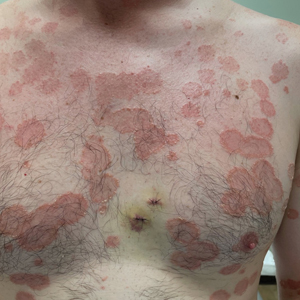
User login
FDA puts REMS requirements on hold to ensure continuity of care
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
In a Nov. 2 notice on its website, the FDA said it is aware that health care professionals and patients continue to experience ongoing difficulties with the clozapine REMS program, including issues with patient access to clozapine following discharge from inpatient care.
A chief concern is that inpatient pharmacies are only allowed to dispense a 7-days’ supply of clozapine to the patient upon discharge.
To address this issue, the FDA said it will now (temporarily) not object if inpatient pharmacies dispense a days’ supply of clozapine that aligns with the patient’s monitoring frequency.
For example, a 7-days’ supply for weekly monitoring, a 14-days’ supply for twice-monthly monitoring, and a 30-days’ supply for monthly monitoring upon discharge from an inpatient facility.
Clozapine is a second-generation (atypical) antipsychotic used to treat schizophrenia that is not well controlled with standard antipsychotics.
While clozapine can be highly effective in some patients, it also carries serious risks, including a decrease in neutrophil count, which can lead to severe neutropenia, serious infections, and death.
As a result, those taking the drug must undergo regular absolute neutrophil count monitoring. Clozapine REMS is intended to maximize the benefits of the drug and minimize risk.
The FDA says it will continue to exercise earlier enforcement discretion regarding the clozapine REMS program announced back in November 2021. This includes allowing pharmacists to dispense clozapine without a REMS dispense authorization and allowing wholesalers to ship clozapine to pharmacies and health care settings without confirming enrollment in the REMS.
“We understand that difficulties with the clozapine REMS program have caused frustration and have led to problems with patient access to clozapine. FDA takes these concerns seriously. Continuity of care, patient access to clozapine, and patient safety are our highest priorities,” the FDA says.
The agency is working closely with the clozapine REMS program administrators to address these challenges and avoid interruptions in patient care.
The FDA encourages pharmacists and prescribers to continue working with the clozapine REMS to complete certification and prescribers to enroll patients in the program.
A version of this article first appeared on Medscape.com.
What does it take for men to embrace cosmetic treatments?
with the same gusto as women.
However, this could be changing as millennials – who tend to be more proactive about efforts to prevent skin aging – are getting older.
At a virtual course on laser and aesthetic skin therapy, Dr. Carruthers referred to the results of an online survey of 600 men aged 30-65 years conducted by Jared Jagdeo, MD, and colleagues in 2016, to gauge attitudes toward age-related changes of their facial features and their preferences for prioritizing treatment. The top five barriers to treatment cited by the respondents were: “I don’t think I need it yet” (47%); “concerned about safety/side effects” (46%); “concerned about injecting a foreign substance into my body” (45%); “cost” (42%), and “concerned my face won’t look natural” (41%).
“Since then, millennials took over as the largest portion of our workforce in North America,” said Dr. Carruthers who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox). “Millennials are interested in how they look and how to keep their aesthetic the best it can possibly be,” she said, so there may be “a generational aspect to this.”
Another factor that may affect the uptake of cosmetic procedures among men is the number of hours they spend gazing at their own image on a computer screen. Since the beginning of the COVID-19 pandemic, men have spent an increasing number of hours on video-conferencing calls via Zoom and other platforms, causing them to rethink how they view their appearance, Dr. Carruthers added. “Zoom dysmorphia” is the term that describes the phenomenon that developed during the pandemic where more patients expressed a desire to make changes to their appearance, including nose jobs and smoothing out forehead wrinkles.
“When we’re on a Zoom call, we’re spending 40% of our time looking at ourselves,” said Dr. Carruthers, clinical professor of ophthalmology and visual sciences at the University of British Columbia in Vancouver. “This would hint that the looking glass is not as powerful as the computer screen to motivate men” to pursue aesthetic treatments.
According to data from the American Society of Plastic Surgeons, the top 5 cosmetic surgical procedures performed in men in 2020 were nose shaping, eyelid surgery, cheek implants, liposuction, and ear surgery. The top 5 minimally-invasive procedures in men were botulinum toxin type A, followed by laser skin resurfacing, laser hair removal, soft tissue fillers, and microdermabrasion.
Why might men consider an injectable instead of surgery? Dr. Carruthers asked. “According to the 2016 survey by Dr. Jagdeo and colleagues, it’s to appear more youthful and to appear good for their age.”
From a clinical standpoint, success comes from understanding the subtle differences between treating men and women, she added.
In a 2022 article about optimizing skin tightening in aesthetics in men, Christian A. Albornoz, MD, and colleagues noted that in contrast to women, men “tend to have higher levels of collagen density and greater skin thickness, but these begin to decrease earlier on. They can also more frequently have severe photodamage”.
In another article published in 2018, Terrence Keaney, MD, and colleagues reviewed the objective data available on male aging and aesthetics. They stated that a “communication gap exists for men, as evidenced by the lack of information available online or by word of mouth about injectable treatments” and concluded that “educating men about available aesthetic treatments and about the safety and side effects associated with each treatment, as well as addressing concerns about their treatment results looking natural, are key considerations.”
That sentiment resonates with Dr. Carruthers. Part of the reason why men have not sought cosmetic treatments along with their female partners and friends seeking cosmetic treatments “is that they haven’t had anything in their cup,” she said. “Maybe this is something we need to think about, to try and help men come in and enjoy the positive benefits of aesthetic, noninvasive cosmetic treatments.”
The course was sponsored by Harvard Medical School, Massachusetts General Hospital, and Wellman Center for Photomedicine.
Dr. Carruthers disclosed that she is a consultant and researcher for Alastin, Appiell, Allergan Aesthetics, Avari Medical, Bonti, Evolus, Fount Bio, Jeune Aesthetics, Merz, and Revance Biopharma.
with the same gusto as women.
However, this could be changing as millennials – who tend to be more proactive about efforts to prevent skin aging – are getting older.
At a virtual course on laser and aesthetic skin therapy, Dr. Carruthers referred to the results of an online survey of 600 men aged 30-65 years conducted by Jared Jagdeo, MD, and colleagues in 2016, to gauge attitudes toward age-related changes of their facial features and their preferences for prioritizing treatment. The top five barriers to treatment cited by the respondents were: “I don’t think I need it yet” (47%); “concerned about safety/side effects” (46%); “concerned about injecting a foreign substance into my body” (45%); “cost” (42%), and “concerned my face won’t look natural” (41%).
“Since then, millennials took over as the largest portion of our workforce in North America,” said Dr. Carruthers who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox). “Millennials are interested in how they look and how to keep their aesthetic the best it can possibly be,” she said, so there may be “a generational aspect to this.”
Another factor that may affect the uptake of cosmetic procedures among men is the number of hours they spend gazing at their own image on a computer screen. Since the beginning of the COVID-19 pandemic, men have spent an increasing number of hours on video-conferencing calls via Zoom and other platforms, causing them to rethink how they view their appearance, Dr. Carruthers added. “Zoom dysmorphia” is the term that describes the phenomenon that developed during the pandemic where more patients expressed a desire to make changes to their appearance, including nose jobs and smoothing out forehead wrinkles.
“When we’re on a Zoom call, we’re spending 40% of our time looking at ourselves,” said Dr. Carruthers, clinical professor of ophthalmology and visual sciences at the University of British Columbia in Vancouver. “This would hint that the looking glass is not as powerful as the computer screen to motivate men” to pursue aesthetic treatments.
According to data from the American Society of Plastic Surgeons, the top 5 cosmetic surgical procedures performed in men in 2020 were nose shaping, eyelid surgery, cheek implants, liposuction, and ear surgery. The top 5 minimally-invasive procedures in men were botulinum toxin type A, followed by laser skin resurfacing, laser hair removal, soft tissue fillers, and microdermabrasion.
Why might men consider an injectable instead of surgery? Dr. Carruthers asked. “According to the 2016 survey by Dr. Jagdeo and colleagues, it’s to appear more youthful and to appear good for their age.”
From a clinical standpoint, success comes from understanding the subtle differences between treating men and women, she added.
In a 2022 article about optimizing skin tightening in aesthetics in men, Christian A. Albornoz, MD, and colleagues noted that in contrast to women, men “tend to have higher levels of collagen density and greater skin thickness, but these begin to decrease earlier on. They can also more frequently have severe photodamage”.
In another article published in 2018, Terrence Keaney, MD, and colleagues reviewed the objective data available on male aging and aesthetics. They stated that a “communication gap exists for men, as evidenced by the lack of information available online or by word of mouth about injectable treatments” and concluded that “educating men about available aesthetic treatments and about the safety and side effects associated with each treatment, as well as addressing concerns about their treatment results looking natural, are key considerations.”
That sentiment resonates with Dr. Carruthers. Part of the reason why men have not sought cosmetic treatments along with their female partners and friends seeking cosmetic treatments “is that they haven’t had anything in their cup,” she said. “Maybe this is something we need to think about, to try and help men come in and enjoy the positive benefits of aesthetic, noninvasive cosmetic treatments.”
The course was sponsored by Harvard Medical School, Massachusetts General Hospital, and Wellman Center for Photomedicine.
Dr. Carruthers disclosed that she is a consultant and researcher for Alastin, Appiell, Allergan Aesthetics, Avari Medical, Bonti, Evolus, Fount Bio, Jeune Aesthetics, Merz, and Revance Biopharma.
with the same gusto as women.
However, this could be changing as millennials – who tend to be more proactive about efforts to prevent skin aging – are getting older.
At a virtual course on laser and aesthetic skin therapy, Dr. Carruthers referred to the results of an online survey of 600 men aged 30-65 years conducted by Jared Jagdeo, MD, and colleagues in 2016, to gauge attitudes toward age-related changes of their facial features and their preferences for prioritizing treatment. The top five barriers to treatment cited by the respondents were: “I don’t think I need it yet” (47%); “concerned about safety/side effects” (46%); “concerned about injecting a foreign substance into my body” (45%); “cost” (42%), and “concerned my face won’t look natural” (41%).
“Since then, millennials took over as the largest portion of our workforce in North America,” said Dr. Carruthers who, with her husband, Alastair Carruthers, MD, pioneered the cosmetic use of onabotulinumtoxinA (Botox). “Millennials are interested in how they look and how to keep their aesthetic the best it can possibly be,” she said, so there may be “a generational aspect to this.”
Another factor that may affect the uptake of cosmetic procedures among men is the number of hours they spend gazing at their own image on a computer screen. Since the beginning of the COVID-19 pandemic, men have spent an increasing number of hours on video-conferencing calls via Zoom and other platforms, causing them to rethink how they view their appearance, Dr. Carruthers added. “Zoom dysmorphia” is the term that describes the phenomenon that developed during the pandemic where more patients expressed a desire to make changes to their appearance, including nose jobs and smoothing out forehead wrinkles.
