User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Chronic Retiform Purpura of the Abdomen and Thighs: A Fatal Case of Intravascular Large Cell Lymphoma
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).
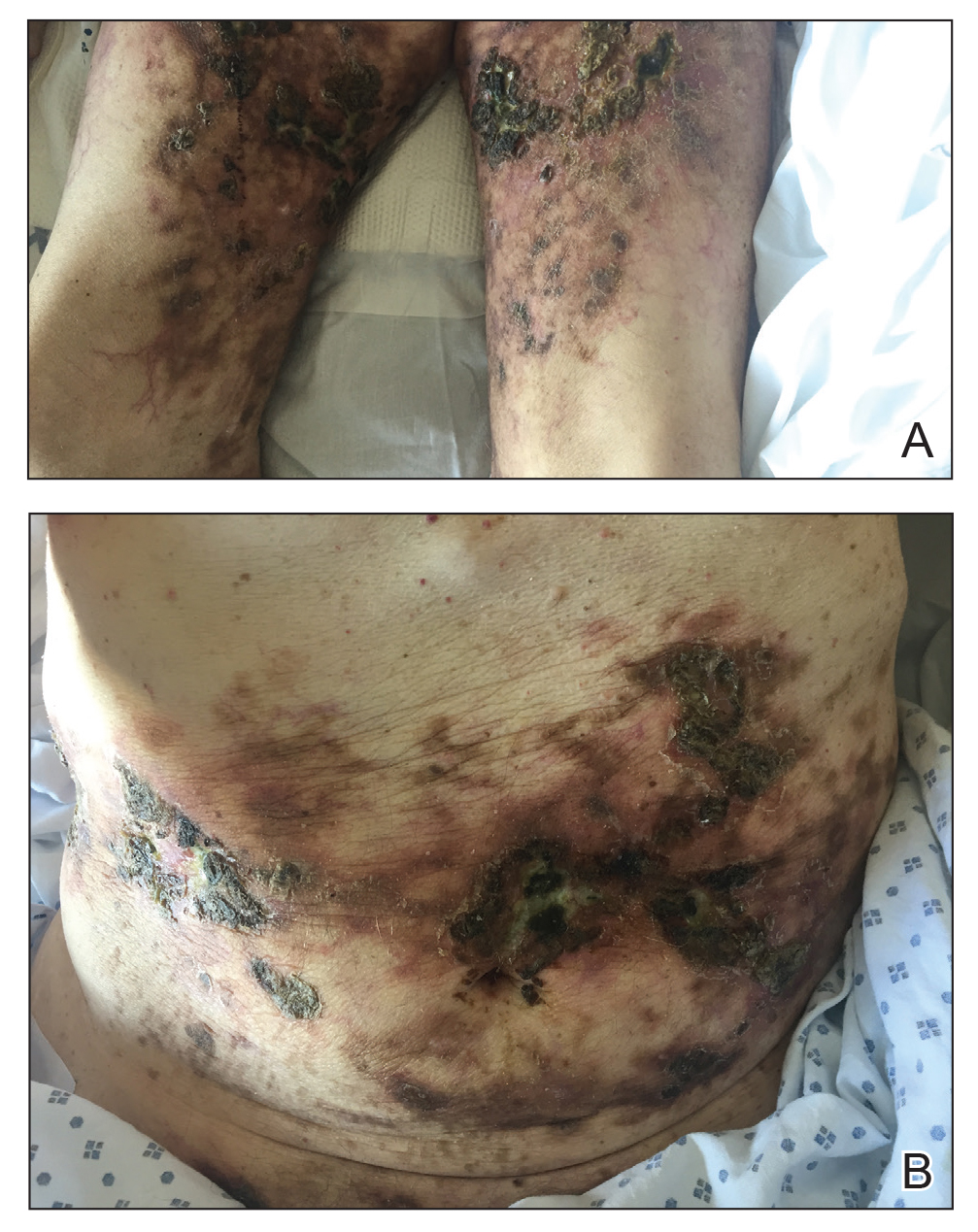
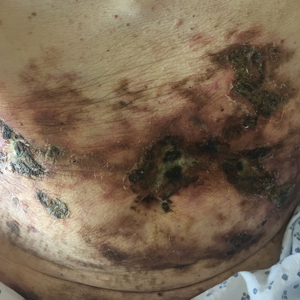
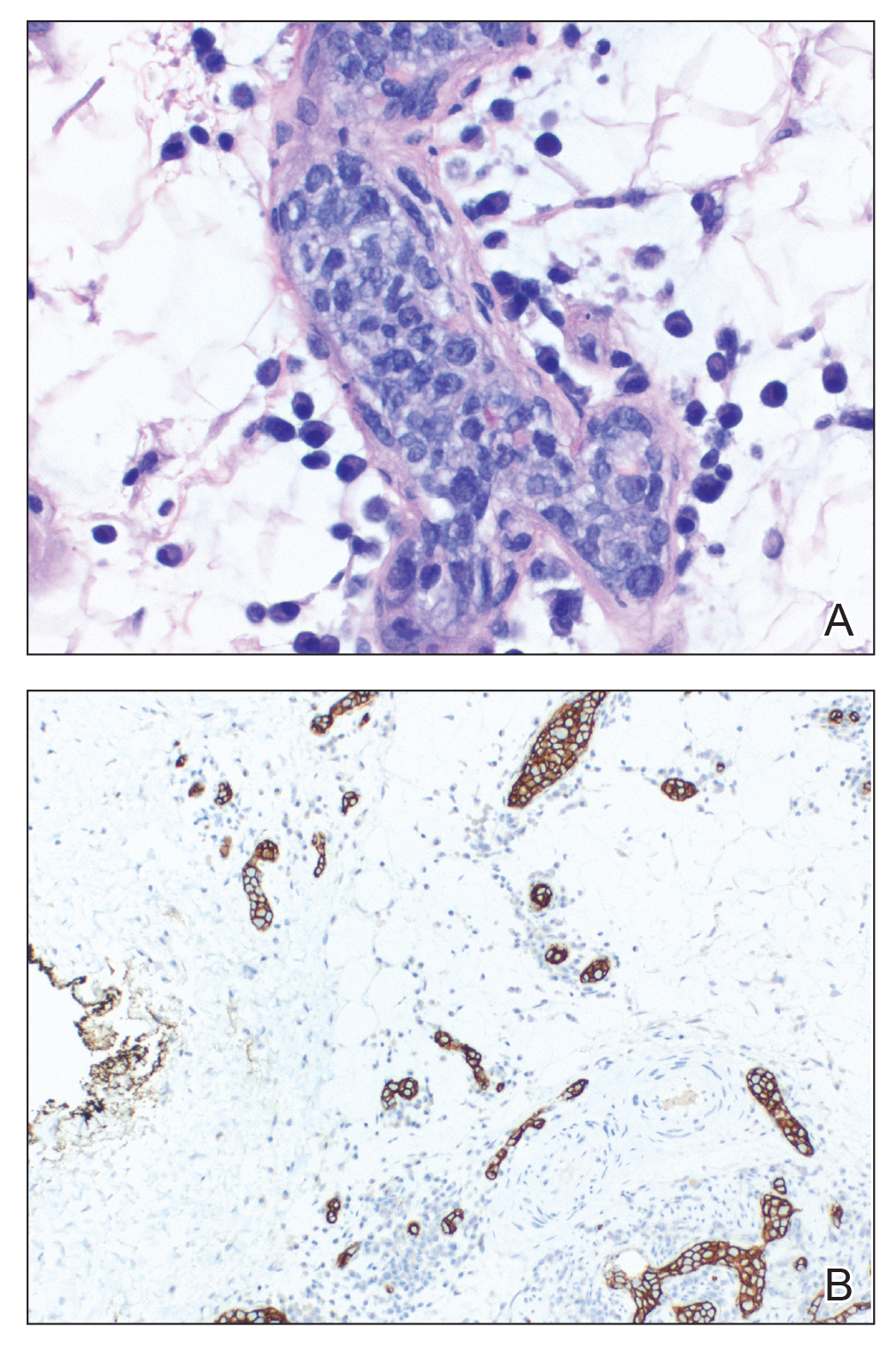
A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.
The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).

A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.

The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).

A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.

The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
Practice Points
- Intravascular large cell lymphoma (ILCL) is a life-threatening malignancy that can present with retiform purpura and other symptoms of vascular occlusion.
- The diagnosis of ILCL can be challenging because of the presence of distractors, and multiple biopsies may be required to establish pathology.
Tumor Necrosis Factor α Inhibitor–Induced Lupuslike Syndrome in a Patient Prescribed Certolizumab Pegol
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.
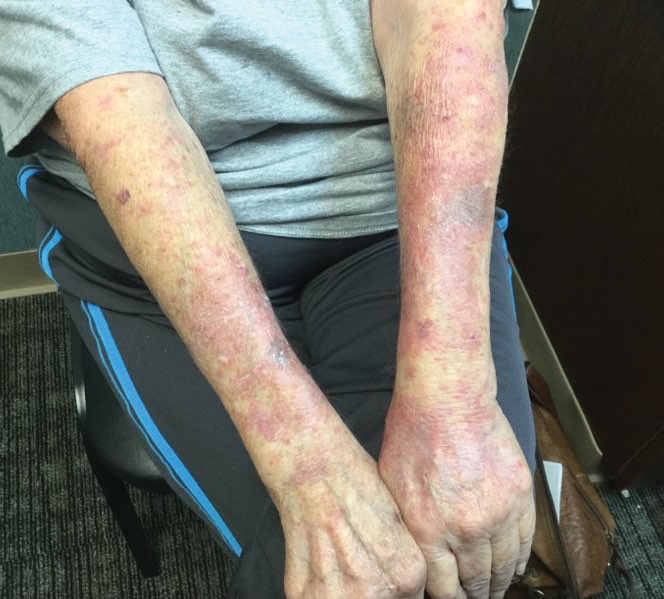
A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.
Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
Practice Points
- Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a form of drug-induced lupus specific to patients on anti–TNF-α therapy.
- The underlying mechanism of disease development is unique compared to other types of drug-induced lupus.
- TAILS most commonly is associated with the use of infliximab and etanercept but also has been reported with adalimumab, golimumab, and certolizumab pegol.
Nodules on the Anterior Neck Following Poly-L-lactic Acid Injection
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
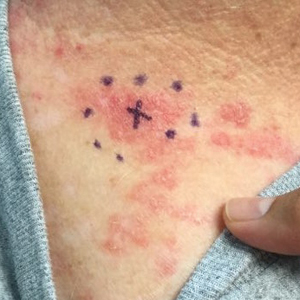
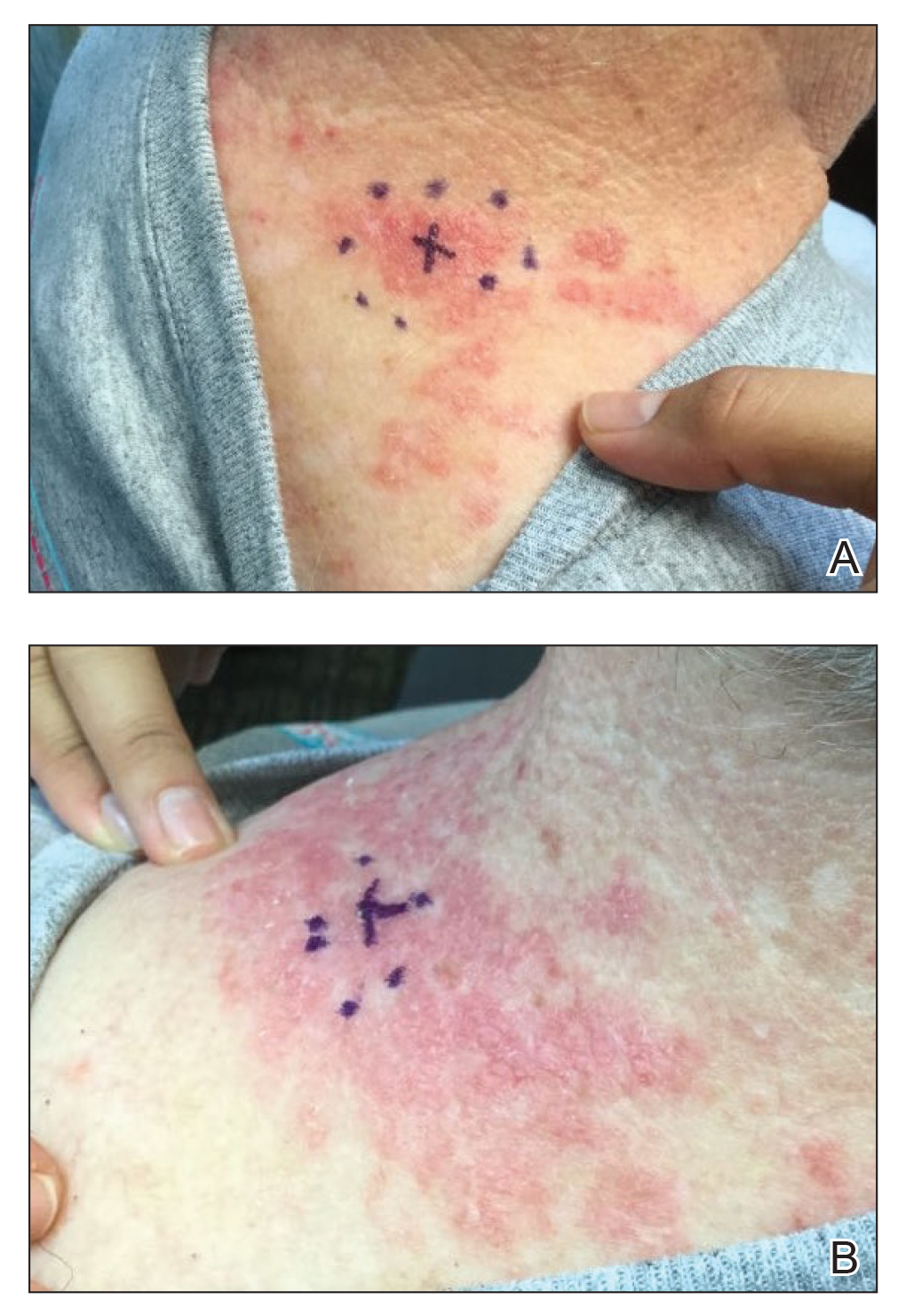
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

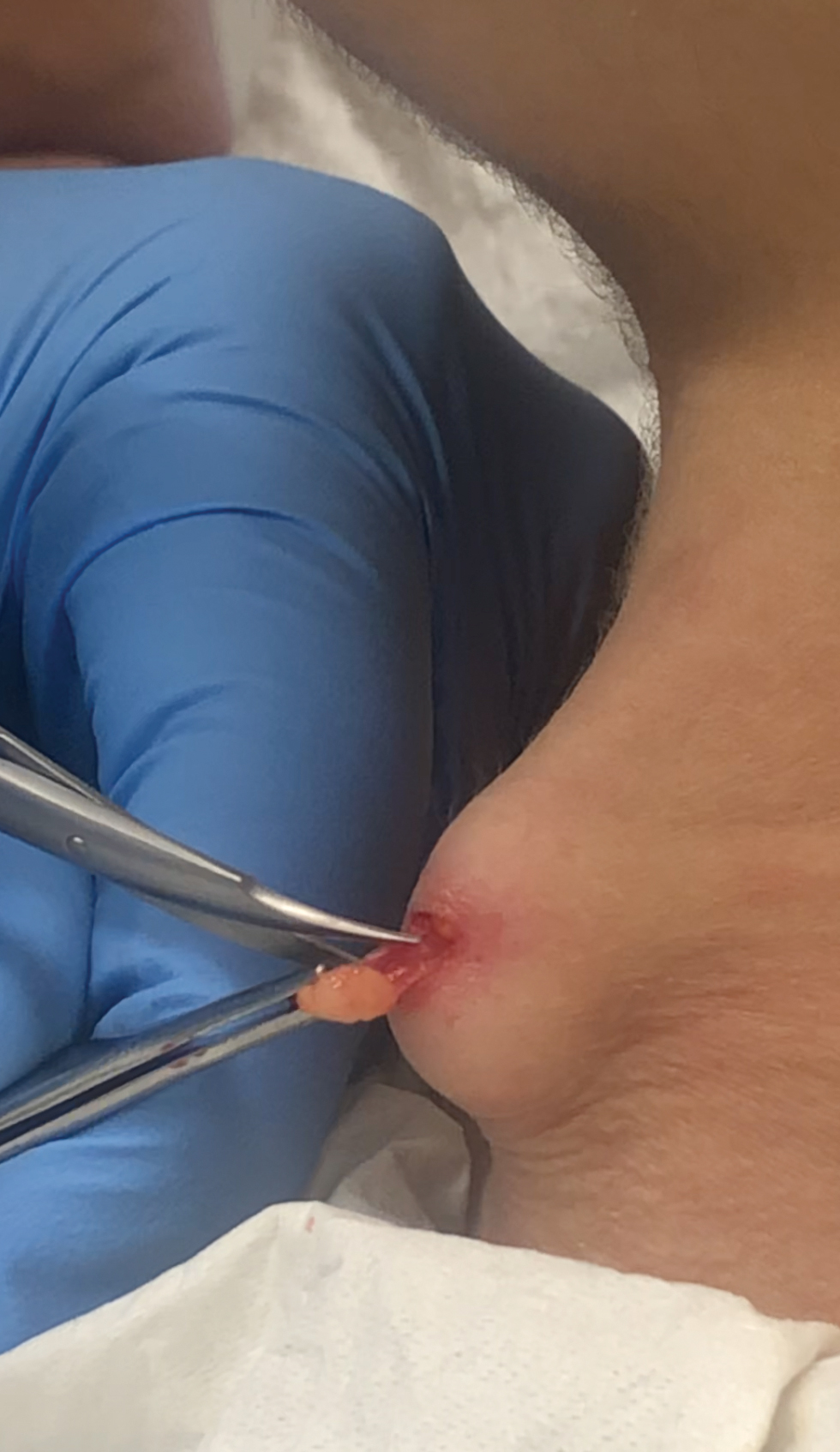
The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).
Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Practice Points
- Injecting poly-L-lactic acid (PLLA) into the anterior neck is an off-label procedure and may cause a higher incidence of nodule formation.
- Most nodules from PLLA can be treated with injections of 5-fluorouracil, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals.
- Treatment-resistant nodules may require surgical excision.
Melanoma incidence is up, but death rates are down
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
AT ASCO 2022
LGBTQ students would get new protections under Biden plan
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
Combo of excision, cryosurgery found to benefit keloid scar outcomes
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
Treating keloid scars by combining excision and contact cryosurgery is a plausible way to decrease the volume of scars, results from a single-center observational study suggest.
“There is currently no consensus regarding the best treatment of keloid scars,” corresponding author Manon Artz, of the department of plastic, reconstructive, and aesthetic surgery at University Hospital of Brest (France), and colleagues wrote in a research letter published online in JAMA Dermatology.
“Earlier studies report a decreased scar volume and a substantial reduction of recurrence in keloid scars treated by cryosurgery,” they wrote. “In this study, our objective was to assess whether intramarginal excision (shaving) of the keloid scar followed by an immediate single session of contact cryosurgery is associated with decreased scar volume.”
Between March 2014 and May 2020, the researchers evaluated the approach in 31 patients with 40 keloid scars who were treated at University Hospital of Brest. Of these study participants, four were lost to follow-up, leaving 27 patients with 35 keloid scars in the final analysis. Their mean age was 24 years, 60% were female, and there was fairly even distribution of Fitzpatrick skin types II-VI.
Most of the keloid scars were located on the ear (69%) and the chest (23%), while the rest were on the head and neck. The primary outcome was reduction of keloid scar volume after 12 months, which was measured with the Vancouver scar scale. The researchers defined 80%-100% reduction in scar volume as “major,” a 50%-80% reduction as “substantial,” and a 0%-50% reduction or recurrence as “moderate.”
After 12 months, 19 scars (54%) showed a major reduction in volume, while 6 (17%) had a substantial reduction, and seven (20%) experienced no reduction. Across all keloid scars, the median scar volume decreased significantly by 81.9%.
Scar volume reduction differed by anatomical location. Specifically, 84% of ear scars showed major or substantial reduction, while 60% of scars on the chest showed a moderate reduction in scar volume or recurrence. In another key finding, the Vancouver scar scale score was reduced overall in 25 scars by 71.4%, from 7 before treatment to 5 after treatment.
“There remains no silver bullet for the treatment of keloids, but this study adds invaluable evidence that tangential excision followed by contact cryosurgery can be a viable treatment regimen with low recurrence rates,” said Marcus G. Tan, MD, who recently completed his dermatology residency at the University of Ottawa and who was asked to comment on the study. “Clinicians should exercise caution especially when treating individuals with darker skin phototypes due to their increased risk of scarring and dyspigmentation.”
Limitations of this study, he said, include a smaller study population with some patient dropouts and a lack of adverse effects reported.
The researchers and Dr. Tan reported having no financial conflicts.
FROM JAMA DERMATOLOGY
Treat-to-target strategy with tapering proves effective in PsA and axSpA
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
FROM THE EULAR 2022 CONGRESS
Why it’s so hard to prevent physician suicide
Kip Wenger, DO, an emergency physician and systems medical director of Team Health, Knoxville, Tenn., was asked to see a patient in the emergency department. He was shocked when he realized who the patient was – a 33-year-old female physician friend and colleague.
She was bleeding from multiple self-inflicted injuries and ultimately died. “I was devastated and couldn’t wrap my head around what had just happened,” Dr. Wenger told this news organization.
It’s important for physicians to be aware of warning signs in their colleagues, such as showing up late, being irritable and short-tempered with staff, missing shifts, making mistakes, or receiving an increasing number of patient complaints, Dr. Wenger says.
Dr. Wenger had had dinner with her several weeks earlier and saw some subtle changes. He had known her as a “positive, upbeat person,” but her demeanor was different during dinner.
“There were no typical telltale signs – she was talking about her plans for the future, including buying a new bicycle – but she wasn’t herself and seemed to become tearful when I hugged her at the end of the evening,” he said. He later heard from another colleague that she had shared feeling “hopeless.”
The scope of the problem
According to the American Society for Suicide Prevention, roughly 300-400 physicians die by suicide annually. Although one study suggests a lower number, official reports likely underestimate suicides, study author Katherine Gold, MD, MSW, associate professor of family medicine, obstetrics, and gynecology, Michigan Medicine, University of Michigan, Ann Arbor, said in an interview.
Peter Yellowlees, MD, MBBS, professor of psychiatry, University of California, Davis, concurs, suggesting that some single-car accidents involving physicians might be suicides. Perry Lin, MD, assistant clinical professor, Heritage College of Osteopathic Medicine, Ohio University, Athens, and national co-chair of the Physician Suicide Awareness Committee of the American Association of Suicidology, says that some death certificates state that the deceased died of “accidental causes” because the physician who completes the certificate, possibly a colleague, is reluctant to list the actual cause of death to protect his colleague’s memory or the family’s feelings.
In general, and among physicians, White men older than 65 “represent the largest percentage of people who die from suicide nationwide,” says Dr. Lin.
But younger people are also susceptible, Dr. Lin adds. One of the most vulnerable periods for potential suicide is during the first few months of residency. This dovetails with the findings of Medscape’s 2022 report Suicide: A Tragedy of the Profession. In that report, a difference was found between frequency of suicidal thoughts in younger physicians, compared with older physicians (14% in those < 35 years vs. 8% for those ≥ 45 years).
Hurdles to preventing physician suicide
“The best thing that can happen in our profession is upstream intervention – if people seek help before they get to the point of suicidality, recognizing they’re under stress and duress and that they might be going down a bad pathway,” says Dr. Lin. But research suggests that many physicians don’t do so.
Gary Price, MD, attending surgeon and clinical assistant professor of surgery, Yale–New Haven Hospital, Connecticut, and president of the Physicians Foundation, says his organization has identified barriers that prevent physicians from seeking help.
Physicians feel they may put their licensure at risk if they admit to receiving help for mental issues. These concerns were expressed by respondents in Medscape’s above mentioned 2022 report, many of whom didn’t seek treatment for depression, burnout, or suicidal thoughts lest it affect their professional standing when renewing their license or seeking credentialing.
Although organizations and societies are advocating against these questions, a recent study found that almost 70% of U.S. states and territories continue to ask physicians about their mental health, and 28% ask for diagnoses (beyond current impairments) – a violation of the Americans With Disabilities Act.
“Mental health illness is different from mental health impairment,” Ryan Mire, MD, a Nashville, Tenn.–based internist, said in an interview. “As physicians, we’re comfortable with licensing boards asking whether the physician has any condition that might impair their care for patients, but not about a history of mental illness.”
The second barrier, says Dr. Price, is that hospital credentialing committees sometimes ask similar questions, as do commercial and malpractice insurers.
Another roadblock is that in some states, undergoing treatment for a mental health problem could be subject to discovery by a plaintiff’s attorney in a malpractice case, even if the physician’s mental health history had no effect on patient care. But that’s uncommon, says Daniel Shapiro, PhD, author of “Delivering Doctor Amelia,” a book about his treatment of a suicidal physician who underwent a malpractice lawsuit. “I’ve never seen that happen.”
A final barrier is that many employers require employees to receive treatment within their own institution or health system. “Physicians may be reluctant to get help where they work, with colleagues and friends knowing about their illness or being involved with their care,” says Dr. Price.
In 2022, the American College of Physicians (ACP) issued a toolkit to help members encourage licensing and credentialing boards to remove questions about mental health on applications and include language that supports receiving treatment, Dr. Mire says.
Layers of vulnerability
There are few data regarding relative risk among particular races or ethnicities, “but we know racism is a social stressor,” says Dr. Mire. “Obviously, people from historically disadvantaged populations tend to have societal stressors like discrimination and racism that add an extra layer of burden.”
Intersectionality – having multiple intersecting risk factors – may confer even higher risk. “For example, if you’re a female physician from a historically marginalized race and a resident dealing with the ‘hidden curriculum’ of trying to be resilient, you have multiple layers of vulnerability.”
There are also limited data regarding which specialties or work environments are associated with highest risk. “Obviously, challenges exist in every segment of medicine and at different ages, stages, and work environments, and they intersect with each individual physician’s personal risk factors,” says Dr. Mire, president of the ACP and assistant clinical professor of clinical medical education, University of Tennessee Health Science Center, Memphis.
Pamela Wible, MD, is an Oregon-based retired physician who herself went through a suicidal period about 11 years into her career that motivated her to embrace a new vision of clinical practice and change her practice model. After a series of physician suicides in her area, she began to speak and write openly about physician suicide, and since her retirement from clinical practice, she makes herself available on a full-time basis to distressed physicians. “When I address a conference of a particular medical specialty or a group in a particular geographical region, I focus on the specific vulnerabilities in that specialty or region,” she says.
What increases the chances of suicide?
“Many factors, both within and outside the professional setting, affect someone’s decision to die by suicide – after all, physicians have the same stressors as other people, like family, finances, and their own health,” Dr. Mire says. When it comes to non–work-related factors, marital stressors and comorbid psychiatric illness particularly raise the risk, says Dr. Lin.
But certain drivers are specific to the practice of medicine, with burnout and depression first in line.
Dr. Shapiro, who is vice dean for faculty and administrative affairs, Penn State University, Hershey, and the Garner James Cline Professor of Medical Humanism, conducts burnout evaluations throughout the country. “Simple depression screeners prior to the pandemic showed about a 10% major depression rate in physicians,” he told this news organization. “Now, we’re seeing a 30%-33% depression rate, even in those who weren’t frontline providers during the pandemic.”
Dr. Price agrees, noting that burnout in physicians has gone from 40% to 60% since the pandemic. But burnout doesn’t always lead to suicide. It’s when burnout progresses to depression, becomes more severe, and is untreated that the suicidal risk arises, he emphasizes.
Additionally, being a doctor isn’t “just a profession” but a “calling and identity,” says Dr. Gold. Job-related problems (for example, a malpractice suit, complaints to the medical board, loss of autonomy, changing work demands) can raise suicidal risk.
And job-related problems can inform the location of suicide, says Dr. Wible, who is the author of “Physicians Suicide Letters – Answered.”
“A work-related catalyst makes it more likely that the person will attempt or complete suicide in the work setting. Physicians have stepped off hospital rooftops, shot or stabbed themselves in hospital parking lots, or [hanged] themselves in hospital chapels. Perhaps it’s because they’re choosing to die in the place where they’ve been most wounded.”
You are not at fault
“If you’re feeling suicidal, you might feel utterly alone, but if there’s one message I can give you, it’s that you’re not alone, and there are many things you can do to mitigate your pain and despair,” Dr. Wible says. “And you’re not defective. It’s the health care system that’s defective. You have nothing to be ashamed of.”
Some institutions have a “buddy system” that pairs clinicians to provide mutual peer support. A partner who notices concerning signs can refer the other partner for help. Physicians can also be paired with a “buddy,” even without a formal institutional structure.
A “buddy” is a step in the right direction, but Dr. Shapiro cautions it might be necessary to consult a trained professional for serious depression or suicidality. Several states provide connection to local resources. Employee assistance programs (EAPs) might be helpful, although many physicians don’t trust their institution’s EAP. Or physicians can ask colleagues to recommend a “doctor’s doctor” who specializes in treating physicians, suggests Dr. Yellowlees, author of “Physician Suicide: Cases and Commentaries.”
In Medscape’s 2022 report, almost all respondents who reported having suicidal colleagues said they offered help, including emotional support, practical assistance, referrals, speaking to family members, or even personally taking the colleague to the ED or to a therapist.
To enhance physicians’ ability to help each other, Dr. Lin recommends “gatekeeper training,” which has been shown to reduce suicide. “This strategy utilizes a peer-to-peer model, but, rather than a single ‘peer buddy,’ everyone is a ‘gatekeeper’ trained in approaches, such as QPR – Question, Persuade, Refer. ‘Gatekeepers’ are taught how to recognize warning signs of suicide, question the potentially suicidal individual, persuade him/her to get help, and provide referrals.”
Other ways to prevent suicide
Dr. Lin advises physicians to “create a personalized safety plan and write down signs and clues that they may be going down the wrong path and what they can do – like breathing exercises, relaxation – and identifying people to talk to, places to go, or phone numbers to call, if those initial measures aren’t enough.” The plan is private and allows the physician to determine at what point help is needed and who should be consulted. “Sometimes, when a person is in acute stress, even looking up a phone number can seem insurmountable. But having it on paper lowers the barrier, making it more achievable.”
Resources should be posted in places where physicians gather so that those who don’t already have a safety plan have easy access to that information, he suggests.
In addition, consideration may be given to reaching out for support if a colleague has died by suicide, experts suggest. Whether offered by one’s institution, a peer arrangement, spiritual counseling, or psychotherapy, one may need help dealing with the trauma, guilt, and grief that often accompany this type of loss.
A version of this article first appeared on Medscape.com.
Kip Wenger, DO, an emergency physician and systems medical director of Team Health, Knoxville, Tenn., was asked to see a patient in the emergency department. He was shocked when he realized who the patient was – a 33-year-old female physician friend and colleague.
She was bleeding from multiple self-inflicted injuries and ultimately died. “I was devastated and couldn’t wrap my head around what had just happened,” Dr. Wenger told this news organization.
It’s important for physicians to be aware of warning signs in their colleagues, such as showing up late, being irritable and short-tempered with staff, missing shifts, making mistakes, or receiving an increasing number of patient complaints, Dr. Wenger says.
Dr. Wenger had had dinner with her several weeks earlier and saw some subtle changes. He had known her as a “positive, upbeat person,” but her demeanor was different during dinner.
“There were no typical telltale signs – she was talking about her plans for the future, including buying a new bicycle – but she wasn’t herself and seemed to become tearful when I hugged her at the end of the evening,” he said. He later heard from another colleague that she had shared feeling “hopeless.”
The scope of the problem
According to the American Society for Suicide Prevention, roughly 300-400 physicians die by suicide annually. Although one study suggests a lower number, official reports likely underestimate suicides, study author Katherine Gold, MD, MSW, associate professor of family medicine, obstetrics, and gynecology, Michigan Medicine, University of Michigan, Ann Arbor, said in an interview.
Peter Yellowlees, MD, MBBS, professor of psychiatry, University of California, Davis, concurs, suggesting that some single-car accidents involving physicians might be suicides. Perry Lin, MD, assistant clinical professor, Heritage College of Osteopathic Medicine, Ohio University, Athens, and national co-chair of the Physician Suicide Awareness Committee of the American Association of Suicidology, says that some death certificates state that the deceased died of “accidental causes” because the physician who completes the certificate, possibly a colleague, is reluctant to list the actual cause of death to protect his colleague’s memory or the family’s feelings.
In general, and among physicians, White men older than 65 “represent the largest percentage of people who die from suicide nationwide,” says Dr. Lin.
But younger people are also susceptible, Dr. Lin adds. One of the most vulnerable periods for potential suicide is during the first few months of residency. This dovetails with the findings of Medscape’s 2022 report Suicide: A Tragedy of the Profession. In that report, a difference was found between frequency of suicidal thoughts in younger physicians, compared with older physicians (14% in those < 35 years vs. 8% for those ≥ 45 years).
Hurdles to preventing physician suicide
“The best thing that can happen in our profession is upstream intervention – if people seek help before they get to the point of suicidality, recognizing they’re under stress and duress and that they might be going down a bad pathway,” says Dr. Lin. But research suggests that many physicians don’t do so.
Gary Price, MD, attending surgeon and clinical assistant professor of surgery, Yale–New Haven Hospital, Connecticut, and president of the Physicians Foundation, says his organization has identified barriers that prevent physicians from seeking help.
Physicians feel they may put their licensure at risk if they admit to receiving help for mental issues. These concerns were expressed by respondents in Medscape’s above mentioned 2022 report, many of whom didn’t seek treatment for depression, burnout, or suicidal thoughts lest it affect their professional standing when renewing their license or seeking credentialing.
Although organizations and societies are advocating against these questions, a recent study found that almost 70% of U.S. states and territories continue to ask physicians about their mental health, and 28% ask for diagnoses (beyond current impairments) – a violation of the Americans With Disabilities Act.
“Mental health illness is different from mental health impairment,” Ryan Mire, MD, a Nashville, Tenn.–based internist, said in an interview. “As physicians, we’re comfortable with licensing boards asking whether the physician has any condition that might impair their care for patients, but not about a history of mental illness.”
The second barrier, says Dr. Price, is that hospital credentialing committees sometimes ask similar questions, as do commercial and malpractice insurers.
Another roadblock is that in some states, undergoing treatment for a mental health problem could be subject to discovery by a plaintiff’s attorney in a malpractice case, even if the physician’s mental health history had no effect on patient care. But that’s uncommon, says Daniel Shapiro, PhD, author of “Delivering Doctor Amelia,” a book about his treatment of a suicidal physician who underwent a malpractice lawsuit. “I’ve never seen that happen.”
A final barrier is that many employers require employees to receive treatment within their own institution or health system. “Physicians may be reluctant to get help where they work, with colleagues and friends knowing about their illness or being involved with their care,” says Dr. Price.
In 2022, the American College of Physicians (ACP) issued a toolkit to help members encourage licensing and credentialing boards to remove questions about mental health on applications and include language that supports receiving treatment, Dr. Mire says.
Layers of vulnerability
There are few data regarding relative risk among particular races or ethnicities, “but we know racism is a social stressor,” says Dr. Mire. “Obviously, people from historically disadvantaged populations tend to have societal stressors like discrimination and racism that add an extra layer of burden.”
Intersectionality – having multiple intersecting risk factors – may confer even higher risk. “For example, if you’re a female physician from a historically marginalized race and a resident dealing with the ‘hidden curriculum’ of trying to be resilient, you have multiple layers of vulnerability.”
There are also limited data regarding which specialties or work environments are associated with highest risk. “Obviously, challenges exist in every segment of medicine and at different ages, stages, and work environments, and they intersect with each individual physician’s personal risk factors,” says Dr. Mire, president of the ACP and assistant clinical professor of clinical medical education, University of Tennessee Health Science Center, Memphis.
Pamela Wible, MD, is an Oregon-based retired physician who herself went through a suicidal period about 11 years into her career that motivated her to embrace a new vision of clinical practice and change her practice model. After a series of physician suicides in her area, she began to speak and write openly about physician suicide, and since her retirement from clinical practice, she makes herself available on a full-time basis to distressed physicians. “When I address a conference of a particular medical specialty or a group in a particular geographical region, I focus on the specific vulnerabilities in that specialty or region,” she says.
What increases the chances of suicide?
“Many factors, both within and outside the professional setting, affect someone’s decision to die by suicide – after all, physicians have the same stressors as other people, like family, finances, and their own health,” Dr. Mire says. When it comes to non–work-related factors, marital stressors and comorbid psychiatric illness particularly raise the risk, says Dr. Lin.
But certain drivers are specific to the practice of medicine, with burnout and depression first in line.
Dr. Shapiro, who is vice dean for faculty and administrative affairs, Penn State University, Hershey, and the Garner James Cline Professor of Medical Humanism, conducts burnout evaluations throughout the country. “Simple depression screeners prior to the pandemic showed about a 10% major depression rate in physicians,” he told this news organization. “Now, we’re seeing a 30%-33% depression rate, even in those who weren’t frontline providers during the pandemic.”
Dr. Price agrees, noting that burnout in physicians has gone from 40% to 60% since the pandemic. But burnout doesn’t always lead to suicide. It’s when burnout progresses to depression, becomes more severe, and is untreated that the suicidal risk arises, he emphasizes.
Additionally, being a doctor isn’t “just a profession” but a “calling and identity,” says Dr. Gold. Job-related problems (for example, a malpractice suit, complaints to the medical board, loss of autonomy, changing work demands) can raise suicidal risk.
And job-related problems can inform the location of suicide, says Dr. Wible, who is the author of “Physicians Suicide Letters – Answered.”
“A work-related catalyst makes it more likely that the person will attempt or complete suicide in the work setting. Physicians have stepped off hospital rooftops, shot or stabbed themselves in hospital parking lots, or [hanged] themselves in hospital chapels. Perhaps it’s because they’re choosing to die in the place where they’ve been most wounded.”
You are not at fault
“If you’re feeling suicidal, you might feel utterly alone, but if there’s one message I can give you, it’s that you’re not alone, and there are many things you can do to mitigate your pain and despair,” Dr. Wible says. “And you’re not defective. It’s the health care system that’s defective. You have nothing to be ashamed of.”
Some institutions have a “buddy system” that pairs clinicians to provide mutual peer support. A partner who notices concerning signs can refer the other partner for help. Physicians can also be paired with a “buddy,” even without a formal institutional structure.
A “buddy” is a step in the right direction, but Dr. Shapiro cautions it might be necessary to consult a trained professional for serious depression or suicidality. Several states provide connection to local resources. Employee assistance programs (EAPs) might be helpful, although many physicians don’t trust their institution’s EAP. Or physicians can ask colleagues to recommend a “doctor’s doctor” who specializes in treating physicians, suggests Dr. Yellowlees, author of “Physician Suicide: Cases and Commentaries.”
In Medscape’s 2022 report, almost all respondents who reported having suicidal colleagues said they offered help, including emotional support, practical assistance, referrals, speaking to family members, or even personally taking the colleague to the ED or to a therapist.
To enhance physicians’ ability to help each other, Dr. Lin recommends “gatekeeper training,” which has been shown to reduce suicide. “This strategy utilizes a peer-to-peer model, but, rather than a single ‘peer buddy,’ everyone is a ‘gatekeeper’ trained in approaches, such as QPR – Question, Persuade, Refer. ‘Gatekeepers’ are taught how to recognize warning signs of suicide, question the potentially suicidal individual, persuade him/her to get help, and provide referrals.”
Other ways to prevent suicide
Dr. Lin advises physicians to “create a personalized safety plan and write down signs and clues that they may be going down the wrong path and what they can do – like breathing exercises, relaxation – and identifying people to talk to, places to go, or phone numbers to call, if those initial measures aren’t enough.” The plan is private and allows the physician to determine at what point help is needed and who should be consulted. “Sometimes, when a person is in acute stress, even looking up a phone number can seem insurmountable. But having it on paper lowers the barrier, making it more achievable.”
Resources should be posted in places where physicians gather so that those who don’t already have a safety plan have easy access to that information, he suggests.
In addition, consideration may be given to reaching out for support if a colleague has died by suicide, experts suggest. Whether offered by one’s institution, a peer arrangement, spiritual counseling, or psychotherapy, one may need help dealing with the trauma, guilt, and grief that often accompany this type of loss.
A version of this article first appeared on Medscape.com.
Kip Wenger, DO, an emergency physician and systems medical director of Team Health, Knoxville, Tenn., was asked to see a patient in the emergency department. He was shocked when he realized who the patient was – a 33-year-old female physician friend and colleague.
She was bleeding from multiple self-inflicted injuries and ultimately died. “I was devastated and couldn’t wrap my head around what had just happened,” Dr. Wenger told this news organization.
It’s important for physicians to be aware of warning signs in their colleagues, such as showing up late, being irritable and short-tempered with staff, missing shifts, making mistakes, or receiving an increasing number of patient complaints, Dr. Wenger says.
Dr. Wenger had had dinner with her several weeks earlier and saw some subtle changes. He had known her as a “positive, upbeat person,” but her demeanor was different during dinner.
“There were no typical telltale signs – she was talking about her plans for the future, including buying a new bicycle – but she wasn’t herself and seemed to become tearful when I hugged her at the end of the evening,” he said. He later heard from another colleague that she had shared feeling “hopeless.”
The scope of the problem
According to the American Society for Suicide Prevention, roughly 300-400 physicians die by suicide annually. Although one study suggests a lower number, official reports likely underestimate suicides, study author Katherine Gold, MD, MSW, associate professor of family medicine, obstetrics, and gynecology, Michigan Medicine, University of Michigan, Ann Arbor, said in an interview.
Peter Yellowlees, MD, MBBS, professor of psychiatry, University of California, Davis, concurs, suggesting that some single-car accidents involving physicians might be suicides. Perry Lin, MD, assistant clinical professor, Heritage College of Osteopathic Medicine, Ohio University, Athens, and national co-chair of the Physician Suicide Awareness Committee of the American Association of Suicidology, says that some death certificates state that the deceased died of “accidental causes” because the physician who completes the certificate, possibly a colleague, is reluctant to list the actual cause of death to protect his colleague’s memory or the family’s feelings.
In general, and among physicians, White men older than 65 “represent the largest percentage of people who die from suicide nationwide,” says Dr. Lin.
But younger people are also susceptible, Dr. Lin adds. One of the most vulnerable periods for potential suicide is during the first few months of residency. This dovetails with the findings of Medscape’s 2022 report Suicide: A Tragedy of the Profession. In that report, a difference was found between frequency of suicidal thoughts in younger physicians, compared with older physicians (14% in those < 35 years vs. 8% for those ≥ 45 years).
Hurdles to preventing physician suicide
“The best thing that can happen in our profession is upstream intervention – if people seek help before they get to the point of suicidality, recognizing they’re under stress and duress and that they might be going down a bad pathway,” says Dr. Lin. But research suggests that many physicians don’t do so.
Gary Price, MD, attending surgeon and clinical assistant professor of surgery, Yale–New Haven Hospital, Connecticut, and president of the Physicians Foundation, says his organization has identified barriers that prevent physicians from seeking help.
Physicians feel they may put their licensure at risk if they admit to receiving help for mental issues. These concerns were expressed by respondents in Medscape’s above mentioned 2022 report, many of whom didn’t seek treatment for depression, burnout, or suicidal thoughts lest it affect their professional standing when renewing their license or seeking credentialing.
Although organizations and societies are advocating against these questions, a recent study found that almost 70% of U.S. states and territories continue to ask physicians about their mental health, and 28% ask for diagnoses (beyond current impairments) – a violation of the Americans With Disabilities Act.
“Mental health illness is different from mental health impairment,” Ryan Mire, MD, a Nashville, Tenn.–based internist, said in an interview. “As physicians, we’re comfortable with licensing boards asking whether the physician has any condition that might impair their care for patients, but not about a history of mental illness.”
The second barrier, says Dr. Price, is that hospital credentialing committees sometimes ask similar questions, as do commercial and malpractice insurers.
Another roadblock is that in some states, undergoing treatment for a mental health problem could be subject to discovery by a plaintiff’s attorney in a malpractice case, even if the physician’s mental health history had no effect on patient care. But that’s uncommon, says Daniel Shapiro, PhD, author of “Delivering Doctor Amelia,” a book about his treatment of a suicidal physician who underwent a malpractice lawsuit. “I’ve never seen that happen.”
A final barrier is that many employers require employees to receive treatment within their own institution or health system. “Physicians may be reluctant to get help where they work, with colleagues and friends knowing about their illness or being involved with their care,” says Dr. Price.
In 2022, the American College of Physicians (ACP) issued a toolkit to help members encourage licensing and credentialing boards to remove questions about mental health on applications and include language that supports receiving treatment, Dr. Mire says.
Layers of vulnerability
There are few data regarding relative risk among particular races or ethnicities, “but we know racism is a social stressor,” says Dr. Mire. “Obviously, people from historically disadvantaged populations tend to have societal stressors like discrimination and racism that add an extra layer of burden.”
Intersectionality – having multiple intersecting risk factors – may confer even higher risk. “For example, if you’re a female physician from a historically marginalized race and a resident dealing with the ‘hidden curriculum’ of trying to be resilient, you have multiple layers of vulnerability.”
There are also limited data regarding which specialties or work environments are associated with highest risk. “Obviously, challenges exist in every segment of medicine and at different ages, stages, and work environments, and they intersect with each individual physician’s personal risk factors,” says Dr. Mire, president of the ACP and assistant clinical professor of clinical medical education, University of Tennessee Health Science Center, Memphis.
Pamela Wible, MD, is an Oregon-based retired physician who herself went through a suicidal period about 11 years into her career that motivated her to embrace a new vision of clinical practice and change her practice model. After a series of physician suicides in her area, she began to speak and write openly about physician suicide, and since her retirement from clinical practice, she makes herself available on a full-time basis to distressed physicians. “When I address a conference of a particular medical specialty or a group in a particular geographical region, I focus on the specific vulnerabilities in that specialty or region,” she says.
What increases the chances of suicide?
“Many factors, both within and outside the professional setting, affect someone’s decision to die by suicide – after all, physicians have the same stressors as other people, like family, finances, and their own health,” Dr. Mire says. When it comes to non–work-related factors, marital stressors and comorbid psychiatric illness particularly raise the risk, says Dr. Lin.
But certain drivers are specific to the practice of medicine, with burnout and depression first in line.
Dr. Shapiro, who is vice dean for faculty and administrative affairs, Penn State University, Hershey, and the Garner James Cline Professor of Medical Humanism, conducts burnout evaluations throughout the country. “Simple depression screeners prior to the pandemic showed about a 10% major depression rate in physicians,” he told this news organization. “Now, we’re seeing a 30%-33% depression rate, even in those who weren’t frontline providers during the pandemic.”
Dr. Price agrees, noting that burnout in physicians has gone from 40% to 60% since the pandemic. But burnout doesn’t always lead to suicide. It’s when burnout progresses to depression, becomes more severe, and is untreated that the suicidal risk arises, he emphasizes.
Additionally, being a doctor isn’t “just a profession” but a “calling and identity,” says Dr. Gold. Job-related problems (for example, a malpractice suit, complaints to the medical board, loss of autonomy, changing work demands) can raise suicidal risk.
And job-related problems can inform the location of suicide, says Dr. Wible, who is the author of “Physicians Suicide Letters – Answered.”
“A work-related catalyst makes it more likely that the person will attempt or complete suicide in the work setting. Physicians have stepped off hospital rooftops, shot or stabbed themselves in hospital parking lots, or [hanged] themselves in hospital chapels. Perhaps it’s because they’re choosing to die in the place where they’ve been most wounded.”
You are not at fault
“If you’re feeling suicidal, you might feel utterly alone, but if there’s one message I can give you, it’s that you’re not alone, and there are many things you can do to mitigate your pain and despair,” Dr. Wible says. “And you’re not defective. It’s the health care system that’s defective. You have nothing to be ashamed of.”
Some institutions have a “buddy system” that pairs clinicians to provide mutual peer support. A partner who notices concerning signs can refer the other partner for help. Physicians can also be paired with a “buddy,” even without a formal institutional structure.
A “buddy” is a step in the right direction, but Dr. Shapiro cautions it might be necessary to consult a trained professional for serious depression or suicidality. Several states provide connection to local resources. Employee assistance programs (EAPs) might be helpful, although many physicians don’t trust their institution’s EAP. Or physicians can ask colleagues to recommend a “doctor’s doctor” who specializes in treating physicians, suggests Dr. Yellowlees, author of “Physician Suicide: Cases and Commentaries.”
In Medscape’s 2022 report, almost all respondents who reported having suicidal colleagues said they offered help, including emotional support, practical assistance, referrals, speaking to family members, or even personally taking the colleague to the ED or to a therapist.
To enhance physicians’ ability to help each other, Dr. Lin recommends “gatekeeper training,” which has been shown to reduce suicide. “This strategy utilizes a peer-to-peer model, but, rather than a single ‘peer buddy,’ everyone is a ‘gatekeeper’ trained in approaches, such as QPR – Question, Persuade, Refer. ‘Gatekeepers’ are taught how to recognize warning signs of suicide, question the potentially suicidal individual, persuade him/her to get help, and provide referrals.”
Other ways to prevent suicide
Dr. Lin advises physicians to “create a personalized safety plan and write down signs and clues that they may be going down the wrong path and what they can do – like breathing exercises, relaxation – and identifying people to talk to, places to go, or phone numbers to call, if those initial measures aren’t enough.” The plan is private and allows the physician to determine at what point help is needed and who should be consulted. “Sometimes, when a person is in acute stress, even looking up a phone number can seem insurmountable. But having it on paper lowers the barrier, making it more achievable.”
Resources should be posted in places where physicians gather so that those who don’t already have a safety plan have easy access to that information, he suggests.
In addition, consideration may be given to reaching out for support if a colleague has died by suicide, experts suggest. Whether offered by one’s institution, a peer arrangement, spiritual counseling, or psychotherapy, one may need help dealing with the trauma, guilt, and grief that often accompany this type of loss.
A version of this article first appeared on Medscape.com.
Antibiotics during pregnancy may increase child’s risk for asthma and other atopic diseases
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY
Roe v. Wade overturned, ending 50 years of abortion protections
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.