User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
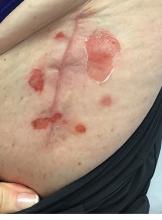
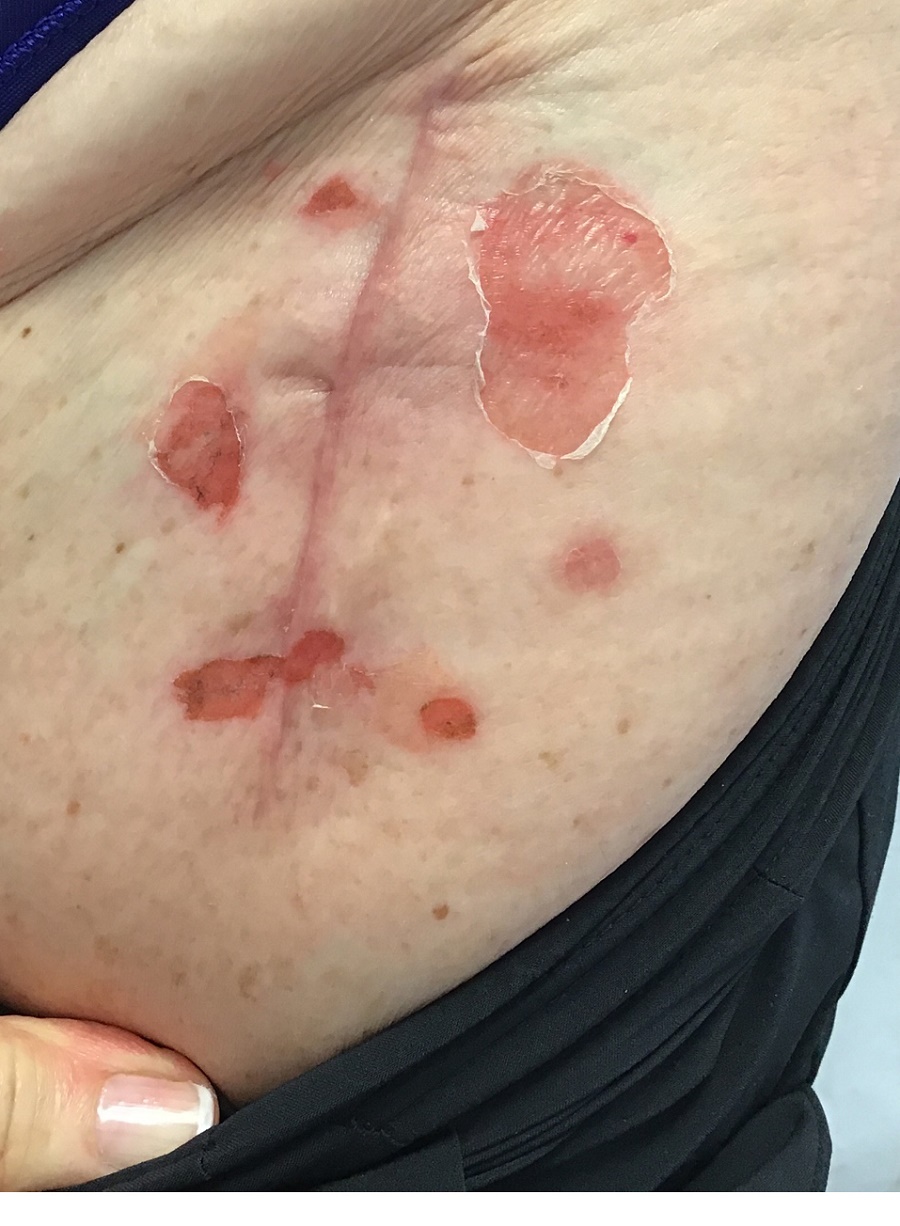
A 71-year-old White female developed erosions after hip replacement surgery 2 months prior to presentation
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
Bimekizumab Gains FDA Approval for Psoriatic Arthritis, Axial Spondyloarthritis
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
Biomarkers in Cord Blood May Predict AD Onset in Newborns, Study Suggests
TOPLINE:
and interleukin (IL) 31.
METHODOLOGY:
- Researchers conducted a prospective study to evaluate the predictive role of serologic biomarkers and cutaneous markers and the development of AD in 40 full-term newborns from a university hospital in Italy.
- Cord blood was collected at birth and analyzed for serum biomarkers such as CCL17/TARC and IL-31.
- TEWL and skin hydration rates were measured at 1, 6, and 12 months, and dermatological features such as dryness, cradle cap, and eczematous lesions were also monitored during visits.
TAKEAWAY:
- At 6 months, 16 infants had symptoms of AD, which included dry skin, pruritus, and keratosis pilaris, which persisted at 12 months. Their mean Eczema Area and Severity Index score was 6.6 at 6 months and 2.9 at 12 months.
- Infants with signs of AD had significantly higher TEWL levels at the anterior cubital fossa at 1, 6, and 12 months than those without AD.
- Cord blood levels of CCL17/TARC and IL-31 were significantly higher in infants with AD.
- A correlation was found between TEWL values and CCL17 levels at 1, 6, and 12 months.
IN PRACTICE:
“,” the authors wrote. “Stratified interventions based on these variables, family history, FLG [filaggrin] variations, and other biomarkers could offer more targeted approaches to AD prevention and management, especially during the first year of life,” they added.
SOURCE:
The study was led by Angelo Massimiliano D’Erme, MD, PhD, of the Dermatology Unit, in the Department of Medical and Oncology, University of Pisa, Pisa, Italy, and was published online in JAMA Dermatology.
LIMITATIONS:
The limitations included the observational design and small sample size, and it was a single-center study.
DISCLOSURES:
The authors did not disclose any funding information. One author disclosed receiving personal fees from various pharmaceutical companies and serving as a founder and chairman of a nonprofit organization.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
TOPLINE:
and interleukin (IL) 31.
METHODOLOGY:
- Researchers conducted a prospective study to evaluate the predictive role of serologic biomarkers and cutaneous markers and the development of AD in 40 full-term newborns from a university hospital in Italy.
- Cord blood was collected at birth and analyzed for serum biomarkers such as CCL17/TARC and IL-31.
- TEWL and skin hydration rates were measured at 1, 6, and 12 months, and dermatological features such as dryness, cradle cap, and eczematous lesions were also monitored during visits.
TAKEAWAY:
- At 6 months, 16 infants had symptoms of AD, which included dry skin, pruritus, and keratosis pilaris, which persisted at 12 months. Their mean Eczema Area and Severity Index score was 6.6 at 6 months and 2.9 at 12 months.
- Infants with signs of AD had significantly higher TEWL levels at the anterior cubital fossa at 1, 6, and 12 months than those without AD.
- Cord blood levels of CCL17/TARC and IL-31 were significantly higher in infants with AD.
- A correlation was found between TEWL values and CCL17 levels at 1, 6, and 12 months.
IN PRACTICE:
“,” the authors wrote. “Stratified interventions based on these variables, family history, FLG [filaggrin] variations, and other biomarkers could offer more targeted approaches to AD prevention and management, especially during the first year of life,” they added.
SOURCE:
The study was led by Angelo Massimiliano D’Erme, MD, PhD, of the Dermatology Unit, in the Department of Medical and Oncology, University of Pisa, Pisa, Italy, and was published online in JAMA Dermatology.
LIMITATIONS:
The limitations included the observational design and small sample size, and it was a single-center study.
DISCLOSURES:
The authors did not disclose any funding information. One author disclosed receiving personal fees from various pharmaceutical companies and serving as a founder and chairman of a nonprofit organization.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
TOPLINE:
and interleukin (IL) 31.
METHODOLOGY:
- Researchers conducted a prospective study to evaluate the predictive role of serologic biomarkers and cutaneous markers and the development of AD in 40 full-term newborns from a university hospital in Italy.
- Cord blood was collected at birth and analyzed for serum biomarkers such as CCL17/TARC and IL-31.
- TEWL and skin hydration rates were measured at 1, 6, and 12 months, and dermatological features such as dryness, cradle cap, and eczematous lesions were also monitored during visits.
TAKEAWAY:
- At 6 months, 16 infants had symptoms of AD, which included dry skin, pruritus, and keratosis pilaris, which persisted at 12 months. Their mean Eczema Area and Severity Index score was 6.6 at 6 months and 2.9 at 12 months.
- Infants with signs of AD had significantly higher TEWL levels at the anterior cubital fossa at 1, 6, and 12 months than those without AD.
- Cord blood levels of CCL17/TARC and IL-31 were significantly higher in infants with AD.
- A correlation was found between TEWL values and CCL17 levels at 1, 6, and 12 months.
IN PRACTICE:
“,” the authors wrote. “Stratified interventions based on these variables, family history, FLG [filaggrin] variations, and other biomarkers could offer more targeted approaches to AD prevention and management, especially during the first year of life,” they added.
SOURCE:
The study was led by Angelo Massimiliano D’Erme, MD, PhD, of the Dermatology Unit, in the Department of Medical and Oncology, University of Pisa, Pisa, Italy, and was published online in JAMA Dermatology.
LIMITATIONS:
The limitations included the observational design and small sample size, and it was a single-center study.
DISCLOSURES:
The authors did not disclose any funding information. One author disclosed receiving personal fees from various pharmaceutical companies and serving as a founder and chairman of a nonprofit organization.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
Trial Looks at Early Use of Mycophenolate to Reduce Flares, Nephritis
Early use of mycophenolate mofetil (MMF), a drug used to dampen the immune system in organ transplant recipients, may reduce the risk for severe flares in patients with newly diagnosed systemic lupus erythematosus (SLE), according to results from a randomized, open-label, observer-blinded clinical trial.
In interviews, two SLE specialists who were not involved with the study said the research is preliminary but promising. However, another specialist criticized the paper’s reliance on unusual doses of prednisone and MMF, saying it “puts people on a treatment regimen that nobody ever uses.”
The Lupus Foundation of America estimates that about 16,000 people in the United States are diagnosed with lupus each year. “Our current treatment paradigm is to go pretty slowly and start treatment for new-onset, mild SLE with glucocorticoids, if necessary, and hydroxychloroquine,” said Karen H. Costenbader, MD, MPH, of Harvard Medical School and Harvard School of Public Health, Boston, Massachusetts.
Stronger immunosuppressive agents may be added as patients progress, she said.
Off-label use of MMF, which is approved by the Food and Drug Administration only for patients with certain organ transplants, may be appropriate in some cases, she said. “There is a big push to start immunosuppressives earlier, but we currently would reserve mycophenolate for those with severe manifestations — lupus nephritis; vasculitis; or lung, brain, or heart inflammation.”
In the trial, adult patients who received oral prednisone (starting at 0.5 mg/kg per day) and hydroxychloroquine sulfate (5 mg/kg per day) plus MMF (500 mg twice daily) for 96 weeks were less likely to develop severe flares than those who took the regimen without MMF (relative risk [RR], 0.39; 95% CI, 0.17-0.87; P = .01). Severe flares occurred in 10.8% of the MMF group (7 of 65 patients) and in 27.7% of the control group (18 of 65), Yijun You, MD, of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and colleagues reported in JAMA Network Open.
Patients in the MMF group also had 89% lower risk for lupus nephritis than those in the control group (RR, 0.11; 95% CI, 0.01-0.85; P = .008), with kidney involvement occurring in 1.5% (1 of 65) vs 13.8% (9 of 65).
During 2018-2021, researchers recruited 130 patients in China aged 18-65 years with newly diagnosed SLE, a high titer of anti–double-stranded DNA (dsDNA) antibodies, and no major organ involvement (mean age, 34.5 years; 86.2% women). Patients’ initial 0.5–mg/kg per day prednisone dose was maintained for 4 weeks, then tapered by 5.0 mg every 2 weeks, and when the dose had been reduced to 20.0 mg/day, it was tapered by 5 mg every month and then gradually to 0.1-0.2 mg/kg per day. If patients had severe flares, they stopped taking MMF. (The study authors did not respond to requests for comment on the study.)
‘A Treatment Regimen That Nobody Ever Uses’
While Dr. Costenbader called the study “very interesting” and said “every person diagnosing or taking care of patients with lupus should be familiar” with it, she noted that the prednisone doses were high. “I am wondering why they used quite so much glucocorticoid for everyone. This may have masked some of the MMF effect and biased toward the null. They also used a low dose of MMF and did not ramp it up as we would normally to a full dose. That being said, it is remarkable that it was well-tolerated and resulted in better outcomes over the period of the trial.”
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center, Los Angeles, California, and the University of California, Los Angeles, also highlighted the high doses of prednisone and low doses of MMF. “It’s a useless paper that puts people on a treatment regimen that nobody ever uses,” he said.
The rates of mild to moderate flares were similar between the control and intervention groups (38.5% vs 36.9%, respectively; RR, 0.96; P = .90). This finding is surprising, said Judith A. James, MD, PhD, executive vice president, chief medical officer, and head of the rheumatology clinic and Arthritis and Clinical Immunology Research Program at the Oklahoma Medical Research Foundation in Oklahoma City and also the Associate Vice Provost of Clinical & Translational Science, professor of medicine, and George Lynn Cross Research Professor at the University of Oklahoma Health Sciences Center in Oklahoma City. “It may be that mild flares have a different mechanism or are caused by noninflammatory endotypes that don’t respond to MMF.”
Dr. Costenbader noted that a risk-benefit analysis will need to be done to take the risks of MMF into account. “However, every time that a person flares or is not in lupus low-disease activity state, potentially permanent organ damage is done and the patient suffers,” she said. “Preventing lupus nephritis de novo was also seen — nine cases potentially prevented — and that is also really interesting. It would be amazing if we could completely avoid that life-threatening complication.”
MMF can cause miscarriage and boost the risk for birth defects, and the manufacturer says it can lower the effectiveness of birth control pills. It can also boost the risk for some cancers such as lymphoma and increase the risk for infections.
Surprisingly, the number of adverse events in the control and intervention groups were similar (35.4% vs 46.2%, respectively; RR, 1.30; 95% CI, 0.86-1.99; P = .20). They included infection (30.8% vs 33.8%, respectively; P = .70) and gastrointestinal tract events (16.9% for both; P > .99).
“There were overall pretty similar rates of side effects, but maybe this was because MMF dose was pretty low in the treated group, or the glucocorticoid dose was not so low in both groups,” Dr. Costenbader said. She also noted that “the risk of malignancy with MMF is longer term than this study. It may not show up for 5-10 or even more years, but we know it exists. Infections are also increased with MMF — some of which can be avoided with vaccines for COVID, pneumonia, influenza, shingles, etc. MMF also causes gastrointestinal intolerance, and people often are not able to take it because of nausea, vomiting, diarrhea, and elevated liver function tests.”
Dr. James said the infection rates “may be due to the higher doses of steroids patients in both groups are on for several months at the beginning of the study.”
A total of 12 patients in the MMF group discontinued the intervention for various reasons, and 6 were lost to follow-up. In the control group, 20 discontinued the intervention and two were lost to follow-up. However, all 130 patients in the trial were included in the primary and secondary outcome analyses.
Should clinicians consider prescribing MMF to patients with new-onset SLE? “We usually wait until later when there are indications of more severe disease, but here they started it from the time of diagnosis if the patient was anti-dsDNA positive. Given insurance restrictions in this country, we would be unlikely to be able to do that for many patients,” Dr. Costenbader said. “They likely also overtreated a lot of patients who didn’t need it. Due to our lack of more specific biomarkers and precision medicine for lupus, we do currently undertreat a lot of patients, as this study highlights, as well as overtreat others.”
How Much Might Cost Factor Into Treatment Decisions?
The study did not examine cost. Prednisone and hydroxychloroquine sulfate are inexpensive, but Dr. James said MMF can cost about $450 a month at the study dosage. However, “the average hospitalization without an ICU [intensive care unit] visit for an SLE patient is about $15,000-$20,000. If you can avoid one hospitalization, you can pay for nearly 4 years of MMF. More importantly, from a financial perspective, if you can convert a severe lupus patient to a mild/moderate lupus patient, then the annual costs of lupus decrease nearly by half, from about $52,000 per year to $25,000 per year.”
The study authors noted various limitations such as the small number of subjects, the need for a longer trial “to determine the advantages and disadvantages of early application of MMF,” and the fact that all subjects were Asian. The authors also called for confirmation via a double-blind, placebo-controlled study.
