User login
Nicotine blocks estrogen production in women’s brains
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
VIENNA – The production of estrogen in the thalamus appears to be curtailed by just one dose of nicotine, equivalent to that in a cigarette, reveals a whole brain analysis of healthy women in the first study of its kind.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
The researchers performed both MRI and positron emission tomography (PET) scans in 10 healthy women using a tracer that binds to aromatase, also known as estrogen synthase.
They found that, following an intranasal spray delivering 1 mg of nicotine, there was a significant reduction in estrogen synthase in both the right and left thalamus.
“For the first time, we can see that nicotine works to shut down the estrogen production mechanism in the brains of women,” said lead researcher Erika Comasco, PhD, department of neuroscience, Uppsala University, Sweden, in a release.
“We were surprised to see that this effect could be seen even with a single dose of nicotine, equivalent to just one cigarette, showing how powerful the effects of smoking are on a woman’s brain.”
Emphasizing the preliminary nature of the study and the need for a larger sample, she added: “We’re still not sure what the behavioral or cognitive outcomes are, only that nicotine acts on this area of the brain.
“However, we note that the affected brain system is a target for addictive drugs, such as nicotine.”
Previous research has revealed that women are less successful at quitting smoking than men, and appear to be more resistant to nicotine replacement therapy, and experience more relapses.
There is evidence to suggest that there is a complex interaction between sex and steroid hormones and the reward effect of nicotine, modulated by the dopaminergic system.
Moreover, women who smoke enter menopause earlier than nonsmokers, and have lower plasma estrogen levels, Dr. Camasco told this news organization.
Dr. Comasco explained that “besides its role in reproductive function and sexual behavior, estrogen has an impact on the brain wherever there are receptors, which is basically regions that are related to emotional regulation, cognitive function, and so on.”
Estrogen, she continued, has two main mechanisms of action, via dopaminergic and serotonergic signaling. However, levels of the hormone cannot be measured directly in the brain.
The researchers therefore turned to estrogen synthase, which regulates the synthesis of estrogen, and is highly expressed in the limbic system, a brain region associated with addiction.
Moreover, estrogen synthase levels can be measured in vivo, and previous animal studies have indicated that nicotine inhibits estrogen synthase.
To investigate its impact in humans, the researchers performed structural MRI and two 11C-cetrozole PET scans in 10 healthy women.
The assessments were performed before and after the nasal administration of 1 mg of nicotine, the dose contained in one cigarette, via two sprays of a nasal spray each containing 0.5 mg of nicotine.
A whole brain analysis was then used to determine changes in nondisplaceable binding potential of 11C-cetrozole to estrogen synthase between the two scans to indicate the availability of the enzyme at the two time points.
The results showed that, at baseline, high availability of estrogen synthase was observed in the thalamus, hypothalamus, and amygdala, with the highest levels in the right and left thalamus.
However, nicotine exposure was associated with a significant reduction in estrogen binding bilaterally in the thalamus when averaged across the participants (P < .01).
Region-of-interest analysis using within-individual voxel-wise comparison confirmed reduced estrogen synthase levels in both the right and left thalamus (P < .05), as well as in the subthalamic area.
Next, Dr. Comasco would like to test the impact of nicotine on estrogen synthase in men.
While men have lower levels of estrogen then women, “the reaction will take place anyway,” she said, although the “impact would be different.”
She would also like to look at the behavioral effects of reductions in estrogen synthase, and look at the effect of nicotine from a functional point of view.
Wim van den Brink, MD, PhD, professor of psychiatry and addiction at the Academic Medical Center, University of Amsterdam, commented that this is an “important first finding.”
“Smoking has many adverse effects in men and in women, but this particular effect of nicotine on the reduction of estrogen production in women was not known before,” he added in the release.
However, he underlined that tobacco addition is a “complex disorder” and it is “unlikely that this specific effect of nicotine on the thalamus explains all the observed differences in the development, treatment, and outcomes between male and female smokers.”
“It is still a long way from a nicotine-induced reduction in estrogen production to a reduced risk of nicotine addiction and negative effects of treatment and relapse in female cigarette smokers, but this work merits further investigation,” Dr. van den Brink said.
The study was funded by the Science for Life Laboratory/Uppsala University.
No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
Sexual assault–related visits to the ED are on the rise
Data from the Federal Bureau of Investigation show an increase in reported rapes and sexual assaults (SAs) since 2006, and studies of victims show an increased risk of conditions such as suicidal ideation, PTSD, depression, substance use, and chronic conditions, write Emily L. Vogt of the University of Michigan, Ann Arbor, and colleagues.
However, trends and disparities in ED use by adults seeking care following SA have not been explored, they said.
For a study that was published in JAMA Network Open, researchers reviewed data from the Nationwide Emergency Department Sample (NEDS), a large, nationally representative database managed by the Agency for Healthcare Research and Quality. The dataset consisted of 120 million to 143 million weighted ED visits reported annually from 2006 through 2016. The study population included adults aged 18-65 years who had made an ED visit that was recorded in the NEDS and that was coded as an SA. SA was defined using ICD-9 codes until the fourth quarter of 2015, at which time ICD-10 codes came into use.
Overall, the number of SA-related ED visits increased by 1,533.0% during the study period, from 3,607 in 2006 to 55,296 in 2019. The average annual percentage change was 23.0% (P < .001). The greatest increase occurred from 2015 to 2016, when annual visits increased from 17,709 to 47,732. This increase likely reflected the updated ICD-10 codes, in which there are categories for suspected adult rape, confirmed adult rape, and adult forced sexual exploitation, the researchers note.
Patients presenting to the ED after an SA were mainly women (91.5%). Individuals aged 18-25 years accounted for nearly half of the presentations. Individuals in the lowest and second-lowest income quartiles also were overrepresented.
Despite the increased presentation to EDs, admission rates for SA decreased, from 12.6% to 4.3%, the researchers note. Patients who were older and were insured through Medicaid were more likely to be admitted than persons of other demographic groups.
The researchers also found that increases in ED presentations outpaced increases in SA reports to law enforcement. They compared the ED trends with FBI-reported rapes/SAs from 2015 to 2019 and found increases of 7% and 22% during the times of ICD-9 and ICD-10 codes, respectively. However, in 2019, the number of SA survivors who sought ED care remained below the number who reported to law enforcement (55,296 vs. 139,815, as determined on the basis of revised SA definitions).
“Although the association between increased coding specificity and documentation of SA is still unclear, ICD-10 likely contributed to increased ED documentation of SA,” but the data show steady increases that are independent of the coding change, the researchers write.
The study findings were limited by several factors, including the potential for multiple representations of patients, coding errors associated with the NEDS database, and the reliance on voluntary reports in the NEDS and FBI datasets, the researchers note. The results were strengthened by the large, diverse sample size and by the inclusion of hospital admissions and crime data for comparison, they say.
“As few as 21% of survivors seek medical care after SA, meaning that the survivors captured in this study represent a fraction of total SA-related care need,” the researchers write. “Our finding that most SA ED visits are by young, female, and low-income survivors can inform policy changes to better support these individuals,” which could include the development of outpatient and longitudinal care settings to better serve these populations, they conclude.
Better understanding not only of the trends underlying SA reporting but also of the demographics of survivors who seek treatment and evaluation after SA is vital, said Robert Glatter, MD, in an interview.
“Being able to better understand how social and societal movements affect a patient’s comfort in reporting an SA is vital in tracking the numbers of people who seek care in the ED,” said Dr. Glatter, an emergency medicine physician at Lenox Hill Hospital at Northwell Health, New York, and also of Hofstra University, Hempstead, N.Y.
Dr. Glatter said he was not surprised by the significant increase in sexual assault presentations, especially in light of increased awareness and the influence of the #MeToo movement and other social justice movements over the past decade.
“While I believe that victims of sexual violence may now feel more empowered to report an assault, the volume of SA that go unreported remains a serious public health issue and concern” in the United States and globally, he emphasized.
A key message from the current study is that there is a need for investment in “compassionate and comprehensive care for all survivors of SA,” Dr. Glatter said. “This includes recognition of the extensive mental health consequences of SA that can lead to not only depression, PTSD, and anxiety but also to suicidal ideation and suicide. The longer-term medical effects become life altering, permeating families and future generations,” he emphasized.
“As a society, we must also place a strong emphasis on caring for all SA survivors, but particularly those who come from economically or socially disadvantaged backgrounds who are uninsured or underinsured,” Dr. Glatter said. Issues of race, gender identity, and sexual identity among SA survivors also must be taken into consideration, he added.
“We need to better understand how our health care system can provide more nuanced follow-up care and reporting for survivors in outpatient settings. … Making access easier, while ensuring confidentiality, will allow more survivors of SA to seek treatment and care,” he said. “We also need to understand how using forensic nurses in this capacity, and beyond the ED, can better serve minority and racially diverse communities” and to increase the recruitment and training of such specialized nurses to care for SA victims, Dr. Glatter noted.
The study was supported by internal funding from the University of Michigan and the department of obstetrics and gynecology. Corresponding author Erica C. Marsh, MD, has received personal fees from Myovant Sciences and Pfizer unrelated to the current study. Dr. Glatter has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the Federal Bureau of Investigation show an increase in reported rapes and sexual assaults (SAs) since 2006, and studies of victims show an increased risk of conditions such as suicidal ideation, PTSD, depression, substance use, and chronic conditions, write Emily L. Vogt of the University of Michigan, Ann Arbor, and colleagues.
However, trends and disparities in ED use by adults seeking care following SA have not been explored, they said.
For a study that was published in JAMA Network Open, researchers reviewed data from the Nationwide Emergency Department Sample (NEDS), a large, nationally representative database managed by the Agency for Healthcare Research and Quality. The dataset consisted of 120 million to 143 million weighted ED visits reported annually from 2006 through 2016. The study population included adults aged 18-65 years who had made an ED visit that was recorded in the NEDS and that was coded as an SA. SA was defined using ICD-9 codes until the fourth quarter of 2015, at which time ICD-10 codes came into use.
Overall, the number of SA-related ED visits increased by 1,533.0% during the study period, from 3,607 in 2006 to 55,296 in 2019. The average annual percentage change was 23.0% (P < .001). The greatest increase occurred from 2015 to 2016, when annual visits increased from 17,709 to 47,732. This increase likely reflected the updated ICD-10 codes, in which there are categories for suspected adult rape, confirmed adult rape, and adult forced sexual exploitation, the researchers note.
Patients presenting to the ED after an SA were mainly women (91.5%). Individuals aged 18-25 years accounted for nearly half of the presentations. Individuals in the lowest and second-lowest income quartiles also were overrepresented.
Despite the increased presentation to EDs, admission rates for SA decreased, from 12.6% to 4.3%, the researchers note. Patients who were older and were insured through Medicaid were more likely to be admitted than persons of other demographic groups.
The researchers also found that increases in ED presentations outpaced increases in SA reports to law enforcement. They compared the ED trends with FBI-reported rapes/SAs from 2015 to 2019 and found increases of 7% and 22% during the times of ICD-9 and ICD-10 codes, respectively. However, in 2019, the number of SA survivors who sought ED care remained below the number who reported to law enforcement (55,296 vs. 139,815, as determined on the basis of revised SA definitions).
“Although the association between increased coding specificity and documentation of SA is still unclear, ICD-10 likely contributed to increased ED documentation of SA,” but the data show steady increases that are independent of the coding change, the researchers write.
The study findings were limited by several factors, including the potential for multiple representations of patients, coding errors associated with the NEDS database, and the reliance on voluntary reports in the NEDS and FBI datasets, the researchers note. The results were strengthened by the large, diverse sample size and by the inclusion of hospital admissions and crime data for comparison, they say.
“As few as 21% of survivors seek medical care after SA, meaning that the survivors captured in this study represent a fraction of total SA-related care need,” the researchers write. “Our finding that most SA ED visits are by young, female, and low-income survivors can inform policy changes to better support these individuals,” which could include the development of outpatient and longitudinal care settings to better serve these populations, they conclude.
Better understanding not only of the trends underlying SA reporting but also of the demographics of survivors who seek treatment and evaluation after SA is vital, said Robert Glatter, MD, in an interview.
“Being able to better understand how social and societal movements affect a patient’s comfort in reporting an SA is vital in tracking the numbers of people who seek care in the ED,” said Dr. Glatter, an emergency medicine physician at Lenox Hill Hospital at Northwell Health, New York, and also of Hofstra University, Hempstead, N.Y.
Dr. Glatter said he was not surprised by the significant increase in sexual assault presentations, especially in light of increased awareness and the influence of the #MeToo movement and other social justice movements over the past decade.
“While I believe that victims of sexual violence may now feel more empowered to report an assault, the volume of SA that go unreported remains a serious public health issue and concern” in the United States and globally, he emphasized.
A key message from the current study is that there is a need for investment in “compassionate and comprehensive care for all survivors of SA,” Dr. Glatter said. “This includes recognition of the extensive mental health consequences of SA that can lead to not only depression, PTSD, and anxiety but also to suicidal ideation and suicide. The longer-term medical effects become life altering, permeating families and future generations,” he emphasized.
“As a society, we must also place a strong emphasis on caring for all SA survivors, but particularly those who come from economically or socially disadvantaged backgrounds who are uninsured or underinsured,” Dr. Glatter said. Issues of race, gender identity, and sexual identity among SA survivors also must be taken into consideration, he added.
“We need to better understand how our health care system can provide more nuanced follow-up care and reporting for survivors in outpatient settings. … Making access easier, while ensuring confidentiality, will allow more survivors of SA to seek treatment and care,” he said. “We also need to understand how using forensic nurses in this capacity, and beyond the ED, can better serve minority and racially diverse communities” and to increase the recruitment and training of such specialized nurses to care for SA victims, Dr. Glatter noted.
The study was supported by internal funding from the University of Michigan and the department of obstetrics and gynecology. Corresponding author Erica C. Marsh, MD, has received personal fees from Myovant Sciences and Pfizer unrelated to the current study. Dr. Glatter has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the Federal Bureau of Investigation show an increase in reported rapes and sexual assaults (SAs) since 2006, and studies of victims show an increased risk of conditions such as suicidal ideation, PTSD, depression, substance use, and chronic conditions, write Emily L. Vogt of the University of Michigan, Ann Arbor, and colleagues.
However, trends and disparities in ED use by adults seeking care following SA have not been explored, they said.
For a study that was published in JAMA Network Open, researchers reviewed data from the Nationwide Emergency Department Sample (NEDS), a large, nationally representative database managed by the Agency for Healthcare Research and Quality. The dataset consisted of 120 million to 143 million weighted ED visits reported annually from 2006 through 2016. The study population included adults aged 18-65 years who had made an ED visit that was recorded in the NEDS and that was coded as an SA. SA was defined using ICD-9 codes until the fourth quarter of 2015, at which time ICD-10 codes came into use.
Overall, the number of SA-related ED visits increased by 1,533.0% during the study period, from 3,607 in 2006 to 55,296 in 2019. The average annual percentage change was 23.0% (P < .001). The greatest increase occurred from 2015 to 2016, when annual visits increased from 17,709 to 47,732. This increase likely reflected the updated ICD-10 codes, in which there are categories for suspected adult rape, confirmed adult rape, and adult forced sexual exploitation, the researchers note.
Patients presenting to the ED after an SA were mainly women (91.5%). Individuals aged 18-25 years accounted for nearly half of the presentations. Individuals in the lowest and second-lowest income quartiles also were overrepresented.
Despite the increased presentation to EDs, admission rates for SA decreased, from 12.6% to 4.3%, the researchers note. Patients who were older and were insured through Medicaid were more likely to be admitted than persons of other demographic groups.
The researchers also found that increases in ED presentations outpaced increases in SA reports to law enforcement. They compared the ED trends with FBI-reported rapes/SAs from 2015 to 2019 and found increases of 7% and 22% during the times of ICD-9 and ICD-10 codes, respectively. However, in 2019, the number of SA survivors who sought ED care remained below the number who reported to law enforcement (55,296 vs. 139,815, as determined on the basis of revised SA definitions).
“Although the association between increased coding specificity and documentation of SA is still unclear, ICD-10 likely contributed to increased ED documentation of SA,” but the data show steady increases that are independent of the coding change, the researchers write.
The study findings were limited by several factors, including the potential for multiple representations of patients, coding errors associated with the NEDS database, and the reliance on voluntary reports in the NEDS and FBI datasets, the researchers note. The results were strengthened by the large, diverse sample size and by the inclusion of hospital admissions and crime data for comparison, they say.
“As few as 21% of survivors seek medical care after SA, meaning that the survivors captured in this study represent a fraction of total SA-related care need,” the researchers write. “Our finding that most SA ED visits are by young, female, and low-income survivors can inform policy changes to better support these individuals,” which could include the development of outpatient and longitudinal care settings to better serve these populations, they conclude.
Better understanding not only of the trends underlying SA reporting but also of the demographics of survivors who seek treatment and evaluation after SA is vital, said Robert Glatter, MD, in an interview.
“Being able to better understand how social and societal movements affect a patient’s comfort in reporting an SA is vital in tracking the numbers of people who seek care in the ED,” said Dr. Glatter, an emergency medicine physician at Lenox Hill Hospital at Northwell Health, New York, and also of Hofstra University, Hempstead, N.Y.
Dr. Glatter said he was not surprised by the significant increase in sexual assault presentations, especially in light of increased awareness and the influence of the #MeToo movement and other social justice movements over the past decade.
“While I believe that victims of sexual violence may now feel more empowered to report an assault, the volume of SA that go unreported remains a serious public health issue and concern” in the United States and globally, he emphasized.
A key message from the current study is that there is a need for investment in “compassionate and comprehensive care for all survivors of SA,” Dr. Glatter said. “This includes recognition of the extensive mental health consequences of SA that can lead to not only depression, PTSD, and anxiety but also to suicidal ideation and suicide. The longer-term medical effects become life altering, permeating families and future generations,” he emphasized.
“As a society, we must also place a strong emphasis on caring for all SA survivors, but particularly those who come from economically or socially disadvantaged backgrounds who are uninsured or underinsured,” Dr. Glatter said. Issues of race, gender identity, and sexual identity among SA survivors also must be taken into consideration, he added.
“We need to better understand how our health care system can provide more nuanced follow-up care and reporting for survivors in outpatient settings. … Making access easier, while ensuring confidentiality, will allow more survivors of SA to seek treatment and care,” he said. “We also need to understand how using forensic nurses in this capacity, and beyond the ED, can better serve minority and racially diverse communities” and to increase the recruitment and training of such specialized nurses to care for SA victims, Dr. Glatter noted.
The study was supported by internal funding from the University of Michigan and the department of obstetrics and gynecology. Corresponding author Erica C. Marsh, MD, has received personal fees from Myovant Sciences and Pfizer unrelated to the current study. Dr. Glatter has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
ObesityWeek 2022: What’s stopping effective treatment of obesity?
ObesityWeek 2022 is the largest international conference on obesity, with over 100 sessions, and coincides with the 40th anniversary of the Obesity Society. Being held Nov. 1-4, it is a hybrid meeting that participants can attend onsite in sunny San Diego or virtually.
“The meeting offers a wide perspective, from basic science, all the way to public policy on studies of treatment and prevention of obesity,” program planning chair for ObesityWeek, Kelly C. Allison, PhD, said in an interview.
The Presidential Plenary session on Nov. 1 will kick off the meeting with “a series of 10-minute rapid talks on cutting-edge topics in the field,” noted Dr. Allison, who is also director, Center for Weight and Eating Disorders, Hospital of the University of Pennsylvania, and professor of psychiatry, University of Pennsylvania, both in Philadelphia.
Among others, Ania M. Jastreboff, MD, PhD, will speak about “New developments in anti-obesity pharmacotherapy,” and Theodore K. Kyle, RPh, MBA, will discuss “Reducing barriers to treatment: Insurance coverage.”
“We’re seeing some pretty effective antiobesity medication, but still they are not being covered by many insurances,” said Dr. Allison. Some clinicians might be hesitant to prescribe antiobesity medications, remembering older drugs that were pulled from the market for health concerns, and some patients may also have concerns, she speculated. There is a need for greater education about the current antiobesity drugs.
In his presidential address, Dan Bessesen, MD, professor of medicine at the University of Colorado at Denver, Aurora, will discuss “Regulation of body weight and adaptive responses to weight loss.”
Pediatric obesity is a major focus of this year›s conference too, Allison noted.
At 8 a.m on Nov. 3, The Obesity Society, the World Obesity Federation, the European Association for the Study of Obesity, and Obesity Canada will present a joint symposium, “International innovations in pediatric obesity,” with speakers from Canada, Australia, and Ireland discussing ongoing paradigm shifts in the prevention and treatment of pediatric obesity.
Two hours later, at a joint symposium by the American Academy of Pediatrics/The Obesity Society, attendees will get a behind-the-scenes look at the making of the new AAP Obesity Clinical Practice Guideline for children and adolescents with obesity.
The conference tracks reflect the broad scope of this event: Track 1: Metabolism and Integrative Physiology; Track 2: Neuroscience; Track 3: Interventional and Clinical Studies; Track 4: Population Health; Track 5: Clinical/Professional Practice; Track 6: Policy/Public Health, and a subtrack: Eradicating Treatment Barriers.
Dr. Allison highlighted the following oral presentations and posters about antiobesity drugs:
- “Once-weekly subcutaneous semaglutide 2.4 mg in adolescents with overweight or obesity,” with an extended Q&A session, Nov. 2.
- “Clinical outcomes with medication use in tertiary pediatric weight management program,” by Enayet and colleagues. Poster 030.
- “The metabolically healthy obese paradigm and liver fat content in the Fels longitudinal study,” by Garza and colleagues Oral 055, Nov. 2.
- “Phase 3 clinical trial of metformin for treatment of COVID-19 in adults with overweight and obesity,” by Bramante and colleagues. Oral 067, Nov. 3. This trial was published in the (N Engl J Med. 2022;387:599-610).
- “Glucagon/GLP-1 receptor dual agonist BI 456906 reduces bodyweight in patients with type 2 diabetes,” by Rosenstock and colleagues. Oral-063, Nov. 3.
- “A randomized controlled trial of naltrexone and bupropion and behavior therapy for binge-eating disorder,” by Grilo and colleagues. Oral 066, Nov. 3.
And on Nov. 4, researchers will present four oral abstracts about the dual glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1) receptor agonist tirzepatide (Mounjaro), which is approved for type 2 diabetes and now has fast track designation for weight loss from the Food and Drug Administration. Oral abstracts 109, 110, 111, and 112 cover weight loss with tirzepatide across different age groups, body mass indexes, and comorbidities, as well as quality of life.
Dr. Allison also highlighted the following presentations that cover other diverse topics:
- Family-based treatment: “Pilot study to inform a randomized controlled trial of HeLP: Obesity prevention & treatment for the entire Hispanic family,” by Haemer and colleagues. Oral 029. November 2.
- Bariatric surgery: “Long-term outcomes of laparoscopic sleeve gastrectomy from 2010-2016: A nationwide cohort study,” Oral 014. Nov. 2.
- Prevention/public health: “Impact of positive and negative front-of-package food labels in a randomized experiment,” by Grummon and colleagues. Oral 068. Nov. 3.
- Time-restricted eating: “Effects of 8-hour time restricted eating for weight loss over 12 months,” by Gabel and colleagues. Oral 102. Nov. 4.
- Patient management: “Identifying interprofessional drivers of practice gaps in the management of patients with obesity,” by Robinson and colleagues. Poster 055.
On Nov. 4, researchers will present five winning papers that will be published in the December issue of the Obesity journal about GLP-1 agonists versus bariatric surgery; monoacylglycerol O-acyltransferase 1 in mice; a behavioral weight-loss intervention; the Canberra Obesity Management Service; and macronutrient (im)balance in an obesogenic environment.
“I’m always excited to hear some talks that are outside of my comfort area to understand the mechanisms of obesity better,” concluded Dr. Allison.
A version of this article first appeared on Medscape.com.
ObesityWeek 2022 is the largest international conference on obesity, with over 100 sessions, and coincides with the 40th anniversary of the Obesity Society. Being held Nov. 1-4, it is a hybrid meeting that participants can attend onsite in sunny San Diego or virtually.
“The meeting offers a wide perspective, from basic science, all the way to public policy on studies of treatment and prevention of obesity,” program planning chair for ObesityWeek, Kelly C. Allison, PhD, said in an interview.
The Presidential Plenary session on Nov. 1 will kick off the meeting with “a series of 10-minute rapid talks on cutting-edge topics in the field,” noted Dr. Allison, who is also director, Center for Weight and Eating Disorders, Hospital of the University of Pennsylvania, and professor of psychiatry, University of Pennsylvania, both in Philadelphia.
Among others, Ania M. Jastreboff, MD, PhD, will speak about “New developments in anti-obesity pharmacotherapy,” and Theodore K. Kyle, RPh, MBA, will discuss “Reducing barriers to treatment: Insurance coverage.”
“We’re seeing some pretty effective antiobesity medication, but still they are not being covered by many insurances,” said Dr. Allison. Some clinicians might be hesitant to prescribe antiobesity medications, remembering older drugs that were pulled from the market for health concerns, and some patients may also have concerns, she speculated. There is a need for greater education about the current antiobesity drugs.
In his presidential address, Dan Bessesen, MD, professor of medicine at the University of Colorado at Denver, Aurora, will discuss “Regulation of body weight and adaptive responses to weight loss.”
Pediatric obesity is a major focus of this year›s conference too, Allison noted.
At 8 a.m on Nov. 3, The Obesity Society, the World Obesity Federation, the European Association for the Study of Obesity, and Obesity Canada will present a joint symposium, “International innovations in pediatric obesity,” with speakers from Canada, Australia, and Ireland discussing ongoing paradigm shifts in the prevention and treatment of pediatric obesity.
Two hours later, at a joint symposium by the American Academy of Pediatrics/The Obesity Society, attendees will get a behind-the-scenes look at the making of the new AAP Obesity Clinical Practice Guideline for children and adolescents with obesity.
The conference tracks reflect the broad scope of this event: Track 1: Metabolism and Integrative Physiology; Track 2: Neuroscience; Track 3: Interventional and Clinical Studies; Track 4: Population Health; Track 5: Clinical/Professional Practice; Track 6: Policy/Public Health, and a subtrack: Eradicating Treatment Barriers.
Dr. Allison highlighted the following oral presentations and posters about antiobesity drugs:
- “Once-weekly subcutaneous semaglutide 2.4 mg in adolescents with overweight or obesity,” with an extended Q&A session, Nov. 2.
- “Clinical outcomes with medication use in tertiary pediatric weight management program,” by Enayet and colleagues. Poster 030.
- “The metabolically healthy obese paradigm and liver fat content in the Fels longitudinal study,” by Garza and colleagues Oral 055, Nov. 2.
- “Phase 3 clinical trial of metformin for treatment of COVID-19 in adults with overweight and obesity,” by Bramante and colleagues. Oral 067, Nov. 3. This trial was published in the (N Engl J Med. 2022;387:599-610).
- “Glucagon/GLP-1 receptor dual agonist BI 456906 reduces bodyweight in patients with type 2 diabetes,” by Rosenstock and colleagues. Oral-063, Nov. 3.
- “A randomized controlled trial of naltrexone and bupropion and behavior therapy for binge-eating disorder,” by Grilo and colleagues. Oral 066, Nov. 3.
And on Nov. 4, researchers will present four oral abstracts about the dual glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1) receptor agonist tirzepatide (Mounjaro), which is approved for type 2 diabetes and now has fast track designation for weight loss from the Food and Drug Administration. Oral abstracts 109, 110, 111, and 112 cover weight loss with tirzepatide across different age groups, body mass indexes, and comorbidities, as well as quality of life.
Dr. Allison also highlighted the following presentations that cover other diverse topics:
- Family-based treatment: “Pilot study to inform a randomized controlled trial of HeLP: Obesity prevention & treatment for the entire Hispanic family,” by Haemer and colleagues. Oral 029. November 2.
- Bariatric surgery: “Long-term outcomes of laparoscopic sleeve gastrectomy from 2010-2016: A nationwide cohort study,” Oral 014. Nov. 2.
- Prevention/public health: “Impact of positive and negative front-of-package food labels in a randomized experiment,” by Grummon and colleagues. Oral 068. Nov. 3.
- Time-restricted eating: “Effects of 8-hour time restricted eating for weight loss over 12 months,” by Gabel and colleagues. Oral 102. Nov. 4.
- Patient management: “Identifying interprofessional drivers of practice gaps in the management of patients with obesity,” by Robinson and colleagues. Poster 055.
On Nov. 4, researchers will present five winning papers that will be published in the December issue of the Obesity journal about GLP-1 agonists versus bariatric surgery; monoacylglycerol O-acyltransferase 1 in mice; a behavioral weight-loss intervention; the Canberra Obesity Management Service; and macronutrient (im)balance in an obesogenic environment.
“I’m always excited to hear some talks that are outside of my comfort area to understand the mechanisms of obesity better,” concluded Dr. Allison.
A version of this article first appeared on Medscape.com.
ObesityWeek 2022 is the largest international conference on obesity, with over 100 sessions, and coincides with the 40th anniversary of the Obesity Society. Being held Nov. 1-4, it is a hybrid meeting that participants can attend onsite in sunny San Diego or virtually.
“The meeting offers a wide perspective, from basic science, all the way to public policy on studies of treatment and prevention of obesity,” program planning chair for ObesityWeek, Kelly C. Allison, PhD, said in an interview.
The Presidential Plenary session on Nov. 1 will kick off the meeting with “a series of 10-minute rapid talks on cutting-edge topics in the field,” noted Dr. Allison, who is also director, Center for Weight and Eating Disorders, Hospital of the University of Pennsylvania, and professor of psychiatry, University of Pennsylvania, both in Philadelphia.
Among others, Ania M. Jastreboff, MD, PhD, will speak about “New developments in anti-obesity pharmacotherapy,” and Theodore K. Kyle, RPh, MBA, will discuss “Reducing barriers to treatment: Insurance coverage.”
“We’re seeing some pretty effective antiobesity medication, but still they are not being covered by many insurances,” said Dr. Allison. Some clinicians might be hesitant to prescribe antiobesity medications, remembering older drugs that were pulled from the market for health concerns, and some patients may also have concerns, she speculated. There is a need for greater education about the current antiobesity drugs.
In his presidential address, Dan Bessesen, MD, professor of medicine at the University of Colorado at Denver, Aurora, will discuss “Regulation of body weight and adaptive responses to weight loss.”
Pediatric obesity is a major focus of this year›s conference too, Allison noted.
At 8 a.m on Nov. 3, The Obesity Society, the World Obesity Federation, the European Association for the Study of Obesity, and Obesity Canada will present a joint symposium, “International innovations in pediatric obesity,” with speakers from Canada, Australia, and Ireland discussing ongoing paradigm shifts in the prevention and treatment of pediatric obesity.
Two hours later, at a joint symposium by the American Academy of Pediatrics/The Obesity Society, attendees will get a behind-the-scenes look at the making of the new AAP Obesity Clinical Practice Guideline for children and adolescents with obesity.
The conference tracks reflect the broad scope of this event: Track 1: Metabolism and Integrative Physiology; Track 2: Neuroscience; Track 3: Interventional and Clinical Studies; Track 4: Population Health; Track 5: Clinical/Professional Practice; Track 6: Policy/Public Health, and a subtrack: Eradicating Treatment Barriers.
Dr. Allison highlighted the following oral presentations and posters about antiobesity drugs:
- “Once-weekly subcutaneous semaglutide 2.4 mg in adolescents with overweight or obesity,” with an extended Q&A session, Nov. 2.
- “Clinical outcomes with medication use in tertiary pediatric weight management program,” by Enayet and colleagues. Poster 030.
- “The metabolically healthy obese paradigm and liver fat content in the Fels longitudinal study,” by Garza and colleagues Oral 055, Nov. 2.
- “Phase 3 clinical trial of metformin for treatment of COVID-19 in adults with overweight and obesity,” by Bramante and colleagues. Oral 067, Nov. 3. This trial was published in the (N Engl J Med. 2022;387:599-610).
- “Glucagon/GLP-1 receptor dual agonist BI 456906 reduces bodyweight in patients with type 2 diabetes,” by Rosenstock and colleagues. Oral-063, Nov. 3.
- “A randomized controlled trial of naltrexone and bupropion and behavior therapy for binge-eating disorder,” by Grilo and colleagues. Oral 066, Nov. 3.
And on Nov. 4, researchers will present four oral abstracts about the dual glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1) receptor agonist tirzepatide (Mounjaro), which is approved for type 2 diabetes and now has fast track designation for weight loss from the Food and Drug Administration. Oral abstracts 109, 110, 111, and 112 cover weight loss with tirzepatide across different age groups, body mass indexes, and comorbidities, as well as quality of life.
Dr. Allison also highlighted the following presentations that cover other diverse topics:
- Family-based treatment: “Pilot study to inform a randomized controlled trial of HeLP: Obesity prevention & treatment for the entire Hispanic family,” by Haemer and colleagues. Oral 029. November 2.
- Bariatric surgery: “Long-term outcomes of laparoscopic sleeve gastrectomy from 2010-2016: A nationwide cohort study,” Oral 014. Nov. 2.
- Prevention/public health: “Impact of positive and negative front-of-package food labels in a randomized experiment,” by Grummon and colleagues. Oral 068. Nov. 3.
- Time-restricted eating: “Effects of 8-hour time restricted eating for weight loss over 12 months,” by Gabel and colleagues. Oral 102. Nov. 4.
- Patient management: “Identifying interprofessional drivers of practice gaps in the management of patients with obesity,” by Robinson and colleagues. Poster 055.
On Nov. 4, researchers will present five winning papers that will be published in the December issue of the Obesity journal about GLP-1 agonists versus bariatric surgery; monoacylglycerol O-acyltransferase 1 in mice; a behavioral weight-loss intervention; the Canberra Obesity Management Service; and macronutrient (im)balance in an obesogenic environment.
“I’m always excited to hear some talks that are outside of my comfort area to understand the mechanisms of obesity better,” concluded Dr. Allison.
A version of this article first appeared on Medscape.com.
Itchy Red-Brown Spots on a Child
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.
Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
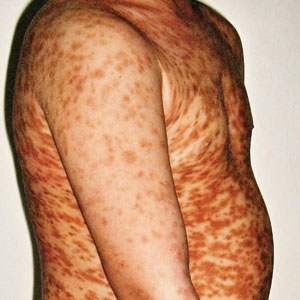
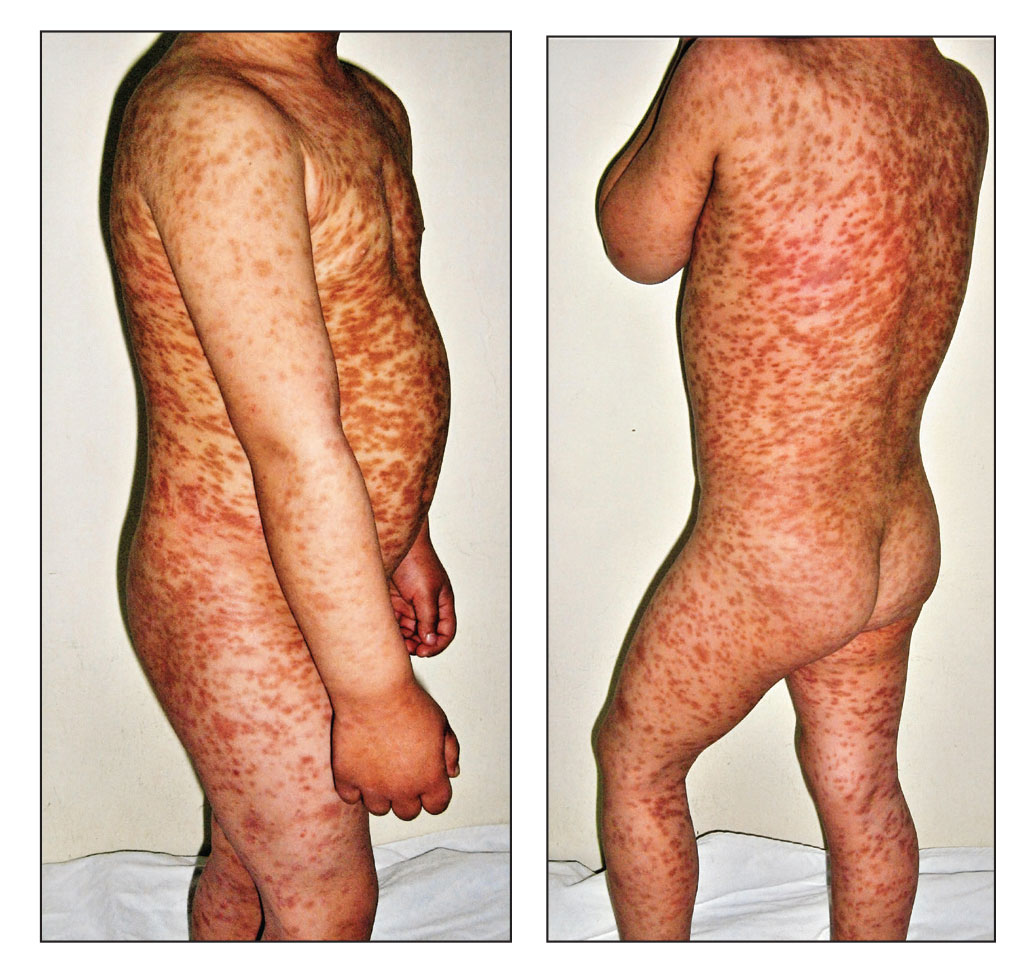
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
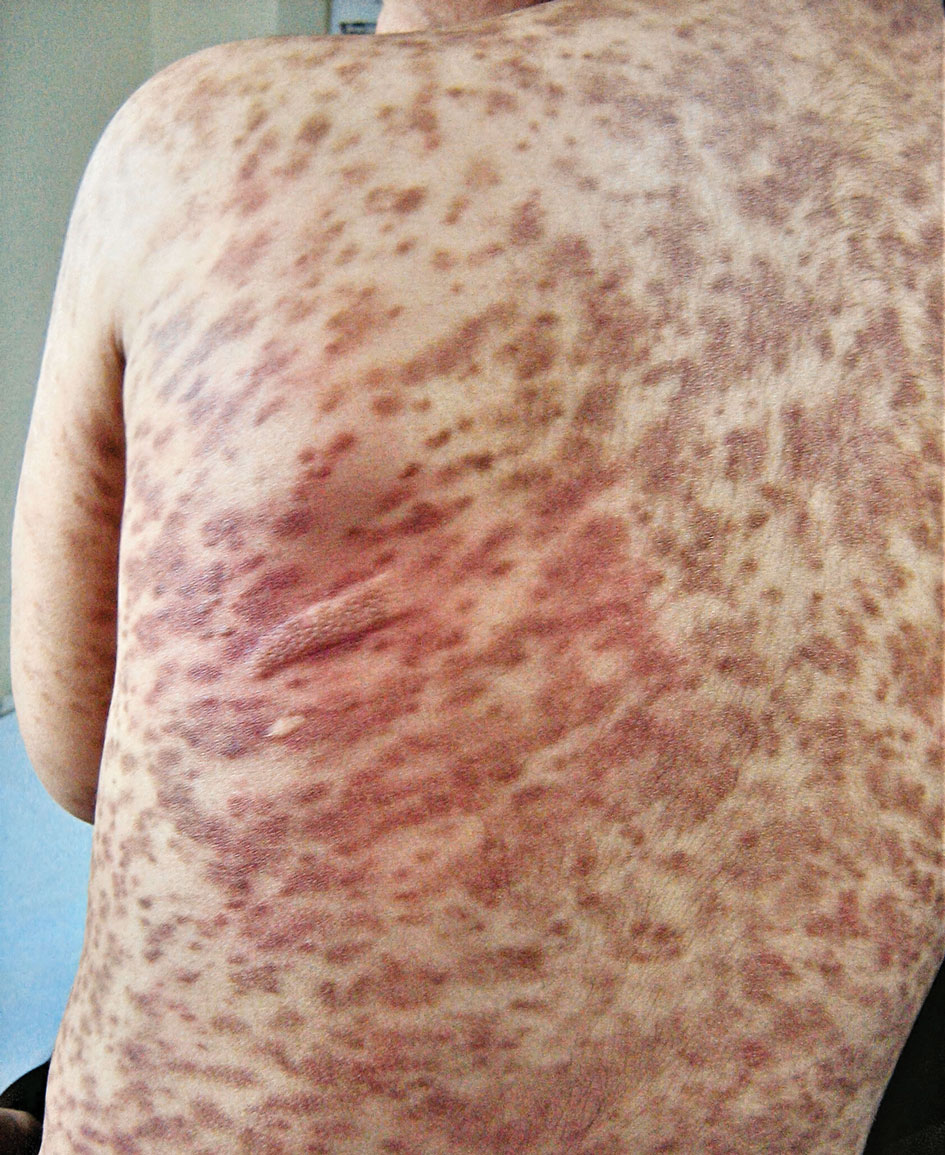
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.

Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.

Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
A 5-year-old boy presented with red-brown spots diffusely spread over the body that were present since birth. There were no subjective symptoms, except for rare instances of flushing, itching, and urtication following hot baths and abrasive scrubs. Dermatologic examination revealed widespread brown polymorphic macules and papules of varying sizes on the forehead, neck, torso, and extremities. Physical examination was otherwise normal.
Emergency contraception for psychiatric patients
Ms. A, age 22, is a college student who presents for an initial psychiatric evaluation. Her body mass index (BMI) is 20 (normal range: 18.5 to 24.9), and her medical history is positive only for childhood asthma. She has been treated for major depressive disorder with venlafaxine by her previous psychiatrist. While this antidepressant has been effective for some symptoms, she has experienced adverse effects and is interested in a different medication. During the evaluation, Ms. A remarks that she had a “scare” last night when the condom broke while having sex with her boyfriend. She says that she is interested in having children at some point, but not at present; she is concerned that getting pregnant now would cause her depression to “spiral out of control.”
Unwanted or mistimed pregnancies account for 45% of all pregnancies.1 While there are ramifications for any unintended pregnancy, the risks for patients with mental illness are greater and include potential adverse effects on the neonate from both psychiatric disease and psychiatric medication use, worse obstetrical outcomes for patients with untreated mental illness, and worsening of psychiatric symptoms and suicide risk in the peripartum period.2 These risks become even more pronounced when psychiatric medications are reflexively discontinued or reduced in pregnancy, which is commonly done contrary to best practice recommendations. In the United States, the recent Supreme Court decision in Dobbs v Jackson Women’s Health Organization has erased federal protections for abortion previously conferred by Roe v Wade. As a result, as of early October 2022, abortion had been made illegal in 11 states, and was likely to be banned in many others, most commonly in states where there is limited support for either parents or children. Thus, preventing unplanned pregnancies should be a treatment consideration for all medical disciplines.3
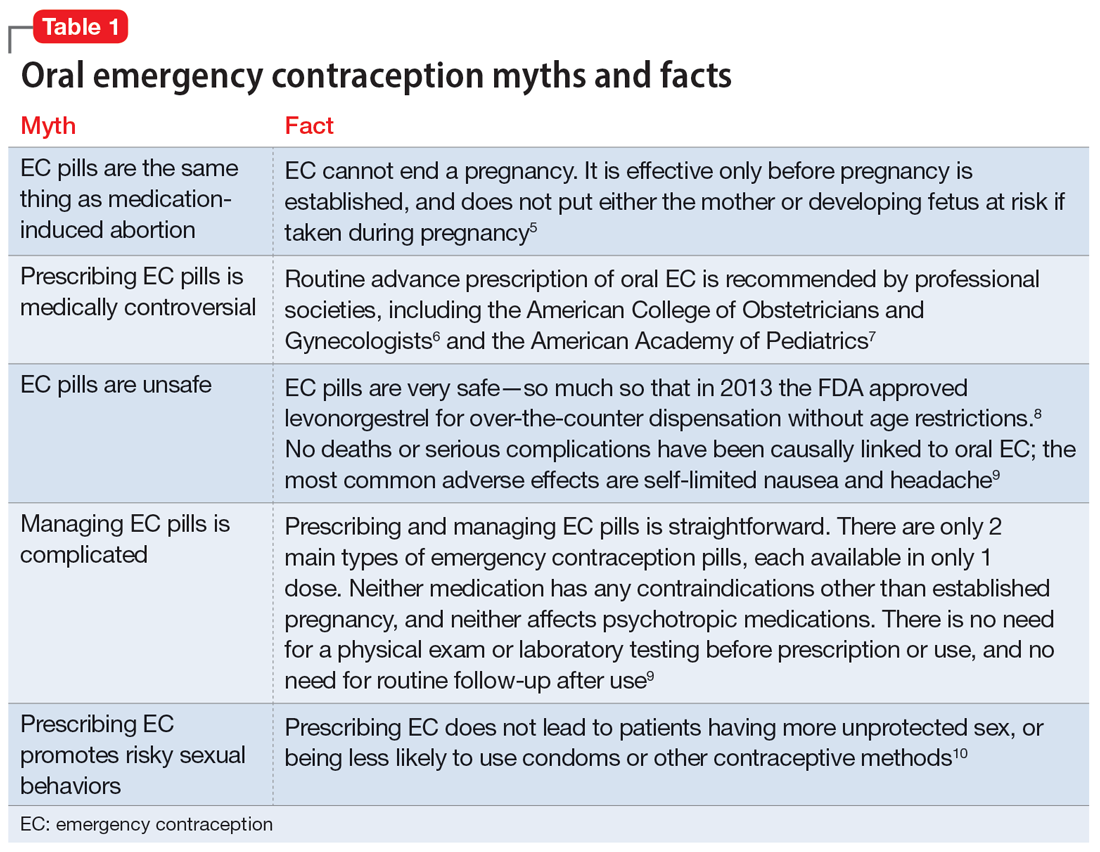
Psychiatrists may hesitate to prescribe emergency contraception (EC) due to fears it falls outside the scope of their practice. However, psychiatry has already moved towards prescribing nonpsychiatric medications when doing so clearly benefits the patient. One example is prescribing metformin to address metabolic syndrome related to the use of second-generation antipsychotics. Emergency contraceptives have strong safety profiles and are easy to prescribe. Unfortunately, there are many barriers to increasing access to emergency contraceptives for psychiatric patients.4 These include the erroneous belief that laboratory and physical exams are needed before starting EC, cost and/or limited stock of emergency contraceptives at pharmacies, and general confusion regarding what constitutes EC vs an oral abortive (Table 15-10). Psychiatrists are particularly well-positioned to support the reproductive autonomy and well-being of patients who struggle to engage with other clinicians. This article aims to help psychiatrists better understand EC so they can comfortably prescribe it before their patients need it.
What is emergency contraception?
EC is medications or devices that patients can use after sexual intercourse to prevent pregnancy. They do not impede the development of an established pregnancy and thus are not abortifacients. EC is not recommended as a primary means of contraception,9 but it can be extremely valuable to reduce pregnancy risk after unprotected intercourse or contraceptive failures such as broken condoms or missed doses of birth control pills. EC can prevent ≥95% of pregnancies when taken within 5 days of at-risk intercourse.11
Methods of EC fall into 2 categories: oral medications (sometimes referred to as “morning after pills”) and intrauterine devices (IUDs). IUDs are the most effective means of EC, especially for patients with higher BMIs or who may be taking medications such as cytochrome P450 (CYP)3A4 inducers that could interfere with the effectiveness of oral methods. IUDs also have the advantage of providing highly effective ongoing contraception.6 However, IUDs require in-office placement by a trained clinician, and patients may experience difficulty obtaining placement within 5 days of unprotected sex. Therefore, oral medication is the most common form of EC.
Oral EC is safe and effective, and professional societies (including the American College of Obstetricians and Gynecologists6 and the American Academy of Pediatrics7) recommend routinely prescribing oral EC for patients in advance of need. Advance prescribing eliminates barriers to accessing EC, increases the use of EC, and does not encourage risky sexual behaviors.10
Overview of oral emergency contraception
Two medications are FDA-approved for use as oral EC: ulipristal acetate and levonorgestrel. Both are available in generic and branded versions. While many common birth control pills can also be safely used off-label as emergency contraception (an approach known as the Yuzpe method), they are less effective, not as well-tolerated, and require knowledge of the specific type of pill the patient has available.9 Oral EC appears to work primarily through delay or inhibition of ovulation, and is unlikely to prevent implantation of a fertilized egg.9
Continue to: Ulipristal acetate
Ulipristal acetate (UPA) is an oral progesterone receptor agonist-antagonist taken as a single 30 mg dose up to 5 days after unprotected sex. Pregnancy rates from a single act of unprotected sex followed by UPA use range from 0% to 1.8%.4 Many pharmacies stock UPA, and others (especially chain pharmacies) report being able to order and fill it within 24 hours.12
Levonorgestrel (LNG) is an oral progestin that is available by prescription and has also been approved for over-the-counter sale to patients of all ages and sexes (without the need to show identification) since 2013.8 It is administered as a single 1.5 mg dose taken as soon as possible up to 3 days after unprotected sex, although it may continue to provide benefits when taken within 5 days. Pregnancy rates from a single act of unprotected sex followed by LNG use range from 0.3% to 2.6%, with much higher odds among women who are obese.4 LNG is available both by prescription or over-the-counter,13 although it is often kept in a locked cabinet or behind the counter, and staff are often misinformed regarding the lack of age restrictions for sale without a prescription.14
Safety and adverse effects. According to the CDC, there are no conditions for which the risks outweigh the advantages of use of either UPA or LNG,5 and patients for whom hormonal birth control is otherwise contraindicated can still use them safely. If a pregnancy has already occurred, taking EC will not harm the developing fetus; it is also safe to use when breastfeeding.5 Both medications are generally well-tolerated—neither has been causally linked to deaths or serious complications,5 and the most common adverse effects are headache (approximately 19%) and nausea (approximately 12%), in addition to irregular bleeding, fatigue, dizziness, and abdominal pain.15 Oral EC may be used more than once, even within the same menstrual cycle. Patients who use EC repeatedly should be encouraged to discuss more efficacious contraceptive options with their primary physician or gynecologist.
Will oral EC affect psychiatric treatment?
Oral EC is unlikely to have a meaningful effect on psychiatric symptoms or management, particularly when compared to the significant impacts of unintended pregnancies. Neither medication is known to have any clinically significant impacts on the pharmacokinetics or pharmacodynamics of psychotropic medications, although the effectiveness of both medications can be impaired by CYP3A4 inducers such as carbamazepine.5 In addition, while research has not specifically examined the impact of EC on psychiatric symptoms, the broader literature on hormonal contraception indicates that most patients with psychiatric disorders generally report similar or lower rates of mood symptoms associated with their use.16 Some women treated with hormonal contraceptives do develop dysphoric mood,16 but any such effects resulting from LNG would likely be transient. Mood disruptions or other psychiatric symptoms have not been associated with UPA use.
How to prescribe oral emergency contraception
Who and when. Women of reproductive age should be counseled about EC as part of anticipatory guidance, regardless of their current intentions for sexual behaviors. Patients do not need a physical examination or pregnancy test before being prescribed or using oral EC.9 Much like how intranasal naloxone is prescribed, prescriptions should be provided in advance of need, with multiple refills to facilitate ready access when needed.
Continue to: Which to prescribe
Which to prescribe. UPA is more effective in preventing pregnancy than LNG at all time points up to 120 hours after sex, including for women who are overweight or obese.15 As such, it is recommended as the first-line choice. However, because LNG is available without prescription and is more readily available (including via online order), it may be a good choice for patients who need rapid EC or who prefer a medication that does not require a prescription (Table 24,5,8,9,15).
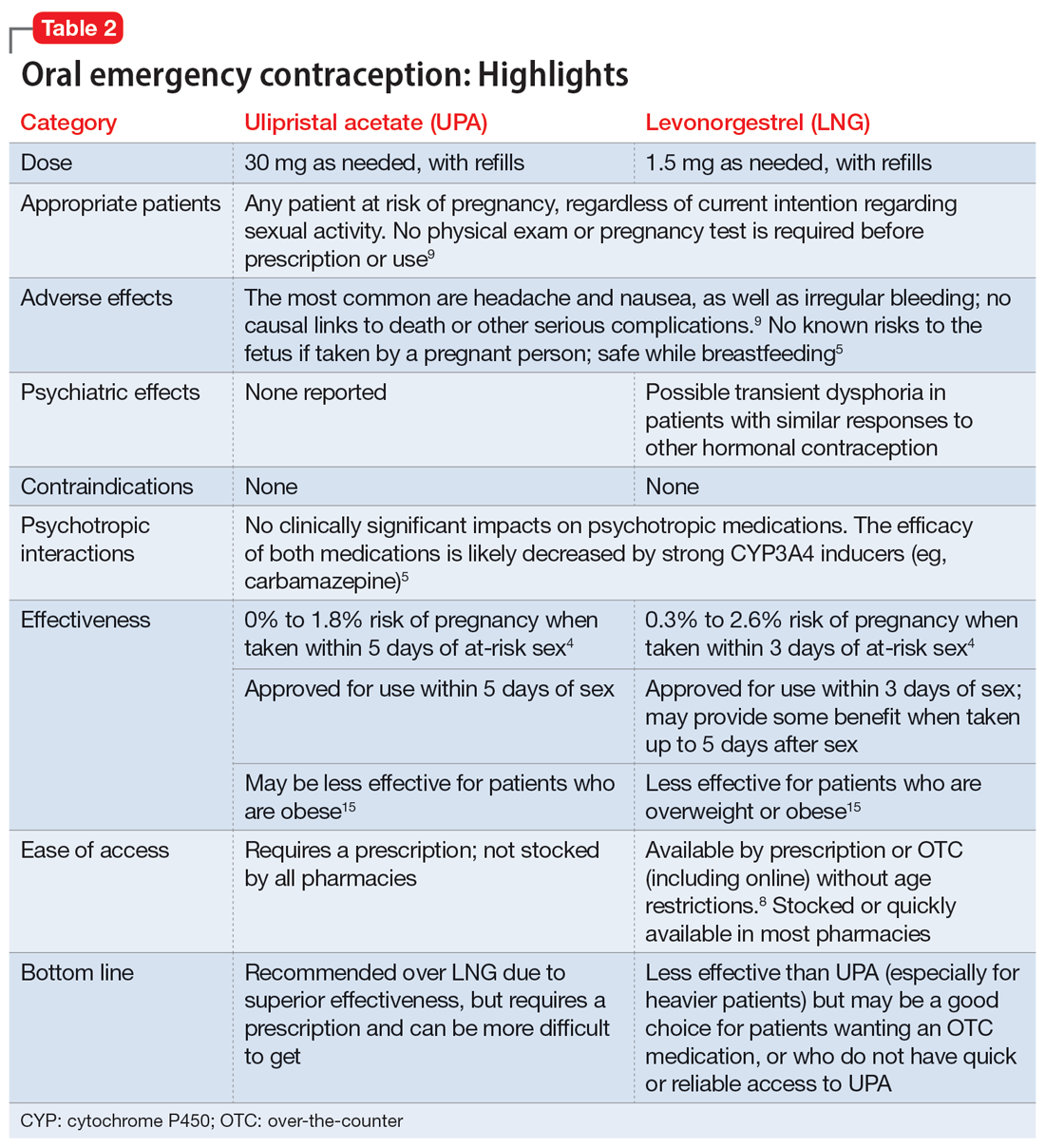
What to tell patients. Patients should be instructed to fill their prescription before they expect to use it, to ensure ready availability when desired (Table 35,9). Oral EC is shelf stable for at least 3 years when stored in a cool, dry environment. Patients should take the medication as soon as possible following at-risk sexual intercourse (Table 4). Tell them that if they vomit within 3 hours of taking the medication, they should take a second dose. Remind patients that EC does not protect against sexually transmitted infections, or from sex that occurs after the medication is taken (in fact, they can increase the possibility of pregnancy later in that menstrual cycle due to delayed ovulation).9 Counsel patients to abstain from sex or to use barrier contraception for 7 days after use. Those who take birth control pills can resume use immediately after using LNG; they should wait 5 days after taking UPA.

No routine follow-up is needed after taking UPA or LNG. However, patients should get a pregnancy test if their period does not start within 3 weeks, and should seek medical evaluation if they experience significant lower abdominal pain or persistent irregular bleeding in order to rule out pregnancy-related complications. Patients who use EC repeatedly should be recommended to pursue routine contraceptive care.

Billing. Counseling your patients about contraception can increase the reimbursement you receive by adding to the complexity of the encounter (regardless of whether you prescribe a medication) through use of the ICD-10 code Z30.0.
Emergency contraception for special populations
Some patients face additional challenges to effective EC that should be considered when counseling and prescribing. Table 54,5,7,15,17-21 discusses the use of EC in these special populations. Of particular importance for psychiatrists, LNG is less effective at preventing undesired pregnancy among patients who are overweight or obese,15,17,18 and strong CYP3A4-inducing agents may decrease the effectiveness of both LNG and UPA.5 Keep in mind, however, that the advantages of using either UPA or LNG outweigh the risks for all populations.5 Patients must be aware of appropriate information in order to make informed decisions, but should not be discouraged from using EC.

Continue to: Other groups of patients...
Other groups of patients may face barriers due to some clinicians’ hesitancy regarding their ability to consent to reproductive care. Most patients with psychiatric illnesses have decision-making capacity regarding reproductive issues.22 Although EC is supported by the American Academy of Pediatrics,7 patients age <18 have varying rights to consent across states,21 and merit special consideration.
CASE CONTINUED
Ms. A does not wish to get pregnant at this time, and expresses fears that her recent contraceptive failure could lead to an unintended pregnancy. In addition to her psychiatric treatment, her psychiatrist should discuss EC options with her. She has a healthy BMI and had inadequately protected sex <1 day ago, so her clinician may prescribe LNG (to ensure rapid access for immediate use) in addition to UPA for her to have available in case of future “scares.” The psychiatrist should consider pharmacologic treatment with an antidepressant with a relatively safe reproductive record (eg, sertraline).23 This is considered preventive ethics, since Ms. A is of reproductive age, even if she is not presently planning to get pregnant, due to the aforementioned high rate of unplanned pregnancy.23,24 It is also important for the psychiatrist to continue the dialogue in future sessions about preventing unintended pregnancy. Since Ms. A has benefited from a psychotropic medication when not pregnant, it will be important to discuss with her the risks and benefits of medication should she plan a pregnancy.
Bottom Line
Patients with mental illnesses are at increased risk of adverse outcomes resulting from unintended pregnancies. Clinicians should counsel patients about emergency contraception (EC) as a part of routine psychiatric care, and should prescribe oral EC in advance of patient need to facilitate effective use.
Related Resources
- American College of Obstetricians and Gynecologists Practice Bulletin on Emergency Contraception. https://www.acog.org/clinical/clinical-guidance/practice-bulletin/ articles/2015/09/emergency-contraception
- State policies on emergency contraception. https://www.guttmacher.org/state-policy/explore/emergency-contraception
- State policies on minors’ access to contraceptive services. https://www.guttmacher.org/state-policy/explore/minors-access-contraceptive-services
- Patient-oriented contraceptive education materials (in English and Spanish). https://shop.powertodecide.org/ptd-category/educational-materials
Drug Brand Names
Carbamazepine • Tegretol
Levonorgestrel • Plan B One-Step, Fallback
Metformin • Glucophage
Naloxone • Narcan
Norethindrone • Aygestin
Sertraline • Zoloft
Topiramate • Topamax
Ulipristal acetate • Ella
Venlafaxine • Effexor
1. Grossman D. Expanding access to short-acting hormonal contraceptive methods in the United States. JAMA Intern Med. 2019;179:1209-1210.
2. Gur TL, Kim DR, Epperson CN. Central nervous system effects of prenatal selective serotonin reuptake inhibitors: sensing the signal through the noise. Psychopharmacology (Berl). 2013;227:567-582.
3. Ross N, Landess J, Kaempf A, et al. Pregnancy termination: what psychiatrists need to know. Current Psychiatry. 2022;21:8-9.
4. Haeger KO, Lamme J, Cleland K. State of emergency contraception in the US, 2018. Contracept Reprod Med. 2018;3:20.
5. Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-3.
6. American College of Obstetricians and Gynecologists. Committee Opinion No 707: Access to emergency contraception. Obstet Gynecol. 2017;130:e48-e52.
7. Upadhya KK, Breuner CC, Alderman EM, et al. Emergency contraception. Pediatrics. 2019;144:e20193149.
8. Rowan A. Obama administration yields to the courts and the evidence, allows emergency contraception to be sold without restrictions. Guttmacher Institute. Published June 25, 2013. Accessed July 31, 2022. https://www.guttmacher.org/gpr/2013/06/obama-administration-yields-courts-and-evidence-allows-emergency-contraception-be-sold#
9. American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
10. Rodriguez MI, Curtis KM, Gaffield ML, et al. Advance supply of emergency contraception: a systematic review. Contraception. 2013;87:590-601.
11. World Health Organization. Emergency contraception. Published November 9, 2021. Accessed August 4, 2022. https://www.who.int/news-room/fact-sheets/detail/emergency-contraception
12. Shigesato M, Elia J, Tschann M, et al. Pharmacy access to ulipristal acetate in major cities throughout the United States. Contraception. 2018;97:264-269.
13. Wilkinson TA, Clark P, Rafie S, et al. Access to emergency contraception after removal of age restrictions. Pediatrics. 2017;140:e20164262.
14. Cleland K, Bass J, Doci F, et al. Access to emergency contraception in the over-the-counter era. Women’s Health Issues. 2016;26:622-627.
15. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
16. McCloskey LR, Wisner KL, Cattan MK, et al. Contraception for women with psychiatric disorders. Am J Psychiatry. 2021;178:247-255.
17. Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91:97-104.
18. Festin MP, Peregoudov A, Seuc A, et al. Effect of BMI and body weight on pregnancy rates with LNG as emergency contraception: analysis of four WHO HRP studies. Contraception. 2017;95:50-54.
19. Edelman AB, Hennebold JD, Bond K, et al. Double dosing levonorgestrel-based emergency contraception for individuals with obesity: a randomized controlled trial. Obstet Gynecol. 2022;140(1):48-54.
20. FSRH Clinical Effectiveness Unit. FSRH clinical guideline: Emergency contraception. Published March 2017. Amended December 2020. Faculty of Sexual & Reproductive Healthcare. Accessed August 4, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-emergency-contraception-march-2017/
21. Guttmacher Institute. Minors’ access to contraceptive services. Guttmacher Institute. Accessed August 4, 2022. https://www.guttmacher.org/state-policy/explore/minors-access-contraceptive-services
22. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022;63:61-70.
23. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20:30-36.
24. Friedman SH. The ethics of treating depression in pregnancy. J Primary Healthcare. 2015;7:81-83.
Ms. A, age 22, is a college student who presents for an initial psychiatric evaluation. Her body mass index (BMI) is 20 (normal range: 18.5 to 24.9), and her medical history is positive only for childhood asthma. She has been treated for major depressive disorder with venlafaxine by her previous psychiatrist. While this antidepressant has been effective for some symptoms, she has experienced adverse effects and is interested in a different medication. During the evaluation, Ms. A remarks that she had a “scare” last night when the condom broke while having sex with her boyfriend. She says that she is interested in having children at some point, but not at present; she is concerned that getting pregnant now would cause her depression to “spiral out of control.”
Unwanted or mistimed pregnancies account for 45% of all pregnancies.1 While there are ramifications for any unintended pregnancy, the risks for patients with mental illness are greater and include potential adverse effects on the neonate from both psychiatric disease and psychiatric medication use, worse obstetrical outcomes for patients with untreated mental illness, and worsening of psychiatric symptoms and suicide risk in the peripartum period.2 These risks become even more pronounced when psychiatric medications are reflexively discontinued or reduced in pregnancy, which is commonly done contrary to best practice recommendations. In the United States, the recent Supreme Court decision in Dobbs v Jackson Women’s Health Organization has erased federal protections for abortion previously conferred by Roe v Wade. As a result, as of early October 2022, abortion had been made illegal in 11 states, and was likely to be banned in many others, most commonly in states where there is limited support for either parents or children. Thus, preventing unplanned pregnancies should be a treatment consideration for all medical disciplines.3
Psychiatrists may hesitate to prescribe emergency contraception (EC) due to fears it falls outside the scope of their practice. However, psychiatry has already moved towards prescribing nonpsychiatric medications when doing so clearly benefits the patient. One example is prescribing metformin to address metabolic syndrome related to the use of second-generation antipsychotics. Emergency contraceptives have strong safety profiles and are easy to prescribe. Unfortunately, there are many barriers to increasing access to emergency contraceptives for psychiatric patients.4 These include the erroneous belief that laboratory and physical exams are needed before starting EC, cost and/or limited stock of emergency contraceptives at pharmacies, and general confusion regarding what constitutes EC vs an oral abortive (Table 15-10). Psychiatrists are particularly well-positioned to support the reproductive autonomy and well-being of patients who struggle to engage with other clinicians. This article aims to help psychiatrists better understand EC so they can comfortably prescribe it before their patients need it.

What is emergency contraception?
EC is medications or devices that patients can use after sexual intercourse to prevent pregnancy. They do not impede the development of an established pregnancy and thus are not abortifacients. EC is not recommended as a primary means of contraception,9 but it can be extremely valuable to reduce pregnancy risk after unprotected intercourse or contraceptive failures such as broken condoms or missed doses of birth control pills. EC can prevent ≥95% of pregnancies when taken within 5 days of at-risk intercourse.11
Methods of EC fall into 2 categories: oral medications (sometimes referred to as “morning after pills”) and intrauterine devices (IUDs). IUDs are the most effective means of EC, especially for patients with higher BMIs or who may be taking medications such as cytochrome P450 (CYP)3A4 inducers that could interfere with the effectiveness of oral methods. IUDs also have the advantage of providing highly effective ongoing contraception.6 However, IUDs require in-office placement by a trained clinician, and patients may experience difficulty obtaining placement within 5 days of unprotected sex. Therefore, oral medication is the most common form of EC.
Oral EC is safe and effective, and professional societies (including the American College of Obstetricians and Gynecologists6 and the American Academy of Pediatrics7) recommend routinely prescribing oral EC for patients in advance of need. Advance prescribing eliminates barriers to accessing EC, increases the use of EC, and does not encourage risky sexual behaviors.10
Overview of oral emergency contraception
Two medications are FDA-approved for use as oral EC: ulipristal acetate and levonorgestrel. Both are available in generic and branded versions. While many common birth control pills can also be safely used off-label as emergency contraception (an approach known as the Yuzpe method), they are less effective, not as well-tolerated, and require knowledge of the specific type of pill the patient has available.9 Oral EC appears to work primarily through delay or inhibition of ovulation, and is unlikely to prevent implantation of a fertilized egg.9
Continue to: Ulipristal acetate
Ulipristal acetate (UPA) is an oral progesterone receptor agonist-antagonist taken as a single 30 mg dose up to 5 days after unprotected sex. Pregnancy rates from a single act of unprotected sex followed by UPA use range from 0% to 1.8%.4 Many pharmacies stock UPA, and others (especially chain pharmacies) report being able to order and fill it within 24 hours.12
Levonorgestrel (LNG) is an oral progestin that is available by prescription and has also been approved for over-the-counter sale to patients of all ages and sexes (without the need to show identification) since 2013.8 It is administered as a single 1.5 mg dose taken as soon as possible up to 3 days after unprotected sex, although it may continue to provide benefits when taken within 5 days. Pregnancy rates from a single act of unprotected sex followed by LNG use range from 0.3% to 2.6%, with much higher odds among women who are obese.4 LNG is available both by prescription or over-the-counter,13 although it is often kept in a locked cabinet or behind the counter, and staff are often misinformed regarding the lack of age restrictions for sale without a prescription.14
Safety and adverse effects. According to the CDC, there are no conditions for which the risks outweigh the advantages of use of either UPA or LNG,5 and patients for whom hormonal birth control is otherwise contraindicated can still use them safely. If a pregnancy has already occurred, taking EC will not harm the developing fetus; it is also safe to use when breastfeeding.5 Both medications are generally well-tolerated—neither has been causally linked to deaths or serious complications,5 and the most common adverse effects are headache (approximately 19%) and nausea (approximately 12%), in addition to irregular bleeding, fatigue, dizziness, and abdominal pain.15 Oral EC may be used more than once, even within the same menstrual cycle. Patients who use EC repeatedly should be encouraged to discuss more efficacious contraceptive options with their primary physician or gynecologist.
Will oral EC affect psychiatric treatment?
Oral EC is unlikely to have a meaningful effect on psychiatric symptoms or management, particularly when compared to the significant impacts of unintended pregnancies. Neither medication is known to have any clinically significant impacts on the pharmacokinetics or pharmacodynamics of psychotropic medications, although the effectiveness of both medications can be impaired by CYP3A4 inducers such as carbamazepine.5 In addition, while research has not specifically examined the impact of EC on psychiatric symptoms, the broader literature on hormonal contraception indicates that most patients with psychiatric disorders generally report similar or lower rates of mood symptoms associated with their use.16 Some women treated with hormonal contraceptives do develop dysphoric mood,16 but any such effects resulting from LNG would likely be transient. Mood disruptions or other psychiatric symptoms have not been associated with UPA use.
How to prescribe oral emergency contraception
Who and when. Women of reproductive age should be counseled about EC as part of anticipatory guidance, regardless of their current intentions for sexual behaviors. Patients do not need a physical examination or pregnancy test before being prescribed or using oral EC.9 Much like how intranasal naloxone is prescribed, prescriptions should be provided in advance of need, with multiple refills to facilitate ready access when needed.
Continue to: Which to prescribe
Which to prescribe. UPA is more effective in preventing pregnancy than LNG at all time points up to 120 hours after sex, including for women who are overweight or obese.15 As such, it is recommended as the first-line choice. However, because LNG is available without prescription and is more readily available (including via online order), it may be a good choice for patients who need rapid EC or who prefer a medication that does not require a prescription (Table 24,5,8,9,15).

What to tell patients. Patients should be instructed to fill their prescription before they expect to use it, to ensure ready availability when desired (Table 35,9). Oral EC is shelf stable for at least 3 years when stored in a cool, dry environment. Patients should take the medication as soon as possible following at-risk sexual intercourse (Table 4). Tell them that if they vomit within 3 hours of taking the medication, they should take a second dose. Remind patients that EC does not protect against sexually transmitted infections, or from sex that occurs after the medication is taken (in fact, they can increase the possibility of pregnancy later in that menstrual cycle due to delayed ovulation).9 Counsel patients to abstain from sex or to use barrier contraception for 7 days after use. Those who take birth control pills can resume use immediately after using LNG; they should wait 5 days after taking UPA.

No routine follow-up is needed after taking UPA or LNG. However, patients should get a pregnancy test if their period does not start within 3 weeks, and should seek medical evaluation if they experience significant lower abdominal pain or persistent irregular bleeding in order to rule out pregnancy-related complications. Patients who use EC repeatedly should be recommended to pursue routine contraceptive care.

Billing. Counseling your patients about contraception can increase the reimbursement you receive by adding to the complexity of the encounter (regardless of whether you prescribe a medication) through use of the ICD-10 code Z30.0.
Emergency contraception for special populations
Some patients face additional challenges to effective EC that should be considered when counseling and prescribing. Table 54,5,7,15,17-21 discusses the use of EC in these special populations. Of particular importance for psychiatrists, LNG is less effective at preventing undesired pregnancy among patients who are overweight or obese,15,17,18 and strong CYP3A4-inducing agents may decrease the effectiveness of both LNG and UPA.5 Keep in mind, however, that the advantages of using either UPA or LNG outweigh the risks for all populations.5 Patients must be aware of appropriate information in order to make informed decisions, but should not be discouraged from using EC.

Continue to: Other groups of patients...
Other groups of patients may face barriers due to some clinicians’ hesitancy regarding their ability to consent to reproductive care. Most patients with psychiatric illnesses have decision-making capacity regarding reproductive issues.22 Although EC is supported by the American Academy of Pediatrics,7 patients age <18 have varying rights to consent across states,21 and merit special consideration.
CASE CONTINUED
Ms. A does not wish to get pregnant at this time, and expresses fears that her recent contraceptive failure could lead to an unintended pregnancy. In addition to her psychiatric treatment, her psychiatrist should discuss EC options with her. She has a healthy BMI and had inadequately protected sex <1 day ago, so her clinician may prescribe LNG (to ensure rapid access for immediate use) in addition to UPA for her to have available in case of future “scares.” The psychiatrist should consider pharmacologic treatment with an antidepressant with a relatively safe reproductive record (eg, sertraline).23 This is considered preventive ethics, since Ms. A is of reproductive age, even if she is not presently planning to get pregnant, due to the aforementioned high rate of unplanned pregnancy.23,24 It is also important for the psychiatrist to continue the dialogue in future sessions about preventing unintended pregnancy. Since Ms. A has benefited from a psychotropic medication when not pregnant, it will be important to discuss with her the risks and benefits of medication should she plan a pregnancy.
Bottom Line
Patients with mental illnesses are at increased risk of adverse outcomes resulting from unintended pregnancies. Clinicians should counsel patients about emergency contraception (EC) as a part of routine psychiatric care, and should prescribe oral EC in advance of patient need to facilitate effective use.
Related Resources
- American College of Obstetricians and Gynecologists Practice Bulletin on Emergency Contraception. https://www.acog.org/clinical/clinical-guidance/practice-bulletin/ articles/2015/09/emergency-contraception
- State policies on emergency contraception. https://www.guttmacher.org/state-policy/explore/emergency-contraception
- State policies on minors’ access to contraceptive services. https://www.guttmacher.org/state-policy/explore/minors-access-contraceptive-services
- Patient-oriented contraceptive education materials (in English and Spanish). https://shop.powertodecide.org/ptd-category/educational-materials
Drug Brand Names
Carbamazepine • Tegretol
Levonorgestrel • Plan B One-Step, Fallback
Metformin • Glucophage
Naloxone • Narcan
Norethindrone • Aygestin
Sertraline • Zoloft
Topiramate • Topamax
Ulipristal acetate • Ella
Venlafaxine • Effexor
Ms. A, age 22, is a college student who presents for an initial psychiatric evaluation. Her body mass index (BMI) is 20 (normal range: 18.5 to 24.9), and her medical history is positive only for childhood asthma. She has been treated for major depressive disorder with venlafaxine by her previous psychiatrist. While this antidepressant has been effective for some symptoms, she has experienced adverse effects and is interested in a different medication. During the evaluation, Ms. A remarks that she had a “scare” last night when the condom broke while having sex with her boyfriend. She says that she is interested in having children at some point, but not at present; she is concerned that getting pregnant now would cause her depression to “spiral out of control.”
Unwanted or mistimed pregnancies account for 45% of all pregnancies.1 While there are ramifications for any unintended pregnancy, the risks for patients with mental illness are greater and include potential adverse effects on the neonate from both psychiatric disease and psychiatric medication use, worse obstetrical outcomes for patients with untreated mental illness, and worsening of psychiatric symptoms and suicide risk in the peripartum period.2 These risks become even more pronounced when psychiatric medications are reflexively discontinued or reduced in pregnancy, which is commonly done contrary to best practice recommendations. In the United States, the recent Supreme Court decision in Dobbs v Jackson Women’s Health Organization has erased federal protections for abortion previously conferred by Roe v Wade. As a result, as of early October 2022, abortion had been made illegal in 11 states, and was likely to be banned in many others, most commonly in states where there is limited support for either parents or children. Thus, preventing unplanned pregnancies should be a treatment consideration for all medical disciplines.3
Psychiatrists may hesitate to prescribe emergency contraception (EC) due to fears it falls outside the scope of their practice. However, psychiatry has already moved towards prescribing nonpsychiatric medications when doing so clearly benefits the patient. One example is prescribing metformin to address metabolic syndrome related to the use of second-generation antipsychotics. Emergency contraceptives have strong safety profiles and are easy to prescribe. Unfortunately, there are many barriers to increasing access to emergency contraceptives for psychiatric patients.4 These include the erroneous belief that laboratory and physical exams are needed before starting EC, cost and/or limited stock of emergency contraceptives at pharmacies, and general confusion regarding what constitutes EC vs an oral abortive (Table 15-10). Psychiatrists are particularly well-positioned to support the reproductive autonomy and well-being of patients who struggle to engage with other clinicians. This article aims to help psychiatrists better understand EC so they can comfortably prescribe it before their patients need it.

What is emergency contraception?
EC is medications or devices that patients can use after sexual intercourse to prevent pregnancy. They do not impede the development of an established pregnancy and thus are not abortifacients. EC is not recommended as a primary means of contraception,9 but it can be extremely valuable to reduce pregnancy risk after unprotected intercourse or contraceptive failures such as broken condoms or missed doses of birth control pills. EC can prevent ≥95% of pregnancies when taken within 5 days of at-risk intercourse.11
Methods of EC fall into 2 categories: oral medications (sometimes referred to as “morning after pills”) and intrauterine devices (IUDs). IUDs are the most effective means of EC, especially for patients with higher BMIs or who may be taking medications such as cytochrome P450 (CYP)3A4 inducers that could interfere with the effectiveness of oral methods. IUDs also have the advantage of providing highly effective ongoing contraception.6 However, IUDs require in-office placement by a trained clinician, and patients may experience difficulty obtaining placement within 5 days of unprotected sex. Therefore, oral medication is the most common form of EC.
Oral EC is safe and effective, and professional societies (including the American College of Obstetricians and Gynecologists6 and the American Academy of Pediatrics7) recommend routinely prescribing oral EC for patients in advance of need. Advance prescribing eliminates barriers to accessing EC, increases the use of EC, and does not encourage risky sexual behaviors.10
Overview of oral emergency contraception
Two medications are FDA-approved for use as oral EC: ulipristal acetate and levonorgestrel. Both are available in generic and branded versions. While many common birth control pills can also be safely used off-label as emergency contraception (an approach known as the Yuzpe method), they are less effective, not as well-tolerated, and require knowledge of the specific type of pill the patient has available.9 Oral EC appears to work primarily through delay or inhibition of ovulation, and is unlikely to prevent implantation of a fertilized egg.9
Continue to: Ulipristal acetate
Ulipristal acetate (UPA) is an oral progesterone receptor agonist-antagonist taken as a single 30 mg dose up to 5 days after unprotected sex. Pregnancy rates from a single act of unprotected sex followed by UPA use range from 0% to 1.8%.4 Many pharmacies stock UPA, and others (especially chain pharmacies) report being able to order and fill it within 24 hours.12
Levonorgestrel (LNG) is an oral progestin that is available by prescription and has also been approved for over-the-counter sale to patients of all ages and sexes (without the need to show identification) since 2013.8 It is administered as a single 1.5 mg dose taken as soon as possible up to 3 days after unprotected sex, although it may continue to provide benefits when taken within 5 days. Pregnancy rates from a single act of unprotected sex followed by LNG use range from 0.3% to 2.6%, with much higher odds among women who are obese.4 LNG is available both by prescription or over-the-counter,13 although it is often kept in a locked cabinet or behind the counter, and staff are often misinformed regarding the lack of age restrictions for sale without a prescription.14
Safety and adverse effects. According to the CDC, there are no conditions for which the risks outweigh the advantages of use of either UPA or LNG,5 and patients for whom hormonal birth control is otherwise contraindicated can still use them safely. If a pregnancy has already occurred, taking EC will not harm the developing fetus; it is also safe to use when breastfeeding.5 Both medications are generally well-tolerated—neither has been causally linked to deaths or serious complications,5 and the most common adverse effects are headache (approximately 19%) and nausea (approximately 12%), in addition to irregular bleeding, fatigue, dizziness, and abdominal pain.15 Oral EC may be used more than once, even within the same menstrual cycle. Patients who use EC repeatedly should be encouraged to discuss more efficacious contraceptive options with their primary physician or gynecologist.
Will oral EC affect psychiatric treatment?
Oral EC is unlikely to have a meaningful effect on psychiatric symptoms or management, particularly when compared to the significant impacts of unintended pregnancies. Neither medication is known to have any clinically significant impacts on the pharmacokinetics or pharmacodynamics of psychotropic medications, although the effectiveness of both medications can be impaired by CYP3A4 inducers such as carbamazepine.5 In addition, while research has not specifically examined the impact of EC on psychiatric symptoms, the broader literature on hormonal contraception indicates that most patients with psychiatric disorders generally report similar or lower rates of mood symptoms associated with their use.16 Some women treated with hormonal contraceptives do develop dysphoric mood,16 but any such effects resulting from LNG would likely be transient. Mood disruptions or other psychiatric symptoms have not been associated with UPA use.
How to prescribe oral emergency contraception
Who and when. Women of reproductive age should be counseled about EC as part of anticipatory guidance, regardless of their current intentions for sexual behaviors. Patients do not need a physical examination or pregnancy test before being prescribed or using oral EC.9 Much like how intranasal naloxone is prescribed, prescriptions should be provided in advance of need, with multiple refills to facilitate ready access when needed.
Continue to: Which to prescribe
Which to prescribe. UPA is more effective in preventing pregnancy than LNG at all time points up to 120 hours after sex, including for women who are overweight or obese.15 As such, it is recommended as the first-line choice. However, because LNG is available without prescription and is more readily available (including via online order), it may be a good choice for patients who need rapid EC or who prefer a medication that does not require a prescription (Table 24,5,8,9,15).

What to tell patients. Patients should be instructed to fill their prescription before they expect to use it, to ensure ready availability when desired (Table 35,9). Oral EC is shelf stable for at least 3 years when stored in a cool, dry environment. Patients should take the medication as soon as possible following at-risk sexual intercourse (Table 4). Tell them that if they vomit within 3 hours of taking the medication, they should take a second dose. Remind patients that EC does not protect against sexually transmitted infections, or from sex that occurs after the medication is taken (in fact, they can increase the possibility of pregnancy later in that menstrual cycle due to delayed ovulation).9 Counsel patients to abstain from sex or to use barrier contraception for 7 days after use. Those who take birth control pills can resume use immediately after using LNG; they should wait 5 days after taking UPA.

No routine follow-up is needed after taking UPA or LNG. However, patients should get a pregnancy test if their period does not start within 3 weeks, and should seek medical evaluation if they experience significant lower abdominal pain or persistent irregular bleeding in order to rule out pregnancy-related complications. Patients who use EC repeatedly should be recommended to pursue routine contraceptive care.

Billing. Counseling your patients about contraception can increase the reimbursement you receive by adding to the complexity of the encounter (regardless of whether you prescribe a medication) through use of the ICD-10 code Z30.0.
Emergency contraception for special populations
Some patients face additional challenges to effective EC that should be considered when counseling and prescribing. Table 54,5,7,15,17-21 discusses the use of EC in these special populations. Of particular importance for psychiatrists, LNG is less effective at preventing undesired pregnancy among patients who are overweight or obese,15,17,18 and strong CYP3A4-inducing agents may decrease the effectiveness of both LNG and UPA.5 Keep in mind, however, that the advantages of using either UPA or LNG outweigh the risks for all populations.5 Patients must be aware of appropriate information in order to make informed decisions, but should not be discouraged from using EC.

Continue to: Other groups of patients...
Other groups of patients may face barriers due to some clinicians’ hesitancy regarding their ability to consent to reproductive care. Most patients with psychiatric illnesses have decision-making capacity regarding reproductive issues.22 Although EC is supported by the American Academy of Pediatrics,7 patients age <18 have varying rights to consent across states,21 and merit special consideration.
CASE CONTINUED
Ms. A does not wish to get pregnant at this time, and expresses fears that her recent contraceptive failure could lead to an unintended pregnancy. In addition to her psychiatric treatment, her psychiatrist should discuss EC options with her. She has a healthy BMI and had inadequately protected sex <1 day ago, so her clinician may prescribe LNG (to ensure rapid access for immediate use) in addition to UPA for her to have available in case of future “scares.” The psychiatrist should consider pharmacologic treatment with an antidepressant with a relatively safe reproductive record (eg, sertraline).23 This is considered preventive ethics, since Ms. A is of reproductive age, even if she is not presently planning to get pregnant, due to the aforementioned high rate of unplanned pregnancy.23,24 It is also important for the psychiatrist to continue the dialogue in future sessions about preventing unintended pregnancy. Since Ms. A has benefited from a psychotropic medication when not pregnant, it will be important to discuss with her the risks and benefits of medication should she plan a pregnancy.
Bottom Line
Patients with mental illnesses are at increased risk of adverse outcomes resulting from unintended pregnancies. Clinicians should counsel patients about emergency contraception (EC) as a part of routine psychiatric care, and should prescribe oral EC in advance of patient need to facilitate effective use.
Related Resources
- American College of Obstetricians and Gynecologists Practice Bulletin on Emergency Contraception. https://www.acog.org/clinical/clinical-guidance/practice-bulletin/ articles/2015/09/emergency-contraception
- State policies on emergency contraception. https://www.guttmacher.org/state-policy/explore/emergency-contraception
- State policies on minors’ access to contraceptive services. https://www.guttmacher.org/state-policy/explore/minors-access-contraceptive-services
- Patient-oriented contraceptive education materials (in English and Spanish). https://shop.powertodecide.org/ptd-category/educational-materials
Drug Brand Names
Carbamazepine • Tegretol
Levonorgestrel • Plan B One-Step, Fallback
Metformin • Glucophage
Naloxone • Narcan
Norethindrone • Aygestin
Sertraline • Zoloft
Topiramate • Topamax
Ulipristal acetate • Ella
Venlafaxine • Effexor
1. Grossman D. Expanding access to short-acting hormonal contraceptive methods in the United States. JAMA Intern Med. 2019;179:1209-1210.
2. Gur TL, Kim DR, Epperson CN. Central nervous system effects of prenatal selective serotonin reuptake inhibitors: sensing the signal through the noise. Psychopharmacology (Berl). 2013;227:567-582.
3. Ross N, Landess J, Kaempf A, et al. Pregnancy termination: what psychiatrists need to know. Current Psychiatry. 2022;21:8-9.
4. Haeger KO, Lamme J, Cleland K. State of emergency contraception in the US, 2018. Contracept Reprod Med. 2018;3:20.
5. Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-3.
6. American College of Obstetricians and Gynecologists. Committee Opinion No 707: Access to emergency contraception. Obstet Gynecol. 2017;130:e48-e52.
7. Upadhya KK, Breuner CC, Alderman EM, et al. Emergency contraception. Pediatrics. 2019;144:e20193149.
8. Rowan A. Obama administration yields to the courts and the evidence, allows emergency contraception to be sold without restrictions. Guttmacher Institute. Published June 25, 2013. Accessed July 31, 2022. https://www.guttmacher.org/gpr/2013/06/obama-administration-yields-courts-and-evidence-allows-emergency-contraception-be-sold#
9. American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
10. Rodriguez MI, Curtis KM, Gaffield ML, et al. Advance supply of emergency contraception: a systematic review. Contraception. 2013;87:590-601.
11. World Health Organization. Emergency contraception. Published November 9, 2021. Accessed August 4, 2022. https://www.who.int/news-room/fact-sheets/detail/emergency-contraception
12. Shigesato M, Elia J, Tschann M, et al. Pharmacy access to ulipristal acetate in major cities throughout the United States. Contraception. 2018;97:264-269.
13. Wilkinson TA, Clark P, Rafie S, et al. Access to emergency contraception after removal of age restrictions. Pediatrics. 2017;140:e20164262.
14. Cleland K, Bass J, Doci F, et al. Access to emergency contraception in the over-the-counter era. Women’s Health Issues. 2016;26:622-627.
15. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
16. McCloskey LR, Wisner KL, Cattan MK, et al. Contraception for women with psychiatric disorders. Am J Psychiatry. 2021;178:247-255.
17. Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91:97-104.
18. Festin MP, Peregoudov A, Seuc A, et al. Effect of BMI and body weight on pregnancy rates with LNG as emergency contraception: analysis of four WHO HRP studies. Contraception. 2017;95:50-54.
19. Edelman AB, Hennebold JD, Bond K, et al. Double dosing levonorgestrel-based emergency contraception for individuals with obesity: a randomized controlled trial. Obstet Gynecol. 2022;140(1):48-54.
20. FSRH Clinical Effectiveness Unit. FSRH clinical guideline: Emergency contraception. Published March 2017. Amended December 2020. Faculty of Sexual & Reproductive Healthcare. Accessed August 4, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-emergency-contraception-march-2017/
21. Guttmacher Institute. Minors’ access to contraceptive services. Guttmacher Institute. Accessed August 4, 2022. https://www.guttmacher.org/state-policy/explore/minors-access-contraceptive-services
22. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022;63:61-70.
23. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20:30-36.
24. Friedman SH. The ethics of treating depression in pregnancy. J Primary Healthcare. 2015;7:81-83.
1. Grossman D. Expanding access to short-acting hormonal contraceptive methods in the United States. JAMA Intern Med. 2019;179:1209-1210.
2. Gur TL, Kim DR, Epperson CN. Central nervous system effects of prenatal selective serotonin reuptake inhibitors: sensing the signal through the noise. Psychopharmacology (Berl). 2013;227:567-582.
3. Ross N, Landess J, Kaempf A, et al. Pregnancy termination: what psychiatrists need to know. Current Psychiatry. 2022;21:8-9.
4. Haeger KO, Lamme J, Cleland K. State of emergency contraception in the US, 2018. Contracept Reprod Med. 2018;3:20.
5. Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-3.
6. American College of Obstetricians and Gynecologists. Committee Opinion No 707: Access to emergency contraception. Obstet Gynecol. 2017;130:e48-e52.
7. Upadhya KK, Breuner CC, Alderman EM, et al. Emergency contraception. Pediatrics. 2019;144:e20193149.
8. Rowan A. Obama administration yields to the courts and the evidence, allows emergency contraception to be sold without restrictions. Guttmacher Institute. Published June 25, 2013. Accessed July 31, 2022. https://www.guttmacher.org/gpr/2013/06/obama-administration-yields-courts-and-evidence-allows-emergency-contraception-be-sold#
9. American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126:e1-e11.
10. Rodriguez MI, Curtis KM, Gaffield ML, et al. Advance supply of emergency contraception: a systematic review. Contraception. 2013;87:590-601.
11. World Health Organization. Emergency contraception. Published November 9, 2021. Accessed August 4, 2022. https://www.who.int/news-room/fact-sheets/detail/emergency-contraception
12. Shigesato M, Elia J, Tschann M, et al. Pharmacy access to ulipristal acetate in major cities throughout the United States. Contraception. 2018;97:264-269.
13. Wilkinson TA, Clark P, Rafie S, et al. Access to emergency contraception after removal of age restrictions. Pediatrics. 2017;140:e20164262.
14. Cleland K, Bass J, Doci F, et al. Access to emergency contraception in the over-the-counter era. Women’s Health Issues. 2016;26:622-627.
15. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
16. McCloskey LR, Wisner KL, Cattan MK, et al. Contraception for women with psychiatric disorders. Am J Psychiatry. 2021;178:247-255.
17. Kapp N, Abitbol JL, Mathé H, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception. 2015;91:97-104.
18. Festin MP, Peregoudov A, Seuc A, et al. Effect of BMI and body weight on pregnancy rates with LNG as emergency contraception: analysis of four WHO HRP studies. Contraception. 2017;95:50-54.
19. Edelman AB, Hennebold JD, Bond K, et al. Double dosing levonorgestrel-based emergency contraception for individuals with obesity: a randomized controlled trial. Obstet Gynecol. 2022;140(1):48-54.
20. FSRH Clinical Effectiveness Unit. FSRH clinical guideline: Emergency contraception. Published March 2017. Amended December 2020. Faculty of Sexual & Reproductive Healthcare. Accessed August 4, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-emergency-contraception-march-2017/
21. Guttmacher Institute. Minors’ access to contraceptive services. Guttmacher Institute. Accessed August 4, 2022. https://www.guttmacher.org/state-policy/explore/minors-access-contraceptive-services
22. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022;63:61-70.
23. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20:30-36.
24. Friedman SH. The ethics of treating depression in pregnancy. J Primary Healthcare. 2015;7:81-83.
Incorporating positive psychiatry with children and adolescents
The principles and practices of positive psychiatry are especially well-suited for work with children, adolescents, and families. Positive psychiatry is “the science and practice of psychiatry that seeks to understand and promote well-being through assessments and interventions aimed at enhancing positive psychosocial factors among people who have or are at risk for developing mental or physical illnesses.”1 The concept sprung from the momentum of positive psychology, which originated from Seligman et al.2 Importantly, the standards and techniques of positive psychiatry are designed as an enhancement, perhaps even as a completion, of more traditional psychiatry, rather than an alternative.3 They come from an acknowledgment that to be most effective as a mental health professional, it is important for clinicians to be experts in the full range of mental functioning.4,5
For most clinicians currently practicing “traditional” child and adolescent psychiatry, adapting at least some of the principles of positive psychiatry within one’s routine practice will not necessarily involve a radical transformation of thought or effort. Indeed, upon hearing about positive psychiatry principles, many nonprofessionals express surprise that this is not already considered routine practice. This article briefly outlines some of the basic tenets of positive child psychiatry and describes practical initial steps that can be readily incorporated into one’s day-to-day approach.
Defining pediatric positive psychiatry
There remains a fair amount of discussion and debate regarding what positive psychiatry is and isn’t, and how it fits into routine practice. While there is no official doctrine as to what “counts” as the practice of positive psychiatry, one can arguably divide most of its interventions into 2 main areas. The first is paying additional clinical attention to behaviors commonly associated with wellness or health promotion in youth. These include domains such as exercise, sleep habits, an authoritative parenting style, screen limits, and nutrition. The second area relates to specific techniques or procedures designed to cultivate positive emotions and mindsets; these often are referred to as positive psychology interventions (PPIs).6 Examples include gratitude exercises, practicing forgiveness, and activities that build optimism and hope. Many of the latter procedures share poorly defined boundaries with “tried and true” cognitive-behavioral therapy techniques, while others are more distinct to positive psychology and psychiatry. For both health promotion and PPIs, the goal of these interventions is to go beyond response and even remission for a patient to actual mental well-being, which is a construct that has also proven to be somewhat elusive and difficult to define. One well-described model by Seligman7 that has been gaining traction is the PERMA model, which breaks down well-being into 5 main components: positive emotions, engagement, relationships, meaning, and accomplishment.
Positive psychiatry: The evidence base
One myth about positive psychiatry is that it involves the pursuit of fringe and scientifically suspect techniques that have fallen under the expanding umbrella of “wellness.” Sadly, numerous unscientific and ineffective remedies have been widely promoted under the guise of wellness, leaving many families and clinicians uncertain about which areas have a solid evidence base and which are scientifically on shakier ground. While the lines delineating what are often referred to as PPI and more traditional psychotherapeutic techniques are blurry, there is increasing evidence supporting the use of PPI.8 A recent meta-analysis indicated that these techniques have larger effect sizes for children and young adults compared to older adults.9 More research, however, is needed, particularly for youth with diagnosable mental health conditions and for younger children.10
The evidence supporting the role of wellness and health promotion in preventing and treating pediatric mental health conditions has a quite robust research base. For example, a recent randomized controlled trial found greater reductions in multiple areas of emotional-behavior problems in children treated in a primary care setting with a wellness and health promotion model (the Vermont Family Based Approach) compared to those in a control condition.11 Another study examining the course of attention-deficit/hyperactivity disorder (ADHD) showed a 62% reduction of diagnosis among children who met 7 of 9 health promotion recommendations in areas such as nutrition, physical activity, and screen time, compared to those who met just 1 to 3 of these recommendations.12 Techniques such as mindfulness also have been found to be useful for adolescents with anxiety disorders.13 While a full review of the evidence is beyond the scope of this article, it is fair to say that many health promotion areas (such as exercise, nutrition, sleep habits, positive parenting skills, and some types of mindfulness) have strong scientific support—arguably at a level that is comparable to or even exceeds that of the off-label use of many psychiatric medications. The American Academy of Child and Adolescent Psychiatry has published a brief document that summarizes many age-related health promotion recommendations.14 The studies that underlie many of these recommendations contradict the misperception that wellness activities are only for already healthy individuals who want to become healthier, and show their utility for patients with more significant and chronic mental health conditions.
Incorporating core principles of positive psychiatry
Table 1 summarizes the core principles of positive child and adolescent psychiatry. There is no official procedure or certification one must complete to be considered a “positive psychiatrist,” and the term itself is somewhat debatable. Incorporating many of the principles of positive psychiatry into one’s daily routine does not necessitate a practice overhaul, and clinicians can integrate as many of these ideas as they deem clinically appropriate. That said, some adjustments to one’s perspective, approach, and workflow are likely needed, and the practice of positive psychiatry is arguably difficult to accomplish within the common “med check” model that emphasizes high volumes of short appointments that focus primarily on symptoms and adverse effects of medications.

Contrary to another misconception about positive psychiatry, working within a positive psychiatry framework does not involve encouraging patients to “put on a happy face” and ignore the very real suffering and trauma that many of them have experienced. Further, adhering to positive psychiatry does not entail abandoning the use of psychopharmacology (although careful prescribing is generally recommended) or applying gimmicks to superficially cover a person’s emotional pain.
Continue to: Rather, incorporating positive psychiatry...
Rather, incorporating positive psychiatry is best viewed as the creation of a supplementary toolbox that allows clinicians an expanded set of focus areas that can be used along with traditional psychotherapy and pharmacotherapy to help patients achieve a more robust and sustained response to treatment.4,5,15 The positive psychiatrist looks beyond the individual to examine a youth’s entire environment, and beyond areas of challenge to assess strengths, hopes, and aspirations.16 While many of these values are already in the formal description of a child psychiatrist, these priorities can take a back seat when trying to get through a busy day. For some, being a positive child psychiatrist means prescribing exercise rather than a sleep medication, assessing a child’s character strengths in addition to their behavioral challenges, or discussing the concept of parental warmth and how a struggling mother or father can replenish their tank when it feels like there is little left to give. It can mean reading literature on subjects such as happiness and optimal parenting practices in addition to depression and child maltreatment, and seeing oneself as an expert in mental health rather than just mental illness.
I have published a previous case example of positive psychiatry.17 Here I provide a brief vignette to further illustrate these concepts, and to compare traditional vs positive child psychiatry (Table 2).
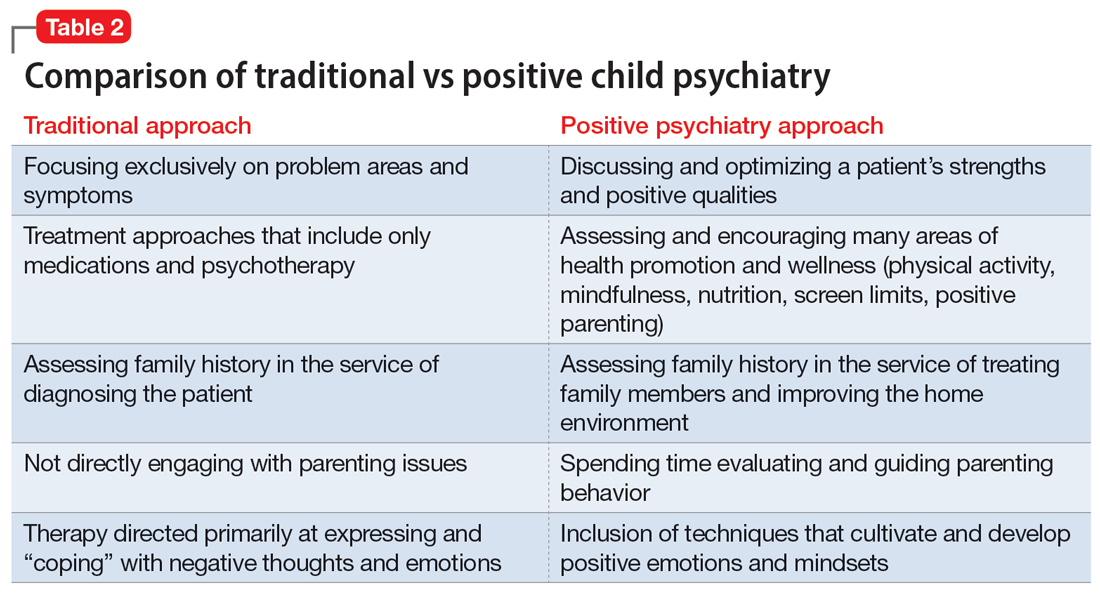
CASE REPORT
Tyler, age 7, presents to a child and adolescent psychiatrist for refractory ADHD problems, continued defiance, and aggressive outbursts. Approximately 1 year ago, Tyler’s pediatrician had diagnosed him with fairly classic ADHD symptoms and prescribed long-acting methylphenidate. Tyler’s attention has improved somewhat at school, but there remains a significant degree of conflict and dysregulation at home. Tyler remains easily frustrated and is often very negative. The pediatrician is looking for additional treatment recommendations.
Traditional approach
The child psychiatrist assesses Tyler and gathers data from the patient, his parents, and his school. She confirms the diagnosis of ADHD, but in reviewing other potential conditions also discovers that Tyler meets DSM-5 criteria for oppositional defiant disorder. The clinician suspects there may also be a co-occurring learning disability and notices that Tyler has chronic difficulties getting to sleep. She also hypothesizes the stimulant medication is wearing off at about the time Tyler gets home from school. The psychiatrist recommends adding an immediate-release formulation of methylphenidate upon return from school, melatonin at night, a school psychoeducational assessment, and behavioral therapy for Tyler and his parents to focus on his disrespectful and oppositional behavior.
Three months later, there has been incremental improvement with the additional medication and a school individualized education plan. Tyler is also working with a therapist, who does some play therapy with Tyler and works on helping his parents create incentives for prosocial behavior, but progress has been slow and the amount of improvement in this area is minimal. Further, the initial positive effect of the melatonin on sleep has waned lately, and the parents now ask about “something stronger.”
Continue to: Positive psychiatry approach
Positive psychiatry approach
In addition to assessing problem areas and DSM-5 criteria, the psychiatrist assesses a number of other domains. She finds that most of the interaction between Tyler and his parents are negative to the point that his parents often just stay out of his way. She also discovers that Tyler does little in the way of structured activities and spends most of his time at home playing video games, sometimes well into the evening. He gets little to no physical activity outside of school. He also is a very selective eater and often skips breakfast entirely due to the usually chaotic home scene in the morning. A brief mental health screen of the parents further reveals that the mother would also likely meet criteria for ADHD, and the father may be experiencing depression.
The psychiatrist prescribes an additional immediate-release formulation stimulant for the afternoon but holds off on prescribing sleep medication. Instead, she discusses a plan in which Tyler can earn his screen time by reading or exercising, and urges the parents to do some regular physical activity together. She discusses the findings of her screenings of the parents and helps them get a more thorough assessment. She also encourages more family time and introduces them to the “rose, thorn, bud” exercise where each family member discusses a success, challenge, and opportunity of the day.
Three months later, Tyler’s attention and negativity have decreased. His increased physical activity has helped his sleep, and ADHD treatment for the mother has made the mornings much smoother, allowing Tyler to eat a regular breakfast. Both improvements contribute further to Tyler’s improved attention during the day. Challenges remain, but the increased positive family experiences are helping the parents feel less depleted. As a result, they engage with Tyler more productively, and he has responded with more confidence and enthusiasm.
A natural extension of traditional work
The principles and practices associated with positive psychiatry represent a natural and highly needed extension of traditional work within child and adolescent psychiatry. Its emphasis on health promotion activities, family functioning, parental mental health, and utilization of strengths align closely with the growing scientific knowledge base that supports the complex interplay between the many genetic and environmental factors that underlie mental and physical health across the lifespan. For most psychiatrists, incorporating these important concepts and approaches will not require a radical transformation of one’s outlook or methodology, although some adjustments to practice and knowledge base augmentations are often needed. Clinicians interested in supplementing their skill set and working toward becoming an expert in the full range of mental functioning are encouraged to begin taking some of the steps outlined in this article to further their proficiency in the emerging discipline of positive psychiatry.
Bottom Line
Positive psychiatry is an important development that complements traditional approaches to child and adolescent mental health treatment through health promotion and cultivation of positive emotions and qualities. Incorporating it into routine practice is well within reach.
Related Resources
- Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Positive Psychology Center. University of Pennsylvania School of Arts and Sciences. https://ppc.sas.upenn.edu/
- Rettew DC. Building healthy brains: a brief tip sheet for parents and schools. American Academy of Child & Adolescent Psychiatry. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/schools/Wellness_Dev_Tips.pdf
Drug Brand Names
Methylphenidate extended-release • Concerta, Ritalin LA
1. Jeste DV, Palmer BW. Introduction: What is positive psychiatry? In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:1-16.
2. Seligman MEP, Csikszentmihalyi M. Positive psychology: an introduction. Am Psychol. 2000;55:5-14.
3. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76:675-683.
4. Rettew DC. Better than better: the new focus on well-being in child psychiatry. Child Adolesc Psychiatr Clin N Am. 2019;28:127-135.
5. Rettew DC. Positive child psychiatry. In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:285-304.
6. Parks AC, Kleiman EM, Kashdan TB, et al. Positive psychotherapeutic and behavioral interventions. In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:147-165.
7. Seligman MEP. Flourish: A Visionary New Understanding of Happiness and Well-Being. Simon & Shuster; 2012.
8. Brunwasser SM, Gillham JE, Kim ES. A meta-analytic review of the Penn Resiliency Program’s effect on depressive symptoms. J Consult Clin Psychol. 2009;77:1042-1054.
9. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Pos Psychol. 2021:16:749-769.
10. Benoit V, Gabola P. Effects of positive psychology interventions on the well-being of young children: a systematic literature review. Int J Environ Res Public Health. 2021;18:12065.
11. Ivanova MY, Hall A, Weinberger S, et al. The Vermont family based approach in primary care pediatrics: effects on children’s and parents’ emotional and behavioral problems and parents’ health-related quality of life. Child Psychiatry Hum Dev. Published online March 4, 2022. doi: 10.1007/s10578-022-01329-4
12. Lowen OK, Maximova K, Ekwaru JP, et al. Adherence to life-style recommendations and attention-deficit/hyperactivity disorder. Psychosom Med. 2020;82:305-315.
13. Zhou X, Guo J, et al. Effects of mindfulness-based stress reduction on anxiety symptoms in young people: a systematic review and meta-analysis. Psychiatry Res. 2020;289:113002.
14. Rettew DC. Building health brains: a brief tip sheet for parents and schools. American Academy of Child & Adolescent Psychiatry. Accessed May 11, 2022. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/schools/Wellness_Dev_Tips.pdf
15. Pustilnik S. Adapting well-being into outpatient child psychiatry. Child Adolesc Psychiatry Clin N Am. 2019;28:221-235.
16. Schlechter AD, O’Brien KH, Stewart C. The positive assessment: a model for integrating well-being and strengths-based approaches into the child and adolescent psychiatry clinical evaluation. Child Adolesc Psychiatry Clin N Am. 2019;28:157-169.
17. Rettew DC. A family- and wellness-based approach to child emotional-behavioral problems. In: RF Summers, Jeste DV, eds. Positive Psychiatry: A Casebook. American Psychiatric Association Publishing; 2019:29-44.
The principles and practices of positive psychiatry are especially well-suited for work with children, adolescents, and families. Positive psychiatry is “the science and practice of psychiatry that seeks to understand and promote well-being through assessments and interventions aimed at enhancing positive psychosocial factors among people who have or are at risk for developing mental or physical illnesses.”1 The concept sprung from the momentum of positive psychology, which originated from Seligman et al.2 Importantly, the standards and techniques of positive psychiatry are designed as an enhancement, perhaps even as a completion, of more traditional psychiatry, rather than an alternative.3 They come from an acknowledgment that to be most effective as a mental health professional, it is important for clinicians to be experts in the full range of mental functioning.4,5
For most clinicians currently practicing “traditional” child and adolescent psychiatry, adapting at least some of the principles of positive psychiatry within one’s routine practice will not necessarily involve a radical transformation of thought or effort. Indeed, upon hearing about positive psychiatry principles, many nonprofessionals express surprise that this is not already considered routine practice. This article briefly outlines some of the basic tenets of positive child psychiatry and describes practical initial steps that can be readily incorporated into one’s day-to-day approach.
Defining pediatric positive psychiatry
There remains a fair amount of discussion and debate regarding what positive psychiatry is and isn’t, and how it fits into routine practice. While there is no official doctrine as to what “counts” as the practice of positive psychiatry, one can arguably divide most of its interventions into 2 main areas. The first is paying additional clinical attention to behaviors commonly associated with wellness or health promotion in youth. These include domains such as exercise, sleep habits, an authoritative parenting style, screen limits, and nutrition. The second area relates to specific techniques or procedures designed to cultivate positive emotions and mindsets; these often are referred to as positive psychology interventions (PPIs).6 Examples include gratitude exercises, practicing forgiveness, and activities that build optimism and hope. Many of the latter procedures share poorly defined boundaries with “tried and true” cognitive-behavioral therapy techniques, while others are more distinct to positive psychology and psychiatry. For both health promotion and PPIs, the goal of these interventions is to go beyond response and even remission for a patient to actual mental well-being, which is a construct that has also proven to be somewhat elusive and difficult to define. One well-described model by Seligman7 that has been gaining traction is the PERMA model, which breaks down well-being into 5 main components: positive emotions, engagement, relationships, meaning, and accomplishment.
Positive psychiatry: The evidence base
One myth about positive psychiatry is that it involves the pursuit of fringe and scientifically suspect techniques that have fallen under the expanding umbrella of “wellness.” Sadly, numerous unscientific and ineffective remedies have been widely promoted under the guise of wellness, leaving many families and clinicians uncertain about which areas have a solid evidence base and which are scientifically on shakier ground. While the lines delineating what are often referred to as PPI and more traditional psychotherapeutic techniques are blurry, there is increasing evidence supporting the use of PPI.8 A recent meta-analysis indicated that these techniques have larger effect sizes for children and young adults compared to older adults.9 More research, however, is needed, particularly for youth with diagnosable mental health conditions and for younger children.10
The evidence supporting the role of wellness and health promotion in preventing and treating pediatric mental health conditions has a quite robust research base. For example, a recent randomized controlled trial found greater reductions in multiple areas of emotional-behavior problems in children treated in a primary care setting with a wellness and health promotion model (the Vermont Family Based Approach) compared to those in a control condition.11 Another study examining the course of attention-deficit/hyperactivity disorder (ADHD) showed a 62% reduction of diagnosis among children who met 7 of 9 health promotion recommendations in areas such as nutrition, physical activity, and screen time, compared to those who met just 1 to 3 of these recommendations.12 Techniques such as mindfulness also have been found to be useful for adolescents with anxiety disorders.13 While a full review of the evidence is beyond the scope of this article, it is fair to say that many health promotion areas (such as exercise, nutrition, sleep habits, positive parenting skills, and some types of mindfulness) have strong scientific support—arguably at a level that is comparable to or even exceeds that of the off-label use of many psychiatric medications. The American Academy of Child and Adolescent Psychiatry has published a brief document that summarizes many age-related health promotion recommendations.14 The studies that underlie many of these recommendations contradict the misperception that wellness activities are only for already healthy individuals who want to become healthier, and show their utility for patients with more significant and chronic mental health conditions.
Incorporating core principles of positive psychiatry
Table 1 summarizes the core principles of positive child and adolescent psychiatry. There is no official procedure or certification one must complete to be considered a “positive psychiatrist,” and the term itself is somewhat debatable. Incorporating many of the principles of positive psychiatry into one’s daily routine does not necessitate a practice overhaul, and clinicians can integrate as many of these ideas as they deem clinically appropriate. That said, some adjustments to one’s perspective, approach, and workflow are likely needed, and the practice of positive psychiatry is arguably difficult to accomplish within the common “med check” model that emphasizes high volumes of short appointments that focus primarily on symptoms and adverse effects of medications.

Contrary to another misconception about positive psychiatry, working within a positive psychiatry framework does not involve encouraging patients to “put on a happy face” and ignore the very real suffering and trauma that many of them have experienced. Further, adhering to positive psychiatry does not entail abandoning the use of psychopharmacology (although careful prescribing is generally recommended) or applying gimmicks to superficially cover a person’s emotional pain.
Continue to: Rather, incorporating positive psychiatry...
Rather, incorporating positive psychiatry is best viewed as the creation of a supplementary toolbox that allows clinicians an expanded set of focus areas that can be used along with traditional psychotherapy and pharmacotherapy to help patients achieve a more robust and sustained response to treatment.4,5,15 The positive psychiatrist looks beyond the individual to examine a youth’s entire environment, and beyond areas of challenge to assess strengths, hopes, and aspirations.16 While many of these values are already in the formal description of a child psychiatrist, these priorities can take a back seat when trying to get through a busy day. For some, being a positive child psychiatrist means prescribing exercise rather than a sleep medication, assessing a child’s character strengths in addition to their behavioral challenges, or discussing the concept of parental warmth and how a struggling mother or father can replenish their tank when it feels like there is little left to give. It can mean reading literature on subjects such as happiness and optimal parenting practices in addition to depression and child maltreatment, and seeing oneself as an expert in mental health rather than just mental illness.
I have published a previous case example of positive psychiatry.17 Here I provide a brief vignette to further illustrate these concepts, and to compare traditional vs positive child psychiatry (Table 2).

CASE REPORT
Tyler, age 7, presents to a child and adolescent psychiatrist for refractory ADHD problems, continued defiance, and aggressive outbursts. Approximately 1 year ago, Tyler’s pediatrician had diagnosed him with fairly classic ADHD symptoms and prescribed long-acting methylphenidate. Tyler’s attention has improved somewhat at school, but there remains a significant degree of conflict and dysregulation at home. Tyler remains easily frustrated and is often very negative. The pediatrician is looking for additional treatment recommendations.
Traditional approach
The child psychiatrist assesses Tyler and gathers data from the patient, his parents, and his school. She confirms the diagnosis of ADHD, but in reviewing other potential conditions also discovers that Tyler meets DSM-5 criteria for oppositional defiant disorder. The clinician suspects there may also be a co-occurring learning disability and notices that Tyler has chronic difficulties getting to sleep. She also hypothesizes the stimulant medication is wearing off at about the time Tyler gets home from school. The psychiatrist recommends adding an immediate-release formulation of methylphenidate upon return from school, melatonin at night, a school psychoeducational assessment, and behavioral therapy for Tyler and his parents to focus on his disrespectful and oppositional behavior.
Three months later, there has been incremental improvement with the additional medication and a school individualized education plan. Tyler is also working with a therapist, who does some play therapy with Tyler and works on helping his parents create incentives for prosocial behavior, but progress has been slow and the amount of improvement in this area is minimal. Further, the initial positive effect of the melatonin on sleep has waned lately, and the parents now ask about “something stronger.”
Continue to: Positive psychiatry approach
Positive psychiatry approach
In addition to assessing problem areas and DSM-5 criteria, the psychiatrist assesses a number of other domains. She finds that most of the interaction between Tyler and his parents are negative to the point that his parents often just stay out of his way. She also discovers that Tyler does little in the way of structured activities and spends most of his time at home playing video games, sometimes well into the evening. He gets little to no physical activity outside of school. He also is a very selective eater and often skips breakfast entirely due to the usually chaotic home scene in the morning. A brief mental health screen of the parents further reveals that the mother would also likely meet criteria for ADHD, and the father may be experiencing depression.
The psychiatrist prescribes an additional immediate-release formulation stimulant for the afternoon but holds off on prescribing sleep medication. Instead, she discusses a plan in which Tyler can earn his screen time by reading or exercising, and urges the parents to do some regular physical activity together. She discusses the findings of her screenings of the parents and helps them get a more thorough assessment. She also encourages more family time and introduces them to the “rose, thorn, bud” exercise where each family member discusses a success, challenge, and opportunity of the day.
Three months later, Tyler’s attention and negativity have decreased. His increased physical activity has helped his sleep, and ADHD treatment for the mother has made the mornings much smoother, allowing Tyler to eat a regular breakfast. Both improvements contribute further to Tyler’s improved attention during the day. Challenges remain, but the increased positive family experiences are helping the parents feel less depleted. As a result, they engage with Tyler more productively, and he has responded with more confidence and enthusiasm.
A natural extension of traditional work
The principles and practices associated with positive psychiatry represent a natural and highly needed extension of traditional work within child and adolescent psychiatry. Its emphasis on health promotion activities, family functioning, parental mental health, and utilization of strengths align closely with the growing scientific knowledge base that supports the complex interplay between the many genetic and environmental factors that underlie mental and physical health across the lifespan. For most psychiatrists, incorporating these important concepts and approaches will not require a radical transformation of one’s outlook or methodology, although some adjustments to practice and knowledge base augmentations are often needed. Clinicians interested in supplementing their skill set and working toward becoming an expert in the full range of mental functioning are encouraged to begin taking some of the steps outlined in this article to further their proficiency in the emerging discipline of positive psychiatry.
Bottom Line
Positive psychiatry is an important development that complements traditional approaches to child and adolescent mental health treatment through health promotion and cultivation of positive emotions and qualities. Incorporating it into routine practice is well within reach.
Related Resources
- Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Positive Psychology Center. University of Pennsylvania School of Arts and Sciences. https://ppc.sas.upenn.edu/
- Rettew DC. Building healthy brains: a brief tip sheet for parents and schools. American Academy of Child & Adolescent Psychiatry. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/schools/Wellness_Dev_Tips.pdf
Drug Brand Names
Methylphenidate extended-release • Concerta, Ritalin LA
The principles and practices of positive psychiatry are especially well-suited for work with children, adolescents, and families. Positive psychiatry is “the science and practice of psychiatry that seeks to understand and promote well-being through assessments and interventions aimed at enhancing positive psychosocial factors among people who have or are at risk for developing mental or physical illnesses.”1 The concept sprung from the momentum of positive psychology, which originated from Seligman et al.2 Importantly, the standards and techniques of positive psychiatry are designed as an enhancement, perhaps even as a completion, of more traditional psychiatry, rather than an alternative.3 They come from an acknowledgment that to be most effective as a mental health professional, it is important for clinicians to be experts in the full range of mental functioning.4,5
For most clinicians currently practicing “traditional” child and adolescent psychiatry, adapting at least some of the principles of positive psychiatry within one’s routine practice will not necessarily involve a radical transformation of thought or effort. Indeed, upon hearing about positive psychiatry principles, many nonprofessionals express surprise that this is not already considered routine practice. This article briefly outlines some of the basic tenets of positive child psychiatry and describes practical initial steps that can be readily incorporated into one’s day-to-day approach.
Defining pediatric positive psychiatry
There remains a fair amount of discussion and debate regarding what positive psychiatry is and isn’t, and how it fits into routine practice. While there is no official doctrine as to what “counts” as the practice of positive psychiatry, one can arguably divide most of its interventions into 2 main areas. The first is paying additional clinical attention to behaviors commonly associated with wellness or health promotion in youth. These include domains such as exercise, sleep habits, an authoritative parenting style, screen limits, and nutrition. The second area relates to specific techniques or procedures designed to cultivate positive emotions and mindsets; these often are referred to as positive psychology interventions (PPIs).6 Examples include gratitude exercises, practicing forgiveness, and activities that build optimism and hope. Many of the latter procedures share poorly defined boundaries with “tried and true” cognitive-behavioral therapy techniques, while others are more distinct to positive psychology and psychiatry. For both health promotion and PPIs, the goal of these interventions is to go beyond response and even remission for a patient to actual mental well-being, which is a construct that has also proven to be somewhat elusive and difficult to define. One well-described model by Seligman7 that has been gaining traction is the PERMA model, which breaks down well-being into 5 main components: positive emotions, engagement, relationships, meaning, and accomplishment.
Positive psychiatry: The evidence base
One myth about positive psychiatry is that it involves the pursuit of fringe and scientifically suspect techniques that have fallen under the expanding umbrella of “wellness.” Sadly, numerous unscientific and ineffective remedies have been widely promoted under the guise of wellness, leaving many families and clinicians uncertain about which areas have a solid evidence base and which are scientifically on shakier ground. While the lines delineating what are often referred to as PPI and more traditional psychotherapeutic techniques are blurry, there is increasing evidence supporting the use of PPI.8 A recent meta-analysis indicated that these techniques have larger effect sizes for children and young adults compared to older adults.9 More research, however, is needed, particularly for youth with diagnosable mental health conditions and for younger children.10
The evidence supporting the role of wellness and health promotion in preventing and treating pediatric mental health conditions has a quite robust research base. For example, a recent randomized controlled trial found greater reductions in multiple areas of emotional-behavior problems in children treated in a primary care setting with a wellness and health promotion model (the Vermont Family Based Approach) compared to those in a control condition.11 Another study examining the course of attention-deficit/hyperactivity disorder (ADHD) showed a 62% reduction of diagnosis among children who met 7 of 9 health promotion recommendations in areas such as nutrition, physical activity, and screen time, compared to those who met just 1 to 3 of these recommendations.12 Techniques such as mindfulness also have been found to be useful for adolescents with anxiety disorders.13 While a full review of the evidence is beyond the scope of this article, it is fair to say that many health promotion areas (such as exercise, nutrition, sleep habits, positive parenting skills, and some types of mindfulness) have strong scientific support—arguably at a level that is comparable to or even exceeds that of the off-label use of many psychiatric medications. The American Academy of Child and Adolescent Psychiatry has published a brief document that summarizes many age-related health promotion recommendations.14 The studies that underlie many of these recommendations contradict the misperception that wellness activities are only for already healthy individuals who want to become healthier, and show their utility for patients with more significant and chronic mental health conditions.
Incorporating core principles of positive psychiatry
Table 1 summarizes the core principles of positive child and adolescent psychiatry. There is no official procedure or certification one must complete to be considered a “positive psychiatrist,” and the term itself is somewhat debatable. Incorporating many of the principles of positive psychiatry into one’s daily routine does not necessitate a practice overhaul, and clinicians can integrate as many of these ideas as they deem clinically appropriate. That said, some adjustments to one’s perspective, approach, and workflow are likely needed, and the practice of positive psychiatry is arguably difficult to accomplish within the common “med check” model that emphasizes high volumes of short appointments that focus primarily on symptoms and adverse effects of medications.

Contrary to another misconception about positive psychiatry, working within a positive psychiatry framework does not involve encouraging patients to “put on a happy face” and ignore the very real suffering and trauma that many of them have experienced. Further, adhering to positive psychiatry does not entail abandoning the use of psychopharmacology (although careful prescribing is generally recommended) or applying gimmicks to superficially cover a person’s emotional pain.
Continue to: Rather, incorporating positive psychiatry...
Rather, incorporating positive psychiatry is best viewed as the creation of a supplementary toolbox that allows clinicians an expanded set of focus areas that can be used along with traditional psychotherapy and pharmacotherapy to help patients achieve a more robust and sustained response to treatment.4,5,15 The positive psychiatrist looks beyond the individual to examine a youth’s entire environment, and beyond areas of challenge to assess strengths, hopes, and aspirations.16 While many of these values are already in the formal description of a child psychiatrist, these priorities can take a back seat when trying to get through a busy day. For some, being a positive child psychiatrist means prescribing exercise rather than a sleep medication, assessing a child’s character strengths in addition to their behavioral challenges, or discussing the concept of parental warmth and how a struggling mother or father can replenish their tank when it feels like there is little left to give. It can mean reading literature on subjects such as happiness and optimal parenting practices in addition to depression and child maltreatment, and seeing oneself as an expert in mental health rather than just mental illness.
I have published a previous case example of positive psychiatry.17 Here I provide a brief vignette to further illustrate these concepts, and to compare traditional vs positive child psychiatry (Table 2).

CASE REPORT
Tyler, age 7, presents to a child and adolescent psychiatrist for refractory ADHD problems, continued defiance, and aggressive outbursts. Approximately 1 year ago, Tyler’s pediatrician had diagnosed him with fairly classic ADHD symptoms and prescribed long-acting methylphenidate. Tyler’s attention has improved somewhat at school, but there remains a significant degree of conflict and dysregulation at home. Tyler remains easily frustrated and is often very negative. The pediatrician is looking for additional treatment recommendations.
Traditional approach
The child psychiatrist assesses Tyler and gathers data from the patient, his parents, and his school. She confirms the diagnosis of ADHD, but in reviewing other potential conditions also discovers that Tyler meets DSM-5 criteria for oppositional defiant disorder. The clinician suspects there may also be a co-occurring learning disability and notices that Tyler has chronic difficulties getting to sleep. She also hypothesizes the stimulant medication is wearing off at about the time Tyler gets home from school. The psychiatrist recommends adding an immediate-release formulation of methylphenidate upon return from school, melatonin at night, a school psychoeducational assessment, and behavioral therapy for Tyler and his parents to focus on his disrespectful and oppositional behavior.
Three months later, there has been incremental improvement with the additional medication and a school individualized education plan. Tyler is also working with a therapist, who does some play therapy with Tyler and works on helping his parents create incentives for prosocial behavior, but progress has been slow and the amount of improvement in this area is minimal. Further, the initial positive effect of the melatonin on sleep has waned lately, and the parents now ask about “something stronger.”
Continue to: Positive psychiatry approach
Positive psychiatry approach
In addition to assessing problem areas and DSM-5 criteria, the psychiatrist assesses a number of other domains. She finds that most of the interaction between Tyler and his parents are negative to the point that his parents often just stay out of his way. She also discovers that Tyler does little in the way of structured activities and spends most of his time at home playing video games, sometimes well into the evening. He gets little to no physical activity outside of school. He also is a very selective eater and often skips breakfast entirely due to the usually chaotic home scene in the morning. A brief mental health screen of the parents further reveals that the mother would also likely meet criteria for ADHD, and the father may be experiencing depression.
The psychiatrist prescribes an additional immediate-release formulation stimulant for the afternoon but holds off on prescribing sleep medication. Instead, she discusses a plan in which Tyler can earn his screen time by reading or exercising, and urges the parents to do some regular physical activity together. She discusses the findings of her screenings of the parents and helps them get a more thorough assessment. She also encourages more family time and introduces them to the “rose, thorn, bud” exercise where each family member discusses a success, challenge, and opportunity of the day.
Three months later, Tyler’s attention and negativity have decreased. His increased physical activity has helped his sleep, and ADHD treatment for the mother has made the mornings much smoother, allowing Tyler to eat a regular breakfast. Both improvements contribute further to Tyler’s improved attention during the day. Challenges remain, but the increased positive family experiences are helping the parents feel less depleted. As a result, they engage with Tyler more productively, and he has responded with more confidence and enthusiasm.
A natural extension of traditional work
The principles and practices associated with positive psychiatry represent a natural and highly needed extension of traditional work within child and adolescent psychiatry. Its emphasis on health promotion activities, family functioning, parental mental health, and utilization of strengths align closely with the growing scientific knowledge base that supports the complex interplay between the many genetic and environmental factors that underlie mental and physical health across the lifespan. For most psychiatrists, incorporating these important concepts and approaches will not require a radical transformation of one’s outlook or methodology, although some adjustments to practice and knowledge base augmentations are often needed. Clinicians interested in supplementing their skill set and working toward becoming an expert in the full range of mental functioning are encouraged to begin taking some of the steps outlined in this article to further their proficiency in the emerging discipline of positive psychiatry.
Bottom Line
Positive psychiatry is an important development that complements traditional approaches to child and adolescent mental health treatment through health promotion and cultivation of positive emotions and qualities. Incorporating it into routine practice is well within reach.
Related Resources
- Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015.
- Positive Psychology Center. University of Pennsylvania School of Arts and Sciences. https://ppc.sas.upenn.edu/
- Rettew DC. Building healthy brains: a brief tip sheet for parents and schools. American Academy of Child & Adolescent Psychiatry. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/schools/Wellness_Dev_Tips.pdf
Drug Brand Names
Methylphenidate extended-release • Concerta, Ritalin LA
1. Jeste DV, Palmer BW. Introduction: What is positive psychiatry? In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:1-16.
2. Seligman MEP, Csikszentmihalyi M. Positive psychology: an introduction. Am Psychol. 2000;55:5-14.
3. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76:675-683.
4. Rettew DC. Better than better: the new focus on well-being in child psychiatry. Child Adolesc Psychiatr Clin N Am. 2019;28:127-135.
5. Rettew DC. Positive child psychiatry. In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:285-304.
6. Parks AC, Kleiman EM, Kashdan TB, et al. Positive psychotherapeutic and behavioral interventions. In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:147-165.
7. Seligman MEP. Flourish: A Visionary New Understanding of Happiness and Well-Being. Simon & Shuster; 2012.
8. Brunwasser SM, Gillham JE, Kim ES. A meta-analytic review of the Penn Resiliency Program’s effect on depressive symptoms. J Consult Clin Psychol. 2009;77:1042-1054.
9. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Pos Psychol. 2021:16:749-769.
10. Benoit V, Gabola P. Effects of positive psychology interventions on the well-being of young children: a systematic literature review. Int J Environ Res Public Health. 2021;18:12065.
11. Ivanova MY, Hall A, Weinberger S, et al. The Vermont family based approach in primary care pediatrics: effects on children’s and parents’ emotional and behavioral problems and parents’ health-related quality of life. Child Psychiatry Hum Dev. Published online March 4, 2022. doi: 10.1007/s10578-022-01329-4
12. Lowen OK, Maximova K, Ekwaru JP, et al. Adherence to life-style recommendations and attention-deficit/hyperactivity disorder. Psychosom Med. 2020;82:305-315.
13. Zhou X, Guo J, et al. Effects of mindfulness-based stress reduction on anxiety symptoms in young people: a systematic review and meta-analysis. Psychiatry Res. 2020;289:113002.
14. Rettew DC. Building health brains: a brief tip sheet for parents and schools. American Academy of Child & Adolescent Psychiatry. Accessed May 11, 2022. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/schools/Wellness_Dev_Tips.pdf
15. Pustilnik S. Adapting well-being into outpatient child psychiatry. Child Adolesc Psychiatry Clin N Am. 2019;28:221-235.
16. Schlechter AD, O’Brien KH, Stewart C. The positive assessment: a model for integrating well-being and strengths-based approaches into the child and adolescent psychiatry clinical evaluation. Child Adolesc Psychiatry Clin N Am. 2019;28:157-169.
17. Rettew DC. A family- and wellness-based approach to child emotional-behavioral problems. In: RF Summers, Jeste DV, eds. Positive Psychiatry: A Casebook. American Psychiatric Association Publishing; 2019:29-44.
1. Jeste DV, Palmer BW. Introduction: What is positive psychiatry? In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:1-16.
2. Seligman MEP, Csikszentmihalyi M. Positive psychology: an introduction. Am Psychol. 2000;55:5-14.
3. Jeste DV, Palmer BW, Rettew DC, et al. Positive psychiatry: its time has come. J Clin Psychiatry. 2015;76:675-683.
4. Rettew DC. Better than better: the new focus on well-being in child psychiatry. Child Adolesc Psychiatr Clin N Am. 2019;28:127-135.
5. Rettew DC. Positive child psychiatry. In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:285-304.
6. Parks AC, Kleiman EM, Kashdan TB, et al. Positive psychotherapeutic and behavioral interventions. In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. American Psychiatric Publishing; 2015:147-165.
7. Seligman MEP. Flourish: A Visionary New Understanding of Happiness and Well-Being. Simon & Shuster; 2012.
8. Brunwasser SM, Gillham JE, Kim ES. A meta-analytic review of the Penn Resiliency Program’s effect on depressive symptoms. J Consult Clin Psychol. 2009;77:1042-1054.
9. Carr A, Cullen K, Keeney C, et al. Effectiveness of positive psychology interventions: a systematic review and meta-analysis. J Pos Psychol. 2021:16:749-769.
10. Benoit V, Gabola P. Effects of positive psychology interventions on the well-being of young children: a systematic literature review. Int J Environ Res Public Health. 2021;18:12065.
11. Ivanova MY, Hall A, Weinberger S, et al. The Vermont family based approach in primary care pediatrics: effects on children’s and parents’ emotional and behavioral problems and parents’ health-related quality of life. Child Psychiatry Hum Dev. Published online March 4, 2022. doi: 10.1007/s10578-022-01329-4
12. Lowen OK, Maximova K, Ekwaru JP, et al. Adherence to life-style recommendations and attention-deficit/hyperactivity disorder. Psychosom Med. 2020;82:305-315.
13. Zhou X, Guo J, et al. Effects of mindfulness-based stress reduction on anxiety symptoms in young people: a systematic review and meta-analysis. Psychiatry Res. 2020;289:113002.
14. Rettew DC. Building health brains: a brief tip sheet for parents and schools. American Academy of Child & Adolescent Psychiatry. Accessed May 11, 2022. https://www.aacap.org/App_Themes/AACAP/Docs/resource_centers/schools/Wellness_Dev_Tips.pdf
15. Pustilnik S. Adapting well-being into outpatient child psychiatry. Child Adolesc Psychiatry Clin N Am. 2019;28:221-235.
16. Schlechter AD, O’Brien KH, Stewart C. The positive assessment: a model for integrating well-being and strengths-based approaches into the child and adolescent psychiatry clinical evaluation. Child Adolesc Psychiatry Clin N Am. 2019;28:157-169.
17. Rettew DC. A family- and wellness-based approach to child emotional-behavioral problems. In: RF Summers, Jeste DV, eds. Positive Psychiatry: A Casebook. American Psychiatric Association Publishing; 2019:29-44.
Complex trauma in the perinatal period
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5
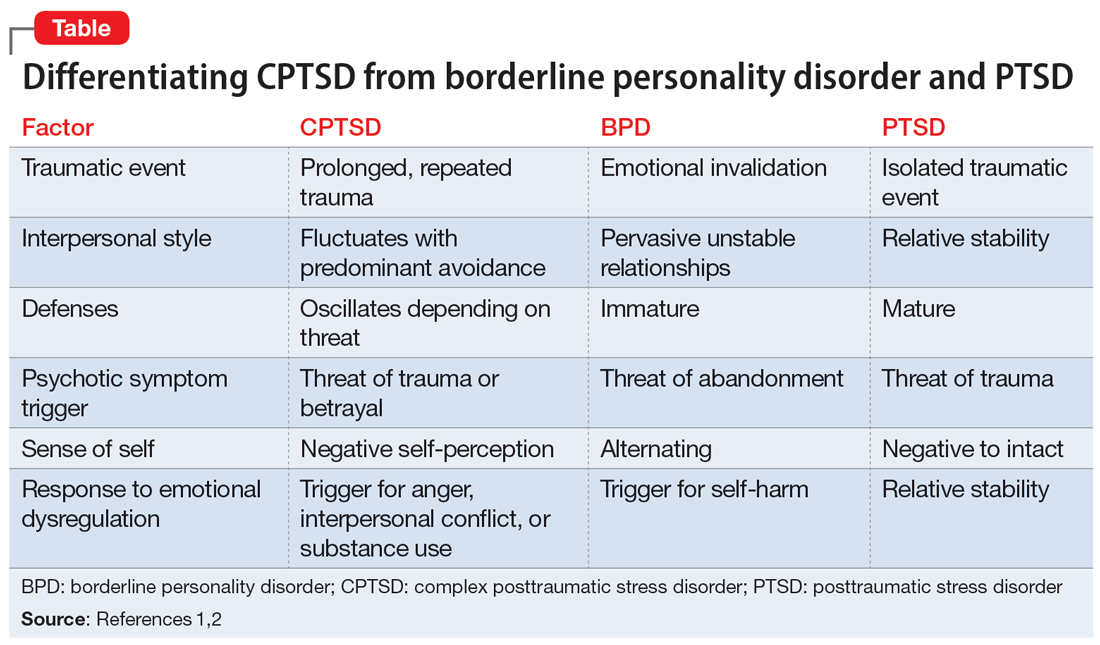
Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.
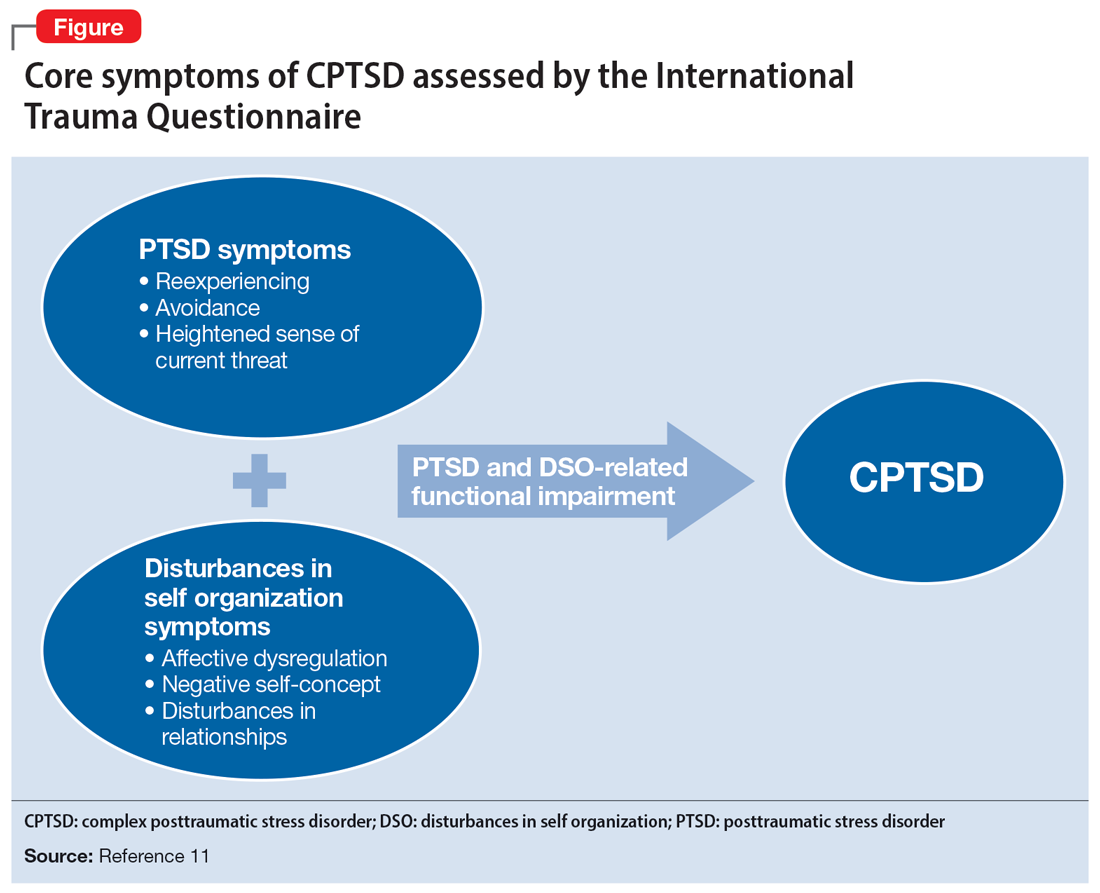
Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5

Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.

Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5

Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.

Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
Is evolution’s greatest triumph its worst blunder?
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
Of all the dazzling achievements of evolution, the most glorious by far is the emergence of the advanced human brain, especially the prefrontal cortex. Homo sapiens (the wise humans) are without doubt the most transformative development in the consequential annals of evolution. It was evolution’s spectacular “moonshot.” Ironically, it may also have been the seed of its destruction.
The unprecedented growth of the human brain over the past 7 million years (tripling in size) was a monumental tipping point in evolution that ultimately disrupted the entire orderly cascade of evolution on Planet Earth. Because of their superior intelligence, Homo sapiens have substantially “tinkered” with the foundations of evolution, such as “natural selection” and “survival of the fittest,” and may eventually change the course of evolution, or even reverse it. It should also be recognized that 20% of the human genome is Neanderthal, and the 2022 Nobel Prize in Physiology or Medicine was awarded to Svante Pääbo, the founder of the field of paleogenetics, who demonstrated genetically that Homo sapiens interbred with Homo neanderthalensis (who disappeared 30,000 years ago).
The majestic evolution of the human brain, in both size and complexity, led to monumental changes in the history of humankind compared to their primitive predecessors. Thanks to a superior cerebral cortex, humans developed traits and abilities that were nonexistent, even unimaginable, in the rest of animal kingdom, including primates and other mammals. These include thoughts; speech (hundreds of languages), spoken and written, to communicate among themselves; composed music and created numerous instruments to play it; invented mathematics, physics, and chemistry; developed agriculture to sustain and feed the masses; built homes, palaces, and pyramids, with water and sewage systems; hatched hundreds of religions and built thousands of houses of worship; built machines to transport themselves (cars, trains, ships, planes, and space shuttles); paved airports and countless miles of roads and railways; established companies, universities, hospitals, and research laboratories; built sports facilities such as stadiums for Olympic games and all its athletics; created hotels, restaurants, coffee shops, newspapers, and magazines; discovered the amazing DNA double helix and its genome with 23,000 coding genes containing instructions to build the brain and 200 other body tissues; developed surgeries and invented medications for diseases that would have killed millions every year; and established paper money to replace gold and silver coins. Humans established governments that included monarchies, dictatorships, democracies, and pseudodemocracies; stipulated constitutions, laws, and regulations to maintain various societies; and created several civilizations around the world that thrived and then faded. Over the past century, the advanced human brain elevated human existence to a higher sophistication with technologies such as electricity, phones, computers, internet, artificial intelligence, and machine learning. Using powerful rockets and space stations, humans have begun to expand their influence to the moon and planets of the solar system. Humans are very likely to continue achieving what evolution could never have done without evolving the human brain to become the most powerful force in nature.
The key ingredient of the brain that has enabled humans to achieve so much is the development of an advanced cognition, with superior functions that far exceed those of other living organisms. These include neurocognitive functions such as memory and attention, and executive functions that include planning, problem-solving, decision-making, abstract thinking, and insight. Those cognitive functions generate lofty prose, splendiferous poetry, and heavenly symphonies that inspire those who create it and others. The human brain also developed social cognition, with empathy, theory of mind, recognition of facial expressions, and courtship rituals that can trigger infatuation and love. Homo sapiens can experience a wide range of emotions in addition to love and attachment (necessary for procreation), including shame, guilt, surprise, embarrassment, disgust, and indifference, and a unique sense of right and wrong.
Perhaps the most distinctive human attribute, generated by an advanced prefrontal cortex, is a belief system that includes philosophy, politics, religion, and faith. Hundreds of different religions sprouted throughout human history (each claiming a monopoly on “the truth”), mandating rituals and behaviors, but also promoting a profound and unshakable belief in a divine “higher being” and an afterlife that mitigates the fear of death. Humans, unlike other animals, are painfully aware of mortality and the inevitability of death. Faith is an antidote for thanatophobia. Unfortunately, religious beliefs often generated severe and protracted schisms and warfare, with fatal consequences for their followers.
The anti-evolution aspect of the advanced brain
Despite remarkable talents and achievements, the unprecedented evolutionary expansion of the human brain also has a detrimental downside. The same intellectual power that led to astonishing positive accomplishments has a wicked side as well. While most animals have a predator, humans have become the “omni-predator” that preys on all living things. The balanced ecosystems of animals and plants has been dominated and disrupted by humans. Thousands of species that evolution had so ingeniously spawned became extinct because of human actions. The rainforests, jewels of nature’s plantation system, were victimized by human indifference to the deleterious effects on nature and climate. The excavation of coal and oil, exploited as necessary sources of energy for societal infrastructure, came back to haunt humans with climate consequences. In many ways, human “progress” corrupted evolution and dismantled its components. Survival of the fittest among various species was whittled down to “survival of humans” (and their domesticated animals) at the expense of all other organisms, animals, or plants.
Among Homo sapiens, momentous scientific, medical, and technological advances completely undermined the principle of survival of the fittest. Very premature infants, who would have certainly died, were kept alive. Children with disabling genetic disorders who would have perished in childhood were kept alive into the age of procreation, perpetuating the genetic mutations. The discovery of antibiotic and antiviral medications, and especially vaccines, ensured the survival of millions of humans who would have succumbed to infections. With evolution’s natural selection, humans who survived severe infections without medications would have passed on their “infection-resistant genes” to their progeny. The triumph of human medical progress can be conceptualized as a setback for the principles of evolution.
Continue to: The most malignant...
The most malignant consequence of the exceptional human brain is the evil of which it is capable. Human ingenuity led to the development of weapons of individual killing (guns), large-scale murder (machine guns), and massive destruction (nuclear weapons). And because aggression and warfare are an inherent part of human nature, the most potent predator for a human is another human. The history of humans is riddled with conflict and death on a large scale. Ironically, many wars were instigated by various religious groups around the world, who developed intense hostility towards one another.
There are other downsides to the advanced human brain. It can channel its talents and skills into unimaginably wicked and depraved behaviors, such as premeditated and well-planned murder, slavery, cults, child abuse, domestic abuse, pornography, fascism, dictatorships, and political corruption. Astonishingly, the same brain that can be loving, kind, friendly, and empathetic can suddenly become hateful, vengeful, cruel, vile, sinister, vicious, diabolical, and capable of unimaginable violence and atrocities. The advanced human brain definitely has a very dark side.
Finally, unlike other members of the animal kingdom, the human brain generates its virtual counterpart: the highly complex human mind, which is prone to various maladies, labeled as “psychiatric disorders.” No other animal species develops delusions, hallucinations, thought disorders, melancholia, mania, obsessive-compulsive disorder, generalized anxiety, panic attacks, posttraumatic stress disorder, psychopathy, narcissistic and borderline personality disorders, alcohol addiction, and drug abuse. Homo sapiens are the only species whose members decide to end their own life in large numbers. About 25% of human minds are afflicted with one or more of those psychiatric ailments.1,2 The redeeming grace of the large human brain is that it led to the development of pharmacologic and somatic treatments for most of them, including psychotherapy, which is a uniquely human treatment strategy that can mend many psychiatric disorders.
Evolution may not realize what it hath wrought when it evolved the dramatically expanded human brain, with its extraordinary cognition. This awe-inspiring “biological computer” can be creative and adaptive, with superlative survival abilities, but it can also degenerate and become nefarious, villainous, murderous, and even demonic. The human brain has essentially brought evolution to a screeching halt and may at some point end up destroying Earth and all of its Homo sapien inhabitants, who may foolishly use their weapons of mass destruction. The historic achievement of evolution has become the ultimate example of “the law of unintended consequences.”
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
1. Robin LN, Regier DA. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Free Press; 1990.
2. Johns Hopkins Medicine. Mental Health Disorder Statistics. Accessed October 12, 2022. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
Warning: Watch out for ‘medication substitution reaction’
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
I (MZP) recently started medical school, and one of the first things we learned in our Human Dimension class was to listen to our patients. While this may seem prosaic to seasoned practitioners, I quickly realized the important, real-world consequences of doing so.
Clinicians rightfully presume that when they send a prescription to a pharmacy, the patient will receive what they have ordered or the generic equivalent unless it is ordered “Dispense as written.” Unfortunately, a confluence of increased demand and supply chain disruptions has produced nationwide shortages of generic Adderall extended-release (XR) and Adderall, which are commonly prescribed to patients with attention-deficit/hyperactivity disorder (ADHD).1 While pharmacies should notify patients when they do not have these medications in stock, we have encountered numerous cases where due to shortages, prescriptions for generic dextroamphetamine/amphetamine salts XR or immediate-release (IR) have been filled with the same milligrams of only dextroamphetamine XR or IR, respectively, without notifying the patient or the prescribing clinician. Pharmacies have included several national chains and local independent stores in the New York/New Jersey region.
Over the past several months, we have encountered patients who had been well stabilized on their ADHD medication regimen who began to report anxiety, jitteriness, agitation, fatigue, poor concentration, and/or hyperactivity, and who also reported that their pills “look different.” First, we considered their symptoms could be attributed to a switch between generic manufacturers. However, upon further inspection, we discovered that the medication name printed on the label was different from what had been prescribed. We confirmed this by checking the Prescription Monitoring Program database.
Pharmacists have recently won prescribing privileges for nirmatrelvir/ritonavir (Paxlovid) to treat COVID-19, but they certainly are not permitted to fill prescriptions for psychoactive controlled substances that have different pharmacologic profiles than the medication the clinician ordered. Adderall contains D-amphetamine and L-amphetamine in a ratio of 3:1, which makes it different in potency from dextroamphetamine alone and requires adjustment to the dosage and potentially to the frequency to achieve near equivalency.
Once we realized the issue and helped our patients locate a pharmacy that had generic Adderall XR and Adderall in stock so they could resume their previous regimen, their symptoms resolved.
It is important for all clinicians to add “medication substitution reaction” to their differential diagnosis of new-onset ADHD-related symptoms in previously stable patients.
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
1. Pharmaceutical Commerce. Innovative solutions for pandemic-driven pharmacy drug shortages. Published February 28, 2022. Accessed September 8, 2022. https://www.pharmaceuticalcommerce.com/view/innovative-solutions-for-pandemic-driven-pharmacy-drug-shortages
Psychotropic medications for chronic pain
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.
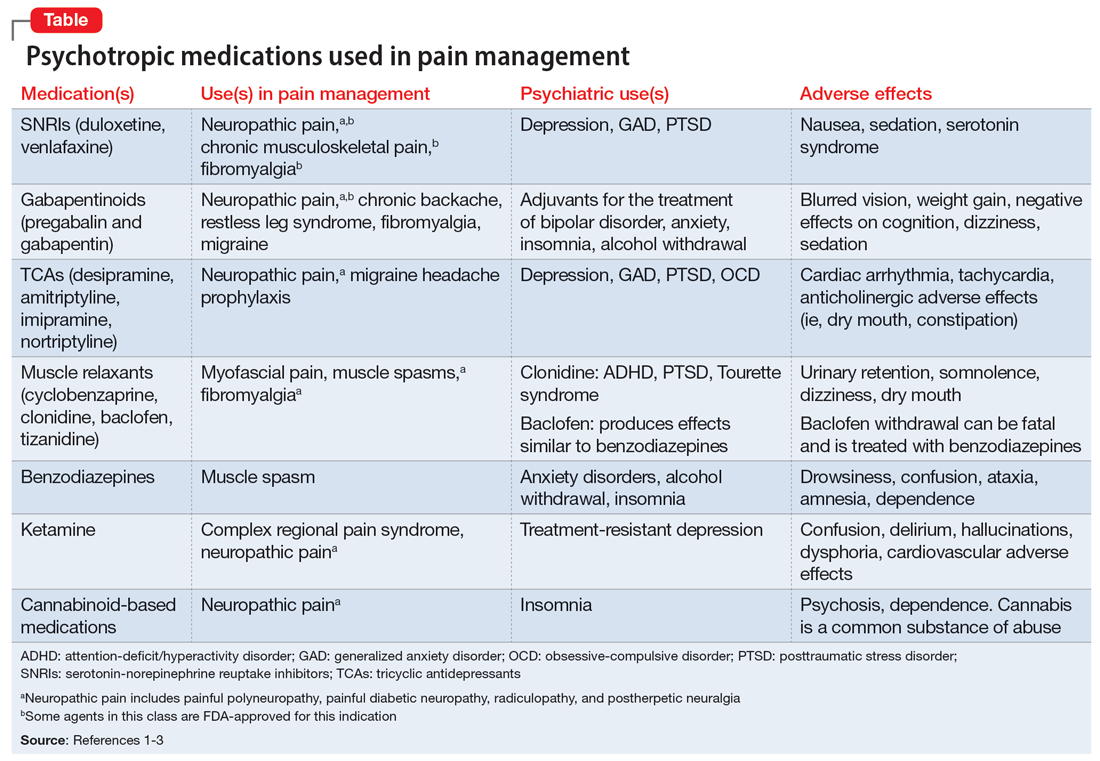
In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.

In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
The opioid crisis presents a need to consider alternative options for treating chronic pain. There is significant overlap in neuroanatomical circuits that process pain, emotions, and motivation. Neurotransmitters modulated by psychotropic medications are also involved in regulating the pain pathways.1,2 In light of this, psychotropics can be considered for treating chronic pain in certain patients. The Table1-3 outlines various uses and adverse effects of select psychotropic medications used to treat pain, as well as their psychiatric uses.

In addition to its psychiatric indications, the serotonin-norepinephrine reuptake inhibitor duloxetine is FDA-approved for treating fibromyalgia and diabetic neuropathic pain. It is often prescribed in the treatment of multiple pain disorders. Tricyclic antidepressants (TCAs) have the largest effect size in the treatment of neuropathic pain.2 Cyclobenzaprine is a TCA used to treat muscle spasms. Gabapentinoids (alpha-2 delta-1 calcium channel inhibition) are FDA-approved for treating postherpetic neuralgia, fibromyalgia, and diabetic neuropathy.1,2
Ketamine is an anesthetic with analgesic and antidepressant properties used as an IV infusion to manage several pain disorders.2 The alpha-2 adrenergic agonists tizanidine and clonidine are muscle relaxants2; the latter is used to treat attention-deficit/hyperactivity disorder and Tourette syndrome. Benzodiazepines (GABA-A agonists) are used for short-term treatment of anxiety disorders, insomnia, and muscle spasms.1,2 Baclofen (GABA-B receptor agonist) is used to treat spasticity.2 Medical cannabis (tetrahydrocannabinol/cannabidiol) is also gaining popularity for treating chronic pain and insomnia.1-3
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228
1. Sutherland AM, Nicholls J, Bao J, et al. Overlaps in pharmacology for the treatment of chronic pain and mental health disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):290-297.
2. Bajwa ZH, Wootton RJ, Warfield CA. Principles and Practice of Pain Medicine. 3rd ed. McGraw Hill; 2016.
3. McDonagh MS, Selph SS, Buckley DI, et al. Nonopioid Pharmacologic Treatments for Chronic Pain. Comparative Effectiveness Review No. 228. Agency for Healthcare Research and Quality; 2020. doi:10.23970/AHRQEPCCER228