User login
Depression and substance abuse
The crisis of poor physical health and early mortality of psychiatric patients
It is well established that general medical conditions can be associated with various psychiatric disorders. But the reverse is less recognized: That serious mental illness is associated with many physical maladies, often leading to early mortality. Thus, it is a bidirectional medical reality.
The multisystem adverse effects of psychotropic medications, such as metabolic dysregulation, often are blamed for the serious medical problems afflicting psychiatrically ill patients. However, evidence is mounting that while iatrogenic effects play a role, the larger effect appears to be due to a genetic link between psychiatric disorders and cardiovascular risk.1 Unhealthy lifestyles, including sedentary living, poor dietary habits, smoking, and alcohol/substance use, also play a role in the rapid deterioration of physical health and early mortality of individuals afflicted by mood disorders, psychotic disorders, and anxiety disorders. The mantra of “healthy body, healthy mind” is well known, but “unhealthy mind, unhealthy body” should be equally emphasized as a reason for high morbidity and premature mortality in patients with serious mental disorders.
Consider the following alarming findings:
- A recent study revealed that even before the onset of the first psychotic episode, young patients with schizophrenia already suffer from a wide variety of medical conditions.2 In a large sample of 954,351 Danish persons followed from birth to adulthood, of whom 4,371 developed schizophrenia, 95.6% of patients with schizophrenia had a history of hospitalization for somatic problems, including gastrointestinal, endocrine, genitourinary, metabolic, and circulatory system diseases; cancer; and epilepsy. Those findings suggest genetic, physiological, immunological, or developmental overlap between schizophrenia and medical conditions.
- A survey of 67,609 individuals with mood, anxiety, eating, impulse control, or substance use disorders followed for 10 years found that persons with those psychiatric disorders had a significantly higher risk of chronic medical conditions, including heart disease, stroke, hypertension, diabetes, asthma, arthritis, lung disease, peptic ulcer, and cancer.3
- A 7-year follow-up study of 1,138,853 individuals with schizophrenia in the United States found a 350% increase in mortality among this group of patients, who ranged in age from 20 to 64 years, compared with the general population, matched for age, sex, race, ethnicity, and geographic regions.4 An editorial accompanying this study urged psychiatrists to urgently address the “deadly consequences” of major psychiatric disorders.5
- A study of 18,380 individuals with schizophrenia, schizoaffective disorder, or bipolar disorder in London found that these patients were frequently hospitalized for general medical conditions, most commonly urinary, digestive, respiratory, endocrine/metabolic, hematologic, neurologic, dermatologic, and infectious disorders, neoplasm, and poisoning.6 The authors attributed those nonpsychiatric hospitalizations to self-neglect, self-harm, and poor health care access, as well as to “medically unexplained” causes.
- An extremely elevated mortality rate (24-fold higher than the general population) was reported in a 12-month study of young individuals (age 16 to 30 years) diagnosed with psychosis.7 The investigators also found that 61% of the cohort did not fill their antipsychotic prescriptions during that year, and 62% had ≥1 hospitalizations and/or emergency room visits during that year. The relationship between high mortality and lack of treatment with antipsychotics in schizophrenia was confirmed by another recent study,8 a 7-year follow-up of 29,823 persons with schizophrenia in Sweden that measured all-cause mortality. These researchers found the highest mortality among patients not receiving any antipsychotics, while the lowest mortality was among those receiving a long-acting injectable second-generation antipsychotic.
- A recent systematic review of 16 studies that examined glucose homeostasis in first-episode psychosis9 revealed that even at the onset of schizophrenia, glucose homeostasis was already altered, suggesting that predisposition to type 2 diabetes mellitus is a medical condition associated with schizophrenia, and not simply an iatrogenic effect of antipsychotic pharmacotherapy. This adds fodder to the possibility of a genetic overlap between schizophrenia and somatic disorders, including diabetes.10
- In a meta-analysis of 47 studies of young people at “ultra-high risk” for schizophrenia, cardiovascular risk was found to be high, mostly as a result of lifestyle factors such as low levels of physical activity and high rates of smoking and alcohol use, even before the onset of psychosis.11
- The risk of stroke was found to be higher in 80,569 patients with schizophrenia compared with 241,707 age- and sex-matched control subjects.12
- A meta-analysis of the risk of stroke in 6 cohorts with schizophrenia found that there is a higher risk for stroke in schizophrenia, and that this may be related to natural history of the illness itself, not just due to comorbid metabolic risk factors.13
- The high rate of cardiovascular disease in depression has been attributed to neuroinflammation14 or possibly to increased platelet reactivity.15
Continue to: As psychiatric physicians...
As psychiatric physicians, we always screen our patients for past and current medical conditions that are comorbid with their psychiatric disorders. We are aware of the lifestyle factors that increase these patients’ physical morbidity and mortality, above and beyond their suicide-related mortality. Our patients with schizophrenia and mood disorders have triple the smoking rates of the general population, and they tend to be sedentary with poor eating habits that lead to obesity, obstructive sleep apnea, diabetes, hypertension, and dyslipidemia, which increases their risk for heart attack, stroke, and cancer. Self-neglect during acute episodes of depression or psychosis increases the risk of infection, malnutrition, and tooth decay. We also see skin damage in obsessive-compulsive disorder patients who are compelled to wash their hands numerous times a day, the life-threatening effects of anorexia nervosa, and various types of medical ailments caused by incomplete suicidal attempts. Poverty and substance use among chronically mentally ill patients also increase the odds of physical ailments.
So we need to act diligently to reduce the alarming medical morbidity and mortality of the psychiatric population. Collaborative care with a primary care provider is a must, not an option, for every patient, because studies indicate that without collaborative care, patients receive inadequate primary care.16 Providing rapid access to standard medical care is the single most critical step for the prevention or amelioration of physical disorders in our psychiatric patients, concurrently with stabilizing their ailing brains and minds. If we focus only on treating psychopathology, then we will win the battle against mental illness, but lose the war of life and death.
1. Azad MC, Shoesmith WD, Al Mamun M, et al. Cardiovascular diseases among patients with schizophrenia. Asian J Psychiatr. 2016;19:28-36.
2. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900 000 individuals. Schizophr Bull. 2015;41(2):513-521.
3. Scott KM, Lim C, Al-Hamzawi A, et al. Association of mental disorders with subsequent chronic physical conditions: world mental health surveys from 17 countries. JAMA Psychiatry. 2016;73(2):150-158.
4. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
5. Suetani S, Whiteford HA, McGrath JJ. An urgent call to address the deadly consequences of serious mental disorders. JAMA Psychiatry. 2015;72(12):1166-1167.
6. Jayatilleke N, Hayes RD, Chang CK, et al. Acute general hospital admissions in people with serious mental illness [published online February 28, 2018]. Psychol Med. 2018;1-8.
7. Schoenbaum M, Sutherland JM, Chappel A, et al. Twelve-month health care use and mortality in commercially insured young people with incident psychosis in the United States. Schizophr Bull. 2017;43(6):1262-1272.
8. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia [published online December 20, 2017]. Schizophr Res. pii: S0920-9964(17)30762-4. doi: 10.1016/j.schres.2017.12.010.
9. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
10. Dieset I, Andreassen OA, Haukvik UK. Somatic comorbidity in schizophrenia: some possible biological mechanisms across the life span. Schizophr Bull. 2016;42(6):1316-1319.
11. Carney R, Cotter J, Bradshaw T, et al. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2016;170(2-3):290-300.
12. Tsai KY, Lee CC, Chou YM, et al. The incidence and relative risk of stroke in patients with schizophrenia: a five-year follow-up study. Schizophr Res. 2012;138(1):41-47.
13. Li M, Fan YL, Tang ZY, et al. Schizophrenia and risk of stroke: a meta-analysis of cohort studies. Int J Cardiol. 2014;173(3):588-590.
14. Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45-70.
15. Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140(suppl 4):57-62.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
It is well established that general medical conditions can be associated with various psychiatric disorders. But the reverse is less recognized: That serious mental illness is associated with many physical maladies, often leading to early mortality. Thus, it is a bidirectional medical reality.
The multisystem adverse effects of psychotropic medications, such as metabolic dysregulation, often are blamed for the serious medical problems afflicting psychiatrically ill patients. However, evidence is mounting that while iatrogenic effects play a role, the larger effect appears to be due to a genetic link between psychiatric disorders and cardiovascular risk.1 Unhealthy lifestyles, including sedentary living, poor dietary habits, smoking, and alcohol/substance use, also play a role in the rapid deterioration of physical health and early mortality of individuals afflicted by mood disorders, psychotic disorders, and anxiety disorders. The mantra of “healthy body, healthy mind” is well known, but “unhealthy mind, unhealthy body” should be equally emphasized as a reason for high morbidity and premature mortality in patients with serious mental disorders.
Consider the following alarming findings:
- A recent study revealed that even before the onset of the first psychotic episode, young patients with schizophrenia already suffer from a wide variety of medical conditions.2 In a large sample of 954,351 Danish persons followed from birth to adulthood, of whom 4,371 developed schizophrenia, 95.6% of patients with schizophrenia had a history of hospitalization for somatic problems, including gastrointestinal, endocrine, genitourinary, metabolic, and circulatory system diseases; cancer; and epilepsy. Those findings suggest genetic, physiological, immunological, or developmental overlap between schizophrenia and medical conditions.
- A survey of 67,609 individuals with mood, anxiety, eating, impulse control, or substance use disorders followed for 10 years found that persons with those psychiatric disorders had a significantly higher risk of chronic medical conditions, including heart disease, stroke, hypertension, diabetes, asthma, arthritis, lung disease, peptic ulcer, and cancer.3
- A 7-year follow-up study of 1,138,853 individuals with schizophrenia in the United States found a 350% increase in mortality among this group of patients, who ranged in age from 20 to 64 years, compared with the general population, matched for age, sex, race, ethnicity, and geographic regions.4 An editorial accompanying this study urged psychiatrists to urgently address the “deadly consequences” of major psychiatric disorders.5
- A study of 18,380 individuals with schizophrenia, schizoaffective disorder, or bipolar disorder in London found that these patients were frequently hospitalized for general medical conditions, most commonly urinary, digestive, respiratory, endocrine/metabolic, hematologic, neurologic, dermatologic, and infectious disorders, neoplasm, and poisoning.6 The authors attributed those nonpsychiatric hospitalizations to self-neglect, self-harm, and poor health care access, as well as to “medically unexplained” causes.
- An extremely elevated mortality rate (24-fold higher than the general population) was reported in a 12-month study of young individuals (age 16 to 30 years) diagnosed with psychosis.7 The investigators also found that 61% of the cohort did not fill their antipsychotic prescriptions during that year, and 62% had ≥1 hospitalizations and/or emergency room visits during that year. The relationship between high mortality and lack of treatment with antipsychotics in schizophrenia was confirmed by another recent study,8 a 7-year follow-up of 29,823 persons with schizophrenia in Sweden that measured all-cause mortality. These researchers found the highest mortality among patients not receiving any antipsychotics, while the lowest mortality was among those receiving a long-acting injectable second-generation antipsychotic.
- A recent systematic review of 16 studies that examined glucose homeostasis in first-episode psychosis9 revealed that even at the onset of schizophrenia, glucose homeostasis was already altered, suggesting that predisposition to type 2 diabetes mellitus is a medical condition associated with schizophrenia, and not simply an iatrogenic effect of antipsychotic pharmacotherapy. This adds fodder to the possibility of a genetic overlap between schizophrenia and somatic disorders, including diabetes.10
- In a meta-analysis of 47 studies of young people at “ultra-high risk” for schizophrenia, cardiovascular risk was found to be high, mostly as a result of lifestyle factors such as low levels of physical activity and high rates of smoking and alcohol use, even before the onset of psychosis.11
- The risk of stroke was found to be higher in 80,569 patients with schizophrenia compared with 241,707 age- and sex-matched control subjects.12
- A meta-analysis of the risk of stroke in 6 cohorts with schizophrenia found that there is a higher risk for stroke in schizophrenia, and that this may be related to natural history of the illness itself, not just due to comorbid metabolic risk factors.13
- The high rate of cardiovascular disease in depression has been attributed to neuroinflammation14 or possibly to increased platelet reactivity.15
Continue to: As psychiatric physicians...
As psychiatric physicians, we always screen our patients for past and current medical conditions that are comorbid with their psychiatric disorders. We are aware of the lifestyle factors that increase these patients’ physical morbidity and mortality, above and beyond their suicide-related mortality. Our patients with schizophrenia and mood disorders have triple the smoking rates of the general population, and they tend to be sedentary with poor eating habits that lead to obesity, obstructive sleep apnea, diabetes, hypertension, and dyslipidemia, which increases their risk for heart attack, stroke, and cancer. Self-neglect during acute episodes of depression or psychosis increases the risk of infection, malnutrition, and tooth decay. We also see skin damage in obsessive-compulsive disorder patients who are compelled to wash their hands numerous times a day, the life-threatening effects of anorexia nervosa, and various types of medical ailments caused by incomplete suicidal attempts. Poverty and substance use among chronically mentally ill patients also increase the odds of physical ailments.
So we need to act diligently to reduce the alarming medical morbidity and mortality of the psychiatric population. Collaborative care with a primary care provider is a must, not an option, for every patient, because studies indicate that without collaborative care, patients receive inadequate primary care.16 Providing rapid access to standard medical care is the single most critical step for the prevention or amelioration of physical disorders in our psychiatric patients, concurrently with stabilizing their ailing brains and minds. If we focus only on treating psychopathology, then we will win the battle against mental illness, but lose the war of life and death.
It is well established that general medical conditions can be associated with various psychiatric disorders. But the reverse is less recognized: That serious mental illness is associated with many physical maladies, often leading to early mortality. Thus, it is a bidirectional medical reality.
The multisystem adverse effects of psychotropic medications, such as metabolic dysregulation, often are blamed for the serious medical problems afflicting psychiatrically ill patients. However, evidence is mounting that while iatrogenic effects play a role, the larger effect appears to be due to a genetic link between psychiatric disorders and cardiovascular risk.1 Unhealthy lifestyles, including sedentary living, poor dietary habits, smoking, and alcohol/substance use, also play a role in the rapid deterioration of physical health and early mortality of individuals afflicted by mood disorders, psychotic disorders, and anxiety disorders. The mantra of “healthy body, healthy mind” is well known, but “unhealthy mind, unhealthy body” should be equally emphasized as a reason for high morbidity and premature mortality in patients with serious mental disorders.
Consider the following alarming findings:
- A recent study revealed that even before the onset of the first psychotic episode, young patients with schizophrenia already suffer from a wide variety of medical conditions.2 In a large sample of 954,351 Danish persons followed from birth to adulthood, of whom 4,371 developed schizophrenia, 95.6% of patients with schizophrenia had a history of hospitalization for somatic problems, including gastrointestinal, endocrine, genitourinary, metabolic, and circulatory system diseases; cancer; and epilepsy. Those findings suggest genetic, physiological, immunological, or developmental overlap between schizophrenia and medical conditions.
- A survey of 67,609 individuals with mood, anxiety, eating, impulse control, or substance use disorders followed for 10 years found that persons with those psychiatric disorders had a significantly higher risk of chronic medical conditions, including heart disease, stroke, hypertension, diabetes, asthma, arthritis, lung disease, peptic ulcer, and cancer.3
- A 7-year follow-up study of 1,138,853 individuals with schizophrenia in the United States found a 350% increase in mortality among this group of patients, who ranged in age from 20 to 64 years, compared with the general population, matched for age, sex, race, ethnicity, and geographic regions.4 An editorial accompanying this study urged psychiatrists to urgently address the “deadly consequences” of major psychiatric disorders.5
- A study of 18,380 individuals with schizophrenia, schizoaffective disorder, or bipolar disorder in London found that these patients were frequently hospitalized for general medical conditions, most commonly urinary, digestive, respiratory, endocrine/metabolic, hematologic, neurologic, dermatologic, and infectious disorders, neoplasm, and poisoning.6 The authors attributed those nonpsychiatric hospitalizations to self-neglect, self-harm, and poor health care access, as well as to “medically unexplained” causes.
- An extremely elevated mortality rate (24-fold higher than the general population) was reported in a 12-month study of young individuals (age 16 to 30 years) diagnosed with psychosis.7 The investigators also found that 61% of the cohort did not fill their antipsychotic prescriptions during that year, and 62% had ≥1 hospitalizations and/or emergency room visits during that year. The relationship between high mortality and lack of treatment with antipsychotics in schizophrenia was confirmed by another recent study,8 a 7-year follow-up of 29,823 persons with schizophrenia in Sweden that measured all-cause mortality. These researchers found the highest mortality among patients not receiving any antipsychotics, while the lowest mortality was among those receiving a long-acting injectable second-generation antipsychotic.
- A recent systematic review of 16 studies that examined glucose homeostasis in first-episode psychosis9 revealed that even at the onset of schizophrenia, glucose homeostasis was already altered, suggesting that predisposition to type 2 diabetes mellitus is a medical condition associated with schizophrenia, and not simply an iatrogenic effect of antipsychotic pharmacotherapy. This adds fodder to the possibility of a genetic overlap between schizophrenia and somatic disorders, including diabetes.10
- In a meta-analysis of 47 studies of young people at “ultra-high risk” for schizophrenia, cardiovascular risk was found to be high, mostly as a result of lifestyle factors such as low levels of physical activity and high rates of smoking and alcohol use, even before the onset of psychosis.11
- The risk of stroke was found to be higher in 80,569 patients with schizophrenia compared with 241,707 age- and sex-matched control subjects.12
- A meta-analysis of the risk of stroke in 6 cohorts with schizophrenia found that there is a higher risk for stroke in schizophrenia, and that this may be related to natural history of the illness itself, not just due to comorbid metabolic risk factors.13
- The high rate of cardiovascular disease in depression has been attributed to neuroinflammation14 or possibly to increased platelet reactivity.15
Continue to: As psychiatric physicians...
As psychiatric physicians, we always screen our patients for past and current medical conditions that are comorbid with their psychiatric disorders. We are aware of the lifestyle factors that increase these patients’ physical morbidity and mortality, above and beyond their suicide-related mortality. Our patients with schizophrenia and mood disorders have triple the smoking rates of the general population, and they tend to be sedentary with poor eating habits that lead to obesity, obstructive sleep apnea, diabetes, hypertension, and dyslipidemia, which increases their risk for heart attack, stroke, and cancer. Self-neglect during acute episodes of depression or psychosis increases the risk of infection, malnutrition, and tooth decay. We also see skin damage in obsessive-compulsive disorder patients who are compelled to wash their hands numerous times a day, the life-threatening effects of anorexia nervosa, and various types of medical ailments caused by incomplete suicidal attempts. Poverty and substance use among chronically mentally ill patients also increase the odds of physical ailments.
So we need to act diligently to reduce the alarming medical morbidity and mortality of the psychiatric population. Collaborative care with a primary care provider is a must, not an option, for every patient, because studies indicate that without collaborative care, patients receive inadequate primary care.16 Providing rapid access to standard medical care is the single most critical step for the prevention or amelioration of physical disorders in our psychiatric patients, concurrently with stabilizing their ailing brains and minds. If we focus only on treating psychopathology, then we will win the battle against mental illness, but lose the war of life and death.
1. Azad MC, Shoesmith WD, Al Mamun M, et al. Cardiovascular diseases among patients with schizophrenia. Asian J Psychiatr. 2016;19:28-36.
2. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900 000 individuals. Schizophr Bull. 2015;41(2):513-521.
3. Scott KM, Lim C, Al-Hamzawi A, et al. Association of mental disorders with subsequent chronic physical conditions: world mental health surveys from 17 countries. JAMA Psychiatry. 2016;73(2):150-158.
4. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
5. Suetani S, Whiteford HA, McGrath JJ. An urgent call to address the deadly consequences of serious mental disorders. JAMA Psychiatry. 2015;72(12):1166-1167.
6. Jayatilleke N, Hayes RD, Chang CK, et al. Acute general hospital admissions in people with serious mental illness [published online February 28, 2018]. Psychol Med. 2018;1-8.
7. Schoenbaum M, Sutherland JM, Chappel A, et al. Twelve-month health care use and mortality in commercially insured young people with incident psychosis in the United States. Schizophr Bull. 2017;43(6):1262-1272.
8. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia [published online December 20, 2017]. Schizophr Res. pii: S0920-9964(17)30762-4. doi: 10.1016/j.schres.2017.12.010.
9. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
10. Dieset I, Andreassen OA, Haukvik UK. Somatic comorbidity in schizophrenia: some possible biological mechanisms across the life span. Schizophr Bull. 2016;42(6):1316-1319.
11. Carney R, Cotter J, Bradshaw T, et al. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2016;170(2-3):290-300.
12. Tsai KY, Lee CC, Chou YM, et al. The incidence and relative risk of stroke in patients with schizophrenia: a five-year follow-up study. Schizophr Res. 2012;138(1):41-47.
13. Li M, Fan YL, Tang ZY, et al. Schizophrenia and risk of stroke: a meta-analysis of cohort studies. Int J Cardiol. 2014;173(3):588-590.
14. Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45-70.
15. Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140(suppl 4):57-62.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
1. Azad MC, Shoesmith WD, Al Mamun M, et al. Cardiovascular diseases among patients with schizophrenia. Asian J Psychiatr. 2016;19:28-36.
2. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900 000 individuals. Schizophr Bull. 2015;41(2):513-521.
3. Scott KM, Lim C, Al-Hamzawi A, et al. Association of mental disorders with subsequent chronic physical conditions: world mental health surveys from 17 countries. JAMA Psychiatry. 2016;73(2):150-158.
4. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
5. Suetani S, Whiteford HA, McGrath JJ. An urgent call to address the deadly consequences of serious mental disorders. JAMA Psychiatry. 2015;72(12):1166-1167.
6. Jayatilleke N, Hayes RD, Chang CK, et al. Acute general hospital admissions in people with serious mental illness [published online February 28, 2018]. Psychol Med. 2018;1-8.
7. Schoenbaum M, Sutherland JM, Chappel A, et al. Twelve-month health care use and mortality in commercially insured young people with incident psychosis in the United States. Schizophr Bull. 2017;43(6):1262-1272.
8. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia [published online December 20, 2017]. Schizophr Res. pii: S0920-9964(17)30762-4. doi: 10.1016/j.schres.2017.12.010.
9. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261-269.
10. Dieset I, Andreassen OA, Haukvik UK. Somatic comorbidity in schizophrenia: some possible biological mechanisms across the life span. Schizophr Bull. 2016;42(6):1316-1319.
11. Carney R, Cotter J, Bradshaw T, et al. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2016;170(2-3):290-300.
12. Tsai KY, Lee CC, Chou YM, et al. The incidence and relative risk of stroke in patients with schizophrenia: a five-year follow-up study. Schizophr Res. 2012;138(1):41-47.
13. Li M, Fan YL, Tang ZY, et al. Schizophrenia and risk of stroke: a meta-analysis of cohort studies. Int J Cardiol. 2014;173(3):588-590.
14. Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45-70.
15. Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140(suppl 4):57-62.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
How precision psychiatry helped my patient; Ketamine: The next ‘opioid crisis’?
How precision psychiatry helped my patient
I applaud Dr. Nasrallah’s editorial “The dawn of precision psychiatry” (From the Editor,
Ms. G, age 14, presented with periodic emotional “meltdowns,” which would occur in any setting, and I determined that they were precipitated by a high glycemic intake. By carefully controlling her glycemic intake and starting her on caprylic acid (a medium-chain triglyceride, which was used to maintain a ketotic state), 1 tablespoon 3 times daily, we were able to reduce the frequency of her episodes by 80% to 90%. Using data from commercially available DNA testing, I determined that she had single nucleotide polymorphisms (SNPs) in an alpha-ketoglutarate dehydrogenase gene, which is primarily located in the prefrontal cortex (PFC), and whose function is supported by thiamine and impaired by high glycemic intake.1 After adding oral thiamine hydrochloride, 100 mg twice a day, and correcting other abnormalities (eg, she was hypothyroid), her episodes are now rare. She is functioning well, has been getting high grades, and recently wrote a 40-page short story.
Once she improved, she was able to describe having a partial seizure, with a rising sensation, which often improves with ketosis. Clearly, disruption of her PFC energetics due to the SNPs described above contributed to the disinhibition of the temporal lobe structures. Furthermore, she has an APOE3/4 status, which puts her at risk for Alzheimer’s disease. Her mother was educated about the importance of good health habits, which is personalized and preventative medicine.
Robert Hedaya, MD, DLFAPA
Clinical Professor
MedStar Georgetown University Hospital
Washington, DC
Faculty
Institute for Functional Medicine
Gig Harbor, Washington, DC
Founder
National Center for Whole Psychiatry
Rockville, Maryland
Reference
1. Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2335-2345.
Dr. Nasrallah responds
My thanks to Dr. Hedaya for his letter and for providing an excellent example of precision psychiatry. His brief case vignette brings it to life! I commend him for practicing on the cutting-edge of psychiatry’s scientific frontier.
Continue to: Ketamine: The next 'opioid crisis'?
Ketamine: The next ‘opioid crisis’?
The chief of the FDA, Scott Gottlieb, MD, recently discussed the
There are many similarities between the use of opioids to treat pain and the potential use of ketamine to treat suicidality. Physical and mental pain are subjective, qualitative, and difficult to quantify, which makes it difficult to develop accurate measurements of symptom severity. Chronic physical pain and suicidality are not illnesses, but symptoms of myriad types of pathologies with differing etiologies and treatment options.5 Due to the ambiguous and subjective experience of physical and mental pain, we tend to lump them together as 1 pathological category without understanding pathophysiologic differences. The most commonly reported types of pain include low back pain, migraine/headache, neck pain, and facial pain.6 However, each of these pain types would likely have a different pathophysiology and treatment. Likewise, suicide can be associated with various psychiatric conditions,7 and suicidality resulting from these conditions may require a different etiology and treatment.
We already know that both opioids and ketamine are addictive. For example, there is a report of a nurse stealing a hospital’s supply of ketamine and self-treating for depression, which led to an inpatient detox admission after she developed toxicity and addiction.8 Some ketamine research supports its safe use, but it may be biased due to conflicts of interest. For example, several authors of a recent study proclaiming the effectiveness of a single dose of ketamine in treating suicidal ideation
Warnings stating how both opioid and ketamine should be used were published years ago but have since been ignored. For example, a 1977 article advocated that opioids should only be used for a “short duration and limited to patients with acute diseases or inoperable or metastatic cancer who require long-term relief.”10 The rationale for this distinction was foretelling of the current opioid epidemic: “Continued and prolonged use of narcotics in patients with chronic benign pain is not recommended because of serious behavioral consequences, the development of tolerance, and addiction liability. Long-term use of analgesic drugs in chronic pain usually produces negative behavioral complications that are more difficult to manage than the pain it was desired to eliminate.”10 We knew better then.
The earliest report of ketamine dependency I could find was published in 1987, which predates its classification as a controlled substance.11 More recently, ketamine dependency has been associated with adverse effects that are similar to “not only cocaine and amphetamine but also with opiates, alcohol and cannabis, as well as the psychological attractions of its distinctive psychedelic properties.”12 We should consider ourselves warned.
Michael Shapiro, MD
Assistant Professor
Department of Psychiatry
University of Florida
Gainesville, Florida
References
1. Jayne O’Donnell. FDA chief supports opioid prescription limits, regrets agency’s prior inaction. USA TODAY. https://www.usatoday.com/story/news/politics/2017/10/23/fda-chief-supports-opioid-prescription-limits-regrets-agencys-prior-inaction/774007001. Published October 23, 2017. Accessed January 25, 2018.
2. Bill Whitaker. Ex-DEA agent: opioid crisis fueled by drug industry and Congress. CBS News. https://www.cbsnews.com/news/ex-dea-agent-opioid-crisis-fueled-by-drug-industry-and-congress. Published October 15, 2017. Accessed January 25, 2018.
3. Drug Enforcement Administration. Diversion of Control Division. Ketamine. https://www.deadiversion.usdoj.gov/drug_chem_info/ketamine.pdf. Published August 2013. Accessed January 25, 2018.
4. Bell RF. Ketamine for chronic noncancer pain: concerns regarding toxicity. Curr Opin Support Palliat Care. 2012;6(2):183-187.
5. Barzilay S, Apter A. Psychological models of suicide. Arch Suicide Res. 2014;18(4):295-312.
6. American Academy of Pain Medicine. AAPM facts and figures on pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx.
7. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
8. Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47(4):276-85.
9. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2017. https://doi.org/10.1176/appi.ajp.2017.17040472
10. Halpern LM. Analgesic drugs in the management of pain. Arch Surg. 1977;112(7):861-869.
11. Kamaya H, Krishna PR. Anesthesiology. 1987;67(5):861-862.
12. Jansen KL, Darracot-Cankovic R. The nonmedical use of ketamine, part two: a review of problem use and dependence. J Psychoactive Drugs. 2001;33(2):151-158.
How precision psychiatry helped my patient
I applaud Dr. Nasrallah’s editorial “The dawn of precision psychiatry” (From the Editor,
Ms. G, age 14, presented with periodic emotional “meltdowns,” which would occur in any setting, and I determined that they were precipitated by a high glycemic intake. By carefully controlling her glycemic intake and starting her on caprylic acid (a medium-chain triglyceride, which was used to maintain a ketotic state), 1 tablespoon 3 times daily, we were able to reduce the frequency of her episodes by 80% to 90%. Using data from commercially available DNA testing, I determined that she had single nucleotide polymorphisms (SNPs) in an alpha-ketoglutarate dehydrogenase gene, which is primarily located in the prefrontal cortex (PFC), and whose function is supported by thiamine and impaired by high glycemic intake.1 After adding oral thiamine hydrochloride, 100 mg twice a day, and correcting other abnormalities (eg, she was hypothyroid), her episodes are now rare. She is functioning well, has been getting high grades, and recently wrote a 40-page short story.
Once she improved, she was able to describe having a partial seizure, with a rising sensation, which often improves with ketosis. Clearly, disruption of her PFC energetics due to the SNPs described above contributed to the disinhibition of the temporal lobe structures. Furthermore, she has an APOE3/4 status, which puts her at risk for Alzheimer’s disease. Her mother was educated about the importance of good health habits, which is personalized and preventative medicine.
Robert Hedaya, MD, DLFAPA
Clinical Professor
MedStar Georgetown University Hospital
Washington, DC
Faculty
Institute for Functional Medicine
Gig Harbor, Washington, DC
Founder
National Center for Whole Psychiatry
Rockville, Maryland
Reference
1. Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2335-2345.
Dr. Nasrallah responds
My thanks to Dr. Hedaya for his letter and for providing an excellent example of precision psychiatry. His brief case vignette brings it to life! I commend him for practicing on the cutting-edge of psychiatry’s scientific frontier.
Continue to: Ketamine: The next 'opioid crisis'?
Ketamine: The next ‘opioid crisis’?
The chief of the FDA, Scott Gottlieb, MD, recently discussed the
There are many similarities between the use of opioids to treat pain and the potential use of ketamine to treat suicidality. Physical and mental pain are subjective, qualitative, and difficult to quantify, which makes it difficult to develop accurate measurements of symptom severity. Chronic physical pain and suicidality are not illnesses, but symptoms of myriad types of pathologies with differing etiologies and treatment options.5 Due to the ambiguous and subjective experience of physical and mental pain, we tend to lump them together as 1 pathological category without understanding pathophysiologic differences. The most commonly reported types of pain include low back pain, migraine/headache, neck pain, and facial pain.6 However, each of these pain types would likely have a different pathophysiology and treatment. Likewise, suicide can be associated with various psychiatric conditions,7 and suicidality resulting from these conditions may require a different etiology and treatment.
We already know that both opioids and ketamine are addictive. For example, there is a report of a nurse stealing a hospital’s supply of ketamine and self-treating for depression, which led to an inpatient detox admission after she developed toxicity and addiction.8 Some ketamine research supports its safe use, but it may be biased due to conflicts of interest. For example, several authors of a recent study proclaiming the effectiveness of a single dose of ketamine in treating suicidal ideation
Warnings stating how both opioid and ketamine should be used were published years ago but have since been ignored. For example, a 1977 article advocated that opioids should only be used for a “short duration and limited to patients with acute diseases or inoperable or metastatic cancer who require long-term relief.”10 The rationale for this distinction was foretelling of the current opioid epidemic: “Continued and prolonged use of narcotics in patients with chronic benign pain is not recommended because of serious behavioral consequences, the development of tolerance, and addiction liability. Long-term use of analgesic drugs in chronic pain usually produces negative behavioral complications that are more difficult to manage than the pain it was desired to eliminate.”10 We knew better then.
The earliest report of ketamine dependency I could find was published in 1987, which predates its classification as a controlled substance.11 More recently, ketamine dependency has been associated with adverse effects that are similar to “not only cocaine and amphetamine but also with opiates, alcohol and cannabis, as well as the psychological attractions of its distinctive psychedelic properties.”12 We should consider ourselves warned.
Michael Shapiro, MD
Assistant Professor
Department of Psychiatry
University of Florida
Gainesville, Florida
References
1. Jayne O’Donnell. FDA chief supports opioid prescription limits, regrets agency’s prior inaction. USA TODAY. https://www.usatoday.com/story/news/politics/2017/10/23/fda-chief-supports-opioid-prescription-limits-regrets-agencys-prior-inaction/774007001. Published October 23, 2017. Accessed January 25, 2018.
2. Bill Whitaker. Ex-DEA agent: opioid crisis fueled by drug industry and Congress. CBS News. https://www.cbsnews.com/news/ex-dea-agent-opioid-crisis-fueled-by-drug-industry-and-congress. Published October 15, 2017. Accessed January 25, 2018.
3. Drug Enforcement Administration. Diversion of Control Division. Ketamine. https://www.deadiversion.usdoj.gov/drug_chem_info/ketamine.pdf. Published August 2013. Accessed January 25, 2018.
4. Bell RF. Ketamine for chronic noncancer pain: concerns regarding toxicity. Curr Opin Support Palliat Care. 2012;6(2):183-187.
5. Barzilay S, Apter A. Psychological models of suicide. Arch Suicide Res. 2014;18(4):295-312.
6. American Academy of Pain Medicine. AAPM facts and figures on pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx.
7. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
8. Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47(4):276-85.
9. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2017. https://doi.org/10.1176/appi.ajp.2017.17040472
10. Halpern LM. Analgesic drugs in the management of pain. Arch Surg. 1977;112(7):861-869.
11. Kamaya H, Krishna PR. Anesthesiology. 1987;67(5):861-862.
12. Jansen KL, Darracot-Cankovic R. The nonmedical use of ketamine, part two: a review of problem use and dependence. J Psychoactive Drugs. 2001;33(2):151-158.
How precision psychiatry helped my patient
I applaud Dr. Nasrallah’s editorial “The dawn of precision psychiatry” (From the Editor,
Ms. G, age 14, presented with periodic emotional “meltdowns,” which would occur in any setting, and I determined that they were precipitated by a high glycemic intake. By carefully controlling her glycemic intake and starting her on caprylic acid (a medium-chain triglyceride, which was used to maintain a ketotic state), 1 tablespoon 3 times daily, we were able to reduce the frequency of her episodes by 80% to 90%. Using data from commercially available DNA testing, I determined that she had single nucleotide polymorphisms (SNPs) in an alpha-ketoglutarate dehydrogenase gene, which is primarily located in the prefrontal cortex (PFC), and whose function is supported by thiamine and impaired by high glycemic intake.1 After adding oral thiamine hydrochloride, 100 mg twice a day, and correcting other abnormalities (eg, she was hypothyroid), her episodes are now rare. She is functioning well, has been getting high grades, and recently wrote a 40-page short story.
Once she improved, she was able to describe having a partial seizure, with a rising sensation, which often improves with ketosis. Clearly, disruption of her PFC energetics due to the SNPs described above contributed to the disinhibition of the temporal lobe structures. Furthermore, she has an APOE3/4 status, which puts her at risk for Alzheimer’s disease. Her mother was educated about the importance of good health habits, which is personalized and preventative medicine.
Robert Hedaya, MD, DLFAPA
Clinical Professor
MedStar Georgetown University Hospital
Washington, DC
Faculty
Institute for Functional Medicine
Gig Harbor, Washington, DC
Founder
National Center for Whole Psychiatry
Rockville, Maryland
Reference
1. Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2335-2345.
Dr. Nasrallah responds
My thanks to Dr. Hedaya for his letter and for providing an excellent example of precision psychiatry. His brief case vignette brings it to life! I commend him for practicing on the cutting-edge of psychiatry’s scientific frontier.
Continue to: Ketamine: The next 'opioid crisis'?
Ketamine: The next ‘opioid crisis’?
The chief of the FDA, Scott Gottlieb, MD, recently discussed the
There are many similarities between the use of opioids to treat pain and the potential use of ketamine to treat suicidality. Physical and mental pain are subjective, qualitative, and difficult to quantify, which makes it difficult to develop accurate measurements of symptom severity. Chronic physical pain and suicidality are not illnesses, but symptoms of myriad types of pathologies with differing etiologies and treatment options.5 Due to the ambiguous and subjective experience of physical and mental pain, we tend to lump them together as 1 pathological category without understanding pathophysiologic differences. The most commonly reported types of pain include low back pain, migraine/headache, neck pain, and facial pain.6 However, each of these pain types would likely have a different pathophysiology and treatment. Likewise, suicide can be associated with various psychiatric conditions,7 and suicidality resulting from these conditions may require a different etiology and treatment.
We already know that both opioids and ketamine are addictive. For example, there is a report of a nurse stealing a hospital’s supply of ketamine and self-treating for depression, which led to an inpatient detox admission after she developed toxicity and addiction.8 Some ketamine research supports its safe use, but it may be biased due to conflicts of interest. For example, several authors of a recent study proclaiming the effectiveness of a single dose of ketamine in treating suicidal ideation
Warnings stating how both opioid and ketamine should be used were published years ago but have since been ignored. For example, a 1977 article advocated that opioids should only be used for a “short duration and limited to patients with acute diseases or inoperable or metastatic cancer who require long-term relief.”10 The rationale for this distinction was foretelling of the current opioid epidemic: “Continued and prolonged use of narcotics in patients with chronic benign pain is not recommended because of serious behavioral consequences, the development of tolerance, and addiction liability. Long-term use of analgesic drugs in chronic pain usually produces negative behavioral complications that are more difficult to manage than the pain it was desired to eliminate.”10 We knew better then.
The earliest report of ketamine dependency I could find was published in 1987, which predates its classification as a controlled substance.11 More recently, ketamine dependency has been associated with adverse effects that are similar to “not only cocaine and amphetamine but also with opiates, alcohol and cannabis, as well as the psychological attractions of its distinctive psychedelic properties.”12 We should consider ourselves warned.
Michael Shapiro, MD
Assistant Professor
Department of Psychiatry
University of Florida
Gainesville, Florida
References
1. Jayne O’Donnell. FDA chief supports opioid prescription limits, regrets agency’s prior inaction. USA TODAY. https://www.usatoday.com/story/news/politics/2017/10/23/fda-chief-supports-opioid-prescription-limits-regrets-agencys-prior-inaction/774007001. Published October 23, 2017. Accessed January 25, 2018.
2. Bill Whitaker. Ex-DEA agent: opioid crisis fueled by drug industry and Congress. CBS News. https://www.cbsnews.com/news/ex-dea-agent-opioid-crisis-fueled-by-drug-industry-and-congress. Published October 15, 2017. Accessed January 25, 2018.
3. Drug Enforcement Administration. Diversion of Control Division. Ketamine. https://www.deadiversion.usdoj.gov/drug_chem_info/ketamine.pdf. Published August 2013. Accessed January 25, 2018.
4. Bell RF. Ketamine for chronic noncancer pain: concerns regarding toxicity. Curr Opin Support Palliat Care. 2012;6(2):183-187.
5. Barzilay S, Apter A. Psychological models of suicide. Arch Suicide Res. 2014;18(4):295-312.
6. American Academy of Pain Medicine. AAPM facts and figures on pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx.
7. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
8. Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47(4):276-85.
9. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2017. https://doi.org/10.1176/appi.ajp.2017.17040472
10. Halpern LM. Analgesic drugs in the management of pain. Arch Surg. 1977;112(7):861-869.
11. Kamaya H, Krishna PR. Anesthesiology. 1987;67(5):861-862.
12. Jansen KL, Darracot-Cankovic R. The nonmedical use of ketamine, part two: a review of problem use and dependence. J Psychoactive Drugs. 2001;33(2):151-158.
A 10-year-old boy with ‘voices in my head’: Is it a psychotic disorder?
CASE Auditory hallucinations?
M, age 10, has had multiple visits to the pediatric emergency department (PED) with the chief concern of excessive urinary frequency. At each visit, the medical workup has been negative and he was discharged home. After a few months, M’s parents bring their son back to the PED because he reports hearing “voices in my head” and “feeling tense and scared.” When these feelings become too overwhelming, M stops eating and experiences substantial fear and anxiety that require his mother’s repeated reassurances. M’s mother reports that 2 weeks before his most recent PED visit, he became increasingly anxious and disturbed, and said he was afraid most of the time, and worried about the safety of his family for no apparent reason.
The psychiatrist evaluates M in the PED and diagnoses him with unspecified schizophrenia spectrum and other psychotic disorder based on his persistent report of auditory and tactile hallucinations, including hearing a voice of a man telling him he was going to choke on his food and feeling someone touch his arm to soothe him during his anxious moments. M does not meet criteria for acute inpatient hospitalization, and is discharged home with referral to follow-up at our child and adolescent psychiatry outpatient clinic.
On subsequent evaluation in our clinic, M reports most of the same about his experience hearing “voices in my head” that repeatedly suggest “I might choke on my food and end up seriously ill in the hospital.” He started to hear the “voices” after he witnessed his sister choke while eating a few days earlier. He also mentions that the “voices” tell him “you have to use the restroom.” As a result, he uses the restroom several times before leaving for home and is frequently late for school. His parents accommodate his behavior—his mother allows him to use the bathroom multiple times, and his father overlooks the behavior as part of school anxiety.
At school, his teacher reports a concern for attention-deficit/hyperactivity disorder (ADHD) based on M’s continuous inattentiveness in class and dropping grades. He asks for bathroom breaks up to 15 times a day, which disrupts his class work.
These behaviors have led to a gradual 1-year decline in his overall functioning, including difficulty at school for requesting too many bathroom breaks; having to repeat the 3rd grade; and incurring multiple hospital visits for evaluation of his various complaints. M has become socially isolated and withdrawn from friends and family.
M’s developmental history is normal and his family history is negative for any psychiatric disorder. Medical history and physical examination are unremarkable. CT scan of his head is unremarkable, and all hematologic and biochemistry laboratory test values are within normal range.
[polldaddy:9971376]
Continue to: The authors' observations
The authors’ observations
Several factors may contribute to an increased chance of misdiagnosis of a psychiatric illness
On closer sequential evaluations with M and his family, we determined that the “voices” he was hearing were actually intrusive thoughts, and not hallucinations. M clarified this by saying that first he feels a “pressure”-like sensation in his head, followed by repeated intrusive thoughts of voiding his bladder that compel him to go to the restroom to try to urinate. He feels temporary relief after complying with the urge, even when he passes only a small amount of urine or just washes his hands. After a brief period of relief, this process repeats itself. Further, he was able to clarify his experience while eating food, where he first felt a “pressure”-like sensation in his head, followed by intrusive thoughts of choking that result in him not eating.

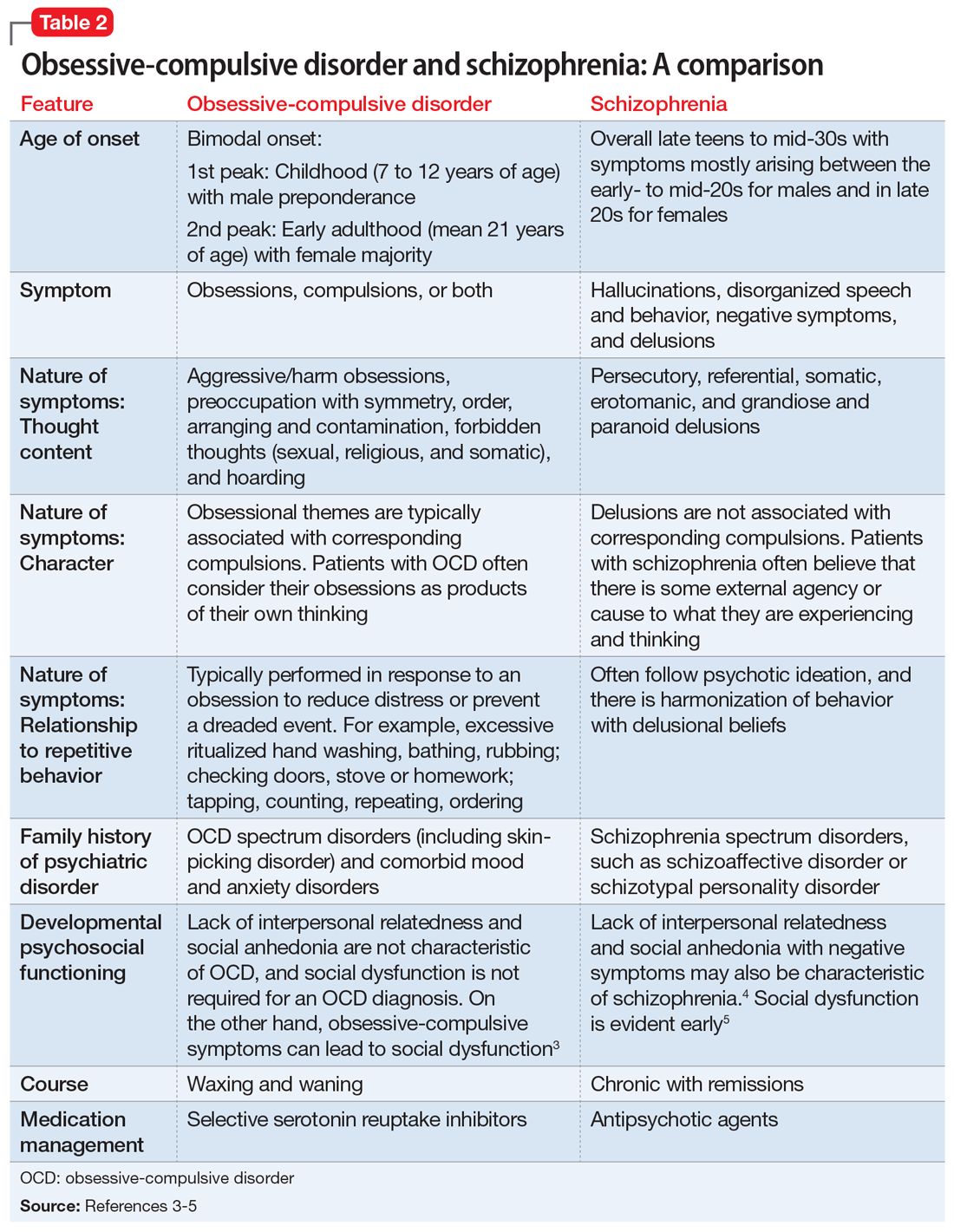
This led us to a more appropriate diagnosis of OCD (Table 11). The incidence of OCD has 2 peaks, with different gender distributions. The first peak occurs in childhood, with symptoms mostly arising between 7 and 12 years of age and affecting boys more often than girls. The second peak occurs in early adulthood, at a mean age of 21 years, with a slight female majority.2 However, OCD is often under recognized and undertreated, perhaps due to its extensive heterogeneity; symptom presentations and comorbidity patterns can vary noticeably between individual patients as well as age groups.
OCD is characterized by the presence of obsessions or compulsions that wax and wane in severity, are time-consuming (at least 1 hour per day), and cause subjective distress or interfere with life of the patient or the family. Adults with OCD recognize at some level that the obsessions and/or compulsions are excessive and unreasonable, although children are not required to have this insight to meet criteria for the diagnosis.1 Rating scales, such as the Children’s Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, and Family Accommodation Scale, are useful to obtain detailed information regarding OCD symptoms, tics, and other factors relevant to the diagnosis.
Continue to: M's symptomatology...
M’s symptomatology did not appear to be psychotic. He was screened for positive or negative symptoms of psychosis, which he and his family clearly denied. Moreover, M’s compulsions (going to the restroom) were typically performed in response to his obsessions (urge to void his bladder) to reduce his distress, which is different from schizophrenia, in which repetitive behaviors are performed in response to psychotic ideation, and not obsessions (Table 23-5).
M’s inattentiveness in the classroom was found to be related to his obsessions and compulsions, and not part of a symptom cluster characterizing ADHD. Teachers often interpret inattention and poor classroom performance as ADHD, but having detailed conversations with teachers often is helpful in understanding the nature of a child’s symptomology and making the appropriate diagnosis.
Establishing the correct clinical diagnosis is critical because it is the starting point in treatment. First-line medication for one condition may exacerbate the symptoms of others. For example, in addition to having a large adverse-effect burden, antipsychotics can induce de novo obsessive–compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors (SSRIs) may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.6 Similarly, stimulant medications for ADHD may exacerbate OCS and may even induce them on their own.7,8
[polldaddy:9971377]
Continue to: The authors' observations
The authors’ observations
Studies have reported an average of 2.5 years from the onset of OCD symptoms to diagnosis in the United States.9 A key reason for this delay, which is more frequently encountered in pediatric patients, is secrecy. Children often feel embarrassed about their symptoms and conceal them until the interference with their functioning becomes extremely disabling. In some cases, symptoms may closely resemble normal childhood routines. In fact, some repetitive behaviors may be normal in some developmental stages, and OCD could be conceptualized as a pathological condition with continuity of normal behaviors during different developmental periods.10
Also, symptoms may go unnoticed for quite some time as unsuspecting and well-intentioned parents and family members become overly involved in the child’s rituals (eg, allowing for increasing frequent prolonged bathroom breaks or frequent change of clothing, etc.). This well-established phenomenon, termed accommodation, is defined as participation of family members in a child’s OCD–related rituals.11 Especially when symptoms are mild or the child is functioning well, accommodation can make it difficult for parents to realize the presence or nature of a problem, as they might tend to minimize their child’s symptoms as representing a unique personality trait or a special “quirk.” Parents generally will seek treatment when their child’s symptoms become more impairing and begin to interfere with social functioning, school performance, or family functioning.
The clinical picture is further complicated by comorbidity. Approximately 60% to 80% of children and adolescents with OCD have ≥1 comorbid psychiatric disorders. Some of the most common include tic disorders, ADHD, anxiety disorders, and mood or eating disorders.9
[polldaddy:9971379]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
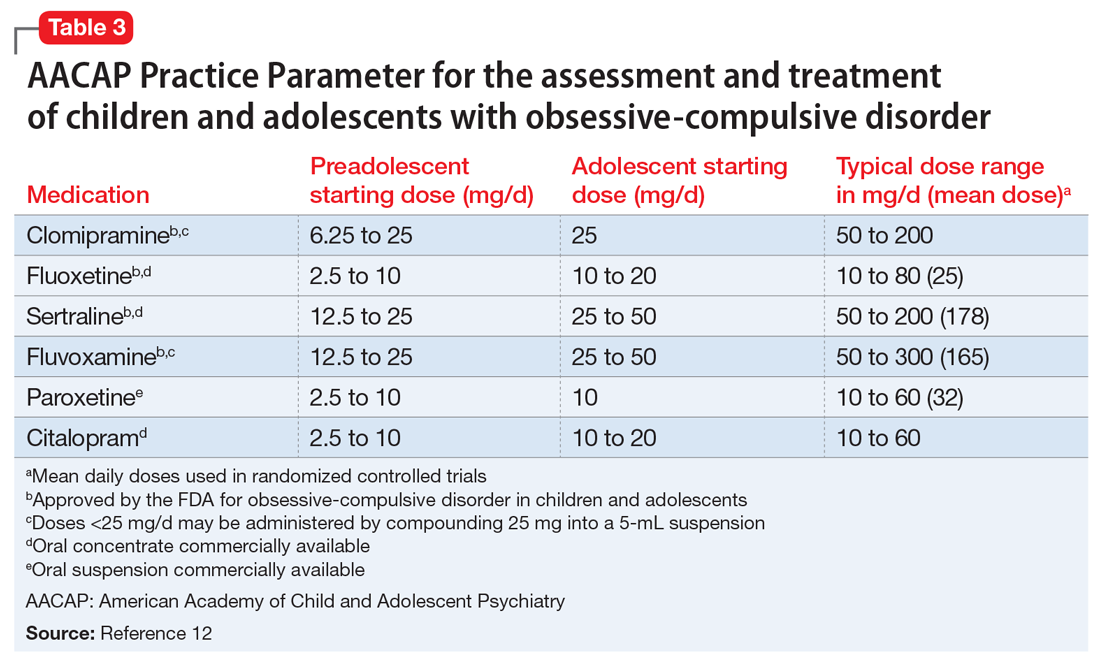
In keeping with American Academy of Child and Adolescent Psychiatry guidelines on treating OCD (Table 312), we start M on fluoxetine 10 mg/d. He also begins CBT. Fluoxetine is slowly titrated to 40 mg/d while M engages in learning and utilizing CBT techniques to manage his OCD.
The authors’ observations
The combination of CBT and medication has been suggested as the treatment of choice for moderate and severe OCD.12 The Pediatric OCD Treatment Study, a 5-year, 3-site outcome study designed to compare placebo, sertraline, CBT, and combined CBT and sertraline, concluded that the combined treatment (CBT plus sertraline) was more effective than CBT alone or sertraline alone.13 The effect sizes for the combined treatment, CBT alone, and sertraline alone were 1.4, 0.97, and 0.67, respectively. Remission rates for SSRIs alone are <33%.13,14
SSRIs are the first-line medication for OCD in children, adolescents, and adults (Table 312). Well-designed clinical trials have demonstrated the efficacy and safety of the SSRIs fluoxetine, sertraline, and fluvoxamine (alone or combined with CBT) in children and adolescents with OCD.13 Other SSRIs, such as citalopram, paroxetine, and escitalopram, also have demonstrated efficacy in children and adolescents with OCD, even though the FDA has not yet approved their use in pediatric patients.12 Despite a positive trial of paroxetine in pediatric OCD,12 there have been concerns related to its higher rates of treatment-emergent suicidality,15 lower likelihood of treatment response,16 and its particularly short half-life in pediatric patients.17
Clomipramine is a tricyclic antidepressant with serotonergic properties that is used alone or to boost the effect of an SSRI when there is a partial response. It should be introduced at a low dose in pediatric patients (before age 12) and closely monitored for anticholinergic and cardiac adverse effects. A systemic review and meta-analysis of early treatment responses of SSRIs and clomipramine in pediatric OCD indicated that the greatest benefits occurred early in treatment.18 Clomipramine was associated with a greater measured benefit compared with placebo than SSRIs; there was no evidence of a relationship between SSRI dosing and treatment effect, although data were limited. Adults and children with OCD demonstrated a similar degree and time course of response to SSRIs in OCD.18
Treatment should start with a low dose to reduce the risk of adverse effects with an adequate trial for 10 to 16 weeks at adequate doses. Most experts suggest that treatment should continue for at least 12 months after symptom resolution or stabilization, followed by a very gradual cessation.19
Continue to: OUTCOME Improvement in functioning
OUTCOME Improvement in functioning
After 12 months of combined CBT and fluoxetine, M’s global assessment of functioning (GAF) scale score improves from 35 to 80, indicating major improvement in overall functional level.
Acknowledgement
The authors thank Uzoma Osuchukwu, MD, ex-fellow, Department of Child and Adolescent Psychiatry, Columbia University College of Physicians and Surgeons, Harlem Hospital Center, New York, New York, for his assistance with this article.
Bottom Line
Obsessive-compulsive disorder may masquerade as a schizophrenia spectrum disorder, particularly in younger patients. Accurate differentiation is crucial because antipsychotics can induce de novo obsessive-compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.
Related Resource
- Raveendranathan D, Shiva L, Sharma E, et al. Obsessive compulsive disorder masquerading as psychosis. Indian J Psychol Med. 2012;34(2):179-180.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Paroxetine • Paxil
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry.1998;37(4):420-427.
3. Huppert JD, Simpson HB, Nissenson KJ, et al. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39-45.
4. Sobel W, Wolski R, Cancro R, et al. Interpersonal relatedness and paranoid schizophrenia. Am J Psychiatry.1996;153(8):1084-1087.
5. Meares A. The diagnosis of prepsychotic schizophrenia. Lancet. 1959;1(7063):55-58.
6. Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs. 2004;18(14):989-1010.
7. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry. 1998;37(2):135.
8. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry. 2003;160(1):183.
9. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am. 2006;29(2):352-370.
10. Evans DW, Milanak ME, Medeiros B, et al. Magical beliefs and rituals in young children. Child Psychiatry Hum Dev. 2002;33(1):43-58.
11. Amir N, Freshman M, Foa E. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
12. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
13. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
14. Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: The Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224-1232.
15. Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5-10.
16. Sakolsky DJ, Perel JM, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the treatment of resistant depression in adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92-97.
17. Findling RL. How (not) to dose antidepressants and antipsychotics for children. Current Psychiatry. 2007;6(6):79-83.
18. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):851-859.e2.
19. Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol. 2010;20(4):299-308.
CASE Auditory hallucinations?
M, age 10, has had multiple visits to the pediatric emergency department (PED) with the chief concern of excessive urinary frequency. At each visit, the medical workup has been negative and he was discharged home. After a few months, M’s parents bring their son back to the PED because he reports hearing “voices in my head” and “feeling tense and scared.” When these feelings become too overwhelming, M stops eating and experiences substantial fear and anxiety that require his mother’s repeated reassurances. M’s mother reports that 2 weeks before his most recent PED visit, he became increasingly anxious and disturbed, and said he was afraid most of the time, and worried about the safety of his family for no apparent reason.
The psychiatrist evaluates M in the PED and diagnoses him with unspecified schizophrenia spectrum and other psychotic disorder based on his persistent report of auditory and tactile hallucinations, including hearing a voice of a man telling him he was going to choke on his food and feeling someone touch his arm to soothe him during his anxious moments. M does not meet criteria for acute inpatient hospitalization, and is discharged home with referral to follow-up at our child and adolescent psychiatry outpatient clinic.
On subsequent evaluation in our clinic, M reports most of the same about his experience hearing “voices in my head” that repeatedly suggest “I might choke on my food and end up seriously ill in the hospital.” He started to hear the “voices” after he witnessed his sister choke while eating a few days earlier. He also mentions that the “voices” tell him “you have to use the restroom.” As a result, he uses the restroom several times before leaving for home and is frequently late for school. His parents accommodate his behavior—his mother allows him to use the bathroom multiple times, and his father overlooks the behavior as part of school anxiety.
At school, his teacher reports a concern for attention-deficit/hyperactivity disorder (ADHD) based on M’s continuous inattentiveness in class and dropping grades. He asks for bathroom breaks up to 15 times a day, which disrupts his class work.
These behaviors have led to a gradual 1-year decline in his overall functioning, including difficulty at school for requesting too many bathroom breaks; having to repeat the 3rd grade; and incurring multiple hospital visits for evaluation of his various complaints. M has become socially isolated and withdrawn from friends and family.
M’s developmental history is normal and his family history is negative for any psychiatric disorder. Medical history and physical examination are unremarkable. CT scan of his head is unremarkable, and all hematologic and biochemistry laboratory test values are within normal range.
[polldaddy:9971376]
Continue to: The authors' observations
The authors’ observations
Several factors may contribute to an increased chance of misdiagnosis of a psychiatric illness
On closer sequential evaluations with M and his family, we determined that the “voices” he was hearing were actually intrusive thoughts, and not hallucinations. M clarified this by saying that first he feels a “pressure”-like sensation in his head, followed by repeated intrusive thoughts of voiding his bladder that compel him to go to the restroom to try to urinate. He feels temporary relief after complying with the urge, even when he passes only a small amount of urine or just washes his hands. After a brief period of relief, this process repeats itself. Further, he was able to clarify his experience while eating food, where he first felt a “pressure”-like sensation in his head, followed by intrusive thoughts of choking that result in him not eating.

