User login
Emapalumab produces major responses in macrophage activation syndrome
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
MADRID – Initial results of an ongoing small pilot study of the anti-interferon gamma (IFN-gamma) monoclonal antibody emapalumab has demonstrated efficacy in the treatment of glucocorticoid-refractory macrophage activation syndrome (MAS) in patients with systemic juvenile idiopathic arthritis (SJIA).
“By week 4 there was a complete response in all six patients treated. All of them had failed conventional therapy,” Fabrizio De Benedetti, MD, PhD, head of the division of rheumatology at IRCCS Ospedale Pediatrico Bambino Gesù, Rome, reported at the European Congress of Rheumatology.
The results are the first of a multicenter, pilot study with twin protocols in Europe and North America. Emapalumab (Gamifant) is already approved by the Food and Drug Administration for the treatment of primary hemophagocytic lymphohistiocytosis (HLH) unresponsive to conventional therapy.
The study is enrolling children with MAS complicating SJIA that is unresponsive to high-dose intravenous glucocorticoids. Emapalumab, which has been shown to neutralize IFN-gamma in animal models, is being administered in an initial dose of 6 mg/kg followed by doses of 3 mg/kg every 3 days for 4 weeks.
MAS is a common complication of rheumatic diseases, particularly SJIA, according to Dr. De Benedetti. It has been characterized as a secondary form of HLH involving an excessive activation and expansion of macrophages as well as T cells. It can produce a wide variety of complications, including hepatosplenomegaly, liver dysfunction, and coagulation abnormalities. If uncontrolled, it can lead to organ failure and death.
Among the first six patients, four had confirmed SJIA and two had presumptive SJIA. The average age was 11 years with a range of 2 to 25 years. Four of the patients were female.
Many of the patients had failed therapies in addition to glucocorticoids, such as cyclosporine and anakinra. A diagnosis of HLH and prior treatment with a biologic therapy were exclusion criteria.
By 8 weeks, six had a complete response, which included the resolution of symptoms by normalization of ferritin, liver enzymes, and D-dimers. In three of the six patients, a complete response was achieved by week 4.
“Steroid tapering by investigator discretion was permitted, and four of the six patients had a meaningful tapering of steroids within 8 weeks,” Dr. De Benedetti reported.
Of the three serious adverse events recorded so far, only reactivation of cytomegalovirus (CMV) infection was attributed to emapalumab. This infection resolved with treatment. Several other infections observed over the course of the study were not thought to be related to treatment.
The initial results have encouraged an expansion of the study protocol in Europe where several treatment centers are expected to begin enrolling patients shortly. A second parallel study protocol will begin soon in North America, but no patient had been treated at the time that Dr. De Benedetti presented these initial findings.
Based on evidence that IFN-gamma drives hyperinflammation and hypercytokinemia in MAS, the initial results with emapalumab are encouraging, according to Dr. De Benedetti. He said the results not only provide evidence that emapalumab is active in MAS but support the pathogenic role of IFN-gamma in this disease.
Dr. De Benedetti reported financial relationships with multiple pharmaceutical companies, including SOBI, the sponsor of this study.
SOURCE: De Benedetti F et al. Ann Rheum Dis. Jun 2019;78(Suppl2):178. Abstract OPO204, doi: 10.1136/annrheumdis-2019-eular.3341.
REPORTING FROM EULAR 2019 CONGRESS
Drug efficacy in phase 2 RA trials often missing in phase 3
MADRID – The efficacy results from new rheumatoid arthritis drugs tested in many phase 2 trials run over the past couple of decades have routinely overestimated the efficacy of many of the drugs tested when compared with how the same agents performed in subsequent phase 3 testing, according to an analysis of published results from 44 pairs of phase 2 and 3 trials.
Based on the percentage of rheumatoid arthritis (RA) patients who showed an American College of Rheumatology 20% improvement (ACR20) in their joint symptoms, the 44 phase 2 trials overestimated efficacy by an average of 39% when compared with the ACR20 responses seen in paired phase 3 trials, Andreas Kerschbaumer, MD, said at the European Congress of Rheumatology. The ACR50 results overstated efficacy by an average of 34% when compared with phase 3 results for the same drugs, and the ACR70 endpoint showed drug efficacy that averaged 39% better during phase 2 studies than it did in the phase 3 trials. All three between-group differences were statistically significant, said Dr. Kerschbaumer, a rheumatologist at the Medical University of Vienna.
“Active treatment arms of phase 2 studies systematically overestimated efficacy when compared with subsequent phase 3 studies,” he said.
“Many researchers had already seen this, and realized that phase 2 trials often overestimated [efficacy], but no one has ever shown this systematically,” Dr. Kerschbaumer said in an interview. The same problem also appears to affect trials of oncology drugs, he noted, but a unique feature of RA studies allowed him and his colleagues to examine the ubiquity and persistence of this phase 2 bias in rheumatology trials over time: ongoing reliance during more than 2 decades of experience on the ACR20 response as the primary endpoint of RA drug trials. Even with this advantage, which allowed inclusion of 44 pairs of studies, “it was very surprising to find a statistically significant effect in the meta-analysis,” he said.
He and his associates also ran a further analysis that looked for measured parameters that showed significant correlation with mismatch of the phase 2 and 3 results, and this identified two apparently causal factors: having a low number of swollen and tender joints as an inclusion criterion for patients and using a 28-joint count rather than a 66-joint count for assessing disease activity during the study. The analysis lacked enough information to provide clear evidence on why these two aspects of patient assessment could lead to misleading phase 2 results, but Dr. Kerschbaumer believed the findings were clear enough to influence future trial design.
Going forward, trialists “should be very careful of how you include patients [in studies]. This is what our study shows. And they should not use 28 joints but 66. The higher the number of swollen and tender joints detected for study inclusion, the less the possibility to overestimate [efficacy]. It’s easier to achieve ACR20 when you look at fewer joints.”
Until now, companies that sponsor drug trials had an incentive to use a lower minimum number of swollen and tender joints for enrolled patients because it made enrollment easier, he noted. The downside, in addition to overstating efficacy at the phase 2 stage, is subjecting patients to treatments in phase 3 trials with a reduced likelihood for success.
Dr. Kerschbaumer offered two examples of drugs that showed promising RA efficacy based on ACR20 responses in phase 2 trial results that were followed by neutral phase 3 trial outcomes. One episode involved tabalumab (Ann Rheum Dis. 2015 Aug;74[8]:1567-70), and a second was a trial of fostamatinib (Arthritis Rheumatol. 2014 Dec;66[12]:3255-64).
The systematic review he led identified 44 study pairs run since the late 1990s that met all the study criteria, which covered 19 different drugs tested in more than 17,000 RA patients.
What the results showed was “just an association, so we must be careful not to overstate the results, but we saw something that may explain what people have seen [anecdotally] over the past 20 years,” he said. “Some people get very excited by phase 2 results, but we need to be careful about interpreting these outcomes.” Validation of the finding would require analysis of patient-level data, something that would be hard to obtain for a large number of phase 2 and 3 trials, Dr. Kerschbaumer noted.
Dr. Kerschbaumer has been a speaker on behalf of Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, and Pfizer.
SOURCE: Kerschbaumer A et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):191-2. Abstract OP0229, doi: 10.1136/annrheumdis-2019-eular.5161.
MADRID – The efficacy results from new rheumatoid arthritis drugs tested in many phase 2 trials run over the past couple of decades have routinely overestimated the efficacy of many of the drugs tested when compared with how the same agents performed in subsequent phase 3 testing, according to an analysis of published results from 44 pairs of phase 2 and 3 trials.
Based on the percentage of rheumatoid arthritis (RA) patients who showed an American College of Rheumatology 20% improvement (ACR20) in their joint symptoms, the 44 phase 2 trials overestimated efficacy by an average of 39% when compared with the ACR20 responses seen in paired phase 3 trials, Andreas Kerschbaumer, MD, said at the European Congress of Rheumatology. The ACR50 results overstated efficacy by an average of 34% when compared with phase 3 results for the same drugs, and the ACR70 endpoint showed drug efficacy that averaged 39% better during phase 2 studies than it did in the phase 3 trials. All three between-group differences were statistically significant, said Dr. Kerschbaumer, a rheumatologist at the Medical University of Vienna.
“Active treatment arms of phase 2 studies systematically overestimated efficacy when compared with subsequent phase 3 studies,” he said.
“Many researchers had already seen this, and realized that phase 2 trials often overestimated [efficacy], but no one has ever shown this systematically,” Dr. Kerschbaumer said in an interview. The same problem also appears to affect trials of oncology drugs, he noted, but a unique feature of RA studies allowed him and his colleagues to examine the ubiquity and persistence of this phase 2 bias in rheumatology trials over time: ongoing reliance during more than 2 decades of experience on the ACR20 response as the primary endpoint of RA drug trials. Even with this advantage, which allowed inclusion of 44 pairs of studies, “it was very surprising to find a statistically significant effect in the meta-analysis,” he said.
He and his associates also ran a further analysis that looked for measured parameters that showed significant correlation with mismatch of the phase 2 and 3 results, and this identified two apparently causal factors: having a low number of swollen and tender joints as an inclusion criterion for patients and using a 28-joint count rather than a 66-joint count for assessing disease activity during the study. The analysis lacked enough information to provide clear evidence on why these two aspects of patient assessment could lead to misleading phase 2 results, but Dr. Kerschbaumer believed the findings were clear enough to influence future trial design.
Going forward, trialists “should be very careful of how you include patients [in studies]. This is what our study shows. And they should not use 28 joints but 66. The higher the number of swollen and tender joints detected for study inclusion, the less the possibility to overestimate [efficacy]. It’s easier to achieve ACR20 when you look at fewer joints.”
