User login
Ancient Viruses in Our DNA Hold Clues to Cancer Treatment
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
according to a fascinating new study in Science Advances. Targeting these viral remnants still lingering in our DNA could lead to more effective cancer treatment with fewer side effects, the researchers said.
The study “gives a better understanding of how gene regulation can be impacted by these ancient retroviral sequences,” said Dixie Mager, PhD, scientist emeritus at the Terry Fox Laboratory at the British Columbia Cancer Research Institute, Vancouver, British Columbia, Canada. (Mager was not involved in the study.)
Long thought to be “junk” DNA with no biologic function, “endogenous retroviruses,” which have mutated over time and lost their ability to create the virus, are now known to regulate genes — allowing some genes to turn on and off. Research in recent years suggests they may play a role in diseases like cancer.
But scientists weren’t exactly sure what that role was, said senior study author Edward Chuong, PhD, a genome biologist at the University of Colorado Boulder.
Most studies have looked at whether endogenous retroviruses code for proteins that influence cancer. But these ancient viral strands usually don’t code for proteins at all.
Dr. Chuong took a different approach. Inspired by scientists who’ve studied how viral remnants regulate positive processes (immunity, brain development, or placenta development), he and his team explored whether some might regulate genes that, once activated, help cancer thrive.
Borrowing from epigenomic analysis data (data on molecules that alter gene expression) for 21 cancers mapped by the Cancer Genome Atlas, the researchers identified 19 virus-derived DNA sequences that bind to regulatory proteins more in cancer cells than in healthy cells. All of these could potentially act as gene regulators that promote cancer.
The researchers homed in on one sequence, called LTR10, because it showed especially high activity in several cancers, including lung and colorectal cancer. This DNA segment comes from a virus that entered our ancestors’ genome 30 million years ago, and it’s activated in a third of colorectal cancers.
Using the gene editing technology clustered regularly interspaced short palindromic repeats (CRISPR), Dr. Chuong’s team silenced LTR10 in colorectal cancer cells, altering the gene sequence so it couldn’t bind to regulatory proteins. Doing so dampened the activity of nearby cancer-promoting genes.
“They still behaved like cancer cells,” Dr. Chuong said. But “it made the cancer cells more susceptible to radiation. That would imply that the presence of that viral ‘switch’ actually helped those cancer cells survive radiation therapy.”
Previously, two studies had found that viral regulators play a role in promoting two types of cancer: Leukemia and prostate cancer. The new study shows these two cases weren’t flukes. All 21 cancers they looked at had at least one of those 19 viral elements, presumably working as cancer enhancers.
The study also identified what activates LTR10 to make it promote cancer. The culprit is a regulator protein called mitogen-activated protein (MAP) kinase, which is overactivated in about 40% of all human cancers.
Some cancer drugs — MAP kinase inhibitors — already target MAP kinase, and they’re often the first ones prescribed when a patient is diagnosed with cancer, Dr. Chuong said. As with many cancer treatments, doctors don’t know why they work, just that they do.
“By understanding the mechanisms in the cell, we might be able to make them work better or further optimize their treatment,” he said.
“MAP kinase inhibitors are really like a sledgehammer to the cell,” Dr. Chuong said — meaning they affect many cellular processes, not just those related to cancer.
“If we’re able to say that these viral switches are what’s important, then that could potentially help us develop a more targeted therapy that uses something like CRISPR to silence these viral elements,” he said. Or it could help providers choose a MAP kinase inhibitor from among the dozens available best suited to treat an individual patient and avoid side effects.
Still, whether the findings translate to real cancer patients remains to be seen. “It’s very, very hard to go the final step of showing in a patient that these actually make a difference in the cancer,” Dr. Mager said.
More lab research, human trials, and at least a few years will be needed before this discovery could help treat cancer. “Directly targeting these elements as a therapy would be at least 5 years out,” Dr. Chuong said, “partly because that application would rely on CRISPR epigenome editing technology that is still being developed for clinical use.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Anti-Smith and Anti–Double-Stranded DNA Antibodies in a Patient With Henoch-Schönlein Purpura Following COVID-19 Vaccination
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
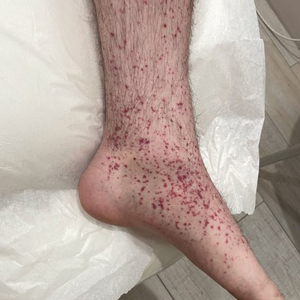
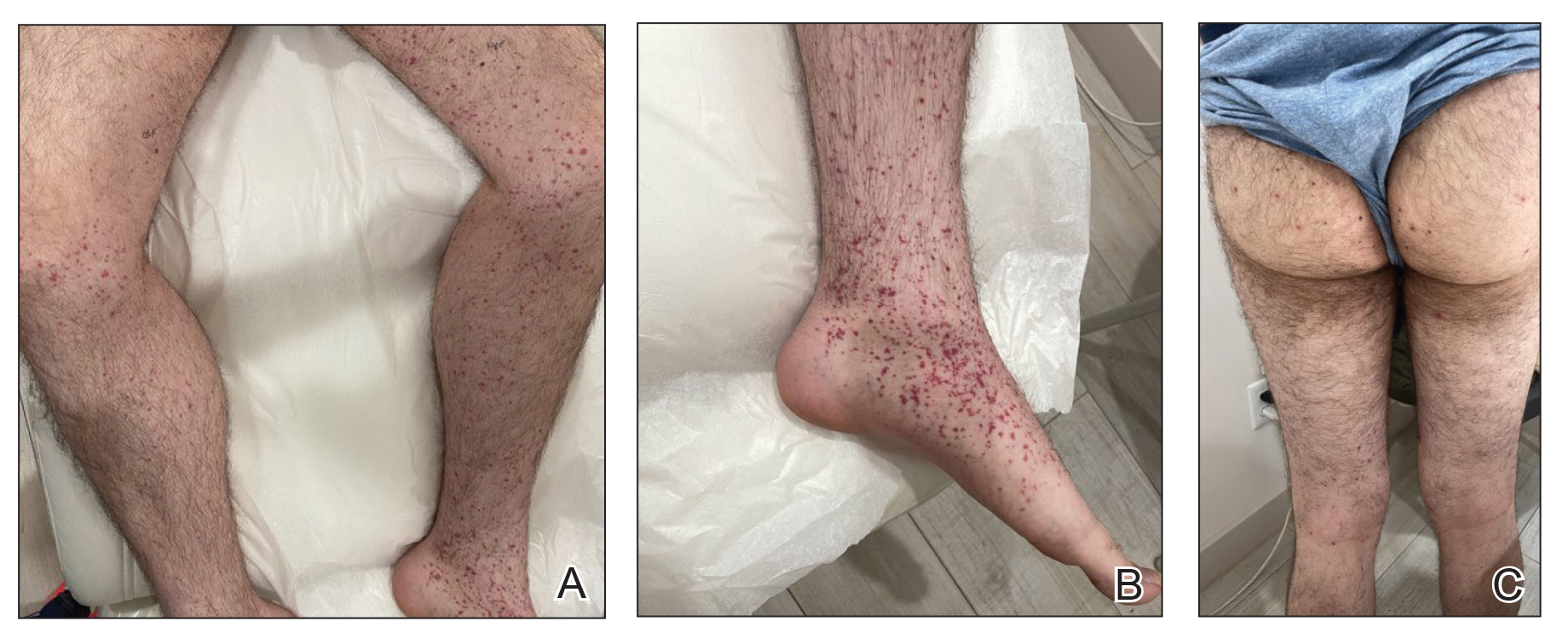
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
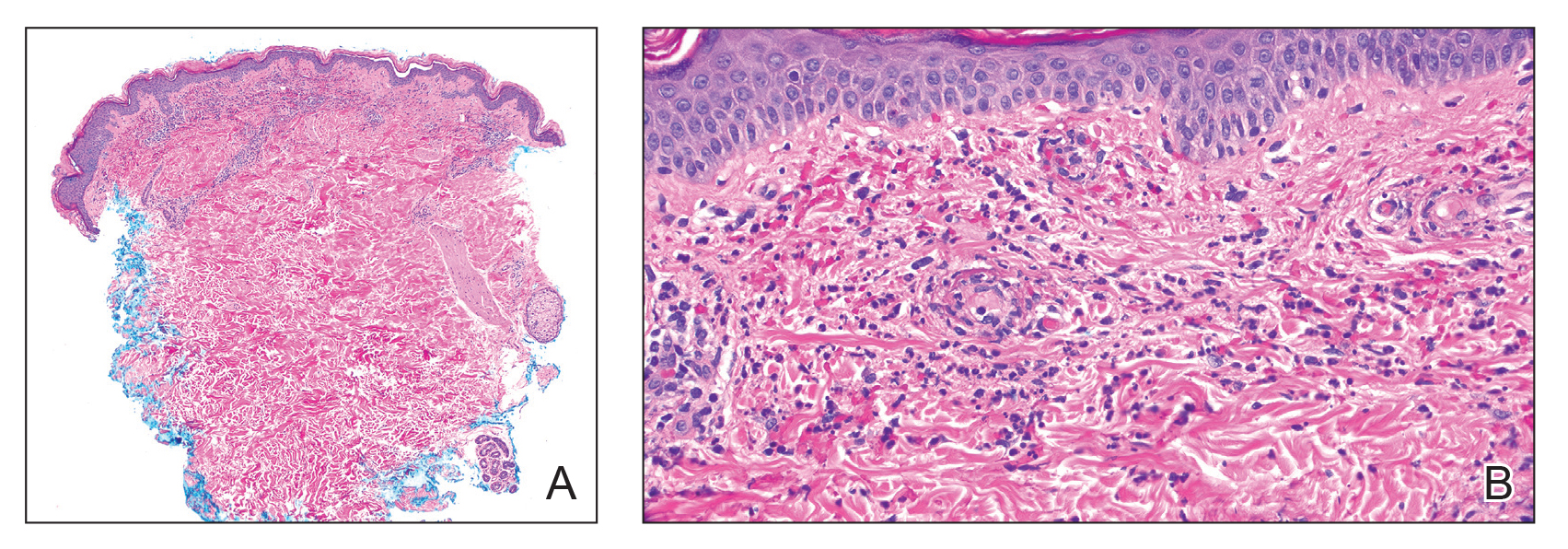
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.
Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
Practice Points
- Dermatologists should be vigilant for Henoch-Schönlein purpura (HSP) despite negative direct immunofluorescence of IgA deposition and unusual antibodies.
- Messenger RNA–based COVID-19 vaccines are associated with various cutaneous reactions, including HSP.
- Anti-Smith and anti–double-stranded DNA antibodies typically are not associated with HSP but may be seen in patients with coexisting systemic lupus erythematosus.
Infinite Learning
Dear Friends,
This issue of The New Gastroenterologist marks my first year completed as faculty. It has been both the best year and the HARDEST year. I celebrated many successes, felt intellectually and emotionally drained by difficult and complicated cases, and learned that there is so much more I still do not know. But that’s the beauty of our field — we are constantly learning to be better physicians for our patients. To trainees and my fellow gastroenterologists in practice, never stop asking questions!
