User login
Why VADS is gaining ground in pediatrics
The miniaturization of continuous-flow ventricular assist devices has launched the era of continuous-flow VAD support in pediatric patients, and the trend may accelerate with the introduction of a continuous-flow device designed specifically for small children. In an expert opinion in the Journal of Thoracic and Cardiovascular Surgery, Iki Adachi, MD, of Baylor College of Medicine in Houston, said the emerging science of continuous-flow VADs in children promises to solve problems like device size mismatch, hospital-only VADs, and chronic therapy (J Thorac Cardiovasc Surg. 2017 Oct;154:1358-61). “With ongoing device miniaturization, enthusiasm has been growing among pediatric physicians for the use of continuous-flow VADs in children,” Dr. Adachi said. He noted the introduction of a continuous-flow device for small children, the Infant Jarvik 2015, “may further accelerate the trend.”
Dr. Adachi cited PediMACS reports that stated that more than half of the long-term devices now registered are continuous-flow devices, and that continuous-flow VADs comprised 62% of all durable VAD implants in the third quarter of 2016. “With the encouraging results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing,” he said.
Miniaturization has addressed the problem of size mismatch when using continuous-flow VAD devices in children, he said, noting that use of the Infant Jarvik device may be expanded even further to children as small at 8 kg or less. The PumpKIN trial(Pumps for Kids, Infants, and Neonates), which is evaluating the Infant Jarvik 2015 vs. the Berlin Heart EXCOR, could provide answers on the feasibility of continuous-flow VADs in small children.
“Based on experience with the chronic animal model, I believe that the Infant Jarvik device will properly fit the patients included in the trial,” he said.
Continuous-flow VAD in children also holds potential for managing these patients outside the hospital setting. “Outpatient management of children with continuous-flow VADs has been shown to be feasible,” he said, adding that the PediMACS registry has reported that only 45% of patients have been managed this way. “Nonetheless, with maturation of the pediatric field, outpatient management will become routine rather than the exception,” he said.
Greater use of continuous-flow VADs also may create opportunities to improve the status and suitability for transplantation of children with severe heart failure, he said. He gave as an example his group’s practice at Houston’s Texas Children’s Hospital, which is to deactivate patients on the transplant wait list for 3 months once they start continuous-flow VAD support. “A postoperative ‘grace period’ affords protected opportunities for both physical and psychological recovery,” he said. This timeout of sorts also affords the care team time to assess the myocardium for possible functional recovery.
In patients who are not good candidates for transplantation, durable continuous-flow VADs may provide chronic therapy, and in time, these patients may become suitable transplant candidates, said Dr. Adachi. “Bypassing such an unfavorable period for transplantation with prolonged VAD support may be a reasonable approach,” he said.
Patients with failing single-ventricle circulation also may benefit from VAD support, although the challenges facing this population are more profound than in other groups, Dr. Adachi said. VAD support for single-ventricle disease is sparse, but these patients require careful evaluation of the nature of their condition. “If systolic dysfunction is the predominant cause of circulatory failure, then VAD support for the failing systemic ventricle will likely improve hemodynamics,” said Dr. Adachi. VAD support also could help the patient move through the various stages of palliation.
“Again, the emphasis is not just on simply keeping the patient alive until a donor organ becomes available; rather, attention is refocused on overall health beyond survival, which may eventually affect transplantation candidacy and even post transplantation outcome,” Dr. Adachi concluded.
Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare, and as a consultant for the New England Research Institute related to the PumpKIN trial.
The miniaturization of continuous-flow ventricular assist devices has launched the era of continuous-flow VAD support in pediatric patients, and the trend may accelerate with the introduction of a continuous-flow device designed specifically for small children. In an expert opinion in the Journal of Thoracic and Cardiovascular Surgery, Iki Adachi, MD, of Baylor College of Medicine in Houston, said the emerging science of continuous-flow VADs in children promises to solve problems like device size mismatch, hospital-only VADs, and chronic therapy (J Thorac Cardiovasc Surg. 2017 Oct;154:1358-61). “With ongoing device miniaturization, enthusiasm has been growing among pediatric physicians for the use of continuous-flow VADs in children,” Dr. Adachi said. He noted the introduction of a continuous-flow device for small children, the Infant Jarvik 2015, “may further accelerate the trend.”
Dr. Adachi cited PediMACS reports that stated that more than half of the long-term devices now registered are continuous-flow devices, and that continuous-flow VADs comprised 62% of all durable VAD implants in the third quarter of 2016. “With the encouraging results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing,” he said.
Miniaturization has addressed the problem of size mismatch when using continuous-flow VAD devices in children, he said, noting that use of the Infant Jarvik device may be expanded even further to children as small at 8 kg or less. The PumpKIN trial(Pumps for Kids, Infants, and Neonates), which is evaluating the Infant Jarvik 2015 vs. the Berlin Heart EXCOR, could provide answers on the feasibility of continuous-flow VADs in small children.
“Based on experience with the chronic animal model, I believe that the Infant Jarvik device will properly fit the patients included in the trial,” he said.
Continuous-flow VAD in children also holds potential for managing these patients outside the hospital setting. “Outpatient management of children with continuous-flow VADs has been shown to be feasible,” he said, adding that the PediMACS registry has reported that only 45% of patients have been managed this way. “Nonetheless, with maturation of the pediatric field, outpatient management will become routine rather than the exception,” he said.
Greater use of continuous-flow VADs also may create opportunities to improve the status and suitability for transplantation of children with severe heart failure, he said. He gave as an example his group’s practice at Houston’s Texas Children’s Hospital, which is to deactivate patients on the transplant wait list for 3 months once they start continuous-flow VAD support. “A postoperative ‘grace period’ affords protected opportunities for both physical and psychological recovery,” he said. This timeout of sorts also affords the care team time to assess the myocardium for possible functional recovery.
In patients who are not good candidates for transplantation, durable continuous-flow VADs may provide chronic therapy, and in time, these patients may become suitable transplant candidates, said Dr. Adachi. “Bypassing such an unfavorable period for transplantation with prolonged VAD support may be a reasonable approach,” he said.
Patients with failing single-ventricle circulation also may benefit from VAD support, although the challenges facing this population are more profound than in other groups, Dr. Adachi said. VAD support for single-ventricle disease is sparse, but these patients require careful evaluation of the nature of their condition. “If systolic dysfunction is the predominant cause of circulatory failure, then VAD support for the failing systemic ventricle will likely improve hemodynamics,” said Dr. Adachi. VAD support also could help the patient move through the various stages of palliation.
“Again, the emphasis is not just on simply keeping the patient alive until a donor organ becomes available; rather, attention is refocused on overall health beyond survival, which may eventually affect transplantation candidacy and even post transplantation outcome,” Dr. Adachi concluded.
Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare, and as a consultant for the New England Research Institute related to the PumpKIN trial.
The miniaturization of continuous-flow ventricular assist devices has launched the era of continuous-flow VAD support in pediatric patients, and the trend may accelerate with the introduction of a continuous-flow device designed specifically for small children. In an expert opinion in the Journal of Thoracic and Cardiovascular Surgery, Iki Adachi, MD, of Baylor College of Medicine in Houston, said the emerging science of continuous-flow VADs in children promises to solve problems like device size mismatch, hospital-only VADs, and chronic therapy (J Thorac Cardiovasc Surg. 2017 Oct;154:1358-61). “With ongoing device miniaturization, enthusiasm has been growing among pediatric physicians for the use of continuous-flow VADs in children,” Dr. Adachi said. He noted the introduction of a continuous-flow device for small children, the Infant Jarvik 2015, “may further accelerate the trend.”
Dr. Adachi cited PediMACS reports that stated that more than half of the long-term devices now registered are continuous-flow devices, and that continuous-flow VADs comprised 62% of all durable VAD implants in the third quarter of 2016. “With the encouraging results recorded to date, the use of continuous-flow devices in the pediatric population is rapidly increasing,” he said.
Miniaturization has addressed the problem of size mismatch when using continuous-flow VAD devices in children, he said, noting that use of the Infant Jarvik device may be expanded even further to children as small at 8 kg or less. The PumpKIN trial(Pumps for Kids, Infants, and Neonates), which is evaluating the Infant Jarvik 2015 vs. the Berlin Heart EXCOR, could provide answers on the feasibility of continuous-flow VADs in small children.
“Based on experience with the chronic animal model, I believe that the Infant Jarvik device will properly fit the patients included in the trial,” he said.
Continuous-flow VAD in children also holds potential for managing these patients outside the hospital setting. “Outpatient management of children with continuous-flow VADs has been shown to be feasible,” he said, adding that the PediMACS registry has reported that only 45% of patients have been managed this way. “Nonetheless, with maturation of the pediatric field, outpatient management will become routine rather than the exception,” he said.
Greater use of continuous-flow VADs also may create opportunities to improve the status and suitability for transplantation of children with severe heart failure, he said. He gave as an example his group’s practice at Houston’s Texas Children’s Hospital, which is to deactivate patients on the transplant wait list for 3 months once they start continuous-flow VAD support. “A postoperative ‘grace period’ affords protected opportunities for both physical and psychological recovery,” he said. This timeout of sorts also affords the care team time to assess the myocardium for possible functional recovery.
In patients who are not good candidates for transplantation, durable continuous-flow VADs may provide chronic therapy, and in time, these patients may become suitable transplant candidates, said Dr. Adachi. “Bypassing such an unfavorable period for transplantation with prolonged VAD support may be a reasonable approach,” he said.
Patients with failing single-ventricle circulation also may benefit from VAD support, although the challenges facing this population are more profound than in other groups, Dr. Adachi said. VAD support for single-ventricle disease is sparse, but these patients require careful evaluation of the nature of their condition. “If systolic dysfunction is the predominant cause of circulatory failure, then VAD support for the failing systemic ventricle will likely improve hemodynamics,” said Dr. Adachi. VAD support also could help the patient move through the various stages of palliation.
“Again, the emphasis is not just on simply keeping the patient alive until a donor organ becomes available; rather, attention is refocused on overall health beyond survival, which may eventually affect transplantation candidacy and even post transplantation outcome,” Dr. Adachi concluded.
Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare, and as a consultant for the New England Research Institute related to the PumpKIN trial.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Advances in continuous-flow ventricular assist devices (VADs) promise a paradigm shift in pediatrics.
Major finding: Device miniaturization is solving problems such as size mismatch, inpatients on VADs, and chronic therapy.
Data source: Expert opinion drawing on PediMACS reports and published trials of continuous-flow VAD.
Disclosures: Dr. Adachi serves as a consultant and proctor for Berlin Heart and HeartWare and as a consultant for the New England Research Institute related to the PumpKIN trial.
Obesity-Related Cancer Is on the Rise
Overweight and obesity are associated with an increased risk of 13 types of cancer—and those cancers account for about 40% of all cancers diagnosed in 2014, according to the CDC.
The 13 cancers are meningioma, adenocarcinoma of the esophagus, multiple myeloma, kidney, uterine, ovarian, thyroid, breast, liver, gallbladder, upper stomach, pancreas, and colorectal. About 630,000 people were diagnosed with 1 of those cancers in 2014; 2 in 3 cancers were in adults aged 50 to 74 years. In 2013-2014, about 2 of every 3 American adults were overweight or obese.
Related: The Impact of Obesity on Simvastatin for Lowering LDL-C Among Veterans
Overall, the rate of new cancer cases has been on the decline since the 1990s, the report says, but increases in overweight- and obesity-related cancers “are likely slowing this progress.” Obesity-related cancers (not including colorectal cancer, which declined by 23%) increased by 7% between 2005 and 2014, while the rates of nonobesity–related cancers declined 13%.
Health care providers can help, the CDC says, by counseling patients on healthy weight and its role in cancer prevention, referring obese patients to intensive management programs and connecting patients and their families to community services that give them easier access to healthful food. The National Comprehensive Cancer Control Program (https://www.cdc.gov/cancer/ncccp/index.htm) funds cancer coalitions across the U.S., which include strategies to prevent and control overweight and obesity.
Related: The Impact of Obesity on the Recovery of Patients With Cancer
“When people ask me if there’s a cure for cancer, I say, ‘Yes, good health is the best prescription for preventing chronic diseases, including cancer,” said Lisa Richardson, MD, director of CDC’s Division of Cancer Prevention and Control. That means, she says, giving people the information they need to make healthy choices where they live, work, learn, and play.
Overweight and obesity are associated with an increased risk of 13 types of cancer—and those cancers account for about 40% of all cancers diagnosed in 2014, according to the CDC.
The 13 cancers are meningioma, adenocarcinoma of the esophagus, multiple myeloma, kidney, uterine, ovarian, thyroid, breast, liver, gallbladder, upper stomach, pancreas, and colorectal. About 630,000 people were diagnosed with 1 of those cancers in 2014; 2 in 3 cancers were in adults aged 50 to 74 years. In 2013-2014, about 2 of every 3 American adults were overweight or obese.
Related: The Impact of Obesity on Simvastatin for Lowering LDL-C Among Veterans
Overall, the rate of new cancer cases has been on the decline since the 1990s, the report says, but increases in overweight- and obesity-related cancers “are likely slowing this progress.” Obesity-related cancers (not including colorectal cancer, which declined by 23%) increased by 7% between 2005 and 2014, while the rates of nonobesity–related cancers declined 13%.
Health care providers can help, the CDC says, by counseling patients on healthy weight and its role in cancer prevention, referring obese patients to intensive management programs and connecting patients and their families to community services that give them easier access to healthful food. The National Comprehensive Cancer Control Program (https://www.cdc.gov/cancer/ncccp/index.htm) funds cancer coalitions across the U.S., which include strategies to prevent and control overweight and obesity.
Related: The Impact of Obesity on the Recovery of Patients With Cancer
“When people ask me if there’s a cure for cancer, I say, ‘Yes, good health is the best prescription for preventing chronic diseases, including cancer,” said Lisa Richardson, MD, director of CDC’s Division of Cancer Prevention and Control. That means, she says, giving people the information they need to make healthy choices where they live, work, learn, and play.
Overweight and obesity are associated with an increased risk of 13 types of cancer—and those cancers account for about 40% of all cancers diagnosed in 2014, according to the CDC.
The 13 cancers are meningioma, adenocarcinoma of the esophagus, multiple myeloma, kidney, uterine, ovarian, thyroid, breast, liver, gallbladder, upper stomach, pancreas, and colorectal. About 630,000 people were diagnosed with 1 of those cancers in 2014; 2 in 3 cancers were in adults aged 50 to 74 years. In 2013-2014, about 2 of every 3 American adults were overweight or obese.
Related: The Impact of Obesity on Simvastatin for Lowering LDL-C Among Veterans
Overall, the rate of new cancer cases has been on the decline since the 1990s, the report says, but increases in overweight- and obesity-related cancers “are likely slowing this progress.” Obesity-related cancers (not including colorectal cancer, which declined by 23%) increased by 7% between 2005 and 2014, while the rates of nonobesity–related cancers declined 13%.
Health care providers can help, the CDC says, by counseling patients on healthy weight and its role in cancer prevention, referring obese patients to intensive management programs and connecting patients and their families to community services that give them easier access to healthful food. The National Comprehensive Cancer Control Program (https://www.cdc.gov/cancer/ncccp/index.htm) funds cancer coalitions across the U.S., which include strategies to prevent and control overweight and obesity.
Related: The Impact of Obesity on the Recovery of Patients With Cancer
“When people ask me if there’s a cure for cancer, I say, ‘Yes, good health is the best prescription for preventing chronic diseases, including cancer,” said Lisa Richardson, MD, director of CDC’s Division of Cancer Prevention and Control. That means, she says, giving people the information they need to make healthy choices where they live, work, learn, and play.
Team identifies HSCs that rapidly reconstitute hematopoiesis
Researchers say they have identified a subpopulation of hematopoietic stem cells (HSCs) that immediately contributes to long-term, multilineage hematopoietic reconstitution after transplant.
These HSCs were discovered in macaques, but the cells are similar to a subset of HSCs found in humans.
The researchers found the 2 sets of cells behaved identically when tested in vitro.
The team believes their findings will increase the efficiency of future efforts for HSC transplants, gene therapies, and gene editing.
Hans-Peter Kiem, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues reported these findings in Science Translational Medicine.
The researchers performed HSC transplants in pig-tailed macaques, following hundreds of thousands of cells immediately after transplant and over the course of 7.5 years.
Previous reports had suggested that successive waves of progenitor cells expand and contract to establish the new bone marrow after transplant.
However, Dr Kiem and his colleagues homed in on a distinct group of HSCs that took hold early after transplant and went on to produce all cell lineages that constitute a complete blood system.
“These findings came as a surprise,” Dr Kiem said. “We had thought that there were multiple types of blood stem cells that take on different roles in rebuilding a blood and immune system. This population does it all.”
The population is a subset of CD34+ cells expressing CD90 and lacking CD45RA markers.
“The gold standard target cell population for stem cell gene therapy are cells with the marker CD34,” said study author Stefan Radtke, PhD, of the Fred Hutchinson Cancer Research Center.
“But we used 2 additional markers to further distinguish the population from the other blood stem cells.”
The researchers noted that the CD34+ CD45RA- CD90+ HSCs started repopulating the hematopoietic system within 10 days of being infused in macaques undergoing transplant.
A year later, the researchers found strong molecular traces of the cells, suggesting they were responsible for the ongoing maintenance of the newly transplanted system.
The team also determined the minimum numbers of CD34+ CD45RA- CD90+ HSCs that were necessary for successful transplant (defined as sustained neutrophil and platelet recovery).
And the researchers found similar gene expression profiles between macaque and human CD34+ CD45RA- CD90+ HSCs.
The team therefore believes these findings could have implications for HSC transplants in humans.
The researchers are now working to move their findings into the clinic with the hopes of integrating them in ongoing clinical trials. The team is currently looking for commercial partners. ![]()
Researchers say they have identified a subpopulation of hematopoietic stem cells (HSCs) that immediately contributes to long-term, multilineage hematopoietic reconstitution after transplant.
These HSCs were discovered in macaques, but the cells are similar to a subset of HSCs found in humans.
The researchers found the 2 sets of cells behaved identically when tested in vitro.
The team believes their findings will increase the efficiency of future efforts for HSC transplants, gene therapies, and gene editing.
Hans-Peter Kiem, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues reported these findings in Science Translational Medicine.
The researchers performed HSC transplants in pig-tailed macaques, following hundreds of thousands of cells immediately after transplant and over the course of 7.5 years.
Previous reports had suggested that successive waves of progenitor cells expand and contract to establish the new bone marrow after transplant.
However, Dr Kiem and his colleagues homed in on a distinct group of HSCs that took hold early after transplant and went on to produce all cell lineages that constitute a complete blood system.
“These findings came as a surprise,” Dr Kiem said. “We had thought that there were multiple types of blood stem cells that take on different roles in rebuilding a blood and immune system. This population does it all.”
The population is a subset of CD34+ cells expressing CD90 and lacking CD45RA markers.
“The gold standard target cell population for stem cell gene therapy are cells with the marker CD34,” said study author Stefan Radtke, PhD, of the Fred Hutchinson Cancer Research Center.
“But we used 2 additional markers to further distinguish the population from the other blood stem cells.”
The researchers noted that the CD34+ CD45RA- CD90+ HSCs started repopulating the hematopoietic system within 10 days of being infused in macaques undergoing transplant.
A year later, the researchers found strong molecular traces of the cells, suggesting they were responsible for the ongoing maintenance of the newly transplanted system.
The team also determined the minimum numbers of CD34+ CD45RA- CD90+ HSCs that were necessary for successful transplant (defined as sustained neutrophil and platelet recovery).
And the researchers found similar gene expression profiles between macaque and human CD34+ CD45RA- CD90+ HSCs.
The team therefore believes these findings could have implications for HSC transplants in humans.
The researchers are now working to move their findings into the clinic with the hopes of integrating them in ongoing clinical trials. The team is currently looking for commercial partners. ![]()
Researchers say they have identified a subpopulation of hematopoietic stem cells (HSCs) that immediately contributes to long-term, multilineage hematopoietic reconstitution after transplant.
These HSCs were discovered in macaques, but the cells are similar to a subset of HSCs found in humans.
The researchers found the 2 sets of cells behaved identically when tested in vitro.
The team believes their findings will increase the efficiency of future efforts for HSC transplants, gene therapies, and gene editing.
Hans-Peter Kiem, MD, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues reported these findings in Science Translational Medicine.
The researchers performed HSC transplants in pig-tailed macaques, following hundreds of thousands of cells immediately after transplant and over the course of 7.5 years.
Previous reports had suggested that successive waves of progenitor cells expand and contract to establish the new bone marrow after transplant.
However, Dr Kiem and his colleagues homed in on a distinct group of HSCs that took hold early after transplant and went on to produce all cell lineages that constitute a complete blood system.
“These findings came as a surprise,” Dr Kiem said. “We had thought that there were multiple types of blood stem cells that take on different roles in rebuilding a blood and immune system. This population does it all.”
The population is a subset of CD34+ cells expressing CD90 and lacking CD45RA markers.
“The gold standard target cell population for stem cell gene therapy are cells with the marker CD34,” said study author Stefan Radtke, PhD, of the Fred Hutchinson Cancer Research Center.
“But we used 2 additional markers to further distinguish the population from the other blood stem cells.”
The researchers noted that the CD34+ CD45RA- CD90+ HSCs started repopulating the hematopoietic system within 10 days of being infused in macaques undergoing transplant.
A year later, the researchers found strong molecular traces of the cells, suggesting they were responsible for the ongoing maintenance of the newly transplanted system.
The team also determined the minimum numbers of CD34+ CD45RA- CD90+ HSCs that were necessary for successful transplant (defined as sustained neutrophil and platelet recovery).
And the researchers found similar gene expression profiles between macaque and human CD34+ CD45RA- CD90+ HSCs.
The team therefore believes these findings could have implications for HSC transplants in humans.
The researchers are now working to move their findings into the clinic with the hopes of integrating them in ongoing clinical trials. The team is currently looking for commercial partners. ![]()
FDA grants product breakthrough designation for MM
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to GSK2857916, an anti-B-cell maturation antigen (BCMA) monoclonal antibody conjugated to the cytotoxic agent monomethyl auristatin-F via a non-cleavable linker.
The designation is for GSK2857916 as monotherapy for patients with multiple myeloma (MM) who have failed at least 3 prior lines of therapy, including an anti-CD38 antibody, and who are refractory to a proteasome inhibitor and an immunomodulatory agent.
The designation is based on results from a phase 1, dose-escalation and expansion study in patients with relapsed/refractory MM, irrespective of BCMA expression (NCT02064387).
Data from this ongoing trial are scheduled to be presented December 11 in an oral presentation at the 59th Annual Meeting of the American Society of Hematology (ASH) in Atlanta, Georgia.
GSK2857916 has also received orphan drug designation from the FDA and the European Medicines Agency (EMA) as well as PRIME designation from the EMA.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Orphan and PRIME designations
The FDA grants orphan designation to therapies intended to treat conditions that affect fewer than 200,000 people in the US. The designation qualifies a drug’s sponsor for various development incentives of the Orphan Drug Act, including tax credits for qualified clinical testing and 7 years of market exclusivity.
In Europe, sponsors who obtain orphan designation for a potential new medicine benefit from a range of incentives, including protocol assistance, access to the centralized procedure, 10 years of market exclusivity, and fee reductions.
The EMA grants PRIME designation to enhance support for the development of medicines that target an unmet medical need. The designation is based on enhanced interaction between sponsor companies and the EMA to optimize development plans and speed up evaluation so these medicines can reach patients earlier. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to GSK2857916, an anti-B-cell maturation antigen (BCMA) monoclonal antibody conjugated to the cytotoxic agent monomethyl auristatin-F via a non-cleavable linker.
The designation is for GSK2857916 as monotherapy for patients with multiple myeloma (MM) who have failed at least 3 prior lines of therapy, including an anti-CD38 antibody, and who are refractory to a proteasome inhibitor and an immunomodulatory agent.
The designation is based on results from a phase 1, dose-escalation and expansion study in patients with relapsed/refractory MM, irrespective of BCMA expression (NCT02064387).
Data from this ongoing trial are scheduled to be presented December 11 in an oral presentation at the 59th Annual Meeting of the American Society of Hematology (ASH) in Atlanta, Georgia.
GSK2857916 has also received orphan drug designation from the FDA and the European Medicines Agency (EMA) as well as PRIME designation from the EMA.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Orphan and PRIME designations
The FDA grants orphan designation to therapies intended to treat conditions that affect fewer than 200,000 people in the US. The designation qualifies a drug’s sponsor for various development incentives of the Orphan Drug Act, including tax credits for qualified clinical testing and 7 years of market exclusivity.
In Europe, sponsors who obtain orphan designation for a potential new medicine benefit from a range of incentives, including protocol assistance, access to the centralized procedure, 10 years of market exclusivity, and fee reductions.
The EMA grants PRIME designation to enhance support for the development of medicines that target an unmet medical need. The designation is based on enhanced interaction between sponsor companies and the EMA to optimize development plans and speed up evaluation so these medicines can reach patients earlier. ![]()
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation to GSK2857916, an anti-B-cell maturation antigen (BCMA) monoclonal antibody conjugated to the cytotoxic agent monomethyl auristatin-F via a non-cleavable linker.
The designation is for GSK2857916 as monotherapy for patients with multiple myeloma (MM) who have failed at least 3 prior lines of therapy, including an anti-CD38 antibody, and who are refractory to a proteasome inhibitor and an immunomodulatory agent.
The designation is based on results from a phase 1, dose-escalation and expansion study in patients with relapsed/refractory MM, irrespective of BCMA expression (NCT02064387).
Data from this ongoing trial are scheduled to be presented December 11 in an oral presentation at the 59th Annual Meeting of the American Society of Hematology (ASH) in Atlanta, Georgia.
GSK2857916 has also received orphan drug designation from the FDA and the European Medicines Agency (EMA) as well as PRIME designation from the EMA.
About breakthrough designation
The FDA’s breakthrough designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions. The designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
Orphan and PRIME designations
The FDA grants orphan designation to therapies intended to treat conditions that affect fewer than 200,000 people in the US. The designation qualifies a drug’s sponsor for various development incentives of the Orphan Drug Act, including tax credits for qualified clinical testing and 7 years of market exclusivity.
In Europe, sponsors who obtain orphan designation for a potential new medicine benefit from a range of incentives, including protocol assistance, access to the centralized procedure, 10 years of market exclusivity, and fee reductions.
The EMA grants PRIME designation to enhance support for the development of medicines that target an unmet medical need. The designation is based on enhanced interaction between sponsor companies and the EMA to optimize development plans and speed up evaluation so these medicines can reach patients earlier. ![]()
Cancer patients prefer computer-free interactions
SAN DIEGO—A new study suggests patients with advanced cancer may prefer doctors who do not use a computer while communicating with them.
Most of the 120 patients studied said they preferred face-to-face consultations in which a doctor used a notepad rather than a computer.
Doctors who did not use a computer were perceived as more compassionate, communicative, and professional.
These findings were presented at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 26*).
“To our knowledge, this is the only study that compares exam room interactions between people with advanced cancer and their physicians, with or without a computer present,” said study investigator Ali Haider, MD, of the University of Texas MD Anderson Cancer Center in Houston.
For this study, Dr Haider and his colleagues enrolled 120 patients with localized, recurrent, or metastatic disease. The patients’ median ECOG performance status was 2.
All patients were English speakers, they had a median age of 58 (range, 44-66), and 55% were female. Sixty-seven percent of patients were white, 18% were Hispanic, 13% were African American, and 2% were “other.” Forty-one percent of patients had completed college.
According to the Edmonton Symptom Assessment System, patients’ median pain score was 5 (range, 2-7), and their median fatigue score was 4 (range, 3-7). According to the Hospital Anxiety and Depression Scale, patients’ median anxiety score was 6 (range, 4-8), and their median depression score was 6 (range, 4-9).
The intervention
The investigators randomly assigned patients to watch different videos showing doctor-patient interactions with and without computer use. The team had filmed 4 short videos that featured actors playing the parts of doctor and patient.
All study participants were blinded to the hypothesis of the study. The actors were carefully scripted and used the same gestures, expressions, and other nonverbal communication in each video to minimize bias.
Video 1 involved Doctor A in a face-to-face consultation with just a notepad in hand, and Video 2 involved Doctor A in a consultation using a computer.
Video 3 involved Doctor B in a face-to-face consultation with just a notepad in hand, and Video 4 involved Doctor B in a consultation using a computer.
Doctors A and B looked similar, which was intended to minimize bias.
After viewing their first video, patients completed a validated questionnaire rating the doctor’s communication skills, professionalism, and compassion.
Subsequently, each group was assigned to a video topic (face-to-face or computer) they had not viewed previously featuring the doctor they had not viewed in the first video.
A follow-up questionnaire was given after this round of viewing, and the patients were also asked to rate their overall physician preference.
Results
After the first round of viewing, the patients gave better ratings to doctors (A or B) in the face-to-face videos than in the computer videos. Face-to-face doctors were rated significantly higher for compassion (P=0.0003), communication skills (P=0.0012), and professionalism (P=0.0001).
After patients had watched both videos, doctors in the face-to-face videos still had better scores for compassion, communication, and professionalism (P<0.001 for all).
Most patients (72%) said they preferred the face-to-face consultation, while 8% said they preferred the computer consultation, and 20% said they had no preference.
Dr Haider said a possible explanation for these findings is that patients with serious chronic illnesses might value undivided attention from their physicians, and patients might perceive providers using computers as more distracted or multitasking during visits.
“We know that having a good rapport with patients can be extremely beneficial for their health,” Dr Haider said. “Patients with advanced disease need the cues that come with direct interaction to help them along with their care.”
However, Dr Haider also noted that additional research is needed to confirm these results. And he said perceptions might be different in a younger population with higher computer literacy. ![]()
*Data in the abstract differ from the presentation.
SAN DIEGO—A new study suggests patients with advanced cancer may prefer doctors who do not use a computer while communicating with them.
Most of the 120 patients studied said they preferred face-to-face consultations in which a doctor used a notepad rather than a computer.
Doctors who did not use a computer were perceived as more compassionate, communicative, and professional.
These findings were presented at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 26*).
“To our knowledge, this is the only study that compares exam room interactions between people with advanced cancer and their physicians, with or without a computer present,” said study investigator Ali Haider, MD, of the University of Texas MD Anderson Cancer Center in Houston.
For this study, Dr Haider and his colleagues enrolled 120 patients with localized, recurrent, or metastatic disease. The patients’ median ECOG performance status was 2.
All patients were English speakers, they had a median age of 58 (range, 44-66), and 55% were female. Sixty-seven percent of patients were white, 18% were Hispanic, 13% were African American, and 2% were “other.” Forty-one percent of patients had completed college.
According to the Edmonton Symptom Assessment System, patients’ median pain score was 5 (range, 2-7), and their median fatigue score was 4 (range, 3-7). According to the Hospital Anxiety and Depression Scale, patients’ median anxiety score was 6 (range, 4-8), and their median depression score was 6 (range, 4-9).
The intervention
The investigators randomly assigned patients to watch different videos showing doctor-patient interactions with and without computer use. The team had filmed 4 short videos that featured actors playing the parts of doctor and patient.
All study participants were blinded to the hypothesis of the study. The actors were carefully scripted and used the same gestures, expressions, and other nonverbal communication in each video to minimize bias.
Video 1 involved Doctor A in a face-to-face consultation with just a notepad in hand, and Video 2 involved Doctor A in a consultation using a computer.
Video 3 involved Doctor B in a face-to-face consultation with just a notepad in hand, and Video 4 involved Doctor B in a consultation using a computer.
Doctors A and B looked similar, which was intended to minimize bias.
After viewing their first video, patients completed a validated questionnaire rating the doctor’s communication skills, professionalism, and compassion.
Subsequently, each group was assigned to a video topic (face-to-face or computer) they had not viewed previously featuring the doctor they had not viewed in the first video.
A follow-up questionnaire was given after this round of viewing, and the patients were also asked to rate their overall physician preference.
Results
After the first round of viewing, the patients gave better ratings to doctors (A or B) in the face-to-face videos than in the computer videos. Face-to-face doctors were rated significantly higher for compassion (P=0.0003), communication skills (P=0.0012), and professionalism (P=0.0001).
After patients had watched both videos, doctors in the face-to-face videos still had better scores for compassion, communication, and professionalism (P<0.001 for all).
Most patients (72%) said they preferred the face-to-face consultation, while 8% said they preferred the computer consultation, and 20% said they had no preference.
Dr Haider said a possible explanation for these findings is that patients with serious chronic illnesses might value undivided attention from their physicians, and patients might perceive providers using computers as more distracted or multitasking during visits.
“We know that having a good rapport with patients can be extremely beneficial for their health,” Dr Haider said. “Patients with advanced disease need the cues that come with direct interaction to help them along with their care.”
However, Dr Haider also noted that additional research is needed to confirm these results. And he said perceptions might be different in a younger population with higher computer literacy. ![]()
*Data in the abstract differ from the presentation.
SAN DIEGO—A new study suggests patients with advanced cancer may prefer doctors who do not use a computer while communicating with them.
Most of the 120 patients studied said they preferred face-to-face consultations in which a doctor used a notepad rather than a computer.
Doctors who did not use a computer were perceived as more compassionate, communicative, and professional.
These findings were presented at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 26*).
“To our knowledge, this is the only study that compares exam room interactions between people with advanced cancer and their physicians, with or without a computer present,” said study investigator Ali Haider, MD, of the University of Texas MD Anderson Cancer Center in Houston.
For this study, Dr Haider and his colleagues enrolled 120 patients with localized, recurrent, or metastatic disease. The patients’ median ECOG performance status was 2.
All patients were English speakers, they had a median age of 58 (range, 44-66), and 55% were female. Sixty-seven percent of patients were white, 18% were Hispanic, 13% were African American, and 2% were “other.” Forty-one percent of patients had completed college.
According to the Edmonton Symptom Assessment System, patients’ median pain score was 5 (range, 2-7), and their median fatigue score was 4 (range, 3-7). According to the Hospital Anxiety and Depression Scale, patients’ median anxiety score was 6 (range, 4-8), and their median depression score was 6 (range, 4-9).
The intervention
The investigators randomly assigned patients to watch different videos showing doctor-patient interactions with and without computer use. The team had filmed 4 short videos that featured actors playing the parts of doctor and patient.
All study participants were blinded to the hypothesis of the study. The actors were carefully scripted and used the same gestures, expressions, and other nonverbal communication in each video to minimize bias.
Video 1 involved Doctor A in a face-to-face consultation with just a notepad in hand, and Video 2 involved Doctor A in a consultation using a computer.
Video 3 involved Doctor B in a face-to-face consultation with just a notepad in hand, and Video 4 involved Doctor B in a consultation using a computer.
Doctors A and B looked similar, which was intended to minimize bias.
After viewing their first video, patients completed a validated questionnaire rating the doctor’s communication skills, professionalism, and compassion.
Subsequently, each group was assigned to a video topic (face-to-face or computer) they had not viewed previously featuring the doctor they had not viewed in the first video.
A follow-up questionnaire was given after this round of viewing, and the patients were also asked to rate their overall physician preference.
Results
After the first round of viewing, the patients gave better ratings to doctors (A or B) in the face-to-face videos than in the computer videos. Face-to-face doctors were rated significantly higher for compassion (P=0.0003), communication skills (P=0.0012), and professionalism (P=0.0001).
After patients had watched both videos, doctors in the face-to-face videos still had better scores for compassion, communication, and professionalism (P<0.001 for all).
Most patients (72%) said they preferred the face-to-face consultation, while 8% said they preferred the computer consultation, and 20% said they had no preference.
Dr Haider said a possible explanation for these findings is that patients with serious chronic illnesses might value undivided attention from their physicians, and patients might perceive providers using computers as more distracted or multitasking during visits.
“We know that having a good rapport with patients can be extremely beneficial for their health,” Dr Haider said. “Patients with advanced disease need the cues that come with direct interaction to help them along with their care.”
However, Dr Haider also noted that additional research is needed to confirm these results. And he said perceptions might be different in a younger population with higher computer literacy. ![]()
*Data in the abstract differ from the presentation.
Acute Management of Severe Asymptomatic Hypertension
IN THIS ARTICLE
- Patient history; what to ask
- Cardiovascular risk factors
- Disposition pathway
- Oral medications
Approximately one in three US adults, or about 75 million people, have high blood pressure (BP), which has been defined as a BP of 140/90 mm Hg or higher.1 Unfortunately, only about half (54%) of those affected have their condition under optimal control.1 From an epidemiologic standpoint, hypertension has the distinction of being the most common chronic condition in the US, affecting about 54% of persons ages 55 to 64 and about 73% of those 75 and older.2,3 It is the number one reason patients schedule office visits with physicians; it accounts for the most prescriptions; and it is a major risk factor for heart disease and stroke, as well as a significant contributor to mortality throughout the world.4
HYPERTENSIVE URGENCY VS EMERGENCY
Hypertensive urgencies and emergencies account for approximately 27% of all medical emergencies and 2% to 3% of all annual visits to the emergency department (ED).5 Hypertensive urgency, or severe asymptomatic hypertension, is a common complaint in urgent care clinics and primary care offices as well. It is often defined as a systolic BP (SBP) of ≥ 160 mm Hg and/or a diastolic BP (DBP) ≥ 100 mm Hg with no associated end-organ damage.5-7 Patients may experience hypertensive urgency if they have been noncompliant with their antihypertensive drug regimen; present with pain; have white-coat hypertension or anxiety; or use recreational drugs (eg, sympathomimetics).5,8-10
Alternatively, hypertensive emergency, also known as hypertensive crisis, is generally defined as elevated BP > 180/120 mm Hg. Equally important, it is associated with signs, symptoms, or laboratory values indicative of target end-organ damage, such as cerebrovascular accident, myocardial infarction (MI), aortic dissection, acute left ventricular failure, acute pulmonary edema, acute renal failure, acute mental status changes (hypertensive encephalopathy), and eclampsia.5,7,8,11,12
Determining appropriate management for patients with hypertensive urgency is controversial among clinicians. Practice patterns range from full screening and “rule-outs”—with prompt initiation of antihypertensive agents, regardless of whether the patient is symptomatic—to sending the patient home with minimal screening, laboratory testing, or treatment.
This article offers a guided approach to managing patients with hypertensive urgency in a logical fashion, based on risk stratification, thereby avoiding both extremes (extensive unnecessary workup or discharge without workup resulting in adverse outcomes). It is vital to differentiate between patients with hypertensive emergency, in which BP should be lowered in minutes, and patients with hypertensive urgency, in which BP can be lowered more slowly.12
PATHOPHYSIOLOGY
Normally, when BP increases, blood vessel diameter changes in response; this autoregulation serves to limit damage. However, when BP increases abruptly, the body’s ability to hemodynamically calibrate to such a rapid change is impeded, thus allowing for potential end-organ damage.5,12 The increased vascular resistance observed in many patients with hypertension appears to be an autoregulatory process that helps to maintain a normal or viable level of tissue blood flow and organ perfusion despite the increased BP, rather than a primary cause of the hypertension.13
The exact physiology of hypertensive urgencies is not clearly understood, because of the multifactorial nature of the process. One leading theory is that circulating humoral vasoconstrictors cause an abrupt increase in systemic vascular resistance, which in turn causes mechanical shear stress to the endothelial wall. This endothelial damage promotes more vasoconstriction, platelet aggregation, and activation of the renin-angiotensin-aldosterone system, which thereby increases release of angiotensin II and various cytokines.14
HISTORY AND PHYSICAL
A detailed medical history is of utmost importance in distinguishing patients who present with asymptomatic hypertensive urgency from those experiencing a hypertensive emergency. In addition, obtain a full medication list, including any nutritional supplements or illicit drugs the patient may be taking. Question the patient regarding medication adherence; some may not be taking antihypertensive agents as prescribed or may have altered the dosing frequency in an effort to extend the duration of their prescription.5,8 Table 1 lists pertinent questions to ask at presentation; the answers will dictate who needs further workup and possible admission as well as who will require screening for end-organ damage.7
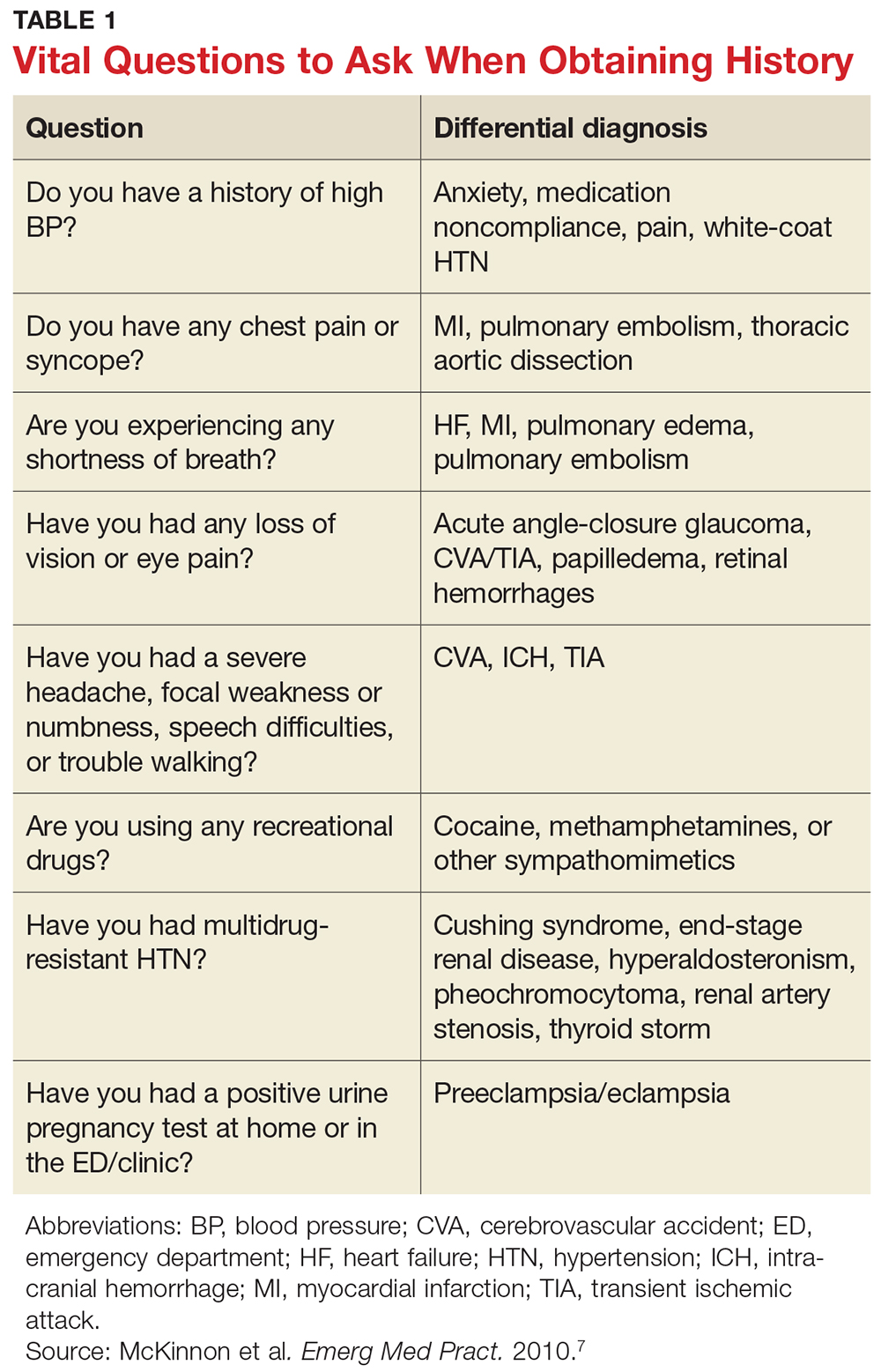
The physical exam should focus primarily on a thorough cardiopulmonary and neurologic examination, as well as funduscopic examination, if needed. A complete set of vital signs should be recorded upon the patient’s arrival to the ED or clinic and should be repeated on the opposite arm for verification. Beginning with the eyes, conduct a thorough funduscopic examination to evaluate for papilledema or hemorrhages.5 During the cardiopulmonary exam, attention should be focused on signs of congestive heart failure and/or pulmonary edema, such as increased jugular vein distension, an S3 gallop, peripheral edema, and pulmonary rales. The neurologic exam is essential in evaluating for cerebrovascular accident, transient ischemic attack, or intracranial hemorrhage. A full cranial nerve examination is necessary, in addition to motor and sensory testing, at minimum.5,9
RISK STRATIFICATION
According to the 2013 Task Force of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC), several risk factors contribute to overall cardiovascular risk in asymptomatic patients presenting with severe hypertension (see Table 2).8 This report has been monumental in linking grades of hypertension directly to cardiovascular risk factors, but it differs from that recently published by the Eighth Joint National Committee (JNC 8), which offers evidence-based guidelines for the management of high BP in the general population of adults (with some modifications for individuals with diabetes or chronic kidney disease or of black ethnicity).15
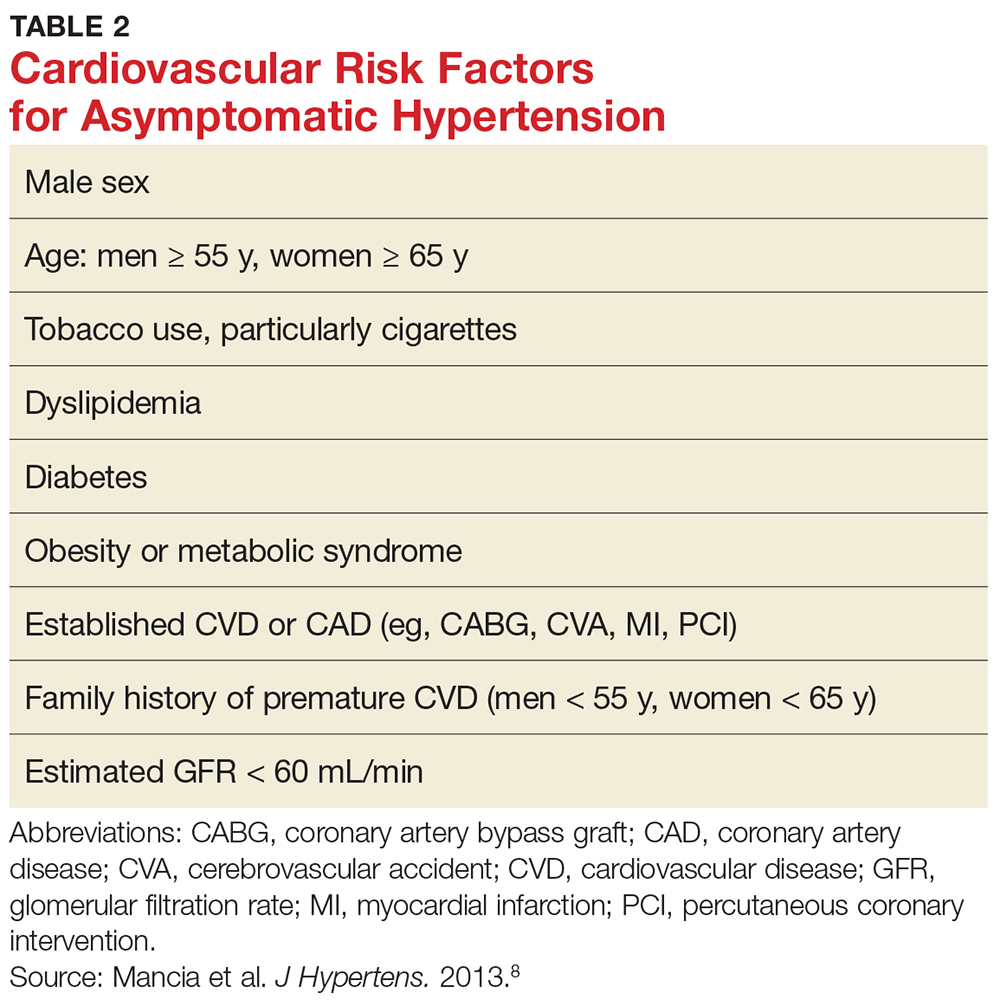
According to the ESH/ESC study, patients with one or two risk factors who have grade 1 hypertension (SBP 140-159 mm Hg) are at moderate risk for cardiovascular disease (CVD) and patients with grade 2 (SBP 160-179 mm Hg) or grade 3 (SBP ≥ 180 mm Hg) hypertension are at moderate-to-high risk and high risk, respectively.8 Patients with three or more risk factors, or who already have end-organ damage, diabetes, or chronic kidney disease, enter the high-risk category for CVD even at grade 1 hypertension.8
These cardiovascular risk factors can and should be used as guidelines for deciding who needs further screening and who may have benign causes of severe hypertension (eg, white-coat hypertension, anxiety) that can be managed safely in an outpatient setting. In the author’s opinion, patients with known cardiovascular risk factors, those with signs or symptoms of end-organ damage, and those with test results suggestive of end-organ damage should have a more immediate treatment strategy initiated.
Numerous observational studies have shown a direct relationship between systemic hypertension and CVD risk in men and women of various ages, races, and ethnicities, regardless of other risk factors for CVD.12 In patients with diabetes, uncontrolled hypertension is a strong predictor of cardiovascular morbidity and mortality and of progressive nephropathy leading to chronic kidney disease.8
SCREENING
Results from the following tests may provide useful clues in the workup of a patient with hypertensive urgency.
Basic metabolic panel. Many EDs and primary care offices offer point-of-care testing that can typically give a rapid (< 10 min) result of a basic metabolic panel. This useful, quick screening tool can identify renal failure due to chronic untreated hypertension, acute renal failure, or other disease states that cause electrolyte abnormalities such as hyperaldosteronism (hypertension with hypokalemia) or Cushing syndrome (hypertension with hypernatremia and hyperkalemia).7
Cardiac enzymes. Measurement of cardiac troponins (T or I) may provide confirmatory evidence of myocardial necrosis within two to three hours of suspected acute MI.16,17 These tests are now available in most EDs and some clinics with point-of-care testing. A variety of current guidelines advocate repeat cardiac enzyme measurements at various time points, depending on results of initial testing and concomitant risk factors. These protocols vary by facility.
ECG. Obtaining an ECG is another quick, easy, and useful way to screen patients presenting with severe hypertensive urgency. Evidence of left ventricular hypertrophy suggests an increased risk for MI, stroke, heart failure, and sudden death.7,18-20 The Cornell criteria of summing the R wave in aVL and the S wave in V3, with a cutoff of 2.8 mV in men and 2.0 mV in women, has been shown to be the best predictor of future cardiovascular mortality.7 While an isolated finding of left ventricular hypertrophy on an ECG—in and of itself—may have limited value for an individual patient, this finding coupled with other risk factors may alter the provider’s assessment.
Chest radiograph. A chest radiograph can be helpful when used in conjunction with physical exam findings that suggest pulmonary edema and cardiomegaly.7 Widened mediastinum and tortuous aorta may also be evident on chest x-ray, necessitating further workup and imaging.
Urinalysis. In a patient presenting with asymptomatic hypertensive urgency, a urine dipstick result that shows new-onset proteinuria, while not definitive for diagnosis of nephrotic syndrome, may certainly prove helpful in the patient’s workup.5,13
Urine drug screen. In patients without a history of hypertension who present with asymptomatic hypertensive urgency, the urine drug screen may ascertain exposure to cocaine, amphetamine, or phencyclidine.
Pregnancy test. A pregnancy test is essential for any female patient of childbearing age presenting to the ED, and a positive result may be concerning for preeclampsia in a hypertensive patient with no prior history of the condition.7
TREATMENT
Knowing who to treat and when is a vast area of debate among emergency and primary care providers. Patients with hypertension who have established risk factors are known to have worse outcomes than those who may be otherwise healthy. Some clinicians believe that patients presenting with hypertensive urgency should be discharged home without screening and/or treatment. However, because uncontrolled severe hypertension can lead to acute complications (eg, MI, cerebrovascular accident), in practice, many providers are unwilling to send the patient home without workup.12 The patient’s condition must be viewed in the context of the entire disease spectrum, including risk factors.
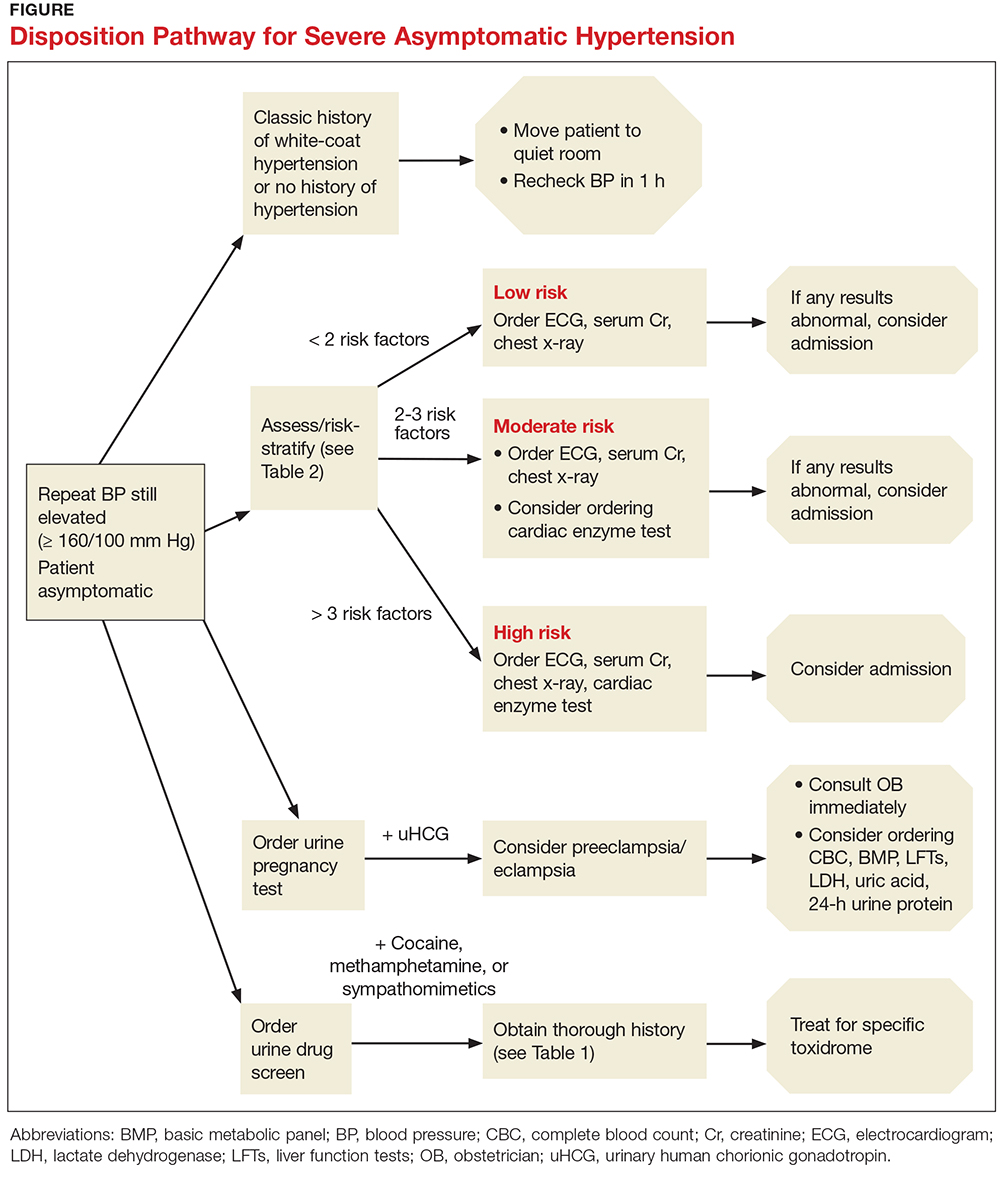
The Figure offers a disposition pathway of recommendations based on risk stratification as well as screening tools for some of the less common causes of hypertensive urgency. Regardless of the results of screening tests or the decision to treat, affected patients require close primary care follow-up. Many of these patients may need further testing and careful management of their BP medication regimen.
How to treat
For patients with severe asymptomatic hypertension, if the history, physical, and screening tests do not show evidence of end-organ damage, BP can be controlled within 24 to 48 hours.5,10,11,21 In adults with hypertensive urgency, the most reasonable goal is to reduce the BP to ≤ 160/100 mm Hg5-7; however, the mean arterial pressure should not be lowered by more than 25% within the first two to three hours.13
Patients at high risk for imminent neurovascular, cardiovascular, renovascular, or pulmonary events should have their BP lowered over a period of hours, not minutes. In fact, there is evidence that rapid lowering of BP in asymptomatic patients may cause adverse outcomes.6 For example, in patients with acute ischemic stroke, increases in cerebral perfusion pressure promote an increase in vascular resistance—but decreasing the cerebral perfusion pressure abruptly will thereby decrease the cerebral blood flow, potentially causing cerebral ischemia or a worsening of the stroke.9,14
Treatment options
A broad spectrum of therapeutic options has proven helpful in lowering BP over a short period of time, including oral captopril, clonidine, hydralazine, labetalol, and hydrochlorothiazide (see Table 3).7,9,12,15 Nifedipine is contraindicated because of the abrupt and often unpredictable reduction in BP and associated myocardial ischemia, especially in patients with MI or left ventricular hypertrophy.14,22,23 In cases of hypertensive urgency secondary to cocaine abuse, benzodiazepines would be the drug of choice and ß-blockers should be avoided due to the risk for coronary vasoconstriction.7
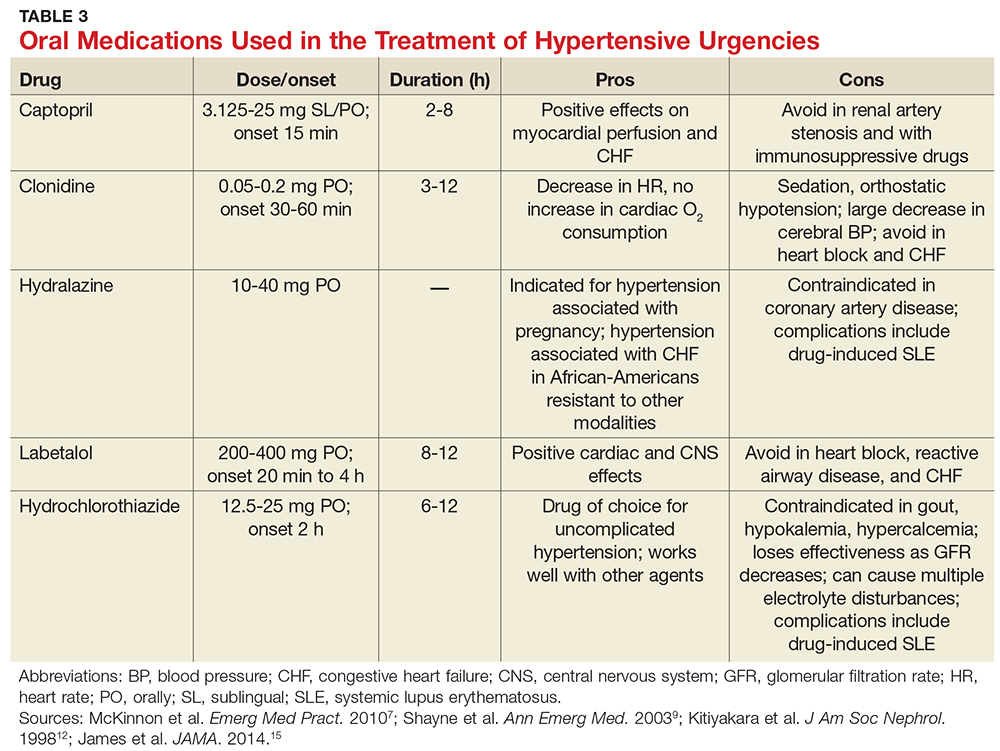
For patients with previously treated hypertension, the following options are reasonable: Increase the dose of the current antihypertensive medication; add another agent; reinstitute prior antihypertensive medications in nonadherent patients; or add a diuretic.
In patients with previously untreated hypertension, no clear evidence supports using one particular agent over another. However, initial treatment options that are generally considered safe include an ACE inhibitor, an angiotensin receptor blocker, a calcium channel blocker, or a thiazide diuretic.15 A few examples of medications within these categories include lisinopril (10 mg PO qd), losartan (50 mg PO qd), amlodipine (2.5 mg PO qd), or hydrochlorothiazide (25 mg PO qd).
Close follow-up is essential when an antihypertensive medication is started or reinstituted. Encourage the patient to reestablish care with their primary care provider (if you do not fill that role). You may need to refer the patient to a new provider or, in some cases, have the patient return to the ED for a repeat BP check.
CONCLUSION
The challenges of managing patients with hypertensive urgency are complicated by low follow-up rates with primary physicians, difficulty in obtaining referrals and follow-up for the patient, and hesitancy of providers to start patients on new BP medications. This article clarifies a well-defined algorithm for how to screen and risk-stratify patients who present to the ED or primary care office with hypertensive urgency.
1. CDC. High blood pressure fact sheet. www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_bloodpressure.htm. Accessed September 26, 2017.
2. Decker WW, Godwin SA, Hess EP, et al; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-249.
3. CDC. High blood pressure facts. www.cdc.gov/bloodpressure/facts.htm. Accessed October 19, 2017.
4. World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: WHO; 2009. www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. Accessed October 19, 2017.
5. Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006;33(3):613-623.
6. Wolf SJ, Lo B, Shih RD, et al; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68.
7. McKinnon M, O’Neill JM. Hypertension in the emergency department: treat now, later, or not at all. Emerg Med Pract. 2010;12(6):1-22.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7): 1281-1357.
9. Shayne PH, Pitts SR. Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41(4): 513-529.
10. Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
11. Houston MC. The comparative effects of clonidine hydrochloride and nifedipine in the treatment of hypertensive crises. Am Heart J. 1998;115(1 pt 1):152-159.
12. Kitiyakara C, Guaman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol. 1998;9(1):133-142.
13. Elliott WJ. Hypertensive emergencies. Crit Care Clin. 2001;17(2):435-451.
14. Papadopoulos DP, Mourouzis I, Thomopoulos C, et al. Hypertension crisis. Blood Press. 2010;19(6):328-336.
15. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
16. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868-877.
17. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858-867.
18. Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17(6):1277-1282.
19. Bang CN, Soliman EZ, Simpson LM, et al. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: the ALLHAT study. Am J Hypertens. 2017;30(9):914-922.
20. Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J. 2005;150(1):161-167.
21. Kinsella K, Baraff LJ. Initiation of therapy for asymptomatic hypertension in the emergency department. Ann Emerg Med. 2009;54(6):791-792.
22. O’Mailia JJ, Sander GE, Giles TD. Nifedipine-associated myocardial ischemia or infarction in the treatment of hypertensive urgencies. Ann Intern Med. 1987;107(2):185-186.
23. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331.
IN THIS ARTICLE
- Patient history; what to ask
- Cardiovascular risk factors
- Disposition pathway
- Oral medications
Approximately one in three US adults, or about 75 million people, have high blood pressure (BP), which has been defined as a BP of 140/90 mm Hg or higher.1 Unfortunately, only about half (54%) of those affected have their condition under optimal control.1 From an epidemiologic standpoint, hypertension has the distinction of being the most common chronic condition in the US, affecting about 54% of persons ages 55 to 64 and about 73% of those 75 and older.2,3 It is the number one reason patients schedule office visits with physicians; it accounts for the most prescriptions; and it is a major risk factor for heart disease and stroke, as well as a significant contributor to mortality throughout the world.4
HYPERTENSIVE URGENCY VS EMERGENCY
Hypertensive urgencies and emergencies account for approximately 27% of all medical emergencies and 2% to 3% of all annual visits to the emergency department (ED).5 Hypertensive urgency, or severe asymptomatic hypertension, is a common complaint in urgent care clinics and primary care offices as well. It is often defined as a systolic BP (SBP) of ≥ 160 mm Hg and/or a diastolic BP (DBP) ≥ 100 mm Hg with no associated end-organ damage.5-7 Patients may experience hypertensive urgency if they have been noncompliant with their antihypertensive drug regimen; present with pain; have white-coat hypertension or anxiety; or use recreational drugs (eg, sympathomimetics).5,8-10
Alternatively, hypertensive emergency, also known as hypertensive crisis, is generally defined as elevated BP > 180/120 mm Hg. Equally important, it is associated with signs, symptoms, or laboratory values indicative of target end-organ damage, such as cerebrovascular accident, myocardial infarction (MI), aortic dissection, acute left ventricular failure, acute pulmonary edema, acute renal failure, acute mental status changes (hypertensive encephalopathy), and eclampsia.5,7,8,11,12
Determining appropriate management for patients with hypertensive urgency is controversial among clinicians. Practice patterns range from full screening and “rule-outs”—with prompt initiation of antihypertensive agents, regardless of whether the patient is symptomatic—to sending the patient home with minimal screening, laboratory testing, or treatment.
This article offers a guided approach to managing patients with hypertensive urgency in a logical fashion, based on risk stratification, thereby avoiding both extremes (extensive unnecessary workup or discharge without workup resulting in adverse outcomes). It is vital to differentiate between patients with hypertensive emergency, in which BP should be lowered in minutes, and patients with hypertensive urgency, in which BP can be lowered more slowly.12

PATHOPHYSIOLOGY
Normally, when BP increases, blood vessel diameter changes in response; this autoregulation serves to limit damage. However, when BP increases abruptly, the body’s ability to hemodynamically calibrate to such a rapid change is impeded, thus allowing for potential end-organ damage.5,12 The increased vascular resistance observed in many patients with hypertension appears to be an autoregulatory process that helps to maintain a normal or viable level of tissue blood flow and organ perfusion despite the increased BP, rather than a primary cause of the hypertension.13
The exact physiology of hypertensive urgencies is not clearly understood, because of the multifactorial nature of the process. One leading theory is that circulating humoral vasoconstrictors cause an abrupt increase in systemic vascular resistance, which in turn causes mechanical shear stress to the endothelial wall. This endothelial damage promotes more vasoconstriction, platelet aggregation, and activation of the renin-angiotensin-aldosterone system, which thereby increases release of angiotensin II and various cytokines.14
HISTORY AND PHYSICAL
A detailed medical history is of utmost importance in distinguishing patients who present with asymptomatic hypertensive urgency from those experiencing a hypertensive emergency. In addition, obtain a full medication list, including any nutritional supplements or illicit drugs the patient may be taking. Question the patient regarding medication adherence; some may not be taking antihypertensive agents as prescribed or may have altered the dosing frequency in an effort to extend the duration of their prescription.5,8 Table 1 lists pertinent questions to ask at presentation; the answers will dictate who needs further workup and possible admission as well as who will require screening for end-organ damage.7

The physical exam should focus primarily on a thorough cardiopulmonary and neurologic examination, as well as funduscopic examination, if needed. A complete set of vital signs should be recorded upon the patient’s arrival to the ED or clinic and should be repeated on the opposite arm for verification. Beginning with the eyes, conduct a thorough funduscopic examination to evaluate for papilledema or hemorrhages.5 During the cardiopulmonary exam, attention should be focused on signs of congestive heart failure and/or pulmonary edema, such as increased jugular vein distension, an S3 gallop, peripheral edema, and pulmonary rales. The neurologic exam is essential in evaluating for cerebrovascular accident, transient ischemic attack, or intracranial hemorrhage. A full cranial nerve examination is necessary, in addition to motor and sensory testing, at minimum.5,9
RISK STRATIFICATION
According to the 2013 Task Force of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC), several risk factors contribute to overall cardiovascular risk in asymptomatic patients presenting with severe hypertension (see Table 2).8 This report has been monumental in linking grades of hypertension directly to cardiovascular risk factors, but it differs from that recently published by the Eighth Joint National Committee (JNC 8), which offers evidence-based guidelines for the management of high BP in the general population of adults (with some modifications for individuals with diabetes or chronic kidney disease or of black ethnicity).15

According to the ESH/ESC study, patients with one or two risk factors who have grade 1 hypertension (SBP 140-159 mm Hg) are at moderate risk for cardiovascular disease (CVD) and patients with grade 2 (SBP 160-179 mm Hg) or grade 3 (SBP ≥ 180 mm Hg) hypertension are at moderate-to-high risk and high risk, respectively.8 Patients with three or more risk factors, or who already have end-organ damage, diabetes, or chronic kidney disease, enter the high-risk category for CVD even at grade 1 hypertension.8
These cardiovascular risk factors can and should be used as guidelines for deciding who needs further screening and who may have benign causes of severe hypertension (eg, white-coat hypertension, anxiety) that can be managed safely in an outpatient setting. In the author’s opinion, patients with known cardiovascular risk factors, those with signs or symptoms of end-organ damage, and those with test results suggestive of end-organ damage should have a more immediate treatment strategy initiated.
Numerous observational studies have shown a direct relationship between systemic hypertension and CVD risk in men and women of various ages, races, and ethnicities, regardless of other risk factors for CVD.12 In patients with diabetes, uncontrolled hypertension is a strong predictor of cardiovascular morbidity and mortality and of progressive nephropathy leading to chronic kidney disease.8
SCREENING
Results from the following tests may provide useful clues in the workup of a patient with hypertensive urgency.
Basic metabolic panel. Many EDs and primary care offices offer point-of-care testing that can typically give a rapid (< 10 min) result of a basic metabolic panel. This useful, quick screening tool can identify renal failure due to chronic untreated hypertension, acute renal failure, or other disease states that cause electrolyte abnormalities such as hyperaldosteronism (hypertension with hypokalemia) or Cushing syndrome (hypertension with hypernatremia and hyperkalemia).7
Cardiac enzymes. Measurement of cardiac troponins (T or I) may provide confirmatory evidence of myocardial necrosis within two to three hours of suspected acute MI.16,17 These tests are now available in most EDs and some clinics with point-of-care testing. A variety of current guidelines advocate repeat cardiac enzyme measurements at various time points, depending on results of initial testing and concomitant risk factors. These protocols vary by facility.
ECG. Obtaining an ECG is another quick, easy, and useful way to screen patients presenting with severe hypertensive urgency. Evidence of left ventricular hypertrophy suggests an increased risk for MI, stroke, heart failure, and sudden death.7,18-20 The Cornell criteria of summing the R wave in aVL and the S wave in V3, with a cutoff of 2.8 mV in men and 2.0 mV in women, has been shown to be the best predictor of future cardiovascular mortality.7 While an isolated finding of left ventricular hypertrophy on an ECG—in and of itself—may have limited value for an individual patient, this finding coupled with other risk factors may alter the provider’s assessment.
Chest radiograph. A chest radiograph can be helpful when used in conjunction with physical exam findings that suggest pulmonary edema and cardiomegaly.7 Widened mediastinum and tortuous aorta may also be evident on chest x-ray, necessitating further workup and imaging.
Urinalysis. In a patient presenting with asymptomatic hypertensive urgency, a urine dipstick result that shows new-onset proteinuria, while not definitive for diagnosis of nephrotic syndrome, may certainly prove helpful in the patient’s workup.5,13
Urine drug screen. In patients without a history of hypertension who present with asymptomatic hypertensive urgency, the urine drug screen may ascertain exposure to cocaine, amphetamine, or phencyclidine.
Pregnancy test. A pregnancy test is essential for any female patient of childbearing age presenting to the ED, and a positive result may be concerning for preeclampsia in a hypertensive patient with no prior history of the condition.7
TREATMENT
Knowing who to treat and when is a vast area of debate among emergency and primary care providers. Patients with hypertension who have established risk factors are known to have worse outcomes than those who may be otherwise healthy. Some clinicians believe that patients presenting with hypertensive urgency should be discharged home without screening and/or treatment. However, because uncontrolled severe hypertension can lead to acute complications (eg, MI, cerebrovascular accident), in practice, many providers are unwilling to send the patient home without workup.12 The patient’s condition must be viewed in the context of the entire disease spectrum, including risk factors.
The Figure offers a disposition pathway of recommendations based on risk stratification as well as screening tools for some of the less common causes of hypertensive urgency. Regardless of the results of screening tests or the decision to treat, affected patients require close primary care follow-up. Many of these patients may need further testing and careful management of their BP medication regimen.

How to treat
For patients with severe asymptomatic hypertension, if the history, physical, and screening tests do not show evidence of end-organ damage, BP can be controlled within 24 to 48 hours.5,10,11,21 In adults with hypertensive urgency, the most reasonable goal is to reduce the BP to ≤ 160/100 mm Hg5-7; however, the mean arterial pressure should not be lowered by more than 25% within the first two to three hours.13
Patients at high risk for imminent neurovascular, cardiovascular, renovascular, or pulmonary events should have their BP lowered over a period of hours, not minutes. In fact, there is evidence that rapid lowering of BP in asymptomatic patients may cause adverse outcomes.6 For example, in patients with acute ischemic stroke, increases in cerebral perfusion pressure promote an increase in vascular resistance—but decreasing the cerebral perfusion pressure abruptly will thereby decrease the cerebral blood flow, potentially causing cerebral ischemia or a worsening of the stroke.9,14
Treatment options
A broad spectrum of therapeutic options has proven helpful in lowering BP over a short period of time, including oral captopril, clonidine, hydralazine, labetalol, and hydrochlorothiazide (see Table 3).7,9,12,15 Nifedipine is contraindicated because of the abrupt and often unpredictable reduction in BP and associated myocardial ischemia, especially in patients with MI or left ventricular hypertrophy.14,22,23 In cases of hypertensive urgency secondary to cocaine abuse, benzodiazepines would be the drug of choice and ß-blockers should be avoided due to the risk for coronary vasoconstriction.7

For patients with previously treated hypertension, the following options are reasonable: Increase the dose of the current antihypertensive medication; add another agent; reinstitute prior antihypertensive medications in nonadherent patients; or add a diuretic.
In patients with previously untreated hypertension, no clear evidence supports using one particular agent over another. However, initial treatment options that are generally considered safe include an ACE inhibitor, an angiotensin receptor blocker, a calcium channel blocker, or a thiazide diuretic.15 A few examples of medications within these categories include lisinopril (10 mg PO qd), losartan (50 mg PO qd), amlodipine (2.5 mg PO qd), or hydrochlorothiazide (25 mg PO qd).
Close follow-up is essential when an antihypertensive medication is started or reinstituted. Encourage the patient to reestablish care with their primary care provider (if you do not fill that role). You may need to refer the patient to a new provider or, in some cases, have the patient return to the ED for a repeat BP check.
CONCLUSION
The challenges of managing patients with hypertensive urgency are complicated by low follow-up rates with primary physicians, difficulty in obtaining referrals and follow-up for the patient, and hesitancy of providers to start patients on new BP medications. This article clarifies a well-defined algorithm for how to screen and risk-stratify patients who present to the ED or primary care office with hypertensive urgency.
IN THIS ARTICLE
- Patient history; what to ask
- Cardiovascular risk factors
- Disposition pathway
- Oral medications
Approximately one in three US adults, or about 75 million people, have high blood pressure (BP), which has been defined as a BP of 140/90 mm Hg or higher.1 Unfortunately, only about half (54%) of those affected have their condition under optimal control.1 From an epidemiologic standpoint, hypertension has the distinction of being the most common chronic condition in the US, affecting about 54% of persons ages 55 to 64 and about 73% of those 75 and older.2,3 It is the number one reason patients schedule office visits with physicians; it accounts for the most prescriptions; and it is a major risk factor for heart disease and stroke, as well as a significant contributor to mortality throughout the world.4
HYPERTENSIVE URGENCY VS EMERGENCY
Hypertensive urgencies and emergencies account for approximately 27% of all medical emergencies and 2% to 3% of all annual visits to the emergency department (ED).5 Hypertensive urgency, or severe asymptomatic hypertension, is a common complaint in urgent care clinics and primary care offices as well. It is often defined as a systolic BP (SBP) of ≥ 160 mm Hg and/or a diastolic BP (DBP) ≥ 100 mm Hg with no associated end-organ damage.5-7 Patients may experience hypertensive urgency if they have been noncompliant with their antihypertensive drug regimen; present with pain; have white-coat hypertension or anxiety; or use recreational drugs (eg, sympathomimetics).5,8-10
Alternatively, hypertensive emergency, also known as hypertensive crisis, is generally defined as elevated BP > 180/120 mm Hg. Equally important, it is associated with signs, symptoms, or laboratory values indicative of target end-organ damage, such as cerebrovascular accident, myocardial infarction (MI), aortic dissection, acute left ventricular failure, acute pulmonary edema, acute renal failure, acute mental status changes (hypertensive encephalopathy), and eclampsia.5,7,8,11,12
Determining appropriate management for patients with hypertensive urgency is controversial among clinicians. Practice patterns range from full screening and “rule-outs”—with prompt initiation of antihypertensive agents, regardless of whether the patient is symptomatic—to sending the patient home with minimal screening, laboratory testing, or treatment.
This article offers a guided approach to managing patients with hypertensive urgency in a logical fashion, based on risk stratification, thereby avoiding both extremes (extensive unnecessary workup or discharge without workup resulting in adverse outcomes). It is vital to differentiate between patients with hypertensive emergency, in which BP should be lowered in minutes, and patients with hypertensive urgency, in which BP can be lowered more slowly.12

PATHOPHYSIOLOGY
Normally, when BP increases, blood vessel diameter changes in response; this autoregulation serves to limit damage. However, when BP increases abruptly, the body’s ability to hemodynamically calibrate to such a rapid change is impeded, thus allowing for potential end-organ damage.5,12 The increased vascular resistance observed in many patients with hypertension appears to be an autoregulatory process that helps to maintain a normal or viable level of tissue blood flow and organ perfusion despite the increased BP, rather than a primary cause of the hypertension.13
The exact physiology of hypertensive urgencies is not clearly understood, because of the multifactorial nature of the process. One leading theory is that circulating humoral vasoconstrictors cause an abrupt increase in systemic vascular resistance, which in turn causes mechanical shear stress to the endothelial wall. This endothelial damage promotes more vasoconstriction, platelet aggregation, and activation of the renin-angiotensin-aldosterone system, which thereby increases release of angiotensin II and various cytokines.14
HISTORY AND PHYSICAL
A detailed medical history is of utmost importance in distinguishing patients who present with asymptomatic hypertensive urgency from those experiencing a hypertensive emergency. In addition, obtain a full medication list, including any nutritional supplements or illicit drugs the patient may be taking. Question the patient regarding medication adherence; some may not be taking antihypertensive agents as prescribed or may have altered the dosing frequency in an effort to extend the duration of their prescription.5,8 Table 1 lists pertinent questions to ask at presentation; the answers will dictate who needs further workup and possible admission as well as who will require screening for end-organ damage.7

The physical exam should focus primarily on a thorough cardiopulmonary and neurologic examination, as well as funduscopic examination, if needed. A complete set of vital signs should be recorded upon the patient’s arrival to the ED or clinic and should be repeated on the opposite arm for verification. Beginning with the eyes, conduct a thorough funduscopic examination to evaluate for papilledema or hemorrhages.5 During the cardiopulmonary exam, attention should be focused on signs of congestive heart failure and/or pulmonary edema, such as increased jugular vein distension, an S3 gallop, peripheral edema, and pulmonary rales. The neurologic exam is essential in evaluating for cerebrovascular accident, transient ischemic attack, or intracranial hemorrhage. A full cranial nerve examination is necessary, in addition to motor and sensory testing, at minimum.5,9
RISK STRATIFICATION
According to the 2013 Task Force of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC), several risk factors contribute to overall cardiovascular risk in asymptomatic patients presenting with severe hypertension (see Table 2).8 This report has been monumental in linking grades of hypertension directly to cardiovascular risk factors, but it differs from that recently published by the Eighth Joint National Committee (JNC 8), which offers evidence-based guidelines for the management of high BP in the general population of adults (with some modifications for individuals with diabetes or chronic kidney disease or of black ethnicity).15

According to the ESH/ESC study, patients with one or two risk factors who have grade 1 hypertension (SBP 140-159 mm Hg) are at moderate risk for cardiovascular disease (CVD) and patients with grade 2 (SBP 160-179 mm Hg) or grade 3 (SBP ≥ 180 mm Hg) hypertension are at moderate-to-high risk and high risk, respectively.8 Patients with three or more risk factors, or who already have end-organ damage, diabetes, or chronic kidney disease, enter the high-risk category for CVD even at grade 1 hypertension.8
These cardiovascular risk factors can and should be used as guidelines for deciding who needs further screening and who may have benign causes of severe hypertension (eg, white-coat hypertension, anxiety) that can be managed safely in an outpatient setting. In the author’s opinion, patients with known cardiovascular risk factors, those with signs or symptoms of end-organ damage, and those with test results suggestive of end-organ damage should have a more immediate treatment strategy initiated.
Numerous observational studies have shown a direct relationship between systemic hypertension and CVD risk in men and women of various ages, races, and ethnicities, regardless of other risk factors for CVD.12 In patients with diabetes, uncontrolled hypertension is a strong predictor of cardiovascular morbidity and mortality and of progressive nephropathy leading to chronic kidney disease.8
SCREENING
Results from the following tests may provide useful clues in the workup of a patient with hypertensive urgency.
Basic metabolic panel. Many EDs and primary care offices offer point-of-care testing that can typically give a rapid (< 10 min) result of a basic metabolic panel. This useful, quick screening tool can identify renal failure due to chronic untreated hypertension, acute renal failure, or other disease states that cause electrolyte abnormalities such as hyperaldosteronism (hypertension with hypokalemia) or Cushing syndrome (hypertension with hypernatremia and hyperkalemia).7
Cardiac enzymes. Measurement of cardiac troponins (T or I) may provide confirmatory evidence of myocardial necrosis within two to three hours of suspected acute MI.16,17 These tests are now available in most EDs and some clinics with point-of-care testing. A variety of current guidelines advocate repeat cardiac enzyme measurements at various time points, depending on results of initial testing and concomitant risk factors. These protocols vary by facility.
ECG. Obtaining an ECG is another quick, easy, and useful way to screen patients presenting with severe hypertensive urgency. Evidence of left ventricular hypertrophy suggests an increased risk for MI, stroke, heart failure, and sudden death.7,18-20 The Cornell criteria of summing the R wave in aVL and the S wave in V3, with a cutoff of 2.8 mV in men and 2.0 mV in women, has been shown to be the best predictor of future cardiovascular mortality.7 While an isolated finding of left ventricular hypertrophy on an ECG—in and of itself—may have limited value for an individual patient, this finding coupled with other risk factors may alter the provider’s assessment.
Chest radiograph. A chest radiograph can be helpful when used in conjunction with physical exam findings that suggest pulmonary edema and cardiomegaly.7 Widened mediastinum and tortuous aorta may also be evident on chest x-ray, necessitating further workup and imaging.
Urinalysis. In a patient presenting with asymptomatic hypertensive urgency, a urine dipstick result that shows new-onset proteinuria, while not definitive for diagnosis of nephrotic syndrome, may certainly prove helpful in the patient’s workup.5,13
Urine drug screen. In patients without a history of hypertension who present with asymptomatic hypertensive urgency, the urine drug screen may ascertain exposure to cocaine, amphetamine, or phencyclidine.
Pregnancy test. A pregnancy test is essential for any female patient of childbearing age presenting to the ED, and a positive result may be concerning for preeclampsia in a hypertensive patient with no prior history of the condition.7
TREATMENT
Knowing who to treat and when is a vast area of debate among emergency and primary care providers. Patients with hypertension who have established risk factors are known to have worse outcomes than those who may be otherwise healthy. Some clinicians believe that patients presenting with hypertensive urgency should be discharged home without screening and/or treatment. However, because uncontrolled severe hypertension can lead to acute complications (eg, MI, cerebrovascular accident), in practice, many providers are unwilling to send the patient home without workup.12 The patient’s condition must be viewed in the context of the entire disease spectrum, including risk factors.
The Figure offers a disposition pathway of recommendations based on risk stratification as well as screening tools for some of the less common causes of hypertensive urgency. Regardless of the results of screening tests or the decision to treat, affected patients require close primary care follow-up. Many of these patients may need further testing and careful management of their BP medication regimen.

How to treat
For patients with severe asymptomatic hypertension, if the history, physical, and screening tests do not show evidence of end-organ damage, BP can be controlled within 24 to 48 hours.5,10,11,21 In adults with hypertensive urgency, the most reasonable goal is to reduce the BP to ≤ 160/100 mm Hg5-7; however, the mean arterial pressure should not be lowered by more than 25% within the first two to three hours.13
Patients at high risk for imminent neurovascular, cardiovascular, renovascular, or pulmonary events should have their BP lowered over a period of hours, not minutes. In fact, there is evidence that rapid lowering of BP in asymptomatic patients may cause adverse outcomes.6 For example, in patients with acute ischemic stroke, increases in cerebral perfusion pressure promote an increase in vascular resistance—but decreasing the cerebral perfusion pressure abruptly will thereby decrease the cerebral blood flow, potentially causing cerebral ischemia or a worsening of the stroke.9,14
Treatment options
A broad spectrum of therapeutic options has proven helpful in lowering BP over a short period of time, including oral captopril, clonidine, hydralazine, labetalol, and hydrochlorothiazide (see Table 3).7,9,12,15 Nifedipine is contraindicated because of the abrupt and often unpredictable reduction in BP and associated myocardial ischemia, especially in patients with MI or left ventricular hypertrophy.14,22,23 In cases of hypertensive urgency secondary to cocaine abuse, benzodiazepines would be the drug of choice and ß-blockers should be avoided due to the risk for coronary vasoconstriction.7

For patients with previously treated hypertension, the following options are reasonable: Increase the dose of the current antihypertensive medication; add another agent; reinstitute prior antihypertensive medications in nonadherent patients; or add a diuretic.
In patients with previously untreated hypertension, no clear evidence supports using one particular agent over another. However, initial treatment options that are generally considered safe include an ACE inhibitor, an angiotensin receptor blocker, a calcium channel blocker, or a thiazide diuretic.15 A few examples of medications within these categories include lisinopril (10 mg PO qd), losartan (50 mg PO qd), amlodipine (2.5 mg PO qd), or hydrochlorothiazide (25 mg PO qd).
Close follow-up is essential when an antihypertensive medication is started or reinstituted. Encourage the patient to reestablish care with their primary care provider (if you do not fill that role). You may need to refer the patient to a new provider or, in some cases, have the patient return to the ED for a repeat BP check.
CONCLUSION
The challenges of managing patients with hypertensive urgency are complicated by low follow-up rates with primary physicians, difficulty in obtaining referrals and follow-up for the patient, and hesitancy of providers to start patients on new BP medications. This article clarifies a well-defined algorithm for how to screen and risk-stratify patients who present to the ED or primary care office with hypertensive urgency.
1. CDC. High blood pressure fact sheet. www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_bloodpressure.htm. Accessed September 26, 2017.
2. Decker WW, Godwin SA, Hess EP, et al; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-249.
3. CDC. High blood pressure facts. www.cdc.gov/bloodpressure/facts.htm. Accessed October 19, 2017.
4. World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: WHO; 2009. www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. Accessed October 19, 2017.
5. Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006;33(3):613-623.
6. Wolf SJ, Lo B, Shih RD, et al; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68.
7. McKinnon M, O’Neill JM. Hypertension in the emergency department: treat now, later, or not at all. Emerg Med Pract. 2010;12(6):1-22.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7): 1281-1357.
9. Shayne PH, Pitts SR. Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41(4): 513-529.
10. Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
11. Houston MC. The comparative effects of clonidine hydrochloride and nifedipine in the treatment of hypertensive crises. Am Heart J. 1998;115(1 pt 1):152-159.
12. Kitiyakara C, Guaman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol. 1998;9(1):133-142.
13. Elliott WJ. Hypertensive emergencies. Crit Care Clin. 2001;17(2):435-451.
14. Papadopoulos DP, Mourouzis I, Thomopoulos C, et al. Hypertension crisis. Blood Press. 2010;19(6):328-336.
15. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
16. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868-877.
17. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858-867.
18. Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17(6):1277-1282.
19. Bang CN, Soliman EZ, Simpson LM, et al. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: the ALLHAT study. Am J Hypertens. 2017;30(9):914-922.
20. Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J. 2005;150(1):161-167.
21. Kinsella K, Baraff LJ. Initiation of therapy for asymptomatic hypertension in the emergency department. Ann Emerg Med. 2009;54(6):791-792.
22. O’Mailia JJ, Sander GE, Giles TD. Nifedipine-associated myocardial ischemia or infarction in the treatment of hypertensive urgencies. Ann Intern Med. 1987;107(2):185-186.
23. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331.
1. CDC. High blood pressure fact sheet. www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_bloodpressure.htm. Accessed September 26, 2017.
2. Decker WW, Godwin SA, Hess EP, et al; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-249.
3. CDC. High blood pressure facts. www.cdc.gov/bloodpressure/facts.htm. Accessed October 19, 2017.
4. World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: WHO; 2009. www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. Accessed October 19, 2017.
5. Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006;33(3):613-623.
6. Wolf SJ, Lo B, Shih RD, et al; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68.
7. McKinnon M, O’Neill JM. Hypertension in the emergency department: treat now, later, or not at all. Emerg Med Pract. 2010;12(6):1-22.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7): 1281-1357.
9. Shayne PH, Pitts SR. Severely increased blood pressure in the emergency department. Ann Emerg Med. 2003;41(4): 513-529.
10. Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
11. Houston MC. The comparative effects of clonidine hydrochloride and nifedipine in the treatment of hypertensive crises. Am Heart J. 1998;115(1 pt 1):152-159.
12. Kitiyakara C, Guaman NJ. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol. 1998;9(1):133-142.
13. Elliott WJ. Hypertensive emergencies. Crit Care Clin. 2001;17(2):435-451.
14. Papadopoulos DP, Mourouzis I, Thomopoulos C, et al. Hypertension crisis. Blood Press. 2010;19(6):328-336.
15. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
16. Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868-877.
17. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858-867.
18. Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17(6):1277-1282.
19. Bang CN, Soliman EZ, Simpson LM, et al. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: the ALLHAT study. Am J Hypertens. 2017;30(9):914-922.
20. Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J. 2005;150(1):161-167.
21. Kinsella K, Baraff LJ. Initiation of therapy for asymptomatic hypertension in the emergency department. Ann Emerg Med. 2009;54(6):791-792.
22. O’Mailia JJ, Sander GE, Giles TD. Nifedipine-associated myocardial ischemia or infarction in the treatment of hypertensive urgencies. Ann Intern Med. 1987;107(2):185-186.
23. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331.
Sebum inhibition steps up against acne
In the future, topical agents that target sebum production could play a greater role in acne management.
Linda F. Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit highlighted several studies of these investigational therapies in a presentation on acne at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
• SB204, a topical agent that releases nitric oxide.
• DRM01, a topical sebum inhibitor that inhibits acetyl coenzyme-A carboxylase, an enzyme involved in the synthesis of fatty acids.
• Cortexolone 17a-propionate (CB-03-01) 1% cream, a topical antiandrogen.
A 4% gel formulation of SB204 once a day was compared with a vehicle, also applied topically once a day, in a pair of phase 3 randomized, multicenter pivotal trials of 2,639 patients aged 9 years and older with moderate to severe acne, Dr. Stein Gold said. At 12 weeks, the absolute change in number of noninflammatory lesions among those treated with SB204 was a reduction by 15.4 vs. 13.4 among those treated with a vehicle in one study (P = .03) and by 14.9 vs. 12.3 with a vehicle in the other study (P = .001). The absolute change in the number of inflammatory lesions among those using SB204 in the first study was not significantly different from those seen among patients using the vehicle (a reduction of 12.1 vs. 11.1, respectively) but was significant in the second study (a reduction of 12.9 vs. 10.6 for vehicle; P less than .001).
No new safety signals were observed and treatment was “generally safe and well tolerated,” with fewer than 2% of patients discontinuing treatment because of treatment-emergent adverse events, she noted.
In a phase 2 study, those treated with a 4% formulation of SB204 had a significant reduction in inflammatory and noninflammatory lesions at 12 weeks, with mild local irritation as the main adverse effect, she said (J. Drugs Dermatol. 2016 Dec 1;15[12]:1496-527). The treatment was generally safe and well tolerated, said Dr. Stein Gold, one of the study authors.
In a phase 2 randomized, multicenter, double-blind study of 108 patients with moderate or severe acne, she continued, those treated with DRM01 7.5% applied to the face twice a day for 12 weeks, those treated with DRM01 showed significant improvement across all efficacy measures at 12 weeks, including a significantly greater reduction in both inflammatory and noninflammatory lesions, and on measures of investigator’s global assessment – after 12 weeks of twice-daily treatment. Treatment was well tolerated, and no serious adverse events related to the treatment were reported, Dr. Stein Gold said.
(In October, the manufacturer, Dermira, announced that patient enrollment in two phase 3 trials of DRM01– now known as olumacostat glasaretil – in patients aged 9 years and older with facial acne had been completed.)
Phase 3 studies of cortexolone 17a-propionate (CB-03-01) 1% cream are underway, Dr. Stein Gold said.
Dr. Stein Gold disclosed relationships with multiple companies including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promius, Anacor, and Medimetriks.
SDEF and this news organization are owned by the same parent company.
In the future, topical agents that target sebum production could play a greater role in acne management.
Linda F. Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit highlighted several studies of these investigational therapies in a presentation on acne at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
• SB204, a topical agent that releases nitric oxide.
• DRM01, a topical sebum inhibitor that inhibits acetyl coenzyme-A carboxylase, an enzyme involved in the synthesis of fatty acids.
• Cortexolone 17a-propionate (CB-03-01) 1% cream, a topical antiandrogen.
A 4% gel formulation of SB204 once a day was compared with a vehicle, also applied topically once a day, in a pair of phase 3 randomized, multicenter pivotal trials of 2,639 patients aged 9 years and older with moderate to severe acne, Dr. Stein Gold said. At 12 weeks, the absolute change in number of noninflammatory lesions among those treated with SB204 was a reduction by 15.4 vs. 13.4 among those treated with a vehicle in one study (P = .03) and by 14.9 vs. 12.3 with a vehicle in the other study (P = .001). The absolute change in the number of inflammatory lesions among those using SB204 in the first study was not significantly different from those seen among patients using the vehicle (a reduction of 12.1 vs. 11.1, respectively) but was significant in the second study (a reduction of 12.9 vs. 10.6 for vehicle; P less than .001).
No new safety signals were observed and treatment was “generally safe and well tolerated,” with fewer than 2% of patients discontinuing treatment because of treatment-emergent adverse events, she noted.
In a phase 2 study, those treated with a 4% formulation of SB204 had a significant reduction in inflammatory and noninflammatory lesions at 12 weeks, with mild local irritation as the main adverse effect, she said (J. Drugs Dermatol. 2016 Dec 1;15[12]:1496-527). The treatment was generally safe and well tolerated, said Dr. Stein Gold, one of the study authors.
In a phase 2 randomized, multicenter, double-blind study of 108 patients with moderate or severe acne, she continued, those treated with DRM01 7.5% applied to the face twice a day for 12 weeks, those treated with DRM01 showed significant improvement across all efficacy measures at 12 weeks, including a significantly greater reduction in both inflammatory and noninflammatory lesions, and on measures of investigator’s global assessment – after 12 weeks of twice-daily treatment. Treatment was well tolerated, and no serious adverse events related to the treatment were reported, Dr. Stein Gold said.
(In October, the manufacturer, Dermira, announced that patient enrollment in two phase 3 trials of DRM01– now known as olumacostat glasaretil – in patients aged 9 years and older with facial acne had been completed.)
Phase 3 studies of cortexolone 17a-propionate (CB-03-01) 1% cream are underway, Dr. Stein Gold said.
Dr. Stein Gold disclosed relationships with multiple companies including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promius, Anacor, and Medimetriks.
SDEF and this news organization are owned by the same parent company.
In the future, topical agents that target sebum production could play a greater role in acne management.
Linda F. Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit highlighted several studies of these investigational therapies in a presentation on acne at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
• SB204, a topical agent that releases nitric oxide.
• DRM01, a topical sebum inhibitor that inhibits acetyl coenzyme-A carboxylase, an enzyme involved in the synthesis of fatty acids.
• Cortexolone 17a-propionate (CB-03-01) 1% cream, a topical antiandrogen.
A 4% gel formulation of SB204 once a day was compared with a vehicle, also applied topically once a day, in a pair of phase 3 randomized, multicenter pivotal trials of 2,639 patients aged 9 years and older with moderate to severe acne, Dr. Stein Gold said. At 12 weeks, the absolute change in number of noninflammatory lesions among those treated with SB204 was a reduction by 15.4 vs. 13.4 among those treated with a vehicle in one study (P = .03) and by 14.9 vs. 12.3 with a vehicle in the other study (P = .001). The absolute change in the number of inflammatory lesions among those using SB204 in the first study was not significantly different from those seen among patients using the vehicle (a reduction of 12.1 vs. 11.1, respectively) but was significant in the second study (a reduction of 12.9 vs. 10.6 for vehicle; P less than .001).
No new safety signals were observed and treatment was “generally safe and well tolerated,” with fewer than 2% of patients discontinuing treatment because of treatment-emergent adverse events, she noted.
In a phase 2 study, those treated with a 4% formulation of SB204 had a significant reduction in inflammatory and noninflammatory lesions at 12 weeks, with mild local irritation as the main adverse effect, she said (J. Drugs Dermatol. 2016 Dec 1;15[12]:1496-527). The treatment was generally safe and well tolerated, said Dr. Stein Gold, one of the study authors.
In a phase 2 randomized, multicenter, double-blind study of 108 patients with moderate or severe acne, she continued, those treated with DRM01 7.5% applied to the face twice a day for 12 weeks, those treated with DRM01 showed significant improvement across all efficacy measures at 12 weeks, including a significantly greater reduction in both inflammatory and noninflammatory lesions, and on measures of investigator’s global assessment – after 12 weeks of twice-daily treatment. Treatment was well tolerated, and no serious adverse events related to the treatment were reported, Dr. Stein Gold said.
(In October, the manufacturer, Dermira, announced that patient enrollment in two phase 3 trials of DRM01– now known as olumacostat glasaretil – in patients aged 9 years and older with facial acne had been completed.)
Phase 3 studies of cortexolone 17a-propionate (CB-03-01) 1% cream are underway, Dr. Stein Gold said.
Dr. Stein Gold disclosed relationships with multiple companies including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promius, Anacor, and Medimetriks.
SDEF and this news organization are owned by the same parent company.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
VIDEO: Various treatments for acne in clinical trials
LAS VEGAS – “It’s an exciting time in the treatment of acne,” Linda F. Stein Gold, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In the interview, Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit, discussed several new developments in the field of acne, including a once-daily oral antibiotic, sarecycline, and topical minocycline drugs in different stages in the research pipeline.
Scarring can occur with any degree of acne severity, but new data suggest that patients struggling with scars may benefit from a fixed combination of adapalene gel 0.3% and benzoyl peroxide gel 2.5%, Dr. Stein Gold noted.
She disclosed relationships with multiple companies including Allergan, Anacor, Celgene, Dermira, Foamix, Galderma, LEO, Medimetriks, Novan, Novartis, Promius, Sol-gel, and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – “It’s an exciting time in the treatment of acne,” Linda F. Stein Gold, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In the interview, Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit, discussed several new developments in the field of acne, including a once-daily oral antibiotic, sarecycline, and topical minocycline drugs in different stages in the research pipeline.
Scarring can occur with any degree of acne severity, but new data suggest that patients struggling with scars may benefit from a fixed combination of adapalene gel 0.3% and benzoyl peroxide gel 2.5%, Dr. Stein Gold noted.
She disclosed relationships with multiple companies including Allergan, Anacor, Celgene, Dermira, Foamix, Galderma, LEO, Medimetriks, Novan, Novartis, Promius, Sol-gel, and Valeant.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – “It’s an exciting time in the treatment of acne,” Linda F. Stein Gold, MD, said in a video interview at Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In the interview, Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit, discussed several new developments in the field of acne, including a once-daily oral antibiotic, sarecycline, and topical minocycline drugs in different stages in the research pipeline.
Scarring can occur with any degree of acne severity, but new data suggest that patients struggling with scars may benefit from a fixed combination of adapalene gel 0.3% and benzoyl peroxide gel 2.5%, Dr. Stein Gold noted.
She disclosed relationships with multiple companies including Allergan, Anacor, Celgene, Dermira, Foamix, Galderma, LEO, Medimetriks, Novan, Novartis, Promius, Sol-gel, and Valeant.
SDEF and this news organization are owned by the same parent company.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Challenges of validating cerebral protection for TAVI
While using cerebral embolic protection during transcatheter aortic valve implantation (TAVI) seems appealing to reduce the risk of stroke, which has been reported to be higher than in open aortic valve replacement, the challenge of developing practical CEP devices and then designing appropriate trials may be insurmountable, according to a featured expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154;880-3).
Dr. Messé and Dr. Furie reviewed completed trials of five different CEP devices in 630 patients, noting that the trials confirmed the difficulty of “designing a trial that can prove a clinical benefit.” They noted 30-day stroke rates ranged from 4% to 6.7%, although prospective, nonrandomized European registries reported rates of 3.4% to 4.1%, and a large U.S. registry reported a rate of 2.5%. These results suggest “that some neurologic complications are going undetected or underreported in routine clinical practice.”
That may be a function of the different methods the trials used to determine complications. “There are little data to define best practice, but direct comprehensive assessment by a neurologist is likely the most accurate and sensitive method for detecting clinical stroke,” they added.
Another important factor is the timing of the assessment. They pointed out that half of all 30-day stroke events in TAVI are detected within 2 days of the procedure, but mild or transient symptoms can be missed if the only evaluation occurs just before discharge. “Unfortunately, in most studies this is when the neurologic assessment is performed,” Dr. Messé and Dr. Furie wrote. They added that long-term effects of these strokes have not been well studied.
What’s more, many TAVI patients have subclinical ischemic injury that only neuroimaging can detect. “Studies of MRI performed early after TAVI have demonstrated acute infarcts in 68% to 97% of patients,” the stated. While small and multiple, these microinfarcts may not be totally silent. “Additional studies to assess the long-term implications of clinically silent infarcts are clearly needed,” the coauthors said.
They also noted that a trial of stenting vs. endarterectomy for carotid stenosis raises caution about CEP devices (Lancet Neurol. 2010;9:353-62), as patients who had angioplasty and stenting and were treated with a CEP device had higher rates of acute infarct detected on MRI than those who did not have the CEP. Placing the CEP device through a severely stenosed and symptomatic carotid artery may have led to additional cerebral emboli.
“Placing a cerebral protection device in the aorta for a TAVI procedure also could be problematic in the presence of severe aortic arch disease or variant anatomy,” Dr. Messé and Dr. Furie commented. With two large trials of embolic protection in TAVI currently underway, the coauthors said, “the field eagerly awaits these results.”
Dr. Messé has received research support from GlaxoSmithKline and Direct Flow Medical. Both Dr. Messé and Dr. Furie have participated in the National Institutes of Health/National Heart, Lung, and Blood Institute/National Institute of Neurologic Disorders and Stroke–sponsored Cardiothoracic Surgery Network.
Dr. Messé and Dr. Furie pointed out the difficulty of quantifying the incidence of stroke during TAVI. But the long-term impact of stroke is more important because TAVI, as opposed to surgical aortic valve replacement, is more likely to be performed in a younger, healthier population, John Bozinovski, MD, of the University of British Columbia in Victoria said in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:484-5).
While Dr. Messé and Dr. Furie make a valid point that clinically significant stroke, as opposed to diagnostically apparent stroke, is an important outcome of trials of embolic protection in TAVI, the long-term impact of silent strokes identified only with neuroimaging is unknown, “As such, ‘clinically significant’ stroke carries a nebulous definition,” Dr. Bozinovski said.
Nonetheless, CEP devices may become standard “even without good evidence” to support their use, Dr. Bozinovski said, “or perhaps they will be used infrequently or not at all.” Their uptake by cardiac surgeons will depend on the supporting evidence and their treatment effect. “Not only is it difficult to design an effective device, it is possible that we may not know whether a device is effective or what is the size of that effect,” he said. As Dr. Messé and Dr. Furie point out, “Designing and successfully conducting the trial to do so might never occur.”
Dr. Bozinovski disclosed he was a paid consultant with Edwards Life Sciences.
Dr. Messé and Dr. Furie pointed out the difficulty of quantifying the incidence of stroke during TAVI. But the long-term impact of stroke is more important because TAVI, as opposed to surgical aortic valve replacement, is more likely to be performed in a younger, healthier population, John Bozinovski, MD, of the University of British Columbia in Victoria said in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:484-5).
While Dr. Messé and Dr. Furie make a valid point that clinically significant stroke, as opposed to diagnostically apparent stroke, is an important outcome of trials of embolic protection in TAVI, the long-term impact of silent strokes identified only with neuroimaging is unknown, “As such, ‘clinically significant’ stroke carries a nebulous definition,” Dr. Bozinovski said.
Nonetheless, CEP devices may become standard “even without good evidence” to support their use, Dr. Bozinovski said, “or perhaps they will be used infrequently or not at all.” Their uptake by cardiac surgeons will depend on the supporting evidence and their treatment effect. “Not only is it difficult to design an effective device, it is possible that we may not know whether a device is effective or what is the size of that effect,” he said. As Dr. Messé and Dr. Furie point out, “Designing and successfully conducting the trial to do so might never occur.”
Dr. Bozinovski disclosed he was a paid consultant with Edwards Life Sciences.
Dr. Messé and Dr. Furie pointed out the difficulty of quantifying the incidence of stroke during TAVI. But the long-term impact of stroke is more important because TAVI, as opposed to surgical aortic valve replacement, is more likely to be performed in a younger, healthier population, John Bozinovski, MD, of the University of British Columbia in Victoria said in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:484-5).
While Dr. Messé and Dr. Furie make a valid point that clinically significant stroke, as opposed to diagnostically apparent stroke, is an important outcome of trials of embolic protection in TAVI, the long-term impact of silent strokes identified only with neuroimaging is unknown, “As such, ‘clinically significant’ stroke carries a nebulous definition,” Dr. Bozinovski said.
Nonetheless, CEP devices may become standard “even without good evidence” to support their use, Dr. Bozinovski said, “or perhaps they will be used infrequently or not at all.” Their uptake by cardiac surgeons will depend on the supporting evidence and their treatment effect. “Not only is it difficult to design an effective device, it is possible that we may not know whether a device is effective or what is the size of that effect,” he said. As Dr. Messé and Dr. Furie point out, “Designing and successfully conducting the trial to do so might never occur.”
Dr. Bozinovski disclosed he was a paid consultant with Edwards Life Sciences.
While using cerebral embolic protection during transcatheter aortic valve implantation (TAVI) seems appealing to reduce the risk of stroke, which has been reported to be higher than in open aortic valve replacement, the challenge of developing practical CEP devices and then designing appropriate trials may be insurmountable, according to a featured expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154;880-3).
Dr. Messé and Dr. Furie reviewed completed trials of five different CEP devices in 630 patients, noting that the trials confirmed the difficulty of “designing a trial that can prove a clinical benefit.” They noted 30-day stroke rates ranged from 4% to 6.7%, although prospective, nonrandomized European registries reported rates of 3.4% to 4.1%, and a large U.S. registry reported a rate of 2.5%. These results suggest “that some neurologic complications are going undetected or underreported in routine clinical practice.”
That may be a function of the different methods the trials used to determine complications. “There are little data to define best practice, but direct comprehensive assessment by a neurologist is likely the most accurate and sensitive method for detecting clinical stroke,” they added.
Another important factor is the timing of the assessment. They pointed out that half of all 30-day stroke events in TAVI are detected within 2 days of the procedure, but mild or transient symptoms can be missed if the only evaluation occurs just before discharge. “Unfortunately, in most studies this is when the neurologic assessment is performed,” Dr. Messé and Dr. Furie wrote. They added that long-term effects of these strokes have not been well studied.
What’s more, many TAVI patients have subclinical ischemic injury that only neuroimaging can detect. “Studies of MRI performed early after TAVI have demonstrated acute infarcts in 68% to 97% of patients,” the stated. While small and multiple, these microinfarcts may not be totally silent. “Additional studies to assess the long-term implications of clinically silent infarcts are clearly needed,” the coauthors said.
They also noted that a trial of stenting vs. endarterectomy for carotid stenosis raises caution about CEP devices (Lancet Neurol. 2010;9:353-62), as patients who had angioplasty and stenting and were treated with a CEP device had higher rates of acute infarct detected on MRI than those who did not have the CEP. Placing the CEP device through a severely stenosed and symptomatic carotid artery may have led to additional cerebral emboli.
“Placing a cerebral protection device in the aorta for a TAVI procedure also could be problematic in the presence of severe aortic arch disease or variant anatomy,” Dr. Messé and Dr. Furie commented. With two large trials of embolic protection in TAVI currently underway, the coauthors said, “the field eagerly awaits these results.”
Dr. Messé has received research support from GlaxoSmithKline and Direct Flow Medical. Both Dr. Messé and Dr. Furie have participated in the National Institutes of Health/National Heart, Lung, and Blood Institute/National Institute of Neurologic Disorders and Stroke–sponsored Cardiothoracic Surgery Network.
While using cerebral embolic protection during transcatheter aortic valve implantation (TAVI) seems appealing to reduce the risk of stroke, which has been reported to be higher than in open aortic valve replacement, the challenge of developing practical CEP devices and then designing appropriate trials may be insurmountable, according to a featured expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154;880-3).
Dr. Messé and Dr. Furie reviewed completed trials of five different CEP devices in 630 patients, noting that the trials confirmed the difficulty of “designing a trial that can prove a clinical benefit.” They noted 30-day stroke rates ranged from 4% to 6.7%, although prospective, nonrandomized European registries reported rates of 3.4% to 4.1%, and a large U.S. registry reported a rate of 2.5%. These results suggest “that some neurologic complications are going undetected or underreported in routine clinical practice.”
That may be a function of the different methods the trials used to determine complications. “There are little data to define best practice, but direct comprehensive assessment by a neurologist is likely the most accurate and sensitive method for detecting clinical stroke,” they added.
Another important factor is the timing of the assessment. They pointed out that half of all 30-day stroke events in TAVI are detected within 2 days of the procedure, but mild or transient symptoms can be missed if the only evaluation occurs just before discharge. “Unfortunately, in most studies this is when the neurologic assessment is performed,” Dr. Messé and Dr. Furie wrote. They added that long-term effects of these strokes have not been well studied.
What’s more, many TAVI patients have subclinical ischemic injury that only neuroimaging can detect. “Studies of MRI performed early after TAVI have demonstrated acute infarcts in 68% to 97% of patients,” the stated. While small and multiple, these microinfarcts may not be totally silent. “Additional studies to assess the long-term implications of clinically silent infarcts are clearly needed,” the coauthors said.
They also noted that a trial of stenting vs. endarterectomy for carotid stenosis raises caution about CEP devices (Lancet Neurol. 2010;9:353-62), as patients who had angioplasty and stenting and were treated with a CEP device had higher rates of acute infarct detected on MRI than those who did not have the CEP. Placing the CEP device through a severely stenosed and symptomatic carotid artery may have led to additional cerebral emboli.
“Placing a cerebral protection device in the aorta for a TAVI procedure also could be problematic in the presence of severe aortic arch disease or variant anatomy,” Dr. Messé and Dr. Furie commented. With two large trials of embolic protection in TAVI currently underway, the coauthors said, “the field eagerly awaits these results.”
Dr. Messé has received research support from GlaxoSmithKline and Direct Flow Medical. Both Dr. Messé and Dr. Furie have participated in the National Institutes of Health/National Heart, Lung, and Blood Institute/National Institute of Neurologic Disorders and Stroke–sponsored Cardiothoracic Surgery Network.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Cerebral embolic protection during TAVI is an appealing concept that faces challenges.
Major finding: Developing practical and safe devices and testing them in adequately powered trials are daunting tasks.
Data source: Review of five completed and two ongoing clinical trials of 603 and 613 patients, respectively.
Disclosures: Dr. Messé has received research support from GlaxoSmithKline and Direct Flow Medical. Both Dr. Messé and Dr. Furie have participated in the NIH/NHLBI/NINDS-sponsored Cardiothoracic Surgery Network.
MS Treatment Decisions Based on Milestones That Matter
PARIS—Tracking disease impact in patients with multiple sclerosis (MS) by predictable loss of economically important milestones trajectory, beyond what can be documented by EDSS, MRI, or apparent or reported relapse, can be accomplished by use of objective multidomain cognitive testing, according to a report presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. Such a strategy can provide patient-centric information such as predicting likely loss of economically impactful abilities that are not completely dependent upon EDSS nor currently obtained in the course of traditional MS care or clinical trials. “This objective approach might provide a pathway towards actionable change by objectively monitoring disease progression in a way that EDSS and MRI are unable and that will likely impact therapy choice as well as timing of disease-modifying treatment change,” said Mark Gudesblatt, MD, Medical Director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Islip, New York, on behalf of his coauthors. “This approach can be incorporated into routine care and also can be utilized to easily and quantitatively track examiner independent multidomain cognitive impact longitudinally in a patient-centric manner in people with MS to perhaps improve care outcomes and reduce economic costs that accompany such increased disease burden.”
MS, which is usually characterized by relapses and progression, is traditionally measured by relapse rate reduction, changes in EDSS, and MRI findings. “EDSS change is primarily driven by physical findings or walking impairment, neither of which accounts for cognitive impact or reserve or accumulation of cognitive impairment,” Dr. Gudesblatt said. Cognitive impairment, he added, is not typically quantified or tracked in patients with MS in routine care or clinical trials. EDSS is also insensitive to the degree or types of cognitive impairment. Cognitive impairment, Dr. Gudesblatt and colleagues posit, impacts economically important abilities (eg, employment, ability to drive, and freedom from falls for both simple and complex daily activities) that are not addressed by traditional metrics.
Another layer of complexity is treatment choice. There are multiple available disease-modifying therapies of varied routes, dosing frequency, and efficacy. This makes individual treatment choice and timing of disease-modifying therapy change problematic. “A patient-centric objective analysis of disease trajectory and loss of economically important milestones relating to predictive loss of ability can supplement and perhaps improve alternative approaches to guide treatment choice, change, and timing,” Dr. Gudesblatt said.
An objective, quantitative, patient-centric, and granular EDSS-independent approach of likely disability trajectory might improve decision making regarding disease-modifying therapy choice and timing of change, offer a path to compare outcome measures across clinical trials, and possibly provide an opportunity to preempt the appearance of important disabilities that result in significantly increased cost of care and reduced quality of life. “Objective comprehensive analytics documenting unseen disease impact and change offer unique opportunities to improve care,” Dr. Gudesblatt said.
Toward this end, Dr. Gudesblatt and colleagues conducted a cross-sectional review of a prospective digital MS registry obtained in the course of routine care utilizing standardized computerized cognitive testing (NeuroTrax) to evaluate the relationship of cognitive impairment to disability. Cognitive impairment was defined as number of cognitive domains impaired (CDI) more than one standard deviation from age/education normal. Disability domains assessed were unemployment, loss of driving, and freedom from falls. Patients with an EDSS of less than 6 were included in the study cohort (ie, no one was included who was disabled to the point of requiring a cane to ambulate).
The researchers found that increasing accumulated number of CDI in patients with MS and an EDSS less than 6 is associated with likely progressive loss of:
- Employment (n = 543, CDI-0 = 61%, CDI-1 = 50%, CDI-2 = 43%, CDI-3 = 32%)
- Driving (n = 115, CDI-0 = 100%, CDI-1 = 66%, CDI-2 = 53%, CDI-3 = 21%)
- Freedom from falls (n = 159) for simple daily activities (CDI-0 = 77%, CDI-1 = 65%, CDI-2 = 37%, CDI-3 = 39%) and reduced freedom from falls for complex daily activities (CDI-0 = 72%, CDI-1 = 58%, CDI-2 = 36%, CDI-3 = 33%).
Increased risk of falls and reduced likelihood of employment and driving all represent significant impact on quality of life and result in increased economic burden and long-term costs of the disease, Dr. Gudesblatt and colleagues said.
PARIS—Tracking disease impact in patients with multiple sclerosis (MS) by predictable loss of economically important milestones trajectory, beyond what can be documented by EDSS, MRI, or apparent or reported relapse, can be accomplished by use of objective multidomain cognitive testing, according to a report presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. Such a strategy can provide patient-centric information such as predicting likely loss of economically impactful abilities that are not completely dependent upon EDSS nor currently obtained in the course of traditional MS care or clinical trials. “This objective approach might provide a pathway towards actionable change by objectively monitoring disease progression in a way that EDSS and MRI are unable and that will likely impact therapy choice as well as timing of disease-modifying treatment change,” said Mark Gudesblatt, MD, Medical Director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Islip, New York, on behalf of his coauthors. “This approach can be incorporated into routine care and also can be utilized to easily and quantitatively track examiner independent multidomain cognitive impact longitudinally in a patient-centric manner in people with MS to perhaps improve care outcomes and reduce economic costs that accompany such increased disease burden.”
MS, which is usually characterized by relapses and progression, is traditionally measured by relapse rate reduction, changes in EDSS, and MRI findings. “EDSS change is primarily driven by physical findings or walking impairment, neither of which accounts for cognitive impact or reserve or accumulation of cognitive impairment,” Dr. Gudesblatt said. Cognitive impairment, he added, is not typically quantified or tracked in patients with MS in routine care or clinical trials. EDSS is also insensitive to the degree or types of cognitive impairment. Cognitive impairment, Dr. Gudesblatt and colleagues posit, impacts economically important abilities (eg, employment, ability to drive, and freedom from falls for both simple and complex daily activities) that are not addressed by traditional metrics.
Another layer of complexity is treatment choice. There are multiple available disease-modifying therapies of varied routes, dosing frequency, and efficacy. This makes individual treatment choice and timing of disease-modifying therapy change problematic. “A patient-centric objective analysis of disease trajectory and loss of economically important milestones relating to predictive loss of ability can supplement and perhaps improve alternative approaches to guide treatment choice, change, and timing,” Dr. Gudesblatt said.
An objective, quantitative, patient-centric, and granular EDSS-independent approach of likely disability trajectory might improve decision making regarding disease-modifying therapy choice and timing of change, offer a path to compare outcome measures across clinical trials, and possibly provide an opportunity to preempt the appearance of important disabilities that result in significantly increased cost of care and reduced quality of life. “Objective comprehensive analytics documenting unseen disease impact and change offer unique opportunities to improve care,” Dr. Gudesblatt said.
Toward this end, Dr. Gudesblatt and colleagues conducted a cross-sectional review of a prospective digital MS registry obtained in the course of routine care utilizing standardized computerized cognitive testing (NeuroTrax) to evaluate the relationship of cognitive impairment to disability. Cognitive impairment was defined as number of cognitive domains impaired (CDI) more than one standard deviation from age/education normal. Disability domains assessed were unemployment, loss of driving, and freedom from falls. Patients with an EDSS of less than 6 were included in the study cohort (ie, no one was included who was disabled to the point of requiring a cane to ambulate).
The researchers found that increasing accumulated number of CDI in patients with MS and an EDSS less than 6 is associated with likely progressive loss of:
- Employment (n = 543, CDI-0 = 61%, CDI-1 = 50%, CDI-2 = 43%, CDI-3 = 32%)
- Driving (n = 115, CDI-0 = 100%, CDI-1 = 66%, CDI-2 = 53%, CDI-3 = 21%)
- Freedom from falls (n = 159) for simple daily activities (CDI-0 = 77%, CDI-1 = 65%, CDI-2 = 37%, CDI-3 = 39%) and reduced freedom from falls for complex daily activities (CDI-0 = 72%, CDI-1 = 58%, CDI-2 = 36%, CDI-3 = 33%).
Increased risk of falls and reduced likelihood of employment and driving all represent significant impact on quality of life and result in increased economic burden and long-term costs of the disease, Dr. Gudesblatt and colleagues said.
PARIS—Tracking disease impact in patients with multiple sclerosis (MS) by predictable loss of economically important milestones trajectory, beyond what can be documented by EDSS, MRI, or apparent or reported relapse, can be accomplished by use of objective multidomain cognitive testing, according to a report presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. Such a strategy can provide patient-centric information such as predicting likely loss of economically impactful abilities that are not completely dependent upon EDSS nor currently obtained in the course of traditional MS care or clinical trials. “This objective approach might provide a pathway towards actionable change by objectively monitoring disease progression in a way that EDSS and MRI are unable and that will likely impact therapy choice as well as timing of disease-modifying treatment change,” said Mark Gudesblatt, MD, Medical Director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Islip, New York, on behalf of his coauthors. “This approach can be incorporated into routine care and also can be utilized to easily and quantitatively track examiner independent multidomain cognitive impact longitudinally in a patient-centric manner in people with MS to perhaps improve care outcomes and reduce economic costs that accompany such increased disease burden.”
MS, which is usually characterized by relapses and progression, is traditionally measured by relapse rate reduction, changes in EDSS, and MRI findings. “EDSS change is primarily driven by physical findings or walking impairment, neither of which accounts for cognitive impact or reserve or accumulation of cognitive impairment,” Dr. Gudesblatt said. Cognitive impairment, he added, is not typically quantified or tracked in patients with MS in routine care or clinical trials. EDSS is also insensitive to the degree or types of cognitive impairment. Cognitive impairment, Dr. Gudesblatt and colleagues posit, impacts economically important abilities (eg, employment, ability to drive, and freedom from falls for both simple and complex daily activities) that are not addressed by traditional metrics.
Another layer of complexity is treatment choice. There are multiple available disease-modifying therapies of varied routes, dosing frequency, and efficacy. This makes individual treatment choice and timing of disease-modifying therapy change problematic. “A patient-centric objective analysis of disease trajectory and loss of economically important milestones relating to predictive loss of ability can supplement and perhaps improve alternative approaches to guide treatment choice, change, and timing,” Dr. Gudesblatt said.
An objective, quantitative, patient-centric, and granular EDSS-independent approach of likely disability trajectory might improve decision making regarding disease-modifying therapy choice and timing of change, offer a path to compare outcome measures across clinical trials, and possibly provide an opportunity to preempt the appearance of important disabilities that result in significantly increased cost of care and reduced quality of life. “Objective comprehensive analytics documenting unseen disease impact and change offer unique opportunities to improve care,” Dr. Gudesblatt said.
Toward this end, Dr. Gudesblatt and colleagues conducted a cross-sectional review of a prospective digital MS registry obtained in the course of routine care utilizing standardized computerized cognitive testing (NeuroTrax) to evaluate the relationship of cognitive impairment to disability. Cognitive impairment was defined as number of cognitive domains impaired (CDI) more than one standard deviation from age/education normal. Disability domains assessed were unemployment, loss of driving, and freedom from falls. Patients with an EDSS of less than 6 were included in the study cohort (ie, no one was included who was disabled to the point of requiring a cane to ambulate).
The researchers found that increasing accumulated number of CDI in patients with MS and an EDSS less than 6 is associated with likely progressive loss of:
- Employment (n = 543, CDI-0 = 61%, CDI-1 = 50%, CDI-2 = 43%, CDI-3 = 32%)
- Driving (n = 115, CDI-0 = 100%, CDI-1 = 66%, CDI-2 = 53%, CDI-3 = 21%)
- Freedom from falls (n = 159) for simple daily activities (CDI-0 = 77%, CDI-1 = 65%, CDI-2 = 37%, CDI-3 = 39%) and reduced freedom from falls for complex daily activities (CDI-0 = 72%, CDI-1 = 58%, CDI-2 = 36%, CDI-3 = 33%).
Increased risk of falls and reduced likelihood of employment and driving all represent significant impact on quality of life and result in increased economic burden and long-term costs of the disease, Dr. Gudesblatt and colleagues said.













