User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
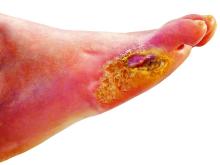
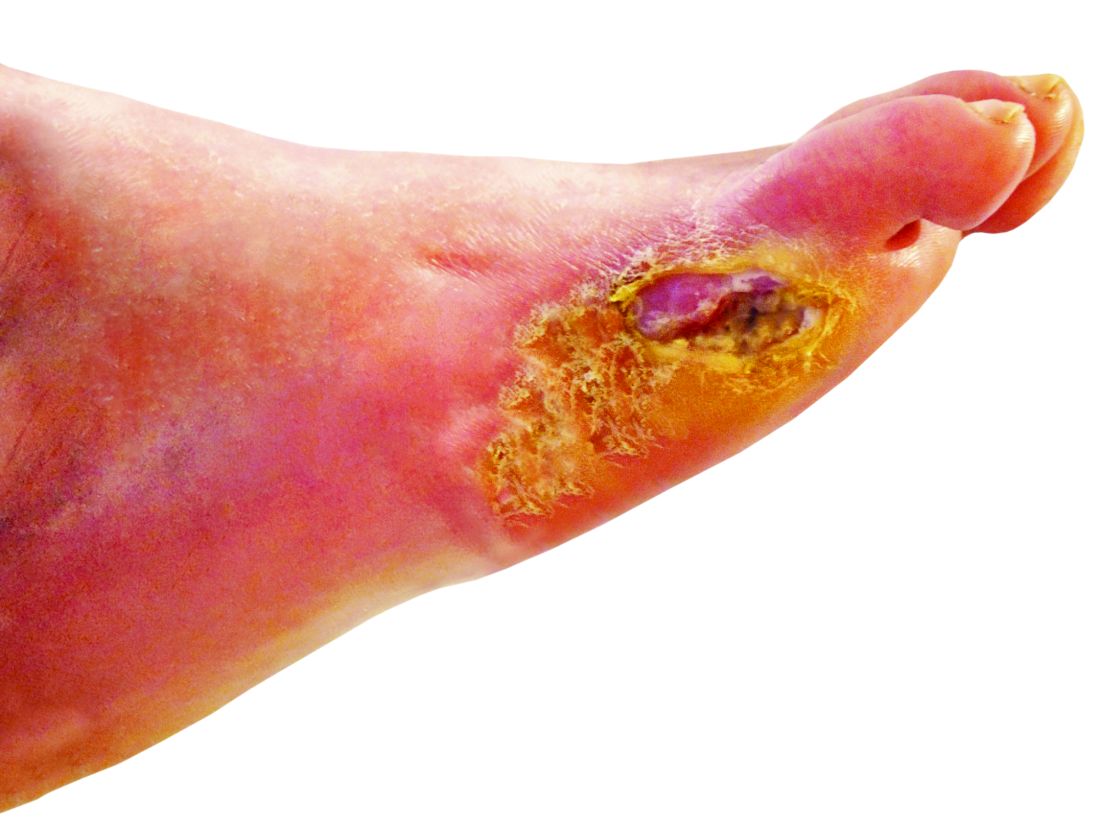
‘Amazing’ data for cheap beta-blocker gel for diabetic foot ulcers
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2022
Apremilast alleviates severe psoriasis in some children, data show
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
not controlled by topical therapy, according to the results of a phase 3 trial.
“Unfortunately, there are limited treatment options for pediatric patients with moderate to severe plaque psoriasis” who do not respond to or cannot use topical therapy, said study investigator Anna Belloni Fortina, MD, speaking at the annual meeting of the European Academy of Dermatology and Venereology.
“In this randomized, placebo-controlled trial, oral apremilast demonstrated effectiveness and was well tolerated,” added Dr. Belloni Fortina, of Azienda Ospedale Università Padova (Italy). “I underline oral because for children, oral administration is better than the injection treatment.”
Key findings
Dubbed the SPROUT study, the trial set a primary endpoint of the percentage of children with a Physician’s Global Assessment (sPGA) response after 16 weeks of treatment or placebo. The sPGA is a 5-point scale ranging from 0 (clear) to 4 (severe). The study enrolled children with an sPGA greater than or equal to 3. Response was defined as a sPGA score of 0 or 1, indicating clear or almost clear skin, with at least a 2-point reduction from baseline values.
At week 16, the primary endpoint was met by 33% of 163 children treated with apremilast versus 11% of 82 children who had been given a placebo, a treatment difference of 21.7% (95% confidence interval, 11.2%-32.1%).
A greater proportion of children treated with apremilast also achieved a major secondary endpoint, a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI-75) (45.4% vs. 16.1%), a treatment difference of 29.4% (95% CI, 17.8%-40.9%).
Results unaffected by weight and age
Regarding apremilast, “it’s important to underline that patients were dosed according to their weight,” Dr. Belloni Fortina said.
A dose of 20 mg twice daily was given to children who weighed between 20 kg and less than 50 kg, and a 30-mg twice-daily dose was given to those who weighed greater than or equal to 50 kg.
When the data were analyzed according to weight, proportionately more children on apremilast saw a sPGA response: 47.4% versus 21.8% in the lower weight and dose range and 19.2% versus 1.6% in the higher weight and dose range.
As for PASI-75, a greater proportion of children on apremilast also responded in both the lower and upper weight ranges, a respective 52.4% and 38.7% of patients, compared with 21.4% and 11% of those treated with placebo.
Data were also evaluated according to age, with a younger (aged 6-11 years) and older (age 12-17 years) group. The mean age of children was 12 years overall. Results showed a similar pattern for weight: The psoriasis of more children treated with apremilast was reduced by both measures, sPGA response, and PASI-75.
Safety of apremilast in children
“The overall safety profile during the placebo-controlled phase was comparable with the known safety profile of apremilast,” Dr. Belloni Fontina reported. “No new safety signals were identified.”
The rate of any adverse event was substantially higher in children given the active treatment, however, at 65% versus 41.3% for placebo.
Rates of severe and serious adverse events were low, at around 1.3%, and similar between the groups.
There was also a low rate of withdrawal because of side effects, although this was higher in the apremilast group (3.1% vs. 1.3%).
The primary reason for withdrawal of apremilast treatment were the most commonly reported adverse events: gastrointestinal disorders, including diarrhea, nausea, upper and lower abdominal pain, and vomiting. Headache, pyrexia, and nasopharyngitis were also reported.
Despite being common, most treatment-related adverse effects resolved within 3 days, Dr. Belloni Fontina said.
Expect further data
Further data from the trial are to be expected, because only the 16-week primary endpoint results have been released so far. The trial also included a 36-week extension phase, during which all children who had originally been randomly assigned to placebo were now eligible to be treated with apremilast, and all those who were originally given the active treatment were able to continue. This extension treatment period means that data will be available for a full year of treatment, and there will also be a further 2-week observational follow-up at the end of the trial.
The study was funded by Amgen. Dr. Belloni Fontina reported acting as an investigator and advisory board member for and receiving honoraria from Amgen, Galderma, Leo Pharma, and Pfizer. She also reported speaking on behalf of Pierre-Fabre and Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2022
Uncombable hair syndrome: One gene, variants responsible for many cases
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that manifests during infancy, investigators have reported.
The findings are from a cohort study published in JAMA Dermatology, which involved 107 unrelated children and adults suspected of having UHS, as well as family members, all of whom were recruited from January 2013 to December 2021. Genetic analyses were conducted in Germany from January 2014 to December 2021 with exome sequencing.
Study builds on prior research
Senior author Regina C. Betz, MD, professor of dermatogenetics at the Institute of Human Genetics, University Hospital Bonn, Germany, said that in 2016, she and her coinvestigators authored a study on the molecular genetics of UHS. That study, which involved 18 people with UHS, identified variants in three genes – PADI3, TCHH, and TGM3 – that encode proteins that play a role in the formation of the hair shaft. The investigators described how a deficiency in the shaping and mechanical strengthening of the hair shaft occurs in the UHS phenotype, which is characterized by dry, frizzy, and wiry hair that cannot be combed flat.
As a result of that previous work, “we base the assignment or confirmation of a clinical diagnosis of UHS on molecular genetic diagnostics,” the authors write in the new study, rather than on the clinical appearance of the hair and the physical examination of the patient, with confirmation on microscopical examination of the hair shaft.
Social media as instrument in finding study participants
Following the 2016 study, Dr. Betz and colleagues were contacted by many clinicians and by the public through Facebook and other social media platforms with details about possible cases of UHS, an autosomal recessive disorder. Through these contacts, blood samples, saliva, or DNA was sent to the investigators’ laboratory from 89 unrelated index patients (69 female patients, 20 male patients) suspected of having UHS. This resulted in the identification of pathogenic variants in 69 cases, the investigators write.
“In the first study, we had 18 patients, and then we tried to collect as many as possible” to determine the main mechanism behind UHS, Dr. Betz said. One question is whether there are additional genes responsible for UHS, she noted. “Even now, we are not sure, because in 25% [of cases in the new study], we didn’t find any mutation in the three known genes.”
The current study resulted in the discovery of eight novel pathogenic variants in PADI3, which are responsible for 71.0% (76) of the 107 cases. Of those, “6 were single observations and 2 were observed in 3 and 2 individuals, respectively,” the investigators write.
Children can grow out of this disorder, but it can also persist into adulthood, Dr. Betz noted. Communication that investigators had with parents of the children with UHS revealed that these children are often the targets of bullying by other children, she added.
She and her and colleagues will continue this research and are currently studying adults who have UHS.
Research leads to possible treatment pathways
Jeff Donovan, MD, FRCPC, FAAD, a dermatologist and medical director of the Donovan Hair Clinic in Whistler, British Columbia, described these findings as fundamental to understanding UHS and creating pathways to possible treatments.
