User login
Product can improve joint health in hemophilia A
New research suggests prophylaxis with a recombinant factor VIII Fc fusion protein (rFVIIIFc) can improve joint health over time in patients with hemophilia A.
Patients saw continuous improvement in joint health over a nearly 3-year period, regardless of prior treatment regimen, severity of joint damage, or target joints.
“Gradual joint destruction, which is the leading cause of morbidity for people with hemophilia, remains a significant challenge in the treatment of hemophilia A,” said Johannes Oldenburg, MD, of University Clinic Bonn in Germany.
“This is the first study to show that functional joint health can continue to improve using prophylactic treatment with an extended half-life factor therapy, even for those who have severe joint disease at the start of treatment.”
Dr Oldenburg and his colleagues reported these findings in Haemophilia. The research was sponsored by Biogen/Bioverativ and Sobi, the companies marketing rFVIIIFc (or efmoroctocog alfa) as Eloctate or Elocta.
This interim post hoc analysis was an evaluation of joint health in adults and adolescents who received rFVIIIFc prophylaxis in the A-LONG and ASPIRE studies.
In A-LONG, patients age 12 and older who had severe hemophilia A received rFVIIIFc at 25-65 IU/kg every 3 to 5 days (arm 1), at 65 IU/kg weekly (arm 2), or as episodic treatment (arm 3). Patients who completed A-LONG could then enroll in the ASPIRE extension study.
For the current analysis, Dr Oldenburg and his colleagues assessed joint health in ASPIRE enrollees using a modified version of the Hemophilia Joint Health Score (mHJHS). This tool grades joints by specific domains, including swelling, muscle atrophy, alignment, range of motion, joint pain, strength, and global gait.
The researchers examined mHJHS measurements (a decrease in score reflecting improvement) taken at A-LONG baseline, ASPIRE baseline, and annually thereafter for roughly 2.8 years of treatment.
There were 47 patients who had mHJHS data at both study baselines, ASPIRE year 1, and ASPIRE year 2.
These patients had a mean improvement in joint health score of -4.1 at ASPIRE year 2, compared with A-LONG baseline (P=0.001).
The mean improvement was -2.4 (P=0.09) for patients who received pre-study prophylaxis and -7.2 (P=0.003) for those who received pre-study episodic treatment.
The mean improvement was -5.6 (P=0.005) in patients with target joints and -8.8 (P=0.02) in those with severe joint destruction.
The mHJHS components with the greatest improvement at ASPIRE year 2 were swelling (-1.4, P=0.008), range of motion (-1.1, P=0.03), and strength (-0.8, P=0.04). ![]()
New research suggests prophylaxis with a recombinant factor VIII Fc fusion protein (rFVIIIFc) can improve joint health over time in patients with hemophilia A.
Patients saw continuous improvement in joint health over a nearly 3-year period, regardless of prior treatment regimen, severity of joint damage, or target joints.
“Gradual joint destruction, which is the leading cause of morbidity for people with hemophilia, remains a significant challenge in the treatment of hemophilia A,” said Johannes Oldenburg, MD, of University Clinic Bonn in Germany.
“This is the first study to show that functional joint health can continue to improve using prophylactic treatment with an extended half-life factor therapy, even for those who have severe joint disease at the start of treatment.”
Dr Oldenburg and his colleagues reported these findings in Haemophilia. The research was sponsored by Biogen/Bioverativ and Sobi, the companies marketing rFVIIIFc (or efmoroctocog alfa) as Eloctate or Elocta.
This interim post hoc analysis was an evaluation of joint health in adults and adolescents who received rFVIIIFc prophylaxis in the A-LONG and ASPIRE studies.
In A-LONG, patients age 12 and older who had severe hemophilia A received rFVIIIFc at 25-65 IU/kg every 3 to 5 days (arm 1), at 65 IU/kg weekly (arm 2), or as episodic treatment (arm 3). Patients who completed A-LONG could then enroll in the ASPIRE extension study.
For the current analysis, Dr Oldenburg and his colleagues assessed joint health in ASPIRE enrollees using a modified version of the Hemophilia Joint Health Score (mHJHS). This tool grades joints by specific domains, including swelling, muscle atrophy, alignment, range of motion, joint pain, strength, and global gait.
The researchers examined mHJHS measurements (a decrease in score reflecting improvement) taken at A-LONG baseline, ASPIRE baseline, and annually thereafter for roughly 2.8 years of treatment.
There were 47 patients who had mHJHS data at both study baselines, ASPIRE year 1, and ASPIRE year 2.
These patients had a mean improvement in joint health score of -4.1 at ASPIRE year 2, compared with A-LONG baseline (P=0.001).
The mean improvement was -2.4 (P=0.09) for patients who received pre-study prophylaxis and -7.2 (P=0.003) for those who received pre-study episodic treatment.
The mean improvement was -5.6 (P=0.005) in patients with target joints and -8.8 (P=0.02) in those with severe joint destruction.
The mHJHS components with the greatest improvement at ASPIRE year 2 were swelling (-1.4, P=0.008), range of motion (-1.1, P=0.03), and strength (-0.8, P=0.04). ![]()
New research suggests prophylaxis with a recombinant factor VIII Fc fusion protein (rFVIIIFc) can improve joint health over time in patients with hemophilia A.
Patients saw continuous improvement in joint health over a nearly 3-year period, regardless of prior treatment regimen, severity of joint damage, or target joints.
“Gradual joint destruction, which is the leading cause of morbidity for people with hemophilia, remains a significant challenge in the treatment of hemophilia A,” said Johannes Oldenburg, MD, of University Clinic Bonn in Germany.
“This is the first study to show that functional joint health can continue to improve using prophylactic treatment with an extended half-life factor therapy, even for those who have severe joint disease at the start of treatment.”
Dr Oldenburg and his colleagues reported these findings in Haemophilia. The research was sponsored by Biogen/Bioverativ and Sobi, the companies marketing rFVIIIFc (or efmoroctocog alfa) as Eloctate or Elocta.
This interim post hoc analysis was an evaluation of joint health in adults and adolescents who received rFVIIIFc prophylaxis in the A-LONG and ASPIRE studies.
In A-LONG, patients age 12 and older who had severe hemophilia A received rFVIIIFc at 25-65 IU/kg every 3 to 5 days (arm 1), at 65 IU/kg weekly (arm 2), or as episodic treatment (arm 3). Patients who completed A-LONG could then enroll in the ASPIRE extension study.
For the current analysis, Dr Oldenburg and his colleagues assessed joint health in ASPIRE enrollees using a modified version of the Hemophilia Joint Health Score (mHJHS). This tool grades joints by specific domains, including swelling, muscle atrophy, alignment, range of motion, joint pain, strength, and global gait.
The researchers examined mHJHS measurements (a decrease in score reflecting improvement) taken at A-LONG baseline, ASPIRE baseline, and annually thereafter for roughly 2.8 years of treatment.
There were 47 patients who had mHJHS data at both study baselines, ASPIRE year 1, and ASPIRE year 2.
These patients had a mean improvement in joint health score of -4.1 at ASPIRE year 2, compared with A-LONG baseline (P=0.001).
The mean improvement was -2.4 (P=0.09) for patients who received pre-study prophylaxis and -7.2 (P=0.003) for those who received pre-study episodic treatment.
The mean improvement was -5.6 (P=0.005) in patients with target joints and -8.8 (P=0.02) in those with severe joint destruction.
The mHJHS components with the greatest improvement at ASPIRE year 2 were swelling (-1.4, P=0.008), range of motion (-1.1, P=0.03), and strength (-0.8, P=0.04). ![]()
Birthdays, Booze, and … Broken Bones?
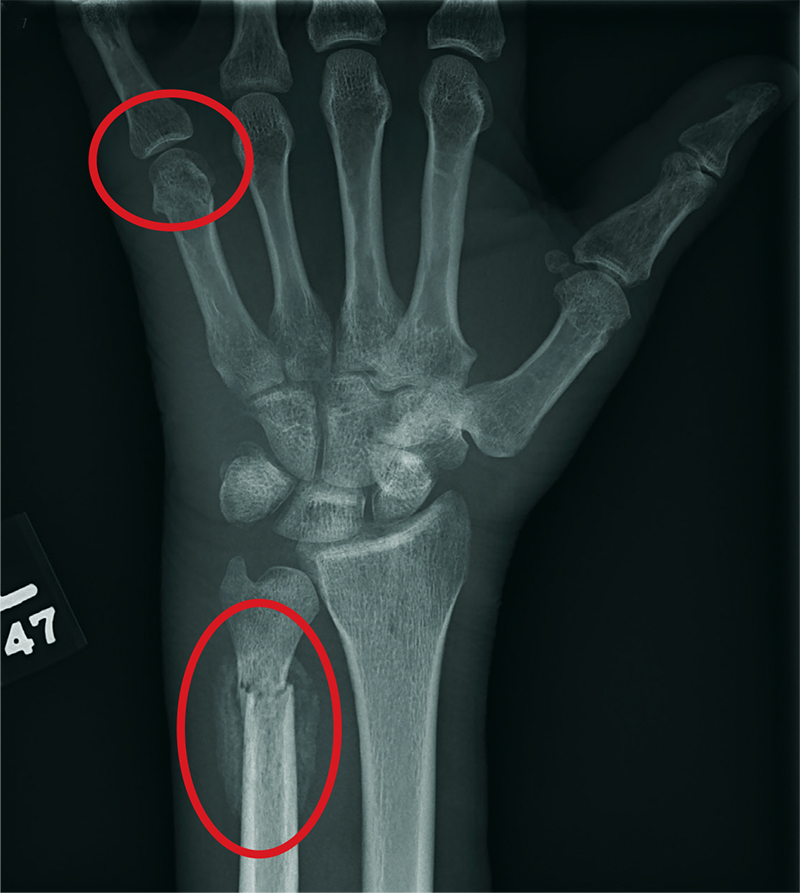
ANSWER
The radiograph shows two fractures: one within the distal ulna and one within the fifth metacarpal. On closer examination, you can see that a bony callous surrounds each of the fracture lines, making these injuries more likely to be subacute or remote than acute.
Review of the patient's electronic health record showed he had presented three months earlier for a left hand and wrist injury, at which time an acute fracture was diagnosed. Nonetheless, he was placed in a splint and referred to orthopedics for outpatient follow-up.

ANSWER
The radiograph shows two fractures: one within the distal ulna and one within the fifth metacarpal. On closer examination, you can see that a bony callous surrounds each of the fracture lines, making these injuries more likely to be subacute or remote than acute.
Review of the patient's electronic health record showed he had presented three months earlier for a left hand and wrist injury, at which time an acute fracture was diagnosed. Nonetheless, he was placed in a splint and referred to orthopedics for outpatient follow-up.

ANSWER
The radiograph shows two fractures: one within the distal ulna and one within the fifth metacarpal. On closer examination, you can see that a bony callous surrounds each of the fracture lines, making these injuries more likely to be subacute or remote than acute.
Review of the patient's electronic health record showed he had presented three months earlier for a left hand and wrist injury, at which time an acute fracture was diagnosed. Nonetheless, he was placed in a splint and referred to orthopedics for outpatient follow-up.
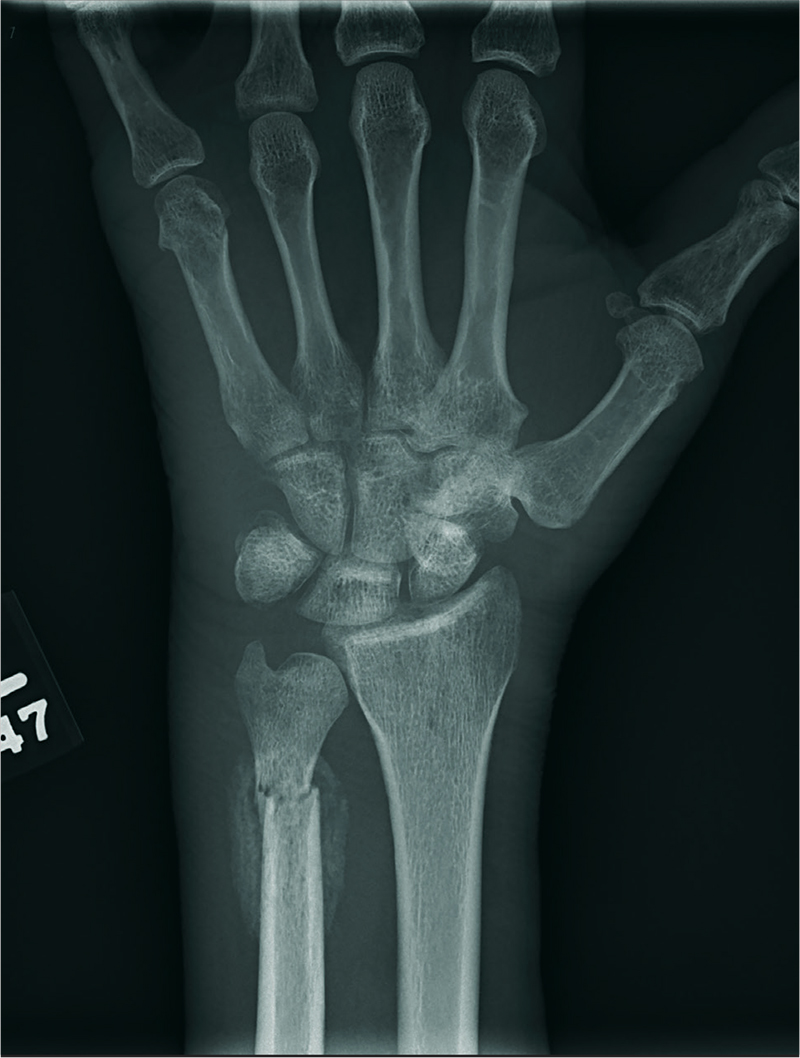
A 60-year-old man is brought to your facility emergently for decreased consciousness secondary to alcohol intoxication. He is somewhat incoherent, but from what you gather, he was attending a birthday celebration. He does not know how much he drank.
The patient complains of a headache and pain in his left wrist. You ask if he fell or was assaulted, but he does not respond. His medical history is otherwise unknown.
His initial vital signs are stable, and primary survey does not show any major injuries. He appears to spontaneously move all four extremities.
Closer examination of his left wrist shows no deformity or swelling, but the dorsolateral aspect of his hand is tender. Radiograph of his left wrist is obtained (shown). What is your impression?
VIDEO: Treating vascular lesions in children
LAS VEGAS – Clinicians should not shy away from light-based treatment of vascular lesions in children, for reasons that include achieving better results when treated early, according to Kristen M. Kelly, MD.
Special considerations include addressing children’s fears. “One of the strategies we use is we have child life specialists who help us” create a friendly and welcoming environment, Dr. Kelly said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Consequently, “many of our children actually come in, they’re excited about their visit ... and are looking forward to seeing us at the next visit,” she noted.
Which type of anesthesia to use is another important consideration when treating children, said Dr. Kelly of the University of California, Irvine, in Orange. “For a larger procedure ... one definitely could consider general anesthesia,” but there are risks and benefits to general anesthesia in very young children, and options should be discussed with patients and their families, she said.
Dr. Kelly disclosed relationships with multiple companies including Allergan, MundiPharma, Syneron-Candela, Light Sciences Oncology, Novartis, Sciton, and ThermiRF.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Clinicians should not shy away from light-based treatment of vascular lesions in children, for reasons that include achieving better results when treated early, according to Kristen M. Kelly, MD.
Special considerations include addressing children’s fears. “One of the strategies we use is we have child life specialists who help us” create a friendly and welcoming environment, Dr. Kelly said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Consequently, “many of our children actually come in, they’re excited about their visit ... and are looking forward to seeing us at the next visit,” she noted.
Which type of anesthesia to use is another important consideration when treating children, said Dr. Kelly of the University of California, Irvine, in Orange. “For a larger procedure ... one definitely could consider general anesthesia,” but there are risks and benefits to general anesthesia in very young children, and options should be discussed with patients and their families, she said.
Dr. Kelly disclosed relationships with multiple companies including Allergan, MundiPharma, Syneron-Candela, Light Sciences Oncology, Novartis, Sciton, and ThermiRF.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Clinicians should not shy away from light-based treatment of vascular lesions in children, for reasons that include achieving better results when treated early, according to Kristen M. Kelly, MD.
Special considerations include addressing children’s fears. “One of the strategies we use is we have child life specialists who help us” create a friendly and welcoming environment, Dr. Kelly said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. Consequently, “many of our children actually come in, they’re excited about their visit ... and are looking forward to seeing us at the next visit,” she noted.
Which type of anesthesia to use is another important consideration when treating children, said Dr. Kelly of the University of California, Irvine, in Orange. “For a larger procedure ... one definitely could consider general anesthesia,” but there are risks and benefits to general anesthesia in very young children, and options should be discussed with patients and their families, she said.
Dr. Kelly disclosed relationships with multiple companies including Allergan, MundiPharma, Syneron-Candela, Light Sciences Oncology, Novartis, Sciton, and ThermiRF.
SDEF and this news organization are owned by the same parent company.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Your patient has a large symptomatic fibroid: Tools for decision making
VIDEO: A challenging case of lichen planus
LAS VEGAS – For challenging cases of oral or cutaneous lichen planus, bullous pemphigoid, or lupus, Miriam S. Bettencourt, MD, recommends thinking outside the box and considering off-label treatments.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Bettencourt discussed such cases, including a series of patients with oral lichen planus who improved with apremilast, an oral phosphodiesterase 4 inhibitor approved for psoriasis.
In a video interview at the meeting, she described one of those patients, a 73-year-old woman with mouth ulcers who was diagnosed with oral lichen planus. Multiple topical and oral therapies proved unsuccessful, and her condition was eventually controlled with apremilast, and the patient is doing well, “with occasional flares,” said Dr. Bettencourt, of the University of Nevada, Las Vegas.
She described this case in her annual presentation at the meeting, titled “Great Cases From the Las Vegas Dermatology Society.”
Dr. Bettencourt disclosed relationships with multiple companies including AbbVie, Aclaris, Celgene, IntraDerm, Pfizer, Promium, Sun Pharma, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – For challenging cases of oral or cutaneous lichen planus, bullous pemphigoid, or lupus, Miriam S. Bettencourt, MD, recommends thinking outside the box and considering off-label treatments.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Bettencourt discussed such cases, including a series of patients with oral lichen planus who improved with apremilast, an oral phosphodiesterase 4 inhibitor approved for psoriasis.
In a video interview at the meeting, she described one of those patients, a 73-year-old woman with mouth ulcers who was diagnosed with oral lichen planus. Multiple topical and oral therapies proved unsuccessful, and her condition was eventually controlled with apremilast, and the patient is doing well, “with occasional flares,” said Dr. Bettencourt, of the University of Nevada, Las Vegas.
She described this case in her annual presentation at the meeting, titled “Great Cases From the Las Vegas Dermatology Society.”
Dr. Bettencourt disclosed relationships with multiple companies including AbbVie, Aclaris, Celgene, IntraDerm, Pfizer, Promium, Sun Pharma, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – For challenging cases of oral or cutaneous lichen planus, bullous pemphigoid, or lupus, Miriam S. Bettencourt, MD, recommends thinking outside the box and considering off-label treatments.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Dr. Bettencourt discussed such cases, including a series of patients with oral lichen planus who improved with apremilast, an oral phosphodiesterase 4 inhibitor approved for psoriasis.
In a video interview at the meeting, she described one of those patients, a 73-year-old woman with mouth ulcers who was diagnosed with oral lichen planus. Multiple topical and oral therapies proved unsuccessful, and her condition was eventually controlled with apremilast, and the patient is doing well, “with occasional flares,” said Dr. Bettencourt, of the University of Nevada, Las Vegas.
She described this case in her annual presentation at the meeting, titled “Great Cases From the Las Vegas Dermatology Society.”
Dr. Bettencourt disclosed relationships with multiple companies including AbbVie, Aclaris, Celgene, IntraDerm, Pfizer, Promium, Sun Pharma, and Valeant.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Aducanumab continues to rack up positive numbers in phase 1b open-label extension
BOSTON – The antiamyloid antibody aducanumab continued its slide around the bases this week, revealing more positive imaging and cognitive data at 36 months into the phase 1b PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study improved the most on two measures of cognition, the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–sum of boxes (CDR-sb). On the pathology side, at least some of the 10-mg/kg patients dropped below the threshold of PET amyloid positivity by 24 months and stayed at that low level up to 36 months, Samantha Budd Haeberlein, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
The 36-month data support the continued development of aducanumab, she said. The antibody is now being tested in two phase 3 studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD is good news for safety and good news for the signals we need to see in the phase 3 trials,” Maria Carillo, PhD, chief science officer of the Alzheimer’s Association said when asked to comment on the latest data. “These are hopeful signs, but – based on what we’ve learned from past Alzheimer’s studies – we need to wait for the phase 3 trial results.”
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaque in the brain.
PRIME enrolled 165 patients with prodromal or mild Alzheimer’s disease. Importantly, all of the subjects had brain amyloid proven by PET imaging. PRIME is the first randomized trial of an antiamyloid compound to enroll a purely PET-proven amyloid-positive cohort. These subjects were randomized to placebo or aducanumab at 1, 3, 6, or 10 mg/kg for 1 year. This was followed by a 2-year open-label extension period. Patients who were randomized to placebo or 1 mg/kg were switched to aducanumab 3 mg/kg or to a 3- to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3, 6 or 10 mg/kg or titration in the placebo-controlled period continued in the same dose group.
Dr. Haeberlein presented only the fixed-dose data; the titration group data will be presented later in the conference.
PRIME’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, not usually assessed in a phase 1b study, are exploratory. This is important to remember, Dr. Haeberlein said. She also stressed that the numbers in each dosing group are quite small. Of the original cohort, 117 entered the extension study and just 50 made it to 166 weeks, at which time 10-16 patients were in each of the dosage cohorts.
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan. The 6-mg/kg group approached the threshold, but did not fall below it. The 1- and 3-mg/kg groups declined similarly to each other, although not as dramatically as the higher-dose group.
Everyone in the trial declined on both cognitive measures, the MMSE and CDR-sb. However, the decline was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who took 10 mg/kg. The average decline from baseline on the CDR-sb was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-sb were:
- 5.28 points in those who switched from placebo to 3 mg/kg.
- 6.11 points in those who switched from 1 mg/kg to 3 mg/kg.
- 3.86 points in the 3-mg/kg treatment group.
- 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were:
- 7.98 points in those who switched from placebo to 3 mg/kg.
- 6.35 points in those who switched from 1 mg/kg to 3 mg/kg.
- 4.83 points in the 3-mg/kg treatment group.
- 8.97 points in the 6 mg/kg treatment group.
During the presentation, Dr. Haeberlein said these differences were not statistically significant. In an interview, she said, “In this extension trial, we aren’t talking about statistical significance. We are beyond that.”
The incidence of ARIA (Amyloid-Related Imaging Abnormalities), however, did not follow this dose-dependent pattern. All eight cases of ARIA-E (the edematous form) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg or in the 1-mg/kg group that titrated up to 3 mg/kg. All cases occurred early in the extension phase, with no new cases during the last year, and all but one occurred in APOE4 allele carriers.
Hemorrhagic ARIA was more sporadic, occurring in two placebo switchers, five taking 3 mg/kg, two taking 6 mg/kg, and one patient taking the highest 10 mg/kg dose. Again, these cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously.
In all of PRIME, 46 patients have experienced ARIA, with 6 experiencing more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died – one taking 6 mg/kg and one taking 10 mg/kg. Neither death was related to the study medication.
[email protected]
On Twitter @alz_gal
BOSTON – The antiamyloid antibody aducanumab continued its slide around the bases this week, revealing more positive imaging and cognitive data at 36 months into the phase 1b PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study improved the most on two measures of cognition, the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–sum of boxes (CDR-sb). On the pathology side, at least some of the 10-mg/kg patients dropped below the threshold of PET amyloid positivity by 24 months and stayed at that low level up to 36 months, Samantha Budd Haeberlein, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
The 36-month data support the continued development of aducanumab, she said. The antibody is now being tested in two phase 3 studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD is good news for safety and good news for the signals we need to see in the phase 3 trials,” Maria Carillo, PhD, chief science officer of the Alzheimer’s Association said when asked to comment on the latest data. “These are hopeful signs, but – based on what we’ve learned from past Alzheimer’s studies – we need to wait for the phase 3 trial results.”
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaque in the brain.
PRIME enrolled 165 patients with prodromal or mild Alzheimer’s disease. Importantly, all of the subjects had brain amyloid proven by PET imaging. PRIME is the first randomized trial of an antiamyloid compound to enroll a purely PET-proven amyloid-positive cohort. These subjects were randomized to placebo or aducanumab at 1, 3, 6, or 10 mg/kg for 1 year. This was followed by a 2-year open-label extension period. Patients who were randomized to placebo or 1 mg/kg were switched to aducanumab 3 mg/kg or to a 3- to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3, 6 or 10 mg/kg or titration in the placebo-controlled period continued in the same dose group.
Dr. Haeberlein presented only the fixed-dose data; the titration group data will be presented later in the conference.
PRIME’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, not usually assessed in a phase 1b study, are exploratory. This is important to remember, Dr. Haeberlein said. She also stressed that the numbers in each dosing group are quite small. Of the original cohort, 117 entered the extension study and just 50 made it to 166 weeks, at which time 10-16 patients were in each of the dosage cohorts.
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan. The 6-mg/kg group approached the threshold, but did not fall below it. The 1- and 3-mg/kg groups declined similarly to each other, although not as dramatically as the higher-dose group.
