: a case report. Medicine (Baltimore). 2021;100:E25971. doi:10.1097/MD.0000000000025971
- Sharma M, Singh M, Gupta K, et al. Eccrine poroma of the eyelid. Indian J Ophthalmol. 2020;68:2522.
- Rasool MN, Hawary MB. Benign eccrine poroma in the palm of the hand. Ann Saudi Med. 2004;24:46-47.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms [published online April 2, 2014]. Int J Dermatol. 2014;53:1053-1061. doi:10.1111/ijd.12448
- López-Sánchez C, Ferguson P, Collgros H. Basal cell carcinoma of the palm: an unusual presentation of a common tumour [published online August 6, 2019]. Australas J Dermatol. 2020;61:69-70. doi:10.1111/ajd.13129
- Berk DR, Böer A, Bauschard FD, et al. Circumscribed acral hypokeratosis [published online April 6, 2007]. J Am Acad Dermatol. 2007;57:292-296. doi:10.1016/j.jaad.2007.02.022
- Majluf-Cáceres P, Vera-Kellet C, González-Bombardiere S. New dermoscopic keys for circumscribed acral hypokeratosis: report of four cases. Dermatol Pract Concept. 2021;11:E2021010. doi:10.5826/dpc.1102a10
- Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: role of iron filings in its development? Br J Dermatol. 2003;148:1061-1063. doi:10.1046/j.1365-2133.2003.05331.x
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Al Zolibani AA, Al Robaee AA. Primary palmoplantar Kaposi’s sarcoma: an unusual presentation. Skinmed. 2006;5:248-249. doi:10.1111/j.1540-9740.2006.04662.x
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Etemad SA, Dewan AK. Kaposi sarcoma updates [published online July 10, 2019]. Dermatol Clin. 2019;37:505-517. doi:10.1016/j. det.2019.05.008
- Murthy SC, Nagaraj A. Pyogenic granuloma. Indian Pediatr. 2012;49:855. doi:10.1007/s13312-012-0184-4
- Wankhade V, Singh R, Sadhwani V, et al. Eccrine poroma. Indian Dermatol Online J. 2015;6:304-305.
- Yorulmaz A, Aksoy GG, Ozhamam EU. A growing mass under the nail: subungual eccrine poroma. Skin Appendage Disord. 2020;6:254-257.
- Wang Y, Liu M, Zheng Y, et al. Eccrine poroma presented as spindleshaped plaque: a case report. Medicine (Baltimore). 2021;100:E25971. doi:10.1097/MD.0000000000025971
- Sharma M, Singh M, Gupta K, et al. Eccrine poroma of the eyelid. Indian J Ophthalmol. 2020;68:2522.
- Rasool MN, Hawary MB. Benign eccrine poroma in the palm of the hand. Ann Saudi Med. 2004;24:46-47.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms [published online April 2, 2014]. Int J Dermatol. 2014;53:1053-1061. doi:10.1111/ijd.12448
- López-Sánchez C, Ferguson P, Collgros H. Basal cell carcinoma of the palm: an unusual presentation of a common tumour [published online August 6, 2019]. Australas J Dermatol. 2020;61:69-70. doi:10.1111/ajd.13129
- Berk DR, Böer A, Bauschard FD, et al. Circumscribed acral hypokeratosis [published online April 6, 2007]. J Am Acad Dermatol. 2007;57:292-296. doi:10.1016/j.jaad.2007.02.022
- Majluf-Cáceres P, Vera-Kellet C, González-Bombardiere S. New dermoscopic keys for circumscribed acral hypokeratosis: report of four cases. Dermatol Pract Concept. 2021;11:E2021010. doi:10.5826/dpc.1102a10
- Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: role of iron filings in its development? Br J Dermatol. 2003;148:1061-1063. doi:10.1046/j.1365-2133.2003.05331.x
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Al Zolibani AA, Al Robaee AA. Primary palmoplantar Kaposi’s sarcoma: an unusual presentation. Skinmed. 2006;5:248-249. doi:10.1111/j.1540-9740.2006.04662.x
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Etemad SA, Dewan AK. Kaposi sarcoma updates [published online July 10, 2019]. Dermatol Clin. 2019;37:505-517. doi:10.1016/j. det.2019.05.008
- Murthy SC, Nagaraj A. Pyogenic granuloma. Indian Pediatr. 2012;49:855. doi:10.1007/s13312-012-0184-4
A 43-year-old woman presented with a painful lesion on the palm of 30 years’ duration that had grown in size. Physical examination revealed an oval, brown, lobulated plaque with a hyperkeratotic rim on the left palm. She reported bleeding and pain. A shallow cup-shaped depression was noted within the plaque. A 4-mm punch biopsy was performed.
Abrocitinib remains effective at 96 weeks, in older as well as younger adults
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
AT RAD 2023
IL-17 inhibitor approved in Europe for hidradenitis suppurativa
The biologic is the first interleukin-17A (IL-17A) inhibitor to be approved for the treatment of moderate to severe HS. The manufacturer, Novartis, expects a regulatory decision from the U.S. Food and Drug Administration later this year, according to a company press release announcing the approval.
The European approval is based on the results from the phase 3 SUNSHINE and SUNRISE trials, which evaluated the efficacy, safety, and tolerability of the drug. The multicenter, randomized, placebo-controlled, double-blind trials enrolled a total of more than 1,000 adults with moderate to severe HS.
Patients were randomly assigned either to receive subcutaneous secukinumab 300 mg every 2 weeks or 4 weeks or to receive placebo. The treatment was effective at improving the symptoms of HS when given every 2 weeks, according to results recently published in The Lancet.
The primary outcome measure for both trials was HS clinical response – defined as a decrease in abscess and inflammatory nodule count by 50% or more with no increase in the number of abscesses or draining fistulae, compared with baseline.
In the studies, 42% and 45% of patients treated with secukinumab every 2 weeks in the SUNRISE and SUNSHINE trials, respectively, had a clinical response at 16 weeks, compared with 31% and 34% among those who received placebo, which were statistically significant differences. A significant clinical response was seen at week 4 in the SUNSHINE trial and in week 2 in the SUNRISE trial. In both trials, clinical efficacy was sustained to the end of the trial, at week 52.
