User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Moderate drinking shows more benefit for older vs. younger adults
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
FROM THE LANCET
Amazon involved with new cancer vaccine clinical trial
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
The trial is aimed at finding “personalized vaccines” to treat breast cancer and melanoma. The phase 1 trial is recruiting 20 people over the age of 18 to study the safety of the vaccines, according to CNBC.
The Fred Hutchinson Cancer Research Center and University of Washington Cancer Consortium are listed as the researchers of the clinical trial, and Amazon is listed as a collaborator, according to a filing on the ClinicalTrials.gov database.
“Amazon is contributing scientific and machine learning expertise to a partnership with Fred Hutch to explore the development of a personalized treatment for certain forms of cancer,” an Amazon spokesperson told CNBC.
“It’s very early, but Fred Hutch recently received permission from the U.S. Food and Drug Administration to proceed with a phase 1 clinical trial, and it’s unclear whether it will be successful,” the spokesperson said. “This will be a long, multiyear process – should it progress, we would be open to working with other organizations in health care and life sciences that might also be interested in similar efforts.”
In recent years, Amazon has grown its presence in the health care industry, CNBC reported. The company launched an online pharmacy in 2020, developed a telehealth service called Amazon Care, and released its own COVID-19 test during the pandemic.
A research and development group inside Amazon, known as Grand Challenge, oversaw the company’s early cancer vaccine effort, according to Business Insider. It’s now under the purview of a cancer research team that reports to Robert Williams, the company’s vice president of devices.
The study was first posted on ClinicalTrials.gov in October 2021 and began recruiting patients on June 9, according to the filing. The phase 1 trial is expected to run through November 2023.
The phase 1 trial will study the safety of personalized vaccines to treat patients with late-stage melanoma or hormone receptor-positive HER2-negative breast cancer which has either spread to other parts of the body or doesn’t respond to treatment.
More information about the study can be found on ClinicalTrials.gov under the identifier NCT05098210.
A version of this article first appeared on WebMD.com.
Don’t wait for a cyberattack; know what coverage you have now
Barbara L. McAneny, MD, CEO of New Mexico Oncology Hematology Consultants, experienced a data breach about 10 years ago, when a laptop was stolen from her large practice.
She and the other physicians were upset and worried that the individual would attempt to log in to the computer system and hack their patients’ private health information.
Dr. McAneny was also worried that the practice would have to pay a hefty fine to the government for having unsecured private health information on a laptop. She could have paid from $50,000 to more than $1.9 million for lost and stolen devices (although that didn’t happen).
Dr. McAneny had a standard cyber liability benefit in her med-mal policy that covered up to $50,000 of the data breach costs. That covered the legal advice The Doctors Company provided about state and federal reporting requirements when a data breach occurs and the costs the practice incurred from mailing letters to all of its patients notifying them of the data breach, says Dr. McAneny.
“The data breach taught me a lot. Our practice spent a lot of money on increasing our internal controls, cybersecurity, and monitoring. Our IT department started testing our computer firewalls periodically, and that’s how we discovered that cybercriminals were attempting to break into our computer system at least 100 times daily,” says Dr. McAneny.
That discovery changed how she thought about insurance. “I decided the med-mal benefit wasn’t enough. I bought the best cybersecurity policy we could afford to protect against future breaches, especially malware or ransomware attacks.”
Her practice also had to make its electronic health records (EHRs) more secure to comply with the Department of Health & Human Services Office of Civil Rights standards for protected health information. The cost of increased security wasn’t covered by her cyber benefit.
Cyberattacks increasing in health care
Despite having comprehensive coverage, Dr. McAneny worries that the cybercriminals are a step ahead of the cybersecurity experts and her practice will eventually have another data breach.
“The policy only covers things that we know about today. As we upgrade our defenses, criminals are finding new ways to breach firewalls and work around our defenses,” she says.
So far this year, nearly 200 medical groups have reported cyberattacks involving 500 or more of their patients’ medical records to the federal government.
EHRs are valuable targets to cybercriminals because of the protected health information they contain. Cybercriminals grab information such as Social Security numbers, dates of birth, medical procedures and results, and in some cases billing and financial information and sell it on the dark web.
They typically bundle the information and sell it to other criminals who later use it for various kinds of fraud and extortion such as banking and credit fraud, health care fraud, identity theft, and ransom extortion.
What do most doctors have?
The vast majority (82%) of doctors polled by the Medical Group Management Association last year said they had cyber insurance, compared with 54% in 2018.
For those who answered “yes,” many said they have coverage through their malpractice insurance carrier.
David Zetter, president of Zetter HealthCare Management Consultants, recommends that physicians speak with their malpractice carrier to determine what coverage they have, if any, within their malpractice policy.
A typical cybersecurity benefit is limited to what is needed to fix and resolve the hacking incident, says Raj Shah, senior regulatory attorney and policyholder advisor at MagMutual, which insures medical practices for malpractice and cyber liability.
That usually covers investigating the cause of the breach and the extent of the damage, legal advice about federal and state reporting requirements, whether to pay a ransom, and a public relations professional to handle patient communication, says Mr. Shah.
The benefit doesn’t cover lost patient revenue when practices have to shut down their operations, the cost of replacing damaged computers, or the ransom payment, he says.
Mr. Zetter advises doctors to consider buying cybersecurity coverage. “I recommend that they speak with an insurance broker who is experienced with cybersecurity policies sold to health care professionals to determine what type of coverage and how much coverage they may need. Their malpractice carrier may also be able to provide some answers,” says Mr. Zetter.
The physician will need to be able to answer questions about their network and how many staff they have and may need to involve their IT vendor too, he adds.
How does comprehensive coverage compare?
Ransomware attacks continue to be one of the most frequent types of attacks, and the amount criminals are demanding has risen significantly. The median ransom payment was $5,000 in the fourth quarter of 2018, compared with over $300,000 during the fourth quarter of 2021.
Cybercriminals now engage in “double extortion” – demanding a ransom payment to hand over the code that will unlock their encrypted data – and then another ransom payment to not post patients’ sensitive medical information they copied onto the dark web.
Comprehensive cybersecurity insurance will cover “double extortion” payments, legal costs that may arise from defending against patient lawsuits, and the costs of meeting federal and state privacy requirements, including notifying patients of the data breach and regulatory investigations, says Michael Carr, head of risk engineering for North America for Coalition, a cyber insurance firm.
Cyber insurers also contract with vendors who sell bitcoin, which is the currency cybercriminals typically demand for ransom payments, and work with ransom negotiators.
For example, once Coalition decided to pay the ransom on behalf of a health care client, it negotiated the ransom demand down by nearly 75% from $750,000 to $200,000, and proceeded to help the company restore all of its data.
The costs to respond to the incident, to recover lost data, and to pay the extortion, together with the lost business income resulting from the incident, were covered by Coalition’s cyber insurance policy.
Other clients have had their funds retrieved before a fraudulent wire transfer was completed. “Medical practices have vendors they pay regularly. A cybercriminal may compromise your email or take over a bank account and then impersonate a vendor asking to be paid for services they didn’t provide,” says Mr. Carr.
How much coverage do you need? Cost?
Dr. McAneny has increased her cybersecurity coverage every year. “It’s expensive, but I think it’s worth it. But you can never buy enough protection due to the coverage limits.”
She worries that the costs could exceed the limits if a ransomware attack disrupts her practice for days, weeks, or longer, or if the Office for Civil Rights fines her practice $10,000 per patient chart – the practice has 100,000 health records. “That can run several millions of dollars and ruin a practice,” she says.
Health systems and hospitals need massive amounts of coverage, which often runs from $20 million to $30 million, says Mr. Shah. However, practices insured through MagMutual have lower coverage limits that range from $1 million to $5 million, he says.
“A large practice does not necessarily need more than $1,000,000 in coverage if they have limited loss in this area and strong internal processes and controls. Most large practices also have a dedicated information security director, which reduces their risk, so they may be comfortable with $1,000,000 in coverage,” says Mr. Shah.
