User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA approves topical tapinarof for plaque psoriasis
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
The the manufacturer announced.
Tapinarof is an aryl hydrocarbon receptor agonist and is the first FDA-approved steroid-free topical medication in this class, according to a press release from the manufacturer, Dermavant.
Approval was based on results of three studies in a phase 3 clinical trial program (PSOARING 1, PSOARING 2), and an open-label extension study, (PSOARING 3), the company release said. In PSOARING 1 and 2, approximately 1,000 adults aged 18-75 years (median age, 51 years) with plaque psoriasis were randomized to once-daily topical tapinarof or placebo for up to 12 weeks; 85% were White and 57% were men. The study findings were published in the New England Journal of Medicine in December 2021.
The primary endpoint for both trials was the proportion of patients who achieved Physician Global Assessment (PGA) scores score of “clear” (0) or “almost clear” (1) and improvement of at least two grades from baseline.
After 12 weeks, 36% of the patients in PSOARING 1 and 40% in PSOARING 2 who received tapinarof met the primary outcome, compared with 6% of patients on placebo (P < .001 for both studies). Of these, a total of 73 patients from both studies who achieved PGA scores of 0 were entered in PSOARING 3, a 40-week open-label extension study, in which they stopped tapinarof treatment and retained PGA scores of 0 or 1 for approximately 4 months off treatment. An additional 312 patients who were enrolled in the PSOARING 3 extension study achieved PGA scores of 0 at least once during the study period, with “remittive” effects lasting a mean of 130 days off of treatment.
In addition, patients who received tapinarof in the PSOARING 1 and 2 studies showed significant improvement from baseline, compared with patients on placebo, across a range of secondary endpoints including a 75% or greater improvement in Psoriasis Area and Severity Index score (PASI 75).
In PSOARING 1, and 2, respectively, 36.1% and 47.6% of those on tapinarof achieved a PASI 75 response at week 12, compared with 10.2% and 6.9% of those on the vehicle (P < .001 for both).
Across all three studies, the majority adverse events were mild to moderate, and limited to the application site.
The most common adverse events reported by patients in the tapinarof groups were folliculitis, nasopharyngitis, and contact dermatitis. Headaches were more common among those treated with tapinarof than those on vehicle in the studies (3.8% vs. 2.4% in PSOARING 1, and 3.8% vs. 0.6% in PSOARING 2), leading to only three treatment discontinuations.
At the end of the PSOARING 3 study (at either week 40 or early termination), 599 participants responded to satisfaction questionnaires. Of these, 83.6% said they were satisfied with the results of tapinarof treatment, and 81.7% said it was more effective than previous topical treatments they had used, according to the company’s release.
Tapinarof cream can be used on all areas of the body, including the face, skin folds, neck, genitalia, anal crux, inflammatory areas, and axillae, according to the company release.
Full prescribing information is available here.
Monkeypox quarantines not needed in U.S., Biden says
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
He said the United States has enough vaccine doses available to stop any serious outbreaks and to “deal with the likelihood of the problem,” according to The Washington Post .
“I just don’t think it rises to the level of the kind of concern that existed with COVID-19, and the smallpox vaccine works for it,” Biden said during a news conference in Japan.
The World Health Organization has identified monkeypox cases in at least a dozen countries where the disease isn’t typically considered endemic. Generally found in Central and West Africa, the illness has been reported in several European countries, as well as the United States, Canada, and Australia.
On Sunday, Biden told reporters that monkeypox is a “concern in that if it were to spread, it would be consequential.” Administration officials have said the president has been briefed on the disease, the newspaper reported.
Monkeypox spreads through droplets and bodily fluids but doesn’t pass easily between humans and is less contagious than the coronavirus, the Post reported. The CDC has reported that the smallpox vaccine is 85% effective against monkeypox, and the U.S. has licensed two smallpox vaccines that could help in potential outbreaks, including one that specifically targets monkeypox.
Mandatory monkeypox quarantine in Belgium
Belgium is the first country to put a mandatory 21-day quarantine in place for monkeypox patients as cases spread globally, according to CNBC. Health authorities announced the quarantine on Friday after the country recorded its third case.
The quarantine only applies to patients with a confirmed infection. Close contacts aren’t required to self-isolate but are encouraged to be careful and watch for symptoms, especially if they spend time with vulnerable people who could contract a serious illness, CNBC reported.
The United Kingdom has published guidelines to assess risks of monkeypox infection and provide guidance on self-isolation and monitoring. Health officials have said that those who have high exposure risks should self-isolate for 21 days, which includes household contacts or medical professionals who have worked with infected patients.
As of Saturday, the WHO has received reports of 92 confirmed monkeypox cases and 28 suspected cases across 12 countries where the virus isn’t typically found. No deaths linked to the cases have been reported so far.
The outbreaks have caused concern among health officials because most cases don’t have travel links to endemic countries. So far, many cases have spread between men who have sex with men, and the cases have been identified as patients seek care in primary care and sexual health clinics, the WHO reported.
“The identification of confirmed and suspected cases of monkeypox with no direct travel links to an endemic area represents a highly unusual event,” the WHO said. “Available information suggests that human-to-human transmission is occurring among people in close physical contact with cases who are symptomatic.”
The WHO said Saturday that more outbreaks will be reported as health officials uncover new information. The fast growth in community cases, especially in urban areas, suggests that a wider outbreak could be possible.
“To have it appear now – more than 100 cases in 12 different countries with no obvious connection – means we have to figure out exactly what’s happening,” Seth Berkley, MD, the CEO of global vaccine alliance Gavi, told CNBC.
“The truth is, we don’t know what that is and therefore how severe it’s going to be,” he said. “But it’s likely that we’re going to see more cases.”
White House health official doesn’t foresee major outbreak
Ashish Jha, MD, a top Biden administration health official who serves as the White House COVID-19 response coordinator, said Sunday that he doesn’t expect monkeypox to have widespread effects in the U.S.
“I feel like this is a virus we understand,” he said on ABC News’s This Week.
The virus has been monitored for decades, and there are treatments for it, Dr. Jha said.
“We have vaccines against it. We have treatments against it,” he said. “It’s not as contagious as COVID. So, I am confident we’re going to be able to keep our arms around it.”
At the same time, Dr. Jha agreed that health officials should keep an eye on the situation. Cases have been confirmed in recent days in several countries, as well as the United States.
“I would not be surprised if we see a few more cases in the upcoming days,” he said. “Any time we have an infectious outbreak like this, we should all be paying attention.”
Dr. Jha also stressed ongoing caution amid the COVID-19 pandemic as cases once again surpass 100,000 daily infections. Variants will continue to evolve, he said, and ongoing outbreaks will reinfect people who have been vaccinated or had a previous infection.
