User login
U.S. News & World Report releases best hospitals for maternity care with changes, few high performing
U.S. News & World Report has released its Best Hospitals for Maternity Care rankings for 2022. The rankings are intended to assist expectant mothers in making informed decisions about maternal health care for uncomplicated pregnancies.
The ratings assess eight aspects of care. Three categories are new – rates of episiotomy; transparency for racial and ethnic disparities; and adherence to federal guidelines for birthing friendly practices, which include efforts by staff to reduce maternal morbidity and mortality.
Of the 649 hospitals reviewed, 297 received a mark of “high performing.” Hospitals included in the high-performing category were Thomas Hospital, Fairhope, Ala.; Kaiser Permanente Los Angeles Medical Center; and Northwestern Memorial Hospital, Chicago. Over 300 hospitals received a ranking of “not high performing.”
Min Hee Seo, a senior health data analyst at U.S. News & World Report, said the new additions to the ranking system will help parents make more informed decisions about their maternal care. The information on racial and ethnic disparities could help patients make decisions about the equity of their care, Dr. Seo also said.
“By validating hospitals solely on their objective data and performance, we are providing more information to patients or families who are in need,” she said.
To produce the maternity care rankings – which first appeared in 2021 – the magazine focused on data from 2020 for each hospital it evaluated. The data were derived from government sources and through surveys of hospitals that provide maternity care.
In addition to the three new measures, the five indicators in the rankings are rates of cesarean delivery in lower-risk pregnancies, newborn complications, exclusive breast milk feeding, early elective delivery, and options for vaginal birth after cesarean delivery.
The U.S. News & World Report rankings for education have come under scrutiny recently, and some schools are no longer participating in the popular feature. However, Dr. Seo said the controversy does not affect the hospital rankings. She said expectant mothers and doctors frequently use the data in hospital rankings to improve quality of care and to have conversations about care with patients.
“Providers can use these rankings to make references and transfer patients to where they will receive the best care,” she said.
A version of this article first appeared on Medscape.com.
U.S. News & World Report has released its Best Hospitals for Maternity Care rankings for 2022. The rankings are intended to assist expectant mothers in making informed decisions about maternal health care for uncomplicated pregnancies.
The ratings assess eight aspects of care. Three categories are new – rates of episiotomy; transparency for racial and ethnic disparities; and adherence to federal guidelines for birthing friendly practices, which include efforts by staff to reduce maternal morbidity and mortality.
Of the 649 hospitals reviewed, 297 received a mark of “high performing.” Hospitals included in the high-performing category were Thomas Hospital, Fairhope, Ala.; Kaiser Permanente Los Angeles Medical Center; and Northwestern Memorial Hospital, Chicago. Over 300 hospitals received a ranking of “not high performing.”
Min Hee Seo, a senior health data analyst at U.S. News & World Report, said the new additions to the ranking system will help parents make more informed decisions about their maternal care. The information on racial and ethnic disparities could help patients make decisions about the equity of their care, Dr. Seo also said.
“By validating hospitals solely on their objective data and performance, we are providing more information to patients or families who are in need,” she said.
To produce the maternity care rankings – which first appeared in 2021 – the magazine focused on data from 2020 for each hospital it evaluated. The data were derived from government sources and through surveys of hospitals that provide maternity care.
In addition to the three new measures, the five indicators in the rankings are rates of cesarean delivery in lower-risk pregnancies, newborn complications, exclusive breast milk feeding, early elective delivery, and options for vaginal birth after cesarean delivery.
The U.S. News & World Report rankings for education have come under scrutiny recently, and some schools are no longer participating in the popular feature. However, Dr. Seo said the controversy does not affect the hospital rankings. She said expectant mothers and doctors frequently use the data in hospital rankings to improve quality of care and to have conversations about care with patients.
“Providers can use these rankings to make references and transfer patients to where they will receive the best care,” she said.
A version of this article first appeared on Medscape.com.
U.S. News & World Report has released its Best Hospitals for Maternity Care rankings for 2022. The rankings are intended to assist expectant mothers in making informed decisions about maternal health care for uncomplicated pregnancies.
The ratings assess eight aspects of care. Three categories are new – rates of episiotomy; transparency for racial and ethnic disparities; and adherence to federal guidelines for birthing friendly practices, which include efforts by staff to reduce maternal morbidity and mortality.
Of the 649 hospitals reviewed, 297 received a mark of “high performing.” Hospitals included in the high-performing category were Thomas Hospital, Fairhope, Ala.; Kaiser Permanente Los Angeles Medical Center; and Northwestern Memorial Hospital, Chicago. Over 300 hospitals received a ranking of “not high performing.”
Min Hee Seo, a senior health data analyst at U.S. News & World Report, said the new additions to the ranking system will help parents make more informed decisions about their maternal care. The information on racial and ethnic disparities could help patients make decisions about the equity of their care, Dr. Seo also said.
“By validating hospitals solely on their objective data and performance, we are providing more information to patients or families who are in need,” she said.
To produce the maternity care rankings – which first appeared in 2021 – the magazine focused on data from 2020 for each hospital it evaluated. The data were derived from government sources and through surveys of hospitals that provide maternity care.
In addition to the three new measures, the five indicators in the rankings are rates of cesarean delivery in lower-risk pregnancies, newborn complications, exclusive breast milk feeding, early elective delivery, and options for vaginal birth after cesarean delivery.
The U.S. News & World Report rankings for education have come under scrutiny recently, and some schools are no longer participating in the popular feature. However, Dr. Seo said the controversy does not affect the hospital rankings. She said expectant mothers and doctors frequently use the data in hospital rankings to improve quality of care and to have conversations about care with patients.
“Providers can use these rankings to make references and transfer patients to where they will receive the best care,” she said.
A version of this article first appeared on Medscape.com.
Tackling oral health in primary care: A task that’s worth the time
Tooth decay can be easy to overlook – particularly for pediatricians and family physicians, who may be neglecting a crucial aspect of childhood health.
Left untreated, it can lead to serious and even fatal medical problems. The incorporation of preventive oral health care services like the application of fluoride varnish into primary care may be helping protect kids’ smiles and improving their overall physical well-being, according to doctors and a recent government report.
‘We don’t deal with that in pediatrics’
Physicians historically were not trained to examine teeth. That was the dentist’s job.
But dental caries is one of the most common chronic diseases in children, and many children do not regularly see a dentist.
“I stumbled across the statistic that oral health problems in children are five times as common as asthma,” said Susan A. Fisher-Owens, MD, MPH, professor of pediatrics at the University of California, San Francisco. “And I said to myself, ‘Well, that can’t be. We don’t deal with that in pediatrics.’ And then I realized, ‘Oh my goodness, we don’t deal with that in pediatrics!’ ”
Children should see a dentist, of course. Physicians should refer families to a dentist by age 1 for routine care, Dr. Fisher-Owens said. The sooner kids are seen, the more likely they are to stay healthy and avoid the need for costlier care, she said.
But the receipt of dental care has gaps.
“About half of all American children do not receive regular dental care because of social, economic, and geographic obstacles,” according to a 2021 fact sheet from the National Institute of Dental and Craniofacial Research. “Integrating dental care within family and pediatric medical care settings is improving children’s oral health.”
Many children do not start to see a dentist when they are supposed to, acknowledged Kami Hoss, DDS, MS, founder of a large dental practice in California and the author of a new book, “If Your Mouth Could Talk,” that examines links between oral health and physical disease.
Although the American Academy of Pediatric Dentistry says every child should see a pediatric dentist by the time their first baby teeth come in, usually at around 6 months or no later than age 1 year, that does not always happen.
Indeed, only about 16% of children adhere to that guidance, Dr. Hoss said, “which means 84% of parents rely on their pediatricians for oral health advice.”
At older ages, oral health problems like gum disease are linked to almost every chronic disease, Dr. Hoss said.
“We love to bridge the gap, to build bridges between medicine and dentistry,” he said. “After all, your mouth is part of your body.”
A 2021 NIDCR report similarly describes the stakes: “Although caries is largely preventable, if untreated it can lead to pain, inflammation, and the spread of infection to bone and soft tissue. Children may suffer from difficulty eating, poor nutrition, delayed physical development, and poor self-image and socialization. Even academic performance can be affected.”
In November, the World Health Organization published a report showing that about 45% of the world’s population – 3.5 billion people – have oral diseases, including 2.5 billion people with untreated dental caries.
Oral health care is often neglected in public health research, and often entails high out-of-pocket costs for families, the organization notes.
‘Strep tooth’
Dental cavities are caused by bacteria – mainly Streptococcus mutans – that eat sugars or carbohydrates in the mouth. That process causes acid, which can erode teeth. In that way, the development of caries is a fully preventable infectious disease process, Dr. Fisher-Owens said.
“I think if people looked at this disease as ‘strep tooth,’ it would get a lot more people interested,” she said.
Bacteria that cause caries can spread from caregiver to child, such as when a parent tries to clean a dropped pacifier in their own mouth, or from child to child.
Caries can be prevented with proper diet and oral hygiene: toothbrushing and then applying fluoride to strengthen teeth or restrengthen teeth that have been weakened by the disease process, Dr. Fisher-Owens said.
The biggest risk factor for having cavities in adult teeth is having them in primary teeth, she said. “There is a common misconception that it doesn’t matter what happens with baby teeth. They’ll fall out,” she said. “But actually it does because it puts you on a trajectory of having cavities in the adult teeth and worse outcomes with other adult conditions, such as diabetes.”
At the 2022 annual meeting of the American Academy of Pediatrics in Anaheim in October, Dr. Fisher-Owens and Jean Calvo, DDS, MPH, also with the University of California, San Francisco, trained colleagues to apply fluoride varnish to primary teeth – so-called baby teeth – in the doctor’s office. This session is a regular feature at these conferences.
Since 2014, the U.S. Preventive Services Task Force has recommended that primary care clinicians apply fluoride varnish to the primary teeth of all infants and children.
Many pediatricians may not do this regularly, however.
Researchers recently reported that, despite insurance coverage, less than 5% of well-child visits for privately insured young children between 2016 and 2018 included the service.
Nevertheless, the practice may be helping, according to the NIDCR report.
Since 2000, untreated tooth decay in primary teeth among children younger than 12 years has fallen from 23% to 15%, according to the report. For children aged 2-5 years, untreated tooth decay decreased from at least 19% to 10%. For children aged 6-11 years, the prevalence of dental cavities in permanent teeth fell from 25% to 18%, the report states.
“Fluoridated water, toothpastes, and varnish – as well as dental sealants – can work together to dramatically reduce the incidence of caries,” according to the NIDCR. “Integrating dental care within family and pediatric medical care settings has been another important advancement. The delivery of preventive oral health services, such as fluoride varnish, during well-child visits in medical offices is showing promise in reducing dental caries among preschool-age children.”
Integrating oral health care with medical primary care has met challenges, however, including “resistance by providers, lack of training, and the need for insurance reimbursement for services,” the report notes.
Clinicians can already bill for the application of fluoride varnish using Current Procedural Terminology (CPT) code 99188, and additional oral health care procedures may be on the horizon.
The American Medical Association this fall established a new Category III CPT code for the application of silver diamine fluoride to dental cavities.
Silver diamine fluoride is a newer product that was approved as a desensitizing agent by the Food and Drug Administration in 2014. It has antimicrobial and remineralizing properties, and researchers have found that it can stop the progression of early tooth decay and is more effective than fluoride varnish in preventing cavities.
Several dental groups supported the creation of this new code, which is expected to be made available by electronic health records vendors in July.
Some dentists have reservations, however. The Academy of General Dentistry in October expressed concerns that allowing “nondental health care workers to administer silver diamine fluoride is a temporary solution to a growing oral health crisis.”
Silver diamine fluoride may stop about 80% of cavities. Although the CPT code for silver diamine fluoride has been established, whether insurers will reimburse health care professionals for the service is another matter, said Richard Niederman, DMD, professor and chair of epidemiology and health promotion at New York University College of Dentistry.
Fatal consequences
Disparities in oral health in children may be greater than with almost any other disease process. The rate of caries in children who are poor is about twice that for children who are not poor, Dr. Fisher-Owens said. Disparities by race or ethnicity compound these differences.
In 2007, 12-year-old Deamonte Driver died after bacteria from a dental abscess spread to his brain. He had needed a tooth extraction, but his family lacked insurance and had had trouble finding dentists that accepted Medicaid near where they lived in Maryland.
After two emergency brain surgeries, 2 weeks in a hospital, and another 6 weeks in a hospital for rehabilitation, Deamonte died from the infection. The case sparked calls to fix the dental health system.
Physicians may notice more oral health problems in their patients, including dental abscesses, once they start looking for them, Dr. Fisher-Owens said.
She recalled one instance where a child with an underlying seizure disorder was hospitalized at an academic center because they appeared to be having more seizures.
“They eventually discharged the kid because they looked at all of the things related to seizures. None of them were there,” she said.
When Dr. Fisher-Owens saw the child for a discharge exam, she looked in the mouth and saw a whopping dental abscess.
“I realized that this kid wasn’t seizing but was actually rigoring in pain,” she said. No one else on the medical team had seen the true problem despite multiple examinations. The child started antibiotics, was referred to a dentist to have the abscess drained, and had a good outcome.
Suzanne C. Boulter, MD, adjunct clinical professor of pediatrics and community health at the Geisel School of Medicine at Dartmouth, Hanover, N.H., had noticed that many of her poorer patients had oral health problems, but many pediatric dentists were not able or willing to see them to provide treatment.
“Taking it one step further, you really want to prevent early childhood caries,” Dr. Boulter said. She started speaking up about oral health at pediatric meetings and became an early adopter of preventive interventions, including the use of fluoride varnish.
“Fluoride varnish is a sticky substance that has a very high concentration of fluoride in it,and it’s a very powerful reducer of oral childhood caries, by maybe 35%,” Dr. Boulter said.
Applying the varnish is fairly simple, but it had never been part of the well-child exam. Dr. Boulter started using it around 2005.
Initially, convincing other pediatricians to adopt this procedure – when visits are already time-constrained – was not always easy, she said.
Now that fluoride varnish is recommended for all children and is part of Bright Futures recommendations and is covered in the Affordable Care Act, “it became more the norm,” Dr. Boulter said. But there is room for improvement.
“There is still not a high enough percentage of pediatricians and family physicians who actually have incorporated application of fluoride varnish into their practice,” she said.
Brush, book, bed
Clinicians can take other steps to counsel parents about protecting their child’s teeth, like making sure that their teeth get brushed before bed, encouraging kids to drink tap water, especially if it’s fluoridated, and avoiding juice. The AAP has a program called Brush, Book, Bed to promote oral health, along with reading and good sleep habits.
Dr. Hoss noted that parents, and even dentists, may have misconceptions about optimal oral hygiene. “For example, you’re supposed to brush your teeth before breakfast, not after breakfast. But I’ve seen dentists even tell their patients, brush after breakfast,” he said.
In addition, people should brush gently but thoroughly using high-quality toothbrushes with soft bristles – “not scrubbing the teeth away with a coarse toothbrush,” he said.
Dr. Niederman has studied ways to prevent caries in underserved communities and is co-CEO of CariedAway, an organization that brings free cavity-prevention programs to schools.
In an average classroom of 24 students, about 6 children would be expected to have untreated tooth decay, Dr. Niederman said. And about 10% of the children with untreated tooth decay experience a toothache. So in two classrooms, at least one child would be expected to be experiencing pain, while the other students with caries might feel a lesser degree of discomfort. “That reduces presenteeism in the classroom and certainly presenteeism for the kid with a toothache,” Dr. Niederman said.
In communities with less access to dental care, including rural areas, the number of students with tooth decay might be double.
The new WHO report shows that the prevalence of caries in permanent teeth in various countries has remained at roughly 30%, regardless of a country’s income level, and despite efforts to bolster the dental workforce, said Dr. Niederman.
“The dental system is similar globally and focused on drilling and filling rather than prevention,” he said.
A 2019 Lancet series on oral health called for radical change in dental care, Dr. Niederman noted. “One of those radical changes would be primary care physicians or their offices participating in outreach programs to deliver care in schools,” he said.
Dr. Fisher-Owens is on a data and safety monitoring board for research by Colgate. Dr. Boulter serves on the editorial advisory board of Pediatric News. Dr. Hoss is the author of “If Your Mouth Could Talk” and the founder and CEO of SuperMouth, which markets children’s oral care products. Dr. Niederman’s research has used toothbrushes, toothpaste, and fluoride varnish donated by Colgate; silver diamine fluoride provided by Elevate Oral Health; and glass ionomer provided by GC America.
Tooth decay can be easy to overlook – particularly for pediatricians and family physicians, who may be neglecting a crucial aspect of childhood health.
Left untreated, it can lead to serious and even fatal medical problems. The incorporation of preventive oral health care services like the application of fluoride varnish into primary care may be helping protect kids’ smiles and improving their overall physical well-being, according to doctors and a recent government report.
‘We don’t deal with that in pediatrics’
Physicians historically were not trained to examine teeth. That was the dentist’s job.
But dental caries is one of the most common chronic diseases in children, and many children do not regularly see a dentist.
“I stumbled across the statistic that oral health problems in children are five times as common as asthma,” said Susan A. Fisher-Owens, MD, MPH, professor of pediatrics at the University of California, San Francisco. “And I said to myself, ‘Well, that can’t be. We don’t deal with that in pediatrics.’ And then I realized, ‘Oh my goodness, we don’t deal with that in pediatrics!’ ”
Children should see a dentist, of course. Physicians should refer families to a dentist by age 1 for routine care, Dr. Fisher-Owens said. The sooner kids are seen, the more likely they are to stay healthy and avoid the need for costlier care, she said.
But the receipt of dental care has gaps.
“About half of all American children do not receive regular dental care because of social, economic, and geographic obstacles,” according to a 2021 fact sheet from the National Institute of Dental and Craniofacial Research. “Integrating dental care within family and pediatric medical care settings is improving children’s oral health.”
Many children do not start to see a dentist when they are supposed to, acknowledged Kami Hoss, DDS, MS, founder of a large dental practice in California and the author of a new book, “If Your Mouth Could Talk,” that examines links between oral health and physical disease.
Although the American Academy of Pediatric Dentistry says every child should see a pediatric dentist by the time their first baby teeth come in, usually at around 6 months or no later than age 1 year, that does not always happen.
Indeed, only about 16% of children adhere to that guidance, Dr. Hoss said, “which means 84% of parents rely on their pediatricians for oral health advice.”
At older ages, oral health problems like gum disease are linked to almost every chronic disease, Dr. Hoss said.
“We love to bridge the gap, to build bridges between medicine and dentistry,” he said. “After all, your mouth is part of your body.”
A 2021 NIDCR report similarly describes the stakes: “Although caries is largely preventable, if untreated it can lead to pain, inflammation, and the spread of infection to bone and soft tissue. Children may suffer from difficulty eating, poor nutrition, delayed physical development, and poor self-image and socialization. Even academic performance can be affected.”
In November, the World Health Organization published a report showing that about 45% of the world’s population – 3.5 billion people – have oral diseases, including 2.5 billion people with untreated dental caries.
Oral health care is often neglected in public health research, and often entails high out-of-pocket costs for families, the organization notes.
‘Strep tooth’
Dental cavities are caused by bacteria – mainly Streptococcus mutans – that eat sugars or carbohydrates in the mouth. That process causes acid, which can erode teeth. In that way, the development of caries is a fully preventable infectious disease process, Dr. Fisher-Owens said.
“I think if people looked at this disease as ‘strep tooth,’ it would get a lot more people interested,” she said.
Bacteria that cause caries can spread from caregiver to child, such as when a parent tries to clean a dropped pacifier in their own mouth, or from child to child.
Caries can be prevented with proper diet and oral hygiene: toothbrushing and then applying fluoride to strengthen teeth or restrengthen teeth that have been weakened by the disease process, Dr. Fisher-Owens said.
The biggest risk factor for having cavities in adult teeth is having them in primary teeth, she said. “There is a common misconception that it doesn’t matter what happens with baby teeth. They’ll fall out,” she said. “But actually it does because it puts you on a trajectory of having cavities in the adult teeth and worse outcomes with other adult conditions, such as diabetes.”
At the 2022 annual meeting of the American Academy of Pediatrics in Anaheim in October, Dr. Fisher-Owens and Jean Calvo, DDS, MPH, also with the University of California, San Francisco, trained colleagues to apply fluoride varnish to primary teeth – so-called baby teeth – in the doctor’s office. This session is a regular feature at these conferences.
Since 2014, the U.S. Preventive Services Task Force has recommended that primary care clinicians apply fluoride varnish to the primary teeth of all infants and children.
Many pediatricians may not do this regularly, however.
Researchers recently reported that, despite insurance coverage, less than 5% of well-child visits for privately insured young children between 2016 and 2018 included the service.
Nevertheless, the practice may be helping, according to the NIDCR report.
Since 2000, untreated tooth decay in primary teeth among children younger than 12 years has fallen from 23% to 15%, according to the report. For children aged 2-5 years, untreated tooth decay decreased from at least 19% to 10%. For children aged 6-11 years, the prevalence of dental cavities in permanent teeth fell from 25% to 18%, the report states.
“Fluoridated water, toothpastes, and varnish – as well as dental sealants – can work together to dramatically reduce the incidence of caries,” according to the NIDCR. “Integrating dental care within family and pediatric medical care settings has been another important advancement. The delivery of preventive oral health services, such as fluoride varnish, during well-child visits in medical offices is showing promise in reducing dental caries among preschool-age children.”
Integrating oral health care with medical primary care has met challenges, however, including “resistance by providers, lack of training, and the need for insurance reimbursement for services,” the report notes.
Clinicians can already bill for the application of fluoride varnish using Current Procedural Terminology (CPT) code 99188, and additional oral health care procedures may be on the horizon.
The American Medical Association this fall established a new Category III CPT code for the application of silver diamine fluoride to dental cavities.
Silver diamine fluoride is a newer product that was approved as a desensitizing agent by the Food and Drug Administration in 2014. It has antimicrobial and remineralizing properties, and researchers have found that it can stop the progression of early tooth decay and is more effective than fluoride varnish in preventing cavities.
Several dental groups supported the creation of this new code, which is expected to be made available by electronic health records vendors in July.
Some dentists have reservations, however. The Academy of General Dentistry in October expressed concerns that allowing “nondental health care workers to administer silver diamine fluoride is a temporary solution to a growing oral health crisis.”
Silver diamine fluoride may stop about 80% of cavities. Although the CPT code for silver diamine fluoride has been established, whether insurers will reimburse health care professionals for the service is another matter, said Richard Niederman, DMD, professor and chair of epidemiology and health promotion at New York University College of Dentistry.
Fatal consequences
Disparities in oral health in children may be greater than with almost any other disease process. The rate of caries in children who are poor is about twice that for children who are not poor, Dr. Fisher-Owens said. Disparities by race or ethnicity compound these differences.
In 2007, 12-year-old Deamonte Driver died after bacteria from a dental abscess spread to his brain. He had needed a tooth extraction, but his family lacked insurance and had had trouble finding dentists that accepted Medicaid near where they lived in Maryland.
After two emergency brain surgeries, 2 weeks in a hospital, and another 6 weeks in a hospital for rehabilitation, Deamonte died from the infection. The case sparked calls to fix the dental health system.
Physicians may notice more oral health problems in their patients, including dental abscesses, once they start looking for them, Dr. Fisher-Owens said.
She recalled one instance where a child with an underlying seizure disorder was hospitalized at an academic center because they appeared to be having more seizures.
“They eventually discharged the kid because they looked at all of the things related to seizures. None of them were there,” she said.
When Dr. Fisher-Owens saw the child for a discharge exam, she looked in the mouth and saw a whopping dental abscess.
“I realized that this kid wasn’t seizing but was actually rigoring in pain,” she said. No one else on the medical team had seen the true problem despite multiple examinations. The child started antibiotics, was referred to a dentist to have the abscess drained, and had a good outcome.
Suzanne C. Boulter, MD, adjunct clinical professor of pediatrics and community health at the Geisel School of Medicine at Dartmouth, Hanover, N.H., had noticed that many of her poorer patients had oral health problems, but many pediatric dentists were not able or willing to see them to provide treatment.
“Taking it one step further, you really want to prevent early childhood caries,” Dr. Boulter said. She started speaking up about oral health at pediatric meetings and became an early adopter of preventive interventions, including the use of fluoride varnish.
“Fluoride varnish is a sticky substance that has a very high concentration of fluoride in it,and it’s a very powerful reducer of oral childhood caries, by maybe 35%,” Dr. Boulter said.
Applying the varnish is fairly simple, but it had never been part of the well-child exam. Dr. Boulter started using it around 2005.
Initially, convincing other pediatricians to adopt this procedure – when visits are already time-constrained – was not always easy, she said.
Now that fluoride varnish is recommended for all children and is part of Bright Futures recommendations and is covered in the Affordable Care Act, “it became more the norm,” Dr. Boulter said. But there is room for improvement.
“There is still not a high enough percentage of pediatricians and family physicians who actually have incorporated application of fluoride varnish into their practice,” she said.
Brush, book, bed
Clinicians can take other steps to counsel parents about protecting their child’s teeth, like making sure that their teeth get brushed before bed, encouraging kids to drink tap water, especially if it’s fluoridated, and avoiding juice. The AAP has a program called Brush, Book, Bed to promote oral health, along with reading and good sleep habits.
Dr. Hoss noted that parents, and even dentists, may have misconceptions about optimal oral hygiene. “For example, you’re supposed to brush your teeth before breakfast, not after breakfast. But I’ve seen dentists even tell their patients, brush after breakfast,” he said.
In addition, people should brush gently but thoroughly using high-quality toothbrushes with soft bristles – “not scrubbing the teeth away with a coarse toothbrush,” he said.
Dr. Niederman has studied ways to prevent caries in underserved communities and is co-CEO of CariedAway, an organization that brings free cavity-prevention programs to schools.
In an average classroom of 24 students, about 6 children would be expected to have untreated tooth decay, Dr. Niederman said. And about 10% of the children with untreated tooth decay experience a toothache. So in two classrooms, at least one child would be expected to be experiencing pain, while the other students with caries might feel a lesser degree of discomfort. “That reduces presenteeism in the classroom and certainly presenteeism for the kid with a toothache,” Dr. Niederman said.
In communities with less access to dental care, including rural areas, the number of students with tooth decay might be double.
The new WHO report shows that the prevalence of caries in permanent teeth in various countries has remained at roughly 30%, regardless of a country’s income level, and despite efforts to bolster the dental workforce, said Dr. Niederman.
“The dental system is similar globally and focused on drilling and filling rather than prevention,” he said.
A 2019 Lancet series on oral health called for radical change in dental care, Dr. Niederman noted. “One of those radical changes would be primary care physicians or their offices participating in outreach programs to deliver care in schools,” he said.
Dr. Fisher-Owens is on a data and safety monitoring board for research by Colgate. Dr. Boulter serves on the editorial advisory board of Pediatric News. Dr. Hoss is the author of “If Your Mouth Could Talk” and the founder and CEO of SuperMouth, which markets children’s oral care products. Dr. Niederman’s research has used toothbrushes, toothpaste, and fluoride varnish donated by Colgate; silver diamine fluoride provided by Elevate Oral Health; and glass ionomer provided by GC America.
Tooth decay can be easy to overlook – particularly for pediatricians and family physicians, who may be neglecting a crucial aspect of childhood health.
Left untreated, it can lead to serious and even fatal medical problems. The incorporation of preventive oral health care services like the application of fluoride varnish into primary care may be helping protect kids’ smiles and improving their overall physical well-being, according to doctors and a recent government report.
‘We don’t deal with that in pediatrics’
Physicians historically were not trained to examine teeth. That was the dentist’s job.
But dental caries is one of the most common chronic diseases in children, and many children do not regularly see a dentist.
“I stumbled across the statistic that oral health problems in children are five times as common as asthma,” said Susan A. Fisher-Owens, MD, MPH, professor of pediatrics at the University of California, San Francisco. “And I said to myself, ‘Well, that can’t be. We don’t deal with that in pediatrics.’ And then I realized, ‘Oh my goodness, we don’t deal with that in pediatrics!’ ”
Children should see a dentist, of course. Physicians should refer families to a dentist by age 1 for routine care, Dr. Fisher-Owens said. The sooner kids are seen, the more likely they are to stay healthy and avoid the need for costlier care, she said.
But the receipt of dental care has gaps.
“About half of all American children do not receive regular dental care because of social, economic, and geographic obstacles,” according to a 2021 fact sheet from the National Institute of Dental and Craniofacial Research. “Integrating dental care within family and pediatric medical care settings is improving children’s oral health.”
Many children do not start to see a dentist when they are supposed to, acknowledged Kami Hoss, DDS, MS, founder of a large dental practice in California and the author of a new book, “If Your Mouth Could Talk,” that examines links between oral health and physical disease.
Although the American Academy of Pediatric Dentistry says every child should see a pediatric dentist by the time their first baby teeth come in, usually at around 6 months or no later than age 1 year, that does not always happen.
Indeed, only about 16% of children adhere to that guidance, Dr. Hoss said, “which means 84% of parents rely on their pediatricians for oral health advice.”
At older ages, oral health problems like gum disease are linked to almost every chronic disease, Dr. Hoss said.
“We love to bridge the gap, to build bridges between medicine and dentistry,” he said. “After all, your mouth is part of your body.”
A 2021 NIDCR report similarly describes the stakes: “Although caries is largely preventable, if untreated it can lead to pain, inflammation, and the spread of infection to bone and soft tissue. Children may suffer from difficulty eating, poor nutrition, delayed physical development, and poor self-image and socialization. Even academic performance can be affected.”
In November, the World Health Organization published a report showing that about 45% of the world’s population – 3.5 billion people – have oral diseases, including 2.5 billion people with untreated dental caries.
Oral health care is often neglected in public health research, and often entails high out-of-pocket costs for families, the organization notes.
‘Strep tooth’
Dental cavities are caused by bacteria – mainly Streptococcus mutans – that eat sugars or carbohydrates in the mouth. That process causes acid, which can erode teeth. In that way, the development of caries is a fully preventable infectious disease process, Dr. Fisher-Owens said.
“I think if people looked at this disease as ‘strep tooth,’ it would get a lot more people interested,” she said.
Bacteria that cause caries can spread from caregiver to child, such as when a parent tries to clean a dropped pacifier in their own mouth, or from child to child.
Caries can be prevented with proper diet and oral hygiene: toothbrushing and then applying fluoride to strengthen teeth or restrengthen teeth that have been weakened by the disease process, Dr. Fisher-Owens said.
The biggest risk factor for having cavities in adult teeth is having them in primary teeth, she said. “There is a common misconception that it doesn’t matter what happens with baby teeth. They’ll fall out,” she said. “But actually it does because it puts you on a trajectory of having cavities in the adult teeth and worse outcomes with other adult conditions, such as diabetes.”
At the 2022 annual meeting of the American Academy of Pediatrics in Anaheim in October, Dr. Fisher-Owens and Jean Calvo, DDS, MPH, also with the University of California, San Francisco, trained colleagues to apply fluoride varnish to primary teeth – so-called baby teeth – in the doctor’s office. This session is a regular feature at these conferences.
Since 2014, the U.S. Preventive Services Task Force has recommended that primary care clinicians apply fluoride varnish to the primary teeth of all infants and children.
Many pediatricians may not do this regularly, however.
Researchers recently reported that, despite insurance coverage, less than 5% of well-child visits for privately insured young children between 2016 and 2018 included the service.
Nevertheless, the practice may be helping, according to the NIDCR report.
Since 2000, untreated tooth decay in primary teeth among children younger than 12 years has fallen from 23% to 15%, according to the report. For children aged 2-5 years, untreated tooth decay decreased from at least 19% to 10%. For children aged 6-11 years, the prevalence of dental cavities in permanent teeth fell from 25% to 18%, the report states.
“Fluoridated water, toothpastes, and varnish – as well as dental sealants – can work together to dramatically reduce the incidence of caries,” according to the NIDCR. “Integrating dental care within family and pediatric medical care settings has been another important advancement. The delivery of preventive oral health services, such as fluoride varnish, during well-child visits in medical offices is showing promise in reducing dental caries among preschool-age children.”
Integrating oral health care with medical primary care has met challenges, however, including “resistance by providers, lack of training, and the need for insurance reimbursement for services,” the report notes.
Clinicians can already bill for the application of fluoride varnish using Current Procedural Terminology (CPT) code 99188, and additional oral health care procedures may be on the horizon.
The American Medical Association this fall established a new Category III CPT code for the application of silver diamine fluoride to dental cavities.
Silver diamine fluoride is a newer product that was approved as a desensitizing agent by the Food and Drug Administration in 2014. It has antimicrobial and remineralizing properties, and researchers have found that it can stop the progression of early tooth decay and is more effective than fluoride varnish in preventing cavities.
Several dental groups supported the creation of this new code, which is expected to be made available by electronic health records vendors in July.
Some dentists have reservations, however. The Academy of General Dentistry in October expressed concerns that allowing “nondental health care workers to administer silver diamine fluoride is a temporary solution to a growing oral health crisis.”
Silver diamine fluoride may stop about 80% of cavities. Although the CPT code for silver diamine fluoride has been established, whether insurers will reimburse health care professionals for the service is another matter, said Richard Niederman, DMD, professor and chair of epidemiology and health promotion at New York University College of Dentistry.
Fatal consequences
Disparities in oral health in children may be greater than with almost any other disease process. The rate of caries in children who are poor is about twice that for children who are not poor, Dr. Fisher-Owens said. Disparities by race or ethnicity compound these differences.
In 2007, 12-year-old Deamonte Driver died after bacteria from a dental abscess spread to his brain. He had needed a tooth extraction, but his family lacked insurance and had had trouble finding dentists that accepted Medicaid near where they lived in Maryland.
After two emergency brain surgeries, 2 weeks in a hospital, and another 6 weeks in a hospital for rehabilitation, Deamonte died from the infection. The case sparked calls to fix the dental health system.
Physicians may notice more oral health problems in their patients, including dental abscesses, once they start looking for them, Dr. Fisher-Owens said.
She recalled one instance where a child with an underlying seizure disorder was hospitalized at an academic center because they appeared to be having more seizures.
“They eventually discharged the kid because they looked at all of the things related to seizures. None of them were there,” she said.
When Dr. Fisher-Owens saw the child for a discharge exam, she looked in the mouth and saw a whopping dental abscess.
“I realized that this kid wasn’t seizing but was actually rigoring in pain,” she said. No one else on the medical team had seen the true problem despite multiple examinations. The child started antibiotics, was referred to a dentist to have the abscess drained, and had a good outcome.
Suzanne C. Boulter, MD, adjunct clinical professor of pediatrics and community health at the Geisel School of Medicine at Dartmouth, Hanover, N.H., had noticed that many of her poorer patients had oral health problems, but many pediatric dentists were not able or willing to see them to provide treatment.
“Taking it one step further, you really want to prevent early childhood caries,” Dr. Boulter said. She started speaking up about oral health at pediatric meetings and became an early adopter of preventive interventions, including the use of fluoride varnish.
“Fluoride varnish is a sticky substance that has a very high concentration of fluoride in it,and it’s a very powerful reducer of oral childhood caries, by maybe 35%,” Dr. Boulter said.
Applying the varnish is fairly simple, but it had never been part of the well-child exam. Dr. Boulter started using it around 2005.
Initially, convincing other pediatricians to adopt this procedure – when visits are already time-constrained – was not always easy, she said.
Now that fluoride varnish is recommended for all children and is part of Bright Futures recommendations and is covered in the Affordable Care Act, “it became more the norm,” Dr. Boulter said. But there is room for improvement.
“There is still not a high enough percentage of pediatricians and family physicians who actually have incorporated application of fluoride varnish into their practice,” she said.
Brush, book, bed
Clinicians can take other steps to counsel parents about protecting their child’s teeth, like making sure that their teeth get brushed before bed, encouraging kids to drink tap water, especially if it’s fluoridated, and avoiding juice. The AAP has a program called Brush, Book, Bed to promote oral health, along with reading and good sleep habits.
Dr. Hoss noted that parents, and even dentists, may have misconceptions about optimal oral hygiene. “For example, you’re supposed to brush your teeth before breakfast, not after breakfast. But I’ve seen dentists even tell their patients, brush after breakfast,” he said.
In addition, people should brush gently but thoroughly using high-quality toothbrushes with soft bristles – “not scrubbing the teeth away with a coarse toothbrush,” he said.
Dr. Niederman has studied ways to prevent caries in underserved communities and is co-CEO of CariedAway, an organization that brings free cavity-prevention programs to schools.
In an average classroom of 24 students, about 6 children would be expected to have untreated tooth decay, Dr. Niederman said. And about 10% of the children with untreated tooth decay experience a toothache. So in two classrooms, at least one child would be expected to be experiencing pain, while the other students with caries might feel a lesser degree of discomfort. “That reduces presenteeism in the classroom and certainly presenteeism for the kid with a toothache,” Dr. Niederman said.
In communities with less access to dental care, including rural areas, the number of students with tooth decay might be double.
The new WHO report shows that the prevalence of caries in permanent teeth in various countries has remained at roughly 30%, regardless of a country’s income level, and despite efforts to bolster the dental workforce, said Dr. Niederman.
“The dental system is similar globally and focused on drilling and filling rather than prevention,” he said.
A 2019 Lancet series on oral health called for radical change in dental care, Dr. Niederman noted. “One of those radical changes would be primary care physicians or their offices participating in outreach programs to deliver care in schools,” he said.
Dr. Fisher-Owens is on a data and safety monitoring board for research by Colgate. Dr. Boulter serves on the editorial advisory board of Pediatric News. Dr. Hoss is the author of “If Your Mouth Could Talk” and the founder and CEO of SuperMouth, which markets children’s oral care products. Dr. Niederman’s research has used toothbrushes, toothpaste, and fluoride varnish donated by Colgate; silver diamine fluoride provided by Elevate Oral Health; and glass ionomer provided by GC America.
EHR alerts to both doc and patient may boost statin prescribing
Automated alerts to aid clinical decision-making are designed with the best of intentions but can be easy to ignore or overlook. But a randomized trial testing such electronic alerts or “nudges” for promoting statin prescribing may have identified a few design features that help their success, researchers say.
In the trial’s primary finding, for example, reminders displayed to primary care physicians in the electronic health record worked best when the system also reached out to the patient.
Reminders sent only to the clinician also boosted statin prescribing, but not as well, and nudging only the patient didn’t work at all, compared to a nudge-free usual care approach. The patient-only nudges consisted of text messages explaining why a statin prescription may figure in their upcoming appointment.
Nudge trustworthiness
Importantly, the clinician nudges were more than simply reminders to consider a statin prescription, Mitesh S. Patel, MD, MBA, Ascension Health, St. Louis, told this news organization. They also displayed the patient’s atherosclerotic cardiovascular disease (ASCVD) 10-year risk score and explained why a statin may be appropriate. He thinks that information, often left out of such clinical decision support alerts, increases physician trust in them.
In another key feature, Dr. Patel said, the EHR nudges themselves were actionable – that is, they were functional in ways that streamlined the prescribing process. In particular, they include checkbox shortcuts to prescribing statins at appropriate patient-specific dosages, making the entire process “faster and easier,” said Dr. Patel, who is senior author on the study published in JAMA Cardiology with lead author Srinath Adusumalli, MD, University of Pennsylvania, Philadelphia.
The timing may matter as well, he observed. In previous iterations of the study’s EHR nudge system, the nudge would appear “when you open the chart,” he said. “Now, it’s when you go to the orders section, which is when you’re going to be in the mindset of ordering prescriptions and tests.”
Prescription rates were higher with the doctor-patient nudges than with the doctor-only approach, Dr. Patel speculates, largely because the decision process for initiating statins is shared. “The most effective intervention is going to recognize that and try to bring the two groups together.”
Two text messages
The trial, with 158 participating physicians in 28 primary care practices, randomly assigned 4,131 patients to three intervention groups and one control group. Nudges were sent only to the physician, only to the patient, or to both physician and patient; and there was a no-nudge usual-care group.
Patient nudges consisted of two text messages, one 4 days and another 15 minutes before the appointment, announcing that prescription of a statin “to reduce the chance of a heart attack” would be discussed with the physician, the report states.
Statins are grossly underprescribed nationally, it notes, and that was reflected in prescription rates seen during the study’s initial 12-month, no-intervention period of observation. Rates ranged from only 4.7% up to 6% of patients across the four assignment groups.
During the subsequent 6-month intervention period, however, the rates climbed in the doctor-only and doctor-plus-patient nudge groups compared with usual care, by 5.5 (P = .01) and 7.2 (P = .001) absolute percentage points, respectively.
The overall cohort’s mean age was 65.5. About half were male, 29% were Black, 66% were White, and 22.6% already had a cardiovascular disease diagnosis. The analysis was adjusted for calendar month and preintervention statin prescribing rates. Further adjustment for demographics, insurance type, household income, and comorbidities yielded results similar to the primary analysis, the report states.
The results in context
“Although the differences in the combined clinician and patient and clinician-only arms were small, this outcome needs to be interpreted in the context of the population in which the study was performed,” an editorial accompanying the published report states.
For example, “the majority of untreated patients were candidates for primary, not secondary, prevention, making this group of patients particularly challenging for seeing large effect sizes of interventions.”
Moreover, “There was a high baseline prescription rate of statins in the statin-eligible population (approximately 70%) and a high rate of already established patients,” write Faraz S. Ahmad, MD, and Stephen D. Persell, MD, of Northwestern University, Chicago.
Among the approximately 30% of patients who had not previously been prescribed statins, the true target of the nudge interventions, the published trial report states, about 98% were not seeing the physician for the first time.
So “this may not have been the first opportunity to discuss statins,” they write. “It is possible that many of these patients were resistant to statins in the past, which could have created a ceiling effect for prescribing rates.”
Dr. Patel reports owning and receiving personal fees from Catalyst Health and serving on an advisory board for and receiving personal fees from Humana. Dr. Adusumalli reports having been employed by CVS Health. Dr. Ahmad reports receiving consulting fees from Teladoc Livongo and Pfizer. Dr. Persell discloses receiving grants from Omron Healthcare.
A version of this article first appeared on Medscape.com.
Automated alerts to aid clinical decision-making are designed with the best of intentions but can be easy to ignore or overlook. But a randomized trial testing such electronic alerts or “nudges” for promoting statin prescribing may have identified a few design features that help their success, researchers say.
In the trial’s primary finding, for example, reminders displayed to primary care physicians in the electronic health record worked best when the system also reached out to the patient.
Reminders sent only to the clinician also boosted statin prescribing, but not as well, and nudging only the patient didn’t work at all, compared to a nudge-free usual care approach. The patient-only nudges consisted of text messages explaining why a statin prescription may figure in their upcoming appointment.
Nudge trustworthiness
Importantly, the clinician nudges were more than simply reminders to consider a statin prescription, Mitesh S. Patel, MD, MBA, Ascension Health, St. Louis, told this news organization. They also displayed the patient’s atherosclerotic cardiovascular disease (ASCVD) 10-year risk score and explained why a statin may be appropriate. He thinks that information, often left out of such clinical decision support alerts, increases physician trust in them.
In another key feature, Dr. Patel said, the EHR nudges themselves were actionable – that is, they were functional in ways that streamlined the prescribing process. In particular, they include checkbox shortcuts to prescribing statins at appropriate patient-specific dosages, making the entire process “faster and easier,” said Dr. Patel, who is senior author on the study published in JAMA Cardiology with lead author Srinath Adusumalli, MD, University of Pennsylvania, Philadelphia.
The timing may matter as well, he observed. In previous iterations of the study’s EHR nudge system, the nudge would appear “when you open the chart,” he said. “Now, it’s when you go to the orders section, which is when you’re going to be in the mindset of ordering prescriptions and tests.”
Prescription rates were higher with the doctor-patient nudges than with the doctor-only approach, Dr. Patel speculates, largely because the decision process for initiating statins is shared. “The most effective intervention is going to recognize that and try to bring the two groups together.”
Two text messages
The trial, with 158 participating physicians in 28 primary care practices, randomly assigned 4,131 patients to three intervention groups and one control group. Nudges were sent only to the physician, only to the patient, or to both physician and patient; and there was a no-nudge usual-care group.
Patient nudges consisted of two text messages, one 4 days and another 15 minutes before the appointment, announcing that prescription of a statin “to reduce the chance of a heart attack” would be discussed with the physician, the report states.
Statins are grossly underprescribed nationally, it notes, and that was reflected in prescription rates seen during the study’s initial 12-month, no-intervention period of observation. Rates ranged from only 4.7% up to 6% of patients across the four assignment groups.
During the subsequent 6-month intervention period, however, the rates climbed in the doctor-only and doctor-plus-patient nudge groups compared with usual care, by 5.5 (P = .01) and 7.2 (P = .001) absolute percentage points, respectively.
The overall cohort’s mean age was 65.5. About half were male, 29% were Black, 66% were White, and 22.6% already had a cardiovascular disease diagnosis. The analysis was adjusted for calendar month and preintervention statin prescribing rates. Further adjustment for demographics, insurance type, household income, and comorbidities yielded results similar to the primary analysis, the report states.
The results in context
“Although the differences in the combined clinician and patient and clinician-only arms were small, this outcome needs to be interpreted in the context of the population in which the study was performed,” an editorial accompanying the published report states.
For example, “the majority of untreated patients were candidates for primary, not secondary, prevention, making this group of patients particularly challenging for seeing large effect sizes of interventions.”
Moreover, “There was a high baseline prescription rate of statins in the statin-eligible population (approximately 70%) and a high rate of already established patients,” write Faraz S. Ahmad, MD, and Stephen D. Persell, MD, of Northwestern University, Chicago.
Among the approximately 30% of patients who had not previously been prescribed statins, the true target of the nudge interventions, the published trial report states, about 98% were not seeing the physician for the first time.
So “this may not have been the first opportunity to discuss statins,” they write. “It is possible that many of these patients were resistant to statins in the past, which could have created a ceiling effect for prescribing rates.”
Dr. Patel reports owning and receiving personal fees from Catalyst Health and serving on an advisory board for and receiving personal fees from Humana. Dr. Adusumalli reports having been employed by CVS Health. Dr. Ahmad reports receiving consulting fees from Teladoc Livongo and Pfizer. Dr. Persell discloses receiving grants from Omron Healthcare.
A version of this article first appeared on Medscape.com.
Automated alerts to aid clinical decision-making are designed with the best of intentions but can be easy to ignore or overlook. But a randomized trial testing such electronic alerts or “nudges” for promoting statin prescribing may have identified a few design features that help their success, researchers say.
In the trial’s primary finding, for example, reminders displayed to primary care physicians in the electronic health record worked best when the system also reached out to the patient.
Reminders sent only to the clinician also boosted statin prescribing, but not as well, and nudging only the patient didn’t work at all, compared to a nudge-free usual care approach. The patient-only nudges consisted of text messages explaining why a statin prescription may figure in their upcoming appointment.
Nudge trustworthiness
Importantly, the clinician nudges were more than simply reminders to consider a statin prescription, Mitesh S. Patel, MD, MBA, Ascension Health, St. Louis, told this news organization. They also displayed the patient’s atherosclerotic cardiovascular disease (ASCVD) 10-year risk score and explained why a statin may be appropriate. He thinks that information, often left out of such clinical decision support alerts, increases physician trust in them.
In another key feature, Dr. Patel said, the EHR nudges themselves were actionable – that is, they were functional in ways that streamlined the prescribing process. In particular, they include checkbox shortcuts to prescribing statins at appropriate patient-specific dosages, making the entire process “faster and easier,” said Dr. Patel, who is senior author on the study published in JAMA Cardiology with lead author Srinath Adusumalli, MD, University of Pennsylvania, Philadelphia.
The timing may matter as well, he observed. In previous iterations of the study’s EHR nudge system, the nudge would appear “when you open the chart,” he said. “Now, it’s when you go to the orders section, which is when you’re going to be in the mindset of ordering prescriptions and tests.”
Prescription rates were higher with the doctor-patient nudges than with the doctor-only approach, Dr. Patel speculates, largely because the decision process for initiating statins is shared. “The most effective intervention is going to recognize that and try to bring the two groups together.”
Two text messages
The trial, with 158 participating physicians in 28 primary care practices, randomly assigned 4,131 patients to three intervention groups and one control group. Nudges were sent only to the physician, only to the patient, or to both physician and patient; and there was a no-nudge usual-care group.
Patient nudges consisted of two text messages, one 4 days and another 15 minutes before the appointment, announcing that prescription of a statin “to reduce the chance of a heart attack” would be discussed with the physician, the report states.
Statins are grossly underprescribed nationally, it notes, and that was reflected in prescription rates seen during the study’s initial 12-month, no-intervention period of observation. Rates ranged from only 4.7% up to 6% of patients across the four assignment groups.
During the subsequent 6-month intervention period, however, the rates climbed in the doctor-only and doctor-plus-patient nudge groups compared with usual care, by 5.5 (P = .01) and 7.2 (P = .001) absolute percentage points, respectively.
The overall cohort’s mean age was 65.5. About half were male, 29% were Black, 66% were White, and 22.6% already had a cardiovascular disease diagnosis. The analysis was adjusted for calendar month and preintervention statin prescribing rates. Further adjustment for demographics, insurance type, household income, and comorbidities yielded results similar to the primary analysis, the report states.
The results in context
“Although the differences in the combined clinician and patient and clinician-only arms were small, this outcome needs to be interpreted in the context of the population in which the study was performed,” an editorial accompanying the published report states.
For example, “the majority of untreated patients were candidates for primary, not secondary, prevention, making this group of patients particularly challenging for seeing large effect sizes of interventions.”
Moreover, “There was a high baseline prescription rate of statins in the statin-eligible population (approximately 70%) and a high rate of already established patients,” write Faraz S. Ahmad, MD, and Stephen D. Persell, MD, of Northwestern University, Chicago.
Among the approximately 30% of patients who had not previously been prescribed statins, the true target of the nudge interventions, the published trial report states, about 98% were not seeing the physician for the first time.
So “this may not have been the first opportunity to discuss statins,” they write. “It is possible that many of these patients were resistant to statins in the past, which could have created a ceiling effect for prescribing rates.”
Dr. Patel reports owning and receiving personal fees from Catalyst Health and serving on an advisory board for and receiving personal fees from Humana. Dr. Adusumalli reports having been employed by CVS Health. Dr. Ahmad reports receiving consulting fees from Teladoc Livongo and Pfizer. Dr. Persell discloses receiving grants from Omron Healthcare.
A version of this article first appeared on Medscape.com.
ASH 2022: New clinical data challenge long-held assumptions
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
FROM ASH 2022
Novel PCI screening approach detects diffuse CAD
A novel approach for stratifying patients into one of two phenotypes for coronary artery disease (CAD) helped differentiate those who would benefit from percutaneous coronary intervention (PCI) from those who wouldn’t, researchers in Belgium reported in a subanalysis of a single-center, randomized clinical trial.
“What this study adds is that we are actually creating a refined definition of the appropriateness criteria for PCI,” lead study author Carlos Collet, MD, PhD, of the Cardiovascular Center at OLV Hospital in Aalst, Belgium, said in an interview. “We have been too long implanting stents in diffuse disease that actually have no benefit for the patient.”
The study found that patients with diffuse CAD were almost twice as likely to have residual angina 3 months after PCI than patients with focal CAD, with respective rates of 51.9% vs. 27.5% after PCI (P = .02).
The researchers analyzed 103 patients from the TARGET-FFR (Trial of Angiography vs. pressure-Ratio-Guided Enhancement Techniques–Fractional Flow Reserve) conducted at the Golden Jubilee National Hospital in Glasgow. Study patients completed the 7-item Seattle Angina Questionnaire at baseline and at 3 months after PCI, which provided the researchers information on outcomes.
The study, published in JACC: Cardiovascular Interventions, used median pullback pressure gradient (PPG) to define focal and diffuse CAD. The operators used the PressureWire X Guidewire (Abbott Vascular) to measure fractional flow reserve (FFR).
The procedure involved administering a 200-mcg bolus of intracoronary nitrate and then positioning the pressure wire sensor at the tip of the guide catheter equalized with aortic pressure. The pressure wire was then advanced to the position sensor in the distal third of the vessel. After hyperemia was induced, coronary flow reserve was assessed using bolus thermodilution. Manual FFR pullback maneuvers were done at a constant speed for 20-30 seconds. The PPG index was calculated post hoc from the manual FFR pullback recordings obtained pre-PCI.
In this study, patients with low PPG needed longer (48 mm vs. 37 mm; P = .015) and more (1.5 vs. 1.0; P = .036) stents during PCI, Dr. Collet and colleagues reported. They concluded that patients with low PPG can be treated with medical therapy.
“The beauty of the PPG is that everything happens before you implant the stent,” Dr. Collet said. “We’re starting to understand that we cannot treat diffuse disease with a focal disease therapy.”
The challenge with differentiating diffuse from focal CAD has been that it relies on visual assessment. “It’s subject to operator variability, and that’s the reason why there are no trials with focal or diffuse disease specifically because, until now, we didn’t have any metric that quantified the diffuseness or the focality of the disease,” Dr. Collet said.
The PPG itself isn’t novel, Dr. Collet said. “The novelty is that for first time we can quantify in a reproducible way the information from the pullback,” he added.
“What this study tells us is that once you have a patient with diffuse coronary artery disease, don’t try PCI because it will not help half of them,” Patrick W. Serruys, MD, PhD, a cardiologist at the National University of Ireland, Galway, and author of the accompanying editorial, said in an interview.
He noted that one limitation of the study was that Dr. Collet and colleagues used mechanical PPG to measure the pressure gradient. “We use now a surrogate, which is angiography,” Dr. Serruys said. “It’s not exactly the same as a measurement of pressure with the pressure wire, but we know from many, many studies that it’s quite a good surrogate.” Future research should focus on use of angiography without the pressure wire to evaluate the pressure gradient.
The ongoing PPG Global registry will aim to further validate findings from the subanalysis, Dr. Collet said, and the PPG Primetime study will evaluate deferring PCI in patients with low PPG.
Dr. Collet disclosed relationships with Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, OpSens, Abbott Vascular and Philips Volcano. Dr. Serruys disclosed relationships with Sinomedical Sciences Technology, Sahajanand Medical Technological, Philips Volcano, Xeltis and HeartFlow.
A novel approach for stratifying patients into one of two phenotypes for coronary artery disease (CAD) helped differentiate those who would benefit from percutaneous coronary intervention (PCI) from those who wouldn’t, researchers in Belgium reported in a subanalysis of a single-center, randomized clinical trial.
“What this study adds is that we are actually creating a refined definition of the appropriateness criteria for PCI,” lead study author Carlos Collet, MD, PhD, of the Cardiovascular Center at OLV Hospital in Aalst, Belgium, said in an interview. “We have been too long implanting stents in diffuse disease that actually have no benefit for the patient.”
The study found that patients with diffuse CAD were almost twice as likely to have residual angina 3 months after PCI than patients with focal CAD, with respective rates of 51.9% vs. 27.5% after PCI (P = .02).
The researchers analyzed 103 patients from the TARGET-FFR (Trial of Angiography vs. pressure-Ratio-Guided Enhancement Techniques–Fractional Flow Reserve) conducted at the Golden Jubilee National Hospital in Glasgow. Study patients completed the 7-item Seattle Angina Questionnaire at baseline and at 3 months after PCI, which provided the researchers information on outcomes.
The study, published in JACC: Cardiovascular Interventions, used median pullback pressure gradient (PPG) to define focal and diffuse CAD. The operators used the PressureWire X Guidewire (Abbott Vascular) to measure fractional flow reserve (FFR).
The procedure involved administering a 200-mcg bolus of intracoronary nitrate and then positioning the pressure wire sensor at the tip of the guide catheter equalized with aortic pressure. The pressure wire was then advanced to the position sensor in the distal third of the vessel. After hyperemia was induced, coronary flow reserve was assessed using bolus thermodilution. Manual FFR pullback maneuvers were done at a constant speed for 20-30 seconds. The PPG index was calculated post hoc from the manual FFR pullback recordings obtained pre-PCI.
In this study, patients with low PPG needed longer (48 mm vs. 37 mm; P = .015) and more (1.5 vs. 1.0; P = .036) stents during PCI, Dr. Collet and colleagues reported. They concluded that patients with low PPG can be treated with medical therapy.
“The beauty of the PPG is that everything happens before you implant the stent,” Dr. Collet said. “We’re starting to understand that we cannot treat diffuse disease with a focal disease therapy.”
The challenge with differentiating diffuse from focal CAD has been that it relies on visual assessment. “It’s subject to operator variability, and that’s the reason why there are no trials with focal or diffuse disease specifically because, until now, we didn’t have any metric that quantified the diffuseness or the focality of the disease,” Dr. Collet said.
The PPG itself isn’t novel, Dr. Collet said. “The novelty is that for first time we can quantify in a reproducible way the information from the pullback,” he added.
“What this study tells us is that once you have a patient with diffuse coronary artery disease, don’t try PCI because it will not help half of them,” Patrick W. Serruys, MD, PhD, a cardiologist at the National University of Ireland, Galway, and author of the accompanying editorial, said in an interview.
He noted that one limitation of the study was that Dr. Collet and colleagues used mechanical PPG to measure the pressure gradient. “We use now a surrogate, which is angiography,” Dr. Serruys said. “It’s not exactly the same as a measurement of pressure with the pressure wire, but we know from many, many studies that it’s quite a good surrogate.” Future research should focus on use of angiography without the pressure wire to evaluate the pressure gradient.
The ongoing PPG Global registry will aim to further validate findings from the subanalysis, Dr. Collet said, and the PPG Primetime study will evaluate deferring PCI in patients with low PPG.
Dr. Collet disclosed relationships with Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, OpSens, Abbott Vascular and Philips Volcano. Dr. Serruys disclosed relationships with Sinomedical Sciences Technology, Sahajanand Medical Technological, Philips Volcano, Xeltis and HeartFlow.
A novel approach for stratifying patients into one of two phenotypes for coronary artery disease (CAD) helped differentiate those who would benefit from percutaneous coronary intervention (PCI) from those who wouldn’t, researchers in Belgium reported in a subanalysis of a single-center, randomized clinical trial.
“What this study adds is that we are actually creating a refined definition of the appropriateness criteria for PCI,” lead study author Carlos Collet, MD, PhD, of the Cardiovascular Center at OLV Hospital in Aalst, Belgium, said in an interview. “We have been too long implanting stents in diffuse disease that actually have no benefit for the patient.”
The study found that patients with diffuse CAD were almost twice as likely to have residual angina 3 months after PCI than patients with focal CAD, with respective rates of 51.9% vs. 27.5% after PCI (P = .02).
The researchers analyzed 103 patients from the TARGET-FFR (Trial of Angiography vs. pressure-Ratio-Guided Enhancement Techniques–Fractional Flow Reserve) conducted at the Golden Jubilee National Hospital in Glasgow. Study patients completed the 7-item Seattle Angina Questionnaire at baseline and at 3 months after PCI, which provided the researchers information on outcomes.
The study, published in JACC: Cardiovascular Interventions, used median pullback pressure gradient (PPG) to define focal and diffuse CAD. The operators used the PressureWire X Guidewire (Abbott Vascular) to measure fractional flow reserve (FFR).
The procedure involved administering a 200-mcg bolus of intracoronary nitrate and then positioning the pressure wire sensor at the tip of the guide catheter equalized with aortic pressure. The pressure wire was then advanced to the position sensor in the distal third of the vessel. After hyperemia was induced, coronary flow reserve was assessed using bolus thermodilution. Manual FFR pullback maneuvers were done at a constant speed for 20-30 seconds. The PPG index was calculated post hoc from the manual FFR pullback recordings obtained pre-PCI.
In this study, patients with low PPG needed longer (48 mm vs. 37 mm; P = .015) and more (1.5 vs. 1.0; P = .036) stents during PCI, Dr. Collet and colleagues reported. They concluded that patients with low PPG can be treated with medical therapy.
“The beauty of the PPG is that everything happens before you implant the stent,” Dr. Collet said. “We’re starting to understand that we cannot treat diffuse disease with a focal disease therapy.”
The challenge with differentiating diffuse from focal CAD has been that it relies on visual assessment. “It’s subject to operator variability, and that’s the reason why there are no trials with focal or diffuse disease specifically because, until now, we didn’t have any metric that quantified the diffuseness or the focality of the disease,” Dr. Collet said.
The PPG itself isn’t novel, Dr. Collet said. “The novelty is that for first time we can quantify in a reproducible way the information from the pullback,” he added.
“What this study tells us is that once you have a patient with diffuse coronary artery disease, don’t try PCI because it will not help half of them,” Patrick W. Serruys, MD, PhD, a cardiologist at the National University of Ireland, Galway, and author of the accompanying editorial, said in an interview.
He noted that one limitation of the study was that Dr. Collet and colleagues used mechanical PPG to measure the pressure gradient. “We use now a surrogate, which is angiography,” Dr. Serruys said. “It’s not exactly the same as a measurement of pressure with the pressure wire, but we know from many, many studies that it’s quite a good surrogate.” Future research should focus on use of angiography without the pressure wire to evaluate the pressure gradient.
The ongoing PPG Global registry will aim to further validate findings from the subanalysis, Dr. Collet said, and the PPG Primetime study will evaluate deferring PCI in patients with low PPG.
Dr. Collet disclosed relationships with Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, OpSens, Abbott Vascular and Philips Volcano. Dr. Serruys disclosed relationships with Sinomedical Sciences Technology, Sahajanand Medical Technological, Philips Volcano, Xeltis and HeartFlow.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Review gives weight to supplements for hair loss
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
Knee lesion that bleeds
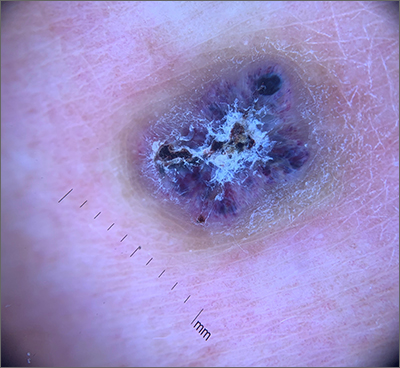
This combination of vascular features with excess keratin fit perfectly with the name of the diagnosis: angiokeratoma. The dark color of the lesion on magnification, or in this case with dermoscopy, showed the lacunar pattern of dilated vessels. The overlying keratin was likely accentuated because it was on an extensor surface; the rim of hyperpigmentation is common for these lesions.
Angiokeratomas result from dilation of the blood vessels underneath the epidermis. There are different inciting events that lead to the 5 different types of angiokeratomas. The overlying epidermal changes are secondary to the underlying process of capillary ectasia.1 This lesion was not part of a cluster, so it was characterized as a solitary angiokeratoma. Smaller lesions are usually less keratinized and are commonly seen on the scrotum and vulva, where there are usually multiple lesions (referred to as angiokeratoma of Fordyce).
Zaballos2 studied the dermoscopic characteristics of 32 solitary angiokeratomas and reported 6 findings in at least half of the solitary lesions. The most common features were dark lacunae in 94% of the lesions, white veil in 91%, and erythema in 69%. Peripheral erythema, red lacunae, and hemorrhagic crusts were all seen at a rate of 53%. The most common location was the lower extremities.
This patient’s previous pathology report from a shave biopsy was found, confirming that the original diagnosis was angiokeratoma. Since the patient’s lesion had not resolved and was symptomatic from minor trauma, he was scheduled to come back in for an elliptical excision to remove the lesion.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282. doi: 10.1159/000246270
2. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318–325. doi:10.1001/archderm.143.3.318

This combination of vascular features with excess keratin fit perfectly with the name of the diagnosis: angiokeratoma. The dark color of the lesion on magnification, or in this case with dermoscopy, showed the lacunar pattern of dilated vessels. The overlying keratin was likely accentuated because it was on an extensor surface; the rim of hyperpigmentation is common for these lesions.
Angiokeratomas result from dilation of the blood vessels underneath the epidermis. There are different inciting events that lead to the 5 different types of angiokeratomas. The overlying epidermal changes are secondary to the underlying process of capillary ectasia.1 This lesion was not part of a cluster, so it was characterized as a solitary angiokeratoma. Smaller lesions are usually less keratinized and are commonly seen on the scrotum and vulva, where there are usually multiple lesions (referred to as angiokeratoma of Fordyce).
Zaballos2 studied the dermoscopic characteristics of 32 solitary angiokeratomas and reported 6 findings in at least half of the solitary lesions. The most common features were dark lacunae in 94% of the lesions, white veil in 91%, and erythema in 69%. Peripheral erythema, red lacunae, and hemorrhagic crusts were all seen at a rate of 53%. The most common location was the lower extremities.
This patient’s previous pathology report from a shave biopsy was found, confirming that the original diagnosis was angiokeratoma. Since the patient’s lesion had not resolved and was symptomatic from minor trauma, he was scheduled to come back in for an elliptical excision to remove the lesion.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This combination of vascular features with excess keratin fit perfectly with the name of the diagnosis: angiokeratoma. The dark color of the lesion on magnification, or in this case with dermoscopy, showed the lacunar pattern of dilated vessels. The overlying keratin was likely accentuated because it was on an extensor surface; the rim of hyperpigmentation is common for these lesions.
Angiokeratomas result from dilation of the blood vessels underneath the epidermis. There are different inciting events that lead to the 5 different types of angiokeratomas. The overlying epidermal changes are secondary to the underlying process of capillary ectasia.1 This lesion was not part of a cluster, so it was characterized as a solitary angiokeratoma. Smaller lesions are usually less keratinized and are commonly seen on the scrotum and vulva, where there are usually multiple lesions (referred to as angiokeratoma of Fordyce).
Zaballos2 studied the dermoscopic characteristics of 32 solitary angiokeratomas and reported 6 findings in at least half of the solitary lesions. The most common features were dark lacunae in 94% of the lesions, white veil in 91%, and erythema in 69%. Peripheral erythema, red lacunae, and hemorrhagic crusts were all seen at a rate of 53%. The most common location was the lower extremities.
This patient’s previous pathology report from a shave biopsy was found, confirming that the original diagnosis was angiokeratoma. Since the patient’s lesion had not resolved and was symptomatic from minor trauma, he was scheduled to come back in for an elliptical excision to remove the lesion.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282. doi: 10.1159/000246270
2. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318–325. doi:10.1001/archderm.143.3.318
1. Schiller PI, Itin PH. Angiokeratomas: an update. Dermatology. 1996;193:275-282. doi: 10.1159/000246270
2. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318–325. doi:10.1001/archderm.143.3.318
Erythrasma
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
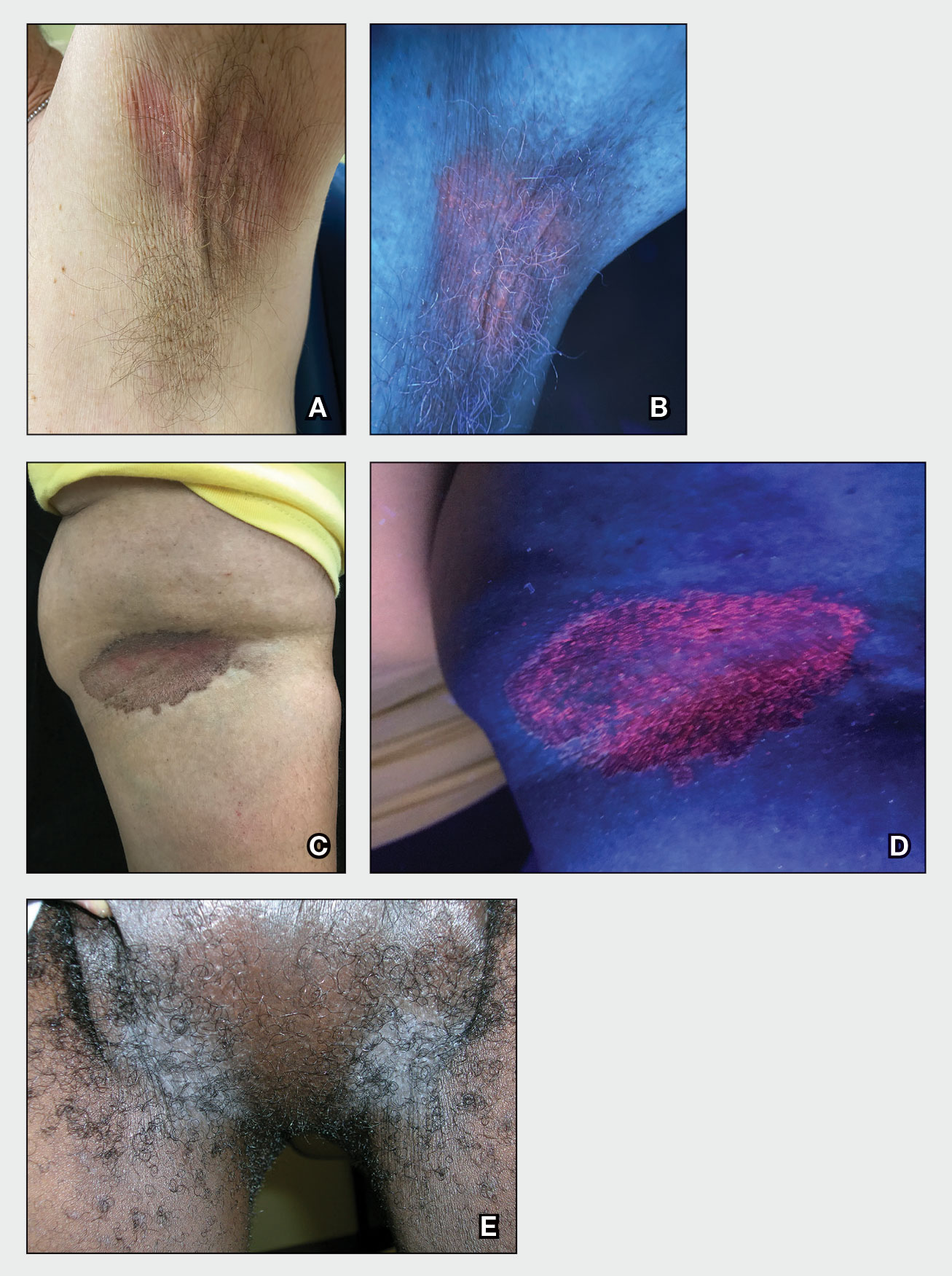
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
Analysis suggests CV benefits for some antioxidant supplements
Other antioxidant supplements that showed some evidence of reducing cardiovascular risk were omega-6 fatty acids, L-arginine, L-citrulline, magnesium, zinc, alpha-lipoic acid, melatonin, catechin, curcumin, flavanol, genistein, and quercetin.
No effect was seen with vitamin C, vitamin D, vitamin E, or selenium, and beta-carotene supplementation was linked to an increase in all-cause mortality in the analysis.
The study is published in the Journal of the American College of Cardiology and was also published online.
“Our systematic assessment and quantification of multiple differential effects of a wide variety of micronutrients and phytochemicals on cardiometabolic health indicate that an optimal nutritional strategy to promote cardiometabolic health will likely involve personalized combinations of these nutrients,” the authors, led by Peng An, PhD, China Agricultural University, Beijing, conclude.
“Identifying the optimal mixture of micronutrients is important, as not all are beneficial, and some may even have harmful effects,” senior author Simin Liu, MD, professor of epidemiology and medicine at Brown University, Providence, R.I., said in an American College of Cardiology press release.
“The micronutrients identified require further validation in large, high-quality interventional trials to establish clinical efficacy to determine their long-term balance of risks and benefits,” the authors add.
Experts cautious
Experts in the field of cardiovascular risk and preventative medicine have urged caution in interpreting these results.
JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, told this news organization that she has concerns that some of the results in the meta-analysis may be inflated by publication bias and some are chance findings that haven’t been well replicated.
“Although this meta-analysis of micronutrients and cardiometabolic health was based on randomized clinical trials, the quality of randomized trials on this subject varies widely,” she noted.
“The study is informative, but the conclusions are only as good as the quality of the evidence. Some of the trials are limited by short duration, and included trials have a wide range of quality, dosing, inclusion criteria, imperfect blinding, and few of them focus on hard clinical events,” Dr. Manson said. “Also, with trials of this nature, the potential for publication bias warrants consideration, because many of the smaller trials with unfavorable or neutral results may remain unpublished or not even be submitted for publication.”