Premiums are based on the number of patient health records per practice, which translates into higher premiums for larger practices.
Other factors that come into play include the underlying coverage, risk controls the practice has implemented, and its claims history, says Mr. Shah.
However, the cost for cyber liability insurance has increased, and practices can expect to pay higher premiums and deductibles. For example, a practice that paid $10,000 in premiums for a new policy last year will have to pay $20,000 this year, says Dan Hanson, senior vice president of management liability and client experience at Marsh & McLennon Agency, a risk management firm that sells cyber insurance policies.
“We saw 71% of our self-insured clients experience higher deductibles over last year due to increased claim activity and the lack of capacity in the market. The carriers are saying they will set limits, but you are going to pay a lot more, and you are going to participate more in losses through the higher deductibles,” says Mr. Hanson.
Are you eligible?
Cyber insurance companies have a vested interest in avoiding claims. With increasing cyberattacks and larger payouts, many insurers are requiring practices to implement some defensive measures before they insure them. Some insurers, such as Coalition, say they may still insure small practices for comprehensive coverage, but it may impact the pricing or what’s covered, says Mr. Carr.
Here are some of the security measures that cyber insurers are looking for:
- Multifactorial authentication (MFA) requires an extra layer of security to access the system. For example, when logging into your organization’s EHR platform, instead of just using a username and password to access the platform, MFA would require you to input an additional unique login credential before you can access the EHR. A secondary login credential may include security questions, a one-time PIN, or biometrics.
- Removing a terminated employee’s login credentials quickly from the computer system. “One of the most damaging and expensive types of attacks are by disgruntled employees who still have their login credentials and take revenge by logging back into the system and planting malware,” says Mr. Shah.
- Automatic system updates (patches). “Phishing email compromises usually result from a failure to fix vulnerabilities. When a system needs to restart, it should be set to automatically update any potential security loopholes within programs or products,” says Mr. Carr. The firewall settings should also be updated.
- Prior hacking incidents: Are the attackers out of your system? Once criminals hack into the system, your practice is vulnerable to repeat attacks. “If a cyberattack is not completely addressed, threat actors will maintain access to or a presence on the compromised network. In general, we will work with the insured to ensure that the initial point of compromise has been addressed and that any threat actor presence in the network has been removed,” says Mr. Carr.
When doctors compare cybersecurity policies, experts recommend avoiding companies that may offer lower prices but lack a proven track record of handling claims and do not offer resources that can detect a threat, such as ongoing network monitoring and employee training with simulated exercises.
“Practices tend to think, ‘It won’t happen to me.’ Every practice needs to take this seriously,” says Dr. McAneny.
A version of this article first appeared on Medscape.com.
Barbara L. McAneny, MD, CEO of New Mexico Oncology Hematology Consultants, experienced a data breach about 10 years ago, when a laptop was stolen from her large practice.
She and the other physicians were upset and worried that the individual would attempt to log in to the computer system and hack their patients’ private health information.
Dr. McAneny was also worried that the practice would have to pay a hefty fine to the government for having unsecured private health information on a laptop. She could have paid from $50,000 to more than $1.9 million for lost and stolen devices (although that didn’t happen).
Dr. McAneny had a standard cyber liability benefit in her med-mal policy that covered up to $50,000 of the data breach costs. That covered the legal advice The Doctors Company provided about state and federal reporting requirements when a data breach occurs and the costs the practice incurred from mailing letters to all of its patients notifying them of the data breach, says Dr. McAneny.
“The data breach taught me a lot. Our practice spent a lot of money on increasing our internal controls, cybersecurity, and monitoring. Our IT department started testing our computer firewalls periodically, and that’s how we discovered that cybercriminals were attempting to break into our computer system at least 100 times daily,” says Dr. McAneny.
That discovery changed how she thought about insurance. “I decided the med-mal benefit wasn’t enough. I bought the best cybersecurity policy we could afford to protect against future breaches, especially malware or ransomware attacks.”