Everyone in the trial declined on both cognitive measures, the MMSE and CDR-sb. However, the decline was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who took 10 mg/kg. The average decline from baseline on the CDR-sb was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-sb were:
- 5.28 points in those who switched from placebo to 3 mg/kg.
- 6.11 points in those who switched from 1 mg/kg to 3 mg/kg.
- 3.86 points in the 3-mg/kg treatment group.
- 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were:
- 7.98 points in those who switched from placebo to 3 mg/kg.
- 6.35 points in those who switched from 1 mg/kg to 3 mg/kg.
- 4.83 points in the 3-mg/kg treatment group.
- 8.97 points in the 6 mg/kg treatment group.
During the presentation, Dr. Haeberlein said these differences were not statistically significant. In an interview, she said, “In this extension trial, we aren’t talking about statistical significance. We are beyond that.”
The incidence of ARIA (Amyloid-Related Imaging Abnormalities), however, did not follow this dose-dependent pattern. All eight cases of ARIA-E (the edematous form) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg or in the 1-mg/kg group that titrated up to 3 mg/kg. All cases occurred early in the extension phase, with no new cases during the last year, and all but one occurred in APOE4 allele carriers.
Hemorrhagic ARIA was more sporadic, occurring in two placebo switchers, five taking 3 mg/kg, two taking 6 mg/kg, and one patient taking the highest 10 mg/kg dose. Again, these cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously.
In all of PRIME, 46 patients have experienced ARIA, with 6 experiencing more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died – one taking 6 mg/kg and one taking 10 mg/kg. Neither death was related to the study medication.
[email protected]
On Twitter @alz_gal
BOSTON – The antiamyloid antibody aducanumab continued its slide around the bases this week, revealing more positive imaging and cognitive data at 36 months into the phase 1b PRIME trial.
Patients who have been taking the highest dose of aducanumab, 10 mg/kg, for the duration of the study improved the most on two measures of cognition, the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating Scale–sum of boxes (CDR-sb). On the pathology side, at least some of the 10-mg/kg patients dropped below the threshold of PET amyloid positivity by 24 months and stayed at that low level up to 36 months, Samantha Budd Haeberlein, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
The 36-month data support the continued development of aducanumab, she said. The antibody is now being tested in two phase 3 studies, ENGAGE and EMERGE.
“The aducanumab data reported at CTAD is good news for safety and good news for the signals we need to see in the phase 3 trials,” Maria Carillo, PhD, chief science officer of the Alzheimer’s Association said when asked to comment on the latest data. “These are hopeful signs, but – based on what we’ve learned from past Alzheimer’s studies – we need to wait for the phase 3 trial results.”
Aducanumab is a monoclonal human antibody derived from B cells collected from a cohort of cognitively normal elderly subjects and cognitively impaired elderly subjects who exhibited unusually slow decline, according to Biogen. It binds to fibrillar and oligomeric amyloid aggregates, thus directly reducing amyloid plaque in the brain.
PRIME enrolled 165 patients with prodromal or mild Alzheimer’s disease. Importantly, all of the subjects had brain amyloid proven by PET imaging. PRIME is the first randomized trial of an antiamyloid compound to enroll a purely PET-proven amyloid-positive cohort. These subjects were randomized to placebo or aducanumab at 1, 3, 6, or 10 mg/kg for 1 year. This was followed by a 2-year open-label extension period. Patients who were randomized to placebo or 1 mg/kg were switched to aducanumab 3 mg/kg or to a 3- to 6-mg/kg titration regimen in the long-term extension. Patients randomized to aducanumab at 3, 6 or 10 mg/kg or titration in the placebo-controlled period continued in the same dose group.
Dr. Haeberlein presented only the fixed-dose data; the titration group data will be presented later in the conference.
PRIME’s primary outcomes are safety and tolerability. The cognitive and functional outcomes, not usually assessed in a phase 1b study, are exploratory. This is important to remember, Dr. Haeberlein said. She also stressed that the numbers in each dosing group are quite small. Of the original cohort, 117 entered the extension study and just 50 made it to 166 weeks, at which time 10-16 patients were in each of the dosage cohorts.
At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan. The 6-mg/kg group approached the threshold, but did not fall below it. The 1- and 3-mg/kg groups declined similarly to each other, although not as dramatically as the higher-dose group.
Everyone in the trial declined on both cognitive measures, the MMSE and CDR-sb. However, the decline was clearly attenuated in some of the active groups, where the best results were seen in the 10 patients who took 10 mg/kg. The average decline from baseline on the CDR-sb was 2.84 points among those patients. In the other groups, declines from baseline on the CDR-sb were:
- 5.28 points in those who switched from placebo to 3 mg/kg.
- 6.11 points in those who switched from 1 mg/kg to 3 mg/kg.
- 3.86 points in the 3-mg/kg treatment group.
- 4.49 points in the 6-mg/kg treatment group.
Patients taking 10 mg/kg also fared best on the MMSE, declining 4.10 points on average. Declines in the other groups were:
- 7.98 points in those who switched from placebo to 3 mg/kg.
- 6.35 points in those who switched from 1 mg/kg to 3 mg/kg.
- 4.83 points in the 3-mg/kg treatment group.
- 8.97 points in the 6 mg/kg treatment group.
During the presentation, Dr. Haeberlein said these differences were not statistically significant. In an interview, she said, “In this extension trial, we aren’t talking about statistical significance. We are beyond that.”
The incidence of ARIA (Amyloid-Related Imaging Abnormalities), however, did not follow this dose-dependent pattern. All eight cases of ARIA-E (the edematous form) in the long-term extension phase occurred in the placebo group that switched to 1 mg/kg or in the 1-mg/kg group that titrated up to 3 mg/kg. All cases occurred early in the extension phase, with no new cases during the last year, and all but one occurred in APOE4 allele carriers.
Hemorrhagic ARIA was more sporadic, occurring in two placebo switchers, five taking 3 mg/kg, two taking 6 mg/kg, and one patient taking the highest 10 mg/kg dose. Again, these cases occurred early in the trial. All of the ARIA cases, regardless of etiology, were considered mild and resolved spontaneously.
In all of PRIME, 46 patients have experienced ARIA, with 6 experiencing more than one episode.
The most common adverse events in the long-term extension phase were falls, headache, and ARIA. Two patients in the extension phase died – one taking 6 mg/kg and one taking 10 mg/kg. Neither death was related to the study medication.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM CTAD
Key clinical point:
Major finding: At 36 months, the mean change in amyloid plaque level was greatest for the 10-mg/kg group, which, on average, fell below the threshold of amyloid positivity on florbetapir PET scan.
Data source: 3-year extension phase data from the phase 1B PRIME trial.
Disclosures: The PRIME trial is sponsored by Biogen. The presenter is an employee of the company.
VIDEO: Metabolic regulator FGF21 improves fibrosis in NASH patients
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
WASHINGTON – Fibroblast growth factor 21 (FGF21), a nonmitogenic hormone, improved fibrosis, liver injury, and steatosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the American Association for the Study of Liver Disease’s annual meeting.
There is no drug therapy currently available for NASH, the most advanced form of nonalcoholic fatty liver disease (NAFLD), creating a strong need for effective treatments, according to Arun Sanyal, MD, of the Virginia Commonwealth University, Richmond, said in a video interview.
This treatment “relative to placebo was associated with improvements in biomarkers of fibrosis, metabolic parameters, and markers of hepatic injury,” said Dr. Sanyal. “These results suggest BMS-986036 [FGF21] has beneficial effects on steatosis, liver injury, and fibrosis in NASH.”
Investigators conducted a phase 2 multicenter, double-blind, placebo-controlled study of 74 NASH patients to test BMS-986036, a pegylated version of FGF21.
Patients were an average of 51 years old, most were women (64%), who were predominantly white (96%), with a mean hepatic fat fraction of 19%.
Patients received either a 10-mg treatment daily, a 20-mg treatment weekly, or placebo, over the course of 16 weeks, with patients distributed equally among the three arms.
Overall hepatic fat fraction among the daily and weekly treatment groups reduced by 6.8% and 5.2%, respectively, compared with the placebo group, which reduced by 1.3% (P less than .001).
Patients in the treatment arms also saw improvement in average adiponectin levels, growing 15.3% in the daily arm and 15.7% in the weekly arm. Meanwhile, adiponectin levels dropped by an average of 3.5% in the placebo group.
In investigating serum Pro-C3 levels, which are associated with fibrosis, patients in the daily and weekly treatment group saw an average drop of 29% and 19%, respectively, as opposed to an increase of 2% in the placebo group (P less than .0001).
Patients in the treatment groups saw no serious adverse effects, and no patients died during the study.
Dr. Sanyal received funding for this study from Bristol-Myers Squibb and reported receiving financial compensation from Pfizer, Nimbus, Novartis, AstraZeneca, and other similar companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
AT THE LIVER MEETING 2017
More physicians excluded from MIPS under final CMS rule
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
Dopamine synthesis capacity appears linked to psychosis in bipolar disorder
Increased dopamine synthesis – a feature classically associated with schizophrenia – might underlie bipolar psychosis, a new study suggests.
To conduct the cross-sectional case-control study, Sameer Jauhar, MRCPsych, and his associates recruited 60 people from first-episode psychosis services in London – 22 with bipolar psychosis, 16 with schizophrenia, and 22 matched controls. Of the 22 with bipolar psychosis, 18 were antipsychotic-naïve or free, and of the 16 patients with schizophrenia, 14 were antipsychotic-naïve (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2943). The researchers used fluorodihydroxyphenyl-L-alanine ([18F]-DOPA) positron emission tomography to study dopamine synthesis capacity in the participants.
The study showed that mean dopamine synthesis capacity in the striatum was significantly higher both in the bipolar group and the schizophrenia group, compared with controls – even after excluding individuals taking antipsychotic medication.
“These results extend previous findings that dopamine synthesis capacity is elevated in schizophrenia and psychosis associated with temporal lobe epilepsy and increases with the onset of psychosis, suggesting that presynaptic dopamine dysfunction is associated with psychosis across diagnostic categories,” wrote Dr. Jauhar, who is affiliated with the Institute of Psychiatry, Psychology & Neuroscience at King’s College, London, and his coauthors.
In addition, Dr. Jauhar and his coauthors found a significant relationship between mean whole striatal dopamine synthesis capacity and Positive and Negative Syndrome Scale (PANSS) scores in the group of patients who was experiencing a psychotic episode at the time of the study, which overall explained 27% of the variance.
In the bipolar disorder group, there was a nonsignificant relationship between whole striatal dopamine synthesis capacity and the PANSS positive subscale symptom severity score. However, this became significant when the analysis was restricted to patients who were experiencing a current psychotic episode and accounted for 36% of the variance in psychotic symptoms.
But this effect was not seen in patients with schizophrenia – all of whom were experiencing a psychotic episode at the time.
“Our finding of a relationship between positive psychotic symptoms and dopamine synthesis capacity in the combined bipolar and schizophrenia sample but not in the schizophrenia group could be due to a lack of power or inclusion of more patients with longer illness durations in the schizophrenia group,” the researchers reported.
Overall, no significant difference was found in mean dopamine synthesis capacity between patients with bipolar disorder and those with schizophrenia.
The authors also controlled for duration of illness, given that those in the schizophrenia group had a longer duration of illness than those in the bipolar group, with no effect on mean dopamine synthesis capacity differences between the two. The effect was seen in the whole striatum, the associative striatum, the limbic striatum, and the sensorimotor striatum.
“Relative to controls, the subregional analyses showed significant elevations in all three functional striatal subdivisions in the bipolar group but only a suggestion in the associative striatum in the schizophrenia group, with no differences in the substantia nigra for either group,” the authors wrote.
The authors acknowledged one concern with using patients experiencing a first episode of psychosis was that their diagnoses may change over time. But even after a minimum of 18 months’ follow-up, none of the original diagnoses had changed, and they had even been strengthened by the difference in negative but not positive symptoms.
The outcome measure used – KiCER – was an index for the uptake of [18F]-DOPA into dopamine neurons, and its conversion into [18F]-dopamine and storage in terminals. “Therefore, the increased KiCER we report likely reflects an increase in one or more of these processes, as well as a net increase in dopamine synthesis capacity,” they wrote.
“This finding provides a potential neurobiological explanation for why antipsychotic drugs, which are all dopamine D2/D3 receptor blockers, are effective in bipolar psychosis and schizophrenia and identifies the regulation of dopamine synthesis as a potential novel drug target for bipolar disorder and schizophrenia.” Furthermore, they said, the findings suggest that dopamine synthesis capacity might be a drug target for bipolar disorder and schizophrenia.
The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
Some empirical evidence suggests that “schizophrenia and bipolar disorder exist along a psychosis continuum, with some patients having more affective features and others having more psychotic features,” Dost Öngür, MD, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2330). However, the study by Dr. Jauhar and his associates show that both illnesses are similar when it comes to dopaminergic dysfunction.
“Clinical experience is perhaps more consistent with a model including not one but two dimensions, namely, affective and psychotic,” Dr. Öngür wrote. “This is because more severe psychosis does not always indicate less severe affective illness; affective syndromes of variable intensity are seen among patients with severe psychosis and vice versa.”
Future studies could seek to quantify the psychosis dimension with the ultimate goal of “developing a more valid classification system for psychotic disorders,” he wrote.
Dr. Öngür is affiliated with the department of psychiatry at McLean Hospital and Harvard Medical School, both in Boston. He reported serving on a scientific advisory board for Neurocrine Biosciences.
Some empirical evidence suggests that “schizophrenia and bipolar disorder exist along a psychosis continuum, with some patients having more affective features and others having more psychotic features,” Dost Öngür, MD, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2330). However, the study by Dr. Jauhar and his associates show that both illnesses are similar when it comes to dopaminergic dysfunction.
“Clinical experience is perhaps more consistent with a model including not one but two dimensions, namely, affective and psychotic,” Dr. Öngür wrote. “This is because more severe psychosis does not always indicate less severe affective illness; affective syndromes of variable intensity are seen among patients with severe psychosis and vice versa.”
Future studies could seek to quantify the psychosis dimension with the ultimate goal of “developing a more valid classification system for psychotic disorders,” he wrote.
Dr. Öngür is affiliated with the department of psychiatry at McLean Hospital and Harvard Medical School, both in Boston. He reported serving on a scientific advisory board for Neurocrine Biosciences.
Some empirical evidence suggests that “schizophrenia and bipolar disorder exist along a psychosis continuum, with some patients having more affective features and others having more psychotic features,” Dost Öngür, MD, PhD, wrote in an accompanying editorial (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2330). However, the study by Dr. Jauhar and his associates show that both illnesses are similar when it comes to dopaminergic dysfunction.
“Clinical experience is perhaps more consistent with a model including not one but two dimensions, namely, affective and psychotic,” Dr. Öngür wrote. “This is because more severe psychosis does not always indicate less severe affective illness; affective syndromes of variable intensity are seen among patients with severe psychosis and vice versa.”
Future studies could seek to quantify the psychosis dimension with the ultimate goal of “developing a more valid classification system for psychotic disorders,” he wrote.
Dr. Öngür is affiliated with the department of psychiatry at McLean Hospital and Harvard Medical School, both in Boston. He reported serving on a scientific advisory board for Neurocrine Biosciences.
Increased dopamine synthesis – a feature classically associated with schizophrenia – might underlie bipolar psychosis, a new study suggests.
To conduct the cross-sectional case-control study, Sameer Jauhar, MRCPsych, and his associates recruited 60 people from first-episode psychosis services in London – 22 with bipolar psychosis, 16 with schizophrenia, and 22 matched controls. Of the 22 with bipolar psychosis, 18 were antipsychotic-naïve or free, and of the 16 patients with schizophrenia, 14 were antipsychotic-naïve (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2943). The researchers used fluorodihydroxyphenyl-L-alanine ([18F]-DOPA) positron emission tomography to study dopamine synthesis capacity in the participants.
The study showed that mean dopamine synthesis capacity in the striatum was significantly higher both in the bipolar group and the schizophrenia group, compared with controls – even after excluding individuals taking antipsychotic medication.
“These results extend previous findings that dopamine synthesis capacity is elevated in schizophrenia and psychosis associated with temporal lobe epilepsy and increases with the onset of psychosis, suggesting that presynaptic dopamine dysfunction is associated with psychosis across diagnostic categories,” wrote Dr. Jauhar, who is affiliated with the Institute of Psychiatry, Psychology & Neuroscience at King’s College, London, and his coauthors.
In addition, Dr. Jauhar and his coauthors found a significant relationship between mean whole striatal dopamine synthesis capacity and Positive and Negative Syndrome Scale (PANSS) scores in the group of patients who was experiencing a psychotic episode at the time of the study, which overall explained 27% of the variance.
In the bipolar disorder group, there was a nonsignificant relationship between whole striatal dopamine synthesis capacity and the PANSS positive subscale symptom severity score. However, this became significant when the analysis was restricted to patients who were experiencing a current psychotic episode and accounted for 36% of the variance in psychotic symptoms.
But this effect was not seen in patients with schizophrenia – all of whom were experiencing a psychotic episode at the time.
“Our finding of a relationship between positive psychotic symptoms and dopamine synthesis capacity in the combined bipolar and schizophrenia sample but not in the schizophrenia group could be due to a lack of power or inclusion of more patients with longer illness durations in the schizophrenia group,” the researchers reported.
Overall, no significant difference was found in mean dopamine synthesis capacity between patients with bipolar disorder and those with schizophrenia.
The authors also controlled for duration of illness, given that those in the schizophrenia group had a longer duration of illness than those in the bipolar group, with no effect on mean dopamine synthesis capacity differences between the two. The effect was seen in the whole striatum, the associative striatum, the limbic striatum, and the sensorimotor striatum.
“Relative to controls, the subregional analyses showed significant elevations in all three functional striatal subdivisions in the bipolar group but only a suggestion in the associative striatum in the schizophrenia group, with no differences in the substantia nigra for either group,” the authors wrote.
The authors acknowledged one concern with using patients experiencing a first episode of psychosis was that their diagnoses may change over time. But even after a minimum of 18 months’ follow-up, none of the original diagnoses had changed, and they had even been strengthened by the difference in negative but not positive symptoms.
The outcome measure used – KiCER – was an index for the uptake of [18F]-DOPA into dopamine neurons, and its conversion into [18F]-dopamine and storage in terminals. “Therefore, the increased KiCER we report likely reflects an increase in one or more of these processes, as well as a net increase in dopamine synthesis capacity,” they wrote.
“This finding provides a potential neurobiological explanation for why antipsychotic drugs, which are all dopamine D2/D3 receptor blockers, are effective in bipolar psychosis and schizophrenia and identifies the regulation of dopamine synthesis as a potential novel drug target for bipolar disorder and schizophrenia.” Furthermore, they said, the findings suggest that dopamine synthesis capacity might be a drug target for bipolar disorder and schizophrenia.
The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
Increased dopamine synthesis – a feature classically associated with schizophrenia – might underlie bipolar psychosis, a new study suggests.
To conduct the cross-sectional case-control study, Sameer Jauhar, MRCPsych, and his associates recruited 60 people from first-episode psychosis services in London – 22 with bipolar psychosis, 16 with schizophrenia, and 22 matched controls. Of the 22 with bipolar psychosis, 18 were antipsychotic-naïve or free, and of the 16 patients with schizophrenia, 14 were antipsychotic-naïve (JAMA Psychiatry. 2017 Oct 11. doi: 10.1001/jamapsychiatry.2017.2943). The researchers used fluorodihydroxyphenyl-L-alanine ([18F]-DOPA) positron emission tomography to study dopamine synthesis capacity in the participants.
The study showed that mean dopamine synthesis capacity in the striatum was significantly higher both in the bipolar group and the schizophrenia group, compared with controls – even after excluding individuals taking antipsychotic medication.
“These results extend previous findings that dopamine synthesis capacity is elevated in schizophrenia and psychosis associated with temporal lobe epilepsy and increases with the onset of psychosis, suggesting that presynaptic dopamine dysfunction is associated with psychosis across diagnostic categories,” wrote Dr. Jauhar, who is affiliated with the Institute of Psychiatry, Psychology & Neuroscience at King’s College, London, and his coauthors.
In addition, Dr. Jauhar and his coauthors found a significant relationship between mean whole striatal dopamine synthesis capacity and Positive and Negative Syndrome Scale (PANSS) scores in the group of patients who was experiencing a psychotic episode at the time of the study, which overall explained 27% of the variance.
In the bipolar disorder group, there was a nonsignificant relationship between whole striatal dopamine synthesis capacity and the PANSS positive subscale symptom severity score. However, this became significant when the analysis was restricted to patients who were experiencing a current psychotic episode and accounted for 36% of the variance in psychotic symptoms.
But this effect was not seen in patients with schizophrenia – all of whom were experiencing a psychotic episode at the time.
“Our finding of a relationship between positive psychotic symptoms and dopamine synthesis capacity in the combined bipolar and schizophrenia sample but not in the schizophrenia group could be due to a lack of power or inclusion of more patients with longer illness durations in the schizophrenia group,” the researchers reported.
Overall, no significant difference was found in mean dopamine synthesis capacity between patients with bipolar disorder and those with schizophrenia.
The authors also controlled for duration of illness, given that those in the schizophrenia group had a longer duration of illness than those in the bipolar group, with no effect on mean dopamine synthesis capacity differences between the two. The effect was seen in the whole striatum, the associative striatum, the limbic striatum, and the sensorimotor striatum.
“Relative to controls, the subregional analyses showed significant elevations in all three functional striatal subdivisions in the bipolar group but only a suggestion in the associative striatum in the schizophrenia group, with no differences in the substantia nigra for either group,” the authors wrote.
The authors acknowledged one concern with using patients experiencing a first episode of psychosis was that their diagnoses may change over time. But even after a minimum of 18 months’ follow-up, none of the original diagnoses had changed, and they had even been strengthened by the difference in negative but not positive symptoms.
The outcome measure used – KiCER – was an index for the uptake of [18F]-DOPA into dopamine neurons, and its conversion into [18F]-dopamine and storage in terminals. “Therefore, the increased KiCER we report likely reflects an increase in one or more of these processes, as well as a net increase in dopamine synthesis capacity,” they wrote.
“This finding provides a potential neurobiological explanation for why antipsychotic drugs, which are all dopamine D2/D3 receptor blockers, are effective in bipolar psychosis and schizophrenia and identifies the regulation of dopamine synthesis as a potential novel drug target for bipolar disorder and schizophrenia.” Furthermore, they said, the findings suggest that dopamine synthesis capacity might be a drug target for bipolar disorder and schizophrenia.
The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
FROM JAMA PSYCHIATRY
Key clinical point: Dopamine synthesis capacity is elevated in individuals with bipolar disorder, particularly those experiencing a psychotic episode.
Major finding: Individuals with bipolar disorder have a similarly elevated dopamine synthesis capacity as individuals with schizophrenia.
Data source: Positron emission tomography study in 22 individuals with bipolar disorder, 16 with schizophrenia, and 22 controls.
Disclosures: The study was supported by several entities, including the Medical Research Council, the U.S. Brain & Behavior Research Foundation, the Wellcome Trust, and the National Institute for Health Research Biomedical Research Centre at South London. Three authors declared research funding, advisory or speaker engagements, or lecture payments from a variety of pharmaceutical companies. No other conflicts of interest were declared.
VIDEO: New herpes zoster vaccine may boost vaccination rate
LAS VEGAS – One of the benefits of the recently approved inactivated herpes zoster is its efficacy in older adults, Kenneth J. Tomecki, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
In addition, the vaccine will be recommended not only for healthy adults, but for ill adults aged 50 years and older, said Dr. Tomecki of the department of dermatology at the Cleveland Clinic. “Efficacy is greater than 90% for zoster and postherpetic neuralgia” with the new vaccine, he added.
Vaccination rates among eligible adults with the current vaccine, which is highly effective, are low, but ideally, the advent of the new vaccine will boost vaccination rates, especially in older adults, he noted.
for preventing herpes zoster in adults aged 50 years and older. The currently available herpes zoster vaccine, Zostavax, a live attenuated virus vaccine, was approved by the FDA in 2006.
Dr. Tomecki had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – One of the benefits of the recently approved inactivated herpes zoster is its efficacy in older adults, Kenneth J. Tomecki, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
In addition, the vaccine will be recommended not only for healthy adults, but for ill adults aged 50 years and older, said Dr. Tomecki of the department of dermatology at the Cleveland Clinic. “Efficacy is greater than 90% for zoster and postherpetic neuralgia” with the new vaccine, he added.
Vaccination rates among eligible adults with the current vaccine, which is highly effective, are low, but ideally, the advent of the new vaccine will boost vaccination rates, especially in older adults, he noted.
for preventing herpes zoster in adults aged 50 years and older. The currently available herpes zoster vaccine, Zostavax, a live attenuated virus vaccine, was approved by the FDA in 2006.
Dr. Tomecki had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – One of the benefits of the recently approved inactivated herpes zoster is its efficacy in older adults, Kenneth J. Tomecki, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
In addition, the vaccine will be recommended not only for healthy adults, but for ill adults aged 50 years and older, said Dr. Tomecki of the department of dermatology at the Cleveland Clinic. “Efficacy is greater than 90% for zoster and postherpetic neuralgia” with the new vaccine, he added.
Vaccination rates among eligible adults with the current vaccine, which is highly effective, are low, but ideally, the advent of the new vaccine will boost vaccination rates, especially in older adults, he noted.
for preventing herpes zoster in adults aged 50 years and older. The currently available herpes zoster vaccine, Zostavax, a live attenuated virus vaccine, was approved by the FDA in 2006.
Dr. Tomecki had no financial conflicts to disclose.
SDEF and this news organization are owned by the same parent company.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR