User login
Vitamin D status may play a pivotal role in colon cancer prevention
This is according to an observational study published in the journal Gastroenterology. The study included 94,205 women (aged 25-42 years) who were followed between 1991 and 2015 during which 111 incident cases of early-onset colorectal cancer were diagnosed. Among 29,186 women who had at least one lower endoscopy from 1991 to 2011, 1,439 newly diagnosed conventional adenomas and 1,878 serrated polyps were found.
Women who consumed the highest average levels of total vitamin D of 450 IU per day, compared with those consuming less than 300 IU per day, showed a significantly reduced risk of early-onset colorectal cancer. Consuming 400 IU each day was associated with a 54% reduced risk of early-onset colorectal cancer.
“If confirmed, our findings could potentially lead to recommendations for higher vitamin D intake as an inexpensive low-risk complement to colorectal cancer screening as a prevention strategy for adults younger than age 50,” wrote the study authors, led by Edward L. Giovannucci, MD, ScD, of the Harvard School of Public Health, Boston.
Associations between vitamin D levels and colorectal cancer have been documented in review articles over the years. The link is the subject of 10 recently completed or ongoing clinical trials. Few studies have focused on early colorectal cancer and vitamin D intake. Unlike advanced colorectal cancer, the early-onset form of the disease is not as strongly associated with the traditional risk factors of a family history of colorectal cancer and it is therefore believed to be more strongly linked to other factors, such as lifestyle and diet – including vitamin D supplementation.
The evidence is in, but it’s incomplete
In addition to the new study in Gastroenterology, other observational studies, as well as laboratory and animal studies, suggest that vitamin D plays a role in inhibiting carcinogenesis. Vitamin D, researchers theorize, contains anti-inflammatory, immunomodulatory, and tumor angiogenesis properties that can slow the growth of tumors, but the evidence is mixed.
A meta-analysis of 137,567 patients published in 2013 in Preventive Medicine found an inverse association between 25-hydroxyvitamin D (25[OH]D) and total cancer mortality in women, but not among men. Three meta-analyses published in 2014 and 2019 found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12%-13%.
In 2019, researchers led by Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research for the American Cancer Society, described a causal relationship between circulating vitamin D and colorectal cancer risk among 17 cohorts from a pooled analysis. “Our study suggests that optimal circulating 25(OH)D concentrations for colorectal cancer risk reduction are 75-100 nmol/L, [which is] higher than current Institute of Medicine recommendations for bone health,” she and colleagues wrote. Their findings were published in the Journal of the National Cancer Institute.
The Vitamin D and Omega-3 Trial (VITAL) published in 2019 in the New England Journal of Medicine, showed no significant effect of vitamin D3 supplementation of 2,000 IU/day in lowering the risk of invasive cancer or cardiovascular events.
Despite the mixed results, studies offer valuable insights into cancer risks, said Scott Kopetz, MD, PhD, codirector of the colorectal cancer moon shot research program at the University of Texas MD Anderson Cancer Center, Houston.
The Gastroenterology study is noteworthy because it focuses on early-onset colorectal cancer, he said.
“[The authors] demonstrate for the first time that there is an association of vitamin D intake with early-onset colorectal incidence, especially in the left side of the colon and rectum where the increase in early onset colorectal cancer manifests,” Dr. Kopetz said. “The analysis suggests that it may require long-term vitamin D intake to derive the benefit, which may explain why some shorter-term randomized studies failed to demonstrate.”
In animal models, vitamin D3 is “estimated to lower the incidence of colorectal cancer by 50%,” according to Lidija Klampfer, PhD, formerly a molecular biologist and senior research scientist with the Southern Research Institute, Birmingham, Ala.
Dr. Klampfer, a founding partner of ProteXase Therapeutics, is the author of an article on vitamin D and colon cancer published in 2014 in the World Journal of Gastrointestinal Oncology.
“The levels of vitamin D3 appear to be an essential determinant for the development and progression of colon cancer and supplementation with vitamin D3 is effective in suppressing intestinal tumorigenesis in animal models,” she wrote. “Studies have shown that 1,25 dihydroxyvitamin D3 can inhibit tumor-promoting inflammation leading to the development and progression of colon cancer.”
The hazards of a vitamin D deficiency
A severe vitamin D deficiency is associated with compromised bone and muscle health, calcium absorption, immunity, heart function and it can affect mood. Other studies have linked vitamin D deficiency to colorectal cancer, blood cancers, and bowel cancer.
Serum 25(OH)D is the primary circulating form of vitamin D and is considered the best marker for assessing vitamin D status, says Karin Amrein, MD, MSc, an endocrinologist with the Medical University of Graz (Austria). She was the lead author of a review on vitamin D deficiency published in January 2020 in the European Journal of Clinical Nutrition.
The Global Consensus Recommendations define vitamin D insufficiency as 12-20 ng/mL (30-50 nmol/L) and a deficiency as a serum 25OHD concentration less than 12 ng/mL (30 nmol/L). A deficiency in adults is usually treated with 50,000 IU of vitamin D2 or D3 once weekly for 8 weeks followed by maintenance dosages of cholecalciferol (vitamin D3) at 800-1,000 IU daily from dietary and supplemental sources.
Screening is recommended for individuals who exhibit symptoms and conditions associated with a vitamin D deficiency, but there is little agreement on recommended serum levels because every individual is different, according to the U.S. Preventive Services Task Force which updated its vitamin D recommendations in April for the first time in 7 years.
This is according to an observational study published in the journal Gastroenterology. The study included 94,205 women (aged 25-42 years) who were followed between 1991 and 2015 during which 111 incident cases of early-onset colorectal cancer were diagnosed. Among 29,186 women who had at least one lower endoscopy from 1991 to 2011, 1,439 newly diagnosed conventional adenomas and 1,878 serrated polyps were found.
Women who consumed the highest average levels of total vitamin D of 450 IU per day, compared with those consuming less than 300 IU per day, showed a significantly reduced risk of early-onset colorectal cancer. Consuming 400 IU each day was associated with a 54% reduced risk of early-onset colorectal cancer.
“If confirmed, our findings could potentially lead to recommendations for higher vitamin D intake as an inexpensive low-risk complement to colorectal cancer screening as a prevention strategy for adults younger than age 50,” wrote the study authors, led by Edward L. Giovannucci, MD, ScD, of the Harvard School of Public Health, Boston.
Associations between vitamin D levels and colorectal cancer have been documented in review articles over the years. The link is the subject of 10 recently completed or ongoing clinical trials. Few studies have focused on early colorectal cancer and vitamin D intake. Unlike advanced colorectal cancer, the early-onset form of the disease is not as strongly associated with the traditional risk factors of a family history of colorectal cancer and it is therefore believed to be more strongly linked to other factors, such as lifestyle and diet – including vitamin D supplementation.
The evidence is in, but it’s incomplete
In addition to the new study in Gastroenterology, other observational studies, as well as laboratory and animal studies, suggest that vitamin D plays a role in inhibiting carcinogenesis. Vitamin D, researchers theorize, contains anti-inflammatory, immunomodulatory, and tumor angiogenesis properties that can slow the growth of tumors, but the evidence is mixed.
A meta-analysis of 137,567 patients published in 2013 in Preventive Medicine found an inverse association between 25-hydroxyvitamin D (25[OH]D) and total cancer mortality in women, but not among men. Three meta-analyses published in 2014 and 2019 found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12%-13%.
In 2019, researchers led by Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research for the American Cancer Society, described a causal relationship between circulating vitamin D and colorectal cancer risk among 17 cohorts from a pooled analysis. “Our study suggests that optimal circulating 25(OH)D concentrations for colorectal cancer risk reduction are 75-100 nmol/L, [which is] higher than current Institute of Medicine recommendations for bone health,” she and colleagues wrote. Their findings were published in the Journal of the National Cancer Institute.
The Vitamin D and Omega-3 Trial (VITAL) published in 2019 in the New England Journal of Medicine, showed no significant effect of vitamin D3 supplementation of 2,000 IU/day in lowering the risk of invasive cancer or cardiovascular events.
Despite the mixed results, studies offer valuable insights into cancer risks, said Scott Kopetz, MD, PhD, codirector of the colorectal cancer moon shot research program at the University of Texas MD Anderson Cancer Center, Houston.
The Gastroenterology study is noteworthy because it focuses on early-onset colorectal cancer, he said.
“[The authors] demonstrate for the first time that there is an association of vitamin D intake with early-onset colorectal incidence, especially in the left side of the colon and rectum where the increase in early onset colorectal cancer manifests,” Dr. Kopetz said. “The analysis suggests that it may require long-term vitamin D intake to derive the benefit, which may explain why some shorter-term randomized studies failed to demonstrate.”
In animal models, vitamin D3 is “estimated to lower the incidence of colorectal cancer by 50%,” according to Lidija Klampfer, PhD, formerly a molecular biologist and senior research scientist with the Southern Research Institute, Birmingham, Ala.
Dr. Klampfer, a founding partner of ProteXase Therapeutics, is the author of an article on vitamin D and colon cancer published in 2014 in the World Journal of Gastrointestinal Oncology.
“The levels of vitamin D3 appear to be an essential determinant for the development and progression of colon cancer and supplementation with vitamin D3 is effective in suppressing intestinal tumorigenesis in animal models,” she wrote. “Studies have shown that 1,25 dihydroxyvitamin D3 can inhibit tumor-promoting inflammation leading to the development and progression of colon cancer.”
The hazards of a vitamin D deficiency
A severe vitamin D deficiency is associated with compromised bone and muscle health, calcium absorption, immunity, heart function and it can affect mood. Other studies have linked vitamin D deficiency to colorectal cancer, blood cancers, and bowel cancer.
Serum 25(OH)D is the primary circulating form of vitamin D and is considered the best marker for assessing vitamin D status, says Karin Amrein, MD, MSc, an endocrinologist with the Medical University of Graz (Austria). She was the lead author of a review on vitamin D deficiency published in January 2020 in the European Journal of Clinical Nutrition.
The Global Consensus Recommendations define vitamin D insufficiency as 12-20 ng/mL (30-50 nmol/L) and a deficiency as a serum 25OHD concentration less than 12 ng/mL (30 nmol/L). A deficiency in adults is usually treated with 50,000 IU of vitamin D2 or D3 once weekly for 8 weeks followed by maintenance dosages of cholecalciferol (vitamin D3) at 800-1,000 IU daily from dietary and supplemental sources.
Screening is recommended for individuals who exhibit symptoms and conditions associated with a vitamin D deficiency, but there is little agreement on recommended serum levels because every individual is different, according to the U.S. Preventive Services Task Force which updated its vitamin D recommendations in April for the first time in 7 years.
This is according to an observational study published in the journal Gastroenterology. The study included 94,205 women (aged 25-42 years) who were followed between 1991 and 2015 during which 111 incident cases of early-onset colorectal cancer were diagnosed. Among 29,186 women who had at least one lower endoscopy from 1991 to 2011, 1,439 newly diagnosed conventional adenomas and 1,878 serrated polyps were found.
Women who consumed the highest average levels of total vitamin D of 450 IU per day, compared with those consuming less than 300 IU per day, showed a significantly reduced risk of early-onset colorectal cancer. Consuming 400 IU each day was associated with a 54% reduced risk of early-onset colorectal cancer.
“If confirmed, our findings could potentially lead to recommendations for higher vitamin D intake as an inexpensive low-risk complement to colorectal cancer screening as a prevention strategy for adults younger than age 50,” wrote the study authors, led by Edward L. Giovannucci, MD, ScD, of the Harvard School of Public Health, Boston.
Associations between vitamin D levels and colorectal cancer have been documented in review articles over the years. The link is the subject of 10 recently completed or ongoing clinical trials. Few studies have focused on early colorectal cancer and vitamin D intake. Unlike advanced colorectal cancer, the early-onset form of the disease is not as strongly associated with the traditional risk factors of a family history of colorectal cancer and it is therefore believed to be more strongly linked to other factors, such as lifestyle and diet – including vitamin D supplementation.
The evidence is in, but it’s incomplete
In addition to the new study in Gastroenterology, other observational studies, as well as laboratory and animal studies, suggest that vitamin D plays a role in inhibiting carcinogenesis. Vitamin D, researchers theorize, contains anti-inflammatory, immunomodulatory, and tumor angiogenesis properties that can slow the growth of tumors, but the evidence is mixed.
A meta-analysis of 137,567 patients published in 2013 in Preventive Medicine found an inverse association between 25-hydroxyvitamin D (25[OH]D) and total cancer mortality in women, but not among men. Three meta-analyses published in 2014 and 2019 found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12%-13%.
In 2019, researchers led by Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research for the American Cancer Society, described a causal relationship between circulating vitamin D and colorectal cancer risk among 17 cohorts from a pooled analysis. “Our study suggests that optimal circulating 25(OH)D concentrations for colorectal cancer risk reduction are 75-100 nmol/L, [which is] higher than current Institute of Medicine recommendations for bone health,” she and colleagues wrote. Their findings were published in the Journal of the National Cancer Institute.
The Vitamin D and Omega-3 Trial (VITAL) published in 2019 in the New England Journal of Medicine, showed no significant effect of vitamin D3 supplementation of 2,000 IU/day in lowering the risk of invasive cancer or cardiovascular events.
Despite the mixed results, studies offer valuable insights into cancer risks, said Scott Kopetz, MD, PhD, codirector of the colorectal cancer moon shot research program at the University of Texas MD Anderson Cancer Center, Houston.
The Gastroenterology study is noteworthy because it focuses on early-onset colorectal cancer, he said.
“[The authors] demonstrate for the first time that there is an association of vitamin D intake with early-onset colorectal incidence, especially in the left side of the colon and rectum where the increase in early onset colorectal cancer manifests,” Dr. Kopetz said. “The analysis suggests that it may require long-term vitamin D intake to derive the benefit, which may explain why some shorter-term randomized studies failed to demonstrate.”
In animal models, vitamin D3 is “estimated to lower the incidence of colorectal cancer by 50%,” according to Lidija Klampfer, PhD, formerly a molecular biologist and senior research scientist with the Southern Research Institute, Birmingham, Ala.
Dr. Klampfer, a founding partner of ProteXase Therapeutics, is the author of an article on vitamin D and colon cancer published in 2014 in the World Journal of Gastrointestinal Oncology.
“The levels of vitamin D3 appear to be an essential determinant for the development and progression of colon cancer and supplementation with vitamin D3 is effective in suppressing intestinal tumorigenesis in animal models,” she wrote. “Studies have shown that 1,25 dihydroxyvitamin D3 can inhibit tumor-promoting inflammation leading to the development and progression of colon cancer.”
The hazards of a vitamin D deficiency
A severe vitamin D deficiency is associated with compromised bone and muscle health, calcium absorption, immunity, heart function and it can affect mood. Other studies have linked vitamin D deficiency to colorectal cancer, blood cancers, and bowel cancer.
Serum 25(OH)D is the primary circulating form of vitamin D and is considered the best marker for assessing vitamin D status, says Karin Amrein, MD, MSc, an endocrinologist with the Medical University of Graz (Austria). She was the lead author of a review on vitamin D deficiency published in January 2020 in the European Journal of Clinical Nutrition.
The Global Consensus Recommendations define vitamin D insufficiency as 12-20 ng/mL (30-50 nmol/L) and a deficiency as a serum 25OHD concentration less than 12 ng/mL (30 nmol/L). A deficiency in adults is usually treated with 50,000 IU of vitamin D2 or D3 once weekly for 8 weeks followed by maintenance dosages of cholecalciferol (vitamin D3) at 800-1,000 IU daily from dietary and supplemental sources.
Screening is recommended for individuals who exhibit symptoms and conditions associated with a vitamin D deficiency, but there is little agreement on recommended serum levels because every individual is different, according to the U.S. Preventive Services Task Force which updated its vitamin D recommendations in April for the first time in 7 years.
FROM GASTROENTEROLOGY
Association of Healthcare Access With Intensive Care Unit Utilization and Mortality in Patients of Hispanic Ethnicity Hospitalized With COVID-19
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).
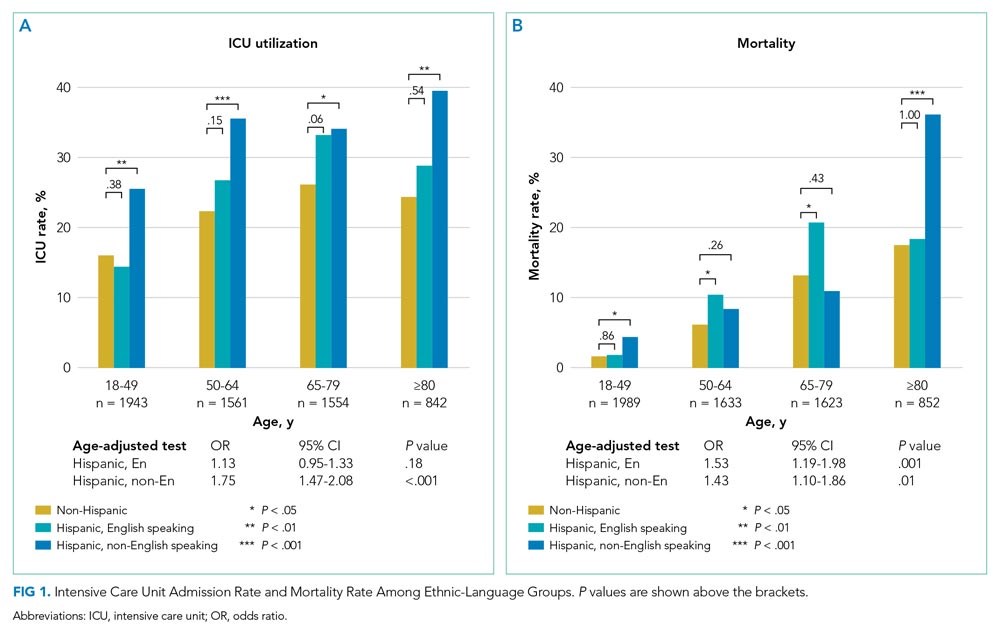
To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

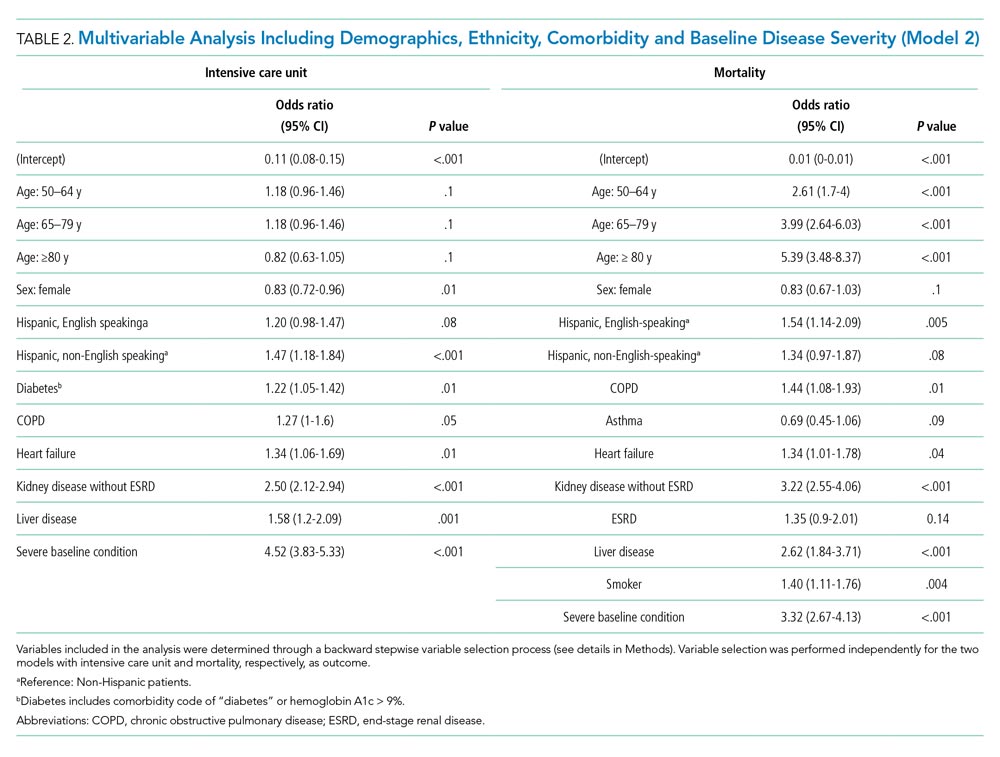
We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.
Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).

To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.

Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).

To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.

Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
© 2021 Society of Hospital Medicine
Top case
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Vikrant Parihar, MD, wrote the following in “COVID-19 and UC”:
A 43-year-old man with an index presentation of distal colitis (Montreal E2) (Mayo endoscopic score 2-3) was discharged home on tapering doses of oral steroids. He was being worked up to commence anti-TNF likely initially as combo therapy. Fully vaccinated against COVID – had both doses of vaccine way back in May. Attended a match and looks to have got mild symptoms and on testing turned out to be COVID+. Rx himself by self-quarantine.
What would be the optimal strategy?
1. Stop steroids completely and immediately given the adverse signal in registry data?
2. When can anti-TNF’s be safely started?
3. How to manage him in the interim?
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25172.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Vikrant Parihar, MD, wrote the following in “COVID-19 and UC”:
A 43-year-old man with an index presentation of distal colitis (Montreal E2) (Mayo endoscopic score 2-3) was discharged home on tapering doses of oral steroids. He was being worked up to commence anti-TNF likely initially as combo therapy. Fully vaccinated against COVID – had both doses of vaccine way back in May. Attended a match and looks to have got mild symptoms and on testing turned out to be COVID+. Rx himself by self-quarantine.
What would be the optimal strategy?
1. Stop steroids completely and immediately given the adverse signal in registry data?
2. When can anti-TNF’s be safely started?
3. How to manage him in the interim?
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25172.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Vikrant Parihar, MD, wrote the following in “COVID-19 and UC”:
A 43-year-old man with an index presentation of distal colitis (Montreal E2) (Mayo endoscopic score 2-3) was discharged home on tapering doses of oral steroids. He was being worked up to commence anti-TNF likely initially as combo therapy. Fully vaccinated against COVID – had both doses of vaccine way back in May. Attended a match and looks to have got mild symptoms and on testing turned out to be COVID+. Rx himself by self-quarantine.
What would be the optimal strategy?
1. Stop steroids completely and immediately given the adverse signal in registry data?
2. When can anti-TNF’s be safely started?
3. How to manage him in the interim?
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25172.
Sleep time ‘sweet spot’ to slow cognitive decline identified?
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
From Brain
SURPASS-4: ‘Twincretin’ tirzepatide surpasses insulin glargine in pivotal trial
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
FROM THE LANCET
Researchers parse which patients with T2D need SGLT2 inhibition
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
FROM DIABETES CARE
PRP injections don’t top placebo for ankle osteoarthritis
Platelet-rich plasma (PRP) injections did not significantly improve pain or function when compared with placebo injections in patients with ankle osteoarthritis (OA), a new study has found.
“Previous evidence for PRP injections in ankle osteoarthritis was limited to 4 small case series with methodological flaws,” wrote Liam D. A. Paget, MD, of the University of Amsterdam, and coauthors. The study was published online Oct. 26 in JAMA.
To assess the value of PRP injections as a treatment for ankle OA, the researchers launched a double-blind, randomized clinical trial of Dutch patients with notable ankle pain and tibiotalar joint space narrowing. From six sites in the Netherlands, 100 patients (45% women, mean age 56 years) were split into two groups: one that received two intra-articular injections of PRP 6 weeks apart (n = 48) and one that received two injections of saline placebo (n = 52).
At baseline, mean American Orthopaedic Foot and Ankle Society (AOFAS) scores were 63 in the PRP group and 64 in the placebo group (range 0-100, with higher scores indicating less pain and more function). At 26-week follow-up, the mean AOFAS score improved by 10 points in the PRP group (95% confidence interval, 6-14; P < .001) and by 11 points in the placebo group (95% CI, 7-15; P < .001). The adjusted between-group difference for AOFAS improvement over 26 weeks was –1 point (95% CI, –6 to 3; P = .56).
There was one serious adverse event in the placebo group – a transient ischemic attack 3 weeks after the first injection – but it was deemed unrelated.
Searching for answers regarding PRP and osteoarthritis
“From my standpoint, this study is a great step forward to where the field needs to be, which is honing in on longer-term studies that are standardizing PRP and teasing out its effects,” Prathap Jayaram, MD, director of regenerative sports medicine at the Baylor College of Medicine in Houston, said in an interview.
He highlighted the authors’ acknowledgment of previous studies in which PRP injections appeared effective in treating knee OA, including their statement that the “results reported here for ankle osteoarthritis were not consistent with these potentially beneficial effects in knee osteoarthritis.”
“They’re acknowledging that this does have some benefit in knees,” he said. “Could that translate toward the ankle?”
“PRP did lead to an improvement,” he added. “There just wasn’t a big enough difference to say one was superior to the other.”
Citing his team’s recent preclinical study that was published in Osteoarthritis and Cartilage, Dr. Jayaram emphasized the possibility that PRP could have much-needed disease-modifying effects in osteoarthritis. More work is needed to pin down the details.
“We need more mechanistic studies to be done so we can actually identify the therapeutic properties in PRP and leverage them to track reproducible outcomes,” he said, adding that “simply put, your blood and my blood might be different. There is going to be heterogeneity there. The analogy I give my patients is, when they take an antibiotic, we have a specific dose, a specific drug, and a specific duration. It’s very standardized. We’re just not there yet with PRP.”
The authors acknowledged their study’s limitations, including a likely inability to generalize their results to other platelet-rich blood products as well as a lack of composition analysis of the PRP they used. That said, they added that this particular PRP has been “analyzed previously” for another trial and noted that such analysis is not typically performed in a clinical setting.
The study was supported by a grant from the Dutch Arthritis Society. Its authors reported several potential conflicts of interest, including receiving their own grants from the Dutch Arthritis Society and other organizations, as well as accepting loaned Hettich centrifuges from a medical device company for the study.
A version of this article first appeared on Medscape.com.
Platelet-rich plasma (PRP) injections did not significantly improve pain or function when compared with placebo injections in patients with ankle osteoarthritis (OA), a new study has found.
“Previous evidence for PRP injections in ankle osteoarthritis was limited to 4 small case series with methodological flaws,” wrote Liam D. A. Paget, MD, of the University of Amsterdam, and coauthors. The study was published online Oct. 26 in JAMA.
To assess the value of PRP injections as a treatment for ankle OA, the researchers launched a double-blind, randomized clinical trial of Dutch patients with notable ankle pain and tibiotalar joint space narrowing. From six sites in the Netherlands, 100 patients (45% women, mean age 56 years) were split into two groups: one that received two intra-articular injections of PRP 6 weeks apart (n = 48) and one that received two injections of saline placebo (n = 52).
At baseline, mean American Orthopaedic Foot and Ankle Society (AOFAS) scores were 63 in the PRP group and 64 in the placebo group (range 0-100, with higher scores indicating less pain and more function). At 26-week follow-up, the mean AOFAS score improved by 10 points in the PRP group (95% confidence interval, 6-14; P < .001) and by 11 points in the placebo group (95% CI, 7-15; P < .001). The adjusted between-group difference for AOFAS improvement over 26 weeks was –1 point (95% CI, –6 to 3; P = .56).
There was one serious adverse event in the placebo group – a transient ischemic attack 3 weeks after the first injection – but it was deemed unrelated.
Searching for answers regarding PRP and osteoarthritis
“From my standpoint, this study is a great step forward to where the field needs to be, which is honing in on longer-term studies that are standardizing PRP and teasing out its effects,” Prathap Jayaram, MD, director of regenerative sports medicine at the Baylor College of Medicine in Houston, said in an interview.
He highlighted the authors’ acknowledgment of previous studies in which PRP injections appeared effective in treating knee OA, including their statement that the “results reported here for ankle osteoarthritis were not consistent with these potentially beneficial effects in knee osteoarthritis.”
“They’re acknowledging that this does have some benefit in knees,” he said. “Could that translate toward the ankle?”
“PRP did lead to an improvement,” he added. “There just wasn’t a big enough difference to say one was superior to the other.”
Citing his team’s recent preclinical study that was published in Osteoarthritis and Cartilage, Dr. Jayaram emphasized the possibility that PRP could have much-needed disease-modifying effects in osteoarthritis. More work is needed to pin down the details.
“We need more mechanistic studies to be done so we can actually identify the therapeutic properties in PRP and leverage them to track reproducible outcomes,” he said, adding that “simply put, your blood and my blood might be different. There is going to be heterogeneity there. The analogy I give my patients is, when they take an antibiotic, we have a specific dose, a specific drug, and a specific duration. It’s very standardized. We’re just not there yet with PRP.”
The authors acknowledged their study’s limitations, including a likely inability to generalize their results to other platelet-rich blood products as well as a lack of composition analysis of the PRP they used. That said, they added that this particular PRP has been “analyzed previously” for another trial and noted that such analysis is not typically performed in a clinical setting.
The study was supported by a grant from the Dutch Arthritis Society. Its authors reported several potential conflicts of interest, including receiving their own grants from the Dutch Arthritis Society and other organizations, as well as accepting loaned Hettich centrifuges from a medical device company for the study.
A version of this article first appeared on Medscape.com.
Platelet-rich plasma (PRP) injections did not significantly improve pain or function when compared with placebo injections in patients with ankle osteoarthritis (OA), a new study has found.
“Previous evidence for PRP injections in ankle osteoarthritis was limited to 4 small case series with methodological flaws,” wrote Liam D. A. Paget, MD, of the University of Amsterdam, and coauthors. The study was published online Oct. 26 in JAMA.
To assess the value of PRP injections as a treatment for ankle OA, the researchers launched a double-blind, randomized clinical trial of Dutch patients with notable ankle pain and tibiotalar joint space narrowing. From six sites in the Netherlands, 100 patients (45% women, mean age 56 years) were split into two groups: one that received two intra-articular injections of PRP 6 weeks apart (n = 48) and one that received two injections of saline placebo (n = 52).
At baseline, mean American Orthopaedic Foot and Ankle Society (AOFAS) scores were 63 in the PRP group and 64 in the placebo group (range 0-100, with higher scores indicating less pain and more function). At 26-week follow-up, the mean AOFAS score improved by 10 points in the PRP group (95% confidence interval, 6-14; P < .001) and by 11 points in the placebo group (95% CI, 7-15; P < .001). The adjusted between-group difference for AOFAS improvement over 26 weeks was –1 point (95% CI, –6 to 3; P = .56).
There was one serious adverse event in the placebo group – a transient ischemic attack 3 weeks after the first injection – but it was deemed unrelated.
Searching for answers regarding PRP and osteoarthritis
“From my standpoint, this study is a great step forward to where the field needs to be, which is honing in on longer-term studies that are standardizing PRP and teasing out its effects,” Prathap Jayaram, MD, director of regenerative sports medicine at the Baylor College of Medicine in Houston, said in an interview.
He highlighted the authors’ acknowledgment of previous studies in which PRP injections appeared effective in treating knee OA, including their statement that the “results reported here for ankle osteoarthritis were not consistent with these potentially beneficial effects in knee osteoarthritis.”
“They’re acknowledging that this does have some benefit in knees,” he said. “Could that translate toward the ankle?”
“PRP did lead to an improvement,” he added. “There just wasn’t a big enough difference to say one was superior to the other.”
Citing his team’s recent preclinical study that was published in Osteoarthritis and Cartilage, Dr. Jayaram emphasized the possibility that PRP could have much-needed disease-modifying effects in osteoarthritis. More work is needed to pin down the details.
“We need more mechanistic studies to be done so we can actually identify the therapeutic properties in PRP and leverage them to track reproducible outcomes,” he said, adding that “simply put, your blood and my blood might be different. There is going to be heterogeneity there. The analogy I give my patients is, when they take an antibiotic, we have a specific dose, a specific drug, and a specific duration. It’s very standardized. We’re just not there yet with PRP.”
The authors acknowledged their study’s limitations, including a likely inability to generalize their results to other platelet-rich blood products as well as a lack of composition analysis of the PRP they used. That said, they added that this particular PRP has been “analyzed previously” for another trial and noted that such analysis is not typically performed in a clinical setting.
The study was supported by a grant from the Dutch Arthritis Society. Its authors reported several potential conflicts of interest, including receiving their own grants from the Dutch Arthritis Society and other organizations, as well as accepting loaned Hettich centrifuges from a medical device company for the study.
A version of this article first appeared on Medscape.com.
Opioid-induced adrenal insufficiency for the hospitalist
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Consider OIAI, even among patients with common infections
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Major increase seen in cosmeceutical alternatives to topical hydroquinone
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
along with new strategies to improve their efficacy, according to a report at the Skin of Color Update 2021.
“Ten or 15 years ago, I was showing a slide with five [alternatives to hydroquinone]. Now there are dozens,” reported Heather Woolery-Lloyd, MD, director of the skin of color division in the department of dermatology at the University of Miami.
The growth in alternatives to hydroquinone is timely. After threats to do so for more than a decade, the Food and Drug Administration finally banned hydroquinone from OTC products in 2020. The ban was folded into the Coronavirus Aid, Relief, and Economic Security (CARES) Act passed in March of 2020 and then implemented the following September.
Until the ban of hydroquinone, OTC products with this compound were widely sought by many individuals with darker skin tones to self-treat melasma and other forms of hyperpigmentation, according to Dr. Woolery-Lloyd. Hydroquinone is still available in prescription products, but she is often asked for OTC alternatives, and she says the list is long and getting longer.
Niacinamide
Detailing the products she has been recommending most frequently as substitutes, Dr. Woolery-Lloyd reported that several are supported by high quality studies. One example is niacinamide.
Of the several controlled studies she cited, one double-blind randomized trial found niacinamide to be equivalent to hydroquinone for melasma on the basis of colorimetric measures. The study compared 4% niacinamide cream applied on one side of the face with 4% hydroquinone cream applied on the other side in 27 patients with melasma. Although the proportion of responses rated good or excellent on a subjective basis was lower with niacinamide (44% vs. 55%), the difference was not statistically significant and niacinamide cream was clearly active, producing objective improvements in mast cell infiltrate and solar elastosis in melasma skin as well. Both were well tolerated.
In other studies, niacinamide has been shown to be effective in the treatment of melasma when combined with other active agents such as tranexamic acid, said Dr. Woolery-Lloyd, who added that OTC products containing niacinamide are now “among my favorites” when directing patients to cosmeceuticals for hyperpigmentation.
Topical vitamin C
Topical vitamin C or ascorbic acid is another. Like niacinamide, topical vitamin C has also been compared with hydroquinone in a double-blind, randomized trial. Although the niacinamide trial and this study were performed 10 or more years ago, these data have new relevance with the ban of OTC hydroquinone.
In the study, 5% ascorbic acid cream on one side of the face was compared with 4% hydroquinone cream, applied on the other side, in 16 women with melasma. Again, there were no statistical differences in colorimetric measures, but good to excellent results were reported for 93% of the sides of the face treated with hydroquinone versus 62.5% of the sides treated with vitamin C (P < .05). “Hydroquinone performed better, but the vitamin C was active and very well tolerated,” Dr. Woolery-Lloyd said.
However, the ascorbic acid cream was better tolerated, with a far lower rate of adverse events (6.2% vs. 68.7%), an advantage that makes it easy to recommend to patients, said Dr. Woolery-Lloyd, who now uses it frequently in her own practice.
Liquiritin, a licorice extract, is another lightening agent increasingly included in OTC products that she also recommends. In two older studies in medical journals published in Pakistan, both the 2% and 4% strengths of liquiritin cream outperformed hydroquinone on the basis of a Melasma Area and Severity Index (MASI) rating. The liquiritin cream was well tolerated in both studies.
Azelaic acid, tranexamic acid
OTC products containing azelaic acid are also effective for hyperpigmentation based on published trials in which they were compared with hydroquinone for treating melasma. In one study of 29 women with melasma cited by Dr. Woolery-Lloyd, 20% azelaic acid cream was more effective than hydroquinone 4% cream after 2 months of treatment on the basis of the mean MASI score (6.2 vs. 3.8).
The list also includes cysteamine, silymarin, and tranexamic acid.
In the case of tranexamic acid, Dr. Woolery-Lloyd cited a relatively recent study of 60 patients with melasma, comparing two strategies for applying tranexamic acid to treatment with hydroquinone over 12 weeks. Compared with 2% hydroquinone (applied nightly) or 1.8% liposomal tranexamic acid (applied twice a day), 5% tranexamic acid solution with microneedling (weekly) had a slightly greater rate of success defined as more than a 50% improvement in hyperpigmentation in an Asian population (30%, 27.8%, and 33.3%, respectively).
“Microneedling is a newer technology that appears to be effective at improving absorption,” said Dr. Woolery-Lloyd. She predicts that microneedling will be used with increasing frequency in combination with topical cosmeceuticals.
She also predicted that these topical agents will be increasingly employed in combinations as the field of cosmeceuticals becomes increasingly more sophisticated. “When it comes to skin quality, cosmeceuticals remain our first-line therapy, especially in skin of color,” she said.
The rapid growth and utility of OTC cosmeceuticals is an area that dermatologists need to be following, according to Darius Mehregan, MD, chair of the department of dermatology, Wayne State University, Detroit, who was senior author of an article published last year that reviewed the ingredients of popular OTC cosmeceuticals.
“Our patients have a great interest in cosmeceuticals and are looking to us for guidance. I think we have a responsibility to help them identify products supported by evidence and to warn them about potential side effects,” Dr. Mehregan, who was not at the meeting, said in an interview.
He agreed that the removal of hydroquinone from OTC products will create a specific need in the area of cosmeceuticals.
“Hydroquinone has for a long time been one of the most effective agents in OTC products for melasma, so patients are going to be looking for alternatives. Identifying which drugs have shown efficacy in controlled studies will be very helpful,” he said.
Dr. Woolery-Lloyd reports financial relationships with Ortho Dermatologics, L’Oréal, Galderma, Allergan, and Somabella Laboratories. Dr. Mehregan reports no potential conflicts of interest.
FROM SOC 2021
Forming specialized immune cell structures could combat pancreatic cancer
In a new study, researchers stimulated immune cells to assemble into tertiary lymphoid structures that improved the efficacy of chemotherapy in a preclinical model of pancreatic cancer.
Overall, the evidence generated by the study supports the notion that induction of tertiary lymphoid structures may potentiate chemotherapy’s antitumor activity, at least in a murine model of pancreatic ductal adenocarcinoma (PDAC). A more detailed understanding of tertiary lymphoid structure “kinetics and their induction, owing to multiple host and tumor factors, may help design personalized therapies harnessing the potential of immuno-oncology,” Francesca Delvecchio of Queen Mary University of London and colleagues wrote in Cellular and Molecular Gastroenterology and Hepatology.
While the immune system can play a role in combating cancer, a dense stroma surrounds pancreatic cancer centers, often blocking the immune cells’ ability to access the tumor. As shown by Young and colleagues, this leads immunotherapies to have very little success in the management of pancreatic cancer, despite the efficacy of these therapies in other types of cancer.
In a proportion of patients with pancreatic cancer, clusters of immune cells known as tertiary lymphoid structures can assemble within the stroma. These structures are associated with improved survival in PDAC. In the study, Mr. Delvecchio and colleagues sought to further elucidate the role of tertiary lymphoid structures in PDAC, particularly the structures’ antitumor activity.
The investigators analyzed donated tissue samples from patients to identify the presence of the structures within chemotherapy-naive human pancreatic cancer. Tertiary lymphoid structures were defined by the presence of tissue zones that were rich in T cells, B cells, and dendritic cells. Staining techniques were used to visualize the various cell types in the samples, revealing tertiary lymphoid structures in approximately 30% of tissue microarrays and 42% of the full section.
Multicolor immunofluorescence and immunohistochemistry were also used to characterize tertiary lymphoid structures in murine models of pancreatic cancer. Additionally, the investigators developed the orthotopic murine model to assess the development of the structures and the effect of a combined chemotherapy and immunotherapy regimen on tumor growth. While tertiary lymphoid structures were not initially present in the preclinical murine model, B cells and T cells subsequently infiltrated into the tumor site following injection of lymphoid chemokines. These cells consequently assembled into the tertiary lymphoid structures.
In addition, the researchers combined chemotherapy gemcitabine with the intratumoral lymphoid chemokine and injected this combination treatment into orthotopic tumors. Following injection, the researchers observed “altered immune cell infiltration,” which facilitated the induction of tertiary lymphoid structures and potentiated antitumor activity of the chemotherapy. As a result, there was a significant reduction in the tumors, an effect the researchers did not find following the use of either treatment alone.
According to the investigators, the antitumor activity observed following induction of the tertiary lymphoid structures within the cancer is associated with B cell–mediated activation of dendritic cells, a requirement for the initiation of the immune response.
Based on the findings, the researchers concluded that the combination of chemotherapy and lymphoid chemokines could be a viable strategy for promoting an antitumor immune response in pancreatic cancer. In turn, the researchers suggest this strategy may result in better clinical outcomes for patients with the disease. Additionally, the researchers wrote that mature tertiary lymphoid structures in PDAC prior to an immune treatment could “be used as a biomarker to define inclusion criteria of patients in immunotherapy protocols, with the aim to boost the ongoing antitumor immune response.”
Given that the study relied on a mouse model, the findings may currently lack generalizability across humans. In the context of PDAC, the researchers wrote that further investigation and understanding of the formation of tertiary lymphoid structures may support the development of tailored treatments, including those that take advantage of the body’s immune system, to combat cancer and improve patient outcomes.
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
In a new study, researchers stimulated immune cells to assemble into tertiary lymphoid structures that improved the efficacy of chemotherapy in a preclinical model of pancreatic cancer.
Overall, the evidence generated by the study supports the notion that induction of tertiary lymphoid structures may potentiate chemotherapy’s antitumor activity, at least in a murine model of pancreatic ductal adenocarcinoma (PDAC). A more detailed understanding of tertiary lymphoid structure “kinetics and their induction, owing to multiple host and tumor factors, may help design personalized therapies harnessing the potential of immuno-oncology,” Francesca Delvecchio of Queen Mary University of London and colleagues wrote in Cellular and Molecular Gastroenterology and Hepatology.
While the immune system can play a role in combating cancer, a dense stroma surrounds pancreatic cancer centers, often blocking the immune cells’ ability to access the tumor. As shown by Young and colleagues, this leads immunotherapies to have very little success in the management of pancreatic cancer, despite the efficacy of these therapies in other types of cancer.
In a proportion of patients with pancreatic cancer, clusters of immune cells known as tertiary lymphoid structures can assemble within the stroma. These structures are associated with improved survival in PDAC. In the study, Mr. Delvecchio and colleagues sought to further elucidate the role of tertiary lymphoid structures in PDAC, particularly the structures’ antitumor activity.
The investigators analyzed donated tissue samples from patients to identify the presence of the structures within chemotherapy-naive human pancreatic cancer. Tertiary lymphoid structures were defined by the presence of tissue zones that were rich in T cells, B cells, and dendritic cells. Staining techniques were used to visualize the various cell types in the samples, revealing tertiary lymphoid structures in approximately 30% of tissue microarrays and 42% of the full section.
Multicolor immunofluorescence and immunohistochemistry were also used to characterize tertiary lymphoid structures in murine models of pancreatic cancer. Additionally, the investigators developed the orthotopic murine model to assess the development of the structures and the effect of a combined chemotherapy and immunotherapy regimen on tumor growth. While tertiary lymphoid structures were not initially present in the preclinical murine model, B cells and T cells subsequently infiltrated into the tumor site following injection of lymphoid chemokines. These cells consequently assembled into the tertiary lymphoid structures.
In addition, the researchers combined chemotherapy gemcitabine with the intratumoral lymphoid chemokine and injected this combination treatment into orthotopic tumors. Following injection, the researchers observed “altered immune cell infiltration,” which facilitated the induction of tertiary lymphoid structures and potentiated antitumor activity of the chemotherapy. As a result, there was a significant reduction in the tumors, an effect the researchers did not find following the use of either treatment alone.
According to the investigators, the antitumor activity observed following induction of the tertiary lymphoid structures within the cancer is associated with B cell–mediated activation of dendritic cells, a requirement for the initiation of the immune response.
Based on the findings, the researchers concluded that the combination of chemotherapy and lymphoid chemokines could be a viable strategy for promoting an antitumor immune response in pancreatic cancer. In turn, the researchers suggest this strategy may result in better clinical outcomes for patients with the disease. Additionally, the researchers wrote that mature tertiary lymphoid structures in PDAC prior to an immune treatment could “be used as a biomarker to define inclusion criteria of patients in immunotherapy protocols, with the aim to boost the ongoing antitumor immune response.”
Given that the study relied on a mouse model, the findings may currently lack generalizability across humans. In the context of PDAC, the researchers wrote that further investigation and understanding of the formation of tertiary lymphoid structures may support the development of tailored treatments, including those that take advantage of the body’s immune system, to combat cancer and improve patient outcomes.
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
In a new study, researchers stimulated immune cells to assemble into tertiary lymphoid structures that improved the efficacy of chemotherapy in a preclinical model of pancreatic cancer.
Overall, the evidence generated by the study supports the notion that induction of tertiary lymphoid structures may potentiate chemotherapy’s antitumor activity, at least in a murine model of pancreatic ductal adenocarcinoma (PDAC). A more detailed understanding of tertiary lymphoid structure “kinetics and their induction, owing to multiple host and tumor factors, may help design personalized therapies harnessing the potential of immuno-oncology,” Francesca Delvecchio of Queen Mary University of London and colleagues wrote in Cellular and Molecular Gastroenterology and Hepatology.
While the immune system can play a role in combating cancer, a dense stroma surrounds pancreatic cancer centers, often blocking the immune cells’ ability to access the tumor. As shown by Young and colleagues, this leads immunotherapies to have very little success in the management of pancreatic cancer, despite the efficacy of these therapies in other types of cancer.
In a proportion of patients with pancreatic cancer, clusters of immune cells known as tertiary lymphoid structures can assemble within the stroma. These structures are associated with improved survival in PDAC. In the study, Mr. Delvecchio and colleagues sought to further elucidate the role of tertiary lymphoid structures in PDAC, particularly the structures’ antitumor activity.
The investigators analyzed donated tissue samples from patients to identify the presence of the structures within chemotherapy-naive human pancreatic cancer. Tertiary lymphoid structures were defined by the presence of tissue zones that were rich in T cells, B cells, and dendritic cells. Staining techniques were used to visualize the various cell types in the samples, revealing tertiary lymphoid structures in approximately 30% of tissue microarrays and 42% of the full section.
Multicolor immunofluorescence and immunohistochemistry were also used to characterize tertiary lymphoid structures in murine models of pancreatic cancer. Additionally, the investigators developed the orthotopic murine model to assess the development of the structures and the effect of a combined chemotherapy and immunotherapy regimen on tumor growth. While tertiary lymphoid structures were not initially present in the preclinical murine model, B cells and T cells subsequently infiltrated into the tumor site following injection of lymphoid chemokines. These cells consequently assembled into the tertiary lymphoid structures.
In addition, the researchers combined chemotherapy gemcitabine with the intratumoral lymphoid chemokine and injected this combination treatment into orthotopic tumors. Following injection, the researchers observed “altered immune cell infiltration,” which facilitated the induction of tertiary lymphoid structures and potentiated antitumor activity of the chemotherapy. As a result, there was a significant reduction in the tumors, an effect the researchers did not find following the use of either treatment alone.
According to the investigators, the antitumor activity observed following induction of the tertiary lymphoid structures within the cancer is associated with B cell–mediated activation of dendritic cells, a requirement for the initiation of the immune response.
Based on the findings, the researchers concluded that the combination of chemotherapy and lymphoid chemokines could be a viable strategy for promoting an antitumor immune response in pancreatic cancer. In turn, the researchers suggest this strategy may result in better clinical outcomes for patients with the disease. Additionally, the researchers wrote that mature tertiary lymphoid structures in PDAC prior to an immune treatment could “be used as a biomarker to define inclusion criteria of patients in immunotherapy protocols, with the aim to boost the ongoing antitumor immune response.”
Given that the study relied on a mouse model, the findings may currently lack generalizability across humans. In the context of PDAC, the researchers wrote that further investigation and understanding of the formation of tertiary lymphoid structures may support the development of tailored treatments, including those that take advantage of the body’s immune system, to combat cancer and improve patient outcomes.
The researchers reported no conflicts of interest with the pharmaceutical industry. No funding was reported for the study.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
















