User login
Recognizing and Preventing Arbovirus Infections
What do patients need to know about arboviruses?
Dengue is the most common arbovirus worldwide, with more than 300 million individuals infected each year, most of them asymptomatic carriers. It is the most common febrile illness in travelers returning from Southeast Asia, South America, and the Caribbean. Dengue symptoms typically begin 3 to 12 days after exposure and may include fever; headache; conjunctivitis; and a biphasic rash that begins with blanching macular erythema, which patients may mistake for sunburn, followed by a morbilliform to petechial rash with islands of sparing (white islands in a sea of red). Severe dengue (formerly known as dengue hemorrhagic fever) may present with dramatic skin and mucosal hemorrhage including purpura, hemorrhagic bullae, and bleeding from orifices and injection sites, with associated thrombocytopenia and hypotension (dengue shock syndrome). Patients with onset of such symptoms need to go to the emergency department for inpatient management.
Individuals living in the United States should be particularly aware of West Nile virus, with infections reported in all US states in 2017, except Alaska and Hawaii thus far. Transmitted by the bite of the Culex mosquito, most infections are symptomatic; however, up to 20% of patients may present with nonspecific symptoms such as mild febrile or flulike symptoms, nonspecific morbilliform rash, and headaches, and up to 1% of patients may develop encephalitis or meningitis, with approximately 10% mortality rates.
What are your go-to treatments?
Arboviral infections generally are self-limited and there are no specific treatments available. Supportive care, including fluid resuscitation, and analgesia (if needed for joint, muscle, or bone pain) are the mainstays of management. Diagnoses generally are confirmed via viral polymerase chain reaction from serum (<7 days), IgM enzyme-linked immunosorbent assay (>4 days), or IgG serologies for later presentations.
Patients should avoid the use of nonsteroidal anti-inflammatory drugs if there is a possibility of dengue virus infection, as they may potentiate the risk for hemorrhagic complications in patients with severe dengue. Instead, acetaminophen is recommended for analgesia and antipyretic purposes, if needed.
How do you recommend patients prevent infection while traveling?
Primary prevention of infection and secondary prevention of transmission are important. Although mosquito bed netting is helpful in preventing some mosquito-borne viruses, many arboviruses (ie, dengue, Zika, chikun-gunya) are transmitted by primarily daytime-biting Aedes mosquitoes. In an endemic area, travelers should try to stay within air-conditioned buildings with intact window and door screens. When outdoors, wear long sleeves and pants and use Environmental Protection Agency-registered mosquito repellents. Conventional repellents include the following:
- DEET: concentrations 10% to 30% are safe for children 2 months and older and pregnant women; concentrations around 10% are effective for periods of approximately 2 hours; as the concentration of DEET increases, the duration of protection increases.
- Picaridin: concentrations of 5% to 20%; effective for 4 to 8 hours depending on the concentration; most effective concentration is 20%; is not effective against ticks.
Biopesticide repellents include the following:
- IR3535 (ethyl butylacetylaminopropionate): concentration 10% to 30% has been used as an insect repellent in Europe for 20 years with no substantial adverse effects.
- 2-undecanone: a natural compound from leaves and stems of the wild tomato plant; is a biopesticide product less toxic than conventional pesticides.
- Oil of lemon eucalyptus (OLE) or PMD (the synthesized version of OLE): concentration 30%; "pure" oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended.
- Natural oils (eg, soybean, lemongrass, citronella, cedar, peppermint, lavender, geranium) are exempted from Environmental Protection Agency registration; duration of effectiveness is estimated between 30 minutes and 2 hours.
Products that combine sunscreen and repellent are not recommended because sunscreen may need to be reapplied, increasing the toxicity of the repellent. Use separate products, applying sunscreen first and then applying the repellent.
Permethrin-treated clothing may provide an additional measure of protection, though in some endemic areas, resistance has been reported.
Because sexual transmission has been reported for Zika virus (between both male and female partners), any known or possibly infected persons should use condoms. Durations of abstinence or protected sex recommendations vary by situation and more detailed recommendations can be found from the Centers for Disease Control and Prevention. Pregnant women and women trying to get pregnant should take preventive measures with respect to Zika due to the possibility of the virus causing severe birth defects (congenital Zika syndrome) including microcephaly, joint deformities, ocular damage, and hypertonia.
What do patients need to know about arboviruses?
Dengue is the most common arbovirus worldwide, with more than 300 million individuals infected each year, most of them asymptomatic carriers. It is the most common febrile illness in travelers returning from Southeast Asia, South America, and the Caribbean. Dengue symptoms typically begin 3 to 12 days after exposure and may include fever; headache; conjunctivitis; and a biphasic rash that begins with blanching macular erythema, which patients may mistake for sunburn, followed by a morbilliform to petechial rash with islands of sparing (white islands in a sea of red). Severe dengue (formerly known as dengue hemorrhagic fever) may present with dramatic skin and mucosal hemorrhage including purpura, hemorrhagic bullae, and bleeding from orifices and injection sites, with associated thrombocytopenia and hypotension (dengue shock syndrome). Patients with onset of such symptoms need to go to the emergency department for inpatient management.
Individuals living in the United States should be particularly aware of West Nile virus, with infections reported in all US states in 2017, except Alaska and Hawaii thus far. Transmitted by the bite of the Culex mosquito, most infections are symptomatic; however, up to 20% of patients may present with nonspecific symptoms such as mild febrile or flulike symptoms, nonspecific morbilliform rash, and headaches, and up to 1% of patients may develop encephalitis or meningitis, with approximately 10% mortality rates.
What are your go-to treatments?
Arboviral infections generally are self-limited and there are no specific treatments available. Supportive care, including fluid resuscitation, and analgesia (if needed for joint, muscle, or bone pain) are the mainstays of management. Diagnoses generally are confirmed via viral polymerase chain reaction from serum (<7 days), IgM enzyme-linked immunosorbent assay (>4 days), or IgG serologies for later presentations.
Patients should avoid the use of nonsteroidal anti-inflammatory drugs if there is a possibility of dengue virus infection, as they may potentiate the risk for hemorrhagic complications in patients with severe dengue. Instead, acetaminophen is recommended for analgesia and antipyretic purposes, if needed.
How do you recommend patients prevent infection while traveling?
Primary prevention of infection and secondary prevention of transmission are important. Although mosquito bed netting is helpful in preventing some mosquito-borne viruses, many arboviruses (ie, dengue, Zika, chikun-gunya) are transmitted by primarily daytime-biting Aedes mosquitoes. In an endemic area, travelers should try to stay within air-conditioned buildings with intact window and door screens. When outdoors, wear long sleeves and pants and use Environmental Protection Agency-registered mosquito repellents. Conventional repellents include the following:
- DEET: concentrations 10% to 30% are safe for children 2 months and older and pregnant women; concentrations around 10% are effective for periods of approximately 2 hours; as the concentration of DEET increases, the duration of protection increases.
- Picaridin: concentrations of 5% to 20%; effective for 4 to 8 hours depending on the concentration; most effective concentration is 20%; is not effective against ticks.
Biopesticide repellents include the following:
- IR3535 (ethyl butylacetylaminopropionate): concentration 10% to 30% has been used as an insect repellent in Europe for 20 years with no substantial adverse effects.
- 2-undecanone: a natural compound from leaves and stems of the wild tomato plant; is a biopesticide product less toxic than conventional pesticides.
- Oil of lemon eucalyptus (OLE) or PMD (the synthesized version of OLE): concentration 30%; "pure" oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended.
- Natural oils (eg, soybean, lemongrass, citronella, cedar, peppermint, lavender, geranium) are exempted from Environmental Protection Agency registration; duration of effectiveness is estimated between 30 minutes and 2 hours.
Products that combine sunscreen and repellent are not recommended because sunscreen may need to be reapplied, increasing the toxicity of the repellent. Use separate products, applying sunscreen first and then applying the repellent.
Permethrin-treated clothing may provide an additional measure of protection, though in some endemic areas, resistance has been reported.
Because sexual transmission has been reported for Zika virus (between both male and female partners), any known or possibly infected persons should use condoms. Durations of abstinence or protected sex recommendations vary by situation and more detailed recommendations can be found from the Centers for Disease Control and Prevention. Pregnant women and women trying to get pregnant should take preventive measures with respect to Zika due to the possibility of the virus causing severe birth defects (congenital Zika syndrome) including microcephaly, joint deformities, ocular damage, and hypertonia.
What do patients need to know about arboviruses?
Dengue is the most common arbovirus worldwide, with more than 300 million individuals infected each year, most of them asymptomatic carriers. It is the most common febrile illness in travelers returning from Southeast Asia, South America, and the Caribbean. Dengue symptoms typically begin 3 to 12 days after exposure and may include fever; headache; conjunctivitis; and a biphasic rash that begins with blanching macular erythema, which patients may mistake for sunburn, followed by a morbilliform to petechial rash with islands of sparing (white islands in a sea of red). Severe dengue (formerly known as dengue hemorrhagic fever) may present with dramatic skin and mucosal hemorrhage including purpura, hemorrhagic bullae, and bleeding from orifices and injection sites, with associated thrombocytopenia and hypotension (dengue shock syndrome). Patients with onset of such symptoms need to go to the emergency department for inpatient management.
Individuals living in the United States should be particularly aware of West Nile virus, with infections reported in all US states in 2017, except Alaska and Hawaii thus far. Transmitted by the bite of the Culex mosquito, most infections are symptomatic; however, up to 20% of patients may present with nonspecific symptoms such as mild febrile or flulike symptoms, nonspecific morbilliform rash, and headaches, and up to 1% of patients may develop encephalitis or meningitis, with approximately 10% mortality rates.
What are your go-to treatments?
Arboviral infections generally are self-limited and there are no specific treatments available. Supportive care, including fluid resuscitation, and analgesia (if needed for joint, muscle, or bone pain) are the mainstays of management. Diagnoses generally are confirmed via viral polymerase chain reaction from serum (<7 days), IgM enzyme-linked immunosorbent assay (>4 days), or IgG serologies for later presentations.
Patients should avoid the use of nonsteroidal anti-inflammatory drugs if there is a possibility of dengue virus infection, as they may potentiate the risk for hemorrhagic complications in patients with severe dengue. Instead, acetaminophen is recommended for analgesia and antipyretic purposes, if needed.
How do you recommend patients prevent infection while traveling?
Primary prevention of infection and secondary prevention of transmission are important. Although mosquito bed netting is helpful in preventing some mosquito-borne viruses, many arboviruses (ie, dengue, Zika, chikun-gunya) are transmitted by primarily daytime-biting Aedes mosquitoes. In an endemic area, travelers should try to stay within air-conditioned buildings with intact window and door screens. When outdoors, wear long sleeves and pants and use Environmental Protection Agency-registered mosquito repellents. Conventional repellents include the following:
- DEET: concentrations 10% to 30% are safe for children 2 months and older and pregnant women; concentrations around 10% are effective for periods of approximately 2 hours; as the concentration of DEET increases, the duration of protection increases.
- Picaridin: concentrations of 5% to 20%; effective for 4 to 8 hours depending on the concentration; most effective concentration is 20%; is not effective against ticks.
Biopesticide repellents include the following:
- IR3535 (ethyl butylacetylaminopropionate): concentration 10% to 30% has been used as an insect repellent in Europe for 20 years with no substantial adverse effects.
- 2-undecanone: a natural compound from leaves and stems of the wild tomato plant; is a biopesticide product less toxic than conventional pesticides.
- Oil of lemon eucalyptus (OLE) or PMD (the synthesized version of OLE): concentration 30%; "pure" oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended.
- Natural oils (eg, soybean, lemongrass, citronella, cedar, peppermint, lavender, geranium) are exempted from Environmental Protection Agency registration; duration of effectiveness is estimated between 30 minutes and 2 hours.
Products that combine sunscreen and repellent are not recommended because sunscreen may need to be reapplied, increasing the toxicity of the repellent. Use separate products, applying sunscreen first and then applying the repellent.
Permethrin-treated clothing may provide an additional measure of protection, though in some endemic areas, resistance has been reported.
Because sexual transmission has been reported for Zika virus (between both male and female partners), any known or possibly infected persons should use condoms. Durations of abstinence or protected sex recommendations vary by situation and more detailed recommendations can be found from the Centers for Disease Control and Prevention. Pregnant women and women trying to get pregnant should take preventive measures with respect to Zika due to the possibility of the virus causing severe birth defects (congenital Zika syndrome) including microcephaly, joint deformities, ocular damage, and hypertonia.
Durable Improvements in Clinical Outcomes With Alemtuzumab: Seven-Year Follow-Up
PARIS—Clinical efficacy of alemtuzumab was maintained for seven years in patients who had inadequate response to prior therapy, despite 47% receiving no additional treatment since the initial two courses of alemtuzumab, according to study data presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. In addition, 44% of patients showed improvement in disability, researchers reported. “These findings suggest that alemtuzumab may provide a unique treatment approach for patients with relapsing-remitting multiple sclerosis (RRMS), offering durable efficacy in the absence of continuous treatment,” said Barry Singer, MD, Director of the MS Center for Innovations in Care, Missouri Baptist Medical Center, St. Louis.
Alemtuzumab Treatment: Then
In the CARE-MS II trial, alemtuzumab significantly improved clinical outcomes compared with subcutaneous interferon beta-1a over two years in patients with active RRMS and an inadequate response to prior therapy. Durable efficacy of alemtuzumab was demonstrated over six years in a completed extension study in the absence of continuous treatment. Patients in the CARE-MS II study received two courses of alemtuzumab 12 mg/day (five days of therapy at baseline and three days of therapy 12 months later). In the extension study, patients could receive as-needed alemtuzumab retreatment (12 mg/day on three consecutive days at least 12 months after a previous course for relapse or MRI activity) or other disease-modifying therapy per investigator discretion. Patients completing at least 48 months of the extension could enroll in the five-year TOPAZ study for further long-term evaluation.
Alemtuzumab Treatment: Now
The goal of the TOPAZ study was to evaluate the seven-year efficacy and safety of alemtuzumab in patients with RRMS who received alemtuzumab in the CARE-MS II trial. In TOPAZ, patients could receive alemtuzumab retreatment 12 months or more after a previous course or other disease-modifying therapy at any time point (both per investigator discretion; no criteria). MRI scans were done annually. Annualized relapse rate, six-month confirmed disability worsening, six-month confirmed disability improvement, no evidence of disease activity (NEDA), and adverse events were analyzed in TOPAZ.
In total, 338 of 393 (86%) CARE-MS II patients who entered the extension remained on study until the end of year six and then entered TOPAZ; 317 (94%) remained on study through year seven. Annualized release rate remained low (0.14 at year seven) and the proportion of patients with stable or improved Expanded Disability Status Scale score remained high (73% at year seven). Through year seven, 69% of patients were free from six-month confirmed disability worsening, 44% achieved six-month confirmed disability improvement, and the majority achieved NEDA each year. These effects were achieved with 47% of patients receiving no additional treatment (alemtuzumab or other disease-modifying treatment) after their initial two courses of alemtuzumab. Incidences of overall adverse events, infusion-associated reactions, and infections decreased over time and were reduced, compared with those in the two-year core study. Thyroid adverse events incidence peaked at year three and then declined.
PARIS—Clinical efficacy of alemtuzumab was maintained for seven years in patients who had inadequate response to prior therapy, despite 47% receiving no additional treatment since the initial two courses of alemtuzumab, according to study data presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. In addition, 44% of patients showed improvement in disability, researchers reported. “These findings suggest that alemtuzumab may provide a unique treatment approach for patients with relapsing-remitting multiple sclerosis (RRMS), offering durable efficacy in the absence of continuous treatment,” said Barry Singer, MD, Director of the MS Center for Innovations in Care, Missouri Baptist Medical Center, St. Louis.
Alemtuzumab Treatment: Then
In the CARE-MS II trial, alemtuzumab significantly improved clinical outcomes compared with subcutaneous interferon beta-1a over two years in patients with active RRMS and an inadequate response to prior therapy. Durable efficacy of alemtuzumab was demonstrated over six years in a completed extension study in the absence of continuous treatment. Patients in the CARE-MS II study received two courses of alemtuzumab 12 mg/day (five days of therapy at baseline and three days of therapy 12 months later). In the extension study, patients could receive as-needed alemtuzumab retreatment (12 mg/day on three consecutive days at least 12 months after a previous course for relapse or MRI activity) or other disease-modifying therapy per investigator discretion. Patients completing at least 48 months of the extension could enroll in the five-year TOPAZ study for further long-term evaluation.
Alemtuzumab Treatment: Now
The goal of the TOPAZ study was to evaluate the seven-year efficacy and safety of alemtuzumab in patients with RRMS who received alemtuzumab in the CARE-MS II trial. In TOPAZ, patients could receive alemtuzumab retreatment 12 months or more after a previous course or other disease-modifying therapy at any time point (both per investigator discretion; no criteria). MRI scans were done annually. Annualized relapse rate, six-month confirmed disability worsening, six-month confirmed disability improvement, no evidence of disease activity (NEDA), and adverse events were analyzed in TOPAZ.
In total, 338 of 393 (86%) CARE-MS II patients who entered the extension remained on study until the end of year six and then entered TOPAZ; 317 (94%) remained on study through year seven. Annualized release rate remained low (0.14 at year seven) and the proportion of patients with stable or improved Expanded Disability Status Scale score remained high (73% at year seven). Through year seven, 69% of patients were free from six-month confirmed disability worsening, 44% achieved six-month confirmed disability improvement, and the majority achieved NEDA each year. These effects were achieved with 47% of patients receiving no additional treatment (alemtuzumab or other disease-modifying treatment) after their initial two courses of alemtuzumab. Incidences of overall adverse events, infusion-associated reactions, and infections decreased over time and were reduced, compared with those in the two-year core study. Thyroid adverse events incidence peaked at year three and then declined.
PARIS—Clinical efficacy of alemtuzumab was maintained for seven years in patients who had inadequate response to prior therapy, despite 47% receiving no additional treatment since the initial two courses of alemtuzumab, according to study data presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. In addition, 44% of patients showed improvement in disability, researchers reported. “These findings suggest that alemtuzumab may provide a unique treatment approach for patients with relapsing-remitting multiple sclerosis (RRMS), offering durable efficacy in the absence of continuous treatment,” said Barry Singer, MD, Director of the MS Center for Innovations in Care, Missouri Baptist Medical Center, St. Louis.
Alemtuzumab Treatment: Then
In the CARE-MS II trial, alemtuzumab significantly improved clinical outcomes compared with subcutaneous interferon beta-1a over two years in patients with active RRMS and an inadequate response to prior therapy. Durable efficacy of alemtuzumab was demonstrated over six years in a completed extension study in the absence of continuous treatment. Patients in the CARE-MS II study received two courses of alemtuzumab 12 mg/day (five days of therapy at baseline and three days of therapy 12 months later). In the extension study, patients could receive as-needed alemtuzumab retreatment (12 mg/day on three consecutive days at least 12 months after a previous course for relapse or MRI activity) or other disease-modifying therapy per investigator discretion. Patients completing at least 48 months of the extension could enroll in the five-year TOPAZ study for further long-term evaluation.
Alemtuzumab Treatment: Now
The goal of the TOPAZ study was to evaluate the seven-year efficacy and safety of alemtuzumab in patients with RRMS who received alemtuzumab in the CARE-MS II trial. In TOPAZ, patients could receive alemtuzumab retreatment 12 months or more after a previous course or other disease-modifying therapy at any time point (both per investigator discretion; no criteria). MRI scans were done annually. Annualized relapse rate, six-month confirmed disability worsening, six-month confirmed disability improvement, no evidence of disease activity (NEDA), and adverse events were analyzed in TOPAZ.
In total, 338 of 393 (86%) CARE-MS II patients who entered the extension remained on study until the end of year six and then entered TOPAZ; 317 (94%) remained on study through year seven. Annualized release rate remained low (0.14 at year seven) and the proportion of patients with stable or improved Expanded Disability Status Scale score remained high (73% at year seven). Through year seven, 69% of patients were free from six-month confirmed disability worsening, 44% achieved six-month confirmed disability improvement, and the majority achieved NEDA each year. These effects were achieved with 47% of patients receiving no additional treatment (alemtuzumab or other disease-modifying treatment) after their initial two courses of alemtuzumab. Incidences of overall adverse events, infusion-associated reactions, and infections decreased over time and were reduced, compared with those in the two-year core study. Thyroid adverse events incidence peaked at year three and then declined.
Analysis of failed Alzheimer’s trials gives two antiamyloid antibodies new momentum
BOSTON – Despite years of frustrating failures, Alzheimer’s researchers keep punching away at beta-amyloid brain plaques, now apparently with a highly focused one-two of “more drug, given sooner.”
Solanezumab and gantenerumab – both of which failed in earlier phase 3 studies – will be pushed forward now at much higher doses, the Alzheimer’s disease research community learned at the opening session of the Clinical Trials on Alzheimer’s Disease conference.
Both drugs are antiamyloid antibodies. In their prior trials, both effectively cleared amyloid plaques, but neither significantly improved cognition in patients with mild-moderate disease. This has been a common theme of every antiamyloid study: Although these drugs stimulate different mechanisms of plaque removal, none has ever significantly improved thinking or memory.
In discussing these failures, the Alzheimer’s research community has collectively wondered whether the doses were high enough. Drug companies have erred on the side of caution in large part because antiamyloid antibodies can cause a syndrome called ARIA (Amyloid-Related Imaging Abnormalities), an inflammatory response of brain edema or microhemorrhages. Concern over this side effect has moderated as researchers accumulate more adverse event data. Most cases are asymptomatic and resolve spontaneously. New open-label extension data from the Scarlet Road and Marguerite Road trials of gantenerumab, plus a new titration model by Roche, have also increased confidence that patients will tolerate the antibody at subcutaneous doses of up to 1,200 mg.
The other fear that plagues researchers is therapeutic timing. It’s increasingly apparent that plaque eradication does not rescue cognition. As heart disease must be attacked before cardiac damage occurs, it seems likely that Alzheimer’s disease must be attacked before amyloid and its attendant protein, tau, wreak havoc in the hippocampus and neocortex.
After reevaluating the high-profile solanezumab and gantenerumab failures, researchers now hope that higher doses delivered much earlier in the disease process might be effective, not at restoring lost cognition, but at preventing cognitive decline in the first place.
“One of the greatest advances in this field over the past 10 years is the recognition that Alzheimer’s disease is a continuum that likely begins well before the stage we recognize as dementia, and even before the stages of mild cognitive impairment [MCI] and prodromal Alzheimer’s,” said Dr. Sperling of Brigham and Women’s Hospital, Boston. “Treating in the presymptomatic phase may be the best opportunity to bend this curve back toward the trajectory of normal aging.”
But amyloid is only part of the Alzheimer’s disease story. Tau is a key player and, some say, the prime antagonist, since it is the main driver of memory and thought decline. Tau is present deep in the brains of most cognitively aging normal people, but something about amyloid deposition spurs its devastating spread into the neocortex. Preventing amyloid accumulation may prevent dementia, not just by keeping amyloid at bay, but by preventing it from igniting the spread of tau.
Dr. Sperling cited unpublished data showing very subtle cognitive decline in cognitively normal patients who have both amyloid and tau in the brain. Although the scores stayed within normal, subjects with both declined over 2 years on specific measures of memory and were more likely to progress to MCI.
“This is very striking to me and made me a little worried about the critical window of intervention,” she said. “What is also striking is that, even though we restricted the eligibility criteria of A4 to those with normal memory and normal cognition, we do see that tau positivity at baseline is associated with lower baseline performance. Again we have this suggestion that amyloid is associated with tau and tau is associated with poor memory even in normal people.”
Although hard to swallow, digesting solanezumab’s failure in the series of EXPEDITION studies has now refined the A4 protocol. “To be honest, I didn’t sleep for months following the release of EXPEDITION 3 data, because I was really concerned about how it should guide us about changes to A4. We think solanezumab has an increased chance of success here [compared with EXPEDITION] because we’re employing it 10-15 years earlier in the disease. But we also want to maximize its chances.”
Thus, she said, investigators and Eli Lilly have now decided to quadruple the dose in A4. Subjects will titrate from 600 mg to 800 mg for 2 months and then go up to 1,600 mg every 4 weeks. A safety cohort of 200 patients will be monitored for any adverse events, with a particular eye out for hemorrhagic or edematous ARIA. “We are also extending the double-blind phase to 240 weeks, which allows everyone to dose-escalate and increases our power to detect small effect sizes,” she said.
Right now, recruitment stands at 1,151; Dr. Sperling expects the full 1,200-subject cohort to be randomized by the end of 2017.
Gantenerumab is also experiencing a rebirth after a deep dive into open-label extension data from both the Scarlet Road and Marguerite Road studies, Dr. Klein said. Patients in these studies were randomized to either 105 or 225 mg of the antibody. While there were no significant cognitive benefits, there were trends toward improvement with the higher dose, as well as dose-dependent plaque clearance. This encouraged researchers to look at higher doses in 52-week open-label extensions of each study.
Dr. Klein presented new imaging data for these studies. Between both, 40 patients were maintained for 6-9 months on the highest doses (900-1,200 mg). Of these, 17 had almost total clearance of their amyloid burden. Their scans, Dr. Klein said, read as traces of amyloid or as amyloid negative. The effect was consistent regardless of the amount of amyloid at baseline.
“These are very encouraging biomarker data,” he said. “We are going into our new phase 3 studies, Graduate I and II, very optimistic.”
Little information is available about these studies. According to a press release, they will target patients with prodromal-mild disease at the higher doses. Emails to Roche and its German partner, MorphoSys, were not returned by press time. But from Dr. Klein’s comments, it seems clear that gantenerumab has not reached the end of its road.
Dr. Sperling disclosed relationships with numerous pharmaceutical companies. Dr. Klein is an employee of Roche.
[email protected]
On Twitter @alz_gal
BOSTON – Despite years of frustrating failures, Alzheimer’s researchers keep punching away at beta-amyloid brain plaques, now apparently with a highly focused one-two of “more drug, given sooner.”
Solanezumab and gantenerumab – both of which failed in earlier phase 3 studies – will be pushed forward now at much higher doses, the Alzheimer’s disease research community learned at the opening session of the Clinical Trials on Alzheimer’s Disease conference.
Both drugs are antiamyloid antibodies. In their prior trials, both effectively cleared amyloid plaques, but neither significantly improved cognition in patients with mild-moderate disease. This has been a common theme of every antiamyloid study: Although these drugs stimulate different mechanisms of plaque removal, none has ever significantly improved thinking or memory.
In discussing these failures, the Alzheimer’s research community has collectively wondered whether the doses were high enough. Drug companies have erred on the side of caution in large part because antiamyloid antibodies can cause a syndrome called ARIA (Amyloid-Related Imaging Abnormalities), an inflammatory response of brain edema or microhemorrhages. Concern over this side effect has moderated as researchers accumulate more adverse event data. Most cases are asymptomatic and resolve spontaneously. New open-label extension data from the Scarlet Road and Marguerite Road trials of gantenerumab, plus a new titration model by Roche, have also increased confidence that patients will tolerate the antibody at subcutaneous doses of up to 1,200 mg.
The other fear that plagues researchers is therapeutic timing. It’s increasingly apparent that plaque eradication does not rescue cognition. As heart disease must be attacked before cardiac damage occurs, it seems likely that Alzheimer’s disease must be attacked before amyloid and its attendant protein, tau, wreak havoc in the hippocampus and neocortex.
After reevaluating the high-profile solanezumab and gantenerumab failures, researchers now hope that higher doses delivered much earlier in the disease process might be effective, not at restoring lost cognition, but at preventing cognitive decline in the first place.
“One of the greatest advances in this field over the past 10 years is the recognition that Alzheimer’s disease is a continuum that likely begins well before the stage we recognize as dementia, and even before the stages of mild cognitive impairment [MCI] and prodromal Alzheimer’s,” said Dr. Sperling of Brigham and Women’s Hospital, Boston. “Treating in the presymptomatic phase may be the best opportunity to bend this curve back toward the trajectory of normal aging.”
But amyloid is only part of the Alzheimer’s disease story. Tau is a key player and, some say, the prime antagonist, since it is the main driver of memory and thought decline. Tau is present deep in the brains of most cognitively aging normal people, but something about amyloid deposition spurs its devastating spread into the neocortex. Preventing amyloid accumulation may prevent dementia, not just by keeping amyloid at bay, but by preventing it from igniting the spread of tau.
Dr. Sperling cited unpublished data showing very subtle cognitive decline in cognitively normal patients who have both amyloid and tau in the brain. Although the scores stayed within normal, subjects with both declined over 2 years on specific measures of memory and were more likely to progress to MCI.
“This is very striking to me and made me a little worried about the critical window of intervention,” she said. “What is also striking is that, even though we restricted the eligibility criteria of A4 to those with normal memory and normal cognition, we do see that tau positivity at baseline is associated with lower baseline performance. Again we have this suggestion that amyloid is associated with tau and tau is associated with poor memory even in normal people.”
Although hard to swallow, digesting solanezumab’s failure in the series of EXPEDITION studies has now refined the A4 protocol. “To be honest, I didn’t sleep for months following the release of EXPEDITION 3 data, because I was really concerned about how it should guide us about changes to A4. We think solanezumab has an increased chance of success here [compared with EXPEDITION] because we’re employing it 10-15 years earlier in the disease. But we also want to maximize its chances.”
Thus, she said, investigators and Eli Lilly have now decided to quadruple the dose in A4. Subjects will titrate from 600 mg to 800 mg for 2 months and then go up to 1,600 mg every 4 weeks. A safety cohort of 200 patients will be monitored for any adverse events, with a particular eye out for hemorrhagic or edematous ARIA. “We are also extending the double-blind phase to 240 weeks, which allows everyone to dose-escalate and increases our power to detect small effect sizes,” she said.
Right now, recruitment stands at 1,151; Dr. Sperling expects the full 1,200-subject cohort to be randomized by the end of 2017.
Gantenerumab is also experiencing a rebirth after a deep dive into open-label extension data from both the Scarlet Road and Marguerite Road studies, Dr. Klein said. Patients in these studies were randomized to either 105 or 225 mg of the antibody. While there were no significant cognitive benefits, there were trends toward improvement with the higher dose, as well as dose-dependent plaque clearance. This encouraged researchers to look at higher doses in 52-week open-label extensions of each study.
Dr. Klein presented new imaging data for these studies. Between both, 40 patients were maintained for 6-9 months on the highest doses (900-1,200 mg). Of these, 17 had almost total clearance of their amyloid burden. Their scans, Dr. Klein said, read as traces of amyloid or as amyloid negative. The effect was consistent regardless of the amount of amyloid at baseline.
“These are very encouraging biomarker data,” he said. “We are going into our new phase 3 studies, Graduate I and II, very optimistic.”
Little information is available about these studies. According to a press release, they will target patients with prodromal-mild disease at the higher doses. Emails to Roche and its German partner, MorphoSys, were not returned by press time. But from Dr. Klein’s comments, it seems clear that gantenerumab has not reached the end of its road.
Dr. Sperling disclosed relationships with numerous pharmaceutical companies. Dr. Klein is an employee of Roche.
[email protected]
On Twitter @alz_gal
BOSTON – Despite years of frustrating failures, Alzheimer’s researchers keep punching away at beta-amyloid brain plaques, now apparently with a highly focused one-two of “more drug, given sooner.”
Solanezumab and gantenerumab – both of which failed in earlier phase 3 studies – will be pushed forward now at much higher doses, the Alzheimer’s disease research community learned at the opening session of the Clinical Trials on Alzheimer’s Disease conference.
Both drugs are antiamyloid antibodies. In their prior trials, both effectively cleared amyloid plaques, but neither significantly improved cognition in patients with mild-moderate disease. This has been a common theme of every antiamyloid study: Although these drugs stimulate different mechanisms of plaque removal, none has ever significantly improved thinking or memory.
In discussing these failures, the Alzheimer’s research community has collectively wondered whether the doses were high enough. Drug companies have erred on the side of caution in large part because antiamyloid antibodies can cause a syndrome called ARIA (Amyloid-Related Imaging Abnormalities), an inflammatory response of brain edema or microhemorrhages. Concern over this side effect has moderated as researchers accumulate more adverse event data. Most cases are asymptomatic and resolve spontaneously. New open-label extension data from the Scarlet Road and Marguerite Road trials of gantenerumab, plus a new titration model by Roche, have also increased confidence that patients will tolerate the antibody at subcutaneous doses of up to 1,200 mg.
The other fear that plagues researchers is therapeutic timing. It’s increasingly apparent that plaque eradication does not rescue cognition. As heart disease must be attacked before cardiac damage occurs, it seems likely that Alzheimer’s disease must be attacked before amyloid and its attendant protein, tau, wreak havoc in the hippocampus and neocortex.
After reevaluating the high-profile solanezumab and gantenerumab failures, researchers now hope that higher doses delivered much earlier in the disease process might be effective, not at restoring lost cognition, but at preventing cognitive decline in the first place.
“One of the greatest advances in this field over the past 10 years is the recognition that Alzheimer’s disease is a continuum that likely begins well before the stage we recognize as dementia, and even before the stages of mild cognitive impairment [MCI] and prodromal Alzheimer’s,” said Dr. Sperling of Brigham and Women’s Hospital, Boston. “Treating in the presymptomatic phase may be the best opportunity to bend this curve back toward the trajectory of normal aging.”
But amyloid is only part of the Alzheimer’s disease story. Tau is a key player and, some say, the prime antagonist, since it is the main driver of memory and thought decline. Tau is present deep in the brains of most cognitively aging normal people, but something about amyloid deposition spurs its devastating spread into the neocortex. Preventing amyloid accumulation may prevent dementia, not just by keeping amyloid at bay, but by preventing it from igniting the spread of tau.
Dr. Sperling cited unpublished data showing very subtle cognitive decline in cognitively normal patients who have both amyloid and tau in the brain. Although the scores stayed within normal, subjects with both declined over 2 years on specific measures of memory and were more likely to progress to MCI.
“This is very striking to me and made me a little worried about the critical window of intervention,” she said. “What is also striking is that, even though we restricted the eligibility criteria of A4 to those with normal memory and normal cognition, we do see that tau positivity at baseline is associated with lower baseline performance. Again we have this suggestion that amyloid is associated with tau and tau is associated with poor memory even in normal people.”
Although hard to swallow, digesting solanezumab’s failure in the series of EXPEDITION studies has now refined the A4 protocol. “To be honest, I didn’t sleep for months following the release of EXPEDITION 3 data, because I was really concerned about how it should guide us about changes to A4. We think solanezumab has an increased chance of success here [compared with EXPEDITION] because we’re employing it 10-15 years earlier in the disease. But we also want to maximize its chances.”
Thus, she said, investigators and Eli Lilly have now decided to quadruple the dose in A4. Subjects will titrate from 600 mg to 800 mg for 2 months and then go up to 1,600 mg every 4 weeks. A safety cohort of 200 patients will be monitored for any adverse events, with a particular eye out for hemorrhagic or edematous ARIA. “We are also extending the double-blind phase to 240 weeks, which allows everyone to dose-escalate and increases our power to detect small effect sizes,” she said.
Right now, recruitment stands at 1,151; Dr. Sperling expects the full 1,200-subject cohort to be randomized by the end of 2017.
Gantenerumab is also experiencing a rebirth after a deep dive into open-label extension data from both the Scarlet Road and Marguerite Road studies, Dr. Klein said. Patients in these studies were randomized to either 105 or 225 mg of the antibody. While there were no significant cognitive benefits, there were trends toward improvement with the higher dose, as well as dose-dependent plaque clearance. This encouraged researchers to look at higher doses in 52-week open-label extensions of each study.
Dr. Klein presented new imaging data for these studies. Between both, 40 patients were maintained for 6-9 months on the highest doses (900-1,200 mg). Of these, 17 had almost total clearance of their amyloid burden. Their scans, Dr. Klein said, read as traces of amyloid or as amyloid negative. The effect was consistent regardless of the amount of amyloid at baseline.
“These are very encouraging biomarker data,” he said. “We are going into our new phase 3 studies, Graduate I and II, very optimistic.”
Little information is available about these studies. According to a press release, they will target patients with prodromal-mild disease at the higher doses. Emails to Roche and its German partner, MorphoSys, were not returned by press time. But from Dr. Klein’s comments, it seems clear that gantenerumab has not reached the end of its road.
Dr. Sperling disclosed relationships with numerous pharmaceutical companies. Dr. Klein is an employee of Roche.
[email protected]
On Twitter @alz_gal
AT CTAD
A Case of Leprosy in Central Florida
Case Report
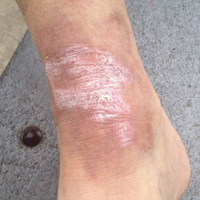
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.
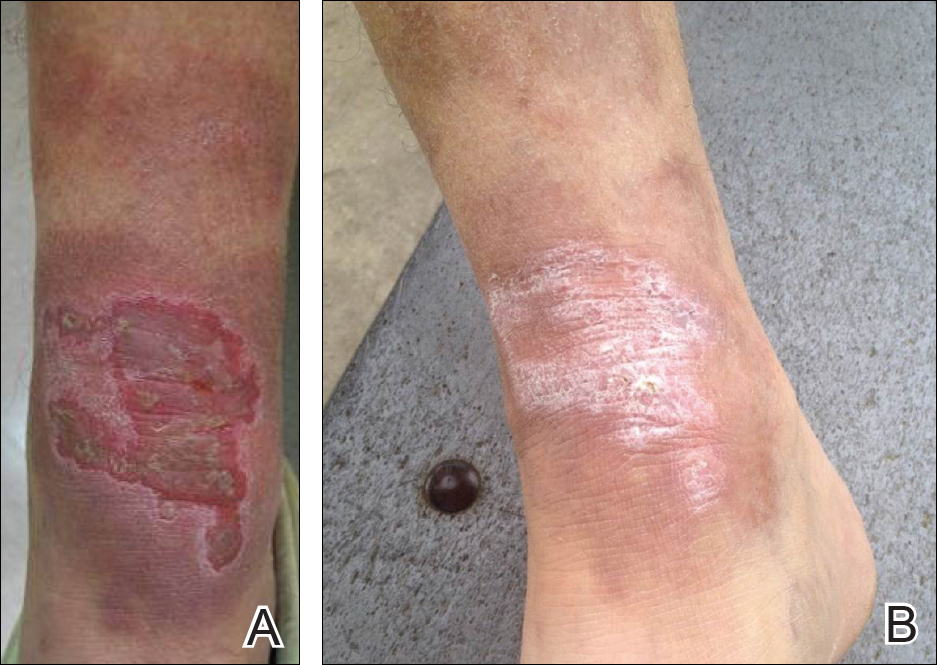
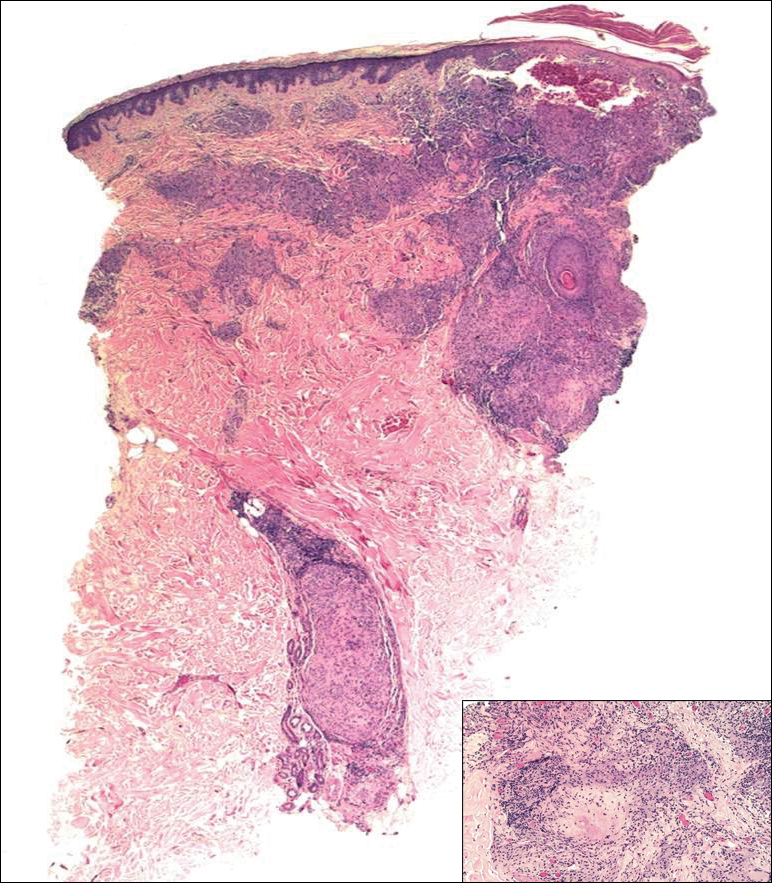
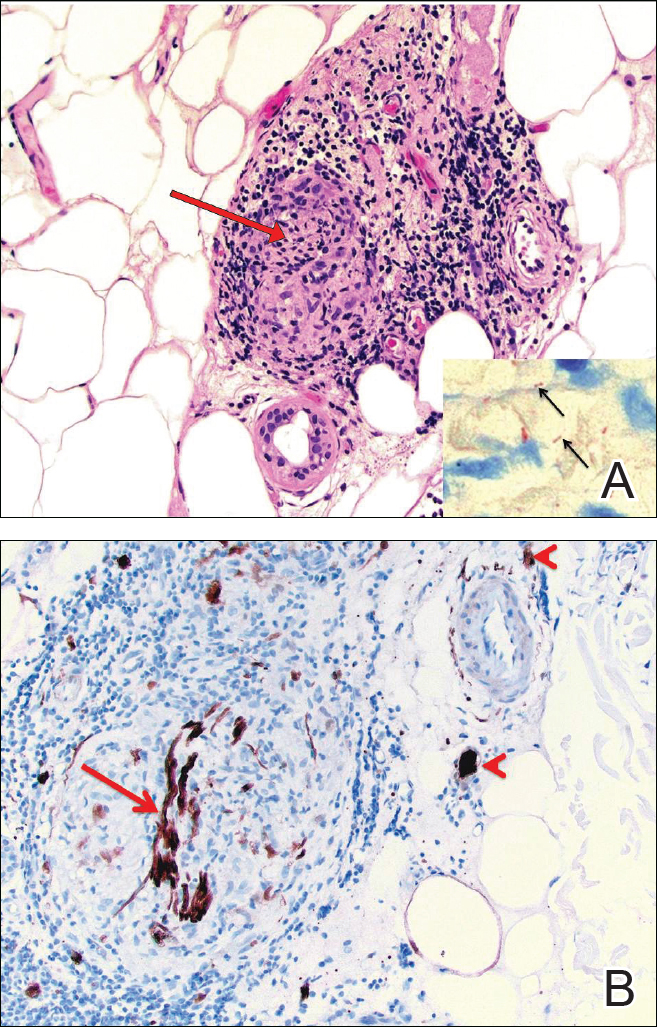
The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Practice Points
- A majority of leprosy cases in the United States have been reported in Florida, California, Texas, Louisiana, Hawaii, and New York.
- Leprosy should be included in the differential diagnosis for annular plaques, particularly those not responding to traditional treatment.
Event-free survival at 24 months predicts outcomes in peripheral T-cell lymphomas
Event-free survival at 24 months (EFS24) is predictive of survival in patients with peripheral T-cell lymphomas (PTCLs), according to new findings published in the Journal of Clinical Oncology.
Patients who were event free 2 years after diagnosis had a more favorable outcome, compared with those who relapsed within that time period. Some patients who remained event free for 24 months were potentially cured; conversely, events within 2 years were associated with an early death in almost all of those patients.
“Thus, EFS24 is a dichotomous end point that allows individualized risk prediction in patients with PTCL and can help inform patient counseling, biomarker discovery, clinical trial design, and precision medicine approaches,” wrote Matthew J. Maurer, MS, of the Mayo Clinic, Rochester, MN, and his coauthors (J Clin Oncol. 2017 Oct 26. doi: 10.1200/JCO.2017.73.8195).
PTCL is an uncommon and heterogeneous group of non-Hodgkin lymphomas that carry a very poor prognosis; most systemic cases are treated with anthracycline-based combination chemotherapy. Previous studies have reported that achieving EFS24 is predictive of excellent long-term outcomes, independent of baseline prognostic factors.
In this study Mr. Maurer and his coauthors assessed the association between EFS24 and overall survival in 775 patients with newly systemic PTCL who were diagnosed during 2000-2012 and received treatment with curative intent.
Among the entire cohort, 36% of patients achieved EFS24 while 64% did not, and the median overall survival following progression within that 2-year time period was 4.9 months (95% confidence interval, 3.8-5.9 months). The 5-year overall survival in the group that relapsed was 11%, with a standardized mortality ratio of 46.4 (95% CI, 41.8-51.3).
Conversely, among patients with EFS24, the median overall survival was not reached, and the 5-year overall survival was 78% (95% CI, 73%-84%). In this group, the 5-year risk of subsequent lymphoma relapse was 23%, and survival following a late relapse was generally poor (median of 10.3 months; 95% CI, 5.7-19.1 months). The best outcomes after achieving EFS24 were observed among patients aged 60 years or younger: These patients had a 5-year overall survival of 91%.
“The use of a dichotomous end point that allows individualized risk prediction is particularly important in rare diseases such as PTCL, where limited numbers of patients may make formal surrogate end point analysis difficult,” wrote the authors.
The study was supported by grants from the National Cancer Institute, the Terry Fox Research Institute, and the BC Cancer Foundation. Dr. Maurer reported research funding from Kite Pharma and Celgene, and several of the coauthors reported relationships with industry.
Event-free survival at 24 months (EFS24) is predictive of survival in patients with peripheral T-cell lymphomas (PTCLs), according to new findings published in the Journal of Clinical Oncology.
Patients who were event free 2 years after diagnosis had a more favorable outcome, compared with those who relapsed within that time period. Some patients who remained event free for 24 months were potentially cured; conversely, events within 2 years were associated with an early death in almost all of those patients.
“Thus, EFS24 is a dichotomous end point that allows individualized risk prediction in patients with PTCL and can help inform patient counseling, biomarker discovery, clinical trial design, and precision medicine approaches,” wrote Matthew J. Maurer, MS, of the Mayo Clinic, Rochester, MN, and his coauthors (J Clin Oncol. 2017 Oct 26. doi: 10.1200/JCO.2017.73.8195).
PTCL is an uncommon and heterogeneous group of non-Hodgkin lymphomas that carry a very poor prognosis; most systemic cases are treated with anthracycline-based combination chemotherapy. Previous studies have reported that achieving EFS24 is predictive of excellent long-term outcomes, independent of baseline prognostic factors.
In this study Mr. Maurer and his coauthors assessed the association between EFS24 and overall survival in 775 patients with newly systemic PTCL who were diagnosed during 2000-2012 and received treatment with curative intent.
Among the entire cohort, 36% of patients achieved EFS24 while 64% did not, and the median overall survival following progression within that 2-year time period was 4.9 months (95% confidence interval, 3.8-5.9 months). The 5-year overall survival in the group that relapsed was 11%, with a standardized mortality ratio of 46.4 (95% CI, 41.8-51.3).
Conversely, among patients with EFS24, the median overall survival was not reached, and the 5-year overall survival was 78% (95% CI, 73%-84%). In this group, the 5-year risk of subsequent lymphoma relapse was 23%, and survival following a late relapse was generally poor (median of 10.3 months; 95% CI, 5.7-19.1 months). The best outcomes after achieving EFS24 were observed among patients aged 60 years or younger: These patients had a 5-year overall survival of 91%.
“The use of a dichotomous end point that allows individualized risk prediction is particularly important in rare diseases such as PTCL, where limited numbers of patients may make formal surrogate end point analysis difficult,” wrote the authors.
The study was supported by grants from the National Cancer Institute, the Terry Fox Research Institute, and the BC Cancer Foundation. Dr. Maurer reported research funding from Kite Pharma and Celgene, and several of the coauthors reported relationships with industry.
Event-free survival at 24 months (EFS24) is predictive of survival in patients with peripheral T-cell lymphomas (PTCLs), according to new findings published in the Journal of Clinical Oncology.
Patients who were event free 2 years after diagnosis had a more favorable outcome, compared with those who relapsed within that time period. Some patients who remained event free for 24 months were potentially cured; conversely, events within 2 years were associated with an early death in almost all of those patients.
“Thus, EFS24 is a dichotomous end point that allows individualized risk prediction in patients with PTCL and can help inform patient counseling, biomarker discovery, clinical trial design, and precision medicine approaches,” wrote Matthew J. Maurer, MS, of the Mayo Clinic, Rochester, MN, and his coauthors (J Clin Oncol. 2017 Oct 26. doi: 10.1200/JCO.2017.73.8195).
PTCL is an uncommon and heterogeneous group of non-Hodgkin lymphomas that carry a very poor prognosis; most systemic cases are treated with anthracycline-based combination chemotherapy. Previous studies have reported that achieving EFS24 is predictive of excellent long-term outcomes, independent of baseline prognostic factors.
In this study Mr. Maurer and his coauthors assessed the association between EFS24 and overall survival in 775 patients with newly systemic PTCL who were diagnosed during 2000-2012 and received treatment with curative intent.
Among the entire cohort, 36% of patients achieved EFS24 while 64% did not, and the median overall survival following progression within that 2-year time period was 4.9 months (95% confidence interval, 3.8-5.9 months). The 5-year overall survival in the group that relapsed was 11%, with a standardized mortality ratio of 46.4 (95% CI, 41.8-51.3).
Conversely, among patients with EFS24, the median overall survival was not reached, and the 5-year overall survival was 78% (95% CI, 73%-84%). In this group, the 5-year risk of subsequent lymphoma relapse was 23%, and survival following a late relapse was generally poor (median of 10.3 months; 95% CI, 5.7-19.1 months). The best outcomes after achieving EFS24 were observed among patients aged 60 years or younger: These patients had a 5-year overall survival of 91%.
“The use of a dichotomous end point that allows individualized risk prediction is particularly important in rare diseases such as PTCL, where limited numbers of patients may make formal surrogate end point analysis difficult,” wrote the authors.
The study was supported by grants from the National Cancer Institute, the Terry Fox Research Institute, and the BC Cancer Foundation. Dr. Maurer reported research funding from Kite Pharma and Celgene, and several of the coauthors reported relationships with industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Event-free survival at 24 months (EFS24) stratifies outcomes in peripheral T-cell lymphomas.
Major finding: Five-year overall survival for those who achieved EFS24 was 78% vs. 11% for those who did not.
Data source: Multinational cohort study that included 775 patients with newly diagnosed PTCL who were evaluated for EFS24 as a predictive endpoint.
Disclosures: The study was supported by grants from the National Cancer Institute, the Terry Fox Research Institute, and the BC Cancer Foundation. Dr. Maurer reported research funding from Kite Pharma and Celgene, and several of the coauthors reported relationships with industry.
TAVR wallops SAVR in cost-effectiveness for intermediate-risk patients
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
AT TCT 2017
Key clinical point:
Major finding: The total cost of TAVR with the Sapien 3 valve in intermediate-risk patients, including the index hospitalization and costs incurred during the first year after, averaged $80,977, compared with $96,489 per SAVR patient.
Data source: This patient-level formal cost-effectiveness analysis included nearly 2,000 patients in the PARTNER 2A trial and more than 1,700 in a registry of recipients of the Sapien 3 TAVR valve.
Disclosures: The cost-effectiveness analysis was funded by Edwards Lifesciences. The presenter reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
IGRA preferred test for latent TB diagnosis
TORONTO – U.S.-based pulmonary and infectious disease specialists prefer interferon-gamma release assays (IGRA) over tuberculin skin tests (TST) for the diagnosis of latent TB infection, but may not fully understand how to use and interpret the test results, according to survey results presented at the CHEST annual meeting.
Adam G. Green, MD, conducted the research while he was a fellow in pulmonology/critical care at Montefiore Medical Center in New York. Dr. Green told attendees that about one-third of the world’s population are infected with TB and about 15 million of those live in the United States. Two-thirds of U.S. cases are seen in foreign-born individuals and are clustered in four states—New, York, California, Florida, and Texas.
Among 304 clinicians who responded to an invitation to an online questionnaire, 78% said they preferred to use IGRA over TST and 91% said they had a “good understanding” of how to use and interpret IGRA. However, when queried further on how to best use and interpret IGRAs according to current guidelines, their answers to 11 knowledge-based questions told a somewhat different story, said Dr. Green, who is an intensivist at Cooper University Health Care in Camden, N.J.
While 96% knew IGRAs are not helpful in monitoring response to TB treatment, 20% erroneously thought that a positive IGRA predicts latent TB infection reactivation in the future.
Most respondents correctly answered two “fundamental” questions on cross-reactivity of IGRAs with Mycobacterium avium complex and bacilli Calmette-Guérin (BCG) vaccination (84% and 96%, respectively). “While 80% sounds good, I think we’re talking about ID and pulmonary docs at the best institutions across the United States, so I would have expected much higher,” Dr. Green said.
Only one-third of respondents knew that the T-SPOT.TB test, an IGRA, had the highest sensitivity for identifying those with latent TB infection. And only about half were able to appropriately identify the need to initiate therapy for latent TB in a scenario in which the patient was at “high risk for latent tuberculosis with a positive tuberculin skin test and a negative interferon-gamma release assay.”
Fellows comprised 42.5% of respondents and the remainder were attendings of varying levels of seniority. About half of respondents were pulmonologists and the other half infectious disease specialists. The majority (91%) were practicing or training in university hospitals.
One major limitation of the study, said Dr. Green, is the low response rate. “I would have liked 3,000 responses,” he said, rather than just over 300.
To disseminate the questionnaire, he contacted pulmonary and infectious disease academic program directors and coordinators and asked them to forward the survey invitation to their full-time faculty and fellows. Dr. Green also acknowledged that his project missed those physicians not working in academic centers.
“I would like to think that the reason people didn’t do as well as I had hoped is because of the conflicting literature out there and using not necessarily the guidelines but rather their current knowledge on what was most recently published,” said Dr. Green. “But maybe there is a true misunderstanding.”
The authors reported there were no product or funding disclosures relevant to this study.
TORONTO – U.S.-based pulmonary and infectious disease specialists prefer interferon-gamma release assays (IGRA) over tuberculin skin tests (TST) for the diagnosis of latent TB infection, but may not fully understand how to use and interpret the test results, according to survey results presented at the CHEST annual meeting.
Adam G. Green, MD, conducted the research while he was a fellow in pulmonology/critical care at Montefiore Medical Center in New York. Dr. Green told attendees that about one-third of the world’s population are infected with TB and about 15 million of those live in the United States. Two-thirds of U.S. cases are seen in foreign-born individuals and are clustered in four states—New, York, California, Florida, and Texas.
Among 304 clinicians who responded to an invitation to an online questionnaire, 78% said they preferred to use IGRA over TST and 91% said they had a “good understanding” of how to use and interpret IGRA. However, when queried further on how to best use and interpret IGRAs according to current guidelines, their answers to 11 knowledge-based questions told a somewhat different story, said Dr. Green, who is an intensivist at Cooper University Health Care in Camden, N.J.
While 96% knew IGRAs are not helpful in monitoring response to TB treatment, 20% erroneously thought that a positive IGRA predicts latent TB infection reactivation in the future.
Most respondents correctly answered two “fundamental” questions on cross-reactivity of IGRAs with Mycobacterium avium complex and bacilli Calmette-Guérin (BCG) vaccination (84% and 96%, respectively). “While 80% sounds good, I think we’re talking about ID and pulmonary docs at the best institutions across the United States, so I would have expected much higher,” Dr. Green said.
Only one-third of respondents knew that the T-SPOT.TB test, an IGRA, had the highest sensitivity for identifying those with latent TB infection. And only about half were able to appropriately identify the need to initiate therapy for latent TB in a scenario in which the patient was at “high risk for latent tuberculosis with a positive tuberculin skin test and a negative interferon-gamma release assay.”
Fellows comprised 42.5% of respondents and the remainder were attendings of varying levels of seniority. About half of respondents were pulmonologists and the other half infectious disease specialists. The majority (91%) were practicing or training in university hospitals.
One major limitation of the study, said Dr. Green, is the low response rate. “I would have liked 3,000 responses,” he said, rather than just over 300.
To disseminate the questionnaire, he contacted pulmonary and infectious disease academic program directors and coordinators and asked them to forward the survey invitation to their full-time faculty and fellows. Dr. Green also acknowledged that his project missed those physicians not working in academic centers.
“I would like to think that the reason people didn’t do as well as I had hoped is because of the conflicting literature out there and using not necessarily the guidelines but rather their current knowledge on what was most recently published,” said Dr. Green. “But maybe there is a true misunderstanding.”
The authors reported there were no product or funding disclosures relevant to this study.
TORONTO – U.S.-based pulmonary and infectious disease specialists prefer interferon-gamma release assays (IGRA) over tuberculin skin tests (TST) for the diagnosis of latent TB infection, but may not fully understand how to use and interpret the test results, according to survey results presented at the CHEST annual meeting.
Adam G. Green, MD, conducted the research while he was a fellow in pulmonology/critical care at Montefiore Medical Center in New York. Dr. Green told attendees that about one-third of the world’s population are infected with TB and about 15 million of those live in the United States. Two-thirds of U.S. cases are seen in foreign-born individuals and are clustered in four states—New, York, California, Florida, and Texas.
Among 304 clinicians who responded to an invitation to an online questionnaire, 78% said they preferred to use IGRA over TST and 91% said they had a “good understanding” of how to use and interpret IGRA. However, when queried further on how to best use and interpret IGRAs according to current guidelines, their answers to 11 knowledge-based questions told a somewhat different story, said Dr. Green, who is an intensivist at Cooper University Health Care in Camden, N.J.
While 96% knew IGRAs are not helpful in monitoring response to TB treatment, 20% erroneously thought that a positive IGRA predicts latent TB infection reactivation in the future.
Most respondents correctly answered two “fundamental” questions on cross-reactivity of IGRAs with Mycobacterium avium complex and bacilli Calmette-Guérin (BCG) vaccination (84% and 96%, respectively). “While 80% sounds good, I think we’re talking about ID and pulmonary docs at the best institutions across the United States, so I would have expected much higher,” Dr. Green said.
Only one-third of respondents knew that the T-SPOT.TB test, an IGRA, had the highest sensitivity for identifying those with latent TB infection. And only about half were able to appropriately identify the need to initiate therapy for latent TB in a scenario in which the patient was at “high risk for latent tuberculosis with a positive tuberculin skin test and a negative interferon-gamma release assay.”
Fellows comprised 42.5% of respondents and the remainder were attendings of varying levels of seniority. About half of respondents were pulmonologists and the other half infectious disease specialists. The majority (91%) were practicing or training in university hospitals.
One major limitation of the study, said Dr. Green, is the low response rate. “I would have liked 3,000 responses,” he said, rather than just over 300.
To disseminate the questionnaire, he contacted pulmonary and infectious disease academic program directors and coordinators and asked them to forward the survey invitation to their full-time faculty and fellows. Dr. Green also acknowledged that his project missed those physicians not working in academic centers.
“I would like to think that the reason people didn’t do as well as I had hoped is because of the conflicting literature out there and using not necessarily the guidelines but rather their current knowledge on what was most recently published,” said Dr. Green. “But maybe there is a true misunderstanding.”
The authors reported there were no product or funding disclosures relevant to this study.
AT CHEST 2017
Key clinical point: Most physicians queried preferred IGRAs over TST for the detection of latent TB infection.
Major finding: Of the 304 respondents to a survey, 78% said they preferred IGRAs over TST for TB testing and 91% reported having a good understanding of how to use and interpret IGRAs.
Data source: Online survey of 304 pulmonary and infectious disease attending physicians and fellows from U.S.-based academic programs.
Disclosures: The authors reported there were no product or funding disclosures relevant to this study.
Strict OR attire policy had no impact on SSI rate
SAN DIEGO – Implementation of strict operating room (OR) attire policies did not reduce the rates of superficial surgical site infections (SSIs), according to an analysis of more than 6,500 patients.
“SSIs are the most common cause of health care–associated infections in the U.S.,” study author Sandra Farach, MD, said at the annual clinical congress of the American College of Surgeons. “It’s estimated that SSIs occur in 2%-5% of patients undergoing inpatient surgery. They’re associated with significant patient morbidity and mortality and are a significant burden to the health care system, accounting for an estimated $3.5 to $10 billion in health care expenditures.”
In February 2015, the Association for periOperative Registered Nurses published recommendations on operating room attire, providing a guideline for modifying facility policies and regulatory requirements. It included stringent policies designed to minimize the exposed areas of skin and hair of operating room staff. “New attire policies were met with some criticism as there is a paucity of evidence-based data to support these recommendations,” said Dr. Farach, who helped conduct the study during her tenure as chief resident of general surgery at the University of Rochester (N.Y.) Medical Center.
A total of 6,517 patients were included in the analysis: 3,077 in the preimplementation group and 3,440 patients in the postimplementation group. The postimplementation group tended to be older and had significantly higher rates of hypertension, dialysis treatments, steroid use, and systemic inflammatory response syndrome, as well as higher American Society of Anesthesiologists classification scores. “However, they had a significantly lower BMI, incidence of smoking and COPD, and a higher incidence of clean wounds, which would theoretically leave them less exposed to SSIs,” said Dr. Farach, who is now a pediatric surgical critical care fellow at Le Bonheur Children’s Hospital in Memphis.
Overall, the rate of SSIs by wound class increased between the preimplementation and postimplementation time periods: The percent of change was 0.6%, 0.9%, 2.3%, and 3.8% in the clean, clean-contaminated, contaminated, and dirty/infected cases, respectively. When the review was limited to clean or clean-contaminated cases, SSI increased slightly, from 0.7% to 0.8% (P = .085). There were no significant differences in the complication rate, 30-day mortality, unplanned return to the OR, or length of stay between preimplementation or postimplementation at either hospital.
When Dr. Farach and her associates examined the overall infection rate, they observed no significant differences preimplementation and postimplementation in the rates of incisional SSI (0.97% vs. 0.96%, respectively; P = .949), organ space SSI (1.20% vs. 0.81%; P = .115), and total SSIs (2.11% vs. 1.77%; P = .321). Multivariate analysis showed that implementation of OR changes was not associated with an increased risk of SSIs. Factors that did predict high SSI rates included preoperative SSI (adjusted odds ratio 23.04), long operative time (AOR 3.4), preoperative open wound (AOR 2.94), contaminated/dirty wound classes (AOR 2.32), and morbid obesity (AOR 1.8).
“A hypothetical analysis revealed that a sample of over 495,000 patients would be required to demonstrate a 10% incisional SSI reduction among patients with clean or clean-contaminated wounds,” Dr. Farach noted. “Nevertheless, the study showed a numerical increase in SSI during the study period. Policies regarding OR attire were universally unpopular. As a result, OR governance is now working to repeal these new policies at both hospitals.”
“Given the rarity of SSI in the population subset which is relevant to the OR attire question (clean and clean-contaminated wounds, 0.7%), designing a study to prove effectiveness of an intervention (i.e., a 10% improvement) is totally impractical to conduct as this would require nearly a half a million cases,” said Jacob Moalem, MD, the lead author of the study who is an endocrine surgeon at the University of Rochester. At the meeting, a discussant suggested that conducting such a study is feasible; however, “I would strongly argue that putting that many people through such a study, when we know that these attire rules have a deleterious effect on surgeon comfort and OR team dynamics and morale, would not be prudent,” Dr. Moalem said. “We know that surgeon comfort, ability to focus on the task at hand, and minimizing distractions in the OR are critically important in reducing errors. In my opinion, by continuing to focus on these unfounded attire restrictions, one would be far more likely to actually cause injury to a patient than to prevent a wound infection.”
The researchers reported having no financial disclosures.
SAN DIEGO – Implementation of strict operating room (OR) attire policies did not reduce the rates of superficial surgical site infections (SSIs), according to an analysis of more than 6,500 patients.
“SSIs are the most common cause of health care–associated infections in the U.S.,” study author Sandra Farach, MD, said at the annual clinical congress of the American College of Surgeons. “It’s estimated that SSIs occur in 2%-5% of patients undergoing inpatient surgery. They’re associated with significant patient morbidity and mortality and are a significant burden to the health care system, accounting for an estimated $3.5 to $10 billion in health care expenditures.”
In February 2015, the Association for periOperative Registered Nurses published recommendations on operating room attire, providing a guideline for modifying facility policies and regulatory requirements. It included stringent policies designed to minimize the exposed areas of skin and hair of operating room staff. “New attire policies were met with some criticism as there is a paucity of evidence-based data to support these recommendations,” said Dr. Farach, who helped conduct the study during her tenure as chief resident of general surgery at the University of Rochester (N.Y.) Medical Center.
A total of 6,517 patients were included in the analysis: 3,077 in the preimplementation group and 3,440 patients in the postimplementation group. The postimplementation group tended to be older and had significantly higher rates of hypertension, dialysis treatments, steroid use, and systemic inflammatory response syndrome, as well as higher American Society of Anesthesiologists classification scores. “However, they had a significantly lower BMI, incidence of smoking and COPD, and a higher incidence of clean wounds, which would theoretically leave them less exposed to SSIs,” said Dr. Farach, who is now a pediatric surgical critical care fellow at Le Bonheur Children’s Hospital in Memphis.
Overall, the rate of SSIs by wound class increased between the preimplementation and postimplementation time periods: The percent of change was 0.6%, 0.9%, 2.3%, and 3.8% in the clean, clean-contaminated, contaminated, and dirty/infected cases, respectively. When the review was limited to clean or clean-contaminated cases, SSI increased slightly, from 0.7% to 0.8% (P = .085). There were no significant differences in the complication rate, 30-day mortality, unplanned return to the OR, or length of stay between preimplementation or postimplementation at either hospital.
When Dr. Farach and her associates examined the overall infection rate, they observed no significant differences preimplementation and postimplementation in the rates of incisional SSI (0.97% vs. 0.96%, respectively; P = .949), organ space SSI (1.20% vs. 0.81%; P = .115), and total SSIs (2.11% vs. 1.77%; P = .321). Multivariate analysis showed that implementation of OR changes was not associated with an increased risk of SSIs. Factors that did predict high SSI rates included preoperative SSI (adjusted odds ratio 23.04), long operative time (AOR 3.4), preoperative open wound (AOR 2.94), contaminated/dirty wound classes (AOR 2.32), and morbid obesity (AOR 1.8).
“A hypothetical analysis revealed that a sample of over 495,000 patients would be required to demonstrate a 10% incisional SSI reduction among patients with clean or clean-contaminated wounds,” Dr. Farach noted. “Nevertheless, the study showed a numerical increase in SSI during the study period. Policies regarding OR attire were universally unpopular. As a result, OR governance is now working to repeal these new policies at both hospitals.”
“Given the rarity of SSI in the population subset which is relevant to the OR attire question (clean and clean-contaminated wounds, 0.7%), designing a study to prove effectiveness of an intervention (i.e., a 10% improvement) is totally impractical to conduct as this would require nearly a half a million cases,” said Jacob Moalem, MD, the lead author of the study who is an endocrine surgeon at the University of Rochester. At the meeting, a discussant suggested that conducting such a study is feasible; however, “I would strongly argue that putting that many people through such a study, when we know that these attire rules have a deleterious effect on surgeon comfort and OR team dynamics and morale, would not be prudent,” Dr. Moalem said. “We know that surgeon comfort, ability to focus on the task at hand, and minimizing distractions in the OR are critically important in reducing errors. In my opinion, by continuing to focus on these unfounded attire restrictions, one would be far more likely to actually cause injury to a patient than to prevent a wound infection.”
The researchers reported having no financial disclosures.
SAN DIEGO – Implementation of strict operating room (OR) attire policies did not reduce the rates of superficial surgical site infections (SSIs), according to an analysis of more than 6,500 patients.
“SSIs are the most common cause of health care–associated infections in the U.S.,” study author Sandra Farach, MD, said at the annual clinical congress of the American College of Surgeons. “It’s estimated that SSIs occur in 2%-5% of patients undergoing inpatient surgery. They’re associated with significant patient morbidity and mortality and are a significant burden to the health care system, accounting for an estimated $3.5 to $10 billion in health care expenditures.”
In February 2015, the Association for periOperative Registered Nurses published recommendations on operating room attire, providing a guideline for modifying facility policies and regulatory requirements. It included stringent policies designed to minimize the exposed areas of skin and hair of operating room staff. “New attire policies were met with some criticism as there is a paucity of evidence-based data to support these recommendations,” said Dr. Farach, who helped conduct the study during her tenure as chief resident of general surgery at the University of Rochester (N.Y.) Medical Center.
A total of 6,517 patients were included in the analysis: 3,077 in the preimplementation group and 3,440 patients in the postimplementation group. The postimplementation group tended to be older and had significantly higher rates of hypertension, dialysis treatments, steroid use, and systemic inflammatory response syndrome, as well as higher American Society of Anesthesiologists classification scores. “However, they had a significantly lower BMI, incidence of smoking and COPD, and a higher incidence of clean wounds, which would theoretically leave them less exposed to SSIs,” said Dr. Farach, who is now a pediatric surgical critical care fellow at Le Bonheur Children’s Hospital in Memphis.
Overall, the rate of SSIs by wound class increased between the preimplementation and postimplementation time periods: The percent of change was 0.6%, 0.9%, 2.3%, and 3.8% in the clean, clean-contaminated, contaminated, and dirty/infected cases, respectively. When the review was limited to clean or clean-contaminated cases, SSI increased slightly, from 0.7% to 0.8% (P = .085). There were no significant differences in the complication rate, 30-day mortality, unplanned return to the OR, or length of stay between preimplementation or postimplementation at either hospital.
When Dr. Farach and her associates examined the overall infection rate, they observed no significant differences preimplementation and postimplementation in the rates of incisional SSI (0.97% vs. 0.96%, respectively; P = .949), organ space SSI (1.20% vs. 0.81%; P = .115), and total SSIs (2.11% vs. 1.77%; P = .321). Multivariate analysis showed that implementation of OR changes was not associated with an increased risk of SSIs. Factors that did predict high SSI rates included preoperative SSI (adjusted odds ratio 23.04), long operative time (AOR 3.4), preoperative open wound (AOR 2.94), contaminated/dirty wound classes (AOR 2.32), and morbid obesity (AOR 1.8).
“A hypothetical analysis revealed that a sample of over 495,000 patients would be required to demonstrate a 10% incisional SSI reduction among patients with clean or clean-contaminated wounds,” Dr. Farach noted. “Nevertheless, the study showed a numerical increase in SSI during the study period. Policies regarding OR attire were universally unpopular. As a result, OR governance is now working to repeal these new policies at both hospitals.”
“Given the rarity of SSI in the population subset which is relevant to the OR attire question (clean and clean-contaminated wounds, 0.7%), designing a study to prove effectiveness of an intervention (i.e., a 10% improvement) is totally impractical to conduct as this would require nearly a half a million cases,” said Jacob Moalem, MD, the lead author of the study who is an endocrine surgeon at the University of Rochester. At the meeting, a discussant suggested that conducting such a study is feasible; however, “I would strongly argue that putting that many people through such a study, when we know that these attire rules have a deleterious effect on surgeon comfort and OR team dynamics and morale, would not be prudent,” Dr. Moalem said. “We know that surgeon comfort, ability to focus on the task at hand, and minimizing distractions in the OR are critically important in reducing errors. In my opinion, by continuing to focus on these unfounded attire restrictions, one would be far more likely to actually cause injury to a patient than to prevent a wound infection.”
The researchers reported having no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Implementation of stringent operating room attire policies do not reduce rates superficial surgical site infections (SSIs).
Major finding: The researchers observed no significant differences preimplementation and postimplementation of OR attire policies in the rates of incisional SSI (0.97 vs. 0.96, respectively; P = .949), organ space SSI (1.20 vs 0.81; P = .115), and total SSIs (2.11 vs. 1.77; P = .321).
Study details: A study of 6,517 patients who underwent surgery at two tertiary care teaching hospitals.
Disclosures: The researchers reported having no financial disclosures.
New and Noteworthy Information—November 2017
Can a Fatty Diet Increase Relapse Risk in Children With MS?
In children with multiple sclerosis (MS), high energy intake from fat, especially saturated fat, may increase the hazard of relapse, while vegetable intake may be independently protective, according to a study published online ahead of print October 9 in the Journal of Neurology, Neurosurgery & Psychiatry. A total of 219 patients with pediatric relapsing-remitting MS or clinically isolated syndrome with disease onset before age 18 and duration of less than four years were enrolled in a multicenter study that was completed at 11 pediatric MS centers. Investigators used the Block Kids Food Screener to examine dietary intake during the week before enrollment. Each 10% increase in energy intake from fat increased the hazard of relapse by 56%, and each 10% increase in saturated fat tripled this hazard.
Azary S, Schreiner T, Graves J, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017 Oct 9 [Epub ahead of print].
Cooling Reduces Risk of Epilepsy After Perinatal Asphyxia
Administering therapeutic hypothermia to babies with perinatal asphyxia can reduce their risk of epilepsy in childhood, according to a study published online ahead of print September 29 in Epilepsia. From 2006 to 2013, 151 infants with perinatal asphyxia underwent 72 hours of cooling. Clinical and amplitude-integrated EEG with single-channel EEG-verified neonatal seizures were treated with antiepileptic drugs (AEDs). MRI was assessed using a severity score that ranged from 0 to 11. One hundred thirty-four children were assessed at 18–24 months. Babies born after 2007 who received therapeutic hypothermia had a lower rate of epilepsy than those born before this method was introduced. At two years, 6% of the children had epilepsy, and 2% were receiving AEDs. Before therapeutic hypothermia was introduced, the rate of death or moderate or severe disability was about 66%.
Liu X, Jary S, Cowan F, Thoresen M. Reduced infancy and childhood epilepsy following hypothermia-treated neonatal encephalopathy. Epilepsia. 2017 Sep 29 [Epub ahead of print].
Risk Factors Are Increasing in People With Stroke
The prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse in acute ischemic stroke increased between 2004 and 2014, according to a study published online ahead of print October 11 in Neurology. Researchers used the National Inpatient Sample to identify 922,451 adult hospitalizations for ischemic stroke. In all, 92.5% of patients with stroke had one or more risk factors. Age- and sex-adjusted prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse were 79%, 34%, 47%, 15%, and 2%, respectively. The prevalence of carotid stenosis, chronic renal failure, and coronary artery disease were 13%, 12%, and 27%, respectively. Risk factor prevalence varied by age, race, and sex. The prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse increased annually by 1.4%, 2%, 7%, 5%, and 7%, respectively.
Otite FO, Liaw N, Khandelwal P, et al. Increasing prevalence of vascular risk factors in patients with stroke: a call to action. Neurology. 2017 Oct 11 [Epub ahead of print].
Transcranial Direct-Current Stimulation for MS-Related Fatigue
People with multiple sclerosis (MS) who undergo a noninvasive form of electrical brain stimulation have significantly reduced fatigue, according to a study published online ahead of print September 1 in the Multiple Sclerosis Journal. Twenty-seven people with MS were randomized to receive transcranial direct-current stimulation or a placebo while playing a cognitive training game that targets processing speed and working memory. After 20 sessions, participants reported their level of fatigue using the Patient-Reported Outcomes Measurement Information System. Higher numbers correlated with greater fatigue. The researchers reported a statistically significant reduction in the group that underwent transcranial direct-current stimulation, compared with the placebo group. Intervention participants had a 5.6-point drop in fatigue on average, while control participants had a 0.9-point increase in fatigue.
Charvet LE, Dobbs B, Shaw MT, et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. 2017 Sep 1 [Epub ahead of print].
New Genetic Risk Variants for RLS Identified
Thirteen previously unknown genetic risk variants for restless legs syndrome (RLS) have been identified, according to a study published in the November issue of Lancet Neurology. Researchers combined three genome-wide association studies’ datasets with diagnosis data collected from 2003 to 2017. The latter data came from interviews and questionnaires and included 15,126 cases and 95,725 controls. Significant genome-wide signals were tested for replication in an independent genome-wide association study of 30,770 cases and 286,913 controls. Investigators identified and replicated 13 new risk loci for RLS and confirmed six previously identified risk loci. MEIS1 was confirmed as the strongest genetic risk factor for RLS. Gene prioritization, enrichment, and genetic correlation analyses showed that identified pathways were related to neurodevelopment and highlighted genes linked to axon guidance, synapse formation, and neuronal specification.
Schormair B, Zhao C, Bell S, et al. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16(11):898-907.
High Blood Pressure Associated With Increased Dementia Risk in Women
Hypertension is more common in men, but is only associated with dementia risk in women, according to a study published online ahead of print October 4 in Neurology. Researchers evaluated 5,646 members of a diverse integrated health care delivery system who had clinical examinations and health survey data from 1964 to 1973 (mean age, 32.7) and 1978 to 1985 (mean age, 44.3) and were members as of January 1, 1996 (mean age, 59.8). A total of 532 people were diagnosed with dementia. Mid-adulthood (circa age 44) hypertension was associated with 65% increased dementia risk among women, but not among men. Onset of hypertension in mid-adulthood predicted 73% higher dementia risk in women, compared with stable normotension. There was no evidence that hypertension or changes in hypertension increased dementia risk among men.
Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017 Oct 4 [Epub ahead of print].
A Risk Factor for Major Bleeding During Treatment With NOACs
Among patients taking non-vitamin K oral anticoagulants (NOACs) for nonvalvular atrial fibrillation, concurrent use of amiodarone, fluconazole, rifampin, and phenytoin, compared with the use of NOACs alone, is associated with increased risk of major bleeding, according to a study published October 3 in JAMA. Researchers retrospectively examined data for 91,330 patients with nonvalvular atrial fibrillation who received at least one NOAC prescription of dabigatran, rivaroxaban, or apixaban from 2012 through 2016. They analyzed the bleeding risk associated with the concurrent use of 12 commonly prescribed medications. A total of 4,770 major bleeding events occurred. The incidence rate of major bleeding was significantly lower for concurrent use of atorvastatin, digoxin, and erythromycin or clarithromycin. Physicians prescribing NOACs should consider the potential risks associated with concomitant use of other drugs, the researchers said.
Chang SH, Chou IJ, Yeh YH, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318(13):1250-1259.
Discontinuing Aspirin Therapy May Increase Cardiovascular Risk
In long-term users, discontinuation of low-dose aspirin in the absence of major surgery or bleeding is associated with a greater-than-30% increased risk of cardiovascular events, according to a study published September 26 in Circulation. Researchers performed a cohort study of 601,527 participants taking low-dose aspirin for heart attack and stroke prevention between 2005 and 2009. Participants were older than 40, cancer-free, and had an adherence rate of 80% or greater during the first year of treatment. During a median of 3.0 years of follow-up, 62,690 cardiovascular events were reported. Patients who discontinued aspirin had a higher rate of cardiovascular events than those who continued (multivariable-adjusted hazard ratio, 1.37), corresponding to an additional cardiovascular event observed per year in one of every 74 patients who discontinued aspirin.
Sundström J, Hedberg J, Thuresson M, et al. Low-dose aspirin discontinuation and risk of cardiovascular events: a Swedish nationwide, population-based cohort study. Circulation. 2017;136(13):1183-1192.
FDA Approves 80-mg Ingrezza Capsule for Tardive Dyskinesia
The FDA has approved an 80-mg capsule of Ingrezza (valbenazine) for the treatment of adults with tardive dyskinesia. In clinical studies, Ingrezza 80 mg provided significant, rapid, and meaningful improvement in tardive dyskinesia severity, compared with placebo, at six weeks. Results were seen as early as two weeks, and continued reductions were observed through 48 weeks of treatment. The drug was FDA-approved in April and has been available as 40-mg capsules. Neurocrine Biosciences markets Ingrezza.
FDA Approves Generic Version of Copaxone
The FDA has approved Mylan’s glatiramer acetate injection 40 mg/mL for thrice-weekly injection. The drug is a substitutable generic version of Teva’s Copaxone 40 mg/mL. The agency also approved Mylan’s glatiramer acetate injection 20 mg/mL for once-daily injection, a substitutable generic version of Teva’s Copaxone 20 mg/mL. Both products are indicated for the treatment of patients with relapsing forms of multiple sclerosis. As part of its applications, Mylan submitted side-by-side analyses demonstrating that its glatiramer acetate injections have the same active ingredient, dosage form, route of administration, and strength as their branded counterpart.
FDA Approves Gocovri for Dyskinesia in Parkinson’s Disease
The FDA has approved Gocovri (amantadine) extended-release capsules for the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy with or without concomitant dopaminergic medications. Gocovri, previously granted orphan drug status by the FDA, is the first medicine approved by the FDA for this indication. Gocovri is a high-dose (274 mg) formulation of amantadine (equivalent to 340 mg of amantadine HCl) taken once-daily at bedtime that delivers consistently high levels of amantadine from the morning and throughout the day. Adamas Pharmaceuticals markets the drug.
FDA Has Approved Lyrica CR for Two Indications
The FDA has approved Lyrica CR (pregabalin) extended-release tablets CV as once-daily therapy for the management of neuropathic pain associated with diabetic peripheral neuropathy and the management of postherpetic neuralgia. The efficacy and safety of Lyrica CR in postherpetic neuralgia was established in a randomized placebo-controlled clinical trial conducted in 801 patients with postherpetic neuralgia who entered single-blind treatment with Lyrica CR. In the study, 73.6% of patients in the Lyrica CR group achieved at least 50% improvement in pain intensity, compared with 54.6% in the placebo group. The postherpetic neuralgia data also supported the diabetic peripheral neuropathy indication. Pfizer markets Lyrica CR.
—Kimberly Williams
Can a Fatty Diet Increase Relapse Risk in Children With MS?
In children with multiple sclerosis (MS), high energy intake from fat, especially saturated fat, may increase the hazard of relapse, while vegetable intake may be independently protective, according to a study published online ahead of print October 9 in the Journal of Neurology, Neurosurgery & Psychiatry. A total of 219 patients with pediatric relapsing-remitting MS or clinically isolated syndrome with disease onset before age 18 and duration of less than four years were enrolled in a multicenter study that was completed at 11 pediatric MS centers. Investigators used the Block Kids Food Screener to examine dietary intake during the week before enrollment. Each 10% increase in energy intake from fat increased the hazard of relapse by 56%, and each 10% increase in saturated fat tripled this hazard.
Azary S, Schreiner T, Graves J, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017 Oct 9 [Epub ahead of print].
Cooling Reduces Risk of Epilepsy After Perinatal Asphyxia
Administering therapeutic hypothermia to babies with perinatal asphyxia can reduce their risk of epilepsy in childhood, according to a study published online ahead of print September 29 in Epilepsia. From 2006 to 2013, 151 infants with perinatal asphyxia underwent 72 hours of cooling. Clinical and amplitude-integrated EEG with single-channel EEG-verified neonatal seizures were treated with antiepileptic drugs (AEDs). MRI was assessed using a severity score that ranged from 0 to 11. One hundred thirty-four children were assessed at 18–24 months. Babies born after 2007 who received therapeutic hypothermia had a lower rate of epilepsy than those born before this method was introduced. At two years, 6% of the children had epilepsy, and 2% were receiving AEDs. Before therapeutic hypothermia was introduced, the rate of death or moderate or severe disability was about 66%.
Liu X, Jary S, Cowan F, Thoresen M. Reduced infancy and childhood epilepsy following hypothermia-treated neonatal encephalopathy. Epilepsia. 2017 Sep 29 [Epub ahead of print].
Risk Factors Are Increasing in People With Stroke
The prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse in acute ischemic stroke increased between 2004 and 2014, according to a study published online ahead of print October 11 in Neurology. Researchers used the National Inpatient Sample to identify 922,451 adult hospitalizations for ischemic stroke. In all, 92.5% of patients with stroke had one or more risk factors. Age- and sex-adjusted prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse were 79%, 34%, 47%, 15%, and 2%, respectively. The prevalence of carotid stenosis, chronic renal failure, and coronary artery disease were 13%, 12%, and 27%, respectively. Risk factor prevalence varied by age, race, and sex. The prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse increased annually by 1.4%, 2%, 7%, 5%, and 7%, respectively.
Otite FO, Liaw N, Khandelwal P, et al. Increasing prevalence of vascular risk factors in patients with stroke: a call to action. Neurology. 2017 Oct 11 [Epub ahead of print].
Transcranial Direct-Current Stimulation for MS-Related Fatigue
People with multiple sclerosis (MS) who undergo a noninvasive form of electrical brain stimulation have significantly reduced fatigue, according to a study published online ahead of print September 1 in the Multiple Sclerosis Journal. Twenty-seven people with MS were randomized to receive transcranial direct-current stimulation or a placebo while playing a cognitive training game that targets processing speed and working memory. After 20 sessions, participants reported their level of fatigue using the Patient-Reported Outcomes Measurement Information System. Higher numbers correlated with greater fatigue. The researchers reported a statistically significant reduction in the group that underwent transcranial direct-current stimulation, compared with the placebo group. Intervention participants had a 5.6-point drop in fatigue on average, while control participants had a 0.9-point increase in fatigue.
Charvet LE, Dobbs B, Shaw MT, et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. 2017 Sep 1 [Epub ahead of print].
New Genetic Risk Variants for RLS Identified
Thirteen previously unknown genetic risk variants for restless legs syndrome (RLS) have been identified, according to a study published in the November issue of Lancet Neurology. Researchers combined three genome-wide association studies’ datasets with diagnosis data collected from 2003 to 2017. The latter data came from interviews and questionnaires and included 15,126 cases and 95,725 controls. Significant genome-wide signals were tested for replication in an independent genome-wide association study of 30,770 cases and 286,913 controls. Investigators identified and replicated 13 new risk loci for RLS and confirmed six previously identified risk loci. MEIS1 was confirmed as the strongest genetic risk factor for RLS. Gene prioritization, enrichment, and genetic correlation analyses showed that identified pathways were related to neurodevelopment and highlighted genes linked to axon guidance, synapse formation, and neuronal specification.
Schormair B, Zhao C, Bell S, et al. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16(11):898-907.
High Blood Pressure Associated With Increased Dementia Risk in Women
Hypertension is more common in men, but is only associated with dementia risk in women, according to a study published online ahead of print October 4 in Neurology. Researchers evaluated 5,646 members of a diverse integrated health care delivery system who had clinical examinations and health survey data from 1964 to 1973 (mean age, 32.7) and 1978 to 1985 (mean age, 44.3) and were members as of January 1, 1996 (mean age, 59.8). A total of 532 people were diagnosed with dementia. Mid-adulthood (circa age 44) hypertension was associated with 65% increased dementia risk among women, but not among men. Onset of hypertension in mid-adulthood predicted 73% higher dementia risk in women, compared with stable normotension. There was no evidence that hypertension or changes in hypertension increased dementia risk among men.
Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017 Oct 4 [Epub ahead of print].
A Risk Factor for Major Bleeding During Treatment With NOACs
Among patients taking non-vitamin K oral anticoagulants (NOACs) for nonvalvular atrial fibrillation, concurrent use of amiodarone, fluconazole, rifampin, and phenytoin, compared with the use of NOACs alone, is associated with increased risk of major bleeding, according to a study published October 3 in JAMA. Researchers retrospectively examined data for 91,330 patients with nonvalvular atrial fibrillation who received at least one NOAC prescription of dabigatran, rivaroxaban, or apixaban from 2012 through 2016. They analyzed the bleeding risk associated with the concurrent use of 12 commonly prescribed medications. A total of 4,770 major bleeding events occurred. The incidence rate of major bleeding was significantly lower for concurrent use of atorvastatin, digoxin, and erythromycin or clarithromycin. Physicians prescribing NOACs should consider the potential risks associated with concomitant use of other drugs, the researchers said.
Chang SH, Chou IJ, Yeh YH, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318(13):1250-1259.
Discontinuing Aspirin Therapy May Increase Cardiovascular Risk
In long-term users, discontinuation of low-dose aspirin in the absence of major surgery or bleeding is associated with a greater-than-30% increased risk of cardiovascular events, according to a study published September 26 in Circulation. Researchers performed a cohort study of 601,527 participants taking low-dose aspirin for heart attack and stroke prevention between 2005 and 2009. Participants were older than 40, cancer-free, and had an adherence rate of 80% or greater during the first year of treatment. During a median of 3.0 years of follow-up, 62,690 cardiovascular events were reported. Patients who discontinued aspirin had a higher rate of cardiovascular events than those who continued (multivariable-adjusted hazard ratio, 1.37), corresponding to an additional cardiovascular event observed per year in one of every 74 patients who discontinued aspirin.
Sundström J, Hedberg J, Thuresson M, et al. Low-dose aspirin discontinuation and risk of cardiovascular events: a Swedish nationwide, population-based cohort study. Circulation. 2017;136(13):1183-1192.
FDA Approves 80-mg Ingrezza Capsule for Tardive Dyskinesia
The FDA has approved an 80-mg capsule of Ingrezza (valbenazine) for the treatment of adults with tardive dyskinesia. In clinical studies, Ingrezza 80 mg provided significant, rapid, and meaningful improvement in tardive dyskinesia severity, compared with placebo, at six weeks. Results were seen as early as two weeks, and continued reductions were observed through 48 weeks of treatment. The drug was FDA-approved in April and has been available as 40-mg capsules. Neurocrine Biosciences markets Ingrezza.
FDA Approves Generic Version of Copaxone
The FDA has approved Mylan’s glatiramer acetate injection 40 mg/mL for thrice-weekly injection. The drug is a substitutable generic version of Teva’s Copaxone 40 mg/mL. The agency also approved Mylan’s glatiramer acetate injection 20 mg/mL for once-daily injection, a substitutable generic version of Teva’s Copaxone 20 mg/mL. Both products are indicated for the treatment of patients with relapsing forms of multiple sclerosis. As part of its applications, Mylan submitted side-by-side analyses demonstrating that its glatiramer acetate injections have the same active ingredient, dosage form, route of administration, and strength as their branded counterpart.
FDA Approves Gocovri for Dyskinesia in Parkinson’s Disease
The FDA has approved Gocovri (amantadine) extended-release capsules for the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy with or without concomitant dopaminergic medications. Gocovri, previously granted orphan drug status by the FDA, is the first medicine approved by the FDA for this indication. Gocovri is a high-dose (274 mg) formulation of amantadine (equivalent to 340 mg of amantadine HCl) taken once-daily at bedtime that delivers consistently high levels of amantadine from the morning and throughout the day. Adamas Pharmaceuticals markets the drug.
FDA Has Approved Lyrica CR for Two Indications
The FDA has approved Lyrica CR (pregabalin) extended-release tablets CV as once-daily therapy for the management of neuropathic pain associated with diabetic peripheral neuropathy and the management of postherpetic neuralgia. The efficacy and safety of Lyrica CR in postherpetic neuralgia was established in a randomized placebo-controlled clinical trial conducted in 801 patients with postherpetic neuralgia who entered single-blind treatment with Lyrica CR. In the study, 73.6% of patients in the Lyrica CR group achieved at least 50% improvement in pain intensity, compared with 54.6% in the placebo group. The postherpetic neuralgia data also supported the diabetic peripheral neuropathy indication. Pfizer markets Lyrica CR.
—Kimberly Williams
Can a Fatty Diet Increase Relapse Risk in Children With MS?
In children with multiple sclerosis (MS), high energy intake from fat, especially saturated fat, may increase the hazard of relapse, while vegetable intake may be independently protective, according to a study published online ahead of print October 9 in the Journal of Neurology, Neurosurgery & Psychiatry. A total of 219 patients with pediatric relapsing-remitting MS or clinically isolated syndrome with disease onset before age 18 and duration of less than four years were enrolled in a multicenter study that was completed at 11 pediatric MS centers. Investigators used the Block Kids Food Screener to examine dietary intake during the week before enrollment. Each 10% increase in energy intake from fat increased the hazard of relapse by 56%, and each 10% increase in saturated fat tripled this hazard.
Azary S, Schreiner T, Graves J, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017 Oct 9 [Epub ahead of print].
Cooling Reduces Risk of Epilepsy After Perinatal Asphyxia
Administering therapeutic hypothermia to babies with perinatal asphyxia can reduce their risk of epilepsy in childhood, according to a study published online ahead of print September 29 in Epilepsia. From 2006 to 2013, 151 infants with perinatal asphyxia underwent 72 hours of cooling. Clinical and amplitude-integrated EEG with single-channel EEG-verified neonatal seizures were treated with antiepileptic drugs (AEDs). MRI was assessed using a severity score that ranged from 0 to 11. One hundred thirty-four children were assessed at 18–24 months. Babies born after 2007 who received therapeutic hypothermia had a lower rate of epilepsy than those born before this method was introduced. At two years, 6% of the children had epilepsy, and 2% were receiving AEDs. Before therapeutic hypothermia was introduced, the rate of death or moderate or severe disability was about 66%.
Liu X, Jary S, Cowan F, Thoresen M. Reduced infancy and childhood epilepsy following hypothermia-treated neonatal encephalopathy. Epilepsia. 2017 Sep 29 [Epub ahead of print].
Risk Factors Are Increasing in People With Stroke
The prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse in acute ischemic stroke increased between 2004 and 2014, according to a study published online ahead of print October 11 in Neurology. Researchers used the National Inpatient Sample to identify 922,451 adult hospitalizations for ischemic stroke. In all, 92.5% of patients with stroke had one or more risk factors. Age- and sex-adjusted prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse were 79%, 34%, 47%, 15%, and 2%, respectively. The prevalence of carotid stenosis, chronic renal failure, and coronary artery disease were 13%, 12%, and 27%, respectively. Risk factor prevalence varied by age, race, and sex. The prevalence of hypertension, diabetes, dyslipidemia, smoking, and drug abuse increased annually by 1.4%, 2%, 7%, 5%, and 7%, respectively.
Otite FO, Liaw N, Khandelwal P, et al. Increasing prevalence of vascular risk factors in patients with stroke: a call to action. Neurology. 2017 Oct 11 [Epub ahead of print].
Transcranial Direct-Current Stimulation for MS-Related Fatigue
People with multiple sclerosis (MS) who undergo a noninvasive form of electrical brain stimulation have significantly reduced fatigue, according to a study published online ahead of print September 1 in the Multiple Sclerosis Journal. Twenty-seven people with MS were randomized to receive transcranial direct-current stimulation or a placebo while playing a cognitive training game that targets processing speed and working memory. After 20 sessions, participants reported their level of fatigue using the Patient-Reported Outcomes Measurement Information System. Higher numbers correlated with greater fatigue. The researchers reported a statistically significant reduction in the group that underwent transcranial direct-current stimulation, compared with the placebo group. Intervention participants had a 5.6-point drop in fatigue on average, while control participants had a 0.9-point increase in fatigue.
Charvet LE, Dobbs B, Shaw MT, et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. 2017 Sep 1 [Epub ahead of print].
New Genetic Risk Variants for RLS Identified
Thirteen previously unknown genetic risk variants for restless legs syndrome (RLS) have been identified, according to a study published in the November issue of Lancet Neurology. Researchers combined three genome-wide association studies’ datasets with diagnosis data collected from 2003 to 2017. The latter data came from interviews and questionnaires and included 15,126 cases and 95,725 controls. Significant genome-wide signals were tested for replication in an independent genome-wide association study of 30,770 cases and 286,913 controls. Investigators identified and replicated 13 new risk loci for RLS and confirmed six previously identified risk loci. MEIS1 was confirmed as the strongest genetic risk factor for RLS. Gene prioritization, enrichment, and genetic correlation analyses showed that identified pathways were related to neurodevelopment and highlighted genes linked to axon guidance, synapse formation, and neuronal specification.
Schormair B, Zhao C, Bell S, et al. Identification of novel risk loci for restless legs syndrome in genome-wide association studies in individuals of European ancestry: a meta-analysis. Lancet Neurol. 2017;16(11):898-907.
High Blood Pressure Associated With Increased Dementia Risk in Women
Hypertension is more common in men, but is only associated with dementia risk in women, according to a study published online ahead of print October 4 in Neurology. Researchers evaluated 5,646 members of a diverse integrated health care delivery system who had clinical examinations and health survey data from 1964 to 1973 (mean age, 32.7) and 1978 to 1985 (mean age, 44.3) and were members as of January 1, 1996 (mean age, 59.8). A total of 532 people were diagnosed with dementia. Mid-adulthood (circa age 44) hypertension was associated with 65% increased dementia risk among women, but not among men. Onset of hypertension in mid-adulthood predicted 73% higher dementia risk in women, compared with stable normotension. There was no evidence that hypertension or changes in hypertension increased dementia risk among men.
Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017 Oct 4 [Epub ahead of print].
A Risk Factor for Major Bleeding During Treatment With NOACs
Among patients taking non-vitamin K oral anticoagulants (NOACs) for nonvalvular atrial fibrillation, concurrent use of amiodarone, fluconazole, rifampin, and phenytoin, compared with the use of NOACs alone, is associated with increased risk of major bleeding, according to a study published October 3 in JAMA. Researchers retrospectively examined data for 91,330 patients with nonvalvular atrial fibrillation who received at least one NOAC prescription of dabigatran, rivaroxaban, or apixaban from 2012 through 2016. They analyzed the bleeding risk associated with the concurrent use of 12 commonly prescribed medications. A total of 4,770 major bleeding events occurred. The incidence rate of major bleeding was significantly lower for concurrent use of atorvastatin, digoxin, and erythromycin or clarithromycin. Physicians prescribing NOACs should consider the potential risks associated with concomitant use of other drugs, the researchers said.
Chang SH, Chou IJ, Yeh YH, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318(13):1250-1259.
Discontinuing Aspirin Therapy May Increase Cardiovascular Risk
In long-term users, discontinuation of low-dose aspirin in the absence of major surgery or bleeding is associated with a greater-than-30% increased risk of cardiovascular events, according to a study published September 26 in Circulation. Researchers performed a cohort study of 601,527 participants taking low-dose aspirin for heart attack and stroke prevention between 2005 and 2009. Participants were older than 40, cancer-free, and had an adherence rate of 80% or greater during the first year of treatment. During a median of 3.0 years of follow-up, 62,690 cardiovascular events were reported. Patients who discontinued aspirin had a higher rate of cardiovascular events than those who continued (multivariable-adjusted hazard ratio, 1.37), corresponding to an additional cardiovascular event observed per year in one of every 74 patients who discontinued aspirin.
Sundström J, Hedberg J, Thuresson M, et al. Low-dose aspirin discontinuation and risk of cardiovascular events: a Swedish nationwide, population-based cohort study. Circulation. 2017;136(13):1183-1192.
FDA Approves 80-mg Ingrezza Capsule for Tardive Dyskinesia
The FDA has approved an 80-mg capsule of Ingrezza (valbenazine) for the treatment of adults with tardive dyskinesia. In clinical studies, Ingrezza 80 mg provided significant, rapid, and meaningful improvement in tardive dyskinesia severity, compared with placebo, at six weeks. Results were seen as early as two weeks, and continued reductions were observed through 48 weeks of treatment. The drug was FDA-approved in April and has been available as 40-mg capsules. Neurocrine Biosciences markets Ingrezza.
FDA Approves Generic Version of Copaxone
The FDA has approved Mylan’s glatiramer acetate injection 40 mg/mL for thrice-weekly injection. The drug is a substitutable generic version of Teva’s Copaxone 40 mg/mL. The agency also approved Mylan’s glatiramer acetate injection 20 mg/mL for once-daily injection, a substitutable generic version of Teva’s Copaxone 20 mg/mL. Both products are indicated for the treatment of patients with relapsing forms of multiple sclerosis. As part of its applications, Mylan submitted side-by-side analyses demonstrating that its glatiramer acetate injections have the same active ingredient, dosage form, route of administration, and strength as their branded counterpart.
FDA Approves Gocovri for Dyskinesia in Parkinson’s Disease
The FDA has approved Gocovri (amantadine) extended-release capsules for the treatment of dyskinesia in patients with Parkinson’s disease receiving levodopa-based therapy with or without concomitant dopaminergic medications. Gocovri, previously granted orphan drug status by the FDA, is the first medicine approved by the FDA for this indication. Gocovri is a high-dose (274 mg) formulation of amantadine (equivalent to 340 mg of amantadine HCl) taken once-daily at bedtime that delivers consistently high levels of amantadine from the morning and throughout the day. Adamas Pharmaceuticals markets the drug.
FDA Has Approved Lyrica CR for Two Indications
The FDA has approved Lyrica CR (pregabalin) extended-release tablets CV as once-daily therapy for the management of neuropathic pain associated with diabetic peripheral neuropathy and the management of postherpetic neuralgia. The efficacy and safety of Lyrica CR in postherpetic neuralgia was established in a randomized placebo-controlled clinical trial conducted in 801 patients with postherpetic neuralgia who entered single-blind treatment with Lyrica CR. In the study, 73.6% of patients in the Lyrica CR group achieved at least 50% improvement in pain intensity, compared with 54.6% in the placebo group. The postherpetic neuralgia data also supported the diabetic peripheral neuropathy indication. Pfizer markets Lyrica CR.
—Kimberly Williams
Weight recidivism after bariatric surgery: What constitutes failure?
NATIONAL HARBOR, MD. – A standard definition of bariatric surgery failure based on weight regain is needed to assess long-term outcomes in place of the seemingly arbitrary thresholds now in use, according to discussion generated by long-term outcome studies presented at Obesity Week 2017.
In another study, presented by Colin Martyn, MD, a general surgery resident at Texas Tech University Health Sciences Center, El Paso, the bariatric surgery failure rate at 11 years was characterized as an “alarming” 33.9%. In this study, bariatric surgery was considered a failure if the patient did not maintain excess weight loss (EWL) of 50% or greater.
The problem with this definition, like many others, is that “it fails to recognize that there could be significant health benefits and improvements in quality of life with less weight loss,” according to Philip Schauer, MD, director of the Cleveland Clinic Bariatric and Metabolic Institute. As the invited discussant for the data presented by Dr. Martyn, Dr. Schauer acknowledged that 50% EWL has been used by others as the dividing line between success and failure, but he called it “obsolete.”
This definition was one of several applied to weight recidivism in the study presented by Dr. Morell. Others included weight regain of more than 25% EWL over the postoperative nadir, an increase in body mass index to more than 35 kg/m2 after achieving a lower BMI, and a postsurgical BMI increase of more than 5 mg/m2. Not surprisingly, weight recidivism “varied widely with regard to the definitions used,” Dr. Morell reported.
Dr. Morell’s study involved evaluation of 1,766 patients with at least 1 year of follow-up after bariatric procedure. Most (1,490 patients) underwent laparoscopic Roux-en-y gastric bypass. After 2 years of follow-up, 93% achieved at least the 50% EWL threshold of treatment success, but Dr. Morell reported that the proportion above this or any threshold progressively diminished over time. For a definition of treatment success, Dr. Morell favors maintenance of at least 20% total weight loss as a threshold of long-term clinical success, a threshold met by 75% of patients at 5 years, in his analysis.
As has been shown in these studies and reported previously, the regaining of weight over time after bariatric surgery is common and progressive, but both studies ignited controversy about what measure is meaningful for declaring that bariatric surgery has failed over the long term. None of the current thresholds for failure are based on evidence that clinical benefit has been lost. Rather, it appears that these are simply accepted conventions.
“It bothers me to hear the word failure in these presentations, because I think the paradigm is changing from success and failure to that of treating chronic disease,” said Stacy Brethauer, MD, a staff surgeon in the Cleveland Clinic Digestive Disease Institute. Dr. Brethauer, the moderator of the session at Obesity Week where both long-term follow-up papers were presented, agreed that the at least 50% EWL benchmark is “flawed.” He suggested that more clinically meaningful methods of evaluating long-term outcome are needed for both clinical and research purposes.
The discussant of Dr. Morell’s paper, Samer G. Mattar, MD, a bariatric surgeon at the Swedish Medical Center, Seattle, also called for metrics based on clinical benefit rather than on weight alone.
“I would caution against this overall emphasis that we seem to place on weight gain and weight loss as a benchmark and predominant objective for what we do,” he said. “Our nonsurgeon colleagues have repeatedly demonstrated clinical benefits from total body weight loss of 10% or even 5%. So let’s not beat up ourselves over trying to maintain a greater than 50% EWL in all our patients.”
NATIONAL HARBOR, MD. – A standard definition of bariatric surgery failure based on weight regain is needed to assess long-term outcomes in place of the seemingly arbitrary thresholds now in use, according to discussion generated by long-term outcome studies presented at Obesity Week 2017.
In another study, presented by Colin Martyn, MD, a general surgery resident at Texas Tech University Health Sciences Center, El Paso, the bariatric surgery failure rate at 11 years was characterized as an “alarming” 33.9%. In this study, bariatric surgery was considered a failure if the patient did not maintain excess weight loss (EWL) of 50% or greater.
The problem with this definition, like many others, is that “it fails to recognize that there could be significant health benefits and improvements in quality of life with less weight loss,” according to Philip Schauer, MD, director of the Cleveland Clinic Bariatric and Metabolic Institute. As the invited discussant for the data presented by Dr. Martyn, Dr. Schauer acknowledged that 50% EWL has been used by others as the dividing line between success and failure, but he called it “obsolete.”
This definition was one of several applied to weight recidivism in the study presented by Dr. Morell. Others included weight regain of more than 25% EWL over the postoperative nadir, an increase in body mass index to more than 35 kg/m2 after achieving a lower BMI, and a postsurgical BMI increase of more than 5 mg/m2. Not surprisingly, weight recidivism “varied widely with regard to the definitions used,” Dr. Morell reported.
Dr. Morell’s study involved evaluation of 1,766 patients with at least 1 year of follow-up after bariatric procedure. Most (1,490 patients) underwent laparoscopic Roux-en-y gastric bypass. After 2 years of follow-up, 93% achieved at least the 50% EWL threshold of treatment success, but Dr. Morell reported that the proportion above this or any threshold progressively diminished over time. For a definition of treatment success, Dr. Morell favors maintenance of at least 20% total weight loss as a threshold of long-term clinical success, a threshold met by 75% of patients at 5 years, in his analysis.
As has been shown in these studies and reported previously, the regaining of weight over time after bariatric surgery is common and progressive, but both studies ignited controversy about what measure is meaningful for declaring that bariatric surgery has failed over the long term. None of the current thresholds for failure are based on evidence that clinical benefit has been lost. Rather, it appears that these are simply accepted conventions.
“It bothers me to hear the word failure in these presentations, because I think the paradigm is changing from success and failure to that of treating chronic disease,” said Stacy Brethauer, MD, a staff surgeon in the Cleveland Clinic Digestive Disease Institute. Dr. Brethauer, the moderator of the session at Obesity Week where both long-term follow-up papers were presented, agreed that the at least 50% EWL benchmark is “flawed.” He suggested that more clinically meaningful methods of evaluating long-term outcome are needed for both clinical and research purposes.
The discussant of Dr. Morell’s paper, Samer G. Mattar, MD, a bariatric surgeon at the Swedish Medical Center, Seattle, also called for metrics based on clinical benefit rather than on weight alone.
“I would caution against this overall emphasis that we seem to place on weight gain and weight loss as a benchmark and predominant objective for what we do,” he said. “Our nonsurgeon colleagues have repeatedly demonstrated clinical benefits from total body weight loss of 10% or even 5%. So let’s not beat up ourselves over trying to maintain a greater than 50% EWL in all our patients.”
NATIONAL HARBOR, MD. – A standard definition of bariatric surgery failure based on weight regain is needed to assess long-term outcomes in place of the seemingly arbitrary thresholds now in use, according to discussion generated by long-term outcome studies presented at Obesity Week 2017.
In another study, presented by Colin Martyn, MD, a general surgery resident at Texas Tech University Health Sciences Center, El Paso, the bariatric surgery failure rate at 11 years was characterized as an “alarming” 33.9%. In this study, bariatric surgery was considered a failure if the patient did not maintain excess weight loss (EWL) of 50% or greater.
The problem with this definition, like many others, is that “it fails to recognize that there could be significant health benefits and improvements in quality of life with less weight loss,” according to Philip Schauer, MD, director of the Cleveland Clinic Bariatric and Metabolic Institute. As the invited discussant for the data presented by Dr. Martyn, Dr. Schauer acknowledged that 50% EWL has been used by others as the dividing line between success and failure, but he called it “obsolete.”
This definition was one of several applied to weight recidivism in the study presented by Dr. Morell. Others included weight regain of more than 25% EWL over the postoperative nadir, an increase in body mass index to more than 35 kg/m2 after achieving a lower BMI, and a postsurgical BMI increase of more than 5 mg/m2. Not surprisingly, weight recidivism “varied widely with regard to the definitions used,” Dr. Morell reported.
Dr. Morell’s study involved evaluation of 1,766 patients with at least 1 year of follow-up after bariatric procedure. Most (1,490 patients) underwent laparoscopic Roux-en-y gastric bypass. After 2 years of follow-up, 93% achieved at least the 50% EWL threshold of treatment success, but Dr. Morell reported that the proportion above this or any threshold progressively diminished over time. For a definition of treatment success, Dr. Morell favors maintenance of at least 20% total weight loss as a threshold of long-term clinical success, a threshold met by 75% of patients at 5 years, in his analysis.
As has been shown in these studies and reported previously, the regaining of weight over time after bariatric surgery is common and progressive, but both studies ignited controversy about what measure is meaningful for declaring that bariatric surgery has failed over the long term. None of the current thresholds for failure are based on evidence that clinical benefit has been lost. Rather, it appears that these are simply accepted conventions.
“It bothers me to hear the word failure in these presentations, because I think the paradigm is changing from success and failure to that of treating chronic disease,” said Stacy Brethauer, MD, a staff surgeon in the Cleveland Clinic Digestive Disease Institute. Dr. Brethauer, the moderator of the session at Obesity Week where both long-term follow-up papers were presented, agreed that the at least 50% EWL benchmark is “flawed.” He suggested that more clinically meaningful methods of evaluating long-term outcome are needed for both clinical and research purposes.
The discussant of Dr. Morell’s paper, Samer G. Mattar, MD, a bariatric surgeon at the Swedish Medical Center, Seattle, also called for metrics based on clinical benefit rather than on weight alone.
“I would caution against this overall emphasis that we seem to place on weight gain and weight loss as a benchmark and predominant objective for what we do,” he said. “Our nonsurgeon colleagues have repeatedly demonstrated clinical benefits from total body weight loss of 10% or even 5%. So let’s not beat up ourselves over trying to maintain a greater than 50% EWL in all our patients.”
AT OBESITY WEEK 2017
Key clinical point: Many patients regain weight after bariatric surgery, but experts argue over the definition of long-term treatment failure, for which there is no standard.
Major finding: After 5 or more years of follow-up, failure rates range from 25% to 70% depending on definition of unacceptable weight regain.
Data source: A retrospective review.
Disclosures: Dr. Morell and Dr. Martyn reported no financial relationships relevant to this topic.