User login
After warning, codeine use after tonsillectomy drops, doesn’t stop
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
– and there has been inappropriate substitution of other opioids rather than nonopioid pain relievers such as ibuprofen, said Kao-Ping Chua, MD, PhD, of the University of Chicago, and his associates.
Codeine has been one of the most commonly prescribed analgesics for children after tonsillectomies and adenoidectomies because it was considered safe.
The FDA issued a boxed warning Feb. 20, 2013, recommending against codeine use in all children undergoing tonsillectomy and/or adenoidectomy. Also, children with obstructive sleep apnea are at risk of opioid-related respiratory depression.
A retrospective study of 362,992 children aged 0-18 years who underwent tonsillectomy and/or adenoidectomy was undertaken in 2010-2015, prior to and after the FDA issued the boxed warning against codeine use. In January 2010, codeine products were prescribed in 47% of cases, compared with 48% for hydrocodone, 4% for oxycodone, and less than 1% for other opioid products. In December 2015, codeine was prescribed in 9% of cases, compared with 73% for hydrocodone, 17% for oxycodone, and less than 1% for other opioid products.
“The unadjusted proportion of children receiving alternative opioids rose substantially during the study period, presumably because of factors other than the investigation itself,” said Dr. Chua and his associates. “This increase deserves further examination, given the abuse liability associated with higher-potency opioids, as well as the growing evidence of genetic variability in the metabolism of many alternative opioids, including oxycodone and tramadol.
“Future quality-improvement efforts should focus on eliminating this residual inappropriate codeine prescribing, and on encouraging the use of effective nonopioid medications such as ibuprofen,” the investigators said.
Read more in Pediatrics (2017 Nov 16. doi: 10.1542/ peds.2017-1765).
FROM PEDIATRICS
Extensive rash and joint pain
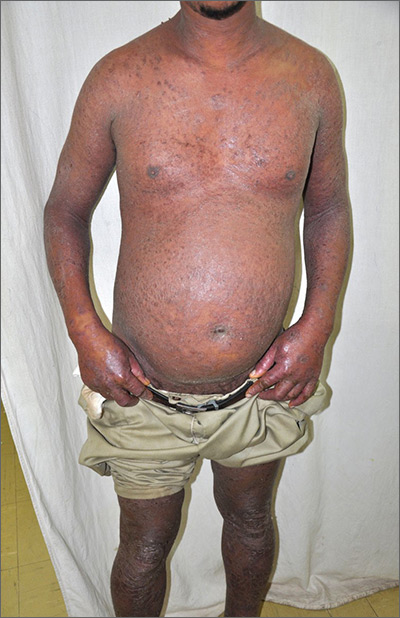
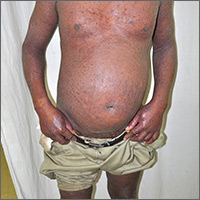
Based on the patient’s tender finger and wrist joints and exfoliation of skin, the FP diagnosed erythrodermic psoriasis, although the patient’s dark skin made the erythema less visible. The patient was not systemically ill; however, the FP recognized that he would need systemic therapy for the arthritis and severe skin manifestations and referred the patient to a dermatology specialist.
The dermatology specialist agreed to see the patient the next day. She ordered the following lab tests: complete blood count (CBC), chemistry panel, QuantiFERON TB gold, hepatitis C antibody, and hepatitis B surface antigen, surface antibody, and core antibody. She also ordered bilateral hand films, which showed early juxta-articular erosions (consistent with psoriatic arthritis). Because of the strong skin, nail, and joint evidence, the dermatology specialist determined a biopsy was not necessary.
The patient was instructed to apply 0.1% triamcinolone ointment to his entire body using the wet pajama technique overnight. At follow-up, he said his skin felt better, but his joints did not. The dermatology specialist reviewed the lab results and explained to the patient that the 2 best options were oral methotrexate weekly or an expensive injectable biologic agent. Because most health insurance companies now require that the patient fail a less expensive agent such as methotrexate before trying the biologic, the decision was made to start with methotrexate. Within a month, the patient's skin was remarkably better and his joint pain and stiffness had improved significantly.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D. Erythroderma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 915-921.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Based on the patient’s tender finger and wrist joints and exfoliation of skin, the FP diagnosed erythrodermic psoriasis, although the patient’s dark skin made the erythema less visible. The patient was not systemically ill; however, the FP recognized that he would need systemic therapy for the arthritis and severe skin manifestations and referred the patient to a dermatology specialist.
The dermatology specialist agreed to see the patient the next day. She ordered the following lab tests: complete blood count (CBC), chemistry panel, QuantiFERON TB gold, hepatitis C antibody, and hepatitis B surface antigen, surface antibody, and core antibody. She also ordered bilateral hand films, which showed early juxta-articular erosions (consistent with psoriatic arthritis). Because of the strong skin, nail, and joint evidence, the dermatology specialist determined a biopsy was not necessary.
The patient was instructed to apply 0.1% triamcinolone ointment to his entire body using the wet pajama technique overnight. At follow-up, he said his skin felt better, but his joints did not. The dermatology specialist reviewed the lab results and explained to the patient that the 2 best options were oral methotrexate weekly or an expensive injectable biologic agent. Because most health insurance companies now require that the patient fail a less expensive agent such as methotrexate before trying the biologic, the decision was made to start with methotrexate. Within a month, the patient's skin was remarkably better and his joint pain and stiffness had improved significantly.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D. Erythroderma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 915-921.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Based on the patient’s tender finger and wrist joints and exfoliation of skin, the FP diagnosed erythrodermic psoriasis, although the patient’s dark skin made the erythema less visible. The patient was not systemically ill; however, the FP recognized that he would need systemic therapy for the arthritis and severe skin manifestations and referred the patient to a dermatology specialist.
The dermatology specialist agreed to see the patient the next day. She ordered the following lab tests: complete blood count (CBC), chemistry panel, QuantiFERON TB gold, hepatitis C antibody, and hepatitis B surface antigen, surface antibody, and core antibody. She also ordered bilateral hand films, which showed early juxta-articular erosions (consistent with psoriatic arthritis). Because of the strong skin, nail, and joint evidence, the dermatology specialist determined a biopsy was not necessary.
The patient was instructed to apply 0.1% triamcinolone ointment to his entire body using the wet pajama technique overnight. At follow-up, he said his skin felt better, but his joints did not. The dermatology specialist reviewed the lab results and explained to the patient that the 2 best options were oral methotrexate weekly or an expensive injectable biologic agent. Because most health insurance companies now require that the patient fail a less expensive agent such as methotrexate before trying the biologic, the decision was made to start with methotrexate. Within a month, the patient's skin was remarkably better and his joint pain and stiffness had improved significantly.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Henderson D. Erythroderma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 915-921.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
VIDEO: Bariatric experts discuss recent experience with gastric balloon devices
Two experts on bariatric surgery spoke at the Minimally Invasive Surgery Symposium, held in Las Vegas. Jaime Ponce, MD, FACS, and John Morton, MD, FACS, discussed several different types of gastric balloon devices and the factors guiding their use for patients. Dr. Morton suggested that the balloon devices could serve as an intermediate treatment between medications and surgery.

Two experts on bariatric surgery spoke at the Minimally Invasive Surgery Symposium, held in Las Vegas. Jaime Ponce, MD, FACS, and John Morton, MD, FACS, discussed several different types of gastric balloon devices and the factors guiding their use for patients. Dr. Morton suggested that the balloon devices could serve as an intermediate treatment between medications and surgery.

Two experts on bariatric surgery spoke at the Minimally Invasive Surgery Symposium, held in Las Vegas. Jaime Ponce, MD, FACS, and John Morton, MD, FACS, discussed several different types of gastric balloon devices and the factors guiding their use for patients. Dr. Morton suggested that the balloon devices could serve as an intermediate treatment between medications and surgery.

Carefully monitor cannabis-using patients in opioid-agonist therapy
Canada has passed legislation that will lead to legalization of cannabis for recreational use. Researchers disagree about the public health effects that legalization will have. Alexandra M. Franklyn and her coinvestigators at the Northern Ontario School of Medicine, Sudbury, designed their study to look at one population that might be at risk of cannabis-related harms.
Of 644 patients, 328 (50.9%) were baseline cannabis users. Baseline users were 38.9% more likely to drop out of OAT. Heavy cannabis users (n = 256; 39.8%) were 48.1% more likely to drop out of OAT than those who weren’t heavy users. Heavy users are “a group that should be carefully monitored throughout treatment,” regardless of whether the cannabis use can be considered another substance use disorder or if it is intended to self-medicate another condition that may make dropout more likely, Ms. Franklyn and her colleagues concluded.
“While studies have shown a potential for cannabis legalization to be a positive change for the population as a whole, there may be unique implications for those patients receiving OAT. ... These patients should receive education surrounding the potential harms of cannabis use, including worsened OAT outcomes,” the researchers wrote (PLoS One. 2017 Nov 8;12[11]:e0187633).
Neither Ms. Franklyn nor her colleagues had conflicts of interest to disclose.
Canada has passed legislation that will lead to legalization of cannabis for recreational use. Researchers disagree about the public health effects that legalization will have. Alexandra M. Franklyn and her coinvestigators at the Northern Ontario School of Medicine, Sudbury, designed their study to look at one population that might be at risk of cannabis-related harms.
Of 644 patients, 328 (50.9%) were baseline cannabis users. Baseline users were 38.9% more likely to drop out of OAT. Heavy cannabis users (n = 256; 39.8%) were 48.1% more likely to drop out of OAT than those who weren’t heavy users. Heavy users are “a group that should be carefully monitored throughout treatment,” regardless of whether the cannabis use can be considered another substance use disorder or if it is intended to self-medicate another condition that may make dropout more likely, Ms. Franklyn and her colleagues concluded.
“While studies have shown a potential for cannabis legalization to be a positive change for the population as a whole, there may be unique implications for those patients receiving OAT. ... These patients should receive education surrounding the potential harms of cannabis use, including worsened OAT outcomes,” the researchers wrote (PLoS One. 2017 Nov 8;12[11]:e0187633).
Neither Ms. Franklyn nor her colleagues had conflicts of interest to disclose.
Canada has passed legislation that will lead to legalization of cannabis for recreational use. Researchers disagree about the public health effects that legalization will have. Alexandra M. Franklyn and her coinvestigators at the Northern Ontario School of Medicine, Sudbury, designed their study to look at one population that might be at risk of cannabis-related harms.
Of 644 patients, 328 (50.9%) were baseline cannabis users. Baseline users were 38.9% more likely to drop out of OAT. Heavy cannabis users (n = 256; 39.8%) were 48.1% more likely to drop out of OAT than those who weren’t heavy users. Heavy users are “a group that should be carefully monitored throughout treatment,” regardless of whether the cannabis use can be considered another substance use disorder or if it is intended to self-medicate another condition that may make dropout more likely, Ms. Franklyn and her colleagues concluded.
“While studies have shown a potential for cannabis legalization to be a positive change for the population as a whole, there may be unique implications for those patients receiving OAT. ... These patients should receive education surrounding the potential harms of cannabis use, including worsened OAT outcomes,” the researchers wrote (PLoS One. 2017 Nov 8;12[11]:e0187633).
Neither Ms. Franklyn nor her colleagues had conflicts of interest to disclose.
FROM PLOS ONE
Infliximab useful in a case of concurrent RA/PBC
The anti–tumor necrosis factor alpha agent infliximab is a beneficial option in the treatment of concurrent rheumatoid arthritis (RA) and primary biliary cholangitis (PBC), according to A.K. Ben, MD, and associates.
In a case report, a 56-year-old woman presented with 5 years of evolving symmetrical polyarthritis involving her large and small joints, and was diagnosed with RA. Methotrexate was initially used as treatment, but after 6 months of continued high disease activity, infliximab was added to treatment. The patient showed good clinical response to the combination treatment.
The combination treatment was stopped after the patient showed abnormal liver function. Antimitochondrial antibody testing was positive, and after a liver biopsy to confirm, the patient was diagnosed with PBC. Ursodeoxycholic acid was prescribed and liver function was normalized.
The patient was restarted on methotrexate and 3 mg/kg infliximab when the RA flared after 6 months and was persistent until the dosage of infliximab was increased to 5 mg/kg. This was effective in the patient, who has experienced disease decline for 5 years.
“Additional studies may be considered to better explore the therapeutic role (dosage and molecule disparities) of TNF [tumor necrosis factor] blockers on clinical and morphological course of PBC associated with RA,” the investigators concluded.
Find the full case report in Internal Medicine: Open Access (doi: 10.4172/2165-8048.1000250).
The anti–tumor necrosis factor alpha agent infliximab is a beneficial option in the treatment of concurrent rheumatoid arthritis (RA) and primary biliary cholangitis (PBC), according to A.K. Ben, MD, and associates.
In a case report, a 56-year-old woman presented with 5 years of evolving symmetrical polyarthritis involving her large and small joints, and was diagnosed with RA. Methotrexate was initially used as treatment, but after 6 months of continued high disease activity, infliximab was added to treatment. The patient showed good clinical response to the combination treatment.
The combination treatment was stopped after the patient showed abnormal liver function. Antimitochondrial antibody testing was positive, and after a liver biopsy to confirm, the patient was diagnosed with PBC. Ursodeoxycholic acid was prescribed and liver function was normalized.
The patient was restarted on methotrexate and 3 mg/kg infliximab when the RA flared after 6 months and was persistent until the dosage of infliximab was increased to 5 mg/kg. This was effective in the patient, who has experienced disease decline for 5 years.
“Additional studies may be considered to better explore the therapeutic role (dosage and molecule disparities) of TNF [tumor necrosis factor] blockers on clinical and morphological course of PBC associated with RA,” the investigators concluded.
Find the full case report in Internal Medicine: Open Access (doi: 10.4172/2165-8048.1000250).
The anti–tumor necrosis factor alpha agent infliximab is a beneficial option in the treatment of concurrent rheumatoid arthritis (RA) and primary biliary cholangitis (PBC), according to A.K. Ben, MD, and associates.
In a case report, a 56-year-old woman presented with 5 years of evolving symmetrical polyarthritis involving her large and small joints, and was diagnosed with RA. Methotrexate was initially used as treatment, but after 6 months of continued high disease activity, infliximab was added to treatment. The patient showed good clinical response to the combination treatment.
The combination treatment was stopped after the patient showed abnormal liver function. Antimitochondrial antibody testing was positive, and after a liver biopsy to confirm, the patient was diagnosed with PBC. Ursodeoxycholic acid was prescribed and liver function was normalized.
The patient was restarted on methotrexate and 3 mg/kg infliximab when the RA flared after 6 months and was persistent until the dosage of infliximab was increased to 5 mg/kg. This was effective in the patient, who has experienced disease decline for 5 years.
“Additional studies may be considered to better explore the therapeutic role (dosage and molecule disparities) of TNF [tumor necrosis factor] blockers on clinical and morphological course of PBC associated with RA,” the investigators concluded.
Find the full case report in Internal Medicine: Open Access (doi: 10.4172/2165-8048.1000250).
FROM INTERNAL MEDICINE: OPEN ACCESS
Higher BMI linked to lower risk of hysterectomy reoperation
NATIONAL HARBOR, MD. – Women with a greater body mass index (BMI) were less likely to need reoperation after hysterectomy, according to findings presented at the AAGL Global Congress.
“What’s unusual is women who are considered overweight or obese are generally thought to be at higher risk of any complication, including reoperation,” Janelle Moulder, MD, of the department of ob.gyn. at the University of Tennessee, Knoxville, said in an interview prior to the meeting. “We don’t have enough data to say what exactly might be protective. And to see that women who are at normal or below normal BMI were at increased risk makes you pause as to what could potentially put them at risk.”
Dr. Moulder and her colleagues analyzed data on 28,487 women who underwent an abdominal, vaginal, or laparoscopic hysterectomy from 2014 to 2015. The data came from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database.
Patients were excluded if they had cancer, their surgery was not performed by a gynecologist, or their BMI data was missing.
A majority of patients (13,000) had a BMI of 30 kg/m2 or greater.
Compared with patients with a normal BMI of 24 kg/m2, patients with a BMI of 39 kg/m2 had the lowest odds of reoperation (adjusted odds ratio, 0.73; P = .02). Patients with BMIs of 29 kg/m2 and 34 kg/m2 were also at lower odds of reoperation, with adjusted odds ratios of 0.83 (P = .003) and 0.75 (P = .005), respectively.
Patients with a low normal BMI of 18.5 kg/m2 were at a higher risk of reoperation (aOR = 1.33; P = .001).
Researchers were unable to comment on women with a BMI of 45 kg/m2 or greater, due to the limited number of women in this group.
Researchers did not have access to the reason for reoperation, which may have limited the scope of the study.
“The next thing to be evaluated is what is the protective effect of the increasing BMI on reoperation and also look at variables that may put low normal BMI women at risk for reoperation,” Dr. Moulder said.
The researchers reported having no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
NATIONAL HARBOR, MD. – Women with a greater body mass index (BMI) were less likely to need reoperation after hysterectomy, according to findings presented at the AAGL Global Congress.
“What’s unusual is women who are considered overweight or obese are generally thought to be at higher risk of any complication, including reoperation,” Janelle Moulder, MD, of the department of ob.gyn. at the University of Tennessee, Knoxville, said in an interview prior to the meeting. “We don’t have enough data to say what exactly might be protective. And to see that women who are at normal or below normal BMI were at increased risk makes you pause as to what could potentially put them at risk.”
Dr. Moulder and her colleagues analyzed data on 28,487 women who underwent an abdominal, vaginal, or laparoscopic hysterectomy from 2014 to 2015. The data came from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database.
Patients were excluded if they had cancer, their surgery was not performed by a gynecologist, or their BMI data was missing.
A majority of patients (13,000) had a BMI of 30 kg/m2 or greater.
Compared with patients with a normal BMI of 24 kg/m2, patients with a BMI of 39 kg/m2 had the lowest odds of reoperation (adjusted odds ratio, 0.73; P = .02). Patients with BMIs of 29 kg/m2 and 34 kg/m2 were also at lower odds of reoperation, with adjusted odds ratios of 0.83 (P = .003) and 0.75 (P = .005), respectively.
Patients with a low normal BMI of 18.5 kg/m2 were at a higher risk of reoperation (aOR = 1.33; P = .001).
Researchers were unable to comment on women with a BMI of 45 kg/m2 or greater, due to the limited number of women in this group.
Researchers did not have access to the reason for reoperation, which may have limited the scope of the study.
“The next thing to be evaluated is what is the protective effect of the increasing BMI on reoperation and also look at variables that may put low normal BMI women at risk for reoperation,” Dr. Moulder said.
The researchers reported having no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
NATIONAL HARBOR, MD. – Women with a greater body mass index (BMI) were less likely to need reoperation after hysterectomy, according to findings presented at the AAGL Global Congress.
“What’s unusual is women who are considered overweight or obese are generally thought to be at higher risk of any complication, including reoperation,” Janelle Moulder, MD, of the department of ob.gyn. at the University of Tennessee, Knoxville, said in an interview prior to the meeting. “We don’t have enough data to say what exactly might be protective. And to see that women who are at normal or below normal BMI were at increased risk makes you pause as to what could potentially put them at risk.”
Dr. Moulder and her colleagues analyzed data on 28,487 women who underwent an abdominal, vaginal, or laparoscopic hysterectomy from 2014 to 2015. The data came from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database.
Patients were excluded if they had cancer, their surgery was not performed by a gynecologist, or their BMI data was missing.
A majority of patients (13,000) had a BMI of 30 kg/m2 or greater.
Compared with patients with a normal BMI of 24 kg/m2, patients with a BMI of 39 kg/m2 had the lowest odds of reoperation (adjusted odds ratio, 0.73; P = .02). Patients with BMIs of 29 kg/m2 and 34 kg/m2 were also at lower odds of reoperation, with adjusted odds ratios of 0.83 (P = .003) and 0.75 (P = .005), respectively.
Patients with a low normal BMI of 18.5 kg/m2 were at a higher risk of reoperation (aOR = 1.33; P = .001).
Researchers were unable to comment on women with a BMI of 45 kg/m2 or greater, due to the limited number of women in this group.
Researchers did not have access to the reason for reoperation, which may have limited the scope of the study.
“The next thing to be evaluated is what is the protective effect of the increasing BMI on reoperation and also look at variables that may put low normal BMI women at risk for reoperation,” Dr. Moulder said.
The researchers reported having no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
AT AAGL 2017
Key clinical point:
Major finding: Patients with a BMI of 39 kg/m2 less likely to need a reoperation after hysterectomy (aOR, .73; P = .02).
Data source: Retrospective study of 28,487 women who underwent a hysterectomy from 2014 to 2015 from the American College of Surgeons National Surgical Quality Improvement Program database.
Disclosures: The researchers reported having no relevant financial disclosures.
FDA issues alert on illegal silicone injections
The Food and Drug Administration has issued a warning regarding the use of injectable silicone or other products illegally marketed as dermal fillers for body contouring.
“We have significant concerns with unsafe injectable silicone that’s being marketed for body contouring by unlicensed providers,” FDA Commissioner Scott Gottlieb, MD, said in a statement on Nov. 14. “We’ve seen serious adverse events result from products, which are sometimes industrial-grade silicone, being used for these unapproved medical purposes,” he said.
Simply injecting silicone into various parts of the body for contouring purposes is not approved by the FDA. Side effects of such a procedure can occur immediately, or may appear after days, weeks, months, or years, according to the statement. Side effects include pain, scarring, disfigurement, life-threatening embolism, stroke, or infection, the FDA emphasized.
The FDA continues to take action against unlicensed practitioners found guilty of treating patients with unapproved silicone for body contouring. “In addition to prosecuting the criminals who take advantage of consumers, the FDA is taking action to educate consumers in order to prevent the serious injuries resulting from these injections,” Melinda Plaisier, associate commissioner for regulatory affairs at the FDA, said in the statement. “We hope to raise public awareness about the short- and long-term risks of injecting silicone directly into the body, and encourage consumers to choose FDA-approved products and licensed providers when considering any type of cosmetic enhancement,” she said.
The FDA will continue to monitor adverse event reports related to silicone, and encourages clinicians or consumers with information about the use of injectable silicone by unlicensed providers to use the “Report Suspected Criminal Activity” form on the FDA website to report those cases.
The Food and Drug Administration has issued a warning regarding the use of injectable silicone or other products illegally marketed as dermal fillers for body contouring.
“We have significant concerns with unsafe injectable silicone that’s being marketed for body contouring by unlicensed providers,” FDA Commissioner Scott Gottlieb, MD, said in a statement on Nov. 14. “We’ve seen serious adverse events result from products, which are sometimes industrial-grade silicone, being used for these unapproved medical purposes,” he said.
Simply injecting silicone into various parts of the body for contouring purposes is not approved by the FDA. Side effects of such a procedure can occur immediately, or may appear after days, weeks, months, or years, according to the statement. Side effects include pain, scarring, disfigurement, life-threatening embolism, stroke, or infection, the FDA emphasized.
The FDA continues to take action against unlicensed practitioners found guilty of treating patients with unapproved silicone for body contouring. “In addition to prosecuting the criminals who take advantage of consumers, the FDA is taking action to educate consumers in order to prevent the serious injuries resulting from these injections,” Melinda Plaisier, associate commissioner for regulatory affairs at the FDA, said in the statement. “We hope to raise public awareness about the short- and long-term risks of injecting silicone directly into the body, and encourage consumers to choose FDA-approved products and licensed providers when considering any type of cosmetic enhancement,” she said.
The FDA will continue to monitor adverse event reports related to silicone, and encourages clinicians or consumers with information about the use of injectable silicone by unlicensed providers to use the “Report Suspected Criminal Activity” form on the FDA website to report those cases.
The Food and Drug Administration has issued a warning regarding the use of injectable silicone or other products illegally marketed as dermal fillers for body contouring.
“We have significant concerns with unsafe injectable silicone that’s being marketed for body contouring by unlicensed providers,” FDA Commissioner Scott Gottlieb, MD, said in a statement on Nov. 14. “We’ve seen serious adverse events result from products, which are sometimes industrial-grade silicone, being used for these unapproved medical purposes,” he said.
Simply injecting silicone into various parts of the body for contouring purposes is not approved by the FDA. Side effects of such a procedure can occur immediately, or may appear after days, weeks, months, or years, according to the statement. Side effects include pain, scarring, disfigurement, life-threatening embolism, stroke, or infection, the FDA emphasized.
The FDA continues to take action against unlicensed practitioners found guilty of treating patients with unapproved silicone for body contouring. “In addition to prosecuting the criminals who take advantage of consumers, the FDA is taking action to educate consumers in order to prevent the serious injuries resulting from these injections,” Melinda Plaisier, associate commissioner for regulatory affairs at the FDA, said in the statement. “We hope to raise public awareness about the short- and long-term risks of injecting silicone directly into the body, and encourage consumers to choose FDA-approved products and licensed providers when considering any type of cosmetic enhancement,” she said.
The FDA will continue to monitor adverse event reports related to silicone, and encourages clinicians or consumers with information about the use of injectable silicone by unlicensed providers to use the “Report Suspected Criminal Activity” form on the FDA website to report those cases.
FDA clears first-ever neurostimulation device for opioid withdrawal symptoms
The Food and Drug Administration has cleared a medical device that applies electrical stimulation to cranial nerves in order to reduce opioid withdrawal symptoms.
The clearance was granted amid the opioid crisis, which is killing 175 Americans each day, according to the recent report by The President’s Commission on Combating Drug Addiction and the Opioid Crisis. Currently, opioid addiction is treatable by three approved medications, said FDA Commissioner Scott Gottlieb, MD, in a press statement announcing the agency’s decision to permit marketing of the NSS-2 Bridge device.
“While we continue to pursue better medicines for the treatment of opioid use disorder, we also need to look to devices that can assist in this therapy,” Dr. Gottlieb said.
The NSS-2 Bridge device was cleared as an acupuncture aid in 2014. The new use of the device to reduce the symptoms of opioid withdrawal required a new clearance, which was granted based on a single, one-arm clinical study. In the study, 73 patients who were experiencing physical withdrawal from opioids used the neurostimulation device. They reported an initial mean score of 20.1 on the Clinical Opiate Withdrawal Score (COWS). All patients using the device had a reduction of at least 31% on the COWS within 30 minutes of beginning use of the NSS-2 Bridge. A total of 88% of participating patients transitioned to medication-assisted treatment after 5 days of using the device. Additional medications used to treat specific symptoms, such as nausea and vomiting, were permitted during the trial.
The physician-placed battery-powered device sits behind the ear and uses three percutaneous electrode arrays and one single-point needle to provide neurostimulation. The electrode placement is assisted with a transillumination technique, and also is based on known neuroanatomic landmarks for branches of cranial nerves V, VII, and IX, along with branches of the occipital nerve (Clin Med Diagnostics. 2015;5[4]:70-9).
The single-use device is designed to be used for up to 5 days during acute opioid withdrawal and is contraindicated for patients with hemophilia, patients with cardiac pacemakers, or those diagnosed with psoriasis vulgaris. The NSS-2 Bridge device requires a prescription and was cleared through the de novo premarket review pathway. This, said the FDA in the press statement, is “a regulatory pathway for some low- to moderate-risk devices that are novel and for which there is no legally marketed predicate device to which the device can claim substantial equivalence.”
The NSS-2 Bridge device will be marketed by Innovative Health Solutions.
[email protected]
On Twitter @karioakes
The Food and Drug Administration has cleared a medical device that applies electrical stimulation to cranial nerves in order to reduce opioid withdrawal symptoms.
The clearance was granted amid the opioid crisis, which is killing 175 Americans each day, according to the recent report by The President’s Commission on Combating Drug Addiction and the Opioid Crisis. Currently, opioid addiction is treatable by three approved medications, said FDA Commissioner Scott Gottlieb, MD, in a press statement announcing the agency’s decision to permit marketing of the NSS-2 Bridge device.
“While we continue to pursue better medicines for the treatment of opioid use disorder, we also need to look to devices that can assist in this therapy,” Dr. Gottlieb said.
The NSS-2 Bridge device was cleared as an acupuncture aid in 2014. The new use of the device to reduce the symptoms of opioid withdrawal required a new clearance, which was granted based on a single, one-arm clinical study. In the study, 73 patients who were experiencing physical withdrawal from opioids used the neurostimulation device. They reported an initial mean score of 20.1 on the Clinical Opiate Withdrawal Score (COWS). All patients using the device had a reduction of at least 31% on the COWS within 30 minutes of beginning use of the NSS-2 Bridge. A total of 88% of participating patients transitioned to medication-assisted treatment after 5 days of using the device. Additional medications used to treat specific symptoms, such as nausea and vomiting, were permitted during the trial.
The physician-placed battery-powered device sits behind the ear and uses three percutaneous electrode arrays and one single-point needle to provide neurostimulation. The electrode placement is assisted with a transillumination technique, and also is based on known neuroanatomic landmarks for branches of cranial nerves V, VII, and IX, along with branches of the occipital nerve (Clin Med Diagnostics. 2015;5[4]:70-9).
The single-use device is designed to be used for up to 5 days during acute opioid withdrawal and is contraindicated for patients with hemophilia, patients with cardiac pacemakers, or those diagnosed with psoriasis vulgaris. The NSS-2 Bridge device requires a prescription and was cleared through the de novo premarket review pathway. This, said the FDA in the press statement, is “a regulatory pathway for some low- to moderate-risk devices that are novel and for which there is no legally marketed predicate device to which the device can claim substantial equivalence.”
The NSS-2 Bridge device will be marketed by Innovative Health Solutions.
[email protected]
On Twitter @karioakes
The Food and Drug Administration has cleared a medical device that applies electrical stimulation to cranial nerves in order to reduce opioid withdrawal symptoms.
The clearance was granted amid the opioid crisis, which is killing 175 Americans each day, according to the recent report by The President’s Commission on Combating Drug Addiction and the Opioid Crisis. Currently, opioid addiction is treatable by three approved medications, said FDA Commissioner Scott Gottlieb, MD, in a press statement announcing the agency’s decision to permit marketing of the NSS-2 Bridge device.
“While we continue to pursue better medicines for the treatment of opioid use disorder, we also need to look to devices that can assist in this therapy,” Dr. Gottlieb said.
The NSS-2 Bridge device was cleared as an acupuncture aid in 2014. The new use of the device to reduce the symptoms of opioid withdrawal required a new clearance, which was granted based on a single, one-arm clinical study. In the study, 73 patients who were experiencing physical withdrawal from opioids used the neurostimulation device. They reported an initial mean score of 20.1 on the Clinical Opiate Withdrawal Score (COWS). All patients using the device had a reduction of at least 31% on the COWS within 30 minutes of beginning use of the NSS-2 Bridge. A total of 88% of participating patients transitioned to medication-assisted treatment after 5 days of using the device. Additional medications used to treat specific symptoms, such as nausea and vomiting, were permitted during the trial.
The physician-placed battery-powered device sits behind the ear and uses three percutaneous electrode arrays and one single-point needle to provide neurostimulation. The electrode placement is assisted with a transillumination technique, and also is based on known neuroanatomic landmarks for branches of cranial nerves V, VII, and IX, along with branches of the occipital nerve (Clin Med Diagnostics. 2015;5[4]:70-9).
The single-use device is designed to be used for up to 5 days during acute opioid withdrawal and is contraindicated for patients with hemophilia, patients with cardiac pacemakers, or those diagnosed with psoriasis vulgaris. The NSS-2 Bridge device requires a prescription and was cleared through the de novo premarket review pathway. This, said the FDA in the press statement, is “a regulatory pathway for some low- to moderate-risk devices that are novel and for which there is no legally marketed predicate device to which the device can claim substantial equivalence.”
The NSS-2 Bridge device will be marketed by Innovative Health Solutions.
[email protected]
On Twitter @karioakes
Heart transplantation: Preop LVAD erases adverse impact of pulmonary hypertension
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.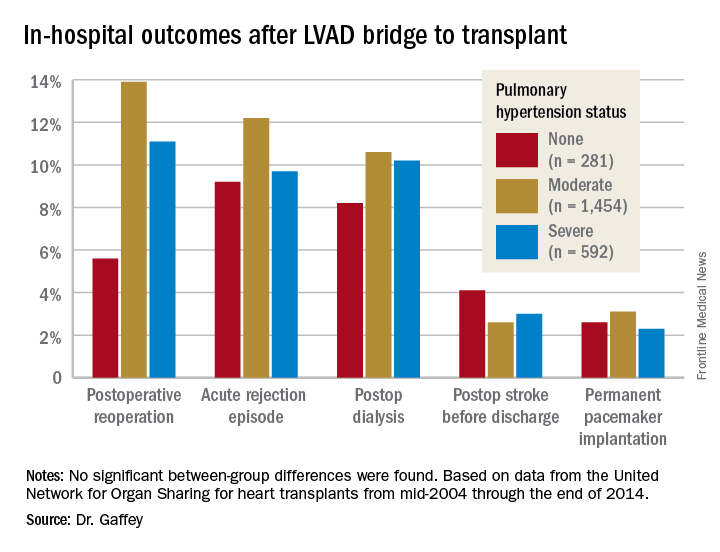
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
COLORADO SPRINGS – Reconsideration of the role of pulmonary hypertension in heart transplant outcomes is appropriate in the emerging era of the use of left ventricular assist devices (LVADs) as bridge to transplant, according to Ann C. Gaffey, MD, of the University of Pennsylvania, Philadelphia.
“Pulmonary hypertension secondary to congestive heart failure more than likely can be reversed to the values acceptable for heart transplant by the use of an LVAD. For bridge-to-transplant patients, pretransplant pulmonary hypertension does not affect recipient outcomes post transplantation,” she said at the annual meeting of the Western Thoracic Surgical Association.
Vasodilators are prescribed in an effort to reduce PH; however, 40% of patients with PH are unresponsive to the medications and have therefore been excluded from consideration as potential candidates for a donor heart.
But the growing use of LVADs as a bridge to transplant has changed all that, Dr. Gaffey said. As supporting evidence, she presented a retrospective analysis of the United Network for Organ Sharing database on adult heart transplants from mid-2004 through the end of 2014.
The review turned up 3,951 heart transplant recipients who had been bridged to transplant with an LVAD. Dr. Gaffey and her coinvestigators divided them into three groups: 281 patients without pretransplant PH; 1,454 with moderate PH as defined by 1-3 Wood units; and 592 with severe PH and more than 3 Wood units.
The three groups didn’t differ in terms of age, sex, wait-list time, or the prevalence of diabetes or renal, liver, or cerebrovascular disease. Nor did their donors differ in age, sex, left ventricular function, or allograft ischemic time.
Key in-hospital outcomes were similar between the groups with no, mild, and severe PH.
Moreover, there was no between-group difference in the rate of rejection at 1 year. Five-year survival rates were closely similar in the three groups, in the mid-70s.
Audience member Nahush A. Mokadam, MD, rose to praise Dr. Gaffey’s report.
“This is a great and important study. I think as a group we have been too conservative with pulmonary hypertension, so thank you for shining a good light on it,” said Dr. Mokadam of the University of Washington, Seattle.
Dr. Gaffey reported having no financial conflicts regarding the study, which was conducted free of commercial support.
AT THE WTSA ANNUAL MEETING
Key clinical point:
Major finding: It’s time to reconsider the practice of excluding patients with pulmonary hypertension from consideration for a donor heart.
Data source: A retrospective analysis of the United Network for Organ Sharing database including outcomes out to 5 years on 3,951 heart transplant recipients who had been bridged to transplant with an LVAD, most of whom had moderate or severe pulmonary hypertension before transplant.
Disclosures: This study was conducted free of commercial support. The presenter reported having no relevant financial conflicts of interest.
Incidence of adenomyosis in hysterectomy patients higher than previously reported
NATIONAL HARBOR, MD – Patients who experience chronic pelvic pain have higher rates of adenomyosis than previously thought, suggest new findings presented at the AAGL Global Congress.
Samantha P. Nadella, MD, and her associates conducted a retrospective cohort study analyzing 101 women with chronic pelvic pain who underwent hysterectomy between April 2014 and December 2016. In total, 51 patients (50.5%) were found to have adenomyosis. Previous studies of adenomyosis had suggested an overall incidence rate of 20%-35%. Dr. Nadella, currently of Kaiser Permanente South Bay Medical Center in Harbor City, Calif., conducted the study during her fellowship in minimally invasive gynecology surgery at St. Joseph’s Hospital and Medical Center in Phoenix.
“Anytime women have pelvic pain, or abdominal pain, they are always sent to their gynecologist,” Dr. Nadella said in an interview. However, there are many disorders that may present with pelvic pain but are not related to uterine pathology, including pelvic floor muscle dysfunction, interstitial cystitis, and irritable bowel syndrome. As suggested in this study, pelvic pain is frequently present in patients with IBS, both with and without adenomyosis.
“When we’re talking about hysterectomy and surgical management for pelvic pain, we need to do our due diligence to make sure we are not missing more treatable disorders, especially those with conservative treatments,” Dr. Nadella said. “It is important to counsel patients who are undergoing surgery for pelvic pain by explaining what will happen and what they can expect, including postsurgical pain and need for ongoing treatments. Additionally, cross-disciplinary communication is key in delivering more effective and personalized treatment of pelvic pain.”
Dr. Nadella reported having no financial disclosures.
NATIONAL HARBOR, MD – Patients who experience chronic pelvic pain have higher rates of adenomyosis than previously thought, suggest new findings presented at the AAGL Global Congress.
Samantha P. Nadella, MD, and her associates conducted a retrospective cohort study analyzing 101 women with chronic pelvic pain who underwent hysterectomy between April 2014 and December 2016. In total, 51 patients (50.5%) were found to have adenomyosis. Previous studies of adenomyosis had suggested an overall incidence rate of 20%-35%. Dr. Nadella, currently of Kaiser Permanente South Bay Medical Center in Harbor City, Calif., conducted the study during her fellowship in minimally invasive gynecology surgery at St. Joseph’s Hospital and Medical Center in Phoenix.
“Anytime women have pelvic pain, or abdominal pain, they are always sent to their gynecologist,” Dr. Nadella said in an interview. However, there are many disorders that may present with pelvic pain but are not related to uterine pathology, including pelvic floor muscle dysfunction, interstitial cystitis, and irritable bowel syndrome. As suggested in this study, pelvic pain is frequently present in patients with IBS, both with and without adenomyosis.
“When we’re talking about hysterectomy and surgical management for pelvic pain, we need to do our due diligence to make sure we are not missing more treatable disorders, especially those with conservative treatments,” Dr. Nadella said. “It is important to counsel patients who are undergoing surgery for pelvic pain by explaining what will happen and what they can expect, including postsurgical pain and need for ongoing treatments. Additionally, cross-disciplinary communication is key in delivering more effective and personalized treatment of pelvic pain.”
Dr. Nadella reported having no financial disclosures.
NATIONAL HARBOR, MD – Patients who experience chronic pelvic pain have higher rates of adenomyosis than previously thought, suggest new findings presented at the AAGL Global Congress.
Samantha P. Nadella, MD, and her associates conducted a retrospective cohort study analyzing 101 women with chronic pelvic pain who underwent hysterectomy between April 2014 and December 2016. In total, 51 patients (50.5%) were found to have adenomyosis. Previous studies of adenomyosis had suggested an overall incidence rate of 20%-35%. Dr. Nadella, currently of Kaiser Permanente South Bay Medical Center in Harbor City, Calif., conducted the study during her fellowship in minimally invasive gynecology surgery at St. Joseph’s Hospital and Medical Center in Phoenix.
“Anytime women have pelvic pain, or abdominal pain, they are always sent to their gynecologist,” Dr. Nadella said in an interview. However, there are many disorders that may present with pelvic pain but are not related to uterine pathology, including pelvic floor muscle dysfunction, interstitial cystitis, and irritable bowel syndrome. As suggested in this study, pelvic pain is frequently present in patients with IBS, both with and without adenomyosis.
“When we’re talking about hysterectomy and surgical management for pelvic pain, we need to do our due diligence to make sure we are not missing more treatable disorders, especially those with conservative treatments,” Dr. Nadella said. “It is important to counsel patients who are undergoing surgery for pelvic pain by explaining what will happen and what they can expect, including postsurgical pain and need for ongoing treatments. Additionally, cross-disciplinary communication is key in delivering more effective and personalized treatment of pelvic pain.”
Dr. Nadella reported having no financial disclosures.
AT AAGL 2017
Key clinical point:
Major finding: In total, 51 of 101 (50.5%) patients undergoing hysterectomy had adenomyosis.
Data source: Retrospective cohort study of 101 patients with chronic pain undergoing hysterectomy between April 2014 and December 2016 at a community hospital.
Disclosures: Dr. Nadella reported having no financial disclosures.