User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Crohn’s disease research goes to the dogs
Why it might be better to be a dog person
Here’s that old debate again: Dogs or cats? You probably have your own opinion, but research presented at this year’s Digestive Disease Week may have tipped the scale by showing that children who lived with dogs may be less likely to have Crohn’s disease as adults.
The research was done by having approximately 4,300 people closely related to patients with Crohn’s disease fill out an environmental questionnaire. Using these data, the research team looked into environmental factors such as size of the families, where the home was, how many bathrooms the homes had, and quality of drinking water.
The researchers found that those who had or were exposed to dogs between the ages of 5 and 15 years were more likely to have healthy gut permeability and balanced microbes, which increased their protection against Crohn’s disease.
“Our study seems to add to others that have explored the ‘hygiene hypothesis’ which suggests that the lack of exposure to microbes early in life may lead to lack of immune regulation toward environmental microbes,” senior author Williams Turpin, PhD, said in the written statement.
The researchers aren’t sure why they didn’t get the same findings with cats, but Dr. Turpin theorized that dog owners tend to be outside more with their dogs or live in places with more green space, which are good protectors against Crohn’s disease.
It’s all good for dog owners, but do their pets’ parasites make you more attractive? Just more fuel for the ongoing debate.
Come for the history, stay for the fossilized parasites
Another week, another analysis of old British poop. LOTME really is your one-stop shop for all the important, hard-hitting news about historic parasites. You’re welcome, Internet.
The news this week is from Stonehenge, which is apparently kind of a big deal. Rocks in a circle, celestial calendar, cultural significance, whatever. We’re not here to talk about rocks. We’re here to talk about, uh, rocks. Smaller rocks. Specifically, coprolites, which are essentially poop turned into a rock. (Though now we’re imagining Stonehenge made out of fossilized poop rocks. Would it still be a big tourist destination? We can see both sides of the argument on that one.)
Archaeologists from the University of Cambridge have conducted an analysis of coprolites from Durrington Walls, a Neolithic settlement just a few kilometers from Stonehenge. The town dates to the same time that Stonehenge was constructed, and it’s believed that the residents were responsible for building the landmark. These coprolites, depending on what’s inside, can tell us a lot about how the builders of Stonehenge lived and, more specifically, how they ate.
In this case, the coprolites of one human and three dogs contained capillariid worm eggs. These worms come from cows, and when a human is typically infected, the eggs embed in the liver and do not pass through the body. Finding them in excrement indicates that the people were eating raw cow organs and feeding leftovers to their dogs. This is interesting, because a preponderance of pottery and cooking implements also found at the site indicates that the residents of Durrington Walls were spit-roasting or boiling their beef and pork. So the meat was cooked, but not the organs. That is an interesting dietary decision, ancient British people. Then again, modern British cuisine exists. At least now we know where they got it from.
This new research raises one other very important question: When are we going to get a full-on guided tour of all the important coprolite sites in Britain? They’ve clearly got plenty of them, and the tourist demand for ancient parasites must be sky-high. Come on, capitalism, follow through on this. We’d go.
Everyone lies: Food intake edition
Do you have any patients on special diets? Do you ask them if they are following those diets? Don’t bother, because they’re lying. Everyone lies about the food they eat. Everyone. Obese people lie, and nonobese people lie.
Investigators at the University of Essex in England asked 221 adults to keep food diaries, and then they checked on energy consumption by analyzing radioactive water levels in the participants’ urine over a 10-day period.
Underreporting of food consumption was rampant, even among those who were not obese. The obese subjects did underreport by a greater extent (1,200 calories per day) than did those who were not obese, who were off by only 800 calories, but the obese participants burned about 400 calories more each day than did the nonobese, so the difference was a wash.
Everyone ended up underreporting their calorie consumption by an average of about 900 calories, and the investigators were good enough to provide some food equivalents, tops on the list being three MacDonald’s cheeseburgers.
“Public health recommendations have historically relied heavily on self-reported energy intake values,” senior author Gavin Sandercock, PhD, said in a EurekAlert statement, and “recognising that the measures of energy intake are incorrect might result in the setting of more realistic targets.”
Maybe you can be more realistic with your patients, too. Go ahead and ask Mr. Smith about the burger sticking out of his coat pocket, because there are probably two more you can’t see. We’ve each got 900 calories hiding on us somewhere. Ours is usually pizza.
The art of the gallbladder
Ever thought you would see a portrait of a gallbladder hanging up in a gallery? Not just an artist’s rendition, but an actual photo from an actual patient? Well, you can at the Soloway Gallery in Brooklyn, N.Y., at least until June 12.
The artist? K.C. Joseph, MD, a general surgeon from St. Marie, Pa., who died in 2015. His daughter Melissa is the curator of the show and told ARTnews about the interesting connection her father had with art and surgery.
In 2010, Dr. Joseph gave his daughter a box of photos and said “Make me a famous artist,” she recalled. At first, “I was like, ‘These are weird,’ and then I put them under my bed for 10 years.”
Apparently he had been making art with his patients’ organs for about 15 years and had a system in which he put each one together. Before a surgery Dr. Joseph would make a note card with the patient’s name handwritten in calligraphy with a couple of pages taken out of the magazine from the waiting room as the backdrop. Afterward, when the patient was in recovery, the removed organ would be placed among the pages and the name card. A photo was taken with the same endoscope that was used for the procedure.
After the show’s debut, people reached out expressing their love for their photos. “I wish, before he died, I had asked him more questions about it,” Ms. Joseph told ARTnews. “I’m regretting it so much now, kicking myself.”
Who gets to take home an artsy photo of their gallbladder after getting it removed? Not us, that’s who. Each collage is a one-of-a-kind piece. They definitely should be framed and shown in an art gallery. Oh, right. Never mind.
Why it might be better to be a dog person
Here’s that old debate again: Dogs or cats? You probably have your own opinion, but research presented at this year’s Digestive Disease Week may have tipped the scale by showing that children who lived with dogs may be less likely to have Crohn’s disease as adults.
The research was done by having approximately 4,300 people closely related to patients with Crohn’s disease fill out an environmental questionnaire. Using these data, the research team looked into environmental factors such as size of the families, where the home was, how many bathrooms the homes had, and quality of drinking water.
The researchers found that those who had or were exposed to dogs between the ages of 5 and 15 years were more likely to have healthy gut permeability and balanced microbes, which increased their protection against Crohn’s disease.
“Our study seems to add to others that have explored the ‘hygiene hypothesis’ which suggests that the lack of exposure to microbes early in life may lead to lack of immune regulation toward environmental microbes,” senior author Williams Turpin, PhD, said in the written statement.
The researchers aren’t sure why they didn’t get the same findings with cats, but Dr. Turpin theorized that dog owners tend to be outside more with their dogs or live in places with more green space, which are good protectors against Crohn’s disease.
It’s all good for dog owners, but do their pets’ parasites make you more attractive? Just more fuel for the ongoing debate.
Come for the history, stay for the fossilized parasites
Another week, another analysis of old British poop. LOTME really is your one-stop shop for all the important, hard-hitting news about historic parasites. You’re welcome, Internet.
The news this week is from Stonehenge, which is apparently kind of a big deal. Rocks in a circle, celestial calendar, cultural significance, whatever. We’re not here to talk about rocks. We’re here to talk about, uh, rocks. Smaller rocks. Specifically, coprolites, which are essentially poop turned into a rock. (Though now we’re imagining Stonehenge made out of fossilized poop rocks. Would it still be a big tourist destination? We can see both sides of the argument on that one.)
Archaeologists from the University of Cambridge have conducted an analysis of coprolites from Durrington Walls, a Neolithic settlement just a few kilometers from Stonehenge. The town dates to the same time that Stonehenge was constructed, and it’s believed that the residents were responsible for building the landmark. These coprolites, depending on what’s inside, can tell us a lot about how the builders of Stonehenge lived and, more specifically, how they ate.
In this case, the coprolites of one human and three dogs contained capillariid worm eggs. These worms come from cows, and when a human is typically infected, the eggs embed in the liver and do not pass through the body. Finding them in excrement indicates that the people were eating raw cow organs and feeding leftovers to their dogs. This is interesting, because a preponderance of pottery and cooking implements also found at the site indicates that the residents of Durrington Walls were spit-roasting or boiling their beef and pork. So the meat was cooked, but not the organs. That is an interesting dietary decision, ancient British people. Then again, modern British cuisine exists. At least now we know where they got it from.
This new research raises one other very important question: When are we going to get a full-on guided tour of all the important coprolite sites in Britain? They’ve clearly got plenty of them, and the tourist demand for ancient parasites must be sky-high. Come on, capitalism, follow through on this. We’d go.