Headaches were the most common side effect. They affected approximately 1 in 10 patients in both trials.
HS, also called acne inversa, is a chronic skin condition that causes painful lesions. The condition affects 1%- 2% of the U.S. population, according to the nonprofit Hidradenitis Suppurativa Foundation. It also disproportionately affects young adults, women, and Black patients.
In Europe, about 200,000 people live with moderate to severe stages of the condition, according to the Novartis press release.
Secukinumab inhibits IL-17A, a cytokine involved in the inflammation of psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, and nonradiographic axial spondylarthritis. It has been approved for the treatment of those conditions, as well as for the treatment of juvenile idiopathic arthritis and enthesitis-related arthritis in the United States and the European Union.
The only other approved biologic therapy for HS is the tumor necrosis factor inhibitor adalimumab.
Novartis is investigating the potential application of secukinumab for the treatment of lupus nephritis and giant cell arteritis, as well as polymyalgia rheumatica and rotator cuff tendinopathy, according to the company press release.
The study published in The Lancet was funded by Novartis.
A version of this article first appeared on Medscape.com.
The biologic is the first interleukin-17A (IL-17A) inhibitor to be approved for the treatment of moderate to severe HS. The manufacturer, Novartis, expects a regulatory decision from the U.S. Food and Drug Administration later this year, according to a company press release announcing the approval.
The European approval is based on the results from the phase 3 SUNSHINE and SUNRISE trials, which evaluated the efficacy, safety, and tolerability of the drug. The multicenter, randomized, placebo-controlled, double-blind trials enrolled a total of more than 1,000 adults with moderate to severe HS.
Patients were randomly assigned either to receive subcutaneous secukinumab 300 mg every 2 weeks or 4 weeks or to receive placebo. The treatment was effective at improving the symptoms of HS when given every 2 weeks, according to results recently published in The Lancet.
The primary outcome measure for both trials was HS clinical response – defined as a decrease in abscess and inflammatory nodule count by 50% or more with no increase in the number of abscesses or draining fistulae, compared with baseline.
In the studies, 42% and 45% of patients treated with secukinumab every 2 weeks in the SUNRISE and SUNSHINE trials, respectively, had a clinical response at 16 weeks, compared with 31% and 34% among those who received placebo, which were statistically significant differences. A significant clinical response was seen at week 4 in the SUNSHINE trial and in week 2 in the SUNRISE trial. In both trials, clinical efficacy was sustained to the end of the trial, at week 52.
Headaches were the most common side effect. They affected approximately 1 in 10 patients in both trials.
HS, also called acne inversa, is a chronic skin condition that causes painful lesions. The condition affects 1%- 2% of the U.S. population, according to the nonprofit Hidradenitis Suppurativa Foundation. It also disproportionately affects young adults, women, and Black patients.
In Europe, about 200,000 people live with moderate to severe stages of the condition, according to the Novartis press release.
Secukinumab inhibits IL-17A, a cytokine involved in the inflammation of psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, and nonradiographic axial spondylarthritis. It has been approved for the treatment of those conditions, as well as for the treatment of juvenile idiopathic arthritis and enthesitis-related arthritis in the United States and the European Union.
The only other approved biologic therapy for HS is the tumor necrosis factor inhibitor adalimumab.
Novartis is investigating the potential application of secukinumab for the treatment of lupus nephritis and giant cell arteritis, as well as polymyalgia rheumatica and rotator cuff tendinopathy, according to the company press release.
The study published in The Lancet was funded by Novartis.
A version of this article first appeared on Medscape.com.
The biologic is the first interleukin-17A (IL-17A) inhibitor to be approved for the treatment of moderate to severe HS. The manufacturer, Novartis, expects a regulatory decision from the U.S. Food and Drug Administration later this year, according to a company press release announcing the approval.
The European approval is based on the results from the phase 3 SUNSHINE and SUNRISE trials, which evaluated the efficacy, safety, and tolerability of the drug. The multicenter, randomized, placebo-controlled, double-blind trials enrolled a total of more than 1,000 adults with moderate to severe HS.
Patients were randomly assigned either to receive subcutaneous secukinumab 300 mg every 2 weeks or 4 weeks or to receive placebo. The treatment was effective at improving the symptoms of HS when given every 2 weeks, according to results recently published in The Lancet.
The primary outcome measure for both trials was HS clinical response – defined as a decrease in abscess and inflammatory nodule count by 50% or more with no increase in the number of abscesses or draining fistulae, compared with baseline.
In the studies, 42% and 45% of patients treated with secukinumab every 2 weeks in the SUNRISE and SUNSHINE trials, respectively, had a clinical response at 16 weeks, compared with 31% and 34% among those who received placebo, which were statistically significant differences. A significant clinical response was seen at week 4 in the SUNSHINE trial and in week 2 in the SUNRISE trial. In both trials, clinical efficacy was sustained to the end of the trial, at week 52.
Headaches were the most common side effect. They affected approximately 1 in 10 patients in both trials.
HS, also called acne inversa, is a chronic skin condition that causes painful lesions. The condition affects 1%- 2% of the U.S. population, according to the nonprofit Hidradenitis Suppurativa Foundation. It also disproportionately affects young adults, women, and Black patients.
In Europe, about 200,000 people live with moderate to severe stages of the condition, according to the Novartis press release.
Secukinumab inhibits IL-17A, a cytokine involved in the inflammation of psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, and nonradiographic axial spondylarthritis. It has been approved for the treatment of those conditions, as well as for the treatment of juvenile idiopathic arthritis and enthesitis-related arthritis in the United States and the European Union.
The only other approved biologic therapy for HS is the tumor necrosis factor inhibitor adalimumab.
Novartis is investigating the potential application of secukinumab for the treatment of lupus nephritis and giant cell arteritis, as well as polymyalgia rheumatica and rotator cuff tendinopathy, according to the company press release.
The study published in The Lancet was funded by Novartis.
A version of this article first appeared on Medscape.com.
