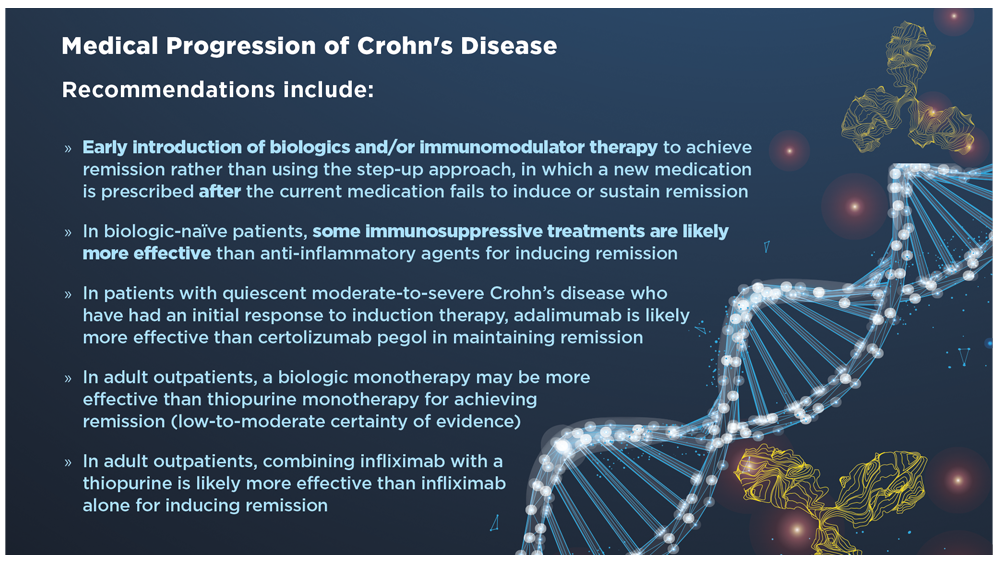
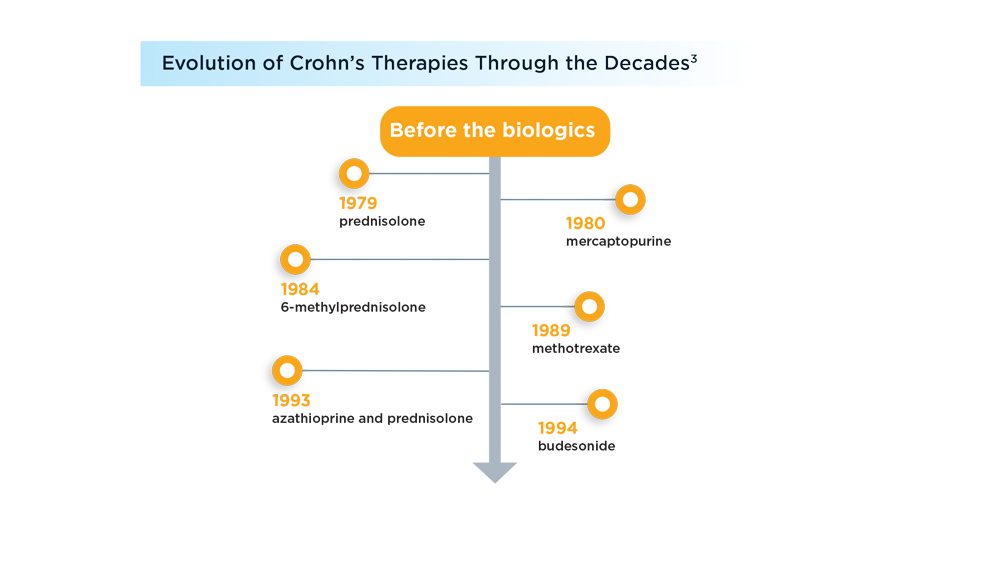
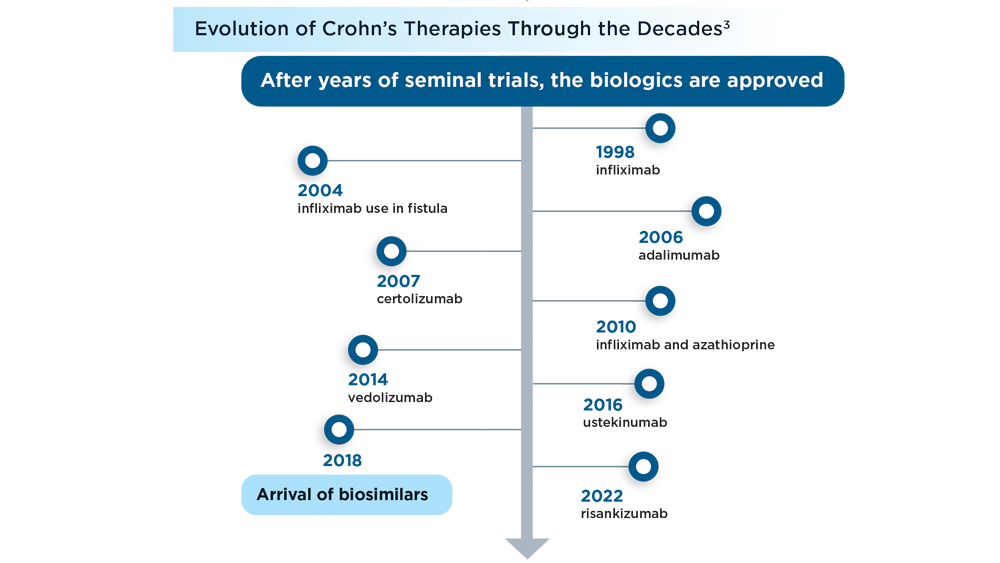
User login
Daily aspirin fails to reduce risk of fractures in older adults
Previous research suggests that aspirin may reduce the risk of fragility fractures by delaying bone loss, but the direct effects of aspirin on bone microarchitecture and the association between aspirin use and fracture risk in humans has not been explored, corresponding author Anna L. Barker, PhD, and colleagues wrote in their paper published in JAMA Internal Medicine.
Dr. Barker, who is executive director of research and innovation for Silverchain (a senior care program), said, in an interview, that she and her coauthors hypothesized “that aspirin could reduce both falls and fractures by reducing cardiovascular-associated physical and cognitive impairments and the anti-inflammatory properties mediating bone remodeling.”
Study methods and results
In the ASPREE-FRACTURE substudy, the authors examined the impact of daily low-dose aspirin (100 mg) on incidence of any fracture in more than 16,000 community-dwelling adults aged 70 years and older. A secondary endpoint was the incidence of serious falls, defined as falls requiring a hospital visit. Individuals with chronic illness and cardiovascular or cerebrovascular disease were excluded, as were those with dementia or other cognitive impairment, or a physical disability.
The study population included 16,703 participants enrolled in the larger Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial between 2010 and 2014. Of these, 8,322 were randomized to aspirin and 8,381 to a placebo. The median age was 74 years, and 55% of the participants were women.
Over a median follow-up of 4.6 years (76,219 total person-years), the risk of first fracture was similar between the aspirin and placebo groups (hazard ratio, 0.97), but the risk of serious falls was significantly higher in the aspirin group (884 falls vs. 804 falls, P = .01).
The incidence of first fracture was similar between the aspirin and placebo groups (813 vs. 718), as was the incidence of all fractures (1,394 and 1,471, respectively).
The results for both fractures and falls were essentially unchanged in a multivariate analysis controlling for variables known to affect fracture and fall risk and remained similar for different types of fractures (hip, trauma-related, nonpathological) as well, the researchers noted.
In their discussion, the researchers wrote that the clinical significance of the study is the inability of aspirin to reduce the risk of fractures in otherwise healthy older adults. They expressed surprise at the increase in serious falls, citing their hypothesis that the antiplatelet effects of aspirin may reduce cardiovascular and cerebrovascular events, thereby slowing physical decline and decreasing fall risk.
The increased risk of serious falls was not accompanied by an increase in fractures, and the increased fall risk was similar across subgroups of aspirin users, the researchers said.
Low-dose aspirin’s failure to reduce the risk of fractures but increasing the risk of serious falls adds to evidence that this agent provides little favorable benefit in a healthy, White older adult population.
The study findings were limited by several factors including the relatively homogeneous older and healthy population, and possible insufficient study duration to allow for changes in fracture and fall risk, the researchers noted. Other potential limitations include that the dose of aspirin used in the study was too low to affect bone remodeling and the lack of data on bone density, rheumatoid arthritis, and osteoporosis, they said.
However, the results were strengthened by the large sample size and high participant retention rate, and represent the first known examination of data from a randomized, controlled trial of the effect of aspirin on fractures, they added.
Setting the stage for more research
Overall, “This study adds to the growing body of evidence from other studies that the use of aspirin in people who do not have a risk of cardiovascular disease or stroke provides little benefit,” said Dr. Barker, who is also a professor at Monash University, Melbourne, Australia. However, “Older adults with a medical reason to take aspirin should continue to do so,” she emphasized.
“The most important thing the study showed is the primary endpoint, which was that aspirin use does not have an effect on fracture risk,” said Neil Skolnik, MD, of Sidney Kimmel Medical College, Philadelphia, in an interview.
“The increase in serious falls, as defined by a fall resulting in a visit to a hospital, is likely due to an increased risk of bleeding after a fall on aspirin,” said Dr. Skolnik, who was not involved in the study. Dr. Skolnik added that the current study findings support the current recommendations of the United States Preventive Services Task Force, which he quoted as follows, “The USPSTF recommends against initiating low-dose aspirin use for the primary prevention of CVD in adults 60 years or older.”
The study was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health; the National Health and Medical Research Council (Australia); Monash University; and the Victorian Cancer Agency. Lead author Dr. Barker was supported in part by the NHMRC and also disclosed grants from the NHMRC outside the current study. The ASPREE substudy also was supported by the University of Pittsburgh Claude D. Pepper Older American Independence Center and the Wake Forest University Claude D. Pepper Older Americans Independence Center. Bayer AG provided the aspirin used in the study but had no other role. Dr. Skolnik had no financial conflicts to disclose, but he serves on the editorial advisory board of Family Practice News.
Previous research suggests that aspirin may reduce the risk of fragility fractures by delaying bone loss, but the direct effects of aspirin on bone microarchitecture and the association between aspirin use and fracture risk in humans has not been explored, corresponding author Anna L. Barker, PhD, and colleagues wrote in their paper published in JAMA Internal Medicine.
Dr. Barker, who is executive director of research and innovation for Silverchain (a senior care program), said, in an interview, that she and her coauthors hypothesized “that aspirin could reduce both falls and fractures by reducing cardiovascular-associated physical and cognitive impairments and the anti-inflammatory properties mediating bone remodeling.”
Study methods and results
In the ASPREE-FRACTURE substudy, the authors examined the impact of daily low-dose aspirin (100 mg) on incidence of any fracture in more than 16,000 community-dwelling adults aged 70 years and older. A secondary endpoint was the incidence of serious falls, defined as falls requiring a hospital visit. Individuals with chronic illness and cardiovascular or cerebrovascular disease were excluded, as were those with dementia or other cognitive impairment, or a physical disability.
The study population included 16,703 participants enrolled in the larger Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial between 2010 and 2014. Of these, 8,322 were randomized to aspirin and 8,381 to a placebo. The median age was 74 years, and 55% of the participants were women.
Over a median follow-up of 4.6 years (76,219 total person-years), the risk of first fracture was similar between the aspirin and placebo groups (hazard ratio, 0.97), but the risk of serious falls was significantly higher in the aspirin group (884 falls vs. 804 falls, P = .01).
The incidence of first fracture was similar between the aspirin and placebo groups (813 vs. 718), as was the incidence of all fractures (1,394 and 1,471, respectively).
The results for both fractures and falls were essentially unchanged in a multivariate analysis controlling for variables known to affect fracture and fall risk and remained similar for different types of fractures (hip, trauma-related, nonpathological) as well, the researchers noted.
In their discussion, the researchers wrote that the clinical significance of the study is the inability of aspirin to reduce the risk of fractures in otherwise healthy older adults. They expressed surprise at the increase in serious falls, citing their hypothesis that the antiplatelet effects of aspirin may reduce cardiovascular and cerebrovascular events, thereby slowing physical decline and decreasing fall risk.
The increased risk of serious falls was not accompanied by an increase in fractures, and the increased fall risk was similar across subgroups of aspirin users, the researchers said.
Low-dose aspirin’s failure to reduce the risk of fractures but increasing the risk of serious falls adds to evidence that this agent provides little favorable benefit in a healthy, White older adult population.
The study findings were limited by several factors including the relatively homogeneous older and healthy population, and possible insufficient study duration to allow for changes in fracture and fall risk, the researchers noted. Other potential limitations include that the dose of aspirin used in the study was too low to affect bone remodeling and the lack of data on bone density, rheumatoid arthritis, and osteoporosis, they said.
However, the results were strengthened by the large sample size and high participant retention rate, and represent the first known examination of data from a randomized, controlled trial of the effect of aspirin on fractures, they added.
Setting the stage for more research
Overall, “This study adds to the growing body of evidence from other studies that the use of aspirin in people who do not have a risk of cardiovascular disease or stroke provides little benefit,” said Dr. Barker, who is also a professor at Monash University, Melbourne, Australia. However, “Older adults with a medical reason to take aspirin should continue to do so,” she emphasized.
“The most important thing the study showed is the primary endpoint, which was that aspirin use does not have an effect on fracture risk,” said Neil Skolnik, MD, of Sidney Kimmel Medical College, Philadelphia, in an interview.
“The increase in serious falls, as defined by a fall resulting in a visit to a hospital, is likely due to an increased risk of bleeding after a fall on aspirin,” said Dr. Skolnik, who was not involved in the study. Dr. Skolnik added that the current study findings support the current recommendations of the United States Preventive Services Task Force, which he quoted as follows, “The USPSTF recommends against initiating low-dose aspirin use for the primary prevention of CVD in adults 60 years or older.”
The study was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health; the National Health and Medical Research Council (Australia); Monash University; and the Victorian Cancer Agency. Lead author Dr. Barker was supported in part by the NHMRC and also disclosed grants from the NHMRC outside the current study. The ASPREE substudy also was supported by the University of Pittsburgh Claude D. Pepper Older American Independence Center and the Wake Forest University Claude D. Pepper Older Americans Independence Center. Bayer AG provided the aspirin used in the study but had no other role. Dr. Skolnik had no financial conflicts to disclose, but he serves on the editorial advisory board of Family Practice News.
Previous research suggests that aspirin may reduce the risk of fragility fractures by delaying bone loss, but the direct effects of aspirin on bone microarchitecture and the association between aspirin use and fracture risk in humans has not been explored, corresponding author Anna L. Barker, PhD, and colleagues wrote in their paper published in JAMA Internal Medicine.
Dr. Barker, who is executive director of research and innovation for Silverchain (a senior care program), said, in an interview, that she and her coauthors hypothesized “that aspirin could reduce both falls and fractures by reducing cardiovascular-associated physical and cognitive impairments and the anti-inflammatory properties mediating bone remodeling.”
Study methods and results
In the ASPREE-FRACTURE substudy, the authors examined the impact of daily low-dose aspirin (100 mg) on incidence of any fracture in more than 16,000 community-dwelling adults aged 70 years and older. A secondary endpoint was the incidence of serious falls, defined as falls requiring a hospital visit. Individuals with chronic illness and cardiovascular or cerebrovascular disease were excluded, as were those with dementia or other cognitive impairment, or a physical disability.
The study population included 16,703 participants enrolled in the larger Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial between 2010 and 2014. Of these, 8,322 were randomized to aspirin and 8,381 to a placebo. The median age was 74 years, and 55% of the participants were women.
Over a median follow-up of 4.6 years (76,219 total person-years), the risk of first fracture was similar between the aspirin and placebo groups (hazard ratio, 0.97), but the risk of serious falls was significantly higher in the aspirin group (884 falls vs. 804 falls, P = .01).
The incidence of first fracture was similar between the aspirin and placebo groups (813 vs. 718), as was the incidence of all fractures (1,394 and 1,471, respectively).
The results for both fractures and falls were essentially unchanged in a multivariate analysis controlling for variables known to affect fracture and fall risk and remained similar for different types of fractures (hip, trauma-related, nonpathological) as well, the researchers noted.
In their discussion, the researchers wrote that the clinical significance of the study is the inability of aspirin to reduce the risk of fractures in otherwise healthy older adults. They expressed surprise at the increase in serious falls, citing their hypothesis that the antiplatelet effects of aspirin may reduce cardiovascular and cerebrovascular events, thereby slowing physical decline and decreasing fall risk.
The increased risk of serious falls was not accompanied by an increase in fractures, and the increased fall risk was similar across subgroups of aspirin users, the researchers said.
Low-dose aspirin’s failure to reduce the risk of fractures but increasing the risk of serious falls adds to evidence that this agent provides little favorable benefit in a healthy, White older adult population.
The study findings were limited by several factors including the relatively homogeneous older and healthy population, and possible insufficient study duration to allow for changes in fracture and fall risk, the researchers noted. Other potential limitations include that the dose of aspirin used in the study was too low to affect bone remodeling and the lack of data on bone density, rheumatoid arthritis, and osteoporosis, they said.
However, the results were strengthened by the large sample size and high participant retention rate, and represent the first known examination of data from a randomized, controlled trial of the effect of aspirin on fractures, they added.
Setting the stage for more research
Overall, “This study adds to the growing body of evidence from other studies that the use of aspirin in people who do not have a risk of cardiovascular disease or stroke provides little benefit,” said Dr. Barker, who is also a professor at Monash University, Melbourne, Australia. However, “Older adults with a medical reason to take aspirin should continue to do so,” she emphasized.
“The most important thing the study showed is the primary endpoint, which was that aspirin use does not have an effect on fracture risk,” said Neil Skolnik, MD, of Sidney Kimmel Medical College, Philadelphia, in an interview.
“The increase in serious falls, as defined by a fall resulting in a visit to a hospital, is likely due to an increased risk of bleeding after a fall on aspirin,” said Dr. Skolnik, who was not involved in the study. Dr. Skolnik added that the current study findings support the current recommendations of the United States Preventive Services Task Force, which he quoted as follows, “The USPSTF recommends against initiating low-dose aspirin use for the primary prevention of CVD in adults 60 years or older.”
The study was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health; the National Health and Medical Research Council (Australia); Monash University; and the Victorian Cancer Agency. Lead author Dr. Barker was supported in part by the NHMRC and also disclosed grants from the NHMRC outside the current study. The ASPREE substudy also was supported by the University of Pittsburgh Claude D. Pepper Older American Independence Center and the Wake Forest University Claude D. Pepper Older Americans Independence Center. Bayer AG provided the aspirin used in the study but had no other role. Dr. Skolnik had no financial conflicts to disclose, but he serves on the editorial advisory board of Family Practice News.
FROM JAMA INTERNAL MEDICINE
Botanical Briefs: Toxicodendron Dermatitis
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
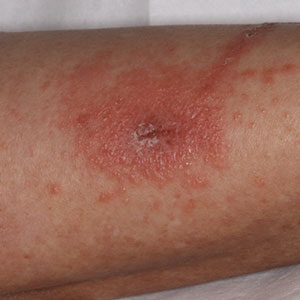
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7
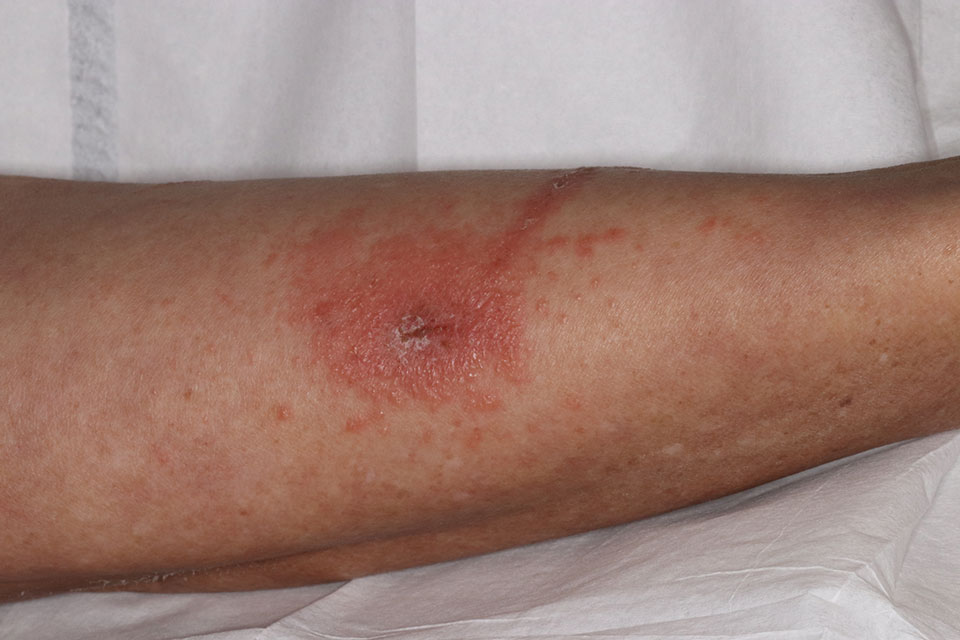
When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Practice Points
- Toxicodendron dermatitis is a pruritic vesicular eruption in areas of contact with the plant.
- Identification and avoidance are primary methods of preventing Toxicodendron dermatitis.
Dietary Triggers for Atopic Dermatitis in Children
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
Practice Points
- The perception of dietary triggers is so entrenched and widespread that it should be addressed even when thought to be irrelevant.
- It is important not to dismiss food as a factor in atopic dermatitis (AD), as it can play a number of roles in the condition.
- On the other hand, education about the wide range of food reactions and the relative rarity of true food-driven AD along with the potential risks of dietary modification may enhance both rapport and understanding between the clinician and patient.
Disaster Preparedness in Dermatology Residency Programs
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).
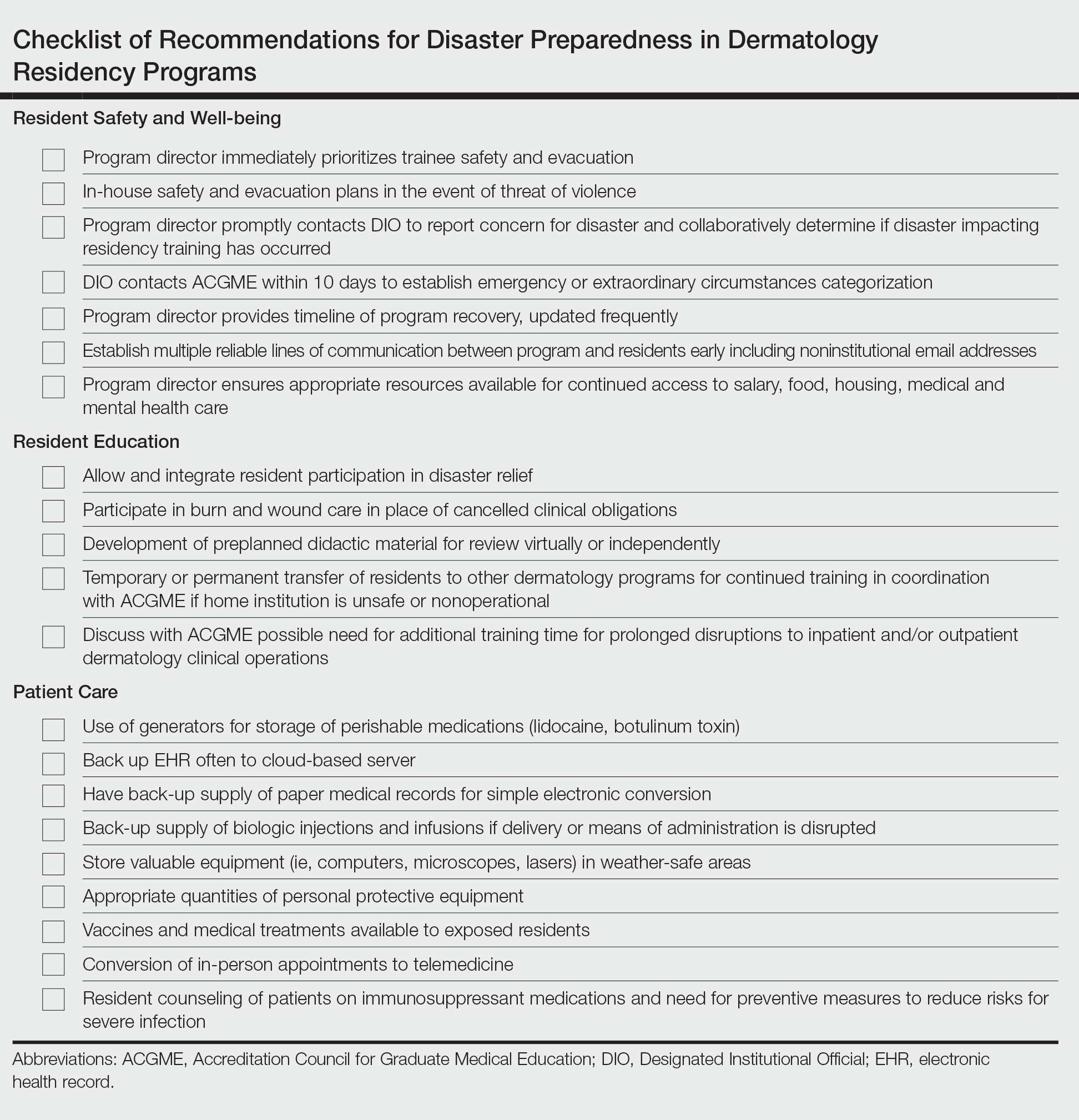
Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).

Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
In an age of changing climate and emerging global pandemics, the ability of residency programs to prepare for and adapt to potential disasters may be paramount in preserving the training of physicians. The current literature regarding residency program disaster preparedness, which focuses predominantly on hurricanes and COVID-19,1-8 is lacking in recommendations specific to dermatology residency programs. Likewise, the Accreditation Council for Graduate Medical Education (ACGME) guidelines9 do not address dermatology-specific concerns in disaster preparedness or response. Herein, we propose recommendations to mitigate the impact of various types of disasters on dermatology residency programs and their trainees with regard to resident safety and wellness, resident education, and patient care (Table).

Resident Safety and Wellness
Role of the Program Director—The role of the program director is critical, serving as a figure of structure and reassurance.4,7,10 Once concern of disaster arises, the program director should contact the Designated Institutional Official (DIO) to express concerns about possible disruptions to resident training. The DIO should then contact the ACGME within 10 days to report the disaster and submit a request for emergency (eg, pandemic) or extraordinary circumstances (eg, natural disaster) categorization.4,9 Program directors should promptly prepare plans for program reconfiguration and resident transfers in alignment with ACGME requirements to maintain evaluation and completion of core competencies of training during disasters.9 Program directors should prioritize the safety of trainees during the immediate threat with clear guidelines on sheltering, evacuations, or quarantines; a timeline of program recovery based on communication with residents, faculty, and administration should then be established.10,11
Communication—Establishing a strong line of communication between program directors and residents is paramount. Collection of emergency noninstitutional contact information, establishment of a centralized website for information dissemination, use of noninstitutional email and proxy servers outside of the location of impact, social media updates, on-site use of 2-way radios, and program-wide conference calls when possible should be strongly considered as part of the disaster response.2-4,12,13
Resident Accommodations and Mental Health—If training is disrupted, residents should be reassured of continued access to salary, housing, food, or other resources as necessary.3,4,11 There should be clear contingency plans if residents need to leave the program for extended periods of time due to injury, illness, or personal circumstances. Although relevant in all types of disasters, resident mental health and response to trauma also must be addressed. Access to counseling, morale-building opportunities (eg, resident social events), and screening for depression or posttraumatic stress disorder may help promote well-being among residents following traumatic events.14
Resident Education
Participation in Disaster Relief—Residents may seek to aid in the disaster response, which may prove challenging in the setting of programs with high patient volume.4 In coordination with the ACGME and graduate medical education governing bodies, program directors should consider how residents may fulfill dermatology training requirements in conjunction with disaster relief efforts, such as working in an inpatient setting or providing wound care.10
Continued Didactic Education—The use of online learning and conference calls for continuing the dermatology curriculum is an efficient means to maintaining resident education when meeting in person poses risks to residents.15 Projections of microscopy images, clinical photographs, or other instructional materials allow for continued instruction on resident examination, histopathology, and diagnostic skills.
Continued Clinical Training—If the home institution cannot support the operation of dermatology clinics, residents should be guaranteed continued training at other institutions. Agreements with other dermatology programs, community hospitals, or private dermatology practices should be established in advance, with consideration given to the number of residents a program can support, funding transfers, and credentialing requirements.2,4,5
Prolonged Disruptions—Nonessential departments of medical institutions may cease to function during war or mass casualty disasters, and it may be unsafe to send dermatology residents to other institutions or clinical areas. If the threat is prolonged, programs may need to consider allowing current residents a longer duration of training despite potential overlap with incoming dermatology residents.7
Patient Care
Disruptions to Clinic Operations—Regarding threats of violence, dangerous exposures, or natural disasters, there should be clear guidelines on sheltering in the clinical setting or stabilizing patients during a procedure.11 Equipment used by residents such as laptops, microscopes, and treatment devices (eg, lasers) should be stored in weather-safe locations that would not be notably impacted by moisture or structural damage to the clinic building. If electricity or internet access are compromised, paper medical records should be available to residents to continue clinical operations. Electronic health records used by residents should regularly be backed up on remote servers or cloud storage to allow continued access to patient health information if on-site servers are not functional.12 If disruptions are prolonged, residency program administration should coordinate with the institution to ensure there is adequate supply and storage of medications (eg, lidocaine, botulinum toxin) as well as a continued means of delivering biologic medications to patients and an ability to obtain laboratory or dermatopathology services.
In-Person Appointments vs Telemedicine—There are benefits to both residency training and patient care when physicians are able to perform in-person examinations, biopsies, and in-office treatments.16 Programs should ensure an adequate supply of personal protective equipment to continue in-office appointments, vaccinations, and medical care if a resident or other members of the team are exposed to an infectious disease.7 If in-person appointments are limited or impossible, telemedicine capabilities may still allow residents to meet program requirements.7,10,15 However, reduced patient volume due to decreased elective visits or procedures may complicate the fulfillment of clinical requirements, which may need to be adjusted in the wake of a disaster.7
Use of Immunosuppressive Therapies—Residency programs should address the risks of prescribing immunosuppressive therapies (eg, biologics) during an infectious threat with their residents and encourage trainees to counsel patients on the importance of preventative measures to reduce risks for severe infection.17
Final Thoughts
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
- Davis W. Hurricane Katrina: the challenge to graduate medical education. Ochsner J. 2006;6:39.
- Cefalu CA, Schwartz RS. Salvaging a geriatric medicine academic program in disaster mode—the LSU training program post-Katrina.J Natl Med Assoc. 2007;99:590-596.
- Ayyala R. Lessons from Katrina: a program director’s perspective. Ophthalmology. 2007;114:1425-1426.
- Wiese JG. Leadership in graduate medical education: eleven steps instrumental in recovering residency programs after a disaster. Am J Med Sci. 2008;336:168-173.
- Griffies WS. Post-Katrina stabilization of the LSU/Ochsner Psychiatry Residency Program: caveats for disaster preparedness. Acad Psychiatry. 2009;33:418-422.
- Kearns DG, Chat VS, Uppal S, et al. Applying to dermatology residency during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:1214-1215.
- Matthews JB, Blair PG, Ellison EC, et al. Checklist framework for surgical education disaster plans. J Am Coll Surg. 2021;233:557-563.
- Litchman GH, Marson JW, Rigel DS. The continuing impact of COVID-19 on dermatology practice: office workflow, economics, and future implications. J Am Acad Dermatol. 2021;84:576-579.
- Accreditation Council for Graduate Medical Education. Sponsoring institution emergency categorization. Accessed October 20, 2022. https://www.acgme.org/covid-19/sponsoring-institution-emergency-categorization/
- Li YM, Galimberti F, Abrouk M, et al. US dermatology resident responses about the COVID-19 pandemic: results from a nationwide survey. South Med J. 2020;113:462-465.
- Newman B, Gallion C. Hurricane Harvey: firsthand perspectives for disaster preparedness in graduate medical education. Acad Med. 2019;94:1267-1269.
- Pero CD, Pou AM, Arriaga MA, et al. Post-Katrina: study in crisis-related program adaptability. Otolaryngol Head Neck Surg. 2008;138:394-397.
- Hattaway R, Singh N, Rais-Bahrami S, et al. Adaptations of dermatology residency programs to changes in medical education amid the COVID-19 pandemic: virtual opportunities and social media. SKIN. 2021;5:94-100.
- Hillier K, Paskaradevan J, Wilkes JK, et al. Disaster plans: resident involvement and well-being during Hurricane Harvey. J Grad Med Educ. 2019;11:129-131.
- Samimi S, Choi J, Rosman IS, et al. Impact of COVID-19 on dermatology residency. Dermatol Clin. 2021;39:609-618.
- Bastola M, Locatis C, Fontelo P. Diagnostic reliability of in-person versus remote dermatology: a meta-analysis. Telemed J E Health. 2021;27:247-250.
- Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic? J Dermatolog Treat. 2020;31:317-318.
Practice Points
- Dermatology residency programs should prioritize the development of disaster preparedness plans prior to the onset of disasters.
- Comprehensive disaster preparedness addresses many possible disruptions to dermatology resident training and clinic operations, including natural and manmade disasters and threats of widespread infectious disease.
- Safety being paramount, dermatology residency programs may be tasked with maintaining resident wellness, continuing resident education—potentially in unconventional ways—and adapting clinical operations to continue patient care.
Simplify Postoperative Self-removal of Bandages for Isolated Patients With Limited Range of Motion Using Pull Tabs
Practice Gap
A male patient presented with 2 concerning lesions, which histopathology revealed were invasive squamous cell carcinoma (SCC) on the right medial chest and SCC in situ on the right upper scapular region. Both were treated with wide local excision; margins were clear in our office the same day.
This case highlighted a practice gap in postoperative care. Two factors posed a challenge to proper postoperative wound care for our patient:
• Because of the high risk of transmission of SARS-CoV-2, the patient hoped to limit exposure by avoiding an office visit to remove the bandage.
• The patient did not have someone at home to serve as an immediate support system, which made it impossible for him to rely on others for postoperative wound care.
Previously, the patient had to ask a friend to remove a bandage for melanoma in situ on the inner aspect of the left upper arm. Therefore, after this procedure, the patient asked if the bandage could be fashioned in a manner that would allow him to remove it without assistance (Figure 1).
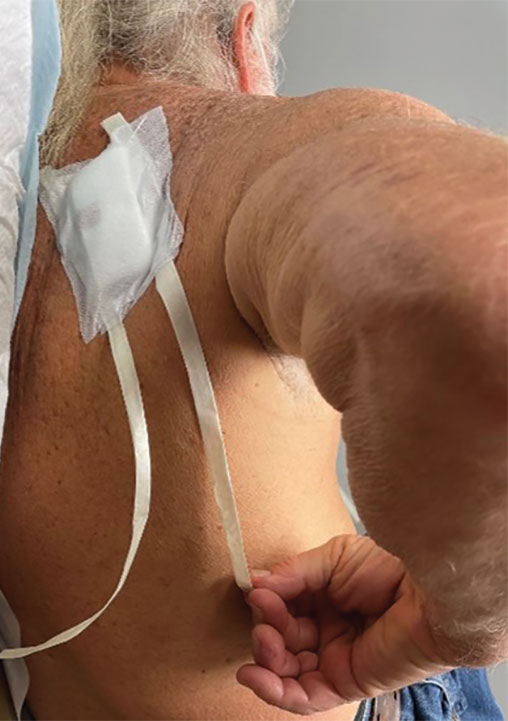
Technique
In constructing a bandage that is easier to remove, some necessary pressure that is provided by the bandage often is sacrificed by making it looser. Considering that our patient had moderate bleeding during the procedure—in part because he took low-dose aspirin (81 mg/d)—it was important to maintain firm pressure under the bandage postoperatively to help prevent untoward bleeding. Furthermore, because of the location of the treated site and the patient’s limited range of motion, it was not feasible for him to reach the area on the scapula and remove the bandage.1
For easy self-removal, we designed a bandage with a pull tab that was within the patient’s reach. Suitable materials for the pull tab bandage included surgical tape, bandaging tape with adequate stretch, sterile nonadhesive gauze, fenestrated surgical gauze, and a topical emollient such as petroleum jelly or antibacterial ointment.
To clean the site and decrease the amount of oil that would reduce the effectiveness of the adhesive, the wound was prepared with 70% alcohol. The site was then treated with petroleum jelly.
Next, we designed 2 pull tab bandage prototypes that allowed easy self-removal. For both prototypes, sterile nonadhesive gauze was applied to the wound along with folded and fenestrated gauze, which provided pressure. We used prototype #1 in our patient, and prototype #2 was demonstrated as an option.
Prototype #1—We created 2 tabs—each 2-feet long—using bandaging tape that was folded on itself once horizontally (Figure 2). The tabs were aligned on either side of the wound, the tops of which sat approximately 2 inches above the top of the first layer of adhesive bandage. An initial layer of adhesive surgical dressing was applied to cover the wound; 1 inch of the dressing was left exposed on the top of each tab. In addition, there were 2 “feet” running on the bottom.
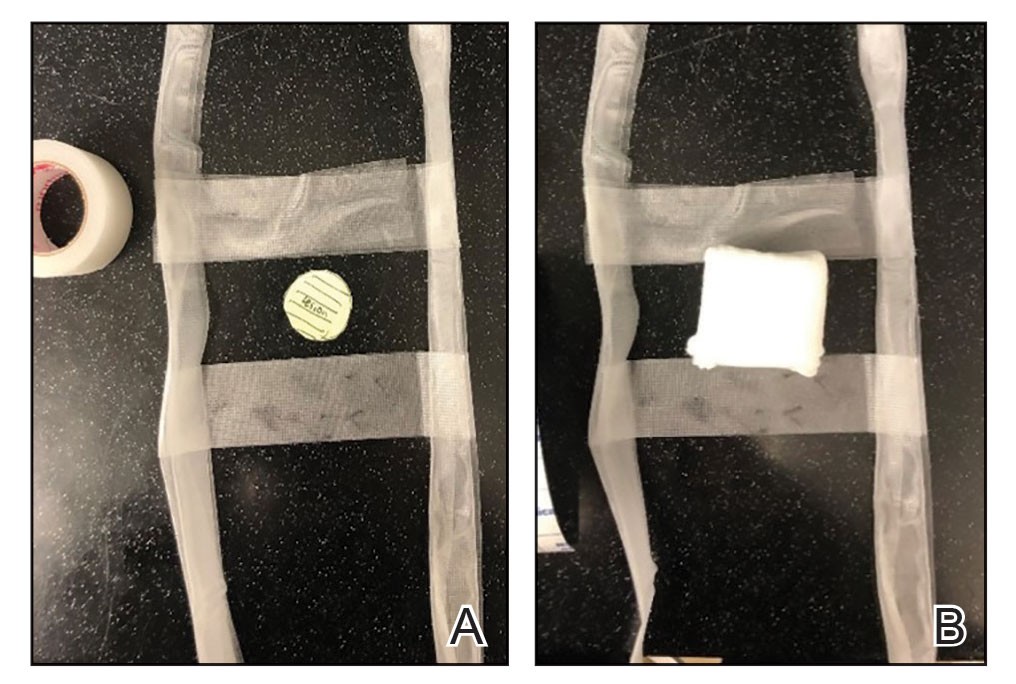
The tops of the tabs were folded back over the adhesive tape, creating a type of “hook.” An additional final layer of adhesive tape was applied to ensure adequate pressure on the surgical site.
The patient was instructed to remove the bandage 2 days after the procedure. The outcome was qualified through a 3-day postoperative telephone call. The patient was asked about postoperative pain and his level of satisfaction with treatment. He was asked if he had any changes such as bleeding, swelling, signs of infection, or increased pain in the days after surgery or perceived postoperative complications, such as irritation. We asked the patient about the relative ease of removing the bandage and if removal was painful. He reported that the bandage was easy to remove, and that doing so was not painful; furthermore, he did not have problems with the bandage or healing and did not experience any medical changes. He found the bandage to be comfortable. The patient stated that the hanging feet of the prototype #1 bandage were not bothersome and were sturdy for the time that the bandage was on.
Prototype #2—We prepared a bandage using surgical packing as the tab (Figure 3). The packing was slowly placed around the site, which was already covered with nonadhesive gauze and fenestrated surgical gauze, with adequate spacing between each loop (for a total of 3 loops), 1 of which crossed over the third loop so that the adhesive bandaging tape could be removed easily. This allowed for a single tab that could be removed by a single pull. A final layer of adhesive tape was applied to ensure adequate pressure, similar to prototype #1. The same postoperative protocol was employed to provide a consistent standard of care. We recommend use of this prototype when surgical tape is not available, and surgical packing can be used as a substitute.

Practice Implications
Patients have a better appreciation for avoiding excess visits to medical offices due to the COVID-19 pandemic. The risk for exposure to SARS-CoV-2 infection is greater when patients who lack a support system must return to the office for aftercare or to have a bandage removed. Although protection offered by the COVID-19 vaccine alleviates concern, many patients have realized the benefits of only visiting medical offices in person when necessary.
The concept of pull tab bandages that can be removed by the patient at home has other applications. For example, patients who travel a long distance to see their physician will benefit from easier aftercare and avoid additional follow-up visits when provided with a self-removable bandage.
- Stathokostas, L, McDonald MW, Little RMD, et al. Flexibility of older adults aged 55-86 years and the influence of physical activity. J Aging Res. 2013;2013:1-8. doi:10.1155/2013/743843
Practice Gap
A male patient presented with 2 concerning lesions, which histopathology revealed were invasive squamous cell carcinoma (SCC) on the right medial chest and SCC in situ on the right upper scapular region. Both were treated with wide local excision; margins were clear in our office the same day.
This case highlighted a practice gap in postoperative care. Two factors posed a challenge to proper postoperative wound care for our patient:
• Because of the high risk of transmission of SARS-CoV-2, the patient hoped to limit exposure by avoiding an office visit to remove the bandage.
• The patient did not have someone at home to serve as an immediate support system, which made it impossible for him to rely on others for postoperative wound care.
Previously, the patient had to ask a friend to remove a bandage for melanoma in situ on the inner aspect of the left upper arm. Therefore, after this procedure, the patient asked if the bandage could be fashioned in a manner that would allow him to remove it without assistance (Figure 1).

Technique
In constructing a bandage that is easier to remove, some necessary pressure that is provided by the bandage often is sacrificed by making it looser. Considering that our patient had moderate bleeding during the procedure—in part because he took low-dose aspirin (81 mg/d)—it was important to maintain firm pressure under the bandage postoperatively to help prevent untoward bleeding. Furthermore, because of the location of the treated site and the patient’s limited range of motion, it was not feasible for him to reach the area on the scapula and remove the bandage.1
For easy self-removal, we designed a bandage with a pull tab that was within the patient’s reach. Suitable materials for the pull tab bandage included surgical tape, bandaging tape with adequate stretch, sterile nonadhesive gauze, fenestrated surgical gauze, and a topical emollient such as petroleum jelly or antibacterial ointment.
To clean the site and decrease the amount of oil that would reduce the effectiveness of the adhesive, the wound was prepared with 70% alcohol. The site was then treated with petroleum jelly.
Next, we designed 2 pull tab bandage prototypes that allowed easy self-removal. For both prototypes, sterile nonadhesive gauze was applied to the wound along with folded and fenestrated gauze, which provided pressure. We used prototype #1 in our patient, and prototype #2 was demonstrated as an option.
Prototype #1—We created 2 tabs—each 2-feet long—using bandaging tape that was folded on itself once horizontally (Figure 2). The tabs were aligned on either side of the wound, the tops of which sat approximately 2 inches above the top of the first layer of adhesive bandage. An initial layer of adhesive surgical dressing was applied to cover the wound; 1 inch of the dressing was left exposed on the top of each tab. In addition, there were 2 “feet” running on the bottom.

The tops of the tabs were folded back over the adhesive tape, creating a type of “hook.” An additional final layer of adhesive tape was applied to ensure adequate pressure on the surgical site.
The patient was instructed to remove the bandage 2 days after the procedure. The outcome was qualified through a 3-day postoperative telephone call. The patient was asked about postoperative pain and his level of satisfaction with treatment. He was asked if he had any changes such as bleeding, swelling, signs of infection, or increased pain in the days after surgery or perceived postoperative complications, such as irritation. We asked the patient about the relative ease of removing the bandage and if removal was painful. He reported that the bandage was easy to remove, and that doing so was not painful; furthermore, he did not have problems with the bandage or healing and did not experience any medical changes. He found the bandage to be comfortable. The patient stated that the hanging feet of the prototype #1 bandage were not bothersome and were sturdy for the time that the bandage was on.
Prototype #2—We prepared a bandage using surgical packing as the tab (Figure 3). The packing was slowly placed around the site, which was already covered with nonadhesive gauze and fenestrated surgical gauze, with adequate spacing between each loop (for a total of 3 loops), 1 of which crossed over the third loop so that the adhesive bandaging tape could be removed easily. This allowed for a single tab that could be removed by a single pull. A final layer of adhesive tape was applied to ensure adequate pressure, similar to prototype #1. The same postoperative protocol was employed to provide a consistent standard of care. We recommend use of this prototype when surgical tape is not available, and surgical packing can be used as a substitute.

Practice Implications
Patients have a better appreciation for avoiding excess visits to medical offices due to the COVID-19 pandemic. The risk for exposure to SARS-CoV-2 infection is greater when patients who lack a support system must return to the office for aftercare or to have a bandage removed. Although protection offered by the COVID-19 vaccine alleviates concern, many patients have realized the benefits of only visiting medical offices in person when necessary.
The concept of pull tab bandages that can be removed by the patient at home has other applications. For example, patients who travel a long distance to see their physician will benefit from easier aftercare and avoid additional follow-up visits when provided with a self-removable bandage.
Practice Gap
A male patient presented with 2 concerning lesions, which histopathology revealed were invasive squamous cell carcinoma (SCC) on the right medial chest and SCC in situ on the right upper scapular region. Both were treated with wide local excision; margins were clear in our office the same day.
This case highlighted a practice gap in postoperative care. Two factors posed a challenge to proper postoperative wound care for our patient:
• Because of the high risk of transmission of SARS-CoV-2, the patient hoped to limit exposure by avoiding an office visit to remove the bandage.
• The patient did not have someone at home to serve as an immediate support system, which made it impossible for him to rely on others for postoperative wound care.
Previously, the patient had to ask a friend to remove a bandage for melanoma in situ on the inner aspect of the left upper arm. Therefore, after this procedure, the patient asked if the bandage could be fashioned in a manner that would allow him to remove it without assistance (Figure 1).

Technique
In constructing a bandage that is easier to remove, some necessary pressure that is provided by the bandage often is sacrificed by making it looser. Considering that our patient had moderate bleeding during the procedure—in part because he took low-dose aspirin (81 mg/d)—it was important to maintain firm pressure under the bandage postoperatively to help prevent untoward bleeding. Furthermore, because of the location of the treated site and the patient’s limited range of motion, it was not feasible for him to reach the area on the scapula and remove the bandage.1
For easy self-removal, we designed a bandage with a pull tab that was within the patient’s reach. Suitable materials for the pull tab bandage included surgical tape, bandaging tape with adequate stretch, sterile nonadhesive gauze, fenestrated surgical gauze, and a topical emollient such as petroleum jelly or antibacterial ointment.
To clean the site and decrease the amount of oil that would reduce the effectiveness of the adhesive, the wound was prepared with 70% alcohol. The site was then treated with petroleum jelly.
Next, we designed 2 pull tab bandage prototypes that allowed easy self-removal. For both prototypes, sterile nonadhesive gauze was applied to the wound along with folded and fenestrated gauze, which provided pressure. We used prototype #1 in our patient, and prototype #2 was demonstrated as an option.
Prototype #1—We created 2 tabs—each 2-feet long—using bandaging tape that was folded on itself once horizontally (Figure 2). The tabs were aligned on either side of the wound, the tops of which sat approximately 2 inches above the top of the first layer of adhesive bandage. An initial layer of adhesive surgical dressing was applied to cover the wound; 1 inch of the dressing was left exposed on the top of each tab. In addition, there were 2 “feet” running on the bottom.

The tops of the tabs were folded back over the adhesive tape, creating a type of “hook.” An additional final layer of adhesive tape was applied to ensure adequate pressure on the surgical site.
The patient was instructed to remove the bandage 2 days after the procedure. The outcome was qualified through a 3-day postoperative telephone call. The patient was asked about postoperative pain and his level of satisfaction with treatment. He was asked if he had any changes such as bleeding, swelling, signs of infection, or increased pain in the days after surgery or perceived postoperative complications, such as irritation. We asked the patient about the relative ease of removing the bandage and if removal was painful. He reported that the bandage was easy to remove, and that doing so was not painful; furthermore, he did not have problems with the bandage or healing and did not experience any medical changes. He found the bandage to be comfortable. The patient stated that the hanging feet of the prototype #1 bandage were not bothersome and were sturdy for the time that the bandage was on.
Prototype #2—We prepared a bandage using surgical packing as the tab (Figure 3). The packing was slowly placed around the site, which was already covered with nonadhesive gauze and fenestrated surgical gauze, with adequate spacing between each loop (for a total of 3 loops), 1 of which crossed over the third loop so that the adhesive bandaging tape could be removed easily. This allowed for a single tab that could be removed by a single pull. A final layer of adhesive tape was applied to ensure adequate pressure, similar to prototype #1. The same postoperative protocol was employed to provide a consistent standard of care. We recommend use of this prototype when surgical tape is not available, and surgical packing can be used as a substitute.

Practice Implications
Patients have a better appreciation for avoiding excess visits to medical offices due to the COVID-19 pandemic. The risk for exposure to SARS-CoV-2 infection is greater when patients who lack a support system must return to the office for aftercare or to have a bandage removed. Although protection offered by the COVID-19 vaccine alleviates concern, many patients have realized the benefits of only visiting medical offices in person when necessary.
The concept of pull tab bandages that can be removed by the patient at home has other applications. For example, patients who travel a long distance to see their physician will benefit from easier aftercare and avoid additional follow-up visits when provided with a self-removable bandage.
- Stathokostas, L, McDonald MW, Little RMD, et al. Flexibility of older adults aged 55-86 years and the influence of physical activity. J Aging Res. 2013;2013:1-8. doi:10.1155/2013/743843
- Stathokostas, L, McDonald MW, Little RMD, et al. Flexibility of older adults aged 55-86 years and the influence of physical activity. J Aging Res. 2013;2013:1-8. doi:10.1155/2013/743843
Evolving Therapeutic Goals in Crohn’s Disease Management
- Dorrington AM, Selinger CP, Parkes GC, Smith M, Pollok RC, Raine T. The historical role and contemporary use of corticosteroids in inflammatory bowel disease. J Crohns Colitis. 2020;14(9):1316-1329. doi:10.1093/ecco-jcc/jjaa053
- Melsheimer R, Geldhof A, Apaolaza I, Schaible T. Remicade® (infliximab): 20 years of contributions to science and medicine. Biologics. 2019;13:139-178. doi:10.2147/BTT.S207246
- Kumar A, Cole A, Segal J, Smith P, Limdi JK. A review of the therapeutic management of Crohn’s disease. Therap Adv Gastroenterol. 2022;15:17562848221078456. doi:10.1177/17562848221078456
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779-2789. doi:10.1016/S0140-6736(17)32641-7
- Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology. 2020;159(1):139-147. doi:10.1053/j.gastro.2020.03.039
- Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021;19(10):2031-2045.e11. doi:10.1016/j.cgh.2020.10.039
- Chapman S, Sibelli A, St-Clair Jones A, Forbes A, Chater A, Horne R. Personalised adherence support for maintenance treatment of inflammatory bowel disease: a tailored digital intervention to change adherence-related beliefs and barriers. J Crohns Colitis. 2020;14(10):1394-1404. doi:10.1093/ecco-jcc/jjz034
- Turner D, Ricciuto A, Lewis A, et al; for the International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi:10.1053/j.gastro.2020.12.031
- Rozich JJ, Dulai PS, Fumery M, Sandborn WJ, Singh S. Progression of elderly onset inflammatory bowel diseases: a systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2020;18(11):2437-2447.e6. doi:10.1016/j.cgh.2020.02.048
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166-1169.doi:10.15585/mmwr.mm6542a3
- M’Koma AE. Inflammatory bowel disease: clinical diagnosis and surgical treatment-overview. Medicina (Kaunas). 2022;58(5):567. doi:10.3390/medicina58050567
- Weissman S, Patel K, Kolli S, et al. Obesity in inflammatory bowel disease is associated with early readmissions characterised by an increased systems and patient-level burden. J Crohns Colitis. 2021;15(11):1807-1815. doi:10.1093/ecco-jcc/jjab088
- Agrawal M, Spencer EA, Colombel JF, Ungaro RC. Approach to the management of recently diagnosed inflammatory bowel disease patients: a user’s guide for adult and pediatric gastroenterologists. Gastroenterology. 2021;161(1):47-65. doi:10.1053/j.gastro.2021.04.063
- Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47(4):506-513. doi:10.1136/gut.47.4.506.
- Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA technical review on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology. 2021;160(7):2512-2556.e9. doi:10.1053/j.gastro.2021.04.023
- Dorrington AM, Selinger CP, Parkes GC, Smith M, Pollok RC, Raine T. The historical role and contemporary use of corticosteroids in inflammatory bowel disease. J Crohns Colitis. 2020;14(9):1316-1329. doi:10.1093/ecco-jcc/jjaa053
- Melsheimer R, Geldhof A, Apaolaza I, Schaible T. Remicade® (infliximab): 20 years of contributions to science and medicine. Biologics. 2019;13:139-178. doi:10.2147/BTT.S207246
- Kumar A, Cole A, Segal J, Smith P, Limdi JK. A review of the therapeutic management of Crohn’s disease. Therap Adv Gastroenterol. 2022;15:17562848221078456. doi:10.1177/17562848221078456
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779-2789. doi:10.1016/S0140-6736(17)32641-7
- Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology. 2020;159(1):139-147. doi:10.1053/j.gastro.2020.03.039
- Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021;19(10):2031-2045.e11. doi:10.1016/j.cgh.2020.10.039
- Chapman S, Sibelli A, St-Clair Jones A, Forbes A, Chater A, Horne R. Personalised adherence support for maintenance treatment of inflammatory bowel disease: a tailored digital intervention to change adherence-related beliefs and barriers. J Crohns Colitis. 2020;14(10):1394-1404. doi:10.1093/ecco-jcc/jjz034
- Turner D, Ricciuto A, Lewis A, et al; for the International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi:10.1053/j.gastro.2020.12.031
- Rozich JJ, Dulai PS, Fumery M, Sandborn WJ, Singh S. Progression of elderly onset inflammatory bowel diseases: a systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2020;18(11):2437-2447.e6. doi:10.1016/j.cgh.2020.02.048
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166-1169.doi:10.15585/mmwr.mm6542a3
- M’Koma AE. Inflammatory bowel disease: clinical diagnosis and surgical treatment-overview. Medicina (Kaunas). 2022;58(5):567. doi:10.3390/medicina58050567
- Weissman S, Patel K, Kolli S, et al. Obesity in inflammatory bowel disease is associated with early readmissions characterised by an increased systems and patient-level burden. J Crohns Colitis. 2021;15(11):1807-1815. doi:10.1093/ecco-jcc/jjab088
- Agrawal M, Spencer EA, Colombel JF, Ungaro RC. Approach to the management of recently diagnosed inflammatory bowel disease patients: a user’s guide for adult and pediatric gastroenterologists. Gastroenterology. 2021;161(1):47-65. doi:10.1053/j.gastro.2021.04.063
- Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47(4):506-513. doi:10.1136/gut.47.4.506.
- Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA technical review on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology. 2021;160(7):2512-2556.e9. doi:10.1053/j.gastro.2021.04.023
- Dorrington AM, Selinger CP, Parkes GC, Smith M, Pollok RC, Raine T. The historical role and contemporary use of corticosteroids in inflammatory bowel disease. J Crohns Colitis. 2020;14(9):1316-1329. doi:10.1093/ecco-jcc/jjaa053
- Melsheimer R, Geldhof A, Apaolaza I, Schaible T. Remicade® (infliximab): 20 years of contributions to science and medicine. Biologics. 2019;13:139-178. doi:10.2147/BTT.S207246
- Kumar A, Cole A, Segal J, Smith P, Limdi JK. A review of the therapeutic management of Crohn’s disease. Therap Adv Gastroenterol. 2022;15:17562848221078456. doi:10.1177/17562848221078456
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779-2789. doi:10.1016/S0140-6736(17)32641-7
- Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology. 2020;159(1):139-147. doi:10.1053/j.gastro.2020.03.039
- Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021;19(10):2031-2045.e11. doi:10.1016/j.cgh.2020.10.039
- Chapman S, Sibelli A, St-Clair Jones A, Forbes A, Chater A, Horne R. Personalised adherence support for maintenance treatment of inflammatory bowel disease: a tailored digital intervention to change adherence-related beliefs and barriers. J Crohns Colitis. 2020;14(10):1394-1404. doi:10.1093/ecco-jcc/jjz034
- Turner D, Ricciuto A, Lewis A, et al; for the International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi:10.1053/j.gastro.2020.12.031
- Rozich JJ, Dulai PS, Fumery M, Sandborn WJ, Singh S. Progression of elderly onset inflammatory bowel diseases: a systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2020;18(11):2437-2447.e6. doi:10.1016/j.cgh.2020.02.048
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged ≥18 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166-1169.doi:10.15585/mmwr.mm6542a3
- M’Koma AE. Inflammatory bowel disease: clinical diagnosis and surgical treatment-overview. Medicina (Kaunas). 2022;58(5):567. doi:10.3390/medicina58050567
- Weissman S, Patel K, Kolli S, et al. Obesity in inflammatory bowel disease is associated with early readmissions characterised by an increased systems and patient-level burden. J Crohns Colitis. 2021;15(11):1807-1815. doi:10.1093/ecco-jcc/jjab088
- Agrawal M, Spencer EA, Colombel JF, Ungaro RC. Approach to the management of recently diagnosed inflammatory bowel disease patients: a user’s guide for adult and pediatric gastroenterologists. Gastroenterology. 2021;161(1):47-65. doi:10.1053/j.gastro.2021.04.063
- Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47(4):506-513. doi:10.1136/gut.47.4.506.
- Singh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA technical review on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology. 2021;160(7):2512-2556.e9. doi:10.1053/j.gastro.2021.04.023
Children and COVID: New cases increase for second straight week
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.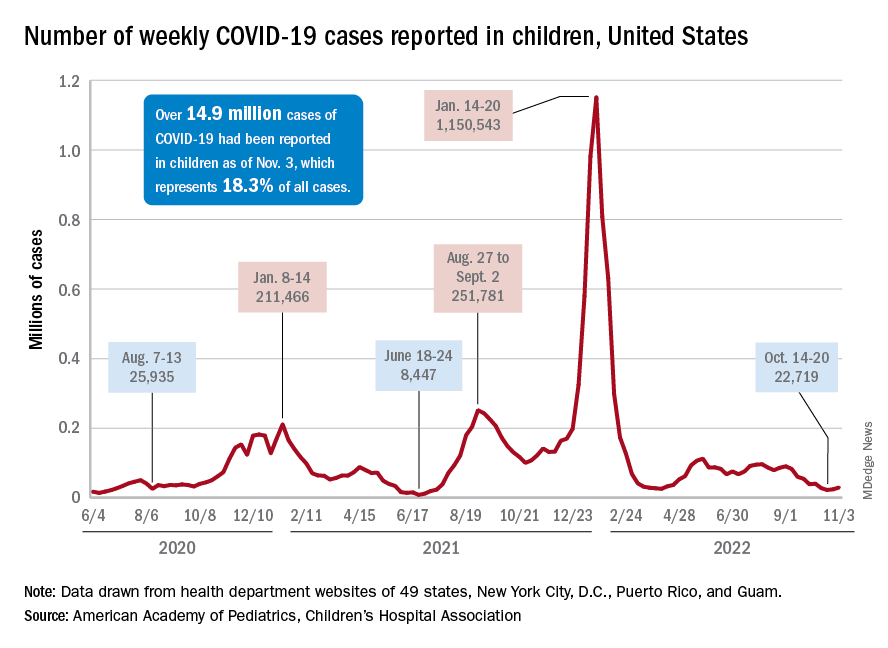
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
Clozapine underutilized in treatment-resistant schizophrenia
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
, and when it is used, the drug is often delayed by several crucial years, reducing chances of efficacy.
“Despite being the only pharmacological therapy approved for treatment-resistant schizophrenia, clozapine is underutilized globally, even in developed countries, where only about 30% of patients who would benefit from the drug receive it,” said John M. Kane, MD, of the department of psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, N.Y., in a presentation on the subject at the 21st Annual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists in Cincinnati, Ohio.
Clozapine, a tricyclic dibenzodiazepine available in branded and various generic versions, is approved by the U.S. Food and Drug Administration as a third-line therapy for severe, treatment-resistant schizophrenia, with studies showing benefits exceeding those of any other antipsychotics for the indication.
But while recommendations suggest use after a trial of two or more antipsychotics, with at least one being an atypical antipsychotic, one recent review finds delays in clozapine commencement ranging from 19.3 weeks to 5.5 years, and the duration of illness prior to clozapine use ranging from 1.1 to 9.7 years.
Blood monitoring, side effects
The key deterrents preventing many clinicians and patients from trying clozapine sooner are the drug’s safety and tolerability profiles, and notably the requirement of regular blood testing due to an increased risk of agranulocytosis.
Specifically, the blood testing is required every week for 6 months, then every other week for the next 6 months, and then once a month after that; however, “many of us think that that’s excessive at this point in time,” Dr. Kane noted.
Various other potential side effects are also of concern, including myocarditis, seizures, constipation, arrhythmia, hypersalivation, pneumonia, and metabolic symptoms including diabetes.
In terms of the common strategies that clinicians turn to when patients fail to respond to their current antipsychotic, including increasing doses, combining agents, or treatment switching, “none of the strategies likely rival clozapine in terms of efficacy,” Dr. Kane said.
Regarding higher dosing: “There is very little data suggesting that higher doses of antipsychotic drugs will work when the moderate or recommended dose has not worked,” he said.
Combination therapy strategies may provide benefits, but “they’re not a substitute for clozapine,” Dr. Kane added, noting that the combinations that do appear to be the most effective involve clozapine.
And regarding drug switching, studies suggest the likelihood of response in switching from one drug to another is “actually very low,” Dr. Kane added.
Clozapine also doesn’t work for all – the response rate runs between about 30% and 60%, Dr. Kane said, but when it is effective, the benefits can be profound.
“There are some patients who have a very pronounced response to clozapine – some patients describe it as life-changing,” he said.
Treatment delays reduce efficacy
Importantly, the delays before receiving clozapine are not inconsequential – data show that each outpatient antipsychotic trial prior to clozapine reduces the likelihood of response by 8%-11%, and each hospital admission further reduces the likelihood of response by 4%-8%, underscoring the need to identify treatment resistance as early as possible, Dr. Kane said.
“It’s critically important to try to identify treatment resistance earlier than we usually do because if we can get it under control sooner, we have a better chance of improving the patient’s outcome, and this has been shown in a number of studies,” he said.
“The longer you wait, the less likely you are to see a good response even to clozapine.”
Despite the concerns about clozapine, Dr. Kane notes that even the blood monitoring does not appear to be a big complaint for patients, especially they are improving.
“In our experience, the patients who benefit from clozapine don’t really have a problem with the monitoring,” he said.
“In fact, patients who benefit from clozapine are much more adherent to the medication than other patients that we see, which is understandable, because if you feel you’re really getting a benefit from medicine, you’re going to be much more motivated to take it even if it has side effects.”
A recent systematic review of 13 studies and 1,487 patients backs that up, concluding that “patients generally have a favorable experience when being treated with clozapine,” with the caveat that “conclusions are limited by the risk of bias, particularly survivorship bias.”
Preference for clozapine over other antipsychotic medications was reported by 54%-86% of patients in the review, with specific improvements in mood (11%-78%) and cognition (5%-68%).
Clinicians the biggest ‘obstacle’
Dr. Kane notes that an important factor in underutilization could indeed be the manner in which clinicians discuss clozapine with their patients – often opening the discussion by focusing on the negative aspects that, without the context of the potential benefits, can be deal-breakers for patient from the start.
“The clinicians in my opinion are really the obstacle,” Dr. Kane said. “What we always hear from clinicians is ‘I can’t do it because the patient refuses, or the patient doesn’t like the side effects’.”
Dr. Kane notes that most side effects can indeed be managed – regarding the risk for metabolic syndrome, for instance, he recommends that patients should be given metformin from the beginning when they’re started on clozapine.
He adds that in most cases, a 3-month trial is enough to answer the question of whether clozapine is working or not.
“Three months is a good trial, but it may not even tell you the total response to clozapine because that may actually accrue over time,” he said. “We’ve seen patients who actually get better and better beyond 3 months.”
Not offering the drug to patients, however, is doing them a serious disservice, Dr. Kane added.
“What I tell patients and families is that it would be a shame to miss this opportunity for a potential treatment that could be life-changing,” he said. “Does it have potential side effects? Yes. Do you have to get blood tests? Yes. And I can’t tell by evaluating a patient’s history or examining that patient whether or not they’re going to be a good responder. But would you really want to miss an opportunity to find that out?”
“To me the argument is – let’s try this drug for 3 months and see what effect it has, and at that point you’ll be in a much better position to make a decision about the benefits versus risk,” Dr. Kane said.
The only FDA-approved drug for treatment-resistant schizophrenia
Remarkably, clozapine isn’t just the only drug to currently have approval from the FDA for treatment-resistant schizophrenia – it has been for the last 3 decades.
“There have been attempts to develop medications with similar efficacy, but they have not succeeded,” Dr. Kane said in an interview. “We are still uncertain as to what accounts for clozapine’s unique qualities.”
Yet, with treatment-resistant schizophrenia patients representing some of the most dire mental illness cases clinicians may face, the need for better treatment decisions – and additional options – is pressing, Dr. Kane said.
“[The lack of any other drugs] is a big embarrassment to our field, in my opinion,” he said. “I’m a big proponent of clozapine, but we should have found another substance by now that could substitute for clozapine, which obviously has a lot of side effects and is not the easiest drug to use.”
Dr. Kane reported relationships either as a speaker or consultant/advisory board member and/or receives research grant support from Alkermes, Allergan, Click Therapeutics, Dainippon Sumitomo, H. Lundbeck, HLS Therapeutics, Indivior, Intra-Cellular Therapies, Janssen Pharmaceutical, Johnson & Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Neumora Therapeutics, Novartis Pharmaceuticals, Otsuka, Reviva, Roche, Saladax, Sunovion, Takeda, and Teva. Dr. Kane receives non-mutual funds stock ownership/stock options from LB Pharmaceuticals, Vanguard Research Group, and North Shore Therapeutics, and receives patent holder/royalties paid by UpToDate.
The Psychopharmacology Update was sponsored by Medscape Live. Medscape Live and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Airways Disorders Network
Pediatric Chest Medicine Section
CPAP for pediatric OSA: “Off-label” use
Pediatric providers are well aware of the “off-label” uses of medications/devices. While it’s not a stretch to apply “adult” diagnostic and therapeutic criteria to older adolescents, more careful consideration is needed for our younger patients. Typically, adenotonsillectomy is first-line treatment for pediatric OSA, but CPAP can be essential for those for whom surgical intervention is not an option, not an option yet, or has been insufficient (residual OSA). Unfortunately, standard CPAP devices are not approved for use in children, and often have a minimum weight requirement of 30 kg. There are respiratory assist devices and home mechanical ventilators that are approved for use in pediatric patients (minimum weight 13 kg or 5 kg) and designed for more complex ventilatory support, and that also are capable of providing continuous pressure. Alternatively, pediatric providers may proceed with the “off-label” use of simpler CPAP-only medical devices and face obstacles in attaining insurance approval. The recent American Academy of Sleep Medicine position statement (Amos, et al. J Clin Sleep Med. 2022;18[8]:2041-3) acknowledges that CPAP therapy can be safe and effective when management is guided by a pediatric specialist and is typically initiated in a monitored setting (inpatient or polysomnogram). The authors bring up excellent points regarding unique considerations for pediatric CPAP therapy, including the need for desensitization and facial development monitoring, lack of technical/software designed for younger/smaller patients, and limited published data (small and diverse cohorts). Ultimately, evaluation of effectiveness and safety, while distinct, must both be seriously considered in this risk-benefit analysis of care.
Pallavi P. Patwari, MD, FAAP, FAASM
Member-at-Large
Pediatric Chest Medicine Section
CPAP for pediatric OSA: “Off-label” use
Pediatric providers are well aware of the “off-label” uses of medications/devices. While it’s not a stretch to apply “adult” diagnostic and therapeutic criteria to older adolescents, more careful consideration is needed for our younger patients. Typically, adenotonsillectomy is first-line treatment for pediatric OSA, but CPAP can be essential for those for whom surgical intervention is not an option, not an option yet, or has been insufficient (residual OSA). Unfortunately, standard CPAP devices are not approved for use in children, and often have a minimum weight requirement of 30 kg. There are respiratory assist devices and home mechanical ventilators that are approved for use in pediatric patients (minimum weight 13 kg or 5 kg) and designed for more complex ventilatory support, and that also are capable of providing continuous pressure. Alternatively, pediatric providers may proceed with the “off-label” use of simpler CPAP-only medical devices and face obstacles in attaining insurance approval. The recent American Academy of Sleep Medicine position statement (Amos, et al. J Clin Sleep Med. 2022;18[8]:2041-3) acknowledges that CPAP therapy can be safe and effective when management is guided by a pediatric specialist and is typically initiated in a monitored setting (inpatient or polysomnogram). The authors bring up excellent points regarding unique considerations for pediatric CPAP therapy, including the need for desensitization and facial development monitoring, lack of technical/software designed for younger/smaller patients, and limited published data (small and diverse cohorts). Ultimately, evaluation of effectiveness and safety, while distinct, must both be seriously considered in this risk-benefit analysis of care.
Pallavi P. Patwari, MD, FAAP, FAASM
Member-at-Large
Pediatric Chest Medicine Section
CPAP for pediatric OSA: “Off-label” use
Pediatric providers are well aware of the “off-label” uses of medications/devices. While it’s not a stretch to apply “adult” diagnostic and therapeutic criteria to older adolescents, more careful consideration is needed for our younger patients. Typically, adenotonsillectomy is first-line treatment for pediatric OSA, but CPAP can be essential for those for whom surgical intervention is not an option, not an option yet, or has been insufficient (residual OSA). Unfortunately, standard CPAP devices are not approved for use in children, and often have a minimum weight requirement of 30 kg. There are respiratory assist devices and home mechanical ventilators that are approved for use in pediatric patients (minimum weight 13 kg or 5 kg) and designed for more complex ventilatory support, and that also are capable of providing continuous pressure. Alternatively, pediatric providers may proceed with the “off-label” use of simpler CPAP-only medical devices and face obstacles in attaining insurance approval. The recent American Academy of Sleep Medicine position statement (Amos, et al. J Clin Sleep Med. 2022;18[8]:2041-3) acknowledges that CPAP therapy can be safe and effective when management is guided by a pediatric specialist and is typically initiated in a monitored setting (inpatient or polysomnogram). The authors bring up excellent points regarding unique considerations for pediatric CPAP therapy, including the need for desensitization and facial development monitoring, lack of technical/software designed for younger/smaller patients, and limited published data (small and diverse cohorts). Ultimately, evaluation of effectiveness and safety, while distinct, must both be seriously considered in this risk-benefit analysis of care.
Pallavi P. Patwari, MD, FAAP, FAASM
Member-at-Large
EHR-based thromboembolism risk tool boosted prophylaxis
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
AT AHA 2022