User login
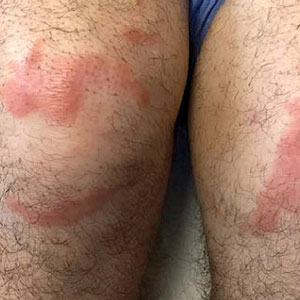
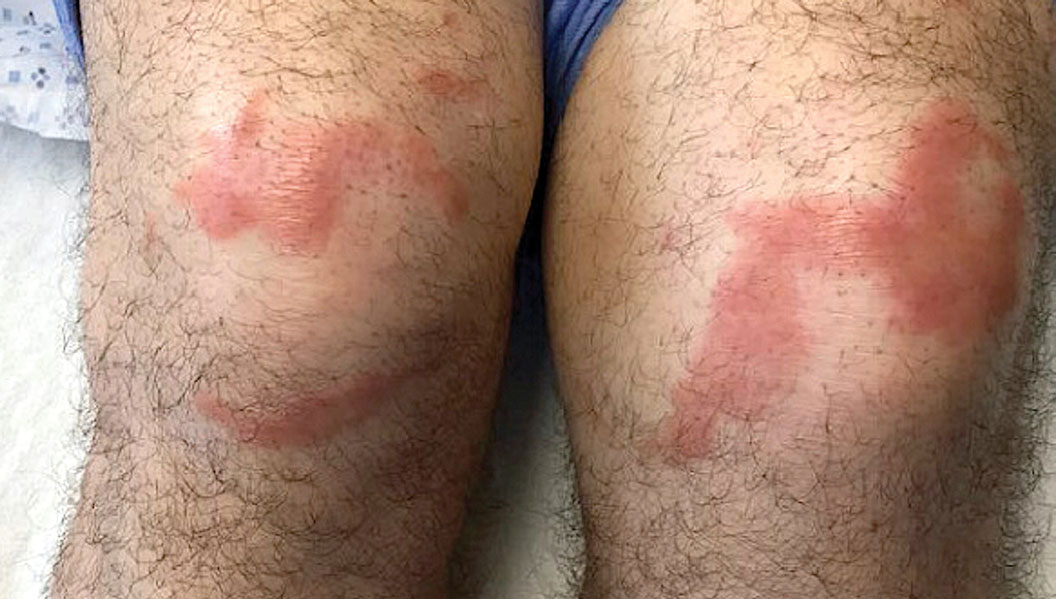
Multiple Annular Erythematous Plaques
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
A 59-year-old man was admitted to the medical ward with multiple annular erythematous plaques and polyarthralgia of several months’ duration. His medical history included low-grade stage IIA follicular lymphoma diagnosed 6 months prior to presentation, substance abuse with opiates and cocaine, coronary artery disease, ascending aortic aneurysm, and chronic lower back pain. Physical examination revealed multiple red to red-brown papules and plaques, some in an annular configuration, that were distributed on the cheeks, left wrist, knees, dorsal feet, and soles. Bilateral inguinal lymphadenopathy also was noted. Serological testing for HIV, hepatitis B and C viruses, Treponema pallidum, Borrelia burgdorferi, and tuberculosis assay were negative. Arthrocentesis of the left wrist 1 week prior to admission noted 5333 nucleated cells/μL (reference range, <3000 cells/μL) and no crystals; culture of the fluid was sterile. Skin biopsies of plaques on the left wrist, left dorsal foot, and right knee were obtained for histopathologic analysis.
Dialing down the negativity
I don’t do email. Or texting. You want to talk to me and my staff? Pick up a phone.
Some people say I’m old fashioned, or not patient-friendly, or whatever.
I don’t care.
To me there are too many issues with things that can get missed in emails, too many security concerns, too many ways to alter them so it looks like something different was said.
Now, a recent study of an EHR system found that 3% of emails from patients had negative, if not downright nasty, sentiments expressed to their physicians.
Here’s some examples:
“I hope and expect that you will spend eternity in hell. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
The study also noted that the most common expletive used by patients is the F-bomb, and that words with violent connotations, such as “shoot,” “fight,” and “kill” were often used in such emails. The last are definitely concerning in an era of increased violence directed at doctors and other health care workers who are just trying to do their jobs.
Now, I know doctors are a microcosm of society. Like patients, most are decent people trying their best, but a few are ... not particularly nice.
But still, I don’t think we, or anyone for that matter, need to be getting emails of this nature. It certainly doesn’t put anyone in a good position, or allow for objective, unbiased, care. Even if they’re only 3% of emails, that can still be quite a few.
Who needs that?
One of the issues with email is that it’s easy to type something nasty and hit “send,” then later have it occur to you that maybe you should have calmed down first. Granted, that sort of thing can (and does) happen when talking to another person (by phone or in person), but it’s harder.
Direct personal contact, especially face-to-face, appears to lessen impulsive reactions for most. The other person isn’t an invisible email address, they’re someone you’re talking to. You can read tone-of-voice and facial expressions. Again, I’m aware people still can lose their cool in person, but it’s harder.
and running into the next exam room. Plus, it ensures that all noncritical patient interactions occur during business hours, when we’re in doctor mode, rather than at 2:45 a.m. when we look at the iPhone while waiting for the dog to come back in. That’s a terrible time to receive and send medical (or any) emails for both doctor and patient.
A lot rides on every one of my patient interactions, and that’s why I still want them done directly. If that makes me old-fashioned, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t do email. Or texting. You want to talk to me and my staff? Pick up a phone.
Some people say I’m old fashioned, or not patient-friendly, or whatever.
I don’t care.
To me there are too many issues with things that can get missed in emails, too many security concerns, too many ways to alter them so it looks like something different was said.
Now, a recent study of an EHR system found that 3% of emails from patients had negative, if not downright nasty, sentiments expressed to their physicians.
Here’s some examples:
“I hope and expect that you will spend eternity in hell. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
The study also noted that the most common expletive used by patients is the F-bomb, and that words with violent connotations, such as “shoot,” “fight,” and “kill” were often used in such emails. The last are definitely concerning in an era of increased violence directed at doctors and other health care workers who are just trying to do their jobs.
Now, I know doctors are a microcosm of society. Like patients, most are decent people trying their best, but a few are ... not particularly nice.
But still, I don’t think we, or anyone for that matter, need to be getting emails of this nature. It certainly doesn’t put anyone in a good position, or allow for objective, unbiased, care. Even if they’re only 3% of emails, that can still be quite a few.
Who needs that?
One of the issues with email is that it’s easy to type something nasty and hit “send,” then later have it occur to you that maybe you should have calmed down first. Granted, that sort of thing can (and does) happen when talking to another person (by phone or in person), but it’s harder.
Direct personal contact, especially face-to-face, appears to lessen impulsive reactions for most. The other person isn’t an invisible email address, they’re someone you’re talking to. You can read tone-of-voice and facial expressions. Again, I’m aware people still can lose their cool in person, but it’s harder.
and running into the next exam room. Plus, it ensures that all noncritical patient interactions occur during business hours, when we’re in doctor mode, rather than at 2:45 a.m. when we look at the iPhone while waiting for the dog to come back in. That’s a terrible time to receive and send medical (or any) emails for both doctor and patient.
A lot rides on every one of my patient interactions, and that’s why I still want them done directly. If that makes me old-fashioned, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t do email. Or texting. You want to talk to me and my staff? Pick up a phone.
Some people say I’m old fashioned, or not patient-friendly, or whatever.
I don’t care.
To me there are too many issues with things that can get missed in emails, too many security concerns, too many ways to alter them so it looks like something different was said.
Now, a recent study of an EHR system found that 3% of emails from patients had negative, if not downright nasty, sentiments expressed to their physicians.
Here’s some examples:
“I hope and expect that you will spend eternity in hell. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
The study also noted that the most common expletive used by patients is the F-bomb, and that words with violent connotations, such as “shoot,” “fight,” and “kill” were often used in such emails. The last are definitely concerning in an era of increased violence directed at doctors and other health care workers who are just trying to do their jobs.
Now, I know doctors are a microcosm of society. Like patients, most are decent people trying their best, but a few are ... not particularly nice.
But still, I don’t think we, or anyone for that matter, need to be getting emails of this nature. It certainly doesn’t put anyone in a good position, or allow for objective, unbiased, care. Even if they’re only 3% of emails, that can still be quite a few.
Who needs that?
One of the issues with email is that it’s easy to type something nasty and hit “send,” then later have it occur to you that maybe you should have calmed down first. Granted, that sort of thing can (and does) happen when talking to another person (by phone or in person), but it’s harder.
Direct personal contact, especially face-to-face, appears to lessen impulsive reactions for most. The other person isn’t an invisible email address, they’re someone you’re talking to. You can read tone-of-voice and facial expressions. Again, I’m aware people still can lose their cool in person, but it’s harder.
and running into the next exam room. Plus, it ensures that all noncritical patient interactions occur during business hours, when we’re in doctor mode, rather than at 2:45 a.m. when we look at the iPhone while waiting for the dog to come back in. That’s a terrible time to receive and send medical (or any) emails for both doctor and patient.
A lot rides on every one of my patient interactions, and that’s why I still want them done directly. If that makes me old-fashioned, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
RSV surge stuns parents and strains providers, but doctors offer help
RSV cases peaked in mid-November, according to the latest Centers for Disease Control and Prevention data, with RSV-associated hospitalizations in the United States among patients 0-4 years having maxed out five times higher than they were at the same time in 2021. These surges strained providers and left parents scrambling for care. Fortunately, pediatric hospitalizations appear to be subsiding.
In interviews, the parents of the child who had a severe case of RSV reflected on their son’s bout with the illness, and doctors described challenges to dealing with the surge in RSV cases this season. The physicians also offered advice on how recognize and respond to future cases of the virus.
Sebastian Witt’s story
“I didn’t even know what RSV was,” said Malte Witt, whose son, Sebastian, 2, was recently hospitalized for RSV in Denver.
Mr. Witt and his wife, Emily Witt, both 32, thought they were dealing with a typical cold until Sebastian’s condition dramatically deteriorated about 36 hours after symptom onset.
“He basically just slumped over and collapsed, coughing uncontrollably,” Mr. Witt said in an interview. “He couldn’t catch his breath.”
The Witts rushed Sebastian to the ED at Children’s Hospital Colorado, expecting to see a doctor immediately. Instead, they spent the night in an overcrowded waiting room alongside many other families in the same situation.
“There was no room for anyone to sit anywhere,” Mr. Witt said. “There were people sitting on the floor. I counted maybe six children hooked up to oxygen when we walked in.”
After waiting approximately 45 minutes, a nurse checked Sebastian’s oxygen saturation. The readings were 79%-83%. This range is significantly below thresholds for supplemental oxygen described by most pediatric guidelines, which range from 90 to 94%.
The nurse connected Sebastian to bottled oxygen in the waiting room, and a recheck 4 hours later showed that his oxygen saturation had improved.
But the improvement didn’t last.
“At roughly hour 10 in the waiting room – it was 4 in the morning – you could tell that Seb was exhausted, really not acting like himself,” Mr. Witt said. “We thought maybe it’s just late at night, he hasn’t really slept. But then Emily noticed that his oxygen tank had run out.”
Mr. Witt told a nurse, and after another check revealed low oxygen saturation, Sebastian was finally admitted.
Early RSV surge strains pediatric providers
With RSV-associated hospitalizations peaking at 48 per 100,000 children, Colorado has been among the states hardest hit by the virus. New Mexico – where hospitalizations peaked at 56.4 per 100,000 children – comes in second. Even in states like California, where hospitalization rates have been almost 10-fold lower than New Mexico, pediatric providers have been stretched to their limits.
“Many hospitals are really being overwhelmed with admissions for RSV, both routine RSV – relatively mild hospitalizations with bronchiolitis – as well as kids in the ICU with more severe cases,” said Dean Blumberg, MD, chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, said in an interview.
Dr. Blumberg believes the severity of the 2022-2023 RSV season is likely COVID related.
“All community-associated respiratory viral infections are out of whack because of the pandemic, and all the masking and social distancing that was occurring,” he said.
This may also explain why older kids are coming down with more severe cases of RSV.
“Some children are getting RSV for the first time as older children,” Dr. Blumberg said, noting that, historically, most children were infected in the first 2 years of life. “There are reports of children 3 or 4 years of age being admitted with their first episode of RSV because of the [COVID] pandemic.”
This year’s RSV season is also notable for arriving early, potentially catching the community off guard, according to Jennifer D. Kusma, MD, a primary care pediatrician at Ann & Robert H. Lurie Children’s Hospital of Chicago.
“People who should have been protected often weren’t protected yet,” Dr. Kusma said in an interview.
Treatments new, old, and unproven
On Nov. 17, in the midst of the RSV surge, the American Academy of Pediatrics issued updated guidance for palivizumab, an RSV-targeting monoclonal antibody labeled for children at risk of severe RSV, including those with pre-existing lung or heart conditions, and infants with a history of premature birth (less than or equal to 35 weeks’ gestational age).
“If RSV disease activity persists at high levels in a given region through the fall and winter, the AAP supports providing more than five consecutive doses of palivizumab to eligible children,” the update stated.
Insurance companies appear to be responding in kind, covering additional doses for children in need.
“[Payers] have agreed that, if [palivizumab] needs to be given for an additional month or 2 or 3, then they’re making a commitment that they’ll reimburse hospitals for providing that,” Dr. Blumberg said.
For ineligible patients, such as Sebastian, who was born prematurely at 36 weeks – 1 week shy of the label requirement – treatment relies upon supportive care with oxygen and IV fluids.