The study was funded by grants to the authors by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Natural Science Foundation of Shanghai, Five-Year National Key R&D Program, and Ruijin–Zhongmei Huadong Lupus Funding. The authors had no disclosures. Dr. Costenbader disclosed consulting/research collaboration relationships with AstraZeneca, Amgen, Biogen, Bristol-Myers Squibb, GSK, Merck, Gilead, and Cabaletta. Dr. James and Dr. Wallace had no disclosures.
A version of this article first appeared on Medscape.com.
Early use of mycophenolate mofetil (MMF), a drug used to dampen the immune system in organ transplant recipients, may reduce the risk for severe flares in patients with newly diagnosed systemic lupus erythematosus (SLE), according to results from a randomized, open-label, observer-blinded clinical trial.
In interviews, two SLE specialists who were not involved with the study said the research is preliminary but promising. However, another specialist criticized the paper’s reliance on unusual doses of prednisone and MMF, saying it “puts people on a treatment regimen that nobody ever uses.”
The Lupus Foundation of America estimates that about 16,000 people in the United States are diagnosed with lupus each year. “Our current treatment paradigm is to go pretty slowly and start treatment for new-onset, mild SLE with glucocorticoids, if necessary, and hydroxychloroquine,” said Karen H. Costenbader, MD, MPH, of Harvard Medical School and Harvard School of Public Health, Boston, Massachusetts.
Stronger immunosuppressive agents may be added as patients progress, she said.
Off-label use of MMF, which is approved by the Food and Drug Administration only for patients with certain organ transplants, may be appropriate in some cases, she said. “There is a big push to start immunosuppressives earlier, but we currently would reserve mycophenolate for those with severe manifestations — lupus nephritis; vasculitis; or lung, brain, or heart inflammation.”
In the trial, adult patients who received oral prednisone (starting at 0.5 mg/kg per day) and hydroxychloroquine sulfate (5 mg/kg per day) plus MMF (500 mg twice daily) for 96 weeks were less likely to develop severe flares than those who took the regimen without MMF (relative risk [RR], 0.39; 95% CI, 0.17-0.87; P = .01). Severe flares occurred in 10.8% of the MMF group (7 of 65 patients) and in 27.7% of the control group (18 of 65), Yijun You, MD, of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and colleagues reported in JAMA Network Open.
Patients in the MMF group also had 89% lower risk for lupus nephritis than those in the control group (RR, 0.11; 95% CI, 0.01-0.85; P = .008), with kidney involvement occurring in 1.5% (1 of 65) vs 13.8% (9 of 65).
During 2018-2021, researchers recruited 130 patients in China aged 18-65 years with newly diagnosed SLE, a high titer of anti–double-stranded DNA (dsDNA) antibodies, and no major organ involvement (mean age, 34.5 years; 86.2% women). Patients’ initial 0.5–mg/kg per day prednisone dose was maintained for 4 weeks, then tapered by 5.0 mg every 2 weeks, and when the dose had been reduced to 20.0 mg/day, it was tapered by 5 mg every month and then gradually to 0.1-0.2 mg/kg per day. If patients had severe flares, they stopped taking MMF. (The study authors did not respond to requests for comment on the study.)
‘A Treatment Regimen That Nobody Ever Uses’
While Dr. Costenbader called the study “very interesting” and said “every person diagnosing or taking care of patients with lupus should be familiar” with it, she noted that the prednisone doses were high. “I am wondering why they used quite so much glucocorticoid for everyone. This may have masked some of the MMF effect and biased toward the null. They also used a low dose of MMF and did not ramp it up as we would normally to a full dose. That being said, it is remarkable that it was well-tolerated and resulted in better outcomes over the period of the trial.”
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center, Los Angeles, California, and the University of California, Los Angeles, also highlighted the high doses of prednisone and low doses of MMF. “It’s a useless paper that puts people on a treatment regimen that nobody ever uses,” he said.
The rates of mild to moderate flares were similar between the control and intervention groups (38.5% vs 36.9%, respectively; RR, 0.96; P = .90). This finding is surprising, said Judith A. James, MD, PhD, executive vice president, chief medical officer, and head of the rheumatology clinic and Arthritis and Clinical Immunology Research Program at the Oklahoma Medical Research Foundation in Oklahoma City and also the Associate Vice Provost of Clinical & Translational Science, professor of medicine, and George Lynn Cross Research Professor at the University of Oklahoma Health Sciences Center in Oklahoma City. “It may be that mild flares have a different mechanism or are caused by noninflammatory endotypes that don’t respond to MMF.”
Dr. Costenbader noted that a risk-benefit analysis will need to be done to take the risks of MMF into account. “However, every time that a person flares or is not in lupus low-disease activity state, potentially permanent organ damage is done and the patient suffers,” she said. “Preventing lupus nephritis de novo was also seen — nine cases potentially prevented — and that is also really interesting. It would be amazing if we could completely avoid that life-threatening complication.”
MMF can cause miscarriage and boost the risk for birth defects, and the manufacturer says it can lower the effectiveness of birth control pills. It can also boost the risk for some cancers such as lymphoma and increase the risk for infections.
Surprisingly, the number of adverse events in the control and intervention groups were similar (35.4% vs 46.2%, respectively; RR, 1.30; 95% CI, 0.86-1.99; P = .20). They included infection (30.8% vs 33.8%, respectively; P = .70) and gastrointestinal tract events (16.9% for both; P > .99).
“There were overall pretty similar rates of side effects, but maybe this was because MMF dose was pretty low in the treated group, or the glucocorticoid dose was not so low in both groups,” Dr. Costenbader said. She also noted that “the risk of malignancy with MMF is longer term than this study. It may not show up for 5-10 or even more years, but we know it exists. Infections are also increased with MMF — some of which can be avoided with vaccines for COVID, pneumonia, influenza, shingles, etc. MMF also causes gastrointestinal intolerance, and people often are not able to take it because of nausea, vomiting, diarrhea, and elevated liver function tests.”
Dr. James said the infection rates “may be due to the higher doses of steroids patients in both groups are on for several months at the beginning of the study.”
A total of 12 patients in the MMF group discontinued the intervention for various reasons, and 6 were lost to follow-up. In the control group, 20 discontinued the intervention and two were lost to follow-up. However, all 130 patients in the trial were included in the primary and secondary outcome analyses.
Should clinicians consider prescribing MMF to patients with new-onset SLE? “We usually wait until later when there are indications of more severe disease, but here they started it from the time of diagnosis if the patient was anti-dsDNA positive. Given insurance restrictions in this country, we would be unlikely to be able to do that for many patients,” Dr. Costenbader said. “They likely also overtreated a lot of patients who didn’t need it. Due to our lack of more specific biomarkers and precision medicine for lupus, we do currently undertreat a lot of patients, as this study highlights, as well as overtreat others.”
How Much Might Cost Factor Into Treatment Decisions?
The study did not examine cost. Prednisone and hydroxychloroquine sulfate are inexpensive, but Dr. James said MMF can cost about $450 a month at the study dosage. However, “the average hospitalization without an ICU [intensive care unit] visit for an SLE patient is about $15,000-$20,000. If you can avoid one hospitalization, you can pay for nearly 4 years of MMF. More importantly, from a financial perspective, if you can convert a severe lupus patient to a mild/moderate lupus patient, then the annual costs of lupus decrease nearly by half, from about $52,000 per year to $25,000 per year.”
The study authors noted various limitations such as the small number of subjects, the need for a longer trial “to determine the advantages and disadvantages of early application of MMF,” and the fact that all subjects were Asian. The authors also called for confirmation via a double-blind, placebo-controlled study.
The study was funded by grants to the authors by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Natural Science Foundation of Shanghai, Five-Year National Key R&D Program, and Ruijin–Zhongmei Huadong Lupus Funding. The authors had no disclosures. Dr. Costenbader disclosed consulting/research collaboration relationships with AstraZeneca, Amgen, Biogen, Bristol-Myers Squibb, GSK, Merck, Gilead, and Cabaletta. Dr. James and Dr. Wallace had no disclosures.
A version of this article first appeared on Medscape.com.
Early use of mycophenolate mofetil (MMF), a drug used to dampen the immune system in organ transplant recipients, may reduce the risk for severe flares in patients with newly diagnosed systemic lupus erythematosus (SLE), according to results from a randomized, open-label, observer-blinded clinical trial.
In interviews, two SLE specialists who were not involved with the study said the research is preliminary but promising. However, another specialist criticized the paper’s reliance on unusual doses of prednisone and MMF, saying it “puts people on a treatment regimen that nobody ever uses.”
The Lupus Foundation of America estimates that about 16,000 people in the United States are diagnosed with lupus each year. “Our current treatment paradigm is to go pretty slowly and start treatment for new-onset, mild SLE with glucocorticoids, if necessary, and hydroxychloroquine,” said Karen H. Costenbader, MD, MPH, of Harvard Medical School and Harvard School of Public Health, Boston, Massachusetts.
Stronger immunosuppressive agents may be added as patients progress, she said.
Off-label use of MMF, which is approved by the Food and Drug Administration only for patients with certain organ transplants, may be appropriate in some cases, she said. “There is a big push to start immunosuppressives earlier, but we currently would reserve mycophenolate for those with severe manifestations — lupus nephritis; vasculitis; or lung, brain, or heart inflammation.”
In the trial, adult patients who received oral prednisone (starting at 0.5 mg/kg per day) and hydroxychloroquine sulfate (5 mg/kg per day) plus MMF (500 mg twice daily) for 96 weeks were less likely to develop severe flares than those who took the regimen without MMF (relative risk [RR], 0.39; 95% CI, 0.17-0.87; P = .01). Severe flares occurred in 10.8% of the MMF group (7 of 65 patients) and in 27.7% of the control group (18 of 65), Yijun You, MD, of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, and colleagues reported in JAMA Network Open.
Patients in the MMF group also had 89% lower risk for lupus nephritis than those in the control group (RR, 0.11; 95% CI, 0.01-0.85; P = .008), with kidney involvement occurring in 1.5% (1 of 65) vs 13.8% (9 of 65).
During 2018-2021, researchers recruited 130 patients in China aged 18-65 years with newly diagnosed SLE, a high titer of anti–double-stranded DNA (dsDNA) antibodies, and no major organ involvement (mean age, 34.5 years; 86.2% women). Patients’ initial 0.5–mg/kg per day prednisone dose was maintained for 4 weeks, then tapered by 5.0 mg every 2 weeks, and when the dose had been reduced to 20.0 mg/day, it was tapered by 5 mg every month and then gradually to 0.1-0.2 mg/kg per day. If patients had severe flares, they stopped taking MMF. (The study authors did not respond to requests for comment on the study.)
‘A Treatment Regimen That Nobody Ever Uses’
While Dr. Costenbader called the study “very interesting” and said “every person diagnosing or taking care of patients with lupus should be familiar” with it, she noted that the prednisone doses were high. “I am wondering why they used quite so much glucocorticoid for everyone. This may have masked some of the MMF effect and biased toward the null. They also used a low dose of MMF and did not ramp it up as we would normally to a full dose. That being said, it is remarkable that it was well-tolerated and resulted in better outcomes over the period of the trial.”
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center, Los Angeles, California, and the University of California, Los Angeles, also highlighted the high doses of prednisone and low doses of MMF. “It’s a useless paper that puts people on a treatment regimen that nobody ever uses,” he said.
The rates of mild to moderate flares were similar between the control and intervention groups (38.5% vs 36.9%, respectively; RR, 0.96; P = .90). This finding is surprising, said Judith A. James, MD, PhD, executive vice president, chief medical officer, and head of the rheumatology clinic and Arthritis and Clinical Immunology Research Program at the Oklahoma Medical Research Foundation in Oklahoma City and also the Associate Vice Provost of Clinical & Translational Science, professor of medicine, and George Lynn Cross Research Professor at the University of Oklahoma Health Sciences Center in Oklahoma City. “It may be that mild flares have a different mechanism or are caused by noninflammatory endotypes that don’t respond to MMF.”
Dr. Costenbader noted that a risk-benefit analysis will need to be done to take the risks of MMF into account. “However, every time that a person flares or is not in lupus low-disease activity state, potentially permanent organ damage is done and the patient suffers,” she said. “Preventing lupus nephritis de novo was also seen — nine cases potentially prevented — and that is also really interesting. It would be amazing if we could completely avoid that life-threatening complication.”
MMF can cause miscarriage and boost the risk for birth defects, and the manufacturer says it can lower the effectiveness of birth control pills. It can also boost the risk for some cancers such as lymphoma and increase the risk for infections.
Surprisingly, the number of adverse events in the control and intervention groups were similar (35.4% vs 46.2%, respectively; RR, 1.30; 95% CI, 0.86-1.99; P = .20). They included infection (30.8% vs 33.8%, respectively; P = .70) and gastrointestinal tract events (16.9% for both; P > .99).
“There were overall pretty similar rates of side effects, but maybe this was because MMF dose was pretty low in the treated group, or the glucocorticoid dose was not so low in both groups,” Dr. Costenbader said. She also noted that “the risk of malignancy with MMF is longer term than this study. It may not show up for 5-10 or even more years, but we know it exists. Infections are also increased with MMF — some of which can be avoided with vaccines for COVID, pneumonia, influenza, shingles, etc. MMF also causes gastrointestinal intolerance, and people often are not able to take it because of nausea, vomiting, diarrhea, and elevated liver function tests.”
Dr. James said the infection rates “may be due to the higher doses of steroids patients in both groups are on for several months at the beginning of the study.”
A total of 12 patients in the MMF group discontinued the intervention for various reasons, and 6 were lost to follow-up. In the control group, 20 discontinued the intervention and two were lost to follow-up. However, all 130 patients in the trial were included in the primary and secondary outcome analyses.
Should clinicians consider prescribing MMF to patients with new-onset SLE? “We usually wait until later when there are indications of more severe disease, but here they started it from the time of diagnosis if the patient was anti-dsDNA positive. Given insurance restrictions in this country, we would be unlikely to be able to do that for many patients,” Dr. Costenbader said. “They likely also overtreated a lot of patients who didn’t need it. Due to our lack of more specific biomarkers and precision medicine for lupus, we do currently undertreat a lot of patients, as this study highlights, as well as overtreat others.”
How Much Might Cost Factor Into Treatment Decisions?
The study did not examine cost. Prednisone and hydroxychloroquine sulfate are inexpensive, but Dr. James said MMF can cost about $450 a month at the study dosage. However, “the average hospitalization without an ICU [intensive care unit] visit for an SLE patient is about $15,000-$20,000. If you can avoid one hospitalization, you can pay for nearly 4 years of MMF. More importantly, from a financial perspective, if you can convert a severe lupus patient to a mild/moderate lupus patient, then the annual costs of lupus decrease nearly by half, from about $52,000 per year to $25,000 per year.”
The study authors noted various limitations such as the small number of subjects, the need for a longer trial “to determine the advantages and disadvantages of early application of MMF,” and the fact that all subjects were Asian. The authors also called for confirmation via a double-blind, placebo-controlled study.
The study was funded by grants to the authors by the National Natural Science Foundation of China, Shanghai Rising-Star Program, Natural Science Foundation of Shanghai, Five-Year National Key R&D Program, and Ruijin–Zhongmei Huadong Lupus Funding. The authors had no disclosures. Dr. Costenbader disclosed consulting/research collaboration relationships with AstraZeneca, Amgen, Biogen, Bristol-Myers Squibb, GSK, Merck, Gilead, and Cabaletta. Dr. James and Dr. Wallace had no disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Inspection of Deep Tumor Margins for Accurate Cutaneous Squamous Cell Carcinoma Staging
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.
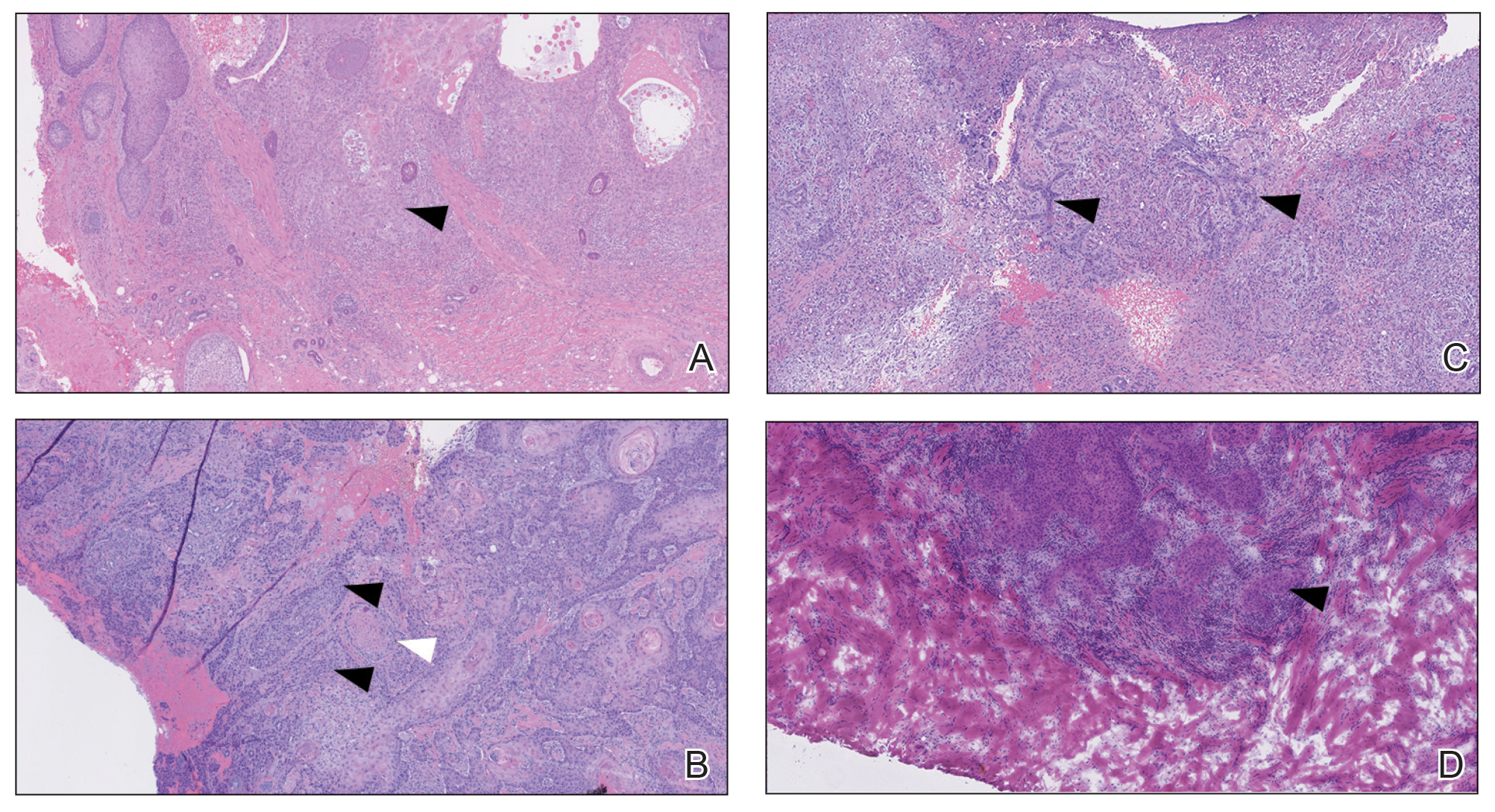
A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
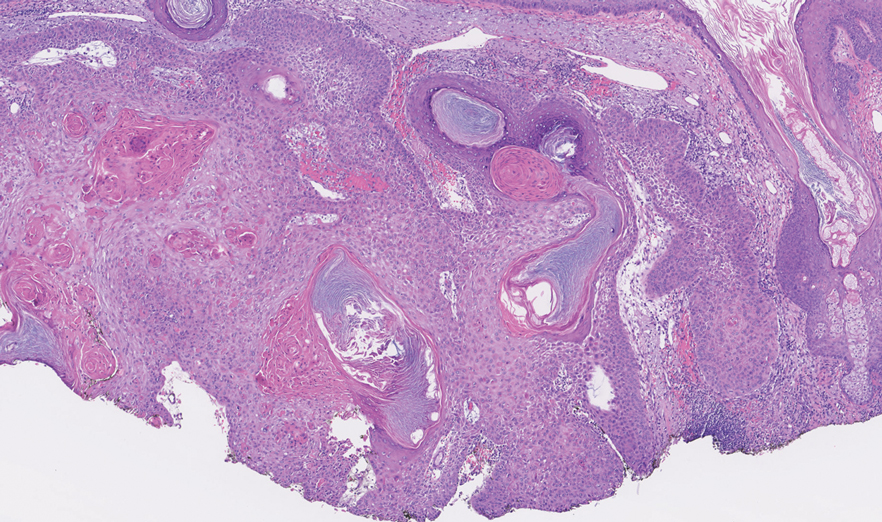
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.
Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
Practice Points
- A proportion of cutaneous squamous cell carcinomas are upgraded from the initial biopsy during Mohs micrographic surgery due to evidence of perineural invasion, bony invasion, or lesser differentiation noted on Mohs stages or debulk analysis.
- Thorough inspection of the deep tumor margins may be required for accurate tumor staging and evaluation of metastatic risk. Cells at the deep margin of the tumor may demonstrate poorer differentiation and/or other higher-risk tumor features than those closer to the surface.
- Tumor staging may be incomplete until the deep margins are assessed to find the most dysplastic and likely clinically relevant cells, which may be missed without evaluation of the debulked tumor.
New Options for Treating Atopic Dermatitis Available, and in Development
HUNTINGTON BEACH, CALIFORNIA — If the number of recent drug approvals for atopic dermatitis (AD) is overwhelming, the future is unlikely to be any less challenging: According to the National Eczema Association, the current pipeline for AD includes 39 injectable medications, 21 oral agents, and 49 topicals, some with novel targets, like human umbilical cord blood derived stem cells.
“It’s amazing how many drugs are coming out for AD,” Robert Sidbury, MD, MPH, said at the annual meeting of the Pacific Dermatologic Association (PDA). and is approved in Europe for the treatment of moderate to severe AD in patients aged ≥ 12 years. (On September 13, after the PDA meeting, lebrikizumab was approved by the Food and Drug Administration [FDA] for treatment of moderate to severe AD in adults and adolescents aged ≥ 12 years.)
In two identical phase 3 trials known as ADvocate 1 and ADvocate 2, researchers randomly assigned 851 patients with moderate to severe AD in a 2:1 ratio to receive either lebrikizumab at a dose of 250 mg (loading dose of 500 mg at baseline and week 2) or placebo, administered subcutaneously every 2 weeks, through week 16. The primary outcome was an Investigator’s Global Assessment (IGA) score of 0 or 1, indicating clear or almost clear skin. The researchers reported that an IGA score of 0 or 1 was achieved by 43.1% of patients in the lebrikizumab arm compared with 12.7% of those in the placebo arm.
“Those are good numbers,” said Dr. Sidbury, who was not involved with the study. Conjunctivitis occurred more often in those who received lebrikizumab compared with those who received placebo (7.4% vs 2.8%, respectively), “which is not surprising because it is an IL-13 agent,” he said.
In a subsequent study presented during the Revolutionizing Atopic Dermatitis meeting in the fall of 2023, researchers presented data on Eczema Severity and Area Index (EASI)-90 responses in the ADvocate trial participants, showing EASI-90 responses were sustained up to 38 weeks after lebrikizumab withdrawal, while serum concentrations were negligible. They found that between week 14 and week 32, approximately five serum concentration half-lives of the medication had elapsed since patients randomized to the withdrawal arm received their last dose of lebrikizumab, extending to approximately 11 half-lives by week 52. “That durability of response with next to no blood levels of drug in many of the study participants is interesting,” said Dr. Sidbury, who cochairs the current iteration of the American Academy of Dermatology Atopic Dermatitis Guidelines.
Nemolizumab is a neuroimmune response modulator that inhibits the IL-31 receptor and is approved in Japan for the treatment of itch associated with AD in patients aged ≥ 13 years. Results from two identical phase 3, randomized, controlled trials known as ARCADIA 1 and ARCADIA 2 found that 36% of patients in ARCADIA 1 and 38% in ARCADIA 2 achieved clear skin, compared with 25% and 26% of patients in the placebo group, respectively. (Nemolizumab was recently approved by the FDA for treating prurigo nodularis and is under FDA review for AD.)
