User login
Myelodysplastic syndromes: etiologies, evaluation, and therapy
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
Estrogen patch counters eating disorders in women athletes
CHICAGO – It’s a good idea to look for eating disorders in young, athletic women who present with oligomenorrhea; they are at high risk for them and can be helped with estrogen replacement, especially with a patch, according to investigators from Massachusetts General Hospital, Boston.
Estrogen replacement will also help with stress fractures and other bone problems, but young, athletic women might resist the idea. They worry that it might hurt their performance and are often proud that they work out hard enough to stop their periods, according to investigator Vibha Singhal, MD, a pediatric endocrinologist and eating disorder specialist at the hospital.
Earlier research done at Massachusetts General had shown that replacing estrogen to physiological levels helped reduce anxiety and body dissatisfaction in anorexia nervosa. Both are key drivers of the condition, along with the drive for thinness, lead investigator Franziska Plessow, PhD, a neuroendocrine researcher at the hospital, said at the Endocrine Society’s annual meeting.
But the researchers wondered whether estrogen also helps healthy weight women with nascent eating disorders, so the investigators turned to female athletes aged 14-25 years who had stopped menstruating or were about to.
The 117 oligomenorrheic athletes (OA) they investigated – none of whom had formally diagnosed eating disorders – scored significantly higher on measures of body dissatisfaction, drive for thinness, perfectionism, and “cognitive restraint of eating,” compared with 50 female athletes and 41 nonathletic women, both with normal periods. For instance, OA women scored a mean of 4.21 on the drive for thinness scale of the Eating Disorder Inventory–2, a low score, but still significantly higher than the 1.66 points in eumenorrheic athletes and 1.61 points in nonathletes (P = .0005).
Oligomenorrheic female athletes “show more disordered eating behavior and psychopathology at the subclinical level,” Dr. Plessow said.
Seventy OA women were then randomized to three groups: 25 to physiological estrogen replacement with an estradiol patch and cyclic progesterone for 12 days/month; 19 to replacement with contraceptive pills on the standard monthly schedule; and 26 to no replacement.
Over 12 months, women on the patch dropped their drive for thinness, body dissatisfaction, and uncontrolled eating scores. Body dissatisfaction scores on the Eating Disorder Inventory–2, for example, fell about two points, compared with staying about the same in the pill group and increasing by about two points in the no-estrogen group.
The pill seemed to help a bit on some measures, too, but the benefit of the pill versus that of no estrogen wasn’t generally statistically significant. Meanwhile, symptoms worsened in women who didn’t get estrogen. “The practice right now is the pill. We are shifting our practice with these data to the patch,” Dr. Singhal said.
Overall, “these findings emphasize the importance of normalizing estrogen levels in this population,” she and her colleagues concluded.
It’s a mystery why estrogen helps with eating disorders. Maybe it has something to do with the estrogen receptors in the appetite centers of the brain. Maybe the patch works better than the pill because there’s no first-pass through the liver, Dr. Singhal said.
Subjects were aged about 20 years, on average. OA women had a mean body mass index of 20.6 kg/m2 and a mean estradiol level of 45 pg/mL. Subjects with regular periods had mean BMIs of about 22 kg/m2; mean estradiol levels were 70.2 pg/mL in eumenorrheic athletes and 83.6 pg/mL in nonathletic women.
The work was funded by the National Institutes of Health. The investigators didn’t have any relevant disclosures.
SOURCE: Plessow F et al. ENDO 2018, Abstract SAT-290.
CHICAGO – It’s a good idea to look for eating disorders in young, athletic women who present with oligomenorrhea; they are at high risk for them and can be helped with estrogen replacement, especially with a patch, according to investigators from Massachusetts General Hospital, Boston.
Estrogen replacement will also help with stress fractures and other bone problems, but young, athletic women might resist the idea. They worry that it might hurt their performance and are often proud that they work out hard enough to stop their periods, according to investigator Vibha Singhal, MD, a pediatric endocrinologist and eating disorder specialist at the hospital.
Earlier research done at Massachusetts General had shown that replacing estrogen to physiological levels helped reduce anxiety and body dissatisfaction in anorexia nervosa. Both are key drivers of the condition, along with the drive for thinness, lead investigator Franziska Plessow, PhD, a neuroendocrine researcher at the hospital, said at the Endocrine Society’s annual meeting.
But the researchers wondered whether estrogen also helps healthy weight women with nascent eating disorders, so the investigators turned to female athletes aged 14-25 years who had stopped menstruating or were about to.
The 117 oligomenorrheic athletes (OA) they investigated – none of whom had formally diagnosed eating disorders – scored significantly higher on measures of body dissatisfaction, drive for thinness, perfectionism, and “cognitive restraint of eating,” compared with 50 female athletes and 41 nonathletic women, both with normal periods. For instance, OA women scored a mean of 4.21 on the drive for thinness scale of the Eating Disorder Inventory–2, a low score, but still significantly higher than the 1.66 points in eumenorrheic athletes and 1.61 points in nonathletes (P = .0005).
Oligomenorrheic female athletes “show more disordered eating behavior and psychopathology at the subclinical level,” Dr. Plessow said.
Seventy OA women were then randomized to three groups: 25 to physiological estrogen replacement with an estradiol patch and cyclic progesterone for 12 days/month; 19 to replacement with contraceptive pills on the standard monthly schedule; and 26 to no replacement.
Over 12 months, women on the patch dropped their drive for thinness, body dissatisfaction, and uncontrolled eating scores. Body dissatisfaction scores on the Eating Disorder Inventory–2, for example, fell about two points, compared with staying about the same in the pill group and increasing by about two points in the no-estrogen group.
The pill seemed to help a bit on some measures, too, but the benefit of the pill versus that of no estrogen wasn’t generally statistically significant. Meanwhile, symptoms worsened in women who didn’t get estrogen. “The practice right now is the pill. We are shifting our practice with these data to the patch,” Dr. Singhal said.
Overall, “these findings emphasize the importance of normalizing estrogen levels in this population,” she and her colleagues concluded.
It’s a mystery why estrogen helps with eating disorders. Maybe it has something to do with the estrogen receptors in the appetite centers of the brain. Maybe the patch works better than the pill because there’s no first-pass through the liver, Dr. Singhal said.
Subjects were aged about 20 years, on average. OA women had a mean body mass index of 20.6 kg/m2 and a mean estradiol level of 45 pg/mL. Subjects with regular periods had mean BMIs of about 22 kg/m2; mean estradiol levels were 70.2 pg/mL in eumenorrheic athletes and 83.6 pg/mL in nonathletic women.
The work was funded by the National Institutes of Health. The investigators didn’t have any relevant disclosures.
SOURCE: Plessow F et al. ENDO 2018, Abstract SAT-290.
CHICAGO – It’s a good idea to look for eating disorders in young, athletic women who present with oligomenorrhea; they are at high risk for them and can be helped with estrogen replacement, especially with a patch, according to investigators from Massachusetts General Hospital, Boston.
Estrogen replacement will also help with stress fractures and other bone problems, but young, athletic women might resist the idea. They worry that it might hurt their performance and are often proud that they work out hard enough to stop their periods, according to investigator Vibha Singhal, MD, a pediatric endocrinologist and eating disorder specialist at the hospital.
Earlier research done at Massachusetts General had shown that replacing estrogen to physiological levels helped reduce anxiety and body dissatisfaction in anorexia nervosa. Both are key drivers of the condition, along with the drive for thinness, lead investigator Franziska Plessow, PhD, a neuroendocrine researcher at the hospital, said at the Endocrine Society’s annual meeting.
But the researchers wondered whether estrogen also helps healthy weight women with nascent eating disorders, so the investigators turned to female athletes aged 14-25 years who had stopped menstruating or were about to.
The 117 oligomenorrheic athletes (OA) they investigated – none of whom had formally diagnosed eating disorders – scored significantly higher on measures of body dissatisfaction, drive for thinness, perfectionism, and “cognitive restraint of eating,” compared with 50 female athletes and 41 nonathletic women, both with normal periods. For instance, OA women scored a mean of 4.21 on the drive for thinness scale of the Eating Disorder Inventory–2, a low score, but still significantly higher than the 1.66 points in eumenorrheic athletes and 1.61 points in nonathletes (P = .0005).
Oligomenorrheic female athletes “show more disordered eating behavior and psychopathology at the subclinical level,” Dr. Plessow said.
Seventy OA women were then randomized to three groups: 25 to physiological estrogen replacement with an estradiol patch and cyclic progesterone for 12 days/month; 19 to replacement with contraceptive pills on the standard monthly schedule; and 26 to no replacement.
Over 12 months, women on the patch dropped their drive for thinness, body dissatisfaction, and uncontrolled eating scores. Body dissatisfaction scores on the Eating Disorder Inventory–2, for example, fell about two points, compared with staying about the same in the pill group and increasing by about two points in the no-estrogen group.
The pill seemed to help a bit on some measures, too, but the benefit of the pill versus that of no estrogen wasn’t generally statistically significant. Meanwhile, symptoms worsened in women who didn’t get estrogen. “The practice right now is the pill. We are shifting our practice with these data to the patch,” Dr. Singhal said.
Overall, “these findings emphasize the importance of normalizing estrogen levels in this population,” she and her colleagues concluded.
It’s a mystery why estrogen helps with eating disorders. Maybe it has something to do with the estrogen receptors in the appetite centers of the brain. Maybe the patch works better than the pill because there’s no first-pass through the liver, Dr. Singhal said.
Subjects were aged about 20 years, on average. OA women had a mean body mass index of 20.6 kg/m2 and a mean estradiol level of 45 pg/mL. Subjects with regular periods had mean BMIs of about 22 kg/m2; mean estradiol levels were 70.2 pg/mL in eumenorrheic athletes and 83.6 pg/mL in nonathletic women.
The work was funded by the National Institutes of Health. The investigators didn’t have any relevant disclosures.
SOURCE: Plessow F et al. ENDO 2018, Abstract SAT-290.
REPORTING FROM ENDO 2018
Key clinical point: It’s a good idea to look for eating disorders in young, athletic women who present with oligo-amenorrhea; they’re at high risk for them and can be helped with estrogen replacement, especially with a patch.
Major finding: Over 12 months, body dissatisfaction scores fell about 2 points among women on the patch, versus staying about the same in the pill group, and increasing about 2 points in the no-estrogen group.
Study details: Combined cross-sectional and randomized investigation
Disclosures: The work was supported by the National Institutes of Health. The investigators didn’t have any disclosures.
Source: Plessow F et al. ENDO 2018 abstract SAT-290
Complication rates rise after decline in uterine fibroid morcellation
The rate of major and minor 30-day complications from the treatment of uterine fibroids has increased significantly since the Food and Drug Administration’s black-box warning against the use of power morcellation, data suggest.
Researchers examined the incidence of 30-day posthysterectomy complications in 75,487 women who underwent treatment for benign gynecologic indications before and after November 2014, when the FDA’s edict was issued over concerns about the risk of disseminating benign or malignant disease. Of these women, 25,571 had uterine fibroids as the indication for hysterectomy.
The retrospective cohort study, published online April 11 in JAMA Surgery, showed that while the overall rate of complications in the cohort was relatively unchanged before and after the FDA’s warning, complication rates increased significantly in women undergoing treatment for uterine fibroids.
Before the FDA’s announcement, the 30-day major complication rate in women undergoing hysterectomy for uterine fibroids was 1.9%, which increased to 2.4% after the FDA’s warning (odds ratio, 1.23; 95% confidence interval, 1.04-1.47; P = .02). Similarly, the rate of minor 30-day complications increased from 2.7% before the warning to 3.3% after the warning (OR, 1.21; 95% CI, 1.04-1.40; P = .01), after adjustment for factors such as age, body mass index, comorbidities, and other associated procedures.
“This 20% increase in the odds of major and minor complications could translate into a large number of additional complications among the 200,000 hysterectomies performed annually for uterine fibroids in the United States,” wrote Francesco Multinu, MD, of the department of obstetrics and gynecology at the Mayo Clinic, Rochester, Minn., and his coauthors.
Overall, the researchers saw a much higher rate of major complications in women undergoing open abdominal surgery, compared with women who underwent minimally invasive surgery or vaginal hysterectomy (3.5% vs. 1.7% vs. 1.7%). A similar pattern was seen in the subgroup of women who underwent hysterectomy for uterine fibroids (2.8% vs. 1.8% vs. 1.8%).
However, minor 30-day complication rates were higher in women who underwent vaginal hysterectomy (4.5%), compared with open abdominal surgery (4.1%) and minimally invasive surgery (3.2%). In women with uterine fibroids, the minor complication rates were slightly higher in those who underwent open hysterectomy or vaginal hysterectomy than in those who had minimally invasive surgery, but this was not statistically significant.
The type of surgery in women with uterine fibroids changed significantly after the FDA announcement. Before the black-box warning against power morcellation was issued, 37.2% of hysterectomy procedures for uterine fibroids were open, 56.1% were minimally invasive, and 6.7% were vaginal. After the announcement, the percentage that were open procedures increased to 43%, minimally invasive procedures decreased to 49.7%, and vaginal hysterectomies increased to 7.3% (P less than .001).
A similar but less pronounced trend was seen across all hysterectomies for benign gynecological indications.
The authors noted that the study analyzed data on 30-day complications, so they weren’t able to draw conclusions about longer-term outcomes.
“Although caution is required to avoid morcellation of unexpected uterine malignant neoplasms, our results should be considered by women and clinicians during the process of shared decision making and by medical societies and regulatory bodies when issuing safety communications,” the authors wrote.
The study was supported by a grant from the National Center for Advancing Translational Sciences, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
SOURCE: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
The rate of major and minor 30-day complications from the treatment of uterine fibroids has increased significantly since the Food and Drug Administration’s black-box warning against the use of power morcellation, data suggest.
Researchers examined the incidence of 30-day posthysterectomy complications in 75,487 women who underwent treatment for benign gynecologic indications before and after November 2014, when the FDA’s edict was issued over concerns about the risk of disseminating benign or malignant disease. Of these women, 25,571 had uterine fibroids as the indication for hysterectomy.
The retrospective cohort study, published online April 11 in JAMA Surgery, showed that while the overall rate of complications in the cohort was relatively unchanged before and after the FDA’s warning, complication rates increased significantly in women undergoing treatment for uterine fibroids.
Before the FDA’s announcement, the 30-day major complication rate in women undergoing hysterectomy for uterine fibroids was 1.9%, which increased to 2.4% after the FDA’s warning (odds ratio, 1.23; 95% confidence interval, 1.04-1.47; P = .02). Similarly, the rate of minor 30-day complications increased from 2.7% before the warning to 3.3% after the warning (OR, 1.21; 95% CI, 1.04-1.40; P = .01), after adjustment for factors such as age, body mass index, comorbidities, and other associated procedures.
“This 20% increase in the odds of major and minor complications could translate into a large number of additional complications among the 200,000 hysterectomies performed annually for uterine fibroids in the United States,” wrote Francesco Multinu, MD, of the department of obstetrics and gynecology at the Mayo Clinic, Rochester, Minn., and his coauthors.
Overall, the researchers saw a much higher rate of major complications in women undergoing open abdominal surgery, compared with women who underwent minimally invasive surgery or vaginal hysterectomy (3.5% vs. 1.7% vs. 1.7%). A similar pattern was seen in the subgroup of women who underwent hysterectomy for uterine fibroids (2.8% vs. 1.8% vs. 1.8%).