However, she added, “despite these limitations, this is an important contribution to the literature on micronutrients and health – and goes a long way in separating the wheat from the chaff.”
Steve Nissen, MD, chief academic officer of the Heart Vascular and Thoracic Institute at the Cleveland Clinic, was more critical of the meta-analysis.
“This study does not make sense. Some of the ‘micronutrients’ in this meta-analysis have undergone thorough testing in large randomized clinical trials that showed different results. I am skeptical whether any of the purported benefits of these supplements would be confirmed in a high-quality randomized controlled trial,” he said.
Dr. Nissen added that many of the included studies are low in quality. “I must quote [renowned cardiologist, Dr.] Franz Messerli: ‘A meta-analysis is like making bouillabaisse. ... One rotten fish can spoil the broth.’ This type of analysis does not override high-quality large, randomized trials.”
In the JACC paper, the study investigators note that the American Heart Association now recommends dietary patterns, including the Mediterranean diet and DASH (the Dietary Approach to Stop Hypertension), as preventive or treatment approaches for cardiovascular disease. A common feature of these dietary patterns is that they are low in saturated fat and sodium and rich in micronutrients such as phytochemicals, unsaturated fatty acids, antioxidant vitamins, and minerals.
“To personalize cardiometabolic preventive and therapeutic dietary practices, it is of critical importance to have a comprehensive and in-depth understanding of the balance of benefits and risks associated with constituent micronutrients in diverse dietary patterns,” they note.
They therefore conducted the current systematic review and meta-analyses of all available randomized controlled trials investigating the effect of micronutrients with antioxidant properties on cardiovascular risk factors and events in diverse populations.
The meta-analysis included a total of 884 randomized trials evaluating 27 types of micronutrients among 883,627 participants.
Results showed that supplementation with n-3 fatty acids, n-6 fatty acids, L-arginine, L-citrulline, folic acid, magnesium, zinc, alpha-lipoic acid, coenzyme Q10, melatonin, catechin, curcumin, flavanol, genistein, and quercetin had “moderate-to high-quality evidence” for reducing cardiovascular risk factors.
Specifically, n-3 fatty acid supplementation was linked to reduced rates of cardiovascular mortality (relative risk, 0.93), myocardial infarction (RR, 0.85), and coronary heart disease events (RR, 0.86). Folic acid supplementation was linked to a decreased stroke risk (RR, 0.84) and coenzyme Q10 was associated with a lower rate of all-cause mortality (RR, 0.68).
“The current study represents the first attempt in providing a comprehensive and most up-to-date evidence map that systematically assessed the quality and quantity of all randomized trials linking the effects of a wide variety of micronutrients on cardiovascular risk factors,” the authors say.
“The comprehensive evidence map presented here highlights the importance of micronutrient diversity and the balance of benefits and risks in the design of whole food–based dietary patterns to promote cardiometabolic health, which may require cultural adaptations to apply globally,” they conclude.
Commenting on some of the specific beneficial findings, Dr. Manson said: “I do believe that the marine omega-3s confer heart benefits, but results are not consistent and vary by dose and formulation.”
However, she pointed out that, regarding folic acid, a previous meta-analysis including eight large randomized trials in more than 37,000 participants found no reduction in coronary events, stroke, or major cardiovascular events with folic acid supplementation, compared with placebo, “so the reported stroke benefit would need further confirmation.”
In an accompanying editorial, Juan Gormaz, PhD, University of Chile, and Rodrigo Carrasco, MD, Chilean Society of Cardiology and Cardiovascular Surgery, both in Santiago, state: “Given that the compounds with more pleiotropic properties produced the better outcomes, the antioxidant paradigm on cardiovascular prevention can be challenged. For example, inasmuch as n-3 fatty acids have antiplatelet and anti-inflammatory properties, they are too complex to enable attribution of the observed benefits solely to their antioxidant capacity.”
The editorialists note that from a research point of view, “although the current information opens interesting perspectives for future consolidation of some antioxidants in preventive cardiology, there is still a long way to go in terms of generating evidence.”
They add that the challenge now for some compounds is to begin establishing consensus in definitions of dose and combinations, as well as continue strengthening the evidence of effectiveness.
“Regarding routine clinical practice, these results begin to open spaces for the integration of new tools into the therapeutic arsenal aimed at cardiovascular prevention in selected populations, which could be easily accessible and, with specific exceptions, would present a low frequency of adverse effects,” they conclude.
This work was partly supported by the United States’ Fulbright Program and by the Beijing Advanced Innovation Center for Food Nutrition and Human Health, the National Natural Science Foundation of China, the Chinese Universities Scientific Fund, and the Beijing Municipal Natural Science Foundation.
Dr. Liu has received honoraria for scientific presentations or reviews at Johns Hopkins University, Fred Hutchinson Cancer Center, Harvard University, University of Buffalo, Guangdong General Hospital, Fuwai Hospital, Chinese Academy of Medical Sciences, and the National Institutes of Health; he is a member of the Data Safety and Monitoring Board for several trials, including the SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) trial sponsored by Novo Nordisk and a trial of pulmonary hypertension in diabetes patients sponsored by Massachusetts General Hospital; he has received royalties from UpToDate and has received an honorarium from the American Society for Nutrition for his duties as Associate Editor. Co-author Jeffrey Mechanick, MD, has received honoraria from Abbott Nutrition for lectures and serves on the advisory boards of Aveta.Life, L-Nutra, and Twin Health. The other authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Other antioxidant supplements that showed some evidence of reducing cardiovascular risk were omega-6 fatty acids, L-arginine, L-citrulline, magnesium, zinc, alpha-lipoic acid, melatonin, catechin, curcumin, flavanol, genistein, and quercetin.
No effect was seen with vitamin C, vitamin D, vitamin E, or selenium, and beta-carotene supplementation was linked to an increase in all-cause mortality in the analysis.
The study is published in the Journal of the American College of Cardiology and was also published online.
“Our systematic assessment and quantification of multiple differential effects of a wide variety of micronutrients and phytochemicals on cardiometabolic health indicate that an optimal nutritional strategy to promote cardiometabolic health will likely involve personalized combinations of these nutrients,” the authors, led by Peng An, PhD, China Agricultural University, Beijing, conclude.
“Identifying the optimal mixture of micronutrients is important, as not all are beneficial, and some may even have harmful effects,” senior author Simin Liu, MD, professor of epidemiology and medicine at Brown University, Providence, R.I., said in an American College of Cardiology press release.
“The micronutrients identified require further validation in large, high-quality interventional trials to establish clinical efficacy to determine their long-term balance of risks and benefits,” the authors add.
Experts cautious
Experts in the field of cardiovascular risk and preventative medicine have urged caution in interpreting these results.
JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, told this news organization that she has concerns that some of the results in the meta-analysis may be inflated by publication bias and some are chance findings that haven’t been well replicated.
“Although this meta-analysis of micronutrients and cardiometabolic health was based on randomized clinical trials, the quality of randomized trials on this subject varies widely,” she noted.
“The study is informative, but the conclusions are only as good as the quality of the evidence. Some of the trials are limited by short duration, and included trials have a wide range of quality, dosing, inclusion criteria, imperfect blinding, and few of them focus on hard clinical events,” Dr. Manson said. “Also, with trials of this nature, the potential for publication bias warrants consideration, because many of the smaller trials with unfavorable or neutral results may remain unpublished or not even be submitted for publication.”
However, she added, “despite these limitations, this is an important contribution to the literature on micronutrients and health – and goes a long way in separating the wheat from the chaff.”
Steve Nissen, MD, chief academic officer of the Heart Vascular and Thoracic Institute at the Cleveland Clinic, was more critical of the meta-analysis.
“This study does not make sense. Some of the ‘micronutrients’ in this meta-analysis have undergone thorough testing in large randomized clinical trials that showed different results. I am skeptical whether any of the purported benefits of these supplements would be confirmed in a high-quality randomized controlled trial,” he said.
Dr. Nissen added that many of the included studies are low in quality. “I must quote [renowned cardiologist, Dr.] Franz Messerli: ‘A meta-analysis is like making bouillabaisse. ... One rotten fish can spoil the broth.’ This type of analysis does not override high-quality large, randomized trials.”
In the JACC paper, the study investigators note that the American Heart Association now recommends dietary patterns, including the Mediterranean diet and DASH (the Dietary Approach to Stop Hypertension), as preventive or treatment approaches for cardiovascular disease. A common feature of these dietary patterns is that they are low in saturated fat and sodium and rich in micronutrients such as phytochemicals, unsaturated fatty acids, antioxidant vitamins, and minerals.
“To personalize cardiometabolic preventive and therapeutic dietary practices, it is of critical importance to have a comprehensive and in-depth understanding of the balance of benefits and risks associated with constituent micronutrients in diverse dietary patterns,” they note.
They therefore conducted the current systematic review and meta-analyses of all available randomized controlled trials investigating the effect of micronutrients with antioxidant properties on cardiovascular risk factors and events in diverse populations.
The meta-analysis included a total of 884 randomized trials evaluating 27 types of micronutrients among 883,627 participants.
Results showed that supplementation with n-3 fatty acids, n-6 fatty acids, L-arginine, L-citrulline, folic acid, magnesium, zinc, alpha-lipoic acid, coenzyme Q10, melatonin, catechin, curcumin, flavanol, genistein, and quercetin had “moderate-to high-quality evidence” for reducing cardiovascular risk factors.
Specifically, n-3 fatty acid supplementation was linked to reduced rates of cardiovascular mortality (relative risk, 0.93), myocardial infarction (RR, 0.85), and coronary heart disease events (RR, 0.86). Folic acid supplementation was linked to a decreased stroke risk (RR, 0.84) and coenzyme Q10 was associated with a lower rate of all-cause mortality (RR, 0.68).
“The current study represents the first attempt in providing a comprehensive and most up-to-date evidence map that systematically assessed the quality and quantity of all randomized trials linking the effects of a wide variety of micronutrients on cardiovascular risk factors,” the authors say.
“The comprehensive evidence map presented here highlights the importance of micronutrient diversity and the balance of benefits and risks in the design of whole food–based dietary patterns to promote cardiometabolic health, which may require cultural adaptations to apply globally,” they conclude.
Commenting on some of the specific beneficial findings, Dr. Manson said: “I do believe that the marine omega-3s confer heart benefits, but results are not consistent and vary by dose and formulation.”
However, she pointed out that, regarding folic acid, a previous meta-analysis including eight large randomized trials in more than 37,000 participants found no reduction in coronary events, stroke, or major cardiovascular events with folic acid supplementation, compared with placebo, “so the reported stroke benefit would need further confirmation.”
In an accompanying editorial, Juan Gormaz, PhD, University of Chile, and Rodrigo Carrasco, MD, Chilean Society of Cardiology and Cardiovascular Surgery, both in Santiago, state: “Given that the compounds with more pleiotropic properties produced the better outcomes, the antioxidant paradigm on cardiovascular prevention can be challenged. For example, inasmuch as n-3 fatty acids have antiplatelet and anti-inflammatory properties, they are too complex to enable attribution of the observed benefits solely to their antioxidant capacity.”
The editorialists note that from a research point of view, “although the current information opens interesting perspectives for future consolidation of some antioxidants in preventive cardiology, there is still a long way to go in terms of generating evidence.”
They add that the challenge now for some compounds is to begin establishing consensus in definitions of dose and combinations, as well as continue strengthening the evidence of effectiveness.
“Regarding routine clinical practice, these results begin to open spaces for the integration of new tools into the therapeutic arsenal aimed at cardiovascular prevention in selected populations, which could be easily accessible and, with specific exceptions, would present a low frequency of adverse effects,” they conclude.
This work was partly supported by the United States’ Fulbright Program and by the Beijing Advanced Innovation Center for Food Nutrition and Human Health, the National Natural Science Foundation of China, the Chinese Universities Scientific Fund, and the Beijing Municipal Natural Science Foundation.