Until now, companies that sponsor drug trials had an incentive to use a lower minimum number of swollen and tender joints for enrolled patients because it made enrollment easier, he noted. The downside, in addition to overstating efficacy at the phase 2 stage, is subjecting patients to treatments in phase 3 trials with a reduced likelihood for success.
Dr. Kerschbaumer offered two examples of drugs that showed promising RA efficacy based on ACR20 responses in phase 2 trial results that were followed by neutral phase 3 trial outcomes. One episode involved tabalumab (Ann Rheum Dis. 2015 Aug;74[8]:1567-70), and a second was a trial of fostamatinib (Arthritis Rheumatol. 2014 Dec;66[12]:3255-64).
The systematic review he led identified 44 study pairs run since the late 1990s that met all the study criteria, which covered 19 different drugs tested in more than 17,000 RA patients.
What the results showed was “just an association, so we must be careful not to overstate the results, but we saw something that may explain what people have seen [anecdotally] over the past 20 years,” he said. “Some people get very excited by phase 2 results, but we need to be careful about interpreting these outcomes.” Validation of the finding would require analysis of patient-level data, something that would be hard to obtain for a large number of phase 2 and 3 trials, Dr. Kerschbaumer noted.
Dr. Kerschbaumer has been a speaker on behalf of Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, and Pfizer.
SOURCE: Kerschbaumer A et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):191-2. Abstract OP0229, doi: 10.1136/annrheumdis-2019-eular.5161.
MADRID – The efficacy results from new rheumatoid arthritis drugs tested in many phase 2 trials run over the past couple of decades have routinely overestimated the efficacy of many of the drugs tested when compared with how the same agents performed in subsequent phase 3 testing, according to an analysis of published results from 44 pairs of phase 2 and 3 trials.
Based on the percentage of rheumatoid arthritis (RA) patients who showed an American College of Rheumatology 20% improvement (ACR20) in their joint symptoms, the 44 phase 2 trials overestimated efficacy by an average of 39% when compared with the ACR20 responses seen in paired phase 3 trials, Andreas Kerschbaumer, MD, said at the European Congress of Rheumatology. The ACR50 results overstated efficacy by an average of 34% when compared with phase 3 results for the same drugs, and the ACR70 endpoint showed drug efficacy that averaged 39% better during phase 2 studies than it did in the phase 3 trials. All three between-group differences were statistically significant, said Dr. Kerschbaumer, a rheumatologist at the Medical University of Vienna.
“Active treatment arms of phase 2 studies systematically overestimated efficacy when compared with subsequent phase 3 studies,” he said.
“Many researchers had already seen this, and realized that phase 2 trials often overestimated [efficacy], but no one has ever shown this systematically,” Dr. Kerschbaumer said in an interview. The same problem also appears to affect trials of oncology drugs, he noted, but a unique feature of RA studies allowed him and his colleagues to examine the ubiquity and persistence of this phase 2 bias in rheumatology trials over time: ongoing reliance during more than 2 decades of experience on the ACR20 response as the primary endpoint of RA drug trials. Even with this advantage, which allowed inclusion of 44 pairs of studies, “it was very surprising to find a statistically significant effect in the meta-analysis,” he said.
He and his associates also ran a further analysis that looked for measured parameters that showed significant correlation with mismatch of the phase 2 and 3 results, and this identified two apparently causal factors: having a low number of swollen and tender joints as an inclusion criterion for patients and using a 28-joint count rather than a 66-joint count for assessing disease activity during the study. The analysis lacked enough information to provide clear evidence on why these two aspects of patient assessment could lead to misleading phase 2 results, but Dr. Kerschbaumer believed the findings were clear enough to influence future trial design.
Going forward, trialists “should be very careful of how you include patients [in studies]. This is what our study shows. And they should not use 28 joints but 66. The higher the number of swollen and tender joints detected for study inclusion, the less the possibility to overestimate [efficacy]. It’s easier to achieve ACR20 when you look at fewer joints.”
Until now, companies that sponsor drug trials had an incentive to use a lower minimum number of swollen and tender joints for enrolled patients because it made enrollment easier, he noted. The downside, in addition to overstating efficacy at the phase 2 stage, is subjecting patients to treatments in phase 3 trials with a reduced likelihood for success.
Dr. Kerschbaumer offered two examples of drugs that showed promising RA efficacy based on ACR20 responses in phase 2 trial results that were followed by neutral phase 3 trial outcomes. One episode involved tabalumab (Ann Rheum Dis. 2015 Aug;74[8]:1567-70), and a second was a trial of fostamatinib (Arthritis Rheumatol. 2014 Dec;66[12]:3255-64).
The systematic review he led identified 44 study pairs run since the late 1990s that met all the study criteria, which covered 19 different drugs tested in more than 17,000 RA patients.
What the results showed was “just an association, so we must be careful not to overstate the results, but we saw something that may explain what people have seen [anecdotally] over the past 20 years,” he said. “Some people get very excited by phase 2 results, but we need to be careful about interpreting these outcomes.” Validation of the finding would require analysis of patient-level data, something that would be hard to obtain for a large number of phase 2 and 3 trials, Dr. Kerschbaumer noted.
Dr. Kerschbaumer has been a speaker on behalf of Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, and Pfizer.
SOURCE: Kerschbaumer A et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):191-2. Abstract OP0229, doi: 10.1136/annrheumdis-2019-eular.5161.
REPORTING FROM EULAR 2019 CONGRESS
Hospitalist movers and shakers – July 2019
Christopher Moriates, MD, has been named executive director of the nonprofit health care organization Costs of Care (Boston). He replaces Neel Shah, MD, who was tabbed chairperson of the board.
Dr. Moriates serves a number of roles at the University of Texas at Austin. He is the assistant dean for health care value; associate chair for quality, safety and value; and associate professor of internal medicine.
In his role at Costs of Care, Dr. Moriates will direct an organization that uses feedback and stories from frontline physicians to help health systems provide high-quality care at lower costs.
Kai Mebust, MD, was recently named the new associate chief of medicine at Bassett Hospital (Cooperstown, N.Y.), where he has worked the past 15 years as a hospitalist and internist, serving as chief of hospitalists for the last decade. Dr. Mebust also completed his internship and residency at Bassett, and he is a fellow with the Society of Hospital Medicine.
Dr. Mebust will work closely with Dr. Charles Hyman, the center’s physician in chief, who is leaving the role at the end of the calendar year. Dr. Mebust will oversee inpatient services and be part of the transition process when Dr. Hyman departs.
Ronak Bhimani, MD, has been appointed chief medical officer at Lower Bucks Hospital (Bristol, Pa.). Dr. Bhimani moves over from Suburban Community Hospital (Norristown, Pa.), where he served as an academic hospitalist the past 2 years.
Previously, Dr. Bhimani was medical director of Kindred/Avalon Hospice and a core faculty member in the internal medicine program for residents at Suburban Community.
Danielle Prince, MD, was recently named associate medical director at St. Luke’s Siouxland PACE (Sioux City, Iowa), an affiliate of UnityPoint Health. Dr. Prince is a practicing hospitalist at UnityPoint Health St. Luke’s and served previously in as a family physician while working as chief medical informatics officer at Mercy Medical Center (Sioux City).
At Siouxland PACE, Dr. Prince will assist in managing the full-service care of elderly patients, including home health, specialty care, medications, transportation, and other therapies.
Alex Rankin, MD, has been named the new associate chief medical officer for the University of New Mexico Health Transfer Center and Patient Throughput in Albuquerque. A hospitalist with UNMH’s Family and Community Medicine department, Dr. Rankin was previously the medical director at the system’s 3 North facility since 2014.
Dr. Rankin came to UNMH after working for hospitals in Colorado and Nebraska and is a founding member of the UNMH patient flow committee, striving to improve patient care processes throughout the institution.
Tom Guirkin, MD, has been appointed vice president of medical affairs for Virginia Commonwealth University Community Memorial Hospital (South Hill, Va.). Dr. Guirkin, a Virginia native, returns to his home state after most recently overseeing the hospitalist group at Saint Francis Health System (Tulsa, Okla.).
Dr. Guirkin will have the opportunity to continue practicing medicine at CMH while helping to manage the quality management side of the business. He received his MBA from Virginia Commonwealth, working for James River Hospitalist Group in Richmond at the same time.
Alteon Health (Germantown, Md.) has become the manager of hospitalist services for three facilities in Maryland and Ohio, including Carroll Hospital (Westminster, Md.), Washington Adventist Hospital (Takoma Park, Md.), and University Hospitals Cleveland Medical Center.
At Carroll, Alteon physicians will provide critical care services in addition to hospitalist duties. Alteon has been Carroll’s emergency medicine provider for more than two decades.
At Washington Adventist, Alteon will take over the hospitalist program, adding to the emergency medicine services it has provided since 1991 and critical care services it has managed since 1996.
At UH Cleveland, Alteon will assume hospitalist management at its third University Hospitals facility. Alteon controls emergency medicine at 14 UH locations as well. UH Cleveland is an affiliate of Case Western Reserve University.
Christopher Moriates, MD, has been named executive director of the nonprofit health care organization Costs of Care (Boston). He replaces Neel Shah, MD, who was tabbed chairperson of the board.
Dr. Moriates serves a number of roles at the University of Texas at Austin. He is the assistant dean for health care value; associate chair for quality, safety and value; and associate professor of internal medicine.
In his role at Costs of Care, Dr. Moriates will direct an organization that uses feedback and stories from frontline physicians to help health systems provide high-quality care at lower costs.
Kai Mebust, MD, was recently named the new associate chief of medicine at Bassett Hospital (Cooperstown, N.Y.), where he has worked the past 15 years as a hospitalist and internist, serving as chief of hospitalists for the last decade. Dr. Mebust also completed his internship and residency at Bassett, and he is a fellow with the Society of Hospital Medicine.