However, minor 30-day complication rates were higher in women who underwent vaginal hysterectomy (4.5%), compared with open abdominal surgery (4.1%) and minimally invasive surgery (3.2%). In women with uterine fibroids, the minor complication rates were slightly higher in those who underwent open hysterectomy or vaginal hysterectomy than in those who had minimally invasive surgery, but this was not statistically significant.
The type of surgery in women with uterine fibroids changed significantly after the FDA announcement. Before the black-box warning against power morcellation was issued, 37.2% of hysterectomy procedures for uterine fibroids were open, 56.1% were minimally invasive, and 6.7% were vaginal. After the announcement, the percentage that were open procedures increased to 43%, minimally invasive procedures decreased to 49.7%, and vaginal hysterectomies increased to 7.3% (P less than .001).
A similar but less pronounced trend was seen across all hysterectomies for benign gynecological indications.
The authors noted that the study analyzed data on 30-day complications, so they weren’t able to draw conclusions about longer-term outcomes.
“Although caution is required to avoid morcellation of unexpected uterine malignant neoplasms, our results should be considered by women and clinicians during the process of shared decision making and by medical societies and regulatory bodies when issuing safety communications,” the authors wrote.
The study was supported by a grant from the National Center for Advancing Translational Sciences, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
SOURCE: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
The rate of major and minor 30-day complications from the treatment of uterine fibroids has increased significantly since the Food and Drug Administration’s black-box warning against the use of power morcellation, data suggest.
Researchers examined the incidence of 30-day posthysterectomy complications in 75,487 women who underwent treatment for benign gynecologic indications before and after November 2014, when the FDA’s edict was issued over concerns about the risk of disseminating benign or malignant disease. Of these women, 25,571 had uterine fibroids as the indication for hysterectomy.
The retrospective cohort study, published online April 11 in JAMA Surgery, showed that while the overall rate of complications in the cohort was relatively unchanged before and after the FDA’s warning, complication rates increased significantly in women undergoing treatment for uterine fibroids.
Before the FDA’s announcement, the 30-day major complication rate in women undergoing hysterectomy for uterine fibroids was 1.9%, which increased to 2.4% after the FDA’s warning (odds ratio, 1.23; 95% confidence interval, 1.04-1.47; P = .02). Similarly, the rate of minor 30-day complications increased from 2.7% before the warning to 3.3% after the warning (OR, 1.21; 95% CI, 1.04-1.40; P = .01), after adjustment for factors such as age, body mass index, comorbidities, and other associated procedures.
“This 20% increase in the odds of major and minor complications could translate into a large number of additional complications among the 200,000 hysterectomies performed annually for uterine fibroids in the United States,” wrote Francesco Multinu, MD, of the department of obstetrics and gynecology at the Mayo Clinic, Rochester, Minn., and his coauthors.
Overall, the researchers saw a much higher rate of major complications in women undergoing open abdominal surgery, compared with women who underwent minimally invasive surgery or vaginal hysterectomy (3.5% vs. 1.7% vs. 1.7%). A similar pattern was seen in the subgroup of women who underwent hysterectomy for uterine fibroids (2.8% vs. 1.8% vs. 1.8%).
However, minor 30-day complication rates were higher in women who underwent vaginal hysterectomy (4.5%), compared with open abdominal surgery (4.1%) and minimally invasive surgery (3.2%). In women with uterine fibroids, the minor complication rates were slightly higher in those who underwent open hysterectomy or vaginal hysterectomy than in those who had minimally invasive surgery, but this was not statistically significant.
The type of surgery in women with uterine fibroids changed significantly after the FDA announcement. Before the black-box warning against power morcellation was issued, 37.2% of hysterectomy procedures for uterine fibroids were open, 56.1% were minimally invasive, and 6.7% were vaginal. After the announcement, the percentage that were open procedures increased to 43%, minimally invasive procedures decreased to 49.7%, and vaginal hysterectomies increased to 7.3% (P less than .001).
A similar but less pronounced trend was seen across all hysterectomies for benign gynecological indications.
The authors noted that the study analyzed data on 30-day complications, so they weren’t able to draw conclusions about longer-term outcomes.
“Although caution is required to avoid morcellation of unexpected uterine malignant neoplasms, our results should be considered by women and clinicians during the process of shared decision making and by medical societies and regulatory bodies when issuing safety communications,” the authors wrote.
The study was supported by a grant from the National Center for Advancing Translational Sciences, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
SOURCE: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
FROM JAMA SURGERY
Key clinical point: Major complications from uterine fibroid hysterectomies have increased with more open surgeries.
Major finding: The rate of 30-day complications after uterine fibroid hysterectomy has increased by 20%.
Study details: A retrospective cohort study of 75,487 women.
Disclosures: The study was supported by a grant from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, and one author was supported by the University of Insubria, and by Fondo Miglierina, Varese, Italy. No conflicts of interest were declared.
Source: Multinu F et al. JAMA Surg. 2018 Apr 11. doi: 10.1001/jamasurg.2018.0141.
Risk of ED visit/hospitalization increases when brand-name angiotensin receptor blockers (ARB) are switched to generic versions
Clinical question: Does switching from a brand name ARB to its generic lead to more ED visits and hospitalizations?
Background: Once a brand name drug’s patent expires, its generic form is commercialized and patients may be switched to the generic version. The drug equivalence of the generic vs. the brand name product may be substantial enough to affect clinically what is happening to the patient. Very few studies exist on the impact of the differences between brand-name and generic ARBs; those that do exist show conflicting results on clinical outcomes for the patient.
Study design: Observational retrospective interrupted time–series analysis.
Setting: Quebec Integrated Chronic Disease Surveillance System in Quebec.
Synopsis: The study analyzed 136,177 patients older than 66 years old with multiple comorbidities during the transition from brand-name to generic versions of losartan, valsartan, and candesartan. The authors compared ER visits or hospitalization of the brand-name users for 24 months before and 12 months after being transitioned from a brand-name ARB to a generic. All three groups were found to have higher rates of adverse events after switching to generics (8% for losartan, 11.7% for valsartan, and 16.6% for candesartan). The study was limited as the authors did not have access to the reason for the ER visits/admissions or the ability to determine which generic version was used (e.g., losartan has eight generic versions). The study highlights the need for further evaluation by risk and survival analysis to control confounders when switching to a generic formulation.
Bottom line: Switching patients from a brand-name to a generic ARB may lead to more ED consultations and hospital admissions.
Citation: Leclerc J et al. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec Canada. Circ Cardiovasc Qual Outcomes. 2017 Oct 3. pii 10e003891. doi: 10.1161/circoutcomes.117.003891.
Dr. Smith is assistant professor of medicine in the Division of Hospital Medicine, Emory University, Atlanta.
Clinical question: Does switching from a brand name ARB to its generic lead to more ED visits and hospitalizations?
Background: Once a brand name drug’s patent expires, its generic form is commercialized and patients may be switched to the generic version. The drug equivalence of the generic vs. the brand name product may be substantial enough to affect clinically what is happening to the patient. Very few studies exist on the impact of the differences between brand-name and generic ARBs; those that do exist show conflicting results on clinical outcomes for the patient.
Study design: Observational retrospective interrupted time–series analysis.
Setting: Quebec Integrated Chronic Disease Surveillance System in Quebec.
Synopsis: The study analyzed 136,177 patients older than 66 years old with multiple comorbidities during the transition from brand-name to generic versions of losartan, valsartan, and candesartan. The authors compared ER visits or hospitalization of the brand-name users for 24 months before and 12 months after being transitioned from a brand-name ARB to a generic. All three groups were found to have higher rates of adverse events after switching to generics (8% for losartan, 11.7% for valsartan, and 16.6% for candesartan). The study was limited as the authors did not have access to the reason for the ER visits/admissions or the ability to determine which generic version was used (e.g., losartan has eight generic versions). The study highlights the need for further evaluation by risk and survival analysis to control confounders when switching to a generic formulation.
Bottom line: Switching patients from a brand-name to a generic ARB may lead to more ED consultations and hospital admissions.
Citation: Leclerc J et al. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec Canada. Circ Cardiovasc Qual Outcomes. 2017 Oct 3. pii 10e003891. doi: 10.1161/circoutcomes.117.003891.
Dr. Smith is assistant professor of medicine in the Division of Hospital Medicine, Emory University, Atlanta.
Clinical question: Does switching from a brand name ARB to its generic lead to more ED visits and hospitalizations?
Background: Once a brand name drug’s patent expires, its generic form is commercialized and patients may be switched to the generic version. The drug equivalence of the generic vs. the brand name product may be substantial enough to affect clinically what is happening to the patient. Very few studies exist on the impact of the differences between brand-name and generic ARBs; those that do exist show conflicting results on clinical outcomes for the patient.
Study design: Observational retrospective interrupted time–series analysis.
Setting: Quebec Integrated Chronic Disease Surveillance System in Quebec.
Synopsis: The study analyzed 136,177 patients older than 66 years old with multiple comorbidities during the transition from brand-name to generic versions of losartan, valsartan, and candesartan. The authors compared ER visits or hospitalization of the brand-name users for 24 months before and 12 months after being transitioned from a brand-name ARB to a generic. All three groups were found to have higher rates of adverse events after switching to generics (8% for losartan, 11.7% for valsartan, and 16.6% for candesartan). The study was limited as the authors did not have access to the reason for the ER visits/admissions or the ability to determine which generic version was used (e.g., losartan has eight generic versions). The study highlights the need for further evaluation by risk and survival analysis to control confounders when switching to a generic formulation.
Bottom line: Switching patients from a brand-name to a generic ARB may lead to more ED consultations and hospital admissions.
Citation: Leclerc J et al. Impact of the commercialization of three generic angiotensin II receptor blockers on adverse events in Quebec Canada. Circ Cardiovasc Qual Outcomes. 2017 Oct 3. pii 10e003891. doi: 10.1161/circoutcomes.117.003891.
Dr. Smith is assistant professor of medicine in the Division of Hospital Medicine, Emory University, Atlanta.
Can adhesive small bowel obstructions be addressed laparoscopically?
LAS VEGAS – whether they are open or laparoscopic. Small bowel obstructions can result, and management decisions can be complex. What if the patient resolves? Should you offer elective adhesiolysis? If it doesn’t resolve, can the surgery be done laparoscopically or should the procedure be open?
Bradley R. Davis, MD, FACS, discussed some of these options, and the case circumstances that inform the surgeon’s choices at the Annual Minimally Invasive Surgery Symposium by -Global Academy for Medical Education. Dr. Davis is chief of general surgery and of rectal surgery at Carolinas Medical Center, Charlotte, N.C.
Laparoscopic surgeries are associated with significantly lower rates of small bowel obstruction, but it can still happen. More often, patients will have had a previous open surgery, and this should be a selling point for doing first-time surgeries using minimally invasive techniques. “That’s one thing I tell my patients when I see them in the office. There’s a real reduction in adhesive small bowel obstruction and certainly in hernia formation, so there are long-term benefits that I don’t think we talk enough about,” said Dr. Davis.
Although the majority of obstructions are caused by adhesions, some are the result of malignancies or hernias, and Dr. Davis encourages his residents to do exams to determine if a hernia is to blame. “That’s harder and harder to do now that everyone does a CT scan, but that’s always an interesting question to ask a resident,” he said. Inflammatory bowel disease is sometimes also a cause, but that’s rare.
CT scans are the diagnostic mode of choice for small bowel obstructions. Some believe that oral contrast agents may help resolve obstructions, but Dr. Davis mentioned evidence from a study showing that contrast agents don’t change the course of obstructions or reduce laparotomy rates. However, contrast agents can help predict the clinical course of an obstruction. If the contrast agent is present in the colon at 24 hours, then that predicts that the patient will resolve with conservative treatment. “You have a pretty good idea that the patient is going to get better,” said Dr. Davis.
The American Association for the Surgery of Trauma severity grade is helpful for adhesive small bowel obstructions. Grade 2 cases involve intestinal distension and possibly a transition zone, some passage of contrast on follow-up films, and no evidence of intestinal compromise. Grade 3 cases have no distal contrast flow and evidence of complete obstruction or impending bowel compromise. In the latter cases, “we’re scratching our heads wondering whether to take the patient to the operating room. Certainly most of these cases we’ll manage initially nonoperatively, but those patients will end up getting an earlier operation,” said Dr. Davis.
The majority of surgeries are adhesiolysis, sometimes with a bowel resection. Whether or not the surgery can be performed laparoscopically or as an open surgery depends on several factors. If the index operation was done laparoscopically, chances are good that the adhesiolysis can be performed the same way. On the other hand, “if a patient has a known hostile abdomen, I wouldn’t even try. I would basically go straight to an open procedure,” said Dr. Davis.
Generally speaking, though, reoperative surgeries can be attempted laparoscopically and then converted to open procedures if needed, he added. The most common reasons for conversion are dense adhesions and ischemia-related resection.
However, iatrogenic injuries can also occur as a result of trocar access. “Just keep in mind that if you put the trocar into the bowel, the worst thing you can do is take it out because you won’t always find that hole. Just leave the trocar in the bowel, convert to an open procedure, and find the hole and fix it,” said Dr. Davis.
Cases are particularly challenging when the transition zone is in the pelvis. Those procedures are difficult to do laparoscopically because of a difficult angle, and they are more likely to convert to open surgery. “To be honest, that’s not an easy operation to open either, so beware that transition zone in the pelvis can be a difficult case.
“I don’t try to do anything heroic laparoscopically. If you put a camera in and you find it’s going to be a massive adhesiolysis laparoscopically, you might just be better off to open,” said Dr. Davis. In cases like that it can be hard to find the transition zone, which must be identified in order to ensure that the underlying problem is fixed.
An aggressive option in difficult cases is to use a PEEK Port, which starts with a 6-8 cm incision. The surgeon can open up a minimum of disposables and put a hand in to assist the laparoscopic view and determine if the procedure can be completed laparoscopically. “If you encounter extensive adhesions, you just convert to a laparotomy and you haven’t lost any time or spent any money in terms of disposables,” he said.
Dr. Davis had no disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – whether they are open or laparoscopic. Small bowel obstructions can result, and management decisions can be complex. What if the patient resolves? Should you offer elective adhesiolysis? If it doesn’t resolve, can the surgery be done laparoscopically or should the procedure be open?
Bradley R. Davis, MD, FACS, discussed some of these options, and the case circumstances that inform the surgeon’s choices at the Annual Minimally Invasive Surgery Symposium by -Global Academy for Medical Education. Dr. Davis is chief of general surgery and of rectal surgery at Carolinas Medical Center, Charlotte, N.C.
Laparoscopic surgeries are associated with significantly lower rates of small bowel obstruction, but it can still happen. More often, patients will have had a previous open surgery, and this should be a selling point for doing first-time surgeries using minimally invasive techniques. “That’s one thing I tell my patients when I see them in the office. There’s a real reduction in adhesive small bowel obstruction and certainly in hernia formation, so there are long-term benefits that I don’t think we talk enough about,” said Dr. Davis.
Although the majority of obstructions are caused by adhesions, some are the result of malignancies or hernias, and Dr. Davis encourages his residents to do exams to determine if a hernia is to blame. “That’s harder and harder to do now that everyone does a CT scan, but that’s always an interesting question to ask a resident,” he said. Inflammatory bowel disease is sometimes also a cause, but that’s rare.
CT scans are the diagnostic mode of choice for small bowel obstructions. Some believe that oral contrast agents may help resolve obstructions, but Dr. Davis mentioned evidence from a study showing that contrast agents don’t change the course of obstructions or reduce laparotomy rates. However, contrast agents can help predict the clinical course of an obstruction. If the contrast agent is present in the colon at 24 hours, then that predicts that the patient will resolve with conservative treatment. “You have a pretty good idea that the patient is going to get better,” said Dr. Davis.
The American Association for the Surgery of Trauma severity grade is helpful for adhesive small bowel obstructions. Grade 2 cases involve intestinal distension and possibly a transition zone, some passage of contrast on follow-up films, and no evidence of intestinal compromise. Grade 3 cases have no distal contrast flow and evidence of complete obstruction or impending bowel compromise. In the latter cases, “we’re scratching our heads wondering whether to take the patient to the operating room. Certainly most of these cases we’ll manage initially nonoperatively, but those patients will end up getting an earlier operation,” said Dr. Davis.
The majority of surgeries are adhesiolysis, sometimes with a bowel resection. Whether or not the surgery can be performed laparoscopically or as an open surgery depends on several factors. If the index operation was done laparoscopically, chances are good that the adhesiolysis can be performed the same way. On the other hand, “if a patient has a known hostile abdomen, I wouldn’t even try. I would basically go straight to an open procedure,” said Dr. Davis.
Generally speaking, though, reoperative surgeries can be attempted laparoscopically and then converted to open procedures if needed, he added. The most common reasons for conversion are dense adhesions and ischemia-related resection.
However, iatrogenic injuries can also occur as a result of trocar access. “Just keep in mind that if you put the trocar into the bowel, the worst thing you can do is take it out because you won’t always find that hole. Just leave the trocar in the bowel, convert to an open procedure, and find the hole and fix it,” said Dr. Davis.
Cases are particularly challenging when the transition zone is in the pelvis. Those procedures are difficult to do laparoscopically because of a difficult angle, and they are more likely to convert to open surgery. “To be honest, that’s not an easy operation to open either, so beware that transition zone in the pelvis can be a difficult case.
“I don’t try to do anything heroic laparoscopically. If you put a camera in and you find it’s going to be a massive adhesiolysis laparoscopically, you might just be better off to open,” said Dr. Davis. In cases like that it can be hard to find the transition zone, which must be identified in order to ensure that the underlying problem is fixed.
An aggressive option in difficult cases is to use a PEEK Port, which starts with a 6-8 cm incision. The surgeon can open up a minimum of disposables and put a hand in to assist the laparoscopic view and determine if the procedure can be completed laparoscopically. “If you encounter extensive adhesions, you just convert to a laparotomy and you haven’t lost any time or spent any money in terms of disposables,” he said.
Dr. Davis had no disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – whether they are open or laparoscopic. Small bowel obstructions can result, and management decisions can be complex. What if the patient resolves? Should you offer elective adhesiolysis? If it doesn’t resolve, can the surgery be done laparoscopically or should the procedure be open?
Bradley R. Davis, MD, FACS, discussed some of these options, and the case circumstances that inform the surgeon’s choices at the Annual Minimally Invasive Surgery Symposium by -Global Academy for Medical Education. Dr. Davis is chief of general surgery and of rectal surgery at Carolinas Medical Center, Charlotte, N.C.
Laparoscopic surgeries are associated with significantly lower rates of small bowel obstruction, but it can still happen. More often, patients will have had a previous open surgery, and this should be a selling point for doing first-time surgeries using minimally invasive techniques. “That’s one thing I tell my patients when I see them in the office. There’s a real reduction in adhesive small bowel obstruction and certainly in hernia formation, so there are long-term benefits that I don’t think we talk enough about,” said Dr. Davis.
Although the majority of obstructions are caused by adhesions, some are the result of malignancies or hernias, and Dr. Davis encourages his residents to do exams to determine if a hernia is to blame. “That’s harder and harder to do now that everyone does a CT scan, but that’s always an interesting question to ask a resident,” he said. Inflammatory bowel disease is sometimes also a cause, but that’s rare.
CT scans are the diagnostic mode of choice for small bowel obstructions. Some believe that oral contrast agents may help resolve obstructions, but Dr. Davis mentioned evidence from a study showing that contrast agents don’t change the course of obstructions or reduce laparotomy rates. However, contrast agents can help predict the clinical course of an obstruction. If the contrast agent is present in the colon at 24 hours, then that predicts that the patient will resolve with conservative treatment. “You have a pretty good idea that the patient is going to get better,” said Dr. Davis.
The American Association for the Surgery of Trauma severity grade is helpful for adhesive small bowel obstructions. Grade 2 cases involve intestinal distension and possibly a transition zone, some passage of contrast on follow-up films, and no evidence of intestinal compromise. Grade 3 cases have no distal contrast flow and evidence of complete obstruction or impending bowel compromise. In the latter cases, “we’re scratching our heads wondering whether to take the patient to the operating room. Certainly most of these cases we’ll manage initially nonoperatively, but those patients will end up getting an earlier operation,” said Dr. Davis.
The majority of surgeries are adhesiolysis, sometimes with a bowel resection. Whether or not the surgery can be performed laparoscopically or as an open surgery depends on several factors. If the index operation was done laparoscopically, chances are good that the adhesiolysis can be performed the same way. On the other hand, “if a patient has a known hostile abdomen, I wouldn’t even try. I would basically go straight to an open procedure,” said Dr. Davis.
Generally speaking, though, reoperative surgeries can be attempted laparoscopically and then converted to open procedures if needed, he added. The most common reasons for conversion are dense adhesions and ischemia-related resection.
However, iatrogenic injuries can also occur as a result of trocar access. “Just keep in mind that if you put the trocar into the bowel, the worst thing you can do is take it out because you won’t always find that hole. Just leave the trocar in the bowel, convert to an open procedure, and find the hole and fix it,” said Dr. Davis.
Cases are particularly challenging when the transition zone is in the pelvis. Those procedures are difficult to do laparoscopically because of a difficult angle, and they are more likely to convert to open surgery. “To be honest, that’s not an easy operation to open either, so beware that transition zone in the pelvis can be a difficult case.
“I don’t try to do anything heroic laparoscopically. If you put a camera in and you find it’s going to be a massive adhesiolysis laparoscopically, you might just be better off to open,” said Dr. Davis. In cases like that it can be hard to find the transition zone, which must be identified in order to ensure that the underlying problem is fixed.
An aggressive option in difficult cases is to use a PEEK Port, which starts with a 6-8 cm incision. The surgeon can open up a minimum of disposables and put a hand in to assist the laparoscopic view and determine if the procedure can be completed laparoscopically. “If you encounter extensive adhesions, you just convert to a laparotomy and you haven’t lost any time or spent any money in terms of disposables,” he said.
Dr. Davis had no disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM MISS
New PsA questionnaire fails to beat existing early screening methods
A new screening tool for detecting early psoriatic arthritis in patients with psoriasis that combined the “most discriminative questions” from several other questionnaires performed as well as the Psoriasis Epidemiology Screening Tool (PEST) for detecting the disease.
“The CONTEST questionnaire was developed using the best performing items from three other screening questionnaires in the hope that it would perform better than its originators,” Laura Coates, MBChB, PhD, of the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences at the University of Oxford, England, and her coauthors wrote in the Journal of the European Academy of Dermatology and Venereology. “In development this was partly correct but the current study does not support this – statistically there was no difference between PEST and CONTEST in terms of ability to detect psoriatic arthritis [PsA] in patients with psoriasis.”
The researchers found 27 patients (17%; 95% confidence interval, 12.3%-21.7%) with previously undiagnosed PsA, 71 with a different musculoskeletal disease, and 61 without musculoskeletal disease. Patients with PsA tended to be male, older, with “worse functional ability,” a similar age at onset of psoriasis, and had similar skin and nail disease severity. The sensitivity for PEST was 0.60 (95% CI, 0.42-0.78) and the specificity was 0.76 (95% CI, 0.69-0.83), while for CONTEST, the sensitivity was 0.53 (95% CI, 0.34-0.72) and the specificity was 0.71 (95% CI, 0.63-0.79). The area under the receiver operating curve confidence intervals for both screening tools were similar, with PEST having an AUC of 0.72 (95% CI, 0.61-0.84) and CONTEST having an AUC of 0.66 (95% CI, 0.54-0.77).
“The relative simplicity of the PEST questionnaire has raised concerns that the tool is not able to detect pure axial forms of the disease,” Dr. Coates and her colleagues wrote. “The CONTEST questionnaire includes items specific to back and neck pain, and so it was hoped it would better detect this subgroup. In this study this is not the case, although the numbers were small and imaging of the spine was not part of the study.”
AbbVie supported this study with an educational grant. The study was supported by the National Institute for Health Research Leeds Biomedical Research Centre. Some of the authors reported potential conflicts of interest.
SOURCE: Coates L et al. J Eur Acad Dermatol Venereol. 2018 Mar 26. doi: 10.1111/jdv.14971.
A new screening tool for detecting early psoriatic arthritis in patients with psoriasis that combined the “most discriminative questions” from several other questionnaires performed as well as the Psoriasis Epidemiology Screening Tool (PEST) for detecting the disease.
“The CONTEST questionnaire was developed using the best performing items from three other screening questionnaires in the hope that it would perform better than its originators,” Laura Coates, MBChB, PhD, of the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences at the University of Oxford, England, and her coauthors wrote in the Journal of the European Academy of Dermatology and Venereology. “In development this was partly correct but the current study does not support this – statistically there was no difference between PEST and CONTEST in terms of ability to detect psoriatic arthritis [PsA] in patients with psoriasis.”
The researchers found 27 patients (17%; 95% confidence interval, 12.3%-21.7%) with previously undiagnosed PsA, 71 with a different musculoskeletal disease, and 61 without musculoskeletal disease. Patients with PsA tended to be male, older, with “worse functional ability,” a similar age at onset of psoriasis, and had similar skin and nail disease severity. The sensitivity for PEST was 0.60 (95% CI, 0.42-0.78) and the specificity was 0.76 (95% CI, 0.69-0.83), while for CONTEST, the sensitivity was 0.53 (95% CI, 0.34-0.72) and the specificity was 0.71 (95% CI, 0.63-0.79). The area under the receiver operating curve confidence intervals for both screening tools were similar, with PEST having an AUC of 0.72 (95% CI, 0.61-0.84) and CONTEST having an AUC of 0.66 (95% CI, 0.54-0.77).
“The relative simplicity of the PEST questionnaire has raised concerns that the tool is not able to detect pure axial forms of the disease,” Dr. Coates and her colleagues wrote. “The CONTEST questionnaire includes items specific to back and neck pain, and so it was hoped it would better detect this subgroup. In this study this is not the case, although the numbers were small and imaging of the spine was not part of the study.”
AbbVie supported this study with an educational grant. The study was supported by the National Institute for Health Research Leeds Biomedical Research Centre. Some of the authors reported potential conflicts of interest.
SOURCE: Coates L et al. J Eur Acad Dermatol Venereol. 2018 Mar 26. doi: 10.1111/jdv.14971.
A new screening tool for detecting early psoriatic arthritis in patients with psoriasis that combined the “most discriminative questions” from several other questionnaires performed as well as the Psoriasis Epidemiology Screening Tool (PEST) for detecting the disease.
“The CONTEST questionnaire was developed using the best performing items from three other screening questionnaires in the hope that it would perform better than its originators,” Laura Coates, MBChB, PhD, of the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences at the University of Oxford, England, and her coauthors wrote in the Journal of the European Academy of Dermatology and Venereology. “In development this was partly correct but the current study does not support this – statistically there was no difference between PEST and CONTEST in terms of ability to detect psoriatic arthritis [PsA] in patients with psoriasis.”
The researchers found 27 patients (17%; 95% confidence interval, 12.3%-21.7%) with previously undiagnosed PsA, 71 with a different musculoskeletal disease, and 61 without musculoskeletal disease. Patients with PsA tended to be male, older, with “worse functional ability,” a similar age at onset of psoriasis, and had similar skin and nail disease severity. The sensitivity for PEST was 0.60 (95% CI, 0.42-0.78) and the specificity was 0.76 (95% CI, 0.69-0.83), while for CONTEST, the sensitivity was 0.53 (95% CI, 0.34-0.72) and the specificity was 0.71 (95% CI, 0.63-0.79). The area under the receiver operating curve confidence intervals for both screening tools were similar, with PEST having an AUC of 0.72 (95% CI, 0.61-0.84) and CONTEST having an AUC of 0.66 (95% CI, 0.54-0.77).
“The relative simplicity of the PEST questionnaire has raised concerns that the tool is not able to detect pure axial forms of the disease,” Dr. Coates and her colleagues wrote. “The CONTEST questionnaire includes items specific to back and neck pain, and so it was hoped it would better detect this subgroup. In this study this is not the case, although the numbers were small and imaging of the spine was not part of the study.”
AbbVie supported this study with an educational grant. The study was supported by the National Institute for Health Research Leeds Biomedical Research Centre. Some of the authors reported potential conflicts of interest.
SOURCE: Coates L et al. J Eur Acad Dermatol Venereol. 2018 Mar 26. doi: 10.1111/jdv.14971.
FROM JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: The CONTEST screening tool detected psoriatic arthritis in psoriasis patients as accurately as the Psoriasis Epidemiology Screening Tool.
Major finding: The sensitivity and specificity of CONTEST was 0.53 and 0.71, respectively, while PEST had a sensitivity of 0.60 and specificity of 0.76.
Study details: An observational, cross-sectional study of 159 psoriasis patients at four secondary care dermatology centers in the United Kingdom from November 2013 to March 2017.
Disclosures: AbbVie supported this study with an educational grant. The study was supported by the National Institute for Health Research Leeds Biomedical Research Centre. Some of the authors reported potential conflicts of interest.
Source: Coates L et al. J Eur Acad Dermatol Venereol. 2018 Mar 26. doi: 10.1111/jdv.14971.
The Rash That Outlasts
A 63-year-old man says the 20-year-old rash on his face first appeared one summer. Although it slackens a bit each winter, it flares up again when the weather warms—despite a bevy of OTC and prescription topical treatments.
The patient has consulted many providers, including several dermatologists, who have diagnosed the butterfly rash of lupus. But blood tests failed to bear out that theory, and no one has ever biopsied it.
The patient spent many years working in the sun with minimal to no protection. He denies fever, malaise, joint pain, or other illness. He denies having a similar rash elsewhere on his body.
EXAMINATION
A symmetrical, strikingly red, extensive rash covers most of both sides of the patient’s face. There is epidermal scaling and roughness and large areas of obvious follicular enlargement, atrophy, and telangiectasias.
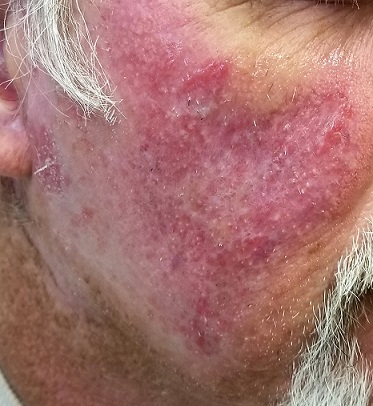
Punch biopsy shows a multitude of changes: atrophic epithelium, basal vacuolar changes, an intense dermal lymphocytic infiltrate, liquefaction degeneration, and apoptotic keratinocytes. Compact orthokeratosis is noted on the surface, and increased mucin formation in the dermis.
What is the diagnosis?
DISCUSSION
These skin changes, in conjunction with the biopsy findings, are consistent with a diagnosis of discoid lupus erythematosus (DLE). The vast majority of affected patients do not have systemic lupus erythematosus (SLE), although nearly 17% eventually progress to it. This patient’s seronegative status had puzzled his previous providers—a common mistake that frequently delays diagnosis and treatment.
DLE is the most common type of chronic cutaneous lupus, occurring in about 17 to 48 of every 100,000 people in the general population. It affects far more women than men, mostly those ages 20 to 40. More blacks than whites are affected by DLE, which is thought to be a polygenic autoimmune disease linked to various human leukocyte antigen groups.
DLE affects sun-exposed areas, including the scalp (where it often goes undiagnosed, leading to scarring alopecia). It can also occur in the mouth, manifesting as annular eroded areas. In this patient’s case, the combination of UV exposure and apparent genetic predisposition caused his malady.
Following workup to rule out systemic disease, the patient was started on hydroxychloroquine HCL (200 mg bid) and given strict instructions to protect himself from the sun. This should yield considerable improvement, although the scarring on large sections of his face (eg, the posterior cheeks) will be permanent. He will also be monitored for possible progression to SLE.
TAKE-HOME LEARNING POINTS
- Discoid lupus erythematosus (DLE) is a form of chronic cutaneous lupus caused by overexposure to the sun in a genetically susceptible individual.
- Most DLE patients never develop systemic lupus, though it’s far from unknown (17%).
- DLE, an autoimmune disease, is far more common in women (ages 20 – 40) than in men.
- Diagnosis is often made clinically, but biopsy reveals characteristic findings that confirm the disease.
- Besides sun protection, DLE is treated with the oral antimalarial hydroxychloroquine HCL (200 mg bid) and topical steroids as needed.
A 63-year-old man says the 20-year-old rash on his face first appeared one summer. Although it slackens a bit each winter, it flares up again when the weather warms—despite a bevy of OTC and prescription topical treatments.
The patient has consulted many providers, including several dermatologists, who have diagnosed the butterfly rash of lupus. But blood tests failed to bear out that theory, and no one has ever biopsied it.
The patient spent many years working in the sun with minimal to no protection. He denies fever, malaise, joint pain, or other illness. He denies having a similar rash elsewhere on his body.
EXAMINATION
A symmetrical, strikingly red, extensive rash covers most of both sides of the patient’s face. There is epidermal scaling and roughness and large areas of obvious follicular enlargement, atrophy, and telangiectasias.

Punch biopsy shows a multitude of changes: atrophic epithelium, basal vacuolar changes, an intense dermal lymphocytic infiltrate, liquefaction degeneration, and apoptotic keratinocytes. Compact orthokeratosis is noted on the surface, and increased mucin formation in the dermis.
What is the diagnosis?
DISCUSSION
These skin changes, in conjunction with the biopsy findings, are consistent with a diagnosis of discoid lupus erythematosus (DLE). The vast majority of affected patients do not have systemic lupus erythematosus (SLE), although nearly 17% eventually progress to it. This patient’s seronegative status had puzzled his previous providers—a common mistake that frequently delays diagnosis and treatment.
DLE is the most common type of chronic cutaneous lupus, occurring in about 17 to 48 of every 100,000 people in the general population. It affects far more women than men, mostly those ages 20 to 40. More blacks than whites are affected by DLE, which is thought to be a polygenic autoimmune disease linked to various human leukocyte antigen groups.
DLE affects sun-exposed areas, including the scalp (where it often goes undiagnosed, leading to scarring alopecia). It can also occur in the mouth, manifesting as annular eroded areas. In this patient’s case, the combination of UV exposure and apparent genetic predisposition caused his malady.
Following workup to rule out systemic disease, the patient was started on hydroxychloroquine HCL (200 mg bid) and given strict instructions to protect himself from the sun. This should yield considerable improvement, although the scarring on large sections of his face (eg, the posterior cheeks) will be permanent. He will also be monitored for possible progression to SLE.
TAKE-HOME LEARNING POINTS
- Discoid lupus erythematosus (DLE) is a form of chronic cutaneous lupus caused by overexposure to the sun in a genetically susceptible individual.
- Most DLE patients never develop systemic lupus, though it’s far from unknown (17%).
- DLE, an autoimmune disease, is far more common in women (ages 20 – 40) than in men.
- Diagnosis is often made clinically, but biopsy reveals characteristic findings that confirm the disease.
- Besides sun protection, DLE is treated with the oral antimalarial hydroxychloroquine HCL (200 mg bid) and topical steroids as needed.
A 63-year-old man says the 20-year-old rash on his face first appeared one summer. Although it slackens a bit each winter, it flares up again when the weather warms—despite a bevy of OTC and prescription topical treatments.
The patient has consulted many providers, including several dermatologists, who have diagnosed the butterfly rash of lupus. But blood tests failed to bear out that theory, and no one has ever biopsied it.
The patient spent many years working in the sun with minimal to no protection. He denies fever, malaise, joint pain, or other illness. He denies having a similar rash elsewhere on his body.
EXAMINATION
A symmetrical, strikingly red, extensive rash covers most of both sides of the patient’s face. There is epidermal scaling and roughness and large areas of obvious follicular enlargement, atrophy, and telangiectasias.

Punch biopsy shows a multitude of changes: atrophic epithelium, basal vacuolar changes, an intense dermal lymphocytic infiltrate, liquefaction degeneration, and apoptotic keratinocytes. Compact orthokeratosis is noted on the surface, and increased mucin formation in the dermis.
What is the diagnosis?
DISCUSSION
These skin changes, in conjunction with the biopsy findings, are consistent with a diagnosis of discoid lupus erythematosus (DLE). The vast majority of affected patients do not have systemic lupus erythematosus (SLE), although nearly 17% eventually progress to it. This patient’s seronegative status had puzzled his previous providers—a common mistake that frequently delays diagnosis and treatment.
DLE is the most common type of chronic cutaneous lupus, occurring in about 17 to 48 of every 100,000 people in the general population. It affects far more women than men, mostly those ages 20 to 40. More blacks than whites are affected by DLE, which is thought to be a polygenic autoimmune disease linked to various human leukocyte antigen groups.
DLE affects sun-exposed areas, including the scalp (where it often goes undiagnosed, leading to scarring alopecia). It can also occur in the mouth, manifesting as annular eroded areas. In this patient’s case, the combination of UV exposure and apparent genetic predisposition caused his malady.
Following workup to rule out systemic disease, the patient was started on hydroxychloroquine HCL (200 mg bid) and given strict instructions to protect himself from the sun. This should yield considerable improvement, although the scarring on large sections of his face (eg, the posterior cheeks) will be permanent. He will also be monitored for possible progression to SLE.
TAKE-HOME LEARNING POINTS
- Discoid lupus erythematosus (DLE) is a form of chronic cutaneous lupus caused by overexposure to the sun in a genetically susceptible individual.
- Most DLE patients never develop systemic lupus, though it’s far from unknown (17%).
- DLE, an autoimmune disease, is far more common in women (ages 20 – 40) than in men.
- Diagnosis is often made clinically, but biopsy reveals characteristic findings that confirm the disease.
- Besides sun protection, DLE is treated with the oral antimalarial hydroxychloroquine HCL (200 mg bid) and topical steroids as needed.
Blinatumomab triggers complete MRD response in ALL
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
The study by Dr. Gökbuget and her colleagues provides “strong evidence” that blinatumomab immunotherapy eliminates residual B-cell acute lymphoblastic leukemia (ALL) cells, thereby preventing relapse and improving survival, according to Patrick Brown, MD.
“This addresses the most important unsolved clinical problem in adults with B-ALL: the development of chemotherapy-resistant relapsed disease,” Dr. Brown wrote in an editorial.
Persistence of minimal residual disease (MRD) is the strongest independent predictor of outcomes in B-cell ALL, and is seen in up to 50% of adult patients after chemotherapy, according to Dr. Brown.
The “well-designed and well-executed” multicenter phase 2 study demonstrated an MRD clearance rate of 78% after one cycle of blinatumomab with modest adverse effects, according to Dr. Brown. Moreover, the results show a doubling of overall survival and tripling of relapse-free survival in MRD responders versus nonresponders, he said.
“An important caveat, however, is that, although the MRD clearance rate was no lower in the 35% of patients who had already relapsed once before enrolling, these patients had a substantially inferior RFS [relapse-free survival] and OS [overall survival], compared with those treated in first remission,” he added. “The clear lesson is that the impact of immunotherapeutic clearance of MRD on survival is greatest when applied early in the disease course.
The “most pressing question” not answered by this study is the impact of hematopoietic stem cell transplantation after complete MRD response, since the study allowed optional HSCT.
Patrick A. Brown, MD, is with Johns Hopkins University, Baltimore. These comments are adapted from his editorial in Blood (2018;131:1497-8). Dr. Brown reported having no competing financial interests related to his editorial.
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
After treatment with blinatumomab, most patients with minimal residual disease–positive acute lymphoblastic leukemia (ALL) achieved complete MRD response, according to results of a single-arm phase 2 study.
Achieving complete MRD response was associated with significantly longer relapse-free and overall survival in the patients, who were already in hematologic complete remission, researchers reported in the journal Blood.
“Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies,” Nicola Gökbuget, MD, University Hospital, Frankfurt, Germany, and her coauthors wrote.
This is the first international multicenter study to specifically enroll MRD-positive ALL patients and evaluate them for an MRD-based primary outcome in a cohort of MRD-positive ALL patients, according to the authors.
Preemptively treating low but measurable disease in ALL in remission, instead of waiting for overt relapse, is a strategy that may prolong overall survival, Dr. Gökbuget and her colleagues said in describing the rationale for their study. While there is no standard therapy yet for ALL patients with detectable MRD after intensive chemotherapy, hematopoietic stem cell transplantation (HSCT) is recommended, based on data that it may improve outcomes in patients with persistent MRD. However, other studies suggest detectable MRD before HSCT is associated with higher relapse rates, and many patients relapse while waiting for HSCT, the researchers noted.
To test an MRD-directed treatment strategy, Dr. Gökbuget and colleagues at 46 centers in Europe and Russia conducted an open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission. Patients in the study received up to four cycles of blinatumomab, a bispecific, T cell–engager antibody construct that enables T cells to recognize and eliminate CD19-positive cells.
Of 113 evaluable patients, 88 (78%) achieved complete MRD response after one cycle, the primary end point of the study. Relapse-free survival at 18 months was estimated at 54% and median overall survival was 36.5 months in the subset of 110 patients with Philadelphia chromosome–negative ALL in hematologic remission.
Complete MRD responders had improved relapse-free survival versus MRD nonresponders (23.6 vs. 5.7 months; P = .002), they reported. Likewise, overall survival was improved for MRD responders (38.9 vs. 12.5 months; P = .002).
Adverse events were consistent with what was previously reported for blinatumomab and included grade 3 and 4 neurologic events in 12 patients (10%) and 3 patients (3%), respectively. Cytokine-release syndrome was seen in four patients, with grade 1 and grade 3 cases.
The study was not designed to assess the impact of HSCT, which most patients (n = 76) underwent. However, a number of patients with complete MRD response but no HSCT remained in long-term remission, confirming results of an earlier blinatumomab pilot study, according to the researchers.
“This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients,” Dr. Gökbuget and her coauthors wrote.
Additional studies are needed to clarify the role and indications for HSCT in this setting, they added.
The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
SOURCE: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
FROM BLOOD
Key clinical point:
Major finding: Complete MRD response, seen in 78% of blinatumomab-treated patients, was associated with improved relapse-free and overall survival.
Study details: An open-label, single-arm, phase 2 study including 116 patients with B-cell precursor ALL in hematologic complete remission, conducted at 46 centers in Europe and Russia.
Disclosures: The study was designed by Amgen Research in collaboration with the researchers. Dr. Gökbuget reported financial relationships with Amgen and Pfizer. Other authors reported ties to various pharmaceutical companies.
Source: Gökbuget N et al. Blood. 2018 Apr 5;131(14):1522-31.
MDedge Daily News: Is rosacea a red flag for deeper dangers?
MedPAC wants to curb low-value care. Statins cut sepsis patients’ risk of death. And why reading aloud to kids can cut hyperactivity.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
MedPAC wants to curb low-value care. Statins cut sepsis patients’ risk of death. And why reading aloud to kids can cut hyperactivity.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
MedPAC wants to curb low-value care. Statins cut sepsis patients’ risk of death. And why reading aloud to kids can cut hyperactivity.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Studies Look at Monoclonal Antibodies for Resistant Infection
As more hospitalized patients develop infections that are immune to antibiotics, researchers are looking into new preventive therapies. National Institute of Allergy and Infectious-supported researchers are studying monoclonal antibodies and their effects on Pseudomonas aeruginosa (P aeruginosa) and Staphylococcus aureus (S aureus), which are among the antibiotic-resistant bacteria that the World Health Organization says pose the greatest risk to human health.
The monoclonal antibodies can be administered along with standard antibiotic therapy. Monoclonal antibodies have been used in cancer, Ebola, and respiratory syncytial virus but rarely have been used to target bacterial pathogens, National Institute of Health says.
One trial, EVADE, will evaluate the safety of the investigational medicine MEDI3902 and whether it can prevent pneumonia caused by P aeruginosa. The other study, SAATELLITE, will test the safety and efficacy of another investigational medicine, suvratoxumab, against S aureus. The researchers hope to enroll 30 patients from 15 intensive care units.
As more hospitalized patients develop infections that are immune to antibiotics, researchers are looking into new preventive therapies. National Institute of Allergy and Infectious-supported researchers are studying monoclonal antibodies and their effects on Pseudomonas aeruginosa (P aeruginosa) and Staphylococcus aureus (S aureus), which are among the antibiotic-resistant bacteria that the World Health Organization says pose the greatest risk to human health.
The monoclonal antibodies can be administered along with standard antibiotic therapy. Monoclonal antibodies have been used in cancer, Ebola, and respiratory syncytial virus but rarely have been used to target bacterial pathogens, National Institute of Health says.
One trial, EVADE, will evaluate the safety of the investigational medicine MEDI3902 and whether it can prevent pneumonia caused by P aeruginosa. The other study, SAATELLITE, will test the safety and efficacy of another investigational medicine, suvratoxumab, against S aureus. The researchers hope to enroll 30 patients from 15 intensive care units.
As more hospitalized patients develop infections that are immune to antibiotics, researchers are looking into new preventive therapies. National Institute of Allergy and Infectious-supported researchers are studying monoclonal antibodies and their effects on Pseudomonas aeruginosa (P aeruginosa) and Staphylococcus aureus (S aureus), which are among the antibiotic-resistant bacteria that the World Health Organization says pose the greatest risk to human health.
The monoclonal antibodies can be administered along with standard antibiotic therapy. Monoclonal antibodies have been used in cancer, Ebola, and respiratory syncytial virus but rarely have been used to target bacterial pathogens, National Institute of Health says.
One trial, EVADE, will evaluate the safety of the investigational medicine MEDI3902 and whether it can prevent pneumonia caused by P aeruginosa. The other study, SAATELLITE, will test the safety and efficacy of another investigational medicine, suvratoxumab, against S aureus. The researchers hope to enroll 30 patients from 15 intensive care units.