In this issue’s “In Focus,” Dr. Rajan Singh and Dr. Baharak Moshiree describe a practical approach to patients with bloating by evaluating and investigating the pathophysiology and etiology of bloating, such as food intolerances, visceral hypersensitivity, pelvic floor dysfunction, abdominophrenic dyssynergia, gut dysmotility, and small intestinal bacterial overgrowth, as well as treatment management. In the “Short Clinical Review” section, Dr. Ahmad Bazarbashi and his colleagues review when to refer complex polyps to an advanced endoscopist and the different techniques of advanced tissue resection, including endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling endoscopic resection, and full thickness resection.
Locum practices have become more popular among gastroenterologists. Dr. Catherine Bartholomew is a retired professor of medicine who was chief of gastroenterology at an academic institution, and is now working as a GI locum after retirement. She details what a locum tenens is, the role of the company, being an independent contractor, and the benefits.
Navigating and negotiating maternity and paternity leave may be challenging in private practice. Dr. Marybeth Spanarkel gives her opinion on the nuances of maternity/paternity leave in private practices, what it may mean financially, and things to inquire of the practice if planning to have children.
As we move from joining non-traditional practices and navigating family planning with private practices, Dr. Vasu Appalaneni shares her experiences with financial planning for retirement. She describes ways to financially plan a retirement, but also to consider aspects that affect financial well-being during retirement, including healthcare coverage, lifestyle and traveling, legal and estate, professional development, and emotional and social support.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), communications/managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are not without appreciating where we were: The first colonic polypectomy using an electrosurgical snare was performed by Dr. Hiromi Shinya at Beth Israel Medical Center in New York City, in 1969.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
This issue of The New Gastroenterologist marks my first year completed as faculty. It has been both the best year and the HARDEST year. I celebrated many successes, felt intellectually and emotionally drained by difficult and complicated cases, and learned that there is so much more I still do not know. But that’s the beauty of our field — we are constantly learning to be better physicians for our patients. To trainees and my fellow gastroenterologists in practice, never stop asking questions!
In this issue’s “In Focus,” Dr. Rajan Singh and Dr. Baharak Moshiree describe a practical approach to patients with bloating by evaluating and investigating the pathophysiology and etiology of bloating, such as food intolerances, visceral hypersensitivity, pelvic floor dysfunction, abdominophrenic dyssynergia, gut dysmotility, and small intestinal bacterial overgrowth, as well as treatment management. In the “Short Clinical Review” section, Dr. Ahmad Bazarbashi and his colleagues review when to refer complex polyps to an advanced endoscopist and the different techniques of advanced tissue resection, including endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling endoscopic resection, and full thickness resection.
Locum practices have become more popular among gastroenterologists. Dr. Catherine Bartholomew is a retired professor of medicine who was chief of gastroenterology at an academic institution, and is now working as a GI locum after retirement. She details what a locum tenens is, the role of the company, being an independent contractor, and the benefits.
Navigating and negotiating maternity and paternity leave may be challenging in private practice. Dr. Marybeth Spanarkel gives her opinion on the nuances of maternity/paternity leave in private practices, what it may mean financially, and things to inquire of the practice if planning to have children.
As we move from joining non-traditional practices and navigating family planning with private practices, Dr. Vasu Appalaneni shares her experiences with financial planning for retirement. She describes ways to financially plan a retirement, but also to consider aspects that affect financial well-being during retirement, including healthcare coverage, lifestyle and traveling, legal and estate, professional development, and emotional and social support.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), communications/managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are not without appreciating where we were: The first colonic polypectomy using an electrosurgical snare was performed by Dr. Hiromi Shinya at Beth Israel Medical Center in New York City, in 1969.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
This issue of The New Gastroenterologist marks my first year completed as faculty. It has been both the best year and the HARDEST year. I celebrated many successes, felt intellectually and emotionally drained by difficult and complicated cases, and learned that there is so much more I still do not know. But that’s the beauty of our field — we are constantly learning to be better physicians for our patients. To trainees and my fellow gastroenterologists in practice, never stop asking questions!
In this issue’s “In Focus,” Dr. Rajan Singh and Dr. Baharak Moshiree describe a practical approach to patients with bloating by evaluating and investigating the pathophysiology and etiology of bloating, such as food intolerances, visceral hypersensitivity, pelvic floor dysfunction, abdominophrenic dyssynergia, gut dysmotility, and small intestinal bacterial overgrowth, as well as treatment management. In the “Short Clinical Review” section, Dr. Ahmad Bazarbashi and his colleagues review when to refer complex polyps to an advanced endoscopist and the different techniques of advanced tissue resection, including endoscopic mucosal resection, endoscopic submucosal dissection, submucosal tunneling endoscopic resection, and full thickness resection.
Locum practices have become more popular among gastroenterologists. Dr. Catherine Bartholomew is a retired professor of medicine who was chief of gastroenterology at an academic institution, and is now working as a GI locum after retirement. She details what a locum tenens is, the role of the company, being an independent contractor, and the benefits.
Navigating and negotiating maternity and paternity leave may be challenging in private practice. Dr. Marybeth Spanarkel gives her opinion on the nuances of maternity/paternity leave in private practices, what it may mean financially, and things to inquire of the practice if planning to have children.
As we move from joining non-traditional practices and navigating family planning with private practices, Dr. Vasu Appalaneni shares her experiences with financial planning for retirement. She describes ways to financially plan a retirement, but also to consider aspects that affect financial well-being during retirement, including healthcare coverage, lifestyle and traveling, legal and estate, professional development, and emotional and social support.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Danielle Kiefer ([email protected]), communications/managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are not without appreciating where we were: The first colonic polypectomy using an electrosurgical snare was performed by Dr. Hiromi Shinya at Beth Israel Medical Center in New York City, in 1969.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Consider Risks, Toxicity of Some Topical Ingredients in Infants, Young Children
TORONTO — Lawrence A. Schachner, MD, would like pediatric dermatologists to adopt a “toxic agent of the year” to raise awareness about the potential harm related to certain topical treatments in babies and young children.
Dr. Schachner, director of the Division of Pediatric Dermatology in the Department of Dermatology & Cutaneous Surgery at the University of Miami, Coral Gables, Florida, said he got the idea from the American Contact Dermatitis Society, which annually names the “Allergen of the Year.”
, said Dr. Schachner, professor of pediatrics and dermatology at the University of Miami.
“Any one of those would be excellent toxic substances of the year” that could be the focus of an educational campaign, he told this news organization following his presentation on “Toxicology of Topical Ingredients in Pediatric Dermatology” at the annual meeting of the Society for Pediatric Dermatology on July 14.
Benzene might also be a good candidate for the list, although the jury seems to be still out on its toxicity, said Dr. Schachner.
He talked about the “four Ps” of poisoning — the physician, pharmacy, parents, and pharmaceutical manufacturing — which all have some responsibility for errors that lead to adverse outcomes but can also take steps to prevent them.
During his presentation, Dr. Schachner discussed how babies are especially sensitive to topical therapies, noting that a baby’s skin is thinner and more permeable than that of an adult. And children have a greater body surface-to-weight ratio, so they absorb more substances through their skin.
He also noted that babies lack natural moisturizing factors, and their skin barrier isn’t mature until about age 3-5 years, stressing the need for extreme care when applying a topical agent to a baby’s skin.
Tragic Stories
Dr. Schachner pointed to some instances of mishaps related to toxic topical substances in children. There was the outbreak in the early 1980s of accidental hexachlorophene poisoning among children in France exposed to talc “baby powder.” Of the 204 affected children, 36 died.
The cause was a manufacturing error; the product contained 6.3% hexachlorophene, as opposed to the 0.1% limit recommended by the US Food and Drug Administration (FDA).
Local anesthetics, including lidocaine, dibucaine, and prilocaine, can cause local anesthetic systemic toxicity, a syndrome with symptoms that include central nervous system depression, seizures, and cardiotoxicity. Dr. Schachner described the case of a 3-year-old who developed methemoglobinemia, with seizures, after treatment with an excessive amount of eutectic mixture of local anesthetics (EMLA) cream, which contains both lidocaine and prilocaine.
EMLA shouldn’t be used with methemoglobinemia-inducing agents, such as some antimalarials, analgesics, anesthetics, and antineoplastic agents. It’s not recommended in neonates or for those under 12 months if receiving methemoglobinemia-inducing agents, “and I would keep an eye on it after 12 months of age,” said Dr. Schachner.
He cited a retrospective review of topical lidocaine toxicity in pediatric patients reported to the National Poison Data System from 2000 to 2020. It found 37 cases of toxicity, the most common from application prior to dermatologic procedures (37.5%), which led to two deaths.
Not Benign Agents
“These are not benign agents; we have to use them correctly,” Dr. Schachner stressed. When discussing alcohols and antiseptics, he noted that phenol is found in a variety of household disinfectants, gargling products, ointments, and lip balms. Phenol can be used as a chemical peel and is the antiseptic component of Castellani paint. He also referred to cases of alcohol intoxication linked to umbilical care in newborns.
Benzene at elevated levels has been found in some topical benzoyl peroxide acne products and in some sunscreens. There have been suggestions, not strongly substantiated, that benzene may increase the risk for cancer, especially leukemias.
But there is sparse data on the absorption and toxicity of benzene exposure with sunscreen use. The data, he said, include an analysis of National Health and Nutrition Examination Survey data, which found that people who regularly used sunscreens were less likely to have elevated benzene levels compared with those who didn’t use sunscreens.
Turning to insecticides, Dr. Schachner discussed N,N-diethyl-m-toluamide (DEET), the active ingredient in many insect repellents. It helps avoid “some terrible diseases,” including mosquito-borne illnesses such as malaria and tick-borne conditions such as Lyme disease, and is available in several convenient formulations, he said.
When used on children, the American Academy of Pediatrics (AAP) recommends products with no more than 30% DEET. And insect repellents are not recommended for children younger than 2 months, or under clothing or damaged skin, he said.
Dr. Schachner referred to a case series of 18 children who developed DEET-induced encephalopathy; 13 (72%) involved dermal exposure. Three of those with cutaneous exposure died, mostly from neurologic, respiratory, and cardiac issues. “What’s very striking is that 55% of the kids were exposed to DEET of 20% or less, even though the AAP approves DEET at 30%, so maybe that’s something we have to look at,” he said.
Medication Patches
With medication patches, especially fentanyl transdermal patches, much can go wrong when it comes to children. This was highlighted by the cases Schachner cited, including an infant who developed acute cytotoxic cerebellar edema from fentanyl patch intoxication.
In another case, emergency room staff found a fentanyl patch stuck to the back of a 3-year-old girl. A CT scan showed global cerebral edema, and the patient progressed to brain death. “This is not a unique case; there have been over 10 such cases in the United States,” said Dr. Schachner. “We should be doing better with fentanyl.”