In terms of safety, Dr. Sidbury, who is a member of the steering committee for the ARCADIA trials, said that nemolizumab has been “generally well tolerated;” with 1%-3% of study participants experiencing at least one serious treatment-emergent adverse event that included asthma exacerbation, facial edema, and peripheral edema. “The latest data are reassuring but we are watching these safety concerns carefully,” he said.
Dr. Sidbury disclosed that he is an investigator for Regeneron, Pfizer, Galderma, UCB, and Castle; a consultant for Lilly, Leo, Arcutis, and Dermavant; and a member of the speaker’s bureau for Beiersdorf.
A version of this article appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — If the number of recent drug approvals for atopic dermatitis (AD) is overwhelming, the future is unlikely to be any less challenging: According to the National Eczema Association, the current pipeline for AD includes 39 injectable medications, 21 oral agents, and 49 topicals, some with novel targets, like human umbilical cord blood derived stem cells.
“It’s amazing how many drugs are coming out for AD,” Robert Sidbury, MD, MPH, said at the annual meeting of the Pacific Dermatologic Association (PDA). and is approved in Europe for the treatment of moderate to severe AD in patients aged ≥ 12 years. (On September 13, after the PDA meeting, lebrikizumab was approved by the Food and Drug Administration [FDA] for treatment of moderate to severe AD in adults and adolescents aged ≥ 12 years.)
In two identical phase 3 trials known as ADvocate 1 and ADvocate 2, researchers randomly assigned 851 patients with moderate to severe AD in a 2:1 ratio to receive either lebrikizumab at a dose of 250 mg (loading dose of 500 mg at baseline and week 2) or placebo, administered subcutaneously every 2 weeks, through week 16. The primary outcome was an Investigator’s Global Assessment (IGA) score of 0 or 1, indicating clear or almost clear skin. The researchers reported that an IGA score of 0 or 1 was achieved by 43.1% of patients in the lebrikizumab arm compared with 12.7% of those in the placebo arm.
“Those are good numbers,” said Dr. Sidbury, who was not involved with the study. Conjunctivitis occurred more often in those who received lebrikizumab compared with those who received placebo (7.4% vs 2.8%, respectively), “which is not surprising because it is an IL-13 agent,” he said.
In a subsequent study presented during the Revolutionizing Atopic Dermatitis meeting in the fall of 2023, researchers presented data on Eczema Severity and Area Index (EASI)-90 responses in the ADvocate trial participants, showing EASI-90 responses were sustained up to 38 weeks after lebrikizumab withdrawal, while serum concentrations were negligible. They found that between week 14 and week 32, approximately five serum concentration half-lives of the medication had elapsed since patients randomized to the withdrawal arm received their last dose of lebrikizumab, extending to approximately 11 half-lives by week 52. “That durability of response with next to no blood levels of drug in many of the study participants is interesting,” said Dr. Sidbury, who cochairs the current iteration of the American Academy of Dermatology Atopic Dermatitis Guidelines.
Nemolizumab is a neuroimmune response modulator that inhibits the IL-31 receptor and is approved in Japan for the treatment of itch associated with AD in patients aged ≥ 13 years. Results from two identical phase 3, randomized, controlled trials known as ARCADIA 1 and ARCADIA 2 found that 36% of patients in ARCADIA 1 and 38% in ARCADIA 2 achieved clear skin, compared with 25% and 26% of patients in the placebo group, respectively. (Nemolizumab was recently approved by the FDA for treating prurigo nodularis and is under FDA review for AD.)
In terms of safety, Dr. Sidbury, who is a member of the steering committee for the ARCADIA trials, said that nemolizumab has been “generally well tolerated;” with 1%-3% of study participants experiencing at least one serious treatment-emergent adverse event that included asthma exacerbation, facial edema, and peripheral edema. “The latest data are reassuring but we are watching these safety concerns carefully,” he said.
Dr. Sidbury disclosed that he is an investigator for Regeneron, Pfizer, Galderma, UCB, and Castle; a consultant for Lilly, Leo, Arcutis, and Dermavant; and a member of the speaker’s bureau for Beiersdorf.
A version of this article appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — If the number of recent drug approvals for atopic dermatitis (AD) is overwhelming, the future is unlikely to be any less challenging: According to the National Eczema Association, the current pipeline for AD includes 39 injectable medications, 21 oral agents, and 49 topicals, some with novel targets, like human umbilical cord blood derived stem cells.
“It’s amazing how many drugs are coming out for AD,” Robert Sidbury, MD, MPH, said at the annual meeting of the Pacific Dermatologic Association (PDA). and is approved in Europe for the treatment of moderate to severe AD in patients aged ≥ 12 years. (On September 13, after the PDA meeting, lebrikizumab was approved by the Food and Drug Administration [FDA] for treatment of moderate to severe AD in adults and adolescents aged ≥ 12 years.)
In two identical phase 3 trials known as ADvocate 1 and ADvocate 2, researchers randomly assigned 851 patients with moderate to severe AD in a 2:1 ratio to receive either lebrikizumab at a dose of 250 mg (loading dose of 500 mg at baseline and week 2) or placebo, administered subcutaneously every 2 weeks, through week 16. The primary outcome was an Investigator’s Global Assessment (IGA) score of 0 or 1, indicating clear or almost clear skin. The researchers reported that an IGA score of 0 or 1 was achieved by 43.1% of patients in the lebrikizumab arm compared with 12.7% of those in the placebo arm.
“Those are good numbers,” said Dr. Sidbury, who was not involved with the study. Conjunctivitis occurred more often in those who received lebrikizumab compared with those who received placebo (7.4% vs 2.8%, respectively), “which is not surprising because it is an IL-13 agent,” he said.
In a subsequent study presented during the Revolutionizing Atopic Dermatitis meeting in the fall of 2023, researchers presented data on Eczema Severity and Area Index (EASI)-90 responses in the ADvocate trial participants, showing EASI-90 responses were sustained up to 38 weeks after lebrikizumab withdrawal, while serum concentrations were negligible. They found that between week 14 and week 32, approximately five serum concentration half-lives of the medication had elapsed since patients randomized to the withdrawal arm received their last dose of lebrikizumab, extending to approximately 11 half-lives by week 52. “That durability of response with next to no blood levels of drug in many of the study participants is interesting,” said Dr. Sidbury, who cochairs the current iteration of the American Academy of Dermatology Atopic Dermatitis Guidelines.
Nemolizumab is a neuroimmune response modulator that inhibits the IL-31 receptor and is approved in Japan for the treatment of itch associated with AD in patients aged ≥ 13 years. Results from two identical phase 3, randomized, controlled trials known as ARCADIA 1 and ARCADIA 2 found that 36% of patients in ARCADIA 1 and 38% in ARCADIA 2 achieved clear skin, compared with 25% and 26% of patients in the placebo group, respectively. (Nemolizumab was recently approved by the FDA for treating prurigo nodularis and is under FDA review for AD.)
In terms of safety, Dr. Sidbury, who is a member of the steering committee for the ARCADIA trials, said that nemolizumab has been “generally well tolerated;” with 1%-3% of study participants experiencing at least one serious treatment-emergent adverse event that included asthma exacerbation, facial edema, and peripheral edema. “The latest data are reassuring but we are watching these safety concerns carefully,” he said.
Dr. Sidbury disclosed that he is an investigator for Regeneron, Pfizer, Galderma, UCB, and Castle; a consultant for Lilly, Leo, Arcutis, and Dermavant; and a member of the speaker’s bureau for Beiersdorf.
A version of this article appeared on Medscape.com.
