User login
Pyzchiva Receives FDA Approval as Third Ustekinumab Biosimilar
The Food and Drug Administration has approved ustekinumab-ttwe (Pyzchiva) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions.
In addition, the agency “provisionally determined” that the medication would be interchangeable with the reference product but that designation would not take hold until the interchangeability exclusivity period for the first approved biosimilar ustekinumab-auub (Wezlana) expires, according to a press release. This designation would, depending on state law, allow a pharmacist to substitute the biosimilar for the reference product without involving the prescribing clinician. It’s unclear when ustekinumab-auub’s interchangeability exclusivity ends.
Ustekinumab-ttwe, a human interleukin (IL)-12 and IL-23 antagonist, is indicated for the treatment of:
- Moderate to severe plaque psoriasis in adults and pediatric patients aged 6 years or older who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis in adults and pediatric patients aged 6 years or older with moderately to severely active Crohn’s disease or ulcerative colitis
It is administered via subcutaneous injection in 45 mg/0.5 mL and 90 mg/mL prefilled syringes or via intravenous infusion in 130 mg/26 mL (5 mg/mL) single-dose vial.
Developed by Samsung Bioepis, ustekinumab-ttwe will be commercialized by Sandoz in the United States. Besides ustekinumab-auub, the other ustekinumab biosimilar is ustekinumab-aekn (Selarsdi).
Ustekinumab-ttwe is expected to launch in February 2025 “in accordance with the settlement and license agreement with Janssen Biotech,” which manufacturers the reference product, Sandoz said. The other approved ustekinumab biosimilars will launch within a similar time frame.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved ustekinumab-ttwe (Pyzchiva) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions.
In addition, the agency “provisionally determined” that the medication would be interchangeable with the reference product but that designation would not take hold until the interchangeability exclusivity period for the first approved biosimilar ustekinumab-auub (Wezlana) expires, according to a press release. This designation would, depending on state law, allow a pharmacist to substitute the biosimilar for the reference product without involving the prescribing clinician. It’s unclear when ustekinumab-auub’s interchangeability exclusivity ends.
Ustekinumab-ttwe, a human interleukin (IL)-12 and IL-23 antagonist, is indicated for the treatment of:
- Moderate to severe plaque psoriasis in adults and pediatric patients aged 6 years or older who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis in adults and pediatric patients aged 6 years or older with moderately to severely active Crohn’s disease or ulcerative colitis
It is administered via subcutaneous injection in 45 mg/0.5 mL and 90 mg/mL prefilled syringes or via intravenous infusion in 130 mg/26 mL (5 mg/mL) single-dose vial.
Developed by Samsung Bioepis, ustekinumab-ttwe will be commercialized by Sandoz in the United States. Besides ustekinumab-auub, the other ustekinumab biosimilar is ustekinumab-aekn (Selarsdi).
Ustekinumab-ttwe is expected to launch in February 2025 “in accordance with the settlement and license agreement with Janssen Biotech,” which manufacturers the reference product, Sandoz said. The other approved ustekinumab biosimilars will launch within a similar time frame.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved ustekinumab-ttwe (Pyzchiva) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions.
In addition, the agency “provisionally determined” that the medication would be interchangeable with the reference product but that designation would not take hold until the interchangeability exclusivity period for the first approved biosimilar ustekinumab-auub (Wezlana) expires, according to a press release. This designation would, depending on state law, allow a pharmacist to substitute the biosimilar for the reference product without involving the prescribing clinician. It’s unclear when ustekinumab-auub’s interchangeability exclusivity ends.
Ustekinumab-ttwe, a human interleukin (IL)-12 and IL-23 antagonist, is indicated for the treatment of:
- Moderate to severe plaque psoriasis in adults and pediatric patients aged 6 years or older who are candidates for phototherapy or systemic therapy
- Active psoriatic arthritis in adults and pediatric patients aged 6 years or older with moderately to severely active Crohn’s disease or ulcerative colitis
It is administered via subcutaneous injection in 45 mg/0.5 mL and 90 mg/mL prefilled syringes or via intravenous infusion in 130 mg/26 mL (5 mg/mL) single-dose vial.
Developed by Samsung Bioepis, ustekinumab-ttwe will be commercialized by Sandoz in the United States. Besides ustekinumab-auub, the other ustekinumab biosimilar is ustekinumab-aekn (Selarsdi).
Ustekinumab-ttwe is expected to launch in February 2025 “in accordance with the settlement and license agreement with Janssen Biotech,” which manufacturers the reference product, Sandoz said. The other approved ustekinumab biosimilars will launch within a similar time frame.
A version of this article appeared on Medscape.com.
Is Anxiety a Prodromal Feature of Parkinson’s Disease?
new research suggested.
Investigators drew on 10-year data from primary care registry to compare almost 110,000 patients who developed anxiety after the age of 50 years with close to 900,000 matched controls without anxiety.
After adjusting for a variety of sociodemographic, lifestyle, psychiatric, and neurological factors, they found that the risk of developing Parkinson’s disease was double in those with anxiety, compared with controls.
“Anxiety is known to be a feature of the early stages of Parkinson’s disease, but prior to our study, the prospective risk of Parkinson’s in those over the age of 50 with new-onset anxiety was unknown,” colead author Juan Bazo Alvarez, a senior research fellow in the Division of Epidemiology and Health at University College London, London, England, said in a news release.
The study was published online in the British Journal of General Practice.
The presence of anxiety is increased in prodromal Parkinson’s disease, but the prospective risk for Parkinson’s disease in those aged 50 years or older with new-onset anxiety was largely unknown.
Investigators analyzed data from a large UK primary care dataset that includes all people aged between 50 and 99 years who were registered with a participating practice from Jan. 1, 2008, to Dec. 31, 2018.
They identified 109,435 people (35% men) with more than one anxiety record in the database but no previous record of anxiety for 1 year or more and 878,256 people (37% men) with no history of anxiety (control group).
Features of Parkinson’s disease such as sleep problems, depression, tremor, and impaired balance were then tracked from the point of the anxiety diagnosis until 1 year before the Parkinson’s disease diagnosis.
Among those with anxiety, 331 developed Parkinson’s disease during the follow-up period, with a median time to diagnosis of 4.9 years after the first recorded episode of anxiety.
The incidence of Parkinson’s disease was 1.2 per 1000 person-years (95% CI, 0.92-1.13) in those with anxiety versus 0.49 (95% CI, 0.47-0.52) in those without anxiety.
After adjustment for age, sex, social deprivation, lifestyle factors, severe mental illness, head trauma, and dementia, the risk for Parkinson’s disease was double in those with anxiety, compared with the non-anxiety group (hazard ratio, 2.1; 95% CI, 1.9-2.4).
Individuals without anxiety also developed Parkinson’s disease later than those with anxiety.
The researchers identified specific symptoms that were associated with later development of Parkinson’s disease in those with anxiety, including depression, sleep disturbance, fatigue, and cognitive impairment, among other symptoms.
“The results suggest that there is a strong association between anxiety and diagnosis of Parkinson’s disease in patients aged over 50 years who present with a new diagnosis of anxiety,” the authors wrote. “This provides evidence for anxiety as a prodromal presentation of Parkinson’s disease.”
Future research “should explore anxiety in relation to other prodromal symptoms and how this symptom complex is associated with the incidence of Parkinson’s disease,” the researchers wrote. Doing so “may lead to earlier diagnosis and better management of Parkinson’s disease.”
This study was funded by the European Union. Specific authors received funding from the National Institute for Health and Care Research and the Alzheimer’s Society Clinical Training Fellowship program. The authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggested.
Investigators drew on 10-year data from primary care registry to compare almost 110,000 patients who developed anxiety after the age of 50 years with close to 900,000 matched controls without anxiety.
After adjusting for a variety of sociodemographic, lifestyle, psychiatric, and neurological factors, they found that the risk of developing Parkinson’s disease was double in those with anxiety, compared with controls.
“Anxiety is known to be a feature of the early stages of Parkinson’s disease, but prior to our study, the prospective risk of Parkinson’s in those over the age of 50 with new-onset anxiety was unknown,” colead author Juan Bazo Alvarez, a senior research fellow in the Division of Epidemiology and Health at University College London, London, England, said in a news release.
The study was published online in the British Journal of General Practice.
The presence of anxiety is increased in prodromal Parkinson’s disease, but the prospective risk for Parkinson’s disease in those aged 50 years or older with new-onset anxiety was largely unknown.
Investigators analyzed data from a large UK primary care dataset that includes all people aged between 50 and 99 years who were registered with a participating practice from Jan. 1, 2008, to Dec. 31, 2018.
They identified 109,435 people (35% men) with more than one anxiety record in the database but no previous record of anxiety for 1 year or more and 878,256 people (37% men) with no history of anxiety (control group).
Features of Parkinson’s disease such as sleep problems, depression, tremor, and impaired balance were then tracked from the point of the anxiety diagnosis until 1 year before the Parkinson’s disease diagnosis.
Among those with anxiety, 331 developed Parkinson’s disease during the follow-up period, with a median time to diagnosis of 4.9 years after the first recorded episode of anxiety.
The incidence of Parkinson’s disease was 1.2 per 1000 person-years (95% CI, 0.92-1.13) in those with anxiety versus 0.49 (95% CI, 0.47-0.52) in those without anxiety.
After adjustment for age, sex, social deprivation, lifestyle factors, severe mental illness, head trauma, and dementia, the risk for Parkinson’s disease was double in those with anxiety, compared with the non-anxiety group (hazard ratio, 2.1; 95% CI, 1.9-2.4).
Individuals without anxiety also developed Parkinson’s disease later than those with anxiety.
The researchers identified specific symptoms that were associated with later development of Parkinson’s disease in those with anxiety, including depression, sleep disturbance, fatigue, and cognitive impairment, among other symptoms.
“The results suggest that there is a strong association between anxiety and diagnosis of Parkinson’s disease in patients aged over 50 years who present with a new diagnosis of anxiety,” the authors wrote. “This provides evidence for anxiety as a prodromal presentation of Parkinson’s disease.”
Future research “should explore anxiety in relation to other prodromal symptoms and how this symptom complex is associated with the incidence of Parkinson’s disease,” the researchers wrote. Doing so “may lead to earlier diagnosis and better management of Parkinson’s disease.”
This study was funded by the European Union. Specific authors received funding from the National Institute for Health and Care Research and the Alzheimer’s Society Clinical Training Fellowship program. The authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggested.
Investigators drew on 10-year data from primary care registry to compare almost 110,000 patients who developed anxiety after the age of 50 years with close to 900,000 matched controls without anxiety.
After adjusting for a variety of sociodemographic, lifestyle, psychiatric, and neurological factors, they found that the risk of developing Parkinson’s disease was double in those with anxiety, compared with controls.
“Anxiety is known to be a feature of the early stages of Parkinson’s disease, but prior to our study, the prospective risk of Parkinson’s in those over the age of 50 with new-onset anxiety was unknown,” colead author Juan Bazo Alvarez, a senior research fellow in the Division of Epidemiology and Health at University College London, London, England, said in a news release.
The study was published online in the British Journal of General Practice.
The presence of anxiety is increased in prodromal Parkinson’s disease, but the prospective risk for Parkinson’s disease in those aged 50 years or older with new-onset anxiety was largely unknown.
Investigators analyzed data from a large UK primary care dataset that includes all people aged between 50 and 99 years who were registered with a participating practice from Jan. 1, 2008, to Dec. 31, 2018.
They identified 109,435 people (35% men) with more than one anxiety record in the database but no previous record of anxiety for 1 year or more and 878,256 people (37% men) with no history of anxiety (control group).
Features of Parkinson’s disease such as sleep problems, depression, tremor, and impaired balance were then tracked from the point of the anxiety diagnosis until 1 year before the Parkinson’s disease diagnosis.
Among those with anxiety, 331 developed Parkinson’s disease during the follow-up period, with a median time to diagnosis of 4.9 years after the first recorded episode of anxiety.
The incidence of Parkinson’s disease was 1.2 per 1000 person-years (95% CI, 0.92-1.13) in those with anxiety versus 0.49 (95% CI, 0.47-0.52) in those without anxiety.
After adjustment for age, sex, social deprivation, lifestyle factors, severe mental illness, head trauma, and dementia, the risk for Parkinson’s disease was double in those with anxiety, compared with the non-anxiety group (hazard ratio, 2.1; 95% CI, 1.9-2.4).
Individuals without anxiety also developed Parkinson’s disease later than those with anxiety.
The researchers identified specific symptoms that were associated with later development of Parkinson’s disease in those with anxiety, including depression, sleep disturbance, fatigue, and cognitive impairment, among other symptoms.
“The results suggest that there is a strong association between anxiety and diagnosis of Parkinson’s disease in patients aged over 50 years who present with a new diagnosis of anxiety,” the authors wrote. “This provides evidence for anxiety as a prodromal presentation of Parkinson’s disease.”
Future research “should explore anxiety in relation to other prodromal symptoms and how this symptom complex is associated with the incidence of Parkinson’s disease,” the researchers wrote. Doing so “may lead to earlier diagnosis and better management of Parkinson’s disease.”
This study was funded by the European Union. Specific authors received funding from the National Institute for Health and Care Research and the Alzheimer’s Society Clinical Training Fellowship program. The authors declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF GENERAL PRACTICE
Benzos Are Hard on the Brain, But Do They Raise Dementia Risk?
The study of more than 5000 older adults found that benzodiazepine use was associated with an accelerated reduction in the volume of the hippocampus and amygdala — brain regions involved in memory and mood regulation. However, benzodiazepine use overall was not associated with an increased risk for dementia.
The findings suggest that benzodiazepine use “may have subtle, long-term impact on brain health,” lead investigator Frank Wolters, MD, PhD, with Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues wrote.
The study was published online in BMC Medicine.
Conflicting Evidence
Benzodiazepines are commonly prescribed in older adults for anxiety and sleep disorders. Though the short-term cognitive side effects are well documented, the long-term impact on neurodegeneration and dementia risk remains unclear. Some studies have linked benzodiazepine use to an increased risk for dementia, whereas others have not.
Dr. Wolters and colleagues assessed the effect of benzodiazepine use on long-term dementia risk and on imaging markers of neurodegeneration in 5443 cognitively healthy adults (mean age, 71 years; 57% women) from the population-based Rotterdam Study.
Benzodiazepine use between 1991 and 2008 was determined using pharmacy dispensing records, and dementia incidence was determined from medical records.
Half of the participants had used benzodiazepines at any time in the 15 years before baseline (2005-2008); 47% used anxiolytics, 20% used sedative-hypnotics, 34% used both, and 13% were still using the drugs at the baseline assessment.
During an average follow-up of 11 years, 13% of participants developed dementia.
Overall, use of benzodiazepines was not associated with dementia risk, compared with never-use (hazard ratio [HR], 1.06), irrespective of cumulative dose.
The risk for dementia was somewhat higher with any use of anxiolytics than with sedative-hypnotics (HR, 1.17 vs HR, 0.92), although neither was statistically significant. The highest risk estimates were observed for high cumulative dose of anxiolytics (HR, 1.33).
Sensitivity analyses of the two most commonly used anxiolytics found no differences in risk between use of short half-life oxazepam and long half-life diazepam (HR, 1.01 and HR, 1.06, respectively, for ever-use, compared with never-use for oxazepam and diazepam).
Brain Atrophy
The researchers investigated potential associations between benzodiazepine use and brain volumes using brain MRI imaging from 4836 participants.
They found that current use of a benzodiazepine at baseline was significantly associated with lower total brain volume — as well as lower hippocampus, amygdala, and thalamus volume cross-sectionally — and with accelerated volume loss of the hippocampus and, to a lesser extent, amygdala longitudinally.
Imaging findings did not differ by type of benzodiazepine used or cumulative dose.
“Given the availability of effective alternative pharmacological and nonpharmacological treatments for anxiety and sleep problems, it is important to carefully consider the necessity of prolonged benzodiazepine use in light of potential detrimental effects on brain health,” the authors wrote.
Risks Go Beyond the Brain
Commenting on the study, Shaheen Lakhan, MD, PhD, a neurologist and researcher based in Miami, Florida, noted that “chronic benzodiazepine use may reduce neuroplasticity, potentially interfering with the brain’s ability to form new connections and adapt.
“Long-term use can lead to down-regulation of GABA receptors, altering the brain’s natural inhibitory mechanisms and potentially contributing to tolerance and withdrawal symptoms. Prolonged use can also disrupt the balance of various neurotransmitter systems beyond just GABA, potentially affecting mood, cognition, and overall brain function,” said Dr. Lakhan, who was not involved in the study.
“While the literature is mixed on chronic benzodiazepine use and dementia risk, prolonged use has consistently been associated with accelerated volume loss in certain brain regions, particularly the hippocampus and amygdala,” which are responsible for memory, learning, and emotional regulation, he noted.
“Beyond cognitive impairments and brain volume loss, chronic benzodiazepine use is associated with tolerance and dependence, potential for abuse, interactions with other drugs, and increased fall risk, especially in older adults,” Dr. Lakhan added.
Current guidelines discourage long-term use of benzodiazepines because of risk for psychological and physical dependence; falls; and cognitive impairment, especially in older adults. Nevertheless, research shows that 30%-40% of older benzodiazepine users stay on the medication beyond the recommended period of several weeks.
Donovan T. Maust, MD, Department of Psychiatry, University of Michigan Medical School, Ann Arbor, said in an interview these new findings are consistent with other recently published observational research that suggest benzodiazepine use is not linked to dementia risk.
“I realize that such meta-analyses that find a positive relationship between benzodiazepines and dementia are out there, but they include older, less rigorous studies,” said Dr. Maust, who was not part of the new study. “In my opinion, the jury is not still out on this topic. However, there are plenty of other reasons to avoid them — and in particular, starting them — in older adults, most notably the increased risk of fall injury as well as increased overdose risk when taken along with opioids.”
A version of this article first appeared on Medscape.com.
The study of more than 5000 older adults found that benzodiazepine use was associated with an accelerated reduction in the volume of the hippocampus and amygdala — brain regions involved in memory and mood regulation. However, benzodiazepine use overall was not associated with an increased risk for dementia.
The findings suggest that benzodiazepine use “may have subtle, long-term impact on brain health,” lead investigator Frank Wolters, MD, PhD, with Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues wrote.
The study was published online in BMC Medicine.
Conflicting Evidence
Benzodiazepines are commonly prescribed in older adults for anxiety and sleep disorders. Though the short-term cognitive side effects are well documented, the long-term impact on neurodegeneration and dementia risk remains unclear. Some studies have linked benzodiazepine use to an increased risk for dementia, whereas others have not.
Dr. Wolters and colleagues assessed the effect of benzodiazepine use on long-term dementia risk and on imaging markers of neurodegeneration in 5443 cognitively healthy adults (mean age, 71 years; 57% women) from the population-based Rotterdam Study.
Benzodiazepine use between 1991 and 2008 was determined using pharmacy dispensing records, and dementia incidence was determined from medical records.
Half of the participants had used benzodiazepines at any time in the 15 years before baseline (2005-2008); 47% used anxiolytics, 20% used sedative-hypnotics, 34% used both, and 13% were still using the drugs at the baseline assessment.
During an average follow-up of 11 years, 13% of participants developed dementia.
Overall, use of benzodiazepines was not associated with dementia risk, compared with never-use (hazard ratio [HR], 1.06), irrespective of cumulative dose.
The risk for dementia was somewhat higher with any use of anxiolytics than with sedative-hypnotics (HR, 1.17 vs HR, 0.92), although neither was statistically significant. The highest risk estimates were observed for high cumulative dose of anxiolytics (HR, 1.33).
Sensitivity analyses of the two most commonly used anxiolytics found no differences in risk between use of short half-life oxazepam and long half-life diazepam (HR, 1.01 and HR, 1.06, respectively, for ever-use, compared with never-use for oxazepam and diazepam).
Brain Atrophy
The researchers investigated potential associations between benzodiazepine use and brain volumes using brain MRI imaging from 4836 participants.
They found that current use of a benzodiazepine at baseline was significantly associated with lower total brain volume — as well as lower hippocampus, amygdala, and thalamus volume cross-sectionally — and with accelerated volume loss of the hippocampus and, to a lesser extent, amygdala longitudinally.
Imaging findings did not differ by type of benzodiazepine used or cumulative dose.
“Given the availability of effective alternative pharmacological and nonpharmacological treatments for anxiety and sleep problems, it is important to carefully consider the necessity of prolonged benzodiazepine use in light of potential detrimental effects on brain health,” the authors wrote.
Risks Go Beyond the Brain
Commenting on the study, Shaheen Lakhan, MD, PhD, a neurologist and researcher based in Miami, Florida, noted that “chronic benzodiazepine use may reduce neuroplasticity, potentially interfering with the brain’s ability to form new connections and adapt.
“Long-term use can lead to down-regulation of GABA receptors, altering the brain’s natural inhibitory mechanisms and potentially contributing to tolerance and withdrawal symptoms. Prolonged use can also disrupt the balance of various neurotransmitter systems beyond just GABA, potentially affecting mood, cognition, and overall brain function,” said Dr. Lakhan, who was not involved in the study.
“While the literature is mixed on chronic benzodiazepine use and dementia risk, prolonged use has consistently been associated with accelerated volume loss in certain brain regions, particularly the hippocampus and amygdala,” which are responsible for memory, learning, and emotional regulation, he noted.
“Beyond cognitive impairments and brain volume loss, chronic benzodiazepine use is associated with tolerance and dependence, potential for abuse, interactions with other drugs, and increased fall risk, especially in older adults,” Dr. Lakhan added.
Current guidelines discourage long-term use of benzodiazepines because of risk for psychological and physical dependence; falls; and cognitive impairment, especially in older adults. Nevertheless, research shows that 30%-40% of older benzodiazepine users stay on the medication beyond the recommended period of several weeks.
Donovan T. Maust, MD, Department of Psychiatry, University of Michigan Medical School, Ann Arbor, said in an interview these new findings are consistent with other recently published observational research that suggest benzodiazepine use is not linked to dementia risk.
“I realize that such meta-analyses that find a positive relationship between benzodiazepines and dementia are out there, but they include older, less rigorous studies,” said Dr. Maust, who was not part of the new study. “In my opinion, the jury is not still out on this topic. However, there are plenty of other reasons to avoid them — and in particular, starting them — in older adults, most notably the increased risk of fall injury as well as increased overdose risk when taken along with opioids.”
A version of this article first appeared on Medscape.com.
The study of more than 5000 older adults found that benzodiazepine use was associated with an accelerated reduction in the volume of the hippocampus and amygdala — brain regions involved in memory and mood regulation. However, benzodiazepine use overall was not associated with an increased risk for dementia.
The findings suggest that benzodiazepine use “may have subtle, long-term impact on brain health,” lead investigator Frank Wolters, MD, PhD, with Erasmus University Medical Center, Rotterdam, the Netherlands, and colleagues wrote.
The study was published online in BMC Medicine.
Conflicting Evidence
Benzodiazepines are commonly prescribed in older adults for anxiety and sleep disorders. Though the short-term cognitive side effects are well documented, the long-term impact on neurodegeneration and dementia risk remains unclear. Some studies have linked benzodiazepine use to an increased risk for dementia, whereas others have not.
Dr. Wolters and colleagues assessed the effect of benzodiazepine use on long-term dementia risk and on imaging markers of neurodegeneration in 5443 cognitively healthy adults (mean age, 71 years; 57% women) from the population-based Rotterdam Study.
Benzodiazepine use between 1991 and 2008 was determined using pharmacy dispensing records, and dementia incidence was determined from medical records.
Half of the participants had used benzodiazepines at any time in the 15 years before baseline (2005-2008); 47% used anxiolytics, 20% used sedative-hypnotics, 34% used both, and 13% were still using the drugs at the baseline assessment.
During an average follow-up of 11 years, 13% of participants developed dementia.
Overall, use of benzodiazepines was not associated with dementia risk, compared with never-use (hazard ratio [HR], 1.06), irrespective of cumulative dose.
The risk for dementia was somewhat higher with any use of anxiolytics than with sedative-hypnotics (HR, 1.17 vs HR, 0.92), although neither was statistically significant. The highest risk estimates were observed for high cumulative dose of anxiolytics (HR, 1.33).
Sensitivity analyses of the two most commonly used anxiolytics found no differences in risk between use of short half-life oxazepam and long half-life diazepam (HR, 1.01 and HR, 1.06, respectively, for ever-use, compared with never-use for oxazepam and diazepam).
Brain Atrophy
The researchers investigated potential associations between benzodiazepine use and brain volumes using brain MRI imaging from 4836 participants.
They found that current use of a benzodiazepine at baseline was significantly associated with lower total brain volume — as well as lower hippocampus, amygdala, and thalamus volume cross-sectionally — and with accelerated volume loss of the hippocampus and, to a lesser extent, amygdala longitudinally.
Imaging findings did not differ by type of benzodiazepine used or cumulative dose.
“Given the availability of effective alternative pharmacological and nonpharmacological treatments for anxiety and sleep problems, it is important to carefully consider the necessity of prolonged benzodiazepine use in light of potential detrimental effects on brain health,” the authors wrote.
Risks Go Beyond the Brain
Commenting on the study, Shaheen Lakhan, MD, PhD, a neurologist and researcher based in Miami, Florida, noted that “chronic benzodiazepine use may reduce neuroplasticity, potentially interfering with the brain’s ability to form new connections and adapt.
“Long-term use can lead to down-regulation of GABA receptors, altering the brain’s natural inhibitory mechanisms and potentially contributing to tolerance and withdrawal symptoms. Prolonged use can also disrupt the balance of various neurotransmitter systems beyond just GABA, potentially affecting mood, cognition, and overall brain function,” said Dr. Lakhan, who was not involved in the study.
“While the literature is mixed on chronic benzodiazepine use and dementia risk, prolonged use has consistently been associated with accelerated volume loss in certain brain regions, particularly the hippocampus and amygdala,” which are responsible for memory, learning, and emotional regulation, he noted.
“Beyond cognitive impairments and brain volume loss, chronic benzodiazepine use is associated with tolerance and dependence, potential for abuse, interactions with other drugs, and increased fall risk, especially in older adults,” Dr. Lakhan added.
Current guidelines discourage long-term use of benzodiazepines because of risk for psychological and physical dependence; falls; and cognitive impairment, especially in older adults. Nevertheless, research shows that 30%-40% of older benzodiazepine users stay on the medication beyond the recommended period of several weeks.
Donovan T. Maust, MD, Department of Psychiatry, University of Michigan Medical School, Ann Arbor, said in an interview these new findings are consistent with other recently published observational research that suggest benzodiazepine use is not linked to dementia risk.
“I realize that such meta-analyses that find a positive relationship between benzodiazepines and dementia are out there, but they include older, less rigorous studies,” said Dr. Maust, who was not part of the new study. “In my opinion, the jury is not still out on this topic. However, there are plenty of other reasons to avoid them — and in particular, starting them — in older adults, most notably the increased risk of fall injury as well as increased overdose risk when taken along with opioids.”
A version of this article first appeared on Medscape.com.
FROM BMC MEDICINE
Medication Overuse in Mental Health Facilities: Not the Answer, Regardless of Consent, Says Ethicist
This transcript has been edited for clarity.
There’s a growing scandal in mental health care. Recent studies are showing that certain medications that basically are used to, if you will, quiet patients — antipsychotic drugs — are being overused, particularly in facilities that serve poorer people and people who are minorities. This situation is utterly, ethically unacceptable and it’s something that we are starting to get really pressed to solve.
Part of this is due to the fact that numbers of caregivers are in short supply. We need to get more people trained. We need to get more mental health providers at all levels into facilities in order to provide care, and not substitute that inability to have a provider present and minimize risk to patients by having drug-induced sleepiness, soporific behavior, or, if you will, snowing them just because we don’t have enough people to keep an eye on them. Furthermore, we can’t let them engage in some activities, even things like walking around, because we’re worried about falls. The nursing homes or mental health facilities don’t want anybody to get injured, much less killed, because that’s going to really bring government agencies down on them.
What do we do, aside from trying to get more numbers in there? California came up with a law not too long ago that basically put the burden of using these drugs on consent. They passed a law that said the patient, before going under and being administered any type of psychoactive drug, has to consent; or if they’re really unable to do that, their relative or next of kin should have to consent.
California law now puts the burden on getting consent from the patient in order to use these drugs. It’s not a good solution. It still permits the use of the drugs to substitute for the inability to provide adequate numbers of people to provide care in safe environments. It’s almost like saying, “We know you’re going into a dangerous place. We can’t really reduce the danger, so we’re going to make sure that you stay in your seat. You better consent to that because otherwise things could not go well for you in this mental institution.”
That’s not a sound argument for the use of informed consent. Moreover, I’m very skeptical that many of these people in mental institutions do have the capacity to either say, “Fine, give me psychoactive drugs if I have to stay here,” or “No, I don’t want that. I’ll take my chances.”
They’re vulnerable people. Many of them may not be fully incompetent, but they often have compromised competency. Relatives may be thinking, Well, the right thing to do is just to make sure they don’t get hurt or injure themselves. Yes, give them the drugs.
Consent, while I support it, is not the solution to what is fundamentally an infrastructure problem, a personnel problem, and one of the shames of American healthcare, which is lousy long-term mental health care. For too many people, their care is in the street. For too many people, their care is taking place in institutions that have dangerous designs where people either get injured, can’t provide enough spacing, or just don’t have the people to do it.
Let’s move to fix the mental health care system and not be in a situation where we say to people, “The system stinks and you’re at risk. Is it okay with you if we drug you because we can’t think of any other way to keep you safe, given the rotten nature of the institutions that we’ve got?”
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and serves as a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a growing scandal in mental health care. Recent studies are showing that certain medications that basically are used to, if you will, quiet patients — antipsychotic drugs — are being overused, particularly in facilities that serve poorer people and people who are minorities. This situation is utterly, ethically unacceptable and it’s something that we are starting to get really pressed to solve.
Part of this is due to the fact that numbers of caregivers are in short supply. We need to get more people trained. We need to get more mental health providers at all levels into facilities in order to provide care, and not substitute that inability to have a provider present and minimize risk to patients by having drug-induced sleepiness, soporific behavior, or, if you will, snowing them just because we don’t have enough people to keep an eye on them. Furthermore, we can’t let them engage in some activities, even things like walking around, because we’re worried about falls. The nursing homes or mental health facilities don’t want anybody to get injured, much less killed, because that’s going to really bring government agencies down on them.
What do we do, aside from trying to get more numbers in there? California came up with a law not too long ago that basically put the burden of using these drugs on consent. They passed a law that said the patient, before going under and being administered any type of psychoactive drug, has to consent; or if they’re really unable to do that, their relative or next of kin should have to consent.
California law now puts the burden on getting consent from the patient in order to use these drugs. It’s not a good solution. It still permits the use of the drugs to substitute for the inability to provide adequate numbers of people to provide care in safe environments. It’s almost like saying, “We know you’re going into a dangerous place. We can’t really reduce the danger, so we’re going to make sure that you stay in your seat. You better consent to that because otherwise things could not go well for you in this mental institution.”
That’s not a sound argument for the use of informed consent. Moreover, I’m very skeptical that many of these people in mental institutions do have the capacity to either say, “Fine, give me psychoactive drugs if I have to stay here,” or “No, I don’t want that. I’ll take my chances.”
They’re vulnerable people. Many of them may not be fully incompetent, but they often have compromised competency. Relatives may be thinking, Well, the right thing to do is just to make sure they don’t get hurt or injure themselves. Yes, give them the drugs.
Consent, while I support it, is not the solution to what is fundamentally an infrastructure problem, a personnel problem, and one of the shames of American healthcare, which is lousy long-term mental health care. For too many people, their care is in the street. For too many people, their care is taking place in institutions that have dangerous designs where people either get injured, can’t provide enough spacing, or just don’t have the people to do it.
Let’s move to fix the mental health care system and not be in a situation where we say to people, “The system stinks and you’re at risk. Is it okay with you if we drug you because we can’t think of any other way to keep you safe, given the rotten nature of the institutions that we’ve got?”
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and serves as a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a growing scandal in mental health care. Recent studies are showing that certain medications that basically are used to, if you will, quiet patients — antipsychotic drugs — are being overused, particularly in facilities that serve poorer people and people who are minorities. This situation is utterly, ethically unacceptable and it’s something that we are starting to get really pressed to solve.
Part of this is due to the fact that numbers of caregivers are in short supply. We need to get more people trained. We need to get more mental health providers at all levels into facilities in order to provide care, and not substitute that inability to have a provider present and minimize risk to patients by having drug-induced sleepiness, soporific behavior, or, if you will, snowing them just because we don’t have enough people to keep an eye on them. Furthermore, we can’t let them engage in some activities, even things like walking around, because we’re worried about falls. The nursing homes or mental health facilities don’t want anybody to get injured, much less killed, because that’s going to really bring government agencies down on them.
What do we do, aside from trying to get more numbers in there? California came up with a law not too long ago that basically put the burden of using these drugs on consent. They passed a law that said the patient, before going under and being administered any type of psychoactive drug, has to consent; or if they’re really unable to do that, their relative or next of kin should have to consent.
California law now puts the burden on getting consent from the patient in order to use these drugs. It’s not a good solution. It still permits the use of the drugs to substitute for the inability to provide adequate numbers of people to provide care in safe environments. It’s almost like saying, “We know you’re going into a dangerous place. We can’t really reduce the danger, so we’re going to make sure that you stay in your seat. You better consent to that because otherwise things could not go well for you in this mental institution.”
That’s not a sound argument for the use of informed consent. Moreover, I’m very skeptical that many of these people in mental institutions do have the capacity to either say, “Fine, give me psychoactive drugs if I have to stay here,” or “No, I don’t want that. I’ll take my chances.”
They’re vulnerable people. Many of them may not be fully incompetent, but they often have compromised competency. Relatives may be thinking, Well, the right thing to do is just to make sure they don’t get hurt or injure themselves. Yes, give them the drugs.
Consent, while I support it, is not the solution to what is fundamentally an infrastructure problem, a personnel problem, and one of the shames of American healthcare, which is lousy long-term mental health care. For too many people, their care is in the street. For too many people, their care is taking place in institutions that have dangerous designs where people either get injured, can’t provide enough spacing, or just don’t have the people to do it.
Let’s move to fix the mental health care system and not be in a situation where we say to people, “The system stinks and you’re at risk. Is it okay with you if we drug you because we can’t think of any other way to keep you safe, given the rotten nature of the institutions that we’ve got?”
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He disclosed ties with Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and serves as a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
Could Tuberculosis Medication Management Be as Simple as Monitoring Sweat?
Analysis of finger sweat detected isoniazid in adults with tuberculosis (TB) for ≤ 6 hours after administration, based on data from a new pilot study.
Katherine Longman, a PhD student at the University of Surrey, Guildford, England, and colleagues wrote.
Although TB is treatable, “it is well known that insufficient drug dosing leads to treatment failure and drug resistance, and so ensuring that patients have sufficient drug exposure is important,” said corresponding author Melanie J. Bailey, PhD, also of the University of Surrey.
“This can be carried out using blood, but blood is painful to collect and difficult to transport. Finger sweat offers a completely noninvasive way to sample patients,” but its use to determine medication adherence has not been examined, she said.
In a pilot study published in the International Journal of Antimicrobial Agents, the researchers reviewed data from 10 adults with TB who provided finger sweat, blood, and saliva samples at several time points ≤ 6 hours after receiving a controlled dose of isoniazid (median of 300 mg daily). They used liquid chromatography–mass spectrometry to examine the samples.
Overall, “isoniazid and acetyl isoniazid were detected in at least one finger sweat sample from all patients,” with detection rates of 96% and 77%, respectively, the researchers wrote. Given the short half-life of isoniazid, they used a window of 1-6 hours after administration. Isoniazid was consistently detected between 1 and 6 hours after administration, while acetyl isoniazid had a noticeably higher detection rate at 6 hours.
The researchers also examined creatinine to account for variability in volume of sweat samples, and found that finger sweat was significantly correlated to isoniazid concentration. The maximum isoniazid to creatinine ratio in finger sweat occurred mainly in the first hour after drug administration, and the activity of isoniazid in finger sweat over time reflected isoniazid concentration in serum more closely after normalization to creatinine, they said. The Pearson’s correlation coefficient (r) was 0.98 (P < .001; one-tailed), with normalization to creatinine, compared with r = 0.52 without normalization (P = .051).
The study findings were limited by several factors including the lack of knowledge of the last drug dose and lack of confirmation testing with an established method of analysis, the researchers noted. However, the results support the potential of the finger sweat test as a screening tool to indicate patients’ nonadherence or to identify patients at risk of low medication exposure.
“We were surprised that we were able to detect the drug in so many patient samples because the sample volume is so low, and so detection is challenging,” said Dr. Bailey. “We were also surprised that fingerprint and drug levels correlated so well after normalizing to creatinine. This is exciting as it unlocks the possibility to test drug levels, as well as providing a yes/no test.”
In practice, the finger sweat technique could reduce the burden on clinics by offering a completely noninvasive way to test a patient’s medication adherence. Looking ahead, more research is needed to explore whether creatinine normalization is widely applicable, such as whether it works for patients with abnormal kidney function, she added.
Noninvasive Option May Mitigate Treatment Challenges
The current study presents a strategy that might address current limitations in TB management, said Krishna Thavarajah, MD, a pulmonologist and director of the interstitial lung disease program at Henry Ford Hospital, Detroit, Michigan, in an interview.
Both self-administered treatment and directly observed therapy (DOT) for TB therapy have limitations, including adherence as low as 50% for TB regimens, she said. In addition, “DOT availability and efficacy can be limited by cost, personnel availability from an administration perspective, and by distrust of those being treated.”
In the current study, “I was struck by the correlation between the sweat and serum values of [isoniazid] and by the level of sophistication of noninvasive testing, being able to normalize for creatinine to account for different volumes of sweat,” said Dr. Thavarajah. In clinical practice, finger sweat isoniazid could potentially serve as an adjunct or alternative to DOT in patients with TB.
Although adherence to the sampling protocol and possible patient distrust of the process (such as concerns over what else is being collected in their sweat) might be barriers to the use of a finger sweat strategy in the clinical setting, appropriate patient selection, patient training, and encouraging clinicians to incorporate this testing into practice could overcome these barriers, said Dr. Thavarajah.
However, more research is needed to study the finger sweat strategy in larger, real-world samples and to study accuracy and treatment adherence with monitoring in a population undergoing DOT, she said.
The study was supported by the Engineering & Physical Sciences Research Council and by Santander PhD Mobility Awards 2019. The researchers had no financial conflicts to disclose. Dr. Thavarajah had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Analysis of finger sweat detected isoniazid in adults with tuberculosis (TB) for ≤ 6 hours after administration, based on data from a new pilot study.
Katherine Longman, a PhD student at the University of Surrey, Guildford, England, and colleagues wrote.
Although TB is treatable, “it is well known that insufficient drug dosing leads to treatment failure and drug resistance, and so ensuring that patients have sufficient drug exposure is important,” said corresponding author Melanie J. Bailey, PhD, also of the University of Surrey.
“This can be carried out using blood, but blood is painful to collect and difficult to transport. Finger sweat offers a completely noninvasive way to sample patients,” but its use to determine medication adherence has not been examined, she said.
In a pilot study published in the International Journal of Antimicrobial Agents, the researchers reviewed data from 10 adults with TB who provided finger sweat, blood, and saliva samples at several time points ≤ 6 hours after receiving a controlled dose of isoniazid (median of 300 mg daily). They used liquid chromatography–mass spectrometry to examine the samples.
Overall, “isoniazid and acetyl isoniazid were detected in at least one finger sweat sample from all patients,” with detection rates of 96% and 77%, respectively, the researchers wrote. Given the short half-life of isoniazid, they used a window of 1-6 hours after administration. Isoniazid was consistently detected between 1 and 6 hours after administration, while acetyl isoniazid had a noticeably higher detection rate at 6 hours.
The researchers also examined creatinine to account for variability in volume of sweat samples, and found that finger sweat was significantly correlated to isoniazid concentration. The maximum isoniazid to creatinine ratio in finger sweat occurred mainly in the first hour after drug administration, and the activity of isoniazid in finger sweat over time reflected isoniazid concentration in serum more closely after normalization to creatinine, they said. The Pearson’s correlation coefficient (r) was 0.98 (P < .001; one-tailed), with normalization to creatinine, compared with r = 0.52 without normalization (P = .051).
The study findings were limited by several factors including the lack of knowledge of the last drug dose and lack of confirmation testing with an established method of analysis, the researchers noted. However, the results support the potential of the finger sweat test as a screening tool to indicate patients’ nonadherence or to identify patients at risk of low medication exposure.
“We were surprised that we were able to detect the drug in so many patient samples because the sample volume is so low, and so detection is challenging,” said Dr. Bailey. “We were also surprised that fingerprint and drug levels correlated so well after normalizing to creatinine. This is exciting as it unlocks the possibility to test drug levels, as well as providing a yes/no test.”
In practice, the finger sweat technique could reduce the burden on clinics by offering a completely noninvasive way to test a patient’s medication adherence. Looking ahead, more research is needed to explore whether creatinine normalization is widely applicable, such as whether it works for patients with abnormal kidney function, she added.
Noninvasive Option May Mitigate Treatment Challenges
The current study presents a strategy that might address current limitations in TB management, said Krishna Thavarajah, MD, a pulmonologist and director of the interstitial lung disease program at Henry Ford Hospital, Detroit, Michigan, in an interview.
Both self-administered treatment and directly observed therapy (DOT) for TB therapy have limitations, including adherence as low as 50% for TB regimens, she said. In addition, “DOT availability and efficacy can be limited by cost, personnel availability from an administration perspective, and by distrust of those being treated.”
In the current study, “I was struck by the correlation between the sweat and serum values of [isoniazid] and by the level of sophistication of noninvasive testing, being able to normalize for creatinine to account for different volumes of sweat,” said Dr. Thavarajah. In clinical practice, finger sweat isoniazid could potentially serve as an adjunct or alternative to DOT in patients with TB.
Although adherence to the sampling protocol and possible patient distrust of the process (such as concerns over what else is being collected in their sweat) might be barriers to the use of a finger sweat strategy in the clinical setting, appropriate patient selection, patient training, and encouraging clinicians to incorporate this testing into practice could overcome these barriers, said Dr. Thavarajah.
However, more research is needed to study the finger sweat strategy in larger, real-world samples and to study accuracy and treatment adherence with monitoring in a population undergoing DOT, she said.
The study was supported by the Engineering & Physical Sciences Research Council and by Santander PhD Mobility Awards 2019. The researchers had no financial conflicts to disclose. Dr. Thavarajah had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Analysis of finger sweat detected isoniazid in adults with tuberculosis (TB) for ≤ 6 hours after administration, based on data from a new pilot study.
Katherine Longman, a PhD student at the University of Surrey, Guildford, England, and colleagues wrote.
Although TB is treatable, “it is well known that insufficient drug dosing leads to treatment failure and drug resistance, and so ensuring that patients have sufficient drug exposure is important,” said corresponding author Melanie J. Bailey, PhD, also of the University of Surrey.
“This can be carried out using blood, but blood is painful to collect and difficult to transport. Finger sweat offers a completely noninvasive way to sample patients,” but its use to determine medication adherence has not been examined, she said.
In a pilot study published in the International Journal of Antimicrobial Agents, the researchers reviewed data from 10 adults with TB who provided finger sweat, blood, and saliva samples at several time points ≤ 6 hours after receiving a controlled dose of isoniazid (median of 300 mg daily). They used liquid chromatography–mass spectrometry to examine the samples.
Overall, “isoniazid and acetyl isoniazid were detected in at least one finger sweat sample from all patients,” with detection rates of 96% and 77%, respectively, the researchers wrote. Given the short half-life of isoniazid, they used a window of 1-6 hours after administration. Isoniazid was consistently detected between 1 and 6 hours after administration, while acetyl isoniazid had a noticeably higher detection rate at 6 hours.
The researchers also examined creatinine to account for variability in volume of sweat samples, and found that finger sweat was significantly correlated to isoniazid concentration. The maximum isoniazid to creatinine ratio in finger sweat occurred mainly in the first hour after drug administration, and the activity of isoniazid in finger sweat over time reflected isoniazid concentration in serum more closely after normalization to creatinine, they said. The Pearson’s correlation coefficient (r) was 0.98 (P < .001; one-tailed), with normalization to creatinine, compared with r = 0.52 without normalization (P = .051).
The study findings were limited by several factors including the lack of knowledge of the last drug dose and lack of confirmation testing with an established method of analysis, the researchers noted. However, the results support the potential of the finger sweat test as a screening tool to indicate patients’ nonadherence or to identify patients at risk of low medication exposure.
“We were surprised that we were able to detect the drug in so many patient samples because the sample volume is so low, and so detection is challenging,” said Dr. Bailey. “We were also surprised that fingerprint and drug levels correlated so well after normalizing to creatinine. This is exciting as it unlocks the possibility to test drug levels, as well as providing a yes/no test.”
In practice, the finger sweat technique could reduce the burden on clinics by offering a completely noninvasive way to test a patient’s medication adherence. Looking ahead, more research is needed to explore whether creatinine normalization is widely applicable, such as whether it works for patients with abnormal kidney function, she added.
Noninvasive Option May Mitigate Treatment Challenges
The current study presents a strategy that might address current limitations in TB management, said Krishna Thavarajah, MD, a pulmonologist and director of the interstitial lung disease program at Henry Ford Hospital, Detroit, Michigan, in an interview.
Both self-administered treatment and directly observed therapy (DOT) for TB therapy have limitations, including adherence as low as 50% for TB regimens, she said. In addition, “DOT availability and efficacy can be limited by cost, personnel availability from an administration perspective, and by distrust of those being treated.”
In the current study, “I was struck by the correlation between the sweat and serum values of [isoniazid] and by the level of sophistication of noninvasive testing, being able to normalize for creatinine to account for different volumes of sweat,” said Dr. Thavarajah. In clinical practice, finger sweat isoniazid could potentially serve as an adjunct or alternative to DOT in patients with TB.
Although adherence to the sampling protocol and possible patient distrust of the process (such as concerns over what else is being collected in their sweat) might be barriers to the use of a finger sweat strategy in the clinical setting, appropriate patient selection, patient training, and encouraging clinicians to incorporate this testing into practice could overcome these barriers, said Dr. Thavarajah.
However, more research is needed to study the finger sweat strategy in larger, real-world samples and to study accuracy and treatment adherence with monitoring in a population undergoing DOT, she said.
The study was supported by the Engineering & Physical Sciences Research Council and by Santander PhD Mobility Awards 2019. The researchers had no financial conflicts to disclose. Dr. Thavarajah had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
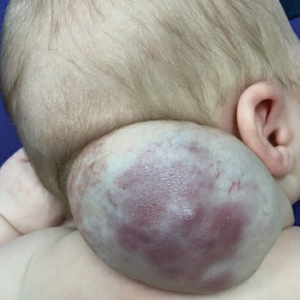
Vascular Mass on the Posterior Neck in a Newborn
The Diagnosis: Congenital Hemangioma
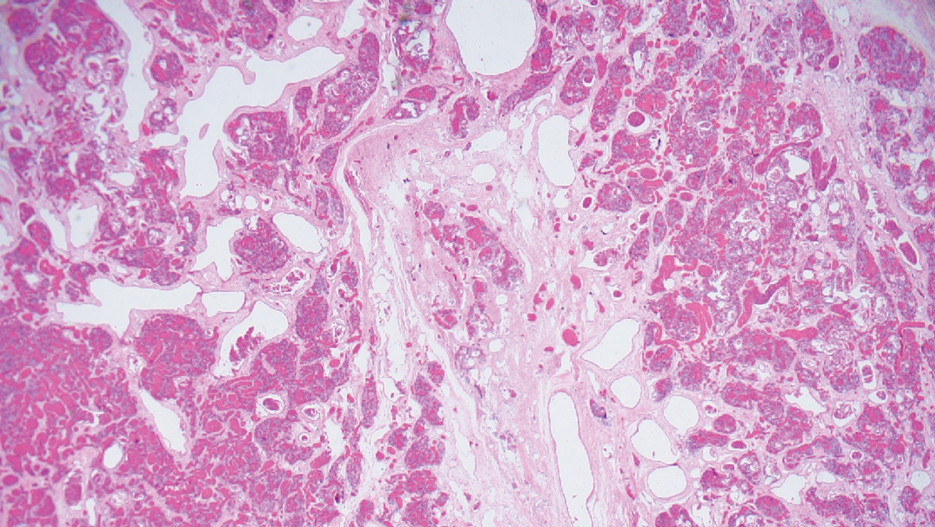
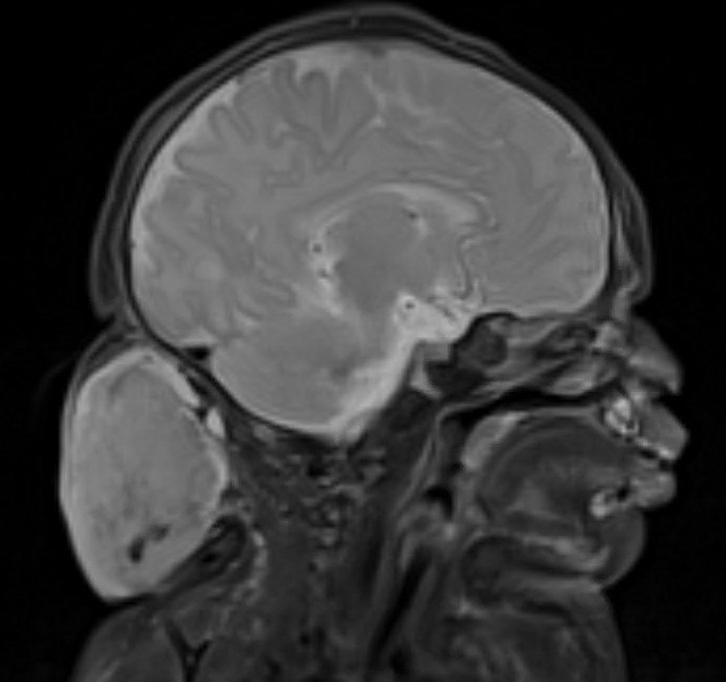
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.

Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18
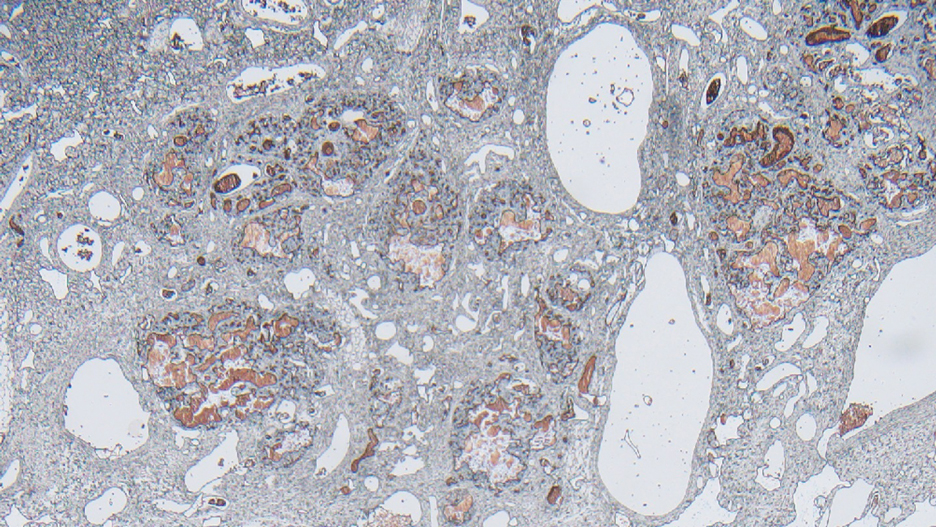
Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.


Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18


Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
The Diagnosis: Congenital Hemangioma
Surgical resection of the mass was performed at 4 months of age without complication (Figure 1). Histopathology revealed a lobular endothelial cell proliferation within a densely fibrotic stroma, multiple thin-walled vessels, and negative immunoreactivity to glucose transporter type 1 (GLUT-1)(Figures 2 and 3). Combined with the patient’s clinical history and findings on imaging (Figure 4), the most accurate diagnosis was a congenital hemangioma (CH). The mass was determined to be a noninvoluting congenital hemangioma (NICH).
A variety of vascular anomalies manifest in newborns and can be differentiated by the patient’s clinical history—particularly whether the lesion is present at birth or develops after birth. Imaging and histopathology of the lesion(s) may be utilized when clinical examination alone is not sufficient to make a diagnosis. Histopathology and immunohistochemistry further aid in differentiating the type of vascular lesion.


Overall, vascular anomalies are classified broadly into 2 categories based on their pathogenesis: tumors and malformations. Vascular tumors are composed of proliferating endothelial cells that have the potential to resolve spontaneously over time. Examples include CH, infantile hemangioma (IH), kaposiform hemangioendothelioma (KHE), and tufted angioma (TA). In contrast, vascular malformations (ie, arteriovenous malformations) are composed of dysplastic vessels with normal endothelial cell turnover and do not resolve without intervention.1
Congenital hemangiomas are rare vascular tumors that are fully developed at birth. These tumors proliferate in utero, enabling prenatal detection via ultrasonography as early as 12 weeks’ gestation for large heterogeneous vascular masses.2-4 Congenital hemangiomas are described as solitary, well-circumscribed, raised, violaceous lesions most commonly located in the head and neck region.4-6 Histopathologically, they are characterized by lobules of proliferating capillaries surrounded by fibrous stroma and dysplastic vascular channels.6,7
Congenital hemangiomas are categorized based on their postnatal involution patterns.2 Fetally involuting CH both develops and begins regression in utero and often is completely regressed at birth.8 Rapidly involuting CH begins regression in the first few weeks of life and usually is completely involuted by 14 months of age.6,9-11 Conversely, NICH does not regress, often requiring surgical excision due to functional and cosmetic issues.12,13 Partially involuting CH is intermediary, beginning as rapidly involuting but not involuting completely and persisting as lesions that resemble NICH.14-16 Although generally benign and asymptomatic, these tumors can cause transient thrombocytopenia and coagulopathy at birth, as seen in our patient.17,18


Infantile hemangioma is the most common vascular tumor of infancy.19-21 Although a precursor lesion may be present at birth, generally this tumor becomes apparent after the first few weeks of life as a solitary vascular plaque or nodule with a predilection for the head and neck.22-25 Once it arises, IH quickly enters a period of rapid growth, followed by a period of slower continued growth, with most reaching maximum size by 3 months.22 Thereafter, IH enters a slow period of involution (range, 3–9 years)26; more recent data suggest near resolution by 5 years of age.27 Infantile hemangioma is categorized based on its depth in the skin and subcutaneous tissues and can be classified as superficial, mixed, or deep.22,24,28,29 Superficial IH appears as a red plaque and may exhibit lobulation, while deep IH can be identified as flesh-colored or blue subcutaneous masses. Mixed IH may manifest with both superficial and deep features depending on the extent of its involvement in the dermal and subcutaneous layers. The pattern of involvement may be focal, segmental, or indeterminate.24 In contrast, CH typically is a solitary vascular mass with prominent telangiectases, nodules, and radiating veins.6 Histologically, IH is composed of proliferative plump endothelial cells that form capillaries, and the lesion stains positively for GLUT-1, whereas CH does not.30
Kaposiform hemangioendothelioma is classified as a locally aggressive vascular tumor that manifests either prenatally or in early infancy.31 It is described as a solitary, ill-defined, firm, purple plaque most commonly located on the extremities and retroperitoneum.32-34 Histopathologically, these lesions are characterized by dilated lymphatic channels and irregular sheets or lobules of spindle-shaped endothelial cells infiltrating the dermis and subcutaneous fat.33,35 In contrast to CH, KHE lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein.36,37 Notably, 70% of these tumors are complicated by the presence of Kasabach-Merritt phenomenon, a potentially life-threatening emergency that occurs when platelets are trapped within a vascular tumor, leading to the consumption of clotting factors, intralesional bleeding, and rapid enlargement of the tumor.32 The Kasabach-Merritt phenomenon manifests clinically as microangiopathic hemolytic anemia, severe thrombocytopenia, and disseminated intravascular coagulation. 38 Although CH lesions also can be associated with thrombocytopenia and coagulopathy, they generally are mild and self-limited.18
Tufted angioma is a vascular tumor that arises within the first 5 years of life as firm violaceous papules or plaques, often with associated hyperhidrosis or hypertrichosis.39,40 Although TA grows slowly for a period of time, it eventually stabilizes and persists, rarely regressing completely.41 These tumors share many similarities with KHE, and it has been suggested that they may be part of the same spectrum. 42 As with KHE, TA lesions show immunoreactivity to the markers podoplanin, lymphatic vessel endothelial receptor 1, and prospero homeobox 1 protein, which are negative in CH.36,37 Although TA also can be complicated by Kasabach-Merritt phenomenon, the incidence is much lower (up to 38%).43,44 As such, TAs tend to be recognized as more superficial benign lesions. However, they still can cause notable cosmetic and functional impairment and should be monitored closely, especially in the presence of associated symptoms or complications.
Arteriovenous malformation is a vascular lesion that results from errors during the embryonic development of vascular channels.45 Although present at birth, it may not become clinically apparent until later in life. Arteriovenous malformations enlarge postnatally, and their growth is proportional to the developmental growth of the affected individual rather than the result of endothelial proliferation.46 In infants, AVM may manifest as a faint vascular stain that can evolve over time into a pink patch associated with a palpable thrill during adolescence. 4 On Doppler flow imaging, AVMs are identified as fast-flow anomalies arising from an abnormal communication between high-pressure arterial systems and low-pressure venous systems without the presence of a capillary bed.47 One of the differentiating factors between AVM and CH is that AVMs do not regress spontaneously and tend to have high recurrence rates, even with intervention. 48 In contrast, CH can be categorized based on its postnatal involution pattern. Another distinguishing factor is that AVMs tend to be larger and more invasive than CHs.46 Therefore, early diagnosis and intervention are crucial to prevent complications such as bleeding, seizures, or neurologic deficits associated with AVMs.1
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
- Enjolras O, Wassef M, Chapot R. Introduction: ISSVA Classification. In: Enjolras O, Wassef M, Chapot R, eds. Color Atlas of Vascular Tumors and Vascular Malformations. Cambridge University Press; 2007:3-11.
- Fadell MF, Jones BV, Adams DM. Prenatal diagnosis and postnatal follow-up of rapidly involuting congenital hemangioma (RICH). Pediatr Radiol. 2011;41:1057-1060.
- Feygin T, Khalek N, Moldenhauer JS. Fetal brain, head, and neck tumors: prenatal imaging and management. Prenat Diagn. 2020;40:1203-1219.
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr. 2015;62:227-255.
- Bruder E, Alaggio R, Kozakewich HPW, et al. Vascular and perivascular lesions of skin and soft tissues in children and adolescents. Pediatr Dev Pathol. 2012;15:26-61.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Maguiness S, Uihlein LC, Liang MG, et al. Rapidly involuting congenital hemangioma with fetal involution. Pediatr Dermatol. 2015;32:321-326.
- Keating LJ, Soares GM, Muratore CS. Rapidly involuting congenital hemangioma. Med Health R I. 2012;95:149-152.
- Schafer F, Tapia M, Pinto C. Rapidly involuting congenital haemangioma. Arch Dis Child Fetal Neonatal Ed. 2014;99:F422.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:E203-E214.
- Boull C, Maguiness SM. Congenital hemangiomas. Semin Cutan Med Surg. 2016;35:124-127.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Baselga E, Cordisco MR, Garzon M, et al. Rapidly involuting congenital haemangioma associated with transient thrombocytopenia and coagulopathy: a case series. Br J Dermatol. 2008;158:1363-1370.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367.
- Hidano A, Nakajima S. Earliest features of the strawberry mark in the newborn. Br J Dermatol. 1972;87:138-144.
- Martinez-Perez D, Fein NA, Boon LM, et al. Not all hemangiomas look like strawberries: uncommon presentations of the most common tumor of infancy. Pediatr Dermatol. 1995;12:1-6.
- Payne MM, Moyer F, Marcks KM, et al. The precursor to the hemangioma. Plast Reconstr Surg. 1966;38:64-67.
- Bowers RE, Graham EA, Tomlinson KM. The natural history of the strawberry nevus. Arch Dermatol. 1960;82:667-680.
- Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619-624.
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173-181.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Gruman A, Liang MG, Mulliken JB, et al. Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon. J Am Acad Dermatol. 2005;52:616-622.
- Croteau SE, Liang MG, Kozakewich HP, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach- Merritt phenomenon in 107 referrals. J Pediatr. 2013;162:142-147.
- Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. an aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321-328.
- Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol. 2001;14:1087-1092.
- O’Rafferty C, O’Regan GM, Irvine AD, et al. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51.
- Le Huu AR, Jokinen CH, Rubin BP, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563-1573.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immuno-histochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454-1460.
- Haisley-Royster C, Enjolras O, Frieden IJ, et al. Kasabach-Merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459-462.
- Wilmer A, Kaatz M, Bocker T, et al. Tufted angioma. Eur J Dermatol. 1999;9:51-53.
- Herron MD, Coffin CM, Vanderhooft SL. Tufted angiomas: variability of the clinical morphology. Pediatr Dermatol. 2002;19:394-401.
- North PE. Pediatric vascular tumors and malformations. Surg Pathol Clin. 2010,3:455-494.
- Chu CY, Hsiao CH, Chiu HC. Transformation between kaposiform hemangioendothelioma and tufted angioma. Dermatology. 2003;206:334-337.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Johnson EF, Davis DM, Tollefson MM, et al. Vascular tumors in infants: case report and review of clinical, histopathologic, and immunohistochemical characteristics of infantile hemangioma, pyogenic granuloma, noninvoluting congenital hemangioma, tufted angioma, and kaposiform hemangioendothelioma. Am J Dermatopathol. 2018;40:231-239.
- Christison-Lagay ER, Fishman SJ. Vascular anomalies. Surg Clin North Am. 2006;86:393-425.
- Liu AS, Mulliken JB, Zurakowski D, et al. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194.
- Young AE, Mulliken JB. Arteriovenous malformations. In: Mulliken JB, Young AE, eds. Vascular Birthmarks: Haemangiomas and Malformations. WB Saunders; 1988:228-245.
- Duggan EM, Fishman SJ. Vascular anomalies. In: Holcomb GW III, Murphy JP, St Peter SD, eds. Holcomb and Ashcraft’s Pediatric Surgery. 7th edition. Elsevier; 2019:1147-1170.
A newborn male was delivered via cesarean section at 38 weeks 5 days’ gestation with a large vascular mass on the posterior neck. The mass previously had been identified on a 23-week prenatal ultrasound. Physical examination by dermatology at birth revealed a well-defined violaceous mass measuring 6×5 cm with prominent radiating veins, coarse telangiectases, and a pale rim. Magnetic resonance imaging demonstrated a well-circumscribed mass with avid arterial phase enhancement. The patient experienced transient thrombocytopenia that resolved following administration of methylprednisolone. No evidence of rapid involution was noted after 3 months of observation.
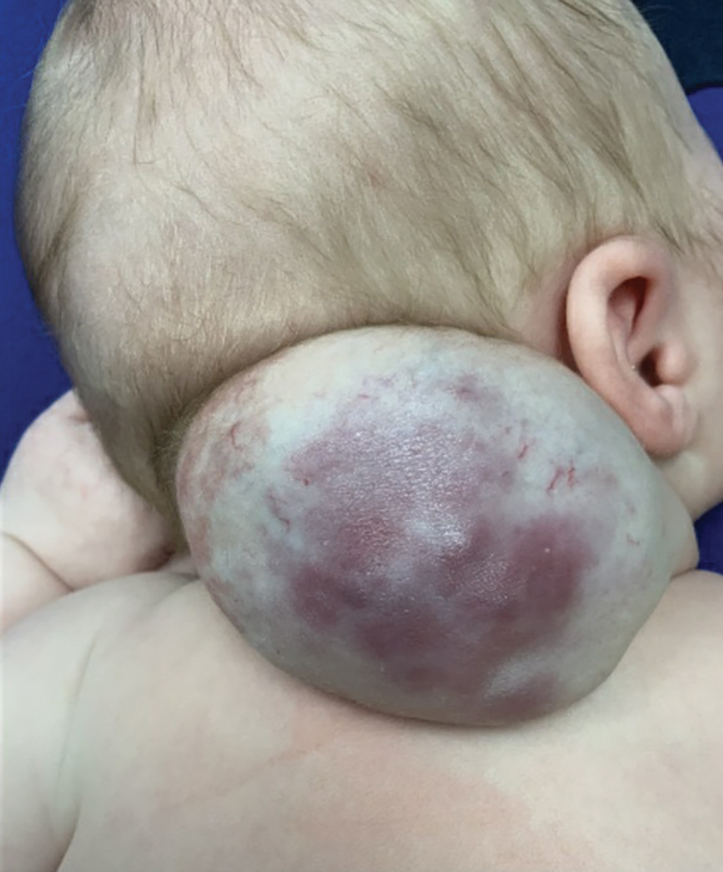
The Future of Obesity
I am not planning on having a headstone on my grave, or even having a grave for that matter. However, if my heirs decide to ignore my wishes and opt for some pithy observation chiseled into a tastefully sized granite block, I suspect they might choose “He always knew which way the wind was blowing ... but wasn’t so sure about the tides.” Which aptly describes both my navigational deficiencies they have observed here over my six decades on the Maine coast as well as my general inability to predict the future. Nonetheless, I am going to throw caution to the wind and take this opportunity to ponder where obesity in this country will go over the next couple of decades.
In March of last year the London-based World Obesity Federation published its World Obesity Atlas. In the summary the authors predict that based on current trends “obesity will cost the global economy of US $4 trillion of potential income in 2035, nearly 3% of current global domestic product (GDP).” They envision the “rising prevalence of obesity to be steepest among children and adolescents rising from 10% to 20% of the world’s boys during the period 2029 to 2035, and rising fro 8% to 18% of the world’s girls.”
These dire predictions assume no significant measures to reverse this trajectory such as universal health coverage. Nor do the authors attempt to predict the effect of the growing use of GLP-1 agonists. This omission is surprising and somewhat refreshing given the fact that the project was funded by an unrestricted grant from Novo Nordisk, a major producer of one of these drugs.
Unfortunately, I think it is unlikely that over the next couple of decades any large countries who do not already have a functioning universal health care system will find the political will to develop one capable of reversing the trend toward obesity. Certainly, I don’t see it in the cards for this country.
On the other hand, I can foresee the availability and ease of administration for GLP-1 agonists and similar drugs improving over the near term. However, the cost and availability will continue to widen the separation between the haves and the have-nots, both globally and within each country. This will mean that the countries and population subgroups that already experience the bulk of the economic and health consequences of obesity will continue to shoulder an outsized burden of this “disease.”
It is unclear how much this widening of the fat-getting-fatter dynamic will add to the global and national political unrest that already seems to be tracking the effects of climate change. However, I can’t imaging it is going to be a calming or uniting force.
Narrowing our focus from an international to an individual resource-rich country such as the United States, let’s consider what the significant growth in availability and affordability of GLP-1 agonist drugs will mean. There will certainly be short-term improvements in the morbidity and mortality of some of the obesity related diseases. However, for other conditions it may take longer than two decades for us to notice an effect. While it is tempting to consider these declines as a financial boon for the country that already spends a high percentage of its GDP on healthcare. However, as the well-known Saturday Night Live pundit Roseanne Roseannadanna often observed, ”it’s always something ... if it’s not one thing it’s another.” There may be other non-obesity conditions that surge to fill the gap, leaving us still with a substantial financial burden for healthcare.
Patients taking GLP-1 agonists lose weight because they feel full and eat less food. While currently the number of patients taking these drugs is relatively small, the effect on this country’s food consumption is too small to calculate. However, let’s assume that 20 years from now half of the obese patients are taking appetite blunting medication. Using today’s statistics this means that 50 million adults will be eating significantly less food. Will the agriculturists have gradually adjusted to produce less food? Will this mean there is more food for the those experiencing “food insecurity”? I doubt it. Most food insecurity seems to be a problem of distribution and inequality, not supply.
Physicians now caution patients taking GLP-1 agonists to eat a healthy and balanced diet. When the drugs are more commonly available, will this caution be heeded by the majority? Will we see a population that may no longer be obese but nonetheless malnourished because of bad choices?
And, finally, in a similar vein, will previously obese individuals suddenly or gradually begin to be more physically active once the appetite blunting medicines have helped them lose weight? Here, I have my doubts. Of course, some leaner individuals begin to take advantage of their new body morphology. But, I fear that old sedentary habits will die very slowly for most, and not at all for many. We have built a vehicle-centric society in which being physically active requires making a conscious effort. Electronic devices and sedentary entertainment options are not going to disappear just because a significant percentage of the population is no longer obese.
So there you have it. I suspect that I am correct about which way some of the winds are blowing as the obesity becomes moves into its treatable “disease” phase. But, as always, I haven’t a clue which way the tide is running.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I am not planning on having a headstone on my grave, or even having a grave for that matter. However, if my heirs decide to ignore my wishes and opt for some pithy observation chiseled into a tastefully sized granite block, I suspect they might choose “He always knew which way the wind was blowing ... but wasn’t so sure about the tides.” Which aptly describes both my navigational deficiencies they have observed here over my six decades on the Maine coast as well as my general inability to predict the future. Nonetheless, I am going to throw caution to the wind and take this opportunity to ponder where obesity in this country will go over the next couple of decades.
In March of last year the London-based World Obesity Federation published its World Obesity Atlas. In the summary the authors predict that based on current trends “obesity will cost the global economy of US $4 trillion of potential income in 2035, nearly 3% of current global domestic product (GDP).” They envision the “rising prevalence of obesity to be steepest among children and adolescents rising from 10% to 20% of the world’s boys during the period 2029 to 2035, and rising fro 8% to 18% of the world’s girls.”
These dire predictions assume no significant measures to reverse this trajectory such as universal health coverage. Nor do the authors attempt to predict the effect of the growing use of GLP-1 agonists. This omission is surprising and somewhat refreshing given the fact that the project was funded by an unrestricted grant from Novo Nordisk, a major producer of one of these drugs.
Unfortunately, I think it is unlikely that over the next couple of decades any large countries who do not already have a functioning universal health care system will find the political will to develop one capable of reversing the trend toward obesity. Certainly, I don’t see it in the cards for this country.
On the other hand, I can foresee the availability and ease of administration for GLP-1 agonists and similar drugs improving over the near term. However, the cost and availability will continue to widen the separation between the haves and the have-nots, both globally and within each country. This will mean that the countries and population subgroups that already experience the bulk of the economic and health consequences of obesity will continue to shoulder an outsized burden of this “disease.”
It is unclear how much this widening of the fat-getting-fatter dynamic will add to the global and national political unrest that already seems to be tracking the effects of climate change. However, I can’t imaging it is going to be a calming or uniting force.
Narrowing our focus from an international to an individual resource-rich country such as the United States, let’s consider what the significant growth in availability and affordability of GLP-1 agonist drugs will mean. There will certainly be short-term improvements in the morbidity and mortality of some of the obesity related diseases. However, for other conditions it may take longer than two decades for us to notice an effect. While it is tempting to consider these declines as a financial boon for the country that already spends a high percentage of its GDP on healthcare. However, as the well-known Saturday Night Live pundit Roseanne Roseannadanna often observed, ”it’s always something ... if it’s not one thing it’s another.” There may be other non-obesity conditions that surge to fill the gap, leaving us still with a substantial financial burden for healthcare.
Patients taking GLP-1 agonists lose weight because they feel full and eat less food. While currently the number of patients taking these drugs is relatively small, the effect on this country’s food consumption is too small to calculate. However, let’s assume that 20 years from now half of the obese patients are taking appetite blunting medication. Using today’s statistics this means that 50 million adults will be eating significantly less food. Will the agriculturists have gradually adjusted to produce less food? Will this mean there is more food for the those experiencing “food insecurity”? I doubt it. Most food insecurity seems to be a problem of distribution and inequality, not supply.
Physicians now caution patients taking GLP-1 agonists to eat a healthy and balanced diet. When the drugs are more commonly available, will this caution be heeded by the majority? Will we see a population that may no longer be obese but nonetheless malnourished because of bad choices?
And, finally, in a similar vein, will previously obese individuals suddenly or gradually begin to be more physically active once the appetite blunting medicines have helped them lose weight? Here, I have my doubts. Of course, some leaner individuals begin to take advantage of their new body morphology. But, I fear that old sedentary habits will die very slowly for most, and not at all for many. We have built a vehicle-centric society in which being physically active requires making a conscious effort. Electronic devices and sedentary entertainment options are not going to disappear just because a significant percentage of the population is no longer obese.
So there you have it. I suspect that I am correct about which way some of the winds are blowing as the obesity becomes moves into its treatable “disease” phase. But, as always, I haven’t a clue which way the tide is running.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I am not planning on having a headstone on my grave, or even having a grave for that matter. However, if my heirs decide to ignore my wishes and opt for some pithy observation chiseled into a tastefully sized granite block, I suspect they might choose “He always knew which way the wind was blowing ... but wasn’t so sure about the tides.” Which aptly describes both my navigational deficiencies they have observed here over my six decades on the Maine coast as well as my general inability to predict the future. Nonetheless, I am going to throw caution to the wind and take this opportunity to ponder where obesity in this country will go over the next couple of decades.
In March of last year the London-based World Obesity Federation published its World Obesity Atlas. In the summary the authors predict that based on current trends “obesity will cost the global economy of US $4 trillion of potential income in 2035, nearly 3% of current global domestic product (GDP).” They envision the “rising prevalence of obesity to be steepest among children and adolescents rising from 10% to 20% of the world’s boys during the period 2029 to 2035, and rising fro 8% to 18% of the world’s girls.”
These dire predictions assume no significant measures to reverse this trajectory such as universal health coverage. Nor do the authors attempt to predict the effect of the growing use of GLP-1 agonists. This omission is surprising and somewhat refreshing given the fact that the project was funded by an unrestricted grant from Novo Nordisk, a major producer of one of these drugs.
Unfortunately, I think it is unlikely that over the next couple of decades any large countries who do not already have a functioning universal health care system will find the political will to develop one capable of reversing the trend toward obesity. Certainly, I don’t see it in the cards for this country.
On the other hand, I can foresee the availability and ease of administration for GLP-1 agonists and similar drugs improving over the near term. However, the cost and availability will continue to widen the separation between the haves and the have-nots, both globally and within each country. This will mean that the countries and population subgroups that already experience the bulk of the economic and health consequences of obesity will continue to shoulder an outsized burden of this “disease.”
It is unclear how much this widening of the fat-getting-fatter dynamic will add to the global and national political unrest that already seems to be tracking the effects of climate change. However, I can’t imaging it is going to be a calming or uniting force.
Narrowing our focus from an international to an individual resource-rich country such as the United States, let’s consider what the significant growth in availability and affordability of GLP-1 agonist drugs will mean. There will certainly be short-term improvements in the morbidity and mortality of some of the obesity related diseases. However, for other conditions it may take longer than two decades for us to notice an effect. While it is tempting to consider these declines as a financial boon for the country that already spends a high percentage of its GDP on healthcare. However, as the well-known Saturday Night Live pundit Roseanne Roseannadanna often observed, ”it’s always something ... if it’s not one thing it’s another.” There may be other non-obesity conditions that surge to fill the gap, leaving us still with a substantial financial burden for healthcare.
Patients taking GLP-1 agonists lose weight because they feel full and eat less food. While currently the number of patients taking these drugs is relatively small, the effect on this country’s food consumption is too small to calculate. However, let’s assume that 20 years from now half of the obese patients are taking appetite blunting medication. Using today’s statistics this means that 50 million adults will be eating significantly less food. Will the agriculturists have gradually adjusted to produce less food? Will this mean there is more food for the those experiencing “food insecurity”? I doubt it. Most food insecurity seems to be a problem of distribution and inequality, not supply.
Physicians now caution patients taking GLP-1 agonists to eat a healthy and balanced diet. When the drugs are more commonly available, will this caution be heeded by the majority? Will we see a population that may no longer be obese but nonetheless malnourished because of bad choices?
And, finally, in a similar vein, will previously obese individuals suddenly or gradually begin to be more physically active once the appetite blunting medicines have helped them lose weight? Here, I have my doubts. Of course, some leaner individuals begin to take advantage of their new body morphology. But, I fear that old sedentary habits will die very slowly for most, and not at all for many. We have built a vehicle-centric society in which being physically active requires making a conscious effort. Electronic devices and sedentary entertainment options are not going to disappear just because a significant percentage of the population is no longer obese.
So there you have it. I suspect that I am correct about which way some of the winds are blowing as the obesity becomes moves into its treatable “disease” phase. But, as always, I haven’t a clue which way the tide is running.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Reducing Unnecessary Antibiotics for Conjunctivitis
TOPLINE:
More than two thirds of children with conjunctivitis received antibiotics within a day of their initial ambulatory care visit; however,
METHODOLOGY:
- Researchers evaluated the frequency of topical antibiotic treatment and its association with subsequent health care use among commercially insured children with acute infectious conjunctivitis in the United States.
- This cohort study analyzed data from the 2021 MarketScan Commercial Claims and Encounters Database, including 44,793 children with conjunctivitis (median age, 5 years; 47% girls) and ambulatory care encounters.
- The primary exposure was a topical antibiotic prescription dispensed within 1 day of an ambulatory care visit, with outcomes assessed 2-14 days after the visit.
- The primary outcomes were ambulatory care revisits for conjunctivitis and same-day dispensation of a new topical antibiotic, and secondary outcomes included emergency department revisits and hospitalizations.
TAKEAWAY:
- Topical antibiotics were dispensed within a day of an ambulatory care visit in 69% of the cases; however, they were less frequently dispensed following visits to eye clinics (34%), for children aged 6-11 years (66%), and for those with viral conjunctivitis (28%).
- Ambulatory care revisits for conjunctivitis within 2 weeks occurred in only 3.2% of children who had received antibiotics (adjusted odds ratio [aOR], 1.11; 95% CI, 0.99-1.25).
- Similarly, revisits with same-day dispensation of a new antibiotic were also rare (1.4%), with no significant association between antibiotic treatment and revisits (aOR, 1.10; 95% CI, 0.92-1.33).
- Hospitalizations for conjunctivitis occurred in 0.03% of cases, and emergency department revisits occurred in 0.12%, with no differences between children who received antibiotics and those who did not.
IN PRACTICE:
“Given that antibiotics may not be associated with improved outcomes or change in subsequent health care use and are associated with adverse effects and antibiotic resistance, efforts to reduce overtreatment of acute infectious conjunctivitis are warranted,” the authors wrote.
SOURCE:
The study was led by Daniel J. Shapiro, MD, MPH, of the Department of Emergency Medicine at the University of California, San Francisco, and published online on June 27, 2024, in JAMA Ophthalmology.
LIMITATIONS:
The major limitations of the study included the inability to distinguish scheduled visits from unscheduled revisits, incomplete clinical data such as rare complications of conjunctivitis, and the inability to confirm the accuracy of the coded diagnosis of infectious conjunctivitis, especially in children who did not receive a thorough eye examination.
DISCLOSURES:
This study did not declare receiving funding from any sources. One author reported receiving grants from several sources outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
More than two thirds of children with conjunctivitis received antibiotics within a day of their initial ambulatory care visit; however,
METHODOLOGY:
- Researchers evaluated the frequency of topical antibiotic treatment and its association with subsequent health care use among commercially insured children with acute infectious conjunctivitis in the United States.
- This cohort study analyzed data from the 2021 MarketScan Commercial Claims and Encounters Database, including 44,793 children with conjunctivitis (median age, 5 years; 47% girls) and ambulatory care encounters.
- The primary exposure was a topical antibiotic prescription dispensed within 1 day of an ambulatory care visit, with outcomes assessed 2-14 days after the visit.
- The primary outcomes were ambulatory care revisits for conjunctivitis and same-day dispensation of a new topical antibiotic, and secondary outcomes included emergency department revisits and hospitalizations.
TAKEAWAY:
- Topical antibiotics were dispensed within a day of an ambulatory care visit in 69% of the cases; however, they were less frequently dispensed following visits to eye clinics (34%), for children aged 6-11 years (66%), and for those with viral conjunctivitis (28%).
- Ambulatory care revisits for conjunctivitis within 2 weeks occurred in only 3.2% of children who had received antibiotics (adjusted odds ratio [aOR], 1.11; 95% CI, 0.99-1.25).
- Similarly, revisits with same-day dispensation of a new antibiotic were also rare (1.4%), with no significant association between antibiotic treatment and revisits (aOR, 1.10; 95% CI, 0.92-1.33).
- Hospitalizations for conjunctivitis occurred in 0.03% of cases, and emergency department revisits occurred in 0.12%, with no differences between children who received antibiotics and those who did not.
IN PRACTICE:
“Given that antibiotics may not be associated with improved outcomes or change in subsequent health care use and are associated with adverse effects and antibiotic resistance, efforts to reduce overtreatment of acute infectious conjunctivitis are warranted,” the authors wrote.
SOURCE:
The study was led by Daniel J. Shapiro, MD, MPH, of the Department of Emergency Medicine at the University of California, San Francisco, and published online on June 27, 2024, in JAMA Ophthalmology.
LIMITATIONS:
The major limitations of the study included the inability to distinguish scheduled visits from unscheduled revisits, incomplete clinical data such as rare complications of conjunctivitis, and the inability to confirm the accuracy of the coded diagnosis of infectious conjunctivitis, especially in children who did not receive a thorough eye examination.
DISCLOSURES:
This study did not declare receiving funding from any sources. One author reported receiving grants from several sources outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
More than two thirds of children with conjunctivitis received antibiotics within a day of their initial ambulatory care visit; however,
METHODOLOGY:
- Researchers evaluated the frequency of topical antibiotic treatment and its association with subsequent health care use among commercially insured children with acute infectious conjunctivitis in the United States.
- This cohort study analyzed data from the 2021 MarketScan Commercial Claims and Encounters Database, including 44,793 children with conjunctivitis (median age, 5 years; 47% girls) and ambulatory care encounters.
- The primary exposure was a topical antibiotic prescription dispensed within 1 day of an ambulatory care visit, with outcomes assessed 2-14 days after the visit.
- The primary outcomes were ambulatory care revisits for conjunctivitis and same-day dispensation of a new topical antibiotic, and secondary outcomes included emergency department revisits and hospitalizations.
TAKEAWAY:
- Topical antibiotics were dispensed within a day of an ambulatory care visit in 69% of the cases; however, they were less frequently dispensed following visits to eye clinics (34%), for children aged 6-11 years (66%), and for those with viral conjunctivitis (28%).
- Ambulatory care revisits for conjunctivitis within 2 weeks occurred in only 3.2% of children who had received antibiotics (adjusted odds ratio [aOR], 1.11; 95% CI, 0.99-1.25).
- Similarly, revisits with same-day dispensation of a new antibiotic were also rare (1.4%), with no significant association between antibiotic treatment and revisits (aOR, 1.10; 95% CI, 0.92-1.33).
- Hospitalizations for conjunctivitis occurred in 0.03% of cases, and emergency department revisits occurred in 0.12%, with no differences between children who received antibiotics and those who did not.
IN PRACTICE:
“Given that antibiotics may not be associated with improved outcomes or change in subsequent health care use and are associated with adverse effects and antibiotic resistance, efforts to reduce overtreatment of acute infectious conjunctivitis are warranted,” the authors wrote.
SOURCE:
The study was led by Daniel J. Shapiro, MD, MPH, of the Department of Emergency Medicine at the University of California, San Francisco, and published online on June 27, 2024, in JAMA Ophthalmology.
LIMITATIONS:
The major limitations of the study included the inability to distinguish scheduled visits from unscheduled revisits, incomplete clinical data such as rare complications of conjunctivitis, and the inability to confirm the accuracy of the coded diagnosis of infectious conjunctivitis, especially in children who did not receive a thorough eye examination.
DISCLOSURES:
This study did not declare receiving funding from any sources. One author reported receiving grants from several sources outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Exercise Plus GLP-1 RAs Upped Weight Loss, Bone Retention
TOPLINE:
People with obesity who exercise while taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs; liraglutide) showed increased weight loss and preserved bone health, according to a study published in JAMA Network Open.
METHODOLOGY:
- Patients were placed on an initial diet that consisted of no more than 800 calories per day for 8 weeks. Those who lost at least 5% of their starting weight were then placed into a 1-year program.
- Participants included 195 adults aged between 18 and 65 years with obesity and no diabetes, 64% of whom were women.
- They were split into four groups of interventions: Exercise only (48 patients), liraglutide only (49 patients), a combination of both (49 participants), and placebo (49 participants), for a 1-year period.
- Patients received liraglutide or volume-matched placebo as daily injections starting at 0.6 mg/d with a weekly increase until 3 mg/d was reached; exercise entailed 30-minute sessions for 4 days a week.
- Researchers studied bone health at each patient’s hip, spine, and forearm after they lost weight, by measuring bone mineral density (BMD).
TAKEAWAY:
- The overall average change in weight loss over the course of 52 weeks was 7.03 kg in the placebo group, 11.19 kg in the exercise group, 13.74 kg in the liraglutide group, and 16.88 kg in the combination group.
- BMD did not change in the combination group in comparison to the placebo group at the hip (mean change, −0.006 g/cm2; 95% CI, −0.017 to 0.004 g/cm2; P = .24) or spine (−0.010 g/cm2; 95% CI, −0.025 to 0.005 g/cm2; P = .20).
- BMD of the spine in the liraglutide group decreased in comparison to the exercise group (mean change, −0.016 g/cm2; 95% CI, −0.032 to −0.001 g/cm2; P = .04) and the placebo group, in addition to decreases in the hip.
IN PRACTICE:
“Our results show that the combination of exercise and GLP-1 RA was the most effective weight loss strategy while preserving bone health,” study authors wrote.
SOURCE:
The study was led by Simon Birk Kjær Jensen, PhD, of the Department of Biomedical Sciences and Faculty of Health and Medical Sciences at the University of Copenhagen in Denmark, and published on June 25 in JAMA Network Open.
LIMITATIONS:
The study only included adults aged between 18 and 65 years without other chronic diseases and may not apply to patients who are older or have diabetes. The study sample was diverse but was conducted in Denmark, with a population of generally similar ancestry.
DISCLOSURES:
One study author reported serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Bayer, and Amgen, among others. Other authors reported various financial interests, including grants, personal fees, and salaries, from Amgen, Novo Nordisk, and Abbott Lab, among others.
A version of this article first appeared on Medscape.com.
TOPLINE:
People with obesity who exercise while taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs; liraglutide) showed increased weight loss and preserved bone health, according to a study published in JAMA Network Open.
METHODOLOGY:
- Patients were placed on an initial diet that consisted of no more than 800 calories per day for 8 weeks. Those who lost at least 5% of their starting weight were then placed into a 1-year program.
- Participants included 195 adults aged between 18 and 65 years with obesity and no diabetes, 64% of whom were women.
- They were split into four groups of interventions: Exercise only (48 patients), liraglutide only (49 patients), a combination of both (49 participants), and placebo (49 participants), for a 1-year period.
- Patients received liraglutide or volume-matched placebo as daily injections starting at 0.6 mg/d with a weekly increase until 3 mg/d was reached; exercise entailed 30-minute sessions for 4 days a week.
- Researchers studied bone health at each patient’s hip, spine, and forearm after they lost weight, by measuring bone mineral density (BMD).
TAKEAWAY:
- The overall average change in weight loss over the course of 52 weeks was 7.03 kg in the placebo group, 11.19 kg in the exercise group, 13.74 kg in the liraglutide group, and 16.88 kg in the combination group.
- BMD did not change in the combination group in comparison to the placebo group at the hip (mean change, −0.006 g/cm2; 95% CI, −0.017 to 0.004 g/cm2; P = .24) or spine (−0.010 g/cm2; 95% CI, −0.025 to 0.005 g/cm2; P = .20).
- BMD of the spine in the liraglutide group decreased in comparison to the exercise group (mean change, −0.016 g/cm2; 95% CI, −0.032 to −0.001 g/cm2; P = .04) and the placebo group, in addition to decreases in the hip.
IN PRACTICE:
“Our results show that the combination of exercise and GLP-1 RA was the most effective weight loss strategy while preserving bone health,” study authors wrote.
SOURCE:
The study was led by Simon Birk Kjær Jensen, PhD, of the Department of Biomedical Sciences and Faculty of Health and Medical Sciences at the University of Copenhagen in Denmark, and published on June 25 in JAMA Network Open.
LIMITATIONS:
The study only included adults aged between 18 and 65 years without other chronic diseases and may not apply to patients who are older or have diabetes. The study sample was diverse but was conducted in Denmark, with a population of generally similar ancestry.
DISCLOSURES:
One study author reported serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Bayer, and Amgen, among others. Other authors reported various financial interests, including grants, personal fees, and salaries, from Amgen, Novo Nordisk, and Abbott Lab, among others.
A version of this article first appeared on Medscape.com.
TOPLINE:
People with obesity who exercise while taking glucagon-like peptide 1 receptor agonists (GLP-1 RAs; liraglutide) showed increased weight loss and preserved bone health, according to a study published in JAMA Network Open.
METHODOLOGY:
- Patients were placed on an initial diet that consisted of no more than 800 calories per day for 8 weeks. Those who lost at least 5% of their starting weight were then placed into a 1-year program.
- Participants included 195 adults aged between 18 and 65 years with obesity and no diabetes, 64% of whom were women.
- They were split into four groups of interventions: Exercise only (48 patients), liraglutide only (49 patients), a combination of both (49 participants), and placebo (49 participants), for a 1-year period.
- Patients received liraglutide or volume-matched placebo as daily injections starting at 0.6 mg/d with a weekly increase until 3 mg/d was reached; exercise entailed 30-minute sessions for 4 days a week.
- Researchers studied bone health at each patient’s hip, spine, and forearm after they lost weight, by measuring bone mineral density (BMD).
TAKEAWAY:
- The overall average change in weight loss over the course of 52 weeks was 7.03 kg in the placebo group, 11.19 kg in the exercise group, 13.74 kg in the liraglutide group, and 16.88 kg in the combination group.
- BMD did not change in the combination group in comparison to the placebo group at the hip (mean change, −0.006 g/cm2; 95% CI, −0.017 to 0.004 g/cm2; P = .24) or spine (−0.010 g/cm2; 95% CI, −0.025 to 0.005 g/cm2; P = .20).
- BMD of the spine in the liraglutide group decreased in comparison to the exercise group (mean change, −0.016 g/cm2; 95% CI, −0.032 to −0.001 g/cm2; P = .04) and the placebo group, in addition to decreases in the hip.
IN PRACTICE:
“Our results show that the combination of exercise and GLP-1 RA was the most effective weight loss strategy while preserving bone health,” study authors wrote.
SOURCE:
The study was led by Simon Birk Kjær Jensen, PhD, of the Department of Biomedical Sciences and Faculty of Health and Medical Sciences at the University of Copenhagen in Denmark, and published on June 25 in JAMA Network Open.
LIMITATIONS:
The study only included adults aged between 18 and 65 years without other chronic diseases and may not apply to patients who are older or have diabetes. The study sample was diverse but was conducted in Denmark, with a population of generally similar ancestry.
DISCLOSURES:
One study author reported serving on advisory boards for AstraZeneca, Boehringer Ingelheim, Bayer, and Amgen, among others. Other authors reported various financial interests, including grants, personal fees, and salaries, from Amgen, Novo Nordisk, and Abbott Lab, among others.
A version of this article first appeared on Medscape.com.
Does Semaglutide Reduce Inflammation?
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.
LYON, FRANCE — The anti-obesity drug semaglutide is associated with significant reductions in the inflammatory marker high-sensitivity C-reactive protein (CRP), even in patients who do not lose substantial amounts of weight with the drug, according to data from the SELECT clinical trial.
The research, presented at the European Atherosclerosis Society 2024, involved over 17,600 patients with overweight or obesity and had established cardiovascular disease but not diabetes.
“Weight loss was associated with greater high-sensitivity CRP reduction in both treatment groups,” said study presenter Jorge Plutzky, MD, director of Preventive Cardiology at Brigham and Women’s Hospital, Boston, but “with increased high-sensitivity CRP reductions in those receiving semaglutide.”
The drug also “significantly reduced high-sensitivity CRP early,” he said, “prior to major weight loss and in those who did not lose significant amounts of weight.” The reductions reached approximately 12% at 4 weeks and around 20% at 8 weeks, when the weight loss “was still quite modest,” at 2% and 3% of body weight, respectively. Even among patients who achieved weight loss of less than 2% body weight, semaglutide was associated with a reduction in high-sensitivity CRP levels.
In the SELECT trial, semaglutide also resulted in a consistent reduction of around 20% vs placebo in major adverse cardiovascular events such as cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke.
But Naveed Sattar, MD, PhD, professor of cardiometabolic medicine at the University of Glasgow, Scotland, said in an interview that body weight “is probably the major driver” of CRP levels in the population, accounting for between 20% and 30% of the variation.
Dr. Sattar, who was not involved in the study, said that because drugs like semaglutide lower weight but also have anti-inflammatory effects, the question becomes: “Could the anti-inflammatory effects be part of the mechanisms by which these drugs affect the risk of major adverse cardiovascular events?”
Reducing Cardiovascular Events
The current analysis, however, cannot answer the question, he said. “All it tells us is about associations.”
“What we do know is semaglutide, predominantly by lowering weight, is lowering CRP levels and equally, we know that when you lose weight, you improve blood pressure, you improve lipids, and you reduce the risk of diabetes,” he said.
Dr. Sattar also took issue with the researchers’ conclusion that the high-sensitivity CRP reductions seen in SELECT occurred prior to major weight loss because the “pattern of CRP reduction and weight reduction is almost identical.”
Dr. Sattar also pointed out in a recent editorial that the drug appears to have a direct effect on blood vessels and the heart, which may lead to improvements in systemic inflammation. Consequently, he said, any assertion that semaglutide is genuinely anti-inflammatory is, at this stage, “speculation.”
Dr. Plutzky said that “systemic, chronic inflammation is implicated as a potential mechanism and therapeutic target in atherosclerosis and major adverse cardiovascular events, as well as obesity,” and high-sensitivity CRP levels are an “established biomarker of inflammation and have been shown to predict cardiovascular risk.”
However, the relationship between high-sensitivity CRP, responses to glucagon-like peptide 1 receptor agonists like semaglutide, and cardiovascular outcomes in obesity “remains incompletely understood,” said Dr. Plutzky.
A version of this article appeared on Medscape.com.