“What we know is that this virus is evolving very quickly, and every iteration of it has more and more immune escape,” he said. “That makes it harder for this virus to be contained unless we continue vaccinating people and keeping people up to date.”
Third possible U.S. monkeypox case found in Florida
The CDC said Sunday that it may have found a third monkeypox case in the United States and is running tests on a patient in South Florida, according to Reuters.
The person is in Broward County and remains isolated. The case appears to be related to international travel, the CDC told Reuters.
Health officials are doing tests to confirm if the patient has the disease, with results expected “soon.” No other cases have been identified in Florida so far.
The first monkeypox case in the United States was reported in Massachusetts last week. The patient had recently traveled to Canada.
The second U.S. case was reported in a New York City resident who tested positive on Friday.
The disease, which is like human smallpox but milder, is a viral infection that was first found in the Democratic Republic of Congo in the 1970s. Symptoms include fever, headaches, and a skin rash across the body.
A version of this article first appeared on WebMD.com.
How to manage drug interactions with Paxlovid for COVID-19
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Expect Ellacor’s applications to be wide-ranging, expert predicts
SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
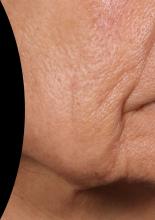
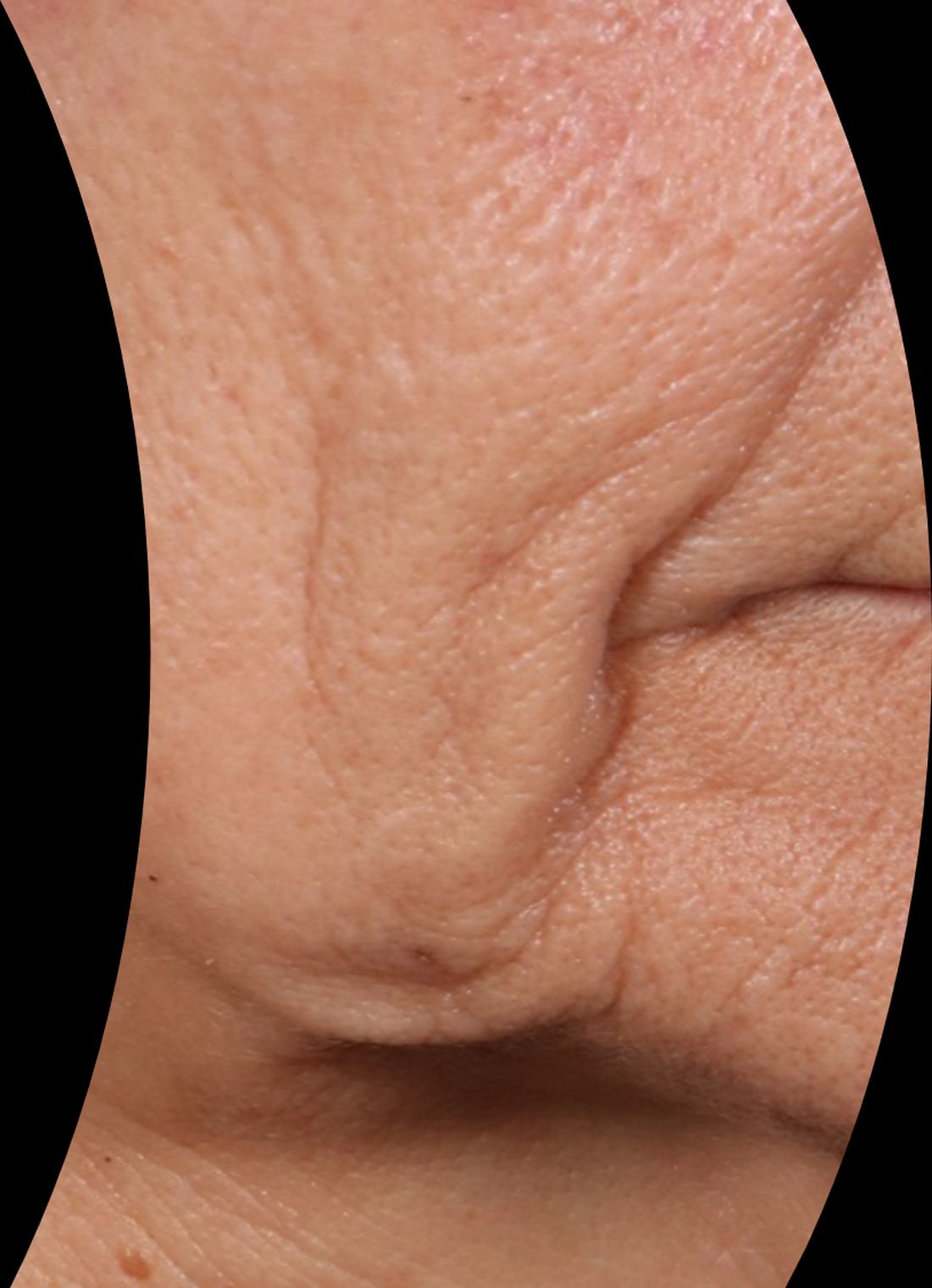
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.
SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.
SAN DIEGO – When the Food and Drug Administration gave the nod to a first-in-class tissue removal device in July of 2021, clearance was limited to the treatment of moderate to severe wrinkles in the mid to lower face.
Jill S. Waibel, MD, a dermatologist with the Miami Dermatology and Laser Institute, predicted at the annual conference of the American Society for Laser Medicine and Surgery. “I’m using it in my practice more for laxity and jowls,” she said. Eventually, “I think it’s going to be preventative for 30- to 50-year-olds but that hasn’t been studied. I think it’s going to have a pre- and postrhytidectomy role, so I think the plastic surgeons are going to love this in their practice.”
Developed by Cytrellis, and based on research conducted by William G. Austen Jr., MD, chief of plastic and reconstructive surgery at Massachusetts General Hospital (MGH), Boston, and R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH, the company’s scientific founders, the device uses hollow needles contained in a handpiece to create thousands of microexcisions to physically remove small cores of skin – a process known as microcoring. Dr. Austin and Dr. Anderson were the senior authors, respectively, of seminal trials of the device in swine, published in 2013 and 2015.
This can result in immediate physical hole closures (mechanical closure), which may lead to skin tightening.
“We’re removing dermis and epidermis,” said Dr. Waibel, who noted that the technology has been studied mostly for skin laxity and rhytids. “There are no other devices that are doing this.”
The immediate closure of tiny holes in the skin results in a quantitative and directional reduction in the treated area of skin, she said, which leads to wrinkle improvement, tightening, and smoothing of lax skin. The device contains three 22-guage needles that are less than 500 micrometers in diameter. “Based on optical coherence tomography work we did, these channels of treated skin stay open for about 1.5 minutes,” she added, noting that the tunable depth of the device ranges from 0 to 4 mm. “I tend to treat only with the 4-mm depth,” said Dr. Waibel, who is subsection chief of dermatology at Baptist Hospital of Miami.
The device features a disposable tip that can remove up to 24,000 cores of skin, and the amount of skin removed in a treated area can be 1%, 3%, 5%, 7%, and 8%. “The more cores you do, the more wrinkle improvement we saw in pivotal trials,” she said. “A minimal core count of 12,000 per treatment is recommended for the mid and lower face. Interestingly, higher core counts do not result in more patient downtime.” In her office, she said that the treatment takes about 20 minutes. Recommended postoperative care involves application of petrolatum or Aquaphor over the treated area for 24 hours, or until the holes have closed. “There is very low postoperative downtime,” she noted.
According to pivotal clinical data from Cytrellis on 51 patients treated with Ellacor, patients experienced a mean 1.3-grade improvement on the Lemperle Rating Scale, 86% said that they were satisfied with the procedure, and investigators rated their Global Aesthetic Improvement as 90%.
To date, Dr. Waibel and her colleagues in Miami have treated 102 patients with Ellacor, mostly for wrinkles and skin laxity. In these patients, the minimal downtime experienced was 3.8 days, 75% of patients did not miss any work, and 46% did not miss any social activities. The worst part for patients is the preprocedure numbing, she said. “We do lidocaine injections. Some people do nerve blocks. Once you do the lidocaine injection, the average pain is about 0.36 on a scale of 1-10 during treatment and 0 for all subsequent time points.”
At the meeting, she presented a set of before and after photos that showed improvement of moderate facial wrinkles in a female patient 90 days after one treatment with Ellacor, which removed about 5% of skin in the area of the jowls. “It’s pretty incredible,” Dr. Waibel remarked.
“I don’t have anything in my practice that can help with that kind of laxity other than sending them to a plastic surgeon, and I have about 80 devices.”
At the 2019 meeting of the American Society for Dermatologic Surgery, Dr. Waibel and Roy G. Geronemus, MD, director of the Laser & Skin Surgery Center of New York, presented a small pilot study on the successful use of Ellacor for acne scars and striae. Dr. Waibel said that she and her colleagues in Miami have been using the device to treat skin laxity in several anatomical areas, including the neck, nose, inner thigh, above the knee, elbow, and the axillary region. They have used the device to treat tattoos, rhytidectomy scars, abdominal striae, acne and surgical scars, and idiopathic guttate hypomelanosis, she added.
“We do a lot of combinations with other devices on the same day, and I think this list will increase over the next few years,” she said. “Probably my favorite use in the past 5 months has been doing microcoring and, separated by a month, doing resurfacing.”
Clinical trials of Ellacor were conducted in patients with types I-IV skin, but she has treated several patients with types V-VI skin “with absolutely no safety issues,” which includes treatment of epidermal nevi.
Which variables are the most important for patient selection and procedural success remain unclear, she continued, including patient age, elastic recoil, body mass index, history of a prior procedure (such as radiofrequency or ultrasound), the amount of laxity and rhytids, and overall health, which have not been studied, Dr. Waibel said.
“We have patients that don’t have the same response as others. For the modest improvement seen in some patients, is that their elastic recoil or are we choosing the wrong patients? Do they need more treatments? We are also still learning about the ideal treatment for scars and other indications.”
The device is expected to launch in fourth quarter of 2022.
Dr. Waibel disclosed that she is an advisory board member for Cytrellis. She has conducted clinical trials for and is a consultant to many pharmaceutical and device companies.
AT ASLMS 2022
20th anniversary and history of cosmetic botulinum toxin type A
The timeline of botulinum toxin discovery began with deadly outbreaks related to contaminated food across Europe in the late 1700s, the largest of which occurred in 1793 in Wildebrad, in southern Germany. In 1811, “prussic acid” was named as the culprit in what was referred to as sausage poisoning. Between 1817 and 1822, German physician Justinus Kerner noted that the active substance interrupted signals from motor nerves to muscles, but spared sensory and cognitive abilities, accurately describing botulism. He hypothesized that this substance could possibly be used as treatment for medical conditions when ingested orally. It wasn’t until 1895 that microbiologist Emile Pierre Van Ermengem, a professor of bacteriology in Belgium, identified the bacterium responsible as Bacillus botulinus, later renamed C. botulinum.
In 1905, it was discovered that C. botulinum produced a substance that affected neurotransmitter function, and between 1895 and 1915, seven toxin serotypes were recognized. In 1928, Herman Sommer, PhD, at the Hooper Foundation, at the University of California, San Francisco, isolated the most potent serotype: botulinum toxin type A (BoNT-A).
In 1946, Carl Lamanna and James Duff developed concentration and crystallization techniques that were subsequently used by Edward Schantz, PhD, at Fort Detrick, Md., for a possible biologic weapon. In 1972, Dr. Schantz took his research to the University of Wisconsin, where he produced a large batch of BoNT-A that remained in clinical use until December 1997.
In the late 1960s and early 1970s, an ophthalmologist in San Francisco, Alan Scott, MD, began animal studies with BoNT-A supplied by Dr. Schantz, as a possible treatment for strabismus, publishing his first report of BoNT-A in primates in 1973. In 1978, the Food and Drug Administration granted approval to begin testing small amounts of the toxin in human volunteers. In 1980, a landmark paper was published demonstrating that BoNT-A corrects gaze misalignment in humans. By 1989, it was approved as Oculinum by the FDA for the treatment of strabismus and blepharospasm.
Keen clinical observation and a serendipitous discovery led to botulinum toxin’s first uses for cosmetic purposes. In the mid-1980s, Jean Carruthers, MD, an ophthalmologist in Vancouver, noted an unexpected concomitant improvement of glabellar rhytids in a patient treated with BoNT for blepharospasm. The result of the treatment was a more serene, untroubled expression. Dr. Carruthers discussed the observation with her dermatologist spouse, Alastair Carruthers, MD, who was attempting to use soft tissue–augmenting agents available at the time to soften forehead wrinkles. Intrigued by the possibilities, the Carruthers subsequently injected a small amount of BoNT-A between the eyebrows of their assistant, Cathy Bickerton Swann, also now known as “patient zero” and awaited the results.
Seventeen additional patients followed, aged 34-51, who collectively, would become part of the first report on the efficacy of BoNT-A for cosmetic use – for the treatment of glabellar frown lines – published in 1992.
Between 1992 and 1997, the popularity of off-label use of BoNT-A grew so rapidly that Allergan’s supply temporarily ran out. By 2002, safety and efficacy profiles of use in medical conditions such as strabismus, blepharospasm, hemifacial spasm, cervical dystonia, cerebral palsy, poststroke spasticity, hyperhidrosis, headache, and back pain had been well-established, facilitating the comfort and use for cosmetic purposes.
By 2002, open-label studies of more than 800 patients confirmed the efficacy and safety of BoNT for the treatment of dynamic facial rhytids. And in April 2002, the FDA granted approval of BoNT for the nonsurgical reduction of glabellar rhytids. The rest, some would say, is history. On this 20th-year anniversary of the approval of botulinum toxin for cosmetic use, special recognition is given here for the physicians and scientists who were astute enough to make this discovery, as botulinum toxin use remains one of the most popular and effective nonsurgical cosmetic procedures today.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. Dr. Wesley disclosed that she has been a clinical investigator and consultant for Botox manufacturer Allergan in the past, and manufacturers of other brands of botulinum toxins available for cosmetic use; Dysport (Galderma), Xeomin (Merz), and Jeuveau (Evolus). Dr. Talakoub had no disclosures.
Reference
“Botulinum Toxin: Procedures in Cosmetic Dermatology Series 3rd Edition” (Philadelphia: Saunders Elsevier, 2013)
The timeline of botulinum toxin discovery began with deadly outbreaks related to contaminated food across Europe in the late 1700s, the largest of which occurred in 1793 in Wildebrad, in southern Germany. In 1811, “prussic acid” was named as the culprit in what was referred to as sausage poisoning. Between 1817 and 1822, German physician Justinus Kerner noted that the active substance interrupted signals from motor nerves to muscles, but spared sensory and cognitive abilities, accurately describing botulism. He hypothesized that this substance could possibly be used as treatment for medical conditions when ingested orally. It wasn’t until 1895 that microbiologist Emile Pierre Van Ermengem, a professor of bacteriology in Belgium, identified the bacterium responsible as Bacillus botulinus, later renamed C. botulinum.
In 1905, it was discovered that C. botulinum produced a substance that affected neurotransmitter function, and between 1895 and 1915, seven toxin serotypes were recognized. In 1928, Herman Sommer, PhD, at the Hooper Foundation, at the University of California, San Francisco, isolated the most potent serotype: botulinum toxin type A (BoNT-A).
In 1946, Carl Lamanna and James Duff developed concentration and crystallization techniques that were subsequently used by Edward Schantz, PhD, at Fort Detrick, Md., for a possible biologic weapon. In 1972, Dr. Schantz took his research to the University of Wisconsin, where he produced a large batch of BoNT-A that remained in clinical use until December 1997.
In the late 1960s and early 1970s, an ophthalmologist in San Francisco, Alan Scott, MD, began animal studies with BoNT-A supplied by Dr. Schantz, as a possible treatment for strabismus, publishing his first report of BoNT-A in primates in 1973. In 1978, the Food and Drug Administration granted approval to begin testing small amounts of the toxin in human volunteers. In 1980, a landmark paper was published demonstrating that BoNT-A corrects gaze misalignment in humans. By 1989, it was approved as Oculinum by the FDA for the treatment of strabismus and blepharospasm.
Keen clinical observation and a serendipitous discovery led to botulinum toxin’s first uses for cosmetic purposes. In the mid-1980s, Jean Carruthers, MD, an ophthalmologist in Vancouver, noted an unexpected concomitant improvement of glabellar rhytids in a patient treated with BoNT for blepharospasm. The result of the treatment was a more serene, untroubled expression. Dr. Carruthers discussed the observation with her dermatologist spouse, Alastair Carruthers, MD, who was attempting to use soft tissue–augmenting agents available at the time to soften forehead wrinkles. Intrigued by the possibilities, the Carruthers subsequently injected a small amount of BoNT-A between the eyebrows of their assistant, Cathy Bickerton Swann, also now known as “patient zero” and awaited the results.
Seventeen additional patients followed, aged 34-51, who collectively, would become part of the first report on the efficacy of BoNT-A for cosmetic use – for the treatment of glabellar frown lines – published in 1992.
Between 1992 and 1997, the popularity of off-label use of BoNT-A grew so rapidly that Allergan’s supply temporarily ran out. By 2002, safety and efficacy profiles of use in medical conditions such as strabismus, blepharospasm, hemifacial spasm, cervical dystonia, cerebral palsy, poststroke spasticity, hyperhidrosis, headache, and back pain had been well-established, facilitating the comfort and use for cosmetic purposes.
By 2002, open-label studies of more than 800 patients confirmed the efficacy and safety of BoNT for the treatment of dynamic facial rhytids. And in April 2002, the FDA granted approval of BoNT for the nonsurgical reduction of glabellar rhytids. The rest, some would say, is history. On this 20th-year anniversary of the approval of botulinum toxin for cosmetic use, special recognition is given here for the physicians and scientists who were astute enough to make this discovery, as botulinum toxin use remains one of the most popular and effective nonsurgical cosmetic procedures today.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. Dr. Wesley disclosed that she has been a clinical investigator and consultant for Botox manufacturer Allergan in the past, and manufacturers of other brands of botulinum toxins available for cosmetic use; Dysport (Galderma), Xeomin (Merz), and Jeuveau (Evolus). Dr. Talakoub had no disclosures.
Reference
“Botulinum Toxin: Procedures in Cosmetic Dermatology Series 3rd Edition” (Philadelphia: Saunders Elsevier, 2013)
The timeline of botulinum toxin discovery began with deadly outbreaks related to contaminated food across Europe in the late 1700s, the largest of which occurred in 1793 in Wildebrad, in southern Germany. In 1811, “prussic acid” was named as the culprit in what was referred to as sausage poisoning. Between 1817 and 1822, German physician Justinus Kerner noted that the active substance interrupted signals from motor nerves to muscles, but spared sensory and cognitive abilities, accurately describing botulism. He hypothesized that this substance could possibly be used as treatment for medical conditions when ingested orally. It wasn’t until 1895 that microbiologist Emile Pierre Van Ermengem, a professor of bacteriology in Belgium, identified the bacterium responsible as Bacillus botulinus, later renamed C. botulinum.
In 1905, it was discovered that C. botulinum produced a substance that affected neurotransmitter function, and between 1895 and 1915, seven toxin serotypes were recognized. In 1928, Herman Sommer, PhD, at the Hooper Foundation, at the University of California, San Francisco, isolated the most potent serotype: botulinum toxin type A (BoNT-A).
In 1946, Carl Lamanna and James Duff developed concentration and crystallization techniques that were subsequently used by Edward Schantz, PhD, at Fort Detrick, Md., for a possible biologic weapon. In 1972, Dr. Schantz took his research to the University of Wisconsin, where he produced a large batch of BoNT-A that remained in clinical use until December 1997.
In the late 1960s and early 1970s, an ophthalmologist in San Francisco, Alan Scott, MD, began animal studies with BoNT-A supplied by Dr. Schantz, as a possible treatment for strabismus, publishing his first report of BoNT-A in primates in 1973. In 1978, the Food and Drug Administration granted approval to begin testing small amounts of the toxin in human volunteers. In 1980, a landmark paper was published demonstrating that BoNT-A corrects gaze misalignment in humans. By 1989, it was approved as Oculinum by the FDA for the treatment of strabismus and blepharospasm.
Keen clinical observation and a serendipitous discovery led to botulinum toxin’s first uses for cosmetic purposes. In the mid-1980s, Jean Carruthers, MD, an ophthalmologist in Vancouver, noted an unexpected concomitant improvement of glabellar rhytids in a patient treated with BoNT for blepharospasm. The result of the treatment was a more serene, untroubled expression. Dr. Carruthers discussed the observation with her dermatologist spouse, Alastair Carruthers, MD, who was attempting to use soft tissue–augmenting agents available at the time to soften forehead wrinkles. Intrigued by the possibilities, the Carruthers subsequently injected a small amount of BoNT-A between the eyebrows of their assistant, Cathy Bickerton Swann, also now known as “patient zero” and awaited the results.
Seventeen additional patients followed, aged 34-51, who collectively, would become part of the first report on the efficacy of BoNT-A for cosmetic use – for the treatment of glabellar frown lines – published in 1992.
Between 1992 and 1997, the popularity of off-label use of BoNT-A grew so rapidly that Allergan’s supply temporarily ran out. By 2002, safety and efficacy profiles of use in medical conditions such as strabismus, blepharospasm, hemifacial spasm, cervical dystonia, cerebral palsy, poststroke spasticity, hyperhidrosis, headache, and back pain had been well-established, facilitating the comfort and use for cosmetic purposes.
By 2002, open-label studies of more than 800 patients confirmed the efficacy and safety of BoNT for the treatment of dynamic facial rhytids. And in April 2002, the FDA granted approval of BoNT for the nonsurgical reduction of glabellar rhytids. The rest, some would say, is history. On this 20th-year anniversary of the approval of botulinum toxin for cosmetic use, special recognition is given here for the physicians and scientists who were astute enough to make this discovery, as botulinum toxin use remains one of the most popular and effective nonsurgical cosmetic procedures today.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. Dr. Wesley disclosed that she has been a clinical investigator and consultant for Botox manufacturer Allergan in the past, and manufacturers of other brands of botulinum toxins available for cosmetic use; Dysport (Galderma), Xeomin (Merz), and Jeuveau (Evolus). Dr. Talakoub had no disclosures.
Reference
“Botulinum Toxin: Procedures in Cosmetic Dermatology Series 3rd Edition” (Philadelphia: Saunders Elsevier, 2013)
European committee recommends approval of baricitinib for severe alopecia areata
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
The European Medicines Agency’s ( (AA).
The development, which was announced in a May 20, 2022, press release from the manufacturer, Eli Lilly and Incyte, marks the first step toward European regulatory approval of baricitinib (Olumiant) for patients with severe AA, and it is now referred to the European Commission for final action. A decision is expected within the next 2 months.
The committee based its positive opinion on the results of the phase 3 BRAVE-AA1 and BRAVE-AA2 trials, recently published in the New England Journal of Medicine, which evaluated the efficacy and safety of baricitinib in 1,200 patients with severe AA, according to the press release. The primary endpoint was the proportion of patients achieving a Severity of Alopecia Tool (SALT) score of ≤20 at week 36. In both studies, 1 out of 3 patients treated with baricitinib 4-mg achieved 80% or more scalp hair coverage, compared with 1 out of 20 patients and 1 out of 50 patients taking placebo in BRAVE-AA1 and BRAVE-AA2, respectively (P ≤ .001 for all comparisons to placebo).
According to safety profile information from the phase 3 BRAVE-AA clinical program, few patients discontinued treatment because of adverse events (2.6% or less across both studies), and most treatment-emergent adverse events were mild or moderate in severity.
In February 2022, the Food and Drug Administration granted priority review for baricitinib in adults with severe AA. Lilly expects additional regulatory decisions in the United States and Japan in 2022.
Baricitinib is approved in the United States as a treatment for adults with moderate to severe rheumatoid arthritis. Prescribing information can be viewed here.
Climate change, medical education, and dermatology
The recent article on including the impact of climate on health in medical education programs shines an important light on the challenge – and urgent need – of integrating climate change training into medical education. These nascent efforts are just getting underway across the country, with some programs – notably Harvard’s C-CHANGE (Center for Climate, Health, and the Global Environment) program, mentioned in the article, and others, such as the University of Colorado’s Climate Medicine diploma course – leading the way. A number of publications, such as the editorial titled “A planetary health curriculum for medicine” published in 2021 in the BMJ, offer a roadmap to do so.
Medical schools, residency programs, and other medical specialty programs – including those for advanced practice providers, dentists, nurses, and more – should be incorporating climate change and its myriad of health impacts into their training pathways. The medical student group, Medical Students for a Sustainable Future, has put forth a planetary health report card that evaluates training programs on the strength of their focus on the intersections between climate and health.
While the article did not specifically focus on dermatology, these impacts are true in our field as well. The article notes that “at least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change.” Notably in dermatology, the International Journal of Women’s Dermatology devoted an entire 124-page themed issue to climate change and dermatology in January, 2021, while JAMA Dermatology editor Kanade Shinkai, MD, PhD, called out climate change as one of the journal’s priorities in her annual editorial, stating, “Another priority for the journal is to better understand the effect of climate change on human health, specifically skin disease.”
The impacts of climate change in dermatology range from heat-related illness (a major cause of climate-associated mortality, with the skin serving as an essential thermoregulatory organ) to changing patterns of vector-borne illnesses to pollution and wildfire smoke flaring inflammatory skin diseases, to an increase in skin cancer, and more. While incorporation of health issues relating to climate change is important at a medical school level, it is also critical at the residency training – and board exam/certification – level as well.
Beyond the importance of building climate education into undergraduate and graduate medical education, it is also important that practicing physicians, post-residency training, remain up to date and keep abreast of changing patterns of disease in our rapidly changing climate. Lyme disease now occurs in Canada – and both earlier and later in the year even in places that are geographically used to seeing it. Early recognition is essential, but unprepared physicians may miss the early erythema migrans rash, and patients may suffer more severe sequelae as a result.
Finally, it’s important that medical organizations are aware of not just the health implications of climate change, but also potential policy impacts. Health care is a major emitter of CO2, and assistant secretary for health for the U.S. Department of Health and Human Services, Admiral Rachel L. Levine, MD, with the National Academy of Medicine, has appropriately pledged to reduce health care carbon emissions as part of the necessary steps that we must all take to avert the worst impacts of a warming world. The field of medicine and individual providers should educate themselves and actively work toward sustainability in health care, to improve the health of their patients, populations, and future generations.
Dr. Rosenbach is associate professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, and is the founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues. Dr. Rosenbach is speaking on behalf of himself and not the AAD.
The recent article on including the impact of climate on health in medical education programs shines an important light on the challenge – and urgent need – of integrating climate change training into medical education. These nascent efforts are just getting underway across the country, with some programs – notably Harvard’s C-CHANGE (Center for Climate, Health, and the Global Environment) program, mentioned in the article, and others, such as the University of Colorado’s Climate Medicine diploma course – leading the way. A number of publications, such as the editorial titled “A planetary health curriculum for medicine” published in 2021 in the BMJ, offer a roadmap to do so.
Medical schools, residency programs, and other medical specialty programs – including those for advanced practice providers, dentists, nurses, and more – should be incorporating climate change and its myriad of health impacts into their training pathways. The medical student group, Medical Students for a Sustainable Future, has put forth a planetary health report card that evaluates training programs on the strength of their focus on the intersections between climate and health.
While the article did not specifically focus on dermatology, these impacts are true in our field as well. The article notes that “at least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change.” Notably in dermatology, the International Journal of Women’s Dermatology devoted an entire 124-page themed issue to climate change and dermatology in January, 2021, while JAMA Dermatology editor Kanade Shinkai, MD, PhD, called out climate change as one of the journal’s priorities in her annual editorial, stating, “Another priority for the journal is to better understand the effect of climate change on human health, specifically skin disease.”
The impacts of climate change in dermatology range from heat-related illness (a major cause of climate-associated mortality, with the skin serving as an essential thermoregulatory organ) to changing patterns of vector-borne illnesses to pollution and wildfire smoke flaring inflammatory skin diseases, to an increase in skin cancer, and more. While incorporation of health issues relating to climate change is important at a medical school level, it is also critical at the residency training – and board exam/certification – level as well.
Beyond the importance of building climate education into undergraduate and graduate medical education, it is also important that practicing physicians, post-residency training, remain up to date and keep abreast of changing patterns of disease in our rapidly changing climate. Lyme disease now occurs in Canada – and both earlier and later in the year even in places that are geographically used to seeing it. Early recognition is essential, but unprepared physicians may miss the early erythema migrans rash, and patients may suffer more severe sequelae as a result.
Finally, it’s important that medical organizations are aware of not just the health implications of climate change, but also potential policy impacts. Health care is a major emitter of CO2, and assistant secretary for health for the U.S. Department of Health and Human Services, Admiral Rachel L. Levine, MD, with the National Academy of Medicine, has appropriately pledged to reduce health care carbon emissions as part of the necessary steps that we must all take to avert the worst impacts of a warming world. The field of medicine and individual providers should educate themselves and actively work toward sustainability in health care, to improve the health of their patients, populations, and future generations.
Dr. Rosenbach is associate professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, and is the founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues. Dr. Rosenbach is speaking on behalf of himself and not the AAD.
The recent article on including the impact of climate on health in medical education programs shines an important light on the challenge – and urgent need – of integrating climate change training into medical education. These nascent efforts are just getting underway across the country, with some programs – notably Harvard’s C-CHANGE (Center for Climate, Health, and the Global Environment) program, mentioned in the article, and others, such as the University of Colorado’s Climate Medicine diploma course – leading the way. A number of publications, such as the editorial titled “A planetary health curriculum for medicine” published in 2021 in the BMJ, offer a roadmap to do so.
Medical schools, residency programs, and other medical specialty programs – including those for advanced practice providers, dentists, nurses, and more – should be incorporating climate change and its myriad of health impacts into their training pathways. The medical student group, Medical Students for a Sustainable Future, has put forth a planetary health report card that evaluates training programs on the strength of their focus on the intersections between climate and health.
While the article did not specifically focus on dermatology, these impacts are true in our field as well. The article notes that “at least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change.” Notably in dermatology, the International Journal of Women’s Dermatology devoted an entire 124-page themed issue to climate change and dermatology in January, 2021, while JAMA Dermatology editor Kanade Shinkai, MD, PhD, called out climate change as one of the journal’s priorities in her annual editorial, stating, “Another priority for the journal is to better understand the effect of climate change on human health, specifically skin disease.”
The impacts of climate change in dermatology range from heat-related illness (a major cause of climate-associated mortality, with the skin serving as an essential thermoregulatory organ) to changing patterns of vector-borne illnesses to pollution and wildfire smoke flaring inflammatory skin diseases, to an increase in skin cancer, and more. While incorporation of health issues relating to climate change is important at a medical school level, it is also critical at the residency training – and board exam/certification – level as well.
Beyond the importance of building climate education into undergraduate and graduate medical education, it is also important that practicing physicians, post-residency training, remain up to date and keep abreast of changing patterns of disease in our rapidly changing climate. Lyme disease now occurs in Canada – and both earlier and later in the year even in places that are geographically used to seeing it. Early recognition is essential, but unprepared physicians may miss the early erythema migrans rash, and patients may suffer more severe sequelae as a result.
Finally, it’s important that medical organizations are aware of not just the health implications of climate change, but also potential policy impacts. Health care is a major emitter of CO2, and assistant secretary for health for the U.S. Department of Health and Human Services, Admiral Rachel L. Levine, MD, with the National Academy of Medicine, has appropriately pledged to reduce health care carbon emissions as part of the necessary steps that we must all take to avert the worst impacts of a warming world. The field of medicine and individual providers should educate themselves and actively work toward sustainability in health care, to improve the health of their patients, populations, and future generations.
Dr. Rosenbach is associate professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, and is the founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues. Dr. Rosenbach is speaking on behalf of himself and not the AAD.
Rabies: CDC updates and simplifies preexposure prophylaxis vaccination recommendations
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Each year, there are about 59,000 deaths from rabies globally. Most of these occur outside the United States and are the result of dog bites. Since infection with rabies is almost always fatal, there has been considerable attention given to vaccinating people at high risk before likely exposure and responding immediately to those bitten by a rabid animal.
The Centers for Disease Control and Prevention recently revised its preexposure prophylaxis (PrEP) recommendations for rabies. Under the previous 2008 guidelines, PrEP injections were given on days 0, 7, and 21 and cost more than $1,100.
The first two groups are those with very high risk of occupational exposures – either working with rabies virus in the laboratory or working with or having contact with bats or performing animal necropsies. They are now advised to get two doses of rabies vaccine on days 0 and 7. The lab workers should have titers checked every 6 months to ensure that they remain adequately protected. And a booster should be given if the titer drops to < 0.5 IU/mL. The second group, with bat exposures, should have titers checked every 2 years.
Risk category 3 is those with long-term (> 3 years) exposure to mammals other than bats that might be rabid. This group would include veterinarians, wildlife biologists, animal control officers, and spelunkers (cavers). Category 3 also includes travelers who may encounter rabid dogs, which is not a risk in the United States. They would get the same initial two doses. The new recommendations for a third dose are based either on a titer drawn 1-3 years later being < 0.5 IU/mL or choosing to give a booster between 3 weeks and 3 years after the second dose.
The same groups are covered in risk group 4, but these are expected to have less than 3 years of potential exposure after PrEP. They would receive two doses on days 0 and 7.
Finally, group 5, at the lowest risk, includes most of the U.S. population. They do not require any PrEP.
Agam Rao, MD, CAPT, U.S. Public Health Service, CDC, told this news organization that the CDC’s Advisory Committee on Immunization Practices (ACIP) has been working on updating the 2008 rabies PrEP recommendations for several years. The committee wanted the new guideline to be “as easily followable as possible but also based on the evidence itself.”
There were two significant problems the committee tried to address. “One was that travelers who book their travel on kind of short notice don’t have enough time to get that third dose, which at the earliest can be given on day 21,” Dr. Rao said.
The second problem is that “a three-dose series [is] just really expensive. And what we found from data that had been published since the last ACIP recommendations is that fewer people than we recommend get vaccinated were getting vaccinated. So hopefully, the two-dose series helps with that.”
The ACIP used an adapted Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to determine the certainty of the evidence for immunogenicity. The ACIP also used an evidence to recommendations (EtR) framework. “This incorporates a lot of other factors like the acceptability, usability, equity, all of these other variables that are important to the evidence being translated into recommendations,” Dr. Rao said. A table details their analysis.
Rabies expert Thiravat Hemachudha, MD, professor of neurology at WHO Collaborating Centre for Research and Training on Viral Zoonoses, Chulalongkorn University Hospital, Bangkok, told this news organization via email that “the ACIP relies mostly on serology, whereas the rest of the world cannot afford the test or testing may not be available.”
He added: “The issue of ‘long-term immunogenicity’ after receiving [PrEP is] an anamnestic response. All standard tissue culture rabies vaccines with appropriate dosage and route of delivery, either IM or ID, are considered safe and effective. There are many studies in Asian countries confirming that with only one primary series of PrEP, ID or IM with reduced doses, can produce immunity for as long as 20 years. Therefore, serology check is not necessary in general populations in rabies endemic countries where most of the rabies deaths occur. Investigation of all death cases was performed in Thailand and did not reveal any failure. Cases with PrEP in the past who died did not receive a booster after exposure.”
Dr. Rao offered one additional suggestion to clinicians faced with an urgent need to get a rabies titer: “They really should reach out to the lab (with all the information) before they send the specimen for the titer check ... so that the testing can be facilitated. All of these laboratories have the capacity to do stat and ASAP testing ... Clinicians do not know that they can call laboratories directly and expedite this sort of testing.”
Dr. Rao emphasized that PrEP does not eliminate the need for postexposure prophylaxis (PEP). Still, it eliminates the need for rabies immunoglobulin and decreases the number of vaccine doses required for PEP. “I hope more people will take advantage of the titer checks and potentially save the patient some money,” she concluded.
Dr. Rao and Dr. Hemachudha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pancreatic involvement in COVID-19: What do we know?
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
Doc faces U.S. federal charges for hacking, ransomware
According to a statement from the U.S. Department of Justice, 55-year-old Moises Luis Zagala Gonzalez, MD, is charged with creating and distributing ransomware with a “doomsday” clock and sharing in profits from ransomware attacks.
Dr. Zagala, also known as “Nosophoros,” “Aesculapius,” and “Nebuchadnezzar,” is a citizen of France and Venezuela who currently lives in Ciudad Bolivar, Venezuela.
Breon Peace, U.S. attorney for the Eastern District of New York, and Michael J. Driscoll, assistant director in charge of the Federal Bureau of Investigaton’s New York Field Office, announced the charges.
“As alleged, the multitasking doctor treated patients, created and named his cyber tool after death, profited from a global ransomware ecosystem in which he sold the tools for conducting ransomware attacks, trained the attackers about how to extort victims, and then boasted about successful attacks, including by malicious actors associated with the government of Iran,” Mr. Peace said in the news release from the DOJ.
“We allege Zagala not only created and sold ransomware products to hackers, but also trained them in their use. Our actions today will prevent Zagala from further victimizing users,” Mr. Driscoll said. “However, many other malicious criminals are searching for businesses and organizations that haven’t taken steps to protect their systems – which is an incredibly vital step in stopping the next ransomware attack.”
Ransomware tools are malicious software that cybercriminals use to extort money from companies, nonprofits, and other institutions by encrypting their files and then demanding a ransom for the decryption keys.
One of Dr. Zagala’s early ransomware tools, called “Jigsaw v. 2,” had what Dr. Zagala described as a doomsday counter that kept track of how many times the user tried to remove the ransomware. “If the user kills the ransomware too many times, then it’s clear he won’t pay so better erase the whole hard drive,” Dr. Zagala wrote.
According to the DOJ, beginning in late 2019, Dr. Zagala began advertising a new tool as a “private ransomware builder,” which he called Thanos. The name appears to be in reference to a fictional villain responsible for destroying half of all life in the universe and to “Thanatos” from Greek mythology, who is associated with death.
Dr. Zagala’s Thanos software allows users to create their own unique ransomware software for personal use or to rent to other cybercriminals.
Dr. Zagala allegedly not only sold or rented out his ransomware tools to cybercriminals, but he also taught users how to deploy the tools, steal passwords from victim computers, and set up a Bitcoin address for ransom payments.
Dr. Zagala’s customers were happy with his products, the DOJ release noted. In a message posted in July 2020, one user said the ransomware was “very powerful” and claimed that he had used it to infect a network of roughly 3,000 computers.
In December 2020, another user wrote a post in Russian: “We have been working with this product for over a month now, we have a good profit! Best support I’ve met.”
Earlier in May, law enforcement agents interviewed a relative of Dr. Zagala who lives in Florida and whose PayPal account was used by Dr. Zagala to receive illicit proceeds.
According to the DOJ, the relative confirmed that Dr. Zagala lives in Venezuela and had taught himself computer programming. The relative also showed agents contact information for Dr. Zagala that matched the registered email for malicious infrastructure associated with the Thanos ransomware.
Dr. Zagala, who remains in Venezuela, faces up to 10 years in prison for attempted computer intrusions and conspiracy charges if brought to justice in the United States.
A version of this article first appeared on Medscape.com.
According to a statement from the U.S. Department of Justice, 55-year-old Moises Luis Zagala Gonzalez, MD, is charged with creating and distributing ransomware with a “doomsday” clock and sharing in profits from ransomware attacks.
Dr. Zagala, also known as “Nosophoros,” “Aesculapius,” and “Nebuchadnezzar,” is a citizen of France and Venezuela who currently lives in Ciudad Bolivar, Venezuela.
Breon Peace, U.S. attorney for the Eastern District of New York, and Michael J. Driscoll, assistant director in charge of the Federal Bureau of Investigaton’s New York Field Office, announced the charges.
“As alleged, the multitasking doctor treated patients, created and named his cyber tool after death, profited from a global ransomware ecosystem in which he sold the tools for conducting ransomware attacks, trained the attackers about how to extort victims, and then boasted about successful attacks, including by malicious actors associated with the government of Iran,” Mr. Peace said in the news release from the DOJ.
“We allege Zagala not only created and sold ransomware products to hackers, but also trained them in their use. Our actions today will prevent Zagala from further victimizing users,” Mr. Driscoll said. “However, many other malicious criminals are searching for businesses and organizations that haven’t taken steps to protect their systems – which is an incredibly vital step in stopping the next ransomware attack.”
Ransomware tools are malicious software that cybercriminals use to extort money from companies, nonprofits, and other institutions by encrypting their files and then demanding a ransom for the decryption keys.
One of Dr. Zagala’s early ransomware tools, called “Jigsaw v. 2,” had what Dr. Zagala described as a doomsday counter that kept track of how many times the user tried to remove the ransomware. “If the user kills the ransomware too many times, then it’s clear he won’t pay so better erase the whole hard drive,” Dr. Zagala wrote.
According to the DOJ, beginning in late 2019, Dr. Zagala began advertising a new tool as a “private ransomware builder,” which he called Thanos. The name appears to be in reference to a fictional villain responsible for destroying half of all life in the universe and to “Thanatos” from Greek mythology, who is associated with death.
Dr. Zagala’s Thanos software allows users to create their own unique ransomware software for personal use or to rent to other cybercriminals.
Dr. Zagala allegedly not only sold or rented out his ransomware tools to cybercriminals, but he also taught users how to deploy the tools, steal passwords from victim computers, and set up a Bitcoin address for ransom payments.
Dr. Zagala’s customers were happy with his products, the DOJ release noted. In a message posted in July 2020, one user said the ransomware was “very powerful” and claimed that he had used it to infect a network of roughly 3,000 computers.
In December 2020, another user wrote a post in Russian: “We have been working with this product for over a month now, we have a good profit! Best support I’ve met.”
Earlier in May, law enforcement agents interviewed a relative of Dr. Zagala who lives in Florida and whose PayPal account was used by Dr. Zagala to receive illicit proceeds.
According to the DOJ, the relative confirmed that Dr. Zagala lives in Venezuela and had taught himself computer programming. The relative also showed agents contact information for Dr. Zagala that matched the registered email for malicious infrastructure associated with the Thanos ransomware.
Dr. Zagala, who remains in Venezuela, faces up to 10 years in prison for attempted computer intrusions and conspiracy charges if brought to justice in the United States.
A version of this article first appeared on Medscape.com.
According to a statement from the U.S. Department of Justice, 55-year-old Moises Luis Zagala Gonzalez, MD, is charged with creating and distributing ransomware with a “doomsday” clock and sharing in profits from ransomware attacks.
Dr. Zagala, also known as “Nosophoros,” “Aesculapius,” and “Nebuchadnezzar,” is a citizen of France and Venezuela who currently lives in Ciudad Bolivar, Venezuela.
Breon Peace, U.S. attorney for the Eastern District of New York, and Michael J. Driscoll, assistant director in charge of the Federal Bureau of Investigaton’s New York Field Office, announced the charges.
“As alleged, the multitasking doctor treated patients, created and named his cyber tool after death, profited from a global ransomware ecosystem in which he sold the tools for conducting ransomware attacks, trained the attackers about how to extort victims, and then boasted about successful attacks, including by malicious actors associated with the government of Iran,” Mr. Peace said in the news release from the DOJ.
“We allege Zagala not only created and sold ransomware products to hackers, but also trained them in their use. Our actions today will prevent Zagala from further victimizing users,” Mr. Driscoll said. “However, many other malicious criminals are searching for businesses and organizations that haven’t taken steps to protect their systems – which is an incredibly vital step in stopping the next ransomware attack.”
Ransomware tools are malicious software that cybercriminals use to extort money from companies, nonprofits, and other institutions by encrypting their files and then demanding a ransom for the decryption keys.
One of Dr. Zagala’s early ransomware tools, called “Jigsaw v. 2,” had what Dr. Zagala described as a doomsday counter that kept track of how many times the user tried to remove the ransomware. “If the user kills the ransomware too many times, then it’s clear he won’t pay so better erase the whole hard drive,” Dr. Zagala wrote.
According to the DOJ, beginning in late 2019, Dr. Zagala began advertising a new tool as a “private ransomware builder,” which he called Thanos. The name appears to be in reference to a fictional villain responsible for destroying half of all life in the universe and to “Thanatos” from Greek mythology, who is associated with death.
Dr. Zagala’s Thanos software allows users to create their own unique ransomware software for personal use or to rent to other cybercriminals.
Dr. Zagala allegedly not only sold or rented out his ransomware tools to cybercriminals, but he also taught users how to deploy the tools, steal passwords from victim computers, and set up a Bitcoin address for ransom payments.
Dr. Zagala’s customers were happy with his products, the DOJ release noted. In a message posted in July 2020, one user said the ransomware was “very powerful” and claimed that he had used it to infect a network of roughly 3,000 computers.
In December 2020, another user wrote a post in Russian: “We have been working with this product for over a month now, we have a good profit! Best support I’ve met.”
Earlier in May, law enforcement agents interviewed a relative of Dr. Zagala who lives in Florida and whose PayPal account was used by Dr. Zagala to receive illicit proceeds.
According to the DOJ, the relative confirmed that Dr. Zagala lives in Venezuela and had taught himself computer programming. The relative also showed agents contact information for Dr. Zagala that matched the registered email for malicious infrastructure associated with the Thanos ransomware.
Dr. Zagala, who remains in Venezuela, faces up to 10 years in prison for attempted computer intrusions and conspiracy charges if brought to justice in the United States.
A version of this article first appeared on Medscape.com.