“When we’re on a Zoom call, we’re spending 40% of our time looking at ourselves,” said Dr. Carruthers, clinical professor of ophthalmology and visual sciences at the University of British Columbia in Vancouver. “This would hint that the looking glass is not as powerful as the computer screen to motivate men” to pursue aesthetic treatments.
According to data from the American Society of Plastic Surgeons, the top 5 cosmetic surgical procedures performed in men in 2020 were nose shaping, eyelid surgery, cheek implants, liposuction, and ear surgery. The top 5 minimally-invasive procedures in men were botulinum toxin type A, followed by laser skin resurfacing, laser hair removal, soft tissue fillers, and microdermabrasion.
Why might men consider an injectable instead of surgery? Dr. Carruthers asked. “According to the 2016 survey by Dr. Jagdeo and colleagues, it’s to appear more youthful and to appear good for their age.”
From a clinical standpoint, success comes from understanding the subtle differences between treating men and women, she added.
In a 2022 article about optimizing skin tightening in aesthetics in men, Christian A. Albornoz, MD, and colleagues noted that in contrast to women, men “tend to have higher levels of collagen density and greater skin thickness, but these begin to decrease earlier on. They can also more frequently have severe photodamage”.
In another article published in 2018, Terrence Keaney, MD, and colleagues reviewed the objective data available on male aging and aesthetics. They stated that a “communication gap exists for men, as evidenced by the lack of information available online or by word of mouth about injectable treatments” and concluded that “educating men about available aesthetic treatments and about the safety and side effects associated with each treatment, as well as addressing concerns about their treatment results looking natural, are key considerations.”
That sentiment resonates with Dr. Carruthers. Part of the reason why men have not sought cosmetic treatments along with their female partners and friends seeking cosmetic treatments “is that they haven’t had anything in their cup,” she said. “Maybe this is something we need to think about, to try and help men come in and enjoy the positive benefits of aesthetic, noninvasive cosmetic treatments.”
The course was sponsored by Harvard Medical School, Massachusetts General Hospital, and Wellman Center for Photomedicine.
Dr. Carruthers disclosed that she is a consultant and researcher for Alastin, Appiell, Allergan Aesthetics, Avari Medical, Bonti, Evolus, Fount Bio, Jeune Aesthetics, Merz, and Revance Biopharma.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
World falls short on HBV, HCV elimination targets
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vaccination campaigns in more than 80 nations have successfully reduced the prevalence of hepatitis B virus (HBV) surface antigen. That’s the good news.
Less good is the news that reported Sarah Blach, MHS, associate director of the Center for Disease Analysis Foundation, based in Lafayette, Colo.
“As countries progress toward eliminating hepatitis B and C, we really need to do more to expand political will and financing of national elimination programs. It’s great to see that it’s happening in some of these countries, but we really need that to expand,” she said at the annual meeting of the American Association for the Study of Liver Diseases.
Ms. Blach presented data from the foundation’s Polaris Observatory, an initiative that provides epidemiological data, modeling tools, training, and decision analytics to support eliminating HBV and HCV globally by 2030.
The investigators used mathematical disease burden models for HBV and HCV to assess worldwide trends toward viral elimination. They also evaluated HBV and HCV elimination policies as reported by authorities in various countries.
They forecast the year in which each country or territory would meet each of the World Health Organization’s four elimination targets from 110 HCV models and 166 HBV models. The targets are 90% diagnosed, 80% of the eligible population treated, 65% reduction in mortality, and 80% incidence reduction for HCV and either 95% incidence reduction or prevalence of 0.1% or less in children aged 5 years and younger for HBV.
Investigators summarized the results across countries by disease area and time period of elimination; that is, elimination before 2030, between 2031 and 2050, or after 2050.
Results for HCV and HBV targets
The 11 nations on track to achieve all absolute or relative (programmatic) targets for HCV by 2030 are Australia, Canada, Denmark, Egypt, Finland, France, Georgia, Japan, Norway, Spain, and the United Kingdom.
An additional 24 countries are on track to meet their goals for HCV between 2031 and 2050.
But the rest, including the United States, much of sub-Saharan Africa, China, and South Asia, are not on track to meet their goals for HCV by 2050.
No countries are on track to achieve the absolute or relative (programmatic) targets for elimination of HBV, Ms. Blach said.
However, 83 countries or territories, including the United States, are on track for achieving the HBV surface antigen prevalence target of less than 0.1% in children aged 5 years and younger by 2030.
Ms. Blach and colleagues also looked at results of quantitative policy surveys submitted by 61 countries. The respondents were asked to report on linkage to care, awareness and screening, monitoring and evaluation, ability to expand capacity, harm-reduction programs, financing of national programs, and political will to achieve targets.
The investigators scored countries on a scale of 1-10, with 10 being the highest score, in each category. For HCV, 25 countries (42%) had high scores, defined as 9 or 10, for political will, and 33 countries (54%) had high scores for national funding. For HBV, 17 countries (30%) received the high scores for political will, and 30 (51%) received the high scores for financing the national program.
The big picture
Most countries have not expanded HBV or HCV treatment beyond specialists, and HBV policies appear to lag behind policies directed toward HCV elimination, Ms. Blach noted.
“We do need to expand screening and treatment for hepatitis B moving forward,” she said.
The United States and the rest of the world need to do better, especially regarding HBV elimination, but the United States does appear to be making progress, said Richard Sterling, MD, MSc, from Virginia Commonwealth University, Richmond, who comoderated the session where Ms. Blach reported the data.
“My impression is that we’re doing a pretty good job with [HBV] vaccinations in the United States,” Dr. Sterling, who was not involved in the study, said in an interview.
One way to make progress, he said, may be to expand eligibility for HBV vaccines beyond the current upper age limit of 59 years.
Implementing simpler dosing regimens – the currently available vaccine is split into three doses – could improve vaccine compliance and lower costs, Dr. Sterling added.
During the session, Brian Conway, MD, medical director of the Vancouver Infectious Disease Centre, said it seems hard to use a composite set of data to determine a yes/no answer about whether a country is on track to reach targets.
“When you take my country of Canada, we have absolutely no national program, no hope of a national program, very little funding, and yet we make the cut. So how do you balance all these different variables to arrive at a yes/no answer and is there a way of putting a bit more subtlety into it?” Dr. Conway asked Ms. Blach.
Ms. Blach replied that the data are fluid, and countries can move closer or farther from reaching targets over time as conditions change.
Some countries seem to be improving efforts and “just need a bit more” work, Ms. Blach said.
“But we also saw some countries who we thought were going to be a shoo-in, and as time progressed the number of treatments just dropped in shocking ways. The reality is that a lot of countries are struggling to treat patients,” she said.
Canada “has a really great health system. It’s not a fragmented health system, and so even if you don’t have some of that push for elimination from the government level, having access to treatment, having access to those services, means that at least patients can come in and get what they need,” Ms. Blach said.
The study data are available for free on the Center for Disease Analysis Foundation’s Polaris website.
The study was funded by grants from the John C. Martin Foundation, ZeShan Foundation, EndHep2030, Gilead Sciences, and AbbVie. Ms. Blach is employed by the Center for Disease Analysis Foundation, which receives research grants from Gilead and AbbVie. Dr. Sterling and Dr. Conway reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
NAFLD patients with diabetes have higher fibrosis progression rate
Among people with nonalcoholic fatty liver disease (NAFLD), the fibrosis progression rate was higher among those who also had diabetes, according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
NAFLD patients with type 2 diabetes progressed by one stage about every 6 years, compared with one stage about every 8 years among patients without diabetes, said Daniel Huang, MBBS, a visiting scholar at the University of California San Diego (UCSD) NAFLD Research Center and a transplant hepatologist at National University Hospital in Singapore.
“We now know that fibrosis stage is a major determinant of liver-related outcomes in NAFLD, as well as overall mortality,” he said. “Liver fibrosis progresses by approximately one stage every 7 years for individuals with NASH (nonalcoholic steatohepatitis).”
Recent UCSD data have indicated that about 14% of patients over age 50 with type 2 diabetes have NAFLD with advanced fibrosis, he noted. Previous studies have shown that diabetes is associated with higher rates of advanced fibrosis, cirrhosis, and hepatocellular carcinoma, but limited data exist around whether the fibrosis progression rate is higher among diabetics.
National study cohort
Dr. Huang and colleagues conducted a multicenter, multiethnic prospective cohort study within the NASH Clinical Research Network consortium to examine the fibrosis progression rate and the fibrosis regression rate among patients with or without diabetes. The study included adult participants at eight sites across the United States who had biopsy-confirmed NAFLD and available paired liver biopsies that were at least 1 year apart.
Clinical and laboratory data were obtained at enrollment and prospectively at 48-week intervals and recorded at the time of any liver biopsies. A central pathology committee conducted the liver histology assessment, and the entire pathology committee was blinded to clinical data and the sequence of liver biopsy. The fibrosis progression and regression rates were defined as the change in fibrosis stage over time between biopsies, measured in years.
The study comprised 447 adult participants with NAFLD: 208 patients with type 2 diabetes and 239 patients without diabetes, Dr. Huang said. The mean age was 51, and the mean body mass index was 34.7. The patients with diabetes were more likely to be older, to be women, and to have metabolic syndrome, NASH, and a higher fibrosis stage.
Notably, the median HbA1c among patients with diabetes was 6.8%, indicating a cohort with fairly well-controlled blood sugar. The median time between biopsies was 3.3 years.
Difference in progression, not regression
Overall, 151 participants (34%) experienced fibrosis progression, the primary study outcome. In a secondary outcome, 102 participants (23%) had fibrosis regression. The remaining 194 participants (43%) had no change in fibrosis stage. About 26% of patients with types 2 diabetes progressed to advanced fibrosis, as compared with 14.1% of patients without diabetes.
Among all those with fibrosis progression, the rate was 0.15 stages per year, with an average progression rate of one stage over 6.7 years. For patients with diabetes, the progression rate was significantly higher at 0.17 stages per year, compared with 0.13 stages per year among patients without diabetes, Dr. Huang said. That translated to an average progression of one stage over 5.9 years for patients with diabetes and 7.7 years for patients without diabetes.
In contrast, the regression rate was similar between those with or without diabetes at baseline, at –0.13 stages per year for those with diabetes versus –0.14 stages per year for those without diabetes. The similar outcome translated to an average regression of one stage over 7.7 years among those with diabetes and 7.1 years among those without diabetes.
Type 2 diabetes was an independent predictor of fibrosis progression in NAFLD, in both unadjusted and multivariable adjusted models, including baseline fibrosis stage, Dr. Huang said. In addition, patients with diabetes had a significantly higher cumulative incidence of fibrosis progression at 4 years (23% versus 19%), 8 years (59% versus 49%), and 12 years (93% versus 76%).
The research team didn’t find a significant difference in HbA1c as a predictor of fibrosis progression when using a cutoff of 7%.
“It is possible that poor glycemic control may accelerate fibrosis further, but we need studies to validate this,” Dr. Huang said. “These data have important implications for clinical practice and clinical trial design. Patients with NAFLD and diabetes may require more frequent monitoring for disease progression.”
The NASH Clinical Research Network consortium is sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Huang has served on an advisory board for Eisai. The other authors declared various research support and advisory roles with numerous pharmaceutical companies.
Among people with nonalcoholic fatty liver disease (NAFLD), the fibrosis progression rate was higher among those who also had diabetes, according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
NAFLD patients with type 2 diabetes progressed by one stage about every 6 years, compared with one stage about every 8 years among patients without diabetes, said Daniel Huang, MBBS, a visiting scholar at the University of California San Diego (UCSD) NAFLD Research Center and a transplant hepatologist at National University Hospital in Singapore.
“We now know that fibrosis stage is a major determinant of liver-related outcomes in NAFLD, as well as overall mortality,” he said. “Liver fibrosis progresses by approximately one stage every 7 years for individuals with NASH (nonalcoholic steatohepatitis).”
Recent UCSD data have indicated that about 14% of patients over age 50 with type 2 diabetes have NAFLD with advanced fibrosis, he noted. Previous studies have shown that diabetes is associated with higher rates of advanced fibrosis, cirrhosis, and hepatocellular carcinoma, but limited data exist around whether the fibrosis progression rate is higher among diabetics.
National study cohort
Dr. Huang and colleagues conducted a multicenter, multiethnic prospective cohort study within the NASH Clinical Research Network consortium to examine the fibrosis progression rate and the fibrosis regression rate among patients with or without diabetes. The study included adult participants at eight sites across the United States who had biopsy-confirmed NAFLD and available paired liver biopsies that were at least 1 year apart.
Clinical and laboratory data were obtained at enrollment and prospectively at 48-week intervals and recorded at the time of any liver biopsies. A central pathology committee conducted the liver histology assessment, and the entire pathology committee was blinded to clinical data and the sequence of liver biopsy. The fibrosis progression and regression rates were defined as the change in fibrosis stage over time between biopsies, measured in years.
The study comprised 447 adult participants with NAFLD: 208 patients with type 2 diabetes and 239 patients without diabetes, Dr. Huang said. The mean age was 51, and the mean body mass index was 34.7. The patients with diabetes were more likely to be older, to be women, and to have metabolic syndrome, NASH, and a higher fibrosis stage.
Notably, the median HbA1c among patients with diabetes was 6.8%, indicating a cohort with fairly well-controlled blood sugar. The median time between biopsies was 3.3 years.
Difference in progression, not regression
Overall, 151 participants (34%) experienced fibrosis progression, the primary study outcome. In a secondary outcome, 102 participants (23%) had fibrosis regression. The remaining 194 participants (43%) had no change in fibrosis stage. About 26% of patients with types 2 diabetes progressed to advanced fibrosis, as compared with 14.1% of patients without diabetes.
Among all those with fibrosis progression, the rate was 0.15 stages per year, with an average progression rate of one stage over 6.7 years. For patients with diabetes, the progression rate was significantly higher at 0.17 stages per year, compared with 0.13 stages per year among patients without diabetes, Dr. Huang said. That translated to an average progression of one stage over 5.9 years for patients with diabetes and 7.7 years for patients without diabetes.
In contrast, the regression rate was similar between those with or without diabetes at baseline, at –0.13 stages per year for those with diabetes versus –0.14 stages per year for those without diabetes. The similar outcome translated to an average regression of one stage over 7.7 years among those with diabetes and 7.1 years among those without diabetes.
Type 2 diabetes was an independent predictor of fibrosis progression in NAFLD, in both unadjusted and multivariable adjusted models, including baseline fibrosis stage, Dr. Huang said. In addition, patients with diabetes had a significantly higher cumulative incidence of fibrosis progression at 4 years (23% versus 19%), 8 years (59% versus 49%), and 12 years (93% versus 76%).
The research team didn’t find a significant difference in HbA1c as a predictor of fibrosis progression when using a cutoff of 7%.
“It is possible that poor glycemic control may accelerate fibrosis further, but we need studies to validate this,” Dr. Huang said. “These data have important implications for clinical practice and clinical trial design. Patients with NAFLD and diabetes may require more frequent monitoring for disease progression.”
The NASH Clinical Research Network consortium is sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Huang has served on an advisory board for Eisai. The other authors declared various research support and advisory roles with numerous pharmaceutical companies.
Among people with nonalcoholic fatty liver disease (NAFLD), the fibrosis progression rate was higher among those who also had diabetes, according to new findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
NAFLD patients with type 2 diabetes progressed by one stage about every 6 years, compared with one stage about every 8 years among patients without diabetes, said Daniel Huang, MBBS, a visiting scholar at the University of California San Diego (UCSD) NAFLD Research Center and a transplant hepatologist at National University Hospital in Singapore.
“We now know that fibrosis stage is a major determinant of liver-related outcomes in NAFLD, as well as overall mortality,” he said. “Liver fibrosis progresses by approximately one stage every 7 years for individuals with NASH (nonalcoholic steatohepatitis).”
Recent UCSD data have indicated that about 14% of patients over age 50 with type 2 diabetes have NAFLD with advanced fibrosis, he noted. Previous studies have shown that diabetes is associated with higher rates of advanced fibrosis, cirrhosis, and hepatocellular carcinoma, but limited data exist around whether the fibrosis progression rate is higher among diabetics.
National study cohort
Dr. Huang and colleagues conducted a multicenter, multiethnic prospective cohort study within the NASH Clinical Research Network consortium to examine the fibrosis progression rate and the fibrosis regression rate among patients with or without diabetes. The study included adult participants at eight sites across the United States who had biopsy-confirmed NAFLD and available paired liver biopsies that were at least 1 year apart.
Clinical and laboratory data were obtained at enrollment and prospectively at 48-week intervals and recorded at the time of any liver biopsies. A central pathology committee conducted the liver histology assessment, and the entire pathology committee was blinded to clinical data and the sequence of liver biopsy. The fibrosis progression and regression rates were defined as the change in fibrosis stage over time between biopsies, measured in years.
The study comprised 447 adult participants with NAFLD: 208 patients with type 2 diabetes and 239 patients without diabetes, Dr. Huang said. The mean age was 51, and the mean body mass index was 34.7. The patients with diabetes were more likely to be older, to be women, and to have metabolic syndrome, NASH, and a higher fibrosis stage.
Notably, the median HbA1c among patients with diabetes was 6.8%, indicating a cohort with fairly well-controlled blood sugar. The median time between biopsies was 3.3 years.
Difference in progression, not regression
Overall, 151 participants (34%) experienced fibrosis progression, the primary study outcome. In a secondary outcome, 102 participants (23%) had fibrosis regression. The remaining 194 participants (43%) had no change in fibrosis stage. About 26% of patients with types 2 diabetes progressed to advanced fibrosis, as compared with 14.1% of patients without diabetes.
Among all those with fibrosis progression, the rate was 0.15 stages per year, with an average progression rate of one stage over 6.7 years. For patients with diabetes, the progression rate was significantly higher at 0.17 stages per year, compared with 0.13 stages per year among patients without diabetes, Dr. Huang said. That translated to an average progression of one stage over 5.9 years for patients with diabetes and 7.7 years for patients without diabetes.
In contrast, the regression rate was similar between those with or without diabetes at baseline, at –0.13 stages per year for those with diabetes versus –0.14 stages per year for those without diabetes. The similar outcome translated to an average regression of one stage over 7.7 years among those with diabetes and 7.1 years among those without diabetes.
Type 2 diabetes was an independent predictor of fibrosis progression in NAFLD, in both unadjusted and multivariable adjusted models, including baseline fibrosis stage, Dr. Huang said. In addition, patients with diabetes had a significantly higher cumulative incidence of fibrosis progression at 4 years (23% versus 19%), 8 years (59% versus 49%), and 12 years (93% versus 76%).
The research team didn’t find a significant difference in HbA1c as a predictor of fibrosis progression when using a cutoff of 7%.
“It is possible that poor glycemic control may accelerate fibrosis further, but we need studies to validate this,” Dr. Huang said. “These data have important implications for clinical practice and clinical trial design. Patients with NAFLD and diabetes may require more frequent monitoring for disease progression.”
The NASH Clinical Research Network consortium is sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Huang has served on an advisory board for Eisai. The other authors declared various research support and advisory roles with numerous pharmaceutical companies.
FROM THE LIVER MEETING
Many moms don’t remember well-child nutrition advice
Recent findings from a study examining mothers’ recall of doctors’ advice on early-child nutrition suggest that key feeding messages may not be heard, remembered, or even delivered.
During a typical child wellness visit, pediatricians provide parents with anticipatory guidance on all aspects of child development and safety, up to the age of 5 years.
The analysis of data from a subset of 1,302 mothers participating in the 2017-2019 National Survey of Family Growth showed that those older than 31 years of age and those who identified as non-Hispanic White were more likely to recall discussion of certain child nutrition topics compared with younger mothers or those who identified as Hispanic.
Of the six child-feeding topics referenced from the American Academy of Pediatrics’ “Bright Futures Guidelines,” less than half of the mothers, all of whom had a child between the ages of 6 months and 5 years, recalled guidance on limiting meals in front of the television or other electronic devices. Similarly, fewer than 50% remembered being told not to force their child to finish a bottle or food, the analysis showed.
When it came to the best time to introduce solid foods, 37% didn’t recall being told to wait at least 4 months and preferably, 6 months. In fact, these mothers reported being advised to introduce solid foods before 6 months, said Andrea McGowan, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
The study was published in the Journal of Nutrition Education and Behavior.
“All in all, this research draws attention to certain nutrition guidance topics or subpopulations that might be prioritized to improve receipt and recall of guidance,” said Ms. McGowan, now a first-year medical student at the University of Michigan, Ann Arbor, in a podcast. “This research ... implores us to consider ways to revamp the existing standard practice for pediatric well-child care to improve recall of messages.”
The analysis also included data on mothers’ recall of advice on offering foods with different tastes and textures; offering a variety of fruits and vegetables; and limiting added sugar. More than half of mothers remembered discussing four or five child nutrition topics, but 31% recalled talking about only one or two. Offering a variety of fruits and vegetables had the highest percentage of recall.
The study wasn’t powered to determine whether the nutrition guidance provided at a well-child visit was not remembered or not provided, Ms. McGowan said, adding: “So exploring this is definitely the goal of future research.”
However, pediatricians report spending an average of 18 minutes with children and their parents, she noted. “This is definitely not enough time to cover every single topic a pediatrician or a parent might want to discuss.” Other barriers, such as a lack of insurance or transportation, may limit parents’ access to this kind of anticipatory guidance, the researchers said.
Priority should be given to certain topics and to certain mothers, they suggested. “Innovative strategies tailored to families’ needs might alleviate the HCP [health care provider] burden and could enhance parental recall, especially when messaging is culturally relevant and personalized,” Ms. McGowan said.
Two independent experts agreed in interviews. Pediatricians must do their best to tailor advice to each particular family so that parents can engage in the conversation, said Lauren Fiechtner, MD, director of the center for pediatric nutrition at Mass General for Children, Boston. “As the authors suggest, we should seek to understand the cultural relevance of our recommendations and to understand the barriers our patient families might face in implementing our advice,” said Dr. Fiechtner, who is also an assistant professor at Harvard Medical School, also in Boston.
“Much of the instructions we as pediatricians give to parents must be repeated and reinforced,” said Rebecca S. Fisk, MD, a pediatrician at Lenox Hill Hospital, Northwell Health, in New York. Often, the doctor’s advice runs counter to what family and friends recommend, she pointed out. Some parents may believe that “the baby who starts solid food earlier will sleep through the night earlier or that eating in front of the TV relaxes the child or allows them to eat more,” Dr. Fisk explained. In her practice, a nurse goes over her instructions, answers questions, and provides specific examples and written information.
Sometimes, even that’s not enough, Dr. Fisk admitted. “I, myself, have fielded many repeated questions about feeding, when to start, how much to give, and so on, despite printed guidance given to parents at well-child visits.”
This study was funded by the U.S. Centers for Disease Control and Prevention. Ms. McGowan and study coauthors reported having no potential conflicts of interest. Dr. Fiechtner and Dr. Fisk disclosed having no potential conflicts of interest.
Recent findings from a study examining mothers’ recall of doctors’ advice on early-child nutrition suggest that key feeding messages may not be heard, remembered, or even delivered.
During a typical child wellness visit, pediatricians provide parents with anticipatory guidance on all aspects of child development and safety, up to the age of 5 years.
The analysis of data from a subset of 1,302 mothers participating in the 2017-2019 National Survey of Family Growth showed that those older than 31 years of age and those who identified as non-Hispanic White were more likely to recall discussion of certain child nutrition topics compared with younger mothers or those who identified as Hispanic.
Of the six child-feeding topics referenced from the American Academy of Pediatrics’ “Bright Futures Guidelines,” less than half of the mothers, all of whom had a child between the ages of 6 months and 5 years, recalled guidance on limiting meals in front of the television or other electronic devices. Similarly, fewer than 50% remembered being told not to force their child to finish a bottle or food, the analysis showed.
When it came to the best time to introduce solid foods, 37% didn’t recall being told to wait at least 4 months and preferably, 6 months. In fact, these mothers reported being advised to introduce solid foods before 6 months, said Andrea McGowan, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
The study was published in the Journal of Nutrition Education and Behavior.
“All in all, this research draws attention to certain nutrition guidance topics or subpopulations that might be prioritized to improve receipt and recall of guidance,” said Ms. McGowan, now a first-year medical student at the University of Michigan, Ann Arbor, in a podcast. “This research ... implores us to consider ways to revamp the existing standard practice for pediatric well-child care to improve recall of messages.”
The analysis also included data on mothers’ recall of advice on offering foods with different tastes and textures; offering a variety of fruits and vegetables; and limiting added sugar. More than half of mothers remembered discussing four or five child nutrition topics, but 31% recalled talking about only one or two. Offering a variety of fruits and vegetables had the highest percentage of recall.
The study wasn’t powered to determine whether the nutrition guidance provided at a well-child visit was not remembered or not provided, Ms. McGowan said, adding: “So exploring this is definitely the goal of future research.”
However, pediatricians report spending an average of 18 minutes with children and their parents, she noted. “This is definitely not enough time to cover every single topic a pediatrician or a parent might want to discuss.” Other barriers, such as a lack of insurance or transportation, may limit parents’ access to this kind of anticipatory guidance, the researchers said.
Priority should be given to certain topics and to certain mothers, they suggested. “Innovative strategies tailored to families’ needs might alleviate the HCP [health care provider] burden and could enhance parental recall, especially when messaging is culturally relevant and personalized,” Ms. McGowan said.
Two independent experts agreed in interviews. Pediatricians must do their best to tailor advice to each particular family so that parents can engage in the conversation, said Lauren Fiechtner, MD, director of the center for pediatric nutrition at Mass General for Children, Boston. “As the authors suggest, we should seek to understand the cultural relevance of our recommendations and to understand the barriers our patient families might face in implementing our advice,” said Dr. Fiechtner, who is also an assistant professor at Harvard Medical School, also in Boston.
“Much of the instructions we as pediatricians give to parents must be repeated and reinforced,” said Rebecca S. Fisk, MD, a pediatrician at Lenox Hill Hospital, Northwell Health, in New York. Often, the doctor’s advice runs counter to what family and friends recommend, she pointed out. Some parents may believe that “the baby who starts solid food earlier will sleep through the night earlier or that eating in front of the TV relaxes the child or allows them to eat more,” Dr. Fisk explained. In her practice, a nurse goes over her instructions, answers questions, and provides specific examples and written information.
Sometimes, even that’s not enough, Dr. Fisk admitted. “I, myself, have fielded many repeated questions about feeding, when to start, how much to give, and so on, despite printed guidance given to parents at well-child visits.”
This study was funded by the U.S. Centers for Disease Control and Prevention. Ms. McGowan and study coauthors reported having no potential conflicts of interest. Dr. Fiechtner and Dr. Fisk disclosed having no potential conflicts of interest.
Recent findings from a study examining mothers’ recall of doctors’ advice on early-child nutrition suggest that key feeding messages may not be heard, remembered, or even delivered.
During a typical child wellness visit, pediatricians provide parents with anticipatory guidance on all aspects of child development and safety, up to the age of 5 years.
The analysis of data from a subset of 1,302 mothers participating in the 2017-2019 National Survey of Family Growth showed that those older than 31 years of age and those who identified as non-Hispanic White were more likely to recall discussion of certain child nutrition topics compared with younger mothers or those who identified as Hispanic.
Of the six child-feeding topics referenced from the American Academy of Pediatrics’ “Bright Futures Guidelines,” less than half of the mothers, all of whom had a child between the ages of 6 months and 5 years, recalled guidance on limiting meals in front of the television or other electronic devices. Similarly, fewer than 50% remembered being told not to force their child to finish a bottle or food, the analysis showed.
When it came to the best time to introduce solid foods, 37% didn’t recall being told to wait at least 4 months and preferably, 6 months. In fact, these mothers reported being advised to introduce solid foods before 6 months, said Andrea McGowan, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
The study was published in the Journal of Nutrition Education and Behavior.
“All in all, this research draws attention to certain nutrition guidance topics or subpopulations that might be prioritized to improve receipt and recall of guidance,” said Ms. McGowan, now a first-year medical student at the University of Michigan, Ann Arbor, in a podcast. “This research ... implores us to consider ways to revamp the existing standard practice for pediatric well-child care to improve recall of messages.”
The analysis also included data on mothers’ recall of advice on offering foods with different tastes and textures; offering a variety of fruits and vegetables; and limiting added sugar. More than half of mothers remembered discussing four or five child nutrition topics, but 31% recalled talking about only one or two. Offering a variety of fruits and vegetables had the highest percentage of recall.
The study wasn’t powered to determine whether the nutrition guidance provided at a well-child visit was not remembered or not provided, Ms. McGowan said, adding: “So exploring this is definitely the goal of future research.”
However, pediatricians report spending an average of 18 minutes with children and their parents, she noted. “This is definitely not enough time to cover every single topic a pediatrician or a parent might want to discuss.” Other barriers, such as a lack of insurance or transportation, may limit parents’ access to this kind of anticipatory guidance, the researchers said.
Priority should be given to certain topics and to certain mothers, they suggested. “Innovative strategies tailored to families’ needs might alleviate the HCP [health care provider] burden and could enhance parental recall, especially when messaging is culturally relevant and personalized,” Ms. McGowan said.
Two independent experts agreed in interviews. Pediatricians must do their best to tailor advice to each particular family so that parents can engage in the conversation, said Lauren Fiechtner, MD, director of the center for pediatric nutrition at Mass General for Children, Boston. “As the authors suggest, we should seek to understand the cultural relevance of our recommendations and to understand the barriers our patient families might face in implementing our advice,” said Dr. Fiechtner, who is also an assistant professor at Harvard Medical School, also in Boston.
“Much of the instructions we as pediatricians give to parents must be repeated and reinforced,” said Rebecca S. Fisk, MD, a pediatrician at Lenox Hill Hospital, Northwell Health, in New York. Often, the doctor’s advice runs counter to what family and friends recommend, she pointed out. Some parents may believe that “the baby who starts solid food earlier will sleep through the night earlier or that eating in front of the TV relaxes the child or allows them to eat more,” Dr. Fisk explained. In her practice, a nurse goes over her instructions, answers questions, and provides specific examples and written information.
Sometimes, even that’s not enough, Dr. Fisk admitted. “I, myself, have fielded many repeated questions about feeding, when to start, how much to give, and so on, despite printed guidance given to parents at well-child visits.”
This study was funded by the U.S. Centers for Disease Control and Prevention. Ms. McGowan and study coauthors reported having no potential conflicts of interest. Dr. Fiechtner and Dr. Fisk disclosed having no potential conflicts of interest.
With a little help from your friends
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Painful and Pruritic Eruptions on the Entire Body
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.
IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
A 36-year-old man presented with painful tender blisters and rashes on the entire body, including the ears and tongue. The rash began as a few pinpointed red dots on the abdomen, which subsequently increased in size and spread over the last week. He initially felt red and flushed and noticed new lesions appearing throughout the day. He did not attempt any specific treatment for these lesions. The patient tested positive for COVID-19 four months prior to the skin eruption. He denied systemic symptoms, smoking, or recent travel. He had no history of skin cancer, skin disorders, HIV, or hepatitis. He had no known medication allergies. Physical examination revealed multiple disseminated pustules on the ears, superficial ulcerations on the tongue, and blisters on the right lip. Few lesions were tender to the touch and drained clear fluid. Bacterial, viral, HIV, herpes, and rapid plasma reagin culture and laboratory screenings were negative. He was started on valaciclovir and cephalexin; however, no improvement was noticed. Punch biopsies were taken from the blisters on the chest and perilesional area.
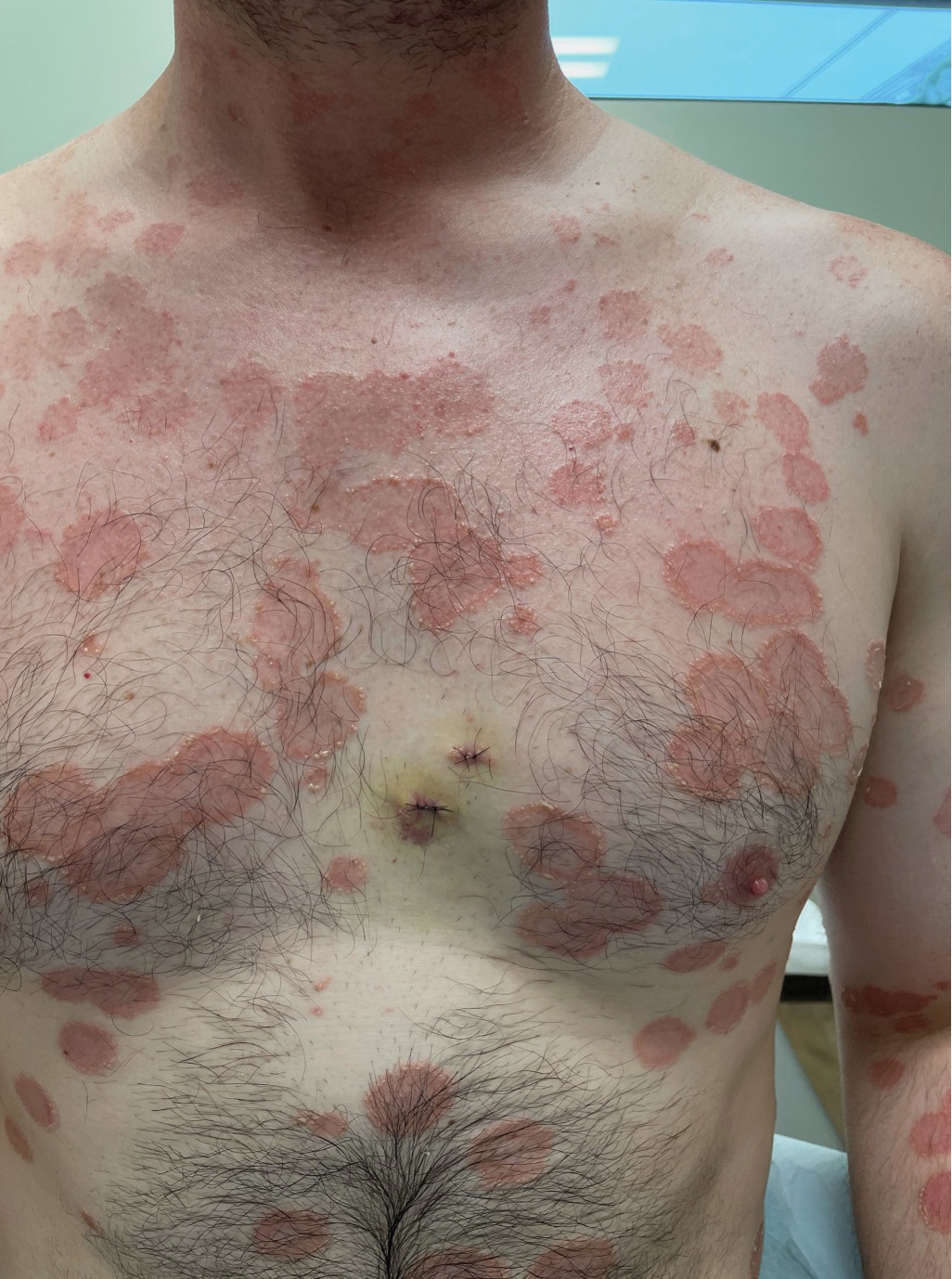
In rheumatoid arthritis, reducing inflammation reduces dementia risk
The incidence of dementia in patients with rheumatoid arthritis who took either a biologic disease-modifying antirheumatic drug (bDMARD) or targeted synthetic DMARD (tsDMARD) was significantly lower than the rate observed in patients who take only a conventional synthetic DMARD (csDMARD) in a national database study.
The work builds on previous research indicating a higher risk of Alzheimer’s disease and related dementias in people with RA. While joint pain and swelling are the cardinal symptoms of RA, its systemic inflammation leads to multiple systemic manifestations, offering biologically plausible links with cognitive decline. In addition, patients with RA have high prevalence of cardiovascular disease, diabetes, depression, disability, and physical inactivity, all of which are risk factors for dementia.
Chronic neuroinflammation secondary to either intrinsic or systemic stimuli is thought to play a key role in dementia development, especially Alzheimer’s dementia (AD). Research showing a role of tumor necrosis factor–alpha (TNF-alpha) in the development of dementia has piqued interest in a potential protective effect of TNF inhibitors. “TNF-alpha is thought to have an important role in different stages of the pathophysiology and disease progression of Alzheimer’s disease,” study first author Sebastian E. Sattui, MD, assistant professor of medicine at the University of Pittsburgh and director of the University of Pittsburgh Vasculitis Center, said in an interview. “Animal models have shown that TNF inhibition reduces microgliosis, neuronal loss, and tau phosphorylation. Cognitive improvement has been seen in two trials with Alzheimer’s disease patients, but were not in rheumatoid arthritis patients.”
In the newest study, published online in Seminars in Arthritis and Rheumatism, Dr. Sattui and colleagues suggest that a lower risk for dementia seen with bDMARDs and tsDMARDs may be attributable to an overall greater decrease in inflammation rather than any mechanism of action specific to these drugs.
In the study of Centers for Medicare & Medicaid Services claims during 2006-2017 for 141,326 adult patients with RA, the crude incident rates were 2.0 per 100 person-years (95% confidence interval, 1.9-2.1) for patients on csDMARDs and 1.3 (95% CI, 1.2-1.4) for patients on any b/tsDMARD. There were 3,794 cases of incident dementia during follow-up among 233,271 initiations of any DMARD. The adjusted risk for dementia among users of bDMARDs or tsDMARDs was 19% lower than the adjusted risk for patients on csDMARDs (hazard ratio, 0.81; 95% CI, 0.76-0.87). No significant differences were found between classes of bDMARDs or tsDMARDs.
Dr. Sattui and coauthors’ investigation included adults aged at least 40 years with two RA diagnoses by a rheumatologist more than 7 and less than 365 days apart. Those with prior dementia diagnoses were excluded. Their analysis found the risk of incident dementia to be comparable between patients receiving TNF inhibitors (HR, 0.86; 95% CI, 0.80-0.93), non-TNFi bDMARDs (HR, 0.76; 95% CI, 0.70-0.83), and tsDMARDs (HR, 0.69; 95% CI, 0.53-0.90), with csDMARDs as the referent. A second subgroup analysis looking at patients with prior methotrexate use who were taking bDMARDs or tsDMARDs revealed similar decreases in risk of incident dementia, compared with patients taking bDMARDs or tsDMARDs along with methotrexate at baseline.
“NSAIDs and glucocorticoids have been studied in RCTs [randomized, controlled trials],” Dr. Sattui said in the interview. “Despite initial observational data that showed some signal for improvement, no benefit was observed in either of the RCTs. Other agents with possible anti-inflammatory effects and more benign profiles, such as curcumin, are being studied. There are also ongoing trials looking into the use of JAK [Janus kinase] inhibitors or [interleukin]-1 inhibition in dementia.”
He added: “There is a need to better study the association between cognition and disease activity, as well as treat-to-target strategies, prospectively in patients with RA. It is important to also acknowledge that any of these findings might be just specific for RA, so extrapolation to non-RA individuals might be limited.”
In commenting on the findings of the study, Rishi J. Desai, PhD, assistant professor of medicine in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, said that “superior inflammation control with biologics or targeted DMARDs is an interesting hypothesis explaining the observed findings. It merits further investigation and replication in diverse populations.” He added: “It should be noted that a key challenge in evaluating this hypothesis using insurance claims data is unavailability of some important factors such as socioeconomic status and patient frailty. These may be driving treatment selection between conventional DMARDs, which are cheaper with more benign adverse-event profiles, and biologic or targeted DMARDs, which are more expensive with a less favorable adverse-event profile.”
Prior research
Several studies have investigated the effect of DMARDs, including bDMARDs like tumor necrosis factor inhibitors, on incident dementia in patients with RA.
Among this research is a study by Dr. Desai and colleagues that looked at comparative risk of AD and related dementia in 22,569 Medicare beneficiaries receiving tofacitinib (a JAK inhibitor), tocilizumab (an IL-6 inhibitor), or TNF inhibitors in comparison with abatacept (a T-cell activation inhibitor). No differentiating risk associations were found in this cohort study.
Other past studies include:
- A study comparing about 21,000 patients with RA and a non-RA cohort of about 62,000 found a 37% reduction in dementia development among RA patients receiving DMARDs. The effect was dose dependent, greater with high cumulative dosages, and was found in both men and women and in subgroups younger and older than 65 years.
- A retrospective study of electronic health records from 56 million adult patients identified a subset of patients with RA, psoriasis, ankylosing spondylitis, ulcerative colitis, or Crohn’s disease in whom systemic inflammation increased risk for AD through a mechanism involving TNF. The risk for AD in patients was lowered by treatment with etanercept, adalimumab, infliximab, or methotrexate, with larger reductions observed in younger patients than in older patients receiving TNF blockers.
- A propensity score–matched retrospective cohort study in 2,510 U.S. veterans with RA found that use of a TNF inhibitor reduced the risk of dementia by 36%, compared with control patients (HR, 0.64; 95% CI, 0.52-0.80), and the effect was consistent over 5-20 years post RA diagnosis.
- In a retrospective, multinational, matched, case-control study of patients older than 50 years with RA, prior methotrexate use was associated with lower dementia risk (OR, 0.71; 95% CI, 0.52-0.98). Use of methotrexate longer than 4 years demonstrated the lowest dementia risk (odds ratio, 0.37; 95% CI, 0.17-0.79).
These past studies, Dr. Sattui and colleagues pointed out, have multiple shortcomings, including case-control design, different definitions of exposure or outcomes, and inadequate control of confounders, underscoring the need for more rigorous studies.
Several authors of the CMS claims study disclosed research support, grants, and consulting fees from pharmaceutical companies. The research was supported by a grant from the National Institutes of Health. Dr. Desai disclosed that he has received funding from the National Institute on Aging for drug repurposing studies of dementia.
The incidence of dementia in patients with rheumatoid arthritis who took either a biologic disease-modifying antirheumatic drug (bDMARD) or targeted synthetic DMARD (tsDMARD) was significantly lower than the rate observed in patients who take only a conventional synthetic DMARD (csDMARD) in a national database study.
The work builds on previous research indicating a higher risk of Alzheimer’s disease and related dementias in people with RA. While joint pain and swelling are the cardinal symptoms of RA, its systemic inflammation leads to multiple systemic manifestations, offering biologically plausible links with cognitive decline. In addition, patients with RA have high prevalence of cardiovascular disease, diabetes, depression, disability, and physical inactivity, all of which are risk factors for dementia.
Chronic neuroinflammation secondary to either intrinsic or systemic stimuli is thought to play a key role in dementia development, especially Alzheimer’s dementia (AD). Research showing a role of tumor necrosis factor–alpha (TNF-alpha) in the development of dementia has piqued interest in a potential protective effect of TNF inhibitors. “TNF-alpha is thought to have an important role in different stages of the pathophysiology and disease progression of Alzheimer’s disease,” study first author Sebastian E. Sattui, MD, assistant professor of medicine at the University of Pittsburgh and director of the University of Pittsburgh Vasculitis Center, said in an interview. “Animal models have shown that TNF inhibition reduces microgliosis, neuronal loss, and tau phosphorylation. Cognitive improvement has been seen in two trials with Alzheimer’s disease patients, but were not in rheumatoid arthritis patients.”
In the newest study, published online in Seminars in Arthritis and Rheumatism, Dr. Sattui and colleagues suggest that a lower risk for dementia seen with bDMARDs and tsDMARDs may be attributable to an overall greater decrease in inflammation rather than any mechanism of action specific to these drugs.
In the study of Centers for Medicare & Medicaid Services claims during 2006-2017 for 141,326 adult patients with RA, the crude incident rates were 2.0 per 100 person-years (95% confidence interval, 1.9-2.1) for patients on csDMARDs and 1.3 (95% CI, 1.2-1.4) for patients on any b/tsDMARD. There were 3,794 cases of incident dementia during follow-up among 233,271 initiations of any DMARD. The adjusted risk for dementia among users of bDMARDs or tsDMARDs was 19% lower than the adjusted risk for patients on csDMARDs (hazard ratio, 0.81; 95% CI, 0.76-0.87). No significant differences were found between classes of bDMARDs or tsDMARDs.
Dr. Sattui and coauthors’ investigation included adults aged at least 40 years with two RA diagnoses by a rheumatologist more than 7 and less than 365 days apart. Those with prior dementia diagnoses were excluded. Their analysis found the risk of incident dementia to be comparable between patients receiving TNF inhibitors (HR, 0.86; 95% CI, 0.80-0.93), non-TNFi bDMARDs (HR, 0.76; 95% CI, 0.70-0.83), and tsDMARDs (HR, 0.69; 95% CI, 0.53-0.90), with csDMARDs as the referent. A second subgroup analysis looking at patients with prior methotrexate use who were taking bDMARDs or tsDMARDs revealed similar decreases in risk of incident dementia, compared with patients taking bDMARDs or tsDMARDs along with methotrexate at baseline.
“NSAIDs and glucocorticoids have been studied in RCTs [randomized, controlled trials],” Dr. Sattui said in the interview. “Despite initial observational data that showed some signal for improvement, no benefit was observed in either of the RCTs. Other agents with possible anti-inflammatory effects and more benign profiles, such as curcumin, are being studied. There are also ongoing trials looking into the use of JAK [Janus kinase] inhibitors or [interleukin]-1 inhibition in dementia.”
He added: “There is a need to better study the association between cognition and disease activity, as well as treat-to-target strategies, prospectively in patients with RA. It is important to also acknowledge that any of these findings might be just specific for RA, so extrapolation to non-RA individuals might be limited.”
In commenting on the findings of the study, Rishi J. Desai, PhD, assistant professor of medicine in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, said that “superior inflammation control with biologics or targeted DMARDs is an interesting hypothesis explaining the observed findings. It merits further investigation and replication in diverse populations.” He added: “It should be noted that a key challenge in evaluating this hypothesis using insurance claims data is unavailability of some important factors such as socioeconomic status and patient frailty. These may be driving treatment selection between conventional DMARDs, which are cheaper with more benign adverse-event profiles, and biologic or targeted DMARDs, which are more expensive with a less favorable adverse-event profile.”
Prior research
Several studies have investigated the effect of DMARDs, including bDMARDs like tumor necrosis factor inhibitors, on incident dementia in patients with RA.
Among this research is a study by Dr. Desai and colleagues that looked at comparative risk of AD and related dementia in 22,569 Medicare beneficiaries receiving tofacitinib (a JAK inhibitor), tocilizumab (an IL-6 inhibitor), or TNF inhibitors in comparison with abatacept (a T-cell activation inhibitor). No differentiating risk associations were found in this cohort study.
Other past studies include:
- A study comparing about 21,000 patients with RA and a non-RA cohort of about 62,000 found a 37% reduction in dementia development among RA patients receiving DMARDs. The effect was dose dependent, greater with high cumulative dosages, and was found in both men and women and in subgroups younger and older than 65 years.
- A retrospective study of electronic health records from 56 million adult patients identified a subset of patients with RA, psoriasis, ankylosing spondylitis, ulcerative colitis, or Crohn’s disease in whom systemic inflammation increased risk for AD through a mechanism involving TNF. The risk for AD in patients was lowered by treatment with etanercept, adalimumab, infliximab, or methotrexate, with larger reductions observed in younger patients than in older patients receiving TNF blockers.
- A propensity score–matched retrospective cohort study in 2,510 U.S. veterans with RA found that use of a TNF inhibitor reduced the risk of dementia by 36%, compared with control patients (HR, 0.64; 95% CI, 0.52-0.80), and the effect was consistent over 5-20 years post RA diagnosis.
- In a retrospective, multinational, matched, case-control study of patients older than 50 years with RA, prior methotrexate use was associated with lower dementia risk (OR, 0.71; 95% CI, 0.52-0.98). Use of methotrexate longer than 4 years demonstrated the lowest dementia risk (odds ratio, 0.37; 95% CI, 0.17-0.79).
These past studies, Dr. Sattui and colleagues pointed out, have multiple shortcomings, including case-control design, different definitions of exposure or outcomes, and inadequate control of confounders, underscoring the need for more rigorous studies.
Several authors of the CMS claims study disclosed research support, grants, and consulting fees from pharmaceutical companies. The research was supported by a grant from the National Institutes of Health. Dr. Desai disclosed that he has received funding from the National Institute on Aging for drug repurposing studies of dementia.
The incidence of dementia in patients with rheumatoid arthritis who took either a biologic disease-modifying antirheumatic drug (bDMARD) or targeted synthetic DMARD (tsDMARD) was significantly lower than the rate observed in patients who take only a conventional synthetic DMARD (csDMARD) in a national database study.
The work builds on previous research indicating a higher risk of Alzheimer’s disease and related dementias in people with RA. While joint pain and swelling are the cardinal symptoms of RA, its systemic inflammation leads to multiple systemic manifestations, offering biologically plausible links with cognitive decline. In addition, patients with RA have high prevalence of cardiovascular disease, diabetes, depression, disability, and physical inactivity, all of which are risk factors for dementia.
Chronic neuroinflammation secondary to either intrinsic or systemic stimuli is thought to play a key role in dementia development, especially Alzheimer’s dementia (AD). Research showing a role of tumor necrosis factor–alpha (TNF-alpha) in the development of dementia has piqued interest in a potential protective effect of TNF inhibitors. “TNF-alpha is thought to have an important role in different stages of the pathophysiology and disease progression of Alzheimer’s disease,” study first author Sebastian E. Sattui, MD, assistant professor of medicine at the University of Pittsburgh and director of the University of Pittsburgh Vasculitis Center, said in an interview. “Animal models have shown that TNF inhibition reduces microgliosis, neuronal loss, and tau phosphorylation. Cognitive improvement has been seen in two trials with Alzheimer’s disease patients, but were not in rheumatoid arthritis patients.”
In the newest study, published online in Seminars in Arthritis and Rheumatism, Dr. Sattui and colleagues suggest that a lower risk for dementia seen with bDMARDs and tsDMARDs may be attributable to an overall greater decrease in inflammation rather than any mechanism of action specific to these drugs.
In the study of Centers for Medicare & Medicaid Services claims during 2006-2017 for 141,326 adult patients with RA, the crude incident rates were 2.0 per 100 person-years (95% confidence interval, 1.9-2.1) for patients on csDMARDs and 1.3 (95% CI, 1.2-1.4) for patients on any b/tsDMARD. There were 3,794 cases of incident dementia during follow-up among 233,271 initiations of any DMARD. The adjusted risk for dementia among users of bDMARDs or tsDMARDs was 19% lower than the adjusted risk for patients on csDMARDs (hazard ratio, 0.81; 95% CI, 0.76-0.87). No significant differences were found between classes of bDMARDs or tsDMARDs.
Dr. Sattui and coauthors’ investigation included adults aged at least 40 years with two RA diagnoses by a rheumatologist more than 7 and less than 365 days apart. Those with prior dementia diagnoses were excluded. Their analysis found the risk of incident dementia to be comparable between patients receiving TNF inhibitors (HR, 0.86; 95% CI, 0.80-0.93), non-TNFi bDMARDs (HR, 0.76; 95% CI, 0.70-0.83), and tsDMARDs (HR, 0.69; 95% CI, 0.53-0.90), with csDMARDs as the referent. A second subgroup analysis looking at patients with prior methotrexate use who were taking bDMARDs or tsDMARDs revealed similar decreases in risk of incident dementia, compared with patients taking bDMARDs or tsDMARDs along with methotrexate at baseline.
“NSAIDs and glucocorticoids have been studied in RCTs [randomized, controlled trials],” Dr. Sattui said in the interview. “Despite initial observational data that showed some signal for improvement, no benefit was observed in either of the RCTs. Other agents with possible anti-inflammatory effects and more benign profiles, such as curcumin, are being studied. There are also ongoing trials looking into the use of JAK [Janus kinase] inhibitors or [interleukin]-1 inhibition in dementia.”
He added: “There is a need to better study the association between cognition and disease activity, as well as treat-to-target strategies, prospectively in patients with RA. It is important to also acknowledge that any of these findings might be just specific for RA, so extrapolation to non-RA individuals might be limited.”
In commenting on the findings of the study, Rishi J. Desai, PhD, assistant professor of medicine in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston, said that “superior inflammation control with biologics or targeted DMARDs is an interesting hypothesis explaining the observed findings. It merits further investigation and replication in diverse populations.” He added: “It should be noted that a key challenge in evaluating this hypothesis using insurance claims data is unavailability of some important factors such as socioeconomic status and patient frailty. These may be driving treatment selection between conventional DMARDs, which are cheaper with more benign adverse-event profiles, and biologic or targeted DMARDs, which are more expensive with a less favorable adverse-event profile.”
Prior research
Several studies have investigated the effect of DMARDs, including bDMARDs like tumor necrosis factor inhibitors, on incident dementia in patients with RA.
Among this research is a study by Dr. Desai and colleagues that looked at comparative risk of AD and related dementia in 22,569 Medicare beneficiaries receiving tofacitinib (a JAK inhibitor), tocilizumab (an IL-6 inhibitor), or TNF inhibitors in comparison with abatacept (a T-cell activation inhibitor). No differentiating risk associations were found in this cohort study.
Other past studies include:
- A study comparing about 21,000 patients with RA and a non-RA cohort of about 62,000 found a 37% reduction in dementia development among RA patients receiving DMARDs. The effect was dose dependent, greater with high cumulative dosages, and was found in both men and women and in subgroups younger and older than 65 years.
- A retrospective study of electronic health records from 56 million adult patients identified a subset of patients with RA, psoriasis, ankylosing spondylitis, ulcerative colitis, or Crohn’s disease in whom systemic inflammation increased risk for AD through a mechanism involving TNF. The risk for AD in patients was lowered by treatment with etanercept, adalimumab, infliximab, or methotrexate, with larger reductions observed in younger patients than in older patients receiving TNF blockers.
- A propensity score–matched retrospective cohort study in 2,510 U.S. veterans with RA found that use of a TNF inhibitor reduced the risk of dementia by 36%, compared with control patients (HR, 0.64; 95% CI, 0.52-0.80), and the effect was consistent over 5-20 years post RA diagnosis.
- In a retrospective, multinational, matched, case-control study of patients older than 50 years with RA, prior methotrexate use was associated with lower dementia risk (OR, 0.71; 95% CI, 0.52-0.98). Use of methotrexate longer than 4 years demonstrated the lowest dementia risk (odds ratio, 0.37; 95% CI, 0.17-0.79).
These past studies, Dr. Sattui and colleagues pointed out, have multiple shortcomings, including case-control design, different definitions of exposure or outcomes, and inadequate control of confounders, underscoring the need for more rigorous studies.
Several authors of the CMS claims study disclosed research support, grants, and consulting fees from pharmaceutical companies. The research was supported by a grant from the National Institutes of Health. Dr. Desai disclosed that he has received funding from the National Institute on Aging for drug repurposing studies of dementia.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Red blood cells made to deliver antibiotics to bacteria
Over several years, “we developed experimental and computational techniques to study how proteins and drugs interact with membranes,” Hannah Krivić, a graduate student, and Maikel C. Rheinstädter, PhD, a professor of physics, both at McMaster University in Hamilton, Ont., told this news organization.
In earlier work, these researchers investigated how antibiotics target bacterial membranes and how those membranes enable the development of antibiotic resistance. Then, they said, “we started to ... manipulate membranes by tuning their properties [with] synthetic lipid molecules to create ‘hybrid’ membranes – that is, functionalized biological membranes with optimized properties.
“We are now using this approach to functionalize red blood cells by using them as drug carriers. We optimize these cells to carry certain loads, such as drug molecules, and anchor proteins in their membranes that target receptors in bacteria to selectively and efficiently deliver that load.”
The strategy, they said, “has become a universal red blood cell–based delivery platform that we call ‘smart blood’ ... that can safely and selectively deliver antibiotics to certain bacterial targets.”
The platform currently is being tested in vitro, and in vivo testing is slated to begin in early 2023. Their study was published online in ACS Infectious Diseases.
Optimizing dosing
Polymyxin B (PmB) is one of a few potent antibiotics with promising efficacy against drug-resistant bacteria such as E. coli. PmB, however, is widely considered a treatment of last resort because of its toxic side effects (which include nephrotoxicity, neurotoxicity, and neuromuscular blockade) particularly at higher doses.
The researchers hypothesized that targeted delivery of PmB might lead to optimized dosing and potentially reduce the need for higher or repeated doses. In the current study, they tested the ability of the smart blood platform to deliver PmB to E. coli.
Creating “erythro-PmBs” involves removing the inner components of red blood cells, loading the cells with PmB, and coating the cell membranes (liposomes) with antibacterial (in this case, anti–E. coli) antibodies.
The in vitro experiments showed that the cells could be loaded with PmB and retain and selectively deliver the drug to E. coli, with no apparent hemolytic activity or nephrotoxicity. Specifically, the erythro-PmBs had a loading efficiency of approximately 90% and delivered PmB to E. coli with values for the minimum inhibitory concentration that were comparable with those of free PmB.
“In contrast to drug-delivery systems based on synthetic carriers, our erythrocytes have a high biocompatibility and can stay in circulation in the body for several weeks to provide a sustained and targeted release of the drug,” said Ms. Krivić and Dr. Rheinstädter. “This [profile] can make existing drugs safer by, for instance, increasing their efficacy while at the same time lowering the required dosage, thereby reducing side effects.”
The researchers are now exploring the ability of the smart blood platform to deliver neurotrophic factors across the blood-brain barrier to potentially treat neurodegenerative diseases. Their approach is identical to that used in the current study, they said, except in this case, the red blood cell membranes are designed to deliver neurotrophic factors specifically to the blood-brain barrier.
‘Certainly promising’
David W. Deamer, PhD, research professor of biomolecular engineering at the University of California, Santa Cruz, said in a comment: “This is certainly promising. The erythro-PmBs have a greater loading capacity and longer circulation time than ordinary liposomes used for drug delivery. They can also be prepared with specific antibodies so that the antibiotic is delivered more directly when they bind to bacterial pathogens.”
The effect on bacterial growth, however, was tested in a model system, not in an actual infection, he said, adding that an important next step will be to perform animal testing. “One of the simplest tests is induced sepsis in mice, which mimics a burst appendix. If the erythro-PmBs can treat sepsis effectively, it will be an encouraging sign that they have potential therapeutic value. It will also be interesting to see whether the antigens responsible for ABO blood groups are retained on the surfaces of the erythro-PmBs. If they are, it might be necessary to match donor blood to that of the recipient.
“Getting a product ready for clinical use will require partnership with a major pharmaceutical firm, several years of animal testing, and then several more years carrying out phase 1, 2, and 3 clinical trials in human patients,” Dr. Deamer concluded.
No commercial funding was disclosed. Ms. Krivić, Dr. Rheinstädter, and Dr. Deamer reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Over several years, “we developed experimental and computational techniques to study how proteins and drugs interact with membranes,” Hannah Krivić, a graduate student, and Maikel C. Rheinstädter, PhD, a professor of physics, both at McMaster University in Hamilton, Ont., told this news organization.
In earlier work, these researchers investigated how antibiotics target bacterial membranes and how those membranes enable the development of antibiotic resistance. Then, they said, “we started to ... manipulate membranes by tuning their properties [with] synthetic lipid molecules to create ‘hybrid’ membranes – that is, functionalized biological membranes with optimized properties.
“We are now using this approach to functionalize red blood cells by using them as drug carriers. We optimize these cells to carry certain loads, such as drug molecules, and anchor proteins in their membranes that target receptors in bacteria to selectively and efficiently deliver that load.”
The strategy, they said, “has become a universal red blood cell–based delivery platform that we call ‘smart blood’ ... that can safely and selectively deliver antibiotics to certain bacterial targets.”
The platform currently is being tested in vitro, and in vivo testing is slated to begin in early 2023. Their study was published online in ACS Infectious Diseases.
Optimizing dosing
Polymyxin B (PmB) is one of a few potent antibiotics with promising efficacy against drug-resistant bacteria such as E. coli. PmB, however, is widely considered a treatment of last resort because of its toxic side effects (which include nephrotoxicity, neurotoxicity, and neuromuscular blockade) particularly at higher doses.
The researchers hypothesized that targeted delivery of PmB might lead to optimized dosing and potentially reduce the need for higher or repeated doses. In the current study, they tested the ability of the smart blood platform to deliver PmB to E. coli.
Creating “erythro-PmBs” involves removing the inner components of red blood cells, loading the cells with PmB, and coating the cell membranes (liposomes) with antibacterial (in this case, anti–E. coli) antibodies.
The in vitro experiments showed that the cells could be loaded with PmB and retain and selectively deliver the drug to E. coli, with no apparent hemolytic activity or nephrotoxicity. Specifically, the erythro-PmBs had a loading efficiency of approximately 90% and delivered PmB to E. coli with values for the minimum inhibitory concentration that were comparable with those of free PmB.
“In contrast to drug-delivery systems based on synthetic carriers, our erythrocytes have a high biocompatibility and can stay in circulation in the body for several weeks to provide a sustained and targeted release of the drug,” said Ms. Krivić and Dr. Rheinstädter. “This [profile] can make existing drugs safer by, for instance, increasing their efficacy while at the same time lowering the required dosage, thereby reducing side effects.”
The researchers are now exploring the ability of the smart blood platform to deliver neurotrophic factors across the blood-brain barrier to potentially treat neurodegenerative diseases. Their approach is identical to that used in the current study, they said, except in this case, the red blood cell membranes are designed to deliver neurotrophic factors specifically to the blood-brain barrier.
‘Certainly promising’
David W. Deamer, PhD, research professor of biomolecular engineering at the University of California, Santa Cruz, said in a comment: “This is certainly promising. The erythro-PmBs have a greater loading capacity and longer circulation time than ordinary liposomes used for drug delivery. They can also be prepared with specific antibodies so that the antibiotic is delivered more directly when they bind to bacterial pathogens.”
The effect on bacterial growth, however, was tested in a model system, not in an actual infection, he said, adding that an important next step will be to perform animal testing. “One of the simplest tests is induced sepsis in mice, which mimics a burst appendix. If the erythro-PmBs can treat sepsis effectively, it will be an encouraging sign that they have potential therapeutic value. It will also be interesting to see whether the antigens responsible for ABO blood groups are retained on the surfaces of the erythro-PmBs. If they are, it might be necessary to match donor blood to that of the recipient.
“Getting a product ready for clinical use will require partnership with a major pharmaceutical firm, several years of animal testing, and then several more years carrying out phase 1, 2, and 3 clinical trials in human patients,” Dr. Deamer concluded.
No commercial funding was disclosed. Ms. Krivić, Dr. Rheinstädter, and Dr. Deamer reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Over several years, “we developed experimental and computational techniques to study how proteins and drugs interact with membranes,” Hannah Krivić, a graduate student, and Maikel C. Rheinstädter, PhD, a professor of physics, both at McMaster University in Hamilton, Ont., told this news organization.
In earlier work, these researchers investigated how antibiotics target bacterial membranes and how those membranes enable the development of antibiotic resistance. Then, they said, “we started to ... manipulate membranes by tuning their properties [with] synthetic lipid molecules to create ‘hybrid’ membranes – that is, functionalized biological membranes with optimized properties.
“We are now using this approach to functionalize red blood cells by using them as drug carriers. We optimize these cells to carry certain loads, such as drug molecules, and anchor proteins in their membranes that target receptors in bacteria to selectively and efficiently deliver that load.”
The strategy, they said, “has become a universal red blood cell–based delivery platform that we call ‘smart blood’ ... that can safely and selectively deliver antibiotics to certain bacterial targets.”
The platform currently is being tested in vitro, and in vivo testing is slated to begin in early 2023. Their study was published online in ACS Infectious Diseases.
Optimizing dosing
Polymyxin B (PmB) is one of a few potent antibiotics with promising efficacy against drug-resistant bacteria such as E. coli. PmB, however, is widely considered a treatment of last resort because of its toxic side effects (which include nephrotoxicity, neurotoxicity, and neuromuscular blockade) particularly at higher doses.
The researchers hypothesized that targeted delivery of PmB might lead to optimized dosing and potentially reduce the need for higher or repeated doses. In the current study, they tested the ability of the smart blood platform to deliver PmB to E. coli.
Creating “erythro-PmBs” involves removing the inner components of red blood cells, loading the cells with PmB, and coating the cell membranes (liposomes) with antibacterial (in this case, anti–E. coli) antibodies.
The in vitro experiments showed that the cells could be loaded with PmB and retain and selectively deliver the drug to E. coli, with no apparent hemolytic activity or nephrotoxicity. Specifically, the erythro-PmBs had a loading efficiency of approximately 90% and delivered PmB to E. coli with values for the minimum inhibitory concentration that were comparable with those of free PmB.
“In contrast to drug-delivery systems based on synthetic carriers, our erythrocytes have a high biocompatibility and can stay in circulation in the body for several weeks to provide a sustained and targeted release of the drug,” said Ms. Krivić and Dr. Rheinstädter. “This [profile] can make existing drugs safer by, for instance, increasing their efficacy while at the same time lowering the required dosage, thereby reducing side effects.”
The researchers are now exploring the ability of the smart blood platform to deliver neurotrophic factors across the blood-brain barrier to potentially treat neurodegenerative diseases. Their approach is identical to that used in the current study, they said, except in this case, the red blood cell membranes are designed to deliver neurotrophic factors specifically to the blood-brain barrier.
‘Certainly promising’
David W. Deamer, PhD, research professor of biomolecular engineering at the University of California, Santa Cruz, said in a comment: “This is certainly promising. The erythro-PmBs have a greater loading capacity and longer circulation time than ordinary liposomes used for drug delivery. They can also be prepared with specific antibodies so that the antibiotic is delivered more directly when they bind to bacterial pathogens.”
The effect on bacterial growth, however, was tested in a model system, not in an actual infection, he said, adding that an important next step will be to perform animal testing. “One of the simplest tests is induced sepsis in mice, which mimics a burst appendix. If the erythro-PmBs can treat sepsis effectively, it will be an encouraging sign that they have potential therapeutic value. It will also be interesting to see whether the antigens responsible for ABO blood groups are retained on the surfaces of the erythro-PmBs. If they are, it might be necessary to match donor blood to that of the recipient.
“Getting a product ready for clinical use will require partnership with a major pharmaceutical firm, several years of animal testing, and then several more years carrying out phase 1, 2, and 3 clinical trials in human patients,” Dr. Deamer concluded.
No commercial funding was disclosed. Ms. Krivić, Dr. Rheinstädter, and Dr. Deamer reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ACS INFECTIOUS DISEASES
Doc trains family physicians in vasectomy care
One physician has made it his mission to help.
Charles W. Monteith Jr., MD, medical director of his own practice in Raleigh, N.C., said that before the Court’s decision in Dobbs v. Jackson , he was booked “2-3 weeks” in advance for vasectomies.
“Now I am booked out 3 months,” he said.
In September, Dr. Monteith launched a training program for physicians interested in providing vasectomies in their offices. The course, which Dr. Monteith conceived in 2021 before the Supreme Court’s latest ruling, offers one-on-one training and mentorship for physicians who want to learn to perform minimally invasive vasectomies under local anesthesia.
In addition to training, Dr. Monteith provides all the necessary equipment, including eye loupes, exam room surgical furniture, and instrument sterilization system. The program can be completed over 4 weekends and costs $38,000; participants typically perform 40 vasectomy procedures during the training period.
Dr. Monteith, who trained in obstetrics and gynecology, said that he has performed over 7,000 no-scalpel vasectomies since 2008.
Requests for vasectomy consultations at the end of June – when the Dobbs decision was announced – came from men of all ages but particularly from younger men with fewer than two children, Dr. Monteith said.
Prior to the ruling, men with no children accounted for 10% of his patient roster; now, he added, “some days, it is 80%.”
With the increase in demand came a unique opportunity for more doctors to offer the service. The majority of vasectomies in the United States, around 75%, are performed by urologists, but 25% are performed by specialists in family medicine or general surgery.
Some research shows that urologists are typically unwilling to train family physicians on the procedure, citing concerns over competition and not enough cases to go around. But Doug Stein, MD, a urologist and director of Vasectomy and Reversal Centers of Florida in Tampa, offers a similar training for physicians, most of whom are family physicians. Opening the door for more men to get a vasectomy may be a net good, according to Dr. Stein.
“There’s a lot of trust required for vasectomy,” Dr. Stein noted. “Men are probably more likely to go to their family medicine doctor,” that they have a rapport with than a specialist they’ve never met.
Alex Shteynshlyuger, MD, director of urology at New York Urology Specialists, said that he supports family physicians performing vasectomies. However, he cautioned that like any other procedure, complications can arise, and thorough training is essential.
“While complications are not common, they do occur, including pain, bleeding, infection, granuloma formation, and fistula tract,” Dr. Shteynshlyuger said. Family physicians must also know when to refer patients to a specialist.
Dr. Monteith said that safety considerations are why he designed his training program for clinicians who want to offer 10-20 vasectomies per week.
Dr. Monteith sees his work in teaching family care physicians on how to perform vasectomies similar to his previous role as medical director of Planned Parenthood of Central North Carolina. There, he helped provide family planning options, mostly to women. Now, he offers the options to men.
“Most of our public health efforts seem to be focused on female reproduction,” Dr. Monteith said. “It is never a good idea to let specialists be the main providers of a preventive healthcare treatment or service, kind of like only allowing patients to go to a cardiologist to get a prescription for cholesterol medication. I needed to do what I could do to increase the number of providers offering easier access to vasectomy.”
A version of this article first appeared on Medscape.com.
One physician has made it his mission to help.
Charles W. Monteith Jr., MD, medical director of his own practice in Raleigh, N.C., said that before the Court’s decision in Dobbs v. Jackson , he was booked “2-3 weeks” in advance for vasectomies.
“Now I am booked out 3 months,” he said.
In September, Dr. Monteith launched a training program for physicians interested in providing vasectomies in their offices. The course, which Dr. Monteith conceived in 2021 before the Supreme Court’s latest ruling, offers one-on-one training and mentorship for physicians who want to learn to perform minimally invasive vasectomies under local anesthesia.
In addition to training, Dr. Monteith provides all the necessary equipment, including eye loupes, exam room surgical furniture, and instrument sterilization system. The program can be completed over 4 weekends and costs $38,000; participants typically perform 40 vasectomy procedures during the training period.
Dr. Monteith, who trained in obstetrics and gynecology, said that he has performed over 7,000 no-scalpel vasectomies since 2008.
Requests for vasectomy consultations at the end of June – when the Dobbs decision was announced – came from men of all ages but particularly from younger men with fewer than two children, Dr. Monteith said.
Prior to the ruling, men with no children accounted for 10% of his patient roster; now, he added, “some days, it is 80%.”
With the increase in demand came a unique opportunity for more doctors to offer the service. The majority of vasectomies in the United States, around 75%, are performed by urologists, but 25% are performed by specialists in family medicine or general surgery.
Some research shows that urologists are typically unwilling to train family physicians on the procedure, citing concerns over competition and not enough cases to go around. But Doug Stein, MD, a urologist and director of Vasectomy and Reversal Centers of Florida in Tampa, offers a similar training for physicians, most of whom are family physicians. Opening the door for more men to get a vasectomy may be a net good, according to Dr. Stein.
“There’s a lot of trust required for vasectomy,” Dr. Stein noted. “Men are probably more likely to go to their family medicine doctor,” that they have a rapport with than a specialist they’ve never met.
Alex Shteynshlyuger, MD, director of urology at New York Urology Specialists, said that he supports family physicians performing vasectomies. However, he cautioned that like any other procedure, complications can arise, and thorough training is essential.
“While complications are not common, they do occur, including pain, bleeding, infection, granuloma formation, and fistula tract,” Dr. Shteynshlyuger said. Family physicians must also know when to refer patients to a specialist.
Dr. Monteith said that safety considerations are why he designed his training program for clinicians who want to offer 10-20 vasectomies per week.
Dr. Monteith sees his work in teaching family care physicians on how to perform vasectomies similar to his previous role as medical director of Planned Parenthood of Central North Carolina. There, he helped provide family planning options, mostly to women. Now, he offers the options to men.
“Most of our public health efforts seem to be focused on female reproduction,” Dr. Monteith said. “It is never a good idea to let specialists be the main providers of a preventive healthcare treatment or service, kind of like only allowing patients to go to a cardiologist to get a prescription for cholesterol medication. I needed to do what I could do to increase the number of providers offering easier access to vasectomy.”
A version of this article first appeared on Medscape.com.
One physician has made it his mission to help.
Charles W. Monteith Jr., MD, medical director of his own practice in Raleigh, N.C., said that before the Court’s decision in Dobbs v. Jackson , he was booked “2-3 weeks” in advance for vasectomies.
“Now I am booked out 3 months,” he said.
In September, Dr. Monteith launched a training program for physicians interested in providing vasectomies in their offices. The course, which Dr. Monteith conceived in 2021 before the Supreme Court’s latest ruling, offers one-on-one training and mentorship for physicians who want to learn to perform minimally invasive vasectomies under local anesthesia.
In addition to training, Dr. Monteith provides all the necessary equipment, including eye loupes, exam room surgical furniture, and instrument sterilization system. The program can be completed over 4 weekends and costs $38,000; participants typically perform 40 vasectomy procedures during the training period.
Dr. Monteith, who trained in obstetrics and gynecology, said that he has performed over 7,000 no-scalpel vasectomies since 2008.
Requests for vasectomy consultations at the end of June – when the Dobbs decision was announced – came from men of all ages but particularly from younger men with fewer than two children, Dr. Monteith said.
Prior to the ruling, men with no children accounted for 10% of his patient roster; now, he added, “some days, it is 80%.”
With the increase in demand came a unique opportunity for more doctors to offer the service. The majority of vasectomies in the United States, around 75%, are performed by urologists, but 25% are performed by specialists in family medicine or general surgery.
Some research shows that urologists are typically unwilling to train family physicians on the procedure, citing concerns over competition and not enough cases to go around. But Doug Stein, MD, a urologist and director of Vasectomy and Reversal Centers of Florida in Tampa, offers a similar training for physicians, most of whom are family physicians. Opening the door for more men to get a vasectomy may be a net good, according to Dr. Stein.
“There’s a lot of trust required for vasectomy,” Dr. Stein noted. “Men are probably more likely to go to their family medicine doctor,” that they have a rapport with than a specialist they’ve never met.
Alex Shteynshlyuger, MD, director of urology at New York Urology Specialists, said that he supports family physicians performing vasectomies. However, he cautioned that like any other procedure, complications can arise, and thorough training is essential.
“While complications are not common, they do occur, including pain, bleeding, infection, granuloma formation, and fistula tract,” Dr. Shteynshlyuger said. Family physicians must also know when to refer patients to a specialist.
Dr. Monteith said that safety considerations are why he designed his training program for clinicians who want to offer 10-20 vasectomies per week.
Dr. Monteith sees his work in teaching family care physicians on how to perform vasectomies similar to his previous role as medical director of Planned Parenthood of Central North Carolina. There, he helped provide family planning options, mostly to women. Now, he offers the options to men.
“Most of our public health efforts seem to be focused on female reproduction,” Dr. Monteith said. “It is never a good idea to let specialists be the main providers of a preventive healthcare treatment or service, kind of like only allowing patients to go to a cardiologist to get a prescription for cholesterol medication. I needed to do what I could do to increase the number of providers offering easier access to vasectomy.”
A version of this article first appeared on Medscape.com.