Everyone lies: Food intake edition
Do you have any patients on special diets? Do you ask them if they are following those diets? Don’t bother, because they’re lying. Everyone lies about the food they eat. Everyone. Obese people lie, and nonobese people lie.
Investigators at the University of Essex in England asked 221 adults to keep food diaries, and then they checked on energy consumption by analyzing radioactive water levels in the participants’ urine over a 10-day period.
Underreporting of food consumption was rampant, even among those who were not obese. The obese subjects did underreport by a greater extent (1,200 calories per day) than did those who were not obese, who were off by only 800 calories, but the obese participants burned about 400 calories more each day than did the nonobese, so the difference was a wash.
Everyone ended up underreporting their calorie consumption by an average of about 900 calories, and the investigators were good enough to provide some food equivalents, tops on the list being three MacDonald’s cheeseburgers.
“Public health recommendations have historically relied heavily on self-reported energy intake values,” senior author Gavin Sandercock, PhD, said in a EurekAlert statement, and “recognising that the measures of energy intake are incorrect might result in the setting of more realistic targets.”
Maybe you can be more realistic with your patients, too. Go ahead and ask Mr. Smith about the burger sticking out of his coat pocket, because there are probably two more you can’t see. We’ve each got 900 calories hiding on us somewhere. Ours is usually pizza.
The art of the gallbladder
Ever thought you would see a portrait of a gallbladder hanging up in a gallery? Not just an artist’s rendition, but an actual photo from an actual patient? Well, you can at the Soloway Gallery in Brooklyn, N.Y., at least until June 12.
The artist? K.C. Joseph, MD, a general surgeon from St. Marie, Pa., who died in 2015. His daughter Melissa is the curator of the show and told ARTnews about the interesting connection her father had with art and surgery.
In 2010, Dr. Joseph gave his daughter a box of photos and said “Make me a famous artist,” she recalled. At first, “I was like, ‘These are weird,’ and then I put them under my bed for 10 years.”
Apparently he had been making art with his patients’ organs for about 15 years and had a system in which he put each one together. Before a surgery Dr. Joseph would make a note card with the patient’s name handwritten in calligraphy with a couple of pages taken out of the magazine from the waiting room as the backdrop. Afterward, when the patient was in recovery, the removed organ would be placed among the pages and the name card. A photo was taken with the same endoscope that was used for the procedure.
After the show’s debut, people reached out expressing their love for their photos. “I wish, before he died, I had asked him more questions about it,” Ms. Joseph told ARTnews. “I’m regretting it so much now, kicking myself.”
Who gets to take home an artsy photo of their gallbladder after getting it removed? Not us, that’s who. Each collage is a one-of-a-kind piece. They definitely should be framed and shown in an art gallery. Oh, right. Never mind.
Why it might be better to be a dog person
Here’s that old debate again: Dogs or cats? You probably have your own opinion, but research presented at this year’s Digestive Disease Week may have tipped the scale by showing that children who lived with dogs may be less likely to have Crohn’s disease as adults.
The research was done by having approximately 4,300 people closely related to patients with Crohn’s disease fill out an environmental questionnaire. Using these data, the research team looked into environmental factors such as size of the families, where the home was, how many bathrooms the homes had, and quality of drinking water.
The researchers found that those who had or were exposed to dogs between the ages of 5 and 15 years were more likely to have healthy gut permeability and balanced microbes, which increased their protection against Crohn’s disease.
“Our study seems to add to others that have explored the ‘hygiene hypothesis’ which suggests that the lack of exposure to microbes early in life may lead to lack of immune regulation toward environmental microbes,” senior author Williams Turpin, PhD, said in the written statement.
The researchers aren’t sure why they didn’t get the same findings with cats, but Dr. Turpin theorized that dog owners tend to be outside more with their dogs or live in places with more green space, which are good protectors against Crohn’s disease.
It’s all good for dog owners, but do their pets’ parasites make you more attractive? Just more fuel for the ongoing debate.
Come for the history, stay for the fossilized parasites
Another week, another analysis of old British poop. LOTME really is your one-stop shop for all the important, hard-hitting news about historic parasites. You’re welcome, Internet.
The news this week is from Stonehenge, which is apparently kind of a big deal. Rocks in a circle, celestial calendar, cultural significance, whatever. We’re not here to talk about rocks. We’re here to talk about, uh, rocks. Smaller rocks. Specifically, coprolites, which are essentially poop turned into a rock. (Though now we’re imagining Stonehenge made out of fossilized poop rocks. Would it still be a big tourist destination? We can see both sides of the argument on that one.)
Archaeologists from the University of Cambridge have conducted an analysis of coprolites from Durrington Walls, a Neolithic settlement just a few kilometers from Stonehenge. The town dates to the same time that Stonehenge was constructed, and it’s believed that the residents were responsible for building the landmark. These coprolites, depending on what’s inside, can tell us a lot about how the builders of Stonehenge lived and, more specifically, how they ate.
In this case, the coprolites of one human and three dogs contained capillariid worm eggs. These worms come from cows, and when a human is typically infected, the eggs embed in the liver and do not pass through the body. Finding them in excrement indicates that the people were eating raw cow organs and feeding leftovers to their dogs. This is interesting, because a preponderance of pottery and cooking implements also found at the site indicates that the residents of Durrington Walls were spit-roasting or boiling their beef and pork. So the meat was cooked, but not the organs. That is an interesting dietary decision, ancient British people. Then again, modern British cuisine exists. At least now we know where they got it from.
This new research raises one other very important question: When are we going to get a full-on guided tour of all the important coprolite sites in Britain? They’ve clearly got plenty of them, and the tourist demand for ancient parasites must be sky-high. Come on, capitalism, follow through on this. We’d go.
Everyone lies: Food intake edition
Do you have any patients on special diets? Do you ask them if they are following those diets? Don’t bother, because they’re lying. Everyone lies about the food they eat. Everyone. Obese people lie, and nonobese people lie.
Investigators at the University of Essex in England asked 221 adults to keep food diaries, and then they checked on energy consumption by analyzing radioactive water levels in the participants’ urine over a 10-day period.
Underreporting of food consumption was rampant, even among those who were not obese. The obese subjects did underreport by a greater extent (1,200 calories per day) than did those who were not obese, who were off by only 800 calories, but the obese participants burned about 400 calories more each day than did the nonobese, so the difference was a wash.
Everyone ended up underreporting their calorie consumption by an average of about 900 calories, and the investigators were good enough to provide some food equivalents, tops on the list being three MacDonald’s cheeseburgers.
“Public health recommendations have historically relied heavily on self-reported energy intake values,” senior author Gavin Sandercock, PhD, said in a EurekAlert statement, and “recognising that the measures of energy intake are incorrect might result in the setting of more realistic targets.”
Maybe you can be more realistic with your patients, too. Go ahead and ask Mr. Smith about the burger sticking out of his coat pocket, because there are probably two more you can’t see. We’ve each got 900 calories hiding on us somewhere. Ours is usually pizza.
The art of the gallbladder
Ever thought you would see a portrait of a gallbladder hanging up in a gallery? Not just an artist’s rendition, but an actual photo from an actual patient? Well, you can at the Soloway Gallery in Brooklyn, N.Y., at least until June 12.
The artist? K.C. Joseph, MD, a general surgeon from St. Marie, Pa., who died in 2015. His daughter Melissa is the curator of the show and told ARTnews about the interesting connection her father had with art and surgery.
In 2010, Dr. Joseph gave his daughter a box of photos and said “Make me a famous artist,” she recalled. At first, “I was like, ‘These are weird,’ and then I put them under my bed for 10 years.”
Apparently he had been making art with his patients’ organs for about 15 years and had a system in which he put each one together. Before a surgery Dr. Joseph would make a note card with the patient’s name handwritten in calligraphy with a couple of pages taken out of the magazine from the waiting room as the backdrop. Afterward, when the patient was in recovery, the removed organ would be placed among the pages and the name card. A photo was taken with the same endoscope that was used for the procedure.
After the show’s debut, people reached out expressing their love for their photos. “I wish, before he died, I had asked him more questions about it,” Ms. Joseph told ARTnews. “I’m regretting it so much now, kicking myself.”
Who gets to take home an artsy photo of their gallbladder after getting it removed? Not us, that’s who. Each collage is a one-of-a-kind piece. They definitely should be framed and shown in an art gallery. Oh, right. Never mind.
