User login
Novel vaccine approach halts disease after 23 years of breast cancer
A recent 6-month follow-up showed no evidence of new or recurrent disease, and scans showed regression of a distant bulky left adrenal metastasis, as well as at other sites.
A small site of residual hypermetabolism remains in the sternum, but this is thought to be related to scar tissue.
The patient, Stephanie Gangi, told Medscape Medical News that, before she entered into the trial for the novel cancer vaccine, she was “mentally and physically exhausted.” She had benefited from being diagnosed with hormone-positive breast cancer just as its treatment was evolving and progressing, which meant that, every time a treatment failed, “there was the next thing to try, which was great and kept me going.”
“But I will admit that, by age 66, and more than 20 years of cancer treatments, I was exhausted.”
Ms. Gangi, a New York City-based poet, essayist, and fiction writer, said she was “cautiously optimistic” about the cancer vaccine, but the “overriding thought was I wanted to avoid chemotherapy.”
“I was not really signing on for great outcomes, I was signing on for something that might keep chemo at bay. The biggest impact so far for me has been that, for the first time in more than a decade, I am not on any medication. That’s really amazing…and that means no side effects,” she said.
Ms. Gangi stopped the vaccine treatment this past July, and just over 3 months later, she is still “wrapping her head around” the fact that her cancer has regressed. “I’ve had breast cancer a long time,” she said, “and you can’t just snap your fingers and be fine.”
Although the two scans that she has had since the trial ended have been “astonishing,” she underlined that this is not about a ‘cure,’ but rather “clearing tumors for the first time in many years.”
“Cancer is sneaky and sinister, and it figures out how to circumvent all kinds of treatments,” she said, adding nevertheless that she is “happy and hopeful, and my family is thrilled, of course.”
Ms. Gangi was classed as having had a partial response to the cancer vaccine, one of a few in a small phase 1/2 trial at the Icahn School of Medicine at Mount Sinai in New York. One other patient also had a partial response, and one patient had a complete response.
However, six patients have progressive disease, and one has stable disease.
These results come from an interim analysis of 10 patients from the trial, and show a 30% response rate. They were presented at the recent annual meeting of the Society for Immunotherapy of Cancer.
The vaccine that was being tested combines local low-dose radiation, intramural Flt3L, which stimulates dendritic cells, and intravenous poly-ICLC, an immune stimulating factor, with the PD-1 inhibitor pembrolizumab (Keytruda).
The result is that, instead of making a vaccine in a laboratory and administering it, “we’re actually formulating it within the body,” lead author Thomas Marron, MD, PhD, professor of medicine (hematology and medical oncology) at Mount Sinai, said in an interview.
“What people don’t realize,” he said, is that bulky tumor sites contain “a lot of dead tumor, because they grow so fast and in a haphazard way.” This means that the immune system can be recruited to recognize the dead tumor and “gobble up the dead stuff that’s already there,” he added.
The hope is that the immune system will then kill not only “the tumor you are injecting into, but also tumors elsewhere in the body,” Dr. Marron said. “So you’re basically using your body’s own immune system and on and off switches to vaccinate the patient against their cancer.”
Another patient in the trial who had a complete response to the vaccine was William Morrison, with non-Hodgkin lymphoma (NHL).
Mr. Morrison was diagnosed in 2017, at which time he was enrolled onto a phase 1 trial of an earlier version of this novel vaccine treatment regimen. “Basically, they didn’t get the results they were hoping for, and I still had the lymphoma,” he said. In 2018, his indolent follicular lymphoma transformed into an aggressive diffuse large B-cell lymphoma, for which Mr. Morrison was given six cycles of chemotherapy. This put him into remission and cleared his lymphoma.
“But the remission lasted for maybe a little over a year,” he said.
The cancer came back, and at that point he was given the opportunity to enroll in the Mount Sinai trial. At the end of the treatment, “everything was clear.”
“I’ve been for PET scans every 6 months, and I just had a scan done the other week, and everything has been fine…I’ve been pretty excited. I was pretty lucky.”
“This recent one really has worked wonders,” he said, “When they gave me the good news the other day. I felt like a big weight had been lifted.”
Mr. Morrison also said that he did not experience any serious adverse events while being treated with the vaccine. “Other than a few minor things, I tolerated it pretty well,” he said.
In contrast, Ms. Gangi said she experienced “intense” flu-like symptoms that started in the first few days after the treatment and lasted for a couple of days.
Need to improve response rate
The current trial achieved responses in 30% of patients, which “is great, [but] we want to be at 100%,” said Dr. Marron.
“What we’re doing in the laboratory right now is using this as an opportunity to study what it is that’s special about those three people who responded and what’s not happening in the other seven people, and we have some initial data that we’re analyzing,” he said.
“We are seeing that the patients who responded have a much more robust response to the Ft3L in particular…and that could suggest that maybe we need a better Ft3L, or we could think about other ways to potentially manipulate this vaccine.
“Most of the patients who are referred to me are people who have run out of options…and that usually means they’ve had many different types of chemotherapy,” Dr. Marron commented. For example, Ms. Gangi had already been through 12 different chemotherapy regimens.
Chemotherapy suppresses the immune system, but it’s not only that — also having an effect are all the other treatments aimed at reducing nausea and allergic reactions to the anti-cancer therapy, Dr. Marron explained.
“By the time that I see a patient,” Dr. Marron said, “oftentimes their immune system is not optimal. So another way in which we would hope to see better responses is by moving this vaccine earlier in the treatment paradigm, and administering it to patients as their first or second treatment.”
Senior author Joshua Brody, MD, director of the Lymphoma Immunotherapy Program at Mount Sinai’s Tisch Cancer Institute, added that it “might be easy” to incorporate the vaccine into earlier lines of therapy.
He said in an interview that both immunotherapy and radiation therapy are “standard” treatments, and the key is “adding multiple ingredients together that don’t have cumulative toxicity.”
“You can’t just chemo one plus chemo two, because they have some of the same toxicities, but the delightful thing here is this therapy had been quite safe.
“So in theory it would be fairly easy to incorporate this into earlier lines of therapy, once we can get a bit more proof of principle,” Dr. Brody said.
Approached for comment, Ann W. Silk, MD, said that the results are “particularly impressive because we know anti-PD-1 plus radiation therapy does not work in hormone-positive breast cancer or lymphoma.”
Dr. Silk, an oncologist at the Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School in Boston, said in an interview that one advantage of this vaccine is that it “is not restricted to a certain number of antigens and does not rely on an algorithm.”
“I would love to see more data in hormone-positive metastatic breast cancer patients,” she added. “I would use this approach after the hormonal treatments stop working, but before chemotherapy.”
Dr. Silk also said that the safety profile “looks quite good, and I imagine this approach would result in a much better quality of life for patients as compared to chemotherapy.”
Details of the trial and results
The Mount Sinai researchers had previously developed a personalized genomic cancer vaccine, PGV-001, which showed promise in a phase 1 trial in 13 patients with solid tumors or multiple myeloma and a high risk of recurrence after surgery or autologous stem cell transplant.
Next, they worked to develop the concept further to turn the tumor into its own vaccine, which involved inducing anti-tumor responses in indolent NHL, which typically responds poorly to checkpoint blockade, by combining Ft3L, low-dose irradiation, and poly-ICLC.
The next phase 1 trial showed that this approach was feasible, but preclinical modeling suggested that the addition of PD-1 blockade could improve the cure rates. The researchers therefore conducted the current trial, recruiting 10 patients with indolent NHL, metastatic breast cancer, or head and neck squamous cell carcinoma (HNSCC).
Patients were given local radiation therapy on days 1 and 2, and intramural Ft3L to the same tumor on day 9, followed by eight intravenous injections of poly-ICLC over 6 weeks. On day 23, they received their first of eight doses of pembrolizumab.
Dr. Marron explained that the radiotherapy increases the amount of dead material for the immune system to work on by “killing some of the tumor cells,” adding: “We’re not trying to kill the whole tumor with the radiation…it just starts the process of releasing some more of that dead stuff.”
He explained that Ft3L is a human growth factor that simulates dendritic cells, “which I always say are the professor cells of the immune system,” as they tell the body “what’s good and what’s bad.”
The poly-ICLC is “basically like a fake virus,” Dr. Marron said, as it “turns on those immune cells that have taken up the tumor antigen in the neighborhood” of the tumor, so they “teach the immune system that there is something bad”.
Finally, the pembrolizumab is there to “take the foot off the brake of the immune system” and “grease the wheels a bit more,” he added, even though it does not work in all patients, or in all tumor types, including indolent NHL.
The trial was planned in two phases. In the first part, six patients were enrolled to assess the safety of the approach; the phase 2 stage of the trial followed a Simon’s Two-Stage design, with the aim of recruiting seven patients of each tumor type, followed by a further 12 patients if they showed a response.
The current interim analysis that was presented at the SITC meeting focused on the first 10 patients in the phase 2 part, who were enrolled between April 2019 and July 2022. This included six patients with metastatic breast cancer, three with indolent NHL, and one with HNSCC, all of whom completed their first disease response assessment.
All patients experienced treatment-related adverse events, largely comprising low-grade injection site reactions and flu-like symptoms linked to the poly-ICLC injections.
One patient experienced grade 3 pembrolizumab-related colitis, while another had self-resolving grade 3 fever following poly-ICLC injection.
The study was sponsored by Icahn School of Medicine at Mount Sinai and conducted in collaboration with Merck Sharp & Dohme LLC and Celldex Therapeutics. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
A recent 6-month follow-up showed no evidence of new or recurrent disease, and scans showed regression of a distant bulky left adrenal metastasis, as well as at other sites.
A small site of residual hypermetabolism remains in the sternum, but this is thought to be related to scar tissue.
The patient, Stephanie Gangi, told Medscape Medical News that, before she entered into the trial for the novel cancer vaccine, she was “mentally and physically exhausted.” She had benefited from being diagnosed with hormone-positive breast cancer just as its treatment was evolving and progressing, which meant that, every time a treatment failed, “there was the next thing to try, which was great and kept me going.”
“But I will admit that, by age 66, and more than 20 years of cancer treatments, I was exhausted.”
Ms. Gangi, a New York City-based poet, essayist, and fiction writer, said she was “cautiously optimistic” about the cancer vaccine, but the “overriding thought was I wanted to avoid chemotherapy.”
“I was not really signing on for great outcomes, I was signing on for something that might keep chemo at bay. The biggest impact so far for me has been that, for the first time in more than a decade, I am not on any medication. That’s really amazing…and that means no side effects,” she said.
Ms. Gangi stopped the vaccine treatment this past July, and just over 3 months later, she is still “wrapping her head around” the fact that her cancer has regressed. “I’ve had breast cancer a long time,” she said, “and you can’t just snap your fingers and be fine.”
Although the two scans that she has had since the trial ended have been “astonishing,” she underlined that this is not about a ‘cure,’ but rather “clearing tumors for the first time in many years.”
“Cancer is sneaky and sinister, and it figures out how to circumvent all kinds of treatments,” she said, adding nevertheless that she is “happy and hopeful, and my family is thrilled, of course.”
Ms. Gangi was classed as having had a partial response to the cancer vaccine, one of a few in a small phase 1/2 trial at the Icahn School of Medicine at Mount Sinai in New York. One other patient also had a partial response, and one patient had a complete response.
However, six patients have progressive disease, and one has stable disease.
These results come from an interim analysis of 10 patients from the trial, and show a 30% response rate. They were presented at the recent annual meeting of the Society for Immunotherapy of Cancer.
The vaccine that was being tested combines local low-dose radiation, intramural Flt3L, which stimulates dendritic cells, and intravenous poly-ICLC, an immune stimulating factor, with the PD-1 inhibitor pembrolizumab (Keytruda).
The result is that, instead of making a vaccine in a laboratory and administering it, “we’re actually formulating it within the body,” lead author Thomas Marron, MD, PhD, professor of medicine (hematology and medical oncology) at Mount Sinai, said in an interview.
“What people don’t realize,” he said, is that bulky tumor sites contain “a lot of dead tumor, because they grow so fast and in a haphazard way.” This means that the immune system can be recruited to recognize the dead tumor and “gobble up the dead stuff that’s already there,” he added.
The hope is that the immune system will then kill not only “the tumor you are injecting into, but also tumors elsewhere in the body,” Dr. Marron said. “So you’re basically using your body’s own immune system and on and off switches to vaccinate the patient against their cancer.”
Another patient in the trial who had a complete response to the vaccine was William Morrison, with non-Hodgkin lymphoma (NHL).
Mr. Morrison was diagnosed in 2017, at which time he was enrolled onto a phase 1 trial of an earlier version of this novel vaccine treatment regimen. “Basically, they didn’t get the results they were hoping for, and I still had the lymphoma,” he said. In 2018, his indolent follicular lymphoma transformed into an aggressive diffuse large B-cell lymphoma, for which Mr. Morrison was given six cycles of chemotherapy. This put him into remission and cleared his lymphoma.
“But the remission lasted for maybe a little over a year,” he said.
The cancer came back, and at that point he was given the opportunity to enroll in the Mount Sinai trial. At the end of the treatment, “everything was clear.”
“I’ve been for PET scans every 6 months, and I just had a scan done the other week, and everything has been fine…I’ve been pretty excited. I was pretty lucky.”
“This recent one really has worked wonders,” he said, “When they gave me the good news the other day. I felt like a big weight had been lifted.”
Mr. Morrison also said that he did not experience any serious adverse events while being treated with the vaccine. “Other than a few minor things, I tolerated it pretty well,” he said.
In contrast, Ms. Gangi said she experienced “intense” flu-like symptoms that started in the first few days after the treatment and lasted for a couple of days.
Need to improve response rate
The current trial achieved responses in 30% of patients, which “is great, [but] we want to be at 100%,” said Dr. Marron.
“What we’re doing in the laboratory right now is using this as an opportunity to study what it is that’s special about those three people who responded and what’s not happening in the other seven people, and we have some initial data that we’re analyzing,” he said.
“We are seeing that the patients who responded have a much more robust response to the Ft3L in particular…and that could suggest that maybe we need a better Ft3L, or we could think about other ways to potentially manipulate this vaccine.
