User login
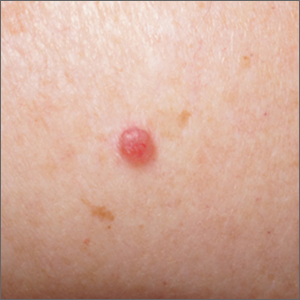
Pink shoulder lesion
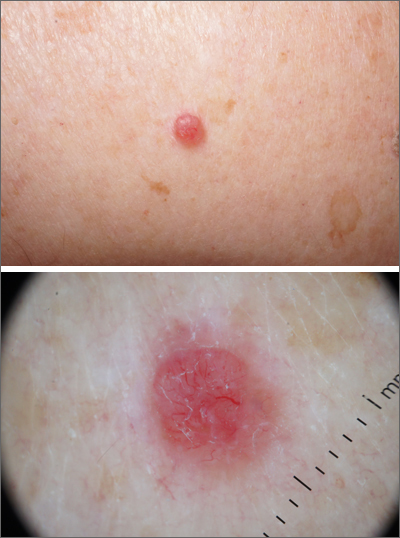
A scoop shave biopsy was performed and histology was consistent with a nodular basal cell carcinoma. BCC is the most common skin cancer in the United States, occurring in approximately 30% of patients with skin types I and II.1 In patients who are Black, squamous cell carcinoma is more common than BCC.2 The overall incidence of BCC is increasing by 4% to 8% every year in the United States.1
BCC most often affects sun-damaged areas—especially on the head and neck—and frequently causes significant tissue damage. It is, however, associated with a low risk of metastasis and mortality.
BCCs may appear as a pink, brown, blue, or white papule or macule. The surface is frequently shiny or pearly in appearance with a rolled border. Dilated, angulated, tree-branch like vessels termed “arborizing vessels” are common. Infiltrative BCC subtypes may look like melted candlewax and extend beyond the area that is clinically apparent.
Partial shave biopsies of a lesion can confirm the diagnosis. A punch biopsy can make it easier to evaluate flat (or even sunken) lesions.
The patient described here was treated with electrodessication and curettage (EDC)—a fast, economical, and effective treatment for the low-risk subtypes of superficial or nodular BCCs on the trunk or extremities. EDC should be avoided with higher risk subtypes of micronodular and infiltrative BCC. With these subtypes, excision (with 4- to 6-mm margins) or Mohs microsurgery is recommended.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME. References
1. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24. doi:10.1016/j.hoc.2018.09.004
2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.

A scoop shave biopsy was performed and histology was consistent with a nodular basal cell carcinoma. BCC is the most common skin cancer in the United States, occurring in approximately 30% of patients with skin types I and II.1 In patients who are Black, squamous cell carcinoma is more common than BCC.2 The overall incidence of BCC is increasing by 4% to 8% every year in the United States.1
BCC most often affects sun-damaged areas—especially on the head and neck—and frequently causes significant tissue damage. It is, however, associated with a low risk of metastasis and mortality.
BCCs may appear as a pink, brown, blue, or white papule or macule. The surface is frequently shiny or pearly in appearance with a rolled border. Dilated, angulated, tree-branch like vessels termed “arborizing vessels” are common. Infiltrative BCC subtypes may look like melted candlewax and extend beyond the area that is clinically apparent.
Partial shave biopsies of a lesion can confirm the diagnosis. A punch biopsy can make it easier to evaluate flat (or even sunken) lesions.
The patient described here was treated with electrodessication and curettage (EDC)—a fast, economical, and effective treatment for the low-risk subtypes of superficial or nodular BCCs on the trunk or extremities. EDC should be avoided with higher risk subtypes of micronodular and infiltrative BCC. With these subtypes, excision (with 4- to 6-mm margins) or Mohs microsurgery is recommended.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME. References

A scoop shave biopsy was performed and histology was consistent with a nodular basal cell carcinoma. BCC is the most common skin cancer in the United States, occurring in approximately 30% of patients with skin types I and II.1 In patients who are Black, squamous cell carcinoma is more common than BCC.2 The overall incidence of BCC is increasing by 4% to 8% every year in the United States.1
BCC most often affects sun-damaged areas—especially on the head and neck—and frequently causes significant tissue damage. It is, however, associated with a low risk of metastasis and mortality.
BCCs may appear as a pink, brown, blue, or white papule or macule. The surface is frequently shiny or pearly in appearance with a rolled border. Dilated, angulated, tree-branch like vessels termed “arborizing vessels” are common. Infiltrative BCC subtypes may look like melted candlewax and extend beyond the area that is clinically apparent.
Partial shave biopsies of a lesion can confirm the diagnosis. A punch biopsy can make it easier to evaluate flat (or even sunken) lesions.
The patient described here was treated with electrodessication and curettage (EDC)—a fast, economical, and effective treatment for the low-risk subtypes of superficial or nodular BCCs on the trunk or extremities. EDC should be avoided with higher risk subtypes of micronodular and infiltrative BCC. With these subtypes, excision (with 4- to 6-mm margins) or Mohs microsurgery is recommended.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME. References
1. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24. doi:10.1016/j.hoc.2018.09.004
2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
1. Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24. doi:10.1016/j.hoc.2018.09.004
2. Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
Florida medical boards ban transgender care for minors
Florida’s two main medical bodies have voted to stop gender-affirming treatment of children, including the use of puberty blockers, cross-sex hormones, and surgery, other than in minors who are already receiving such care.
The move, which is unprecedented, makes Florida one of several U.S. states to restrict gender-affirming care for adolescents, but the first to do so via an administrative process, through the actions of its Board of Medicine and Board of Osteopathic Medicine.
“I appreciate the integrity of the Boards for ruling in the best interest of children in Florida despite facing tremendous pressure to permit these unproven and risky treatments,” Florida Surgeon General Joseph Ladapo, MD, PhD, said in a statement.
In a statement, The Endocrine Society criticizes the decision as “blatantly discriminatory” and not based on medical evidence.
During a meeting on Oct. 28 that involved testimonies from doctors, parents of transgender children, detransitioners, and patients, board members referred to similar changes in Europe, where some countries have pushed psychotherapy instead of surgery or hormone treatment.
Then, on Nov. 4, the boards each set slightly different instructions, with the Board of Osteopathic Medicine voting to restrict care for new patients but allowing an exception for children enrolled in clinical studies, which “must include long-term longitudinal assessments of the patients’ physiologic and psychologic outcomes,” according to the Florida Department of Health.
The Board of Medicine did not allow the latter.
The proposed rules are open to public comment before finalization.
Arkansas was the first state to enact such a ban on gender-affirming care, with Republican lawmakers in 2021 overriding GOP Gov. Asa Hutchinson’s veto of the legislation. Alabama Republicans in 2022 approved legislation to outlaw gender-affirming medications for transgender youths. Both laws have been paused amid unfolding legal battles, according to Associated Press.
Oklahoma Gov. Kevin Stitt, a Republican, signed a bill in October that bars federal funds earmarked for the University of Oklahoma Medical Center from being used for gender reassignment treatments for minors. Gov. Stitt also called for the legislature to ban some of those gender reassignment treatments statewide when it returns in February.
Top Tennessee Republicans also have vowed to push for strict antitransgender policies. The state already bans doctors from providing gender-confirming hormone treatment to prepubescent minors. To date, no one has legally challenged the law as medical experts maintain no doctor in Tennessee does so.
A version of this article first appeared on Medscape.com.
Florida’s two main medical bodies have voted to stop gender-affirming treatment of children, including the use of puberty blockers, cross-sex hormones, and surgery, other than in minors who are already receiving such care.
The move, which is unprecedented, makes Florida one of several U.S. states to restrict gender-affirming care for adolescents, but the first to do so via an administrative process, through the actions of its Board of Medicine and Board of Osteopathic Medicine.
“I appreciate the integrity of the Boards for ruling in the best interest of children in Florida despite facing tremendous pressure to permit these unproven and risky treatments,” Florida Surgeon General Joseph Ladapo, MD, PhD, said in a statement.
In a statement, The Endocrine Society criticizes the decision as “blatantly discriminatory” and not based on medical evidence.
During a meeting on Oct. 28 that involved testimonies from doctors, parents of transgender children, detransitioners, and patients, board members referred to similar changes in Europe, where some countries have pushed psychotherapy instead of surgery or hormone treatment.
Then, on Nov. 4, the boards each set slightly different instructions, with the Board of Osteopathic Medicine voting to restrict care for new patients but allowing an exception for children enrolled in clinical studies, which “must include long-term longitudinal assessments of the patients’ physiologic and psychologic outcomes,” according to the Florida Department of Health.
The Board of Medicine did not allow the latter.
The proposed rules are open to public comment before finalization.
Arkansas was the first state to enact such a ban on gender-affirming care, with Republican lawmakers in 2021 overriding GOP Gov. Asa Hutchinson’s veto of the legislation. Alabama Republicans in 2022 approved legislation to outlaw gender-affirming medications for transgender youths. Both laws have been paused amid unfolding legal battles, according to Associated Press.
Oklahoma Gov. Kevin Stitt, a Republican, signed a bill in October that bars federal funds earmarked for the University of Oklahoma Medical Center from being used for gender reassignment treatments for minors. Gov. Stitt also called for the legislature to ban some of those gender reassignment treatments statewide when it returns in February.
Top Tennessee Republicans also have vowed to push for strict antitransgender policies. The state already bans doctors from providing gender-confirming hormone treatment to prepubescent minors. To date, no one has legally challenged the law as medical experts maintain no doctor in Tennessee does so.
A version of this article first appeared on Medscape.com.
Florida’s two main medical bodies have voted to stop gender-affirming treatment of children, including the use of puberty blockers, cross-sex hormones, and surgery, other than in minors who are already receiving such care.
The move, which is unprecedented, makes Florida one of several U.S. states to restrict gender-affirming care for adolescents, but the first to do so via an administrative process, through the actions of its Board of Medicine and Board of Osteopathic Medicine.
“I appreciate the integrity of the Boards for ruling in the best interest of children in Florida despite facing tremendous pressure to permit these unproven and risky treatments,” Florida Surgeon General Joseph Ladapo, MD, PhD, said in a statement.
In a statement, The Endocrine Society criticizes the decision as “blatantly discriminatory” and not based on medical evidence.
During a meeting on Oct. 28 that involved testimonies from doctors, parents of transgender children, detransitioners, and patients, board members referred to similar changes in Europe, where some countries have pushed psychotherapy instead of surgery or hormone treatment.
Then, on Nov. 4, the boards each set slightly different instructions, with the Board of Osteopathic Medicine voting to restrict care for new patients but allowing an exception for children enrolled in clinical studies, which “must include long-term longitudinal assessments of the patients’ physiologic and psychologic outcomes,” according to the Florida Department of Health.
The Board of Medicine did not allow the latter.
The proposed rules are open to public comment before finalization.
Arkansas was the first state to enact such a ban on gender-affirming care, with Republican lawmakers in 2021 overriding GOP Gov. Asa Hutchinson’s veto of the legislation. Alabama Republicans in 2022 approved legislation to outlaw gender-affirming medications for transgender youths. Both laws have been paused amid unfolding legal battles, according to Associated Press.
Oklahoma Gov. Kevin Stitt, a Republican, signed a bill in October that bars federal funds earmarked for the University of Oklahoma Medical Center from being used for gender reassignment treatments for minors. Gov. Stitt also called for the legislature to ban some of those gender reassignment treatments statewide when it returns in February.
Top Tennessee Republicans also have vowed to push for strict antitransgender policies. The state already bans doctors from providing gender-confirming hormone treatment to prepubescent minors. To date, no one has legally challenged the law as medical experts maintain no doctor in Tennessee does so.
A version of this article first appeared on Medscape.com.
Study finds high rate of psychiatric burden in cosmetic dermatology patients
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Living donor liver transplants on rise for most urgent need
Living donor liver transplants (LDLT) for recipients with the most urgent need for a liver transplant in the next 3 months – a model for end-stage liver disease (MELD) score of 25 or higher – have become more frequent during the past decade, according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Among LDLT recipients, researchers found comparable patient and graft survival at low and high MELD scores. But among patients with high MELD scores, researchers found lower adjusted graft survival and a higher transplant rate among those with living donors, compared with recipients of deceased donor liver transplantation (DDLT).
The findings suggest certain advantages of LDLT over DDLT may be lost in the high-MELD setting in terms of graft survival, said Benjamin Rosenthal, MD, an internal medicine resident focused on transplant hepatology at the Hospital of the University of Pennsylvania, Philadelphia.
“Historically, in the United States especially, living donor liver transplantation has been offered to patients with low or moderate MELD,” he said. “The outcomes of LDLT at high MELD are currently unknown.”
Previous data from the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) found that LDLT offered a survival benefit versus remaining on the wait list, independent of MELD score, he said. A recent study also has demonstrated a survival benefit across MELD scores of 11-26, but findings for MELD scores of 25 and higher have been mixed.
Trends and outcomes in LDLT at high MELD scores
Dr. Rosenthal and colleagues conducted a retrospective cohort study of adult LDLT recipients from 2010 to 2021 using data from the Organ Procurement and Transplantation Network (OPTN), the U.S. donation and transplantation system.
In baseline characteristics among LDLT transplant recipients, there weren’t significant differences in age, sex, race, and ethnicity for MELD scores below 25 or at 25 and higher. There also weren’t significant differences in donor age, relationship, use of nondirected grafts, or percentage of right and left lobe donors for LDLT recipients. However, recipients with high MELD scores had more nonalcoholic steatohepatitis (29.5% versus 24.6%) and alcohol-assisted cirrhosis (21.6% versus 14.3%).
