User login
StopRA trial: Hydroxychloroquine doesn’t prevent or delay onset of rheumatoid arthritis
PHILADELPHIA – Hydroxychloroquine (HCQ) isn’t any more effective at preventing or delaying the onset of rheumatoid arthritis than placebo, based on interim results of a randomized clinical trial reported at the annual meeting of the American College of Rheumatology. Despite that futility, the percentage of patients who actually went on to develop clinical RA was lower than investigators expected, and the trial supports the use of a key biomarker for identifying RA.
While the StopRA trial was halted early because of futility of the treatment, investigators are continuing to mine the gathered data to deepen their understanding of disease progression and the potential of HCQ to improve symptoms in RA patients, said lead study author Kevin D. Deane, MD, PhD, of the University of Colorado at Denver, Aurora.
Overall, around 35% of the study participants on average developed RA, Dr. Deane said. “We were expecting somewhat more,” he said. “Teasing out who’s really going to progress to RA during a study and who’s not is going to be incredibly important.”
StopRA enrolled 144 adults who had elevated anti–cyclic citrullinated peptide antibodies (CCP3) levels of at least 40 units (about twice the normal level) but no history if inflammatory arthritis, randomizing them on a 1:1 basis to either HCQ (200-400 mg a day based on weight) or placebo for a 1-year treatment regimen.
The study identified participants through rheumatology clinics, testing of first-degree relatives with established RA, health fairs, blood donors, and biobanks. The interim findings are based on 2 years of follow-up after the last dose.
The study focused on HCQ because it has a relatively low risk profile with good safety and tolerability, is easy to administer, and is relatively low cost, Dr. Deane said.
StopRA study failed to meet its primary endpoint: to determine if 1 year of treatment with HCQ reduced the risk of developing inflammatory arthritis and classifiable RA at the end of 3 years in the study population. At the time of the interim analysis, 34% of patients in the HCQ arm and 36% in the placebo arm had developed RA (P = .844), Dr. Deane said. Baseline characteristics were balanced in both treatment arms.
The findings also support the use of CCP3 as a biomarker for RA, Dr. Deane said.
Now that the trial has been terminated, Dr. Deane said investigators are going to review the final data and perform secondary analyses for further clarity on the impact HCQ may have on RA.
“The future analysis should hopefully say if this treatment actually changes symptoms,” he said in an interview. “Because, if somebody felt better on the drug or had a milder form of rheumatoid arthritis once they developed it, that could potentially be a benefit.”
Dr. Deane noted the TREAT EARLIER trial similarly found that a 1-year course of methotrexate didn’t prevent the onset of clinical arthritis, but it did alter the disease course as measured in MRI-detected inflammation, related symptoms, and impairment.
“We’re hoping to look at those things and hopefully look at biologic changes over time,” Dr. Deane said of the extended analysis. “We’re not sure if the drug was associated with changes in biomarkers yet still didn’t halt progression to RA. That might be interesting, because those biomarkers might not be fundamentally related to the disease, but other mechanisms may be. That could give us some insights.”
Session moderator Ted Mikuls, MD, a professor of rheumatology at the University of Nebraska Medical Center, Omaha, said further mining of the study data is warranted.
“It’s common in a study like that, which took a lot of time and investment, to really take a deep dive into the data to make sure there aren’t signals that we’re missing,” he said in an interview.
One of the challenges with the study may have been patient enrollment, Dr. Mikuls noted. “I wonder about the study population in terms of where they recruit patients from. Who’s more likely to get RA? Is it patients who already have symptoms? Is it asymptomatic patients from biobanks? If it’s arthralgia joint pain patients, maybe by the time you have joint and autoantibody positivity it’s too late to have an intervention.”
The National Institute of Allergy and Infectious Diseases sponsored the study. Dr. Deane disclosed a relationship with Werfen. Dr. Mikuls has no relevant disclosures.
PHILADELPHIA – Hydroxychloroquine (HCQ) isn’t any more effective at preventing or delaying the onset of rheumatoid arthritis than placebo, based on interim results of a randomized clinical trial reported at the annual meeting of the American College of Rheumatology. Despite that futility, the percentage of patients who actually went on to develop clinical RA was lower than investigators expected, and the trial supports the use of a key biomarker for identifying RA.
While the StopRA trial was halted early because of futility of the treatment, investigators are continuing to mine the gathered data to deepen their understanding of disease progression and the potential of HCQ to improve symptoms in RA patients, said lead study author Kevin D. Deane, MD, PhD, of the University of Colorado at Denver, Aurora.
Overall, around 35% of the study participants on average developed RA, Dr. Deane said. “We were expecting somewhat more,” he said. “Teasing out who’s really going to progress to RA during a study and who’s not is going to be incredibly important.”
StopRA enrolled 144 adults who had elevated anti–cyclic citrullinated peptide antibodies (CCP3) levels of at least 40 units (about twice the normal level) but no history if inflammatory arthritis, randomizing them on a 1:1 basis to either HCQ (200-400 mg a day based on weight) or placebo for a 1-year treatment regimen.
The study identified participants through rheumatology clinics, testing of first-degree relatives with established RA, health fairs, blood donors, and biobanks. The interim findings are based on 2 years of follow-up after the last dose.
The study focused on HCQ because it has a relatively low risk profile with good safety and tolerability, is easy to administer, and is relatively low cost, Dr. Deane said.
StopRA study failed to meet its primary endpoint: to determine if 1 year of treatment with HCQ reduced the risk of developing inflammatory arthritis and classifiable RA at the end of 3 years in the study population. At the time of the interim analysis, 34% of patients in the HCQ arm and 36% in the placebo arm had developed RA (P = .844), Dr. Deane said. Baseline characteristics were balanced in both treatment arms.
The findings also support the use of CCP3 as a biomarker for RA, Dr. Deane said.
Now that the trial has been terminated, Dr. Deane said investigators are going to review the final data and perform secondary analyses for further clarity on the impact HCQ may have on RA.
“The future analysis should hopefully say if this treatment actually changes symptoms,” he said in an interview. “Because, if somebody felt better on the drug or had a milder form of rheumatoid arthritis once they developed it, that could potentially be a benefit.”
Dr. Deane noted the TREAT EARLIER trial similarly found that a 1-year course of methotrexate didn’t prevent the onset of clinical arthritis, but it did alter the disease course as measured in MRI-detected inflammation, related symptoms, and impairment.
“We’re hoping to look at those things and hopefully look at biologic changes over time,” Dr. Deane said of the extended analysis. “We’re not sure if the drug was associated with changes in biomarkers yet still didn’t halt progression to RA. That might be interesting, because those biomarkers might not be fundamentally related to the disease, but other mechanisms may be. That could give us some insights.”
Session moderator Ted Mikuls, MD, a professor of rheumatology at the University of Nebraska Medical Center, Omaha, said further mining of the study data is warranted.
“It’s common in a study like that, which took a lot of time and investment, to really take a deep dive into the data to make sure there aren’t signals that we’re missing,” he said in an interview.
One of the challenges with the study may have been patient enrollment, Dr. Mikuls noted. “I wonder about the study population in terms of where they recruit patients from. Who’s more likely to get RA? Is it patients who already have symptoms? Is it asymptomatic patients from biobanks? If it’s arthralgia joint pain patients, maybe by the time you have joint and autoantibody positivity it’s too late to have an intervention.”
The National Institute of Allergy and Infectious Diseases sponsored the study. Dr. Deane disclosed a relationship with Werfen. Dr. Mikuls has no relevant disclosures.
PHILADELPHIA – Hydroxychloroquine (HCQ) isn’t any more effective at preventing or delaying the onset of rheumatoid arthritis than placebo, based on interim results of a randomized clinical trial reported at the annual meeting of the American College of Rheumatology. Despite that futility, the percentage of patients who actually went on to develop clinical RA was lower than investigators expected, and the trial supports the use of a key biomarker for identifying RA.
While the StopRA trial was halted early because of futility of the treatment, investigators are continuing to mine the gathered data to deepen their understanding of disease progression and the potential of HCQ to improve symptoms in RA patients, said lead study author Kevin D. Deane, MD, PhD, of the University of Colorado at Denver, Aurora.
Overall, around 35% of the study participants on average developed RA, Dr. Deane said. “We were expecting somewhat more,” he said. “Teasing out who’s really going to progress to RA during a study and who’s not is going to be incredibly important.”
StopRA enrolled 144 adults who had elevated anti–cyclic citrullinated peptide antibodies (CCP3) levels of at least 40 units (about twice the normal level) but no history if inflammatory arthritis, randomizing them on a 1:1 basis to either HCQ (200-400 mg a day based on weight) or placebo for a 1-year treatment regimen.
The study identified participants through rheumatology clinics, testing of first-degree relatives with established RA, health fairs, blood donors, and biobanks. The interim findings are based on 2 years of follow-up after the last dose.
The study focused on HCQ because it has a relatively low risk profile with good safety and tolerability, is easy to administer, and is relatively low cost, Dr. Deane said.
StopRA study failed to meet its primary endpoint: to determine if 1 year of treatment with HCQ reduced the risk of developing inflammatory arthritis and classifiable RA at the end of 3 years in the study population. At the time of the interim analysis, 34% of patients in the HCQ arm and 36% in the placebo arm had developed RA (P = .844), Dr. Deane said. Baseline characteristics were balanced in both treatment arms.
The findings also support the use of CCP3 as a biomarker for RA, Dr. Deane said.
Now that the trial has been terminated, Dr. Deane said investigators are going to review the final data and perform secondary analyses for further clarity on the impact HCQ may have on RA.
“The future analysis should hopefully say if this treatment actually changes symptoms,” he said in an interview. “Because, if somebody felt better on the drug or had a milder form of rheumatoid arthritis once they developed it, that could potentially be a benefit.”
Dr. Deane noted the TREAT EARLIER trial similarly found that a 1-year course of methotrexate didn’t prevent the onset of clinical arthritis, but it did alter the disease course as measured in MRI-detected inflammation, related symptoms, and impairment.
“We’re hoping to look at those things and hopefully look at biologic changes over time,” Dr. Deane said of the extended analysis. “We’re not sure if the drug was associated with changes in biomarkers yet still didn’t halt progression to RA. That might be interesting, because those biomarkers might not be fundamentally related to the disease, but other mechanisms may be. That could give us some insights.”
Session moderator Ted Mikuls, MD, a professor of rheumatology at the University of Nebraska Medical Center, Omaha, said further mining of the study data is warranted.
“It’s common in a study like that, which took a lot of time and investment, to really take a deep dive into the data to make sure there aren’t signals that we’re missing,” he said in an interview.
One of the challenges with the study may have been patient enrollment, Dr. Mikuls noted. “I wonder about the study population in terms of where they recruit patients from. Who’s more likely to get RA? Is it patients who already have symptoms? Is it asymptomatic patients from biobanks? If it’s arthralgia joint pain patients, maybe by the time you have joint and autoantibody positivity it’s too late to have an intervention.”
The National Institute of Allergy and Infectious Diseases sponsored the study. Dr. Deane disclosed a relationship with Werfen. Dr. Mikuls has no relevant disclosures.
AT ACR 2022
‘A huge deal’: Millions have long COVID, and more are expected
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
with symptoms that have lasted 3 months or longer, according to the latest U.S. government survey done in October. More than a quarter say their condition is severe enough to significantly limit their day-to-day activities – yet the problem is only barely starting to get the attention of employers, the health care system, and policymakers.
With no cure or treatment in sight, long COVID is already burdening not only the health care system, but also the economy – and that burden is set to grow. Many experts worry about the possible long-term ripple effects, from increased spending on medical care costs to lost wages due to not being able to work, as well as the policy implications that come with addressing these issues.
“At this point, anyone who’s looking at this seriously would say this is a huge deal,” says senior Brookings Institution fellow Katie Bach, the author of a study that analyzed long COVID’s impact on the labor market.
“We need a real concerted focus on treating these people, which means both research and the clinical side, and figuring out how to build a labor market that is more inclusive of people with disabilities,” she said.
It’s not only that many people are affected. It’s that they are often affected for months and possibly even years.
The U.S. government figures suggest more than 18 million people could have symptoms of long COVID right now. The latest Household Pulse Survey by the Census Bureau and the National Center for Health Statistics takes data from 41,415 people.
A preprint of a study by researchers from City University of New York, posted on medRxiv in September and based on a similar population survey done between June 30 and July 2, drew comparable results. The study has not been peer reviewed.
More than 7% of all those who answered said they had long COVID at the time of the survey, which the researchers said corresponded to approximately 18.5 million U.S. adults. The same study found that a quarter of those, or an estimated 4.7 million adults, said their daily activities were impacted “a lot.”
This can translate into pain not only for the patients, but for governments and employers, too.
In high-income countries around the world, government surveys and other studies are shedding light on the extent to which post-COVID-19 symptoms – commonly known as long COVID – are affecting populations. While results vary, they generally fall within similar ranges.
The World Health Organization estimates that between 10% and 20% of those with COVID-19 go on to have an array of medium- to long-term post-COVID-19 symptoms that range from mild to debilitating. The U.S. Government Accountability Office puts that estimate at 10% to 30%; one of the latest studies published at the end of October in The Journal of the American Medical Association found that 15% of U.S. adults who had tested positive for COVID-19 reported current long COVID symptoms. Elsewhere, a study from the Netherlands published in The Lancet in August found that one in eight COVID-19 cases, or 12.7%, were likely to become long COVID.
“It’s very clear that the condition is devastating people’s lives and livelihoods,” WHO Director-General Tedros Adhanom Ghebreyesus wrote in an article for The Guardian newspaper in October.
“The world has already lost a significant number of the workforce to illness, death, fatigue, unplanned retirement due to an increase in long-term disability, which not only impacts the health system, but is a hit to the overarching economy … the impact of long COVID for all countries is very serious and needs immediate and sustained action equivalent to its scale.”
Global snapshot: Lasting symptoms, impact on activities
Patients describe a spectrum of persistent issues, with extreme fatigue, brain fog or cognitive problems, and shortness of breath among the most common complaints. Many also have manageable symptoms that worsen significantly after even mild physical or mental exertion.
Women appear almost twice as likely as men to get long COVID. Many patients have other medical conditions and disabilities that make them more vulnerable to the condition. Those who face greater obstacles accessing health care due to discrimination or socioeconomic inequity are at higher risk as well.
While many are older, a large number are also in their prime working age. The Census Bureau data show that people ages 40-49 are more likely than any other group to get long COVID, which has broader implications for labor markets and the global economy. Already, experts have estimated that long COVID is likely to cost the U.S. trillions of dollars and affect multiple industries.
“Whether they’re in the financial world, the medical system, lawyers, they’re telling me they’re sitting at the computer screen and they’re unable to process the data,” said Zachary Schwartz, MD, medical director for Vancouver General Hospital’s Post-COVID-19 Recovery Clinic.
“That is what’s most distressing for people, in that they’re not working, they’re not making money, and they don’t know when, or if, they’re going to get better.”
Nearly a third of respondents in the Census Bureau’s Household Pulse Survey who said they have had COVID-19 reported symptoms that lasted 3 months or longer. People between the ages of 30 and 59 were the most affected, with about 32% reporting symptoms. Across the entire adult U.S. population, the survey found that 1 in 7 adults have had long COVID at some point during the pandemic, with about 1 in 18 saying it limited their activity to some degree, and 1 in 50 saying they have faced “a lot” of limits on their activities. Any way these numbers are dissected, long COVID has impacted a large swath of the population.
Yet research into the causes and possible treatments of long COVID is just getting underway.
“The amount of energy and time devoted to it is way, way less than it should, given how many people are likely affected,” said David Cutler, PhD, professor of economics at Harvard University, Cambridge, Mass., who has written about the economic cost of long COVID. “We’re way, way underdoing it here. And I think that’s really a terrible thing.”
Population surveys and studies from around the world show that long COVID lives up to its name, with people reporting serious symptoms for months on end.
In October, Statistics Canada and the Public Health Agency of Canada published early results from a questionnaire done between spring and summer 2022 that found just under 15% of adults who had a confirmed or suspected case of COVID-19 went on to have new or continuing symptoms 3 or more months later. Nearly half, or 47.3%, dealt with symptoms that lasted a year or more. More than one in five said their symptoms “often or always” limited their day-to-day activities, which included routine tasks such as preparing meals, doing errands and chores, and basic functions such as personal care and moving around in their homes.
Nearly three-quarters of workers or students said they missed an average of 20 days of work or school.
“We haven’t yet been able to determine exactly when symptoms resolve,” said Rainu Kaushal, MD, the senior associate dean for clinical research at Weill Cornell Medicine in New York. She is co-leading a national study on long COVID in adults and children, funded by the National Institutes of Health RECOVER Initiative.
“But there does seem to be, for many of the milder symptoms, resolution at about 4-6 weeks. There seems to be a second point of resolution around 6 months for certain symptoms, and then some symptoms do seem to be permanent, and those tend to be patients who have underlying conditions,” she said.
Reducing the risk
Given all the data so far, experts recommend urgent policy changes to help people with long COVID.
“The population needs to be prepared, that understanding long COVID is going to be a very long and difficult process,” said Alexander Charney, MD, PhD, associate professor and the lead principal investigator of the RECOVER adult cohort at Icahn School of Medicine at Mount Sinai in New York. He said the government can do a great deal to help, including setting up a network of connected clinics treating long COVID, standardizing best practices, and sharing information.
“That would go a long way towards making sure that every person feels like they’re not too far away from a clinic where they can get treated for this particular condition,” he said.
But the only known way to prevent long COVID is to prevent COVID-19 infections in the first place, experts say. That means equitable access to tests, therapeutics, and vaccines.
“I will say that avoiding COVID remains the best treatment in the arsenal right now,” said Dr. Kaushal. This means masking, avoiding crowded places with poor ventilation and high exposure risk, and being up to date on vaccinations, she said.
A number of papers – including a large U.K. study published in May 2022, another one from July, and the JAMA study from October – all suggest that vaccinations can help reduce the risk of long COVID.
“I am absolutely of the belief that vaccination has reduced the incidence and overall amount of long COVID … [and is] still by far the best thing the public can do,” said Dr. Schwartz.
A version of this article first appeared on WebMD.com.
Make room for continuous glucose monitoring in type 2 diabetes management
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
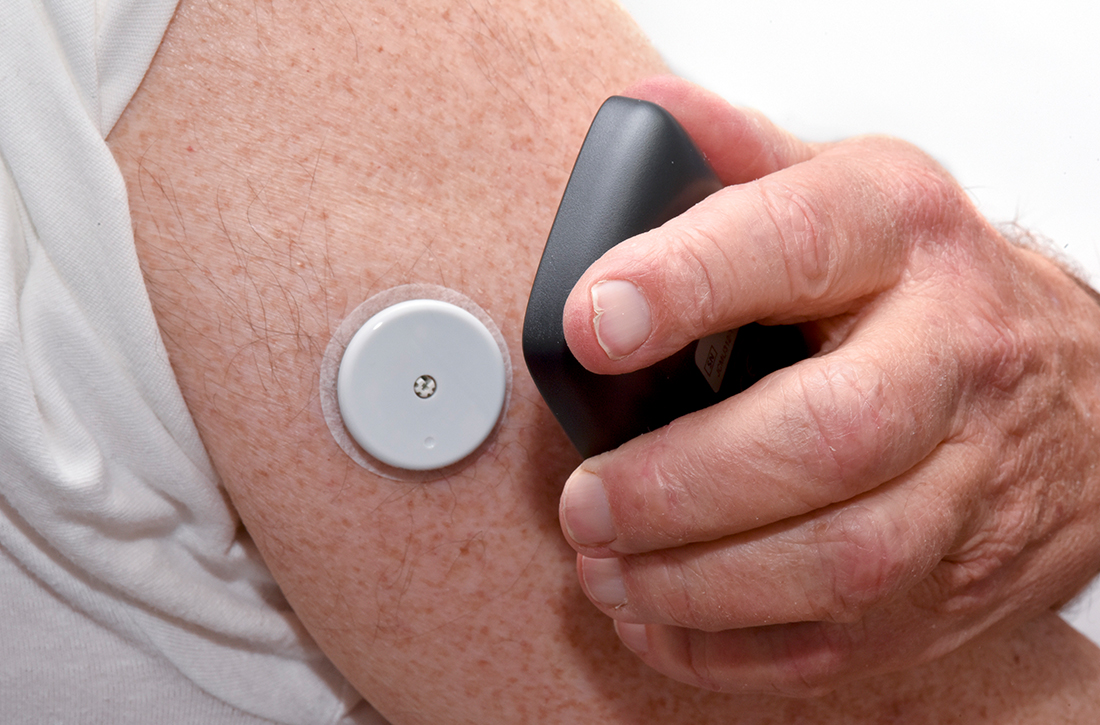
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.
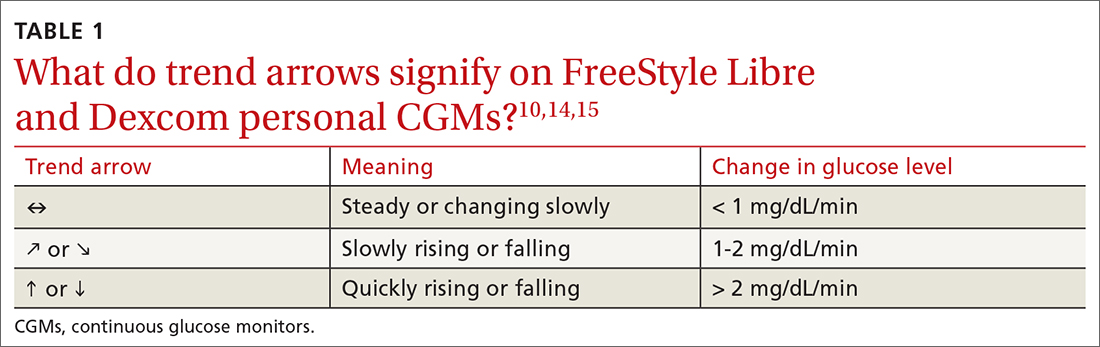
For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
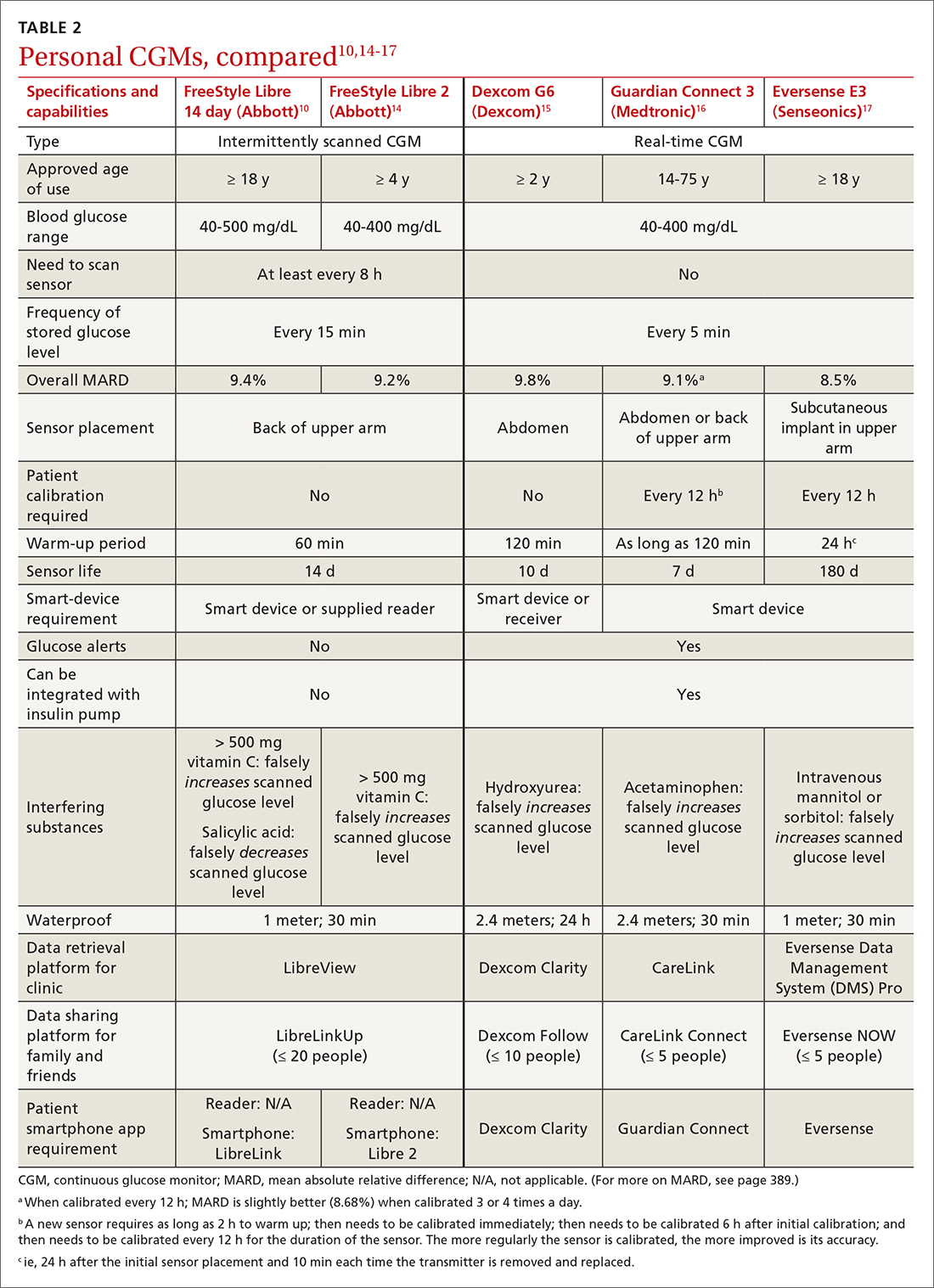
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17
The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
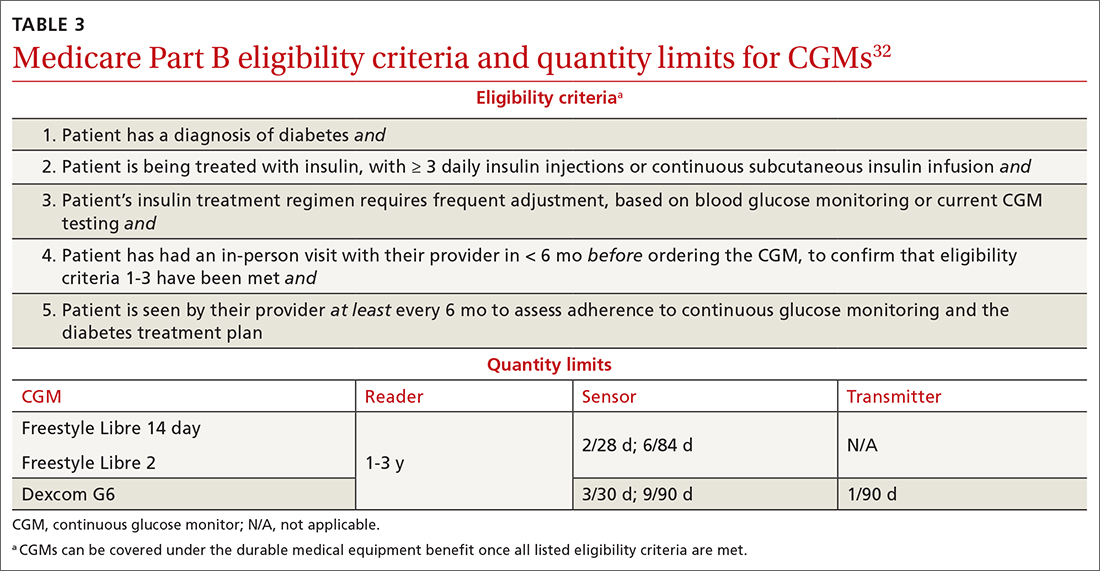
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32
Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
PRACTICE RECOMMENDATIONS
› Initiate continuous glucose monitoring early in the disease process, based on a patient’s needs or preferences. C
› Interpret a continuous glucose monitor (CGM) report with the understanding that time within target range is the most important factor to evaluate. Minimizing or eliminating time below range is of paramount importance. B
› Advise patients who use a CGM to continue to have access to a glucometer and instruct them on appropriate times when such confirmation might be necessary. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
U.S. dementia rate drops as education, women’s employment rises
new research shows. New data from the Health and Retirement Study, a nationally representative survey, show that the prevalence of dementia among individuals aged 65 and older dropped from 12.2% in 2000 to 8.5% in 2016 – a 30.1% decrease. In men, the prevalence of dementia fell from 10.2% to 7.0%, while for women, it declined from 13.6% to 9.7%, researchers reported. Their finding were published online in PNAS.
The study also revealed that the proportion of college-educated men in the sample increased from 21.5% in 2000 to 33.7% in 2016, while the proportion of college-educated women increased from 12.3% in 2000 to 23% in 2016.
The findings also show a decline in the dementia prevalence in non-Hispanic Black men, which dropped from 17.2% to 9.9%, a decrease of 42.6%. In non-Hispanic White men, dementia declined 9.3% to 6.6%, or 29.0%.
The investigators also found a substantial increase in the level of education between 2000 and 2016. In addition, they found that, among 74- to 84-year-old women in 2000, 29.5% had worked for more than 30 years during their lifetime versus 59.0% in 2016.
The investigators speculated that the decline in dementia prevalence reflects larger socioeconomic changes in the United States as well as prevention strategies to reduce cardiovascular disease.
A person born around 1920, for example, would have had greater exposure to the Great Depression, while someone born in 1936 would have benefited more from the changes in living standards in the years following World War II, they noted.
“There’s a need for more research on the effect of employment on cognitive reserve. It’s plausible that working is good for your mental cognitive abilities,” said study investigator Péter Hudomiet, PhD, from the RAND Corporation, adding that there may also be benefits that extend beyond working years. It’s possible that women’s greater participation in the workforce gives them more chances to establish relationships that in some cases last well into retirement and provide essential social connection. It’s well known that social isolation has a negative impact on cognition.
“It’s plausible that working is good for your mental cognitive abilities,” he added.
The investigators noted that it is beyond the scope of their study to draw definitive conclusions about the causes of the decline, but they observed that positive trends in employment and standard of living make sense. “They would suggest that as schooling levels continue to rise in the U.S. population in younger generations, the prevalence of dementia would continue to decrease.
The investigators report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows. New data from the Health and Retirement Study, a nationally representative survey, show that the prevalence of dementia among individuals aged 65 and older dropped from 12.2% in 2000 to 8.5% in 2016 – a 30.1% decrease. In men, the prevalence of dementia fell from 10.2% to 7.0%, while for women, it declined from 13.6% to 9.7%, researchers reported. Their finding were published online in PNAS.
The study also revealed that the proportion of college-educated men in the sample increased from 21.5% in 2000 to 33.7% in 2016, while the proportion of college-educated women increased from 12.3% in 2000 to 23% in 2016.
The findings also show a decline in the dementia prevalence in non-Hispanic Black men, which dropped from 17.2% to 9.9%, a decrease of 42.6%. In non-Hispanic White men, dementia declined 9.3% to 6.6%, or 29.0%.
The investigators also found a substantial increase in the level of education between 2000 and 2016. In addition, they found that, among 74- to 84-year-old women in 2000, 29.5% had worked for more than 30 years during their lifetime versus 59.0% in 2016.
The investigators speculated that the decline in dementia prevalence reflects larger socioeconomic changes in the United States as well as prevention strategies to reduce cardiovascular disease.
A person born around 1920, for example, would have had greater exposure to the Great Depression, while someone born in 1936 would have benefited more from the changes in living standards in the years following World War II, they noted.
“There’s a need for more research on the effect of employment on cognitive reserve. It’s plausible that working is good for your mental cognitive abilities,” said study investigator Péter Hudomiet, PhD, from the RAND Corporation, adding that there may also be benefits that extend beyond working years. It’s possible that women’s greater participation in the workforce gives them more chances to establish relationships that in some cases last well into retirement and provide essential social connection. It’s well known that social isolation has a negative impact on cognition.
“It’s plausible that working is good for your mental cognitive abilities,” he added.
The investigators noted that it is beyond the scope of their study to draw definitive conclusions about the causes of the decline, but they observed that positive trends in employment and standard of living make sense. “They would suggest that as schooling levels continue to rise in the U.S. population in younger generations, the prevalence of dementia would continue to decrease.
The investigators report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research shows. New data from the Health and Retirement Study, a nationally representative survey, show that the prevalence of dementia among individuals aged 65 and older dropped from 12.2% in 2000 to 8.5% in 2016 – a 30.1% decrease. In men, the prevalence of dementia fell from 10.2% to 7.0%, while for women, it declined from 13.6% to 9.7%, researchers reported. Their finding were published online in PNAS.
The study also revealed that the proportion of college-educated men in the sample increased from 21.5% in 2000 to 33.7% in 2016, while the proportion of college-educated women increased from 12.3% in 2000 to 23% in 2016.
The findings also show a decline in the dementia prevalence in non-Hispanic Black men, which dropped from 17.2% to 9.9%, a decrease of 42.6%. In non-Hispanic White men, dementia declined 9.3% to 6.6%, or 29.0%.
The investigators also found a substantial increase in the level of education between 2000 and 2016. In addition, they found that, among 74- to 84-year-old women in 2000, 29.5% had worked for more than 30 years during their lifetime versus 59.0% in 2016.
The investigators speculated that the decline in dementia prevalence reflects larger socioeconomic changes in the United States as well as prevention strategies to reduce cardiovascular disease.
A person born around 1920, for example, would have had greater exposure to the Great Depression, while someone born in 1936 would have benefited more from the changes in living standards in the years following World War II, they noted.
“There’s a need for more research on the effect of employment on cognitive reserve. It’s plausible that working is good for your mental cognitive abilities,” said study investigator Péter Hudomiet, PhD, from the RAND Corporation, adding that there may also be benefits that extend beyond working years. It’s possible that women’s greater participation in the workforce gives them more chances to establish relationships that in some cases last well into retirement and provide essential social connection. It’s well known that social isolation has a negative impact on cognition.
“It’s plausible that working is good for your mental cognitive abilities,” he added.
The investigators noted that it is beyond the scope of their study to draw definitive conclusions about the causes of the decline, but they observed that positive trends in employment and standard of living make sense. “They would suggest that as schooling levels continue to rise in the U.S. population in younger generations, the prevalence of dementia would continue to decrease.
The investigators report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From PNAS
Opioids increase risk for all-cause deaths in RA vs. NSAIDs
PHILADELPHIA – For patients with rheumatoid arthritis who are already at increased risk for major adverse cardiovascular events (MACE), NSAIDs may be safer than opioids, results of a new-user active comparator study suggest.
Among 6,866 patients with RA who started on opioids and 13,698 patients who started on NSAIDs for pain, the use of both weak and strong opioids was associated with a 33% increase in risk for all-cause mortality and a trend toward higher rates of venous thromboembolism (VTE), compared with NSAID use, reported Gulsen Ozen, MD, of the University of Nebraska Medical Center, Omaha.
“Pain in RA is a very complex process, and we know that it’s not solely dependent on the disease activity, but there is no evidence that opioids have any benefit in long-term pain management, and it can even cause hyperalgesia. And as we show, it’s not safer than NSAIDs,” she said in an oral abstract session at the annual meeting of the American College of Rheumatology.
She stressed that patients should be assessed for non-RA causes of pain and should use nonpharmacologic methods when possible.
“If a pharmacological treatment is needed and NSAIDs are contraindicated, the lowest possible dose of weak opioids can be used for a very limited time for acute pain only,” she said.
Pain despite disease control
Even when their disease is well controlled, approximately 60% of patients with RA still report pain. NSAIDs are commonly used to treat pain in patients with RA, but they are associated with modest increases in risk for cardiovascular disease (CVD), gastrointestinal bleeding, renal injury, and hypertension.
Some providers are leery of NSAIDs and will instead prescribe either regular or intermittent opioids for pain control in their patients.
Disease-modifying antirheumatic drugs have only minimal pain-relieving benefits, “and even worse, opioids can delay initiation of DMARDs in RA,” Dr. Ozen said.
Opioids have been shown to increase oxidative stress, platelet aggregation, and myocardial fibrosis, as well as hypogonadism, weight gain, and CVD risk factors.
There is little evidence, however, on whether opioids are associated with cardiovascular events in patients with RA. This dearth of data prompted Dr. Ozen and colleagues to study the relative risks for MACE in patients with RA starting on opioids or NSAIDs for pain.
Matched cohorts
They used data from FORWARD, a joint Canadian and U.S. databank for rheumatic diseases, to conduct a new-user active comparator cohort study. The cohort included adults with RA without cancer who participated in FORWARD for a minimum of 1 year between 1998 and 2021.
The patients were followed either from drug initiation until 3 months after the end of treatment, defined as either discontinuation or a switch to a different analgesic, end of study follow-up, or the development of a MACE outcome.
The investigators used propensity score matching to compare each opioid initiator with two NSAID initiators. The participants were matched by age, sex, body mass index, smoking, alcohol, RA duration, disease activity, Health Assessment Questionnaire, visual analog scale for pain, joint surgeries, prior CVD and VTE, hypertension, diabetes, rheumatic diseases comorbidity index, osteoporosis/fractures, thyroid, chronic liver, kidney, lung and mental health diseases, hospitalizations, 36-Item Short Form Health Survey scores, and sleep scores.
The two groups were well matched, except for a slightly higher incidence of VTE in opioid initiators, although incidence rates were low in both groups (0.9% vs. 0.6% of NSAID initiators).
Higher death rate in opioid users
The incidence rate of MACE among opioid initiators was 20.6% versus18.9% among NSAID initiators, a difference that was not statistically significant. There were also no significant differences in incidence rates of the individual components of the MACE composite outcome: myocardial infarction, stroke, heart failure, CVD death, or VTE.
There were, however, significantly more deaths from any cause among patients in the opioid group, with an incidence rate of 13.5% versus 10.8% in the NSAID group.
An analysis of the associaion of drug type with outcomes, adjusted for propensity score weight and prior VTE showed that patients on opioids had a statistically significant hazard ratio for death from any cause of 1.33 (95% confidence interval, 1.06-1.67).
The increased risk for all-cause mortality occurred both in patients starting on weak opioids (hydrocodone, tramadol, codeine, pentazocine, and propoxyphene) and on strong opioids (hydromorphone, dihydromorphinone, oxymorphone, butorphanol, methadone, morphine, oxycodone, meperidine, and fentanyl).
As noted before, there was a trend toward an increased risk for VTE among opioid initiators, but this was not statistically significant.
The increase in risk was higher among patients on strong versus weak opioids, suggesting a dose-dependent relationship, Dr. Ozen said.
A comparison of opioid-associated risk for all-cause mortality vs. NSAIDs according to type (nonselective or selective) showed that most of the increase in risk was relative to selective cycloxygenase-2 inhibitors.
‘Beautiful’ analysis
“This is a beautiful piece of analysis on a really difficult question to address because the confounding is really hard to unpick,” commented James Galloway, MBBS, deputy head of the center for rheumatic diseases at King’s College London and consulting rheumatologist at King’s College Hospital, also in London.
“The headline message is that there didn’t appear to be a clear signal that NSAIDs were worse, which is what I thought the preexisting view might have been. And so, people may have paradoxically prescribed opioids in favor of NSAIDs in a person with cardiovascular risk,” he said in an interview. Dr. Galloway attended the oral abstract session but was not involved in the study.
The study was supported by a grant to Dr. Ozen from the Rheumatology Research Foundation. Dr. Galloway reported having no relevant disclosures.
PHILADELPHIA – For patients with rheumatoid arthritis who are already at increased risk for major adverse cardiovascular events (MACE), NSAIDs may be safer than opioids, results of a new-user active comparator study suggest.
Among 6,866 patients with RA who started on opioids and 13,698 patients who started on NSAIDs for pain, the use of both weak and strong opioids was associated with a 33% increase in risk for all-cause mortality and a trend toward higher rates of venous thromboembolism (VTE), compared with NSAID use, reported Gulsen Ozen, MD, of the University of Nebraska Medical Center, Omaha.
“Pain in RA is a very complex process, and we know that it’s not solely dependent on the disease activity, but there is no evidence that opioids have any benefit in long-term pain management, and it can even cause hyperalgesia. And as we show, it’s not safer than NSAIDs,” she said in an oral abstract session at the annual meeting of the American College of Rheumatology.
She stressed that patients should be assessed for non-RA causes of pain and should use nonpharmacologic methods when possible.
“If a pharmacological treatment is needed and NSAIDs are contraindicated, the lowest possible dose of weak opioids can be used for a very limited time for acute pain only,” she said.
Pain despite disease control
Even when their disease is well controlled, approximately 60% of patients with RA still report pain. NSAIDs are commonly used to treat pain in patients with RA, but they are associated with modest increases in risk for cardiovascular disease (CVD), gastrointestinal bleeding, renal injury, and hypertension.
Some providers are leery of NSAIDs and will instead prescribe either regular or intermittent opioids for pain control in their patients.
Disease-modifying antirheumatic drugs have only minimal pain-relieving benefits, “and even worse, opioids can delay initiation of DMARDs in RA,” Dr. Ozen said.
Opioids have been shown to increase oxidative stress, platelet aggregation, and myocardial fibrosis, as well as hypogonadism, weight gain, and CVD risk factors.
There is little evidence, however, on whether opioids are associated with cardiovascular events in patients with RA. This dearth of data prompted Dr. Ozen and colleagues to study the relative risks for MACE in patients with RA starting on opioids or NSAIDs for pain.
Matched cohorts
They used data from FORWARD, a joint Canadian and U.S. databank for rheumatic diseases, to conduct a new-user active comparator cohort study. The cohort included adults with RA without cancer who participated in FORWARD for a minimum of 1 year between 1998 and 2021.
The patients were followed either from drug initiation until 3 months after the end of treatment, defined as either discontinuation or a switch to a different analgesic, end of study follow-up, or the development of a MACE outcome.
The investigators used propensity score matching to compare each opioid initiator with two NSAID initiators. The participants were matched by age, sex, body mass index, smoking, alcohol, RA duration, disease activity, Health Assessment Questionnaire, visual analog scale for pain, joint surgeries, prior CVD and VTE, hypertension, diabetes, rheumatic diseases comorbidity index, osteoporosis/fractures, thyroid, chronic liver, kidney, lung and mental health diseases, hospitalizations, 36-Item Short Form Health Survey scores, and sleep scores.
The two groups were well matched, except for a slightly higher incidence of VTE in opioid initiators, although incidence rates were low in both groups (0.9% vs. 0.6% of NSAID initiators).
Higher death rate in opioid users
The incidence rate of MACE among opioid initiators was 20.6% versus18.9% among NSAID initiators, a difference that was not statistically significant. There were also no significant differences in incidence rates of the individual components of the MACE composite outcome: myocardial infarction, stroke, heart failure, CVD death, or VTE.
There were, however, significantly more deaths from any cause among patients in the opioid group, with an incidence rate of 13.5% versus 10.8% in the NSAID group.
An analysis of the associaion of drug type with outcomes, adjusted for propensity score weight and prior VTE showed that patients on opioids had a statistically significant hazard ratio for death from any cause of 1.33 (95% confidence interval, 1.06-1.67).
The increased risk for all-cause mortality occurred both in patients starting on weak opioids (hydrocodone, tramadol, codeine, pentazocine, and propoxyphene) and on strong opioids (hydromorphone, dihydromorphinone, oxymorphone, butorphanol, methadone, morphine, oxycodone, meperidine, and fentanyl).
As noted before, there was a trend toward an increased risk for VTE among opioid initiators, but this was not statistically significant.
The increase in risk was higher among patients on strong versus weak opioids, suggesting a dose-dependent relationship, Dr. Ozen said.
A comparison of opioid-associated risk for all-cause mortality vs. NSAIDs according to type (nonselective or selective) showed that most of the increase in risk was relative to selective cycloxygenase-2 inhibitors.
‘Beautiful’ analysis
“This is a beautiful piece of analysis on a really difficult question to address because the confounding is really hard to unpick,” commented James Galloway, MBBS, deputy head of the center for rheumatic diseases at King’s College London and consulting rheumatologist at King’s College Hospital, also in London.
“The headline message is that there didn’t appear to be a clear signal that NSAIDs were worse, which is what I thought the preexisting view might have been. And so, people may have paradoxically prescribed opioids in favor of NSAIDs in a person with cardiovascular risk,” he said in an interview. Dr. Galloway attended the oral abstract session but was not involved in the study.
The study was supported by a grant to Dr. Ozen from the Rheumatology Research Foundation. Dr. Galloway reported having no relevant disclosures.
PHILADELPHIA – For patients with rheumatoid arthritis who are already at increased risk for major adverse cardiovascular events (MACE), NSAIDs may be safer than opioids, results of a new-user active comparator study suggest.
Among 6,866 patients with RA who started on opioids and 13,698 patients who started on NSAIDs for pain, the use of both weak and strong opioids was associated with a 33% increase in risk for all-cause mortality and a trend toward higher rates of venous thromboembolism (VTE), compared with NSAID use, reported Gulsen Ozen, MD, of the University of Nebraska Medical Center, Omaha.
“Pain in RA is a very complex process, and we know that it’s not solely dependent on the disease activity, but there is no evidence that opioids have any benefit in long-term pain management, and it can even cause hyperalgesia. And as we show, it’s not safer than NSAIDs,” she said in an oral abstract session at the annual meeting of the American College of Rheumatology.
She stressed that patients should be assessed for non-RA causes of pain and should use nonpharmacologic methods when possible.
“If a pharmacological treatment is needed and NSAIDs are contraindicated, the lowest possible dose of weak opioids can be used for a very limited time for acute pain only,” she said.
Pain despite disease control
Even when their disease is well controlled, approximately 60% of patients with RA still report pain. NSAIDs are commonly used to treat pain in patients with RA, but they are associated with modest increases in risk for cardiovascular disease (CVD), gastrointestinal bleeding, renal injury, and hypertension.
Some providers are leery of NSAIDs and will instead prescribe either regular or intermittent opioids for pain control in their patients.
Disease-modifying antirheumatic drugs have only minimal pain-relieving benefits, “and even worse, opioids can delay initiation of DMARDs in RA,” Dr. Ozen said.
Opioids have been shown to increase oxidative stress, platelet aggregation, and myocardial fibrosis, as well as hypogonadism, weight gain, and CVD risk factors.
There is little evidence, however, on whether opioids are associated with cardiovascular events in patients with RA. This dearth of data prompted Dr. Ozen and colleagues to study the relative risks for MACE in patients with RA starting on opioids or NSAIDs for pain.
Matched cohorts
They used data from FORWARD, a joint Canadian and U.S. databank for rheumatic diseases, to conduct a new-user active comparator cohort study. The cohort included adults with RA without cancer who participated in FORWARD for a minimum of 1 year between 1998 and 2021.
The patients were followed either from drug initiation until 3 months after the end of treatment, defined as either discontinuation or a switch to a different analgesic, end of study follow-up, or the development of a MACE outcome.
The investigators used propensity score matching to compare each opioid initiator with two NSAID initiators. The participants were matched by age, sex, body mass index, smoking, alcohol, RA duration, disease activity, Health Assessment Questionnaire, visual analog scale for pain, joint surgeries, prior CVD and VTE, hypertension, diabetes, rheumatic diseases comorbidity index, osteoporosis/fractures, thyroid, chronic liver, kidney, lung and mental health diseases, hospitalizations, 36-Item Short Form Health Survey scores, and sleep scores.
The two groups were well matched, except for a slightly higher incidence of VTE in opioid initiators, although incidence rates were low in both groups (0.9% vs. 0.6% of NSAID initiators).
Higher death rate in opioid users
The incidence rate of MACE among opioid initiators was 20.6% versus18.9% among NSAID initiators, a difference that was not statistically significant. There were also no significant differences in incidence rates of the individual components of the MACE composite outcome: myocardial infarction, stroke, heart failure, CVD death, or VTE.
There were, however, significantly more deaths from any cause among patients in the opioid group, with an incidence rate of 13.5% versus 10.8% in the NSAID group.
An analysis of the associaion of drug type with outcomes, adjusted for propensity score weight and prior VTE showed that patients on opioids had a statistically significant hazard ratio for death from any cause of 1.33 (95% confidence interval, 1.06-1.67).
The increased risk for all-cause mortality occurred both in patients starting on weak opioids (hydrocodone, tramadol, codeine, pentazocine, and propoxyphene) and on strong opioids (hydromorphone, dihydromorphinone, oxymorphone, butorphanol, methadone, morphine, oxycodone, meperidine, and fentanyl).
As noted before, there was a trend toward an increased risk for VTE among opioid initiators, but this was not statistically significant.
The increase in risk was higher among patients on strong versus weak opioids, suggesting a dose-dependent relationship, Dr. Ozen said.
A comparison of opioid-associated risk for all-cause mortality vs. NSAIDs according to type (nonselective or selective) showed that most of the increase in risk was relative to selective cycloxygenase-2 inhibitors.
‘Beautiful’ analysis
“This is a beautiful piece of analysis on a really difficult question to address because the confounding is really hard to unpick,” commented James Galloway, MBBS, deputy head of the center for rheumatic diseases at King’s College London and consulting rheumatologist at King’s College Hospital, also in London.
“The headline message is that there didn’t appear to be a clear signal that NSAIDs were worse, which is what I thought the preexisting view might have been. And so, people may have paradoxically prescribed opioids in favor of NSAIDs in a person with cardiovascular risk,” he said in an interview. Dr. Galloway attended the oral abstract session but was not involved in the study.
The study was supported by a grant to Dr. Ozen from the Rheumatology Research Foundation. Dr. Galloway reported having no relevant disclosures.
AT ACR 2022
Fungi inside cancer cells: ‘A new and emerging hallmark’
The investigators characterized the cancer mycobiome within 17,401 tissue, blood, and plasma samples from four international cohorts, revealing new information about fungi distribution, association with immune cells, and potential prognostic value.
Fungi were detected in all cancer types studied and were often intracellular, reported Lian Narunsky-Haziza, PhD, of Weizmann Institute of Science, Rehovot, Israel, and colleagues.
Additionally, multiple fungal-bacterial-immune ecologies were detected across tumors, and intratumoral fungi stratified clinical outcomes, including immunotherapy response, they noted. Also, cell-free fungal DNA diagnosed healthy and cancer patients in early-stage disease.
The findings, published online in the journal Cell, have potential implications for cancer detection, diagnosis, and treatment, the researchers suggested.
The existence of fungi in most human cancers “is both a surprise and to be expected,” study coauthor Rob Knight, PhD, a professor at the University of California, San Diego, stated in a press release. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected, because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
Exploration of the associations between cancer and microbes are nothing new, but cancer-associated fungi have rarely been examined, the authors noted.
The findings from this pan-cancer analysis, which suggested “prognostic and diagnostic capacities of the tissue and plasma mycobiomes, even in stage I cancers,” complement current “understanding of the interaction between cancer cells and the bacteria that exist in tumors alongside fungi, bacteria that have been shown to affect cancer growth, metastasis, and response to therapy,” they explained.
Of note, the study revealed multiple correlations between the presence of specific fungi in tumors and conditions related to treatment. For example, patients with breast cancer whose tumors contained Malassezia globosa – a fungus found naturally on the skin – had a much lower survival rate than those whose tumors did not contain the fungus. Furthermore, specific fungi were more prevalent in breast tumors from older vs. younger patients, in lung tumors of smokers vs. nonsmokers, and in melanoma tumors that responded to immunotherapy vs. those that did not respond.
These findings suggest that fungal activity is “a new and emerging hallmark of cancer,” stated study coleader Ravid Straussman, PhD, of the Weizmann molecular cell biology department. “These findings should drive us to better explore the potential effects of tumor fungi and to re-examine almost everything we know about cancer through a ‘microbiome lens.’ ”
Unique relationships observed between fungi and bacteria – for example, tumors that contain Aspergillus fungi tended to have specific bacteria in them, whereas tumors that contain Malassezia fungi tended to have other bacteria in them – may have implications for treatment, as they correlated with both tumor immunity and patient survival, according to the authors.
“This study sheds new light on the complex biological environment within tumors, and future research will reveal how fungi affect cancerous growth,” said coauthor Yitzhak Pilpel, PhD, a principal investigator at the Weizmann molecular genetics department. “The fact that fungi can be found not only in cancer cells but also in immune cells implies that, in the future, we’ll probably find that fungi have some effect not only on the cancer cells but also on immune cells and their activity.”
A further finding related to the presence of fungal and bacterial DNA in human blood further suggests that measuring microbial DNA in the blood could lead to early detection of cancer, the authors noted.
Dr. Straussman’s research is supported by the Swiss Society Institute for Cancer Prevention Research, the Fabricant-Morse Families Research Fund for Humanity, the Dr. Chantal d’Adesky Scheinberg Research Fund, and the Dr. Dvora and Haim Teitelbaum Endowment Fund.
A version of this article first appeared on Medscape.com.
The investigators characterized the cancer mycobiome within 17,401 tissue, blood, and plasma samples from four international cohorts, revealing new information about fungi distribution, association with immune cells, and potential prognostic value.
Fungi were detected in all cancer types studied and were often intracellular, reported Lian Narunsky-Haziza, PhD, of Weizmann Institute of Science, Rehovot, Israel, and colleagues.
Additionally, multiple fungal-bacterial-immune ecologies were detected across tumors, and intratumoral fungi stratified clinical outcomes, including immunotherapy response, they noted. Also, cell-free fungal DNA diagnosed healthy and cancer patients in early-stage disease.
The findings, published online in the journal Cell, have potential implications for cancer detection, diagnosis, and treatment, the researchers suggested.
The existence of fungi in most human cancers “is both a surprise and to be expected,” study coauthor Rob Knight, PhD, a professor at the University of California, San Diego, stated in a press release. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected, because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
Exploration of the associations between cancer and microbes are nothing new, but cancer-associated fungi have rarely been examined, the authors noted.
The findings from this pan-cancer analysis, which suggested “prognostic and diagnostic capacities of the tissue and plasma mycobiomes, even in stage I cancers,” complement current “understanding of the interaction between cancer cells and the bacteria that exist in tumors alongside fungi, bacteria that have been shown to affect cancer growth, metastasis, and response to therapy,” they explained.
Of note, the study revealed multiple correlations between the presence of specific fungi in tumors and conditions related to treatment. For example, patients with breast cancer whose tumors contained Malassezia globosa – a fungus found naturally on the skin – had a much lower survival rate than those whose tumors did not contain the fungus. Furthermore, specific fungi were more prevalent in breast tumors from older vs. younger patients, in lung tumors of smokers vs. nonsmokers, and in melanoma tumors that responded to immunotherapy vs. those that did not respond.
These findings suggest that fungal activity is “a new and emerging hallmark of cancer,” stated study coleader Ravid Straussman, PhD, of the Weizmann molecular cell biology department. “These findings should drive us to better explore the potential effects of tumor fungi and to re-examine almost everything we know about cancer through a ‘microbiome lens.’ ”
Unique relationships observed between fungi and bacteria – for example, tumors that contain Aspergillus fungi tended to have specific bacteria in them, whereas tumors that contain Malassezia fungi tended to have other bacteria in them – may have implications for treatment, as they correlated with both tumor immunity and patient survival, according to the authors.
“This study sheds new light on the complex biological environment within tumors, and future research will reveal how fungi affect cancerous growth,” said coauthor Yitzhak Pilpel, PhD, a principal investigator at the Weizmann molecular genetics department. “The fact that fungi can be found not only in cancer cells but also in immune cells implies that, in the future, we’ll probably find that fungi have some effect not only on the cancer cells but also on immune cells and their activity.”
A further finding related to the presence of fungal and bacterial DNA in human blood further suggests that measuring microbial DNA in the blood could lead to early detection of cancer, the authors noted.
Dr. Straussman’s research is supported by the Swiss Society Institute for Cancer Prevention Research, the Fabricant-Morse Families Research Fund for Humanity, the Dr. Chantal d’Adesky Scheinberg Research Fund, and the Dr. Dvora and Haim Teitelbaum Endowment Fund.
A version of this article first appeared on Medscape.com.
The investigators characterized the cancer mycobiome within 17,401 tissue, blood, and plasma samples from four international cohorts, revealing new information about fungi distribution, association with immune cells, and potential prognostic value.
Fungi were detected in all cancer types studied and were often intracellular, reported Lian Narunsky-Haziza, PhD, of Weizmann Institute of Science, Rehovot, Israel, and colleagues.
Additionally, multiple fungal-bacterial-immune ecologies were detected across tumors, and intratumoral fungi stratified clinical outcomes, including immunotherapy response, they noted. Also, cell-free fungal DNA diagnosed healthy and cancer patients in early-stage disease.
The findings, published online in the journal Cell, have potential implications for cancer detection, diagnosis, and treatment, the researchers suggested.
The existence of fungi in most human cancers “is both a surprise and to be expected,” study coauthor Rob Knight, PhD, a professor at the University of California, San Diego, stated in a press release. “It is surprising because we don’t know how fungi could get into tumors throughout the body. But it is also expected, because it fits the pattern of healthy microbiomes throughout the body, including the gut, mouth, and skin, where bacteria and fungi interact as part of a complex community.”
Exploration of the associations between cancer and microbes are nothing new, but cancer-associated fungi have rarely been examined, the authors noted.
The findings from this pan-cancer analysis, which suggested “prognostic and diagnostic capacities of the tissue and plasma mycobiomes, even in stage I cancers,” complement current “understanding of the interaction between cancer cells and the bacteria that exist in tumors alongside fungi, bacteria that have been shown to affect cancer growth, metastasis, and response to therapy,” they explained.
Of note, the study revealed multiple correlations between the presence of specific fungi in tumors and conditions related to treatment. For example, patients with breast cancer whose tumors contained Malassezia globosa – a fungus found naturally on the skin – had a much lower survival rate than those whose tumors did not contain the fungus. Furthermore, specific fungi were more prevalent in breast tumors from older vs. younger patients, in lung tumors of smokers vs. nonsmokers, and in melanoma tumors that responded to immunotherapy vs. those that did not respond.
These findings suggest that fungal activity is “a new and emerging hallmark of cancer,” stated study coleader Ravid Straussman, PhD, of the Weizmann molecular cell biology department. “These findings should drive us to better explore the potential effects of tumor fungi and to re-examine almost everything we know about cancer through a ‘microbiome lens.’ ”
Unique relationships observed between fungi and bacteria – for example, tumors that contain Aspergillus fungi tended to have specific bacteria in them, whereas tumors that contain Malassezia fungi tended to have other bacteria in them – may have implications for treatment, as they correlated with both tumor immunity and patient survival, according to the authors.
“This study sheds new light on the complex biological environment within tumors, and future research will reveal how fungi affect cancerous growth,” said coauthor Yitzhak Pilpel, PhD, a principal investigator at the Weizmann molecular genetics department. “The fact that fungi can be found not only in cancer cells but also in immune cells implies that, in the future, we’ll probably find that fungi have some effect not only on the cancer cells but also on immune cells and their activity.”
A further finding related to the presence of fungal and bacterial DNA in human blood further suggests that measuring microbial DNA in the blood could lead to early detection of cancer, the authors noted.
Dr. Straussman’s research is supported by the Swiss Society Institute for Cancer Prevention Research, the Fabricant-Morse Families Research Fund for Humanity, the Dr. Chantal d’Adesky Scheinberg Research Fund, and the Dr. Dvora and Haim Teitelbaum Endowment Fund.
A version of this article first appeared on Medscape.com.
FROM CELL
Migraine Workup
Doctors urge screening for autoimmune disorders for patients with celiac disease
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Hiccups in patients with cancer often overlooked, undertreated
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF HOSPICE AND PALLIATIVE MEDICINE
Patient harm, not malpractice, top of mind for emergency medicine physicians
according to a study published in JAMA Network Open.
The cross-sectional study was conducted by researchers from Soroka University Medical Center, Israel; the University of Massachusetts, Worcester; Beth Israel Deaconess Medical Center; Harvard Medical School, Boston; and the University of Massachusetts, Amherst.
Online survey responses were collected from 1,222 emergency department attending physicians and advanced practice clinicians (APCs) in acute care hospitals throughout Massachusetts from January to September 2020.
Participants were asked to rank their level of agreement – from “strongly disagree” to “strongly agree” – with two statements: “In my day-to-day practice, I am fearful of making a mistake which results in [1] harm to the patient” (fear of harm) and [2] “being sued” (fear of suit).
The average age of the participants was about 44 years; 54.2% were men, 45.1% were women, and 0.7% were of other gender. Approximately 70% of responses were from MDs or DOs, and the remainder were from nurse practitioners and physician assistants. Participants had between 5 and 19 years of experience (median, 10 years).
The study found that the mean score was greater with regard to fear of harm than to fear of suit, regardless of clinician type, experience, or sex and whether the survey was completed before or after the start of the COVID-19 pandemic. There was no significant difference in mean scores regarding fear of suit before the pandemic and after it.
“Our data show a significantly greater fear of harming a patient than a fear of a malpractice suit,” Linda Isbell, PhD, professor of psychology at the University of Massachusetts, Amherst, who is one of the study’s authors, told this news organization. “There is a genuine concern and fear of harming patients and a desire to provide the best care for the patient’s well-being.”
In general, fear-of-harm and fear-of-suit scores decreased as providers gained experience. Those with less than 5 years of experience reported the highest levels of both.
“Although our data do not specifically provide reasons why age may impact [fear] levels, it is possible that with more practice experience ... providers have a better sense of the likelihood of patient harm and malpractice and how to manage such outcomes should they happen,” says Dr. Isbell. She noted that a longitudinal study is necessary to confirm this hypothesis.
One exception was female APCs, whose fear-of-harm scores remained relatively steady across all experience levels. Among male APCs, fear of causing patient harm decreased among those with 5-14 years of experience but increased slightly at 14-44 years of experience.
While previous research typically focused on fear of malpractice as a significant driver of defensive medicine, such as testing excessively, this study examined providers’ fear of harming patients because of a medical error.
The findings suggest “that fear of harm should be considered with, and may be more consequential than, fear of suit in medical decision-making,” the authors note.
“[F]ear can motivate people to engage in more careful and thorough information processing, which can drive behaviors in systematic ways,” says Dr. Isbell. “It is possible that one’s fear of harming a patient is triggering a high level of vigilance, reflected in the practice of defensive medicine across different types of patients – some of whom may be better off with less testing and referrals.”
Rade B. Vukmir, MD, JD, FACEP, an emergency medicine physician and spokesman for the American College of Emergency Physicians, says defensive medicine is common in the specialty and that it occurs 20%-40% of the time.
“Early in practice, the proverbial worst sin is missing a diagnosis, so that’s where the overtesting mentality comes from,” he says. In addition, “there are cities where you can’t drive a mile without seeing a half dozen legal advertisements. That imposes a cost burden on the system, [adding] roughly 20% to the cost of overall care.”
Emergency medicine providers attempt to minimize testing, but between their role as “America’s safety net” and the difficult circumstances they often face when treating patients, it takes a while to strike a balance, Dr. Vukmir acknowledges.
“There’s a training correlation, which showed up [in this study]; as people got further advanced in training, they felt more comfortable and felt the need to do it less,” says Dr. Vukmir.
The study was funded by a grant from the Agency for Healthcare Research and Quality. Dr. Isbell reports no conflicts of interest. Dr. Vukmir has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Network Open.
The cross-sectional study was conducted by researchers from Soroka University Medical Center, Israel; the University of Massachusetts, Worcester; Beth Israel Deaconess Medical Center; Harvard Medical School, Boston; and the University of Massachusetts, Amherst.
Online survey responses were collected from 1,222 emergency department attending physicians and advanced practice clinicians (APCs) in acute care hospitals throughout Massachusetts from January to September 2020.
Participants were asked to rank their level of agreement – from “strongly disagree” to “strongly agree” – with two statements: “In my day-to-day practice, I am fearful of making a mistake which results in [1] harm to the patient” (fear of harm) and [2] “being sued” (fear of suit).
The average age of the participants was about 44 years; 54.2% were men, 45.1% were women, and 0.7% were of other gender. Approximately 70% of responses were from MDs or DOs, and the remainder were from nurse practitioners and physician assistants. Participants had between 5 and 19 years of experience (median, 10 years).
The study found that the mean score was greater with regard to fear of harm than to fear of suit, regardless of clinician type, experience, or sex and whether the survey was completed before or after the start of the COVID-19 pandemic. There was no significant difference in mean scores regarding fear of suit before the pandemic and after it.
“Our data show a significantly greater fear of harming a patient than a fear of a malpractice suit,” Linda Isbell, PhD, professor of psychology at the University of Massachusetts, Amherst, who is one of the study’s authors, told this news organization. “There is a genuine concern and fear of harming patients and a desire to provide the best care for the patient’s well-being.”
In general, fear-of-harm and fear-of-suit scores decreased as providers gained experience. Those with less than 5 years of experience reported the highest levels of both.
“Although our data do not specifically provide reasons why age may impact [fear] levels, it is possible that with more practice experience ... providers have a better sense of the likelihood of patient harm and malpractice and how to manage such outcomes should they happen,” says Dr. Isbell. She noted that a longitudinal study is necessary to confirm this hypothesis.
One exception was female APCs, whose fear-of-harm scores remained relatively steady across all experience levels. Among male APCs, fear of causing patient harm decreased among those with 5-14 years of experience but increased slightly at 14-44 years of experience.
While previous research typically focused on fear of malpractice as a significant driver of defensive medicine, such as testing excessively, this study examined providers’ fear of harming patients because of a medical error.
The findings suggest “that fear of harm should be considered with, and may be more consequential than, fear of suit in medical decision-making,” the authors note.
“[F]ear can motivate people to engage in more careful and thorough information processing, which can drive behaviors in systematic ways,” says Dr. Isbell. “It is possible that one’s fear of harming a patient is triggering a high level of vigilance, reflected in the practice of defensive medicine across different types of patients – some of whom may be better off with less testing and referrals.”
Rade B. Vukmir, MD, JD, FACEP, an emergency medicine physician and spokesman for the American College of Emergency Physicians, says defensive medicine is common in the specialty and that it occurs 20%-40% of the time.
“Early in practice, the proverbial worst sin is missing a diagnosis, so that’s where the overtesting mentality comes from,” he says. In addition, “there are cities where you can’t drive a mile without seeing a half dozen legal advertisements. That imposes a cost burden on the system, [adding] roughly 20% to the cost of overall care.”
Emergency medicine providers attempt to minimize testing, but between their role as “America’s safety net” and the difficult circumstances they often face when treating patients, it takes a while to strike a balance, Dr. Vukmir acknowledges.
“There’s a training correlation, which showed up [in this study]; as people got further advanced in training, they felt more comfortable and felt the need to do it less,” says Dr. Vukmir.
The study was funded by a grant from the Agency for Healthcare Research and Quality. Dr. Isbell reports no conflicts of interest. Dr. Vukmir has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Network Open.
The cross-sectional study was conducted by researchers from Soroka University Medical Center, Israel; the University of Massachusetts, Worcester; Beth Israel Deaconess Medical Center; Harvard Medical School, Boston; and the University of Massachusetts, Amherst.
Online survey responses were collected from 1,222 emergency department attending physicians and advanced practice clinicians (APCs) in acute care hospitals throughout Massachusetts from January to September 2020.
Participants were asked to rank their level of agreement – from “strongly disagree” to “strongly agree” – with two statements: “In my day-to-day practice, I am fearful of making a mistake which results in [1] harm to the patient” (fear of harm) and [2] “being sued” (fear of suit).
The average age of the participants was about 44 years; 54.2% were men, 45.1% were women, and 0.7% were of other gender. Approximately 70% of responses were from MDs or DOs, and the remainder were from nurse practitioners and physician assistants. Participants had between 5 and 19 years of experience (median, 10 years).
The study found that the mean score was greater with regard to fear of harm than to fear of suit, regardless of clinician type, experience, or sex and whether the survey was completed before or after the start of the COVID-19 pandemic. There was no significant difference in mean scores regarding fear of suit before the pandemic and after it.
“Our data show a significantly greater fear of harming a patient than a fear of a malpractice suit,” Linda Isbell, PhD, professor of psychology at the University of Massachusetts, Amherst, who is one of the study’s authors, told this news organization. “There is a genuine concern and fear of harming patients and a desire to provide the best care for the patient’s well-being.”
In general, fear-of-harm and fear-of-suit scores decreased as providers gained experience. Those with less than 5 years of experience reported the highest levels of both.
“Although our data do not specifically provide reasons why age may impact [fear] levels, it is possible that with more practice experience ... providers have a better sense of the likelihood of patient harm and malpractice and how to manage such outcomes should they happen,” says Dr. Isbell. She noted that a longitudinal study is necessary to confirm this hypothesis.
One exception was female APCs, whose fear-of-harm scores remained relatively steady across all experience levels. Among male APCs, fear of causing patient harm decreased among those with 5-14 years of experience but increased slightly at 14-44 years of experience.
While previous research typically focused on fear of malpractice as a significant driver of defensive medicine, such as testing excessively, this study examined providers’ fear of harming patients because of a medical error.
The findings suggest “that fear of harm should be considered with, and may be more consequential than, fear of suit in medical decision-making,” the authors note.
“[F]ear can motivate people to engage in more careful and thorough information processing, which can drive behaviors in systematic ways,” says Dr. Isbell. “It is possible that one’s fear of harming a patient is triggering a high level of vigilance, reflected in the practice of defensive medicine across different types of patients – some of whom may be better off with less testing and referrals.”
Rade B. Vukmir, MD, JD, FACEP, an emergency medicine physician and spokesman for the American College of Emergency Physicians, says defensive medicine is common in the specialty and that it occurs 20%-40% of the time.
“Early in practice, the proverbial worst sin is missing a diagnosis, so that’s where the overtesting mentality comes from,” he says. In addition, “there are cities where you can’t drive a mile without seeing a half dozen legal advertisements. That imposes a cost burden on the system, [adding] roughly 20% to the cost of overall care.”
Emergency medicine providers attempt to minimize testing, but between their role as “America’s safety net” and the difficult circumstances they often face when treating patients, it takes a while to strike a balance, Dr. Vukmir acknowledges.
“There’s a training correlation, which showed up [in this study]; as people got further advanced in training, they felt more comfortable and felt the need to do it less,” says Dr. Vukmir.
The study was funded by a grant from the Agency for Healthcare Research and Quality. Dr. Isbell reports no conflicts of interest. Dr. Vukmir has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN