User login
What are the benefits and risks of daily low-dose aspirin for primary prevention of CV events?
EVIDENCE SUMMARY
A 2013 systematic review of RCTs, systematic reviews, and meta-analyses examined the prophylactic use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) among adults 18 years and older.1 Twenty-seven papers met inclusion criteria; the total number of patients wasn’t reported.
A composite finding of nonfatal MI, nonfatal stroke, and CVD death indicated a number needed to treat (NNT) of 138 over 10 years of therapy (relative risk [RR]=0.90; 95% confidence interval [CI], 0.85-0.96). CVD death wasn’t disaggregated from this composite, but an analysis of all-cause mortality didn’t reach statistical significance (RR=0.94; 95% CI, 0.88-1.00). RR for nonfatal stroke alone also wasn’t disaggregated.
Risk of gastrointestinal (GI) bleeding was found to be a number needed to harm (NNH) of 108 over 10 years (RR=1.37; 95% CI, 1.15-1.62) whereas risk of hemorrhagic stroke didn’t reach statistical significance (RR=1.32; 95% CI, 1.00-1.74). This population-level review didn’t report disaggregated findings by age or baseline atherosclerotic cardiovascular disease (ASCVD) risk.
Another review finds benefit only for prevention of nonfatal MI
A 2016 systematic review included 2 good-quality and 9 fair-quality RCTs evaluating the benefits of low-dose aspirin compared with placebo or no treatment for primary prevention of CVD events in 118,445 patients ages 40 years and older.2 The review found benefit only for nonfatal MI, with an NNT of 126 over 10 years (RR=0.78; 95% CI, 0.71-0.87). There was no change in RR for nonfatal stroke (RR=0.95; 95% CI, 0.85-1.06); negligible impact on all-cause mortality (RR=0.95; 95% CI, 0.89-0.99); and no statistically significant benefit for CVD-specific mortality (RR=0.94; 95% CI, 0.86-1.03).
Aspirin carries risk of GI hemorrhage, but not hemorrhagic stroke
A companion 2016 systematic review of 16 RCTs, cohort studies, and meta-analyses evaluated the risk of serious bleeding in patients using low-dose aspirin for primary prevention of either CVD or cancer.3 The review (number of patients not reported) found that estimated excess bleeding events differed substantially depending on varying sources for baseline bleeding rates in aspirin nonusers.
The most conservative comparison yielded an NNH of 72 over 10 years of therapy (1.39 excess major GI bleeding events per 1000 person-years, 95% CI, 0.70-2.28). Comparison with other baseline bleeding rates in trial data yielded less risk of harm, with an NNH of 357 over 10 years (0.28 excess major GI bleeding events per 1000 person-years; 95% CI, 0.14-0.46). Excess risk for hemorrhagic stroke was not statistically significant (0.32 excess events per 1000 person-years; 95% CI, −0.05 to 0.82).
RECOMMENDATIONS
The US Preventive Services Task Force gives a Grade B recommendation (recommended, based on moderate to substantial benefit) to the use of aspirin to prevent CVD among adults ages 50 to 59 years with an ASCVD risk ≥10% who don’t have increased bleeding risk and are capable of 10 years of pharmacologic adherence with a similar expected longevity.4 The Task Force assigns a Grade C recommendation (individual and professional choice) to patients 60 to 69 years of age with the same constellation of risk factors and health status. Insufficient evidence was available to make recommendations for other age cohorts.
The American College of Chest Physicians recommends 75 to 100 mg of aspirin daily for adults 50 years or older who have moderate to high CV risk, defined as ≥10%.5
A working group of the European Society of Cardiology (ESC) released a statement in 2014 recommending aspirin for primary prevention in adults with a CV risk ≥20% and no risk factors for bleeding. For patients with a CVD risk between 10% and 20%, the ESC recommends deferring to patient preference.6
1. Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8:e81970.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Bibbins-Domingo K, US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836-845.
5. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S.
6. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Working Group on Thrombosis. J Am Coll Cardiol. 2014;64:319-327.
EVIDENCE SUMMARY
A 2013 systematic review of RCTs, systematic reviews, and meta-analyses examined the prophylactic use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) among adults 18 years and older.1 Twenty-seven papers met inclusion criteria; the total number of patients wasn’t reported.
A composite finding of nonfatal MI, nonfatal stroke, and CVD death indicated a number needed to treat (NNT) of 138 over 10 years of therapy (relative risk [RR]=0.90; 95% confidence interval [CI], 0.85-0.96). CVD death wasn’t disaggregated from this composite, but an analysis of all-cause mortality didn’t reach statistical significance (RR=0.94; 95% CI, 0.88-1.00). RR for nonfatal stroke alone also wasn’t disaggregated.
Risk of gastrointestinal (GI) bleeding was found to be a number needed to harm (NNH) of 108 over 10 years (RR=1.37; 95% CI, 1.15-1.62) whereas risk of hemorrhagic stroke didn’t reach statistical significance (RR=1.32; 95% CI, 1.00-1.74). This population-level review didn’t report disaggregated findings by age or baseline atherosclerotic cardiovascular disease (ASCVD) risk.
Another review finds benefit only for prevention of nonfatal MI
A 2016 systematic review included 2 good-quality and 9 fair-quality RCTs evaluating the benefits of low-dose aspirin compared with placebo or no treatment for primary prevention of CVD events in 118,445 patients ages 40 years and older.2 The review found benefit only for nonfatal MI, with an NNT of 126 over 10 years (RR=0.78; 95% CI, 0.71-0.87). There was no change in RR for nonfatal stroke (RR=0.95; 95% CI, 0.85-1.06); negligible impact on all-cause mortality (RR=0.95; 95% CI, 0.89-0.99); and no statistically significant benefit for CVD-specific mortality (RR=0.94; 95% CI, 0.86-1.03).
Aspirin carries risk of GI hemorrhage, but not hemorrhagic stroke
A companion 2016 systematic review of 16 RCTs, cohort studies, and meta-analyses evaluated the risk of serious bleeding in patients using low-dose aspirin for primary prevention of either CVD or cancer.3 The review (number of patients not reported) found that estimated excess bleeding events differed substantially depending on varying sources for baseline bleeding rates in aspirin nonusers.
The most conservative comparison yielded an NNH of 72 over 10 years of therapy (1.39 excess major GI bleeding events per 1000 person-years, 95% CI, 0.70-2.28). Comparison with other baseline bleeding rates in trial data yielded less risk of harm, with an NNH of 357 over 10 years (0.28 excess major GI bleeding events per 1000 person-years; 95% CI, 0.14-0.46). Excess risk for hemorrhagic stroke was not statistically significant (0.32 excess events per 1000 person-years; 95% CI, −0.05 to 0.82).
RECOMMENDATIONS
The US Preventive Services Task Force gives a Grade B recommendation (recommended, based on moderate to substantial benefit) to the use of aspirin to prevent CVD among adults ages 50 to 59 years with an ASCVD risk ≥10% who don’t have increased bleeding risk and are capable of 10 years of pharmacologic adherence with a similar expected longevity.4 The Task Force assigns a Grade C recommendation (individual and professional choice) to patients 60 to 69 years of age with the same constellation of risk factors and health status. Insufficient evidence was available to make recommendations for other age cohorts.
The American College of Chest Physicians recommends 75 to 100 mg of aspirin daily for adults 50 years or older who have moderate to high CV risk, defined as ≥10%.5
A working group of the European Society of Cardiology (ESC) released a statement in 2014 recommending aspirin for primary prevention in adults with a CV risk ≥20% and no risk factors for bleeding. For patients with a CVD risk between 10% and 20%, the ESC recommends deferring to patient preference.6
EVIDENCE SUMMARY
A 2013 systematic review of RCTs, systematic reviews, and meta-analyses examined the prophylactic use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) among adults 18 years and older.1 Twenty-seven papers met inclusion criteria; the total number of patients wasn’t reported.
A composite finding of nonfatal MI, nonfatal stroke, and CVD death indicated a number needed to treat (NNT) of 138 over 10 years of therapy (relative risk [RR]=0.90; 95% confidence interval [CI], 0.85-0.96). CVD death wasn’t disaggregated from this composite, but an analysis of all-cause mortality didn’t reach statistical significance (RR=0.94; 95% CI, 0.88-1.00). RR for nonfatal stroke alone also wasn’t disaggregated.
Risk of gastrointestinal (GI) bleeding was found to be a number needed to harm (NNH) of 108 over 10 years (RR=1.37; 95% CI, 1.15-1.62) whereas risk of hemorrhagic stroke didn’t reach statistical significance (RR=1.32; 95% CI, 1.00-1.74). This population-level review didn’t report disaggregated findings by age or baseline atherosclerotic cardiovascular disease (ASCVD) risk.
Another review finds benefit only for prevention of nonfatal MI
A 2016 systematic review included 2 good-quality and 9 fair-quality RCTs evaluating the benefits of low-dose aspirin compared with placebo or no treatment for primary prevention of CVD events in 118,445 patients ages 40 years and older.2 The review found benefit only for nonfatal MI, with an NNT of 126 over 10 years (RR=0.78; 95% CI, 0.71-0.87). There was no change in RR for nonfatal stroke (RR=0.95; 95% CI, 0.85-1.06); negligible impact on all-cause mortality (RR=0.95; 95% CI, 0.89-0.99); and no statistically significant benefit for CVD-specific mortality (RR=0.94; 95% CI, 0.86-1.03).
Aspirin carries risk of GI hemorrhage, but not hemorrhagic stroke
A companion 2016 systematic review of 16 RCTs, cohort studies, and meta-analyses evaluated the risk of serious bleeding in patients using low-dose aspirin for primary prevention of either CVD or cancer.3 The review (number of patients not reported) found that estimated excess bleeding events differed substantially depending on varying sources for baseline bleeding rates in aspirin nonusers.
The most conservative comparison yielded an NNH of 72 over 10 years of therapy (1.39 excess major GI bleeding events per 1000 person-years, 95% CI, 0.70-2.28). Comparison with other baseline bleeding rates in trial data yielded less risk of harm, with an NNH of 357 over 10 years (0.28 excess major GI bleeding events per 1000 person-years; 95% CI, 0.14-0.46). Excess risk for hemorrhagic stroke was not statistically significant (0.32 excess events per 1000 person-years; 95% CI, −0.05 to 0.82).
RECOMMENDATIONS
The US Preventive Services Task Force gives a Grade B recommendation (recommended, based on moderate to substantial benefit) to the use of aspirin to prevent CVD among adults ages 50 to 59 years with an ASCVD risk ≥10% who don’t have increased bleeding risk and are capable of 10 years of pharmacologic adherence with a similar expected longevity.4 The Task Force assigns a Grade C recommendation (individual and professional choice) to patients 60 to 69 years of age with the same constellation of risk factors and health status. Insufficient evidence was available to make recommendations for other age cohorts.
The American College of Chest Physicians recommends 75 to 100 mg of aspirin daily for adults 50 years or older who have moderate to high CV risk, defined as ≥10%.5
A working group of the European Society of Cardiology (ESC) released a statement in 2014 recommending aspirin for primary prevention in adults with a CV risk ≥20% and no risk factors for bleeding. For patients with a CVD risk between 10% and 20%, the ESC recommends deferring to patient preference.6
1. Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8:e81970.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Bibbins-Domingo K, US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836-845.
5. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S.
6. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Working Group on Thrombosis. J Am Coll Cardiol. 2014;64:319-327.
1. Sutcliffe P, Connock M, Gurung T, et al. Aspirin in primary prevention of cardiovascular disease and cancer: a systematic review of the balance of evidence from reviews of randomized trials. PLoS One. 2013;8:e81970.
2. Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:804-813.
3. Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2016;164:826-835.
4. Bibbins-Domingo K, US Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836-845.
5. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e637S-e668S.
6. Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology Working Group on Thrombosis. J Am Coll Cardiol. 2014;64:319-327.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE BASED ANSWER:
One nonfatal myocardial infarction (MI) will be avoided for every 126 to 138 adults who take daily aspirin for 10 years (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of multiple randomized controlled trials [RCTs]).
Taking low-dose aspirin for primary prevention shows no clear mortality benefit. A benefit for primary prevention of stroke is less certain. Although no evidence establishes increased risk of hemorrhagic stroke from daily low-dose aspirin, one gastrointestinal hemorrhage will occur for every 72 to 357 adults who take aspirin for longer than 10 years (SOR: A, systematic reviews and meta-analyses of multiple RCTs and cohort studies).
We need to treat gun violence like an epidemic
In an interesting bit of timing, just one month before the tragic shooting at the Marjory Stoneman Douglas High School in Parkland, Florida, the AMA Journal of Ethics devoted its entire January issue to the role of physicians in preventing violence. Part of the discussion centered on the idea of treating gun violence as an infectious disease epidemic.1
Dr. Gary Slutkin, an infectious disease specialist and former Centers for Disease Control and Prevention epidemiologist, is a proponent of this approach. His research has demonstrated that epidemic disease control measures are effective in reducing violence and violence-related deaths.2-5
Just look at incidence. Violent deaths in the United States are at an epidemic proportion, just like deaths due to narcotic overdoses. In 2015, there were approximately 33,091 deaths due to narcotic overdoses and 36,252 deaths due to gun violence.6,7
Geographic and social factors. Like infectious disease epidemics, violence tends to cluster in certain geographic areas and social networks. The cause of violence is multifactorial, just like other infectious disease epidemics, such as tuberculosis. Poverty, poor education, and inadequate family structure act as modulating factors that increase the rate of violence in those exposed to it.
Enlisting the community. This contagious disease prevention approach uses community health workers to map areas of high transmission, reach out to those exposed, and intervene to reduce risk factors. For example, gang-related deaths are often due to retaliation. A thorough investigation of a patient who arrives in the emergency department (ED) with a gunshot wound can reveal the next likely perpetrators and victims. Then community violence prevention workers can go directly to these people and others in their social networks, such as parents and friends, to attempt to prevent the next shooting. This approach, dubbed “Cure Violence” (CureViolence.org), has resulted in up to a 70% decrease in violence in some areas of Chicago.2 Some neighborhoods of Baltimore and New York have seen similar reductions.3-5
What can family practitioners do? Dr. Slutkin believes his approach could be expanded from EDs to other health care settings, like primary care, where we can identify people at risk and refer them to community violence prevention resources. Imagine it—a day when violence goes the way of polio.
1. Slutkin G, Ransford C, Zvetina D. How the health sector can reduce violence by treating it as a contagion. AMA J Ethics. 2018;20:47-55.
2. Skogan WG, Hartnett SM, Bump N, et al. Evaluation of CeaseFire-Chicago. Evanston, IL: Northwestern University Institute for Policy Research; 2008. Available at: https://www.ncjrs.gov/pdffiles1/nij/grants/227181.pdf. Accessed September 11, 2017.
3. Webster DW, Whitehill JM, Vernick JS, et al. Evaluation of Baltimore’s Safe Streets program: effects on attitudes, participants’ experiences, and gun violence. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; January 11, 2012. Available at: http://baltimorehealth.org/wp-content/uploads/2016/06/2012_01_10_JHSPH_Safe_Streets_evaluation.pdf. Accessed September 11, 2017.
4. Delgado SA, Alsabahi L, Wolff K, et al. Demoralizing violence: the effects of Cure Violence in the South Bronx and East New York, Brooklyn. John Jay College of Criminal Justice Research and Evaluation Center. Available at: https://johnjayrec.nyc/2017/10/02/cvinsobronxeastny/. Published October 2, 2017. Accessed November 15, 2017.
5. Picard-Fritsche S, Cerniglia L. Testing a public approach to gun violence: an evaluation of Crown Heights Save Our Streets, a replication of the Cure Violence Model. Center for Court Innovation; 2013. Available at: https://www.courtinnovation.org/sites/default/files/documents/SOS_Evaluation.pdf. Accessed November 28, 2017.
6. Murphy SL, Xu J, Kochanek KD, et al. Deaths: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1-75.
7. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
In an interesting bit of timing, just one month before the tragic shooting at the Marjory Stoneman Douglas High School in Parkland, Florida, the AMA Journal of Ethics devoted its entire January issue to the role of physicians in preventing violence. Part of the discussion centered on the idea of treating gun violence as an infectious disease epidemic.1
Dr. Gary Slutkin, an infectious disease specialist and former Centers for Disease Control and Prevention epidemiologist, is a proponent of this approach. His research has demonstrated that epidemic disease control measures are effective in reducing violence and violence-related deaths.2-5
Just look at incidence. Violent deaths in the United States are at an epidemic proportion, just like deaths due to narcotic overdoses. In 2015, there were approximately 33,091 deaths due to narcotic overdoses and 36,252 deaths due to gun violence.6,7
Geographic and social factors. Like infectious disease epidemics, violence tends to cluster in certain geographic areas and social networks. The cause of violence is multifactorial, just like other infectious disease epidemics, such as tuberculosis. Poverty, poor education, and inadequate family structure act as modulating factors that increase the rate of violence in those exposed to it.
Enlisting the community. This contagious disease prevention approach uses community health workers to map areas of high transmission, reach out to those exposed, and intervene to reduce risk factors. For example, gang-related deaths are often due to retaliation. A thorough investigation of a patient who arrives in the emergency department (ED) with a gunshot wound can reveal the next likely perpetrators and victims. Then community violence prevention workers can go directly to these people and others in their social networks, such as parents and friends, to attempt to prevent the next shooting. This approach, dubbed “Cure Violence” (CureViolence.org), has resulted in up to a 70% decrease in violence in some areas of Chicago.2 Some neighborhoods of Baltimore and New York have seen similar reductions.3-5
What can family practitioners do? Dr. Slutkin believes his approach could be expanded from EDs to other health care settings, like primary care, where we can identify people at risk and refer them to community violence prevention resources. Imagine it—a day when violence goes the way of polio.
In an interesting bit of timing, just one month before the tragic shooting at the Marjory Stoneman Douglas High School in Parkland, Florida, the AMA Journal of Ethics devoted its entire January issue to the role of physicians in preventing violence. Part of the discussion centered on the idea of treating gun violence as an infectious disease epidemic.1
Dr. Gary Slutkin, an infectious disease specialist and former Centers for Disease Control and Prevention epidemiologist, is a proponent of this approach. His research has demonstrated that epidemic disease control measures are effective in reducing violence and violence-related deaths.2-5
Just look at incidence. Violent deaths in the United States are at an epidemic proportion, just like deaths due to narcotic overdoses. In 2015, there were approximately 33,091 deaths due to narcotic overdoses and 36,252 deaths due to gun violence.6,7
Geographic and social factors. Like infectious disease epidemics, violence tends to cluster in certain geographic areas and social networks. The cause of violence is multifactorial, just like other infectious disease epidemics, such as tuberculosis. Poverty, poor education, and inadequate family structure act as modulating factors that increase the rate of violence in those exposed to it.
Enlisting the community. This contagious disease prevention approach uses community health workers to map areas of high transmission, reach out to those exposed, and intervene to reduce risk factors. For example, gang-related deaths are often due to retaliation. A thorough investigation of a patient who arrives in the emergency department (ED) with a gunshot wound can reveal the next likely perpetrators and victims. Then community violence prevention workers can go directly to these people and others in their social networks, such as parents and friends, to attempt to prevent the next shooting. This approach, dubbed “Cure Violence” (CureViolence.org), has resulted in up to a 70% decrease in violence in some areas of Chicago.2 Some neighborhoods of Baltimore and New York have seen similar reductions.3-5
What can family practitioners do? Dr. Slutkin believes his approach could be expanded from EDs to other health care settings, like primary care, where we can identify people at risk and refer them to community violence prevention resources. Imagine it—a day when violence goes the way of polio.
1. Slutkin G, Ransford C, Zvetina D. How the health sector can reduce violence by treating it as a contagion. AMA J Ethics. 2018;20:47-55.
2. Skogan WG, Hartnett SM, Bump N, et al. Evaluation of CeaseFire-Chicago. Evanston, IL: Northwestern University Institute for Policy Research; 2008. Available at: https://www.ncjrs.gov/pdffiles1/nij/grants/227181.pdf. Accessed September 11, 2017.
3. Webster DW, Whitehill JM, Vernick JS, et al. Evaluation of Baltimore’s Safe Streets program: effects on attitudes, participants’ experiences, and gun violence. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; January 11, 2012. Available at: http://baltimorehealth.org/wp-content/uploads/2016/06/2012_01_10_JHSPH_Safe_Streets_evaluation.pdf. Accessed September 11, 2017.
4. Delgado SA, Alsabahi L, Wolff K, et al. Demoralizing violence: the effects of Cure Violence in the South Bronx and East New York, Brooklyn. John Jay College of Criminal Justice Research and Evaluation Center. Available at: https://johnjayrec.nyc/2017/10/02/cvinsobronxeastny/. Published October 2, 2017. Accessed November 15, 2017.
5. Picard-Fritsche S, Cerniglia L. Testing a public approach to gun violence: an evaluation of Crown Heights Save Our Streets, a replication of the Cure Violence Model. Center for Court Innovation; 2013. Available at: https://www.courtinnovation.org/sites/default/files/documents/SOS_Evaluation.pdf. Accessed November 28, 2017.
6. Murphy SL, Xu J, Kochanek KD, et al. Deaths: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1-75.
7. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
1. Slutkin G, Ransford C, Zvetina D. How the health sector can reduce violence by treating it as a contagion. AMA J Ethics. 2018;20:47-55.
2. Skogan WG, Hartnett SM, Bump N, et al. Evaluation of CeaseFire-Chicago. Evanston, IL: Northwestern University Institute for Policy Research; 2008. Available at: https://www.ncjrs.gov/pdffiles1/nij/grants/227181.pdf. Accessed September 11, 2017.
3. Webster DW, Whitehill JM, Vernick JS, et al. Evaluation of Baltimore’s Safe Streets program: effects on attitudes, participants’ experiences, and gun violence. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; January 11, 2012. Available at: http://baltimorehealth.org/wp-content/uploads/2016/06/2012_01_10_JHSPH_Safe_Streets_evaluation.pdf. Accessed September 11, 2017.
4. Delgado SA, Alsabahi L, Wolff K, et al. Demoralizing violence: the effects of Cure Violence in the South Bronx and East New York, Brooklyn. John Jay College of Criminal Justice Research and Evaluation Center. Available at: https://johnjayrec.nyc/2017/10/02/cvinsobronxeastny/. Published October 2, 2017. Accessed November 15, 2017.
5. Picard-Fritsche S, Cerniglia L. Testing a public approach to gun violence: an evaluation of Crown Heights Save Our Streets, a replication of the Cure Violence Model. Center for Court Innovation; 2013. Available at: https://www.courtinnovation.org/sites/default/files/documents/SOS_Evaluation.pdf. Accessed November 28, 2017.
6. Murphy SL, Xu J, Kochanek KD, et al. Deaths: Final Data for 2015. Natl Vital Stat Rep. 2017;66:1-75.
7. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
Bilateral nonpitting edema and xerotic skin
A 60-year-old African American woman who had congestive heart failure (CHF) with reduced ejection fraction, untreated hepatitis C virus infection, and chronic kidney disease presented to the emergency department (ED) with a 6-month history of bilateral lower extremity edema. Use of diuretics and antibiotic therapy for suspected CHF exacerbation and cellulitis, directed by her primary care physician, had no effect. In the month prior to presenting to the ED, the patient took 2 different antibiotics, each for 10 days: clindamycin 300 mg every 6 hours and doxycycline 100 mg every 12 hours. Additionally, she was taking furosemide 40 mg/d with good urine output, but no appreciable improvement in lower extremity edema.
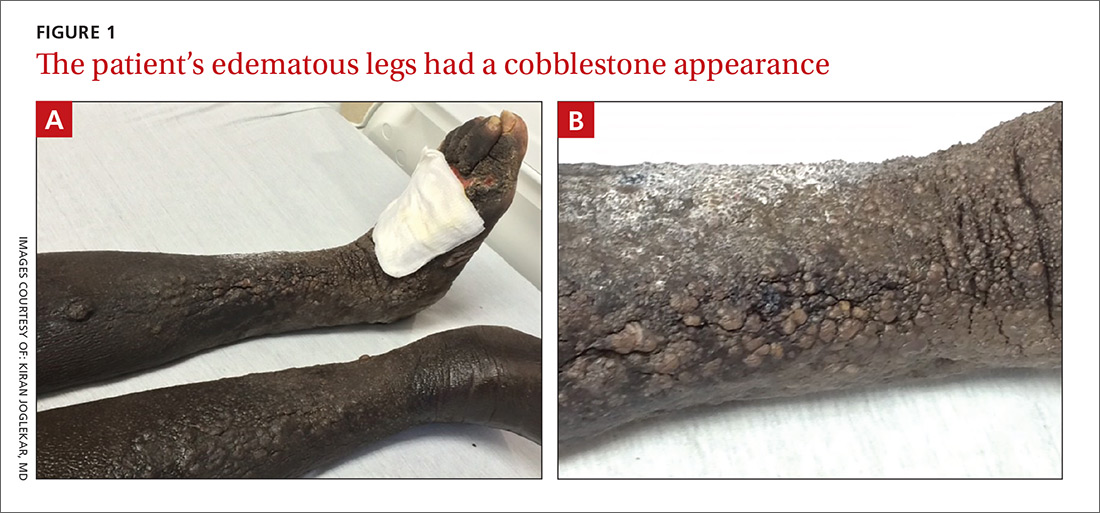
The physical examination revealed bilateral nonpitting edema. Weeping pearly papules, xerotic skin, and a cobblestone appearance extended from the dorsa of the patient’s feet to her knees (FIGURES 1A and 1B). The patient underwent Doppler ultrasound of the lower extremities and a skin biopsy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Elephantiasis nostras verrucosa
The Doppler ultrasound was negative, and the biopsy ruled out malignancy and infection; however, the pathology report was histologically consistent with a diagnosis of elephantiasis nostras verrucosa (ENV).
ENV is a disfiguring, nonfilarial lymphedema that affects the lower extremities and is characterized by progressive cobblestoning and verrucous distortion of gravity-dependent areas.1 The skin changes are caused by lymphatic damage and obstruction from an accumulation of protein-rich fluid in the dermis and subcutaneous tissues.1,2
The term ENV was first coined by Aldo Castellani in 1934 to differentiate the condition from elephantiasis tropica (filariasis), which is caused by parasitic Wuchereria worms.3 ENV is also known as lymphangitis recurrens elephantogenica, elephantiasis verrucosa, elephantiasis nostra, mossy leg, and elephantiasis of the temperate zone.1
ENV is notably uncommon; its exact incidence is unknown. The etiology is multifactorial but can include obesity, chronic lymphedema, CHF, and recurrent cellulitis (the latter 2 were noted in our patient’s history).1
Although the diagnosis can be made based on patient history and physical examination alone, skin biopsy is warranted to rule out underlying malignancy or fungal infection.1,4 Histologic findings suggestive of ENV include pseudoepitheliomatous hyperplasia, lymph channel dilation, widened tissue spaces, and loss of dermal papillae.1 In our patient’s case, the pathology report revealed dermal fibrosis, dilated lymph channels, and a mixed inflammatory infiltrate. Her lab work, which included a complete blood count and basic metabolic panel, was significant for neutrophilic leukocytosis (white blood cell count, 30,000 cells/mcL), chronic kidney disease, and elevated inflammatory markers.
The differential includes other types of edema and infections
Several other diseases must be differentiated from ENV, including:
Venous stasis dermatitis. Unlike ENV, this condition involves pitting edema with erythema and does not have a verrucous appearance.2,4
Lipedema. Histologically, lipedema shows no changes. It typically spares the feet, has an early age of onset, and is associated with a positive family history.1,2,4
Lipodermatosclerosis. This condition is caused by venous stasis with swelling of the proximal lower extremity and fibrosis of the distal parts. The affected leg develops an “inverted wine bottle” appearance.2,4
Pretibial myxedema. Patients with pretibial myxedema will have thyroid function test abnormalities and exhibit other signs of hyperthyroidism. If suspected, the laboratory evaluation should include thyroid-stimulating hormone levels.2,4
Filariasis. Endemic to tropical regions, filariasis is a parasitic infection. A travel history helps to differentiate this from ENV. If suspected, include a Giemsa blood smear in the laboratory evaluation.2
Chromoblastomycosis. This chronic fungal infection is typically contracted in rural tropical or subtropical regions. The causative fungi, which are present in soil, enter the skin through minor wounds (eg, thorns or splinters). The wounds are typically forgotten by the time the patient seeks medical attention. Biopsy can effectively rule out this condition.1,2,5
Treatment centers on preserving function, preventing complications
Currently, no standard treatment exists for ENV.1,4 Therapies are aimed at treating the underlying cause, preserving function in the affected limb, and preventing complications. Conservative therapy includes elevation of the affected limb and use of compression devices for edema. Antibiotics can be administered for associated cellulitis. There have been few case reports of successful treatment with oral retinoids. If medical therapy fails, surgical debridement serves as a last resort.1,4,6
Our patient improved after a week with antibiotic therapy (IV piperacillin/tazobactam 3.375 g every 6 hours) and other conservative measures, such as leg elevation.
CORRESPONDENCE
Kavita Natrajan, MBBS, George Washington University/Medical Faculty Associates, Division of Hematology and Oncology, 2150 Pennsylvania Avenue NW, DC 20037; [email protected].
1. Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
2. Liaw FY, Huang CF, Wu YC, et al. Elephantiasis nostras verrucosa: swelling with verrucose appearance of lower limbs. Can Fam Physician. 2012;58:e551-e553.
3. Castellani A. Researches on elephantiasis nostras and elephantiasis tropica with special regard to their initial stage of recurring lymphangitis (lymphangitis recurrens elephantogenica). J Trop Med Hyg. 1969;72:89-97.
4. Baird D, Bode D, Akers T, et al. Elephantiasis nostras verrucosa (ENV): a complication of congestive heart failure and obesity. J Am Board Fam Med. 2010;23:413-417.
5. Queiroz-Telles F, Fahal AH, Falci R, et. al. Neglected endemic mycoses. Lancet Inf Dis. 2017;17:e367-e377.
6. Han HH, Lim SY, Oh DY. Successful surgical treatment for elephantiasis nostras verrucosa using a new designed column flap. Int J Low Extrem Wounds. 2015;14:299-302.
A 60-year-old African American woman who had congestive heart failure (CHF) with reduced ejection fraction, untreated hepatitis C virus infection, and chronic kidney disease presented to the emergency department (ED) with a 6-month history of bilateral lower extremity edema. Use of diuretics and antibiotic therapy for suspected CHF exacerbation and cellulitis, directed by her primary care physician, had no effect. In the month prior to presenting to the ED, the patient took 2 different antibiotics, each for 10 days: clindamycin 300 mg every 6 hours and doxycycline 100 mg every 12 hours. Additionally, she was taking furosemide 40 mg/d with good urine output, but no appreciable improvement in lower extremity edema.
The physical examination revealed bilateral nonpitting edema. Weeping pearly papules, xerotic skin, and a cobblestone appearance extended from the dorsa of the patient’s feet to her knees (FIGURES 1A and 1B). The patient underwent Doppler ultrasound of the lower extremities and a skin biopsy.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Elephantiasis nostras verrucosa
The Doppler ultrasound was negative, and the biopsy ruled out malignancy and infection; however, the pathology report was histologically consistent with a diagnosis of elephantiasis nostras verrucosa (ENV).
ENV is a disfiguring, nonfilarial lymphedema that affects the lower extremities and is characterized by progressive cobblestoning and verrucous distortion of gravity-dependent areas.1 The skin changes are caused by lymphatic damage and obstruction from an accumulation of protein-rich fluid in the dermis and subcutaneous tissues.1,2
The term ENV was first coined by Aldo Castellani in 1934 to differentiate the condition from elephantiasis tropica (filariasis), which is caused by parasitic Wuchereria worms.3 ENV is also known as lymphangitis recurrens elephantogenica, elephantiasis verrucosa, elephantiasis nostra, mossy leg, and elephantiasis of the temperate zone.1
ENV is notably uncommon; its exact incidence is unknown. The etiology is multifactorial but can include obesity, chronic lymphedema, CHF, and recurrent cellulitis (the latter 2 were noted in our patient’s history).1
Although the diagnosis can be made based on patient history and physical examination alone, skin biopsy is warranted to rule out underlying malignancy or fungal infection.1,4 Histologic findings suggestive of ENV include pseudoepitheliomatous hyperplasia, lymph channel dilation, widened tissue spaces, and loss of dermal papillae.1 In our patient’s case, the pathology report revealed dermal fibrosis, dilated lymph channels, and a mixed inflammatory infiltrate. Her lab work, which included a complete blood count and basic metabolic panel, was significant for neutrophilic leukocytosis (white blood cell count, 30,000 cells/mcL), chronic kidney disease, and elevated inflammatory markers.
The differential includes other types of edema and infections
Several other diseases must be differentiated from ENV, including:
Venous stasis dermatitis. Unlike ENV, this condition involves pitting edema with erythema and does not have a verrucous appearance.2,4
Lipedema. Histologically, lipedema shows no changes. It typically spares the feet, has an early age of onset, and is associated with a positive family history.1,2,4
Lipodermatosclerosis. This condition is caused by venous stasis with swelling of the proximal lower extremity and fibrosis of the distal parts. The affected leg develops an “inverted wine bottle” appearance.2,4
Pretibial myxedema. Patients with pretibial myxedema will have thyroid function test abnormalities and exhibit other signs of hyperthyroidism. If suspected, the laboratory evaluation should include thyroid-stimulating hormone levels.2,4
Filariasis. Endemic to tropical regions, filariasis is a parasitic infection. A travel history helps to differentiate this from ENV. If suspected, include a Giemsa blood smear in the laboratory evaluation.2
Chromoblastomycosis. This chronic fungal infection is typically contracted in rural tropical or subtropical regions. The causative fungi, which are present in soil, enter the skin through minor wounds (eg, thorns or splinters). The wounds are typically forgotten by the time the patient seeks medical attention. Biopsy can effectively rule out this condition.1,2,5
Treatment centers on preserving function, preventing complications
Currently, no standard treatment exists for ENV.1,4 Therapies are aimed at treating the underlying cause, preserving function in the affected limb, and preventing complications. Conservative therapy includes elevation of the affected limb and use of compression devices for edema. Antibiotics can be administered for associated cellulitis. There have been few case reports of successful treatment with oral retinoids. If medical therapy fails, surgical debridement serves as a last resort.1,4,6
Our patient improved after a week with antibiotic therapy (IV piperacillin/tazobactam 3.375 g every 6 hours) and other conservative measures, such as leg elevation.
CORRESPONDENCE
Kavita Natrajan, MBBS, George Washington University/Medical Faculty Associates, Division of Hematology and Oncology, 2150 Pennsylvania Avenue NW, DC 20037; [email protected].
A 60-year-old African American woman who had congestive heart failure (CHF) with reduced ejection fraction, untreated hepatitis C virus infection, and chronic kidney disease presented to the emergency department (ED) with a 6-month history of bilateral lower extremity edema. Use of diuretics and antibiotic therapy for suspected CHF exacerbation and cellulitis, directed by her primary care physician, had no effect. In the month prior to presenting to the ED, the patient took 2 different antibiotics, each for 10 days: clindamycin 300 mg every 6 hours and doxycycline 100 mg every 12 hours. Additionally, she was taking furosemide 40 mg/d with good urine output, but no appreciable improvement in lower extremity edema.
The physical examination revealed bilateral nonpitting edema. Weeping pearly papules, xerotic skin, and a cobblestone appearance extended from the dorsa of the patient’s feet to her knees (FIGURES 1A and 1B). The patient underwent Doppler ultrasound of the lower extremities and a skin biopsy.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Elephantiasis nostras verrucosa
The Doppler ultrasound was negative, and the biopsy ruled out malignancy and infection; however, the pathology report was histologically consistent with a diagnosis of elephantiasis nostras verrucosa (ENV).
ENV is a disfiguring, nonfilarial lymphedema that affects the lower extremities and is characterized by progressive cobblestoning and verrucous distortion of gravity-dependent areas.1 The skin changes are caused by lymphatic damage and obstruction from an accumulation of protein-rich fluid in the dermis and subcutaneous tissues.1,2
The term ENV was first coined by Aldo Castellani in 1934 to differentiate the condition from elephantiasis tropica (filariasis), which is caused by parasitic Wuchereria worms.3 ENV is also known as lymphangitis recurrens elephantogenica, elephantiasis verrucosa, elephantiasis nostra, mossy leg, and elephantiasis of the temperate zone.1
ENV is notably uncommon; its exact incidence is unknown. The etiology is multifactorial but can include obesity, chronic lymphedema, CHF, and recurrent cellulitis (the latter 2 were noted in our patient’s history).1
Although the diagnosis can be made based on patient history and physical examination alone, skin biopsy is warranted to rule out underlying malignancy or fungal infection.1,4 Histologic findings suggestive of ENV include pseudoepitheliomatous hyperplasia, lymph channel dilation, widened tissue spaces, and loss of dermal papillae.1 In our patient’s case, the pathology report revealed dermal fibrosis, dilated lymph channels, and a mixed inflammatory infiltrate. Her lab work, which included a complete blood count and basic metabolic panel, was significant for neutrophilic leukocytosis (white blood cell count, 30,000 cells/mcL), chronic kidney disease, and elevated inflammatory markers.
