User login
First Results From Laser-Related Adverse Events Registry Reported
BALTIMORE — A relatively . But the process of reporting AEs to the registry needs to be made easier to attract more cases and provide a more complete picture of complications after dermatologic procedures, a researcher and observer said.
The Cutaneous Procedures Adverse Events Reporting Registry (CAPER) was established in 2021 to track AEs from dermatologic procedures. Since then, it has logged a total of 81 cases and 147 AEs from 27 unique procedures, Eric Koza, MD, a postdoctoral research fellow in the Department of Dermatology at Northwestern University, Chicago, reported at the annual conference of the American Society for Laser Medicine and Surgery.
“The takeaways from this project is that 20 laser and energy device treatments have been reported to the registry, half of which were nonablative laser treatments,” Dr. Koza said in presenting the results. “Of the adverse events reported, nonphysicians and non-dermatologic physicians were more likely to be associated with severe or persistent adverse events.”
The American Society for Dermatologic Surgery Association and the Northwestern University Department of Dermatology launched CAPER. Previously, Dr. Koza said, AEs were typically reported only through the Food and Drug Administration’s AE reporting system. He noted that CAPER is the only voluntary national reporting registry for AEs from dermatologic procedures.
What the Registry Shows So Far
The registry matched 72 of the 81 cases with type of provider, with dermatologist-conducted procedures (51, 70.8%) comprising the majority, followed by nonphysician-conducted procedures (14, 19.4%) and nondermatologist physician–conducted procedures (7, 9.7%).
Of the 81 total cases, the following reports were related to laser and energy device treatments: 12 (14.3%) from nonablative laser treatments, five (6%) from light treatments, and three (3.6%) from ablative laser treatments, Dr. Koza said.
Among nonablative laser treatments, the most common AE was blistering (six reports, 50%). Scar, pain, and hypopigmentation accounted for two cases each (16.67%). Dermatologists performed seven of these cases (58.3%); nonphysicians, four (33.3%); and a non-dermatologist physician, one (8.3%).
For intense pulsed-light treatments, burns were the most common AEs (three reports, 60%), with swelling and inflammation each accounting for one case (20%). Three of these cases (75%) were confirmed to have been performed by nonphysicians.
The ablative laser treatment AEs included one case each of hypopigmentation, scar, and erythema. Two of the three cases were confirmed to have been performed by dermatologists.
Dr. Koza acknowledged the low number of cases is a limitation of this analysis of registry reports. A future goal for CAPER is to publicize it more, he said. “The registry is only 3 years old,” he told this news organization. “Hopefully, we can get more data as time goes on. We’ve been getting more and more each year.” CAPER adapted data entry forms used in other registries.
Submitting a case to the registry takes about 15 minutes of the provider’s time, Dr. Koza said. “We can streamline that to make it easier for people to submit their adverse events,” he said in an interview.
Only registry staff have access to the reports, and when reported, the data “is de-identified and any identifying information pertaining to the patient or reporter is removed,” according to a statement on the CAPER website.
‘Needs a Little Help’
Jennifer Lin, MD, a dermatologist at Brigham and Women’s Hospital and Dana-Farber Cancer Institute in Boston, who was at the meeting, commented on the onerous reporting process and the “low” enrollment. “It’s such an important initiative and with everyone over-logging e-mails, a 15-minute entry just is not going to cut it,” she told this news organization.
For providers, reporting AEs is stressful, she said. “As it is, it’s hard to voluntarily submit an adverse event,” Dr. Lin continued. “There’s a feeling of shame. Hospitals require it in order to monitor adverse events, but there’s no monitoring when you’re out in your own private practice.”
“The idea is excellent, but I think to facilitate better enrollment, the word has to get out at all these meetings” and make it easier to submit cases, Dr. Lin added. “It’s a good idea, but it needs a little help.”
Information on submitting AE reports to CAPER is available on the CAPER website.
Dr. Koza and Dr. Lin had no relevant relationships to disclose.
A version of this article first appeared on Medscape.com.
BALTIMORE — A relatively . But the process of reporting AEs to the registry needs to be made easier to attract more cases and provide a more complete picture of complications after dermatologic procedures, a researcher and observer said.
The Cutaneous Procedures Adverse Events Reporting Registry (CAPER) was established in 2021 to track AEs from dermatologic procedures. Since then, it has logged a total of 81 cases and 147 AEs from 27 unique procedures, Eric Koza, MD, a postdoctoral research fellow in the Department of Dermatology at Northwestern University, Chicago, reported at the annual conference of the American Society for Laser Medicine and Surgery.
“The takeaways from this project is that 20 laser and energy device treatments have been reported to the registry, half of which were nonablative laser treatments,” Dr. Koza said in presenting the results. “Of the adverse events reported, nonphysicians and non-dermatologic physicians were more likely to be associated with severe or persistent adverse events.”
The American Society for Dermatologic Surgery Association and the Northwestern University Department of Dermatology launched CAPER. Previously, Dr. Koza said, AEs were typically reported only through the Food and Drug Administration’s AE reporting system. He noted that CAPER is the only voluntary national reporting registry for AEs from dermatologic procedures.
What the Registry Shows So Far
The registry matched 72 of the 81 cases with type of provider, with dermatologist-conducted procedures (51, 70.8%) comprising the majority, followed by nonphysician-conducted procedures (14, 19.4%) and nondermatologist physician–conducted procedures (7, 9.7%).
Of the 81 total cases, the following reports were related to laser and energy device treatments: 12 (14.3%) from nonablative laser treatments, five (6%) from light treatments, and three (3.6%) from ablative laser treatments, Dr. Koza said.
Among nonablative laser treatments, the most common AE was blistering (six reports, 50%). Scar, pain, and hypopigmentation accounted for two cases each (16.67%). Dermatologists performed seven of these cases (58.3%); nonphysicians, four (33.3%); and a non-dermatologist physician, one (8.3%).
For intense pulsed-light treatments, burns were the most common AEs (three reports, 60%), with swelling and inflammation each accounting for one case (20%). Three of these cases (75%) were confirmed to have been performed by nonphysicians.
The ablative laser treatment AEs included one case each of hypopigmentation, scar, and erythema. Two of the three cases were confirmed to have been performed by dermatologists.
Dr. Koza acknowledged the low number of cases is a limitation of this analysis of registry reports. A future goal for CAPER is to publicize it more, he said. “The registry is only 3 years old,” he told this news organization. “Hopefully, we can get more data as time goes on. We’ve been getting more and more each year.” CAPER adapted data entry forms used in other registries.
Submitting a case to the registry takes about 15 minutes of the provider’s time, Dr. Koza said. “We can streamline that to make it easier for people to submit their adverse events,” he said in an interview.
Only registry staff have access to the reports, and when reported, the data “is de-identified and any identifying information pertaining to the patient or reporter is removed,” according to a statement on the CAPER website.
‘Needs a Little Help’
Jennifer Lin, MD, a dermatologist at Brigham and Women’s Hospital and Dana-Farber Cancer Institute in Boston, who was at the meeting, commented on the onerous reporting process and the “low” enrollment. “It’s such an important initiative and with everyone over-logging e-mails, a 15-minute entry just is not going to cut it,” she told this news organization.
For providers, reporting AEs is stressful, she said. “As it is, it’s hard to voluntarily submit an adverse event,” Dr. Lin continued. “There’s a feeling of shame. Hospitals require it in order to monitor adverse events, but there’s no monitoring when you’re out in your own private practice.”
“The idea is excellent, but I think to facilitate better enrollment, the word has to get out at all these meetings” and make it easier to submit cases, Dr. Lin added. “It’s a good idea, but it needs a little help.”
Information on submitting AE reports to CAPER is available on the CAPER website.
Dr. Koza and Dr. Lin had no relevant relationships to disclose.
A version of this article first appeared on Medscape.com.
BALTIMORE — A relatively . But the process of reporting AEs to the registry needs to be made easier to attract more cases and provide a more complete picture of complications after dermatologic procedures, a researcher and observer said.
The Cutaneous Procedures Adverse Events Reporting Registry (CAPER) was established in 2021 to track AEs from dermatologic procedures. Since then, it has logged a total of 81 cases and 147 AEs from 27 unique procedures, Eric Koza, MD, a postdoctoral research fellow in the Department of Dermatology at Northwestern University, Chicago, reported at the annual conference of the American Society for Laser Medicine and Surgery.
“The takeaways from this project is that 20 laser and energy device treatments have been reported to the registry, half of which were nonablative laser treatments,” Dr. Koza said in presenting the results. “Of the adverse events reported, nonphysicians and non-dermatologic physicians were more likely to be associated with severe or persistent adverse events.”
The American Society for Dermatologic Surgery Association and the Northwestern University Department of Dermatology launched CAPER. Previously, Dr. Koza said, AEs were typically reported only through the Food and Drug Administration’s AE reporting system. He noted that CAPER is the only voluntary national reporting registry for AEs from dermatologic procedures.
What the Registry Shows So Far
The registry matched 72 of the 81 cases with type of provider, with dermatologist-conducted procedures (51, 70.8%) comprising the majority, followed by nonphysician-conducted procedures (14, 19.4%) and nondermatologist physician–conducted procedures (7, 9.7%).
Of the 81 total cases, the following reports were related to laser and energy device treatments: 12 (14.3%) from nonablative laser treatments, five (6%) from light treatments, and three (3.6%) from ablative laser treatments, Dr. Koza said.
Among nonablative laser treatments, the most common AE was blistering (six reports, 50%). Scar, pain, and hypopigmentation accounted for two cases each (16.67%). Dermatologists performed seven of these cases (58.3%); nonphysicians, four (33.3%); and a non-dermatologist physician, one (8.3%).
For intense pulsed-light treatments, burns were the most common AEs (three reports, 60%), with swelling and inflammation each accounting for one case (20%). Three of these cases (75%) were confirmed to have been performed by nonphysicians.
The ablative laser treatment AEs included one case each of hypopigmentation, scar, and erythema. Two of the three cases were confirmed to have been performed by dermatologists.
Dr. Koza acknowledged the low number of cases is a limitation of this analysis of registry reports. A future goal for CAPER is to publicize it more, he said. “The registry is only 3 years old,” he told this news organization. “Hopefully, we can get more data as time goes on. We’ve been getting more and more each year.” CAPER adapted data entry forms used in other registries.
Submitting a case to the registry takes about 15 minutes of the provider’s time, Dr. Koza said. “We can streamline that to make it easier for people to submit their adverse events,” he said in an interview.
Only registry staff have access to the reports, and when reported, the data “is de-identified and any identifying information pertaining to the patient or reporter is removed,” according to a statement on the CAPER website.
‘Needs a Little Help’
Jennifer Lin, MD, a dermatologist at Brigham and Women’s Hospital and Dana-Farber Cancer Institute in Boston, who was at the meeting, commented on the onerous reporting process and the “low” enrollment. “It’s such an important initiative and with everyone over-logging e-mails, a 15-minute entry just is not going to cut it,” she told this news organization.
For providers, reporting AEs is stressful, she said. “As it is, it’s hard to voluntarily submit an adverse event,” Dr. Lin continued. “There’s a feeling of shame. Hospitals require it in order to monitor adverse events, but there’s no monitoring when you’re out in your own private practice.”
“The idea is excellent, but I think to facilitate better enrollment, the word has to get out at all these meetings” and make it easier to submit cases, Dr. Lin added. “It’s a good idea, but it needs a little help.”
Information on submitting AE reports to CAPER is available on the CAPER website.
Dr. Koza and Dr. Lin had no relevant relationships to disclose.
A version of this article first appeared on Medscape.com.
FROM ASLMS 2024
A Welcome Trade-off
At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.
It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.
Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.
There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.
But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.
On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.
There’s some revenue lost in the deal, but I’ll still take the trade-off.
It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.
Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.
They probably have more energy, too.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.
It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.
Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.
There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.
But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.
On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.
There’s some revenue lost in the deal, but I’ll still take the trade-off.
It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.
Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.
They probably have more energy, too.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
At the end of March, in an anniversary no one but I noticed, I passed 4 years since I’d last rounded at the hospital.
It’s hard to comprehend that. I was at the hospital regularly for the first 22 years of my career, though admittedly it had dwindled from daily (1998-2011) to 1-2 weekends a month at the end.
Looking back, I still don’t miss it, and have no desire to go back. That’s not to say I don’t keep up on inpatient neurology, in case circumstances change, but at this point, honestly, I don’t want to. I’ve become accustomed to my non-hospital world, no late-night consults, no weekends spent rounding, no taking separate cars to restaurants or family events in case I get called in.
There are certainly things I miss about it. As odd as it may seem (and as much as I’d complain about it) I liked the wee hours of the really late night and early morning. It was quieter. Less chasing patients to tests or therapy. Pleasant idle chatter with staff and the few others docs around. Sitting at the computer and trying to think out a case on the fly. There was always junk food lying around.
But at this point in my life I’ll take the quiet of being home and my routine office hours. I know when my office day starts and ends. Aside from the occasional stop at Costco, I won’t be going anywhere else on my way home. I still get the occasional after-hours call, but none that require me to run to the ER.
On Fridays I’m glad the week is over, and don’t dread the 5:00 answering service switchover, or my call partner giving me the patient list.
There’s some revenue lost in the deal, but I’ll still take the trade-off.
It’s not like I ever had some grand plan to leave the hospital — I actually had thought I’d be there, at least occasionally, until retirement. But here I am.
Not to say there aren’t docs my age (and older) who still do it. Certainly our experience makes us good at it. But younger docs are closer to residency, which is primarily inpatient, so it’s an easier transition for many.
They probably have more energy, too.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Commentary: Comparisons Among PsA Therapies, May 2024
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Can Rectal Cancer Patients Benefit from Deintensification of Treatment?
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
FROM NCCN 2024
Commentary: Studies Often Do Not Answer Clinical Questions in AD, May 2024
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
In "Atopic Dermatitis in Early Childhood and Risk of Inflammatory Bowel Disease: A Scandinavian Birth Cohort Study," Lerchova and colleagues found a statistically significant increased risk for inflammatory bowel disease (IBD) in children with atopic dermatitis. The study had a large patient population, giving it the power to identify very small differences. The researchers found increased risks for IBD, Crohn's disease, and ulcerative colitis (UC) in children with atopic dermatitis; UC had the greatest relative risk. But I don't think this risk was clinically meaningful. About 2 in every 1000 children with atopic dermatitis had UC, whereas about 1 in every 1000 children without atopic dermatitis had UC. Even if the increased absolute risk of 1 in 1000 children was due to atopic dermatitis and not to other factors, I don't think it justifies the authors' conclusion that "these findings might be useful in identifying at-risk individuals for IBD."
Sometimes reviewing articles makes me feel like a crotchety old man. A study by Guttman-Yassky and colleagues, "Targeting IL-13 With Tralokinumab Normalizes Type 2 Inflammation in Atopic Dermatitis Both Early and at 2 Years," didn't seem to test any specific hypothesis. The researchers just looked at a variety of inflammation markers in patients with atopic dermatitis treated with tralokinumab, an interleukin-13 (IL-13) antagonist. In these patients, as expected, the atopic dermatitis improved; so did the inflammatory markers. Did we learn anything clinically useful? I don't think so. We already know that IL-13 is important in atopic dermatitis because when we block IL-13, atopic dermatitis improves.
Vitamin D supplementation doesn't appear to improve atopic dermatitis, as reported by Borzutzky and colleagues in "Effect of Weekly Vitamin D Supplementation on the Severity of Atopic Dermatitis and Type 2 Immunity Biomarkers in Children: A Randomized Controlled Trial." A group of 101 children with atopic dermatitis were randomly assigned to receive oral vitamin D supplementation or placebo. The two groups improved to a similar extent. If you know me, you know I'm wondering whether they took the medication. It appears that they did, because at baseline most of the children were vitamin D deficient, and vitamin D levels improved greatly in the group treated with vitamin D but not in the placebo group.
Journals such as the Journal of the American Academy of Dermatology should require articles to report absolute risk. In "Risk of Lymphoma in Patients With Atopic Dermatitis: A Case-Control Study in the All of Us Database," Powers and colleagues tell us that atopic dermatitis is associated with a statistically significantly increased risk for lymphoma. This means that increased risk wasn't likely due to chance alone. The article says nothing, as far as I could tell, about how big the risk is. Does everyone get lymphoma? Or is it a one in a million risk? Without knowing the absolute risk, the relative risk doesn't tell us whether there is a clinically meaningful increased risk or not. I suspect the increased risk is small. If the incidence of lymphoma is about 2 in 10,000 and peripheral T-cell lymphomas (PTCL) account for 10% of those, even a fourfold increase in the risk for PTCL (the form of lymphoma with the highest relative risk) would not amount to much.
Traidl and colleagues report in "Treatment of Moderate-to-Severe Atopic Dermatitis With Baricitinib: Results From an Interim Analysis of the TREATgermany Registry" that the Janus kinase inhibitor baricitinib makes atopic dermatitis better.
In "Dupilumab Therapy for Atopic Dermatitis Is Associated With Increased Risk of Cutaneous T Cell Lymphoma," Hasan and colleagues report that "it requires 738 prescriptions of dupilumab to produce one case of CTCL [cutaneous T-cell lymphoma]." It seems that this finding could easily be due to 1 in 738 people with a rash thought to be severe atopic dermatitis needing dupilumab having CTCL, not atopic dermatitis, to begin with. If we were to wonder whether dupilumab causes CTCL, perhaps it would be better to study asthma patients treated with or without dupilumab.
Are Direct-to-Consumer Microbiome Tests Clinically Useful?
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
GLP-1s May Increase Post-Endoscopy Aspiration Pneumonia Risk
, according to a new large population-based study.
In June 2023, the American Society of Anesthesiologists (ASA) recommended holding GLP-1 RAs before an endoscopic or surgical procedure to reduce the risk for complications associated with anesthesia and delayed stomach emptying.
In response, the American Gastroenterological Association (AGA) published a rapid clinical practice update in November 2023 that found insufficient evidence to support patients stopping the medications before endoscopic procedures.
“It is known that GLP-1 RAs significantly reduce the motility of the stomach and small bowel. As more and more patients are being started on GLP-1 RAs at higher doses and longer half-life, the question became whether the current recommended fasting durations are enough to reasonably assume the stomach is empty prior to procedures that require sedation,” said senior author Ali Rezaie, MD, medical director of the GI Motility Program at Cedars-Sinai Medical Center in Los Angeles.
“We wanted to see if these medications in fact increased the chance of aspiration before the ASA suggestion went into effect,” he said. “However, this is not an easy task, as aspiration is a rare event and a large sample size is needed to confidently answer that question. That is why we evaluated nearly 1 million cases.”
The study was published online in Gastroenterology.
Analyzing GLP-1 RA Use
Dr. Rezaie and colleagues conducted a population-based, retrospective cohort study of the TriNetX dataset, which includes 114 million deidentified individual health records from 80 healthcare organizations. The research team analyzed nearly 1 million records for adult patients between ages 21 and 70 who underwent upper and lower endoscopies between January 2018 and December 2020.
The researchers defined GLP-1 RA users as those who had the medication for more than 6 months and two or more refills within 6 months before the procedure. They adjusted for 59 factors that could affect gut motility or aspiration risks, such as obesity, numerous chronic diseases, and dozens of medications. The primary outcome was aspiration pneumonia within a month after the procedure.
Among 963,184 patients who underwent endoscopy, 46,935 (4.9%) were considered GLP-1 RA users. Among those, 20,099 GLP-1 RA users met the inclusion criteria and had their results compared with non-GLP-1 RA users.
After propensity score matching for the 59 potential confounders, GLP-1 RA use had a higher incidence rate of aspiration pneumonia (0.83% vs 0.63%) and was associated with a significantly higher risk for aspiration pneumonia, with a hazard ratio (HR) of 1.33.
An even higher risk was seen among patients with propofol-assisted endoscopies (HR, 1.49) but not among those without propofol (HR, 1.31).
In a subgroup analysis based on endoscopy type, an elevated risk was observed among patients who underwent upper endoscopy (HR, 1.82) and combined upper and lower endoscopy (HR, 2.26) but not lower endoscopy (HR, 0.56).
“The results were not necessarily surprising given the mechanism of action of GLP-1 RAs. However, for the first time, this was shown with a clinically relevant outcome, such as aspiration pneumonia,” Dr. Rezaie said. “Aspiration during sedation can have devastating consequences, and the 0.2% difference in risk of aspiration can have a significant effect on healthcare as well.”
More than 20 million endoscopies are performed across the United States annually. Based on the assumption that about 3% of those patients are taking GLP-1 RAs, about 1200 aspiration cases per year can be prevented by raising awareness, he said.
Considering Next Steps
The varying risk profiles observed with separate sedation and endoscopy types point to a need for more tailored guidance in managing GLP-1 RA use before a procedure, the study authors wrote.
Although holding the medications before endoscopy may disrupt diabetes management, the potential increased risk for aspiration could justify a change in practice, particularly for upper endoscopy and propofol-associated procedures, they added.
At the same time, additional studies are needed to understand the optimal drug withholding windows before endoscopies and other procedures, they concluded.
“We will need more data on what is the optimal duration of holding GLP-1 RAs,” Dr. Rezaie said. “But given our data and current ASA guidance, stopping these medications prior to elective procedures is the safe thing to do.”
For now, AGA guidance remains the same as offered in the November 2023 update, suggesting an individual approach for each patient on a GLP-1 RA rather than a “blanket statement” on how to manage all patients taking these medications.
“Overall, I believe that this study is important, but we require more high-level data to inform clinical decision-making regarding patients using GLP-1 receptor agonists prior to gastrointestinal endoscopy,” said Andrew Y. Wang, MD, AGAF, chief of gastroenterology and hepatology and director of interventional endoscopy at the University of Virginia in Charlottesville.
Dr. Wang, who wasn’t involved with this study, coauthored the AGA rapid clinical practice update. He and colleagues advised continuing with a procedure as planned for patients on GLP-1 RAs who followed standard preprocedure fasting instructions and didn’t have nausea, vomiting, dyspepsia, or abdominal distention.
Among patients with symptoms that suggest retained gastric contents, rapid sequence intubation may be considered, though it may not be possible in ambulatory or office-based endoscopy settings, Dr. Wang and colleagues wrote. As another option in lieu of stopping GLP-1 RAs, patients can be placed on a liquid diet for 1 day before the procedure.
“While this study found a signal suggesting that patients using GLP-1 RAs had an increased risk of aspiration pneumonia within 1 month following upper endoscopy or combined upper and lower endoscopy, it does not inform us if having patients stop GLP-1 RAs before endoscopic procedures — especially for a single dose — will mitigate this potential risk,” Dr. Wang said.
“It was also interesting that these investigators found that patients taking GLP-1 RAs who underwent lower endoscopy alone were not at increased risk for aspiration pneumonia,” Dr. Wang noted.
The authors didn’t report a funding source and disclosed no potential conflicts. Dr. Wang reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new large population-based study.
In June 2023, the American Society of Anesthesiologists (ASA) recommended holding GLP-1 RAs before an endoscopic or surgical procedure to reduce the risk for complications associated with anesthesia and delayed stomach emptying.
In response, the American Gastroenterological Association (AGA) published a rapid clinical practice update in November 2023 that found insufficient evidence to support patients stopping the medications before endoscopic procedures.
“It is known that GLP-1 RAs significantly reduce the motility of the stomach and small bowel. As more and more patients are being started on GLP-1 RAs at higher doses and longer half-life, the question became whether the current recommended fasting durations are enough to reasonably assume the stomach is empty prior to procedures that require sedation,” said senior author Ali Rezaie, MD, medical director of the GI Motility Program at Cedars-Sinai Medical Center in Los Angeles.
“We wanted to see if these medications in fact increased the chance of aspiration before the ASA suggestion went into effect,” he said. “However, this is not an easy task, as aspiration is a rare event and a large sample size is needed to confidently answer that question. That is why we evaluated nearly 1 million cases.”
The study was published online in Gastroenterology.
Analyzing GLP-1 RA Use
Dr. Rezaie and colleagues conducted a population-based, retrospective cohort study of the TriNetX dataset, which includes 114 million deidentified individual health records from 80 healthcare organizations. The research team analyzed nearly 1 million records for adult patients between ages 21 and 70 who underwent upper and lower endoscopies between January 2018 and December 2020.
The researchers defined GLP-1 RA users as those who had the medication for more than 6 months and two or more refills within 6 months before the procedure. They adjusted for 59 factors that could affect gut motility or aspiration risks, such as obesity, numerous chronic diseases, and dozens of medications. The primary outcome was aspiration pneumonia within a month after the procedure.
Among 963,184 patients who underwent endoscopy, 46,935 (4.9%) were considered GLP-1 RA users. Among those, 20,099 GLP-1 RA users met the inclusion criteria and had their results compared with non-GLP-1 RA users.
After propensity score matching for the 59 potential confounders, GLP-1 RA use had a higher incidence rate of aspiration pneumonia (0.83% vs 0.63%) and was associated with a significantly higher risk for aspiration pneumonia, with a hazard ratio (HR) of 1.33.
An even higher risk was seen among patients with propofol-assisted endoscopies (HR, 1.49) but not among those without propofol (HR, 1.31).
In a subgroup analysis based on endoscopy type, an elevated risk was observed among patients who underwent upper endoscopy (HR, 1.82) and combined upper and lower endoscopy (HR, 2.26) but not lower endoscopy (HR, 0.56).
“The results were not necessarily surprising given the mechanism of action of GLP-1 RAs. However, for the first time, this was shown with a clinically relevant outcome, such as aspiration pneumonia,” Dr. Rezaie said. “Aspiration during sedation can have devastating consequences, and the 0.2% difference in risk of aspiration can have a significant effect on healthcare as well.”
More than 20 million endoscopies are performed across the United States annually. Based on the assumption that about 3% of those patients are taking GLP-1 RAs, about 1200 aspiration cases per year can be prevented by raising awareness, he said.
Considering Next Steps
The varying risk profiles observed with separate sedation and endoscopy types point to a need for more tailored guidance in managing GLP-1 RA use before a procedure, the study authors wrote.
Although holding the medications before endoscopy may disrupt diabetes management, the potential increased risk for aspiration could justify a change in practice, particularly for upper endoscopy and propofol-associated procedures, they added.
At the same time, additional studies are needed to understand the optimal drug withholding windows before endoscopies and other procedures, they concluded.
“We will need more data on what is the optimal duration of holding GLP-1 RAs,” Dr. Rezaie said. “But given our data and current ASA guidance, stopping these medications prior to elective procedures is the safe thing to do.”
For now, AGA guidance remains the same as offered in the November 2023 update, suggesting an individual approach for each patient on a GLP-1 RA rather than a “blanket statement” on how to manage all patients taking these medications.
“Overall, I believe that this study is important, but we require more high-level data to inform clinical decision-making regarding patients using GLP-1 receptor agonists prior to gastrointestinal endoscopy,” said Andrew Y. Wang, MD, AGAF, chief of gastroenterology and hepatology and director of interventional endoscopy at the University of Virginia in Charlottesville.
Dr. Wang, who wasn’t involved with this study, coauthored the AGA rapid clinical practice update. He and colleagues advised continuing with a procedure as planned for patients on GLP-1 RAs who followed standard preprocedure fasting instructions and didn’t have nausea, vomiting, dyspepsia, or abdominal distention.
Among patients with symptoms that suggest retained gastric contents, rapid sequence intubation may be considered, though it may not be possible in ambulatory or office-based endoscopy settings, Dr. Wang and colleagues wrote. As another option in lieu of stopping GLP-1 RAs, patients can be placed on a liquid diet for 1 day before the procedure.
“While this study found a signal suggesting that patients using GLP-1 RAs had an increased risk of aspiration pneumonia within 1 month following upper endoscopy or combined upper and lower endoscopy, it does not inform us if having patients stop GLP-1 RAs before endoscopic procedures — especially for a single dose — will mitigate this potential risk,” Dr. Wang said.
“It was also interesting that these investigators found that patients taking GLP-1 RAs who underwent lower endoscopy alone were not at increased risk for aspiration pneumonia,” Dr. Wang noted.
The authors didn’t report a funding source and disclosed no potential conflicts. Dr. Wang reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new large population-based study.
In June 2023, the American Society of Anesthesiologists (ASA) recommended holding GLP-1 RAs before an endoscopic or surgical procedure to reduce the risk for complications associated with anesthesia and delayed stomach emptying.
In response, the American Gastroenterological Association (AGA) published a rapid clinical practice update in November 2023 that found insufficient evidence to support patients stopping the medications before endoscopic procedures.
“It is known that GLP-1 RAs significantly reduce the motility of the stomach and small bowel. As more and more patients are being started on GLP-1 RAs at higher doses and longer half-life, the question became whether the current recommended fasting durations are enough to reasonably assume the stomach is empty prior to procedures that require sedation,” said senior author Ali Rezaie, MD, medical director of the GI Motility Program at Cedars-Sinai Medical Center in Los Angeles.
“We wanted to see if these medications in fact increased the chance of aspiration before the ASA suggestion went into effect,” he said. “However, this is not an easy task, as aspiration is a rare event and a large sample size is needed to confidently answer that question. That is why we evaluated nearly 1 million cases.”
The study was published online in Gastroenterology.
Analyzing GLP-1 RA Use
Dr. Rezaie and colleagues conducted a population-based, retrospective cohort study of the TriNetX dataset, which includes 114 million deidentified individual health records from 80 healthcare organizations. The research team analyzed nearly 1 million records for adult patients between ages 21 and 70 who underwent upper and lower endoscopies between January 2018 and December 2020.
The researchers defined GLP-1 RA users as those who had the medication for more than 6 months and two or more refills within 6 months before the procedure. They adjusted for 59 factors that could affect gut motility or aspiration risks, such as obesity, numerous chronic diseases, and dozens of medications. The primary outcome was aspiration pneumonia within a month after the procedure.
Among 963,184 patients who underwent endoscopy, 46,935 (4.9%) were considered GLP-1 RA users. Among those, 20,099 GLP-1 RA users met the inclusion criteria and had their results compared with non-GLP-1 RA users.
After propensity score matching for the 59 potential confounders, GLP-1 RA use had a higher incidence rate of aspiration pneumonia (0.83% vs 0.63%) and was associated with a significantly higher risk for aspiration pneumonia, with a hazard ratio (HR) of 1.33.
An even higher risk was seen among patients with propofol-assisted endoscopies (HR, 1.49) but not among those without propofol (HR, 1.31).
In a subgroup analysis based on endoscopy type, an elevated risk was observed among patients who underwent upper endoscopy (HR, 1.82) and combined upper and lower endoscopy (HR, 2.26) but not lower endoscopy (HR, 0.56).
“The results were not necessarily surprising given the mechanism of action of GLP-1 RAs. However, for the first time, this was shown with a clinically relevant outcome, such as aspiration pneumonia,” Dr. Rezaie said. “Aspiration during sedation can have devastating consequences, and the 0.2% difference in risk of aspiration can have a significant effect on healthcare as well.”
More than 20 million endoscopies are performed across the United States annually. Based on the assumption that about 3% of those patients are taking GLP-1 RAs, about 1200 aspiration cases per year can be prevented by raising awareness, he said.
Considering Next Steps
The varying risk profiles observed with separate sedation and endoscopy types point to a need for more tailored guidance in managing GLP-1 RA use before a procedure, the study authors wrote.
Although holding the medications before endoscopy may disrupt diabetes management, the potential increased risk for aspiration could justify a change in practice, particularly for upper endoscopy and propofol-associated procedures, they added.
At the same time, additional studies are needed to understand the optimal drug withholding windows before endoscopies and other procedures, they concluded.
“We will need more data on what is the optimal duration of holding GLP-1 RAs,” Dr. Rezaie said. “But given our data and current ASA guidance, stopping these medications prior to elective procedures is the safe thing to do.”
For now, AGA guidance remains the same as offered in the November 2023 update, suggesting an individual approach for each patient on a GLP-1 RA rather than a “blanket statement” on how to manage all patients taking these medications.
“Overall, I believe that this study is important, but we require more high-level data to inform clinical decision-making regarding patients using GLP-1 receptor agonists prior to gastrointestinal endoscopy,” said Andrew Y. Wang, MD, AGAF, chief of gastroenterology and hepatology and director of interventional endoscopy at the University of Virginia in Charlottesville.
Dr. Wang, who wasn’t involved with this study, coauthored the AGA rapid clinical practice update. He and colleagues advised continuing with a procedure as planned for patients on GLP-1 RAs who followed standard preprocedure fasting instructions and didn’t have nausea, vomiting, dyspepsia, or abdominal distention.
Among patients with symptoms that suggest retained gastric contents, rapid sequence intubation may be considered, though it may not be possible in ambulatory or office-based endoscopy settings, Dr. Wang and colleagues wrote. As another option in lieu of stopping GLP-1 RAs, patients can be placed on a liquid diet for 1 day before the procedure.
“While this study found a signal suggesting that patients using GLP-1 RAs had an increased risk of aspiration pneumonia within 1 month following upper endoscopy or combined upper and lower endoscopy, it does not inform us if having patients stop GLP-1 RAs before endoscopic procedures — especially for a single dose — will mitigate this potential risk,” Dr. Wang said.
“It was also interesting that these investigators found that patients taking GLP-1 RAs who underwent lower endoscopy alone were not at increased risk for aspiration pneumonia,” Dr. Wang noted.
The authors didn’t report a funding source and disclosed no potential conflicts. Dr. Wang reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Dermatologic Care for Refugees: Effective Management of Scabies and Pediculosis
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.
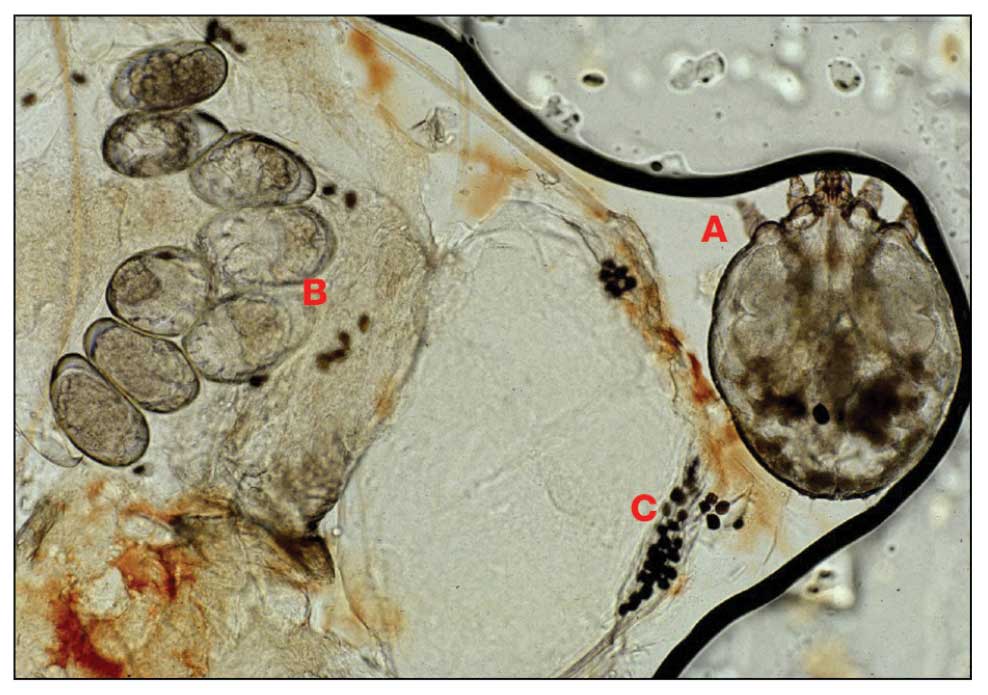
The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25 Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27
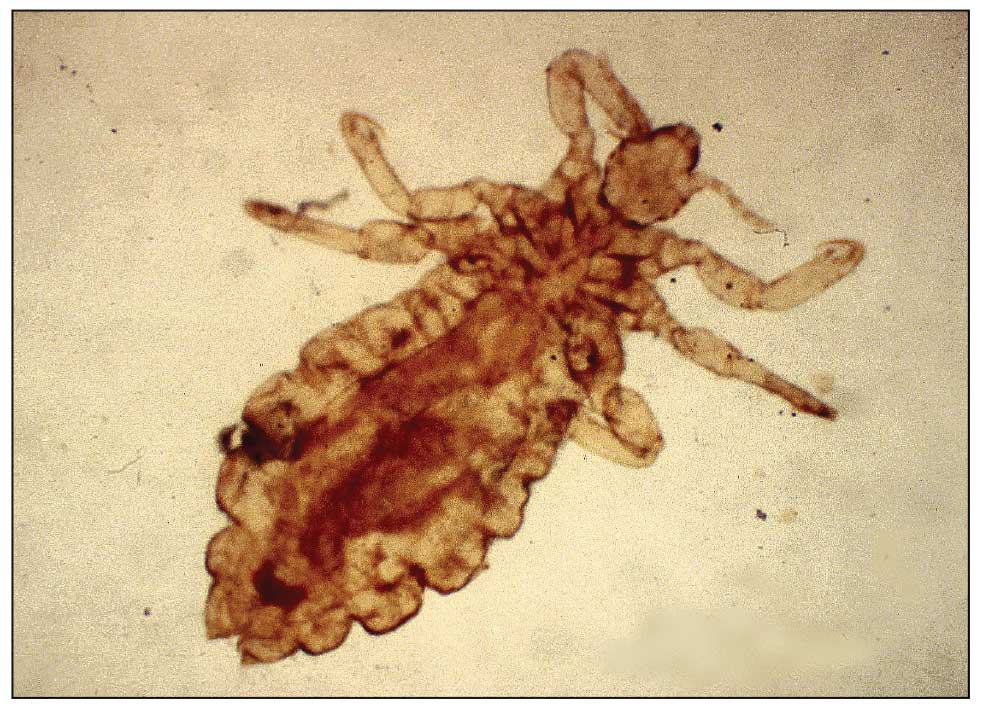
Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32
Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31
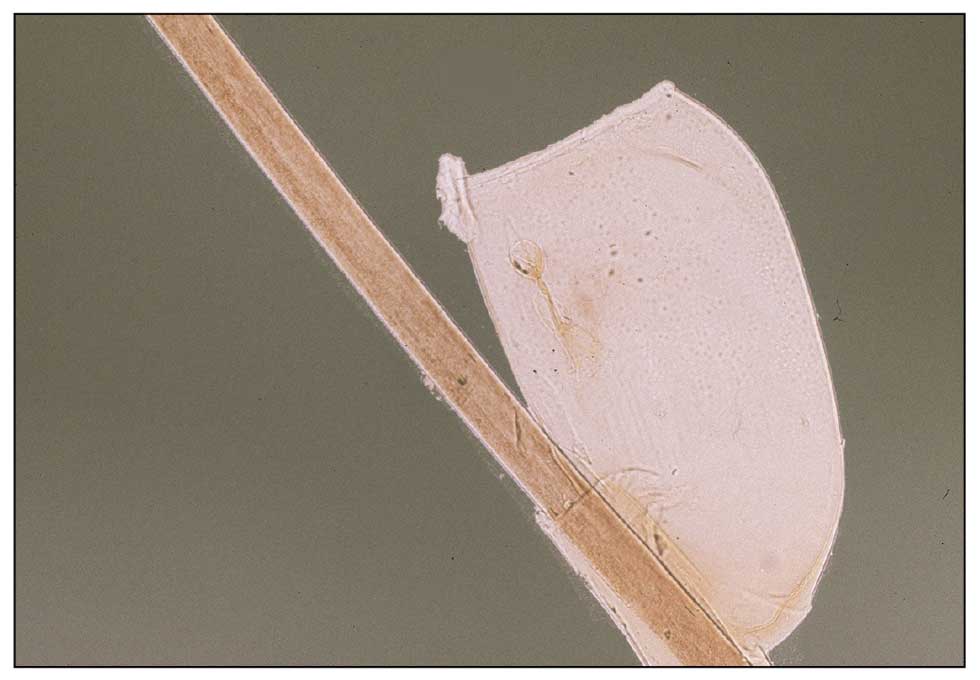
Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.
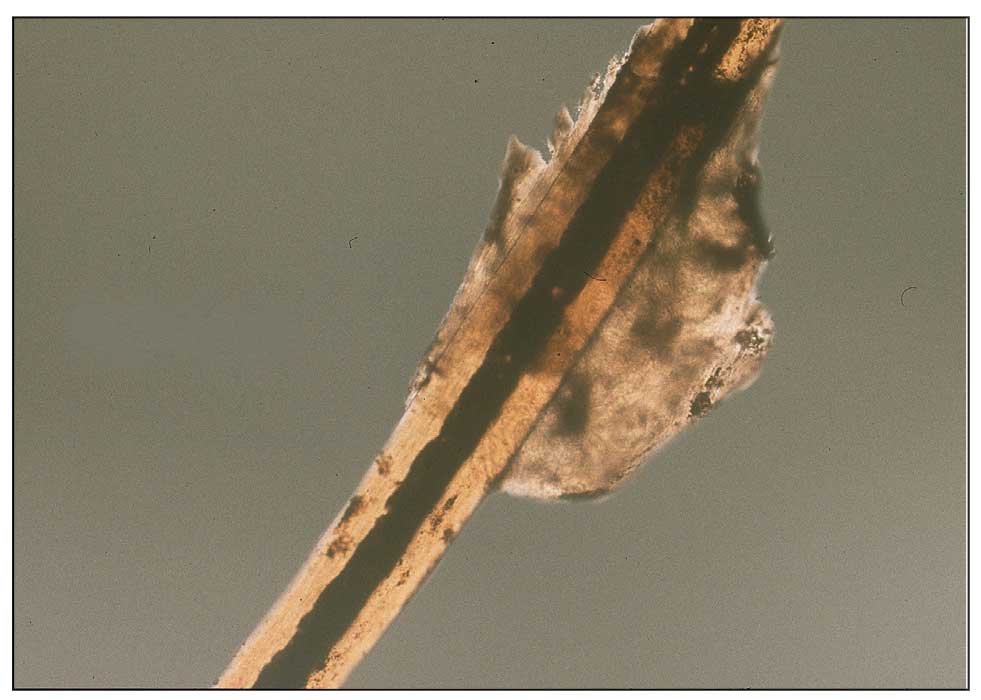
Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25 Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
Approximately 108 million individuals have been forcibly displaced across the globe as of 2022, 35 million of whom are formally designated as refugees.1,2 The United States has coordinated resettlement of more refugee populations than any other country; the most common countries of origin are the Democratic Republic of the Congo, Syria, Afghanistan, and Myanmar.3 In 2021, policy to increase the number of refugees resettled in the United States by more than 700% (from 15,000 up to 125,000) was established; since enactment, the United States has seen more than double the refugee arrivals in 2023 than the prior year, making medical care for this population increasingly relevant for the dermatologist.4
Understanding how to care for this population begins with an accurate understanding of the term refugee. The United Nations defines a refugee as a person who is unwilling or unable to return to their country of nationality because of persecution or well-founded fear of persecution due to race, religion, nationality, membership in a particular social group, or political opinion. This term grants a protected status under international law and encompasses access to travel assistance, housing, cultural orientation, and medical evaluation upon resettlement.5,6
The burden of treatable dermatologic conditions in refugee populations ranges from 19% to 96% in the literature7,8 and varies from inflammatory disorders to infectious and parasitic diseases.9 In one study of 6899 displaced individuals in Greece, the prevalence of dermatologic conditions was higher than traumatic injury, cardiac disease, psychological conditions, and dental disease.10
When outlining differential diagnoses for parasitic infestations of the skin that affect refugee populations, helpful considerations include the individual’s country of origin, route traveled, and method of travel.11 Parasitic infestations specifically are more common in refugee populations when there are barriers to basic hygiene, crowded living or travel conditions, or lack of access to health care, which they may experience at any point in their home country, during travel, or in resettlement housing.8
Even with limited examination and diagnostic resources, the skin is the most accessible first indication of patients’ overall well-being and often provides simple diagnostic clues—in combination with contextualization of the patient’s unique circumstances—necessary for successful diagnosis and treatment of scabies and pediculosis.12 The dermatologist working with refugee populations may be the first set of eyes available and trained to discern skin infestations and therefore has the potential to improve overall outcomes.
Some parasitic infestations in refugee populations may fall under the category of neglected tropical diseases, including scabies, ascariasis, trypanosomiasis, leishmaniasis, and schistosomiasis; they affect an estimated 1 billion individuals across the globe but historically have been underrepresented in the literature and in health policy due in part to limited access to care.13 This review will focus on infestations by the scabies mite (Sarcoptes scabiei var hominis) and the human louse, as these frequently are encountered, easily diagnosed, and treatable by trained clinicians, even in resource-limited settings.
Scabies
Scabies is a parasitic skin infestation caused by the 8-legged mite Sarcoptes scabiei var hominis. The female mite begins the infestation process via penetration of the epidermis, particularly the stratum corneum, and commences laying eggs (Figure 1). The subsequent larvae emerge 48 to 72 hours later and remain burrowed in the epidermis. The larvae mature over the next 10 to 14 days and continue the reproductive cycle.14,15 Symptoms of infestation occurs due to a hypersensitivity reaction to the mite and its by-products.16 Transmission of the mite primarily occurs via direct (skin-to-skin) contact with infected individuals or environmental surfaces for 24 to36 hours in specific conditions, though the latter source has been debated in the literature.

The method of transmission is particularly important when considering care for refugee populations. Scabies is found most often in those living in or traveling from tropical regions including East Asia, Southeast Asia, Oceania, and Latin America.17 In displaced or refugee populations, a lack of access to basic hygiene, extended travel in close quarters, and suboptimal health care access all may lead to an increased incidence of untreated scabies infestations.18 Scabies is more prevalent in children, with increased potential for secondary bacterial infections with Streptococcus and Staphylococcus species due to excoriation in unsanitary conditions. Secondary infection with Streptococcus pyogenes can lead to acute poststreptococcal glomerulonephritis, which accounts for a large burden of chronic kidney disease in affected populations.19 However, scabies may be found in any population, regardless of hygiene or health care access. Treating health care providers should keep a broad differential.
Presentation—The latency of scabies symptoms is 2 to 6 weeks in a primary outbreak and may be as short as 1 to 3 days with re-infestation, following the course of delayed-type hypersensitivity.20 The initial hallmark symptom is pruritus with increased severity in the evening. Visible lesions, excoriations, and burrows associated with scattered vesicles or pustules may be seen over the web spaces of the hands and feet, volar surfaces of the wrists, axillae, waist, genitalia, inner thighs, or buttocks.19 Chronic infestation often manifests with genital nodules. In populations with limited access to health care, there are reports of a sensitization phenomenon in which the individual may become less symptomatic after 4 to 6 weeks and yet be a potential carrier of the mite.21
Those with compromised immune function, such as individuals living with HIV or severe malnutrition, may present with crusted scabies, a variant that manifests as widespread hyperkeratotic scaling with more pronounced involvement of the head, neck, and acral areas. In contrast to classic scabies, crusted scabies is associated with minimal pruritus.22
Diagnosis—The diagnosis of scabies is largely clinical with confirmation through skin scrapings. The International Alliance for Control of Scabies has established diagnostic criteria that include a combination of clinical findings, history, and visualization of mites.23 A dermatologist working with refugee populations may employ any combination of history (eg, nocturnal itch, exposure to an affected individual) or clinical findings along with a high degree of suspicion in those with elevated risk. Visualization of mites is helpful to confirm the diagnosis and may be completed with the application of mineral oil at the terminal end of a burrow, skin scraping with a surgical blade or needle, and examination under light microscopy.
Treatment—First-line treatment for scabies consists of application of permethrin cream 5% on the skin of the neck to the soles of the feet, which is to be left on for 8 to 14 hours followed by rinsing. Re-application is recommended in 1 to 2 weeks. Oral ivermectin is a reasonable alternative to permethrin cream due to its low cost and easy administration in large affected groups. It is not labeled for use in pregnant women or children weighing less than 15 kg but has no selective fetal toxicity. Treatment of scabies with ivermectin has the benefit of treating many other parasitic infections. Both medications are on the World Health Organization Model List of Essential Medications and are widely available for treating providers, even in resource-limited settings.24
Much of the world still uses benzyl benzoate or precipitated sulfur ointment to treat scabies, and some botanicals used in folk medicine have genuine antiscabetic properties. Pruritus may persist for 1 to 4 weeks following treatment and does not indicate treatment failure. Topical camphor and menthol preparations, low-potency topical corticosteroids, or emollients all may be employed for relief.25 Sarna is a Spanish term for scabies and has become the proprietary name for topical antipruritic agents. Additional methods of treatment and prevention include washing clothes and linens in hot water and drying on high heat. If machine washing is not available, clothing and linens may be sealed in a plastic bag for 72 hours.
Pediculosis
Pediculosis is an infestation caused by the ectoparasite Pediculus humanus, an obligate, sesame seed–sized louse that feeds exclusively on the blood of its host (Figure 2).26 Of the lice species, 2 require humans as hosts; one is P humanus and the other is Pthirus pubis (pubic lice). Pediculus humanus may be further classified into morphologies based largely on the affected area: body (P humanus corporis) or head (P humanus capitis), both of which will be discussed.27

Lice primarily attach to clothing and hair shafts, then transfer to the skin for blood feeds. Females lay eggs that hatch 6 to 10 days later, subsequently maturing into adults. The lifespan of these parasites with regular access to a host is 1 to 3 months for head lice and 18 days for body lice vs only 3 to 5 days without a host.28 Transmission of P humanus capitis primarily occurs via direct contact with affected individuals, either head-to-head contact or sharing of items such as brushes and headscarves; P humanus corporis also may be transmitted via direct contact with affected individuals or clothing.
Pediculosis is an important infestation to consider when providing care for refugee populations. Risk factors include lack of access to basic hygiene, including regular bathing or laundering of clothing, and crowded conditions that make direct person-to-person contact with affected individuals more likely.29 Body lice are associated more often with domestic turbulence and displaced populations30 in comparison to head lice, which have broad demographic variables, most often affecting females and children.28 Fatty acids in adult male sebum make the scalp less hospitable to lice.
Presentation—The most common clinical manifestation of pediculosis is pruritus. Cutaneous findings can include papules, wheals, or hemorrhagic puncta secondary to the louse bite. Due to the Tyndall effect of deep hemosiderin pigment, blue-grey macules termed maculae ceruleae (Figure 3) also may be present in chronic infestations of pediculosis pubis, in contrast to pediculosis capitis or corporis.31 Body louse infestation is associated with a general pruritus concentrated on the neck, shoulders, and waist—areas where clothing makes the most direct contact. Lesions may be visible and include eczematous patches with excoriation and possible secondary bacterial infection. Chronic infestation may exhibit lichenification or hyperpigmentation in associated areas. Head lice most often manifest with localized scalp pruritus and associated excoriation and cervical or occipital lymphadenopathy.32

Diagnosis—The diagnosis of pediculosis is clinical, with confirmation requiring direct examination of the insect or nits (the egg case of the parasite)(Figure 4). Body lice and associated nits can be visualized on clothing seams near areas of highest body temperature, particularly the waistband. Head lice may be visualized crawling on hair shafts or on a louse comb. Nits are firmly attached to hair shafts and are visible to the naked eye, whereas pseudonits slide freely along the hair shaft and are not a manifestation of louse infestation (Figure 5).31

Treatment—Treatment varies by affected area. Pediculosis corporis may be treated with permethrin cream 5% applied to the entire body and left on for 8 to 10 hours, but this may not be necessary if facilities are available to wash and dry clothing.33 The use of oral ivermectin and permethrin-impregnated underwear both have been proposed.34,35 Treatment of pediculosis capitis may be accomplished with a variety of topical pediculicides including permethrin, pyrethrum with piperonyl butoxide, dimethicone, malathion, benzyl alcohol, spinosad, and topical ivermectin.22 Topical corticosteroids or emollients may be employed for residual pruritus.

Equally important is environmental elimination of infestation. Clothing should be discarded if possible or washed and dried using high heat. If neither approach is possible or appropriate, clothing may be sealed in a plastic bag for 2 weeks or treated with a pediculicide. Nit combing is an important adjunct in the treatment of pediculosis capitis.36 It is important to encourage return to work and/or school immediately after treatment. “No nit” policies are more harmful to education than helpful for prevention of investation.37
Pediculosis corporis may transmit infectious agents including Bartonella quintana, (trench fever, endocarditis, bacillary angiomatosis), Borrelia recurrentis (louse-borne relapsing fever), and Rickettsia prowazekii (epidemic typhus).31,38,39 Additionally, severe pediculosis infestations have the potential to cause chronic blood loss in affected populations. In a study of patients with active pediculosis infestation, mean hemoglobin values were found to be 2.5 g/dL lower than a matched population without infestation.40 It is important to consider pediculosis as a risk for iron-deficiency anemia in populations who are known to lack access to regular medical evaluation.41
Future Considerations
Increased access to tools and education for clinicians treating refugee populations is key to reducing the burden of parasitic skin disease and related morbidity and mortality in vulnerable groups both domestically and globally. One such tool, the Skin NTDs App, was launched by the World Health Organization in 2020. It is available for free for Android and iOS devices to assist clinicians in the field with the diagnosis and treatment of neglected tropical diseases—including scabies—that may affect refugee populations.42
Additionally, to both improve access and limit preventable sequelae, future investigations into appropriate models of community-based care are paramount. The model of community-based care is centered on the idea of care provision that prioritizes safety, accessibility, affordability, and acceptability in an environment closest to vulnerable populations. The largest dermatologic society, the International League of Dermatological Societies, formed a Migrant Health Dermatology Working Group that prioritizes understanding and improving care for refugee and migrant populations; this group hosted a summit in 2022, bringing together international subject matter leaders to discuss such models of care and set goals for the creation of tool kits for patients, frontline health care workers, and dermatologists.43
Conclusion
Improvement in dermatologic care of refugee populations includes provision of culturally and linguistically appropriate care by trained clinicians, adequate access to the most essential medications, and basic physical or legal access to health care systems in general.8,11,44 Parasitic infestations have the potential to remain asymptomatic for extended periods of time and result in spread to potentially nonendemic regions of resettlement.45 Additionally, the psychosocial well-being of refugee populations upon resettlement may be negatively affected by stigma of disease processes such as scabies and pediculosis, leading to additional barriers to successful re-entry into the patient’s new environment.46 Therefore, proper screening, diagnosis, and treatment of the most common parasitic infestations in this population have great potential to improve outcomes for large groups across the globe.
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
- Monin K, Batalova J, Lai T. Refugees and Asylees in the United States. Migration Information Source. Published May 13, 2021. Accessed April 4, 2024. https://www.migrationpolicy.org/article/refugees-and-asylees-united-states-2021
- UNHCR. Figures at a Glance. UNHCR USA. Update June 14, 2023. Accessed April 4, 2024. https://www.unhcr.org/en-us/figures-at-a-glance.html
- UNHCR. Refugee resettlement facts. Published October 2023. Accessed April 8, 2024. https://www.unhcr.org/us/media/refugee-resettlement-facts
- US Department of State. Report to Congress on Proposed Refugee Admissions for Fiscal Year 2024. Published November 3, 2023. Accessed April 8, 2024. https://www.state.gov/report-to-congress-on-proposed-refugee-admissions-for-fiscal-year-2024/
- UNHCR. Compact for Migration: Definitions. United Nations. Accessed April 4, 2024. https://refugeesmigrants.un.org/definitions
- United Nations High Commissioner for Refugees (UNHCR). Convention and Protocol Relating to the Status of Refugees. Published December 2010. Accessed January 11, 2024. https://www.unhcr.org/us/media/convention-and-protocol-relating-status-refugees
- Kibar Öztürk M. Skin diseases in rural Nyala, Sudan (in a rural hospital, in 12 orphanages, and in two refugee camps). Int J Dermatol. 2019;58:1341-1349. doi:10.1111/ijd.14619
- Padovese V, Knapp A. Challenges of managing skin diseases in refugees and migrants. Dermatol Clin. 2021;39:101-115. doi:10.1016/j.det.2020.08.010
- Saikal SL, Ge L, Mir A, et al. Skin disease profile of Syrian refugees in Jordan: a field-mission assessment. J Eur Acad Dermatol Venereol. 2020;34:419-425. doi:10.1111/jdv.15909
- Eonomopoulou A, Pavli A, Stasinopoulou P, et al. Migrant screening: lessons learned from the migrant holding level at the Greek-Turkish borders. J Infect Public Health. 2017;10:177-184. doi:10.1016/j.jiph.2016.04.012
- Marano N, Angelo KM, Merrill RD, et al. Expanding travel medicine in the 21st century to address the health needs of the world’s migrants.J Travel Med. 2018;25. doi:10.1093/jtm/tay067
- Hay RJ, Asiedu K. Skin-related neglected tropical diseases (skin NTDs)—a new challenge. Trop Med Infect Dis. 2018;4. doi:10.3390/tropicalmed4010004
- NIAID. Neglected tropical diseases. Updated July 11, 2016. Accessed April 4, 2024. https://www.niaid.nih.gov/research/neglected-tropical-diseases
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Arlian LG, Runyan RA, Achar S, et al. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J Am Acad Dermatol. 1984;11(2 pt 1):210-215. doi:10.1016/s0190-9622(84)70151-4
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967. doi:10.1016/S1473-3099(15)00132-2
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Mellanby K, Johnson CG, Bartley WC. Treatment of scabies. Br Med J. 1942;2:1-4. doi:10.1136/bmj.2.4252.1
- Walton SF. The immunology of susceptibility and resistance to scabies. Parasit Immunol. 2010;32:532-540. doi:10.1111/j.1365-3024.2010.01218.x
- Coates SJ, Thomas C, Chosidow O, et al. Ectoparasites: pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551-569. doi:10.1016/j.jaad.2019.05.110
- Engelman D, Fuller LC, Steer AC; International Alliance for the Control of Scabies Delphi p. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis. 2018;12:E0006549. doi:10.1371/journal.pntd.0006549
- World Health Organization. WHO Model Lists of Essential Medicines—23rd list, 2023. Updated July 26, 2023. Accessed April 8, 2024. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Badiaga S, Brouqui P. Human louse-transmitted infectious diseases. Clin Microbiol Infect. 2012;18:332-337. doi:10.1111/j.1469-0691.2012.03778.x
- Leo NP, Campbell NJH, Yang X, et al. Evidence from mitochondrial DNA that head lice and body lice of humans (Phthiraptera: Pediculidae) are conspecific. J Med Entomol. 2002;39:662-666. doi:10.1603/0022-2585-39.4.662
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826. doi:10.1016/S0140-6736(99)09458-1
- Arnaud A, Chosidow O, Détrez M-A, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol. 2016;174:104-112. doi:10.1111/bjd.14226
- Brouqui P. Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol. 2011;56:357-374. doi:10.1146/annurev-ento-120709-144739
- Ko CJ, Elston DM. Pediculosis. J Am Acad Dermatol. 2004;50:1-12. doi:10.1016/S0190-9622(03)02729-4
- Bloomfield D. Head lice. Pediatr Rev. 2002;23:34-35; discussion 34-35. doi:10.1542/pir.23-1-34
- Stone SP GJ, Bacelieri RE. Scabies, other mites, and pediculosis. In: Wolf K GL, Katz SI, et al (eds). Fitzpatrick’s Dermatology in General Medicine. McGraw Hill; 2008:2029.
- Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476. doi:10.1086/499279
- Benkouiten S, Drali R, Badiaga S, et al. Effect of permethrin-impregnated underwear on body lice in sheltered homeless persons: a randomized controlled trial. JAMA Dermatol. 2014;150:273-279. doi:10.1001/jamadermatol.2013.6398
- CDC. Parasites: Treatment. Updated October 15, 2019. Accessed April 4, 2024. https://www.cdc.gov/parasites/lice/head/treatment.html
- Devore CD, Schutze GE; Council on School Health and Committee on Infectious Diseases, American Academy of Pediatrics. Head lice. Pediatrics. 2015;135:e1355-e1365. doi:10.1542/peds.2015-0746
- Ohl ME, Spach DH. Bartonella quintana and urban trench fever. Clin Infect Dis. 2000;31:131-135. doi:10.1086/313890
- Drali R, Sangaré AK, Boutellis A, et al. Bartonella quintana in body lice from scalp hair of homeless persons, France. Emerg Infect Dis. 2014;20:907-908. doi:10.3201/eid2005.131242
- Rudd N, Zakaria A, Kohn MA, et al. Association of body lice infestation with hemoglobin values in hospitalized dermatology patients. JAMA Dermatol. 2022;158:691-693. doi:10.1001/jamadermatol.2022.0818
- Guss DA, Koenig M, Castillo EM. Severe iron deficiency anemia and lice infestation. J Emergency Med. 2011;41:362-365. doi:10.1016/j.jemermed.2010.05.030
- Neglected tropical diseases of the skin: WHO launches mobile application to facilitate diagnosis. News release. World Health Organization; July 16, 2020. Accessed April 4, 2024. https://www.who.int/news/item/16-07-2020-neglected-tropical-diseases-of-the-skin-who-launches-mobile-application-to-facilitate-diagnosis
- Padovese V, Fuller LC, Griffiths CEM, et al; Migrant Health Dermatology Working Group of the International Foundation for Dermatology. Migrant skin health: perspectives from the Migrant Health Summit, Malta, 2022. Br J Dermatology. 2023;188:553-554. doi:10.1093/bjd/ljad001
- Knapp AP, Rehmus W, Chang AY. Skin diseases in displaced populations: a review of contributing factors, challenges, and approaches to care. Int J Dermatol. 2020;59:1299-1311. doi:10.1111/ijd.15063
- Norman FF, Comeche B, Chamorro S, et al. Overcoming challenges in the diagnosis and treatment of parasitic infectious diseases in migrants. Expert Rev Anti-infective Therapy. 2020;18:127-143. doi:10.1080/14787210.2020.1713099
- Skin NTDs: prioritizing integrated approaches to reduce suffering, psychosocial impact and stigmatization. News release. World Health Organization; October 29, 2020. Accessed April 4, 2024. https://www.who.int/news/item/29-10-2020-skin-ntds-prioritizing-integrated-approaches-to-reduce-suffering-psychosocial-impact-and-stigmatization
Practice Points
- War and natural disasters displace populations and disrupt infrastructure and access to medical care.
- Infestations and cutaneous infections are common among refugee populations, and impetigo often is a sign of underlying scabies infestation.
- Body lice are important disease vectors inrefugee populations.
Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Microbiome Alterations Linked to Growth Hormone Deficiency
, said Chinese researchers.
The research, published recently in Pediatric Research, involved more than 80 children and showed that those with GHD had alterations in microbial populations that have been linked to longevity, as well as a microbial and metabolite signature that allowed accurate discrimination from ISS.
“These findings provide novel insights into potential early diagnosis and innovative treatment alternatives, such as fecal microbiota transplantation, for short stature with varying growth hormone levels,” the authors wrote.
Andrew Dauber, MD, MMSc, chief of endocrinology, Children’s National Hospital, Washington, who was not involved in the study, said that while this is “a really interesting area of research,” he expressed “hesitancy about getting too excited about this data yet.”
“One of the problems is how you define growth hormone deficiency,” as it is “not a black and white diagnosis,” and the etiology and child’s growth trajectory also need to be considered, Dr. Dauber told said.
He explained: “The problem is that, when you rely on the growth hormone stimulation test alone, there’s so many false positives and so much overlap between patients with true growth hormone deficiency and those without. And I think that this article fell prey to that.”
He added: “It would be really, really interesting and helpful to have a microbiome signature that allows you to distinguish between true growth hormone deficiency and patients with idiopathic short stature.”
“But you have to make sure that your groups are very well defined for this study to be really valid. And that’s one of my concerns here.”
Dr. Dauber continued: “Now, that being said, they did find some associations that correlated with growth hormone peak levels,” some which replicate previous findings, “so I do think that there are kernels of important findings here.”
‘Tease Out Influences’ to Isolate the Interaction
He pointed out that there are “many factors that influence the microbiome,” such as the use of antibiotics, diet, age, and geographic location. Therefore, a study that could truly tease out all these influences and isolate the interaction with growth hormone levels would need to be “very thoughtfully designed.”
A number of factors contribute to short stature, lead author Lan Li, MD, Department of Radiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China, and colleagues.
These include genetic factors, environmental factors, and conditions such as being small for gestational age at birth, familial short stature, and chronic systemic diseases, as well as GHD and ISS.
Recent animal studies have suggested that there may be a bidirectional relationship between the gut microbiota and the growth hormone/insulin-like growth factor 1 axis, and it has been shown that individuals with GHD have significant alterations in their gut microbiota compared with healthy controls.
To investigate, they studied 36 children diagnosed with GHD, 32 with ISS, and 16 age- and sex-matched healthy controls, all of whom were recruited between February 2019 and June 2021 from the Pediatric Endocrinology Department of The Second Affiliated Hospital of Wenzhou Medical University.
Fecal samples obtained from the children underwent microbiome analysis using 16S ribosomal RNA gene sequencing, alongside nuclear MRI analysis of the metabolome, or the entire complement of small molecules in the samples.
Patients with GHD had a significantly higher body mass index than those with ISS (P < .05), and their peak growth hormone level was significantly lower (P < .001). Patients with GHD also had significantly higher total cholesterol and low-density lipoprotein cholesterol levels than patients with ISS (P < .05).
The team reports that the alpha diversity of the fecal microbiome, which measures the microbial diversity within a fecal sample, was similar between the three groups.
However, there was significant variation between the groups in the beta diversity, which quantifies the similarity or dissimilarity between two samples, and allows the overall taxonomic or functional diversity pattern to be linked to environmental features.
Compared with the healthy control group, the abundance of Pelomonas, Rodentibacter, and Rothia was significantly decreased in GHD and patients with ISS, while the abundance of Prevotellaceae_NK3B31_group was increased in the two patient groups, particularly in those with GHD.
In addition, the researchers found a decreased Firmicutes/Bacteroidota (F/B) ratio in participants with short stature, particularly in the GHD group. They noted that “emerging evidence suggests the F/B ratio may play a role in longevity.”
Nocardioides was substantially more common in the ISS group vs both patients with GHD and healthy controls, while Fusobacterium mortiferum was characteristic of GHD. The team suggests this “may serve as a critical intestinal factor contributing to the short stature observed in GHD.”
The metabolome analysis revealed that glucose, pyruvate, and pyrimidine metabolism may also play a significant role in distinguishing between patients with GHD and ISS and healthy control groups.
Finally, the team demonstrated that a panel combining 13 microbiome and metabolome markers was able to discriminate between GHD and ISS at an area under the receiver operating characteristic curve of 0.945, with a sensitivity of 87% and a specificity of 91%.
The study was supported by grants from the National Natural Science Foundation of China and Wenzhou Science and Technology Bureau in China. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
, said Chinese researchers.
The research, published recently in Pediatric Research, involved more than 80 children and showed that those with GHD had alterations in microbial populations that have been linked to longevity, as well as a microbial and metabolite signature that allowed accurate discrimination from ISS.
“These findings provide novel insights into potential early diagnosis and innovative treatment alternatives, such as fecal microbiota transplantation, for short stature with varying growth hormone levels,” the authors wrote.
Andrew Dauber, MD, MMSc, chief of endocrinology, Children’s National Hospital, Washington, who was not involved in the study, said that while this is “a really interesting area of research,” he expressed “hesitancy about getting too excited about this data yet.”
“One of the problems is how you define growth hormone deficiency,” as it is “not a black and white diagnosis,” and the etiology and child’s growth trajectory also need to be considered, Dr. Dauber told said.
He explained: “The problem is that, when you rely on the growth hormone stimulation test alone, there’s so many false positives and so much overlap between patients with true growth hormone deficiency and those without. And I think that this article fell prey to that.”
He added: “It would be really, really interesting and helpful to have a microbiome signature that allows you to distinguish between true growth hormone deficiency and patients with idiopathic short stature.”
“But you have to make sure that your groups are very well defined for this study to be really valid. And that’s one of my concerns here.”
Dr. Dauber continued: “Now, that being said, they did find some associations that correlated with growth hormone peak levels,” some which replicate previous findings, “so I do think that there are kernels of important findings here.”
‘Tease Out Influences’ to Isolate the Interaction
He pointed out that there are “many factors that influence the microbiome,” such as the use of antibiotics, diet, age, and geographic location. Therefore, a study that could truly tease out all these influences and isolate the interaction with growth hormone levels would need to be “very thoughtfully designed.”
A number of factors contribute to short stature, lead author Lan Li, MD, Department of Radiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China, and colleagues.
These include genetic factors, environmental factors, and conditions such as being small for gestational age at birth, familial short stature, and chronic systemic diseases, as well as GHD and ISS.
Recent animal studies have suggested that there may be a bidirectional relationship between the gut microbiota and the growth hormone/insulin-like growth factor 1 axis, and it has been shown that individuals with GHD have significant alterations in their gut microbiota compared with healthy controls.
To investigate, they studied 36 children diagnosed with GHD, 32 with ISS, and 16 age- and sex-matched healthy controls, all of whom were recruited between February 2019 and June 2021 from the Pediatric Endocrinology Department of The Second Affiliated Hospital of Wenzhou Medical University.
Fecal samples obtained from the children underwent microbiome analysis using 16S ribosomal RNA gene sequencing, alongside nuclear MRI analysis of the metabolome, or the entire complement of small molecules in the samples.
Patients with GHD had a significantly higher body mass index than those with ISS (P < .05), and their peak growth hormone level was significantly lower (P < .001). Patients with GHD also had significantly higher total cholesterol and low-density lipoprotein cholesterol levels than patients with ISS (P < .05).
The team reports that the alpha diversity of the fecal microbiome, which measures the microbial diversity within a fecal sample, was similar between the three groups.
However, there was significant variation between the groups in the beta diversity, which quantifies the similarity or dissimilarity between two samples, and allows the overall taxonomic or functional diversity pattern to be linked to environmental features.
Compared with the healthy control group, the abundance of Pelomonas, Rodentibacter, and Rothia was significantly decreased in GHD and patients with ISS, while the abundance of Prevotellaceae_NK3B31_group was increased in the two patient groups, particularly in those with GHD.
In addition, the researchers found a decreased Firmicutes/Bacteroidota (F/B) ratio in participants with short stature, particularly in the GHD group. They noted that “emerging evidence suggests the F/B ratio may play a role in longevity.”
Nocardioides was substantially more common in the ISS group vs both patients with GHD and healthy controls, while Fusobacterium mortiferum was characteristic of GHD. The team suggests this “may serve as a critical intestinal factor contributing to the short stature observed in GHD.”
The metabolome analysis revealed that glucose, pyruvate, and pyrimidine metabolism may also play a significant role in distinguishing between patients with GHD and ISS and healthy control groups.
Finally, the team demonstrated that a panel combining 13 microbiome and metabolome markers was able to discriminate between GHD and ISS at an area under the receiver operating characteristic curve of 0.945, with a sensitivity of 87% and a specificity of 91%.
The study was supported by grants from the National Natural Science Foundation of China and Wenzhou Science and Technology Bureau in China. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
, said Chinese researchers.
The research, published recently in Pediatric Research, involved more than 80 children and showed that those with GHD had alterations in microbial populations that have been linked to longevity, as well as a microbial and metabolite signature that allowed accurate discrimination from ISS.
“These findings provide novel insights into potential early diagnosis and innovative treatment alternatives, such as fecal microbiota transplantation, for short stature with varying growth hormone levels,” the authors wrote.
Andrew Dauber, MD, MMSc, chief of endocrinology, Children’s National Hospital, Washington, who was not involved in the study, said that while this is “a really interesting area of research,” he expressed “hesitancy about getting too excited about this data yet.”
“One of the problems is how you define growth hormone deficiency,” as it is “not a black and white diagnosis,” and the etiology and child’s growth trajectory also need to be considered, Dr. Dauber told said.
He explained: “The problem is that, when you rely on the growth hormone stimulation test alone, there’s so many false positives and so much overlap between patients with true growth hormone deficiency and those without. And I think that this article fell prey to that.”
He added: “It would be really, really interesting and helpful to have a microbiome signature that allows you to distinguish between true growth hormone deficiency and patients with idiopathic short stature.”
“But you have to make sure that your groups are very well defined for this study to be really valid. And that’s one of my concerns here.”
Dr. Dauber continued: “Now, that being said, they did find some associations that correlated with growth hormone peak levels,” some which replicate previous findings, “so I do think that there are kernels of important findings here.”
‘Tease Out Influences’ to Isolate the Interaction
He pointed out that there are “many factors that influence the microbiome,” such as the use of antibiotics, diet, age, and geographic location. Therefore, a study that could truly tease out all these influences and isolate the interaction with growth hormone levels would need to be “very thoughtfully designed.”
A number of factors contribute to short stature, lead author Lan Li, MD, Department of Radiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China, and colleagues.
These include genetic factors, environmental factors, and conditions such as being small for gestational age at birth, familial short stature, and chronic systemic diseases, as well as GHD and ISS.
Recent animal studies have suggested that there may be a bidirectional relationship between the gut microbiota and the growth hormone/insulin-like growth factor 1 axis, and it has been shown that individuals with GHD have significant alterations in their gut microbiota compared with healthy controls.
To investigate, they studied 36 children diagnosed with GHD, 32 with ISS, and 16 age- and sex-matched healthy controls, all of whom were recruited between February 2019 and June 2021 from the Pediatric Endocrinology Department of The Second Affiliated Hospital of Wenzhou Medical University.
Fecal samples obtained from the children underwent microbiome analysis using 16S ribosomal RNA gene sequencing, alongside nuclear MRI analysis of the metabolome, or the entire complement of small molecules in the samples.
Patients with GHD had a significantly higher body mass index than those with ISS (P < .05), and their peak growth hormone level was significantly lower (P < .001). Patients with GHD also had significantly higher total cholesterol and low-density lipoprotein cholesterol levels than patients with ISS (P < .05).
The team reports that the alpha diversity of the fecal microbiome, which measures the microbial diversity within a fecal sample, was similar between the three groups.
However, there was significant variation between the groups in the beta diversity, which quantifies the similarity or dissimilarity between two samples, and allows the overall taxonomic or functional diversity pattern to be linked to environmental features.
Compared with the healthy control group, the abundance of Pelomonas, Rodentibacter, and Rothia was significantly decreased in GHD and patients with ISS, while the abundance of Prevotellaceae_NK3B31_group was increased in the two patient groups, particularly in those with GHD.
In addition, the researchers found a decreased Firmicutes/Bacteroidota (F/B) ratio in participants with short stature, particularly in the GHD group. They noted that “emerging evidence suggests the F/B ratio may play a role in longevity.”
Nocardioides was substantially more common in the ISS group vs both patients with GHD and healthy controls, while Fusobacterium mortiferum was characteristic of GHD. The team suggests this “may serve as a critical intestinal factor contributing to the short stature observed in GHD.”
The metabolome analysis revealed that glucose, pyruvate, and pyrimidine metabolism may also play a significant role in distinguishing between patients with GHD and ISS and healthy control groups.
Finally, the team demonstrated that a panel combining 13 microbiome and metabolome markers was able to discriminate between GHD and ISS at an area under the receiver operating characteristic curve of 0.945, with a sensitivity of 87% and a specificity of 91%.
The study was supported by grants from the National Natural Science Foundation of China and Wenzhou Science and Technology Bureau in China. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM PEDIATRIC RESEARCH