User login
-
Residents react: Has residency become easier or overly difficult?
Medical residents have cleared many hurdles to get where they are, as detailed in Medscape’s Residents Salary and Debt Report 2022 which explains their challenges with compensation and school loans as well as long hours and problematic personal relationships.
Whereas 72% of residents described themselves as “very satisfied” or “satisfied” with their professional training experience, only 27% felt that highly about how well they’re paid. Satisfaction levels increased somewhat farther into residency, reaching 35% in year 5.
Do residents have it easier today?
If so, is that rite of passage getting any easier? You’ll get different answers from residents and physicians.
Medscape asked respondents whether their journey to residency was made easier once the Step 1 exam was converted to pass-fail, and interviews brought online, because of the COVID-19 pandemic.
Many residents conceded their journey became easier, less stressful, and less expensive under the new Step 1 formats. One respondent said he was freed up to focus more intently on higher-yield academic goals such as research.
Another respondent called the pass/fail change a “total game-changer,” as it lets applicants apply to all specialties while having other qualifications than test scores considered. A resident who took Step 1 before pass/fail was instituted described the “insurmountable stress associated with studying for Step 1 to get the highest score you possibly could.”
But not all residents liked the difficulty in being able to differentiate themselves, beyond med school pedigrees, in the absence of Step 1 scores.
Meanwhile, some doctors posting comments to the Medscape report strongly disagreed with the idea that residency life is getting harder. They depict residency as a rite of passage under the best of circumstances.
“Whatever issues there may be [today’s residents] are still making eight times what I got and, from what I’ve seen, we had a lot more independent responsibilities,” one physician commenter said.
Other doctors were more sympathetic and worried about the future price to be paid for hardships during residency. “Compensation should not be tied to the willingness to sacrifice the most beautiful years of life,” one commentator wrote.
Online interviews: Pros and cons
Many resident respondents celebrated the opportunity to interview for residency programs online. Some who traveled to in-person interviews before the pandemic said they racked up as much as $10,000 in travel costs, adding to their debt loads.
But not everyone was a fan. Other residents sniped that peers can apply to more residencies and “hoard” interviews, making the competition that much harder.
And how useful are online interviews to a prospective resident? “Virtual interviews are terrible for getting a true sense for a program or even the people,” a 1st-year family medicine resident complained. And it’s harder for an applicant “to shine when you’re on Zoom,” a 1st-year internal medicine resident opined.
Whether to report harassment
In survey, respondents were asked whether they ever witnessed sexual abuse, harassment, or misconduct; and if so, what they did about it. Among those who did, many opted to take no action, fearing retaliation or retribution. “I saw a resident made out to be a ‘problem resident’ when reporting it and then ultimately fired,” one respondent recounted.
Other residents said they felt unsure about the protocol, whom to report to, or even what constituted harassment or misconduct. “I didn’t realize [an incident] was harassment until later,” one resident said. Others thought “minor” or “subtle” incidents did not warrant action; “they are typically microaggressions and appear accepted within the culture of the institution.”
Residents’ confusion heightened when the perpetrator was a patient. “I’m not sure what to do about that,” a respondent acknowledged. An emergency medicine resident added, “most of the time … it is the patients who are acting inappropriately, saying inappropriate things, etc. There is no way to file a complaint like that.”
Rewards and challenges for residents
Among the most rewarding parts of residency that respondents described were developing specific skills such as surgical techniques, job security, and “learning a little day by day” in the words of a 1st-year gastroenterology resident.
Others felt gratified by the chances to help patients and families, their teams, and to advance social justice and health equity.
But challenges abound – chiefly money struggles. A 3rd-year psychiatry resident lamented “being financially strapped in the prime of my life from student loans and low wages.”
Stress and emotional fatigue also came up often as major challenges. “Constantly being told to do more, more presentations, more papers, more research, more studying,” a 5th-year neurosurgery resident bemoaned. “Being expected to be at the top of my game despite being sleep-deprived, depressed, and burned out,” a 3rd-year ob.gyn. resident groused.
But some physician commenters urged residents to look for long-term growth behind the challenges. “Yes, it was hard, but the experience was phenomenal, and I am glad I did it,” one doctor said.
A version of this article first appeared on Medscape.com.
Medical residents have cleared many hurdles to get where they are, as detailed in Medscape’s Residents Salary and Debt Report 2022 which explains their challenges with compensation and school loans as well as long hours and problematic personal relationships.
Whereas 72% of residents described themselves as “very satisfied” or “satisfied” with their professional training experience, only 27% felt that highly about how well they’re paid. Satisfaction levels increased somewhat farther into residency, reaching 35% in year 5.
Do residents have it easier today?
If so, is that rite of passage getting any easier? You’ll get different answers from residents and physicians.
Medscape asked respondents whether their journey to residency was made easier once the Step 1 exam was converted to pass-fail, and interviews brought online, because of the COVID-19 pandemic.
Many residents conceded their journey became easier, less stressful, and less expensive under the new Step 1 formats. One respondent said he was freed up to focus more intently on higher-yield academic goals such as research.
Another respondent called the pass/fail change a “total game-changer,” as it lets applicants apply to all specialties while having other qualifications than test scores considered. A resident who took Step 1 before pass/fail was instituted described the “insurmountable stress associated with studying for Step 1 to get the highest score you possibly could.”
But not all residents liked the difficulty in being able to differentiate themselves, beyond med school pedigrees, in the absence of Step 1 scores.
Meanwhile, some doctors posting comments to the Medscape report strongly disagreed with the idea that residency life is getting harder. They depict residency as a rite of passage under the best of circumstances.
“Whatever issues there may be [today’s residents] are still making eight times what I got and, from what I’ve seen, we had a lot more independent responsibilities,” one physician commenter said.
Other doctors were more sympathetic and worried about the future price to be paid for hardships during residency. “Compensation should not be tied to the willingness to sacrifice the most beautiful years of life,” one commentator wrote.
Online interviews: Pros and cons
Many resident respondents celebrated the opportunity to interview for residency programs online. Some who traveled to in-person interviews before the pandemic said they racked up as much as $10,000 in travel costs, adding to their debt loads.
But not everyone was a fan. Other residents sniped that peers can apply to more residencies and “hoard” interviews, making the competition that much harder.
And how useful are online interviews to a prospective resident? “Virtual interviews are terrible for getting a true sense for a program or even the people,” a 1st-year family medicine resident complained. And it’s harder for an applicant “to shine when you’re on Zoom,” a 1st-year internal medicine resident opined.
Whether to report harassment
In survey, respondents were asked whether they ever witnessed sexual abuse, harassment, or misconduct; and if so, what they did about it. Among those who did, many opted to take no action, fearing retaliation or retribution. “I saw a resident made out to be a ‘problem resident’ when reporting it and then ultimately fired,” one respondent recounted.
Other residents said they felt unsure about the protocol, whom to report to, or even what constituted harassment or misconduct. “I didn’t realize [an incident] was harassment until later,” one resident said. Others thought “minor” or “subtle” incidents did not warrant action; “they are typically microaggressions and appear accepted within the culture of the institution.”
Residents’ confusion heightened when the perpetrator was a patient. “I’m not sure what to do about that,” a respondent acknowledged. An emergency medicine resident added, “most of the time … it is the patients who are acting inappropriately, saying inappropriate things, etc. There is no way to file a complaint like that.”
Rewards and challenges for residents
Among the most rewarding parts of residency that respondents described were developing specific skills such as surgical techniques, job security, and “learning a little day by day” in the words of a 1st-year gastroenterology resident.
Others felt gratified by the chances to help patients and families, their teams, and to advance social justice and health equity.
But challenges abound – chiefly money struggles. A 3rd-year psychiatry resident lamented “being financially strapped in the prime of my life from student loans and low wages.”
Stress and emotional fatigue also came up often as major challenges. “Constantly being told to do more, more presentations, more papers, more research, more studying,” a 5th-year neurosurgery resident bemoaned. “Being expected to be at the top of my game despite being sleep-deprived, depressed, and burned out,” a 3rd-year ob.gyn. resident groused.
But some physician commenters urged residents to look for long-term growth behind the challenges. “Yes, it was hard, but the experience was phenomenal, and I am glad I did it,” one doctor said.
A version of this article first appeared on Medscape.com.
Medical residents have cleared many hurdles to get where they are, as detailed in Medscape’s Residents Salary and Debt Report 2022 which explains their challenges with compensation and school loans as well as long hours and problematic personal relationships.
Whereas 72% of residents described themselves as “very satisfied” or “satisfied” with their professional training experience, only 27% felt that highly about how well they’re paid. Satisfaction levels increased somewhat farther into residency, reaching 35% in year 5.
Do residents have it easier today?
If so, is that rite of passage getting any easier? You’ll get different answers from residents and physicians.
Medscape asked respondents whether their journey to residency was made easier once the Step 1 exam was converted to pass-fail, and interviews brought online, because of the COVID-19 pandemic.
Many residents conceded their journey became easier, less stressful, and less expensive under the new Step 1 formats. One respondent said he was freed up to focus more intently on higher-yield academic goals such as research.
Another respondent called the pass/fail change a “total game-changer,” as it lets applicants apply to all specialties while having other qualifications than test scores considered. A resident who took Step 1 before pass/fail was instituted described the “insurmountable stress associated with studying for Step 1 to get the highest score you possibly could.”
But not all residents liked the difficulty in being able to differentiate themselves, beyond med school pedigrees, in the absence of Step 1 scores.
Meanwhile, some doctors posting comments to the Medscape report strongly disagreed with the idea that residency life is getting harder. They depict residency as a rite of passage under the best of circumstances.
“Whatever issues there may be [today’s residents] are still making eight times what I got and, from what I’ve seen, we had a lot more independent responsibilities,” one physician commenter said.
Other doctors were more sympathetic and worried about the future price to be paid for hardships during residency. “Compensation should not be tied to the willingness to sacrifice the most beautiful years of life,” one commentator wrote.
Online interviews: Pros and cons
Many resident respondents celebrated the opportunity to interview for residency programs online. Some who traveled to in-person interviews before the pandemic said they racked up as much as $10,000 in travel costs, adding to their debt loads.
But not everyone was a fan. Other residents sniped that peers can apply to more residencies and “hoard” interviews, making the competition that much harder.
And how useful are online interviews to a prospective resident? “Virtual interviews are terrible for getting a true sense for a program or even the people,” a 1st-year family medicine resident complained. And it’s harder for an applicant “to shine when you’re on Zoom,” a 1st-year internal medicine resident opined.
Whether to report harassment
In survey, respondents were asked whether they ever witnessed sexual abuse, harassment, or misconduct; and if so, what they did about it. Among those who did, many opted to take no action, fearing retaliation or retribution. “I saw a resident made out to be a ‘problem resident’ when reporting it and then ultimately fired,” one respondent recounted.
Other residents said they felt unsure about the protocol, whom to report to, or even what constituted harassment or misconduct. “I didn’t realize [an incident] was harassment until later,” one resident said. Others thought “minor” or “subtle” incidents did not warrant action; “they are typically microaggressions and appear accepted within the culture of the institution.”
Residents’ confusion heightened when the perpetrator was a patient. “I’m not sure what to do about that,” a respondent acknowledged. An emergency medicine resident added, “most of the time … it is the patients who are acting inappropriately, saying inappropriate things, etc. There is no way to file a complaint like that.”
Rewards and challenges for residents
Among the most rewarding parts of residency that respondents described were developing specific skills such as surgical techniques, job security, and “learning a little day by day” in the words of a 1st-year gastroenterology resident.
Others felt gratified by the chances to help patients and families, their teams, and to advance social justice and health equity.
But challenges abound – chiefly money struggles. A 3rd-year psychiatry resident lamented “being financially strapped in the prime of my life from student loans and low wages.”
Stress and emotional fatigue also came up often as major challenges. “Constantly being told to do more, more presentations, more papers, more research, more studying,” a 5th-year neurosurgery resident bemoaned. “Being expected to be at the top of my game despite being sleep-deprived, depressed, and burned out,” a 3rd-year ob.gyn. resident groused.
But some physician commenters urged residents to look for long-term growth behind the challenges. “Yes, it was hard, but the experience was phenomenal, and I am glad I did it,” one doctor said.
A version of this article first appeared on Medscape.com.
A plane crash interrupts a doctor’s vacation
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Emergencies happen anywhere, anytime – and sometimes physicians find themselves in situations where they are the only ones who can help. “Is There a Doctor in the House?” is a new series telling these stories.
When the plane crashed, I was asleep. I had arrived the evening before with my wife and three sons at a house on Kezar Lake on the Maine–New Hampshire border. I jumped out of bed and ran downstairs. My kids had been watching a float plane circling and gliding along the lake. It had crashed into the water and flipped upside down. My oldest brother-in-law jumped into his ski boat and we sped out to the scene.
All we can see are the plane’s pontoons. The rest is underwater. A woman has already surfaced, screaming. I dive in.
I find the woman’s husband and 3-year-old son struggling to get free from the plane through the smashed windshield. They manage to get to the surface. The pilot is dead, impaled through the chest by the left wing strut.
The big problem: A little girl, whom I would learn later is named Lauren, remained trapped. The water is murky but I can see her, a 5- or 6-year-old girl with this long hair, strapped in upside down and unconscious.
The mom and I dive down over and over, pulling and ripping at the door. We cannot get it open. Finally, I’m able to bend the door open enough where I can reach in, but I can’t undo the seatbelt. In my mind, I’m debating, should I try and go through the front windshield? I’m getting really tired, I can tell there’s fuel in the water, and I don’t want to drown in the plane. So I pop up to the surface and yell, “Does anyone have a knife?”
My brother-in-law shoots back to shore in the boat, screaming, “Get a knife!” My niece gets in the boat with one. I’m standing on the pontoon, and my niece is in the front of the boat calling, “Uncle Todd! Uncle Todd!” and she throws the knife. It goes way over my head. I can’t even jump for it, it’s so high.
I have to get the knife. So, I dive into the water to try and find it. Somehow, the black knife has landed on the white wing, 4 or 5 feet under the water. Pure luck. It could have sunk down a hundred feet into the lake. I grab the knife and hand it to the mom, Beth. She’s able to cut the seatbelt, and we both pull Lauren to the surface.
I lay her out on the pontoon. She has no pulse and her pupils are fixed and dilated. Her mom is yelling, “She’s dead, isn’t she?” I start CPR. My skin and eyes are burning from the airplane fuel in the water. I get her breathing, and her heart comes back very quickly. Lauren starts to vomit and I’m trying to keep her airway clear. She’s breathing spontaneously and she has a pulse, so I decide it’s time to move her to shore.
We pull the boat up to the dock and Lauren’s now having anoxic seizures. Her brain has been without oxygen, and now she’s getting perfused again. We get her to shore and lay her on the lawn. I’m still doing mouth-to-mouth, but she’s seizing like crazy, and I don’t have any way to control that. Beth is crying and wants to hold her daughter gently while I’m working.
Someone had called 911, and finally this dude shows up with an ambulance, and it’s like something out of World War II. All he has is an oxygen tank, but the mask is old and cracked. It’s too big for Lauren, but it sort of fits me, so I’m sucking in oxygen and blowing it into the girl’s mouth. I’m doing whatever I can, but I don’t have an IV to start. I have no fluids. I got nothing.
As it happens, I’d done my emergency medicine training at Maine Medical Center, so I tell someone to call them and get a Life Flight chopper. We have to drive somewhere where the chopper can land, so we take the ambulance to the parking lot of the closest store called the Wicked Good Store. That’s a common thing in Maine. Everything is “wicked good.”
The whole town is there by that point. The chopper arrives. The ambulance doors pop open and a woman says, “Todd?” And I say, “Heather?”
Heather is an emergency flight nurse whom I’d trained with many years ago. There’s immediate trust. She has all the right equipment. We put in breathing tubes and IVs. We stop Lauren from seizing. The kid is soon stable.
There is only one extra seat in the chopper, so I tell Beth to go. They take off.
Suddenly, I begin to doubt my decision. Lauren had been underwater for 15 minutes at minimum. I know how long that is. Did I do the right thing? Did I resuscitate a brain-dead child? I didn’t think about it at the time, but if that patient had come to me in the emergency department, I’m honestly not sure what I would have done.
So, I go home. And I don’t get a call. The FAA and sheriff arrive to take statements from us. I don’t hear from anyone.
The next day I start calling. No one will tell me anything, so I finally get to one of the pediatric ICU attendings who had trained me. He says Lauren literally woke up and said, “I have to go pee.” And that was it. She was 100% normal. I couldn’t believe it.
Here’s a theory: In kids, there’s something called the glottic reflex. I think her glottic reflex went off as soon as she hit the water, which basically closed her airway. So when she passed out, she could never get enough water in her lungs and still had enough air in there to keep her alive. Later, I got a call from her uncle. He could barely get the words out because he was in tears. He said Lauren was doing beautifully.
Three days later, I drove to Lauren’s house with my wife and kids. I had her read to me. I watched her play on the jungle gym for motor function. All sorts of stuff. She was totally normal.
Beth told us that the night before the accident, her mother had given the women in her family what she called a “miracle bracelet,” a bracelet that is supposed to give you one miracle in your life. Beth said she had the bracelet on her wrist the day of the accident, and now it’s gone. “Saving Lauren’s life was my miracle,” she said.
Funny thing: For 20 years, I ran all the EMS, police, fire, ambulance, in Boulder, Colo., where I live. I wrote all the protocols, and I would never advise any of my paramedics to dive into jet fuel to save someone. That was risky. But at the time, it was totally automatic. I think it taught me not to give up in certain situations, because you really don’t know.
Dr. Dorfman is an emergency medicine physician in Boulder, Colo., and medical director at Cedalion Health.
A version of this article first appeared on Medscape.com.
Give bacterial diversity a chance: The antibiotic dichotomy
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Fungi inside cancer cells: ‘A new and emerging hallmark’
The investigators characterized the cancer mycobiome within 17,401 tissue, blood, and plasma samples from four international cohorts, revealing new information about fungi distribution, association with immune cells, and potential prognostic value.
Fungi were detected in all cancer types studied and were often intracellular, reported Lian Narunsky-Haziza, PhD, of Weizmann Institute of Science, Rehovot, Israel, and colleagues.
Additionally, multiple fungal-bacterial-immune ecologies were detected across tumors, and intratumoral fungi stratified clinical outcomes, including immunotherapy response, they noted. Also, cell-free fungal DNA diagnosed healthy and cancer patients in early-stage disease.
The findings, published online in the journal Cell, have potential implications for cancer detection, diagnosis, and treatment, the researchers suggested.
The existence of fungi in most human cancers “is both a surprise and to be expected,” study coauthor Rob Knight, PhD, a professor at the University of California, San Diego, stated in a press release. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected, because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
Exploration of the associations between cancer and microbes are nothing new, but cancer-associated fungi have rarely been examined, the authors noted.
The findings from this pan-cancer analysis, which suggested “prognostic and diagnostic capacities of the tissue and plasma mycobiomes, even in stage I cancers,” complement current “understanding of the interaction between cancer cells and the bacteria that exist in tumors alongside fungi, bacteria that have been shown to affect cancer growth, metastasis, and response to therapy,” they explained.
Of note, the study revealed multiple correlations between the presence of specific fungi in tumors and conditions related to treatment. For example, patients with breast cancer whose tumors contained Malassezia globosa – a fungus found naturally on the skin – had a much lower survival rate than those whose tumors did not contain the fungus. Furthermore, specific fungi were more prevalent in breast tumors from older vs. younger patients, in lung tumors of smokers vs. nonsmokers, and in melanoma tumors that responded to immunotherapy vs. those that did not respond.
These findings suggest that fungal activity is “a new and emerging hallmark of cancer,” stated study coleader Ravid Straussman, PhD, of the Weizmann molecular cell biology department. “These findings should drive us to better explore the potential effects of tumor fungi and to re-examine almost everything we know about cancer through a ‘microbiome lens.’ ”
Unique relationships observed between fungi and bacteria – for example, tumors that contain Aspergillus fungi tended to have specific bacteria in them, whereas tumors that contain Malassezia fungi tended to have other bacteria in them – may have implications for treatment, as they correlated with both tumor immunity and patient survival, according to the authors.
“This study sheds new light on the complex biological environment within tumors, and future research will reveal how fungi affect cancerous growth,” said coauthor Yitzhak Pilpel, PhD, a principal investigator at the Weizmann molecular genetics department. “The fact that fungi can be found not only in cancer cells but also in immune cells implies that, in the future, we’ll probably find that fungi have some effect not only on the cancer cells but also on immune cells and their activity.”
A further finding related to the presence of fungal and bacterial DNA in human blood further suggests that measuring microbial DNA in the blood could lead to early detection of cancer, the authors noted.
Dr. Straussman’s research is supported by the Swiss Society Institute for Cancer Prevention Research, the Fabricant-Morse Families Research Fund for Humanity, the Dr. Chantal d’Adesky Scheinberg Research Fund, and the Dr. Dvora and Haim Teitelbaum Endowment Fund.
A version of this article first appeared on Medscape.com.
The investigators characterized the cancer mycobiome within 17,401 tissue, blood, and plasma samples from four international cohorts, revealing new information about fungi distribution, association with immune cells, and potential prognostic value.
Fungi were detected in all cancer types studied and were often intracellular, reported Lian Narunsky-Haziza, PhD, of Weizmann Institute of Science, Rehovot, Israel, and colleagues.
Additionally, multiple fungal-bacterial-immune ecologies were detected across tumors, and intratumoral fungi stratified clinical outcomes, including immunotherapy response, they noted. Also, cell-free fungal DNA diagnosed healthy and cancer patients in early-stage disease.
The findings, published online in the journal Cell, have potential implications for cancer detection, diagnosis, and treatment, the researchers suggested.
The existence of fungi in most human cancers “is both a surprise and to be expected,” study coauthor Rob Knight, PhD, a professor at the University of California, San Diego, stated in a press release. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected, because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
Exploration of the associations between cancer and microbes are nothing new, but cancer-associated fungi have rarely been examined, the authors noted.
The findings from this pan-cancer analysis, which suggested “prognostic and diagnostic capacities of the tissue and plasma mycobiomes, even in stage I cancers,” complement current “understanding of the interaction between cancer cells and the bacteria that exist in tumors alongside fungi, bacteria that have been shown to affect cancer growth, metastasis, and response to therapy,” they explained.
Of note, the study revealed multiple correlations between the presence of specific fungi in tumors and conditions related to treatment. For example, patients with breast cancer whose tumors contained Malassezia globosa – a fungus found naturally on the skin – had a much lower survival rate than those whose tumors did not contain the fungus. Furthermore, specific fungi were more prevalent in breast tumors from older vs. younger patients, in lung tumors of smokers vs. nonsmokers, and in melanoma tumors that responded to immunotherapy vs. those that did not respond.
These findings suggest that fungal activity is “a new and emerging hallmark of cancer,” stated study coleader Ravid Straussman, PhD, of the Weizmann molecular cell biology department. “These findings should drive us to better explore the potential effects of tumor fungi and to re-examine almost everything we know about cancer through a ‘microbiome lens.’ ”
Unique relationships observed between fungi and bacteria – for example, tumors that contain Aspergillus fungi tended to have specific bacteria in them, whereas tumors that contain Malassezia fungi tended to have other bacteria in them – may have implications for treatment, as they correlated with both tumor immunity and patient survival, according to the authors.
“This study sheds new light on the complex biological environment within tumors, and future research will reveal how fungi affect cancerous growth,” said coauthor Yitzhak Pilpel, PhD, a principal investigator at the Weizmann molecular genetics department. “The fact that fungi can be found not only in cancer cells but also in immune cells implies that, in the future, we’ll probably find that fungi have some effect not only on the cancer cells but also on immune cells and their activity.”
A further finding related to the presence of fungal and bacterial DNA in human blood further suggests that measuring microbial DNA in the blood could lead to early detection of cancer, the authors noted.
Dr. Straussman’s research is supported by the Swiss Society Institute for Cancer Prevention Research, the Fabricant-Morse Families Research Fund for Humanity, the Dr. Chantal d’Adesky Scheinberg Research Fund, and the Dr. Dvora and Haim Teitelbaum Endowment Fund.
A version of this article first appeared on Medscape.com.
The investigators characterized the cancer mycobiome within 17,401 tissue, blood, and plasma samples from four international cohorts, revealing new information about fungi distribution, association with immune cells, and potential prognostic value.
Fungi were detected in all cancer types studied and were often intracellular, reported Lian Narunsky-Haziza, PhD, of Weizmann Institute of Science, Rehovot, Israel, and colleagues.
Additionally, multiple fungal-bacterial-immune ecologies were detected across tumors, and intratumoral fungi stratified clinical outcomes, including immunotherapy response, they noted. Also, cell-free fungal DNA diagnosed healthy and cancer patients in early-stage disease.
The findings, published online in the journal Cell, have potential implications for cancer detection, diagnosis, and treatment, the researchers suggested.
The existence of fungi in most human cancers “is both a surprise and to be expected,” study coauthor Rob Knight, PhD, a professor at the University of California, San Diego, stated in a press release. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected, because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
Exploration of the associations between cancer and microbes are nothing new, but cancer-associated fungi have rarely been examined, the authors noted.
The findings from this pan-cancer analysis, which suggested “prognostic and diagnostic capacities of the tissue and plasma mycobiomes, even in stage I cancers,” complement current “understanding of the interaction between cancer cells and the bacteria that exist in tumors alongside fungi, bacteria that have been shown to affect cancer growth, metastasis, and response to therapy,” they explained.
Of note, the study revealed multiple correlations between the presence of specific fungi in tumors and conditions related to treatment. For example, patients with breast cancer whose tumors contained Malassezia globosa – a fungus found naturally on the skin – had a much lower survival rate than those whose tumors did not contain the fungus. Furthermore, specific fungi were more prevalent in breast tumors from older vs. younger patients, in lung tumors of smokers vs. nonsmokers, and in melanoma tumors that responded to immunotherapy vs. those that did not respond.
These findings suggest that fungal activity is “a new and emerging hallmark of cancer,” stated study coleader Ravid Straussman, PhD, of the Weizmann molecular cell biology department. “These findings should drive us to better explore the potential effects of tumor fungi and to re-examine almost everything we know about cancer through a ‘microbiome lens.’ ”
Unique relationships observed between fungi and bacteria – for example, tumors that contain Aspergillus fungi tended to have specific bacteria in them, whereas tumors that contain Malassezia fungi tended to have other bacteria in them – may have implications for treatment, as they correlated with both tumor immunity and patient survival, according to the authors.
“This study sheds new light on the complex biological environment within tumors, and future research will reveal how fungi affect cancerous growth,” said coauthor Yitzhak Pilpel, PhD, a principal investigator at the Weizmann molecular genetics department. “The fact that fungi can be found not only in cancer cells but also in immune cells implies that, in the future, we’ll probably find that fungi have some effect not only on the cancer cells but also on immune cells and their activity.”
A further finding related to the presence of fungal and bacterial DNA in human blood further suggests that measuring microbial DNA in the blood could lead to early detection of cancer, the authors noted.
Dr. Straussman’s research is supported by the Swiss Society Institute for Cancer Prevention Research, the Fabricant-Morse Families Research Fund for Humanity, the Dr. Chantal d’Adesky Scheinberg Research Fund, and the Dr. Dvora and Haim Teitelbaum Endowment Fund.
A version of this article first appeared on Medscape.com.
FROM CELL
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.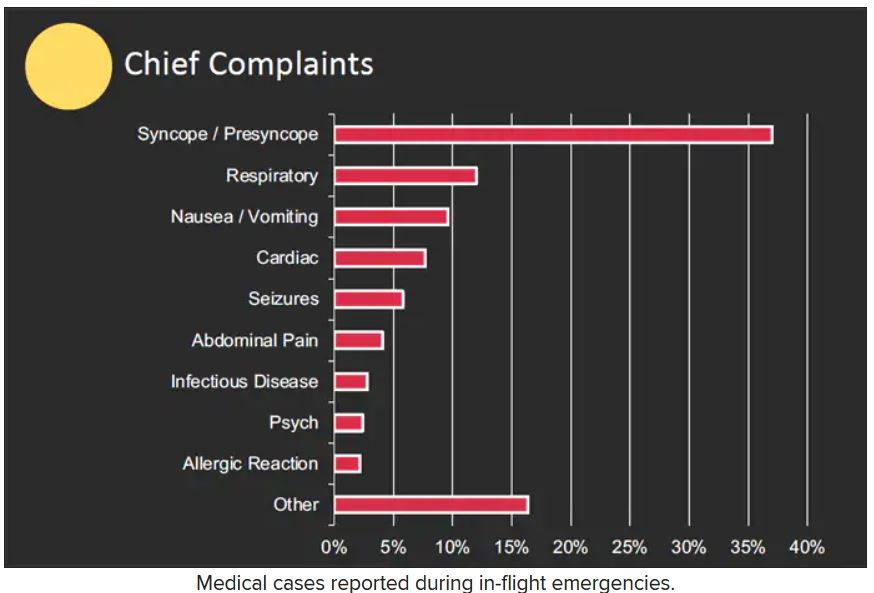
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Teclistamab for MM: Lifesaver or 'cause of death'?
Following “unprecedented” results in a phase 1/2 study, teclistamab (Tecvayli, Janssen Biotech) received accelerated approval from the Food and Drug Administration for adults with relapsed/refractory multiple myeloma who had received at least four lines of therapy. Typically, patients in this situation have just a few weeks to live.
This is “unprecedented” said Nikhil Munshi, MD, professor of medicine at Harvard Medical School, Boston, who was not involved with the study. “Pomalidomide got approved with 30% response rate, carfilzomib got approved with 29% response rate, selinexor got approved with 31% response rate and so on and on. ... So here is teclistamab with [this] response rate in patients having five, six lines of treatment. ...[It’s] going to be so much in demand because it’s a great drug.”
The first cut of the data appeared in the New England Journal of Medicine.
At the 6-month mark, 90.6% of patients who responded had no progression of their disease, and at 9 months, 66.5% of patients were still holding steady.
Senior investigator in the trial, Saad Usmani, MD, of Memorial Sloan Kettering Cancer Center, New York, said: “What was most striking was the high response rates and the durability of response.”
Dr. Usmani said ease of administration was the other aspect of teclistamab that impressed him. The drug is given by subcutaneous injection weekly after a short ramp-up period.
He contrasted this regimen with that of chimeric antigen receptor (CAR) T-cell therapy, the only alternative with similar efficacy in such sick patients: “I can prescribe [teclistamab] today, and my patient gets it tomorrow,” Dr. Usmani said. “With CAR T, I prescribe today and it will take 4-6 weeks for us to collect T cells and another 6-7 weeks for the product to come back.” Dr. Usmani said many patients die before CAR T reaches them.
Community oncology will benefit greatly from teclistamab, especially patients for whom CAR T isn’t feasible, said Kashyap Patel, MD, president of the Community Oncology Alliance. “My patients are most of them underserved minority-class populations with myeloma, and they cannot travel many miles to go to a CAR T center. With sub[cutaneous] injection, the patient can have [teclistamab] administered in their doctor’s office and continue to live their normal life.”
However, how should the wider oncology community make sense of a drug approval based solely on response in a single-arm, phase 1/2 study, with no survival data?
Dr. Patel said, “Phase 1 plus phase 2 data is probably a little bit quick, but time will tell eventually.” He cited melflufen as a cautionary tale: a product given accelerated approval for multiple myeloma, then withdrawn when new data showed that it increased the risk of death.
When Dr. Munshi was asked about trial design for accelerated approvals, he responded, “you are touching a topic very close to my heart, a topic of great significance currently.”
He went on to say that overall survival (OS) is no longer a viable trial endpoint in diseases like multiple myeloma for several reasons. Most significantly, he noted: “Survival has gone up to 10 or 15 years [so] today, if you randomize between one [drug] versus another, there are going to be seven or eight more treatments before the patient dies.”
Similarly, progression-free survival (PFS) in multiple myeloma is now as much as 5 years, Dr. Munshi said. “Do we want a patient to wait 5 years to get a very good new drug?”
For these reasons and others, Dr, Munshi observed, myeloma researchers are increasingly relying on a surrogate called “negative minimal residual disease” (negative MRD) – in other words, a situation in which myeloma cells can no longer be detected in the bone marrow. MRD is hunted out using next-generation flow or next-generation sequencing of myeloma-cell DNA from bone-marrow aspirate to levels as low as 1 in 100,000 or 1,000,000 cells.
In 2020, Dr. Munshi and colleagues published a large meta-analysis showing that a negative MRD in a patient with multiple myeloma was significantly prognostic for both progression-free survival (hazard ratio, 0.33; P < .001) and overall survival (HR, 0.45; P < .001). The team concluded: “MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in [multiple myeloma].”
In MajesTEC-1 overall, 26.7% of patients on teclistamab had no signs of residual disease at a threshold of 1 in 100,000. Among patients who showed a “complete response” by International Myeloma Working Group criteria, 46% had no residual disease.
Dr. Munshi stressed that such patients are not necessarily “cured.” It will take a few more years to prove that. He noted: “Simply, physiologically, [negative MRD] means that if a patient has one [myeloma] cell in a million, that cell is going to take a much longer time to grow up to be myeloma.”
On Nov. 8 and 9, the FDA and the International Myeloma Society held a workshop to discuss the vexed question of surrogate endpoints and single-arm studies for drug approvals entitled the “Future of Drug Development in Multiple Myeloma.” Dr. Munshi was cochair.
A panelist at the meeting who was a senior investigator in the MajesTEC-1 trial, Ajai Chari, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, summed up the dilemma: “No one disagrees that randomized studies are the best way of doing things. The question is, if you’re a patient who’s exhausted all available therapies, do you have that time to wait? ... The role of accelerated approval is to get the drug to the patient faster. But what does it not pick up? How do we make these accelerated approvals more meaningful and not have to retract for safety?”
Jonathon Vallejo, also on the panel, agreed that safety was the key worry. The ideal scenario for accelerated approval would be a drug that was better than available therapy, and “in some sense, it’s much safer.” However, such situations are rare.
“Most of the time, we don’t have these products that come in that have no toxicity signals,” he said. “So one thing we have to think carefully about in the single-arm trial setting is, what are the toxicities? How do they stack up?”
Dr. Chari said that, for his part, he wanted to see more transparency around “cause of death” in all studies that lead to accelerated approvals. He said he was “tired” of seeing a death labeled as “not attributed” to the drug by the investigator or the drug company.
“Let me decide. Show me the deaths, and show me the myeloma status at that point,” Dr. Chari said. “That’s a signal – if you’re a responding patient and dying, then the FDA should be a little bit more cautious.”
The FDA has added a boxed warning to the teclistamab product information concerning cytokine-release syndrome and neurologic toxicity.
Cytokine-release syndrome, the most common side effect overall, showed up in 72% of patients, typically 2 days after the first step-up dose.
Neurologic toxicity occurred in 57% of patients, including headache (25%), motor dysfunction (16%), sensory neuropathy (15%) and encephalopathy (13%). About 6%of patients developed a serious, life-threatening neurologic condition called immune effector cell–associated neurotoxicity syndrome.
Overall, serious adverse reactions occurred in 54% of participants in MajesTEC-1, and 5% of people in the trial died from adverse reactions during the study, most commonly infections.
Because of its safety profile, teclistamab is available only through a restricted program called TECVAYLI Risk Evaluation and Mitigation Strategy.
The continued approval of teclistamab for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
To that end, eight more studies of teclistamab are underway, aiming for approximately 1,300 multiple myeloma patients around the world. Three of these trials are in newly diagnosed patients. Four more studies are planned to come online in the next 3 months, raising the final tally of patients testing out teclistamab to approximately 4,700. The trials will look at teclistamab in sequence or in combination with standards such as bortezomib and pomalidomide. All studies are open label.
Dr. Patel believes that, until these trials say otherwise, the benefits of teclistamab outweigh the risks. “I’m very happy we have one more option in this space, particularly the fourth or fifth line for patients who want to continue to fight the disease,” Dr. Patel concluded.
Dr. Munshi disclosed advisory board/consultant work for Adaptive, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Legend, Millennium, Novartis, Oncopep, and Pfizer and is the scientific founder of Oncopep and DCT. The 2020 meta-analysis by Dr. Munshi and colleagues was funded by Janssen-Cilag. Dr. Patel declared funding from Janssen for a diversity-equity initiative and membership of the South Carolina Medicaid P & T Committee. Dr. Usmani declared conflicts of interest with Amgen, BMS/Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Abbvie, Genentech, Gilead, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio.
Following “unprecedented” results in a phase 1/2 study, teclistamab (Tecvayli, Janssen Biotech) received accelerated approval from the Food and Drug Administration for adults with relapsed/refractory multiple myeloma who had received at least four lines of therapy. Typically, patients in this situation have just a few weeks to live.
This is “unprecedented” said Nikhil Munshi, MD, professor of medicine at Harvard Medical School, Boston, who was not involved with the study. “Pomalidomide got approved with 30% response rate, carfilzomib got approved with 29% response rate, selinexor got approved with 31% response rate and so on and on. ... So here is teclistamab with [this] response rate in patients having five, six lines of treatment. ...[It’s] going to be so much in demand because it’s a great drug.”
The first cut of the data appeared in the New England Journal of Medicine.
At the 6-month mark, 90.6% of patients who responded had no progression of their disease, and at 9 months, 66.5% of patients were still holding steady.
Senior investigator in the trial, Saad Usmani, MD, of Memorial Sloan Kettering Cancer Center, New York, said: “What was most striking was the high response rates and the durability of response.”
Dr. Usmani said ease of administration was the other aspect of teclistamab that impressed him. The drug is given by subcutaneous injection weekly after a short ramp-up period.
He contrasted this regimen with that of chimeric antigen receptor (CAR) T-cell therapy, the only alternative with similar efficacy in such sick patients: “I can prescribe [teclistamab] today, and my patient gets it tomorrow,” Dr. Usmani said. “With CAR T, I prescribe today and it will take 4-6 weeks for us to collect T cells and another 6-7 weeks for the product to come back.” Dr. Usmani said many patients die before CAR T reaches them.
Community oncology will benefit greatly from teclistamab, especially patients for whom CAR T isn’t feasible, said Kashyap Patel, MD, president of the Community Oncology Alliance. “My patients are most of them underserved minority-class populations with myeloma, and they cannot travel many miles to go to a CAR T center. With sub[cutaneous] injection, the patient can have [teclistamab] administered in their doctor’s office and continue to live their normal life.”
However, how should the wider oncology community make sense of a drug approval based solely on response in a single-arm, phase 1/2 study, with no survival data?
Dr. Patel said, “Phase 1 plus phase 2 data is probably a little bit quick, but time will tell eventually.” He cited melflufen as a cautionary tale: a product given accelerated approval for multiple myeloma, then withdrawn when new data showed that it increased the risk of death.
When Dr. Munshi was asked about trial design for accelerated approvals, he responded, “you are touching a topic very close to my heart, a topic of great significance currently.”
He went on to say that overall survival (OS) is no longer a viable trial endpoint in diseases like multiple myeloma for several reasons. Most significantly, he noted: “Survival has gone up to 10 or 15 years [so] today, if you randomize between one [drug] versus another, there are going to be seven or eight more treatments before the patient dies.”
Similarly, progression-free survival (PFS) in multiple myeloma is now as much as 5 years, Dr. Munshi said. “Do we want a patient to wait 5 years to get a very good new drug?”
For these reasons and others, Dr, Munshi observed, myeloma researchers are increasingly relying on a surrogate called “negative minimal residual disease” (negative MRD) – in other words, a situation in which myeloma cells can no longer be detected in the bone marrow. MRD is hunted out using next-generation flow or next-generation sequencing of myeloma-cell DNA from bone-marrow aspirate to levels as low as 1 in 100,000 or 1,000,000 cells.
In 2020, Dr. Munshi and colleagues published a large meta-analysis showing that a negative MRD in a patient with multiple myeloma was significantly prognostic for both progression-free survival (hazard ratio, 0.33; P < .001) and overall survival (HR, 0.45; P < .001). The team concluded: “MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in [multiple myeloma].”
In MajesTEC-1 overall, 26.7% of patients on teclistamab had no signs of residual disease at a threshold of 1 in 100,000. Among patients who showed a “complete response” by International Myeloma Working Group criteria, 46% had no residual disease.
Dr. Munshi stressed that such patients are not necessarily “cured.” It will take a few more years to prove that. He noted: “Simply, physiologically, [negative MRD] means that if a patient has one [myeloma] cell in a million, that cell is going to take a much longer time to grow up to be myeloma.”
On Nov. 8 and 9, the FDA and the International Myeloma Society held a workshop to discuss the vexed question of surrogate endpoints and single-arm studies for drug approvals entitled the “Future of Drug Development in Multiple Myeloma.” Dr. Munshi was cochair.
A panelist at the meeting who was a senior investigator in the MajesTEC-1 trial, Ajai Chari, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, summed up the dilemma: “No one disagrees that randomized studies are the best way of doing things. The question is, if you’re a patient who’s exhausted all available therapies, do you have that time to wait? ... The role of accelerated approval is to get the drug to the patient faster. But what does it not pick up? How do we make these accelerated approvals more meaningful and not have to retract for safety?”
Jonathon Vallejo, also on the panel, agreed that safety was the key worry. The ideal scenario for accelerated approval would be a drug that was better than available therapy, and “in some sense, it’s much safer.” However, such situations are rare.
“Most of the time, we don’t have these products that come in that have no toxicity signals,” he said. “So one thing we have to think carefully about in the single-arm trial setting is, what are the toxicities? How do they stack up?”
Dr. Chari said that, for his part, he wanted to see more transparency around “cause of death” in all studies that lead to accelerated approvals. He said he was “tired” of seeing a death labeled as “not attributed” to the drug by the investigator or the drug company.
“Let me decide. Show me the deaths, and show me the myeloma status at that point,” Dr. Chari said. “That’s a signal – if you’re a responding patient and dying, then the FDA should be a little bit more cautious.”
The FDA has added a boxed warning to the teclistamab product information concerning cytokine-release syndrome and neurologic toxicity.
Cytokine-release syndrome, the most common side effect overall, showed up in 72% of patients, typically 2 days after the first step-up dose.
Neurologic toxicity occurred in 57% of patients, including headache (25%), motor dysfunction (16%), sensory neuropathy (15%) and encephalopathy (13%). About 6%of patients developed a serious, life-threatening neurologic condition called immune effector cell–associated neurotoxicity syndrome.
Overall, serious adverse reactions occurred in 54% of participants in MajesTEC-1, and 5% of people in the trial died from adverse reactions during the study, most commonly infections.
Because of its safety profile, teclistamab is available only through a restricted program called TECVAYLI Risk Evaluation and Mitigation Strategy.
The continued approval of teclistamab for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
To that end, eight more studies of teclistamab are underway, aiming for approximately 1,300 multiple myeloma patients around the world. Three of these trials are in newly diagnosed patients. Four more studies are planned to come online in the next 3 months, raising the final tally of patients testing out teclistamab to approximately 4,700. The trials will look at teclistamab in sequence or in combination with standards such as bortezomib and pomalidomide. All studies are open label.
Dr. Patel believes that, until these trials say otherwise, the benefits of teclistamab outweigh the risks. “I’m very happy we have one more option in this space, particularly the fourth or fifth line for patients who want to continue to fight the disease,” Dr. Patel concluded.
Dr. Munshi disclosed advisory board/consultant work for Adaptive, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Legend, Millennium, Novartis, Oncopep, and Pfizer and is the scientific founder of Oncopep and DCT. The 2020 meta-analysis by Dr. Munshi and colleagues was funded by Janssen-Cilag. Dr. Patel declared funding from Janssen for a diversity-equity initiative and membership of the South Carolina Medicaid P & T Committee. Dr. Usmani declared conflicts of interest with Amgen, BMS/Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Abbvie, Genentech, Gilead, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio.
Following “unprecedented” results in a phase 1/2 study, teclistamab (Tecvayli, Janssen Biotech) received accelerated approval from the Food and Drug Administration for adults with relapsed/refractory multiple myeloma who had received at least four lines of therapy. Typically, patients in this situation have just a few weeks to live.
This is “unprecedented” said Nikhil Munshi, MD, professor of medicine at Harvard Medical School, Boston, who was not involved with the study. “Pomalidomide got approved with 30% response rate, carfilzomib got approved with 29% response rate, selinexor got approved with 31% response rate and so on and on. ... So here is teclistamab with [this] response rate in patients having five, six lines of treatment. ...[It’s] going to be so much in demand because it’s a great drug.”
The first cut of the data appeared in the New England Journal of Medicine.
At the 6-month mark, 90.6% of patients who responded had no progression of their disease, and at 9 months, 66.5% of patients were still holding steady.
Senior investigator in the trial, Saad Usmani, MD, of Memorial Sloan Kettering Cancer Center, New York, said: “What was most striking was the high response rates and the durability of response.”
Dr. Usmani said ease of administration was the other aspect of teclistamab that impressed him. The drug is given by subcutaneous injection weekly after a short ramp-up period.
He contrasted this regimen with that of chimeric antigen receptor (CAR) T-cell therapy, the only alternative with similar efficacy in such sick patients: “I can prescribe [teclistamab] today, and my patient gets it tomorrow,” Dr. Usmani said. “With CAR T, I prescribe today and it will take 4-6 weeks for us to collect T cells and another 6-7 weeks for the product to come back.” Dr. Usmani said many patients die before CAR T reaches them.
Community oncology will benefit greatly from teclistamab, especially patients for whom CAR T isn’t feasible, said Kashyap Patel, MD, president of the Community Oncology Alliance. “My patients are most of them underserved minority-class populations with myeloma, and they cannot travel many miles to go to a CAR T center. With sub[cutaneous] injection, the patient can have [teclistamab] administered in their doctor’s office and continue to live their normal life.”
However, how should the wider oncology community make sense of a drug approval based solely on response in a single-arm, phase 1/2 study, with no survival data?
Dr. Patel said, “Phase 1 plus phase 2 data is probably a little bit quick, but time will tell eventually.” He cited melflufen as a cautionary tale: a product given accelerated approval for multiple myeloma, then withdrawn when new data showed that it increased the risk of death.
When Dr. Munshi was asked about trial design for accelerated approvals, he responded, “you are touching a topic very close to my heart, a topic of great significance currently.”
He went on to say that overall survival (OS) is no longer a viable trial endpoint in diseases like multiple myeloma for several reasons. Most significantly, he noted: “Survival has gone up to 10 or 15 years [so] today, if you randomize between one [drug] versus another, there are going to be seven or eight more treatments before the patient dies.”
Similarly, progression-free survival (PFS) in multiple myeloma is now as much as 5 years, Dr. Munshi said. “Do we want a patient to wait 5 years to get a very good new drug?”
For these reasons and others, Dr, Munshi observed, myeloma researchers are increasingly relying on a surrogate called “negative minimal residual disease” (negative MRD) – in other words, a situation in which myeloma cells can no longer be detected in the bone marrow. MRD is hunted out using next-generation flow or next-generation sequencing of myeloma-cell DNA from bone-marrow aspirate to levels as low as 1 in 100,000 or 1,000,000 cells.
In 2020, Dr. Munshi and colleagues published a large meta-analysis showing that a negative MRD in a patient with multiple myeloma was significantly prognostic for both progression-free survival (hazard ratio, 0.33; P < .001) and overall survival (HR, 0.45; P < .001). The team concluded: “MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in [multiple myeloma].”
In MajesTEC-1 overall, 26.7% of patients on teclistamab had no signs of residual disease at a threshold of 1 in 100,000. Among patients who showed a “complete response” by International Myeloma Working Group criteria, 46% had no residual disease.
Dr. Munshi stressed that such patients are not necessarily “cured.” It will take a few more years to prove that. He noted: “Simply, physiologically, [negative MRD] means that if a patient has one [myeloma] cell in a million, that cell is going to take a much longer time to grow up to be myeloma.”
On Nov. 8 and 9, the FDA and the International Myeloma Society held a workshop to discuss the vexed question of surrogate endpoints and single-arm studies for drug approvals entitled the “Future of Drug Development in Multiple Myeloma.” Dr. Munshi was cochair.
A panelist at the meeting who was a senior investigator in the MajesTEC-1 trial, Ajai Chari, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, summed up the dilemma: “No one disagrees that randomized studies are the best way of doing things. The question is, if you’re a patient who’s exhausted all available therapies, do you have that time to wait? ... The role of accelerated approval is to get the drug to the patient faster. But what does it not pick up? How do we make these accelerated approvals more meaningful and not have to retract for safety?”
Jonathon Vallejo, also on the panel, agreed that safety was the key worry. The ideal scenario for accelerated approval would be a drug that was better than available therapy, and “in some sense, it’s much safer.” However, such situations are rare.
“Most of the time, we don’t have these products that come in that have no toxicity signals,” he said. “So one thing we have to think carefully about in the single-arm trial setting is, what are the toxicities? How do they stack up?”
Dr. Chari said that, for his part, he wanted to see more transparency around “cause of death” in all studies that lead to accelerated approvals. He said he was “tired” of seeing a death labeled as “not attributed” to the drug by the investigator or the drug company.
“Let me decide. Show me the deaths, and show me the myeloma status at that point,” Dr. Chari said. “That’s a signal – if you’re a responding patient and dying, then the FDA should be a little bit more cautious.”
The FDA has added a boxed warning to the teclistamab product information concerning cytokine-release syndrome and neurologic toxicity.
Cytokine-release syndrome, the most common side effect overall, showed up in 72% of patients, typically 2 days after the first step-up dose.
Neurologic toxicity occurred in 57% of patients, including headache (25%), motor dysfunction (16%), sensory neuropathy (15%) and encephalopathy (13%). About 6%of patients developed a serious, life-threatening neurologic condition called immune effector cell–associated neurotoxicity syndrome.
Overall, serious adverse reactions occurred in 54% of participants in MajesTEC-1, and 5% of people in the trial died from adverse reactions during the study, most commonly infections.
Because of its safety profile, teclistamab is available only through a restricted program called TECVAYLI Risk Evaluation and Mitigation Strategy.
The continued approval of teclistamab for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
To that end, eight more studies of teclistamab are underway, aiming for approximately 1,300 multiple myeloma patients around the world. Three of these trials are in newly diagnosed patients. Four more studies are planned to come online in the next 3 months, raising the final tally of patients testing out teclistamab to approximately 4,700. The trials will look at teclistamab in sequence or in combination with standards such as bortezomib and pomalidomide. All studies are open label.
Dr. Patel believes that, until these trials say otherwise, the benefits of teclistamab outweigh the risks. “I’m very happy we have one more option in this space, particularly the fourth or fifth line for patients who want to continue to fight the disease,” Dr. Patel concluded.
Dr. Munshi disclosed advisory board/consultant work for Adaptive, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Legend, Millennium, Novartis, Oncopep, and Pfizer and is the scientific founder of Oncopep and DCT. The 2020 meta-analysis by Dr. Munshi and colleagues was funded by Janssen-Cilag. Dr. Patel declared funding from Janssen for a diversity-equity initiative and membership of the South Carolina Medicaid P & T Committee. Dr. Usmani declared conflicts of interest with Amgen, BMS/Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Abbvie, Genentech, Gilead, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
No benefit of rivaroxaban in COVID outpatients: PREVENT-HD
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
A new U.S. randomized trial has failed to show benefit of a 35-day course of oral anticoagulation with rivaroxaban for the prevention of thrombotic events in outpatients with symptomatic COVID-19.
The PREVENT-HD trial was presented at the American Heart Association scientific sessions by Gregory Piazza, MD, Brigham and Women’s Hospital, Boston.
“With the caveat that the trial was underpowered to provide a definitive conclusion, these data do not support routine antithrombotic prophylaxis in nonhospitalized patients with symptomatic COVID-19,” Dr. Piazza concluded.
PREVENT-HD is the largest randomized study to look at anticoagulation in nonhospitalized COVID-19 patients and joins a long list of smaller trials that have also shown no benefit with this approach.
However, anticoagulation is recommended in patients who are hospitalized with COVID-19.
Dr. Piazza noted that the issue of anticoagulation in COVID-19 has focused mainly on hospitalized patients, but most COVID-19 cases are treated as outpatients, who are also suspected to be at risk for venous and arterial thrombotic events, especially if they have additional risk factors. Histopathological evidence also suggests that at least part of the deterioration in lung function leading to hospitalization may be attributable to in situ pulmonary artery thrombosis.
The PREVENT-HD trial explored the question of whether early initiation of thromboprophylaxis dosing of rivaroxaban in higher-risk outpatients with COVID-19 may lower the incidence of venous and arterial thrombotic events, reduce in situ pulmonary thrombosis and the worsening of pulmonary function that may lead to hospitalization, and reduce all-cause mortality.
The trial included 1,284 outpatients with a positive test for COVID-19 and who were within 14 days of symptom onset. They also had to have at least one of the following additional risk factors: age over 60 years; prior history of venous thromboembolism (VTE), thrombophilia, coronary artery disease, peripheral artery disease, cardiovascular disease or ischemic stroke, cancer, diabetes, heart failure, obesity (body mass index ≥ 35 kg/m2) or D-dimer > upper limit of normal. Around 35% of the study population had two or more of these risk factors.
Patients were randomized to rivaroxaban 10 mg daily for 35 days or placebo.
The primary efficacy endpoint was time to first occurrence of a composite of symptomatic VTE, myocardial infarction, ischemic stroke, acute limb ischemia, non–central nervous system systemic embolization, all-cause hospitalization, and all-cause mortality up to day 35.
The primary safety endpoint was time to first occurrence of International Society on Thrombosis and Hemostasis (ISTH) critical-site and fatal bleeding.
A modified intention-to-treat analysis (all participants taking at least one dose of study intervention) was also planned.
The trial was stopped early in April this year because of a lower than expected event incidence (3.2%), compared with the planned rate (8.5%), giving a very low likelihood of being able to achieve the required number of events.
Dr. Piazza said reasons contributing to the low event rate included a falling COVID-19 death and hospitalization rate nationwide, and increased use of effective vaccines.
Results of the main intention-to-treat analysis (in 1,284 patients) showed no significant difference in the primary efficacy composite endpoint, which occurred in 3.4% of the rivaroxaban group versus 3.0% of the placebo group.
In the modified intention-to-treat analysis (which included 1,197 patients who actually took at least one dose of the study medication) there was shift in the directionality of the point estimate (rivaroxaban 2.0% vs. placebo 2.7%), which Dr. Piazza said was related to a higher number of patients hospitalized before receiving study drug in the rivaroxaban group. However, the difference was still nonsignificant.
The first major secondary outcome of symptomatic VTE, arterial thrombotic events, and all-cause mortality occurred in 0.3% of rivaroxaban patients versus 1.1% of placebo patients, but this difference did not reach statistical significance.
However, a post hoc exploratory analysis did show a significant reduction in the outcome of symptomatic VTE and arterial thrombotic events.
In terms of safety, there were no fatal critical-site bleeding events, and there was no difference in ISTH major bleeding, which occurred in one patient in the rivaroxaban group versus no patients in the placebo group.
There was, however, a significant increase in nonmajor clinically relevant bleeding with rivaroxaban, which occurred in nine patients (1.5%) versus one patient (0.2%) in the placebo group.
Trivial bleeding was also increased in the rivaroxaban group, occurring in 17 patients (2.8%) versus 5 patients (0.8%) in the placebo group.
Discussant for the study, Renato Lopes, MD, Duke University Medical Center, Durham, N.C., noted that the relationship between COVID-19 and thrombosis has been an important issue since the beginning of the pandemic, with many proposed mechanisms to explain the COVID-19–associated coagulopathy, which is a major cause of death and disability.
While observational data at the beginning of the pandemic suggested patients with COVID-19 might benefit from anticoagulation, looking at all the different randomized trials that have tested anticoagulation in COVID-19 outpatients, there is no treatment effect on the various different primary outcomes in those studies and also no effect on all-cause mortality, Dr. Lopes said.
He pointed out that PREVENT-HD was stopped prematurely with only about one-third of the planned number of patients enrolled, “just like every other outpatient COVID-19 trial.”
He also drew attention to the low rates of vaccination in the trial population, which does not reflect the current vaccination rate in the United States, and said the different direction of the results between the main intention-to-treat and modified intention-to-treat analyses deserve further investigation.
However, Dr. Lopes concluded, “The results of this trial, in line with the body of evidence in this field, do not support the routine use of any antithrombotic therapy for outpatients with COVID-19.”
The PREVENT-HD trial was sponsored by Janssen. Dr. Piazza has reported receiving research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, and Boston Scientific, and consulting fees from Bristol-Myers Squibb/Pfizer Alliance, Boston Scientific, Janssen, NAMSA, Prairie Education and Research Cooperative, Boston Clinical Research Institute, and Amgen.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Novel co-admin of CAR T cells achieves 99% remission in leukemia
In this trial, the largest study to date of a CAR T-cell therapy for such patients, the researchers co-administered two CAR T-cell therapies, one targeting CD19 and the other targeting CD22.
The results showed that 192 of 194 patients (99%) achieved a complete remission.
The combined overall 12-month event-free survival was 73.5%.
The study was published online in the Journal of Clinical Oncology.
These results are better than what has been reported for CAR T cells that are already on the market. These products, which target CD19, have achieved complete remission in 85.5% of cases and a 12-month event-free survival of 52.4% in children with B-ALL.
“We do believe [this approach] will become standard of care,” said study author Ching-Hon Pui, MD, of the departments of oncology, pathology, and global pediatric medicine, St. Jude Children’s Research Hospital, Memphis.
He noted that this work builds on the huge success that has already been achieved in this field with CAR T-cell products directed at CD19. The first of these products to reach the market was tisagenlecleucel-T (Novartis).
“To put this study in context, the first child who received CAR T-cell therapy for B-ALL after multiple relapses has recently celebrated her 10-year cancer-free survival milestone, and we hope that our finding will result in many more such milestones,” he said.
These new results are very impressive, said Stephen P. Hunger, MD, an expert commenting for the American Society of Clinical Oncology, which highlighted the research in a press release. “They were also able to treat almost 200 patients in a relatively short time.”
Hunger pointed out that dual administration and targeting is not a new idea and is one of the strategies that is currently under investigation. But it is too early to consider this to be the standard of care, he said. “We want to see it replicated in other centers and to see longer follow-up,” said Dr. Hunger, who is Distinguished Chair in Pediatrics and director of the center for childhood cancer research at Children’s Hospital of Philadelphia. “We can establish this as a first step down the road, and we will see if others will achieve similar results.”
Strategy of dual targeting
Despite the success CAR T-cell therapy in childhood leukemia, the currently available products have limitations, Dr. Pui and colleagues note.
About half of patients treated with CD19 CAR T cells experience relapse within 1 year, owing either to loss of CAR T-cell persistence or to loss of CD19 antigen because of splice variants, acquired genetic mutations, or lineage switch.
With further treatment with CAR T cells directed against CD22, 70%-80% of patients who failed CD19 CAR T will achieve into complete remission. However, most will experience relapse.
Recent efforts in the field have turned to exploring the safety and feasibility of CAR T cells that target both CD19 and CD22. The results were not superior to those of the CD19 CAR T-cell therapy given alone, although sequential treatment has yielded promising response rates, the authors note.
They hypothesized that co-administration of CD19- and CD22-targeted CAR T cells would improve efficacy, as it could forestall the development of drug resistance.
Achieved 99% remission
Dr. Pui and colleagues conducted a phase 2 trial that included 225 evaluable patients aged 20 years or younger who were being treated at five urban hospitals in and near Shanghai, China. Of this group, 194 had refractory disease or hematologic relapse, and 31 patients had isolated extramedullary relapse.
A safety run-in stage to determine the recommended dose was initially conducted. An interim analysis of the first 30 patients who were treated (27 at the recommended dose) showed that the approach was safe and effective. Additional patients were then enrolled.
The 192 patients (of 194) who achieved complete remission attained negative minimal residual disease status.
At a median follow-up of 11 months, 43 patients experienced relapse (24 with CD191/CD221 relapse, 16 with CD19– /CD221, one with CD19– /CD22– , and two unknown), for a cumulative risk of 22.2%.
Transplant and relapse options
In an interview, Dr. Pui noted that various treatment options were available for the children who experienced relapse. “For patients who were in good clinical condition, we will treat them with molecular therapeutics, allogeneic CAR T cells from donor, or even repeated humanized CD19 and/or CD22 CAR T cells with or without CD20 CAR T cells in an attempt to induce a remission for allogeneic transplantation,” he said.
The site-specific 12-month event-free survival rate in the trial was 69.2% for patients who did not receive a transplant, 95% for those children who had an isolated relapse to the testicles, and 68.6% for those who had an isolated central nervous system relapse.
After censoring 78 patients for consolidative transplantation, the 12-month overall survival was 87.7%.
Consolidative transplantation was performed in 24 of the 37 patients with KMT2A-rearranged or ZNF384-rearranged ALL and in 54 patients because of parental request. The reason for this was that patients with these two genetic subtypes of leukemia (KMT2A-rearranged and ZNF384-rearranged), under the pressure of phenotype-specific treatment (such as CAR T cells or blinatumomab) are at risk of lineage switch and development of secondary acute myeloid leukemia, explained Dr. Pui. “That is an even more resistant form of leukemia, and up to 5%-10% of the patients have been reported to develop this complication.
“We performed consolidation transplantation in these patients to avoid the risk of lineage switch but would accept the parental request not to perform allogeneic transplant after they were clearly informed of the risk,” he told this news organization.
He also suggested that this approach of co-administration of two types of CAR T cells would be especially suitable for “patients with extramedullary involvement, because most of them will be spared of local irradiation so that they can preserve their neurocognitive function and fertility and avoid radiation-induced second cancer, such as brain tumor,” he said.
Lower toxicity
With regard to toxicity, the majority of patients (n = 98, 88%) developed cytokine release syndrome, which was grade ≥3 in 64 (28.4%) patients and fatal in one. Neurotoxicity occurred in 47 (20.9%) patients, was of grade ≥3 in 9 (4.0%) patients, and was fatal in 2 patients who received 12 x 106 and 5.6 x 106 CAR T cells/kg.
In addition, grade 3 or 4 seizure developed in 14.2% of the patients; it was more common in those who had presented with isolated or combined CNS leukemia. Grade 3 or 4 hypotension occurred in 40.9% of the patients. About three-quarters of the patients were treated with tocilizumab (n = 67, 74.2%), and 79 (35.1%) were treated with corticosteroids.
“In general, CD19 and CD22 CAR T cells were less toxic than CD19 CAR T cells, the historical controls, in our experience,” said Dr. Pui. “There were three fatal complications, a rate not excessive considering a large number of patients were treated.”
Future studies needed
The researchers note that in this trial, the CD22 CAR T cells did not expand as robustly or persist as long as did the CD19 CAR T cells, and they hope that future studies will elucidate whether enhancing CD22 CAR T-cell persistence and activity would further improve outcomes.
The study was supported in part by the National Natural Science Foundation of China, the Shanghai Collaborative Innovation Center for Translational Medicine, the Research Programs of Shanghai Science, the Technology Commission Foundation, the U.S. National Cancer Institute, the VIVA China Children’s Cancer Foundation, and the American Lebanese Syrian Associated Charities.
A version of this article first appeared on Medscape.com.
In this trial, the largest study to date of a CAR T-cell therapy for such patients, the researchers co-administered two CAR T-cell therapies, one targeting CD19 and the other targeting CD22.
The results showed that 192 of 194 patients (99%) achieved a complete remission.
The combined overall 12-month event-free survival was 73.5%.
The study was published online in the Journal of Clinical Oncology.
These results are better than what has been reported for CAR T cells that are already on the market. These products, which target CD19, have achieved complete remission in 85.5% of cases and a 12-month event-free survival of 52.4% in children with B-ALL.
“We do believe [this approach] will become standard of care,” said study author Ching-Hon Pui, MD, of the departments of oncology, pathology, and global pediatric medicine, St. Jude Children’s Research Hospital, Memphis.
He noted that this work builds on the huge success that has already been achieved in this field with CAR T-cell products directed at CD19. The first of these products to reach the market was tisagenlecleucel-T (Novartis).
“To put this study in context, the first child who received CAR T-cell therapy for B-ALL after multiple relapses has recently celebrated her 10-year cancer-free survival milestone, and we hope that our finding will result in many more such milestones,” he said.
These new results are very impressive, said Stephen P. Hunger, MD, an expert commenting for the American Society of Clinical Oncology, which highlighted the research in a press release. “They were also able to treat almost 200 patients in a relatively short time.”
Hunger pointed out that dual administration and targeting is not a new idea and is one of the strategies that is currently under investigation. But it is too early to consider this to be the standard of care, he said. “We want to see it replicated in other centers and to see longer follow-up,” said Dr. Hunger, who is Distinguished Chair in Pediatrics and director of the center for childhood cancer research at Children’s Hospital of Philadelphia. “We can establish this as a first step down the road, and we will see if others will achieve similar results.”
Strategy of dual targeting
Despite the success CAR T-cell therapy in childhood leukemia, the currently available products have limitations, Dr. Pui and colleagues note.
About half of patients treated with CD19 CAR T cells experience relapse within 1 year, owing either to loss of CAR T-cell persistence or to loss of CD19 antigen because of splice variants, acquired genetic mutations, or lineage switch.
With further treatment with CAR T cells directed against CD22, 70%-80% of patients who failed CD19 CAR T will achieve into complete remission. However, most will experience relapse.
Recent efforts in the field have turned to exploring the safety and feasibility of CAR T cells that target both CD19 and CD22. The results were not superior to those of the CD19 CAR T-cell therapy given alone, although sequential treatment has yielded promising response rates, the authors note.
They hypothesized that co-administration of CD19- and CD22-targeted CAR T cells would improve efficacy, as it could forestall the development of drug resistance.
Achieved 99% remission
Dr. Pui and colleagues conducted a phase 2 trial that included 225 evaluable patients aged 20 years or younger who were being treated at five urban hospitals in and near Shanghai, China. Of this group, 194 had refractory disease or hematologic relapse, and 31 patients had isolated extramedullary relapse.
A safety run-in stage to determine the recommended dose was initially conducted. An interim analysis of the first 30 patients who were treated (27 at the recommended dose) showed that the approach was safe and effective. Additional patients were then enrolled.
The 192 patients (of 194) who achieved complete remission attained negative minimal residual disease status.
At a median follow-up of 11 months, 43 patients experienced relapse (24 with CD191/CD221 relapse, 16 with CD19– /CD221, one with CD19– /CD22– , and two unknown), for a cumulative risk of 22.2%.
Transplant and relapse options
In an interview, Dr. Pui noted that various treatment options were available for the children who experienced relapse. “For patients who were in good clinical condition, we will treat them with molecular therapeutics, allogeneic CAR T cells from donor, or even repeated humanized CD19 and/or CD22 CAR T cells with or without CD20 CAR T cells in an attempt to induce a remission for allogeneic transplantation,” he said.
The site-specific 12-month event-free survival rate in the trial was 69.2% for patients who did not receive a transplant, 95% for those children who had an isolated relapse to the testicles, and 68.6% for those who had an isolated central nervous system relapse.
After censoring 78 patients for consolidative transplantation, the 12-month overall survival was 87.7%.
Consolidative transplantation was performed in 24 of the 37 patients with KMT2A-rearranged or ZNF384-rearranged ALL and in 54 patients because of parental request. The reason for this was that patients with these two genetic subtypes of leukemia (KMT2A-rearranged and ZNF384-rearranged), under the pressure of phenotype-specific treatment (such as CAR T cells or blinatumomab) are at risk of lineage switch and development of secondary acute myeloid leukemia, explained Dr. Pui. “That is an even more resistant form of leukemia, and up to 5%-10% of the patients have been reported to develop this complication.
“We performed consolidation transplantation in these patients to avoid the risk of lineage switch but would accept the parental request not to perform allogeneic transplant after they were clearly informed of the risk,” he told this news organization.
He also suggested that this approach of co-administration of two types of CAR T cells would be especially suitable for “patients with extramedullary involvement, because most of them will be spared of local irradiation so that they can preserve their neurocognitive function and fertility and avoid radiation-induced second cancer, such as brain tumor,” he said.
Lower toxicity
With regard to toxicity, the majority of patients (n = 98, 88%) developed cytokine release syndrome, which was grade ≥3 in 64 (28.4%) patients and fatal in one. Neurotoxicity occurred in 47 (20.9%) patients, was of grade ≥3 in 9 (4.0%) patients, and was fatal in 2 patients who received 12 x 106 and 5.6 x 106 CAR T cells/kg.
In addition, grade 3 or 4 seizure developed in 14.2% of the patients; it was more common in those who had presented with isolated or combined CNS leukemia. Grade 3 or 4 hypotension occurred in 40.9% of the patients. About three-quarters of the patients were treated with tocilizumab (n = 67, 74.2%), and 79 (35.1%) were treated with corticosteroids.
“In general, CD19 and CD22 CAR T cells were less toxic than CD19 CAR T cells, the historical controls, in our experience,” said Dr. Pui. “There were three fatal complications, a rate not excessive considering a large number of patients were treated.”
Future studies needed
The researchers note that in this trial, the CD22 CAR T cells did not expand as robustly or persist as long as did the CD19 CAR T cells, and they hope that future studies will elucidate whether enhancing CD22 CAR T-cell persistence and activity would further improve outcomes.
The study was supported in part by the National Natural Science Foundation of China, the Shanghai Collaborative Innovation Center for Translational Medicine, the Research Programs of Shanghai Science, the Technology Commission Foundation, the U.S. National Cancer Institute, the VIVA China Children’s Cancer Foundation, and the American Lebanese Syrian Associated Charities.
A version of this article first appeared on Medscape.com.
In this trial, the largest study to date of a CAR T-cell therapy for such patients, the researchers co-administered two CAR T-cell therapies, one targeting CD19 and the other targeting CD22.
The results showed that 192 of 194 patients (99%) achieved a complete remission.
The combined overall 12-month event-free survival was 73.5%.
The study was published online in the Journal of Clinical Oncology.
These results are better than what has been reported for CAR T cells that are already on the market. These products, which target CD19, have achieved complete remission in 85.5% of cases and a 12-month event-free survival of 52.4% in children with B-ALL.
“We do believe [this approach] will become standard of care,” said study author Ching-Hon Pui, MD, of the departments of oncology, pathology, and global pediatric medicine, St. Jude Children’s Research Hospital, Memphis.
He noted that this work builds on the huge success that has already been achieved in this field with CAR T-cell products directed at CD19. The first of these products to reach the market was tisagenlecleucel-T (Novartis).
“To put this study in context, the first child who received CAR T-cell therapy for B-ALL after multiple relapses has recently celebrated her 10-year cancer-free survival milestone, and we hope that our finding will result in many more such milestones,” he said.
These new results are very impressive, said Stephen P. Hunger, MD, an expert commenting for the American Society of Clinical Oncology, which highlighted the research in a press release. “They were also able to treat almost 200 patients in a relatively short time.”
Hunger pointed out that dual administration and targeting is not a new idea and is one of the strategies that is currently under investigation. But it is too early to consider this to be the standard of care, he said. “We want to see it replicated in other centers and to see longer follow-up,” said Dr. Hunger, who is Distinguished Chair in Pediatrics and director of the center for childhood cancer research at Children’s Hospital of Philadelphia. “We can establish this as a first step down the road, and we will see if others will achieve similar results.”
Strategy of dual targeting
Despite the success CAR T-cell therapy in childhood leukemia, the currently available products have limitations, Dr. Pui and colleagues note.
About half of patients treated with CD19 CAR T cells experience relapse within 1 year, owing either to loss of CAR T-cell persistence or to loss of CD19 antigen because of splice variants, acquired genetic mutations, or lineage switch.
With further treatment with CAR T cells directed against CD22, 70%-80% of patients who failed CD19 CAR T will achieve into complete remission. However, most will experience relapse.
Recent efforts in the field have turned to exploring the safety and feasibility of CAR T cells that target both CD19 and CD22. The results were not superior to those of the CD19 CAR T-cell therapy given alone, although sequential treatment has yielded promising response rates, the authors note.
They hypothesized that co-administration of CD19- and CD22-targeted CAR T cells would improve efficacy, as it could forestall the development of drug resistance.
Achieved 99% remission
Dr. Pui and colleagues conducted a phase 2 trial that included 225 evaluable patients aged 20 years or younger who were being treated at five urban hospitals in and near Shanghai, China. Of this group, 194 had refractory disease or hematologic relapse, and 31 patients had isolated extramedullary relapse.
A safety run-in stage to determine the recommended dose was initially conducted. An interim analysis of the first 30 patients who were treated (27 at the recommended dose) showed that the approach was safe and effective. Additional patients were then enrolled.
The 192 patients (of 194) who achieved complete remission attained negative minimal residual disease status.
At a median follow-up of 11 months, 43 patients experienced relapse (24 with CD191/CD221 relapse, 16 with CD19– /CD221, one with CD19– /CD22– , and two unknown), for a cumulative risk of 22.2%.
Transplant and relapse options
In an interview, Dr. Pui noted that various treatment options were available for the children who experienced relapse. “For patients who were in good clinical condition, we will treat them with molecular therapeutics, allogeneic CAR T cells from donor, or even repeated humanized CD19 and/or CD22 CAR T cells with or without CD20 CAR T cells in an attempt to induce a remission for allogeneic transplantation,” he said.
The site-specific 12-month event-free survival rate in the trial was 69.2% for patients who did not receive a transplant, 95% for those children who had an isolated relapse to the testicles, and 68.6% for those who had an isolated central nervous system relapse.
After censoring 78 patients for consolidative transplantation, the 12-month overall survival was 87.7%.
Consolidative transplantation was performed in 24 of the 37 patients with KMT2A-rearranged or ZNF384-rearranged ALL and in 54 patients because of parental request. The reason for this was that patients with these two genetic subtypes of leukemia (KMT2A-rearranged and ZNF384-rearranged), under the pressure of phenotype-specific treatment (such as CAR T cells or blinatumomab) are at risk of lineage switch and development of secondary acute myeloid leukemia, explained Dr. Pui. “That is an even more resistant form of leukemia, and up to 5%-10% of the patients have been reported to develop this complication.
“We performed consolidation transplantation in these patients to avoid the risk of lineage switch but would accept the parental request not to perform allogeneic transplant after they were clearly informed of the risk,” he told this news organization.
He also suggested that this approach of co-administration of two types of CAR T cells would be especially suitable for “patients with extramedullary involvement, because most of them will be spared of local irradiation so that they can preserve their neurocognitive function and fertility and avoid radiation-induced second cancer, such as brain tumor,” he said.
Lower toxicity
With regard to toxicity, the majority of patients (n = 98, 88%) developed cytokine release syndrome, which was grade ≥3 in 64 (28.4%) patients and fatal in one. Neurotoxicity occurred in 47 (20.9%) patients, was of grade ≥3 in 9 (4.0%) patients, and was fatal in 2 patients who received 12 x 106 and 5.6 x 106 CAR T cells/kg.
In addition, grade 3 or 4 seizure developed in 14.2% of the patients; it was more common in those who had presented with isolated or combined CNS leukemia. Grade 3 or 4 hypotension occurred in 40.9% of the patients. About three-quarters of the patients were treated with tocilizumab (n = 67, 74.2%), and 79 (35.1%) were treated with corticosteroids.
“In general, CD19 and CD22 CAR T cells were less toxic than CD19 CAR T cells, the historical controls, in our experience,” said Dr. Pui. “There were three fatal complications, a rate not excessive considering a large number of patients were treated.”
Future studies needed
The researchers note that in this trial, the CD22 CAR T cells did not expand as robustly or persist as long as did the CD19 CAR T cells, and they hope that future studies will elucidate whether enhancing CD22 CAR T-cell persistence and activity would further improve outcomes.
The study was supported in part by the National Natural Science Foundation of China, the Shanghai Collaborative Innovation Center for Translational Medicine, the Research Programs of Shanghai Science, the Technology Commission Foundation, the U.S. National Cancer Institute, the VIVA China Children’s Cancer Foundation, and the American Lebanese Syrian Associated Charities.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ONCOLOGY
Have you heard the one about the emergency dept. that called 911?
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.