User login
Myocarditis shows causal role in frequent PVCs
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
I was quite taken by this study, which produced results that raise shock and alarm. I believe that the clinical condition that this study highlights is a real biological phenomenon that affects patients who were not on my radar screen.
From now on, I will certainly be more alert for and concerned about patients whom I see with an abrupt onset of frequent premature ventricular contractions, especially those who also have a reduced left ventricular ejection fraction. However the potential need to use serial PET examinations to identify and then follow these patients also raises concern about the cumulative radiation exposure patients could receive from serial PET studies.
David J. Callans, MD , is professor of medicine and associate director of electrophysiology at the University of Pennsylvania in Philadelphia. He has been a consultant to Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, and St. Jude. He made these comments as designated discussant for the report.
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
BOSTON – About half of patients who present with a new onset of frequent premature ventricular contractions without obvious underlying heart disease had an underlying myocardial inflammation that was often responsive to immunosuppressive treatment, according to a single-center series of 107 patients.
“Early diagnosis and appropriate treatment with immunosuppressive therapy can significantly affect the clinical course,” although large-scale, multicenter, randomized trials must confirm this as an effective management approach, Dhanunjaya Lakkireddy, MD, said at the annual scientific sessions of the Heart Rhythm Society. He stressed that the anecdotal efficacy seen in this series with immunosuppressive therapy and selected use of ablation treatment for the premature ventricular contractions (PVCs) applies only to patients with new-onset PVCs that occur at a rate of at least 5,000 during 24 hours who also have myocardial inflammation identified by a PET scan showing increased fluorodeoxyglucose (FDG) uptake.
The apparent impact of immunosuppressive treatment was “profound,” Dr. Lakkireddy said. The treatment usually involved prednisone and, in many patients, a second immunosuppressant agent such as azathioprine, cyclophosphamide, or methotrexate. The results suggest “a unique opportunity to intervene early with immunosuppression to change the natural course of the disease. PVCs may be the earliest sign of a disease process” featuring myocardial inflammation.
The data came from the Myocarditis and Ventricular Arrhythmia (MAVERIC) registry that Dr. Lakkireddy and his associates started because “we began seeing patients referred for ablations without underlying heart disease who had suddenly presented with a lot of PVCs,” he recalled, an observation that led them to systematically study these patients in an “arduous” process that involved several tests. One hundred seven patients met the registry’s inclusion criteria for new onset of frequent PVCs without apparent underlying heart disease, and roughly half of these patients showed clear evidence of myocardial inflammation by FDG and PET imaging. “If the PET is negative, I don’t worry about myocarditis, “ Dr. Lakkireddy said.
The 55 patients with apparent myocarditis on PET imaging, out of the 107 patients examined generally, had lower left ventricular (LV) ejection fractions averaging 46%, compared with 51% among the patients without myocarditis The patients with myocarditis further subdivided into 27 with preserved LV function, with an average ejection fraction of 60%, and 28 with a reduced LV function, with an average ejection fraction of 40%. The researchers saw an optimal response to immunosuppressive therapy in 18 of the 23 patients (78%) with preserved ejection fractions who received this treatment and in 13 of the 24 patients (54%) with diminished LV ejection fractions who got immunosuppressive therapy.
Twenty-eight of the 55 patients with myocarditis on PET imaging underwent a right-sided biopsy during their work-up, and 13 of these 28 biopsies (46%) showed a lymphocytic infiltrate of a type often seen in patients with postviral myocarditis. Seven of the 28 biopsied patients (25%) had completely normal-appearing cardiac tissue.
Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
SOURCE: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
REPORTING FROM HEART RHYTHM 2018
Key clinical point:
Major finding: Immunosuppressive therapy resolved myocarditis in two-thirds of 51% of patients with new-onset, frequents PVCs.
Study details: Single-center series with 107 patients.
Disclosures: Dr. Lakkireddy has been a consultant to or has received research support from Biosense Webster, Boehinger Ingelheim, Bristol-Myers Squibb, Estech, Janssen, Pfizer, SentreHeart, and St. Jude.
Source: Lakkireddy D et al. Heart Rhythm 2018, Abstract B-LBCT02-02.
New models of gastroenterology practice
The variety of employment models available to gastroenterologists reflects the dynamic changes we are experiencing in medicine today. Delivery of gastrointestinal (GI) care in the United States continues to evolve in light of health care reform and the Affordable Care Act.1 Within the past decade, as health systems and payers continue to consolidate, regulatory pressures have increased steadily and new policies such as electronic documentation and mandatory quality metrics reporting have added new challenges to the emerging generation of gastroenterologists.2 Although the lay press tends to focus on health care costs, coverage, physician reimbursement, provider burnout, health system consolidation, and value-based payment models, relatively less has been published about emerging employment and practice models.
Here,
Background
When the senior author graduated from fellowship in 1983 (J.I.A.), gastroenterology practice model choices were limited to essentially 4: independent community-based, single-specialty, physician-owned practice (solo or small group); independent multispecialty physician-owned practice; hospital or health system–owned multispecialty practice; and academic practice (including the Veterans Administration Medical Centers).
In the private sector, young community gastroenterologists typically would join a physician-owned practice and spend time (2–5 y) as an employed physician in a partnership track. During this time, his/her salary was subsidized while he/she built a practice base. Then, they would buy into the Professional Association with cash or equity equivalents and become a partner. As a partner, he/she then had the opportunity to share in ancillary revenue streams such as facility fees derived from a practice-owned ambulatory endoscopy center (AEC). By contrast, young academic faculty would be hired as an instructor and, if successful, climb the traditional ladder track to assistant, associate, and professor of medicine in an academic medical center (AMC).
In the 1980s, a typical community GI practice comprised 1 to 8 physicians, with most having been formed by 1 or 2 male gastroenterologists in the early 1970s when flexible endoscopy moved into clinical practice. The three practices that eventually would become Minnesota Gastroenterology (where J.I.A. practiced) opened in 1972. In 1996, the three practices merged into a single group of 38 physicians with ownership in three AECs. Advanced practice nurses and physician assistants were not yet part of the equation. Colonoscopy represented 48% of procedure volume, accounts receivable (time between submitting an insurance claim and being paid) averaged 88 days, and physicians averaged 9000 work relative value units (wRVUs) per partner annually. By comparison, median wRVUs for a full-time community GI in 1996 was 10,422 according to the Medical Group Management Association.3 Annual gross revenue (before expenses) per physician was approximately $400,000, and overhead reached 38% and 47% of revenue (there were 2 divisions). Partner incomes were at the 12% level of the Medical Group Management Association for gastroenterologists (personal management notes of J.I.A.). Minnesota Gastroenterology was the largest single-specialty GI practice in 1996 and its consolidation foreshadowed a trend that has accelerated over the ensuing generation.
When one of the authors (N.K.) graduated from the University of California Los Angeles in 2017, the GI employment landscape had evolved considerably. At least five new models of GI practice had emerged: individual incorporation with a Professional Services Agreement (PSA), a clinician track within an AMC, large single-specialty group practice (partnership or employee), private equity-backed multistate practice, and locum tenens (Figure 1).
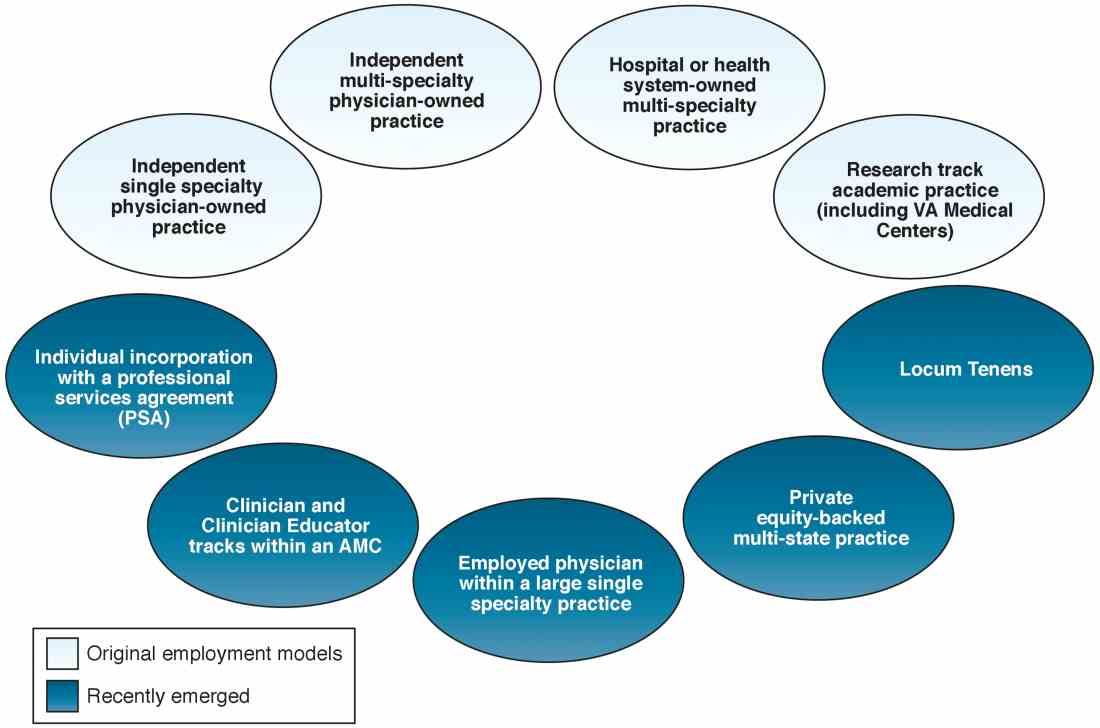
Employment models (light blue) available in the 1980s and those that have emerged as common models in the last decade (dark blue).
An individual corporation with a professional services agreement
For gastroenterologists at any career stage, the prospect of employment within a corporate entity, be it an academic university, hospital system, or private practice group, can be daunting. To that end, one central question facing nearly all gastroenterologists is: how much independence and flexibility, both clinically and financially, do I really want, and what can I do to realize my ideal job description?
An interesting alternative to direct health system employment occurs when a physician forms a solo corporation and then contracts with a hospital or health system under a PSA. Here, the physician provides professional services on a contractual basis, but retains control of finances and has more autonomy compared with employment. Essentially, the physician is a corporation of one, with hospital alignment rather than employment. For full disclosure, this is the employment model of one of the authors (N.K.).
A PSA arrangement is common for larger independent GI practices. Many practices have PSA arrangements with hospitals ranging from call coverage to full professional services. For an individual working within a PSA, income is not the traditional W-2 Internal Revenue Service arrangement in which taxes are removed automatically. Income derived from a PSA usually falls under an Internal Revenue Service Form 1099. The physician actually is employed through their practice corporation and relates to the hospital as an independent contractor.
There are four common variants of the PSA model.4 A Global Payment PSA is when a hospital contracts with the physician practice for specific services and pays a global rate linked to wRVUs. The rate is negotiated to encompass physician compensation, benefits, and practice overhead. The practice retains control of its own office functions and staff.
In a traditional PSA, the hospital contracts with physicians and pays them based on RVU production, but the hospital owns the administrative part of the practice (staff, billing, collections, equipment, and supplies).
A practice management arrangement occurs when the hospital employs the physician who provides professional services and a separate third party manages the practice via a separate management contract. Finally, a Carve-Out PSA can use any of the earlier-described PSA arrangements and certain services are carved out under line-item provisions. For example, a hospital could contract with a private GI group for endoscopic services or night call and write a PSA expressly for these purposes.
Some notable benefits of the PSA are that physicians can maintain financial and employment independence from the hospital and have more control over benefits packages, retirement savings options, and health insurance. Physicians also can provide services outside of the hospital (e.g., telemedicine or locums tenens — see later) without institutional restrictions or conflicts. Finally, physicians benefit from tax advantages of self-employment (with associated business-related tax deductions) through their corporation. The potential downsides of a PSA contract are the subtle expansion of services demanded (known as scope creep) or the possibility of contract termination (or nonrenewal) by the hospital. In addition, medical training does not equip physicians with the knowledge to navigate personal and corporate finances, benefits packages, and tax structures, so the learning curve can be quite steep. Nevertheless, PSAs can be an innovative employment model for gastroenterologists who wish to preserve autonomy and financial flexibility. In this model, legal advice by an attorney skilled in employment law is mandatory.
Academic clinicians track
Until recently, clinically oriented academic faculty were channeled into the traditional ladder faculty model in which advancement was contingent on publications, national recognition, grant support, and teaching. As competition for market share has intensified among regional health systems, many AMCs have developed purely clinical tracks in which research, publication, and teaching are not expected; salaries are linked to clinical productivity; and income may approximate the professional (but not ancillary) income of a community gastroenterologist.
Various models of this arrangement exist as well. For example, clinicians can be employed within a group that has a board and management structure distinct from the faculty group practice, as in the case of the Northeast Medical Group at Yale New Haven Health System5 and the University of Maryland Community Medical Group. In addition, clinicians can form an operating group separate from the faculty practice but as a controlled subsidiary (such as the University of Pittsburgh Community Medicine), separate operating group for primary care but specialists are employed within their respective departments (Emory Specialty Associates) or as a distinct clinical department within a faculty practice (University of California Los Angeles Medical Group Staff Physicians).
Irrespective of the employment model, these clinicians essentially work similar to community gastroenterologists but within the umbrella of an AMC. For young faculty whose interest is not in research or teaching, this can be an attractive option that maintains a tie to a university health system. For a seasoned clinician in community practice, this is an option to return to an academic environment. Usually, productivity expectations within the clinician track approximate those of a community practice gastroenterologist, but again total compensation may not be as great because ancillary income streams usually are not available. We expect this AMC employment track to become more prevalent as universities expand their footprints and acquire practices, hospitals, and ambulatory facilities distant from the main campus.
Large single-specialty practice
Consolidation of independent practices has been evident for 20 years and has accelerated as physicians in smaller practices have aged and burdens of practice have increased. Now, most urban centers have large mega-sized practices or super groups that have grown through practice mergers, acquisitions, and successful recruitment. Large practices can be modeled as a single integrated corporation (with ancillary components such as an AEC or infusion center) or as individual business units that are grouped under a single corporate entity.6
Within these large and mega-sized practices, differing employment options have emerged in addition to the traditional partnership track. These include payment on a per-diem basis, annual salary, or a mix of both. As opposed to partnership, the employment track avoids responsibility for governance and corporate liability, although not individual liability, and usually does not involve after-hours call. An employed physician usually does not benefit from ancillary income that derives from AEC facility fees, infusion centers, and pathology and anesthesia services.
Private equity ownership of gastroenterology practices
In June 2016, private equity entered the GI space with the investment of the Audax Group in a community GI practice based in Miami, Florida. The term private equity refers to capital that is not reported in public forums and comprises funds that investors directly invest into private companies or use to buy out public companies and turn them private.
According to their website, when the Audax Group invests in a medical practice, they provide capital for substantial infrastructure support, business experience, and acumen, but retain medical practice leaders as their clinical decision makers. They also bring proven expertise and economies of scale to resource-intensive aspects of a medical practice including information technology, regulation compliance, human resources, revenue cycle management, payroll, benefits, rents, and lease as examples. These components can be difficult to manage efficiently within independent medical practices, so many maturing practices are selling their practices to regional health systems. This multistate equity-backed medical practice is an alternative to health system acquisition, and may help physicians feel more in control of their practices and potentially share in the equity investment.
It is important to understand the employment structure and associations of any practice you are contemplating joining. The model devised by this group is meant to retain physician authority and responsibility while providing capital to support innovation and the development of needed infrastructure. Growth of market share and revenues can accrue back to physician owners. This is distinct from practices that are part of a health system in which there may be more of a corporate feeling and centralized governance.
Locum tenens
Locum tenens is a Latin phrase that means “to hold the place of.” According to the website of a large locum tenens company, this practice model originated in the 1970s when the federal government provided a grant to the University of Utah to provide physician services for underserved areas in the Western United States. The program proved so successful that hospital administrators who had difficulty recruiting staff physicians began asking for staffing assistance.
Today, a substantial number of physicians at all stages of their careers are working as locum tenens. They work as independent contractors so that income taxes are not withheld and benefits are the responsibility of the individual. As with the PSA arrangement, a physician would meet with both an accountant and labor lawyer to establish him or herself as a corporate entity for tax advantages and limited liability from litigation.
Early stage physicians who might be following a significant other or spouse to specific locations sometimes consider a locum tenens as a bridge to permanent positions. Late-stage physicians who no longer want to be tied to a small group or solo practice have become locum tenens physicians who enjoy multiple temporary employment positions nationwide. This pathway no longer is unusual and can be a satisfying means to expand employment horizons. As with all employment situations, due diligence is mandatory before signing with any locum tenens company.
Conclusions
The employment spectrum for gastroenterologists and other medical professionals has expanded greatly between the time the senior author and the junior author entered the workforce. Change is now the one constant in medicine, and medicine today largely is fast-paced, corporatized, and highly regulated. Finding an employment model that is comfortable for current physicians, whose life situations are quite diverse, can be challenging. but a variety of opportunities now exist.
Think carefully about what you truly desire as a medical professional and how you might shape your employment to realize your goals. Options are available for those with an open mind and persistence.
References
1. Sheen E, Dorn SD, Brill JV, et al. Health care reform and the road ahead for gastroenterology. Clin Gastroenterol Hepatol. 2012;10:1062-5.
2. Kosinski LR. Meaningful use and electronic medical records for the gastroenterology practice. Clin Gastroenterol Hepatol. 2010;8:494-7.
3. Medical Group Management Association (MGMA). Accessed January 20, 2017.
4. The Coker Group. PSAs as an Alternative to Employment: A Contemporary Option for Alignment and Integration. In: The Coker Group Thought Leadership – White Papers. March 2016.
5. Houston R, McGinnis T. Accountable care organizations: looking back and moving forward. Centers for Health Care Strategies Inc. Brief. January 2016. Accessed January 20, 2017.
6. Pallardy C. 7 gastroenterologists leading GI mega-practices. Becker’s GI and endoscopy 2015. Accessed January 20, 2017.
Dr. Allen is in the division of gastroenterology and hepatology, department of medicine, University of Michigan School of Medicine, Ann Arbor; he is also the Editor in Chief of GI & Hepatology News. Dr. Kaushal is in the division of gastroenterology, Adventist Health Systems, Sonora, Calif. The authors disclose no conflicts.
The variety of employment models available to gastroenterologists reflects the dynamic changes we are experiencing in medicine today. Delivery of gastrointestinal (GI) care in the United States continues to evolve in light of health care reform and the Affordable Care Act.1 Within the past decade, as health systems and payers continue to consolidate, regulatory pressures have increased steadily and new policies such as electronic documentation and mandatory quality metrics reporting have added new challenges to the emerging generation of gastroenterologists.2 Although the lay press tends to focus on health care costs, coverage, physician reimbursement, provider burnout, health system consolidation, and value-based payment models, relatively less has been published about emerging employment and practice models.
Here,
Background
When the senior author graduated from fellowship in 1983 (J.I.A.), gastroenterology practice model choices were limited to essentially 4: independent community-based, single-specialty, physician-owned practice (solo or small group); independent multispecialty physician-owned practice; hospital or health system–owned multispecialty practice; and academic practice (including the Veterans Administration Medical Centers).
In the private sector, young community gastroenterologists typically would join a physician-owned practice and spend time (2–5 y) as an employed physician in a partnership track. During this time, his/her salary was subsidized while he/she built a practice base. Then, they would buy into the Professional Association with cash or equity equivalents and become a partner. As a partner, he/she then had the opportunity to share in ancillary revenue streams such as facility fees derived from a practice-owned ambulatory endoscopy center (AEC). By contrast, young academic faculty would be hired as an instructor and, if successful, climb the traditional ladder track to assistant, associate, and professor of medicine in an academic medical center (AMC).
In the 1980s, a typical community GI practice comprised 1 to 8 physicians, with most having been formed by 1 or 2 male gastroenterologists in the early 1970s when flexible endoscopy moved into clinical practice. The three practices that eventually would become Minnesota Gastroenterology (where J.I.A. practiced) opened in 1972. In 1996, the three practices merged into a single group of 38 physicians with ownership in three AECs. Advanced practice nurses and physician assistants were not yet part of the equation. Colonoscopy represented 48% of procedure volume, accounts receivable (time between submitting an insurance claim and being paid) averaged 88 days, and physicians averaged 9000 work relative value units (wRVUs) per partner annually. By comparison, median wRVUs for a full-time community GI in 1996 was 10,422 according to the Medical Group Management Association.3 Annual gross revenue (before expenses) per physician was approximately $400,000, and overhead reached 38% and 47% of revenue (there were 2 divisions). Partner incomes were at the 12% level of the Medical Group Management Association for gastroenterologists (personal management notes of J.I.A.). Minnesota Gastroenterology was the largest single-specialty GI practice in 1996 and its consolidation foreshadowed a trend that has accelerated over the ensuing generation.
When one of the authors (N.K.) graduated from the University of California Los Angeles in 2017, the GI employment landscape had evolved considerably. At least five new models of GI practice had emerged: individual incorporation with a Professional Services Agreement (PSA), a clinician track within an AMC, large single-specialty group practice (partnership or employee), private equity-backed multistate practice, and locum tenens (Figure 1).

Employment models (light blue) available in the 1980s and those that have emerged as common models in the last decade (dark blue).
An individual corporation with a professional services agreement
For gastroenterologists at any career stage, the prospect of employment within a corporate entity, be it an academic university, hospital system, or private practice group, can be daunting. To that end, one central question facing nearly all gastroenterologists is: how much independence and flexibility, both clinically and financially, do I really want, and what can I do to realize my ideal job description?
An interesting alternative to direct health system employment occurs when a physician forms a solo corporation and then contracts with a hospital or health system under a PSA. Here, the physician provides professional services on a contractual basis, but retains control of finances and has more autonomy compared with employment. Essentially, the physician is a corporation of one, with hospital alignment rather than employment. For full disclosure, this is the employment model of one of the authors (N.K.).
A PSA arrangement is common for larger independent GI practices. Many practices have PSA arrangements with hospitals ranging from call coverage to full professional services. For an individual working within a PSA, income is not the traditional W-2 Internal Revenue Service arrangement in which taxes are removed automatically. Income derived from a PSA usually falls under an Internal Revenue Service Form 1099. The physician actually is employed through their practice corporation and relates to the hospital as an independent contractor.
There are four common variants of the PSA model.4 A Global Payment PSA is when a hospital contracts with the physician practice for specific services and pays a global rate linked to wRVUs. The rate is negotiated to encompass physician compensation, benefits, and practice overhead. The practice retains control of its own office functions and staff.
In a traditional PSA, the hospital contracts with physicians and pays them based on RVU production, but the hospital owns the administrative part of the practice (staff, billing, collections, equipment, and supplies).
A practice management arrangement occurs when the hospital employs the physician who provides professional services and a separate third party manages the practice via a separate management contract. Finally, a Carve-Out PSA can use any of the earlier-described PSA arrangements and certain services are carved out under line-item provisions. For example, a hospital could contract with a private GI group for endoscopic services or night call and write a PSA expressly for these purposes.
Some notable benefits of the PSA are that physicians can maintain financial and employment independence from the hospital and have more control over benefits packages, retirement savings options, and health insurance. Physicians also can provide services outside of the hospital (e.g., telemedicine or locums tenens — see later) without institutional restrictions or conflicts. Finally, physicians benefit from tax advantages of self-employment (with associated business-related tax deductions) through their corporation. The potential downsides of a PSA contract are the subtle expansion of services demanded (known as scope creep) or the possibility of contract termination (or nonrenewal) by the hospital. In addition, medical training does not equip physicians with the knowledge to navigate personal and corporate finances, benefits packages, and tax structures, so the learning curve can be quite steep. Nevertheless, PSAs can be an innovative employment model for gastroenterologists who wish to preserve autonomy and financial flexibility. In this model, legal advice by an attorney skilled in employment law is mandatory.
Academic clinicians track
Until recently, clinically oriented academic faculty were channeled into the traditional ladder faculty model in which advancement was contingent on publications, national recognition, grant support, and teaching. As competition for market share has intensified among regional health systems, many AMCs have developed purely clinical tracks in which research, publication, and teaching are not expected; salaries are linked to clinical productivity; and income may approximate the professional (but not ancillary) income of a community gastroenterologist.
Various models of this arrangement exist as well. For example, clinicians can be employed within a group that has a board and management structure distinct from the faculty group practice, as in the case of the Northeast Medical Group at Yale New Haven Health System5 and the University of Maryland Community Medical Group. In addition, clinicians can form an operating group separate from the faculty practice but as a controlled subsidiary (such as the University of Pittsburgh Community Medicine), separate operating group for primary care but specialists are employed within their respective departments (Emory Specialty Associates) or as a distinct clinical department within a faculty practice (University of California Los Angeles Medical Group Staff Physicians).
Irrespective of the employment model, these clinicians essentially work similar to community gastroenterologists but within the umbrella of an AMC. For young faculty whose interest is not in research or teaching, this can be an attractive option that maintains a tie to a university health system. For a seasoned clinician in community practice, this is an option to return to an academic environment. Usually, productivity expectations within the clinician track approximate those of a community practice gastroenterologist, but again total compensation may not be as great because ancillary income streams usually are not available. We expect this AMC employment track to become more prevalent as universities expand their footprints and acquire practices, hospitals, and ambulatory facilities distant from the main campus.
Large single-specialty practice
Consolidation of independent practices has been evident for 20 years and has accelerated as physicians in smaller practices have aged and burdens of practice have increased. Now, most urban centers have large mega-sized practices or super groups that have grown through practice mergers, acquisitions, and successful recruitment. Large practices can be modeled as a single integrated corporation (with ancillary components such as an AEC or infusion center) or as individual business units that are grouped under a single corporate entity.6
Within these large and mega-sized practices, differing employment options have emerged in addition to the traditional partnership track. These include payment on a per-diem basis, annual salary, or a mix of both. As opposed to partnership, the employment track avoids responsibility for governance and corporate liability, although not individual liability, and usually does not involve after-hours call. An employed physician usually does not benefit from ancillary income that derives from AEC facility fees, infusion centers, and pathology and anesthesia services.
Private equity ownership of gastroenterology practices
In June 2016, private equity entered the GI space with the investment of the Audax Group in a community GI practice based in Miami, Florida. The term private equity refers to capital that is not reported in public forums and comprises funds that investors directly invest into private companies or use to buy out public companies and turn them private.
According to their website, when the Audax Group invests in a medical practice, they provide capital for substantial infrastructure support, business experience, and acumen, but retain medical practice leaders as their clinical decision makers. They also bring proven expertise and economies of scale to resource-intensive aspects of a medical practice including information technology, regulation compliance, human resources, revenue cycle management, payroll, benefits, rents, and lease as examples. These components can be difficult to manage efficiently within independent medical practices, so many maturing practices are selling their practices to regional health systems. This multistate equity-backed medical practice is an alternative to health system acquisition, and may help physicians feel more in control of their practices and potentially share in the equity investment.
It is important to understand the employment structure and associations of any practice you are contemplating joining. The model devised by this group is meant to retain physician authority and responsibility while providing capital to support innovation and the development of needed infrastructure. Growth of market share and revenues can accrue back to physician owners. This is distinct from practices that are part of a health system in which there may be more of a corporate feeling and centralized governance.
Locum tenens
Locum tenens is a Latin phrase that means “to hold the place of.” According to the website of a large locum tenens company, this practice model originated in the 1970s when the federal government provided a grant to the University of Utah to provide physician services for underserved areas in the Western United States. The program proved so successful that hospital administrators who had difficulty recruiting staff physicians began asking for staffing assistance.
Today, a substantial number of physicians at all stages of their careers are working as locum tenens. They work as independent contractors so that income taxes are not withheld and benefits are the responsibility of the individual. As with the PSA arrangement, a physician would meet with both an accountant and labor lawyer to establish him or herself as a corporate entity for tax advantages and limited liability from litigation.
Early stage physicians who might be following a significant other or spouse to specific locations sometimes consider a locum tenens as a bridge to permanent positions. Late-stage physicians who no longer want to be tied to a small group or solo practice have become locum tenens physicians who enjoy multiple temporary employment positions nationwide. This pathway no longer is unusual and can be a satisfying means to expand employment horizons. As with all employment situations, due diligence is mandatory before signing with any locum tenens company.
Conclusions
The employment spectrum for gastroenterologists and other medical professionals has expanded greatly between the time the senior author and the junior author entered the workforce. Change is now the one constant in medicine, and medicine today largely is fast-paced, corporatized, and highly regulated. Finding an employment model that is comfortable for current physicians, whose life situations are quite diverse, can be challenging. but a variety of opportunities now exist.
Think carefully about what you truly desire as a medical professional and how you might shape your employment to realize your goals. Options are available for those with an open mind and persistence.
References
1. Sheen E, Dorn SD, Brill JV, et al. Health care reform and the road ahead for gastroenterology. Clin Gastroenterol Hepatol. 2012;10:1062-5.
2. Kosinski LR. Meaningful use and electronic medical records for the gastroenterology practice. Clin Gastroenterol Hepatol. 2010;8:494-7.
3. Medical Group Management Association (MGMA). Accessed January 20, 2017.
4. The Coker Group. PSAs as an Alternative to Employment: A Contemporary Option for Alignment and Integration. In: The Coker Group Thought Leadership – White Papers. March 2016.
5. Houston R, McGinnis T. Accountable care organizations: looking back and moving forward. Centers for Health Care Strategies Inc. Brief. January 2016. Accessed January 20, 2017.
6. Pallardy C. 7 gastroenterologists leading GI mega-practices. Becker’s GI and endoscopy 2015. Accessed January 20, 2017.
Dr. Allen is in the division of gastroenterology and hepatology, department of medicine, University of Michigan School of Medicine, Ann Arbor; he is also the Editor in Chief of GI & Hepatology News. Dr. Kaushal is in the division of gastroenterology, Adventist Health Systems, Sonora, Calif. The authors disclose no conflicts.
The variety of employment models available to gastroenterologists reflects the dynamic changes we are experiencing in medicine today. Delivery of gastrointestinal (GI) care in the United States continues to evolve in light of health care reform and the Affordable Care Act.1 Within the past decade, as health systems and payers continue to consolidate, regulatory pressures have increased steadily and new policies such as electronic documentation and mandatory quality metrics reporting have added new challenges to the emerging generation of gastroenterologists.2 Although the lay press tends to focus on health care costs, coverage, physician reimbursement, provider burnout, health system consolidation, and value-based payment models, relatively less has been published about emerging employment and practice models.
Here,
Background
When the senior author graduated from fellowship in 1983 (J.I.A.), gastroenterology practice model choices were limited to essentially 4: independent community-based, single-specialty, physician-owned practice (solo or small group); independent multispecialty physician-owned practice; hospital or health system–owned multispecialty practice; and academic practice (including the Veterans Administration Medical Centers).
In the private sector, young community gastroenterologists typically would join a physician-owned practice and spend time (2–5 y) as an employed physician in a partnership track. During this time, his/her salary was subsidized while he/she built a practice base. Then, they would buy into the Professional Association with cash or equity equivalents and become a partner. As a partner, he/she then had the opportunity to share in ancillary revenue streams such as facility fees derived from a practice-owned ambulatory endoscopy center (AEC). By contrast, young academic faculty would be hired as an instructor and, if successful, climb the traditional ladder track to assistant, associate, and professor of medicine in an academic medical center (AMC).
In the 1980s, a typical community GI practice comprised 1 to 8 physicians, with most having been formed by 1 or 2 male gastroenterologists in the early 1970s when flexible endoscopy moved into clinical practice. The three practices that eventually would become Minnesota Gastroenterology (where J.I.A. practiced) opened in 1972. In 1996, the three practices merged into a single group of 38 physicians with ownership in three AECs. Advanced practice nurses and physician assistants were not yet part of the equation. Colonoscopy represented 48% of procedure volume, accounts receivable (time between submitting an insurance claim and being paid) averaged 88 days, and physicians averaged 9000 work relative value units (wRVUs) per partner annually. By comparison, median wRVUs for a full-time community GI in 1996 was 10,422 according to the Medical Group Management Association.3 Annual gross revenue (before expenses) per physician was approximately $400,000, and overhead reached 38% and 47% of revenue (there were 2 divisions). Partner incomes were at the 12% level of the Medical Group Management Association for gastroenterologists (personal management notes of J.I.A.). Minnesota Gastroenterology was the largest single-specialty GI practice in 1996 and its consolidation foreshadowed a trend that has accelerated over the ensuing generation.
When one of the authors (N.K.) graduated from the University of California Los Angeles in 2017, the GI employment landscape had evolved considerably. At least five new models of GI practice had emerged: individual incorporation with a Professional Services Agreement (PSA), a clinician track within an AMC, large single-specialty group practice (partnership or employee), private equity-backed multistate practice, and locum tenens (Figure 1).

Employment models (light blue) available in the 1980s and those that have emerged as common models in the last decade (dark blue).
An individual corporation with a professional services agreement
For gastroenterologists at any career stage, the prospect of employment within a corporate entity, be it an academic university, hospital system, or private practice group, can be daunting. To that end, one central question facing nearly all gastroenterologists is: how much independence and flexibility, both clinically and financially, do I really want, and what can I do to realize my ideal job description?
An interesting alternative to direct health system employment occurs when a physician forms a solo corporation and then contracts with a hospital or health system under a PSA. Here, the physician provides professional services on a contractual basis, but retains control of finances and has more autonomy compared with employment. Essentially, the physician is a corporation of one, with hospital alignment rather than employment. For full disclosure, this is the employment model of one of the authors (N.K.).
A PSA arrangement is common for larger independent GI practices. Many practices have PSA arrangements with hospitals ranging from call coverage to full professional services. For an individual working within a PSA, income is not the traditional W-2 Internal Revenue Service arrangement in which taxes are removed automatically. Income derived from a PSA usually falls under an Internal Revenue Service Form 1099. The physician actually is employed through their practice corporation and relates to the hospital as an independent contractor.
There are four common variants of the PSA model.4 A Global Payment PSA is when a hospital contracts with the physician practice for specific services and pays a global rate linked to wRVUs. The rate is negotiated to encompass physician compensation, benefits, and practice overhead. The practice retains control of its own office functions and staff.
In a traditional PSA, the hospital contracts with physicians and pays them based on RVU production, but the hospital owns the administrative part of the practice (staff, billing, collections, equipment, and supplies).