The research team evaluated graft survival among LDLT recipients by MELD below 25 and at 25 or higher. They also compared posttransplant patient and graft survival between LDLT and DDLT recipients with a MELD of 25 or higher. They excluded transplant candidates on the wait list for Status 1/1A, redo transplant, or multiorgan transplant.
Among the 3,590 patients who had LDLT between 2010 and 2021, 342 patients (9.5%) had a MELD of 25 or higher at transplant. There was some progression during the waiting period, Dr. Rosenthal noted, with a median listing MELD score of 19 among those who had a MELD of 25 or higher at transplant and 21 among those who had a MELD of 30 or higher at transplant.
For LDLT recipients with MELD scores above or below 25, researchers found no significant differences in adjusted patient survival or adjusted graft survival.
Then the team compared outcomes of LDLT and DDLT in high-MELD recipients. Among the 67,279-patient DDLT comparator group, 27,552 patients (41%) had a MELD of 25 or higher at transplant.
In terms of LDLT versus DDLT, unadjusted and adjusted patient survival were no different for patients with MELD of 25 or higher. In addition, unadjusted graft survival was no different.
However, adjusted graft survival was worse for LDLT recipients with high MELD scores. In addition, the retransplant rate was higher in LDLT recipients, at 5.7% versus 2.4%.
The reason why graft survival may be worse remains unclear, Dr. Rosenthal said. One hypothesis is that a low graft-to-recipient weight ratio in LDLT can cause small-for-size syndrome. However, these ratios were not available from OPTN.
“Further studies should be done to see what the benefit is, with graft-to-recipient weight ratios included,” he said. “The differences between DDLT and LDLT in this setting should be further explored as well.”
The research team also described temporal and transplant center trends for LDLT by MELD group. For temporal trends, they expanded the study period from 2002-2021.
The found a marked U.S. increase in the percentage of LDLT with a MELD of 25 or higher, particularly in the last decade and especially in the last 5 years. But the percentage of LDLT with high MELD remains lower than 15%, even in recent years, Dr. Rosenthal noted.
Across transplant centers, there was a trend toward centers with increasing LDLT volume having a greater proportion of LDLT recipients with a MELD of 25 or higher. At the 19.6% of centers performing 10 or fewer LDLT during the study period, none of the LDLT recipients had a MELD of 25 or higher, Dr. Rosenthal said.
The authors didn’t report a funding source. The authors declared no relevant disclosures.
Living donor liver transplants (LDLT) for recipients with the most urgent need for a liver transplant in the next 3 months – a model for end-stage liver disease (MELD) score of 25 or higher – have become more frequent during the past decade, according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Among LDLT recipients, researchers found comparable patient and graft survival at low and high MELD scores. But among patients with high MELD scores, researchers found lower adjusted graft survival and a higher transplant rate among those with living donors, compared with recipients of deceased donor liver transplantation (DDLT).
The findings suggest certain advantages of LDLT over DDLT may be lost in the high-MELD setting in terms of graft survival, said Benjamin Rosenthal, MD, an internal medicine resident focused on transplant hepatology at the Hospital of the University of Pennsylvania, Philadelphia.
“Historically, in the United States especially, living donor liver transplantation has been offered to patients with low or moderate MELD,” he said. “The outcomes of LDLT at high MELD are currently unknown.”
Previous data from the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) found that LDLT offered a survival benefit versus remaining on the wait list, independent of MELD score, he said. A recent study also has demonstrated a survival benefit across MELD scores of 11-26, but findings for MELD scores of 25 and higher have been mixed.
Trends and outcomes in LDLT at high MELD scores
Dr. Rosenthal and colleagues conducted a retrospective cohort study of adult LDLT recipients from 2010 to 2021 using data from the Organ Procurement and Transplantation Network (OPTN), the U.S. donation and transplantation system.
In baseline characteristics among LDLT transplant recipients, there weren’t significant differences in age, sex, race, and ethnicity for MELD scores below 25 or at 25 and higher. There also weren’t significant differences in donor age, relationship, use of nondirected grafts, or percentage of right and left lobe donors for LDLT recipients. However, recipients with high MELD scores had more nonalcoholic steatohepatitis (29.5% versus 24.6%) and alcohol-assisted cirrhosis (21.6% versus 14.3%).
The research team evaluated graft survival among LDLT recipients by MELD below 25 and at 25 or higher. They also compared posttransplant patient and graft survival between LDLT and DDLT recipients with a MELD of 25 or higher. They excluded transplant candidates on the wait list for Status 1/1A, redo transplant, or multiorgan transplant.
Among the 3,590 patients who had LDLT between 2010 and 2021, 342 patients (9.5%) had a MELD of 25 or higher at transplant. There was some progression during the waiting period, Dr. Rosenthal noted, with a median listing MELD score of 19 among those who had a MELD of 25 or higher at transplant and 21 among those who had a MELD of 30 or higher at transplant.
For LDLT recipients with MELD scores above or below 25, researchers found no significant differences in adjusted patient survival or adjusted graft survival.
Then the team compared outcomes of LDLT and DDLT in high-MELD recipients. Among the 67,279-patient DDLT comparator group, 27,552 patients (41%) had a MELD of 25 or higher at transplant.
In terms of LDLT versus DDLT, unadjusted and adjusted patient survival were no different for patients with MELD of 25 or higher. In addition, unadjusted graft survival was no different.
However, adjusted graft survival was worse for LDLT recipients with high MELD scores. In addition, the retransplant rate was higher in LDLT recipients, at 5.7% versus 2.4%.
The reason why graft survival may be worse remains unclear, Dr. Rosenthal said. One hypothesis is that a low graft-to-recipient weight ratio in LDLT can cause small-for-size syndrome. However, these ratios were not available from OPTN.
“Further studies should be done to see what the benefit is, with graft-to-recipient weight ratios included,” he said. “The differences between DDLT and LDLT in this setting should be further explored as well.”
The research team also described temporal and transplant center trends for LDLT by MELD group. For temporal trends, they expanded the study period from 2002-2021.
The found a marked U.S. increase in the percentage of LDLT with a MELD of 25 or higher, particularly in the last decade and especially in the last 5 years. But the percentage of LDLT with high MELD remains lower than 15%, even in recent years, Dr. Rosenthal noted.
Across transplant centers, there was a trend toward centers with increasing LDLT volume having a greater proportion of LDLT recipients with a MELD of 25 or higher. At the 19.6% of centers performing 10 or fewer LDLT during the study period, none of the LDLT recipients had a MELD of 25 or higher, Dr. Rosenthal said.
The authors didn’t report a funding source. The authors declared no relevant disclosures.
Living donor liver transplants (LDLT) for recipients with the most urgent need for a liver transplant in the next 3 months – a model for end-stage liver disease (MELD) score of 25 or higher – have become more frequent during the past decade, according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
Among LDLT recipients, researchers found comparable patient and graft survival at low and high MELD scores. But among patients with high MELD scores, researchers found lower adjusted graft survival and a higher transplant rate among those with living donors, compared with recipients of deceased donor liver transplantation (DDLT).
The findings suggest certain advantages of LDLT over DDLT may be lost in the high-MELD setting in terms of graft survival, said Benjamin Rosenthal, MD, an internal medicine resident focused on transplant hepatology at the Hospital of the University of Pennsylvania, Philadelphia.
“Historically, in the United States especially, living donor liver transplantation has been offered to patients with low or moderate MELD,” he said. “The outcomes of LDLT at high MELD are currently unknown.”
Previous data from the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) found that LDLT offered a survival benefit versus remaining on the wait list, independent of MELD score, he said. A recent study also has demonstrated a survival benefit across MELD scores of 11-26, but findings for MELD scores of 25 and higher have been mixed.
Trends and outcomes in LDLT at high MELD scores
Dr. Rosenthal and colleagues conducted a retrospective cohort study of adult LDLT recipients from 2010 to 2021 using data from the Organ Procurement and Transplantation Network (OPTN), the U.S. donation and transplantation system.
In baseline characteristics among LDLT transplant recipients, there weren’t significant differences in age, sex, race, and ethnicity for MELD scores below 25 or at 25 and higher. There also weren’t significant differences in donor age, relationship, use of nondirected grafts, or percentage of right and left lobe donors for LDLT recipients. However, recipients with high MELD scores had more nonalcoholic steatohepatitis (29.5% versus 24.6%) and alcohol-assisted cirrhosis (21.6% versus 14.3%).
The research team evaluated graft survival among LDLT recipients by MELD below 25 and at 25 or higher. They also compared posttransplant patient and graft survival between LDLT and DDLT recipients with a MELD of 25 or higher. They excluded transplant candidates on the wait list for Status 1/1A, redo transplant, or multiorgan transplant.
Among the 3,590 patients who had LDLT between 2010 and 2021, 342 patients (9.5%) had a MELD of 25 or higher at transplant. There was some progression during the waiting period, Dr. Rosenthal noted, with a median listing MELD score of 19 among those who had a MELD of 25 or higher at transplant and 21 among those who had a MELD of 30 or higher at transplant.
For LDLT recipients with MELD scores above or below 25, researchers found no significant differences in adjusted patient survival or adjusted graft survival.
Then the team compared outcomes of LDLT and DDLT in high-MELD recipients. Among the 67,279-patient DDLT comparator group, 27,552 patients (41%) had a MELD of 25 or higher at transplant.
In terms of LDLT versus DDLT, unadjusted and adjusted patient survival were no different for patients with MELD of 25 or higher. In addition, unadjusted graft survival was no different.
However, adjusted graft survival was worse for LDLT recipients with high MELD scores. In addition, the retransplant rate was higher in LDLT recipients, at 5.7% versus 2.4%.
The reason why graft survival may be worse remains unclear, Dr. Rosenthal said. One hypothesis is that a low graft-to-recipient weight ratio in LDLT can cause small-for-size syndrome. However, these ratios were not available from OPTN.
“Further studies should be done to see what the benefit is, with graft-to-recipient weight ratios included,” he said. “The differences between DDLT and LDLT in this setting should be further explored as well.”
The research team also described temporal and transplant center trends for LDLT by MELD group. For temporal trends, they expanded the study period from 2002-2021.
The found a marked U.S. increase in the percentage of LDLT with a MELD of 25 or higher, particularly in the last decade and especially in the last 5 years. But the percentage of LDLT with high MELD remains lower than 15%, even in recent years, Dr. Rosenthal noted.
Across transplant centers, there was a trend toward centers with increasing LDLT volume having a greater proportion of LDLT recipients with a MELD of 25 or higher. At the 19.6% of centers performing 10 or fewer LDLT during the study period, none of the LDLT recipients had a MELD of 25 or higher, Dr. Rosenthal said.
The authors didn’t report a funding source. The authors declared no relevant disclosures.
FROM THE LIVER MEETING
Have you heard the one about the emergency dept. that called 911?
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
A Patient Presenting With Shortness of Breath, Fever, and Eosinophilia
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?
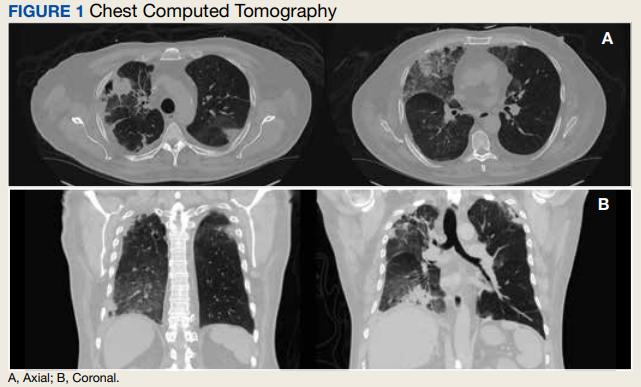
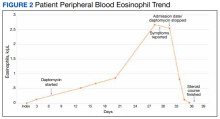
In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
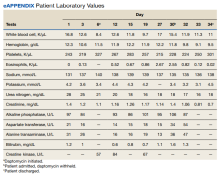
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
A 70-year-old veteran with a history notable for type 2 diabetes mellitus, complicated by peripheral neuropathy and bilateral foot ulceration, and previous pulmonary tuberculosis (treated in June 2013) presented to an outside medical facility with bilateral worsening foot pain, swelling, and drainage of preexisting ulcers. He received a diagnosis of bilateral fifth toe osteomyelitis and was discharged with a 6-week course of IV daptomycin 600 mg (8 mg/kg) and ertapenem 1 g/d. At discharge, the patient was in stable condition. Follow-up was done by our outpatient parenteral antimicrobial therapy (OPAT) team, which consists of an infectious disease pharmacist and the physician director of antimicrobial stewardship who monitor veterans receiving outpatient IV antibiotic therapy.1
Three weeks later as part of the regular OPAT surveillance, the patient reported via telephone that his foot osteomyelitis was stable, but he had a 101 °F fever and a new cough. He was instructed to come to the emergency department (ED) immediately. On arrival,
- What is your diagnosis?
- How would you treat this patient?

In the ED, the patient was given a provisional diagnosis of multifocal bacterial pneumonia and was admitted to the hospital for further management. His outpatient regimen of IV daptomycin and ertapenem was adjusted to IV vancomycin and meropenem. The infectious disease service was consulted within 24 hours of admission, and based on the new onset chest infiltrates, therapy with daptomycin and notable peripheral blood eosinophilia, a presumptive diagnosis of daptomycin-related acute eosinophilic pneumonia was made. A medication list review yielded no other potential etiologic agents for drug-related eosinophilia, and the patient did not have any remote or recent pertinent travel history concerning for parasitic disease.
The patient was treated with oral prednisone 40 mg (0.5 mg/kg) daily and the daptomycin was not restarted. Within 24 hours, the patient’s fevers, oxygen requirements, and cough subsided. Laboratory values
Discussion
Daptomycin is a commonly used cyclic lipopeptide IV antibiotic with broad activity against gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE). Daptomycin has emerged as a convenient alternative for infections typically treated with IV vancomycin: shorter infusion time (2-30 minutes vs 60-180 minutes), daily administration, and less need for dose adjustments. A recent survey reported higher satisfaction and less disruption in patients receiving daptomycin compared with vancomycin.2 The main daptomycin-specific adverse effect (AE) that warrants close monitoring is elevated creatine kinase (CK) levels and skeletal muscle breakdown (reversible after holding medication).3 Other rarely reported AEs include drug reaction with eosinophilia and systemic symptoms (DRESS), acute eosinophilic pneumonitis, hepatitis, and peripheral neuropathy.4-6 Consequently, weekly monitoring for this drug should include symptom inquiry for cough and muscle pain, and laboratory testing with CBC with differential, comprehensive metabolic panel (CMP), and CK.
Daptomycin-induced eosinophilic pneumonia has been described in several case reports and in a recent study, the frequency of this event was almost 5% in those receiving long-term daptomycin therapy.7 The most common symptoms include dyspnea, fever, infiltrates/opacities on chest imaging, and peripheral eosinophilia. It is theorized that the chemical structure of daptomycin causes immune-mediated pulmonary epithelial cell injury with eosinophils, resulting in increased peripheral eosinophilia.3 Risk factors that have been identified for daptomycin-induced eosinophilia include age > 70 years; the presence of comorbidities of heart and pulmonary disease; duration of daptomycin beyond 2 weeks; and cumulative doses over 10 g. Average onset of illness from initiation of daptomycin has been reported to be about 3 weeks.7,8 The diagnosis of daptomycin-induced eosinophilic pneumonitis is made on several criteria per the FDA. These include exposure to daptomycin, fever, dyspnea with oxygen requirement, new infiltrates on imaging, bronchoalveolar lavage with > 25% eosinophils, and last, clinical improvement on removal of the drug.9 However, as bronchoscopy is an invasive diagnostic modality, it is not always performed or necessary as seen in this case. Furthermore, not all patients will have peripheral eosinophilia, with only 77% of patients having that finding in a systematic review.10 Taken together, the overall true incidence of daptomycin-induced eosinophilia may be underestimated. Treatment involves discontinuation of the daptomycin and initiation of steroids. In a review of 35 cases, the majority did receive systemic steroids, usually 60 to 125 mg of IV methylprednisolone every 6 hours, which was converted to oral steroids and tapered over 2 to 6 weeks.10 However, all patients including those who did not receive steroids had symptom improvement or complete resolution, highlighting that prompt discontinuation of daptomycin is the most crucial intervention.
Conclusions
As home IV antibiotic therapy becomes increasingly used to facilitate shorter lengths of stay in hospitals and enable more patients to receive their infectious disease care at home, the general practitioner must be aware of the potential AEs of commonly used IV antibiotics. While acute cutaneous reactions and disturbances in renal and liver function are commonly recognized entities of adverse drug reactions, symptoms of fever and cough are more likely to be interpreted as acute viral or bacterial respiratory infections. A high index of clinical suspicion is needed for eosinophilic pneumonitis secondary to daptomycin. A simple and readily available test, such as a CBC with differential may facilitate the identification of this potentially serious AE, allowing prompt discontinuation of the drug.
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
1. Kent M, Kouma M, Jodlowski T, Cutrell JB. 755. Outpatient parenteral antimicrobial therapy program evaluation within a large Veterans Affairs healthcare system. Open Forum Infect Dis. 2019;6(suppl 2):S337. Published 2019 Oct 23. doi:10.1093/ofid/ofz360.823
2. Wu KH, Sakoulas G, Geriak M. Vancomycin or daptomycin for outpatient parenteral antibiotic therapy: does it make a difference in patient satisfaction? Open Forum Infect Dis. 2021;8(8):ofab418. Published 2021 Aug 30. doi:10.1093/ofid/ofab418
3. Gonzalez-Ruiz A, Seaton RA, Hamed K. Daptomycin: an evidence-based review of its role in the treatment of gram-positive infections. Infect Drug Resist. 2016;9:47-58. Published 2016 Apr 15. doi:10.2147/IDR.S99046
4. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S. Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a literature review. Eur J Clin Pharmacol. 2021;77(3):275-289. doi:10.1007/s00228-020-03005-9
5. Mo Y, Nehring F, Jung AH, Housman ST. Possible hepatotoxicity associated with daptomycin: a case report and literature review. J Pharm Pract. 2016;29(3):253-256. doi:10.1177/0897190015625403
6. Villaverde Piñeiro L, Rabuñal Rey R, García Sabina A, Monte Secades R, García Pais MJ. Paralysis of the external popliteal sciatic nerve associated with daptomycin administration. J Clin Pharm Ther. 2018;43(4):578-580. doi:10.1111/jcpt.12666
7. Soldevila-Boixader L, Villanueva B, Ulldemolins M, et al. Risk factors of daptomycin-induced eosinophilic pneumonia in a population with osteoarticular infection. Antibiotics (Basel). 2021;10(4):446. Published 2021 Apr 16. doi:10.3390/antibiotics10040446
8. Kumar S, Acosta-Sanchez I, Rajagopalan N. Daptomycin-induced acute eosinophilic pneumonia. Cureus. 2018;10(6):e2899. Published 2018 Jun 30. doi:10.7759/cureus.2899
9. Center for Drug Evaluation and Research. Eosinophilic pneumonia associated with the use of cubicin. U.S. Food and Drug Administration. Updated August 3, 2017. Accessed October 10, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-eosinophilic-pneumonia-associated-use-cubicin-daptomycin
10. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia—a systematic review. Antimicrob Resist Infect Control. 2016;5:55. Published 2016 Dec 12. doi:10.1186/s13756-016-0158-8
Medicaid coverage of HPV vaccine in adults: Implications in dermatology
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
, according to the authors of a review of Medicaid policies across all 50 states.
The human papillomavirus (HPV) vaccine is approved for people aged 9-45 years, for preventing genital, cervical, anal, and oropharyngeal cancers, and genital warts. And the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommends routine vaccination with the HPV vaccine for individuals aged 9-26 years, with “shared clinical decision-making” recommended for vaccination of those aged 27-45 years, wrote Nathaniel Goldman of New York Medical College, Valhalla, and coauthors, from the University of Missouri–Kansas City and Harvard Medical School, Boston.
A total of 33 states offered formal statewide Medicaid coverage policies that were accessible online or through the state’s Medicaid office. Another 11 states provided coverage through Medicaid managed care organizations, and 4 states had HPV vaccination as part of their formal Medicaid adult vaccination programs.
Overall, 43 states covered HPV vaccination through age 45 years with no need for prior authorization, and another 4 states (Ohio, Maine, Nebraska, and New York) provided coverage with prior authorization for adults older than 26 years.
The study findings were limited by the use of Medicaid coverage only, the researchers noted. Consequently, patients eligible for HPV vaccination who are uninsured or have other types of insurance may face additional barriers in the form of high costs, given that the current retail price is $250-$350 per shot for the three-shot series, the researchers noted.
However, the results suggest that Medicaid coverage for HPV vaccination may inform dermatologists’ recommendations for patients at increased risk, they said. More research is needed to “better identify dermatology patients at risk for new HPV infection and ways to improve vaccination rates in these vulnerable individuals,” they added.
Vaccine discussions are important in dermatology
“Dermatologists care for patients who may be an increased risk of vaccine-preventable illnesses, either from a skin disease or a dermatology medication,” corresponding author Megan H. Noe, MD, a dermatologist at Brigham and Women’s Hospital, and assistant professor of dermatology, Harvard Medical School, Boston, said in an interview. “Over the last several years, we have seen that all physicians, whether they provide vaccinations or not, can play an important role in discussing vaccines with their patients,” she said.
“Vaccines can be cost-prohibitive for patients without insurance coverage, so we hope that dermatologists will be more likely to recommend the HPV vaccine to patients 27-45 years of age if they know that it is likely covered by insurance,” Dr. Noe noted.
However, “time may be a barrier for many dermatologists who have many important things to discuss with patients during their appointments,” she said. “We are currently working on developing educational information to help facilitate this conversation,” she added.
Looking ahead, she said that “additional research is necessary to create vaccine guidelines specific to dermatology patients and dermatology medications, so we can provide clear recommendations to our patients and ensure appropriate insurance coverage for all necessary vaccines.”
Vaccine discussions
“I think it’s great that many Medicaid plans are covering HPV vaccination,” said Karl Saardi, MD, of the department of dermatology, George Washington University, Washington, who was asked to comment on the study. “I routinely recommend [vaccination] for patients who have viral warts, since it does lead to improvement in some cases,” Dr. Saardi, who was not involved in the current study, said in an interview. “Although we don’t have the HPV vaccines in our clinic for administration, my experience has been that patients are very open to discussing it with their primary care doctors.”
Although the upper age range continues to rise, “I think getting younger people vaccinated will also prove to be important,” said Dr. Saardi, director of the inpatient dermatology service at the George Washington University Hospital.
The point made in the current study about the importance of HPV vaccination in patients with hidradenitis suppurativa is also crucial, he added. “Since chronic skin inflammation in hidradenitis drives squamous cell carcinoma, reducing the impact of HPV on such cancers makes perfect sense.”
The study received no outside funding. Dr. Noe disclosed grants from Boehringer Ingelheim unrelated to the current study. Dr. Saardi had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Chronic hepatitis B infections associated with a range of liver malignancies
, shows a new study conducted in South Korea.
In this study, which was published in the Journal of Clinical Oncology, researchers found that long-term treatment with nucleos(t)ide analogues (NAs) for patients with chronic hepatitis B lowered their risk of developing extrahepatic cancer types.
In addition to lowering the risk of liver cancers, treatment with nucleos(t)ide analogues, including tenofovir disoproxil fumarate, entecavir, lamivudine, telbivudine, adefovir, and clevudine, lowered the risk of developing cancer of the pancreas and prostate, but increased the risk of breast cancer.
By controlling chronic hepatitis B infections (CHB), NAs have been known to reduce the risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. About half of the 700,000 people who die each year from chronic hepatitis B infections also have an intrahepatic malignancy.
But extrahepatic cholangiocarcinoma, in which tumors grow outside of the liver in the bile ducts, is exceedingly rare, affecting only 8,000 people each year in the United States.
The study was led by Jeong-Hoon Lee, MD, PhD, Seoul National University, South Korea.
The study details
Researchers sought to understand whether CHB treatment with NA drugs could reduce the risk of extrahepatic cancer. The study is based on an analysis of South Korean medical insurance claims data that included 90,944 patients (6,539 treated with NAs) with a newly diagnosed chronic hepatitis B infection, and 685,436 controls. The median age of the groups ranged from 47 to 51, and the percentage of men ranged from 51.3% to 62.5%.
Over the median 47.4-month study period, 3.9% (30,413) of subjects developed cancer outside the liver. Patients with CHB who weren’t treated with NAs had a higher overall risk vs. the NA-treatment group (adjusted subdistribution hazard ratio = 1.28; 95% confidence interval, 1.12-1.45; P < .001) and vs. controls (aSHR = 1.22; 95% CI, 1.18-1.26; P < .001).
The researchers write that “the direction of the original result was maintained” even after adjustment for cancer risk factors such as smoking and alcohol consumption. “Randomized controlled trials might be warranted to explore whether NA treatment will reduce the risk of extrahepatic malignancy in patients with CHB outside the current treatment indication,” they wrote.
In an accompanying commentary, Lewis R. Roberts, MBChB, PhD, of Mayo Clinic, Rochester, Minn., said that what is perhaps “the most controversial result ... one that is not the direct subject of their study, the observation that NA treatment was not associated with a decrease in risk of primary intrahepatic malignancy – hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma. The observed decrease in risk of intrahepatic malignancy was 12%, with an adjusted subdistribution hazard ratio of 0.88 (95% CI, 0.77-1.01; P = .08).”
As Dr. Roberts wrote, the authors suggested this could be related to the low prevalence of cirrhosis in the study group. “This explanation is plausible, as it has previously been shown that the major impact of NA treatment in reducing HCC incidence is in those with CHB-induced cirrhosis,” he wrote.
Dr. Roberts added that randomized trials of NA in CHB would be difficult because the drugs are so effective. “The most important implication of this study may be the observation that CHB is associated with a higher risk of a range of extrahepatic malignancies, and the opportunity to advise patients with CHB to adhere to current recommendations for screening for the major cancer types.”
The study was publicly funded, but several study authors report numerous disclosures including relationships with Yuhan Corporation, Bayer, Gilead Sciences, Bristol Myers Squibb, and others. Dr. Roberts reports numerous personal and institutional disclosures including relationships with Bayer, Gilead Sciences, Medscape, Roche, and others plus a patent and royalties.
, shows a new study conducted in South Korea.
In this study, which was published in the Journal of Clinical Oncology, researchers found that long-term treatment with nucleos(t)ide analogues (NAs) for patients with chronic hepatitis B lowered their risk of developing extrahepatic cancer types.
In addition to lowering the risk of liver cancers, treatment with nucleos(t)ide analogues, including tenofovir disoproxil fumarate, entecavir, lamivudine, telbivudine, adefovir, and clevudine, lowered the risk of developing cancer of the pancreas and prostate, but increased the risk of breast cancer.
By controlling chronic hepatitis B infections (CHB), NAs have been known to reduce the risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. About half of the 700,000 people who die each year from chronic hepatitis B infections also have an intrahepatic malignancy.
But extrahepatic cholangiocarcinoma, in which tumors grow outside of the liver in the bile ducts, is exceedingly rare, affecting only 8,000 people each year in the United States.
The study was led by Jeong-Hoon Lee, MD, PhD, Seoul National University, South Korea.
The study details
Researchers sought to understand whether CHB treatment with NA drugs could reduce the risk of extrahepatic cancer. The study is based on an analysis of South Korean medical insurance claims data that included 90,944 patients (6,539 treated with NAs) with a newly diagnosed chronic hepatitis B infection, and 685,436 controls. The median age of the groups ranged from 47 to 51, and the percentage of men ranged from 51.3% to 62.5%.
Over the median 47.4-month study period, 3.9% (30,413) of subjects developed cancer outside the liver. Patients with CHB who weren’t treated with NAs had a higher overall risk vs. the NA-treatment group (adjusted subdistribution hazard ratio = 1.28; 95% confidence interval, 1.12-1.45; P < .001) and vs. controls (aSHR = 1.22; 95% CI, 1.18-1.26; P < .001).
The researchers write that “the direction of the original result was maintained” even after adjustment for cancer risk factors such as smoking and alcohol consumption. “Randomized controlled trials might be warranted to explore whether NA treatment will reduce the risk of extrahepatic malignancy in patients with CHB outside the current treatment indication,” they wrote.
In an accompanying commentary, Lewis R. Roberts, MBChB, PhD, of Mayo Clinic, Rochester, Minn., said that what is perhaps “the most controversial result ... one that is not the direct subject of their study, the observation that NA treatment was not associated with a decrease in risk of primary intrahepatic malignancy – hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma. The observed decrease in risk of intrahepatic malignancy was 12%, with an adjusted subdistribution hazard ratio of 0.88 (95% CI, 0.77-1.01; P = .08).”
As Dr. Roberts wrote, the authors suggested this could be related to the low prevalence of cirrhosis in the study group. “This explanation is plausible, as it has previously been shown that the major impact of NA treatment in reducing HCC incidence is in those with CHB-induced cirrhosis,” he wrote.
Dr. Roberts added that randomized trials of NA in CHB would be difficult because the drugs are so effective. “The most important implication of this study may be the observation that CHB is associated with a higher risk of a range of extrahepatic malignancies, and the opportunity to advise patients with CHB to adhere to current recommendations for screening for the major cancer types.”
The study was publicly funded, but several study authors report numerous disclosures including relationships with Yuhan Corporation, Bayer, Gilead Sciences, Bristol Myers Squibb, and others. Dr. Roberts reports numerous personal and institutional disclosures including relationships with Bayer, Gilead Sciences, Medscape, Roche, and others plus a patent and royalties.
, shows a new study conducted in South Korea.
In this study, which was published in the Journal of Clinical Oncology, researchers found that long-term treatment with nucleos(t)ide analogues (NAs) for patients with chronic hepatitis B lowered their risk of developing extrahepatic cancer types.
In addition to lowering the risk of liver cancers, treatment with nucleos(t)ide analogues, including tenofovir disoproxil fumarate, entecavir, lamivudine, telbivudine, adefovir, and clevudine, lowered the risk of developing cancer of the pancreas and prostate, but increased the risk of breast cancer.
By controlling chronic hepatitis B infections (CHB), NAs have been known to reduce the risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. About half of the 700,000 people who die each year from chronic hepatitis B infections also have an intrahepatic malignancy.
But extrahepatic cholangiocarcinoma, in which tumors grow outside of the liver in the bile ducts, is exceedingly rare, affecting only 8,000 people each year in the United States.
The study was led by Jeong-Hoon Lee, MD, PhD, Seoul National University, South Korea.
The study details
Researchers sought to understand whether CHB treatment with NA drugs could reduce the risk of extrahepatic cancer. The study is based on an analysis of South Korean medical insurance claims data that included 90,944 patients (6,539 treated with NAs) with a newly diagnosed chronic hepatitis B infection, and 685,436 controls. The median age of the groups ranged from 47 to 51, and the percentage of men ranged from 51.3% to 62.5%.
Over the median 47.4-month study period, 3.9% (30,413) of subjects developed cancer outside the liver. Patients with CHB who weren’t treated with NAs had a higher overall risk vs. the NA-treatment group (adjusted subdistribution hazard ratio = 1.28; 95% confidence interval, 1.12-1.45; P < .001) and vs. controls (aSHR = 1.22; 95% CI, 1.18-1.26; P < .001).
The researchers write that “the direction of the original result was maintained” even after adjustment for cancer risk factors such as smoking and alcohol consumption. “Randomized controlled trials might be warranted to explore whether NA treatment will reduce the risk of extrahepatic malignancy in patients with CHB outside the current treatment indication,” they wrote.
In an accompanying commentary, Lewis R. Roberts, MBChB, PhD, of Mayo Clinic, Rochester, Minn., said that what is perhaps “the most controversial result ... one that is not the direct subject of their study, the observation that NA treatment was not associated with a decrease in risk of primary intrahepatic malignancy – hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma. The observed decrease in risk of intrahepatic malignancy was 12%, with an adjusted subdistribution hazard ratio of 0.88 (95% CI, 0.77-1.01; P = .08).”
As Dr. Roberts wrote, the authors suggested this could be related to the low prevalence of cirrhosis in the study group. “This explanation is plausible, as it has previously been shown that the major impact of NA treatment in reducing HCC incidence is in those with CHB-induced cirrhosis,” he wrote.
Dr. Roberts added that randomized trials of NA in CHB would be difficult because the drugs are so effective. “The most important implication of this study may be the observation that CHB is associated with a higher risk of a range of extrahepatic malignancies, and the opportunity to advise patients with CHB to adhere to current recommendations for screening for the major cancer types.”
The study was publicly funded, but several study authors report numerous disclosures including relationships with Yuhan Corporation, Bayer, Gilead Sciences, Bristol Myers Squibb, and others. Dr. Roberts reports numerous personal and institutional disclosures including relationships with Bayer, Gilead Sciences, Medscape, Roche, and others plus a patent and royalties.
FROM JOURNAL OF CLINICAL ONCOLOGY
The Long Arc of Justice for Veteran Benefits
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
This Veterans Day we honor the passing of the largest expansion of veterans benefits and services in history. On August 10, 2022, President Biden signed the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act. This act was named for a combat medic who died of a rare form of lung cancer believed to be the result of a toxic military exposure. His widow was present during the President's State of the Union address that urged Congress to pass the legislation.2
Like all other congressional bills and government regulations, the PACT Act is complex in its details and still a work in progress. Simply put, the PACT Act expands and/or extends enrollment for a group of previously ineligible veterans. Eligibility will no longer require that veterans demonstrate a service-connected disability due to toxic exposure, including those from burn pits. This has long been a barrier for many veterans seeking benefits and not just related to toxic exposures. Logistical barriers and documentary losses have prevented many service members from establishing a clean chain of evidence for the injuries or illnesses they sustained while in uniform.
The new process is a massive step forward by the US Department of Veterans Affairs (VA) to establish high standards of procedural justice for settling beneficiary claims. The PACT Act removes the burden from the shoulders of the veteran and places it squarely on the VA to demonstrate that > 20 different medical conditions--primarily cancers and respiratory illnesses--are linked to toxic exposure. The VA must establish that exposure occurred to cohorts of service members in specific theaters and time frames. A veteran who served in that area and period and has one of the indexed illnesses is presumed to have been exposed in the line of duty.3,4
As a result, the VA instituted a new screening process to determine that toxic military exposures (a) led to illness; and (b) both exposure and illness are connected to service. According to the VA, the new process is evidence based, transparent, and allows the VA to fast-track policy decisions related to exposures. The PACT Act includes a provision intended to promote sustained implementation and prevent the program from succumbing as so many new initiatives have to inadequate adoption. VA is required to deploy its considerable internal research capacity to collaborate with external partners in and outside government to study military members with toxic exposures.4
Congress had initially proposed that the provisions of the PACT ACT would take effect in 2026, providing time to ramp up the process. The White House and VA telescoped that time line so veterans can begin now to apply for benefits that they could foreseeably receive in 2023. However, a long-standing problem for the VA has been unfunded agency or congressional mandates. These have often end in undermining the legislative intention or policy purpose of the program undermining their legislative intention or policy purpose through staffing shortages, leading to lack of or delayed access. The PACT Act promises to eschew the infamous Phoenix problem by providing increased personnel, training infrastructure, and technology resources for both the Veterans Benefit Administration and the Veterans Health Administration. Ironically, many seasoned VA observers expect the PACT expansion will lead to even larger backlogs of claims as hundreds of newly eligible veterans are added to the extant rolls of those seeking benefits.5
An estimated 1 in 5 veterans may be entitled to PACT benefits. The PACT Act is the latest of a long uneven movement toward distributive justice for veteran benefits and services. It is fitting in the month of Veterans Day 2022 to trace that trajectory. Congress first passed veteran benefits legislation in 1917, focused on soldiers with disabilities. This resulted in a massive investment in building hospitals. Ironically, part of the impetus for VA health care was an earlier toxic military exposure. World War I service members suffered from the detrimental effects of mustard gas among other chemical byproducts. In 1924, VA benefits and services underwent a momentous opening to include individuals with non-service-connected disabilities. Four years later, the VA tent became even bigger, welcoming women, National Guard, and militia members to receive care under its auspices.6
The PACT Act is a fitting memorial for Veterans Day as an increasingly divided country presents a unified response to veterans and their survivors exposed to a variety of toxins across multiple wars. The PACT Act was hard won with veterans and their advocates having to fight years of political bickering, government abdication of accountability, and scientific sparring before this bipartisan legislation passed.7 It covers Vietnam War veterans with several conditions due to Agent Orange exposure; Gulf War and post-9/11 veterans with cancer and respiratory conditions; and the service members deployed to Afghanistan and Iraq afflicted with illnesses due to the smoke of burn pits and other toxins.
As many areas of the country roll back LGBTQ+ rights to health care and social services, the VA has emerged as a leader in the movement for diversity and inclusion. VA Secretary McDonough provided a pathway to VA eligibility for other than honorably discharged veterans, including those LGBTQ+ persons discharged under Don't Ask, Don't Tell.8 Lest we take this new inclusivity for granted, we should never forget that this journey toward equity for the military and VA has been long, slow, and uneven. There are many difficult miles yet to travel if we are to achieve liberty and justice for veteran members of racial minorities, women, and other marginalized populations. Even the PACT Act does not cover all putative exposures to toxins.9 Yet it is a significant step closer to fulfilling the motto of the VA LGBTQ+ program: to serve all who served.10
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
- Parker T. Of justice and the conscience. In: Ten Sermons of Religion. Crosby, Nichols and Company; 1853:66-85.
- The White House. Fact sheet: President Biden signs the PACT Act and delivers on his promise to America's veterans. August 9, 2022. Accessed October 24, 2022. https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/10/fact-sheet-president-biden-signs-the-pact-act-and-delivers-on-his-promise-to-americas-veterans
- Shane L. Vets can apply for all PACT benefits now after VA speeds up law. Military Times. September 1, 2022. Accessed October 24, 2022. https://www.militarytimes.com/news/burn-pits/2022/09/01/vets-can-apply-for-all-pact-act-benefits-now-after-va-speeds-up-law
- US Department of Veterans Affairs. The PACT Act and your VA benefits. Updated September 28, 2022. Accessed October 24, 2022. https://www.va.gov/resources/the-pact-act-and-your-va-benefits
- Wentling N. Discharged LGBTQ+ veterans now eligible for benefits under new guidance issued by VA. Stars & Stripes. September 20, 2021. Accessed October 24, 2022. https://www.stripes.com/veterans/2021-09-20/veterans-affairs-dont-ask-dont-tell-benefits-lgbt-discharges-2956761.html
- US Department of Veterans Affairs, VA History Office. History--Department of Veterans Affairs (VA). Updated May 27, 2021. Accessed October 24, 2022. https://www.va.gov/HISTORY/VA_History/Overview.asp
- Atkins D, Kilbourne A, Lipson L. Health equity research in the Veterans Health Administration: we've come far but aren't there yet. Am J Public Health. 2014;104(suppl 4):S525-S526. doi:10.2105/AJPH.2014.302216
- Stack MK. The soldiers came home sick. The government denied it was responsible. New York Times. Updated January 16, 2022. Accessed October 24, 2022. https://www.nytimes.com/2022/01/11/magazine/military-burn-pits.html
- Namaz A, Sagalyn D. VA secretary discusses health care overhaul helping veterans exposed to toxic burn pits. PBS NewsHour. September 1, 2022. Accessed October 24, 2022. https://www.pbs.org/newshour/show/va-secretary-discusses-health-care-overhaul-helping-veterans-exposed-to-toxic-burn-pits
- US Department of Veterans Affairs, Patient Care Services. VHA LGBTQ+ health program. Updated September 13, 2022. Accessed October 31, 2022. https://www.patientcare.va.gov/lgbt
Leukocytoclastic Vasculitis Masquerading as Chronic ITP
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033