Nicotine patches can also be dangerous to children, he added. As for other topical agents, there have been reports of toxicity and deaths linked to salicylic acid, commonly used by dermatologists because of its bacteriostatic, fungicidal, keratolytic, and photoprotective properties.
Dr. Schachner cited the case of a 2-month-old where the pediatrician prescribed 50% salicylic acid for seborrheic dermatitis of the scalp, under occlusion. “It’s amazing this child survived; that’s clearly a physician error,” he said.
Henna, a reddish-brown dye derived from the crushed leaves of Lawsonia alba, is used cosmetically for the hair, skin, and nails. Many henna products are mixed with additives, including para-phenylenediamine, which has been associated with dermatitis, asthma, renal failure, and permanent vision loss.
Asked to comment on the presentation, Sheilagh Maguiness, MD, professor of dermatology and pediatrics and chair of pediatric dermatology at the University of Minnesota, Minneapolis, recalled a particularly concerning story in 2008, when the FDA issued a warning about Mommy’s Bliss, a cream containing chlorphenesin and phenoxyethanol as preservatives, promoted to nursing mothers for soothing cracked nipples. There were reports of the cream causing respiratory distress, vomiting, and diarrhea in nursing infants.
Dr. Schachner is chair of Stiefel Laboratories and is an investigator with: Astellas, Berg Pharma, Celgene, Ferndale Labs, Lilly, Medimetriks Pharmaceuticals, Novartis, Organogenesis, Pfizer, Sciton; is a consultant for: Alphyn, Amryt Pharma, Beiersdorf, Brickell, Cutanea, Hoth, Lexington, Mustela, TopMD, Noble Pharma; a speaker for: Novartis, Sanofi-Regeneron, CeraVe; is on the advisory boards of: Almirall, Alphyn, Apogee, Aslan, Biofrontera, CeraVe, Krystal Biotech, Mustela, Noble Pharma, Pfizer, Pierre Fabre, Sanofi-Regeneron; and owns stocks in: TopMD and Alphyn. Dr. Maguiness had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TORONTO — Lawrence A. Schachner, MD, would like pediatric dermatologists to adopt a “toxic agent of the year” to raise awareness about the potential harm related to certain topical treatments in babies and young children.
Dr. Schachner, director of the Division of Pediatric Dermatology in the Department of Dermatology & Cutaneous Surgery at the University of Miami, Coral Gables, Florida, said he got the idea from the American Contact Dermatitis Society, which annually names the “Allergen of the Year.”
, said Dr. Schachner, professor of pediatrics and dermatology at the University of Miami.
“Any one of those would be excellent toxic substances of the year” that could be the focus of an educational campaign, he told this news organization following his presentation on “Toxicology of Topical Ingredients in Pediatric Dermatology” at the annual meeting of the Society for Pediatric Dermatology on July 14.
Benzene might also be a good candidate for the list, although the jury seems to be still out on its toxicity, said Dr. Schachner.
He talked about the “four Ps” of poisoning — the physician, pharmacy, parents, and pharmaceutical manufacturing — which all have some responsibility for errors that lead to adverse outcomes but can also take steps to prevent them.
During his presentation, Dr. Schachner discussed how babies are especially sensitive to topical therapies, noting that a baby’s skin is thinner and more permeable than that of an adult. And children have a greater body surface-to-weight ratio, so they absorb more substances through their skin.
He also noted that babies lack natural moisturizing factors, and their skin barrier isn’t mature until about age 3-5 years, stressing the need for extreme care when applying a topical agent to a baby’s skin.
Tragic Stories
Dr. Schachner pointed to some instances of mishaps related to toxic topical substances in children. There was the outbreak in the early 1980s of accidental hexachlorophene poisoning among children in France exposed to talc “baby powder.” Of the 204 affected children, 36 died.
The cause was a manufacturing error; the product contained 6.3% hexachlorophene, as opposed to the 0.1% limit recommended by the US Food and Drug Administration (FDA).
Local anesthetics, including lidocaine, dibucaine, and prilocaine, can cause local anesthetic systemic toxicity, a syndrome with symptoms that include central nervous system depression, seizures, and cardiotoxicity. Dr. Schachner described the case of a 3-year-old who developed methemoglobinemia, with seizures, after treatment with an excessive amount of eutectic mixture of local anesthetics (EMLA) cream, which contains both lidocaine and prilocaine.
EMLA shouldn’t be used with methemoglobinemia-inducing agents, such as some antimalarials, analgesics, anesthetics, and antineoplastic agents. It’s not recommended in neonates or for those under 12 months if receiving methemoglobinemia-inducing agents, “and I would keep an eye on it after 12 months of age,” said Dr. Schachner.
He cited a retrospective review of topical lidocaine toxicity in pediatric patients reported to the National Poison Data System from 2000 to 2020. It found 37 cases of toxicity, the most common from application prior to dermatologic procedures (37.5%), which led to two deaths.
Not Benign Agents
“These are not benign agents; we have to use them correctly,” Dr. Schachner stressed. When discussing alcohols and antiseptics, he noted that phenol is found in a variety of household disinfectants, gargling products, ointments, and lip balms. Phenol can be used as a chemical peel and is the antiseptic component of Castellani paint. He also referred to cases of alcohol intoxication linked to umbilical care in newborns.
Benzene at elevated levels has been found in some topical benzoyl peroxide acne products and in some sunscreens. There have been suggestions, not strongly substantiated, that benzene may increase the risk for cancer, especially leukemias.
But there is sparse data on the absorption and toxicity of benzene exposure with sunscreen use. The data, he said, include an analysis of National Health and Nutrition Examination Survey data, which found that people who regularly used sunscreens were less likely to have elevated benzene levels compared with those who didn’t use sunscreens.
Turning to insecticides, Dr. Schachner discussed N,N-diethyl-m-toluamide (DEET), the active ingredient in many insect repellents. It helps avoid “some terrible diseases,” including mosquito-borne illnesses such as malaria and tick-borne conditions such as Lyme disease, and is available in several convenient formulations, he said.
When used on children, the American Academy of Pediatrics (AAP) recommends products with no more than 30% DEET. And insect repellents are not recommended for children younger than 2 months, or under clothing or damaged skin, he said.
Dr. Schachner referred to a case series of 18 children who developed DEET-induced encephalopathy; 13 (72%) involved dermal exposure. Three of those with cutaneous exposure died, mostly from neurologic, respiratory, and cardiac issues. “What’s very striking is that 55% of the kids were exposed to DEET of 20% or less, even though the AAP approves DEET at 30%, so maybe that’s something we have to look at,” he said.
Medication Patches
With medication patches, especially fentanyl transdermal patches, much can go wrong when it comes to children. This was highlighted by the cases Schachner cited, including an infant who developed acute cytotoxic cerebellar edema from fentanyl patch intoxication.
In another case, emergency room staff found a fentanyl patch stuck to the back of a 3-year-old girl. A CT scan showed global cerebral edema, and the patient progressed to brain death. “This is not a unique case; there have been over 10 such cases in the United States,” said Dr. Schachner. “We should be doing better with fentanyl.”
Nicotine patches can also be dangerous to children, he added. As for other topical agents, there have been reports of toxicity and deaths linked to salicylic acid, commonly used by dermatologists because of its bacteriostatic, fungicidal, keratolytic, and photoprotective properties.
Dr. Schachner cited the case of a 2-month-old where the pediatrician prescribed 50% salicylic acid for seborrheic dermatitis of the scalp, under occlusion. “It’s amazing this child survived; that’s clearly a physician error,” he said.
Henna, a reddish-brown dye derived from the crushed leaves of Lawsonia alba, is used cosmetically for the hair, skin, and nails. Many henna products are mixed with additives, including para-phenylenediamine, which has been associated with dermatitis, asthma, renal failure, and permanent vision loss.
Asked to comment on the presentation, Sheilagh Maguiness, MD, professor of dermatology and pediatrics and chair of pediatric dermatology at the University of Minnesota, Minneapolis, recalled a particularly concerning story in 2008, when the FDA issued a warning about Mommy’s Bliss, a cream containing chlorphenesin and phenoxyethanol as preservatives, promoted to nursing mothers for soothing cracked nipples. There were reports of the cream causing respiratory distress, vomiting, and diarrhea in nursing infants.
Dr. Schachner is chair of Stiefel Laboratories and is an investigator with: Astellas, Berg Pharma, Celgene, Ferndale Labs, Lilly, Medimetriks Pharmaceuticals, Novartis, Organogenesis, Pfizer, Sciton; is a consultant for: Alphyn, Amryt Pharma, Beiersdorf, Brickell, Cutanea, Hoth, Lexington, Mustela, TopMD, Noble Pharma; a speaker for: Novartis, Sanofi-Regeneron, CeraVe; is on the advisory boards of: Almirall, Alphyn, Apogee, Aslan, Biofrontera, CeraVe, Krystal Biotech, Mustela, Noble Pharma, Pfizer, Pierre Fabre, Sanofi-Regeneron; and owns stocks in: TopMD and Alphyn. Dr. Maguiness had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TORONTO — Lawrence A. Schachner, MD, would like pediatric dermatologists to adopt a “toxic agent of the year” to raise awareness about the potential harm related to certain topical treatments in babies and young children.
Dr. Schachner, director of the Division of Pediatric Dermatology in the Department of Dermatology & Cutaneous Surgery at the University of Miami, Coral Gables, Florida, said he got the idea from the American Contact Dermatitis Society, which annually names the “Allergen of the Year.”
, said Dr. Schachner, professor of pediatrics and dermatology at the University of Miami.
“Any one of those would be excellent toxic substances of the year” that could be the focus of an educational campaign, he told this news organization following his presentation on “Toxicology of Topical Ingredients in Pediatric Dermatology” at the annual meeting of the Society for Pediatric Dermatology on July 14.
Benzene might also be a good candidate for the list, although the jury seems to be still out on its toxicity, said Dr. Schachner.
He talked about the “four Ps” of poisoning — the physician, pharmacy, parents, and pharmaceutical manufacturing — which all have some responsibility for errors that lead to adverse outcomes but can also take steps to prevent them.
During his presentation, Dr. Schachner discussed how babies are especially sensitive to topical therapies, noting that a baby’s skin is thinner and more permeable than that of an adult. And children have a greater body surface-to-weight ratio, so they absorb more substances through their skin.
He also noted that babies lack natural moisturizing factors, and their skin barrier isn’t mature until about age 3-5 years, stressing the need for extreme care when applying a topical agent to a baby’s skin.
Tragic Stories
Dr. Schachner pointed to some instances of mishaps related to toxic topical substances in children. There was the outbreak in the early 1980s of accidental hexachlorophene poisoning among children in France exposed to talc “baby powder.” Of the 204 affected children, 36 died.
The cause was a manufacturing error; the product contained 6.3% hexachlorophene, as opposed to the 0.1% limit recommended by the US Food and Drug Administration (FDA).
Local anesthetics, including lidocaine, dibucaine, and prilocaine, can cause local anesthetic systemic toxicity, a syndrome with symptoms that include central nervous system depression, seizures, and cardiotoxicity. Dr. Schachner described the case of a 3-year-old who developed methemoglobinemia, with seizures, after treatment with an excessive amount of eutectic mixture of local anesthetics (EMLA) cream, which contains both lidocaine and prilocaine.
EMLA shouldn’t be used with methemoglobinemia-inducing agents, such as some antimalarials, analgesics, anesthetics, and antineoplastic agents. It’s not recommended in neonates or for those under 12 months if receiving methemoglobinemia-inducing agents, “and I would keep an eye on it after 12 months of age,” said Dr. Schachner.
He cited a retrospective review of topical lidocaine toxicity in pediatric patients reported to the National Poison Data System from 2000 to 2020. It found 37 cases of toxicity, the most common from application prior to dermatologic procedures (37.5%), which led to two deaths.
Not Benign Agents
“These are not benign agents; we have to use them correctly,” Dr. Schachner stressed. When discussing alcohols and antiseptics, he noted that phenol is found in a variety of household disinfectants, gargling products, ointments, and lip balms. Phenol can be used as a chemical peel and is the antiseptic component of Castellani paint. He also referred to cases of alcohol intoxication linked to umbilical care in newborns.
Benzene at elevated levels has been found in some topical benzoyl peroxide acne products and in some sunscreens. There have been suggestions, not strongly substantiated, that benzene may increase the risk for cancer, especially leukemias.
But there is sparse data on the absorption and toxicity of benzene exposure with sunscreen use. The data, he said, include an analysis of National Health and Nutrition Examination Survey data, which found that people who regularly used sunscreens were less likely to have elevated benzene levels compared with those who didn’t use sunscreens.
Turning to insecticides, Dr. Schachner discussed N,N-diethyl-m-toluamide (DEET), the active ingredient in many insect repellents. It helps avoid “some terrible diseases,” including mosquito-borne illnesses such as malaria and tick-borne conditions such as Lyme disease, and is available in several convenient formulations, he said.
When used on children, the American Academy of Pediatrics (AAP) recommends products with no more than 30% DEET. And insect repellents are not recommended for children younger than 2 months, or under clothing or damaged skin, he said.
Dr. Schachner referred to a case series of 18 children who developed DEET-induced encephalopathy; 13 (72%) involved dermal exposure. Three of those with cutaneous exposure died, mostly from neurologic, respiratory, and cardiac issues. “What’s very striking is that 55% of the kids were exposed to DEET of 20% or less, even though the AAP approves DEET at 30%, so maybe that’s something we have to look at,” he said.
Medication Patches
With medication patches, especially fentanyl transdermal patches, much can go wrong when it comes to children. This was highlighted by the cases Schachner cited, including an infant who developed acute cytotoxic cerebellar edema from fentanyl patch intoxication.
In another case, emergency room staff found a fentanyl patch stuck to the back of a 3-year-old girl. A CT scan showed global cerebral edema, and the patient progressed to brain death. “This is not a unique case; there have been over 10 such cases in the United States,” said Dr. Schachner. “We should be doing better with fentanyl.”
Nicotine patches can also be dangerous to children, he added. As for other topical agents, there have been reports of toxicity and deaths linked to salicylic acid, commonly used by dermatologists because of its bacteriostatic, fungicidal, keratolytic, and photoprotective properties.
Dr. Schachner cited the case of a 2-month-old where the pediatrician prescribed 50% salicylic acid for seborrheic dermatitis of the scalp, under occlusion. “It’s amazing this child survived; that’s clearly a physician error,” he said.
Henna, a reddish-brown dye derived from the crushed leaves of Lawsonia alba, is used cosmetically for the hair, skin, and nails. Many henna products are mixed with additives, including para-phenylenediamine, which has been associated with dermatitis, asthma, renal failure, and permanent vision loss.
Asked to comment on the presentation, Sheilagh Maguiness, MD, professor of dermatology and pediatrics and chair of pediatric dermatology at the University of Minnesota, Minneapolis, recalled a particularly concerning story in 2008, when the FDA issued a warning about Mommy’s Bliss, a cream containing chlorphenesin and phenoxyethanol as preservatives, promoted to nursing mothers for soothing cracked nipples. There were reports of the cream causing respiratory distress, vomiting, and diarrhea in nursing infants.
Dr. Schachner is chair of Stiefel Laboratories and is an investigator with: Astellas, Berg Pharma, Celgene, Ferndale Labs, Lilly, Medimetriks Pharmaceuticals, Novartis, Organogenesis, Pfizer, Sciton; is a consultant for: Alphyn, Amryt Pharma, Beiersdorf, Brickell, Cutanea, Hoth, Lexington, Mustela, TopMD, Noble Pharma; a speaker for: Novartis, Sanofi-Regeneron, CeraVe; is on the advisory boards of: Almirall, Alphyn, Apogee, Aslan, Biofrontera, CeraVe, Krystal Biotech, Mustela, Noble Pharma, Pfizer, Pierre Fabre, Sanofi-Regeneron; and owns stocks in: TopMD and Alphyn. Dr. Maguiness had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM SPD 2024
August 2024 – ICYMI
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Too Much Coffee Linked to Accelerated Cognitive Decline
PHILADELPHIA – results from a large study suggest.
Investigators examined the impact of different amounts of coffee and tea on fluid intelligence — a measure of cognitive functions including abstract reasoning, pattern recognition, and logical thinking.
“It’s the old adage that too much of anything isn’t good. It’s all about balance, so moderate coffee consumption is okay but too much is probably not recommended,” said study investigator Kelsey R. Sewell, PhD, Advent Health Research Institute, Orlando, Florida.
The findings of the study were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
One of the World’s Most Widely Consumed Beverages
Coffee is one of the most widely consumed beverages around the world. The beans contain a range of bioactive compounds, including caffeine, chlorogenic acid, and small amounts of vitamins and minerals.
Consistent evidence from observational and epidemiologic studies indicates that intake of both coffee and tea has beneficial effects on stroke, heart failure, cancers, diabetes, and Parkinson’s disease.
Several studies also suggest that coffee may reduce the risk for Alzheimer’s disease, said Dr. Sewell. However, there are limited longitudinal data on associations between coffee and tea intake and cognitive decline, particularly in distinct cognitive domains.
Dr. Sewell’s group previously published a study of cognitively unimpaired older adults that found greater coffee consumption was associated with slower cognitive decline and slower accumulation of brain beta-amyloid.
Their current study extends some of the prior findings and investigates the relationship between both coffee and tea intake and cognitive decline over time in a larger sample of older adults.
This new study included 8451 mostly female (60%) and White (97%) cognitively unimpaired adults older than 60 (mean age, 67.8 years) in the UK Biobank, a large-scale research resource containing in-depth, deidentified genetic and health information from half a million UK participants. Study subjects had a mean body mass index (BMI) of 26, and about 26% were apolipoprotein epsilon 4 (APOE e4) gene carriers.
Researchers divided coffee and tea consumption into tertiles: high, moderate, and no consumption.
For daily coffee consumption, 18% reported drinking four or more cups (high consumption), 58% reported drinking one to three cups (moderate consumption), and 25% reported that they never drink coffee. For daily tea consumption, 47% reported drinking four or more cups (high consumption), 38% reported drinking one to three cups (moderate consumption), and 15% reported that they never drink tea.
The study assessed cognitive function at baseline and at least two additional patient visits.
Researchers used linear mixed models to assess the relationships between coffee and tea intake and cognitive outcomes. The models adjusted for age, sex, Townsend deprivation index (reflecting socioeconomic status), ethnicity, APOE e4 status, and BMI.
Steeper Decline
Compared with high coffee consumption (four or more cups daily), people who never consumed coffee (beta, 0.06; standard error [SE], 0.02; P = .005) and those with moderate consumption (beta, 0.07; SE, 0.02; P = < .001) had slower decline in fluid intelligence after an average of 8.83 years of follow-up.
“We can see that those with high coffee consumption showed the steepest decline in fluid intelligence across the follow up, compared to those with moderate coffee consumption and those never consuming coffee,” said Dr. Sewell, referring to illustrative graphs.
At the same time, “our data suggest that across this time period, moderate coffee consumption can serve as some kind of protective factor against cognitive decline,” she added.
For tea, there was a somewhat different pattern. People who never drank tea had a greater decline in fluid intelligence, compared with those who had moderate consumption (beta, 0.06; SE, 0.02; P = .0090) or high consumption (beta, 0.06; SE, 0.02; P = .003).
Because this is an observational study, “we still need randomized controlled trials to better understand the neuroprotective mechanism of coffee and tea compounds,” said Dr. Sewell.
Responding later to a query from a meeting delegate about how moderate coffee drinking could be protective, Dr. Sewell said there are probably “different levels of mechanisms,” including at the molecular level (possibly involving amyloid toxicity) and the behavioral level (possibly involving sleep patterns).
Dr. Sewell said that she hopes this line of investigation will lead to new avenues of research in preventive strategies for Alzheimer’s disease.
“We hope that coffee and tea intake could contribute to the development of a safe and inexpensive strategy for delaying the onset and reducing the incidence for Alzheimer’s disease.”
A limitation of the study is possible recall bias, because coffee and tea consumption were self-reported. However, this may not be much of an issue because coffee and tea consumption “is usually quite a habitual behavior,” said Dr. Sewell.
The study also had no data on midlife coffee or tea consumption and did not compare the effect of different preparation methods or types of coffee and tea — for example, green tea versus black tea.
When asked if the study controlled for smoking, Dr. Sewell said it didn’t but added that it would be interesting to explore its impact on cognition.
Dr. Sewell reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – results from a large study suggest.
Investigators examined the impact of different amounts of coffee and tea on fluid intelligence — a measure of cognitive functions including abstract reasoning, pattern recognition, and logical thinking.
“It’s the old adage that too much of anything isn’t good. It’s all about balance, so moderate coffee consumption is okay but too much is probably not recommended,” said study investigator Kelsey R. Sewell, PhD, Advent Health Research Institute, Orlando, Florida.
The findings of the study were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
One of the World’s Most Widely Consumed Beverages
Coffee is one of the most widely consumed beverages around the world. The beans contain a range of bioactive compounds, including caffeine, chlorogenic acid, and small amounts of vitamins and minerals.
Consistent evidence from observational and epidemiologic studies indicates that intake of both coffee and tea has beneficial effects on stroke, heart failure, cancers, diabetes, and Parkinson’s disease.
Several studies also suggest that coffee may reduce the risk for Alzheimer’s disease, said Dr. Sewell. However, there are limited longitudinal data on associations between coffee and tea intake and cognitive decline, particularly in distinct cognitive domains.
Dr. Sewell’s group previously published a study of cognitively unimpaired older adults that found greater coffee consumption was associated with slower cognitive decline and slower accumulation of brain beta-amyloid.
Their current study extends some of the prior findings and investigates the relationship between both coffee and tea intake and cognitive decline over time in a larger sample of older adults.
This new study included 8451 mostly female (60%) and White (97%) cognitively unimpaired adults older than 60 (mean age, 67.8 years) in the UK Biobank, a large-scale research resource containing in-depth, deidentified genetic and health information from half a million UK participants. Study subjects had a mean body mass index (BMI) of 26, and about 26% were apolipoprotein epsilon 4 (APOE e4) gene carriers.
