User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
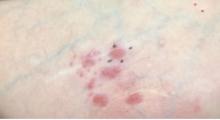
A 64-year-old woman presents with a history of asymptomatic erythematous grouped papules on the right breast
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
Experts urge stopping melanoma trial because of failure and harm
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Are physician white coats becoming obsolete? How docs dress for work now
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Myositis guidelines aim to standardize adult and pediatric care
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
FROM BSR 2022
ED staff speak out about workplace violence, ask for mitigation
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
WASHINGTON – Speaker after speaker, veteran emergency department physicians and nurses approached the podium for a May 4 press conference on the U.S. Capitol lawn across from the East Senate steps to describe violent incidents – being bitten, punched, slapped, kicked, choked, spat on, threatened – that they have both observed and have been subject to while working in EDs.
The press conference was cosponsored by the American College of Emergency Physicians and the Emergency Nurses Association, which have partnered since 2019 on the No Silence on ED Violence campaign.
The numbers confirm their experience. A 2018 poll of 3,500 ED physicians nationwide, which was conducted by Marketing General and was reported at ACEP’s annual meeting, found that nearly half of respondents had been assaulted at work; 27% of them were injured from the assault. Nurses, who spend more time with patients, may face even higher rates.
Incidence was reported to be increasing in 2018, and that was before the social and psychological upheavals imposed by the COVID pandemic caused assaults on staff in the hospital to go up an estimated 200%-300%.
But what really grated was that more than 95% of such cases, mostly perpetrated by patients, were never prosecuted, said Jennifer Casaletto, MD, FACEP, a North Carolina emergency physician and president of the state’s ACEP chapter. “Hospital and law enforcement see violence as just part of the job in our EDs.”
It’s no secret that workplace violence is increasing, Dr. Casaletto said. Four weeks ago, she stitched up the face of a charge nurse who had been assaulted. The nurse didn’t report the incident because she didn’t believe anything would change.
“Listening to my colleagues, I know the terror they have felt in the moment – for themselves, their colleagues, their patients. I know that raw fear of being attacked, and the complex emotions that follow. I’ve been hit, bit, and punched and watched colleagues getting choked.”
Dr. Casaletto was present in the ED when an out-of-control patient clubbed a nurse with an IV pole as she tried to close the doors to other patients’ rooms. “Instinctively, I pulled my stethoscope from around my neck, hoping I wouldn’t be strangled with it.”
Tennessee emergency nurse Todd Haines, MSN, RN, AEMT, CEN, said he has stepped in to help pull patients off coworkers. “I’ve seen some staff so severely injured they could not return to the bedside. I’ve been verbally threatened. My family has been threatened by patients and their families,” he reported. “We’ve all seen it. And COVID has made some people even meaner. They just lose their minds, and ED staff take the brunt of their aggression. But then to report these incidents and hear: ‘It’s just part of your job,’ well, it’s not part of my job.”
Mr. Haines spent 10 years in law enforcement with a sheriff’s department in middle Tennessee and was on its special tactical response team before becoming an ED nurse. He said he saw many more verbal and physical assaults in 11 years in the ED than during his police career.
“I love emergency nursing at the bedside, but it got to the point where I took the first chance to leave the bedside. And I’m not alone. Other nurses are leaving in droves.” Mr. Haines now has a job directing a trauma program, and he volunteers on policy issues for the Tennessee ENA. But he worries about the toll of this violence on the ED workforce, with so many professionals already mulling over leaving the field because of job stress and burnout.
“We have to do something to keep experienced hospital emergency staff at the bedside.”
What’s the answer?
Also speaking at the press conference was Senator Tammy Baldwin (D-Wis.), who pledged to introduce the Workplace Violence Prevention for Health Care and Social Services Workers Act, which passed the House in April. This bill would direct the Occupational Health and Safety Administration to issue a standard requiring employers in health care and social services to develop and implement workplace violence prevention plans. It would cover a variety of health facilities but not doctor’s offices or home-based services.
An interim final standard would be due within a year of enactment, with a final version to follow. Covered employers would have 6 months to develop and implement their own comprehensive workplace violence prevention plans, with the meaningful participation of direct care employees, tailored for and specific to the conditions and hazards of their facility, informed by past violent incidents, and subject to the size and complexity of the setting.
The plan would also name an individual responsible for its implementation, would include staff training and education, and would require facilities to track incidents and prohibit retaliation against employees who reported incidents of workplace violence.
On Wednesday, Sen. Baldwin called for unanimous consent on the Senate floor to fast-track this bill, but that was opposed by Senator Mike Braun (R-Ind.). She will soon introduce legislation similar to HR 1195, which the House passed.
“This bill will provide long overdue protections and safety standards,” she said. It will ensure that workplaces adopt proven protection techniques, such as those in OSHA’s 2015 guideline for preventing health care workplace violence. The American Hospital Association opposed the House bill on the grounds that hospitals have already implemented policies and programs specifically tailored to address workplace violence, so the OSHA standards required by the bill are not warranted.
Another speaker at the press conference, Aisha Terry, MD, MPH, FACEP, an emergency physician for George Washington University and Veterans Affairs in Washington, D.C., and current vice president of ACEP, described an incident that occurred when she was at work. A patient punched the nurse caring for him in the face, knocking her unconscious to the floor. “I’ll never forget that sound,” Dr. Terry said. “To this day, it has impacted her career. She hasn’t known what to do.”
Many people don’t realize how bad workplace violence really is, Dr. Terry added. “You assume you can serve as the safety net of this country, taking care of patients in the context of the pandemic, and feel safe – and not have to worry about your own safety. It’s past due that we put an end to this.”
Biggest win
Mr. Haines called the workplace violence bill a game changer for ED professionals, now and into the future. “We’re not going to totally eliminate violence in the emergency department. That is part of our business. But this legislation will support us and give a safer environment for us to do the work we love,” he said.
“The biggest win for this legislation is that it will create a supportive, nonretaliatory environment. It will give us as nurses a structured way to report things.” And, when these incidents do get reported, staff will get the help they need, Mr. Haines said. “The legislation will help show the importance of implementing systems and processes in emergency settings to address the risks and hazards that makes us all vulnerable to violence.”
No relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
TikTok challenge hits Taco Bell right in its ‘Stuft Nacho’
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Medical education programs tell how climate change affects health
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Unique residency track focuses on rural placement of graduates
BOSTON – As a former active-duty cavalry officer in the U.S. Army who served a 15-month tour in Iraq in 2003, Adam C. Byrd, MD, isn’t easily rattled.
On any given day, as the only dermatologist in his hometown of Louisville, Miss., which has a population of about 6,500, he sees 35-40 patients who present with conditions ranging from an infantile hemangioma to dermatomyositis and porphyria cutanea tarda. Being the go-to specialist for hundreds of miles with no on-site lab and no immediate personal access to Mohs surgeons and other subspecialists might unnerve some dermatologists, but not him.

“They’re a text message away, but they’re not in my office,” he said during a session on rural dermatology at the annual meeting of the American Academy of Dermatology. “I don’t have a mid-level practitioner, either. It’s just me and the residents, so it can be somewhat isolating. But in a rural area, you’re doing your patients a disservice if you can’t handle broad-spectrum medical dermatology. I consider myself a family dermatologist; I do a little bit of everything.” This includes prescribing treatments ranging from methotrexate for psoriasis, psoriatic arthritis, eczema, and other conditions; cyclosporine and azathioprine for pediatric eczema; propranolol for infantile hemangiomas; to IV infusions for dermatomyositis; phlebotomy for porphyria cutanea tarda; and biologics.
With no on-site pathology lab, Dr. Byrd sends specimens twice a week to the University of Mississippi Medical Center in Jackson via FedEx to be read. “I have to wait 3 days for results instead of 2,” he said. At the end of each workday, he personally carries microbiology samples to Winston Medical Center in Louisville – the area’s only hospital and where he was born – for processing.
After completing a 5-year integrated internal medicine-dermatology residency at the University of Minnesota in 2016, Dr. Byrd worked with Robert T. Brodell, MD, who chairs the department of dermatology at UMMC, and other university officials to open a satellite clinic in Louisville, where he provides full-spectrum skin care for Northern Mississippians. The clinic, located about 95 miles from UMMC’s “mothership” in Jackson, has become a vital training ground for the university, which created the only rural-specific dermatology residency of the 142 accredited dermatology programs in the United States. Of the three to four residents accepted per year, one is a rural track resident who spends 3-month–long rotations at rural clinic sites such as Dr. Byrd’s during each of the 3 years of general dermatology training, and the remaining 9 months of each year alongside their non–rural track coresidents.

One of the program’s rural track residents, Joshua R. Ortego, MD, worked in Dr. Byrd’s clinic during PGY-2. “It’s unique for one attending and one resident to work together for 3 months straight,” said Dr. Ortego, who grew up in Bay St. Louis on the Gulf Coast of Mississippi, which has a population of about 9,200. “Dr. Byrd learns our weaknesses and knows our strengths and areas for improvement. You get close. And there’s continuity; you see some patients back. With all the shuffling in the traditional dermatology residency model, sometimes you’re not seeing patients for follow-up appointments. But here you do.”
Rural dermatology track residents who rotate through Dr. Byrd’s Louisville clinic spend each Monday at the main campus in Jackson for a continuity clinic and didactics with non–rural track residents, “which allows for collegiality,” Dr. Ortego said. “My coresidents are like family; it would be hard to spend 3 months or even a year away from family like that.” The department foots the cost of lodging in a Louisville hotel 4 nights per week during these 3 months of training.
Dr. Ortego said that he performed a far greater number of procedures during PGY-2, compared with the averages performed in UMMC’s general dermatology rotation: 75 excisions (vs. 17), 71 repairs (vs. 15), and 23 excisions on the face or scalp (vs. none). He also cared for patients who presented with advanced disease because of access issues, and others with rare conditions. For example, in one afternoon clinic he and Dr. Byrd saw two patients with porphyria cutanea tarda, and one case each of dermatomyositis, bullous pemphigoid, and pyoderma gangrenosum. “We have an autoimmune blistering disease clinic in Jackson, but patients don’t want to drive there,” he said.

Then there are the perks that come with practicing in a rural area, including ready access to hiking, fishing, hunting, and spending time with family and friends. “Rural residents should be comfortable with the lifestyle,” he said. “Some cities don’t have the same amenities as San Francisco or Boston, but not everyone requires that. They just love where they’re from.”
The residency’s structure is designed to address the dire shortage of rural-based dermatologists in the United States. A study published in 2018 found that the difference in dermatologist density between metropolitan and rural counties in the United States increased from 3.41 per 100,000 people (3.47 vs. 0.065 per 100,000 people) in 1995 to 4.03 per 100, 000 people (4.11 vs. 0.085 per 100,000 people in 2013; P = .053). That’s about 40 times the number of dermatologists in metro areas, compared with rural areas.
Residents enrolled in UMMC’s rural dermatology track are expected to serve at least 3 years at a rural location upon graduation at a site mutually agreed upon by the resident and the UMMC. Dr. Ortego plans to practice in Bay St. Louis after completing his residency. “The idea is that you’re happy, that you’re in your hometown,” he said.
According to Dr. Byrd, the 3-year commitment brings job security to rural track residents in their preferred location while meeting the demands of an underserved population. “We are still tweaking this,” he said of the residency track, which includes plans to establish more satellite clinics in other areas of rural Mississippi. “Our department chair does not have 100% control over hiring and office expansion. We are subject to the Mississippi Institutions of Higher Learning, which is a branch of the state government. This has to be addressed at the council of chairs and university chancellor level and even state government. It can be done, but you really must be dedicated.”
Meanwhile, the effect that dermatologists like Dr. Byrd have on citizens of his area of rural Mississippi is palpable. Many refuse to travel outside of Louisville city limits to see a specialist, so when surgery for a suspicious lesion is indicated, they tell him, “You’re going to do it, or it’s not going to get done,” said Dr. Byrd, who continues to serve in the Mississippi Army National Guard as a field surgeon. “I don’t say ‘no’ a whole lot.” He refers patients to Mohs micrographic surgery colleagues in Jackson daily and is transparent with patients who hesitate to elect Mohs surgery. “I’ll say, ‘I can do the job, but there’s a higher risk of positive margins, and a Mohs surgeon could do a much better job.’”
He acknowledged that rural dermatology “isn’t for everyone. It requires a physician that has a good training foundation in medical and surgical dermatology, someone with a ‘can do’ attitude and a healthy level of confidence. I try to do the best for my patients. It’s endearing when they trust you.”
Mary Logue, MD, who practices dermatology in Minot, N.D., finds the structure of UMMC’s rural dermatology track inspiring. Upon completing her dermatology residency at the University of New Mexico, where she remains on the volunteer faculty, she had hoped to return to serve the community of Gallup, N.M., and help bridge the gap in dermatology health care access for residents of rural New Mexico, especially those on Native American reservations. That opportunity never transpired, but Dr. Logue was able to pursue her passion for rural medicine in North Dakota.
“It is my hope that more programs will implement a similar structure to UMMC’s rural dermatology track and get more dermatologists practicing in rural areas,” Dr. Logue told this news organization. “They have developed a very practical and financially sustainable model, which I think every state could benefit from.”
She added that the UMMC “has found a way to bring dermatology to disadvantaged rural communities while also addressing the problem of underrepresented minorities in medicine. Medical students of color and medical students from rural communities are the least represented groups in dermatology, but the most likely to return to their communities to practice. Every day I see patients with adverse dermatologic outcomes as a direct result of lack of access to a dermatologist. This is happening across the country, which is why the efforts of UMMC Dermatology and their department chair, Dr. Brodell, are so important.”
BOSTON – As a former active-duty cavalry officer in the U.S. Army who served a 15-month tour in Iraq in 2003, Adam C. Byrd, MD, isn’t easily rattled.
On any given day, as the only dermatologist in his hometown of Louisville, Miss., which has a population of about 6,500, he sees 35-40 patients who present with conditions ranging from an infantile hemangioma to dermatomyositis and porphyria cutanea tarda. Being the go-to specialist for hundreds of miles with no on-site lab and no immediate personal access to Mohs surgeons and other subspecialists might unnerve some dermatologists, but not him.

“They’re a text message away, but they’re not in my office,” he said during a session on rural dermatology at the annual meeting of the American Academy of Dermatology. “I don’t have a mid-level practitioner, either. It’s just me and the residents, so it can be somewhat isolating. But in a rural area, you’re doing your patients a disservice if you can’t handle broad-spectrum medical dermatology. I consider myself a family dermatologist; I do a little bit of everything.” This includes prescribing treatments ranging from methotrexate for psoriasis, psoriatic arthritis, eczema, and other conditions; cyclosporine and azathioprine for pediatric eczema; propranolol for infantile hemangiomas; to IV infusions for dermatomyositis; phlebotomy for porphyria cutanea tarda; and biologics.
With no on-site pathology lab, Dr. Byrd sends specimens twice a week to the University of Mississippi Medical Center in Jackson via FedEx to be read. “I have to wait 3 days for results instead of 2,” he said. At the end of each workday, he personally carries microbiology samples to Winston Medical Center in Louisville – the area’s only hospital and where he was born – for processing.
After completing a 5-year integrated internal medicine-dermatology residency at the University of Minnesota in 2016, Dr. Byrd worked with Robert T. Brodell, MD, who chairs the department of dermatology at UMMC, and other university officials to open a satellite clinic in Louisville, where he provides full-spectrum skin care for Northern Mississippians. The clinic, located about 95 miles from UMMC’s “mothership” in Jackson, has become a vital training ground for the university, which created the only rural-specific dermatology residency of the 142 accredited dermatology programs in the United States. Of the three to four residents accepted per year, one is a rural track resident who spends 3-month–long rotations at rural clinic sites such as Dr. Byrd’s during each of the 3 years of general dermatology training, and the remaining 9 months of each year alongside their non–rural track coresidents.

One of the program’s rural track residents, Joshua R. Ortego, MD, worked in Dr. Byrd’s clinic during PGY-2. “It’s unique for one attending and one resident to work together for 3 months straight,” said Dr. Ortego, who grew up in Bay St. Louis on the Gulf Coast of Mississippi, which has a population of about 9,200. “Dr. Byrd learns our weaknesses and knows our strengths and areas for improvement. You get close. And there’s continuity; you see some patients back. With all the shuffling in the traditional dermatology residency model, sometimes you’re not seeing patients for follow-up appointments. But here you do.”
Rural dermatology track residents who rotate through Dr. Byrd’s Louisville clinic spend each Monday at the main campus in Jackson for a continuity clinic and didactics with non–rural track residents, “which allows for collegiality,” Dr. Ortego said. “My coresidents are like family; it would be hard to spend 3 months or even a year away from family like that.” The department foots the cost of lodging in a Louisville hotel 4 nights per week during these 3 months of training.
Dr. Ortego said that he performed a far greater number of procedures during PGY-2, compared with the averages performed in UMMC’s general dermatology rotation: 75 excisions (vs. 17), 71 repairs (vs. 15), and 23 excisions on the face or scalp (vs. none). He also cared for patients who presented with advanced disease because of access issues, and others with rare conditions. For example, in one afternoon clinic he and Dr. Byrd saw two patients with porphyria cutanea tarda, and one case each of dermatomyositis, bullous pemphigoid, and pyoderma gangrenosum. “We have an autoimmune blistering disease clinic in Jackson, but patients don’t want to drive there,” he said.

Then there are the perks that come with practicing in a rural area, including ready access to hiking, fishing, hunting, and spending time with family and friends. “Rural residents should be comfortable with the lifestyle,” he said. “Some cities don’t have the same amenities as San Francisco or Boston, but not everyone requires that. They just love where they’re from.”
The residency’s structure is designed to address the dire shortage of rural-based dermatologists in the United States. A study published in 2018 found that the difference in dermatologist density between metropolitan and rural counties in the United States increased from 3.41 per 100,000 people (3.47 vs. 0.065 per 100,000 people) in 1995 to 4.03 per 100, 000 people (4.11 vs. 0.085 per 100,000 people in 2013; P = .053). That’s about 40 times the number of dermatologists in metro areas, compared with rural areas.
Residents enrolled in UMMC’s rural dermatology track are expected to serve at least 3 years at a rural location upon graduation at a site mutually agreed upon by the resident and the UMMC. Dr. Ortego plans to practice in Bay St. Louis after completing his residency. “The idea is that you’re happy, that you’re in your hometown,” he said.
According to Dr. Byrd, the 3-year commitment brings job security to rural track residents in their preferred location while meeting the demands of an underserved population. “We are still tweaking this,” he said of the residency track, which includes plans to establish more satellite clinics in other areas of rural Mississippi. “Our department chair does not have 100% control over hiring and office expansion. We are subject to the Mississippi Institutions of Higher Learning, which is a branch of the state government. This has to be addressed at the council of chairs and university chancellor level and even state government. It can be done, but you really must be dedicated.”
Meanwhile, the effect that dermatologists like Dr. Byrd have on citizens of his area of rural Mississippi is palpable. Many refuse to travel outside of Louisville city limits to see a specialist, so when surgery for a suspicious lesion is indicated, they tell him, “You’re going to do it, or it’s not going to get done,” said Dr. Byrd, who continues to serve in the Mississippi Army National Guard as a field surgeon. “I don’t say ‘no’ a whole lot.” He refers patients to Mohs micrographic surgery colleagues in Jackson daily and is transparent with patients who hesitate to elect Mohs surgery. “I’ll say, ‘I can do the job, but there’s a higher risk of positive margins, and a Mohs surgeon could do a much better job.’”
He acknowledged that rural dermatology “isn’t for everyone. It requires a physician that has a good training foundation in medical and surgical dermatology, someone with a ‘can do’ attitude and a healthy level of confidence. I try to do the best for my patients. It’s endearing when they trust you.”
Mary Logue, MD, who practices dermatology in Minot, N.D., finds the structure of UMMC’s rural dermatology track inspiring. Upon completing her dermatology residency at the University of New Mexico, where she remains on the volunteer faculty, she had hoped to return to serve the community of Gallup, N.M., and help bridge the gap in dermatology health care access for residents of rural New Mexico, especially those on Native American reservations. That opportunity never transpired, but Dr. Logue was able to pursue her passion for rural medicine in North Dakota.
“It is my hope that more programs will implement a similar structure to UMMC’s rural dermatology track and get more dermatologists practicing in rural areas,” Dr. Logue told this news organization. “They have developed a very practical and financially sustainable model, which I think every state could benefit from.”
She added that the UMMC “has found a way to bring dermatology to disadvantaged rural communities while also addressing the problem of underrepresented minorities in medicine. Medical students of color and medical students from rural communities are the least represented groups in dermatology, but the most likely to return to their communities to practice. Every day I see patients with adverse dermatologic outcomes as a direct result of lack of access to a dermatologist. This is happening across the country, which is why the efforts of UMMC Dermatology and their department chair, Dr. Brodell, are so important.”
BOSTON – As a former active-duty cavalry officer in the U.S. Army who served a 15-month tour in Iraq in 2003, Adam C. Byrd, MD, isn’t easily rattled.
On any given day, as the only dermatologist in his hometown of Louisville, Miss., which has a population of about 6,500, he sees 35-40 patients who present with conditions ranging from an infantile hemangioma to dermatomyositis and porphyria cutanea tarda. Being the go-to specialist for hundreds of miles with no on-site lab and no immediate personal access to Mohs surgeons and other subspecialists might unnerve some dermatologists, but not him.

“They’re a text message away, but they’re not in my office,” he said during a session on rural dermatology at the annual meeting of the American Academy of Dermatology. “I don’t have a mid-level practitioner, either. It’s just me and the residents, so it can be somewhat isolating. But in a rural area, you’re doing your patients a disservice if you can’t handle broad-spectrum medical dermatology. I consider myself a family dermatologist; I do a little bit of everything.” This includes prescribing treatments ranging from methotrexate for psoriasis, psoriatic arthritis, eczema, and other conditions; cyclosporine and azathioprine for pediatric eczema; propranolol for infantile hemangiomas; to IV infusions for dermatomyositis; phlebotomy for porphyria cutanea tarda; and biologics.
With no on-site pathology lab, Dr. Byrd sends specimens twice a week to the University of Mississippi Medical Center in Jackson via FedEx to be read. “I have to wait 3 days for results instead of 2,” he said. At the end of each workday, he personally carries microbiology samples to Winston Medical Center in Louisville – the area’s only hospital and where he was born – for processing.
After completing a 5-year integrated internal medicine-dermatology residency at the University of Minnesota in 2016, Dr. Byrd worked with Robert T. Brodell, MD, who chairs the department of dermatology at UMMC, and other university officials to open a satellite clinic in Louisville, where he provides full-spectrum skin care for Northern Mississippians. The clinic, located about 95 miles from UMMC’s “mothership” in Jackson, has become a vital training ground for the university, which created the only rural-specific dermatology residency of the 142 accredited dermatology programs in the United States. Of the three to four residents accepted per year, one is a rural track resident who spends 3-month–long rotations at rural clinic sites such as Dr. Byrd’s during each of the 3 years of general dermatology training, and the remaining 9 months of each year alongside their non–rural track coresidents.

One of the program’s rural track residents, Joshua R. Ortego, MD, worked in Dr. Byrd’s clinic during PGY-2. “It’s unique for one attending and one resident to work together for 3 months straight,” said Dr. Ortego, who grew up in Bay St. Louis on the Gulf Coast of Mississippi, which has a population of about 9,200. “Dr. Byrd learns our weaknesses and knows our strengths and areas for improvement. You get close. And there’s continuity; you see some patients back. With all the shuffling in the traditional dermatology residency model, sometimes you’re not seeing patients for follow-up appointments. But here you do.”
Rural dermatology track residents who rotate through Dr. Byrd’s Louisville clinic spend each Monday at the main campus in Jackson for a continuity clinic and didactics with non–rural track residents, “which allows for collegiality,” Dr. Ortego said. “My coresidents are like family; it would be hard to spend 3 months or even a year away from family like that.” The department foots the cost of lodging in a Louisville hotel 4 nights per week during these 3 months of training.
Dr. Ortego said that he performed a far greater number of procedures during PGY-2, compared with the averages performed in UMMC’s general dermatology rotation: 75 excisions (vs. 17), 71 repairs (vs. 15), and 23 excisions on the face or scalp (vs. none). He also cared for patients who presented with advanced disease because of access issues, and others with rare conditions. For example, in one afternoon clinic he and Dr. Byrd saw two patients with porphyria cutanea tarda, and one case each of dermatomyositis, bullous pemphigoid, and pyoderma gangrenosum. “We have an autoimmune blistering disease clinic in Jackson, but patients don’t want to drive there,” he said.

Then there are the perks that come with practicing in a rural area, including ready access to hiking, fishing, hunting, and spending time with family and friends. “Rural residents should be comfortable with the lifestyle,” he said. “Some cities don’t have the same amenities as San Francisco or Boston, but not everyone requires that. They just love where they’re from.”
The residency’s structure is designed to address the dire shortage of rural-based dermatologists in the United States. A study published in 2018 found that the difference in dermatologist density between metropolitan and rural counties in the United States increased from 3.41 per 100,000 people (3.47 vs. 0.065 per 100,000 people) in 1995 to 4.03 per 100, 000 people (4.11 vs. 0.085 per 100,000 people in 2013; P = .053). That’s about 40 times the number of dermatologists in metro areas, compared with rural areas.
Residents enrolled in UMMC’s rural dermatology track are expected to serve at least 3 years at a rural location upon graduation at a site mutually agreed upon by the resident and the UMMC. Dr. Ortego plans to practice in Bay St. Louis after completing his residency. “The idea is that you’re happy, that you’re in your hometown,” he said.
According to Dr. Byrd, the 3-year commitment brings job security to rural track residents in their preferred location while meeting the demands of an underserved population. “We are still tweaking this,” he said of the residency track, which includes plans to establish more satellite clinics in other areas of rural Mississippi. “Our department chair does not have 100% control over hiring and office expansion. We are subject to the Mississippi Institutions of Higher Learning, which is a branch of the state government. This has to be addressed at the council of chairs and university chancellor level and even state government. It can be done, but you really must be dedicated.”
Meanwhile, the effect that dermatologists like Dr. Byrd have on citizens of his area of rural Mississippi is palpable. Many refuse to travel outside of Louisville city limits to see a specialist, so when surgery for a suspicious lesion is indicated, they tell him, “You’re going to do it, or it’s not going to get done,” said Dr. Byrd, who continues to serve in the Mississippi Army National Guard as a field surgeon. “I don’t say ‘no’ a whole lot.” He refers patients to Mohs micrographic surgery colleagues in Jackson daily and is transparent with patients who hesitate to elect Mohs surgery. “I’ll say, ‘I can do the job, but there’s a higher risk of positive margins, and a Mohs surgeon could do a much better job.’”
He acknowledged that rural dermatology “isn’t for everyone. It requires a physician that has a good training foundation in medical and surgical dermatology, someone with a ‘can do’ attitude and a healthy level of confidence. I try to do the best for my patients. It’s endearing when they trust you.”
Mary Logue, MD, who practices dermatology in Minot, N.D., finds the structure of UMMC’s rural dermatology track inspiring. Upon completing her dermatology residency at the University of New Mexico, where she remains on the volunteer faculty, she had hoped to return to serve the community of Gallup, N.M., and help bridge the gap in dermatology health care access for residents of rural New Mexico, especially those on Native American reservations. That opportunity never transpired, but Dr. Logue was able to pursue her passion for rural medicine in North Dakota.
“It is my hope that more programs will implement a similar structure to UMMC’s rural dermatology track and get more dermatologists practicing in rural areas,” Dr. Logue told this news organization. “They have developed a very practical and financially sustainable model, which I think every state could benefit from.”
She added that the UMMC “has found a way to bring dermatology to disadvantaged rural communities while also addressing the problem of underrepresented minorities in medicine. Medical students of color and medical students from rural communities are the least represented groups in dermatology, but the most likely to return to their communities to practice. Every day I see patients with adverse dermatologic outcomes as a direct result of lack of access to a dermatologist. This is happening across the country, which is why the efforts of UMMC Dermatology and their department chair, Dr. Brodell, are so important.”
At AAD 22
Reduced-frequency methotrexate monitoring causes no harm
Reducing the frequency of routine blood monitoring for methotrexate in patients with rheumatoid arthritis during the COVID-19 pandemic was associated with no adverse outcomes for patients, British researchers have found.
Similar laboratory results were recorded in patients who were switched from testing once per month to once every 3 or 5 months, Natasha Wood, a general practice trainee at North Devon District Hospital in Barnstaple, England, reported at the annual meeting of the British Society for Rheumatology.
“Less frequent monitoring did not result in patient harm,” she said.
“There’s an increasing evidence base; we wonder whether now’s the time to reconsider our DMARD-monitoring strategy,” Ms. Wood said.
Changes in monitoring because of pandemic
Methotrexate monitoring is important to minimize the risk of harm to patients, and it is recommended that standard laboratory tests, such as a complete blood count, creatinine, and liver enzymes are measured regularly. Indeed, both the BSR and the American College of Rheumatology have specific recommendations on the monitoring of methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDS).
“The BSR used to advise for monthly blood tests in patients taking methotrexate,” Ms. Wood said, but the BSR moved to recommend testing patients on a stable dose every 3 months in 2017.
“Things of course changed again rapidly with COVID, with the BSR quickly updating their guidelines advising for less frequent monitoring in this patient group,” Ms. Wood said.
As a result, the North Devon Clinical Commissioning Group, which covers the hospital where Ms. Wood works, agreed to allow testing every 6 months for patients on a stable methotrexate dose. “This was across specialties, so not just rheumatology, but dermatology and gastroenterology as well,” she said.
“This provided us with a really exciting and unique opportunity to look at this patient group and see what happened,” Ms. Wood explained.
Effect of less frequent monitoring
At the meeting, Ms. Wood presented the results of an audit of 854 patients found via a search of hospital pathology records who were stable on methotrexate monotherapy for at least 12 months.
Two subanalyses were performed: One looked at patients who had changed from blood testing once every month to once every 3 months (n = 229) and the other looking at a group of 120 patients who had gone from testing once every 3 months to approximately every 5 months.
The mean age of patients was 67 for monthly testing, 69 for testing every 3 months, and 66 for testing about every 5 months, with around two-thirds of patients being of female sex.
A comparison of the number of blood tests performed to the end of April 2020 with the number performed to the end of April 2021 showed that there had mainly been a shift from testing once per month to once every 3 months, with some patients being tested in line with the revised BSR guidelines at around 5 months.
“Interestingly, a third of this group had no changed monitoring frequency despite the change in guidelines,” Ms. Wood said.
“Prepandemic, most patients [were] having monthly bloods despite BSR advice from 2017, and despite the pandemic with the updated shared care guidelines,” patients were still having blood drawn every 3 months, Ms. Wood noted. This perhaps needs further investigation and consideration to understand why recommended changes to the frequency of testing are not being adhered to.
The overall distribution of laboratory findings was similar among those who went from testing once per month to once every 3 months and from every 3 months to every 5 months. This included the distribution of neutrophils, whole blood counts, and alanine aminotransferase. There were some changes for platelets, mean cell volume, and the estimated glomerular filtration rate, but these were not clinically significant.
“Abnormal blood results aren’t common in stable methotrexate monotherapy patients,” Ms. Wood reported. “Where abnormalities did occur, it was in the context of patients being concurrently unwell and symptomatic.”
Time for patient-initiated testing?
There are several advantages of less frequent methotrexate monitoring, Ms. Wood said. One is the practicalities of getting to and from appointments, particularly in remote locations, such as where she works.
In addition to reducing workloads and pressure on already busy hospitals and primary care, this could have a huge environmental impact, she suggested.
Moreover, “moderate-quality evidence” supports the current monitoring frequency recommendation.
“We know that our numbers are small – we’re a small center – but our findings are consistent with much larger studies across the U.K.,” Ms. Wood said.
“We wonder whether there’s the possibility of moving towards annual monitoring with good safety netting and patient education for additional blood tests if they are unwell,” she said, adding that “now may be the time for patient-initiated methotrexate monitoring.”
Ms. Wood disclosed Janssen sponsorship for attending the BSR 2022 annual meeting.
Reducing the frequency of routine blood monitoring for methotrexate in patients with rheumatoid arthritis during the COVID-19 pandemic was associated with no adverse outcomes for patients, British researchers have found.
Similar laboratory results were recorded in patients who were switched from testing once per month to once every 3 or 5 months, Natasha Wood, a general practice trainee at North Devon District Hospital in Barnstaple, England, reported at the annual meeting of the British Society for Rheumatology.
“Less frequent monitoring did not result in patient harm,” she said.
“There’s an increasing evidence base; we wonder whether now’s the time to reconsider our DMARD-monitoring strategy,” Ms. Wood said.
Changes in monitoring because of pandemic
Methotrexate monitoring is important to minimize the risk of harm to patients, and it is recommended that standard laboratory tests, such as a complete blood count, creatinine, and liver enzymes are measured regularly. Indeed, both the BSR and the American College of Rheumatology have specific recommendations on the monitoring of methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDS).
“The BSR used to advise for monthly blood tests in patients taking methotrexate,” Ms. Wood said, but the BSR moved to recommend testing patients on a stable dose every 3 months in 2017.
“Things of course changed again rapidly with COVID, with the BSR quickly updating their guidelines advising for less frequent monitoring in this patient group,” Ms. Wood said.
As a result, the North Devon Clinical Commissioning Group, which covers the hospital where Ms. Wood works, agreed to allow testing every 6 months for patients on a stable methotrexate dose. “This was across specialties, so not just rheumatology, but dermatology and gastroenterology as well,” she said.
“This provided us with a really exciting and unique opportunity to look at this patient group and see what happened,” Ms. Wood explained.
Effect of less frequent monitoring
At the meeting, Ms. Wood presented the results of an audit of 854 patients found via a search of hospital pathology records who were stable on methotrexate monotherapy for at least 12 months.
Two subanalyses were performed: One looked at patients who had changed from blood testing once every month to once every 3 months (n = 229) and the other looking at a group of 120 patients who had gone from testing once every 3 months to approximately every 5 months.
The mean age of patients was 67 for monthly testing, 69 for testing every 3 months, and 66 for testing about every 5 months, with around two-thirds of patients being of female sex.
A comparison of the number of blood tests performed to the end of April 2020 with the number performed to the end of April 2021 showed that there had mainly been a shift from testing once per month to once every 3 months, with some patients being tested in line with the revised BSR guidelines at around 5 months.
“Interestingly, a third of this group had no changed monitoring frequency despite the change in guidelines,” Ms. Wood said.
“Prepandemic, most patients [were] having monthly bloods despite BSR advice from 2017, and despite the pandemic with the updated shared care guidelines,” patients were still having blood drawn every 3 months, Ms. Wood noted. This perhaps needs further investigation and consideration to understand why recommended changes to the frequency of testing are not being adhered to.
The overall distribution of laboratory findings was similar among those who went from testing once per month to once every 3 months and from every 3 months to every 5 months. This included the distribution of neutrophils, whole blood counts, and alanine aminotransferase. There were some changes for platelets, mean cell volume, and the estimated glomerular filtration rate, but these were not clinically significant.
“Abnormal blood results aren’t common in stable methotrexate monotherapy patients,” Ms. Wood reported. “Where abnormalities did occur, it was in the context of patients being concurrently unwell and symptomatic.”
Time for patient-initiated testing?
There are several advantages of less frequent methotrexate monitoring, Ms. Wood said. One is the practicalities of getting to and from appointments, particularly in remote locations, such as where she works.
In addition to reducing workloads and pressure on already busy hospitals and primary care, this could have a huge environmental impact, she suggested.
Moreover, “moderate-quality evidence” supports the current monitoring frequency recommendation.
“We know that our numbers are small – we’re a small center – but our findings are consistent with much larger studies across the U.K.,” Ms. Wood said.
“We wonder whether there’s the possibility of moving towards annual monitoring with good safety netting and patient education for additional blood tests if they are unwell,” she said, adding that “now may be the time for patient-initiated methotrexate monitoring.”
Ms. Wood disclosed Janssen sponsorship for attending the BSR 2022 annual meeting.
Reducing the frequency of routine blood monitoring for methotrexate in patients with rheumatoid arthritis during the COVID-19 pandemic was associated with no adverse outcomes for patients, British researchers have found.
Similar laboratory results were recorded in patients who were switched from testing once per month to once every 3 or 5 months, Natasha Wood, a general practice trainee at North Devon District Hospital in Barnstaple, England, reported at the annual meeting of the British Society for Rheumatology.
“Less frequent monitoring did not result in patient harm,” she said.
“There’s an increasing evidence base; we wonder whether now’s the time to reconsider our DMARD-monitoring strategy,” Ms. Wood said.
Changes in monitoring because of pandemic
Methotrexate monitoring is important to minimize the risk of harm to patients, and it is recommended that standard laboratory tests, such as a complete blood count, creatinine, and liver enzymes are measured regularly. Indeed, both the BSR and the American College of Rheumatology have specific recommendations on the monitoring of methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDS).
“The BSR used to advise for monthly blood tests in patients taking methotrexate,” Ms. Wood said, but the BSR moved to recommend testing patients on a stable dose every 3 months in 2017.
“Things of course changed again rapidly with COVID, with the BSR quickly updating their guidelines advising for less frequent monitoring in this patient group,” Ms. Wood said.
As a result, the North Devon Clinical Commissioning Group, which covers the hospital where Ms. Wood works, agreed to allow testing every 6 months for patients on a stable methotrexate dose. “This was across specialties, so not just rheumatology, but dermatology and gastroenterology as well,” she said.
“This provided us with a really exciting and unique opportunity to look at this patient group and see what happened,” Ms. Wood explained.
Effect of less frequent monitoring
At the meeting, Ms. Wood presented the results of an audit of 854 patients found via a search of hospital pathology records who were stable on methotrexate monotherapy for at least 12 months.
Two subanalyses were performed: One looked at patients who had changed from blood testing once every month to once every 3 months (n = 229) and the other looking at a group of 120 patients who had gone from testing once every 3 months to approximately every 5 months.
The mean age of patients was 67 for monthly testing, 69 for testing every 3 months, and 66 for testing about every 5 months, with around two-thirds of patients being of female sex.
A comparison of the number of blood tests performed to the end of April 2020 with the number performed to the end of April 2021 showed that there had mainly been a shift from testing once per month to once every 3 months, with some patients being tested in line with the revised BSR guidelines at around 5 months.
“Interestingly, a third of this group had no changed monitoring frequency despite the change in guidelines,” Ms. Wood said.
“Prepandemic, most patients [were] having monthly bloods despite BSR advice from 2017, and despite the pandemic with the updated shared care guidelines,” patients were still having blood drawn every 3 months, Ms. Wood noted. This perhaps needs further investigation and consideration to understand why recommended changes to the frequency of testing are not being adhered to.
The overall distribution of laboratory findings was similar among those who went from testing once per month to once every 3 months and from every 3 months to every 5 months. This included the distribution of neutrophils, whole blood counts, and alanine aminotransferase. There were some changes for platelets, mean cell volume, and the estimated glomerular filtration rate, but these were not clinically significant.
“Abnormal blood results aren’t common in stable methotrexate monotherapy patients,” Ms. Wood reported. “Where abnormalities did occur, it was in the context of patients being concurrently unwell and symptomatic.”
Time for patient-initiated testing?
There are several advantages of less frequent methotrexate monitoring, Ms. Wood said. One is the practicalities of getting to and from appointments, particularly in remote locations, such as where she works.
In addition to reducing workloads and pressure on already busy hospitals and primary care, this could have a huge environmental impact, she suggested.
Moreover, “moderate-quality evidence” supports the current monitoring frequency recommendation.
“We know that our numbers are small – we’re a small center – but our findings are consistent with much larger studies across the U.K.,” Ms. Wood said.
“We wonder whether there’s the possibility of moving towards annual monitoring with good safety netting and patient education for additional blood tests if they are unwell,” she said, adding that “now may be the time for patient-initiated methotrexate monitoring.”
Ms. Wood disclosed Janssen sponsorship for attending the BSR 2022 annual meeting.
FROM BSR 2022
My choice? Unvaccinated pose outsize risk to vaccinated
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL