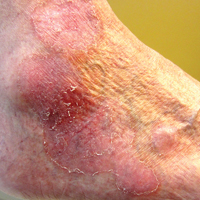
User login
Diversity in Dermatology: A Society Devoted to Skin of Color
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
Practice Points
- The mission of the Skin of Color Society (SOCS) is to improve education of young dermatologists relevant to skin of color patients.
- Educational resources on many different diseases important to patients with skin of color are available to patients and providers on the SOCS website.
Restoring the promise of (really) meaningful use
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
When we started publishing the EHR Report several years ago, our very first column was a brief overview of a new federal incentive program known as Meaningful Use. At that time, the prospect of receiving thousands of dollars to adopt an electronic health record seemed exciting, and our dream of health care’s digital future appeared to be coming true.
Best of all, we as physicians would be paid to simply embrace it!
Unfortunately, it wasn’t long before that dream (for many at least) devolved into a nightmare. Electronic health records hadn’t been designed to fit into physicians’ long-established work flows, and just weren’t up to the challenge of increasing efficiency. In fact, EHRs quickly became virtual taskmasters, leaving physicians mired in a sea of clicks and slow-moving screens.
Frankly speaking, Meaningful Use hasn’t lived up to its promises. With measures obligating users to fill in a myriad of check-boxes and document often irrelevant information, the program has seemed less like an incentive and more like a penance.
To top it off, the all-or-nothing requirement has meant that – after a year of hard work – providers missing even one goal receive no payments at all, and instead are assessed financial penalties!
All of this has appropriately led physicians to become jaded – not excited – about the digital future.
Thankfully, there is reason for hope: 2017 marks the end of Meaningful Use under Medicare.
What’s new for 2017?
MACRA has a much grander scope and sets an even loftier goal: transforming care delivery to achieve better value and ultimately healthier patients.
Now, in case you’re not already confused by the number of programs cited above, there is one more we need to mention to explain the future of EHR incentives: the Merit-based Incentive Payment System, or MIPS, one of two tracks in the Quality Payment Program.
The majority of Medicare providers will choose this track, which focuses on four major components to determine reimbursement incentives: quality, improvement activities, advancing care information, and cost.
Depending on performance in each of these areas, participants will see a variable payment adjustment (upward or downward) in subsequent years (this is a percentage of Medicare payments that increases annually, beginning with a possible +/– 4% in 2019, to a maximum of +/– 9% in 2022).
Providers under MIPS who choose to attest for this year can select from three levels of participation:
1. Test: submission of only a minimal amount of 2017 data (such as one or two measures) to avoid penalty.
2. Partial: submission of 90 days’ worth of data, which may result in a neutral or positive payment adjustment (and may even earn the max adjustment).
3. Full: submission of a full year of data.
Here’s an example of how this will work: A provider who attests in March 2018 for the full 2017 year and does really well could see up to a 4% incentive bonus on Medicare payments in 2019. A provider who chooses not to attest would receive a penalty of 4%.
It’s worth noting here that MIPS expands upon the inclusion criteria set for Meaningful Use under Medicare. Medicare Part B clinicians are eligible to participate if they bill $30,000 in charges and see at least 100 Medicare patients annually. MIPS also broadens the list of eligible provider types. Physicians, nurse practitioners, physician assistants, clinical nurse specialists, and certified registered nurse anesthetists are all able to attest.
Advancing Care Information
Under MIPS, Meaningful Use is replaced by an initiative called Advancing Care Information, or ACI. In this new incarnation, there are fewer required measures, and they are much less onerous than they were under the former program.
Also, there are a number of optional measures. A provider may choose to attest to these nonrequired metrics to improve his or her chances of achieving the maximum incentive, but it isn’t necessary. There are also bonus measures involving public health registry reporting. These are optional but a sure bet to increase incentives. In all, the ACI component composes 25% of a provider’s final MIPS score.
For 2017, participants are able to choose one of two tracks in the ACI program, depending on their EHR’s certification year. (If you are confused by this or don’t know the status of your product, check with your vendor or go to https://chpl.healthit.gov to figure it out).
Providers with technology certified to the 2015 edition (or a combination of technologies from the 2014 and 2015 editions) can fully attest to the ACI objectives and measures or elect to use the transition objectives and measures. Those with 2014 edition software must choose the transition measures.
We will cover the specific measures in a future column, but for now we’ll note that both tracks are very similar and focus on protecting patient data, encouraging patient access to their own records, and sharing information electronically with other providers.
Rekindling the dream
We are certain that changing legislation won’t solve all of the problems inherent in current EHR systems, but we are always encouraged by any attempt to reduce the documentation burden on physicians. By eschewing thresholds, eliminating the all-or-nothing requirement, and reducing the number of required measures, the ACI program does seem to shift the focus away from volume and toward value.
That alone has the potential to restore our hope of a brighter future, and make our use of electronic health records significantly more meaningful.
Note: To learn more about Quality Payment Program and MIPS, we highly recommend an online resource published by the Centers for Medicare & Medicaid Services that is easy to follow and is full of useful information. It can be found at https://qpp.cms.gov.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Early-stage HL patients fare well 10 years after lower-intensity regimens
Lower-intensity radiation regimens for patients with early-stage Hodgkin lymphoma (HL) did not shorten progression-free survival (PFS), according to a long-term analysis. Further, for patients with unfavorable early-stage disease, a more intense chemotherapy or radiation regimen conferred no survival benefit.
The German Hodgkin Study Group included patients with early-stage HL who had both early-stage favorable HL and early-stage unfavorable HL. Stephanie Sasse, MD, and her study group colleagues published long-term follow-up findings from multiple trials, conducted from 1993 to 2003, that evaluated risk-adapted treatment strategies to reduce radiation field size and chemotherapy intensity, “aiming at achieving sufficient tumor control while potentially reducing treatment-associated toxicity,” wrote Dr. Sasse and her colleagues of the University Hospital of Cologne (Ger.) (J Clin Oncol. 2017 Apr 18. doi: JCO2016709410).
Trials in favorable HL
Of the 627 patients in the HD7 trial in patients with favorable HL, combined-modality therapy resulted in better rates of PFS (73%) over a 15-year period, compared with extended-field radiotherapy (RT) alone (52%) (hazard ratio, 0.5; 95% confidence interval, 0.3-0.6; P less than 0.001). Another study, called HD10, was in early-stage favorable HL patients. It compared a lower-intensity regimen of two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus 20 Gy involved-field RT with a four-cycle ABVD regimen combined with 30 Gy involved-field RT. The 1,190-patient study achieved a median follow-up of 98 months, finding that the less-intense regimen was not inferior with an identical 10-year PFS of 87% in both arms (HR 1.0; 95% CI 0.6-1.5). Overall survival (OS) was nearly identical as well, at 94% in each arm (HR 0.9; 95% CI, 0.5-1.6).
Both trials HD7 and HD10 tracked the incidence of secondary neoplasias and detected no significant differences between groups, though there was a nonsignificant trend toward more secondary neoplasias for the HD7 patients who received extended-field radiotherapy. These analyses “strongly support the current risk-adapted treatment strategy in early-stage favorable HL,” wrote Dr. Sasse and her coinvestigators.
Trials in unfavorable HL
The HD8 trial enrolled 1,064 patients and followed them for a median 153 months to compare the efficacy of involved-field RT with extended-field RT, finding involved-field RT noninferior for PFS (HR, 1.0; 95% CI, 0.8-1.2). However, the overall 15-year PFS rate of 74% and OS rate of 82% “leave room for improvement,” said the investigators.
Finally, trial HD11 compared two different chemotherapy regimens and two different radiation doses. Patients received four cycles of either ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone at baseline dosage (BEACOPPbaseline), followed by 20 or 30 Gy involved-field RT. The study, which followed 1,395 patients for a median of 106 months, had a 2x2 factorial design.
Following the HD11 cohort longitudinally showed that BEACOPPbaseline did not confer a PFS advantage over ABVD for patients receiving the 30 Gy RT regimen (HR 1.1; 95% CI, 0.7-1.5). Nor did patients who received 20 Gy RT have significantly longer PFS with the more intense BEACOPPbaseline chemotherapy regimen (HR 0.8; 95% CI, 0.6-1.1).
Overall survival and the incidence of secondary neoplasias did not differ between trial arms in HD11, said Dr. Sasse and her coinvestigators.
To further explore whether more intense chemotherapy might result in better PFS rates for patients with early-stage unfavorable HL, Dr. Sasse and her colleagues are following long-term results from more recent trial, HD14, that combined two cycles of BEACOPPescalated and two cycles of ABVD. More short-term toxicity was seen, but patients in this trial arm have significantly better 5-year PFS rates than do those receiving four cycles of ABVD. “The improved tumor control is a relevant outcome parameter for patients,” wrote Dr. Sasse and her colleagues.
The investigators are reserving judgment about whether more radiation exposure and higher doses of alkylating agents and etoposide may eventually result in higher rates of secondary neoplasms. “Subsequent analyses with even longer follow-up will have to confirm that the reduction of RT field size or dose indeed translates into a reduced risk of [secondary neoplasms],” they wrote.
Several of the authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
[email protected]
On Twitter @karioakes
Lower-intensity radiation regimens for patients with early-stage Hodgkin lymphoma (HL) did not shorten progression-free survival (PFS), according to a long-term analysis. Further, for patients with unfavorable early-stage disease, a more intense chemotherapy or radiation regimen conferred no survival benefit.
The German Hodgkin Study Group included patients with early-stage HL who had both early-stage favorable HL and early-stage unfavorable HL. Stephanie Sasse, MD, and her study group colleagues published long-term follow-up findings from multiple trials, conducted from 1993 to 2003, that evaluated risk-adapted treatment strategies to reduce radiation field size and chemotherapy intensity, “aiming at achieving sufficient tumor control while potentially reducing treatment-associated toxicity,” wrote Dr. Sasse and her colleagues of the University Hospital of Cologne (Ger.) (J Clin Oncol. 2017 Apr 18. doi: JCO2016709410).
Trials in favorable HL
Of the 627 patients in the HD7 trial in patients with favorable HL, combined-modality therapy resulted in better rates of PFS (73%) over a 15-year period, compared with extended-field radiotherapy (RT) alone (52%) (hazard ratio, 0.5; 95% confidence interval, 0.3-0.6; P less than 0.001). Another study, called HD10, was in early-stage favorable HL patients. It compared a lower-intensity regimen of two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus 20 Gy involved-field RT with a four-cycle ABVD regimen combined with 30 Gy involved-field RT. The 1,190-patient study achieved a median follow-up of 98 months, finding that the less-intense regimen was not inferior with an identical 10-year PFS of 87% in both arms (HR 1.0; 95% CI 0.6-1.5). Overall survival (OS) was nearly identical as well, at 94% in each arm (HR 0.9; 95% CI, 0.5-1.6).
Both trials HD7 and HD10 tracked the incidence of secondary neoplasias and detected no significant differences between groups, though there was a nonsignificant trend toward more secondary neoplasias for the HD7 patients who received extended-field radiotherapy. These analyses “strongly support the current risk-adapted treatment strategy in early-stage favorable HL,” wrote Dr. Sasse and her coinvestigators.
Trials in unfavorable HL
The HD8 trial enrolled 1,064 patients and followed them for a median 153 months to compare the efficacy of involved-field RT with extended-field RT, finding involved-field RT noninferior for PFS (HR, 1.0; 95% CI, 0.8-1.2). However, the overall 15-year PFS rate of 74% and OS rate of 82% “leave room for improvement,” said the investigators.
Finally, trial HD11 compared two different chemotherapy regimens and two different radiation doses. Patients received four cycles of either ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone at baseline dosage (BEACOPPbaseline), followed by 20 or 30 Gy involved-field RT. The study, which followed 1,395 patients for a median of 106 months, had a 2x2 factorial design.
Following the HD11 cohort longitudinally showed that BEACOPPbaseline did not confer a PFS advantage over ABVD for patients receiving the 30 Gy RT regimen (HR 1.1; 95% CI, 0.7-1.5). Nor did patients who received 20 Gy RT have significantly longer PFS with the more intense BEACOPPbaseline chemotherapy regimen (HR 0.8; 95% CI, 0.6-1.1).
Overall survival and the incidence of secondary neoplasias did not differ between trial arms in HD11, said Dr. Sasse and her coinvestigators.
To further explore whether more intense chemotherapy might result in better PFS rates for patients with early-stage unfavorable HL, Dr. Sasse and her colleagues are following long-term results from more recent trial, HD14, that combined two cycles of BEACOPPescalated and two cycles of ABVD. More short-term toxicity was seen, but patients in this trial arm have significantly better 5-year PFS rates than do those receiving four cycles of ABVD. “The improved tumor control is a relevant outcome parameter for patients,” wrote Dr. Sasse and her colleagues.
The investigators are reserving judgment about whether more radiation exposure and higher doses of alkylating agents and etoposide may eventually result in higher rates of secondary neoplasms. “Subsequent analyses with even longer follow-up will have to confirm that the reduction of RT field size or dose indeed translates into a reduced risk of [secondary neoplasms],” they wrote.
Several of the authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
[email protected]
On Twitter @karioakes
Lower-intensity radiation regimens for patients with early-stage Hodgkin lymphoma (HL) did not shorten progression-free survival (PFS), according to a long-term analysis. Further, for patients with unfavorable early-stage disease, a more intense chemotherapy or radiation regimen conferred no survival benefit.
The German Hodgkin Study Group included patients with early-stage HL who had both early-stage favorable HL and early-stage unfavorable HL. Stephanie Sasse, MD, and her study group colleagues published long-term follow-up findings from multiple trials, conducted from 1993 to 2003, that evaluated risk-adapted treatment strategies to reduce radiation field size and chemotherapy intensity, “aiming at achieving sufficient tumor control while potentially reducing treatment-associated toxicity,” wrote Dr. Sasse and her colleagues of the University Hospital of Cologne (Ger.) (J Clin Oncol. 2017 Apr 18. doi: JCO2016709410).
Trials in favorable HL
Of the 627 patients in the HD7 trial in patients with favorable HL, combined-modality therapy resulted in better rates of PFS (73%) over a 15-year period, compared with extended-field radiotherapy (RT) alone (52%) (hazard ratio, 0.5; 95% confidence interval, 0.3-0.6; P less than 0.001). Another study, called HD10, was in early-stage favorable HL patients. It compared a lower-intensity regimen of two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) plus 20 Gy involved-field RT with a four-cycle ABVD regimen combined with 30 Gy involved-field RT. The 1,190-patient study achieved a median follow-up of 98 months, finding that the less-intense regimen was not inferior with an identical 10-year PFS of 87% in both arms (HR 1.0; 95% CI 0.6-1.5). Overall survival (OS) was nearly identical as well, at 94% in each arm (HR 0.9; 95% CI, 0.5-1.6).
Both trials HD7 and HD10 tracked the incidence of secondary neoplasias and detected no significant differences between groups, though there was a nonsignificant trend toward more secondary neoplasias for the HD7 patients who received extended-field radiotherapy. These analyses “strongly support the current risk-adapted treatment strategy in early-stage favorable HL,” wrote Dr. Sasse and her coinvestigators.
Trials in unfavorable HL
The HD8 trial enrolled 1,064 patients and followed them for a median 153 months to compare the efficacy of involved-field RT with extended-field RT, finding involved-field RT noninferior for PFS (HR, 1.0; 95% CI, 0.8-1.2). However, the overall 15-year PFS rate of 74% and OS rate of 82% “leave room for improvement,” said the investigators.
Finally, trial HD11 compared two different chemotherapy regimens and two different radiation doses. Patients received four cycles of either ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone at baseline dosage (BEACOPPbaseline), followed by 20 or 30 Gy involved-field RT. The study, which followed 1,395 patients for a median of 106 months, had a 2x2 factorial design.
Following the HD11 cohort longitudinally showed that BEACOPPbaseline did not confer a PFS advantage over ABVD for patients receiving the 30 Gy RT regimen (HR 1.1; 95% CI, 0.7-1.5). Nor did patients who received 20 Gy RT have significantly longer PFS with the more intense BEACOPPbaseline chemotherapy regimen (HR 0.8; 95% CI, 0.6-1.1).
Overall survival and the incidence of secondary neoplasias did not differ between trial arms in HD11, said Dr. Sasse and her coinvestigators.
To further explore whether more intense chemotherapy might result in better PFS rates for patients with early-stage unfavorable HL, Dr. Sasse and her colleagues are following long-term results from more recent trial, HD14, that combined two cycles of BEACOPPescalated and two cycles of ABVD. More short-term toxicity was seen, but patients in this trial arm have significantly better 5-year PFS rates than do those receiving four cycles of ABVD. “The improved tumor control is a relevant outcome parameter for patients,” wrote Dr. Sasse and her colleagues.
The investigators are reserving judgment about whether more radiation exposure and higher doses of alkylating agents and etoposide may eventually result in higher rates of secondary neoplasms. “Subsequent analyses with even longer follow-up will have to confirm that the reduction of RT field size or dose indeed translates into a reduced risk of [secondary neoplasms],” they wrote.
Several of the authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
[email protected]
On Twitter @karioakes
FROM JCO
Key clinical point:
Major finding: Early-stage favorable HL patients had identical progression-free survival, whether they received a more or less intense chemotherapy and radiation regimen (10-year PFS, 87% in each arm).
Data source: Long-term follow-up data from 4,276 patients in four arms of the German Hodgkin Study Group trials.
Disclosures: Several study authors reported multiple relationships with pharmaceutical companies. The study was funded by a grant from the German Cancer Aid.
Biomarkers come up empty for ribociclib targeting
BRUSSELS – The inability of researchers to find a biomarker that could flag a subgroup of breast cancer patients with increased responsiveness to ribociclib plus an aromatase inhibitor was “somewhat disappointing,” Philippe Bedard, MD, said at a breast cancer conference sponsored by the European Society for Medical Oncology.
Many patients who are candidates for this combination treatment, approved by the Food and Drug Administration in March 2017 for postmenopausal women with advanced breast cancer that is hormone receptor positive and HER2 negative, could also do just as well on an aromatase inhibitor alone, said Dr. Bedard, of the Princess Margaret Cancer Centre in Toronto.
“The only validated marker for response to a cyclin-dependent kinase 4/6 inhibitor [such as ribociclib] is hormone receptor positivity. The bottom line is that you can’t target to a more specific subgroup with a biomarker,” Dr. Bedard said in an interview.
The researchers who ran the study collected tumor specimens at baseline from all participants, and Fabrice André, MD reported results from analyses run for seven different biomarkers. The analysis looked at protein levels for three biomarkers – Rb, Ki67, and p16 – in 479, 463, and 405 patients respectively; messenger RNA levels for CCND1, CDKN2A, and ESR1 in 386 patients; and DNA mutational status for the PIKC3A gene in 573 patients. In all seven cases, responsiveness to ribociclib was roughly similar regardless of protein or RNA expression levels or the type of PIKC3A (wild or mutated) the tumor carried, reported Dr. André of the Gustave Roussy Cancer Institute in Villejuif, France. Additional biomarker studies on the MONALEESA-2 specimens are ongoing, he said.
[email protected]
On Twitter @mitchelzoler
Correction, 5/6/17: An earlier version of this article misstated the citation.
BRUSSELS – The inability of researchers to find a biomarker that could flag a subgroup of breast cancer patients with increased responsiveness to ribociclib plus an aromatase inhibitor was “somewhat disappointing,” Philippe Bedard, MD, said at a breast cancer conference sponsored by the European Society for Medical Oncology.
Many patients who are candidates for this combination treatment, approved by the Food and Drug Administration in March 2017 for postmenopausal women with advanced breast cancer that is hormone receptor positive and HER2 negative, could also do just as well on an aromatase inhibitor alone, said Dr. Bedard, of the Princess Margaret Cancer Centre in Toronto.
“The only validated marker for response to a cyclin-dependent kinase 4/6 inhibitor [such as ribociclib] is hormone receptor positivity. The bottom line is that you can’t target to a more specific subgroup with a biomarker,” Dr. Bedard said in an interview.
The researchers who ran the study collected tumor specimens at baseline from all participants, and Fabrice André, MD reported results from analyses run for seven different biomarkers. The analysis looked at protein levels for three biomarkers – Rb, Ki67, and p16 – in 479, 463, and 405 patients respectively; messenger RNA levels for CCND1, CDKN2A, and ESR1 in 386 patients; and DNA mutational status for the PIKC3A gene in 573 patients. In all seven cases, responsiveness to ribociclib was roughly similar regardless of protein or RNA expression levels or the type of PIKC3A (wild or mutated) the tumor carried, reported Dr. André of the Gustave Roussy Cancer Institute in Villejuif, France. Additional biomarker studies on the MONALEESA-2 specimens are ongoing, he said.
[email protected]
On Twitter @mitchelzoler
Correction, 5/6/17: An earlier version of this article misstated the citation.
BRUSSELS – The inability of researchers to find a biomarker that could flag a subgroup of breast cancer patients with increased responsiveness to ribociclib plus an aromatase inhibitor was “somewhat disappointing,” Philippe Bedard, MD, said at a breast cancer conference sponsored by the European Society for Medical Oncology.
Many patients who are candidates for this combination treatment, approved by the Food and Drug Administration in March 2017 for postmenopausal women with advanced breast cancer that is hormone receptor positive and HER2 negative, could also do just as well on an aromatase inhibitor alone, said Dr. Bedard, of the Princess Margaret Cancer Centre in Toronto.
“The only validated marker for response to a cyclin-dependent kinase 4/6 inhibitor [such as ribociclib] is hormone receptor positivity. The bottom line is that you can’t target to a more specific subgroup with a biomarker,” Dr. Bedard said in an interview.
The researchers who ran the study collected tumor specimens at baseline from all participants, and Fabrice André, MD reported results from analyses run for seven different biomarkers. The analysis looked at protein levels for three biomarkers – Rb, Ki67, and p16 – in 479, 463, and 405 patients respectively; messenger RNA levels for CCND1, CDKN2A, and ESR1 in 386 patients; and DNA mutational status for the PIKC3A gene in 573 patients. In all seven cases, responsiveness to ribociclib was roughly similar regardless of protein or RNA expression levels or the type of PIKC3A (wild or mutated) the tumor carried, reported Dr. André of the Gustave Roussy Cancer Institute in Villejuif, France. Additional biomarker studies on the MONALEESA-2 specimens are ongoing, he said.
[email protected]
On Twitter @mitchelzoler
Correction, 5/6/17: An earlier version of this article misstated the citation.
EXPERT ANALYSIS FROM IMPAKT 2017 BREAST CANCER CONFERENCE
Key clinical point:
Major finding: Hazard ratio benefits from ribociclib plus letrozole, compared with letrozole alone, were similar regardless of the levels of seven biomarkers.
Data source: Exploratory analysis of tumor specimens collected from 668 patients with breast cancer enrolled in the MONALEESA-2 study.
Disclosures: MONALEESA-2 was sponsored by Novartis, the company that markets ribociclib (Kisqali). Dr. Bedard has received honoraria from Novartis, Pfizer, Roche, and Sanofi, and he has received research funding from Novartis and several other companies. Dr. André has received research funding from Novartis, AstraZeneca, Lilly, and Pfizer.
Breast density is no reason to perform MRI
LAS VEGAS – In women with higher density (HD) breasts, preoperative MRI revealed more abnormalities than were seen in women with lower density (LD) breasts, but there was no difference in the number of secondary cancers detected or long-term recurrence rates.
Breast density is often cited by radiologists as a reason to conduct a preoperative MRI, but the study suggests that it should not be a driving factor. “It’s a real challenge when our radiologists provide us reports that say, ‘Due to increased density, we recommend MRI,’ because it’s really hard to then disregard that. I think this is very important data,” said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, MN, who moderated the session at the annual meeting of the American Society of Breast Surgeons where the research was presented.
“MRI is a valuable tool, and we’re still trying to figure out who it should be performed in,” said lead author Sarah McLaughlin, MD, of the Mayo Clinic, Jacksonville, FL, in an interview.
The researchers retrospectively analyzed data from 683 women at their institution who underwent preoperative MRI between 2007 and 2011. They grouped them by mammography results into LD (33%; Breast Imaging–Reporting and Data System density, 1 and 2) and HD (67%; BI-RADS density, 3 and 4).
Patients in the HD group more often had ipsilateral MRI findings (42% vs. 31%; P = .005), but ,of those with MRI findings, a similar number of patients in each group needed a second site biopsy (HD 65% vs. LD 67%; P = .78).
In all patients who had an additional MRI finding, the odds of detecting an additional ipsilateral cancer were not statistically significant between HD (32%) and LD (23%; P = .15) patients.
HD patients were also more likely to have abnormalities in the contralateral breast (25% vs. 14%; P = .009), but there were no statistically significant differences in rates of second-site biopsy recommendations or in the percentages of abnormalities that turned out to be cancerous (HD 6% vs. LD 3%; P = 1.0).
Following MRI, 70% of LD patients expressed a preference for breast-conserving surgery, compared with 53% of HD patients (P = .0001).
Over a median 7 years of follow-up, there was no difference in freedom from recurrence rates between the two groups (91% in LD vs. 90% in HD; P = .57).
“To me, it says that you don’t have to order an MRI just because they have cancer in a high density breast. You can feel reassured by your surgical plan and treatment recommendations based on conventional imaging,” said Dr. McLaughlin.
The researchers can’t determine if having an MRI done increased patient worry and potentially led to the higher rate of mastectomies chosen by women in the HD group. “Is that a result of the MRI? I don’t think we can say that, but there’s this whole other discussion piece that goes into it. You definitely see patients who say, ‘But it found these other things, and I’m going to have a mastectomy.’ So, there’s that patient preference and worry piece,” said Dr. McLaughlin.
The study results should offer some reassurance to patients. “There were no differences in local recurrence rates according to density. Maybe the next angle is allaying some of that fear, because the outcomes were the same. It’s really driven more by tumor biology and multimodality therapy,” said Dr. McLaughlin.
The study doesn’t provide the final word on breast density and MRI, according to Dr. Boughey. “I think this is an area that needs to be studied more with a clinical trial. There are several going on in different countries, and this is an area where we need level 1 data. This study does fit with what many other studies have shown, which is that MRI probably doesn’t have as much benefit as patients believe it does, so our role really is to try to help educate patients.”
LAS VEGAS – In women with higher density (HD) breasts, preoperative MRI revealed more abnormalities than were seen in women with lower density (LD) breasts, but there was no difference in the number of secondary cancers detected or long-term recurrence rates.
Breast density is often cited by radiologists as a reason to conduct a preoperative MRI, but the study suggests that it should not be a driving factor. “It’s a real challenge when our radiologists provide us reports that say, ‘Due to increased density, we recommend MRI,’ because it’s really hard to then disregard that. I think this is very important data,” said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, MN, who moderated the session at the annual meeting of the American Society of Breast Surgeons where the research was presented.
“MRI is a valuable tool, and we’re still trying to figure out who it should be performed in,” said lead author Sarah McLaughlin, MD, of the Mayo Clinic, Jacksonville, FL, in an interview.
The researchers retrospectively analyzed data from 683 women at their institution who underwent preoperative MRI between 2007 and 2011. They grouped them by mammography results into LD (33%; Breast Imaging–Reporting and Data System density, 1 and 2) and HD (67%; BI-RADS density, 3 and 4).
Patients in the HD group more often had ipsilateral MRI findings (42% vs. 31%; P = .005), but ,of those with MRI findings, a similar number of patients in each group needed a second site biopsy (HD 65% vs. LD 67%; P = .78).
In all patients who had an additional MRI finding, the odds of detecting an additional ipsilateral cancer were not statistically significant between HD (32%) and LD (23%; P = .15) patients.
HD patients were also more likely to have abnormalities in the contralateral breast (25% vs. 14%; P = .009), but there were no statistically significant differences in rates of second-site biopsy recommendations or in the percentages of abnormalities that turned out to be cancerous (HD 6% vs. LD 3%; P = 1.0).
Following MRI, 70% of LD patients expressed a preference for breast-conserving surgery, compared with 53% of HD patients (P = .0001).
Over a median 7 years of follow-up, there was no difference in freedom from recurrence rates between the two groups (91% in LD vs. 90% in HD; P = .57).
“To me, it says that you don’t have to order an MRI just because they have cancer in a high density breast. You can feel reassured by your surgical plan and treatment recommendations based on conventional imaging,” said Dr. McLaughlin.
The researchers can’t determine if having an MRI done increased patient worry and potentially led to the higher rate of mastectomies chosen by women in the HD group. “Is that a result of the MRI? I don’t think we can say that, but there’s this whole other discussion piece that goes into it. You definitely see patients who say, ‘But it found these other things, and I’m going to have a mastectomy.’ So, there’s that patient preference and worry piece,” said Dr. McLaughlin.
The study results should offer some reassurance to patients. “There were no differences in local recurrence rates according to density. Maybe the next angle is allaying some of that fear, because the outcomes were the same. It’s really driven more by tumor biology and multimodality therapy,” said Dr. McLaughlin.
The study doesn’t provide the final word on breast density and MRI, according to Dr. Boughey. “I think this is an area that needs to be studied more with a clinical trial. There are several going on in different countries, and this is an area where we need level 1 data. This study does fit with what many other studies have shown, which is that MRI probably doesn’t have as much benefit as patients believe it does, so our role really is to try to help educate patients.”
LAS VEGAS – In women with higher density (HD) breasts, preoperative MRI revealed more abnormalities than were seen in women with lower density (LD) breasts, but there was no difference in the number of secondary cancers detected or long-term recurrence rates.
Breast density is often cited by radiologists as a reason to conduct a preoperative MRI, but the study suggests that it should not be a driving factor. “It’s a real challenge when our radiologists provide us reports that say, ‘Due to increased density, we recommend MRI,’ because it’s really hard to then disregard that. I think this is very important data,” said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, MN, who moderated the session at the annual meeting of the American Society of Breast Surgeons where the research was presented.
“MRI is a valuable tool, and we’re still trying to figure out who it should be performed in,” said lead author Sarah McLaughlin, MD, of the Mayo Clinic, Jacksonville, FL, in an interview.
The researchers retrospectively analyzed data from 683 women at their institution who underwent preoperative MRI between 2007 and 2011. They grouped them by mammography results into LD (33%; Breast Imaging–Reporting and Data System density, 1 and 2) and HD (67%; BI-RADS density, 3 and 4).
Patients in the HD group more often had ipsilateral MRI findings (42% vs. 31%; P = .005), but ,of those with MRI findings, a similar number of patients in each group needed a second site biopsy (HD 65% vs. LD 67%; P = .78).
In all patients who had an additional MRI finding, the odds of detecting an additional ipsilateral cancer were not statistically significant between HD (32%) and LD (23%; P = .15) patients.
HD patients were also more likely to have abnormalities in the contralateral breast (25% vs. 14%; P = .009), but there were no statistically significant differences in rates of second-site biopsy recommendations or in the percentages of abnormalities that turned out to be cancerous (HD 6% vs. LD 3%; P = 1.0).
Following MRI, 70% of LD patients expressed a preference for breast-conserving surgery, compared with 53% of HD patients (P = .0001).
Over a median 7 years of follow-up, there was no difference in freedom from recurrence rates between the two groups (91% in LD vs. 90% in HD; P = .57).
“To me, it says that you don’t have to order an MRI just because they have cancer in a high density breast. You can feel reassured by your surgical plan and treatment recommendations based on conventional imaging,” said Dr. McLaughlin.
The researchers can’t determine if having an MRI done increased patient worry and potentially led to the higher rate of mastectomies chosen by women in the HD group. “Is that a result of the MRI? I don’t think we can say that, but there’s this whole other discussion piece that goes into it. You definitely see patients who say, ‘But it found these other things, and I’m going to have a mastectomy.’ So, there’s that patient preference and worry piece,” said Dr. McLaughlin.
The study results should offer some reassurance to patients. “There were no differences in local recurrence rates according to density. Maybe the next angle is allaying some of that fear, because the outcomes were the same. It’s really driven more by tumor biology and multimodality therapy,” said Dr. McLaughlin.
The study doesn’t provide the final word on breast density and MRI, according to Dr. Boughey. “I think this is an area that needs to be studied more with a clinical trial. There are several going on in different countries, and this is an area where we need level 1 data. This study does fit with what many other studies have shown, which is that MRI probably doesn’t have as much benefit as patients believe it does, so our role really is to try to help educate patients.”
AT ASBS 2017
Key clinical point: High breast density is probably not cause enough to order preoperative MRI.
Major finding: Freedom from recurrence rates were 90% in high density and 91% in low.
Data source: Retrospective analysis of 683 women at a single institution.
Disclosures: The study was funded internally. Dr. McLaughlin and Dr. Boughey reported having no relevant financial relationships.
Radiation bests mastectomy for occult breast cancer
LAS VEGAS – Overall survival was better when women with occult breast cancer had axillary lymph node dissection and radiation, instead of mastectomy, in a database review of 934 cases by the University of Maryland Medical Center, Baltimore, the largest review to date of how best to handle the problem.
Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation, according to an analysis of the National Cancer Database from 2004-2013. The results were presented at the annual meeting of the American Society of Breast Surgeons.
ALND plus radiation was independently associated with overall survival on multivariate analysis (HR 0.51, 95% CI 0.32-0.81, P = .004), and was associated with fewer comorbidities, use of chemotherapy, number of positive nodes, and number of nodes examined, compared with mastectomy.
Women treated with ALND plus radiation “had significantly better overall survival than those treated with mastectomy, even after adjusting for other covariates. We believe the study supports overall use of this treatment approach in patients with occult breast cancer,” said lead investigator, Lindsay Hessler, MD, of University of Maryland, Baltimore.
Occult breast cancer – axillary lymph node metastases without clinical or radiologic evidence of a primary breast tumor – is rare and accounted for less than 0.1% of the 2.03 million breast cancer cases in the database. It’s been unclear how best to treat it; most of the previous investigations were small single-center series and case reports.
The only other significant review was smaller, with 750 women in the Surveillance, Epidemiology, and End Results database treated from 1983 to 2006, the “vast majority” before routine use of breast MRI. It showed that “definitive locoregional treatment with either mastectomy or [radiation therapy] improves [overall survival] in patients with occult breast cancer and axillary metastasis who undergo ALND,” but it didn’t suggest which option is best. The National Comprehensive Cancer Network recommends either approach (Cancer. 2010 Sep 1;116[17]:4000-6).
The new University of Maryland findings “confirm that women do not need to have a mastectomy if you can’t find the cancer in their breast. Women do better if you radiate the breast instead of removing it. A lot of academic centers are doing this now, but some people don’t know about it. This needs to be implemented in a more widespread fashion,” said Shelley Hwang, MD, a surgical oncologist at Duke University, Durham, N.C., who moderated Dr. Hessler’s presentation.
Indeed, patients were most likely to be treated with radiation and ALND at an academic center (OR 2.03, 95% CI 1.5-2.74, P less than .001), the only factor on multivariate analysis related to treatment choice.
The review excluded women with only internal mammary lymph node involvement, those with lumpectomies, and women who had less than four nodes recovered on ALND. Mastectomy and radiation patients were similar in nodal stage, race, income, insurance, estrogen receptor status, comorbidities, and year of diagnosis. On pathology, a tumor was found in about a third of the patients who had mastectomies. MRI use and recurrence rates were unavailable in the National Cancer Database.
The findings are subject to all the limits of database reviews, including the possible confounder that women treated at university hospitals might also have had more optimal systemic therapy, as an audience member noted.
The investigators said they had no financial disclosures.
LAS VEGAS – Overall survival was better when women with occult breast cancer had axillary lymph node dissection and radiation, instead of mastectomy, in a database review of 934 cases by the University of Maryland Medical Center, Baltimore, the largest review to date of how best to handle the problem.
Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation, according to an analysis of the National Cancer Database from 2004-2013. The results were presented at the annual meeting of the American Society of Breast Surgeons.
ALND plus radiation was independently associated with overall survival on multivariate analysis (HR 0.51, 95% CI 0.32-0.81, P = .004), and was associated with fewer comorbidities, use of chemotherapy, number of positive nodes, and number of nodes examined, compared with mastectomy.
Women treated with ALND plus radiation “had significantly better overall survival than those treated with mastectomy, even after adjusting for other covariates. We believe the study supports overall use of this treatment approach in patients with occult breast cancer,” said lead investigator, Lindsay Hessler, MD, of University of Maryland, Baltimore.
Occult breast cancer – axillary lymph node metastases without clinical or radiologic evidence of a primary breast tumor – is rare and accounted for less than 0.1% of the 2.03 million breast cancer cases in the database. It’s been unclear how best to treat it; most of the previous investigations were small single-center series and case reports.
The only other significant review was smaller, with 750 women in the Surveillance, Epidemiology, and End Results database treated from 1983 to 2006, the “vast majority” before routine use of breast MRI. It showed that “definitive locoregional treatment with either mastectomy or [radiation therapy] improves [overall survival] in patients with occult breast cancer and axillary metastasis who undergo ALND,” but it didn’t suggest which option is best. The National Comprehensive Cancer Network recommends either approach (Cancer. 2010 Sep 1;116[17]:4000-6).
The new University of Maryland findings “confirm that women do not need to have a mastectomy if you can’t find the cancer in their breast. Women do better if you radiate the breast instead of removing it. A lot of academic centers are doing this now, but some people don’t know about it. This needs to be implemented in a more widespread fashion,” said Shelley Hwang, MD, a surgical oncologist at Duke University, Durham, N.C., who moderated Dr. Hessler’s presentation.
Indeed, patients were most likely to be treated with radiation and ALND at an academic center (OR 2.03, 95% CI 1.5-2.74, P less than .001), the only factor on multivariate analysis related to treatment choice.
The review excluded women with only internal mammary lymph node involvement, those with lumpectomies, and women who had less than four nodes recovered on ALND. Mastectomy and radiation patients were similar in nodal stage, race, income, insurance, estrogen receptor status, comorbidities, and year of diagnosis. On pathology, a tumor was found in about a third of the patients who had mastectomies. MRI use and recurrence rates were unavailable in the National Cancer Database.
The findings are subject to all the limits of database reviews, including the possible confounder that women treated at university hospitals might also have had more optimal systemic therapy, as an audience member noted.
The investigators said they had no financial disclosures.
LAS VEGAS – Overall survival was better when women with occult breast cancer had axillary lymph node dissection and radiation, instead of mastectomy, in a database review of 934 cases by the University of Maryland Medical Center, Baltimore, the largest review to date of how best to handle the problem.
Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation, according to an analysis of the National Cancer Database from 2004-2013. The results were presented at the annual meeting of the American Society of Breast Surgeons.
ALND plus radiation was independently associated with overall survival on multivariate analysis (HR 0.51, 95% CI 0.32-0.81, P = .004), and was associated with fewer comorbidities, use of chemotherapy, number of positive nodes, and number of nodes examined, compared with mastectomy.
Women treated with ALND plus radiation “had significantly better overall survival than those treated with mastectomy, even after adjusting for other covariates. We believe the study supports overall use of this treatment approach in patients with occult breast cancer,” said lead investigator, Lindsay Hessler, MD, of University of Maryland, Baltimore.
Occult breast cancer – axillary lymph node metastases without clinical or radiologic evidence of a primary breast tumor – is rare and accounted for less than 0.1% of the 2.03 million breast cancer cases in the database. It’s been unclear how best to treat it; most of the previous investigations were small single-center series and case reports.
The only other significant review was smaller, with 750 women in the Surveillance, Epidemiology, and End Results database treated from 1983 to 2006, the “vast majority” before routine use of breast MRI. It showed that “definitive locoregional treatment with either mastectomy or [radiation therapy] improves [overall survival] in patients with occult breast cancer and axillary metastasis who undergo ALND,” but it didn’t suggest which option is best. The National Comprehensive Cancer Network recommends either approach (Cancer. 2010 Sep 1;116[17]:4000-6).
The new University of Maryland findings “confirm that women do not need to have a mastectomy if you can’t find the cancer in their breast. Women do better if you radiate the breast instead of removing it. A lot of academic centers are doing this now, but some people don’t know about it. This needs to be implemented in a more widespread fashion,” said Shelley Hwang, MD, a surgical oncologist at Duke University, Durham, N.C., who moderated Dr. Hessler’s presentation.
Indeed, patients were most likely to be treated with radiation and ALND at an academic center (OR 2.03, 95% CI 1.5-2.74, P less than .001), the only factor on multivariate analysis related to treatment choice.
The review excluded women with only internal mammary lymph node involvement, those with lumpectomies, and women who had less than four nodes recovered on ALND. Mastectomy and radiation patients were similar in nodal stage, race, income, insurance, estrogen receptor status, comorbidities, and year of diagnosis. On pathology, a tumor was found in about a third of the patients who had mastectomies. MRI use and recurrence rates were unavailable in the National Cancer Database.
The findings are subject to all the limits of database reviews, including the possible confounder that women treated at university hospitals might also have had more optimal systemic therapy, as an audience member noted.
The investigators said they had no financial disclosures.
AT ASBS 2017
Key clinical point:
Major finding: Five- and 10-year overall survival was 90.8% and 84.8%, respectively, among the 342 women treated with axillary lymph node dissection (ALND) plus adjuvant radiation, versus 80.0% and 69.8% among the 592 who had ALND and mastectomies, plus or minus radiation.
Data source: Review of 934 cases in the National Cancer Database.
Disclosures: The investigators said they had no financial disclosures.
Novel agent varlilumab shows activity in RCC
A novel, first-in-class, agonist anti-CD27 monoclonal antibody was found to be clinically and biologically active, according to early findings published in the Journal of Clinical Oncology.
In this phase I first-in-human study of varlilumab, doses at 0.1 to 10 mg/kg were well tolerated by patients with advanced solid tumors. In addition, antitumor activity was also observed. One heavily pretreated patient with stage IV disease renal cell carcinoma (RCC) experienced durable and significant tumor regression, with 78% tumor shrinkage and a partial response that persisted for 2.3 years without additional anticancer therapy, reported Howard A. Burris, MD, of the Sarah Cannon Research Institute, Tennessee Oncology, Nashville, and his colleagues (J Clin Oncol. 2017 May 2. doi: 10.1200/JCO.2016.70.1508).
In addition, eight patients achieved stable disease for more than 3 months while on the study.
“This phase I study of varlilumab provides proof of concept and a rationale for further study in combination with immunotherapies and traditional therapies,” wrote Dr. Burris and his colleagues. “Therapy that targets multiple nonredundant pathways that regulate immune responses may be synergistic and enhance antitumor immune responses.”
The study was conducted to assess the safety, pharmacokinetics, pharmacodynamics, and activity of varlilumab when used as a single agent in patients with advanced solid tumors.
The entire cohort included 56 patients enrolled at nine centers, and the dose escalation component included 25 patients. The expansion part of the trial included 16 patients with melanoma and 15 patients with RCC.
The most common cancer type in the dose-escalation phase was colorectal cancer (40%) followed by melanoma (28%). All patients had stage IV disease and, in general, were heavily pretreated.
A median of 4 doses was administered to the cohort (1-21), and, in the dose escalation phase, the 10 mg/kg was reached without identifying a maximum tolerated dose. Only one patient experienced a dose limiting toxicity, which was grade 3 asymptomatic hyponatremia (129 mmol/L) that occurred at the 1.0-mg/kg dose level.
A total of eight patients achieved stable disease, including four with RCC (duration of stable disease: 5.3, 5.6, 9.3, and 47.3 months), three with melanoma (3.8, 7.3, and 11.5 months), and one patient with colorectal adenocarcinoma (5.7 months).
A novel, first-in-class, agonist anti-CD27 monoclonal antibody was found to be clinically and biologically active, according to early findings published in the Journal of Clinical Oncology.
In this phase I first-in-human study of varlilumab, doses at 0.1 to 10 mg/kg were well tolerated by patients with advanced solid tumors. In addition, antitumor activity was also observed. One heavily pretreated patient with stage IV disease renal cell carcinoma (RCC) experienced durable and significant tumor regression, with 78% tumor shrinkage and a partial response that persisted for 2.3 years without additional anticancer therapy, reported Howard A. Burris, MD, of the Sarah Cannon Research Institute, Tennessee Oncology, Nashville, and his colleagues (J Clin Oncol. 2017 May 2. doi: 10.1200/JCO.2016.70.1508).
In addition, eight patients achieved stable disease for more than 3 months while on the study.
“This phase I study of varlilumab provides proof of concept and a rationale for further study in combination with immunotherapies and traditional therapies,” wrote Dr. Burris and his colleagues. “Therapy that targets multiple nonredundant pathways that regulate immune responses may be synergistic and enhance antitumor immune responses.”
The study was conducted to assess the safety, pharmacokinetics, pharmacodynamics, and activity of varlilumab when used as a single agent in patients with advanced solid tumors.
The entire cohort included 56 patients enrolled at nine centers, and the dose escalation component included 25 patients. The expansion part of the trial included 16 patients with melanoma and 15 patients with RCC.
The most common cancer type in the dose-escalation phase was colorectal cancer (40%) followed by melanoma (28%). All patients had stage IV disease and, in general, were heavily pretreated.
A median of 4 doses was administered to the cohort (1-21), and, in the dose escalation phase, the 10 mg/kg was reached without identifying a maximum tolerated dose. Only one patient experienced a dose limiting toxicity, which was grade 3 asymptomatic hyponatremia (129 mmol/L) that occurred at the 1.0-mg/kg dose level.
A total of eight patients achieved stable disease, including four with RCC (duration of stable disease: 5.3, 5.6, 9.3, and 47.3 months), three with melanoma (3.8, 7.3, and 11.5 months), and one patient with colorectal adenocarcinoma (5.7 months).
A novel, first-in-class, agonist anti-CD27 monoclonal antibody was found to be clinically and biologically active, according to early findings published in the Journal of Clinical Oncology.
In this phase I first-in-human study of varlilumab, doses at 0.1 to 10 mg/kg were well tolerated by patients with advanced solid tumors. In addition, antitumor activity was also observed. One heavily pretreated patient with stage IV disease renal cell carcinoma (RCC) experienced durable and significant tumor regression, with 78% tumor shrinkage and a partial response that persisted for 2.3 years without additional anticancer therapy, reported Howard A. Burris, MD, of the Sarah Cannon Research Institute, Tennessee Oncology, Nashville, and his colleagues (J Clin Oncol. 2017 May 2. doi: 10.1200/JCO.2016.70.1508).
In addition, eight patients achieved stable disease for more than 3 months while on the study.
“This phase I study of varlilumab provides proof of concept and a rationale for further study in combination with immunotherapies and traditional therapies,” wrote Dr. Burris and his colleagues. “Therapy that targets multiple nonredundant pathways that regulate immune responses may be synergistic and enhance antitumor immune responses.”
The study was conducted to assess the safety, pharmacokinetics, pharmacodynamics, and activity of varlilumab when used as a single agent in patients with advanced solid tumors.
The entire cohort included 56 patients enrolled at nine centers, and the dose escalation component included 25 patients. The expansion part of the trial included 16 patients with melanoma and 15 patients with RCC.
The most common cancer type in the dose-escalation phase was colorectal cancer (40%) followed by melanoma (28%). All patients had stage IV disease and, in general, were heavily pretreated.
A median of 4 doses was administered to the cohort (1-21), and, in the dose escalation phase, the 10 mg/kg was reached without identifying a maximum tolerated dose. Only one patient experienced a dose limiting toxicity, which was grade 3 asymptomatic hyponatremia (129 mmol/L) that occurred at the 1.0-mg/kg dose level.
A total of eight patients achieved stable disease, including four with RCC (duration of stable disease: 5.3, 5.6, 9.3, and 47.3 months), three with melanoma (3.8, 7.3, and 11.5 months), and one patient with colorectal adenocarcinoma (5.7 months).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A novel, first-in-class, agonist anti-CD27 monoclonal antibody showed activity in solid tumors when used as monotherapy.
Major finding: Two patients with advanced renal cell carcinoma experienced tumor shrinkage and durable responses while eight achieved stable disease.
Data source: Phase I first-in-human study comprising 56 patients with advanced colorectal cancer, renal cell carcinoma, and melanoma.
Disclosures: The study was supported by Celldex Therapeutics. Dr. Burris has no disclosures, and several coauthors have declared relationships with the industry.
Low-histamine diet reduces disease activity in chronic urticaria
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
A low-histamine diet could decrease symptoms and improve the quality of life for people with chronic spontaneous urticaria (CsU), according to Nicola Wagner, MD, of the department of dermatology at the Clinical Center Darmstadt (Germany) GmbH, Darmstadt, and her coauthors.
In their prospective study of 56 patients with a 3-month history of CsU (average 25 months) who followed a low-histamine diet for 3 weeks, 42 (75%) showed improvements in the urticaria activity score (UAS), compared to baseline. In nine patients (16%), disease activity remained the same, and five patients (9%) experienced worsening symptoms.
The primary endpoint, which was at least a three-point improvement in urticaria activity score, was reached by 34 patients (61%). Among these 34 patients, the average reduction in the UAS score during the last 4 days of the diet compared with the 4 days before starting the diet was 8.59 points (P less than .001), and dropped from 9.05 to 4.23 (P = .004) across the entire group (J Eur Acad Dermatol Venereol. 2017 Apr;31[4]:650-5).
The low-histamine diet omitted food such as cheese, preserved meats, strawberries, raspberries, citrus fruit, bananas, kiwis, plums, papaya, and alcohol, and included foods such as dairy, vegetables, fresh meat, eggs, bread, pasta, rice, and certain varieties of fish. “Many patients with CsU complain of worsening of symptoms by consuming histamine-rich food, like red wine or matured cheese, but to the best of our knowledge, until now no studies were available supporting these observations,” the authors wrote.
The low-histamine diet was also associated with an average improvement in Dermatological Life Quality Instrument Questionnaire from baseline of 2.08 points across all participants, while Chronic Urticaria Quality of Life Questionnaire scores improved by an average of 5.46 score points in the 52 patients for whom these evaluations were available.
“Quality of life showed an improvement by a low-histamine diet, which was surprising, because dieting may decrease quality of life,” the authors noted.
There was also a reduction in antihistamine use among participants taking antihistamines at baseline, with 39% (22 of 56) reducing their intake and just over half of these patients (12 of the 22) stopping antihistamines altogether.
The study measured levels of diamine oxidase – the enzyme responsible for disintegrating histamine – both before and after, and saw no significant change across the patient group. However, patients with higher urticaria activity scores at baseline did show a decline in diamine oxidase activity with the diet.
Addressing concerns that these improvements could simply reflect spontaneous remission of the disease, the authors pointed out that a previous study had found a remission rate of just 3.4% over 6 months.
Based on the results, they concluded that a low-histamine diet “might be recommended for a period of 3-4 weeks in CsU patients in order to reduce symptoms and antihistamine intake as well as to improve quality of life.”
The authors had no funding source or conflicts of interest to declare.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: A low-histamine diet for 3-4 weeks may be a simple therapeutic option for patients suffering with chronic spontaneous urticaria (CsU).
Major finding: Three-quarters of patients with CsU showed an improvement in disease activity score after 3 weeks on a low-histamine diet.
Data source: A prospective 3-week study evaluating the impact of a low-histamine diet on 56 patients with CsU.
Disclosures: No conflicts of interest or study funding source were declared.
Recalcitrant Solitary Erythematous Scaly Patch on the Foot
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.
Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
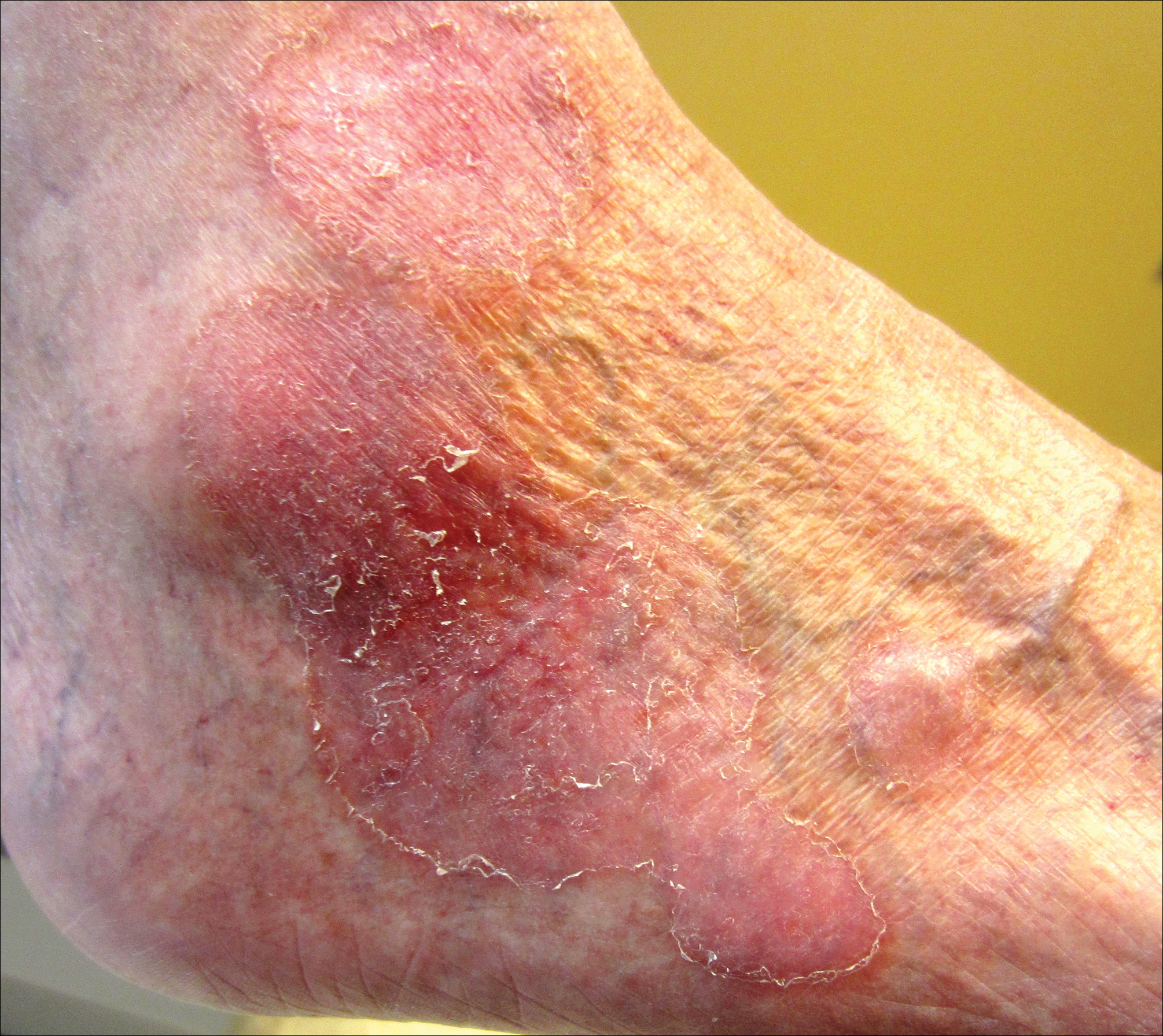
An 80-year-old man with a history of malignant melanoma and squamous cell carcinoma presented to the dermatology clinic with a chronic rash of 20 years' duration on the right ankle that extended to the instep of the right foot. His medical history was notable for hypertension and hyperlipidemia. Family history was unremarkable. The patient described the rash as red and scaly but denied associated pain or pruritus. Over the last 2 to 3 years he had tried treating the affected area with petroleum jelly, topical and oral antifungals, and mild topical steroids with minimal improvement. Complete review of systems was performed and was negative other than some mild constipation. Physical examination revealed an erythematous scaly patch on the dorsal aspect of the right ankle. Potassium hydroxide preparation and fungal culture swab yielded negative results, and a shave biopsy was performed.