Researchers divided coffee and tea consumption into tertiles: high, moderate, and no consumption.
For daily coffee consumption, 18% reported drinking four or more cups (high consumption), 58% reported drinking one to three cups (moderate consumption), and 25% reported that they never drink coffee. For daily tea consumption, 47% reported drinking four or more cups (high consumption), 38% reported drinking one to three cups (moderate consumption), and 15% reported that they never drink tea.
The study assessed cognitive function at baseline and at least two additional patient visits.
Researchers used linear mixed models to assess the relationships between coffee and tea intake and cognitive outcomes. The models adjusted for age, sex, Townsend deprivation index (reflecting socioeconomic status), ethnicity, APOE e4 status, and BMI.
Steeper Decline
Compared with high coffee consumption (four or more cups daily), people who never consumed coffee (beta, 0.06; standard error [SE], 0.02; P = .005) and those with moderate consumption (beta, 0.07; SE, 0.02; P = < .001) had slower decline in fluid intelligence after an average of 8.83 years of follow-up.
“We can see that those with high coffee consumption showed the steepest decline in fluid intelligence across the follow up, compared to those with moderate coffee consumption and those never consuming coffee,” said Dr. Sewell, referring to illustrative graphs.
At the same time, “our data suggest that across this time period, moderate coffee consumption can serve as some kind of protective factor against cognitive decline,” she added.
For tea, there was a somewhat different pattern. People who never drank tea had a greater decline in fluid intelligence, compared with those who had moderate consumption (beta, 0.06; SE, 0.02; P = .0090) or high consumption (beta, 0.06; SE, 0.02; P = .003).
Because this is an observational study, “we still need randomized controlled trials to better understand the neuroprotective mechanism of coffee and tea compounds,” said Dr. Sewell.
Responding later to a query from a meeting delegate about how moderate coffee drinking could be protective, Dr. Sewell said there are probably “different levels of mechanisms,” including at the molecular level (possibly involving amyloid toxicity) and the behavioral level (possibly involving sleep patterns).
Dr. Sewell said that she hopes this line of investigation will lead to new avenues of research in preventive strategies for Alzheimer’s disease.
“We hope that coffee and tea intake could contribute to the development of a safe and inexpensive strategy for delaying the onset and reducing the incidence for Alzheimer’s disease.”
A limitation of the study is possible recall bias, because coffee and tea consumption were self-reported. However, this may not be much of an issue because coffee and tea consumption “is usually quite a habitual behavior,” said Dr. Sewell.
The study also had no data on midlife coffee or tea consumption and did not compare the effect of different preparation methods or types of coffee and tea — for example, green tea versus black tea.
When asked if the study controlled for smoking, Dr. Sewell said it didn’t but added that it would be interesting to explore its impact on cognition.
Dr. Sewell reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – results from a large study suggest.
Investigators examined the impact of different amounts of coffee and tea on fluid intelligence — a measure of cognitive functions including abstract reasoning, pattern recognition, and logical thinking.
“It’s the old adage that too much of anything isn’t good. It’s all about balance, so moderate coffee consumption is okay but too much is probably not recommended,” said study investigator Kelsey R. Sewell, PhD, Advent Health Research Institute, Orlando, Florida.
The findings of the study were presented at the 2024 Alzheimer’s Association International Conference (AAIC).
One of the World’s Most Widely Consumed Beverages
Coffee is one of the most widely consumed beverages around the world. The beans contain a range of bioactive compounds, including caffeine, chlorogenic acid, and small amounts of vitamins and minerals.
Consistent evidence from observational and epidemiologic studies indicates that intake of both coffee and tea has beneficial effects on stroke, heart failure, cancers, diabetes, and Parkinson’s disease.
Several studies also suggest that coffee may reduce the risk for Alzheimer’s disease, said Dr. Sewell. However, there are limited longitudinal data on associations between coffee and tea intake and cognitive decline, particularly in distinct cognitive domains.
Dr. Sewell’s group previously published a study of cognitively unimpaired older adults that found greater coffee consumption was associated with slower cognitive decline and slower accumulation of brain beta-amyloid.
Their current study extends some of the prior findings and investigates the relationship between both coffee and tea intake and cognitive decline over time in a larger sample of older adults.
This new study included 8451 mostly female (60%) and White (97%) cognitively unimpaired adults older than 60 (mean age, 67.8 years) in the UK Biobank, a large-scale research resource containing in-depth, deidentified genetic and health information from half a million UK participants. Study subjects had a mean body mass index (BMI) of 26, and about 26% were apolipoprotein epsilon 4 (APOE e4) gene carriers.
Researchers divided coffee and tea consumption into tertiles: high, moderate, and no consumption.
For daily coffee consumption, 18% reported drinking four or more cups (high consumption), 58% reported drinking one to three cups (moderate consumption), and 25% reported that they never drink coffee. For daily tea consumption, 47% reported drinking four or more cups (high consumption), 38% reported drinking one to three cups (moderate consumption), and 15% reported that they never drink tea.
The study assessed cognitive function at baseline and at least two additional patient visits.
Researchers used linear mixed models to assess the relationships between coffee and tea intake and cognitive outcomes. The models adjusted for age, sex, Townsend deprivation index (reflecting socioeconomic status), ethnicity, APOE e4 status, and BMI.
Steeper Decline
Compared with high coffee consumption (four or more cups daily), people who never consumed coffee (beta, 0.06; standard error [SE], 0.02; P = .005) and those with moderate consumption (beta, 0.07; SE, 0.02; P = < .001) had slower decline in fluid intelligence after an average of 8.83 years of follow-up.
“We can see that those with high coffee consumption showed the steepest decline in fluid intelligence across the follow up, compared to those with moderate coffee consumption and those never consuming coffee,” said Dr. Sewell, referring to illustrative graphs.
At the same time, “our data suggest that across this time period, moderate coffee consumption can serve as some kind of protective factor against cognitive decline,” she added.
For tea, there was a somewhat different pattern. People who never drank tea had a greater decline in fluid intelligence, compared with those who had moderate consumption (beta, 0.06; SE, 0.02; P = .0090) or high consumption (beta, 0.06; SE, 0.02; P = .003).
Because this is an observational study, “we still need randomized controlled trials to better understand the neuroprotective mechanism of coffee and tea compounds,” said Dr. Sewell.
Responding later to a query from a meeting delegate about how moderate coffee drinking could be protective, Dr. Sewell said there are probably “different levels of mechanisms,” including at the molecular level (possibly involving amyloid toxicity) and the behavioral level (possibly involving sleep patterns).
Dr. Sewell said that she hopes this line of investigation will lead to new avenues of research in preventive strategies for Alzheimer’s disease.
“We hope that coffee and tea intake could contribute to the development of a safe and inexpensive strategy for delaying the onset and reducing the incidence for Alzheimer’s disease.”
A limitation of the study is possible recall bias, because coffee and tea consumption were self-reported. However, this may not be much of an issue because coffee and tea consumption “is usually quite a habitual behavior,” said Dr. Sewell.
The study also had no data on midlife coffee or tea consumption and did not compare the effect of different preparation methods or types of coffee and tea — for example, green tea versus black tea.
When asked if the study controlled for smoking, Dr. Sewell said it didn’t but added that it would be interesting to explore its impact on cognition.
Dr. Sewell reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM AAIC 2024
Early Knee Osteoarthritis: Exercise Therapy’s Golden Window
TOPLINE:
People with knee osteoarthritis and symptoms for less than 1 year benefit more from exercise therapy than do those with longer symptom duration, especially when long-term outcomes are considered.
METHODOLOGY:
- Researchers conducted an individual participant data meta-analysis using data from the OA Trial Bank, including 1769 participants (mean age, 65.1 years; 66% women) with knee osteoarthritis from 10 randomized controlled trials.
- The participants were categorized on the basis of their symptom duration: ≤ 1 year, > 1 and ≤ 2 years, and > 2 years.
- This study included an exercise therapy group comprising land- and water-based therapeutic exercise interventions and a control group comprising no exercise or sham treatment.
- The primary outcomes were self-reported pain and physical function, standardized to a 0-100 scale, at short-term (closest to 3 months) and long-term (closest to 12 months) follow-ups.
TAKEAWAY:
- The overall pain and physical function associated with osteoarthritis improved in the exercise therapy group at both short- and long-term follow-ups compared with in the control group.
- Exercise therapy led to a greater improvement in short-term (mean difference [MD], −3.57; P = .028) and long-term (MD, −8.33; P < .001) pain among participants with a symptom duration ≤ 1 year vs > 1 year.
- Similarly, those with a symptom duration ≤ 2 years vs > 2 years who underwent exercise therapy showed greater benefits in terms of short-term (P = .001) and long-term (P < .001) pain.
- Exercise therapy improved long-term physical function in those with a symptom duration ≤ 1 year vs > 1 year (MD, −5.46; P = .005) and ≤ 2 years vs > 2 years (MD, −4.56; P = .001).
IN PRACTICE:
“Exercise should be encouraged as early as possible once symptoms emerge in the disease process to take advantage of its effects in potentially [slowing] disease progression within the suggested ‘window of opportunity,’ ” the authors wrote.
SOURCE:
The study was led by Marienke van Middelkoop, PhD, Erasmus MC Medical University, Rotterdam, the Netherlands. It was published online in Osteoarthritis and Cartilage.
LIMITATIONS:
The dataset of most studies included in the meta-analysis lacked information on the radiographic severity of osteoarthritis. The relatively short follow-up time hindered interpreting the impact of exercise on the long-term progression of osteoarthritis. The reliance on patient recall for recording symptom duration may have led to misclassification.
DISCLOSURES:
The Netherlands Organisation for Health Research and Development supported this study. Three authors received funding from the Dutch Arthritis Society for the program grant Center of Excellence “OA prevention and early treatment – OA Pearl.” One author declared receiving royalties for the UpToDate knee osteoarthritis clinical guidelines.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
People with knee osteoarthritis and symptoms for less than 1 year benefit more from exercise therapy than do those with longer symptom duration, especially when long-term outcomes are considered.
METHODOLOGY:
- Researchers conducted an individual participant data meta-analysis using data from the OA Trial Bank, including 1769 participants (mean age, 65.1 years; 66% women) with knee osteoarthritis from 10 randomized controlled trials.
- The participants were categorized on the basis of their symptom duration: ≤ 1 year, > 1 and ≤ 2 years, and > 2 years.
- This study included an exercise therapy group comprising land- and water-based therapeutic exercise interventions and a control group comprising no exercise or sham treatment.
- The primary outcomes were self-reported pain and physical function, standardized to a 0-100 scale, at short-term (closest to 3 months) and long-term (closest to 12 months) follow-ups.
TAKEAWAY:
- The overall pain and physical function associated with osteoarthritis improved in the exercise therapy group at both short- and long-term follow-ups compared with in the control group.
- Exercise therapy led to a greater improvement in short-term (mean difference [MD], −3.57; P = .028) and long-term (MD, −8.33; P < .001) pain among participants with a symptom duration ≤ 1 year vs > 1 year.
- Similarly, those with a symptom duration ≤ 2 years vs > 2 years who underwent exercise therapy showed greater benefits in terms of short-term (P = .001) and long-term (P < .001) pain.
- Exercise therapy improved long-term physical function in those with a symptom duration ≤ 1 year vs > 1 year (MD, −5.46; P = .005) and ≤ 2 years vs > 2 years (MD, −4.56; P = .001).
IN PRACTICE:
“Exercise should be encouraged as early as possible once symptoms emerge in the disease process to take advantage of its effects in potentially [slowing] disease progression within the suggested ‘window of opportunity,’ ” the authors wrote.
SOURCE:
The study was led by Marienke van Middelkoop, PhD, Erasmus MC Medical University, Rotterdam, the Netherlands. It was published online in Osteoarthritis and Cartilage.
LIMITATIONS:
The dataset of most studies included in the meta-analysis lacked information on the radiographic severity of osteoarthritis. The relatively short follow-up time hindered interpreting the impact of exercise on the long-term progression of osteoarthritis. The reliance on patient recall for recording symptom duration may have led to misclassification.
DISCLOSURES:
The Netherlands Organisation for Health Research and Development supported this study. Three authors received funding from the Dutch Arthritis Society for the program grant Center of Excellence “OA prevention and early treatment – OA Pearl.” One author declared receiving royalties for the UpToDate knee osteoarthritis clinical guidelines.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
People with knee osteoarthritis and symptoms for less than 1 year benefit more from exercise therapy than do those with longer symptom duration, especially when long-term outcomes are considered.
METHODOLOGY:
- Researchers conducted an individual participant data meta-analysis using data from the OA Trial Bank, including 1769 participants (mean age, 65.1 years; 66% women) with knee osteoarthritis from 10 randomized controlled trials.
- The participants were categorized on the basis of their symptom duration: ≤ 1 year, > 1 and ≤ 2 years, and > 2 years.
- This study included an exercise therapy group comprising land- and water-based therapeutic exercise interventions and a control group comprising no exercise or sham treatment.
- The primary outcomes were self-reported pain and physical function, standardized to a 0-100 scale, at short-term (closest to 3 months) and long-term (closest to 12 months) follow-ups.
TAKEAWAY:
- The overall pain and physical function associated with osteoarthritis improved in the exercise therapy group at both short- and long-term follow-ups compared with in the control group.
- Exercise therapy led to a greater improvement in short-term (mean difference [MD], −3.57; P = .028) and long-term (MD, −8.33; P < .001) pain among participants with a symptom duration ≤ 1 year vs > 1 year.
- Similarly, those with a symptom duration ≤ 2 years vs > 2 years who underwent exercise therapy showed greater benefits in terms of short-term (P = .001) and long-term (P < .001) pain.
- Exercise therapy improved long-term physical function in those with a symptom duration ≤ 1 year vs > 1 year (MD, −5.46; P = .005) and ≤ 2 years vs > 2 years (MD, −4.56; P = .001).
IN PRACTICE:
“Exercise should be encouraged as early as possible once symptoms emerge in the disease process to take advantage of its effects in potentially [slowing] disease progression within the suggested ‘window of opportunity,’ ” the authors wrote.
SOURCE:
The study was led by Marienke van Middelkoop, PhD, Erasmus MC Medical University, Rotterdam, the Netherlands. It was published online in Osteoarthritis and Cartilage.
LIMITATIONS:
The dataset of most studies included in the meta-analysis lacked information on the radiographic severity of osteoarthritis. The relatively short follow-up time hindered interpreting the impact of exercise on the long-term progression of osteoarthritis. The reliance on patient recall for recording symptom duration may have led to misclassification.
DISCLOSURES:
The Netherlands Organisation for Health Research and Development supported this study. Three authors received funding from the Dutch Arthritis Society for the program grant Center of Excellence “OA prevention and early treatment – OA Pearl.” One author declared receiving royalties for the UpToDate knee osteoarthritis clinical guidelines.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Expands Darzalex Faspro Indication in Myeloma
Approval followed priority review and was based on efficacy and safety findings from the open-label PERSEUS trial involving 709 patients under age 70 years who were randomized to receive bortezomib, lenalidomide, and dexamethasone alone or in combination with daratumumab and hyaluronidase-fihj, according to the FDA.
Compared with bortezomib, lenalidomide, and dexamethasone alone, the addition of daratumumab and hyaluronidase-fihj resulted in a 60% reduction in the risk for disease progression or death (hazard ratio, 0.40). Median progression-free survival was not reached in either group.
Adverse reactions occurring in ≥ 20% of patients were peripheral neuropathy, fatigue, edema, pyrexia, upper respiratory infection, constipation, diarrhea, musculoskeletal pain, insomnia, and rash.
The recommended dosage for this indication is 1800 mg daratumumab and 30,000 units hyaluronidase, according to the full prescribing information.
Daratumumab and hyaluronidase-fihj, which was first approved in 2020, has a range of other indications in multiple myeloma.
A version of this article appeared on Medscape.com.
Approval followed priority review and was based on efficacy and safety findings from the open-label PERSEUS trial involving 709 patients under age 70 years who were randomized to receive bortezomib, lenalidomide, and dexamethasone alone or in combination with daratumumab and hyaluronidase-fihj, according to the FDA.
Compared with bortezomib, lenalidomide, and dexamethasone alone, the addition of daratumumab and hyaluronidase-fihj resulted in a 60% reduction in the risk for disease progression or death (hazard ratio, 0.40). Median progression-free survival was not reached in either group.
Adverse reactions occurring in ≥ 20% of patients were peripheral neuropathy, fatigue, edema, pyrexia, upper respiratory infection, constipation, diarrhea, musculoskeletal pain, insomnia, and rash.
The recommended dosage for this indication is 1800 mg daratumumab and 30,000 units hyaluronidase, according to the full prescribing information.
Daratumumab and hyaluronidase-fihj, which was first approved in 2020, has a range of other indications in multiple myeloma.
A version of this article appeared on Medscape.com.
Approval followed priority review and was based on efficacy and safety findings from the open-label PERSEUS trial involving 709 patients under age 70 years who were randomized to receive bortezomib, lenalidomide, and dexamethasone alone or in combination with daratumumab and hyaluronidase-fihj, according to the FDA.
Compared with bortezomib, lenalidomide, and dexamethasone alone, the addition of daratumumab and hyaluronidase-fihj resulted in a 60% reduction in the risk for disease progression or death (hazard ratio, 0.40). Median progression-free survival was not reached in either group.
Adverse reactions occurring in ≥ 20% of patients were peripheral neuropathy, fatigue, edema, pyrexia, upper respiratory infection, constipation, diarrhea, musculoskeletal pain, insomnia, and rash.
The recommended dosage for this indication is 1800 mg daratumumab and 30,000 units hyaluronidase, according to the full prescribing information.
Daratumumab and hyaluronidase-fihj, which was first approved in 2020, has a range of other indications in multiple myeloma.
A version of this article appeared on Medscape.com.
Insurers’ Rules and AI for Preauthorization: ‘Ethically Nuts,’ Says Ethicist
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the Division of Medical Ethics at New York University Grossman School of Medicine in New York City.
There are many things screwy with our healthcare system. Many of you [reading] this are dealing with bureaucracy, paperwork, all sorts of constraints, restraints, and requirements that sometimes make the practice of medicine, or even nursing, difficult.
I don’t think I’ve seen anything screwier, from a moral point of view, than the system we have that allows for preauthorization by third-party payers, or insurers, in order to give care to patients. It’s pretty clear that a third-party payer has a conflict of interest. It’s simple: They don’t want to spend money.
Their goal as profit-making companies is to reduce what it is that they’re going to authorize. That clearly is driving how the preauthorization process works. or somebody saying, this is the standard of care and this is what ought to happen.
We’re letting the people who have the pocketbooks and the wallets have prior approval of what the doctor thinks is correct. That is really not the way to practice medicine.
We now have more evidence about what really is going on. A doctor was recently interviewed by ProPublica and said that she had worked for Cigna as a reviewer. Basically, the message she got from that insurer was to speed it up, go fast, and basically “deny, deny, deny” when she got requests. Those are her words, not mine.
We get a peek under the tent of how this works, and Dr. Day is basically saying she had to leave because she just didn’t feel that it was evidence-driven. It was driven by concerns about who’s going to lose money or make money.
If you want to check to see whether something is appropriate, the question becomes, who ought to do prior review?
Who does it now? Sometimes doctors. Sometimes nurses who aren’t in the specialty where the request is coming in for preapproval. I’ve even seen situations where some companies use nurses in other countries, such as the Philippines, to do preapproval. They send them information, like a clip, to use to deny things that basically is boilerplate language, whatever the request is.
Looming up now, some insurers are starting to think, well, maybe artificial intelligence could do it. Just review the written request, trigger certain responses on the part of the artificial intelligence — it can deny the claims just as well as a human — and maybe it’s even cheaper to set up that system for the insurer.
This is ethically nuts. We need to have a system where doctors’ judgments drive what patients get. You listen to doctors, as I do, about preapproval access and they say patients sometimes give up trying to get what they think is needed. Continuity of care is interrupted if they have to keep making requests all the time.
There are adverse events when the thing that the doctor thought was most appropriate isn’t approved and something else is used that is less safe or less efficacious. It isn’t in patient interest to have the person with the wallet saying, this is what we think you need, and then having unqualified people or even automated intelligence with no accountability and no transparency get involved in preauthorization.
This system costs us money because middlemen are doing all this work. It basically becomes one of the huge scandals, in my view, of our health system, that doctors don’t ultimately decide what the patient needs. A preauthorizing third party or robot, without transparency, without accountability, and behind closed doors second-guesses what’s going on.
I’m Art Caplan at the Division of Medical Ethics at the New York University Grossman School of Medicine.
Arthur L. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York, New York, has disclosed the following relevant financial relationships: Served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position). Serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
Lipedema: Current Diagnostic and Treatment Evidence
Lipedema affects about 11% of cisgender women, according to the Brazilian Society of Angiology and Vascular Surgery. Yet the condition remains wrapped in uncertainties. Despite significant advancements in understanding its physiology, diagnosis, and treatment, more clarity is needed as awareness and diagnoses increase.
At the latest International Congress on Obesity (ICO) in São Paulo, Brazil, Philipp Scherer, PhD, director of the Touchstone Diabetes Center, discussed the complexities of lipedema. “It is an extremely frustrating condition for someone like me, who has spent a lifetime studying functional and dysfunctional adipose tissue. We are trying to understand the physiology of this pathology, but it is challenging, and so far, we have not been able to find a concrete answer,” he noted.
Lipedema is characterized by the abnormal accumulation of subcutaneous adipose tissue, especially in the lower limbs, and almost exclusively affects cisgender women. The reason for this gender disparity is unclear. It could be an intrinsic characteristic of the disease or a result from clinicians’ lack of familiarity with lipedema, which often leads to misdiagnosis as obesity. This misdiagnosis results in fewer men seeking treatment.
Research has predominantly focused on women, and evidence suggests that hormones play a crucial role in the disease’s pathophysiology. Lipedema typically manifests during periods of hormonal changes, such as puberty, pregnancy, menopause, and hormone replacement therapies, reinforcing the idea that hormones significantly influence the condition’s development and progression.
Main Symptoms
Jonathan Kartt, CEO of the Lipedema Foundation, emphasized that intense pain in the areas of adipose tissue accumulation is a hallmark symptom of lipedema, setting it apart from obesity. Pain levels can vary widely among patients, ranging from moderate to severe, with unbearable peaks on certain days. Mr. Kartt stressed the importance of recognizing and addressing this often underestimated symptom.
Lipedema is characterized by a bilateral, symmetrical increase in mass compared with the rest of the body. This is commonly distinguished by the “cuff sign,” a separation between normal tissue in the feet and abnormal tissue from the ankle upward. Other frequent symptoms include a feeling of heaviness, discomfort, fatigue, frequent bruising, and tiredness. A notable sign is the presence of subcutaneous nodules with a texture similar to that of rice grains, which are crucial for differentiating lipedema from other conditions. Palpation during anamnesis is essential to identify these nodules and confirm the diagnosis.
“It is crucial to investigate the family history for genetic predisposition. Additionally, it is fundamental to ask whether, even with weight loss, the affected areas retain accumulated fat. Hormonal changes, pain symptoms, and impact on quality of life should also be carefully evaluated,” advised Mr. Kartt.
Diagnostic Tools
André Murad, MD, a clinical consultant at the Instituto Lipedema Brazil, has been exploring new diagnostic approaches for lipedema beyond traditional anamnesis. During his presentation at the ICO, he shared studies on the efficacy of imaging exams such as ultrasound, tomography, and MRI in diagnosing the characteristic lipedema-associated increase in subcutaneous tissue.
He also discussed lymphangiography and lymphoscintigraphy, highlighting the use of magnetic resonance lymphangiography to evaluate dilated lymphatic vessels often observed in patients with lipedema. “By injecting contrast into the feet, this technique allows the evaluation of vessels, which are usually dilated, indicating characteristic lymphatic system overload in lipedema. Lymphoscintigraphy is crucial for detecting associated lymphedema, revealing delayed lymphatic flow and asymmetry between limbs in cases of lipedema without lymphedema,” he explained.
Despite the various diagnostic options, Dr. Murad highlighted two highly effective studies. A Brazilian study used ultrasound to establish a cutoff point of 11.7 mm in the pretibial subcutaneous tissue thickness, achieving 96% specificity for diagnosis. Another study emphasized the value of dual-energy x-ray absorptiometry (DXA), which demonstrated 95% sensitivity. This method assesses fat distribution by correlating the amount present in the legs with the total body, providing a cost-effective and accessible option for specialists.
“DXA allows for a precise mathematical evaluation of fat distribution relative to the total body. A ratio of 0.38 in the leg-to-body relationship is a significant indicator of high suspicion of lipedema,” highlighted Dr. Murad. “In clinical practice, many patients self-diagnose with lipedema, but the clinical exam often reveals no disproportion, with the leg-to-body ratio below 0.38 being common in these cases,” he added.
Treatment Approaches
Treatments for lipedema are still evolving, with considerable debate about the best approach. While some specialists advocate exclusively for conservative treatment, others recommend combining these methods with surgical interventions, depending on the stage of the disease. The relative novelty of lipedema and the scarcity of robust, long-term studies contribute to the uncertainty around treatment efficacy.
Conservative treatment typically includes compression, lymphatic drainage techniques, and pressure therapy. An active lifestyle and a healthy diet are also recommended. Although these measures do not prevent the accumulation of adipose tissue, they help reduce inflammation and improve quality of life. “Even though the causes of lipedema are not fully known, lifestyle management is essential for controlling symptoms, starting with an anti-inflammatory diet,” emphasized Dr. Murad.
Because insulin promotes lipogenesis, a diet that avoids spikes in glycemic and insulin levels is advisable. Insulin resistance can exacerbate edema formation, so a Mediterranean diet may be beneficial. This diet limits fast-absorbing carbohydrates, such as added sugar, refined grains, and ultraprocessed foods, while promoting complex carbohydrates from whole grains and legumes.
Dr. Murad also presented a study evaluating the potential benefits of a low-carbohydrate, high-fat diet for patients with lipedema. The study demonstrated weight loss, reduced body fat, controlled leg volume, and, notably, pain relief.
For more advanced stages of lipedema, plastic surgery is often considered when conservative approaches do not yield satisfactory results. Some specialists advocate for surgery as an effective way to remove diseased adipose cells and reduce excess fat accumulation, which can improve physical appearance and associated pain. There is a growing consensus that surgical intervention should be performed early, ideally in stage I of IV, to maximize efficacy and prevent disease progression.
Fábio Masato Kamamoto, MD, a plastic surgeon and director of the Instituto Lipedema Brazil, shared insights into surgical treatments for lipedema. He discussed techniques from liposuction to advanced skin retraction and dermolipectomy, crucial for addressing more advanced stages of the condition. “It’s a complex process that demands precision to protect the lymphatic system, especially considering the characteristic nodules of lipedema,” he noted.
Dr. Kamamoto discussed a former patient with stage III lipedema. In the initial stage, he performed liposuction, removing 8 L of fat and 3.4 kg of skin. After 6 months, a follow-up procedure resulted in a total removal of 15 kg. Complementary procedures, such as microneedling, were performed to stimulate collagen production and reduce skin sagging. In addition to cosmetic improvements, the procedure also removed the distinctive lipedema nodules, which Mr. Kartt described as feeling like “rice grains.” Removing these nodules significantly alleviates pain, according to Dr. Kamamoto.
The benefits of surgical treatment for lipedema can be long lasting. Dr. Kamamoto noted that fat tends not to reaccumulate in treated areas, with patients often experiencing lower weight, reduced edema, and decreased pain over time. “While we hope that patients do not regain weight, the benefits of surgery persist even if weight is regained. Therefore, combining conservative and surgical treatments remains a valid and effective approach,” he concluded.
Dr. Scherer highlighted that despite various approaches, there is still no definitive “magic signature” that fully explains lipedema. This lack of clarity directly affects the effectiveness of diagnoses and treatments. He expressed hope that future integration of data from different studies and approaches will lead to the identification of a clinically useful molecular signature. “The true cause of lipedema remains unknown, requiring more speculation, hypothesis formulation, and testing for significant discoveries. This situation is frustrating, as the disease affects many women who lack a clear diagnosis that differentiates them from patients with obesity, as well as evidence-based recommendations,” he concluded.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Lipedema affects about 11% of cisgender women, according to the Brazilian Society of Angiology and Vascular Surgery. Yet the condition remains wrapped in uncertainties. Despite significant advancements in understanding its physiology, diagnosis, and treatment, more clarity is needed as awareness and diagnoses increase.
At the latest International Congress on Obesity (ICO) in São Paulo, Brazil, Philipp Scherer, PhD, director of the Touchstone Diabetes Center, discussed the complexities of lipedema. “It is an extremely frustrating condition for someone like me, who has spent a lifetime studying functional and dysfunctional adipose tissue. We are trying to understand the physiology of this pathology, but it is challenging, and so far, we have not been able to find a concrete answer,” he noted.
Lipedema is characterized by the abnormal accumulation of subcutaneous adipose tissue, especially in the lower limbs, and almost exclusively affects cisgender women. The reason for this gender disparity is unclear. It could be an intrinsic characteristic of the disease or a result from clinicians’ lack of familiarity with lipedema, which often leads to misdiagnosis as obesity. This misdiagnosis results in fewer men seeking treatment.
Research has predominantly focused on women, and evidence suggests that hormones play a crucial role in the disease’s pathophysiology. Lipedema typically manifests during periods of hormonal changes, such as puberty, pregnancy, menopause, and hormone replacement therapies, reinforcing the idea that hormones significantly influence the condition’s development and progression.
Main Symptoms
Jonathan Kartt, CEO of the Lipedema Foundation, emphasized that intense pain in the areas of adipose tissue accumulation is a hallmark symptom of lipedema, setting it apart from obesity. Pain levels can vary widely among patients, ranging from moderate to severe, with unbearable peaks on certain days. Mr. Kartt stressed the importance of recognizing and addressing this often underestimated symptom.
Lipedema is characterized by a bilateral, symmetrical increase in mass compared with the rest of the body. This is commonly distinguished by the “cuff sign,” a separation between normal tissue in the feet and abnormal tissue from the ankle upward. Other frequent symptoms include a feeling of heaviness, discomfort, fatigue, frequent bruising, and tiredness. A notable sign is the presence of subcutaneous nodules with a texture similar to that of rice grains, which are crucial for differentiating lipedema from other conditions. Palpation during anamnesis is essential to identify these nodules and confirm the diagnosis.
“It is crucial to investigate the family history for genetic predisposition. Additionally, it is fundamental to ask whether, even with weight loss, the affected areas retain accumulated fat. Hormonal changes, pain symptoms, and impact on quality of life should also be carefully evaluated,” advised Mr. Kartt.
Diagnostic Tools
André Murad, MD, a clinical consultant at the Instituto Lipedema Brazil, has been exploring new diagnostic approaches for lipedema beyond traditional anamnesis. During his presentation at the ICO, he shared studies on the efficacy of imaging exams such as ultrasound, tomography, and MRI in diagnosing the characteristic lipedema-associated increase in subcutaneous tissue.
He also discussed lymphangiography and lymphoscintigraphy, highlighting the use of magnetic resonance lymphangiography to evaluate dilated lymphatic vessels often observed in patients with lipedema. “By injecting contrast into the feet, this technique allows the evaluation of vessels, which are usually dilated, indicating characteristic lymphatic system overload in lipedema. Lymphoscintigraphy is crucial for detecting associated lymphedema, revealing delayed lymphatic flow and asymmetry between limbs in cases of lipedema without lymphedema,” he explained.
Despite the various diagnostic options, Dr. Murad highlighted two highly effective studies. A Brazilian study used ultrasound to establish a cutoff point of 11.7 mm in the pretibial subcutaneous tissue thickness, achieving 96% specificity for diagnosis. Another study emphasized the value of dual-energy x-ray absorptiometry (DXA), which demonstrated 95% sensitivity. This method assesses fat distribution by correlating the amount present in the legs with the total body, providing a cost-effective and accessible option for specialists.
“DXA allows for a precise mathematical evaluation of fat distribution relative to the total body. A ratio of 0.38 in the leg-to-body relationship is a significant indicator of high suspicion of lipedema,” highlighted Dr. Murad. “In clinical practice, many patients self-diagnose with lipedema, but the clinical exam often reveals no disproportion, with the leg-to-body ratio below 0.38 being common in these cases,” he added.
Treatment Approaches
Treatments for lipedema are still evolving, with considerable debate about the best approach. While some specialists advocate exclusively for conservative treatment, others recommend combining these methods with surgical interventions, depending on the stage of the disease. The relative novelty of lipedema and the scarcity of robust, long-term studies contribute to the uncertainty around treatment efficacy.
Conservative treatment typically includes compression, lymphatic drainage techniques, and pressure therapy. An active lifestyle and a healthy diet are also recommended. Although these measures do not prevent the accumulation of adipose tissue, they help reduce inflammation and improve quality of life. “Even though the causes of lipedema are not fully known, lifestyle management is essential for controlling symptoms, starting with an anti-inflammatory diet,” emphasized Dr. Murad.
Because insulin promotes lipogenesis, a diet that avoids spikes in glycemic and insulin levels is advisable. Insulin resistance can exacerbate edema formation, so a Mediterranean diet may be beneficial. This diet limits fast-absorbing carbohydrates, such as added sugar, refined grains, and ultraprocessed foods, while promoting complex carbohydrates from whole grains and legumes.
Dr. Murad also presented a study evaluating the potential benefits of a low-carbohydrate, high-fat diet for patients with lipedema. The study demonstrated weight loss, reduced body fat, controlled leg volume, and, notably, pain relief.
For more advanced stages of lipedema, plastic surgery is often considered when conservative approaches do not yield satisfactory results. Some specialists advocate for surgery as an effective way to remove diseased adipose cells and reduce excess fat accumulation, which can improve physical appearance and associated pain. There is a growing consensus that surgical intervention should be performed early, ideally in stage I of IV, to maximize efficacy and prevent disease progression.
Fábio Masato Kamamoto, MD, a plastic surgeon and director of the Instituto Lipedema Brazil, shared insights into surgical treatments for lipedema. He discussed techniques from liposuction to advanced skin retraction and dermolipectomy, crucial for addressing more advanced stages of the condition. “It’s a complex process that demands precision to protect the lymphatic system, especially considering the characteristic nodules of lipedema,” he noted.
Dr. Kamamoto discussed a former patient with stage III lipedema. In the initial stage, he performed liposuction, removing 8 L of fat and 3.4 kg of skin. After 6 months, a follow-up procedure resulted in a total removal of 15 kg. Complementary procedures, such as microneedling, were performed to stimulate collagen production and reduce skin sagging. In addition to cosmetic improvements, the procedure also removed the distinctive lipedema nodules, which Mr. Kartt described as feeling like “rice grains.” Removing these nodules significantly alleviates pain, according to Dr. Kamamoto.
The benefits of surgical treatment for lipedema can be long lasting. Dr. Kamamoto noted that fat tends not to reaccumulate in treated areas, with patients often experiencing lower weight, reduced edema, and decreased pain over time. “While we hope that patients do not regain weight, the benefits of surgery persist even if weight is regained. Therefore, combining conservative and surgical treatments remains a valid and effective approach,” he concluded.
Dr. Scherer highlighted that despite various approaches, there is still no definitive “magic signature” that fully explains lipedema. This lack of clarity directly affects the effectiveness of diagnoses and treatments. He expressed hope that future integration of data from different studies and approaches will lead to the identification of a clinically useful molecular signature. “The true cause of lipedema remains unknown, requiring more speculation, hypothesis formulation, and testing for significant discoveries. This situation is frustrating, as the disease affects many women who lack a clear diagnosis that differentiates them from patients with obesity, as well as evidence-based recommendations,” he concluded.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Lipedema affects about 11% of cisgender women, according to the Brazilian Society of Angiology and Vascular Surgery. Yet the condition remains wrapped in uncertainties. Despite significant advancements in understanding its physiology, diagnosis, and treatment, more clarity is needed as awareness and diagnoses increase.
At the latest International Congress on Obesity (ICO) in São Paulo, Brazil, Philipp Scherer, PhD, director of the Touchstone Diabetes Center, discussed the complexities of lipedema. “It is an extremely frustrating condition for someone like me, who has spent a lifetime studying functional and dysfunctional adipose tissue. We are trying to understand the physiology of this pathology, but it is challenging, and so far, we have not been able to find a concrete answer,” he noted.
Lipedema is characterized by the abnormal accumulation of subcutaneous adipose tissue, especially in the lower limbs, and almost exclusively affects cisgender women. The reason for this gender disparity is unclear. It could be an intrinsic characteristic of the disease or a result from clinicians’ lack of familiarity with lipedema, which often leads to misdiagnosis as obesity. This misdiagnosis results in fewer men seeking treatment.
Research has predominantly focused on women, and evidence suggests that hormones play a crucial role in the disease’s pathophysiology. Lipedema typically manifests during periods of hormonal changes, such as puberty, pregnancy, menopause, and hormone replacement therapies, reinforcing the idea that hormones significantly influence the condition’s development and progression.
Main Symptoms
Jonathan Kartt, CEO of the Lipedema Foundation, emphasized that intense pain in the areas of adipose tissue accumulation is a hallmark symptom of lipedema, setting it apart from obesity. Pain levels can vary widely among patients, ranging from moderate to severe, with unbearable peaks on certain days. Mr. Kartt stressed the importance of recognizing and addressing this often underestimated symptom.
Lipedema is characterized by a bilateral, symmetrical increase in mass compared with the rest of the body. This is commonly distinguished by the “cuff sign,” a separation between normal tissue in the feet and abnormal tissue from the ankle upward. Other frequent symptoms include a feeling of heaviness, discomfort, fatigue, frequent bruising, and tiredness. A notable sign is the presence of subcutaneous nodules with a texture similar to that of rice grains, which are crucial for differentiating lipedema from other conditions. Palpation during anamnesis is essential to identify these nodules and confirm the diagnosis.
“It is crucial to investigate the family history for genetic predisposition. Additionally, it is fundamental to ask whether, even with weight loss, the affected areas retain accumulated fat. Hormonal changes, pain symptoms, and impact on quality of life should also be carefully evaluated,” advised Mr. Kartt.
Diagnostic Tools
André Murad, MD, a clinical consultant at the Instituto Lipedema Brazil, has been exploring new diagnostic approaches for lipedema beyond traditional anamnesis. During his presentation at the ICO, he shared studies on the efficacy of imaging exams such as ultrasound, tomography, and MRI in diagnosing the characteristic lipedema-associated increase in subcutaneous tissue.
He also discussed lymphangiography and lymphoscintigraphy, highlighting the use of magnetic resonance lymphangiography to evaluate dilated lymphatic vessels often observed in patients with lipedema. “By injecting contrast into the feet, this technique allows the evaluation of vessels, which are usually dilated, indicating characteristic lymphatic system overload in lipedema. Lymphoscintigraphy is crucial for detecting associated lymphedema, revealing delayed lymphatic flow and asymmetry between limbs in cases of lipedema without lymphedema,” he explained.
Despite the various diagnostic options, Dr. Murad highlighted two highly effective studies. A Brazilian study used ultrasound to establish a cutoff point of 11.7 mm in the pretibial subcutaneous tissue thickness, achieving 96% specificity for diagnosis. Another study emphasized the value of dual-energy x-ray absorptiometry (DXA), which demonstrated 95% sensitivity. This method assesses fat distribution by correlating the amount present in the legs with the total body, providing a cost-effective and accessible option for specialists.
“DXA allows for a precise mathematical evaluation of fat distribution relative to the total body. A ratio of 0.38 in the leg-to-body relationship is a significant indicator of high suspicion of lipedema,” highlighted Dr. Murad. “In clinical practice, many patients self-diagnose with lipedema, but the clinical exam often reveals no disproportion, with the leg-to-body ratio below 0.38 being common in these cases,” he added.
Treatment Approaches
Treatments for lipedema are still evolving, with considerable debate about the best approach. While some specialists advocate exclusively for conservative treatment, others recommend combining these methods with surgical interventions, depending on the stage of the disease. The relative novelty of lipedema and the scarcity of robust, long-term studies contribute to the uncertainty around treatment efficacy.
Conservative treatment typically includes compression, lymphatic drainage techniques, and pressure therapy. An active lifestyle and a healthy diet are also recommended. Although these measures do not prevent the accumulation of adipose tissue, they help reduce inflammation and improve quality of life. “Even though the causes of lipedema are not fully known, lifestyle management is essential for controlling symptoms, starting with an anti-inflammatory diet,” emphasized Dr. Murad.
Because insulin promotes lipogenesis, a diet that avoids spikes in glycemic and insulin levels is advisable. Insulin resistance can exacerbate edema formation, so a Mediterranean diet may be beneficial. This diet limits fast-absorbing carbohydrates, such as added sugar, refined grains, and ultraprocessed foods, while promoting complex carbohydrates from whole grains and legumes.
Dr. Murad also presented a study evaluating the potential benefits of a low-carbohydrate, high-fat diet for patients with lipedema. The study demonstrated weight loss, reduced body fat, controlled leg volume, and, notably, pain relief.
For more advanced stages of lipedema, plastic surgery is often considered when conservative approaches do not yield satisfactory results. Some specialists advocate for surgery as an effective way to remove diseased adipose cells and reduce excess fat accumulation, which can improve physical appearance and associated pain. There is a growing consensus that surgical intervention should be performed early, ideally in stage I of IV, to maximize efficacy and prevent disease progression.
Fábio Masato Kamamoto, MD, a plastic surgeon and director of the Instituto Lipedema Brazil, shared insights into surgical treatments for lipedema. He discussed techniques from liposuction to advanced skin retraction and dermolipectomy, crucial for addressing more advanced stages of the condition. “It’s a complex process that demands precision to protect the lymphatic system, especially considering the characteristic nodules of lipedema,” he noted.
Dr. Kamamoto discussed a former patient with stage III lipedema. In the initial stage, he performed liposuction, removing 8 L of fat and 3.4 kg of skin. After 6 months, a follow-up procedure resulted in a total removal of 15 kg. Complementary procedures, such as microneedling, were performed to stimulate collagen production and reduce skin sagging. In addition to cosmetic improvements, the procedure also removed the distinctive lipedema nodules, which Mr. Kartt described as feeling like “rice grains.” Removing these nodules significantly alleviates pain, according to Dr. Kamamoto.
The benefits of surgical treatment for lipedema can be long lasting. Dr. Kamamoto noted that fat tends not to reaccumulate in treated areas, with patients often experiencing lower weight, reduced edema, and decreased pain over time. “While we hope that patients do not regain weight, the benefits of surgery persist even if weight is regained. Therefore, combining conservative and surgical treatments remains a valid and effective approach,” he concluded.
Dr. Scherer highlighted that despite various approaches, there is still no definitive “magic signature” that fully explains lipedema. This lack of clarity directly affects the effectiveness of diagnoses and treatments. He expressed hope that future integration of data from different studies and approaches will lead to the identification of a clinically useful molecular signature. “The true cause of lipedema remains unknown, requiring more speculation, hypothesis formulation, and testing for significant discoveries. This situation is frustrating, as the disease affects many women who lack a clear diagnosis that differentiates them from patients with obesity, as well as evidence-based recommendations,” he concluded.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.