At home, parents are left with simpler options.
Dr. Blumberg and Dr. Kusma recommended keeping children hydrated, maintaining humidified air, and using saline nose drops with bulb suction to clear mucus.
In the Witts’ experience, that last step may be easier said than done.
“Every time a nurse would walk into the room, Sebastian would yell: ‘Go away, doctor! I don’t want snot sucker!’” Mr. Witt said.
“If you over snot-suck, that’s really uncomfortable for the kid, and really hard for you,” Ms. Witt said. “And it doesn’t make much of a difference. It’s just very hard to find a middle ground, where you’re helping and keeping them comfortable.”
Some parents are turning to novel strategies, such as nebulized hypertonic saline, currently marketed on Amazon for children with RSV.
Although the AAP offers a weak recommendation for nebulized hypertonic saline in children hospitalized more than 72 hours, they advise against it in the emergency setting, citing inconsistent findings in clinical trials.
To any parents tempted by thousands of positive Amazon reviews, Dr. Blumberg said, “I wouldn’t waste my money on that.”
Dr. Kusma agreed.
“[Nebulized hypertonic saline] can be irritating,” she said. “It’s saltwater, essentially. If a parent is in the position where they’re worried about their child’s breathing to the point that they think they need to use it, I would err on the side of calling your pediatrician and being seen.”
Going in, coming home
Dr. Kusma said parents should seek medical attention if a child is breathing faster and working harder to get air. Increased work of breathing is characterized by pulling of the skin at the notch where the throat meets the chest bone (tracheal tugging), and flattening of the belly that makes the ribcage more prominent.
Mr. Witt saw these signs in Sebastian. He knew they were significant, because a friend who is a nurse had previously shown him some examples of children who exhibited these symptoms online.
“That’s how I knew that things were actually really dangerous,” Mr. Witt said. “Had she not shown me those videos a month and a half before this happened, I don’t know that we would have hit the alarm bell as quickly as we did.”
After spending their second night and the following day in a cramped preoperative room converted to manage overflow from the emergency department, Sebastian’s condition improved, and he was discharged. The Witts are relieved to be home, but frustrations from their ordeal remain, especially considering the estimated $5,000 in out-of-pocket costs they expect to pay.
“How is this our health care system?” Ms. Witt asked. “This is unbelievable.”
An optimistic outlook
RSV seasons typically demonstrate a clear peak, followed by a decline through the rest of the season, suggesting better times lie ahead; however, this season has been anything but typical.
“I’m hopeful that it will just go away and stay away,” Dr. Kusma said, citing this trend. “But I can’t know for sure.”
To anxious parents, Dr. Blumberg offered an optimistic view of RSV seasons to come.
“There’s hope,” he said. “There are vaccines that are being developed that are very close to FDA approval. So, it’s possible that this time next year, we might have widespread RSV vaccination available for children so that we don’t have to go through this nightmare again.”
Dr. Blumberg and Dr. Kusma disclosed no relevant conflicts of interest.
RSV cases peaked in mid-November, according to the latest Centers for Disease Control and Prevention data, with RSV-associated hospitalizations in the United States among patients 0-4 years having maxed out five times higher than they were at the same time in 2021. These surges strained providers and left parents scrambling for care. Fortunately, pediatric hospitalizations appear to be subsiding.
In interviews, the parents of the child who had a severe case of RSV reflected on their son’s bout with the illness, and doctors described challenges to dealing with the surge in RSV cases this season. The physicians also offered advice on how recognize and respond to future cases of the virus.
Sebastian Witt’s story
“I didn’t even know what RSV was,” said Malte Witt, whose son, Sebastian, 2, was recently hospitalized for RSV in Denver.
Mr. Witt and his wife, Emily Witt, both 32, thought they were dealing with a typical cold until Sebastian’s condition dramatically deteriorated about 36 hours after symptom onset.
“He basically just slumped over and collapsed, coughing uncontrollably,” Mr. Witt said in an interview. “He couldn’t catch his breath.”
The Witts rushed Sebastian to the ED at Children’s Hospital Colorado, expecting to see a doctor immediately. Instead, they spent the night in an overcrowded waiting room alongside many other families in the same situation.
“There was no room for anyone to sit anywhere,” Mr. Witt said. “There were people sitting on the floor. I counted maybe six children hooked up to oxygen when we walked in.”
After waiting approximately 45 minutes, a nurse checked Sebastian’s oxygen saturation. The readings were 79%-83%. This range is significantly below thresholds for supplemental oxygen described by most pediatric guidelines, which range from 90 to 94%.
The nurse connected Sebastian to bottled oxygen in the waiting room, and a recheck 4 hours later showed that his oxygen saturation had improved.
But the improvement didn’t last.
“At roughly hour 10 in the waiting room – it was 4 in the morning – you could tell that Seb was exhausted, really not acting like himself,” Mr. Witt said. “We thought maybe it’s just late at night, he hasn’t really slept. But then Emily noticed that his oxygen tank had run out.”
Mr. Witt told a nurse, and after another check revealed low oxygen saturation, Sebastian was finally admitted.
Early RSV surge strains pediatric providers
With RSV-associated hospitalizations peaking at 48 per 100,000 children, Colorado has been among the states hardest hit by the virus. New Mexico – where hospitalizations peaked at 56.4 per 100,000 children – comes in second. Even in states like California, where hospitalization rates have been almost 10-fold lower than New Mexico, pediatric providers have been stretched to their limits.
“Many hospitals are really being overwhelmed with admissions for RSV, both routine RSV – relatively mild hospitalizations with bronchiolitis – as well as kids in the ICU with more severe cases,” said Dean Blumberg, MD, chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, said in an interview.
Dr. Blumberg believes the severity of the 2022-2023 RSV season is likely COVID related.
“All community-associated respiratory viral infections are out of whack because of the pandemic, and all the masking and social distancing that was occurring,” he said.
This may also explain why older kids are coming down with more severe cases of RSV.
“Some children are getting RSV for the first time as older children,” Dr. Blumberg said, noting that, historically, most children were infected in the first 2 years of life. “There are reports of children 3 or 4 years of age being admitted with their first episode of RSV because of the [COVID] pandemic.”
This year’s RSV season is also notable for arriving early, potentially catching the community off guard, according to Jennifer D. Kusma, MD, a primary care pediatrician at Ann & Robert H. Lurie Children’s Hospital of Chicago.
“People who should have been protected often weren’t protected yet,” Dr. Kusma said in an interview.
Treatments new, old, and unproven
On Nov. 17, in the midst of the RSV surge, the American Academy of Pediatrics issued updated guidance for palivizumab, an RSV-targeting monoclonal antibody labeled for children at risk of severe RSV, including those with pre-existing lung or heart conditions, and infants with a history of premature birth (less than or equal to 35 weeks’ gestational age).
“If RSV disease activity persists at high levels in a given region through the fall and winter, the AAP supports providing more than five consecutive doses of palivizumab to eligible children,” the update stated.
Insurance companies appear to be responding in kind, covering additional doses for children in need.
“[Payers] have agreed that, if [palivizumab] needs to be given for an additional month or 2 or 3, then they’re making a commitment that they’ll reimburse hospitals for providing that,” Dr. Blumberg said.
For ineligible patients, such as Sebastian, who was born prematurely at 36 weeks – 1 week shy of the label requirement – treatment relies upon supportive care with oxygen and IV fluids.
At home, parents are left with simpler options.
Dr. Blumberg and Dr. Kusma recommended keeping children hydrated, maintaining humidified air, and using saline nose drops with bulb suction to clear mucus.
In the Witts’ experience, that last step may be easier said than done.
“Every time a nurse would walk into the room, Sebastian would yell: ‘Go away, doctor! I don’t want snot sucker!’” Mr. Witt said.
“If you over snot-suck, that’s really uncomfortable for the kid, and really hard for you,” Ms. Witt said. “And it doesn’t make much of a difference. It’s just very hard to find a middle ground, where you’re helping and keeping them comfortable.”
Some parents are turning to novel strategies, such as nebulized hypertonic saline, currently marketed on Amazon for children with RSV.
Although the AAP offers a weak recommendation for nebulized hypertonic saline in children hospitalized more than 72 hours, they advise against it in the emergency setting, citing inconsistent findings in clinical trials.
To any parents tempted by thousands of positive Amazon reviews, Dr. Blumberg said, “I wouldn’t waste my money on that.”
Dr. Kusma agreed.
“[Nebulized hypertonic saline] can be irritating,” she said. “It’s saltwater, essentially. If a parent is in the position where they’re worried about their child’s breathing to the point that they think they need to use it, I would err on the side of calling your pediatrician and being seen.”
Going in, coming home
Dr. Kusma said parents should seek medical attention if a child is breathing faster and working harder to get air. Increased work of breathing is characterized by pulling of the skin at the notch where the throat meets the chest bone (tracheal tugging), and flattening of the belly that makes the ribcage more prominent.
Mr. Witt saw these signs in Sebastian. He knew they were significant, because a friend who is a nurse had previously shown him some examples of children who exhibited these symptoms online.
“That’s how I knew that things were actually really dangerous,” Mr. Witt said. “Had she not shown me those videos a month and a half before this happened, I don’t know that we would have hit the alarm bell as quickly as we did.”
After spending their second night and the following day in a cramped preoperative room converted to manage overflow from the emergency department, Sebastian’s condition improved, and he was discharged. The Witts are relieved to be home, but frustrations from their ordeal remain, especially considering the estimated $5,000 in out-of-pocket costs they expect to pay.
“How is this our health care system?” Ms. Witt asked. “This is unbelievable.”
An optimistic outlook
RSV seasons typically demonstrate a clear peak, followed by a decline through the rest of the season, suggesting better times lie ahead; however, this season has been anything but typical.
“I’m hopeful that it will just go away and stay away,” Dr. Kusma said, citing this trend. “But I can’t know for sure.”
To anxious parents, Dr. Blumberg offered an optimistic view of RSV seasons to come.
“There’s hope,” he said. “There are vaccines that are being developed that are very close to FDA approval. So, it’s possible that this time next year, we might have widespread RSV vaccination available for children so that we don’t have to go through this nightmare again.”
Dr. Blumberg and Dr. Kusma disclosed no relevant conflicts of interest.
RSV cases peaked in mid-November, according to the latest Centers for Disease Control and Prevention data, with RSV-associated hospitalizations in the United States among patients 0-4 years having maxed out five times higher than they were at the same time in 2021. These surges strained providers and left parents scrambling for care. Fortunately, pediatric hospitalizations appear to be subsiding.
In interviews, the parents of the child who had a severe case of RSV reflected on their son’s bout with the illness, and doctors described challenges to dealing with the surge in RSV cases this season. The physicians also offered advice on how recognize and respond to future cases of the virus.
Sebastian Witt’s story
“I didn’t even know what RSV was,” said Malte Witt, whose son, Sebastian, 2, was recently hospitalized for RSV in Denver.
Mr. Witt and his wife, Emily Witt, both 32, thought they were dealing with a typical cold until Sebastian’s condition dramatically deteriorated about 36 hours after symptom onset.
“He basically just slumped over and collapsed, coughing uncontrollably,” Mr. Witt said in an interview. “He couldn’t catch his breath.”
The Witts rushed Sebastian to the ED at Children’s Hospital Colorado, expecting to see a doctor immediately. Instead, they spent the night in an overcrowded waiting room alongside many other families in the same situation.
“There was no room for anyone to sit anywhere,” Mr. Witt said. “There were people sitting on the floor. I counted maybe six children hooked up to oxygen when we walked in.”
After waiting approximately 45 minutes, a nurse checked Sebastian’s oxygen saturation. The readings were 79%-83%. This range is significantly below thresholds for supplemental oxygen described by most pediatric guidelines, which range from 90 to 94%.
The nurse connected Sebastian to bottled oxygen in the waiting room, and a recheck 4 hours later showed that his oxygen saturation had improved.
But the improvement didn’t last.
“At roughly hour 10 in the waiting room – it was 4 in the morning – you could tell that Seb was exhausted, really not acting like himself,” Mr. Witt said. “We thought maybe it’s just late at night, he hasn’t really slept. But then Emily noticed that his oxygen tank had run out.”
Mr. Witt told a nurse, and after another check revealed low oxygen saturation, Sebastian was finally admitted.
Early RSV surge strains pediatric providers
With RSV-associated hospitalizations peaking at 48 per 100,000 children, Colorado has been among the states hardest hit by the virus. New Mexico – where hospitalizations peaked at 56.4 per 100,000 children – comes in second. Even in states like California, where hospitalization rates have been almost 10-fold lower than New Mexico, pediatric providers have been stretched to their limits.
“Many hospitals are really being overwhelmed with admissions for RSV, both routine RSV – relatively mild hospitalizations with bronchiolitis – as well as kids in the ICU with more severe cases,” said Dean Blumberg, MD, chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, said in an interview.
Dr. Blumberg believes the severity of the 2022-2023 RSV season is likely COVID related.
“All community-associated respiratory viral infections are out of whack because of the pandemic, and all the masking and social distancing that was occurring,” he said.
This may also explain why older kids are coming down with more severe cases of RSV.
“Some children are getting RSV for the first time as older children,” Dr. Blumberg said, noting that, historically, most children were infected in the first 2 years of life. “There are reports of children 3 or 4 years of age being admitted with their first episode of RSV because of the [COVID] pandemic.”
This year’s RSV season is also notable for arriving early, potentially catching the community off guard, according to Jennifer D. Kusma, MD, a primary care pediatrician at Ann & Robert H. Lurie Children’s Hospital of Chicago.
“People who should have been protected often weren’t protected yet,” Dr. Kusma said in an interview.
Treatments new, old, and unproven
On Nov. 17, in the midst of the RSV surge, the American Academy of Pediatrics issued updated guidance for palivizumab, an RSV-targeting monoclonal antibody labeled for children at risk of severe RSV, including those with pre-existing lung or heart conditions, and infants with a history of premature birth (less than or equal to 35 weeks’ gestational age).
“If RSV disease activity persists at high levels in a given region through the fall and winter, the AAP supports providing more than five consecutive doses of palivizumab to eligible children,” the update stated.
Insurance companies appear to be responding in kind, covering additional doses for children in need.
“[Payers] have agreed that, if [palivizumab] needs to be given for an additional month or 2 or 3, then they’re making a commitment that they’ll reimburse hospitals for providing that,” Dr. Blumberg said.
For ineligible patients, such as Sebastian, who was born prematurely at 36 weeks – 1 week shy of the label requirement – treatment relies upon supportive care with oxygen and IV fluids.
At home, parents are left with simpler options.
Dr. Blumberg and Dr. Kusma recommended keeping children hydrated, maintaining humidified air, and using saline nose drops with bulb suction to clear mucus.
In the Witts’ experience, that last step may be easier said than done.
“Every time a nurse would walk into the room, Sebastian would yell: ‘Go away, doctor! I don’t want snot sucker!’” Mr. Witt said.
“If you over snot-suck, that’s really uncomfortable for the kid, and really hard for you,” Ms. Witt said. “And it doesn’t make much of a difference. It’s just very hard to find a middle ground, where you’re helping and keeping them comfortable.”
Some parents are turning to novel strategies, such as nebulized hypertonic saline, currently marketed on Amazon for children with RSV.
Although the AAP offers a weak recommendation for nebulized hypertonic saline in children hospitalized more than 72 hours, they advise against it in the emergency setting, citing inconsistent findings in clinical trials.
To any parents tempted by thousands of positive Amazon reviews, Dr. Blumberg said, “I wouldn’t waste my money on that.”
Dr. Kusma agreed.
“[Nebulized hypertonic saline] can be irritating,” she said. “It’s saltwater, essentially. If a parent is in the position where they’re worried about their child’s breathing to the point that they think they need to use it, I would err on the side of calling your pediatrician and being seen.”
Going in, coming home
Dr. Kusma said parents should seek medical attention if a child is breathing faster and working harder to get air. Increased work of breathing is characterized by pulling of the skin at the notch where the throat meets the chest bone (tracheal tugging), and flattening of the belly that makes the ribcage more prominent.
Mr. Witt saw these signs in Sebastian. He knew they were significant, because a friend who is a nurse had previously shown him some examples of children who exhibited these symptoms online.
“That’s how I knew that things were actually really dangerous,” Mr. Witt said. “Had she not shown me those videos a month and a half before this happened, I don’t know that we would have hit the alarm bell as quickly as we did.”
After spending their second night and the following day in a cramped preoperative room converted to manage overflow from the emergency department, Sebastian’s condition improved, and he was discharged. The Witts are relieved to be home, but frustrations from their ordeal remain, especially considering the estimated $5,000 in out-of-pocket costs they expect to pay.
“How is this our health care system?” Ms. Witt asked. “This is unbelievable.”
An optimistic outlook
RSV seasons typically demonstrate a clear peak, followed by a decline through the rest of the season, suggesting better times lie ahead; however, this season has been anything but typical.
“I’m hopeful that it will just go away and stay away,” Dr. Kusma said, citing this trend. “But I can’t know for sure.”
To anxious parents, Dr. Blumberg offered an optimistic view of RSV seasons to come.
“There’s hope,” he said. “There are vaccines that are being developed that are very close to FDA approval. So, it’s possible that this time next year, we might have widespread RSV vaccination available for children so that we don’t have to go through this nightmare again.”
Dr. Blumberg and Dr. Kusma disclosed no relevant conflicts of interest.
Virtual yoga program appears to improve IBS symptoms, fatigue, stress
Participants reported a decrease in IBS-related symptoms and improvements in quality of life, fatigue, and perceived stress.
“IBS affects upwards of 15%-20% of the North American population, and despite our advances in the area, we have very limited options to offer our patients,” Maitreyi Raman, MD, an associate professor of medicine at the University of Calgary (Alta.), said in an interview.
“Often, we are focused on treating symptoms but not addressing the underlying cause,” said Dr. Raman, who is director of Alberta’s Collaboration of Excellence for Nutrition in Digestive Diseases. “With advances around the gut microbiome and the evolving science on the brain-gut axis, mind-body interventions could offer a therapeutic option that patients can use to improve the overall course of their disease.”
The study was published online in the American Journal of Gastroenterology.
Online yoga program vs. IBS advice only
IBS often involves alterations of the gut-brain axis and can be affected by psychological or physiological stress, the study authors write. Previous studies have found that in-person yoga programs can manage IBS symptoms and improve physiological, psychological, and emotional health.
During the COVID-19 pandemic, yoga programs had to switch to a virtual format – a delivery method that could remain relevant due to limited health care resources. However, the efficacy, feasibility, and safety of virtual yoga for people with IBS were unknown.
Dr. Raman and colleagues conducted a randomized, two-group, controlled clinical trial at the University of Calgary (Alta.) between March 2021 and December 2022. The 79 participants weren’t blinded to the trial arms – an online yoga program or an advice-only control group.
The eligible participants had a diagnosis of IBS, scored at least 75 out of 500 points on the IBS Symptoms Severity Scale (IBS-SSS) for mild IBS, and were on stable doses of medications for IBS. They were instructed to continue with their current therapies during the study but didn’t start new medications or make major changes to their diet or physical patterns.
The yoga program was based on Upa Yoga, a subtype of Hatha Yoga developed by the Isha Foundation of Inner Sciences. The program was delivered by a certified yoga facilitator from the Isha Foundation and included directional movements, neck rotations, breathing practices, breath watching, and mantra meditation with aum/om chanting.
The online classes of three to seven participants were delivered in 60-minute sessions for 8 weeks. The participants were also asked to practice at home daily with the support of yoga videos.
The advice-only control group included a 10-minute video with general education on IBS, the mind-gut connection in IBS, and the role of mind-body therapies in managing IBS. The participants received a list of IBS-related resources from the Canadian Digestive Health Foundation, a link to an IBS patient support group, and information about physical activity guidelines from the World Health Organization.
The research team looked for a primary endpoint of at least a 50-point reduction on the IBS-SSS, which is considered clinically meaningful.
They also measured for secondary outcomes, such as quality of life, anxiety, depression, perceived stress, COVID-19–related stress, fatigue, somatic symptoms, self-compassion, and intention to practice yoga.
Among the 79 participants, 38 were randomized to the yoga program and 41 were randomized to the advice-only control group. The average age was 45 years. Most (92%) were women, and 81% were White. The average IBS duration since diagnosis was 11.5 years.
The overall average IBS-SSS was moderate, at 245.3, at the beginning of the program, and dropped to 207.9 at week 8. The score decreased from 255.2 to 200.5 in the yoga group and from 236.1 to 213.5 in the control group. The difference between the groups was 32 points, which wasn’t statistically significant, though symptom improvement began after 4 weeks in the yoga group.
In the yoga group, 14 participants (37%) met the target decrease of 50 points or more, compared with eight participants (20%) in the control group. These 22 “responders” reported improvements in IBS symptoms, quality of life, perceived stress, and COVID-19–related stress.
Specifically, among the 14 responders in the yoga group, there were significant improvements in IBS symptoms, quality of life, fatigue, somatic symptoms, self-compassion, and COVID-19–related stress. In the control group, there were significant improvements in IBS symptoms and COVID-19–related stress.
Using an intent-to-treat analysis, the research team found that the yoga group had improved quality of life, fatigue, and perceived stress. In the control group, improvements were seen only in COVID-19–related stress.
No significant improvements were found in anxiety or depression between the groups, although the changes in depression scores were in favor of the yoga group. The intention to practice yoga dropped in both groups during the study period, but it wasn’t associated with the actual yoga practice minutes or change in IBS-SSS scores.
“We saw a surprising improvement in quality of life,” Dr. Raman said. “Although we talk about quality of life as an important endpoint, it can be hard to show in studies, so that was a nice finding to demonstrate in this study.”
The yoga intervention was feasible in terms of adherence (79%), attrition rate (20%), and high program satisfaction, the researchers write. Safety was demonstrated by the absence of any adverse events.
Future program considerations
Dr. Raman and colleagues are interested in understanding the mechanisms that underlie the efficacy of mind-body interventions. They also plan to test the virtual yoga program in a mobile app, called LyfeMD, which is intended to support patients with digestive diseases through evidence-based dietary programs and mind-body interventions, such as guided meditation, breathing exercises, and cognitive behavioral therapy.
“We know that patients are looking for all possible resources,” Dr. Raman said. “Our next goal is to better understand how an app-based intervention can be effective, even without a live instructor.”
Future studies should also consider clinicians’ perspectives, she noted. In previous studies, Dr. Raman and colleagues have found that physicians are open to recommending yoga as a therapeutic option for patients, but some are unsure how to prescribe a recommended dose, frequency, or type of yoga.
“When treating patients with IBS, it is important to think broadly and creatively about all our treatment options,” said Elyse Thakur, PhD, a clinical health psychologist at Atrium Health Gastroenterology and Hepatology, Charlotte, N.C.
Dr. Thakur, who wasn’t involved with this study, specializes in gastrointestinal health psychology. She and colleagues use numerous complementary and alternative medicine options with patients.
“We have to remember that people may respond differently to available treatment options,” she said. “It is imperative to understand the evidence so we can have productive conversations with our patients about the pros and cons and the potential benefits and limitations.”
The study did not receive a specific grant from a funding agency. The authors and Dr. Thakur declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Participants reported a decrease in IBS-related symptoms and improvements in quality of life, fatigue, and perceived stress.
“IBS affects upwards of 15%-20% of the North American population, and despite our advances in the area, we have very limited options to offer our patients,” Maitreyi Raman, MD, an associate professor of medicine at the University of Calgary (Alta.), said in an interview.
“Often, we are focused on treating symptoms but not addressing the underlying cause,” said Dr. Raman, who is director of Alberta’s Collaboration of Excellence for Nutrition in Digestive Diseases. “With advances around the gut microbiome and the evolving science on the brain-gut axis, mind-body interventions could offer a therapeutic option that patients can use to improve the overall course of their disease.”
The study was published online in the American Journal of Gastroenterology.
Online yoga program vs. IBS advice only
IBS often involves alterations of the gut-brain axis and can be affected by psychological or physiological stress, the study authors write. Previous studies have found that in-person yoga programs can manage IBS symptoms and improve physiological, psychological, and emotional health.
During the COVID-19 pandemic, yoga programs had to switch to a virtual format – a delivery method that could remain relevant due to limited health care resources. However, the efficacy, feasibility, and safety of virtual yoga for people with IBS were unknown.
Dr. Raman and colleagues conducted a randomized, two-group, controlled clinical trial at the University of Calgary (Alta.) between March 2021 and December 2022. The 79 participants weren’t blinded to the trial arms – an online yoga program or an advice-only control group.
The eligible participants had a diagnosis of IBS, scored at least 75 out of 500 points on the IBS Symptoms Severity Scale (IBS-SSS) for mild IBS, and were on stable doses of medications for IBS. They were instructed to continue with their current therapies during the study but didn’t start new medications or make major changes to their diet or physical patterns.
The yoga program was based on Upa Yoga, a subtype of Hatha Yoga developed by the Isha Foundation of Inner Sciences. The program was delivered by a certified yoga facilitator from the Isha Foundation and included directional movements, neck rotations, breathing practices, breath watching, and mantra meditation with aum/om chanting.
The online classes of three to seven participants were delivered in 60-minute sessions for 8 weeks. The participants were also asked to practice at home daily with the support of yoga videos.
The advice-only control group included a 10-minute video with general education on IBS, the mind-gut connection in IBS, and the role of mind-body therapies in managing IBS. The participants received a list of IBS-related resources from the Canadian Digestive Health Foundation, a link to an IBS patient support group, and information about physical activity guidelines from the World Health Organization.
The research team looked for a primary endpoint of at least a 50-point reduction on the IBS-SSS, which is considered clinically meaningful.
They also measured for secondary outcomes, such as quality of life, anxiety, depression, perceived stress, COVID-19–related stress, fatigue, somatic symptoms, self-compassion, and intention to practice yoga.
Among the 79 participants, 38 were randomized to the yoga program and 41 were randomized to the advice-only control group. The average age was 45 years. Most (92%) were women, and 81% were White. The average IBS duration since diagnosis was 11.5 years.
The overall average IBS-SSS was moderate, at 245.3, at the beginning of the program, and dropped to 207.9 at week 8. The score decreased from 255.2 to 200.5 in the yoga group and from 236.1 to 213.5 in the control group. The difference between the groups was 32 points, which wasn’t statistically significant, though symptom improvement began after 4 weeks in the yoga group.
In the yoga group, 14 participants (37%) met the target decrease of 50 points or more, compared with eight participants (20%) in the control group. These 22 “responders” reported improvements in IBS symptoms, quality of life, perceived stress, and COVID-19–related stress.
Specifically, among the 14 responders in the yoga group, there were significant improvements in IBS symptoms, quality of life, fatigue, somatic symptoms, self-compassion, and COVID-19–related stress. In the control group, there were significant improvements in IBS symptoms and COVID-19–related stress.
Using an intent-to-treat analysis, the research team found that the yoga group had improved quality of life, fatigue, and perceived stress. In the control group, improvements were seen only in COVID-19–related stress.
No significant improvements were found in anxiety or depression between the groups, although the changes in depression scores were in favor of the yoga group. The intention to practice yoga dropped in both groups during the study period, but it wasn’t associated with the actual yoga practice minutes or change in IBS-SSS scores.
“We saw a surprising improvement in quality of life,” Dr. Raman said. “Although we talk about quality of life as an important endpoint, it can be hard to show in studies, so that was a nice finding to demonstrate in this study.”
The yoga intervention was feasible in terms of adherence (79%), attrition rate (20%), and high program satisfaction, the researchers write. Safety was demonstrated by the absence of any adverse events.
Future program considerations
Dr. Raman and colleagues are interested in understanding the mechanisms that underlie the efficacy of mind-body interventions. They also plan to test the virtual yoga program in a mobile app, called LyfeMD, which is intended to support patients with digestive diseases through evidence-based dietary programs and mind-body interventions, such as guided meditation, breathing exercises, and cognitive behavioral therapy.
“We know that patients are looking for all possible resources,” Dr. Raman said. “Our next goal is to better understand how an app-based intervention can be effective, even without a live instructor.”
Future studies should also consider clinicians’ perspectives, she noted. In previous studies, Dr. Raman and colleagues have found that physicians are open to recommending yoga as a therapeutic option for patients, but some are unsure how to prescribe a recommended dose, frequency, or type of yoga.
“When treating patients with IBS, it is important to think broadly and creatively about all our treatment options,” said Elyse Thakur, PhD, a clinical health psychologist at Atrium Health Gastroenterology and Hepatology, Charlotte, N.C.
Dr. Thakur, who wasn’t involved with this study, specializes in gastrointestinal health psychology. She and colleagues use numerous complementary and alternative medicine options with patients.
“We have to remember that people may respond differently to available treatment options,” she said. “It is imperative to understand the evidence so we can have productive conversations with our patients about the pros and cons and the potential benefits and limitations.”
The study did not receive a specific grant from a funding agency. The authors and Dr. Thakur declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Participants reported a decrease in IBS-related symptoms and improvements in quality of life, fatigue, and perceived stress.
“IBS affects upwards of 15%-20% of the North American population, and despite our advances in the area, we have very limited options to offer our patients,” Maitreyi Raman, MD, an associate professor of medicine at the University of Calgary (Alta.), said in an interview.
“Often, we are focused on treating symptoms but not addressing the underlying cause,” said Dr. Raman, who is director of Alberta’s Collaboration of Excellence for Nutrition in Digestive Diseases. “With advances around the gut microbiome and the evolving science on the brain-gut axis, mind-body interventions could offer a therapeutic option that patients can use to improve the overall course of their disease.”
The study was published online in the American Journal of Gastroenterology.
Online yoga program vs. IBS advice only
IBS often involves alterations of the gut-brain axis and can be affected by psychological or physiological stress, the study authors write. Previous studies have found that in-person yoga programs can manage IBS symptoms and improve physiological, psychological, and emotional health.
During the COVID-19 pandemic, yoga programs had to switch to a virtual format – a delivery method that could remain relevant due to limited health care resources. However, the efficacy, feasibility, and safety of virtual yoga for people with IBS were unknown.
Dr. Raman and colleagues conducted a randomized, two-group, controlled clinical trial at the University of Calgary (Alta.) between March 2021 and December 2022. The 79 participants weren’t blinded to the trial arms – an online yoga program or an advice-only control group.
The eligible participants had a diagnosis of IBS, scored at least 75 out of 500 points on the IBS Symptoms Severity Scale (IBS-SSS) for mild IBS, and were on stable doses of medications for IBS. They were instructed to continue with their current therapies during the study but didn’t start new medications or make major changes to their diet or physical patterns.
The yoga program was based on Upa Yoga, a subtype of Hatha Yoga developed by the Isha Foundation of Inner Sciences. The program was delivered by a certified yoga facilitator from the Isha Foundation and included directional movements, neck rotations, breathing practices, breath watching, and mantra meditation with aum/om chanting.
The online classes of three to seven participants were delivered in 60-minute sessions for 8 weeks. The participants were also asked to practice at home daily with the support of yoga videos.
The advice-only control group included a 10-minute video with general education on IBS, the mind-gut connection in IBS, and the role of mind-body therapies in managing IBS. The participants received a list of IBS-related resources from the Canadian Digestive Health Foundation, a link to an IBS patient support group, and information about physical activity guidelines from the World Health Organization.
The research team looked for a primary endpoint of at least a 50-point reduction on the IBS-SSS, which is considered clinically meaningful.
They also measured for secondary outcomes, such as quality of life, anxiety, depression, perceived stress, COVID-19–related stress, fatigue, somatic symptoms, self-compassion, and intention to practice yoga.
Among the 79 participants, 38 were randomized to the yoga program and 41 were randomized to the advice-only control group. The average age was 45 years. Most (92%) were women, and 81% were White. The average IBS duration since diagnosis was 11.5 years.
The overall average IBS-SSS was moderate, at 245.3, at the beginning of the program, and dropped to 207.9 at week 8. The score decreased from 255.2 to 200.5 in the yoga group and from 236.1 to 213.5 in the control group. The difference between the groups was 32 points, which wasn’t statistically significant, though symptom improvement began after 4 weeks in the yoga group.
In the yoga group, 14 participants (37%) met the target decrease of 50 points or more, compared with eight participants (20%) in the control group. These 22 “responders” reported improvements in IBS symptoms, quality of life, perceived stress, and COVID-19–related stress.
Specifically, among the 14 responders in the yoga group, there were significant improvements in IBS symptoms, quality of life, fatigue, somatic symptoms, self-compassion, and COVID-19–related stress. In the control group, there were significant improvements in IBS symptoms and COVID-19–related stress.
Using an intent-to-treat analysis, the research team found that the yoga group had improved quality of life, fatigue, and perceived stress. In the control group, improvements were seen only in COVID-19–related stress.
No significant improvements were found in anxiety or depression between the groups, although the changes in depression scores were in favor of the yoga group. The intention to practice yoga dropped in both groups during the study period, but it wasn’t associated with the actual yoga practice minutes or change in IBS-SSS scores.
“We saw a surprising improvement in quality of life,” Dr. Raman said. “Although we talk about quality of life as an important endpoint, it can be hard to show in studies, so that was a nice finding to demonstrate in this study.”
The yoga intervention was feasible in terms of adherence (79%), attrition rate (20%), and high program satisfaction, the researchers write. Safety was demonstrated by the absence of any adverse events.
Future program considerations
Dr. Raman and colleagues are interested in understanding the mechanisms that underlie the efficacy of mind-body interventions. They also plan to test the virtual yoga program in a mobile app, called LyfeMD, which is intended to support patients with digestive diseases through evidence-based dietary programs and mind-body interventions, such as guided meditation, breathing exercises, and cognitive behavioral therapy.
“We know that patients are looking for all possible resources,” Dr. Raman said. “Our next goal is to better understand how an app-based intervention can be effective, even without a live instructor.”
Future studies should also consider clinicians’ perspectives, she noted. In previous studies, Dr. Raman and colleagues have found that physicians are open to recommending yoga as a therapeutic option for patients, but some are unsure how to prescribe a recommended dose, frequency, or type of yoga.
“When treating patients with IBS, it is important to think broadly and creatively about all our treatment options,” said Elyse Thakur, PhD, a clinical health psychologist at Atrium Health Gastroenterology and Hepatology, Charlotte, N.C.
Dr. Thakur, who wasn’t involved with this study, specializes in gastrointestinal health psychology. She and colleagues use numerous complementary and alternative medicine options with patients.
“We have to remember that people may respond differently to available treatment options,” she said. “It is imperative to understand the evidence so we can have productive conversations with our patients about the pros and cons and the potential benefits and limitations.”
The study did not receive a specific grant from a funding agency. The authors and Dr. Thakur declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Transplant provides no clear survival benefit in real-world MCL study
In younger patients with mantle cell lymphoma treated in U.S. community oncology settings in recent years, use of autologous transplant was not associated with improved survival, results of a large observational study show.
Autologous stem-cell transplant (ASCT) use was not linked overall survival (OS), according to the authors of the retrospective analysis of patients diagnosed with mantle cell lymphoma (MCL) between 2011 and 2021.
This lack of a clear survival benefit with use of ASCT is an “apparent contradiction” with prospective data from earlier clinical trials, authors wrote in the Journal of Clinical Oncology
However, they added, the finding is consistent with several recent registry analyses that also do not support a link between ASCT and overall survival in patients with MCL.
Although these findings are limited by the retrospective nature of the study, the results at least suggest that it is ethical to do research that doesn’t involve ASCT, study author Peter Martin, MD, said in an interview.
Furthermore, emerging data from the randomized TRIANGLE study from the European MCL Network suggest the potential for ASCT to be replaced by maintenance therapy with the Bruton's tyrosine kinase inhibitor ibrutinib, according to Dr. Martin, associate professor with Weill Cornell Medicine, New York.
“There are probably a lot of questions that will come up there, but essentially the barriers to research that do not include ASCT have been moved away, and we can go ahead and study non-ASCT (approaches) in younger patients,” Dr. Martin said.
No clear OS benefit
In current guidelines, recommended initial therapy for MCL patients younger than 65 years includes use of high-dose cytarabine-containing chemoimmunotherapy induction, followed by ASCT as consolidation, and then rituximab maintenance, Dr. Martin and coauthors say in their report.
Their primary analysis was based on the Flatiron Health database, which is derived from electronic medical records, mostly in U.S. community oncology practices, according to the report.
The researchers identified 1,274 patients under the age of 65 with a record of first-line treatment for MCL, and of those, 962 (or 76%) were considered eligible for ASCT.
Among ASCT-eligible patients, there was no significant association between receipt of ASCT and OS, with a hazard ratio of 0.86 (95% confidence interval, 0.63-1.18). The 3-year OS was 88% for patients receiving ASCT and similarly, 84% for those who did not, according to authors.
Likewise, there was no association between ASCT and real-world time to next treatment, an endpoint defined as time from start of first-line therapy to subsequent treatment or death, the report says.
Findings in perspective
The lack of clear survival benefit with ASCT in this and other recent observational studies may be explained in part by improvements in induction regimens, according to Timothy Fenske, MD, professor in the department of medicine at the Medical College of Wisconsin, Milwaukee.
“As our induction regimens have improved, it is very possible that the benefit for autologous transplantation will become less apparent,” Dr. Fenske said in an interview.
The discussion over ASCT in MCL is expected to evolve further in light of findings from TRIANGLE and EA4151, a randomized phase 3 trial of rituximab with or without ASCT specifically in patients with minimal residual disease (MRD)–negative MCL in first complete remission.
“If that study shows that the MRD-negative patients do not have much benefit from autologous transplantation,” Dr. Fenske said, “I think these studies will all be giving the same message – that autologous transplantation was beneficial back when induction regimens were poor (for example, CHOP without rituximab), but will have much less benefit in patients receiving modern inductions, which by and large will get more patients to be MRD negative.”
However, subgroup analyses of those TRIANGLE will be important, he added, since some patients may still benefit from ASCT, such as younger patients who remain MRD positive, or who have certain other high-risk molecular features.
Dr. Martin reported consulting or advisory roles with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda. He reported institutional research funding from Karyopharm Therapeutics.
In younger patients with mantle cell lymphoma treated in U.S. community oncology settings in recent years, use of autologous transplant was not associated with improved survival, results of a large observational study show.
Autologous stem-cell transplant (ASCT) use was not linked overall survival (OS), according to the authors of the retrospective analysis of patients diagnosed with mantle cell lymphoma (MCL) between 2011 and 2021.
This lack of a clear survival benefit with use of ASCT is an “apparent contradiction” with prospective data from earlier clinical trials, authors wrote in the Journal of Clinical Oncology
However, they added, the finding is consistent with several recent registry analyses that also do not support a link between ASCT and overall survival in patients with MCL.
Although these findings are limited by the retrospective nature of the study, the results at least suggest that it is ethical to do research that doesn’t involve ASCT, study author Peter Martin, MD, said in an interview.
Furthermore, emerging data from the randomized TRIANGLE study from the European MCL Network suggest the potential for ASCT to be replaced by maintenance therapy with the Bruton's tyrosine kinase inhibitor ibrutinib, according to Dr. Martin, associate professor with Weill Cornell Medicine, New York.
“There are probably a lot of questions that will come up there, but essentially the barriers to research that do not include ASCT have been moved away, and we can go ahead and study non-ASCT (approaches) in younger patients,” Dr. Martin said.
No clear OS benefit
In current guidelines, recommended initial therapy for MCL patients younger than 65 years includes use of high-dose cytarabine-containing chemoimmunotherapy induction, followed by ASCT as consolidation, and then rituximab maintenance, Dr. Martin and coauthors say in their report.
Their primary analysis was based on the Flatiron Health database, which is derived from electronic medical records, mostly in U.S. community oncology practices, according to the report.
The researchers identified 1,274 patients under the age of 65 with a record of first-line treatment for MCL, and of those, 962 (or 76%) were considered eligible for ASCT.
Among ASCT-eligible patients, there was no significant association between receipt of ASCT and OS, with a hazard ratio of 0.86 (95% confidence interval, 0.63-1.18). The 3-year OS was 88% for patients receiving ASCT and similarly, 84% for those who did not, according to authors.
Likewise, there was no association between ASCT and real-world time to next treatment, an endpoint defined as time from start of first-line therapy to subsequent treatment or death, the report says.
Findings in perspective
The lack of clear survival benefit with ASCT in this and other recent observational studies may be explained in part by improvements in induction regimens, according to Timothy Fenske, MD, professor in the department of medicine at the Medical College of Wisconsin, Milwaukee.
“As our induction regimens have improved, it is very possible that the benefit for autologous transplantation will become less apparent,” Dr. Fenske said in an interview.
The discussion over ASCT in MCL is expected to evolve further in light of findings from TRIANGLE and EA4151, a randomized phase 3 trial of rituximab with or without ASCT specifically in patients with minimal residual disease (MRD)–negative MCL in first complete remission.
“If that study shows that the MRD-negative patients do not have much benefit from autologous transplantation,” Dr. Fenske said, “I think these studies will all be giving the same message – that autologous transplantation was beneficial back when induction regimens were poor (for example, CHOP without rituximab), but will have much less benefit in patients receiving modern inductions, which by and large will get more patients to be MRD negative.”
However, subgroup analyses of those TRIANGLE will be important, he added, since some patients may still benefit from ASCT, such as younger patients who remain MRD positive, or who have certain other high-risk molecular features.
Dr. Martin reported consulting or advisory roles with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda. He reported institutional research funding from Karyopharm Therapeutics.
In younger patients with mantle cell lymphoma treated in U.S. community oncology settings in recent years, use of autologous transplant was not associated with improved survival, results of a large observational study show.
Autologous stem-cell transplant (ASCT) use was not linked overall survival (OS), according to the authors of the retrospective analysis of patients diagnosed with mantle cell lymphoma (MCL) between 2011 and 2021.
This lack of a clear survival benefit with use of ASCT is an “apparent contradiction” with prospective data from earlier clinical trials, authors wrote in the Journal of Clinical Oncology
However, they added, the finding is consistent with several recent registry analyses that also do not support a link between ASCT and overall survival in patients with MCL.
Although these findings are limited by the retrospective nature of the study, the results at least suggest that it is ethical to do research that doesn’t involve ASCT, study author Peter Martin, MD, said in an interview.
Furthermore, emerging data from the randomized TRIANGLE study from the European MCL Network suggest the potential for ASCT to be replaced by maintenance therapy with the Bruton's tyrosine kinase inhibitor ibrutinib, according to Dr. Martin, associate professor with Weill Cornell Medicine, New York.
“There are probably a lot of questions that will come up there, but essentially the barriers to research that do not include ASCT have been moved away, and we can go ahead and study non-ASCT (approaches) in younger patients,” Dr. Martin said.
No clear OS benefit
In current guidelines, recommended initial therapy for MCL patients younger than 65 years includes use of high-dose cytarabine-containing chemoimmunotherapy induction, followed by ASCT as consolidation, and then rituximab maintenance, Dr. Martin and coauthors say in their report.
Their primary analysis was based on the Flatiron Health database, which is derived from electronic medical records, mostly in U.S. community oncology practices, according to the report.
The researchers identified 1,274 patients under the age of 65 with a record of first-line treatment for MCL, and of those, 962 (or 76%) were considered eligible for ASCT.
Among ASCT-eligible patients, there was no significant association between receipt of ASCT and OS, with a hazard ratio of 0.86 (95% confidence interval, 0.63-1.18). The 3-year OS was 88% for patients receiving ASCT and similarly, 84% for those who did not, according to authors.
Likewise, there was no association between ASCT and real-world time to next treatment, an endpoint defined as time from start of first-line therapy to subsequent treatment or death, the report says.
Findings in perspective
The lack of clear survival benefit with ASCT in this and other recent observational studies may be explained in part by improvements in induction regimens, according to Timothy Fenske, MD, professor in the department of medicine at the Medical College of Wisconsin, Milwaukee.
“As our induction regimens have improved, it is very possible that the benefit for autologous transplantation will become less apparent,” Dr. Fenske said in an interview.
The discussion over ASCT in MCL is expected to evolve further in light of findings from TRIANGLE and EA4151, a randomized phase 3 trial of rituximab with or without ASCT specifically in patients with minimal residual disease (MRD)–negative MCL in first complete remission.
“If that study shows that the MRD-negative patients do not have much benefit from autologous transplantation,” Dr. Fenske said, “I think these studies will all be giving the same message – that autologous transplantation was beneficial back when induction regimens were poor (for example, CHOP without rituximab), but will have much less benefit in patients receiving modern inductions, which by and large will get more patients to be MRD negative.”
However, subgroup analyses of those TRIANGLE will be important, he added, since some patients may still benefit from ASCT, such as younger patients who remain MRD positive, or who have certain other high-risk molecular features.
Dr. Martin reported consulting or advisory roles with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda. He reported institutional research funding from Karyopharm Therapeutics.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
FDA approves olutasidenib for some AML patients
Specifically, the drug is approved for use in patients who have R/R AML with a susceptible isocitrate dehydrogenase 1 (IDH1) mutation as detected by an FDA-approved test.
The FDA also approved the Abbott RealTime IDH1 Assay to select patients for treatment.
Olutasidenib is an oral inhibitor of mutated IDH1 that has been designed to bind and inhibit mutated IDH1 to reduce hydroxyglutarate levels and restore cellular differentiation of myeloid cells, says the manufacturer, Rigel.
About half of all patients with AML have relapse after treatment and remission, and about 10%-40% have refractory cases and do not achieve remission even after intensive treatment, the company noted.
“Given the limited treatment options for adult patients with mIDH1 R/R AML, who typically have a poor prognosis, olutasidenib may provide an effective new treatment option with a well-characterized safety profile,” Jorges Cortes, MD, director of the Georgia Cancer Center, Augusta, commented in the company press release. He was an investigator on the phase 2 trial that led to the drug’s approval.
This was Study 2102-HEM-101 (NCT02719574), an open-label, single-arm, multicenter clinical trial that included 147 adult patients with relapsed or refractory AML with an IDH1 mutation confirmed using the Abbott assay.
Olutasidenib was given orally at 150 mg twice daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplantation (performed in 16 patients [11%]). The median treatment duration was 4.7 months (range, 0.1-26 months).
The FDA noted that efficacy was established on the rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to independence.
The CR+CRh rate was 35% (95% confidence interval, 27%-43%), including 32% CR and 2.7% CRh. The median time to CR+CRh was 1.9 months (range, 0.9-5.6 months), and the median duration of CR+CRh was 25.9 months (95% CI, 13.5 months to not reached).
Commenting on these results in the company statement, Dr. Cortes noted that among the patients who responded, more than 90% were experiencing incomplete remission. He added that the “25.9 months median duration of CR+CRh is a clinically meaningful improvement for AML patients and appears to be longer than currently available treatment options.”
The FDA also noted that among the 86 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 29 (34%) became independent of RBC and platelet transfusions during any 56-day postbaseline period.
Of the 61 patients who were independent of both RBC and platelet transfusions at baseline, 39 (64%) remained transfusion-independent during any 56-day post-baseline period.
The most common adverse reactions (≥ 20%) were nausea, fatigue/malaise, arthralgia, constipation, leukocytosis, dyspnea, fever, rash, mucositis, diarrhea, and transaminitis.
The prescribing information contains a boxed warning about the risk for differentiation syndrome, which can be fatal.
Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal, the company explained. Symptoms may include leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain.
In the trial, differentiation syndrome was observed in 16% of patients, with grade 3 or 4 occurring in 8% of patients treated and death in 1% of patients. It occurred as early as 1 day and up to 18 months after starting treatment.
In most cases, differentiation syndrome was manageable with dose interruption and corticosteroids, the company said. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption.
Further details are available in the full prescribing information.
A version of this article first appeared on Medscape.com.
Specifically, the drug is approved for use in patients who have R/R AML with a susceptible isocitrate dehydrogenase 1 (IDH1) mutation as detected by an FDA-approved test.
The FDA also approved the Abbott RealTime IDH1 Assay to select patients for treatment.
Olutasidenib is an oral inhibitor of mutated IDH1 that has been designed to bind and inhibit mutated IDH1 to reduce hydroxyglutarate levels and restore cellular differentiation of myeloid cells, says the manufacturer, Rigel.
About half of all patients with AML have relapse after treatment and remission, and about 10%-40% have refractory cases and do not achieve remission even after intensive treatment, the company noted.
“Given the limited treatment options for adult patients with mIDH1 R/R AML, who typically have a poor prognosis, olutasidenib may provide an effective new treatment option with a well-characterized safety profile,” Jorges Cortes, MD, director of the Georgia Cancer Center, Augusta, commented in the company press release. He was an investigator on the phase 2 trial that led to the drug’s approval.
This was Study 2102-HEM-101 (NCT02719574), an open-label, single-arm, multicenter clinical trial that included 147 adult patients with relapsed or refractory AML with an IDH1 mutation confirmed using the Abbott assay.
Olutasidenib was given orally at 150 mg twice daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplantation (performed in 16 patients [11%]). The median treatment duration was 4.7 months (range, 0.1-26 months).
The FDA noted that efficacy was established on the rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to independence.
The CR+CRh rate was 35% (95% confidence interval, 27%-43%), including 32% CR and 2.7% CRh. The median time to CR+CRh was 1.9 months (range, 0.9-5.6 months), and the median duration of CR+CRh was 25.9 months (95% CI, 13.5 months to not reached).
Commenting on these results in the company statement, Dr. Cortes noted that among the patients who responded, more than 90% were experiencing incomplete remission. He added that the “25.9 months median duration of CR+CRh is a clinically meaningful improvement for AML patients and appears to be longer than currently available treatment options.”
The FDA also noted that among the 86 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 29 (34%) became independent of RBC and platelet transfusions during any 56-day postbaseline period.
Of the 61 patients who were independent of both RBC and platelet transfusions at baseline, 39 (64%) remained transfusion-independent during any 56-day post-baseline period.
The most common adverse reactions (≥ 20%) were nausea, fatigue/malaise, arthralgia, constipation, leukocytosis, dyspnea, fever, rash, mucositis, diarrhea, and transaminitis.
The prescribing information contains a boxed warning about the risk for differentiation syndrome, which can be fatal.
Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal, the company explained. Symptoms may include leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain.
In the trial, differentiation syndrome was observed in 16% of patients, with grade 3 or 4 occurring in 8% of patients treated and death in 1% of patients. It occurred as early as 1 day and up to 18 months after starting treatment.
In most cases, differentiation syndrome was manageable with dose interruption and corticosteroids, the company said. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption.
Further details are available in the full prescribing information.
A version of this article first appeared on Medscape.com.
Specifically, the drug is approved for use in patients who have R/R AML with a susceptible isocitrate dehydrogenase 1 (IDH1) mutation as detected by an FDA-approved test.
The FDA also approved the Abbott RealTime IDH1 Assay to select patients for treatment.
Olutasidenib is an oral inhibitor of mutated IDH1 that has been designed to bind and inhibit mutated IDH1 to reduce hydroxyglutarate levels and restore cellular differentiation of myeloid cells, says the manufacturer, Rigel.
About half of all patients with AML have relapse after treatment and remission, and about 10%-40% have refractory cases and do not achieve remission even after intensive treatment, the company noted.
“Given the limited treatment options for adult patients with mIDH1 R/R AML, who typically have a poor prognosis, olutasidenib may provide an effective new treatment option with a well-characterized safety profile,” Jorges Cortes, MD, director of the Georgia Cancer Center, Augusta, commented in the company press release. He was an investigator on the phase 2 trial that led to the drug’s approval.
This was Study 2102-HEM-101 (NCT02719574), an open-label, single-arm, multicenter clinical trial that included 147 adult patients with relapsed or refractory AML with an IDH1 mutation confirmed using the Abbott assay.
Olutasidenib was given orally at 150 mg twice daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplantation (performed in 16 patients [11%]). The median treatment duration was 4.7 months (range, 0.1-26 months).
The FDA noted that efficacy was established on the rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to independence.
The CR+CRh rate was 35% (95% confidence interval, 27%-43%), including 32% CR and 2.7% CRh. The median time to CR+CRh was 1.9 months (range, 0.9-5.6 months), and the median duration of CR+CRh was 25.9 months (95% CI, 13.5 months to not reached).
Commenting on these results in the company statement, Dr. Cortes noted that among the patients who responded, more than 90% were experiencing incomplete remission. He added that the “25.9 months median duration of CR+CRh is a clinically meaningful improvement for AML patients and appears to be longer than currently available treatment options.”
The FDA also noted that among the 86 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 29 (34%) became independent of RBC and platelet transfusions during any 56-day postbaseline period.
Of the 61 patients who were independent of both RBC and platelet transfusions at baseline, 39 (64%) remained transfusion-independent during any 56-day post-baseline period.
The most common adverse reactions (≥ 20%) were nausea, fatigue/malaise, arthralgia, constipation, leukocytosis, dyspnea, fever, rash, mucositis, diarrhea, and transaminitis.
The prescribing information contains a boxed warning about the risk for differentiation syndrome, which can be fatal.
Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal, the company explained. Symptoms may include leukocytosis, dyspnea, pulmonary infiltrates/pleuropericardial effusion, kidney injury, hypotension, fever, and weight gain.
In the trial, differentiation syndrome was observed in 16% of patients, with grade 3 or 4 occurring in 8% of patients treated and death in 1% of patients. It occurred as early as 1 day and up to 18 months after starting treatment.
In most cases, differentiation syndrome was manageable with dose interruption and corticosteroids, the company said. Of the 25 patients who experienced differentiation syndrome, 19 (76%) recovered after treatment or after dose interruption.
Further details are available in the full prescribing information.
A version of this article first appeared on Medscape.com.
Commentary: Risk factors and treatment for pediatric migraine, December 2022
This month, we will take a look at three new studies investigating risk factors and treatments for headache in children.
Stress has long been noted to be one of the most consistent triggers for migraine attacks. Much has been written and studied regarding the effect of migraine on mood in adults; however, few studies have done the same in the pediatric and adolescent population. Childhood trauma has been associated with the development of chronic migraine as an adult, and behavioral treatments, such as cognitive-behavioral therapy and biofeedback, are considered as effective or more effective for migraine prevention in children compared with preventive medications. Falla and colleagues have quantified the risk for anxiety and depression in children and adolescents with migraine.
The "internalization of symptoms" is defined by the authors as an individual's tendency to react to stress with physical symptoms, including anxiety and depression. These are thought to be elevated in children and adolescents with many effects, including migraine. However, no correlation has yet been shown. Beyond the internalization of symptoms, specific psychiatric diagnoses may also be more prominent in this population.
This study was a meta-analysis of data pooled from studies that assessed migraine-related symptoms as they relate to disorders on the spectrum of anxiety, depression, and trauma-related disorders. Any studies with participants older than 18 years were excluded from this analysis. A total of 80 studies were included. Anxiety symptoms were seen to be significantly higher in children and adolescents with migraine compared to controls and the odds of having an anxiety disorder were higher among those with migraine compared with controls. Depressive symptoms were also significantly higher; however, this effect size was much smaller. The incidence of migraine was not different than that of other headache disorders.
Many patients describe stress as a trigger for migraine and other headache attacks. Mood disorders and childhood trauma are associated with the development of chronic migraine as an adult. This study reveals a two-way connection between mood disorders and headache diagnoses in children. Screening for the underlying symptoms of depression and anxiety should be done when evaluating children and adolescents for headache disorders, and there should be a focus on the treatment of these conditions in addition to treating the headache symptoms.
There are, unfortunately, very few acute pediatric migraine trials. Only a handful of medications have actually been investigated for the treatment of migraine in children younger than 12 years, and only one migraine-specific medication, rizatriptan, is approved in the United States for pediatric use. Because of this, many argue that children and adolescents with migraines end up overusing over-the-counter medication options, increasing the risk for medication overuse headache. In addition, many patients need nonoral acute migraine treatments due to nausea and vomiting or rapid onset migraine attacks.
Yonker and colleagues conducted a phase 3, randomized, double-blind, multicenter trial that only enrolled patients aged 6-11 years, all of whom had a diagnosis of episodic migraine (< 15 days of migraine per month). Children that weighed < 50 kg were given a randomly assigned lower dose of 1 mg or 2.5 mg zolmitriptan nasal spray, and those who weighed > 50 kg were randomly assigned to either 2.5 mg or 5 mg, which is the standard adult dose.
The primary outcome was a standard 2-hour pain freedom level; secondary outcomes included the proportion of improvement at 0.5, 1, and 24 hours post-dose, as well as sustained headache response for the following 2-24 hours and time to rescue medication use. Although 300 patients were enrolled and taken through the run-in process, 114 were discontinued either due to placebo response or because they had no treated migraine during the run-in phase. The mean age was 9.2 years and half of the patients were girls (of note, this is considered an appropriate proportion for pediatric migraine).
The primary endpoint of 2-hour pain freedom was not met; however, more patients in the high-dose treatment group were pain-free after 2 hours than in the placebo group. Several secondary endpoints did achieve statistical significance, including pain-free status at 1 hour post-dose, as well as headache response at 0.5, 1, and 2 hours — all of which were lower in the high-dose treatment group. The lower-dose treatment group was statistically similar to placebo for all the timepoints noted above. Treatment-related adverse events were rare in all groups.
This study did not meet its primary efficacy endpoint. However, it does show safety and effectiveness, enough at least to broaden the use of zolmitriptan for pediatric migraine. Many more effective medications for migraine treatment in adults should follow the lead of this group to find better and more specific treatments for children with migraine.
As we noted above, childhood trauma is associated with the development of chronic migraine in adulthood. Prior studies have defined childhood trauma as physical or emotional abuse primarily, and the correlation between childhood illnesses and headache disorder has not previously been determined. Davidsson and colleagues published a study in the Journal of Cancer Epidemiology that reviewed nationwide database registers longitudinally to better understand this potential risk factor.
The Danish Cancer Register was reviewed for the purpose of identifying anyone in the general Danish population who developed a diagnosis of cancer before age 20 years. The individual identification numbers in that register were linked to data in other nationwide registers for the purpose of determining whether those individuals initiated migraine-specific medications or were admitted for an inpatient hospitalization for migraine. Study participants were also grouped based on cancer type, specifically hematologic cancers involving chemotherapy and central nervous system-directed therapies, brain tumors needing intracranial surgery or radiation to the brain, blastomas or other solid tumors outside of the central nervous system, sarcomas treated with an alkylating chemotherapy, and all other carcinomas.
Among all individuals diagnosed with a childhood cancer, there was a significant increase in overall risk for the need to initiate antimigraine medication. Of interest, this was higher in those diagnosed in their teenage years. Migraine hospitalization was also noted to be higher in nearly all strata, with the exception of those diagnosed with cancer prior to the age of 5 years. The highest risk was also noted in individuals with hematologic cancers, blastomas, and brain tumors as opposed to those with sarcomas and other carcinomas. The highest cumulative risk for migraine remains in those who were diagnosed with cancer between ages 15 and 19 years.
This month, we will take a look at three new studies investigating risk factors and treatments for headache in children.
Stress has long been noted to be one of the most consistent triggers for migraine attacks. Much has been written and studied regarding the effect of migraine on mood in adults; however, few studies have done the same in the pediatric and adolescent population. Childhood trauma has been associated with the development of chronic migraine as an adult, and behavioral treatments, such as cognitive-behavioral therapy and biofeedback, are considered as effective or more effective for migraine prevention in children compared with preventive medications. Falla and colleagues have quantified the risk for anxiety and depression in children and adolescents with migraine.
The "internalization of symptoms" is defined by the authors as an individual's tendency to react to stress with physical symptoms, including anxiety and depression. These are thought to be elevated in children and adolescents with many effects, including migraine. However, no correlation has yet been shown. Beyond the internalization of symptoms, specific psychiatric diagnoses may also be more prominent in this population.
This study was a meta-analysis of data pooled from studies that assessed migraine-related symptoms as they relate to disorders on the spectrum of anxiety, depression, and trauma-related disorders. Any studies with participants older than 18 years were excluded from this analysis. A total of 80 studies were included. Anxiety symptoms were seen to be significantly higher in children and adolescents with migraine compared to controls and the odds of having an anxiety disorder were higher among those with migraine compared with controls. Depressive symptoms were also significantly higher; however, this effect size was much smaller. The incidence of migraine was not different than that of other headache disorders.
Many patients describe stress as a trigger for migraine and other headache attacks. Mood disorders and childhood trauma are associated with the development of chronic migraine as an adult. This study reveals a two-way connection between mood disorders and headache diagnoses in children. Screening for the underlying symptoms of depression and anxiety should be done when evaluating children and adolescents for headache disorders, and there should be a focus on the treatment of these conditions in addition to treating the headache symptoms.
There are, unfortunately, very few acute pediatric migraine trials. Only a handful of medications have actually been investigated for the treatment of migraine in children younger than 12 years, and only one migraine-specific medication, rizatriptan, is approved in the United States for pediatric use. Because of this, many argue that children and adolescents with migraines end up overusing over-the-counter medication options, increasing the risk for medication overuse headache. In addition, many patients need nonoral acute migraine treatments due to nausea and vomiting or rapid onset migraine attacks.
Yonker and colleagues conducted a phase 3, randomized, double-blind, multicenter trial that only enrolled patients aged 6-11 years, all of whom had a diagnosis of episodic migraine (< 15 days of migraine per month). Children that weighed < 50 kg were given a randomly assigned lower dose of 1 mg or 2.5 mg zolmitriptan nasal spray, and those who weighed > 50 kg were randomly assigned to either 2.5 mg or 5 mg, which is the standard adult dose.
The primary outcome was a standard 2-hour pain freedom level; secondary outcomes included the proportion of improvement at 0.5, 1, and 24 hours post-dose, as well as sustained headache response for the following 2-24 hours and time to rescue medication use. Although 300 patients were enrolled and taken through the run-in process, 114 were discontinued either due to placebo response or because they had no treated migraine during the run-in phase. The mean age was 9.2 years and half of the patients were girls (of note, this is considered an appropriate proportion for pediatric migraine).
The primary endpoint of 2-hour pain freedom was not met; however, more patients in the high-dose treatment group were pain-free after 2 hours than in the placebo group. Several secondary endpoints did achieve statistical significance, including pain-free status at 1 hour post-dose, as well as headache response at 0.5, 1, and 2 hours — all of which were lower in the high-dose treatment group. The lower-dose treatment group was statistically similar to placebo for all the timepoints noted above. Treatment-related adverse events were rare in all groups.
This study did not meet its primary efficacy endpoint. However, it does show safety and effectiveness, enough at least to broaden the use of zolmitriptan for pediatric migraine. Many more effective medications for migraine treatment in adults should follow the lead of this group to find better and more specific treatments for children with migraine.
As we noted above, childhood trauma is associated with the development of chronic migraine in adulthood. Prior studies have defined childhood trauma as physical or emotional abuse primarily, and the correlation between childhood illnesses and headache disorder has not previously been determined. Davidsson and colleagues published a study in the Journal of Cancer Epidemiology that reviewed nationwide database registers longitudinally to better understand this potential risk factor.
The Danish Cancer Register was reviewed for the purpose of identifying anyone in the general Danish population who developed a diagnosis of cancer before age 20 years. The individual identification numbers in that register were linked to data in other nationwide registers for the purpose of determining whether those individuals initiated migraine-specific medications or were admitted for an inpatient hospitalization for migraine. Study participants were also grouped based on cancer type, specifically hematologic cancers involving chemotherapy and central nervous system-directed therapies, brain tumors needing intracranial surgery or radiation to the brain, blastomas or other solid tumors outside of the central nervous system, sarcomas treated with an alkylating chemotherapy, and all other carcinomas.
Among all individuals diagnosed with a childhood cancer, there was a significant increase in overall risk for the need to initiate antimigraine medication. Of interest, this was higher in those diagnosed in their teenage years. Migraine hospitalization was also noted to be higher in nearly all strata, with the exception of those diagnosed with cancer prior to the age of 5 years. The highest risk was also noted in individuals with hematologic cancers, blastomas, and brain tumors as opposed to those with sarcomas and other carcinomas. The highest cumulative risk for migraine remains in those who were diagnosed with cancer between ages 15 and 19 years.
This month, we will take a look at three new studies investigating risk factors and treatments for headache in children.
Stress has long been noted to be one of the most consistent triggers for migraine attacks. Much has been written and studied regarding the effect of migraine on mood in adults; however, few studies have done the same in the pediatric and adolescent population. Childhood trauma has been associated with the development of chronic migraine as an adult, and behavioral treatments, such as cognitive-behavioral therapy and biofeedback, are considered as effective or more effective for migraine prevention in children compared with preventive medications. Falla and colleagues have quantified the risk for anxiety and depression in children and adolescents with migraine.
The "internalization of symptoms" is defined by the authors as an individual's tendency to react to stress with physical symptoms, including anxiety and depression. These are thought to be elevated in children and adolescents with many effects, including migraine. However, no correlation has yet been shown. Beyond the internalization of symptoms, specific psychiatric diagnoses may also be more prominent in this population.
This study was a meta-analysis of data pooled from studies that assessed migraine-related symptoms as they relate to disorders on the spectrum of anxiety, depression, and trauma-related disorders. Any studies with participants older than 18 years were excluded from this analysis. A total of 80 studies were included. Anxiety symptoms were seen to be significantly higher in children and adolescents with migraine compared to controls and the odds of having an anxiety disorder were higher among those with migraine compared with controls. Depressive symptoms were also significantly higher; however, this effect size was much smaller. The incidence of migraine was not different than that of other headache disorders.
Many patients describe stress as a trigger for migraine and other headache attacks. Mood disorders and childhood trauma are associated with the development of chronic migraine as an adult. This study reveals a two-way connection between mood disorders and headache diagnoses in children. Screening for the underlying symptoms of depression and anxiety should be done when evaluating children and adolescents for headache disorders, and there should be a focus on the treatment of these conditions in addition to treating the headache symptoms.
There are, unfortunately, very few acute pediatric migraine trials. Only a handful of medications have actually been investigated for the treatment of migraine in children younger than 12 years, and only one migraine-specific medication, rizatriptan, is approved in the United States for pediatric use. Because of this, many argue that children and adolescents with migraines end up overusing over-the-counter medication options, increasing the risk for medication overuse headache. In addition, many patients need nonoral acute migraine treatments due to nausea and vomiting or rapid onset migraine attacks.
Yonker and colleagues conducted a phase 3, randomized, double-blind, multicenter trial that only enrolled patients aged 6-11 years, all of whom had a diagnosis of episodic migraine (< 15 days of migraine per month). Children that weighed < 50 kg were given a randomly assigned lower dose of 1 mg or 2.5 mg zolmitriptan nasal spray, and those who weighed > 50 kg were randomly assigned to either 2.5 mg or 5 mg, which is the standard adult dose.
The primary outcome was a standard 2-hour pain freedom level; secondary outcomes included the proportion of improvement at 0.5, 1, and 24 hours post-dose, as well as sustained headache response for the following 2-24 hours and time to rescue medication use. Although 300 patients were enrolled and taken through the run-in process, 114 were discontinued either due to placebo response or because they had no treated migraine during the run-in phase. The mean age was 9.2 years and half of the patients were girls (of note, this is considered an appropriate proportion for pediatric migraine).
The primary endpoint of 2-hour pain freedom was not met; however, more patients in the high-dose treatment group were pain-free after 2 hours than in the placebo group. Several secondary endpoints did achieve statistical significance, including pain-free status at 1 hour post-dose, as well as headache response at 0.5, 1, and 2 hours — all of which were lower in the high-dose treatment group. The lower-dose treatment group was statistically similar to placebo for all the timepoints noted above. Treatment-related adverse events were rare in all groups.
This study did not meet its primary efficacy endpoint. However, it does show safety and effectiveness, enough at least to broaden the use of zolmitriptan for pediatric migraine. Many more effective medications for migraine treatment in adults should follow the lead of this group to find better and more specific treatments for children with migraine.
As we noted above, childhood trauma is associated with the development of chronic migraine in adulthood. Prior studies have defined childhood trauma as physical or emotional abuse primarily, and the correlation between childhood illnesses and headache disorder has not previously been determined. Davidsson and colleagues published a study in the Journal of Cancer Epidemiology that reviewed nationwide database registers longitudinally to better understand this potential risk factor.
The Danish Cancer Register was reviewed for the purpose of identifying anyone in the general Danish population who developed a diagnosis of cancer before age 20 years. The individual identification numbers in that register were linked to data in other nationwide registers for the purpose of determining whether those individuals initiated migraine-specific medications or were admitted for an inpatient hospitalization for migraine. Study participants were also grouped based on cancer type, specifically hematologic cancers involving chemotherapy and central nervous system-directed therapies, brain tumors needing intracranial surgery or radiation to the brain, blastomas or other solid tumors outside of the central nervous system, sarcomas treated with an alkylating chemotherapy, and all other carcinomas.
Among all individuals diagnosed with a childhood cancer, there was a significant increase in overall risk for the need to initiate antimigraine medication. Of interest, this was higher in those diagnosed in their teenage years. Migraine hospitalization was also noted to be higher in nearly all strata, with the exception of those diagnosed with cancer prior to the age of 5 years. The highest risk was also noted in individuals with hematologic cancers, blastomas, and brain tumors as opposed to those with sarcomas and other carcinomas. The highest cumulative risk for migraine remains in those who were diagnosed with cancer between ages 15 and 19 years.
Tips and tricks for a successful rollerball endometrial ablation
How can clinicians distinguish food allergy and GERD in young kids?
The debate about a possible link between food allergy (FA) and pediatric gastroesophageal reflux disease (GERD) continues, and more, better-designed research is needed, a position paper by the European Academy of Allergy and Clinical Immunology reports.
The report offers consensus-based recommendations and a graphical decision pathway to guide providers through assessing and treating food allergy–related GERD. And the authors call for further, better-designed related research.
Food allergy and GERD are common in babies under 1 year of age and can lead to bothersome GERD, the authors write.
“An extensive literature search has found that whilst food proteins, in particular cow milk protein, can be a contributing factor to FA-associated” GERD, distinguishing between FA and non–FA-associated GERD is difficult, lead author Rosan Meyer, RD, PhD, senior lecturer at Imperial College London, and colleagues from the Academy task force on non-IgE mediated allergy, write in Pediatric Allergy and Immunology.
Consensus despite limited data
Dr. Meyer and colleagues developed clinical questions that addressed various aspects of the relationship between food allergy and GERD – pathophysiology, symptoms, diagnosis, dietary and medical management, prevalence, and impact on quality of life.
To address these issues, they systematically searched the literature for randomized controlled, observational, case-control, and retrospective studies of infants and children diagnosed with non-IgE gastrointestinal food allergies and GERD, published in English until February 2021.
Because of limited data in many of these areas, they used a modified Delphi method to reach consensus and provide practical advice on food allergy–associated GERD management.
The task force concludes:
- Food proteins, especially cow’s milk protein, can contribute to food allergy–associated GERD. The confirmation of food allergy is based on the elimination diet, always followed by reintroducing the offending allergen, and the diagnosis and treatment pathway should consider effects on quality of life.
- Breastfeeding should be supported in food allergy–associated GERD, and dietary advice should consider the potential nutritional impact on the breastfeeding mother. When breast milk is not available or is insufficient, formula and dietary advice to counteract the child’s nutrient deficiencies should be considered.
- Although some clarity exists about when GERD medications may be considered, they are often used inappropriately and may harm patients, especially infants.
Rigorous research needed
“Clinicians can use this algorithm to help them identify patients who may be affected by food allergy–related GERD,” Jonathan Tam, MD, medical director of the Gores Family Allergy Center at Children’s Hospital Los Angeles, told this news organization by email.
“Clinicians who suspect their patients may have food allergy–related GERD now have clearer guidance on how to systemically evaluate their patients,” added Dr. Tam, who was not involved in developing the report.
“Many allergists fear that patients may be labeled with a food allergy unnecessarily. Because no biomarkers or tests for food allergy–related GERD are available, elimination diets are a crucial part of the evaluation,” he said.
Dr. Tam added that the authors point out two key parts of a trial elimination: First, the trial should last at least 2 weeks, but full resolution may not occur until 6 weeks. Second, targeted elimination must be followed by reintroduction to confirm that the food was causing the symptoms, not that time itself may have been responsible for the clinical change.
“The authors’ note on allergy testing is important,” he said. “Allergy testing is not necessary when a clinician is concerned about food allergy–related GERD unless there are other associated atopic comorbidities, like eczema or IgE-mediated immediate food allergies.”
Jonathan M. Spergel, MD, PhD, chief of the allergy section at Children’s Hospital of Philadelphia, said in an email that families often ask whether food allergy is causing their child’s reflux.
“Both conditions are common and, in most cases, may not be related. As the report highlights, the risk of food allergy is increased if the patient has other atopic disease (atopic dermatitis), and standard allergy testing (skin testing, specific IgE) and IgG4 testing are not recommended,” he explained. “Food allergy in a patient with reflux can be considered if standard therapy is failing.”
Expert opinion on diagnosis and management
Dr. Spergel, also not involved in the report, joined the authors in advocating further, stronger studies. “While the expert opinion is a major strength,” he said, “with most available studies being neither randomized nor placebo-controlled, the true prevalence of food allergy reflux is unknown.”
Jay M. Portnoy, MD, specialist in pediatric allergy and immunology and medical director of telemedicine at Children’s Mercy Kansas City, said: “While most physicians believe that food allergy contributes to GERD, the evidence for the relationship is minimal. Reflux often occurs regardless of what food is eaten,” said Dr. Portnoy, who was not associated with the research.
Before removing a food from the diet, it’s important to determine whether that food is causing the problem, he urged.
“Blaming a food is easy. Food allergy is often suspected to cause symptoms it does not cause,” he said. “This unfounded blame can lead to unnecessary avoidance, reduce a family’s quality of life, and cause malnutrition.
“How a child is evaluated and treated depends as much on which physician they see as on whether the food is the culprit,” Dr. Portnoy said. “This report is an attempt to clarify the issues and to standardize an approach to the condition, so each provider evaluates and manages the condition in a consistent and evidence-based manner.
“It is important to see how well this report is incorporated into practice and whether following its guidance actually improves patient care,” Dr. Portnoy noted.
Funding information was not provided. Dr. Meyer and two coauthors report financial relationships with the nutritional health care industry. The full list can be found with the original article. The remaining authors and Dr. Tam, Dr. Spergel, and Dr. Portnoy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate about a possible link between food allergy (FA) and pediatric gastroesophageal reflux disease (GERD) continues, and more, better-designed research is needed, a position paper by the European Academy of Allergy and Clinical Immunology reports.
The report offers consensus-based recommendations and a graphical decision pathway to guide providers through assessing and treating food allergy–related GERD. And the authors call for further, better-designed related research.
Food allergy and GERD are common in babies under 1 year of age and can lead to bothersome GERD, the authors write.
“An extensive literature search has found that whilst food proteins, in particular cow milk protein, can be a contributing factor to FA-associated” GERD, distinguishing between FA and non–FA-associated GERD is difficult, lead author Rosan Meyer, RD, PhD, senior lecturer at Imperial College London, and colleagues from the Academy task force on non-IgE mediated allergy, write in Pediatric Allergy and Immunology.
Consensus despite limited data
Dr. Meyer and colleagues developed clinical questions that addressed various aspects of the relationship between food allergy and GERD – pathophysiology, symptoms, diagnosis, dietary and medical management, prevalence, and impact on quality of life.
To address these issues, they systematically searched the literature for randomized controlled, observational, case-control, and retrospective studies of infants and children diagnosed with non-IgE gastrointestinal food allergies and GERD, published in English until February 2021.
Because of limited data in many of these areas, they used a modified Delphi method to reach consensus and provide practical advice on food allergy–associated GERD management.
The task force concludes:
- Food proteins, especially cow’s milk protein, can contribute to food allergy–associated GERD. The confirmation of food allergy is based on the elimination diet, always followed by reintroducing the offending allergen, and the diagnosis and treatment pathway should consider effects on quality of life.
- Breastfeeding should be supported in food allergy–associated GERD, and dietary advice should consider the potential nutritional impact on the breastfeeding mother. When breast milk is not available or is insufficient, formula and dietary advice to counteract the child’s nutrient deficiencies should be considered.
- Although some clarity exists about when GERD medications may be considered, they are often used inappropriately and may harm patients, especially infants.
Rigorous research needed
“Clinicians can use this algorithm to help them identify patients who may be affected by food allergy–related GERD,” Jonathan Tam, MD, medical director of the Gores Family Allergy Center at Children’s Hospital Los Angeles, told this news organization by email.
“Clinicians who suspect their patients may have food allergy–related GERD now have clearer guidance on how to systemically evaluate their patients,” added Dr. Tam, who was not involved in developing the report.
“Many allergists fear that patients may be labeled with a food allergy unnecessarily. Because no biomarkers or tests for food allergy–related GERD are available, elimination diets are a crucial part of the evaluation,” he said.
Dr. Tam added that the authors point out two key parts of a trial elimination: First, the trial should last at least 2 weeks, but full resolution may not occur until 6 weeks. Second, targeted elimination must be followed by reintroduction to confirm that the food was causing the symptoms, not that time itself may have been responsible for the clinical change.
“The authors’ note on allergy testing is important,” he said. “Allergy testing is not necessary when a clinician is concerned about food allergy–related GERD unless there are other associated atopic comorbidities, like eczema or IgE-mediated immediate food allergies.”
Jonathan M. Spergel, MD, PhD, chief of the allergy section at Children’s Hospital of Philadelphia, said in an email that families often ask whether food allergy is causing their child’s reflux.
“Both conditions are common and, in most cases, may not be related. As the report highlights, the risk of food allergy is increased if the patient has other atopic disease (atopic dermatitis), and standard allergy testing (skin testing, specific IgE) and IgG4 testing are not recommended,” he explained. “Food allergy in a patient with reflux can be considered if standard therapy is failing.”
Expert opinion on diagnosis and management
Dr. Spergel, also not involved in the report, joined the authors in advocating further, stronger studies. “While the expert opinion is a major strength,” he said, “with most available studies being neither randomized nor placebo-controlled, the true prevalence of food allergy reflux is unknown.”
Jay M. Portnoy, MD, specialist in pediatric allergy and immunology and medical director of telemedicine at Children’s Mercy Kansas City, said: “While most physicians believe that food allergy contributes to GERD, the evidence for the relationship is minimal. Reflux often occurs regardless of what food is eaten,” said Dr. Portnoy, who was not associated with the research.
Before removing a food from the diet, it’s important to determine whether that food is causing the problem, he urged.
“Blaming a food is easy. Food allergy is often suspected to cause symptoms it does not cause,” he said. “This unfounded blame can lead to unnecessary avoidance, reduce a family’s quality of life, and cause malnutrition.
“How a child is evaluated and treated depends as much on which physician they see as on whether the food is the culprit,” Dr. Portnoy said. “This report is an attempt to clarify the issues and to standardize an approach to the condition, so each provider evaluates and manages the condition in a consistent and evidence-based manner.
“It is important to see how well this report is incorporated into practice and whether following its guidance actually improves patient care,” Dr. Portnoy noted.
Funding information was not provided. Dr. Meyer and two coauthors report financial relationships with the nutritional health care industry. The full list can be found with the original article. The remaining authors and Dr. Tam, Dr. Spergel, and Dr. Portnoy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The debate about a possible link between food allergy (FA) and pediatric gastroesophageal reflux disease (GERD) continues, and more, better-designed research is needed, a position paper by the European Academy of Allergy and Clinical Immunology reports.
The report offers consensus-based recommendations and a graphical decision pathway to guide providers through assessing and treating food allergy–related GERD. And the authors call for further, better-designed related research.
Food allergy and GERD are common in babies under 1 year of age and can lead to bothersome GERD, the authors write.
“An extensive literature search has found that whilst food proteins, in particular cow milk protein, can be a contributing factor to FA-associated” GERD, distinguishing between FA and non–FA-associated GERD is difficult, lead author Rosan Meyer, RD, PhD, senior lecturer at Imperial College London, and colleagues from the Academy task force on non-IgE mediated allergy, write in Pediatric Allergy and Immunology.
Consensus despite limited data
Dr. Meyer and colleagues developed clinical questions that addressed various aspects of the relationship between food allergy and GERD – pathophysiology, symptoms, diagnosis, dietary and medical management, prevalence, and impact on quality of life.
To address these issues, they systematically searched the literature for randomized controlled, observational, case-control, and retrospective studies of infants and children diagnosed with non-IgE gastrointestinal food allergies and GERD, published in English until February 2021.
Because of limited data in many of these areas, they used a modified Delphi method to reach consensus and provide practical advice on food allergy–associated GERD management.
The task force concludes:
- Food proteins, especially cow’s milk protein, can contribute to food allergy–associated GERD. The confirmation of food allergy is based on the elimination diet, always followed by reintroducing the offending allergen, and the diagnosis and treatment pathway should consider effects on quality of life.
- Breastfeeding should be supported in food allergy–associated GERD, and dietary advice should consider the potential nutritional impact on the breastfeeding mother. When breast milk is not available or is insufficient, formula and dietary advice to counteract the child’s nutrient deficiencies should be considered.
- Although some clarity exists about when GERD medications may be considered, they are often used inappropriately and may harm patients, especially infants.
Rigorous research needed
“Clinicians can use this algorithm to help them identify patients who may be affected by food allergy–related GERD,” Jonathan Tam, MD, medical director of the Gores Family Allergy Center at Children’s Hospital Los Angeles, told this news organization by email.
“Clinicians who suspect their patients may have food allergy–related GERD now have clearer guidance on how to systemically evaluate their patients,” added Dr. Tam, who was not involved in developing the report.
“Many allergists fear that patients may be labeled with a food allergy unnecessarily. Because no biomarkers or tests for food allergy–related GERD are available, elimination diets are a crucial part of the evaluation,” he said.
Dr. Tam added that the authors point out two key parts of a trial elimination: First, the trial should last at least 2 weeks, but full resolution may not occur until 6 weeks. Second, targeted elimination must be followed by reintroduction to confirm that the food was causing the symptoms, not that time itself may have been responsible for the clinical change.
“The authors’ note on allergy testing is important,” he said. “Allergy testing is not necessary when a clinician is concerned about food allergy–related GERD unless there are other associated atopic comorbidities, like eczema or IgE-mediated immediate food allergies.”
Jonathan M. Spergel, MD, PhD, chief of the allergy section at Children’s Hospital of Philadelphia, said in an email that families often ask whether food allergy is causing their child’s reflux.
“Both conditions are common and, in most cases, may not be related. As the report highlights, the risk of food allergy is increased if the patient has other atopic disease (atopic dermatitis), and standard allergy testing (skin testing, specific IgE) and IgG4 testing are not recommended,” he explained. “Food allergy in a patient with reflux can be considered if standard therapy is failing.”
Expert opinion on diagnosis and management
Dr. Spergel, also not involved in the report, joined the authors in advocating further, stronger studies. “While the expert opinion is a major strength,” he said, “with most available studies being neither randomized nor placebo-controlled, the true prevalence of food allergy reflux is unknown.”
Jay M. Portnoy, MD, specialist in pediatric allergy and immunology and medical director of telemedicine at Children’s Mercy Kansas City, said: “While most physicians believe that food allergy contributes to GERD, the evidence for the relationship is minimal. Reflux often occurs regardless of what food is eaten,” said Dr. Portnoy, who was not associated with the research.
Before removing a food from the diet, it’s important to determine whether that food is causing the problem, he urged.
“Blaming a food is easy. Food allergy is often suspected to cause symptoms it does not cause,” he said. “This unfounded blame can lead to unnecessary avoidance, reduce a family’s quality of life, and cause malnutrition.
“How a child is evaluated and treated depends as much on which physician they see as on whether the food is the culprit,” Dr. Portnoy said. “This report is an attempt to clarify the issues and to standardize an approach to the condition, so each provider evaluates and manages the condition in a consistent and evidence-based manner.
“It is important to see how well this report is incorporated into practice and whether following its guidance actually improves patient care,” Dr. Portnoy noted.
Funding information was not provided. Dr. Meyer and two coauthors report financial relationships with the nutritional health care industry. The full list can be found with the original article. The remaining authors and Dr. Tam, Dr. Spergel, and Dr. Portnoy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Deductibles a threat to more imaging after abnormal mammogram
CHICAGO – One in five women will skip further imaging after an abnormal mammogram if they have to pay out of pocket before their deductible is met, new data indicate.
“The ACA [Affordable Care Act] removed out-of-pocket costs for screening mammograms under most health plans to encourage women to partake in this important preventative health care measure,” Michael Ngo, MD, a radiology resident at Boston Medical Center and Boston University, said in a statement.
However, the screening mammogram is only the first step. If it’s abnormal, additional tests and a biopsy help determine whether the patient has cancer. The ACA does not mandate coverage for those, Dr. Ngo noted.
Dr. Ngo was lead author of the study presented at the annual meeting of the Radiological Society of North America.
Researchers collected 932 surveys. Asked whether they would skip follow-up imaging if they knew that they would have to pay a deductible, 151 of 714 (21.2%) said that they would skip the imaging; 424 (59.4%) said that they would not skip further imaging; and 139 (19.5%) were undecided. Responses differed by race, education level, household income, and insurance payer.
Groups most likely to forgo further tests
The groups with the highest percentage of persons who would skip additional imaging were Hispanic persons (33%); persons whose level of education was high school or less (31.0%); persons with a household income of less than $35,000 (27%); and those covered by Medicaid or who were uninsured (31.5%).
Wendie Berg, MD, PhD, professor of radiology at the University of Pittsburgh, who was not part of the study, said that because insurance companies had to cover initial mammograms fully under the ACA, “they generally increased deductibles. That resulted in more charges to patients when they came in for additional testing.
“It caught a lot of women by surprise,” she told this news organization.
The out-of-pocket charges can escalate with each step – more images, a biopsy, then more if they do have cancer, she said. This puts patients on the hook for hundreds, if not thousands, of dollars in medical bills.
However, Dr. Berg said, “The vast majority of women – 95% – who are called back for additional testing don’t have cancer. It is a problem that a lot of women will experience the cost and don’t have any benefit.”
Reducing false-positive recalls
The study highlights several things, she said. One is that “it’s incumbent on all of us to reduce false-positive recalls, which is one of the benefits of 3-D mammogram.”
Physicians who order additional tests must also consider the financial burden for patients, she said.
Some states have tackled the issue, she said. “Seven states do require insurance to cover diagnostic testing.” But those states differ in the extent of the coverage. DenseBreast-info.org, a website she helps with on a volunteer basis, explains the benefits by state.
Further compounding the problem is that not every insurer is subject to state law, she said.
Many states have programs that cover the cost for those who meet income requirements, although, she noted, some women make too much to qualify.
“It would be great to have a federal law that is inclusive,” she said.
Education efforts may help
Brian N. Dontchos, MD, with the University of Washington in Seattle, who was not part of the study, views the data another way. He told this news organization, “It is encouraging from the study that the majority of women would pursue additional imaging after an abnormal screening mammogram despite incurring more cost.”
He said that since direct patient education “has been shown to be effective in improving patient participation in screening programs, it is possible that, with more education of patients and providers, that advocacy could influence payers to support downstream imaging and biopsies that result from screening programs.”
Dr. Ngo, Dr. Berg, and Dr. Dontchos report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – One in five women will skip further imaging after an abnormal mammogram if they have to pay out of pocket before their deductible is met, new data indicate.
“The ACA [Affordable Care Act] removed out-of-pocket costs for screening mammograms under most health plans to encourage women to partake in this important preventative health care measure,” Michael Ngo, MD, a radiology resident at Boston Medical Center and Boston University, said in a statement.
However, the screening mammogram is only the first step. If it’s abnormal, additional tests and a biopsy help determine whether the patient has cancer. The ACA does not mandate coverage for those, Dr. Ngo noted.
Dr. Ngo was lead author of the study presented at the annual meeting of the Radiological Society of North America.
Researchers collected 932 surveys. Asked whether they would skip follow-up imaging if they knew that they would have to pay a deductible, 151 of 714 (21.2%) said that they would skip the imaging; 424 (59.4%) said that they would not skip further imaging; and 139 (19.5%) were undecided. Responses differed by race, education level, household income, and insurance payer.
Groups most likely to forgo further tests
The groups with the highest percentage of persons who would skip additional imaging were Hispanic persons (33%); persons whose level of education was high school or less (31.0%); persons with a household income of less than $35,000 (27%); and those covered by Medicaid or who were uninsured (31.5%).
Wendie Berg, MD, PhD, professor of radiology at the University of Pittsburgh, who was not part of the study, said that because insurance companies had to cover initial mammograms fully under the ACA, “they generally increased deductibles. That resulted in more charges to patients when they came in for additional testing.
“It caught a lot of women by surprise,” she told this news organization.
The out-of-pocket charges can escalate with each step – more images, a biopsy, then more if they do have cancer, she said. This puts patients on the hook for hundreds, if not thousands, of dollars in medical bills.
However, Dr. Berg said, “The vast majority of women – 95% – who are called back for additional testing don’t have cancer. It is a problem that a lot of women will experience the cost and don’t have any benefit.”
Reducing false-positive recalls
The study highlights several things, she said. One is that “it’s incumbent on all of us to reduce false-positive recalls, which is one of the benefits of 3-D mammogram.”
Physicians who order additional tests must also consider the financial burden for patients, she said.
Some states have tackled the issue, she said. “Seven states do require insurance to cover diagnostic testing.” But those states differ in the extent of the coverage. DenseBreast-info.org, a website she helps with on a volunteer basis, explains the benefits by state.
Further compounding the problem is that not every insurer is subject to state law, she said.
Many states have programs that cover the cost for those who meet income requirements, although, she noted, some women make too much to qualify.
“It would be great to have a federal law that is inclusive,” she said.
Education efforts may help
Brian N. Dontchos, MD, with the University of Washington in Seattle, who was not part of the study, views the data another way. He told this news organization, “It is encouraging from the study that the majority of women would pursue additional imaging after an abnormal screening mammogram despite incurring more cost.”
He said that since direct patient education “has been shown to be effective in improving patient participation in screening programs, it is possible that, with more education of patients and providers, that advocacy could influence payers to support downstream imaging and biopsies that result from screening programs.”
Dr. Ngo, Dr. Berg, and Dr. Dontchos report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – One in five women will skip further imaging after an abnormal mammogram if they have to pay out of pocket before their deductible is met, new data indicate.
“The ACA [Affordable Care Act] removed out-of-pocket costs for screening mammograms under most health plans to encourage women to partake in this important preventative health care measure,” Michael Ngo, MD, a radiology resident at Boston Medical Center and Boston University, said in a statement.
However, the screening mammogram is only the first step. If it’s abnormal, additional tests and a biopsy help determine whether the patient has cancer. The ACA does not mandate coverage for those, Dr. Ngo noted.
Dr. Ngo was lead author of the study presented at the annual meeting of the Radiological Society of North America.
Researchers collected 932 surveys. Asked whether they would skip follow-up imaging if they knew that they would have to pay a deductible, 151 of 714 (21.2%) said that they would skip the imaging; 424 (59.4%) said that they would not skip further imaging; and 139 (19.5%) were undecided. Responses differed by race, education level, household income, and insurance payer.
Groups most likely to forgo further tests
The groups with the highest percentage of persons who would skip additional imaging were Hispanic persons (33%); persons whose level of education was high school or less (31.0%); persons with a household income of less than $35,000 (27%); and those covered by Medicaid or who were uninsured (31.5%).
Wendie Berg, MD, PhD, professor of radiology at the University of Pittsburgh, who was not part of the study, said that because insurance companies had to cover initial mammograms fully under the ACA, “they generally increased deductibles. That resulted in more charges to patients when they came in for additional testing.
“It caught a lot of women by surprise,” she told this news organization.
The out-of-pocket charges can escalate with each step – more images, a biopsy, then more if they do have cancer, she said. This puts patients on the hook for hundreds, if not thousands, of dollars in medical bills.
However, Dr. Berg said, “The vast majority of women – 95% – who are called back for additional testing don’t have cancer. It is a problem that a lot of women will experience the cost and don’t have any benefit.”
Reducing false-positive recalls
The study highlights several things, she said. One is that “it’s incumbent on all of us to reduce false-positive recalls, which is one of the benefits of 3-D mammogram.”
Physicians who order additional tests must also consider the financial burden for patients, she said.
Some states have tackled the issue, she said. “Seven states do require insurance to cover diagnostic testing.” But those states differ in the extent of the coverage. DenseBreast-info.org, a website she helps with on a volunteer basis, explains the benefits by state.
Further compounding the problem is that not every insurer is subject to state law, she said.
Many states have programs that cover the cost for those who meet income requirements, although, she noted, some women make too much to qualify.
“It would be great to have a federal law that is inclusive,” she said.
Education efforts may help
Brian N. Dontchos, MD, with the University of Washington in Seattle, who was not part of the study, views the data another way. He told this news organization, “It is encouraging from the study that the majority of women would pursue additional imaging after an abnormal screening mammogram despite incurring more cost.”
He said that since direct patient education “has been shown to be effective in improving patient participation in screening programs, it is possible that, with more education of patients and providers, that advocacy could influence payers to support downstream imaging and biopsies that result from screening programs.”
Dr. Ngo, Dr. Berg, and Dr. Dontchos report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT RSNA 2022