User login
Rimegepant looks safe in migraine patients with cardiovascular risk
Results from a 1-year, open-label safety study suggest that Patients who fall into this category may be ineligible for treatment with triptans.
There are mechanistic concerns with rimegepant and related CGRP receptor antagonists. They block CGRP’s effect in the central nervous system, but CGRP is also active in blood vessels and the heart, leading to the possibility that countering its vasodilating effect could expose organs to risk of ischemia.
The Food and Drug Administration approved rimegepant in 2020 for treatment of acute migraine attacks. Sponsor Biohaven is also seeking approval for migraine prevention after a successful phase 3 study published January 2021 in The Lancet.
Susan Hutchinson, MD, who is a headache specialist at Orange County Migraine & Headache Center in Irvine, Calif., presented the results at the American Academy of Neurology’s 2021 annual meeting. The open-label study suggested that rimegepant is generally safe. “The proportion of subjects reporting at least one adverse event was similar among subjects whether they had zero, one, or two or more cardiovascular disease risk factors, and also among those with low and moderate to high 10-year cardiovascular risk, as determined by the Framingham Risk Score,” said Dr. Hutchinson during her presentation.
Still, there was one concerning case: A 53-year-old man experienced an attack of angina. But he already had angina prior to the study, was being treated for hypercholesterolemia, and had current or former exposure to statins. “This adverse event was deemed by the investigator to not be related to rimegepant,” said Dr. Hutchinson.
During the following question-and-answer session, an attendee pressed Dr. Hutchinson about the case, and she admitted to some initial doubts. “That was my concern when I saw those slides. I’m like, ‘oh, my goodness.’ ” She clarified that the man’s angina history dated to 2016, which was several years before the trial, and the episode of angina occurred 7 months after the first dose of rimegepant. “He was treated with nitroglycerin and taken out of the trial,” said Dr. Hutchinson.
Proper patient selection is key
The research adds to the literature on rimegepant by providing data on multiple uses, as opposed to the phase 3 study, which only looked at single use, according to Olivia Begasse de Dhaem, MD, who is a neurology attending physician at Stamford (Conn.) Health and was the session moderator. Rimegepant and other oral CGRP receptor antagonists, including the FDA-approved ubrogepant and the investigative drug atogepant, will help fill the gap of patients who don’t tolerate or are ineligible for triptans, she said.
Dr. Begasse de Dhaem pointed out that patient selection remains important. “I think the main thing for patient care is to look at whether the patient we are treating would fit within the inclusion criteria, or would have been excluded from this study,” said Dr. Begasse de Dhaem. Specifically, according to its clinicaltrials.gov page, the trial excluded patients with hemiplegic and basilar migraine, as well as patients with uncontrolled, unstable, or recently diagnosed cardiovascular disease, those with a body mass index of 30 kg/m2 or higher, and hemoglobin A1c levels of 6.5% or higher. “This also looked at people with less than 15 migraine days per month, so it’s limited in how much we can extrapolate to people with chronic migraine who may take more than 7.7 rimegepant [doses, the mean value taken by trial participants] per month,” Dr. Begasse de Dhaem added.
She also applauded the inclusion of older patients in the study, noting that most migraine studies have an upper age limit.
The study included subjects who experienced 2-14 moderate or severe migraine attacks per month, and they were allowed to take other migraine medications. Cardiovascular risk factors did not prevent entry to the trial and, like the previous pivotal trial, the long-term safety study admitted subjects older than 65. Among the study cohort, 1,514 participants were told to treat migraine pain of any intensity with 75 mg rimegepant up to once per day on an as-needed basis (PRN), and a second group of 286 were told to take 75 mg rimegepant every other day for 12 weeks, along with PRN dosing on nonscheduled treatment days.
Nearly 90% of subjects were female, the mean age was 43.1 years, and 3.7% were age 65 or older. Among the study participants, 40.8% had cardiovascular risk factors, including 28.8% with one risk factor, and 12.1% with two or more. About 7% had a moderate to high (≥10%) 10-year cardiovascular risk by Framingham Risk Score, 23.6% had a family history of coronary artery disease, 11.7% were being treated for hypertension, 10.4% smoked, 8.3% were being treated with a statin, and 3.0% had a history of diabetes.
In total, subjects were exposed to 112,014 doses of rimegepant, a mean of 7.7 doses per 4-week period. The exposure was similar across all risk groups, which included zero risk factors, one risk factor, and two or more risk factors; FRS of less than 10%; and FRS of 10% or greater. The most common adverse events were upper respiratory tract infection (8.8%), nasopharyngitis (6.8%), and sinusitis (5.1%). The frequency of one or more adverse events was similar among those with zero cardiovascular risk factors (59.6%), one risk factor (61.4%), two or more risk factors (62.2%), FRS less than 10% (59.9%), and FRS of 10% or greater (59.9%).
The study was funded by Biohaven Pharmaceuticals. Dr. Hutchinson has been a consultant or advisory board member for Biohaven, Alder, Allergan, Amgen, Avanir, electroCore, Lilly, Novartis, Promius, Supernus, and Teva. She has been on the speaker’s bureau for Allergan, Amgen, Avanir, electroCore, Lilly, Novartis, Promius, Supernus, and Teva. Dr. Begasse de Dhaem has no relevant financial disclosures.
Results from a 1-year, open-label safety study suggest that Patients who fall into this category may be ineligible for treatment with triptans.
There are mechanistic concerns with rimegepant and related CGRP receptor antagonists. They block CGRP’s effect in the central nervous system, but CGRP is also active in blood vessels and the heart, leading to the possibility that countering its vasodilating effect could expose organs to risk of ischemia.
The Food and Drug Administration approved rimegepant in 2020 for treatment of acute migraine attacks. Sponsor Biohaven is also seeking approval for migraine prevention after a successful phase 3 study published January 2021 in The Lancet.
Susan Hutchinson, MD, who is a headache specialist at Orange County Migraine & Headache Center in Irvine, Calif., presented the results at the American Academy of Neurology’s 2021 annual meeting. The open-label study suggested that rimegepant is generally safe. “The proportion of subjects reporting at least one adverse event was similar among subjects whether they had zero, one, or two or more cardiovascular disease risk factors, and also among those with low and moderate to high 10-year cardiovascular risk, as determined by the Framingham Risk Score,” said Dr. Hutchinson during her presentation.
Still, there was one concerning case: A 53-year-old man experienced an attack of angina. But he already had angina prior to the study, was being treated for hypercholesterolemia, and had current or former exposure to statins. “This adverse event was deemed by the investigator to not be related to rimegepant,” said Dr. Hutchinson.
During the following question-and-answer session, an attendee pressed Dr. Hutchinson about the case, and she admitted to some initial doubts. “That was my concern when I saw those slides. I’m like, ‘oh, my goodness.’ ” She clarified that the man’s angina history dated to 2016, which was several years before the trial, and the episode of angina occurred 7 months after the first dose of rimegepant. “He was treated with nitroglycerin and taken out of the trial,” said Dr. Hutchinson.
Proper patient selection is key
The research adds to the literature on rimegepant by providing data on multiple uses, as opposed to the phase 3 study, which only looked at single use, according to Olivia Begasse de Dhaem, MD, who is a neurology attending physician at Stamford (Conn.) Health and was the session moderator. Rimegepant and other oral CGRP receptor antagonists, including the FDA-approved ubrogepant and the investigative drug atogepant, will help fill the gap of patients who don’t tolerate or are ineligible for triptans, she said.
Dr. Begasse de Dhaem pointed out that patient selection remains important. “I think the main thing for patient care is to look at whether the patient we are treating would fit within the inclusion criteria, or would have been excluded from this study,” said Dr. Begasse de Dhaem. Specifically, according to its clinicaltrials.gov page, the trial excluded patients with hemiplegic and basilar migraine, as well as patients with uncontrolled, unstable, or recently diagnosed cardiovascular disease, those with a body mass index of 30 kg/m2 or higher, and hemoglobin A1c levels of 6.5% or higher. “This also looked at people with less than 15 migraine days per month, so it’s limited in how much we can extrapolate to people with chronic migraine who may take more than 7.7 rimegepant [doses, the mean value taken by trial participants] per month,” Dr. Begasse de Dhaem added.
She also applauded the inclusion of older patients in the study, noting that most migraine studies have an upper age limit.
The study included subjects who experienced 2-14 moderate or severe migraine attacks per month, and they were allowed to take other migraine medications. Cardiovascular risk factors did not prevent entry to the trial and, like the previous pivotal trial, the long-term safety study admitted subjects older than 65. Among the study cohort, 1,514 participants were told to treat migraine pain of any intensity with 75 mg rimegepant up to once per day on an as-needed basis (PRN), and a second group of 286 were told to take 75 mg rimegepant every other day for 12 weeks, along with PRN dosing on nonscheduled treatment days.
Nearly 90% of subjects were female, the mean age was 43.1 years, and 3.7% were age 65 or older. Among the study participants, 40.8% had cardiovascular risk factors, including 28.8% with one risk factor, and 12.1% with two or more. About 7% had a moderate to high (≥10%) 10-year cardiovascular risk by Framingham Risk Score, 23.6% had a family history of coronary artery disease, 11.7% were being treated for hypertension, 10.4% smoked, 8.3% were being treated with a statin, and 3.0% had a history of diabetes.
In total, subjects were exposed to 112,014 doses of rimegepant, a mean of 7.7 doses per 4-week period. The exposure was similar across all risk groups, which included zero risk factors, one risk factor, and two or more risk factors; FRS of less than 10%; and FRS of 10% or greater. The most common adverse events were upper respiratory tract infection (8.8%), nasopharyngitis (6.8%), and sinusitis (5.1%). The frequency of one or more adverse events was similar among those with zero cardiovascular risk factors (59.6%), one risk factor (61.4%), two or more risk factors (62.2%), FRS less than 10% (59.9%), and FRS of 10% or greater (59.9%).
The study was funded by Biohaven Pharmaceuticals. Dr. Hutchinson has been a consultant or advisory board member for Biohaven, Alder, Allergan, Amgen, Avanir, electroCore, Lilly, Novartis, Promius, Supernus, and Teva. She has been on the speaker’s bureau for Allergan, Amgen, Avanir, electroCore, Lilly, Novartis, Promius, Supernus, and Teva. Dr. Begasse de Dhaem has no relevant financial disclosures.
Results from a 1-year, open-label safety study suggest that Patients who fall into this category may be ineligible for treatment with triptans.
There are mechanistic concerns with rimegepant and related CGRP receptor antagonists. They block CGRP’s effect in the central nervous system, but CGRP is also active in blood vessels and the heart, leading to the possibility that countering its vasodilating effect could expose organs to risk of ischemia.
The Food and Drug Administration approved rimegepant in 2020 for treatment of acute migraine attacks. Sponsor Biohaven is also seeking approval for migraine prevention after a successful phase 3 study published January 2021 in The Lancet.
Susan Hutchinson, MD, who is a headache specialist at Orange County Migraine & Headache Center in Irvine, Calif., presented the results at the American Academy of Neurology’s 2021 annual meeting. The open-label study suggested that rimegepant is generally safe. “The proportion of subjects reporting at least one adverse event was similar among subjects whether they had zero, one, or two or more cardiovascular disease risk factors, and also among those with low and moderate to high 10-year cardiovascular risk, as determined by the Framingham Risk Score,” said Dr. Hutchinson during her presentation.
Still, there was one concerning case: A 53-year-old man experienced an attack of angina. But he already had angina prior to the study, was being treated for hypercholesterolemia, and had current or former exposure to statins. “This adverse event was deemed by the investigator to not be related to rimegepant,” said Dr. Hutchinson.
During the following question-and-answer session, an attendee pressed Dr. Hutchinson about the case, and she admitted to some initial doubts. “That was my concern when I saw those slides. I’m like, ‘oh, my goodness.’ ” She clarified that the man’s angina history dated to 2016, which was several years before the trial, and the episode of angina occurred 7 months after the first dose of rimegepant. “He was treated with nitroglycerin and taken out of the trial,” said Dr. Hutchinson.
Proper patient selection is key
The research adds to the literature on rimegepant by providing data on multiple uses, as opposed to the phase 3 study, which only looked at single use, according to Olivia Begasse de Dhaem, MD, who is a neurology attending physician at Stamford (Conn.) Health and was the session moderator. Rimegepant and other oral CGRP receptor antagonists, including the FDA-approved ubrogepant and the investigative drug atogepant, will help fill the gap of patients who don’t tolerate or are ineligible for triptans, she said.
Dr. Begasse de Dhaem pointed out that patient selection remains important. “I think the main thing for patient care is to look at whether the patient we are treating would fit within the inclusion criteria, or would have been excluded from this study,” said Dr. Begasse de Dhaem. Specifically, according to its clinicaltrials.gov page, the trial excluded patients with hemiplegic and basilar migraine, as well as patients with uncontrolled, unstable, or recently diagnosed cardiovascular disease, those with a body mass index of 30 kg/m2 or higher, and hemoglobin A1c levels of 6.5% or higher. “This also looked at people with less than 15 migraine days per month, so it’s limited in how much we can extrapolate to people with chronic migraine who may take more than 7.7 rimegepant [doses, the mean value taken by trial participants] per month,” Dr. Begasse de Dhaem added.
She also applauded the inclusion of older patients in the study, noting that most migraine studies have an upper age limit.
The study included subjects who experienced 2-14 moderate or severe migraine attacks per month, and they were allowed to take other migraine medications. Cardiovascular risk factors did not prevent entry to the trial and, like the previous pivotal trial, the long-term safety study admitted subjects older than 65. Among the study cohort, 1,514 participants were told to treat migraine pain of any intensity with 75 mg rimegepant up to once per day on an as-needed basis (PRN), and a second group of 286 were told to take 75 mg rimegepant every other day for 12 weeks, along with PRN dosing on nonscheduled treatment days.
Nearly 90% of subjects were female, the mean age was 43.1 years, and 3.7% were age 65 or older. Among the study participants, 40.8% had cardiovascular risk factors, including 28.8% with one risk factor, and 12.1% with two or more. About 7% had a moderate to high (≥10%) 10-year cardiovascular risk by Framingham Risk Score, 23.6% had a family history of coronary artery disease, 11.7% were being treated for hypertension, 10.4% smoked, 8.3% were being treated with a statin, and 3.0% had a history of diabetes.
In total, subjects were exposed to 112,014 doses of rimegepant, a mean of 7.7 doses per 4-week period. The exposure was similar across all risk groups, which included zero risk factors, one risk factor, and two or more risk factors; FRS of less than 10%; and FRS of 10% or greater. The most common adverse events were upper respiratory tract infection (8.8%), nasopharyngitis (6.8%), and sinusitis (5.1%). The frequency of one or more adverse events was similar among those with zero cardiovascular risk factors (59.6%), one risk factor (61.4%), two or more risk factors (62.2%), FRS less than 10% (59.9%), and FRS of 10% or greater (59.9%).
The study was funded by Biohaven Pharmaceuticals. Dr. Hutchinson has been a consultant or advisory board member for Biohaven, Alder, Allergan, Amgen, Avanir, electroCore, Lilly, Novartis, Promius, Supernus, and Teva. She has been on the speaker’s bureau for Allergan, Amgen, Avanir, electroCore, Lilly, Novartis, Promius, Supernus, and Teva. Dr. Begasse de Dhaem has no relevant financial disclosures.
FROM AAN 2021
Do ObGyns plan on getting a 9vHPV vaccine?
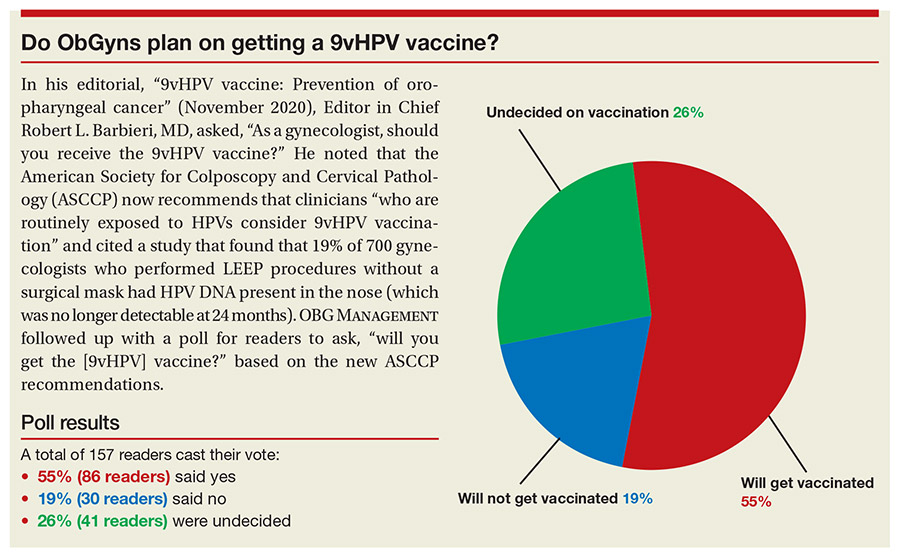
In his editorial, "9vHPV vaccine: Prevention of oropharyngeal cancer" (November 2020), Editor in Chief Robert L. Barbieri, MD, asked, "As a gynecologist, should you receive the 9vHPV vaccine?" He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) now recommends that clinicians "who are routinely exposed to HPVs consider 9vHPV vaccination" and cited a study that found that 19% of 700 gynecologists who performed LEEP procedures without a surgical mask had HPV DNA present in the nose (which was no longer detectable at 24 months). OBG MANAGEMENT followed up with a poll for readers to ask, "will you get the [9vHPV] vaccine?" based on the new ASCCP recommendations.
A total of 157 readers cast their vote:
55% (86 readers) said yes
19% (30 readers) said no
26% (41 readers) were undecided
In his editorial, "9vHPV vaccine: Prevention of oropharyngeal cancer" (November 2020), Editor in Chief Robert L. Barbieri, MD, asked, "As a gynecologist, should you receive the 9vHPV vaccine?" He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) now recommends that clinicians "who are routinely exposed to HPVs consider 9vHPV vaccination" and cited a study that found that 19% of 700 gynecologists who performed LEEP procedures without a surgical mask had HPV DNA present in the nose (which was no longer detectable at 24 months). OBG MANAGEMENT followed up with a poll for readers to ask, "will you get the [9vHPV] vaccine?" based on the new ASCCP recommendations.
A total of 157 readers cast their vote:
55% (86 readers) said yes
19% (30 readers) said no
26% (41 readers) were undecided

In his editorial, "9vHPV vaccine: Prevention of oropharyngeal cancer" (November 2020), Editor in Chief Robert L. Barbieri, MD, asked, "As a gynecologist, should you receive the 9vHPV vaccine?" He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) now recommends that clinicians "who are routinely exposed to HPVs consider 9vHPV vaccination" and cited a study that found that 19% of 700 gynecologists who performed LEEP procedures without a surgical mask had HPV DNA present in the nose (which was no longer detectable at 24 months). OBG MANAGEMENT followed up with a poll for readers to ask, "will you get the [9vHPV] vaccine?" based on the new ASCCP recommendations.
A total of 157 readers cast their vote:
55% (86 readers) said yes
19% (30 readers) said no
26% (41 readers) were undecided

Dr. G. Gayle Stephens was a teacher, progressive force, and ‘poet laureate of family medicine’
G. Gayle Stephens, MD, who is roundly regarded as one of the founders of family medicine, gave his talk “Family Medicine as Counterculture” at the Society of Teachers of Family Medicine annual conference in 1979, 10 years after the specialty’s establishment.
The speech was then published, republished 10 years later, and, like many of Dr. Stephen’s other essays and articles, remains very much alive in the minds of practicing family physicians, in the teachings of FP academicians, and in the Google searches of budding FPs.
The late Dr. Stephens saw family medicine as a counterculture within medicine, rooted in social change. In his speech he examined these roots – in reform initiatives in the 1960s, and in certain philosophies and “minority” movements such as agrarianism and the preservation of rural life, utopianism, humanism, consumerism, and feminism.
He also looked forward, challenging the specialty to remain true to itself and its roots – to its belief in “uninhibited access” to medical care for everyone, for instance, and to continual whole-person and family-oriented care – and cautioned against moving to resemble the “rest of the medical bureaucracy.”
“Clearly we have been on the side of change in American life. We have identified ourselves with certain minorities and minority positions ... [and] been counter to many of the dominant forces in society,” Dr. Stephens said in his talk. Family practice “succeeded in the decade just past because we were identified with reforms that are more pervasive and powerful than ourselves.”
The family practice movement has “more in common with [the] counterculture than it does with the dominant scientific medical establishment,” he said.
A teacher and founder of medical education programs
Larry A. Green, MD, who was pursuing his own residency training as Dr. Stephens was leading a department of family practice, said
“It was from this philosophical position that he became a synthesizer and observer and interpreter of what was going on in the development of family medicine,” said Dr. Green, Distinguished Professor and Epperson Zorn Chair for Innovation in Family Medicine and Primary Care at the University of Colorado at Denver, Aurora.
Dr. Stephens, who died at home in 2014 at the age of 85, was “probably the most important person in exposing what I now consider to be a fact – that family medicine was the product of social changes ... of social movements related to women’s rights, civil rights, and social responsibility,” Dr. Green said. “He could recall lessons from the past and forecast the challenges of the future. And there was no one more effective in clarifying the importance of personal [doctor-patient] relationships in family medicine.”
After years of general practice in rural Wichita, Kan., his wife Eula Jean’s hometown, Dr. Stephens founded and led one of the first family medicine residencies at Wesley Hospital in Wichita in 1967. His core principles, as described on today’s Wesley Family Medicine Residency website, included that a family physician consider the whole person, be honest, have a full scope of training including behavioral and mental health, and be “reflective about him/herself ... [learning about] his/her assets, liabilities, foibles, and idiosyncrasies.” Dr. Stephens, who had grown up in rural Ashburn, Mo., later became the founding dean of the School of Primary Medical Care at the University of Alabama in Huntsville and then chaired the department of family practice at the University of Alabama at Birmingham.
A thought leader for family medicine
He held numerous state and national leadership positions, and initiated what became the Keystone Conference Series – an invitational gathering of leaders in family medicine that examined and discuss the specialty’s ongoing development. In 2006, he was elected to the Institute of Medicine of the National Academies of Science.
Dr. Stephens authored a textbook, The Intellectual Basis of Family Medicine (Tucson, Ariz.: Winter Publishing Company, 1982), and authored essays, which Dr. Green said will stand the test of time.
“Some of us refer to him as the poet laureate of family medicine,” Dr. Green noted.
In a 1974 article on clinical wisdom, Dr. Stephens wrote that “it is not enough to determine what condition the patient has, but also what patient has the condition.” In another of these essays, which was published in 1979, Dr. Stephens wrote that “physicians need to keep in touch with their own tradition and with public welfare if they are to be considered moral by the society that sponsors them, and from which they take their strength and privilege.”
These excerpts are featured in an article by John P. Geyman, MD, published in 2011 in Family Medicine, called “G. Gayle Stephens Festschrift”.
A ‘progressive force’
Linda Prine, MD, professor of family and community medicine at the Icahn School of Medicine at Mount Sinai, New York, knows of Dr. Stephens from her teachers. “The people I looked up to when I was a younger physician were quoting his Counterculture article,” she said.
“It’s not that I studied him. But whenever I heard someone speak about the values of family medicine, his name would come up [and] the values of universal health care and community care and putting the patients’ interests first ahead of the insurance companies and being a doctor for the whole family,” Dr. Prine said. Dr. Stephens was a “progressive force that our specialty has not always lived up to.”
Dr. Stephens voiced serious concerns about the impact of managed care in the 1980s and of “gatekeeping,” a practice intended to control access to specialists and reduce costs.
“He was many times not welcomed by family medicine [for his warnings] against the temptations that managed care presented,” said Dr. Green, the founding director of the Robert Graham Center, Washington. “He saw the conflict of interest of being a gatekeeper, how that would erode trust in a personal relationship with your personal doctor.”
“Gayle thought it was a disaster waiting to happen, and it was,” he said, referring to the eventual rejection by the public of barriers to direct access to specialists.
Through the 1990s and more recently, Dr. Stephens expressed frustration with the “medical-industrial complex” and the decline of family medicine after its surge in the 1970s and 1980s, Dr. Green said. “But in my opinion, near the end of his life, he was encouraged by young leaders who he saw grasped the important ideas from the ages.”
Dr. Stephens’ interest in medical education extended to nurses and nurse practitioners (the latter of whom had begun their discipline in the mid-1960s), and to optometrists, for whom he taught a recurring course in “physical diagnosis.”
A listener and proponent of listening
Linda Tompkins, RN, FNP, of Newton, Kan., trained with Dr. Stephens at part of a year-long nurse education program in the early 1970s at Wichita (Kan.) State University, where he was leading the department of family practice (prior to moving to Alabama). “You couldn’t ask too many questions,” she said. “And he never talked down to us, he wasn’t condescending. There were not a lot of doctors like that.”
Dr. Stephens spoke and wrote often about the importance of listening –about how it was vital to the “durable clinical relationship.” It was also vital to his writing and to his impact on the teachers of family medicine, said Dan Ostergaard, MD, who served as a residency director and in various staff leadership positions at the American Academy of Family Physicians, including in its division of education.
“He created a lot of aha moments for me, about where we came from and what we really need to be [as a specialty] and where we need to go,” said Dr. Ostergaard. “To be such a great thinker and a great writer, you have to be a great listener.”
“I can just visualize him,” he said, “leaning back in his chair while we were talking about residency criteria [or other issues], with a half-smile on his face and his reading glasses down his note, smoking his pipe and just looking at all of us, listening.”
Dr. Stephens’ papers are housed in the Center for the History of Family Medicine, a project of the AAFP Foundation.
G. Gayle Stephens, MD, who is roundly regarded as one of the founders of family medicine, gave his talk “Family Medicine as Counterculture” at the Society of Teachers of Family Medicine annual conference in 1979, 10 years after the specialty’s establishment.
The speech was then published, republished 10 years later, and, like many of Dr. Stephen’s other essays and articles, remains very much alive in the minds of practicing family physicians, in the teachings of FP academicians, and in the Google searches of budding FPs.
The late Dr. Stephens saw family medicine as a counterculture within medicine, rooted in social change. In his speech he examined these roots – in reform initiatives in the 1960s, and in certain philosophies and “minority” movements such as agrarianism and the preservation of rural life, utopianism, humanism, consumerism, and feminism.
He also looked forward, challenging the specialty to remain true to itself and its roots – to its belief in “uninhibited access” to medical care for everyone, for instance, and to continual whole-person and family-oriented care – and cautioned against moving to resemble the “rest of the medical bureaucracy.”
“Clearly we have been on the side of change in American life. We have identified ourselves with certain minorities and minority positions ... [and] been counter to many of the dominant forces in society,” Dr. Stephens said in his talk. Family practice “succeeded in the decade just past because we were identified with reforms that are more pervasive and powerful than ourselves.”
The family practice movement has “more in common with [the] counterculture than it does with the dominant scientific medical establishment,” he said.
A teacher and founder of medical education programs
Larry A. Green, MD, who was pursuing his own residency training as Dr. Stephens was leading a department of family practice, said
“It was from this philosophical position that he became a synthesizer and observer and interpreter of what was going on in the development of family medicine,” said Dr. Green, Distinguished Professor and Epperson Zorn Chair for Innovation in Family Medicine and Primary Care at the University of Colorado at Denver, Aurora.
Dr. Stephens, who died at home in 2014 at the age of 85, was “probably the most important person in exposing what I now consider to be a fact – that family medicine was the product of social changes ... of social movements related to women’s rights, civil rights, and social responsibility,” Dr. Green said. “He could recall lessons from the past and forecast the challenges of the future. And there was no one more effective in clarifying the importance of personal [doctor-patient] relationships in family medicine.”
After years of general practice in rural Wichita, Kan., his wife Eula Jean’s hometown, Dr. Stephens founded and led one of the first family medicine residencies at Wesley Hospital in Wichita in 1967. His core principles, as described on today’s Wesley Family Medicine Residency website, included that a family physician consider the whole person, be honest, have a full scope of training including behavioral and mental health, and be “reflective about him/herself ... [learning about] his/her assets, liabilities, foibles, and idiosyncrasies.” Dr. Stephens, who had grown up in rural Ashburn, Mo., later became the founding dean of the School of Primary Medical Care at the University of Alabama in Huntsville and then chaired the department of family practice at the University of Alabama at Birmingham.
A thought leader for family medicine
He held numerous state and national leadership positions, and initiated what became the Keystone Conference Series – an invitational gathering of leaders in family medicine that examined and discuss the specialty’s ongoing development. In 2006, he was elected to the Institute of Medicine of the National Academies of Science.
Dr. Stephens authored a textbook, The Intellectual Basis of Family Medicine (Tucson, Ariz.: Winter Publishing Company, 1982), and authored essays, which Dr. Green said will stand the test of time.
“Some of us refer to him as the poet laureate of family medicine,” Dr. Green noted.
In a 1974 article on clinical wisdom, Dr. Stephens wrote that “it is not enough to determine what condition the patient has, but also what patient has the condition.” In another of these essays, which was published in 1979, Dr. Stephens wrote that “physicians need to keep in touch with their own tradition and with public welfare if they are to be considered moral by the society that sponsors them, and from which they take their strength and privilege.”
These excerpts are featured in an article by John P. Geyman, MD, published in 2011 in Family Medicine, called “G. Gayle Stephens Festschrift”.
A ‘progressive force’
Linda Prine, MD, professor of family and community medicine at the Icahn School of Medicine at Mount Sinai, New York, knows of Dr. Stephens from her teachers. “The people I looked up to when I was a younger physician were quoting his Counterculture article,” she said.
“It’s not that I studied him. But whenever I heard someone speak about the values of family medicine, his name would come up [and] the values of universal health care and community care and putting the patients’ interests first ahead of the insurance companies and being a doctor for the whole family,” Dr. Prine said. Dr. Stephens was a “progressive force that our specialty has not always lived up to.”
Dr. Stephens voiced serious concerns about the impact of managed care in the 1980s and of “gatekeeping,” a practice intended to control access to specialists and reduce costs.
“He was many times not welcomed by family medicine [for his warnings] against the temptations that managed care presented,” said Dr. Green, the founding director of the Robert Graham Center, Washington. “He saw the conflict of interest of being a gatekeeper, how that would erode trust in a personal relationship with your personal doctor.”
“Gayle thought it was a disaster waiting to happen, and it was,” he said, referring to the eventual rejection by the public of barriers to direct access to specialists.
Through the 1990s and more recently, Dr. Stephens expressed frustration with the “medical-industrial complex” and the decline of family medicine after its surge in the 1970s and 1980s, Dr. Green said. “But in my opinion, near the end of his life, he was encouraged by young leaders who he saw grasped the important ideas from the ages.”
Dr. Stephens’ interest in medical education extended to nurses and nurse practitioners (the latter of whom had begun their discipline in the mid-1960s), and to optometrists, for whom he taught a recurring course in “physical diagnosis.”
A listener and proponent of listening
Linda Tompkins, RN, FNP, of Newton, Kan., trained with Dr. Stephens at part of a year-long nurse education program in the early 1970s at Wichita (Kan.) State University, where he was leading the department of family practice (prior to moving to Alabama). “You couldn’t ask too many questions,” she said. “And he never talked down to us, he wasn’t condescending. There were not a lot of doctors like that.”
Dr. Stephens spoke and wrote often about the importance of listening –about how it was vital to the “durable clinical relationship.” It was also vital to his writing and to his impact on the teachers of family medicine, said Dan Ostergaard, MD, who served as a residency director and in various staff leadership positions at the American Academy of Family Physicians, including in its division of education.
“He created a lot of aha moments for me, about where we came from and what we really need to be [as a specialty] and where we need to go,” said Dr. Ostergaard. “To be such a great thinker and a great writer, you have to be a great listener.”
“I can just visualize him,” he said, “leaning back in his chair while we were talking about residency criteria [or other issues], with a half-smile on his face and his reading glasses down his note, smoking his pipe and just looking at all of us, listening.”
Dr. Stephens’ papers are housed in the Center for the History of Family Medicine, a project of the AAFP Foundation.
G. Gayle Stephens, MD, who is roundly regarded as one of the founders of family medicine, gave his talk “Family Medicine as Counterculture” at the Society of Teachers of Family Medicine annual conference in 1979, 10 years after the specialty’s establishment.
The speech was then published, republished 10 years later, and, like many of Dr. Stephen’s other essays and articles, remains very much alive in the minds of practicing family physicians, in the teachings of FP academicians, and in the Google searches of budding FPs.
The late Dr. Stephens saw family medicine as a counterculture within medicine, rooted in social change. In his speech he examined these roots – in reform initiatives in the 1960s, and in certain philosophies and “minority” movements such as agrarianism and the preservation of rural life, utopianism, humanism, consumerism, and feminism.
He also looked forward, challenging the specialty to remain true to itself and its roots – to its belief in “uninhibited access” to medical care for everyone, for instance, and to continual whole-person and family-oriented care – and cautioned against moving to resemble the “rest of the medical bureaucracy.”
“Clearly we have been on the side of change in American life. We have identified ourselves with certain minorities and minority positions ... [and] been counter to many of the dominant forces in society,” Dr. Stephens said in his talk. Family practice “succeeded in the decade just past because we were identified with reforms that are more pervasive and powerful than ourselves.”
The family practice movement has “more in common with [the] counterculture than it does with the dominant scientific medical establishment,” he said.
A teacher and founder of medical education programs
Larry A. Green, MD, who was pursuing his own residency training as Dr. Stephens was leading a department of family practice, said
“It was from this philosophical position that he became a synthesizer and observer and interpreter of what was going on in the development of family medicine,” said Dr. Green, Distinguished Professor and Epperson Zorn Chair for Innovation in Family Medicine and Primary Care at the University of Colorado at Denver, Aurora.
Dr. Stephens, who died at home in 2014 at the age of 85, was “probably the most important person in exposing what I now consider to be a fact – that family medicine was the product of social changes ... of social movements related to women’s rights, civil rights, and social responsibility,” Dr. Green said. “He could recall lessons from the past and forecast the challenges of the future. And there was no one more effective in clarifying the importance of personal [doctor-patient] relationships in family medicine.”
After years of general practice in rural Wichita, Kan., his wife Eula Jean’s hometown, Dr. Stephens founded and led one of the first family medicine residencies at Wesley Hospital in Wichita in 1967. His core principles, as described on today’s Wesley Family Medicine Residency website, included that a family physician consider the whole person, be honest, have a full scope of training including behavioral and mental health, and be “reflective about him/herself ... [learning about] his/her assets, liabilities, foibles, and idiosyncrasies.” Dr. Stephens, who had grown up in rural Ashburn, Mo., later became the founding dean of the School of Primary Medical Care at the University of Alabama in Huntsville and then chaired the department of family practice at the University of Alabama at Birmingham.
A thought leader for family medicine
He held numerous state and national leadership positions, and initiated what became the Keystone Conference Series – an invitational gathering of leaders in family medicine that examined and discuss the specialty’s ongoing development. In 2006, he was elected to the Institute of Medicine of the National Academies of Science.
Dr. Stephens authored a textbook, The Intellectual Basis of Family Medicine (Tucson, Ariz.: Winter Publishing Company, 1982), and authored essays, which Dr. Green said will stand the test of time.
“Some of us refer to him as the poet laureate of family medicine,” Dr. Green noted.
In a 1974 article on clinical wisdom, Dr. Stephens wrote that “it is not enough to determine what condition the patient has, but also what patient has the condition.” In another of these essays, which was published in 1979, Dr. Stephens wrote that “physicians need to keep in touch with their own tradition and with public welfare if they are to be considered moral by the society that sponsors them, and from which they take their strength and privilege.”
These excerpts are featured in an article by John P. Geyman, MD, published in 2011 in Family Medicine, called “G. Gayle Stephens Festschrift”.
A ‘progressive force’
Linda Prine, MD, professor of family and community medicine at the Icahn School of Medicine at Mount Sinai, New York, knows of Dr. Stephens from her teachers. “The people I looked up to when I was a younger physician were quoting his Counterculture article,” she said.
“It’s not that I studied him. But whenever I heard someone speak about the values of family medicine, his name would come up [and] the values of universal health care and community care and putting the patients’ interests first ahead of the insurance companies and being a doctor for the whole family,” Dr. Prine said. Dr. Stephens was a “progressive force that our specialty has not always lived up to.”
Dr. Stephens voiced serious concerns about the impact of managed care in the 1980s and of “gatekeeping,” a practice intended to control access to specialists and reduce costs.
“He was many times not welcomed by family medicine [for his warnings] against the temptations that managed care presented,” said Dr. Green, the founding director of the Robert Graham Center, Washington. “He saw the conflict of interest of being a gatekeeper, how that would erode trust in a personal relationship with your personal doctor.”
“Gayle thought it was a disaster waiting to happen, and it was,” he said, referring to the eventual rejection by the public of barriers to direct access to specialists.
Through the 1990s and more recently, Dr. Stephens expressed frustration with the “medical-industrial complex” and the decline of family medicine after its surge in the 1970s and 1980s, Dr. Green said. “But in my opinion, near the end of his life, he was encouraged by young leaders who he saw grasped the important ideas from the ages.”
Dr. Stephens’ interest in medical education extended to nurses and nurse practitioners (the latter of whom had begun their discipline in the mid-1960s), and to optometrists, for whom he taught a recurring course in “physical diagnosis.”
A listener and proponent of listening
Linda Tompkins, RN, FNP, of Newton, Kan., trained with Dr. Stephens at part of a year-long nurse education program in the early 1970s at Wichita (Kan.) State University, where he was leading the department of family practice (prior to moving to Alabama). “You couldn’t ask too many questions,” she said. “And he never talked down to us, he wasn’t condescending. There were not a lot of doctors like that.”
Dr. Stephens spoke and wrote often about the importance of listening –about how it was vital to the “durable clinical relationship.” It was also vital to his writing and to his impact on the teachers of family medicine, said Dan Ostergaard, MD, who served as a residency director and in various staff leadership positions at the American Academy of Family Physicians, including in its division of education.
“He created a lot of aha moments for me, about where we came from and what we really need to be [as a specialty] and where we need to go,” said Dr. Ostergaard. “To be such a great thinker and a great writer, you have to be a great listener.”
“I can just visualize him,” he said, “leaning back in his chair while we were talking about residency criteria [or other issues], with a half-smile on his face and his reading glasses down his note, smoking his pipe and just looking at all of us, listening.”
Dr. Stephens’ papers are housed in the Center for the History of Family Medicine, a project of the AAFP Foundation.
Common MS treatment wears off more quickly in Black patients
new research suggests. In a study of almost 200 patients, Black participants with MS or NMOSD showed significantly more rapid B-cell repopulation 6-12 months after receiving anti-CD20 infusion therapy with rituximab or ocrelizumab (Rituxan, Ocrevus, Genentech) than did White participants.
“The results showed that this B-cell targeted therapy wore off more quickly in African Americans,” said study coinvestigator Gregg J. Silverman, MD, a professor at New York University.
He said that, although the study was more observational in design, “over time when people come back to the clinic, it gives you an idea of whether the agent is still working in their bodies.”
Overall, “our findings raise the question of whether the same therapy dose may be equally effective for all people,” coinvestigator Ilya Kister, MD, also from NYU, added in a press release.
Dr. Kister noted that this could have implications for the way Black patients with autoimmune diseases are treated in the future.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
More severe disease in Black patients
Anti-CD20 infusion therapy, or B-cell depletion therapy, is commonly used to treat autoimmune diseases, including MS and NMOSD. “While previous research has shown that this type of infusion therapy is effective for people with these diseases, we also know that Black people tend to have more severe courses of MS,” Dr. Kister said.
“We wanted to compare how quickly the B cells came back in Black people and White people after treatment,” he added.
Dr. Silverman noted that he has been “studying this agent in autoimmune disease for many years. And from all the different studies, I don’t think we had the right population to ask this question. Demographics were just reviewed as they were.”
The current study included 168 participants (mean age, 44 years; 71% women) who had a diagnosis of MS (n = 134) or NMOSD (n = 32) or who were considered to have MS or NMOSD (n = 2). In addition, 36% of the participants self-identified as Black or African American, 36% self-identified as White, and 28% self-identified as another race.
Flow cytometry results were available for all patients after undergoing anti-CD20 infusions at the NYU MS Care Center. Cluster analyses were conducted on the following B-cell subsets: CD19, CD20, IgD, and CD27. “B-cell repopulation was defined as any detectable number of CD19+ cells on flow cytometry,” the investigators reported.
Clinical implications?
Results showed that 29.8% of the full study group showed B-cell repopulation a mean of 6.8 months after infusion. In those with B-cell repopulation, 80.3% had IgD+/CD27– subsets, 11.6% had IgD–/CD27+, 6.2% had IgD–/CD27–, and 1.8% had IgD+/CD27+. These B-cell subset ratios did not differ significantly between the Black and White participants.
Interestingly, no patients showed B-cell repopulation before 4 months after infusion. From 4 to 6 months after infusion, there were no significant differences between the Black and White participants in terms of frequency of B-cell repopulation (20.8% vs. 17.9%, respectively; P = .79).
However, repopulation was significantly more frequent in the Black patients 6-12 months after infusion (76.2% vs. 33.3%; P = .02).
Overall, the findings “may have implications for clinical management of MS/NMOSD” in Black individuals, the investigators wrote.
“I was impressed by the differences we saw in responses of patients that were self-declaring as African Americans versus those who were Whites,” Dr. Silverman said. However, “as we say in science: it gives us an answer but it raises even more questions, which may well be important for helping us understand how the agent works and how the disease affects different people.”
Still, Dr. Silverman noted that the findings give clinicians using the agent “a signal that they should be very vigilant. It was an observation at one center, but we’re asking our colleagues [at other clinics] to think about being more careful as they review data with their patients.”
He added that future multicenter studies will allow these issues to be assessed more comprehensively. “This was a discovery study; it now needs validation; and maybe the next step would be looking into the mechanism.”
Dr. Silverman pointed out that the Food and Drug Administration–approved label for this type of therapy “allows for somewhat more frequent dosing. So that might be indicated if it’s found that it’s wearing off in an individual. Perhaps they should be treated more frequently?”
“At a minimum, this has raised our vigilance – and we’re interested to see what the feedback will be at the [AAN] meeting,” he added.
Real-world data
Commenting on the findings, Eric Klawiter, MD, associate professor of neurology at Harvard Medical School and director of the Multiple Sclerosis and NMO unit at Massachusetts General Hospital, both in Boston, noted that an important study factor was the focus on repopulation to identify specific groups “who may be early repopulators” as it relates to disease activity and disability progression in MS.
“I thought this was a nicely designed study that made good use of real-world data in MS and NMOSD,” added Dr. Klawiter, who was not involved with the research. He pointed out that timing was another interesting aspect of the study. “As we typically use these cell-depleting agents on an ‘every-6-month’ basis, the most pertinent time frame surrounds those that repopulate prior to 6 months.”
If the current study would have shown differences between the Black and White participants at that time point, “I think that would have been most pertinent from a clinical standpoint and a greater opportunity for intervention,” Dr. Klawiter said. “But we saw that, before 4 and 6 months, [the difference] wasn’t significant.”
Still, “after 6 months, the study demonstrates that Black people with MS and NMOSD may repopulate faster,” he added.
“The only real change a clinician could make would be to modify the frequency of the dosing. So if we can identify certain characteristics that would lead you to want to evaluate for the need of redosing sooner, I think that would be useful,” he said.
Specific characteristics identified in previous research include body mass index. “If there are also ethnicity factors, that would be an additional demographic factor that a clinician should pay close attention to,” said Dr. Klawiter.
He noted that his current practice is to check flow cytometry and B-cell counts at the time of a patient’s next infusion. “And if I’m seeing that B-cell levels are repleting at that time point, I am already then making adjustments with their next infusion as to the dosing frequency,” he added.
“This [study] may elucidate some of the potential reasons why we see some people replete their B cells faster than others, but I think additional studies are necessary to make that determination,” Dr. Klawiter concluded.
Genentech provided funding for the study. Dr. Silverman reported no relevant financial relationships. Dr. Klawiter reported having received research funds and consulting fees from Genentech.
A version of this article first appeared on Medscape.com.
new research suggests. In a study of almost 200 patients, Black participants with MS or NMOSD showed significantly more rapid B-cell repopulation 6-12 months after receiving anti-CD20 infusion therapy with rituximab or ocrelizumab (Rituxan, Ocrevus, Genentech) than did White participants.
“The results showed that this B-cell targeted therapy wore off more quickly in African Americans,” said study coinvestigator Gregg J. Silverman, MD, a professor at New York University.
He said that, although the study was more observational in design, “over time when people come back to the clinic, it gives you an idea of whether the agent is still working in their bodies.”
Overall, “our findings raise the question of whether the same therapy dose may be equally effective for all people,” coinvestigator Ilya Kister, MD, also from NYU, added in a press release.
Dr. Kister noted that this could have implications for the way Black patients with autoimmune diseases are treated in the future.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
More severe disease in Black patients
Anti-CD20 infusion therapy, or B-cell depletion therapy, is commonly used to treat autoimmune diseases, including MS and NMOSD. “While previous research has shown that this type of infusion therapy is effective for people with these diseases, we also know that Black people tend to have more severe courses of MS,” Dr. Kister said.
“We wanted to compare how quickly the B cells came back in Black people and White people after treatment,” he added.
Dr. Silverman noted that he has been “studying this agent in autoimmune disease for many years. And from all the different studies, I don’t think we had the right population to ask this question. Demographics were just reviewed as they were.”
The current study included 168 participants (mean age, 44 years; 71% women) who had a diagnosis of MS (n = 134) or NMOSD (n = 32) or who were considered to have MS or NMOSD (n = 2). In addition, 36% of the participants self-identified as Black or African American, 36% self-identified as White, and 28% self-identified as another race.
Flow cytometry results were available for all patients after undergoing anti-CD20 infusions at the NYU MS Care Center. Cluster analyses were conducted on the following B-cell subsets: CD19, CD20, IgD, and CD27. “B-cell repopulation was defined as any detectable number of CD19+ cells on flow cytometry,” the investigators reported.
Clinical implications?
Results showed that 29.8% of the full study group showed B-cell repopulation a mean of 6.8 months after infusion. In those with B-cell repopulation, 80.3% had IgD+/CD27– subsets, 11.6% had IgD–/CD27+, 6.2% had IgD–/CD27–, and 1.8% had IgD+/CD27+. These B-cell subset ratios did not differ significantly between the Black and White participants.
Interestingly, no patients showed B-cell repopulation before 4 months after infusion. From 4 to 6 months after infusion, there were no significant differences between the Black and White participants in terms of frequency of B-cell repopulation (20.8% vs. 17.9%, respectively; P = .79).
However, repopulation was significantly more frequent in the Black patients 6-12 months after infusion (76.2% vs. 33.3%; P = .02).
Overall, the findings “may have implications for clinical management of MS/NMOSD” in Black individuals, the investigators wrote.
“I was impressed by the differences we saw in responses of patients that were self-declaring as African Americans versus those who were Whites,” Dr. Silverman said. However, “as we say in science: it gives us an answer but it raises even more questions, which may well be important for helping us understand how the agent works and how the disease affects different people.”
Still, Dr. Silverman noted that the findings give clinicians using the agent “a signal that they should be very vigilant. It was an observation at one center, but we’re asking our colleagues [at other clinics] to think about being more careful as they review data with their patients.”
He added that future multicenter studies will allow these issues to be assessed more comprehensively. “This was a discovery study; it now needs validation; and maybe the next step would be looking into the mechanism.”
Dr. Silverman pointed out that the Food and Drug Administration–approved label for this type of therapy “allows for somewhat more frequent dosing. So that might be indicated if it’s found that it’s wearing off in an individual. Perhaps they should be treated more frequently?”
“At a minimum, this has raised our vigilance – and we’re interested to see what the feedback will be at the [AAN] meeting,” he added.
Real-world data
Commenting on the findings, Eric Klawiter, MD, associate professor of neurology at Harvard Medical School and director of the Multiple Sclerosis and NMO unit at Massachusetts General Hospital, both in Boston, noted that an important study factor was the focus on repopulation to identify specific groups “who may be early repopulators” as it relates to disease activity and disability progression in MS.
“I thought this was a nicely designed study that made good use of real-world data in MS and NMOSD,” added Dr. Klawiter, who was not involved with the research. He pointed out that timing was another interesting aspect of the study. “As we typically use these cell-depleting agents on an ‘every-6-month’ basis, the most pertinent time frame surrounds those that repopulate prior to 6 months.”
If the current study would have shown differences between the Black and White participants at that time point, “I think that would have been most pertinent from a clinical standpoint and a greater opportunity for intervention,” Dr. Klawiter said. “But we saw that, before 4 and 6 months, [the difference] wasn’t significant.”
Still, “after 6 months, the study demonstrates that Black people with MS and NMOSD may repopulate faster,” he added.
“The only real change a clinician could make would be to modify the frequency of the dosing. So if we can identify certain characteristics that would lead you to want to evaluate for the need of redosing sooner, I think that would be useful,” he said.
Specific characteristics identified in previous research include body mass index. “If there are also ethnicity factors, that would be an additional demographic factor that a clinician should pay close attention to,” said Dr. Klawiter.
He noted that his current practice is to check flow cytometry and B-cell counts at the time of a patient’s next infusion. “And if I’m seeing that B-cell levels are repleting at that time point, I am already then making adjustments with their next infusion as to the dosing frequency,” he added.
“This [study] may elucidate some of the potential reasons why we see some people replete their B cells faster than others, but I think additional studies are necessary to make that determination,” Dr. Klawiter concluded.
Genentech provided funding for the study. Dr. Silverman reported no relevant financial relationships. Dr. Klawiter reported having received research funds and consulting fees from Genentech.
A version of this article first appeared on Medscape.com.
new research suggests. In a study of almost 200 patients, Black participants with MS or NMOSD showed significantly more rapid B-cell repopulation 6-12 months after receiving anti-CD20 infusion therapy with rituximab or ocrelizumab (Rituxan, Ocrevus, Genentech) than did White participants.
“The results showed that this B-cell targeted therapy wore off more quickly in African Americans,” said study coinvestigator Gregg J. Silverman, MD, a professor at New York University.
He said that, although the study was more observational in design, “over time when people come back to the clinic, it gives you an idea of whether the agent is still working in their bodies.”
Overall, “our findings raise the question of whether the same therapy dose may be equally effective for all people,” coinvestigator Ilya Kister, MD, also from NYU, added in a press release.
Dr. Kister noted that this could have implications for the way Black patients with autoimmune diseases are treated in the future.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
More severe disease in Black patients
Anti-CD20 infusion therapy, or B-cell depletion therapy, is commonly used to treat autoimmune diseases, including MS and NMOSD. “While previous research has shown that this type of infusion therapy is effective for people with these diseases, we also know that Black people tend to have more severe courses of MS,” Dr. Kister said.
“We wanted to compare how quickly the B cells came back in Black people and White people after treatment,” he added.
Dr. Silverman noted that he has been “studying this agent in autoimmune disease for many years. And from all the different studies, I don’t think we had the right population to ask this question. Demographics were just reviewed as they were.”
The current study included 168 participants (mean age, 44 years; 71% women) who had a diagnosis of MS (n = 134) or NMOSD (n = 32) or who were considered to have MS or NMOSD (n = 2). In addition, 36% of the participants self-identified as Black or African American, 36% self-identified as White, and 28% self-identified as another race.
Flow cytometry results were available for all patients after undergoing anti-CD20 infusions at the NYU MS Care Center. Cluster analyses were conducted on the following B-cell subsets: CD19, CD20, IgD, and CD27. “B-cell repopulation was defined as any detectable number of CD19+ cells on flow cytometry,” the investigators reported.
Clinical implications?
Results showed that 29.8% of the full study group showed B-cell repopulation a mean of 6.8 months after infusion. In those with B-cell repopulation, 80.3% had IgD+/CD27– subsets, 11.6% had IgD–/CD27+, 6.2% had IgD–/CD27–, and 1.8% had IgD+/CD27+. These B-cell subset ratios did not differ significantly between the Black and White participants.
Interestingly, no patients showed B-cell repopulation before 4 months after infusion. From 4 to 6 months after infusion, there were no significant differences between the Black and White participants in terms of frequency of B-cell repopulation (20.8% vs. 17.9%, respectively; P = .79).
However, repopulation was significantly more frequent in the Black patients 6-12 months after infusion (76.2% vs. 33.3%; P = .02).
Overall, the findings “may have implications for clinical management of MS/NMOSD” in Black individuals, the investigators wrote.
“I was impressed by the differences we saw in responses of patients that were self-declaring as African Americans versus those who were Whites,” Dr. Silverman said. However, “as we say in science: it gives us an answer but it raises even more questions, which may well be important for helping us understand how the agent works and how the disease affects different people.”
Still, Dr. Silverman noted that the findings give clinicians using the agent “a signal that they should be very vigilant. It was an observation at one center, but we’re asking our colleagues [at other clinics] to think about being more careful as they review data with their patients.”
He added that future multicenter studies will allow these issues to be assessed more comprehensively. “This was a discovery study; it now needs validation; and maybe the next step would be looking into the mechanism.”
Dr. Silverman pointed out that the Food and Drug Administration–approved label for this type of therapy “allows for somewhat more frequent dosing. So that might be indicated if it’s found that it’s wearing off in an individual. Perhaps they should be treated more frequently?”
“At a minimum, this has raised our vigilance – and we’re interested to see what the feedback will be at the [AAN] meeting,” he added.
Real-world data
Commenting on the findings, Eric Klawiter, MD, associate professor of neurology at Harvard Medical School and director of the Multiple Sclerosis and NMO unit at Massachusetts General Hospital, both in Boston, noted that an important study factor was the focus on repopulation to identify specific groups “who may be early repopulators” as it relates to disease activity and disability progression in MS.
“I thought this was a nicely designed study that made good use of real-world data in MS and NMOSD,” added Dr. Klawiter, who was not involved with the research. He pointed out that timing was another interesting aspect of the study. “As we typically use these cell-depleting agents on an ‘every-6-month’ basis, the most pertinent time frame surrounds those that repopulate prior to 6 months.”
If the current study would have shown differences between the Black and White participants at that time point, “I think that would have been most pertinent from a clinical standpoint and a greater opportunity for intervention,” Dr. Klawiter said. “But we saw that, before 4 and 6 months, [the difference] wasn’t significant.”
Still, “after 6 months, the study demonstrates that Black people with MS and NMOSD may repopulate faster,” he added.
“The only real change a clinician could make would be to modify the frequency of the dosing. So if we can identify certain characteristics that would lead you to want to evaluate for the need of redosing sooner, I think that would be useful,” he said.
Specific characteristics identified in previous research include body mass index. “If there are also ethnicity factors, that would be an additional demographic factor that a clinician should pay close attention to,” said Dr. Klawiter.
He noted that his current practice is to check flow cytometry and B-cell counts at the time of a patient’s next infusion. “And if I’m seeing that B-cell levels are repleting at that time point, I am already then making adjustments with their next infusion as to the dosing frequency,” he added.
“This [study] may elucidate some of the potential reasons why we see some people replete their B cells faster than others, but I think additional studies are necessary to make that determination,” Dr. Klawiter concluded.
Genentech provided funding for the study. Dr. Silverman reported no relevant financial relationships. Dr. Klawiter reported having received research funds and consulting fees from Genentech.
A version of this article first appeared on Medscape.com.
FROM AAN 2021
Teen tanning bed ban would prevent more than 15,000 melanoma cases
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.
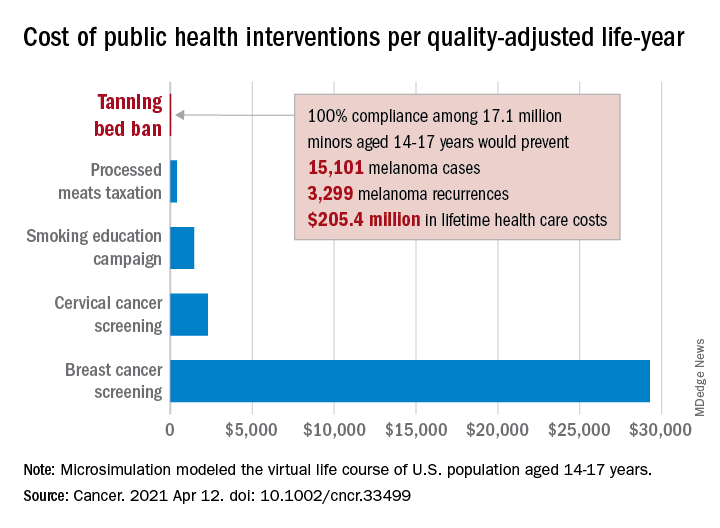
“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.

“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
and cost less than other, well-established public health interventions, according to a microsimulation of that age group’s virtual life course.

“Even with extensive sensitivity analyses on the costs of inspections, noncompliance with a ban, and the risk of developing melanoma in those who have used tanning beds, a ban can be considered highly cost effective,” Antoine Eskander, MD, ScM, of the University of Toronto, and associates said in Cancer.
Compared with no ban, such an intervention could save over $205 million in lifetime health care costs among the 17.1 million young people (based on the 2010 Census population) who would be affected, they said.
The more than 15,000 melanoma cases and 3,300 recurrences prevented would save $12 per average minor after adjusting for societal costs, such as lost productivity, formal and informal health care, economic losses to the tanning bed industry, and the need for monitoring, the investigators reported.
Switching to quality-adjusted life-years shows an improvement of 0.0002 QALYs per child for a ban, based on an overall cost of almost $24.9 per QALY, compared with no ban, they said, which makes it “more cost effective than many well-established public health interventions”:
- Processed meats taxation ($270/QALY).
- Smoking education campaign ($1,337/QALY).
- Cervical cancer screening ($2,166/QALY).
- Breast cancer screening ($29,284/QALY).
- Lung cancer screening ($49,200-$96,700/QALY).
Among the many parameters included in the microsimulation were the odds ratio of developing melanoma from exposure to tanning beds before age 25 (1.35), melanoma stage at presentation, risk of recurrence, and the cost of four annual inspections for each of the nation’s more than 13,000 tanning salons, Dr. Eskander and associates explained.
FROM CANCER
Cortical surface changes linked to sensorimotor abnormalities in schizophrenia
Schizophrenia patients with parkinsonism show unique neurodevelopmental signatures on imaging that involve the sensorimotor system, according to MRI data from 73 adult schizophrenia patients.
Although sensorimotor abnormalities are common in patients with schizophrenia, the neurobiology of parkinsonism in particular is not well understood. Aberrant neurodevelopment is considered a potential mechanism of action for the emergence of such abnormalities, wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a multimodal MRI study published in Schizophrenia Research, the investigators identified 38 adults with schizophrenia and parkinsonism (SZ-P), 35 schizophrenia patients without parkinsonism (SZ-nonP), and 20 healthy controls.
Parkinsonism was defined as scores of 4 or higher on the Simpson-Angus Scale, while non-Parkinsonism schizophrenia patients had scores of 1 or less.
The researchers examined cortical and subcortical gray-matter volume, as well as three cortical surface markers related to neurodevelopment: cortical thickness (CTh), complexity of cortical folding (CCF), and sulcus depth.
Overall, the SZ-P patients showed increased CCF in the left supplementary motor cortex (SMC) and decreased left postcentral sulcus depth, compared with SZ-nonP patients (P < .05). The left SMC also showed increased CCF, compared with healthy controls – but that difference was not significant.
Both SZ-P and SZ-nonP patients showed higher levels of activity in the left SMC, compared with controls, and activity was higher in SZ-nonP patients, compared with SZ-P patients. In addition, Dr. Wolf and colleagues reported.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as CCF and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers said.
The study findings were limited by several factors, including the cross-sectional design, the challenges of using the potential restraint inherent in the Simpson-Angus Scale to diagnose parkinsonism, the inability to gauge the impact of lifetime exposure to antipsychotics, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results suggest the impact of cortical development on parkinsonism in schizophrenia,.
“Cortical surface changes in the sensorimotor system suggest abnormal neurodevelopmental processes that are associated with increased risk for intrinsic sensorimotor abnormalities in SZ and related psychotic disorders,” they concluded.
The study was supported by the German Research Foundation and the German Federal Ministry of Education and Research. The researchers disclosed no financial conflicts.
Schizophrenia patients with parkinsonism show unique neurodevelopmental signatures on imaging that involve the sensorimotor system, according to MRI data from 73 adult schizophrenia patients.
Although sensorimotor abnormalities are common in patients with schizophrenia, the neurobiology of parkinsonism in particular is not well understood. Aberrant neurodevelopment is considered a potential mechanism of action for the emergence of such abnormalities, wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a multimodal MRI study published in Schizophrenia Research, the investigators identified 38 adults with schizophrenia and parkinsonism (SZ-P), 35 schizophrenia patients without parkinsonism (SZ-nonP), and 20 healthy controls.
Parkinsonism was defined as scores of 4 or higher on the Simpson-Angus Scale, while non-Parkinsonism schizophrenia patients had scores of 1 or less.
The researchers examined cortical and subcortical gray-matter volume, as well as three cortical surface markers related to neurodevelopment: cortical thickness (CTh), complexity of cortical folding (CCF), and sulcus depth.
Overall, the SZ-P patients showed increased CCF in the left supplementary motor cortex (SMC) and decreased left postcentral sulcus depth, compared with SZ-nonP patients (P < .05). The left SMC also showed increased CCF, compared with healthy controls – but that difference was not significant.
Both SZ-P and SZ-nonP patients showed higher levels of activity in the left SMC, compared with controls, and activity was higher in SZ-nonP patients, compared with SZ-P patients. In addition, Dr. Wolf and colleagues reported.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as CCF and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers said.
The study findings were limited by several factors, including the cross-sectional design, the challenges of using the potential restraint inherent in the Simpson-Angus Scale to diagnose parkinsonism, the inability to gauge the impact of lifetime exposure to antipsychotics, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results suggest the impact of cortical development on parkinsonism in schizophrenia,.
“Cortical surface changes in the sensorimotor system suggest abnormal neurodevelopmental processes that are associated with increased risk for intrinsic sensorimotor abnormalities in SZ and related psychotic disorders,” they concluded.
The study was supported by the German Research Foundation and the German Federal Ministry of Education and Research. The researchers disclosed no financial conflicts.
Schizophrenia patients with parkinsonism show unique neurodevelopmental signatures on imaging that involve the sensorimotor system, according to MRI data from 73 adult schizophrenia patients.
Although sensorimotor abnormalities are common in patients with schizophrenia, the neurobiology of parkinsonism in particular is not well understood. Aberrant neurodevelopment is considered a potential mechanism of action for the emergence of such abnormalities, wrote Robert Christian Wolf, MD, of Heidelberg (Germany) University, and colleagues.
In a multimodal MRI study published in Schizophrenia Research, the investigators identified 38 adults with schizophrenia and parkinsonism (SZ-P), 35 schizophrenia patients without parkinsonism (SZ-nonP), and 20 healthy controls.
Parkinsonism was defined as scores of 4 or higher on the Simpson-Angus Scale, while non-Parkinsonism schizophrenia patients had scores of 1 or less.
The researchers examined cortical and subcortical gray-matter volume, as well as three cortical surface markers related to neurodevelopment: cortical thickness (CTh), complexity of cortical folding (CCF), and sulcus depth.
Overall, the SZ-P patients showed increased CCF in the left supplementary motor cortex (SMC) and decreased left postcentral sulcus depth, compared with SZ-nonP patients (P < .05). The left SMC also showed increased CCF, compared with healthy controls – but that difference was not significant.
Both SZ-P and SZ-nonP patients showed higher levels of activity in the left SMC, compared with controls, and activity was higher in SZ-nonP patients, compared with SZ-P patients. In addition, Dr. Wolf and colleagues reported.
“Overall, the data support the notion that cortical features of distinct neurodevelopmental origin, particularly cortical folding indices such as CCF and sulcus depth, contribute to the pathogenesis of parkinsonism in SZ,” the researchers said.
The study findings were limited by several factors, including the cross-sectional design, the challenges of using the potential restraint inherent in the Simpson-Angus Scale to diagnose parkinsonism, the inability to gauge the impact of lifetime exposure to antipsychotics, and the inability to identify changes in brain stem nuclei, the researchers noted. However, the results suggest the impact of cortical development on parkinsonism in schizophrenia,.
“Cortical surface changes in the sensorimotor system suggest abnormal neurodevelopmental processes that are associated with increased risk for intrinsic sensorimotor abnormalities in SZ and related psychotic disorders,” they concluded.
The study was supported by the German Research Foundation and the German Federal Ministry of Education and Research. The researchers disclosed no financial conflicts.
FROM SCHIZOPHRENIA RESEARCH
Respiratory Illness Presenteeism in Academic Medicine: A Conceivable COVID-19 Culture Change for the Better
An intern is rotating on a medical ward in January 2018. Influenza is prevalent and hospital admissions are increasing daily. Despite receiving her influenza vaccine in October 2017, she develops fevers and myalgias. Due to time constraints, she does not get tested for influenza. She instead decides to work while sick to avoid payback of shifts.
She is now a hematology/oncology fellow in December 2020. She and her colleagues experienced the harrowing first wave of the COVID-19 pandemic. Unfortunately, community prevalence and hospital admissions are again rising. She adheres to mandatory masking and eye protection at work. Two days after attending a procedural workshop with lunch provided, she develops headache, myalgias, and sore throat. She contacts her supervisor, calls out sick, and initiates home isolation due to a positive result on a COVID-19 test performed through occupational health services (OHS). No patients are affected, but multiple colleagues are required to quarantine and others are pulled to provide coverage.
PRESENTEEISM
Presenteeism, the act of attending work despite personal illness, can adversely affect individuals and organizations.1 In a healthcare setting, transmissible illnesses contribute to complications in patients and missed workdays for staff. Prior to the pandemic, the rate of presenteeism among physicians was as high as 90%.2 Such presenteeism may have contributed to medical errors and decreased work efficiency.3,4 At our hospital in the Bronx, New York, a high annual prevalence of seasonal influenza fueled influenza clusters among patients and trainees, leading to presenteeism.
Our prior work on influenza-related practices in academic medicine revealed that 54% of trainees and 26% of program directors self-reported influenza-like illness (ILI) presenteeism. Drivers included desire to display a strong work ethic, desire not to burden colleagues, concern about colleagues’ negative perceptions, and knowledge gaps in influenza transmission.5
INFLUENCE OF THE COVID-19 PANDEMIC ON PRESENTEEISM
The COVID-19 pandemic has profoundly affected staffing models, infection prevention protocols, use of shared spaces, educational conferences, visitation policies, and other habitual healthcare practices. The experience of post-graduate training during a pandemic has resulted in important mindset and practice changes that may decrease presenteeism. However, health systems need robust mechanisms to accommodate appropriate work absences due to illness. We hypothesize that ILI/COVID-like illness presenteeism will decrease significantly for the following reasons, which will have positive and negative impacts on the organization and individual.
Shift in Accountability and Rewards
Our 2018 study revealed that presenteeism was motivated by a desire not to burden colleagues with extra clinical duties and to display conscientiousness. Despite a back-up call system, house staff were concerned about colleagues’ negative perceptions. Accountability was perceived as fulfilling one’s assigned clinical duties rather than protecting others from illness.
More recently, staff have experienced personal or family illness with COVID-19 or witnessed its rapid spread through the healthcare system. Forty-two percent (103 of 245) of our internal medicine residents had work absences resulting in 875 total missed workdays between February 29 and May 22, 2020. At the peak of the pandemic’s first wave in the spring of 2020, 16% (38 of 245) were out sick.6 We hypothesize that this experience resulted in a modified sense of accountability to peers and patients which manifested as a desire not to expose them to illness. Staying home while ill is now positively reinforced by supervisors, and presenteeism is recognized as harmful rather than commendable. However, increased utilization of the back-up call system to meet patient care demands is a secondary consequence.
Consequences of Exposures
While trainees and program faculty acknowledged that presenteeism puts patients and coworkers at risk,5 there was insufficient individual or institutional motivation to prevent it or fear its consequences pre-pandemic. An individual infected with influenza A may spread illness to one or two others, and several outpatient influenza treatments exist. Also, current trainees did not experience the prior respiratory viral pandemic (2009 H1N1 influenza A) as healthcare workers (HCWs).
In contrast, SARS-CoV-2 is more transmissible, with one infection resulting in two to three additional cases.7 Hence, mandatory quarantine and isolation policies are more stringent than those for influenza. While reinfection with SARS-CoV-2 is rare,8 HCWs can be exposed and quarantined multiple times, which potentially impacts paid sick leave for HCWs and elective-time for house staff pursuing fellowships. COVID-19 among HCWs also impacts secondary contacts, resulting in missed work and school days and strain on families. Hospital resource utilization for contact tracing and testing postexposure is significant. Unlike influenza, there are currently no oral antiviral treatments for outpatient COVID-19, and illness has been linked to chronic disabling symptoms.9 Finally, absences due to illness or quarantine may disrupt education, training, and fulfillment of competencies and experiences necessary for advancement.
We predict that these potentially sweeping consequences will reduce presenteeism. An important aspect of health system pandemic planning must include adequate staffing to account for work absences due to illness or quarantine.
Access to Occupational Health Services
Previously, staff reported barriers to seeking care from OHS. Therefore, this step was skipped, and HCWs managed their own symptoms, tested and treated each other for influenza, and returned to work at an arbitrary interval, without coordination with OHS protocols. OHS processes have since greatly improved. Employees with a COVID-19 exposure or concerning symptoms call the OHS hotline, are referred for same-day testing, and are given specific instructions regarding home quarantine or isolation and return to work. Follow-up to confirm fitness for duty is provided. In September 2020, an electronic screening tool assessing COVID-19 symptoms, exposures, and high-risk travel was implemented at our institution. Associates must present their clearance at hospital entrances.
Protection From Vaccine
Survey results indicated that all house staff and program faculty received the annual influenza vaccine.5 In New York State, public health regulations ensure a high rate of annual influenza vaccination among HCWs.10 It is possible that house staff did not perceive that ILI symptoms were caused by influenza after vaccination, and that vaccinated colleagues were at lower risk of illness. The influenza vaccine also has a well-established safety record, contributing to good uptake among HCWs.
At the time of writing of this article, HCWs have been prioritized for COVID-19 vaccination. Studies are in progress pertaining to the degree of protection after one dose, incidence of new infections after first and second doses, and secondary transmissions from vaccinated individuals. Vaccination is likely to influence HCW behaviors as well as occupational health policies. We suggest that the impact of COVID-19 vaccination on subsequent HCW presenteeism be given precedence in future studies.
Consistent Messaging and Communication
Prior to the pandemic, regular communication to staff on transmissible disease outbreaks scarcely occurred. Likewise, recurring training on infection prevention and personal protective equipment (PPE) protocols did not occur, and hospital policies regarding personal illness were not emphasized. Harms of presenteeism were infrequently addressed outside of nosocomial outbreaks. The pandemic has positively impacted communication from hospital leadership. Infection control and occupational health guidelines are continually revised and disseminated. Program directors send regular COVID-19 updates to trainees. The infectious diseases program director serves as a graduate medical education liaison to hospital leadership. All staff are regularly updated on evolving policies and given resources to assist with personal illness. While many positive practice changes have occurred, a decrease in presenteeism may exhaust sick coverage and compromise patient care. We suggest that health systems create safer work environments and ensure adequate staffing to accommodate illnesses and quarantines.
STRATEGIES TO CREATE SAFER WORK ENVIRONMENTS
- Conduct recurring staff PPE simulations spanning a range of communicable illnesses.
- Ensure adequate PPE for surge conditions.
- Implement occupancy limits for shared spaces, distanced seating, staggered mealtimes, plexiglass barriers, and portable air-filtration systems in rooms lacking windows.
- Invest in large-scale, serial testing of asymptomatic HCWs to identify early cases and enact quarantines prior to excess exposures.
STRATEGIES TO ADDRESS STAFFING CONSTRAINTS IN ACADEMIC MEDICAL CENTERS
- Adopt nonpunitive coverage systems, reducing presenteeism by removing expectations to “pay-back” colleagues later.
- Establish a third-party notification system, reducing strain on house staff to find coverage. This will enable strategic use of the jeopardy pool by training program leadership.
- Establish a backup coverage pool populated by hospitalists and third-year residents who have completed fellowship match. Ideally, health systems should be prepared to compensate physicians for extra shifts.
- Engage nondeployed physician assistants or nurse practitioners to provide coverage for residents on a per diem basis.
- At a federal level, funding for trainee workforce expansion can occur to ensure staffing redundancy. The appropriate number of trainees should be determined by program leadership, balancing surge needs with education and autonomy. Likewise, training extensions due to COVID-related absences or deployments away from research or electives should be federally funded.
- Inpatient and community COVID-19 surges can result in large-scale furloughs of HCWs; hospital leadership should expediently implement public health recommendations allowing fully immunized HCWs to work after exposures while maximally adhering to infection prevention protocols.
The COVID-19 pandemic has profoundly impacted academic medicine. It is imperative to explore solutions to balance workplace safety, education, and training with staffing constraints and patient care needs. Resource investment and executive leadership support are required to achieve this balance.
1. Webster RK, Liu R, Karimullina K, Hall I, Amlôt R, Rubin GJ. A systematic review of infectious illness Presenteeism: prevalence, reasons and risk factors. BMC Public Health. 2019;19:799. https://doi.org/10.1186/s12889-019-7138-x
2. Bergström G, Bodin L, Hagberg J, Aronsson G, Josephson M. Sickness presenteeism today, sickness absenteeism tomorrow? A prospective study on sickness presenteeism and future sickness absenteeism. J Occup Environ Med. 2009;51(6):629-638. https://doi.org/10.1097/JOM.0b013e3181a8281b
3. Al Nuhait M, Al Harbi K, Al Jarboa A, et al. Sickness presenteeism among health care providers in an academic tertiary care center in Riyadh. J Infect Public Health. 2017;10(6):711-715. https://doi.org/10.1016/j.jiph.2016.09.019
4. Brborovic H, Brborovic O. Patient safety culture shapes presenteeism and absenteeism: a cross-sectional study among Croatian healthcare workers. Arh Hig Rada Toksikol. 2017;68(3):185-189. https://doi.org/10.1515/aiht-2017-68-2957
5. Cowman K, Mittal J, Weston G, et al. Understanding drivers of influenza-like illness presenteeism within training programs: a survey of trainees and their program directors. Am J Infect Control. 2019;47(8):895-901. https://doi.org/10.1016/j.ajic.2019.02.004
6. Merkin R, Kruger A, Bhardwaj G, Kajita GR, Shapiro L, Galen BT. Internal medicine resident work absence during the COVID-19 pandemic at a large academic medical center in New York City. J Grad Med Educ. 2020;12(6):682-685. https://doi.org/10.4300/JGME-D-20-00657.1
7. Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238-244. https://doi.org/10.1016/S1473-3099(20)30484-9
8. Reinfection with COVID-19. Centers for Disease Control and Prevention. Updated October 27, 2020. Accessed March 31, 2021. https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html
9. Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324(14):1381-1383. https://doi.org/10.1001/jama.2020.17709
10. Regulation for prevention of influenza transmission by healthcare and residential facility and agency personnel. New York State Department of Health. Revised March 2021. Accessed December 7, 2020. https://www.health.ny.gov/diseases/communicable/influenza/seasonal/providers/prevention_of_influenza_transmission/
An intern is rotating on a medical ward in January 2018. Influenza is prevalent and hospital admissions are increasing daily. Despite receiving her influenza vaccine in October 2017, she develops fevers and myalgias. Due to time constraints, she does not get tested for influenza. She instead decides to work while sick to avoid payback of shifts.
She is now a hematology/oncology fellow in December 2020. She and her colleagues experienced the harrowing first wave of the COVID-19 pandemic. Unfortunately, community prevalence and hospital admissions are again rising. She adheres to mandatory masking and eye protection at work. Two days after attending a procedural workshop with lunch provided, she develops headache, myalgias, and sore throat. She contacts her supervisor, calls out sick, and initiates home isolation due to a positive result on a COVID-19 test performed through occupational health services (OHS). No patients are affected, but multiple colleagues are required to quarantine and others are pulled to provide coverage.
PRESENTEEISM
Presenteeism, the act of attending work despite personal illness, can adversely affect individuals and organizations.1 In a healthcare setting, transmissible illnesses contribute to complications in patients and missed workdays for staff. Prior to the pandemic, the rate of presenteeism among physicians was as high as 90%.2 Such presenteeism may have contributed to medical errors and decreased work efficiency.3,4 At our hospital in the Bronx, New York, a high annual prevalence of seasonal influenza fueled influenza clusters among patients and trainees, leading to presenteeism.
Our prior work on influenza-related practices in academic medicine revealed that 54% of trainees and 26% of program directors self-reported influenza-like illness (ILI) presenteeism. Drivers included desire to display a strong work ethic, desire not to burden colleagues, concern about colleagues’ negative perceptions, and knowledge gaps in influenza transmission.5
INFLUENCE OF THE COVID-19 PANDEMIC ON PRESENTEEISM
The COVID-19 pandemic has profoundly affected staffing models, infection prevention protocols, use of shared spaces, educational conferences, visitation policies, and other habitual healthcare practices. The experience of post-graduate training during a pandemic has resulted in important mindset and practice changes that may decrease presenteeism. However, health systems need robust mechanisms to accommodate appropriate work absences due to illness. We hypothesize that ILI/COVID-like illness presenteeism will decrease significantly for the following reasons, which will have positive and negative impacts on the organization and individual.
Shift in Accountability and Rewards
Our 2018 study revealed that presenteeism was motivated by a desire not to burden colleagues with extra clinical duties and to display conscientiousness. Despite a back-up call system, house staff were concerned about colleagues’ negative perceptions. Accountability was perceived as fulfilling one’s assigned clinical duties rather than protecting others from illness.
More recently, staff have experienced personal or family illness with COVID-19 or witnessed its rapid spread through the healthcare system. Forty-two percent (103 of 245) of our internal medicine residents had work absences resulting in 875 total missed workdays between February 29 and May 22, 2020. At the peak of the pandemic’s first wave in the spring of 2020, 16% (38 of 245) were out sick.6 We hypothesize that this experience resulted in a modified sense of accountability to peers and patients which manifested as a desire not to expose them to illness. Staying home while ill is now positively reinforced by supervisors, and presenteeism is recognized as harmful rather than commendable. However, increased utilization of the back-up call system to meet patient care demands is a secondary consequence.
Consequences of Exposures
While trainees and program faculty acknowledged that presenteeism puts patients and coworkers at risk,5 there was insufficient individual or institutional motivation to prevent it or fear its consequences pre-pandemic. An individual infected with influenza A may spread illness to one or two others, and several outpatient influenza treatments exist. Also, current trainees did not experience the prior respiratory viral pandemic (2009 H1N1 influenza A) as healthcare workers (HCWs).
In contrast, SARS-CoV-2 is more transmissible, with one infection resulting in two to three additional cases.7 Hence, mandatory quarantine and isolation policies are more stringent than those for influenza. While reinfection with SARS-CoV-2 is rare,8 HCWs can be exposed and quarantined multiple times, which potentially impacts paid sick leave for HCWs and elective-time for house staff pursuing fellowships. COVID-19 among HCWs also impacts secondary contacts, resulting in missed work and school days and strain on families. Hospital resource utilization for contact tracing and testing postexposure is significant. Unlike influenza, there are currently no oral antiviral treatments for outpatient COVID-19, and illness has been linked to chronic disabling symptoms.9 Finally, absences due to illness or quarantine may disrupt education, training, and fulfillment of competencies and experiences necessary for advancement.
We predict that these potentially sweeping consequences will reduce presenteeism. An important aspect of health system pandemic planning must include adequate staffing to account for work absences due to illness or quarantine.
Access to Occupational Health Services
Previously, staff reported barriers to seeking care from OHS. Therefore, this step was skipped, and HCWs managed their own symptoms, tested and treated each other for influenza, and returned to work at an arbitrary interval, without coordination with OHS protocols. OHS processes have since greatly improved. Employees with a COVID-19 exposure or concerning symptoms call the OHS hotline, are referred for same-day testing, and are given specific instructions regarding home quarantine or isolation and return to work. Follow-up to confirm fitness for duty is provided. In September 2020, an electronic screening tool assessing COVID-19 symptoms, exposures, and high-risk travel was implemented at our institution. Associates must present their clearance at hospital entrances.
Protection From Vaccine
Survey results indicated that all house staff and program faculty received the annual influenza vaccine.5 In New York State, public health regulations ensure a high rate of annual influenza vaccination among HCWs.10 It is possible that house staff did not perceive that ILI symptoms were caused by influenza after vaccination, and that vaccinated colleagues were at lower risk of illness. The influenza vaccine also has a well-established safety record, contributing to good uptake among HCWs.
At the time of writing of this article, HCWs have been prioritized for COVID-19 vaccination. Studies are in progress pertaining to the degree of protection after one dose, incidence of new infections after first and second doses, and secondary transmissions from vaccinated individuals. Vaccination is likely to influence HCW behaviors as well as occupational health policies. We suggest that the impact of COVID-19 vaccination on subsequent HCW presenteeism be given precedence in future studies.
Consistent Messaging and Communication
Prior to the pandemic, regular communication to staff on transmissible disease outbreaks scarcely occurred. Likewise, recurring training on infection prevention and personal protective equipment (PPE) protocols did not occur, and hospital policies regarding personal illness were not emphasized. Harms of presenteeism were infrequently addressed outside of nosocomial outbreaks. The pandemic has positively impacted communication from hospital leadership. Infection control and occupational health guidelines are continually revised and disseminated. Program directors send regular COVID-19 updates to trainees. The infectious diseases program director serves as a graduate medical education liaison to hospital leadership. All staff are regularly updated on evolving policies and given resources to assist with personal illness. While many positive practice changes have occurred, a decrease in presenteeism may exhaust sick coverage and compromise patient care. We suggest that health systems create safer work environments and ensure adequate staffing to accommodate illnesses and quarantines.
STRATEGIES TO CREATE SAFER WORK ENVIRONMENTS
- Conduct recurring staff PPE simulations spanning a range of communicable illnesses.
- Ensure adequate PPE for surge conditions.
- Implement occupancy limits for shared spaces, distanced seating, staggered mealtimes, plexiglass barriers, and portable air-filtration systems in rooms lacking windows.
- Invest in large-scale, serial testing of asymptomatic HCWs to identify early cases and enact quarantines prior to excess exposures.
STRATEGIES TO ADDRESS STAFFING CONSTRAINTS IN ACADEMIC MEDICAL CENTERS
- Adopt nonpunitive coverage systems, reducing presenteeism by removing expectations to “pay-back” colleagues later.
- Establish a third-party notification system, reducing strain on house staff to find coverage. This will enable strategic use of the jeopardy pool by training program leadership.
- Establish a backup coverage pool populated by hospitalists and third-year residents who have completed fellowship match. Ideally, health systems should be prepared to compensate physicians for extra shifts.
- Engage nondeployed physician assistants or nurse practitioners to provide coverage for residents on a per diem basis.
- At a federal level, funding for trainee workforce expansion can occur to ensure staffing redundancy. The appropriate number of trainees should be determined by program leadership, balancing surge needs with education and autonomy. Likewise, training extensions due to COVID-related absences or deployments away from research or electives should be federally funded.
- Inpatient and community COVID-19 surges can result in large-scale furloughs of HCWs; hospital leadership should expediently implement public health recommendations allowing fully immunized HCWs to work after exposures while maximally adhering to infection prevention protocols.
The COVID-19 pandemic has profoundly impacted academic medicine. It is imperative to explore solutions to balance workplace safety, education, and training with staffing constraints and patient care needs. Resource investment and executive leadership support are required to achieve this balance.
An intern is rotating on a medical ward in January 2018. Influenza is prevalent and hospital admissions are increasing daily. Despite receiving her influenza vaccine in October 2017, she develops fevers and myalgias. Due to time constraints, she does not get tested for influenza. She instead decides to work while sick to avoid payback of shifts.
She is now a hematology/oncology fellow in December 2020. She and her colleagues experienced the harrowing first wave of the COVID-19 pandemic. Unfortunately, community prevalence and hospital admissions are again rising. She adheres to mandatory masking and eye protection at work. Two days after attending a procedural workshop with lunch provided, she develops headache, myalgias, and sore throat. She contacts her supervisor, calls out sick, and initiates home isolation due to a positive result on a COVID-19 test performed through occupational health services (OHS). No patients are affected, but multiple colleagues are required to quarantine and others are pulled to provide coverage.
PRESENTEEISM
Presenteeism, the act of attending work despite personal illness, can adversely affect individuals and organizations.1 In a healthcare setting, transmissible illnesses contribute to complications in patients and missed workdays for staff. Prior to the pandemic, the rate of presenteeism among physicians was as high as 90%.2 Such presenteeism may have contributed to medical errors and decreased work efficiency.3,4 At our hospital in the Bronx, New York, a high annual prevalence of seasonal influenza fueled influenza clusters among patients and trainees, leading to presenteeism.
Our prior work on influenza-related practices in academic medicine revealed that 54% of trainees and 26% of program directors self-reported influenza-like illness (ILI) presenteeism. Drivers included desire to display a strong work ethic, desire not to burden colleagues, concern about colleagues’ negative perceptions, and knowledge gaps in influenza transmission.5
INFLUENCE OF THE COVID-19 PANDEMIC ON PRESENTEEISM
The COVID-19 pandemic has profoundly affected staffing models, infection prevention protocols, use of shared spaces, educational conferences, visitation policies, and other habitual healthcare practices. The experience of post-graduate training during a pandemic has resulted in important mindset and practice changes that may decrease presenteeism. However, health systems need robust mechanisms to accommodate appropriate work absences due to illness. We hypothesize that ILI/COVID-like illness presenteeism will decrease significantly for the following reasons, which will have positive and negative impacts on the organization and individual.
Shift in Accountability and Rewards
Our 2018 study revealed that presenteeism was motivated by a desire not to burden colleagues with extra clinical duties and to display conscientiousness. Despite a back-up call system, house staff were concerned about colleagues’ negative perceptions. Accountability was perceived as fulfilling one’s assigned clinical duties rather than protecting others from illness.
More recently, staff have experienced personal or family illness with COVID-19 or witnessed its rapid spread through the healthcare system. Forty-two percent (103 of 245) of our internal medicine residents had work absences resulting in 875 total missed workdays between February 29 and May 22, 2020. At the peak of the pandemic’s first wave in the spring of 2020, 16% (38 of 245) were out sick.6 We hypothesize that this experience resulted in a modified sense of accountability to peers and patients which manifested as a desire not to expose them to illness. Staying home while ill is now positively reinforced by supervisors, and presenteeism is recognized as harmful rather than commendable. However, increased utilization of the back-up call system to meet patient care demands is a secondary consequence.
Consequences of Exposures
While trainees and program faculty acknowledged that presenteeism puts patients and coworkers at risk,5 there was insufficient individual or institutional motivation to prevent it or fear its consequences pre-pandemic. An individual infected with influenza A may spread illness to one or two others, and several outpatient influenza treatments exist. Also, current trainees did not experience the prior respiratory viral pandemic (2009 H1N1 influenza A) as healthcare workers (HCWs).
In contrast, SARS-CoV-2 is more transmissible, with one infection resulting in two to three additional cases.7 Hence, mandatory quarantine and isolation policies are more stringent than those for influenza. While reinfection with SARS-CoV-2 is rare,8 HCWs can be exposed and quarantined multiple times, which potentially impacts paid sick leave for HCWs and elective-time for house staff pursuing fellowships. COVID-19 among HCWs also impacts secondary contacts, resulting in missed work and school days and strain on families. Hospital resource utilization for contact tracing and testing postexposure is significant. Unlike influenza, there are currently no oral antiviral treatments for outpatient COVID-19, and illness has been linked to chronic disabling symptoms.9 Finally, absences due to illness or quarantine may disrupt education, training, and fulfillment of competencies and experiences necessary for advancement.
We predict that these potentially sweeping consequences will reduce presenteeism. An important aspect of health system pandemic planning must include adequate staffing to account for work absences due to illness or quarantine.
Access to Occupational Health Services
Previously, staff reported barriers to seeking care from OHS. Therefore, this step was skipped, and HCWs managed their own symptoms, tested and treated each other for influenza, and returned to work at an arbitrary interval, without coordination with OHS protocols. OHS processes have since greatly improved. Employees with a COVID-19 exposure or concerning symptoms call the OHS hotline, are referred for same-day testing, and are given specific instructions regarding home quarantine or isolation and return to work. Follow-up to confirm fitness for duty is provided. In September 2020, an electronic screening tool assessing COVID-19 symptoms, exposures, and high-risk travel was implemented at our institution. Associates must present their clearance at hospital entrances.
Protection From Vaccine
Survey results indicated that all house staff and program faculty received the annual influenza vaccine.5 In New York State, public health regulations ensure a high rate of annual influenza vaccination among HCWs.10 It is possible that house staff did not perceive that ILI symptoms were caused by influenza after vaccination, and that vaccinated colleagues were at lower risk of illness. The influenza vaccine also has a well-established safety record, contributing to good uptake among HCWs.
At the time of writing of this article, HCWs have been prioritized for COVID-19 vaccination. Studies are in progress pertaining to the degree of protection after one dose, incidence of new infections after first and second doses, and secondary transmissions from vaccinated individuals. Vaccination is likely to influence HCW behaviors as well as occupational health policies. We suggest that the impact of COVID-19 vaccination on subsequent HCW presenteeism be given precedence in future studies.
Consistent Messaging and Communication
Prior to the pandemic, regular communication to staff on transmissible disease outbreaks scarcely occurred. Likewise, recurring training on infection prevention and personal protective equipment (PPE) protocols did not occur, and hospital policies regarding personal illness were not emphasized. Harms of presenteeism were infrequently addressed outside of nosocomial outbreaks. The pandemic has positively impacted communication from hospital leadership. Infection control and occupational health guidelines are continually revised and disseminated. Program directors send regular COVID-19 updates to trainees. The infectious diseases program director serves as a graduate medical education liaison to hospital leadership. All staff are regularly updated on evolving policies and given resources to assist with personal illness. While many positive practice changes have occurred, a decrease in presenteeism may exhaust sick coverage and compromise patient care. We suggest that health systems create safer work environments and ensure adequate staffing to accommodate illnesses and quarantines.
STRATEGIES TO CREATE SAFER WORK ENVIRONMENTS
- Conduct recurring staff PPE simulations spanning a range of communicable illnesses.
- Ensure adequate PPE for surge conditions.
- Implement occupancy limits for shared spaces, distanced seating, staggered mealtimes, plexiglass barriers, and portable air-filtration systems in rooms lacking windows.
- Invest in large-scale, serial testing of asymptomatic HCWs to identify early cases and enact quarantines prior to excess exposures.
STRATEGIES TO ADDRESS STAFFING CONSTRAINTS IN ACADEMIC MEDICAL CENTERS
- Adopt nonpunitive coverage systems, reducing presenteeism by removing expectations to “pay-back” colleagues later.
- Establish a third-party notification system, reducing strain on house staff to find coverage. This will enable strategic use of the jeopardy pool by training program leadership.
- Establish a backup coverage pool populated by hospitalists and third-year residents who have completed fellowship match. Ideally, health systems should be prepared to compensate physicians for extra shifts.
- Engage nondeployed physician assistants or nurse practitioners to provide coverage for residents on a per diem basis.
- At a federal level, funding for trainee workforce expansion can occur to ensure staffing redundancy. The appropriate number of trainees should be determined by program leadership, balancing surge needs with education and autonomy. Likewise, training extensions due to COVID-related absences or deployments away from research or electives should be federally funded.
- Inpatient and community COVID-19 surges can result in large-scale furloughs of HCWs; hospital leadership should expediently implement public health recommendations allowing fully immunized HCWs to work after exposures while maximally adhering to infection prevention protocols.
The COVID-19 pandemic has profoundly impacted academic medicine. It is imperative to explore solutions to balance workplace safety, education, and training with staffing constraints and patient care needs. Resource investment and executive leadership support are required to achieve this balance.
1. Webster RK, Liu R, Karimullina K, Hall I, Amlôt R, Rubin GJ. A systematic review of infectious illness Presenteeism: prevalence, reasons and risk factors. BMC Public Health. 2019;19:799. https://doi.org/10.1186/s12889-019-7138-x
2. Bergström G, Bodin L, Hagberg J, Aronsson G, Josephson M. Sickness presenteeism today, sickness absenteeism tomorrow? A prospective study on sickness presenteeism and future sickness absenteeism. J Occup Environ Med. 2009;51(6):629-638. https://doi.org/10.1097/JOM.0b013e3181a8281b
3. Al Nuhait M, Al Harbi K, Al Jarboa A, et al. Sickness presenteeism among health care providers in an academic tertiary care center in Riyadh. J Infect Public Health. 2017;10(6):711-715. https://doi.org/10.1016/j.jiph.2016.09.019
4. Brborovic H, Brborovic O. Patient safety culture shapes presenteeism and absenteeism: a cross-sectional study among Croatian healthcare workers. Arh Hig Rada Toksikol. 2017;68(3):185-189. https://doi.org/10.1515/aiht-2017-68-2957
5. Cowman K, Mittal J, Weston G, et al. Understanding drivers of influenza-like illness presenteeism within training programs: a survey of trainees and their program directors. Am J Infect Control. 2019;47(8):895-901. https://doi.org/10.1016/j.ajic.2019.02.004
6. Merkin R, Kruger A, Bhardwaj G, Kajita GR, Shapiro L, Galen BT. Internal medicine resident work absence during the COVID-19 pandemic at a large academic medical center in New York City. J Grad Med Educ. 2020;12(6):682-685. https://doi.org/10.4300/JGME-D-20-00657.1
7. Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238-244. https://doi.org/10.1016/S1473-3099(20)30484-9
8. Reinfection with COVID-19. Centers for Disease Control and Prevention. Updated October 27, 2020. Accessed March 31, 2021. https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html
9. Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324(14):1381-1383. https://doi.org/10.1001/jama.2020.17709
10. Regulation for prevention of influenza transmission by healthcare and residential facility and agency personnel. New York State Department of Health. Revised March 2021. Accessed December 7, 2020. https://www.health.ny.gov/diseases/communicable/influenza/seasonal/providers/prevention_of_influenza_transmission/
1. Webster RK, Liu R, Karimullina K, Hall I, Amlôt R, Rubin GJ. A systematic review of infectious illness Presenteeism: prevalence, reasons and risk factors. BMC Public Health. 2019;19:799. https://doi.org/10.1186/s12889-019-7138-x
2. Bergström G, Bodin L, Hagberg J, Aronsson G, Josephson M. Sickness presenteeism today, sickness absenteeism tomorrow? A prospective study on sickness presenteeism and future sickness absenteeism. J Occup Environ Med. 2009;51(6):629-638. https://doi.org/10.1097/JOM.0b013e3181a8281b
3. Al Nuhait M, Al Harbi K, Al Jarboa A, et al. Sickness presenteeism among health care providers in an academic tertiary care center in Riyadh. J Infect Public Health. 2017;10(6):711-715. https://doi.org/10.1016/j.jiph.2016.09.019
4. Brborovic H, Brborovic O. Patient safety culture shapes presenteeism and absenteeism: a cross-sectional study among Croatian healthcare workers. Arh Hig Rada Toksikol. 2017;68(3):185-189. https://doi.org/10.1515/aiht-2017-68-2957
5. Cowman K, Mittal J, Weston G, et al. Understanding drivers of influenza-like illness presenteeism within training programs: a survey of trainees and their program directors. Am J Infect Control. 2019;47(8):895-901. https://doi.org/10.1016/j.ajic.2019.02.004
6. Merkin R, Kruger A, Bhardwaj G, Kajita GR, Shapiro L, Galen BT. Internal medicine resident work absence during the COVID-19 pandemic at a large academic medical center in New York City. J Grad Med Educ. 2020;12(6):682-685. https://doi.org/10.4300/JGME-D-20-00657.1
7. Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238-244. https://doi.org/10.1016/S1473-3099(20)30484-9
8. Reinfection with COVID-19. Centers for Disease Control and Prevention. Updated October 27, 2020. Accessed March 31, 2021. https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html
9. Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324(14):1381-1383. https://doi.org/10.1001/jama.2020.17709
10. Regulation for prevention of influenza transmission by healthcare and residential facility and agency personnel. New York State Department of Health. Revised March 2021. Accessed December 7, 2020. https://www.health.ny.gov/diseases/communicable/influenza/seasonal/providers/prevention_of_influenza_transmission/
© 2021 Society of Hospital Medicine
Still Burning
A celebratory mood pervaded the last week of service for my ward team at the end of the academic year. As the attending, it was just another day, but it was hard not to be caught up in the general feeling of a milestone flying past. Like most days in a hospital, this one passed in a rhythm of alternating mundanity and crisis. Late in the afternoon, one of the residents called me to a bedside for help. Imagining that my diagnostic skills were urgently required, I took the stairs. The problem, as it turned out, was not strictly medical.
I could hear the yelling before I cleared the locked entry doors to the ward. It doesn’t really matter what the yelling was about, just that there is often yelling and there is always very little I can do about the root cause of it. As I stepped into the middle of the conflagration, I remembered the story an intern told me about the night earlier in the month when it fell to her to wheel the same patient’s intoxicated parent down to the emergency department. After sleeping it off, the parent was diagnosed with an “allergic reaction” and given a prescription for diphenhydramine. We all knew the diagnosis was fantasy, and yet we all went along with it because there was simply no help available for the root cause of the problem. State social services was already involved, and we had a “safety” plan in place for discharge. As meager as that may have been, we had done the best we could to balance the risk with the available resources… or so we told ourselves.
As a nation, we have chosen not to provide much of a social safety net for our citizens who suffer from addiction and/or mental illness and, most importantly, for those who’ve just not had a leg up on the economic ladder. As a hospital-based clinician, I know that people in distress lose their cool and yell sometimes. Ironically, they may yell most loudly at people who sincerely want to help, simply because others do not engage them. Medical schools don’t teach us how to handle the yelling, though many would say it is part of the hidden curriculum. One thing that distinguishes many pediatricians like myself is a willingness to listen to the yelling, to engage with it, and to try to help. Not surprisingly, our reputation around the hospital is that we skew a bit naive.
It is worth asking, though: Are pediatricians naive? Sure, we make funny faces. We clown. We baby-talk. Those things are largely true, but there are other true things about pediatricians. Chief among them is the fact that we come to know some of the worst things there are to know about human beings. Everybody knows people can be awful, but we know exactly how awful they are to defenseless children in precise detail. For instance, I’ve seen a 4-year-old who was repeatedly starved as a regular punishment. She was so hungry she ate her hair, which turned out to be lucky for her because it caused an intestinal blockage that led to the discovery of the abuse. I gave her an apple one day and she immediately hid it under her shirt. Where you see a scab on a child’s inner arm, I see a cigarette burn. I’ve resuscitated a baby whose parents dipped his pacifier in heroin to stop his crying—the remarkable part of the story being that it was heroin cooked in the hospital bathroom. And then there are the things that I cannot even bring myself to write down.
Carrying this knowledge hollows out a gap between pediatricians and the rest of the world. The divergence between our expectations of how a society should treat its children and the reality of our daily experience grinds away any naivete. The gap becomes a canyon for some of us. We live with the sense that nobody would believe the things we’ve seen, so we rarely talk about them. Years ago, I was testifying in a (for me) routine child-abuse case where this fact hit home. It is common for juries to disbelieve that a caregiver could do the things we allege. I say allege, but if you work as a pediatrician long enough, the space between allegation and fact narrows. It is simply pattern recognition to you—abuse happens so consistently that we accept it as a diagnostic category. The case in question was a submersion burn, which is an almost unmistakable pattern. The other piece of the story is that it happens to toddlers during toilet training as caregivers lose their self-control and punish children for soiling themselves. For me, simple pattern recognition; for the jury, simply unbelievable. We lost the case.
We are almost always losing the case as pediatricians. Spending on children makes up less than 10% of the federal budget, whereas spending on the elderly, including Social Security, Medicare, and the adult component of Medicaid, dominates that budget.1 Moreover, twice as many children as adults over age 65 are living in poverty in the United States. The Temporary Assistance for Needy Families program is often debated in this country and frequently criticized as wasteful. However, what is not debatable is that the allocated budget ($16.5 billion) hasn’t changed since 1996, resulting in a functional 40% decrease due to inflation.2 Life, for poor children, gets a little tougher every year.
After the resident and I wrapped up our day, we talked a little about how hard it is to witness some of the things you see in a children’s hospital. I could see the gap between her and the outside world widening right in front of me. In my weaker moments, I want to tell trainees like her to run while they can. I want to warn them that they don’t want to know so many of the things we’re going to teach them. I know how the story usually ends. I know that our country doesn’t promise children safety from social deprivation, hunger, or physical abuse. Instead, we’ve created the conditions for those things to occur at embarrassingly high rates, and we prosecute the unlucky after the fact. The children are simply collateral damage.
We stood at our patient’s bedside and tried to imagine a happy future. Even without his medical problems, he would likely need a major investment of resources in order to thrive. Where would those resources come from? I saw the hospital crib, metal bars on all sides and a thick plastic roof to prevent escape, as a metaphor. Later, I took the elevator down and overheard a snippet of conversation between two residents. One of them asked the other, “How do you know when you’ve burned out?” The other replied, “I don’t know, I guess when you’ve stopped burning.” Burnout is a hot topic in medicine, and some may assume the reasons are obvious: long hours and intellectually demanding work. In reality, those drivers may be less important than the repeated exposure to profound injustice inherent to the practice of medicine in our country.
As hospitalists, we address acute decompensation in our patients and send them back out into the world knowing there will soon be a next time. We also know that the next time might be preventable, if only … This cycle sometimes feels inexorable, but it can also prompt us to think about our obligation to work toward a more just society. We have to imagine a better future even as we struggle to believe it is on the way.
Most of our hospitals are trying to help. They have community-engagement programs, they purchase housing for homeless patients, they provide large amounts of uncompensated care and sometimes operate at a loss. Yet none of this addresses the root cause of the problem. Medicine, either in the form of an institution or a doctor, can’t replace a just society, but the truth of this fact does not mean we should not try.
Pediatrics has always been a field disposed toward advocacy. The origin of our largest professional organization in the United States was the intraprofessional conflict within the American Medical Association (AMA) over the Sheppard-Towner Act of 1921, one of this country’s first attempts to address the social determinants of health with legislation.3 The American Academy of Pediatrics was formed in 1930 after the AMA House of Delegates rebuked the Pediatric section for advocating for continuance of the act during the late 1920s.3 Perhaps what Pediatrics has to teach the rest of medicine is the necessity of making advocacy a part of our professional identity. And perhaps that’s the reason that so many pediatricians are still burning and not burned out.
1. Committee for a Responsible Federal Budget. Chartbook: budgeting for the next generation. October 11, 2018. Accessed February 2, 2021. http://www.crfb.org/papers/chartbook-budgeting-next-generation
2. Center on Budget and Policy Priorities. Policy basics: temporary assistance for needy families. Updated March 31, 2021. Accessed February 2, 2021. https://www.cbpp.org/research/family-income-support/temporary-assistance-for-needy-families
3. van Dyck PC. A history of child health equity legislation in the United States. Pediatrics. 2003;112(3 pt 2):727-730.
A celebratory mood pervaded the last week of service for my ward team at the end of the academic year. As the attending, it was just another day, but it was hard not to be caught up in the general feeling of a milestone flying past. Like most days in a hospital, this one passed in a rhythm of alternating mundanity and crisis. Late in the afternoon, one of the residents called me to a bedside for help. Imagining that my diagnostic skills were urgently required, I took the stairs. The problem, as it turned out, was not strictly medical.
I could hear the yelling before I cleared the locked entry doors to the ward. It doesn’t really matter what the yelling was about, just that there is often yelling and there is always very little I can do about the root cause of it. As I stepped into the middle of the conflagration, I remembered the story an intern told me about the night earlier in the month when it fell to her to wheel the same patient’s intoxicated parent down to the emergency department. After sleeping it off, the parent was diagnosed with an “allergic reaction” and given a prescription for diphenhydramine. We all knew the diagnosis was fantasy, and yet we all went along with it because there was simply no help available for the root cause of the problem. State social services was already involved, and we had a “safety” plan in place for discharge. As meager as that may have been, we had done the best we could to balance the risk with the available resources… or so we told ourselves.
As a nation, we have chosen not to provide much of a social safety net for our citizens who suffer from addiction and/or mental illness and, most importantly, for those who’ve just not had a leg up on the economic ladder. As a hospital-based clinician, I know that people in distress lose their cool and yell sometimes. Ironically, they may yell most loudly at people who sincerely want to help, simply because others do not engage them. Medical schools don’t teach us how to handle the yelling, though many would say it is part of the hidden curriculum. One thing that distinguishes many pediatricians like myself is a willingness to listen to the yelling, to engage with it, and to try to help. Not surprisingly, our reputation around the hospital is that we skew a bit naive.
It is worth asking, though: Are pediatricians naive? Sure, we make funny faces. We clown. We baby-talk. Those things are largely true, but there are other true things about pediatricians. Chief among them is the fact that we come to know some of the worst things there are to know about human beings. Everybody knows people can be awful, but we know exactly how awful they are to defenseless children in precise detail. For instance, I’ve seen a 4-year-old who was repeatedly starved as a regular punishment. She was so hungry she ate her hair, which turned out to be lucky for her because it caused an intestinal blockage that led to the discovery of the abuse. I gave her an apple one day and she immediately hid it under her shirt. Where you see a scab on a child’s inner arm, I see a cigarette burn. I’ve resuscitated a baby whose parents dipped his pacifier in heroin to stop his crying—the remarkable part of the story being that it was heroin cooked in the hospital bathroom. And then there are the things that I cannot even bring myself to write down.
Carrying this knowledge hollows out a gap between pediatricians and the rest of the world. The divergence between our expectations of how a society should treat its children and the reality of our daily experience grinds away any naivete. The gap becomes a canyon for some of us. We live with the sense that nobody would believe the things we’ve seen, so we rarely talk about them. Years ago, I was testifying in a (for me) routine child-abuse case where this fact hit home. It is common for juries to disbelieve that a caregiver could do the things we allege. I say allege, but if you work as a pediatrician long enough, the space between allegation and fact narrows. It is simply pattern recognition to you—abuse happens so consistently that we accept it as a diagnostic category. The case in question was a submersion burn, which is an almost unmistakable pattern. The other piece of the story is that it happens to toddlers during toilet training as caregivers lose their self-control and punish children for soiling themselves. For me, simple pattern recognition; for the jury, simply unbelievable. We lost the case.
We are almost always losing the case as pediatricians. Spending on children makes up less than 10% of the federal budget, whereas spending on the elderly, including Social Security, Medicare, and the adult component of Medicaid, dominates that budget.1 Moreover, twice as many children as adults over age 65 are living in poverty in the United States. The Temporary Assistance for Needy Families program is often debated in this country and frequently criticized as wasteful. However, what is not debatable is that the allocated budget ($16.5 billion) hasn’t changed since 1996, resulting in a functional 40% decrease due to inflation.2 Life, for poor children, gets a little tougher every year.
After the resident and I wrapped up our day, we talked a little about how hard it is to witness some of the things you see in a children’s hospital. I could see the gap between her and the outside world widening right in front of me. In my weaker moments, I want to tell trainees like her to run while they can. I want to warn them that they don’t want to know so many of the things we’re going to teach them. I know how the story usually ends. I know that our country doesn’t promise children safety from social deprivation, hunger, or physical abuse. Instead, we’ve created the conditions for those things to occur at embarrassingly high rates, and we prosecute the unlucky after the fact. The children are simply collateral damage.
We stood at our patient’s bedside and tried to imagine a happy future. Even without his medical problems, he would likely need a major investment of resources in order to thrive. Where would those resources come from? I saw the hospital crib, metal bars on all sides and a thick plastic roof to prevent escape, as a metaphor. Later, I took the elevator down and overheard a snippet of conversation between two residents. One of them asked the other, “How do you know when you’ve burned out?” The other replied, “I don’t know, I guess when you’ve stopped burning.” Burnout is a hot topic in medicine, and some may assume the reasons are obvious: long hours and intellectually demanding work. In reality, those drivers may be less important than the repeated exposure to profound injustice inherent to the practice of medicine in our country.
As hospitalists, we address acute decompensation in our patients and send them back out into the world knowing there will soon be a next time. We also know that the next time might be preventable, if only … This cycle sometimes feels inexorable, but it can also prompt us to think about our obligation to work toward a more just society. We have to imagine a better future even as we struggle to believe it is on the way.
Most of our hospitals are trying to help. They have community-engagement programs, they purchase housing for homeless patients, they provide large amounts of uncompensated care and sometimes operate at a loss. Yet none of this addresses the root cause of the problem. Medicine, either in the form of an institution or a doctor, can’t replace a just society, but the truth of this fact does not mean we should not try.
Pediatrics has always been a field disposed toward advocacy. The origin of our largest professional organization in the United States was the intraprofessional conflict within the American Medical Association (AMA) over the Sheppard-Towner Act of 1921, one of this country’s first attempts to address the social determinants of health with legislation.3 The American Academy of Pediatrics was formed in 1930 after the AMA House of Delegates rebuked the Pediatric section for advocating for continuance of the act during the late 1920s.3 Perhaps what Pediatrics has to teach the rest of medicine is the necessity of making advocacy a part of our professional identity. And perhaps that’s the reason that so many pediatricians are still burning and not burned out.
A celebratory mood pervaded the last week of service for my ward team at the end of the academic year. As the attending, it was just another day, but it was hard not to be caught up in the general feeling of a milestone flying past. Like most days in a hospital, this one passed in a rhythm of alternating mundanity and crisis. Late in the afternoon, one of the residents called me to a bedside for help. Imagining that my diagnostic skills were urgently required, I took the stairs. The problem, as it turned out, was not strictly medical.
I could hear the yelling before I cleared the locked entry doors to the ward. It doesn’t really matter what the yelling was about, just that there is often yelling and there is always very little I can do about the root cause of it. As I stepped into the middle of the conflagration, I remembered the story an intern told me about the night earlier in the month when it fell to her to wheel the same patient’s intoxicated parent down to the emergency department. After sleeping it off, the parent was diagnosed with an “allergic reaction” and given a prescription for diphenhydramine. We all knew the diagnosis was fantasy, and yet we all went along with it because there was simply no help available for the root cause of the problem. State social services was already involved, and we had a “safety” plan in place for discharge. As meager as that may have been, we had done the best we could to balance the risk with the available resources… or so we told ourselves.
As a nation, we have chosen not to provide much of a social safety net for our citizens who suffer from addiction and/or mental illness and, most importantly, for those who’ve just not had a leg up on the economic ladder. As a hospital-based clinician, I know that people in distress lose their cool and yell sometimes. Ironically, they may yell most loudly at people who sincerely want to help, simply because others do not engage them. Medical schools don’t teach us how to handle the yelling, though many would say it is part of the hidden curriculum. One thing that distinguishes many pediatricians like myself is a willingness to listen to the yelling, to engage with it, and to try to help. Not surprisingly, our reputation around the hospital is that we skew a bit naive.
It is worth asking, though: Are pediatricians naive? Sure, we make funny faces. We clown. We baby-talk. Those things are largely true, but there are other true things about pediatricians. Chief among them is the fact that we come to know some of the worst things there are to know about human beings. Everybody knows people can be awful, but we know exactly how awful they are to defenseless children in precise detail. For instance, I’ve seen a 4-year-old who was repeatedly starved as a regular punishment. She was so hungry she ate her hair, which turned out to be lucky for her because it caused an intestinal blockage that led to the discovery of the abuse. I gave her an apple one day and she immediately hid it under her shirt. Where you see a scab on a child’s inner arm, I see a cigarette burn. I’ve resuscitated a baby whose parents dipped his pacifier in heroin to stop his crying—the remarkable part of the story being that it was heroin cooked in the hospital bathroom. And then there are the things that I cannot even bring myself to write down.
Carrying this knowledge hollows out a gap between pediatricians and the rest of the world. The divergence between our expectations of how a society should treat its children and the reality of our daily experience grinds away any naivete. The gap becomes a canyon for some of us. We live with the sense that nobody would believe the things we’ve seen, so we rarely talk about them. Years ago, I was testifying in a (for me) routine child-abuse case where this fact hit home. It is common for juries to disbelieve that a caregiver could do the things we allege. I say allege, but if you work as a pediatrician long enough, the space between allegation and fact narrows. It is simply pattern recognition to you—abuse happens so consistently that we accept it as a diagnostic category. The case in question was a submersion burn, which is an almost unmistakable pattern. The other piece of the story is that it happens to toddlers during toilet training as caregivers lose their self-control and punish children for soiling themselves. For me, simple pattern recognition; for the jury, simply unbelievable. We lost the case.
We are almost always losing the case as pediatricians. Spending on children makes up less than 10% of the federal budget, whereas spending on the elderly, including Social Security, Medicare, and the adult component of Medicaid, dominates that budget.1 Moreover, twice as many children as adults over age 65 are living in poverty in the United States. The Temporary Assistance for Needy Families program is often debated in this country and frequently criticized as wasteful. However, what is not debatable is that the allocated budget ($16.5 billion) hasn’t changed since 1996, resulting in a functional 40% decrease due to inflation.2 Life, for poor children, gets a little tougher every year.
After the resident and I wrapped up our day, we talked a little about how hard it is to witness some of the things you see in a children’s hospital. I could see the gap between her and the outside world widening right in front of me. In my weaker moments, I want to tell trainees like her to run while they can. I want to warn them that they don’t want to know so many of the things we’re going to teach them. I know how the story usually ends. I know that our country doesn’t promise children safety from social deprivation, hunger, or physical abuse. Instead, we’ve created the conditions for those things to occur at embarrassingly high rates, and we prosecute the unlucky after the fact. The children are simply collateral damage.
We stood at our patient’s bedside and tried to imagine a happy future. Even without his medical problems, he would likely need a major investment of resources in order to thrive. Where would those resources come from? I saw the hospital crib, metal bars on all sides and a thick plastic roof to prevent escape, as a metaphor. Later, I took the elevator down and overheard a snippet of conversation between two residents. One of them asked the other, “How do you know when you’ve burned out?” The other replied, “I don’t know, I guess when you’ve stopped burning.” Burnout is a hot topic in medicine, and some may assume the reasons are obvious: long hours and intellectually demanding work. In reality, those drivers may be less important than the repeated exposure to profound injustice inherent to the practice of medicine in our country.
As hospitalists, we address acute decompensation in our patients and send them back out into the world knowing there will soon be a next time. We also know that the next time might be preventable, if only … This cycle sometimes feels inexorable, but it can also prompt us to think about our obligation to work toward a more just society. We have to imagine a better future even as we struggle to believe it is on the way.
Most of our hospitals are trying to help. They have community-engagement programs, they purchase housing for homeless patients, they provide large amounts of uncompensated care and sometimes operate at a loss. Yet none of this addresses the root cause of the problem. Medicine, either in the form of an institution or a doctor, can’t replace a just society, but the truth of this fact does not mean we should not try.
Pediatrics has always been a field disposed toward advocacy. The origin of our largest professional organization in the United States was the intraprofessional conflict within the American Medical Association (AMA) over the Sheppard-Towner Act of 1921, one of this country’s first attempts to address the social determinants of health with legislation.3 The American Academy of Pediatrics was formed in 1930 after the AMA House of Delegates rebuked the Pediatric section for advocating for continuance of the act during the late 1920s.3 Perhaps what Pediatrics has to teach the rest of medicine is the necessity of making advocacy a part of our professional identity. And perhaps that’s the reason that so many pediatricians are still burning and not burned out.
1. Committee for a Responsible Federal Budget. Chartbook: budgeting for the next generation. October 11, 2018. Accessed February 2, 2021. http://www.crfb.org/papers/chartbook-budgeting-next-generation
2. Center on Budget and Policy Priorities. Policy basics: temporary assistance for needy families. Updated March 31, 2021. Accessed February 2, 2021. https://www.cbpp.org/research/family-income-support/temporary-assistance-for-needy-families
3. van Dyck PC. A history of child health equity legislation in the United States. Pediatrics. 2003;112(3 pt 2):727-730.
1. Committee for a Responsible Federal Budget. Chartbook: budgeting for the next generation. October 11, 2018. Accessed February 2, 2021. http://www.crfb.org/papers/chartbook-budgeting-next-generation
2. Center on Budget and Policy Priorities. Policy basics: temporary assistance for needy families. Updated March 31, 2021. Accessed February 2, 2021. https://www.cbpp.org/research/family-income-support/temporary-assistance-for-needy-families
3. van Dyck PC. A history of child health equity legislation in the United States. Pediatrics. 2003;112(3 pt 2):727-730.
© 2021 Society of Hospital Medicine
Ableism and Quality of Life During the Coronavirus Pandemic
Michael Hickson was a 46-year-old with a severe acquired disability whose COVID-19 course involved multisystem organ failure, a court-appointed guardian, hospice care, discontinued fluids and tube feeds, and eventual death. While some details have been released by the hospital,1 the recorded conversation between Mr. Hickson’s wife and a treating physician has been shared widely in disability communities.
Physician: “Right now, his quality of life—he doesn’t have much of one.”
Spouse: “What do you mean? Because he’s paralyzed with a brain injury, he doesn’t have a quality of life?”
Physician: “Correct.”
As physiatrists—physicians for patients with disabilities—we heard those words with heavy hearts and sunken stomachs. We can only imagine the anger, fear, and betrayal felt by our patients and other people with disabilities. Or perhaps they feel vindicated, that the quiet sentiments were finally said out loud. The recording expresses what people with disabilities long suspected: physicians don’t always value the lives of persons with disabilities the way they value the nondisabled. Research confirms this.2-4 The privilege of the nondisabled is often expressed as “I would never want to live like that.” People make personal judgments about how they would feel in somebody else’s situation. The usually quiet sentiment, this time said aloud and recorded—“He doesn’t have much [quality of life]”—showed how physicians’ judgments and biases can have a grave impact on others, especially people with disabilities.
Stereotypes, assumptions, and biases about the quality of life of people with disabilities are pervasive throughout healthcare, resulting in the devaluation and disparate treatment of people with disabilities.5 Healthcare providers are not exempt from deficit-based perspectives about people with disabilities,6 and discrimination ensues when healthcare providers make critical decisions from these perspectives.5 Ableist biases are underrecognized among physicians, who often misperceive quality of life for people with disabilities as poor, and fail to recognize that medical judgments can be biased accordingly.5 Consequently, necessary care can be withheld or withdrawn inappropriately.5 An estimated 25% of adults in the United States self-report disability; furthermore, disability is highly correlated with age as well as socioeconomic disadvantages.7 There is also extensive evidence that, as a population, people with disabilities experience healthcare disparities.8 Bias against people with disabilities serves to both restrict and reduce access to healthcare.9
The consequences of the pandemic have disproportionally affected the Black community, in terms of both economic and disease burden. Mr. Hickson, a Black man with disabilities who contracted COVID-19, personifies the intersection of race and disability and demands our concern and attention as physicians. We must appreciate the intrinsic worth of all people and populations, and seek to understand and respect their capacity to be active agents in their own lives, making their own decisions about their quality of life. The lives of Black people have value, but movements such as Black Lives Matter have been needed to highlight this truth, and there still needs to be meaningful action beyond rhetoric. The lives of people with disabilities have value. Healthcare systems and providers similarly need to acknowledge and act in a way that honors the intrinsic worth of people with disabilities.
People with disabilities face long-standing systemic barriers to equitable healthcare,10 as do Black people. During the pandemic, widespread alarm was raised about individual and structural racism in medicine, just as numerous disability rights organizations raised concerns that ableism would lead to undertreatment during the COVID-19 crisis, worsening existing healthcare inequities. In response, the US Department of Health and Human Services Office for Civil Rights in Action released a bulletin that stated, “In this time of emergency, the laudable goal of providing care quickly and efficiently must be guided by the fundamental principles of fairness, equality, and compassion that animate our civil rights laws. This is particularly true with respect to the treatment of persons with disabilities during medical emergencies as they possess the same dignity and worth as everyone else.”11 Using the presence of disabilities to limit or deny a person’s access to health care constitutes a clear violation of nondiscrimination law.12 Hospitals and providers should not limit the care offered to people with disabilities because of their disabilities or utilize quality-of-life judgments when deciding whether or not to provide care.12 While the hospital where Michael Hickson died released a statement claiming that they did not consider his disability status as part of their treatment decision-making, the recorded words of the physician suggest otherwise.
The impact of our words and actions, and not the underlying intent, most affects patients’, families’, and communities’ trust in the institution of medicine, represented by individual providers. The hospital statement indicated “it was not medically possible to save [Mr. Hickson].”1 The phrase “not medically possible” ties Mr. Hickson’s case to one of futility; however, the recording was about quality of life, not futility. The National Council on Disability found that subjective quality-of-life assumptions influence medical futility decisions.5 While the intent of withdrawing care from Mr. Hickson may have been related to futility, the consequences of this decision are far-reaching as people with disabilities have reason to question whether someone else’s judgment about the quality and worth of their life will lead to loss of their life.
Emphasizing perceived quality of life in making treatment decisions, as was implied for Mr. Hickson, is not a rare event and is one that is likely more common when health systems are stressed. Despite having policies and procedures to follow, biases creep into treatment decisions. In Oregon, for example, multiple cases of disability discrimination during the pandemic were brought to the attention of the state Senate by Disability Rights Oregon.13,14
Physicians and healthcare leaders must consider the unique needs of the disability community through health equity efforts in the COVID-19 response. There must be universally accessible approaches when planning and implementing a COVID response to increase impact and ensure systems are reaching all underserved communities. For healthcare institutions and hospitals, disability equity must be emphasized in the development and implementation of COVID-19 policies. The exclusion of people with disabilities from decisions about people with disabilities is problematic. This systemic exclusion means that ableist beliefs and policies are often unchallenged.15 Including people with disabilities on committees creating crisis standards of care protocols or other policies that may purposefully or unintentionally discriminate against people with disabilities is an important step.16 Representation matters, and people with disabilities must be central in the development of all health equity strategies during a pandemic. Furthermore, when system-level decision algorithms exist that value the life of people with disabilities, clinician biases are minimized, leading to more equitable care.
Examples of strategies include accessible formats for essential COVID-19-related communications, such as American Sign Language, large print, or screen reading technology. We must acknowledge that necessary universal mask policies can generate communication barriers for people reading lips. Hospitals and clinics have rapidly expanded virtual care and telemedicine to improve access. This has enhanced access to care for many people with mobility disability, but can exacerbate disparities for those with vision, hearing, communication, or intellectual disability. To better manage this issue, tailored strategies, such as live closed captioning or digital patient navigators, can be implemented.
Additionally, a person with a disability has the legal right to be accompanied by a designated essential support person. Hospital visitor policies must become less restrictive or enable exceptions when a person with a disability requires their personal care attendant. When it comes to outcome data, it is important to highlight the need for better collection of disability data that can be used to identify inequities as well as monitor outcomes of treatment.
As previously acknowledged, people without disabilities tend to have negative attitudes (both implicit and explicit) toward people with disabilities. These attitudes are re-enforced by societal-level institutions, policies, and structures that marginalize people with disabilities.17 We call on all physicians and those working in healthcare to question their biases. When you consider quality of life in your decision-making, ask yourself, “whose life?” Recognize and honor the personal, social, and cultural contexts that affect how an individual experiences “quality of life.” Unless the answer to “whose life?” is your own or that of your incapacitated dependent, it is not your place to make “quality of life” judgments. You can and should describe potential outcomes at the physiological or activity level, but leave quality-of-life decisions where they belong—with the individual or their desi
Social media activity in the disability community indicates that Mr. Hickson’s story is perceived, regardless of the provider’s and healthcare system’s intentions, to be yet another breach of trust by the medical system. It is not the burden of the oppressed and betrayed to repair a broken relationship. It is our obligation, as individual physicians and the greater medical institution, to provide care that demonstrates the value and worth of people with disabilities. An imperative step toward equitable care for people with disabilities is to recognize and address our ableist biases.
1. Anderson D. Statement on the death of Michael Hickson. St David’s HealthCare. July 2, 2020. Accessed July 6, 2020. https://stdavids.com/about/newsroom/statement-on-the-death-of-michael-hickson
2. Amundson R. Disability, ideology, and quality of life: a bias in biomedical ethics. In: Wasserman D, Bickenbach J, Wachbroit R, eds. Quality of Life and Human Difference: Genetic Testing, Health Care, and Disability. Cambridge University Press; 2005:101-124.
3. Dunn DS. Outsider privileges can lead to insider disadvantages: some psychosocial aspects of ableism. J Soc Issues. 2019;75(3):665-682. https://doi.org/10.1111/josi.12331
4. Kothari S. Clinical (mis)judgments of quality of life after disability. J Clin Ethics. 2004;15:300-307.
5. National Council on Disability. Medical futility and disability bias: part of the bioethics and disability series. November 19, 2019. Accessed March 31, 2021. https://www.ncd.gov/sites/default/files/NCD_Medical_Futility_Report_508.pdf
6. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. https://doi.org/10.1377/hlthaff.2020.01452
7. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. https://doi.org/10.15585/mmwr.mm6732a3
8. Meade MA, Mahmoudi E, Lee SY. The intersection of disability and healthcare disparities: a conceptual framework. Disabil Rehabil. 2015;37(7):632-641. https://doi.org/10.3109/09638288.2014.938176
9. Andrews EE, Ayers KB, Brown KS, Dunn DS, Pilarski CR. No body is expendable: medical rationing and disability justice during the COVID-19 pandemic. Am Psychol. Published online July 23, 2020. https://doi.org/10.1037/amp0000709
10. Savin K, Guidry-Grimes L. Confronting disability discrimination during the pandemic. The Hastings Center. April 2, 2020. Accessed March 31, 2021. https://www.thehastingscenter.org/confronting-disability-discrimination-during-the-pandemic/
11. Health and Human Services Office for Civil Rights in Action. Bulletin: civil rights, HIPAA, and the coronavirus disease 2019. March 28, 2020. Accessed March 31, 2021. https://www.hhs.gov/sites/default/files/ocr-bulletin-3-28-20.pdf
12. Preventing discrimination in the treatment of COVID-19 patients: the illegality of medical rationing on the basis of disability. Disability Rights Education & Defense Fund. March 25, 2020. Accessed March 31, 2021. https://dredf.org/wp-content/uploads/2020/03/DREDF-Policy-Statement-on-COVID-19-and-Medical-Rationing-3-25-2020.pdf
13. Oregon hospitals told not to withhold care because of a person’s disability. Transcript. Morning Edition. National Public Radio. December 21, 2020. Accessed March 31, 2021. https://www.npr.org/2020/12/21/948697808/oregon-hospitals-told-not-to-withhold-care-because-of-a-persons-disability
14. As hospitals fear being overwhelmed by COVID-19, do the disabled get the same access? Transcript. Morning Edition. National Public Radio. December 14, 2020. Accessed March 31, 2021. https://www.npr.org/2020/12/14/945056176/as-hospitals-fear-being-overwhelmed-by-covid-19-do-the-disabled-get-the-same-acc
15. Lund EM, Forber-Pratt AJ, Wilson C, Mona LR. The COVID-19 pandemic, stress, and trauma in the disability community: a call to action. Rehabil Psychol. 2020;65(4):313-322. https://doi.org/10.1037/rep0000368
16. Auriemma CL, Molinero AM, Houtrow AJ, Persad G, White DB, Halpern SD. Eliminating categorical exclusion criteria in crisis standards of care frameworks. Am J Bioeth. 2020;20(7):28-36. http://doi.org/10.1080/15265161.2020.1764141
17. Bogart KR, Dunn DS. Ableism special issue introduction. J Soc Issues. 2019;75(3):650-664. https://doi.org/10.1111/josi.12354
Michael Hickson was a 46-year-old with a severe acquired disability whose COVID-19 course involved multisystem organ failure, a court-appointed guardian, hospice care, discontinued fluids and tube feeds, and eventual death. While some details have been released by the hospital,1 the recorded conversation between Mr. Hickson’s wife and a treating physician has been shared widely in disability communities.
Physician: “Right now, his quality of life—he doesn’t have much of one.”
Spouse: “What do you mean? Because he’s paralyzed with a brain injury, he doesn’t have a quality of life?”
Physician: “Correct.”
As physiatrists—physicians for patients with disabilities—we heard those words with heavy hearts and sunken stomachs. We can only imagine the anger, fear, and betrayal felt by our patients and other people with disabilities. Or perhaps they feel vindicated, that the quiet sentiments were finally said out loud. The recording expresses what people with disabilities long suspected: physicians don’t always value the lives of persons with disabilities the way they value the nondisabled. Research confirms this.2-4 The privilege of the nondisabled is often expressed as “I would never want to live like that.” People make personal judgments about how they would feel in somebody else’s situation. The usually quiet sentiment, this time said aloud and recorded—“He doesn’t have much [quality of life]”—showed how physicians’ judgments and biases can have a grave impact on others, especially people with disabilities.
Stereotypes, assumptions, and biases about the quality of life of people with disabilities are pervasive throughout healthcare, resulting in the devaluation and disparate treatment of people with disabilities.5 Healthcare providers are not exempt from deficit-based perspectives about people with disabilities,6 and discrimination ensues when healthcare providers make critical decisions from these perspectives.5 Ableist biases are underrecognized among physicians, who often misperceive quality of life for people with disabilities as poor, and fail to recognize that medical judgments can be biased accordingly.5 Consequently, necessary care can be withheld or withdrawn inappropriately.5 An estimated 25% of adults in the United States self-report disability; furthermore, disability is highly correlated with age as well as socioeconomic disadvantages.7 There is also extensive evidence that, as a population, people with disabilities experience healthcare disparities.8 Bias against people with disabilities serves to both restrict and reduce access to healthcare.9
The consequences of the pandemic have disproportionally affected the Black community, in terms of both economic and disease burden. Mr. Hickson, a Black man with disabilities who contracted COVID-19, personifies the intersection of race and disability and demands our concern and attention as physicians. We must appreciate the intrinsic worth of all people and populations, and seek to understand and respect their capacity to be active agents in their own lives, making their own decisions about their quality of life. The lives of Black people have value, but movements such as Black Lives Matter have been needed to highlight this truth, and there still needs to be meaningful action beyond rhetoric. The lives of people with disabilities have value. Healthcare systems and providers similarly need to acknowledge and act in a way that honors the intrinsic worth of people with disabilities.
People with disabilities face long-standing systemic barriers to equitable healthcare,10 as do Black people. During the pandemic, widespread alarm was raised about individual and structural racism in medicine, just as numerous disability rights organizations raised concerns that ableism would lead to undertreatment during the COVID-19 crisis, worsening existing healthcare inequities. In response, the US Department of Health and Human Services Office for Civil Rights in Action released a bulletin that stated, “In this time of emergency, the laudable goal of providing care quickly and efficiently must be guided by the fundamental principles of fairness, equality, and compassion that animate our civil rights laws. This is particularly true with respect to the treatment of persons with disabilities during medical emergencies as they possess the same dignity and worth as everyone else.”11 Using the presence of disabilities to limit or deny a person’s access to health care constitutes a clear violation of nondiscrimination law.12 Hospitals and providers should not limit the care offered to people with disabilities because of their disabilities or utilize quality-of-life judgments when deciding whether or not to provide care.12 While the hospital where Michael Hickson died released a statement claiming that they did not consider his disability status as part of their treatment decision-making, the recorded words of the physician suggest otherwise.
The impact of our words and actions, and not the underlying intent, most affects patients’, families’, and communities’ trust in the institution of medicine, represented by individual providers. The hospital statement indicated “it was not medically possible to save [Mr. Hickson].”1 The phrase “not medically possible” ties Mr. Hickson’s case to one of futility; however, the recording was about quality of life, not futility. The National Council on Disability found that subjective quality-of-life assumptions influence medical futility decisions.5 While the intent of withdrawing care from Mr. Hickson may have been related to futility, the consequences of this decision are far-reaching as people with disabilities have reason to question whether someone else’s judgment about the quality and worth of their life will lead to loss of their life.
Emphasizing perceived quality of life in making treatment decisions, as was implied for Mr. Hickson, is not a rare event and is one that is likely more common when health systems are stressed. Despite having policies and procedures to follow, biases creep into treatment decisions. In Oregon, for example, multiple cases of disability discrimination during the pandemic were brought to the attention of the state Senate by Disability Rights Oregon.13,14
Physicians and healthcare leaders must consider the unique needs of the disability community through health equity efforts in the COVID-19 response. There must be universally accessible approaches when planning and implementing a COVID response to increase impact and ensure systems are reaching all underserved communities. For healthcare institutions and hospitals, disability equity must be emphasized in the development and implementation of COVID-19 policies. The exclusion of people with disabilities from decisions about people with disabilities is problematic. This systemic exclusion means that ableist beliefs and policies are often unchallenged.15 Including people with disabilities on committees creating crisis standards of care protocols or other policies that may purposefully or unintentionally discriminate against people with disabilities is an important step.16 Representation matters, and people with disabilities must be central in the development of all health equity strategies during a pandemic. Furthermore, when system-level decision algorithms exist that value the life of people with disabilities, clinician biases are minimized, leading to more equitable care.
Examples of strategies include accessible formats for essential COVID-19-related communications, such as American Sign Language, large print, or screen reading technology. We must acknowledge that necessary universal mask policies can generate communication barriers for people reading lips. Hospitals and clinics have rapidly expanded virtual care and telemedicine to improve access. This has enhanced access to care for many people with mobility disability, but can exacerbate disparities for those with vision, hearing, communication, or intellectual disability. To better manage this issue, tailored strategies, such as live closed captioning or digital patient navigators, can be implemented.
Additionally, a person with a disability has the legal right to be accompanied by a designated essential support person. Hospital visitor policies must become less restrictive or enable exceptions when a person with a disability requires their personal care attendant. When it comes to outcome data, it is important to highlight the need for better collection of disability data that can be used to identify inequities as well as monitor outcomes of treatment.
As previously acknowledged, people without disabilities tend to have negative attitudes (both implicit and explicit) toward people with disabilities. These attitudes are re-enforced by societal-level institutions, policies, and structures that marginalize people with disabilities.17 We call on all physicians and those working in healthcare to question their biases. When you consider quality of life in your decision-making, ask yourself, “whose life?” Recognize and honor the personal, social, and cultural contexts that affect how an individual experiences “quality of life.” Unless the answer to “whose life?” is your own or that of your incapacitated dependent, it is not your place to make “quality of life” judgments. You can and should describe potential outcomes at the physiological or activity level, but leave quality-of-life decisions where they belong—with the individual or their desi
Social media activity in the disability community indicates that Mr. Hickson’s story is perceived, regardless of the provider’s and healthcare system’s intentions, to be yet another breach of trust by the medical system. It is not the burden of the oppressed and betrayed to repair a broken relationship. It is our obligation, as individual physicians and the greater medical institution, to provide care that demonstrates the value and worth of people with disabilities. An imperative step toward equitable care for people with disabilities is to recognize and address our ableist biases.
Michael Hickson was a 46-year-old with a severe acquired disability whose COVID-19 course involved multisystem organ failure, a court-appointed guardian, hospice care, discontinued fluids and tube feeds, and eventual death. While some details have been released by the hospital,1 the recorded conversation between Mr. Hickson’s wife and a treating physician has been shared widely in disability communities.
Physician: “Right now, his quality of life—he doesn’t have much of one.”
Spouse: “What do you mean? Because he’s paralyzed with a brain injury, he doesn’t have a quality of life?”
Physician: “Correct.”
As physiatrists—physicians for patients with disabilities—we heard those words with heavy hearts and sunken stomachs. We can only imagine the anger, fear, and betrayal felt by our patients and other people with disabilities. Or perhaps they feel vindicated, that the quiet sentiments were finally said out loud. The recording expresses what people with disabilities long suspected: physicians don’t always value the lives of persons with disabilities the way they value the nondisabled. Research confirms this.2-4 The privilege of the nondisabled is often expressed as “I would never want to live like that.” People make personal judgments about how they would feel in somebody else’s situation. The usually quiet sentiment, this time said aloud and recorded—“He doesn’t have much [quality of life]”—showed how physicians’ judgments and biases can have a grave impact on others, especially people with disabilities.
Stereotypes, assumptions, and biases about the quality of life of people with disabilities are pervasive throughout healthcare, resulting in the devaluation and disparate treatment of people with disabilities.5 Healthcare providers are not exempt from deficit-based perspectives about people with disabilities,6 and discrimination ensues when healthcare providers make critical decisions from these perspectives.5 Ableist biases are underrecognized among physicians, who often misperceive quality of life for people with disabilities as poor, and fail to recognize that medical judgments can be biased accordingly.5 Consequently, necessary care can be withheld or withdrawn inappropriately.5 An estimated 25% of adults in the United States self-report disability; furthermore, disability is highly correlated with age as well as socioeconomic disadvantages.7 There is also extensive evidence that, as a population, people with disabilities experience healthcare disparities.8 Bias against people with disabilities serves to both restrict and reduce access to healthcare.9
The consequences of the pandemic have disproportionally affected the Black community, in terms of both economic and disease burden. Mr. Hickson, a Black man with disabilities who contracted COVID-19, personifies the intersection of race and disability and demands our concern and attention as physicians. We must appreciate the intrinsic worth of all people and populations, and seek to understand and respect their capacity to be active agents in their own lives, making their own decisions about their quality of life. The lives of Black people have value, but movements such as Black Lives Matter have been needed to highlight this truth, and there still needs to be meaningful action beyond rhetoric. The lives of people with disabilities have value. Healthcare systems and providers similarly need to acknowledge and act in a way that honors the intrinsic worth of people with disabilities.
People with disabilities face long-standing systemic barriers to equitable healthcare,10 as do Black people. During the pandemic, widespread alarm was raised about individual and structural racism in medicine, just as numerous disability rights organizations raised concerns that ableism would lead to undertreatment during the COVID-19 crisis, worsening existing healthcare inequities. In response, the US Department of Health and Human Services Office for Civil Rights in Action released a bulletin that stated, “In this time of emergency, the laudable goal of providing care quickly and efficiently must be guided by the fundamental principles of fairness, equality, and compassion that animate our civil rights laws. This is particularly true with respect to the treatment of persons with disabilities during medical emergencies as they possess the same dignity and worth as everyone else.”11 Using the presence of disabilities to limit or deny a person’s access to health care constitutes a clear violation of nondiscrimination law.12 Hospitals and providers should not limit the care offered to people with disabilities because of their disabilities or utilize quality-of-life judgments when deciding whether or not to provide care.12 While the hospital where Michael Hickson died released a statement claiming that they did not consider his disability status as part of their treatment decision-making, the recorded words of the physician suggest otherwise.
The impact of our words and actions, and not the underlying intent, most affects patients’, families’, and communities’ trust in the institution of medicine, represented by individual providers. The hospital statement indicated “it was not medically possible to save [Mr. Hickson].”1 The phrase “not medically possible” ties Mr. Hickson’s case to one of futility; however, the recording was about quality of life, not futility. The National Council on Disability found that subjective quality-of-life assumptions influence medical futility decisions.5 While the intent of withdrawing care from Mr. Hickson may have been related to futility, the consequences of this decision are far-reaching as people with disabilities have reason to question whether someone else’s judgment about the quality and worth of their life will lead to loss of their life.
Emphasizing perceived quality of life in making treatment decisions, as was implied for Mr. Hickson, is not a rare event and is one that is likely more common when health systems are stressed. Despite having policies and procedures to follow, biases creep into treatment decisions. In Oregon, for example, multiple cases of disability discrimination during the pandemic were brought to the attention of the state Senate by Disability Rights Oregon.13,14
Physicians and healthcare leaders must consider the unique needs of the disability community through health equity efforts in the COVID-19 response. There must be universally accessible approaches when planning and implementing a COVID response to increase impact and ensure systems are reaching all underserved communities. For healthcare institutions and hospitals, disability equity must be emphasized in the development and implementation of COVID-19 policies. The exclusion of people with disabilities from decisions about people with disabilities is problematic. This systemic exclusion means that ableist beliefs and policies are often unchallenged.15 Including people with disabilities on committees creating crisis standards of care protocols or other policies that may purposefully or unintentionally discriminate against people with disabilities is an important step.16 Representation matters, and people with disabilities must be central in the development of all health equity strategies during a pandemic. Furthermore, when system-level decision algorithms exist that value the life of people with disabilities, clinician biases are minimized, leading to more equitable care.
Examples of strategies include accessible formats for essential COVID-19-related communications, such as American Sign Language, large print, or screen reading technology. We must acknowledge that necessary universal mask policies can generate communication barriers for people reading lips. Hospitals and clinics have rapidly expanded virtual care and telemedicine to improve access. This has enhanced access to care for many people with mobility disability, but can exacerbate disparities for those with vision, hearing, communication, or intellectual disability. To better manage this issue, tailored strategies, such as live closed captioning or digital patient navigators, can be implemented.
Additionally, a person with a disability has the legal right to be accompanied by a designated essential support person. Hospital visitor policies must become less restrictive or enable exceptions when a person with a disability requires their personal care attendant. When it comes to outcome data, it is important to highlight the need for better collection of disability data that can be used to identify inequities as well as monitor outcomes of treatment.
As previously acknowledged, people without disabilities tend to have negative attitudes (both implicit and explicit) toward people with disabilities. These attitudes are re-enforced by societal-level institutions, policies, and structures that marginalize people with disabilities.17 We call on all physicians and those working in healthcare to question their biases. When you consider quality of life in your decision-making, ask yourself, “whose life?” Recognize and honor the personal, social, and cultural contexts that affect how an individual experiences “quality of life.” Unless the answer to “whose life?” is your own or that of your incapacitated dependent, it is not your place to make “quality of life” judgments. You can and should describe potential outcomes at the physiological or activity level, but leave quality-of-life decisions where they belong—with the individual or their desi
Social media activity in the disability community indicates that Mr. Hickson’s story is perceived, regardless of the provider’s and healthcare system’s intentions, to be yet another breach of trust by the medical system. It is not the burden of the oppressed and betrayed to repair a broken relationship. It is our obligation, as individual physicians and the greater medical institution, to provide care that demonstrates the value and worth of people with disabilities. An imperative step toward equitable care for people with disabilities is to recognize and address our ableist biases.
1. Anderson D. Statement on the death of Michael Hickson. St David’s HealthCare. July 2, 2020. Accessed July 6, 2020. https://stdavids.com/about/newsroom/statement-on-the-death-of-michael-hickson
2. Amundson R. Disability, ideology, and quality of life: a bias in biomedical ethics. In: Wasserman D, Bickenbach J, Wachbroit R, eds. Quality of Life and Human Difference: Genetic Testing, Health Care, and Disability. Cambridge University Press; 2005:101-124.
3. Dunn DS. Outsider privileges can lead to insider disadvantages: some psychosocial aspects of ableism. J Soc Issues. 2019;75(3):665-682. https://doi.org/10.1111/josi.12331
4. Kothari S. Clinical (mis)judgments of quality of life after disability. J Clin Ethics. 2004;15:300-307.
5. National Council on Disability. Medical futility and disability bias: part of the bioethics and disability series. November 19, 2019. Accessed March 31, 2021. https://www.ncd.gov/sites/default/files/NCD_Medical_Futility_Report_508.pdf
6. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. https://doi.org/10.1377/hlthaff.2020.01452
7. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. https://doi.org/10.15585/mmwr.mm6732a3
8. Meade MA, Mahmoudi E, Lee SY. The intersection of disability and healthcare disparities: a conceptual framework. Disabil Rehabil. 2015;37(7):632-641. https://doi.org/10.3109/09638288.2014.938176
9. Andrews EE, Ayers KB, Brown KS, Dunn DS, Pilarski CR. No body is expendable: medical rationing and disability justice during the COVID-19 pandemic. Am Psychol. Published online July 23, 2020. https://doi.org/10.1037/amp0000709
10. Savin K, Guidry-Grimes L. Confronting disability discrimination during the pandemic. The Hastings Center. April 2, 2020. Accessed March 31, 2021. https://www.thehastingscenter.org/confronting-disability-discrimination-during-the-pandemic/
11. Health and Human Services Office for Civil Rights in Action. Bulletin: civil rights, HIPAA, and the coronavirus disease 2019. March 28, 2020. Accessed March 31, 2021. https://www.hhs.gov/sites/default/files/ocr-bulletin-3-28-20.pdf
12. Preventing discrimination in the treatment of COVID-19 patients: the illegality of medical rationing on the basis of disability. Disability Rights Education & Defense Fund. March 25, 2020. Accessed March 31, 2021. https://dredf.org/wp-content/uploads/2020/03/DREDF-Policy-Statement-on-COVID-19-and-Medical-Rationing-3-25-2020.pdf
13. Oregon hospitals told not to withhold care because of a person’s disability. Transcript. Morning Edition. National Public Radio. December 21, 2020. Accessed March 31, 2021. https://www.npr.org/2020/12/21/948697808/oregon-hospitals-told-not-to-withhold-care-because-of-a-persons-disability
14. As hospitals fear being overwhelmed by COVID-19, do the disabled get the same access? Transcript. Morning Edition. National Public Radio. December 14, 2020. Accessed March 31, 2021. https://www.npr.org/2020/12/14/945056176/as-hospitals-fear-being-overwhelmed-by-covid-19-do-the-disabled-get-the-same-acc
15. Lund EM, Forber-Pratt AJ, Wilson C, Mona LR. The COVID-19 pandemic, stress, and trauma in the disability community: a call to action. Rehabil Psychol. 2020;65(4):313-322. https://doi.org/10.1037/rep0000368
16. Auriemma CL, Molinero AM, Houtrow AJ, Persad G, White DB, Halpern SD. Eliminating categorical exclusion criteria in crisis standards of care frameworks. Am J Bioeth. 2020;20(7):28-36. http://doi.org/10.1080/15265161.2020.1764141
17. Bogart KR, Dunn DS. Ableism special issue introduction. J Soc Issues. 2019;75(3):650-664. https://doi.org/10.1111/josi.12354
1. Anderson D. Statement on the death of Michael Hickson. St David’s HealthCare. July 2, 2020. Accessed July 6, 2020. https://stdavids.com/about/newsroom/statement-on-the-death-of-michael-hickson
2. Amundson R. Disability, ideology, and quality of life: a bias in biomedical ethics. In: Wasserman D, Bickenbach J, Wachbroit R, eds. Quality of Life and Human Difference: Genetic Testing, Health Care, and Disability. Cambridge University Press; 2005:101-124.
3. Dunn DS. Outsider privileges can lead to insider disadvantages: some psychosocial aspects of ableism. J Soc Issues. 2019;75(3):665-682. https://doi.org/10.1111/josi.12331
4. Kothari S. Clinical (mis)judgments of quality of life after disability. J Clin Ethics. 2004;15:300-307.
5. National Council on Disability. Medical futility and disability bias: part of the bioethics and disability series. November 19, 2019. Accessed March 31, 2021. https://www.ncd.gov/sites/default/files/NCD_Medical_Futility_Report_508.pdf
6. Iezzoni LI, Rao SR, Ressalam J, et al. Physicians’ perceptions of people with disability and their health care. Health Aff (Millwood). 2021;40(2):297-306. https://doi.org/10.1377/hlthaff.2020.01452
7. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887. https://doi.org/10.15585/mmwr.mm6732a3
8. Meade MA, Mahmoudi E, Lee SY. The intersection of disability and healthcare disparities: a conceptual framework. Disabil Rehabil. 2015;37(7):632-641. https://doi.org/10.3109/09638288.2014.938176
9. Andrews EE, Ayers KB, Brown KS, Dunn DS, Pilarski CR. No body is expendable: medical rationing and disability justice during the COVID-19 pandemic. Am Psychol. Published online July 23, 2020. https://doi.org/10.1037/amp0000709
10. Savin K, Guidry-Grimes L. Confronting disability discrimination during the pandemic. The Hastings Center. April 2, 2020. Accessed March 31, 2021. https://www.thehastingscenter.org/confronting-disability-discrimination-during-the-pandemic/
11. Health and Human Services Office for Civil Rights in Action. Bulletin: civil rights, HIPAA, and the coronavirus disease 2019. March 28, 2020. Accessed March 31, 2021. https://www.hhs.gov/sites/default/files/ocr-bulletin-3-28-20.pdf
12. Preventing discrimination in the treatment of COVID-19 patients: the illegality of medical rationing on the basis of disability. Disability Rights Education & Defense Fund. March 25, 2020. Accessed March 31, 2021. https://dredf.org/wp-content/uploads/2020/03/DREDF-Policy-Statement-on-COVID-19-and-Medical-Rationing-3-25-2020.pdf
13. Oregon hospitals told not to withhold care because of a person’s disability. Transcript. Morning Edition. National Public Radio. December 21, 2020. Accessed March 31, 2021. https://www.npr.org/2020/12/21/948697808/oregon-hospitals-told-not-to-withhold-care-because-of-a-persons-disability
14. As hospitals fear being overwhelmed by COVID-19, do the disabled get the same access? Transcript. Morning Edition. National Public Radio. December 14, 2020. Accessed March 31, 2021. https://www.npr.org/2020/12/14/945056176/as-hospitals-fear-being-overwhelmed-by-covid-19-do-the-disabled-get-the-same-acc
15. Lund EM, Forber-Pratt AJ, Wilson C, Mona LR. The COVID-19 pandemic, stress, and trauma in the disability community: a call to action. Rehabil Psychol. 2020;65(4):313-322. https://doi.org/10.1037/rep0000368
16. Auriemma CL, Molinero AM, Houtrow AJ, Persad G, White DB, Halpern SD. Eliminating categorical exclusion criteria in crisis standards of care frameworks. Am J Bioeth. 2020;20(7):28-36. http://doi.org/10.1080/15265161.2020.1764141
17. Bogart KR, Dunn DS. Ableism special issue introduction. J Soc Issues. 2019;75(3):650-664. https://doi.org/10.1111/josi.12354
© 2021 Society of Hospital Medicine
Supporting Hospitals During a New Wave of COVID-19
The COVID-19 pandemic has put an extraordinary strain on US hospitals.1 In spring 2020, many hospitals had to quickly adapt to treat a surge of patients, and many more had to prepare for a potential surge. Creating reserve capacity meant halting outpatient care and elective surgeries, repurposing inpatient units, and increasing critical care staffing. Hospitals again face these difficult decisions, as COVID-19 resurges and variants of SARS-CoV-2 increasingly circulate, with large financial losses projected for 2021.2 Some large hospital systems may have the financial reserves to weather this storm, but the precarious situation facing others likely requires policy action.
Hospitals’ financial stress emanates from multiple quarters. First, revenue from elective inpatient procedures and outpatient care dropped dramatically, has not fully rebounded,3,4 and is not fully offset by revenue from COVID-19 care. Second, high unemployment may force up to 20% of commercially insured Americans into lower-reimbursing public insurance or the ranks of the uninsured, generating a projected $95 billion annual loss for hospitals.5 Third, under the current payment system, the costs of preparing for a pandemic are not directly reimbursed. Yet—whether or not they ultimately experienced a large COVID-19 caseload—hospitals’ surge preparation has involved purchasing vast quantities of protective personal equipment (PPE) and other supplies and equipment, hiring additional staff, building SARS-CoV-2 testing capacity, and expanding occupational health services. Many expenses persist as “the new normal”: admissions now require SARS-CoV-2 testing, additional staff and PPE, and often, a private room. Physical distancing requirements mean hospitals’ capacity—and thus, revenue—will remain reduced.
Private insurers, by and large, are not volunteering to cover these increased costs, and it is difficult for hospitals to pass them along. Payment terms in many contracts (eg, for Medicare) are not modifiable; even where they are, renegotiating takes time. To date, federal relief payments from the CARES Act do not fully reimburse COVID-19 losses—a particular problem for smaller and safety-net hospitals without large reserves.
This situation raises ethical concerns. For example, it is ethically relevant that COVID-19 resurgence and hospitalizations are linked to states’ decisions to reopen quickly to ease economic burdens on businesses and workers. One result has been to shift some of the pandemic’s economic burden to the healthcare sector. From a fairness perspective, there should be limits on the losses hospitals are forced to shoulder to maintain COVID-19 preparedness and services. Even if hospitals have reserves, spending them threatens funding for other essential activities, such as capital investment.
The current situation is also fraught with perverse incentives that could jeopardize safe care. With elective care remaining at risk of being reduced,6 pressure intensifies to deliver as many services as possible as quickly as possible, which may not align with patients’ best interests. Across hospitals that need to maximize volume to survive, a push to keep elective services open may emerge, even as COVID-19 prevalence may favor a shutdown. Hospitals with a heavy COVID-19 caseload may have greater difficulty reopening than competitors with lower caseloads, potentially impacting quality if patients seek elective care at lower-volume centers or in ways that disrupt continuity of care.
Ethical dilemmas are also raised by the delicate balancing of interests that hospitals have been engaging in among patient groups. How should they balance the needs of COVID-19 patients against potential harms to others who must delay care?
It is wrong to ask hospitals to make such choices when policy solutions are available. With the resurgence of COVID-19 must come a fresh, sustained program of federal financial relief for hospitals. While direct government support is the swiftest path, consideration should be given to the role of private insurers, which have benefited economically from the widespread deferment and forgoing of elective care. Voluntary or mandatory investments by insurers in helping hospitals survive the pandemic and weather the new normal are consonant with insurers’ commitment to providing their members access to high-quality healthcare.
The 200-page National Strategy document released by the Biden administration on January 21, 2021, promises some important assistance to hospitals.7 It includes plans to accelerate the production of PPE and other essential supplies using the Defense Production Act and other federal authorities, to rationalize nationwide distribution of these supplies and take steps to prevent price gouging, and to deploy federal personnel and assets to help surge critical-care personnel.
These steps, if fully funded and implemented, would bring welcome respite from some of the most vexing problems hospitals have encountered during COVID-19 surges. Yet, plans for direct financial relief for hospitals are strikingly absent from the National Strategy. Nor does the recently passed $1.9 trillion federal stimulus package provide dedicated funds for hospitals, though some funds earmarked for vaccine delivery may land at hospitals. These are consequential omissions in otherwise comprehensive, thoughtful pandemic response plans.
Future legislation should include an immediate revenue infusion to reimburse hospitals’ COVID-19 preparations and lost volume and a firm commitment of ongoing financial support for preparedness through the end of the pandemic at a level sufficient to offset COVID-19–related losses. Experience with the CARES Act also suggests specific lessons for statutory design: support for hospitals should be allocated based on actual COVID-19–related burden for preparation and care, unlike CARES Act grants that were allocated based on hospitals’ past revenue and Medicare billing. This resulted in some large payments to relatively well-off hospitals and scant support for others (eg, rural or safety-net hospitals) with substantial COVID-19–related losses, a misstep that should not be repeated.
Hospitals are an integral part of the nation’s public health system. In the context of a pandemic, they should not be forced to serve as a backstop for shortcomings in other parts of the system without assistance. They, and their mission during the pandemic, are too important to fail.
1. Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127-2128. https://doi.org/10.1001/jama.2020.6269
2. Coleman-Lochner L. Hospitals plead for bailout in face of runaway pandemic bills. February 26, 2021. Accessed March 25, 2021. https://www.bloomberg.com/news/articles/2021-02-26/hospitals-plead-for-bailout-in-face-of-runaway-pandemic-bills
3. American Hospital Association. Hospitals and health systems continue to face unprecedented financial challenges due to COVID-19. June 2020. Accessed February 5. 2021. https://www.aha.org/system/files/media/file/2020/06/aha-covid19-financial-impact-report.pdf
4. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
5. Teasdale B, Schulman KA. Are U.S. hospitals still “recession-proof”? N Engl J Med. 2020;383(13):e82. https://doi.org/10.1056/NEJMp2018846
6. Meredith JW, High KP, Freischlag JA. Preserving elective surgeries in the COVID-19 pandemic and the future. JAMA. 2020;324(17):1725-1726. https://doi.org/10.1001/jama.2020.19594
7. Biden JR. National strategy for the COVID-19 response and pandemic preparedness. Bloomberg. January 2021. Accessed February 8, 2021. https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-COVID-19-Response-and-Pandemic-Preparedness.pdf
The COVID-19 pandemic has put an extraordinary strain on US hospitals.1 In spring 2020, many hospitals had to quickly adapt to treat a surge of patients, and many more had to prepare for a potential surge. Creating reserve capacity meant halting outpatient care and elective surgeries, repurposing inpatient units, and increasing critical care staffing. Hospitals again face these difficult decisions, as COVID-19 resurges and variants of SARS-CoV-2 increasingly circulate, with large financial losses projected for 2021.2 Some large hospital systems may have the financial reserves to weather this storm, but the precarious situation facing others likely requires policy action.
Hospitals’ financial stress emanates from multiple quarters. First, revenue from elective inpatient procedures and outpatient care dropped dramatically, has not fully rebounded,3,4 and is not fully offset by revenue from COVID-19 care. Second, high unemployment may force up to 20% of commercially insured Americans into lower-reimbursing public insurance or the ranks of the uninsured, generating a projected $95 billion annual loss for hospitals.5 Third, under the current payment system, the costs of preparing for a pandemic are not directly reimbursed. Yet—whether or not they ultimately experienced a large COVID-19 caseload—hospitals’ surge preparation has involved purchasing vast quantities of protective personal equipment (PPE) and other supplies and equipment, hiring additional staff, building SARS-CoV-2 testing capacity, and expanding occupational health services. Many expenses persist as “the new normal”: admissions now require SARS-CoV-2 testing, additional staff and PPE, and often, a private room. Physical distancing requirements mean hospitals’ capacity—and thus, revenue—will remain reduced.
Private insurers, by and large, are not volunteering to cover these increased costs, and it is difficult for hospitals to pass them along. Payment terms in many contracts (eg, for Medicare) are not modifiable; even where they are, renegotiating takes time. To date, federal relief payments from the CARES Act do not fully reimburse COVID-19 losses—a particular problem for smaller and safety-net hospitals without large reserves.
This situation raises ethical concerns. For example, it is ethically relevant that COVID-19 resurgence and hospitalizations are linked to states’ decisions to reopen quickly to ease economic burdens on businesses and workers. One result has been to shift some of the pandemic’s economic burden to the healthcare sector. From a fairness perspective, there should be limits on the losses hospitals are forced to shoulder to maintain COVID-19 preparedness and services. Even if hospitals have reserves, spending them threatens funding for other essential activities, such as capital investment.
The current situation is also fraught with perverse incentives that could jeopardize safe care. With elective care remaining at risk of being reduced,6 pressure intensifies to deliver as many services as possible as quickly as possible, which may not align with patients’ best interests. Across hospitals that need to maximize volume to survive, a push to keep elective services open may emerge, even as COVID-19 prevalence may favor a shutdown. Hospitals with a heavy COVID-19 caseload may have greater difficulty reopening than competitors with lower caseloads, potentially impacting quality if patients seek elective care at lower-volume centers or in ways that disrupt continuity of care.
Ethical dilemmas are also raised by the delicate balancing of interests that hospitals have been engaging in among patient groups. How should they balance the needs of COVID-19 patients against potential harms to others who must delay care?
It is wrong to ask hospitals to make such choices when policy solutions are available. With the resurgence of COVID-19 must come a fresh, sustained program of federal financial relief for hospitals. While direct government support is the swiftest path, consideration should be given to the role of private insurers, which have benefited economically from the widespread deferment and forgoing of elective care. Voluntary or mandatory investments by insurers in helping hospitals survive the pandemic and weather the new normal are consonant with insurers’ commitment to providing their members access to high-quality healthcare.
The 200-page National Strategy document released by the Biden administration on January 21, 2021, promises some important assistance to hospitals.7 It includes plans to accelerate the production of PPE and other essential supplies using the Defense Production Act and other federal authorities, to rationalize nationwide distribution of these supplies and take steps to prevent price gouging, and to deploy federal personnel and assets to help surge critical-care personnel.
These steps, if fully funded and implemented, would bring welcome respite from some of the most vexing problems hospitals have encountered during COVID-19 surges. Yet, plans for direct financial relief for hospitals are strikingly absent from the National Strategy. Nor does the recently passed $1.9 trillion federal stimulus package provide dedicated funds for hospitals, though some funds earmarked for vaccine delivery may land at hospitals. These are consequential omissions in otherwise comprehensive, thoughtful pandemic response plans.
Future legislation should include an immediate revenue infusion to reimburse hospitals’ COVID-19 preparations and lost volume and a firm commitment of ongoing financial support for preparedness through the end of the pandemic at a level sufficient to offset COVID-19–related losses. Experience with the CARES Act also suggests specific lessons for statutory design: support for hospitals should be allocated based on actual COVID-19–related burden for preparation and care, unlike CARES Act grants that were allocated based on hospitals’ past revenue and Medicare billing. This resulted in some large payments to relatively well-off hospitals and scant support for others (eg, rural or safety-net hospitals) with substantial COVID-19–related losses, a misstep that should not be repeated.
Hospitals are an integral part of the nation’s public health system. In the context of a pandemic, they should not be forced to serve as a backstop for shortcomings in other parts of the system without assistance. They, and their mission during the pandemic, are too important to fail.
The COVID-19 pandemic has put an extraordinary strain on US hospitals.1 In spring 2020, many hospitals had to quickly adapt to treat a surge of patients, and many more had to prepare for a potential surge. Creating reserve capacity meant halting outpatient care and elective surgeries, repurposing inpatient units, and increasing critical care staffing. Hospitals again face these difficult decisions, as COVID-19 resurges and variants of SARS-CoV-2 increasingly circulate, with large financial losses projected for 2021.2 Some large hospital systems may have the financial reserves to weather this storm, but the precarious situation facing others likely requires policy action.
Hospitals’ financial stress emanates from multiple quarters. First, revenue from elective inpatient procedures and outpatient care dropped dramatically, has not fully rebounded,3,4 and is not fully offset by revenue from COVID-19 care. Second, high unemployment may force up to 20% of commercially insured Americans into lower-reimbursing public insurance or the ranks of the uninsured, generating a projected $95 billion annual loss for hospitals.5 Third, under the current payment system, the costs of preparing for a pandemic are not directly reimbursed. Yet—whether or not they ultimately experienced a large COVID-19 caseload—hospitals’ surge preparation has involved purchasing vast quantities of protective personal equipment (PPE) and other supplies and equipment, hiring additional staff, building SARS-CoV-2 testing capacity, and expanding occupational health services. Many expenses persist as “the new normal”: admissions now require SARS-CoV-2 testing, additional staff and PPE, and often, a private room. Physical distancing requirements mean hospitals’ capacity—and thus, revenue—will remain reduced.
Private insurers, by and large, are not volunteering to cover these increased costs, and it is difficult for hospitals to pass them along. Payment terms in many contracts (eg, for Medicare) are not modifiable; even where they are, renegotiating takes time. To date, federal relief payments from the CARES Act do not fully reimburse COVID-19 losses—a particular problem for smaller and safety-net hospitals without large reserves.
This situation raises ethical concerns. For example, it is ethically relevant that COVID-19 resurgence and hospitalizations are linked to states’ decisions to reopen quickly to ease economic burdens on businesses and workers. One result has been to shift some of the pandemic’s economic burden to the healthcare sector. From a fairness perspective, there should be limits on the losses hospitals are forced to shoulder to maintain COVID-19 preparedness and services. Even if hospitals have reserves, spending them threatens funding for other essential activities, such as capital investment.
The current situation is also fraught with perverse incentives that could jeopardize safe care. With elective care remaining at risk of being reduced,6 pressure intensifies to deliver as many services as possible as quickly as possible, which may not align with patients’ best interests. Across hospitals that need to maximize volume to survive, a push to keep elective services open may emerge, even as COVID-19 prevalence may favor a shutdown. Hospitals with a heavy COVID-19 caseload may have greater difficulty reopening than competitors with lower caseloads, potentially impacting quality if patients seek elective care at lower-volume centers or in ways that disrupt continuity of care.
Ethical dilemmas are also raised by the delicate balancing of interests that hospitals have been engaging in among patient groups. How should they balance the needs of COVID-19 patients against potential harms to others who must delay care?
It is wrong to ask hospitals to make such choices when policy solutions are available. With the resurgence of COVID-19 must come a fresh, sustained program of federal financial relief for hospitals. While direct government support is the swiftest path, consideration should be given to the role of private insurers, which have benefited economically from the widespread deferment and forgoing of elective care. Voluntary or mandatory investments by insurers in helping hospitals survive the pandemic and weather the new normal are consonant with insurers’ commitment to providing their members access to high-quality healthcare.
The 200-page National Strategy document released by the Biden administration on January 21, 2021, promises some important assistance to hospitals.7 It includes plans to accelerate the production of PPE and other essential supplies using the Defense Production Act and other federal authorities, to rationalize nationwide distribution of these supplies and take steps to prevent price gouging, and to deploy federal personnel and assets to help surge critical-care personnel.
These steps, if fully funded and implemented, would bring welcome respite from some of the most vexing problems hospitals have encountered during COVID-19 surges. Yet, plans for direct financial relief for hospitals are strikingly absent from the National Strategy. Nor does the recently passed $1.9 trillion federal stimulus package provide dedicated funds for hospitals, though some funds earmarked for vaccine delivery may land at hospitals. These are consequential omissions in otherwise comprehensive, thoughtful pandemic response plans.
Future legislation should include an immediate revenue infusion to reimburse hospitals’ COVID-19 preparations and lost volume and a firm commitment of ongoing financial support for preparedness through the end of the pandemic at a level sufficient to offset COVID-19–related losses. Experience with the CARES Act also suggests specific lessons for statutory design: support for hospitals should be allocated based on actual COVID-19–related burden for preparation and care, unlike CARES Act grants that were allocated based on hospitals’ past revenue and Medicare billing. This resulted in some large payments to relatively well-off hospitals and scant support for others (eg, rural or safety-net hospitals) with substantial COVID-19–related losses, a misstep that should not be repeated.
Hospitals are an integral part of the nation’s public health system. In the context of a pandemic, they should not be forced to serve as a backstop for shortcomings in other parts of the system without assistance. They, and their mission during the pandemic, are too important to fail.
1. Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127-2128. https://doi.org/10.1001/jama.2020.6269
2. Coleman-Lochner L. Hospitals plead for bailout in face of runaway pandemic bills. February 26, 2021. Accessed March 25, 2021. https://www.bloomberg.com/news/articles/2021-02-26/hospitals-plead-for-bailout-in-face-of-runaway-pandemic-bills
3. American Hospital Association. Hospitals and health systems continue to face unprecedented financial challenges due to COVID-19. June 2020. Accessed February 5. 2021. https://www.aha.org/system/files/media/file/2020/06/aha-covid19-financial-impact-report.pdf
4. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
5. Teasdale B, Schulman KA. Are U.S. hospitals still “recession-proof”? N Engl J Med. 2020;383(13):e82. https://doi.org/10.1056/NEJMp2018846
6. Meredith JW, High KP, Freischlag JA. Preserving elective surgeries in the COVID-19 pandemic and the future. JAMA. 2020;324(17):1725-1726. https://doi.org/10.1001/jama.2020.19594
7. Biden JR. National strategy for the COVID-19 response and pandemic preparedness. Bloomberg. January 2021. Accessed February 8, 2021. https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-COVID-19-Response-and-Pandemic-Preparedness.pdf
1. Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127-2128. https://doi.org/10.1001/jama.2020.6269
2. Coleman-Lochner L. Hospitals plead for bailout in face of runaway pandemic bills. February 26, 2021. Accessed March 25, 2021. https://www.bloomberg.com/news/articles/2021-02-26/hospitals-plead-for-bailout-in-face-of-runaway-pandemic-bills
3. American Hospital Association. Hospitals and health systems continue to face unprecedented financial challenges due to COVID-19. June 2020. Accessed February 5. 2021. https://www.aha.org/system/files/media/file/2020/06/aha-covid19-financial-impact-report.pdf
4. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
5. Teasdale B, Schulman KA. Are U.S. hospitals still “recession-proof”? N Engl J Med. 2020;383(13):e82. https://doi.org/10.1056/NEJMp2018846
6. Meredith JW, High KP, Freischlag JA. Preserving elective surgeries in the COVID-19 pandemic and the future. JAMA. 2020;324(17):1725-1726. https://doi.org/10.1001/jama.2020.19594
7. Biden JR. National strategy for the COVID-19 response and pandemic preparedness. Bloomberg. January 2021. Accessed February 8, 2021. https://www.whitehouse.gov/wp-content/uploads/2021/01/National-Strategy-for-the-COVID-19-Response-and-Pandemic-Preparedness.pdf
© 2021 Society of Hospital Medicine