Manufacturer announces FDA approval for molluscum treatment delayed
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pemphigus Vulgaris Aggravated: Rifampicin Found at the Scene of the Crime
Case Report
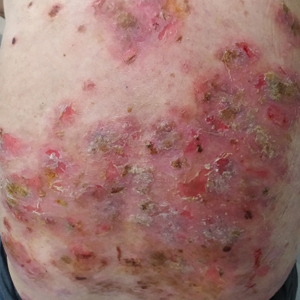
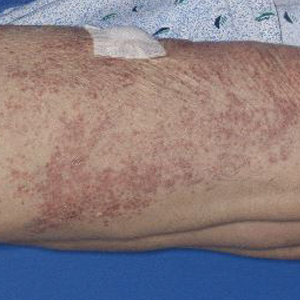
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
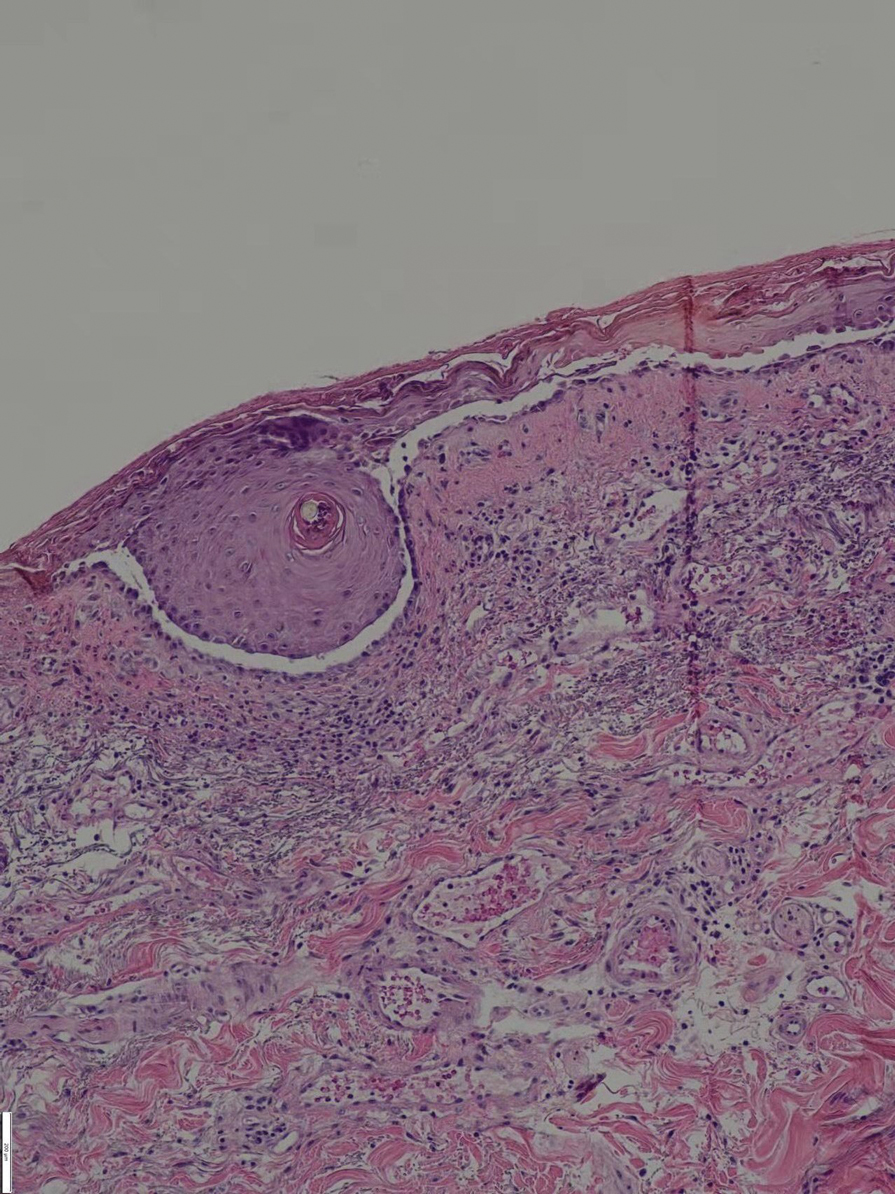
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
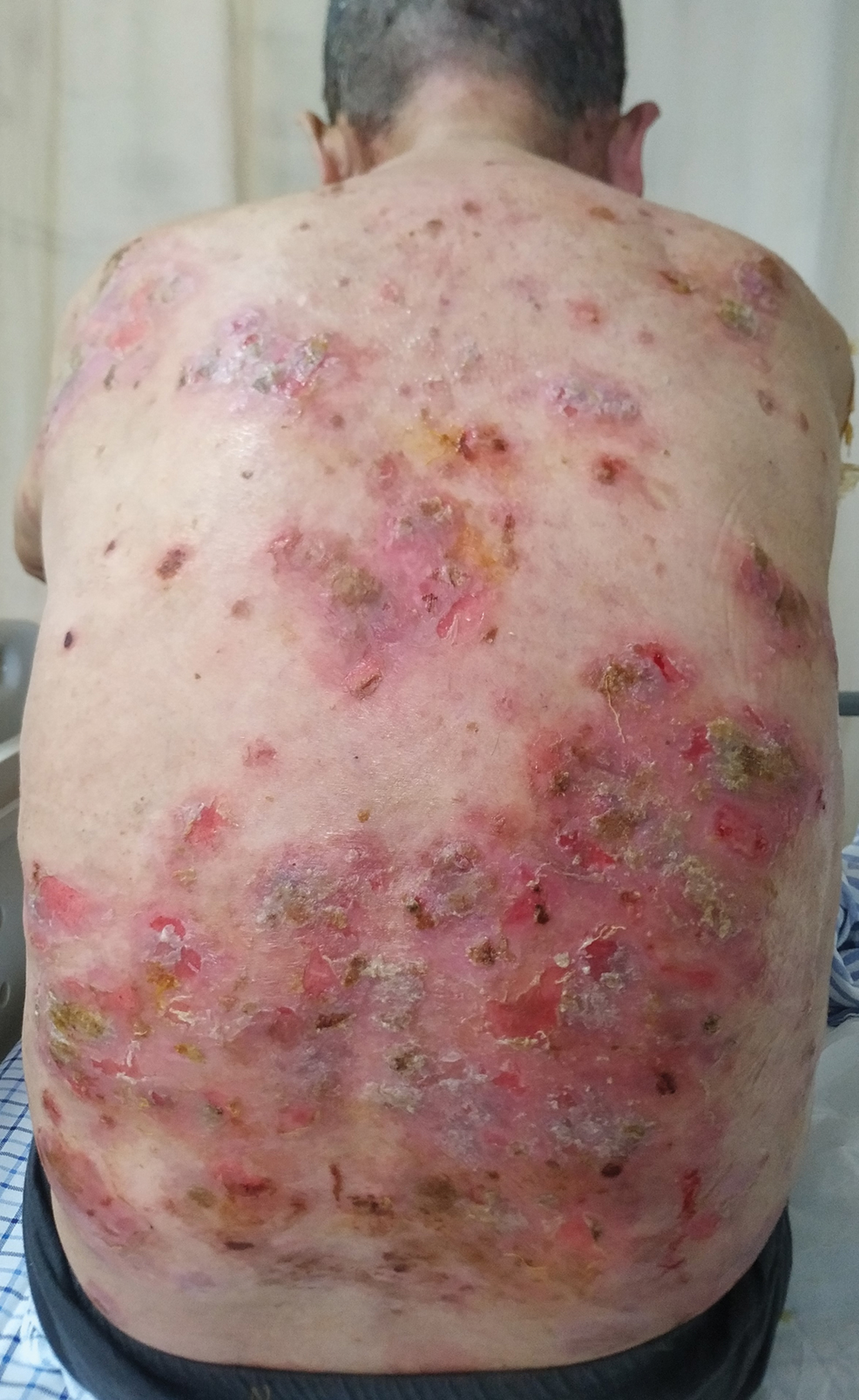
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion

The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.

In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion

The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.

In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Practice Points
- Long-term use of immunosuppressants requires constant attention for infections, especially latent infections in the body.
- Clinicians should carefully inquire with patients about concomitant diseases and medications used, and be vigilant about drug interactions.
Forceps for Milia Extraction
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
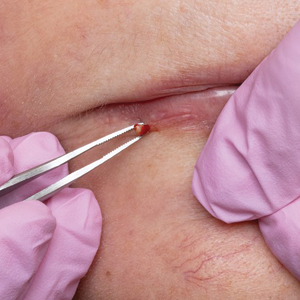
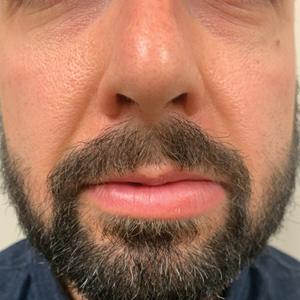
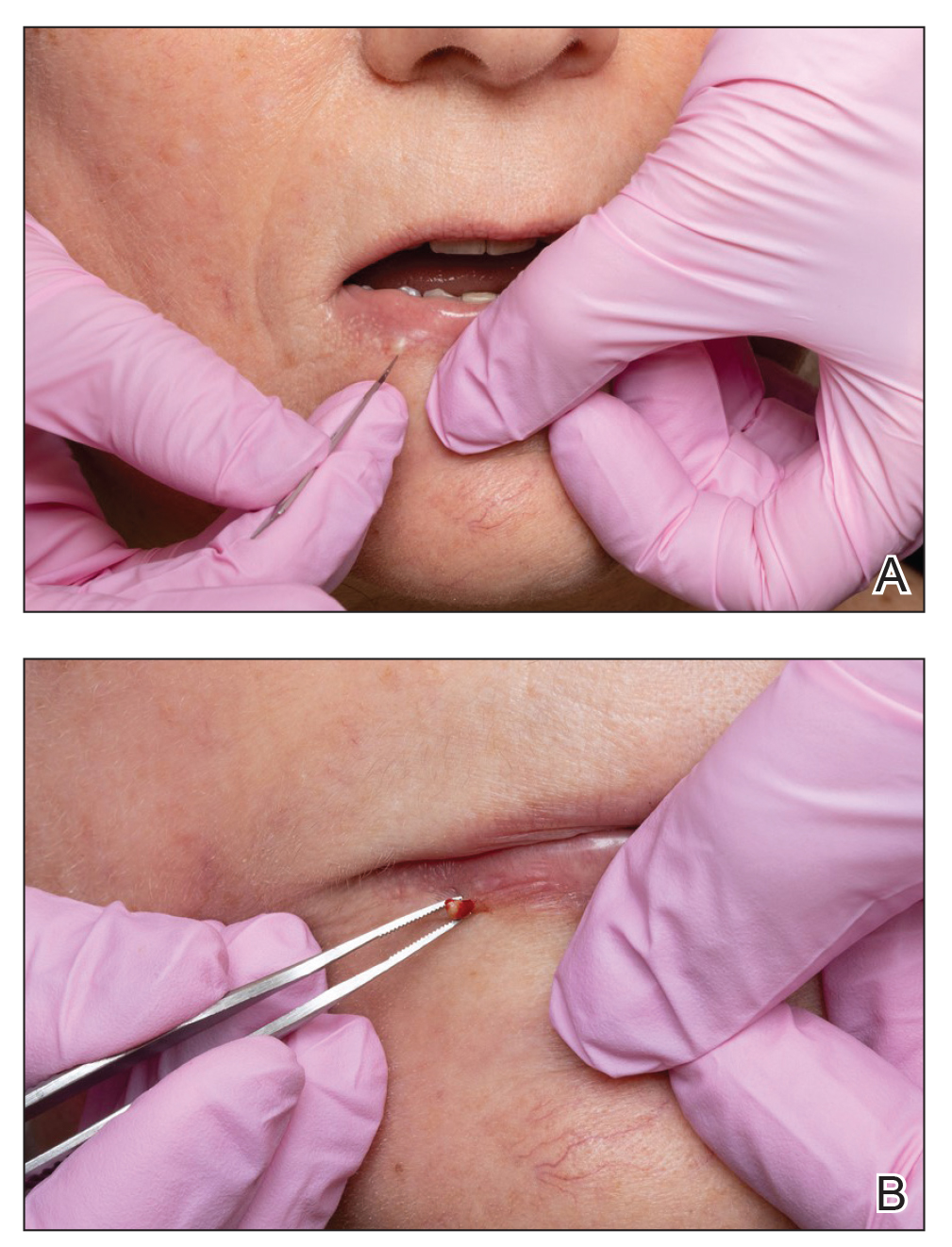
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.

Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.

Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
Practice Points
- Milia are common benign lesions that are cosmetically undesirable to some patients.
- Although some methods of milia removal can be painful, removal with forceps is quick and effective.
Navigating Motherhood and Dermatology Residency
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
Motherhood and dermatology residency are both full-time jobs. The thought that a woman must either be superhuman to succeed at both or that success at one must come at the expense of the other is antiquated. With careful navigation and sufficient support, these two roles can complement and heighten one another. The most recent Accreditation Council for Graduate Medical Education (ACGME) report showed that nearly 60% of dermatology residents are women,1 with most women in training being of childbearing age. One study showed that female dermatologists were most likely to have children during residency (51% of those surveyed), despite residents reporting more barriers to childbearing at this career stage.2 Trainees thinking of starting a family have many considerations to navigate: timing of pregnancy, maternity leave scheduling, breastfeeding while working, and planning for childcare. For the first time in the history of the specialty, most active dermatologists in practice are women.3 Thus, the future of dermatology requires supportive policies and resources for the successful navigation of these issues by today’s trainees.
Timing of Pregnancy
Timing of pregnancy can be a source of stress to the female dermatology resident. Barriers to childbearing during residency include the perception that women who have children during residency training are less committed to their jobs; concerns of overburdening fellow residents; and fear that residency may need to be extended, thereby delaying the ability to sit for the board examination.2 However, the potential increased risk for infertility in delaying pregnancy adds to the stress of pregnancy planning. A 2016 survey of female physicians (N=327) showed that 24.1% of respondents who had attempted conception were diagnosed with infertility, with an average age at diagnosis of 33.7 years.4 This is higher than the national average, with the Centers for Disease Control and Prevention reporting that approximately 19% of women aged 15 to 49 years with no prior births experience infertility.5 In a 1992 survey of female physician residents (N=373) who gave birth during residency, 32% indicated that they would not recommend the experience to others; of the 68% who would recommend the experience, one-third encouraged timing delivery to occur in the last 2 months of residency due to benefits of continued insurance coverage, a decrease in clinic responsibilities, and the potential for extended maternity leave during hiatus between jobs.6 Although this may be a good strategy, studying and sitting for board examinations while caring for a newborn right after graduation may be overly difficult for some. The first year of residency was perceived as the most stressful time to be pregnant, with each subsequent year being less problematic.6 Planning pregnancy for delivery near the end of the second year and beginning of the third year of dermatology residency may be a reasonable choice.
Maternity Leave
The Family and Medical Leave Act entitles eligible employees of covered employers to take unpaid, job-protected leave, with 12 workweeks of leave in a 12-month period for the birth of a child and to care for the newborn child within 1 year of birth.7 The actual length of maternity leave taken by most surveyed female dermatologists (n=96) is shorter: 25% (24/96) took less than 4 weeks, 42.7% (41/96) took 4 to 8 weeks, 25% (24/96) took 9 to 12 weeks, and 7.3% (7/96) were able to take more than 12 weeks of maternity leave.2
The American Board of Dermatology implemented a new Resident Leave policy that went into effect July 1, 2021, stipulating that, within certain parameters, time spent away from training for family and parental leave would not exhaust vacation time or require an extension in training. Under this policy, absence from training exceeding 8 weeks (6 weeks leave, plus 2 weeks of vacation) in a given year should be approved only under exceptional circumstances and may necessitate additional training time to ensure that competency requirements are met.8 Although this policy is a step in the right direction, institutional policies still may vary. Dermatology residents planning to start a family during training should consider their plans for fellowship, as taking an extended maternity leave beyond 8 weeks may jeopardize a July fellowship start date.
Lactation and Residency
The American Academy of Pediatrics recommends exclusive breastfeeding for approximately 6 months, with continuation of breastfeeding for 1 year or longer as mutually desired by the mother and infant.9 Successful lactation and achieving breastfeeding goals can be difficult during medical training. A national cross-sectional survey of female residents (N=312) showed that the median total time of breastfeeding and pumping was 9 months, with 74% continuing after 6 months and 13% continuing past 12 months. Of those surveyed, 73% reported residency limited their ability to lactate, and 37% stopped prior to their desired goal.10 As of July 1, 2020, the ACGME requires that residency programs and sponsoring institutions provide clean and private facilities for lactation that have refrigeration capabilities, with proximity appropriate for safe patient care.11 There has been a call to dermatology program leadership to support breastfeeding residents by providing sufficient time and space to pump; a breastfeeding resident will need a 20- to 30-minute break to express milk approximately every 3 hours during the work day.12 One innovative initiative to meet the ACGME lactation requirement reported by the Kansas University Medical Center Graduate Medical Education program (Kansas City, Kansas) was the purchase of wearable breast pumps to loan to residents. The benefits of wearable breast pumps are that they are discreet and can allow mothers to express milk inconspicuously while working, can increase milk supply, require less set up and expression time than traditional pumps, and can allow the mother to manage time more efficiently.13 Breastfeeding plans and goals should be discussed with program leadership before return from leave to strategize and anticipate gaps in clinic scheduling to accommodate the lactating resident.
