User login
This month in the journal CHEST®
Editor’s picks
ORIGINAL RESEARCH
The Saint Georges Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared to the classic definition. By Dr. V. Kim, et al.
Confocal laser endomicroscopy (CLE) as a guidance tool for pleural biopsies in malignant pleural mesothelioma. By Dr. L. Wijmans, et al.
Association of Angiotensin Modulators With the Course of Idiopathic Pulmonary Fibrosis. By Dr. M. Kreuter, et al.
Age-Stratified National Trends in Pulmonary Embolism Admissions. By Dr. E. D. Pauley, et al.
COMMENTARY
Solving the Opioid Crisis: Respiratory Depression by Opioids as Critical Endpoint.By Dr. G. Montandon and Dr. A. S. Slutsky.
Editor’s picks
Editor’s picks
ORIGINAL RESEARCH
The Saint Georges Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared to the classic definition. By Dr. V. Kim, et al.
Confocal laser endomicroscopy (CLE) as a guidance tool for pleural biopsies in malignant pleural mesothelioma. By Dr. L. Wijmans, et al.
Association of Angiotensin Modulators With the Course of Idiopathic Pulmonary Fibrosis. By Dr. M. Kreuter, et al.
Age-Stratified National Trends in Pulmonary Embolism Admissions. By Dr. E. D. Pauley, et al.
COMMENTARY
Solving the Opioid Crisis: Respiratory Depression by Opioids as Critical Endpoint.By Dr. G. Montandon and Dr. A. S. Slutsky.
ORIGINAL RESEARCH
The Saint Georges Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared to the classic definition. By Dr. V. Kim, et al.
Confocal laser endomicroscopy (CLE) as a guidance tool for pleural biopsies in malignant pleural mesothelioma. By Dr. L. Wijmans, et al.
Association of Angiotensin Modulators With the Course of Idiopathic Pulmonary Fibrosis. By Dr. M. Kreuter, et al.
Age-Stratified National Trends in Pulmonary Embolism Admissions. By Dr. E. D. Pauley, et al.
COMMENTARY
Solving the Opioid Crisis: Respiratory Depression by Opioids as Critical Endpoint.By Dr. G. Montandon and Dr. A. S. Slutsky.
E-cigarette-associated respiratory diseases: Ask your patients about vaping substances via e-cigarettes
E-cigarettes arrived in the U.S. market between 2005 and 2007. Vaping via e-cigarettes involves inhaling substances such as nicotine, flavorings, chemicals, and, sometimes, marijuana and/or other substances deep into the lungs. While the use of these devices is prevalent, the long-term effects are not known. We, as clinicians, need to specifically ask our patients about their use of substances via e-cigarettes because of alarming cases of severe, life-threatening respiratory illnesses recently being reported throughout the United States in young, otherwise healthy, individuals.
As of September 11, 2019, over 380 cases have been reported to the Centers for Disease Control and Prevention (CDC), where young, healthy people from 33 states and one US territory were hospitalized with severe respiratory disease. There have been at least six confirmed deaths and approximately one-third of those who survived required aggressive support with intubation and mechanical ventilation. The number of reported cases is rapidly rising (from 215 possible cases on August 27, 2019). The common theme in these cases is that every patient reported using an e-cigarette product within 90 days of the onset of symptoms, and most within the prior 2 weeks. By definition, other etiologies of respiratory failure, such as infections, collagen vascular, immunologic diseases, and malignancies were excluded.
Between 90% and 98% of patients presented to the hospital with respiratory symptoms, such as shortness of breath, cough, hemoptysis, and/or chest pain. The most common reported e-cigarette product exposure among these case patients is tetrahydrocannabinol, THC (in approximately 80% to 85%); however, some used only nicotine-based products (15% to 20%). In addition, approximately 45% to 50% reported using THC and nicotine-based products. One concerning fact that requires special attention is that some affected patients initially presented with nonrespiratory complaints, such as GI symptoms of nausea, vomiting, and/or diarrhea; constitutional symptoms such as fever (up to 104oF), fatigue, and/or weight loss; and neurologic symptoms such as headaches and even seizures. Many of these symptoms preceded the respiratory symptoms by up to 2 weeks. Therefore, a few of these patients initially presented without significant respiratory symptoms and with normal chest radiographs – but progressed over days to weeks to acute hypoxemic respiratory failure.
Up to 75% of the affected patients who ultimately required hospitalization for e-cigarette-associated respiratory disease initially presented to a primary care clinic or ED and were sent home due to nonspecific signs and symptoms, which mimic common viral illnesses. Therefore, it is critical for all health-care professionals to have a high clinical suspicion for e-cigarette-associated respiratory disease, particularly while more data are being gathered. When suspected, the CDC recommends asking patients about specific substances inhaled, the manufacturer, where the products/cartridges were obtained, type of device(s) used, and method used (ie, aerosolization, dabbing, dripping, etc).
The most common types of imaging and pathologic patterns attributed to e-cigarette use reported to date include lipoid pneumonia, diffuse alveolar damage, acute
respiratory distress syndrome (ARDS), diffuse alveolar hemorrhage (DAH), acute eosinophilic pneumonia, hypersensitivity pneumonitis, and organizing pneumonia. The most common patterns on imaging include basilar-predominant consolidation and ground-glass opacities with areas of subpleural sparing. In addition, approximately 10% to 15% of the reported cases had a spontaneous pneumothorax, pneumomediastinum, and/or associated pleural effusions. Bronchoscopy specimens, such as bronchoalveolar lavage (BAL) and transbronchial biopsies (TBBx), were often but not always obtained. In patients who underwent bronchoscopy; many were found to have lipid-laden alveolar macrophages. These findings were discovered by staining fresh (ie, those not placed in fixative) specimens from BAL and/or TBBx for lipids with oil red O or another stain to specifically detect fat within the samples. Other etiologies of these radiographic/pathologic patterns and conditions should be excluded, as listed above.
The clinical course varies widely among these reported cases of vaping and e-cigarette-associated respiratory diseases. A minority of the reported patients spontaneously improved, and others required significant supportive care – from supplemental oxygen to complete support with ECMO. Some were treated with systemic corticosteroids with a wide range of responses and with various dosages: from prednisone of 0.5 to 1 mg/kg up to pulse-dose steroids with 1 g methylprednisolone for 3 days with a slow taper.
The information and data reported about these e-cigarette-associated respiratory diseases are clearly evolving quickly and vary from center to center and state to state. All suspected cases should be reported to your state health department. Similar to other inhalational injuries, it is critical to monitor these patients following recovery from the acute illness to help determine the long-term pulmonary effects and clinical courses of these individuals. Offering assistance and treatment for addiction is also important in these patients to help reduce their chances of recurrent respiratory problems from ongoing exposure to these substances in e-cigarettes. The bottom line is that cases of e-cigarette-associated respiratory diseases are increasing rapidly throughout the United States. Therefore, we should all be vigilant about asking our patients about their use of these substances and providing clear and strong messages for each of our patients to avoid vaping any substances through e-cigarettes.
Dr. Adams is Professor of Medicine, Pulmonary/Critical Care Division, Distinguished Teaching Professor, UT Health San Antonio; Staff Physician, South Texas Veterans Health Care System, San Antonio, Texas
References
Centers for Disease Control and Prevention. Severe pulmonary disease associated with using e-cigarette products. Health Alert Network. August 30, 2019. CDCHAN-00421. .
https://emergency.cdc.gov/han/han00421.asp
Centers for Disease Control and Prevention. Outbreak of lung illness associated with using e-cigarette products. Investigation Notice. September 6, 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
Henry TS et al. Imaging of vaping-associated lung disease. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMc1911995. [Epub ahead of print].
Layden JE et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin – preliminary report. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMoa1911614. [Epub ahead of print].
Maddock SD et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMc1912038. [Epub ahead of print].
E-cigarettes arrived in the U.S. market between 2005 and 2007. Vaping via e-cigarettes involves inhaling substances such as nicotine, flavorings, chemicals, and, sometimes, marijuana and/or other substances deep into the lungs. While the use of these devices is prevalent, the long-term effects are not known. We, as clinicians, need to specifically ask our patients about their use of substances via e-cigarettes because of alarming cases of severe, life-threatening respiratory illnesses recently being reported throughout the United States in young, otherwise healthy, individuals.
As of September 11, 2019, over 380 cases have been reported to the Centers for Disease Control and Prevention (CDC), where young, healthy people from 33 states and one US territory were hospitalized with severe respiratory disease. There have been at least six confirmed deaths and approximately one-third of those who survived required aggressive support with intubation and mechanical ventilation. The number of reported cases is rapidly rising (from 215 possible cases on August 27, 2019). The common theme in these cases is that every patient reported using an e-cigarette product within 90 days of the onset of symptoms, and most within the prior 2 weeks. By definition, other etiologies of respiratory failure, such as infections, collagen vascular, immunologic diseases, and malignancies were excluded.
Between 90% and 98% of patients presented to the hospital with respiratory symptoms, such as shortness of breath, cough, hemoptysis, and/or chest pain. The most common reported e-cigarette product exposure among these case patients is tetrahydrocannabinol, THC (in approximately 80% to 85%); however, some used only nicotine-based products (15% to 20%). In addition, approximately 45% to 50% reported using THC and nicotine-based products. One concerning fact that requires special attention is that some affected patients initially presented with nonrespiratory complaints, such as GI symptoms of nausea, vomiting, and/or diarrhea; constitutional symptoms such as fever (up to 104oF), fatigue, and/or weight loss; and neurologic symptoms such as headaches and even seizures. Many of these symptoms preceded the respiratory symptoms by up to 2 weeks. Therefore, a few of these patients initially presented without significant respiratory symptoms and with normal chest radiographs – but progressed over days to weeks to acute hypoxemic respiratory failure.
Up to 75% of the affected patients who ultimately required hospitalization for e-cigarette-associated respiratory disease initially presented to a primary care clinic or ED and were sent home due to nonspecific signs and symptoms, which mimic common viral illnesses. Therefore, it is critical for all health-care professionals to have a high clinical suspicion for e-cigarette-associated respiratory disease, particularly while more data are being gathered. When suspected, the CDC recommends asking patients about specific substances inhaled, the manufacturer, where the products/cartridges were obtained, type of device(s) used, and method used (ie, aerosolization, dabbing, dripping, etc).
The most common types of imaging and pathologic patterns attributed to e-cigarette use reported to date include lipoid pneumonia, diffuse alveolar damage, acute
respiratory distress syndrome (ARDS), diffuse alveolar hemorrhage (DAH), acute eosinophilic pneumonia, hypersensitivity pneumonitis, and organizing pneumonia. The most common patterns on imaging include basilar-predominant consolidation and ground-glass opacities with areas of subpleural sparing. In addition, approximately 10% to 15% of the reported cases had a spontaneous pneumothorax, pneumomediastinum, and/or associated pleural effusions. Bronchoscopy specimens, such as bronchoalveolar lavage (BAL) and transbronchial biopsies (TBBx), were often but not always obtained. In patients who underwent bronchoscopy; many were found to have lipid-laden alveolar macrophages. These findings were discovered by staining fresh (ie, those not placed in fixative) specimens from BAL and/or TBBx for lipids with oil red O or another stain to specifically detect fat within the samples. Other etiologies of these radiographic/pathologic patterns and conditions should be excluded, as listed above.
The clinical course varies widely among these reported cases of vaping and e-cigarette-associated respiratory diseases. A minority of the reported patients spontaneously improved, and others required significant supportive care – from supplemental oxygen to complete support with ECMO. Some were treated with systemic corticosteroids with a wide range of responses and with various dosages: from prednisone of 0.5 to 1 mg/kg up to pulse-dose steroids with 1 g methylprednisolone for 3 days with a slow taper.
The information and data reported about these e-cigarette-associated respiratory diseases are clearly evolving quickly and vary from center to center and state to state. All suspected cases should be reported to your state health department. Similar to other inhalational injuries, it is critical to monitor these patients following recovery from the acute illness to help determine the long-term pulmonary effects and clinical courses of these individuals. Offering assistance and treatment for addiction is also important in these patients to help reduce their chances of recurrent respiratory problems from ongoing exposure to these substances in e-cigarettes. The bottom line is that cases of e-cigarette-associated respiratory diseases are increasing rapidly throughout the United States. Therefore, we should all be vigilant about asking our patients about their use of these substances and providing clear and strong messages for each of our patients to avoid vaping any substances through e-cigarettes.
Dr. Adams is Professor of Medicine, Pulmonary/Critical Care Division, Distinguished Teaching Professor, UT Health San Antonio; Staff Physician, South Texas Veterans Health Care System, San Antonio, Texas
References
Centers for Disease Control and Prevention. Severe pulmonary disease associated with using e-cigarette products. Health Alert Network. August 30, 2019. CDCHAN-00421. .
https://emergency.cdc.gov/han/han00421.asp
Centers for Disease Control and Prevention. Outbreak of lung illness associated with using e-cigarette products. Investigation Notice. September 6, 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
Henry TS et al. Imaging of vaping-associated lung disease. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMc1911995. [Epub ahead of print].
Layden JE et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin – preliminary report. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMoa1911614. [Epub ahead of print].
Maddock SD et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMc1912038. [Epub ahead of print].
E-cigarettes arrived in the U.S. market between 2005 and 2007. Vaping via e-cigarettes involves inhaling substances such as nicotine, flavorings, chemicals, and, sometimes, marijuana and/or other substances deep into the lungs. While the use of these devices is prevalent, the long-term effects are not known. We, as clinicians, need to specifically ask our patients about their use of substances via e-cigarettes because of alarming cases of severe, life-threatening respiratory illnesses recently being reported throughout the United States in young, otherwise healthy, individuals.
As of September 11, 2019, over 380 cases have been reported to the Centers for Disease Control and Prevention (CDC), where young, healthy people from 33 states and one US territory were hospitalized with severe respiratory disease. There have been at least six confirmed deaths and approximately one-third of those who survived required aggressive support with intubation and mechanical ventilation. The number of reported cases is rapidly rising (from 215 possible cases on August 27, 2019). The common theme in these cases is that every patient reported using an e-cigarette product within 90 days of the onset of symptoms, and most within the prior 2 weeks. By definition, other etiologies of respiratory failure, such as infections, collagen vascular, immunologic diseases, and malignancies were excluded.
Between 90% and 98% of patients presented to the hospital with respiratory symptoms, such as shortness of breath, cough, hemoptysis, and/or chest pain. The most common reported e-cigarette product exposure among these case patients is tetrahydrocannabinol, THC (in approximately 80% to 85%); however, some used only nicotine-based products (15% to 20%). In addition, approximately 45% to 50% reported using THC and nicotine-based products. One concerning fact that requires special attention is that some affected patients initially presented with nonrespiratory complaints, such as GI symptoms of nausea, vomiting, and/or diarrhea; constitutional symptoms such as fever (up to 104oF), fatigue, and/or weight loss; and neurologic symptoms such as headaches and even seizures. Many of these symptoms preceded the respiratory symptoms by up to 2 weeks. Therefore, a few of these patients initially presented without significant respiratory symptoms and with normal chest radiographs – but progressed over days to weeks to acute hypoxemic respiratory failure.
Up to 75% of the affected patients who ultimately required hospitalization for e-cigarette-associated respiratory disease initially presented to a primary care clinic or ED and were sent home due to nonspecific signs and symptoms, which mimic common viral illnesses. Therefore, it is critical for all health-care professionals to have a high clinical suspicion for e-cigarette-associated respiratory disease, particularly while more data are being gathered. When suspected, the CDC recommends asking patients about specific substances inhaled, the manufacturer, where the products/cartridges were obtained, type of device(s) used, and method used (ie, aerosolization, dabbing, dripping, etc).
The most common types of imaging and pathologic patterns attributed to e-cigarette use reported to date include lipoid pneumonia, diffuse alveolar damage, acute
respiratory distress syndrome (ARDS), diffuse alveolar hemorrhage (DAH), acute eosinophilic pneumonia, hypersensitivity pneumonitis, and organizing pneumonia. The most common patterns on imaging include basilar-predominant consolidation and ground-glass opacities with areas of subpleural sparing. In addition, approximately 10% to 15% of the reported cases had a spontaneous pneumothorax, pneumomediastinum, and/or associated pleural effusions. Bronchoscopy specimens, such as bronchoalveolar lavage (BAL) and transbronchial biopsies (TBBx), were often but not always obtained. In patients who underwent bronchoscopy; many were found to have lipid-laden alveolar macrophages. These findings were discovered by staining fresh (ie, those not placed in fixative) specimens from BAL and/or TBBx for lipids with oil red O or another stain to specifically detect fat within the samples. Other etiologies of these radiographic/pathologic patterns and conditions should be excluded, as listed above.
The clinical course varies widely among these reported cases of vaping and e-cigarette-associated respiratory diseases. A minority of the reported patients spontaneously improved, and others required significant supportive care – from supplemental oxygen to complete support with ECMO. Some were treated with systemic corticosteroids with a wide range of responses and with various dosages: from prednisone of 0.5 to 1 mg/kg up to pulse-dose steroids with 1 g methylprednisolone for 3 days with a slow taper.
The information and data reported about these e-cigarette-associated respiratory diseases are clearly evolving quickly and vary from center to center and state to state. All suspected cases should be reported to your state health department. Similar to other inhalational injuries, it is critical to monitor these patients following recovery from the acute illness to help determine the long-term pulmonary effects and clinical courses of these individuals. Offering assistance and treatment for addiction is also important in these patients to help reduce their chances of recurrent respiratory problems from ongoing exposure to these substances in e-cigarettes. The bottom line is that cases of e-cigarette-associated respiratory diseases are increasing rapidly throughout the United States. Therefore, we should all be vigilant about asking our patients about their use of these substances and providing clear and strong messages for each of our patients to avoid vaping any substances through e-cigarettes.
Dr. Adams is Professor of Medicine, Pulmonary/Critical Care Division, Distinguished Teaching Professor, UT Health San Antonio; Staff Physician, South Texas Veterans Health Care System, San Antonio, Texas
References
Centers for Disease Control and Prevention. Severe pulmonary disease associated with using e-cigarette products. Health Alert Network. August 30, 2019. CDCHAN-00421. .
https://emergency.cdc.gov/han/han00421.asp
Centers for Disease Control and Prevention. Outbreak of lung illness associated with using e-cigarette products. Investigation Notice. September 6, 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
Henry TS et al. Imaging of vaping-associated lung disease. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMc1911995. [Epub ahead of print].
Layden JE et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin – preliminary report. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMoa1911614. [Epub ahead of print].
Maddock SD et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMc1912038. [Epub ahead of print].
Coding changes coming soon
There may be some positive changes coming to evaluation and management (E/M) services effective January 1, 2021. In the proposed calendar year 2020 Physician Fee Schedule (CY 2020 PFS), the Centers for Medicare & Medicaid Services (CMS) suggested a number of coding, payment, and documentation changes for office/outpatient E/M visits, Current Procedural Terminology (CPT®) codes 99201-99215. A summary of these changes include:
• Separate payment for the five levels of office/outpatient E/M visit CPT codes, as revised by the CPT Editorial Panel effective January 1, 2021. This would include deletion of CPT code 99201 (Level 1 new patient office/outpatient E/M visit) and adoption of the revised CPT code descriptors for CPT codes 99202-99215;
• Elimination of the use of history and/or physical exam to select among code levels;
• Choice of time or medical decision making to decide the level of office/outpatient E/M visit (using the revised CPT interpretive guidelines for medical decision making);
• Payment for prolonged office/outpatient E/M visits using the revised CPT code for such services, including separate payment for new CPT code 99XXX and deletion of Healthcare Common Procedure Coding System (HCPCS) code GPRO1 (extended office/outpatient E/M visit) that was previously finalized for 2021;
• Revise the descriptor for HCPCS code GPC1X and delete HCPCS code GCG0X; and
• Increase in value for HCPCS code GPC1X and allowing it to be reported with all office/outpatient E/M visit levels.
These changes were recommended by CMS to improve payment accuracy, reduce the administrative burden, and better reflect the current practice of medicine. These changes are predicted to result in a simplification of physician documentation and a redistribution of payments favoring providers who deliver primary care or care to more complex patients.
In CY 2019 PFS, CMS proposed to pay a single (blended) rate for office/outpatient visits 2-4, but due to comments from stakeholders, including specialty societies, CMS proposed to accept alternate recommendations by AMA/CPT. These recommendations include using medical decision making or time to determine the level of a visit, rather than the schema that was based on history and physical exam and outlined in the 1995/1997 guidelines. This resulted in elimination of CPT code 99201 and changes to the descriptors of 99202-99215. These codes were resurveyed by the Relative Value Update Committee (RUC) resulting in new values and times. (See Table 1).
One can see that there has been an incremental increase in time and value for most codes. When selecting a code based upon time, there is a range that is defined for each code,and additional information about the codes, including the descriptors and ranges, can be found on the AMA website https://www.ama-assn.org/cpt-evaluation-and-management.
For CPT codes 99205 and 99215 (level 5 codes), an add-on code has also been proposed that would account for additional time spent above the new levels defined in the codes. The descriptor for CPT 99XXX (the final numbers have not yet been assigned) reads Prolonged office or other outpatient evaluation and management service(s) (beyond the total time of the primary procedure which has been selected using total time), requiring total time with or without direct patient contact beyond the usual service, on the date of the primary service; each 15 minutes (List separately in addition to codes 99205, 99215 for office or other outpatient Evaluation and Management services). 99XXX is similar to CPT add-on code 99292 in that it may be used multiple times for a single encounter. This is illustrated in Table 2.
However, 99XXX is only used with level 5 codes. It will replace HCPCS code GPRO1, which had been finalized in the CY 2019 PFS. The proposed code will have a value of 0.61 RVU.
Finally, there is a proposal to revise the descriptor for HCPCS code GPC1X and eliminate HCPCS code GCG0X. The new descriptor for GPC1X Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex chronic condition. (Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established) is being updated to simplify the coding and, with the elimination of GCG0X, to remove the perception that the code is primary care or specialty specific. The value of GPC1X is also being increased to 0.33 RVU.
It must be made clear that these changes are proposals only, and CMS is still reviewing stakeholder and public comments. Any actual changes will not be codified until publication of the CY2020 PFS later this year. Additional information regarding the proposed rule can be found by accessing https://federalregister.gov/d/2019-16041.
There may be some positive changes coming to evaluation and management (E/M) services effective January 1, 2021. In the proposed calendar year 2020 Physician Fee Schedule (CY 2020 PFS), the Centers for Medicare & Medicaid Services (CMS) suggested a number of coding, payment, and documentation changes for office/outpatient E/M visits, Current Procedural Terminology (CPT®) codes 99201-99215. A summary of these changes include:
• Separate payment for the five levels of office/outpatient E/M visit CPT codes, as revised by the CPT Editorial Panel effective January 1, 2021. This would include deletion of CPT code 99201 (Level 1 new patient office/outpatient E/M visit) and adoption of the revised CPT code descriptors for CPT codes 99202-99215;
• Elimination of the use of history and/or physical exam to select among code levels;
• Choice of time or medical decision making to decide the level of office/outpatient E/M visit (using the revised CPT interpretive guidelines for medical decision making);
• Payment for prolonged office/outpatient E/M visits using the revised CPT code for such services, including separate payment for new CPT code 99XXX and deletion of Healthcare Common Procedure Coding System (HCPCS) code GPRO1 (extended office/outpatient E/M visit) that was previously finalized for 2021;
• Revise the descriptor for HCPCS code GPC1X and delete HCPCS code GCG0X; and
• Increase in value for HCPCS code GPC1X and allowing it to be reported with all office/outpatient E/M visit levels.
These changes were recommended by CMS to improve payment accuracy, reduce the administrative burden, and better reflect the current practice of medicine. These changes are predicted to result in a simplification of physician documentation and a redistribution of payments favoring providers who deliver primary care or care to more complex patients.
In CY 2019 PFS, CMS proposed to pay a single (blended) rate for office/outpatient visits 2-4, but due to comments from stakeholders, including specialty societies, CMS proposed to accept alternate recommendations by AMA/CPT. These recommendations include using medical decision making or time to determine the level of a visit, rather than the schema that was based on history and physical exam and outlined in the 1995/1997 guidelines. This resulted in elimination of CPT code 99201 and changes to the descriptors of 99202-99215. These codes were resurveyed by the Relative Value Update Committee (RUC) resulting in new values and times. (See Table 1).
One can see that there has been an incremental increase in time and value for most codes. When selecting a code based upon time, there is a range that is defined for each code,and additional information about the codes, including the descriptors and ranges, can be found on the AMA website https://www.ama-assn.org/cpt-evaluation-and-management.
For CPT codes 99205 and 99215 (level 5 codes), an add-on code has also been proposed that would account for additional time spent above the new levels defined in the codes. The descriptor for CPT 99XXX (the final numbers have not yet been assigned) reads Prolonged office or other outpatient evaluation and management service(s) (beyond the total time of the primary procedure which has been selected using total time), requiring total time with or without direct patient contact beyond the usual service, on the date of the primary service; each 15 minutes (List separately in addition to codes 99205, 99215 for office or other outpatient Evaluation and Management services). 99XXX is similar to CPT add-on code 99292 in that it may be used multiple times for a single encounter. This is illustrated in Table 2.
However, 99XXX is only used with level 5 codes. It will replace HCPCS code GPRO1, which had been finalized in the CY 2019 PFS. The proposed code will have a value of 0.61 RVU.
Finally, there is a proposal to revise the descriptor for HCPCS code GPC1X and eliminate HCPCS code GCG0X. The new descriptor for GPC1X Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex chronic condition. (Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established) is being updated to simplify the coding and, with the elimination of GCG0X, to remove the perception that the code is primary care or specialty specific. The value of GPC1X is also being increased to 0.33 RVU.
It must be made clear that these changes are proposals only, and CMS is still reviewing stakeholder and public comments. Any actual changes will not be codified until publication of the CY2020 PFS later this year. Additional information regarding the proposed rule can be found by accessing https://federalregister.gov/d/2019-16041.
There may be some positive changes coming to evaluation and management (E/M) services effective January 1, 2021. In the proposed calendar year 2020 Physician Fee Schedule (CY 2020 PFS), the Centers for Medicare & Medicaid Services (CMS) suggested a number of coding, payment, and documentation changes for office/outpatient E/M visits, Current Procedural Terminology (CPT®) codes 99201-99215. A summary of these changes include:
• Separate payment for the five levels of office/outpatient E/M visit CPT codes, as revised by the CPT Editorial Panel effective January 1, 2021. This would include deletion of CPT code 99201 (Level 1 new patient office/outpatient E/M visit) and adoption of the revised CPT code descriptors for CPT codes 99202-99215;
• Elimination of the use of history and/or physical exam to select among code levels;
• Choice of time or medical decision making to decide the level of office/outpatient E/M visit (using the revised CPT interpretive guidelines for medical decision making);
• Payment for prolonged office/outpatient E/M visits using the revised CPT code for such services, including separate payment for new CPT code 99XXX and deletion of Healthcare Common Procedure Coding System (HCPCS) code GPRO1 (extended office/outpatient E/M visit) that was previously finalized for 2021;
• Revise the descriptor for HCPCS code GPC1X and delete HCPCS code GCG0X; and
• Increase in value for HCPCS code GPC1X and allowing it to be reported with all office/outpatient E/M visit levels.
These changes were recommended by CMS to improve payment accuracy, reduce the administrative burden, and better reflect the current practice of medicine. These changes are predicted to result in a simplification of physician documentation and a redistribution of payments favoring providers who deliver primary care or care to more complex patients.
In CY 2019 PFS, CMS proposed to pay a single (blended) rate for office/outpatient visits 2-4, but due to comments from stakeholders, including specialty societies, CMS proposed to accept alternate recommendations by AMA/CPT. These recommendations include using medical decision making or time to determine the level of a visit, rather than the schema that was based on history and physical exam and outlined in the 1995/1997 guidelines. This resulted in elimination of CPT code 99201 and changes to the descriptors of 99202-99215. These codes were resurveyed by the Relative Value Update Committee (RUC) resulting in new values and times. (See Table 1).
One can see that there has been an incremental increase in time and value for most codes. When selecting a code based upon time, there is a range that is defined for each code,and additional information about the codes, including the descriptors and ranges, can be found on the AMA website https://www.ama-assn.org/cpt-evaluation-and-management.
For CPT codes 99205 and 99215 (level 5 codes), an add-on code has also been proposed that would account for additional time spent above the new levels defined in the codes. The descriptor for CPT 99XXX (the final numbers have not yet been assigned) reads Prolonged office or other outpatient evaluation and management service(s) (beyond the total time of the primary procedure which has been selected using total time), requiring total time with or without direct patient contact beyond the usual service, on the date of the primary service; each 15 minutes (List separately in addition to codes 99205, 99215 for office or other outpatient Evaluation and Management services). 99XXX is similar to CPT add-on code 99292 in that it may be used multiple times for a single encounter. This is illustrated in Table 2.
However, 99XXX is only used with level 5 codes. It will replace HCPCS code GPRO1, which had been finalized in the CY 2019 PFS. The proposed code will have a value of 0.61 RVU.
Finally, there is a proposal to revise the descriptor for HCPCS code GPC1X and eliminate HCPCS code GCG0X. The new descriptor for GPC1X Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious, or complex chronic condition. (Add-on code, list separately in addition to office/outpatient evaluation and management visit, new or established) is being updated to simplify the coding and, with the elimination of GCG0X, to remove the perception that the code is primary care or specialty specific. The value of GPC1X is also being increased to 0.33 RVU.
It must be made clear that these changes are proposals only, and CMS is still reviewing stakeholder and public comments. Any actual changes will not be codified until publication of the CY2020 PFS later this year. Additional information regarding the proposed rule can be found by accessing https://federalregister.gov/d/2019-16041.
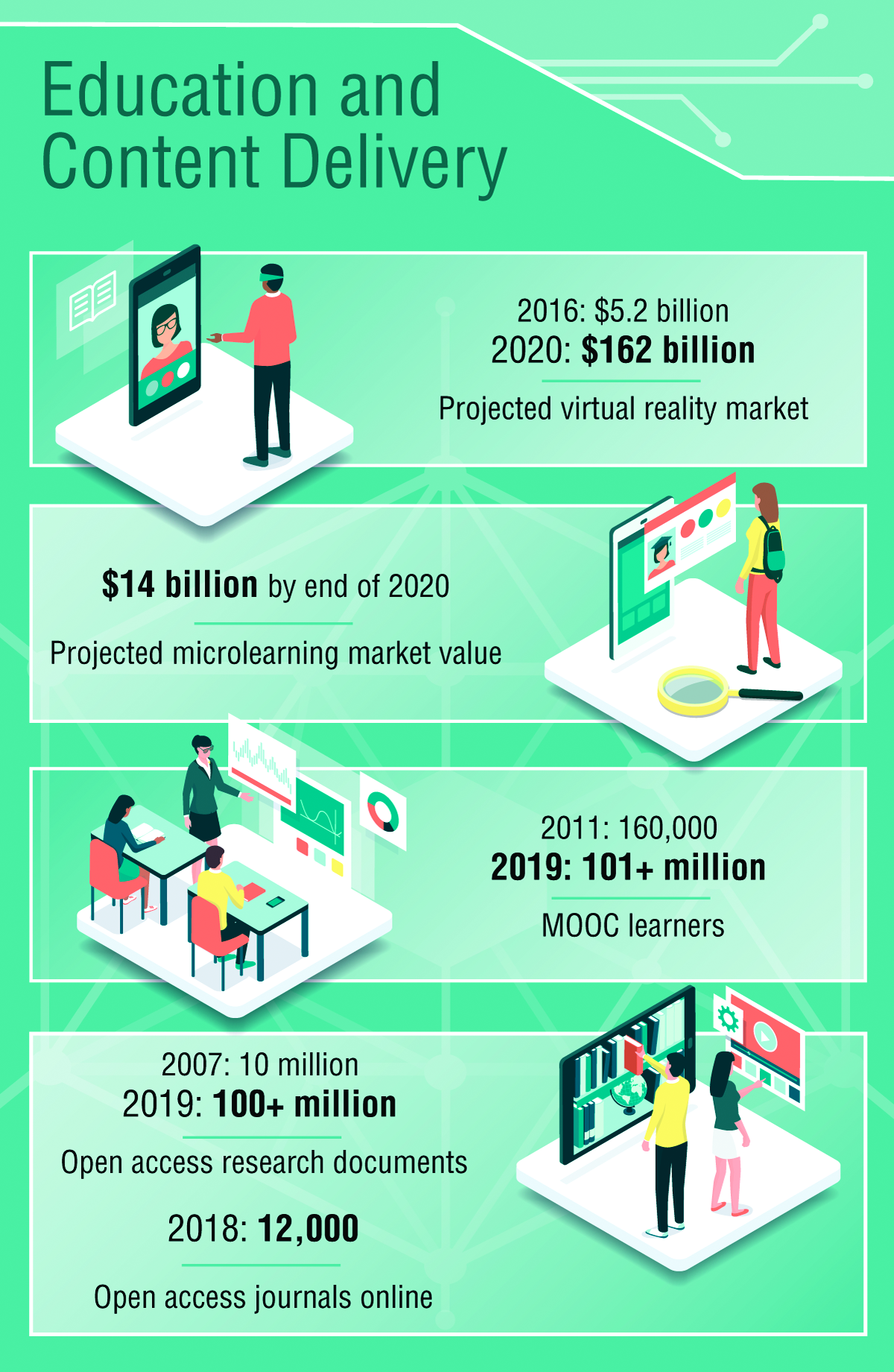
Environmental Scan: Drivers of change in education, content delivery, and career advancement
Keeping up to date and maintaining currency on developments in medicine are a routine part of medical practice, but the means by which this is accomplished are changing rapidly. Training, maintenance of certification, continuing education, mentoring, and career development will all be transformed in the coming years because of new technology and changing needs of physicians. Traditional learning channels such as print media and in-person courses will give way to options that emphasize ease of access, collaboration with fellow learners, and digitally optimized content.
Education and content delivery
The primary distribution channels for keeping medical professionals current in their specialty will continue to shift away from print publications and expand to digital outlets including podcasts, video, and online access to content.1 Individuals seeking to keep up professionally will increasingly turn to resources that can be found quickly and easily, for example, through voice search. Content that has been optimized to appear quickly and with a clear layout adapted to a wide variety of devices will most likely be consumed at a higher rate than resources from well-established organizations that have not transformed their continuing education content. There is already a growing demand for video and audiocasts accessible via mobile device.2
John D. Buckley, MD, FCCP, professor of medicine and vice chair for education at Indiana University, Indianapolis, sees the transformation of content delivery as a net plus for physicians, with a couple of caveats. He noted, “Whether it is conducting an in-depth literature search, reading/streaming a review lecture, or simply confirming a medical fact, quick access can enhance patient care and advance learning in a manner that meets an individual’s learning style. One potential downside is the risk of unreliable information, so accessing trustworthy sources is essential. Another potential downside is that, while accessing the answer to a very specific question can be done very easily, this might compromise additional learning of related material that used to occur when you had to read an entire book chapter to answer your question. Not only did you answer your question, you learned a lot of other relevant information along the way.”
Online learning is now a vast industry and has been harnessed by millions to further professional learning opportunities. Massive Open Online Courses (MOOCs) are free online courses available for anyone to enroll.3 MOOCs have been established at Harvard, MIT, Microsoft, and other top universities and institutions in subjects like computer science, data science, business, and more. MOOCs are being replicated in conventional universities and are projected to be a model for adult learning in the coming decade.4
Another trend is the growing interest in microlearning, defined as short educational activities that deal with relatively small learning units utilized at the point where the learner will actually need the information.5
Dr. Buckley sees potential in microlearning for continuing medical education. “It is unlikely that microlearning would be eligible for CME currently unless there were a mechanism for aggregating multiple events into a substantive unit of credit. But the ACCME [Accreditation Council for Continuing Medical Education] has been very adaptive to various forms of learning, so aggregate microlearning for CME credit may be possible in the future.” He added that the benefits of rapid and reliable access of specific information from a trusted source are significant, and the opportunities for microlearning for chest physicians are almost limitless. “Whether searching for the most updated review of a medical topic, or checking to see if your ICU patient’s sedating medication can cause serotonin syndrome, microlearning is already playing a large role in physician education, just less formal that what’s been used historically,” he said.
Institutions for which professional development learning modules are an important revenue stream will increasingly be challenged to compete with open-access courses of varying quality.
A key trend identified in 2018 is accelerating higher-education technology adoption and a growing focus on measured outcomes and learning.5 Individuals are interested in personalized learning plans and adaptive learning systems that can provide real-time assessments and immediate feedback. It is expected that learning modules and curricula will be most successful if they are easily accessed, attractively presented, and incorporate immediate feedback on learning progress. Driving technology adoption in higher education in the next 3-5 years will be the proliferation of open educational resources and the rise of new forms of interdisciplinary studies. As the environment for providing and accessing content shifts from pay-to-access to open-access, organizations will need to identify a new value proposition if they wish to grow or maintain related revenue streams.6
The implications of these changes in demand are profound for creators of continuing education content for medical professionals. Major investment will be needed in new, possibly costly platforms that deliver high-quality content with accessibility and interactive elements to meet the demands of professionals, the younger generation in particular.7 The market will continue to develop new technology to serve continuing education needs and preferences of users, thus fueling competition among stakeholders. With the proliferation of free and low-cost online and virtual programs, continuing education providers may experience a negative impact on an important revenue stream if they don’t identify a competitive advantage that meets the needs of tomorrow’s workforce. However, educational programs and courses that use artificial intelligence, virtual reality, and augmented reality to enhance the learning experience are likely to experience higher levels of use in the coming years.8
Workforce diversity and mentoring
A global economy requires organizations to seek a diverse workforce. Diversity can also lead to higher levels of profitability and employee satisfaction. As such, it will be essential for organizations to increase opportunities for individuals from diverse backgrounds to join the workforce. Creating a diverse workforce will mean removing barriers of time and location to skill building through online learning opportunities and facilitation of interdisciplinary career paths.
A critical piece of the emerging model of career development will be mentoring. Many professionals in today’s workforce view mentoring as an opportunity to gain immediate skills and knowledge quickly and effectively. Mentoring has evolved from pairing young professionals with seasoned veterans to creating relationships that match individuals with others who have the skills and knowledge they desire to learn about – regardless of age and experience. Institutions striving to develop a diverse workforce will need many individuals to serve as both mentors and mentees. When searching for solutions to work-related challenges, individuals will increasingly turn to knowledge management and collaboration systems (virtual mentoring) that provide them with the opportunity to match their needs in an efficient and effective manner.
Dr. Buckley values peer-to-peer mentoring as a means of accessing and sharing niche expertise among colleagues, but he acknowledges the difficulties in incorporating it into everyday practice. “The biggest obstacles are probably time and access. More and more learners and mentors are recognizing the tremendous value of effective mentorship, so convincing people is less of an issue than finding time,” he said.
Mentorship will continue to play a central role in the advancement of one’s career, yet women and minorities find it increasingly difficult to match with a mentor within the workplace. These candidates are likely to seek external opportunities. Individuals will evaluate the experience, opportunities for career advancement and the level of diversity and inclusion when seeking and accepting a new job.
Dr. Buckley sees both progress and remaining challenges in reducing barriers to underrepresented groups in medical institutions. “There continues to be a need for ongoing training to help individuals and institutions recognize and eliminate their barriers and biases, both conscious and subconscious, that interfere with achieving diversity and inclusion. Another important limitation is the pipeline of underrepresented groups that are pursuing careers in medicine. We need to do more empowerment, encouragement, and recruitment of underrepresented groups at a very early stage in their education if we ever expect to achieve our goals.”
Future challenges
The transformations described above will require a large investment by physicians aiming to maintain professional currency, by creators of continuing education content, and by employers seeking a diversified workforce. All these stakeholders have an interest in the future direction of continuing education and professional training. The development of new platforms for delivery of content that is easily accessible, formatted for a wide variety of devices, and built with real-time feedback functions will require a significant commitment of resources.
References
1. IDC Trackers. “Worldwide semiannual augmented and virtual reality spending guide.” Accessed Sept. 3, 2019.
2. ASAE. “Foresight Works: User’s Guide.” ASAE Foundation, 2018.
3. Online Course Report. “The State of MOOC 2016: A year of massive landscape change for massive open online courses.” Accessed Sept. 3, 2019.
4. Bill & Melinda Gates Foundation. “Postsecondary Success: Data and Information.” Accessed Sept. 4, 2019.
5. QYReports. “The Microlearning Market Report, 2018.” Accessed Sept. 4, 2019.
6. Adams S et al. “NMC Horizon Report: 2018 Higher Education Edition.” Louisville, CO: EDUCAUSE, 2018.
7. An M. “Content trends: Preferences emerge along generational fault lines.” Hubspot: Nov. 6, 2017; updated Dec 14, 2018.
8. Grajek S and Grama J. “Higher education’s 2018 trend watch and top 10 strategic technologies.” EDUCAUSE Review, Jan 29, 2018.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Keeping up to date and maintaining currency on developments in medicine are a routine part of medical practice, but the means by which this is accomplished are changing rapidly. Training, maintenance of certification, continuing education, mentoring, and career development will all be transformed in the coming years because of new technology and changing needs of physicians. Traditional learning channels such as print media and in-person courses will give way to options that emphasize ease of access, collaboration with fellow learners, and digitally optimized content.
Education and content delivery
The primary distribution channels for keeping medical professionals current in their specialty will continue to shift away from print publications and expand to digital outlets including podcasts, video, and online access to content.1 Individuals seeking to keep up professionally will increasingly turn to resources that can be found quickly and easily, for example, through voice search. Content that has been optimized to appear quickly and with a clear layout adapted to a wide variety of devices will most likely be consumed at a higher rate than resources from well-established organizations that have not transformed their continuing education content. There is already a growing demand for video and audiocasts accessible via mobile device.2
John D. Buckley, MD, FCCP, professor of medicine and vice chair for education at Indiana University, Indianapolis, sees the transformation of content delivery as a net plus for physicians, with a couple of caveats. He noted, “Whether it is conducting an in-depth literature search, reading/streaming a review lecture, or simply confirming a medical fact, quick access can enhance patient care and advance learning in a manner that meets an individual’s learning style. One potential downside is the risk of unreliable information, so accessing trustworthy sources is essential. Another potential downside is that, while accessing the answer to a very specific question can be done very easily, this might compromise additional learning of related material that used to occur when you had to read an entire book chapter to answer your question. Not only did you answer your question, you learned a lot of other relevant information along the way.”
Online learning is now a vast industry and has been harnessed by millions to further professional learning opportunities. Massive Open Online Courses (MOOCs) are free online courses available for anyone to enroll.3 MOOCs have been established at Harvard, MIT, Microsoft, and other top universities and institutions in subjects like computer science, data science, business, and more. MOOCs are being replicated in conventional universities and are projected to be a model for adult learning in the coming decade.4
Another trend is the growing interest in microlearning, defined as short educational activities that deal with relatively small learning units utilized at the point where the learner will actually need the information.5
Dr. Buckley sees potential in microlearning for continuing medical education. “It is unlikely that microlearning would be eligible for CME currently unless there were a mechanism for aggregating multiple events into a substantive unit of credit. But the ACCME [Accreditation Council for Continuing Medical Education] has been very adaptive to various forms of learning, so aggregate microlearning for CME credit may be possible in the future.” He added that the benefits of rapid and reliable access of specific information from a trusted source are significant, and the opportunities for microlearning for chest physicians are almost limitless. “Whether searching for the most updated review of a medical topic, or checking to see if your ICU patient’s sedating medication can cause serotonin syndrome, microlearning is already playing a large role in physician education, just less formal that what’s been used historically,” he said.
Institutions for which professional development learning modules are an important revenue stream will increasingly be challenged to compete with open-access courses of varying quality.
A key trend identified in 2018 is accelerating higher-education technology adoption and a growing focus on measured outcomes and learning.5 Individuals are interested in personalized learning plans and adaptive learning systems that can provide real-time assessments and immediate feedback. It is expected that learning modules and curricula will be most successful if they are easily accessed, attractively presented, and incorporate immediate feedback on learning progress. Driving technology adoption in higher education in the next 3-5 years will be the proliferation of open educational resources and the rise of new forms of interdisciplinary studies. As the environment for providing and accessing content shifts from pay-to-access to open-access, organizations will need to identify a new value proposition if they wish to grow or maintain related revenue streams.6
The implications of these changes in demand are profound for creators of continuing education content for medical professionals. Major investment will be needed in new, possibly costly platforms that deliver high-quality content with accessibility and interactive elements to meet the demands of professionals, the younger generation in particular.7 The market will continue to develop new technology to serve continuing education needs and preferences of users, thus fueling competition among stakeholders. With the proliferation of free and low-cost online and virtual programs, continuing education providers may experience a negative impact on an important revenue stream if they don’t identify a competitive advantage that meets the needs of tomorrow’s workforce. However, educational programs and courses that use artificial intelligence, virtual reality, and augmented reality to enhance the learning experience are likely to experience higher levels of use in the coming years.8
Workforce diversity and mentoring
A global economy requires organizations to seek a diverse workforce. Diversity can also lead to higher levels of profitability and employee satisfaction. As such, it will be essential for organizations to increase opportunities for individuals from diverse backgrounds to join the workforce. Creating a diverse workforce will mean removing barriers of time and location to skill building through online learning opportunities and facilitation of interdisciplinary career paths.
A critical piece of the emerging model of career development will be mentoring. Many professionals in today’s workforce view mentoring as an opportunity to gain immediate skills and knowledge quickly and effectively. Mentoring has evolved from pairing young professionals with seasoned veterans to creating relationships that match individuals with others who have the skills and knowledge they desire to learn about – regardless of age and experience. Institutions striving to develop a diverse workforce will need many individuals to serve as both mentors and mentees. When searching for solutions to work-related challenges, individuals will increasingly turn to knowledge management and collaboration systems (virtual mentoring) that provide them with the opportunity to match their needs in an efficient and effective manner.
Dr. Buckley values peer-to-peer mentoring as a means of accessing and sharing niche expertise among colleagues, but he acknowledges the difficulties in incorporating it into everyday practice. “The biggest obstacles are probably time and access. More and more learners and mentors are recognizing the tremendous value of effective mentorship, so convincing people is less of an issue than finding time,” he said.
Mentorship will continue to play a central role in the advancement of one’s career, yet women and minorities find it increasingly difficult to match with a mentor within the workplace. These candidates are likely to seek external opportunities. Individuals will evaluate the experience, opportunities for career advancement and the level of diversity and inclusion when seeking and accepting a new job.
Dr. Buckley sees both progress and remaining challenges in reducing barriers to underrepresented groups in medical institutions. “There continues to be a need for ongoing training to help individuals and institutions recognize and eliminate their barriers and biases, both conscious and subconscious, that interfere with achieving diversity and inclusion. Another important limitation is the pipeline of underrepresented groups that are pursuing careers in medicine. We need to do more empowerment, encouragement, and recruitment of underrepresented groups at a very early stage in their education if we ever expect to achieve our goals.”
Future challenges
The transformations described above will require a large investment by physicians aiming to maintain professional currency, by creators of continuing education content, and by employers seeking a diversified workforce. All these stakeholders have an interest in the future direction of continuing education and professional training. The development of new platforms for delivery of content that is easily accessible, formatted for a wide variety of devices, and built with real-time feedback functions will require a significant commitment of resources.
References
1. IDC Trackers. “Worldwide semiannual augmented and virtual reality spending guide.” Accessed Sept. 3, 2019.
2. ASAE. “Foresight Works: User’s Guide.” ASAE Foundation, 2018.
3. Online Course Report. “The State of MOOC 2016: A year of massive landscape change for massive open online courses.” Accessed Sept. 3, 2019.
4. Bill & Melinda Gates Foundation. “Postsecondary Success: Data and Information.” Accessed Sept. 4, 2019.
5. QYReports. “The Microlearning Market Report, 2018.” Accessed Sept. 4, 2019.
6. Adams S et al. “NMC Horizon Report: 2018 Higher Education Edition.” Louisville, CO: EDUCAUSE, 2018.
7. An M. “Content trends: Preferences emerge along generational fault lines.” Hubspot: Nov. 6, 2017; updated Dec 14, 2018.
8. Grajek S and Grama J. “Higher education’s 2018 trend watch and top 10 strategic technologies.” EDUCAUSE Review, Jan 29, 2018.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Keeping up to date and maintaining currency on developments in medicine are a routine part of medical practice, but the means by which this is accomplished are changing rapidly. Training, maintenance of certification, continuing education, mentoring, and career development will all be transformed in the coming years because of new technology and changing needs of physicians. Traditional learning channels such as print media and in-person courses will give way to options that emphasize ease of access, collaboration with fellow learners, and digitally optimized content.
Education and content delivery
The primary distribution channels for keeping medical professionals current in their specialty will continue to shift away from print publications and expand to digital outlets including podcasts, video, and online access to content.1 Individuals seeking to keep up professionally will increasingly turn to resources that can be found quickly and easily, for example, through voice search. Content that has been optimized to appear quickly and with a clear layout adapted to a wide variety of devices will most likely be consumed at a higher rate than resources from well-established organizations that have not transformed their continuing education content. There is already a growing demand for video and audiocasts accessible via mobile device.2
John D. Buckley, MD, FCCP, professor of medicine and vice chair for education at Indiana University, Indianapolis, sees the transformation of content delivery as a net plus for physicians, with a couple of caveats. He noted, “Whether it is conducting an in-depth literature search, reading/streaming a review lecture, or simply confirming a medical fact, quick access can enhance patient care and advance learning in a manner that meets an individual’s learning style. One potential downside is the risk of unreliable information, so accessing trustworthy sources is essential. Another potential downside is that, while accessing the answer to a very specific question can be done very easily, this might compromise additional learning of related material that used to occur when you had to read an entire book chapter to answer your question. Not only did you answer your question, you learned a lot of other relevant information along the way.”
Online learning is now a vast industry and has been harnessed by millions to further professional learning opportunities. Massive Open Online Courses (MOOCs) are free online courses available for anyone to enroll.3 MOOCs have been established at Harvard, MIT, Microsoft, and other top universities and institutions in subjects like computer science, data science, business, and more. MOOCs are being replicated in conventional universities and are projected to be a model for adult learning in the coming decade.4
Another trend is the growing interest in microlearning, defined as short educational activities that deal with relatively small learning units utilized at the point where the learner will actually need the information.5
Dr. Buckley sees potential in microlearning for continuing medical education. “It is unlikely that microlearning would be eligible for CME currently unless there were a mechanism for aggregating multiple events into a substantive unit of credit. But the ACCME [Accreditation Council for Continuing Medical Education] has been very adaptive to various forms of learning, so aggregate microlearning for CME credit may be possible in the future.” He added that the benefits of rapid and reliable access of specific information from a trusted source are significant, and the opportunities for microlearning for chest physicians are almost limitless. “Whether searching for the most updated review of a medical topic, or checking to see if your ICU patient’s sedating medication can cause serotonin syndrome, microlearning is already playing a large role in physician education, just less formal that what’s been used historically,” he said.
Institutions for which professional development learning modules are an important revenue stream will increasingly be challenged to compete with open-access courses of varying quality.
A key trend identified in 2018 is accelerating higher-education technology adoption and a growing focus on measured outcomes and learning.5 Individuals are interested in personalized learning plans and adaptive learning systems that can provide real-time assessments and immediate feedback. It is expected that learning modules and curricula will be most successful if they are easily accessed, attractively presented, and incorporate immediate feedback on learning progress. Driving technology adoption in higher education in the next 3-5 years will be the proliferation of open educational resources and the rise of new forms of interdisciplinary studies. As the environment for providing and accessing content shifts from pay-to-access to open-access, organizations will need to identify a new value proposition if they wish to grow or maintain related revenue streams.6
The implications of these changes in demand are profound for creators of continuing education content for medical professionals. Major investment will be needed in new, possibly costly platforms that deliver high-quality content with accessibility and interactive elements to meet the demands of professionals, the younger generation in particular.7 The market will continue to develop new technology to serve continuing education needs and preferences of users, thus fueling competition among stakeholders. With the proliferation of free and low-cost online and virtual programs, continuing education providers may experience a negative impact on an important revenue stream if they don’t identify a competitive advantage that meets the needs of tomorrow’s workforce. However, educational programs and courses that use artificial intelligence, virtual reality, and augmented reality to enhance the learning experience are likely to experience higher levels of use in the coming years.8
Workforce diversity and mentoring
A global economy requires organizations to seek a diverse workforce. Diversity can also lead to higher levels of profitability and employee satisfaction. As such, it will be essential for organizations to increase opportunities for individuals from diverse backgrounds to join the workforce. Creating a diverse workforce will mean removing barriers of time and location to skill building through online learning opportunities and facilitation of interdisciplinary career paths.
A critical piece of the emerging model of career development will be mentoring. Many professionals in today’s workforce view mentoring as an opportunity to gain immediate skills and knowledge quickly and effectively. Mentoring has evolved from pairing young professionals with seasoned veterans to creating relationships that match individuals with others who have the skills and knowledge they desire to learn about – regardless of age and experience. Institutions striving to develop a diverse workforce will need many individuals to serve as both mentors and mentees. When searching for solutions to work-related challenges, individuals will increasingly turn to knowledge management and collaboration systems (virtual mentoring) that provide them with the opportunity to match their needs in an efficient and effective manner.
Dr. Buckley values peer-to-peer mentoring as a means of accessing and sharing niche expertise among colleagues, but he acknowledges the difficulties in incorporating it into everyday practice. “The biggest obstacles are probably time and access. More and more learners and mentors are recognizing the tremendous value of effective mentorship, so convincing people is less of an issue than finding time,” he said.
Mentorship will continue to play a central role in the advancement of one’s career, yet women and minorities find it increasingly difficult to match with a mentor within the workplace. These candidates are likely to seek external opportunities. Individuals will evaluate the experience, opportunities for career advancement and the level of diversity and inclusion when seeking and accepting a new job.
Dr. Buckley sees both progress and remaining challenges in reducing barriers to underrepresented groups in medical institutions. “There continues to be a need for ongoing training to help individuals and institutions recognize and eliminate their barriers and biases, both conscious and subconscious, that interfere with achieving diversity and inclusion. Another important limitation is the pipeline of underrepresented groups that are pursuing careers in medicine. We need to do more empowerment, encouragement, and recruitment of underrepresented groups at a very early stage in their education if we ever expect to achieve our goals.”
Future challenges
The transformations described above will require a large investment by physicians aiming to maintain professional currency, by creators of continuing education content, and by employers seeking a diversified workforce. All these stakeholders have an interest in the future direction of continuing education and professional training. The development of new platforms for delivery of content that is easily accessible, formatted for a wide variety of devices, and built with real-time feedback functions will require a significant commitment of resources.
References
1. IDC Trackers. “Worldwide semiannual augmented and virtual reality spending guide.” Accessed Sept. 3, 2019.
2. ASAE. “Foresight Works: User’s Guide.” ASAE Foundation, 2018.
3. Online Course Report. “The State of MOOC 2016: A year of massive landscape change for massive open online courses.” Accessed Sept. 3, 2019.
4. Bill & Melinda Gates Foundation. “Postsecondary Success: Data and Information.” Accessed Sept. 4, 2019.
5. QYReports. “The Microlearning Market Report, 2018.” Accessed Sept. 4, 2019.
6. Adams S et al. “NMC Horizon Report: 2018 Higher Education Edition.” Louisville, CO: EDUCAUSE, 2018.
7. An M. “Content trends: Preferences emerge along generational fault lines.” Hubspot: Nov. 6, 2017; updated Dec 14, 2018.
8. Grajek S and Grama J. “Higher education’s 2018 trend watch and top 10 strategic technologies.” EDUCAUSE Review, Jan 29, 2018.
Note: Background research performed by Avenue M Group.
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: The CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
New practice guideline: CRC screening isn’t necessary for low-risk patients aged 50-75 years
Patients 50-79 years old with a demonstrably low risk of developing the disease within 15 years probably don’t need to be screened for colorectal cancer. But if their risk of disease is at least 3% over 15 years, patients should be screened, Lise M. Helsingen, MD, and colleagues wrote in BMJ (2019;367:l5515 doi: 10.1136/bmj.l5515).
For these patients, “We suggest screening with one of the four screening options: fecal immunochemical test (FIT) every year, FIT every 2 years, a single sigmoidoscopy, or a single colonoscopy,” wrote Dr. Helsingen of the University of Oslo, and her team.
She chaired a 22-member international panel that developed a collaborative effort from the MAGIC research and innovation program as a part of the BMJ Rapid Recommendations project. The team reviewed 12 research papers comprising almost 1.4 million patients from Denmark, Italy, the Netherlands, Norway, Poland, Spain, Sweden, the United Kingdom, and the United States. Follow-up ranged from 0 to 19.5 years for colorectal cancer incidence and up to 30 years for mortality.
Because of the dearth of relevant data in some studies, however, the projected outcomes had to be simulated, with benefits and harms calculations based on 100% screening adherence. However, the team noted, it’s impossible to achieve complete adherence. Most studies of colorectal screening don’t exceed a 50% adherence level.
“All the modeling data are of low certainty. It is a useful indication, but there is a high chance that new evidence will show a smaller or larger benefit, which in turn may alter these recommendations.”
Compared with no screening, all four screening models reduced the risk of colorectal cancer mortality to a similar level.
- FIT every year, 59%.
- FIT every 2 years, 50%.
- Single sigmoidoscopy, 52%.
- Single colonoscopy, 67%.
Screening had less of an impact on reducing the incidence of colorectal cancer:
- FIT every 2 years, 0.05%.
- FIT every year, 0.15%.
- Single sigmoidoscopy, 27%.
- Single colonoscopy, 34%.
The panel also assessed potential harms. Among almost 1 million patients, the colonoscopy-related mortality rate was 0.03 per 1,000 procedures. The perforation rate was 0.8 per 1,000 colonoscopies after a positive fecal test, and 1.4 per 1,000 screened with sigmoidoscopy. The bleeding rate was 1.9 per 1,000 colonoscopies performed after a positive fecal test, and 3-4 per 1,000 screened with sigmoidoscopy.
Successful implementation of these recommendations hinges on accurate risk assessment, however. The team recommended the QCancer platform as “one of the best performing models for both men and women.”
The calculator includes age, sex, ethnicity, smoking status, alcohol use, family history of gastrointestinal cancer, personal history of other cancers, diabetes, ulcerative colitis, colonic polyps, and body mass index.
“We suggest this model because it is available as an online calculator; includes only risk factors available in routine health care; has been validated in a population separate from the derivation population; has reasonable discriminatory ability; and has a good fit between predicted and observed outcomes. In addition, it is the only online risk calculator we know of that predicts risk over a 15-year time horizon.”
The team stressed that their recommendations can’t be applied to all patients. Because evidence for both screening recommendations was weak – largely because of the dearth of supporting data – patients and physicians should cocreate a personalized screening plan.
“Several factors influence individuals’ decisions whether to be screened, even when they are presented with the same information,” the authors said. These include variation in an individual’s values and preferences, a close balance of benefits versus harms and burdens, and personal preference.
“Some individuals may value a minimally invasive test such as FIT, and the possibility of invasive screening with colonoscopy might put them off screening altogether. Those who most value preventing colorectal cancer or avoiding repeated testing are likely to choose sigmoidoscopy or colonoscopy.”
The authors had no financial conflicts of interest.
SOURCE: BMJ 2019;367:l5515. doi: 10.1136/bmj.l5515.
There is compelling evidence that CRC screening of average-risk individuals is effective – screening with one of several modalities can reduce CRC incidence and mortality in average-risk individuals. Various guidelines throughout the world have recommended screening, usually beginning at age 50 years, in a one-size-fits-all manner. Despite our knowledge that different people have a different lifetime risk of CRC, no prior guidelines have suggested that risk stratification be built into the decision making.
All of the recommendations in this practice guideline are weak because they are derived from models that lack adequate precision. Nevertheless, the authors have proposed a new approach to CRC screening, similar to management plans for patients with cardiovascular disease. Before adopting such an approach, we need to be more comfortable with the precision of the risk estimates. These estimates, derived entirely from demographic and clinical information, may be enhanced by genomic data to achieve more precision. Further data on the willingness of the public to accept no screening if their risk is below a certain threshold needs to be evaluated. Despite these issues, the guideline presents a provocative approach which demands our attention.
David Lieberman, MD, AGAF, is professor of medicine and chief of the division of gastroenterology and hepatology, Oregon Health & Science University, Portland. He is Past President of the AGA Institute. He has no conflicts of interest.
There is compelling evidence that CRC screening of average-risk individuals is effective – screening with one of several modalities can reduce CRC incidence and mortality in average-risk individuals. Various guidelines throughout the world have recommended screening, usually beginning at age 50 years, in a one-size-fits-all manner. Despite our knowledge that different people have a different lifetime risk of CRC, no prior guidelines have suggested that risk stratification be built into the decision making.
All of the recommendations in this practice guideline are weak because they are derived from models that lack adequate precision. Nevertheless, the authors have proposed a new approach to CRC screening, similar to management plans for patients with cardiovascular disease. Before adopting such an approach, we need to be more comfortable with the precision of the risk estimates. These estimates, derived entirely from demographic and clinical information, may be enhanced by genomic data to achieve more precision. Further data on the willingness of the public to accept no screening if their risk is below a certain threshold needs to be evaluated. Despite these issues, the guideline presents a provocative approach which demands our attention.
David Lieberman, MD, AGAF, is professor of medicine and chief of the division of gastroenterology and hepatology, Oregon Health & Science University, Portland. He is Past President of the AGA Institute. He has no conflicts of interest.
There is compelling evidence that CRC screening of average-risk individuals is effective – screening with one of several modalities can reduce CRC incidence and mortality in average-risk individuals. Various guidelines throughout the world have recommended screening, usually beginning at age 50 years, in a one-size-fits-all manner. Despite our knowledge that different people have a different lifetime risk of CRC, no prior guidelines have suggested that risk stratification be built into the decision making.
All of the recommendations in this practice guideline are weak because they are derived from models that lack adequate precision. Nevertheless, the authors have proposed a new approach to CRC screening, similar to management plans for patients with cardiovascular disease. Before adopting such an approach, we need to be more comfortable with the precision of the risk estimates. These estimates, derived entirely from demographic and clinical information, may be enhanced by genomic data to achieve more precision. Further data on the willingness of the public to accept no screening if their risk is below a certain threshold needs to be evaluated. Despite these issues, the guideline presents a provocative approach which demands our attention.
David Lieberman, MD, AGAF, is professor of medicine and chief of the division of gastroenterology and hepatology, Oregon Health & Science University, Portland. He is Past President of the AGA Institute. He has no conflicts of interest.
Patients 50-79 years old with a demonstrably low risk of developing the disease within 15 years probably don’t need to be screened for colorectal cancer. But if their risk of disease is at least 3% over 15 years, patients should be screened, Lise M. Helsingen, MD, and colleagues wrote in BMJ (2019;367:l5515 doi: 10.1136/bmj.l5515).
For these patients, “We suggest screening with one of the four screening options: fecal immunochemical test (FIT) every year, FIT every 2 years, a single sigmoidoscopy, or a single colonoscopy,” wrote Dr. Helsingen of the University of Oslo, and her team.
She chaired a 22-member international panel that developed a collaborative effort from the MAGIC research and innovation program as a part of the BMJ Rapid Recommendations project. The team reviewed 12 research papers comprising almost 1.4 million patients from Denmark, Italy, the Netherlands, Norway, Poland, Spain, Sweden, the United Kingdom, and the United States. Follow-up ranged from 0 to 19.5 years for colorectal cancer incidence and up to 30 years for mortality.
Because of the dearth of relevant data in some studies, however, the projected outcomes had to be simulated, with benefits and harms calculations based on 100% screening adherence. However, the team noted, it’s impossible to achieve complete adherence. Most studies of colorectal screening don’t exceed a 50% adherence level.
“All the modeling data are of low certainty. It is a useful indication, but there is a high chance that new evidence will show a smaller or larger benefit, which in turn may alter these recommendations.”
Compared with no screening, all four screening models reduced the risk of colorectal cancer mortality to a similar level.
- FIT every year, 59%.
- FIT every 2 years, 50%.
- Single sigmoidoscopy, 52%.
- Single colonoscopy, 67%.
Screening had less of an impact on reducing the incidence of colorectal cancer:
- FIT every 2 years, 0.05%.
- FIT every year, 0.15%.
- Single sigmoidoscopy, 27%.
- Single colonoscopy, 34%.
The panel also assessed potential harms. Among almost 1 million patients, the colonoscopy-related mortality rate was 0.03 per 1,000 procedures. The perforation rate was 0.8 per 1,000 colonoscopies after a positive fecal test, and 1.4 per 1,000 screened with sigmoidoscopy. The bleeding rate was 1.9 per 1,000 colonoscopies performed after a positive fecal test, and 3-4 per 1,000 screened with sigmoidoscopy.
Successful implementation of these recommendations hinges on accurate risk assessment, however. The team recommended the QCancer platform as “one of the best performing models for both men and women.”
The calculator includes age, sex, ethnicity, smoking status, alcohol use, family history of gastrointestinal cancer, personal history of other cancers, diabetes, ulcerative colitis, colonic polyps, and body mass index.
“We suggest this model because it is available as an online calculator; includes only risk factors available in routine health care; has been validated in a population separate from the derivation population; has reasonable discriminatory ability; and has a good fit between predicted and observed outcomes. In addition, it is the only online risk calculator we know of that predicts risk over a 15-year time horizon.”
The team stressed that their recommendations can’t be applied to all patients. Because evidence for both screening recommendations was weak – largely because of the dearth of supporting data – patients and physicians should cocreate a personalized screening plan.
“Several factors influence individuals’ decisions whether to be screened, even when they are presented with the same information,” the authors said. These include variation in an individual’s values and preferences, a close balance of benefits versus harms and burdens, and personal preference.
“Some individuals may value a minimally invasive test such as FIT, and the possibility of invasive screening with colonoscopy might put them off screening altogether. Those who most value preventing colorectal cancer or avoiding repeated testing are likely to choose sigmoidoscopy or colonoscopy.”
The authors had no financial conflicts of interest.
SOURCE: BMJ 2019;367:l5515. doi: 10.1136/bmj.l5515.
Patients 50-79 years old with a demonstrably low risk of developing the disease within 15 years probably don’t need to be screened for colorectal cancer. But if their risk of disease is at least 3% over 15 years, patients should be screened, Lise M. Helsingen, MD, and colleagues wrote in BMJ (2019;367:l5515 doi: 10.1136/bmj.l5515).
For these patients, “We suggest screening with one of the four screening options: fecal immunochemical test (FIT) every year, FIT every 2 years, a single sigmoidoscopy, or a single colonoscopy,” wrote Dr. Helsingen of the University of Oslo, and her team.
She chaired a 22-member international panel that developed a collaborative effort from the MAGIC research and innovation program as a part of the BMJ Rapid Recommendations project. The team reviewed 12 research papers comprising almost 1.4 million patients from Denmark, Italy, the Netherlands, Norway, Poland, Spain, Sweden, the United Kingdom, and the United States. Follow-up ranged from 0 to 19.5 years for colorectal cancer incidence and up to 30 years for mortality.
Because of the dearth of relevant data in some studies, however, the projected outcomes had to be simulated, with benefits and harms calculations based on 100% screening adherence. However, the team noted, it’s impossible to achieve complete adherence. Most studies of colorectal screening don’t exceed a 50% adherence level.
“All the modeling data are of low certainty. It is a useful indication, but there is a high chance that new evidence will show a smaller or larger benefit, which in turn may alter these recommendations.”
Compared with no screening, all four screening models reduced the risk of colorectal cancer mortality to a similar level.
- FIT every year, 59%.
- FIT every 2 years, 50%.
- Single sigmoidoscopy, 52%.
- Single colonoscopy, 67%.
Screening had less of an impact on reducing the incidence of colorectal cancer:
- FIT every 2 years, 0.05%.
- FIT every year, 0.15%.
- Single sigmoidoscopy, 27%.
- Single colonoscopy, 34%.
The panel also assessed potential harms. Among almost 1 million patients, the colonoscopy-related mortality rate was 0.03 per 1,000 procedures. The perforation rate was 0.8 per 1,000 colonoscopies after a positive fecal test, and 1.4 per 1,000 screened with sigmoidoscopy. The bleeding rate was 1.9 per 1,000 colonoscopies performed after a positive fecal test, and 3-4 per 1,000 screened with sigmoidoscopy.
Successful implementation of these recommendations hinges on accurate risk assessment, however. The team recommended the QCancer platform as “one of the best performing models for both men and women.”
The calculator includes age, sex, ethnicity, smoking status, alcohol use, family history of gastrointestinal cancer, personal history of other cancers, diabetes, ulcerative colitis, colonic polyps, and body mass index.
“We suggest this model because it is available as an online calculator; includes only risk factors available in routine health care; has been validated in a population separate from the derivation population; has reasonable discriminatory ability; and has a good fit between predicted and observed outcomes. In addition, it is the only online risk calculator we know of that predicts risk over a 15-year time horizon.”
The team stressed that their recommendations can’t be applied to all patients. Because evidence for both screening recommendations was weak – largely because of the dearth of supporting data – patients and physicians should cocreate a personalized screening plan.
“Several factors influence individuals’ decisions whether to be screened, even when they are presented with the same information,” the authors said. These include variation in an individual’s values and preferences, a close balance of benefits versus harms and burdens, and personal preference.
“Some individuals may value a minimally invasive test such as FIT, and the possibility of invasive screening with colonoscopy might put them off screening altogether. Those who most value preventing colorectal cancer or avoiding repeated testing are likely to choose sigmoidoscopy or colonoscopy.”
The authors had no financial conflicts of interest.
SOURCE: BMJ 2019;367:l5515. doi: 10.1136/bmj.l5515.
FROM BMJ
An emerging role for physicians in health policy advocacy
The American Board of Internal Medicine has called for “a commitment to the promotion of public health and preventative medicine, as well as public advocacy on the part of each physician.”1 In our responsibility to preserve and promote human life, physicians are not only uniquely positioned for advocacy but also inherently assume the role of becoming health care activists.
The American Medical Association has defined physician advocacy as promoting “social, economic, educational, and political changes that ameliorate suffering and contribute to human well being.”2 For health care professionals, this translates into ensuring the concerns and best interests of patients are at the core of all decisions.3 For generations, physicians have taken extra steps for patient care in daily practice, including submitting prior authorizations, performing peer review, and taking part in family meetings. Many doctors also participate on hospital committees and boards for quality improvement measures and are leaders in designing strategies to improve patient safety and health care experiences. Although these examples may be viewed as a fundamental part of daily practice, in fact, these roles are consistent with advocacy on a local level. A significant number of physicians participate in medical education, research, and societal duties, which include formulating and reviewing guidelines for medical practice. Participation in conference organizing committees and reviewing medical journals are likewise not uncommon roles among medical practitioners. These efforts to provide education to improve patient care are also forms of advocacy on a national or regional level but often viewed as a standard in professionalism.4
It is on the federal and political level in advocacy where physician representation is critical. Health legislation is enacted by Congress and signed into law by the president of the United States.5 These laws can drastically affect clinical practice and patient care, especially in the realm of preventive medicine and pharmaceuticals. Gastroenterology is a unique field in which a large portion of practice is dedicated to cancer prevention, by screening age-appropriate individuals and monitoring high-risk patients. The field is rapidly expanding in the pharmaceutical area with new medications for inflammatory bowel disease and groundbreaking treatments for viral hepatitis. The breadth of practice in gastroenterology calls for antiquated laws to be changed to accommodate the development of patient care guidelines. With physicians representing less than 3% of Congress,6 the rules that govern our practice are largely left to those unfamiliar with the delivery of health care.
Lack of experience, limited time, and a tradition in medicine that prefers physicians to be apolitical are each contributing factors for reduced participation in federal advocacy.7 Professional GI societies, including the American Gastroenterological Association, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, have a presence in public policy to educate lawmakers and promote statutes in gastroenterology. The involvement of these organizations in legislation is critical since public policy directly affects the interests and well-being of patients.
The priority public policy issues for GI societies are listed as follows:
- Reducing the administrative burden of prior authorizations.
- Implementing timely appeals for non–first-line therapies as determined by payers (step therapy).
- Eliminating surprise billing and cost-sharing for screening colonoscopy.
- Preserving patient protections, including for preexisting conditions and preventive services.
- Increasing federal funding and research appropriations for gastrointestinal research.
Communication with and development of relationships with legislators are essential to effective advocacy.7,8 Health professionals should be well-informed resources for members of Congress and therefore it is pivotal to provide factual information when presenting topics. There are various ways to reach congressional representatives, including personal visits, writing letters, making phone calls, or attending town halls.
Of the aforementioned, in-person meetings are the best way to directly connect with legislators. These allow for time to discuss a legislative issue, including the background and societal impact, proposed initiative, and personal accounts relating to the topic. Attending town halls also will give face-time with legislators, although the format to ask questions often is abbreviated. GI societies use letter writing as a way to increase support for a proposed bill or measure. The efficacy of letter writing increases with higher involvement. Letters are often generated in an online forum that requires the user’s zip code (so the letter can be routed to the appropriate legislator) and name with electronic signature, which are designed for easy use to boost participation.
Understanding that physicians are advocates in daily practice and that federal initiatives have significant impact on patients and clinical practice is the first step to getting involved. Participation at the local level includes connecting with the district offices of congressional leaders through letter writing, making phone calls, or in-person visits. On regional and national levels, involvement with state legislators, GI societies, or personal like-minded groups are ways to initiate federal advocacy. GI societies have federal policy committees, political action committees, and opportunities for early-career gastroenterologists to become involved in advocacy, including the Congressional Advocates Program from the AGA and the Young Physician Leadership Scholars Program from the ACG. Be sure to visit AGA’s Advocacy & Policy page to keep informed about current and future opportunities.
As the population grows and human life expectancy increases, the practice of medicine is a prime target for legislative changes, which ultimately affect patient care and clinical practice. Physicians are respected members of society, have expansive knowledge in disease processes and the delivery of health care to patients, and are naturally patient advocates. For these reasons, it is imperative for doctors to rise to the calling of federal advocacy, to continue to preserve the best interests and dignity of our patients.
References
1. ABIM Foundation. Ann Intern Med. 2002;136:243-6.
2. Earnest MA et al. Academic Med. 2010;85(1):63-7.
3. Schwartz L. J Med Ethics. 2002;28:37-40.
4. Howell BA et al. J Gen Intern Med. 2019 Aug 5. https://doi.org/10.1007/s11606-019-05184-3. [epub ahead of print]
5. The House of Representatives.
6. AGA News: https://www.gastro.org/news/new-congress-includes-22-health-care-providers
7. Kupfer SS et al. Gastroenterology. 2019;156(4)8:834-7.
8. Grace ND and LB Dennis. Hepatology. 2007;45(6):1337-9.
Dr. Abbasi is a gastroenterologist who works in inflammatory bowel diseases at Cedars-Sinai Medical Center and Santa Monica Gastroenterology, Calif.
The American Board of Internal Medicine has called for “a commitment to the promotion of public health and preventative medicine, as well as public advocacy on the part of each physician.”1 In our responsibility to preserve and promote human life, physicians are not only uniquely positioned for advocacy but also inherently assume the role of becoming health care activists.
The American Medical Association has defined physician advocacy as promoting “social, economic, educational, and political changes that ameliorate suffering and contribute to human well being.”2 For health care professionals, this translates into ensuring the concerns and best interests of patients are at the core of all decisions.3 For generations, physicians have taken extra steps for patient care in daily practice, including submitting prior authorizations, performing peer review, and taking part in family meetings. Many doctors also participate on hospital committees and boards for quality improvement measures and are leaders in designing strategies to improve patient safety and health care experiences. Although these examples may be viewed as a fundamental part of daily practice, in fact, these roles are consistent with advocacy on a local level. A significant number of physicians participate in medical education, research, and societal duties, which include formulating and reviewing guidelines for medical practice. Participation in conference organizing committees and reviewing medical journals are likewise not uncommon roles among medical practitioners. These efforts to provide education to improve patient care are also forms of advocacy on a national or regional level but often viewed as a standard in professionalism.4
It is on the federal and political level in advocacy where physician representation is critical. Health legislation is enacted by Congress and signed into law by the president of the United States.5 These laws can drastically affect clinical practice and patient care, especially in the realm of preventive medicine and pharmaceuticals. Gastroenterology is a unique field in which a large portion of practice is dedicated to cancer prevention, by screening age-appropriate individuals and monitoring high-risk patients. The field is rapidly expanding in the pharmaceutical area with new medications for inflammatory bowel disease and groundbreaking treatments for viral hepatitis. The breadth of practice in gastroenterology calls for antiquated laws to be changed to accommodate the development of patient care guidelines. With physicians representing less than 3% of Congress,6 the rules that govern our practice are largely left to those unfamiliar with the delivery of health care.
Lack of experience, limited time, and a tradition in medicine that prefers physicians to be apolitical are each contributing factors for reduced participation in federal advocacy.7 Professional GI societies, including the American Gastroenterological Association, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, have a presence in public policy to educate lawmakers and promote statutes in gastroenterology. The involvement of these organizations in legislation is critical since public policy directly affects the interests and well-being of patients.
The priority public policy issues for GI societies are listed as follows:
- Reducing the administrative burden of prior authorizations.
- Implementing timely appeals for non–first-line therapies as determined by payers (step therapy).
- Eliminating surprise billing and cost-sharing for screening colonoscopy.
- Preserving patient protections, including for preexisting conditions and preventive services.
- Increasing federal funding and research appropriations for gastrointestinal research.
Communication with and development of relationships with legislators are essential to effective advocacy.7,8 Health professionals should be well-informed resources for members of Congress and therefore it is pivotal to provide factual information when presenting topics. There are various ways to reach congressional representatives, including personal visits, writing letters, making phone calls, or attending town halls.
Of the aforementioned, in-person meetings are the best way to directly connect with legislators. These allow for time to discuss a legislative issue, including the background and societal impact, proposed initiative, and personal accounts relating to the topic. Attending town halls also will give face-time with legislators, although the format to ask questions often is abbreviated. GI societies use letter writing as a way to increase support for a proposed bill or measure. The efficacy of letter writing increases with higher involvement. Letters are often generated in an online forum that requires the user’s zip code (so the letter can be routed to the appropriate legislator) and name with electronic signature, which are designed for easy use to boost participation.
Understanding that physicians are advocates in daily practice and that federal initiatives have significant impact on patients and clinical practice is the first step to getting involved. Participation at the local level includes connecting with the district offices of congressional leaders through letter writing, making phone calls, or in-person visits. On regional and national levels, involvement with state legislators, GI societies, or personal like-minded groups are ways to initiate federal advocacy. GI societies have federal policy committees, political action committees, and opportunities for early-career gastroenterologists to become involved in advocacy, including the Congressional Advocates Program from the AGA and the Young Physician Leadership Scholars Program from the ACG. Be sure to visit AGA’s Advocacy & Policy page to keep informed about current and future opportunities.
As the population grows and human life expectancy increases, the practice of medicine is a prime target for legislative changes, which ultimately affect patient care and clinical practice. Physicians are respected members of society, have expansive knowledge in disease processes and the delivery of health care to patients, and are naturally patient advocates. For these reasons, it is imperative for doctors to rise to the calling of federal advocacy, to continue to preserve the best interests and dignity of our patients.
References
1. ABIM Foundation. Ann Intern Med. 2002;136:243-6.
2. Earnest MA et al. Academic Med. 2010;85(1):63-7.
3. Schwartz L. J Med Ethics. 2002;28:37-40.
4. Howell BA et al. J Gen Intern Med. 2019 Aug 5. https://doi.org/10.1007/s11606-019-05184-3. [epub ahead of print]
5. The House of Representatives.
6. AGA News: https://www.gastro.org/news/new-congress-includes-22-health-care-providers
7. Kupfer SS et al. Gastroenterology. 2019;156(4)8:834-7.
8. Grace ND and LB Dennis. Hepatology. 2007;45(6):1337-9.
Dr. Abbasi is a gastroenterologist who works in inflammatory bowel diseases at Cedars-Sinai Medical Center and Santa Monica Gastroenterology, Calif.
The American Board of Internal Medicine has called for “a commitment to the promotion of public health and preventative medicine, as well as public advocacy on the part of each physician.”1 In our responsibility to preserve and promote human life, physicians are not only uniquely positioned for advocacy but also inherently assume the role of becoming health care activists.
The American Medical Association has defined physician advocacy as promoting “social, economic, educational, and political changes that ameliorate suffering and contribute to human well being.”2 For health care professionals, this translates into ensuring the concerns and best interests of patients are at the core of all decisions.3 For generations, physicians have taken extra steps for patient care in daily practice, including submitting prior authorizations, performing peer review, and taking part in family meetings. Many doctors also participate on hospital committees and boards for quality improvement measures and are leaders in designing strategies to improve patient safety and health care experiences. Although these examples may be viewed as a fundamental part of daily practice, in fact, these roles are consistent with advocacy on a local level. A significant number of physicians participate in medical education, research, and societal duties, which include formulating and reviewing guidelines for medical practice. Participation in conference organizing committees and reviewing medical journals are likewise not uncommon roles among medical practitioners. These efforts to provide education to improve patient care are also forms of advocacy on a national or regional level but often viewed as a standard in professionalism.4
It is on the federal and political level in advocacy where physician representation is critical. Health legislation is enacted by Congress and signed into law by the president of the United States.5 These laws can drastically affect clinical practice and patient care, especially in the realm of preventive medicine and pharmaceuticals. Gastroenterology is a unique field in which a large portion of practice is dedicated to cancer prevention, by screening age-appropriate individuals and monitoring high-risk patients. The field is rapidly expanding in the pharmaceutical area with new medications for inflammatory bowel disease and groundbreaking treatments for viral hepatitis. The breadth of practice in gastroenterology calls for antiquated laws to be changed to accommodate the development of patient care guidelines. With physicians representing less than 3% of Congress,6 the rules that govern our practice are largely left to those unfamiliar with the delivery of health care.
Lack of experience, limited time, and a tradition in medicine that prefers physicians to be apolitical are each contributing factors for reduced participation in federal advocacy.7 Professional GI societies, including the American Gastroenterological Association, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, have a presence in public policy to educate lawmakers and promote statutes in gastroenterology. The involvement of these organizations in legislation is critical since public policy directly affects the interests and well-being of patients.
The priority public policy issues for GI societies are listed as follows:
- Reducing the administrative burden of prior authorizations.
- Implementing timely appeals for non–first-line therapies as determined by payers (step therapy).
- Eliminating surprise billing and cost-sharing for screening colonoscopy.
- Preserving patient protections, including for preexisting conditions and preventive services.
- Increasing federal funding and research appropriations for gastrointestinal research.
Communication with and development of relationships with legislators are essential to effective advocacy.7,8 Health professionals should be well-informed resources for members of Congress and therefore it is pivotal to provide factual information when presenting topics. There are various ways to reach congressional representatives, including personal visits, writing letters, making phone calls, or attending town halls.
Of the aforementioned, in-person meetings are the best way to directly connect with legislators. These allow for time to discuss a legislative issue, including the background and societal impact, proposed initiative, and personal accounts relating to the topic. Attending town halls also will give face-time with legislators, although the format to ask questions often is abbreviated. GI societies use letter writing as a way to increase support for a proposed bill or measure. The efficacy of letter writing increases with higher involvement. Letters are often generated in an online forum that requires the user’s zip code (so the letter can be routed to the appropriate legislator) and name with electronic signature, which are designed for easy use to boost participation.
Understanding that physicians are advocates in daily practice and that federal initiatives have significant impact on patients and clinical practice is the first step to getting involved. Participation at the local level includes connecting with the district offices of congressional leaders through letter writing, making phone calls, or in-person visits. On regional and national levels, involvement with state legislators, GI societies, or personal like-minded groups are ways to initiate federal advocacy. GI societies have federal policy committees, political action committees, and opportunities for early-career gastroenterologists to become involved in advocacy, including the Congressional Advocates Program from the AGA and the Young Physician Leadership Scholars Program from the ACG. Be sure to visit AGA’s Advocacy & Policy page to keep informed about current and future opportunities.
As the population grows and human life expectancy increases, the practice of medicine is a prime target for legislative changes, which ultimately affect patient care and clinical practice. Physicians are respected members of society, have expansive knowledge in disease processes and the delivery of health care to patients, and are naturally patient advocates. For these reasons, it is imperative for doctors to rise to the calling of federal advocacy, to continue to preserve the best interests and dignity of our patients.
References
1. ABIM Foundation. Ann Intern Med. 2002;136:243-6.
2. Earnest MA et al. Academic Med. 2010;85(1):63-7.
3. Schwartz L. J Med Ethics. 2002;28:37-40.
4. Howell BA et al. J Gen Intern Med. 2019 Aug 5. https://doi.org/10.1007/s11606-019-05184-3. [epub ahead of print]
5. The House of Representatives.
6. AGA News: https://www.gastro.org/news/new-congress-includes-22-health-care-providers
7. Kupfer SS et al. Gastroenterology. 2019;156(4)8:834-7.
8. Grace ND and LB Dennis. Hepatology. 2007;45(6):1337-9.
Dr. Abbasi is a gastroenterologist who works in inflammatory bowel diseases at Cedars-Sinai Medical Center and Santa Monica Gastroenterology, Calif.
Follow-up shows favorable results with acalabrutinib in MCL
Acalabrutinib monotherapy can produce durable responses in relapsed/refractory mantle cell lymphoma (MCL), according to updated results from a phase 2 trial.
The drug produced an overall response rate (ORR) of 81%, and the median duration of response was 26 months.
These are the highest such figures reported “among all approved single-agent therapies for the treatment of relapsed/refractory MCL,” Michael Wang, MD, of the MD Anderson Cancer Center at the University of Texas in Houston and colleagues wrote in a letter in Leukemia.
Dr. Wang and colleagues reported updated results in 124 patients treated on the ACE-LY-004 trial. At baseline, the patients had a median age of 68 years (range, 42-90 years), and 80% were men. Three-quarters of patients had stage IV disease, 72% had extranodal disease, 21% had blastoid/pleomorphic MCL, and 26% had a Ki-67 proliferation index of 50% or greater.
At a median follow-up of 26 months, 40% (n = 49) of patients were still on acalabrutinib, and 61% (n = 76) were still in follow-up for survival. Six patients went on to allogeneic transplant at a median of 19 days after stopping acalabrutinib.
The ORR was 81% (100/124), and the complete response (CR) rate was 43% (n = 53). Four patients who initially had a partial response converted to a CR with longer follow-up. The estimated 24-month duration of response was 52.4%.
“ORR was consistent across patients with refractory disease and those with blastoid/pleomorphic MCL, despite those patients having a higher mean Ki-67 index [of 50% or greater], suggesting that some patients with poorer prognosis may also benefit from acalabrutinib,” Dr. Wang and colleagues wrote.
There were 29 patients evaluable for minimal residual disease (MRD) assessment. Seven patients (24%) had MRD-negative disease in the peripheral blood after they achieved a CR. An additional patient with a CR became MRD negative when a second blood sample was taken about 6 months after the first.
“Despite limited samples, these results demonstrate that continued use of acalabrutinib can lead to undetectable MRD in patients with CR,” Dr. Wang and his colleagues wrote. “Since most patients with MRD data are still on treatment (27/29), relationships between MRD negativity and durability of response cannot be made at this time.”
The median progression-free survival was 20 months, and the median overall survival was not reached. The estimated 24-month progression-free survival rate was 49.0%, and the estimated 24-month overall survival rate was 72.4%. Patients with low/intermediate Mantle Cell Lymphoma International Prognostic Index scores, classical MCL, and a Ki-67 index less than 50% had a longer duration of response and survival.
The adverse event profile was “largely consistent with earlier reporting,” Dr. Wang and colleagues wrote. The most frequent adverse events were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). The most common grade 3/4 adverse events were anemia (10%), neutropenia (10%), and pneumonia (6%).
Ten patients developed second primary cancers. There were no new atrial fibrillation events and no new hypertension events. The frequency of infections decreased over time, as did the number of bleeding events. However, two of three major hemorrhage events occurred after the previous report was published.
There were 43 deaths (35%), 29 of them because of disease progression. Six patients died of adverse events, two died of unknown causes, and two died of secondary acute myeloid leukemia. Other causes of death included multiorgan failure, intestinal obstruction, lung cancer, and graft-versus-host disease.
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The researchers reported relationships with AstraZeneca/Acerta Pharma and many other companies.
SOURCE: Wang M et al. Leukemia. 2019 Sep 26. doi: 10.1038/s41375-019-0575-9.
Acalabrutinib monotherapy can produce durable responses in relapsed/refractory mantle cell lymphoma (MCL), according to updated results from a phase 2 trial.
The drug produced an overall response rate (ORR) of 81%, and the median duration of response was 26 months.
These are the highest such figures reported “among all approved single-agent therapies for the treatment of relapsed/refractory MCL,” Michael Wang, MD, of the MD Anderson Cancer Center at the University of Texas in Houston and colleagues wrote in a letter in Leukemia.
Dr. Wang and colleagues reported updated results in 124 patients treated on the ACE-LY-004 trial. At baseline, the patients had a median age of 68 years (range, 42-90 years), and 80% were men. Three-quarters of patients had stage IV disease, 72% had extranodal disease, 21% had blastoid/pleomorphic MCL, and 26% had a Ki-67 proliferation index of 50% or greater.
At a median follow-up of 26 months, 40% (n = 49) of patients were still on acalabrutinib, and 61% (n = 76) were still in follow-up for survival. Six patients went on to allogeneic transplant at a median of 19 days after stopping acalabrutinib.
The ORR was 81% (100/124), and the complete response (CR) rate was 43% (n = 53). Four patients who initially had a partial response converted to a CR with longer follow-up. The estimated 24-month duration of response was 52.4%.
“ORR was consistent across patients with refractory disease and those with blastoid/pleomorphic MCL, despite those patients having a higher mean Ki-67 index [of 50% or greater], suggesting that some patients with poorer prognosis may also benefit from acalabrutinib,” Dr. Wang and colleagues wrote.
There were 29 patients evaluable for minimal residual disease (MRD) assessment. Seven patients (24%) had MRD-negative disease in the peripheral blood after they achieved a CR. An additional patient with a CR became MRD negative when a second blood sample was taken about 6 months after the first.
“Despite limited samples, these results demonstrate that continued use of acalabrutinib can lead to undetectable MRD in patients with CR,” Dr. Wang and his colleagues wrote. “Since most patients with MRD data are still on treatment (27/29), relationships between MRD negativity and durability of response cannot be made at this time.”
The median progression-free survival was 20 months, and the median overall survival was not reached. The estimated 24-month progression-free survival rate was 49.0%, and the estimated 24-month overall survival rate was 72.4%. Patients with low/intermediate Mantle Cell Lymphoma International Prognostic Index scores, classical MCL, and a Ki-67 index less than 50% had a longer duration of response and survival.
The adverse event profile was “largely consistent with earlier reporting,” Dr. Wang and colleagues wrote. The most frequent adverse events were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). The most common grade 3/4 adverse events were anemia (10%), neutropenia (10%), and pneumonia (6%).
Ten patients developed second primary cancers. There were no new atrial fibrillation events and no new hypertension events. The frequency of infections decreased over time, as did the number of bleeding events. However, two of three major hemorrhage events occurred after the previous report was published.
There were 43 deaths (35%), 29 of them because of disease progression. Six patients died of adverse events, two died of unknown causes, and two died of secondary acute myeloid leukemia. Other causes of death included multiorgan failure, intestinal obstruction, lung cancer, and graft-versus-host disease.
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The researchers reported relationships with AstraZeneca/Acerta Pharma and many other companies.
SOURCE: Wang M et al. Leukemia. 2019 Sep 26. doi: 10.1038/s41375-019-0575-9.
Acalabrutinib monotherapy can produce durable responses in relapsed/refractory mantle cell lymphoma (MCL), according to updated results from a phase 2 trial.
The drug produced an overall response rate (ORR) of 81%, and the median duration of response was 26 months.
These are the highest such figures reported “among all approved single-agent therapies for the treatment of relapsed/refractory MCL,” Michael Wang, MD, of the MD Anderson Cancer Center at the University of Texas in Houston and colleagues wrote in a letter in Leukemia.
Dr. Wang and colleagues reported updated results in 124 patients treated on the ACE-LY-004 trial. At baseline, the patients had a median age of 68 years (range, 42-90 years), and 80% were men. Three-quarters of patients had stage IV disease, 72% had extranodal disease, 21% had blastoid/pleomorphic MCL, and 26% had a Ki-67 proliferation index of 50% or greater.
At a median follow-up of 26 months, 40% (n = 49) of patients were still on acalabrutinib, and 61% (n = 76) were still in follow-up for survival. Six patients went on to allogeneic transplant at a median of 19 days after stopping acalabrutinib.
The ORR was 81% (100/124), and the complete response (CR) rate was 43% (n = 53). Four patients who initially had a partial response converted to a CR with longer follow-up. The estimated 24-month duration of response was 52.4%.
“ORR was consistent across patients with refractory disease and those with blastoid/pleomorphic MCL, despite those patients having a higher mean Ki-67 index [of 50% or greater], suggesting that some patients with poorer prognosis may also benefit from acalabrutinib,” Dr. Wang and colleagues wrote.
There were 29 patients evaluable for minimal residual disease (MRD) assessment. Seven patients (24%) had MRD-negative disease in the peripheral blood after they achieved a CR. An additional patient with a CR became MRD negative when a second blood sample was taken about 6 months after the first.
“Despite limited samples, these results demonstrate that continued use of acalabrutinib can lead to undetectable MRD in patients with CR,” Dr. Wang and his colleagues wrote. “Since most patients with MRD data are still on treatment (27/29), relationships between MRD negativity and durability of response cannot be made at this time.”
The median progression-free survival was 20 months, and the median overall survival was not reached. The estimated 24-month progression-free survival rate was 49.0%, and the estimated 24-month overall survival rate was 72.4%. Patients with low/intermediate Mantle Cell Lymphoma International Prognostic Index scores, classical MCL, and a Ki-67 index less than 50% had a longer duration of response and survival.
The adverse event profile was “largely consistent with earlier reporting,” Dr. Wang and colleagues wrote. The most frequent adverse events were headache (38%), diarrhea (36%), fatigue (28%), cough (22%), and myalgia (21%). The most common grade 3/4 adverse events were anemia (10%), neutropenia (10%), and pneumonia (6%).
Ten patients developed second primary cancers. There were no new atrial fibrillation events and no new hypertension events. The frequency of infections decreased over time, as did the number of bleeding events. However, two of three major hemorrhage events occurred after the previous report was published.
There were 43 deaths (35%), 29 of them because of disease progression. Six patients died of adverse events, two died of unknown causes, and two died of secondary acute myeloid leukemia. Other causes of death included multiorgan failure, intestinal obstruction, lung cancer, and graft-versus-host disease.
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The researchers reported relationships with AstraZeneca/Acerta Pharma and many other companies.
SOURCE: Wang M et al. Leukemia. 2019 Sep 26. doi: 10.1038/s41375-019-0575-9.
FROM LEUKEMIA
FDA approves afamelanotide for treatment of rare condition with light-induced pain
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
Too few pregnant women receive both influenza and Tdap vaccines
according to a Morbidity and Mortality Weekly Report published by the Centers for Disease Control and Prevention.
The CDC recommends that all pregnant women receive the Tdap vaccine, preferably between 27 and 36 weeks’ gestation. The flu vaccine is recommended for all women at any point in pregnancy if the pregnancy falls within flu season. Women do not need a second flu shot if they received the vaccine before pregnancy in the same influenza season. Both vaccines provide protection to infants after birth.
“Clinicians caring for women who are pregnant have a huge role in helping women understand risks and benefits and the value of vaccines,” Anne Schuchat, MD, principal deputy director of the CDC, Atlanta, said in a telebriefing about the new report. “A lot of women are worried about taking any extra medicine or getting shots during pregnancy, and clinicians can let them know about the large data available showing the safety of the vaccine as well as the effectiveness. We also think it’s important to let people know about the risk of not vaccinating.”
Pregnant women are at higher risk for influenza complications and represent a disproportionate number of flu-related hospitalizations. From the 2010-2011 to 2017-2018 influenza seasons, 24%-34% of influenza hospitalizations each season were pregnant women aged 15-44, yet only 9% of women in this age group are pregnant at any point each year, according to the report.
Similarly, infants under 6 months have the greatest risk of hospitalization from influenza, and half of pertussis hospitalizations and 69% of pertussis deaths occur in infants under 2 months old. But a fetus receives protective maternal antibodies from flu and pertussis vaccines about 2 weeks after the mother is vaccinated.
Influenza hospitalization is 40% lower among pregnant women vaccinated against flu and 72% lower in infants under 6 months who received maternal influenza antibodies during gestation. Similarly, Tdap vaccination during the third trimester of pregnancy reduces pertussis infection risk by 78% and pertussis hospitalization by 91% in infants under 2 months.
“Infant protection can motivate pregnant women to receive recommended vaccines, and intention to vaccinate is higher among women who perceive more serious consequences of influenza or pertussis disease for their own or their infant’s health,” Megan C. Lindley, MPH, of the CDC’s Immunization Services Division, and colleagues wrote in the MMWR report.
In March-April 2019, Ms. Lindley and associates conducted an Internet survey about flu and Tdap immunizations among women aged 18-49 who had been pregnant at any point since August 1, 2018. A total of 2,626 women completed the survey of 2,762 invitations (95% response rate).
Among 817 women who knew their Tdap status during pregnancy, 55% received the Tdap vaccine. Among 2,097 women who reported a pregnancy between October 2018 and January 2019, 54% received the flu vaccine before or during pregnancy.
But many women received one vaccine without the other: 65% of women surveyed had not received both vaccines during pregnancy. Higher immunization rates occurred among women whose clinicians recommended the vaccines: 66% received a flu shot and 71% received Tdap.
“We’re learning a lot about improved communication between clinicians and patients. One thing we suggest is to begin the conversations early.” Dr Schuchat said. “If you begin talking early in the pregnancy about the things you’ll be looking forward to and provide information, by the time it is flu season or it is that third trimester, they’re prepared to make a good choice.”
Most women surveyed (75%) said their providers did offer a flu or Tdap vaccine in the office or a referral for one. Yet more than 30% of these women did not get the recommended vaccine.
The most common reason for not getting the Tdap during pregnancy, cited by 38% of women who didn’t receive it, was not knowing about the recommendation. Those who did not receive flu vaccination, however, cited concerns about effectiveness (18%) or safety for the baby (16%). A similar proportion of women cited safety concerns for not getting the Tdap (17%).
Sharing information early and engaging respectfully with patients are key to successful provider recommendations, Dr Schuchat said.
“It’s really important for clinicians to begin by listening to women, asking, ‘Can I answer your questions? What are the concerns that you have?’ ” she said. “We find that, when a clinician validates a patient’s concerns and really shows that they’re listening, they can build trust and respect.”
Providers’ sharing their personal experience can help as well, Dr Schuchat added. Clinicians can let patients know if they themselves, or their partner, received the vaccines during pregnancy.
Rates for turning down vaccines were higher for black women: 47% received the flu vaccine after a recommendation, compared with 69% of white women. Among those receiving a Tdap recommendation, 53% of black women accepted it, compared with 77% of white women and 66% of Latina women. The authors noted a past study showing black adults had a higher distrust of flu vaccination, their doctor, and CDC information than white adults.
“Differential effects of provider vaccination offers or referrals might also be explained by less patient-centered provider communication with black patients,” Ms. Lindley and associates wrote.
SOURCE: Lindley MC. MMWR Morb Mortal Wkly Rep. 2019 Oct 8. doi: 10.15585/mmwr.mm6840e1.
according to a Morbidity and Mortality Weekly Report published by the Centers for Disease Control and Prevention.
The CDC recommends that all pregnant women receive the Tdap vaccine, preferably between 27 and 36 weeks’ gestation. The flu vaccine is recommended for all women at any point in pregnancy if the pregnancy falls within flu season. Women do not need a second flu shot if they received the vaccine before pregnancy in the same influenza season. Both vaccines provide protection to infants after birth.
“Clinicians caring for women who are pregnant have a huge role in helping women understand risks and benefits and the value of vaccines,” Anne Schuchat, MD, principal deputy director of the CDC, Atlanta, said in a telebriefing about the new report. “A lot of women are worried about taking any extra medicine or getting shots during pregnancy, and clinicians can let them know about the large data available showing the safety of the vaccine as well as the effectiveness. We also think it’s important to let people know about the risk of not vaccinating.”
Pregnant women are at higher risk for influenza complications and represent a disproportionate number of flu-related hospitalizations. From the 2010-2011 to 2017-2018 influenza seasons, 24%-34% of influenza hospitalizations each season were pregnant women aged 15-44, yet only 9% of women in this age group are pregnant at any point each year, according to the report.
Similarly, infants under 6 months have the greatest risk of hospitalization from influenza, and half of pertussis hospitalizations and 69% of pertussis deaths occur in infants under 2 months old. But a fetus receives protective maternal antibodies from flu and pertussis vaccines about 2 weeks after the mother is vaccinated.
Influenza hospitalization is 40% lower among pregnant women vaccinated against flu and 72% lower in infants under 6 months who received maternal influenza antibodies during gestation. Similarly, Tdap vaccination during the third trimester of pregnancy reduces pertussis infection risk by 78% and pertussis hospitalization by 91% in infants under 2 months.
“Infant protection can motivate pregnant women to receive recommended vaccines, and intention to vaccinate is higher among women who perceive more serious consequences of influenza or pertussis disease for their own or their infant’s health,” Megan C. Lindley, MPH, of the CDC’s Immunization Services Division, and colleagues wrote in the MMWR report.
In March-April 2019, Ms. Lindley and associates conducted an Internet survey about flu and Tdap immunizations among women aged 18-49 who had been pregnant at any point since August 1, 2018. A total of 2,626 women completed the survey of 2,762 invitations (95% response rate).
Among 817 women who knew their Tdap status during pregnancy, 55% received the Tdap vaccine. Among 2,097 women who reported a pregnancy between October 2018 and January 2019, 54% received the flu vaccine before or during pregnancy.
But many women received one vaccine without the other: 65% of women surveyed had not received both vaccines during pregnancy. Higher immunization rates occurred among women whose clinicians recommended the vaccines: 66% received a flu shot and 71% received Tdap.
“We’re learning a lot about improved communication between clinicians and patients. One thing we suggest is to begin the conversations early.” Dr Schuchat said. “If you begin talking early in the pregnancy about the things you’ll be looking forward to and provide information, by the time it is flu season or it is that third trimester, they’re prepared to make a good choice.”
Most women surveyed (75%) said their providers did offer a flu or Tdap vaccine in the office or a referral for one. Yet more than 30% of these women did not get the recommended vaccine.
The most common reason for not getting the Tdap during pregnancy, cited by 38% of women who didn’t receive it, was not knowing about the recommendation. Those who did not receive flu vaccination, however, cited concerns about effectiveness (18%) or safety for the baby (16%). A similar proportion of women cited safety concerns for not getting the Tdap (17%).
Sharing information early and engaging respectfully with patients are key to successful provider recommendations, Dr Schuchat said.
“It’s really important for clinicians to begin by listening to women, asking, ‘Can I answer your questions? What are the concerns that you have?’ ” she said. “We find that, when a clinician validates a patient’s concerns and really shows that they’re listening, they can build trust and respect.”
Providers’ sharing their personal experience can help as well, Dr Schuchat added. Clinicians can let patients know if they themselves, or their partner, received the vaccines during pregnancy.
Rates for turning down vaccines were higher for black women: 47% received the flu vaccine after a recommendation, compared with 69% of white women. Among those receiving a Tdap recommendation, 53% of black women accepted it, compared with 77% of white women and 66% of Latina women. The authors noted a past study showing black adults had a higher distrust of flu vaccination, their doctor, and CDC information than white adults.
“Differential effects of provider vaccination offers or referrals might also be explained by less patient-centered provider communication with black patients,” Ms. Lindley and associates wrote.
SOURCE: Lindley MC. MMWR Morb Mortal Wkly Rep. 2019 Oct 8. doi: 10.15585/mmwr.mm6840e1.
according to a Morbidity and Mortality Weekly Report published by the Centers for Disease Control and Prevention.
The CDC recommends that all pregnant women receive the Tdap vaccine, preferably between 27 and 36 weeks’ gestation. The flu vaccine is recommended for all women at any point in pregnancy if the pregnancy falls within flu season. Women do not need a second flu shot if they received the vaccine before pregnancy in the same influenza season. Both vaccines provide protection to infants after birth.
“Clinicians caring for women who are pregnant have a huge role in helping women understand risks and benefits and the value of vaccines,” Anne Schuchat, MD, principal deputy director of the CDC, Atlanta, said in a telebriefing about the new report. “A lot of women are worried about taking any extra medicine or getting shots during pregnancy, and clinicians can let them know about the large data available showing the safety of the vaccine as well as the effectiveness. We also think it’s important to let people know about the risk of not vaccinating.”
Pregnant women are at higher risk for influenza complications and represent a disproportionate number of flu-related hospitalizations. From the 2010-2011 to 2017-2018 influenza seasons, 24%-34% of influenza hospitalizations each season were pregnant women aged 15-44, yet only 9% of women in this age group are pregnant at any point each year, according to the report.
Similarly, infants under 6 months have the greatest risk of hospitalization from influenza, and half of pertussis hospitalizations and 69% of pertussis deaths occur in infants under 2 months old. But a fetus receives protective maternal antibodies from flu and pertussis vaccines about 2 weeks after the mother is vaccinated.
Influenza hospitalization is 40% lower among pregnant women vaccinated against flu and 72% lower in infants under 6 months who received maternal influenza antibodies during gestation. Similarly, Tdap vaccination during the third trimester of pregnancy reduces pertussis infection risk by 78% and pertussis hospitalization by 91% in infants under 2 months.
“Infant protection can motivate pregnant women to receive recommended vaccines, and intention to vaccinate is higher among women who perceive more serious consequences of influenza or pertussis disease for their own or their infant’s health,” Megan C. Lindley, MPH, of the CDC’s Immunization Services Division, and colleagues wrote in the MMWR report.
In March-April 2019, Ms. Lindley and associates conducted an Internet survey about flu and Tdap immunizations among women aged 18-49 who had been pregnant at any point since August 1, 2018. A total of 2,626 women completed the survey of 2,762 invitations (95% response rate).
Among 817 women who knew their Tdap status during pregnancy, 55% received the Tdap vaccine. Among 2,097 women who reported a pregnancy between October 2018 and January 2019, 54% received the flu vaccine before or during pregnancy.
But many women received one vaccine without the other: 65% of women surveyed had not received both vaccines during pregnancy. Higher immunization rates occurred among women whose clinicians recommended the vaccines: 66% received a flu shot and 71% received Tdap.
“We’re learning a lot about improved communication between clinicians and patients. One thing we suggest is to begin the conversations early.” Dr Schuchat said. “If you begin talking early in the pregnancy about the things you’ll be looking forward to and provide information, by the time it is flu season or it is that third trimester, they’re prepared to make a good choice.”
Most women surveyed (75%) said their providers did offer a flu or Tdap vaccine in the office or a referral for one. Yet more than 30% of these women did not get the recommended vaccine.
The most common reason for not getting the Tdap during pregnancy, cited by 38% of women who didn’t receive it, was not knowing about the recommendation. Those who did not receive flu vaccination, however, cited concerns about effectiveness (18%) or safety for the baby (16%). A similar proportion of women cited safety concerns for not getting the Tdap (17%).
Sharing information early and engaging respectfully with patients are key to successful provider recommendations, Dr Schuchat said.
“It’s really important for clinicians to begin by listening to women, asking, ‘Can I answer your questions? What are the concerns that you have?’ ” she said. “We find that, when a clinician validates a patient’s concerns and really shows that they’re listening, they can build trust and respect.”
Providers’ sharing their personal experience can help as well, Dr Schuchat added. Clinicians can let patients know if they themselves, or their partner, received the vaccines during pregnancy.
Rates for turning down vaccines were higher for black women: 47% received the flu vaccine after a recommendation, compared with 69% of white women. Among those receiving a Tdap recommendation, 53% of black women accepted it, compared with 77% of white women and 66% of Latina women. The authors noted a past study showing black adults had a higher distrust of flu vaccination, their doctor, and CDC information than white adults.
“Differential effects of provider vaccination offers or referrals might also be explained by less patient-centered provider communication with black patients,” Ms. Lindley and associates wrote.
SOURCE: Lindley MC. MMWR Morb Mortal Wkly Rep. 2019 Oct 8. doi: 10.15585/mmwr.mm6840e1.
FROM MMWR TELEBRIEFING
Using AI safely in the clinical setting
Understanding limitations of technology is key
Artificial intelligence (AI) and machine learning (ML) are promoted as the solution to many health care problems, but the area risks becoming technology led – with only secondary consideration to the safe clinical application of the technology, says Robert Challen, PhD.
Dr. Challen, of the University of Exeter (England), is the lead author of a recent paper that examines the short-, medium-, and long-term issues with medical applications of AI. “In the short term, AI systems will effectively function like laboratory screening tests, identifying patients who are at higher risk than others of disease, or who could benefit more from a particular treatment,” Dr. Challen said. “We usually accept that laboratory tests are useful to help make a diagnosis; however, clinicians are aware that they might not always be accurate and interpret their output in the clinical context. AI systems are no different in that they will be a useful tool so long as they are designed with safety in mind and used with a pragmatic attitude to their interpretation.”
The paper also suggests a set of short-and medium-term clinical safety issues that need addressing when bringing these systems from laboratory to bedside.
In the longer term, as more continuously learning and autonomous systems are developed, the safety risks will need to be continuously reevaluated, he added. “Any new technology comes with limitations and understanding those limitations is key to safe use of that technology. In the same way a new screening test has limitations on its sensitivity and specificity that define how it can be used, AL and ML systems have limitations on accuracy and which patients they can be used on,” Dr. Challen said. If hospitalists understand these limitations, they can participate better in their development.
Dr. Challen recommends that hospitalists help the development of AI tools by participating in studies that assess AI applications in the clinical environment. “Try to make sure that where AI research is taking place, there is strong clinical involvement.”
Reference
1. Challen R et al. Artificial intelligence, bias and clinical safety. BMJ Qual Saf. 2019 Jan 12. doi: 10.1136/bmjqs-2018-008370.
Understanding limitations of technology is key
Understanding limitations of technology is key
Artificial intelligence (AI) and machine learning (ML) are promoted as the solution to many health care problems, but the area risks becoming technology led – with only secondary consideration to the safe clinical application of the technology, says Robert Challen, PhD.
Dr. Challen, of the University of Exeter (England), is the lead author of a recent paper that examines the short-, medium-, and long-term issues with medical applications of AI. “In the short term, AI systems will effectively function like laboratory screening tests, identifying patients who are at higher risk than others of disease, or who could benefit more from a particular treatment,” Dr. Challen said. “We usually accept that laboratory tests are useful to help make a diagnosis; however, clinicians are aware that they might not always be accurate and interpret their output in the clinical context. AI systems are no different in that they will be a useful tool so long as they are designed with safety in mind and used with a pragmatic attitude to their interpretation.”
The paper also suggests a set of short-and medium-term clinical safety issues that need addressing when bringing these systems from laboratory to bedside.
In the longer term, as more continuously learning and autonomous systems are developed, the safety risks will need to be continuously reevaluated, he added. “Any new technology comes with limitations and understanding those limitations is key to safe use of that technology. In the same way a new screening test has limitations on its sensitivity and specificity that define how it can be used, AL and ML systems have limitations on accuracy and which patients they can be used on,” Dr. Challen said. If hospitalists understand these limitations, they can participate better in their development.
Dr. Challen recommends that hospitalists help the development of AI tools by participating in studies that assess AI applications in the clinical environment. “Try to make sure that where AI research is taking place, there is strong clinical involvement.”
Reference
1. Challen R et al. Artificial intelligence, bias and clinical safety. BMJ Qual Saf. 2019 Jan 12. doi: 10.1136/bmjqs-2018-008370.
Artificial intelligence (AI) and machine learning (ML) are promoted as the solution to many health care problems, but the area risks becoming technology led – with only secondary consideration to the safe clinical application of the technology, says Robert Challen, PhD.
Dr. Challen, of the University of Exeter (England), is the lead author of a recent paper that examines the short-, medium-, and long-term issues with medical applications of AI. “In the short term, AI systems will effectively function like laboratory screening tests, identifying patients who are at higher risk than others of disease, or who could benefit more from a particular treatment,” Dr. Challen said. “We usually accept that laboratory tests are useful to help make a diagnosis; however, clinicians are aware that they might not always be accurate and interpret their output in the clinical context. AI systems are no different in that they will be a useful tool so long as they are designed with safety in mind and used with a pragmatic attitude to their interpretation.”
The paper also suggests a set of short-and medium-term clinical safety issues that need addressing when bringing these systems from laboratory to bedside.
In the longer term, as more continuously learning and autonomous systems are developed, the safety risks will need to be continuously reevaluated, he added. “Any new technology comes with limitations and understanding those limitations is key to safe use of that technology. In the same way a new screening test has limitations on its sensitivity and specificity that define how it can be used, AL and ML systems have limitations on accuracy and which patients they can be used on,” Dr. Challen said. If hospitalists understand these limitations, they can participate better in their development.
Dr. Challen recommends that hospitalists help the development of AI tools by participating in studies that assess AI applications in the clinical environment. “Try to make sure that where AI research is taking place, there is strong clinical involvement.”
Reference
1. Challen R et al. Artificial intelligence, bias and clinical safety. BMJ Qual Saf. 2019 Jan 12. doi: 10.1136/bmjqs-2018-008370.