This led us to a more appropriate diagnosis of OCD (Table 11). The incidence of OCD has 2 peaks, with different gender distributions. The first peak occurs in childhood, with symptoms mostly arising between 7 and 12 years of age and affecting boys more often than girls. The second peak occurs in early adulthood, at a mean age of 21 years, with a slight female majority.2 However, OCD is often under recognized and undertreated, perhaps due to its extensive heterogeneity; symptom presentations and comorbidity patterns can vary noticeably between individual patients as well as age groups.
OCD is characterized by the presence of obsessions or compulsions that wax and wane in severity, are time-consuming (at least 1 hour per day), and cause subjective distress or interfere with life of the patient or the family. Adults with OCD recognize at some level that the obsessions and/or compulsions are excessive and unreasonable, although children are not required to have this insight to meet criteria for the diagnosis.1 Rating scales, such as the Children’s Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, and Family Accommodation Scale, are useful to obtain detailed information regarding OCD symptoms, tics, and other factors relevant to the diagnosis.
Continue to: M's symptomatology...
M’s symptomatology did not appear to be psychotic. He was screened for positive or negative symptoms of psychosis, which he and his family clearly denied. Moreover, M’s compulsions (going to the restroom) were typically performed in response to his obsessions (urge to void his bladder) to reduce his distress, which is different from schizophrenia, in which repetitive behaviors are performed in response to psychotic ideation, and not obsessions (Table 23-5).

M’s inattentiveness in the classroom was found to be related to his obsessions and compulsions, and not part of a symptom cluster characterizing ADHD. Teachers often interpret inattention and poor classroom performance as ADHD, but having detailed conversations with teachers often is helpful in understanding the nature of a child’s symptomology and making the appropriate diagnosis.
Establishing the correct clinical diagnosis is critical because it is the starting point in treatment. First-line medication for one condition may exacerbate the symptoms of others. For example, in addition to having a large adverse-effect burden, antipsychotics can induce de novo obsessive–compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors (SSRIs) may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.6 Similarly, stimulant medications for ADHD may exacerbate OCS and may even induce them on their own.7,8
[polldaddy:9971377]
Continue to: The authors' observations
The authors’ observations
Studies have reported an average of 2.5 years from the onset of OCD symptoms to diagnosis in the United States.9 A key reason for this delay, which is more frequently encountered in pediatric patients, is secrecy. Children often feel embarrassed about their symptoms and conceal them until the interference with their functioning becomes extremely disabling. In some cases, symptoms may closely resemble normal childhood routines. In fact, some repetitive behaviors may be normal in some developmental stages, and OCD could be conceptualized as a pathological condition with continuity of normal behaviors during different developmental periods.10
Also, symptoms may go unnoticed for quite some time as unsuspecting and well-intentioned parents and family members become overly involved in the child’s rituals (eg, allowing for increasing frequent prolonged bathroom breaks or frequent change of clothing, etc.). This well-established phenomenon, termed accommodation, is defined as participation of family members in a child’s OCD–related rituals.11 Especially when symptoms are mild or the child is functioning well, accommodation can make it difficult for parents to realize the presence or nature of a problem, as they might tend to minimize their child’s symptoms as representing a unique personality trait or a special “quirk.” Parents generally will seek treatment when their child’s symptoms become more impairing and begin to interfere with social functioning, school performance, or family functioning.
The clinical picture is further complicated by comorbidity. Approximately 60% to 80% of children and adolescents with OCD have ≥1 comorbid psychiatric disorders. Some of the most common include tic disorders, ADHD, anxiety disorders, and mood or eating disorders.9
[polldaddy:9971379]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
In keeping with American Academy of Child and Adolescent Psychiatry guidelines on treating OCD (Table 312), we start M on fluoxetine 10 mg/d. He also begins CBT. Fluoxetine is slowly titrated to 40 mg/d while M engages in learning and utilizing CBT techniques to manage his OCD.

The authors’ observations
The combination of CBT and medication has been suggested as the treatment of choice for moderate and severe OCD.12 The Pediatric OCD Treatment Study, a 5-year, 3-site outcome study designed to compare placebo, sertraline, CBT, and combined CBT and sertraline, concluded that the combined treatment (CBT plus sertraline) was more effective than CBT alone or sertraline alone.13 The effect sizes for the combined treatment, CBT alone, and sertraline alone were 1.4, 0.97, and 0.67, respectively. Remission rates for SSRIs alone are <33%.13,14
SSRIs are the first-line medication for OCD in children, adolescents, and adults (Table 312). Well-designed clinical trials have demonstrated the efficacy and safety of the SSRIs fluoxetine, sertraline, and fluvoxamine (alone or combined with CBT) in children and adolescents with OCD.13 Other SSRIs, such as citalopram, paroxetine, and escitalopram, also have demonstrated efficacy in children and adolescents with OCD, even though the FDA has not yet approved their use in pediatric patients.12 Despite a positive trial of paroxetine in pediatric OCD,12 there have been concerns related to its higher rates of treatment-emergent suicidality,15 lower likelihood of treatment response,16 and its particularly short half-life in pediatric patients.17
Clomipramine is a tricyclic antidepressant with serotonergic properties that is used alone or to boost the effect of an SSRI when there is a partial response. It should be introduced at a low dose in pediatric patients (before age 12) and closely monitored for anticholinergic and cardiac adverse effects. A systemic review and meta-analysis of early treatment responses of SSRIs and clomipramine in pediatric OCD indicated that the greatest benefits occurred early in treatment.18 Clomipramine was associated with a greater measured benefit compared with placebo than SSRIs; there was no evidence of a relationship between SSRI dosing and treatment effect, although data were limited. Adults and children with OCD demonstrated a similar degree and time course of response to SSRIs in OCD.18
Treatment should start with a low dose to reduce the risk of adverse effects with an adequate trial for 10 to 16 weeks at adequate doses. Most experts suggest that treatment should continue for at least 12 months after symptom resolution or stabilization, followed by a very gradual cessation.19
Continue to: OUTCOME Improvement in functioning
OUTCOME Improvement in functioning
After 12 months of combined CBT and fluoxetine, M’s global assessment of functioning (GAF) scale score improves from 35 to 80, indicating major improvement in overall functional level.
Acknowledgement
The authors thank Uzoma Osuchukwu, MD, ex-fellow, Department of Child and Adolescent Psychiatry, Columbia University College of Physicians and Surgeons, Harlem Hospital Center, New York, New York, for his assistance with this article.
Bottom Line
Obsessive-compulsive disorder may masquerade as a schizophrenia spectrum disorder, particularly in younger patients. Accurate differentiation is crucial because antipsychotics can induce de novo obsessive-compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.
Related Resource
- Raveendranathan D, Shiva L, Sharma E, et al. Obsessive compulsive disorder masquerading as psychosis. Indian J Psychol Med. 2012;34(2):179-180.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Paroxetine • Paxil
Sertraline • Zoloft
CASE Auditory hallucinations?
M, age 10, has had multiple visits to the pediatric emergency department (PED) with the chief concern of excessive urinary frequency. At each visit, the medical workup has been negative and he was discharged home. After a few months, M’s parents bring their son back to the PED because he reports hearing “voices in my head” and “feeling tense and scared.” When these feelings become too overwhelming, M stops eating and experiences substantial fear and anxiety that require his mother’s repeated reassurances. M’s mother reports that 2 weeks before his most recent PED visit, he became increasingly anxious and disturbed, and said he was afraid most of the time, and worried about the safety of his family for no apparent reason.
The psychiatrist evaluates M in the PED and diagnoses him with unspecified schizophrenia spectrum and other psychotic disorder based on his persistent report of auditory and tactile hallucinations, including hearing a voice of a man telling him he was going to choke on his food and feeling someone touch his arm to soothe him during his anxious moments. M does not meet criteria for acute inpatient hospitalization, and is discharged home with referral to follow-up at our child and adolescent psychiatry outpatient clinic.
On subsequent evaluation in our clinic, M reports most of the same about his experience hearing “voices in my head” that repeatedly suggest “I might choke on my food and end up seriously ill in the hospital.” He started to hear the “voices” after he witnessed his sister choke while eating a few days earlier. He also mentions that the “voices” tell him “you have to use the restroom.” As a result, he uses the restroom several times before leaving for home and is frequently late for school. His parents accommodate his behavior—his mother allows him to use the bathroom multiple times, and his father overlooks the behavior as part of school anxiety.
At school, his teacher reports a concern for attention-deficit/hyperactivity disorder (ADHD) based on M’s continuous inattentiveness in class and dropping grades. He asks for bathroom breaks up to 15 times a day, which disrupts his class work.
These behaviors have led to a gradual 1-year decline in his overall functioning, including difficulty at school for requesting too many bathroom breaks; having to repeat the 3rd grade; and incurring multiple hospital visits for evaluation of his various complaints. M has become socially isolated and withdrawn from friends and family.
M’s developmental history is normal and his family history is negative for any psychiatric disorder. Medical history and physical examination are unremarkable. CT scan of his head is unremarkable, and all hematologic and biochemistry laboratory test values are within normal range.
[polldaddy:9971376]
Continue to: The authors' observations
The authors’ observations
Several factors may contribute to an increased chance of misdiagnosis of a psychiatric illness
On closer sequential evaluations with M and his family, we determined that the “voices” he was hearing were actually intrusive thoughts, and not hallucinations. M clarified this by saying that first he feels a “pressure”-like sensation in his head, followed by repeated intrusive thoughts of voiding his bladder that compel him to go to the restroom to try to urinate. He feels temporary relief after complying with the urge, even when he passes only a small amount of urine or just washes his hands. After a brief period of relief, this process repeats itself. Further, he was able to clarify his experience while eating food, where he first felt a “pressure”-like sensation in his head, followed by intrusive thoughts of choking that result in him not eating.

This led us to a more appropriate diagnosis of OCD (Table 11). The incidence of OCD has 2 peaks, with different gender distributions. The first peak occurs in childhood, with symptoms mostly arising between 7 and 12 years of age and affecting boys more often than girls. The second peak occurs in early adulthood, at a mean age of 21 years, with a slight female majority.2 However, OCD is often under recognized and undertreated, perhaps due to its extensive heterogeneity; symptom presentations and comorbidity patterns can vary noticeably between individual patients as well as age groups.
OCD is characterized by the presence of obsessions or compulsions that wax and wane in severity, are time-consuming (at least 1 hour per day), and cause subjective distress or interfere with life of the patient or the family. Adults with OCD recognize at some level that the obsessions and/or compulsions are excessive and unreasonable, although children are not required to have this insight to meet criteria for the diagnosis.1 Rating scales, such as the Children’s Yale-Brown Obsessive-Compulsive Scale, Dimensional Yale-Brown Obsessive-Compulsive Scale, and Family Accommodation Scale, are useful to obtain detailed information regarding OCD symptoms, tics, and other factors relevant to the diagnosis.
Continue to: M's symptomatology...
M’s symptomatology did not appear to be psychotic. He was screened for positive or negative symptoms of psychosis, which he and his family clearly denied. Moreover, M’s compulsions (going to the restroom) were typically performed in response to his obsessions (urge to void his bladder) to reduce his distress, which is different from schizophrenia, in which repetitive behaviors are performed in response to psychotic ideation, and not obsessions (Table 23-5).

M’s inattentiveness in the classroom was found to be related to his obsessions and compulsions, and not part of a symptom cluster characterizing ADHD. Teachers often interpret inattention and poor classroom performance as ADHD, but having detailed conversations with teachers often is helpful in understanding the nature of a child’s symptomology and making the appropriate diagnosis.
Establishing the correct clinical diagnosis is critical because it is the starting point in treatment. First-line medication for one condition may exacerbate the symptoms of others. For example, in addition to having a large adverse-effect burden, antipsychotics can induce de novo obsessive–compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors (SSRIs) may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.6 Similarly, stimulant medications for ADHD may exacerbate OCS and may even induce them on their own.7,8
[polldaddy:9971377]
Continue to: The authors' observations
The authors’ observations
Studies have reported an average of 2.5 years from the onset of OCD symptoms to diagnosis in the United States.9 A key reason for this delay, which is more frequently encountered in pediatric patients, is secrecy. Children often feel embarrassed about their symptoms and conceal them until the interference with their functioning becomes extremely disabling. In some cases, symptoms may closely resemble normal childhood routines. In fact, some repetitive behaviors may be normal in some developmental stages, and OCD could be conceptualized as a pathological condition with continuity of normal behaviors during different developmental periods.10
Also, symptoms may go unnoticed for quite some time as unsuspecting and well-intentioned parents and family members become overly involved in the child’s rituals (eg, allowing for increasing frequent prolonged bathroom breaks or frequent change of clothing, etc.). This well-established phenomenon, termed accommodation, is defined as participation of family members in a child’s OCD–related rituals.11 Especially when symptoms are mild or the child is functioning well, accommodation can make it difficult for parents to realize the presence or nature of a problem, as they might tend to minimize their child’s symptoms as representing a unique personality trait or a special “quirk.” Parents generally will seek treatment when their child’s symptoms become more impairing and begin to interfere with social functioning, school performance, or family functioning.
The clinical picture is further complicated by comorbidity. Approximately 60% to 80% of children and adolescents with OCD have ≥1 comorbid psychiatric disorders. Some of the most common include tic disorders, ADHD, anxiety disorders, and mood or eating disorders.9
[polldaddy:9971379]
Continue to: TREATMENT Combination therapy
TREATMENT Combination therapy
In keeping with American Academy of Child and Adolescent Psychiatry guidelines on treating OCD (Table 312), we start M on fluoxetine 10 mg/d. He also begins CBT. Fluoxetine is slowly titrated to 40 mg/d while M engages in learning and utilizing CBT techniques to manage his OCD.

The authors’ observations
The combination of CBT and medication has been suggested as the treatment of choice for moderate and severe OCD.12 The Pediatric OCD Treatment Study, a 5-year, 3-site outcome study designed to compare placebo, sertraline, CBT, and combined CBT and sertraline, concluded that the combined treatment (CBT plus sertraline) was more effective than CBT alone or sertraline alone.13 The effect sizes for the combined treatment, CBT alone, and sertraline alone were 1.4, 0.97, and 0.67, respectively. Remission rates for SSRIs alone are <33%.13,14
SSRIs are the first-line medication for OCD in children, adolescents, and adults (Table 312). Well-designed clinical trials have demonstrated the efficacy and safety of the SSRIs fluoxetine, sertraline, and fluvoxamine (alone or combined with CBT) in children and adolescents with OCD.13 Other SSRIs, such as citalopram, paroxetine, and escitalopram, also have demonstrated efficacy in children and adolescents with OCD, even though the FDA has not yet approved their use in pediatric patients.12 Despite a positive trial of paroxetine in pediatric OCD,12 there have been concerns related to its higher rates of treatment-emergent suicidality,15 lower likelihood of treatment response,16 and its particularly short half-life in pediatric patients.17
Clomipramine is a tricyclic antidepressant with serotonergic properties that is used alone or to boost the effect of an SSRI when there is a partial response. It should be introduced at a low dose in pediatric patients (before age 12) and closely monitored for anticholinergic and cardiac adverse effects. A systemic review and meta-analysis of early treatment responses of SSRIs and clomipramine in pediatric OCD indicated that the greatest benefits occurred early in treatment.18 Clomipramine was associated with a greater measured benefit compared with placebo than SSRIs; there was no evidence of a relationship between SSRI dosing and treatment effect, although data were limited. Adults and children with OCD demonstrated a similar degree and time course of response to SSRIs in OCD.18
Treatment should start with a low dose to reduce the risk of adverse effects with an adequate trial for 10 to 16 weeks at adequate doses. Most experts suggest that treatment should continue for at least 12 months after symptom resolution or stabilization, followed by a very gradual cessation.19
Continue to: OUTCOME Improvement in functioning
OUTCOME Improvement in functioning
After 12 months of combined CBT and fluoxetine, M’s global assessment of functioning (GAF) scale score improves from 35 to 80, indicating major improvement in overall functional level.
Acknowledgement
The authors thank Uzoma Osuchukwu, MD, ex-fellow, Department of Child and Adolescent Psychiatry, Columbia University College of Physicians and Surgeons, Harlem Hospital Center, New York, New York, for his assistance with this article.
Bottom Line
Obsessive-compulsive disorder may masquerade as a schizophrenia spectrum disorder, particularly in younger patients. Accurate differentiation is crucial because antipsychotics can induce de novo obsessive-compulsive symptoms (OCS) or exacerbate preexisting OCS, and selective serotonin reuptake inhibitors may exacerbate psychosis in schizo-obsessive patients with a history of impulsivity and aggressiveness.
Related Resource
- Raveendranathan D, Shiva L, Sharma E, et al. Obsessive compulsive disorder masquerading as psychosis. Indian J Psychol Med. 2012;34(2):179-180.
Drug Brand Names
Citalopram • Celexa
Clomipramine • Anafranil
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Paroxetine • Paxil
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry.1998;37(4):420-427.
3. Huppert JD, Simpson HB, Nissenson KJ, et al. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39-45.
4. Sobel W, Wolski R, Cancro R, et al. Interpersonal relatedness and paranoid schizophrenia. Am J Psychiatry.1996;153(8):1084-1087.
5. Meares A. The diagnosis of prepsychotic schizophrenia. Lancet. 1959;1(7063):55-58.
6. Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs. 2004;18(14):989-1010.
7. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry. 1998;37(2):135.
8. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry. 2003;160(1):183.
9. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am. 2006;29(2):352-370.
10. Evans DW, Milanak ME, Medeiros B, et al. Magical beliefs and rituals in young children. Child Psychiatry Hum Dev. 2002;33(1):43-58.
11. Amir N, Freshman M, Foa E. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
12. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
13. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
14. Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: The Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224-1232.
15. Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5-10.
16. Sakolsky DJ, Perel JM, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the treatment of resistant depression in adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92-97.
17. Findling RL. How (not) to dose antidepressants and antipsychotics for children. Current Psychiatry. 2007;6(6):79-83.
18. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):851-859.e2.
19. Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol. 2010;20(4):299-308.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Geller D, Biederman J, Jones J, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry.1998;37(4):420-427.
3. Huppert JD, Simpson HB, Nissenson KJ, et al. Quality of life and functional impairment in obsessive-compulsive disorder: A comparison of patients with and without comorbidity, patients in remission, and healthy controls. Depress Anxiety. 2009;26(1):39-45.
4. Sobel W, Wolski R, Cancro R, et al. Interpersonal relatedness and paranoid schizophrenia. Am J Psychiatry.1996;153(8):1084-1087.
5. Meares A. The diagnosis of prepsychotic schizophrenia. Lancet. 1959;1(7063):55-58.
6. Poyurovsky M, Weizman A, Weizman R. Obsessive-compulsive disorder in schizophrenia: Clinical characteristics and treatment. CNS Drugs. 2004;18(14):989-1010.
7. Kouris S. Methylphenidate-induced obsessive-compulsiveness. J Am Acad Child Adolesc Psychiatry. 1998;37(2):135.
8. Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. Am J Psychiatry. 2003;160(1):183.
9. Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatr Clin N Am. 2006;29(2):352-370.
10. Evans DW, Milanak ME, Medeiros B, et al. Magical beliefs and rituals in young children. Child Psychiatry Hum Dev. 2002;33(1):43-58.
11. Amir N, Freshman M, Foa E. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
12. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):98-113.
13. Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969-1976.
14. Franklin ME, Sapyta J, Freeman JB, et al. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: The Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA. 2011;306(11):1224-1232.
15. Wagner KD, Asarnow JR, Vitiello B, et al. Out of the black box: treatment of resistant depression in adolescents and the antidepressant controversy. J Child Adolesc Psychopharmacol. 2012;22(1):5-10.
16. Sakolsky DJ, Perel JM, Emslie GJ, et al. Antidepressant exposure as a predictor of clinical outcomes in the treatment of resistant depression in adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31(1):92-97.
17. Findling RL. How (not) to dose antidepressants and antipsychotics for children. Current Psychiatry. 2007;6(6):79-83.
18. Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):851-859.e2.
19. Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol. 2010;20(4):299-308.
Psychiatric consults: Documenting 6 essential elements
Written communication is an essential skill for a consultation-liaison (C-L) psychiatrist, but unfortunately, how to write a consultation note is not part of formal didactics in medical school or residency training.1 Documentation of a consultation note is a permanent medical record entry that conveys current physician-to-physician information. While considerable literature describes the consultation process, little has been published about composing a consultation note.1,2 Residents and clinicians who do not have frequent consultations may be unfamiliar with the consultation environment and their role as an expert consultant. Therefore, more explicit guidance on documentation and optimal formatting of the consultation note is needed.
The Box provides an outline for completing the Recommendations/Treatment Plan section of psychiatric consultation notes. When providing your recommendations, it is best to use bullet points, numbering, or bold text; do not bury the information in a dense paragraph.3 Be sure to address each of the following 6 elements.
1. Primary consult concern. The first section of the Recommendations section should include the reason for the consult, which may be the most important part of the consultation process.1,2 It is important to recognize that an unclear consult question may be a sign of the primary team’s knowledge gap in psychiatry. The role of the C-L psychiatrist is to help the primary team organize their thoughts and concerns regarding their patient to decide on the final consult question.1 The active consult question may change as clinical issues evolve.
2. Safety and critical issues. Include an assessment of or recommendation on safety and critical issues. An important consideration is whether to recommend a patient sitter and to provide a reason for this recommendation. Occasionally, critical issues are more pressing than the primary consult concern. For example, there are several situations in which abnormal laboratory values and acute medical issues manifest as psychiatric symptoms, including hyponatremia, hypoglycemia, hypotension, low oxygen saturation, or infection. The connection between the 2 may not be clear to the primary treatment team; thus, include a statement to draw their attention to this.
3. Nonpharmacologic recommendations.
4. Psychopharmacology. In this section, the C-L psychiatrist should provide information on the use of any psychotropic medications and an explanation of their indications. If there are discrepancies between a patient’s home and hospital-ordered medications, clarify which medications the patient should be taking while hospitalized. If the C-L treatment team recommends initiating a new medication, provide details regarding the specific medication, dose, route, administration time, and titration schedule, as well as the specific situation for any as-needed medications. It is important to include the indication for any recommended medications, as well as any potential adverse effects. If psychotropic medications are not indicated, add a statement to emphasize this.
5. Social work support. Document any issues that need to be clarified by social work. This might include clarification of a patient’s insurance coverage, current living situation, or durable power of attorney. Also, document how the treatment team would prefer social work to assist with the patient’s care by (for example) providing the patient with resources for outpatient mental health and/or substance abuse treatment or housing options.
Continue to: Disposition
6. Disposition. Finally, include a recommendation regarding disposition. If transfer to a psychiatric facility is not indicated, provide a statement to affirm this. If transfer to a psychiatric facility is recommended, include a discussion of the patient’s appropriateness in the assessment and recommendations. It is helpful to inform the primary team of criteria that may or may not allow the patient to transfer to or be accepted by a psychiatry unit (eg, the patient will need to be off IV medications and able to tolerate oral intake prior to transfer). When transfer is not possible, communicate the reason to the primary treatment team and other ancillary staff.
Communicating responsibilities and expectations
After concluding the Recommendations section, end the consultation note with a brief sentence of gratitude (eg, “Thank you for this consultation and allowing us to assist in the care of your patient.”) and a comment regarding the C-L treatment team’s plan for follow-up. Also, include your contact information in case the primary treatment team has any questions or concerns.
The Recommendations section of a psychiatric consultation note is vital to convey current physician-to-physician recommendations. With the potential complexities in assessing and caring for a medically ill patient with comorbid psychiatric diagnoses, psychiatrists with less C-L experience may be unfamiliar with the essential elements of a consultation note. It is helpful to use a Template to ensure that the consultation and documentation are complete.
1. Garrick TR, Stotland, NL. How to write a psychiatric consultation. Am J Psychiatry. 1982;139(7):849-855.
2. Alexander T, Bloch S. The written report in consultation-liaison psychiatry: a proposed schema. Aust N Z J Psychiatry. 2002;36(2):251-258.
3. von Gunten CF, Weissman DE. Writing the consultation note #267. J Palliat Med. 2013;16(5):579-580.
Written communication is an essential skill for a consultation-liaison (C-L) psychiatrist, but unfortunately, how to write a consultation note is not part of formal didactics in medical school or residency training.1 Documentation of a consultation note is a permanent medical record entry that conveys current physician-to-physician information. While considerable literature describes the consultation process, little has been published about composing a consultation note.1,2 Residents and clinicians who do not have frequent consultations may be unfamiliar with the consultation environment and their role as an expert consultant. Therefore, more explicit guidance on documentation and optimal formatting of the consultation note is needed.
The Box provides an outline for completing the Recommendations/Treatment Plan section of psychiatric consultation notes. When providing your recommendations, it is best to use bullet points, numbering, or bold text; do not bury the information in a dense paragraph.3 Be sure to address each of the following 6 elements.
1. Primary consult concern. The first section of the Recommendations section should include the reason for the consult, which may be the most important part of the consultation process.1,2 It is important to recognize that an unclear consult question may be a sign of the primary team’s knowledge gap in psychiatry. The role of the C-L psychiatrist is to help the primary team organize their thoughts and concerns regarding their patient to decide on the final consult question.1 The active consult question may change as clinical issues evolve.
2. Safety and critical issues. Include an assessment of or recommendation on safety and critical issues. An important consideration is whether to recommend a patient sitter and to provide a reason for this recommendation. Occasionally, critical issues are more pressing than the primary consult concern. For example, there are several situations in which abnormal laboratory values and acute medical issues manifest as psychiatric symptoms, including hyponatremia, hypoglycemia, hypotension, low oxygen saturation, or infection. The connection between the 2 may not be clear to the primary treatment team; thus, include a statement to draw their attention to this.
3. Nonpharmacologic recommendations.
4. Psychopharmacology. In this section, the C-L psychiatrist should provide information on the use of any psychotropic medications and an explanation of their indications. If there are discrepancies between a patient’s home and hospital-ordered medications, clarify which medications the patient should be taking while hospitalized. If the C-L treatment team recommends initiating a new medication, provide details regarding the specific medication, dose, route, administration time, and titration schedule, as well as the specific situation for any as-needed medications. It is important to include the indication for any recommended medications, as well as any potential adverse effects. If psychotropic medications are not indicated, add a statement to emphasize this.
5. Social work support. Document any issues that need to be clarified by social work. This might include clarification of a patient’s insurance coverage, current living situation, or durable power of attorney. Also, document how the treatment team would prefer social work to assist with the patient’s care by (for example) providing the patient with resources for outpatient mental health and/or substance abuse treatment or housing options.
Continue to: Disposition
6. Disposition. Finally, include a recommendation regarding disposition. If transfer to a psychiatric facility is not indicated, provide a statement to affirm this. If transfer to a psychiatric facility is recommended, include a discussion of the patient’s appropriateness in the assessment and recommendations. It is helpful to inform the primary team of criteria that may or may not allow the patient to transfer to or be accepted by a psychiatry unit (eg, the patient will need to be off IV medications and able to tolerate oral intake prior to transfer). When transfer is not possible, communicate the reason to the primary treatment team and other ancillary staff.
Communicating responsibilities and expectations
After concluding the Recommendations section, end the consultation note with a brief sentence of gratitude (eg, “Thank you for this consultation and allowing us to assist in the care of your patient.”) and a comment regarding the C-L treatment team’s plan for follow-up. Also, include your contact information in case the primary treatment team has any questions or concerns.
The Recommendations section of a psychiatric consultation note is vital to convey current physician-to-physician recommendations. With the potential complexities in assessing and caring for a medically ill patient with comorbid psychiatric diagnoses, psychiatrists with less C-L experience may be unfamiliar with the essential elements of a consultation note. It is helpful to use a Template to ensure that the consultation and documentation are complete.
Written communication is an essential skill for a consultation-liaison (C-L) psychiatrist, but unfortunately, how to write a consultation note is not part of formal didactics in medical school or residency training.1 Documentation of a consultation note is a permanent medical record entry that conveys current physician-to-physician information. While considerable literature describes the consultation process, little has been published about composing a consultation note.1,2 Residents and clinicians who do not have frequent consultations may be unfamiliar with the consultation environment and their role as an expert consultant. Therefore, more explicit guidance on documentation and optimal formatting of the consultation note is needed.
The Box provides an outline for completing the Recommendations/Treatment Plan section of psychiatric consultation notes. When providing your recommendations, it is best to use bullet points, numbering, or bold text; do not bury the information in a dense paragraph.3 Be sure to address each of the following 6 elements.
1. Primary consult concern. The first section of the Recommendations section should include the reason for the consult, which may be the most important part of the consultation process.1,2 It is important to recognize that an unclear consult question may be a sign of the primary team’s knowledge gap in psychiatry. The role of the C-L psychiatrist is to help the primary team organize their thoughts and concerns regarding their patient to decide on the final consult question.1 The active consult question may change as clinical issues evolve.
2. Safety and critical issues. Include an assessment of or recommendation on safety and critical issues. An important consideration is whether to recommend a patient sitter and to provide a reason for this recommendation. Occasionally, critical issues are more pressing than the primary consult concern. For example, there are several situations in which abnormal laboratory values and acute medical issues manifest as psychiatric symptoms, including hyponatremia, hypoglycemia, hypotension, low oxygen saturation, or infection. The connection between the 2 may not be clear to the primary treatment team; thus, include a statement to draw their attention to this.
3. Nonpharmacologic recommendations.
4. Psychopharmacology. In this section, the C-L psychiatrist should provide information on the use of any psychotropic medications and an explanation of their indications. If there are discrepancies between a patient’s home and hospital-ordered medications, clarify which medications the patient should be taking while hospitalized. If the C-L treatment team recommends initiating a new medication, provide details regarding the specific medication, dose, route, administration time, and titration schedule, as well as the specific situation for any as-needed medications. It is important to include the indication for any recommended medications, as well as any potential adverse effects. If psychotropic medications are not indicated, add a statement to emphasize this.
5. Social work support. Document any issues that need to be clarified by social work. This might include clarification of a patient’s insurance coverage, current living situation, or durable power of attorney. Also, document how the treatment team would prefer social work to assist with the patient’s care by (for example) providing the patient with resources for outpatient mental health and/or substance abuse treatment or housing options.
Continue to: Disposition
6. Disposition. Finally, include a recommendation regarding disposition. If transfer to a psychiatric facility is not indicated, provide a statement to affirm this. If transfer to a psychiatric facility is recommended, include a discussion of the patient’s appropriateness in the assessment and recommendations. It is helpful to inform the primary team of criteria that may or may not allow the patient to transfer to or be accepted by a psychiatry unit (eg, the patient will need to be off IV medications and able to tolerate oral intake prior to transfer). When transfer is not possible, communicate the reason to the primary treatment team and other ancillary staff.
Communicating responsibilities and expectations
After concluding the Recommendations section, end the consultation note with a brief sentence of gratitude (eg, “Thank you for this consultation and allowing us to assist in the care of your patient.”) and a comment regarding the C-L treatment team’s plan for follow-up. Also, include your contact information in case the primary treatment team has any questions or concerns.
The Recommendations section of a psychiatric consultation note is vital to convey current physician-to-physician recommendations. With the potential complexities in assessing and caring for a medically ill patient with comorbid psychiatric diagnoses, psychiatrists with less C-L experience may be unfamiliar with the essential elements of a consultation note. It is helpful to use a Template to ensure that the consultation and documentation are complete.
1. Garrick TR, Stotland, NL. How to write a psychiatric consultation. Am J Psychiatry. 1982;139(7):849-855.
2. Alexander T, Bloch S. The written report in consultation-liaison psychiatry: a proposed schema. Aust N Z J Psychiatry. 2002;36(2):251-258.
3. von Gunten CF, Weissman DE. Writing the consultation note #267. J Palliat Med. 2013;16(5):579-580.
1. Garrick TR, Stotland, NL. How to write a psychiatric consultation. Am J Psychiatry. 1982;139(7):849-855.
2. Alexander T, Bloch S. The written report in consultation-liaison psychiatry: a proposed schema. Aust N Z J Psychiatry. 2002;36(2):251-258.
3. von Gunten CF, Weissman DE. Writing the consultation note #267. J Palliat Med. 2013;16(5):579-580.
Tardive dyskinesia: 5 Steps for prevention
Tardive dyskinesia (TD) is an elusive-to-treat adverse effect of antipsychotics that has caused extreme discomfort (in a literal and figurative sense) for patients and their psychiatrists. In 2017, the prevalence of TD as a result of exposure to dopamine antagonists was approximately 30% with first-generation antipsychotics and 20% with second-generation antipsychotics.1 There have been several effective attempts at reducing rates of TD, including lowering the dosing, shifting to second-generation antipsychotics, and using recently introduced pharmacologic treatments for TD. The past 2 years have seen increased efforts at treating this often-irreversible adverse effect with pharmacotherapy, such as the recently marketed vesicular monoamine transporter-2 (VMAT2) inhibitors valbenazine and deutetrabenazine, as well as the supplement Ginkgo biloba,2 although issues with cost, adverse effects, or drug–drug interactions could limit the benefits of these agents.
Despite these strategies, one approach has been largely overlooked: prevention. Although it is included in many guidelines and literature reports, prevention has become less of a standard of practice and more of a cliché. Prevention is the key strategy for lowering the rate of TD, and it should be the assumed responsibility of each clinician in every prescription they write throughout the entire continuum of care. Here, we provide steps to take to help prevent TD, and what to consider when TD occurs.
1. Realize that we are all responsible for TD. We know TD exists, but we often feel that this adverse effect is not our fault. Avoid adapting a philosophy of “someone else caused it,” “they didn’t cause it yet,” or “it’s going to happen anyway.” We must remember that every unnecessary exposure to a dopamine antagonist increases the risk of TD, even if we don’t see the adverse effect firsthand.
2. Treat first-episode psychosis early and aggressively. Doing so may prevent chronicity of the illness, which would save a patient from long-term, high-dose exposure to antipsychotics. Lower the risk of TD with atypical antipsychotics and offer long-acting injectables when possible to improve medication adherence.
3. Treat both acute and chronic symptoms of psychosis throughout the continuum of care. The choice of medication and dose should be reevaluated at each interaction to enhance improvement of acute symptoms and to minimize chronic adverse effects. Always recognize the differences in aggressive treatment of an acute episode of psychosis vs maintenance treatment of baseline symptoms. Also, assess for TD by conducting abnormal involuntary movement scale (AIMS) examinations at baseline and at least biannually.
4. Use clozapine instead of 2 antipsychotics in chronic, refractory patients when possible. Clozapine is largely underutilize
5. Consider pharmacotherapy if TD has already occurred. Psychiatrists have been waiting for pharmacologic options for treating TD for quite some time. Explore using VMAT2 inhibitors and other agents when it is too late to implement prevention or when a patient’s symptoms are refractory to other treatments. However, avoid anticholinergic medications; there is insufficient data to support the use of these agents in the treatment of TD.5
1. Carbon M, Hsieh C, Kane J, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Zheng W, Xiang Y, Ng H, et al. Extract of ginkgo biloba for tardive dyskinesia: meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3):107-111.
3. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693.
4. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
5. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
Tardive dyskinesia (TD) is an elusive-to-treat adverse effect of antipsychotics that has caused extreme discomfort (in a literal and figurative sense) for patients and their psychiatrists. In 2017, the prevalence of TD as a result of exposure to dopamine antagonists was approximately 30% with first-generation antipsychotics and 20% with second-generation antipsychotics.1 There have been several effective attempts at reducing rates of TD, including lowering the dosing, shifting to second-generation antipsychotics, and using recently introduced pharmacologic treatments for TD. The past 2 years have seen increased efforts at treating this often-irreversible adverse effect with pharmacotherapy, such as the recently marketed vesicular monoamine transporter-2 (VMAT2) inhibitors valbenazine and deutetrabenazine, as well as the supplement Ginkgo biloba,2 although issues with cost, adverse effects, or drug–drug interactions could limit the benefits of these agents.
Despite these strategies, one approach has been largely overlooked: prevention. Although it is included in many guidelines and literature reports, prevention has become less of a standard of practice and more of a cliché. Prevention is the key strategy for lowering the rate of TD, and it should be the assumed responsibility of each clinician in every prescription they write throughout the entire continuum of care. Here, we provide steps to take to help prevent TD, and what to consider when TD occurs.
1. Realize that we are all responsible for TD. We know TD exists, but we often feel that this adverse effect is not our fault. Avoid adapting a philosophy of “someone else caused it,” “they didn’t cause it yet,” or “it’s going to happen anyway.” We must remember that every unnecessary exposure to a dopamine antagonist increases the risk of TD, even if we don’t see the adverse effect firsthand.
2. Treat first-episode psychosis early and aggressively. Doing so may prevent chronicity of the illness, which would save a patient from long-term, high-dose exposure to antipsychotics. Lower the risk of TD with atypical antipsychotics and offer long-acting injectables when possible to improve medication adherence.
3. Treat both acute and chronic symptoms of psychosis throughout the continuum of care. The choice of medication and dose should be reevaluated at each interaction to enhance improvement of acute symptoms and to minimize chronic adverse effects. Always recognize the differences in aggressive treatment of an acute episode of psychosis vs maintenance treatment of baseline symptoms. Also, assess for TD by conducting abnormal involuntary movement scale (AIMS) examinations at baseline and at least biannually.
4. Use clozapine instead of 2 antipsychotics in chronic, refractory patients when possible. Clozapine is largely underutilize
5. Consider pharmacotherapy if TD has already occurred. Psychiatrists have been waiting for pharmacologic options for treating TD for quite some time. Explore using VMAT2 inhibitors and other agents when it is too late to implement prevention or when a patient’s symptoms are refractory to other treatments. However, avoid anticholinergic medications; there is insufficient data to support the use of these agents in the treatment of TD.5
Tardive dyskinesia (TD) is an elusive-to-treat adverse effect of antipsychotics that has caused extreme discomfort (in a literal and figurative sense) for patients and their psychiatrists. In 2017, the prevalence of TD as a result of exposure to dopamine antagonists was approximately 30% with first-generation antipsychotics and 20% with second-generation antipsychotics.1 There have been several effective attempts at reducing rates of TD, including lowering the dosing, shifting to second-generation antipsychotics, and using recently introduced pharmacologic treatments for TD. The past 2 years have seen increased efforts at treating this often-irreversible adverse effect with pharmacotherapy, such as the recently marketed vesicular monoamine transporter-2 (VMAT2) inhibitors valbenazine and deutetrabenazine, as well as the supplement Ginkgo biloba,2 although issues with cost, adverse effects, or drug–drug interactions could limit the benefits of these agents.
Despite these strategies, one approach has been largely overlooked: prevention. Although it is included in many guidelines and literature reports, prevention has become less of a standard of practice and more of a cliché. Prevention is the key strategy for lowering the rate of TD, and it should be the assumed responsibility of each clinician in every prescription they write throughout the entire continuum of care. Here, we provide steps to take to help prevent TD, and what to consider when TD occurs.
1. Realize that we are all responsible for TD. We know TD exists, but we often feel that this adverse effect is not our fault. Avoid adapting a philosophy of “someone else caused it,” “they didn’t cause it yet,” or “it’s going to happen anyway.” We must remember that every unnecessary exposure to a dopamine antagonist increases the risk of TD, even if we don’t see the adverse effect firsthand.
2. Treat first-episode psychosis early and aggressively. Doing so may prevent chronicity of the illness, which would save a patient from long-term, high-dose exposure to antipsychotics. Lower the risk of TD with atypical antipsychotics and offer long-acting injectables when possible to improve medication adherence.
3. Treat both acute and chronic symptoms of psychosis throughout the continuum of care. The choice of medication and dose should be reevaluated at each interaction to enhance improvement of acute symptoms and to minimize chronic adverse effects. Always recognize the differences in aggressive treatment of an acute episode of psychosis vs maintenance treatment of baseline symptoms. Also, assess for TD by conducting abnormal involuntary movement scale (AIMS) examinations at baseline and at least biannually.
4. Use clozapine instead of 2 antipsychotics in chronic, refractory patients when possible. Clozapine is largely underutilize
5. Consider pharmacotherapy if TD has already occurred. Psychiatrists have been waiting for pharmacologic options for treating TD for quite some time. Explore using VMAT2 inhibitors and other agents when it is too late to implement prevention or when a patient’s symptoms are refractory to other treatments. However, avoid anticholinergic medications; there is insufficient data to support the use of these agents in the treatment of TD.5
1. Carbon M, Hsieh C, Kane J, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Zheng W, Xiang Y, Ng H, et al. Extract of ginkgo biloba for tardive dyskinesia: meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3):107-111.
3. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693.
4. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
5. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
1. Carbon M, Hsieh C, Kane J, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278.
2. Zheng W, Xiang Y, Ng H, et al. Extract of ginkgo biloba for tardive dyskinesia: meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2016;49(3):107-111.
3. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693.
4. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
5. Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
Do You Trust Your Employer?
Recently, I was talking with a colleague who works for a large hospital and health care system. While discussing his experiences over the past five years, he suddenly stopped and blurted out, “I don’t trust this organization. Nobody trusts this organization!”
Taken aback, I asked what made him say that.
First of all, he explained, there is a complete and pervasive lack of transparency as to both short- and long-term goals for the organization. Information is treated as proprietary thinking by nonclinical “corporate folks” and not released to the boots-on-the-ground clinician—which makes it difficult to work toward goals efficiently.
Furthermore, he related, there is consistent failure to provide accurate financial data or any plans to improve the organization’s financial position in the marketplace. This prevents providers from making a positive impact on cost containment. No one is invested. Provider compensation packages are neither universal nor simple. The financial folks devise complex formulas that do not account for the vagaries and complexities of health care. This health care organization views every patient as a Financial Information Number and makes no allowance for the fact that many have complex illnesses requiring significant time and attention.
Lastly, he described a systematic and insidious elimination of support staff at all levels—but particularly bedside nurses. The traditional “nursing safety net”—especially relevant in academic institutions—is in tatters, which threatens to undermine day-to-day success in patient care. Staffing of ancillary providers (those in physical, occupational, or speech-language therapy) has been cut back, which means patients wait longer to see these specialists and primary medical providers are frustrated by the lack of progress their patients make.
Stunned by his comments, I started thinking: How many of us recognize some or all of this description? How many trust the organization we work for? Realizing that a huge percentage of NPs, PAs, and physicians work for large entities, these are important questions.
Trust is central to human interaction on both personal and professional levels. Tschannen-Moran defines it as “one’s willingness to be vulnerable to another, based on the confidence that the other is benevolent, honest, open, reliable, and competent.”1
Continue to: A more focused definition of organizational trust
Organizational trust may require a broader and yet more focused definition—such as that of Cummings and Bromley, who stipulate that trust is a belief, held by an individual or groups of individuals, that another individual or group
- Makes a good faith effort to behave in accordance with any (explicit or implicit) commitments
- Is honest
- Does not take excessive advantage of another, even when the opportunity to do so exists.2
Thus, organizational (or collective) trust refers to the propensity of workgroups, administrators, and employees to trust others within the organization.
But does it really matter if we experience this kind of trust for our employer? Can’t we just show up and do our jobs? Frankly, no (at least, if we truly care about the work that we do).
Research has demonstrated that trust is a critical part of creating a shared vision; employees tend to help one another and work collaboratively when trust is present.3,4 Trust is also the foundation for flexibility and innovation.5 Employees are generally happier, more satisfied, and less stressed in high-trust organizations—and it has been shown that organizations benefit, too.6
By contrast, low-trust organizations usually create barriers to effective performance. In the absence of trust, people create rules and restrictions that mandate how others should act.4 Valuable time is then spent studying, enforcing, discussing, and rewriting rules. This yields low-flexibility results and leaves employees to simply follow and enforce policies. Another outcome is high transaction costs and less efficient work—meaning that processes become slower and more restricted by policies and paperwork.4 Low trust is also a barrier to change.7
Although we recognize organizational trust as an essential component of effective leadership, it remains an issue—one that can make or break an organization’s culture. Lack of trust, particularly between management and employees, creates a hostile work environment in which stress levels are high and productivity is reduced.
Continue to: Three dimensions of trust
There are three dimensions of trust, according to the Grunig Relationship Instrument:
Competence: The belief that an organization has the ability to do what it says it will do (this includes effectiveness and survivability in the marketplace).
Integrity: The belief that an organization is fair and just.
Dependability/reliability: The belief that an organization will do what it says it will do (ie, acts consistently and dependably).8
These concepts have been integrated into a “trust measurement questionnaire” that assists in the assessment of an organization’s trustworthiness. While this tool has been used in a variety of industries and has even been used to assess business-to-business relationships, some of the most relevant items for individual employees are outlined in the Table.8

But measuring trust is only effective if it leads to action. Once you’ve realized you don’t trust your employer, what should you do about it? Unfortunately, the answer is often “push for change or leave!” Aside from voicing your concerns or requesting more information (or leaving), the onus is really on the leaders of the organization to improve communication (among other things).
Continue to: 7 ways leaders can improve trust within their organization
According to Gleeson, there are seven ways leaders can improve trust within their organization, which include
- Having the right people in the right job, since trust must be demonstrated from top to bottom and vice versa
- Being transparent
- Sharing information with all vested parties, from industry partners to customers to employees
- Providing resources to all parties in an equitable manner
- Offering feedback to employees at all levels, perhaps through regular “status update” meetings
- Facing challenges head-on, using teamwork to promote trust and positive attitudes
- Leading by example—the organization’s values and mission should be exemplified by everyone.9
If we want to be leaders, not only within our professions but within our workplaces, we must nurture the ideas of trust, transparency, and communication. I am very interested in hearing from you about organizations that you feel are trustworthy and what makes them so—and what experiences you’ve had that led you to avoid or leave employment situations (you need not “name names,” of course). You can reach me at [email protected].
1. Tschannen-Moran M. Trust Matters: Leadership for Successful Schools. San Francisco, CA: Jossey-Bass; 2004.
2. Cummings LL, Bromley P. The organizational trust inventory (OTI): development and validation. In: Kramer R, Tyler E, eds. Trust in Organizations: Frontiers of Theory and Research. Thousand Oaks, CA: Sage; 1996:302, 330, 429.
3. Roueche JE, Baker GA, Rose RR, eds. Shared Vision: Transformational Leadership in American Community Colleges. Washington, DC: American Association of Community and Junior Colleges; 1989.
4. Henkin AB, Dee JR. The power of trust: teams and collective action in self-managed schools. J School Leadership. 2001;11(1):48-62.
5. Dervitsiotis KN. Building trust for excellence in performance and adaptation to change. Total Qual Manage Bus Excellence. 2006;17(7):795-810.
6. Costa AC, Roe RA, Taillieu T. Trust within teams: the relation with performance effectiveness. Eur J Work Org Psychol. 2001;10(3):225.
7. Kesler R, Perry C, Shay G. So they are resistant to change? Strategies for moving an immovable object. In: The Olympics of Leadership: Overcoming Obstacles, Balancing Skills, Taking Risks: Proceedings of the Annual International Conference of the National Community College Chair Academy (5th, Phoenix, Arizona, February 14-17, 1996). Mesa, AZ: National Community College Chair Academy; 1996.
8. The Institute for Public Relations Commission on PR Measurement and Evaluation, University of Florida. Guidelines for measuring trust in organizations. 2013. http://painepublishing.com/wp-content/uploads/2013/10/Grunig-relationship-instrument.pdf. Accessed March 13, 2018.
9. Gleeson B. 7 ways leaders can improve trust within their organizations. Published June 24, 2015. Inc.com. www.inc.com/brent-gleeson/7-ways-leaders-can-improve-trust-within-their-organizations.html. Accessed March 13, 2018.
Recently, I was talking with a colleague who works for a large hospital and health care system. While discussing his experiences over the past five years, he suddenly stopped and blurted out, “I don’t trust this organization. Nobody trusts this organization!”
Taken aback, I asked what made him say that.
First of all, he explained, there is a complete and pervasive lack of transparency as to both short- and long-term goals for the organization. Information is treated as proprietary thinking by nonclinical “corporate folks” and not released to the boots-on-the-ground clinician—which makes it difficult to work toward goals efficiently.
Furthermore, he related, there is consistent failure to provide accurate financial data or any plans to improve the organization’s financial position in the marketplace. This prevents providers from making a positive impact on cost containment. No one is invested. Provider compensation packages are neither universal nor simple. The financial folks devise complex formulas that do not account for the vagaries and complexities of health care. This health care organization views every patient as a Financial Information Number and makes no allowance for the fact that many have complex illnesses requiring significant time and attention.
Lastly, he described a systematic and insidious elimination of support staff at all levels—but particularly bedside nurses. The traditional “nursing safety net”—especially relevant in academic institutions—is in tatters, which threatens to undermine day-to-day success in patient care. Staffing of ancillary providers (those in physical, occupational, or speech-language therapy) has been cut back, which means patients wait longer to see these specialists and primary medical providers are frustrated by the lack of progress their patients make.
Stunned by his comments, I started thinking: How many of us recognize some or all of this description? How many trust the organization we work for? Realizing that a huge percentage of NPs, PAs, and physicians work for large entities, these are important questions.
Trust is central to human interaction on both personal and professional levels. Tschannen-Moran defines it as “one’s willingness to be vulnerable to another, based on the confidence that the other is benevolent, honest, open, reliable, and competent.”1
Continue to: A more focused definition of organizational trust
Organizational trust may require a broader and yet more focused definition—such as that of Cummings and Bromley, who stipulate that trust is a belief, held by an individual or groups of individuals, that another individual or group
- Makes a good faith effort to behave in accordance with any (explicit or implicit) commitments
- Is honest
- Does not take excessive advantage of another, even when the opportunity to do so exists.2
Thus, organizational (or collective) trust refers to the propensity of workgroups, administrators, and employees to trust others within the organization.
But does it really matter if we experience this kind of trust for our employer? Can’t we just show up and do our jobs? Frankly, no (at least, if we truly care about the work that we do).
Research has demonstrated that trust is a critical part of creating a shared vision; employees tend to help one another and work collaboratively when trust is present.3,4 Trust is also the foundation for flexibility and innovation.5 Employees are generally happier, more satisfied, and less stressed in high-trust organizations—and it has been shown that organizations benefit, too.6
By contrast, low-trust organizations usually create barriers to effective performance. In the absence of trust, people create rules and restrictions that mandate how others should act.4 Valuable time is then spent studying, enforcing, discussing, and rewriting rules. This yields low-flexibility results and leaves employees to simply follow and enforce policies. Another outcome is high transaction costs and less efficient work—meaning that processes become slower and more restricted by policies and paperwork.4 Low trust is also a barrier to change.7
Although we recognize organizational trust as an essential component of effective leadership, it remains an issue—one that can make or break an organization’s culture. Lack of trust, particularly between management and employees, creates a hostile work environment in which stress levels are high and productivity is reduced.
Continue to: Three dimensions of trust
There are three dimensions of trust, according to the Grunig Relationship Instrument:
Competence: The belief that an organization has the ability to do what it says it will do (this includes effectiveness and survivability in the marketplace).
Integrity: The belief that an organization is fair and just.
Dependability/reliability: The belief that an organization will do what it says it will do (ie, acts consistently and dependably).8
These concepts have been integrated into a “trust measurement questionnaire” that assists in the assessment of an organization’s trustworthiness. While this tool has been used in a variety of industries and has even been used to assess business-to-business relationships, some of the most relevant items for individual employees are outlined in the Table.8

But measuring trust is only effective if it leads to action. Once you’ve realized you don’t trust your employer, what should you do about it? Unfortunately, the answer is often “push for change or leave!” Aside from voicing your concerns or requesting more information (or leaving), the onus is really on the leaders of the organization to improve communication (among other things).
Continue to: 7 ways leaders can improve trust within their organization
According to Gleeson, there are seven ways leaders can improve trust within their organization, which include
- Having the right people in the right job, since trust must be demonstrated from top to bottom and vice versa
- Being transparent
- Sharing information with all vested parties, from industry partners to customers to employees
- Providing resources to all parties in an equitable manner
- Offering feedback to employees at all levels, perhaps through regular “status update” meetings
- Facing challenges head-on, using teamwork to promote trust and positive attitudes
- Leading by example—the organization’s values and mission should be exemplified by everyone.9
If we want to be leaders, not only within our professions but within our workplaces, we must nurture the ideas of trust, transparency, and communication. I am very interested in hearing from you about organizations that you feel are trustworthy and what makes them so—and what experiences you’ve had that led you to avoid or leave employment situations (you need not “name names,” of course). You can reach me at [email protected].
Recently, I was talking with a colleague who works for a large hospital and health care system. While discussing his experiences over the past five years, he suddenly stopped and blurted out, “I don’t trust this organization. Nobody trusts this organization!”
Taken aback, I asked what made him say that.
First of all, he explained, there is a complete and pervasive lack of transparency as to both short- and long-term goals for the organization. Information is treated as proprietary thinking by nonclinical “corporate folks” and not released to the boots-on-the-ground clinician—which makes it difficult to work toward goals efficiently.
Furthermore, he related, there is consistent failure to provide accurate financial data or any plans to improve the organization’s financial position in the marketplace. This prevents providers from making a positive impact on cost containment. No one is invested. Provider compensation packages are neither universal nor simple. The financial folks devise complex formulas that do not account for the vagaries and complexities of health care. This health care organization views every patient as a Financial Information Number and makes no allowance for the fact that many have complex illnesses requiring significant time and attention.
Lastly, he described a systematic and insidious elimination of support staff at all levels—but particularly bedside nurses. The traditional “nursing safety net”—especially relevant in academic institutions—is in tatters, which threatens to undermine day-to-day success in patient care. Staffing of ancillary providers (those in physical, occupational, or speech-language therapy) has been cut back, which means patients wait longer to see these specialists and primary medical providers are frustrated by the lack of progress their patients make.
Stunned by his comments, I started thinking: How many of us recognize some or all of this description? How many trust the organization we work for? Realizing that a huge percentage of NPs, PAs, and physicians work for large entities, these are important questions.
Trust is central to human interaction on both personal and professional levels. Tschannen-Moran defines it as “one’s willingness to be vulnerable to another, based on the confidence that the other is benevolent, honest, open, reliable, and competent.”1
Continue to: A more focused definition of organizational trust
Organizational trust may require a broader and yet more focused definition—such as that of Cummings and Bromley, who stipulate that trust is a belief, held by an individual or groups of individuals, that another individual or group
- Makes a good faith effort to behave in accordance with any (explicit or implicit) commitments
- Is honest
- Does not take excessive advantage of another, even when the opportunity to do so exists.2
Thus, organizational (or collective) trust refers to the propensity of workgroups, administrators, and employees to trust others within the organization.
But does it really matter if we experience this kind of trust for our employer? Can’t we just show up and do our jobs? Frankly, no (at least, if we truly care about the work that we do).
Research has demonstrated that trust is a critical part of creating a shared vision; employees tend to help one another and work collaboratively when trust is present.3,4 Trust is also the foundation for flexibility and innovation.5 Employees are generally happier, more satisfied, and less stressed in high-trust organizations—and it has been shown that organizations benefit, too.6
By contrast, low-trust organizations usually create barriers to effective performance. In the absence of trust, people create rules and restrictions that mandate how others should act.4 Valuable time is then spent studying, enforcing, discussing, and rewriting rules. This yields low-flexibility results and leaves employees to simply follow and enforce policies. Another outcome is high transaction costs and less efficient work—meaning that processes become slower and more restricted by policies and paperwork.4 Low trust is also a barrier to change.7
Although we recognize organizational trust as an essential component of effective leadership, it remains an issue—one that can make or break an organization’s culture. Lack of trust, particularly between management and employees, creates a hostile work environment in which stress levels are high and productivity is reduced.
Continue to: Three dimensions of trust
There are three dimensions of trust, according to the Grunig Relationship Instrument:
Competence: The belief that an organization has the ability to do what it says it will do (this includes effectiveness and survivability in the marketplace).
Integrity: The belief that an organization is fair and just.
Dependability/reliability: The belief that an organization will do what it says it will do (ie, acts consistently and dependably).8
These concepts have been integrated into a “trust measurement questionnaire” that assists in the assessment of an organization’s trustworthiness. While this tool has been used in a variety of industries and has even been used to assess business-to-business relationships, some of the most relevant items for individual employees are outlined in the Table.8

But measuring trust is only effective if it leads to action. Once you’ve realized you don’t trust your employer, what should you do about it? Unfortunately, the answer is often “push for change or leave!” Aside from voicing your concerns or requesting more information (or leaving), the onus is really on the leaders of the organization to improve communication (among other things).
Continue to: 7 ways leaders can improve trust within their organization
According to Gleeson, there are seven ways leaders can improve trust within their organization, which include
- Having the right people in the right job, since trust must be demonstrated from top to bottom and vice versa
- Being transparent
- Sharing information with all vested parties, from industry partners to customers to employees
- Providing resources to all parties in an equitable manner
- Offering feedback to employees at all levels, perhaps through regular “status update” meetings
- Facing challenges head-on, using teamwork to promote trust and positive attitudes
- Leading by example—the organization’s values and mission should be exemplified by everyone.9
If we want to be leaders, not only within our professions but within our workplaces, we must nurture the ideas of trust, transparency, and communication. I am very interested in hearing from you about organizations that you feel are trustworthy and what makes them so—and what experiences you’ve had that led you to avoid or leave employment situations (you need not “name names,” of course). You can reach me at [email protected].
1. Tschannen-Moran M. Trust Matters: Leadership for Successful Schools. San Francisco, CA: Jossey-Bass; 2004.
2. Cummings LL, Bromley P. The organizational trust inventory (OTI): development and validation. In: Kramer R, Tyler E, eds. Trust in Organizations: Frontiers of Theory and Research. Thousand Oaks, CA: Sage; 1996:302, 330, 429.
3. Roueche JE, Baker GA, Rose RR, eds. Shared Vision: Transformational Leadership in American Community Colleges. Washington, DC: American Association of Community and Junior Colleges; 1989.
4. Henkin AB, Dee JR. The power of trust: teams and collective action in self-managed schools. J School Leadership. 2001;11(1):48-62.
5. Dervitsiotis KN. Building trust for excellence in performance and adaptation to change. Total Qual Manage Bus Excellence. 2006;17(7):795-810.
6. Costa AC, Roe RA, Taillieu T. Trust within teams: the relation with performance effectiveness. Eur J Work Org Psychol. 2001;10(3):225.
7. Kesler R, Perry C, Shay G. So they are resistant to change? Strategies for moving an immovable object. In: The Olympics of Leadership: Overcoming Obstacles, Balancing Skills, Taking Risks: Proceedings of the Annual International Conference of the National Community College Chair Academy (5th, Phoenix, Arizona, February 14-17, 1996). Mesa, AZ: National Community College Chair Academy; 1996.
8. The Institute for Public Relations Commission on PR Measurement and Evaluation, University of Florida. Guidelines for measuring trust in organizations. 2013. http://painepublishing.com/wp-content/uploads/2013/10/Grunig-relationship-instrument.pdf. Accessed March 13, 2018.
9. Gleeson B. 7 ways leaders can improve trust within their organizations. Published June 24, 2015. Inc.com. www.inc.com/brent-gleeson/7-ways-leaders-can-improve-trust-within-their-organizations.html. Accessed March 13, 2018.
1. Tschannen-Moran M. Trust Matters: Leadership for Successful Schools. San Francisco, CA: Jossey-Bass; 2004.
2. Cummings LL, Bromley P. The organizational trust inventory (OTI): development and validation. In: Kramer R, Tyler E, eds. Trust in Organizations: Frontiers of Theory and Research. Thousand Oaks, CA: Sage; 1996:302, 330, 429.
3. Roueche JE, Baker GA, Rose RR, eds. Shared Vision: Transformational Leadership in American Community Colleges. Washington, DC: American Association of Community and Junior Colleges; 1989.
4. Henkin AB, Dee JR. The power of trust: teams and collective action in self-managed schools. J School Leadership. 2001;11(1):48-62.
5. Dervitsiotis KN. Building trust for excellence in performance and adaptation to change. Total Qual Manage Bus Excellence. 2006;17(7):795-810.
6. Costa AC, Roe RA, Taillieu T. Trust within teams: the relation with performance effectiveness. Eur J Work Org Psychol. 2001;10(3):225.
7. Kesler R, Perry C, Shay G. So they are resistant to change? Strategies for moving an immovable object. In: The Olympics of Leadership: Overcoming Obstacles, Balancing Skills, Taking Risks: Proceedings of the Annual International Conference of the National Community College Chair Academy (5th, Phoenix, Arizona, February 14-17, 1996). Mesa, AZ: National Community College Chair Academy; 1996.
8. The Institute for Public Relations Commission on PR Measurement and Evaluation, University of Florida. Guidelines for measuring trust in organizations. 2013. http://painepublishing.com/wp-content/uploads/2013/10/Grunig-relationship-instrument.pdf. Accessed March 13, 2018.
9. Gleeson B. 7 ways leaders can improve trust within their organizations. Published June 24, 2015. Inc.com. www.inc.com/brent-gleeson/7-ways-leaders-can-improve-trust-within-their-organizations.html. Accessed March 13, 2018.
A guide to providing wide-ranging care to newborns
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.
Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8
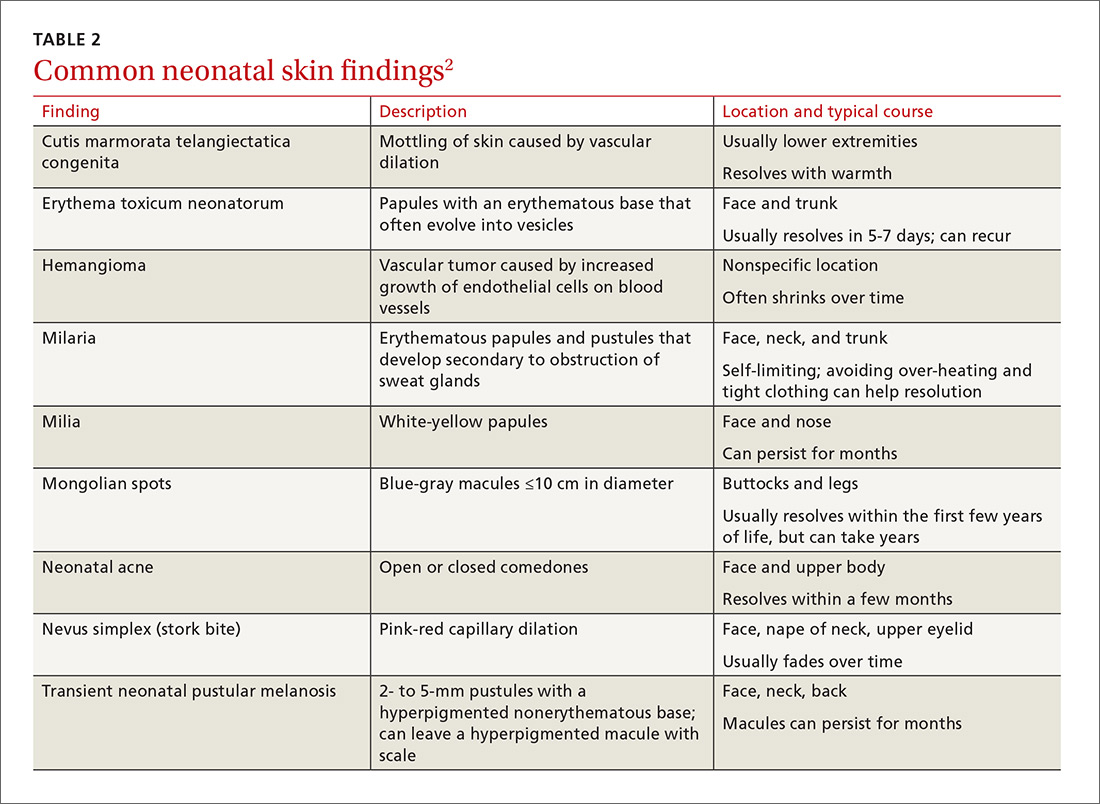
Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1
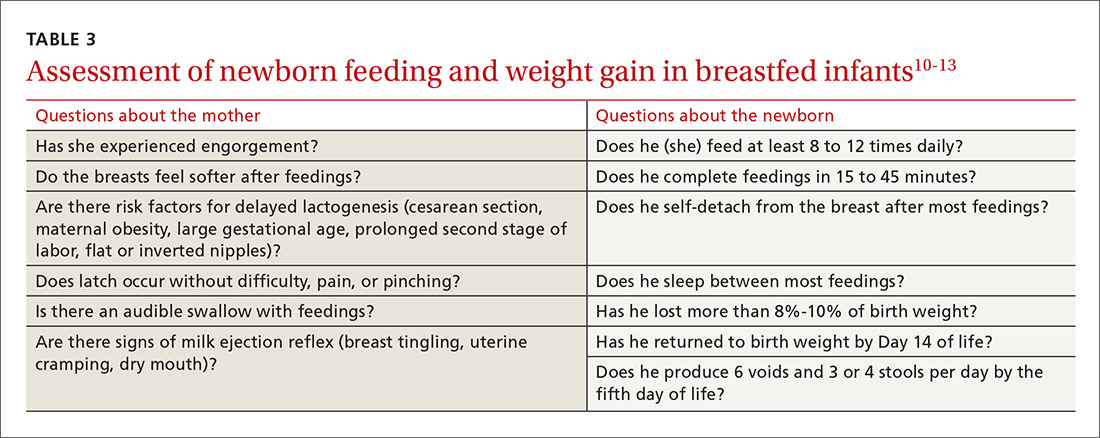
Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38
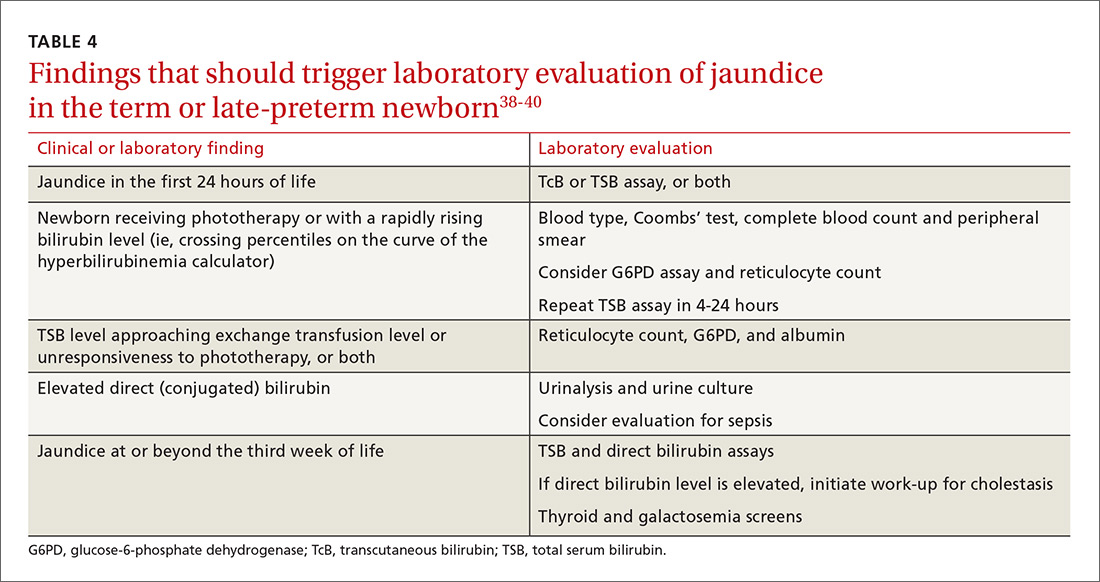
Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.
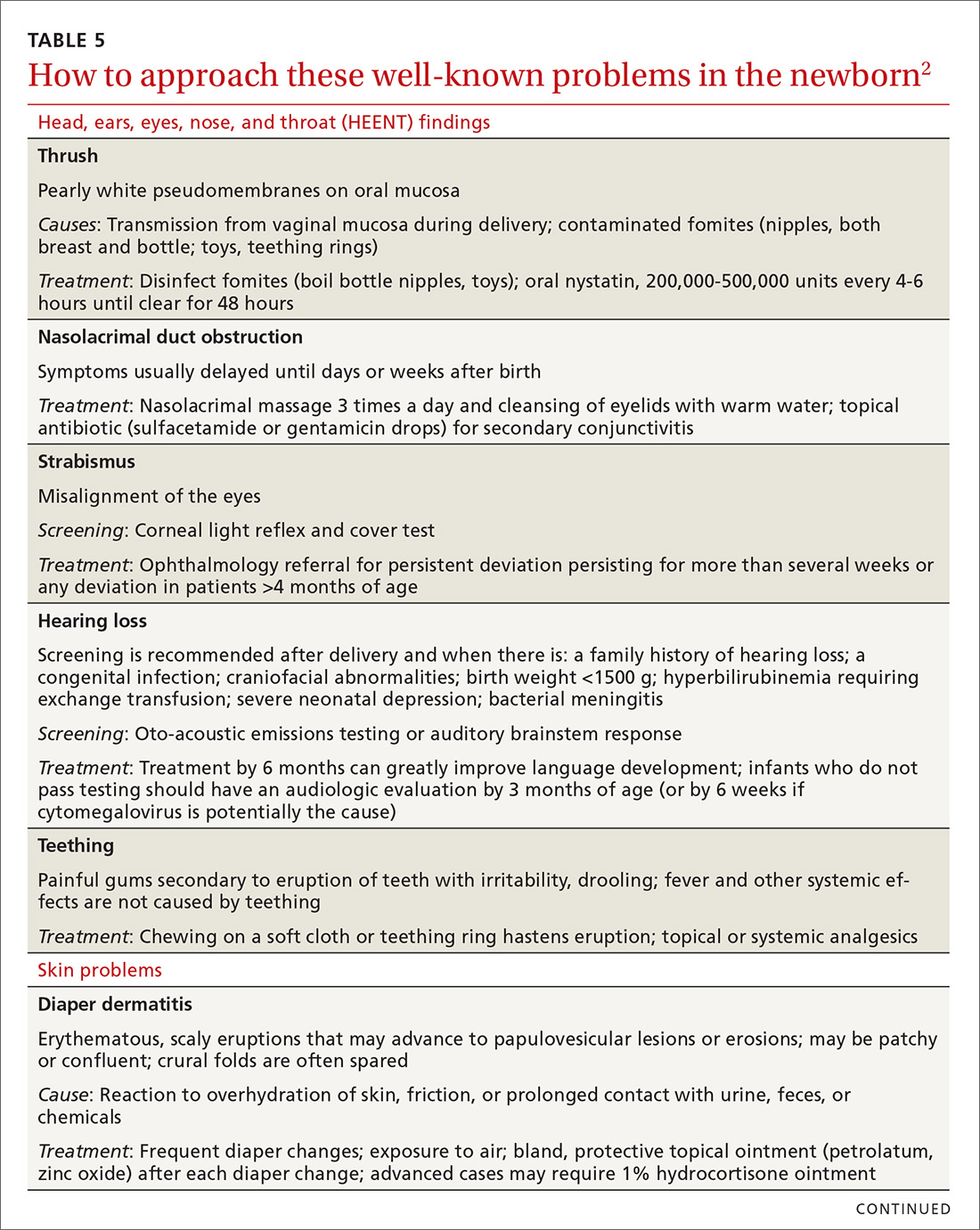
To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57

Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.
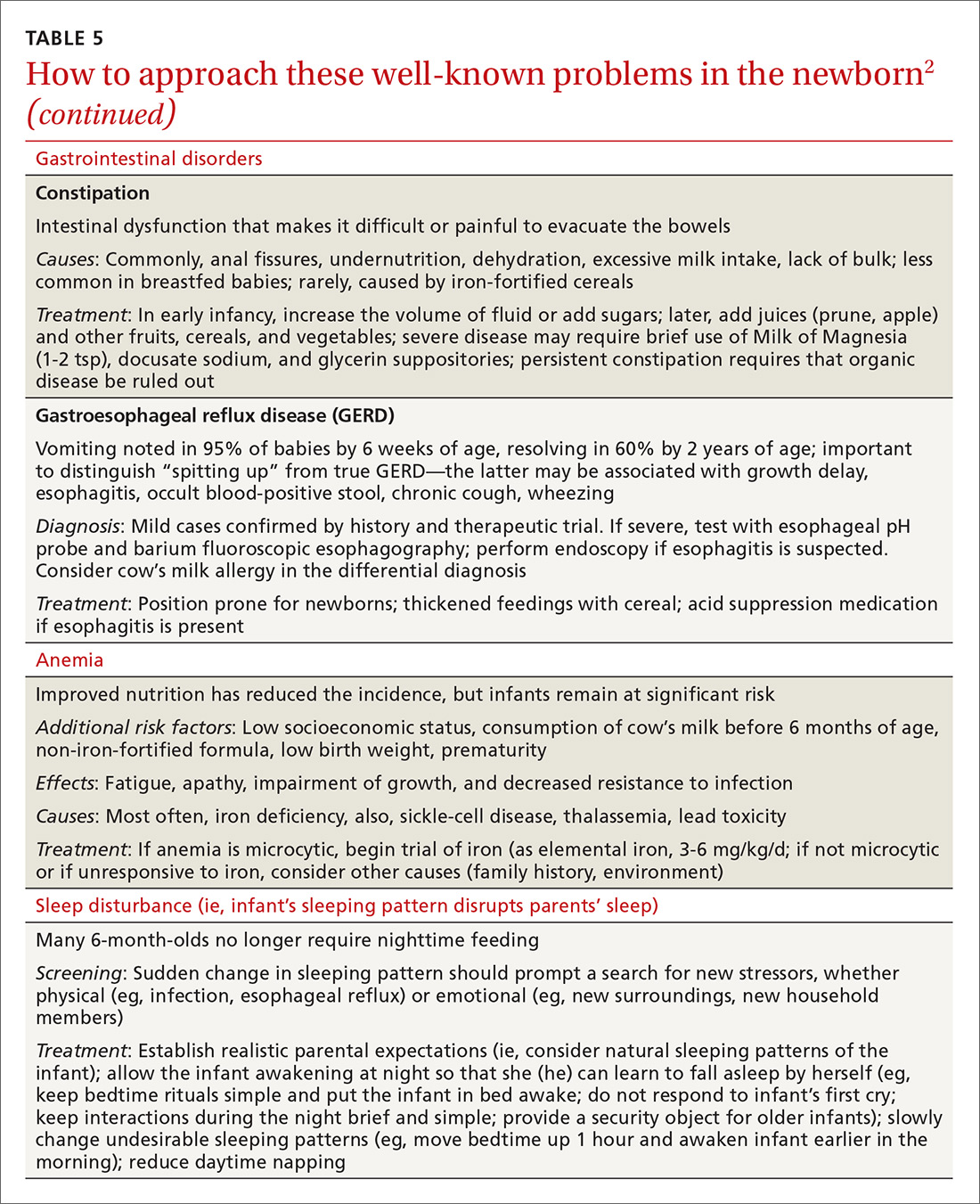
CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.

Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8

Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1

Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38

Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.

To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57

Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.

CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.

Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8

Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1

Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38

Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.

To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57

Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.

CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
From The Journal of Family Practice | 2018;67(4):E4-E15.
PRACTICE RECOMMENDATIONS
› Include a full work-up and empiric antibiotics in the initial management of all febrile infants ≤28 days of age. A
› Recommend that newborns breastfeed exclusively (in the absence of contraindications) for 6 months and continue some breastfeeding until the baby is at least 12 to 24 months of age. A
› Screen all newborns for jaundice before discharge by 1) clinical assessment or 2) testing for total serum bilirubin (TSB) or transcutaneous bilirubin (TcB); measurement of TcB provides a reasonable estimate of the TSB level in healthy newborns at levels <15 mg/dL. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bilateral thigh and knee pain • leg weakness • no history of trauma • Dx?
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
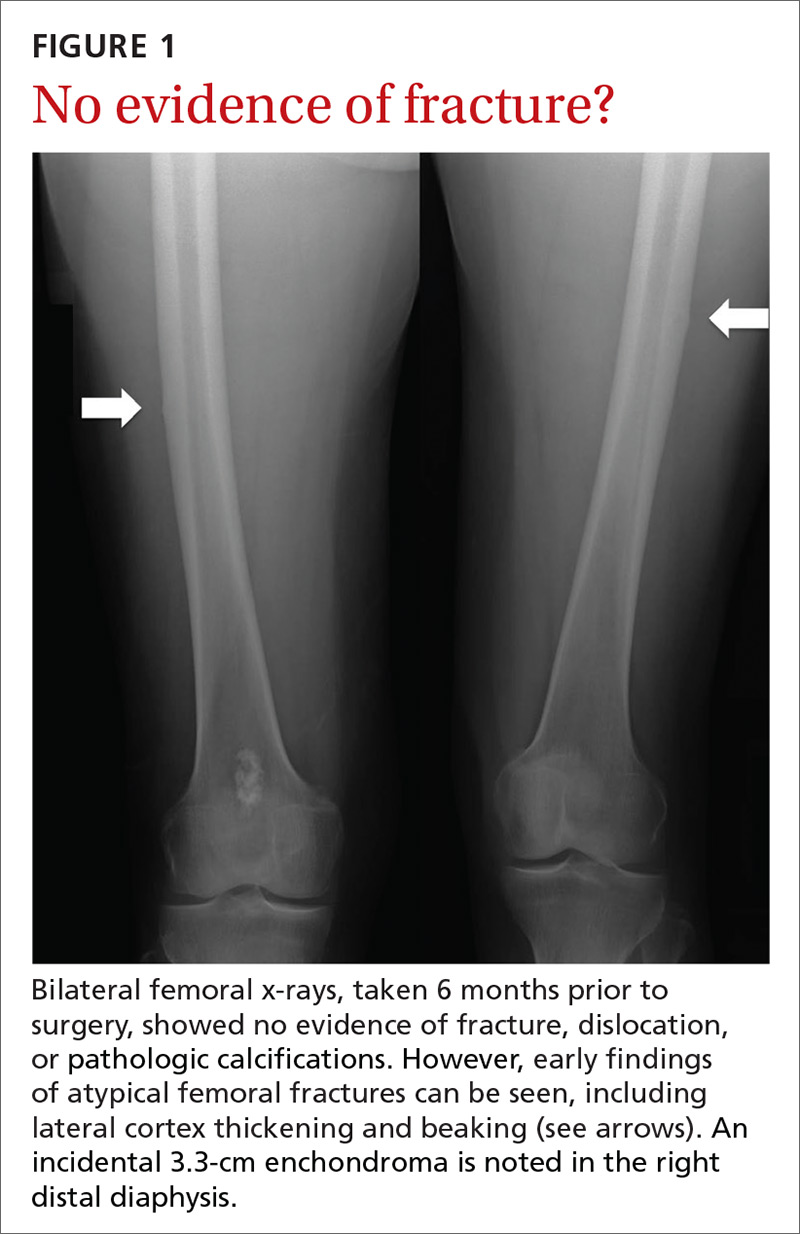
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
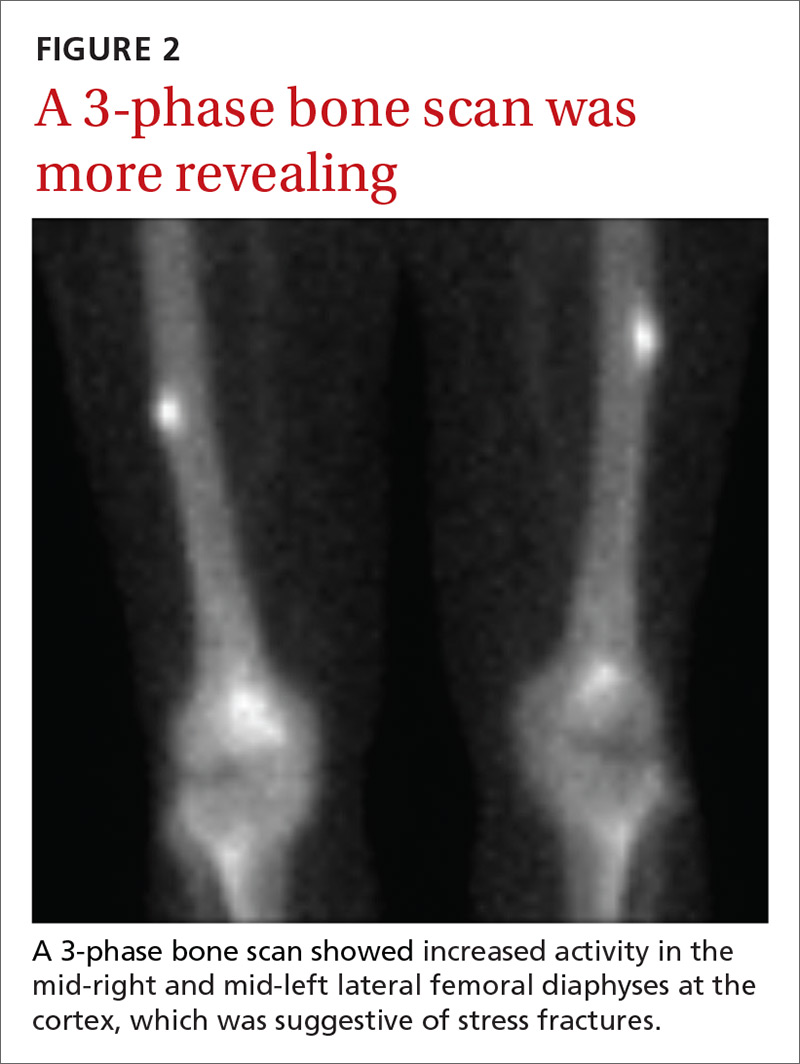
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
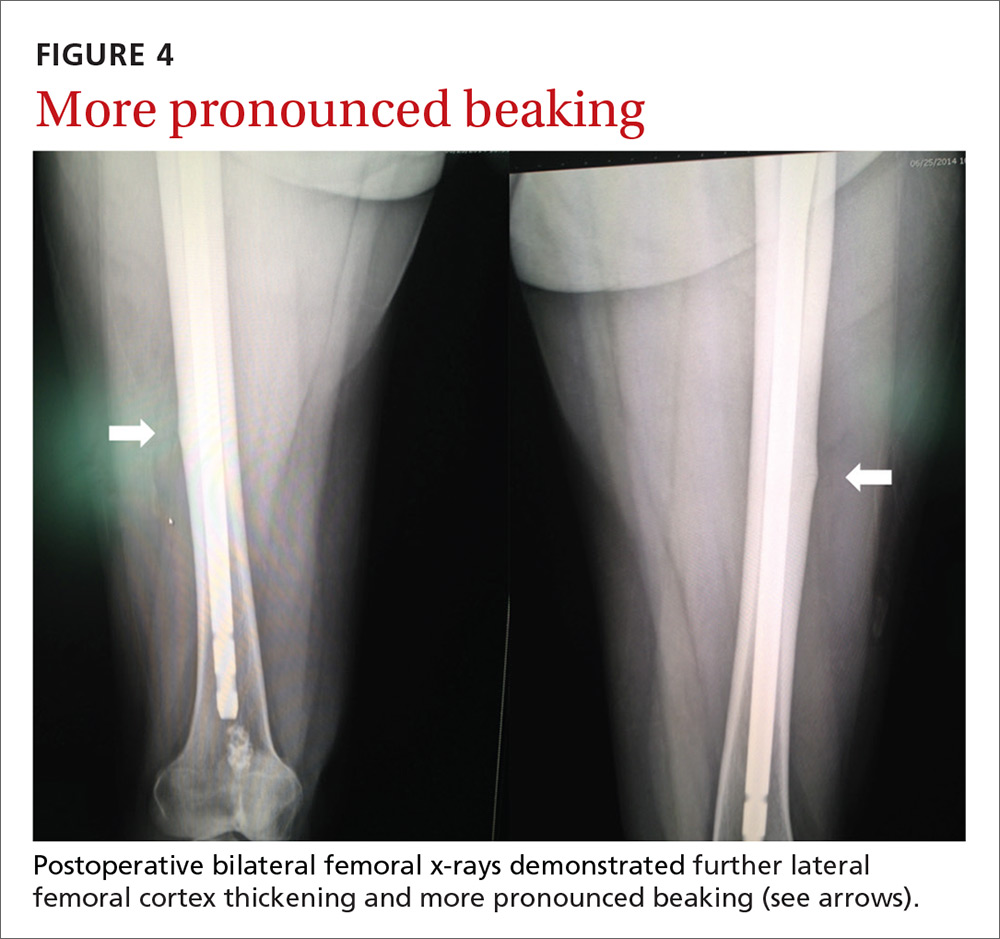
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.

Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).

DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.

Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.

THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.

Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).

DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.

Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.

THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
How well do POLST forms assure that patients get the end-of-life care they requested?
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).

A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3

One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).

A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3

One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).

A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3

One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Quite well, for cardiopulmonary resuscitation (CPR). Most patients (91%-100%) who select “do not resuscitate” (DNR) on their physician’s orders for life-sustaining treatment (POLST) forms are allowed a natural death without attempted CPR across a variety of settings (community, skilled nursing facilities, emergency medical services, and hospice). Few patients (6%) who select “comfort measures only” die in the hospital, whereas more (22%) who choose “limited interventions,” and still more (34%) without a POLST form, die in the hospital (strength of recommendation [SOR]: B, large, consistent cross-sectional and cohort studies).
Most patients (84%) who select “attempt resuscitation” receive resuscitation for out-of-hospital cardiac arrest in emergency services settings (SOR: B, small retrospective cohort study).
POLST orders declining other services (intravenous fluids, intensive care, intubation, feeding tubes) are carried out in most (84%-100%) cases. POLST orders regarding antibiotic treatments are less effectively implemented (SOR: B, moderate-sized retrospective chart review).