Dupilumab outcomes stable at end of open label atopic dermatitis study
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
AT RAD 2023
EULAR systemic sclerosis recommendations now include immunosuppressants
MILAN – Targeted synthetic and biologic therapies are recommended as disease-modifying agents for key fibrotic manifestations of systemic sclerosis for the first time in the 2023 update of European Alliance of Associations for Rheumatology recommendations for the treatment of systemic sclerosis.
Reflecting important advances over the past 8 years, mostly relating to the use of new treatments being made available to patients, the recommendations provide an update on the 2017 recommendations, which relied on evidence published through 2014. Of note, these include the use of immunosuppressive agents, for example, the monoclonal antibody rituximab (Rituxan) for skin and lung fibrosis.
“For the first time, synthetic and targeted treatments are recommended for the treatment of systemic sclerosis–interstitial lung disease, including mycophenolate mofetil [Cellcept], nintedanib [Ofev], rituximab, and tocilizumab [Actemra]. None of these were present in 2017. Mycophenolate mofetil is also recommended for the treatment of skin fibrosis, and this was not present in 2017,” Francesco Del Galdo, MD, rheumatology consultant at Leeds Teaching Hospital NHS Trust, Leeds, England, and member of the 2023 recommendations task force, said in an interview. He gave an overview of the preliminary recommendations at the annual European Congress of Rheumatology.
“Phosphodiesterase-5 [PDE-5] inhibitors and endothelin receptor antagonist [ERA] monotherapy are also recommended for up-front combination use for digital ulcers and pulmonary hypertension, and this is new for 2023 and was not present in the 2017 recommendations,” Dr. Del Galdo added.
The new recommendations also note that iloprost is categorized as having grade A evidence for use in Raynaud’s phenomenon and digital ulcers, while it has grade B evidence for pulmonary hypertension.
“We are not allowed to share the final table [of recommendations] today because the wording has only very recently been agreed” upon, Dr. Del Galdo said, but he provided a summary representation and reflected on some changes, noting that the task force is aiming to publish the 2023 recommendations by the end of the year.
Consideration and discussion of both established and new evidence highlighted a need for more evidence on the use of immunosuppressive agents in vascular manifestations of systemic sclerosis, as well as for gastrointestinal and musculoskeletal ones.
In this update to the 2017 recommendations, high-grade evidence was identified for use of immunosuppressants in skin and lung fibrosis. Grade A evidence has been accepted for the use of rituximab in skin fibrosis; for interstitial lung disease, rituximab, cyclophosphamide, and nintedanib also have grade A evidence, which is a change from the 2017 recommendations.
A total of 20 updated recommendations were agreed on, an increase from 16 in 2017. These were grouped into eight disease domains: Raynaud’s phenomenon, digital ulcers, pulmonary arterial hypertension, skin fibrosis, interstitial lung disease, musculoskeletal and gastrointestinal manifestations, and renal crisis. Interventions were then graded A-D based on the evidence reviewed.
“This approach allowed us to see clearly that there were patterns of similar recommendations in different organ manifestations, reflecting an understanding of common pathogenic pathways,” said Dr. Del Galdo.
He also noted that the development of the recommendations highlighted certain gaps in research that limit treatment options. “By grouping the recommendations in blocks – for example, skin fibrosis or vascular [manifestations] – we show that immunosuppressive treatments have only been studied in skin and lung, while vascular manifestations have very little evidence for immunosuppression. They might be effective but there’s no evidence yet [hence no recommendation in vascular manifestations].”
“Also, there’s no grade A evidence at all for musculoskeletal and gastrointestinal manifestations, and this should help to define the research agenda going forward,” Dr. Del Galdo said.
The 2023 recommendations task force comprised 28 members from 14 countries, including 18 rheumatologists, 1 EULAR methodologist, 1 health professional representative, 5 rheumatology fellows, 1 librarian, and 2 patient representatives. They used a consensual approach incorporating the views of 101 European Scleroderma Trials and Research group (EUSTAR) centers, sourced via a survey in which questions were advanced to an extensive systematic review if there was 70% or greater agreement.
Eventually, 31 questions on interventions were chosen, and the task force reviewed 12,500 abstracts (up to December 2022) related to interventions and outcomes that were either included in the 2017 recommendations or were totally new.
Dr. Del Galdo said that the three vascular manifestations of scleroderma – Raynaud’s, pulmonary arterial hypertension, and digital ulcers – were treated with the same drugs, all with a similar grade of evidence. “This suggests two things – firstly there’s a vascular disease continuum in the disease, and secondly, we’ve borrowed these drugs from vascular community, but we have not yet tested synthetic and biologic targeted treatments in these manifestations, and we should.
“Treating one manifestation may benefit the other, and this is important time wise because pulmonary hypertension usually comes around 10 years after the first phenomena so by treating digital ulcers and Raynaud’s phenomena, we may prevent pulmonary hypertension, but a study is needed,” added Dr. Del Galdo, who is also president of EUSTAR.
Finally, he pointed out that research remains particularly open for nonpharmacologic treatments for digital ulcers and severe gastrointestinal involvement. “Patients can now ask for studies into this because of the current lack of evidence.”
Moderator Ariane Herrick, PhD, professor of rheumatology at the University of Manchester (England), shared her thoughts on the guidelines. “These recommendations have been long awaited by the scleroderma community because there has been some exciting progress in recent years, and the new recommendations reflect these new developments.”
Commenting on the paucity of evidence in some areas, she added that “there do remain some huge areas of unmet need that are difficult to address, and these are musculoskeletal, gastrointestinal, and calcinosis, for which there have been no trials at all.”
Dr. Del Galdo declared disclosures relating to AstraZeneca, Janssen, Boehringer Ingelheim, Capella, Chemomab, GlaxoSmithKline, and Mitsubishi-Tanabe. Dr. Herrick disclosed serving as a consultant for Boehringer Ingelheim and Janssen.
MILAN – Targeted synthetic and biologic therapies are recommended as disease-modifying agents for key fibrotic manifestations of systemic sclerosis for the first time in the 2023 update of European Alliance of Associations for Rheumatology recommendations for the treatment of systemic sclerosis.
Reflecting important advances over the past 8 years, mostly relating to the use of new treatments being made available to patients, the recommendations provide an update on the 2017 recommendations, which relied on evidence published through 2014. Of note, these include the use of immunosuppressive agents, for example, the monoclonal antibody rituximab (Rituxan) for skin and lung fibrosis.
“For the first time, synthetic and targeted treatments are recommended for the treatment of systemic sclerosis–interstitial lung disease, including mycophenolate mofetil [Cellcept], nintedanib [Ofev], rituximab, and tocilizumab [Actemra]. None of these were present in 2017. Mycophenolate mofetil is also recommended for the treatment of skin fibrosis, and this was not present in 2017,” Francesco Del Galdo, MD, rheumatology consultant at Leeds Teaching Hospital NHS Trust, Leeds, England, and member of the 2023 recommendations task force, said in an interview. He gave an overview of the preliminary recommendations at the annual European Congress of Rheumatology.
“Phosphodiesterase-5 [PDE-5] inhibitors and endothelin receptor antagonist [ERA] monotherapy are also recommended for up-front combination use for digital ulcers and pulmonary hypertension, and this is new for 2023 and was not present in the 2017 recommendations,” Dr. Del Galdo added.
The new recommendations also note that iloprost is categorized as having grade A evidence for use in Raynaud’s phenomenon and digital ulcers, while it has grade B evidence for pulmonary hypertension.
“We are not allowed to share the final table [of recommendations] today because the wording has only very recently been agreed” upon, Dr. Del Galdo said, but he provided a summary representation and reflected on some changes, noting that the task force is aiming to publish the 2023 recommendations by the end of the year.
Consideration and discussion of both established and new evidence highlighted a need for more evidence on the use of immunosuppressive agents in vascular manifestations of systemic sclerosis, as well as for gastrointestinal and musculoskeletal ones.
In this update to the 2017 recommendations, high-grade evidence was identified for use of immunosuppressants in skin and lung fibrosis. Grade A evidence has been accepted for the use of rituximab in skin fibrosis; for interstitial lung disease, rituximab, cyclophosphamide, and nintedanib also have grade A evidence, which is a change from the 2017 recommendations.
A total of 20 updated recommendations were agreed on, an increase from 16 in 2017. These were grouped into eight disease domains: Raynaud’s phenomenon, digital ulcers, pulmonary arterial hypertension, skin fibrosis, interstitial lung disease, musculoskeletal and gastrointestinal manifestations, and renal crisis. Interventions were then graded A-D based on the evidence reviewed.
“This approach allowed us to see clearly that there were patterns of similar recommendations in different organ manifestations, reflecting an understanding of common pathogenic pathways,” said Dr. Del Galdo.
He also noted that the development of the recommendations highlighted certain gaps in research that limit treatment options. “By grouping the recommendations in blocks – for example, skin fibrosis or vascular [manifestations] – we show that immunosuppressive treatments have only been studied in skin and lung, while vascular manifestations have very little evidence for immunosuppression. They might be effective but there’s no evidence yet [hence no recommendation in vascular manifestations].”
“Also, there’s no grade A evidence at all for musculoskeletal and gastrointestinal manifestations, and this should help to define the research agenda going forward,” Dr. Del Galdo said.
The 2023 recommendations task force comprised 28 members from 14 countries, including 18 rheumatologists, 1 EULAR methodologist, 1 health professional representative, 5 rheumatology fellows, 1 librarian, and 2 patient representatives. They used a consensual approach incorporating the views of 101 European Scleroderma Trials and Research group (EUSTAR) centers, sourced via a survey in which questions were advanced to an extensive systematic review if there was 70% or greater agreement.
Eventually, 31 questions on interventions were chosen, and the task force reviewed 12,500 abstracts (up to December 2022) related to interventions and outcomes that were either included in the 2017 recommendations or were totally new.
Dr. Del Galdo said that the three vascular manifestations of scleroderma – Raynaud’s, pulmonary arterial hypertension, and digital ulcers – were treated with the same drugs, all with a similar grade of evidence. “This suggests two things – firstly there’s a vascular disease continuum in the disease, and secondly, we’ve borrowed these drugs from vascular community, but we have not yet tested synthetic and biologic targeted treatments in these manifestations, and we should.
“Treating one manifestation may benefit the other, and this is important time wise because pulmonary hypertension usually comes around 10 years after the first phenomena so by treating digital ulcers and Raynaud’s phenomena, we may prevent pulmonary hypertension, but a study is needed,” added Dr. Del Galdo, who is also president of EUSTAR.
Finally, he pointed out that research remains particularly open for nonpharmacologic treatments for digital ulcers and severe gastrointestinal involvement. “Patients can now ask for studies into this because of the current lack of evidence.”
Moderator Ariane Herrick, PhD, professor of rheumatology at the University of Manchester (England), shared her thoughts on the guidelines. “These recommendations have been long awaited by the scleroderma community because there has been some exciting progress in recent years, and the new recommendations reflect these new developments.”
Commenting on the paucity of evidence in some areas, she added that “there do remain some huge areas of unmet need that are difficult to address, and these are musculoskeletal, gastrointestinal, and calcinosis, for which there have been no trials at all.”
Dr. Del Galdo declared disclosures relating to AstraZeneca, Janssen, Boehringer Ingelheim, Capella, Chemomab, GlaxoSmithKline, and Mitsubishi-Tanabe. Dr. Herrick disclosed serving as a consultant for Boehringer Ingelheim and Janssen.
MILAN – Targeted synthetic and biologic therapies are recommended as disease-modifying agents for key fibrotic manifestations of systemic sclerosis for the first time in the 2023 update of European Alliance of Associations for Rheumatology recommendations for the treatment of systemic sclerosis.
Reflecting important advances over the past 8 years, mostly relating to the use of new treatments being made available to patients, the recommendations provide an update on the 2017 recommendations, which relied on evidence published through 2014. Of note, these include the use of immunosuppressive agents, for example, the monoclonal antibody rituximab (Rituxan) for skin and lung fibrosis.
“For the first time, synthetic and targeted treatments are recommended for the treatment of systemic sclerosis–interstitial lung disease, including mycophenolate mofetil [Cellcept], nintedanib [Ofev], rituximab, and tocilizumab [Actemra]. None of these were present in 2017. Mycophenolate mofetil is also recommended for the treatment of skin fibrosis, and this was not present in 2017,” Francesco Del Galdo, MD, rheumatology consultant at Leeds Teaching Hospital NHS Trust, Leeds, England, and member of the 2023 recommendations task force, said in an interview. He gave an overview of the preliminary recommendations at the annual European Congress of Rheumatology.
“Phosphodiesterase-5 [PDE-5] inhibitors and endothelin receptor antagonist [ERA] monotherapy are also recommended for up-front combination use for digital ulcers and pulmonary hypertension, and this is new for 2023 and was not present in the 2017 recommendations,” Dr. Del Galdo added.
The new recommendations also note that iloprost is categorized as having grade A evidence for use in Raynaud’s phenomenon and digital ulcers, while it has grade B evidence for pulmonary hypertension.
“We are not allowed to share the final table [of recommendations] today because the wording has only very recently been agreed” upon, Dr. Del Galdo said, but he provided a summary representation and reflected on some changes, noting that the task force is aiming to publish the 2023 recommendations by the end of the year.
Consideration and discussion of both established and new evidence highlighted a need for more evidence on the use of immunosuppressive agents in vascular manifestations of systemic sclerosis, as well as for gastrointestinal and musculoskeletal ones.
In this update to the 2017 recommendations, high-grade evidence was identified for use of immunosuppressants in skin and lung fibrosis. Grade A evidence has been accepted for the use of rituximab in skin fibrosis; for interstitial lung disease, rituximab, cyclophosphamide, and nintedanib also have grade A evidence, which is a change from the 2017 recommendations.
A total of 20 updated recommendations were agreed on, an increase from 16 in 2017. These were grouped into eight disease domains: Raynaud’s phenomenon, digital ulcers, pulmonary arterial hypertension, skin fibrosis, interstitial lung disease, musculoskeletal and gastrointestinal manifestations, and renal crisis. Interventions were then graded A-D based on the evidence reviewed.
“This approach allowed us to see clearly that there were patterns of similar recommendations in different organ manifestations, reflecting an understanding of common pathogenic pathways,” said Dr. Del Galdo.
He also noted that the development of the recommendations highlighted certain gaps in research that limit treatment options. “By grouping the recommendations in blocks – for example, skin fibrosis or vascular [manifestations] – we show that immunosuppressive treatments have only been studied in skin and lung, while vascular manifestations have very little evidence for immunosuppression. They might be effective but there’s no evidence yet [hence no recommendation in vascular manifestations].”
“Also, there’s no grade A evidence at all for musculoskeletal and gastrointestinal manifestations, and this should help to define the research agenda going forward,” Dr. Del Galdo said.
The 2023 recommendations task force comprised 28 members from 14 countries, including 18 rheumatologists, 1 EULAR methodologist, 1 health professional representative, 5 rheumatology fellows, 1 librarian, and 2 patient representatives. They used a consensual approach incorporating the views of 101 European Scleroderma Trials and Research group (EUSTAR) centers, sourced via a survey in which questions were advanced to an extensive systematic review if there was 70% or greater agreement.
Eventually, 31 questions on interventions were chosen, and the task force reviewed 12,500 abstracts (up to December 2022) related to interventions and outcomes that were either included in the 2017 recommendations or were totally new.
Dr. Del Galdo said that the three vascular manifestations of scleroderma – Raynaud’s, pulmonary arterial hypertension, and digital ulcers – were treated with the same drugs, all with a similar grade of evidence. “This suggests two things – firstly there’s a vascular disease continuum in the disease, and secondly, we’ve borrowed these drugs from vascular community, but we have not yet tested synthetic and biologic targeted treatments in these manifestations, and we should.
“Treating one manifestation may benefit the other, and this is important time wise because pulmonary hypertension usually comes around 10 years after the first phenomena so by treating digital ulcers and Raynaud’s phenomena, we may prevent pulmonary hypertension, but a study is needed,” added Dr. Del Galdo, who is also president of EUSTAR.
Finally, he pointed out that research remains particularly open for nonpharmacologic treatments for digital ulcers and severe gastrointestinal involvement. “Patients can now ask for studies into this because of the current lack of evidence.”
Moderator Ariane Herrick, PhD, professor of rheumatology at the University of Manchester (England), shared her thoughts on the guidelines. “These recommendations have been long awaited by the scleroderma community because there has been some exciting progress in recent years, and the new recommendations reflect these new developments.”
Commenting on the paucity of evidence in some areas, she added that “there do remain some huge areas of unmet need that are difficult to address, and these are musculoskeletal, gastrointestinal, and calcinosis, for which there have been no trials at all.”
Dr. Del Galdo declared disclosures relating to AstraZeneca, Janssen, Boehringer Ingelheim, Capella, Chemomab, GlaxoSmithKline, and Mitsubishi-Tanabe. Dr. Herrick disclosed serving as a consultant for Boehringer Ingelheim and Janssen.
AT EULAR 2023
As Medicaid purge begins, ‘staggering numbers’ of Americans lose coverage
More than 600,000 Americans have lost Medicaid coverage since pandemic protections ended on April 1. And a KFF Health News analysis of state data shows the vast majority were removed from state rolls for not completing paperwork.
Under normal circumstances, states review their Medicaid enrollment lists regularly to ensure every recipient qualifies for coverage. But because of a nationwide pause in those reviews during the pandemic, the health insurance program for low-income and disabled Americans kept people covered even if they no longer qualified.
Now, in what’s known as the Medicaid unwinding, states are combing through rolls and deciding who stays and who goes. People who are no longer eligible or don’t complete paperwork in time will be dropped.
The overwhelming majority of people who have lost coverage in most states were dropped because of technicalities, not because state officials determined they no longer meet Medicaid income limits. Four out of every five people dropped so far either never returned the paperwork or omitted required documents, according to a KFF Health News analysis of data from 11 states that provided details on recent cancellations. Now, lawmakers and advocates are expressing alarm over the volume of people losing coverage and, in some states, calling to pause the process.
KFF Health News sought data from the 19 states that started cancellations by May 1. Based on records from 14 states that provided detailed numbers, either in response to a public records request or by posting online, 36% of people whose eligibility was reviewed have been disenrolled.
In Indiana, 53,000 residents lost coverage in the first month of the unwinding, 89% for procedural reasons like not returning renewal forms. State Rep. Ed Clere, a Republican, expressed dismay at those “staggering numbers” in a May 24 Medicaid advisory group meeting, repeatedly questioning state officials about forms mailed to out-of-date addresses and urging them to give people more than 2 weeks’ notice before canceling their coverage.
Rep. Clere warned that the cancellations set in motion an avoidable revolving door. Some people dropped from Medicaid will have to forgo filling prescriptions and cancel doctor visits because they can’t afford care. Months down the line, after untreated chronic illnesses spiral out of control, they’ll end up in the emergency room where social workers will need to again help them join the program, he said.
Before the unwinding, more than one in four Americans – 93 million – were covered by Medicaid or CHIP, the Children’s Health Insurance Program, according to KFF Health News’ analysis of the latest enrollment data. Half of all kids are covered by the programs.
About 15 million people will be dropped over the next year as states review participants’ eligibility in monthly tranches.
Most people will find health coverage through new jobs or qualify for subsidized plans through the Affordable Care Act. But millions of others, including many children, will become uninsured and unable to afford basic prescriptions or preventive care. The uninsured rate among those under 65 is projected to rise from a historical low of 8.3% today to 9.3% next year, according to the Congressional Budget Office.
Because each state is handling the unwinding differently, the share of enrollees dropped in the first weeks varies widely.
Several states are first reviewing people officials believe are no longer eligible or who haven’t recently used their insurance. High cancellation rates in those states should level out as the agencies move on to people who likely still qualify.
In Utah, nearly 56% of people included in early reviews were dropped. In New Hampshire, 44% received cancellation letters within the first 2 months – almost all for procedural reasons, like not returning paperwork.
But New Hampshire officials found that thousands of people who didn’t fill out the forms indeed earn too much to qualify, according to Henry Lipman, the state’s Medicaid director. They would have been denied anyway. Even so, more people than he expected are not returning renewal forms. “That tells us that we need to change up our strategy,” said Mr. Lipman.
In other states, like Virginia and Nebraska, which aren’t prioritizing renewals by likely eligibility, about 90% have been renewed.
Because of the 3-year pause in renewals, many people on Medicaid have never been through the process or aren’t aware they may need to fill out long verification forms, as a recent KFF poll found. Some people moved and didn’t update their contact information.
And while agencies are required to assist enrollees who don’t speak English well, many are sending the forms in only a few common languages.
Tens of thousands of children are losing coverage, as researchers have warned, even though some may still qualify for Medicaid or CHIP. In its first month of reviews, South Dakota ended coverage for 10% of all Medicaid and CHIP enrollees in the state. More than half of them were children. In Arkansas, about 40% were kids.
Many parents don’t know that limits on household income are significantly higher for children than adults. Parents should fill out renewal forms even if they don’t qualify themselves, said Joan Alker, executive director of the Georgetown University Center for Children and Families, Washington.
New Hampshire has moved most families with children to the end of the review process. Mr. Lipman said his biggest worry is that a child will end up uninsured. Florida also planned to push kids with serious health conditions and other vulnerable groups to the end of the review line.
But according to Miriam Harmatz, advocacy director and founder of the Florida Health Justice Project, state officials sent cancellation letters to several clients with disabled children who probably still qualify. She’s helping those families appeal.
Nearly 250,000 Floridians reviewed in the first month of the unwinding lost coverage, 82% of them for reasons like incomplete paperwork, the state reported to federal authorities. House Democrats from the state petitioned Republican Gov. Ron DeSantis to pause the unwinding.
Advocacy coalitions in both Florida and Arkansas also have called for investigations into the review process and a pause on cancellations.
The state is contacting enrollees by phone, email, and text, and continues to process late applications, said Tori Cuddy, a spokesperson for the Florida Department of Children and Families. Ms. Cuddy did not respond to questions about issues raised in the petitions.
Federal officials are investigating those complaints and any other problems that emerge, said Dan Tsai, director of the Center for Medicaid & CHIP Services. “If we find that the rules are not being followed, we will take action.”
His agency has directed states to automatically reenroll residents using data from other government programs like unemployment and food assistance when possible. Anyone who can’t be approved through that process must act quickly.
“For the past 3 years, people have been told to ignore the mail around this, that the renewal was not going to lead to a termination.” Suddenly that mail matters, he said.
Federal law requires states to tell people why they’re losing Medicaid coverage and how to appeal the decision.
Ms. Harmatz said some cancellation notices in Florida are vague and could violate due process rules. Letters that she’s seen say “your Medicaid for this period is ending” rather than providing a specific reason for disenrollment, like having too high an income or incomplete paperwork.
If a person requests a hearing before their cancellation takes effect, they can stay covered during the appeals process. Even after being disenrolled, many still have a 90-day window to restore coverage.
In New Hampshire, 13% of people deemed ineligible in the first month have asked for extra time to provide the necessary records. “If you’re eligible for Medicaid, we don’t want you to lose it,” said Mr. Lipman.
Rep. Clere pushed Indiana’s Medicaid officials during the May meeting to immediately make changes to avoid people unnecessarily becoming uninsured. One official responded that they’ll learn and improve over time.
“I’m just concerned that we’re going to be ‘learning’ as a result of people losing coverage,” Rep. Clere replied. “So I don’t want to learn at their expense.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
More than 600,000 Americans have lost Medicaid coverage since pandemic protections ended on April 1. And a KFF Health News analysis of state data shows the vast majority were removed from state rolls for not completing paperwork.
Under normal circumstances, states review their Medicaid enrollment lists regularly to ensure every recipient qualifies for coverage. But because of a nationwide pause in those reviews during the pandemic, the health insurance program for low-income and disabled Americans kept people covered even if they no longer qualified.
Now, in what’s known as the Medicaid unwinding, states are combing through rolls and deciding who stays and who goes. People who are no longer eligible or don’t complete paperwork in time will be dropped.
The overwhelming majority of people who have lost coverage in most states were dropped because of technicalities, not because state officials determined they no longer meet Medicaid income limits. Four out of every five people dropped so far either never returned the paperwork or omitted required documents, according to a KFF Health News analysis of data from 11 states that provided details on recent cancellations. Now, lawmakers and advocates are expressing alarm over the volume of people losing coverage and, in some states, calling to pause the process.
KFF Health News sought data from the 19 states that started cancellations by May 1. Based on records from 14 states that provided detailed numbers, either in response to a public records request or by posting online, 36% of people whose eligibility was reviewed have been disenrolled.
In Indiana, 53,000 residents lost coverage in the first month of the unwinding, 89% for procedural reasons like not returning renewal forms. State Rep. Ed Clere, a Republican, expressed dismay at those “staggering numbers” in a May 24 Medicaid advisory group meeting, repeatedly questioning state officials about forms mailed to out-of-date addresses and urging them to give people more than 2 weeks’ notice before canceling their coverage.
Rep. Clere warned that the cancellations set in motion an avoidable revolving door. Some people dropped from Medicaid will have to forgo filling prescriptions and cancel doctor visits because they can’t afford care. Months down the line, after untreated chronic illnesses spiral out of control, they’ll end up in the emergency room where social workers will need to again help them join the program, he said.
Before the unwinding, more than one in four Americans – 93 million – were covered by Medicaid or CHIP, the Children’s Health Insurance Program, according to KFF Health News’ analysis of the latest enrollment data. Half of all kids are covered by the programs.
About 15 million people will be dropped over the next year as states review participants’ eligibility in monthly tranches.
Most people will find health coverage through new jobs or qualify for subsidized plans through the Affordable Care Act. But millions of others, including many children, will become uninsured and unable to afford basic prescriptions or preventive care. The uninsured rate among those under 65 is projected to rise from a historical low of 8.3% today to 9.3% next year, according to the Congressional Budget Office.
Because each state is handling the unwinding differently, the share of enrollees dropped in the first weeks varies widely.
Several states are first reviewing people officials believe are no longer eligible or who haven’t recently used their insurance. High cancellation rates in those states should level out as the agencies move on to people who likely still qualify.
In Utah, nearly 56% of people included in early reviews were dropped. In New Hampshire, 44% received cancellation letters within the first 2 months – almost all for procedural reasons, like not returning paperwork.
But New Hampshire officials found that thousands of people who didn’t fill out the forms indeed earn too much to qualify, according to Henry Lipman, the state’s Medicaid director. They would have been denied anyway. Even so, more people than he expected are not returning renewal forms. “That tells us that we need to change up our strategy,” said Mr. Lipman.
In other states, like Virginia and Nebraska, which aren’t prioritizing renewals by likely eligibility, about 90% have been renewed.
Because of the 3-year pause in renewals, many people on Medicaid have never been through the process or aren’t aware they may need to fill out long verification forms, as a recent KFF poll found. Some people moved and didn’t update their contact information.
And while agencies are required to assist enrollees who don’t speak English well, many are sending the forms in only a few common languages.
Tens of thousands of children are losing coverage, as researchers have warned, even though some may still qualify for Medicaid or CHIP. In its first month of reviews, South Dakota ended coverage for 10% of all Medicaid and CHIP enrollees in the state. More than half of them were children. In Arkansas, about 40% were kids.
Many parents don’t know that limits on household income are significantly higher for children than adults. Parents should fill out renewal forms even if they don’t qualify themselves, said Joan Alker, executive director of the Georgetown University Center for Children and Families, Washington.
New Hampshire has moved most families with children to the end of the review process. Mr. Lipman said his biggest worry is that a child will end up uninsured. Florida also planned to push kids with serious health conditions and other vulnerable groups to the end of the review line.
But according to Miriam Harmatz, advocacy director and founder of the Florida Health Justice Project, state officials sent cancellation letters to several clients with disabled children who probably still qualify. She’s helping those families appeal.
Nearly 250,000 Floridians reviewed in the first month of the unwinding lost coverage, 82% of them for reasons like incomplete paperwork, the state reported to federal authorities. House Democrats from the state petitioned Republican Gov. Ron DeSantis to pause the unwinding.
Advocacy coalitions in both Florida and Arkansas also have called for investigations into the review process and a pause on cancellations.
The state is contacting enrollees by phone, email, and text, and continues to process late applications, said Tori Cuddy, a spokesperson for the Florida Department of Children and Families. Ms. Cuddy did not respond to questions about issues raised in the petitions.
Federal officials are investigating those complaints and any other problems that emerge, said Dan Tsai, director of the Center for Medicaid & CHIP Services. “If we find that the rules are not being followed, we will take action.”
His agency has directed states to automatically reenroll residents using data from other government programs like unemployment and food assistance when possible. Anyone who can’t be approved through that process must act quickly.
“For the past 3 years, people have been told to ignore the mail around this, that the renewal was not going to lead to a termination.” Suddenly that mail matters, he said.
Federal law requires states to tell people why they’re losing Medicaid coverage and how to appeal the decision.
Ms. Harmatz said some cancellation notices in Florida are vague and could violate due process rules. Letters that she’s seen say “your Medicaid for this period is ending” rather than providing a specific reason for disenrollment, like having too high an income or incomplete paperwork.
If a person requests a hearing before their cancellation takes effect, they can stay covered during the appeals process. Even after being disenrolled, many still have a 90-day window to restore coverage.
In New Hampshire, 13% of people deemed ineligible in the first month have asked for extra time to provide the necessary records. “If you’re eligible for Medicaid, we don’t want you to lose it,” said Mr. Lipman.
Rep. Clere pushed Indiana’s Medicaid officials during the May meeting to immediately make changes to avoid people unnecessarily becoming uninsured. One official responded that they’ll learn and improve over time.
“I’m just concerned that we’re going to be ‘learning’ as a result of people losing coverage,” Rep. Clere replied. “So I don’t want to learn at their expense.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
More than 600,000 Americans have lost Medicaid coverage since pandemic protections ended on April 1. And a KFF Health News analysis of state data shows the vast majority were removed from state rolls for not completing paperwork.
Under normal circumstances, states review their Medicaid enrollment lists regularly to ensure every recipient qualifies for coverage. But because of a nationwide pause in those reviews during the pandemic, the health insurance program for low-income and disabled Americans kept people covered even if they no longer qualified.
Now, in what’s known as the Medicaid unwinding, states are combing through rolls and deciding who stays and who goes. People who are no longer eligible or don’t complete paperwork in time will be dropped.
The overwhelming majority of people who have lost coverage in most states were dropped because of technicalities, not because state officials determined they no longer meet Medicaid income limits. Four out of every five people dropped so far either never returned the paperwork or omitted required documents, according to a KFF Health News analysis of data from 11 states that provided details on recent cancellations. Now, lawmakers and advocates are expressing alarm over the volume of people losing coverage and, in some states, calling to pause the process.
KFF Health News sought data from the 19 states that started cancellations by May 1. Based on records from 14 states that provided detailed numbers, either in response to a public records request or by posting online, 36% of people whose eligibility was reviewed have been disenrolled.
In Indiana, 53,000 residents lost coverage in the first month of the unwinding, 89% for procedural reasons like not returning renewal forms. State Rep. Ed Clere, a Republican, expressed dismay at those “staggering numbers” in a May 24 Medicaid advisory group meeting, repeatedly questioning state officials about forms mailed to out-of-date addresses and urging them to give people more than 2 weeks’ notice before canceling their coverage.
Rep. Clere warned that the cancellations set in motion an avoidable revolving door. Some people dropped from Medicaid will have to forgo filling prescriptions and cancel doctor visits because they can’t afford care. Months down the line, after untreated chronic illnesses spiral out of control, they’ll end up in the emergency room where social workers will need to again help them join the program, he said.
Before the unwinding, more than one in four Americans – 93 million – were covered by Medicaid or CHIP, the Children’s Health Insurance Program, according to KFF Health News’ analysis of the latest enrollment data. Half of all kids are covered by the programs.
About 15 million people will be dropped over the next year as states review participants’ eligibility in monthly tranches.
Most people will find health coverage through new jobs or qualify for subsidized plans through the Affordable Care Act. But millions of others, including many children, will become uninsured and unable to afford basic prescriptions or preventive care. The uninsured rate among those under 65 is projected to rise from a historical low of 8.3% today to 9.3% next year, according to the Congressional Budget Office.
Because each state is handling the unwinding differently, the share of enrollees dropped in the first weeks varies widely.
Several states are first reviewing people officials believe are no longer eligible or who haven’t recently used their insurance. High cancellation rates in those states should level out as the agencies move on to people who likely still qualify.
In Utah, nearly 56% of people included in early reviews were dropped. In New Hampshire, 44% received cancellation letters within the first 2 months – almost all for procedural reasons, like not returning paperwork.
But New Hampshire officials found that thousands of people who didn’t fill out the forms indeed earn too much to qualify, according to Henry Lipman, the state’s Medicaid director. They would have been denied anyway. Even so, more people than he expected are not returning renewal forms. “That tells us that we need to change up our strategy,” said Mr. Lipman.
In other states, like Virginia and Nebraska, which aren’t prioritizing renewals by likely eligibility, about 90% have been renewed.
Because of the 3-year pause in renewals, many people on Medicaid have never been through the process or aren’t aware they may need to fill out long verification forms, as a recent KFF poll found. Some people moved and didn’t update their contact information.
And while agencies are required to assist enrollees who don’t speak English well, many are sending the forms in only a few common languages.
Tens of thousands of children are losing coverage, as researchers have warned, even though some may still qualify for Medicaid or CHIP. In its first month of reviews, South Dakota ended coverage for 10% of all Medicaid and CHIP enrollees in the state. More than half of them were children. In Arkansas, about 40% were kids.
Many parents don’t know that limits on household income are significantly higher for children than adults. Parents should fill out renewal forms even if they don’t qualify themselves, said Joan Alker, executive director of the Georgetown University Center for Children and Families, Washington.
New Hampshire has moved most families with children to the end of the review process. Mr. Lipman said his biggest worry is that a child will end up uninsured. Florida also planned to push kids with serious health conditions and other vulnerable groups to the end of the review line.
But according to Miriam Harmatz, advocacy director and founder of the Florida Health Justice Project, state officials sent cancellation letters to several clients with disabled children who probably still qualify. She’s helping those families appeal.
Nearly 250,000 Floridians reviewed in the first month of the unwinding lost coverage, 82% of them for reasons like incomplete paperwork, the state reported to federal authorities. House Democrats from the state petitioned Republican Gov. Ron DeSantis to pause the unwinding.
Advocacy coalitions in both Florida and Arkansas also have called for investigations into the review process and a pause on cancellations.
The state is contacting enrollees by phone, email, and text, and continues to process late applications, said Tori Cuddy, a spokesperson for the Florida Department of Children and Families. Ms. Cuddy did not respond to questions about issues raised in the petitions.
Federal officials are investigating those complaints and any other problems that emerge, said Dan Tsai, director of the Center for Medicaid & CHIP Services. “If we find that the rules are not being followed, we will take action.”
His agency has directed states to automatically reenroll residents using data from other government programs like unemployment and food assistance when possible. Anyone who can’t be approved through that process must act quickly.
“For the past 3 years, people have been told to ignore the mail around this, that the renewal was not going to lead to a termination.” Suddenly that mail matters, he said.
Federal law requires states to tell people why they’re losing Medicaid coverage and how to appeal the decision.
Ms. Harmatz said some cancellation notices in Florida are vague and could violate due process rules. Letters that she’s seen say “your Medicaid for this period is ending” rather than providing a specific reason for disenrollment, like having too high an income or incomplete paperwork.
If a person requests a hearing before their cancellation takes effect, they can stay covered during the appeals process. Even after being disenrolled, many still have a 90-day window to restore coverage.
In New Hampshire, 13% of people deemed ineligible in the first month have asked for extra time to provide the necessary records. “If you’re eligible for Medicaid, we don’t want you to lose it,” said Mr. Lipman.
Rep. Clere pushed Indiana’s Medicaid officials during the May meeting to immediately make changes to avoid people unnecessarily becoming uninsured. One official responded that they’ll learn and improve over time.
“I’m just concerned that we’re going to be ‘learning’ as a result of people losing coverage,” Rep. Clere replied. “So I don’t want to learn at their expense.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.