Her practice also had to make its electronic health records (EHRs) more secure to comply with the Department of Health & Human Services Office of Civil Rights standards for protected health information. The cost of increased security wasn’t covered by her cyber benefit.
Cyberattacks increasing in health care
Despite having comprehensive coverage, Dr. McAneny worries that the cybercriminals are a step ahead of the cybersecurity experts and her practice will eventually have another data breach.
“The policy only covers things that we know about today. As we upgrade our defenses, criminals are finding new ways to breach firewalls and work around our defenses,” she says.
So far this year, nearly 200 medical groups have reported cyberattacks involving 500 or more of their patients’ medical records to the federal government.
EHRs are valuable targets to cybercriminals because of the protected health information they contain. Cybercriminals grab information such as Social Security numbers, dates of birth, medical procedures and results, and in some cases billing and financial information and sell it on the dark web.
They typically bundle the information and sell it to other criminals who later use it for various kinds of fraud and extortion such as banking and credit fraud, health care fraud, identity theft, and ransom extortion.
What do most doctors have?
The vast majority (82%) of doctors polled by the Medical Group Management Association last year said they had cyber insurance, compared with 54% in 2018.
For those who answered “yes,” many said they have coverage through their malpractice insurance carrier.
David Zetter, president of Zetter HealthCare Management Consultants, recommends that physicians speak with their malpractice carrier to determine what coverage they have, if any, within their malpractice policy.
A typical cybersecurity benefit is limited to what is needed to fix and resolve the hacking incident, says Raj Shah, senior regulatory attorney and policyholder advisor at MagMutual, which insures medical practices for malpractice and cyber liability.
That usually covers investigating the cause of the breach and the extent of the damage, legal advice about federal and state reporting requirements, whether to pay a ransom, and a public relations professional to handle patient communication, says Mr. Shah.
The benefit doesn’t cover lost patient revenue when practices have to shut down their operations, the cost of replacing damaged computers, or the ransom payment, he says.
Mr. Zetter advises doctors to consider buying cybersecurity coverage. “I recommend that they speak with an insurance broker who is experienced with cybersecurity policies sold to health care professionals to determine what type of coverage and how much coverage they may need. Their malpractice carrier may also be able to provide some answers,” says Mr. Zetter.
The physician will need to be able to answer questions about their network and how many staff they have and may need to involve their IT vendor too, he adds.
How does comprehensive coverage compare?
Ransomware attacks continue to be one of the most frequent types of attacks, and the amount criminals are demanding has risen significantly. The median ransom payment was $5,000 in the fourth quarter of 2018, compared with over $300,000 during the fourth quarter of 2021.
Cybercriminals now engage in “double extortion” – demanding a ransom payment to hand over the code that will unlock their encrypted data – and then another ransom payment to not post patients’ sensitive medical information they copied onto the dark web.
Comprehensive cybersecurity insurance will cover “double extortion” payments, legal costs that may arise from defending against patient lawsuits, and the costs of meeting federal and state privacy requirements, including notifying patients of the data breach and regulatory investigations, says Michael Carr, head of risk engineering for North America for Coalition, a cyber insurance firm.
Cyber insurers also contract with vendors who sell bitcoin, which is the currency cybercriminals typically demand for ransom payments, and work with ransom negotiators.
For example, once Coalition decided to pay the ransom on behalf of a health care client, it negotiated the ransom demand down by nearly 75% from $750,000 to $200,000, and proceeded to help the company restore all of its data.
The costs to respond to the incident, to recover lost data, and to pay the extortion, together with the lost business income resulting from the incident, were covered by Coalition’s cyber insurance policy.
Other clients have had their funds retrieved before a fraudulent wire transfer was completed. “Medical practices have vendors they pay regularly. A cybercriminal may compromise your email or take over a bank account and then impersonate a vendor asking to be paid for services they didn’t provide,” says Mr. Carr.
How much coverage do you need? Cost?
Dr. McAneny has increased her cybersecurity coverage every year. “It’s expensive, but I think it’s worth it. But you can never buy enough protection due to the coverage limits.”
