User login
Interventional cardiology pioneer reflects on field’s past, future
SNOWMASS, COLO. – When Spencer B. King III, MD, shared his thoughts about the future of interventional cardiology at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology, he felt compelled to offer a cautionary note about his past accuracy as a prognosticator.
It was way back at a poster session during the 1976 annual meeting of the American Heart Association in Miami Beach that he first met Andreas Gruentzig, MD, the father of percutaneous coronary intervention (PCI), who was presenting his initial revolutionary work on what he called “coronary transluminal angioplasty” in dogs.
“I looked at the poster and told him it would never work,” recalled Dr. King, professor emeritus of medicine at Emory University in Atlanta.
He soon changed his mind, however, because, to great acclaim, Dr. Gruentzig performed his successful first in-human coronary angioplasty the next year.
He noted that the Snowmass conference has played a significant role in the development of interventional cardiology in the United States. Dr. Gruentzig attended the conference in 1980, and Dr. King and others took that opportunity to persuade him to leave the bureaucratic confines of Zurich and join him at Emory later that year. The two cardiologists worked closely thereafter, refining angioplasty and conducting clinical trials until Dr. Gruentzig’s death in an airplane crash in Georgia in 1985 at age 46 years.
Turning to the future, Dr. King addressed a number of recent developments in interventional cardiology and rated their chances of significantly improving outcomes in patients with stable ischemic heart disease. He graded the innovations’ potential with use of a four-bar schema, akin to the WiFi signal power rating on a cell phone.
Noninvasive diagnostics to assess anatomy and physiology
“I think coronary CT angiography [CTA] will become the new diagnostic angiogram,” he predicted. “CTA has gotten much better. Outside the United States, in Europe and particularly in Japan and increasingly in China, CTA is becoming extremely common.”
Dr. King cited a recent multicenter study of blinded heart team treatment decision making on the basis of either CTA or conventional invasive angiography in 223 patients with left main or triple-vessel coronary artery disease (CAD). The level of agreement was impressively high: Coronary artery bypass grafting (CABG) was recommended for 28% of patients on the basis of CTA and 26% with conventional angiography, which suggests the feasibility of treatment decision making based solely on noninvasive imaging, history, and clinical examination (Eur Heart J. 2018 Nov 1;39[41]:3689-98).
“The other thing I like about the potential for noninvasive imaging to guide our interventions is that it may [replace] the diagnostic angiogram, which has largely become extinct,” the cardiologist continued. “If you think about it, patients are referred for an angiogram, and as far as informed consent is concerned, the patient is told to pack his bags, go off to some other city, get in the cath lab, and take the family because of what they might do to you. They might put stents in you, they might operate on you. We don’t have any idea because we don’t know what you have. And the patient has to buy into this. With CTA, the potential is there for people to actually know what you’re going to do to them before you do it.”
Coronary artery calcium scoring for primary risk assessment has taken on a prominent role in the latest practice guidelines. “I think it’s mostly helpful in getting people out of the system because they don’t have any calcium,” in Dr. King’s view.
PET and MRI will remain secondary noninvasive technologies. They will be used mostly to diagnose microvascular disease, but that’s information that doesn’t have much influence on whether interventional procedures are performed.
Overall, he gave noninvasive diagnostic tools high marks for their potential to improve outcomes in patients with stable ischemic heart disease.
“I give it a pretty robust three bars. Maybe you could give it four,” he said.
New pharmacologic therapies
Citing in particular the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and the sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. King declared, “It may be that the biggest, newest device in interventional cardiology going forward is not a device at all, it’s medical therapy.”
Interventional cardiologists either need to become expert in advanced medical therapies or else have access to someone in their group who prescribes those medications deftly.
“The future care of our patients will require more than percutaneous coronary intervention,” he emphasized.
So, four bars for the new medical therapies.
PCI and coronary artery bypass surgery
Both get one bar.
“PCI will be a partner of advanced antiatherosclerotic therapies, but will not be replaced by limiting antianginal therapy to medical treatment only,” Dr. King predicted.
Regarding CABG, he highlighted a recent systematic review and pooled analysis of 11,518 patients with stable ischemic heart disease randomized to CABG or PCI using drug-eluting or bare-metal stents in 11 clinical trials. CABG demonstrated a significant mortality benefit over PCI in patients with multivessel disease, particularly among those with diabetes or a higher degree of coronary disease complexity. However, there was no benefit in terms of 5-year all-cause mortality for CABG over PCI in those with left main disease (Lancet. 2018 Mar 10;391[10124]:9399-48).
“CABG will not go away. I predict that about 25% of revascularizations will continue to be done by surgery,” the cardiologist said. “For patients who can have complete revascularization by PCI, it’ll be done with advanced technology, but probably only by a subset of operators. We have a huge number of interventional cardiologists in this country, and some of them do a lot of these kinds of cases and some don’t.”
Endovascular imaging to optimize stent deployment and characterize plaque
Studies suggest that the use of intravascular ultrasound and other endovascular imaging technologies ends up providing better results than when they’re not employed.
“We see greatly increased use of IVUS [intravascular ultrasound], and not so much of optical coherence tomography, because of technical problems. So I give this at least two bars as far as moving practice forward,” according to Dr. King.
Bioresorbable scaffolds
This technology, which he noted “was supposed to solve all of our problems,” has tripped and fallen because of its associated increased risk of scaffold thrombosis. He cited a recent network meta-analysis of 91 randomized, controlled trials comparing bioresorbable scaffolds to current-generation metallic drug-eluting stents in more than 105,000 patients. The bioresorbable scaffolds had a significantly higher rate of scaffold thrombosis in the first 30 days after implantation, as well as from 31 days through 1 year and also beyond 1 year. In fact, there was a rising trend for scaffold thrombosis in the bioresorbable device group after the 1 year mark through a mean 3.7 years of follow-up (EuroIntervention. 2018 Mar 20;13[16]:1904-13).
“The overall impact of bioresorbable scaffolds has been nil. We don’t have them. Bioresorbable scaffolds may become noninferior to the best metal stents, but to become mainstream, they should show superiority,” the cardiologist said.
One bar, based on the uncertain possibility that new bioresorbable scaffolds now in early stages of development ultimately pan out.
Future training needs
PCI operator volumes are low, and that raises a host of issues regarding future training needs. Should fewer interventionalists be trained? Should training in endovascular imaging be a mandatory part of PCI training? Should interventional cardiology be divided into distinct coronary, structural heart, and peripheral vascular subspecialty domains involving different people, a change that is already informally underway in many places? How are operators who are interested in becoming experts in PCI for chronic total occlusion, diffuse disease, left main disease, or other complex cases going to get enough experience to be able to concentrate in those areas?
These are questions that will need to be addressed in the coming years. The answers will surely affect the delivery of interventional cardiology care.
Dr. King reported having no financial conflicts regarding his presentation.
SOURCE: King SB.
SNOWMASS, COLO. – When Spencer B. King III, MD, shared his thoughts about the future of interventional cardiology at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology, he felt compelled to offer a cautionary note about his past accuracy as a prognosticator.
It was way back at a poster session during the 1976 annual meeting of the American Heart Association in Miami Beach that he first met Andreas Gruentzig, MD, the father of percutaneous coronary intervention (PCI), who was presenting his initial revolutionary work on what he called “coronary transluminal angioplasty” in dogs.
“I looked at the poster and told him it would never work,” recalled Dr. King, professor emeritus of medicine at Emory University in Atlanta.
He soon changed his mind, however, because, to great acclaim, Dr. Gruentzig performed his successful first in-human coronary angioplasty the next year.
He noted that the Snowmass conference has played a significant role in the development of interventional cardiology in the United States. Dr. Gruentzig attended the conference in 1980, and Dr. King and others took that opportunity to persuade him to leave the bureaucratic confines of Zurich and join him at Emory later that year. The two cardiologists worked closely thereafter, refining angioplasty and conducting clinical trials until Dr. Gruentzig’s death in an airplane crash in Georgia in 1985 at age 46 years.
Turning to the future, Dr. King addressed a number of recent developments in interventional cardiology and rated their chances of significantly improving outcomes in patients with stable ischemic heart disease. He graded the innovations’ potential with use of a four-bar schema, akin to the WiFi signal power rating on a cell phone.
Noninvasive diagnostics to assess anatomy and physiology
“I think coronary CT angiography [CTA] will become the new diagnostic angiogram,” he predicted. “CTA has gotten much better. Outside the United States, in Europe and particularly in Japan and increasingly in China, CTA is becoming extremely common.”
Dr. King cited a recent multicenter study of blinded heart team treatment decision making on the basis of either CTA or conventional invasive angiography in 223 patients with left main or triple-vessel coronary artery disease (CAD). The level of agreement was impressively high: Coronary artery bypass grafting (CABG) was recommended for 28% of patients on the basis of CTA and 26% with conventional angiography, which suggests the feasibility of treatment decision making based solely on noninvasive imaging, history, and clinical examination (Eur Heart J. 2018 Nov 1;39[41]:3689-98).
“The other thing I like about the potential for noninvasive imaging to guide our interventions is that it may [replace] the diagnostic angiogram, which has largely become extinct,” the cardiologist continued. “If you think about it, patients are referred for an angiogram, and as far as informed consent is concerned, the patient is told to pack his bags, go off to some other city, get in the cath lab, and take the family because of what they might do to you. They might put stents in you, they might operate on you. We don’t have any idea because we don’t know what you have. And the patient has to buy into this. With CTA, the potential is there for people to actually know what you’re going to do to them before you do it.”
Coronary artery calcium scoring for primary risk assessment has taken on a prominent role in the latest practice guidelines. “I think it’s mostly helpful in getting people out of the system because they don’t have any calcium,” in Dr. King’s view.
PET and MRI will remain secondary noninvasive technologies. They will be used mostly to diagnose microvascular disease, but that’s information that doesn’t have much influence on whether interventional procedures are performed.
Overall, he gave noninvasive diagnostic tools high marks for their potential to improve outcomes in patients with stable ischemic heart disease.
“I give it a pretty robust three bars. Maybe you could give it four,” he said.
New pharmacologic therapies
Citing in particular the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and the sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. King declared, “It may be that the biggest, newest device in interventional cardiology going forward is not a device at all, it’s medical therapy.”
Interventional cardiologists either need to become expert in advanced medical therapies or else have access to someone in their group who prescribes those medications deftly.
“The future care of our patients will require more than percutaneous coronary intervention,” he emphasized.
So, four bars for the new medical therapies.
PCI and coronary artery bypass surgery
Both get one bar.
“PCI will be a partner of advanced antiatherosclerotic therapies, but will not be replaced by limiting antianginal therapy to medical treatment only,” Dr. King predicted.
Regarding CABG, he highlighted a recent systematic review and pooled analysis of 11,518 patients with stable ischemic heart disease randomized to CABG or PCI using drug-eluting or bare-metal stents in 11 clinical trials. CABG demonstrated a significant mortality benefit over PCI in patients with multivessel disease, particularly among those with diabetes or a higher degree of coronary disease complexity. However, there was no benefit in terms of 5-year all-cause mortality for CABG over PCI in those with left main disease (Lancet. 2018 Mar 10;391[10124]:9399-48).
“CABG will not go away. I predict that about 25% of revascularizations will continue to be done by surgery,” the cardiologist said. “For patients who can have complete revascularization by PCI, it’ll be done with advanced technology, but probably only by a subset of operators. We have a huge number of interventional cardiologists in this country, and some of them do a lot of these kinds of cases and some don’t.”
Endovascular imaging to optimize stent deployment and characterize plaque
Studies suggest that the use of intravascular ultrasound and other endovascular imaging technologies ends up providing better results than when they’re not employed.
“We see greatly increased use of IVUS [intravascular ultrasound], and not so much of optical coherence tomography, because of technical problems. So I give this at least two bars as far as moving practice forward,” according to Dr. King.
Bioresorbable scaffolds
This technology, which he noted “was supposed to solve all of our problems,” has tripped and fallen because of its associated increased risk of scaffold thrombosis. He cited a recent network meta-analysis of 91 randomized, controlled trials comparing bioresorbable scaffolds to current-generation metallic drug-eluting stents in more than 105,000 patients. The bioresorbable scaffolds had a significantly higher rate of scaffold thrombosis in the first 30 days after implantation, as well as from 31 days through 1 year and also beyond 1 year. In fact, there was a rising trend for scaffold thrombosis in the bioresorbable device group after the 1 year mark through a mean 3.7 years of follow-up (EuroIntervention. 2018 Mar 20;13[16]:1904-13).
“The overall impact of bioresorbable scaffolds has been nil. We don’t have them. Bioresorbable scaffolds may become noninferior to the best metal stents, but to become mainstream, they should show superiority,” the cardiologist said.
One bar, based on the uncertain possibility that new bioresorbable scaffolds now in early stages of development ultimately pan out.
Future training needs
PCI operator volumes are low, and that raises a host of issues regarding future training needs. Should fewer interventionalists be trained? Should training in endovascular imaging be a mandatory part of PCI training? Should interventional cardiology be divided into distinct coronary, structural heart, and peripheral vascular subspecialty domains involving different people, a change that is already informally underway in many places? How are operators who are interested in becoming experts in PCI for chronic total occlusion, diffuse disease, left main disease, or other complex cases going to get enough experience to be able to concentrate in those areas?
These are questions that will need to be addressed in the coming years. The answers will surely affect the delivery of interventional cardiology care.
Dr. King reported having no financial conflicts regarding his presentation.
SOURCE: King SB.
SNOWMASS, COLO. – When Spencer B. King III, MD, shared his thoughts about the future of interventional cardiology at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology, he felt compelled to offer a cautionary note about his past accuracy as a prognosticator.
It was way back at a poster session during the 1976 annual meeting of the American Heart Association in Miami Beach that he first met Andreas Gruentzig, MD, the father of percutaneous coronary intervention (PCI), who was presenting his initial revolutionary work on what he called “coronary transluminal angioplasty” in dogs.
“I looked at the poster and told him it would never work,” recalled Dr. King, professor emeritus of medicine at Emory University in Atlanta.
He soon changed his mind, however, because, to great acclaim, Dr. Gruentzig performed his successful first in-human coronary angioplasty the next year.
He noted that the Snowmass conference has played a significant role in the development of interventional cardiology in the United States. Dr. Gruentzig attended the conference in 1980, and Dr. King and others took that opportunity to persuade him to leave the bureaucratic confines of Zurich and join him at Emory later that year. The two cardiologists worked closely thereafter, refining angioplasty and conducting clinical trials until Dr. Gruentzig’s death in an airplane crash in Georgia in 1985 at age 46 years.
Turning to the future, Dr. King addressed a number of recent developments in interventional cardiology and rated their chances of significantly improving outcomes in patients with stable ischemic heart disease. He graded the innovations’ potential with use of a four-bar schema, akin to the WiFi signal power rating on a cell phone.
Noninvasive diagnostics to assess anatomy and physiology
“I think coronary CT angiography [CTA] will become the new diagnostic angiogram,” he predicted. “CTA has gotten much better. Outside the United States, in Europe and particularly in Japan and increasingly in China, CTA is becoming extremely common.”
Dr. King cited a recent multicenter study of blinded heart team treatment decision making on the basis of either CTA or conventional invasive angiography in 223 patients with left main or triple-vessel coronary artery disease (CAD). The level of agreement was impressively high: Coronary artery bypass grafting (CABG) was recommended for 28% of patients on the basis of CTA and 26% with conventional angiography, which suggests the feasibility of treatment decision making based solely on noninvasive imaging, history, and clinical examination (Eur Heart J. 2018 Nov 1;39[41]:3689-98).
“The other thing I like about the potential for noninvasive imaging to guide our interventions is that it may [replace] the diagnostic angiogram, which has largely become extinct,” the cardiologist continued. “If you think about it, patients are referred for an angiogram, and as far as informed consent is concerned, the patient is told to pack his bags, go off to some other city, get in the cath lab, and take the family because of what they might do to you. They might put stents in you, they might operate on you. We don’t have any idea because we don’t know what you have. And the patient has to buy into this. With CTA, the potential is there for people to actually know what you’re going to do to them before you do it.”
Coronary artery calcium scoring for primary risk assessment has taken on a prominent role in the latest practice guidelines. “I think it’s mostly helpful in getting people out of the system because they don’t have any calcium,” in Dr. King’s view.
PET and MRI will remain secondary noninvasive technologies. They will be used mostly to diagnose microvascular disease, but that’s information that doesn’t have much influence on whether interventional procedures are performed.
Overall, he gave noninvasive diagnostic tools high marks for their potential to improve outcomes in patients with stable ischemic heart disease.
“I give it a pretty robust three bars. Maybe you could give it four,” he said.
New pharmacologic therapies
Citing in particular the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and the sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. King declared, “It may be that the biggest, newest device in interventional cardiology going forward is not a device at all, it’s medical therapy.”
Interventional cardiologists either need to become expert in advanced medical therapies or else have access to someone in their group who prescribes those medications deftly.
“The future care of our patients will require more than percutaneous coronary intervention,” he emphasized.
So, four bars for the new medical therapies.
PCI and coronary artery bypass surgery
Both get one bar.
“PCI will be a partner of advanced antiatherosclerotic therapies, but will not be replaced by limiting antianginal therapy to medical treatment only,” Dr. King predicted.
Regarding CABG, he highlighted a recent systematic review and pooled analysis of 11,518 patients with stable ischemic heart disease randomized to CABG or PCI using drug-eluting or bare-metal stents in 11 clinical trials. CABG demonstrated a significant mortality benefit over PCI in patients with multivessel disease, particularly among those with diabetes or a higher degree of coronary disease complexity. However, there was no benefit in terms of 5-year all-cause mortality for CABG over PCI in those with left main disease (Lancet. 2018 Mar 10;391[10124]:9399-48).
“CABG will not go away. I predict that about 25% of revascularizations will continue to be done by surgery,” the cardiologist said. “For patients who can have complete revascularization by PCI, it’ll be done with advanced technology, but probably only by a subset of operators. We have a huge number of interventional cardiologists in this country, and some of them do a lot of these kinds of cases and some don’t.”
Endovascular imaging to optimize stent deployment and characterize plaque
Studies suggest that the use of intravascular ultrasound and other endovascular imaging technologies ends up providing better results than when they’re not employed.
“We see greatly increased use of IVUS [intravascular ultrasound], and not so much of optical coherence tomography, because of technical problems. So I give this at least two bars as far as moving practice forward,” according to Dr. King.
Bioresorbable scaffolds
This technology, which he noted “was supposed to solve all of our problems,” has tripped and fallen because of its associated increased risk of scaffold thrombosis. He cited a recent network meta-analysis of 91 randomized, controlled trials comparing bioresorbable scaffolds to current-generation metallic drug-eluting stents in more than 105,000 patients. The bioresorbable scaffolds had a significantly higher rate of scaffold thrombosis in the first 30 days after implantation, as well as from 31 days through 1 year and also beyond 1 year. In fact, there was a rising trend for scaffold thrombosis in the bioresorbable device group after the 1 year mark through a mean 3.7 years of follow-up (EuroIntervention. 2018 Mar 20;13[16]:1904-13).
“The overall impact of bioresorbable scaffolds has been nil. We don’t have them. Bioresorbable scaffolds may become noninferior to the best metal stents, but to become mainstream, they should show superiority,” the cardiologist said.
One bar, based on the uncertain possibility that new bioresorbable scaffolds now in early stages of development ultimately pan out.
Future training needs
PCI operator volumes are low, and that raises a host of issues regarding future training needs. Should fewer interventionalists be trained? Should training in endovascular imaging be a mandatory part of PCI training? Should interventional cardiology be divided into distinct coronary, structural heart, and peripheral vascular subspecialty domains involving different people, a change that is already informally underway in many places? How are operators who are interested in becoming experts in PCI for chronic total occlusion, diffuse disease, left main disease, or other complex cases going to get enough experience to be able to concentrate in those areas?
These are questions that will need to be addressed in the coming years. The answers will surely affect the delivery of interventional cardiology care.
Dr. King reported having no financial conflicts regarding his presentation.
SOURCE: King SB.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Destress dermatologic procedures with honesty, distraction, relaxation
according to a report published in Pediatric Dermatology.
For many children, the anticipation of pain and the anxiety about a procedure results in a more painful experience, wrote Andrew M. Armenta of the University of Texas, Galveston, and his colleagues. Preparing children in advance and using cognitive behavioral therapy (CBT) strategies in the moment can help reduce their anxiety.
“CBT is a skill‐based approach that focuses on the present and aims to teach efficient ways of identifying distorted thinking, modifying beliefs, and changing behaviors for a more favorable outcome of real‐life situations,” they wrote.
First, Dr. Armenta and his associates advised, be honest with children about what to expect from a procedure. Evidence does not support phrases such as, “It won’t hurt,” or “It will be over soon,” to reduce anxiety.
Timing the disclosure of a procedure and creating the appropriate setting also can help reduce anxiety. For very young children, short notice of a procedure is often best, with the promise of a small reward or outing afterward. Older children may want some advance notice so they can feel prepared, but their specific concerns should be addressed.
CBT-based techniques include deep breathing and positive coping statements such as “I can do this” for older children, or encouraging them to talk about a family pet or listen to music. Younger children may be distracted with pinwheels, rattles, or songs. “Additionally, in recent years, virtual reality headsets have even proved to be effective distractors, resulting in an overall reduction in both pain and fear,” Dr. Armenta and his associates noted.
Other useful strategies include allowing children to choose their position and location for an injection or procedure when possible. Small children may be able to sit on the lap of an adult, and older children may prefer sitting up to lying down. Avoid physical restraint unless it is absolutely necessary for safety, the researchers emphasized.
Incorporating CBT-based strategies of breathing and distraction with honesty and respectful disclosure of what is being done and why “not only makes practicing pediatric dermatology easier, but also can improve patient adherence to painful procedures,” they said.
No disclosure information was given.
SOURCE: Armenta AM et al. Pediatr Dermatol. 2019. doi: 10.1111/pde.13739.
according to a report published in Pediatric Dermatology.
For many children, the anticipation of pain and the anxiety about a procedure results in a more painful experience, wrote Andrew M. Armenta of the University of Texas, Galveston, and his colleagues. Preparing children in advance and using cognitive behavioral therapy (CBT) strategies in the moment can help reduce their anxiety.
“CBT is a skill‐based approach that focuses on the present and aims to teach efficient ways of identifying distorted thinking, modifying beliefs, and changing behaviors for a more favorable outcome of real‐life situations,” they wrote.
First, Dr. Armenta and his associates advised, be honest with children about what to expect from a procedure. Evidence does not support phrases such as, “It won’t hurt,” or “It will be over soon,” to reduce anxiety.
Timing the disclosure of a procedure and creating the appropriate setting also can help reduce anxiety. For very young children, short notice of a procedure is often best, with the promise of a small reward or outing afterward. Older children may want some advance notice so they can feel prepared, but their specific concerns should be addressed.
CBT-based techniques include deep breathing and positive coping statements such as “I can do this” for older children, or encouraging them to talk about a family pet or listen to music. Younger children may be distracted with pinwheels, rattles, or songs. “Additionally, in recent years, virtual reality headsets have even proved to be effective distractors, resulting in an overall reduction in both pain and fear,” Dr. Armenta and his associates noted.
Other useful strategies include allowing children to choose their position and location for an injection or procedure when possible. Small children may be able to sit on the lap of an adult, and older children may prefer sitting up to lying down. Avoid physical restraint unless it is absolutely necessary for safety, the researchers emphasized.
Incorporating CBT-based strategies of breathing and distraction with honesty and respectful disclosure of what is being done and why “not only makes practicing pediatric dermatology easier, but also can improve patient adherence to painful procedures,” they said.
No disclosure information was given.
SOURCE: Armenta AM et al. Pediatr Dermatol. 2019. doi: 10.1111/pde.13739.
according to a report published in Pediatric Dermatology.
For many children, the anticipation of pain and the anxiety about a procedure results in a more painful experience, wrote Andrew M. Armenta of the University of Texas, Galveston, and his colleagues. Preparing children in advance and using cognitive behavioral therapy (CBT) strategies in the moment can help reduce their anxiety.
“CBT is a skill‐based approach that focuses on the present and aims to teach efficient ways of identifying distorted thinking, modifying beliefs, and changing behaviors for a more favorable outcome of real‐life situations,” they wrote.
First, Dr. Armenta and his associates advised, be honest with children about what to expect from a procedure. Evidence does not support phrases such as, “It won’t hurt,” or “It will be over soon,” to reduce anxiety.
Timing the disclosure of a procedure and creating the appropriate setting also can help reduce anxiety. For very young children, short notice of a procedure is often best, with the promise of a small reward or outing afterward. Older children may want some advance notice so they can feel prepared, but their specific concerns should be addressed.
CBT-based techniques include deep breathing and positive coping statements such as “I can do this” for older children, or encouraging them to talk about a family pet or listen to music. Younger children may be distracted with pinwheels, rattles, or songs. “Additionally, in recent years, virtual reality headsets have even proved to be effective distractors, resulting in an overall reduction in both pain and fear,” Dr. Armenta and his associates noted.
Other useful strategies include allowing children to choose their position and location for an injection or procedure when possible. Small children may be able to sit on the lap of an adult, and older children may prefer sitting up to lying down. Avoid physical restraint unless it is absolutely necessary for safety, the researchers emphasized.
Incorporating CBT-based strategies of breathing and distraction with honesty and respectful disclosure of what is being done and why “not only makes practicing pediatric dermatology easier, but also can improve patient adherence to painful procedures,” they said.
No disclosure information was given.
SOURCE: Armenta AM et al. Pediatr Dermatol. 2019. doi: 10.1111/pde.13739.
FROM PEDIATRIC DERMATOLOGY
Prior authorization revisited: An update from the APA
You may have noticed that one of topics I like to write about in this column is the assault on the practice of medicine: the obligations that steal our time from patients without either the value or outcomes our patients see. It is these extraneous demands on our time and on our psyches that dehumanize medical practice as experienced by our patients and contribute to physician burnout. High on my list is the requirement for prior authorization for medications.
In 2015, I wrote a column, “Prior Authorization for Medications: Who oversees placement of the hoops?” The article followed my unsuccessful 6-week-long endeavor to get modafinil authorized for a patient. My efforts included interactions with the insurance company’s chief medical officer, the insurance commissioners in three states, my U.S. senator, and finally, the American Psychiatric Association’s attorney, Colleen Coyle, who has been working on this issue along with the American Medical Association and other medical specialty organizations for years now. The irony of the article is that, after it came out, a reader informed me that modafinil could be obtained from Costco for $34 for 30 pills, while every pharmacy I had checked was selling the generic medication for nearly $1,000 for the same number of pills!
One might make the case that prior authorization saves money by rationing the most expensive medications. And I might counter that physicians should be willing to try less expensive medications first – if we only knew the price of the medications we are prescribing. There is, however, no clear logic to the tremendous variability in price across pharmacies, which can amount to hundreds of dollars a month in the cost to an uninsured patient, and none of which reflects what might be a negotiated cost for those using health insurance. The newest medications are expensive from any retailer, but when it comes to older generics, it’s a crapshoot.
Years have passed since my prior authorization fiasco. Today, I keep a GoodRx app on my phone and often reference it when prescribing medications that might be expensive at one pharmacy but not at another. The app tells me that I can now buy 30 pills of the modafinil for $40.34 at grocery store pharmacy that is 2.7 miles from my office or for $305.50 at Walgreens, 3.6 miles away. A quick call to Costco shows that the price there has gone up to $40.89. For the prescriptions that I have written since that article, I have told patients that their insurance providers may not authorize the medication and if that is the case, they should price shop, as I don’t have the tenacity to go through the prior authorization process I endured in 2015.
So what progress has the APA made over the past 4 years? I wrote back to the APA’s attorney, Ms. Coyle, to ask for an update. Ms. Coyle was kind enough to respond in some detail. , and there is one less obstacle to obtaining care for a growing number of patients during our overdose epidemic.
Other news, however, is not all good. Medicare plans to increase the use of prior authorization.
Ms. Coyle provided data that confirmed what most physicians suspected. “The AMA did a survey1 and found 92% of physicians report that ‘prior authorizations programs have a negative impact on patient clinical outcomes.’ The AMA study revealed that ‘every week a medical practice completes an average of 29.1 prior authorization requirements per physician, which takes an average of 14.6 hours to process – the equivalent of nearly two business days.’ ”
She recounted the efforts the APA has made on behalf of psychiatrists. “We are working with the AMA and other physician groups to address the issue and recommended [Health and Human Services] address the burden of prior authorization as part of its Patients over Paperwork Initiative.2 Through our website and the helpline, we collected stories from our members about the burden it causes and the negative impact it has on patient care. We’ve shared the stories with the American Medical Association to use in joint advocacy efforts with the administration and private insurers.”
Finally, the APA has written a letter to the Centers for Medicare & Medicaid Services in opposition to a proposal for increased utilization review of Medicare Part D protected classes medications, which include antipsychotics and antidepressants. “We strongly oppose the proposal,” Ms. Coyle noted, “and asked our district branches to also submit letters in opposition.”
What can individual psychiatrists do to advocate? I noted that many of my efforts were futile. My U.S. senator responded with a letter that said my problem was not in his domain.
“As for the best way to approach the issue in the state, we recommend working with the state medical society to push for legislation. The AMA has draft legislation that may be used. It would also be helpful to collect the stories from the members about their challenges with prior authorization and impact on patient care/outcomes to share with state legislatures. It may also be beneficial to share these concerns with the state insurance commissioner and Department of Health and Human Services,” Ms. Coyle wrote. She noted it would be helpful for members to work directly with the district branches of the APA.
I am still left with some sense of futility. In 2014, Danielle Ofri, MD, PhD, a physician and writer, wrote an op-ed3 for the New York Times, “Adventures in ‘Prior Authorization,’ ” where she detailed the egregious practice. In the past 5 years, not much has changed, and insurers have not taken to the idea that preserving physician time for clinical care and face-to-face interactions with patients is the priority that we all might like it to be.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
References
1. AMA Prior Authorization Physician Survey, 2017.
2. CMS Patients Over Paperwork Initiative, CMS.gov.
3. New York Times, Aug. 3, 2014.
You may have noticed that one of topics I like to write about in this column is the assault on the practice of medicine: the obligations that steal our time from patients without either the value or outcomes our patients see. It is these extraneous demands on our time and on our psyches that dehumanize medical practice as experienced by our patients and contribute to physician burnout. High on my list is the requirement for prior authorization for medications.
In 2015, I wrote a column, “Prior Authorization for Medications: Who oversees placement of the hoops?” The article followed my unsuccessful 6-week-long endeavor to get modafinil authorized for a patient. My efforts included interactions with the insurance company’s chief medical officer, the insurance commissioners in three states, my U.S. senator, and finally, the American Psychiatric Association’s attorney, Colleen Coyle, who has been working on this issue along with the American Medical Association and other medical specialty organizations for years now. The irony of the article is that, after it came out, a reader informed me that modafinil could be obtained from Costco for $34 for 30 pills, while every pharmacy I had checked was selling the generic medication for nearly $1,000 for the same number of pills!
One might make the case that prior authorization saves money by rationing the most expensive medications. And I might counter that physicians should be willing to try less expensive medications first – if we only knew the price of the medications we are prescribing. There is, however, no clear logic to the tremendous variability in price across pharmacies, which can amount to hundreds of dollars a month in the cost to an uninsured patient, and none of which reflects what might be a negotiated cost for those using health insurance. The newest medications are expensive from any retailer, but when it comes to older generics, it’s a crapshoot.
Years have passed since my prior authorization fiasco. Today, I keep a GoodRx app on my phone and often reference it when prescribing medications that might be expensive at one pharmacy but not at another. The app tells me that I can now buy 30 pills of the modafinil for $40.34 at grocery store pharmacy that is 2.7 miles from my office or for $305.50 at Walgreens, 3.6 miles away. A quick call to Costco shows that the price there has gone up to $40.89. For the prescriptions that I have written since that article, I have told patients that their insurance providers may not authorize the medication and if that is the case, they should price shop, as I don’t have the tenacity to go through the prior authorization process I endured in 2015.
So what progress has the APA made over the past 4 years? I wrote back to the APA’s attorney, Ms. Coyle, to ask for an update. Ms. Coyle was kind enough to respond in some detail. , and there is one less obstacle to obtaining care for a growing number of patients during our overdose epidemic.
Other news, however, is not all good. Medicare plans to increase the use of prior authorization.
Ms. Coyle provided data that confirmed what most physicians suspected. “The AMA did a survey1 and found 92% of physicians report that ‘prior authorizations programs have a negative impact on patient clinical outcomes.’ The AMA study revealed that ‘every week a medical practice completes an average of 29.1 prior authorization requirements per physician, which takes an average of 14.6 hours to process – the equivalent of nearly two business days.’ ”
She recounted the efforts the APA has made on behalf of psychiatrists. “We are working with the AMA and other physician groups to address the issue and recommended [Health and Human Services] address the burden of prior authorization as part of its Patients over Paperwork Initiative.2 Through our website and the helpline, we collected stories from our members about the burden it causes and the negative impact it has on patient care. We’ve shared the stories with the American Medical Association to use in joint advocacy efforts with the administration and private insurers.”
Finally, the APA has written a letter to the Centers for Medicare & Medicaid Services in opposition to a proposal for increased utilization review of Medicare Part D protected classes medications, which include antipsychotics and antidepressants. “We strongly oppose the proposal,” Ms. Coyle noted, “and asked our district branches to also submit letters in opposition.”
What can individual psychiatrists do to advocate? I noted that many of my efforts were futile. My U.S. senator responded with a letter that said my problem was not in his domain.
“As for the best way to approach the issue in the state, we recommend working with the state medical society to push for legislation. The AMA has draft legislation that may be used. It would also be helpful to collect the stories from the members about their challenges with prior authorization and impact on patient care/outcomes to share with state legislatures. It may also be beneficial to share these concerns with the state insurance commissioner and Department of Health and Human Services,” Ms. Coyle wrote. She noted it would be helpful for members to work directly with the district branches of the APA.
I am still left with some sense of futility. In 2014, Danielle Ofri, MD, PhD, a physician and writer, wrote an op-ed3 for the New York Times, “Adventures in ‘Prior Authorization,’ ” where she detailed the egregious practice. In the past 5 years, not much has changed, and insurers have not taken to the idea that preserving physician time for clinical care and face-to-face interactions with patients is the priority that we all might like it to be.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
References
1. AMA Prior Authorization Physician Survey, 2017.
2. CMS Patients Over Paperwork Initiative, CMS.gov.
3. New York Times, Aug. 3, 2014.
You may have noticed that one of topics I like to write about in this column is the assault on the practice of medicine: the obligations that steal our time from patients without either the value or outcomes our patients see. It is these extraneous demands on our time and on our psyches that dehumanize medical practice as experienced by our patients and contribute to physician burnout. High on my list is the requirement for prior authorization for medications.
In 2015, I wrote a column, “Prior Authorization for Medications: Who oversees placement of the hoops?” The article followed my unsuccessful 6-week-long endeavor to get modafinil authorized for a patient. My efforts included interactions with the insurance company’s chief medical officer, the insurance commissioners in three states, my U.S. senator, and finally, the American Psychiatric Association’s attorney, Colleen Coyle, who has been working on this issue along with the American Medical Association and other medical specialty organizations for years now. The irony of the article is that, after it came out, a reader informed me that modafinil could be obtained from Costco for $34 for 30 pills, while every pharmacy I had checked was selling the generic medication for nearly $1,000 for the same number of pills!
One might make the case that prior authorization saves money by rationing the most expensive medications. And I might counter that physicians should be willing to try less expensive medications first – if we only knew the price of the medications we are prescribing. There is, however, no clear logic to the tremendous variability in price across pharmacies, which can amount to hundreds of dollars a month in the cost to an uninsured patient, and none of which reflects what might be a negotiated cost for those using health insurance. The newest medications are expensive from any retailer, but when it comes to older generics, it’s a crapshoot.
Years have passed since my prior authorization fiasco. Today, I keep a GoodRx app on my phone and often reference it when prescribing medications that might be expensive at one pharmacy but not at another. The app tells me that I can now buy 30 pills of the modafinil for $40.34 at grocery store pharmacy that is 2.7 miles from my office or for $305.50 at Walgreens, 3.6 miles away. A quick call to Costco shows that the price there has gone up to $40.89. For the prescriptions that I have written since that article, I have told patients that their insurance providers may not authorize the medication and if that is the case, they should price shop, as I don’t have the tenacity to go through the prior authorization process I endured in 2015.
So what progress has the APA made over the past 4 years? I wrote back to the APA’s attorney, Ms. Coyle, to ask for an update. Ms. Coyle was kind enough to respond in some detail. , and there is one less obstacle to obtaining care for a growing number of patients during our overdose epidemic.
Other news, however, is not all good. Medicare plans to increase the use of prior authorization.
Ms. Coyle provided data that confirmed what most physicians suspected. “The AMA did a survey1 and found 92% of physicians report that ‘prior authorizations programs have a negative impact on patient clinical outcomes.’ The AMA study revealed that ‘every week a medical practice completes an average of 29.1 prior authorization requirements per physician, which takes an average of 14.6 hours to process – the equivalent of nearly two business days.’ ”
She recounted the efforts the APA has made on behalf of psychiatrists. “We are working with the AMA and other physician groups to address the issue and recommended [Health and Human Services] address the burden of prior authorization as part of its Patients over Paperwork Initiative.2 Through our website and the helpline, we collected stories from our members about the burden it causes and the negative impact it has on patient care. We’ve shared the stories with the American Medical Association to use in joint advocacy efforts with the administration and private insurers.”
Finally, the APA has written a letter to the Centers for Medicare & Medicaid Services in opposition to a proposal for increased utilization review of Medicare Part D protected classes medications, which include antipsychotics and antidepressants. “We strongly oppose the proposal,” Ms. Coyle noted, “and asked our district branches to also submit letters in opposition.”
What can individual psychiatrists do to advocate? I noted that many of my efforts were futile. My U.S. senator responded with a letter that said my problem was not in his domain.
“As for the best way to approach the issue in the state, we recommend working with the state medical society to push for legislation. The AMA has draft legislation that may be used. It would also be helpful to collect the stories from the members about their challenges with prior authorization and impact on patient care/outcomes to share with state legislatures. It may also be beneficial to share these concerns with the state insurance commissioner and Department of Health and Human Services,” Ms. Coyle wrote. She noted it would be helpful for members to work directly with the district branches of the APA.
I am still left with some sense of futility. In 2014, Danielle Ofri, MD, PhD, a physician and writer, wrote an op-ed3 for the New York Times, “Adventures in ‘Prior Authorization,’ ” where she detailed the egregious practice. In the past 5 years, not much has changed, and insurers have not taken to the idea that preserving physician time for clinical care and face-to-face interactions with patients is the priority that we all might like it to be.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
References
1. AMA Prior Authorization Physician Survey, 2017.
2. CMS Patients Over Paperwork Initiative, CMS.gov.
3. New York Times, Aug. 3, 2014.
Guadecitabine may be option for certain MDS/AML patients
New research suggests guadecitabine may be an option for select patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) who have failed treatment with azacitidine.
In a phase 2 trial, eight of 56 patients with high-risk MDS or low-blast-count AML responded to guadecitabine after azacitidine failure. Patients were significantly more likely to respond if they had few or no somatic mutations.
Marie Sébert, MD, of Hôpital Saint Louis in Paris and her colleagues conducted this trial and reported the results in Haematologica.
The trial (NCT02197676) included 56 patients with the following disease types:
- Refractory anemia with excess blasts (RAEB) type 2 (n = 31; 55%).
- RAEB type 1 (n = 11; 20%).
- Low-blast-count AML (n = 11; 20%).
- Refractory cytopenias with multilineage dysplasia (RCMD; n = 2; 4%).
- Chronic myelomonocytic leukemia (n = 1; 2%).
The patients had a median age of 75 years (range, 70-79) at baseline, and 37 (66%) were men. Thirty-four patients (61%) had very-high-risk disease according to the revised International Prognostic Scoring System. Forty-nine patients (87.5%) had at least one somatic mutation. The most commonly mutated genes were ASXL1, RUNX1, TP53, U2AF1, and DNMT3A.
Most patients (n = 41, 73%) had relapsed after azacitidine, and 15 (27%) had primary resistance to the drug. Patients had received a median of 13 azacitidine cycles (range, 6-23).
The patients received guadecitabine subcutaneously at 60 mg/m2 on days 1-5 of a 28-day cycle. They were treated until progression, death, unacceptable toxicity, or no response after six to nine cycles. Patients received a median of three cycles (range, 0-27). One patient died of infection before receiving guadecitabine, but the remaining 55 patients received at least one cycle of treatment. Eighteen patients had a dose reduction.
Eight patients (14.3%) responded to guadecitabine. Two patients achieved a complete response (CR) – one who had RAEB-2 and one with AML. Two patients with RAEB-1 had marrow CRs. Two patients – one with RAEB-2 and one with AML – had marrow CRs with hematologic improvement. A patient with RCMD had hematologic improvement, and a patient with RAEB-2 had a partial response.
The researchers said mutation frequency was the only significant predictor of response. The response rate was significantly higher in patients who did not have somatic mutations (P = .036). The median number of mutations was one (range, zero to three) in responders and two (range, zero to six) in nonresponders (P = .035). None of the patients with TP53 mutations achieved a response.
The median duration of response was 11.5 months. The median overall survival was 17.9 months in responders and 7.1 months in the overall population.
In a multivariate analysis, the following factors were significantly associated with longer survival:
- Having low- to high-risk (vs. very-high-risk) disease (P = .03).
- Having experienced primary (vs. secondary) azacitidine failure (P = .01).
- Having a high rate of demethylation in blood during the first treatment cycle (P = .03).
There were 99 serious adverse events (AEs) reported in 44 patients. Most AEs were hematologic events, and the most common of these was myelosuppression (n = 88; 88%). The most common grade 3/4 nonhematologic AE was pulmonary toxicity (n = 7; 12.5%). Thirteen patients were hospitalized for febrile neutropenia for a median of 14 days.
The researchers said patients reported less pain and fewer secondary lesions with guadecitabine than they had with azacitidine.
This trial was sponsored by Groupe Francophone des Myelodysplasies in collaboration with Astex Pharmaceuticals. The researchers reported having no competing interests.
SOURCE: Sébert M et al. Haematologica. 2019 Feb 7. doi: 0.3324/haematol.2018.207118.
New research suggests guadecitabine may be an option for select patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) who have failed treatment with azacitidine.
In a phase 2 trial, eight of 56 patients with high-risk MDS or low-blast-count AML responded to guadecitabine after azacitidine failure. Patients were significantly more likely to respond if they had few or no somatic mutations.
Marie Sébert, MD, of Hôpital Saint Louis in Paris and her colleagues conducted this trial and reported the results in Haematologica.
The trial (NCT02197676) included 56 patients with the following disease types:
- Refractory anemia with excess blasts (RAEB) type 2 (n = 31; 55%).
- RAEB type 1 (n = 11; 20%).
- Low-blast-count AML (n = 11; 20%).
- Refractory cytopenias with multilineage dysplasia (RCMD; n = 2; 4%).
- Chronic myelomonocytic leukemia (n = 1; 2%).
The patients had a median age of 75 years (range, 70-79) at baseline, and 37 (66%) were men. Thirty-four patients (61%) had very-high-risk disease according to the revised International Prognostic Scoring System. Forty-nine patients (87.5%) had at least one somatic mutation. The most commonly mutated genes were ASXL1, RUNX1, TP53, U2AF1, and DNMT3A.
Most patients (n = 41, 73%) had relapsed after azacitidine, and 15 (27%) had primary resistance to the drug. Patients had received a median of 13 azacitidine cycles (range, 6-23).
The patients received guadecitabine subcutaneously at 60 mg/m2 on days 1-5 of a 28-day cycle. They were treated until progression, death, unacceptable toxicity, or no response after six to nine cycles. Patients received a median of three cycles (range, 0-27). One patient died of infection before receiving guadecitabine, but the remaining 55 patients received at least one cycle of treatment. Eighteen patients had a dose reduction.
Eight patients (14.3%) responded to guadecitabine. Two patients achieved a complete response (CR) – one who had RAEB-2 and one with AML. Two patients with RAEB-1 had marrow CRs. Two patients – one with RAEB-2 and one with AML – had marrow CRs with hematologic improvement. A patient with RCMD had hematologic improvement, and a patient with RAEB-2 had a partial response.
The researchers said mutation frequency was the only significant predictor of response. The response rate was significantly higher in patients who did not have somatic mutations (P = .036). The median number of mutations was one (range, zero to three) in responders and two (range, zero to six) in nonresponders (P = .035). None of the patients with TP53 mutations achieved a response.
The median duration of response was 11.5 months. The median overall survival was 17.9 months in responders and 7.1 months in the overall population.
In a multivariate analysis, the following factors were significantly associated with longer survival:
- Having low- to high-risk (vs. very-high-risk) disease (P = .03).
- Having experienced primary (vs. secondary) azacitidine failure (P = .01).
- Having a high rate of demethylation in blood during the first treatment cycle (P = .03).
There were 99 serious adverse events (AEs) reported in 44 patients. Most AEs were hematologic events, and the most common of these was myelosuppression (n = 88; 88%). The most common grade 3/4 nonhematologic AE was pulmonary toxicity (n = 7; 12.5%). Thirteen patients were hospitalized for febrile neutropenia for a median of 14 days.
The researchers said patients reported less pain and fewer secondary lesions with guadecitabine than they had with azacitidine.
This trial was sponsored by Groupe Francophone des Myelodysplasies in collaboration with Astex Pharmaceuticals. The researchers reported having no competing interests.
SOURCE: Sébert M et al. Haematologica. 2019 Feb 7. doi: 0.3324/haematol.2018.207118.
New research suggests guadecitabine may be an option for select patients with myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) who have failed treatment with azacitidine.
In a phase 2 trial, eight of 56 patients with high-risk MDS or low-blast-count AML responded to guadecitabine after azacitidine failure. Patients were significantly more likely to respond if they had few or no somatic mutations.
Marie Sébert, MD, of Hôpital Saint Louis in Paris and her colleagues conducted this trial and reported the results in Haematologica.
The trial (NCT02197676) included 56 patients with the following disease types:
- Refractory anemia with excess blasts (RAEB) type 2 (n = 31; 55%).
- RAEB type 1 (n = 11; 20%).
- Low-blast-count AML (n = 11; 20%).
- Refractory cytopenias with multilineage dysplasia (RCMD; n = 2; 4%).
- Chronic myelomonocytic leukemia (n = 1; 2%).
The patients had a median age of 75 years (range, 70-79) at baseline, and 37 (66%) were men. Thirty-four patients (61%) had very-high-risk disease according to the revised International Prognostic Scoring System. Forty-nine patients (87.5%) had at least one somatic mutation. The most commonly mutated genes were ASXL1, RUNX1, TP53, U2AF1, and DNMT3A.
Most patients (n = 41, 73%) had relapsed after azacitidine, and 15 (27%) had primary resistance to the drug. Patients had received a median of 13 azacitidine cycles (range, 6-23).
The patients received guadecitabine subcutaneously at 60 mg/m2 on days 1-5 of a 28-day cycle. They were treated until progression, death, unacceptable toxicity, or no response after six to nine cycles. Patients received a median of three cycles (range, 0-27). One patient died of infection before receiving guadecitabine, but the remaining 55 patients received at least one cycle of treatment. Eighteen patients had a dose reduction.
Eight patients (14.3%) responded to guadecitabine. Two patients achieved a complete response (CR) – one who had RAEB-2 and one with AML. Two patients with RAEB-1 had marrow CRs. Two patients – one with RAEB-2 and one with AML – had marrow CRs with hematologic improvement. A patient with RCMD had hematologic improvement, and a patient with RAEB-2 had a partial response.
The researchers said mutation frequency was the only significant predictor of response. The response rate was significantly higher in patients who did not have somatic mutations (P = .036). The median number of mutations was one (range, zero to three) in responders and two (range, zero to six) in nonresponders (P = .035). None of the patients with TP53 mutations achieved a response.
The median duration of response was 11.5 months. The median overall survival was 17.9 months in responders and 7.1 months in the overall population.
In a multivariate analysis, the following factors were significantly associated with longer survival:
- Having low- to high-risk (vs. very-high-risk) disease (P = .03).
- Having experienced primary (vs. secondary) azacitidine failure (P = .01).
- Having a high rate of demethylation in blood during the first treatment cycle (P = .03).
There were 99 serious adverse events (AEs) reported in 44 patients. Most AEs were hematologic events, and the most common of these was myelosuppression (n = 88; 88%). The most common grade 3/4 nonhematologic AE was pulmonary toxicity (n = 7; 12.5%). Thirteen patients were hospitalized for febrile neutropenia for a median of 14 days.
The researchers said patients reported less pain and fewer secondary lesions with guadecitabine than they had with azacitidine.
This trial was sponsored by Groupe Francophone des Myelodysplasies in collaboration with Astex Pharmaceuticals. The researchers reported having no competing interests.
SOURCE: Sébert M et al. Haematologica. 2019 Feb 7. doi: 0.3324/haematol.2018.207118.
REPORTING FROM HAEMATOLOGICA
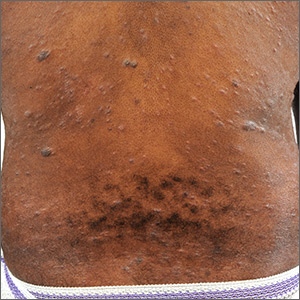
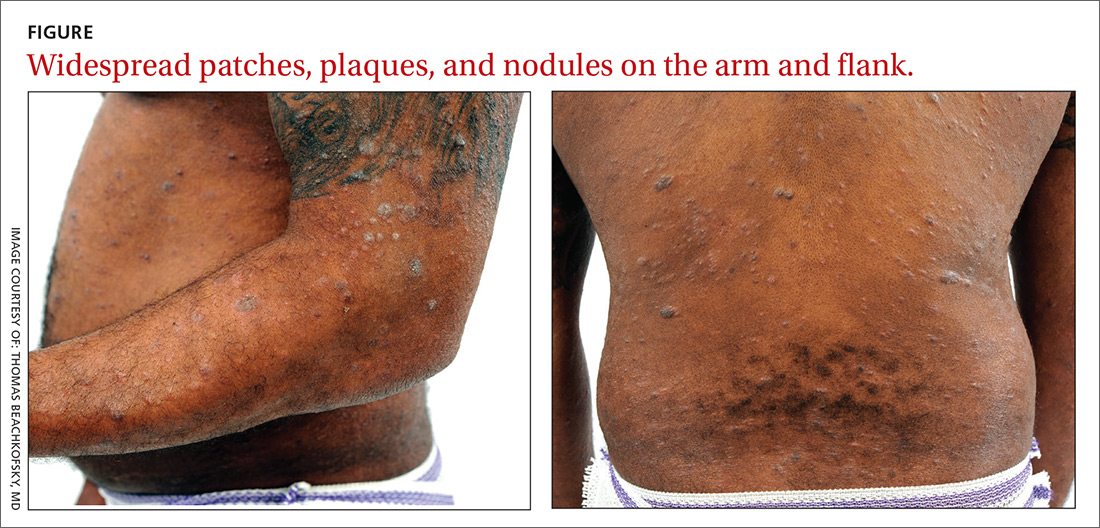
Nodules, tumors, and hyperpigmented patches
A 36-year-old African-American man presented to our clinic for evaluation of a chronic “rash” on his trunk, arms, and legs that had been present for 3 years. Empiric therapy for tinea corporis, nummular eczema, and confluent and reticulated papillomatosis had failed to improve his lesions.
Physical examination revealed a widespread skin eruption consisting of well-demarcated, hyperpigmented, scaly patches and plaques distributed throughout the patient’s trunk and extremities. Initial biopsies of the skin lesions (performed at an outside institution prior to current presentation) were nondiagnostic.
Over the next several months, the patient developed numerous cutaneous nodules and tumors within the background of his persistent patches and plaques (FIGURE). These nodules and tumors intermittently disappeared and recurred, seemingly independent of therapies including psoralen and ultraviolet A phototherapy (PUVA), interferon, and methotrexate.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: CD30+ transformed mycosis fungoides
Repeat biopsies ultimately confirmed a diagnosis of mycosis fungoides (MF), a primary cutaneous non-Hodgkin lymphoma of T-cell origin.
Lymphomas occurring in the skin without evidence of disease in other areas of the body at the time of diagnosis are referred to as primary cutaneous lymphomas. They are broadly divided by their cell of origin into cutaneous T-cell and cutaneous B-cell lymphomas. Differentiating primary cutaneous lymphoma from systemic lymphomas with secondary skin involvement is an important distinction, as primary cutaneous lymphomas have different clinical courses and prognoses and require different treatments.1
MF is the most common type of cutaneous T-cell lymphoma (CTCL), yet it remains an uncommon diagnosis as cutaneous lymphomas comprise only 3.9% of all non-Hodgkin lymphomas.2 The CD30+ transformed variant identified in this patient is a rare subtype of MF that is associated with increased morbidity and mortality.3 Other types of CTCL include primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and adult T-cell leukemia/lymphoma, all of which can resemble MF clinically and are diagnosed by histopathology.
A nonspecific presentation
Patients with MF typically present with a complaint of chronic, slowly progressive skin lesions of various sizes and morphologies including scaly patches, plaques, and tumors, often in skin distributions that are not directly exposed to the sun (often referred to as a “bathing suit” distribution).4 Pruritus typically accompanies these skin lesions, and alopecia and poikiloderma are common findings. Patients with more advanced cases may present with generalized erythroderma. This finding should raise concern for progression to Sézary syndrome, a more aggressive type of CTCL that manifests with leukemic involvement of malignant T-cells matching the T-cell clones found in the skin.2
Alopecia is a common manifestation of MF that can be clinically indistinguishable from alopecia areata.5 While isolated alopecia is unlikely to be a consequence of MF, alopecia in the setting of widespread patches and plaques should raise suspicion for a diagnosis of MF.
Continue to: Diagnostic dilemma
Diagnostic dilemma: Condition mimics benign disorders
This case illustrates the diagnostic dilemma posed by MF due to its propensity to mimic more benign skin disorders, both clinically and histologically.2 One literature review of MF cases in which the diagnosis was not suspected clinically but was eventually confirmed histopathologically found that 25 unique dermatoses can be mimicked clinically by MF.6 The more common conditions that MF can be mistaken for are discussed below. In light of the diagnostic challenge posed by this multitude of morphologic presentations, referral to Dermatology for histopathologic evaluation is a reasonable next step when empiric treatment fails to improve symptoms of these common skin disorders.
Chronic atopic dermatitis (eczema) is characterized by dry skin and severe pruritis, usually associated with lichenified plaques and a history of atopic disease. The pruritic, scaly plaques typical of MF may be misdiagnosed as eczema; however, a distribution involving the “bathing suit” area is more suggestive of MF. Patients with atopic dermatitis are more likely to display a flexural distribution.2
Tinea corporis typically presents as annular, erythematous, pruritic, scaly patches or plaques that are similar to the scaling lesions of MF. Tinea corporis can be distinguished from MF via potassium hydroxide preparation of skin scrapings from an active lesion, which should demonstrate the segmented hyphae characteristic of this infection. If lesions of this type fail to respond to topical antifungal therapy, a skin biopsy may be warranted for further evaluation.
Nummular eczema, like tinea corporis, presents with round, coin-shaped patches that are highly pruritic and can be difficult to distinguish from the pruritic patches of MF. Unlike MF, however, nummular eczema typically is observed in patients with diffusely dry skin7; the lack of this finding should prompt consideration of alternative diagnoses, including MF.
Psoriasis classically presents with symmetrically distributed, well-demarcated plaques with prominent scaling that are usually asymptomatic, but may be associated with pruritis. Psoriatic plaques may be difficult to distinguish clinically from the plaques of MF, but their distribution may offer clues; psoriasis typically follows an extensor surface distribution, whereas MF is more commonly identified in a “bathing suit” or generalized distribution.4
Continue to: Tx based on disease extent, impact on quality of life
Tx based on disease extent, impact on quality of life
Staging of MF is the primary determinant of treatment and involves evaluation of skin, lymph node, viscera, and blood involvement. Early-stage MF has a favorable prognosis and can be effectively managed with skin-directed treatments. These include topical corticosteroids, topical nitrogen mustard agents, topical retinoids, and phototherapy (PUVA).8 Total skin electron beam therapy has been proven effective in the treatment of refractory and extensive MF, and local radiation therapy also is an effective treatment for isolated cutaneous tumors. Prolonged complete remissions of early-stage MF have been achieved using skin-directed treatments.8
In contrast, advanced MF often is treatment refractory and carries a poor prognosis. Systemic treatments such as interferon therapy, oral retinoids, extracorporeal photopheresis, and chemotherapy are added in patients with advanced disease after skin-directed therapy fails to adequately control tumor burden.
Our patient initially was treated with PUVA as well as 6 million U of interferon alfa-2B 3 times weekly, which he eventually elected to discontinue after 8 months because he wanted to father a child. His disease became more widespread after discontinuing these treatments despite interim therapy with clobetasol ointment .05%. His larger nodules and tumors were intermittently treated with local radiation with good response. Methotrexate 15 mg weekly was initiated following his decline after discontinuing PUVA and interferon treatment. Treatment with methotrexate resulted in an overall decrease in his tumor burden and several months of clinical stability.
Approximately 6 months after initiating methotrexate, his condition notably worsened. He developed generalized erythroderma, systemic symptoms including fever, chills, and unintentional 9.07-kg weight loss, in addition to new findings of palmoplantar keratoderma and nail dystrophy. In light of this systemic progression and concern for developing Sézary syndrome, extracorporeal photopheresis, bexarotene (an oral retinoid) 75 mg twice daily, and interferon alfa-2B 5 million U 3 times weekly were initiated. His condition continued to decline despite these therapies, culminating in a hospital admission for sepsis.
The patient recovered, and his regimen was changed to PUVA 3 times weekly in addition to treatment with intravenous brentuximab 1.8 mg/kg, a monoclonal antibody that targets CD30 receptors. Unfortunately, his condition continued to decline on this treatment regimen, which was subsequently abandoned in favor of chemotherapy with gemcitabine 1980 mg/250 mL normal saline. The patient was improving clinically with each cycle of gemcitabine and the plan was to transition him to extracorporeal photopheresis and bexarotene for maintenance therapy; however, the patient succumbed to his disease and passed away at the age of 37.
CORRESPONDENCE
Jonathan Banta, MD, PSC 103, Box 3613, APO, AE 09603 [email protected]
1. Whittaker S, Knobler R, Ortiz P, et al. Major achievements of the EORTC Cutaneous Lymphoma Task Force (CLTF). EJC Suppl. 2012;10:46-50.
2. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16.
3. Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374-376.
4. Pimpinelli N, Olsen EA, Santucci M, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
5. Bi MY, Curry JL, Christiano AM, et al. The spectrum of hair loss in patients with mycosis fungoides and Sézary syndrome. J Am Acad Dermatol. 2011;64:53-63.
6. Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918.
7. Jiamton S, Tangjaturonrusamee C, Kulthanan K. Clinical features and aggravating factors in nummular eczema in Thais. Asian Pac J Allergy Immunol. 2013;31:36-42.
8. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-223.e17.
A 36-year-old African-American man presented to our clinic for evaluation of a chronic “rash” on his trunk, arms, and legs that had been present for 3 years. Empiric therapy for tinea corporis, nummular eczema, and confluent and reticulated papillomatosis had failed to improve his lesions.
Physical examination revealed a widespread skin eruption consisting of well-demarcated, hyperpigmented, scaly patches and plaques distributed throughout the patient’s trunk and extremities. Initial biopsies of the skin lesions (performed at an outside institution prior to current presentation) were nondiagnostic.
Over the next several months, the patient developed numerous cutaneous nodules and tumors within the background of his persistent patches and plaques (FIGURE). These nodules and tumors intermittently disappeared and recurred, seemingly independent of therapies including psoralen and ultraviolet A phototherapy (PUVA), interferon, and methotrexate.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: CD30+ transformed mycosis fungoides
Repeat biopsies ultimately confirmed a diagnosis of mycosis fungoides (MF), a primary cutaneous non-Hodgkin lymphoma of T-cell origin.
Lymphomas occurring in the skin without evidence of disease in other areas of the body at the time of diagnosis are referred to as primary cutaneous lymphomas. They are broadly divided by their cell of origin into cutaneous T-cell and cutaneous B-cell lymphomas. Differentiating primary cutaneous lymphoma from systemic lymphomas with secondary skin involvement is an important distinction, as primary cutaneous lymphomas have different clinical courses and prognoses and require different treatments.1
MF is the most common type of cutaneous T-cell lymphoma (CTCL), yet it remains an uncommon diagnosis as cutaneous lymphomas comprise only 3.9% of all non-Hodgkin lymphomas.2 The CD30+ transformed variant identified in this patient is a rare subtype of MF that is associated with increased morbidity and mortality.3 Other types of CTCL include primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and adult T-cell leukemia/lymphoma, all of which can resemble MF clinically and are diagnosed by histopathology.
A nonspecific presentation
Patients with MF typically present with a complaint of chronic, slowly progressive skin lesions of various sizes and morphologies including scaly patches, plaques, and tumors, often in skin distributions that are not directly exposed to the sun (often referred to as a “bathing suit” distribution).4 Pruritus typically accompanies these skin lesions, and alopecia and poikiloderma are common findings. Patients with more advanced cases may present with generalized erythroderma. This finding should raise concern for progression to Sézary syndrome, a more aggressive type of CTCL that manifests with leukemic involvement of malignant T-cells matching the T-cell clones found in the skin.2
Alopecia is a common manifestation of MF that can be clinically indistinguishable from alopecia areata.5 While isolated alopecia is unlikely to be a consequence of MF, alopecia in the setting of widespread patches and plaques should raise suspicion for a diagnosis of MF.
Continue to: Diagnostic dilemma
Diagnostic dilemma: Condition mimics benign disorders
This case illustrates the diagnostic dilemma posed by MF due to its propensity to mimic more benign skin disorders, both clinically and histologically.2 One literature review of MF cases in which the diagnosis was not suspected clinically but was eventually confirmed histopathologically found that 25 unique dermatoses can be mimicked clinically by MF.6 The more common conditions that MF can be mistaken for are discussed below. In light of the diagnostic challenge posed by this multitude of morphologic presentations, referral to Dermatology for histopathologic evaluation is a reasonable next step when empiric treatment fails to improve symptoms of these common skin disorders.
Chronic atopic dermatitis (eczema) is characterized by dry skin and severe pruritis, usually associated with lichenified plaques and a history of atopic disease. The pruritic, scaly plaques typical of MF may be misdiagnosed as eczema; however, a distribution involving the “bathing suit” area is more suggestive of MF. Patients with atopic dermatitis are more likely to display a flexural distribution.2
Tinea corporis typically presents as annular, erythematous, pruritic, scaly patches or plaques that are similar to the scaling lesions of MF. Tinea corporis can be distinguished from MF via potassium hydroxide preparation of skin scrapings from an active lesion, which should demonstrate the segmented hyphae characteristic of this infection. If lesions of this type fail to respond to topical antifungal therapy, a skin biopsy may be warranted for further evaluation.
Nummular eczema, like tinea corporis, presents with round, coin-shaped patches that are highly pruritic and can be difficult to distinguish from the pruritic patches of MF. Unlike MF, however, nummular eczema typically is observed in patients with diffusely dry skin7; the lack of this finding should prompt consideration of alternative diagnoses, including MF.
Psoriasis classically presents with symmetrically distributed, well-demarcated plaques with prominent scaling that are usually asymptomatic, but may be associated with pruritis. Psoriatic plaques may be difficult to distinguish clinically from the plaques of MF, but their distribution may offer clues; psoriasis typically follows an extensor surface distribution, whereas MF is more commonly identified in a “bathing suit” or generalized distribution.4
Continue to: Tx based on disease extent, impact on quality of life
Tx based on disease extent, impact on quality of life
Staging of MF is the primary determinant of treatment and involves evaluation of skin, lymph node, viscera, and blood involvement. Early-stage MF has a favorable prognosis and can be effectively managed with skin-directed treatments. These include topical corticosteroids, topical nitrogen mustard agents, topical retinoids, and phototherapy (PUVA).8 Total skin electron beam therapy has been proven effective in the treatment of refractory and extensive MF, and local radiation therapy also is an effective treatment for isolated cutaneous tumors. Prolonged complete remissions of early-stage MF have been achieved using skin-directed treatments.8
In contrast, advanced MF often is treatment refractory and carries a poor prognosis. Systemic treatments such as interferon therapy, oral retinoids, extracorporeal photopheresis, and chemotherapy are added in patients with advanced disease after skin-directed therapy fails to adequately control tumor burden.
Our patient initially was treated with PUVA as well as 6 million U of interferon alfa-2B 3 times weekly, which he eventually elected to discontinue after 8 months because he wanted to father a child. His disease became more widespread after discontinuing these treatments despite interim therapy with clobetasol ointment .05%. His larger nodules and tumors were intermittently treated with local radiation with good response. Methotrexate 15 mg weekly was initiated following his decline after discontinuing PUVA and interferon treatment. Treatment with methotrexate resulted in an overall decrease in his tumor burden and several months of clinical stability.
Approximately 6 months after initiating methotrexate, his condition notably worsened. He developed generalized erythroderma, systemic symptoms including fever, chills, and unintentional 9.07-kg weight loss, in addition to new findings of palmoplantar keratoderma and nail dystrophy. In light of this systemic progression and concern for developing Sézary syndrome, extracorporeal photopheresis, bexarotene (an oral retinoid) 75 mg twice daily, and interferon alfa-2B 5 million U 3 times weekly were initiated. His condition continued to decline despite these therapies, culminating in a hospital admission for sepsis.
The patient recovered, and his regimen was changed to PUVA 3 times weekly in addition to treatment with intravenous brentuximab 1.8 mg/kg, a monoclonal antibody that targets CD30 receptors. Unfortunately, his condition continued to decline on this treatment regimen, which was subsequently abandoned in favor of chemotherapy with gemcitabine 1980 mg/250 mL normal saline. The patient was improving clinically with each cycle of gemcitabine and the plan was to transition him to extracorporeal photopheresis and bexarotene for maintenance therapy; however, the patient succumbed to his disease and passed away at the age of 37.
CORRESPONDENCE
Jonathan Banta, MD, PSC 103, Box 3613, APO, AE 09603 [email protected]
A 36-year-old African-American man presented to our clinic for evaluation of a chronic “rash” on his trunk, arms, and legs that had been present for 3 years. Empiric therapy for tinea corporis, nummular eczema, and confluent and reticulated papillomatosis had failed to improve his lesions.
Physical examination revealed a widespread skin eruption consisting of well-demarcated, hyperpigmented, scaly patches and plaques distributed throughout the patient’s trunk and extremities. Initial biopsies of the skin lesions (performed at an outside institution prior to current presentation) were nondiagnostic.
Over the next several months, the patient developed numerous cutaneous nodules and tumors within the background of his persistent patches and plaques (FIGURE). These nodules and tumors intermittently disappeared and recurred, seemingly independent of therapies including psoralen and ultraviolet A phototherapy (PUVA), interferon, and methotrexate.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: CD30+ transformed mycosis fungoides
Repeat biopsies ultimately confirmed a diagnosis of mycosis fungoides (MF), a primary cutaneous non-Hodgkin lymphoma of T-cell origin.
Lymphomas occurring in the skin without evidence of disease in other areas of the body at the time of diagnosis are referred to as primary cutaneous lymphomas. They are broadly divided by their cell of origin into cutaneous T-cell and cutaneous B-cell lymphomas. Differentiating primary cutaneous lymphoma from systemic lymphomas with secondary skin involvement is an important distinction, as primary cutaneous lymphomas have different clinical courses and prognoses and require different treatments.1
MF is the most common type of cutaneous T-cell lymphoma (CTCL), yet it remains an uncommon diagnosis as cutaneous lymphomas comprise only 3.9% of all non-Hodgkin lymphomas.2 The CD30+ transformed variant identified in this patient is a rare subtype of MF that is associated with increased morbidity and mortality.3 Other types of CTCL include primary cutaneous anaplastic large cell lymphoma, lymphomatoid papulosis, and adult T-cell leukemia/lymphoma, all of which can resemble MF clinically and are diagnosed by histopathology.
A nonspecific presentation
Patients with MF typically present with a complaint of chronic, slowly progressive skin lesions of various sizes and morphologies including scaly patches, plaques, and tumors, often in skin distributions that are not directly exposed to the sun (often referred to as a “bathing suit” distribution).4 Pruritus typically accompanies these skin lesions, and alopecia and poikiloderma are common findings. Patients with more advanced cases may present with generalized erythroderma. This finding should raise concern for progression to Sézary syndrome, a more aggressive type of CTCL that manifests with leukemic involvement of malignant T-cells matching the T-cell clones found in the skin.2
Alopecia is a common manifestation of MF that can be clinically indistinguishable from alopecia areata.5 While isolated alopecia is unlikely to be a consequence of MF, alopecia in the setting of widespread patches and plaques should raise suspicion for a diagnosis of MF.
Continue to: Diagnostic dilemma
Diagnostic dilemma: Condition mimics benign disorders
This case illustrates the diagnostic dilemma posed by MF due to its propensity to mimic more benign skin disorders, both clinically and histologically.2 One literature review of MF cases in which the diagnosis was not suspected clinically but was eventually confirmed histopathologically found that 25 unique dermatoses can be mimicked clinically by MF.6 The more common conditions that MF can be mistaken for are discussed below. In light of the diagnostic challenge posed by this multitude of morphologic presentations, referral to Dermatology for histopathologic evaluation is a reasonable next step when empiric treatment fails to improve symptoms of these common skin disorders.
Chronic atopic dermatitis (eczema) is characterized by dry skin and severe pruritis, usually associated with lichenified plaques and a history of atopic disease. The pruritic, scaly plaques typical of MF may be misdiagnosed as eczema; however, a distribution involving the “bathing suit” area is more suggestive of MF. Patients with atopic dermatitis are more likely to display a flexural distribution.2
Tinea corporis typically presents as annular, erythematous, pruritic, scaly patches or plaques that are similar to the scaling lesions of MF. Tinea corporis can be distinguished from MF via potassium hydroxide preparation of skin scrapings from an active lesion, which should demonstrate the segmented hyphae characteristic of this infection. If lesions of this type fail to respond to topical antifungal therapy, a skin biopsy may be warranted for further evaluation.
Nummular eczema, like tinea corporis, presents with round, coin-shaped patches that are highly pruritic and can be difficult to distinguish from the pruritic patches of MF. Unlike MF, however, nummular eczema typically is observed in patients with diffusely dry skin7; the lack of this finding should prompt consideration of alternative diagnoses, including MF.
Psoriasis classically presents with symmetrically distributed, well-demarcated plaques with prominent scaling that are usually asymptomatic, but may be associated with pruritis. Psoriatic plaques may be difficult to distinguish clinically from the plaques of MF, but their distribution may offer clues; psoriasis typically follows an extensor surface distribution, whereas MF is more commonly identified in a “bathing suit” or generalized distribution.4
Continue to: Tx based on disease extent, impact on quality of life
Tx based on disease extent, impact on quality of life
Staging of MF is the primary determinant of treatment and involves evaluation of skin, lymph node, viscera, and blood involvement. Early-stage MF has a favorable prognosis and can be effectively managed with skin-directed treatments. These include topical corticosteroids, topical nitrogen mustard agents, topical retinoids, and phototherapy (PUVA).8 Total skin electron beam therapy has been proven effective in the treatment of refractory and extensive MF, and local radiation therapy also is an effective treatment for isolated cutaneous tumors. Prolonged complete remissions of early-stage MF have been achieved using skin-directed treatments.8
In contrast, advanced MF often is treatment refractory and carries a poor prognosis. Systemic treatments such as interferon therapy, oral retinoids, extracorporeal photopheresis, and chemotherapy are added in patients with advanced disease after skin-directed therapy fails to adequately control tumor burden.
Our patient initially was treated with PUVA as well as 6 million U of interferon alfa-2B 3 times weekly, which he eventually elected to discontinue after 8 months because he wanted to father a child. His disease became more widespread after discontinuing these treatments despite interim therapy with clobetasol ointment .05%. His larger nodules and tumors were intermittently treated with local radiation with good response. Methotrexate 15 mg weekly was initiated following his decline after discontinuing PUVA and interferon treatment. Treatment with methotrexate resulted in an overall decrease in his tumor burden and several months of clinical stability.
Approximately 6 months after initiating methotrexate, his condition notably worsened. He developed generalized erythroderma, systemic symptoms including fever, chills, and unintentional 9.07-kg weight loss, in addition to new findings of palmoplantar keratoderma and nail dystrophy. In light of this systemic progression and concern for developing Sézary syndrome, extracorporeal photopheresis, bexarotene (an oral retinoid) 75 mg twice daily, and interferon alfa-2B 5 million U 3 times weekly were initiated. His condition continued to decline despite these therapies, culminating in a hospital admission for sepsis.
The patient recovered, and his regimen was changed to PUVA 3 times weekly in addition to treatment with intravenous brentuximab 1.8 mg/kg, a monoclonal antibody that targets CD30 receptors. Unfortunately, his condition continued to decline on this treatment regimen, which was subsequently abandoned in favor of chemotherapy with gemcitabine 1980 mg/250 mL normal saline. The patient was improving clinically with each cycle of gemcitabine and the plan was to transition him to extracorporeal photopheresis and bexarotene for maintenance therapy; however, the patient succumbed to his disease and passed away at the age of 37.
CORRESPONDENCE
Jonathan Banta, MD, PSC 103, Box 3613, APO, AE 09603 [email protected]
1. Whittaker S, Knobler R, Ortiz P, et al. Major achievements of the EORTC Cutaneous Lymphoma Task Force (CLTF). EJC Suppl. 2012;10:46-50.
2. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16.
3. Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374-376.
4. Pimpinelli N, Olsen EA, Santucci M, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
5. Bi MY, Curry JL, Christiano AM, et al. The spectrum of hair loss in patients with mycosis fungoides and Sézary syndrome. J Am Acad Dermatol. 2011;64:53-63.
6. Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918.
7. Jiamton S, Tangjaturonrusamee C, Kulthanan K. Clinical features and aggravating factors in nummular eczema in Thais. Asian Pac J Allergy Immunol. 2013;31:36-42.
8. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-223.e17.
1. Whittaker S, Knobler R, Ortiz P, et al. Major achievements of the EORTC Cutaneous Lymphoma Task Force (CLTF). EJC Suppl. 2012;10:46-50.
2. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16.
3. Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2014;70:374-376.
4. Pimpinelli N, Olsen EA, Santucci M, et al; International Society for Cutaneous Lymphoma. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
5. Bi MY, Curry JL, Christiano AM, et al. The spectrum of hair loss in patients with mycosis fungoides and Sézary syndrome. J Am Acad Dermatol. 2011;64:53-63.
6. Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918.
7. Jiamton S, Tangjaturonrusamee C, Kulthanan K. Clinical features and aggravating factors in nummular eczema in Thais. Asian Pac J Allergy Immunol. 2013;31:36-42.
8. Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-223.e17.
Second-generation hydrogel surpasses platinum for brain aneurysm closure
HONOLULU – A second-generation hydrogel coil that’s already approved for U.S. marketing proved superior to bare platinum wire for durably embolizing brain aneurysms in a multicenter, randomized trial with 600 patients.
For the study’s primary endpoint of recurrent intracranial aneurysm during follow-up as long as 18-24 months, the 297 patients treated with the hydrogel coil had a recurrence rate of 4%, compared with a 15% rate among patients treated with platinum wire, Bernard R. Bendok, MD, said at the International Stroke Conference sponsored by the American Heart Association. The hydrogel coil materials were also relatively easy to use – much easier than the first-generation hydrogel materials, according to Dr. Bendok – and the adverse event rate using the hydrogel coils was similar to the rate using platinum wire, the standard material for brain aneurysm embolization.
Given the similar safety and ease of use and superior efficacy, clinicians treating brain aneurysms may now increasingly use hydrogel coil. “I think these results will change practice. It’s compelling data,” Dr. Bendok predicted in an interview. “The problem with platinum is it is not conducive to healing.” When an aneurysm is “closed” by insertion of platinum wire, in reality most of the space within the aneurysm remains unfilled by metal. In contrast, animal models suggest that when hydrogel fills an aneurysm it forms a matrix for collagen deposition that further fills the aneurysm space in a wound-healing process, thereby making recurrence less likely, said Dr. Bendok, professor and chair of neurosurgery at the Mayo Clinic in Phoenix.
The HEAT (Hydrogel Endovascular Aneurysm Treatment) trial enrolled patients at 46 centers in the United States and Canada during June 2012–January 2016. Enrolled patients had a ruptured or unruptured brain aneurysm 3-14 mm in diameter, and averaged 57 years old. A blinded core laboratory reviewed brain scans to identify recurrent aneurysms. Of the 297 patients randomized to hydrogel coil treatment, 254 underwent follow-up at 3-12 months and 222 had additional follow-up at 18-24 months, the study’s prespecified time for the primary endpoint. Among 303 patients treated with platinum, 258 had follow-up after 3-12 months and 231 had follow-up at 18-24 months.
Among patients who entered the study with an unruptured aneurysm (nearly three-quarters of enrolled patients), the recurrence rate was 12% with platinum and 3% with hydrogel. Among the patients with a ruptured aneurysm, the recurrence rate was 24% with platinum and 8% with hydrogel. Adverse events occurred in 25% of those treated with platinum and 22% of those treated with hydrogel coil. Mortality during follow-up was 3% in the platinum arm and 2% in the hydrogel coil arm. The two groups also had similar outcomes as measured by the modified Rankin Scale and by quality-of-life measures.
HEAT was sponsored by MicroVention, the company that markets the tested hydrogel coil. Dr. Bendok had no personal disclosures.
SOURCE: Abi-Aad KR et al. ISC 2019, Abstract LB20.
HONOLULU – A second-generation hydrogel coil that’s already approved for U.S. marketing proved superior to bare platinum wire for durably embolizing brain aneurysms in a multicenter, randomized trial with 600 patients.
For the study’s primary endpoint of recurrent intracranial aneurysm during follow-up as long as 18-24 months, the 297 patients treated with the hydrogel coil had a recurrence rate of 4%, compared with a 15% rate among patients treated with platinum wire, Bernard R. Bendok, MD, said at the International Stroke Conference sponsored by the American Heart Association. The hydrogel coil materials were also relatively easy to use – much easier than the first-generation hydrogel materials, according to Dr. Bendok – and the adverse event rate using the hydrogel coils was similar to the rate using platinum wire, the standard material for brain aneurysm embolization.
Given the similar safety and ease of use and superior efficacy, clinicians treating brain aneurysms may now increasingly use hydrogel coil. “I think these results will change practice. It’s compelling data,” Dr. Bendok predicted in an interview. “The problem with platinum is it is not conducive to healing.” When an aneurysm is “closed” by insertion of platinum wire, in reality most of the space within the aneurysm remains unfilled by metal. In contrast, animal models suggest that when hydrogel fills an aneurysm it forms a matrix for collagen deposition that further fills the aneurysm space in a wound-healing process, thereby making recurrence less likely, said Dr. Bendok, professor and chair of neurosurgery at the Mayo Clinic in Phoenix.
The HEAT (Hydrogel Endovascular Aneurysm Treatment) trial enrolled patients at 46 centers in the United States and Canada during June 2012–January 2016. Enrolled patients had a ruptured or unruptured brain aneurysm 3-14 mm in diameter, and averaged 57 years old. A blinded core laboratory reviewed brain scans to identify recurrent aneurysms. Of the 297 patients randomized to hydrogel coil treatment, 254 underwent follow-up at 3-12 months and 222 had additional follow-up at 18-24 months, the study’s prespecified time for the primary endpoint. Among 303 patients treated with platinum, 258 had follow-up after 3-12 months and 231 had follow-up at 18-24 months.
Among patients who entered the study with an unruptured aneurysm (nearly three-quarters of enrolled patients), the recurrence rate was 12% with platinum and 3% with hydrogel. Among the patients with a ruptured aneurysm, the recurrence rate was 24% with platinum and 8% with hydrogel. Adverse events occurred in 25% of those treated with platinum and 22% of those treated with hydrogel coil. Mortality during follow-up was 3% in the platinum arm and 2% in the hydrogel coil arm. The two groups also had similar outcomes as measured by the modified Rankin Scale and by quality-of-life measures.
HEAT was sponsored by MicroVention, the company that markets the tested hydrogel coil. Dr. Bendok had no personal disclosures.
SOURCE: Abi-Aad KR et al. ISC 2019, Abstract LB20.
HONOLULU – A second-generation hydrogel coil that’s already approved for U.S. marketing proved superior to bare platinum wire for durably embolizing brain aneurysms in a multicenter, randomized trial with 600 patients.
For the study’s primary endpoint of recurrent intracranial aneurysm during follow-up as long as 18-24 months, the 297 patients treated with the hydrogel coil had a recurrence rate of 4%, compared with a 15% rate among patients treated with platinum wire, Bernard R. Bendok, MD, said at the International Stroke Conference sponsored by the American Heart Association. The hydrogel coil materials were also relatively easy to use – much easier than the first-generation hydrogel materials, according to Dr. Bendok – and the adverse event rate using the hydrogel coils was similar to the rate using platinum wire, the standard material for brain aneurysm embolization.
Given the similar safety and ease of use and superior efficacy, clinicians treating brain aneurysms may now increasingly use hydrogel coil. “I think these results will change practice. It’s compelling data,” Dr. Bendok predicted in an interview. “The problem with platinum is it is not conducive to healing.” When an aneurysm is “closed” by insertion of platinum wire, in reality most of the space within the aneurysm remains unfilled by metal. In contrast, animal models suggest that when hydrogel fills an aneurysm it forms a matrix for collagen deposition that further fills the aneurysm space in a wound-healing process, thereby making recurrence less likely, said Dr. Bendok, professor and chair of neurosurgery at the Mayo Clinic in Phoenix.
The HEAT (Hydrogel Endovascular Aneurysm Treatment) trial enrolled patients at 46 centers in the United States and Canada during June 2012–January 2016. Enrolled patients had a ruptured or unruptured brain aneurysm 3-14 mm in diameter, and averaged 57 years old. A blinded core laboratory reviewed brain scans to identify recurrent aneurysms. Of the 297 patients randomized to hydrogel coil treatment, 254 underwent follow-up at 3-12 months and 222 had additional follow-up at 18-24 months, the study’s prespecified time for the primary endpoint. Among 303 patients treated with platinum, 258 had follow-up after 3-12 months and 231 had follow-up at 18-24 months.
Among patients who entered the study with an unruptured aneurysm (nearly three-quarters of enrolled patients), the recurrence rate was 12% with platinum and 3% with hydrogel. Among the patients with a ruptured aneurysm, the recurrence rate was 24% with platinum and 8% with hydrogel. Adverse events occurred in 25% of those treated with platinum and 22% of those treated with hydrogel coil. Mortality during follow-up was 3% in the platinum arm and 2% in the hydrogel coil arm. The two groups also had similar outcomes as measured by the modified Rankin Scale and by quality-of-life measures.
HEAT was sponsored by MicroVention, the company that markets the tested hydrogel coil. Dr. Bendok had no personal disclosures.
SOURCE: Abi-Aad KR et al. ISC 2019, Abstract LB20.
REPORTING FROM ISC 2019
Pruritic rash on chest and back
A 26-year-old woman presented to our clinic with pruritic, hyperpigmented, symmetric edematous plaques on her upper flank, chest, and lower back (FIGURE) 3 weeks after starting a strict ketogenic (high fat/low carbohydrate) diet for postpartum weight loss. The patient was an otherwise healthy stay-at-home mother with an unremarkable medical history.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Prurigo pigmentosa
We recognized that this was a case of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30.1
While the pathophysiology remains unknown, the rash most often is reported in association with ketogenic diets.1 Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
Although prurigo pigmentosa is relatively uncommon (with an unknown incidence), primary care physicians may begin to encounter the characteristic rash more frequently, given the number of articles over the past 5 years in the primary care and nutritional literature highlighting the diet’s health benefits.2 The ketogenic diet is a high-fat, low-carbohydrate diet with preliminary evidence of improved weight loss, cardiovascular health, and glycemic control suggested by a meta-analysis of 13 randomized controlled trials.3 Additionally, the popular press and general public’s rising interest are likely to increase the number of patients on this diet.
A clinical diagnosis
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal. Urinary ketones are present in only 30% to 50% of patients; there is, however, an absence of blood ketones.
Continue to: Other conditions can mimic prurigo pigmentosa
Other conditions can mimic prurigo pigmentosa
Urticaria presents as individual lesions that often have a pale center. The lesions may occur anywhere on the body and generally last less than 24 hours. History may reveal a trigger including drugs, infection, food, or emotional stress in up to 50% of cases.
Irritant contact dermatitis often is associated with a stinging or burning sensation. Irritant and allergic contact dermatitis may have a geometric, or “outside job,” distribution suggestive of external contact, potentially with plants, alkalis, acids, or solvents.5
Confluent and reticulated papillomatosis is a rare asymptomatic dermatosis of unknown etiology that presents as hyperpigmented papules on the upper trunk, neck, and axillae. Most patients lack associated pruritis which is in contrast to prurigo pigmentosa.6
Pityriasis rosea is a viral exanthem that may be associated with constitutional symptoms and often presents initially with a herald patch progressing to a classic “Christmas tree” distribution with a fine collarette of scale. It often is asymptomatic, although some cases may be pruritic.5
Treatment focuses on dietary modification
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade.
Continue to: Pharmaceutical intervention may be necessary
Pharmaceutical intervention may be necessary
If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties.1,4 Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.1,4
Our patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
CORRESPONDENCE
Daniel Croom, MD, 34520 Bob Wilson Dr, Naval Medical Center San Diego, San Diego, CA 92134; [email protected]
1. Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
2. Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA. 2018;319:215-217.
3. Bueno NB, de Melo IS, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187.
4. Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2012;26:1149-1153.
5. James WD, Berger T, Elston D. Diseases of the skin appendages. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Saunders Elsevier; 2015:747-788.
6. Shevchenko A, Valdes-Rodriguez R, Hsu S, et al. Prurigo pigmentosa: case series and differentiation from confluent and reticulated papillomatosis. JAAD Case Rep. 2018;4:77-80.
A 26-year-old woman presented to our clinic with pruritic, hyperpigmented, symmetric edematous plaques on her upper flank, chest, and lower back (FIGURE) 3 weeks after starting a strict ketogenic (high fat/low carbohydrate) diet for postpartum weight loss. The patient was an otherwise healthy stay-at-home mother with an unremarkable medical history.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Prurigo pigmentosa
We recognized that this was a case of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30.1
While the pathophysiology remains unknown, the rash most often is reported in association with ketogenic diets.1 Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
Although prurigo pigmentosa is relatively uncommon (with an unknown incidence), primary care physicians may begin to encounter the characteristic rash more frequently, given the number of articles over the past 5 years in the primary care and nutritional literature highlighting the diet’s health benefits.2 The ketogenic diet is a high-fat, low-carbohydrate diet with preliminary evidence of improved weight loss, cardiovascular health, and glycemic control suggested by a meta-analysis of 13 randomized controlled trials.3 Additionally, the popular press and general public’s rising interest are likely to increase the number of patients on this diet.
A clinical diagnosis
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal. Urinary ketones are present in only 30% to 50% of patients; there is, however, an absence of blood ketones.
Continue to: Other conditions can mimic prurigo pigmentosa
Other conditions can mimic prurigo pigmentosa
Urticaria presents as individual lesions that often have a pale center. The lesions may occur anywhere on the body and generally last less than 24 hours. History may reveal a trigger including drugs, infection, food, or emotional stress in up to 50% of cases.
Irritant contact dermatitis often is associated with a stinging or burning sensation. Irritant and allergic contact dermatitis may have a geometric, or “outside job,” distribution suggestive of external contact, potentially with plants, alkalis, acids, or solvents.5
Confluent and reticulated papillomatosis is a rare asymptomatic dermatosis of unknown etiology that presents as hyperpigmented papules on the upper trunk, neck, and axillae. Most patients lack associated pruritis which is in contrast to prurigo pigmentosa.6
Pityriasis rosea is a viral exanthem that may be associated with constitutional symptoms and often presents initially with a herald patch progressing to a classic “Christmas tree” distribution with a fine collarette of scale. It often is asymptomatic, although some cases may be pruritic.5
Treatment focuses on dietary modification
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade.
Continue to: Pharmaceutical intervention may be necessary
Pharmaceutical intervention may be necessary
If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties.1,4 Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.1,4
Our patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
CORRESPONDENCE
Daniel Croom, MD, 34520 Bob Wilson Dr, Naval Medical Center San Diego, San Diego, CA 92134; [email protected]
A 26-year-old woman presented to our clinic with pruritic, hyperpigmented, symmetric edematous plaques on her upper flank, chest, and lower back (FIGURE) 3 weeks after starting a strict ketogenic (high fat/low carbohydrate) diet for postpartum weight loss. The patient was an otherwise healthy stay-at-home mother with an unremarkable medical history.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Prurigo pigmentosa
We recognized that this was a case of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30.1
While the pathophysiology remains unknown, the rash most often is reported in association with ketogenic diets.1 Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
Although prurigo pigmentosa is relatively uncommon (with an unknown incidence), primary care physicians may begin to encounter the characteristic rash more frequently, given the number of articles over the past 5 years in the primary care and nutritional literature highlighting the diet’s health benefits.2 The ketogenic diet is a high-fat, low-carbohydrate diet with preliminary evidence of improved weight loss, cardiovascular health, and glycemic control suggested by a meta-analysis of 13 randomized controlled trials.3 Additionally, the popular press and general public’s rising interest are likely to increase the number of patients on this diet.
A clinical diagnosis
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal. Urinary ketones are present in only 30% to 50% of patients; there is, however, an absence of blood ketones.
Continue to: Other conditions can mimic prurigo pigmentosa
Other conditions can mimic prurigo pigmentosa
Urticaria presents as individual lesions that often have a pale center. The lesions may occur anywhere on the body and generally last less than 24 hours. History may reveal a trigger including drugs, infection, food, or emotional stress in up to 50% of cases.
Irritant contact dermatitis often is associated with a stinging or burning sensation. Irritant and allergic contact dermatitis may have a geometric, or “outside job,” distribution suggestive of external contact, potentially with plants, alkalis, acids, or solvents.5
Confluent and reticulated papillomatosis is a rare asymptomatic dermatosis of unknown etiology that presents as hyperpigmented papules on the upper trunk, neck, and axillae. Most patients lack associated pruritis which is in contrast to prurigo pigmentosa.6
Pityriasis rosea is a viral exanthem that may be associated with constitutional symptoms and often presents initially with a herald patch progressing to a classic “Christmas tree” distribution with a fine collarette of scale. It often is asymptomatic, although some cases may be pruritic.5
Treatment focuses on dietary modification
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade.
Continue to: Pharmaceutical intervention may be necessary
Pharmaceutical intervention may be necessary
If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties.1,4 Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.1,4
Our patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
CORRESPONDENCE
Daniel Croom, MD, 34520 Bob Wilson Dr, Naval Medical Center San Diego, San Diego, CA 92134; [email protected]
1. Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
2. Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA. 2018;319:215-217.
3. Bueno NB, de Melo IS, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187.
4. Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2012;26:1149-1153.
5. James WD, Berger T, Elston D. Diseases of the skin appendages. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Saunders Elsevier; 2015:747-788.
6. Shevchenko A, Valdes-Rodriguez R, Hsu S, et al. Prurigo pigmentosa: case series and differentiation from confluent and reticulated papillomatosis. JAAD Case Rep. 2018;4:77-80.
1. Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
2. Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA. 2018;319:215-217.
3. Bueno NB, de Melo IS, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187.
4. Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2012;26:1149-1153.
5. James WD, Berger T, Elston D. Diseases of the skin appendages. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: Saunders Elsevier; 2015:747-788.
6. Shevchenko A, Valdes-Rodriguez R, Hsu S, et al. Prurigo pigmentosa: case series and differentiation from confluent and reticulated papillomatosis. JAAD Case Rep. 2018;4:77-80.
First-time, Mild Diverticulitis: Antibiotics or Watchful Waiting?

A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.

A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).

A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
MI, strokes spike during 30 days after cancer diagnosis
HONOLULU – During the first month after a new cancer diagnosis, patients face a substantially elevated risk for an arterial thromboembolic event – an MI or stroke – consistent with the well-described increased risk newly diagnosed cancer patients face from venous thromboembolism, based on findings from a prospective study of more than 4,000 people.
In the new study, 836 patients newly diagnosed with cancer had a 480% increased rate of a fatal or nonfatal MI or stroke during the 30 days following their diagnosis, compared with 3,339 matched people without cancer and after adjustment for baseline differences in demographics and cardiovascular risk factors, Babak B. Navi, MD, said while presenting a poster at the International Stroke Conference sponsored by the American Heart Association.
An additional analysis that focused on 210 of the 836 patients with incident cancer who had any of seven of the cancers known to pose the highest venous thromboembolism risk (lymphoma; gynecologic cancer; or cancer of the pancreas, stomach, lung, bladder, or testes) showed an 1,750% greater rate of incident MI or stroke during the first 30 days after diagnosis, compared with matched people without cancer, reported Dr. Navi, chief of the stroke and hospital neurology at Weill Cornell Medicine, New York.
In contrast, during both the period 1-3 months after the cancer diagnosis and more than 3 months after, the rate of MI or stroke among recently diagnosed cancer patients was not significantly different from the rate in comparator individuals, although the data showed modest trends toward more arterial thromboembolic events after a month, and the lack of statistically significant differences may have been a power issue, Dr. Navi suggested.
The reasons for this acutely increased risk for arterial thromboembolic events, as well as the early spike in venous thromboembolic events, are not completely clear, but they likely result from factors released by tumors, effects from the drugs that patients receive for cancer treatment, stress, and interruption of antithrombotic treatment. Dr. Navi fingered cancer-induced hypercoagulability as likely the biggest culprit. It may now be reasonable to test the idea of treating newly diagnosed cancer patients with agents that could reduce their risk for MI or stroke, such as aspirin or a statin, he said in an interview.
The new analysis used data collected in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, which during 2003-2007 enrolled more than 30,000 U.S. residents who were at least 45 years old. Dr. Navi and his associates used data collected from all REGARDS participants who developed incident cancer and had continuous Medicare coverage for at least 1 year before entering REGARDS, excluding those with cancer before enrollment. They used the Medicare records to identify the cancer diagnoses, and matched each of these people with four similar but cancer-free people enrolled in the study. Follow-up continued through September 2015. The average age of this REGARDS subgroup at enrollment was 72 years old, and nearly half were women. The incident cancers included 640 patients with a solid tumor, 71 with hematologic cancers, 13 with brain tumors, and 112 with an unknown primary cancer site.
Dr. Navi and his associates designed this study to validate previously reported findings of increased arterial thromboembolic events in newly diagnosed cancer patients from studies of insurance claims databases. Although the increased risk for venous thromboembolism in cancer patients is already well established, documenting a similar risk for arterial events is important because they are “generally more impactful for patients than venous thromboembolism,” Dr. Navi said.
REGARDS has received no commercial funding. Dr. Navi reported no disclosures.
SOURCE: Navi BB et al. Stroke. 2019 Feb;50(Suppl_1): Abstract WMP53.
HONOLULU – During the first month after a new cancer diagnosis, patients face a substantially elevated risk for an arterial thromboembolic event – an MI or stroke – consistent with the well-described increased risk newly diagnosed cancer patients face from venous thromboembolism, based on findings from a prospective study of more than 4,000 people.
In the new study, 836 patients newly diagnosed with cancer had a 480% increased rate of a fatal or nonfatal MI or stroke during the 30 days following their diagnosis, compared with 3,339 matched people without cancer and after adjustment for baseline differences in demographics and cardiovascular risk factors, Babak B. Navi, MD, said while presenting a poster at the International Stroke Conference sponsored by the American Heart Association.
An additional analysis that focused on 210 of the 836 patients with incident cancer who had any of seven of the cancers known to pose the highest venous thromboembolism risk (lymphoma; gynecologic cancer; or cancer of the pancreas, stomach, lung, bladder, or testes) showed an 1,750% greater rate of incident MI or stroke during the first 30 days after diagnosis, compared with matched people without cancer, reported Dr. Navi, chief of the stroke and hospital neurology at Weill Cornell Medicine, New York.
In contrast, during both the period 1-3 months after the cancer diagnosis and more than 3 months after, the rate of MI or stroke among recently diagnosed cancer patients was not significantly different from the rate in comparator individuals, although the data showed modest trends toward more arterial thromboembolic events after a month, and the lack of statistically significant differences may have been a power issue, Dr. Navi suggested.
The reasons for this acutely increased risk for arterial thromboembolic events, as well as the early spike in venous thromboembolic events, are not completely clear, but they likely result from factors released by tumors, effects from the drugs that patients receive for cancer treatment, stress, and interruption of antithrombotic treatment. Dr. Navi fingered cancer-induced hypercoagulability as likely the biggest culprit. It may now be reasonable to test the idea of treating newly diagnosed cancer patients with agents that could reduce their risk for MI or stroke, such as aspirin or a statin, he said in an interview.
The new analysis used data collected in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, which during 2003-2007 enrolled more than 30,000 U.S. residents who were at least 45 years old. Dr. Navi and his associates used data collected from all REGARDS participants who developed incident cancer and had continuous Medicare coverage for at least 1 year before entering REGARDS, excluding those with cancer before enrollment. They used the Medicare records to identify the cancer diagnoses, and matched each of these people with four similar but cancer-free people enrolled in the study. Follow-up continued through September 2015. The average age of this REGARDS subgroup at enrollment was 72 years old, and nearly half were women. The incident cancers included 640 patients with a solid tumor, 71 with hematologic cancers, 13 with brain tumors, and 112 with an unknown primary cancer site.
Dr. Navi and his associates designed this study to validate previously reported findings of increased arterial thromboembolic events in newly diagnosed cancer patients from studies of insurance claims databases. Although the increased risk for venous thromboembolism in cancer patients is already well established, documenting a similar risk for arterial events is important because they are “generally more impactful for patients than venous thromboembolism,” Dr. Navi said.
REGARDS has received no commercial funding. Dr. Navi reported no disclosures.
SOURCE: Navi BB et al. Stroke. 2019 Feb;50(Suppl_1): Abstract WMP53.
HONOLULU – During the first month after a new cancer diagnosis, patients face a substantially elevated risk for an arterial thromboembolic event – an MI or stroke – consistent with the well-described increased risk newly diagnosed cancer patients face from venous thromboembolism, based on findings from a prospective study of more than 4,000 people.
In the new study, 836 patients newly diagnosed with cancer had a 480% increased rate of a fatal or nonfatal MI or stroke during the 30 days following their diagnosis, compared with 3,339 matched people without cancer and after adjustment for baseline differences in demographics and cardiovascular risk factors, Babak B. Navi, MD, said while presenting a poster at the International Stroke Conference sponsored by the American Heart Association.
An additional analysis that focused on 210 of the 836 patients with incident cancer who had any of seven of the cancers known to pose the highest venous thromboembolism risk (lymphoma; gynecologic cancer; or cancer of the pancreas, stomach, lung, bladder, or testes) showed an 1,750% greater rate of incident MI or stroke during the first 30 days after diagnosis, compared with matched people without cancer, reported Dr. Navi, chief of the stroke and hospital neurology at Weill Cornell Medicine, New York.
In contrast, during both the period 1-3 months after the cancer diagnosis and more than 3 months after, the rate of MI or stroke among recently diagnosed cancer patients was not significantly different from the rate in comparator individuals, although the data showed modest trends toward more arterial thromboembolic events after a month, and the lack of statistically significant differences may have been a power issue, Dr. Navi suggested.
The reasons for this acutely increased risk for arterial thromboembolic events, as well as the early spike in venous thromboembolic events, are not completely clear, but they likely result from factors released by tumors, effects from the drugs that patients receive for cancer treatment, stress, and interruption of antithrombotic treatment. Dr. Navi fingered cancer-induced hypercoagulability as likely the biggest culprit. It may now be reasonable to test the idea of treating newly diagnosed cancer patients with agents that could reduce their risk for MI or stroke, such as aspirin or a statin, he said in an interview.
The new analysis used data collected in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, which during 2003-2007 enrolled more than 30,000 U.S. residents who were at least 45 years old. Dr. Navi and his associates used data collected from all REGARDS participants who developed incident cancer and had continuous Medicare coverage for at least 1 year before entering REGARDS, excluding those with cancer before enrollment. They used the Medicare records to identify the cancer diagnoses, and matched each of these people with four similar but cancer-free people enrolled in the study. Follow-up continued through September 2015. The average age of this REGARDS subgroup at enrollment was 72 years old, and nearly half were women. The incident cancers included 640 patients with a solid tumor, 71 with hematologic cancers, 13 with brain tumors, and 112 with an unknown primary cancer site.
Dr. Navi and his associates designed this study to validate previously reported findings of increased arterial thromboembolic events in newly diagnosed cancer patients from studies of insurance claims databases. Although the increased risk for venous thromboembolism in cancer patients is already well established, documenting a similar risk for arterial events is important because they are “generally more impactful for patients than venous thromboembolism,” Dr. Navi said.
REGARDS has received no commercial funding. Dr. Navi reported no disclosures.
SOURCE: Navi BB et al. Stroke. 2019 Feb;50(Suppl_1): Abstract WMP53.
REPORTING FROM ISC 2019
Antibiotics gut checkpoint inhibitor efficacy
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
REPORTING FROM ASCO-SITC