A practice management arrangement occurs when the hospital employs the physician who provides professional services and a separate third party manages the practice via a separate management contract. Finally, a Carve-Out PSA can use any of the earlier-described PSA arrangements and certain services are carved out under line-item provisions. For example, a hospital could contract with a private GI group for endoscopic services or night call and write a PSA expressly for these purposes.
Some notable benefits of the PSA are that physicians can maintain financial and employment independence from the hospital and have more control over benefits packages, retirement savings options, and health insurance. Physicians also can provide services outside of the hospital (e.g., telemedicine or locums tenens — see later) without institutional restrictions or conflicts. Finally, physicians benefit from tax advantages of self-employment (with associated business-related tax deductions) through their corporation. The potential downsides of a PSA contract are the subtle expansion of services demanded (known as scope creep) or the possibility of contract termination (or nonrenewal) by the hospital. In addition, medical training does not equip physicians with the knowledge to navigate personal and corporate finances, benefits packages, and tax structures, so the learning curve can be quite steep. Nevertheless, PSAs can be an innovative employment model for gastroenterologists who wish to preserve autonomy and financial flexibility. In this model, legal advice by an attorney skilled in employment law is mandatory.
Academic clinicians track
Until recently, clinically oriented academic faculty were channeled into the traditional ladder faculty model in which advancement was contingent on publications, national recognition, grant support, and teaching. As competition for market share has intensified among regional health systems, many AMCs have developed purely clinical tracks in which research, publication, and teaching are not expected; salaries are linked to clinical productivity; and income may approximate the professional (but not ancillary) income of a community gastroenterologist.
Various models of this arrangement exist as well. For example, clinicians can be employed within a group that has a board and management structure distinct from the faculty group practice, as in the case of the Northeast Medical Group at Yale New Haven Health System5 and the University of Maryland Community Medical Group. In addition, clinicians can form an operating group separate from the faculty practice but as a controlled subsidiary (such as the University of Pittsburgh Community Medicine), separate operating group for primary care but specialists are employed within their respective departments (Emory Specialty Associates) or as a distinct clinical department within a faculty practice (University of California Los Angeles Medical Group Staff Physicians).
Irrespective of the employment model, these clinicians essentially work similar to community gastroenterologists but within the umbrella of an AMC. For young faculty whose interest is not in research or teaching, this can be an attractive option that maintains a tie to a university health system. For a seasoned clinician in community practice, this is an option to return to an academic environment. Usually, productivity expectations within the clinician track approximate those of a community practice gastroenterologist, but again total compensation may not be as great because ancillary income streams usually are not available. We expect this AMC employment track to become more prevalent as universities expand their footprints and acquire practices, hospitals, and ambulatory facilities distant from the main campus.
Large single-specialty practice
Consolidation of independent practices has been evident for 20 years and has accelerated as physicians in smaller practices have aged and burdens of practice have increased. Now, most urban centers have large mega-sized practices or super groups that have grown through practice mergers, acquisitions, and successful recruitment. Large practices can be modeled as a single integrated corporation (with ancillary components such as an AEC or infusion center) or as individual business units that are grouped under a single corporate entity.6
Within these large and mega-sized practices, differing employment options have emerged in addition to the traditional partnership track. These include payment on a per-diem basis, annual salary, or a mix of both. As opposed to partnership, the employment track avoids responsibility for governance and corporate liability, although not individual liability, and usually does not involve after-hours call. An employed physician usually does not benefit from ancillary income that derives from AEC facility fees, infusion centers, and pathology and anesthesia services.
Private equity ownership of gastroenterology practices
In June 2016, private equity entered the GI space with the investment of the Audax Group in a community GI practice based in Miami, Florida. The term private equity refers to capital that is not reported in public forums and comprises funds that investors directly invest into private companies or use to buy out public companies and turn them private.
According to their website, when the Audax Group invests in a medical practice, they provide capital for substantial infrastructure support, business experience, and acumen, but retain medical practice leaders as their clinical decision makers. They also bring proven expertise and economies of scale to resource-intensive aspects of a medical practice including information technology, regulation compliance, human resources, revenue cycle management, payroll, benefits, rents, and lease as examples. These components can be difficult to manage efficiently within independent medical practices, so many maturing practices are selling their practices to regional health systems. This multistate equity-backed medical practice is an alternative to health system acquisition, and may help physicians feel more in control of their practices and potentially share in the equity investment.
It is important to understand the employment structure and associations of any practice you are contemplating joining. The model devised by this group is meant to retain physician authority and responsibility while providing capital to support innovation and the development of needed infrastructure. Growth of market share and revenues can accrue back to physician owners. This is distinct from practices that are part of a health system in which there may be more of a corporate feeling and centralized governance.
Locum tenens
Locum tenens is a Latin phrase that means “to hold the place of.” According to the website of a large locum tenens company, this practice model originated in the 1970s when the federal government provided a grant to the University of Utah to provide physician services for underserved areas in the Western United States. The program proved so successful that hospital administrators who had difficulty recruiting staff physicians began asking for staffing assistance.
Today, a substantial number of physicians at all stages of their careers are working as locum tenens. They work as independent contractors so that income taxes are not withheld and benefits are the responsibility of the individual. As with the PSA arrangement, a physician would meet with both an accountant and labor lawyer to establish him or herself as a corporate entity for tax advantages and limited liability from litigation.
Early stage physicians who might be following a significant other or spouse to specific locations sometimes consider a locum tenens as a bridge to permanent positions. Late-stage physicians who no longer want to be tied to a small group or solo practice have become locum tenens physicians who enjoy multiple temporary employment positions nationwide. This pathway no longer is unusual and can be a satisfying means to expand employment horizons. As with all employment situations, due diligence is mandatory before signing with any locum tenens company.
Conclusions
The employment spectrum for gastroenterologists and other medical professionals has expanded greatly between the time the senior author and the junior author entered the workforce. Change is now the one constant in medicine, and medicine today largely is fast-paced, corporatized, and highly regulated. Finding an employment model that is comfortable for current physicians, whose life situations are quite diverse, can be challenging. but a variety of opportunities now exist.
Think carefully about what you truly desire as a medical professional and how you might shape your employment to realize your goals. Options are available for those with an open mind and persistence.
References
1. Sheen E, Dorn SD, Brill JV, et al. Health care reform and the road ahead for gastroenterology. Clin Gastroenterol Hepatol. 2012;10:1062-5.
2. Kosinski LR. Meaningful use and electronic medical records for the gastroenterology practice. Clin Gastroenterol Hepatol. 2010;8:494-7.
3. Medical Group Management Association (MGMA). Accessed January 20, 2017.
4. The Coker Group. PSAs as an Alternative to Employment: A Contemporary Option for Alignment and Integration. In: The Coker Group Thought Leadership – White Papers. March 2016.
5. Houston R, McGinnis T. Accountable care organizations: looking back and moving forward. Centers for Health Care Strategies Inc. Brief. January 2016. Accessed January 20, 2017.
6. Pallardy C. 7 gastroenterologists leading GI mega-practices. Becker’s GI and endoscopy 2015. Accessed January 20, 2017.
Dr. Allen is in the division of gastroenterology and hepatology, department of medicine, University of Michigan School of Medicine, Ann Arbor; he is also the Editor in Chief of GI & Hepatology News. Dr. Kaushal is in the division of gastroenterology, Adventist Health Systems, Sonora, Calif. The authors disclose no conflicts.
Primary hPTH often goes unnoticed
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
SAN FRANCISCO – Primary hyperparathyroidism was detected in 7% of 742 patients with recurrent kidney stones at a single tertiary care clinic, and the patients’ primary care physicians may have missed the diagnosis because several affected patients’ calcium levels were in the high normal range.
Of the 53 patients diagnosed with primary hyperparathyroidism (hPTH), 72% had high normal serum calcium levels. After examining the charts of those patients, researchers found that 11 of the 53 patients (21%) had been tested for parathyroid hormone and serum calcium levels and could have been identified by their primary care physicians.
None of the 742 patients with kidney stones in the study had vitamin D deficiency or gastrointestinal malabsorption. All were tested for serum calcium and intact serum PTH, and those with hypercalcemia or high normal calcium (greater than 10 mg/dL) and elevated intact serum PTH were diagnosed with primary hPTH.
The findings emphasize “the importance of [looking] for not just outright primary hyperparathyroidism, but the ratio between PTH and calcium levels,” said Mr. Boyd.
The study received no funding. Mr. Boyd declared no relevant financial relationships.
SOURCE: Boyd C et al. AUA 2018, Abstract MP13-03.
REPORTING FROM THE AUA ANNUAL MEETING
Key clinical point: Calcium levels in the high normal range may be confounding diagnoses.
Major finding: About 20% of primary hyperparathyroidism cases could have been spotted by the primary care physician based on tests that had been ordered.
Study details: A retrospective analysis of 742 patients at a tertiary care kidney stone clinic.
Disclosures: The study received no funding. Mr. Boyd declared no relevant financial relationships.
Source: Boyd C et al. AUA 2018, Abstract MP13-03.
When Would a Metal-Backed Component Become Cost-Effective Over an All-Polyethylene Tibia in Total Knee Arthroplasty?
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.
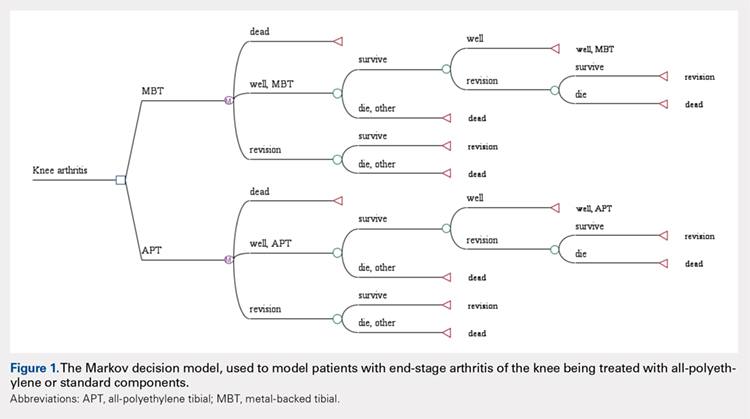
The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
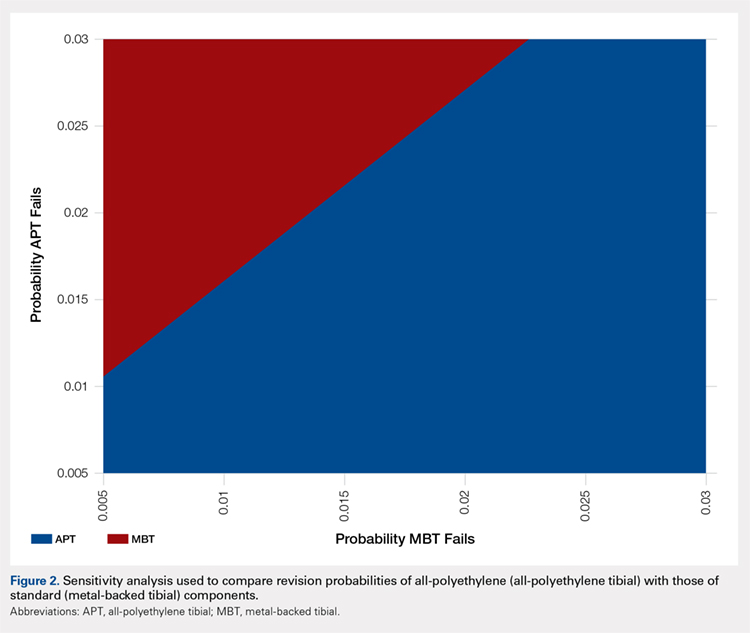
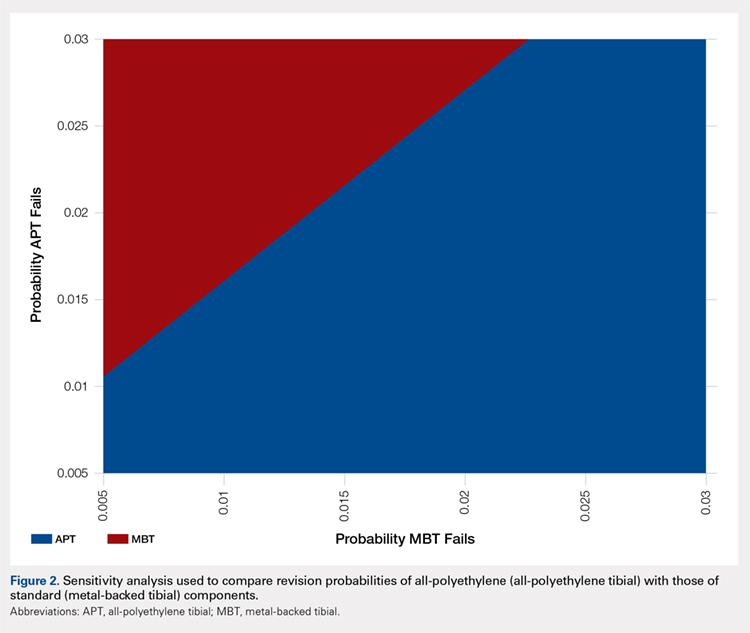
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.
DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
ABSTRACT
The importance of cost control in total knee arthroplasty is increasing in the United States secondary to both changing economic realities of healthcare and the increasing prevalence of joint replacement.
Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. The purpose of this study is to examine the cost-effectiveness of all-polyethylene tibial (APT) components and determine what difference in revision rate would make modular metal-backed tibial (MBT) implants a more cost-effective intervention.
Markov models were constructed using variable implant failure rates and previously published probabilities. Cost data were obtained from both our institution and published United States implant list prices, and modeled with a 3.0% discount rate. The decision tree was continued over a 20-year timeframe.
Using our institutional cost data and model assumptions with a 1.0% annual failure rate for MBT components, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components. A sensitivity analysis was performed with different assumptions for MBT annual failure rates.
Given our assumptions, the APT component is cost-saving if the excess cumulative revision rate increases by <9% in 20 years compared with that of the MBT implant. Surgeons, payers, and hospitals should consider this approach when evaluating implants. Consideration should also be given to the decreased utility associated with revision surgery.
Continue to: All-polythylene tibial implants...
All-polyethylene tibial (APT) implants have been available for use in total knee arthroplasty (TKA) for decades. Except for one particular implant design, APT implants have shown equivalent functional outcome and survivorship to metal-backed tibial (MBT) components.1 Two recent systematic reviews have demonstrated no difference in durability or functional outcome between APT and MBT components.1,2 Despite this data, APT components continue to be used uncommonly in the United States. Improved technical ease and the theoretical advantages of modularity are likely responsible for the continued popularity of MBT implants despite the fact that APT implants cost considerably less than their MBT counterparts.
The importance of cost control in TKA is increasing secondary to changing economic realities of healthcare and increasing prevalence of joint replacement. Payers are seeking ways to ensure quality care at more affordable reimbursement rates. Surgeons play a critical role in cost containment and may soon be incentivized to make cost-effective decisions under proposed gainsharing programs. Implants account for a substantial portion of hospital costs for knee replacement and have been suggested as an essential part of cost control.3 As such, surgeons in the United States will probably need to factor in value when selecting implants and be required to justify the additional cost of “premium” implants.
Given recent systemic reviews concluding both equivalent effectiveness and survivorship, the APT component would appear to be inherently cost-effective when compared with an MBT design. However, the degree to which this implant is cost-effective has been difficult to quantify. The purpose of this study is to take a novel approach to examine the cost-effectiveness of APT components by determining what theoretical difference in revision rate would make modular MBT implants a more cost-effective intervention using our institutional cost data.
MATERIALS AND METHODS
A Markov decision model was used to evaluate the cost-effectiveness of APT components.4 A Markov decision model is a mathematical framework for modeling decision making in situations where outcomes are partly random and partly under the control of a decision maker. They are powerful tools for determining the best solution from all feasible solutions to a given problem. A decision model was constructed (Figure 1) to depict patients with arthritis of the knee being treated with either APT or MBT implants in a fashion similar to previously published models.5 At each point of a patient’s health status in the 20 years following surgery, they are either considered well after total knee replacement, well after revision surgery, or dead. Patients transition through the decision tree and pass through different states according to the probability of each event occurring, a process that is discussed further below. A utility value, measured in quality-adjusted life years (QALYs), and a cost are assigned to every health state and both primary and revision procedures within the model. The model is designed to determine the maximum failure rate for which the APT is the more cost-effective option.

The model probabilities used for survival and mortality following TKA were adapted from those published previously in the literature.5 A utility value was assigned to each health state. The utility after initial surgery was set to 0.83 and utility after revision was set to 0.6.5 These values were obtained from the Swedish Registry and Tufts Cost-Effectiveness Registry, respectively. We also included a disutility of -0.1 for the first year after surgery and -0.2 for the first year after revision, to account for the disutility of undergoing surgery and the post-surgery recovery. Disutilities represent the negative preference patients have for a particular health state or outcome, such as primary or revision knee arthroplasty.5 It is assumed that there is a higher morbidity associated with revision arthroplasty vs primary arthroplasty and, thus, has a higher disutility value assigned to it.
We assumed the age at the initial surgery to be 65 years. Age-specific mortality rates were taken from the 2007 United States Life Tables published by the Centers for Disease Control and Prevention.6 An additional probability of .007 of dying during the surgery or postoperative from the initial surgery and a probability of .011 from the revision was included.
Costs for the surgery were obtained from the University of Virginia’s billing department. We obtained the average cost for the diagnosis-related group in 2012. The cost of primary knee replacement was $17,578.06 with MBT implants. We subtracted institutional cost savings for the APT that could be achieved to obtain a cost of $16,272.10 for the APT. The cost of revision was $21,650.34 and assumed to be the same regardless of the type of initial surgery. A 3% discount rate was used.
The costs, QALYs, and probabilities were then used to compute cost-effectiveness ratios, or the cost per additional QALY, of the 2 options. Unlike previous models published in the orthopedic literature, we assumed a constant probability of revision for the MBT. We initially assumed a 1.0% probability of failure per year for the MBT implant. We then determined what revision rate for the APT would be necessary to be cost equivalent with the MBT. A sensitivity analysis was performed to examine the impact of varying assumptions regarding the rate of revision.
Continue to: Results...
RESULTS
Under our institutional cost data and model assumptions with a 1% annual failure rate for MBT implants, an annual failure rate of 1.6% for APT components would be required to achieve equivalency in cost. Over a 20-year period, a failure rate of >27% for the APT component would be necessary to achieve equivalent cost compared with the proposed failure rate of 18% with MBT components.
A two-way sensitivity analysis for probabilities of failure was performed to compare revision probabilities of the APT with those of MBT components. The preferred strategy graph is included in Figure 2. This graph shows how varying annual revision rates for both the APT and MBT would impact which option would be preferable. For example, on the graph, an annual failure rate of 1.6% for APT implants would be cost equivalent to a 0.1% annual failure rate for MBT implants at 20 years. A 2.0% annual failure rate for the APT would be equivalent to a 1.4% annual failure rate for the MBT, and a 2.5% failure rate for the APT would be equivalent to a 1.8% MBT failure rate. Holding the APT failure rate constant at 2.5%, any MBT failure rate <1.8% would make the MBT the more cost-effective option, whereas a failure rate >1.8% would make the MBT less cost-effective than the APT. For probability combinations that fall in the lower right area of Figure 2, the APT is preferable, and for probability combinations that fall in the upper left area, the MBT is preferable. The line separating the 2 areas is where 1 would be indifferent, such that the cost per additional QALY is the same for both procedures.

DISCUSSION
In light of the current economic climate and push for cost savings in the United States healthcare system, orthopedic surgeons must increasingly understand the realities of cost and the role it plays in the assessment of new technology. This concept is especially true of TKA as it becomes an increasingly common operative intervention. Utilizing cost savings techniques while ensuring quality outcomes is something that needs to be championed by healthcare providers.
Ideally, the introduction of a new medical technology that is more expensive than preexisting technology should lead to improved outcomes. Multiple randomized radiostereometric and clinical outcome studies looking at failure rates of APT compared with MBT have consistently suggested equivalence or superiority of the APT design when modern round-on-round implant designs are utilized.7-17 Two recent systematic reviews demonstrated that APT components were equivalent to MBT components regarding both revision rates and clinical scores.1,18 Given these results, it seems that the increased use of the APT design could save the healthcare system substantial amounts of money without compromising outcomes. For example, in 2006 Muller and colleagues19. proposed a possible cost savings of approximately 39 million dollars per year across England and Wales, if just 50% of the 70,000 TKAs performed annually used APTs. Our study, which helps quantify the potential cost-effectiveness of the APT design in terms of revision rates, should help further support this debate and provide a framework for the evaluation of new technology.
It should be noted that the results of this current study are based on both assumptions and generalizations. Institutional cost data is known to vary widely among institutions and our conclusions regarding comparable revision rates would change with different cost inputs. We are also unable to take into account individual patients, surgeons, or specific implant factors. It is very difficult to place a price on quality-adjusted life years and negative repercussions with revision surgery. Furthermore, speaking specifically about surgical technique, each surgeon has his/her own preference when performing TKA. There is a lack of intraoperative flexibility when using monoblock tibial components that many surgeons may find undesirable. A surgeon is unable to adjust the thickness of the polyethylene insert after cementation of metal implants. Finally, we are aware that cost-effectiveness analyses cannot take the place of rational clinical decision making when evaluating an individual patient for TKA. Patient age, body mass index, and deformity are all factors that may dictate the use of MBTs in an attempt to improve outcomes.
The results of this analysis help quantify the cost-effectiveness of the APT. Given the additional cost, the MBT design would have to lower revision rates substantially when compared with the APT design to be considered cost-effective. Multiple clinical studies have not shown this to be the case. Further studies are required to help guide clinical decision making and define the role of APT components in TKA.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
- Voigt J, Mosier M. Cemented all-polyethylene and metal-backed polyethylene tibial components used for primary total knee arthroplasty: a systematic review of the literature and meta-analysis of randomized controlled trials involving 1798 primary total knee implants. J Bone Joint Surg Am. 2011;93(19):1790-1798. doi:10.2106/JBJS.J.01303.
- Klaas AN, Wiebe CV, Bart GP, Jan WS, Rob GHHN. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Healy WL, Iorio R. Implant selection and cost for total joint arthroplasty: conflict between surgeons and hospitals. Clin Orthop Relat Res. 2007;457:57-63. doi:10.1097/BLO.0b013e31803372e0.
- Hunink MGM, Glasziou PP, Siegel JE, et al. Decision Making in Health and Medicine. Cambridge, UK: Cambridge University Press; 2001.
- Slover JD. Cost effectiveness analysis of custom TK cutting blocks. J Arthroplasty. 2012;27(2):180-185. doi:10.1016/j.arth.2011.04.023.
- Revised United States life tables, 2001-2011. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Accessed January 22, 2013.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. Low-conforming all-polyethylene tibial component not inferior to metal-backed component in cemented total knee arthroplasty: Prospective, randomized radiostereometric analysis study of the AGC total knee prosthesis. J Arthroplasty. 2000;15(6):783-792.
- Adalberth G, Nilsson KG, Byström S, Kolstad K, Milbrink J. All-polyethylene versus metal-backed and stemmed tibial components in cemented total knee arthroplasty: A prospective, randomized RSA study. J Bone Joint Surg Br. 2001;83(6):825-831. doi:10.1302/0301-620X.83B6.0830825
- Gioe TJ, Bowman KR. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin Orthop Relat Res. 2000;380:108-115.
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All-polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 2: completely cemented components. MB not superior to AP components. Acta Orthop. 2005;76(6):778-784. doi:10.1080/17453670510045363
- Hyldahl H, Regnér L, Carlsson L, Kärrholm J, Weidenhielm L. All polyethylene vs. metal-backed tibial component in total knee arthroplasty: a randomized RSA study comparing early fixation of horizontally and completely cemented tibial components. Part 1: horizontally cemented components. AP better fixated than MB. Acta Orthop. 2005;76(6):769-777.
- Norgren B, Dalén T, Nilsson KG. All poly tibial component better than metal backed: a randomized RSA study. Knee. 2004;11(3):189-196. doi:10.1016/S0968-0160(03)00071-1
- Rodriguez JA, Baez N, Rasquinha V, Ranawat CS. Metal-backed and all-polyethylene tibial components in total knee replacement. Clin Orthop Relat Res. 2001;392:174-183. doi:10.1097/00003086-200111000-00021.
- Gioe TJ, Sinner P, Mehle S, Ma W, Killeen KK. Excellent survival of all polyethylene tibial components in a community joint registry. Clin Orthop Relat Res. 2007;464:88-92. doi:10.1097/BLO.0b013e31812f7879.
- Gioe TJ, Stroemer ES, Santos ER. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level I [corrected] evidence study. Clin Orthop Relat Res. 2007;455:212-218. doi:10.1097/01.blo.0000238863.69486.97.
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ER, Singh J. Mobile and fixed bearing (all-polyethylene tibial component) total knee arthroplasty designs: a prospective randomized trial. J Bone Joint Surg Am. 2009;91(9):2104-2112. doi:10.2106/JBJS.H.01442.
- Bettinson KA, Pinder IM, Moran CG, Weir DJ, Lingard EA. All-polyethylene compared with metal-backed tibial components in total knee arthroplasty at ten years: A prospective, randomized controlled trial. J Bone Joint Surg Am. 2009;91(7):1587-1594. doi:10.2106/JBJS.G.01427.
- Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470(12):3549-3559. doi:10.1007/s11999-012-2582-2.
- Muller SD, Deehan DJ, Holland JP, et al. Should we reconsider all-polyethylene tibial implants in total knee replacement? J Bone Joint Surg Br. 2006;88(12):1596-1602. doi:10.1302/0301-620X.88B12.17695.
TAKE-HOME POINTS
- APT components have been shown to be cost-effective when compared to MBT designs in TKA.
- Revision rates would have to be substantially lower in MBT to afford a cost advantage over APT components.
- Given that only a small percentage of surgeons routinely use APT components, factors other than cost-effectiveness must influence the choice of implant.
- Surgeons may find that APT components are more technically demanding to use and they do not allow for modular stems or augmentations.
- Institutional cost data is known to vary widely among institutions, and our conclusions regarding comparable revision rates would change with different cost inputs.
Single Botox treatment cuts AF for 3 years
BOSTON – A single set of four injections with botulinum toxin into neuron-containing cardiac fat pads of patients during open-chest cardiac artery bypass surgery led to a long-term cut in the cumulative incidence of atrial tachyarrhythmias during 3-year follow-up in a pilot, sham-controlled study with 60 patients at two Russian centers.
“Because the favorable reduction of atrial fibrillation [AF] outlasted the anticipated botulinum toxin effects on autonomic nervous system activity, this may represent a form of autonomic reverse remodeling” triggered by just one injection of the paralyzing toxin at each of four intracardiac fat pads, Alexander B. Romanov, MD, said at the annual scientific sessions of the Heart Rhythm Society. Botulinum toxin (BT) blocks neuronal release of acetylcholine, thereby interfering with cholinergic neurotransmission and producing hypothesized neurologic remodeling, explained Dr. Romanov, a researcher at the Meshalkin National Medical Research Center in Novosibirsk, Russia.
The 3-year results also showed statistically significant differences or trends favoring BT injections for several other clinical outcomes. Two deaths and two strokes occurred, all among the control patients. Two patients required a total of three hospitalizations during follow-up in the BT-treated group, compared with 10 patients hospitalized a total of 21 times in the control arm. Clinicians prescribed antiarrhythmic drugs to six of the BT-treated patients and to 15 of the controls.
All patients received an implanted heart rhythm monitor during their bypass surgery, and the researchers measured AF burden – the percentage of time during which AF occurred. After 12 months, 24 months, and 36 months, the AF burden averaged 0.2%, 1.6%, and 1.2%, respectively, in the BT-treated patients and 1.9%, 9.5%, and 6.9% in the sham-control patients.

“We don’t know why this works, but it’s a fascinating new approach that is worthy of further study,” commented Kalyanam Shivkumar, MD, professor and director of the Cardiac Arrhythmia Center at the University of California, Los Angeles, and designated discussant for the report.
“This is an extremely exciting study, but it remains inconclusive because how it works is not fully understood,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver.
BOSTON – A single set of four injections with botulinum toxin into neuron-containing cardiac fat pads of patients during open-chest cardiac artery bypass surgery led to a long-term cut in the cumulative incidence of atrial tachyarrhythmias during 3-year follow-up in a pilot, sham-controlled study with 60 patients at two Russian centers.
“Because the favorable reduction of atrial fibrillation [AF] outlasted the anticipated botulinum toxin effects on autonomic nervous system activity, this may represent a form of autonomic reverse remodeling” triggered by just one injection of the paralyzing toxin at each of four intracardiac fat pads, Alexander B. Romanov, MD, said at the annual scientific sessions of the Heart Rhythm Society. Botulinum toxin (BT) blocks neuronal release of acetylcholine, thereby interfering with cholinergic neurotransmission and producing hypothesized neurologic remodeling, explained Dr. Romanov, a researcher at the Meshalkin National Medical Research Center in Novosibirsk, Russia.
The 3-year results also showed statistically significant differences or trends favoring BT injections for several other clinical outcomes. Two deaths and two strokes occurred, all among the control patients. Two patients required a total of three hospitalizations during follow-up in the BT-treated group, compared with 10 patients hospitalized a total of 21 times in the control arm. Clinicians prescribed antiarrhythmic drugs to six of the BT-treated patients and to 15 of the controls.
All patients received an implanted heart rhythm monitor during their bypass surgery, and the researchers measured AF burden – the percentage of time during which AF occurred. After 12 months, 24 months, and 36 months, the AF burden averaged 0.2%, 1.6%, and 1.2%, respectively, in the BT-treated patients and 1.9%, 9.5%, and 6.9% in the sham-control patients.

“We don’t know why this works, but it’s a fascinating new approach that is worthy of further study,” commented Kalyanam Shivkumar, MD, professor and director of the Cardiac Arrhythmia Center at the University of California, Los Angeles, and designated discussant for the report.
“This is an extremely exciting study, but it remains inconclusive because how it works is not fully understood,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver.
BOSTON – A single set of four injections with botulinum toxin into neuron-containing cardiac fat pads of patients during open-chest cardiac artery bypass surgery led to a long-term cut in the cumulative incidence of atrial tachyarrhythmias during 3-year follow-up in a pilot, sham-controlled study with 60 patients at two Russian centers.
“Because the favorable reduction of atrial fibrillation [AF] outlasted the anticipated botulinum toxin effects on autonomic nervous system activity, this may represent a form of autonomic reverse remodeling” triggered by just one injection of the paralyzing toxin at each of four intracardiac fat pads, Alexander B. Romanov, MD, said at the annual scientific sessions of the Heart Rhythm Society. Botulinum toxin (BT) blocks neuronal release of acetylcholine, thereby interfering with cholinergic neurotransmission and producing hypothesized neurologic remodeling, explained Dr. Romanov, a researcher at the Meshalkin National Medical Research Center in Novosibirsk, Russia.
The 3-year results also showed statistically significant differences or trends favoring BT injections for several other clinical outcomes. Two deaths and two strokes occurred, all among the control patients. Two patients required a total of three hospitalizations during follow-up in the BT-treated group, compared with 10 patients hospitalized a total of 21 times in the control arm. Clinicians prescribed antiarrhythmic drugs to six of the BT-treated patients and to 15 of the controls.
All patients received an implanted heart rhythm monitor during their bypass surgery, and the researchers measured AF burden – the percentage of time during which AF occurred. After 12 months, 24 months, and 36 months, the AF burden averaged 0.2%, 1.6%, and 1.2%, respectively, in the BT-treated patients and 1.9%, 9.5%, and 6.9% in the sham-control patients.

“We don’t know why this works, but it’s a fascinating new approach that is worthy of further study,” commented Kalyanam Shivkumar, MD, professor and director of the Cardiac Arrhythmia Center at the University of California, Los Angeles, and designated discussant for the report.
“This is an extremely exciting study, but it remains inconclusive because how it works is not fully understood,” commented Andrew D. Krahn, MD, professor and chief of cardiology at the University of British Columbia in Vancouver.
REPORTING FROM HEART RHYTHM 2018
Key clinical point:
Major finding: During 3-year follow-up, atrial tachyarrhythmias occurred in 23% of botulinum toxin-treated patients and in 50% of sham controls.
Study details: Randomized, sham-controlled study with 60 patients at two Russian centers.
Disclosures: The study received no commercial funding. Dr. Romanov, Dr. Shivkumar, and Dr. Krahn had no relevant disclosures.
Source: Romanov A et al. Heart Rhythm 2018, Abstract B-LBCT02-01.
Vaccine-related febrile seizures have zero developmental impact
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
MALMO, SWEDEN – Children who experience a febrile seizure in conjunction with a vaccination have developmental outcomes comparable with those of children who have non–vaccine-related febrile seizures and healthy controls who’ve never had a febrile seizure, according to the first prospective case-control cohort study to examine the issue.
This finding has important implications for clinical practice, Lucy Deng, MD, observed at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Febrile seizures associated with a vaccine can decrease parent and provider confidence in vaccine safety,” the pediatrician noted. Based upon her study results, however, physicians now can offer a truly evidence-based message of reassurance.
“If you have a child with a vaccine-related febrile seizure, you can give the same advice to those parents as for anyone else who’s had a febrile seizure, in that there is no difference in the clinical outcomes of vaccine-proximate and non–vaccine-proximate febrile seizures. Vaccine-proximate febrile seizures are usually brief, they don’t require any antiepileptic drugs, their length of stay is usually less than a day, and developmentally at 12-24 months post initial febrile seizure, they’re exactly the same as children who’ve never had a seizure before or who’ve had a non-vaccine-related febrile seizure,” said Dr. Deng of the National Centre for Immunisation Research and Surveillance in Sydney.
The impetus for her study was straightforward: “We all know that most children with a history of febrile seizures have normal behavior, intelligence, and academic achievement and do not later develop epilepsy. What we didn’t know before is if all of these facts apply to vaccine-proximate febrile seizures,” she explained.
The clinical severity analysis portion of this prospective case-control cohort study included 1,085 children with febrile seizures seen at five Australian children’s hospitals. Sixty-eight of them had vaccine-proximate febrile seizures, for a 6.6% rate. The febrile seizures in the other 1,027 children didn’t occur within 2 weeks following a vaccination.
Measles vaccine was implicated in 56 of the 68 children with vaccine-proximate febrile seizures, or 82%. Because Australian children receive their first measles-containing vaccine at age 12 months, the average age of the cohort with vaccine-proximate febrile seizures was 13 months, significantly younger than the 20-month average for children with non–vaccine-related febrile seizures.
In a multivariate analysis adjusted for patient age, gender, and history of prior afebrile seizures, the groups with vaccine-proximate and vaccine-unrelated febrile seizures didn’t differ significantly in terms of the proportion with a hospital length of stay greater than 1 day (20% vs. 15%), ICU admission (1.5% vs. 2.3%), seizure duration of more than 15 minutes (16% vs. 12%), repeat seizures within 24 hours (9% vs. 10%), or discharge on antiepileptic medication (4.4% vs. 4.3%).
In the developmental outcomes analysis, 62 of the children with vaccine-proximate febrile seizures, 70 with vaccine-unrelated febrile seizures, and 85 healthy controls with no seizure history underwent formal assessment using the third edition of the Bayley Scales of Infant and Toddler Development 12-24 months after their initial febrile seizure. Scores adjusted for years of maternal education were closely similar in all three groups across all five test domains: cognitive, language, motor, social-emotional, and general-adaptive.
Dr. Deng reported having no financial conflicts of interest regarding the study, which was partially funded by the Australian National Centre for Immunisation Research and Surveillance.
REPORTING FROM ESPID 2018
Key clinical point: Parents now can confidently be reassured that vaccine-proximate febrile seizures have no long-term consequences.
Major finding: as in controls with no seizure history.
Study details: This prospective case-control study comprised 1,180 children at five Australian children’s hospitals.
Disclosures: The study was partially funded by the Australian National Centre for Immunisation Research and Surveillance. The presenter reported having no financial conflicts.
Reoperation Rates After Cartilage Restoration Procedures in the Knee: Analysis of a Large US Commercial Database
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).
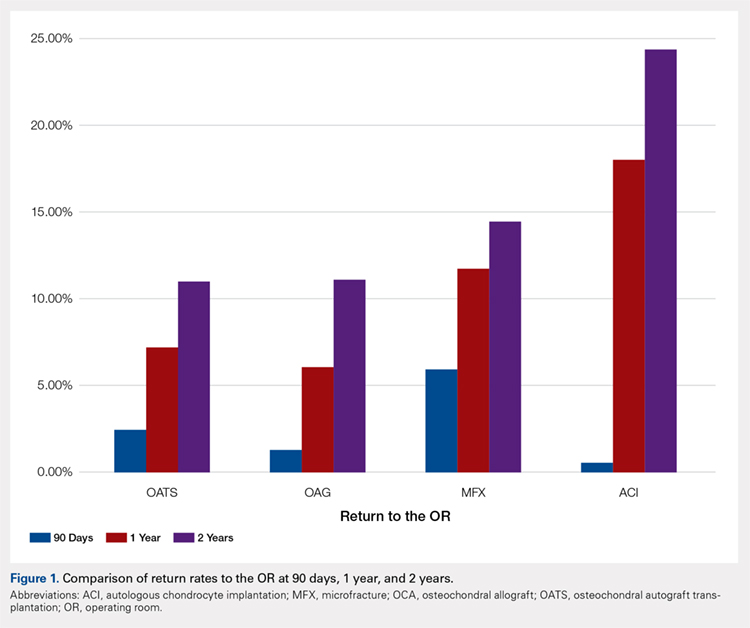
Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
TAKE-HOME POINTS
- With a large US commercial insurance database analyzing techniques for cartilage restoration, reparative procedures were favored for chondral injuries compared to restorative approaches.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 90-day reoperation rate is 6%.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 2-year reoperation rate is 15%.
- Patients undergoing autologous chondrocyte implantation are more likely to experience reoperation at 90 days, 1 year, and 2 years compared to other cartilage restoration techniques including microfracture, osteochondral autograft transfer, and osteochondral allograft transplantation.
- Patients undergoing microfracture are more likely to experience an ultimate conversion to arthroplasty compared to other cartilage restoration techniques including autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation.
Supreme Court case NIFLA v Becerra: What you need to know
On March 20, 2018, the United States Supreme Court heard arguments in National Institute of Family and Life Advocates (NIFLA) v Becerra. The Court is expected to issue its decision in June and the results could shape legislation around the country. Here is what you need to know.
The background
There are more than 4,000 Crisis Pregnancy Centers (CPCs) around the country, vastly out numbering abortion clinics.1 The services offered and the make-up of the staff who work in CPCs can vary. CPCs can be licensed to provide medical services, including urine pregnancy tests and ultrasounds, and may have clinicians on staff. Alternatively, other CPCs may be volunteer-run and provide counseling as well as supplies for women, including diapers and baby formula. Within CPCs, however, women are often given misleading and medically inaccurate information about abortion and contraception and are not provided with appropriate or timely referrals if they seek abortion care.
To ensure women have access to comprehensive reproductive health services, California passed the Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act in 2015. This act requires licensed clinics — which may include some CPCs — to notify patients that they may access state-funded prenatal care, family planning, and abortion services through a county health department phone number. Additionally, facilities that provide pregnancy testing and ultrasounds are required to notify clients if they do not employ a licensed medical professional.
In response, NIFLA sued the state of California, alleging that the law violated their freedom of speech by forcing them to communicate about abortion with women who visited their centers.
The case
NIFLA argues that California is violating CPCs’ freedom of speech by requiring them to post statements about medications and medical procedures they strongly oppose. According to NIFLA, if California wants to promote state-funded options, they should publicize that information and not require the CPCs to post it.
The State of California enacted the law to ensure that California women have timely access to all available health care services, including contraception and abortion, and are made aware that the clinic they visit does not offer licensed medical care. Women may not know of their publicly funded options and, without this law, CPCs could withhold that information or provide misleading information, delaying or preventing women from accessing care.
Possible outcomes
If the Supreme Court strikes down California’s FACT Act as a violation of the First Amendment, CPCs in that state would not be required to provide information about free or low-cost prenatal care, contraception, and abortion services or post, if appropriate, that they were an unlicensed facility. However, such a ruling could call into question laws in 18 other states that require doctors to give women false information about possible side effects and complications of abortion during the consent process. This case could provide precedent for physicians to assert that such requirements violate their freedom of speech.
If the Supreme Court upholds California’s FACT Act, this would likely lead to similar laws around the country requiring CPCs to disclose the availability of affordable contraception and abortion services in their state and the lack of licensed medical providers.
For more information, check out https://www.supremecourt.gov/
Acknowledgement
Special thanks to Sara Needleman Kline, Esq, Chief Legal Officer, American College of Obstetricians and Gynecologists, for aid with this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dias E. The Abortion Battleground: Crisis Pregnancy Centers. Time Magazine. http://content.time.com/time/nation/article/0,8599,2008846,00.html. Published August 5, 2010. Accessed May 16, 2018.
On March 20, 2018, the United States Supreme Court heard arguments in National Institute of Family and Life Advocates (NIFLA) v Becerra. The Court is expected to issue its decision in June and the results could shape legislation around the country. Here is what you need to know.
The background
There are more than 4,000 Crisis Pregnancy Centers (CPCs) around the country, vastly out numbering abortion clinics.1 The services offered and the make-up of the staff who work in CPCs can vary. CPCs can be licensed to provide medical services, including urine pregnancy tests and ultrasounds, and may have clinicians on staff. Alternatively, other CPCs may be volunteer-run and provide counseling as well as supplies for women, including diapers and baby formula. Within CPCs, however, women are often given misleading and medically inaccurate information about abortion and contraception and are not provided with appropriate or timely referrals if they seek abortion care.
To ensure women have access to comprehensive reproductive health services, California passed the Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act in 2015. This act requires licensed clinics — which may include some CPCs — to notify patients that they may access state-funded prenatal care, family planning, and abortion services through a county health department phone number. Additionally, facilities that provide pregnancy testing and ultrasounds are required to notify clients if they do not employ a licensed medical professional.
In response, NIFLA sued the state of California, alleging that the law violated their freedom of speech by forcing them to communicate about abortion with women who visited their centers.
The case
NIFLA argues that California is violating CPCs’ freedom of speech by requiring them to post statements about medications and medical procedures they strongly oppose. According to NIFLA, if California wants to promote state-funded options, they should publicize that information and not require the CPCs to post it.
The State of California enacted the law to ensure that California women have timely access to all available health care services, including contraception and abortion, and are made aware that the clinic they visit does not offer licensed medical care. Women may not know of their publicly funded options and, without this law, CPCs could withhold that information or provide misleading information, delaying or preventing women from accessing care.
Possible outcomes
If the Supreme Court strikes down California’s FACT Act as a violation of the First Amendment, CPCs in that state would not be required to provide information about free or low-cost prenatal care, contraception, and abortion services or post, if appropriate, that they were an unlicensed facility. However, such a ruling could call into question laws in 18 other states that require doctors to give women false information about possible side effects and complications of abortion during the consent process. This case could provide precedent for physicians to assert that such requirements violate their freedom of speech.
If the Supreme Court upholds California’s FACT Act, this would likely lead to similar laws around the country requiring CPCs to disclose the availability of affordable contraception and abortion services in their state and the lack of licensed medical providers.
For more information, check out https://www.supremecourt.gov/
Acknowledgement
Special thanks to Sara Needleman Kline, Esq, Chief Legal Officer, American College of Obstetricians and Gynecologists, for aid with this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
On March 20, 2018, the United States Supreme Court heard arguments in National Institute of Family and Life Advocates (NIFLA) v Becerra. The Court is expected to issue its decision in June and the results could shape legislation around the country. Here is what you need to know.
The background
There are more than 4,000 Crisis Pregnancy Centers (CPCs) around the country, vastly out numbering abortion clinics.1 The services offered and the make-up of the staff who work in CPCs can vary. CPCs can be licensed to provide medical services, including urine pregnancy tests and ultrasounds, and may have clinicians on staff. Alternatively, other CPCs may be volunteer-run and provide counseling as well as supplies for women, including diapers and baby formula. Within CPCs, however, women are often given misleading and medically inaccurate information about abortion and contraception and are not provided with appropriate or timely referrals if they seek abortion care.
To ensure women have access to comprehensive reproductive health services, California passed the Reproductive Freedom, Accountability, Comprehensive Care, and Transparency (FACT) Act in 2015. This act requires licensed clinics — which may include some CPCs — to notify patients that they may access state-funded prenatal care, family planning, and abortion services through a county health department phone number. Additionally, facilities that provide pregnancy testing and ultrasounds are required to notify clients if they do not employ a licensed medical professional.
In response, NIFLA sued the state of California, alleging that the law violated their freedom of speech by forcing them to communicate about abortion with women who visited their centers.
The case
NIFLA argues that California is violating CPCs’ freedom of speech by requiring them to post statements about medications and medical procedures they strongly oppose. According to NIFLA, if California wants to promote state-funded options, they should publicize that information and not require the CPCs to post it.
The State of California enacted the law to ensure that California women have timely access to all available health care services, including contraception and abortion, and are made aware that the clinic they visit does not offer licensed medical care. Women may not know of their publicly funded options and, without this law, CPCs could withhold that information or provide misleading information, delaying or preventing women from accessing care.
Possible outcomes
If the Supreme Court strikes down California’s FACT Act as a violation of the First Amendment, CPCs in that state would not be required to provide information about free or low-cost prenatal care, contraception, and abortion services or post, if appropriate, that they were an unlicensed facility. However, such a ruling could call into question laws in 18 other states that require doctors to give women false information about possible side effects and complications of abortion during the consent process. This case could provide precedent for physicians to assert that such requirements violate their freedom of speech.
If the Supreme Court upholds California’s FACT Act, this would likely lead to similar laws around the country requiring CPCs to disclose the availability of affordable contraception and abortion services in their state and the lack of licensed medical providers.
For more information, check out https://www.supremecourt.gov/
Acknowledgement
Special thanks to Sara Needleman Kline, Esq, Chief Legal Officer, American College of Obstetricians and Gynecologists, for aid with this article.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Dias E. The Abortion Battleground: Crisis Pregnancy Centers. Time Magazine. http://content.time.com/time/nation/article/0,8599,2008846,00.html. Published August 5, 2010. Accessed May 16, 2018.
- Dias E. The Abortion Battleground: Crisis Pregnancy Centers. Time Magazine. http://content.time.com/time/nation/article/0,8599,2008846,00.html. Published August 5, 2010. Accessed May 16, 2018.
ACS: Start colorectal cancer screening at age 45
The American Cancer Society recommends all U.S. adults at average risk of colorectal cancer (CRC) undergo screening starting at age 45 years.
That update to ACS recommendations is based on an increasing burden of CRC in younger individuals, microsimulation modeling results, and a “reasonable expectation” that screening tests will perform as well in adults aged 45-49 years as they do in older adults, members of the ACS Guideline Development Group said in the guideline, which was published in CA: A Cancer Journal for Clinicians.
Starting screening at age 45 contrasts with recommendations from the U.S. Preventive Services Task Force (USPSTF), which in 2016 gave an “A” recommendation for CRC screening from 50 to 75 years of age. At the time, the USPSTF noted a modest increase in life-years gained by starting earlier, based on microsimulation modeling. But it concluded that available evidence best supported starting at age 50.
The updated ACS guidelines are based in part on a modeling study that the authors say extends the previous analysis conducted for the USPSTF.
“The recommendation places a high value on the potential years of life saved, addresses anticipated rising incidence going forward, and is expected to contribute to the reduction in disparities in incidence before age 50 years in some racial groups,” the ACS guideline authors added.
The recommendation to start screening at age 45 is a “qualified” recommendation, the authors said, given the limitations of the current evidence base. Most studies to date have been focused on older individuals, in keeping with long-standing recommendations to start screening at age 50.
The move downward in screening age recommendation acknowledges one of the most “significant and disturbing” developments in CRC, the guideline’s authors said: the marked increase in incidence among younger individuals.
While CRC incidence and mortality have been declining in adults aged 55 years and older, recent studies cited in the ACS guideline document show a 51% increase in incidence from 1994 to 2014 – and an 11% increase in mortality from 2005 to 2015 – for adults younger than 55 years.
The current age-specific incidence rate for adults 45-49 years is 31.4 per 100,000, compared with 58.4 per 100,000 in adults 50-54 years. However, the ACS guideline authors said the higher rate in the older cohort is partly influenced by more frequent screening. “The true underlying risk in adults aged 45-49 years is likely closer to the risk in adults aged 50-54 years than the most recent age-specific rates would suggest,” they wrote.
Since patients in this age range have not been routinely screened before, the ACS recommendation is based on modeling. Now we need to analyze the outcomes of early screening to identify which patients will benefit most. Choices for screening include either a structural examination or a high-sensitivity stool-based test, according to the guideline, which doesn’t state a preference for any particular test.
The AGA, in their statement in response, noted that with CRC rates rising in people younger than age 50, it is appropriate to consider beginning routine screening at age 45. The statement continues “Since patients in this age range have not been previously routinely screened, the ACS recommendation is based on modeling. Now we need to analyze the outcomes of early screening to identify which patients will benefit most.”
In addition to Dr. Wolf, members of the ACS Guideline Development Group received no compensation.
SOURCE: Wolf AMD et al. CA Cancer J Clin. 2018 May 30. doi: 10.3322/caac.21457.
The latest recommendations from the American Cancer Society added individuals 45 years and older to the population for whom CRC screeningshould be performed. The change from a start age of 50 was prompted by the increase in CRC reported in younger adults and was based on a computer simulation that predicted a greater number of life-years saved using an earlier age for initiation of screening among adults at average risk for development of colorectal cancer. It is likely that screening will reduce cancer mortality even in this younger age group; however, several issues should be considered when implementing this policy.
Differences in screening tests: The reason for the increase in CRC in younger adults is not known. Nor is it understood why this increase is far greater for rectal cancer than cancers more proximal in the colon. Based on this observation, however, it is possible that flexible sigmoidoscopy may be a more appropriate test than colonoscopy for younger adults. Conversely, we do not know if the precursor of early-age CRC is more likely to be a flat lesion that is more difficult to detect using endoscopy, or less likely to bleed that may make FIT less able to detect, or have a genetic mechanism different from proximal CRC that is not part of the current DNA stool testing.
Value is the benefit gained with screening compared with the resources required to implement screening. The value of screening is greater in older individuals than in younger individuals because the risk of CRC is increased and for this reason, population-based screening should focus on screening older adults who have not undergone screening. Unfortunately, U.S. population adherence to CRC screening remains below 70% with little improvement since 2010. Only after the older population is fully screened should our attention shift to younger populations.
Disparities: The individuals most likely to undergo screening are unlikely to be the individuals most likely to benefit. African Americans have a higher age-related incidence of CRC but have the lowest screening rates in the U.S. compared with other racial and ethnic groups. This relates to not only reduced access but also reduced utilization. It is a concern that, by increasing the pool of individuals recommended for screening, we may also reduce access to those who may benefit most.
The ACS recommendations to go low may reduce colorectal cancer mortality in younger adults; however, our lack of understanding about the biology of the cancer hampers our ability to recommend the optimal screening strategy, sacrifices value, and may increase disparities in cancer outcomes.
John M. Inadomi, MD, AGAF, is a Cyrus E. Rubin Professor of Medicine and head of the division of gastroenterology at the University of Washington School of Medicine, Seattle. He has no conflicts.
The latest recommendations from the American Cancer Society added individuals 45 years and older to the population for whom CRC screeningshould be performed. The change from a start age of 50 was prompted by the increase in CRC reported in younger adults and was based on a computer simulation that predicted a greater number of life-years saved using an earlier age for initiation of screening among adults at average risk for development of colorectal cancer. It is likely that screening will reduce cancer mortality even in this younger age group; however, several issues should be considered when implementing this policy.
Differences in screening tests: The reason for the increase in CRC in younger adults is not known. Nor is it understood why this increase is far greater for rectal cancer than cancers more proximal in the colon. Based on this observation, however, it is possible that flexible sigmoidoscopy may be a more appropriate test than colonoscopy for younger adults. Conversely, we do not know if the precursor of early-age CRC is more likely to be a flat lesion that is more difficult to detect using endoscopy, or less likely to bleed that may make FIT less able to detect, or have a genetic mechanism different from proximal CRC that is not part of the current DNA stool testing.
Value is the benefit gained with screening compared with the resources required to implement screening. The value of screening is greater in older individuals than in younger individuals because the risk of CRC is increased and for this reason, population-based screening should focus on screening older adults who have not undergone screening. Unfortunately, U.S. population adherence to CRC screening remains below 70% with little improvement since 2010. Only after the older population is fully screened should our attention shift to younger populations.
Disparities: The individuals most likely to undergo screening are unlikely to be the individuals most likely to benefit. African Americans have a higher age-related incidence of CRC but have the lowest screening rates in the U.S. compared with other racial and ethnic groups. This relates to not only reduced access but also reduced utilization. It is a concern that, by increasing the pool of individuals recommended for screening, we may also reduce access to those who may benefit most.
The ACS recommendations to go low may reduce colorectal cancer mortality in younger adults; however, our lack of understanding about the biology of the cancer hampers our ability to recommend the optimal screening strategy, sacrifices value, and may increase disparities in cancer outcomes.
John M. Inadomi, MD, AGAF, is a Cyrus E. Rubin Professor of Medicine and head of the division of gastroenterology at the University of Washington School of Medicine, Seattle. He has no conflicts.
The latest recommendations from the American Cancer Society added individuals 45 years and older to the population for whom CRC screeningshould be performed. The change from a start age of 50 was prompted by the increase in CRC reported in younger adults and was based on a computer simulation that predicted a greater number of life-years saved using an earlier age for initiation of screening among adults at average risk for development of colorectal cancer. It is likely that screening will reduce cancer mortality even in this younger age group; however, several issues should be considered when implementing this policy.
Differences in screening tests: The reason for the increase in CRC in younger adults is not known. Nor is it understood why this increase is far greater for rectal cancer than cancers more proximal in the colon. Based on this observation, however, it is possible that flexible sigmoidoscopy may be a more appropriate test than colonoscopy for younger adults. Conversely, we do not know if the precursor of early-age CRC is more likely to be a flat lesion that is more difficult to detect using endoscopy, or less likely to bleed that may make FIT less able to detect, or have a genetic mechanism different from proximal CRC that is not part of the current DNA stool testing.
Value is the benefit gained with screening compared with the resources required to implement screening. The value of screening is greater in older individuals than in younger individuals because the risk of CRC is increased and for this reason, population-based screening should focus on screening older adults who have not undergone screening. Unfortunately, U.S. population adherence to CRC screening remains below 70% with little improvement since 2010. Only after the older population is fully screened should our attention shift to younger populations.
Disparities: The individuals most likely to undergo screening are unlikely to be the individuals most likely to benefit. African Americans have a higher age-related incidence of CRC but have the lowest screening rates in the U.S. compared with other racial and ethnic groups. This relates to not only reduced access but also reduced utilization. It is a concern that, by increasing the pool of individuals recommended for screening, we may also reduce access to those who may benefit most.
The ACS recommendations to go low may reduce colorectal cancer mortality in younger adults; however, our lack of understanding about the biology of the cancer hampers our ability to recommend the optimal screening strategy, sacrifices value, and may increase disparities in cancer outcomes.
John M. Inadomi, MD, AGAF, is a Cyrus E. Rubin Professor of Medicine and head of the division of gastroenterology at the University of Washington School of Medicine, Seattle. He has no conflicts.
The American Cancer Society recommends all U.S. adults at average risk of colorectal cancer (CRC) undergo screening starting at age 45 years.
That update to ACS recommendations is based on an increasing burden of CRC in younger individuals, microsimulation modeling results, and a “reasonable expectation” that screening tests will perform as well in adults aged 45-49 years as they do in older adults, members of the ACS Guideline Development Group said in the guideline, which was published in CA: A Cancer Journal for Clinicians.
Starting screening at age 45 contrasts with recommendations from the U.S. Preventive Services Task Force (USPSTF), which in 2016 gave an “A” recommendation for CRC screening from 50 to 75 years of age. At the time, the USPSTF noted a modest increase in life-years gained by starting earlier, based on microsimulation modeling. But it concluded that available evidence best supported starting at age 50.
The updated ACS guidelines are based in part on a modeling study that the authors say extends the previous analysis conducted for the USPSTF.
“The recommendation places a high value on the potential years of life saved, addresses anticipated rising incidence going forward, and is expected to contribute to the reduction in disparities in incidence before age 50 years in some racial groups,” the ACS guideline authors added.
The recommendation to start screening at age 45 is a “qualified” recommendation, the authors said, given the limitations of the current evidence base. Most studies to date have been focused on older individuals, in keeping with long-standing recommendations to start screening at age 50.
The move downward in screening age recommendation acknowledges one of the most “significant and disturbing” developments in CRC, the guideline’s authors said: the marked increase in incidence among younger individuals.
While CRC incidence and mortality have been declining in adults aged 55 years and older, recent studies cited in the ACS guideline document show a 51% increase in incidence from 1994 to 2014 – and an 11% increase in mortality from 2005 to 2015 – for adults younger than 55 years.
The current age-specific incidence rate for adults 45-49 years is 31.4 per 100,000, compared with 58.4 per 100,000 in adults 50-54 years. However, the ACS guideline authors said the higher rate in the older cohort is partly influenced by more frequent screening. “The true underlying risk in adults aged 45-49 years is likely closer to the risk in adults aged 50-54 years than the most recent age-specific rates would suggest,” they wrote.
Since patients in this age range have not been routinely screened before, the ACS recommendation is based on modeling. Now we need to analyze the outcomes of early screening to identify which patients will benefit most. Choices for screening include either a structural examination or a high-sensitivity stool-based test, according to the guideline, which doesn’t state a preference for any particular test.
The AGA, in their statement in response, noted that with CRC rates rising in people younger than age 50, it is appropriate to consider beginning routine screening at age 45. The statement continues “Since patients in this age range have not been previously routinely screened, the ACS recommendation is based on modeling. Now we need to analyze the outcomes of early screening to identify which patients will benefit most.”
In addition to Dr. Wolf, members of the ACS Guideline Development Group received no compensation.
SOURCE: Wolf AMD et al. CA Cancer J Clin. 2018 May 30. doi: 10.3322/caac.21457.
The American Cancer Society recommends all U.S. adults at average risk of colorectal cancer (CRC) undergo screening starting at age 45 years.
That update to ACS recommendations is based on an increasing burden of CRC in younger individuals, microsimulation modeling results, and a “reasonable expectation” that screening tests will perform as well in adults aged 45-49 years as they do in older adults, members of the ACS Guideline Development Group said in the guideline, which was published in CA: A Cancer Journal for Clinicians.
Starting screening at age 45 contrasts with recommendations from the U.S. Preventive Services Task Force (USPSTF), which in 2016 gave an “A” recommendation for CRC screening from 50 to 75 years of age. At the time, the USPSTF noted a modest increase in life-years gained by starting earlier, based on microsimulation modeling. But it concluded that available evidence best supported starting at age 50.
The updated ACS guidelines are based in part on a modeling study that the authors say extends the previous analysis conducted for the USPSTF.
“The recommendation places a high value on the potential years of life saved, addresses anticipated rising incidence going forward, and is expected to contribute to the reduction in disparities in incidence before age 50 years in some racial groups,” the ACS guideline authors added.
The recommendation to start screening at age 45 is a “qualified” recommendation, the authors said, given the limitations of the current evidence base. Most studies to date have been focused on older individuals, in keeping with long-standing recommendations to start screening at age 50.
The move downward in screening age recommendation acknowledges one of the most “significant and disturbing” developments in CRC, the guideline’s authors said: the marked increase in incidence among younger individuals.
While CRC incidence and mortality have been declining in adults aged 55 years and older, recent studies cited in the ACS guideline document show a 51% increase in incidence from 1994 to 2014 – and an 11% increase in mortality from 2005 to 2015 – for adults younger than 55 years.
The current age-specific incidence rate for adults 45-49 years is 31.4 per 100,000, compared with 58.4 per 100,000 in adults 50-54 years. However, the ACS guideline authors said the higher rate in the older cohort is partly influenced by more frequent screening. “The true underlying risk in adults aged 45-49 years is likely closer to the risk in adults aged 50-54 years than the most recent age-specific rates would suggest,” they wrote.
Since patients in this age range have not been routinely screened before, the ACS recommendation is based on modeling. Now we need to analyze the outcomes of early screening to identify which patients will benefit most. Choices for screening include either a structural examination or a high-sensitivity stool-based test, according to the guideline, which doesn’t state a preference for any particular test.
The AGA, in their statement in response, noted that with CRC rates rising in people younger than age 50, it is appropriate to consider beginning routine screening at age 45. The statement continues “Since patients in this age range have not been previously routinely screened, the ACS recommendation is based on modeling. Now we need to analyze the outcomes of early screening to identify which patients will benefit most.”
In addition to Dr. Wolf, members of the ACS Guideline Development Group received no compensation.
SOURCE: Wolf AMD et al. CA Cancer J Clin. 2018 May 30. doi: 10.3322/caac.21457.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Evaluation of Seizures and Seizure-like Activity in the Emergency Department: Part 1
Seizures are a common emergency presentation, accounting for approximately 1% of all ED visits.1 Presentations include patients with epilepsy, new-onset or first-time seizure (whether provoked or unprovoked), and other diagnostic entities that can mimic seizure but are not a true epileptic seizure. Even after a detailed and comprehensive evaluation, correctly determining the diagnosis can still be a challenge.2
Seizure Phases
The International League Against Epilepsy (ILAE) defines epileptic seizures as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.”3 There are typically three phases of a seizure—the aural, ictal, and postictal states.
Aural Phase. Patients may or may not experience an aura prior to seizure onset. An aura can manifest as a sense of déjà vu or a rising sensation in the abdomen, abnormal taste or smell, or autonomic changes. These are not warning signs of a seizure but rather an early manifestation of a focal seizure before there has been enough electrical spread to cause cognitive or motor symptoms.
Ictal Phase. The second stage of seizure, the ictal phase, is the typical cognitive or motor manifestations of seizure activity. Seizures can last several seconds to minutes, but the majority has a duration of less than 1 minute.
Postictal Phase. The postictal period occurs after the active phase of seizure and is characterized by confusion, altered mental status, and somnolence. The postictal period can last from several minutes to hours and can result in suppression of function; including cognitive or motor deficits such as Todd’s paralysis wherein a patient experiences transient paralysis confined to one hemisphere.4
Etiology and Classification
Seizures can be subdivided based on two different categories: etiology or origin of abnormal electrical impulses within the brain. To categorize seizures based on etiology, the clinician must determine whether the seizure was brought on by an identifiable cause.
Provoked Seizure
Provoked seizures are also referred to as acute symptomatic seizures, because they present within 7 days of a systemic insult, whether it be secondary to an electrolyte abnormality (eg, hyponatremia, hypoglycemia, hypercalcemia), substance withdrawal (eg, alcohol, benzodiazepines), toxic ingestion, infection, central nervous system lesions, or head injury. The aforementioned does not represent a comprehensive list, but rather some of the more common etiologies of seizures.2,5
Unprovoked Seizure
An unprovoked seizure occurs without an identifiable acute precipitating insult. These types of seizures are generally more consistent with epilepsy or are due to a remote systemic insult greater than 7 days prior. Examples include patients who have a history of stroke, traumatic brain injury, or congenital brain malformation.2,5
Epilepsy is described as a seizure disorder where recurrent, usually unprovoked seizures occur. Determining the probable etiology of a seizure can be important when pursuing proper objective evaluation and work up, as we will discuss in this article.
Seizure Type
Seizures can also be classified as being generalized or focal, depending on the probable origin of the abnormal electrical discharges within the brain. This classification system is widely used and was developed by the ILAE.6
Generalized Seizures
Generalized seizures have bilateral cortical involvement at the onset of presentation and are associated with loss of consciousness. This is determined through electroencephalogram (EEG) monitoring because focal seizures, where the initiation of abnormal electrical discharges are located in one cortical hemisphere or localized area of the brain, may rapidly spread to both hemispheres and appear very similar to a primary generalized seizure.
Tonic-Clonic Seizures. The most colloquial type of generalized seizure is a tonic-clonic seizure. “Tonic” refers to the muscle stiffness or rigidity that occurs during this type of seizure, and “clonic” describes the rhythmic jerking of these muscles.
Nontonic-Clonic Seizures. Other types of generalized seizures include absence seizures (brief staring episodes or an arrest in behavior), atonic seizures (loss of muscle tone), and myoclonic seizures (brief, sudden muscular contractions).5
Focal Seizures
Focal seizures are diagnosed when the history, clinical presentation, and EEG findings support the localization of abnormal electrical neuronal discharges to one hemisphere of the brain. Loss of consciousness does not always occur during a focal seizure, and the ILAE recently updated the terminology in this regard to this distinction in 2017. Instead of classifying focal seizures as simple partial or complex partial in relation to the preservation of consciousness, the terminology has now changed to focal aware (no loss of consciousness) and focal impaired awareness (affected consciousness). Focal seizures can have not only motor manifestations, but may also present with sensory, autonomic, or psychic symptoms, depending on the anatomic location of the abnormal neuronal activity.5-6
Evaluation in the ED Setting
The classification of a seizure does not often change the ED management of seizures, but it is important to be able to recognize that seizures may present with different clinical appearances. It is also important to remember that not all seizure-like activity is due to epilepsy or abnormal neuronal discharges. There are several other conditions that can present with physical symptoms and characteristics similar to seizure, and are often misdiagnosed as seizures. The next section describes several of these seizure mimics and how to recognize or differentiate them from seizures through a careful history, physical examination, and laboratory evaluation; as one diagnostic tool, the EEG, is not routinely available to the emergency physician (EP).
Seizure Mimics
Syncope
Syncope is secondary to decreased cerebral perfusion, which results in brief loss of consciousness and postural tone, and often with brief convulsions. Myoclonic jerking lasting a few seconds can be seen in many syncopal episodes, and if present is termed convulsive syncope. Following any syncopal episode, patients generally return to their baseline mental status without a postictal period. A prodrome of pallor and sweating can be helpful clues to identify a syncopal episode. In addition, a patient’s eyes may remain open during the event.
There are several types of syncope: cardiac, orthostatic, or neurocardiogenic (vasovagal). History and physical examination can help distinguish syncope from seizure.
Cardiac Syncope. Cardiogenic causes of syncope may be seen in elderly patients who lack a prodrome prior to the event, chest pain may have been present, the event may occur with exercise, or there is evidence of underlying heart disease. An electrocardiogram (ECG) should be done to detect cardiac dysrhythmias. Orthostatic Syncope. Vital signs may be useful in assessing for an orthostatic cause of syncope (drop in systolic blood pressure [BP] by 20 mm Hg or more and drop in diastolic BP by 10 mm Hg or more within 3 minutes of standing), though orthostatic hypotension is common in the elderly.7-8 Dysautonomia as a cause of orthostatic hypotension may show a delayed drop in BP after standing 5 to 10 minutes, in contrast to hypovolemia which tends to be present with immediate standing.Neurocardiogenic Syncope. Neurocardiogenic syncope, a somewhat confusing term, is perhaps better described as a reflex syncope, or simple faint. Often this is referred to as “vasovagal” syncope. Typically, there are physical or psychological noxious stimuli prior to the brief loss of consciousness and postural tone. Pain or strong emotions are common triggers.
Convulsive Concussion
Another seizure mimic is convulsive concussion in which the patient exhibits nonepileptic movement following a closed head injury. It is hypothesized that these post-traumatic convulsions are due to transient functional abnormalities, rather than structural brain injury. In one study, 22 cases of concussive convulsions were identified in which tonic-clonic convulsions began within 2 seconds of impact, and lasted for up to 150 seconds. These patients generally have good outcomes and do not require antiepileptic treatment; they also do not need to abstain from sports or other physical activities.9-11
Movement Disorders
Certain movement disorders can appear similar to seizures with sustained muscle contractions, repetitive movements, dystonias, or even abnormal posturing. However, these abnormal movements are generally painful and there is often impairment of consciousness. They may be genetic in nature or secondary to a neurologic disease or medications such as neuroleptics or antipsychotics.
Psychogenic Nonepileptic Seizures
Psychogenic nonepileptic seizures (PNES) are defined as episodes of altered movements or sensations that appear similar to epileptic seizures, but have an underlying psychological etiology rather than abnormal neuronal discharges. Seventy percent of these patients have a psychiatric illness, such as depression, post-traumatic stress disorder, or personality disorders. Features that can help distinguish PNES from epileptic seizures include long duration, fluctuating symptoms, asynchronous or non-rhythmic movements, pelvic thrusting, side-to-side head or body movements, closed eyes, lack of tongue biting, memory recall, crying, or suppression by distraction. Laboratory testing provides little benefit, aside from a lactate level, which if elevated can suggest a possible epileptic etiology.12 These cases may require consultation with neurology and psychiatry or video-EEG monitoring to correctly diagnose.13-14
Other non-epileptic and possible seizure mimic diagnoses to be considered include stroke, transient ischemic attack, migraine headache, and sleep disorders.
Evaluation
When assessing a patient presenting with seizure-like activity or altered mental status, the clinician must keep a broad differential diagnosis. The first step is to evaluate the ABCs. Once that is completed, a blood glucose should be obtained, as it is a quick test and can determine whether hypoglycemia is the likely cause. Intravenous (IV) access should be obtained and routine labs ordered, including a complete blood count (CBC), a comprehensive metabolic profile (CMP), magnesium, urinalysis, ECG, and lactate. Other labs that may be of clinical utility in certain cases include anticonvulsant levels (in patients that are on these medications), toxicology screens, and cerebrospinal fluid studies, if indicated. It is important to note that anticonvulsant reference ranges are trough values, so levels that are drawn within a few hours of the last dose taken
Patient Disposition
The management and ultimate disposition of a patient with a seizure depends on the underlying cause, and whether the patient has neurologic deficits and/or is back to their baseline mental status. For patients presenting with a first-time seizure and have returned to baseline, the American College of Emergency Physicians’ (ACEP) clinical policy states that precipitating medical conditions should be identified and addressed accordingly if it is a provoked seizure.
If patients present with a first-time unprovoked seizure and do not have evidence of brain injury or neurologic disease (ie, persistent altered mental status or abnormal neurologic examination), then the EP does not need to initiate antiepileptic medications. If there is evidence of neurologic disease or brain injury in an unprovoked seizure, then the EP may either choose to initiate antiepileptic medications or choose to defer, pending consultation with neurology. This group of patients (first-time unprovoked seizure back to baseline) do not need to be admitted to the hospital under the premise that they have a negative workup, to include glucose, CT scan (if indicated), ECG, CBC, and CMP. They must also have normal vital signs, be advised regarding seizure precautions such as not to drive until further medical evaluation (with duration perhaps being set by state law), and have good social support. They will require close follow up for further evaluation and definitive diagnosis, which may include head imaging, if not already performed in the ED, and EEG.
Patients with a history of epilepsy can have recurrent seizures that are either provoked or unprovoked. If the seizure appears clinically similar to their previous seizures, then causes that could lower the seizure threshold should be investigated; including compliance with antiepileptic medications (obtain serum levels), infection (urinalysis and/or chest X-ray), sleep deprivation, electrolyte imbalances, or medications known to lower seizure threshold (eg, certain antibiotics such as fluoroquinolones, antidepressants such as bupropion and venlafaxine, and antipsychotics such as clozapine). These underlying causes should be treated accordingly. If a patient has been noncompliant with their medications, a loading dose can be given in the ED, although there are no definitive studies that either support or debunk this practice. If it is a true, unprovoked seizure and the patient is compliant with their antiepileptic medication, it is reasonable to discuss medication regimen changes with the patient’s neurologist.17A patient that presents with seizure-like activity and does not return back to their baseline mental status requires a more immediate and comprehensive evaluation. Persistent altered mental status has a vast differential diagnosis, and is outside the scope of this article, but if seizures were part of the clinical presentation, the possibility of non-convulsive epilepticus should be considered. These patients may require treatment with medications (usually IV benzodiazepines), admission to the hospital, neurology consultation, EEG, imaging (CT vs MRI), and +/- lumbar puncture depending on the clinical scenario.
Status epilepticus will be further discussed in part 2 of this review.
1. Pallin DJ, Goldstein JN, Moussally JS, Pelletier AJ, Green AR, Carmargo CA Jr. Seizure visits in the US emergency departments: epidemiology and potential disparities in care. Int J Emerg Med. 2008;1(2):97-105.
2. Huff JS, Melnick ER, Tomaszewski CA, Thiessen ME, Jagoda AS, Fesmire FM; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2014;63(4):437-447.e15. doi:10.1016/j.annemergmed.2014.01.018.
3. Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470-472.
4. Webb J, Long B, Koyfman A. An emergency medicine-focused review of seizure mimics. J Emerg Med. 2017;52(5):645-653. doi:10.1016/j.jemermed.2016.11.002.
5. Huff JS, Fountain NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin North Am. 2011;29(1):1-13. doi:10.1016/j.emc.2010.08.001.
6. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522-530. doi:10.1111/epi.13670.
7. Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Differentiation of convulsive syncope from epilepsy with an implantable loop recorder. Int J Med Sci. 2009;6(6):296-300.
8. Ozkara C, Metin B, Kucukoglu S. Convulsive syncope: a condition to be differentiated from epilepsy. Epileptic Disord. 2009;11(4):315-319. doi:10.1684/epd.2009.0281.
9. McCrory PR, Berkovic SF. Concussive convulsions. Incidence in sport and treatment recommendations. Sports Med. 1998;25(2);131-136.
10. Ellis MJ, Wennberg R. Convulsions in a 17-year-old boy after a head injury sustained while playing hockey. CMAJ. 2016;188(6):443-445. doi:10.1503/cmaj.150124.
11. McCrory PR, Bladin PF, Berkovic SF. Retrospective study of concussive convulsions in elite Australian rules and rugby league footballers: phenomenology, aetiology, and outcome. BMJ. 1997;314(7075):171-174.
12. Webb JL, Long B. Seizure mimics: pearls and pitfalls. emDocs Web site. http://www.emdocs.net/seizure-mimics-pearls-pitfalls/. Accessed May 15, 2018.
13. Chen DK, LaFrance WC Jr. Diagnosis and treatment of nonepileptic seizures. Continuum (Mineapp Minn). 2016;22(1):116-131. doi:10.1212/CON.0000000000000282.
14. O’Sullivan SS, Redwood RI, Hunt D, McMahon EM, O’Sullivan S. Recognition of psychogenic non-epileptic seizures: a curable neurophobia? J Neurol Neurosurg Psychiatry. 2013;84(2):228-231. doi:10.1136/jnnp-2012-303062.
15. Clinical policy for the initial approach to patients presenting with a chief complaint of seizure, who are not in status epilepticus. Ann Emerg Med. 1993;22(5):875-883.
16. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69(18):1772-1780.
17. Montouris GD, Jagoda AS. Management of breakthrough seizures in the emergency department: continuity of patient care. Curr Med Res Opin. 2007;23(7):1583-1592.
Seizures are a common emergency presentation, accounting for approximately 1% of all ED visits.1 Presentations include patients with epilepsy, new-onset or first-time seizure (whether provoked or unprovoked), and other diagnostic entities that can mimic seizure but are not a true epileptic seizure. Even after a detailed and comprehensive evaluation, correctly determining the diagnosis can still be a challenge.2
Seizure Phases
The International League Against Epilepsy (ILAE) defines epileptic seizures as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.”3 There are typically three phases of a seizure—the aural, ictal, and postictal states.
Aural Phase. Patients may or may not experience an aura prior to seizure onset. An aura can manifest as a sense of déjà vu or a rising sensation in the abdomen, abnormal taste or smell, or autonomic changes. These are not warning signs of a seizure but rather an early manifestation of a focal seizure before there has been enough electrical spread to cause cognitive or motor symptoms.
Ictal Phase. The second stage of seizure, the ictal phase, is the typical cognitive or motor manifestations of seizure activity. Seizures can last several seconds to minutes, but the majority has a duration of less than 1 minute.
Postictal Phase. The postictal period occurs after the active phase of seizure and is characterized by confusion, altered mental status, and somnolence. The postictal period can last from several minutes to hours and can result in suppression of function; including cognitive or motor deficits such as Todd’s paralysis wherein a patient experiences transient paralysis confined to one hemisphere.4
Etiology and Classification
Seizures can be subdivided based on two different categories: etiology or origin of abnormal electrical impulses within the brain. To categorize seizures based on etiology, the clinician must determine whether the seizure was brought on by an identifiable cause.
Provoked Seizure
Provoked seizures are also referred to as acute symptomatic seizures, because they present within 7 days of a systemic insult, whether it be secondary to an electrolyte abnormality (eg, hyponatremia, hypoglycemia, hypercalcemia), substance withdrawal (eg, alcohol, benzodiazepines), toxic ingestion, infection, central nervous system lesions, or head injury. The aforementioned does not represent a comprehensive list, but rather some of the more common etiologies of seizures.2,5
Unprovoked Seizure
An unprovoked seizure occurs without an identifiable acute precipitating insult. These types of seizures are generally more consistent with epilepsy or are due to a remote systemic insult greater than 7 days prior. Examples include patients who have a history of stroke, traumatic brain injury, or congenital brain malformation.2,5
Epilepsy is described as a seizure disorder where recurrent, usually unprovoked seizures occur. Determining the probable etiology of a seizure can be important when pursuing proper objective evaluation and work up, as we will discuss in this article.
Seizure Type
Seizures can also be classified as being generalized or focal, depending on the probable origin of the abnormal electrical discharges within the brain. This classification system is widely used and was developed by the ILAE.6
Generalized Seizures
Generalized seizures have bilateral cortical involvement at the onset of presentation and are associated with loss of consciousness. This is determined through electroencephalogram (EEG) monitoring because focal seizures, where the initiation of abnormal electrical discharges are located in one cortical hemisphere or localized area of the brain, may rapidly spread to both hemispheres and appear very similar to a primary generalized seizure.
Tonic-Clonic Seizures. The most colloquial type of generalized seizure is a tonic-clonic seizure. “Tonic” refers to the muscle stiffness or rigidity that occurs during this type of seizure, and “clonic” describes the rhythmic jerking of these muscles.
Nontonic-Clonic Seizures. Other types of generalized seizures include absence seizures (brief staring episodes or an arrest in behavior), atonic seizures (loss of muscle tone), and myoclonic seizures (brief, sudden muscular contractions).5
Focal Seizures
Focal seizures are diagnosed when the history, clinical presentation, and EEG findings support the localization of abnormal electrical neuronal discharges to one hemisphere of the brain. Loss of consciousness does not always occur during a focal seizure, and the ILAE recently updated the terminology in this regard to this distinction in 2017. Instead of classifying focal seizures as simple partial or complex partial in relation to the preservation of consciousness, the terminology has now changed to focal aware (no loss of consciousness) and focal impaired awareness (affected consciousness). Focal seizures can have not only motor manifestations, but may also present with sensory, autonomic, or psychic symptoms, depending on the anatomic location of the abnormal neuronal activity.5-6
Evaluation in the ED Setting
The classification of a seizure does not often change the ED management of seizures, but it is important to be able to recognize that seizures may present with different clinical appearances. It is also important to remember that not all seizure-like activity is due to epilepsy or abnormal neuronal discharges. There are several other conditions that can present with physical symptoms and characteristics similar to seizure, and are often misdiagnosed as seizures. The next section describes several of these seizure mimics and how to recognize or differentiate them from seizures through a careful history, physical examination, and laboratory evaluation; as one diagnostic tool, the EEG, is not routinely available to the emergency physician (EP).
Seizure Mimics
Syncope
Syncope is secondary to decreased cerebral perfusion, which results in brief loss of consciousness and postural tone, and often with brief convulsions. Myoclonic jerking lasting a few seconds can be seen in many syncopal episodes, and if present is termed convulsive syncope. Following any syncopal episode, patients generally return to their baseline mental status without a postictal period. A prodrome of pallor and sweating can be helpful clues to identify a syncopal episode. In addition, a patient’s eyes may remain open during the event.
There are several types of syncope: cardiac, orthostatic, or neurocardiogenic (vasovagal). History and physical examination can help distinguish syncope from seizure.
Cardiac Syncope. Cardiogenic causes of syncope may be seen in elderly patients who lack a prodrome prior to the event, chest pain may have been present, the event may occur with exercise, or there is evidence of underlying heart disease. An electrocardiogram (ECG) should be done to detect cardiac dysrhythmias. Orthostatic Syncope. Vital signs may be useful in assessing for an orthostatic cause of syncope (drop in systolic blood pressure [BP] by 20 mm Hg or more and drop in diastolic BP by 10 mm Hg or more within 3 minutes of standing), though orthostatic hypotension is common in the elderly.7-8 Dysautonomia as a cause of orthostatic hypotension may show a delayed drop in BP after standing 5 to 10 minutes, in contrast to hypovolemia which tends to be present with immediate standing.Neurocardiogenic Syncope. Neurocardiogenic syncope, a somewhat confusing term, is perhaps better described as a reflex syncope, or simple faint. Often this is referred to as “vasovagal” syncope. Typically, there are physical or psychological noxious stimuli prior to the brief loss of consciousness and postural tone. Pain or strong emotions are common triggers.
Convulsive Concussion
Another seizure mimic is convulsive concussion in which the patient exhibits nonepileptic movement following a closed head injury. It is hypothesized that these post-traumatic convulsions are due to transient functional abnormalities, rather than structural brain injury. In one study, 22 cases of concussive convulsions were identified in which tonic-clonic convulsions began within 2 seconds of impact, and lasted for up to 150 seconds. These patients generally have good outcomes and do not require antiepileptic treatment; they also do not need to abstain from sports or other physical activities.9-11
Movement Disorders
Certain movement disorders can appear similar to seizures with sustained muscle contractions, repetitive movements, dystonias, or even abnormal posturing. However, these abnormal movements are generally painful and there is often impairment of consciousness. They may be genetic in nature or secondary to a neurologic disease or medications such as neuroleptics or antipsychotics.
Psychogenic Nonepileptic Seizures
Psychogenic nonepileptic seizures (PNES) are defined as episodes of altered movements or sensations that appear similar to epileptic seizures, but have an underlying psychological etiology rather than abnormal neuronal discharges. Seventy percent of these patients have a psychiatric illness, such as depression, post-traumatic stress disorder, or personality disorders. Features that can help distinguish PNES from epileptic seizures include long duration, fluctuating symptoms, asynchronous or non-rhythmic movements, pelvic thrusting, side-to-side head or body movements, closed eyes, lack of tongue biting, memory recall, crying, or suppression by distraction. Laboratory testing provides little benefit, aside from a lactate level, which if elevated can suggest a possible epileptic etiology.12 These cases may require consultation with neurology and psychiatry or video-EEG monitoring to correctly diagnose.13-14
Other non-epileptic and possible seizure mimic diagnoses to be considered include stroke, transient ischemic attack, migraine headache, and sleep disorders.
Evaluation
When assessing a patient presenting with seizure-like activity or altered mental status, the clinician must keep a broad differential diagnosis. The first step is to evaluate the ABCs. Once that is completed, a blood glucose should be obtained, as it is a quick test and can determine whether hypoglycemia is the likely cause. Intravenous (IV) access should be obtained and routine labs ordered, including a complete blood count (CBC), a comprehensive metabolic profile (CMP), magnesium, urinalysis, ECG, and lactate. Other labs that may be of clinical utility in certain cases include anticonvulsant levels (in patients that are on these medications), toxicology screens, and cerebrospinal fluid studies, if indicated. It is important to note that anticonvulsant reference ranges are trough values, so levels that are drawn within a few hours of the last dose taken
Patient Disposition
The management and ultimate disposition of a patient with a seizure depends on the underlying cause, and whether the patient has neurologic deficits and/or is back to their baseline mental status. For patients presenting with a first-time seizure and have returned to baseline, the American College of Emergency Physicians’ (ACEP) clinical policy states that precipitating medical conditions should be identified and addressed accordingly if it is a provoked seizure.
If patients present with a first-time unprovoked seizure and do not have evidence of brain injury or neurologic disease (ie, persistent altered mental status or abnormal neurologic examination), then the EP does not need to initiate antiepileptic medications. If there is evidence of neurologic disease or brain injury in an unprovoked seizure, then the EP may either choose to initiate antiepileptic medications or choose to defer, pending consultation with neurology. This group of patients (first-time unprovoked seizure back to baseline) do not need to be admitted to the hospital under the premise that they have a negative workup, to include glucose, CT scan (if indicated), ECG, CBC, and CMP. They must also have normal vital signs, be advised regarding seizure precautions such as not to drive until further medical evaluation (with duration perhaps being set by state law), and have good social support. They will require close follow up for further evaluation and definitive diagnosis, which may include head imaging, if not already performed in the ED, and EEG.
Patients with a history of epilepsy can have recurrent seizures that are either provoked or unprovoked. If the seizure appears clinically similar to their previous seizures, then causes that could lower the seizure threshold should be investigated; including compliance with antiepileptic medications (obtain serum levels), infection (urinalysis and/or chest X-ray), sleep deprivation, electrolyte imbalances, or medications known to lower seizure threshold (eg, certain antibiotics such as fluoroquinolones, antidepressants such as bupropion and venlafaxine, and antipsychotics such as clozapine). These underlying causes should be treated accordingly. If a patient has been noncompliant with their medications, a loading dose can be given in the ED, although there are no definitive studies that either support or debunk this practice. If it is a true, unprovoked seizure and the patient is compliant with their antiepileptic medication, it is reasonable to discuss medication regimen changes with the patient’s neurologist.17A patient that presents with seizure-like activity and does not return back to their baseline mental status requires a more immediate and comprehensive evaluation. Persistent altered mental status has a vast differential diagnosis, and is outside the scope of this article, but if seizures were part of the clinical presentation, the possibility of non-convulsive epilepticus should be considered. These patients may require treatment with medications (usually IV benzodiazepines), admission to the hospital, neurology consultation, EEG, imaging (CT vs MRI), and +/- lumbar puncture depending on the clinical scenario.
Status epilepticus will be further discussed in part 2 of this review.
Seizures are a common emergency presentation, accounting for approximately 1% of all ED visits.1 Presentations include patients with epilepsy, new-onset or first-time seizure (whether provoked or unprovoked), and other diagnostic entities that can mimic seizure but are not a true epileptic seizure. Even after a detailed and comprehensive evaluation, correctly determining the diagnosis can still be a challenge.2
Seizure Phases
The International League Against Epilepsy (ILAE) defines epileptic seizures as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.”3 There are typically three phases of a seizure—the aural, ictal, and postictal states.
Aural Phase. Patients may or may not experience an aura prior to seizure onset. An aura can manifest as a sense of déjà vu or a rising sensation in the abdomen, abnormal taste or smell, or autonomic changes. These are not warning signs of a seizure but rather an early manifestation of a focal seizure before there has been enough electrical spread to cause cognitive or motor symptoms.
Ictal Phase. The second stage of seizure, the ictal phase, is the typical cognitive or motor manifestations of seizure activity. Seizures can last several seconds to minutes, but the majority has a duration of less than 1 minute.
Postictal Phase. The postictal period occurs after the active phase of seizure and is characterized by confusion, altered mental status, and somnolence. The postictal period can last from several minutes to hours and can result in suppression of function; including cognitive or motor deficits such as Todd’s paralysis wherein a patient experiences transient paralysis confined to one hemisphere.4
Etiology and Classification
Seizures can be subdivided based on two different categories: etiology or origin of abnormal electrical impulses within the brain. To categorize seizures based on etiology, the clinician must determine whether the seizure was brought on by an identifiable cause.
Provoked Seizure
Provoked seizures are also referred to as acute symptomatic seizures, because they present within 7 days of a systemic insult, whether it be secondary to an electrolyte abnormality (eg, hyponatremia, hypoglycemia, hypercalcemia), substance withdrawal (eg, alcohol, benzodiazepines), toxic ingestion, infection, central nervous system lesions, or head injury. The aforementioned does not represent a comprehensive list, but rather some of the more common etiologies of seizures.2,5
Unprovoked Seizure
An unprovoked seizure occurs without an identifiable acute precipitating insult. These types of seizures are generally more consistent with epilepsy or are due to a remote systemic insult greater than 7 days prior. Examples include patients who have a history of stroke, traumatic brain injury, or congenital brain malformation.2,5
Epilepsy is described as a seizure disorder where recurrent, usually unprovoked seizures occur. Determining the probable etiology of a seizure can be important when pursuing proper objective evaluation and work up, as we will discuss in this article.
Seizure Type
Seizures can also be classified as being generalized or focal, depending on the probable origin of the abnormal electrical discharges within the brain. This classification system is widely used and was developed by the ILAE.6
Generalized Seizures
Generalized seizures have bilateral cortical involvement at the onset of presentation and are associated with loss of consciousness. This is determined through electroencephalogram (EEG) monitoring because focal seizures, where the initiation of abnormal electrical discharges are located in one cortical hemisphere or localized area of the brain, may rapidly spread to both hemispheres and appear very similar to a primary generalized seizure.
Tonic-Clonic Seizures. The most colloquial type of generalized seizure is a tonic-clonic seizure. “Tonic” refers to the muscle stiffness or rigidity that occurs during this type of seizure, and “clonic” describes the rhythmic jerking of these muscles.
Nontonic-Clonic Seizures. Other types of generalized seizures include absence seizures (brief staring episodes or an arrest in behavior), atonic seizures (loss of muscle tone), and myoclonic seizures (brief, sudden muscular contractions).5
Focal Seizures
Focal seizures are diagnosed when the history, clinical presentation, and EEG findings support the localization of abnormal electrical neuronal discharges to one hemisphere of the brain. Loss of consciousness does not always occur during a focal seizure, and the ILAE recently updated the terminology in this regard to this distinction in 2017. Instead of classifying focal seizures as simple partial or complex partial in relation to the preservation of consciousness, the terminology has now changed to focal aware (no loss of consciousness) and focal impaired awareness (affected consciousness). Focal seizures can have not only motor manifestations, but may also present with sensory, autonomic, or psychic symptoms, depending on the anatomic location of the abnormal neuronal activity.5-6
Evaluation in the ED Setting
The classification of a seizure does not often change the ED management of seizures, but it is important to be able to recognize that seizures may present with different clinical appearances. It is also important to remember that not all seizure-like activity is due to epilepsy or abnormal neuronal discharges. There are several other conditions that can present with physical symptoms and characteristics similar to seizure, and are often misdiagnosed as seizures. The next section describes several of these seizure mimics and how to recognize or differentiate them from seizures through a careful history, physical examination, and laboratory evaluation; as one diagnostic tool, the EEG, is not routinely available to the emergency physician (EP).
Seizure Mimics
Syncope
Syncope is secondary to decreased cerebral perfusion, which results in brief loss of consciousness and postural tone, and often with brief convulsions. Myoclonic jerking lasting a few seconds can be seen in many syncopal episodes, and if present is termed convulsive syncope. Following any syncopal episode, patients generally return to their baseline mental status without a postictal period. A prodrome of pallor and sweating can be helpful clues to identify a syncopal episode. In addition, a patient’s eyes may remain open during the event.
There are several types of syncope: cardiac, orthostatic, or neurocardiogenic (vasovagal). History and physical examination can help distinguish syncope from seizure.
Cardiac Syncope. Cardiogenic causes of syncope may be seen in elderly patients who lack a prodrome prior to the event, chest pain may have been present, the event may occur with exercise, or there is evidence of underlying heart disease. An electrocardiogram (ECG) should be done to detect cardiac dysrhythmias. Orthostatic Syncope. Vital signs may be useful in assessing for an orthostatic cause of syncope (drop in systolic blood pressure [BP] by 20 mm Hg or more and drop in diastolic BP by 10 mm Hg or more within 3 minutes of standing), though orthostatic hypotension is common in the elderly.7-8 Dysautonomia as a cause of orthostatic hypotension may show a delayed drop in BP after standing 5 to 10 minutes, in contrast to hypovolemia which tends to be present with immediate standing.Neurocardiogenic Syncope. Neurocardiogenic syncope, a somewhat confusing term, is perhaps better described as a reflex syncope, or simple faint. Often this is referred to as “vasovagal” syncope. Typically, there are physical or psychological noxious stimuli prior to the brief loss of consciousness and postural tone. Pain or strong emotions are common triggers.
Convulsive Concussion
Another seizure mimic is convulsive concussion in which the patient exhibits nonepileptic movement following a closed head injury. It is hypothesized that these post-traumatic convulsions are due to transient functional abnormalities, rather than structural brain injury. In one study, 22 cases of concussive convulsions were identified in which tonic-clonic convulsions began within 2 seconds of impact, and lasted for up to 150 seconds. These patients generally have good outcomes and do not require antiepileptic treatment; they also do not need to abstain from sports or other physical activities.9-11
Movement Disorders
Certain movement disorders can appear similar to seizures with sustained muscle contractions, repetitive movements, dystonias, or even abnormal posturing. However, these abnormal movements are generally painful and there is often impairment of consciousness. They may be genetic in nature or secondary to a neurologic disease or medications such as neuroleptics or antipsychotics.
Psychogenic Nonepileptic Seizures
Psychogenic nonepileptic seizures (PNES) are defined as episodes of altered movements or sensations that appear similar to epileptic seizures, but have an underlying psychological etiology rather than abnormal neuronal discharges. Seventy percent of these patients have a psychiatric illness, such as depression, post-traumatic stress disorder, or personality disorders. Features that can help distinguish PNES from epileptic seizures include long duration, fluctuating symptoms, asynchronous or non-rhythmic movements, pelvic thrusting, side-to-side head or body movements, closed eyes, lack of tongue biting, memory recall, crying, or suppression by distraction. Laboratory testing provides little benefit, aside from a lactate level, which if elevated can suggest a possible epileptic etiology.12 These cases may require consultation with neurology and psychiatry or video-EEG monitoring to correctly diagnose.13-14
Other non-epileptic and possible seizure mimic diagnoses to be considered include stroke, transient ischemic attack, migraine headache, and sleep disorders.
Evaluation
When assessing a patient presenting with seizure-like activity or altered mental status, the clinician must keep a broad differential diagnosis. The first step is to evaluate the ABCs. Once that is completed, a blood glucose should be obtained, as it is a quick test and can determine whether hypoglycemia is the likely cause. Intravenous (IV) access should be obtained and routine labs ordered, including a complete blood count (CBC), a comprehensive metabolic profile (CMP), magnesium, urinalysis, ECG, and lactate. Other labs that may be of clinical utility in certain cases include anticonvulsant levels (in patients that are on these medications), toxicology screens, and cerebrospinal fluid studies, if indicated. It is important to note that anticonvulsant reference ranges are trough values, so levels that are drawn within a few hours of the last dose taken
Patient Disposition
The management and ultimate disposition of a patient with a seizure depends on the underlying cause, and whether the patient has neurologic deficits and/or is back to their baseline mental status. For patients presenting with a first-time seizure and have returned to baseline, the American College of Emergency Physicians’ (ACEP) clinical policy states that precipitating medical conditions should be identified and addressed accordingly if it is a provoked seizure.
If patients present with a first-time unprovoked seizure and do not have evidence of brain injury or neurologic disease (ie, persistent altered mental status or abnormal neurologic examination), then the EP does not need to initiate antiepileptic medications. If there is evidence of neurologic disease or brain injury in an unprovoked seizure, then the EP may either choose to initiate antiepileptic medications or choose to defer, pending consultation with neurology. This group of patients (first-time unprovoked seizure back to baseline) do not need to be admitted to the hospital under the premise that they have a negative workup, to include glucose, CT scan (if indicated), ECG, CBC, and CMP. They must also have normal vital signs, be advised regarding seizure precautions such as not to drive until further medical evaluation (with duration perhaps being set by state law), and have good social support. They will require close follow up for further evaluation and definitive diagnosis, which may include head imaging, if not already performed in the ED, and EEG.
Patients with a history of epilepsy can have recurrent seizures that are either provoked or unprovoked. If the seizure appears clinically similar to their previous seizures, then causes that could lower the seizure threshold should be investigated; including compliance with antiepileptic medications (obtain serum levels), infection (urinalysis and/or chest X-ray), sleep deprivation, electrolyte imbalances, or medications known to lower seizure threshold (eg, certain antibiotics such as fluoroquinolones, antidepressants such as bupropion and venlafaxine, and antipsychotics such as clozapine). These underlying causes should be treated accordingly. If a patient has been noncompliant with their medications, a loading dose can be given in the ED, although there are no definitive studies that either support or debunk this practice. If it is a true, unprovoked seizure and the patient is compliant with their antiepileptic medication, it is reasonable to discuss medication regimen changes with the patient’s neurologist.17A patient that presents with seizure-like activity and does not return back to their baseline mental status requires a more immediate and comprehensive evaluation. Persistent altered mental status has a vast differential diagnosis, and is outside the scope of this article, but if seizures were part of the clinical presentation, the possibility of non-convulsive epilepticus should be considered. These patients may require treatment with medications (usually IV benzodiazepines), admission to the hospital, neurology consultation, EEG, imaging (CT vs MRI), and +/- lumbar puncture depending on the clinical scenario.
Status epilepticus will be further discussed in part 2 of this review.
1. Pallin DJ, Goldstein JN, Moussally JS, Pelletier AJ, Green AR, Carmargo CA Jr. Seizure visits in the US emergency departments: epidemiology and potential disparities in care. Int J Emerg Med. 2008;1(2):97-105.
2. Huff JS, Melnick ER, Tomaszewski CA, Thiessen ME, Jagoda AS, Fesmire FM; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2014;63(4):437-447.e15. doi:10.1016/j.annemergmed.2014.01.018.
3. Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470-472.
4. Webb J, Long B, Koyfman A. An emergency medicine-focused review of seizure mimics. J Emerg Med. 2017;52(5):645-653. doi:10.1016/j.jemermed.2016.11.002.
5. Huff JS, Fountain NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin North Am. 2011;29(1):1-13. doi:10.1016/j.emc.2010.08.001.
6. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522-530. doi:10.1111/epi.13670.
7. Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Differentiation of convulsive syncope from epilepsy with an implantable loop recorder. Int J Med Sci. 2009;6(6):296-300.
8. Ozkara C, Metin B, Kucukoglu S. Convulsive syncope: a condition to be differentiated from epilepsy. Epileptic Disord. 2009;11(4):315-319. doi:10.1684/epd.2009.0281.
9. McCrory PR, Berkovic SF. Concussive convulsions. Incidence in sport and treatment recommendations. Sports Med. 1998;25(2);131-136.
10. Ellis MJ, Wennberg R. Convulsions in a 17-year-old boy after a head injury sustained while playing hockey. CMAJ. 2016;188(6):443-445. doi:10.1503/cmaj.150124.
11. McCrory PR, Bladin PF, Berkovic SF. Retrospective study of concussive convulsions in elite Australian rules and rugby league footballers: phenomenology, aetiology, and outcome. BMJ. 1997;314(7075):171-174.
12. Webb JL, Long B. Seizure mimics: pearls and pitfalls. emDocs Web site. http://www.emdocs.net/seizure-mimics-pearls-pitfalls/. Accessed May 15, 2018.
13. Chen DK, LaFrance WC Jr. Diagnosis and treatment of nonepileptic seizures. Continuum (Mineapp Minn). 2016;22(1):116-131. doi:10.1212/CON.0000000000000282.
14. O’Sullivan SS, Redwood RI, Hunt D, McMahon EM, O’Sullivan S. Recognition of psychogenic non-epileptic seizures: a curable neurophobia? J Neurol Neurosurg Psychiatry. 2013;84(2):228-231. doi:10.1136/jnnp-2012-303062.
15. Clinical policy for the initial approach to patients presenting with a chief complaint of seizure, who are not in status epilepticus. Ann Emerg Med. 1993;22(5):875-883.
16. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69(18):1772-1780.
17. Montouris GD, Jagoda AS. Management of breakthrough seizures in the emergency department: continuity of patient care. Curr Med Res Opin. 2007;23(7):1583-1592.
1. Pallin DJ, Goldstein JN, Moussally JS, Pelletier AJ, Green AR, Carmargo CA Jr. Seizure visits in the US emergency departments: epidemiology and potential disparities in care. Int J Emerg Med. 2008;1(2):97-105.
2. Huff JS, Melnick ER, Tomaszewski CA, Thiessen ME, Jagoda AS, Fesmire FM; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2014;63(4):437-447.e15. doi:10.1016/j.annemergmed.2014.01.018.
3. Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470-472.
4. Webb J, Long B, Koyfman A. An emergency medicine-focused review of seizure mimics. J Emerg Med. 2017;52(5):645-653. doi:10.1016/j.jemermed.2016.11.002.
5. Huff JS, Fountain NB. Pathophysiology and definitions of seizures and status epilepticus. Emerg Med Clin North Am. 2011;29(1):1-13. doi:10.1016/j.emc.2010.08.001.
6. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522-530. doi:10.1111/epi.13670.
7. Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Differentiation of convulsive syncope from epilepsy with an implantable loop recorder. Int J Med Sci. 2009;6(6):296-300.
8. Ozkara C, Metin B, Kucukoglu S. Convulsive syncope: a condition to be differentiated from epilepsy. Epileptic Disord. 2009;11(4):315-319. doi:10.1684/epd.2009.0281.
9. McCrory PR, Berkovic SF. Concussive convulsions. Incidence in sport and treatment recommendations. Sports Med. 1998;25(2);131-136.
10. Ellis MJ, Wennberg R. Convulsions in a 17-year-old boy after a head injury sustained while playing hockey. CMAJ. 2016;188(6):443-445. doi:10.1503/cmaj.150124.
11. McCrory PR, Bladin PF, Berkovic SF. Retrospective study of concussive convulsions in elite Australian rules and rugby league footballers: phenomenology, aetiology, and outcome. BMJ. 1997;314(7075):171-174.
12. Webb JL, Long B. Seizure mimics: pearls and pitfalls. emDocs Web site. http://www.emdocs.net/seizure-mimics-pearls-pitfalls/. Accessed May 15, 2018.
13. Chen DK, LaFrance WC Jr. Diagnosis and treatment of nonepileptic seizures. Continuum (Mineapp Minn). 2016;22(1):116-131. doi:10.1212/CON.0000000000000282.
14. O’Sullivan SS, Redwood RI, Hunt D, McMahon EM, O’Sullivan S. Recognition of psychogenic non-epileptic seizures: a curable neurophobia? J Neurol Neurosurg Psychiatry. 2013;84(2):228-231. doi:10.1136/jnnp-2012-303062.
15. Clinical policy for the initial approach to patients presenting with a chief complaint of seizure, who are not in status epilepticus. Ann Emerg Med. 1993;22(5):875-883.
16. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2007;69(18):1772-1780.
17. Montouris GD, Jagoda AS. Management of breakthrough seizures in the emergency department: continuity of patient care. Curr Med Res Opin. 2007;23(7):1583-1592.