User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
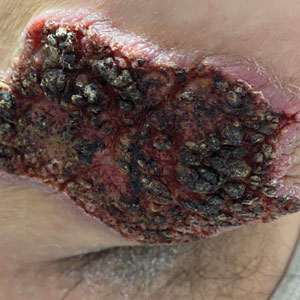
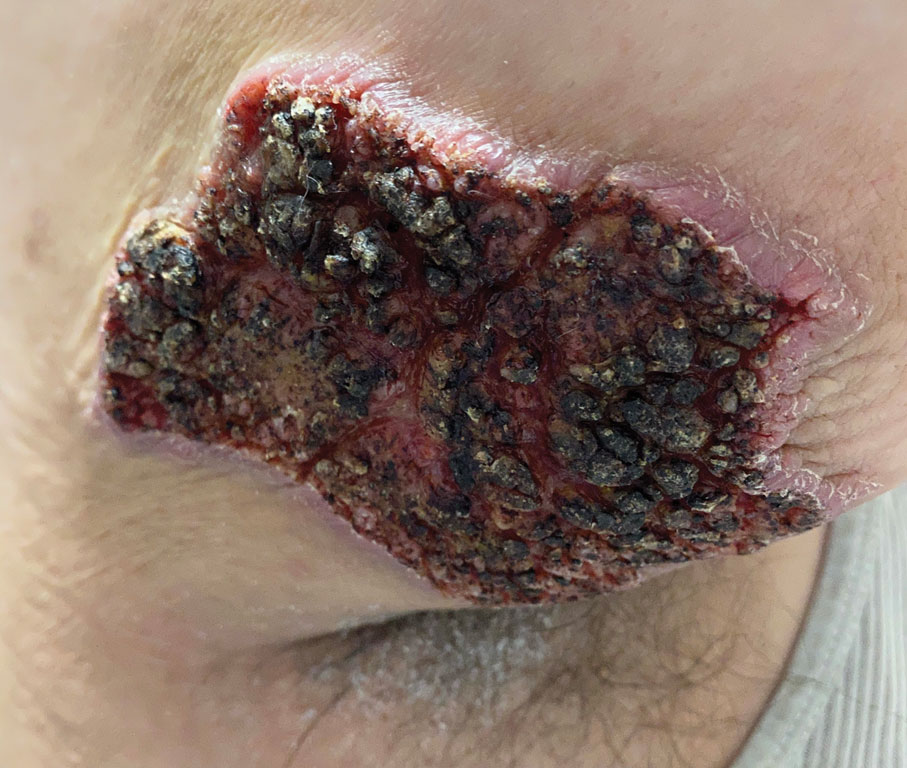
Fungated Eroded Plaque on the Arm
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.
Medical assistants
When I began in private practice several eons ago, I employed only registered nurses (RNs) and licensed practical nurses (LPNs) in my office – as did, I think, most other physicians.
That is still the preferred way to go from an efficiency perspective, as well as the ability to delegate such tasks as blood collection and administering intramuscular injections. Unfortunately,
Given this reality, it makes sense to understand how the use of medical assistants has changed private medical practice, and how the most effective MAs manage their roles and maximize their efficiency in the office.
A recent article by two physicians at the University of Michigan, Ann Arbor, is one of the few published papers to address this issue. It presents the results of a cross-sectional study examining the MA’s experience and key factors that enhance or reduce efficiencies.
The authors sent an email survey to 86 MAs working in six clinics within the department of family medicine at the University of Michigan Medical Center, and received responses from 75 of them, including 61 who completed the entire survey. They then singled out 18 individuals deemed “most efficient” by their peers and conducted face-to-face interviews with them.
The surveys and interviews looked at how MAs identified personal strategies for efficiency, dealt with barriers to implementing those strategies, and navigated interoffice relationships, as well as how all of this affected overall job satisfaction.
All 61 respondents who completed the full survey agreed that the MA role was “very important to keep the clinic functioning” and nearly all said that working in health care was “a calling” for them. About half agreed that their work was very stressful, and about the same percentage reported that there was inadequate MA staffing at their clinic. Others complained of limited pay and promotion opportunities.
The surveyed MAs described important work values that increased their efficiency. These included good communication, strong teamwork, and workload sharing, as well as individual strategies such as multitasking, limiting patient conversations, and completing tasks in a consistent way to improve accuracy.
Other strategies identified as contributing to an efficient operation included preclinic huddles, reviews of patient records before the patient’s arrival, and completing routine office duties before the start of office hours.
Respondents were then asked to identify barriers to clinic efficiency, and most of them involved physicians who barked orders at them, did not complete paperwork or sign orders in a timely manner, and agreed to see late-arriving patients. Some MAs suggested that physicians refrain from “talking down” to them, and teach rather than criticize. They also faulted decisions affecting patient flow made by other staffers without soliciting the MAs’ input.
Despite these barriers, the authors found that most of the surveyed MAs agreed that their work was valued by doctors. “Proper training of managers to provide ... support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial,” they said.
“Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier,” they added.
At the same time, the authors noted that most survey subjects reported that their jobs were “stressful,” and believed that their stress went underrecognized by physicians. They argued that “it’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.”
Since this study involved only MAs in a family practice setting, further studies will be needed to determine whether these results translate to specialty offices – and whether the unique issues inherent in various specialty environments elicit different efficiency contributors and barriers.
Overall, though, “staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients,” the authors wrote. “Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
When I began in private practice several eons ago, I employed only registered nurses (RNs) and licensed practical nurses (LPNs) in my office – as did, I think, most other physicians.
That is still the preferred way to go from an efficiency perspective, as well as the ability to delegate such tasks as blood collection and administering intramuscular injections. Unfortunately,
Given this reality, it makes sense to understand how the use of medical assistants has changed private medical practice, and how the most effective MAs manage their roles and maximize their efficiency in the office.
A recent article by two physicians at the University of Michigan, Ann Arbor, is one of the few published papers to address this issue. It presents the results of a cross-sectional study examining the MA’s experience and key factors that enhance or reduce efficiencies.
The authors sent an email survey to 86 MAs working in six clinics within the department of family medicine at the University of Michigan Medical Center, and received responses from 75 of them, including 61 who completed the entire survey. They then singled out 18 individuals deemed “most efficient” by their peers and conducted face-to-face interviews with them.
The surveys and interviews looked at how MAs identified personal strategies for efficiency, dealt with barriers to implementing those strategies, and navigated interoffice relationships, as well as how all of this affected overall job satisfaction.
All 61 respondents who completed the full survey agreed that the MA role was “very important to keep the clinic functioning” and nearly all said that working in health care was “a calling” for them. About half agreed that their work was very stressful, and about the same percentage reported that there was inadequate MA staffing at their clinic. Others complained of limited pay and promotion opportunities.
The surveyed MAs described important work values that increased their efficiency. These included good communication, strong teamwork, and workload sharing, as well as individual strategies such as multitasking, limiting patient conversations, and completing tasks in a consistent way to improve accuracy.
Other strategies identified as contributing to an efficient operation included preclinic huddles, reviews of patient records before the patient’s arrival, and completing routine office duties before the start of office hours.
Respondents were then asked to identify barriers to clinic efficiency, and most of them involved physicians who barked orders at them, did not complete paperwork or sign orders in a timely manner, and agreed to see late-arriving patients. Some MAs suggested that physicians refrain from “talking down” to them, and teach rather than criticize. They also faulted decisions affecting patient flow made by other staffers without soliciting the MAs’ input.
Despite these barriers, the authors found that most of the surveyed MAs agreed that their work was valued by doctors. “Proper training of managers to provide ... support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial,” they said.
“Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier,” they added.
At the same time, the authors noted that most survey subjects reported that their jobs were “stressful,” and believed that their stress went underrecognized by physicians. They argued that “it’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.”
Since this study involved only MAs in a family practice setting, further studies will be needed to determine whether these results translate to specialty offices – and whether the unique issues inherent in various specialty environments elicit different efficiency contributors and barriers.
Overall, though, “staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients,” the authors wrote. “Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
When I began in private practice several eons ago, I employed only registered nurses (RNs) and licensed practical nurses (LPNs) in my office – as did, I think, most other physicians.
That is still the preferred way to go from an efficiency perspective, as well as the ability to delegate such tasks as blood collection and administering intramuscular injections. Unfortunately,
Given this reality, it makes sense to understand how the use of medical assistants has changed private medical practice, and how the most effective MAs manage their roles and maximize their efficiency in the office.
A recent article by two physicians at the University of Michigan, Ann Arbor, is one of the few published papers to address this issue. It presents the results of a cross-sectional study examining the MA’s experience and key factors that enhance or reduce efficiencies.
The authors sent an email survey to 86 MAs working in six clinics within the department of family medicine at the University of Michigan Medical Center, and received responses from 75 of them, including 61 who completed the entire survey. They then singled out 18 individuals deemed “most efficient” by their peers and conducted face-to-face interviews with them.
The surveys and interviews looked at how MAs identified personal strategies for efficiency, dealt with barriers to implementing those strategies, and navigated interoffice relationships, as well as how all of this affected overall job satisfaction.
All 61 respondents who completed the full survey agreed that the MA role was “very important to keep the clinic functioning” and nearly all said that working in health care was “a calling” for them. About half agreed that their work was very stressful, and about the same percentage reported that there was inadequate MA staffing at their clinic. Others complained of limited pay and promotion opportunities.
The surveyed MAs described important work values that increased their efficiency. These included good communication, strong teamwork, and workload sharing, as well as individual strategies such as multitasking, limiting patient conversations, and completing tasks in a consistent way to improve accuracy.
Other strategies identified as contributing to an efficient operation included preclinic huddles, reviews of patient records before the patient’s arrival, and completing routine office duties before the start of office hours.
Respondents were then asked to identify barriers to clinic efficiency, and most of them involved physicians who barked orders at them, did not complete paperwork or sign orders in a timely manner, and agreed to see late-arriving patients. Some MAs suggested that physicians refrain from “talking down” to them, and teach rather than criticize. They also faulted decisions affecting patient flow made by other staffers without soliciting the MAs’ input.
Despite these barriers, the authors found that most of the surveyed MAs agreed that their work was valued by doctors. “Proper training of managers to provide ... support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial,” they said.
“Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier,” they added.
At the same time, the authors noted that most survey subjects reported that their jobs were “stressful,” and believed that their stress went underrecognized by physicians. They argued that “it’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.”
Since this study involved only MAs in a family practice setting, further studies will be needed to determine whether these results translate to specialty offices – and whether the unique issues inherent in various specialty environments elicit different efficiency contributors and barriers.
Overall, though, “staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients,” the authors wrote. “Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Best meds for insomnia identified?
In a comprehensive comparative-effectiveness analysis, lemborexant and eszopiclone showed the best efficacy, acceptability, and tolerability for acute and long-term insomnia treatment.
However, eszopiclone may cause substantial side effects – and safety data on lemborexant were inconclusive, the researchers note.
Not surprisingly, short-acting, intermediate-acting, and long-acting benzodiazepines were effective in the acute treatment of insomnia, but they have unfavorable tolerability and safety profiles, and there are no long-term data on these issues.
For many insomnia medications, there is a “striking” and “appalling” lack of long-term data, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry, University of Oxford, United Kingdom, noted during a press briefing.
“This is a call for regulators to raise the bar and ask for long-term data when companies submit an application for licensing insomnia drugs,” Dr. Cipriani said.
The findings were published online in The Lancet.
Prevalent, debilitating
Insomnia is highly prevalent, affecting up to 1 in 5 adults, and can have a profound impact on health, well-being, and productivity.
Sleep hygiene and cognitive-behavioral therapy for insomnia (CBT-I) are recommended first-line treatments, but they are often unavailable, which often leads patients and clinicians to turn to medications.
However, “insomnia drugs are not all created equal. Even within the same drug class there are differences,” Dr. Cipriani said.
In a large-scale systematic review and network meta-analysis, the researchers analyzed data from 154 double-blind, randomized controlled trials of medications (licensed or not) used for acute and long-term treatment of insomnia in 44,089 adults (mean age, 51.7 years; 63% women).
Results showed, for the acute treatment of insomnia, benzodiazepines, doxylamine, eszopiclone, lemborexant, seltorexant, zolpidem, and zopiclone were more effective than placebo (standardized mean difference range, 0.36-0.83; high-to-moderate certainty of evidence).
In addition, benzodiazepines, eszopiclone, zolpidem, and zopiclone were more effective than melatonin, ramelteon, and zaleplon (SMD, 0.27-0.71; moderate-to-very low certainty of evidence).
“Our results show that the melatonergic drugs melatonin and ramelteon are not really effective. The data do not support the regular use of these drugs,” co-investigator Phil Cowen, PhD, professor of psychopharmacology, University of Oxford, said at the briefing.
Best available evidence
What little long-term data is available suggest eszopiclone and lemborexant are more effective than placebo. Plus, eszopiclone is more effective than ramelteon and zolpidem but with “very low” certainty of evidence, the researchers report.
“There was insufficient evidence to support the prescription of benzodiazepines and zolpidem in long-term treatment,” they write.
Another problem was lack of data on other important outcomes, they add.
“We wanted to look at hangover effects, daytime sleepiness, [and] rebound effect, but often there was no data reported in trials. We need to collect data about these outcomes because they matter to clinicians and patients,” Dr. Cipriani said.
Summing up, the researchers note the current findings represent the “best available evidence base to guide the choice about pharmacological treatment for insomnia disorder in adults and will assist in shared decisionmaking between patients, carers, and their clinicians, as well as policy makers.”
They caution, however, that all statements comparing the merits of one drug with another “should be tempered by the potential limitations of the current analysis, the quality of the available evidence, the characteristics of the patient populations, and the uncertainties that might result from choice of dose or treatment setting.”
In addition, it is important to also consider nonpharmacologic treatments for insomnia disorder, as they are supported by “high-quality evidence and recommended as first-line treatment by guidelines,” the investigator write.
Shared decisionmaking
In an accompanying editorial, Myrto Samara, MD, University of Thessaly, Larissa, Greece, agrees with the researchers that discussion with patients is key.
“For insomnia treatment, patient-physician shared decisionmaking is crucial to decide when a pharmacological intervention is deemed necessary and which drug [is] to be given by considering the trade-offs for efficacy and side effects,” Dr. Samara writes.
The study was funded by the UK National Institute for Health Research (NIHR) Oxford Health Biomedical Research Center. Dr. Cipriani has received research and consultancy fees from the Italian Network for Pediatric Trials, CARIPLO Foundation, and Angelini Pharma, and is the chief and principal investigator of two trials of seltorexant in depression that are sponsored by Janssen. Dr. Samara has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a comprehensive comparative-effectiveness analysis, lemborexant and eszopiclone showed the best efficacy, acceptability, and tolerability for acute and long-term insomnia treatment.
However, eszopiclone may cause substantial side effects – and safety data on lemborexant were inconclusive, the researchers note.
Not surprisingly, short-acting, intermediate-acting, and long-acting benzodiazepines were effective in the acute treatment of insomnia, but they have unfavorable tolerability and safety profiles, and there are no long-term data on these issues.
For many insomnia medications, there is a “striking” and “appalling” lack of long-term data, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry, University of Oxford, United Kingdom, noted during a press briefing.
“This is a call for regulators to raise the bar and ask for long-term data when companies submit an application for licensing insomnia drugs,” Dr. Cipriani said.
The findings were published online in The Lancet.
Prevalent, debilitating
Insomnia is highly prevalent, affecting up to 1 in 5 adults, and can have a profound impact on health, well-being, and productivity.
Sleep hygiene and cognitive-behavioral therapy for insomnia (CBT-I) are recommended first-line treatments, but they are often unavailable, which often leads patients and clinicians to turn to medications.
However, “insomnia drugs are not all created equal. Even within the same drug class there are differences,” Dr. Cipriani said.
In a large-scale systematic review and network meta-analysis, the researchers analyzed data from 154 double-blind, randomized controlled trials of medications (licensed or not) used for acute and long-term treatment of insomnia in 44,089 adults (mean age, 51.7 years; 63% women).
Results showed, for the acute treatment of insomnia, benzodiazepines, doxylamine, eszopiclone, lemborexant, seltorexant, zolpidem, and zopiclone were more effective than placebo (standardized mean difference range, 0.36-0.83; high-to-moderate certainty of evidence).
In addition, benzodiazepines, eszopiclone, zolpidem, and zopiclone were more effective than melatonin, ramelteon, and zaleplon (SMD, 0.27-0.71; moderate-to-very low certainty of evidence).
“Our results show that the melatonergic drugs melatonin and ramelteon are not really effective. The data do not support the regular use of these drugs,” co-investigator Phil Cowen, PhD, professor of psychopharmacology, University of Oxford, said at the briefing.
Best available evidence
What little long-term data is available suggest eszopiclone and lemborexant are more effective than placebo. Plus, eszopiclone is more effective than ramelteon and zolpidem but with “very low” certainty of evidence, the researchers report.
“There was insufficient evidence to support the prescription of benzodiazepines and zolpidem in long-term treatment,” they write.
Another problem was lack of data on other important outcomes, they add.
“We wanted to look at hangover effects, daytime sleepiness, [and] rebound effect, but often there was no data reported in trials. We need to collect data about these outcomes because they matter to clinicians and patients,” Dr. Cipriani said.
Summing up, the researchers note the current findings represent the “best available evidence base to guide the choice about pharmacological treatment for insomnia disorder in adults and will assist in shared decisionmaking between patients, carers, and their clinicians, as well as policy makers.”
They caution, however, that all statements comparing the merits of one drug with another “should be tempered by the potential limitations of the current analysis, the quality of the available evidence, the characteristics of the patient populations, and the uncertainties that might result from choice of dose or treatment setting.”
In addition, it is important to also consider nonpharmacologic treatments for insomnia disorder, as they are supported by “high-quality evidence and recommended as first-line treatment by guidelines,” the investigator write.
Shared decisionmaking
In an accompanying editorial, Myrto Samara, MD, University of Thessaly, Larissa, Greece, agrees with the researchers that discussion with patients is key.
“For insomnia treatment, patient-physician shared decisionmaking is crucial to decide when a pharmacological intervention is deemed necessary and which drug [is] to be given by considering the trade-offs for efficacy and side effects,” Dr. Samara writes.
The study was funded by the UK National Institute for Health Research (NIHR) Oxford Health Biomedical Research Center. Dr. Cipriani has received research and consultancy fees from the Italian Network for Pediatric Trials, CARIPLO Foundation, and Angelini Pharma, and is the chief and principal investigator of two trials of seltorexant in depression that are sponsored by Janssen. Dr. Samara has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a comprehensive comparative-effectiveness analysis, lemborexant and eszopiclone showed the best efficacy, acceptability, and tolerability for acute and long-term insomnia treatment.
However, eszopiclone may cause substantial side effects – and safety data on lemborexant were inconclusive, the researchers note.
Not surprisingly, short-acting, intermediate-acting, and long-acting benzodiazepines were effective in the acute treatment of insomnia, but they have unfavorable tolerability and safety profiles, and there are no long-term data on these issues.
For many insomnia medications, there is a “striking” and “appalling” lack of long-term data, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry, University of Oxford, United Kingdom, noted during a press briefing.
“This is a call for regulators to raise the bar and ask for long-term data when companies submit an application for licensing insomnia drugs,” Dr. Cipriani said.
The findings were published online in The Lancet.
Prevalent, debilitating
Insomnia is highly prevalent, affecting up to 1 in 5 adults, and can have a profound impact on health, well-being, and productivity.
Sleep hygiene and cognitive-behavioral therapy for insomnia (CBT-I) are recommended first-line treatments, but they are often unavailable, which often leads patients and clinicians to turn to medications.
However, “insomnia drugs are not all created equal. Even within the same drug class there are differences,” Dr. Cipriani said.
In a large-scale systematic review and network meta-analysis, the researchers analyzed data from 154 double-blind, randomized controlled trials of medications (licensed or not) used for acute and long-term treatment of insomnia in 44,089 adults (mean age, 51.7 years; 63% women).
Results showed, for the acute treatment of insomnia, benzodiazepines, doxylamine, eszopiclone, lemborexant, seltorexant, zolpidem, and zopiclone were more effective than placebo (standardized mean difference range, 0.36-0.83; high-to-moderate certainty of evidence).
In addition, benzodiazepines, eszopiclone, zolpidem, and zopiclone were more effective than melatonin, ramelteon, and zaleplon (SMD, 0.27-0.71; moderate-to-very low certainty of evidence).
“Our results show that the melatonergic drugs melatonin and ramelteon are not really effective. The data do not support the regular use of these drugs,” co-investigator Phil Cowen, PhD, professor of psychopharmacology, University of Oxford, said at the briefing.
Best available evidence
What little long-term data is available suggest eszopiclone and lemborexant are more effective than placebo. Plus, eszopiclone is more effective than ramelteon and zolpidem but with “very low” certainty of evidence, the researchers report.
“There was insufficient evidence to support the prescription of benzodiazepines and zolpidem in long-term treatment,” they write.
Another problem was lack of data on other important outcomes, they add.
“We wanted to look at hangover effects, daytime sleepiness, [and] rebound effect, but often there was no data reported in trials. We need to collect data about these outcomes because they matter to clinicians and patients,” Dr. Cipriani said.
Summing up, the researchers note the current findings represent the “best available evidence base to guide the choice about pharmacological treatment for insomnia disorder in adults and will assist in shared decisionmaking between patients, carers, and their clinicians, as well as policy makers.”
They caution, however, that all statements comparing the merits of one drug with another “should be tempered by the potential limitations of the current analysis, the quality of the available evidence, the characteristics of the patient populations, and the uncertainties that might result from choice of dose or treatment setting.”
In addition, it is important to also consider nonpharmacologic treatments for insomnia disorder, as they are supported by “high-quality evidence and recommended as first-line treatment by guidelines,” the investigator write.
Shared decisionmaking
In an accompanying editorial, Myrto Samara, MD, University of Thessaly, Larissa, Greece, agrees with the researchers that discussion with patients is key.
“For insomnia treatment, patient-physician shared decisionmaking is crucial to decide when a pharmacological intervention is deemed necessary and which drug [is] to be given by considering the trade-offs for efficacy and side effects,” Dr. Samara writes.
The study was funded by the UK National Institute for Health Research (NIHR) Oxford Health Biomedical Research Center. Dr. Cipriani has received research and consultancy fees from the Italian Network for Pediatric Trials, CARIPLO Foundation, and Angelini Pharma, and is the chief and principal investigator of two trials of seltorexant in depression that are sponsored by Janssen. Dr. Samara has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Neural networks can distinguish PsA from rheumatoid arthritis on MRI
Hand images are sufficient
NEW YORK – On the basis of MRI images of the hand, a neural network has been trained to distinguish seronegative and seropositive rheumatoid arthritis (RA) from psoriatic arthritis (PsA) as well as from each other, according to a study that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the work so far, the neural network was correct about 70% of the time in the absence of any further clinical analyses, according to David Simon, MD, a rheumatologist in the department of internal medicine at Friedrich-Alexander University, Erlangen, Germany.
Previous to this work, “there has been no study that has exclusively used hand MRI data and deep learning without requiring further expert input for the classification of arthritides,” Dr. Simon said.
In fact, when demographic and clinical data were added, there was no improvement in the performance of patient classification relative to the deep learning classification alone, according to the data presented by Dr. Simon.
The images were evaluated with residual neural networks (ResNet), which represents a sophisticated form of deep learning to facilitate the flow of information across the network layers as they form to improve accuracy in their ability to distinguish one form of disease from the other. The training was performed on images from the T1 coronal, T2 corona1, T1 coronal fat suppressed with contrast, T1 axial fat suppressed with contrast, and T2 fat suppressed axial sequences.
The study included hand MRI scans from 135 patients with seronegative RA, 190 with seropositive RA, 177 with PsA, and 147 with psoriasis. The performance was judged on the basis of area under the receiver operating characteristics curve (AUROC) with and without input of clinical characteristics. Patients who had psoriasis without clinical arthritis were included as a control population.
The AUROC for accuracy was 75% for seropositive RA relative to PsA, 74% for seronegative RA relative to PsA, and 67% for seropositive relative to seronegative RA. Of the patients who had psoriasis without arthritis, 98% were classified as PsA and 2% as RA.
Subsequent to the classification of the patients with psoriasis, 14 of the 147 (9.5%) have developed PsA so far over a relatively short follow-up. All of these were among those identified as PsA by neural network evaluation of the hand MRIs.
This suggests that “a PsA-like pattern may be present early in the course of psoriatic disease,” Dr. Simon said.
In the groups with joint disease, who had mean ages ranging from 56 to 65, the mean disease durations were 2.6 years for those with seropositive RA, 1.3 years for those with seronegative RA, and 0.8 years for those with PsA. The patients with psoriasis were younger (mean age, 40.5 years) but had a longer disease duration (mean 4.2 years).
All of the MRI sequences were relevant for classification, but contrast did not appear to help with accuracy.
“If the images with contrast enhancement were deleted, the loss of performance was only marginal,” Dr. Simon reported.
The accuracy of neural networks increases with data, making it likely that further refinements in methodology will lead to a greater degree of accuracy, according to Dr. Simon. While the methodology is not yet ready for routine use in the clinic, the study demonstrates that neural network analysis of hand MRI to distinguish forms of arthritis “is possible.” Further studies are planned toward the goal of creating a viable clinical tool.
“Of course, if we could create an accurate tool with ultrasound, this would be even more practical,” said Dr. Simon, recognizing the value of an office tool, but he cautioned that this would be far more challenging.
“The precision of MRI is an important factor for effective neural network training,” he said.
Utility: ‘In challenging cases if the accuracy improves’?
A viable method for objectively and rapidly distinguishing inflammatory joint diseases, particularly in patients with an ambiguous clinical presentation, is an unmet need, according to Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center, Seattle.
Although the data presented are promising, Dr. Mease said in an interview that he believes there is a fair amount of work to be done before imaging analysis based on deep learning makes its way into routine clinical care. He is also hoping for methods to distinguish RA from PsA that are easier and less expensive, such as serum biomarkers. However, he agreed that a MRI-based tool could be useful when differentiating disease that is challenging.
“MRI is an expensive way for routine classification of disease, but this approach could be useful in challenging cases if the accuracy improves,” he said.
Meanwhile, other clinical researchers might want to test the principle. “You can try it,” said Dr. Simon, who reported that his team has made the methodology publicly available.
Dr. Simon reported no conflicts of interest. Dr. Mease reported financial relationships with more than 10 pharmaceutical companies, most of which make products used for the treatment of inflammatory joint diseases.
Hand images are sufficient
Hand images are sufficient
NEW YORK – On the basis of MRI images of the hand, a neural network has been trained to distinguish seronegative and seropositive rheumatoid arthritis (RA) from psoriatic arthritis (PsA) as well as from each other, according to a study that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the work so far, the neural network was correct about 70% of the time in the absence of any further clinical analyses, according to David Simon, MD, a rheumatologist in the department of internal medicine at Friedrich-Alexander University, Erlangen, Germany.
Previous to this work, “there has been no study that has exclusively used hand MRI data and deep learning without requiring further expert input for the classification of arthritides,” Dr. Simon said.
In fact, when demographic and clinical data were added, there was no improvement in the performance of patient classification relative to the deep learning classification alone, according to the data presented by Dr. Simon.
The images were evaluated with residual neural networks (ResNet), which represents a sophisticated form of deep learning to facilitate the flow of information across the network layers as they form to improve accuracy in their ability to distinguish one form of disease from the other. The training was performed on images from the T1 coronal, T2 corona1, T1 coronal fat suppressed with contrast, T1 axial fat suppressed with contrast, and T2 fat suppressed axial sequences.
The study included hand MRI scans from 135 patients with seronegative RA, 190 with seropositive RA, 177 with PsA, and 147 with psoriasis. The performance was judged on the basis of area under the receiver operating characteristics curve (AUROC) with and without input of clinical characteristics. Patients who had psoriasis without clinical arthritis were included as a control population.
The AUROC for accuracy was 75% for seropositive RA relative to PsA, 74% for seronegative RA relative to PsA, and 67% for seropositive relative to seronegative RA. Of the patients who had psoriasis without arthritis, 98% were classified as PsA and 2% as RA.
Subsequent to the classification of the patients with psoriasis, 14 of the 147 (9.5%) have developed PsA so far over a relatively short follow-up. All of these were among those identified as PsA by neural network evaluation of the hand MRIs.
This suggests that “a PsA-like pattern may be present early in the course of psoriatic disease,” Dr. Simon said.
In the groups with joint disease, who had mean ages ranging from 56 to 65, the mean disease durations were 2.6 years for those with seropositive RA, 1.3 years for those with seronegative RA, and 0.8 years for those with PsA. The patients with psoriasis were younger (mean age, 40.5 years) but had a longer disease duration (mean 4.2 years).
All of the MRI sequences were relevant for classification, but contrast did not appear to help with accuracy.
“If the images with contrast enhancement were deleted, the loss of performance was only marginal,” Dr. Simon reported.
The accuracy of neural networks increases with data, making it likely that further refinements in methodology will lead to a greater degree of accuracy, according to Dr. Simon. While the methodology is not yet ready for routine use in the clinic, the study demonstrates that neural network analysis of hand MRI to distinguish forms of arthritis “is possible.” Further studies are planned toward the goal of creating a viable clinical tool.
“Of course, if we could create an accurate tool with ultrasound, this would be even more practical,” said Dr. Simon, recognizing the value of an office tool, but he cautioned that this would be far more challenging.
“The precision of MRI is an important factor for effective neural network training,” he said.
Utility: ‘In challenging cases if the accuracy improves’?
A viable method for objectively and rapidly distinguishing inflammatory joint diseases, particularly in patients with an ambiguous clinical presentation, is an unmet need, according to Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center, Seattle.
Although the data presented are promising, Dr. Mease said in an interview that he believes there is a fair amount of work to be done before imaging analysis based on deep learning makes its way into routine clinical care. He is also hoping for methods to distinguish RA from PsA that are easier and less expensive, such as serum biomarkers. However, he agreed that a MRI-based tool could be useful when differentiating disease that is challenging.
“MRI is an expensive way for routine classification of disease, but this approach could be useful in challenging cases if the accuracy improves,” he said.
Meanwhile, other clinical researchers might want to test the principle. “You can try it,” said Dr. Simon, who reported that his team has made the methodology publicly available.
Dr. Simon reported no conflicts of interest. Dr. Mease reported financial relationships with more than 10 pharmaceutical companies, most of which make products used for the treatment of inflammatory joint diseases.
NEW YORK – On the basis of MRI images of the hand, a neural network has been trained to distinguish seronegative and seropositive rheumatoid arthritis (RA) from psoriatic arthritis (PsA) as well as from each other, according to a study that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the work so far, the neural network was correct about 70% of the time in the absence of any further clinical analyses, according to David Simon, MD, a rheumatologist in the department of internal medicine at Friedrich-Alexander University, Erlangen, Germany.
Previous to this work, “there has been no study that has exclusively used hand MRI data and deep learning without requiring further expert input for the classification of arthritides,” Dr. Simon said.
In fact, when demographic and clinical data were added, there was no improvement in the performance of patient classification relative to the deep learning classification alone, according to the data presented by Dr. Simon.
The images were evaluated with residual neural networks (ResNet), which represents a sophisticated form of deep learning to facilitate the flow of information across the network layers as they form to improve accuracy in their ability to distinguish one form of disease from the other. The training was performed on images from the T1 coronal, T2 corona1, T1 coronal fat suppressed with contrast, T1 axial fat suppressed with contrast, and T2 fat suppressed axial sequences.
The study included hand MRI scans from 135 patients with seronegative RA, 190 with seropositive RA, 177 with PsA, and 147 with psoriasis. The performance was judged on the basis of area under the receiver operating characteristics curve (AUROC) with and without input of clinical characteristics. Patients who had psoriasis without clinical arthritis were included as a control population.
The AUROC for accuracy was 75% for seropositive RA relative to PsA, 74% for seronegative RA relative to PsA, and 67% for seropositive relative to seronegative RA. Of the patients who had psoriasis without arthritis, 98% were classified as PsA and 2% as RA.
Subsequent to the classification of the patients with psoriasis, 14 of the 147 (9.5%) have developed PsA so far over a relatively short follow-up. All of these were among those identified as PsA by neural network evaluation of the hand MRIs.
This suggests that “a PsA-like pattern may be present early in the course of psoriatic disease,” Dr. Simon said.
In the groups with joint disease, who had mean ages ranging from 56 to 65, the mean disease durations were 2.6 years for those with seropositive RA, 1.3 years for those with seronegative RA, and 0.8 years for those with PsA. The patients with psoriasis were younger (mean age, 40.5 years) but had a longer disease duration (mean 4.2 years).
All of the MRI sequences were relevant for classification, but contrast did not appear to help with accuracy.
“If the images with contrast enhancement were deleted, the loss of performance was only marginal,” Dr. Simon reported.
The accuracy of neural networks increases with data, making it likely that further refinements in methodology will lead to a greater degree of accuracy, according to Dr. Simon. While the methodology is not yet ready for routine use in the clinic, the study demonstrates that neural network analysis of hand MRI to distinguish forms of arthritis “is possible.” Further studies are planned toward the goal of creating a viable clinical tool.
“Of course, if we could create an accurate tool with ultrasound, this would be even more practical,” said Dr. Simon, recognizing the value of an office tool, but he cautioned that this would be far more challenging.
“The precision of MRI is an important factor for effective neural network training,” he said.
Utility: ‘In challenging cases if the accuracy improves’?
A viable method for objectively and rapidly distinguishing inflammatory joint diseases, particularly in patients with an ambiguous clinical presentation, is an unmet need, according to Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center, Seattle.
Although the data presented are promising, Dr. Mease said in an interview that he believes there is a fair amount of work to be done before imaging analysis based on deep learning makes its way into routine clinical care. He is also hoping for methods to distinguish RA from PsA that are easier and less expensive, such as serum biomarkers. However, he agreed that a MRI-based tool could be useful when differentiating disease that is challenging.
“MRI is an expensive way for routine classification of disease, but this approach could be useful in challenging cases if the accuracy improves,” he said.
Meanwhile, other clinical researchers might want to test the principle. “You can try it,” said Dr. Simon, who reported that his team has made the methodology publicly available.
Dr. Simon reported no conflicts of interest. Dr. Mease reported financial relationships with more than 10 pharmaceutical companies, most of which make products used for the treatment of inflammatory joint diseases.
AT GRAPPA 2022
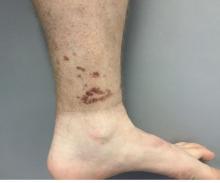
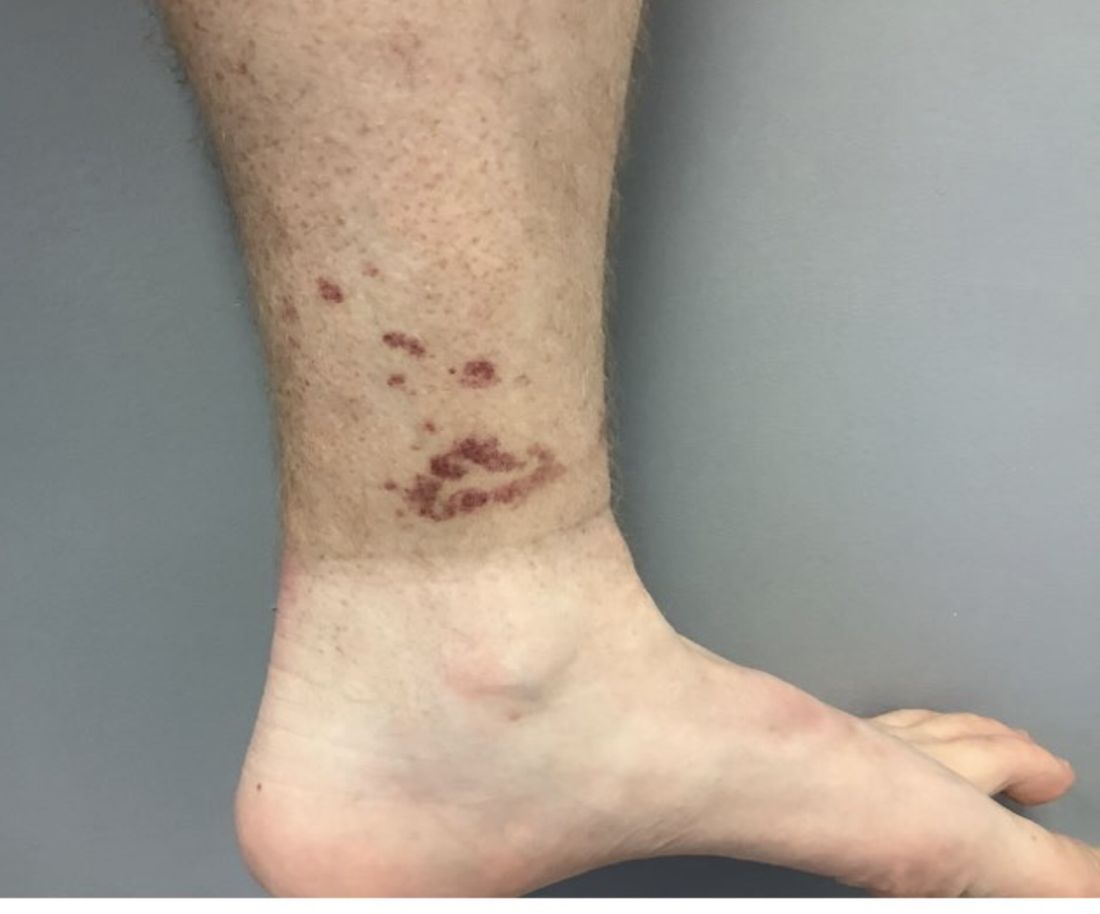
A healthy White male presented with a rash consisting of erythematous to purpuric macules
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. .” A form of EIV has been described in the literature as “Disney dermatitis.” It is often seen in healthy adults after a long day of walking at the parks. Other forms of exercise, such as jogging, hiking, or swimming, may also cause the condition.
Clinically, EIV affects the lower legs and presents as purpuric macules. Edema may be present. Lesions may be asymptomatic or may present with pruritus or burning. Diagnosis is often made clinically. Skin biopsies for H&E and DIF (direct immunofluorescence) can help distinguish the type of vasculitis that is present. Laboratory tests may be needed to exclude other causes of vasculitis. Episodes may be recurrent.
Henoch-Schönlein purpura (HSP), also called anaphylactoid purpura, is a subtype of small-vessel vasculitis where IgA immunoglobulin is deposited in the vessel walls. It is the most common form of vasculitis is children (usually ages 4-8). In addition to skin, organs such as joints, kidneys, and intestines can be involved. Schamberg’s disease, or capillaritis, is also called pigmented purpura. In this benign condition, leakage from capillaries results in erythematous to brown patches on the lower extremities. A true vasculitis is not seen. The brown discoloration is due to hemosiderin deposition. Cryoglobulinemia is a rare condition in which abnormal immunoglobulin complexes deposit in tissues and vessels. Leukocytoclastic vasculitis is present in small vessels. Palpable purpura and livedo may be seen clinically, and systemic symptoms may be present.
Treatment of EIV is largely supportive as lesions will resolve on their own over 3-4 weeks. Postinflammatory hyperpigmentation may result. Temporary cessation of exercise and compression stockings can help speed up the resolution of lesions. Systemic medications used in the treatment of severe vasculitis, such as systemic steroids, dapsone, and colchicine, are not needed in EIV.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. .” A form of EIV has been described in the literature as “Disney dermatitis.” It is often seen in healthy adults after a long day of walking at the parks. Other forms of exercise, such as jogging, hiking, or swimming, may also cause the condition.
Clinically, EIV affects the lower legs and presents as purpuric macules. Edema may be present. Lesions may be asymptomatic or may present with pruritus or burning. Diagnosis is often made clinically. Skin biopsies for H&E and DIF (direct immunofluorescence) can help distinguish the type of vasculitis that is present. Laboratory tests may be needed to exclude other causes of vasculitis. Episodes may be recurrent.
Henoch-Schönlein purpura (HSP), also called anaphylactoid purpura, is a subtype of small-vessel vasculitis where IgA immunoglobulin is deposited in the vessel walls. It is the most common form of vasculitis is children (usually ages 4-8). In addition to skin, organs such as joints, kidneys, and intestines can be involved. Schamberg’s disease, or capillaritis, is also called pigmented purpura. In this benign condition, leakage from capillaries results in erythematous to brown patches on the lower extremities. A true vasculitis is not seen. The brown discoloration is due to hemosiderin deposition. Cryoglobulinemia is a rare condition in which abnormal immunoglobulin complexes deposit in tissues and vessels. Leukocytoclastic vasculitis is present in small vessels. Palpable purpura and livedo may be seen clinically, and systemic symptoms may be present.
Treatment of EIV is largely supportive as lesions will resolve on their own over 3-4 weeks. Postinflammatory hyperpigmentation may result. Temporary cessation of exercise and compression stockings can help speed up the resolution of lesions. Systemic medications used in the treatment of severe vasculitis, such as systemic steroids, dapsone, and colchicine, are not needed in EIV.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Vasculitis is a process in which blood vessels become inflamed and necrotic. Classic small vessel vasculitis reveals a leukocytoclastic vasculitis and most commonly presents as palpable purpura. .” A form of EIV has been described in the literature as “Disney dermatitis.” It is often seen in healthy adults after a long day of walking at the parks. Other forms of exercise, such as jogging, hiking, or swimming, may also cause the condition.
Clinically, EIV affects the lower legs and presents as purpuric macules. Edema may be present. Lesions may be asymptomatic or may present with pruritus or burning. Diagnosis is often made clinically. Skin biopsies for H&E and DIF (direct immunofluorescence) can help distinguish the type of vasculitis that is present. Laboratory tests may be needed to exclude other causes of vasculitis. Episodes may be recurrent.
Henoch-Schönlein purpura (HSP), also called anaphylactoid purpura, is a subtype of small-vessel vasculitis where IgA immunoglobulin is deposited in the vessel walls. It is the most common form of vasculitis is children (usually ages 4-8). In addition to skin, organs such as joints, kidneys, and intestines can be involved. Schamberg’s disease, or capillaritis, is also called pigmented purpura. In this benign condition, leakage from capillaries results in erythematous to brown patches on the lower extremities. A true vasculitis is not seen. The brown discoloration is due to hemosiderin deposition. Cryoglobulinemia is a rare condition in which abnormal immunoglobulin complexes deposit in tissues and vessels. Leukocytoclastic vasculitis is present in small vessels. Palpable purpura and livedo may be seen clinically, and systemic symptoms may be present.
Treatment of EIV is largely supportive as lesions will resolve on their own over 3-4 weeks. Postinflammatory hyperpigmentation may result. Temporary cessation of exercise and compression stockings can help speed up the resolution of lesions. Systemic medications used in the treatment of severe vasculitis, such as systemic steroids, dapsone, and colchicine, are not needed in EIV.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Violent patient throws scalding oil on MD; other patient dangers
Ralph Newman, MD, got a taste of how dangerous medicine could be at age 10, when he witnessed a physician being shot by a patient.
“I was visiting a friend whose father was a psychiatrist,” Dr. Newman recalled. “We were playing in the living room when the doorbell rang. My friend went to the door and opened it. Then I heard a shot. I ran to the front hall and saw my friend’s father slumped at the bottom of the stairs. He had come down the stairs to see who was there. It was a patient armed with a shotgun.”
As a result of the shooting, a large portion of the psychiatrist’s intestines was removed. In spite of this traumatic incident, Dr. Newman went on to become a psychiatrist – who treated many violent prisoners. “I knew it was dangerous,” he said, “but I rationalized that I wouldn’t be attacked because I would be nicer.”
That attitude seemed to work until 2002, when a prisoner threw boiling oil on him. Dr. Newman was working at the Federal Medical Center Butner, a facility for prisoners in North Carolina. “A prisoner I had been treating was denied parole, based on my recommendation,” he said. “From then on, he was looking for a way to exact revenge.”
“One day I was sitting in the nursing station, typing up notes,” Dr. Newman said. “Two new nurses, who were also there, had forgotten to lock the door, and the prisoner noticed that. He heated up some baby oil in a microwave, which was available to prisoners at the time. Then he walked into the office, threw the oil on my back, and came at me with a sharp pencil.”
Dr. Newman said the nurses fled to an adjoining office, locked the door, and wouldn’t let him in. He went into another office and collapsed in exhaustion. He was saved by an inmate who came on the scene, fended off the attacker, and called for help.
“I was taken to the burn unit,” Dr. Newman recalled. “I had second- and third-degree burns on 9% of my body. It was extremely painful. It took me 45 days to recover enough to get back to work.” The two nurses were fired.
Doctors take threats by patients more seriously now
When orthopedic surgeon Preston Phillips, MD, was killed by a patient in Tulsa, Okla., on June 1, Jennifer M. Weiss, MD, recognized the potential danger to physicians.
“The news left me feeling very shaken,” said Dr. Weiss, a pediatric orthopedic surgeon at Southern California Permanente Medical Group, Los Angeles. “Every orthopedic surgeon I talked to about it felt shaken.”
Dr. Weiss said the impact of that event prompted her to take a patient’s abuse more seriously than she might have previously. “Before the killing, my colleagues and I might have swept the incident under the rug, but we reported it to the authorities,” she said.
“What happened was I told a parent of a school-aged child that the child wasn’t ready to go back to sports,” Dr. Weiss says. “This parent was incredibly triggered – screaming and making verbal threats. The parent was standing between me and the door, so I couldn’t get out.”
Coworkers down the hall heard the yelling and helped Dr. Weiss get out of the room. “The parent was escorted out of the building, and the incident was reported to our risk management team,” she said.
Shooters/killers vs. agitated patients
Patients who shoot to kill are very different from agitated patients seen by many doctors on a regular basis – particularly in emergency departments (EDs), psychiatric units, and pain clinics, said Scott Zeller, MD, a psychiatrist who is vice president of Acute Psychiatric Medicine at Vituity, a multistate physician partnership based in Emeryville, California.
“Agitated patients have trouble communicating their needs and can become physically and verbally aggressive,” Dr. Zeller said. He reports that there are 1.7 million such incidents a year in this country, but most of the incidents of verbal aggression can be kept from exploding into physical violence.
Shooters, however, are very hard to stop because they usually plan the action in advance, Dr. Zeller said. He recalled the 2017 murder of Todd Graham, MD, a friend from medical school. Dr. Graham, an orthopedic surgeon in South Bend, Ind., was gunned down by the husband of one of his patients after Dr. Graham declined to prescribe opioids for her.
Playing down the risk of violence
Doctors may play down the risk of violence, even after they have experienced it personally. “Patients can get angry and may make threatening comments,” Dr. Weiss said. “A lot of doctors just brush it off.”
Simple remarks can set off violence-prone patients, as happened to James P. Phillips, MD, director of disaster and operational medicine at George Washington University, Washington. He recalled asking a prisoner who was visiting his hospital to “lower the volume,” and the man exploded. “Even though he was handcuffed to the bed, he heaved an oxygen tank into a window,” Dr. Phillips said. “He said he would be coming back to kill me.”
Sometimes threats or other types of verbal abuse can be as destructive as physical violence. Diann Krywko, MD, an emergency physician at the Medical University of South Carolina (MUSC) Health, Charleston, has had some tough assignments. She worked in EDs in Detroit and Flint, Mich., for a decade before coming to MUSC, where she serves as director of wellness, health, and resilience. One of the incidents that has bothered her the most involved a threat.
It happened when Dr. Krywko denied a patient’s request for narcotics. “She was very angry and said she’d come to my home and cut my children’s heads off,” Dr. Krywko said. “To this day, what she said horrifies me. I still see her smile as she said that.”
Dr. Krywko considered filing for a restraining order against the patient but didn’t because the patient could have learned her address. Dr. Phillips said fear of retaliation is one reason many doctors don’t report threats from patients. “The patient you report knows where you work and may come there to take revenge,” he said. “Also, you may have to continue caring for the person who punched you.”
Online threats also may cause a great deal of angst. Dr. Phillips said he received many online threats when was a medical analyst for CNN in 2020. “Someone sent my address to his Twitter followers, and they shared it with others, so now the whole world knows where I live,” he said. “I had to upgrade security at my home.”
How to deal with volatile patients
Being nice may not always work, but in many cases, it can keep a volatile situation from exploding, according to Dr. Krywko.
“When patients begin to show signs of agitation or are already there, we always try to verbally deescalate the situation, which involves listening,” Dr. Krywko said. “They want someone to hear them out.”
Doctors speak to patients from a position of authority, but Dr. Krywko advises that they should not be too blunt. “Don’t tell patients they’re wrong,” she said. “Even if they may be incorrect, they feel their viewpoint is valid. Encourage a dialogue with words like, ‘Tell me more,’ ” Dr. Krywko said.
Defending yourself
Doctors may have little warning of an impending attack because a patient’s mood can change quickly. This happened several years ago to Jennifer Casaletto, MD, an emergency physician in Charlotte, N.C.
“A man was brought into my ED by ambulance,” she said. “He seemed very calm for a long while, but then he became completely unhinged. A male nurse placed himself between the patient and others and was attacked. He got hurt but was able to continue working.”
Dr. Zeller said health care teams sometimes overreact when patients lash out. “The old-fashioned way to deal with an agitated patient is to call in the cavalry – everyone does a group takedown,” he said. “The patient is put in restraints and heavily sedated. This is not good for anybody. Not only is it likely to injure and traumatize the patient, it can also injure the care team.”
Many hospital EDs have security guards. “I feel safer when a hospital has armed security guards, but they need to be well trained,” Dr. Casaletto said. “Many small hospitals and freestanding EDs do not have security officers at all, or the guards are undertrained or told not to touch anybody.”
In many electronic health record systems, doctors can flag violent patients so future caregivers can be forewarned. However, Dr. Zeller advises against writing about patients’ violence or rudeness in the medical record, because patients can have access to it and might take revenge.
Rising violence from patients
“It feels like it has become much more dangerous to work in the ED,” said Hasan Gokal, MD, an emergency physician working in EDs at the Texas Medical Center. “Just last week, a woman pulled out a gun and fired it in an ED near Houston.”
The statistics back up Dr. Gokal’s assessment. Injuries caused by violent attacks against medical professionals grew by 67% from 2011 to 2018, according to the U.S. Bureau of Labor Statistics. Those levels rose even more during the COVID-19 pandemic – the assault rate in hospitals rose 23% just in 2020.
Dr. Krywko said she had “a patient who said she wanted to hurt the next person who irritated her, and that happened to me. She jumped out of her bed swinging and punching, and I wasn’t ready for it. I yelled for help and the care team came.”
“The rise in violence has to do with a decline in respect for authority,” Dr. Phillips said. “Some people now believe doctors are lying to them about the need for COVID precautions because they are taking money from the vaccine companies. The pandemic has exacerbated violence in every way.”
Dr. Phillips said that a growing lack of resources had led to more anger among patients. “There are fewer nurses and reduced physician coverage,” he said. “That means longer wait times for patients, which increases patients’ frustrations.”
Dr. Weiss said patients have higher expectations. “In sports medicine, the expectations are incredible,” she said. “Parents want their kids to get back to playing as soon as possible.”
“Hospitals in particular are soft targets for violence,” Dr. Phillips said. “People know you can’t assault a flight attendant, because it’s a federal offense, but there is no such federal offense for violence against health care personnel.”
A version of this article first appeared on Medscape.com.
Ralph Newman, MD, got a taste of how dangerous medicine could be at age 10, when he witnessed a physician being shot by a patient.
“I was visiting a friend whose father was a psychiatrist,” Dr. Newman recalled. “We were playing in the living room when the doorbell rang. My friend went to the door and opened it. Then I heard a shot. I ran to the front hall and saw my friend’s father slumped at the bottom of the stairs. He had come down the stairs to see who was there. It was a patient armed with a shotgun.”
As a result of the shooting, a large portion of the psychiatrist’s intestines was removed. In spite of this traumatic incident, Dr. Newman went on to become a psychiatrist – who treated many violent prisoners. “I knew it was dangerous,” he said, “but I rationalized that I wouldn’t be attacked because I would be nicer.”
That attitude seemed to work until 2002, when a prisoner threw boiling oil on him. Dr. Newman was working at the Federal Medical Center Butner, a facility for prisoners in North Carolina. “A prisoner I had been treating was denied parole, based on my recommendation,” he said. “From then on, he was looking for a way to exact revenge.”
“One day I was sitting in the nursing station, typing up notes,” Dr. Newman said. “Two new nurses, who were also there, had forgotten to lock the door, and the prisoner noticed that. He heated up some baby oil in a microwave, which was available to prisoners at the time. Then he walked into the office, threw the oil on my back, and came at me with a sharp pencil.”
Dr. Newman said the nurses fled to an adjoining office, locked the door, and wouldn’t let him in. He went into another office and collapsed in exhaustion. He was saved by an inmate who came on the scene, fended off the attacker, and called for help.
“I was taken to the burn unit,” Dr. Newman recalled. “I had second- and third-degree burns on 9% of my body. It was extremely painful. It took me 45 days to recover enough to get back to work.” The two nurses were fired.
Doctors take threats by patients more seriously now
When orthopedic surgeon Preston Phillips, MD, was killed by a patient in Tulsa, Okla., on June 1, Jennifer M. Weiss, MD, recognized the potential danger to physicians.
“The news left me feeling very shaken,” said Dr. Weiss, a pediatric orthopedic surgeon at Southern California Permanente Medical Group, Los Angeles. “Every orthopedic surgeon I talked to about it felt shaken.”
Dr. Weiss said the impact of that event prompted her to take a patient’s abuse more seriously than she might have previously. “Before the killing, my colleagues and I might have swept the incident under the rug, but we reported it to the authorities,” she said.
“What happened was I told a parent of a school-aged child that the child wasn’t ready to go back to sports,” Dr. Weiss says. “This parent was incredibly triggered – screaming and making verbal threats. The parent was standing between me and the door, so I couldn’t get out.”
Coworkers down the hall heard the yelling and helped Dr. Weiss get out of the room. “The parent was escorted out of the building, and the incident was reported to our risk management team,” she said.
Shooters/killers vs. agitated patients
Patients who shoot to kill are very different from agitated patients seen by many doctors on a regular basis – particularly in emergency departments (EDs), psychiatric units, and pain clinics, said Scott Zeller, MD, a psychiatrist who is vice president of Acute Psychiatric Medicine at Vituity, a multistate physician partnership based in Emeryville, California.
“Agitated patients have trouble communicating their needs and can become physically and verbally aggressive,” Dr. Zeller said. He reports that there are 1.7 million such incidents a year in this country, but most of the incidents of verbal aggression can be kept from exploding into physical violence.
Shooters, however, are very hard to stop because they usually plan the action in advance, Dr. Zeller said. He recalled the 2017 murder of Todd Graham, MD, a friend from medical school. Dr. Graham, an orthopedic surgeon in South Bend, Ind., was gunned down by the husband of one of his patients after Dr. Graham declined to prescribe opioids for her.
Playing down the risk of violence
Doctors may play down the risk of violence, even after they have experienced it personally. “Patients can get angry and may make threatening comments,” Dr. Weiss said. “A lot of doctors just brush it off.”
Simple remarks can set off violence-prone patients, as happened to James P. Phillips, MD, director of disaster and operational medicine at George Washington University, Washington. He recalled asking a prisoner who was visiting his hospital to “lower the volume,” and the man exploded. “Even though he was handcuffed to the bed, he heaved an oxygen tank into a window,” Dr. Phillips said. “He said he would be coming back to kill me.”
Sometimes threats or other types of verbal abuse can be as destructive as physical violence. Diann Krywko, MD, an emergency physician at the Medical University of South Carolina (MUSC) Health, Charleston, has had some tough assignments. She worked in EDs in Detroit and Flint, Mich., for a decade before coming to MUSC, where she serves as director of wellness, health, and resilience. One of the incidents that has bothered her the most involved a threat.
It happened when Dr. Krywko denied a patient’s request for narcotics. “She was very angry and said she’d come to my home and cut my children’s heads off,” Dr. Krywko said. “To this day, what she said horrifies me. I still see her smile as she said that.”
Dr. Krywko considered filing for a restraining order against the patient but didn’t because the patient could have learned her address. Dr. Phillips said fear of retaliation is one reason many doctors don’t report threats from patients. “The patient you report knows where you work and may come there to take revenge,” he said. “Also, you may have to continue caring for the person who punched you.”
Online threats also may cause a great deal of angst. Dr. Phillips said he received many online threats when was a medical analyst for CNN in 2020. “Someone sent my address to his Twitter followers, and they shared it with others, so now the whole world knows where I live,” he said. “I had to upgrade security at my home.”
How to deal with volatile patients
Being nice may not always work, but in many cases, it can keep a volatile situation from exploding, according to Dr. Krywko.
“When patients begin to show signs of agitation or are already there, we always try to verbally deescalate the situation, which involves listening,” Dr. Krywko said. “They want someone to hear them out.”
Doctors speak to patients from a position of authority, but Dr. Krywko advises that they should not be too blunt. “Don’t tell patients they’re wrong,” she said. “Even if they may be incorrect, they feel their viewpoint is valid. Encourage a dialogue with words like, ‘Tell me more,’ ” Dr. Krywko said.
Defending yourself
Doctors may have little warning of an impending attack because a patient’s mood can change quickly. This happened several years ago to Jennifer Casaletto, MD, an emergency physician in Charlotte, N.C.
“A man was brought into my ED by ambulance,” she said. “He seemed very calm for a long while, but then he became completely unhinged. A male nurse placed himself between the patient and others and was attacked. He got hurt but was able to continue working.”
Dr. Zeller said health care teams sometimes overreact when patients lash out. “The old-fashioned way to deal with an agitated patient is to call in the cavalry – everyone does a group takedown,” he said. “The patient is put in restraints and heavily sedated. This is not good for anybody. Not only is it likely to injure and traumatize the patient, it can also injure the care team.”
Many hospital EDs have security guards. “I feel safer when a hospital has armed security guards, but they need to be well trained,” Dr. Casaletto said. “Many small hospitals and freestanding EDs do not have security officers at all, or the guards are undertrained or told not to touch anybody.”
In many electronic health record systems, doctors can flag violent patients so future caregivers can be forewarned. However, Dr. Zeller advises against writing about patients’ violence or rudeness in the medical record, because patients can have access to it and might take revenge.
Rising violence from patients
“It feels like it has become much more dangerous to work in the ED,” said Hasan Gokal, MD, an emergency physician working in EDs at the Texas Medical Center. “Just last week, a woman pulled out a gun and fired it in an ED near Houston.”
The statistics back up Dr. Gokal’s assessment. Injuries caused by violent attacks against medical professionals grew by 67% from 2011 to 2018, according to the U.S. Bureau of Labor Statistics. Those levels rose even more during the COVID-19 pandemic – the assault rate in hospitals rose 23% just in 2020.
Dr. Krywko said she had “a patient who said she wanted to hurt the next person who irritated her, and that happened to me. She jumped out of her bed swinging and punching, and I wasn’t ready for it. I yelled for help and the care team came.”
“The rise in violence has to do with a decline in respect for authority,” Dr. Phillips said. “Some people now believe doctors are lying to them about the need for COVID precautions because they are taking money from the vaccine companies. The pandemic has exacerbated violence in every way.”
Dr. Phillips said that a growing lack of resources had led to more anger among patients. “There are fewer nurses and reduced physician coverage,” he said. “That means longer wait times for patients, which increases patients’ frustrations.”
Dr. Weiss said patients have higher expectations. “In sports medicine, the expectations are incredible,” she said. “Parents want their kids to get back to playing as soon as possible.”
“Hospitals in particular are soft targets for violence,” Dr. Phillips said. “People know you can’t assault a flight attendant, because it’s a federal offense, but there is no such federal offense for violence against health care personnel.”
A version of this article first appeared on Medscape.com.
Ralph Newman, MD, got a taste of how dangerous medicine could be at age 10, when he witnessed a physician being shot by a patient.
“I was visiting a friend whose father was a psychiatrist,” Dr. Newman recalled. “We were playing in the living room when the doorbell rang. My friend went to the door and opened it. Then I heard a shot. I ran to the front hall and saw my friend’s father slumped at the bottom of the stairs. He had come down the stairs to see who was there. It was a patient armed with a shotgun.”
As a result of the shooting, a large portion of the psychiatrist’s intestines was removed. In spite of this traumatic incident, Dr. Newman went on to become a psychiatrist – who treated many violent prisoners. “I knew it was dangerous,” he said, “but I rationalized that I wouldn’t be attacked because I would be nicer.”
That attitude seemed to work until 2002, when a prisoner threw boiling oil on him. Dr. Newman was working at the Federal Medical Center Butner, a facility for prisoners in North Carolina. “A prisoner I had been treating was denied parole, based on my recommendation,” he said. “From then on, he was looking for a way to exact revenge.”
“One day I was sitting in the nursing station, typing up notes,” Dr. Newman said. “Two new nurses, who were also there, had forgotten to lock the door, and the prisoner noticed that. He heated up some baby oil in a microwave, which was available to prisoners at the time. Then he walked into the office, threw the oil on my back, and came at me with a sharp pencil.”
Dr. Newman said the nurses fled to an adjoining office, locked the door, and wouldn’t let him in. He went into another office and collapsed in exhaustion. He was saved by an inmate who came on the scene, fended off the attacker, and called for help.
“I was taken to the burn unit,” Dr. Newman recalled. “I had second- and third-degree burns on 9% of my body. It was extremely painful. It took me 45 days to recover enough to get back to work.” The two nurses were fired.
Doctors take threats by patients more seriously now
When orthopedic surgeon Preston Phillips, MD, was killed by a patient in Tulsa, Okla., on June 1, Jennifer M. Weiss, MD, recognized the potential danger to physicians.
“The news left me feeling very shaken,” said Dr. Weiss, a pediatric orthopedic surgeon at Southern California Permanente Medical Group, Los Angeles. “Every orthopedic surgeon I talked to about it felt shaken.”
Dr. Weiss said the impact of that event prompted her to take a patient’s abuse more seriously than she might have previously. “Before the killing, my colleagues and I might have swept the incident under the rug, but we reported it to the authorities,” she said.
“What happened was I told a parent of a school-aged child that the child wasn’t ready to go back to sports,” Dr. Weiss says. “This parent was incredibly triggered – screaming and making verbal threats. The parent was standing between me and the door, so I couldn’t get out.”
Coworkers down the hall heard the yelling and helped Dr. Weiss get out of the room. “The parent was escorted out of the building, and the incident was reported to our risk management team,” she said.
Shooters/killers vs. agitated patients
Patients who shoot to kill are very different from agitated patients seen by many doctors on a regular basis – particularly in emergency departments (EDs), psychiatric units, and pain clinics, said Scott Zeller, MD, a psychiatrist who is vice president of Acute Psychiatric Medicine at Vituity, a multistate physician partnership based in Emeryville, California.
“Agitated patients have trouble communicating their needs and can become physically and verbally aggressive,” Dr. Zeller said. He reports that there are 1.7 million such incidents a year in this country, but most of the incidents of verbal aggression can be kept from exploding into physical violence.
Shooters, however, are very hard to stop because they usually plan the action in advance, Dr. Zeller said. He recalled the 2017 murder of Todd Graham, MD, a friend from medical school. Dr. Graham, an orthopedic surgeon in South Bend, Ind., was gunned down by the husband of one of his patients after Dr. Graham declined to prescribe opioids for her.
Playing down the risk of violence
Doctors may play down the risk of violence, even after they have experienced it personally. “Patients can get angry and may make threatening comments,” Dr. Weiss said. “A lot of doctors just brush it off.”
Simple remarks can set off violence-prone patients, as happened to James P. Phillips, MD, director of disaster and operational medicine at George Washington University, Washington. He recalled asking a prisoner who was visiting his hospital to “lower the volume,” and the man exploded. “Even though he was handcuffed to the bed, he heaved an oxygen tank into a window,” Dr. Phillips said. “He said he would be coming back to kill me.”
Sometimes threats or other types of verbal abuse can be as destructive as physical violence. Diann Krywko, MD, an emergency physician at the Medical University of South Carolina (MUSC) Health, Charleston, has had some tough assignments. She worked in EDs in Detroit and Flint, Mich., for a decade before coming to MUSC, where she serves as director of wellness, health, and resilience. One of the incidents that has bothered her the most involved a threat.
It happened when Dr. Krywko denied a patient’s request for narcotics. “She was very angry and said she’d come to my home and cut my children’s heads off,” Dr. Krywko said. “To this day, what she said horrifies me. I still see her smile as she said that.”
Dr. Krywko considered filing for a restraining order against the patient but didn’t because the patient could have learned her address. Dr. Phillips said fear of retaliation is one reason many doctors don’t report threats from patients. “The patient you report knows where you work and may come there to take revenge,” he said. “Also, you may have to continue caring for the person who punched you.”
Online threats also may cause a great deal of angst. Dr. Phillips said he received many online threats when was a medical analyst for CNN in 2020. “Someone sent my address to his Twitter followers, and they shared it with others, so now the whole world knows where I live,” he said. “I had to upgrade security at my home.”
How to deal with volatile patients
Being nice may not always work, but in many cases, it can keep a volatile situation from exploding, according to Dr. Krywko.
“When patients begin to show signs of agitation or are already there, we always try to verbally deescalate the situation, which involves listening,” Dr. Krywko said. “They want someone to hear them out.”
Doctors speak to patients from a position of authority, but Dr. Krywko advises that they should not be too blunt. “Don’t tell patients they’re wrong,” she said. “Even if they may be incorrect, they feel their viewpoint is valid. Encourage a dialogue with words like, ‘Tell me more,’ ” Dr. Krywko said.
Defending yourself
Doctors may have little warning of an impending attack because a patient’s mood can change quickly. This happened several years ago to Jennifer Casaletto, MD, an emergency physician in Charlotte, N.C.
“A man was brought into my ED by ambulance,” she said. “He seemed very calm for a long while, but then he became completely unhinged. A male nurse placed himself between the patient and others and was attacked. He got hurt but was able to continue working.”
Dr. Zeller said health care teams sometimes overreact when patients lash out. “The old-fashioned way to deal with an agitated patient is to call in the cavalry – everyone does a group takedown,” he said. “The patient is put in restraints and heavily sedated. This is not good for anybody. Not only is it likely to injure and traumatize the patient, it can also injure the care team.”
Many hospital EDs have security guards. “I feel safer when a hospital has armed security guards, but they need to be well trained,” Dr. Casaletto said. “Many small hospitals and freestanding EDs do not have security officers at all, or the guards are undertrained or told not to touch anybody.”
In many electronic health record systems, doctors can flag violent patients so future caregivers can be forewarned. However, Dr. Zeller advises against writing about patients’ violence or rudeness in the medical record, because patients can have access to it and might take revenge.
Rising violence from patients
“It feels like it has become much more dangerous to work in the ED,” said Hasan Gokal, MD, an emergency physician working in EDs at the Texas Medical Center. “Just last week, a woman pulled out a gun and fired it in an ED near Houston.”
The statistics back up Dr. Gokal’s assessment. Injuries caused by violent attacks against medical professionals grew by 67% from 2011 to 2018, according to the U.S. Bureau of Labor Statistics. Those levels rose even more during the COVID-19 pandemic – the assault rate in hospitals rose 23% just in 2020.
Dr. Krywko said she had “a patient who said she wanted to hurt the next person who irritated her, and that happened to me. She jumped out of her bed swinging and punching, and I wasn’t ready for it. I yelled for help and the care team came.”
“The rise in violence has to do with a decline in respect for authority,” Dr. Phillips said. “Some people now believe doctors are lying to them about the need for COVID precautions because they are taking money from the vaccine companies. The pandemic has exacerbated violence in every way.”
Dr. Phillips said that a growing lack of resources had led to more anger among patients. “There are fewer nurses and reduced physician coverage,” he said. “That means longer wait times for patients, which increases patients’ frustrations.”
Dr. Weiss said patients have higher expectations. “In sports medicine, the expectations are incredible,” she said. “Parents want their kids to get back to playing as soon as possible.”
“Hospitals in particular are soft targets for violence,” Dr. Phillips said. “People know you can’t assault a flight attendant, because it’s a federal offense, but there is no such federal offense for violence against health care personnel.”
A version of this article first appeared on Medscape.com.
Ten steps for clinicians to avoid being racist: The Francis commitment
As a Black man who grew up in this country, I can tell you first-hand what it does to you. The scars never go away, and your status is always in question, no matter your title or uniforms of respect. Eventually it wears you down.
I was born into poverty and the segregation of southwest Louisiana. I experienced the dehumanization intended for me: separate drinking fountains and poor foundational education. I was lucky to attend a historically Black college or university (Southern University, Baton Rouge, La.), that gave me my bearings. I then went to some of the very best, predominantly White institutions.
When I looked for a job after training, there were few integrated medical groups, so I started my own. It included practitioners who were White, Black, Jewish, Asian, Middle Eastern, Muslim, Christian, etc. We cross covered and treated patients from every corner of the globe.
In medicine, we treat human beings with disease. The disease should be the only difference that sets us apart. There is absolutely no place for racism.
It is difficult to be called a racist, and I have met only a handful of people in health care whom I would label as such. But racism is structural and institutionalized so that it is often hidden.
One way to overcome this is to make every effort possible to get to know people as individuals. Only then can we see that there are few real differences between us. I would often seek out a colleague from a different culture or race to have lunch with so I could learn more about them.
We all strive for the same things – validation, happiness, love, family, and a future. We all grieve over the same things.
What some caregivers may not realize is that, just as clinicians have been trained to recognize subtle signs and symptoms of disease, minorities can recognize racism immediately during a medical encounter. Our past experiences make us skilled at picking up a lack of eye contact or body language and tone of voice that are dismissive and disrespectful.
A patient who has felt racism may still return for care because of insurance coverage limitations, location, or a lack of alternatives. But trust and loyalty will never develop on the part of this patient, and empathy will be absent on the part of their caregiver.
To counter this in my own practice, I developed the Francis Commitment to avoid any hint of racism or bias toward my patients.
I commit to the following:
1. I see you.
2. I hear you.
3. I accept who you are.
4. I will try to understand how you must feel (empathy).
5. Treating you is very important to me.
6. I would like to gain your trust that I will do my very best to make you better.
7. I value you as a human being and will treat you as if you are family.
8. I care about what happens to you.
9. I want us to work together to fight this disease.
10. I am grateful that you chose me as your caregiver.
The INOVA health care system where I work has undertaken an initiative called What Matters Most to better understand the needs of every patient. We are currently working on a strategy of patient personalization to not only learn about their medical needs but also to discover who they are as a person. We incorporate Social Determinants of Health in our dealings with patients. We also have participated in a program called “A Long Talk”, where we learned that those of us who remain silent when we see or hear racism are responsible for its persistence and growth.
But we must do more. Racism will propagate if we live in silos surrounded by people whose ideas reflect our own. As long as we have nondiversified board rooms, departments, and staff, the problem will persist.
A lot of the biases that we unconsciously carry in our heads and hearts have no basis in reality and were placed there without our permission by parents, society, and friends. But we can replace these divisive thoughts and impulses.
What’s in your heart can only be known and controlled by you. How tolerant we are of racism is up to us: Do you call out racism; do you challenge any inkling of racism from friends or acquaintances; do you put pressure on institutions where you work to diversify in recruiting and hiring?
Think of all the advances in medicine that were achieved by people from different cultures and races. Racism has no place in what we have all devoted our lives to do – take care of our fellow humans.
A version of this article first appeared on Medscape.com.
As a Black man who grew up in this country, I can tell you first-hand what it does to you. The scars never go away, and your status is always in question, no matter your title or uniforms of respect. Eventually it wears you down.
I was born into poverty and the segregation of southwest Louisiana. I experienced the dehumanization intended for me: separate drinking fountains and poor foundational education. I was lucky to attend a historically Black college or university (Southern University, Baton Rouge, La.), that gave me my bearings. I then went to some of the very best, predominantly White institutions.
When I looked for a job after training, there were few integrated medical groups, so I started my own. It included practitioners who were White, Black, Jewish, Asian, Middle Eastern, Muslim, Christian, etc. We cross covered and treated patients from every corner of the globe.
In medicine, we treat human beings with disease. The disease should be the only difference that sets us apart. There is absolutely no place for racism.
It is difficult to be called a racist, and I have met only a handful of people in health care whom I would label as such. But racism is structural and institutionalized so that it is often hidden.
One way to overcome this is to make every effort possible to get to know people as individuals. Only then can we see that there are few real differences between us. I would often seek out a colleague from a different culture or race to have lunch with so I could learn more about them.
We all strive for the same things – validation, happiness, love, family, and a future. We all grieve over the same things.
What some caregivers may not realize is that, just as clinicians have been trained to recognize subtle signs and symptoms of disease, minorities can recognize racism immediately during a medical encounter. Our past experiences make us skilled at picking up a lack of eye contact or body language and tone of voice that are dismissive and disrespectful.
A patient who has felt racism may still return for care because of insurance coverage limitations, location, or a lack of alternatives. But trust and loyalty will never develop on the part of this patient, and empathy will be absent on the part of their caregiver.
To counter this in my own practice, I developed the Francis Commitment to avoid any hint of racism or bias toward my patients.
I commit to the following:
1. I see you.
2. I hear you.
3. I accept who you are.
4. I will try to understand how you must feel (empathy).
5. Treating you is very important to me.
6. I would like to gain your trust that I will do my very best to make you better.
7. I value you as a human being and will treat you as if you are family.
8. I care about what happens to you.
9. I want us to work together to fight this disease.
10. I am grateful that you chose me as your caregiver.
The INOVA health care system where I work has undertaken an initiative called What Matters Most to better understand the needs of every patient. We are currently working on a strategy of patient personalization to not only learn about their medical needs but also to discover who they are as a person. We incorporate Social Determinants of Health in our dealings with patients. We also have participated in a program called “A Long Talk”, where we learned that those of us who remain silent when we see or hear racism are responsible for its persistence and growth.
But we must do more. Racism will propagate if we live in silos surrounded by people whose ideas reflect our own. As long as we have nondiversified board rooms, departments, and staff, the problem will persist.
A lot of the biases that we unconsciously carry in our heads and hearts have no basis in reality and were placed there without our permission by parents, society, and friends. But we can replace these divisive thoughts and impulses.
What’s in your heart can only be known and controlled by you. How tolerant we are of racism is up to us: Do you call out racism; do you challenge any inkling of racism from friends or acquaintances; do you put pressure on institutions where you work to diversify in recruiting and hiring?
Think of all the advances in medicine that were achieved by people from different cultures and races. Racism has no place in what we have all devoted our lives to do – take care of our fellow humans.
A version of this article first appeared on Medscape.com.
As a Black man who grew up in this country, I can tell you first-hand what it does to you. The scars never go away, and your status is always in question, no matter your title or uniforms of respect. Eventually it wears you down.
I was born into poverty and the segregation of southwest Louisiana. I experienced the dehumanization intended for me: separate drinking fountains and poor foundational education. I was lucky to attend a historically Black college or university (Southern University, Baton Rouge, La.), that gave me my bearings. I then went to some of the very best, predominantly White institutions.
When I looked for a job after training, there were few integrated medical groups, so I started my own. It included practitioners who were White, Black, Jewish, Asian, Middle Eastern, Muslim, Christian, etc. We cross covered and treated patients from every corner of the globe.
In medicine, we treat human beings with disease. The disease should be the only difference that sets us apart. There is absolutely no place for racism.
It is difficult to be called a racist, and I have met only a handful of people in health care whom I would label as such. But racism is structural and institutionalized so that it is often hidden.
One way to overcome this is to make every effort possible to get to know people as individuals. Only then can we see that there are few real differences between us. I would often seek out a colleague from a different culture or race to have lunch with so I could learn more about them.
We all strive for the same things – validation, happiness, love, family, and a future. We all grieve over the same things.
What some caregivers may not realize is that, just as clinicians have been trained to recognize subtle signs and symptoms of disease, minorities can recognize racism immediately during a medical encounter. Our past experiences make us skilled at picking up a lack of eye contact or body language and tone of voice that are dismissive and disrespectful.
A patient who has felt racism may still return for care because of insurance coverage limitations, location, or a lack of alternatives. But trust and loyalty will never develop on the part of this patient, and empathy will be absent on the part of their caregiver.
To counter this in my own practice, I developed the Francis Commitment to avoid any hint of racism or bias toward my patients.
I commit to the following:
1. I see you.
2. I hear you.
3. I accept who you are.
4. I will try to understand how you must feel (empathy).
5. Treating you is very important to me.
6. I would like to gain your trust that I will do my very best to make you better.
7. I value you as a human being and will treat you as if you are family.
8. I care about what happens to you.
9. I want us to work together to fight this disease.
10. I am grateful that you chose me as your caregiver.
The INOVA health care system where I work has undertaken an initiative called What Matters Most to better understand the needs of every patient. We are currently working on a strategy of patient personalization to not only learn about their medical needs but also to discover who they are as a person. We incorporate Social Determinants of Health in our dealings with patients. We also have participated in a program called “A Long Talk”, where we learned that those of us who remain silent when we see or hear racism are responsible for its persistence and growth.
But we must do more. Racism will propagate if we live in silos surrounded by people whose ideas reflect our own. As long as we have nondiversified board rooms, departments, and staff, the problem will persist.
A lot of the biases that we unconsciously carry in our heads and hearts have no basis in reality and were placed there without our permission by parents, society, and friends. But we can replace these divisive thoughts and impulses.
What’s in your heart can only be known and controlled by you. How tolerant we are of racism is up to us: Do you call out racism; do you challenge any inkling of racism from friends or acquaintances; do you put pressure on institutions where you work to diversify in recruiting and hiring?
Think of all the advances in medicine that were achieved by people from different cultures and races. Racism has no place in what we have all devoted our lives to do – take care of our fellow humans.
A version of this article first appeared on Medscape.com.
New algorithm for initial PsA treatment choice is driven by T-cell behavior
T-cell behavior
Biologic selection is cytokine based
NEW YORK – An algorithm in development for psoriatic arthritis (PsA) is showing promise for directing patients to the biologic with the greatest likelihood of producing disease control, according to a proof-of-concept study presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our technique involves a more precise functional assay showing exact T-cell behavior, compared to the previous assessments that only analyzed cellular phenotypes,” reported Gizem Ayan, MD, a fellow in rheumatology at Hacettepe University Faculty of Medicine, Ankara, Turkey.
The concept of precision medicine in PsA as well as other autoimmune diseases is not new. Phenotypes and biomarkers have already shown potential for guiding treatment, according to Dr. Ayan, but she said none are yet guideline recommended or proven to improve patient outcomes.
The principle of the new algorithm that she and her coinvestigators are pursing is based on immunophenotype analysis conducted with a flow-cytometric cytokine secretion assay (FCCSA). In the protocol, monocytes obtained from peripheral blood undergo activation before an FCCSA to distinguish patients by their T-cell behavior.
The treatment decision tree is based on median ratios of tumor necrosis factor (TNF)-alpha, interleukin (IL)–22, IL-17, and interferon-gamma expression among CD4+ and CD8+ cells. Based on a yes-or-no response to specific immune patterns, the patient is funneled to a biologic that inhibits a dominant cytokine.
The proof-of-concept study, which enrolled 8 patients with PsA who were naive to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and 11 patients with PsA who were naive to biologic DMARDs (bDMARDs), was designed to demonstrate feasibility. It did not test clinical benefit, but it did show that immunophenotyping with this methodology can be performed efficiently.
“From the time a blood sample is obtained, the method provided results within 24 hours,” according to Dr. Ayan, who is now planning a randomized trial to test the ability of the algorithm to improve clinical outcomes.
In the decision tree, there are five yes-no pathways to a treatment choice. The first step of the algorithm is to test the ratio of TNF-alpha to interferon-gamma CD4+ T cells. A “yes’ response is produced if the ratio is greater than or equal to 2. These patients are then evaluated for the ratio of TNF-alpha to interferon-gamma CD8+ T cells. A yes response is produced if the ratio is greater than or equal to 0.5. If yes, they are candidates for a TNF-alpha inhibitor. If no, they are directed to an IL-12/23 inhibitor.
If the answer at the first decision point in the algorithm is a “no,” meaning they do not have a TNF-alpha to interferon-gamma CD4+ ratio of 2 or higher, they are evaluated for percentage of CD4+ T cells expressing IL-22 or IL-17. Is it greater than or equal to 2%? If the answer is “no,” they are candidates for an IL-12/23 inhibitor.
If “yes,” they are evaluated for percentage of IL-22 to IL-17 CD4+. If the IL-22 CD4+ percentage is lower than the IL-17 CD4+ percentage, meaning a “yes” to this decision point, they are directed to an IL-17 inhibitor. If the answer at this decision point is “no,” they are directed to an IL-12/23 inhibitor.
Prior to enrollment in this proof-of-concept study, 10 of the bDMARD patients were scheduled to receive an anti-TNF drug and 1 was scheduled to receive an IL-12/23 inhibitor. On the basis of this algorithm, only 5 patients were directed to an anti-TNF drug. Of the remaining, 5 were directed to an IL-17 inhibitor, and 1 was directed to an IL-12/23 inhibitor.
All 19 participants in the proof-of-concept study had peripheral arthritis; their median age was 45 years. Approximately 90% had skin lesions. Axial involvement was present in only one patient. Based on these and other characteristics and the median ratios of the cytokines measured, Dr. Ayan called this a representative population.
Based on the feasibility of this method for subtyping patients by T-cell behavior to guide drug selection, Dr. Ayan anticipates pursuing the additional steps that would show the algorithm makes a difference to patient care, including such adjunctive benefits as more cost-effective treatment selection.
“We aim to develop a treatment decision algorithm that can be implemented in daily practice,” Dr. Ayan said.
Is peripheral blood sampling adequate?
In addition to saying that the algorithm will need to prove that it alters outcomes, Samuel Tzen-yue Hwang, MD, PhD, professor and chair of the department of dermatology at the University of California, Davis, Sacramento, pointed out some potential practical issues.
“Flow cytometry is not typically available as a rapid throughput, and the cost is high,” he said. Moreover, he remains skeptical about performing this algorithm on the basis of peripheral blood samples.
“It is debatable that looking at peripheral cells would provide adequate information about what is taking place at sites of inflammation,” he said. Although it would “be fantastic” to develop an algorithm that required only a peripheral blood sample, he pointed out that “only a fraction of these cells is relevant” to disease activity.
Aspirating fluid from an involved joint “might be more useful,” but it is more work, he added. Yet, Dr. Hwang acknowledged that this approach is intriguing. He agreed that there is considerable heterogeneity among patients with PsA in their response to specific biologics, and a method to better direct patients to the treatment most likely to elicit a response is needed.
Dr. Ayan and Dr. Hwang reported no potential conflicts of interest.
Biologic selection is cytokine based
Biologic selection is cytokine based
NEW YORK – An algorithm in development for psoriatic arthritis (PsA) is showing promise for directing patients to the biologic with the greatest likelihood of producing disease control, according to a proof-of-concept study presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our technique involves a more precise functional assay showing exact T-cell behavior, compared to the previous assessments that only analyzed cellular phenotypes,” reported Gizem Ayan, MD, a fellow in rheumatology at Hacettepe University Faculty of Medicine, Ankara, Turkey.
The concept of precision medicine in PsA as well as other autoimmune diseases is not new. Phenotypes and biomarkers have already shown potential for guiding treatment, according to Dr. Ayan, but she said none are yet guideline recommended or proven to improve patient outcomes.
The principle of the new algorithm that she and her coinvestigators are pursing is based on immunophenotype analysis conducted with a flow-cytometric cytokine secretion assay (FCCSA). In the protocol, monocytes obtained from peripheral blood undergo activation before an FCCSA to distinguish patients by their T-cell behavior.
The treatment decision tree is based on median ratios of tumor necrosis factor (TNF)-alpha, interleukin (IL)–22, IL-17, and interferon-gamma expression among CD4+ and CD8+ cells. Based on a yes-or-no response to specific immune patterns, the patient is funneled to a biologic that inhibits a dominant cytokine.
The proof-of-concept study, which enrolled 8 patients with PsA who were naive to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and 11 patients with PsA who were naive to biologic DMARDs (bDMARDs), was designed to demonstrate feasibility. It did not test clinical benefit, but it did show that immunophenotyping with this methodology can be performed efficiently.
“From the time a blood sample is obtained, the method provided results within 24 hours,” according to Dr. Ayan, who is now planning a randomized trial to test the ability of the algorithm to improve clinical outcomes.
In the decision tree, there are five yes-no pathways to a treatment choice. The first step of the algorithm is to test the ratio of TNF-alpha to interferon-gamma CD4+ T cells. A “yes’ response is produced if the ratio is greater than or equal to 2. These patients are then evaluated for the ratio of TNF-alpha to interferon-gamma CD8+ T cells. A yes response is produced if the ratio is greater than or equal to 0.5. If yes, they are candidates for a TNF-alpha inhibitor. If no, they are directed to an IL-12/23 inhibitor.
If the answer at the first decision point in the algorithm is a “no,” meaning they do not have a TNF-alpha to interferon-gamma CD4+ ratio of 2 or higher, they are evaluated for percentage of CD4+ T cells expressing IL-22 or IL-17. Is it greater than or equal to 2%? If the answer is “no,” they are candidates for an IL-12/23 inhibitor.
If “yes,” they are evaluated for percentage of IL-22 to IL-17 CD4+. If the IL-22 CD4+ percentage is lower than the IL-17 CD4+ percentage, meaning a “yes” to this decision point, they are directed to an IL-17 inhibitor. If the answer at this decision point is “no,” they are directed to an IL-12/23 inhibitor.
Prior to enrollment in this proof-of-concept study, 10 of the bDMARD patients were scheduled to receive an anti-TNF drug and 1 was scheduled to receive an IL-12/23 inhibitor. On the basis of this algorithm, only 5 patients were directed to an anti-TNF drug. Of the remaining, 5 were directed to an IL-17 inhibitor, and 1 was directed to an IL-12/23 inhibitor.
All 19 participants in the proof-of-concept study had peripheral arthritis; their median age was 45 years. Approximately 90% had skin lesions. Axial involvement was present in only one patient. Based on these and other characteristics and the median ratios of the cytokines measured, Dr. Ayan called this a representative population.
Based on the feasibility of this method for subtyping patients by T-cell behavior to guide drug selection, Dr. Ayan anticipates pursuing the additional steps that would show the algorithm makes a difference to patient care, including such adjunctive benefits as more cost-effective treatment selection.
“We aim to develop a treatment decision algorithm that can be implemented in daily practice,” Dr. Ayan said.
Is peripheral blood sampling adequate?
In addition to saying that the algorithm will need to prove that it alters outcomes, Samuel Tzen-yue Hwang, MD, PhD, professor and chair of the department of dermatology at the University of California, Davis, Sacramento, pointed out some potential practical issues.
“Flow cytometry is not typically available as a rapid throughput, and the cost is high,” he said. Moreover, he remains skeptical about performing this algorithm on the basis of peripheral blood samples.
“It is debatable that looking at peripheral cells would provide adequate information about what is taking place at sites of inflammation,” he said. Although it would “be fantastic” to develop an algorithm that required only a peripheral blood sample, he pointed out that “only a fraction of these cells is relevant” to disease activity.
Aspirating fluid from an involved joint “might be more useful,” but it is more work, he added. Yet, Dr. Hwang acknowledged that this approach is intriguing. He agreed that there is considerable heterogeneity among patients with PsA in their response to specific biologics, and a method to better direct patients to the treatment most likely to elicit a response is needed.
Dr. Ayan and Dr. Hwang reported no potential conflicts of interest.
NEW YORK – An algorithm in development for psoriatic arthritis (PsA) is showing promise for directing patients to the biologic with the greatest likelihood of producing disease control, according to a proof-of-concept study presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our technique involves a more precise functional assay showing exact T-cell behavior, compared to the previous assessments that only analyzed cellular phenotypes,” reported Gizem Ayan, MD, a fellow in rheumatology at Hacettepe University Faculty of Medicine, Ankara, Turkey.
The concept of precision medicine in PsA as well as other autoimmune diseases is not new. Phenotypes and biomarkers have already shown potential for guiding treatment, according to Dr. Ayan, but she said none are yet guideline recommended or proven to improve patient outcomes.
The principle of the new algorithm that she and her coinvestigators are pursing is based on immunophenotype analysis conducted with a flow-cytometric cytokine secretion assay (FCCSA). In the protocol, monocytes obtained from peripheral blood undergo activation before an FCCSA to distinguish patients by their T-cell behavior.
The treatment decision tree is based on median ratios of tumor necrosis factor (TNF)-alpha, interleukin (IL)–22, IL-17, and interferon-gamma expression among CD4+ and CD8+ cells. Based on a yes-or-no response to specific immune patterns, the patient is funneled to a biologic that inhibits a dominant cytokine.
The proof-of-concept study, which enrolled 8 patients with PsA who were naive to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and 11 patients with PsA who were naive to biologic DMARDs (bDMARDs), was designed to demonstrate feasibility. It did not test clinical benefit, but it did show that immunophenotyping with this methodology can be performed efficiently.
“From the time a blood sample is obtained, the method provided results within 24 hours,” according to Dr. Ayan, who is now planning a randomized trial to test the ability of the algorithm to improve clinical outcomes.
In the decision tree, there are five yes-no pathways to a treatment choice. The first step of the algorithm is to test the ratio of TNF-alpha to interferon-gamma CD4+ T cells. A “yes’ response is produced if the ratio is greater than or equal to 2. These patients are then evaluated for the ratio of TNF-alpha to interferon-gamma CD8+ T cells. A yes response is produced if the ratio is greater than or equal to 0.5. If yes, they are candidates for a TNF-alpha inhibitor. If no, they are directed to an IL-12/23 inhibitor.
If the answer at the first decision point in the algorithm is a “no,” meaning they do not have a TNF-alpha to interferon-gamma CD4+ ratio of 2 or higher, they are evaluated for percentage of CD4+ T cells expressing IL-22 or IL-17. Is it greater than or equal to 2%? If the answer is “no,” they are candidates for an IL-12/23 inhibitor.
If “yes,” they are evaluated for percentage of IL-22 to IL-17 CD4+. If the IL-22 CD4+ percentage is lower than the IL-17 CD4+ percentage, meaning a “yes” to this decision point, they are directed to an IL-17 inhibitor. If the answer at this decision point is “no,” they are directed to an IL-12/23 inhibitor.
Prior to enrollment in this proof-of-concept study, 10 of the bDMARD patients were scheduled to receive an anti-TNF drug and 1 was scheduled to receive an IL-12/23 inhibitor. On the basis of this algorithm, only 5 patients were directed to an anti-TNF drug. Of the remaining, 5 were directed to an IL-17 inhibitor, and 1 was directed to an IL-12/23 inhibitor.
All 19 participants in the proof-of-concept study had peripheral arthritis; their median age was 45 years. Approximately 90% had skin lesions. Axial involvement was present in only one patient. Based on these and other characteristics and the median ratios of the cytokines measured, Dr. Ayan called this a representative population.
Based on the feasibility of this method for subtyping patients by T-cell behavior to guide drug selection, Dr. Ayan anticipates pursuing the additional steps that would show the algorithm makes a difference to patient care, including such adjunctive benefits as more cost-effective treatment selection.
“We aim to develop a treatment decision algorithm that can be implemented in daily practice,” Dr. Ayan said.
Is peripheral blood sampling adequate?
In addition to saying that the algorithm will need to prove that it alters outcomes, Samuel Tzen-yue Hwang, MD, PhD, professor and chair of the department of dermatology at the University of California, Davis, Sacramento, pointed out some potential practical issues.
“Flow cytometry is not typically available as a rapid throughput, and the cost is high,” he said. Moreover, he remains skeptical about performing this algorithm on the basis of peripheral blood samples.
“It is debatable that looking at peripheral cells would provide adequate information about what is taking place at sites of inflammation,” he said. Although it would “be fantastic” to develop an algorithm that required only a peripheral blood sample, he pointed out that “only a fraction of these cells is relevant” to disease activity.
Aspirating fluid from an involved joint “might be more useful,” but it is more work, he added. Yet, Dr. Hwang acknowledged that this approach is intriguing. He agreed that there is considerable heterogeneity among patients with PsA in their response to specific biologics, and a method to better direct patients to the treatment most likely to elicit a response is needed.
Dr. Ayan and Dr. Hwang reported no potential conflicts of interest.
T-cell behavior
T-cell behavior
AT GRAPPA 2022
Berdazimer gel beats vehicle for molluscum contagiosum in phase 3 study
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Moderate drinking shows more benefit for older vs. younger adults
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
FROM THE LANCET