“Most of the patients who are referred to me are people who have run out of options…and that usually means they’ve had many different types of chemotherapy,” Dr. Marron commented. For example, Ms. Gangi had already been through 12 different chemotherapy regimens.
Chemotherapy suppresses the immune system, but it’s not only that — also having an effect are all the other treatments aimed at reducing nausea and allergic reactions to the anti-cancer therapy, Dr. Marron explained.
“By the time that I see a patient,” Dr. Marron said, “oftentimes their immune system is not optimal. So another way in which we would hope to see better responses is by moving this vaccine earlier in the treatment paradigm, and administering it to patients as their first or second treatment.”
Senior author Joshua Brody, MD, director of the Lymphoma Immunotherapy Program at Mount Sinai’s Tisch Cancer Institute, added that it “might be easy” to incorporate the vaccine into earlier lines of therapy.
He said in an interview that both immunotherapy and radiation therapy are “standard” treatments, and the key is “adding multiple ingredients together that don’t have cumulative toxicity.”
“You can’t just chemo one plus chemo two, because they have some of the same toxicities, but the delightful thing here is this therapy had been quite safe.
“So in theory it would be fairly easy to incorporate this into earlier lines of therapy, once we can get a bit more proof of principle,” Dr. Brody said.
Approached for comment, Ann W. Silk, MD, said that the results are “particularly impressive because we know anti-PD-1 plus radiation therapy does not work in hormone-positive breast cancer or lymphoma.”
Dr. Silk, an oncologist at the Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School in Boston, said in an interview that one advantage of this vaccine is that it “is not restricted to a certain number of antigens and does not rely on an algorithm.”
“I would love to see more data in hormone-positive metastatic breast cancer patients,” she added. “I would use this approach after the hormonal treatments stop working, but before chemotherapy.”
Dr. Silk also said that the safety profile “looks quite good, and I imagine this approach would result in a much better quality of life for patients as compared to chemotherapy.”
Details of the trial and results
The Mount Sinai researchers had previously developed a personalized genomic cancer vaccine, PGV-001, which showed promise in a phase 1 trial in 13 patients with solid tumors or multiple myeloma and a high risk of recurrence after surgery or autologous stem cell transplant.
Next, they worked to develop the concept further to turn the tumor into its own vaccine, which involved inducing anti-tumor responses in indolent NHL, which typically responds poorly to checkpoint blockade, by combining Ft3L, low-dose irradiation, and poly-ICLC.
The next phase 1 trial showed that this approach was feasible, but preclinical modeling suggested that the addition of PD-1 blockade could improve the cure rates. The researchers therefore conducted the current trial, recruiting 10 patients with indolent NHL, metastatic breast cancer, or head and neck squamous cell carcinoma (HNSCC).
Patients were given local radiation therapy on days 1 and 2, and intramural Ft3L to the same tumor on day 9, followed by eight intravenous injections of poly-ICLC over 6 weeks. On day 23, they received their first of eight doses of pembrolizumab.
Dr. Marron explained that the radiotherapy increases the amount of dead material for the immune system to work on by “killing some of the tumor cells,” adding: “We’re not trying to kill the whole tumor with the radiation…it just starts the process of releasing some more of that dead stuff.”
He explained that Ft3L is a human growth factor that simulates dendritic cells, “which I always say are the professor cells of the immune system,” as they tell the body “what’s good and what’s bad.”
The poly-ICLC is “basically like a fake virus,” Dr. Marron said, as it “turns on those immune cells that have taken up the tumor antigen in the neighborhood” of the tumor, so they “teach the immune system that there is something bad”.
Finally, the pembrolizumab is there to “take the foot off the brake of the immune system” and “grease the wheels a bit more,” he added, even though it does not work in all patients, or in all tumor types, including indolent NHL.
The trial was planned in two phases. In the first part, six patients were enrolled to assess the safety of the approach; the phase 2 stage of the trial followed a Simon’s Two-Stage design, with the aim of recruiting seven patients of each tumor type, followed by a further 12 patients if they showed a response.
The current interim analysis that was presented at the SITC meeting focused on the first 10 patients in the phase 2 part, who were enrolled between April 2019 and July 2022. This included six patients with metastatic breast cancer, three with indolent NHL, and one with HNSCC, all of whom completed their first disease response assessment.
All patients experienced treatment-related adverse events, largely comprising low-grade injection site reactions and flu-like symptoms linked to the poly-ICLC injections.
One patient experienced grade 3 pembrolizumab-related colitis, while another had self-resolving grade 3 fever following poly-ICLC injection.
The study was sponsored by Icahn School of Medicine at Mount Sinai and conducted in collaboration with Merck Sharp & Dohme LLC and Celldex Therapeutics. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
A recent 6-month follow-up showed no evidence of new or recurrent disease, and scans showed regression of a distant bulky left adrenal metastasis, as well as at other sites.
A small site of residual hypermetabolism remains in the sternum, but this is thought to be related to scar tissue.
The patient, Stephanie Gangi, told Medscape Medical News that, before she entered into the trial for the novel cancer vaccine, she was “mentally and physically exhausted.” She had benefited from being diagnosed with hormone-positive breast cancer just as its treatment was evolving and progressing, which meant that, every time a treatment failed, “there was the next thing to try, which was great and kept me going.”
“But I will admit that, by age 66, and more than 20 years of cancer treatments, I was exhausted.”
Ms. Gangi, a New York City-based poet, essayist, and fiction writer, said she was “cautiously optimistic” about the cancer vaccine, but the “overriding thought was I wanted to avoid chemotherapy.”
“I was not really signing on for great outcomes, I was signing on for something that might keep chemo at bay. The biggest impact so far for me has been that, for the first time in more than a decade, I am not on any medication. That’s really amazing…and that means no side effects,” she said.
Ms. Gangi stopped the vaccine treatment this past July, and just over 3 months later, she is still “wrapping her head around” the fact that her cancer has regressed. “I’ve had breast cancer a long time,” she said, “and you can’t just snap your fingers and be fine.”
Although the two scans that she has had since the trial ended have been “astonishing,” she underlined that this is not about a ‘cure,’ but rather “clearing tumors for the first time in many years.”
“Cancer is sneaky and sinister, and it figures out how to circumvent all kinds of treatments,” she said, adding nevertheless that she is “happy and hopeful, and my family is thrilled, of course.”
Ms. Gangi was classed as having had a partial response to the cancer vaccine, one of a few in a small phase 1/2 trial at the Icahn School of Medicine at Mount Sinai in New York. One other patient also had a partial response, and one patient had a complete response.
However, six patients have progressive disease, and one has stable disease.
These results come from an interim analysis of 10 patients from the trial, and show a 30% response rate. They were presented at the recent annual meeting of the Society for Immunotherapy of Cancer.
The vaccine that was being tested combines local low-dose radiation, intramural Flt3L, which stimulates dendritic cells, and intravenous poly-ICLC, an immune stimulating factor, with the PD-1 inhibitor pembrolizumab (Keytruda).
The result is that, instead of making a vaccine in a laboratory and administering it, “we’re actually formulating it within the body,” lead author Thomas Marron, MD, PhD, professor of medicine (hematology and medical oncology) at Mount Sinai, said in an interview.
“What people don’t realize,” he said, is that bulky tumor sites contain “a lot of dead tumor, because they grow so fast and in a haphazard way.” This means that the immune system can be recruited to recognize the dead tumor and “gobble up the dead stuff that’s already there,” he added.
The hope is that the immune system will then kill not only “the tumor you are injecting into, but also tumors elsewhere in the body,” Dr. Marron said. “So you’re basically using your body’s own immune system and on and off switches to vaccinate the patient against their cancer.”
Another patient in the trial who had a complete response to the vaccine was William Morrison, with non-Hodgkin lymphoma (NHL).
Mr. Morrison was diagnosed in 2017, at which time he was enrolled onto a phase 1 trial of an earlier version of this novel vaccine treatment regimen. “Basically, they didn’t get the results they were hoping for, and I still had the lymphoma,” he said. In 2018, his indolent follicular lymphoma transformed into an aggressive diffuse large B-cell lymphoma, for which Mr. Morrison was given six cycles of chemotherapy. This put him into remission and cleared his lymphoma.
“But the remission lasted for maybe a little over a year,” he said.
The cancer came back, and at that point he was given the opportunity to enroll in the Mount Sinai trial. At the end of the treatment, “everything was clear.”
“I’ve been for PET scans every 6 months, and I just had a scan done the other week, and everything has been fine…I’ve been pretty excited. I was pretty lucky.”
“This recent one really has worked wonders,” he said, “When they gave me the good news the other day. I felt like a big weight had been lifted.”
Mr. Morrison also said that he did not experience any serious adverse events while being treated with the vaccine. “Other than a few minor things, I tolerated it pretty well,” he said.
In contrast, Ms. Gangi said she experienced “intense” flu-like symptoms that started in the first few days after the treatment and lasted for a couple of days.
Need to improve response rate
The current trial achieved responses in 30% of patients, which “is great, [but] we want to be at 100%,” said Dr. Marron.
“What we’re doing in the laboratory right now is using this as an opportunity to study what it is that’s special about those three people who responded and what’s not happening in the other seven people, and we have some initial data that we’re analyzing,” he said.
“We are seeing that the patients who responded have a much more robust response to the Ft3L in particular…and that could suggest that maybe we need a better Ft3L, or we could think about other ways to potentially manipulate this vaccine.
“Most of the patients who are referred to me are people who have run out of options…and that usually means they’ve had many different types of chemotherapy,” Dr. Marron commented. For example, Ms. Gangi had already been through 12 different chemotherapy regimens.
Chemotherapy suppresses the immune system, but it’s not only that — also having an effect are all the other treatments aimed at reducing nausea and allergic reactions to the anti-cancer therapy, Dr. Marron explained.
“By the time that I see a patient,” Dr. Marron said, “oftentimes their immune system is not optimal. So another way in which we would hope to see better responses is by moving this vaccine earlier in the treatment paradigm, and administering it to patients as their first or second treatment.”
Senior author Joshua Brody, MD, director of the Lymphoma Immunotherapy Program at Mount Sinai’s Tisch Cancer Institute, added that it “might be easy” to incorporate the vaccine into earlier lines of therapy.
He said in an interview that both immunotherapy and radiation therapy are “standard” treatments, and the key is “adding multiple ingredients together that don’t have cumulative toxicity.”
“You can’t just chemo one plus chemo two, because they have some of the same toxicities, but the delightful thing here is this therapy had been quite safe.
“So in theory it would be fairly easy to incorporate this into earlier lines of therapy, once we can get a bit more proof of principle,” Dr. Brody said.
Approached for comment, Ann W. Silk, MD, said that the results are “particularly impressive because we know anti-PD-1 plus radiation therapy does not work in hormone-positive breast cancer or lymphoma.”
Dr. Silk, an oncologist at the Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School in Boston, said in an interview that one advantage of this vaccine is that it “is not restricted to a certain number of antigens and does not rely on an algorithm.”
“I would love to see more data in hormone-positive metastatic breast cancer patients,” she added. “I would use this approach after the hormonal treatments stop working, but before chemotherapy.”
Dr. Silk also said that the safety profile “looks quite good, and I imagine this approach would result in a much better quality of life for patients as compared to chemotherapy.”
Details of the trial and results
The Mount Sinai researchers had previously developed a personalized genomic cancer vaccine, PGV-001, which showed promise in a phase 1 trial in 13 patients with solid tumors or multiple myeloma and a high risk of recurrence after surgery or autologous stem cell transplant.
Next, they worked to develop the concept further to turn the tumor into its own vaccine, which involved inducing anti-tumor responses in indolent NHL, which typically responds poorly to checkpoint blockade, by combining Ft3L, low-dose irradiation, and poly-ICLC.
The next phase 1 trial showed that this approach was feasible, but preclinical modeling suggested that the addition of PD-1 blockade could improve the cure rates. The researchers therefore conducted the current trial, recruiting 10 patients with indolent NHL, metastatic breast cancer, or head and neck squamous cell carcinoma (HNSCC).
Patients were given local radiation therapy on days 1 and 2, and intramural Ft3L to the same tumor on day 9, followed by eight intravenous injections of poly-ICLC over 6 weeks. On day 23, they received their first of eight doses of pembrolizumab.
Dr. Marron explained that the radiotherapy increases the amount of dead material for the immune system to work on by “killing some of the tumor cells,” adding: “We’re not trying to kill the whole tumor with the radiation…it just starts the process of releasing some more of that dead stuff.”
He explained that Ft3L is a human growth factor that simulates dendritic cells, “which I always say are the professor cells of the immune system,” as they tell the body “what’s good and what’s bad.”
The poly-ICLC is “basically like a fake virus,” Dr. Marron said, as it “turns on those immune cells that have taken up the tumor antigen in the neighborhood” of the tumor, so they “teach the immune system that there is something bad”.
Finally, the pembrolizumab is there to “take the foot off the brake of the immune system” and “grease the wheels a bit more,” he added, even though it does not work in all patients, or in all tumor types, including indolent NHL.
The trial was planned in two phases. In the first part, six patients were enrolled to assess the safety of the approach; the phase 2 stage of the trial followed a Simon’s Two-Stage design, with the aim of recruiting seven patients of each tumor type, followed by a further 12 patients if they showed a response.
The current interim analysis that was presented at the SITC meeting focused on the first 10 patients in the phase 2 part, who were enrolled between April 2019 and July 2022. This included six patients with metastatic breast cancer, three with indolent NHL, and one with HNSCC, all of whom completed their first disease response assessment.
All patients experienced treatment-related adverse events, largely comprising low-grade injection site reactions and flu-like symptoms linked to the poly-ICLC injections.
One patient experienced grade 3 pembrolizumab-related colitis, while another had self-resolving grade 3 fever following poly-ICLC injection.
The study was sponsored by Icahn School of Medicine at Mount Sinai and conducted in collaboration with Merck Sharp & Dohme LLC and Celldex Therapeutics. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM SITC 2022
Midodrine may be comparable to albumin for PICD prevention in ACLF
WASHINGTON – , according to the results of a randomized controlled trial.
Albumin protected 80% of patients from PICD 6 days after paracentesis, whereas midodrine protected 84%, a difference that was not statistically significant. However, albumin was associated with a slightly higher incidence of adverse events and higher costs, said Mithun Sharma, MD, during his presentation at the annual meeting of the American Association for the Study of Liver Diseases.
Midodrine may be a safer and cost-effective option for these patients, said Dr. Sharma, of the department of hepatology and liver transplantation, AIG Hospitals, Hyderabad, India.
But he cautioned that given the small size of the open-label study, with only 25 patients in each arm, the results should be considered as proof of concept and need to be validated in larger studies.
PICD common in ACLF
PICD is caused by fluid shift during paracentesis, leading to a decrease in effective circulating blood volume.
The incidence of PICD after large-volume paracentesis in patients receiving albumin ranges from 12% to 20%, Dr. Sharma noted.
Albumin has been shown in several trials to be effective at reducing the incidence of PICD in patients undergoing paracentesis, but this agent requires IV infusion and is comparatively costly, he said.
In contrast, midodrine, a selective alpha-adrenergic agonist usually prescribed for orthostatic hypotension, may help to prevent PICD through its mechanism of action, maintaining mean arterial pressure (MAP).
In two small studies comparing albumin infusion in patients undergoing paracentesis with 8 liters of fluid removal, midodrine was either inferior to albumin or had no beneficial effect, Dr. Sharma said.
Patients with ACLF, however, have paracentesis with much lower fluid volumes, typically with less than 5 liters removed, and may be good candidates for midodrine.
Study details
Dr. Sharma and colleagues tested their hypothesis that in patients with ACLF undergoing modest-volume paracentesis, with fluid removal below 5 liters, midodrine could prevent PICD by increasing MAP, with an efficacy similar to that of intravenous 20% human albumin infusions.
They enrolled 50 patients with ACLF defined by Asian Pacific Association for the Study of the Liver criteria who were undergoing paracentesis with 3- to 4-liter fluid volumes.
They defined PICD as at least a 50% increase in plasma renin activity (PRA) over baseline on the 6th day following paracentesis.
The patients were randomly assigned to receive either intravenous 20% human albumin infusions toward the end of paracentesis or midodrine-hydrochloride 7.5 mg three times daily starting 2 hours before paracentesis. Because of the difference in drug delivery methods, the study could not be blinded to treatment type.
Patients’ mean arterial pressures were recorded daily, renal parameters and serum electrolytes were monitored on days 3 and 6, and blood samples were tested for PRA on day 1 and day 6.
The most common acute and chronic hepatic insults and baseline characteristics of the patients were similar between the groups, with alcohol-related liver disease the most common underlying etiology of cirrhosis.
The incidence of PICD at day 6, the primary endpoint, did not differ significantly between the groups, although mean PRA levels on day 6 were numerically higher in the midodrine group. There was a significant rise in the absolute PRA volume from baseline (P = .006), but this rise did not meet the PICD definition.
Researchers found no significant differences between the two groups in absolute change in PRA, and no significant changes in either group in MAP, creatinine, or sodium levels.
Complications and costs
PICD developed in four patients assigned to the albumin group and five patients assigned to the midodrine group; however, this difference was not significant. Fluid overload occurred in only one patient, in the albumin group.
No cases of hypertension or urinary retention arose in either group.
Grade I/II hepatic encephalopathy occurred 2-3 days after paracentesis in three patients on albumin and in two patients on midodrine.
Acute kidney injury was seen in three patients on albumin and in one patient on midodrine.
At 28 days after paracentesis, three patients in the albumin group had died, all from sepsis and multiorgan failure, while four patients in the midodrine group had died, three from sepsis and multiorgan failure and one from an upper gastrointestinal bleed.
Two patients in the albumin group and one patient in the midodrine group underwent liver transplant 1 month after paracentesis.
A cost-effectiveness analysis showed that the mean cost of albumin infusions was about sixfold higher than that of oral midodrine.
More data needed
Session moderator Shiv K. Sarin, MD, from the Institute of Liver and Biliary Sciences in New Delhi, India, who was not involved in the study, commented that while midodrine is a good drug and generally safe, he would wait to use it in patients who needed modest-volume paracentesis until more data are published.
Dr. Sarin also emphasized that albumin is “mandatory” for protecting patients who require large-volume paracentesis, and that it would be “unethical” not to use it in that clinical situation.
The study was internally supported. Dr. Sharma and Dr. Sarin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , according to the results of a randomized controlled trial.
Albumin protected 80% of patients from PICD 6 days after paracentesis, whereas midodrine protected 84%, a difference that was not statistically significant. However, albumin was associated with a slightly higher incidence of adverse events and higher costs, said Mithun Sharma, MD, during his presentation at the annual meeting of the American Association for the Study of Liver Diseases.
Midodrine may be a safer and cost-effective option for these patients, said Dr. Sharma, of the department of hepatology and liver transplantation, AIG Hospitals, Hyderabad, India.
But he cautioned that given the small size of the open-label study, with only 25 patients in each arm, the results should be considered as proof of concept and need to be validated in larger studies.
PICD common in ACLF
PICD is caused by fluid shift during paracentesis, leading to a decrease in effective circulating blood volume.
The incidence of PICD after large-volume paracentesis in patients receiving albumin ranges from 12% to 20%, Dr. Sharma noted.
Albumin has been shown in several trials to be effective at reducing the incidence of PICD in patients undergoing paracentesis, but this agent requires IV infusion and is comparatively costly, he said.
In contrast, midodrine, a selective alpha-adrenergic agonist usually prescribed for orthostatic hypotension, may help to prevent PICD through its mechanism of action, maintaining mean arterial pressure (MAP).
In two small studies comparing albumin infusion in patients undergoing paracentesis with 8 liters of fluid removal, midodrine was either inferior to albumin or had no beneficial effect, Dr. Sharma said.
Patients with ACLF, however, have paracentesis with much lower fluid volumes, typically with less than 5 liters removed, and may be good candidates for midodrine.
Study details
Dr. Sharma and colleagues tested their hypothesis that in patients with ACLF undergoing modest-volume paracentesis, with fluid removal below 5 liters, midodrine could prevent PICD by increasing MAP, with an efficacy similar to that of intravenous 20% human albumin infusions.
They enrolled 50 patients with ACLF defined by Asian Pacific Association for the Study of the Liver criteria who were undergoing paracentesis with 3- to 4-liter fluid volumes.
They defined PICD as at least a 50% increase in plasma renin activity (PRA) over baseline on the 6th day following paracentesis.
The patients were randomly assigned to receive either intravenous 20% human albumin infusions toward the end of paracentesis or midodrine-hydrochloride 7.5 mg three times daily starting 2 hours before paracentesis. Because of the difference in drug delivery methods, the study could not be blinded to treatment type.
Patients’ mean arterial pressures were recorded daily, renal parameters and serum electrolytes were monitored on days 3 and 6, and blood samples were tested for PRA on day 1 and day 6.
The most common acute and chronic hepatic insults and baseline characteristics of the patients were similar between the groups, with alcohol-related liver disease the most common underlying etiology of cirrhosis.
The incidence of PICD at day 6, the primary endpoint, did not differ significantly between the groups, although mean PRA levels on day 6 were numerically higher in the midodrine group. There was a significant rise in the absolute PRA volume from baseline (P = .006), but this rise did not meet the PICD definition.
Researchers found no significant differences between the two groups in absolute change in PRA, and no significant changes in either group in MAP, creatinine, or sodium levels.
Complications and costs
PICD developed in four patients assigned to the albumin group and five patients assigned to the midodrine group; however, this difference was not significant. Fluid overload occurred in only one patient, in the albumin group.
No cases of hypertension or urinary retention arose in either group.
Grade I/II hepatic encephalopathy occurred 2-3 days after paracentesis in three patients on albumin and in two patients on midodrine.
Acute kidney injury was seen in three patients on albumin and in one patient on midodrine.
At 28 days after paracentesis, three patients in the albumin group had died, all from sepsis and multiorgan failure, while four patients in the midodrine group had died, three from sepsis and multiorgan failure and one from an upper gastrointestinal bleed.
Two patients in the albumin group and one patient in the midodrine group underwent liver transplant 1 month after paracentesis.
A cost-effectiveness analysis showed that the mean cost of albumin infusions was about sixfold higher than that of oral midodrine.
More data needed
Session moderator Shiv K. Sarin, MD, from the Institute of Liver and Biliary Sciences in New Delhi, India, who was not involved in the study, commented that while midodrine is a good drug and generally safe, he would wait to use it in patients who needed modest-volume paracentesis until more data are published.
Dr. Sarin also emphasized that albumin is “mandatory” for protecting patients who require large-volume paracentesis, and that it would be “unethical” not to use it in that clinical situation.
The study was internally supported. Dr. Sharma and Dr. Sarin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON – , according to the results of a randomized controlled trial.
Albumin protected 80% of patients from PICD 6 days after paracentesis, whereas midodrine protected 84%, a difference that was not statistically significant. However, albumin was associated with a slightly higher incidence of adverse events and higher costs, said Mithun Sharma, MD, during his presentation at the annual meeting of the American Association for the Study of Liver Diseases.
Midodrine may be a safer and cost-effective option for these patients, said Dr. Sharma, of the department of hepatology and liver transplantation, AIG Hospitals, Hyderabad, India.
But he cautioned that given the small size of the open-label study, with only 25 patients in each arm, the results should be considered as proof of concept and need to be validated in larger studies.
PICD common in ACLF
PICD is caused by fluid shift during paracentesis, leading to a decrease in effective circulating blood volume.
The incidence of PICD after large-volume paracentesis in patients receiving albumin ranges from 12% to 20%, Dr. Sharma noted.
Albumin has been shown in several trials to be effective at reducing the incidence of PICD in patients undergoing paracentesis, but this agent requires IV infusion and is comparatively costly, he said.
In contrast, midodrine, a selective alpha-adrenergic agonist usually prescribed for orthostatic hypotension, may help to prevent PICD through its mechanism of action, maintaining mean arterial pressure (MAP).
In two small studies comparing albumin infusion in patients undergoing paracentesis with 8 liters of fluid removal, midodrine was either inferior to albumin or had no beneficial effect, Dr. Sharma said.
Patients with ACLF, however, have paracentesis with much lower fluid volumes, typically with less than 5 liters removed, and may be good candidates for midodrine.
Study details
Dr. Sharma and colleagues tested their hypothesis that in patients with ACLF undergoing modest-volume paracentesis, with fluid removal below 5 liters, midodrine could prevent PICD by increasing MAP, with an efficacy similar to that of intravenous 20% human albumin infusions.
They enrolled 50 patients with ACLF defined by Asian Pacific Association for the Study of the Liver criteria who were undergoing paracentesis with 3- to 4-liter fluid volumes.
They defined PICD as at least a 50% increase in plasma renin activity (PRA) over baseline on the 6th day following paracentesis.
The patients were randomly assigned to receive either intravenous 20% human albumin infusions toward the end of paracentesis or midodrine-hydrochloride 7.5 mg three times daily starting 2 hours before paracentesis. Because of the difference in drug delivery methods, the study could not be blinded to treatment type.
Patients’ mean arterial pressures were recorded daily, renal parameters and serum electrolytes were monitored on days 3 and 6, and blood samples were tested for PRA on day 1 and day 6.
The most common acute and chronic hepatic insults and baseline characteristics of the patients were similar between the groups, with alcohol-related liver disease the most common underlying etiology of cirrhosis.
The incidence of PICD at day 6, the primary endpoint, did not differ significantly between the groups, although mean PRA levels on day 6 were numerically higher in the midodrine group. There was a significant rise in the absolute PRA volume from baseline (P = .006), but this rise did not meet the PICD definition.
Researchers found no significant differences between the two groups in absolute change in PRA, and no significant changes in either group in MAP, creatinine, or sodium levels.
Complications and costs
PICD developed in four patients assigned to the albumin group and five patients assigned to the midodrine group; however, this difference was not significant. Fluid overload occurred in only one patient, in the albumin group.
No cases of hypertension or urinary retention arose in either group.
Grade I/II hepatic encephalopathy occurred 2-3 days after paracentesis in three patients on albumin and in two patients on midodrine.
Acute kidney injury was seen in three patients on albumin and in one patient on midodrine.
At 28 days after paracentesis, three patients in the albumin group had died, all from sepsis and multiorgan failure, while four patients in the midodrine group had died, three from sepsis and multiorgan failure and one from an upper gastrointestinal bleed.
Two patients in the albumin group and one patient in the midodrine group underwent liver transplant 1 month after paracentesis.
A cost-effectiveness analysis showed that the mean cost of albumin infusions was about sixfold higher than that of oral midodrine.
More data needed
Session moderator Shiv K. Sarin, MD, from the Institute of Liver and Biliary Sciences in New Delhi, India, who was not involved in the study, commented that while midodrine is a good drug and generally safe, he would wait to use it in patients who needed modest-volume paracentesis until more data are published.
Dr. Sarin also emphasized that albumin is “mandatory” for protecting patients who require large-volume paracentesis, and that it would be “unethical” not to use it in that clinical situation.
The study was internally supported. Dr. Sharma and Dr. Sarin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
Extreme temperature shifts tied to increase in hate speech
, according to researchers from the Potsdam Institute for Climate Impact Research.
What to know
- Analyzing over four billion tweets posted on the social media platform Twitter in the United States, researchers found that hate speech increased across climate zones, income groups, and belief systems when temperatures were too hot or too cold outside.
- The minimum number of hate tweets appears to occur when temperatures are between 15° and 18° C (59° to 65° F). The precise feel-good temperature window varies a little across climate zones, depending on what temperatures are common in those regions.
- When temperatures rose or fell from the feel-good temperature margin, online hate increased up to 12% for colder temperatures and up to 22% for hotter temperatures.
- The United Nations defines hate speech as cases of discriminatory language with reference to a person or a group on the basis of their religion, ethnicity, nationality, race, color, descent, gender, or other identity factor.
- The consequences of more aggressive online behavior can be severe, as hate speech has been found to have negative effects on the mental health of online hate victims, especially for young people and marginalized groups. It can also be predictive of hate crimes in the offline world.
A version of this article first appeared on Medscape.com.
This is a summary of the article, “Temperature Impacts on Hate Speech Online: Evidence From Four Billion Tweets,” published by The Lancet Planetary Health on September 1, 2022. The full article can be found on thelancet.com.
, according to researchers from the Potsdam Institute for Climate Impact Research.
What to know
- Analyzing over four billion tweets posted on the social media platform Twitter in the United States, researchers found that hate speech increased across climate zones, income groups, and belief systems when temperatures were too hot or too cold outside.
- The minimum number of hate tweets appears to occur when temperatures are between 15° and 18° C (59° to 65° F). The precise feel-good temperature window varies a little across climate zones, depending on what temperatures are common in those regions.
- When temperatures rose or fell from the feel-good temperature margin, online hate increased up to 12% for colder temperatures and up to 22% for hotter temperatures.
- The United Nations defines hate speech as cases of discriminatory language with reference to a person or a group on the basis of their religion, ethnicity, nationality, race, color, descent, gender, or other identity factor.
- The consequences of more aggressive online behavior can be severe, as hate speech has been found to have negative effects on the mental health of online hate victims, especially for young people and marginalized groups. It can also be predictive of hate crimes in the offline world.
A version of this article first appeared on Medscape.com.
This is a summary of the article, “Temperature Impacts on Hate Speech Online: Evidence From Four Billion Tweets,” published by The Lancet Planetary Health on September 1, 2022. The full article can be found on thelancet.com.
, according to researchers from the Potsdam Institute for Climate Impact Research.
What to know
- Analyzing over four billion tweets posted on the social media platform Twitter in the United States, researchers found that hate speech increased across climate zones, income groups, and belief systems when temperatures were too hot or too cold outside.
- The minimum number of hate tweets appears to occur when temperatures are between 15° and 18° C (59° to 65° F). The precise feel-good temperature window varies a little across climate zones, depending on what temperatures are common in those regions.
- When temperatures rose or fell from the feel-good temperature margin, online hate increased up to 12% for colder temperatures and up to 22% for hotter temperatures.
- The United Nations defines hate speech as cases of discriminatory language with reference to a person or a group on the basis of their religion, ethnicity, nationality, race, color, descent, gender, or other identity factor.
- The consequences of more aggressive online behavior can be severe, as hate speech has been found to have negative effects on the mental health of online hate victims, especially for young people and marginalized groups. It can also be predictive of hate crimes in the offline world.
A version of this article first appeared on Medscape.com.
This is a summary of the article, “Temperature Impacts on Hate Speech Online: Evidence From Four Billion Tweets,” published by The Lancet Planetary Health on September 1, 2022. The full article can be found on thelancet.com.
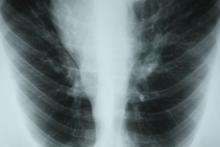
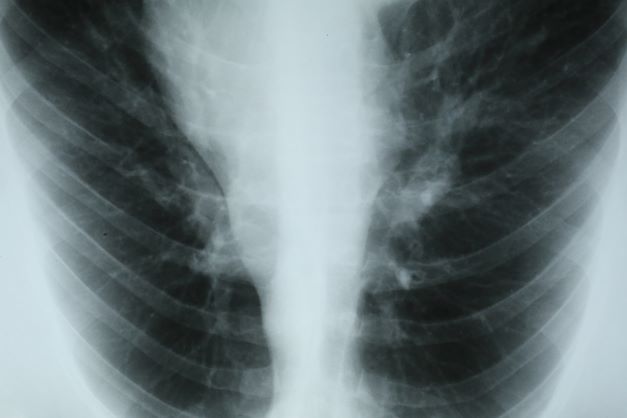
Chest tightness and wheezing
This patient's physical examination and imaging findings are consistent with a diagnosis of acute severe asthma. Agitation, breathlessness during rest, and a respiratory rate > 30 breaths/min are some manifestations of an acute severe episode. During severe episodes, accessory muscles of respiration are usually used, and suprasternal retractions are often present. The heart rate is > 120 beats/min and the respiratory rate is > 30 breaths/min. Loud biphasic (expiratory and inspiratory) wheezing can be heard, and pulsus paradoxus is often present (20-40 mm Hg). Oxyhemoglobin saturation with room air is < 91%. As the severity increases, the patient increasingly assumes a hunched-over sitting position with the hands supporting the torso, termed the tripod position.
Asthma is a chronic, heterogenous inflammatory airway disorder characterized by variable expiratory flow; airway wall thickening; respiratory symptoms; and exacerbations, which sometimes require hospitalization. According to the World Health Organization, asthma affected an estimated 262 million people in 2019. The presence of airway hyperresponsiveness or bronchial hyperreactivity in asthma is an exaggerated response to various exogenous and endogenous stimuli. Mechanisms implicated in the development of asthma include direct stimulation of airway smooth muscle and indirect stimulation by pharmacologically active substances from mediator-secreting cells, such as mast cells or nonmyelinated sensory neurons. The degree of airway hyperresponsiveness is associated with the clinical severity of asthma.
Acute severe asthma is a life-threatening emergency characterized by severe airflow limitation that is unresponsive to the typical appropriate bronchodilator therapy. As a result of pathophysiologic changes, airflow is severely restricted in severe asthma, leading to premature closure of the airway on expiration; impaired gas exchange; and dynamic hyperinflation, or air-trapping. In such cases, urgent action is essential to thwart serious outcomes, including mechanical ventilation and death.
Asthma severity is defined by the level of treatment required to control a patient's symptoms and exacerbations. According to the 2022 Global Initiative for Asthma (GINA) guidelines, a severe asthma exacerbation describes a patient who talks in words (rather than sentences); leans forward; is agitated; uses accessory respiratory muscles; and has a respiratory rate > 30 breaths/min, heart rate > 120 beats/min, oxygen saturation on air < 90%, and peak expiratory flow ≤ 50% of their best or of predicted value. Given the heterogeneity of asthma, patients with acute severe asthma may present with a variety of signs and symptoms, including dyspnea, chest tightness, cough and wheezing, agitation, drowsiness or signs of confusion, and significant breathlessness at rest.
Exposure to external agents, such as indoor and outdoor allergens, air pollutants, and respiratory tract infections (primarily viral), are the most common causes of asthma exacerbations, which vary in severity. Numerous other factors can trigger an asthma exacerbation, including exercise, weather changes, certain foods, additives, drugs, extreme emotional expressions, rhinitis, sinusitis, polyposis, gastroesophageal reflux, menstruation, and pregnancy. Importantly, a patient with known asthma of any level of severity can experience an asthma exacerbation, including patients with mild or well-controlled asthma.
Patients with a history of poorly controlled asthma or a recent exacerbation are at risk for an acute asthma exacerbation. Other risk factors include poor perception of airflow limitation, regular or overuse of short-acting beta agonists, incorrect inhaler technique, and suboptimal adherence to therapy. Comorbidities associated with risk for an acute asthma exacerbation include obesity, chronic rhinosinusitis, inducible laryngeal obstruction (vocal cord dysfunction), gastroesophageal reflux disease, chronic obstructive pulmonary disease, obstructive sleep apnea, bronchiectasis, cardiac disease, and kyphosis due to osteoporosis (followed by corticosteroid overuse). The lack of a written asthma action plan and socioeconomic factors are also associated with increased risk for a severe exacerbation.
In the emergency department setting, pharmacologic therapy of acute severe asthma should consist of a short-acting beta agonist, ipratropium bromide, systemic corticosteroids (oral or intravenous), and controlled oxygen therapy. Clinicians may also consider intravenous magnesium sulfate and high-dose inhaled corticosteroids. Once stable, patients should be treated with optimal asthma-controlling therapy, as outlined in GINA guidelines. Optimizing patients' inhaler technique and adherence to therapy are imperative, and comorbidities should be appropriately managed. Nonpharmacologic interventions, such as smoking cessation, pulmonary rehabilitation, exercise, weight loss, and influenza/COVID-19 vaccination, are also recommended as indicated.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's physical examination and imaging findings are consistent with a diagnosis of acute severe asthma. Agitation, breathlessness during rest, and a respiratory rate > 30 breaths/min are some manifestations of an acute severe episode. During severe episodes, accessory muscles of respiration are usually used, and suprasternal retractions are often present. The heart rate is > 120 beats/min and the respiratory rate is > 30 breaths/min. Loud biphasic (expiratory and inspiratory) wheezing can be heard, and pulsus paradoxus is often present (20-40 mm Hg). Oxyhemoglobin saturation with room air is < 91%. As the severity increases, the patient increasingly assumes a hunched-over sitting position with the hands supporting the torso, termed the tripod position.
Asthma is a chronic, heterogenous inflammatory airway disorder characterized by variable expiratory flow; airway wall thickening; respiratory symptoms; and exacerbations, which sometimes require hospitalization. According to the World Health Organization, asthma affected an estimated 262 million people in 2019. The presence of airway hyperresponsiveness or bronchial hyperreactivity in asthma is an exaggerated response to various exogenous and endogenous stimuli. Mechanisms implicated in the development of asthma include direct stimulation of airway smooth muscle and indirect stimulation by pharmacologically active substances from mediator-secreting cells, such as mast cells or nonmyelinated sensory neurons. The degree of airway hyperresponsiveness is associated with the clinical severity of asthma.
Acute severe asthma is a life-threatening emergency characterized by severe airflow limitation that is unresponsive to the typical appropriate bronchodilator therapy. As a result of pathophysiologic changes, airflow is severely restricted in severe asthma, leading to premature closure of the airway on expiration; impaired gas exchange; and dynamic hyperinflation, or air-trapping. In such cases, urgent action is essential to thwart serious outcomes, including mechanical ventilation and death.
Asthma severity is defined by the level of treatment required to control a patient's symptoms and exacerbations. According to the 2022 Global Initiative for Asthma (GINA) guidelines, a severe asthma exacerbation describes a patient who talks in words (rather than sentences); leans forward; is agitated; uses accessory respiratory muscles; and has a respiratory rate > 30 breaths/min, heart rate > 120 beats/min, oxygen saturation on air < 90%, and peak expiratory flow ≤ 50% of their best or of predicted value. Given the heterogeneity of asthma, patients with acute severe asthma may present with a variety of signs and symptoms, including dyspnea, chest tightness, cough and wheezing, agitation, drowsiness or signs of confusion, and significant breathlessness at rest.
Exposure to external agents, such as indoor and outdoor allergens, air pollutants, and respiratory tract infections (primarily viral), are the most common causes of asthma exacerbations, which vary in severity. Numerous other factors can trigger an asthma exacerbation, including exercise, weather changes, certain foods, additives, drugs, extreme emotional expressions, rhinitis, sinusitis, polyposis, gastroesophageal reflux, menstruation, and pregnancy. Importantly, a patient with known asthma of any level of severity can experience an asthma exacerbation, including patients with mild or well-controlled asthma.
Patients with a history of poorly controlled asthma or a recent exacerbation are at risk for an acute asthma exacerbation. Other risk factors include poor perception of airflow limitation, regular or overuse of short-acting beta agonists, incorrect inhaler technique, and suboptimal adherence to therapy. Comorbidities associated with risk for an acute asthma exacerbation include obesity, chronic rhinosinusitis, inducible laryngeal obstruction (vocal cord dysfunction), gastroesophageal reflux disease, chronic obstructive pulmonary disease, obstructive sleep apnea, bronchiectasis, cardiac disease, and kyphosis due to osteoporosis (followed by corticosteroid overuse). The lack of a written asthma action plan and socioeconomic factors are also associated with increased risk for a severe exacerbation.
In the emergency department setting, pharmacologic therapy of acute severe asthma should consist of a short-acting beta agonist, ipratropium bromide, systemic corticosteroids (oral or intravenous), and controlled oxygen therapy. Clinicians may also consider intravenous magnesium sulfate and high-dose inhaled corticosteroids. Once stable, patients should be treated with optimal asthma-controlling therapy, as outlined in GINA guidelines. Optimizing patients' inhaler technique and adherence to therapy are imperative, and comorbidities should be appropriately managed. Nonpharmacologic interventions, such as smoking cessation, pulmonary rehabilitation, exercise, weight loss, and influenza/COVID-19 vaccination, are also recommended as indicated.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's physical examination and imaging findings are consistent with a diagnosis of acute severe asthma. Agitation, breathlessness during rest, and a respiratory rate > 30 breaths/min are some manifestations of an acute severe episode. During severe episodes, accessory muscles of respiration are usually used, and suprasternal retractions are often present. The heart rate is > 120 beats/min and the respiratory rate is > 30 breaths/min. Loud biphasic (expiratory and inspiratory) wheezing can be heard, and pulsus paradoxus is often present (20-40 mm Hg). Oxyhemoglobin saturation with room air is < 91%. As the severity increases, the patient increasingly assumes a hunched-over sitting position with the hands supporting the torso, termed the tripod position.
Asthma is a chronic, heterogenous inflammatory airway disorder characterized by variable expiratory flow; airway wall thickening; respiratory symptoms; and exacerbations, which sometimes require hospitalization. According to the World Health Organization, asthma affected an estimated 262 million people in 2019. The presence of airway hyperresponsiveness or bronchial hyperreactivity in asthma is an exaggerated response to various exogenous and endogenous stimuli. Mechanisms implicated in the development of asthma include direct stimulation of airway smooth muscle and indirect stimulation by pharmacologically active substances from mediator-secreting cells, such as mast cells or nonmyelinated sensory neurons. The degree of airway hyperresponsiveness is associated with the clinical severity of asthma.
Acute severe asthma is a life-threatening emergency characterized by severe airflow limitation that is unresponsive to the typical appropriate bronchodilator therapy. As a result of pathophysiologic changes, airflow is severely restricted in severe asthma, leading to premature closure of the airway on expiration; impaired gas exchange; and dynamic hyperinflation, or air-trapping. In such cases, urgent action is essential to thwart serious outcomes, including mechanical ventilation and death.
Asthma severity is defined by the level of treatment required to control a patient's symptoms and exacerbations. According to the 2022 Global Initiative for Asthma (GINA) guidelines, a severe asthma exacerbation describes a patient who talks in words (rather than sentences); leans forward; is agitated; uses accessory respiratory muscles; and has a respiratory rate > 30 breaths/min, heart rate > 120 beats/min, oxygen saturation on air < 90%, and peak expiratory flow ≤ 50% of their best or of predicted value. Given the heterogeneity of asthma, patients with acute severe asthma may present with a variety of signs and symptoms, including dyspnea, chest tightness, cough and wheezing, agitation, drowsiness or signs of confusion, and significant breathlessness at rest.
Exposure to external agents, such as indoor and outdoor allergens, air pollutants, and respiratory tract infections (primarily viral), are the most common causes of asthma exacerbations, which vary in severity. Numerous other factors can trigger an asthma exacerbation, including exercise, weather changes, certain foods, additives, drugs, extreme emotional expressions, rhinitis, sinusitis, polyposis, gastroesophageal reflux, menstruation, and pregnancy. Importantly, a patient with known asthma of any level of severity can experience an asthma exacerbation, including patients with mild or well-controlled asthma.
Patients with a history of poorly controlled asthma or a recent exacerbation are at risk for an acute asthma exacerbation. Other risk factors include poor perception of airflow limitation, regular or overuse of short-acting beta agonists, incorrect inhaler technique, and suboptimal adherence to therapy. Comorbidities associated with risk for an acute asthma exacerbation include obesity, chronic rhinosinusitis, inducible laryngeal obstruction (vocal cord dysfunction), gastroesophageal reflux disease, chronic obstructive pulmonary disease, obstructive sleep apnea, bronchiectasis, cardiac disease, and kyphosis due to osteoporosis (followed by corticosteroid overuse). The lack of a written asthma action plan and socioeconomic factors are also associated with increased risk for a severe exacerbation.
In the emergency department setting, pharmacologic therapy of acute severe asthma should consist of a short-acting beta agonist, ipratropium bromide, systemic corticosteroids (oral or intravenous), and controlled oxygen therapy. Clinicians may also consider intravenous magnesium sulfate and high-dose inhaled corticosteroids. Once stable, patients should be treated with optimal asthma-controlling therapy, as outlined in GINA guidelines. Optimizing patients' inhaler technique and adherence to therapy are imperative, and comorbidities should be appropriately managed. Nonpharmacologic interventions, such as smoking cessation, pulmonary rehabilitation, exercise, weight loss, and influenza/COVID-19 vaccination, are also recommended as indicated.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 32-year-old Black man presents to the emergency department with severe dyspnea, chest tightness, and wheezing. The patient is sitting forward in the tripod position and appears agitated and confused. Use of accessory respiratory muscles and suprasternal retractions are noted. He reports an approximate 2-week history of rhinorrhea, cough, and mild fever, for which he has been taking an over-the-counter nonsteroidal anti-inflammatory agent and cough suppressant. His prior medical history is notable for obesity, type 2 diabetes, allergic rhinitis, mild asthma, and hypercholesterolemia. The patient is a current smoker (17 pack-years). Pertinent physical examination reveals a respiratory rate of 48 breaths/min, heart rate of 135 beats/min, 87% oxygen saturation, and peak expiratory flow of 300 L/min. Low biphasic wheezing can be heard. Rapid antigen and PCR tests for SARS-CoV-2 detected by nasopharyngeal swabs both come back negative. Chest radiography is ordered and shows pulmonary hyperinflation with bronchial wall thickening.
Staving off holiday weight gain
Five pounds of weight gain during the holidays is a disproven myth that pops up annually like holiday lights. But before you do a happy dance and pile that extra whipped cream on your pie, you should know two things. One, people do gain weight during the holidays. Two, the extra pounds tend to stick around because most people never lose their holiday weight. Over time, these extra pounds can lead to obesity and weight-related conditions such as diabetes and hypertension.
Let’s be clear. Your weight is one of many markers of your wellness and metabolic health. However, weight changes can indicate that your health is off balance. Holiday weight gain often comes from indulging in increased rich foods, less physical activity, higher stress levels, and sleep disruption.
Optimizing lifestyle factors and trying to lose weight is challenging any time of the year. However, the holiday bustle makes losing weight during this time even more challenging for most people. But maintaining your weight and overall wellness is manageable with three simple shifts in mindset, mindful eating, and meal strategy. Let’s discuss each.
Mindset
From personal and professional experience, I see two primary attitudes regarding holiday eating. They are either “I’ll wait till January to go on a diet” or “I’m on a diet, so I can’t eat anything I like during the holidays.” Both attitude extremes prevent enjoyable and healthy eating during the holidays because they place the focus on food. With both mindsets, food is in control, which leaves you feeling out of control. Rather than having an “all or none” mindset during the holidays, I encourage you to ask yourself:
- “What matters most to me during the holidays?” In a recent survey, 72% of Americans said they look forward to during the holidays. Although food often accompanies family celebrations, it’s the time with family that matters most. Choose to savor sweet time spent with loved ones instead of stuffing yourself with excess sugary sweets.
- “How can I enjoy myself without food or alcoholic beverages?” So often, we eat or drink certain foods out of habit. Shift your mindset from “we always do this” to “what could we do instead?” Asking this question may be the doorway to creating new, non–food-centered traditions.
- “How can I have the foods I love during the holidays and still meet my weight and wellness goals?” This question helps you create opportunities instead of depriving yourself. Rather than depriving yourself, you could cut back on snacking or reduce your sugar intake elsewhere. Or add an extra workout session or stress reduction practice during the holidays.
Mindful eating
The purpose of mindful eating isn’t weight loss. Some studies suggest it may help maintain weight. More importantly, mindfulness can improve your relationship with food and promote wellness. Traditional tips for mindful eating include doing the following as you eat: Being present in the moment, not judging your food, slowing down, and savoring the taste of your food. During the holidays, asking additional questions may enhance mindful eating. For instance:
- “Am I eating to avoid uncomfortable emotions?” The holidays can trigger emotions such as grief, sadness, and anxiety. Also, preexisting can worsen. Decadent foods become a quick fix leading to more emotional eating during this season. Addressing these emotions can help you avoid overeating during the holidays. For mental health resources, visit the
- “What food or drink do I most enjoy during the holidays?” Trying to resist your favorite holiday treats can be an exhausting test of “willpower.” Eventually, and psychological reasons, and you “cheat” on your plan to not eat holiday treats. To prevent this painful battle of treat versus cheat, plan to eat your “indulgence food” in moderation. Savor the foods you enjoy. Then cut out the rest of the food you don’t like or feel you must eat because “Aunty Sarah will feel bad.”
Meal strategy
Many holiday treats and parties are unavoidable unless you plan to hide in a cave for the next few weeks. Rather than torturing yourself nibbling on celery and sipping on sparkling water during your holiday event, create a strategy. For 8 years, I’ve been on my weight loss and wellness journey. I have a holiday strategy that helps my patients, clients, and me maintain our weight and wellness during the holidays. One critical part of the strategy is to anticipate indulgence events. Specifically, look at all the planned holiday events and choose three indulgence events. The rest of the time, do your best to stay on your plan. Knowing your indulgence events to look forward to gives you a sense of control over when you indulge. On non-indulgent days, think, “I can eat it but choose not to” instead of the limiting thought, “I can’t eat that.” Choice is a powerful tool. Once at an indulgence event, I focus on mindful eating and enjoying people around me, which cuts down on overeating just because “I can.”
This holiday season is a reunion time for many people, after enduring long separations from family and friends due to the pandemic. Relishing time with loved ones should be your focus during the holidays – not eating yourself into worse health or worrying about dieting. Even if you choose not to make all the shifts in mindset, mindful eating, and meal strategy mentioned, choosing even one change to focus on can help you both enjoy the holidays and have increased control over your weight and wellness. Whatever you do, may you and your loved ones have a safe, healthy, and enjoyable holiday season.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. She is CEO and lead physician at Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed having no relevant financial relationships. A version of this article first appeared on Medscape.com.
Five pounds of weight gain during the holidays is a disproven myth that pops up annually like holiday lights. But before you do a happy dance and pile that extra whipped cream on your pie, you should know two things. One, people do gain weight during the holidays. Two, the extra pounds tend to stick around because most people never lose their holiday weight. Over time, these extra pounds can lead to obesity and weight-related conditions such as diabetes and hypertension.
Let’s be clear. Your weight is one of many markers of your wellness and metabolic health. However, weight changes can indicate that your health is off balance. Holiday weight gain often comes from indulging in increased rich foods, less physical activity, higher stress levels, and sleep disruption.
Optimizing lifestyle factors and trying to lose weight is challenging any time of the year. However, the holiday bustle makes losing weight during this time even more challenging for most people. But maintaining your weight and overall wellness is manageable with three simple shifts in mindset, mindful eating, and meal strategy. Let’s discuss each.
Mindset
From personal and professional experience, I see two primary attitudes regarding holiday eating. They are either “I’ll wait till January to go on a diet” or “I’m on a diet, so I can’t eat anything I like during the holidays.” Both attitude extremes prevent enjoyable and healthy eating during the holidays because they place the focus on food. With both mindsets, food is in control, which leaves you feeling out of control. Rather than having an “all or none” mindset during the holidays, I encourage you to ask yourself:
- “What matters most to me during the holidays?” In a recent survey, 72% of Americans said they look forward to during the holidays. Although food often accompanies family celebrations, it’s the time with family that matters most. Choose to savor sweet time spent with loved ones instead of stuffing yourself with excess sugary sweets.
- “How can I enjoy myself without food or alcoholic beverages?” So often, we eat or drink certain foods out of habit. Shift your mindset from “we always do this” to “what could we do instead?” Asking this question may be the doorway to creating new, non–food-centered traditions.
- “How can I have the foods I love during the holidays and still meet my weight and wellness goals?” This question helps you create opportunities instead of depriving yourself. Rather than depriving yourself, you could cut back on snacking or reduce your sugar intake elsewhere. Or add an extra workout session or stress reduction practice during the holidays.
Mindful eating
The purpose of mindful eating isn’t weight loss. Some studies suggest it may help maintain weight. More importantly, mindfulness can improve your relationship with food and promote wellness. Traditional tips for mindful eating include doing the following as you eat: Being present in the moment, not judging your food, slowing down, and savoring the taste of your food. During the holidays, asking additional questions may enhance mindful eating. For instance:
- “Am I eating to avoid uncomfortable emotions?” The holidays can trigger emotions such as grief, sadness, and anxiety. Also, preexisting can worsen. Decadent foods become a quick fix leading to more emotional eating during this season. Addressing these emotions can help you avoid overeating during the holidays. For mental health resources, visit the
- “What food or drink do I most enjoy during the holidays?” Trying to resist your favorite holiday treats can be an exhausting test of “willpower.” Eventually, and psychological reasons, and you “cheat” on your plan to not eat holiday treats. To prevent this painful battle of treat versus cheat, plan to eat your “indulgence food” in moderation. Savor the foods you enjoy. Then cut out the rest of the food you don’t like or feel you must eat because “Aunty Sarah will feel bad.”
Meal strategy
Many holiday treats and parties are unavoidable unless you plan to hide in a cave for the next few weeks. Rather than torturing yourself nibbling on celery and sipping on sparkling water during your holiday event, create a strategy. For 8 years, I’ve been on my weight loss and wellness journey. I have a holiday strategy that helps my patients, clients, and me maintain our weight and wellness during the holidays. One critical part of the strategy is to anticipate indulgence events. Specifically, look at all the planned holiday events and choose three indulgence events. The rest of the time, do your best to stay on your plan. Knowing your indulgence events to look forward to gives you a sense of control over when you indulge. On non-indulgent days, think, “I can eat it but choose not to” instead of the limiting thought, “I can’t eat that.” Choice is a powerful tool. Once at an indulgence event, I focus on mindful eating and enjoying people around me, which cuts down on overeating just because “I can.”
This holiday season is a reunion time for many people, after enduring long separations from family and friends due to the pandemic. Relishing time with loved ones should be your focus during the holidays – not eating yourself into worse health or worrying about dieting. Even if you choose not to make all the shifts in mindset, mindful eating, and meal strategy mentioned, choosing even one change to focus on can help you both enjoy the holidays and have increased control over your weight and wellness. Whatever you do, may you and your loved ones have a safe, healthy, and enjoyable holiday season.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. She is CEO and lead physician at Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed having no relevant financial relationships. A version of this article first appeared on Medscape.com.
Five pounds of weight gain during the holidays is a disproven myth that pops up annually like holiday lights. But before you do a happy dance and pile that extra whipped cream on your pie, you should know two things. One, people do gain weight during the holidays. Two, the extra pounds tend to stick around because most people never lose their holiday weight. Over time, these extra pounds can lead to obesity and weight-related conditions such as diabetes and hypertension.
Let’s be clear. Your weight is one of many markers of your wellness and metabolic health. However, weight changes can indicate that your health is off balance. Holiday weight gain often comes from indulging in increased rich foods, less physical activity, higher stress levels, and sleep disruption.
Optimizing lifestyle factors and trying to lose weight is challenging any time of the year. However, the holiday bustle makes losing weight during this time even more challenging for most people. But maintaining your weight and overall wellness is manageable with three simple shifts in mindset, mindful eating, and meal strategy. Let’s discuss each.
Mindset
From personal and professional experience, I see two primary attitudes regarding holiday eating. They are either “I’ll wait till January to go on a diet” or “I’m on a diet, so I can’t eat anything I like during the holidays.” Both attitude extremes prevent enjoyable and healthy eating during the holidays because they place the focus on food. With both mindsets, food is in control, which leaves you feeling out of control. Rather than having an “all or none” mindset during the holidays, I encourage you to ask yourself:
- “What matters most to me during the holidays?” In a recent survey, 72% of Americans said they look forward to during the holidays. Although food often accompanies family celebrations, it’s the time with family that matters most. Choose to savor sweet time spent with loved ones instead of stuffing yourself with excess sugary sweets.
- “How can I enjoy myself without food or alcoholic beverages?” So often, we eat or drink certain foods out of habit. Shift your mindset from “we always do this” to “what could we do instead?” Asking this question may be the doorway to creating new, non–food-centered traditions.
- “How can I have the foods I love during the holidays and still meet my weight and wellness goals?” This question helps you create opportunities instead of depriving yourself. Rather than depriving yourself, you could cut back on snacking or reduce your sugar intake elsewhere. Or add an extra workout session or stress reduction practice during the holidays.
Mindful eating
The purpose of mindful eating isn’t weight loss. Some studies suggest it may help maintain weight. More importantly, mindfulness can improve your relationship with food and promote wellness. Traditional tips for mindful eating include doing the following as you eat: Being present in the moment, not judging your food, slowing down, and savoring the taste of your food. During the holidays, asking additional questions may enhance mindful eating. For instance:
- “Am I eating to avoid uncomfortable emotions?” The holidays can trigger emotions such as grief, sadness, and anxiety. Also, preexisting can worsen. Decadent foods become a quick fix leading to more emotional eating during this season. Addressing these emotions can help you avoid overeating during the holidays. For mental health resources, visit the
- “What food or drink do I most enjoy during the holidays?” Trying to resist your favorite holiday treats can be an exhausting test of “willpower.” Eventually, and psychological reasons, and you “cheat” on your plan to not eat holiday treats. To prevent this painful battle of treat versus cheat, plan to eat your “indulgence food” in moderation. Savor the foods you enjoy. Then cut out the rest of the food you don’t like or feel you must eat because “Aunty Sarah will feel bad.”
Meal strategy
Many holiday treats and parties are unavoidable unless you plan to hide in a cave for the next few weeks. Rather than torturing yourself nibbling on celery and sipping on sparkling water during your holiday event, create a strategy. For 8 years, I’ve been on my weight loss and wellness journey. I have a holiday strategy that helps my patients, clients, and me maintain our weight and wellness during the holidays. One critical part of the strategy is to anticipate indulgence events. Specifically, look at all the planned holiday events and choose three indulgence events. The rest of the time, do your best to stay on your plan. Knowing your indulgence events to look forward to gives you a sense of control over when you indulge. On non-indulgent days, think, “I can eat it but choose not to” instead of the limiting thought, “I can’t eat that.” Choice is a powerful tool. Once at an indulgence event, I focus on mindful eating and enjoying people around me, which cuts down on overeating just because “I can.”
This holiday season is a reunion time for many people, after enduring long separations from family and friends due to the pandemic. Relishing time with loved ones should be your focus during the holidays – not eating yourself into worse health or worrying about dieting. Even if you choose not to make all the shifts in mindset, mindful eating, and meal strategy mentioned, choosing even one change to focus on can help you both enjoy the holidays and have increased control over your weight and wellness. Whatever you do, may you and your loved ones have a safe, healthy, and enjoyable holiday season.
Sylvia Gonsahn-Bollie, MD, DipABOM, is an integrative obesity specialist who specializes in individualized solutions for emotional and biological overeating. She is CEO and lead physician at Embrace You Weight and Wellness, Telehealth & Virtual Counseling. She has disclosed having no relevant financial relationships. A version of this article first appeared on Medscape.com.
Balanced crystalloid fluids surpass saline for kidney transplant
ORLANDO – Using a low-chloride, balanced crystalloid solution for all intravenous fluids received by patients who received a deceased donor kidney transplant resulted in significantly fewer episodes of delayed graft function, compared with patients who received saline as their IV fluids, in a new multicenter trial with 807 randomized and evaluable patients called BEST-Fluids.
“The findings suggest that balanced crystalloids should be the standard-of-care IV fluid in deceased donor kidney transplantations,” Michael G. Collins, MBChB, PhD, said at the annual meeting of the American Society of Nephrology.
“Balanced crystalloids are cheap, readily available worldwide, and this simple change in kidney transplant practice can easily be implemented in global practice ... almost immediately,” said Dr. Collins, a nephrologist at Royal Adelaide Hospital, Australia.
A 1-L bag of balanced crystalloid fluid is more expensive; however, it has a U.S. retail cost of about $2-$5 per bag, compared with about $1 per bag of saline fluid, Dr. Collins added.
Various other commentators had mixed views. Some agreed with Dr. Collins and said the switch could be made immediately, although one researcher wanted to see more trials. Another wondered why balanced crystalloid fluid hadn’t seemed to provide benefit in studies in acute kidney injury.
Treating 10 patients prevents one delayed graft function
The incidence of delayed graft function, defined as the need for dialysis during the 7 days following transplantation, occurred in 30.0% of 404 patients who received balanced crystalloid fluids (Plasma-Lyte 148) and in 39.7% of 403 patients who received saline starting at the time of randomization (prior to surgery) until 48 hours post-surgery, Dr. Collins reported.
This translated into a significant, adjusted relative risk reduction of 26% and a number needed to treat of 10 to result in one avoided episode of delayed graft function.
Preventing delayed graft function is important because it is a “major complication” of deceased donor kidney transplantation that usually occurs in about 30%-50% of people who receive these organs, Dr. Collins explained. Incident delayed graft function leads to higher hospitalization costs because of a prolonged need for dialysis and extended hospital days, as well as increased risk for long-term graft failure and death.
A secondary outcome – the number of dialysis sessions required during the 28 days following transplantation – was 406 sessions among those who received balanced crystalloid fluids and 596 sessions among the controls who received saline, a significant adjusted relative decrease of 30%.
Freedom from need for dialysis by 12 weeks after surgery increased by a significant 10% among those treated with balanced crystalloid fluids, compared with controls. The balanced crystalloid fluids were also significantly linked with an average 1-L increase in urine output during the first 2 days after transplantation, compared with controls.
Chloride is the culprit
“I think this is driven by the harmful effects of saline,” which is currently the standard fluid that kidney transplant patients receive worldwide, said Dr. Collins. Specifically, he cited the chloride content of saline – which contains 0.9% sodium chloride – as the culprit by causing reduced kidney perfusion.
“Some data suggest that saline may be harmful because of chloride acidosis producing vasoconstriction and increasing ischemia,” commented Karen A. Griffin, MD, chief of the renal section at the Edward Hines, Jr. VA Medical Center, Hines, Illinois. But Dr. Griffin said she’d like to see further study of balanced crystalloid fluids in this setting before she’d be comfortable using it routinely as a replacement for saline.
However, Pascale H. Lane, MD, a pediatric nephrologist with Oklahoma University Health, Oklahoma City, predicted that based on these results, “I think it will be rapidly embraced” by U.S. clinicians. Dr. Lane expressed concern about the availability of an adequate supply of balanced crystalloid fluid, but Dr. Collins said he did not believe supply would be an issue based on current availability.
This was “a beautiful study, very well done, with nice results, and a very easy switch to balanced crystalloid fluids without harm,” commented Richard Lafayette, MD, a nephrologist and professor of medicine at Stanford (Calif.) University.
Success attributed to early treatment
But Dr. Lafayette also wondered, “Why should this work for transplant patients when it did not work for patients who develop acute kidney injury in the ICU?” And he found it hard to understand how the impact of the balanced crystalloid fluid could manifest so quickly, with a change in urine output during the first day following surgery.
Dr. Collins attributed the rapid effects and overall success to the early initiation of balanced crystalloid fluids before the transplant occurred.
The BEST-Fluids trial ran at 16 centers in Australia and New Zealand and enrolled patients from January 2018 to August 2020. It enrolled adults and children scheduled to receive a deceased donor kidney, excluding those who weighed less than 20 kg and those who received multiple organs.
Enrolled patients averaged about 55 years old, about 63% were men, and their average duration on dialysis prior to surgery was about 30 months. The study randomized 808 patients who received their transplanted kidney, with 807 included in the efficacy analysis. Patients in each of the two groups showed very close balance for all reported parameters of patient and donor characteristics. During the period of randomized fluid treatment, patients in the balanced crystalloid group received an average of just over 8 L of fluid, while those in the control group received an average of just over 7 L.
During follow-up, serious adverse events were rare and balanced, with three in the balanced crystalloid group and four among controls.
The only significant difference in adverse events was the rate of ICU admissions that required ventilation, which occurred in one patient in the balanced crystalloid group and 12 controls.
BEST-Fluids received balanced crystalloid and saline solutions at no charge from Baxter Healthcare, which markets Plasma-Lyte 148. The study received no other commercial funding. Dr. Collins, Dr. Griffin, and Dr. Lane have reported no relevant financial relationships. Dr. Lafayette has received personal fees and grants from Alexion, Aurinia, Calliditas, Omeros, Pfizer, Roche, Travere, and Vera and has been an advisor to Akahest and Equillium.
A version of this article first appeared on Medscape.com.
ORLANDO – Using a low-chloride, balanced crystalloid solution for all intravenous fluids received by patients who received a deceased donor kidney transplant resulted in significantly fewer episodes of delayed graft function, compared with patients who received saline as their IV fluids, in a new multicenter trial with 807 randomized and evaluable patients called BEST-Fluids.
“The findings suggest that balanced crystalloids should be the standard-of-care IV fluid in deceased donor kidney transplantations,” Michael G. Collins, MBChB, PhD, said at the annual meeting of the American Society of Nephrology.
“Balanced crystalloids are cheap, readily available worldwide, and this simple change in kidney transplant practice can easily be implemented in global practice ... almost immediately,” said Dr. Collins, a nephrologist at Royal Adelaide Hospital, Australia.
A 1-L bag of balanced crystalloid fluid is more expensive; however, it has a U.S. retail cost of about $2-$5 per bag, compared with about $1 per bag of saline fluid, Dr. Collins added.
Various other commentators had mixed views. Some agreed with Dr. Collins and said the switch could be made immediately, although one researcher wanted to see more trials. Another wondered why balanced crystalloid fluid hadn’t seemed to provide benefit in studies in acute kidney injury.
Treating 10 patients prevents one delayed graft function
The incidence of delayed graft function, defined as the need for dialysis during the 7 days following transplantation, occurred in 30.0% of 404 patients who received balanced crystalloid fluids (Plasma-Lyte 148) and in 39.7% of 403 patients who received saline starting at the time of randomization (prior to surgery) until 48 hours post-surgery, Dr. Collins reported.
This translated into a significant, adjusted relative risk reduction of 26% and a number needed to treat of 10 to result in one avoided episode of delayed graft function.
Preventing delayed graft function is important because it is a “major complication” of deceased donor kidney transplantation that usually occurs in about 30%-50% of people who receive these organs, Dr. Collins explained. Incident delayed graft function leads to higher hospitalization costs because of a prolonged need for dialysis and extended hospital days, as well as increased risk for long-term graft failure and death.
A secondary outcome – the number of dialysis sessions required during the 28 days following transplantation – was 406 sessions among those who received balanced crystalloid fluids and 596 sessions among the controls who received saline, a significant adjusted relative decrease of 30%.
Freedom from need for dialysis by 12 weeks after surgery increased by a significant 10% among those treated with balanced crystalloid fluids, compared with controls. The balanced crystalloid fluids were also significantly linked with an average 1-L increase in urine output during the first 2 days after transplantation, compared with controls.
Chloride is the culprit
“I think this is driven by the harmful effects of saline,” which is currently the standard fluid that kidney transplant patients receive worldwide, said Dr. Collins. Specifically, he cited the chloride content of saline – which contains 0.9% sodium chloride – as the culprit by causing reduced kidney perfusion.
“Some data suggest that saline may be harmful because of chloride acidosis producing vasoconstriction and increasing ischemia,” commented Karen A. Griffin, MD, chief of the renal section at the Edward Hines, Jr. VA Medical Center, Hines, Illinois. But Dr. Griffin said she’d like to see further study of balanced crystalloid fluids in this setting before she’d be comfortable using it routinely as a replacement for saline.
However, Pascale H. Lane, MD, a pediatric nephrologist with Oklahoma University Health, Oklahoma City, predicted that based on these results, “I think it will be rapidly embraced” by U.S. clinicians. Dr. Lane expressed concern about the availability of an adequate supply of balanced crystalloid fluid, but Dr. Collins said he did not believe supply would be an issue based on current availability.
This was “a beautiful study, very well done, with nice results, and a very easy switch to balanced crystalloid fluids without harm,” commented Richard Lafayette, MD, a nephrologist and professor of medicine at Stanford (Calif.) University.
Success attributed to early treatment
But Dr. Lafayette also wondered, “Why should this work for transplant patients when it did not work for patients who develop acute kidney injury in the ICU?” And he found it hard to understand how the impact of the balanced crystalloid fluid could manifest so quickly, with a change in urine output during the first day following surgery.
Dr. Collins attributed the rapid effects and overall success to the early initiation of balanced crystalloid fluids before the transplant occurred.
The BEST-Fluids trial ran at 16 centers in Australia and New Zealand and enrolled patients from January 2018 to August 2020. It enrolled adults and children scheduled to receive a deceased donor kidney, excluding those who weighed less than 20 kg and those who received multiple organs.
Enrolled patients averaged about 55 years old, about 63% were men, and their average duration on dialysis prior to surgery was about 30 months. The study randomized 808 patients who received their transplanted kidney, with 807 included in the efficacy analysis. Patients in each of the two groups showed very close balance for all reported parameters of patient and donor characteristics. During the period of randomized fluid treatment, patients in the balanced crystalloid group received an average of just over 8 L of fluid, while those in the control group received an average of just over 7 L.
During follow-up, serious adverse events were rare and balanced, with three in the balanced crystalloid group and four among controls.
The only significant difference in adverse events was the rate of ICU admissions that required ventilation, which occurred in one patient in the balanced crystalloid group and 12 controls.
BEST-Fluids received balanced crystalloid and saline solutions at no charge from Baxter Healthcare, which markets Plasma-Lyte 148. The study received no other commercial funding. Dr. Collins, Dr. Griffin, and Dr. Lane have reported no relevant financial relationships. Dr. Lafayette has received personal fees and grants from Alexion, Aurinia, Calliditas, Omeros, Pfizer, Roche, Travere, and Vera and has been an advisor to Akahest and Equillium.
A version of this article first appeared on Medscape.com.
ORLANDO – Using a low-chloride, balanced crystalloid solution for all intravenous fluids received by patients who received a deceased donor kidney transplant resulted in significantly fewer episodes of delayed graft function, compared with patients who received saline as their IV fluids, in a new multicenter trial with 807 randomized and evaluable patients called BEST-Fluids.
“The findings suggest that balanced crystalloids should be the standard-of-care IV fluid in deceased donor kidney transplantations,” Michael G. Collins, MBChB, PhD, said at the annual meeting of the American Society of Nephrology.
“Balanced crystalloids are cheap, readily available worldwide, and this simple change in kidney transplant practice can easily be implemented in global practice ... almost immediately,” said Dr. Collins, a nephrologist at Royal Adelaide Hospital, Australia.
A 1-L bag of balanced crystalloid fluid is more expensive; however, it has a U.S. retail cost of about $2-$5 per bag, compared with about $1 per bag of saline fluid, Dr. Collins added.
Various other commentators had mixed views. Some agreed with Dr. Collins and said the switch could be made immediately, although one researcher wanted to see more trials. Another wondered why balanced crystalloid fluid hadn’t seemed to provide benefit in studies in acute kidney injury.
Treating 10 patients prevents one delayed graft function
The incidence of delayed graft function, defined as the need for dialysis during the 7 days following transplantation, occurred in 30.0% of 404 patients who received balanced crystalloid fluids (Plasma-Lyte 148) and in 39.7% of 403 patients who received saline starting at the time of randomization (prior to surgery) until 48 hours post-surgery, Dr. Collins reported.
This translated into a significant, adjusted relative risk reduction of 26% and a number needed to treat of 10 to result in one avoided episode of delayed graft function.
Preventing delayed graft function is important because it is a “major complication” of deceased donor kidney transplantation that usually occurs in about 30%-50% of people who receive these organs, Dr. Collins explained. Incident delayed graft function leads to higher hospitalization costs because of a prolonged need for dialysis and extended hospital days, as well as increased risk for long-term graft failure and death.
A secondary outcome – the number of dialysis sessions required during the 28 days following transplantation – was 406 sessions among those who received balanced crystalloid fluids and 596 sessions among the controls who received saline, a significant adjusted relative decrease of 30%.
Freedom from need for dialysis by 12 weeks after surgery increased by a significant 10% among those treated with balanced crystalloid fluids, compared with controls. The balanced crystalloid fluids were also significantly linked with an average 1-L increase in urine output during the first 2 days after transplantation, compared with controls.
Chloride is the culprit
“I think this is driven by the harmful effects of saline,” which is currently the standard fluid that kidney transplant patients receive worldwide, said Dr. Collins. Specifically, he cited the chloride content of saline – which contains 0.9% sodium chloride – as the culprit by causing reduced kidney perfusion.
“Some data suggest that saline may be harmful because of chloride acidosis producing vasoconstriction and increasing ischemia,” commented Karen A. Griffin, MD, chief of the renal section at the Edward Hines, Jr. VA Medical Center, Hines, Illinois. But Dr. Griffin said she’d like to see further study of balanced crystalloid fluids in this setting before she’d be comfortable using it routinely as a replacement for saline.
However, Pascale H. Lane, MD, a pediatric nephrologist with Oklahoma University Health, Oklahoma City, predicted that based on these results, “I think it will be rapidly embraced” by U.S. clinicians. Dr. Lane expressed concern about the availability of an adequate supply of balanced crystalloid fluid, but Dr. Collins said he did not believe supply would be an issue based on current availability.
This was “a beautiful study, very well done, with nice results, and a very easy switch to balanced crystalloid fluids without harm,” commented Richard Lafayette, MD, a nephrologist and professor of medicine at Stanford (Calif.) University.
Success attributed to early treatment
But Dr. Lafayette also wondered, “Why should this work for transplant patients when it did not work for patients who develop acute kidney injury in the ICU?” And he found it hard to understand how the impact of the balanced crystalloid fluid could manifest so quickly, with a change in urine output during the first day following surgery.
Dr. Collins attributed the rapid effects and overall success to the early initiation of balanced crystalloid fluids before the transplant occurred.
The BEST-Fluids trial ran at 16 centers in Australia and New Zealand and enrolled patients from January 2018 to August 2020. It enrolled adults and children scheduled to receive a deceased donor kidney, excluding those who weighed less than 20 kg and those who received multiple organs.
Enrolled patients averaged about 55 years old, about 63% were men, and their average duration on dialysis prior to surgery was about 30 months. The study randomized 808 patients who received their transplanted kidney, with 807 included in the efficacy analysis. Patients in each of the two groups showed very close balance for all reported parameters of patient and donor characteristics. During the period of randomized fluid treatment, patients in the balanced crystalloid group received an average of just over 8 L of fluid, while those in the control group received an average of just over 7 L.
During follow-up, serious adverse events were rare and balanced, with three in the balanced crystalloid group and four among controls.
The only significant difference in adverse events was the rate of ICU admissions that required ventilation, which occurred in one patient in the balanced crystalloid group and 12 controls.
BEST-Fluids received balanced crystalloid and saline solutions at no charge from Baxter Healthcare, which markets Plasma-Lyte 148. The study received no other commercial funding. Dr. Collins, Dr. Griffin, and Dr. Lane have reported no relevant financial relationships. Dr. Lafayette has received personal fees and grants from Alexion, Aurinia, Calliditas, Omeros, Pfizer, Roche, Travere, and Vera and has been an advisor to Akahest and Equillium.
A version of this article first appeared on Medscape.com.
AT KIDNEY WEEK 2022
EHR reminders boost well-child visits, vax rates
Reminder messages sent through electronic health records (EHRs) to patient portals increased rates of scheduling and completion of well-child visits for those overdue for well care, as well as vaccination rates, according to data published today in JAMA Network Open.
Anne E. Berset, BA, with general and community pediatrics at Cincinnati Children’s Hospital, led a randomized clinical trial conducted at three academic primary care practices from July 30 to Oct. 4, 2021.
The practices serve a mostly non-Hispanic Black, low-income population and provide more than 60,000 visits a year to 30,000 patients.
The study population included 945 patients, 62.4% of them non-Hispanic Black and 807 (85.4%) covered by public insurance.
Standard message and tailored message compared
The study population was randomized to get either a standard reminder message, a tailored message, or no message (control group).
The standard messages referenced the patient’s first name and reminded parents their child was due for a well-child visit, and asked them to schedule it using the portal or by calling the number provided.
The tailored message added the date of the last well-child check-up “to address distortions of time perception experienced during the pandemic,” the authors write.
The primary outcome was whether the well-child visit was completed within 8 weeks. Other outcomes included whether a well-child visit was scheduled within 2 weeks of the first reminder message and whether the patient received a COVID-19 vaccine within 8 weeks of the reminder.
Reminders outperform control group
There was a significant increase in scheduling and completion of appointments after parents received the standard and tailored messages compared with controls.
The results with the COVID reminders were particularly striking. While only 3.7% of the eligible patients got a vaccine when they got no message, 17.3% got the vaccine in that time frame when they got the standard message. Interestingly, only 2.7% received the shot after the tailored message.
J. Howard Smart, MD, with Sharp HealthCare in San Diego, told this publication he was not surprised that a patient portal reminder would increase the rate of scheduling overdue well-child visits. “Parents may indeed have lost track of when their children need to have check-ups” during the pandemic, he said.
Reminders without tailored information more effective
He said he was surprised, just as the investigators were, that the reminders without the tailored information seemed to be more effective for some outcomes.
The authors explained that a focus group found “that families with patient portal accounts prefer straightforward, brief, and user-friendly messages.”
That may help explain why the standard message outperformed the tailored message, the authors write.
“Families may have been distracted by the additional information related to the child’s age and date of last WCC [well-child check-up] in the tailored message,” they write.
Dr. Smart said, “Their discussion of why this might be the case sounds reasonable. Simple, short messages may be better received.”
“Evidence like this should stimulate portal software vendors to make this kind of outgoing messaging easy to set up for providers,” he said.
Simple measure, large effect
Tim Joos, MD, a pediatrician in Seattle, told this publication he “was impressed by how such a simple measure increased rates of well-child care completion.”
Minority and low-income patients have traditionally had more access challenges to completing health care visits and vaccinations on schedule, he noted, so he was glad to see positive results from the intervention in this study population.
The authors note that research on portal messages have largely looked at use in non-Hispanic Whites and the privately insured.
Dr. Joos said the intervention also offers convenience in that once patients get into the portal, they can schedule an appointment there without having to wait on the phone or leave a message.
Funding/support was received from the Center for Clinical and Translational Science and Training at the University of Cincinnati, which is funded by the National Institutes of Health. William B. Brinkman, MD, MEd, MSc, one of the study authors, holds common stock in Pfizer, Merck, Abbott Laboratories, Viatris, and Johnson & Johnson outside the submitted work. No other author disclosures were reported. Dr. Smart and Dr. Joos declared no relevant financial relationships.
Reminder messages sent through electronic health records (EHRs) to patient portals increased rates of scheduling and completion of well-child visits for those overdue for well care, as well as vaccination rates, according to data published today in JAMA Network Open.
Anne E. Berset, BA, with general and community pediatrics at Cincinnati Children’s Hospital, led a randomized clinical trial conducted at three academic primary care practices from July 30 to Oct. 4, 2021.
The practices serve a mostly non-Hispanic Black, low-income population and provide more than 60,000 visits a year to 30,000 patients.
The study population included 945 patients, 62.4% of them non-Hispanic Black and 807 (85.4%) covered by public insurance.
Standard message and tailored message compared
The study population was randomized to get either a standard reminder message, a tailored message, or no message (control group).
The standard messages referenced the patient’s first name and reminded parents their child was due for a well-child visit, and asked them to schedule it using the portal or by calling the number provided.
The tailored message added the date of the last well-child check-up “to address distortions of time perception experienced during the pandemic,” the authors write.
The primary outcome was whether the well-child visit was completed within 8 weeks. Other outcomes included whether a well-child visit was scheduled within 2 weeks of the first reminder message and whether the patient received a COVID-19 vaccine within 8 weeks of the reminder.
Reminders outperform control group
There was a significant increase in scheduling and completion of appointments after parents received the standard and tailored messages compared with controls.
The results with the COVID reminders were particularly striking. While only 3.7% of the eligible patients got a vaccine when they got no message, 17.3% got the vaccine in that time frame when they got the standard message. Interestingly, only 2.7% received the shot after the tailored message.
J. Howard Smart, MD, with Sharp HealthCare in San Diego, told this publication he was not surprised that a patient portal reminder would increase the rate of scheduling overdue well-child visits. “Parents may indeed have lost track of when their children need to have check-ups” during the pandemic, he said.
Reminders without tailored information more effective
He said he was surprised, just as the investigators were, that the reminders without the tailored information seemed to be more effective for some outcomes.
The authors explained that a focus group found “that families with patient portal accounts prefer straightforward, brief, and user-friendly messages.”
That may help explain why the standard message outperformed the tailored message, the authors write.
“Families may have been distracted by the additional information related to the child’s age and date of last WCC [well-child check-up] in the tailored message,” they write.
Dr. Smart said, “Their discussion of why this might be the case sounds reasonable. Simple, short messages may be better received.”
“Evidence like this should stimulate portal software vendors to make this kind of outgoing messaging easy to set up for providers,” he said.
Simple measure, large effect
Tim Joos, MD, a pediatrician in Seattle, told this publication he “was impressed by how such a simple measure increased rates of well-child care completion.”
Minority and low-income patients have traditionally had more access challenges to completing health care visits and vaccinations on schedule, he noted, so he was glad to see positive results from the intervention in this study population.
The authors note that research on portal messages have largely looked at use in non-Hispanic Whites and the privately insured.
Dr. Joos said the intervention also offers convenience in that once patients get into the portal, they can schedule an appointment there without having to wait on the phone or leave a message.
Funding/support was received from the Center for Clinical and Translational Science and Training at the University of Cincinnati, which is funded by the National Institutes of Health. William B. Brinkman, MD, MEd, MSc, one of the study authors, holds common stock in Pfizer, Merck, Abbott Laboratories, Viatris, and Johnson & Johnson outside the submitted work. No other author disclosures were reported. Dr. Smart and Dr. Joos declared no relevant financial relationships.
Reminder messages sent through electronic health records (EHRs) to patient portals increased rates of scheduling and completion of well-child visits for those overdue for well care, as well as vaccination rates, according to data published today in JAMA Network Open.
Anne E. Berset, BA, with general and community pediatrics at Cincinnati Children’s Hospital, led a randomized clinical trial conducted at three academic primary care practices from July 30 to Oct. 4, 2021.
The practices serve a mostly non-Hispanic Black, low-income population and provide more than 60,000 visits a year to 30,000 patients.
The study population included 945 patients, 62.4% of them non-Hispanic Black and 807 (85.4%) covered by public insurance.
Standard message and tailored message compared
The study population was randomized to get either a standard reminder message, a tailored message, or no message (control group).
The standard messages referenced the patient’s first name and reminded parents their child was due for a well-child visit, and asked them to schedule it using the portal or by calling the number provided.
The tailored message added the date of the last well-child check-up “to address distortions of time perception experienced during the pandemic,” the authors write.
The primary outcome was whether the well-child visit was completed within 8 weeks. Other outcomes included whether a well-child visit was scheduled within 2 weeks of the first reminder message and whether the patient received a COVID-19 vaccine within 8 weeks of the reminder.
Reminders outperform control group
There was a significant increase in scheduling and completion of appointments after parents received the standard and tailored messages compared with controls.
The results with the COVID reminders were particularly striking. While only 3.7% of the eligible patients got a vaccine when they got no message, 17.3% got the vaccine in that time frame when they got the standard message. Interestingly, only 2.7% received the shot after the tailored message.
J. Howard Smart, MD, with Sharp HealthCare in San Diego, told this publication he was not surprised that a patient portal reminder would increase the rate of scheduling overdue well-child visits. “Parents may indeed have lost track of when their children need to have check-ups” during the pandemic, he said.
Reminders without tailored information more effective
He said he was surprised, just as the investigators were, that the reminders without the tailored information seemed to be more effective for some outcomes.
The authors explained that a focus group found “that families with patient portal accounts prefer straightforward, brief, and user-friendly messages.”
That may help explain why the standard message outperformed the tailored message, the authors write.
“Families may have been distracted by the additional information related to the child’s age and date of last WCC [well-child check-up] in the tailored message,” they write.
Dr. Smart said, “Their discussion of why this might be the case sounds reasonable. Simple, short messages may be better received.”
“Evidence like this should stimulate portal software vendors to make this kind of outgoing messaging easy to set up for providers,” he said.
Simple measure, large effect
Tim Joos, MD, a pediatrician in Seattle, told this publication he “was impressed by how such a simple measure increased rates of well-child care completion.”
Minority and low-income patients have traditionally had more access challenges to completing health care visits and vaccinations on schedule, he noted, so he was glad to see positive results from the intervention in this study population.
The authors note that research on portal messages have largely looked at use in non-Hispanic Whites and the privately insured.
Dr. Joos said the intervention also offers convenience in that once patients get into the portal, they can schedule an appointment there without having to wait on the phone or leave a message.
Funding/support was received from the Center for Clinical and Translational Science and Training at the University of Cincinnati, which is funded by the National Institutes of Health. William B. Brinkman, MD, MEd, MSc, one of the study authors, holds common stock in Pfizer, Merck, Abbott Laboratories, Viatris, and Johnson & Johnson outside the submitted work. No other author disclosures were reported. Dr. Smart and Dr. Joos declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
Medical school culinary medicine programs grow despite limited funding
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
FROM ACADEMIC MEDICINE
Baxdrostat slashes BP in resistant hypertension: BrigHTN
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
CHICAGO – An investigational aldosterone synthase inhibitor could be an effective new treatment to reduce blood pressure in patients with treatment-resistant hypertension, reslts of a phase 2 study suggest.
The BrigHTN trial showed systolic blood pressure fell by an average of 20.3 mm Hg, 17.5 mm Hg, and 12.1 mm Hg with baxdrostat 2 mg, 1 mg, and 0.5 mg after 12 weeks follow-up in 248 patients unable to achieve target blood pressure on stable doses of at least three antihypertensive agents, including a diuretic.
After adjustment for the –9.4 mm Hg change observed in the placebo group, there was a statistically significant difference of 11.0 mm Hg in the 2-mg baxdrostat group (P = .0001) and of 8.1 mm Hg in the 1-mg baxdrostat group (P = .003).
The adjusted change in diastolic blood pressure was significant only for the 2-mg dose (–5.2 mm Hg; P = .004).
Once-daily oral baxdrostat had an acceptable side-effect profile and no patients died.
The study, which was stopped early after meeting criteria for overwhelming efficacy, was presented in the final late-breaking science session at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
Threading the needle
For at least 20 years, researchers have tried to create a drug that would lower aldosterone levels directly by inhibiting hormone synthesis rather than blocking the mineralocorticoid receptor.
What’s made this extraordinarily difficult is that the enzyme that makes aldosterone synthase and the enzyme required for cortisol synthase, 11-beta-hydroxylase, are 93% sequence similar. Baxdrostat, however, is able to selectively block aldosterone synthase, and thus the production of aldosterone, without also blocking the production of cortisol, explained Mason W. Freeman, MD, lead author of the study and executive vice president of clinical development at CinCor Pharma, which is developing the agent.
“We have beautiful biomarker evidence of not only blood pressure lowering but the mechanism by which that blood pressure reduction is occurring,” he said.
Over 12 weeks of follow-up in the new study, the use of baxdrostat led to decreases in serum aldosterone levels ranging from 3.0 ng/dL with the 0.5-mg dose to 4.9 ng/dL with the 2-mg dose. The 24-hour urinary aldosterone levels decreased with all three doses tested.
Baxdrostat increased plasma renin activity by 3.6, 5.0, and 13.8 mg/mL per hr with the 0.5, 1.0, and 2.0 mg doses, respectively, an indicator of its effect on lowering salt and fluid retention, Dr. Freeman said. Serum cortisol levels were not reduced in any of the baxdrostat groups throughout the study.
‘A bright future’
“It seems to have a bright future in the area of resistant hypertension, particularly in patients who are producing too much aldosterone,” said Suzanne Oparil, MD, invited discussant for the study and director of the Vascular Biology and Hypertension program at the University of Alabama at Birmingham.
She noted that aldosterone is a major contributor to the pathogenesis of resistant hypertension, which afflicts about 20% of the hypertensive population. Aldosterone antagonists are considered by many to be the best add-on treatment for resistant hypertension and do lower blood pressure.
“But they have major problems,” Dr. Oparil added. “Spironolactone, for example, causes hyperkalemia in many patients and adverse effects such as gynecomastia, erectile dysfunction, and feminization.”
Baxdrostat was well tolerated with no serious adverse events deemed related to treatment, Dr. Freeman reported. A total of 18 serious adverse events occurred in 10 patients, 6 of which were in a patient with urosepsis.
Ten adverse events of special interest occurred in eight patients, including one case of hypotension, three cases of hyponatremia, and six cases of hyperkalemia.
Potassium levels ranged from 6.0 to 6.3 mmol/L (6.0-6.3 mEq/L) in three patients and between 5.5 and 5.9 mmol/L (5.5-5.9 mEq/L) on at least two consecutive occasions in three others. Four of the patients were able to resume baxdrostat and complete the trial, whereas two patients discontinued treatment, one of whom was the patient with urosepsis.
Dr. Freeman pointed out that the study population was relatively diverse, with 33%-48% of participants of Hispanic or Latinx ethnicity and 23%-32% being Black.
At baseline, all patients had a seated blood pressure of at least 130/80 mm Hg (average 147.8/87.9 mm Hg) on a background therapy that included a diuretic in 100%, an agent targeting the renin-angiotensin-aldosterone system in 91%-96%, a beta-blocker in 52%-68%, and a calcium channel blocker in 64%-70%.
The study was not designed to test the benefits and risks of aldosterone synthase inhibition beyond 12 weeks and baxdrostat was not compared to alternative antihypertensives, he said. Additional limitations are that medication adherence was based on pill counts rather than drug analysis and enrolling only patients with an estimated glomerular filtration rate over 45 mL/min per 1.73m2 reduced the likelihood of hyperkalemia and other adverse events.
Nevertheless, “we think that these data suggest that baxdrostat has the potential to treat disorders associated with aldosterone excess, including hypertension and primary hyperaldosteronism,” Dr. Freeman said.
The intention is to carry the drug forward into additional phase 2 studies in chronic kidney disease and to begin a phase 3 study in hypertension in 2023, he noted.
The study was funded by CinCor Pharma. Dr. Freeman and three coauthors are employees of CinCor and receive stock-based compensation. The remaining authors have a financial relationship with CinRx Pharma, which has an equity stake in CinCor. Dr. Oparil reports grant/research support from Bayer, Higi, and Novartis; and serving on the scientific advisory board/expert committee for CinCor Pharma and Preventric Diagnostics.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Birth method affects microbiome and vaccination response
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
Babies born vaginally have a different microbiome to those born by Caesarean section and have heightened responses to childhood vaccinations, according to a new study heralded as “interesting and important” by experts.
The microbiome is known to play a role in immune responses to vaccination. However, the relationship between early-life effects on intestinal microbiota composition and subsequent childhood vaccine responses had remained poorly understood. In the new study, “the findings suggest that vaginal birthing resulted in a microbiota composition associated with an increase in a specific type of antibody response to two routine childhood vaccines in healthy babies, compared with Caesarean section,” the authors said.
Researchers from the University of Edinburgh, with colleagues at Spaarne Hospital and University Medical Centre in Utrecht, and the National Institute for Public Health and the Environment in The Netherlands, tracked the development of the gut microbiome in a cohort of 120 healthy, full-term infants and assessed their antibody levels following two common childhood vaccinations.
The study, published in Nature Communications, found “a clear relationship between microbes in the gut of those babies and levels of antibodies.” Not only was vaginal birth associated with increased levels of Bifidobacterium and Escherichia coli in the gut microbiome in the first months of life but also with higher IgG antibody responses against both pneumococcal and meningococcal vaccines.
Antibody responses doubled after vaginal birth
The babies were given pneumococcal and meningococcal vaccinations at 8 and 12 weeks, and saliva was collected at follow-up visits at ages 12 and 18 months for antibody measurement. In the 101 babies tested for pneumococcal antibodies, the researchers found that antibody levels were twice as high among babies delivered naturally, compared with those delivered by C-section. High levels of two gut bacteria in particular – Bifidobacterium and E. coli – were associated with high antibody responses to the pneumococcal vaccine, showing that the microbiome mediated the link between mode of delivery and pneumococcal vaccine responses.
In 66 babies tested for anti-meningococcal antibodies, antibodies were 1.7 times higher for vaginally-born babies than those delivered via C-section, and high antibody levels were particularly associated with high levels of E. coli in the babies’ microbiome.
The results were also influenced by breast-feeding, which even among children born vaginally was linked with 3.5 times higher pneumococcal antibody levels, compared with those of formula-fed children. In contrast, levels of antibodies against meningococcus were unaffected by breast-feeding status.
Microbiome ‘sets level of infection protection’
The team said: “The baby acquires Bifidobacterium and E. coli bacteria through natural birth, and human milk is needed to provide the sugars for these bacteria to thrive on.” They explained: “The gut microbiome is seeded at birth, developing rapidly over the first few months of life, and is influenced mostly by delivery mode, breast-feeding, and antibiotic use.” The babies’ microbiome in early life contributes the immune system’s response to vaccines, they said, “and sets the level of protection against certain infections in childhood.”
Study lead Professor Debby Bogaert, chair of pediatric medicine at the University of Edinburgh, said: “I think it is especially interesting that we identified several beneficial microbes to be the link between mode of delivery and vaccine responses. In the future, we may be able to supplement those bacteria to children born by C-section shortly after birth through – for example, mother-to-baby ‘fecal transplants’ or the use of specifically designed probiotics.”
First author Dr. Emma de Koff, a microbiology trainee at the Amsterdam University Medical Center, said: “We expected to find a link between the gut microbiome and the babies’ vaccine responses, however we never thought to find the strongest effects in the first weeks of life.”
The findings “could help to inform conversations about C-sections between expectant mothers and their doctors,” commented the researchers, who said that they could also “shape the design of more tailored vaccination programs.” For example, in the future, vaccination schedules could be adjusted based on the method of delivery or analysis of the baby’s microbiome.
Potential to rectify immune system after Caesarean
Responding to the study, Professor Neil Mabbott, personal chair in immunopathology at the Roslin Institute of the University of Edinburgh, told the Science Media Centre: “This is a very interesting and important study. The authors show that infants delivered by a vaginal birth had higher responses to the two different types of vaccines against bacterial diseases, and this was associated with higher abundances of the potentially beneficial bacteria known as Bifidobacterium and E. coli in their intestines.”
He added: “This study raises the possibility that it may be possible to treat infants, especially Caesarean-delivered infants, with a bacterial supplement, or even a product produced by these beneficial bacteria, to help improve their immune systems, enhance their responses to certain vaccines and reduce their susceptibility to infections.”
The study raises important questions, he said, including whether the increased antibody levels from pneumococcal and meningococcal vaccinations following vaginal birth also leads to increased protection of the infants against infection or serious disease.
Sheena Cruickshank, immunologist and professor in biomedical sciences at the University of Manchester, England, commented: “It is now well established that the microbiome is important in immune development. In turn the mode of delivery and initial method of feeding is important in how the microbiome is first seeded in the baby.”
“However, other factors such as exposure to antibiotics and subsequent diet also play a role in how it then develops, making understanding the way the microbiome develops and changes quite complex. Microbes works as communities, and it can be difficult to determine whether changes in single species are important functionally. Breast milk also plays an important role in protecting the baby via transfer of maternal immunoglobulins, which will wane over a period of 6-12 months in the baby – thus ascertaining whether it’s the baby’s Ig is challenging.
“Given the complexity of the multitude of interactions, it is important that this is accounted for, and group sizes are large enough to ensure data is robust. Whilst this is an interesting study that adds to our knowledge of how the microbiome develops and the possible implications for immune development, it is still very preliminary, and the small group sizes warrant a need for further studies to verify this in larger groups.”
She added: “We will need to understand whether possible impacts of maternal delivery and feeding on immune development or vaccine responses can be restored by, for example, manipulating the microbiome.”
Professor Kim Barrett, vice dean for research at the University of California, Davis School of Medicine, said that, while further research was needed to uncover if and how manipulation of the human microbiome following C-section births might improve vaccine efficacy, “the work should at least lead to prompt additional consideration about an unintended consequence of the ever-increasing use of C-sections that may not be medically-necessary.”
Dr. Marie Lewis, researcher in gut microbiota at the University of Reading, England, said: “We have known for quite some time that the mode of delivery is incredibly important when it comes to the type of bacteria which colonize our guts. We also know that our gut bacteria in early life drive the development of our immune system, and natural births are linked with reduced risks of developing inflammatory conditions, such as asthma. It is therefore perhaps not really surprising that mode of delivery is also linked to responses to vaccinations.”
“The really interesting part here is the extent to which our gut microbiotas are accessible and changeable, and this important work could pave the way for administration of probiotics and prebiotics to improve vaccine responses in Caesarean-born children.”
‘Tantalizing data’
Dr. Chrissie Jones, associate professor of pediatric infectious diseases at the University of Southampton, and Southampton UK and education lead for the British Paediatric Allergy, Immunity, and Infection Group, said: “The link between method of delivery of the infant and the bacteria that live in the gut of the young infant has previously been shown. What is really interesting about this study is that, for the first time, the link between method of delivery (vaginal delivery vs. C-section), differences in bacterial communities of the gut, and differences in responses to vaccines is shown.”
“This study may give us fresh insights into the differences that we see in the amount of protective antibodies made after infant vaccination. It also gives us clues as to ways that we might be able to level the playing field for infants in the future – for instance, giving babies a safe cocktail of ‘friendly bacteria’ as a probiotic, or an additional dose of vaccine.”
“This study is the first step – it shows us a link or association but does not prove cause and effect that differences in the way babies are born alters how the immune system responds to vaccines. To prove this link we will need larger studies, but it is tantalizing data.”
The research was funded by Scotland’s Chief Scientist Office and the Netherlands Organisation for Scientific Research. DB received funding from OM pharma and Sanofi. All of the authors declared no other conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS