User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Poor bone health is a ‘robust’ dementia risk factor
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Longer telomeres tied to better brain health
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Consider life expectancy in surveillance colonoscopy advice
Among nearly 10,000 Medicare beneficiaries, the likelihood of finding advanced polyps or colorectal cancer (CRC) on surveillance colonoscopy was low. Yet, among patients for whom any follow-up recommendation – either for or against colonoscopy – was available, the vast majority (87%) were advised to return for the procedure in the future, even if their life expectancy was limited or there were no significant findings on their surveillance colonoscopy.
“These findings suggest that recommending against future surveillance colonoscopy in older adults with low-risk colonoscopy findings and/or limited life expectancy should be considered more frequently than is currently practiced,” say Audrey Calderwood, MD, with Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and colleagues.
Because of the lack of clear guidance about when to stop recommending colonoscopies to older patients, it is not surprising that physicians recommend surveillance even for patients with low life expectancy, Ziad Gellad, MD, with Duke University Medical Center, Durham, N.C., said in an interview.
“As someone who performs these procedures, I can tell you that it is not easy to tell patients that they are too old to get preventive care, especially patients in whom your only interaction is the procedure itself,” said Dr. Gellad, who wasn’t involved in the study.
The study was published online in JAMA Internal Medicine.
Key findings
For older adults, surveillance after prior findings of colon polyps is the most frequent indication for colonoscopy. Data suggest that an estimated 5.6 million adults older than 75 will undergo follow-up colonoscopy annually by 2024.
For older adults with polyps, current guidelines recommend individualized decision-making about surveillance colonoscopy. That includes weighing the potential benefits (identifying and removing meaningful lesions to prevent CRC) against the burdens and potential harms (such as bleeding or perforation).
While most colon polyps are not harmful, a subset of polyps, if allowed to grow, can develop into cancer over 10-15 years. This long biological time line highlights the importance of considering life expectancy in deciding for whom surveillance colonoscopy should be recommended, Dr. Calderwood and colleagues note.
Using data from the New Hampshire Colonoscopy Registry, which is linked with the Medicare claims database, they evaluated surveillance colonoscopy findings and follow-up advice according to severity of findings and patients’ estimated life expectancy for 9,831 adults (mean age, 73; 54% men).
Life expectancy was 10+ years for 57.5% of patients, 5 to less than 10 years for 35%, and less than 5 years for 7.5%.
Overall, 791 patients (8%) were found to have advanced polyps (7.8%) or CRC (0.2%) on surveillance colonoscopy.
Recommendations to stop or continue future colonoscopy were available for 5,281 patients (53.7%). Among them, 4,588 (86.9%) were recommended to return for future colonoscopy, even when there were no significant colonoscopy findings or the patient’s life expectancy was limited.
Compared with life expectancy of less than 5 years, longer life expectancy was associated with advice to return for future colonoscopy regardless of clinical findings, with adjusted odds ratios of 21.5 and 2.7, respectively, for life expectancy of 10 or more years and of 5 to less than 10 years.
Among patients with no significant findings, 95% of those with life expectancy of 10 or more years were recommended to undergo repeat colonoscopy down the road, compared with 58% of those with estimated life expectancy of less than 5 years.
Among patients expected to live 5 to less than 10 years, future repeat colonoscopy was recommended for 75% who had no significant findings, 82% with one or two small polyps, and 88% with multiple polyps, advanced polyps, or CRC.
The recommended time to repeat colonoscopy was greater than life expectancy for 6.6% of patients with less than 5 years of life expectancy and 6% with 5 to less than 10 years of life expectancy.
Nuanced decisions
The findings “may help refine decision-making” about the potential benefits and harms of pursuing or stopping surveillance colonoscopy for older adults who have a history of polyps, Dr. Calderwood and colleagues say.
The risk for a colonoscopy complication has been estimated at 26 per 1,000 people, they note. That’s nearly 10 times greater than the potential benefits seen in their study (that is, identification of CRC in 23 of 9,831 people, or about 2.3 per 1,000).