Dr. Liu has received honoraria for scientific presentations or reviews at Johns Hopkins University, Fred Hutchinson Cancer Center, Harvard University, University of Buffalo, Guangdong General Hospital, Fuwai Hospital, Chinese Academy of Medical Sciences, and the National Institutes of Health; he is a member of the Data Safety and Monitoring Board for several trials, including the SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) trial sponsored by Novo Nordisk and a trial of pulmonary hypertension in diabetes patients sponsored by Massachusetts General Hospital; he has received royalties from UpToDate and has received an honorarium from the American Society for Nutrition for his duties as Associate Editor. Co-author Jeffrey Mechanick, MD, has received honoraria from Abbott Nutrition for lectures and serves on the advisory boards of Aveta.Life, L-Nutra, and Twin Health. The other authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Other antioxidant supplements that showed some evidence of reducing cardiovascular risk were omega-6 fatty acids, L-arginine, L-citrulline, magnesium, zinc, alpha-lipoic acid, melatonin, catechin, curcumin, flavanol, genistein, and quercetin.
No effect was seen with vitamin C, vitamin D, vitamin E, or selenium, and beta-carotene supplementation was linked to an increase in all-cause mortality in the analysis.
The study is published in the Journal of the American College of Cardiology and was also published online.
“Our systematic assessment and quantification of multiple differential effects of a wide variety of micronutrients and phytochemicals on cardiometabolic health indicate that an optimal nutritional strategy to promote cardiometabolic health will likely involve personalized combinations of these nutrients,” the authors, led by Peng An, PhD, China Agricultural University, Beijing, conclude.
“Identifying the optimal mixture of micronutrients is important, as not all are beneficial, and some may even have harmful effects,” senior author Simin Liu, MD, professor of epidemiology and medicine at Brown University, Providence, R.I., said in an American College of Cardiology press release.
“The micronutrients identified require further validation in large, high-quality interventional trials to establish clinical efficacy to determine their long-term balance of risks and benefits,” the authors add.
Experts cautious
Experts in the field of cardiovascular risk and preventative medicine have urged caution in interpreting these results.
JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, told this news organization that she has concerns that some of the results in the meta-analysis may be inflated by publication bias and some are chance findings that haven’t been well replicated.
“Although this meta-analysis of micronutrients and cardiometabolic health was based on randomized clinical trials, the quality of randomized trials on this subject varies widely,” she noted.
“The study is informative, but the conclusions are only as good as the quality of the evidence. Some of the trials are limited by short duration, and included trials have a wide range of quality, dosing, inclusion criteria, imperfect blinding, and few of them focus on hard clinical events,” Dr. Manson said. “Also, with trials of this nature, the potential for publication bias warrants consideration, because many of the smaller trials with unfavorable or neutral results may remain unpublished or not even be submitted for publication.”
However, she added, “despite these limitations, this is an important contribution to the literature on micronutrients and health – and goes a long way in separating the wheat from the chaff.”
Steve Nissen, MD, chief academic officer of the Heart Vascular and Thoracic Institute at the Cleveland Clinic, was more critical of the meta-analysis.
“This study does not make sense. Some of the ‘micronutrients’ in this meta-analysis have undergone thorough testing in large randomized clinical trials that showed different results. I am skeptical whether any of the purported benefits of these supplements would be confirmed in a high-quality randomized controlled trial,” he said.
Dr. Nissen added that many of the included studies are low in quality. “I must quote [renowned cardiologist, Dr.] Franz Messerli: ‘A meta-analysis is like making bouillabaisse. ... One rotten fish can spoil the broth.’ This type of analysis does not override high-quality large, randomized trials.”
In the JACC paper, the study investigators note that the American Heart Association now recommends dietary patterns, including the Mediterranean diet and DASH (the Dietary Approach to Stop Hypertension), as preventive or treatment approaches for cardiovascular disease. A common feature of these dietary patterns is that they are low in saturated fat and sodium and rich in micronutrients such as phytochemicals, unsaturated fatty acids, antioxidant vitamins, and minerals.
“To personalize cardiometabolic preventive and therapeutic dietary practices, it is of critical importance to have a comprehensive and in-depth understanding of the balance of benefits and risks associated with constituent micronutrients in diverse dietary patterns,” they note.
They therefore conducted the current systematic review and meta-analyses of all available randomized controlled trials investigating the effect of micronutrients with antioxidant properties on cardiovascular risk factors and events in diverse populations.
The meta-analysis included a total of 884 randomized trials evaluating 27 types of micronutrients among 883,627 participants.
Results showed that supplementation with n-3 fatty acids, n-6 fatty acids, L-arginine, L-citrulline, folic acid, magnesium, zinc, alpha-lipoic acid, coenzyme Q10, melatonin, catechin, curcumin, flavanol, genistein, and quercetin had “moderate-to high-quality evidence” for reducing cardiovascular risk factors.
Specifically, n-3 fatty acid supplementation was linked to reduced rates of cardiovascular mortality (relative risk, 0.93), myocardial infarction (RR, 0.85), and coronary heart disease events (RR, 0.86). Folic acid supplementation was linked to a decreased stroke risk (RR, 0.84) and coenzyme Q10 was associated with a lower rate of all-cause mortality (RR, 0.68).
“The current study represents the first attempt in providing a comprehensive and most up-to-date evidence map that systematically assessed the quality and quantity of all randomized trials linking the effects of a wide variety of micronutrients on cardiovascular risk factors,” the authors say.
“The comprehensive evidence map presented here highlights the importance of micronutrient diversity and the balance of benefits and risks in the design of whole food–based dietary patterns to promote cardiometabolic health, which may require cultural adaptations to apply globally,” they conclude.
Commenting on some of the specific beneficial findings, Dr. Manson said: “I do believe that the marine omega-3s confer heart benefits, but results are not consistent and vary by dose and formulation.”
However, she pointed out that, regarding folic acid, a previous meta-analysis including eight large randomized trials in more than 37,000 participants found no reduction in coronary events, stroke, or major cardiovascular events with folic acid supplementation, compared with placebo, “so the reported stroke benefit would need further confirmation.”
In an accompanying editorial, Juan Gormaz, PhD, University of Chile, and Rodrigo Carrasco, MD, Chilean Society of Cardiology and Cardiovascular Surgery, both in Santiago, state: “Given that the compounds with more pleiotropic properties produced the better outcomes, the antioxidant paradigm on cardiovascular prevention can be challenged. For example, inasmuch as n-3 fatty acids have antiplatelet and anti-inflammatory properties, they are too complex to enable attribution of the observed benefits solely to their antioxidant capacity.”
The editorialists note that from a research point of view, “although the current information opens interesting perspectives for future consolidation of some antioxidants in preventive cardiology, there is still a long way to go in terms of generating evidence.”
They add that the challenge now for some compounds is to begin establishing consensus in definitions of dose and combinations, as well as continue strengthening the evidence of effectiveness.
“Regarding routine clinical practice, these results begin to open spaces for the integration of new tools into the therapeutic arsenal aimed at cardiovascular prevention in selected populations, which could be easily accessible and, with specific exceptions, would present a low frequency of adverse effects,” they conclude.
This work was partly supported by the United States’ Fulbright Program and by the Beijing Advanced Innovation Center for Food Nutrition and Human Health, the National Natural Science Foundation of China, the Chinese Universities Scientific Fund, and the Beijing Municipal Natural Science Foundation.
Dr. Liu has received honoraria for scientific presentations or reviews at Johns Hopkins University, Fred Hutchinson Cancer Center, Harvard University, University of Buffalo, Guangdong General Hospital, Fuwai Hospital, Chinese Academy of Medical Sciences, and the National Institutes of Health; he is a member of the Data Safety and Monitoring Board for several trials, including the SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) trial sponsored by Novo Nordisk and a trial of pulmonary hypertension in diabetes patients sponsored by Massachusetts General Hospital; he has received royalties from UpToDate and has received an honorarium from the American Society for Nutrition for his duties as Associate Editor. Co-author Jeffrey Mechanick, MD, has received honoraria from Abbott Nutrition for lectures and serves on the advisory boards of Aveta.Life, L-Nutra, and Twin Health. The other authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC
Mobile Enlarging Scalp Nodule
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6
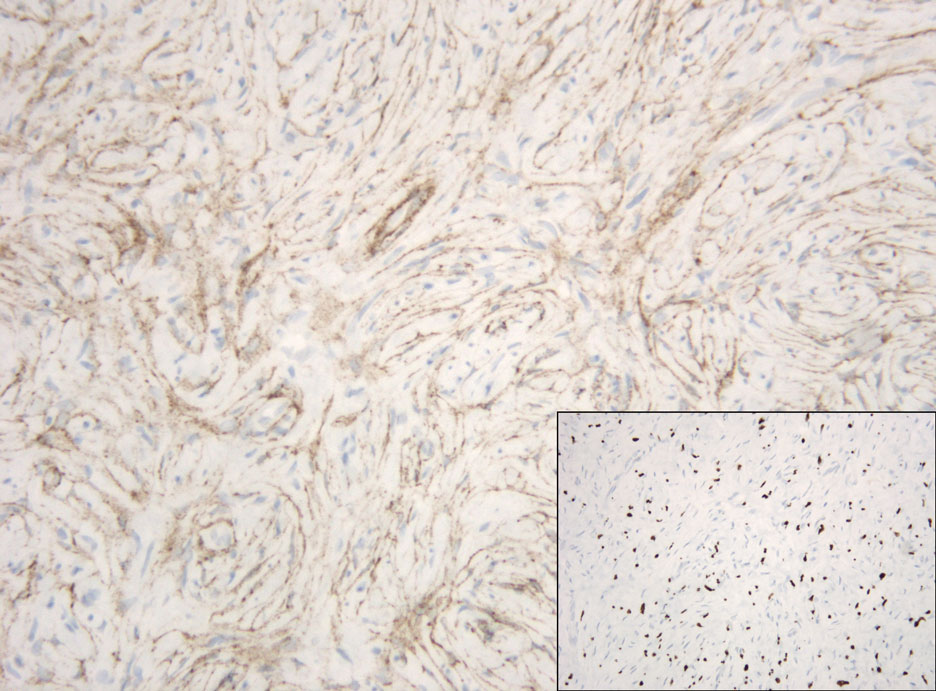
Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9
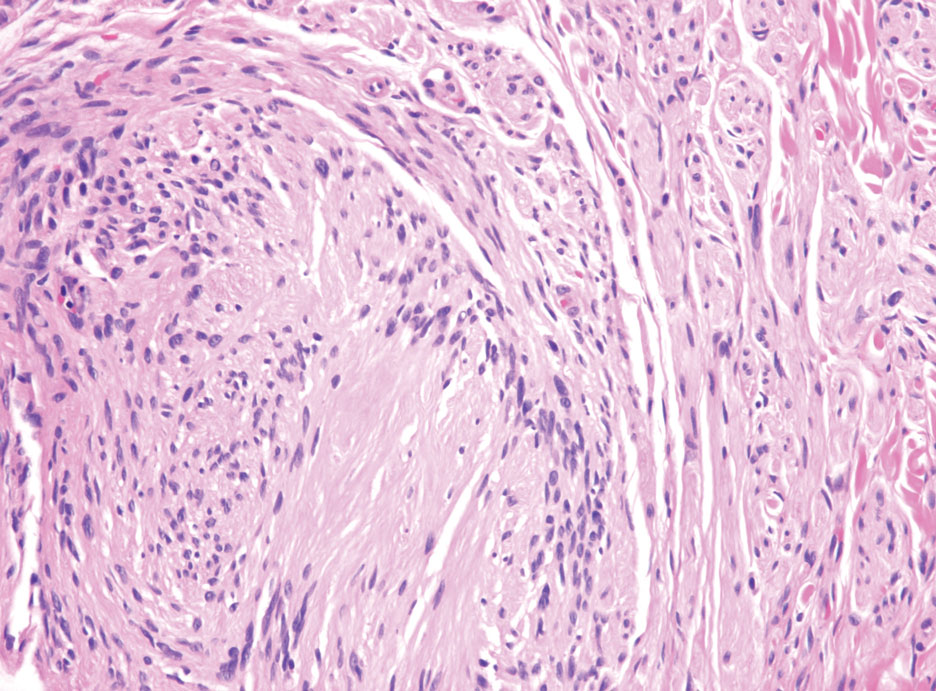
Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7
Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15
Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
A 50-year-old man presented with a 2.5-cm, subcutaneous, freely mobile nodule on the occipital scalp that first appeared 35 years prior but recently had started enlarging. Histologically the lesion was well circumscribed. Immunohistochemical staining was positive for SRY-box transcription factor 10 in some of the spindle cells, and staining for epithelial membrane antigen was positive in a separate population of intermixed spindle cells.