User login
Are Women Better Doctors Than Men?
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.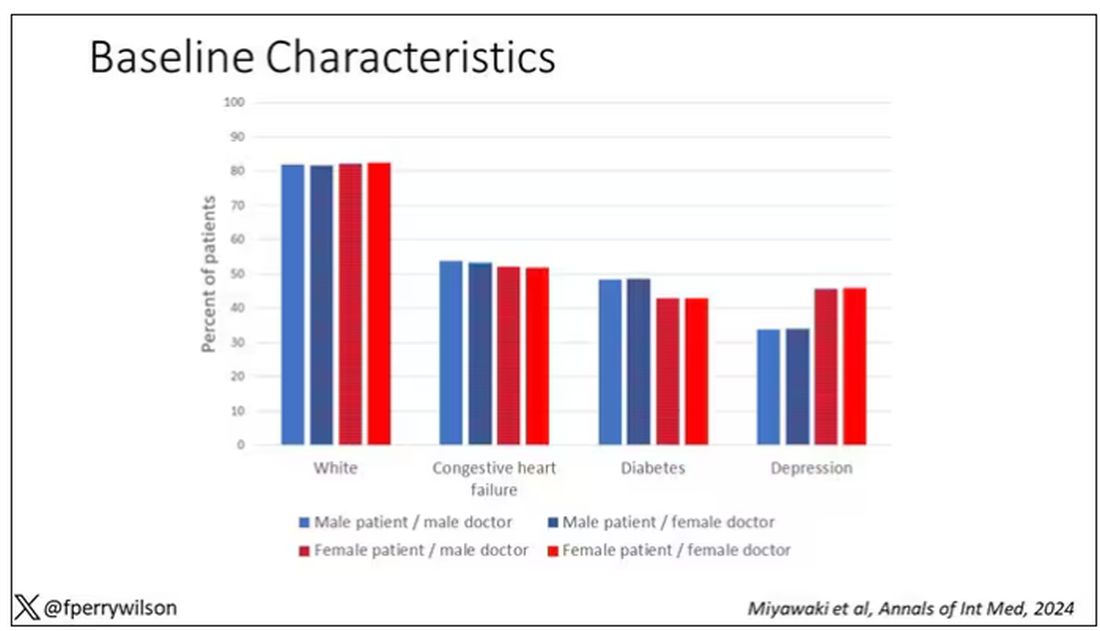
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 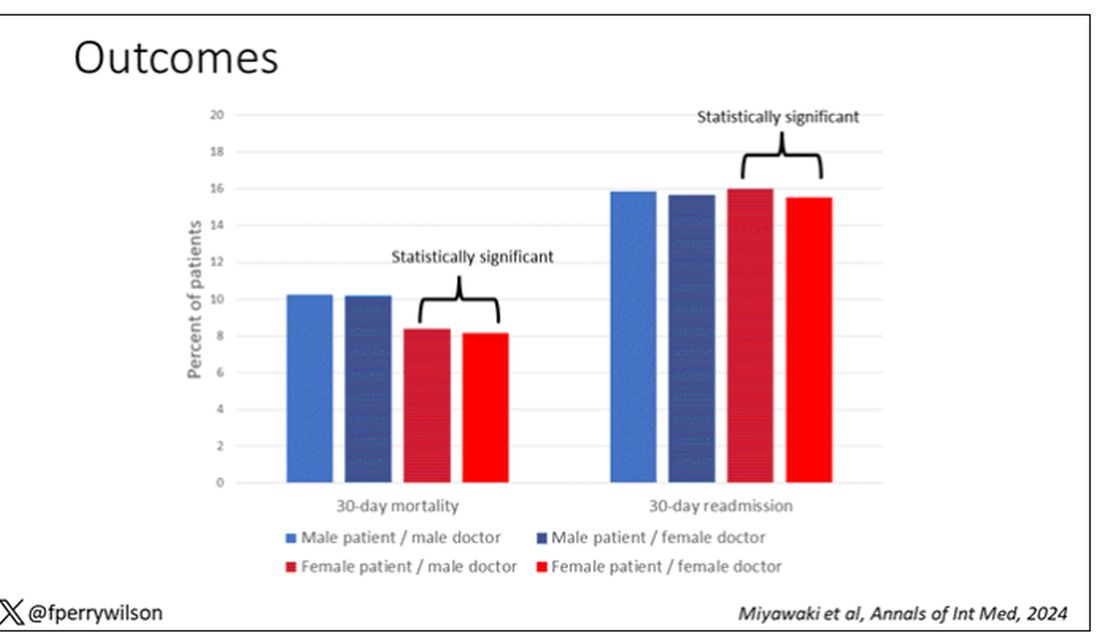
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Avoidance Predicts Worse Long-term Outcomes From Intensive OCD Treatment
BOSTON — , a new analysis shows.
Although avoidant patients with OCD reported symptom improvement immediately after treatment, baseline avoidance was associated with significantly worse outcomes 1 year later.
“Avoidance is often overlooked in OCD,” said lead investigator Michael Wheaton, PhD, an assistant professor of psychology at Barnard College in New York. “It’s really important clinically to focus on that.”
The findings were presented at the Anxiety and Depression Association of America (ADAA) annual conference and published online in the Journal of Obsessive-Compulsive and Related Disorders.
The Avoidance Question
Although ERP is often included in treatment for OCD, between 38% and 60% of patients have residual symptoms after treatment and as many as a quarter don’t respond at all, Dr. Wheaton said.
Severe pretreatment avoidance could affect the efficacy of ERP, which involves exposing patients to situations and stimuli they may usually avoid. But prior research to identify predictors of ERP outcomes have largely excluded severity of pretreatment avoidance as a factor.
The new study analyzed data from 161 Norwegian adults with treatment-resistant OCD who participated in a concentrated ERP therapy called the Bergen 4-day Exposure and Response Prevention (B4DT) treatment. This method delivers intensive treatment over 4 consecutive days in small groups with a 1:1 ratio of therapists to patients.
B4DT is common throughout Norway, with the treatment offered at 55 clinics, and has been trialed in other countries including the United States, Nepal, Ecuador, and Kenya.
Symptom severity was measured using the Yale-Brown Obsessive Compulsive Scale (YBOCS) at baseline, immediately after treatment, and 3 and 12 months later. Functional impairment was measured 12 months after treatment using the Work and Social Adjustment Scale.
Although the formal scoring of the YBOCS does not include any questions about avoidance, one question in the auxiliary items does: “Have you been avoiding doing anything, going anyplace or being with anyone because of obsessional thoughts or out of a need to perform compulsions?”
Dr. Wheaton used this response, which is rated on a five-point scale, to measure avoidance. Overall, 18.8% of participants had no deliberate avoidance, 15% were rated as having mild avoidance, 36% moderate, 23% severe, and 6.8% extreme.
Long-Term Outcomes
Overall, 84% of participants responded to treatment, with a change in mean YBOCS scores from 26.98 at baseline to 12.28 immediately after treatment. Acute outcomes were similar between avoidant and nonavoidant patients.
But at 12-month follow-up, even after controlling for pretreatment OCD severity, patients with more extensive avoidance at baseline had worse long-term outcomes — both more severe OCD symptoms (P = .031) and greater functional impairment (P = .002).
Across all patients, average avoidance decreased significantly immediately after the concentrated ERP treatment. Average avoidance increased somewhat at 3- and 12-month follow-up but remained significantly improved from pretreatment.
Interestingly, patients’ change in avoidance immediately post-treatment to 3 months post-treatment predicted worsening of OCD severity at 12 months. This change could potentially identify people at risk of relapse, Dr. Wheaton said.
Previous research has shown that pretreatment OCD severity, measured using the YBOCS, does not significantly predict ERP outcomes, and this study found the same.
Relapse Prevention
“The fact that they did equally well in the short run I think was great,” Dr. Wheaton said.
Previous research, including 2018 and 2023 papers from Wheaton’s team, has shown that more avoidant patients have worse outcomes from standard 12-week ERP programs.
One possible explanation for this difference is that in the Bergen treatment, most exposures happen during face-to-face time with a therapist instead of as homework, which may be easier to avoid, he said.
“But then the finding was that their symptoms were worsening over time — their avoidance was sliding back into old habits,” said Dr. Wheaton.
He would like to see the study replicated in diverse populations outside Norway and in treatment-naive people. Dr. Wheaton also noted that the study assessed avoidance with only a single item.
Future work is needed to test ways to improve relapse prevention. For example, clinicians may be able to monitor for avoidance behaviors post-treatment, which could be the start of a relapse, said Dr. Wheaton.
Although clinicians consider avoidance when treating phobias, social anxiety disorder, and panic disorder, “somehow avoidance got relegated to item 11 on the YBOCS that isn’t scored,” Helen Blair Simpson, MD, PhD, director of the Center for OCD and Related Disorders at Columbia University, New York, New York, said during the presentation.
A direct implication of Dr. Wheaton’s findings to clinical practice is to “talk to people about their avoidance right up front,” said Dr. Simpson, who was not part of the study.
Clinicians who deliver ERP in their practices “can apply this tomorrow,” Dr. Simpson added.
Dr. Wheaton reported no disclosures. Dr. Simpson reported a stipend from the American Medical Association for serving as associate editor of JAMA Psychiatry and royalties from UpToDate, Inc for articles on OCD and from Cambridge University Press for editing a book on anxiety disorders.
A version of this article appeared on Medscape.com.
BOSTON — , a new analysis shows.
Although avoidant patients with OCD reported symptom improvement immediately after treatment, baseline avoidance was associated with significantly worse outcomes 1 year later.
“Avoidance is often overlooked in OCD,” said lead investigator Michael Wheaton, PhD, an assistant professor of psychology at Barnard College in New York. “It’s really important clinically to focus on that.”
The findings were presented at the Anxiety and Depression Association of America (ADAA) annual conference and published online in the Journal of Obsessive-Compulsive and Related Disorders.
The Avoidance Question
Although ERP is often included in treatment for OCD, between 38% and 60% of patients have residual symptoms after treatment and as many as a quarter don’t respond at all, Dr. Wheaton said.
Severe pretreatment avoidance could affect the efficacy of ERP, which involves exposing patients to situations and stimuli they may usually avoid. But prior research to identify predictors of ERP outcomes have largely excluded severity of pretreatment avoidance as a factor.
The new study analyzed data from 161 Norwegian adults with treatment-resistant OCD who participated in a concentrated ERP therapy called the Bergen 4-day Exposure and Response Prevention (B4DT) treatment. This method delivers intensive treatment over 4 consecutive days in small groups with a 1:1 ratio of therapists to patients.
B4DT is common throughout Norway, with the treatment offered at 55 clinics, and has been trialed in other countries including the United States, Nepal, Ecuador, and Kenya.
Symptom severity was measured using the Yale-Brown Obsessive Compulsive Scale (YBOCS) at baseline, immediately after treatment, and 3 and 12 months later. Functional impairment was measured 12 months after treatment using the Work and Social Adjustment Scale.
Although the formal scoring of the YBOCS does not include any questions about avoidance, one question in the auxiliary items does: “Have you been avoiding doing anything, going anyplace or being with anyone because of obsessional thoughts or out of a need to perform compulsions?”
Dr. Wheaton used this response, which is rated on a five-point scale, to measure avoidance. Overall, 18.8% of participants had no deliberate avoidance, 15% were rated as having mild avoidance, 36% moderate, 23% severe, and 6.8% extreme.
Long-Term Outcomes
Overall, 84% of participants responded to treatment, with a change in mean YBOCS scores from 26.98 at baseline to 12.28 immediately after treatment. Acute outcomes were similar between avoidant and nonavoidant patients.
But at 12-month follow-up, even after controlling for pretreatment OCD severity, patients with more extensive avoidance at baseline had worse long-term outcomes — both more severe OCD symptoms (P = .031) and greater functional impairment (P = .002).
Across all patients, average avoidance decreased significantly immediately after the concentrated ERP treatment. Average avoidance increased somewhat at 3- and 12-month follow-up but remained significantly improved from pretreatment.
Interestingly, patients’ change in avoidance immediately post-treatment to 3 months post-treatment predicted worsening of OCD severity at 12 months. This change could potentially identify people at risk of relapse, Dr. Wheaton said.
Previous research has shown that pretreatment OCD severity, measured using the YBOCS, does not significantly predict ERP outcomes, and this study found the same.
Relapse Prevention
“The fact that they did equally well in the short run I think was great,” Dr. Wheaton said.
Previous research, including 2018 and 2023 papers from Wheaton’s team, has shown that more avoidant patients have worse outcomes from standard 12-week ERP programs.
One possible explanation for this difference is that in the Bergen treatment, most exposures happen during face-to-face time with a therapist instead of as homework, which may be easier to avoid, he said.
“But then the finding was that their symptoms were worsening over time — their avoidance was sliding back into old habits,” said Dr. Wheaton.
He would like to see the study replicated in diverse populations outside Norway and in treatment-naive people. Dr. Wheaton also noted that the study assessed avoidance with only a single item.
Future work is needed to test ways to improve relapse prevention. For example, clinicians may be able to monitor for avoidance behaviors post-treatment, which could be the start of a relapse, said Dr. Wheaton.
Although clinicians consider avoidance when treating phobias, social anxiety disorder, and panic disorder, “somehow avoidance got relegated to item 11 on the YBOCS that isn’t scored,” Helen Blair Simpson, MD, PhD, director of the Center for OCD and Related Disorders at Columbia University, New York, New York, said during the presentation.
A direct implication of Dr. Wheaton’s findings to clinical practice is to “talk to people about their avoidance right up front,” said Dr. Simpson, who was not part of the study.
Clinicians who deliver ERP in their practices “can apply this tomorrow,” Dr. Simpson added.
Dr. Wheaton reported no disclosures. Dr. Simpson reported a stipend from the American Medical Association for serving as associate editor of JAMA Psychiatry and royalties from UpToDate, Inc for articles on OCD and from Cambridge University Press for editing a book on anxiety disorders.
A version of this article appeared on Medscape.com.
BOSTON — , a new analysis shows.
Although avoidant patients with OCD reported symptom improvement immediately after treatment, baseline avoidance was associated with significantly worse outcomes 1 year later.
“Avoidance is often overlooked in OCD,” said lead investigator Michael Wheaton, PhD, an assistant professor of psychology at Barnard College in New York. “It’s really important clinically to focus on that.”
The findings were presented at the Anxiety and Depression Association of America (ADAA) annual conference and published online in the Journal of Obsessive-Compulsive and Related Disorders.
The Avoidance Question
Although ERP is often included in treatment for OCD, between 38% and 60% of patients have residual symptoms after treatment and as many as a quarter don’t respond at all, Dr. Wheaton said.
Severe pretreatment avoidance could affect the efficacy of ERP, which involves exposing patients to situations and stimuli they may usually avoid. But prior research to identify predictors of ERP outcomes have largely excluded severity of pretreatment avoidance as a factor.
The new study analyzed data from 161 Norwegian adults with treatment-resistant OCD who participated in a concentrated ERP therapy called the Bergen 4-day Exposure and Response Prevention (B4DT) treatment. This method delivers intensive treatment over 4 consecutive days in small groups with a 1:1 ratio of therapists to patients.
B4DT is common throughout Norway, with the treatment offered at 55 clinics, and has been trialed in other countries including the United States, Nepal, Ecuador, and Kenya.
Symptom severity was measured using the Yale-Brown Obsessive Compulsive Scale (YBOCS) at baseline, immediately after treatment, and 3 and 12 months later. Functional impairment was measured 12 months after treatment using the Work and Social Adjustment Scale.
Although the formal scoring of the YBOCS does not include any questions about avoidance, one question in the auxiliary items does: “Have you been avoiding doing anything, going anyplace or being with anyone because of obsessional thoughts or out of a need to perform compulsions?”
Dr. Wheaton used this response, which is rated on a five-point scale, to measure avoidance. Overall, 18.8% of participants had no deliberate avoidance, 15% were rated as having mild avoidance, 36% moderate, 23% severe, and 6.8% extreme.
Long-Term Outcomes
Overall, 84% of participants responded to treatment, with a change in mean YBOCS scores from 26.98 at baseline to 12.28 immediately after treatment. Acute outcomes were similar between avoidant and nonavoidant patients.
But at 12-month follow-up, even after controlling for pretreatment OCD severity, patients with more extensive avoidance at baseline had worse long-term outcomes — both more severe OCD symptoms (P = .031) and greater functional impairment (P = .002).
Across all patients, average avoidance decreased significantly immediately after the concentrated ERP treatment. Average avoidance increased somewhat at 3- and 12-month follow-up but remained significantly improved from pretreatment.
Interestingly, patients’ change in avoidance immediately post-treatment to 3 months post-treatment predicted worsening of OCD severity at 12 months. This change could potentially identify people at risk of relapse, Dr. Wheaton said.
Previous research has shown that pretreatment OCD severity, measured using the YBOCS, does not significantly predict ERP outcomes, and this study found the same.
Relapse Prevention
“The fact that they did equally well in the short run I think was great,” Dr. Wheaton said.
Previous research, including 2018 and 2023 papers from Wheaton’s team, has shown that more avoidant patients have worse outcomes from standard 12-week ERP programs.
One possible explanation for this difference is that in the Bergen treatment, most exposures happen during face-to-face time with a therapist instead of as homework, which may be easier to avoid, he said.
“But then the finding was that their symptoms were worsening over time — their avoidance was sliding back into old habits,” said Dr. Wheaton.
He would like to see the study replicated in diverse populations outside Norway and in treatment-naive people. Dr. Wheaton also noted that the study assessed avoidance with only a single item.
Future work is needed to test ways to improve relapse prevention. For example, clinicians may be able to monitor for avoidance behaviors post-treatment, which could be the start of a relapse, said Dr. Wheaton.
Although clinicians consider avoidance when treating phobias, social anxiety disorder, and panic disorder, “somehow avoidance got relegated to item 11 on the YBOCS that isn’t scored,” Helen Blair Simpson, MD, PhD, director of the Center for OCD and Related Disorders at Columbia University, New York, New York, said during the presentation.
A direct implication of Dr. Wheaton’s findings to clinical practice is to “talk to people about their avoidance right up front,” said Dr. Simpson, who was not part of the study.
Clinicians who deliver ERP in their practices “can apply this tomorrow,” Dr. Simpson added.
Dr. Wheaton reported no disclosures. Dr. Simpson reported a stipend from the American Medical Association for serving as associate editor of JAMA Psychiatry and royalties from UpToDate, Inc for articles on OCD and from Cambridge University Press for editing a book on anxiety disorders.
A version of this article appeared on Medscape.com.
FROM ADAA 2024
GLP-1 Receptor Agonists: Which Drug for Which Patient?
With all the excitement about GLP-1 agonists,
Of course, we want to make sure that we’re treating the right condition. If the patient has type 2 diabetes, we tend to give them medication that is indicated for type 2 diabetes. Many GLP-1 agonists are available in a diabetes version and a chronic weight management or obesity version. If a patient has diabetes and obesity, they can receive either one. If a patient has only diabetes but not obesity, they should be prescribed the diabetes version. For obesity without diabetes, we tend to stick with the drugs that are indicated for chronic weight management.
Let’s go through them.
Exenatide. In chronological order of approval, the first GLP-1 drug that was used for diabetes dates back to exenatide (Bydureon). Bydureon had a partner called Byetta (also exenatide), both of which are still on the market but infrequently used. Some patients reported that these medications were inconvenient because they required twice-daily injections and caused painful injection-site nodules.
Diabetes drugs in more common use include liraglutide (Victoza) for type 2 diabetes. It is a daily injection and has various doses. We always start low and increase with tolerance and desired effect for A1c.
Liraglutide. Victoza has an antiobesity counterpart called Saxenda. The Saxenda pen looks very similar to the Victoza pen. It is a daily GLP-1 agonist for chronic weight management. The SCALE trial demonstrated 8%-12% weight loss with Saxenda.
Those are the daily injections: Victoza for diabetes and Saxenda for weight loss.
Our patients are very excited about the advent of weekly injections for diabetes and weight management. Ozempic is very popular. It is a weekly GLP-1 agonist for type 2 diabetes. Many patients come in asking for Ozempic, and we must make sure that we’re moving them in the right direction depending on their condition.
Semaglutide. Ozempic has a few different doses. It is a weekly injection and has been found to be quite efficacious for treating diabetes. The drug’s weight loss counterpart is called Wegovy, which comes in a different pen. Both forms contain the compound semaglutide. While all of these GLP-1 agonists are indicated to treat type 2 diabetes or for weight management, Wegovy has a special indication that none of the others have. In March 2024, Wegovy acquired an indication to decrease cardiac risk in those with a BMI ≥ 27 and a previous cardiac history. This will really change the accessibility of this medication because patients with heart conditions who are on Medicare are expected to have access to Wegovy.
Tirzepatide. Another weekly injection for treatment of type 2 diabetes is called Mounjaro. Its counterpart for weight management is called Zepbound, which was found to have about 20.9% weight loss over 72 weeks. These medications have similar side effects in differing degrees, but the most-often reported are nausea, stool changes, abdominal pain, and reflux. There are some other potential side effects; I recommend that you read the individual prescribing information available for each drug to have more clarity about that.
It is important that we stay on label for using the GLP-1 receptor agonists, for many reasons. One, it increases our patients’ accessibility to the right medication for them, and we can also make sure that we’re treating the patient with the right drug according to the clinical trials. When the clinical trials are done, the study populations demonstrate safety and efficacy for that population. But if we’re prescribing a GLP-1 for a different population, it is considered off-label use.
Dr. Lofton, an obesity medicine specialist, is clinical associate professor of surgery and medicine at NYU Grossman School of Medicine, and director of the medical weight management program at NYU Langone Weight Management Center, New York. She disclosed ties to Novo Nordisk and Eli Lilly. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
With all the excitement about GLP-1 agonists,
Of course, we want to make sure that we’re treating the right condition. If the patient has type 2 diabetes, we tend to give them medication that is indicated for type 2 diabetes. Many GLP-1 agonists are available in a diabetes version and a chronic weight management or obesity version. If a patient has diabetes and obesity, they can receive either one. If a patient has only diabetes but not obesity, they should be prescribed the diabetes version. For obesity without diabetes, we tend to stick with the drugs that are indicated for chronic weight management.
Let’s go through them.
Exenatide. In chronological order of approval, the first GLP-1 drug that was used for diabetes dates back to exenatide (Bydureon). Bydureon had a partner called Byetta (also exenatide), both of which are still on the market but infrequently used. Some patients reported that these medications were inconvenient because they required twice-daily injections and caused painful injection-site nodules.
Diabetes drugs in more common use include liraglutide (Victoza) for type 2 diabetes. It is a daily injection and has various doses. We always start low and increase with tolerance and desired effect for A1c.
Liraglutide. Victoza has an antiobesity counterpart called Saxenda. The Saxenda pen looks very similar to the Victoza pen. It is a daily GLP-1 agonist for chronic weight management. The SCALE trial demonstrated 8%-12% weight loss with Saxenda.
Those are the daily injections: Victoza for diabetes and Saxenda for weight loss.
Our patients are very excited about the advent of weekly injections for diabetes and weight management. Ozempic is very popular. It is a weekly GLP-1 agonist for type 2 diabetes. Many patients come in asking for Ozempic, and we must make sure that we’re moving them in the right direction depending on their condition.
Semaglutide. Ozempic has a few different doses. It is a weekly injection and has been found to be quite efficacious for treating diabetes. The drug’s weight loss counterpart is called Wegovy, which comes in a different pen. Both forms contain the compound semaglutide. While all of these GLP-1 agonists are indicated to treat type 2 diabetes or for weight management, Wegovy has a special indication that none of the others have. In March 2024, Wegovy acquired an indication to decrease cardiac risk in those with a BMI ≥ 27 and a previous cardiac history. This will really change the accessibility of this medication because patients with heart conditions who are on Medicare are expected to have access to Wegovy.
Tirzepatide. Another weekly injection for treatment of type 2 diabetes is called Mounjaro. Its counterpart for weight management is called Zepbound, which was found to have about 20.9% weight loss over 72 weeks. These medications have similar side effects in differing degrees, but the most-often reported are nausea, stool changes, abdominal pain, and reflux. There are some other potential side effects; I recommend that you read the individual prescribing information available for each drug to have more clarity about that.
It is important that we stay on label for using the GLP-1 receptor agonists, for many reasons. One, it increases our patients’ accessibility to the right medication for them, and we can also make sure that we’re treating the patient with the right drug according to the clinical trials. When the clinical trials are done, the study populations demonstrate safety and efficacy for that population. But if we’re prescribing a GLP-1 for a different population, it is considered off-label use.
Dr. Lofton, an obesity medicine specialist, is clinical associate professor of surgery and medicine at NYU Grossman School of Medicine, and director of the medical weight management program at NYU Langone Weight Management Center, New York. She disclosed ties to Novo Nordisk and Eli Lilly. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
With all the excitement about GLP-1 agonists,
Of course, we want to make sure that we’re treating the right condition. If the patient has type 2 diabetes, we tend to give them medication that is indicated for type 2 diabetes. Many GLP-1 agonists are available in a diabetes version and a chronic weight management or obesity version. If a patient has diabetes and obesity, they can receive either one. If a patient has only diabetes but not obesity, they should be prescribed the diabetes version. For obesity without diabetes, we tend to stick with the drugs that are indicated for chronic weight management.
Let’s go through them.
Exenatide. In chronological order of approval, the first GLP-1 drug that was used for diabetes dates back to exenatide (Bydureon). Bydureon had a partner called Byetta (also exenatide), both of which are still on the market but infrequently used. Some patients reported that these medications were inconvenient because they required twice-daily injections and caused painful injection-site nodules.
Diabetes drugs in more common use include liraglutide (Victoza) for type 2 diabetes. It is a daily injection and has various doses. We always start low and increase with tolerance and desired effect for A1c.
Liraglutide. Victoza has an antiobesity counterpart called Saxenda. The Saxenda pen looks very similar to the Victoza pen. It is a daily GLP-1 agonist for chronic weight management. The SCALE trial demonstrated 8%-12% weight loss with Saxenda.
Those are the daily injections: Victoza for diabetes and Saxenda for weight loss.
Our patients are very excited about the advent of weekly injections for diabetes and weight management. Ozempic is very popular. It is a weekly GLP-1 agonist for type 2 diabetes. Many patients come in asking for Ozempic, and we must make sure that we’re moving them in the right direction depending on their condition.
Semaglutide. Ozempic has a few different doses. It is a weekly injection and has been found to be quite efficacious for treating diabetes. The drug’s weight loss counterpart is called Wegovy, which comes in a different pen. Both forms contain the compound semaglutide. While all of these GLP-1 agonists are indicated to treat type 2 diabetes or for weight management, Wegovy has a special indication that none of the others have. In March 2024, Wegovy acquired an indication to decrease cardiac risk in those with a BMI ≥ 27 and a previous cardiac history. This will really change the accessibility of this medication because patients with heart conditions who are on Medicare are expected to have access to Wegovy.
Tirzepatide. Another weekly injection for treatment of type 2 diabetes is called Mounjaro. Its counterpart for weight management is called Zepbound, which was found to have about 20.9% weight loss over 72 weeks. These medications have similar side effects in differing degrees, but the most-often reported are nausea, stool changes, abdominal pain, and reflux. There are some other potential side effects; I recommend that you read the individual prescribing information available for each drug to have more clarity about that.
It is important that we stay on label for using the GLP-1 receptor agonists, for many reasons. One, it increases our patients’ accessibility to the right medication for them, and we can also make sure that we’re treating the patient with the right drug according to the clinical trials. When the clinical trials are done, the study populations demonstrate safety and efficacy for that population. But if we’re prescribing a GLP-1 for a different population, it is considered off-label use.
Dr. Lofton, an obesity medicine specialist, is clinical associate professor of surgery and medicine at NYU Grossman School of Medicine, and director of the medical weight management program at NYU Langone Weight Management Center, New York. She disclosed ties to Novo Nordisk and Eli Lilly. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Is Osimertinib Better Alone or With Chemotherapy in Non–Small Cell Lung Cancer?
SAN DIEGO —
That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) has approved osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.
An answer began to emerge in research presented at the American Association for Cancer Research annual meeting.
An exploratory analysis of the FLAURA2 trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside pemetrexed plus cisplatin or carboplatin had a 9-month PFS advantage compared with those who received osimertinib alone.
Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.
“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator Pasi A. Jänne, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.
The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.
Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.
In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).
In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).
The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.
“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. Jänne said.
Study discussant Marina Chiara Garassino, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.
Patients with baseline EGFR mutations also tended to have larger tumors, more brain metastases, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.
The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.
It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.
The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. Jänne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.
A version of this article appeared on Medscape.com.
SAN DIEGO —
That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) has approved osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.
An answer began to emerge in research presented at the American Association for Cancer Research annual meeting.
An exploratory analysis of the FLAURA2 trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside pemetrexed plus cisplatin or carboplatin had a 9-month PFS advantage compared with those who received osimertinib alone.
Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.
“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator Pasi A. Jänne, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.
The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.
Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.
In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).
In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).
The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.
“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. Jänne said.
Study discussant Marina Chiara Garassino, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.
Patients with baseline EGFR mutations also tended to have larger tumors, more brain metastases, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.
The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.
It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.
The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. Jänne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.
A version of this article appeared on Medscape.com.
SAN DIEGO —
That is a question brewing among some oncologists now that the US Food and Drug Administration (FDA) has approved osimertinib (Tagrisso, AstraZeneca) for both indications in patients with epidermal growth factor receptor (EGFR) mutations.
An answer began to emerge in research presented at the American Association for Cancer Research annual meeting.
An exploratory analysis of the FLAURA2 trial found that, when patients have EGFR mutations on baseline circulating tumor DNA (ctDNA) testing, the combination treatment can extend progression-free survival (PFS). In this patient group, those receiving osimertinib alongside pemetrexed plus cisplatin or carboplatin had a 9-month PFS advantage compared with those who received osimertinib alone.
Conversely, when patients do not have EGFR mutations following baseline ctDNA testing, osimertinib alone appears to offer similar PFS outcomes to the combination therapy, but with less toxicity.
“Baseline detection of plasma EGFR mutations may identify a subgroup of patients who derive most benefit from the addition of platinum-pemetrexed to osimertinib as first-line treatment of EGFR-mutated advance non–small cell lung cancer,” investigator Pasi A. Jänne, MD, PhD, a lung cancer oncologist at the Dana-Farber Cancer Institute, Boston, said during his presentation.
The FLAURA2 trial randomized 557 patients equally to daily osimertinib either alone or with pemetrexed plus cisplatin or carboplatin every 3 weeks for four cycles followed by pemetrexed every 3 weeks until disease progression or unacceptable toxicity.
Patients were tested for Ex19del or L858R EGFR mutations at baseline and at 3 and 6 weeks; baseline mutations were found in 73% of evaluable patients.
In patients with baseline mutations, the median PFS was 24.8 months with the combination therapy vs 13.9 months with osimertinib alone (hazard ratio [HR], 0.60).
In patients without baseline mutations, the median PFS was similar in both groups — 33.3 months with the combination vs 30.3 months with monotherapy (HR, 0.93; 95% CI, 0.51-1.72).
The investigators also found that having baseline mutations was associated with worse outcomes regardless of study arm, and mutation clearance was associated with improved outcomes. Clearance occurred more quickly among patients receiving the combination treatment, but almost 90% of patients in both arms cleared their mutations by week 6.
“As we move forward and think about which of our patients we would treat with the combination ... the presence of baseline EGFR mutations in ctDNA may be one of the features that goes into the conversation,” Dr. Jänne said.
Study discussant Marina Chiara Garassino, MD, a thoracic oncologist at the University of Chicago, agreed that this trial can help oncologists make this kind of treatment decision.
Patients with baseline EGFR mutations also tended to have larger tumors, more brain metastases, and worse performance scores; the combination therapy makes sense when such factors are present in patients with baseline EGFR mutations, Dr. Garassino said.
The wrinkle in the findings is that the study used digital droplet polymerase chain reaction (Biodesix) to test for EGFR mutations, which is not commonly used. Clinicians often use next-generation sequencing, which is less sensitive and can lead to false negatives.
It makes it difficult to know how to apply the findings to everyday practice, but Janne hopes a study will be done to correlate next-generation sequencing detection with outcomes.
The study was funded by AstraZeneca, maker of osimertinib, and researchers included AstraZeneca employees. Dr. Jänne is a consultant for and reported research funding from the company. He is a co-inventor on an EGFR mutations patent. Dr. Garassino is also an AstraZeneca consultant and reported institutional financial interests in the company.
A version of this article appeared on Medscape.com.
FROM AACR
FDA Approves New Bladder Cancer Drug
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Inflammation Affects Association Between Furan Exposure and Chronic Obstructive Pulmonary Disease
TOPLINE:
Exposure to furan, a chemical present in agricultural products, stabilizers, pharmaceuticals, and heat-processed foods, shows a significant positive correlation with the prevalence and respiratory mortality of chronic obstructive pulmonary disease (COPD).
METHODOLOGY:
- The researchers reviewed data from the National Health and Nutrition Examination Survey database from 2013 to 2018 and identified 270 adults with a diagnosis of COPD and 7212 without.
- The researchers used a restricted cubic spline analysis to examine the association between COPD risk and blood furan levels and mediating analysis to explore the impact of inflammation.
- The primary outcome of the study was respiratory mortality.
TAKEAWAY:
- Ten COPD patients died of respiratory diseases; adjusted analysis showed a positive correlation between log10-transformed blood furan levels and respiratory mortality in COPD patients (hazard ratio, 41.00, P = .003).
- In a logistic regression analysis, log10-transformed blood furan levels were significantly associated with increased risk for COPD; individuals in the fifth quartile had significantly increased risk compared with the first quartile (odds ratio, 4.47; P = .006).
- COPD demonstrated a significant positive association with monocytes, neutrophils, and basophils, which showed mediated proportions of 8.73%, 20.90%, and 10.94%, respectively, in the relationship between furan exposure and prevalence of COPD (P < .05 for all).
IN PRACTICE:
“The implication [of the findings] is that reducing exposure to furan in the environment could potentially lower the incidence of COPD and improve the prognosis for COPD patients,” but large-scale prospective cohort studies are needed, the researchers wrote in their conclusion.
SOURCE:
The lead author of the study was Di Sun, MD, of Capital Medical University, Beijing, China. The study was published online in BMC Public Health.
LIMITATIONS:
The cross-sectional design prevented establishment of a causal relationship between furan exposure and COPD; lack of data on the conditions of furan exposure and the reliance on self-reports for COPD diagnosis were among the factors that limited the study findings.
DISCLOSURES:
The study was supported by the High Level Public Health Technology Talent Construction Project and Reform and Development Program of Beijing Institute of Respiratory Medicine. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Exposure to furan, a chemical present in agricultural products, stabilizers, pharmaceuticals, and heat-processed foods, shows a significant positive correlation with the prevalence and respiratory mortality of chronic obstructive pulmonary disease (COPD).
METHODOLOGY:
- The researchers reviewed data from the National Health and Nutrition Examination Survey database from 2013 to 2018 and identified 270 adults with a diagnosis of COPD and 7212 without.
- The researchers used a restricted cubic spline analysis to examine the association between COPD risk and blood furan levels and mediating analysis to explore the impact of inflammation.
- The primary outcome of the study was respiratory mortality.
TAKEAWAY:
- Ten COPD patients died of respiratory diseases; adjusted analysis showed a positive correlation between log10-transformed blood furan levels and respiratory mortality in COPD patients (hazard ratio, 41.00, P = .003).
- In a logistic regression analysis, log10-transformed blood furan levels were significantly associated with increased risk for COPD; individuals in the fifth quartile had significantly increased risk compared with the first quartile (odds ratio, 4.47; P = .006).
- COPD demonstrated a significant positive association with monocytes, neutrophils, and basophils, which showed mediated proportions of 8.73%, 20.90%, and 10.94%, respectively, in the relationship between furan exposure and prevalence of COPD (P < .05 for all).
IN PRACTICE:
“The implication [of the findings] is that reducing exposure to furan in the environment could potentially lower the incidence of COPD and improve the prognosis for COPD patients,” but large-scale prospective cohort studies are needed, the researchers wrote in their conclusion.
SOURCE:
The lead author of the study was Di Sun, MD, of Capital Medical University, Beijing, China. The study was published online in BMC Public Health.
LIMITATIONS:
The cross-sectional design prevented establishment of a causal relationship between furan exposure and COPD; lack of data on the conditions of furan exposure and the reliance on self-reports for COPD diagnosis were among the factors that limited the study findings.
DISCLOSURES:
The study was supported by the High Level Public Health Technology Talent Construction Project and Reform and Development Program of Beijing Institute of Respiratory Medicine. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Exposure to furan, a chemical present in agricultural products, stabilizers, pharmaceuticals, and heat-processed foods, shows a significant positive correlation with the prevalence and respiratory mortality of chronic obstructive pulmonary disease (COPD).
METHODOLOGY:
- The researchers reviewed data from the National Health and Nutrition Examination Survey database from 2013 to 2018 and identified 270 adults with a diagnosis of COPD and 7212 without.
- The researchers used a restricted cubic spline analysis to examine the association between COPD risk and blood furan levels and mediating analysis to explore the impact of inflammation.
- The primary outcome of the study was respiratory mortality.
TAKEAWAY:
- Ten COPD patients died of respiratory diseases; adjusted analysis showed a positive correlation between log10-transformed blood furan levels and respiratory mortality in COPD patients (hazard ratio, 41.00, P = .003).
- In a logistic regression analysis, log10-transformed blood furan levels were significantly associated with increased risk for COPD; individuals in the fifth quartile had significantly increased risk compared with the first quartile (odds ratio, 4.47; P = .006).
- COPD demonstrated a significant positive association with monocytes, neutrophils, and basophils, which showed mediated proportions of 8.73%, 20.90%, and 10.94%, respectively, in the relationship between furan exposure and prevalence of COPD (P < .05 for all).
IN PRACTICE:
“The implication [of the findings] is that reducing exposure to furan in the environment could potentially lower the incidence of COPD and improve the prognosis for COPD patients,” but large-scale prospective cohort studies are needed, the researchers wrote in their conclusion.
SOURCE:
The lead author of the study was Di Sun, MD, of Capital Medical University, Beijing, China. The study was published online in BMC Public Health.
LIMITATIONS:
The cross-sectional design prevented establishment of a causal relationship between furan exposure and COPD; lack of data on the conditions of furan exposure and the reliance on self-reports for COPD diagnosis were among the factors that limited the study findings.
DISCLOSURES:
The study was supported by the High Level Public Health Technology Talent Construction Project and Reform and Development Program of Beijing Institute of Respiratory Medicine. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
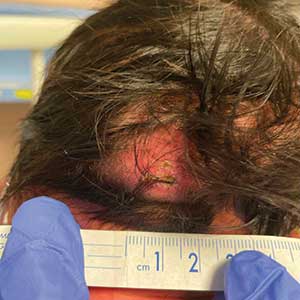
Occipital Scalp Nodule in a Newborn
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.
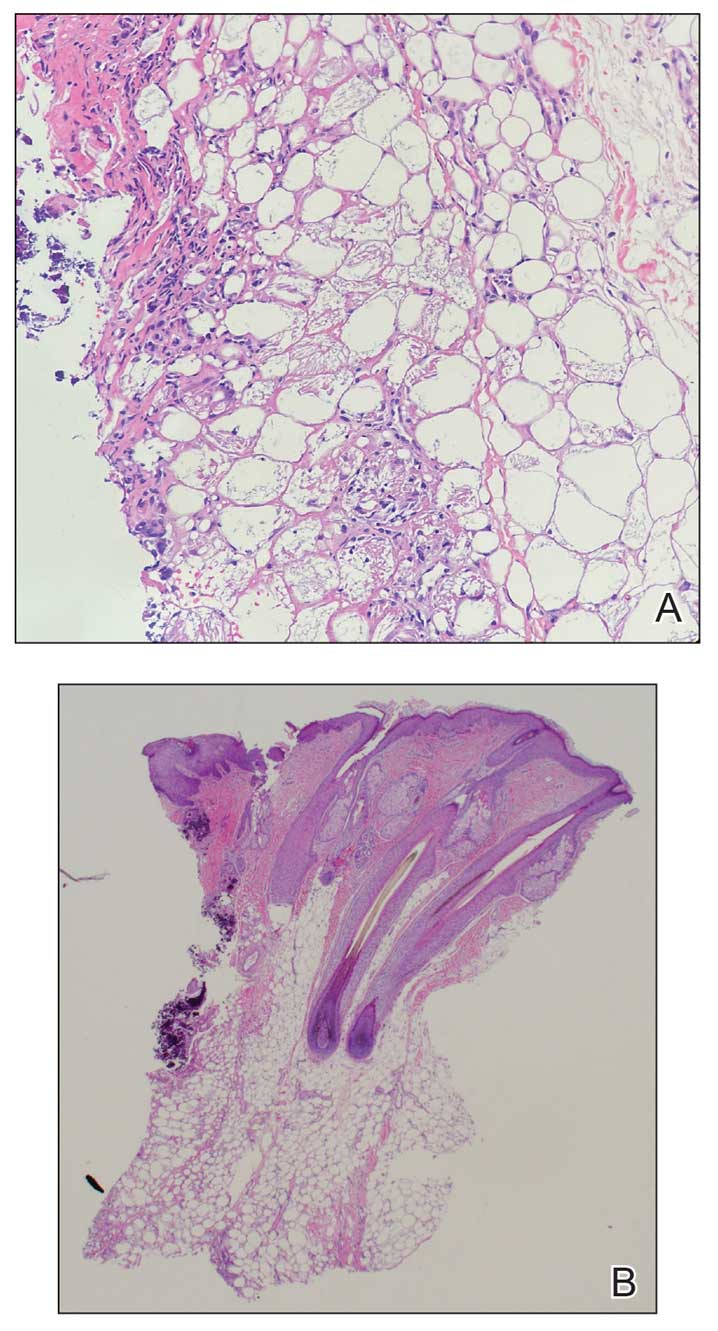
Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
A 4-week-old male infant was referred to dermatology for evaluation of a nodule on the occipital protuberance of 2 weeks’ duration. The patient was born at 36 weeks and 6 days’ gestation via an emergency cesarean delivery due to fetal distress. He later was found to have hypoxic-ischemic encephalopathy, pulmonary hypertension, and hypertrophic cardiomyopathy. He underwent therapeutic hypothermia protocol treatment starting at less than 6 hours after birth. At the current presentation, physical examination showed a 2.5-cm, erythematous, firm, mobile nodule on the occipital scalp with some overlying crusting and minimal surrounding erythema. No other cutaneous features or lesions were present. Initial laboratory findings were remarkable for hypercalcemia at 11 mg/dL (reference range, 8.5-10.5 mg/dL). Magnetic resonance imaging showed a faint abnormality in the subcutaneous tissue in this region without a noted connection to the underlying brain/meningeal matter. A punch biopsy was performed.
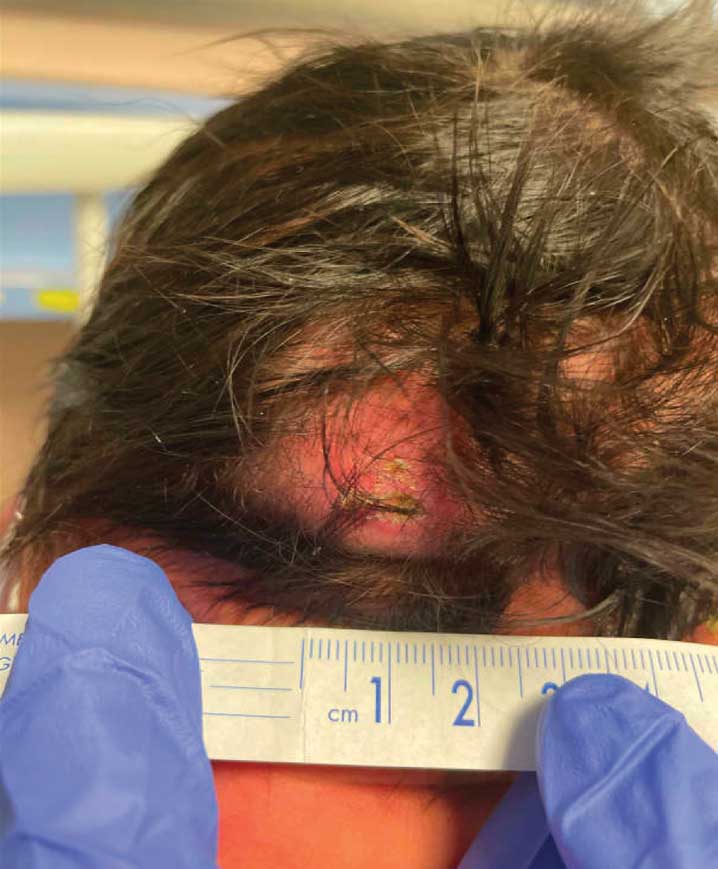
FDA OKs Subcutaneous Vedolizumab for Crohn’s Maintenance Therapy
The move follows the FDA’s approval last year of subcutaneous vedolizumab for maintenance treatment of adults with moderately to severely active ulcerative colitis (UC).
The humanized immunoglobulin G1 monoclonal antibody is available as a single-dose prefilled pen (Entyvio Pen).
The FDA first approved the IV formulation of the biologic in 2014 for patients with moderate to severe UC and CD who cannot tolerate other therapies or in whom such therapies have failed.
The approval of subcutaneous vedolizumab for maintenance treatment of CD is based on the phase 3, randomized, double-blind, placebo-controlled VISIBLE 2 trial.
The trial enrolled 409 adult patients with moderately to severely active CD who had clinical response at week 6 following two doses of open-label IV vedolizumab at weeks 0 and 2.
At week 6, they were randomly allocated in a 2:1 ratio to receive vedolizumab 108 mg administered by subcutaneous injection or placebo every 2 weeks. The primary endpoint was clinical remission at week 52, which was defined as a total Crohn’s Disease Activity Index score ≤ 150.
The results showed that significantly more patients receiving subcutaneous vedolizumab than placebo achieved long-term clinical remission (48% vs 34%; P < .01), the company said in a news release.
The safety profile of subcutaneous vedolizumab is generally consistent with the known safety profile of IV vedolizumab, with the addition of injection-site reactions (including injection-site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria, and edema).
“Crohn’s disease is a complex and usually progressive disease for which an appropriate management plan is critical. My primary goal as a clinician is always to get patients to achieve remission,” Timothy Ritter, MD, senior medical director, GI Alliance Research, and assistant professor of medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, said in the news release.
“In VISIBLE 2, about half of patients treated with Entyvio SC achieved long-term clinical remission. The data from VISIBLE 2 reaffirm the well-established efficacy profile of Entyvio, regardless of route of administration,” Dr. Ritter added.
A version of this article appeared on Medscape.com.
The move follows the FDA’s approval last year of subcutaneous vedolizumab for maintenance treatment of adults with moderately to severely active ulcerative colitis (UC).
The humanized immunoglobulin G1 monoclonal antibody is available as a single-dose prefilled pen (Entyvio Pen).
The FDA first approved the IV formulation of the biologic in 2014 for patients with moderate to severe UC and CD who cannot tolerate other therapies or in whom such therapies have failed.
The approval of subcutaneous vedolizumab for maintenance treatment of CD is based on the phase 3, randomized, double-blind, placebo-controlled VISIBLE 2 trial.
The trial enrolled 409 adult patients with moderately to severely active CD who had clinical response at week 6 following two doses of open-label IV vedolizumab at weeks 0 and 2.
At week 6, they were randomly allocated in a 2:1 ratio to receive vedolizumab 108 mg administered by subcutaneous injection or placebo every 2 weeks. The primary endpoint was clinical remission at week 52, which was defined as a total Crohn’s Disease Activity Index score ≤ 150.
The results showed that significantly more patients receiving subcutaneous vedolizumab than placebo achieved long-term clinical remission (48% vs 34%; P < .01), the company said in a news release.
The safety profile of subcutaneous vedolizumab is generally consistent with the known safety profile of IV vedolizumab, with the addition of injection-site reactions (including injection-site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria, and edema).
“Crohn’s disease is a complex and usually progressive disease for which an appropriate management plan is critical. My primary goal as a clinician is always to get patients to achieve remission,” Timothy Ritter, MD, senior medical director, GI Alliance Research, and assistant professor of medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, said in the news release.
“In VISIBLE 2, about half of patients treated with Entyvio SC achieved long-term clinical remission. The data from VISIBLE 2 reaffirm the well-established efficacy profile of Entyvio, regardless of route of administration,” Dr. Ritter added.
A version of this article appeared on Medscape.com.
The move follows the FDA’s approval last year of subcutaneous vedolizumab for maintenance treatment of adults with moderately to severely active ulcerative colitis (UC).
The humanized immunoglobulin G1 monoclonal antibody is available as a single-dose prefilled pen (Entyvio Pen).
The FDA first approved the IV formulation of the biologic in 2014 for patients with moderate to severe UC and CD who cannot tolerate other therapies or in whom such therapies have failed.
The approval of subcutaneous vedolizumab for maintenance treatment of CD is based on the phase 3, randomized, double-blind, placebo-controlled VISIBLE 2 trial.
The trial enrolled 409 adult patients with moderately to severely active CD who had clinical response at week 6 following two doses of open-label IV vedolizumab at weeks 0 and 2.
At week 6, they were randomly allocated in a 2:1 ratio to receive vedolizumab 108 mg administered by subcutaneous injection or placebo every 2 weeks. The primary endpoint was clinical remission at week 52, which was defined as a total Crohn’s Disease Activity Index score ≤ 150.
The results showed that significantly more patients receiving subcutaneous vedolizumab than placebo achieved long-term clinical remission (48% vs 34%; P < .01), the company said in a news release.
The safety profile of subcutaneous vedolizumab is generally consistent with the known safety profile of IV vedolizumab, with the addition of injection-site reactions (including injection-site erythema, rash, pruritus, swelling, bruising, hematoma, pain, urticaria, and edema).
“Crohn’s disease is a complex and usually progressive disease for which an appropriate management plan is critical. My primary goal as a clinician is always to get patients to achieve remission,” Timothy Ritter, MD, senior medical director, GI Alliance Research, and assistant professor of medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, said in the news release.
“In VISIBLE 2, about half of patients treated with Entyvio SC achieved long-term clinical remission. The data from VISIBLE 2 reaffirm the well-established efficacy profile of Entyvio, regardless of route of administration,” Dr. Ritter added.
A version of this article appeared on Medscape.com.
Approved Therapy for ALS Is Withdrawn When New Study Shows No Benefit
DENVER —
As a result, “PB&TURSO is no longer available for new patients in the United States of Canada,” reported Leonard H. van den Berg, MD, PhD, Direction of the Netherlands ALS Center, UMC Utrecht Brain Center, Utrecht, the Netherlands.
Although the drug is now being withdrawn, patients on therapy as of April 4 who wish to stay on treatment “can be transitioned to a free drug program,” added Dr. van den Berg, who presented the results of this new trial, called PHOENIX, at the 2024 annual meeting of the American Academy of Neurology.
PB&TURSO, marketed as Relyvrio (Amylyx), is a combination of sodium phenylbutyrate (PB) and taurursodiol (TAURO). Having shown promise for preventing neuronal death in experimental and early human studies, it was approved on the basis of the of the double-blind multicenter CENTAUR trial published in The New England Journal of Medicine in 2022.
ALSFRS-R Served as Primary Endpoint in Both Trials
In CENTAUR, like the newly completed PHOENIX, the primary outcome was rate of decline in the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) over 24 weeks. On this endpoint, the rate of change for those randomized to PB&TAURO was –1.24 points per month versus –1.66 points per month on placebo, a difference of 0.42 points that met statistical significance (P = .02).
The CENTAUR trial, which enrolled 177 patients, also showed no differences between those in the experimental and placebo arms for any of the secondary endpoints, including time to tracheostomy, permanent ventilation, or death.
In the much larger and longer PHOENIX trial, 664 ALS patients were randomized in a 3:2 ratio to PB&TURSO or placebo. Fifty-seven percent in each group completed 48 weeks of follow-up. The proportions of patients who withdrew from the study were similar across the reasons, such as adverse events and disease progression.
For the ALSFRS-R primary endpoint at 48 weeks, the decline in both groups was essentially linear and almost completely overlapped with a final change from baseline of –14.98 points in the PB&TURSO group that was statistically indistinguishable from the –15.32 point-change (P = .667) in the placebo group, Dr. van den Berg reported.
Similarly, there were no clinically meaningful or statistically significant differences in the secondary endpoints of mean change in Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) scores or mean change in slow vital capacity (SVC) when compared to baseline or between arms.
As in CENTAUR, the most common side effects associated with PB&TURSO were gastrointestinal, particularly diarrhea (31% vs 10%), but serious adverse events were slightly less common on PT&TURSO (26% vs 28%), and Dr. van der Berg characterized the drug as “generally well tolerated.”
Differences Between Two Trials Were Evaluated
The entry criteria for PHOENIX trial differed modestly from those of the CENTAUR trial. Clinically definite or probable ALS was required in only two or more body regions versus three or more in the earlier trial. Patients were also allowed entry with SVC greater than 60% versus greater than 55% for CENTAUR and have had a longer period since symptom onset (< 24 vs < 18 months). Both studies permitted use of edaravone.
When stratified, patients who entered PHOENIX with CENTAUR-like entry criteria had a similar response to PB&TURSO relative to those who did not. Similarly, there were no meaningful differences between those enrolled in European study sites versus elsewhere. Background edaravone versus no edaravone also had no apparent effect on outcomes.
An ongoing open-label extension of the PHOENIX trial is still collecting data on survival, which was a prespecified endpoint. This endpoint, which requires 70% or more of patients to have died or have been followed for 3 or more years since the last patient was randomized, is not expected until February 2026.
Although “there are further biomarker and subgroup analyses planned,” Dr. van den Berg said that the neutral results of the PHOENIX trial, which he characterized as the largest controlled trial in ALS ever conducted, do not encourage further studies with this agent.
‘Unfortunate’ Results
Robert Bowser, PhD, chief scientific officer and chair of the department of translational neuroscience, Barrow Neurological Institute, Phoenix, called the results “unfortunate.” Just last year, Dr. Bowser published a study showing a reduction in the concentration of biomarkers associated with ALS among patients in the CENTAUR study who were treated with PB&TURSO.
Moreover, the reduction in the serum concentrations of the biomarkers he studied, which included C-reactive protein and YKL-40, correlated with ALSFRS-R total score.
In that paper, he speculated that CRP and YKL-40 might emerge as treatment-sensitive biomarkers in ALS “pending further confirmatory studies, but Dr. Bowser indicated that the PHOENIX study has prompted the correct response from the manufacturers.
“Credit should be given to the leaders at Amylyx for following through with their promise to remove the drug from the market if the PHOENIX study did not confirm the results from the CENTAUR study,” he said.
However, he believes that the study will still have value for better understanding ALS.
“As we move forward, it will be interesting to see biomarker data generated from the biosamples collected during the PHOENIX trial to learn more about treatment impact on biomarkers within those that received the drug,” he said. “I am sure we will continue to learn more from the PHOENIX trial.”
Dr. van den Berg has financial relationships with approximately 10 pharmaceutical companies, including Amylyx, which provided funding for the PHOENIX trial. Dr. Bowser reported no potential conflicts of interest.
DENVER —
As a result, “PB&TURSO is no longer available for new patients in the United States of Canada,” reported Leonard H. van den Berg, MD, PhD, Direction of the Netherlands ALS Center, UMC Utrecht Brain Center, Utrecht, the Netherlands.
Although the drug is now being withdrawn, patients on therapy as of April 4 who wish to stay on treatment “can be transitioned to a free drug program,” added Dr. van den Berg, who presented the results of this new trial, called PHOENIX, at the 2024 annual meeting of the American Academy of Neurology.
PB&TURSO, marketed as Relyvrio (Amylyx), is a combination of sodium phenylbutyrate (PB) and taurursodiol (TAURO). Having shown promise for preventing neuronal death in experimental and early human studies, it was approved on the basis of the of the double-blind multicenter CENTAUR trial published in The New England Journal of Medicine in 2022.
ALSFRS-R Served as Primary Endpoint in Both Trials
In CENTAUR, like the newly completed PHOENIX, the primary outcome was rate of decline in the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) over 24 weeks. On this endpoint, the rate of change for those randomized to PB&TAURO was –1.24 points per month versus –1.66 points per month on placebo, a difference of 0.42 points that met statistical significance (P = .02).
The CENTAUR trial, which enrolled 177 patients, also showed no differences between those in the experimental and placebo arms for any of the secondary endpoints, including time to tracheostomy, permanent ventilation, or death.
In the much larger and longer PHOENIX trial, 664 ALS patients were randomized in a 3:2 ratio to PB&TURSO or placebo. Fifty-seven percent in each group completed 48 weeks of follow-up. The proportions of patients who withdrew from the study were similar across the reasons, such as adverse events and disease progression.
For the ALSFRS-R primary endpoint at 48 weeks, the decline in both groups was essentially linear and almost completely overlapped with a final change from baseline of –14.98 points in the PB&TURSO group that was statistically indistinguishable from the –15.32 point-change (P = .667) in the placebo group, Dr. van den Berg reported.
Similarly, there were no clinically meaningful or statistically significant differences in the secondary endpoints of mean change in Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) scores or mean change in slow vital capacity (SVC) when compared to baseline or between arms.
As in CENTAUR, the most common side effects associated with PB&TURSO were gastrointestinal, particularly diarrhea (31% vs 10%), but serious adverse events were slightly less common on PT&TURSO (26% vs 28%), and Dr. van der Berg characterized the drug as “generally well tolerated.”
Differences Between Two Trials Were Evaluated
The entry criteria for PHOENIX trial differed modestly from those of the CENTAUR trial. Clinically definite or probable ALS was required in only two or more body regions versus three or more in the earlier trial. Patients were also allowed entry with SVC greater than 60% versus greater than 55% for CENTAUR and have had a longer period since symptom onset (< 24 vs < 18 months). Both studies permitted use of edaravone.
When stratified, patients who entered PHOENIX with CENTAUR-like entry criteria had a similar response to PB&TURSO relative to those who did not. Similarly, there were no meaningful differences between those enrolled in European study sites versus elsewhere. Background edaravone versus no edaravone also had no apparent effect on outcomes.
An ongoing open-label extension of the PHOENIX trial is still collecting data on survival, which was a prespecified endpoint. This endpoint, which requires 70% or more of patients to have died or have been followed for 3 or more years since the last patient was randomized, is not expected until February 2026.
Although “there are further biomarker and subgroup analyses planned,” Dr. van den Berg said that the neutral results of the PHOENIX trial, which he characterized as the largest controlled trial in ALS ever conducted, do not encourage further studies with this agent.
‘Unfortunate’ Results
Robert Bowser, PhD, chief scientific officer and chair of the department of translational neuroscience, Barrow Neurological Institute, Phoenix, called the results “unfortunate.” Just last year, Dr. Bowser published a study showing a reduction in the concentration of biomarkers associated with ALS among patients in the CENTAUR study who were treated with PB&TURSO.
Moreover, the reduction in the serum concentrations of the biomarkers he studied, which included C-reactive protein and YKL-40, correlated with ALSFRS-R total score.
In that paper, he speculated that CRP and YKL-40 might emerge as treatment-sensitive biomarkers in ALS “pending further confirmatory studies, but Dr. Bowser indicated that the PHOENIX study has prompted the correct response from the manufacturers.
“Credit should be given to the leaders at Amylyx for following through with their promise to remove the drug from the market if the PHOENIX study did not confirm the results from the CENTAUR study,” he said.
However, he believes that the study will still have value for better understanding ALS.
“As we move forward, it will be interesting to see biomarker data generated from the biosamples collected during the PHOENIX trial to learn more about treatment impact on biomarkers within those that received the drug,” he said. “I am sure we will continue to learn more from the PHOENIX trial.”
Dr. van den Berg has financial relationships with approximately 10 pharmaceutical companies, including Amylyx, which provided funding for the PHOENIX trial. Dr. Bowser reported no potential conflicts of interest.
DENVER —
As a result, “PB&TURSO is no longer available for new patients in the United States of Canada,” reported Leonard H. van den Berg, MD, PhD, Direction of the Netherlands ALS Center, UMC Utrecht Brain Center, Utrecht, the Netherlands.
Although the drug is now being withdrawn, patients on therapy as of April 4 who wish to stay on treatment “can be transitioned to a free drug program,” added Dr. van den Berg, who presented the results of this new trial, called PHOENIX, at the 2024 annual meeting of the American Academy of Neurology.
PB&TURSO, marketed as Relyvrio (Amylyx), is a combination of sodium phenylbutyrate (PB) and taurursodiol (TAURO). Having shown promise for preventing neuronal death in experimental and early human studies, it was approved on the basis of the of the double-blind multicenter CENTAUR trial published in The New England Journal of Medicine in 2022.
ALSFRS-R Served as Primary Endpoint in Both Trials
In CENTAUR, like the newly completed PHOENIX, the primary outcome was rate of decline in the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R) over 24 weeks. On this endpoint, the rate of change for those randomized to PB&TAURO was –1.24 points per month versus –1.66 points per month on placebo, a difference of 0.42 points that met statistical significance (P = .02).
The CENTAUR trial, which enrolled 177 patients, also showed no differences between those in the experimental and placebo arms for any of the secondary endpoints, including time to tracheostomy, permanent ventilation, or death.
In the much larger and longer PHOENIX trial, 664 ALS patients were randomized in a 3:2 ratio to PB&TURSO or placebo. Fifty-seven percent in each group completed 48 weeks of follow-up. The proportions of patients who withdrew from the study were similar across the reasons, such as adverse events and disease progression.
For the ALSFRS-R primary endpoint at 48 weeks, the decline in both groups was essentially linear and almost completely overlapped with a final change from baseline of –14.98 points in the PB&TURSO group that was statistically indistinguishable from the –15.32 point-change (P = .667) in the placebo group, Dr. van den Berg reported.
Similarly, there were no clinically meaningful or statistically significant differences in the secondary endpoints of mean change in Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40) scores or mean change in slow vital capacity (SVC) when compared to baseline or between arms.
As in CENTAUR, the most common side effects associated with PB&TURSO were gastrointestinal, particularly diarrhea (31% vs 10%), but serious adverse events were slightly less common on PT&TURSO (26% vs 28%), and Dr. van der Berg characterized the drug as “generally well tolerated.”
Differences Between Two Trials Were Evaluated
The entry criteria for PHOENIX trial differed modestly from those of the CENTAUR trial. Clinically definite or probable ALS was required in only two or more body regions versus three or more in the earlier trial. Patients were also allowed entry with SVC greater than 60% versus greater than 55% for CENTAUR and have had a longer period since symptom onset (< 24 vs < 18 months). Both studies permitted use of edaravone.
When stratified, patients who entered PHOENIX with CENTAUR-like entry criteria had a similar response to PB&TURSO relative to those who did not. Similarly, there were no meaningful differences between those enrolled in European study sites versus elsewhere. Background edaravone versus no edaravone also had no apparent effect on outcomes.
An ongoing open-label extension of the PHOENIX trial is still collecting data on survival, which was a prespecified endpoint. This endpoint, which requires 70% or more of patients to have died or have been followed for 3 or more years since the last patient was randomized, is not expected until February 2026.
Although “there are further biomarker and subgroup analyses planned,” Dr. van den Berg said that the neutral results of the PHOENIX trial, which he characterized as the largest controlled trial in ALS ever conducted, do not encourage further studies with this agent.
‘Unfortunate’ Results
Robert Bowser, PhD, chief scientific officer and chair of the department of translational neuroscience, Barrow Neurological Institute, Phoenix, called the results “unfortunate.” Just last year, Dr. Bowser published a study showing a reduction in the concentration of biomarkers associated with ALS among patients in the CENTAUR study who were treated with PB&TURSO.
Moreover, the reduction in the serum concentrations of the biomarkers he studied, which included C-reactive protein and YKL-40, correlated with ALSFRS-R total score.
In that paper, he speculated that CRP and YKL-40 might emerge as treatment-sensitive biomarkers in ALS “pending further confirmatory studies, but Dr. Bowser indicated that the PHOENIX study has prompted the correct response from the manufacturers.
“Credit should be given to the leaders at Amylyx for following through with their promise to remove the drug from the market if the PHOENIX study did not confirm the results from the CENTAUR study,” he said.
However, he believes that the study will still have value for better understanding ALS.
“As we move forward, it will be interesting to see biomarker data generated from the biosamples collected during the PHOENIX trial to learn more about treatment impact on biomarkers within those that received the drug,” he said. “I am sure we will continue to learn more from the PHOENIX trial.”
Dr. van den Berg has financial relationships with approximately 10 pharmaceutical companies, including Amylyx, which provided funding for the PHOENIX trial. Dr. Bowser reported no potential conflicts of interest.
FROM AAN 2024
Positive Results From Phase 2 Trial Support Potential New Option for Control of CIDP
DENVER — , according to the results of a phase 2 multinational trial, which were reported at the 2024 annual meeting of the American Academy of Neurology.
“Regardless of prior therapy for CIDP, efgartigimod PH20 was associated with a rapid clinical improvement, and clinical responses have been maintained out to 48 weeks,” said Jeffrey A. Allen, MD, an associate professor of neurology, University of Minnesota, Minneapolis.
Efgartigimod, which reduces circulating IgG immunoglobulin, has been available for the treatment of myasthenia gravis since 2021. In a new trial, called ADHERE, the combination of efgartigimod and rHuPH20 (E-PH20) was tested for CIDP, the most common of the chronic immune-mediated inflammatory polyneuropathies.
ADHERE Called Largest CIDP Trial to Date
In this study, which Dr. Allen called the largest randomized controlled trial ever performed with a CIDP treatment, a run-in stage was required for those candidates who were already on treatment. When these patients went off treatment during this 12-week run-in, clinical deterioration was required to advance to the first of two stages of the trial. Patients with symptomatic CIDP but off treatment at the time of enrollment did not participate in the run-in.
After the run-in, patients who advanced to stage A received 1000 mg of E-PH20 open label for 12 weeks. Of those on treatment prior to the run-in, about half were receiving intravenous immunoglobulins (IVIg). Almost all the remainder had been receiving corticosteroids. About 30% had been off treatment and entered stage A without participating in the run in.
The primary endpoint of stage A was the percentage of patients with evidence of clinical improvement (ECI). Patients who participated in the run-in were allowed to resume their prior treatment for stage A and the subsequent blinded stage B. Stage A was event driven so that it was closed once 88 events were reached,
The ECI endpoint was met by 66.5% of the patients, who thereby met eligibility for the randomized stage B. As the study design excluded those who achieved clinical improvement after the 88-event limit was reached, they were not included among responders. Had they been included, Dr. Allen said that the primary endpoint of stage A would have been reached by 70.4%.
The patterns of improvement in stage A were similar across type of prior CIDP treatment, including no treatment, according to Dr. Allen, who noted that 39.8% of those enrolled in stage A met the primary endpoint within 4 weeks.
There were 322 patients in stage A. Of these, 211 enrolled in stage B. They were randomized in a 1:1 ratio to 1000 mg of E-PH20 or placebo administered weekly by subcutaneous injection. Of those eligible for stage B, 40% had not participated in the run-in.
aINCAT Provided Primary Endpoint for CIDP Trial
For stage B, the primary endpoint was time from baseline to a clinically meaningful limitation of activity. This was evaluated with the adjusted inflammatory neuropathy cause and treatment (aINCAT) disability score.
By the end of 48 weeks of treatment, 27.9% had relapsed on E-PH20 according to the aiNCAT disability score versus 53.6% on those on placebo. By hazard ratio (HR 0.39), the active treatment arm was associated with a highly significant 61% (P = .000039) greater likelihood of avoiding relapse.
When stratified by a background of no therapy, IVIg, subcutaneous immunoglobulins (SCIg), or corticosteroids, all groups in the active treatment arm did better in stage B than any group in the placebo arm, according to Dr. Allen.
In the 48-week deterioration curves, sustained control was observed among responders out to the end of controlled study. Although there appeared to be numerical advantage for those on both E-PH20 and corticosteroids, E-PH20 arms with concomitant IVIg, SCIg, or no treatment also showed sustained control without significant differences between them.
On functional aINCAT scores, 80.9% achieved at least a 1-point improvement. The improvement was at least 2 points in 42.7%, at least 3 points in 28.2%, and at least 4 points in 11.8%.
E-PH20 Is Characterized as Well Tolerated
Injection site erythema (5.4% vs 0%) and injection site bruising (5.4% vs 0.9%) were more common on E-PH20 than placebo, but there was no difference in serious adverse events, and events possibly related to active treatment, such as headache (3.6% vs. 1.8%) were considered to be of mild to moderate severity.
“The safety profile of efgartigimod plus PH20 was consistent with the safety profile of efgartigimod in other autoimmune diseases,” Dr. Allen said.
The weekly subcutaneous injection can be administered within 90 seconds or less, Dr. Allen said. He called this drug a potential “new therapeutic option to reduce treatment burden in patients with CIDP” if it is approved.
There is a need for new options, according to Brett M. Morrison, MD, PhD, associate professor of neurology at the Johns Hopkins School of Medicine, Baltimore, and an expert in neuromuscular disorders. Dr. Morrison was not involved in the study.
“Although there are three currently approved treatments — steroids, IVIg, and plasmapheresis, at least 20% of CIDP patients have minimal or no response” to any of these, Dr. Morrison said. He added that many of those who do respond to standard therapies have a substantial side effect burden that has created a need for alternatives.
Based on the data presented so far, which suggest substantial efficacy and a favorable safety profile, efgartigimod, if and when it becomes available, “would be an important new treatment for CIDP,” according to Dr. Morrison.
Dr. Allen has financial relationships with more than 10 pharmaceutical companies, including Argenx, which provided funding for the ACHIEVE trial. Dr. Morrison reported no potential conflicts of interest.
DENVER — , according to the results of a phase 2 multinational trial, which were reported at the 2024 annual meeting of the American Academy of Neurology.
“Regardless of prior therapy for CIDP, efgartigimod PH20 was associated with a rapid clinical improvement, and clinical responses have been maintained out to 48 weeks,” said Jeffrey A. Allen, MD, an associate professor of neurology, University of Minnesota, Minneapolis.
Efgartigimod, which reduces circulating IgG immunoglobulin, has been available for the treatment of myasthenia gravis since 2021. In a new trial, called ADHERE, the combination of efgartigimod and rHuPH20 (E-PH20) was tested for CIDP, the most common of the chronic immune-mediated inflammatory polyneuropathies.
ADHERE Called Largest CIDP Trial to Date
In this study, which Dr. Allen called the largest randomized controlled trial ever performed with a CIDP treatment, a run-in stage was required for those candidates who were already on treatment. When these patients went off treatment during this 12-week run-in, clinical deterioration was required to advance to the first of two stages of the trial. Patients with symptomatic CIDP but off treatment at the time of enrollment did not participate in the run-in.
After the run-in, patients who advanced to stage A received 1000 mg of E-PH20 open label for 12 weeks. Of those on treatment prior to the run-in, about half were receiving intravenous immunoglobulins (IVIg). Almost all the remainder had been receiving corticosteroids. About 30% had been off treatment and entered stage A without participating in the run in.
The primary endpoint of stage A was the percentage of patients with evidence of clinical improvement (ECI). Patients who participated in the run-in were allowed to resume their prior treatment for stage A and the subsequent blinded stage B. Stage A was event driven so that it was closed once 88 events were reached,
The ECI endpoint was met by 66.5% of the patients, who thereby met eligibility for the randomized stage B. As the study design excluded those who achieved clinical improvement after the 88-event limit was reached, they were not included among responders. Had they been included, Dr. Allen said that the primary endpoint of stage A would have been reached by 70.4%.
The patterns of improvement in stage A were similar across type of prior CIDP treatment, including no treatment, according to Dr. Allen, who noted that 39.8% of those enrolled in stage A met the primary endpoint within 4 weeks.
There were 322 patients in stage A. Of these, 211 enrolled in stage B. They were randomized in a 1:1 ratio to 1000 mg of E-PH20 or placebo administered weekly by subcutaneous injection. Of those eligible for stage B, 40% had not participated in the run-in.
aINCAT Provided Primary Endpoint for CIDP Trial
For stage B, the primary endpoint was time from baseline to a clinically meaningful limitation of activity. This was evaluated with the adjusted inflammatory neuropathy cause and treatment (aINCAT) disability score.
By the end of 48 weeks of treatment, 27.9% had relapsed on E-PH20 according to the aiNCAT disability score versus 53.6% on those on placebo. By hazard ratio (HR 0.39), the active treatment arm was associated with a highly significant 61% (P = .000039) greater likelihood of avoiding relapse.
When stratified by a background of no therapy, IVIg, subcutaneous immunoglobulins (SCIg), or corticosteroids, all groups in the active treatment arm did better in stage B than any group in the placebo arm, according to Dr. Allen.
In the 48-week deterioration curves, sustained control was observed among responders out to the end of controlled study. Although there appeared to be numerical advantage for those on both E-PH20 and corticosteroids, E-PH20 arms with concomitant IVIg, SCIg, or no treatment also showed sustained control without significant differences between them.
On functional aINCAT scores, 80.9% achieved at least a 1-point improvement. The improvement was at least 2 points in 42.7%, at least 3 points in 28.2%, and at least 4 points in 11.8%.
E-PH20 Is Characterized as Well Tolerated
Injection site erythema (5.4% vs 0%) and injection site bruising (5.4% vs 0.9%) were more common on E-PH20 than placebo, but there was no difference in serious adverse events, and events possibly related to active treatment, such as headache (3.6% vs. 1.8%) were considered to be of mild to moderate severity.
“The safety profile of efgartigimod plus PH20 was consistent with the safety profile of efgartigimod in other autoimmune diseases,” Dr. Allen said.
The weekly subcutaneous injection can be administered within 90 seconds or less, Dr. Allen said. He called this drug a potential “new therapeutic option to reduce treatment burden in patients with CIDP” if it is approved.
There is a need for new options, according to Brett M. Morrison, MD, PhD, associate professor of neurology at the Johns Hopkins School of Medicine, Baltimore, and an expert in neuromuscular disorders. Dr. Morrison was not involved in the study.
“Although there are three currently approved treatments — steroids, IVIg, and plasmapheresis, at least 20% of CIDP patients have minimal or no response” to any of these, Dr. Morrison said. He added that many of those who do respond to standard therapies have a substantial side effect burden that has created a need for alternatives.
Based on the data presented so far, which suggest substantial efficacy and a favorable safety profile, efgartigimod, if and when it becomes available, “would be an important new treatment for CIDP,” according to Dr. Morrison.
Dr. Allen has financial relationships with more than 10 pharmaceutical companies, including Argenx, which provided funding for the ACHIEVE trial. Dr. Morrison reported no potential conflicts of interest.
DENVER — , according to the results of a phase 2 multinational trial, which were reported at the 2024 annual meeting of the American Academy of Neurology.
“Regardless of prior therapy for CIDP, efgartigimod PH20 was associated with a rapid clinical improvement, and clinical responses have been maintained out to 48 weeks,” said Jeffrey A. Allen, MD, an associate professor of neurology, University of Minnesota, Minneapolis.
Efgartigimod, which reduces circulating IgG immunoglobulin, has been available for the treatment of myasthenia gravis since 2021. In a new trial, called ADHERE, the combination of efgartigimod and rHuPH20 (E-PH20) was tested for CIDP, the most common of the chronic immune-mediated inflammatory polyneuropathies.
ADHERE Called Largest CIDP Trial to Date
In this study, which Dr. Allen called the largest randomized controlled trial ever performed with a CIDP treatment, a run-in stage was required for those candidates who were already on treatment. When these patients went off treatment during this 12-week run-in, clinical deterioration was required to advance to the first of two stages of the trial. Patients with symptomatic CIDP but off treatment at the time of enrollment did not participate in the run-in.
After the run-in, patients who advanced to stage A received 1000 mg of E-PH20 open label for 12 weeks. Of those on treatment prior to the run-in, about half were receiving intravenous immunoglobulins (IVIg). Almost all the remainder had been receiving corticosteroids. About 30% had been off treatment and entered stage A without participating in the run in.
The primary endpoint of stage A was the percentage of patients with evidence of clinical improvement (ECI). Patients who participated in the run-in were allowed to resume their prior treatment for stage A and the subsequent blinded stage B. Stage A was event driven so that it was closed once 88 events were reached,
The ECI endpoint was met by 66.5% of the patients, who thereby met eligibility for the randomized stage B. As the study design excluded those who achieved clinical improvement after the 88-event limit was reached, they were not included among responders. Had they been included, Dr. Allen said that the primary endpoint of stage A would have been reached by 70.4%.
The patterns of improvement in stage A were similar across type of prior CIDP treatment, including no treatment, according to Dr. Allen, who noted that 39.8% of those enrolled in stage A met the primary endpoint within 4 weeks.
There were 322 patients in stage A. Of these, 211 enrolled in stage B. They were randomized in a 1:1 ratio to 1000 mg of E-PH20 or placebo administered weekly by subcutaneous injection. Of those eligible for stage B, 40% had not participated in the run-in.
aINCAT Provided Primary Endpoint for CIDP Trial
For stage B, the primary endpoint was time from baseline to a clinically meaningful limitation of activity. This was evaluated with the adjusted inflammatory neuropathy cause and treatment (aINCAT) disability score.
By the end of 48 weeks of treatment, 27.9% had relapsed on E-PH20 according to the aiNCAT disability score versus 53.6% on those on placebo. By hazard ratio (HR 0.39), the active treatment arm was associated with a highly significant 61% (P = .000039) greater likelihood of avoiding relapse.
When stratified by a background of no therapy, IVIg, subcutaneous immunoglobulins (SCIg), or corticosteroids, all groups in the active treatment arm did better in stage B than any group in the placebo arm, according to Dr. Allen.
In the 48-week deterioration curves, sustained control was observed among responders out to the end of controlled study. Although there appeared to be numerical advantage for those on both E-PH20 and corticosteroids, E-PH20 arms with concomitant IVIg, SCIg, or no treatment also showed sustained control without significant differences between them.
On functional aINCAT scores, 80.9% achieved at least a 1-point improvement. The improvement was at least 2 points in 42.7%, at least 3 points in 28.2%, and at least 4 points in 11.8%.
E-PH20 Is Characterized as Well Tolerated
Injection site erythema (5.4% vs 0%) and injection site bruising (5.4% vs 0.9%) were more common on E-PH20 than placebo, but there was no difference in serious adverse events, and events possibly related to active treatment, such as headache (3.6% vs. 1.8%) were considered to be of mild to moderate severity.
“The safety profile of efgartigimod plus PH20 was consistent with the safety profile of efgartigimod in other autoimmune diseases,” Dr. Allen said.
The weekly subcutaneous injection can be administered within 90 seconds or less, Dr. Allen said. He called this drug a potential “new therapeutic option to reduce treatment burden in patients with CIDP” if it is approved.
There is a need for new options, according to Brett M. Morrison, MD, PhD, associate professor of neurology at the Johns Hopkins School of Medicine, Baltimore, and an expert in neuromuscular disorders. Dr. Morrison was not involved in the study.
“Although there are three currently approved treatments — steroids, IVIg, and plasmapheresis, at least 20% of CIDP patients have minimal or no response” to any of these, Dr. Morrison said. He added that many of those who do respond to standard therapies have a substantial side effect burden that has created a need for alternatives.
Based on the data presented so far, which suggest substantial efficacy and a favorable safety profile, efgartigimod, if and when it becomes available, “would be an important new treatment for CIDP,” according to Dr. Morrison.
Dr. Allen has financial relationships with more than 10 pharmaceutical companies, including Argenx, which provided funding for the ACHIEVE trial. Dr. Morrison reported no potential conflicts of interest.
FROM AAN 2024