User login
Patients at risk for Barrett’s esophagus rarely screened
Adults with chronic gastroesophageal reflux disease (GERD) are at increased risk for Barrett’s esophagus (BE) – the precursor to esophageal adenocarcinoma (EAC) – but most don’t undergo recommended screening, new research shows.
Jennifer Kolb, MD, with the University of California, Los Angeles, and colleagues surveyed 472 adults with chronic GERD who qualify for BE screening and had a recent visit with their primary care provider.
In this diverse population of people at risk for BE and EAC, only 13% had ever been advised to undergo endoscopy to screen for BE and only 5% actually had a prior screening, the study notes.
“These results make it clear that screening is rarely done,” Dr. Kolb told this news organization.
The results of the survey are published online in the American Journal of Gastroenterology.
Concern high, understanding low
Esophageal cancer and BE have increased among middle-aged adults over roughly the past 5 years, and this increase is not because of better or more frequent screening, as reported previously by this news organization.
In fact, the majority of patients who develop EAC do not have a prior diagnosis of BE, which highlights the failure of current BE screening practices, Dr. Kolb and colleagues point out.
Professional gastroenterology society guidelines recommend screening for BE using upper endoscopy for at-risk individuals, which includes those with chronic GERD, along with other risk factors such as age older than 50 years, male sex, white race, smoking, obesity, and family history of BE or EAC.
In the current survey, most individuals said early detection of BE/EAC is important and leads to better outcomes, but most had poor overall knowledge of risk factors and indications for screening.
Only about two-thirds of respondents correctly identified BE risk factors, and only about 20% believed BE screening was necessary with GERD, the researchers note.
Roughly two-thirds of individuals wanted to prioritize BE screening and felt that getting an upper endoscopy would ease their concern.
Yet, 40% had no prior esophagogastroduodenoscopy. These individuals were less knowledgeable about BE/EAC risk and screening recommendations and identified more barriers to completing endoscopy.
“While minorities were most concerned about developing Barrett’s esophagus and cancer, they reported more barriers to screening compared to White participants,” Dr. Kolb said.
Addressing knowledge gaps
The primary care clinician is often the first line for patients with symptomatic acid reflux and the gateway for preventive cancer screening.
Yet, research has shown that primary care clinicians often have trouble identifying who should be screened for BE, and competing clinical issues make it challenging to implement BE screening.
“As gastroenterologists, we must partner with our primary care colleagues to help increase awareness of this lethal disease and improve recognition of risk factors so that eligible patients can be identified and referred for screening,” Dr. Kolb said.
Reached for comment, Seth Gross, MD, clinical chief of the division of gastroenterology and hepatology at New York University Langone Health, said the results “shed light on the fact that patients with GERD don’t have the knowledge of when they should get medical attention and possibly endoscopy.”
“We may need to do a better job of educating our colleagues and patients to know when to seek specialists to potentially get an endoscopy,” Dr. Gross said.
About 90% of esophageal cancers are diagnosed outside of surveillance programs, noted Prasad G. Iyer, MD, a gastroenterologist at Mayo Clinic in Rochester, Minn.
“Patients didn’t even know that they had Barrett’s [esophagus], so they were never under surveillance. They only come to attention after they have trouble with food sticking, and they can’t swallow solid food,” said Dr. Iyer, who wasn’t involved in the survey.
“Unfortunately, there are just so many cancers and so many issues that primary care providers have to deal with that I think this may not be getting the attention it deserves,” he said.
Access to endoscopy is also likely a barrier, Dr. Iyer noted.
“The waiting list may be several months, and I think providers may focus on other things,” he said.
Less-invasive screening options
Fear of endoscopy may be another issue.
In their survey, Dr. Kolb and colleagues found that 20% of respondents reported fear of discomfort with endoscopy as a barrier to completing screening.
But less-invasive screening options are increasingly available or in development.
This includes Cytosponge, a swallowable capsule containing a compressed sponge attached to a string. When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for BE.
In a guideline released last spring, the American College of Gastroenterology endorsed Cytosponge as a nonendoscopic BE screening modality, as published in the American Journal of Gastroenterology.
“The strength of the recommendation is conditional, but it’s the first time where [the ACG] is saying that this may be an option for people,” Dr. Gross said.
This research was funded by the American College of Gastroenterology. Dr. Kolb, Dr. Gross, and Dr. Iyer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults with chronic gastroesophageal reflux disease (GERD) are at increased risk for Barrett’s esophagus (BE) – the precursor to esophageal adenocarcinoma (EAC) – but most don’t undergo recommended screening, new research shows.
Jennifer Kolb, MD, with the University of California, Los Angeles, and colleagues surveyed 472 adults with chronic GERD who qualify for BE screening and had a recent visit with their primary care provider.
In this diverse population of people at risk for BE and EAC, only 13% had ever been advised to undergo endoscopy to screen for BE and only 5% actually had a prior screening, the study notes.
“These results make it clear that screening is rarely done,” Dr. Kolb told this news organization.
The results of the survey are published online in the American Journal of Gastroenterology.
Concern high, understanding low
Esophageal cancer and BE have increased among middle-aged adults over roughly the past 5 years, and this increase is not because of better or more frequent screening, as reported previously by this news organization.
In fact, the majority of patients who develop EAC do not have a prior diagnosis of BE, which highlights the failure of current BE screening practices, Dr. Kolb and colleagues point out.
Professional gastroenterology society guidelines recommend screening for BE using upper endoscopy for at-risk individuals, which includes those with chronic GERD, along with other risk factors such as age older than 50 years, male sex, white race, smoking, obesity, and family history of BE or EAC.
In the current survey, most individuals said early detection of BE/EAC is important and leads to better outcomes, but most had poor overall knowledge of risk factors and indications for screening.
Only about two-thirds of respondents correctly identified BE risk factors, and only about 20% believed BE screening was necessary with GERD, the researchers note.
Roughly two-thirds of individuals wanted to prioritize BE screening and felt that getting an upper endoscopy would ease their concern.
Yet, 40% had no prior esophagogastroduodenoscopy. These individuals were less knowledgeable about BE/EAC risk and screening recommendations and identified more barriers to completing endoscopy.
“While minorities were most concerned about developing Barrett’s esophagus and cancer, they reported more barriers to screening compared to White participants,” Dr. Kolb said.
Addressing knowledge gaps
The primary care clinician is often the first line for patients with symptomatic acid reflux and the gateway for preventive cancer screening.
Yet, research has shown that primary care clinicians often have trouble identifying who should be screened for BE, and competing clinical issues make it challenging to implement BE screening.
“As gastroenterologists, we must partner with our primary care colleagues to help increase awareness of this lethal disease and improve recognition of risk factors so that eligible patients can be identified and referred for screening,” Dr. Kolb said.
Reached for comment, Seth Gross, MD, clinical chief of the division of gastroenterology and hepatology at New York University Langone Health, said the results “shed light on the fact that patients with GERD don’t have the knowledge of when they should get medical attention and possibly endoscopy.”
“We may need to do a better job of educating our colleagues and patients to know when to seek specialists to potentially get an endoscopy,” Dr. Gross said.
About 90% of esophageal cancers are diagnosed outside of surveillance programs, noted Prasad G. Iyer, MD, a gastroenterologist at Mayo Clinic in Rochester, Minn.
“Patients didn’t even know that they had Barrett’s [esophagus], so they were never under surveillance. They only come to attention after they have trouble with food sticking, and they can’t swallow solid food,” said Dr. Iyer, who wasn’t involved in the survey.
“Unfortunately, there are just so many cancers and so many issues that primary care providers have to deal with that I think this may not be getting the attention it deserves,” he said.
Access to endoscopy is also likely a barrier, Dr. Iyer noted.
“The waiting list may be several months, and I think providers may focus on other things,” he said.
Less-invasive screening options
Fear of endoscopy may be another issue.
In their survey, Dr. Kolb and colleagues found that 20% of respondents reported fear of discomfort with endoscopy as a barrier to completing screening.
But less-invasive screening options are increasingly available or in development.
This includes Cytosponge, a swallowable capsule containing a compressed sponge attached to a string. When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for BE.
In a guideline released last spring, the American College of Gastroenterology endorsed Cytosponge as a nonendoscopic BE screening modality, as published in the American Journal of Gastroenterology.
“The strength of the recommendation is conditional, but it’s the first time where [the ACG] is saying that this may be an option for people,” Dr. Gross said.
This research was funded by the American College of Gastroenterology. Dr. Kolb, Dr. Gross, and Dr. Iyer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adults with chronic gastroesophageal reflux disease (GERD) are at increased risk for Barrett’s esophagus (BE) – the precursor to esophageal adenocarcinoma (EAC) – but most don’t undergo recommended screening, new research shows.
Jennifer Kolb, MD, with the University of California, Los Angeles, and colleagues surveyed 472 adults with chronic GERD who qualify for BE screening and had a recent visit with their primary care provider.
In this diverse population of people at risk for BE and EAC, only 13% had ever been advised to undergo endoscopy to screen for BE and only 5% actually had a prior screening, the study notes.
“These results make it clear that screening is rarely done,” Dr. Kolb told this news organization.
The results of the survey are published online in the American Journal of Gastroenterology.
Concern high, understanding low
Esophageal cancer and BE have increased among middle-aged adults over roughly the past 5 years, and this increase is not because of better or more frequent screening, as reported previously by this news organization.
In fact, the majority of patients who develop EAC do not have a prior diagnosis of BE, which highlights the failure of current BE screening practices, Dr. Kolb and colleagues point out.
Professional gastroenterology society guidelines recommend screening for BE using upper endoscopy for at-risk individuals, which includes those with chronic GERD, along with other risk factors such as age older than 50 years, male sex, white race, smoking, obesity, and family history of BE or EAC.
In the current survey, most individuals said early detection of BE/EAC is important and leads to better outcomes, but most had poor overall knowledge of risk factors and indications for screening.
Only about two-thirds of respondents correctly identified BE risk factors, and only about 20% believed BE screening was necessary with GERD, the researchers note.
Roughly two-thirds of individuals wanted to prioritize BE screening and felt that getting an upper endoscopy would ease their concern.
Yet, 40% had no prior esophagogastroduodenoscopy. These individuals were less knowledgeable about BE/EAC risk and screening recommendations and identified more barriers to completing endoscopy.
“While minorities were most concerned about developing Barrett’s esophagus and cancer, they reported more barriers to screening compared to White participants,” Dr. Kolb said.
Addressing knowledge gaps
The primary care clinician is often the first line for patients with symptomatic acid reflux and the gateway for preventive cancer screening.
Yet, research has shown that primary care clinicians often have trouble identifying who should be screened for BE, and competing clinical issues make it challenging to implement BE screening.
“As gastroenterologists, we must partner with our primary care colleagues to help increase awareness of this lethal disease and improve recognition of risk factors so that eligible patients can be identified and referred for screening,” Dr. Kolb said.
Reached for comment, Seth Gross, MD, clinical chief of the division of gastroenterology and hepatology at New York University Langone Health, said the results “shed light on the fact that patients with GERD don’t have the knowledge of when they should get medical attention and possibly endoscopy.”
“We may need to do a better job of educating our colleagues and patients to know when to seek specialists to potentially get an endoscopy,” Dr. Gross said.
About 90% of esophageal cancers are diagnosed outside of surveillance programs, noted Prasad G. Iyer, MD, a gastroenterologist at Mayo Clinic in Rochester, Minn.
“Patients didn’t even know that they had Barrett’s [esophagus], so they were never under surveillance. They only come to attention after they have trouble with food sticking, and they can’t swallow solid food,” said Dr. Iyer, who wasn’t involved in the survey.
“Unfortunately, there are just so many cancers and so many issues that primary care providers have to deal with that I think this may not be getting the attention it deserves,” he said.
Access to endoscopy is also likely a barrier, Dr. Iyer noted.
“The waiting list may be several months, and I think providers may focus on other things,” he said.
Less-invasive screening options
Fear of endoscopy may be another issue.
In their survey, Dr. Kolb and colleagues found that 20% of respondents reported fear of discomfort with endoscopy as a barrier to completing screening.
But less-invasive screening options are increasingly available or in development.
This includes Cytosponge, a swallowable capsule containing a compressed sponge attached to a string. When withdrawn, the sponge contains esophageal cytology samples that can be used to identify biomarkers for BE.
In a guideline released last spring, the American College of Gastroenterology endorsed Cytosponge as a nonendoscopic BE screening modality, as published in the American Journal of Gastroenterology.