Planning for Childcare
Resident hours can be long and erratic, making choices for childcare difficult. In one survey of female residents, 61% of married or partnered respondents (n=447) were delaying childbearing, and 46% cited lack of access to childcare as a reason.14 Not all dermatology residents are fortunate enough to match to a program near family, but close family support can be an undeniable asset during childrearing and should be weighed heavily when ranking programs. Options for childcare include relying on a stay-at-home spouse or other family member, a live-in or live-out nanny, part-time babysitters, and daycare. It is crucial to have multiple layers and back-up options for childcare available at any given time when working as a resident. Even with a child enrolled in a full-time daycare and a live-in nanny, a daycare closure due to a COVID-19 exposure or sudden medical emergency in the nanny can still leave unpredicted holes in your childcare plan, leaving the resident to potentially miss work to fill the gap. A survey of residents at one institution showed that the most common backup childcare plan for situations in which either the child or the regular caregiver is ill is for the nontrainee parent or spouse to stay home (45%; n=101), with 25% of respondents staying home to care for a sick child themselves, which clearly has an impact on the hospital. The article proposed implementation of on-site or near-site childcare for residents with extended hours or a 24-hour emergency drop-in availability.15 One institution reported success with the development of a departmentally funded childcare supplementation stipend offered to residents to support daycare costs during the first 6 months of a baby’s life.16
Final Thoughts
Due to the competitiveness of the field, dermatology residents are by nature high performing and academically successful. For a high achiever, the idea of potentially disappointing faculty and colleagues by starting a family during residency can be guilt inducing. Concerns about one’s ability to adequately study the breadth of dermatology while simultaneously raising a child can be distressing; however, there are many ways in which motherhood can hone skills to become a better dermatology resident. Through motherhood one can enhance time management skills, increase efficiency, and improve rapport with pediatric patients and trust with their parents/guardians. A dermatology resident may be her own harshest critic, but it is time that the future generation of dermatologists become their own greatest advocates for establishing supportive policies and resources for the successful navigation of motherhood and dermatology residency.
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
- ACGME residents and fellows by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/interactive-data/acgme-residents-and-fellows-sex-and-specialty-2019
- Mattessich S, Shea K, Whitaker-Worth D. Parenting and female dermatologists’ perceptions of work-life balance. Int J Womens Dermatol. 2017;3:127-130. doi:10.1016/j.ijwd.2017.04.001
- Active physicians by sex and specialty, 2019. Association of American Medical Colleges website. Accessed April 21, 2022. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2019
- Stentz NC, Griffith KA, Perkins E, et al. Fertility and childbearing among American female physicians. J Womens Health. 2016;25:1059-1065. doi:10.1089/jwh.2015.5638
- Infertility. Centers for Disease Control and Prevention website. Updated March 1, 2022. Accessed April 21, 2022. https://www.cdc.gov/reproductivehealth/infertility/
- Phelan ST. Sources of stress and support for the pregnant resident. Acad Med. 1992;67:408-410. doi:10.1097/00001888-199206000-00014
- Family and Medical Leave Act. US Department of Labor website. Accessed April 21, 2022. https://www.dol.gov/agencies/whd/fmla
- American Board of Dermatology. Effective July 2021: new family leave policy. Accessed April 21, 2022. https://www.abderm.org/public/announcements/effective-july-2021-new-family-leave-policy.aspx
- Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk. Pediatrics. 2012;129:E827-E841. doi:10.1542/peds.2011-3552
- Peters GW, Kuczmarska-Haas A, Holliday EB, et al. Lactation challenges of resident physicians: results of a national survey. BMC Pregnancy Childbirth. 2020;20:762. doi:10.1186/s12884-020-03436-3
- Common program requirements (residency) sections I-V table of implementation dates. Accreditation Council for Graduate Medical Education website. Accessed April 21, 2022. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/CPRResidencyImplementationTable.pdf
- Gracey LE, Mathes EF, Shinkai K. Supporting breastfeeding mothers during dermatology residency—challenges and best practices. JAMA Dermatol. 2020;156:117-118. doi:10.1001/jamadermatol.2019.3759
- McMillin A, Behravesh B, Byrne P, et al. A GME wearable breast pump program: an innovative method to meet ACGME requirements and federal law. J Grad Med Educ. 2021;13:422-423. doi:10.4300/jgme-d-20-01275.1
- Stack SW, Jagsi R, Biermann JS, et al. Childbearing decisions in residency: a multicenter survey of female residents. Acad Med. 2020;95:1550-1557. doi:10.1097/acm.0000000000003549
- Snyder RA, Tarpley MJ, Phillips SE, et al. The case for on-site child care in residency training and afterward. J Grad Med Educ. 2013;5:365-367. doi:10.4300/jgme-d-12-00294.1
- Key LL. Child care supplementation: aid for residents and advantages for residency programs. J Pediatr. 2008;153:449-450. doi:10.1016/j.jpeds.2008.05.028
Resident Pearl
- Female dermatology residents seeking motherhood during training have many obstacles to navigate, including the timing of pregnancy, maternity leave scheduling, planning for breastfeeding while working, and arranging for childcare. With supportive policies and resources, motherhood and dermatology training can be rewarding complements to one another.
Topical tranexamic acid reduces postop bleeding following Mohs surgery
The use of adjunctive , in a double-blind, randomized, controlled trial.
The findings suggest that “topical TXA application is an inexpensive and easy topical preventative measure to consider adding to the wound care of granulating defects in the setting of Mohs micrographic surgery,” first author Brianna Castillo, MD, chief dermatology resident at the University of Missouri, Columbia, told this news organization.
The study results were presented at the annual meeting of the American College of Mohs Surgery.
In wound healing by second intent after Mohs micrographic surgery, postoperative bleeding is common and can lead to patient distress, as well as return visits or emergency care, resulting in additional health care costs, Dr. Castillo said.
Topical TXA, an antifibrinolytic, synthetic lysine analogue that prevents blood clots from breaking down, is commonly used in surgical settings including cardiothoracic, orthopedic, gynecologic, oral, and trauma surgery, showing no increased risk of thrombotic events. However, its use is relatively new in dermatology.
TXA is approved by the Food and Drug Administration only as an oral formulation for menorrhagia in women and as a short-term preventative measure for hemophilia; however, other formulations are available for topical and subcutaneous uses, Dr. Castillo noted.
To evaluate the potential benefits of the treatment in postsurgical Mohs microsurgery bleeding, Dr. Castillo and colleagues enrolled 124 patients undergoing the surgery between October 2020 and December 2021 who had surgical defects deemed appropriate for second intention healing.
The patients were randomized to groups of 62 patients each to receive normal saline-soaked Telfa pads applied to the wound bed upon completion of surgery or TXA 25 mg/mL at a volume of 1 mL/cm2-soaked Telfa pads to the wound bed upon completion of the surgery.
In both groups, a standard pressure dressing was placed on top of the Telfa pads.
Most participants were men (90 vs. 34 patients), 45 were taking antiplatelet therapy, and 20 were taking anticoagulants, and in all cases, patients were similarly randomized in the two groups. Most of the surgical defects were on the head and neck or an extremity, and most (74) were under 2 cm.
All patients were provided with instructions to apply pressure to their wounds and to report bleeding complications. They were interviewed by phone 3 days following their surgeries regarding postoperative bleeding and any potential issues relating to the TXA treatment.
In follow-up interviews, six patients in the placebo group (9.7%) reported active bleeding from their wounds within 48 hours of surgery, with one patient requiring an intervention, while there were no reports of bleeding in the TXA group (P = .028). No side effects were reported in either group.
In the setting of Mohs micrographic surgery, subcutaneous TXA has previously been studied as an intraoperative hemostatic agent, with bleeding measured prior to the second layer or closure, Dr. Castillo explained. However, “no studies have evaluated topical TXA with the aim to reduce postoperative bleeding in the setting of Mohs micrographic surgery,” she said.