Dr. Mebust will work closely with Dr. Charles Hyman, the center’s physician in chief, who is leaving the role at the end of the calendar year. Dr. Mebust will oversee inpatient services and be part of the transition process when Dr. Hyman departs.
Ronak Bhimani, MD, has been appointed chief medical officer at Lower Bucks Hospital (Bristol, Pa.). Dr. Bhimani moves over from Suburban Community Hospital (Norristown, Pa.), where he served as an academic hospitalist the past 2 years.
Previously, Dr. Bhimani was medical director of Kindred/Avalon Hospice and a core faculty member in the internal medicine program for residents at Suburban Community.
Danielle Prince, MD, was recently named associate medical director at St. Luke’s Siouxland PACE (Sioux City, Iowa), an affiliate of UnityPoint Health. Dr. Prince is a practicing hospitalist at UnityPoint Health St. Luke’s and served previously in as a family physician while working as chief medical informatics officer at Mercy Medical Center (Sioux City).
At Siouxland PACE, Dr. Prince will assist in managing the full-service care of elderly patients, including home health, specialty care, medications, transportation, and other therapies.
Alex Rankin, MD, has been named the new associate chief medical officer for the University of New Mexico Health Transfer Center and Patient Throughput in Albuquerque. A hospitalist with UNMH’s Family and Community Medicine department, Dr. Rankin was previously the medical director at the system’s 3 North facility since 2014.
Dr. Rankin came to UNMH after working for hospitals in Colorado and Nebraska and is a founding member of the UNMH patient flow committee, striving to improve patient care processes throughout the institution.
Tom Guirkin, MD, has been appointed vice president of medical affairs for Virginia Commonwealth University Community Memorial Hospital (South Hill, Va.). Dr. Guirkin, a Virginia native, returns to his home state after most recently overseeing the hospitalist group at Saint Francis Health System (Tulsa, Okla.).
Dr. Guirkin will have the opportunity to continue practicing medicine at CMH while helping to manage the quality management side of the business. He received his MBA from Virginia Commonwealth, working for James River Hospitalist Group in Richmond at the same time.
Alteon Health (Germantown, Md.) has become the manager of hospitalist services for three facilities in Maryland and Ohio, including Carroll Hospital (Westminster, Md.), Washington Adventist Hospital (Takoma Park, Md.), and University Hospitals Cleveland Medical Center.
At Carroll, Alteon physicians will provide critical care services in addition to hospitalist duties. Alteon has been Carroll’s emergency medicine provider for more than two decades.
At Washington Adventist, Alteon will take over the hospitalist program, adding to the emergency medicine services it has provided since 1991 and critical care services it has managed since 1996.
At UH Cleveland, Alteon will assume hospitalist management at its third University Hospitals facility. Alteon controls emergency medicine at 14 UH locations as well. UH Cleveland is an affiliate of Case Western Reserve University.
Christopher Moriates, MD, has been named executive director of the nonprofit health care organization Costs of Care (Boston). He replaces Neel Shah, MD, who was tabbed chairperson of the board.
Dr. Moriates serves a number of roles at the University of Texas at Austin. He is the assistant dean for health care value; associate chair for quality, safety and value; and associate professor of internal medicine.
In his role at Costs of Care, Dr. Moriates will direct an organization that uses feedback and stories from frontline physicians to help health systems provide high-quality care at lower costs.
Kai Mebust, MD, was recently named the new associate chief of medicine at Bassett Hospital (Cooperstown, N.Y.), where he has worked the past 15 years as a hospitalist and internist, serving as chief of hospitalists for the last decade. Dr. Mebust also completed his internship and residency at Bassett, and he is a fellow with the Society of Hospital Medicine.
Dr. Mebust will work closely with Dr. Charles Hyman, the center’s physician in chief, who is leaving the role at the end of the calendar year. Dr. Mebust will oversee inpatient services and be part of the transition process when Dr. Hyman departs.
Ronak Bhimani, MD, has been appointed chief medical officer at Lower Bucks Hospital (Bristol, Pa.). Dr. Bhimani moves over from Suburban Community Hospital (Norristown, Pa.), where he served as an academic hospitalist the past 2 years.
Previously, Dr. Bhimani was medical director of Kindred/Avalon Hospice and a core faculty member in the internal medicine program for residents at Suburban Community.
Danielle Prince, MD, was recently named associate medical director at St. Luke’s Siouxland PACE (Sioux City, Iowa), an affiliate of UnityPoint Health. Dr. Prince is a practicing hospitalist at UnityPoint Health St. Luke’s and served previously in as a family physician while working as chief medical informatics officer at Mercy Medical Center (Sioux City).
At Siouxland PACE, Dr. Prince will assist in managing the full-service care of elderly patients, including home health, specialty care, medications, transportation, and other therapies.
Alex Rankin, MD, has been named the new associate chief medical officer for the University of New Mexico Health Transfer Center and Patient Throughput in Albuquerque. A hospitalist with UNMH’s Family and Community Medicine department, Dr. Rankin was previously the medical director at the system’s 3 North facility since 2014.
Dr. Rankin came to UNMH after working for hospitals in Colorado and Nebraska and is a founding member of the UNMH patient flow committee, striving to improve patient care processes throughout the institution.
Tom Guirkin, MD, has been appointed vice president of medical affairs for Virginia Commonwealth University Community Memorial Hospital (South Hill, Va.). Dr. Guirkin, a Virginia native, returns to his home state after most recently overseeing the hospitalist group at Saint Francis Health System (Tulsa, Okla.).
Dr. Guirkin will have the opportunity to continue practicing medicine at CMH while helping to manage the quality management side of the business. He received his MBA from Virginia Commonwealth, working for James River Hospitalist Group in Richmond at the same time.
Alteon Health (Germantown, Md.) has become the manager of hospitalist services for three facilities in Maryland and Ohio, including Carroll Hospital (Westminster, Md.), Washington Adventist Hospital (Takoma Park, Md.), and University Hospitals Cleveland Medical Center.
At Carroll, Alteon physicians will provide critical care services in addition to hospitalist duties. Alteon has been Carroll’s emergency medicine provider for more than two decades.
At Washington Adventist, Alteon will take over the hospitalist program, adding to the emergency medicine services it has provided since 1991 and critical care services it has managed since 1996.
At UH Cleveland, Alteon will assume hospitalist management at its third University Hospitals facility. Alteon controls emergency medicine at 14 UH locations as well. UH Cleveland is an affiliate of Case Western Reserve University.
Misguided fear is keeping benzodiazepines from elderly
SAN FRANCISCO – Used appropriately, the benefits of benzodiazepines far outweigh the risks in elderly people, according to Carl Salzman, MD, a psychiatry professor at Harvard Medical School, Boston.
Appropriate use means very low doses – 0.5 mg or less every day or b.i.d. – of short-acting benzodiazepines, either lorazepam, oxazepam, or temazepam. There’s no worry of dose escalation or addiction in the elderly, and since the drugs are not metabolized by the cytochrome P450 system, the risk of drug interactions is very small, except for a compounding effect with alcohol and other sedative hypnotics, such as zolpidem (Ambien). The fall risk is lower than it is with antidepressants and antipsychotics (Psychiatr Serv. 2003 Jul;54[7]:1006-1); (Arch Intern Med. 2009 Nov 23;169[21]:1952-60).
In short, the drugs are “wonderful” for geriatric anxiety and anxiety-related insomnia, Dr. Salzman said at the American Psychiatric Association annual meeting.
Even so, it’s “very hard to get doctors and residents to prescribe them.” It’s like the benzodiazepine scare in the 1980s, about valium. “Newspapers were filled with stories about addicts. I’m having a little bit of déjà vu all over again,” he said.
This time around, the problem is a concern that benzodiazepines cause Alzheimer’s disease, plus collateral damage from the opioid crisis. People with addiction to opioids like benzodiazepines, because they boost the high, so they have significant street value, and drug seekers demand them in the clinic. Some clinicians would rather not deal with the drugs at all.
The Alzheimer’s worry stems largely from a widely reported review that found an association between Alzheimer’s disease and previous benzodiazepine use. The finding was based on public health insurance data from Quebec; no patients were seen (BMJ. 2014 Sep 9;349:g5205).
Among many “very large questions” about the study’s validity, people “may have been on benzos because they already had memory impairment and were anxious about it,” a common occurrence. In that case, “it’s not that benzos caused dementia; it was the other way around.” Also, there was no control for substance and alcohol use, Dr. Salzman said (J Clin Psychopharmacol. 2015 Feb;35[1]:1-3).
A more robust study followed patients 65 years and older for a mean of 7.3 years, comparing benzodiazepine users to nonusers. The team found a slightly higher risk of dementia in people with minimal exposure to benzodiazepines but not with the highest level of exposure, and concluded that the finding did “not support a causal association between benzodiazepine use and dementia” (BMJ. 2016 Feb 2;352:i90).
Meanwhile, a recent review of more than a million patients found either no or a minor increased risk of mortality, another concern with benzodiazepines in the elderly. “If a detrimental effect exists, it is likely to be much smaller than previously stated and to have uncertain clinical relevance. Residual confounding likely explains at least part of” it, the investigators concluded (BMJ. 2017 Jul 6;358:j294).
in an upscale nursing home in Boston. A “dramatic” rebound was reported in short-term recall 2 weeks after volunteers tapered off benzodiazepines, mostly lorazepam, compared with those who stayed on them.
“I sat down to have lunch with the discontinuers, and I said to them, ‘Aren’t you glad that you are not taking these horrible drugs anymore, and your memory is so much better? They said, ‘No, what’s to remember? It was true that when we were taking those drugs, we might not have remembered what we watched on television the night before, but if you give a choice between feeling calm in the days, sleeping at night, and remembering what we watch on television, we’ll take the calm and the sleep every time,’ ” Dr. Salzman said.
He had no disclosures.
SAN FRANCISCO – Used appropriately, the benefits of benzodiazepines far outweigh the risks in elderly people, according to Carl Salzman, MD, a psychiatry professor at Harvard Medical School, Boston.
Appropriate use means very low doses – 0.5 mg or less every day or b.i.d. – of short-acting benzodiazepines, either lorazepam, oxazepam, or temazepam. There’s no worry of dose escalation or addiction in the elderly, and since the drugs are not metabolized by the cytochrome P450 system, the risk of drug interactions is very small, except for a compounding effect with alcohol and other sedative hypnotics, such as zolpidem (Ambien). The fall risk is lower than it is with antidepressants and antipsychotics (Psychiatr Serv. 2003 Jul;54[7]:1006-1); (Arch Intern Med. 2009 Nov 23;169[21]:1952-60).
In short, the drugs are “wonderful” for geriatric anxiety and anxiety-related insomnia, Dr. Salzman said at the American Psychiatric Association annual meeting.
Even so, it’s “very hard to get doctors and residents to prescribe them.” It’s like the benzodiazepine scare in the 1980s, about valium. “Newspapers were filled with stories about addicts. I’m having a little bit of déjà vu all over again,” he said.
This time around, the problem is a concern that benzodiazepines cause Alzheimer’s disease, plus collateral damage from the opioid crisis. People with addiction to opioids like benzodiazepines, because they boost the high, so they have significant street value, and drug seekers demand them in the clinic. Some clinicians would rather not deal with the drugs at all.
The Alzheimer’s worry stems largely from a widely reported review that found an association between Alzheimer’s disease and previous benzodiazepine use. The finding was based on public health insurance data from Quebec; no patients were seen (BMJ. 2014 Sep 9;349:g5205).
Among many “very large questions” about the study’s validity, people “may have been on benzos because they already had memory impairment and were anxious about it,” a common occurrence. In that case, “it’s not that benzos caused dementia; it was the other way around.” Also, there was no control for substance and alcohol use, Dr. Salzman said (J Clin Psychopharmacol. 2015 Feb;35[1]:1-3).
A more robust study followed patients 65 years and older for a mean of 7.3 years, comparing benzodiazepine users to nonusers. The team found a slightly higher risk of dementia in people with minimal exposure to benzodiazepines but not with the highest level of exposure, and concluded that the finding did “not support a causal association between benzodiazepine use and dementia” (BMJ. 2016 Feb 2;352:i90).
Meanwhile, a recent review of more than a million patients found either no or a minor increased risk of mortality, another concern with benzodiazepines in the elderly. “If a detrimental effect exists, it is likely to be much smaller than previously stated and to have uncertain clinical relevance. Residual confounding likely explains at least part of” it, the investigators concluded (BMJ. 2017 Jul 6;358:j294).
in an upscale nursing home in Boston. A “dramatic” rebound was reported in short-term recall 2 weeks after volunteers tapered off benzodiazepines, mostly lorazepam, compared with those who stayed on them.
“I sat down to have lunch with the discontinuers, and I said to them, ‘Aren’t you glad that you are not taking these horrible drugs anymore, and your memory is so much better? They said, ‘No, what’s to remember? It was true that when we were taking those drugs, we might not have remembered what we watched on television the night before, but if you give a choice between feeling calm in the days, sleeping at night, and remembering what we watch on television, we’ll take the calm and the sleep every time,’ ” Dr. Salzman said.
He had no disclosures.
SAN FRANCISCO – Used appropriately, the benefits of benzodiazepines far outweigh the risks in elderly people, according to Carl Salzman, MD, a psychiatry professor at Harvard Medical School, Boston.
Appropriate use means very low doses – 0.5 mg or less every day or b.i.d. – of short-acting benzodiazepines, either lorazepam, oxazepam, or temazepam. There’s no worry of dose escalation or addiction in the elderly, and since the drugs are not metabolized by the cytochrome P450 system, the risk of drug interactions is very small, except for a compounding effect with alcohol and other sedative hypnotics, such as zolpidem (Ambien). The fall risk is lower than it is with antidepressants and antipsychotics (Psychiatr Serv. 2003 Jul;54[7]:1006-1); (Arch Intern Med. 2009 Nov 23;169[21]:1952-60).
In short, the drugs are “wonderful” for geriatric anxiety and anxiety-related insomnia, Dr. Salzman said at the American Psychiatric Association annual meeting.
Even so, it’s “very hard to get doctors and residents to prescribe them.” It’s like the benzodiazepine scare in the 1980s, about valium. “Newspapers were filled with stories about addicts. I’m having a little bit of déjà vu all over again,” he said.
This time around, the problem is a concern that benzodiazepines cause Alzheimer’s disease, plus collateral damage from the opioid crisis. People with addiction to opioids like benzodiazepines, because they boost the high, so they have significant street value, and drug seekers demand them in the clinic. Some clinicians would rather not deal with the drugs at all.
The Alzheimer’s worry stems largely from a widely reported review that found an association between Alzheimer’s disease and previous benzodiazepine use. The finding was based on public health insurance data from Quebec; no patients were seen (BMJ. 2014 Sep 9;349:g5205).
Among many “very large questions” about the study’s validity, people “may have been on benzos because they already had memory impairment and were anxious about it,” a common occurrence. In that case, “it’s not that benzos caused dementia; it was the other way around.” Also, there was no control for substance and alcohol use, Dr. Salzman said (J Clin Psychopharmacol. 2015 Feb;35[1]:1-3).
A more robust study followed patients 65 years and older for a mean of 7.3 years, comparing benzodiazepine users to nonusers. The team found a slightly higher risk of dementia in people with minimal exposure to benzodiazepines but not with the highest level of exposure, and concluded that the finding did “not support a causal association between benzodiazepine use and dementia” (BMJ. 2016 Feb 2;352:i90).
Meanwhile, a recent review of more than a million patients found either no or a minor increased risk of mortality, another concern with benzodiazepines in the elderly. “If a detrimental effect exists, it is likely to be much smaller than previously stated and to have uncertain clinical relevance. Residual confounding likely explains at least part of” it, the investigators concluded (BMJ. 2017 Jul 6;358:j294).
in an upscale nursing home in Boston. A “dramatic” rebound was reported in short-term recall 2 weeks after volunteers tapered off benzodiazepines, mostly lorazepam, compared with those who stayed on them.
“I sat down to have lunch with the discontinuers, and I said to them, ‘Aren’t you glad that you are not taking these horrible drugs anymore, and your memory is so much better? They said, ‘No, what’s to remember? It was true that when we were taking those drugs, we might not have remembered what we watched on television the night before, but if you give a choice between feeling calm in the days, sleeping at night, and remembering what we watch on television, we’ll take the calm and the sleep every time,’ ” Dr. Salzman said.
He had no disclosures.
REPORTING FROM APA 2019
Industry payments influence prescription choices
Two studies show a link between industry payments by drug manufacturers to physicians and doctors’ prescribing patterns for certain medications.
In the first study, lead author Taeho Greg Rhee, PhD, of the University of Connecticut, Farmington, and colleagues analyzed Centers for Medicare & Medicaid Services Part D data and Open Payments data for general payments from industry to physicians associated with gabapentinoids.
Specifically, investigators examined data for three brand name products: Gralise (Assertio) and Horizant (Arbor), both of which are extended release formulas approved for the treatment of seizure disorders and postherpetic neuralgia, and Lyrica (Pfizer), which is approved for treatment of seizure disorders, postherpetic neuralgia, neuropathic pain, and fibromyalgia. To evaluate prescribing patterns, researchers estimated physician prescribing as the physician’s proportion of prescription days filled for the three brand-name gabapentinoids in aggregate of all gabapentinoid prescription days filled.
Between 2014 and 2016, manufacturers of the three brand-name gabapentinoids made approximately 510,000 general payments ($11.5 million) to 51,005 physicians, according to Dr. Rhee and colleagues. The doctors represented 14% of physicians who prescribed any gabapentinoid product under Part D during the same time period.
Among physicians who prescribed any gabapentinoid, generic forms of Gralise (gabapentin; 87%) and Lyrica (pregabalin; 12%) were most frequently prescribed. However, physicians receiving payments from industry were more likely to prescribe the three brand-name gabapentinoids than they were gabapentin (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.1082).
Generalist physicians received the majority of payments (62%) payments totaling about $4 million, followed by about $7 million for pain medication specialists and $1 million for other physicians.
The majority of payments were for food and beverages, gifts, or educational materials. In addition, industry payments were most commonly paid to physicians in the southern and eastern regions of the United States.
Among physicians who prescribed gabapentinoids, industry payment was associated with a higher likelihood of prescribing brand-name products than generic gabapentin and that such prescribing patterns increase Medicare spending. Data show that brand name gabapentinoids typically cost account for nearly $2,500 in mean Medicare spending per beneficiary in 2016, compared with less than $20 for a 1-month supply of gabapentin, authors noted.
In the second study, Rishad Khan, MD, of the University of Toronto and colleagues examined the association between industry payments to physicians and Medicare spending on adalimumab (Humira; AbbVie) and certolizumab (Cimzia; Union Chimique Belge), both of which are approved for Crohn’s disease and numerous other indications. Investigators analyzed CMS Part D data and Open Payments data linked to the prescribing of adalimumab and certolizumab. Payments were considered relevant if a gastroenterologist received them from a drug manufacturer the year that the medication was prescribed.
From 2014 to 2016, drug makers made more than $10 million in payments to gastroenterologists prescribing adalimumab or certolizumab, the study found. Investigators found that for every $1 in physician payments, there was a $3.16 increase in spending for adalimumab and a $4.72 increase for certolizumab (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.0999).
For adalimumab, payments totaled $5.5 million for speaking and consulting, $4.9 million for food, travel,and lodging expenses, and $13,000 for education. For certolizumab, payments totaled $180,000 for speaking and consulting, $117,000 for food, travel,and lodging expenses, and $60,000 for education.
Dr. Khan and associates concluded that the findings suggest a significant association between industry payments by drug manufacturers to physicians and Medicare spending.
The studies by Rhee et. al. and Khan et. al. add to previous research finding that marketing to physicians is associated with increased sales of a company’s product and higher Medicare expenditures.
While the analyses do not account for other influences on prescribing, such as direct-to-consumer advertising, the pattern they illustrate is indisputable.
Drug manufacturers market to physicians because they write the prescriptions; however, that marketing can obscure the fact that generic drugs are just as effective and generally less expensive than brand-name medications. When there are choices, the generics should be prescribed.
The growing research demonstrating a link between industry payments and physicians’ prescribing of brand-name medications raise troubling questions about whether such payments are in the best interest of patients.
Robert Steinbrook, MD, is editor at large for JAMA Internal Medicine. His comments are adapted from an editorial (JAMA Intern Med. 2019 July 8. doi:10.1001/jamainternmed.2019.1081) accompanying the studies by Rhee et al. and Khan et al.
The studies by Rhee et. al. and Khan et. al. add to previous research finding that marketing to physicians is associated with increased sales of a company’s product and higher Medicare expenditures.
While the analyses do not account for other influences on prescribing, such as direct-to-consumer advertising, the pattern they illustrate is indisputable.
Drug manufacturers market to physicians because they write the prescriptions; however, that marketing can obscure the fact that generic drugs are just as effective and generally less expensive than brand-name medications. When there are choices, the generics should be prescribed.
The growing research demonstrating a link between industry payments and physicians’ prescribing of brand-name medications raise troubling questions about whether such payments are in the best interest of patients.
Robert Steinbrook, MD, is editor at large for JAMA Internal Medicine. His comments are adapted from an editorial (JAMA Intern Med. 2019 July 8. doi:10.1001/jamainternmed.2019.1081) accompanying the studies by Rhee et al. and Khan et al.
The studies by Rhee et. al. and Khan et. al. add to previous research finding that marketing to physicians is associated with increased sales of a company’s product and higher Medicare expenditures.
While the analyses do not account for other influences on prescribing, such as direct-to-consumer advertising, the pattern they illustrate is indisputable.
Drug manufacturers market to physicians because they write the prescriptions; however, that marketing can obscure the fact that generic drugs are just as effective and generally less expensive than brand-name medications. When there are choices, the generics should be prescribed.
The growing research demonstrating a link between industry payments and physicians’ prescribing of brand-name medications raise troubling questions about whether such payments are in the best interest of patients.
Robert Steinbrook, MD, is editor at large for JAMA Internal Medicine. His comments are adapted from an editorial (JAMA Intern Med. 2019 July 8. doi:10.1001/jamainternmed.2019.1081) accompanying the studies by Rhee et al. and Khan et al.
Two studies show a link between industry payments by drug manufacturers to physicians and doctors’ prescribing patterns for certain medications.
In the first study, lead author Taeho Greg Rhee, PhD, of the University of Connecticut, Farmington, and colleagues analyzed Centers for Medicare & Medicaid Services Part D data and Open Payments data for general payments from industry to physicians associated with gabapentinoids.
Specifically, investigators examined data for three brand name products: Gralise (Assertio) and Horizant (Arbor), both of which are extended release formulas approved for the treatment of seizure disorders and postherpetic neuralgia, and Lyrica (Pfizer), which is approved for treatment of seizure disorders, postherpetic neuralgia, neuropathic pain, and fibromyalgia. To evaluate prescribing patterns, researchers estimated physician prescribing as the physician’s proportion of prescription days filled for the three brand-name gabapentinoids in aggregate of all gabapentinoid prescription days filled.
Between 2014 and 2016, manufacturers of the three brand-name gabapentinoids made approximately 510,000 general payments ($11.5 million) to 51,005 physicians, according to Dr. Rhee and colleagues. The doctors represented 14% of physicians who prescribed any gabapentinoid product under Part D during the same time period.
Among physicians who prescribed any gabapentinoid, generic forms of Gralise (gabapentin; 87%) and Lyrica (pregabalin; 12%) were most frequently prescribed. However, physicians receiving payments from industry were more likely to prescribe the three brand-name gabapentinoids than they were gabapentin (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.1082).
Generalist physicians received the majority of payments (62%) payments totaling about $4 million, followed by about $7 million for pain medication specialists and $1 million for other physicians.
The majority of payments were for food and beverages, gifts, or educational materials. In addition, industry payments were most commonly paid to physicians in the southern and eastern regions of the United States.
Among physicians who prescribed gabapentinoids, industry payment was associated with a higher likelihood of prescribing brand-name products than generic gabapentin and that such prescribing patterns increase Medicare spending. Data show that brand name gabapentinoids typically cost account for nearly $2,500 in mean Medicare spending per beneficiary in 2016, compared with less than $20 for a 1-month supply of gabapentin, authors noted.
In the second study, Rishad Khan, MD, of the University of Toronto and colleagues examined the association between industry payments to physicians and Medicare spending on adalimumab (Humira; AbbVie) and certolizumab (Cimzia; Union Chimique Belge), both of which are approved for Crohn’s disease and numerous other indications. Investigators analyzed CMS Part D data and Open Payments data linked to the prescribing of adalimumab and certolizumab. Payments were considered relevant if a gastroenterologist received them from a drug manufacturer the year that the medication was prescribed.
From 2014 to 2016, drug makers made more than $10 million in payments to gastroenterologists prescribing adalimumab or certolizumab, the study found. Investigators found that for every $1 in physician payments, there was a $3.16 increase in spending for adalimumab and a $4.72 increase for certolizumab (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.0999).
For adalimumab, payments totaled $5.5 million for speaking and consulting, $4.9 million for food, travel,and lodging expenses, and $13,000 for education. For certolizumab, payments totaled $180,000 for speaking and consulting, $117,000 for food, travel,and lodging expenses, and $60,000 for education.
Dr. Khan and associates concluded that the findings suggest a significant association between industry payments by drug manufacturers to physicians and Medicare spending.
Two studies show a link between industry payments by drug manufacturers to physicians and doctors’ prescribing patterns for certain medications.
In the first study, lead author Taeho Greg Rhee, PhD, of the University of Connecticut, Farmington, and colleagues analyzed Centers for Medicare & Medicaid Services Part D data and Open Payments data for general payments from industry to physicians associated with gabapentinoids.
Specifically, investigators examined data for three brand name products: Gralise (Assertio) and Horizant (Arbor), both of which are extended release formulas approved for the treatment of seizure disorders and postherpetic neuralgia, and Lyrica (Pfizer), which is approved for treatment of seizure disorders, postherpetic neuralgia, neuropathic pain, and fibromyalgia. To evaluate prescribing patterns, researchers estimated physician prescribing as the physician’s proportion of prescription days filled for the three brand-name gabapentinoids in aggregate of all gabapentinoid prescription days filled.
Between 2014 and 2016, manufacturers of the three brand-name gabapentinoids made approximately 510,000 general payments ($11.5 million) to 51,005 physicians, according to Dr. Rhee and colleagues. The doctors represented 14% of physicians who prescribed any gabapentinoid product under Part D during the same time period.
Among physicians who prescribed any gabapentinoid, generic forms of Gralise (gabapentin; 87%) and Lyrica (pregabalin; 12%) were most frequently prescribed. However, physicians receiving payments from industry were more likely to prescribe the three brand-name gabapentinoids than they were gabapentin (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.1082).
Generalist physicians received the majority of payments (62%) payments totaling about $4 million, followed by about $7 million for pain medication specialists and $1 million for other physicians.
The majority of payments were for food and beverages, gifts, or educational materials. In addition, industry payments were most commonly paid to physicians in the southern and eastern regions of the United States.
Among physicians who prescribed gabapentinoids, industry payment was associated with a higher likelihood of prescribing brand-name products than generic gabapentin and that such prescribing patterns increase Medicare spending. Data show that brand name gabapentinoids typically cost account for nearly $2,500 in mean Medicare spending per beneficiary in 2016, compared with less than $20 for a 1-month supply of gabapentin, authors noted.
In the second study, Rishad Khan, MD, of the University of Toronto and colleagues examined the association between industry payments to physicians and Medicare spending on adalimumab (Humira; AbbVie) and certolizumab (Cimzia; Union Chimique Belge), both of which are approved for Crohn’s disease and numerous other indications. Investigators analyzed CMS Part D data and Open Payments data linked to the prescribing of adalimumab and certolizumab. Payments were considered relevant if a gastroenterologist received them from a drug manufacturer the year that the medication was prescribed.
From 2014 to 2016, drug makers made more than $10 million in payments to gastroenterologists prescribing adalimumab or certolizumab, the study found. Investigators found that for every $1 in physician payments, there was a $3.16 increase in spending for adalimumab and a $4.72 increase for certolizumab (JAMA Intern Med. 2019 July 8. doi: 10.1001/jamainternmed.2019.0999).
For adalimumab, payments totaled $5.5 million for speaking and consulting, $4.9 million for food, travel,and lodging expenses, and $13,000 for education. For certolizumab, payments totaled $180,000 for speaking and consulting, $117,000 for food, travel,and lodging expenses, and $60,000 for education.
Dr. Khan and associates concluded that the findings suggest a significant association between industry payments by drug manufacturers to physicians and Medicare spending.
Recombinant vaccine cut herpes zoster rate in immunocompromised patients
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT), results of a randomized, placebo-controlled trial indicate.
The incidence of herpes zoster was 30 per 1,000 person-years for patients who received the adjuvanted recombinant zoster vaccine (Shingrix) versus 94 per 1,000 person-years for those who received placebo, according to study results.
Recombinant zoster vaccine induced humoral and cellular responses that were strong and occurring at a rate higher than what was seen in the placebo group, said senior author Keith M. Sullivan, MD, of Duke University Medical Center, Durham, N.C., and coauthors, who reported findings on behalf of the Zoster Efficacy Study in Patients Undergoing HSCT (ZOE-HSCT) Study Group.
“The vaccinations were generally well tolerated, and most symptoms were mild and transient and did not substantially deter participants from receiving their second dose,” Dr. Sullivan and colleagues wrote in JAMA.
The risk of herpes zoster is increased for 2-3 years after autologous HSCT because of diminished T-cell immunity, according to the authors.
“Antiviral prophylaxis is commonly administered to patients after HSCT to prevent such complications, but the efficacy depends on adherence to treatment,” they said.
While vaccines could provide long-term protection, immunocompromised individuals receiving live attenuated vaccine would be at increased risk of varicella caused by spread of the vaccine strain, they added.
There have been a few encouraging recent studies of non-live vaccines in this setting, including one large phase 3 trial of a heat-inactivated varicella-zoster virus vaccine that showed patients undergoing autologous HSCT had a 63.8% estimated efficacy in preventing herpes zoster, investigators from that study said in The Lancet (2018 May 26;391[10135]:2116-27).
A phase 1/2a study of the adjuvanted recombinant zoster vaccine in patients undergoing HSCT demonstrated strong humoral and cell-mediated immunity responses, which provided the rationale for studying the vaccine further in the randomized ZOE-HSCT study, according to Dr. Sullivan and coauthors.
Their study included a total of 1,846 adults who had undergone autologous HSCT. They were randomized to receive two doses of the recombinant zoster vaccine, the first at 50-70 days after the procedure and the second 1-2 months later.
Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, which resulted in overall incidences of 30 and 94 per 1,000 person-years.
The incidence rate ratio of a first episode of herpes zoster was 0.36 for individuals receiving at least one dose, which authors said was equivalent to a vaccine efficacy of 63.7%.
That efficacy rate is “very similar” to the estimated efficacy reported for the heat-inactivated varicella-zoster virus vaccine reported in The Lancet, said Dr. Sullivan and coauthors.
However, the heat-inactivated vaccine achieved that level of protection with a four-dose schedule, including one dose given prior to autologous HSCT.
“An advantage of the short 2-dose posttransplantation schedule is that more patients might complete the vaccination program,” they said in a discussion of the results, noting that 94.7% of the recombinant zoster vaccine recipients completed two doses, compared with 81.9% of recipients who received the heat-inactivated herpes zoster vaccine in the previous report.
The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Dr. Sullivan reported disclosures related to GlaxoSmithKline (GSK), Kiadis Pharmaceutical, Roche Genentech, and the National Institute of Allergy and Infectious Diseases. Coauthors provided disclosures related to GSK, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
SOURCE: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
FROM JAMA
Key clinical point: Two doses of recombinant zoster vaccine significantly reduced incidence of herpes zoster versus placebo in adults who had undergone autologous hematopoietic stem cell transplantation (HSCT).
Major finding: Herpes zoster cases were seen in 49 and 136 individuals in the vaccine and placebo groups, respectively, resulting in overall incidences of 30 and 94 per 1,000 person-years.
Study details: A randomized clinical trial (ZOE-HSCT) including 1,846 adults who had undergone autologous HSCT.
Disclosures: The study was funded and sponsored by GlaxoSmithKline Biologicals SA. Study authors reported disclosures related to GlaxoSmithKline, Kiadis Pharmaceutical, Roche Genentech, AbbVie, Roche, Gilead, Janssen, Pharmacyclics, Morphosys, Helsinn, Celgene, and others.
Source: Bastidas A et al. JAMA. 2019 July 9. doi: 10.1001/jama.2019.9053.
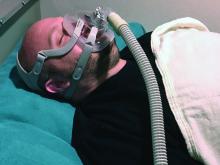
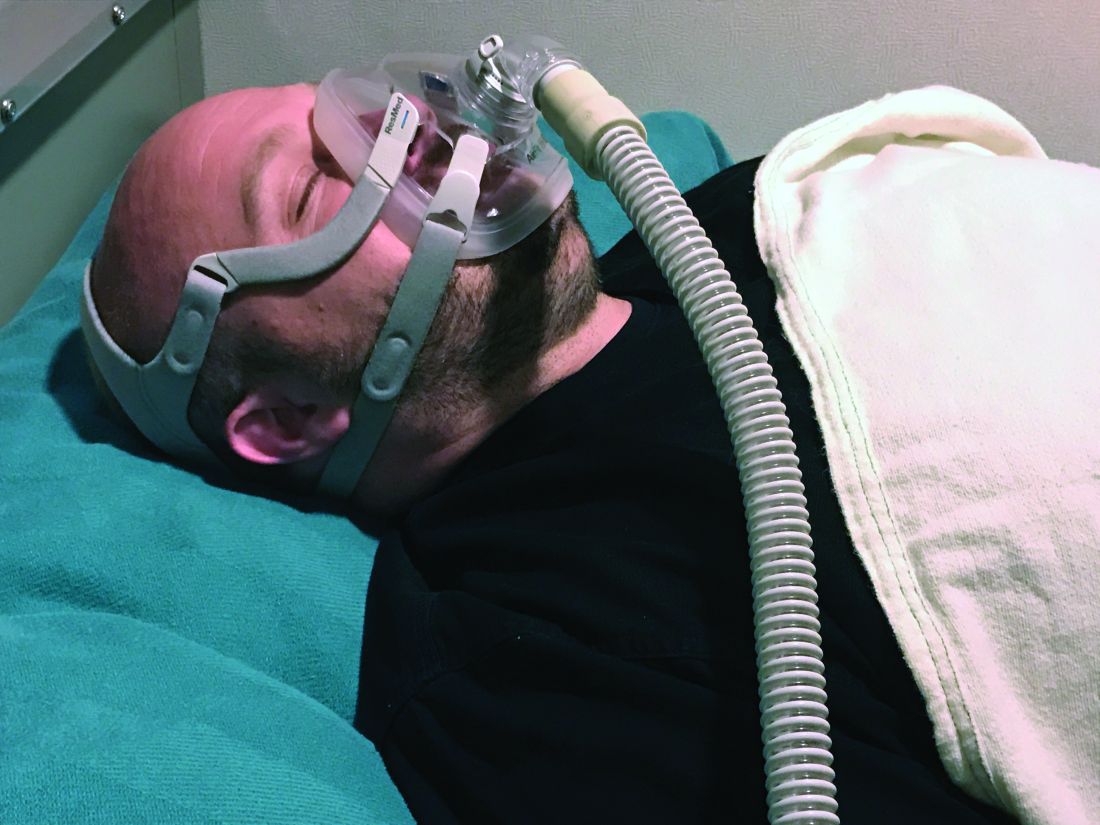
CPAP adherence varies by age, geographic location, study finds
SAN ANTONIO –
However, whether the sources of variability stem from patient factors such as disease severity and socioeconomic status, provider factors, environmental factors, or selection biases in those who are diagnosed with obstructive sleep apnea and treated with CPAP remains to be understood, lead study author Sanjay R. Patel, MD, said at the annual meeting of the Associated Professional Sleep Societies.
In 2015, the American Academy of Sleep Medicine (AASM) endorsed CPAP adherence as a process measure, and the Centers for Medicare and Medicaid Services has used CPAP adherence as an outcome measure to limit long-term coverage of the therapy. It defines CPAP adherence as 4 or more hours of use on greater than 70% of nights in a consecutive 30-day period within the first 90 days. “Strengths of CPAP adherence as an outcome measure include the fact that it is easy to measure and it predicts improvement in sleepiness, quality of life, and blood pressure control,” said Dr. Patel, who directs the University of Pittsburgh’s Center for Sleep and Cardiovascular Outcomes Research. “One issue as to whether we should use CPAP adherence as an outcome-based quality of care measure is, does variability reflect performance at the provider and/or health care system?”
In an effort to describe CPAP adherence rates in general clinical practice as well as sources of variability, Dr. Patel and colleagues evaluated telemonitoring data maintained by Philips Respironics. The study population consisted of 714,270 patients initiated on CPAP therapy between November 2015 and August 2018 who had at least one usage session of CPAP or APAP.
Overall, 90-day adherence to CPAP was 72.5%. Age, sex, and state of residence were all significantly associated with adherence rates (P less than .05). Specifically, adherence rates ranged from 54.8% among those 18-30 years of age to 79.1% among those 61-70 years of age. “There was a plateauing of adherence rates among those in their 70s, and men tended to have a higher adherence level than women across all age groups (73.3% vs. 71.4%, respectively),” he said. “Also, people who got started on CPAP in January had a higher level of adherence than people who got started in May. The differences are relatively small compared to the large age differences, but there was a consistent trend.”
When the researchers carried out age- and sex-adjusted analyses, they observed that adherence rates were lowest in the Northeast and Southwest and highest in the Upper Midwest and Mountain West. Adherence rates ranged from 50.8% in the District of Columbia and 60.5% in New York up to 81.2% in Idaho and 81.9% in South Dakota.
“The question is, is this variability explained by quality measures?” Dr. Patel asked. “We tried to answer this question by seeing whether the variability in adherence by location correlated with other metrics of health care quality.” To accomplish this, they used Dartmouth Atlas, a project that uses Medicare data to understand drivers of health care spending and quality. To understand geographic variability in CPAP adherence, they mapped ZIP codes onto hospital referral regions (HRRs), which are regional health care markets for tertiary medical care. Each HRR has at least one hospital that performs major cardiovascular procedures and neurosurgery. ZIP codes were mapped to 306 HRRs where the majority of residents get their tertiary care.
The researchers observed that Medicare enrollees who saw a primary care physician in the past 12 months had higher rates of adherence, compared with those who did not. “Twenty-three percent of the variance in CPAP adherence across the country can be explained by this measure of having a primary care doctor,” Dr. Patel said. In addition, patients who received care from HRRs located in the middle of the United States had high adherence rates. Top performers were facilities located in Madison, Wis.; Wausau, Wis.; Dubuque, Iowa; and Bloomington, Ill. Poor performers included facilities located in the boroughs of Manhattan and the Bronx, in New York; Muskegon, Mich.; Miami; and Buffalo, N.Y.
“Some of the geographical variability may be due to patient factors such as race, income, and education level,” Dr. Patel said. “That will need to be appropriately addressed in developing a quality of care measure. Nevertheless, some of the geographic variability appears to be related to health care system and provider factors. This variability could be potentially reduced through implementation of a CPAP adherence quality outcome measure.”
Dr. Patel disclosed that he has received grant/research support from Bayer Pharmaceuticals and Philips Respironics, and has served as a consultant to the American Academy of Sleep Medicine.
SOURCE: Patel SR et al. SLEEP 2019, Abstract 0513.
SAN ANTONIO –
However, whether the sources of variability stem from patient factors such as disease severity and socioeconomic status, provider factors, environmental factors, or selection biases in those who are diagnosed with obstructive sleep apnea and treated with CPAP remains to be understood, lead study author Sanjay R. Patel, MD, said at the annual meeting of the Associated Professional Sleep Societies.
In 2015, the American Academy of Sleep Medicine (AASM) endorsed CPAP adherence as a process measure, and the Centers for Medicare and Medicaid Services has used CPAP adherence as an outcome measure to limit long-term coverage of the therapy. It defines CPAP adherence as 4 or more hours of use on greater than 70% of nights in a consecutive 30-day period within the first 90 days. “Strengths of CPAP adherence as an outcome measure include the fact that it is easy to measure and it predicts improvement in sleepiness, quality of life, and blood pressure control,” said Dr. Patel, who directs the University of Pittsburgh’s Center for Sleep and Cardiovascular Outcomes Research. “One issue as to whether we should use CPAP adherence as an outcome-based quality of care measure is, does variability reflect performance at the provider and/or health care system?”
In an effort to describe CPAP adherence rates in general clinical practice as well as sources of variability, Dr. Patel and colleagues evaluated telemonitoring data maintained by Philips Respironics. The study population consisted of 714,270 patients initiated on CPAP therapy between November 2015 and August 2018 who had at least one usage session of CPAP or APAP.
Overall, 90-day adherence to CPAP was 72.5%. Age, sex, and state of residence were all significantly associated with adherence rates (P less than .05). Specifically, adherence rates ranged from 54.8% among those 18-30 years of age to 79.1% among those 61-70 years of age. “There was a plateauing of adherence rates among those in their 70s, and men tended to have a higher adherence level than women across all age groups (73.3% vs. 71.4%, respectively),” he said. “Also, people who got started on CPAP in January had a higher level of adherence than people who got started in May. The differences are relatively small compared to the large age differences, but there was a consistent trend.”
When the researchers carried out age- and sex-adjusted analyses, they observed that adherence rates were lowest in the Northeast and Southwest and highest in the Upper Midwest and Mountain West. Adherence rates ranged from 50.8% in the District of Columbia and 60.5% in New York up to 81.2% in Idaho and 81.9% in South Dakota.
“The question is, is this variability explained by quality measures?” Dr. Patel asked. “We tried to answer this question by seeing whether the variability in adherence by location correlated with other metrics of health care quality.” To accomplish this, they used Dartmouth Atlas, a project that uses Medicare data to understand drivers of health care spending and quality. To understand geographic variability in CPAP adherence, they mapped ZIP codes onto hospital referral regions (HRRs), which are regional health care markets for tertiary medical care. Each HRR has at least one hospital that performs major cardiovascular procedures and neurosurgery. ZIP codes were mapped to 306 HRRs where the majority of residents get their tertiary care.
The researchers observed that Medicare enrollees who saw a primary care physician in the past 12 months had higher rates of adherence, compared with those who did not. “Twenty-three percent of the variance in CPAP adherence across the country can be explained by this measure of having a primary care doctor,” Dr. Patel said. In addition, patients who received care from HRRs located in the middle of the United States had high adherence rates. Top performers were facilities located in Madison, Wis.; Wausau, Wis.; Dubuque, Iowa; and Bloomington, Ill. Poor performers included facilities located in the boroughs of Manhattan and the Bronx, in New York; Muskegon, Mich.; Miami; and Buffalo, N.Y.
“Some of the geographical variability may be due to patient factors such as race, income, and education level,” Dr. Patel said. “That will need to be appropriately addressed in developing a quality of care measure. Nevertheless, some of the geographic variability appears to be related to health care system and provider factors. This variability could be potentially reduced through implementation of a CPAP adherence quality outcome measure.”
Dr. Patel disclosed that he has received grant/research support from Bayer Pharmaceuticals and Philips Respironics, and has served as a consultant to the American Academy of Sleep Medicine.
SOURCE: Patel SR et al. SLEEP 2019, Abstract 0513.
SAN ANTONIO –
However, whether the sources of variability stem from patient factors such as disease severity and socioeconomic status, provider factors, environmental factors, or selection biases in those who are diagnosed with obstructive sleep apnea and treated with CPAP remains to be understood, lead study author Sanjay R. Patel, MD, said at the annual meeting of the Associated Professional Sleep Societies.
In 2015, the American Academy of Sleep Medicine (AASM) endorsed CPAP adherence as a process measure, and the Centers for Medicare and Medicaid Services has used CPAP adherence as an outcome measure to limit long-term coverage of the therapy. It defines CPAP adherence as 4 or more hours of use on greater than 70% of nights in a consecutive 30-day period within the first 90 days. “Strengths of CPAP adherence as an outcome measure include the fact that it is easy to measure and it predicts improvement in sleepiness, quality of life, and blood pressure control,” said Dr. Patel, who directs the University of Pittsburgh’s Center for Sleep and Cardiovascular Outcomes Research. “One issue as to whether we should use CPAP adherence as an outcome-based quality of care measure is, does variability reflect performance at the provider and/or health care system?”
In an effort to describe CPAP adherence rates in general clinical practice as well as sources of variability, Dr. Patel and colleagues evaluated telemonitoring data maintained by Philips Respironics. The study population consisted of 714,270 patients initiated on CPAP therapy between November 2015 and August 2018 who had at least one usage session of CPAP or APAP.
Overall, 90-day adherence to CPAP was 72.5%. Age, sex, and state of residence were all significantly associated with adherence rates (P less than .05). Specifically, adherence rates ranged from 54.8% among those 18-30 years of age to 79.1% among those 61-70 years of age. “There was a plateauing of adherence rates among those in their 70s, and men tended to have a higher adherence level than women across all age groups (73.3% vs. 71.4%, respectively),” he said. “Also, people who got started on CPAP in January had a higher level of adherence than people who got started in May. The differences are relatively small compared to the large age differences, but there was a consistent trend.”
When the researchers carried out age- and sex-adjusted analyses, they observed that adherence rates were lowest in the Northeast and Southwest and highest in the Upper Midwest and Mountain West. Adherence rates ranged from 50.8% in the District of Columbia and 60.5% in New York up to 81.2% in Idaho and 81.9% in South Dakota.
“The question is, is this variability explained by quality measures?” Dr. Patel asked. “We tried to answer this question by seeing whether the variability in adherence by location correlated with other metrics of health care quality.” To accomplish this, they used Dartmouth Atlas, a project that uses Medicare data to understand drivers of health care spending and quality. To understand geographic variability in CPAP adherence, they mapped ZIP codes onto hospital referral regions (HRRs), which are regional health care markets for tertiary medical care. Each HRR has at least one hospital that performs major cardiovascular procedures and neurosurgery. ZIP codes were mapped to 306 HRRs where the majority of residents get their tertiary care.
The researchers observed that Medicare enrollees who saw a primary care physician in the past 12 months had higher rates of adherence, compared with those who did not. “Twenty-three percent of the variance in CPAP adherence across the country can be explained by this measure of having a primary care doctor,” Dr. Patel said. In addition, patients who received care from HRRs located in the middle of the United States had high adherence rates. Top performers were facilities located in Madison, Wis.; Wausau, Wis.; Dubuque, Iowa; and Bloomington, Ill. Poor performers included facilities located in the boroughs of Manhattan and the Bronx, in New York; Muskegon, Mich.; Miami; and Buffalo, N.Y.
“Some of the geographical variability may be due to patient factors such as race, income, and education level,” Dr. Patel said. “That will need to be appropriately addressed in developing a quality of care measure. Nevertheless, some of the geographic variability appears to be related to health care system and provider factors. This variability could be potentially reduced through implementation of a CPAP adherence quality outcome measure.”
Dr. Patel disclosed that he has received grant/research support from Bayer Pharmaceuticals and Philips Respironics, and has served as a consultant to the American Academy of Sleep Medicine.
SOURCE: Patel SR et al. SLEEP 2019, Abstract 0513.
REPORTING FROM SLEEP 2019
IHS and Cherokee Nation Launch HIV-Prevention Project
The Indian Health Service (IHS) and the Cherokee Nation Health Service are launching a new pilot project to help “accelerate progress” toward ending the HIV epidemic in native communities.
The project, which will investigate the most effective prevention strategies and share the findings locally, is part of the initiative Ending the HIV Epidemic: A Plan for America. That plan focuses prevention and treatment efforts on 48 counties and 7 southern states with a higher proportion of HIV diagnosis in rural areas. The Cherokee Nation is in Oklahoma, which has the highest American Indian population among the 7 southern states.
Recent data show new HIV infections at the lowest level yet, but progress in prevention has slowed, in part due to new threats such as the opioid crisis: 10% of new HIV infections are among injectable-drug users.
The Cherokee Nation’s proven track record in hepatitis C prevention and treatment makes it a valuable partner in the project. Half of its health services patients have been screened, and among the 3.2% testing positive, 90% have been cured. The pilot project will use a similar model, IHS says. Current statistics show that 35% of Cherokee National patients using the tribe’s health centers have been screened for HIV, with < 1% testing positive. Of the patients diagnosed with HIV, 90% are receiving care and 90% of those are virally suppressed. The pilot project is aimed at boosting the screening numbers.
The pilot is one of several HHS efforts to jumpstart key activities in select communities using resources from the Minority HIV/AIDS fund. The CDC also is launching projects in select communities.
“Improved health care over multiple generations is our top priority,” said Cherokee Nation Principal Chief Bill John Baker. “If we can collaborate with our federal partners at IHS to raise awareness, increase education, and actively work to prevent new cases of HIV, then we will be creating a healthier future for northeast Oklahoma.”
The Indian Health Service (IHS) and the Cherokee Nation Health Service are launching a new pilot project to help “accelerate progress” toward ending the HIV epidemic in native communities.
The project, which will investigate the most effective prevention strategies and share the findings locally, is part of the initiative Ending the HIV Epidemic: A Plan for America. That plan focuses prevention and treatment efforts on 48 counties and 7 southern states with a higher proportion of HIV diagnosis in rural areas. The Cherokee Nation is in Oklahoma, which has the highest American Indian population among the 7 southern states.
Recent data show new HIV infections at the lowest level yet, but progress in prevention has slowed, in part due to new threats such as the opioid crisis: 10% of new HIV infections are among injectable-drug users.
The Cherokee Nation’s proven track record in hepatitis C prevention and treatment makes it a valuable partner in the project. Half of its health services patients have been screened, and among the 3.2% testing positive, 90% have been cured. The pilot project will use a similar model, IHS says. Current statistics show that 35% of Cherokee National patients using the tribe’s health centers have been screened for HIV, with < 1% testing positive. Of the patients diagnosed with HIV, 90% are receiving care and 90% of those are virally suppressed. The pilot project is aimed at boosting the screening numbers.
The pilot is one of several HHS efforts to jumpstart key activities in select communities using resources from the Minority HIV/AIDS fund. The CDC also is launching projects in select communities.
“Improved health care over multiple generations is our top priority,” said Cherokee Nation Principal Chief Bill John Baker. “If we can collaborate with our federal partners at IHS to raise awareness, increase education, and actively work to prevent new cases of HIV, then we will be creating a healthier future for northeast Oklahoma.”
The Indian Health Service (IHS) and the Cherokee Nation Health Service are launching a new pilot project to help “accelerate progress” toward ending the HIV epidemic in native communities.
The project, which will investigate the most effective prevention strategies and share the findings locally, is part of the initiative Ending the HIV Epidemic: A Plan for America. That plan focuses prevention and treatment efforts on 48 counties and 7 southern states with a higher proportion of HIV diagnosis in rural areas. The Cherokee Nation is in Oklahoma, which has the highest American Indian population among the 7 southern states.
Recent data show new HIV infections at the lowest level yet, but progress in prevention has slowed, in part due to new threats such as the opioid crisis: 10% of new HIV infections are among injectable-drug users.
The Cherokee Nation’s proven track record in hepatitis C prevention and treatment makes it a valuable partner in the project. Half of its health services patients have been screened, and among the 3.2% testing positive, 90% have been cured. The pilot project will use a similar model, IHS says. Current statistics show that 35% of Cherokee National patients using the tribe’s health centers have been screened for HIV, with < 1% testing positive. Of the patients diagnosed with HIV, 90% are receiving care and 90% of those are virally suppressed. The pilot project is aimed at boosting the screening numbers.
The pilot is one of several HHS efforts to jumpstart key activities in select communities using resources from the Minority HIV/AIDS fund. The CDC also is launching projects in select communities.
“Improved health care over multiple generations is our top priority,” said Cherokee Nation Principal Chief Bill John Baker. “If we can collaborate with our federal partners at IHS to raise awareness, increase education, and actively work to prevent new cases of HIV, then we will be creating a healthier future for northeast Oklahoma.”
Round 1: Biologics, JAK inhibitors training up for atopic dermatitis ‘boxing match’
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
MILAN – Get ready for a “boxing match” (AD), Thomas Bieber, MD, PhD, said in an oral presentation at the World Congress of Dermatology.
“In one corner of the ring you will have the biologics, and in the other corner of the ring, you will have the Janus kinase (JAK) inhibitors and some other small molecules,” said Dr. Bieber, medical director of dermatology and allergy, at University Hospital Bonn, Germany.
There will be no winner-take-all scenario, since AD is a heterogeneous disease for which a variety of treatments will be needed, Dr. Bieber noted. However, he said, there are clear differences in mode of action, safety, mode of application, price, and more that will be important as dermatologists weigh the choice of biologics versus JAK inhibitors for a specific patient.
“Currently, dupilumab is the only one that is on the market (in the European Union) and this molecule is really a revolution for us,” Dr. Bieber said at the meeting. “But we will see in the next years, many, many other molecules coming up.”
Beyond dupilumab (Dupixent), the interleukin-4 (IL-4) receptor alpha antagonist approved by the Food and Drug Administration in 2017, biologics under development include tralokinumab, lebrikizumab, and nemolizumab, among others. Development on the small molecule side includes agents such as ZPL-389, a histamine H4 receptor agonist, but is dominated by JAK inhibitors such as baricitinib(Olumiant), approved by the FDA in 2018 for rheumatoid arthritis; upadacitinib; and abrocitinib, according to Dr. Bieber.
There are advantages and disadvantages to each class of molecule, he said. For example, JAK inhibitors act very rapidly to control itch and improve exacerbations, while biologics are “much slower” and provide delayed control of inflammation, he said. In terms of safety, by contrast, biologics probably offer a “better” benefit-to-risk ratio, with no risk for drug-drug interactions and no monitoring needed, while JAK inhibitors appear to have a “narrow” therapeutic window, with a risk for drug-drug interactions and a need for some monitoring, he said.
JAK inhibitors come in a tablet formulation, which, from the patient perspective, may be more convenient and acceptable compared with repeated injections of biologics every 2 or 4 weeks, Dr. Bieber noted. Another point potentially in favor of JAK inhibitors is price: “I think the current situation tells us clearly that the biologics are the more expensive drugs, while the small molecules typically are much less expensive,” he said.
One theoretical advantage of biologics, he said, is the potential for disease modification, while the disease-modifying potential of JAK inhibitors is unclear, as of yet. “We have not had enough experience with all these molecules in order to understand how we can do that particular job, in terms of switching off the disease,” he said.
In terms of the need for patient stratification in the future, it’s a draw between biologics and JAK inhibitors, Dr. Bieber said. “You will not see any of these drugs doing 100 percent of the job in 100 percent of the patients, so to me, currently from my understanding of this disease, there is no one-size-fits-all molecule.”
Toward that end, machine learning and artificial intelligence may aid in evaluating large amounts of data to better understand AD phenotypes. “That’s really something that is a particular kind of challenge in the next years, in order to improve at-the-end drug development, and the management of our patients,” he concluded.
Dr. Bieber reported disclosures related to LEO Pharmaceuticals, Regeneron, and Sanofi.
EXPERT ANALYSIS FROM WCD2019
Clinical Pearl: Benzethonium Chloride for Habit-Tic Nail Deformity
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).

Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).

Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
Practice Gap
Habit-tic nail deformity results from repetitive manipulation of the cuticle and/or proximal nail fold. It most commonly affects one or both thumbnails and presents with a characteristic longitudinal midline furrow with parallel transverse ridges in the nail plate. Complications may include permanent onychodystrophy, frictional melanonychia, and infections. Treatment is challenging, as diagnosis first requires patient insight to the cause of symptoms. Therapeutic options include nonpharmacologic techniques (eg, occlusion of the nails to prevent trauma, cyanoacrylate adhesives, cognitive behavioral therapy) and pharmacologic techniques (eg, N-acetyl cysteine, selective serotonin reuptake inhibitors, tricyclic antidepressants, antipsychotics), with limited supporting data and potential adverse effects.1
The Technique
Benzethonium chloride solution 0.2% is an antiseptic that creates a polymeric layer that binds to the skin. It normally is used to treat small skin erosions and prevent blisters. In patients with habit-tic nail deformity, we recommend once-daily application of benzethonium chloride to the proximal nail fold, thereby artificially recreating the cuticle and forming a sustainable barrier from trauma (Figure, A). Patients should be reminded not to manipulate the cuticle and/or nail fold during treatment. In one 36-year-old man with habit tic nail deformity, we saw clear nail growth after 4 months of treatment (Figure, B).

Practice Implications
Successful treatment of habit-tic nail deformity requires patients to have some insight into their behavior. The benzethonium chloride serves as a reminder for patients to stop picking as an unfamiliar artificial barrier and reminds them to substitute the picking behavior for another more positive behavior. Therefore, benzethonium chloride may be offered to patients as a novel therapy to both protect the cuticle and alter behavior in patients with habit-tic nail deformity, as it can be difficult to treat with few available therapies.
Allergic contact dermatitis to benzethonium chloride is a potential side effect and patients should be cautioned prior to treatment; however, it is extremely rare with 6 cases reported to date based on a PubMed search of articles indexed for MEDLINE using the terms allergic contact dermatitis and benzethonium chloride,2 and much rarer than contact allergy to cyanoacrylates.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.
- Halteh P, Scher RK, Lipner SR. Onychotillomania: diagnosis and management. Am J Clin Dermatol. 2017;18:763-770.
- Hirata Y, Yanagi T, Yamaguchi Y, et al. Ulcerative contact dermatitis caused by benzethonium chloride. Contact Dermatitis. 2017;76:188-190.