User login
Study: Natpara slightly boosts health-related QoL in hypoparathyroidism
A new industry-funded analysis suggests that recombinant human parathyroid hormone, an extraordinarily expensive treatment for hypoparathyroidism, produces slight improvement in some health-related quality of life (HRQoL) domains.
While researchers didn’t find any statistically significant between-group differences vs. a placebo, the study lead author said the positive findings about within-group differences reflect her experiences with some patients. “They’re telling me they feel much better, and they don’t have emergency room visits,” endocrinologist Tamara J. Vokes, MD, of the University of Chicago, said in an interview.
And, she said, as reflected in the findings, she’s seen that those with the lowest HRQoL levels at baseline especially show signs of improvement.
The treatment, known as rhPTH(1-84) or Natpara, was approved by the Food and Drug Administration as a treatment for hypoparathyroidism in 2015. The FDA stated that the drug “is only for people who do not respond well to treatment with calcium and active forms of vitamin D alone, because it may increase the possible risk of bone cancer, known as osteosarcoma.”
Pharmacies list the drug as costing $9,500-$9,900 per month with a coupon or discount. According to the new study, research has shown that quality of life is often impaired in patients who have tried the traditional hypoparathyroidism treatments of calcium supplements and vitamin D. Dr. Vokes and her colleagues aimed to expand upon previous studies of HRQoL that did not reach conclusions or failed to include controls.
They examined findings of a previous multinational, randomized, placebo-controlled study of 122 adults with hypoparathyroidism. Average age was 48 years, and roughly 80% of the patients were women.
After their serum calcium levels were adjusted through medication, the patients were randomly assigned to placebo (n = 39) or rhPTH(1-84) (n = 83, starting dose of 50 mg/d that could be raised to 100 mg/d).
The study, which appears in the Journal of Clinical Endocrinology and Metabolism, analyzes the changes in HRQoL from baseline to 24 weeks per the 36-Item Short-Form Health Survey (SF-36).
The researchers found no significant between-group differences. However, those who took the drug did see statistically significant improvements in 4 of 10 domains: physical component summary score (P = .004), body pain (P = .05), general health (P = .05), and vitality (P less than .001). The changes were small, with the vitality score improving the most, from a mean SF-36 score of 49.5 to 53.
In some cases, she said, she’s seen QoL improve in patients whom she normally wouldn’t consider candidates for the medication. “I would have not have recommended PTH for them, but they insisted on taking it, and they report feeling better.”
This may be a placebo effect, she said. Even so, “if someone doesn’t feel well, it’s worth it at least to try to use PTH and see whether they improve.”
She added that lack of well-being is a preexisting condition for some hypoparathyroidism patients. “I’ve seen quite a number of them who have what we call premorbid personality disorder. They didn’t feel well and weren’t happy, and when you get hypoparathyroidism, you’re more unwell and unhappy.”
With medication, however, “you’re a bit less unhappy but you’re still miserable,” she said.
Carol Greenlee, MD, an endocrinologist in Grand Junction, Colo., said in an interview that she saw a patient in a clinical study who had experienced a marked improvement in QoL. However, she said, “it will be the cost of the PTH that is the burden.”
For her part, Dr. Vokes cautioned that it’s important to take special care with patients taking Natpara. “You can’t just give this injection and say, ‘Goodbye, you will be better.’ It requires following certain protocol, frequent monitoring of the blood levels. Be sure the patient has access to the lab, and insurance that covers the test.”
Dr. Greenlee reported no relevant disclosures. The study was funded by Shire Human Genetic Therapies, and the initial clinical trial was funded by NPS Pharmaceuticals, a wholly owned indirect subsidiary. Dr. Vokes reported consulting for Shire and serving as an investigator for the initial clinical trial. Other study authors reported serving as clinical investigators and/or consulting for Shire, and three authors are employees of Shire.
SOURCE: Vokes, TJ et al. J Clin Endocrinol Metab. 2018 Feb 1;103(2):722-31
A new industry-funded analysis suggests that recombinant human parathyroid hormone, an extraordinarily expensive treatment for hypoparathyroidism, produces slight improvement in some health-related quality of life (HRQoL) domains.
While researchers didn’t find any statistically significant between-group differences vs. a placebo, the study lead author said the positive findings about within-group differences reflect her experiences with some patients. “They’re telling me they feel much better, and they don’t have emergency room visits,” endocrinologist Tamara J. Vokes, MD, of the University of Chicago, said in an interview.
And, she said, as reflected in the findings, she’s seen that those with the lowest HRQoL levels at baseline especially show signs of improvement.
The treatment, known as rhPTH(1-84) or Natpara, was approved by the Food and Drug Administration as a treatment for hypoparathyroidism in 2015. The FDA stated that the drug “is only for people who do not respond well to treatment with calcium and active forms of vitamin D alone, because it may increase the possible risk of bone cancer, known as osteosarcoma.”
Pharmacies list the drug as costing $9,500-$9,900 per month with a coupon or discount. According to the new study, research has shown that quality of life is often impaired in patients who have tried the traditional hypoparathyroidism treatments of calcium supplements and vitamin D. Dr. Vokes and her colleagues aimed to expand upon previous studies of HRQoL that did not reach conclusions or failed to include controls.
They examined findings of a previous multinational, randomized, placebo-controlled study of 122 adults with hypoparathyroidism. Average age was 48 years, and roughly 80% of the patients were women.
After their serum calcium levels were adjusted through medication, the patients were randomly assigned to placebo (n = 39) or rhPTH(1-84) (n = 83, starting dose of 50 mg/d that could be raised to 100 mg/d).
The study, which appears in the Journal of Clinical Endocrinology and Metabolism, analyzes the changes in HRQoL from baseline to 24 weeks per the 36-Item Short-Form Health Survey (SF-36).
The researchers found no significant between-group differences. However, those who took the drug did see statistically significant improvements in 4 of 10 domains: physical component summary score (P = .004), body pain (P = .05), general health (P = .05), and vitality (P less than .001). The changes were small, with the vitality score improving the most, from a mean SF-36 score of 49.5 to 53.
In some cases, she said, she’s seen QoL improve in patients whom she normally wouldn’t consider candidates for the medication. “I would have not have recommended PTH for them, but they insisted on taking it, and they report feeling better.”
This may be a placebo effect, she said. Even so, “if someone doesn’t feel well, it’s worth it at least to try to use PTH and see whether they improve.”
She added that lack of well-being is a preexisting condition for some hypoparathyroidism patients. “I’ve seen quite a number of them who have what we call premorbid personality disorder. They didn’t feel well and weren’t happy, and when you get hypoparathyroidism, you’re more unwell and unhappy.”
With medication, however, “you’re a bit less unhappy but you’re still miserable,” she said.
Carol Greenlee, MD, an endocrinologist in Grand Junction, Colo., said in an interview that she saw a patient in a clinical study who had experienced a marked improvement in QoL. However, she said, “it will be the cost of the PTH that is the burden.”
For her part, Dr. Vokes cautioned that it’s important to take special care with patients taking Natpara. “You can’t just give this injection and say, ‘Goodbye, you will be better.’ It requires following certain protocol, frequent monitoring of the blood levels. Be sure the patient has access to the lab, and insurance that covers the test.”
Dr. Greenlee reported no relevant disclosures. The study was funded by Shire Human Genetic Therapies, and the initial clinical trial was funded by NPS Pharmaceuticals, a wholly owned indirect subsidiary. Dr. Vokes reported consulting for Shire and serving as an investigator for the initial clinical trial. Other study authors reported serving as clinical investigators and/or consulting for Shire, and three authors are employees of Shire.
SOURCE: Vokes, TJ et al. J Clin Endocrinol Metab. 2018 Feb 1;103(2):722-31
A new industry-funded analysis suggests that recombinant human parathyroid hormone, an extraordinarily expensive treatment for hypoparathyroidism, produces slight improvement in some health-related quality of life (HRQoL) domains.
While researchers didn’t find any statistically significant between-group differences vs. a placebo, the study lead author said the positive findings about within-group differences reflect her experiences with some patients. “They’re telling me they feel much better, and they don’t have emergency room visits,” endocrinologist Tamara J. Vokes, MD, of the University of Chicago, said in an interview.
And, she said, as reflected in the findings, she’s seen that those with the lowest HRQoL levels at baseline especially show signs of improvement.
The treatment, known as rhPTH(1-84) or Natpara, was approved by the Food and Drug Administration as a treatment for hypoparathyroidism in 2015. The FDA stated that the drug “is only for people who do not respond well to treatment with calcium and active forms of vitamin D alone, because it may increase the possible risk of bone cancer, known as osteosarcoma.”
Pharmacies list the drug as costing $9,500-$9,900 per month with a coupon or discount. According to the new study, research has shown that quality of life is often impaired in patients who have tried the traditional hypoparathyroidism treatments of calcium supplements and vitamin D. Dr. Vokes and her colleagues aimed to expand upon previous studies of HRQoL that did not reach conclusions or failed to include controls.
They examined findings of a previous multinational, randomized, placebo-controlled study of 122 adults with hypoparathyroidism. Average age was 48 years, and roughly 80% of the patients were women.
After their serum calcium levels were adjusted through medication, the patients were randomly assigned to placebo (n = 39) or rhPTH(1-84) (n = 83, starting dose of 50 mg/d that could be raised to 100 mg/d).
The study, which appears in the Journal of Clinical Endocrinology and Metabolism, analyzes the changes in HRQoL from baseline to 24 weeks per the 36-Item Short-Form Health Survey (SF-36).
The researchers found no significant between-group differences. However, those who took the drug did see statistically significant improvements in 4 of 10 domains: physical component summary score (P = .004), body pain (P = .05), general health (P = .05), and vitality (P less than .001). The changes were small, with the vitality score improving the most, from a mean SF-36 score of 49.5 to 53.
In some cases, she said, she’s seen QoL improve in patients whom she normally wouldn’t consider candidates for the medication. “I would have not have recommended PTH for them, but they insisted on taking it, and they report feeling better.”
This may be a placebo effect, she said. Even so, “if someone doesn’t feel well, it’s worth it at least to try to use PTH and see whether they improve.”
She added that lack of well-being is a preexisting condition for some hypoparathyroidism patients. “I’ve seen quite a number of them who have what we call premorbid personality disorder. They didn’t feel well and weren’t happy, and when you get hypoparathyroidism, you’re more unwell and unhappy.”
With medication, however, “you’re a bit less unhappy but you’re still miserable,” she said.
Carol Greenlee, MD, an endocrinologist in Grand Junction, Colo., said in an interview that she saw a patient in a clinical study who had experienced a marked improvement in QoL. However, she said, “it will be the cost of the PTH that is the burden.”
For her part, Dr. Vokes cautioned that it’s important to take special care with patients taking Natpara. “You can’t just give this injection and say, ‘Goodbye, you will be better.’ It requires following certain protocol, frequent monitoring of the blood levels. Be sure the patient has access to the lab, and insurance that covers the test.”
Dr. Greenlee reported no relevant disclosures. The study was funded by Shire Human Genetic Therapies, and the initial clinical trial was funded by NPS Pharmaceuticals, a wholly owned indirect subsidiary. Dr. Vokes reported consulting for Shire and serving as an investigator for the initial clinical trial. Other study authors reported serving as clinical investigators and/or consulting for Shire, and three authors are employees of Shire.
SOURCE: Vokes, TJ et al. J Clin Endocrinol Metab. 2018 Feb 1;103(2):722-31
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: Health-related quality of life (HRQoL) may improve slightly in patients on recombinant human parathyroid hormone for hypoparathyroidism.
Major finding: In 4 of 10 SF-36 domains, HRQoL improved in within-group analysis. There was no statistically significant improvement vs. placebo.
Study details: A 24-week analysis of previous drug vs. placebo clinical trial of 122 adults with hypoparathyroidism.
Disclosures: Shire Human Genetic Therapies funded the study, and the initial clinical trial was funded by NPS Pharmaceuticals, a wholly owned indirect subsidiary. The researchers reported various relationships to Shire, including employment.
Source: Vokes TJ et al. J Clin Endocrinol Metab. 2018 Feb 1;103(2):722-31.
Evaluations of Medicaid experiments by states, CMS are weak, GAO says
With federal spending on Medicaid experiments soaring in recent years, a congressional watchdog said state and federal governments fail to adequately evaluate if the efforts improve care and save money.
A study by the Government Accountability Office released Feb. 20 found that some states don’t complete evaluation reports for up to 7 years after an experiment begins and often fail to answer vital questions to determine effectiveness. The GAO also slammed the federal Centers for Medicare & Medicaid Services for failing to make results from Medicaid evaluation reports public in a timely manner.
“CMS is missing an opportunity to inform federal and state policy discussions,” the GAO report said.
Joan Alker, executive director of the Georgetown University Center for Children and Families, called the report’s findings “troubling but not surprising.”
“It has been clear for some time that evaluations of Section 1115 waivers are not adequate,” she said. “There is some good work going on in this space at the state level, for example in Michigan and Iowa, but as the report makes clear state’s evaluations are often incomplete and not rigorous enough.”
These experiments are often called “demonstration projects” or “1115 demonstration waivers” – based on the section of the law that allows the federal government to authorize them. They allow federal officials to approve states’ requests to test new approaches to providing coverage. They are used for a wide variety of purposes, including efforts to extend Medicaid to people or services not generally covered or to change payment systems to improve care.
Medicaid demonstration programs often run for a decade or more. Several states that expanded Medicaid eligibility under the Affordable Care Act did so through a demonstration program, including Indiana, Iowa, Arkansas and New Hampshire.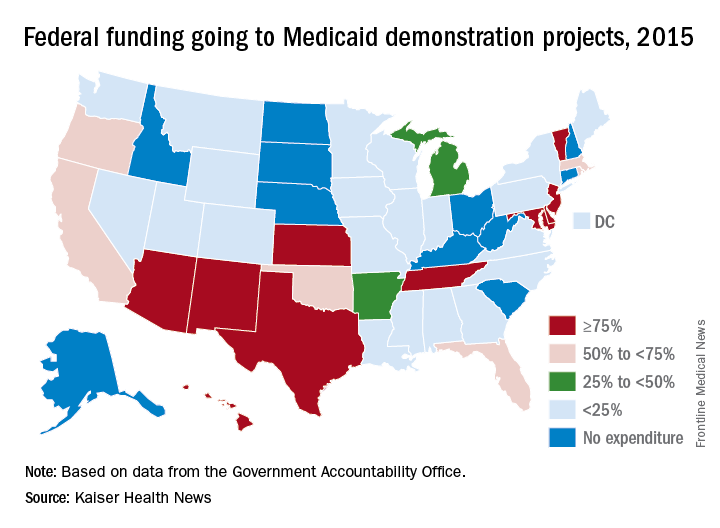
The study, requested by top GOP lawmakers including Sen. Orrin Hatch (R-Utah), reviewed demonstration programs in eight states – Arizona, Arkansas, California, Indiana, Kansas, Maryland, Massachusetts, and New York.
In five of these states, money from their Medicaid demonstration program makes up more than half their total federal Medicaid budgets. Nearly all of Arizona’s funding – 99.7% – is through a demonstration program.
The use of Medicaid demonstration programs accelerated during the 1990s. But, in recent years, the experiments often have reflected the political leanings of state officials or the party controlling the White House. Under a demonstration program, the Trump administration this year approved requests from Indiana and Kentucky to enact work requirements for some adult Medicaid enrollees.
The GAO report noted that states often do not complete their evaluation reports until after the federal government renews their demonstration program. For example, Indiana’s Medicaid expansion demonstration program, which charges premiums and locks some enrollees out of coverage for lack of payment, was renewed in February even though a final evaluation report is not yet complete.
GAO said Indiana’s evaluation of its Medicaid expansion won’t look at the effect of the state’s provision that locks out enrollees for six months if they fail to pay premiums.
“GAO found that selected states’ evaluations of these demonstrations often had significant limitations that affected their usefulness in informing policy decisions,” the report said.
Ms. Alker said that “more sunshine and data are needed” to assess waivers, “especially as they are clearly the vehicle the Trump administration is now using to pursue its ideological objectives for Medicaid.”
While states typically contract with independent groups to evaluate Medicaid demonstration programs, the federal government sometimes does its own review.
But the GAO investigators found Indiana’s Medicaid agency wasn’t willing to work with the federal contractor out of privacy concerns, which halted efforts for a federal review.
Joel Cantor, director of the Center for State Health Policy at Rutgers University in New Brunswick, N.J., said the demonstration programs often have shifted from their intended purpose because they are designed by lawmakers pushing an agenda rather than as a scientific experiment to find better ways to deliver care.
“Demonstration programs have been used since the 1990s to advance policy agenda for whoever holds power in Washington and not designed to test an innovative idea,” he said.
The evaluations often take several years to complete, he said, because of the difficulty of getting patient data from states. His center has done evaluations for New Jersey’s Medicaid program.
GAO recommended that CMS require states to submit a final evaluation report after the end of the waiver period, regardless of whether the experiment is being renewed, and that the federal agency publicly release findings from federal evaluations in a timely manner. Federal officials said they agreed with the recommendations.
Matt Salo, executive director of the National Association of Medicaid Directors, said the report highlighted a need to modernize the law dealing with Medicaid so that successful experiments are quickly incorporated into the overall program.
“The underlying problem is that the Medicaid statute has fundamentally failed to keep up with the changing reality of health care in the 21st century,” he said. “There’s no way to update the rules to make these changes” a permanent part of the program.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
With federal spending on Medicaid experiments soaring in recent years, a congressional watchdog said state and federal governments fail to adequately evaluate if the efforts improve care and save money.
A study by the Government Accountability Office released Feb. 20 found that some states don’t complete evaluation reports for up to 7 years after an experiment begins and often fail to answer vital questions to determine effectiveness. The GAO also slammed the federal Centers for Medicare & Medicaid Services for failing to make results from Medicaid evaluation reports public in a timely manner.
“CMS is missing an opportunity to inform federal and state policy discussions,” the GAO report said.
Joan Alker, executive director of the Georgetown University Center for Children and Families, called the report’s findings “troubling but not surprising.”
“It has been clear for some time that evaluations of Section 1115 waivers are not adequate,” she said. “There is some good work going on in this space at the state level, for example in Michigan and Iowa, but as the report makes clear state’s evaluations are often incomplete and not rigorous enough.”
These experiments are often called “demonstration projects” or “1115 demonstration waivers” – based on the section of the law that allows the federal government to authorize them. They allow federal officials to approve states’ requests to test new approaches to providing coverage. They are used for a wide variety of purposes, including efforts to extend Medicaid to people or services not generally covered or to change payment systems to improve care.
Medicaid demonstration programs often run for a decade or more. Several states that expanded Medicaid eligibility under the Affordable Care Act did so through a demonstration program, including Indiana, Iowa, Arkansas and New Hampshire.
The study, requested by top GOP lawmakers including Sen. Orrin Hatch (R-Utah), reviewed demonstration programs in eight states – Arizona, Arkansas, California, Indiana, Kansas, Maryland, Massachusetts, and New York.
In five of these states, money from their Medicaid demonstration program makes up more than half their total federal Medicaid budgets. Nearly all of Arizona’s funding – 99.7% – is through a demonstration program.
The use of Medicaid demonstration programs accelerated during the 1990s. But, in recent years, the experiments often have reflected the political leanings of state officials or the party controlling the White House. Under a demonstration program, the Trump administration this year approved requests from Indiana and Kentucky to enact work requirements for some adult Medicaid enrollees.
The GAO report noted that states often do not complete their evaluation reports until after the federal government renews their demonstration program. For example, Indiana’s Medicaid expansion demonstration program, which charges premiums and locks some enrollees out of coverage for lack of payment, was renewed in February even though a final evaluation report is not yet complete.
GAO said Indiana’s evaluation of its Medicaid expansion won’t look at the effect of the state’s provision that locks out enrollees for six months if they fail to pay premiums.
“GAO found that selected states’ evaluations of these demonstrations often had significant limitations that affected their usefulness in informing policy decisions,” the report said.
Ms. Alker said that “more sunshine and data are needed” to assess waivers, “especially as they are clearly the vehicle the Trump administration is now using to pursue its ideological objectives for Medicaid.”
While states typically contract with independent groups to evaluate Medicaid demonstration programs, the federal government sometimes does its own review.
But the GAO investigators found Indiana’s Medicaid agency wasn’t willing to work with the federal contractor out of privacy concerns, which halted efforts for a federal review.
Joel Cantor, director of the Center for State Health Policy at Rutgers University in New Brunswick, N.J., said the demonstration programs often have shifted from their intended purpose because they are designed by lawmakers pushing an agenda rather than as a scientific experiment to find better ways to deliver care.
“Demonstration programs have been used since the 1990s to advance policy agenda for whoever holds power in Washington and not designed to test an innovative idea,” he said.
The evaluations often take several years to complete, he said, because of the difficulty of getting patient data from states. His center has done evaluations for New Jersey’s Medicaid program.
GAO recommended that CMS require states to submit a final evaluation report after the end of the waiver period, regardless of whether the experiment is being renewed, and that the federal agency publicly release findings from federal evaluations in a timely manner. Federal officials said they agreed with the recommendations.
Matt Salo, executive director of the National Association of Medicaid Directors, said the report highlighted a need to modernize the law dealing with Medicaid so that successful experiments are quickly incorporated into the overall program.
“The underlying problem is that the Medicaid statute has fundamentally failed to keep up with the changing reality of health care in the 21st century,” he said. “There’s no way to update the rules to make these changes” a permanent part of the program.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
With federal spending on Medicaid experiments soaring in recent years, a congressional watchdog said state and federal governments fail to adequately evaluate if the efforts improve care and save money.
A study by the Government Accountability Office released Feb. 20 found that some states don’t complete evaluation reports for up to 7 years after an experiment begins and often fail to answer vital questions to determine effectiveness. The GAO also slammed the federal Centers for Medicare & Medicaid Services for failing to make results from Medicaid evaluation reports public in a timely manner.
“CMS is missing an opportunity to inform federal and state policy discussions,” the GAO report said.
Joan Alker, executive director of the Georgetown University Center for Children and Families, called the report’s findings “troubling but not surprising.”
“It has been clear for some time that evaluations of Section 1115 waivers are not adequate,” she said. “There is some good work going on in this space at the state level, for example in Michigan and Iowa, but as the report makes clear state’s evaluations are often incomplete and not rigorous enough.”
These experiments are often called “demonstration projects” or “1115 demonstration waivers” – based on the section of the law that allows the federal government to authorize them. They allow federal officials to approve states’ requests to test new approaches to providing coverage. They are used for a wide variety of purposes, including efforts to extend Medicaid to people or services not generally covered or to change payment systems to improve care.
Medicaid demonstration programs often run for a decade or more. Several states that expanded Medicaid eligibility under the Affordable Care Act did so through a demonstration program, including Indiana, Iowa, Arkansas and New Hampshire.
The study, requested by top GOP lawmakers including Sen. Orrin Hatch (R-Utah), reviewed demonstration programs in eight states – Arizona, Arkansas, California, Indiana, Kansas, Maryland, Massachusetts, and New York.
In five of these states, money from their Medicaid demonstration program makes up more than half their total federal Medicaid budgets. Nearly all of Arizona’s funding – 99.7% – is through a demonstration program.
The use of Medicaid demonstration programs accelerated during the 1990s. But, in recent years, the experiments often have reflected the political leanings of state officials or the party controlling the White House. Under a demonstration program, the Trump administration this year approved requests from Indiana and Kentucky to enact work requirements for some adult Medicaid enrollees.
The GAO report noted that states often do not complete their evaluation reports until after the federal government renews their demonstration program. For example, Indiana’s Medicaid expansion demonstration program, which charges premiums and locks some enrollees out of coverage for lack of payment, was renewed in February even though a final evaluation report is not yet complete.
GAO said Indiana’s evaluation of its Medicaid expansion won’t look at the effect of the state’s provision that locks out enrollees for six months if they fail to pay premiums.
“GAO found that selected states’ evaluations of these demonstrations often had significant limitations that affected their usefulness in informing policy decisions,” the report said.
Ms. Alker said that “more sunshine and data are needed” to assess waivers, “especially as they are clearly the vehicle the Trump administration is now using to pursue its ideological objectives for Medicaid.”
While states typically contract with independent groups to evaluate Medicaid demonstration programs, the federal government sometimes does its own review.
But the GAO investigators found Indiana’s Medicaid agency wasn’t willing to work with the federal contractor out of privacy concerns, which halted efforts for a federal review.
Joel Cantor, director of the Center for State Health Policy at Rutgers University in New Brunswick, N.J., said the demonstration programs often have shifted from their intended purpose because they are designed by lawmakers pushing an agenda rather than as a scientific experiment to find better ways to deliver care.
“Demonstration programs have been used since the 1990s to advance policy agenda for whoever holds power in Washington and not designed to test an innovative idea,” he said.
The evaluations often take several years to complete, he said, because of the difficulty of getting patient data from states. His center has done evaluations for New Jersey’s Medicaid program.
GAO recommended that CMS require states to submit a final evaluation report after the end of the waiver period, regardless of whether the experiment is being renewed, and that the federal agency publicly release findings from federal evaluations in a timely manner. Federal officials said they agreed with the recommendations.
Matt Salo, executive director of the National Association of Medicaid Directors, said the report highlighted a need to modernize the law dealing with Medicaid so that successful experiments are quickly incorporated into the overall program.
“The underlying problem is that the Medicaid statute has fundamentally failed to keep up with the changing reality of health care in the 21st century,” he said. “There’s no way to update the rules to make these changes” a permanent part of the program.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
FDA warns against clarithromycin use in patients with heart disease
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
25 Years of Movement Disorders
Peter A. LeWitt, MD
Dr. LeWitt is the Director of the Parkinson Disease and Movement Disorder Program at Henry Ford Hospital, West Bloomfield, Michigan.
For most neurology subspecialties, the past quarter-century was only the latest chapter of a productive history extending through the mid-19th century, but for movement disorders, these years were formative. Before then, movement disorder specialists were few in number, and their medical literature was far more scanty than it is now, with multiple journals and texts now devoted to this topic. Twenty-five years ago, the international society devoted to Parkinson disease and other movement disorders was only a recent arrival. Fortunately, two charismatic and brilliant leaders—the American neurologist Stanley Fahn, MD, and the British neurologist C. David Marsden, MBBS—came forward to inspire a generation of trainees with their broad interests in bridging clinical neurology, experimental neuroscience, and patient advocacy. Today, the impact of these pioneers is still felt by a growing community of movement disorder clinicians and researchers who have transformed movement disorders from an earlier realm of descriptive neurology into a vibrant field exploring disease mechanisms and therapeutics, as well as an expanding array of clinical insights.
When Neurology Reviews was founded in 1993, the majority of significant developments in movement disorders concerned Parkinson’s disease. Strategies to improve upon dopaminergic therapy were being tested in clinical trials, along with ventures into pallidotomy as a neurosurgical treatment. Animal models of parkinsonism and neurochemical analysis of brain and putative biomarkers provided scientific guidance for trials of disease modification. Another milestone from the 1990s was discovery of the first gene leading to autosomal-dominant parkinsonism. This rare mutation in the structural protein alpha-synuclein had a much broader impact on Parkinson’s disease research in that it provided important information about the sporadic disorder’s final common pathway of pathogenesis. Today, alpha-synuclein is the focus of therapeutic intervention utilizing immunologic therapies to halt disease progression. Over the past quarter-century, more than two dozen additional gene mutations associated with parkinsonism have been discovered. These mutations provide additional insight into disease mechanisms. Beyond the science and clinical insights that have propelled advances in understanding Parkinson’s disease and its related disorders, the growth of the patient advocacy and caregiver movement has also been remarkable in expanding what is needed to improve daily life with this disorder.
Since 1993, other movement disorders have also profited from the tremendous growth of molecular neuroscience and its applications. Novel neuroimaging techniques like functional MRI, computation of neural pathways, and scanning using PET and SPECT have helped work out the brain mechanisms of several disorders. Genetic probing of familial disorders like dystonia and myoclonus have helped to unravel the neurochemistry of what has gone wrong to cause certain movement disorders. In turn, transgenic animal models using these human genetic mutations have allowed exploration of what might be done to fix the problems. These developments could have huge implications for making progress in the therapies of the future, since the most common categories of movement disorders—dystonia, myoclonus, ataxia, tremor, restless legs syndrome, Tourette syndrome, choreic disorders, and paroxysmal movement disorders—all can have hereditary patterns. Genetic testing can be valuable for choosing at-risk individuals for participation in trials of disease-modifying drugs for Huntington’s disease, for example, and this strategy may be the way of the future for other late-life emergent disorders. In the past decade, several gene therapy studies have been carried out in patients with Parkinson’s disease. The promise of this approach and the recent gene editing and RNA interference approaches, all part of the molecular biology revolution of the past 25 years, may further change the landscape of movement disorder therapeutics ahead.
Today, movement disorder specialists routinely use therapeutic approaches that were in their infancy in 1993. Botulinum toxin selective denervation has had a major impact by improving quality of life for patients affected with a wide range of movement disorders: dystonic necks, hands, and feet; involuntary facial movements and voice aberrations; tics; spasticity; and drooling. Deep brain stimulation is a routine option for many patients with tremor, Parkinson’s disease, and dystonia. Developments in this field have refined the choice of targets for stimulation and the possibility of treating other disorders with these techniques, like Tourette syndrome. A nonsurgical approach to abolishing tremor, MRI-guided focused ultrasound, recently was approved. In the background of these advances is the increasing knowledge of movement disorders that practitioners have to offer many patients with disorders that in the past were merely neurologic curiosities. Through clinical trial research and serendipity (the major treatment options for essential tremor arose this way), there is an expanding search for repurposing older medications and harnessing new biotechnology to target the mechanisms leading to various movement disorders.
It is impressive how much the neurologic discipline of movement disorders has changed in response to the new era of electronic communications and other technologies. In the past quarter-century, medical students, residents, and practicing neurologists have had instant access to a curriculum that includes resources such as video-recorded movement disorders. Clinical trials are being revolutionized by electronic technologies that record information in ways that improve upon human ratings. A patient with Parkinson’s disease, essential tremor, or restless legs syndrome can now have symptoms monitored remotely by wearable devices that offer a real-world view on how a new therapy is performing. But perhaps the greatest revolution in the recent past has been increasing confidence that progressive disabilities caused by movement disorders might be halted by emerging insights into disease mechanisms. There has been tremendous growth of interest among the basic neuroscience community in human diseases affecting motor control. More than half of research presentations at recent international Parkinson’s disease and movement disorder conferences reflect this trend. These efforts support an optimistic view that, over the nex
Peter A. LeWitt, MD
Dr. LeWitt is the Director of the Parkinson Disease and Movement Disorder Program at Henry Ford Hospital, West Bloomfield, Michigan.
For most neurology subspecialties, the past quarter-century was only the latest chapter of a productive history extending through the mid-19th century, but for movement disorders, these years were formative. Before then, movement disorder specialists were few in number, and their medical literature was far more scanty than it is now, with multiple journals and texts now devoted to this topic. Twenty-five years ago, the international society devoted to Parkinson disease and other movement disorders was only a recent arrival. Fortunately, two charismatic and brilliant leaders—the American neurologist Stanley Fahn, MD, and the British neurologist C. David Marsden, MBBS—came forward to inspire a generation of trainees with their broad interests in bridging clinical neurology, experimental neuroscience, and patient advocacy. Today, the impact of these pioneers is still felt by a growing community of movement disorder clinicians and researchers who have transformed movement disorders from an earlier realm of descriptive neurology into a vibrant field exploring disease mechanisms and therapeutics, as well as an expanding array of clinical insights.
When Neurology Reviews was founded in 1993, the majority of significant developments in movement disorders concerned Parkinson’s disease. Strategies to improve upon dopaminergic therapy were being tested in clinical trials, along with ventures into pallidotomy as a neurosurgical treatment. Animal models of parkinsonism and neurochemical analysis of brain and putative biomarkers provided scientific guidance for trials of disease modification. Another milestone from the 1990s was discovery of the first gene leading to autosomal-dominant parkinsonism. This rare mutation in the structural protein alpha-synuclein had a much broader impact on Parkinson’s disease research in that it provided important information about the sporadic disorder’s final common pathway of pathogenesis. Today, alpha-synuclein is the focus of therapeutic intervention utilizing immunologic therapies to halt disease progression. Over the past quarter-century, more than two dozen additional gene mutations associated with parkinsonism have been discovered. These mutations provide additional insight into disease mechanisms. Beyond the science and clinical insights that have propelled advances in understanding Parkinson’s disease and its related disorders, the growth of the patient advocacy and caregiver movement has also been remarkable in expanding what is needed to improve daily life with this disorder.
Since 1993, other movement disorders have also profited from the tremendous growth of molecular neuroscience and its applications. Novel neuroimaging techniques like functional MRI, computation of neural pathways, and scanning using PET and SPECT have helped work out the brain mechanisms of several disorders. Genetic probing of familial disorders like dystonia and myoclonus have helped to unravel the neurochemistry of what has gone wrong to cause certain movement disorders. In turn, transgenic animal models using these human genetic mutations have allowed exploration of what might be done to fix the problems. These developments could have huge implications for making progress in the therapies of the future, since the most common categories of movement disorders—dystonia, myoclonus, ataxia, tremor, restless legs syndrome, Tourette syndrome, choreic disorders, and paroxysmal movement disorders—all can have hereditary patterns. Genetic testing can be valuable for choosing at-risk individuals for participation in trials of disease-modifying drugs for Huntington’s disease, for example, and this strategy may be the way of the future for other late-life emergent disorders. In the past decade, several gene therapy studies have been carried out in patients with Parkinson’s disease. The promise of this approach and the recent gene editing and RNA interference approaches, all part of the molecular biology revolution of the past 25 years, may further change the landscape of movement disorder therapeutics ahead.
Today, movement disorder specialists routinely use therapeutic approaches that were in their infancy in 1993. Botulinum toxin selective denervation has had a major impact by improving quality of life for patients affected with a wide range of movement disorders: dystonic necks, hands, and feet; involuntary facial movements and voice aberrations; tics; spasticity; and drooling. Deep brain stimulation is a routine option for many patients with tremor, Parkinson’s disease, and dystonia. Developments in this field have refined the choice of targets for stimulation and the possibility of treating other disorders with these techniques, like Tourette syndrome. A nonsurgical approach to abolishing tremor, MRI-guided focused ultrasound, recently was approved. In the background of these advances is the increasing knowledge of movement disorders that practitioners have to offer many patients with disorders that in the past were merely neurologic curiosities. Through clinical trial research and serendipity (the major treatment options for essential tremor arose this way), there is an expanding search for repurposing older medications and harnessing new biotechnology to target the mechanisms leading to various movement disorders.
It is impressive how much the neurologic discipline of movement disorders has changed in response to the new era of electronic communications and other technologies. In the past quarter-century, medical students, residents, and practicing neurologists have had instant access to a curriculum that includes resources such as video-recorded movement disorders. Clinical trials are being revolutionized by electronic technologies that record information in ways that improve upon human ratings. A patient with Parkinson’s disease, essential tremor, or restless legs syndrome can now have symptoms monitored remotely by wearable devices that offer a real-world view on how a new therapy is performing. But perhaps the greatest revolution in the recent past has been increasing confidence that progressive disabilities caused by movement disorders might be halted by emerging insights into disease mechanisms. There has been tremendous growth of interest among the basic neuroscience community in human diseases affecting motor control. More than half of research presentations at recent international Parkinson’s disease and movement disorder conferences reflect this trend. These efforts support an optimistic view that, over the nex
Peter A. LeWitt, MD
Dr. LeWitt is the Director of the Parkinson Disease and Movement Disorder Program at Henry Ford Hospital, West Bloomfield, Michigan.
For most neurology subspecialties, the past quarter-century was only the latest chapter of a productive history extending through the mid-19th century, but for movement disorders, these years were formative. Before then, movement disorder specialists were few in number, and their medical literature was far more scanty than it is now, with multiple journals and texts now devoted to this topic. Twenty-five years ago, the international society devoted to Parkinson disease and other movement disorders was only a recent arrival. Fortunately, two charismatic and brilliant leaders—the American neurologist Stanley Fahn, MD, and the British neurologist C. David Marsden, MBBS—came forward to inspire a generation of trainees with their broad interests in bridging clinical neurology, experimental neuroscience, and patient advocacy. Today, the impact of these pioneers is still felt by a growing community of movement disorder clinicians and researchers who have transformed movement disorders from an earlier realm of descriptive neurology into a vibrant field exploring disease mechanisms and therapeutics, as well as an expanding array of clinical insights.
When Neurology Reviews was founded in 1993, the majority of significant developments in movement disorders concerned Parkinson’s disease. Strategies to improve upon dopaminergic therapy were being tested in clinical trials, along with ventures into pallidotomy as a neurosurgical treatment. Animal models of parkinsonism and neurochemical analysis of brain and putative biomarkers provided scientific guidance for trials of disease modification. Another milestone from the 1990s was discovery of the first gene leading to autosomal-dominant parkinsonism. This rare mutation in the structural protein alpha-synuclein had a much broader impact on Parkinson’s disease research in that it provided important information about the sporadic disorder’s final common pathway of pathogenesis. Today, alpha-synuclein is the focus of therapeutic intervention utilizing immunologic therapies to halt disease progression. Over the past quarter-century, more than two dozen additional gene mutations associated with parkinsonism have been discovered. These mutations provide additional insight into disease mechanisms. Beyond the science and clinical insights that have propelled advances in understanding Parkinson’s disease and its related disorders, the growth of the patient advocacy and caregiver movement has also been remarkable in expanding what is needed to improve daily life with this disorder.
Since 1993, other movement disorders have also profited from the tremendous growth of molecular neuroscience and its applications. Novel neuroimaging techniques like functional MRI, computation of neural pathways, and scanning using PET and SPECT have helped work out the brain mechanisms of several disorders. Genetic probing of familial disorders like dystonia and myoclonus have helped to unravel the neurochemistry of what has gone wrong to cause certain movement disorders. In turn, transgenic animal models using these human genetic mutations have allowed exploration of what might be done to fix the problems. These developments could have huge implications for making progress in the therapies of the future, since the most common categories of movement disorders—dystonia, myoclonus, ataxia, tremor, restless legs syndrome, Tourette syndrome, choreic disorders, and paroxysmal movement disorders—all can have hereditary patterns. Genetic testing can be valuable for choosing at-risk individuals for participation in trials of disease-modifying drugs for Huntington’s disease, for example, and this strategy may be the way of the future for other late-life emergent disorders. In the past decade, several gene therapy studies have been carried out in patients with Parkinson’s disease. The promise of this approach and the recent gene editing and RNA interference approaches, all part of the molecular biology revolution of the past 25 years, may further change the landscape of movement disorder therapeutics ahead.
Today, movement disorder specialists routinely use therapeutic approaches that were in their infancy in 1993. Botulinum toxin selective denervation has had a major impact by improving quality of life for patients affected with a wide range of movement disorders: dystonic necks, hands, and feet; involuntary facial movements and voice aberrations; tics; spasticity; and drooling. Deep brain stimulation is a routine option for many patients with tremor, Parkinson’s disease, and dystonia. Developments in this field have refined the choice of targets for stimulation and the possibility of treating other disorders with these techniques, like Tourette syndrome. A nonsurgical approach to abolishing tremor, MRI-guided focused ultrasound, recently was approved. In the background of these advances is the increasing knowledge of movement disorders that practitioners have to offer many patients with disorders that in the past were merely neurologic curiosities. Through clinical trial research and serendipity (the major treatment options for essential tremor arose this way), there is an expanding search for repurposing older medications and harnessing new biotechnology to target the mechanisms leading to various movement disorders.
It is impressive how much the neurologic discipline of movement disorders has changed in response to the new era of electronic communications and other technologies. In the past quarter-century, medical students, residents, and practicing neurologists have had instant access to a curriculum that includes resources such as video-recorded movement disorders. Clinical trials are being revolutionized by electronic technologies that record information in ways that improve upon human ratings. A patient with Parkinson’s disease, essential tremor, or restless legs syndrome can now have symptoms monitored remotely by wearable devices that offer a real-world view on how a new therapy is performing. But perhaps the greatest revolution in the recent past has been increasing confidence that progressive disabilities caused by movement disorders might be halted by emerging insights into disease mechanisms. There has been tremendous growth of interest among the basic neuroscience community in human diseases affecting motor control. More than half of research presentations at recent international Parkinson’s disease and movement disorder conferences reflect this trend. These efforts support an optimistic view that, over the nex
PFO closure reduces the risk of recurrent stroke compared to antiplatelet therapy alone
Background: Previous research on the use of PFO closure to prevent recurrent stroke has yielded mixed results.
Study design: Gore REDUCE, CLOSE, and RESPECT were all multicenter, randomized, open-label superiority trials, with blinded adjudication of endpoint events. RESPECT data reflected an exploratory analysis of an extended follow up period.
Setting: Gore REDUCE was a multinational study conducted at 63 sites in Europe and North America, from 2008-2015. CLOSE was conducted at 34 sites in France and Germany, from 2007 to 2016. RESPECT was conducted at 69 sites in the United States and Canada, from 2003 to 2011.
Synopsis: Three trials reexamined the impact of PFO closure with standard antiplatelet treatment, with a total of 2,307 patients between the ages of 16 and 60 years. CLOSE included only patients with a PFO and an associated atrial septal aneurysm or a large interatrial shunt. Gore REDUCE and RESPECT were both industry funded. All three trials found a statistically significant reduction in risk of recurrent ischemic stroke associated with PFO closure and antiplatelet therapy compared to antiplatelet therapy alone (CLOSE HR 0.03 [95% CI 0-0.26; P less than .001], RESPECT HR 0.55 [95% CI 0.31-0.999; P = .046], Gore REDUCE HR 0.23 [95% CI 0.09-0.62; P = .002]). Gore REDUCE and CLOSE identified increased rates of postprocedural atrial fibrillation or flutter (6.6% vs. 0.4% [P less than .001], 4.6% vs. 0.9% [P = .02], respectively). Serious adverse events related to the procedure or device ranged from 3.9-5.9%.
Bottom line: PFO closure combined with antiplatelet therapy in patients aged 60 years or younger, particularly in those with significant right-to-left shunts and atrial septal aneurysms, reduced the risk of recurrent ischemic stroke compared to antiplatelet therapy alone.
Citation: Mas JL. Derumeaux B. Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-21.
Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-32.
Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-42.
Background: Previous research on the use of PFO closure to prevent recurrent stroke has yielded mixed results.
Study design: Gore REDUCE, CLOSE, and RESPECT were all multicenter, randomized, open-label superiority trials, with blinded adjudication of endpoint events. RESPECT data reflected an exploratory analysis of an extended follow up period.
Setting: Gore REDUCE was a multinational study conducted at 63 sites in Europe and North America, from 2008-2015. CLOSE was conducted at 34 sites in France and Germany, from 2007 to 2016. RESPECT was conducted at 69 sites in the United States and Canada, from 2003 to 2011.
Synopsis: Three trials reexamined the impact of PFO closure with standard antiplatelet treatment, with a total of 2,307 patients between the ages of 16 and 60 years. CLOSE included only patients with a PFO and an associated atrial septal aneurysm or a large interatrial shunt. Gore REDUCE and RESPECT were both industry funded. All three trials found a statistically significant reduction in risk of recurrent ischemic stroke associated with PFO closure and antiplatelet therapy compared to antiplatelet therapy alone (CLOSE HR 0.03 [95% CI 0-0.26; P less than .001], RESPECT HR 0.55 [95% CI 0.31-0.999; P = .046], Gore REDUCE HR 0.23 [95% CI 0.09-0.62; P = .002]). Gore REDUCE and CLOSE identified increased rates of postprocedural atrial fibrillation or flutter (6.6% vs. 0.4% [P less than .001], 4.6% vs. 0.9% [P = .02], respectively). Serious adverse events related to the procedure or device ranged from 3.9-5.9%.
Bottom line: PFO closure combined with antiplatelet therapy in patients aged 60 years or younger, particularly in those with significant right-to-left shunts and atrial septal aneurysms, reduced the risk of recurrent ischemic stroke compared to antiplatelet therapy alone.
Citation: Mas JL. Derumeaux B. Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-21.
Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-32.
Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-42.
Background: Previous research on the use of PFO closure to prevent recurrent stroke has yielded mixed results.
Study design: Gore REDUCE, CLOSE, and RESPECT were all multicenter, randomized, open-label superiority trials, with blinded adjudication of endpoint events. RESPECT data reflected an exploratory analysis of an extended follow up period.
Setting: Gore REDUCE was a multinational study conducted at 63 sites in Europe and North America, from 2008-2015. CLOSE was conducted at 34 sites in France and Germany, from 2007 to 2016. RESPECT was conducted at 69 sites in the United States and Canada, from 2003 to 2011.
Synopsis: Three trials reexamined the impact of PFO closure with standard antiplatelet treatment, with a total of 2,307 patients between the ages of 16 and 60 years. CLOSE included only patients with a PFO and an associated atrial septal aneurysm or a large interatrial shunt. Gore REDUCE and RESPECT were both industry funded. All three trials found a statistically significant reduction in risk of recurrent ischemic stroke associated with PFO closure and antiplatelet therapy compared to antiplatelet therapy alone (CLOSE HR 0.03 [95% CI 0-0.26; P less than .001], RESPECT HR 0.55 [95% CI 0.31-0.999; P = .046], Gore REDUCE HR 0.23 [95% CI 0.09-0.62; P = .002]). Gore REDUCE and CLOSE identified increased rates of postprocedural atrial fibrillation or flutter (6.6% vs. 0.4% [P less than .001], 4.6% vs. 0.9% [P = .02], respectively). Serious adverse events related to the procedure or device ranged from 3.9-5.9%.
Bottom line: PFO closure combined with antiplatelet therapy in patients aged 60 years or younger, particularly in those with significant right-to-left shunts and atrial septal aneurysms, reduced the risk of recurrent ischemic stroke compared to antiplatelet therapy alone.
Citation: Mas JL. Derumeaux B. Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-21.
Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377(11):1022-32.
Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377(11):1033-42.
Baseline stress signals need for psychological help in CLL
, according to a prospective study of 152 patients.
“These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population,” wrote investigators led by Neha G. Goyal, PhD, a research fellow at Wake Forest University, Winston-Salem, N.C.
The subjects all had relapsed/refractory chronic lymphocytic leukemia (CLL). They filled out mental and physical function questionnaires at baseline, then at months 1, 2, and 5 during a nonrandomized phase 2 trial of ibrutinib (Imbruvica). The findings were published in the Annals of Behavioral Medicine.
Cancer-specific stress – essentially traumatic stress associated with cancer diagnosis, recurrence, and treatment – was assessed by the Impact of Event Scale, a common cancer research tool in which patients rate the intensity of intrusive thoughts and avoidant thoughts and behaviors. A score of 8 – out of a range of 0-64 – was the cut point used to separate patients with low versus high stress; higher scores mean worse symptoms.
“At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes ... higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months,” the investigators said (Ann Behav Med. 2018 Feb 9. doi: 10.1093/abm/kax004).
For instance, high-stress patients started the trial with mean scores of about 4.5 on the 42-point cognitive-affective subscale of the Beck Depression Inventory; scores improved to about 2.5 after 5 months of treatment. Low-stress patients, however, started and ended the study with scores of about 1.5.
Cancer-specific stress has been associated with poorer outcomes in previous cancer studies, but its impact on CLL hasn’t been clear until now. It might be a particularly bad problem in CLL, because the disease is incurable and patients go through multiple relapses and treatment cycles.
“There has been a call to screen cancer patients to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses,” the team noted.
The mean age in the study was 64.1 years; 71% of the subjects were men. The majority were educated beyond high school, and almost all reported significant, supportive relationships. Patients had a median of three prior therapies, but one patients had been through 16.
Dr. Goyal reported having no financial disclosures. One author disclosed ties to Pharmacyclics and Janssen, marketers of ibrutinib. The work was supported by the National Cancer Institute and Pharmacyclics, among others.
, according to a prospective study of 152 patients.
“These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population,” wrote investigators led by Neha G. Goyal, PhD, a research fellow at Wake Forest University, Winston-Salem, N.C.
The subjects all had relapsed/refractory chronic lymphocytic leukemia (CLL). They filled out mental and physical function questionnaires at baseline, then at months 1, 2, and 5 during a nonrandomized phase 2 trial of ibrutinib (Imbruvica). The findings were published in the Annals of Behavioral Medicine.
Cancer-specific stress – essentially traumatic stress associated with cancer diagnosis, recurrence, and treatment – was assessed by the Impact of Event Scale, a common cancer research tool in which patients rate the intensity of intrusive thoughts and avoidant thoughts and behaviors. A score of 8 – out of a range of 0-64 – was the cut point used to separate patients with low versus high stress; higher scores mean worse symptoms.
“At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes ... higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months,” the investigators said (Ann Behav Med. 2018 Feb 9. doi: 10.1093/abm/kax004).
For instance, high-stress patients started the trial with mean scores of about 4.5 on the 42-point cognitive-affective subscale of the Beck Depression Inventory; scores improved to about 2.5 after 5 months of treatment. Low-stress patients, however, started and ended the study with scores of about 1.5.
Cancer-specific stress has been associated with poorer outcomes in previous cancer studies, but its impact on CLL hasn’t been clear until now. It might be a particularly bad problem in CLL, because the disease is incurable and patients go through multiple relapses and treatment cycles.
“There has been a call to screen cancer patients to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses,” the team noted.
The mean age in the study was 64.1 years; 71% of the subjects were men. The majority were educated beyond high school, and almost all reported significant, supportive relationships. Patients had a median of three prior therapies, but one patients had been through 16.
Dr. Goyal reported having no financial disclosures. One author disclosed ties to Pharmacyclics and Janssen, marketers of ibrutinib. The work was supported by the National Cancer Institute and Pharmacyclics, among others.
, according to a prospective study of 152 patients.
“These findings suggest that integration of psychological intervention for patients who have high cancer-specific stress at baseline might be appropriate for this population,” wrote investigators led by Neha G. Goyal, PhD, a research fellow at Wake Forest University, Winston-Salem, N.C.
The subjects all had relapsed/refractory chronic lymphocytic leukemia (CLL). They filled out mental and physical function questionnaires at baseline, then at months 1, 2, and 5 during a nonrandomized phase 2 trial of ibrutinib (Imbruvica). The findings were published in the Annals of Behavioral Medicine.
Cancer-specific stress – essentially traumatic stress associated with cancer diagnosis, recurrence, and treatment – was assessed by the Impact of Event Scale, a common cancer research tool in which patients rate the intensity of intrusive thoughts and avoidant thoughts and behaviors. A score of 8 – out of a range of 0-64 – was the cut point used to separate patients with low versus high stress; higher scores mean worse symptoms.
“At treatment initiation, cancer-specific stress was associated with higher levels of cognitive-affective depressive symptoms, negative mood, fatigue interference, and sleep problems, and lower mental health quality of life. While patients with higher cancer-specific stress at baseline improved more rapidly on these outcomes ... higher cancer-specific stress at baseline was still associated with poorer psychological outcomes, but not physical outcomes, at 5 months,” the investigators said (Ann Behav Med. 2018 Feb 9. doi: 10.1093/abm/kax004).
For instance, high-stress patients started the trial with mean scores of about 4.5 on the 42-point cognitive-affective subscale of the Beck Depression Inventory; scores improved to about 2.5 after 5 months of treatment. Low-stress patients, however, started and ended the study with scores of about 1.5.
Cancer-specific stress has been associated with poorer outcomes in previous cancer studies, but its impact on CLL hasn’t been clear until now. It might be a particularly bad problem in CLL, because the disease is incurable and patients go through multiple relapses and treatment cycles.
“There has been a call to screen cancer patients to determine those who may be at risk for poor outcomes, and assessment of cancer-specific stress may have clinical utility as an individual difference predictor of psychological responses,” the team noted.
The mean age in the study was 64.1 years; 71% of the subjects were men. The majority were educated beyond high school, and almost all reported significant, supportive relationships. Patients had a median of three prior therapies, but one patients had been through 16.
Dr. Goyal reported having no financial disclosures. One author disclosed ties to Pharmacyclics and Janssen, marketers of ibrutinib. The work was supported by the National Cancer Institute and Pharmacyclics, among others.
FROM ANNALS OF BEHAVIORAL MEDICINE
VIDEO: With new therapies available, it’s the ‘decade of eczema,’ researcher says
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
REPORTING FROM AAD 18
For women with alcohol SUD, try gender-specific treatment
TAMPA – The distinct features of substance use disorders (SUDs) in women argue for gender-specific treatment, according to a comprehensive update presented at the annual meeting of the American College of Psychiatrists.
“There is a shorter time between landmarks of SUD progression, and these landmarks are reached at lower doses of alcohol consumed less frequently,” reported Shelly F. Greenfield, MD, chief academic officer at McLean Hospital in Boston.
“For each ounce of alcohol consumed, the blood alcohol concentration is higher with a greater potential for adverse physical consequences,” Dr. Greenfield said. These physiologic differences may account for the more rapid progression of SUD severity in women, a phenomenon that Dr. Greenfield referred to as “telescoping.” She said the same type of telescoping, defined as “an accelerated progression from the initiation of substance use to the onset of dependence and first admission to treatment” (Psychiatr Clin North Am. 2011 Jun 28;33[2]:339-55), also has been seen among women for opioids and stimulants (Drug Alcohol Depend. 2004 Jun 11;74[3]:265-72). These greater risks are reflected in a higher SUD-associated mortality in females, compared with SUD-associated mortality in males.
Relative rates of alcohol SUD among women have been climbing steadily for more than 30 years. In the 1980s, for example, a population survey estimated the male-to-female prevalence ratio of alcohol SUD as 5:1. Citing subsequent surveys, Dr. Greenfield traced a rapid narrowing of the gender gap. In one of the most recent surveys, the rate had fallen below 2:1. Rates of heaving drinking and binge drinking are now about 1.4:1 in individuals aged 18-25 years.
“Each time we look at this gap, it has narrowed further,” Dr. Greenfield said. In another survey she cited, illicit drug use among adolescents between ages 12 and 17 years was higher in boys, but alcohol use in males and females was essentially the same.
Several facts suggest that treatment specific to women will improve outcomes. For one, For another, women are more likely to have co-occurring psychiatric disorders. In one study, anxiety (60.7% vs. 35.8%) and mood disorders (53.5% vs. 28.1%) were nearly twice as common in women with SUDs than in their male counterparts. This is relevant to interventions tailored for females because of evidence showing that treatment for SUDs and co-occurring psychiatric disorders should be integrated rather than addressed independently, according to Dr. Greenfield.
Importantly, “there is evidence of improved treatment outcome in women-focused programs,” Dr. Greenfield said. She suggested that successful programs for alcohol SUD in women not only address gender-specific features but that success can be enhanced further with adjunctive services that address the barriers to treatment, such as child care challenges and stigma – which Dr. Greenfield said is greater in women than in men.
A study called the Women’s Recovery Group (WRG), funded by the National Institute on Drug Abuse and led by Dr. Greenfield, is among those that have reinforced the value of female-specific therapy for SUD (Drug Alcohol Depend. 2014 Sep 1;142:245-53). A manual developed from the study and published in a book she wrote called “Treating Women With Substance Use Disorders: The Women’s Recovery Group Manual” (New York: The Guilford Press, 2016), outlines the principles. Dr. Greenfield said the structured 12-session group therapy for relapse prevention has been effective and well received by women. Some of those women have commented on the reinforcing value of shared experiences.
Up until now, women with alcohol SUD have been commonly treated alongside men, but Dr. Greenfield contended that treatment outcomes with alcohol SUD or other forms of SUDs “can be enhanced by programs that provide services specific to women’s needs.” She believes strategies aimed at addressing the more common histories of sexual or physical trauma and psychiatric comorbidities along with gender-related barriers to treatment have the potential to increase treatment success.
In addition to writing “Treating Women With Substance Use Disorders,” Dr. Greenfield is a coeditor of “Women and Addiction: A Comprehensive Handbook” (New York: Guilford, 2009). She reported no conflicts of interest.
TAMPA – The distinct features of substance use disorders (SUDs) in women argue for gender-specific treatment, according to a comprehensive update presented at the annual meeting of the American College of Psychiatrists.
“There is a shorter time between landmarks of SUD progression, and these landmarks are reached at lower doses of alcohol consumed less frequently,” reported Shelly F. Greenfield, MD, chief academic officer at McLean Hospital in Boston.
“For each ounce of alcohol consumed, the blood alcohol concentration is higher with a greater potential for adverse physical consequences,” Dr. Greenfield said. These physiologic differences may account for the more rapid progression of SUD severity in women, a phenomenon that Dr. Greenfield referred to as “telescoping.” She said the same type of telescoping, defined as “an accelerated progression from the initiation of substance use to the onset of dependence and first admission to treatment” (Psychiatr Clin North Am. 2011 Jun 28;33[2]:339-55), also has been seen among women for opioids and stimulants (Drug Alcohol Depend. 2004 Jun 11;74[3]:265-72). These greater risks are reflected in a higher SUD-associated mortality in females, compared with SUD-associated mortality in males.
Relative rates of alcohol SUD among women have been climbing steadily for more than 30 years. In the 1980s, for example, a population survey estimated the male-to-female prevalence ratio of alcohol SUD as 5:1. Citing subsequent surveys, Dr. Greenfield traced a rapid narrowing of the gender gap. In one of the most recent surveys, the rate had fallen below 2:1. Rates of heaving drinking and binge drinking are now about 1.4:1 in individuals aged 18-25 years.
“Each time we look at this gap, it has narrowed further,” Dr. Greenfield said. In another survey she cited, illicit drug use among adolescents between ages 12 and 17 years was higher in boys, but alcohol use in males and females was essentially the same.
Several facts suggest that treatment specific to women will improve outcomes. For one, For another, women are more likely to have co-occurring psychiatric disorders. In one study, anxiety (60.7% vs. 35.8%) and mood disorders (53.5% vs. 28.1%) were nearly twice as common in women with SUDs than in their male counterparts. This is relevant to interventions tailored for females because of evidence showing that treatment for SUDs and co-occurring psychiatric disorders should be integrated rather than addressed independently, according to Dr. Greenfield.
Importantly, “there is evidence of improved treatment outcome in women-focused programs,” Dr. Greenfield said. She suggested that successful programs for alcohol SUD in women not only address gender-specific features but that success can be enhanced further with adjunctive services that address the barriers to treatment, such as child care challenges and stigma – which Dr. Greenfield said is greater in women than in men.
A study called the Women’s Recovery Group (WRG), funded by the National Institute on Drug Abuse and led by Dr. Greenfield, is among those that have reinforced the value of female-specific therapy for SUD (Drug Alcohol Depend. 2014 Sep 1;142:245-53). A manual developed from the study and published in a book she wrote called “Treating Women With Substance Use Disorders: The Women’s Recovery Group Manual” (New York: The Guilford Press, 2016), outlines the principles. Dr. Greenfield said the structured 12-session group therapy for relapse prevention has been effective and well received by women. Some of those women have commented on the reinforcing value of shared experiences.
Up until now, women with alcohol SUD have been commonly treated alongside men, but Dr. Greenfield contended that treatment outcomes with alcohol SUD or other forms of SUDs “can be enhanced by programs that provide services specific to women’s needs.” She believes strategies aimed at addressing the more common histories of sexual or physical trauma and psychiatric comorbidities along with gender-related barriers to treatment have the potential to increase treatment success.
In addition to writing “Treating Women With Substance Use Disorders,” Dr. Greenfield is a coeditor of “Women and Addiction: A Comprehensive Handbook” (New York: Guilford, 2009). She reported no conflicts of interest.
TAMPA – The distinct features of substance use disorders (SUDs) in women argue for gender-specific treatment, according to a comprehensive update presented at the annual meeting of the American College of Psychiatrists.
“There is a shorter time between landmarks of SUD progression, and these landmarks are reached at lower doses of alcohol consumed less frequently,” reported Shelly F. Greenfield, MD, chief academic officer at McLean Hospital in Boston.
“For each ounce of alcohol consumed, the blood alcohol concentration is higher with a greater potential for adverse physical consequences,” Dr. Greenfield said. These physiologic differences may account for the more rapid progression of SUD severity in women, a phenomenon that Dr. Greenfield referred to as “telescoping.” She said the same type of telescoping, defined as “an accelerated progression from the initiation of substance use to the onset of dependence and first admission to treatment” (Psychiatr Clin North Am. 2011 Jun 28;33[2]:339-55), also has been seen among women for opioids and stimulants (Drug Alcohol Depend. 2004 Jun 11;74[3]:265-72). These greater risks are reflected in a higher SUD-associated mortality in females, compared with SUD-associated mortality in males.
Relative rates of alcohol SUD among women have been climbing steadily for more than 30 years. In the 1980s, for example, a population survey estimated the male-to-female prevalence ratio of alcohol SUD as 5:1. Citing subsequent surveys, Dr. Greenfield traced a rapid narrowing of the gender gap. In one of the most recent surveys, the rate had fallen below 2:1. Rates of heaving drinking and binge drinking are now about 1.4:1 in individuals aged 18-25 years.
“Each time we look at this gap, it has narrowed further,” Dr. Greenfield said. In another survey she cited, illicit drug use among adolescents between ages 12 and 17 years was higher in boys, but alcohol use in males and females was essentially the same.
Several facts suggest that treatment specific to women will improve outcomes. For one, For another, women are more likely to have co-occurring psychiatric disorders. In one study, anxiety (60.7% vs. 35.8%) and mood disorders (53.5% vs. 28.1%) were nearly twice as common in women with SUDs than in their male counterparts. This is relevant to interventions tailored for females because of evidence showing that treatment for SUDs and co-occurring psychiatric disorders should be integrated rather than addressed independently, according to Dr. Greenfield.
Importantly, “there is evidence of improved treatment outcome in women-focused programs,” Dr. Greenfield said. She suggested that successful programs for alcohol SUD in women not only address gender-specific features but that success can be enhanced further with adjunctive services that address the barriers to treatment, such as child care challenges and stigma – which Dr. Greenfield said is greater in women than in men.
A study called the Women’s Recovery Group (WRG), funded by the National Institute on Drug Abuse and led by Dr. Greenfield, is among those that have reinforced the value of female-specific therapy for SUD (Drug Alcohol Depend. 2014 Sep 1;142:245-53). A manual developed from the study and published in a book she wrote called “Treating Women With Substance Use Disorders: The Women’s Recovery Group Manual” (New York: The Guilford Press, 2016), outlines the principles. Dr. Greenfield said the structured 12-session group therapy for relapse prevention has been effective and well received by women. Some of those women have commented on the reinforcing value of shared experiences.
Up until now, women with alcohol SUD have been commonly treated alongside men, but Dr. Greenfield contended that treatment outcomes with alcohol SUD or other forms of SUDs “can be enhanced by programs that provide services specific to women’s needs.” She believes strategies aimed at addressing the more common histories of sexual or physical trauma and psychiatric comorbidities along with gender-related barriers to treatment have the potential to increase treatment success.
In addition to writing “Treating Women With Substance Use Disorders,” Dr. Greenfield is a coeditor of “Women and Addiction: A Comprehensive Handbook” (New York: Guilford, 2009). She reported no conflicts of interest.
REPORTING FROM THE COLLEGE 2018
VIDEO: U.S. melanoma incidence hits all-time high
SAN DIEGO –
“Nobody’s quite sure why the [melanoma] rates are still rising so dramatically,” Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology. The increase remains even after adjustment of incidence rates for the increasing mean age of U.S. adults.
The American Cancer Society reported that the estimated annual incidence rate for invasive melanoma will be 91,270 cases for 2018, following what the society called a rapid rise in the rate for the past 30 years. Add to that the estimate of more than 87,000 U.S. cases of in situ melanoma for an overall annual U.S. rate of 178,560, Dr. Rigel said.
Melanoma has a latency of 5-20 years, “so what we’re seeing right now are the effects of what happened 5, 10, or 20 years ago,” said Dr. Rigel, a dermatologist at New York University.
During a talk at the meeting, Dr. Rigel said that based on current incident levels he projected a lifetime U.S. incidence rate of invasive melanoma of one case for every 40 adults by the end of this decade, and a lifetime incidence rate for either invasive or in situ melanoma of one case for every 20 adults by 2020.
A positive trend is that for the first time, the number of melanoma deaths has started to fall, with an estimated 9,320 deaths from melanoma in 2018 according to American Cancer Society statistics, down from a peak of 10,130 melanoma deaths in 2016, Dr. Rigel said.
SAN DIEGO –
“Nobody’s quite sure why the [melanoma] rates are still rising so dramatically,” Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology. The increase remains even after adjustment of incidence rates for the increasing mean age of U.S. adults.
The American Cancer Society reported that the estimated annual incidence rate for invasive melanoma will be 91,270 cases for 2018, following what the society called a rapid rise in the rate for the past 30 years. Add to that the estimate of more than 87,000 U.S. cases of in situ melanoma for an overall annual U.S. rate of 178,560, Dr. Rigel said.
Melanoma has a latency of 5-20 years, “so what we’re seeing right now are the effects of what happened 5, 10, or 20 years ago,” said Dr. Rigel, a dermatologist at New York University.
During a talk at the meeting, Dr. Rigel said that based on current incident levels he projected a lifetime U.S. incidence rate of invasive melanoma of one case for every 40 adults by the end of this decade, and a lifetime incidence rate for either invasive or in situ melanoma of one case for every 20 adults by 2020.
A positive trend is that for the first time, the number of melanoma deaths has started to fall, with an estimated 9,320 deaths from melanoma in 2018 according to American Cancer Society statistics, down from a peak of 10,130 melanoma deaths in 2016, Dr. Rigel said.
SAN DIEGO –
“Nobody’s quite sure why the [melanoma] rates are still rising so dramatically,” Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology. The increase remains even after adjustment of incidence rates for the increasing mean age of U.S. adults.
The American Cancer Society reported that the estimated annual incidence rate for invasive melanoma will be 91,270 cases for 2018, following what the society called a rapid rise in the rate for the past 30 years. Add to that the estimate of more than 87,000 U.S. cases of in situ melanoma for an overall annual U.S. rate of 178,560, Dr. Rigel said.
Melanoma has a latency of 5-20 years, “so what we’re seeing right now are the effects of what happened 5, 10, or 20 years ago,” said Dr. Rigel, a dermatologist at New York University.
During a talk at the meeting, Dr. Rigel said that based on current incident levels he projected a lifetime U.S. incidence rate of invasive melanoma of one case for every 40 adults by the end of this decade, and a lifetime incidence rate for either invasive or in situ melanoma of one case for every 20 adults by 2020.
A positive trend is that for the first time, the number of melanoma deaths has started to fall, with an estimated 9,320 deaths from melanoma in 2018 according to American Cancer Society statistics, down from a peak of 10,130 melanoma deaths in 2016, Dr. Rigel said.
REPORTING FROM AAD 18
MDedge Daily News: Is SPF 100 really better?
Is 100 really greater than 50 when it comes to sunscreen? A new hepatitis B vaccine gets the nod, a new JAK inhibitor takes aim at atopic dermatitis, and why house cleaning does women’s lungs no favors.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Is 100 really greater than 50 when it comes to sunscreen? A new hepatitis B vaccine gets the nod, a new JAK inhibitor takes aim at atopic dermatitis, and why house cleaning does women’s lungs no favors.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Is 100 really greater than 50 when it comes to sunscreen? A new hepatitis B vaccine gets the nod, a new JAK inhibitor takes aim at atopic dermatitis, and why house cleaning does women’s lungs no favors.
Listen to the MDedge Daily News podcast for all the details on today’s top news.












