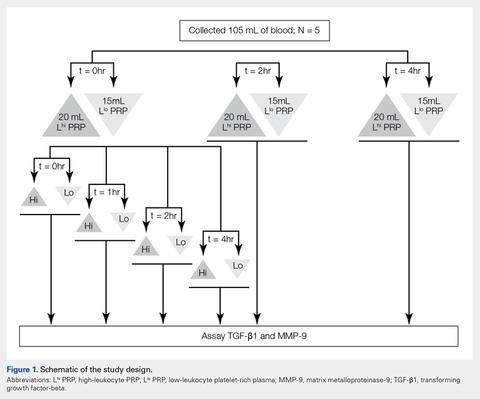
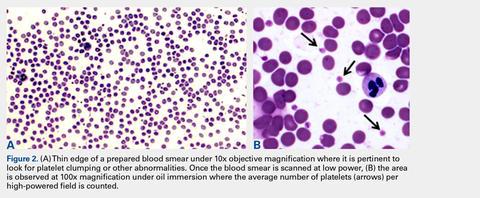
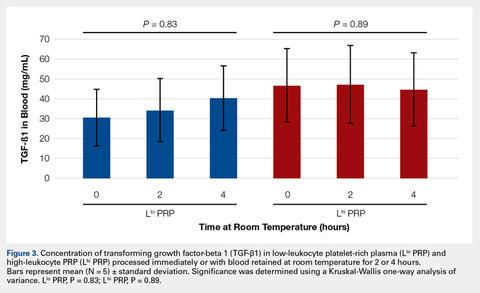
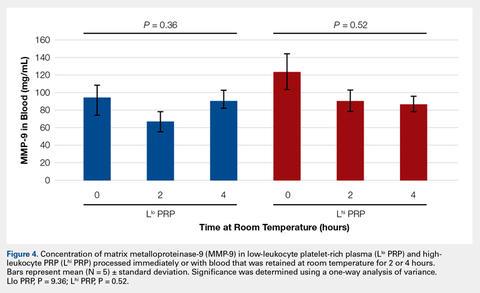
User login
Enhanced recovery led to fewer complications for major oncologic procedures
CHICAGO – Use of an enhanced recovery protocol for oncology patients has been shown to improve outcomes in colorectal surgery but has been largely unproven in other types of major oncology operations. That prompted researchers at the University of Texas MD Anderson Cancer Center in Houston to investigate They found that the enhanced recovery protocol led to a reduction in complication rates and a decrease in hospital stay with no increase in readmissions, according to an analysis of more than 3,000 oncologic operations presented at the Society of Surgical Oncology Annual Cancer Symposium here.
“Patients treated with enhanced recovery did better,” Rebecca Marcus, MD, said in reporting the results. “There were decreased rates of perioperative transfusions, decreased rates of surgical site infections, decreased rates of complications, including severe complications such as wound dehiscence, pneumonia, renal failure, and unintended returns to the operating room.” She noted that the shorter hospital stays – 4 days for patients on the enhanced recovery protocol versus 5 days for those on the traditional postoperative protocol – did not result in increased readmissions.
The study reviewed 3,256 operations performed during 2011-2016 in the MD Anderson institutional American College of Surgeons National Surgical Quality Improvement Program database. The operations were colorectal (20.4%), gynecologic (19.5%), hepatobiliary (8.9%), thoracic (41.9%) and urologic (9.3%). Most employed the traditional postoperative protocol (53.4%). Colorectal and thoracic/vascular surgery were early adopters of the enhanced recovery protocol at MD Anderson.
Dr. Marcus noted that the overall complication rates were 21.9% for those treated with enhanced recovery–protocol versus 33.9% for those treated with traditional postoperative protocol (P less than .0001). The group treated with enhanced recovery protocol also had lower rates of severe complications: 8.7% vs. 11.7% (P =.0048). The study also noted a trend toward reduced National Surgical Quality Improvement Program 30-day mortality with the enhanced recover protocol (0.4% vs. 0.86%; P = .097). Readmission rates were similar between the two groups: 8.3% for enhanced recovery protocol versus 8.9% for traditional postoperative protocol.
The researchers performed a subanalysis of high-magnitude cases that had a relative value unit of 30 or more and involved operations of greater complexity, which constituted 38% of the study population. “In this group, we still saw the benefit of having treatment with an enhanced recovery protocol with decreased rates of preoperative transfusions, complications, and shorter length of stay without any recent readmission,” Dr. Marcus said. Complication rates in the high-magnitude group were 19% for the enhanced recovery protocol versus 26% for the traditional postoperative protocol in colorectal cases, 21% vs. 40% in gynecology, and 19% vs. 28% in thoracic/vascular.
“The beneficial impact of enhanced recovery appears to be maintained across all specialties and to be independent of case magnitude,” Dr. Marcus said.
She said future research of enhanced recovery in surgical oncology should focus on more long-term outcomes, “such as oncologic benefits of these protocols, especially given the known detrimental effect of the delayed return to adjuvant therapy for this patient population.”
Dr. Marcus and her coauthors reported having no financial disclosures.
SOURCE: Marcus RK et al. SSO 2018, Abstract 21.
CHICAGO – Use of an enhanced recovery protocol for oncology patients has been shown to improve outcomes in colorectal surgery but has been largely unproven in other types of major oncology operations. That prompted researchers at the University of Texas MD Anderson Cancer Center in Houston to investigate They found that the enhanced recovery protocol led to a reduction in complication rates and a decrease in hospital stay with no increase in readmissions, according to an analysis of more than 3,000 oncologic operations presented at the Society of Surgical Oncology Annual Cancer Symposium here.
“Patients treated with enhanced recovery did better,” Rebecca Marcus, MD, said in reporting the results. “There were decreased rates of perioperative transfusions, decreased rates of surgical site infections, decreased rates of complications, including severe complications such as wound dehiscence, pneumonia, renal failure, and unintended returns to the operating room.” She noted that the shorter hospital stays – 4 days for patients on the enhanced recovery protocol versus 5 days for those on the traditional postoperative protocol – did not result in increased readmissions.
The study reviewed 3,256 operations performed during 2011-2016 in the MD Anderson institutional American College of Surgeons National Surgical Quality Improvement Program database. The operations were colorectal (20.4%), gynecologic (19.5%), hepatobiliary (8.9%), thoracic (41.9%) and urologic (9.3%). Most employed the traditional postoperative protocol (53.4%). Colorectal and thoracic/vascular surgery were early adopters of the enhanced recovery protocol at MD Anderson.
Dr. Marcus noted that the overall complication rates were 21.9% for those treated with enhanced recovery–protocol versus 33.9% for those treated with traditional postoperative protocol (P less than .0001). The group treated with enhanced recovery protocol also had lower rates of severe complications: 8.7% vs. 11.7% (P =.0048). The study also noted a trend toward reduced National Surgical Quality Improvement Program 30-day mortality with the enhanced recover protocol (0.4% vs. 0.86%; P = .097). Readmission rates were similar between the two groups: 8.3% for enhanced recovery protocol versus 8.9% for traditional postoperative protocol.
The researchers performed a subanalysis of high-magnitude cases that had a relative value unit of 30 or more and involved operations of greater complexity, which constituted 38% of the study population. “In this group, we still saw the benefit of having treatment with an enhanced recovery protocol with decreased rates of preoperative transfusions, complications, and shorter length of stay without any recent readmission,” Dr. Marcus said. Complication rates in the high-magnitude group were 19% for the enhanced recovery protocol versus 26% for the traditional postoperative protocol in colorectal cases, 21% vs. 40% in gynecology, and 19% vs. 28% in thoracic/vascular.
“The beneficial impact of enhanced recovery appears to be maintained across all specialties and to be independent of case magnitude,” Dr. Marcus said.
She said future research of enhanced recovery in surgical oncology should focus on more long-term outcomes, “such as oncologic benefits of these protocols, especially given the known detrimental effect of the delayed return to adjuvant therapy for this patient population.”
Dr. Marcus and her coauthors reported having no financial disclosures.
SOURCE: Marcus RK et al. SSO 2018, Abstract 21.
CHICAGO – Use of an enhanced recovery protocol for oncology patients has been shown to improve outcomes in colorectal surgery but has been largely unproven in other types of major oncology operations. That prompted researchers at the University of Texas MD Anderson Cancer Center in Houston to investigate They found that the enhanced recovery protocol led to a reduction in complication rates and a decrease in hospital stay with no increase in readmissions, according to an analysis of more than 3,000 oncologic operations presented at the Society of Surgical Oncology Annual Cancer Symposium here.
“Patients treated with enhanced recovery did better,” Rebecca Marcus, MD, said in reporting the results. “There were decreased rates of perioperative transfusions, decreased rates of surgical site infections, decreased rates of complications, including severe complications such as wound dehiscence, pneumonia, renal failure, and unintended returns to the operating room.” She noted that the shorter hospital stays – 4 days for patients on the enhanced recovery protocol versus 5 days for those on the traditional postoperative protocol – did not result in increased readmissions.
The study reviewed 3,256 operations performed during 2011-2016 in the MD Anderson institutional American College of Surgeons National Surgical Quality Improvement Program database. The operations were colorectal (20.4%), gynecologic (19.5%), hepatobiliary (8.9%), thoracic (41.9%) and urologic (9.3%). Most employed the traditional postoperative protocol (53.4%). Colorectal and thoracic/vascular surgery were early adopters of the enhanced recovery protocol at MD Anderson.
Dr. Marcus noted that the overall complication rates were 21.9% for those treated with enhanced recovery–protocol versus 33.9% for those treated with traditional postoperative protocol (P less than .0001). The group treated with enhanced recovery protocol also had lower rates of severe complications: 8.7% vs. 11.7% (P =.0048). The study also noted a trend toward reduced National Surgical Quality Improvement Program 30-day mortality with the enhanced recover protocol (0.4% vs. 0.86%; P = .097). Readmission rates were similar between the two groups: 8.3% for enhanced recovery protocol versus 8.9% for traditional postoperative protocol.
The researchers performed a subanalysis of high-magnitude cases that had a relative value unit of 30 or more and involved operations of greater complexity, which constituted 38% of the study population. “In this group, we still saw the benefit of having treatment with an enhanced recovery protocol with decreased rates of preoperative transfusions, complications, and shorter length of stay without any recent readmission,” Dr. Marcus said. Complication rates in the high-magnitude group were 19% for the enhanced recovery protocol versus 26% for the traditional postoperative protocol in colorectal cases, 21% vs. 40% in gynecology, and 19% vs. 28% in thoracic/vascular.
“The beneficial impact of enhanced recovery appears to be maintained across all specialties and to be independent of case magnitude,” Dr. Marcus said.
She said future research of enhanced recovery in surgical oncology should focus on more long-term outcomes, “such as oncologic benefits of these protocols, especially given the known detrimental effect of the delayed return to adjuvant therapy for this patient population.”
Dr. Marcus and her coauthors reported having no financial disclosures.
SOURCE: Marcus RK et al. SSO 2018, Abstract 21.
REPORTING FROM SSO 2018
Key clinical point: Enhanced recovery protocol implementation is feasible in major oncologic surgery.
Major finding: Complication rates were 21.9% for enhanced recovery protocol versus 33.9% for traditional postoperative protocol.
Study details: Analysis of 3,256 oncology operations in an institutional ACS NSQIP database performed from 2011 to 2016.
Disclosures: Dr. Marcus and her coauthors reported having no financial disclosures.
Source: Marcus RK et al. SSO 2018, Abstract 21
High engraftment with new umbilical transplant technique
SALT LAKE CITY – Recipients of hematopoietic cell transplant with umbilical cord blood CD34+ cells expanded with an aryl hydrocarbon receptor (AHR) antagonist had a significantly higher rate of engraftment and comparable survival to a historical cohort of umbilical cord blood recipients.
The robust expansion of donor umbilical cord blood seen with the new technique opens the door for better use of umbilical cord blood inventory with superior human leukocyte antigen (HLA) matching, John Wagner, MD, said at a top abstracts session of the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The new technique still shared the benefits of low rates of graft-versus-host disease (GVHD) and high survival that have been seen in previous umbilical cord blood transplants, with no significant difference in overall survival, relapse, or acute or chronic GVHD.
Compared to historical controls (n = 151), patients receiving the AHR antagonist–expanded umbilical cord blood (UCB) cells with myeloablative conditioning (n = 9) saw complete and more rapid engraftment (100% vs. 89% engraftment at a median 14 days vs. 23 days; P less than .01), reported Dr. Wagner of the University of Minnesota, Minneapolis.
These and other results came from two arms of a phase 2 trial of MGTA-456 (the working name of the AHR-expanded UCB cells). Twenty patients were to receive MGTA-456 derived from partially matched umbilical cord blood units after either myeloablative or nonmyeloablative conditioning; one patient in each arm had low expansion of UCB, so a total of 18 patients received MGTA-456. Each intervention arm was compared with a historical control arm that had received conventional UCB units.
In the myeloablative arm, patient demographics and disease characteristics were similar to the control cohort except that the MGTA-456 patients were significantly heavier (93.8 kg vs. 66.7 kg; P less than .04).
Platelet recovery also rebounded faster with MGTA-456 plus myeloablative conditioning than it did with historical controls: 89% of patients had platelet recovery by a median 46 days, compared with 71% with platelet recovery by a median 64 days in the historical cohort (P less than .01).
Patients achieved rapid complete chimerism if they received myeloablative conditioning, and they had rapid rebound of CD4 counts to at least 200 by 2-3 months posttransplant, Dr. Wagner reported.
The nonmyeloablative arm had a historical control cohort of 132 patients. Characteristics were similar between the two groups except that the MGTA-456 patients were older and more likely to have high-risk disease.
Again, all patients had rapid neutrophil recovery and saw 100% engraftment with MGTA-456. Median time to engraftment was 7 days with MGTA-456 and 15 days for the historical controls (P less than .01). Platelet recovery took longer for the MGTA-456 (median of 47 vs. 107 days), but the difference was not statistically significant.
Complete chimerism was achieved rapidly with the nonmyeloablative regimen as well, and CD4 recovery was brisk, as had been seen with myeloablative conditioning before MGTA-456 transplantation.
Compared with historical controls, “MGTA-456 retains the benefits of low chronic-graft-versus host disease and high survival despite higher disease risk and age” in the study group, Dr. Wagner said. There were no significant differences between the intervention and historical control arms of the nonmyeloablative study in acute or chronic GVHD, relapse, or overall survival.
The use of MGTA-456 occurs against the backdrop of a history of high survival rates with UCB transplantation – about 70% at 5 years, Dr. Wagner said. However, when conventional culture and expansion methods for UCB were used, the median time to engraftment had been reported to be 25 days with a 79% engraftment rate. This contrasts with the mean 13 days to engraftment for peripheral blood transplants and 18 days for bone marrow transplants. All of these transplant sources, regardless of whether the transplant was matched or mismatched, have engraftment rates of 92%-96%, said Dr. Wagner (Lancet Oncol. 2010; 11[7]:653-60).
When an AHR antagonist is used for UCB expansion, hematopoietic stem cell renewal is upped because cell differentiation is blocked, which means expansion is all driven toward hematopoietic stem cell self-renewal, Dr. Wagner said. Of the 36 available samples, MGTA-456 achieved a median 327-fold expansion of CD34+ cells, which enabled investigators to deliver a median CD34+ dose of 17.5 X 106 cells/kg.
The downstream effect of the robust expansion rates is that more cord blood will be available for transplantation, and HLA matches will improve, Dr. Wagner said. Using current expansion techniques, fewer than 5% of cord blood units have a total nucleated cell count sufficient for an adult 80 kg recipient, he said, adding that use of MGTA-456 could make more than 80% of cord blood units available for adults.
According to the UCB transplant history at the University of Minnesota – where Dr. Wagner directs the pediatric blood and marrow transplantation program – of the patients who received 4/6 HLA-matched cord blood, 63% would move to a 5/6 match, and 8% would move to a full HLA match with the MGTA-456 technique. Of patients who received 5/6-matched transplants, almost one in four (23%) would move to a full 6/6 match.
Dr. Wagner and his colleagues had previously shown that adding an AHR antagonist resulted in enhanced T-cell recovery and rapid and sustained engraftment (Science. 2010;329:1345-8).
The researchers then proceeded to a phase 1-2, first-in-human trial of MGTA-456 that used a myeloablative conditioning regimen that met its primary safety endpoint of a lack of infusional toxicity or primary/secondary graft failure (Cell Stem Cell. 2016;18:144-55).
For reasons of safety, this earlier study used a double-transplant platform in which one infusion was uncultured umbilical cord blood and the other was MGTA-456. This study showed rapid neutrophil recovery when MGTA-456 was infused, with median 10.5 days to recovery, compared with a median 26.5 days for historical controls (P less than .001).
Additionally, the study showed a 19-day decrease in duration of the initial hospitalization, and all patients who received MGTA-456 had successful engraftment, Dr. Wagner said. On the strength of these results, the current trials of MGTA-456 alone – with both nonmyeloablative and myeloablative conditioning – were approved.
Multicenter clinical trials of MGTA-456 transplantation are now planned for both malignant and nonmalignant diseases. Enrollment is currently open for a phase 2 clinical trial of MGTA for inherited metabolic disorders (NCT03406962).
The study was funded by Novartis and Magenta Therapeutics. Dr. Wagner reported no other relevant disclosures.
SOURCE: Wagner J et al. 2018 BMT Tandem Meetings, Abstract 4.
SALT LAKE CITY – Recipients of hematopoietic cell transplant with umbilical cord blood CD34+ cells expanded with an aryl hydrocarbon receptor (AHR) antagonist had a significantly higher rate of engraftment and comparable survival to a historical cohort of umbilical cord blood recipients.
The robust expansion of donor umbilical cord blood seen with the new technique opens the door for better use of umbilical cord blood inventory with superior human leukocyte antigen (HLA) matching, John Wagner, MD, said at a top abstracts session of the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The new technique still shared the benefits of low rates of graft-versus-host disease (GVHD) and high survival that have been seen in previous umbilical cord blood transplants, with no significant difference in overall survival, relapse, or acute or chronic GVHD.
Compared to historical controls (n = 151), patients receiving the AHR antagonist–expanded umbilical cord blood (UCB) cells with myeloablative conditioning (n = 9) saw complete and more rapid engraftment (100% vs. 89% engraftment at a median 14 days vs. 23 days; P less than .01), reported Dr. Wagner of the University of Minnesota, Minneapolis.
These and other results came from two arms of a phase 2 trial of MGTA-456 (the working name of the AHR-expanded UCB cells). Twenty patients were to receive MGTA-456 derived from partially matched umbilical cord blood units after either myeloablative or nonmyeloablative conditioning; one patient in each arm had low expansion of UCB, so a total of 18 patients received MGTA-456. Each intervention arm was compared with a historical control arm that had received conventional UCB units.
In the myeloablative arm, patient demographics and disease characteristics were similar to the control cohort except that the MGTA-456 patients were significantly heavier (93.8 kg vs. 66.7 kg; P less than .04).
Platelet recovery also rebounded faster with MGTA-456 plus myeloablative conditioning than it did with historical controls: 89% of patients had platelet recovery by a median 46 days, compared with 71% with platelet recovery by a median 64 days in the historical cohort (P less than .01).
Patients achieved rapid complete chimerism if they received myeloablative conditioning, and they had rapid rebound of CD4 counts to at least 200 by 2-3 months posttransplant, Dr. Wagner reported.
The nonmyeloablative arm had a historical control cohort of 132 patients. Characteristics were similar between the two groups except that the MGTA-456 patients were older and more likely to have high-risk disease.
Again, all patients had rapid neutrophil recovery and saw 100% engraftment with MGTA-456. Median time to engraftment was 7 days with MGTA-456 and 15 days for the historical controls (P less than .01). Platelet recovery took longer for the MGTA-456 (median of 47 vs. 107 days), but the difference was not statistically significant.
Complete chimerism was achieved rapidly with the nonmyeloablative regimen as well, and CD4 recovery was brisk, as had been seen with myeloablative conditioning before MGTA-456 transplantation.
Compared with historical controls, “MGTA-456 retains the benefits of low chronic-graft-versus host disease and high survival despite higher disease risk and age” in the study group, Dr. Wagner said. There were no significant differences between the intervention and historical control arms of the nonmyeloablative study in acute or chronic GVHD, relapse, or overall survival.
The use of MGTA-456 occurs against the backdrop of a history of high survival rates with UCB transplantation – about 70% at 5 years, Dr. Wagner said. However, when conventional culture and expansion methods for UCB were used, the median time to engraftment had been reported to be 25 days with a 79% engraftment rate. This contrasts with the mean 13 days to engraftment for peripheral blood transplants and 18 days for bone marrow transplants. All of these transplant sources, regardless of whether the transplant was matched or mismatched, have engraftment rates of 92%-96%, said Dr. Wagner (Lancet Oncol. 2010; 11[7]:653-60).
When an AHR antagonist is used for UCB expansion, hematopoietic stem cell renewal is upped because cell differentiation is blocked, which means expansion is all driven toward hematopoietic stem cell self-renewal, Dr. Wagner said. Of the 36 available samples, MGTA-456 achieved a median 327-fold expansion of CD34+ cells, which enabled investigators to deliver a median CD34+ dose of 17.5 X 106 cells/kg.
The downstream effect of the robust expansion rates is that more cord blood will be available for transplantation, and HLA matches will improve, Dr. Wagner said. Using current expansion techniques, fewer than 5% of cord blood units have a total nucleated cell count sufficient for an adult 80 kg recipient, he said, adding that use of MGTA-456 could make more than 80% of cord blood units available for adults.
According to the UCB transplant history at the University of Minnesota – where Dr. Wagner directs the pediatric blood and marrow transplantation program – of the patients who received 4/6 HLA-matched cord blood, 63% would move to a 5/6 match, and 8% would move to a full HLA match with the MGTA-456 technique. Of patients who received 5/6-matched transplants, almost one in four (23%) would move to a full 6/6 match.
Dr. Wagner and his colleagues had previously shown that adding an AHR antagonist resulted in enhanced T-cell recovery and rapid and sustained engraftment (Science. 2010;329:1345-8).
The researchers then proceeded to a phase 1-2, first-in-human trial of MGTA-456 that used a myeloablative conditioning regimen that met its primary safety endpoint of a lack of infusional toxicity or primary/secondary graft failure (Cell Stem Cell. 2016;18:144-55).
For reasons of safety, this earlier study used a double-transplant platform in which one infusion was uncultured umbilical cord blood and the other was MGTA-456. This study showed rapid neutrophil recovery when MGTA-456 was infused, with median 10.5 days to recovery, compared with a median 26.5 days for historical controls (P less than .001).
Additionally, the study showed a 19-day decrease in duration of the initial hospitalization, and all patients who received MGTA-456 had successful engraftment, Dr. Wagner said. On the strength of these results, the current trials of MGTA-456 alone – with both nonmyeloablative and myeloablative conditioning – were approved.
Multicenter clinical trials of MGTA-456 transplantation are now planned for both malignant and nonmalignant diseases. Enrollment is currently open for a phase 2 clinical trial of MGTA for inherited metabolic disorders (NCT03406962).
The study was funded by Novartis and Magenta Therapeutics. Dr. Wagner reported no other relevant disclosures.
SOURCE: Wagner J et al. 2018 BMT Tandem Meetings, Abstract 4.
SALT LAKE CITY – Recipients of hematopoietic cell transplant with umbilical cord blood CD34+ cells expanded with an aryl hydrocarbon receptor (AHR) antagonist had a significantly higher rate of engraftment and comparable survival to a historical cohort of umbilical cord blood recipients.
The robust expansion of donor umbilical cord blood seen with the new technique opens the door for better use of umbilical cord blood inventory with superior human leukocyte antigen (HLA) matching, John Wagner, MD, said at a top abstracts session of the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The new technique still shared the benefits of low rates of graft-versus-host disease (GVHD) and high survival that have been seen in previous umbilical cord blood transplants, with no significant difference in overall survival, relapse, or acute or chronic GVHD.
Compared to historical controls (n = 151), patients receiving the AHR antagonist–expanded umbilical cord blood (UCB) cells with myeloablative conditioning (n = 9) saw complete and more rapid engraftment (100% vs. 89% engraftment at a median 14 days vs. 23 days; P less than .01), reported Dr. Wagner of the University of Minnesota, Minneapolis.
These and other results came from two arms of a phase 2 trial of MGTA-456 (the working name of the AHR-expanded UCB cells). Twenty patients were to receive MGTA-456 derived from partially matched umbilical cord blood units after either myeloablative or nonmyeloablative conditioning; one patient in each arm had low expansion of UCB, so a total of 18 patients received MGTA-456. Each intervention arm was compared with a historical control arm that had received conventional UCB units.
In the myeloablative arm, patient demographics and disease characteristics were similar to the control cohort except that the MGTA-456 patients were significantly heavier (93.8 kg vs. 66.7 kg; P less than .04).
Platelet recovery also rebounded faster with MGTA-456 plus myeloablative conditioning than it did with historical controls: 89% of patients had platelet recovery by a median 46 days, compared with 71% with platelet recovery by a median 64 days in the historical cohort (P less than .01).
Patients achieved rapid complete chimerism if they received myeloablative conditioning, and they had rapid rebound of CD4 counts to at least 200 by 2-3 months posttransplant, Dr. Wagner reported.
The nonmyeloablative arm had a historical control cohort of 132 patients. Characteristics were similar between the two groups except that the MGTA-456 patients were older and more likely to have high-risk disease.
Again, all patients had rapid neutrophil recovery and saw 100% engraftment with MGTA-456. Median time to engraftment was 7 days with MGTA-456 and 15 days for the historical controls (P less than .01). Platelet recovery took longer for the MGTA-456 (median of 47 vs. 107 days), but the difference was not statistically significant.
Complete chimerism was achieved rapidly with the nonmyeloablative regimen as well, and CD4 recovery was brisk, as had been seen with myeloablative conditioning before MGTA-456 transplantation.
Compared with historical controls, “MGTA-456 retains the benefits of low chronic-graft-versus host disease and high survival despite higher disease risk and age” in the study group, Dr. Wagner said. There were no significant differences between the intervention and historical control arms of the nonmyeloablative study in acute or chronic GVHD, relapse, or overall survival.
The use of MGTA-456 occurs against the backdrop of a history of high survival rates with UCB transplantation – about 70% at 5 years, Dr. Wagner said. However, when conventional culture and expansion methods for UCB were used, the median time to engraftment had been reported to be 25 days with a 79% engraftment rate. This contrasts with the mean 13 days to engraftment for peripheral blood transplants and 18 days for bone marrow transplants. All of these transplant sources, regardless of whether the transplant was matched or mismatched, have engraftment rates of 92%-96%, said Dr. Wagner (Lancet Oncol. 2010; 11[7]:653-60).
When an AHR antagonist is used for UCB expansion, hematopoietic stem cell renewal is upped because cell differentiation is blocked, which means expansion is all driven toward hematopoietic stem cell self-renewal, Dr. Wagner said. Of the 36 available samples, MGTA-456 achieved a median 327-fold expansion of CD34+ cells, which enabled investigators to deliver a median CD34+ dose of 17.5 X 106 cells/kg.
The downstream effect of the robust expansion rates is that more cord blood will be available for transplantation, and HLA matches will improve, Dr. Wagner said. Using current expansion techniques, fewer than 5% of cord blood units have a total nucleated cell count sufficient for an adult 80 kg recipient, he said, adding that use of MGTA-456 could make more than 80% of cord blood units available for adults.
According to the UCB transplant history at the University of Minnesota – where Dr. Wagner directs the pediatric blood and marrow transplantation program – of the patients who received 4/6 HLA-matched cord blood, 63% would move to a 5/6 match, and 8% would move to a full HLA match with the MGTA-456 technique. Of patients who received 5/6-matched transplants, almost one in four (23%) would move to a full 6/6 match.
Dr. Wagner and his colleagues had previously shown that adding an AHR antagonist resulted in enhanced T-cell recovery and rapid and sustained engraftment (Science. 2010;329:1345-8).
The researchers then proceeded to a phase 1-2, first-in-human trial of MGTA-456 that used a myeloablative conditioning regimen that met its primary safety endpoint of a lack of infusional toxicity or primary/secondary graft failure (Cell Stem Cell. 2016;18:144-55).
For reasons of safety, this earlier study used a double-transplant platform in which one infusion was uncultured umbilical cord blood and the other was MGTA-456. This study showed rapid neutrophil recovery when MGTA-456 was infused, with median 10.5 days to recovery, compared with a median 26.5 days for historical controls (P less than .001).
Additionally, the study showed a 19-day decrease in duration of the initial hospitalization, and all patients who received MGTA-456 had successful engraftment, Dr. Wagner said. On the strength of these results, the current trials of MGTA-456 alone – with both nonmyeloablative and myeloablative conditioning – were approved.
Multicenter clinical trials of MGTA-456 transplantation are now planned for both malignant and nonmalignant diseases. Enrollment is currently open for a phase 2 clinical trial of MGTA for inherited metabolic disorders (NCT03406962).
The study was funded by Novartis and Magenta Therapeutics. Dr. Wagner reported no other relevant disclosures.
SOURCE: Wagner J et al. 2018 BMT Tandem Meetings, Abstract 4.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: The engraftment rate was 100% with both myeloablative and nonmyeloablative conditioning.
Study details: A phase 2 trial of 20 patients receiving MGTA-456, compared with a historical cohort of umbilical cord blood hematopoietic cell transplant recipients.
Disclosures: The study was sponsored by Novartis and Magenta Therapeutics. Dr. Wagner reported no other conflicts of interest.
Source: Wagner J et al. 2018 BMT Tandem Meetings, Abstract 4.
Drug appears to aid chemo in AML
Adding an experimental compound to chemotherapy is a “promising” treatment approach for certain patients with acute myeloid leukemia (AML), according to researchers.
They tested the compound, CPI-613, in combination with high-dose cytarabine and mitoxantrone in a phase 1 trial of patients with relapsed or refractory AML.
The combination produced similar response rates in the overall patient population (50%), patients age 60 and older (47%), and those with poor-risk cytogenetics (46%).
The most common grade 3/4 adverse events (AEs) were hematologic toxicities, and there was 1 fatal AE—hypotension.
Mortality rates in this trial were similar to those observed in historical controls treated with high-dose cytarabine, mitoxantrone, and asparaginase.
“These data are very encouraging, especially for patients 60 years of age or older who have historically done very poorly with this disease,” said Timothy Pardee, MD, PhD, a professor at Wake Forest Baptist Health in Winston-Salem, North Carolina, and chief medical officer of Rafael Pharmaceuticals, Inc., the company developing CPI-613.
Dr Pardee and his colleagues reported these results in Clinical Cancer Research.
The researchers noted that CPI-613 is designed to target mitochondrial metabolism in cancer cells, and preclinical research showed that CPI-613 sensitized AML cells to chemotherapy.
To investigate this further, the team tested CPI-613 in combination with high-dose cytarabine and mitoxantrone in the phase 1 trial. The study included 66 patients with relapsed or refractory AML, as well as a patient with advanced-phase chronic myeloid leukemia (CML) who was mistakenly enrolled.
The patients’ median age was 60 (range, 21-79), and 54% were age 60 and older. Their median percentage of marrow blasts was 43%. Forty percent of patients had poor-risk cytogenetics, and 49% had intermediate-risk cytogenetics.
Most patients (72%) had no prior salvage therapy, 13% had 1 prior line of salvage, 10% had 2 prior lines, and 4% had more than 2. Thirty-one percent of patients had refractory disease.
Seven percent of patients had previously received high-dose cytarabine and mitoxantrone, and 25% had previous salvage including high-dose or intermediate-dose cytarabine.
Treatment
Patients received CPI-613, given over 2 hours, on days 1 to 5 of cycle 1. Doses ranged from 500 mg/m2 to 2750 mg/m2.
Starting on day 3, patients received 5 doses of cytarabine at 3 gm/m2 (for patients younger than 60) or 1.5 gm/m2 (for older patients) in 500 mL normal saline, over 3 hours, every 12 hours.
Patients also received 3 daily doses of mitoxantrone at 6 mg/m2 in 50 mL normal saline, given over 15 minutes, after the first, third, and fifth doses of cytarabine.
Patients were initially assigned to receive 1 cycle of treatment. Those with at least 5% blasts after the first cycle could receive a second course—either a full course or a 3-day course. And patients who responded to the first course could receive up to 2 cycles of the 3-day course.
Safety
There were 2 dose-limiting toxicities when CPI-613 was given at the 2750 mg/m2 dose. One of these toxicities was grade 3 diarrhea that didn’t respond to anti-diarrheals, and the other was grade 3 nausea that didn’t respond to antiemetics.
Because of these events, 2500 mg/m2 was deemed the maximum-tolerated dose. However, the recommended phase 2 dose is 2000 mg/m2.
The most common AEs—occurring in at least 50% of all patients who received CPI-613 (n=67)—included hemoglobin decrease (67%), hyperglycemia (67%), neutropenia (67%), thrombocytopenia (67%), hypomagnesemia (66%), leukopenia (66%), lymphopenia (66%), hypoalbuminemia (65%), hypokalemia (60%), hypocalcemia (57%), and diarrhea (55%).
All cases of neutropenia, thrombocytopenia, leukopenia, and lymphopenia were grade 3/4. Other common grade 3/4 AEs (occurring in at least 20% of patients) included hemoglobin decrease (62%), febrile neutropenia (28%), hypophosphatemia (24%), and hypokalemia (23%).
The only grade 5 AE was hypotension.
The mortality rate was 12% (n=8) at 30 days and 19% (n=13) at 60 days. The researchers said this was similar to the historical experience with high-dose cytarabine, mitoxantrone, and asparaginase. Mortality rates with this regimen were 13% at 30 days and 22% at 60 days.
Efficacy
Sixty-two patients were evaluable for response. Of the 5 patients who were not evaluable, 1 didn’t complete the first cycle of treatment, 1 was the CML patient, and 3 died before assessment.
The overall response rate was 50% (31/62). This included 26 patients with a complete response (CR) and 5 patients who had a CR with incomplete count recovery (CRi).
The rate of CR/CRi was 47% (15/32) in patients older than 60 years of age, 46% (11/24) in patients who had poor-risk cytogenetics, and 53% (8/15) when CPI-613 was given at the recommended phase 2 dose—2000 mg/m2.
The median overall survival (OS) was 6.7 months for all evaluable patients and 13.2 months for patients who achieved a CR/CRi.
The median OS was 6.9 months for patients age 60 and older, which was not significantly different from the median OS in younger patients (P=0.9642).
This study was sponsored by Wake Forest University Health Sciences and the National Cancer Institute.
Adding an experimental compound to chemotherapy is a “promising” treatment approach for certain patients with acute myeloid leukemia (AML), according to researchers.
They tested the compound, CPI-613, in combination with high-dose cytarabine and mitoxantrone in a phase 1 trial of patients with relapsed or refractory AML.
The combination produced similar response rates in the overall patient population (50%), patients age 60 and older (47%), and those with poor-risk cytogenetics (46%).
The most common grade 3/4 adverse events (AEs) were hematologic toxicities, and there was 1 fatal AE—hypotension.
Mortality rates in this trial were similar to those observed in historical controls treated with high-dose cytarabine, mitoxantrone, and asparaginase.
“These data are very encouraging, especially for patients 60 years of age or older who have historically done very poorly with this disease,” said Timothy Pardee, MD, PhD, a professor at Wake Forest Baptist Health in Winston-Salem, North Carolina, and chief medical officer of Rafael Pharmaceuticals, Inc., the company developing CPI-613.
Dr Pardee and his colleagues reported these results in Clinical Cancer Research.
The researchers noted that CPI-613 is designed to target mitochondrial metabolism in cancer cells, and preclinical research showed that CPI-613 sensitized AML cells to chemotherapy.
To investigate this further, the team tested CPI-613 in combination with high-dose cytarabine and mitoxantrone in the phase 1 trial. The study included 66 patients with relapsed or refractory AML, as well as a patient with advanced-phase chronic myeloid leukemia (CML) who was mistakenly enrolled.
The patients’ median age was 60 (range, 21-79), and 54% were age 60 and older. Their median percentage of marrow blasts was 43%. Forty percent of patients had poor-risk cytogenetics, and 49% had intermediate-risk cytogenetics.
Most patients (72%) had no prior salvage therapy, 13% had 1 prior line of salvage, 10% had 2 prior lines, and 4% had more than 2. Thirty-one percent of patients had refractory disease.
Seven percent of patients had previously received high-dose cytarabine and mitoxantrone, and 25% had previous salvage including high-dose or intermediate-dose cytarabine.
Treatment
Patients received CPI-613, given over 2 hours, on days 1 to 5 of cycle 1. Doses ranged from 500 mg/m2 to 2750 mg/m2.
Starting on day 3, patients received 5 doses of cytarabine at 3 gm/m2 (for patients younger than 60) or 1.5 gm/m2 (for older patients) in 500 mL normal saline, over 3 hours, every 12 hours.
Patients also received 3 daily doses of mitoxantrone at 6 mg/m2 in 50 mL normal saline, given over 15 minutes, after the first, third, and fifth doses of cytarabine.
Patients were initially assigned to receive 1 cycle of treatment. Those with at least 5% blasts after the first cycle could receive a second course—either a full course or a 3-day course. And patients who responded to the first course could receive up to 2 cycles of the 3-day course.
Safety
There were 2 dose-limiting toxicities when CPI-613 was given at the 2750 mg/m2 dose. One of these toxicities was grade 3 diarrhea that didn’t respond to anti-diarrheals, and the other was grade 3 nausea that didn’t respond to antiemetics.
Because of these events, 2500 mg/m2 was deemed the maximum-tolerated dose. However, the recommended phase 2 dose is 2000 mg/m2.
The most common AEs—occurring in at least 50% of all patients who received CPI-613 (n=67)—included hemoglobin decrease (67%), hyperglycemia (67%), neutropenia (67%), thrombocytopenia (67%), hypomagnesemia (66%), leukopenia (66%), lymphopenia (66%), hypoalbuminemia (65%), hypokalemia (60%), hypocalcemia (57%), and diarrhea (55%).
All cases of neutropenia, thrombocytopenia, leukopenia, and lymphopenia were grade 3/4. Other common grade 3/4 AEs (occurring in at least 20% of patients) included hemoglobin decrease (62%), febrile neutropenia (28%), hypophosphatemia (24%), and hypokalemia (23%).
The only grade 5 AE was hypotension.
The mortality rate was 12% (n=8) at 30 days and 19% (n=13) at 60 days. The researchers said this was similar to the historical experience with high-dose cytarabine, mitoxantrone, and asparaginase. Mortality rates with this regimen were 13% at 30 days and 22% at 60 days.
Efficacy
Sixty-two patients were evaluable for response. Of the 5 patients who were not evaluable, 1 didn’t complete the first cycle of treatment, 1 was the CML patient, and 3 died before assessment.
The overall response rate was 50% (31/62). This included 26 patients with a complete response (CR) and 5 patients who had a CR with incomplete count recovery (CRi).
The rate of CR/CRi was 47% (15/32) in patients older than 60 years of age, 46% (11/24) in patients who had poor-risk cytogenetics, and 53% (8/15) when CPI-613 was given at the recommended phase 2 dose—2000 mg/m2.
The median overall survival (OS) was 6.7 months for all evaluable patients and 13.2 months for patients who achieved a CR/CRi.
The median OS was 6.9 months for patients age 60 and older, which was not significantly different from the median OS in younger patients (P=0.9642).
This study was sponsored by Wake Forest University Health Sciences and the National Cancer Institute.
Adding an experimental compound to chemotherapy is a “promising” treatment approach for certain patients with acute myeloid leukemia (AML), according to researchers.
They tested the compound, CPI-613, in combination with high-dose cytarabine and mitoxantrone in a phase 1 trial of patients with relapsed or refractory AML.
The combination produced similar response rates in the overall patient population (50%), patients age 60 and older (47%), and those with poor-risk cytogenetics (46%).
The most common grade 3/4 adverse events (AEs) were hematologic toxicities, and there was 1 fatal AE—hypotension.
Mortality rates in this trial were similar to those observed in historical controls treated with high-dose cytarabine, mitoxantrone, and asparaginase.
“These data are very encouraging, especially for patients 60 years of age or older who have historically done very poorly with this disease,” said Timothy Pardee, MD, PhD, a professor at Wake Forest Baptist Health in Winston-Salem, North Carolina, and chief medical officer of Rafael Pharmaceuticals, Inc., the company developing CPI-613.
Dr Pardee and his colleagues reported these results in Clinical Cancer Research.
The researchers noted that CPI-613 is designed to target mitochondrial metabolism in cancer cells, and preclinical research showed that CPI-613 sensitized AML cells to chemotherapy.
To investigate this further, the team tested CPI-613 in combination with high-dose cytarabine and mitoxantrone in the phase 1 trial. The study included 66 patients with relapsed or refractory AML, as well as a patient with advanced-phase chronic myeloid leukemia (CML) who was mistakenly enrolled.
The patients’ median age was 60 (range, 21-79), and 54% were age 60 and older. Their median percentage of marrow blasts was 43%. Forty percent of patients had poor-risk cytogenetics, and 49% had intermediate-risk cytogenetics.
Most patients (72%) had no prior salvage therapy, 13% had 1 prior line of salvage, 10% had 2 prior lines, and 4% had more than 2. Thirty-one percent of patients had refractory disease.
Seven percent of patients had previously received high-dose cytarabine and mitoxantrone, and 25% had previous salvage including high-dose or intermediate-dose cytarabine.
Treatment
Patients received CPI-613, given over 2 hours, on days 1 to 5 of cycle 1. Doses ranged from 500 mg/m2 to 2750 mg/m2.
Starting on day 3, patients received 5 doses of cytarabine at 3 gm/m2 (for patients younger than 60) or 1.5 gm/m2 (for older patients) in 500 mL normal saline, over 3 hours, every 12 hours.
Patients also received 3 daily doses of mitoxantrone at 6 mg/m2 in 50 mL normal saline, given over 15 minutes, after the first, third, and fifth doses of cytarabine.
Patients were initially assigned to receive 1 cycle of treatment. Those with at least 5% blasts after the first cycle could receive a second course—either a full course or a 3-day course. And patients who responded to the first course could receive up to 2 cycles of the 3-day course.
Safety
There were 2 dose-limiting toxicities when CPI-613 was given at the 2750 mg/m2 dose. One of these toxicities was grade 3 diarrhea that didn’t respond to anti-diarrheals, and the other was grade 3 nausea that didn’t respond to antiemetics.
Because of these events, 2500 mg/m2 was deemed the maximum-tolerated dose. However, the recommended phase 2 dose is 2000 mg/m2.
The most common AEs—occurring in at least 50% of all patients who received CPI-613 (n=67)—included hemoglobin decrease (67%), hyperglycemia (67%), neutropenia (67%), thrombocytopenia (67%), hypomagnesemia (66%), leukopenia (66%), lymphopenia (66%), hypoalbuminemia (65%), hypokalemia (60%), hypocalcemia (57%), and diarrhea (55%).
All cases of neutropenia, thrombocytopenia, leukopenia, and lymphopenia were grade 3/4. Other common grade 3/4 AEs (occurring in at least 20% of patients) included hemoglobin decrease (62%), febrile neutropenia (28%), hypophosphatemia (24%), and hypokalemia (23%).
The only grade 5 AE was hypotension.
The mortality rate was 12% (n=8) at 30 days and 19% (n=13) at 60 days. The researchers said this was similar to the historical experience with high-dose cytarabine, mitoxantrone, and asparaginase. Mortality rates with this regimen were 13% at 30 days and 22% at 60 days.
Efficacy
Sixty-two patients were evaluable for response. Of the 5 patients who were not evaluable, 1 didn’t complete the first cycle of treatment, 1 was the CML patient, and 3 died before assessment.
The overall response rate was 50% (31/62). This included 26 patients with a complete response (CR) and 5 patients who had a CR with incomplete count recovery (CRi).
The rate of CR/CRi was 47% (15/32) in patients older than 60 years of age, 46% (11/24) in patients who had poor-risk cytogenetics, and 53% (8/15) when CPI-613 was given at the recommended phase 2 dose—2000 mg/m2.
The median overall survival (OS) was 6.7 months for all evaluable patients and 13.2 months for patients who achieved a CR/CRi.
The median OS was 6.9 months for patients age 60 and older, which was not significantly different from the median OS in younger patients (P=0.9642).
This study was sponsored by Wake Forest University Health Sciences and the National Cancer Institute.
Dermatology practice gaps: improving medication management
KAUAI, HAWAII – Dermatologists don’t ordinarily peruse the ophthalmology literature. So they may be unaware that the American Academy of Ophthalmology has issued Erik J. Stratman, MD, noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Most dermatologists routinely dose hydroxychloroquine at 400 mg/day, regardless of body weight. The former AAO recommendation, which dates back to 2011, called for dosing at up to 6.5 mg/kg of ideal body weight or 400 mg/day, whichever is lower. However, the AAO recommendation has changed in light of a large, retrospective case-control study that suggested this practice may be overdosing thin patients – thereby exposing them to increased risk of retinal toxicity and other drug-related adverse events – while at the same time possibly underdosing some obese patients, said Dr. Stratman, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
This was one of two dermatology practice gaps he highlighted involving suboptimal medication management, the other being most dermatologists’ failure to protect their patients’ gut when prescribing prednisone.
“I think the push over the last 5 years has been ‘protect the bones, protect the bones, protect the bones.’ We’ve done better and better about protecting the bones and getting that into our conversations with patients on prednisone. But we’re not thinking so much about the gut,” the dermatologist said.
Hydroxychloroquine dosing
The former AAO recommendation was revised in response to a retrospective case-control study of retinal toxicity rates in 2,361 patients on the drug continuously for longer than 5 years. The study demonstrated that the risk of retinopathy jumped 5.7-fold with daily consumption of hydroxychloroquine at more than 5.0 mg/kg (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60).
The current AAO recommendation (Ophthalmology. 2016 Jun;123[6]:1386-94) is to dose hydroxychloroquine at a daily maximum of 5.0 mg/kg of real weight, which correlated better with retinopathy risk in the case-control study than did ideal body weight. Hydroxychloroquine doesn’t accumulate well in fat.
Until now, most dermatologists have not routinely measured patients’ body weight in the office or calculated their body mass index. But Dr. Stratman advised against reliance upon a patient’s self-reported body weight, which may diverge substantially from reality. “Get yourself a good office scale – they’re not that expensive – and use it when prescribing drugs with a tight therapeutic window,” he urged.
Another key to minimizing retinopathy risk in patients on hydroxychloroquine is to pay careful attention to how long they’ve been on the drug. As the years go by in patients being treated for cutaneous lupus or other dermatologic disorders where decades-long therapy is often a mainstay, it’s important to check with patients and make sure they’re getting annual ophthalmologic screening for irreversible retinal toxicity by both threshold visual fields and spectral domain optical coherence tomography. In the large, practice-changing retrospective study, patients on hydroxychloroquine at 4.0-5.0 mg/kg daily had a prevalence of retinopathy of less than 2% during the first 10 years of therapy, but the rate shot up to nearly 20% after 20 years of use, Dr. Stratman observed.
He highlighted as helpful an updated review of the use of hydroxychloroquine in dermatology recently published by Anthony P. Fernandez, MD, PhD, of the department of dermatology at the Cleveland Clinic (J Am Acad Dermatol. 2017 Jun;76[6]:1176-82).
Dr. Fernandez recommends following the AAO guidance to dose the drug at 5.0 mg/kg or less of actual body weight in thin or normal-weight patients; however, he departed from the ophthalmologists with regard to treatment of obese patients. Because dosing based on actual weight could potentially lead to relative overdosing in obese patients, in that growing population he recommends calculating the dose based upon 5.0 mg/kg of actual body weight, as well as the dose based on 6.5 mg/kg of ideal body weight, then prescribing the lower of the two, up to a maximum of 400 mg/day.
“The current recommendation is really about not overdosing thin patients. Basically, dosing is not so difficult for obese people because if you weigh more than 175 pounds, you’re going to get 400 mg/day,” Dr. Stratman explained.
That 400 mg/day ceiling is not cast in stone, he continued. The guideline recommends that, if a patient is a nonresponder to several months of hydroxychloroquine at 400 mg/day, it’s worthwhile to order a drug blood level. If it’s not above the efficacy threshold of more than 750 ng/mL, it’s appropriate to titrate up.
Protecting against prednisone-induced gastritis
“We underprotect the gut,” Dr. Stratman asserted.
He referred to a recent comprehensive dermatologic review of the prevention and management of glucocorticoid-related side effects, especially the part on peptic ulcer disease (J Am Acad Dermatol. 2017 Jan;76[1]:11-6). This is an issue that heretofore hadn’t been much emphasized in the dermatology literature.
“I read this and thought, ‘Gosh, I’m not really having a conversation with my patients about a review of systems for gut protection as I should. And I certainly haven’t been thinking about prescribing PPIs [proton pump inhibitors] for my patients,’” he recalled.
Dr. Stratman polled his Hawaii Dermatology Seminar audience as to who had ever prescribed a PPI. Most indicated with their electronic clickers that they had never done so.
“This is what a practice gap is,” he commented. “You read the literature and you say, ‘Oh, I guess that makes sense. Maybe I should be doing that more often, or making sure it gets done.’”
“I don’t want to come across as saying, ‘For everybody we put on prednisone we should be giving vitamin D, calcium, and a PPI.’ That’s not the message. The message is, assess your patient – or make sure your patient is being assessed – for risk of peptic ulcer disease. And if you don’t feel comfortable prescribing a PPI, please get the patient connected with their primary care provider, who should,” Dr. Stratman said.
The authors of the dermatology review made a case for screening for GI risk factors in every patient who is going to receive an oral glucocorticoid. The ones who absolutely should be prescribed a PPI unless contraindicated include patients who are taking daily aspirin or NSAIDs for an essential reason, such as cardiovascular protection or significant arthritic pain. The authors suggest consideration of a PPI in patients with other, less potent risk factors for peptic ulcer disease, including a history of ulcer disease, gastroesophageal reflux disease, Barrett’s esophagus, heavy smoking, heavy alcohol consumption, age greater than 65, and concomitant use of other medications with an associated risk of peptic ulcer disease – such as bisphosphonates, “which you may have just put them on to protect their bones,” Dr. Stratman noted.
Of course, PPIs come with side effects of their own, including increased fracture risk, Clostridium difficile infections, and rebound acid secretion.
Dr. Stratman reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Dermatologists don’t ordinarily peruse the ophthalmology literature. So they may be unaware that the American Academy of Ophthalmology has issued Erik J. Stratman, MD, noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Most dermatologists routinely dose hydroxychloroquine at 400 mg/day, regardless of body weight. The former AAO recommendation, which dates back to 2011, called for dosing at up to 6.5 mg/kg of ideal body weight or 400 mg/day, whichever is lower. However, the AAO recommendation has changed in light of a large, retrospective case-control study that suggested this practice may be overdosing thin patients – thereby exposing them to increased risk of retinal toxicity and other drug-related adverse events – while at the same time possibly underdosing some obese patients, said Dr. Stratman, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
This was one of two dermatology practice gaps he highlighted involving suboptimal medication management, the other being most dermatologists’ failure to protect their patients’ gut when prescribing prednisone.
“I think the push over the last 5 years has been ‘protect the bones, protect the bones, protect the bones.’ We’ve done better and better about protecting the bones and getting that into our conversations with patients on prednisone. But we’re not thinking so much about the gut,” the dermatologist said.
Hydroxychloroquine dosing
The former AAO recommendation was revised in response to a retrospective case-control study of retinal toxicity rates in 2,361 patients on the drug continuously for longer than 5 years. The study demonstrated that the risk of retinopathy jumped 5.7-fold with daily consumption of hydroxychloroquine at more than 5.0 mg/kg (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60).
The current AAO recommendation (Ophthalmology. 2016 Jun;123[6]:1386-94) is to dose hydroxychloroquine at a daily maximum of 5.0 mg/kg of real weight, which correlated better with retinopathy risk in the case-control study than did ideal body weight. Hydroxychloroquine doesn’t accumulate well in fat.
Until now, most dermatologists have not routinely measured patients’ body weight in the office or calculated their body mass index. But Dr. Stratman advised against reliance upon a patient’s self-reported body weight, which may diverge substantially from reality. “Get yourself a good office scale – they’re not that expensive – and use it when prescribing drugs with a tight therapeutic window,” he urged.
Another key to minimizing retinopathy risk in patients on hydroxychloroquine is to pay careful attention to how long they’ve been on the drug. As the years go by in patients being treated for cutaneous lupus or other dermatologic disorders where decades-long therapy is often a mainstay, it’s important to check with patients and make sure they’re getting annual ophthalmologic screening for irreversible retinal toxicity by both threshold visual fields and spectral domain optical coherence tomography. In the large, practice-changing retrospective study, patients on hydroxychloroquine at 4.0-5.0 mg/kg daily had a prevalence of retinopathy of less than 2% during the first 10 years of therapy, but the rate shot up to nearly 20% after 20 years of use, Dr. Stratman observed.
He highlighted as helpful an updated review of the use of hydroxychloroquine in dermatology recently published by Anthony P. Fernandez, MD, PhD, of the department of dermatology at the Cleveland Clinic (J Am Acad Dermatol. 2017 Jun;76[6]:1176-82).
Dr. Fernandez recommends following the AAO guidance to dose the drug at 5.0 mg/kg or less of actual body weight in thin or normal-weight patients; however, he departed from the ophthalmologists with regard to treatment of obese patients. Because dosing based on actual weight could potentially lead to relative overdosing in obese patients, in that growing population he recommends calculating the dose based upon 5.0 mg/kg of actual body weight, as well as the dose based on 6.5 mg/kg of ideal body weight, then prescribing the lower of the two, up to a maximum of 400 mg/day.
“The current recommendation is really about not overdosing thin patients. Basically, dosing is not so difficult for obese people because if you weigh more than 175 pounds, you’re going to get 400 mg/day,” Dr. Stratman explained.
That 400 mg/day ceiling is not cast in stone, he continued. The guideline recommends that, if a patient is a nonresponder to several months of hydroxychloroquine at 400 mg/day, it’s worthwhile to order a drug blood level. If it’s not above the efficacy threshold of more than 750 ng/mL, it’s appropriate to titrate up.
Protecting against prednisone-induced gastritis
“We underprotect the gut,” Dr. Stratman asserted.
He referred to a recent comprehensive dermatologic review of the prevention and management of glucocorticoid-related side effects, especially the part on peptic ulcer disease (J Am Acad Dermatol. 2017 Jan;76[1]:11-6). This is an issue that heretofore hadn’t been much emphasized in the dermatology literature.
“I read this and thought, ‘Gosh, I’m not really having a conversation with my patients about a review of systems for gut protection as I should. And I certainly haven’t been thinking about prescribing PPIs [proton pump inhibitors] for my patients,’” he recalled.
Dr. Stratman polled his Hawaii Dermatology Seminar audience as to who had ever prescribed a PPI. Most indicated with their electronic clickers that they had never done so.
“This is what a practice gap is,” he commented. “You read the literature and you say, ‘Oh, I guess that makes sense. Maybe I should be doing that more often, or making sure it gets done.’”
“I don’t want to come across as saying, ‘For everybody we put on prednisone we should be giving vitamin D, calcium, and a PPI.’ That’s not the message. The message is, assess your patient – or make sure your patient is being assessed – for risk of peptic ulcer disease. And if you don’t feel comfortable prescribing a PPI, please get the patient connected with their primary care provider, who should,” Dr. Stratman said.
The authors of the dermatology review made a case for screening for GI risk factors in every patient who is going to receive an oral glucocorticoid. The ones who absolutely should be prescribed a PPI unless contraindicated include patients who are taking daily aspirin or NSAIDs for an essential reason, such as cardiovascular protection or significant arthritic pain. The authors suggest consideration of a PPI in patients with other, less potent risk factors for peptic ulcer disease, including a history of ulcer disease, gastroesophageal reflux disease, Barrett’s esophagus, heavy smoking, heavy alcohol consumption, age greater than 65, and concomitant use of other medications with an associated risk of peptic ulcer disease – such as bisphosphonates, “which you may have just put them on to protect their bones,” Dr. Stratman noted.
Of course, PPIs come with side effects of their own, including increased fracture risk, Clostridium difficile infections, and rebound acid secretion.
Dr. Stratman reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Dermatologists don’t ordinarily peruse the ophthalmology literature. So they may be unaware that the American Academy of Ophthalmology has issued Erik J. Stratman, MD, noted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Most dermatologists routinely dose hydroxychloroquine at 400 mg/day, regardless of body weight. The former AAO recommendation, which dates back to 2011, called for dosing at up to 6.5 mg/kg of ideal body weight or 400 mg/day, whichever is lower. However, the AAO recommendation has changed in light of a large, retrospective case-control study that suggested this practice may be overdosing thin patients – thereby exposing them to increased risk of retinal toxicity and other drug-related adverse events – while at the same time possibly underdosing some obese patients, said Dr. Stratman, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
This was one of two dermatology practice gaps he highlighted involving suboptimal medication management, the other being most dermatologists’ failure to protect their patients’ gut when prescribing prednisone.
“I think the push over the last 5 years has been ‘protect the bones, protect the bones, protect the bones.’ We’ve done better and better about protecting the bones and getting that into our conversations with patients on prednisone. But we’re not thinking so much about the gut,” the dermatologist said.
Hydroxychloroquine dosing
The former AAO recommendation was revised in response to a retrospective case-control study of retinal toxicity rates in 2,361 patients on the drug continuously for longer than 5 years. The study demonstrated that the risk of retinopathy jumped 5.7-fold with daily consumption of hydroxychloroquine at more than 5.0 mg/kg (JAMA Ophthalmol. 2014 Dec;132[12]:1453-60).
The current AAO recommendation (Ophthalmology. 2016 Jun;123[6]:1386-94) is to dose hydroxychloroquine at a daily maximum of 5.0 mg/kg of real weight, which correlated better with retinopathy risk in the case-control study than did ideal body weight. Hydroxychloroquine doesn’t accumulate well in fat.
Until now, most dermatologists have not routinely measured patients’ body weight in the office or calculated their body mass index. But Dr. Stratman advised against reliance upon a patient’s self-reported body weight, which may diverge substantially from reality. “Get yourself a good office scale – they’re not that expensive – and use it when prescribing drugs with a tight therapeutic window,” he urged.
Another key to minimizing retinopathy risk in patients on hydroxychloroquine is to pay careful attention to how long they’ve been on the drug. As the years go by in patients being treated for cutaneous lupus or other dermatologic disorders where decades-long therapy is often a mainstay, it’s important to check with patients and make sure they’re getting annual ophthalmologic screening for irreversible retinal toxicity by both threshold visual fields and spectral domain optical coherence tomography. In the large, practice-changing retrospective study, patients on hydroxychloroquine at 4.0-5.0 mg/kg daily had a prevalence of retinopathy of less than 2% during the first 10 years of therapy, but the rate shot up to nearly 20% after 20 years of use, Dr. Stratman observed.
He highlighted as helpful an updated review of the use of hydroxychloroquine in dermatology recently published by Anthony P. Fernandez, MD, PhD, of the department of dermatology at the Cleveland Clinic (J Am Acad Dermatol. 2017 Jun;76[6]:1176-82).
Dr. Fernandez recommends following the AAO guidance to dose the drug at 5.0 mg/kg or less of actual body weight in thin or normal-weight patients; however, he departed from the ophthalmologists with regard to treatment of obese patients. Because dosing based on actual weight could potentially lead to relative overdosing in obese patients, in that growing population he recommends calculating the dose based upon 5.0 mg/kg of actual body weight, as well as the dose based on 6.5 mg/kg of ideal body weight, then prescribing the lower of the two, up to a maximum of 400 mg/day.
“The current recommendation is really about not overdosing thin patients. Basically, dosing is not so difficult for obese people because if you weigh more than 175 pounds, you’re going to get 400 mg/day,” Dr. Stratman explained.
That 400 mg/day ceiling is not cast in stone, he continued. The guideline recommends that, if a patient is a nonresponder to several months of hydroxychloroquine at 400 mg/day, it’s worthwhile to order a drug blood level. If it’s not above the efficacy threshold of more than 750 ng/mL, it’s appropriate to titrate up.
Protecting against prednisone-induced gastritis
“We underprotect the gut,” Dr. Stratman asserted.
He referred to a recent comprehensive dermatologic review of the prevention and management of glucocorticoid-related side effects, especially the part on peptic ulcer disease (J Am Acad Dermatol. 2017 Jan;76[1]:11-6). This is an issue that heretofore hadn’t been much emphasized in the dermatology literature.
“I read this and thought, ‘Gosh, I’m not really having a conversation with my patients about a review of systems for gut protection as I should. And I certainly haven’t been thinking about prescribing PPIs [proton pump inhibitors] for my patients,’” he recalled.
Dr. Stratman polled his Hawaii Dermatology Seminar audience as to who had ever prescribed a PPI. Most indicated with their electronic clickers that they had never done so.
“This is what a practice gap is,” he commented. “You read the literature and you say, ‘Oh, I guess that makes sense. Maybe I should be doing that more often, or making sure it gets done.’”
“I don’t want to come across as saying, ‘For everybody we put on prednisone we should be giving vitamin D, calcium, and a PPI.’ That’s not the message. The message is, assess your patient – or make sure your patient is being assessed – for risk of peptic ulcer disease. And if you don’t feel comfortable prescribing a PPI, please get the patient connected with their primary care provider, who should,” Dr. Stratman said.
The authors of the dermatology review made a case for screening for GI risk factors in every patient who is going to receive an oral glucocorticoid. The ones who absolutely should be prescribed a PPI unless contraindicated include patients who are taking daily aspirin or NSAIDs for an essential reason, such as cardiovascular protection or significant arthritic pain. The authors suggest consideration of a PPI in patients with other, less potent risk factors for peptic ulcer disease, including a history of ulcer disease, gastroesophageal reflux disease, Barrett’s esophagus, heavy smoking, heavy alcohol consumption, age greater than 65, and concomitant use of other medications with an associated risk of peptic ulcer disease – such as bisphosphonates, “which you may have just put them on to protect their bones,” Dr. Stratman noted.
Of course, PPIs come with side effects of their own, including increased fracture risk, Clostridium difficile infections, and rebound acid secretion.
Dr. Stratman reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Indoor tanning tax revenues continue to fall
since 2011, despite increased overall spending on services by consumers, according to investigators from the University of Pennsylvania.
Since the Affordable Care Act was passed in 2010 with a 10% excise tax on indoor tanning services, consumer spending, as reflected by collections of that tax, has dropped 13% – from $86.3 million in 2011 to $75.1 in 2016. The amount collected actually increased in 2012 and 2013, when revenue was $91.7 million, making the drop since that peak an even larger 18.1%. Overall personal spending on services, in the meantime, rose steadily from $7.1 billion in 2011 to $8.7 billion in 2016, or 22.6%, Kishore L. Jayakumar and Jules B. Lipoff, MD, said in JAMA Dermatology.
These findings, when combined with earlier surveys demonstrating “that the prevalence of indoor tanning had been increasing as late as 2009 [imply] a trend reversal coinciding with the tax’s implementation,” but they do not definitively show causation and should be considered “secondary to the public health objective of deterring indoor tanning,” the investigators wrote.
Mr. Jayakumar reported receiving “enrolled agent” status from the Internal Revenue Service. They did not report any other conflicts.
SOURCE: Jayakumar KL et al. JAMA Dermatol. 2018 Apr 11. doi: 10.1001/jamadermatol.2018.0161.
since 2011, despite increased overall spending on services by consumers, according to investigators from the University of Pennsylvania.
Since the Affordable Care Act was passed in 2010 with a 10% excise tax on indoor tanning services, consumer spending, as reflected by collections of that tax, has dropped 13% – from $86.3 million in 2011 to $75.1 in 2016. The amount collected actually increased in 2012 and 2013, when revenue was $91.7 million, making the drop since that peak an even larger 18.1%. Overall personal spending on services, in the meantime, rose steadily from $7.1 billion in 2011 to $8.7 billion in 2016, or 22.6%, Kishore L. Jayakumar and Jules B. Lipoff, MD, said in JAMA Dermatology.
These findings, when combined with earlier surveys demonstrating “that the prevalence of indoor tanning had been increasing as late as 2009 [imply] a trend reversal coinciding with the tax’s implementation,” but they do not definitively show causation and should be considered “secondary to the public health objective of deterring indoor tanning,” the investigators wrote.
Mr. Jayakumar reported receiving “enrolled agent” status from the Internal Revenue Service. They did not report any other conflicts.
SOURCE: Jayakumar KL et al. JAMA Dermatol. 2018 Apr 11. doi: 10.1001/jamadermatol.2018.0161.
since 2011, despite increased overall spending on services by consumers, according to investigators from the University of Pennsylvania.
Since the Affordable Care Act was passed in 2010 with a 10% excise tax on indoor tanning services, consumer spending, as reflected by collections of that tax, has dropped 13% – from $86.3 million in 2011 to $75.1 in 2016. The amount collected actually increased in 2012 and 2013, when revenue was $91.7 million, making the drop since that peak an even larger 18.1%. Overall personal spending on services, in the meantime, rose steadily from $7.1 billion in 2011 to $8.7 billion in 2016, or 22.6%, Kishore L. Jayakumar and Jules B. Lipoff, MD, said in JAMA Dermatology.
These findings, when combined with earlier surveys demonstrating “that the prevalence of indoor tanning had been increasing as late as 2009 [imply] a trend reversal coinciding with the tax’s implementation,” but they do not definitively show causation and should be considered “secondary to the public health objective of deterring indoor tanning,” the investigators wrote.
Mr. Jayakumar reported receiving “enrolled agent” status from the Internal Revenue Service. They did not report any other conflicts.
SOURCE: Jayakumar KL et al. JAMA Dermatol. 2018 Apr 11. doi: 10.1001/jamadermatol.2018.0161.
FROM JAMA DERMATOLOGY
Baseline Symptoms May Predict Psychosis in Parkinson’s Disease
REM sleep behavior disorder (RBD), excessive daytime sleepiness (EDS), and a high burden of autonomic symptoms are associated with increased risk of future psychotic symptoms in patients with Parkinson’s disease, according to research published online ahead of print April 4 in Neurology. These symptoms also correlate with low density of the cholinergic nucleus 4 (Ch4) of the basal forebrain.
Data From the Parkinson’s Progression Markers Initiative
Parkinson’s disease psychosis indicates advanced disease and is associated with dementia and increased mortality. Research indicates that degeneration of the nucleus basalis of Meynert is characteristic of Parkinson’s disease and Parkinson’s disease dementia, but this brain region is difficult to measure with MRI because of its size. It is possible, however, to measure Ch4, which incorporates the nucleus basalis of Meynert.
Matthew J. Barrett, MD, Assistant Professor of Neurology at the University of Virginia in Charlottesville, and colleagues studied a cohort of patients with de novo Parkinson’s disease to identify baseline clinical risk factors for future psychotic symptoms and to assess the relationship between baseline Ch4 density and future psychotic symptoms. All participants were untreated at baseline and had enrolled in the Parkinson’s Progression Markers Initiative (PPMI), a prospective, longitudinal, observational study.
Physicians assessed participants with the Movement Disorders Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) at each visit. Item 1.2 assesses hallucinations and psychosis during the previous week. The investigators considered any score above 0 on this item to indicate the presence of psychotic symptoms.
Investigators evaluated patients’ autonomic symptoms with the Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) and administered the RBD screening questionnaire and the Epworth Sleepiness Scale. They determined the density of each participant’s basal forebrain using brain MRI sequences that they obtained from the PPMI database.
Ch4 Density Could Predict Psychotic Symptoms
The investigators included 423 participants in their analysis. The population’s mean age at enrollment was approximately 61.5, and about 35% of the population was female. Mean disease duration was 0.57 years. The population’s median number of clinical visits was 11, and the median last visit occurred at 54 months after baseline.
In all, 138 participants (32.6%) reported psychotic symptoms at least once, and 84 (19.9%) reported them more than once. Dr. Barrett and colleagues identified 17 patients who, having once reported psychotic symptoms, reported these symptoms at each subsequent visit. Among patients who reported psychotic symptoms, approximately 79% reported them at only one visit.
The investigators categorized patients into three groups according to the number of psychotic events. The groups included participants with no psychotic events, those with one psychotic event, and those with two or more psychotic events.
After performing multivariate logistic regression and adjusting the results for age and sex, Dr. Barrett and colleagues found that greater autonomic symptoms (odds ratio [OR], 1.07 for a one-unit change in SCOPA-AUT), the presence of RBD (OR, 1.9), and EDS (OR, 2.5) at baseline were associated with increased risk of reporting psychotic symptoms on two or more occasions, compared to no or one psychotic event.
A logistic regression model adjusted for age and sex indicated that greater autonomic symptoms (OR, 1.08 for a one-unit change in SCOPA-AUT) and EDS (OR, 1.8) at baseline were associated with increased risk of psychotic symptoms on one or more occasions. A Cox regression model adjusted for EDS (hazard ratio [HR], 1.5) indicated that greater autonomic symptoms (HR, 1.03 for a one-unit change in SCOPA-AUT) and presence of RBD (HR, 1.47) were associated with increased risk of having a first psychotic event.
At the last assessment, patients with Parkinson’s disease and two or more psychotic events were more likely to have the postural instability and gait difficulty motor phenotype and lower scores on the Montreal Cognitive Assessment, Letter Number Sequencing test, and Symbol Digit Modalities Test.
The investigators had data about cholinergic nuclei densities at baseline for 228 participants with Parkinson’s disease and 101 controls. Ch4 density in Parkinson’s disease was associated with lower risk of reporting psychotic symptoms on two or more occasions (OR, 0.96 for an increase in density of one unit). Mean Ch4 densities were 1.7% greater for the control group, compared with the Parkinson’s disease group. Participants with Parkinson’s disease and two or more psychotic events had lower baseline Ch4 density, compared with healthy controls, but participants with Parkinson’s disease with no or one psychotic event did not.
In linear regression models adjusted for age and sex, autonomic symptoms were associated with baseline Ch4 density, but EDS and RBD were not. Having comorbid EDS and RBD was associated with lower Ch4 density.
Results Do Not Establish Causation
“Prior studies reported that EDS was associated with hallucinations in Parkinson’s disease, but we are not aware of other studies that found EDS to be a predictor of future psychotic symptoms,” said Dr. Barrett and colleagues.
“In univariate comparisons, participants with Parkinson’s disease with three or more psychotic events had worse visuospatial function, compared with those with two psychotic events. Considering the greater impairment in visuospatial and visuoperceptive function reported in Parkinson’s disease with visual hallucinations, this association should be evaluated in the future in the PPMI Parkinson’s disease cohort as additional follow-up visits occur.”
The current study’s finding of an association between autonomic dysfunction, RBD, and EDS and baseline Ch4 density in Parkinson’s disease is consistent with a recent analysis that found that the same symptoms were associated with a diffuse malignant subtype of Parkinson’s disease, said the authors. “Our finding that these three nonmotor symptoms are linked to future psychotic symptoms validates the prognostic value of these symptoms in predicting worse outcomes early in disease.”
Evidence does not support a causal relationship between lower Ch4 density and autonomic dysfunction, RBD, and EDS. These four characteristics may be associated with widespread subcortical pathology. “The relationship between this triad of clinical symptoms and lower Ch4 density supports the potential utility of this neuroimaging biomarker to identify a diffuse malignant subtype of Parkinson’s disease and to predict more rapid disease progression,” said Dr. Barrett and colleagues.
—Erik Greb
Suggested Reading
Barrett MJ, Blair JC, Sperling SA, et al. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. 2018 Apr 4 [Epub ahead of print].
Bohnen NI, Teipel SJ. Cholinergic forebrain density loss in Parkinson disease: More than just cognitive changes. Neurology. 2018 Apr 4 [Epub ahead of print].
REM sleep behavior disorder (RBD), excessive daytime sleepiness (EDS), and a high burden of autonomic symptoms are associated with increased risk of future psychotic symptoms in patients with Parkinson’s disease, according to research published online ahead of print April 4 in Neurology. These symptoms also correlate with low density of the cholinergic nucleus 4 (Ch4) of the basal forebrain.
Data From the Parkinson’s Progression Markers Initiative
Parkinson’s disease psychosis indicates advanced disease and is associated with dementia and increased mortality. Research indicates that degeneration of the nucleus basalis of Meynert is characteristic of Parkinson’s disease and Parkinson’s disease dementia, but this brain region is difficult to measure with MRI because of its size. It is possible, however, to measure Ch4, which incorporates the nucleus basalis of Meynert.
Matthew J. Barrett, MD, Assistant Professor of Neurology at the University of Virginia in Charlottesville, and colleagues studied a cohort of patients with de novo Parkinson’s disease to identify baseline clinical risk factors for future psychotic symptoms and to assess the relationship between baseline Ch4 density and future psychotic symptoms. All participants were untreated at baseline and had enrolled in the Parkinson’s Progression Markers Initiative (PPMI), a prospective, longitudinal, observational study.
Physicians assessed participants with the Movement Disorders Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) at each visit. Item 1.2 assesses hallucinations and psychosis during the previous week. The investigators considered any score above 0 on this item to indicate the presence of psychotic symptoms.
Investigators evaluated patients’ autonomic symptoms with the Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) and administered the RBD screening questionnaire and the Epworth Sleepiness Scale. They determined the density of each participant’s basal forebrain using brain MRI sequences that they obtained from the PPMI database.
Ch4 Density Could Predict Psychotic Symptoms
The investigators included 423 participants in their analysis. The population’s mean age at enrollment was approximately 61.5, and about 35% of the population was female. Mean disease duration was 0.57 years. The population’s median number of clinical visits was 11, and the median last visit occurred at 54 months after baseline.
In all, 138 participants (32.6%) reported psychotic symptoms at least once, and 84 (19.9%) reported them more than once. Dr. Barrett and colleagues identified 17 patients who, having once reported psychotic symptoms, reported these symptoms at each subsequent visit. Among patients who reported psychotic symptoms, approximately 79% reported them at only one visit.
The investigators categorized patients into three groups according to the number of psychotic events. The groups included participants with no psychotic events, those with one psychotic event, and those with two or more psychotic events.
After performing multivariate logistic regression and adjusting the results for age and sex, Dr. Barrett and colleagues found that greater autonomic symptoms (odds ratio [OR], 1.07 for a one-unit change in SCOPA-AUT), the presence of RBD (OR, 1.9), and EDS (OR, 2.5) at baseline were associated with increased risk of reporting psychotic symptoms on two or more occasions, compared to no or one psychotic event.
A logistic regression model adjusted for age and sex indicated that greater autonomic symptoms (OR, 1.08 for a one-unit change in SCOPA-AUT) and EDS (OR, 1.8) at baseline were associated with increased risk of psychotic symptoms on one or more occasions. A Cox regression model adjusted for EDS (hazard ratio [HR], 1.5) indicated that greater autonomic symptoms (HR, 1.03 for a one-unit change in SCOPA-AUT) and presence of RBD (HR, 1.47) were associated with increased risk of having a first psychotic event.
At the last assessment, patients with Parkinson’s disease and two or more psychotic events were more likely to have the postural instability and gait difficulty motor phenotype and lower scores on the Montreal Cognitive Assessment, Letter Number Sequencing test, and Symbol Digit Modalities Test.
The investigators had data about cholinergic nuclei densities at baseline for 228 participants with Parkinson’s disease and 101 controls. Ch4 density in Parkinson’s disease was associated with lower risk of reporting psychotic symptoms on two or more occasions (OR, 0.96 for an increase in density of one unit). Mean Ch4 densities were 1.7% greater for the control group, compared with the Parkinson’s disease group. Participants with Parkinson’s disease and two or more psychotic events had lower baseline Ch4 density, compared with healthy controls, but participants with Parkinson’s disease with no or one psychotic event did not.
In linear regression models adjusted for age and sex, autonomic symptoms were associated with baseline Ch4 density, but EDS and RBD were not. Having comorbid EDS and RBD was associated with lower Ch4 density.
Results Do Not Establish Causation
“Prior studies reported that EDS was associated with hallucinations in Parkinson’s disease, but we are not aware of other studies that found EDS to be a predictor of future psychotic symptoms,” said Dr. Barrett and colleagues.
“In univariate comparisons, participants with Parkinson’s disease with three or more psychotic events had worse visuospatial function, compared with those with two psychotic events. Considering the greater impairment in visuospatial and visuoperceptive function reported in Parkinson’s disease with visual hallucinations, this association should be evaluated in the future in the PPMI Parkinson’s disease cohort as additional follow-up visits occur.”
The current study’s finding of an association between autonomic dysfunction, RBD, and EDS and baseline Ch4 density in Parkinson’s disease is consistent with a recent analysis that found that the same symptoms were associated with a diffuse malignant subtype of Parkinson’s disease, said the authors. “Our finding that these three nonmotor symptoms are linked to future psychotic symptoms validates the prognostic value of these symptoms in predicting worse outcomes early in disease.”
Evidence does not support a causal relationship between lower Ch4 density and autonomic dysfunction, RBD, and EDS. These four characteristics may be associated with widespread subcortical pathology. “The relationship between this triad of clinical symptoms and lower Ch4 density supports the potential utility of this neuroimaging biomarker to identify a diffuse malignant subtype of Parkinson’s disease and to predict more rapid disease progression,” said Dr. Barrett and colleagues.
—Erik Greb
Suggested Reading
Barrett MJ, Blair JC, Sperling SA, et al. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. 2018 Apr 4 [Epub ahead of print].
Bohnen NI, Teipel SJ. Cholinergic forebrain density loss in Parkinson disease: More than just cognitive changes. Neurology. 2018 Apr 4 [Epub ahead of print].
REM sleep behavior disorder (RBD), excessive daytime sleepiness (EDS), and a high burden of autonomic symptoms are associated with increased risk of future psychotic symptoms in patients with Parkinson’s disease, according to research published online ahead of print April 4 in Neurology. These symptoms also correlate with low density of the cholinergic nucleus 4 (Ch4) of the basal forebrain.
Data From the Parkinson’s Progression Markers Initiative
Parkinson’s disease psychosis indicates advanced disease and is associated with dementia and increased mortality. Research indicates that degeneration of the nucleus basalis of Meynert is characteristic of Parkinson’s disease and Parkinson’s disease dementia, but this brain region is difficult to measure with MRI because of its size. It is possible, however, to measure Ch4, which incorporates the nucleus basalis of Meynert.
Matthew J. Barrett, MD, Assistant Professor of Neurology at the University of Virginia in Charlottesville, and colleagues studied a cohort of patients with de novo Parkinson’s disease to identify baseline clinical risk factors for future psychotic symptoms and to assess the relationship between baseline Ch4 density and future psychotic symptoms. All participants were untreated at baseline and had enrolled in the Parkinson’s Progression Markers Initiative (PPMI), a prospective, longitudinal, observational study.
Physicians assessed participants with the Movement Disorders Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) at each visit. Item 1.2 assesses hallucinations and psychosis during the previous week. The investigators considered any score above 0 on this item to indicate the presence of psychotic symptoms.
Investigators evaluated patients’ autonomic symptoms with the Scales for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT) and administered the RBD screening questionnaire and the Epworth Sleepiness Scale. They determined the density of each participant’s basal forebrain using brain MRI sequences that they obtained from the PPMI database.
Ch4 Density Could Predict Psychotic Symptoms
The investigators included 423 participants in their analysis. The population’s mean age at enrollment was approximately 61.5, and about 35% of the population was female. Mean disease duration was 0.57 years. The population’s median number of clinical visits was 11, and the median last visit occurred at 54 months after baseline.
In all, 138 participants (32.6%) reported psychotic symptoms at least once, and 84 (19.9%) reported them more than once. Dr. Barrett and colleagues identified 17 patients who, having once reported psychotic symptoms, reported these symptoms at each subsequent visit. Among patients who reported psychotic symptoms, approximately 79% reported them at only one visit.
The investigators categorized patients into three groups according to the number of psychotic events. The groups included participants with no psychotic events, those with one psychotic event, and those with two or more psychotic events.
After performing multivariate logistic regression and adjusting the results for age and sex, Dr. Barrett and colleagues found that greater autonomic symptoms (odds ratio [OR], 1.07 for a one-unit change in SCOPA-AUT), the presence of RBD (OR, 1.9), and EDS (OR, 2.5) at baseline were associated with increased risk of reporting psychotic symptoms on two or more occasions, compared to no or one psychotic event.
A logistic regression model adjusted for age and sex indicated that greater autonomic symptoms (OR, 1.08 for a one-unit change in SCOPA-AUT) and EDS (OR, 1.8) at baseline were associated with increased risk of psychotic symptoms on one or more occasions. A Cox regression model adjusted for EDS (hazard ratio [HR], 1.5) indicated that greater autonomic symptoms (HR, 1.03 for a one-unit change in SCOPA-AUT) and presence of RBD (HR, 1.47) were associated with increased risk of having a first psychotic event.
At the last assessment, patients with Parkinson’s disease and two or more psychotic events were more likely to have the postural instability and gait difficulty motor phenotype and lower scores on the Montreal Cognitive Assessment, Letter Number Sequencing test, and Symbol Digit Modalities Test.
The investigators had data about cholinergic nuclei densities at baseline for 228 participants with Parkinson’s disease and 101 controls. Ch4 density in Parkinson’s disease was associated with lower risk of reporting psychotic symptoms on two or more occasions (OR, 0.96 for an increase in density of one unit). Mean Ch4 densities were 1.7% greater for the control group, compared with the Parkinson’s disease group. Participants with Parkinson’s disease and two or more psychotic events had lower baseline Ch4 density, compared with healthy controls, but participants with Parkinson’s disease with no or one psychotic event did not.
In linear regression models adjusted for age and sex, autonomic symptoms were associated with baseline Ch4 density, but EDS and RBD were not. Having comorbid EDS and RBD was associated with lower Ch4 density.
Results Do Not Establish Causation
“Prior studies reported that EDS was associated with hallucinations in Parkinson’s disease, but we are not aware of other studies that found EDS to be a predictor of future psychotic symptoms,” said Dr. Barrett and colleagues.
“In univariate comparisons, participants with Parkinson’s disease with three or more psychotic events had worse visuospatial function, compared with those with two psychotic events. Considering the greater impairment in visuospatial and visuoperceptive function reported in Parkinson’s disease with visual hallucinations, this association should be evaluated in the future in the PPMI Parkinson’s disease cohort as additional follow-up visits occur.”
The current study’s finding of an association between autonomic dysfunction, RBD, and EDS and baseline Ch4 density in Parkinson’s disease is consistent with a recent analysis that found that the same symptoms were associated with a diffuse malignant subtype of Parkinson’s disease, said the authors. “Our finding that these three nonmotor symptoms are linked to future psychotic symptoms validates the prognostic value of these symptoms in predicting worse outcomes early in disease.”
Evidence does not support a causal relationship between lower Ch4 density and autonomic dysfunction, RBD, and EDS. These four characteristics may be associated with widespread subcortical pathology. “The relationship between this triad of clinical symptoms and lower Ch4 density supports the potential utility of this neuroimaging biomarker to identify a diffuse malignant subtype of Parkinson’s disease and to predict more rapid disease progression,” said Dr. Barrett and colleagues.
—Erik Greb
Suggested Reading
Barrett MJ, Blair JC, Sperling SA, et al. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. 2018 Apr 4 [Epub ahead of print].
Bohnen NI, Teipel SJ. Cholinergic forebrain density loss in Parkinson disease: More than just cognitive changes. Neurology. 2018 Apr 4 [Epub ahead of print].
Common infections are potent risk factor for MI, stroke
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
REPORTING FROM ACC 2018
Key clinical point: Once patients have been hospitalized for a respiratory infection or UTI, their postdischarge risk of ischemic stroke is 2.5-fold greater than in those without such a history.
Major finding: Patients with a history of hospitalization for UTI or a respiratory infection who later develop ischemic heart disease or stroke have a threefold higher mortality risk than those without such a hospitalization.
Study details: This was a retrospective study of more than 68,000 subjects in the U.K. ACALM registry study.
Disclosures: The study presenter reported having no financial conflicts of interest.
Source: Carter P. ACC 2018, Abstract 1325M-0.
Carboxytherapy caused small yet transient subcutaneous fat reduction
DALLAS – , a small randomized, controlled trial demonstrated.
“Carboxytherapy has been previously used therapeutically to reduce fat, and studies of carboxytherapy have been done in the past as well,” the study’s senior author, Murad Alam, MD, said in an interview in advance of a poster session at the annual conference of the American Society for Laser Medicine and Surgery Inc. “However, earlier studies have not been controlled trials.”
For the split-body study, he and his associates enrolled 16 adults and randomized one side of their body to receive infusions of 1,000 cc of CO2 every week for 5 weeks in the flank region, while the contralateral side received sham treatments. Outcomes of interest included fat layer thickness using a diagnostic ultrasound, total circumference, and body weight, said Dr. Alam, vice-chair of the department of dermatology and chief of the division of cutaneous and aesthetic surgery at Northwestern University, Chicago. He reported that a significant difference in fat thickness was observed in the carboxytherapy-treated side 1 week after the last treatment (P = .011), but this difference was not maintained at 28 weeks as measured by diagnostic ultrasound. Total circumference decreased nominally but not significantly. Body weights did not significantly change throughout the study.
Dr. Alam acknowledged that the study’s small sample size is a limitation. “We do think we did enough treatments, five per side, to obtain an effect if one were to be seen,” he said. The researchers reported having no financial disclosures.
DALLAS – , a small randomized, controlled trial demonstrated.
“Carboxytherapy has been previously used therapeutically to reduce fat, and studies of carboxytherapy have been done in the past as well,” the study’s senior author, Murad Alam, MD, said in an interview in advance of a poster session at the annual conference of the American Society for Laser Medicine and Surgery Inc. “However, earlier studies have not been controlled trials.”
For the split-body study, he and his associates enrolled 16 adults and randomized one side of their body to receive infusions of 1,000 cc of CO2 every week for 5 weeks in the flank region, while the contralateral side received sham treatments. Outcomes of interest included fat layer thickness using a diagnostic ultrasound, total circumference, and body weight, said Dr. Alam, vice-chair of the department of dermatology and chief of the division of cutaneous and aesthetic surgery at Northwestern University, Chicago. He reported that a significant difference in fat thickness was observed in the carboxytherapy-treated side 1 week after the last treatment (P = .011), but this difference was not maintained at 28 weeks as measured by diagnostic ultrasound. Total circumference decreased nominally but not significantly. Body weights did not significantly change throughout the study.
Dr. Alam acknowledged that the study’s small sample size is a limitation. “We do think we did enough treatments, five per side, to obtain an effect if one were to be seen,” he said. The researchers reported having no financial disclosures.
DALLAS – , a small randomized, controlled trial demonstrated.
“Carboxytherapy has been previously used therapeutically to reduce fat, and studies of carboxytherapy have been done in the past as well,” the study’s senior author, Murad Alam, MD, said in an interview in advance of a poster session at the annual conference of the American Society for Laser Medicine and Surgery Inc. “However, earlier studies have not been controlled trials.”
For the split-body study, he and his associates enrolled 16 adults and randomized one side of their body to receive infusions of 1,000 cc of CO2 every week for 5 weeks in the flank region, while the contralateral side received sham treatments. Outcomes of interest included fat layer thickness using a diagnostic ultrasound, total circumference, and body weight, said Dr. Alam, vice-chair of the department of dermatology and chief of the division of cutaneous and aesthetic surgery at Northwestern University, Chicago. He reported that a significant difference in fat thickness was observed in the carboxytherapy-treated side 1 week after the last treatment (P = .011), but this difference was not maintained at 28 weeks as measured by diagnostic ultrasound. Total circumference decreased nominally but not significantly. Body weights did not significantly change throughout the study.
Dr. Alam acknowledged that the study’s small sample size is a limitation. “We do think we did enough treatments, five per side, to obtain an effect if one were to be seen,” he said. The researchers reported having no financial disclosures.
REPORTING FROM ASLMS 2018
Key clinical point: Carboxytherapy yielded a modest reduction in fat that went away after a few months.
Major finding: A significant difference in fat thickness was observed in the carboxytherapy-treated flank region 1 week after the last treatment (P = .011).
Study details: A randomized, split-body study of 16 adults.
Disclosures: Dr. Alam reported having no financial disclosures.
Short-Term Storage of Platelet-Rich Plasma at Room Temperature Does Not Affect Growth Factor or Catabolic Cytokine Concentration
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 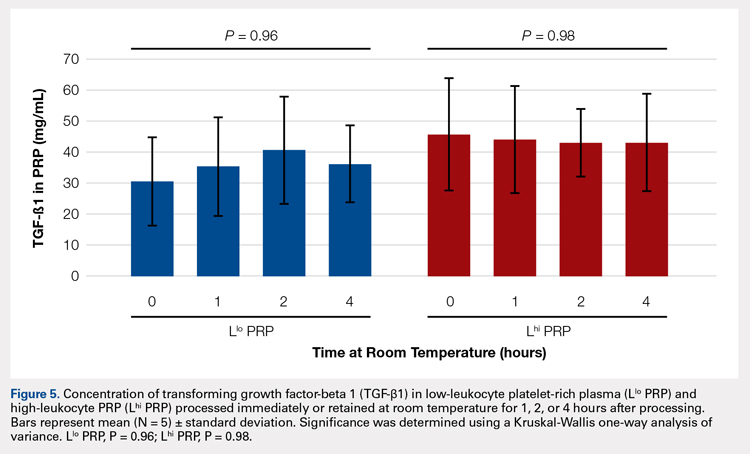
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
TAKE-HOME POINTS
- Blood can be stored at room temperature for up to 4 hours before making PRP without loss in activity.
- PRP can be stored at room temperature for up to 4 hours before administration to a patient without loss in activity.
- Manual, direct smear analysis is as accurate as automated counting for measuring platelet concentration in PRP.
Gene strong predictor of metastasis in melanoma
CHICAGO – Investigators have identified four genes that are overexpressed in primary melanoma, including one, CXCL1, that holds promise as a strong predictor of future metastatic disease, according to study results presented at the Society of Surgical Oncology Annual Cancer Symposium.
The study implicated four genes strongly expressed in primary melanoma tumors of patients who develop distant metastases – CXCL1, CXCL2, CBL, and CD276 – said Jennifer Erdrich, MD, MPH, of Cedars Sinai Medical Center, Los Angeles. However, CXCL1 stood out. “CXCL1 overexpression is an independent predictor of developing metastatic disease. Patients with CXCL1 overexpression in the primary tumor in our study had decreased overall 5-year survival.” CXCL1 may be a useful predictive marker in primary melanoma and a potential target for immunotherapy, she said.
The rationale for analyzing the 79 genes implicated in cancer only rather than the entire array of 22,000 genes was to reduce the odds of a high false-discovery rate from 5% to 0.007%. “This is what strengthens our findings in a cohort of 37 patients,” Dr. Erdrich said.
The study analyzed pathological characteristics of the metastatic and nonmetastatic groups. Most characteristics were similar between the two groups, including location of the primary tumor in the trunk and extremities of 67% and 71%, respectively, and age of 60 years and older. The analysis noted two deviations: primary tumor size was thicker in the metastatic group (2.1 mm vs. 1.05 mm; P = .6), although Dr. Erdrich noted this was “not significantly different”; and a higher rate of ulceration in the metastatic group (50% vs. 13%; P = .05).
The genes CXCL1 and CXCL2 are both chemokines involved in growth and inflammation. “CXCL1 expression was 2.51 times greater in the metastatic group,” Dr. Erdrich said (P less than .001). Overexpression in the other three genes of interest was: CXCL2, 1.68 times greater (P less than .01); CD276, which is involved in T-cell immunity, 1.16 times greater (P = .04); and C-CBL, which is a photo-oncogene involved in the ubiquitin pathway, 1.15 times greater (P = .01). “The overexpression of all four of these was statistically significant,” she said.
Univariate analysis found ulceration of the primary along with overexpression of
the four genes to be significant predictors of metastasis. “However, in our multivariate model, three of the genes dropped out but CXCL1 remained robust,” she said.
Dr. Erdrich noted that CXCL1 is a cytokine located on chromosome 4, is secreted by macrophages, exerts its signal through CXCR2, and is one of five cytokines upregulated in lesions that respond to immunotherapy (Br J Dermatol. 2016;175:966-78).
CXCL1 compares favorably with S100, the existing blood-based biomarker for predicting recurrence in high-risk melanoma, as a predictor of metastases, Dr. Erdrich said, with an area under the curve of 0.80 versus 0.66; sensitivity of 67% versus 77%; specificity of 97% versus 61%; positive predictive value of 80% versus 40%; and negative predictive value of 94% versus 88% (Anticancer Res. 1999;19:2685-90; Cancer. 2003;97:1737-45).
The study also looked at overall survival in patients with low and high expression of CXCL1. “The patients with high expression had 5-year survival of only 50% compared to those of low expression, whose 5-year survival was 97%,” Dr. Erdrich said.
Dr. Erdrich and her coauthors reported having no financial relationships.
SOURCE: Erdrich J et al. SSO 2018, Abstract 82.
CHICAGO – Investigators have identified four genes that are overexpressed in primary melanoma, including one, CXCL1, that holds promise as a strong predictor of future metastatic disease, according to study results presented at the Society of Surgical Oncology Annual Cancer Symposium.
The study implicated four genes strongly expressed in primary melanoma tumors of patients who develop distant metastases – CXCL1, CXCL2, CBL, and CD276 – said Jennifer Erdrich, MD, MPH, of Cedars Sinai Medical Center, Los Angeles. However, CXCL1 stood out. “CXCL1 overexpression is an independent predictor of developing metastatic disease. Patients with CXCL1 overexpression in the primary tumor in our study had decreased overall 5-year survival.” CXCL1 may be a useful predictive marker in primary melanoma and a potential target for immunotherapy, she said.
The rationale for analyzing the 79 genes implicated in cancer only rather than the entire array of 22,000 genes was to reduce the odds of a high false-discovery rate from 5% to 0.007%. “This is what strengthens our findings in a cohort of 37 patients,” Dr. Erdrich said.
The study analyzed pathological characteristics of the metastatic and nonmetastatic groups. Most characteristics were similar between the two groups, including location of the primary tumor in the trunk and extremities of 67% and 71%, respectively, and age of 60 years and older. The analysis noted two deviations: primary tumor size was thicker in the metastatic group (2.1 mm vs. 1.05 mm; P = .6), although Dr. Erdrich noted this was “not significantly different”; and a higher rate of ulceration in the metastatic group (50% vs. 13%; P = .05).
The genes CXCL1 and CXCL2 are both chemokines involved in growth and inflammation. “CXCL1 expression was 2.51 times greater in the metastatic group,” Dr. Erdrich said (P less than .001). Overexpression in the other three genes of interest was: CXCL2, 1.68 times greater (P less than .01); CD276, which is involved in T-cell immunity, 1.16 times greater (P = .04); and C-CBL, which is a photo-oncogene involved in the ubiquitin pathway, 1.15 times greater (P = .01). “The overexpression of all four of these was statistically significant,” she said.
Univariate analysis found ulceration of the primary along with overexpression of
the four genes to be significant predictors of metastasis. “However, in our multivariate model, three of the genes dropped out but CXCL1 remained robust,” she said.
Dr. Erdrich noted that CXCL1 is a cytokine located on chromosome 4, is secreted by macrophages, exerts its signal through CXCR2, and is one of five cytokines upregulated in lesions that respond to immunotherapy (Br J Dermatol. 2016;175:966-78).
CXCL1 compares favorably with S100, the existing blood-based biomarker for predicting recurrence in high-risk melanoma, as a predictor of metastases, Dr. Erdrich said, with an area under the curve of 0.80 versus 0.66; sensitivity of 67% versus 77%; specificity of 97% versus 61%; positive predictive value of 80% versus 40%; and negative predictive value of 94% versus 88% (Anticancer Res. 1999;19:2685-90; Cancer. 2003;97:1737-45).
The study also looked at overall survival in patients with low and high expression of CXCL1. “The patients with high expression had 5-year survival of only 50% compared to those of low expression, whose 5-year survival was 97%,” Dr. Erdrich said.
Dr. Erdrich and her coauthors reported having no financial relationships.
SOURCE: Erdrich J et al. SSO 2018, Abstract 82.
CHICAGO – Investigators have identified four genes that are overexpressed in primary melanoma, including one, CXCL1, that holds promise as a strong predictor of future metastatic disease, according to study results presented at the Society of Surgical Oncology Annual Cancer Symposium.
The study implicated four genes strongly expressed in primary melanoma tumors of patients who develop distant metastases – CXCL1, CXCL2, CBL, and CD276 – said Jennifer Erdrich, MD, MPH, of Cedars Sinai Medical Center, Los Angeles. However, CXCL1 stood out. “CXCL1 overexpression is an independent predictor of developing metastatic disease. Patients with CXCL1 overexpression in the primary tumor in our study had decreased overall 5-year survival.” CXCL1 may be a useful predictive marker in primary melanoma and a potential target for immunotherapy, she said.
The rationale for analyzing the 79 genes implicated in cancer only rather than the entire array of 22,000 genes was to reduce the odds of a high false-discovery rate from 5% to 0.007%. “This is what strengthens our findings in a cohort of 37 patients,” Dr. Erdrich said.
The study analyzed pathological characteristics of the metastatic and nonmetastatic groups. Most characteristics were similar between the two groups, including location of the primary tumor in the trunk and extremities of 67% and 71%, respectively, and age of 60 years and older. The analysis noted two deviations: primary tumor size was thicker in the metastatic group (2.1 mm vs. 1.05 mm; P = .6), although Dr. Erdrich noted this was “not significantly different”; and a higher rate of ulceration in the metastatic group (50% vs. 13%; P = .05).
The genes CXCL1 and CXCL2 are both chemokines involved in growth and inflammation. “CXCL1 expression was 2.51 times greater in the metastatic group,” Dr. Erdrich said (P less than .001). Overexpression in the other three genes of interest was: CXCL2, 1.68 times greater (P less than .01); CD276, which is involved in T-cell immunity, 1.16 times greater (P = .04); and C-CBL, which is a photo-oncogene involved in the ubiquitin pathway, 1.15 times greater (P = .01). “The overexpression of all four of these was statistically significant,” she said.
Univariate analysis found ulceration of the primary along with overexpression of
the four genes to be significant predictors of metastasis. “However, in our multivariate model, three of the genes dropped out but CXCL1 remained robust,” she said.
Dr. Erdrich noted that CXCL1 is a cytokine located on chromosome 4, is secreted by macrophages, exerts its signal through CXCR2, and is one of five cytokines upregulated in lesions that respond to immunotherapy (Br J Dermatol. 2016;175:966-78).
CXCL1 compares favorably with S100, the existing blood-based biomarker for predicting recurrence in high-risk melanoma, as a predictor of metastases, Dr. Erdrich said, with an area under the curve of 0.80 versus 0.66; sensitivity of 67% versus 77%; specificity of 97% versus 61%; positive predictive value of 80% versus 40%; and negative predictive value of 94% versus 88% (Anticancer Res. 1999;19:2685-90; Cancer. 2003;97:1737-45).
The study also looked at overall survival in patients with low and high expression of CXCL1. “The patients with high expression had 5-year survival of only 50% compared to those of low expression, whose 5-year survival was 97%,” Dr. Erdrich said.
Dr. Erdrich and her coauthors reported having no financial relationships.
SOURCE: Erdrich J et al. SSO 2018, Abstract 82.
REPORTING FROM SSO 2018
Key clinical point: The CXCL1 gene may predict metastatic risk in primary melanoma.
Major findings: CXCL1 overexpression yielded 50% 5-year survival, almost half that of underexpression.
Study details: Gene analysis of samples from 37 patients with nonmetastatic primary melanoma who had surgical removal of primary lesion with median follow-up of 38 months.
Disclosures: Dr. Erdrich and her coauthors reported having no financial disclosures.
Source: Erdrich J et al. SSO 2018, Abstract 82.