FROM PDA 2024
Hypnosis May Offer Relief During Sharp Debridement of Skin Ulcers
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Treating Family: Ethicist Discusses Whether It’s Appropriate
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
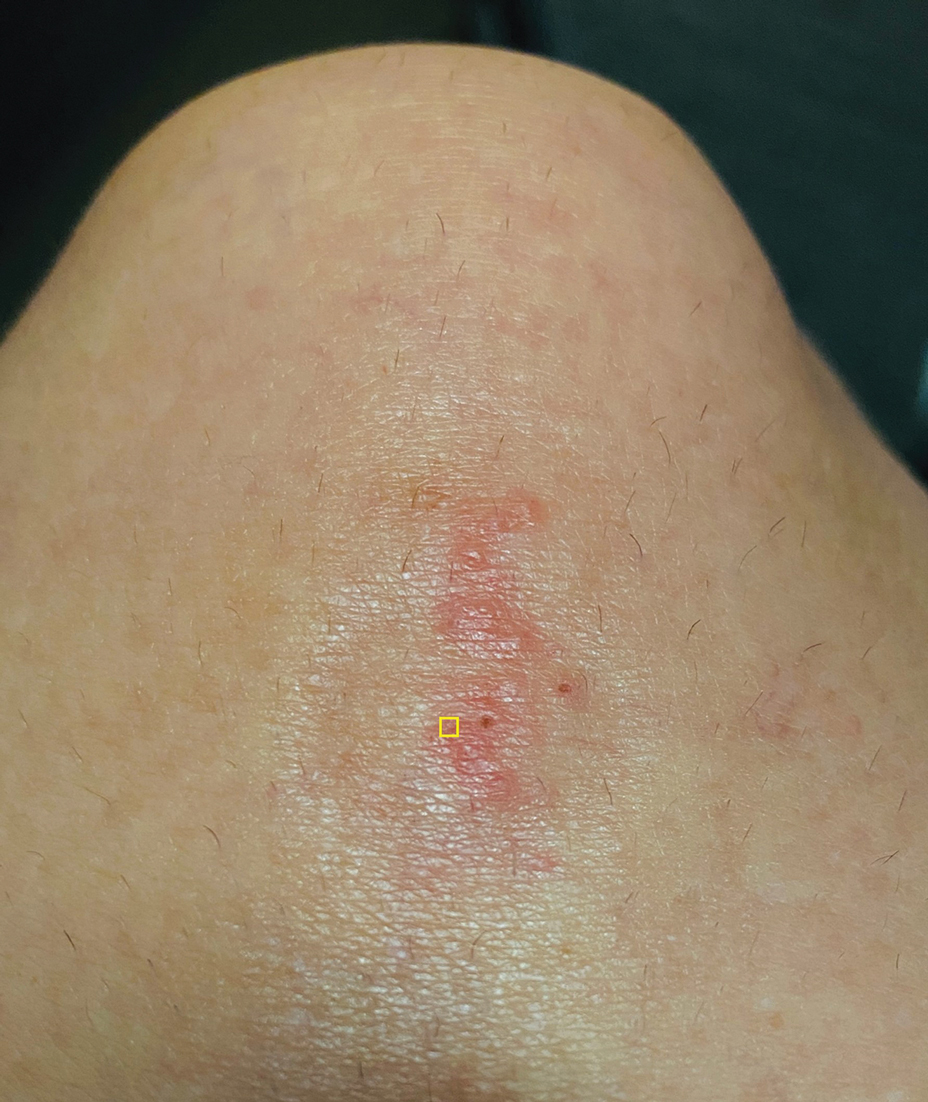
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
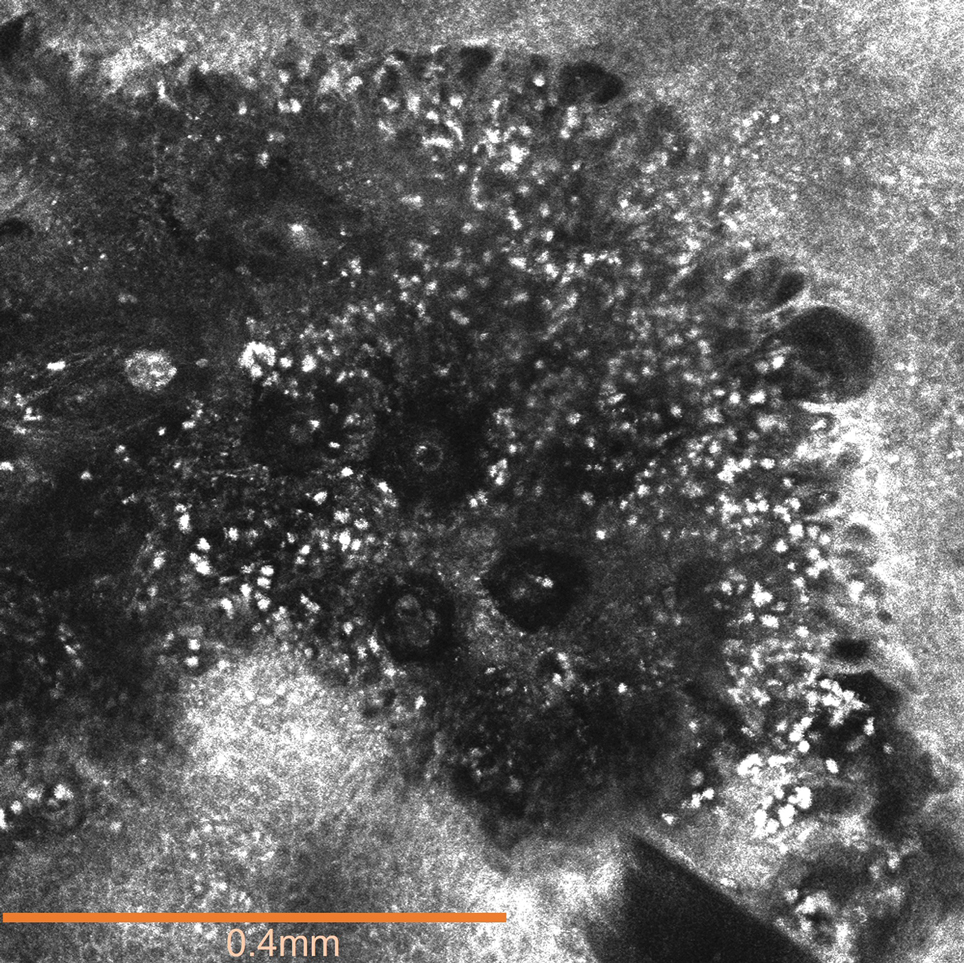
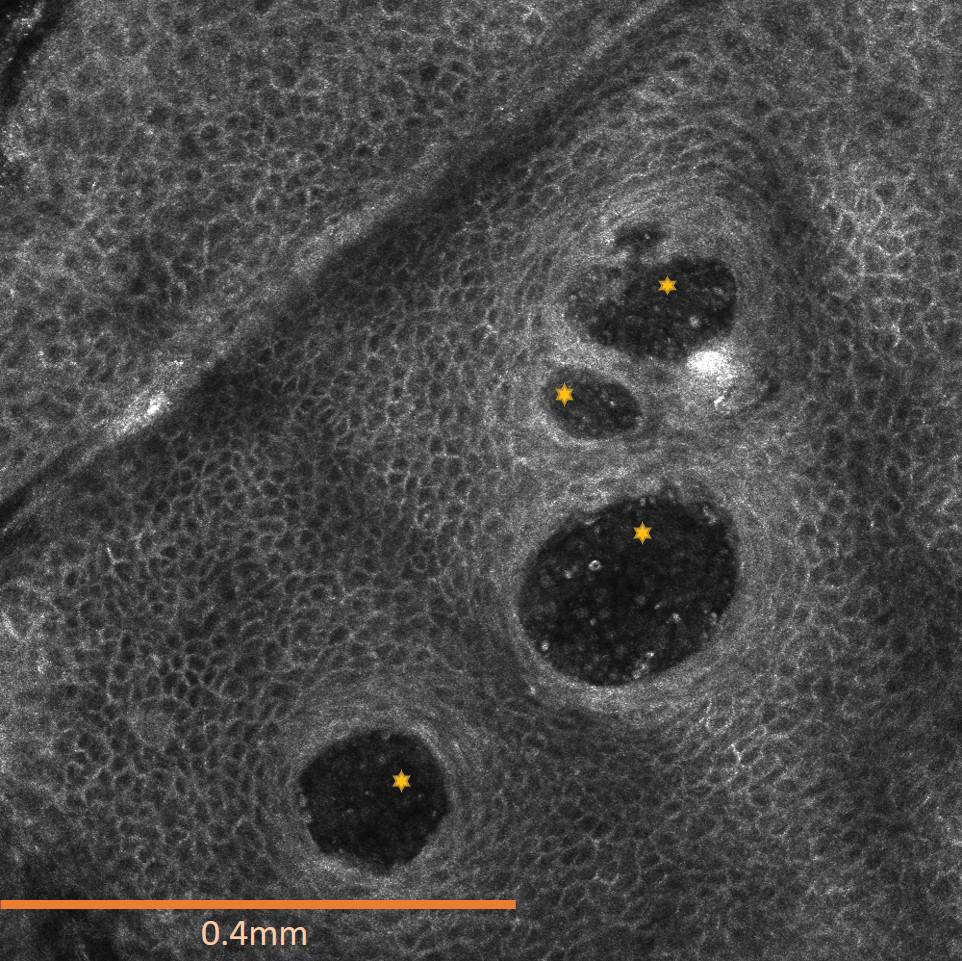
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.
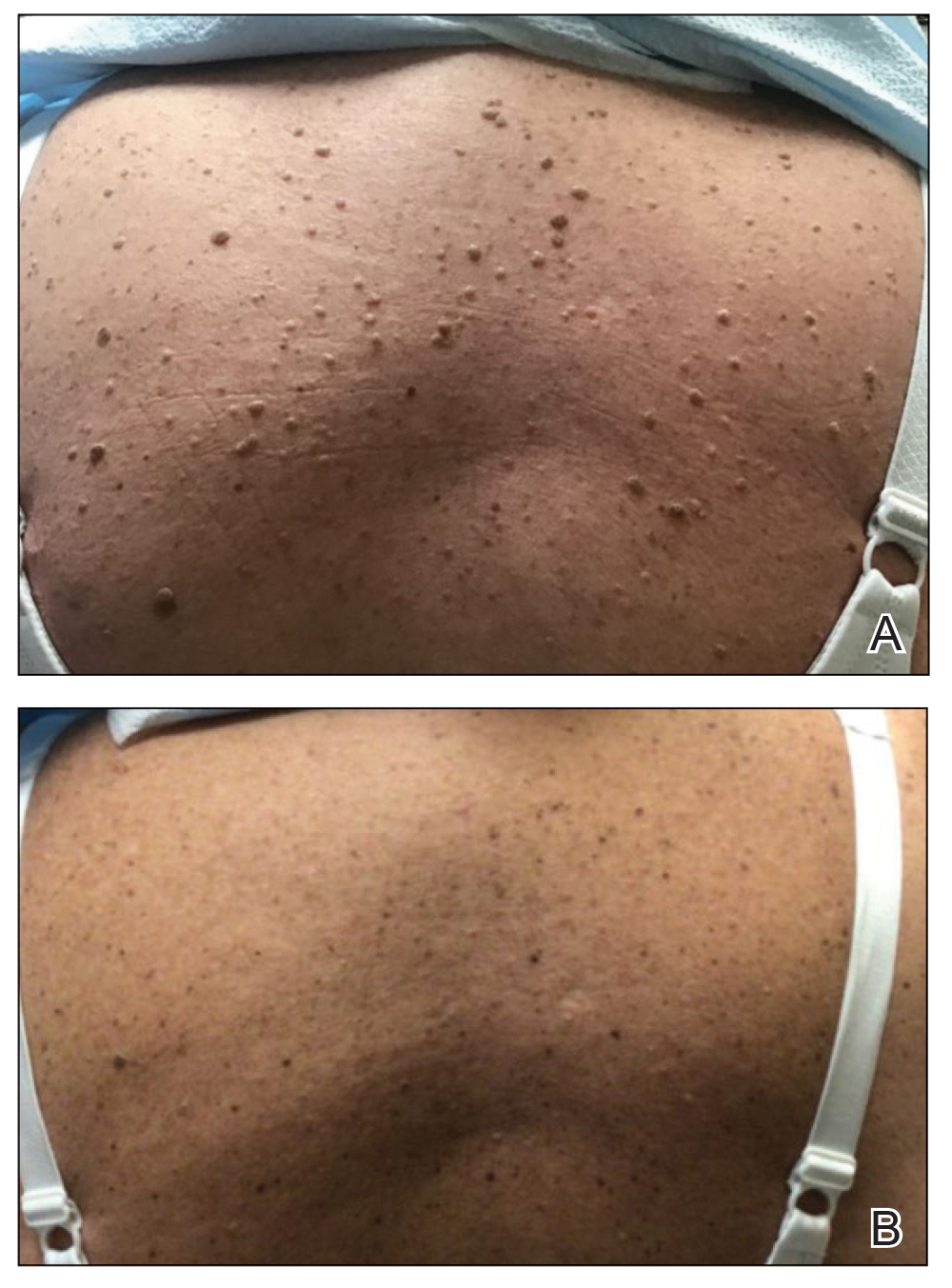
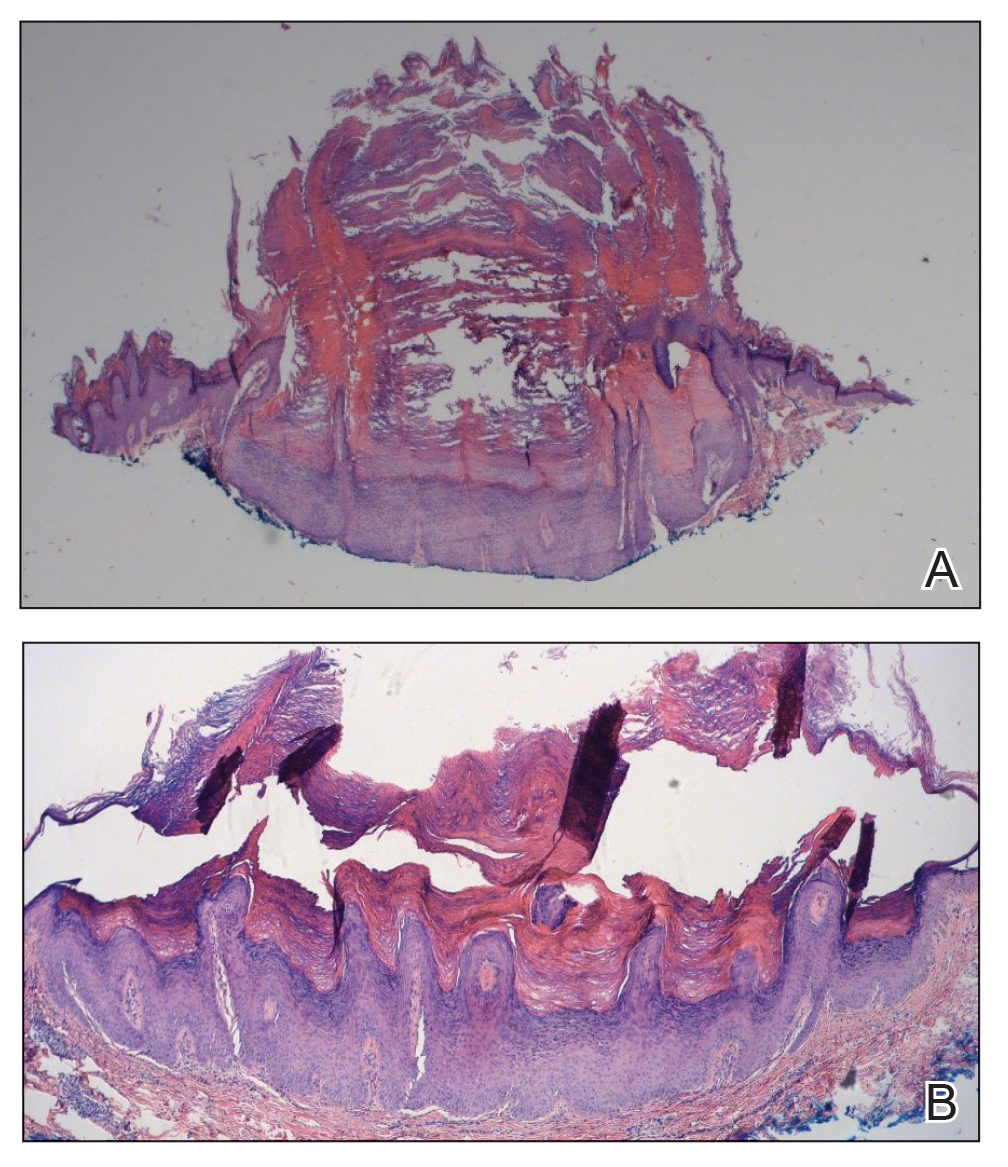
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.