Dr. Castillo noted that topical TXA is relatively inexpensive and typically available in hospital pharmacies. “It’s only about $7 per vial of 10 ccs and we do dilute it,” she noted during the session. “It has a pretty good shelf-life and does not have to be refrigerated.”
“We have implemented this into our practice at the University of Missouri,” she added.
Commenting on the study, M. Laurin Council, MD, associate professor of dermatology in the division of dermatology, department of internal medicine, Washington University, St. Louis, noted that second intention healing is “an excellent option for certain patients after skin cancer removal.
“One problem with this method, however, is that postsurgical wounds may bleed in the hours after a procedure, [and] this can be incredibly distressing to patients and their families,” she told this news organization.
“The study presented here shows great promise for the drug TXA for preventing postsurgical bleeding in this subset of patients,” said Dr. Council, director of dermatologic surgery and director of micrographic surgery and the dermatologic oncology fellowship at Washington University.
Commenting that “the results are impressive,” she noted the study had some limitations. “This is a small pilot study, and we don’t know about confounding factors in each group, such as the proportion of patients who are on blood thinners or who have low platelets, and therefore trouble clotting, for example.”
The authors have reported no relevant financial relationships. Dr. Council has consulted for AbbVie, Castle Biosciences, and Sanofi-Genzyme/Regeneron; however, the consulting was not relevant to the current study.
A version of this article first appeared on Medscape.com.
The use of adjunctive , in a double-blind, randomized, controlled trial.
The findings suggest that “topical TXA application is an inexpensive and easy topical preventative measure to consider adding to the wound care of granulating defects in the setting of Mohs micrographic surgery,” first author Brianna Castillo, MD, chief dermatology resident at the University of Missouri, Columbia, told this news organization.
The study results were presented at the annual meeting of the American College of Mohs Surgery.
In wound healing by second intent after Mohs micrographic surgery, postoperative bleeding is common and can lead to patient distress, as well as return visits or emergency care, resulting in additional health care costs, Dr. Castillo said.
Topical TXA, an antifibrinolytic, synthetic lysine analogue that prevents blood clots from breaking down, is commonly used in surgical settings including cardiothoracic, orthopedic, gynecologic, oral, and trauma surgery, showing no increased risk of thrombotic events. However, its use is relatively new in dermatology.
TXA is approved by the Food and Drug Administration only as an oral formulation for menorrhagia in women and as a short-term preventative measure for hemophilia; however, other formulations are available for topical and subcutaneous uses, Dr. Castillo noted.
To evaluate the potential benefits of the treatment in postsurgical Mohs microsurgery bleeding, Dr. Castillo and colleagues enrolled 124 patients undergoing the surgery between October 2020 and December 2021 who had surgical defects deemed appropriate for second intention healing.
The patients were randomized to groups of 62 patients each to receive normal saline-soaked Telfa pads applied to the wound bed upon completion of surgery or TXA 25 mg/mL at a volume of 1 mL/cm2-soaked Telfa pads to the wound bed upon completion of the surgery.
In both groups, a standard pressure dressing was placed on top of the Telfa pads.
Most participants were men (90 vs. 34 patients), 45 were taking antiplatelet therapy, and 20 were taking anticoagulants, and in all cases, patients were similarly randomized in the two groups. Most of the surgical defects were on the head and neck or an extremity, and most (74) were under 2 cm.
All patients were provided with instructions to apply pressure to their wounds and to report bleeding complications. They were interviewed by phone 3 days following their surgeries regarding postoperative bleeding and any potential issues relating to the TXA treatment.
In follow-up interviews, six patients in the placebo group (9.7%) reported active bleeding from their wounds within 48 hours of surgery, with one patient requiring an intervention, while there were no reports of bleeding in the TXA group (P = .028). No side effects were reported in either group.
In the setting of Mohs micrographic surgery, subcutaneous TXA has previously been studied as an intraoperative hemostatic agent, with bleeding measured prior to the second layer or closure, Dr. Castillo explained. However, “no studies have evaluated topical TXA with the aim to reduce postoperative bleeding in the setting of Mohs micrographic surgery,” she said.
Dr. Castillo noted that topical TXA is relatively inexpensive and typically available in hospital pharmacies. “It’s only about $7 per vial of 10 ccs and we do dilute it,” she noted during the session. “It has a pretty good shelf-life and does not have to be refrigerated.”
“We have implemented this into our practice at the University of Missouri,” she added.
Commenting on the study, M. Laurin Council, MD, associate professor of dermatology in the division of dermatology, department of internal medicine, Washington University, St. Louis, noted that second intention healing is “an excellent option for certain patients after skin cancer removal.
“One problem with this method, however, is that postsurgical wounds may bleed in the hours after a procedure, [and] this can be incredibly distressing to patients and their families,” she told this news organization.
“The study presented here shows great promise for the drug TXA for preventing postsurgical bleeding in this subset of patients,” said Dr. Council, director of dermatologic surgery and director of micrographic surgery and the dermatologic oncology fellowship at Washington University.
Commenting that “the results are impressive,” she noted the study had some limitations. “This is a small pilot study, and we don’t know about confounding factors in each group, such as the proportion of patients who are on blood thinners or who have low platelets, and therefore trouble clotting, for example.”
The authors have reported no relevant financial relationships. Dr. Council has consulted for AbbVie, Castle Biosciences, and Sanofi-Genzyme/Regeneron; however, the consulting was not relevant to the current study.
A version of this article first appeared on Medscape.com.
The use of adjunctive , in a double-blind, randomized, controlled trial.
The findings suggest that “topical TXA application is an inexpensive and easy topical preventative measure to consider adding to the wound care of granulating defects in the setting of Mohs micrographic surgery,” first author Brianna Castillo, MD, chief dermatology resident at the University of Missouri, Columbia, told this news organization.
The study results were presented at the annual meeting of the American College of Mohs Surgery.
In wound healing by second intent after Mohs micrographic surgery, postoperative bleeding is common and can lead to patient distress, as well as return visits or emergency care, resulting in additional health care costs, Dr. Castillo said.
Topical TXA, an antifibrinolytic, synthetic lysine analogue that prevents blood clots from breaking down, is commonly used in surgical settings including cardiothoracic, orthopedic, gynecologic, oral, and trauma surgery, showing no increased risk of thrombotic events. However, its use is relatively new in dermatology.
TXA is approved by the Food and Drug Administration only as an oral formulation for menorrhagia in women and as a short-term preventative measure for hemophilia; however, other formulations are available for topical and subcutaneous uses, Dr. Castillo noted.
To evaluate the potential benefits of the treatment in postsurgical Mohs microsurgery bleeding, Dr. Castillo and colleagues enrolled 124 patients undergoing the surgery between October 2020 and December 2021 who had surgical defects deemed appropriate for second intention healing.
The patients were randomized to groups of 62 patients each to receive normal saline-soaked Telfa pads applied to the wound bed upon completion of surgery or TXA 25 mg/mL at a volume of 1 mL/cm2-soaked Telfa pads to the wound bed upon completion of the surgery.
In both groups, a standard pressure dressing was placed on top of the Telfa pads.
Most participants were men (90 vs. 34 patients), 45 were taking antiplatelet therapy, and 20 were taking anticoagulants, and in all cases, patients were similarly randomized in the two groups. Most of the surgical defects were on the head and neck or an extremity, and most (74) were under 2 cm.
All patients were provided with instructions to apply pressure to their wounds and to report bleeding complications. They were interviewed by phone 3 days following their surgeries regarding postoperative bleeding and any potential issues relating to the TXA treatment.
In follow-up interviews, six patients in the placebo group (9.7%) reported active bleeding from their wounds within 48 hours of surgery, with one patient requiring an intervention, while there were no reports of bleeding in the TXA group (P = .028). No side effects were reported in either group.
In the setting of Mohs micrographic surgery, subcutaneous TXA has previously been studied as an intraoperative hemostatic agent, with bleeding measured prior to the second layer or closure, Dr. Castillo explained. However, “no studies have evaluated topical TXA with the aim to reduce postoperative bleeding in the setting of Mohs micrographic surgery,” she said.
Dr. Castillo noted that topical TXA is relatively inexpensive and typically available in hospital pharmacies. “It’s only about $7 per vial of 10 ccs and we do dilute it,” she noted during the session. “It has a pretty good shelf-life and does not have to be refrigerated.”
“We have implemented this into our practice at the University of Missouri,” she added.
Commenting on the study, M. Laurin Council, MD, associate professor of dermatology in the division of dermatology, department of internal medicine, Washington University, St. Louis, noted that second intention healing is “an excellent option for certain patients after skin cancer removal.