In the study cohort, 10% of patients had comorbid conditions that have been associated with a higher risk for colonoscopy complications. Those with life expectancy of less than 5 years had higher rates of inadequate bowel preparation, which also is associated with increased risk for colonoscopy complications, including perforation.
Dr. Calderwood and colleagues suggest that clinicians use evidence regarding life expectancy and neoplasia progression to modify their recommendations for surveillance colonoscopy for older adults in the following ways:
- If life expectancy is less than 5 years, recommend against surveillance.
- If life expectancy is 5 to less than 10 years and the patient has only low-risk polyps, recommend against surveillance.
- If the patient is healthy with a life expectancy of 10+ years and has recently been found to have advanced polyps, recommend future surveillance colonoscopy, with a caveat that the ultimate decision is dependent on health and priorities at the time the colonoscopy is due to be performed.
- If future health is unknown or unclear, avoid giving definitive recommendations for future surveillance to allow the flexibility of deciding on the basis of risk and benefit when the time comes.
In comments to this news organization, Dr. Gellad noted that an assessment of patient life expectancy “is not readily accessible at the point of care. These are nuanced decisions that require shared decision-making. Sometimes that is best handled outside the procedure setting.”
Support for the study was provided by the National Cancer Institute. The authors have disclosed no relevant financial relationships. Dr. Gellad is a consultant for Merck and Novo Nordisk and is a cofounder of Higgs Boson.
A version of this article originally appeared on Medscape.com.
Among nearly 10,000 Medicare beneficiaries, the likelihood of finding advanced polyps or colorectal cancer (CRC) on surveillance colonoscopy was low. Yet, among patients for whom any follow-up recommendation – either for or against colonoscopy – was available, the vast majority (87%) were advised to return for the procedure in the future, even if their life expectancy was limited or there were no significant findings on their surveillance colonoscopy.
“These findings suggest that recommending against future surveillance colonoscopy in older adults with low-risk colonoscopy findings and/or limited life expectancy should be considered more frequently than is currently practiced,” say Audrey Calderwood, MD, with Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and colleagues.
Because of the lack of clear guidance about when to stop recommending colonoscopies to older patients, it is not surprising that physicians recommend surveillance even for patients with low life expectancy, Ziad Gellad, MD, with Duke University Medical Center, Durham, N.C., said in an interview.
“As someone who performs these procedures, I can tell you that it is not easy to tell patients that they are too old to get preventive care, especially patients in whom your only interaction is the procedure itself,” said Dr. Gellad, who wasn’t involved in the study.
The study was published online in JAMA Internal Medicine.
Key findings
For older adults, surveillance after prior findings of colon polyps is the most frequent indication for colonoscopy. Data suggest that an estimated 5.6 million adults older than 75 will undergo follow-up colonoscopy annually by 2024.
For older adults with polyps, current guidelines recommend individualized decision-making about surveillance colonoscopy. That includes weighing the potential benefits (identifying and removing meaningful lesions to prevent CRC) against the burdens and potential harms (such as bleeding or perforation).
While most colon polyps are not harmful, a subset of polyps, if allowed to grow, can develop into cancer over 10-15 years. This long biological time line highlights the importance of considering life expectancy in deciding for whom surveillance colonoscopy should be recommended, Dr. Calderwood and colleagues note.
Using data from the New Hampshire Colonoscopy Registry, which is linked with the Medicare claims database, they evaluated surveillance colonoscopy findings and follow-up advice according to severity of findings and patients’ estimated life expectancy for 9,831 adults (mean age, 73; 54% men).
Life expectancy was 10+ years for 57.5% of patients, 5 to less than 10 years for 35%, and less than 5 years for 7.5%.
Overall, 791 patients (8%) were found to have advanced polyps (7.8%) or CRC (0.2%) on surveillance colonoscopy.
Recommendations to stop or continue future colonoscopy were available for 5,281 patients (53.7%). Among them, 4,588 (86.9%) were recommended to return for future colonoscopy, even when there were no significant colonoscopy findings or the patient’s life expectancy was limited.
Compared with life expectancy of less than 5 years, longer life expectancy was associated with advice to return for future colonoscopy regardless of clinical findings, with adjusted odds ratios of 21.5 and 2.7, respectively, for life expectancy of 10 or more years and of 5 to less than 10 years.
Among patients with no significant findings, 95% of those with life expectancy of 10 or more years were recommended to undergo repeat colonoscopy down the road, compared with 58% of those with estimated life expectancy of less than 5 years.
Among patients expected to live 5 to less than 10 years, future repeat colonoscopy was recommended for 75% who had no significant findings, 82% with one or two small polyps, and 88% with multiple polyps, advanced polyps, or CRC.
The recommended time to repeat colonoscopy was greater than life expectancy for 6.6% of patients with less than 5 years of life expectancy and 6% with 5 to less than 10 years of life expectancy.
Nuanced decisions
The findings “may help refine decision-making” about the potential benefits and harms of pursuing or stopping surveillance colonoscopy for older adults who have a history of polyps, Dr. Calderwood and colleagues say.
The risk for a colonoscopy complication has been estimated at 26 per 1,000 people, they note. That’s nearly 10 times greater than the potential benefits seen in their study (that is, identification of CRC in 23 of 9,831 people, or about 2.3 per 1,000).
In the study cohort, 10% of patients had comorbid conditions that have been associated with a higher risk for colonoscopy complications. Those with life expectancy of less than 5 years had higher rates of inadequate bowel preparation, which also is associated with increased risk for colonoscopy complications, including perforation.
Dr. Calderwood and colleagues suggest that clinicians use evidence regarding life expectancy and neoplasia progression to modify their recommendations for surveillance colonoscopy for older adults in the following ways:
- If life expectancy is less than 5 years, recommend against surveillance.
- If life expectancy is 5 to less than 10 years and the patient has only low-risk polyps, recommend against surveillance.
- If the patient is healthy with a life expectancy of 10+ years and has recently been found to have advanced polyps, recommend future surveillance colonoscopy, with a caveat that the ultimate decision is dependent on health and priorities at the time the colonoscopy is due to be performed.
- If future health is unknown or unclear, avoid giving definitive recommendations for future surveillance to allow the flexibility of deciding on the basis of risk and benefit when the time comes.
In comments to this news organization, Dr. Gellad noted that an assessment of patient life expectancy “is not readily accessible at the point of care. These are nuanced decisions that require shared decision-making. Sometimes that is best handled outside the procedure setting.”
Support for the study was provided by the National Cancer Institute. The authors have disclosed no relevant financial relationships. Dr. Gellad is a consultant for Merck and Novo Nordisk and is a cofounder of Higgs Boson.
A version of this article originally appeared on Medscape.com.
Among nearly 10,000 Medicare beneficiaries, the likelihood of finding advanced polyps or colorectal cancer (CRC) on surveillance colonoscopy was low. Yet, among patients for whom any follow-up recommendation – either for or against colonoscopy – was available, the vast majority (87%) were advised to return for the procedure in the future, even if their life expectancy was limited or there were no significant findings on their surveillance colonoscopy.
“These findings suggest that recommending against future surveillance colonoscopy in older adults with low-risk colonoscopy findings and/or limited life expectancy should be considered more frequently than is currently practiced,” say Audrey Calderwood, MD, with Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and colleagues.
Because of the lack of clear guidance about when to stop recommending colonoscopies to older patients, it is not surprising that physicians recommend surveillance even for patients with low life expectancy, Ziad Gellad, MD, with Duke University Medical Center, Durham, N.C., said in an interview.
“As someone who performs these procedures, I can tell you that it is not easy to tell patients that they are too old to get preventive care, especially patients in whom your only interaction is the procedure itself,” said Dr. Gellad, who wasn’t involved in the study.
The study was published online in JAMA Internal Medicine.
Key findings
For older adults, surveillance after prior findings of colon polyps is the most frequent indication for colonoscopy. Data suggest that an estimated 5.6 million adults older than 75 will undergo follow-up colonoscopy annually by 2024.