The study “identifies more about the genetic basis of this challenging condition,” said Dr. Donovan, who is also clinical instructor in the department of dermatology at the University of British Columbia, Vancouver, and president of the Canadian Hair Loss Foundation. “We really need this type of information in order to have any sort of clue in terms of how to treat it,” he told this news organization.
“In the hair loss world, it’s pretty clear that if you can understand the genetic basis of things, or the basic science of a condition, whether it’s the basic genetics or the basic immunology, you give yourself the best chance to develop good treatments,” said Dr. Donovan.
The article provides advanced genetic information of the condition, such that geneticists can test for at least three markers if they are suspecting UHS, Dr. Donovan observed.
Condition can lead to bullying
Dr. Donovan also commented that UHS can have a detrimental impact on children with regard to socializing with their peers. “Having hair that sticks out and is very full like this is challenging because kids do get teased,” he said.
“It is often the parents who are the most affected” when a child aged 2-5 years has a hair condition such as UHS. But at age 5-9, “children are developing self-identity and an understanding of various aspects of self-esteem and what they look like and what others look like. And that’s where the teasing really starts. And that’s where it does become troublesome.”
Dr. Betz and Dr. Donovan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Dignity
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Queen Elizabeth is everywhere. She was even on the last slide of a presentation on COVID, monkeypox, and influenza vaccines given by our physician in charge of quality. This was odd. The presenter wasn’t English. The Queen had nothing to do with vaccines. Nor apparently would she have said even if she did have an opinion about them. But there we were, an audience of physicians and staff pausing for a moment of remembrance of her.
I’m not a Monarchist – except perhaps for the Kennedys. I grew up in New England. I don’t have an opinion on whether or not the British Crown should endure. But I do marvel at the astounding effect Queen Elizabeth’s passing had on so many around the world. Her personal qualities, particularly her steadiness and humane sympathy, might explain why so many are sad hearing the news. But also I think there was something in her role that we all wished for: Not the owning of palaces and sceptres, but rather, the respect that was given to her.
She was a stateswoman of “unmatched dignity,” the White House wrote. That was true, but it seems being the Queen might have been the last job on earth where such dignity is still possible. Certainly in politics, education, and even health care, there doesn’t seem to be much left lately.
The same day of that presentation I walked into the room of a patient 22 minutes late, she held her arm forth tapping her watch to indicate the time and my tardiness. Unnecessary, if not impertinent. Covering for one of my female physician colleagues, I read an email from a patient which began, “Dear Julie, With all due respect …” Another patient submitted a photo for us to review that was clearly taken from her car while waiting at a stop light. Hardly the consideration a clinical encounter should be given.
Much has been lost for patients. too. There are patients trying to make appointments lately who are told: “There are none. Call back later.” . There is no dignified way to remove exam paper stuck to your backside before introducing yourself to the doctor. Maybe that last slide of Her Majesty was in fact for us to have a moment of silence for what we’ve all lost.
Walter Bagehot (pronounce it “Baj-et” if you tell this story to your Harlan wine friends) was a political writer and editor of The Economist in the 1860s. He famously said that the secret to the English government was having two kinds of institutions, the dignified and the efficient. The efficient, Parliament, was responsible for all the work. The dignified, the Crown, gives significance and holds everyone’s respect. If medicine ever once was both dignified and efficient, we aren’t lately. We push to reduce backlogs, offer same-time virtual care, work to reduce costs. We’ve driven medicine to the efficient and left little of the dignity it seems.
The Queen will be remembered for her lifelong dedication to the laborious service of others. Even though each of us in medicine pledges the same, we also mourn this week the loss of dignity that once came with it.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Tralokinumab earns EU recommendation to expand age range for atopic dermatitis to include adolescents
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
Tralokinumab has received a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use to extend use to adolescents aged 12 years and older with moderate-to-severe atopic dermatitis (AD) who are candidates for systemic therapy, according to a statement from the manufacturer.
The positive CHMP opinion, issued on Sept. 15, recommends extending the use of tralokinumab (Adtralza), an interleukin-13 antagonist, to adolescents aged 12-17 years in the EU. The positive opinion recommends an initial dose of 600 mg administered subcutaneously followed by 300 mg every other week, the dosing recommended for adults.
In December 2021, tralokinumab was approved for adults with moderate to severe AD in the United States, where it is marketed as Adbry. It is also approved for adults in the EU, Great Britain, Canada, the United Arab Emirates, and Switzerland. It is not currently approved for treatment of adolescents in any country, according to the LEO Pharma statement.
A regulatory filing with the U.S. Food and Drug Administration is in progress, the company said, and an additional study of tralokinumab for individuals aged 12 years and older is underway, according to the manufacturer.
The CHMP opinion was supported by data from a phase 3 study (ECZTRA 6) that assessed safety and efficacy of 150-mg or 300-mg doses of tralokinumab, compared with placebo in adolescents with moderate-to-severe AD, the company statement said. The primary outcomes were an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) and an improvement of at least a 75% on the Eczema Area and Severity Index score (EASI-75). In the study, presented as a poster at a meeting in October 2021, a total of 195 adolescents aged 12-17 with moderate to severe AD who were candidates for systemic therapy were randomly assigned to tralokinumab and 94 to placebo.
At 16 weeks, 21.4% and 17.5% of patients who received 150 mg and 300 mg, respectively, of tralokinumab had IGA scores of 0 or 1, compared with 4.3% of those on placebo (P < .001, P = .002, respectively vs. placebo). In addition, 28.6% and 27.8% of the 150-mg and 300-mg tralokinumab groups, respectively, achieved EASI-75, compared with 6.4% of placebo patients (P < .001, P = .001, respectively, compared with placebo).
Adverse events were similar between the groups, and most were mild or moderate; overall safety profiles were similar to those seen in adult patients.
The European Commission will review the positive opinion and make a final decision.
The research was supported by LEO Pharma.
A version of this article first appeared on Medscape.com.
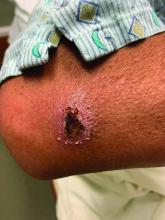
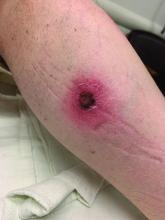
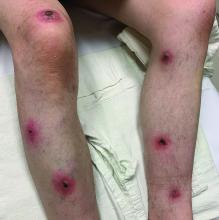
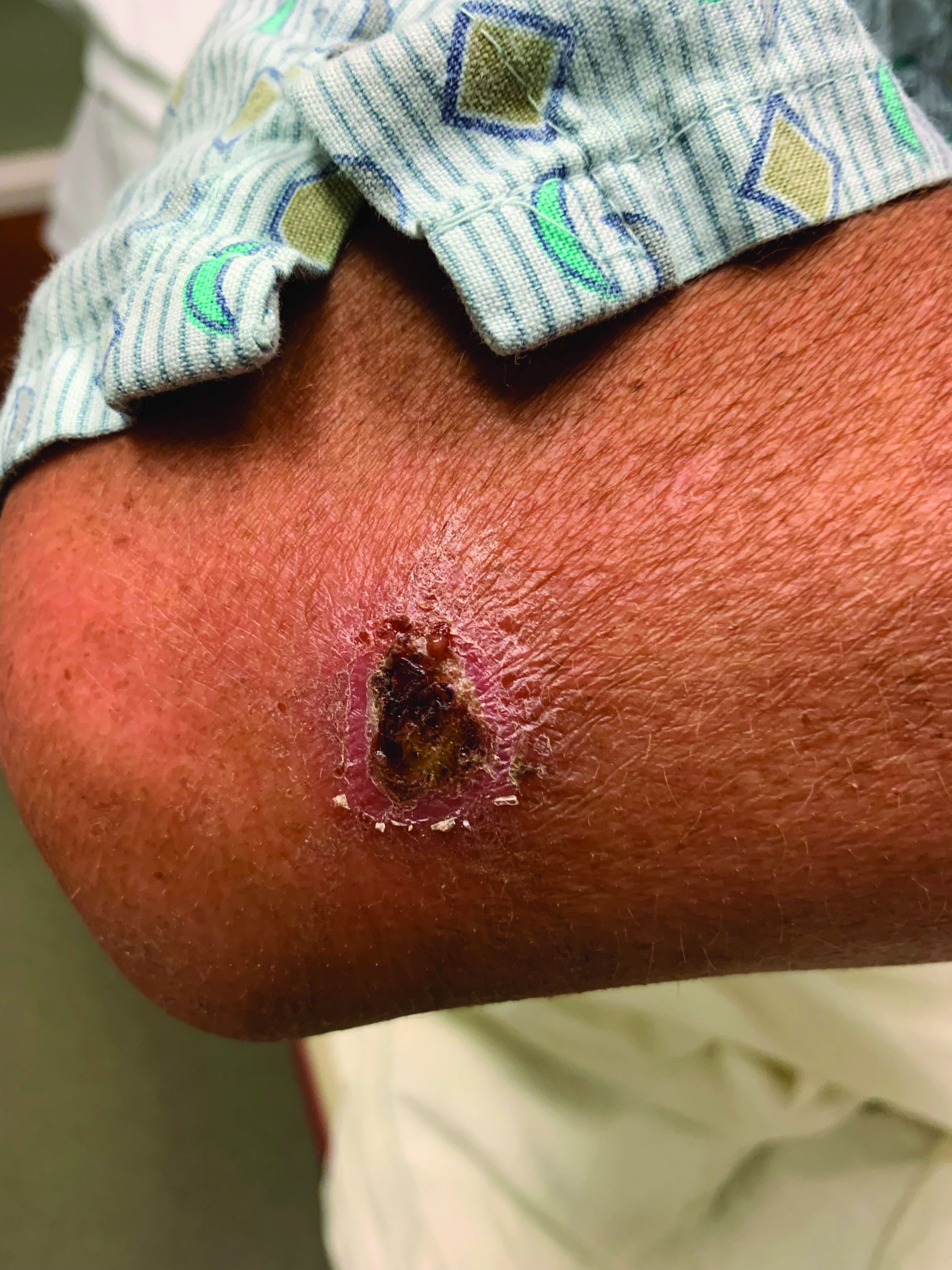
A White male presented with a 1-month history of recurrent, widespread, painful sores
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
Coinfection of staphylococci and streptococci can make it more challenging to treat. Lesions typically begin as a vesicle that enlarges and forms an ulcer with a hemorrhagic crust. Even with treatment, the depth of the lesions may result in scarring. Shins and dorsal feet are nearly always involved. Systemic involvement is rare.
Open wounds, bites, or dermatoses are risk factors for the development of ecthyma. Additionally, poor hygiene and malnutrition play a major role in inoculation and severity of the disease. Poor hygiene may serve as the initiating factor for infection, but malnutrition permits further development because of the body’s inability to mount a sufficient immune response. Intravenous drug users and patients with HIV tend to be affected.
When diagnosing ecthyma, it is important to correlate clinical signs with a bacterial culture. This condition can be difficult to treat because of both coinfection and growing antibiotic resistance in staphylococcal and streptococcal species. Specifically, S. aureus has been found to be resistant to beta-lactam antibiotics for many years, with methicillin-resistant S. aureus (MRSA) being first detected in 1961. While a variety of antibiotics are indicated, the prescription should be tailored to cover the cultured organism.
Topical antibiotics are sufficient for more superficial lesions. Both topical and oral antibiotics may be recommended for ecthyma as the infection can spread more deeply into the skin, eventually causing a cellulitis. Treatment protocol for oral agents varies based on which drug is indicated. This patient was seen in the emergency room. His white blood cell count was elevated at 9 × 109/L. He was started empirically on amoxicillin/clavulanate (Augmentin) and ciprofloxacin. Bacterial cultures grew out Streptococcus pyogenes.
The case and photos were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Susannah Berke, MD, Three Rivers Dermatology, Coraopolis, Pa. Dr. Bilu Martin edited the column. Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kwak Y et al. Infect Chemother. 2017 Dec;49(4):301-25.
2. Pereira LB. An Bras Dermatol. 2014 Mar-Apr;89(2):293-9.
3. Wasserzug O et al. Clin Infect Dis. 2009 May 1;48(9):1213-9.
House passes prior authorization bill, Senate path unclear
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
The path through the U.S. Senate is not yet certain for a bill intended to speed the prior authorization process of insurer-run Medicare Advantage plans, despite the measure having breezed through the House.
House leaders opted to move the Improving Seniors’ Timely Access to Care Act of 2021 (HR 3173) without requiring a roll-call vote. The measure was passed on Sept. 14 by a voice vote, an approach used in general with only uncontroversial measures that have broad support. The bill has 191 Democratic and 135 Republican sponsors, representing about three-quarters of the members of the House.
“There is no reason that patients should be waiting for medically appropriate care, especially when we know that this can lead to worse outcomes,” Rep. Earl Blumenauer (D-Ore.) said in a Sept. 14 speech on the House floor. “The fundamental promise of Medicare Advantage is undermined when people are delaying care, getting sicker, and ultimately costing Medicare more money.”
Rep. Greg Murphy, MD (R-N.C.), spoke on the House floor that day as well, bringing up cases he has seen in his own urology practice in which prior authorization delays disrupted medical care. One patient wound up in the hospital with abscess after an insurer denied an antibiotic prescription, Rep. Murphy said.
But the Senate appears unlikely at this time to move the prior authorization bill as a standalone measure. Instead, the bill may become part of a larger legislative package focused on health care that the Senate Finance Committee intends to prepare later this year.
The House-passed bill would require insurer-run Medicare plans to respond to expedited requests for prior authorization of services within 24 hours and to other requests within 7 days. This bill also would establish an electronic program for prior authorizations and mandate increased transparency as to how insurers use this tool.
CBO: Cost of change would be billions
In seeking to mandate changes in prior authorization, lawmakers likely will need to contend with the issue of a $16 billion cumulative cost estimate for the bill from the Congressional Budget Office. Members of Congress often seek to offset new spending by pairing bills that add to expected costs for the federal government with ones expected to produce savings.
Unlike Rep. Blumenauer, Rep. Murphy, and other backers of the prior authorization streamlining bill, CBO staff estimates that making the mandated changes would raise federal spending, inasmuch as there would be “a greater use of services.”
On Sept. 14, CBO issued a one-page report on the costs of the bill. The CBO report concerns only the bill in question, as is common practice with the office’s estimates.
Prior authorization changes would begin in fiscal 2025 and would add $899 million in spending, or outlays, that year, CBO said. The annual costs from the streamlined prior authorization practices through fiscal 2026 to 2032 range from $1.6 billion to $2.7 billion.
Looking at the CBO estimate against a backdrop of total Medicare Advantage costs, though, may provide important context.
The increases in spending estimated by CBO may suggest that there would be little change in federal spending as a result of streamlining prior authorization practices. These estimates of increased annual spending of $1.6 billion–$2.7 billion are only a small fraction of the current annual cost of insurer-run Medicare, and they represent an even smaller share of the projected expense.
The federal government last year spent about $350 billion on insurer-run plans, excluding Part D drug plan payments, according to the Medicare Advisory Payment Commission (MedPAC).
As of 2021, about 27 million people were enrolled in these plans, accounting for about 46% of the total Medicare population. Enrollment has doubled since 2010, MedPAC said, and it is expected to continue to grow. By 2027, insurer-run Medicare could cover 50% of the program’s population, a figure that may reach 53% by 2031.
Federal payments to these plans will accelerate in the years ahead as insurers attract more people eligible for Medicare as customers. Payments to these private health plans could rise from an expected $418 billion this year to $940.6 billion by 2031, according to the most recent Medicare trustees report.
Good intentions, poor implementation?
Insurer-run Medicare has long enjoyed deep bipartisan support in Congress. That’s due in part to its potential for reducing spending on what are considered low-value treatments, or ones considered unlikely to provide a significant medical benefit, but Rep. Blumenauer is among the members of Congress who see insurer-run Medicare as a path for preserving the giant federal health program. Traditional Medicare has far fewer restrictions on services, which sometimes opens a path for tests and treatments that offer less value for patients.
“I believe that the way traditional fee-for-service Medicare operates is not sustainable and that Medicare Advantage is one of the tools we can use to demonstrate how we can incentivize value,” Rep. Blumenauer said on the House floor. “But this is only possible when the program operates as intended. I have been deeply concerned about the reports of delays in care” caused by the clunky prior authorization processes.
He highlighted a recent report from the internal watchdog group for the Department of Health & Human Services that raises concerns about denials of appropriate care. About 18% of a set of payment denials examined by the Office of Inspector General of HHS in April actually met Medicare coverage rules and plan billing rules.
“For patients and their families, being told that you need to wait longer for care that your doctor tells you that you need is incredibly frustrating and frightening,” Rep. Blumenauer said. “There’s no comfort to be found in the fact that your insurance company needs time to decide if your doctor is right.”
Trends in prior authorization
The CBO report does not provide detail on what kind of medical spending would increase under a streamlined prior authorization process in insurer-run Medicare plans.
From trends reported in prior authorization, though, two factors could be at play in what appear to be relatively small estimated increases in Medicare spending from streamlined prior authorization.
One is the work already underway to create less burdensome electronic systems for these requests, such as the Fast Prior Authorization Technology Highway initiative run by the trade association America’s Health Insurance Plans.
The other factor could be the number of cases in which prior authorization merely causes delays in treatments and tests and thus simply postpones spending while adding to clinicians’ administrative work.
An analysis of prior authorization requests for dermatologic practices affiliated with the University of Utah may represent an extreme example. In a report published in JAMA Dermatology in 2020, researchers described what happened with requests made during 1 month, September 2016.
The approval rate for procedures was 99.6% – 100% (95 of 95) for Mohs surgery, and 96% (130 of 131, with 4 additional cases pending) for excisions. These findings supported calls for simplifying prior authorization procedures, “perhaps first by eliminating unnecessary PAs [prior authorizations] and appeals,” Aaron M. Secrest, MD, PhD, of the University of Utah, Salt Lake City, and coauthors wrote in the article.
Still, there is some evidence that insurer-run Medicare policies reduce the use of low-value care.
In a study published in JAMA Health Forum, Emily Boudreau, PhD, of insurer Humana Inc, and coauthors from Tufts University, Boston, and the University of Pennsylvania, Philadelphia investigated whether insurer-run Medicare could do a better job in reducing the amount of low-value care delivered than the traditional program. They analyzed a set of claims data from 2017 to 2019 for people enrolled in insurer-run and traditional Medicare.
They reported a rate of 23.07 low-value services provided per 100 people in insurer-run Medicare, compared with 25.39 for those in traditional Medicare. Some of the biggest differences reported in the article were in cancer screenings for older people.
As an example, the U.S. Preventive Services Task Force recommends that women older than 65 years not be screened for cervical cancer if they have undergone adequate screening in the past and are not at high risk for cervical cancer. There was an annual count of 1.76 screenings for cervical cancer per 100 women older than 65 in the insurer-run Medicare group versus 3.18 for those in traditional Medicare.
The Better Medicare Alliance issued a statement in favor of the House passage of the Improving Seniors’ Timely Access to Care Act.
In it, the group said the measure would “modernize prior authorization while protecting its essential function in facilitating safe, high-value, evidence-based care.” The alliance promotes use of insurer-run Medicare. The board of the Better Medicare Alliance includes executives who serve with firms that run Advantage plans as well as medical organizations and universities.
“With studies showing that up to one-quarter of all health care expenditures are wasted on services with no benefit to the patient, we need a robust, next-generation prior authorization program to deter low-value, and even harmful, care while protecting access to needed treatment and effective therapies,” said A. Mark Fendrick, MD, director of the University of Michigan’s Center for Value-Based Insurance Design in Ann Arbor, in a statement issued by the Better Medicare Alliance. He is a member of the group’s council of scholars.
On the House floor on September 14, Rep. Ami Bera, MD (D-Calif.), said he has heard from former colleagues and his medical school classmates that they now spend as much as 40% of their time on administrative work. These distractions from patient care are helping drive physicians away from the practice of medicine.
Still, the internist defended the basic premise of prior authorization while strongly appealing for better systems of handling it.
“Yes, there is a role for prior authorization in limited cases. There is also a role to go back and retrospectively look at how care is being delivered,” Rep. Bera said. “But what is happening today is a travesty. It wasn’t the intention of prior authorization. It is a prior authorization process gone awry.”
A version of this article first appeared on Medscape.com.
Meta-analysis demonstrates potential of probiotics in reducing atopic dermatitis disease severity
Key clinical point: The use of probiotic supplementation reduced disease severity in adult patients with atopic dermatitis (AD).
Major finding: Probiotic supplementation vs placebo led to a significant reduction in the Scoring AD index (mean difference −7.90; 95% CI −7.25 to−6.92), but no significant improvements in skin severity and itch severity.
Study details: Findings are from a meta-analysis of six randomized controlled trials including 241 adults with AD, of which 128 received probiotics and 113 received placebo.
Disclosures: This study was funded by a grant from Universitas Airlangga, Indonesia. The authors declared no conflicts of interest.
Source: Umborowati MA et al. The role of probiotics in the treatment of adult atopic dermatitis: a meta-analysis of randomized controlled trials. J Health Popul Nutr. 2022;41:37 (Aug 17). Doi: 10.1186/s41043-022-00318-6
Key clinical point: The use of probiotic supplementation reduced disease severity in adult patients with atopic dermatitis (AD).
Major finding: Probiotic supplementation vs placebo led to a significant reduction in the Scoring AD index (mean difference −7.90; 95% CI −7.25 to−6.92), but no significant improvements in skin severity and itch severity.
Study details: Findings are from a meta-analysis of six randomized controlled trials including 241 adults with AD, of which 128 received probiotics and 113 received placebo.
Disclosures: This study was funded by a grant from Universitas Airlangga, Indonesia. The authors declared no conflicts of interest.
Source: Umborowati MA et al. The role of probiotics in the treatment of adult atopic dermatitis: a meta-analysis of randomized controlled trials. J Health Popul Nutr. 2022;41:37 (Aug 17). Doi: 10.1186/s41043-022-00318-6
Key clinical point: The use of probiotic supplementation reduced disease severity in adult patients with atopic dermatitis (AD).
Major finding: Probiotic supplementation vs placebo led to a significant reduction in the Scoring AD index (mean difference −7.90; 95% CI −7.25 to−6.92), but no significant improvements in skin severity and itch severity.
Study details: Findings are from a meta-analysis of six randomized controlled trials including 241 adults with AD, of which 128 received probiotics and 113 received placebo.
Disclosures: This study was funded by a grant from Universitas Airlangga, Indonesia. The authors declared no conflicts of interest.
Source: Umborowati MA et al. The role of probiotics in the treatment of adult atopic dermatitis: a meta-analysis of randomized controlled trials. J Health Popul Nutr. 2022;41:37 (Aug 17). Doi: 10.1186/s41043-022-00318-6
Real-world characteristics of patients with moderate-to-severe atopic dermatitis receiving dupilumab
Key clinical point: Patients who received dupilumab for atopic dermatitis (AD) had moderate-to-severe disease, long medical history, and high prevalence of coexisting type 2 inflammatory diseases.
Major finding: A majority of patients (66.6%) were diagnosed with AD in childhood, and most patients presented with bordering moderate-to-severe AD (Eczema Area and Severity Index > 21), high prevalence of pruritus (99.6%), and coexisting atopic and type 2 inflammatory diseases (51.8%).
Study details: Findings are from an analysis of PROLEAD, a national, multicenter, prospective, non-interventional study, including 817 patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Three authors declared being employees of or holding stocks in Sanofi. The other authors reported ties with several sources, including Sanofi.
Source: Thaci D et al. Dupilumab treatment of atopic dermatitis in routine clinical care: Baseline characteristics of patients in the PROLEAD prospective, observational study. Dermatol Ther (Heidelb). 2022;12(9):2145-2160 (Aug 19). Doi: 10.1007/s13555-022-00791-1
Key clinical point: Patients who received dupilumab for atopic dermatitis (AD) had moderate-to-severe disease, long medical history, and high prevalence of coexisting type 2 inflammatory diseases.
Major finding: A majority of patients (66.6%) were diagnosed with AD in childhood, and most patients presented with bordering moderate-to-severe AD (Eczema Area and Severity Index > 21), high prevalence of pruritus (99.6%), and coexisting atopic and type 2 inflammatory diseases (51.8%).
Study details: Findings are from an analysis of PROLEAD, a national, multicenter, prospective, non-interventional study, including 817 patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Three authors declared being employees of or holding stocks in Sanofi. The other authors reported ties with several sources, including Sanofi.
Source: Thaci D et al. Dupilumab treatment of atopic dermatitis in routine clinical care: Baseline characteristics of patients in the PROLEAD prospective, observational study. Dermatol Ther (Heidelb). 2022;12(9):2145-2160 (Aug 19). Doi: 10.1007/s13555-022-00791-1
Key clinical point: Patients who received dupilumab for atopic dermatitis (AD) had moderate-to-severe disease, long medical history, and high prevalence of coexisting type 2 inflammatory diseases.
Major finding: A majority of patients (66.6%) were diagnosed with AD in childhood, and most patients presented with bordering moderate-to-severe AD (Eczema Area and Severity Index > 21), high prevalence of pruritus (99.6%), and coexisting atopic and type 2 inflammatory diseases (51.8%).
Study details: Findings are from an analysis of PROLEAD, a national, multicenter, prospective, non-interventional study, including 817 patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Three authors declared being employees of or holding stocks in Sanofi. The other authors reported ties with several sources, including Sanofi.
Source: Thaci D et al. Dupilumab treatment of atopic dermatitis in routine clinical care: Baseline characteristics of patients in the PROLEAD prospective, observational study. Dermatol Ther (Heidelb). 2022;12(9):2145-2160 (Aug 19). Doi: 10.1007/s13555-022-00791-1
Pediatric atopic dermatitis and neuropsychiatric disorders: What is the link?
Key clinical point: Atopic dermatitis (AD) did not increase the incidence risk for most neuropsychiatric disorders in a pediatric cohort.
Major finding: The risks for attention deficit hyperactivity disorder (hazard ratio [HR] 1.02; 95% CI 0.97-1.06), autism (HR 1.02; 95% CI 0.98-1.06), anxiety (HR 1.01; 95% CI 0.99-1.03), and bipolar disorder (HR 1.08; 95% CI 0.85-1.36) were comparable in the AD and non-AD groups. Participants with vs without AD were less likely to develop depression (HR 0.93; 95% CI 0.91-0.95) or schizophrenia (HR 0.72; 95% CI 0.54-0.95) but more likely to develop obsessive compulsive disorder (HR 1.26; 95% CI 1.16-1.37). However, the risks varied with disease severity and patient’s age.
Study details: Findings are from a retrospective population-based cohort study including 409,431 children with AD and 1,809,029 matched children without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. One author declared being an employee of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Wan J et al. Atopic dermatitis and risk of major neuropsychiatric disorders in children: A population-based cohort study. J Eur Acad Dermatol Venereol. 2022 (Aug 26). Doi: 10.1111/jdv.18564
Key clinical point: Atopic dermatitis (AD) did not increase the incidence risk for most neuropsychiatric disorders in a pediatric cohort.
Major finding: The risks for attention deficit hyperactivity disorder (hazard ratio [HR] 1.02; 95% CI 0.97-1.06), autism (HR 1.02; 95% CI 0.98-1.06), anxiety (HR 1.01; 95% CI 0.99-1.03), and bipolar disorder (HR 1.08; 95% CI 0.85-1.36) were comparable in the AD and non-AD groups. Participants with vs without AD were less likely to develop depression (HR 0.93; 95% CI 0.91-0.95) or schizophrenia (HR 0.72; 95% CI 0.54-0.95) but more likely to develop obsessive compulsive disorder (HR 1.26; 95% CI 1.16-1.37). However, the risks varied with disease severity and patient’s age.
Study details: Findings are from a retrospective population-based cohort study including 409,431 children with AD and 1,809,029 matched children without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. One author declared being an employee of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Wan J et al. Atopic dermatitis and risk of major neuropsychiatric disorders in children: A population-based cohort study. J Eur Acad Dermatol Venereol. 2022 (Aug 26). Doi: 10.1111/jdv.18564
Key clinical point: Atopic dermatitis (AD) did not increase the incidence risk for most neuropsychiatric disorders in a pediatric cohort.
Major finding: The risks for attention deficit hyperactivity disorder (hazard ratio [HR] 1.02; 95% CI 0.97-1.06), autism (HR 1.02; 95% CI 0.98-1.06), anxiety (HR 1.01; 95% CI 0.99-1.03), and bipolar disorder (HR 1.08; 95% CI 0.85-1.36) were comparable in the AD and non-AD groups. Participants with vs without AD were less likely to develop depression (HR 0.93; 95% CI 0.91-0.95) or schizophrenia (HR 0.72; 95% CI 0.54-0.95) but more likely to develop obsessive compulsive disorder (HR 1.26; 95% CI 1.16-1.37). However, the risks varied with disease severity and patient’s age.
Study details: Findings are from a retrospective population-based cohort study including 409,431 children with AD and 1,809,029 matched children without AD.
Disclosures: This study was supported by a contract from Pfizer, Inc. One author declared being an employee of Pfizer. The other authors reported ties with several sources, including Pfizer.
Source: Wan J et al. Atopic dermatitis and risk of major neuropsychiatric disorders in children: A population-based cohort study. J Eur Acad Dermatol Venereol. 2022 (Aug 26). Doi: 10.1111/jdv.18564