“The strength of the recommendation is conditional, but it’s the first time where [the ACG] is saying that this may be an option for people,” Dr. Gross said.
This research was funded by the American College of Gastroenterology. Dr. Kolb, Dr. Gross, and Dr. Iyer report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GASTROENTEROLOGY
FDA rejects bulevirtide for hepatitis D
The U.S. Food and Drug Administration (FDA) has declined to approve bulevirtide, Gilead Sciences’ drug for the treatment of hepatitis delta virus (HDV) infection and compensated liver disease.
In a complete response letter, the FDA voiced concerns over the production and delivery of bulevirtide, an investigational, first-in-class HDV entry-inhibitor that received conditional approved in Europe in 2020.
The FDA did not request new studies to evaluate the safety and efficacy of bulevirtide.
As reported previously by this news organization, data from an ongoing phase 3 trial showed that after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable HDV RNA levels and normalized alanine aminotransferase levels.
Chronic HDV infection is the most severe form of viral hepatitis. It is associated with a poor prognosis and high mortality rates.
There are currently no approved treatments for HDV in the United States. Bulevirtide was granted breakthrough therapy and orphan drug designations by the FDA.
Merdad Parsey, MD, PhD, chief medical officer, Gilead Sciences, wrote in a news release that the company looks forward to “continuing our active discussions with FDA so that we may bring bulevirtide to people living with HDV in the U.S. as soon as possible.”
This is the second manufacturing-related complete response letter Gilead has received in the past 8 months. In March, the FDA rejected the long-acting HIV drug lenacapavir. The drug was sanctioned in Europe and the United Kingdom in September.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) has declined to approve bulevirtide, Gilead Sciences’ drug for the treatment of hepatitis delta virus (HDV) infection and compensated liver disease.
In a complete response letter, the FDA voiced concerns over the production and delivery of bulevirtide, an investigational, first-in-class HDV entry-inhibitor that received conditional approved in Europe in 2020.
The FDA did not request new studies to evaluate the safety and efficacy of bulevirtide.
As reported previously by this news organization, data from an ongoing phase 3 trial showed that after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable HDV RNA levels and normalized alanine aminotransferase levels.
Chronic HDV infection is the most severe form of viral hepatitis. It is associated with a poor prognosis and high mortality rates.
There are currently no approved treatments for HDV in the United States. Bulevirtide was granted breakthrough therapy and orphan drug designations by the FDA.
Merdad Parsey, MD, PhD, chief medical officer, Gilead Sciences, wrote in a news release that the company looks forward to “continuing our active discussions with FDA so that we may bring bulevirtide to people living with HDV in the U.S. as soon as possible.”
This is the second manufacturing-related complete response letter Gilead has received in the past 8 months. In March, the FDA rejected the long-acting HIV drug lenacapavir. The drug was sanctioned in Europe and the United Kingdom in September.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) has declined to approve bulevirtide, Gilead Sciences’ drug for the treatment of hepatitis delta virus (HDV) infection and compensated liver disease.
In a complete response letter, the FDA voiced concerns over the production and delivery of bulevirtide, an investigational, first-in-class HDV entry-inhibitor that received conditional approved in Europe in 2020.
The FDA did not request new studies to evaluate the safety and efficacy of bulevirtide.
As reported previously by this news organization, data from an ongoing phase 3 trial showed that after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable HDV RNA levels and normalized alanine aminotransferase levels.
Chronic HDV infection is the most severe form of viral hepatitis. It is associated with a poor prognosis and high mortality rates.
There are currently no approved treatments for HDV in the United States. Bulevirtide was granted breakthrough therapy and orphan drug designations by the FDA.
Merdad Parsey, MD, PhD, chief medical officer, Gilead Sciences, wrote in a news release that the company looks forward to “continuing our active discussions with FDA so that we may bring bulevirtide to people living with HDV in the U.S. as soon as possible.”
This is the second manufacturing-related complete response letter Gilead has received in the past 8 months. In March, the FDA rejected the long-acting HIV drug lenacapavir. The drug was sanctioned in Europe and the United Kingdom in September.
A version of this article first appeared on Medscape.com.
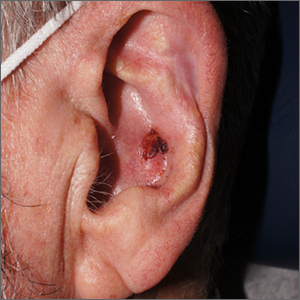
Crusty ear
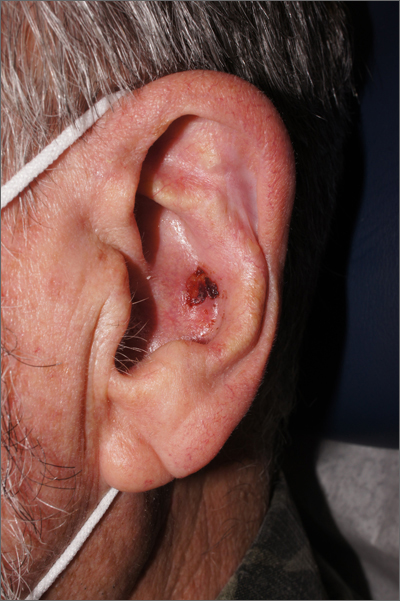
The physician used a curette to perform a shave biopsy; pathology results indicated this was a poorly differentiated squamous cell carcinoma (SCC). Cutaneous SCC is the second most common skin cancer in the United States (after basal cell carcinoma) and increases in frequency with age and cumulative sun damage. It is the most common skin cancer in patients who are Black.
SCC is frequently found on the head and neck, including the ear, but is less commonly found within the conchal bowl (as seen here). Often, SCC manifests as a rough plaque or dome-shaped papule in a sun damaged location, but it may occasionally manifest as an ulcer. While most patients are cured with outpatient surgery, an estimated 8000 patients will develop nodal metastasis and 3000 patients will die from the disease in the United States annually.1 Chronically immunosuppressed patients, such as organ transplant recipients, are at high risk.
This patient underwent Mohs microsurgery (MMS) and clear margins were achieved after 2 stages. The resulting defect was repaired with a full-thickness graft from the postauricular fold. MMS is an excellent technique for keratinocyte carcinomas (SCC and basal cell carcinomas) of the head and neck, recurrent skin cancers on the trunk and extremities, high-risk cancer subtypes, and tumors with indistinct clinical borders. Follow-up for patients with SCCs includes full skin exams every 6 months for 2 years.
The American Academy of Dermatology offers a complimentary Mohs Surgery Appropriate Use Criteria App that assists in determining when Mohs surgery is appropriate, based on multiple tumor characteristics.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001

The physician used a curette to perform a shave biopsy; pathology results indicated this was a poorly differentiated squamous cell carcinoma (SCC). Cutaneous SCC is the second most common skin cancer in the United States (after basal cell carcinoma) and increases in frequency with age and cumulative sun damage. It is the most common skin cancer in patients who are Black.
SCC is frequently found on the head and neck, including the ear, but is less commonly found within the conchal bowl (as seen here). Often, SCC manifests as a rough plaque or dome-shaped papule in a sun damaged location, but it may occasionally manifest as an ulcer. While most patients are cured with outpatient surgery, an estimated 8000 patients will develop nodal metastasis and 3000 patients will die from the disease in the United States annually.1 Chronically immunosuppressed patients, such as organ transplant recipients, are at high risk.
This patient underwent Mohs microsurgery (MMS) and clear margins were achieved after 2 stages. The resulting defect was repaired with a full-thickness graft from the postauricular fold. MMS is an excellent technique for keratinocyte carcinomas (SCC and basal cell carcinomas) of the head and neck, recurrent skin cancers on the trunk and extremities, high-risk cancer subtypes, and tumors with indistinct clinical borders. Follow-up for patients with SCCs includes full skin exams every 6 months for 2 years.
The American Academy of Dermatology offers a complimentary Mohs Surgery Appropriate Use Criteria App that assists in determining when Mohs surgery is appropriate, based on multiple tumor characteristics.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The physician used a curette to perform a shave biopsy; pathology results indicated this was a poorly differentiated squamous cell carcinoma (SCC). Cutaneous SCC is the second most common skin cancer in the United States (after basal cell carcinoma) and increases in frequency with age and cumulative sun damage. It is the most common skin cancer in patients who are Black.
SCC is frequently found on the head and neck, including the ear, but is less commonly found within the conchal bowl (as seen here). Often, SCC manifests as a rough plaque or dome-shaped papule in a sun damaged location, but it may occasionally manifest as an ulcer. While most patients are cured with outpatient surgery, an estimated 8000 patients will develop nodal metastasis and 3000 patients will die from the disease in the United States annually.1 Chronically immunosuppressed patients, such as organ transplant recipients, are at high risk.
This patient underwent Mohs microsurgery (MMS) and clear margins were achieved after 2 stages. The resulting defect was repaired with a full-thickness graft from the postauricular fold. MMS is an excellent technique for keratinocyte carcinomas (SCC and basal cell carcinomas) of the head and neck, recurrent skin cancers on the trunk and extremities, high-risk cancer subtypes, and tumors with indistinct clinical borders. Follow-up for patients with SCCs includes full skin exams every 6 months for 2 years.
The American Academy of Dermatology offers a complimentary Mohs Surgery Appropriate Use Criteria App that assists in determining when Mohs surgery is appropriate, based on multiple tumor characteristics.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
1. Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
Major depression treatments boost brain connectivity
VIENNA – , new research suggests.
In a “repeat” MRI study, adult participants with MDD had significantly lower brain connectivity compared with their healthy peers at baseline – but showed significant improvement at the 6-week follow-up. These improvements were associated with decreases in symptom severity, independent of whether they received electroconvulsive therapy (ECT) or other treatment modalities.
“This means that the brain structure of patients with serious clinical depression is not as fixed as we thought, and we can improve brain structure within a short time frame [of] around 6 weeks,” lead author Jonathan Repple, MD, now professor of predictive psychiatry at the University of Frankfurt, Germany, said in a release.
“This gives hope to patients who believe nothing can change and they have to live with a disease forever because it is ‘set in stone’ in their brain,” he added.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
‘Easily understandable picture’
Dr. Repple said in an interview that the investigators “were surprised to see how plastic” the brain could be.
“I’ve done a lot of imaging studies in the past where we looked at differences in depression vs. healthy controls, and then maybe had tiny effects. But we’ve never seen such a clear and easily understandable picture, where we see a deficit at the beginning and then a significant increase in whatever biomarker we were looking at, that even correlated with how successful the treatment was,” he said.
Dr. Repple noted that “this is the thing everyone is looking for when we’re talking about a biomarker: That we see this exact pattern” – and it is why they are so excited about the results.
However, he cautioned that the study included a “small sample” and the results need to be independently replicated.
“If this can be replicated, this might be a very good target for future intervention studies,” Dr. Repple said.
The investigators noted that altered brain structural connectivity has been implicated before in the pathophysiology of MDD.
However, it is not clear whether these changes are stable over time and indicate a biological predisposition, or are markers of current disease severity and can be altered by effective treatment.
To investigate further, the researchers used gray matter T1-weighted MRI to define nodes in the brain and diffusion-weighted imaging (DWI)-based tractography to determine connections between the nodes, to create a structural connectome or white matter network.
They performed assessments at baseline and at 6 weeks’ follow-up in 123 participants diagnosed with current MDD and receiving inpatient treatment, and 55 participants who acted as the healthy controls group.
Among the patients with MDD, 56 were treated with ECT and 67 received other antidepressant care, including psychological therapy or medications. Some patients had received all three treatment modalities.
Significant interactions
Results showed a significant interaction by group and time between the baseline and 6-week follow-up assessments (P < .05).
This was partly driven by the MDD group having a significantly lower connectivity strength at baseline than the healthy controls group (P < .05).
It was also partly driven by patients showing a significant improvement in connectivity strength between the baseline and follow-up assessments (P < .05), a pattern that was not seen in the nonpatients.
This increase in connectivity strength was associated with a significant decrease in depression symptom severity (P < .05). This was independent of the treatment modality, indicating that it was not linked to the use of ECT.
Dr. Repple acknowledged the relatively short follow-up period of the study, and added that he is not aware of longitudinal studies of the structural connectome with a longer follow-up.
He pointed out that the structural connectivity of the brain decreases with age, but there have been no studies that have assessed patients with depression and “measured the same person again after 2, 4, 6, or 8 years.”
Dr. Repple reported that the investigators will be following up with their participants, “so hopefully in a few years we’ll have more information on that.
“One thing I also need to stress is that, when we’re looking at the MRI brain scans, we see an increase in connectivity strength, but we really can’t say what the molecular mechanisms behind it are,” he said. “This is a black box for us.”
Several unanswered questions
Commenting in the release, Eric Ruhe, MD, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, said this was a “very interesting and difficult study to perform.”
However, Dr. Ruhe, who was not involved in the research, told this news organization that it is “very difficult to connect the lack of brain connectivity to the patient symptomatology because there is a huge gap between them.”
The problem is that, despite “lots of evidence” that they are effective, “we currently don’t know how antidepressant therapies work” in terms of their underlying mechanisms of action, he said.
“We think that these types of therapies all modulate the plasticity of the brain,” said Dr. Ruhe. “What this study showed is there are changes that you can detect even in 6 weeks,” although they may have been observed even sooner with a shorter follow-up.
He noted that big questions are whether the change is specific to the treatment given, and “can you modulate different brain network dysfunctions with different treatments?”
Moreover, he wondered if a brain scan could indicate which type of treatment should be used. “This is, of course, very new and very challenging, and we don’t know yet, but we should be pursuing this,” Dr. Ruhe said.
Another question is whether or not the brain connectivity changes shown in the study represent a persistent change – “and whether this is a persistent change that is associated with a consistent and persistent relief of depression.
“Again, this is something that needs to be followed up,” said Dr. Ruhe.
No funding was declared. The study authors and Dr. Ruhe report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – , new research suggests.
In a “repeat” MRI study, adult participants with MDD had significantly lower brain connectivity compared with their healthy peers at baseline – but showed significant improvement at the 6-week follow-up. These improvements were associated with decreases in symptom severity, independent of whether they received electroconvulsive therapy (ECT) or other treatment modalities.
“This means that the brain structure of patients with serious clinical depression is not as fixed as we thought, and we can improve brain structure within a short time frame [of] around 6 weeks,” lead author Jonathan Repple, MD, now professor of predictive psychiatry at the University of Frankfurt, Germany, said in a release.
“This gives hope to patients who believe nothing can change and they have to live with a disease forever because it is ‘set in stone’ in their brain,” he added.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
‘Easily understandable picture’
Dr. Repple said in an interview that the investigators “were surprised to see how plastic” the brain could be.
“I’ve done a lot of imaging studies in the past where we looked at differences in depression vs. healthy controls, and then maybe had tiny effects. But we’ve never seen such a clear and easily understandable picture, where we see a deficit at the beginning and then a significant increase in whatever biomarker we were looking at, that even correlated with how successful the treatment was,” he said.
Dr. Repple noted that “this is the thing everyone is looking for when we’re talking about a biomarker: That we see this exact pattern” – and it is why they are so excited about the results.
However, he cautioned that the study included a “small sample” and the results need to be independently replicated.
“If this can be replicated, this might be a very good target for future intervention studies,” Dr. Repple said.
The investigators noted that altered brain structural connectivity has been implicated before in the pathophysiology of MDD.
However, it is not clear whether these changes are stable over time and indicate a biological predisposition, or are markers of current disease severity and can be altered by effective treatment.
To investigate further, the researchers used gray matter T1-weighted MRI to define nodes in the brain and diffusion-weighted imaging (DWI)-based tractography to determine connections between the nodes, to create a structural connectome or white matter network.
They performed assessments at baseline and at 6 weeks’ follow-up in 123 participants diagnosed with current MDD and receiving inpatient treatment, and 55 participants who acted as the healthy controls group.
Among the patients with MDD, 56 were treated with ECT and 67 received other antidepressant care, including psychological therapy or medications. Some patients had received all three treatment modalities.
Significant interactions
Results showed a significant interaction by group and time between the baseline and 6-week follow-up assessments (P < .05).
This was partly driven by the MDD group having a significantly lower connectivity strength at baseline than the healthy controls group (P < .05).
It was also partly driven by patients showing a significant improvement in connectivity strength between the baseline and follow-up assessments (P < .05), a pattern that was not seen in the nonpatients.
This increase in connectivity strength was associated with a significant decrease in depression symptom severity (P < .05). This was independent of the treatment modality, indicating that it was not linked to the use of ECT.
Dr. Repple acknowledged the relatively short follow-up period of the study, and added that he is not aware of longitudinal studies of the structural connectome with a longer follow-up.
He pointed out that the structural connectivity of the brain decreases with age, but there have been no studies that have assessed patients with depression and “measured the same person again after 2, 4, 6, or 8 years.”
Dr. Repple reported that the investigators will be following up with their participants, “so hopefully in a few years we’ll have more information on that.
“One thing I also need to stress is that, when we’re looking at the MRI brain scans, we see an increase in connectivity strength, but we really can’t say what the molecular mechanisms behind it are,” he said. “This is a black box for us.”
Several unanswered questions
Commenting in the release, Eric Ruhe, MD, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, said this was a “very interesting and difficult study to perform.”
However, Dr. Ruhe, who was not involved in the research, told this news organization that it is “very difficult to connect the lack of brain connectivity to the patient symptomatology because there is a huge gap between them.”
The problem is that, despite “lots of evidence” that they are effective, “we currently don’t know how antidepressant therapies work” in terms of their underlying mechanisms of action, he said.
“We think that these types of therapies all modulate the plasticity of the brain,” said Dr. Ruhe. “What this study showed is there are changes that you can detect even in 6 weeks,” although they may have been observed even sooner with a shorter follow-up.
He noted that big questions are whether the change is specific to the treatment given, and “can you modulate different brain network dysfunctions with different treatments?”
Moreover, he wondered if a brain scan could indicate which type of treatment should be used. “This is, of course, very new and very challenging, and we don’t know yet, but we should be pursuing this,” Dr. Ruhe said.
Another question is whether or not the brain connectivity changes shown in the study represent a persistent change – “and whether this is a persistent change that is associated with a consistent and persistent relief of depression.
“Again, this is something that needs to be followed up,” said Dr. Ruhe.
No funding was declared. The study authors and Dr. Ruhe report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – , new research suggests.
In a “repeat” MRI study, adult participants with MDD had significantly lower brain connectivity compared with their healthy peers at baseline – but showed significant improvement at the 6-week follow-up. These improvements were associated with decreases in symptom severity, independent of whether they received electroconvulsive therapy (ECT) or other treatment modalities.
“This means that the brain structure of patients with serious clinical depression is not as fixed as we thought, and we can improve brain structure within a short time frame [of] around 6 weeks,” lead author Jonathan Repple, MD, now professor of predictive psychiatry at the University of Frankfurt, Germany, said in a release.
“This gives hope to patients who believe nothing can change and they have to live with a disease forever because it is ‘set in stone’ in their brain,” he added.
The findings were presented at the 35th European College of Neuropsychopharmacology (ECNP) Congress.
‘Easily understandable picture’
Dr. Repple said in an interview that the investigators “were surprised to see how plastic” the brain could be.
“I’ve done a lot of imaging studies in the past where we looked at differences in depression vs. healthy controls, and then maybe had tiny effects. But we’ve never seen such a clear and easily understandable picture, where we see a deficit at the beginning and then a significant increase in whatever biomarker we were looking at, that even correlated with how successful the treatment was,” he said.
Dr. Repple noted that “this is the thing everyone is looking for when we’re talking about a biomarker: That we see this exact pattern” – and it is why they are so excited about the results.
However, he cautioned that the study included a “small sample” and the results need to be independently replicated.
“If this can be replicated, this might be a very good target for future intervention studies,” Dr. Repple said.
The investigators noted that altered brain structural connectivity has been implicated before in the pathophysiology of MDD.
However, it is not clear whether these changes are stable over time and indicate a biological predisposition, or are markers of current disease severity and can be altered by effective treatment.
To investigate further, the researchers used gray matter T1-weighted MRI to define nodes in the brain and diffusion-weighted imaging (DWI)-based tractography to determine connections between the nodes, to create a structural connectome or white matter network.
They performed assessments at baseline and at 6 weeks’ follow-up in 123 participants diagnosed with current MDD and receiving inpatient treatment, and 55 participants who acted as the healthy controls group.
Among the patients with MDD, 56 were treated with ECT and 67 received other antidepressant care, including psychological therapy or medications. Some patients had received all three treatment modalities.
Significant interactions
Results showed a significant interaction by group and time between the baseline and 6-week follow-up assessments (P < .05).
This was partly driven by the MDD group having a significantly lower connectivity strength at baseline than the healthy controls group (P < .05).
It was also partly driven by patients showing a significant improvement in connectivity strength between the baseline and follow-up assessments (P < .05), a pattern that was not seen in the nonpatients.
This increase in connectivity strength was associated with a significant decrease in depression symptom severity (P < .05). This was independent of the treatment modality, indicating that it was not linked to the use of ECT.
Dr. Repple acknowledged the relatively short follow-up period of the study, and added that he is not aware of longitudinal studies of the structural connectome with a longer follow-up.
He pointed out that the structural connectivity of the brain decreases with age, but there have been no studies that have assessed patients with depression and “measured the same person again after 2, 4, 6, or 8 years.”
Dr. Repple reported that the investigators will be following up with their participants, “so hopefully in a few years we’ll have more information on that.
“One thing I also need to stress is that, when we’re looking at the MRI brain scans, we see an increase in connectivity strength, but we really can’t say what the molecular mechanisms behind it are,” he said. “This is a black box for us.”
Several unanswered questions
Commenting in the release, Eric Ruhe, MD, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, said this was a “very interesting and difficult study to perform.”
However, Dr. Ruhe, who was not involved in the research, told this news organization that it is “very difficult to connect the lack of brain connectivity to the patient symptomatology because there is a huge gap between them.”
The problem is that, despite “lots of evidence” that they are effective, “we currently don’t know how antidepressant therapies work” in terms of their underlying mechanisms of action, he said.
“We think that these types of therapies all modulate the plasticity of the brain,” said Dr. Ruhe. “What this study showed is there are changes that you can detect even in 6 weeks,” although they may have been observed even sooner with a shorter follow-up.
He noted that big questions are whether the change is specific to the treatment given, and “can you modulate different brain network dysfunctions with different treatments?”
Moreover, he wondered if a brain scan could indicate which type of treatment should be used. “This is, of course, very new and very challenging, and we don’t know yet, but we should be pursuing this,” Dr. Ruhe said.
Another question is whether or not the brain connectivity changes shown in the study represent a persistent change – “and whether this is a persistent change that is associated with a consistent and persistent relief of depression.
“Again, this is something that needs to be followed up,” said Dr. Ruhe.
No funding was declared. The study authors and Dr. Ruhe report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ECNP 2022
Studies provide compelling momentum for mucosal origins hypothesis of rheumatoid arthritis
A newly discovered strain of bacteria could play a role in the development of rheumatoid arthritis, according to findings recently published in Science Translational Medicine.
Mice colonized with a strain of Subdoligranulum bacteria in their gut – a strain previously unidentified but now named Subdoligranulum didolesgii – developed joint swelling and inflammation as well as antibodies and T-cell responses similar to what is seen in RA, researchers reported.
“This was the first time that anyone has observed arthritis developing in a mouse that was not otherwise immunologically stimulated with an adjuvant of some kind, or genetically manipulated,” said Kristine Kuhn, MD, PhD, associate professor of rheumatology at the University of Colorado at Denver, Aurora, who led a team of researchers that also included investigators from Stanford (Calif.) University and Benaroya Research Institute in Seattle.
The findings offer the latest evidence – and perhaps the most compelling evidence – for the mucosal origins hypothesis, the idea that rheumatoid arthritis can start with an immune response somewhere in the mucosa because of environmental interactions, and then becomes systemic, resulting in symptoms in the joints. Anti-citrullinated protein antibodies (ACPA), hallmarks of RA, have been found at mucosal surfaces in the periodontium and the lungs, and there have been reports of them in the intestine and cervicovaginal mucosa as well.
The latest findings that implicate the new bacterium build on previous findings in which people at risk of RA, but without symptoms yet, had an expansion of B cells producing immunoglobulin A (IgA), an antibody found in the mucosa. A closer look at these B cells, using variable region sequencing, found that they arose from a family that includes both IgA and IgG members. Because IgG antibodies are systemic, this suggested a kind of evolution from an IgA-based, mucosal immune response to one that is systemic and could target the joints.
Researchers mixed monoclonal antibodies from these B cells with a pool of bacteria from the stool of a broad population of people, and then pulled out the bacteria bound by these antibodies, and sequenced them. They found that the antibodies had bound almost exclusively to Ruminococcaceae and Lachnospiraceae.
They then cultured the stool of an individual at risk of developing RA and ended up with five isolates within Ruminococcaceae – “all of which belonged to the Subdoligranulum genus,” Dr. Kuhn said. When they sequenced these, they found that they had a new strain, which was named by Meagan Chriswell, an MD-PhD candidate and member of the Cherokee Nation of Oklahoma, who chose a term based on the Cherokee word for rheumatism.
Researchers at Benaroya then mixed this strain with T cells of people with RA, and those of controls, and only the T cells of those with RA were stimulated by the bacterium, they found.
“When intestines of germ-free mice were colonized with the strain, we found that they were getting arthritis,” Dr. Kuhn said. Photos of the joints show a striking contrast between the swollen joints of the mice given Subdoligranulum didolesgii and those injected with Prevotella copri, another strain suspected of having a link to RA, as well as with another Subdoligranulum strain and a sterile media. Dr. Kuhn noted that the P. copri strain did not come from an RA-affected individual.
“We thought that our results closed the loop nicely to show that these immune responses truly were toward the Subdoligranulum, and also stimulating arthritis,” she said.
The researchers then assessed the prevalence of the strain in people at risk for RA or with RA, and in controls. They found it in 17% of those with or at risk for RA but didn’t see it at all in the healthy control population.
Dr. Kuhn and her research team, she said, are now looking at the prevalence of the strain in a larger population and doing more investigating into the link with RA.
“Does it really associate with the development of immune responses and the development of rheumatoid arthritis?” she said.
Potential etiologic role of P. copri
Another paper, published in Arthritis & Rheumatology by some of the same investigators a week before the study describing the Subdoligranulum findings, tried to ascertain the point at which individuals might develop antibodies to P. copri, which for about a decade has been suspected of having a link to the development of RA.
They found that those with early RA had higher median values of IgG anti–P. copri (Pc) antibodies, compared with matched controls. People with established RA also had higher values of IgA anti-Pc antibodies. Those with ACPA, but not rheumatoid factor (RF), showed a trend toward higher IgG anti-Pc antibodies. Those who were ACPA-positive and RF-positive had significantly increased levels of IgA anti-Pc antibodies and a trend toward higher levels of IgG anti-Pc antibodies, compared with matched controls.
The findings, according to the researchers, “support a potential etiologic role for this microorganism in both RA preclinical evolution and the subsequent pathogenesis of synovitis.”
Dr. Kuhn and others in the field say it’s likely that many microbes play a role in the development of RA, and that the P. copri findings only add evidence of that relationship.
“Maybe the bacteria are involved at different parts of the pathway, and maybe they’re involved in triggering different parts of the immune responses,” she said. “Those are all to be determined.”
Dan Littman, MD, PhD, professor of rheumatology at New York University, who wrote a commentary reflecting on the findings of the Subdoligranulum study, said the results are “another piece of data” adding to the evidence base for the mucosal origins hypothesis.
“It’s by no means proven that this is the way pathogenesis in RA can occur, but it’s certainly a very solid study,” said Dr. Littman, who with colleagues published findings in 2013 linking P. copri to RA. “What makes it most compelling is that they seem to be able to show some evidence of causality in the mouse model.”
Before the findings could lead to therapy, he said, more evidence is needed to show that there is a causal link, and on the mechanism at work, such as whether this is something that occurs at the outset of disease or is something that “fuels the disease” by continually activating immune cells contributing to RA.
“If it’s only something that’s involved in the initiation of the disease, you need to catch it very early,” he said. “But if it’s something that continues to provide fuel for the disease, you may be able to catch it later and still be effective. Those are really critical items.”
Eventually, if these questions are answered, bacteriophages could be developed to snuff out problematic strains, or the regulatory response could be targeted to prevent the activation of the B cells that give rise to autoimmunity, he suggested.
“There are multiple steps to get to a therapeutic here, and I think we’re still a long ways from that,” he said. Still, he said, “I think it’s an important paper because it will encourage more people to look at this mechanism more closely and determine whether it really is representative of what happens in a lot of RA patients.”
The study in Science Translational Medicine was supported by grants from the National Institutes of Health, a Pfizer ASPIRE grant, and a grant from the Rheumatology Research Foundation. The Arthritis & Rheumatology study was supported in part by grants from the National Institutes of Health; the American College of Rheumatology Innovative Grant Program; the Ounsworth-Fitzgerald Foundation; Mathers Foundation; English, Bonter, Mitchell Foundation; Littauer Foundation; Lillian B. Davey Foundation; and the Eshe Fund. None of the researchers in either study had relevant financial disclosures. Dr. Littman is scientific cofounder and member of the scientific advisory board of Vedanta Biosciences, which studies microbiota therapeutics.
A newly discovered strain of bacteria could play a role in the development of rheumatoid arthritis, according to findings recently published in Science Translational Medicine.
Mice colonized with a strain of Subdoligranulum bacteria in their gut – a strain previously unidentified but now named Subdoligranulum didolesgii – developed joint swelling and inflammation as well as antibodies and T-cell responses similar to what is seen in RA, researchers reported.
“This was the first time that anyone has observed arthritis developing in a mouse that was not otherwise immunologically stimulated with an adjuvant of some kind, or genetically manipulated,” said Kristine Kuhn, MD, PhD, associate professor of rheumatology at the University of Colorado at Denver, Aurora, who led a team of researchers that also included investigators from Stanford (Calif.) University and Benaroya Research Institute in Seattle.
The findings offer the latest evidence – and perhaps the most compelling evidence – for the mucosal origins hypothesis, the idea that rheumatoid arthritis can start with an immune response somewhere in the mucosa because of environmental interactions, and then becomes systemic, resulting in symptoms in the joints. Anti-citrullinated protein antibodies (ACPA), hallmarks of RA, have been found at mucosal surfaces in the periodontium and the lungs, and there have been reports of them in the intestine and cervicovaginal mucosa as well.
The latest findings that implicate the new bacterium build on previous findings in which people at risk of RA, but without symptoms yet, had an expansion of B cells producing immunoglobulin A (IgA), an antibody found in the mucosa. A closer look at these B cells, using variable region sequencing, found that they arose from a family that includes both IgA and IgG members. Because IgG antibodies are systemic, this suggested a kind of evolution from an IgA-based, mucosal immune response to one that is systemic and could target the joints.
Researchers mixed monoclonal antibodies from these B cells with a pool of bacteria from the stool of a broad population of people, and then pulled out the bacteria bound by these antibodies, and sequenced them. They found that the antibodies had bound almost exclusively to Ruminococcaceae and Lachnospiraceae.
They then cultured the stool of an individual at risk of developing RA and ended up with five isolates within Ruminococcaceae – “all of which belonged to the Subdoligranulum genus,” Dr. Kuhn said. When they sequenced these, they found that they had a new strain, which was named by Meagan Chriswell, an MD-PhD candidate and member of the Cherokee Nation of Oklahoma, who chose a term based on the Cherokee word for rheumatism.
Researchers at Benaroya then mixed this strain with T cells of people with RA, and those of controls, and only the T cells of those with RA were stimulated by the bacterium, they found.
“When intestines of germ-free mice were colonized with the strain, we found that they were getting arthritis,” Dr. Kuhn said. Photos of the joints show a striking contrast between the swollen joints of the mice given Subdoligranulum didolesgii and those injected with Prevotella copri, another strain suspected of having a link to RA, as well as with another Subdoligranulum strain and a sterile media. Dr. Kuhn noted that the P. copri strain did not come from an RA-affected individual.
“We thought that our results closed the loop nicely to show that these immune responses truly were toward the Subdoligranulum, and also stimulating arthritis,” she said.
The researchers then assessed the prevalence of the strain in people at risk for RA or with RA, and in controls. They found it in 17% of those with or at risk for RA but didn’t see it at all in the healthy control population.
Dr. Kuhn and her research team, she said, are now looking at the prevalence of the strain in a larger population and doing more investigating into the link with RA.
“Does it really associate with the development of immune responses and the development of rheumatoid arthritis?” she said.
Potential etiologic role of P. copri
Another paper, published in Arthritis & Rheumatology by some of the same investigators a week before the study describing the Subdoligranulum findings, tried to ascertain the point at which individuals might develop antibodies to P. copri, which for about a decade has been suspected of having a link to the development of RA.
They found that those with early RA had higher median values of IgG anti–P. copri (Pc) antibodies, compared with matched controls. People with established RA also had higher values of IgA anti-Pc antibodies. Those with ACPA, but not rheumatoid factor (RF), showed a trend toward higher IgG anti-Pc antibodies. Those who were ACPA-positive and RF-positive had significantly increased levels of IgA anti-Pc antibodies and a trend toward higher levels of IgG anti-Pc antibodies, compared with matched controls.
The findings, according to the researchers, “support a potential etiologic role for this microorganism in both RA preclinical evolution and the subsequent pathogenesis of synovitis.”
Dr. Kuhn and others in the field say it’s likely that many microbes play a role in the development of RA, and that the P. copri findings only add evidence of that relationship.
“Maybe the bacteria are involved at different parts of the pathway, and maybe they’re involved in triggering different parts of the immune responses,” she said. “Those are all to be determined.”
Dan Littman, MD, PhD, professor of rheumatology at New York University, who wrote a commentary reflecting on the findings of the Subdoligranulum study, said the results are “another piece of data” adding to the evidence base for the mucosal origins hypothesis.
“It’s by no means proven that this is the way pathogenesis in RA can occur, but it’s certainly a very solid study,” said Dr. Littman, who with colleagues published findings in 2013 linking P. copri to RA. “What makes it most compelling is that they seem to be able to show some evidence of causality in the mouse model.”
Before the findings could lead to therapy, he said, more evidence is needed to show that there is a causal link, and on the mechanism at work, such as whether this is something that occurs at the outset of disease or is something that “fuels the disease” by continually activating immune cells contributing to RA.
“If it’s only something that’s involved in the initiation of the disease, you need to catch it very early,” he said. “But if it’s something that continues to provide fuel for the disease, you may be able to catch it later and still be effective. Those are really critical items.”
Eventually, if these questions are answered, bacteriophages could be developed to snuff out problematic strains, or the regulatory response could be targeted to prevent the activation of the B cells that give rise to autoimmunity, he suggested.
“There are multiple steps to get to a therapeutic here, and I think we’re still a long ways from that,” he said. Still, he said, “I think it’s an important paper because it will encourage more people to look at this mechanism more closely and determine whether it really is representative of what happens in a lot of RA patients.”
The study in Science Translational Medicine was supported by grants from the National Institutes of Health, a Pfizer ASPIRE grant, and a grant from the Rheumatology Research Foundation. The Arthritis & Rheumatology study was supported in part by grants from the National Institutes of Health; the American College of Rheumatology Innovative Grant Program; the Ounsworth-Fitzgerald Foundation; Mathers Foundation; English, Bonter, Mitchell Foundation; Littauer Foundation; Lillian B. Davey Foundation; and the Eshe Fund. None of the researchers in either study had relevant financial disclosures. Dr. Littman is scientific cofounder and member of the scientific advisory board of Vedanta Biosciences, which studies microbiota therapeutics.
A newly discovered strain of bacteria could play a role in the development of rheumatoid arthritis, according to findings recently published in Science Translational Medicine.
Mice colonized with a strain of Subdoligranulum bacteria in their gut – a strain previously unidentified but now named Subdoligranulum didolesgii – developed joint swelling and inflammation as well as antibodies and T-cell responses similar to what is seen in RA, researchers reported.
“This was the first time that anyone has observed arthritis developing in a mouse that was not otherwise immunologically stimulated with an adjuvant of some kind, or genetically manipulated,” said Kristine Kuhn, MD, PhD, associate professor of rheumatology at the University of Colorado at Denver, Aurora, who led a team of researchers that also included investigators from Stanford (Calif.) University and Benaroya Research Institute in Seattle.
The findings offer the latest evidence – and perhaps the most compelling evidence – for the mucosal origins hypothesis, the idea that rheumatoid arthritis can start with an immune response somewhere in the mucosa because of environmental interactions, and then becomes systemic, resulting in symptoms in the joints. Anti-citrullinated protein antibodies (ACPA), hallmarks of RA, have been found at mucosal surfaces in the periodontium and the lungs, and there have been reports of them in the intestine and cervicovaginal mucosa as well.