“One problem with this method, however, is that postsurgical wounds may bleed in the hours after a procedure, [and] this can be incredibly distressing to patients and their families,” she told this news organization.
“The study presented here shows great promise for the drug TXA for preventing postsurgical bleeding in this subset of patients,” said Dr. Council, director of dermatologic surgery and director of micrographic surgery and the dermatologic oncology fellowship at Washington University.
Commenting that “the results are impressive,” she noted the study had some limitations. “This is a small pilot study, and we don’t know about confounding factors in each group, such as the proportion of patients who are on blood thinners or who have low platelets, and therefore trouble clotting, for example.”
The authors have reported no relevant financial relationships. Dr. Council has consulted for AbbVie, Castle Biosciences, and Sanofi-Genzyme/Regeneron; however, the consulting was not relevant to the current study.
A version of this article first appeared on Medscape.com.
FROM THE ACMS ANNUAL MEETING
Commentary: Comparisons of Dupilumab and Other Atopic Dermatitis Treatments, June 2022
Clinicians everywhere are excited to have more options, but they are also reeling from trying to keep up with the enormous amount of data. We are fortunate to have a lot of information for these therapies published in the past month, which provides important insight into how to best use them.
Let's start with some new data on dupilumab, which has emerged as a first-line systemic therapy for moderate to severe AD in the US. Patients who were enrolled in the phase 1-3 randomized clinical trials for dupilumab were allowed to enroll in a long-term open-label extension study where they received 300 mg dupilumab weekly. Beck and colleagues published the interim analysis of the ongoing international, multicenter, long-term extension study of 2677 adults with up to 4 years of dupilumab exposure. They found no major increases in adverse event rates, with high durable efficacy and high rates of drug persistence over time. It is important to note that this was an open-label study without a control group — that is, patients knew exactly what treatment they were receiving. In addition, the analysis presented efficacy among those patients who remained in the study over time, which may not adequately account for loss of efficacy over time in patients who dropped out of the study. Nevertheless, the results suggest that dupilumab can be a good long-term treatment option for patients with chronic AD, with no new major safety concerns.
There is now a large body of evidence generated by phase 4 studies showing the real-world effectiveness of dupilumab. Stingeni and colleagues published the results of a prospective study of 139 adolescents (aged 12-17 years) with moderate-to-severe AD who received dupilumab for 16 weeks. They found significant improvement in AD signs and quality of life overall and across different clinical phenotypes of AD, with robust endpoints achieved most in the diffuse eczema subtype. Despite the previously demonstrated heterogeneity of AD,1,2 these results suggest that dupilumab can be effective across a variety of patient subtypes.
The JAK inhibitors are new additions to our therapeutic armamentarium for AD. Given how new they are to dermatology, we are always craving more data to inform clinical decision-making. Several recent studies provide additional insights into how we can use the JAK inhibitors in clinical practice.
One major question is how well they work in patients in whom dupilumab previously failed. Shi and colleagues published the results of an interesting and clinically relevant phase 3 study (JADE EXTEND), which included 203 patients with moderate-to-severe AD who were randomly assigned to receive 200 mg or 100 mg once-daily abrocitinib after previously receiving dupilumab for 14 weeks in the JADE COMPARE study. They found that at week 12, the majority of dupilumab nonresponders had high Eczema Area and Severity Index (EASI-75) responses with both doses of abrocitinib. Patients who previously had a good clinical response with dupilumab had even higher treatment responses on abrocitinib than those who were dupilumab nonresponders. These data provide important information to support the use of abrocitinib, and perhaps by extension other JAK inhibitors, in patients who previously had inadequate response to dupilumab.
Another major question is how to differentiate the JAK inhibitors from biologics in AD. One consideration is that patients taking JAK inhibitors may achieve more robust clinical responses compared with those on biologics. Stander and colleagues performed a post hoc analysis of pooled phase 2B/3 studies of abrocitinib (942 patients). They found that, at week 12, a higher proportion of patients receiving 200 mg and 100 mg abrocitinib achieved more robust endpoints, such as EASI-90 and EASI-100 scores, compared with placebo recipients. Of note, these data did not include any comparison data with dupilumab. However, on the basis of cross-study comparison, it would seem that abrocitinib, particularly at the higher 200 mg dose, may lead to more robust clinical responses than dupilumab. However, it is very important to acknowledge that this study focused on 12-week data and maximal efficacy with dupilumab may take longer to achieve.
Additional References
1. Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21(2):172-176. Doi: 10.36849/JDD.6408
2. Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Amer Acad Dermatol. 2019;80(2):390-401. Doi: 10.1016/j.jaad.2018.09.035
Clinicians everywhere are excited to have more options, but they are also reeling from trying to keep up with the enormous amount of data. We are fortunate to have a lot of information for these therapies published in the past month, which provides important insight into how to best use them.
Let's start with some new data on dupilumab, which has emerged as a first-line systemic therapy for moderate to severe AD in the US. Patients who were enrolled in the phase 1-3 randomized clinical trials for dupilumab were allowed to enroll in a long-term open-label extension study where they received 300 mg dupilumab weekly. Beck and colleagues published the interim analysis of the ongoing international, multicenter, long-term extension study of 2677 adults with up to 4 years of dupilumab exposure. They found no major increases in adverse event rates, with high durable efficacy and high rates of drug persistence over time. It is important to note that this was an open-label study without a control group — that is, patients knew exactly what treatment they were receiving. In addition, the analysis presented efficacy among those patients who remained in the study over time, which may not adequately account for loss of efficacy over time in patients who dropped out of the study. Nevertheless, the results suggest that dupilumab can be a good long-term treatment option for patients with chronic AD, with no new major safety concerns.
There is now a large body of evidence generated by phase 4 studies showing the real-world effectiveness of dupilumab. Stingeni and colleagues published the results of a prospective study of 139 adolescents (aged 12-17 years) with moderate-to-severe AD who received dupilumab for 16 weeks. They found significant improvement in AD signs and quality of life overall and across different clinical phenotypes of AD, with robust endpoints achieved most in the diffuse eczema subtype. Despite the previously demonstrated heterogeneity of AD,1,2 these results suggest that dupilumab can be effective across a variety of patient subtypes.
The JAK inhibitors are new additions to our therapeutic armamentarium for AD. Given how new they are to dermatology, we are always craving more data to inform clinical decision-making. Several recent studies provide additional insights into how we can use the JAK inhibitors in clinical practice.
One major question is how well they work in patients in whom dupilumab previously failed. Shi and colleagues published the results of an interesting and clinically relevant phase 3 study (JADE EXTEND), which included 203 patients with moderate-to-severe AD who were randomly assigned to receive 200 mg or 100 mg once-daily abrocitinib after previously receiving dupilumab for 14 weeks in the JADE COMPARE study. They found that at week 12, the majority of dupilumab nonresponders had high Eczema Area and Severity Index (EASI-75) responses with both doses of abrocitinib. Patients who previously had a good clinical response with dupilumab had even higher treatment responses on abrocitinib than those who were dupilumab nonresponders. These data provide important information to support the use of abrocitinib, and perhaps by extension other JAK inhibitors, in patients who previously had inadequate response to dupilumab.
Another major question is how to differentiate the JAK inhibitors from biologics in AD. One consideration is that patients taking JAK inhibitors may achieve more robust clinical responses compared with those on biologics. Stander and colleagues performed a post hoc analysis of pooled phase 2B/3 studies of abrocitinib (942 patients). They found that, at week 12, a higher proportion of patients receiving 200 mg and 100 mg abrocitinib achieved more robust endpoints, such as EASI-90 and EASI-100 scores, compared with placebo recipients. Of note, these data did not include any comparison data with dupilumab. However, on the basis of cross-study comparison, it would seem that abrocitinib, particularly at the higher 200 mg dose, may lead to more robust clinical responses than dupilumab. However, it is very important to acknowledge that this study focused on 12-week data and maximal efficacy with dupilumab may take longer to achieve.
Additional References
1. Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21(2):172-176. Doi: 10.36849/JDD.6408
2. Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Amer Acad Dermatol. 2019;80(2):390-401. Doi: 10.1016/j.jaad.2018.09.035
Clinicians everywhere are excited to have more options, but they are also reeling from trying to keep up with the enormous amount of data. We are fortunate to have a lot of information for these therapies published in the past month, which provides important insight into how to best use them.
Let's start with some new data on dupilumab, which has emerged as a first-line systemic therapy for moderate to severe AD in the US. Patients who were enrolled in the phase 1-3 randomized clinical trials for dupilumab were allowed to enroll in a long-term open-label extension study where they received 300 mg dupilumab weekly. Beck and colleagues published the interim analysis of the ongoing international, multicenter, long-term extension study of 2677 adults with up to 4 years of dupilumab exposure. They found no major increases in adverse event rates, with high durable efficacy and high rates of drug persistence over time. It is important to note that this was an open-label study without a control group — that is, patients knew exactly what treatment they were receiving. In addition, the analysis presented efficacy among those patients who remained in the study over time, which may not adequately account for loss of efficacy over time in patients who dropped out of the study. Nevertheless, the results suggest that dupilumab can be a good long-term treatment option for patients with chronic AD, with no new major safety concerns.
There is now a large body of evidence generated by phase 4 studies showing the real-world effectiveness of dupilumab. Stingeni and colleagues published the results of a prospective study of 139 adolescents (aged 12-17 years) with moderate-to-severe AD who received dupilumab for 16 weeks. They found significant improvement in AD signs and quality of life overall and across different clinical phenotypes of AD, with robust endpoints achieved most in the diffuse eczema subtype. Despite the previously demonstrated heterogeneity of AD,1,2 these results suggest that dupilumab can be effective across a variety of patient subtypes.
The JAK inhibitors are new additions to our therapeutic armamentarium for AD. Given how new they are to dermatology, we are always craving more data to inform clinical decision-making. Several recent studies provide additional insights into how we can use the JAK inhibitors in clinical practice.
One major question is how well they work in patients in whom dupilumab previously failed. Shi and colleagues published the results of an interesting and clinically relevant phase 3 study (JADE EXTEND), which included 203 patients with moderate-to-severe AD who were randomly assigned to receive 200 mg or 100 mg once-daily abrocitinib after previously receiving dupilumab for 14 weeks in the JADE COMPARE study. They found that at week 12, the majority of dupilumab nonresponders had high Eczema Area and Severity Index (EASI-75) responses with both doses of abrocitinib. Patients who previously had a good clinical response with dupilumab had even higher treatment responses on abrocitinib than those who were dupilumab nonresponders. These data provide important information to support the use of abrocitinib, and perhaps by extension other JAK inhibitors, in patients who previously had inadequate response to dupilumab.
Another major question is how to differentiate the JAK inhibitors from biologics in AD. One consideration is that patients taking JAK inhibitors may achieve more robust clinical responses compared with those on biologics. Stander and colleagues performed a post hoc analysis of pooled phase 2B/3 studies of abrocitinib (942 patients). They found that, at week 12, a higher proportion of patients receiving 200 mg and 100 mg abrocitinib achieved more robust endpoints, such as EASI-90 and EASI-100 scores, compared with placebo recipients. Of note, these data did not include any comparison data with dupilumab. However, on the basis of cross-study comparison, it would seem that abrocitinib, particularly at the higher 200 mg dose, may lead to more robust clinical responses than dupilumab. However, it is very important to acknowledge that this study focused on 12-week data and maximal efficacy with dupilumab may take longer to achieve.
Additional References
1. Chovatiya R, Silverberg JI. The heterogeneity of atopic dermatitis. J Drugs Dermatol. 2022;21(2):172-176. Doi: 10.36849/JDD.6408
2. Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Amer Acad Dermatol. 2019;80(2):390-401. Doi: 10.1016/j.jaad.2018.09.035
Conjunctival Melanoma of the Left Lower Eyelid
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
Practice Points
- Ophthalmologists should carefully examine palpebral and bulbar conjunctiva at each annual visit paying careful attention to pigmented nevi.
- Conjunctival abnormalities should be thoroughly documented via color photography to accurately follow for suspicious change.
Cutaneous Lupus Erythematosus–like Isotopic Response to Herpes Zoster Infection
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
Practice Points
- Wolf isotopic response describes the occurrence of a new skin condition at the site of a previously healed and unrelated skin disorder; a granulomatous reaction is a commonly reported isotopic response.
- Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses.
Persistent Lip Swelling
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
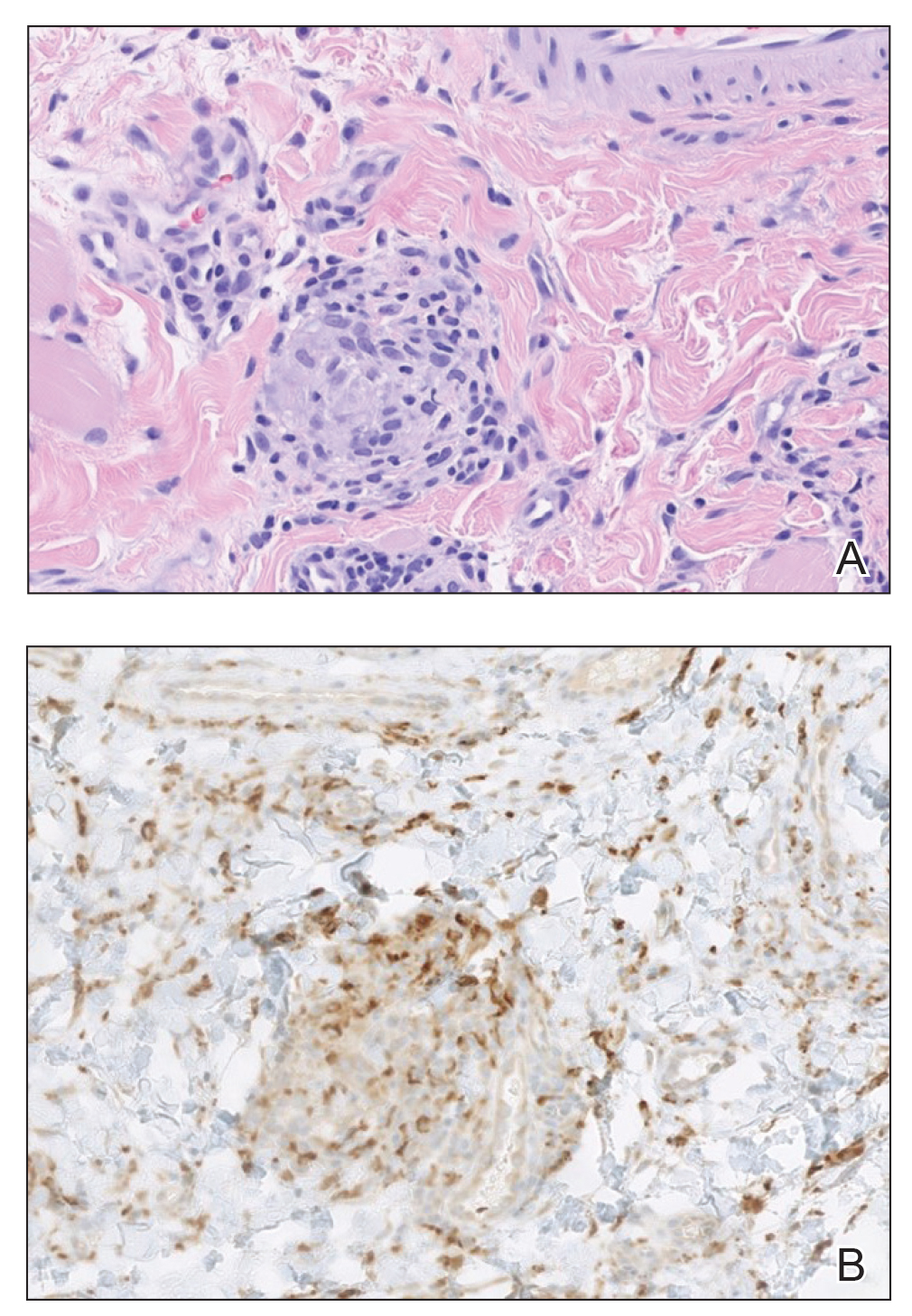
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
A 36-year-old man with allergic rhinitis presented with lower lip swelling of several months’ duration. The swelling was persistent and predominantly on the left side of the lower lip but occasionally spread to the entire lower lip. The episodes of increased swelling would last for several days and were not associated with any apparent triggers. He denied any pain, pruritus, or dryness. He noted more drooling from the affected side but denied any associated breathing difficulty or throat discomfort. Treatment with an oral antihistamine provided no relief. He denied any recent nonsteroidal anti-inflammatory drug or angiotensinconverting enzyme inhibitor use. His family history was notable for lupus in his maternal grandmother and maternal aunt. He denied any personal or family history of inflammatory bowel disease or recent gastrointestinal tract symptoms. Physical examination revealed nontender edema in the left side of the lower lip with no surface changes. No warmth or erythema were noted. The tongue and the rest of the oral cavity were unremarkable.