She worries that the costs could exceed the limits if a ransomware attack disrupts her practice for days, weeks, or longer, or if the Office for Civil Rights fines her practice $10,000 per patient chart – the practice has 100,000 health records. “That can run several millions of dollars and ruin a practice,” she says.
Health systems and hospitals need massive amounts of coverage, which often runs from $20 million to $30 million, says Mr. Shah. However, practices insured through MagMutual have lower coverage limits that range from $1 million to $5 million, he says.
“A large practice does not necessarily need more than $1,000,000 in coverage if they have limited loss in this area and strong internal processes and controls. Most large practices also have a dedicated information security director, which reduces their risk, so they may be comfortable with $1,000,000 in coverage,” says Mr. Shah.
Premiums are based on the number of patient health records per practice, which translates into higher premiums for larger practices.
Other factors that come into play include the underlying coverage, risk controls the practice has implemented, and its claims history, says Mr. Shah.
However, the cost for cyber liability insurance has increased, and practices can expect to pay higher premiums and deductibles. For example, a practice that paid $10,000 in premiums for a new policy last year will have to pay $20,000 this year, says Dan Hanson, senior vice president of management liability and client experience at Marsh & McLennon Agency, a risk management firm that sells cyber insurance policies.
“We saw 71% of our self-insured clients experience higher deductibles over last year due to increased claim activity and the lack of capacity in the market. The carriers are saying they will set limits, but you are going to pay a lot more, and you are going to participate more in losses through the higher deductibles,” says Mr. Hanson.
Are you eligible?
Cyber insurance companies have a vested interest in avoiding claims. With increasing cyberattacks and larger payouts, many insurers are requiring practices to implement some defensive measures before they insure them. Some insurers, such as Coalition, say they may still insure small practices for comprehensive coverage, but it may impact the pricing or what’s covered, says Mr. Carr.
Here are some of the security measures that cyber insurers are looking for:
- Multifactorial authentication (MFA) requires an extra layer of security to access the system. For example, when logging into your organization’s EHR platform, instead of just using a username and password to access the platform, MFA would require you to input an additional unique login credential before you can access the EHR. A secondary login credential may include security questions, a one-time PIN, or biometrics.
- Removing a terminated employee’s login credentials quickly from the computer system. “One of the most damaging and expensive types of attacks are by disgruntled employees who still have their login credentials and take revenge by logging back into the system and planting malware,” says Mr. Shah.
- Automatic system updates (patches). “Phishing email compromises usually result from a failure to fix vulnerabilities. When a system needs to restart, it should be set to automatically update any potential security loopholes within programs or products,” says Mr. Carr. The firewall settings should also be updated.
- Prior hacking incidents: Are the attackers out of your system? Once criminals hack into the system, your practice is vulnerable to repeat attacks. “If a cyberattack is not completely addressed, threat actors will maintain access to or a presence on the compromised network. In general, we will work with the insured to ensure that the initial point of compromise has been addressed and that any threat actor presence in the network has been removed,” says Mr. Carr.
When doctors compare cybersecurity policies, experts recommend avoiding companies that may offer lower prices but lack a proven track record of handling claims and do not offer resources that can detect a threat, such as ongoing network monitoring and employee training with simulated exercises.
“Practices tend to think, ‘It won’t happen to me.’ Every practice needs to take this seriously,” says Dr. McAneny.
A version of this article first appeared on Medscape.com.
Barbara L. McAneny, MD, CEO of New Mexico Oncology Hematology Consultants, experienced a data breach about 10 years ago, when a laptop was stolen from her large practice.
She and the other physicians were upset and worried that the individual would attempt to log in to the computer system and hack their patients’ private health information.
Dr. McAneny was also worried that the practice would have to pay a hefty fine to the government for having unsecured private health information on a laptop. She could have paid from $50,000 to more than $1.9 million for lost and stolen devices (although that didn’t happen).
Dr. McAneny had a standard cyber liability benefit in her med-mal policy that covered up to $50,000 of the data breach costs. That covered the legal advice The Doctors Company provided about state and federal reporting requirements when a data breach occurs and the costs the practice incurred from mailing letters to all of its patients notifying them of the data breach, says Dr. McAneny.
“The data breach taught me a lot. Our practice spent a lot of money on increasing our internal controls, cybersecurity, and monitoring. Our IT department started testing our computer firewalls periodically, and that’s how we discovered that cybercriminals were attempting to break into our computer system at least 100 times daily,” says Dr. McAneny.
That discovery changed how she thought about insurance. “I decided the med-mal benefit wasn’t enough. I bought the best cybersecurity policy we could afford to protect against future breaches, especially malware or ransomware attacks.”
Her practice also had to make its electronic health records (EHRs) more secure to comply with the Department of Health & Human Services Office of Civil Rights standards for protected health information. The cost of increased security wasn’t covered by her cyber benefit.
Cyberattacks increasing in health care
Despite having comprehensive coverage, Dr. McAneny worries that the cybercriminals are a step ahead of the cybersecurity experts and her practice will eventually have another data breach.
“The policy only covers things that we know about today. As we upgrade our defenses, criminals are finding new ways to breach firewalls and work around our defenses,” she says.
So far this year, nearly 200 medical groups have reported cyberattacks involving 500 or more of their patients’ medical records to the federal government.
EHRs are valuable targets to cybercriminals because of the protected health information they contain. Cybercriminals grab information such as Social Security numbers, dates of birth, medical procedures and results, and in some cases billing and financial information and sell it on the dark web.
They typically bundle the information and sell it to other criminals who later use it for various kinds of fraud and extortion such as banking and credit fraud, health care fraud, identity theft, and ransom extortion.
What do most doctors have?
The vast majority (82%) of doctors polled by the Medical Group Management Association last year said they had cyber insurance, compared with 54% in 2018.
For those who answered “yes,” many said they have coverage through their malpractice insurance carrier.
David Zetter, president of Zetter HealthCare Management Consultants, recommends that physicians speak with their malpractice carrier to determine what coverage they have, if any, within their malpractice policy.
A typical cybersecurity benefit is limited to what is needed to fix and resolve the hacking incident, says Raj Shah, senior regulatory attorney and policyholder advisor at MagMutual, which insures medical practices for malpractice and cyber liability.
That usually covers investigating the cause of the breach and the extent of the damage, legal advice about federal and state reporting requirements, whether to pay a ransom, and a public relations professional to handle patient communication, says Mr. Shah.
The benefit doesn’t cover lost patient revenue when practices have to shut down their operations, the cost of replacing damaged computers, or the ransom payment, he says.
Mr. Zetter advises doctors to consider buying cybersecurity coverage. “I recommend that they speak with an insurance broker who is experienced with cybersecurity policies sold to health care professionals to determine what type of coverage and how much coverage they may need. Their malpractice carrier may also be able to provide some answers,” says Mr. Zetter.
The physician will need to be able to answer questions about their network and how many staff they have and may need to involve their IT vendor too, he adds.
How does comprehensive coverage compare?
Ransomware attacks continue to be one of the most frequent types of attacks, and the amount criminals are demanding has risen significantly. The median ransom payment was $5,000 in the fourth quarter of 2018, compared with over $300,000 during the fourth quarter of 2021.
Cybercriminals now engage in “double extortion” – demanding a ransom payment to hand over the code that will unlock their encrypted data – and then another ransom payment to not post patients’ sensitive medical information they copied onto the dark web.
Comprehensive cybersecurity insurance will cover “double extortion” payments, legal costs that may arise from defending against patient lawsuits, and the costs of meeting federal and state privacy requirements, including notifying patients of the data breach and regulatory investigations, says Michael Carr, head of risk engineering for North America for Coalition, a cyber insurance firm.
Cyber insurers also contract with vendors who sell bitcoin, which is the currency cybercriminals typically demand for ransom payments, and work with ransom negotiators.
For example, once Coalition decided to pay the ransom on behalf of a health care client, it negotiated the ransom demand down by nearly 75% from $750,000 to $200,000, and proceeded to help the company restore all of its data.
The costs to respond to the incident, to recover lost data, and to pay the extortion, together with the lost business income resulting from the incident, were covered by Coalition’s cyber insurance policy.
Other clients have had their funds retrieved before a fraudulent wire transfer was completed. “Medical practices have vendors they pay regularly. A cybercriminal may compromise your email or take over a bank account and then impersonate a vendor asking to be paid for services they didn’t provide,” says Mr. Carr.
How much coverage do you need? Cost?
Dr. McAneny has increased her cybersecurity coverage every year. “It’s expensive, but I think it’s worth it. But you can never buy enough protection due to the coverage limits.”
She worries that the costs could exceed the limits if a ransomware attack disrupts her practice for days, weeks, or longer, or if the Office for Civil Rights fines her practice $10,000 per patient chart – the practice has 100,000 health records. “That can run several millions of dollars and ruin a practice,” she says.
Health systems and hospitals need massive amounts of coverage, which often runs from $20 million to $30 million, says Mr. Shah. However, practices insured through MagMutual have lower coverage limits that range from $1 million to $5 million, he says.
“A large practice does not necessarily need more than $1,000,000 in coverage if they have limited loss in this area and strong internal processes and controls. Most large practices also have a dedicated information security director, which reduces their risk, so they may be comfortable with $1,000,000 in coverage,” says Mr. Shah.
Premiums are based on the number of patient health records per practice, which translates into higher premiums for larger practices.
Other factors that come into play include the underlying coverage, risk controls the practice has implemented, and its claims history, says Mr. Shah.
However, the cost for cyber liability insurance has increased, and practices can expect to pay higher premiums and deductibles. For example, a practice that paid $10,000 in premiums for a new policy last year will have to pay $20,000 this year, says Dan Hanson, senior vice president of management liability and client experience at Marsh & McLennon Agency, a risk management firm that sells cyber insurance policies.
“We saw 71% of our self-insured clients experience higher deductibles over last year due to increased claim activity and the lack of capacity in the market. The carriers are saying they will set limits, but you are going to pay a lot more, and you are going to participate more in losses through the higher deductibles,” says Mr. Hanson.
Are you eligible?
Cyber insurance companies have a vested interest in avoiding claims. With increasing cyberattacks and larger payouts, many insurers are requiring practices to implement some defensive measures before they insure them. Some insurers, such as Coalition, say they may still insure small practices for comprehensive coverage, but it may impact the pricing or what’s covered, says Mr. Carr.
Here are some of the security measures that cyber insurers are looking for:
- Multifactorial authentication (MFA) requires an extra layer of security to access the system. For example, when logging into your organization’s EHR platform, instead of just using a username and password to access the platform, MFA would require you to input an additional unique login credential before you can access the EHR. A secondary login credential may include security questions, a one-time PIN, or biometrics.
- Removing a terminated employee’s login credentials quickly from the computer system. “One of the most damaging and expensive types of attacks are by disgruntled employees who still have their login credentials and take revenge by logging back into the system and planting malware,” says Mr. Shah.
- Automatic system updates (patches). “Phishing email compromises usually result from a failure to fix vulnerabilities. When a system needs to restart, it should be set to automatically update any potential security loopholes within programs or products,” says Mr. Carr. The firewall settings should also be updated.
- Prior hacking incidents: Are the attackers out of your system? Once criminals hack into the system, your practice is vulnerable to repeat attacks. “If a cyberattack is not completely addressed, threat actors will maintain access to or a presence on the compromised network. In general, we will work with the insured to ensure that the initial point of compromise has been addressed and that any threat actor presence in the network has been removed,” says Mr. Carr.
When doctors compare cybersecurity policies, experts recommend avoiding companies that may offer lower prices but lack a proven track record of handling claims and do not offer resources that can detect a threat, such as ongoing network monitoring and employee training with simulated exercises.
“Practices tend to think, ‘It won’t happen to me.’ Every practice needs to take this seriously,” says Dr. McAneny.
A version of this article first appeared on Medscape.com.
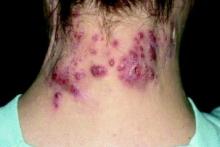
Study eyes characteristics of pediatric patients with hidradenitis suppurativa
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.

“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.

“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
INDIANAPOLIS – in a study presented at the annual meeting of the Society for Pediatric Dermatology.
In addition, 44% presented with scarring, which suggests that HS may be underdiagnosed in this patient population. Those are the key findings from the study, a single-center retrospective chart review presented by Stephanie Sanchez during a poster session at the meeting.

“There is limited research on HS within the pediatric population,” said Ms. Sanchez, a fourth-year medical student at Boston University. “It’s not very well defined or characterized.” The “unusually high number of pediatric patients with HS” at Boston Medical Center provided “a unique opportunity to study this topic.”
Working with her mentor, Lisa Shen, MD, associate medical director of pediatric dermatology at Boston University, Ms. Sanchez and colleagues retrospectively reviewed the medical records of 303 patients aged 4-18 years who were diagnosed with HS at Boston Medical Center from 2012 to 2021. Boston Medical Center is the largest safety net hospital in New England. All data points and outcome measures were collected within 6 months of the patient’s HS diagnosis date.
Of the 303 patients with HS, 84% were female and 16% were male. Complete information about race was available in 286 patients. Of these, 65% were Black/African American, 11% were White, and the rest were from other racial groups. The mean age at symptom onset was 13 years, while the mean age at diagnosis was 15 years, and the mean delay to diagnosis was 2 years. A family history of HS was reported in 36% of patients.
The most common clinical features in these HS patients were pain/tenderness (90%), pustules/papules (65%), discharge/drainage (62%), and deep-seated nodules (51%). Scarring was present in 44% of patients at the time of diagnosis. The three most common sites of involvement were the axillary area (79%), the pubic area (36%), and the inguinal folds/inner thighs (34%).
Obesity was the most common comorbidity at the time of diagnosis, with 64% of patients affected. The next most common comorbidities were acne vulgaris (36%), acanthosis nigricans (25%), depression (18%), being overweight (17%), polycystic ovary syndrome (16%) and anxiety (13%). None had type 1 diabetes or metabolic syndrome.
Referring to the large population of underserved minority patients at Boston Medical Center, Dr. Shen noted, “we have to make sure not to underestimate the prevalence of obesity in this population as they get older. We need to start from a younger age to incorporate multidisciplinary care such as weight management, nutrition, and working with our pediatric surgery colleagues in trying to tackle [HS] because there is data to suggest that the earlier we intervene, the better outcomes they have. That makes sense.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the findings, said that the study “highlights the impressive and concerning gap and delays in diagnosis, not too dissimilar to what the literature shows in adult HS patients, which unfortunately has tremendous ramifications, both physically and emotionally/psychosocially.”
While this single-center study identified potential risk factors, such as obesity and self-identifying as Black, he said, “it is important to note that this condition does not discriminate and therefore it is important not to miss the cases that don’t follow the textbook nor stigmatize this condition as one that only impacts certain demographics.”
The researchers reported having no financial disclosures. Dr. Friedman, who was not involved with the study, reported that he serves as a consultant and/or advisor to numerous pharmaceutical companies. He is a speaker for companies including, Regeneron, Sanofi, AbbVie, Janssen, Incyte, and Brickell Biotech, and has received grants from Pfizer, the Dermatology Foundation, Almirall, Incyte, Galderma, and Janssen.
AT SPD 2022
Shift schedule today could worsen that stroke tomorrow
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Pembrolizumab for melanoma bittersweet, doctor says
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
AT ASCO 2022
What influences a trainee’s decision to choose pediatric dermatology as a career?
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
AT SPD 2022
Physicians urged to write indications on drug scripts as methotrexate users face new barriers with SCOTUS decision
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
Topical gel for epidermolysis bullosa shows ongoing benefit
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.