For older adults with polyps, current guidelines recommend individualized decision-making about surveillance colonoscopy. That includes weighing the potential benefits (identifying and removing meaningful lesions to prevent CRC) against the burdens and potential harms (such as bleeding or perforation).
While most colon polyps are not harmful, a subset of polyps, if allowed to grow, can develop into cancer over 10-15 years. This long biological time line highlights the importance of considering life expectancy in deciding for whom surveillance colonoscopy should be recommended, Dr. Calderwood and colleagues note.
Using data from the New Hampshire Colonoscopy Registry, which is linked with the Medicare claims database, they evaluated surveillance colonoscopy findings and follow-up advice according to severity of findings and patients’ estimated life expectancy for 9,831 adults (mean age, 73; 54% men).
Life expectancy was 10+ years for 57.5% of patients, 5 to less than 10 years for 35%, and less than 5 years for 7.5%.
Overall, 791 patients (8%) were found to have advanced polyps (7.8%) or CRC (0.2%) on surveillance colonoscopy.
Recommendations to stop or continue future colonoscopy were available for 5,281 patients (53.7%). Among them, 4,588 (86.9%) were recommended to return for future colonoscopy, even when there were no significant colonoscopy findings or the patient’s life expectancy was limited.
Compared with life expectancy of less than 5 years, longer life expectancy was associated with advice to return for future colonoscopy regardless of clinical findings, with adjusted odds ratios of 21.5 and 2.7, respectively, for life expectancy of 10 or more years and of 5 to less than 10 years.
Among patients with no significant findings, 95% of those with life expectancy of 10 or more years were recommended to undergo repeat colonoscopy down the road, compared with 58% of those with estimated life expectancy of less than 5 years.
Among patients expected to live 5 to less than 10 years, future repeat colonoscopy was recommended for 75% who had no significant findings, 82% with one or two small polyps, and 88% with multiple polyps, advanced polyps, or CRC.
The recommended time to repeat colonoscopy was greater than life expectancy for 6.6% of patients with less than 5 years of life expectancy and 6% with 5 to less than 10 years of life expectancy.
Nuanced decisions
The findings “may help refine decision-making” about the potential benefits and harms of pursuing or stopping surveillance colonoscopy for older adults who have a history of polyps, Dr. Calderwood and colleagues say.
The risk for a colonoscopy complication has been estimated at 26 per 1,000 people, they note. That’s nearly 10 times greater than the potential benefits seen in their study (that is, identification of CRC in 23 of 9,831 people, or about 2.3 per 1,000).
In the study cohort, 10% of patients had comorbid conditions that have been associated with a higher risk for colonoscopy complications. Those with life expectancy of less than 5 years had higher rates of inadequate bowel preparation, which also is associated with increased risk for colonoscopy complications, including perforation.
Dr. Calderwood and colleagues suggest that clinicians use evidence regarding life expectancy and neoplasia progression to modify their recommendations for surveillance colonoscopy for older adults in the following ways:
- If life expectancy is less than 5 years, recommend against surveillance.
- If life expectancy is 5 to less than 10 years and the patient has only low-risk polyps, recommend against surveillance.
- If the patient is healthy with a life expectancy of 10+ years and has recently been found to have advanced polyps, recommend future surveillance colonoscopy, with a caveat that the ultimate decision is dependent on health and priorities at the time the colonoscopy is due to be performed.
- If future health is unknown or unclear, avoid giving definitive recommendations for future surveillance to allow the flexibility of deciding on the basis of risk and benefit when the time comes.
In comments to this news organization, Dr. Gellad noted that an assessment of patient life expectancy “is not readily accessible at the point of care. These are nuanced decisions that require shared decision-making. Sometimes that is best handled outside the procedure setting.”
Support for the study was provided by the National Cancer Institute. The authors have disclosed no relevant financial relationships. Dr. Gellad is a consultant for Merck and Novo Nordisk and is a cofounder of Higgs Boson.
A version of this article originally appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
eNose knows S. aureus in children with cystic fibrosis
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
FROM THE JOURNAL OF CYSTIC FIBROSIS
Financial navigators saved about $2,500 per cancer patient
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients in the United States face complex financial issues in navigating with medical insurance companies to cover their care. This “financial toxicity” has come to be regarded as a side effect of cancer treatment.
Patients with hematologic malignancies may be particularly vulnerable to financial toxicity, owing to the nature of their treatment, which often includes bone marrow transplantation, lengthy hospital stays, and prolonged intensive follow-up, as well as potential treatment-related complications, such as graft vs. host disease.
The results from this small study suggest that using an oncology financial navigator could be helpful. But not all cancer patients have access to such a person, explained lead author Jean S. Edward, PhD, RN, associate professor in the college of nursing at the University of Kentucky, Lexington.
“Unfortunately, it’s not as common as we would like, especially in underserved areas with patient and caregiver populations that need it the most,” she said. Dr. Edward is hopeful that the results from this study, even though it is small, might help to boost use of this intervention. “OFN [oncology financial navigation] is not necessarily a cutting-edge program or ‘novel’ intervention, but the lack of programs and limitations in implementing in cancer centers does make it a gap in practice,” Dr. Edward told this news organization.
“There are gaps in evidence on how to incorporate an oncology financial navigator in current workflows and sustainability of positions, but as our study has shown, the return on investment to the health care system and/or financial benefits to patients/caregivers could help cover the cost of implementing such programs,” she said.
The study was published in JCO Oncology Practice.
The intervention used in this study, Coverage and Cost-of-Care Links (CC Links), was designed specifically to address financial toxicity among patients with hematologic cancers.
The study’s primary outcomes were defined as improvements in financial distress as well as in physical and mental quality of life.
A total of 54 patients and 32 caregivers completed the intervention and pre-/postintervention surveys. More than half of participants were women. The average age was 63 years. Less than a quarter of the patients were employed (23%), about one-third had income that was below the federal poverty level, and almost all had insurance. About 59% of the caregivers were employed.
The navigators’ functions included screening for financial toxicity using FACIT-Comprehensive Score for Financial Toxicity (COST) and the National Comprehensive Cancer Network’s Distress Thermometer and Problem List. They also helped patients to estimate cost of care, assessed health insurance coverage, and connected patients/caregivers with disease-specific resources and other external assistance programs, among other things.
Participants had an average of three in-person meetings and five telephone interactions with the financial navigator. The most common concern was in regard to high out-of-pocket costs. The most frequently provided services from the navigator were helping with financial assistance programs and grant applications. Overall, the navigator was able to obtain $124,600 in financial benefits for 48 participants, as well as money for travel ($24,000), urgent needs ($16,000), patient financial assistance ($9,100), and copay assistance grants ($75,500).
With regard to scores on the screening tools, the only significant change from pre- to postintervention was in the psychological response score, or COST. It decreased by an average of 2.30 points (P = .019; Hedges’ g = 0.33). For caregivers, there was a significant improvement in COST (average decrease, 2.97 points; P = .021; g = 0.43), material condition scores (average decrease, 0.63 points; P = .031; g = 0.39), and total financial toxicity scores (average decrease, 0.13 points; P = .041; g = 0.37).
Most of the participants gave the intervention high ratings for acceptability (89%) and appropriateness (88%).
“Standardized screening for financial toxicity in cancer care settings is essential to support early identification of financial needs that serve as barriers to care,” the authors conclude. “Close collaboration and coordination with existing services and workflows are essential for the seamless integration of OFN interventions within health systems and to help facilitate contact and communication with participants.”
The study was supported by the National Cancer Institute; the University of Kentucky’s Markey Cancer Center; the Research Communications Office of the Patient Oriented and Population Science Shared Resource Facilities; Joan Scales, LCSW, and the Psych-Oncology Program at the University of Kentucky Markey Cancer Center; and UK HealthCare’s Patient Financial Services. Dr. Edward has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JCO ONCOLOGY PRACTICE
Home-based HPV cervical cancer screening ‘cost effective’
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
For women who are overdue for cervical cancer screening, mailing self-sampling kits for high-risk human papillomavirus (HPV) is a cost-effective means of increasing screening uptake, reveals an analysis of a large U.S. trial.
The finding comes from a randomized trial in almost 20,000 women, which compared women who received a mailed HPV testing kit with those who did not. The results show that mailing was most cost-effective in women aged 50-64 years and in those who were only recently overdue for cervical screening.
The study was published by JAMA Network Open.
“These results support mailing HPV kits as an efficient outreach strategy for increasing screening rates in U.S. health care systems,” say the authors, led by Rachel L. Winer, PhD, MPH, department of epidemiology, University of Washington, Seattle.
They note that their results are consistent with those from previous studies in other health care contexts, but their analysis “benefited from the randomized clinical trial design and a large sample size,” they write.
However, they point out that the trial was conducted “before the beginning of the COVID-19 pandemic,” and it is “well established” that cancer screening rates “decreased substantially during the pandemic.”
They suggest that mailed HPV self-sampling kits could nevertheless be a “means of overcoming screening barriers among underscreened women,” which may have been exacerbated by the “societal consequences of the pandemic.”
Reducing barriers to screening
Cervical screening is associated with “substantial global reductions” in the incidence and mortality of cervical cancer, the authors point out. Because most cases of the disease are consequently preventable, it now occurs “predominantly in individuals who have never or rarely received screening.”
Home-based HPV-only testing reduces the need for office visits and reduces barriers to screening, such as discomfort, embarrassment, and difficulties with scheduling or attending appointments.
Previous studies have shown that the direct mailing of home-based HPV self-collection kits is associated with increased uptake of screening among underscreened women and is cost-effective, although the researchers point out that these previous studies were conducted in countries with “organized national screening programs.”
For their own study, they focused on home-based HPV screening among underscreened individuals in the United States. The team examined data from the Home-based Options to Make cervical cancer screening Easy trial, which has previously showed that mailing kits to women increased screening uptake, compared with usual care alone.
For the current analysis, they conducted an economic evaluation of data on 19,851 trial participants, who were randomized to receive home-based screening or usual care between February 2014 and August 2016 and were followed up to February 2018.
All of the women were aged 30-64 years and had been enrolled in a health plan from Kaiser Permanente Washington (KPW) for at least 3 years and 5 months. They were also required not have undergone a hysterectomy.
Participant-level economic data were collected between June 2019 and March 2021, with intervention delivery costs calculated from the perspective of both the KPW and Medicare health systems and based on the cost of either a wellness visit or Papanicolaou (Pap) test–only visit.
The mean age of the participants was 50.1 years, and the majority (76.7%) were White; 9.7% were Asian and 4.7% were Black or African American.
There were no significant differences in baseline characteristics between the group assigned to usual care, which comprised patient reminders and ad hoc screening outreach, and those in the intervention group, who received usual care and a mailed HPV self-sampling kit.
The researchers report that 1,206 women in the intervention group sent back a mailed HPV kit, with 1,178 (97.7%) meeting the criteria for completed screening uptake.
Overall, screening uptake was higher in the intervention group than in control participants, at 26.3% vs. 17.4%, respectively (relative risk, 1.51).
Intervention participants were also more likely than controls to have a positive test result (relative risk, 1.49) and to receive treatment (relative risk, 1.70).
The incremental cost-effectiveness ratio for increased screening uptake, defined as the incremental difference in cost between the study groups divided by the difference in the number of participants completing screening within 6 months, ranged from $85.84 per additional completed screening to $146.29, depending on the health system and test considered.
In terms of willingness-to-pay (WTP) thresholds for each additional completed screening, the team found that home-based screening achieved a 90% probability of cost-effectiveness, at a WTP of just $148 if the participant’s last Pap test was between 3.4 and 5.0 years before randomization.
A 90% probability of cost-effectiveness was also achieved at a WTP of $198 among participants aged 50-64 years, a threshold that was lower than that among other age groups.
At a WTP threshold of over $350, the intervention was considered to have 100% probability of being cost-effective in all age groups.
The study was supported by a grant from the National Cancer Institute of the National Institutes of Health. Dr. Winer reports a relationship with the National Cancer Institute outside of the submitted work, as do several other authors.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Meet the JCOM Author with Dr. Barkoudah: Leading for High Reliability During the COVID-19 Pandemic
Meet the JCOM Author with Dr. Barkoudah: Residence Characteristics and Nursing Home Compare Quality Measures
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
Relationships Between Residence Characteristics and Nursing Home Compare Database Quality Measures
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18
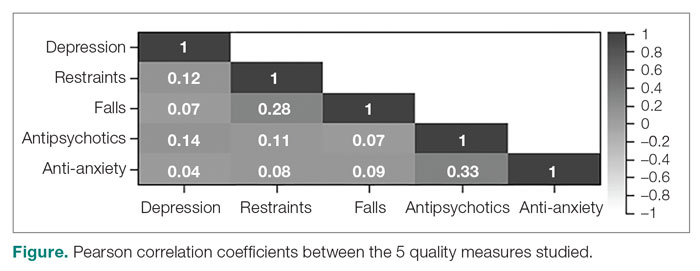
Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).
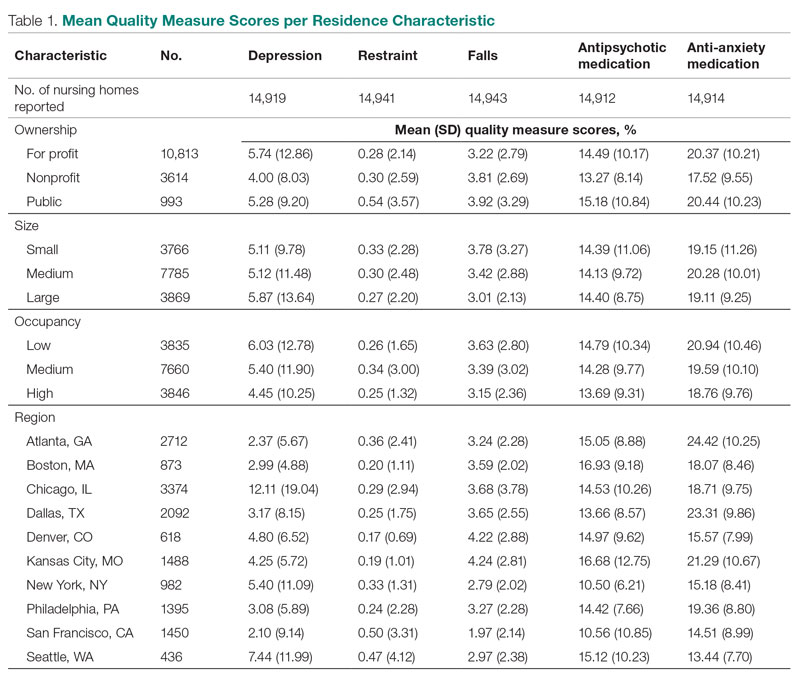
Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.
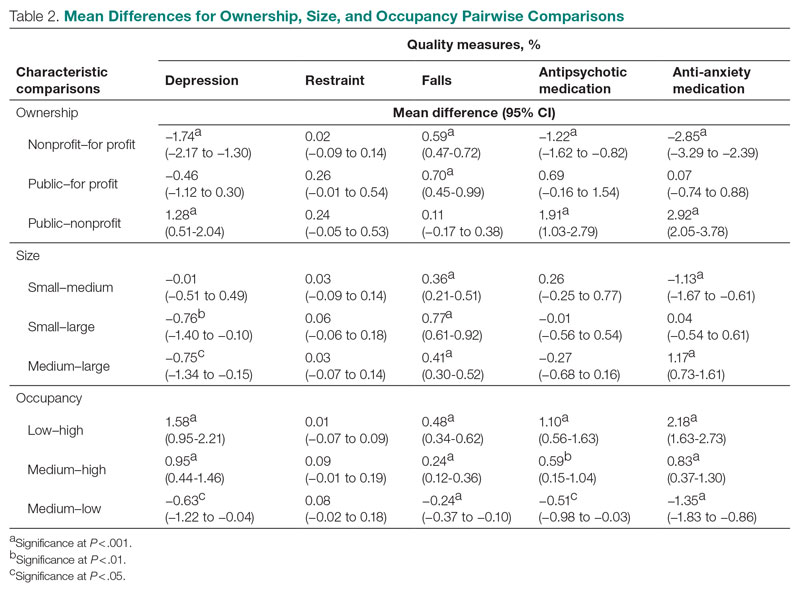
Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.
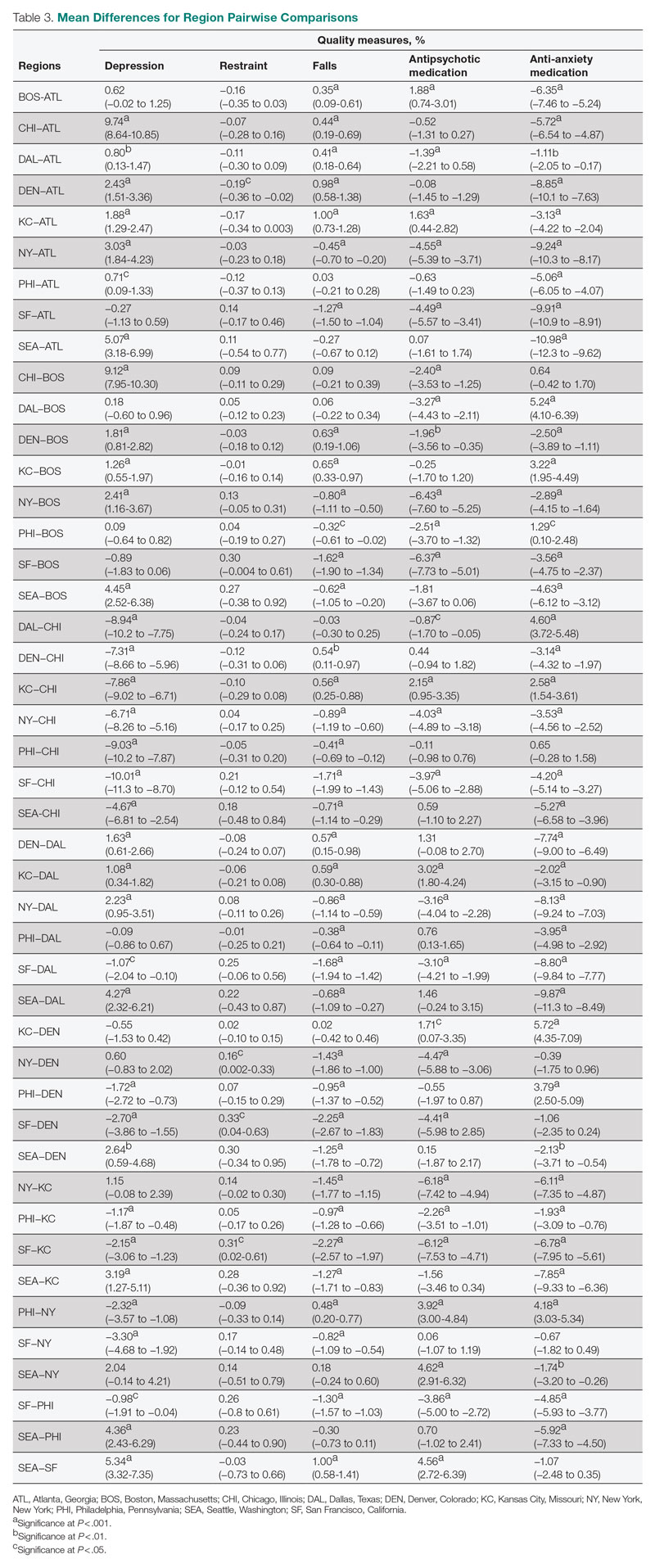
Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, [email protected].
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.