The differential includes other types of edema and infections
Several other diseases must be differentiated from ENV, including:
Venous stasis dermatitis. Unlike ENV, this condition involves pitting edema with erythema and does not have a verrucous appearance.2,4
Lipedema. Histologically, lipedema shows no changes. It typically spares the feet, has an early age of onset, and is associated with a positive family history.1,2,4
Lipodermatosclerosis. This condition is caused by venous stasis with swelling of the proximal lower extremity and fibrosis of the distal parts. The affected leg develops an “inverted wine bottle” appearance.2,4
Pretibial myxedema. Patients with pretibial myxedema will have thyroid function test abnormalities and exhibit other signs of hyperthyroidism. If suspected, the laboratory evaluation should include thyroid-stimulating hormone levels.2,4
Filariasis. Endemic to tropical regions, filariasis is a parasitic infection. A travel history helps to differentiate this from ENV. If suspected, include a Giemsa blood smear in the laboratory evaluation.2
Chromoblastomycosis. This chronic fungal infection is typically contracted in rural tropical or subtropical regions. The causative fungi, which are present in soil, enter the skin through minor wounds (eg, thorns or splinters). The wounds are typically forgotten by the time the patient seeks medical attention. Biopsy can effectively rule out this condition.1,2,5
Treatment centers on preserving function, preventing complications
Currently, no standard treatment exists for ENV.1,4 Therapies are aimed at treating the underlying cause, preserving function in the affected limb, and preventing complications. Conservative therapy includes elevation of the affected limb and use of compression devices for edema. Antibiotics can be administered for associated cellulitis. There have been few case reports of successful treatment with oral retinoids. If medical therapy fails, surgical debridement serves as a last resort.1,4,6
Our patient improved after a week with antibiotic therapy (IV piperacillin/tazobactam 3.375 g every 6 hours) and other conservative measures, such as leg elevation.
CORRESPONDENCE
Kavita Natrajan, MBBS, George Washington University/Medical Faculty Associates, Division of Hematology and Oncology, 2150 Pennsylvania Avenue NW, DC 20037; [email protected].
1. Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
2. Liaw FY, Huang CF, Wu YC, et al. Elephantiasis nostras verrucosa: swelling with verrucose appearance of lower limbs. Can Fam Physician. 2012;58:e551-e553.
3. Castellani A. Researches on elephantiasis nostras and elephantiasis tropica with special regard to their initial stage of recurring lymphangitis (lymphangitis recurrens elephantogenica). J Trop Med Hyg. 1969;72:89-97.
4. Baird D, Bode D, Akers T, et al. Elephantiasis nostras verrucosa (ENV): a complication of congestive heart failure and obesity. J Am Board Fam Med. 2010;23:413-417.
5. Queiroz-Telles F, Fahal AH, Falci R, et. al. Neglected endemic mycoses. Lancet Inf Dis. 2017;17:e367-e377.
6. Han HH, Lim SY, Oh DY. Successful surgical treatment for elephantiasis nostras verrucosa using a new designed column flap. Int J Low Extrem Wounds. 2015;14:299-302.
1. Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
2. Liaw FY, Huang CF, Wu YC, et al. Elephantiasis nostras verrucosa: swelling with verrucose appearance of lower limbs. Can Fam Physician. 2012;58:e551-e553.
3. Castellani A. Researches on elephantiasis nostras and elephantiasis tropica with special regard to their initial stage of recurring lymphangitis (lymphangitis recurrens elephantogenica). J Trop Med Hyg. 1969;72:89-97.
4. Baird D, Bode D, Akers T, et al. Elephantiasis nostras verrucosa (ENV): a complication of congestive heart failure and obesity. J Am Board Fam Med. 2010;23:413-417.
5. Queiroz-Telles F, Fahal AH, Falci R, et. al. Neglected endemic mycoses. Lancet Inf Dis. 2017;17:e367-e377.
6. Han HH, Lim SY, Oh DY. Successful surgical treatment for elephantiasis nostras verrucosa using a new designed column flap. Int J Low Extrem Wounds. 2015;14:299-302.
Treating migraines: It’s different for kids
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Do not prescribe amitriptyline or topiramate as preventive therapy for migraine in children; both drugs are no better than placebo for this population and are associated with increased rates of adverse events.1,2
STRENGTH OF RECOMMENDATION
A: Based on a single double-blind randomized control trial (RCT) and supported by a meta-analysis of 4 RCTs.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
Severe right upper chest pain • tender right sternoclavicular joint • Dx?
THE CASE
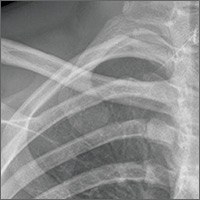
A 16-year-old hockey player presented to our emergency department with sharp pain in his right upper chest after “checking” another player during a game. The pain did not resolve with rest and was worse with movement and breathing. The patient did not have dysphagia, dyspnea, paresthesias, or hoarseness. The physical examination revealed tenderness over the right sternoclavicular joint (SCJ) without swelling or deformity. A distal neurovascular exam was intact, and a chest x-ray showed no evidence of dislocation or fracture (FIGURE 1A). The patient’s pain was refractory to multiple intravenous (IV) pain medications.
THE DIAGNOSIS
A computed tomography (CT) scan with IV contrast of the chest demonstrated posterior and superior dislocation of the right clavicular head. Despite the close proximity of the dislocated head to the brachiocephalic artery (FIGURE 1B-1D), there was no vascular injury.
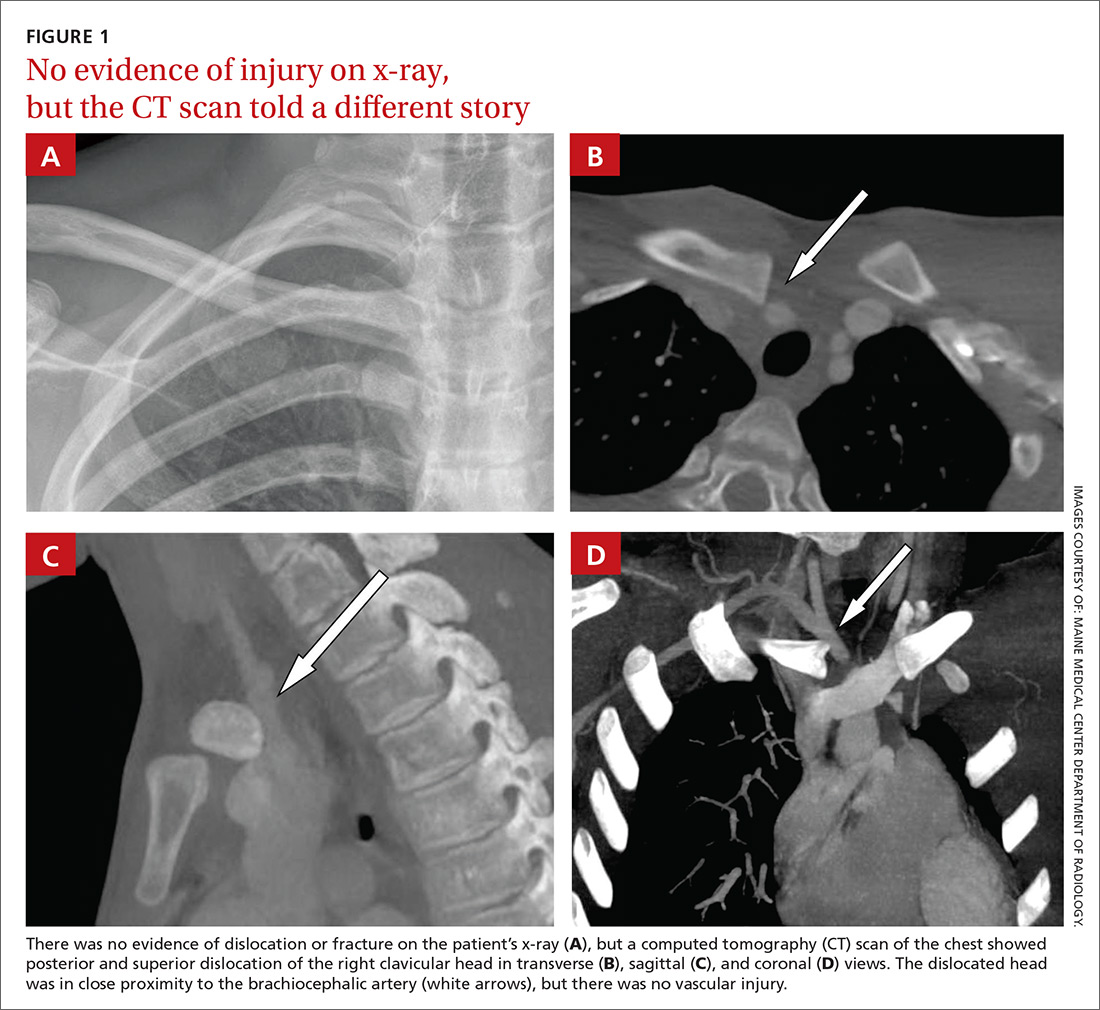
DISCUSSION
Posterior sternoclavicular dislocations (PSCDs) can be difficult to diagnose. Edema can mask the characteristic skin depression that one would expect with a posterior dislocation.1 Chest radiographs are often normal (as was true in this case). Patients may present with an abnormal pulse, paresthesias, hoarseness, dysphagia, and/or dyspnea. However, for more than half of these patients, their only signs and symptoms will be pain, swelling, and limited range of motion.1 As a result, a PSCD may be misdiagnosed as a ligamentous or soft tissue injury.1
An uncommon injury that can result in serious complications
PSCDs represent 3% to 5% of all SCJ dislocations, which comprise <5% of all shoulder girdle injuries.1 Nevertheless, prompt and accurate diagnosis is critical, as these dislocations involve a high risk for injury to the posterior structures, particularly the brachiocephalic vein, right common carotid artery, and aortic arch.
One study found that nearly 75% of patients had a significant structure <1 cm posterior to the SCJ.2 This proximity can result in neurovascular complications—some of which are devastating—in up to 30% of patients with PSCDs.3 A case report from 2011, for example, describes a 19-year-old man who had an undiagnosed PSCD that caused a pseudoaneurysm in his subclavian artery and a subsequent thrombotic cerebrovascular accident.4
Which injuries should raise your suspicions? Injuries in which lateral compression on the shoulder has caused it to roll forward and those in which a posteriorly directed force has been applied to the medial clavicle (as might occur in tackle sports or motor vehicle rollovers) should increase suspicion of a PSCD.1
Proper diagnosis requires CT angiography of the chest to assess the injury and evaluate the underlying structures. If CT is not available, additional chest film views, such as a serendipity view (anteroposterior view with 40° cephalic tilt) or Heinig view (oblique projection perpendicular to SCJ), may be obtained; an ultrasound is also an option.5
PSCD = surgical emergency
Following diagnosis, immediate orthopedic consultation is required. A PSCD is a surgical emergency. Reduction (open or closed) must be performed under general anesthesia with vascular and/or cardiothoracic surgery specialists available, as the reduction itself could injure one of the great vessels. Fortunately, most patients do quite well following surgery, with the majority achieving good-to-excellent results.6
Our patient was admitted to the hospital and underwent orthopedic surgery the following morning. Vascular and cardiothoracic surgeons were consulted and available in the event of a complication. A Salter-Harris type 2 fracture of the medial clavicle was identified intraoperatively, and an open reduction with internal fixation was performed. The patient had an uneventful recovery and resumed his usual activities, including playing hockey.
THE TAKEAWAY
PSCDs, although uncommon, can be life-threatening. Since the physical exam is unreliable and standard radiographs are often normal, accurate diagnosis relies largely on increased clinical suspicion. When there is a history of shoulder trauma, medial clavicle pain, and SCJ edema or tenderness, a PSCD should be suspected.7
Confirm the diagnosis with CT angiogram, and remember that a PSCD is a surgical emergency that requires coordination with orthopedic and vascular/cardiothoracic surgeons.
1. Chaudhry S. Pediatric posterior sternoclavicular joint injuries. J Am Acad Orthop Surg. 2015;23:468-475.
2. Ponce BA, Kundukulam JA, Pflugner R, et al. Sternoclavicular joint surgery: how far does danger lurk below? J Shoulder Elbow Surg. 2013;22:993-999.
3. Daya MR, Bengtzen RR. Shoulder. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:618-642.
4. Marcus MS, Tan V. Cerebrovascular accident in a 19-year-old patient: a case report of posterior sternoclavicular dislocation. J Shoulder Elbow Surg. 2011;20:e1-e4.
5. Morell DJ, Thyagarajan DS. Sternoclavicular joint dislocation and its management: a review of the literature. World J Orthop. 2016;7:244-250.
6. Boesmueller S, Wech M, Tiefenboeck TM, et al. Incidence, characteristics, and long-term follow-up of sternoclavicular injuries: an epidemiologic analysis of 92 cases. J Trauma Acute Care Surg. 2016;80:289-295.
7. Roepke C, Kleiner M, Jhun P, et al. Chest pain bounce-back: posterior sternoclavicular dislocation. Ann Emerg Med. 2015;66:559-561.
THE CASE
A 16-year-old hockey player presented to our emergency department with sharp pain in his right upper chest after “checking” another player during a game. The pain did not resolve with rest and was worse with movement and breathing. The patient did not have dysphagia, dyspnea, paresthesias, or hoarseness. The physical examination revealed tenderness over the right sternoclavicular joint (SCJ) without swelling or deformity. A distal neurovascular exam was intact, and a chest x-ray showed no evidence of dislocation or fracture (FIGURE 1A). The patient’s pain was refractory to multiple intravenous (IV) pain medications.
THE DIAGNOSIS
A computed tomography (CT) scan with IV contrast of the chest demonstrated posterior and superior dislocation of the right clavicular head. Despite the close proximity of the dislocated head to the brachiocephalic artery (FIGURE 1B-1D), there was no vascular injury.

DISCUSSION
Posterior sternoclavicular dislocations (PSCDs) can be difficult to diagnose. Edema can mask the characteristic skin depression that one would expect with a posterior dislocation.1 Chest radiographs are often normal (as was true in this case). Patients may present with an abnormal pulse, paresthesias, hoarseness, dysphagia, and/or dyspnea. However, for more than half of these patients, their only signs and symptoms will be pain, swelling, and limited range of motion.1 As a result, a PSCD may be misdiagnosed as a ligamentous or soft tissue injury.1
An uncommon injury that can result in serious complications
PSCDs represent 3% to 5% of all SCJ dislocations, which comprise <5% of all shoulder girdle injuries.1 Nevertheless, prompt and accurate diagnosis is critical, as these dislocations involve a high risk for injury to the posterior structures, particularly the brachiocephalic vein, right common carotid artery, and aortic arch.
One study found that nearly 75% of patients had a significant structure <1 cm posterior to the SCJ.2 This proximity can result in neurovascular complications—some of which are devastating—in up to 30% of patients with PSCDs.3 A case report from 2011, for example, describes a 19-year-old man who had an undiagnosed PSCD that caused a pseudoaneurysm in his subclavian artery and a subsequent thrombotic cerebrovascular accident.4
Which injuries should raise your suspicions? Injuries in which lateral compression on the shoulder has caused it to roll forward and those in which a posteriorly directed force has been applied to the medial clavicle (as might occur in tackle sports or motor vehicle rollovers) should increase suspicion of a PSCD.1
Proper diagnosis requires CT angiography of the chest to assess the injury and evaluate the underlying structures. If CT is not available, additional chest film views, such as a serendipity view (anteroposterior view with 40° cephalic tilt) or Heinig view (oblique projection perpendicular to SCJ), may be obtained; an ultrasound is also an option.5
PSCD = surgical emergency
Following diagnosis, immediate orthopedic consultation is required. A PSCD is a surgical emergency. Reduction (open or closed) must be performed under general anesthesia with vascular and/or cardiothoracic surgery specialists available, as the reduction itself could injure one of the great vessels. Fortunately, most patients do quite well following surgery, with the majority achieving good-to-excellent results.6
Our patient was admitted to the hospital and underwent orthopedic surgery the following morning. Vascular and cardiothoracic surgeons were consulted and available in the event of a complication. A Salter-Harris type 2 fracture of the medial clavicle was identified intraoperatively, and an open reduction with internal fixation was performed. The patient had an uneventful recovery and resumed his usual activities, including playing hockey.
THE TAKEAWAY
PSCDs, although uncommon, can be life-threatening. Since the physical exam is unreliable and standard radiographs are often normal, accurate diagnosis relies largely on increased clinical suspicion. When there is a history of shoulder trauma, medial clavicle pain, and SCJ edema or tenderness, a PSCD should be suspected.7
Confirm the diagnosis with CT angiogram, and remember that a PSCD is a surgical emergency that requires coordination with orthopedic and vascular/cardiothoracic surgeons.
THE CASE
A 16-year-old hockey player presented to our emergency department with sharp pain in his right upper chest after “checking” another player during a game. The pain did not resolve with rest and was worse with movement and breathing. The patient did not have dysphagia, dyspnea, paresthesias, or hoarseness. The physical examination revealed tenderness over the right sternoclavicular joint (SCJ) without swelling or deformity. A distal neurovascular exam was intact, and a chest x-ray showed no evidence of dislocation or fracture (FIGURE 1A). The patient’s pain was refractory to multiple intravenous (IV) pain medications.
THE DIAGNOSIS
A computed tomography (CT) scan with IV contrast of the chest demonstrated posterior and superior dislocation of the right clavicular head. Despite the close proximity of the dislocated head to the brachiocephalic artery (FIGURE 1B-1D), there was no vascular injury.

DISCUSSION
Posterior sternoclavicular dislocations (PSCDs) can be difficult to diagnose. Edema can mask the characteristic skin depression that one would expect with a posterior dislocation.1 Chest radiographs are often normal (as was true in this case). Patients may present with an abnormal pulse, paresthesias, hoarseness, dysphagia, and/or dyspnea. However, for more than half of these patients, their only signs and symptoms will be pain, swelling, and limited range of motion.1 As a result, a PSCD may be misdiagnosed as a ligamentous or soft tissue injury.1
An uncommon injury that can result in serious complications
PSCDs represent 3% to 5% of all SCJ dislocations, which comprise <5% of all shoulder girdle injuries.1 Nevertheless, prompt and accurate diagnosis is critical, as these dislocations involve a high risk for injury to the posterior structures, particularly the brachiocephalic vein, right common carotid artery, and aortic arch.
One study found that nearly 75% of patients had a significant structure <1 cm posterior to the SCJ.2 This proximity can result in neurovascular complications—some of which are devastating—in up to 30% of patients with PSCDs.3 A case report from 2011, for example, describes a 19-year-old man who had an undiagnosed PSCD that caused a pseudoaneurysm in his subclavian artery and a subsequent thrombotic cerebrovascular accident.4
Which injuries should raise your suspicions? Injuries in which lateral compression on the shoulder has caused it to roll forward and those in which a posteriorly directed force has been applied to the medial clavicle (as might occur in tackle sports or motor vehicle rollovers) should increase suspicion of a PSCD.1
Proper diagnosis requires CT angiography of the chest to assess the injury and evaluate the underlying structures. If CT is not available, additional chest film views, such as a serendipity view (anteroposterior view with 40° cephalic tilt) or Heinig view (oblique projection perpendicular to SCJ), may be obtained; an ultrasound is also an option.5
PSCD = surgical emergency
Following diagnosis, immediate orthopedic consultation is required. A PSCD is a surgical emergency. Reduction (open or closed) must be performed under general anesthesia with vascular and/or cardiothoracic surgery specialists available, as the reduction itself could injure one of the great vessels. Fortunately, most patients do quite well following surgery, with the majority achieving good-to-excellent results.6
Our patient was admitted to the hospital and underwent orthopedic surgery the following morning. Vascular and cardiothoracic surgeons were consulted and available in the event of a complication. A Salter-Harris type 2 fracture of the medial clavicle was identified intraoperatively, and an open reduction with internal fixation was performed. The patient had an uneventful recovery and resumed his usual activities, including playing hockey.
THE TAKEAWAY
PSCDs, although uncommon, can be life-threatening. Since the physical exam is unreliable and standard radiographs are often normal, accurate diagnosis relies largely on increased clinical suspicion. When there is a history of shoulder trauma, medial clavicle pain, and SCJ edema or tenderness, a PSCD should be suspected.7
Confirm the diagnosis with CT angiogram, and remember that a PSCD is a surgical emergency that requires coordination with orthopedic and vascular/cardiothoracic surgeons.
1. Chaudhry S. Pediatric posterior sternoclavicular joint injuries. J Am Acad Orthop Surg. 2015;23:468-475.
2. Ponce BA, Kundukulam JA, Pflugner R, et al. Sternoclavicular joint surgery: how far does danger lurk below? J Shoulder Elbow Surg. 2013;22:993-999.
3. Daya MR, Bengtzen RR. Shoulder. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:618-642.
4. Marcus MS, Tan V. Cerebrovascular accident in a 19-year-old patient: a case report of posterior sternoclavicular dislocation. J Shoulder Elbow Surg. 2011;20:e1-e4.
5. Morell DJ, Thyagarajan DS. Sternoclavicular joint dislocation and its management: a review of the literature. World J Orthop. 2016;7:244-250.
6. Boesmueller S, Wech M, Tiefenboeck TM, et al. Incidence, characteristics, and long-term follow-up of sternoclavicular injuries: an epidemiologic analysis of 92 cases. J Trauma Acute Care Surg. 2016;80:289-295.
7. Roepke C, Kleiner M, Jhun P, et al. Chest pain bounce-back: posterior sternoclavicular dislocation. Ann Emerg Med. 2015;66:559-561.
1. Chaudhry S. Pediatric posterior sternoclavicular joint injuries. J Am Acad Orthop Surg. 2015;23:468-475.
2. Ponce BA, Kundukulam JA, Pflugner R, et al. Sternoclavicular joint surgery: how far does danger lurk below? J Shoulder Elbow Surg. 2013;22:993-999.
3. Daya MR, Bengtzen RR. Shoulder. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:618-642.
4. Marcus MS, Tan V. Cerebrovascular accident in a 19-year-old patient: a case report of posterior sternoclavicular dislocation. J Shoulder Elbow Surg. 2011;20:e1-e4.
5. Morell DJ, Thyagarajan DS. Sternoclavicular joint dislocation and its management: a review of the literature. World J Orthop. 2016;7:244-250.
6. Boesmueller S, Wech M, Tiefenboeck TM, et al. Incidence, characteristics, and long-term follow-up of sternoclavicular injuries: an epidemiologic analysis of 92 cases. J Trauma Acute Care Surg. 2016;80:289-295.
7. Roepke C, Kleiner M, Jhun P, et al. Chest pain bounce-back: posterior sternoclavicular dislocation. Ann Emerg Med. 2015;66:559-561.
Acupuncture for pain: 7 questions answered
An estimated 39.4 million US adults suffer from persistent pain,1 and the National Institutes of Health indicate that pain affects more Americans than diabetes, heart disease, and cancer combined.2
As physicians, we know that conventional options to manage chronic pain leave much to be desired and that more evidence-based treatment options are sorely needed. Patients know this, too, and turn to complementary therapies for pain more than for any other diagnosis.3
Case in point: The use of acupuncture is growing. Its use in the United States tripled between 1997 and 2007.4 In addition, the research base for acupuncture is rapidly expanding. From 1991 to 2009, nearly 4000 acupuncture research studies were published, with studies on pain accounting for 41% of the acupuncture literature.4
But acupuncture is not without controversy. This is due to a lack of a universally accepted biologic mechanism, theories of use and efficacy based in an alternative medical system (traditional Chinese medicine [TCM]), and conflicting views of the evidence.
This article will help make sense of this growing body of knowledge by summarizing the latest evidence and addressing 7 common questions about acupuncture for pain conditions. Applying this information will give you the confidence to counsel patients appropriately and decide if acupuncture fits within their pain management plan.
1. What is acupuncture and how does it work?
Acupuncture, which has a 2000-year history of use, involves inserting needles at various points throughout the body to promote healing and improve function. Although acupuncture represents one piece of TCM (which is a holistic system that also includes herbal medicine, nutrition, meditation, and movement), it is often offered as an independent therapy.
Acupuncture point locations are determined either by using an underlying theoretical framework, such as TCM, or by using anatomic structures, such as muscular trigger points. Providers today often employ a hybrid approach when delivering acupuncture treatment. That is, practitioners may choose point locations based on TCM, but they may combine the practice with local treatments that are based on current knowledge of anatomy. For example, a patient presenting with low back pain may be treated utilizing traditional points located near the ankle and knee, and also by needling active trigger points in the quadratus lumborum muscle.
The mechanism of action. One of the reasons for the continuing controversy surrounding acupuncture is the lack of a clear understanding of its underlying mechanism of action. For centuries the “how” of acupuncture has been explained in poetic terms such as yin, yang, and qi. Only in the past half-century have we begun investigating the potential biologic mechanisms responsible for the physiologic effects seen with acupuncture treatment.
While research has uncovered several interesting theories, how these mechanisms interact to produce therapeutic effects is still unclear. However, looking at various components of the nervous system helps to provide some insight.
Consider the nervous system. One way to conceptualize the mechanisms of acupuncture is to consider the various levels of the nervous system and how each level is affected. In the central nervous system, needling an acupuncture point stimulates the natural endorphin system, altering the pain sensation.5 This effect is reversible with naloxone in animal models, indicating that blocking the endorphin system interferes with the analgesic benefits of acupuncture.5
Serotonergic systems are also involved centrally. Functional magnetic resonance imaging studies have shown that needling specific acupuncture points modulates areas of the brain.
In the spinal cord, the gate control theory is believed to play a role. (The gate control theory puts forth that nonpainful input closes the “gates” to painful input, which prevents pain sensations from traveling to the central nervous system.) Modulation of sensory input occurs at the level of the dorsal horn of the spinal cord during an acupuncture treatment, which can affect the physiologic pain response.6 Opioid receptors are also affected at the spinal cord level.7
Lastly, multiple chemicals released peripherally, including interleukins, substance P, and adenosine, appear to contribute to acupuncture’s analgesia.6 We know this because a local anesthetic injected around a peripheral nerve at an acupoint blocks the analgesic effect of acupuncture.8 Taken together, acupuncture treatment produces physiologic changes in the brain, spinal cord, and at the periphery, making it a truly unique therapeutic modality.
2. Is acupuncture an effective treatment for pain?
Yes, but before we look at the individual studies, it is important to mention some of the shortcomings of the research to date. First, acupuncture trials lack a standard sham control intervention. Some sham treatments involve skin penetration, while others do not. This has led to controversy regarding whether the sham interventions themselves are physiologically active, thus lessening the magnitude of effect for acupuncture. This is a point of contention in the acupuncture literature and a factor to consider when deciding if results have clinical significance.
In addition, the acupuncturist providing treatment in a trial is typically unblinded. This is also true of trials measuring other physical modalities, but it contributes to the debate surrounding the magnitude of placebo response in acupuncture studies.
Finally, many randomized trials involving acupuncture have had low methodologic quality. Fortunately, there are now several high-quality systematic reviews that have attempted to filter out the lower-quality research and provide a better representation of the evidence (TABLE9-14). A discussion of them follows.
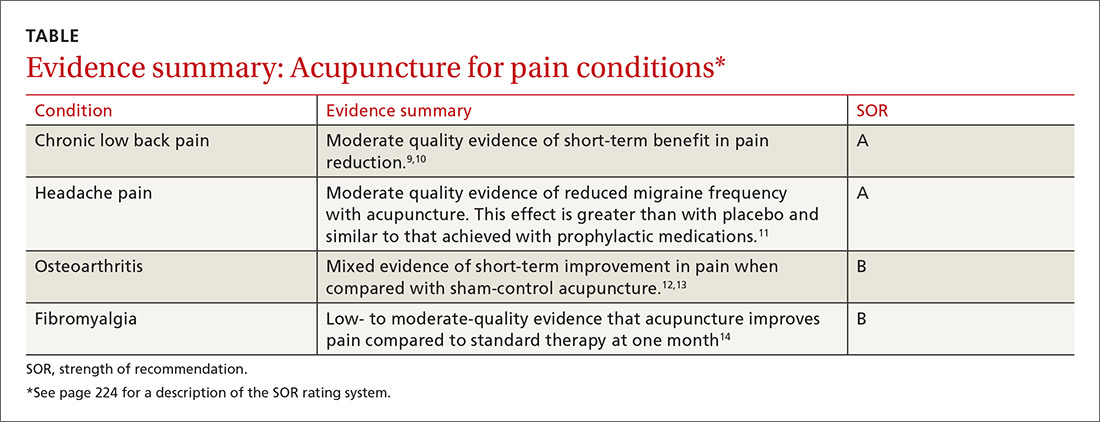
General chronic pain. A 2012 meta-analysis15 evaluated the effectiveness of acupuncture for the treatment of chronic pain with one of 4 etiologies: nonspecific back or neck pain, chronic headache, osteoarthritis, and shoulder pain. This analysis attempted to control for the high variability of study quality in the acupuncture literature by including only studies of high methodologic character. The final analysis included 29 randomized controlled trials (N=17,922). The authors concluded that acupuncture was superior to both no acupuncture and sham (placebo) acupuncture for all pain conditions in the study. The average effect size was 0.5 standard deviations on a 10-point scale. The authors considered this to be clinically relevant, although the magnitude of benefit was modest.15
Low back pain. A 2017 systematic review by Chou et al9 evaluated 32 trials (N=5931) reviewing acupuncture for the treatment of chronic low back pain. This review found acupuncture was associated with lower pain intensity and improved function in the short term when compared with no treatment. And while acupuncture was associated with lower pain intensity when compared with a sham control, there was no difference in function between the 2 groups. Of note, 3 of the included trials compared acupuncture to standard medications used in the treatment of low back pain and found acupuncture to be superior in terms of both pain reduction and improved function.9
The authors of a 2008 systematic review that evaluated 23 trials (N=6359)10 similarly concluded that there is moderate evidence for the use of acupuncture (compared to no treatment) for the treatment of nonspecific low back pain, but did not find evidence that acupuncture was superior to sham controls.10 The 2017 American College of Physicians clinical practice guidelines support the use of acupuncture for the treatment of chronic low back pain.16
In addition to helping with chronic low back pain, acupuncture is also showing promise as a treatment for acute spinal pain. A 2013 systematic review (11 trials, N=1139) showed that acupuncture may be more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) in treating acute low back pain and may cause fewer adverse effects.17
Headache pain. Evidence favoring acupuncture in the management of headache has been fairly consistent over the past decade. An updated Cochrane review on the prevention of migraine headaches was published in 2016.11 Acupuncture was compared with no treatment in 4 trials (n=2199). The authors found moderate quality evidence that acupuncture reduces headache frequency (number needed to treat=4). Acupuncture achieved at least 50% headache reduction in 41% vs 17% in the groups that received no acupuncture. When compared with sham control groups (10 trials, n=1534), acupuncture demonstrated a small but statistically significant improvement in headache frequency. Three trials (n=744) compared acupuncture to medication prophylaxis for migraine headaches and found acupuncture had similar effectiveness with fewer adverse effects.11
Osteoarthritis (OA). Most studies have focused on OA of the knee, and, thus far, have generated conflicting results. A Cochrane review in 2010 included 4 trials (n=884) that had a wait list control and 9 trials (n=1835) that compared acupuncture to a sham control.12 When compared to a wait list control, acupuncture resulted in statistically significant and clinically relevant improvement in pain and function. However, when compared to sham treatment for OA, the review showed statistically significant improvement in pain and function for acupuncture that was unlikely to be clinically relevant.12
A more recent meta-analysis in 2016 evaluated 10 trials (N=2007) investigating acupuncture in the treatment of knee OA.13 The authors found acupuncture improved both pain and functional outcome measures when compared with either no treatment or a sham control.
Fibromyalgia. Systematic reviews in 2007 (5 trials, N=316)18 and 2010 (7 trials, N=385)19 showed that acupuncture did provide short-term pain relief in patients with fibromyalgia, but that the effect was not sustained at follow-up.These reviews were limited by a high risk of bias, which was noted in the studies. The authors of both reviews concluded that acupuncture could not be recommended for the treatment of fibromyalgia.
A more recent Cochrane review published in 2013 (9 trials, N=395) offered low- to moderate-level evidence of benefit for acupuncture compared with no treatment at one month follow-up.14 Of note, there was also evidence of benefit in improved sleep and global well-being, in addition to pain and stiffness measures in this review. The overall magnitude of benefit was small, but clinically significant. Acupuncture also has evidence of benefit in the treatment of conditions commonly seen in conjunction with fibromyalgia, including headaches and low back pain as described earlier.
3. What does a typical acupuncture treatment entail?
In a typical treatment, anywhere from about 5 to 20 needles are inserted into the body. Common areas of needling include the arms and legs, especially below the elbows and knees. Other frequently used areas are the scalp, ears, and structures related to the painful condition.
The needles used are very thin (typically smaller than a 30-gauge needle) and do not have a beveled tip like phlebotomy needles do. Most patients have minimal pain as the needles are inserted. During the treatment, the needles may be left alone or they may be heated or stimulated electrically. An average treatment lasts 30 to 40 minutes; many patients find the sessions relaxing.
4. Are there any adverse effects or complications of treatment?
Acupuncture is generally considered a safe therapy, with most patients experiencing no adverse effects at all. Minor adverse effects can include post-treatment fatigue, minor bruising, or vasovagal reactions from the insertion of the needles. Serious complications, such as pneumothorax, are possible, but are considered rare.20 A 2004 study estimated the incidence of severe complications to be .05 per 10,000 acupuncture treatments.21
Infections are also possible, but most reported cases were due to practitioners reusing needles.22 The standard of care in the United States is to use only sterilized, single-use needles. With this practice, infections due to acupuncture are thought to be rare.
Of note, trials that compare acupuncture to another active therapy often find that acupuncture has fewer adverse effects. This has been the case when acupuncture was compared to NSAIDs for low back pain and to topiramate for headaches.17,23
5. How does acupuncture fit into a patient’s treatment?
The simple answer is that it is often most effectively used as part of a comprehensive management plan for chronic pain.
As our understanding of the complexity of chronic pain deepens, our therapeutic armamentarium for the management of chronic pain needs to broaden. This was summed up well in a 2016 article on the multimodal management of chronic pain when the authors stated, “Many targets need more than one arrow.”24 Effective management of chronic pain involves addressing psychosocial and lifestyle factors in a patient-centered way and finding a combination of treatments that most effectively leads to improved coping and function.
It’s important to note that like medications and injections, acupuncture is a passive therapy. Although there is evidence for efficacy of improved pain with acupuncture in certain conditions, it should be combined with treatments that actively engage patients, such as exercise, behavioral treatments, development of coping skills, sleep hygiene, and educational strategies.
6. To whom do I refer patients for acupuncture treatment?
In the United States, licensed acupuncturists and physicians most commonly perform acupuncture. There are more than 50 schools that train licensed acupuncturists in the United States, and it usually takes 3 years to meet the requirements.25
SIDEBAR
Online resources
American Academy of Medical Acupuncture
www.medicalacupuncture.com
National Center for Complementary and Alternative Medicine
http://nccam.nih.gov/health/acupuncture
National Certification Commission for Acupuncture and Oriental Medicine
www.nccaom.org
Physicians are often trained through continuing medical education (CME) programs that take several months to complete. These programs often combine live lectures, distance learning, and hands-on training and are typically sponsored by a university. Most require 300 hours of CME to complete. Licensure varies by state, but in many states, having an MD or DO degree automatically allows physicians to practice acupuncture. (See “Online resources,” above for links to Web sites that can be useful in finding qualified acupuncturists in your area.)
7. Is acupuncture covered by insurance?
It depends. Insurance coverage of acupuncture is highly variable and based on region and insurance type. Medicare and Medicaid plans do not pay for acupuncture. There are some private insurance plans that do. If covered, there may be limitations regarding diagnosis, number of visits, or provider. It is best for patients to call their insurance plan directly to inquire about coverage and any limitations. If paying out of pocket, patients can expect to pay $75 to $150 per treatment session.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Kennedy J, Roll JM, Schraudner T, et al. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15:979-984.
2. U.S. Department of Health and Human Services. NIH Fact Sheet. Pain management. Available at: https://www.report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed February 12, 2018.
3. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
4. Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1-14.
5. Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
6. Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
7. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482-503.
8. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41-S48.
9. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505.
10. Yuan J, Purepong N, Kerr DP, et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila Pa 1976). 2008;33:E887-E900.
11. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
12. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977.
13. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
14. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;5:CD007070.
15. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
16. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
17. Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29:172-185.
18. Mayhew E, Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46:801-804.
19. Langhorst J, Klose P, Musial F, et al. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxford). 2010;49:778-788.
20. Lao L, Hamilton GR, Fu J, et al. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72-83.
21. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22:122-133.
22. Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013:581203.
23. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009;1:CD001218.
24. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55-64.
25. National Certification Commission for Acupuncture and Oriental Medicine. Available at: www.nccaom.org. Accessed March 20, 2018.
An estimated 39.4 million US adults suffer from persistent pain,1 and the National Institutes of Health indicate that pain affects more Americans than diabetes, heart disease, and cancer combined.2
As physicians, we know that conventional options to manage chronic pain leave much to be desired and that more evidence-based treatment options are sorely needed. Patients know this, too, and turn to complementary therapies for pain more than for any other diagnosis.3
Case in point: The use of acupuncture is growing. Its use in the United States tripled between 1997 and 2007.4 In addition, the research base for acupuncture is rapidly expanding. From 1991 to 2009, nearly 4000 acupuncture research studies were published, with studies on pain accounting for 41% of the acupuncture literature.4
But acupuncture is not without controversy. This is due to a lack of a universally accepted biologic mechanism, theories of use and efficacy based in an alternative medical system (traditional Chinese medicine [TCM]), and conflicting views of the evidence.
This article will help make sense of this growing body of knowledge by summarizing the latest evidence and addressing 7 common questions about acupuncture for pain conditions. Applying this information will give you the confidence to counsel patients appropriately and decide if acupuncture fits within their pain management plan.
1. What is acupuncture and how does it work?
Acupuncture, which has a 2000-year history of use, involves inserting needles at various points throughout the body to promote healing and improve function. Although acupuncture represents one piece of TCM (which is a holistic system that also includes herbal medicine, nutrition, meditation, and movement), it is often offered as an independent therapy.
Acupuncture point locations are determined either by using an underlying theoretical framework, such as TCM, or by using anatomic structures, such as muscular trigger points. Providers today often employ a hybrid approach when delivering acupuncture treatment. That is, practitioners may choose point locations based on TCM, but they may combine the practice with local treatments that are based on current knowledge of anatomy. For example, a patient presenting with low back pain may be treated utilizing traditional points located near the ankle and knee, and also by needling active trigger points in the quadratus lumborum muscle.
The mechanism of action. One of the reasons for the continuing controversy surrounding acupuncture is the lack of a clear understanding of its underlying mechanism of action. For centuries the “how” of acupuncture has been explained in poetic terms such as yin, yang, and qi. Only in the past half-century have we begun investigating the potential biologic mechanisms responsible for the physiologic effects seen with acupuncture treatment.
While research has uncovered several interesting theories, how these mechanisms interact to produce therapeutic effects is still unclear. However, looking at various components of the nervous system helps to provide some insight.
Consider the nervous system. One way to conceptualize the mechanisms of acupuncture is to consider the various levels of the nervous system and how each level is affected. In the central nervous system, needling an acupuncture point stimulates the natural endorphin system, altering the pain sensation.5 This effect is reversible with naloxone in animal models, indicating that blocking the endorphin system interferes with the analgesic benefits of acupuncture.5
Serotonergic systems are also involved centrally. Functional magnetic resonance imaging studies have shown that needling specific acupuncture points modulates areas of the brain.
In the spinal cord, the gate control theory is believed to play a role. (The gate control theory puts forth that nonpainful input closes the “gates” to painful input, which prevents pain sensations from traveling to the central nervous system.) Modulation of sensory input occurs at the level of the dorsal horn of the spinal cord during an acupuncture treatment, which can affect the physiologic pain response.6 Opioid receptors are also affected at the spinal cord level.7
Lastly, multiple chemicals released peripherally, including interleukins, substance P, and adenosine, appear to contribute to acupuncture’s analgesia.6 We know this because a local anesthetic injected around a peripheral nerve at an acupoint blocks the analgesic effect of acupuncture.8 Taken together, acupuncture treatment produces physiologic changes in the brain, spinal cord, and at the periphery, making it a truly unique therapeutic modality.
2. Is acupuncture an effective treatment for pain?
Yes, but before we look at the individual studies, it is important to mention some of the shortcomings of the research to date. First, acupuncture trials lack a standard sham control intervention. Some sham treatments involve skin penetration, while others do not. This has led to controversy regarding whether the sham interventions themselves are physiologically active, thus lessening the magnitude of effect for acupuncture. This is a point of contention in the acupuncture literature and a factor to consider when deciding if results have clinical significance.
In addition, the acupuncturist providing treatment in a trial is typically unblinded. This is also true of trials measuring other physical modalities, but it contributes to the debate surrounding the magnitude of placebo response in acupuncture studies.
Finally, many randomized trials involving acupuncture have had low methodologic quality. Fortunately, there are now several high-quality systematic reviews that have attempted to filter out the lower-quality research and provide a better representation of the evidence (TABLE9-14). A discussion of them follows.

General chronic pain. A 2012 meta-analysis15 evaluated the effectiveness of acupuncture for the treatment of chronic pain with one of 4 etiologies: nonspecific back or neck pain, chronic headache, osteoarthritis, and shoulder pain. This analysis attempted to control for the high variability of study quality in the acupuncture literature by including only studies of high methodologic character. The final analysis included 29 randomized controlled trials (N=17,922). The authors concluded that acupuncture was superior to both no acupuncture and sham (placebo) acupuncture for all pain conditions in the study. The average effect size was 0.5 standard deviations on a 10-point scale. The authors considered this to be clinically relevant, although the magnitude of benefit was modest.15
Low back pain. A 2017 systematic review by Chou et al9 evaluated 32 trials (N=5931) reviewing acupuncture for the treatment of chronic low back pain. This review found acupuncture was associated with lower pain intensity and improved function in the short term when compared with no treatment. And while acupuncture was associated with lower pain intensity when compared with a sham control, there was no difference in function between the 2 groups. Of note, 3 of the included trials compared acupuncture to standard medications used in the treatment of low back pain and found acupuncture to be superior in terms of both pain reduction and improved function.9
The authors of a 2008 systematic review that evaluated 23 trials (N=6359)10 similarly concluded that there is moderate evidence for the use of acupuncture (compared to no treatment) for the treatment of nonspecific low back pain, but did not find evidence that acupuncture was superior to sham controls.10 The 2017 American College of Physicians clinical practice guidelines support the use of acupuncture for the treatment of chronic low back pain.16
In addition to helping with chronic low back pain, acupuncture is also showing promise as a treatment for acute spinal pain. A 2013 systematic review (11 trials, N=1139) showed that acupuncture may be more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) in treating acute low back pain and may cause fewer adverse effects.17
Headache pain. Evidence favoring acupuncture in the management of headache has been fairly consistent over the past decade. An updated Cochrane review on the prevention of migraine headaches was published in 2016.11 Acupuncture was compared with no treatment in 4 trials (n=2199). The authors found moderate quality evidence that acupuncture reduces headache frequency (number needed to treat=4). Acupuncture achieved at least 50% headache reduction in 41% vs 17% in the groups that received no acupuncture. When compared with sham control groups (10 trials, n=1534), acupuncture demonstrated a small but statistically significant improvement in headache frequency. Three trials (n=744) compared acupuncture to medication prophylaxis for migraine headaches and found acupuncture had similar effectiveness with fewer adverse effects.11
Osteoarthritis (OA). Most studies have focused on OA of the knee, and, thus far, have generated conflicting results. A Cochrane review in 2010 included 4 trials (n=884) that had a wait list control and 9 trials (n=1835) that compared acupuncture to a sham control.12 When compared to a wait list control, acupuncture resulted in statistically significant and clinically relevant improvement in pain and function. However, when compared to sham treatment for OA, the review showed statistically significant improvement in pain and function for acupuncture that was unlikely to be clinically relevant.12
A more recent meta-analysis in 2016 evaluated 10 trials (N=2007) investigating acupuncture in the treatment of knee OA.13 The authors found acupuncture improved both pain and functional outcome measures when compared with either no treatment or a sham control.
Fibromyalgia. Systematic reviews in 2007 (5 trials, N=316)18 and 2010 (7 trials, N=385)19 showed that acupuncture did provide short-term pain relief in patients with fibromyalgia, but that the effect was not sustained at follow-up.These reviews were limited by a high risk of bias, which was noted in the studies. The authors of both reviews concluded that acupuncture could not be recommended for the treatment of fibromyalgia.
A more recent Cochrane review published in 2013 (9 trials, N=395) offered low- to moderate-level evidence of benefit for acupuncture compared with no treatment at one month follow-up.14 Of note, there was also evidence of benefit in improved sleep and global well-being, in addition to pain and stiffness measures in this review. The overall magnitude of benefit was small, but clinically significant. Acupuncture also has evidence of benefit in the treatment of conditions commonly seen in conjunction with fibromyalgia, including headaches and low back pain as described earlier.
3. What does a typical acupuncture treatment entail?
In a typical treatment, anywhere from about 5 to 20 needles are inserted into the body. Common areas of needling include the arms and legs, especially below the elbows and knees. Other frequently used areas are the scalp, ears, and structures related to the painful condition.
The needles used are very thin (typically smaller than a 30-gauge needle) and do not have a beveled tip like phlebotomy needles do. Most patients have minimal pain as the needles are inserted. During the treatment, the needles may be left alone or they may be heated or stimulated electrically. An average treatment lasts 30 to 40 minutes; many patients find the sessions relaxing.
4. Are there any adverse effects or complications of treatment?
Acupuncture is generally considered a safe therapy, with most patients experiencing no adverse effects at all. Minor adverse effects can include post-treatment fatigue, minor bruising, or vasovagal reactions from the insertion of the needles. Serious complications, such as pneumothorax, are possible, but are considered rare.20 A 2004 study estimated the incidence of severe complications to be .05 per 10,000 acupuncture treatments.21
Infections are also possible, but most reported cases were due to practitioners reusing needles.22 The standard of care in the United States is to use only sterilized, single-use needles. With this practice, infections due to acupuncture are thought to be rare.
Of note, trials that compare acupuncture to another active therapy often find that acupuncture has fewer adverse effects. This has been the case when acupuncture was compared to NSAIDs for low back pain and to topiramate for headaches.17,23
5. How does acupuncture fit into a patient’s treatment?
The simple answer is that it is often most effectively used as part of a comprehensive management plan for chronic pain.
As our understanding of the complexity of chronic pain deepens, our therapeutic armamentarium for the management of chronic pain needs to broaden. This was summed up well in a 2016 article on the multimodal management of chronic pain when the authors stated, “Many targets need more than one arrow.”24 Effective management of chronic pain involves addressing psychosocial and lifestyle factors in a patient-centered way and finding a combination of treatments that most effectively leads to improved coping and function.
It’s important to note that like medications and injections, acupuncture is a passive therapy. Although there is evidence for efficacy of improved pain with acupuncture in certain conditions, it should be combined with treatments that actively engage patients, such as exercise, behavioral treatments, development of coping skills, sleep hygiene, and educational strategies.
6. To whom do I refer patients for acupuncture treatment?
In the United States, licensed acupuncturists and physicians most commonly perform acupuncture. There are more than 50 schools that train licensed acupuncturists in the United States, and it usually takes 3 years to meet the requirements.25
SIDEBAR
Online resources
American Academy of Medical Acupuncture
www.medicalacupuncture.com
National Center for Complementary and Alternative Medicine
http://nccam.nih.gov/health/acupuncture
National Certification Commission for Acupuncture and Oriental Medicine
www.nccaom.org
Physicians are often trained through continuing medical education (CME) programs that take several months to complete. These programs often combine live lectures, distance learning, and hands-on training and are typically sponsored by a university. Most require 300 hours of CME to complete. Licensure varies by state, but in many states, having an MD or DO degree automatically allows physicians to practice acupuncture. (See “Online resources,” above for links to Web sites that can be useful in finding qualified acupuncturists in your area.)
7. Is acupuncture covered by insurance?
It depends. Insurance coverage of acupuncture is highly variable and based on region and insurance type. Medicare and Medicaid plans do not pay for acupuncture. There are some private insurance plans that do. If covered, there may be limitations regarding diagnosis, number of visits, or provider. It is best for patients to call their insurance plan directly to inquire about coverage and any limitations. If paying out of pocket, patients can expect to pay $75 to $150 per treatment session.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
An estimated 39.4 million US adults suffer from persistent pain,1 and the National Institutes of Health indicate that pain affects more Americans than diabetes, heart disease, and cancer combined.2
As physicians, we know that conventional options to manage chronic pain leave much to be desired and that more evidence-based treatment options are sorely needed. Patients know this, too, and turn to complementary therapies for pain more than for any other diagnosis.3
Case in point: The use of acupuncture is growing. Its use in the United States tripled between 1997 and 2007.4 In addition, the research base for acupuncture is rapidly expanding. From 1991 to 2009, nearly 4000 acupuncture research studies were published, with studies on pain accounting for 41% of the acupuncture literature.4
But acupuncture is not without controversy. This is due to a lack of a universally accepted biologic mechanism, theories of use and efficacy based in an alternative medical system (traditional Chinese medicine [TCM]), and conflicting views of the evidence.
This article will help make sense of this growing body of knowledge by summarizing the latest evidence and addressing 7 common questions about acupuncture for pain conditions. Applying this information will give you the confidence to counsel patients appropriately and decide if acupuncture fits within their pain management plan.
1. What is acupuncture and how does it work?
Acupuncture, which has a 2000-year history of use, involves inserting needles at various points throughout the body to promote healing and improve function. Although acupuncture represents one piece of TCM (which is a holistic system that also includes herbal medicine, nutrition, meditation, and movement), it is often offered as an independent therapy.
Acupuncture point locations are determined either by using an underlying theoretical framework, such as TCM, or by using anatomic structures, such as muscular trigger points. Providers today often employ a hybrid approach when delivering acupuncture treatment. That is, practitioners may choose point locations based on TCM, but they may combine the practice with local treatments that are based on current knowledge of anatomy. For example, a patient presenting with low back pain may be treated utilizing traditional points located near the ankle and knee, and also by needling active trigger points in the quadratus lumborum muscle.
The mechanism of action. One of the reasons for the continuing controversy surrounding acupuncture is the lack of a clear understanding of its underlying mechanism of action. For centuries the “how” of acupuncture has been explained in poetic terms such as yin, yang, and qi. Only in the past half-century have we begun investigating the potential biologic mechanisms responsible for the physiologic effects seen with acupuncture treatment.
While research has uncovered several interesting theories, how these mechanisms interact to produce therapeutic effects is still unclear. However, looking at various components of the nervous system helps to provide some insight.
Consider the nervous system. One way to conceptualize the mechanisms of acupuncture is to consider the various levels of the nervous system and how each level is affected. In the central nervous system, needling an acupuncture point stimulates the natural endorphin system, altering the pain sensation.5 This effect is reversible with naloxone in animal models, indicating that blocking the endorphin system interferes with the analgesic benefits of acupuncture.5
Serotonergic systems are also involved centrally. Functional magnetic resonance imaging studies have shown that needling specific acupuncture points modulates areas of the brain.
In the spinal cord, the gate control theory is believed to play a role. (The gate control theory puts forth that nonpainful input closes the “gates” to painful input, which prevents pain sensations from traveling to the central nervous system.) Modulation of sensory input occurs at the level of the dorsal horn of the spinal cord during an acupuncture treatment, which can affect the physiologic pain response.6 Opioid receptors are also affected at the spinal cord level.7
Lastly, multiple chemicals released peripherally, including interleukins, substance P, and adenosine, appear to contribute to acupuncture’s analgesia.6 We know this because a local anesthetic injected around a peripheral nerve at an acupoint blocks the analgesic effect of acupuncture.8 Taken together, acupuncture treatment produces physiologic changes in the brain, spinal cord, and at the periphery, making it a truly unique therapeutic modality.
2. Is acupuncture an effective treatment for pain?
Yes, but before we look at the individual studies, it is important to mention some of the shortcomings of the research to date. First, acupuncture trials lack a standard sham control intervention. Some sham treatments involve skin penetration, while others do not. This has led to controversy regarding whether the sham interventions themselves are physiologically active, thus lessening the magnitude of effect for acupuncture. This is a point of contention in the acupuncture literature and a factor to consider when deciding if results have clinical significance.
In addition, the acupuncturist providing treatment in a trial is typically unblinded. This is also true of trials measuring other physical modalities, but it contributes to the debate surrounding the magnitude of placebo response in acupuncture studies.
Finally, many randomized trials involving acupuncture have had low methodologic quality. Fortunately, there are now several high-quality systematic reviews that have attempted to filter out the lower-quality research and provide a better representation of the evidence (TABLE9-14). A discussion of them follows.

General chronic pain. A 2012 meta-analysis15 evaluated the effectiveness of acupuncture for the treatment of chronic pain with one of 4 etiologies: nonspecific back or neck pain, chronic headache, osteoarthritis, and shoulder pain. This analysis attempted to control for the high variability of study quality in the acupuncture literature by including only studies of high methodologic character. The final analysis included 29 randomized controlled trials (N=17,922). The authors concluded that acupuncture was superior to both no acupuncture and sham (placebo) acupuncture for all pain conditions in the study. The average effect size was 0.5 standard deviations on a 10-point scale. The authors considered this to be clinically relevant, although the magnitude of benefit was modest.15
Low back pain. A 2017 systematic review by Chou et al9 evaluated 32 trials (N=5931) reviewing acupuncture for the treatment of chronic low back pain. This review found acupuncture was associated with lower pain intensity and improved function in the short term when compared with no treatment. And while acupuncture was associated with lower pain intensity when compared with a sham control, there was no difference in function between the 2 groups. Of note, 3 of the included trials compared acupuncture to standard medications used in the treatment of low back pain and found acupuncture to be superior in terms of both pain reduction and improved function.9
The authors of a 2008 systematic review that evaluated 23 trials (N=6359)10 similarly concluded that there is moderate evidence for the use of acupuncture (compared to no treatment) for the treatment of nonspecific low back pain, but did not find evidence that acupuncture was superior to sham controls.10 The 2017 American College of Physicians clinical practice guidelines support the use of acupuncture for the treatment of chronic low back pain.16
In addition to helping with chronic low back pain, acupuncture is also showing promise as a treatment for acute spinal pain. A 2013 systematic review (11 trials, N=1139) showed that acupuncture may be more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) in treating acute low back pain and may cause fewer adverse effects.17
Headache pain. Evidence favoring acupuncture in the management of headache has been fairly consistent over the past decade. An updated Cochrane review on the prevention of migraine headaches was published in 2016.11 Acupuncture was compared with no treatment in 4 trials (n=2199). The authors found moderate quality evidence that acupuncture reduces headache frequency (number needed to treat=4). Acupuncture achieved at least 50% headache reduction in 41% vs 17% in the groups that received no acupuncture. When compared with sham control groups (10 trials, n=1534), acupuncture demonstrated a small but statistically significant improvement in headache frequency. Three trials (n=744) compared acupuncture to medication prophylaxis for migraine headaches and found acupuncture had similar effectiveness with fewer adverse effects.11
Osteoarthritis (OA). Most studies have focused on OA of the knee, and, thus far, have generated conflicting results. A Cochrane review in 2010 included 4 trials (n=884) that had a wait list control and 9 trials (n=1835) that compared acupuncture to a sham control.12 When compared to a wait list control, acupuncture resulted in statistically significant and clinically relevant improvement in pain and function. However, when compared to sham treatment for OA, the review showed statistically significant improvement in pain and function for acupuncture that was unlikely to be clinically relevant.12
A more recent meta-analysis in 2016 evaluated 10 trials (N=2007) investigating acupuncture in the treatment of knee OA.13 The authors found acupuncture improved both pain and functional outcome measures when compared with either no treatment or a sham control.
Fibromyalgia. Systematic reviews in 2007 (5 trials, N=316)18 and 2010 (7 trials, N=385)19 showed that acupuncture did provide short-term pain relief in patients with fibromyalgia, but that the effect was not sustained at follow-up.These reviews were limited by a high risk of bias, which was noted in the studies. The authors of both reviews concluded that acupuncture could not be recommended for the treatment of fibromyalgia.
A more recent Cochrane review published in 2013 (9 trials, N=395) offered low- to moderate-level evidence of benefit for acupuncture compared with no treatment at one month follow-up.14 Of note, there was also evidence of benefit in improved sleep and global well-being, in addition to pain and stiffness measures in this review. The overall magnitude of benefit was small, but clinically significant. Acupuncture also has evidence of benefit in the treatment of conditions commonly seen in conjunction with fibromyalgia, including headaches and low back pain as described earlier.
3. What does a typical acupuncture treatment entail?
In a typical treatment, anywhere from about 5 to 20 needles are inserted into the body. Common areas of needling include the arms and legs, especially below the elbows and knees. Other frequently used areas are the scalp, ears, and structures related to the painful condition.
The needles used are very thin (typically smaller than a 30-gauge needle) and do not have a beveled tip like phlebotomy needles do. Most patients have minimal pain as the needles are inserted. During the treatment, the needles may be left alone or they may be heated or stimulated electrically. An average treatment lasts 30 to 40 minutes; many patients find the sessions relaxing.
4. Are there any adverse effects or complications of treatment?
Acupuncture is generally considered a safe therapy, with most patients experiencing no adverse effects at all. Minor adverse effects can include post-treatment fatigue, minor bruising, or vasovagal reactions from the insertion of the needles. Serious complications, such as pneumothorax, are possible, but are considered rare.20 A 2004 study estimated the incidence of severe complications to be .05 per 10,000 acupuncture treatments.21
Infections are also possible, but most reported cases were due to practitioners reusing needles.22 The standard of care in the United States is to use only sterilized, single-use needles. With this practice, infections due to acupuncture are thought to be rare.
Of note, trials that compare acupuncture to another active therapy often find that acupuncture has fewer adverse effects. This has been the case when acupuncture was compared to NSAIDs for low back pain and to topiramate for headaches.17,23
5. How does acupuncture fit into a patient’s treatment?
The simple answer is that it is often most effectively used as part of a comprehensive management plan for chronic pain.
As our understanding of the complexity of chronic pain deepens, our therapeutic armamentarium for the management of chronic pain needs to broaden. This was summed up well in a 2016 article on the multimodal management of chronic pain when the authors stated, “Many targets need more than one arrow.”24 Effective management of chronic pain involves addressing psychosocial and lifestyle factors in a patient-centered way and finding a combination of treatments that most effectively leads to improved coping and function.
It’s important to note that like medications and injections, acupuncture is a passive therapy. Although there is evidence for efficacy of improved pain with acupuncture in certain conditions, it should be combined with treatments that actively engage patients, such as exercise, behavioral treatments, development of coping skills, sleep hygiene, and educational strategies.
6. To whom do I refer patients for acupuncture treatment?
In the United States, licensed acupuncturists and physicians most commonly perform acupuncture. There are more than 50 schools that train licensed acupuncturists in the United States, and it usually takes 3 years to meet the requirements.25
SIDEBAR
Online resources
American Academy of Medical Acupuncture
www.medicalacupuncture.com
National Center for Complementary and Alternative Medicine
http://nccam.nih.gov/health/acupuncture
National Certification Commission for Acupuncture and Oriental Medicine
www.nccaom.org
Physicians are often trained through continuing medical education (CME) programs that take several months to complete. These programs often combine live lectures, distance learning, and hands-on training and are typically sponsored by a university. Most require 300 hours of CME to complete. Licensure varies by state, but in many states, having an MD or DO degree automatically allows physicians to practice acupuncture. (See “Online resources,” above for links to Web sites that can be useful in finding qualified acupuncturists in your area.)
7. Is acupuncture covered by insurance?
It depends. Insurance coverage of acupuncture is highly variable and based on region and insurance type. Medicare and Medicaid plans do not pay for acupuncture. There are some private insurance plans that do. If covered, there may be limitations regarding diagnosis, number of visits, or provider. It is best for patients to call their insurance plan directly to inquire about coverage and any limitations. If paying out of pocket, patients can expect to pay $75 to $150 per treatment session.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Kennedy J, Roll JM, Schraudner T, et al. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15:979-984.
2. U.S. Department of Health and Human Services. NIH Fact Sheet. Pain management. Available at: https://www.report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed February 12, 2018.
3. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
4. Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1-14.
5. Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
6. Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
7. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482-503.
8. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41-S48.
9. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505.
10. Yuan J, Purepong N, Kerr DP, et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila Pa 1976). 2008;33:E887-E900.
11. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
12. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977.
13. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
14. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;5:CD007070.
15. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
16. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
17. Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29:172-185.
18. Mayhew E, Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46:801-804.
19. Langhorst J, Klose P, Musial F, et al. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxford). 2010;49:778-788.
20. Lao L, Hamilton GR, Fu J, et al. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72-83.
21. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22:122-133.
22. Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013:581203.
23. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009;1:CD001218.
24. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55-64.
25. National Certification Commission for Acupuncture and Oriental Medicine. Available at: www.nccaom.org. Accessed March 20, 2018.
1. Kennedy J, Roll JM, Schraudner T, et al. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15:979-984.
2. U.S. Department of Health and Human Services. NIH Fact Sheet. Pain management. Available at: https://www.report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed February 12, 2018.
3. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
4. Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1-14.
5. Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
6. Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
7. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482-503.
8. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41-S48.
9. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505.
10. Yuan J, Purepong N, Kerr DP, et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila Pa 1976). 2008;33:E887-E900.
11. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
12. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977.
13. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
14. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;5:CD007070.
15. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
16. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
17. Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29:172-185.
18. Mayhew E, Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46:801-804.
19. Langhorst J, Klose P, Musial F, et al. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxford). 2010;49:778-788.
20. Lao L, Hamilton GR, Fu J, et al. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72-83.
21. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22:122-133.
22. Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013:581203.
23. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009;1:CD001218.
24. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55-64.
25. National Certification Commission for Acupuncture and Oriental Medicine. Available at: www.nccaom.org. Accessed March 20, 2018.
From The Journal of Family Practice | 2018;67(4):224-226,228-230.
PRACTICE RECOMMENDATIONS
› Recommend acupuncture as a prophylactic treatment for migraine headaches. A
› Recommend acupuncture as a treatment option for chronic low back pain. A
› Consider using acupuncture as an adjunctive treatment in the management of fibromyalgia symptoms. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Getting the hypertension Dx right: Patient positioning matters
ABSTRACT
Purpose This study evaluated the effect of patient positioning on the diagnosis of hypertension in a clinic setting and the importance of following guidelines for measuring blood pressure (BP).
Methods In the trial part of this study, we recorded BP measurements by an aneroid sphygmomanometer with patients seated first on an examination table, a commonly observed practice, and second in the standard seated position as defined by the American Heart Association. Two measurements were obtained in each position for 204 patients, and we determined the difference between the average readings in the 2 positions. Factored into the comparison was an estimation of inherent variance of the device and observer achieved by repeated measurements on a healthy individual.
Results This investigation included an initial observational study of 25 regional primary care offices, the results of which showed frequent lack of adherence with accepted guidelines in patient positioning during BP measurement. The overall systolic and diastolic BPs were more than 2 mm Hg lower in the standard seated position compared with the examination table position (P<.001). Noncompliance with the position guideline resulted in misclassification of 15 patients (7.4%) as prehypertensive, when, in fact, they were normotensive. Misclassification of hypertension occurred in 12 patients (5.9%), when, in fact, they were normotensive. Logistic regression using relevant clinical factors did not identify those individuals who were misclassified.
Conclusion This study underscores the importance of patient positioning on BP determinations in order to accurately diagnose hypertension.
The high prevalence of hypertension and its burden of disease in the United States and worldwide are well known.1 Hypertension is a major risk factor for coronary heart disease, congestive heart failure, ischemic and hemorrhagic stroke, chronic kidney disease, and peripheral arterial disease.2 Among all risk factors, hypertension ranked first worldwide in disability-adjusted life-years.3 However, misclassification of an individual’s blood pressure (BP) as prehypertension or hypertension also confers significant health and financial burdens due to unnecessary medical encounters, testing, and treatment, and to increased cost of insurance coverage and out-of-pocket expenses. A correct assessment of BP in the outpatient setting depends on accurate measurement technique.
The diagnosis of hypertension is based on indirect measurement of BP using in-office, ambulatory, or home monitoring. Although office BP measurement is less than ideal, it is used most often to diagnose and monitor hypertension. Furthermore, most published trials of treatment recommendations are based on office BP measurements.4
Automated oscillometric and aneroid sphygmomanometers are common BP measurement devices. Proper technique is particularly important with the aneroid sphygmomanometer to obtain consistent and accurate results.5 Good training and an ability to hear the Korotkoff sounds are crucial.
Expert consensus groups such as the American Heart Association (AHA) publish recommendations for proper technique in reliably measuring BP,6-8 and they emphasize the importance of patient positioning during BP measurement. The individual should be seated comfortably in a chair with both arms and back supported, legs uncrossed, and feet flat on the floor. We’ll refer to this as the “standard position.” Although the proper technique for measuring BP has been widely advocated, a recent literature review for the US Preventive Services Task Force concluded that surprisingly few studies are available on the diagnostic accuracy of office BP practices.9
One paper evaluated the effect of leg crossing on accuracy of BP measurement. No subjects were reclassified as hypertensive, but the study lacked statistical rigor.10 Another study found variable BP readings regardless of body position.11
The purpose of our study was to compare BP measurement in 2 positions: the standard position described above, and the examination table position in which the patient is seated on the edge of the table with back, arms, and feet unsupported.
METHODS
We conducted our literature search across several scientific and medical literature databases, including PubMed, ScienceDirect, and CINAHL. Only English-language articles were reviewed.
We followed the BP measurement guidelines of the AHA. Prior to beginning the study, we provided instructions in proper BP measurement technique to the nurses who would obtain the data. The minimum sample size of patients needed to identify a difference of at least 2 mm Hg was 26, as estimated by power analysis. This was calculated using an alpha of .05 and a beta of .13.
The study population consisted of patients presenting consecutively to a teaching family medicine center. Adult patients, ages 18 and older, were informed about the study and invited to participate. Those who agreed were asked to read and sign an informed consent approved by a regional institutional review board for human subjects. We excluded patients who declined participation for any reason, who were in severe pain or distress that may have prevented them from completing the protocol, or who had limited mobility that could interfere with climbing onto the examination table. Patients considered for the study totaled 250, 28 of whom were ineligible. Another 18 patients declined participation, leaving 204 who completed the protocol.
Before testing began, we estimated the standard deviation of each aneroid sphygmomanometer and the assigned observer by repeatedly measuring the BP of a healthy normotensive individual sitting in the standard position. We obtained 46 measurements over 2 days to avoid subject and operator fatigue. Standard deviation for systolic BP was 3.6 mm Hg; for diastolic it was 3.8 mm Hg.
During testing, nurses recorded BP for each patient twice in the examination table position and twice in the standard position. They entered data into an Excel workbook for subsequent analysis. All examination rooms were equipped with newly purchased aneroid sphygmomanometers, and the appropriate cuff size was selected for each patient. Patients were instructed to remain quiet during the measurements. Patients sat first on the edge of the examination table. After a 5-minute rest, BP was measured twice in the same arm. Measurements were separated by 1 to 2 minutes. Patients then sat in the chair and rested another 2 minutes before BP was again measured twice in the same arm. The arms and back were supported in the chair and the stethoscope placed at heart level.
As per protocol, we obtained 4 BP readings on each patient and calculated the difference between the average systolic and diastolic BP values from the 2 positions. The standard error of the mean of this difference was determined using the equation, where Sd is the standard deviation of the aneroid sphygmomanometer and observer.12 A one-sided, 95% confidence upper bound for the standard error of the difference is 1.65 × SEd. We compared patient-specific differences against this upper bound to identify significant systolic and diastolic BP changes due to positioning. If the patient’s BP difference exceeded the upper bound, it was attributed to the positional change and not to variation inherent to the sphygmomanometer and observer.
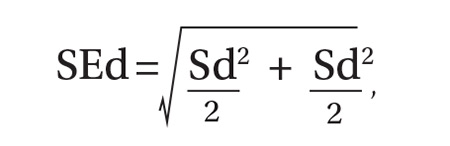
As an example, consider a patient whose average systolic BP readings from the examination-table and standard positions, respectively, were 128 mm Hg and 120 mm Hg. Assuming an SEd of 3.55 and an upper bound of 5.86, the observed 8 mm Hg difference in average systolic BPs would be considered significant. The amount of random variation from the sphygmomanometer and observer would not be expected to exceed 5.86 mm Hg.
In accordance with accepted standards, prehypertension was defined as a BP between 120-139/80-89 mm Hg, and hypertension was defined as a BP ≥140/90 mm Hg.4 BP below 120/80 mm Hg was considered normal. We calculated each patient’s average systolic and diastolic BP values in the 2 positions and thereby classified the individual as normotensive, prehypertensive, or hypertensive. We regarded as misclassified any patient whose BP showed significant lowering between the examination-table and standard positions resulting in a change of classification from prehypertensive or hypertensive to normotensive. For example, a patient with an examination-table position average reading of 126/85 mm Hg and a standard position average reading of 118/78 mm Hg would have been misclassified as prehypertensive.
We reviewed charts and gathered data, including subject age, sex, obesity (defined as a body mass index of ≥30 kg/m2), and history of diabetes, hypertension, or smoking. Other than age, all data were binary. We performed logistic regression analysis using the Excel Add-in Real Statistics Resource Pack software (Release 4.3)13 to determine if these factors could predict significant lowering of BP due to positional change.
Our associated observational study. We also conducted a separate observational study of 25 regional primary care offices to evaluate compliance with the AHA guidelines for measuring BP. The office nurses taking measurements were not informed of the study’s purpose to prevent deviation from their common practice.
Data on 9 guideline criteria were collected to assess supervision of patients before and during measurements, including having the patient sit in a chair in quiet and comfortable surroundings with arms and back supported and feet on the ground. We also noted the type of BP measuring device used. Additionally, observers assessed the technique of the individuals using a manual device, including cuff placement and deflation rate. The observations were conducted during a clinic visit by a medical student knowledgeable in the AHA guidelines for measuring BP by automated oscillometric or aneroid sphygmomanometric devices. We conducted the study over a 2-week period in the second quarter of 2016.
RESULTS
Power analysis performed prior to the study showed that a minimum of 26 patients would be needed to predict a 2 mm Hg difference between BPs obtained in the 2 positions. Of the 204 patients used in the logistic regression analysis, 78 were men and 126 were women. Ages ranged from 18 to 101 years, yielding a mean of 54. One-hundred sixteen had previously received a diagnosis of hypertension, 39 had diabetes, 92 were obese, 22 were current smokers, and 68 were former smokers.
TABLE 1 shows the means and ranges of systolic and diastolic BP for both study positions. With this study population, mean BP recorded in the examination-table position decreased in the standard position by 2.1 and 2.2 mm Hg for systolic and diastolic BP, respectively (P<.001).
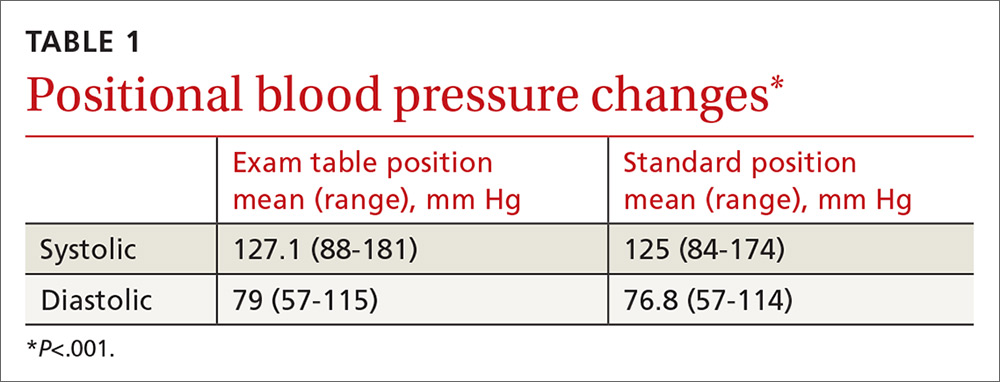
Significant BP lowering—as defined by a one-sided 95% confidence upper bound for the standard error of differences between study positions—was determined to be 5.86 and 6.22 mm Hg for systolic and diastolic pressures, respectively. Significant lowering of BP and misclassification due to positioning are summarized in TABLE 2. Significant lowering of mean systolic or diastolic BP with positional change from table to chair occurred in 62 subjects (30.4%). Misclassification of prehypertension occurred in 7.4% of subjects, and misclassification of hypertension occurred in 5.9%.
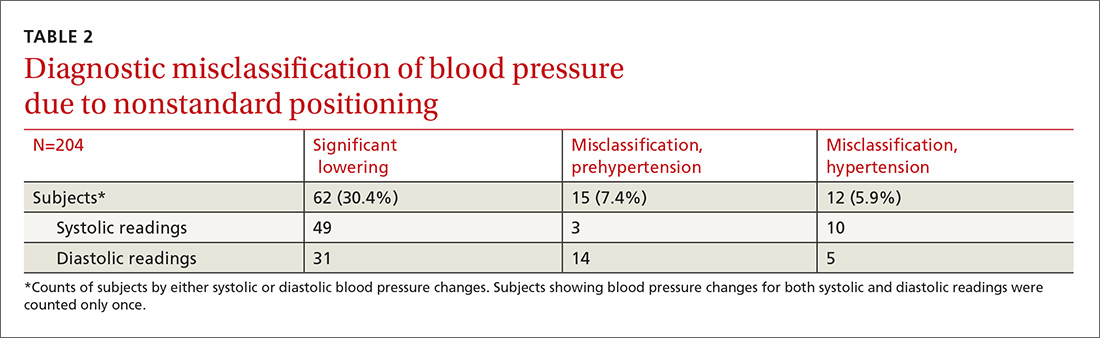
Logistic regression using patient age, sex, obesity, and history of diabetes, hypertension, and smoking as independent factors did not predict significant BP lowering with positional change.
Our observational study revealed that proper positioning in a chair was followed in only 10 of the 25 offices. In the remaining offices, patients were seated on the examination table. A 5-minute rest period before measuring BP was allowed in only 10 of the 25 offices. An automated oscillometric device was used in only 2 of the 25 offices.
DISCUSSION
In this study, 27 subjects (13.2%) were misclassified as prehypertensive or hypertensive as a result of deviating from the standard position in obtaining BP. Although the standard position is universally recommended, the guideline is not always followed in clinical practice.14
One study by Villegas et al found that 60% of physicians and nurses working in a major hospital were measuring BP inaccurately.15 In our initial observational study, 60% of primary care practices visited did not adhere to the recommended patient positioning. These medical offices are located in the community surrounding our facility and are operated by the same health care organization. The misclassification of prehypertension and hypertension observed in our prospective comparison of BP recordings in table and chair positions is, therefore, likely to occur to some degree at these practices, as well.
Similar diagnostic misclassifications have been reported in other medical settings. In a published survey of 114 medical offices, McKay and coworkers noted frequent inconsistencies with published guidelines in measuring BP.16
Common clinical demographic data obtained during this study showed no association with the positional BP change. Increased muscle tension due to lack of body support while sitting on the edge of the examination table could be the cause of elevated BP for this subgroup of individuals. Measuring muscle tension of the arms and back while seated on an exam table and chair was beyond the scope of this study.
In clinical practice, different types of BP measuring devices are used. Calibration and quality control of these devices is often lacking.17 Before starting our study, we determined the statistical variance of the aneroid sphygmomanometers and found it to approximate the manufacturer’s precision specification. Guidelines recommend using the mean of 2 BP readings as representing the patient’s BP for a given clinic visit. Additional readings are recommended if there is more than a 5 mm Hg difference between the initial 2 readings.4
In our study, we used sampling statistics of the BP readings and clinical guideline BP ranges in making diagnostic determinations. The inability to identify those patients whose BP will be affected by positional change highlights the importance of following standard BP measurement guidelines for all patients.
Study limitations. Positional change in BP from examination table to chair lacks a comparison to BP changes in positioning from chair to table. If similar BP changes in the reverse sequence were to be observed, this would add support to the hypothesis that muscle tension of the unsupported body is a cause of BP elevation in certain individuals. We believe, however, that the sequence of BP measurements (from table to chair) did not have a significant impact because all patients were allowed to rest in each position before the BP was measured. The BP was therefore measured in a steady-state in both positions.
Additionally, BP measurement by aneroid sphygmomanometry is highly dependent on observer skill and hearing ability. Furthermore, a disproportionate number of BP measurements recorded in the study ended in zero, suggesting terminal digit bias by the observer. These sources of error may be avoided using an automated oscillometric measuring device.18 Automated devices also allow for repeated independent measurements that minimize the white-coat effect. However, there are also limitations to the accuracy of oscillometric equipment. This is especially true when recording BP in the elderly, a group whose stiff arterial walls may cause erroneous measurements.19
Guideline justification. Nonadherence to standard positioning when measuring BP leads to certain individuals being misclassified as prehypertensive or hypertensive. Misclassification in turn leads to unnecessary medical encounters, testing, and treatment. Misdiagnosis is also likely to increase the cost of an individual’s insurance coverage and out-of-pocket health care expenses.
CORRESPONDENCE
Roy N. Morcos, MD, St. Elizabeth Family Medicine Residency Program, 8423 Market Street, Suite 101, Boardman, Ohio 44512; [email protected].
1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260.
3. Murray CJ, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369:448-457.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
5. Bailey RH, Bauer JH. A review of common errors in the indirect measurement of blood pressure. Sphygmomanometry. Arch Intern Med. 1993;153:2741-2748.
6. Padwal RS, Hemmelgarn BR, McAlister FA, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2007;23:529-538.
7. Campbell NR, Chockalingam A, Fodor JG, et al. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19-24.
8. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens. 2005;7:102-109.
9. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
10. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97-101.
11. Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24:1073-1079.
12. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences (10th Edition). Hoboken, NJ: John Wiley & Sons; 2013.
13. Zaiontz C. Real statistics using Excel. Available at: http://www.real-statistics.com/. Accessed February 20, 2018.
14. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
15. Villegas I, Arias IC, Botero A, et al. Evaluation of the technique used by health-care workers for taking blood pressure. Hypertension. 1995;26:1204-1206.
16. McKay DW, Campbell NR, Parab LS, et al. Clinical assessment of blood pressure. J Hum Hypertens. 1990;4:639-645.
17. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
18. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569-588.
19. Raamat R, Talts J, Jagomägi K, et al. Errors of oscillometric blood pressure measurement as predicted by simulation. Blood Press Monit. 2011;16:238-245.
ABSTRACT
Purpose This study evaluated the effect of patient positioning on the diagnosis of hypertension in a clinic setting and the importance of following guidelines for measuring blood pressure (BP).
Methods In the trial part of this study, we recorded BP measurements by an aneroid sphygmomanometer with patients seated first on an examination table, a commonly observed practice, and second in the standard seated position as defined by the American Heart Association. Two measurements were obtained in each position for 204 patients, and we determined the difference between the average readings in the 2 positions. Factored into the comparison was an estimation of inherent variance of the device and observer achieved by repeated measurements on a healthy individual.
Results This investigation included an initial observational study of 25 regional primary care offices, the results of which showed frequent lack of adherence with accepted guidelines in patient positioning during BP measurement. The overall systolic and diastolic BPs were more than 2 mm Hg lower in the standard seated position compared with the examination table position (P<.001). Noncompliance with the position guideline resulted in misclassification of 15 patients (7.4%) as prehypertensive, when, in fact, they were normotensive. Misclassification of hypertension occurred in 12 patients (5.9%), when, in fact, they were normotensive. Logistic regression using relevant clinical factors did not identify those individuals who were misclassified.
Conclusion This study underscores the importance of patient positioning on BP determinations in order to accurately diagnose hypertension.
The high prevalence of hypertension and its burden of disease in the United States and worldwide are well known.1 Hypertension is a major risk factor for coronary heart disease, congestive heart failure, ischemic and hemorrhagic stroke, chronic kidney disease, and peripheral arterial disease.2 Among all risk factors, hypertension ranked first worldwide in disability-adjusted life-years.3 However, misclassification of an individual’s blood pressure (BP) as prehypertension or hypertension also confers significant health and financial burdens due to unnecessary medical encounters, testing, and treatment, and to increased cost of insurance coverage and out-of-pocket expenses. A correct assessment of BP in the outpatient setting depends on accurate measurement technique.
The diagnosis of hypertension is based on indirect measurement of BP using in-office, ambulatory, or home monitoring. Although office BP measurement is less than ideal, it is used most often to diagnose and monitor hypertension. Furthermore, most published trials of treatment recommendations are based on office BP measurements.4
Automated oscillometric and aneroid sphygmomanometers are common BP measurement devices. Proper technique is particularly important with the aneroid sphygmomanometer to obtain consistent and accurate results.5 Good training and an ability to hear the Korotkoff sounds are crucial.
Expert consensus groups such as the American Heart Association (AHA) publish recommendations for proper technique in reliably measuring BP,6-8 and they emphasize the importance of patient positioning during BP measurement. The individual should be seated comfortably in a chair with both arms and back supported, legs uncrossed, and feet flat on the floor. We’ll refer to this as the “standard position.” Although the proper technique for measuring BP has been widely advocated, a recent literature review for the US Preventive Services Task Force concluded that surprisingly few studies are available on the diagnostic accuracy of office BP practices.9
One paper evaluated the effect of leg crossing on accuracy of BP measurement. No subjects were reclassified as hypertensive, but the study lacked statistical rigor.10 Another study found variable BP readings regardless of body position.11
The purpose of our study was to compare BP measurement in 2 positions: the standard position described above, and the examination table position in which the patient is seated on the edge of the table with back, arms, and feet unsupported.
METHODS
We conducted our literature search across several scientific and medical literature databases, including PubMed, ScienceDirect, and CINAHL. Only English-language articles were reviewed.
We followed the BP measurement guidelines of the AHA. Prior to beginning the study, we provided instructions in proper BP measurement technique to the nurses who would obtain the data. The minimum sample size of patients needed to identify a difference of at least 2 mm Hg was 26, as estimated by power analysis. This was calculated using an alpha of .05 and a beta of .13.
The study population consisted of patients presenting consecutively to a teaching family medicine center. Adult patients, ages 18 and older, were informed about the study and invited to participate. Those who agreed were asked to read and sign an informed consent approved by a regional institutional review board for human subjects. We excluded patients who declined participation for any reason, who were in severe pain or distress that may have prevented them from completing the protocol, or who had limited mobility that could interfere with climbing onto the examination table. Patients considered for the study totaled 250, 28 of whom were ineligible. Another 18 patients declined participation, leaving 204 who completed the protocol.
Before testing began, we estimated the standard deviation of each aneroid sphygmomanometer and the assigned observer by repeatedly measuring the BP of a healthy normotensive individual sitting in the standard position. We obtained 46 measurements over 2 days to avoid subject and operator fatigue. Standard deviation for systolic BP was 3.6 mm Hg; for diastolic it was 3.8 mm Hg.
During testing, nurses recorded BP for each patient twice in the examination table position and twice in the standard position. They entered data into an Excel workbook for subsequent analysis. All examination rooms were equipped with newly purchased aneroid sphygmomanometers, and the appropriate cuff size was selected for each patient. Patients were instructed to remain quiet during the measurements. Patients sat first on the edge of the examination table. After a 5-minute rest, BP was measured twice in the same arm. Measurements were separated by 1 to 2 minutes. Patients then sat in the chair and rested another 2 minutes before BP was again measured twice in the same arm. The arms and back were supported in the chair and the stethoscope placed at heart level.
As per protocol, we obtained 4 BP readings on each patient and calculated the difference between the average systolic and diastolic BP values from the 2 positions. The standard error of the mean of this difference was determined using the equation, where Sd is the standard deviation of the aneroid sphygmomanometer and observer.12 A one-sided, 95% confidence upper bound for the standard error of the difference is 1.65 × SEd. We compared patient-specific differences against this upper bound to identify significant systolic and diastolic BP changes due to positioning. If the patient’s BP difference exceeded the upper bound, it was attributed to the positional change and not to variation inherent to the sphygmomanometer and observer.
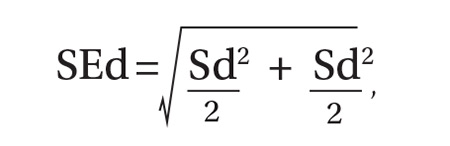
As an example, consider a patient whose average systolic BP readings from the examination-table and standard positions, respectively, were 128 mm Hg and 120 mm Hg. Assuming an SEd of 3.55 and an upper bound of 5.86, the observed 8 mm Hg difference in average systolic BPs would be considered significant. The amount of random variation from the sphygmomanometer and observer would not be expected to exceed 5.86 mm Hg.
In accordance with accepted standards, prehypertension was defined as a BP between 120-139/80-89 mm Hg, and hypertension was defined as a BP ≥140/90 mm Hg.4 BP below 120/80 mm Hg was considered normal. We calculated each patient’s average systolic and diastolic BP values in the 2 positions and thereby classified the individual as normotensive, prehypertensive, or hypertensive. We regarded as misclassified any patient whose BP showed significant lowering between the examination-table and standard positions resulting in a change of classification from prehypertensive or hypertensive to normotensive. For example, a patient with an examination-table position average reading of 126/85 mm Hg and a standard position average reading of 118/78 mm Hg would have been misclassified as prehypertensive.
We reviewed charts and gathered data, including subject age, sex, obesity (defined as a body mass index of ≥30 kg/m2), and history of diabetes, hypertension, or smoking. Other than age, all data were binary. We performed logistic regression analysis using the Excel Add-in Real Statistics Resource Pack software (Release 4.3)13 to determine if these factors could predict significant lowering of BP due to positional change.
Our associated observational study. We also conducted a separate observational study of 25 regional primary care offices to evaluate compliance with the AHA guidelines for measuring BP. The office nurses taking measurements were not informed of the study’s purpose to prevent deviation from their common practice.
Data on 9 guideline criteria were collected to assess supervision of patients before and during measurements, including having the patient sit in a chair in quiet and comfortable surroundings with arms and back supported and feet on the ground. We also noted the type of BP measuring device used. Additionally, observers assessed the technique of the individuals using a manual device, including cuff placement and deflation rate. The observations were conducted during a clinic visit by a medical student knowledgeable in the AHA guidelines for measuring BP by automated oscillometric or aneroid sphygmomanometric devices. We conducted the study over a 2-week period in the second quarter of 2016.
RESULTS
Power analysis performed prior to the study showed that a minimum of 26 patients would be needed to predict a 2 mm Hg difference between BPs obtained in the 2 positions. Of the 204 patients used in the logistic regression analysis, 78 were men and 126 were women. Ages ranged from 18 to 101 years, yielding a mean of 54. One-hundred sixteen had previously received a diagnosis of hypertension, 39 had diabetes, 92 were obese, 22 were current smokers, and 68 were former smokers.
TABLE 1 shows the means and ranges of systolic and diastolic BP for both study positions. With this study population, mean BP recorded in the examination-table position decreased in the standard position by 2.1 and 2.2 mm Hg for systolic and diastolic BP, respectively (P<.001).

Significant BP lowering—as defined by a one-sided 95% confidence upper bound for the standard error of differences between study positions—was determined to be 5.86 and 6.22 mm Hg for systolic and diastolic pressures, respectively. Significant lowering of BP and misclassification due to positioning are summarized in TABLE 2. Significant lowering of mean systolic or diastolic BP with positional change from table to chair occurred in 62 subjects (30.4%). Misclassification of prehypertension occurred in 7.4% of subjects, and misclassification of hypertension occurred in 5.9%.

Logistic regression using patient age, sex, obesity, and history of diabetes, hypertension, and smoking as independent factors did not predict significant BP lowering with positional change.
Our observational study revealed that proper positioning in a chair was followed in only 10 of the 25 offices. In the remaining offices, patients were seated on the examination table. A 5-minute rest period before measuring BP was allowed in only 10 of the 25 offices. An automated oscillometric device was used in only 2 of the 25 offices.
DISCUSSION
In this study, 27 subjects (13.2%) were misclassified as prehypertensive or hypertensive as a result of deviating from the standard position in obtaining BP. Although the standard position is universally recommended, the guideline is not always followed in clinical practice.14
One study by Villegas et al found that 60% of physicians and nurses working in a major hospital were measuring BP inaccurately.15 In our initial observational study, 60% of primary care practices visited did not adhere to the recommended patient positioning. These medical offices are located in the community surrounding our facility and are operated by the same health care organization. The misclassification of prehypertension and hypertension observed in our prospective comparison of BP recordings in table and chair positions is, therefore, likely to occur to some degree at these practices, as well.
Similar diagnostic misclassifications have been reported in other medical settings. In a published survey of 114 medical offices, McKay and coworkers noted frequent inconsistencies with published guidelines in measuring BP.16
Common clinical demographic data obtained during this study showed no association with the positional BP change. Increased muscle tension due to lack of body support while sitting on the edge of the examination table could be the cause of elevated BP for this subgroup of individuals. Measuring muscle tension of the arms and back while seated on an exam table and chair was beyond the scope of this study.
In clinical practice, different types of BP measuring devices are used. Calibration and quality control of these devices is often lacking.17 Before starting our study, we determined the statistical variance of the aneroid sphygmomanometers and found it to approximate the manufacturer’s precision specification. Guidelines recommend using the mean of 2 BP readings as representing the patient’s BP for a given clinic visit. Additional readings are recommended if there is more than a 5 mm Hg difference between the initial 2 readings.4
In our study, we used sampling statistics of the BP readings and clinical guideline BP ranges in making diagnostic determinations. The inability to identify those patients whose BP will be affected by positional change highlights the importance of following standard BP measurement guidelines for all patients.
Study limitations. Positional change in BP from examination table to chair lacks a comparison to BP changes in positioning from chair to table. If similar BP changes in the reverse sequence were to be observed, this would add support to the hypothesis that muscle tension of the unsupported body is a cause of BP elevation in certain individuals. We believe, however, that the sequence of BP measurements (from table to chair) did not have a significant impact because all patients were allowed to rest in each position before the BP was measured. The BP was therefore measured in a steady-state in both positions.
Additionally, BP measurement by aneroid sphygmomanometry is highly dependent on observer skill and hearing ability. Furthermore, a disproportionate number of BP measurements recorded in the study ended in zero, suggesting terminal digit bias by the observer. These sources of error may be avoided using an automated oscillometric measuring device.18 Automated devices also allow for repeated independent measurements that minimize the white-coat effect. However, there are also limitations to the accuracy of oscillometric equipment. This is especially true when recording BP in the elderly, a group whose stiff arterial walls may cause erroneous measurements.19
Guideline justification. Nonadherence to standard positioning when measuring BP leads to certain individuals being misclassified as prehypertensive or hypertensive. Misclassification in turn leads to unnecessary medical encounters, testing, and treatment. Misdiagnosis is also likely to increase the cost of an individual’s insurance coverage and out-of-pocket health care expenses.
CORRESPONDENCE
Roy N. Morcos, MD, St. Elizabeth Family Medicine Residency Program, 8423 Market Street, Suite 101, Boardman, Ohio 44512; [email protected].
ABSTRACT
Purpose This study evaluated the effect of patient positioning on the diagnosis of hypertension in a clinic setting and the importance of following guidelines for measuring blood pressure (BP).
Methods In the trial part of this study, we recorded BP measurements by an aneroid sphygmomanometer with patients seated first on an examination table, a commonly observed practice, and second in the standard seated position as defined by the American Heart Association. Two measurements were obtained in each position for 204 patients, and we determined the difference between the average readings in the 2 positions. Factored into the comparison was an estimation of inherent variance of the device and observer achieved by repeated measurements on a healthy individual.
Results This investigation included an initial observational study of 25 regional primary care offices, the results of which showed frequent lack of adherence with accepted guidelines in patient positioning during BP measurement. The overall systolic and diastolic BPs were more than 2 mm Hg lower in the standard seated position compared with the examination table position (P<.001). Noncompliance with the position guideline resulted in misclassification of 15 patients (7.4%) as prehypertensive, when, in fact, they were normotensive. Misclassification of hypertension occurred in 12 patients (5.9%), when, in fact, they were normotensive. Logistic regression using relevant clinical factors did not identify those individuals who were misclassified.
Conclusion This study underscores the importance of patient positioning on BP determinations in order to accurately diagnose hypertension.
The high prevalence of hypertension and its burden of disease in the United States and worldwide are well known.1 Hypertension is a major risk factor for coronary heart disease, congestive heart failure, ischemic and hemorrhagic stroke, chronic kidney disease, and peripheral arterial disease.2 Among all risk factors, hypertension ranked first worldwide in disability-adjusted life-years.3 However, misclassification of an individual’s blood pressure (BP) as prehypertension or hypertension also confers significant health and financial burdens due to unnecessary medical encounters, testing, and treatment, and to increased cost of insurance coverage and out-of-pocket expenses. A correct assessment of BP in the outpatient setting depends on accurate measurement technique.
The diagnosis of hypertension is based on indirect measurement of BP using in-office, ambulatory, or home monitoring. Although office BP measurement is less than ideal, it is used most often to diagnose and monitor hypertension. Furthermore, most published trials of treatment recommendations are based on office BP measurements.4
Automated oscillometric and aneroid sphygmomanometers are common BP measurement devices. Proper technique is particularly important with the aneroid sphygmomanometer to obtain consistent and accurate results.5 Good training and an ability to hear the Korotkoff sounds are crucial.
Expert consensus groups such as the American Heart Association (AHA) publish recommendations for proper technique in reliably measuring BP,6-8 and they emphasize the importance of patient positioning during BP measurement. The individual should be seated comfortably in a chair with both arms and back supported, legs uncrossed, and feet flat on the floor. We’ll refer to this as the “standard position.” Although the proper technique for measuring BP has been widely advocated, a recent literature review for the US Preventive Services Task Force concluded that surprisingly few studies are available on the diagnostic accuracy of office BP practices.9
One paper evaluated the effect of leg crossing on accuracy of BP measurement. No subjects were reclassified as hypertensive, but the study lacked statistical rigor.10 Another study found variable BP readings regardless of body position.11
The purpose of our study was to compare BP measurement in 2 positions: the standard position described above, and the examination table position in which the patient is seated on the edge of the table with back, arms, and feet unsupported.
METHODS
We conducted our literature search across several scientific and medical literature databases, including PubMed, ScienceDirect, and CINAHL. Only English-language articles were reviewed.
We followed the BP measurement guidelines of the AHA. Prior to beginning the study, we provided instructions in proper BP measurement technique to the nurses who would obtain the data. The minimum sample size of patients needed to identify a difference of at least 2 mm Hg was 26, as estimated by power analysis. This was calculated using an alpha of .05 and a beta of .13.
The study population consisted of patients presenting consecutively to a teaching family medicine center. Adult patients, ages 18 and older, were informed about the study and invited to participate. Those who agreed were asked to read and sign an informed consent approved by a regional institutional review board for human subjects. We excluded patients who declined participation for any reason, who were in severe pain or distress that may have prevented them from completing the protocol, or who had limited mobility that could interfere with climbing onto the examination table. Patients considered for the study totaled 250, 28 of whom were ineligible. Another 18 patients declined participation, leaving 204 who completed the protocol.
Before testing began, we estimated the standard deviation of each aneroid sphygmomanometer and the assigned observer by repeatedly measuring the BP of a healthy normotensive individual sitting in the standard position. We obtained 46 measurements over 2 days to avoid subject and operator fatigue. Standard deviation for systolic BP was 3.6 mm Hg; for diastolic it was 3.8 mm Hg.
During testing, nurses recorded BP for each patient twice in the examination table position and twice in the standard position. They entered data into an Excel workbook for subsequent analysis. All examination rooms were equipped with newly purchased aneroid sphygmomanometers, and the appropriate cuff size was selected for each patient. Patients were instructed to remain quiet during the measurements. Patients sat first on the edge of the examination table. After a 5-minute rest, BP was measured twice in the same arm. Measurements were separated by 1 to 2 minutes. Patients then sat in the chair and rested another 2 minutes before BP was again measured twice in the same arm. The arms and back were supported in the chair and the stethoscope placed at heart level.
As per protocol, we obtained 4 BP readings on each patient and calculated the difference between the average systolic and diastolic BP values from the 2 positions. The standard error of the mean of this difference was determined using the equation, where Sd is the standard deviation of the aneroid sphygmomanometer and observer.12 A one-sided, 95% confidence upper bound for the standard error of the difference is 1.65 × SEd. We compared patient-specific differences against this upper bound to identify significant systolic and diastolic BP changes due to positioning. If the patient’s BP difference exceeded the upper bound, it was attributed to the positional change and not to variation inherent to the sphygmomanometer and observer.
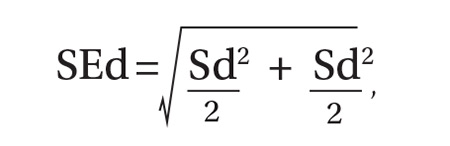
As an example, consider a patient whose average systolic BP readings from the examination-table and standard positions, respectively, were 128 mm Hg and 120 mm Hg. Assuming an SEd of 3.55 and an upper bound of 5.86, the observed 8 mm Hg difference in average systolic BPs would be considered significant. The amount of random variation from the sphygmomanometer and observer would not be expected to exceed 5.86 mm Hg.
In accordance with accepted standards, prehypertension was defined as a BP between 120-139/80-89 mm Hg, and hypertension was defined as a BP ≥140/90 mm Hg.4 BP below 120/80 mm Hg was considered normal. We calculated each patient’s average systolic and diastolic BP values in the 2 positions and thereby classified the individual as normotensive, prehypertensive, or hypertensive. We regarded as misclassified any patient whose BP showed significant lowering between the examination-table and standard positions resulting in a change of classification from prehypertensive or hypertensive to normotensive. For example, a patient with an examination-table position average reading of 126/85 mm Hg and a standard position average reading of 118/78 mm Hg would have been misclassified as prehypertensive.
We reviewed charts and gathered data, including subject age, sex, obesity (defined as a body mass index of ≥30 kg/m2), and history of diabetes, hypertension, or smoking. Other than age, all data were binary. We performed logistic regression analysis using the Excel Add-in Real Statistics Resource Pack software (Release 4.3)13 to determine if these factors could predict significant lowering of BP due to positional change.
Our associated observational study. We also conducted a separate observational study of 25 regional primary care offices to evaluate compliance with the AHA guidelines for measuring BP. The office nurses taking measurements were not informed of the study’s purpose to prevent deviation from their common practice.
Data on 9 guideline criteria were collected to assess supervision of patients before and during measurements, including having the patient sit in a chair in quiet and comfortable surroundings with arms and back supported and feet on the ground. We also noted the type of BP measuring device used. Additionally, observers assessed the technique of the individuals using a manual device, including cuff placement and deflation rate. The observations were conducted during a clinic visit by a medical student knowledgeable in the AHA guidelines for measuring BP by automated oscillometric or aneroid sphygmomanometric devices. We conducted the study over a 2-week period in the second quarter of 2016.
RESULTS
Power analysis performed prior to the study showed that a minimum of 26 patients would be needed to predict a 2 mm Hg difference between BPs obtained in the 2 positions. Of the 204 patients used in the logistic regression analysis, 78 were men and 126 were women. Ages ranged from 18 to 101 years, yielding a mean of 54. One-hundred sixteen had previously received a diagnosis of hypertension, 39 had diabetes, 92 were obese, 22 were current smokers, and 68 were former smokers.
TABLE 1 shows the means and ranges of systolic and diastolic BP for both study positions. With this study population, mean BP recorded in the examination-table position decreased in the standard position by 2.1 and 2.2 mm Hg for systolic and diastolic BP, respectively (P<.001).

Significant BP lowering—as defined by a one-sided 95% confidence upper bound for the standard error of differences between study positions—was determined to be 5.86 and 6.22 mm Hg for systolic and diastolic pressures, respectively. Significant lowering of BP and misclassification due to positioning are summarized in TABLE 2. Significant lowering of mean systolic or diastolic BP with positional change from table to chair occurred in 62 subjects (30.4%). Misclassification of prehypertension occurred in 7.4% of subjects, and misclassification of hypertension occurred in 5.9%.

Logistic regression using patient age, sex, obesity, and history of diabetes, hypertension, and smoking as independent factors did not predict significant BP lowering with positional change.
Our observational study revealed that proper positioning in a chair was followed in only 10 of the 25 offices. In the remaining offices, patients were seated on the examination table. A 5-minute rest period before measuring BP was allowed in only 10 of the 25 offices. An automated oscillometric device was used in only 2 of the 25 offices.
DISCUSSION
In this study, 27 subjects (13.2%) were misclassified as prehypertensive or hypertensive as a result of deviating from the standard position in obtaining BP. Although the standard position is universally recommended, the guideline is not always followed in clinical practice.14
One study by Villegas et al found that 60% of physicians and nurses working in a major hospital were measuring BP inaccurately.15 In our initial observational study, 60% of primary care practices visited did not adhere to the recommended patient positioning. These medical offices are located in the community surrounding our facility and are operated by the same health care organization. The misclassification of prehypertension and hypertension observed in our prospective comparison of BP recordings in table and chair positions is, therefore, likely to occur to some degree at these practices, as well.
Similar diagnostic misclassifications have been reported in other medical settings. In a published survey of 114 medical offices, McKay and coworkers noted frequent inconsistencies with published guidelines in measuring BP.16
Common clinical demographic data obtained during this study showed no association with the positional BP change. Increased muscle tension due to lack of body support while sitting on the edge of the examination table could be the cause of elevated BP for this subgroup of individuals. Measuring muscle tension of the arms and back while seated on an exam table and chair was beyond the scope of this study.
In clinical practice, different types of BP measuring devices are used. Calibration and quality control of these devices is often lacking.17 Before starting our study, we determined the statistical variance of the aneroid sphygmomanometers and found it to approximate the manufacturer’s precision specification. Guidelines recommend using the mean of 2 BP readings as representing the patient’s BP for a given clinic visit. Additional readings are recommended if there is more than a 5 mm Hg difference between the initial 2 readings.4
In our study, we used sampling statistics of the BP readings and clinical guideline BP ranges in making diagnostic determinations. The inability to identify those patients whose BP will be affected by positional change highlights the importance of following standard BP measurement guidelines for all patients.
Study limitations. Positional change in BP from examination table to chair lacks a comparison to BP changes in positioning from chair to table. If similar BP changes in the reverse sequence were to be observed, this would add support to the hypothesis that muscle tension of the unsupported body is a cause of BP elevation in certain individuals. We believe, however, that the sequence of BP measurements (from table to chair) did not have a significant impact because all patients were allowed to rest in each position before the BP was measured. The BP was therefore measured in a steady-state in both positions.
Additionally, BP measurement by aneroid sphygmomanometry is highly dependent on observer skill and hearing ability. Furthermore, a disproportionate number of BP measurements recorded in the study ended in zero, suggesting terminal digit bias by the observer. These sources of error may be avoided using an automated oscillometric measuring device.18 Automated devices also allow for repeated independent measurements that minimize the white-coat effect. However, there are also limitations to the accuracy of oscillometric equipment. This is especially true when recording BP in the elderly, a group whose stiff arterial walls may cause erroneous measurements.19
Guideline justification. Nonadherence to standard positioning when measuring BP leads to certain individuals being misclassified as prehypertensive or hypertensive. Misclassification in turn leads to unnecessary medical encounters, testing, and treatment. Misdiagnosis is also likely to increase the cost of an individual’s insurance coverage and out-of-pocket health care expenses.
CORRESPONDENCE
Roy N. Morcos, MD, St. Elizabeth Family Medicine Residency Program, 8423 Market Street, Suite 101, Boardman, Ohio 44512; [email protected].
1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260.
3. Murray CJ, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369:448-457.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
5. Bailey RH, Bauer JH. A review of common errors in the indirect measurement of blood pressure. Sphygmomanometry. Arch Intern Med. 1993;153:2741-2748.
6. Padwal RS, Hemmelgarn BR, McAlister FA, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2007;23:529-538.
7. Campbell NR, Chockalingam A, Fodor JG, et al. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19-24.
8. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens. 2005;7:102-109.
9. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
10. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97-101.
11. Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24:1073-1079.
12. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences (10th Edition). Hoboken, NJ: John Wiley & Sons; 2013.
13. Zaiontz C. Real statistics using Excel. Available at: http://www.real-statistics.com/. Accessed February 20, 2018.
14. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
15. Villegas I, Arias IC, Botero A, et al. Evaluation of the technique used by health-care workers for taking blood pressure. Hypertension. 1995;26:1204-1206.
16. McKay DW, Campbell NR, Parab LS, et al. Clinical assessment of blood pressure. J Hum Hypertens. 1990;4:639-645.
17. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
18. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569-588.
19. Raamat R, Talts J, Jagomägi K, et al. Errors of oscillometric blood pressure measurement as predicted by simulation. Blood Press Monit. 2011;16:238-245.
1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260.
3. Murray CJ, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369:448-457.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
5. Bailey RH, Bauer JH. A review of common errors in the indirect measurement of blood pressure. Sphygmomanometry. Arch Intern Med. 1993;153:2741-2748.
6. Padwal RS, Hemmelgarn BR, McAlister FA, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2007;23:529-538.
7. Campbell NR, Chockalingam A, Fodor JG, et al. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19-24.
8. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens. 2005;7:102-109.
9. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
10. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97-101.
11. Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24:1073-1079.
12. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences (10th Edition). Hoboken, NJ: John Wiley & Sons; 2013.
13. Zaiontz C. Real statistics using Excel. Available at: http://www.real-statistics.com/. Accessed February 20, 2018.
14. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
15. Villegas I, Arias IC, Botero A, et al. Evaluation of the technique used by health-care workers for taking blood pressure. Hypertension. 1995;26:1204-1206.
16. McKay DW, Campbell NR, Parab LS, et al. Clinical assessment of blood pressure. J Hum Hypertens. 1990;4:639-645.
17. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
18. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569-588.
19. Raamat R, Talts J, Jagomägi K, et al. Errors of oscillometric blood pressure measurement as predicted by simulation. Blood Press Monit. 2011;16:238-245.
Why you shouldn’t screen for ovarian cancer in asymptomatic women
Resources
- US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:588-594.
- Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:595-606.
Resources
- US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:588-594.
- Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:595-606.
Resources
- US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:588-594.
- Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:595-606.
David Henry's JCSO podcast, March-April 2018
In his bimonthly podcast, Dr David Henry, the JCSO Editor-in-Chief, discusses the approval of the biosimilars, bevacizumab-awwb and trastuzumab-dkst, and new therapies for virally associated cancers. Also in the line-up are an interview with Dr Daniel Haller on the latest advances in treating gastrointestinal cancers, and an article on hands-on advice on integrating survivorship care planning in a radiation oncology workflow. Research topics incude how to improve communication between oncology care providers and patient caregivers during hospice; the impact of patient education on enrollment in clinical trials; and organizational barriers to optimal lung cancer care in the community setting. A series of Case Reports that highlight some of the clincial challenges in treating patients with cancer round out the issue.
Listen to the podcast below
In his bimonthly podcast, Dr David Henry, the JCSO Editor-in-Chief, discusses the approval of the biosimilars, bevacizumab-awwb and trastuzumab-dkst, and new therapies for virally associated cancers. Also in the line-up are an interview with Dr Daniel Haller on the latest advances in treating gastrointestinal cancers, and an article on hands-on advice on integrating survivorship care planning in a radiation oncology workflow. Research topics incude how to improve communication between oncology care providers and patient caregivers during hospice; the impact of patient education on enrollment in clinical trials; and organizational barriers to optimal lung cancer care in the community setting. A series of Case Reports that highlight some of the clincial challenges in treating patients with cancer round out the issue.
Listen to the podcast below
In his bimonthly podcast, Dr David Henry, the JCSO Editor-in-Chief, discusses the approval of the biosimilars, bevacizumab-awwb and trastuzumab-dkst, and new therapies for virally associated cancers. Also in the line-up are an interview with Dr Daniel Haller on the latest advances in treating gastrointestinal cancers, and an article on hands-on advice on integrating survivorship care planning in a radiation oncology workflow. Research topics incude how to improve communication between oncology care providers and patient caregivers during hospice; the impact of patient education on enrollment in clinical trials; and organizational barriers to optimal lung cancer care in the community setting. A series of Case Reports that highlight some of the clincial challenges in treating patients with cancer round out the issue.
Listen to the podcast below
Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Click here to read the supplement
CME: Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Earn 1.25 CME Credits.
- Introduction and 2 Case Studies by Genie L. Bailey, MD
- Opioid Use Disorder: The Epidemic is Real by Kevin P. Hill, MD, MHS
- Managing the Opioid Use Disorder Crisis by Richard N. Rosenthal, MD
Click here to read the supplement
Click here to read the supplement
CME: Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Earn 1.25 CME Credits.
- Introduction and 2 Case Studies by Genie L. Bailey, MD
- Opioid Use Disorder: The Epidemic is Real by Kevin P. Hill, MD, MHS
- Managing the Opioid Use Disorder Crisis by Richard N. Rosenthal, MD
Click here to read the supplement
Click here to read the supplement
CME: Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Earn 1.25 CME Credits.
- Introduction and 2 Case Studies by Genie L. Bailey, MD
- Opioid Use Disorder: The Epidemic is Real by Kevin P. Hill, MD, MHS
- Managing the Opioid Use Disorder Crisis by Richard N. Rosenthal, MD