The latest findings that implicate the new bacterium build on previous findings in which people at risk of RA, but without symptoms yet, had an expansion of B cells producing immunoglobulin A (IgA), an antibody found in the mucosa. A closer look at these B cells, using variable region sequencing, found that they arose from a family that includes both IgA and IgG members. Because IgG antibodies are systemic, this suggested a kind of evolution from an IgA-based, mucosal immune response to one that is systemic and could target the joints.
Researchers mixed monoclonal antibodies from these B cells with a pool of bacteria from the stool of a broad population of people, and then pulled out the bacteria bound by these antibodies, and sequenced them. They found that the antibodies had bound almost exclusively to Ruminococcaceae and Lachnospiraceae.
They then cultured the stool of an individual at risk of developing RA and ended up with five isolates within Ruminococcaceae – “all of which belonged to the Subdoligranulum genus,” Dr. Kuhn said. When they sequenced these, they found that they had a new strain, which was named by Meagan Chriswell, an MD-PhD candidate and member of the Cherokee Nation of Oklahoma, who chose a term based on the Cherokee word for rheumatism.
Researchers at Benaroya then mixed this strain with T cells of people with RA, and those of controls, and only the T cells of those with RA were stimulated by the bacterium, they found.
“When intestines of germ-free mice were colonized with the strain, we found that they were getting arthritis,” Dr. Kuhn said. Photos of the joints show a striking contrast between the swollen joints of the mice given Subdoligranulum didolesgii and those injected with Prevotella copri, another strain suspected of having a link to RA, as well as with another Subdoligranulum strain and a sterile media. Dr. Kuhn noted that the P. copri strain did not come from an RA-affected individual.
“We thought that our results closed the loop nicely to show that these immune responses truly were toward the Subdoligranulum, and also stimulating arthritis,” she said.
The researchers then assessed the prevalence of the strain in people at risk for RA or with RA, and in controls. They found it in 17% of those with or at risk for RA but didn’t see it at all in the healthy control population.
Dr. Kuhn and her research team, she said, are now looking at the prevalence of the strain in a larger population and doing more investigating into the link with RA.
“Does it really associate with the development of immune responses and the development of rheumatoid arthritis?” she said.
Potential etiologic role of P. copri
Another paper, published in Arthritis & Rheumatology by some of the same investigators a week before the study describing the Subdoligranulum findings, tried to ascertain the point at which individuals might develop antibodies to P. copri, which for about a decade has been suspected of having a link to the development of RA.
They found that those with early RA had higher median values of IgG anti–P. copri (Pc) antibodies, compared with matched controls. People with established RA also had higher values of IgA anti-Pc antibodies. Those with ACPA, but not rheumatoid factor (RF), showed a trend toward higher IgG anti-Pc antibodies. Those who were ACPA-positive and RF-positive had significantly increased levels of IgA anti-Pc antibodies and a trend toward higher levels of IgG anti-Pc antibodies, compared with matched controls.
The findings, according to the researchers, “support a potential etiologic role for this microorganism in both RA preclinical evolution and the subsequent pathogenesis of synovitis.”
Dr. Kuhn and others in the field say it’s likely that many microbes play a role in the development of RA, and that the P. copri findings only add evidence of that relationship.
“Maybe the bacteria are involved at different parts of the pathway, and maybe they’re involved in triggering different parts of the immune responses,” she said. “Those are all to be determined.”
Dan Littman, MD, PhD, professor of rheumatology at New York University, who wrote a commentary reflecting on the findings of the Subdoligranulum study, said the results are “another piece of data” adding to the evidence base for the mucosal origins hypothesis.
“It’s by no means proven that this is the way pathogenesis in RA can occur, but it’s certainly a very solid study,” said Dr. Littman, who with colleagues published findings in 2013 linking P. copri to RA. “What makes it most compelling is that they seem to be able to show some evidence of causality in the mouse model.”
Before the findings could lead to therapy, he said, more evidence is needed to show that there is a causal link, and on the mechanism at work, such as whether this is something that occurs at the outset of disease or is something that “fuels the disease” by continually activating immune cells contributing to RA.
“If it’s only something that’s involved in the initiation of the disease, you need to catch it very early,” he said. “But if it’s something that continues to provide fuel for the disease, you may be able to catch it later and still be effective. Those are really critical items.”
Eventually, if these questions are answered, bacteriophages could be developed to snuff out problematic strains, or the regulatory response could be targeted to prevent the activation of the B cells that give rise to autoimmunity, he suggested.
“There are multiple steps to get to a therapeutic here, and I think we’re still a long ways from that,” he said. Still, he said, “I think it’s an important paper because it will encourage more people to look at this mechanism more closely and determine whether it really is representative of what happens in a lot of RA patients.”
The study in Science Translational Medicine was supported by grants from the National Institutes of Health, a Pfizer ASPIRE grant, and a grant from the Rheumatology Research Foundation. The Arthritis & Rheumatology study was supported in part by grants from the National Institutes of Health; the American College of Rheumatology Innovative Grant Program; the Ounsworth-Fitzgerald Foundation; Mathers Foundation; English, Bonter, Mitchell Foundation; Littauer Foundation; Lillian B. Davey Foundation; and the Eshe Fund. None of the researchers in either study had relevant financial disclosures. Dr. Littman is scientific cofounder and member of the scientific advisory board of Vedanta Biosciences, which studies microbiota therapeutics.
FROM SCIENCE TRANSLATIONAL MEDICINE AND ARTHRITIS & RHEUMATOLOGY
Locked-in syndrome malpractice case ends with $75 million verdict against docs
The patient, Jonathan Buckelew, was taken to North Fulton Regional Hospital in Roswell, Ga., where imaging revealed that he had suffered a brain stem stroke.
The patient’s attorney, Laura Shamp, alleged in the legal complaint that a series of miscommunications and negligence by multiple providers delayed the diagnosis and treatment of the stroke until the next day, which led to catastrophic brain damage for the patient, who developed locked-in syndrome. The rare neurologic syndrome causes complete paralysis except for the muscles that control eye movements.
“Mr. Buckelew has expended millions of dollars for medical expenses for his care and he will need 24 hour a day care for the rest of his life,” his lawyer said in court documents.
Both physicians’ attorneys denied the claims and said their clients met the standard of care.
The jury attributed 60% fault to ED physician Matthew Womack, MD, and 40% to radiologist James Waldschmidt, MD.
Ms. Shamp alleged that Dr. Womack failed to inform the consulting neurologist of the chiropractic neck adjustment – a known stroke risk factor – and did not adequately communicate the results from CT angiography and lumbar puncture.
In addition, she said Dr. Womack failed to rule out a vertebral artery dissection and that Dr. Waldschmidt did not “[appreciate] an indisputable acute or subacute vertebral-basilar artery occlusion.”
Further allegations were levied against several other members of the patient’s care team, including the neurologist, a critical care physician, a physician assistant, and intensive care unit nurses, but they were not found liable by the jury.
The chiropractor, Michael Axt, DC, was named in the original complaint, but court documents filed earlier in 2022 requested that he be dismissed from the lawsuit, stating that he and the patient had reached an amicable resolution.
“This is a very large verdict,” James B. Edwards, JD, a medical malpractice attorney based in Texas who was not involved in the case, said in an interview. The diagnosis of locked-in syndrome likely contributed to the substantial monetary award.
“The more sympathetic the plaintiff and the situation, the greater risk of a verdict [against] the defendants,” Mr. Edwards said. “Cases that elicit significant and sometimes decisive sympathy include locked-in syndrome, permanent vegetative state, injury to the sexual or reproductive organs, burns, and blindness.”
The effectiveness and safety of chiropractic adjustments often come under fire. Although postmanipulation injuries are not common, they can have near-fatal consequences when they do occur.
In August, a healthy 28-year-old college student experienced four artery dissections after a chiropractic visit for low-back pain. She subsequently had a stroke and went into cardiac arrest. The patient survived but remains paralyzed.
Mr. Buckelew’s legal team said in a statement that his injuries would have been completely avoided had “the slew of health care providers ... acted according to the standard of care, caught and treated his stroke earlier, and communicated more effectively.”
On the day of the chiropractic adjustment, Ms. Shamp said Mr. Axt had documented that Mr. Buckelew’s primary complaints – neck pain, a headache, and bouts of blurred vision and ringing in the ears – began after exercise and had continued for several days.
In his closing statement, Dr. Womack’s attorney said the “chiropractor is solely responsible” for the patient’s injuries because he performed a manipulation despite the patient having a 2-week history of headaches.
Very few medical malpractice verdicts are appealed, though the sizable award in this suit may increase the likelihood that the defense will do so, Mr. Edwards said.
A version of this article first appeared on Medscape.com.
The patient, Jonathan Buckelew, was taken to North Fulton Regional Hospital in Roswell, Ga., where imaging revealed that he had suffered a brain stem stroke.
The patient’s attorney, Laura Shamp, alleged in the legal complaint that a series of miscommunications and negligence by multiple providers delayed the diagnosis and treatment of the stroke until the next day, which led to catastrophic brain damage for the patient, who developed locked-in syndrome. The rare neurologic syndrome causes complete paralysis except for the muscles that control eye movements.
“Mr. Buckelew has expended millions of dollars for medical expenses for his care and he will need 24 hour a day care for the rest of his life,” his lawyer said in court documents.
Both physicians’ attorneys denied the claims and said their clients met the standard of care.
The jury attributed 60% fault to ED physician Matthew Womack, MD, and 40% to radiologist James Waldschmidt, MD.
Ms. Shamp alleged that Dr. Womack failed to inform the consulting neurologist of the chiropractic neck adjustment – a known stroke risk factor – and did not adequately communicate the results from CT angiography and lumbar puncture.
In addition, she said Dr. Womack failed to rule out a vertebral artery dissection and that Dr. Waldschmidt did not “[appreciate] an indisputable acute or subacute vertebral-basilar artery occlusion.”
Further allegations were levied against several other members of the patient’s care team, including the neurologist, a critical care physician, a physician assistant, and intensive care unit nurses, but they were not found liable by the jury.
The chiropractor, Michael Axt, DC, was named in the original complaint, but court documents filed earlier in 2022 requested that he be dismissed from the lawsuit, stating that he and the patient had reached an amicable resolution.
“This is a very large verdict,” James B. Edwards, JD, a medical malpractice attorney based in Texas who was not involved in the case, said in an interview. The diagnosis of locked-in syndrome likely contributed to the substantial monetary award.
“The more sympathetic the plaintiff and the situation, the greater risk of a verdict [against] the defendants,” Mr. Edwards said. “Cases that elicit significant and sometimes decisive sympathy include locked-in syndrome, permanent vegetative state, injury to the sexual or reproductive organs, burns, and blindness.”
The effectiveness and safety of chiropractic adjustments often come under fire. Although postmanipulation injuries are not common, they can have near-fatal consequences when they do occur.
In August, a healthy 28-year-old college student experienced four artery dissections after a chiropractic visit for low-back pain. She subsequently had a stroke and went into cardiac arrest. The patient survived but remains paralyzed.
Mr. Buckelew’s legal team said in a statement that his injuries would have been completely avoided had “the slew of health care providers ... acted according to the standard of care, caught and treated his stroke earlier, and communicated more effectively.”
On the day of the chiropractic adjustment, Ms. Shamp said Mr. Axt had documented that Mr. Buckelew’s primary complaints – neck pain, a headache, and bouts of blurred vision and ringing in the ears – began after exercise and had continued for several days.
In his closing statement, Dr. Womack’s attorney said the “chiropractor is solely responsible” for the patient’s injuries because he performed a manipulation despite the patient having a 2-week history of headaches.
Very few medical malpractice verdicts are appealed, though the sizable award in this suit may increase the likelihood that the defense will do so, Mr. Edwards said.
A version of this article first appeared on Medscape.com.
The patient, Jonathan Buckelew, was taken to North Fulton Regional Hospital in Roswell, Ga., where imaging revealed that he had suffered a brain stem stroke.
The patient’s attorney, Laura Shamp, alleged in the legal complaint that a series of miscommunications and negligence by multiple providers delayed the diagnosis and treatment of the stroke until the next day, which led to catastrophic brain damage for the patient, who developed locked-in syndrome. The rare neurologic syndrome causes complete paralysis except for the muscles that control eye movements.
“Mr. Buckelew has expended millions of dollars for medical expenses for his care and he will need 24 hour a day care for the rest of his life,” his lawyer said in court documents.
Both physicians’ attorneys denied the claims and said their clients met the standard of care.
The jury attributed 60% fault to ED physician Matthew Womack, MD, and 40% to radiologist James Waldschmidt, MD.
Ms. Shamp alleged that Dr. Womack failed to inform the consulting neurologist of the chiropractic neck adjustment – a known stroke risk factor – and did not adequately communicate the results from CT angiography and lumbar puncture.
In addition, she said Dr. Womack failed to rule out a vertebral artery dissection and that Dr. Waldschmidt did not “[appreciate] an indisputable acute or subacute vertebral-basilar artery occlusion.”
Further allegations were levied against several other members of the patient’s care team, including the neurologist, a critical care physician, a physician assistant, and intensive care unit nurses, but they were not found liable by the jury.
The chiropractor, Michael Axt, DC, was named in the original complaint, but court documents filed earlier in 2022 requested that he be dismissed from the lawsuit, stating that he and the patient had reached an amicable resolution.
“This is a very large verdict,” James B. Edwards, JD, a medical malpractice attorney based in Texas who was not involved in the case, said in an interview. The diagnosis of locked-in syndrome likely contributed to the substantial monetary award.
“The more sympathetic the plaintiff and the situation, the greater risk of a verdict [against] the defendants,” Mr. Edwards said. “Cases that elicit significant and sometimes decisive sympathy include locked-in syndrome, permanent vegetative state, injury to the sexual or reproductive organs, burns, and blindness.”
The effectiveness and safety of chiropractic adjustments often come under fire. Although postmanipulation injuries are not common, they can have near-fatal consequences when they do occur.
In August, a healthy 28-year-old college student experienced four artery dissections after a chiropractic visit for low-back pain. She subsequently had a stroke and went into cardiac arrest. The patient survived but remains paralyzed.
Mr. Buckelew’s legal team said in a statement that his injuries would have been completely avoided had “the slew of health care providers ... acted according to the standard of care, caught and treated his stroke earlier, and communicated more effectively.”
On the day of the chiropractic adjustment, Ms. Shamp said Mr. Axt had documented that Mr. Buckelew’s primary complaints – neck pain, a headache, and bouts of blurred vision and ringing in the ears – began after exercise and had continued for several days.
In his closing statement, Dr. Womack’s attorney said the “chiropractor is solely responsible” for the patient’s injuries because he performed a manipulation despite the patient having a 2-week history of headaches.
Very few medical malpractice verdicts are appealed, though the sizable award in this suit may increase the likelihood that the defense will do so, Mr. Edwards said.
A version of this article first appeared on Medscape.com.
Medicare fines for high hospital readmissions drop, but nearly 2,300 facilities are still penalized
resulting in the lightest penalties since 2014.
The Hospital Readmissions Reduction Program has been a mainstay of Medicare’s hospital payment system since it began in 2012. Created by the Affordable Care Act, the program evaluates the frequency with which Medicare patients at most hospitals return within 30 days and lowers future payments to hospitals that had a greater-than-expected rate of return. Hospitals can lose up to 3% of each Medicare payment for a year.
The pandemic threw hospitals into turmoil, inundating them with COVID patients while forcing many to postpone elective surgeries for months. When the Centers for Medicare & Medicaid Services evaluated hospitals’ previous 3 years of readmissions, as it does annually, the government decided to exclude the first half of 2020 because of the chaos caused by the pandemic. CMS also excluded from its calculations Medicare patients who were readmitted with pneumonia across all three years because of the difficulty in distinguishing them from patients with COVID.
Akin Demehin, senior director of quality and patient safety policy at the American Hospital Association, said the changes were warranted. “The COVID pandemic did a lot of really unprecedented things to care patterns of hospitals,” he said.
After making those changes, CMS evaluated 2½ years of readmission cases for Medicare patients who’d had heart failure, heart attacks, chronic obstructive pulmonary disease, coronary artery bypass grafts, and knee and hip replacements. As a result of its analysis, CMS penalized 2,273 hospitals, the fewest since the fiscal year that ended in September 2014, a KHN analysis found.
The average payment reduction was 0.43%, also the lowest since 2014. The reductions will be applied to each Medicare payment to the affected hospitals from Oct. 1 to next September and cost them $320 million over that 12-month period.
Some hospitals will see their penalties greatly reduced from 2021. The penalty on St. Mary’s Hospital in Athens, Ga., is dropping from 2.54% to 0.06%. Saint Joseph East in Lexington, Ky., received the maximum penalty, 3%, in 2021; it will lose 0.78% as of Oct. 1. In Flemington, N.J., the penalty for Hunterdon Medical Center is dropping from 2.29% to 0.12%.
To limit penalties, many hospitals in recent years have instituted new strategies to keep former patients from needing a return visit. Robert Coates, MD, interim chief medical officer at Hunterdon Health, which owns Hunterdon Medical Center, said in a statement that the hospital set up a system to identify patients who visited the emergency room within 30 days of a hospital stay. Instead of readmitting them, Hunterdon helps them set up next-day appointments at a doctor’s office or home monitoring of their health. Hunterdon also calls all discharged patients to ensure they have filled their prescriptions and had a follow-up visit with a clinician within a week of leaving the hospital.
Jessica Satterfield, MD, director of quality and clinical excellence at St. Mary’s Health Care System, which operates St. Mary’s Hospital, said in a statement that the hospital identified patients at risk of readmission when they were first admitted and focused on making sure that their medications were correct and that they had follow-up visits. “We are proud that our efforts are bearing fruit in the form of greatly reduced penalties but, more importantly, as a reflection of the exceptional care our staff and medical staff provide to our patients,” Dr. Satterfield said.
Saint Joseph East did not respond to emails seeking comment.
Despite the changes, 43% of the nation’s 5,236 hospitals were penalized. Of the unpenalized, all but 770 were automatically exempted. The 2,193 exempted hospitals include those that specialize in children, psychiatric patients, or veterans. Rehabilitation and long-term care hospitals are also excluded from the program, as are critical access hospitals, which Medicare pays differently to help them stay open in areas with no other hospitals. The government also exempted Maryland hospitals because that state has a special payment arrangement with Medicare. Of the hospitals that Medicare assessed, 75% were penalized.
For the new fiscal year, Medicare also cited the pandemic in giving hospitals a reprieve from its other major quality-focused effort that assesses penalties: the Hospital-Acquired Condition Reduction Program. It slashes Medicare payments by 1% to the quarter of general hospitals with the highest rates of infections and other potentially preventable patient injuries. For the previous fiscal year, CMS punished 764 hospitals under that program. Those penalties – which would have cost hospitals an estimated $350 million in 2022 – will resume next fiscal year, with adjustments that better take COVID patients into account. CMS will also refine the readmissions penalty program to distinguish pneumonia patients from COVID patients.
“COVID has been a tremendously disruptive force for all aspects of health care, most certainly CMS’ quality measurement programs,” Mr. Demehin said. “It’s probably going to be a couple of volatile years for readmission penalties.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
resulting in the lightest penalties since 2014.
The Hospital Readmissions Reduction Program has been a mainstay of Medicare’s hospital payment system since it began in 2012. Created by the Affordable Care Act, the program evaluates the frequency with which Medicare patients at most hospitals return within 30 days and lowers future payments to hospitals that had a greater-than-expected rate of return. Hospitals can lose up to 3% of each Medicare payment for a year.
The pandemic threw hospitals into turmoil, inundating them with COVID patients while forcing many to postpone elective surgeries for months. When the Centers for Medicare & Medicaid Services evaluated hospitals’ previous 3 years of readmissions, as it does annually, the government decided to exclude the first half of 2020 because of the chaos caused by the pandemic. CMS also excluded from its calculations Medicare patients who were readmitted with pneumonia across all three years because of the difficulty in distinguishing them from patients with COVID.
Akin Demehin, senior director of quality and patient safety policy at the American Hospital Association, said the changes were warranted. “The COVID pandemic did a lot of really unprecedented things to care patterns of hospitals,” he said.
After making those changes, CMS evaluated 2½ years of readmission cases for Medicare patients who’d had heart failure, heart attacks, chronic obstructive pulmonary disease, coronary artery bypass grafts, and knee and hip replacements. As a result of its analysis, CMS penalized 2,273 hospitals, the fewest since the fiscal year that ended in September 2014, a KHN analysis found.
The average payment reduction was 0.43%, also the lowest since 2014. The reductions will be applied to each Medicare payment to the affected hospitals from Oct. 1 to next September and cost them $320 million over that 12-month period.
Some hospitals will see their penalties greatly reduced from 2021. The penalty on St. Mary’s Hospital in Athens, Ga., is dropping from 2.54% to 0.06%. Saint Joseph East in Lexington, Ky., received the maximum penalty, 3%, in 2021; it will lose 0.78% as of Oct. 1. In Flemington, N.J., the penalty for Hunterdon Medical Center is dropping from 2.29% to 0.12%.
To limit penalties, many hospitals in recent years have instituted new strategies to keep former patients from needing a return visit. Robert Coates, MD, interim chief medical officer at Hunterdon Health, which owns Hunterdon Medical Center, said in a statement that the hospital set up a system to identify patients who visited the emergency room within 30 days of a hospital stay. Instead of readmitting them, Hunterdon helps them set up next-day appointments at a doctor’s office or home monitoring of their health. Hunterdon also calls all discharged patients to ensure they have filled their prescriptions and had a follow-up visit with a clinician within a week of leaving the hospital.
Jessica Satterfield, MD, director of quality and clinical excellence at St. Mary’s Health Care System, which operates St. Mary’s Hospital, said in a statement that the hospital identified patients at risk of readmission when they were first admitted and focused on making sure that their medications were correct and that they had follow-up visits. “We are proud that our efforts are bearing fruit in the form of greatly reduced penalties but, more importantly, as a reflection of the exceptional care our staff and medical staff provide to our patients,” Dr. Satterfield said.
Saint Joseph East did not respond to emails seeking comment.
Despite the changes, 43% of the nation’s 5,236 hospitals were penalized. Of the unpenalized, all but 770 were automatically exempted. The 2,193 exempted hospitals include those that specialize in children, psychiatric patients, or veterans. Rehabilitation and long-term care hospitals are also excluded from the program, as are critical access hospitals, which Medicare pays differently to help them stay open in areas with no other hospitals. The government also exempted Maryland hospitals because that state has a special payment arrangement with Medicare. Of the hospitals that Medicare assessed, 75% were penalized.
For the new fiscal year, Medicare also cited the pandemic in giving hospitals a reprieve from its other major quality-focused effort that assesses penalties: the Hospital-Acquired Condition Reduction Program. It slashes Medicare payments by 1% to the quarter of general hospitals with the highest rates of infections and other potentially preventable patient injuries. For the previous fiscal year, CMS punished 764 hospitals under that program. Those penalties – which would have cost hospitals an estimated $350 million in 2022 – will resume next fiscal year, with adjustments that better take COVID patients into account. CMS will also refine the readmissions penalty program to distinguish pneumonia patients from COVID patients.
“COVID has been a tremendously disruptive force for all aspects of health care, most certainly CMS’ quality measurement programs,” Mr. Demehin said. “It’s probably going to be a couple of volatile years for readmission penalties.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
resulting in the lightest penalties since 2014.
The Hospital Readmissions Reduction Program has been a mainstay of Medicare’s hospital payment system since it began in 2012. Created by the Affordable Care Act, the program evaluates the frequency with which Medicare patients at most hospitals return within 30 days and lowers future payments to hospitals that had a greater-than-expected rate of return. Hospitals can lose up to 3% of each Medicare payment for a year.
The pandemic threw hospitals into turmoil, inundating them with COVID patients while forcing many to postpone elective surgeries for months. When the Centers for Medicare & Medicaid Services evaluated hospitals’ previous 3 years of readmissions, as it does annually, the government decided to exclude the first half of 2020 because of the chaos caused by the pandemic. CMS also excluded from its calculations Medicare patients who were readmitted with pneumonia across all three years because of the difficulty in distinguishing them from patients with COVID.
Akin Demehin, senior director of quality and patient safety policy at the American Hospital Association, said the changes were warranted. “The COVID pandemic did a lot of really unprecedented things to care patterns of hospitals,” he said.
After making those changes, CMS evaluated 2½ years of readmission cases for Medicare patients who’d had heart failure, heart attacks, chronic obstructive pulmonary disease, coronary artery bypass grafts, and knee and hip replacements. As a result of its analysis, CMS penalized 2,273 hospitals, the fewest since the fiscal year that ended in September 2014, a KHN analysis found.
The average payment reduction was 0.43%, also the lowest since 2014. The reductions will be applied to each Medicare payment to the affected hospitals from Oct. 1 to next September and cost them $320 million over that 12-month period.
Some hospitals will see their penalties greatly reduced from 2021. The penalty on St. Mary’s Hospital in Athens, Ga., is dropping from 2.54% to 0.06%. Saint Joseph East in Lexington, Ky., received the maximum penalty, 3%, in 2021; it will lose 0.78% as of Oct. 1. In Flemington, N.J., the penalty for Hunterdon Medical Center is dropping from 2.29% to 0.12%.
To limit penalties, many hospitals in recent years have instituted new strategies to keep former patients from needing a return visit. Robert Coates, MD, interim chief medical officer at Hunterdon Health, which owns Hunterdon Medical Center, said in a statement that the hospital set up a system to identify patients who visited the emergency room within 30 days of a hospital stay. Instead of readmitting them, Hunterdon helps them set up next-day appointments at a doctor’s office or home monitoring of their health. Hunterdon also calls all discharged patients to ensure they have filled their prescriptions and had a follow-up visit with a clinician within a week of leaving the hospital.
Jessica Satterfield, MD, director of quality and clinical excellence at St. Mary’s Health Care System, which operates St. Mary’s Hospital, said in a statement that the hospital identified patients at risk of readmission when they were first admitted and focused on making sure that their medications were correct and that they had follow-up visits. “We are proud that our efforts are bearing fruit in the form of greatly reduced penalties but, more importantly, as a reflection of the exceptional care our staff and medical staff provide to our patients,” Dr. Satterfield said.
Saint Joseph East did not respond to emails seeking comment.
Despite the changes, 43% of the nation’s 5,236 hospitals were penalized. Of the unpenalized, all but 770 were automatically exempted. The 2,193 exempted hospitals include those that specialize in children, psychiatric patients, or veterans. Rehabilitation and long-term care hospitals are also excluded from the program, as are critical access hospitals, which Medicare pays differently to help them stay open in areas with no other hospitals. The government also exempted Maryland hospitals because that state has a special payment arrangement with Medicare. Of the hospitals that Medicare assessed, 75% were penalized.
For the new fiscal year, Medicare also cited the pandemic in giving hospitals a reprieve from its other major quality-focused effort that assesses penalties: the Hospital-Acquired Condition Reduction Program. It slashes Medicare payments by 1% to the quarter of general hospitals with the highest rates of infections and other potentially preventable patient injuries. For the previous fiscal year, CMS punished 764 hospitals under that program. Those penalties – which would have cost hospitals an estimated $350 million in 2022 – will resume next fiscal year, with adjustments that better take COVID patients into account. CMS will also refine the readmissions penalty program to distinguish pneumonia patients from COVID patients.
“COVID has been a tremendously disruptive force for all aspects of health care, most certainly CMS’ quality measurement programs,” Mr. Demehin said. “It’s probably going to be a couple of volatile years for readmission penalties.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Exercise later in the day for better blood glucose control?
The data come from 775 participants with a mean body mass index (BMI) of 26.2 kg/m2 in the observational Netherlands Epidemiology of Obesity (NEO) study. Use of activity monitors for four consecutive days showed that performance of MVPA (defined as activity with intensity of > 3 metabolic equivalents of task) in the afternoon or evening was associated with up to 25% reduced insulin resistance compared with an even distribution of activity during the day.
“This is one of the first studies where in humans the relation between timing of physical activity and insulin resistance was examined,” lead author Jeroen van der Velde of the department of clinical epidemiology, Leiden (the Netherlands) University Medical Center, said in an interview.
Moreover, he noted that, while previous intervention studies have shown greater blood glucose reduction with high-intensity exercise performed in the afternoon, compared with the morning, in people with impaired glucose metabolism or type 2 diabetes, “as far as I am aware, we were the first to use a population-based study in a general population to study this.”
Katarina Kos, MD, PhD, senior lecturer in diabetes and obesity, University of Exeter (England), said: “This study is novel in that it relates the timing of physical activity if performed in the morning, afternoon, or evening to insulin resistance and fat content. This is from a cohort of middle-aged Dutch people between ages 45-65 studied 10 years ago and based on self-reports of weight and eating behavior and who were found to be generally overweight.”
Is it down to circadian rhythm?
“The results are of interest in that if the chosen timing was in the afternoon [63% of studied population] or evening (8% of the studied population), it seemed to relate with improved metabolism when compared to the morning exercising [16% of population]. ... Whether this was due to the (timing) of activity is yet to be shown,” Dr. Kos told the UK Science Media Centre.
Mr. van der Velde agrees that the effect may be explained at least in part by the circadian rhythm of the body. “Physical activity may act as ... a cue for the activation of clock genes. Previous research has suggested that our body’s muscular system and oxidative system are also affected by our circadian rhythm and their peak activity seems to be in the late afternoon. So, being mostly active in this time period ... may elicit greater metabolic responses compared to being active in the morning.”
But, he cautioned, “I think it is important to realize that we are just beginning to understand the potential impact of physical activity timing. At this stage, I believe it is most important to be physically active in general. So ... if the morning is the only time of the day to go for a walk or a run, certainly do this.”
Dr. Kos concurred: “As this is not an intervention study, further research is needed to explain the cause of the observed association.”
Mr. van der Velde also added that it’s not yet clear which individuals or subgroups might experience additional benefits from timed activities. That’s the current research focus of a large consortium of several research institutes in the Netherlands and Canada.
Timed exercise reduces insulin resistance but not liver fat
The findings were published online in Diabetologia.
The study population included men and women living in the greater Leiden area in the western Netherlands who were aged 45-65 years and self-reported a BMI of 27 or higher. A second cohort included inhabitants of one municipality who were invited to participate regardless of their BMI. All wore the activity monitors for 4 consecutive days and nights during their usual activities.
Neither sedentary time nor breaks in sedentary time (defined as a period of activity with an acceleration greater than 0.75 m/s2 following a sedentary period) were associated with lower insulin resistance, as calculated by blood sampling.
However, the number of breaks in sedentary time was associated with a significant 22% higher liver fat content, assessed with proton magnetic resonance spectroscopy.
One reason for the lack of effect of breaks on insulin resistance, the authors theorized, is that this was a real-world observational study where regular breaks aren’t common. Alternatively, people might not have been intensively active enough during breaks to make a difference.
After adjustment for total body fat, an additional hour of MVPA was associated with a 5% drop in insulin resistance. An additional hour of MVPA in 5-minute bouts was associated with 9% lower insulin resistance.
Also after adjustments, insulin resistance was reduced significantly in participants who were most active in the afternoon, by 18%, or evening, by 25%, whereas insulin resistance was not affected among those who were most active in the morning (–3%), all compared with people who distributed their MVPA throughout the day.
Timing of MVPA was not associated with liver fat content, and there were no significant differences in liver fat content and insulin resistance between groups based on timing of light physical activity.
“This is just speculation, but perhaps for fat accumulation in the liver the circadian system is less involved. Or perhaps timing of other lifestyle variables are more important here, such as dietary intake,” Mr. van der Velde said.
Finally, he observed, “timing of physical activity is most likely just a piece of the puzzle. Timing of other lifestyle behavior, such as sleep, and food intake are important cues for our circadian system as well, and it is likely that all these behaviors interact with each other.”
The NEO study is supported by Leiden University Medical Center, the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development/Partnership Diabetes/Dutch Diabetes foundation Breakthrough. Mr. van der Velde has reported no further disclosures.
A version of this article first appeared on Medscape.com.
The data come from 775 participants with a mean body mass index (BMI) of 26.2 kg/m2 in the observational Netherlands Epidemiology of Obesity (NEO) study. Use of activity monitors for four consecutive days showed that performance of MVPA (defined as activity with intensity of > 3 metabolic equivalents of task) in the afternoon or evening was associated with up to 25% reduced insulin resistance compared with an even distribution of activity during the day.
“This is one of the first studies where in humans the relation between timing of physical activity and insulin resistance was examined,” lead author Jeroen van der Velde of the department of clinical epidemiology, Leiden (the Netherlands) University Medical Center, said in an interview.
Moreover, he noted that, while previous intervention studies have shown greater blood glucose reduction with high-intensity exercise performed in the afternoon, compared with the morning, in people with impaired glucose metabolism or type 2 diabetes, “as far as I am aware, we were the first to use a population-based study in a general population to study this.”
Katarina Kos, MD, PhD, senior lecturer in diabetes and obesity, University of Exeter (England), said: “This study is novel in that it relates the timing of physical activity if performed in the morning, afternoon, or evening to insulin resistance and fat content. This is from a cohort of middle-aged Dutch people between ages 45-65 studied 10 years ago and based on self-reports of weight and eating behavior and who were found to be generally overweight.”
Is it down to circadian rhythm?
“The results are of interest in that if the chosen timing was in the afternoon [63% of studied population] or evening (8% of the studied population), it seemed to relate with improved metabolism when compared to the morning exercising [16% of population]. ... Whether this was due to the (timing) of activity is yet to be shown,” Dr. Kos told the UK Science Media Centre.
Mr. van der Velde agrees that the effect may be explained at least in part by the circadian rhythm of the body. “Physical activity may act as ... a cue for the activation of clock genes. Previous research has suggested that our body’s muscular system and oxidative system are also affected by our circadian rhythm and their peak activity seems to be in the late afternoon. So, being mostly active in this time period ... may elicit greater metabolic responses compared to being active in the morning.”
But, he cautioned, “I think it is important to realize that we are just beginning to understand the potential impact of physical activity timing. At this stage, I believe it is most important to be physically active in general. So ... if the morning is the only time of the day to go for a walk or a run, certainly do this.”
Dr. Kos concurred: “As this is not an intervention study, further research is needed to explain the cause of the observed association.”
Mr. van der Velde also added that it’s not yet clear which individuals or subgroups might experience additional benefits from timed activities. That’s the current research focus of a large consortium of several research institutes in the Netherlands and Canada.
Timed exercise reduces insulin resistance but not liver fat
The findings were published online in Diabetologia.
The study population included men and women living in the greater Leiden area in the western Netherlands who were aged 45-65 years and self-reported a BMI of 27 or higher. A second cohort included inhabitants of one municipality who were invited to participate regardless of their BMI. All wore the activity monitors for 4 consecutive days and nights during their usual activities.
Neither sedentary time nor breaks in sedentary time (defined as a period of activity with an acceleration greater than 0.75 m/s2 following a sedentary period) were associated with lower insulin resistance, as calculated by blood sampling.
However, the number of breaks in sedentary time was associated with a significant 22% higher liver fat content, assessed with proton magnetic resonance spectroscopy.
One reason for the lack of effect of breaks on insulin resistance, the authors theorized, is that this was a real-world observational study where regular breaks aren’t common. Alternatively, people might not have been intensively active enough during breaks to make a difference.
After adjustment for total body fat, an additional hour of MVPA was associated with a 5% drop in insulin resistance. An additional hour of MVPA in 5-minute bouts was associated with 9% lower insulin resistance.
Also after adjustments, insulin resistance was reduced significantly in participants who were most active in the afternoon, by 18%, or evening, by 25%, whereas insulin resistance was not affected among those who were most active in the morning (–3%), all compared with people who distributed their MVPA throughout the day.
Timing of MVPA was not associated with liver fat content, and there were no significant differences in liver fat content and insulin resistance between groups based on timing of light physical activity.
“This is just speculation, but perhaps for fat accumulation in the liver the circadian system is less involved. Or perhaps timing of other lifestyle variables are more important here, such as dietary intake,” Mr. van der Velde said.
Finally, he observed, “timing of physical activity is most likely just a piece of the puzzle. Timing of other lifestyle behavior, such as sleep, and food intake are important cues for our circadian system as well, and it is likely that all these behaviors interact with each other.”
The NEO study is supported by Leiden University Medical Center, the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development/Partnership Diabetes/Dutch Diabetes foundation Breakthrough. Mr. van der Velde has reported no further disclosures.
A version of this article first appeared on Medscape.com.
The data come from 775 participants with a mean body mass index (BMI) of 26.2 kg/m2 in the observational Netherlands Epidemiology of Obesity (NEO) study. Use of activity monitors for four consecutive days showed that performance of MVPA (defined as activity with intensity of > 3 metabolic equivalents of task) in the afternoon or evening was associated with up to 25% reduced insulin resistance compared with an even distribution of activity during the day.
“This is one of the first studies where in humans the relation between timing of physical activity and insulin resistance was examined,” lead author Jeroen van der Velde of the department of clinical epidemiology, Leiden (the Netherlands) University Medical Center, said in an interview.
Moreover, he noted that, while previous intervention studies have shown greater blood glucose reduction with high-intensity exercise performed in the afternoon, compared with the morning, in people with impaired glucose metabolism or type 2 diabetes, “as far as I am aware, we were the first to use a population-based study in a general population to study this.”
Katarina Kos, MD, PhD, senior lecturer in diabetes and obesity, University of Exeter (England), said: “This study is novel in that it relates the timing of physical activity if performed in the morning, afternoon, or evening to insulin resistance and fat content. This is from a cohort of middle-aged Dutch people between ages 45-65 studied 10 years ago and based on self-reports of weight and eating behavior and who were found to be generally overweight.”
Is it down to circadian rhythm?
“The results are of interest in that if the chosen timing was in the afternoon [63% of studied population] or evening (8% of the studied population), it seemed to relate with improved metabolism when compared to the morning exercising [16% of population]. ... Whether this was due to the (timing) of activity is yet to be shown,” Dr. Kos told the UK Science Media Centre.
Mr. van der Velde agrees that the effect may be explained at least in part by the circadian rhythm of the body. “Physical activity may act as ... a cue for the activation of clock genes. Previous research has suggested that our body’s muscular system and oxidative system are also affected by our circadian rhythm and their peak activity seems to be in the late afternoon. So, being mostly active in this time period ... may elicit greater metabolic responses compared to being active in the morning.”
But, he cautioned, “I think it is important to realize that we are just beginning to understand the potential impact of physical activity timing. At this stage, I believe it is most important to be physically active in general. So ... if the morning is the only time of the day to go for a walk or a run, certainly do this.”
Dr. Kos concurred: “As this is not an intervention study, further research is needed to explain the cause of the observed association.”
Mr. van der Velde also added that it’s not yet clear which individuals or subgroups might experience additional benefits from timed activities. That’s the current research focus of a large consortium of several research institutes in the Netherlands and Canada.
Timed exercise reduces insulin resistance but not liver fat
The findings were published online in Diabetologia.
The study population included men and women living in the greater Leiden area in the western Netherlands who were aged 45-65 years and self-reported a BMI of 27 or higher. A second cohort included inhabitants of one municipality who were invited to participate regardless of their BMI. All wore the activity monitors for 4 consecutive days and nights during their usual activities.
Neither sedentary time nor breaks in sedentary time (defined as a period of activity with an acceleration greater than 0.75 m/s2 following a sedentary period) were associated with lower insulin resistance, as calculated by blood sampling.
However, the number of breaks in sedentary time was associated with a significant 22% higher liver fat content, assessed with proton magnetic resonance spectroscopy.
One reason for the lack of effect of breaks on insulin resistance, the authors theorized, is that this was a real-world observational study where regular breaks aren’t common. Alternatively, people might not have been intensively active enough during breaks to make a difference.
After adjustment for total body fat, an additional hour of MVPA was associated with a 5% drop in insulin resistance. An additional hour of MVPA in 5-minute bouts was associated with 9% lower insulin resistance.
Also after adjustments, insulin resistance was reduced significantly in participants who were most active in the afternoon, by 18%, or evening, by 25%, whereas insulin resistance was not affected among those who were most active in the morning (–3%), all compared with people who distributed their MVPA throughout the day.
Timing of MVPA was not associated with liver fat content, and there were no significant differences in liver fat content and insulin resistance between groups based on timing of light physical activity.
“This is just speculation, but perhaps for fat accumulation in the liver the circadian system is less involved. Or perhaps timing of other lifestyle variables are more important here, such as dietary intake,” Mr. van der Velde said.
Finally, he observed, “timing of physical activity is most likely just a piece of the puzzle. Timing of other lifestyle behavior, such as sleep, and food intake are important cues for our circadian system as well, and it is likely that all these behaviors interact with each other.”
The NEO study is supported by Leiden University Medical Center, the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation, and the Netherlands Organisation for Health Research and Development/Partnership Diabetes/Dutch Diabetes foundation Breakthrough. Mr. van der Velde has reported no further disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
‘Unappreciated’ ties between COVID and gut dysbiosis
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Mycetomalike Skin Infection Due to Gordonia bronchialis in an Immunocompetent Patient
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.
Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.
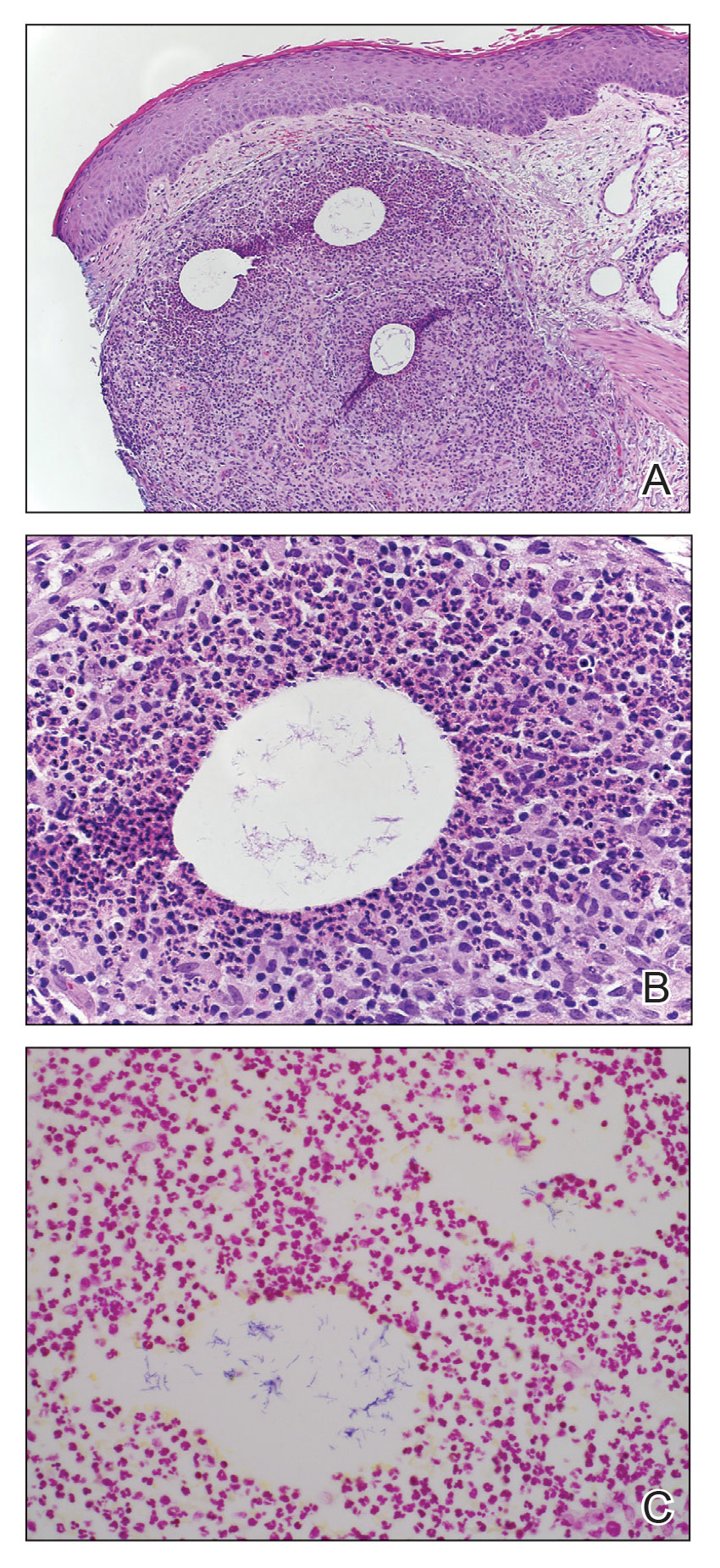
Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.
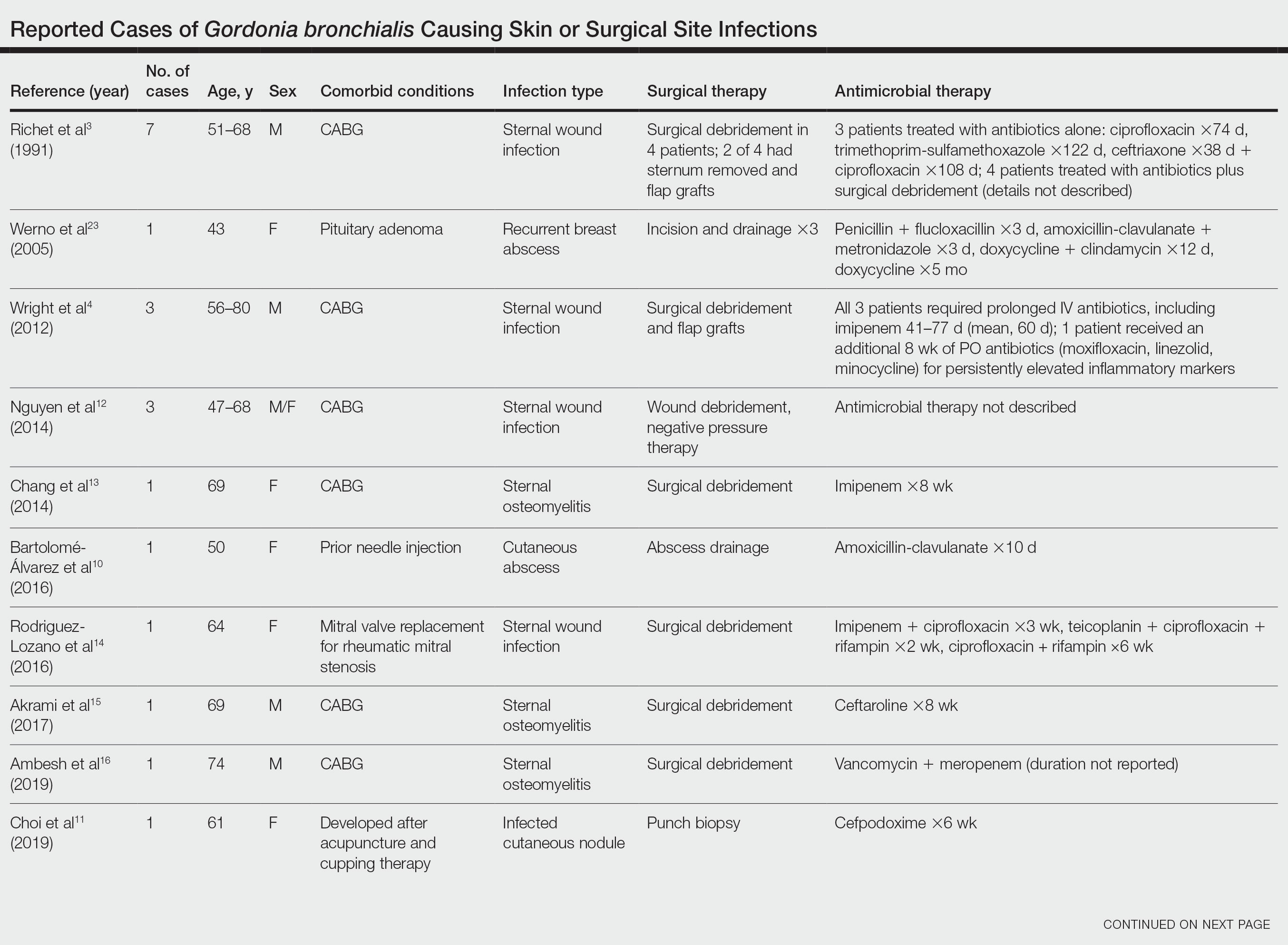
Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.

Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.

Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.


Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.

Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.

Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.


Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
Practice Points
- Gordonia bronchialis is an emerging cause of human skin and soft tissue infection, typically occurring after trauma, inoculation, or surgery.
- Gordonia species can cause a mycetomalike skin infection.
- Increasing use of molecular methods to identify bacteria has improved identification of clinically relevant actinomycetes, such as Helvetica Neue LT StdGordonia, and increases the likelihood that clinicians will see these organisms on culture results.