User login
Beauty sleep: Sleep deprivation and the skin
There are many, many, short-term and long-term consequences of sleep deprivation. The most clinically apparent ones – swollen, sunken eyes; dark circles; and pale, dehydrated skin – are obvious. However the subclinical consequences are not so obvious. Sleep deprivation affects wound healing, collagen growth, skin hydration, and skin texture. Inflammation is also higher in sleep-deprived patients, causing outbreaks of acne, eczema, psoriasis, and skin allergies.
The reduction of sleep time affects the composition and integrity of the skin. Sleep deprivation increases glucocorticoid production. The elevation of cortisol inhibits fibroblast function and increases matrix metalloproteinases (collagenase, gelatinase). Matrix metalloproteinases accelerate collagen and elastin breakdown, which is essential to skin integrity, and hastens the aging process by increasing wrinkles, decreasing skin thickness, inhibiting growth factors, and decreasing skin elasticity.
Are there treatments to reverse these signs? Yes. Treatments to help increase skin collagen production include microneedling, radiofrequency devices, fractionated lasers, and topical agents such as retinoids. However, we cannot readily reverse the impact inflammatory processes, skin barrier dysfunction, or the disruption of the skin biome has on our skin. Beauty sleep is both necessary and irreplaceable.
References
1. Am J Physiol. 1993 Nov;265(5 Pt 2):R1148-54.
2. Am J Physiol Regul Integr Comp Physiol. 2000 Apr;278(4):R905-16.
3. Am J Physiol Regul Integr Comp Physiol. 2005 Feb;288(2):R374-83.
4. Am J Physiol Regul Integr Comp Physiol. 2007 Jul;293(1):R504-9.
5. Med Hypotheses. 2010 Dec;75(6):535-7.
6. Sleep. 2013 Sep 1;36(9):1355-60.
7. BMJ. 2010 Dec 14;341:c6614.
8. Brain Behav Immun. 2009 Nov;23(8):1089-95.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
There are many, many, short-term and long-term consequences of sleep deprivation. The most clinically apparent ones – swollen, sunken eyes; dark circles; and pale, dehydrated skin – are obvious. However the subclinical consequences are not so obvious. Sleep deprivation affects wound healing, collagen growth, skin hydration, and skin texture. Inflammation is also higher in sleep-deprived patients, causing outbreaks of acne, eczema, psoriasis, and skin allergies.
The reduction of sleep time affects the composition and integrity of the skin. Sleep deprivation increases glucocorticoid production. The elevation of cortisol inhibits fibroblast function and increases matrix metalloproteinases (collagenase, gelatinase). Matrix metalloproteinases accelerate collagen and elastin breakdown, which is essential to skin integrity, and hastens the aging process by increasing wrinkles, decreasing skin thickness, inhibiting growth factors, and decreasing skin elasticity.
Are there treatments to reverse these signs? Yes. Treatments to help increase skin collagen production include microneedling, radiofrequency devices, fractionated lasers, and topical agents such as retinoids. However, we cannot readily reverse the impact inflammatory processes, skin barrier dysfunction, or the disruption of the skin biome has on our skin. Beauty sleep is both necessary and irreplaceable.
References
1. Am J Physiol. 1993 Nov;265(5 Pt 2):R1148-54.
2. Am J Physiol Regul Integr Comp Physiol. 2000 Apr;278(4):R905-16.
3. Am J Physiol Regul Integr Comp Physiol. 2005 Feb;288(2):R374-83.
4. Am J Physiol Regul Integr Comp Physiol. 2007 Jul;293(1):R504-9.
5. Med Hypotheses. 2010 Dec;75(6):535-7.
6. Sleep. 2013 Sep 1;36(9):1355-60.
7. BMJ. 2010 Dec 14;341:c6614.
8. Brain Behav Immun. 2009 Nov;23(8):1089-95.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
There are many, many, short-term and long-term consequences of sleep deprivation. The most clinically apparent ones – swollen, sunken eyes; dark circles; and pale, dehydrated skin – are obvious. However the subclinical consequences are not so obvious. Sleep deprivation affects wound healing, collagen growth, skin hydration, and skin texture. Inflammation is also higher in sleep-deprived patients, causing outbreaks of acne, eczema, psoriasis, and skin allergies.
The reduction of sleep time affects the composition and integrity of the skin. Sleep deprivation increases glucocorticoid production. The elevation of cortisol inhibits fibroblast function and increases matrix metalloproteinases (collagenase, gelatinase). Matrix metalloproteinases accelerate collagen and elastin breakdown, which is essential to skin integrity, and hastens the aging process by increasing wrinkles, decreasing skin thickness, inhibiting growth factors, and decreasing skin elasticity.
Are there treatments to reverse these signs? Yes. Treatments to help increase skin collagen production include microneedling, radiofrequency devices, fractionated lasers, and topical agents such as retinoids. However, we cannot readily reverse the impact inflammatory processes, skin barrier dysfunction, or the disruption of the skin biome has on our skin. Beauty sleep is both necessary and irreplaceable.
References
1. Am J Physiol. 1993 Nov;265(5 Pt 2):R1148-54.
2. Am J Physiol Regul Integr Comp Physiol. 2000 Apr;278(4):R905-16.
3. Am J Physiol Regul Integr Comp Physiol. 2005 Feb;288(2):R374-83.
4. Am J Physiol Regul Integr Comp Physiol. 2007 Jul;293(1):R504-9.
5. Med Hypotheses. 2010 Dec;75(6):535-7.
6. Sleep. 2013 Sep 1;36(9):1355-60.
7. BMJ. 2010 Dec 14;341:c6614.
8. Brain Behav Immun. 2009 Nov;23(8):1089-95.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected].
Audiocast: Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Resources
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Resources
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Resources
Clinical rating scales
The New Opioid Epidemic and the Law of Unintended Consequences
In this issue of EM, EP-toxicologists Rama Rao, MD, and Lewis Nelson, MD, review the salient features of the current opioid epidemic in the United States. The authors differentiate this epidemic from prior patterns of heroin and opioid abuse partly by the clinical features that now make timely diagnosis and treatment in the ED more difficult.
According to the CDC, between 2000 and 2015, the number of opioid overdose deaths in this country quadrupled to half a million, or 91 deaths a day (
The road to the current epidemic began to be paved with good intentions in the late 1990s when, soon after the FDA approved the controlled-release form of oxycodone (Oxycontin), the American Pain Society introduced the phrase “pain as the fifth vital sign.” In 1999, the Department of Veterans Affairs embraced the statement, as did other organizations. The Joint Commission standards for pain management in 2001 stated “pain is assessed in all patients” (all was dropped in 2009) and contained a passing reference to pain as the fifth vital sign. In 2012, CMS added to its ED performance core measures timely pain treatment for long bone fractures, emphasizing parenteral medications.
By 2010, the problems created by emphasizing effective pain management had become evident, and measures began to be introduced to restrict the prescribing and availability of pharmaceutical opioids. The restrictions sent many patients to EDs seeking pain meds. Others sought substitutes on the street and ultimately ended up in EDs as overdoses from very potent synthetics. Many EPs began to limit opioid prescriptions to 3 days for acute painful conditions, though not all patients were able to obtain follow-up appointments with PCPs within that time period.
In April 2016, the Joint Commission issued a statement claiming it was not responsible for “pain as the fifth vital sign” or for suggesting that pain be treated with opioids. In June 2016, the AMA urged dropping “pain-as-the-fifth-vital-sign” policies, and in 2014, CMS modified its core measure emphasis on parenteral medication in the timely treatment of long bone fractures. But the damage has been done, leaving many people requiring help managing their pain and others suffering the consequences of opioid dependence.
EPs must continue to deal with victims of overdoses without denying pain treatment to those with acute, acute-on-chronic, and recurrent pain. Increased use of effective non-opioid pain meds such as NSAIDs may help, although not everyone can tolerate them and there are long-term risks. For large, overcrowded, urban EDs where treatment of pain is not always timely or consistent, 24/7 ED pain management teams working with EPs could be a tremendous asset, just as 24/7 ED pharmacists have proven to be. Until both effective pain treatment and the resultant opioid dependence and overdoses can be successfully addressed, regulatory agencies should deemphasize, without completely eliminating, pain treatment questions in scoring patient satisfaction.
In this issue of EM, EP-toxicologists Rama Rao, MD, and Lewis Nelson, MD, review the salient features of the current opioid epidemic in the United States. The authors differentiate this epidemic from prior patterns of heroin and opioid abuse partly by the clinical features that now make timely diagnosis and treatment in the ED more difficult.
According to the CDC, between 2000 and 2015, the number of opioid overdose deaths in this country quadrupled to half a million, or 91 deaths a day (
The road to the current epidemic began to be paved with good intentions in the late 1990s when, soon after the FDA approved the controlled-release form of oxycodone (Oxycontin), the American Pain Society introduced the phrase “pain as the fifth vital sign.” In 1999, the Department of Veterans Affairs embraced the statement, as did other organizations. The Joint Commission standards for pain management in 2001 stated “pain is assessed in all patients” (all was dropped in 2009) and contained a passing reference to pain as the fifth vital sign. In 2012, CMS added to its ED performance core measures timely pain treatment for long bone fractures, emphasizing parenteral medications.
By 2010, the problems created by emphasizing effective pain management had become evident, and measures began to be introduced to restrict the prescribing and availability of pharmaceutical opioids. The restrictions sent many patients to EDs seeking pain meds. Others sought substitutes on the street and ultimately ended up in EDs as overdoses from very potent synthetics. Many EPs began to limit opioid prescriptions to 3 days for acute painful conditions, though not all patients were able to obtain follow-up appointments with PCPs within that time period.
In April 2016, the Joint Commission issued a statement claiming it was not responsible for “pain as the fifth vital sign” or for suggesting that pain be treated with opioids. In June 2016, the AMA urged dropping “pain-as-the-fifth-vital-sign” policies, and in 2014, CMS modified its core measure emphasis on parenteral medication in the timely treatment of long bone fractures. But the damage has been done, leaving many people requiring help managing their pain and others suffering the consequences of opioid dependence.
EPs must continue to deal with victims of overdoses without denying pain treatment to those with acute, acute-on-chronic, and recurrent pain. Increased use of effective non-opioid pain meds such as NSAIDs may help, although not everyone can tolerate them and there are long-term risks. For large, overcrowded, urban EDs where treatment of pain is not always timely or consistent, 24/7 ED pain management teams working with EPs could be a tremendous asset, just as 24/7 ED pharmacists have proven to be. Until both effective pain treatment and the resultant opioid dependence and overdoses can be successfully addressed, regulatory agencies should deemphasize, without completely eliminating, pain treatment questions in scoring patient satisfaction.
In this issue of EM, EP-toxicologists Rama Rao, MD, and Lewis Nelson, MD, review the salient features of the current opioid epidemic in the United States. The authors differentiate this epidemic from prior patterns of heroin and opioid abuse partly by the clinical features that now make timely diagnosis and treatment in the ED more difficult.
According to the CDC, between 2000 and 2015, the number of opioid overdose deaths in this country quadrupled to half a million, or 91 deaths a day (
The road to the current epidemic began to be paved with good intentions in the late 1990s when, soon after the FDA approved the controlled-release form of oxycodone (Oxycontin), the American Pain Society introduced the phrase “pain as the fifth vital sign.” In 1999, the Department of Veterans Affairs embraced the statement, as did other organizations. The Joint Commission standards for pain management in 2001 stated “pain is assessed in all patients” (all was dropped in 2009) and contained a passing reference to pain as the fifth vital sign. In 2012, CMS added to its ED performance core measures timely pain treatment for long bone fractures, emphasizing parenteral medications.
By 2010, the problems created by emphasizing effective pain management had become evident, and measures began to be introduced to restrict the prescribing and availability of pharmaceutical opioids. The restrictions sent many patients to EDs seeking pain meds. Others sought substitutes on the street and ultimately ended up in EDs as overdoses from very potent synthetics. Many EPs began to limit opioid prescriptions to 3 days for acute painful conditions, though not all patients were able to obtain follow-up appointments with PCPs within that time period.
In April 2016, the Joint Commission issued a statement claiming it was not responsible for “pain as the fifth vital sign” or for suggesting that pain be treated with opioids. In June 2016, the AMA urged dropping “pain-as-the-fifth-vital-sign” policies, and in 2014, CMS modified its core measure emphasis on parenteral medication in the timely treatment of long bone fractures. But the damage has been done, leaving many people requiring help managing their pain and others suffering the consequences of opioid dependence.
EPs must continue to deal with victims of overdoses without denying pain treatment to those with acute, acute-on-chronic, and recurrent pain. Increased use of effective non-opioid pain meds such as NSAIDs may help, although not everyone can tolerate them and there are long-term risks. For large, overcrowded, urban EDs where treatment of pain is not always timely or consistent, 24/7 ED pain management teams working with EPs could be a tremendous asset, just as 24/7 ED pharmacists have proven to be. Until both effective pain treatment and the resultant opioid dependence and overdoses can be successfully addressed, regulatory agencies should deemphasize, without completely eliminating, pain treatment questions in scoring patient satisfaction.
Diagnosis of Severe Acute Lower Gastrointestinal Bleeding with CTA
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
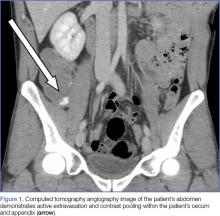
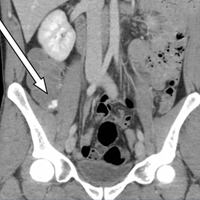
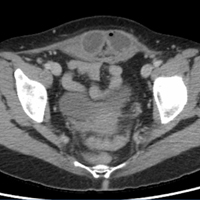
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
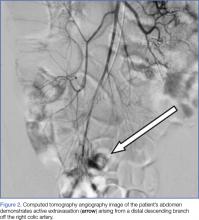
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
Case
A 31-year-old white man presented to the ED with abdominal and rectal pain accompanied by multiple episodes of bloody diarrhea. He stated he had mild rectal pain the previous night but was pain-free and in his usual state of health the morning of his presentation. Approximately 2 hours before presenting to the ED, however, he began experiencing mild stomach pain, then bloody diarrhea which he described as bright red and “filling the toilet bowl with blood.” He had no history of inflammatory bowel disease or other gastrointestinal (GI) disorder, no recent travel, no complaints of nausea or vomiting, and no infectious symptoms. He described a remote history of external hemorrhoids, and review of his family history was significant for multiple paternal relatives with aortic aneurysms. He was not taking any medications and was a nonsmoker with a normal body mass index (24.3 kg/m2).
Upon arrival at the ED, the patient’s vital signs were: heart rate, 112 beats/min; and blood pressure, 139/102 mm Hg; respiratory rate and temperature were normal, as was the patient’s oxygen saturation on room air. Physical examination was notable for no subjective or objective findings of orthostatic hypotension; increased bowel sounds and diffuse mild abdominal tenderness; and no external hemorrhoids, fissures, or rectal tenderness. Laboratory evaluation was significant for hemoglobin (Hgb), 15.0 g/dL; blood urea nitrogen (BUN)-to-creatinine (Cr) ratio, 11.6; and anion gap, 17 mEq/L.
Upon initial presentation, there was some concern for an infection. However, as the patient continued to have bowel movements consisting almost entirely of frank blood and did not have any infectious signs, a vascular etiology was more strongly considered. Given the patient’s relatively stable vital signs, BUN-to-Cr ratio of less than 20, and lack of orthostatic hypotension, there was low concern for an upper GI etiology, and endoscopy was not obtained emergently. The patient instead underwent abdominal computed tomography angiography (CTA), which identified active extravasation and contrast pooling within the cecum and appendix (Figure 1).
Shortly after the patient returned from imaging, repeat laboratory studies were performed, demonstrating an Hgb drop from 15.0 g/dL to 12.3 g/dL, and surgical services was emergently consulted. The surgeon recommended that embolization first be attempted, with surgery as the option of last resort given the poor localization of the bleed on CTA and the long-term consequences of colonic resection in a young, otherwise healthy man.
Interventional radiology was consulted, and the patient was brought immediately to the angiography suite, where he was found to have “active extravasation arising from a distal descending branch off the right colic artery” (Figure 2). Coil embolization resulted in complete resolution of the hemorrhage.
Later that evening, the patient’s Hgb continued to drop, reaching nadir at 7.3 g/dL, and he continued to have severe hematochezia. His falling Hgb was thought to be indicative of the degree of hemorrhage he had sustained prior to embolization, and the clearance of such blood as the source of his ongoing hematochezia. Following transfusion of 2 U of packed red blood cells (PRBCs), the patient’s Hgb improved to 12.0 g/dL, and he did not experience any significant bleeding for the remainder of his hospital stay.
The following morning, the patient underwent an extensive colonoscopy (extending 25 cm into the terminal ileum), which was unable to detect any signs of arteriovenous malformations, angiodysplasia, or any other possible source of bleeding. After 24 hours with stable vital signs and Hgb levels, the patient was discharged home with close surgical and gastroenterological follow-up, with possible genetic testing for connective tissue diseases. The diagnosis at discharge was spontaneous mesenteric hemorrhage of unknown etiology.
Discussion
Acute lower GI bleeding has an estimated annual hospitalization rate of 36 patients per 100,000, or about half the rate for upper GI bleeding.1,2 The majority of patients (>80%) will have spontaneous resolution and can be worked up nonemergently.
Etiology and Work-Up
Assessment of the etiology of hematochezia begins with ruling out an upper GI source of the bleed; 10% to 15% of patients presenting with hematochezia without hematemesis are ultimately diagnosed with an upper GI etiology. 4,5
BUN-to-Cr Ratio. In a study of patients presenting with hematochezia but no hematemesis or renal failure, Srygley et al6 found a BUN-to-Cr ratio greater than 93% to be sensitive for an upper GI source, with a likelihood ratio of 7.5. The proposed etiology is some combination of absorption of digested blood products and prerenal azotemia due to hypovolemia.
Tachycardia and Orthostatic Hypotension. There have been discussions in the literature about other findings to rule in/out upper GI bleeding. While some studies have found statistically significant results between upper and lower GI bleeding for tachycardia and orthostatic hypotension (increased percentage of both in upper GI bleeding), there is disagreement about whether these findings are clinically significant.7-9
Nasogastric Lavage. Although nasogastric (NG) lavage is no longer the standard of care in the ED due to poor sensitivity and marked discomfort to the patient, most current gastroenterology guidelines still recommend its use; therefore, NG may be requested by the GI consultant.10-12
Diagnosis
Once an upper GI source has been ruled out, identification of the lesion is the next step. The differential diagnosis includes common sources such as diverticular disease, angiodysplasia, colitis, anorectal sources, and neoplasm.5 Less common, but associated with a high risk of mortality, is aortoenteric fistula (100% mortality without surgical intervention).5
Colonoscopy. Emergent colonoscopy can be used for both diagnosis and (potential) therapeutic intervention and is therefore the first option of choice.1,3,4,9 However, as seen in our case, some patients experience such profound hemorrhage that visualization of the colon may be difficult or impossible; patients may also be too unstable to await bowel preparation or undergo a procedure.
Computed Tomography Angiography. For patients in whom colonoscopy is contraindicated, CTA is the imaging modality of choice, and has a 91% to 92% sensitivity in identifying active bleeding (>0.35 mL/min).13-16
Computed tomography of the abdomen and pelvis with contrast alone, as opposed to CTA, is insufficient for detecting GI bleeding, as it is timed so that imaging is obtained when the contrast is in the portal venous capillary beds, rather than in the arteries or arterioles. By protocol, though, many institutions require abdominal and pelvic CTA to include both arterial phase and venous phase images, allowing for assessment of both active arterial bleeding and alternative lower GI sources of hematochezia (eg, mesenteric ischemia).
When ordering a CT study, an awareness of local practice is important in understanding the information that will be obtained from the study. Protocols for lower GI bleed that include CTA have reported accuracy and efficiency without worsening of renal function, despite the increased contrast load.17
Triphasic CT Enterography. Another CT modality to consider is triphasic CT enterography, which uses IV and oral contrast. In a preliminary trial, this modality achieved a specificity of 100% (sensitivity 42%) in detecting GI bleeding.18
Red Blood Cell Scintigraphy. An additional imaging modality that has been the subject of much debate in the GI literature is tagged RBC scintigraphy with Technetium-99m. Various studies have found bleeding-site confirmation in 24% to 97% of patients, and correct localization in 41% to 100% of patients. Given the extensive variability within the literature on selection criteria, localization, site confirmation, and other variables, as well as evidence from one prospective trial by Zink et al19 that found a significant disagreement between CTA and scintigraphy, RBC scintigraphy is not recommended as an alternative imaging modality for the rapid diagnosis of an acute lower GI bleed.
Conclusion
Severe hematochezia is a potential surgical emergency with a broad differential diagnosis. While emergent colonoscopy is an excellent first option, in patients with severe hematochezia, there may be too much blood in the colon to obtain adequate visual images; additionally, depending on practice setting, emergency colonoscopy may not be immediately available. In either case, CTA—a readily available, noninvasive, rapid, and repeatable diagnostic tool—should be considered as an alternate to colonoscopy, particularly in patients with brisk hematochezia.
If a patient with severe hematochezia presents to the ED, the emergency physician (EP) must recognize that the degree of hemorrhage may not correlate with the patient’s vital signs or initial laboratory values. For this reason, the EP must have a high index of suspicion, and consider CTA to allow for a rapid definitive diagnosis and prompt discussion between surgical, interventional radiology, and/or gastroenterology teams to improve clinical outcomes and decrease morbidity and mortality.20
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
1. Ghassemi K, Jensen D. Lower GI bleeding: epidemiology and management. Curr Gastroenterol Rep. 2013;15(7):333. doi:10.1007/s11894-013-0333-5.
2. Strate LL, Ayanian JZ, Kotler G, Syngal S. Risk factors for mortality in lower intestinal bleeding. Clin Gastroenterol Hepatol. 2008;6(9):1004-1010. doi:10.1016/j.cgh.2008.03.021.
3. Qayad E, Dagar G, Nanchal R. Lower gastrointestinal hemorrhage. Crit Care Clin. 2016;32(2):241-254. doi:10.1016/j.ccc.2015.12.004.
4. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-664.
5. Goralnick E, Meguerdichian D. Gastrointestinal bleeding. In: Marx J, Hockberger R, Walls R. (Eds.). Rosen’s Emergency Medicine, 8th Edition. Philadelphia, PA: Saunders, 2014;248-253.
6. Srygley FD, Gerando CJ, Tran T, Fisher DA. Does this patient have a severe upper gastrointestinal bleed? JAMA. 2012;307(10):1072-1079. doi:10.1001/jama.2012.253.
7. Whelen C, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: a direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med. 2010;5(3):141-147. doi:10.1002/jhm.606.
8. Sittichanbunch Y, Senasu S, Thongkrau T, Keeratiksikorn C, Sawanyawisuth K. How to differentiate sites of gastrointestinal bleeding in patients with hematochezia by using clinical factors? Gastroenterol Res Pract. 2013;2013:265076. doi:10.1155/2013/265076.
9. Velayos F, Williamson A, Sousa KH, et al. Early predictors of severe lower gastrointestinal bleeding and adverse outcomes: a prospective study. Clin Gastroenterol Hepatol. 2004;2(6):485-490.
10. Palamidessi N, Sinert R, Falzon L, Zehtabchi S. Nasogastric aspiration and lavage in emergency department patients with hematochezia or melena without hematemesis. Acad Emerg Med. 2010;17(2):126-132. doi:10.1111/j.1553-2712.2009.00609.x.
11. Singer AJ, Richman PB, Kowalska A, Thode HC Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
12. Strate L, Gralnek I. ACG clinical guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016;111(4):459-474. doi:10.1038/ajg.2016.41.
13. Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16(31):3957-3963.
14. Geffroy Y, Rodallec MH, Boulay-Coletta I, Julles MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(3):E35-E46.
15. Reis F, Cardia P, D’Ippolito G. Computed tomography angiography in patients with active gastrointestinal bleeding. Radiol Bras. 2015;48(6):381-390. doi:10.1590/0100-3984.2014.0014.
16. Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2015;38(2):329-335. doi:10.1007/s00270-014-0928-8.
17. Jacovides T, Nadolski G, Allen S, et al. Arteriography for lower gastrointestinal hemorrhage: role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surgery. 2015;150(7):650-656. doi:10.1001/jamasurg.2015.97.
18. Hara AK, Walker FB, Silva AC, Leighton JA. Preliminary estimate of triphasic CT enterography performance in hemodynamically stable patients with suspected gastrointestinal bleeding. AJR Am J Roentgenol. 2009;193(5):1252-1260. doi:10.2214/AJR.08.1494.
19. Zink SI, Ohki SK, Stein B, et al. Noninvasive evaluation of active lower gastrointestinal bleeding: comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;91(4):1107-1114. doi:10.2214/AJR.07.3642.
20. Nable J, Graham A. Gastrointestinal bleeding. Emerg Med Clin N Am. 2016;34(2):309-325. doi:10.1016/j.emc.2015.12.001.
Idiopathic Intracranial Hypertension in a 24-Year-Old Woman
Case
A 24-year-old woman presented to the ED for evaluation of a 3-week history of worsening headache and a 5-day history of increasingly blurry vision. The patient stated that she had initially contacted her primary care physician, but instead presented to the ED because he had no open appointments until the following week and recommended that she go to the ED.
The patient described her headache as a pulsating and throbbing pain over her entire head, which only mildly improved after taking over-the-counter (OTC) ibuprofen. She further noted that her headache was somewhat worse when lying down, and reported the sensation of hearing her own pulsating heartbeat in her ears.
The patient had no personal or family history of migraines, tension headaches, aneurysms, clotting disorders, bleeding disorders, or renal disease, and stated that she had never experienced this type of headache before. She denied photophobia, phonophobia, neck stiffness, fever, vomiting, cough, numbness or weakness in her extremities, or pain anywhere else in her body.
Over the past 5 days, the patient noticed her vision had become increasingly blurry. She was not on any prescription medications, stating the only medication she used was occasional OTC ibuprofen. She had no known allergy to medications and denied smoking or recreational drug use; she admitted to occasional alcohol consumption.
The patient resided with her husband, who had no similar symptoms. Physical examination showed an obese woman (height, 5 ft 6 in; weight, 195 lb; body mass index, 32 kg/m2), lying supine in apparent discomfort. Vital signs at presentation were all normal, and oxygen saturation was normal on room air.
A bedside ocular examination showed 20/100 in both eyes while using glasses; no visual field cuts or obvious central scotoma was present. The patient was alert and oriented to time and place. The neurological examination showed intact cranial nerves, 5/5 strength in all extremities, intact sensation in all extremities, no pronator drift, negative Romberg test, and a normal gait. Fundoscopic examination revealed mildly blurred medial optic discs bilaterally. The rest of the physical examination was normal.
Discussion
Pseudotumor cerebri, more commonly referred to as idiopathic intracranial hypertension (IIH), is characterized by increased intracranial pressure (ICP) with no explanatory findings on imaging studies or in cerebrospinal fluid (CSF) analysis, and may be accompanied by symptoms of chronic headache, tinnitus, papilledema and progressive vision loss caused by optic nerve damage.1 Though historically IIH was referred to by several other names, including “benign intracranial hypertension,” the condition is not benign—when untreated, IIH can cause chronic disabling headaches and permanent vision loss.1
Clinical Course
The clinical course of IIH is unpredictable: In some patients, vision loss occurs gradually over the course of several weeks, while in others, loss occurs over a several month period. There are also patients with IIH who do not experience any alteration or loss of vision. Furthermore, some patients will experience permanent resolution of symptoms after a single lumbar puncture (LP); others have symptom recurrence after less than 24 hours; and some patients spontaneously remit on their own with no treatment whatsoever.1-4
Etiology
In the United States, IIH is a rare cause of headache, occurring in just 1 person per 100,000 annually.1 Though 90% of IIH cases occur in obese women of childbearing age, the etiology of IIH is unknown. Lumbar puncture usually alleviates the patient’s headache, but the CSF pressure typically returns to its pre-tap levels after a few hours.4,5 Neither CSF overproduction nor insufficient CSF resorption is responsible for causing IIH. One theory on the etiology of IIH proposes its cause to be due to a congenital malformation of the venous sinuses. This theory would explain why the symptoms so closely mimic those of venous sinus thrombosis, and why some IIH patients experience relief of symptoms after placement of a venous sinus stent.2
Symptoms
As noted previously, the most common symptom of IIH is headache, which patients usually describe as pressure-like and throbbing, and often involving retro-ocular pain. One feature in over half of patients is pulse-synchronous tinnitus (ie, hearing their own heartbeat in their ears). Eye pain, photophobia, blurry vision, and nausea/vomiting are all common symptoms in IIH, but these symptoms are also present in other causes of headache. The IIH headache might be relapsing and remitting, and can last from a few hours to weeks.2-4,6
Diagnosis
Imaging Studies. Noncontrast computed tomography (CT) imaging studies do not typically demonstrate any abnormal findings.1 Magnetic resonance imaging (MRI) studies show some inconsistent and subtle findings, such as flattening of the backs of the eyeballs, empty sella, or tortuous optic nerves.1
Lumbar Puncture. On LP, a very high opening pressure is a hallmark of IIH. An opening pressure <20 cm H2O is generally considered normal, 20 cm to 25 cm H2O is “equivocal,” and >25 cm H2O is abnormal.7 Patients presenting with IIH commonly have an opening pressure that exceeds 200 cm H2O.1-3 Extremely high pressures, however, are not required for the diagnosis, but some elevations in opening pressure will always be present.2,5 With the exception of a high opening pressure, the patient’s CSF analysis is normal.
Differential Diagnosis
Idiopathic intracranial hypertension is essentially a diagnosis of exclusion, one that is made after exclusion of all other potential causes of increased ICP (Table). Since contrast CT and MRI can identify subtle anatomical deformities and small lesions, their absence on these studies can help establish a diagnosis of IIH.
Venous Sinus Thrombosis. Venous sinus thrombosis is a rare but devastating condition that also cannot be diagnosed from a noncontrast CT but must always be considered in the differential diagnosis of IIH.8-10 Venous sinus thrombosis is characterized by a clot in one of the large venous sinuses that drain blood from the brain; the clot causes pressure to back up into the smaller cerebral vasculature, eventually inducing either a hemorrhagic stroke from a stressed vessel rupturing, or an ischemic stroke from lack of blood flow to the affected area of the brain. This condition is even more rare than IIH (0.5 cases per 100,000 population), but it can be devastating if missed, carrying a mortality rate as high as 15% in some studies.11
Risk Factors
Risk factors known to cause cerebral venous clots include genetic thrombophilias, pregnancy or recent pregnancy, oral contraceptive use, inflammatory bowel disease, severe dehydration, local infection/trauma, and substance abuse. Regardless of risk factors, the most recent guidelines of the American Heart Association/American Stroke Association recommend imaging studies of the cerebral venous sinuses for any patient presenting with new-onset symptoms suggestive of IIH (Class 1, Level of Evidence C).11 The two imaging options for evaluation of the cerebral venous sinuses are CT venography or MR venography. Since the 2013 American College of Radiology Appropriateness Criteria do not indicate a preference of one modality over the other, the choice of can be left to your radiologist.12
Patient Disposition
Patients with IIH typically do not require inpatient admission. Only about 3% of IIH patients will have a fulminant course of rapid-onset of vision loss, but even the most severe and acute cases will deteriorate over weeks, not hours or days.13 Nevertheless, close neurology follow-up is essential. If rapid and thorough outpatient neurological care is unavailable, admission is required.
Management
Not every patient with IIH experiences amelioration or resolution of symptoms following an LP; moreover, there is no clear way to differentiate patients who will experience therapeutic effects from LP from those who will not. Serial LPs as treatment for IIH have been discussed in the literature, but a ventriculoperitoneal shunt is a more practical approach in patients who do not respond to an initial LP.2,14
CSF Volume. The volume of CSF that can be removed safely may be 15 to 25 mL or more. A 1974 paper by Johnston and Paterson15 described five pseudotumor patients whose CSF was drained until their pressure had normalized; the amount removed varied from 15 to 25 mL, without adverse effects. A 1975 case series by Weisberg6 described safe removal of up to 30 mL of CSF in pseudotumor patients—the precise amount removed was determined by that which was necessary to lower the CSF pressure into the normal range. In 2007, a case report by Aly and Lawther16 of a pregnant woman with IIH describes twice weekly LP drainage of 30 mL.
There is nothing in the current literature to suggest that removing 10 to 30 mL of CSF instead of the 4 to 8 mL typically drawn in a diagnostic LP is going to pose any risk to the patient. The main complication associated with therapeutic LP is post-LP headache.5,17,18 There are currently no studies documenting outcomes after specific amounts of CSF removal.
Lifestyle Modifications: Weight Loss. No prospective, randomized controlled trials have proven weight loss to be effective in ameliorating the symptoms of IIH; however, several studies have found that rapid weight loss—whether through aggressive dieting or gastric bypass surgery—can improve symptoms dramatically within several months.19,20 One small study by Johnson et al has suggested that a 6% weight reduction is associated with marked improvement in papilledema.21Pharmacotherapy. The accepted first-line medication to alleviate symptoms of IIH is acetazolamide, and its use is supported by a recent randomized controlled trial conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).22 Most neurologists will administer a starting dose of acetazolamide 500 mg twice a day, and then increase the dose until symptoms are controlled or adverse effects appear (eg, fatigue, nausea/vomiting/diarrhea, electrolyte abnormalities, kidney stones) that contraindicate further dosage increases. In the NORDIC trial, patients were given up to 4 g of acetazolamide daily.22
Other medications, including loop diuretics and corticosteroids, should not be used except under the direct supervision of a neurologist.2,14
Refractory Cases
A patient who fails conservative treatment should be referred to a neurosurgeon for placement of a CSF shunt, optic nerve sheath fenestration, or placement of a venous sinus stent.23
Case Conclusion
After a noncontrast CT of the head was interpreted as completely normal, an LP was performed with the patient in the left lateral recumbent position. The opening CSF pressure exceeded 55 cm H2O (the upper limit of the manometer). The CSF was clear, and opening pressure was rechecked after each 5 mL draw. After 15 mL had been removed, the patient reported a sudden, dramatic disappearance of her headache and clearing of her vision. After 19 mL of CSF had been removed, the CSF pressure had dropped into the normal range (<20 cm H2O), and the procedure was ended.
To definitively rule out venous sinus thrombosis, a CT venogram was performed in the ED, and interpreted as normal. All other CSF results (cell count, protein, glucose, and gram stain) were normal. After complete resolution of the patient’s symptoms, she was discharged home with a prescription for acetazolamide 500 mg twice daily and instructions to follow-up with a neurologist within 48 hours. At discharge, the patient also received weight-loss counseling and was instructed to return immediately to the ED if her headache recurred or if she experienced any new neurological symptoms.
Summary
Idiopathic intracranial hypertension, also referred to as pseudotumor cerebri, is a rare but potentially vision-threatening cause of headache. Patients with signs and symptoms of IIH often initially present to the ED for evaluation and management. While the etiology of IIH is poorly understood, its clinical picture is unique: elevated ICP (sometimes markedly so) with no other significant findings on noncontrast head CT or CSF analysis. Venous sinus thrombosis, a life-threatening mimic of IIH, must always be included in the differential diagnosis.
Idiopathic intracranial hypertension is initially treated with rapid weight loss and acetazolamide. Many patients experience instant, though sometimes only transient, symptom relief from LP. No definitive studies to support any specific approach, including “therapeutic lumbar punctures.” The condition is rarely fulminant, and hospital admission is not typically required as long as urgent outpatient neurology follow-up is available.
1. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986-1893. doi:10.3174/ajnr.A2404.
2. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488-494. doi:10.1136/jnnp-2011-302029. 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114(Pt 1A):155-180.
4. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617. doi:10.1016/j.ncl.2010.03.003.
5. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58(10):1551-1553.
6. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
7. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690-1691.
8. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537-1542.
9. Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6(5):601-604.
10. Sylaja PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S. Differential diagnosis of patients with intracranial sinus venous thrombosis related isolated intracranial hypertension from those with idiopathic intracranial hypertension. J Neurol Sci. 2003;215(1-2):9-12.
11. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi:10.1161/STR.0b013e31820a8364.
12. American College of Radiology ACR Appropriateness Criteria: Headache. https://acsearch.acr.org/docs/69482/Narrative/. Updated 2013. Accessed January 19, 2017.
13. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229-232.
14. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380-390. doi:10.1136/practneurol-2014-000821.
15. Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97(2):301-312.
16. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anesthesia. 2007;62(2):178-181.
17. Panikkath R, Welker J, Johnston R, Lado-Abeal J. Intracranial hypertension and intracranial hypotension causing headache in the same patient. Proc (Bayl Univ Med Cent). 2014;27(3):217-218.
18. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778-781. doi:10.1111/j.1460-9592.2004.01529.x.
19. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi:10.1136/bmj.c2701.
20. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-1098.
21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105(12):2313-2317. doi:10.1016/S0161-6420(98)91234-9.
22. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee; Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-1651. doi:10.1001/jama.2014.3312.
23. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol. 2014;98(10):1360-1363. doi:10.1136/bjophthalmol-2014-304953.
Case
A 24-year-old woman presented to the ED for evaluation of a 3-week history of worsening headache and a 5-day history of increasingly blurry vision. The patient stated that she had initially contacted her primary care physician, but instead presented to the ED because he had no open appointments until the following week and recommended that she go to the ED.
The patient described her headache as a pulsating and throbbing pain over her entire head, which only mildly improved after taking over-the-counter (OTC) ibuprofen. She further noted that her headache was somewhat worse when lying down, and reported the sensation of hearing her own pulsating heartbeat in her ears.
The patient had no personal or family history of migraines, tension headaches, aneurysms, clotting disorders, bleeding disorders, or renal disease, and stated that she had never experienced this type of headache before. She denied photophobia, phonophobia, neck stiffness, fever, vomiting, cough, numbness or weakness in her extremities, or pain anywhere else in her body.
Over the past 5 days, the patient noticed her vision had become increasingly blurry. She was not on any prescription medications, stating the only medication she used was occasional OTC ibuprofen. She had no known allergy to medications and denied smoking or recreational drug use; she admitted to occasional alcohol consumption.
The patient resided with her husband, who had no similar symptoms. Physical examination showed an obese woman (height, 5 ft 6 in; weight, 195 lb; body mass index, 32 kg/m2), lying supine in apparent discomfort. Vital signs at presentation were all normal, and oxygen saturation was normal on room air.
A bedside ocular examination showed 20/100 in both eyes while using glasses; no visual field cuts or obvious central scotoma was present. The patient was alert and oriented to time and place. The neurological examination showed intact cranial nerves, 5/5 strength in all extremities, intact sensation in all extremities, no pronator drift, negative Romberg test, and a normal gait. Fundoscopic examination revealed mildly blurred medial optic discs bilaterally. The rest of the physical examination was normal.
Discussion
Pseudotumor cerebri, more commonly referred to as idiopathic intracranial hypertension (IIH), is characterized by increased intracranial pressure (ICP) with no explanatory findings on imaging studies or in cerebrospinal fluid (CSF) analysis, and may be accompanied by symptoms of chronic headache, tinnitus, papilledema and progressive vision loss caused by optic nerve damage.1 Though historically IIH was referred to by several other names, including “benign intracranial hypertension,” the condition is not benign—when untreated, IIH can cause chronic disabling headaches and permanent vision loss.1
Clinical Course
The clinical course of IIH is unpredictable: In some patients, vision loss occurs gradually over the course of several weeks, while in others, loss occurs over a several month period. There are also patients with IIH who do not experience any alteration or loss of vision. Furthermore, some patients will experience permanent resolution of symptoms after a single lumbar puncture (LP); others have symptom recurrence after less than 24 hours; and some patients spontaneously remit on their own with no treatment whatsoever.1-4
Etiology
In the United States, IIH is a rare cause of headache, occurring in just 1 person per 100,000 annually.1 Though 90% of IIH cases occur in obese women of childbearing age, the etiology of IIH is unknown. Lumbar puncture usually alleviates the patient’s headache, but the CSF pressure typically returns to its pre-tap levels after a few hours.4,5 Neither CSF overproduction nor insufficient CSF resorption is responsible for causing IIH. One theory on the etiology of IIH proposes its cause to be due to a congenital malformation of the venous sinuses. This theory would explain why the symptoms so closely mimic those of venous sinus thrombosis, and why some IIH patients experience relief of symptoms after placement of a venous sinus stent.2
Symptoms
As noted previously, the most common symptom of IIH is headache, which patients usually describe as pressure-like and throbbing, and often involving retro-ocular pain. One feature in over half of patients is pulse-synchronous tinnitus (ie, hearing their own heartbeat in their ears). Eye pain, photophobia, blurry vision, and nausea/vomiting are all common symptoms in IIH, but these symptoms are also present in other causes of headache. The IIH headache might be relapsing and remitting, and can last from a few hours to weeks.2-4,6
Diagnosis
Imaging Studies. Noncontrast computed tomography (CT) imaging studies do not typically demonstrate any abnormal findings.1 Magnetic resonance imaging (MRI) studies show some inconsistent and subtle findings, such as flattening of the backs of the eyeballs, empty sella, or tortuous optic nerves.1
Lumbar Puncture. On LP, a very high opening pressure is a hallmark of IIH. An opening pressure <20 cm H2O is generally considered normal, 20 cm to 25 cm H2O is “equivocal,” and >25 cm H2O is abnormal.7 Patients presenting with IIH commonly have an opening pressure that exceeds 200 cm H2O.1-3 Extremely high pressures, however, are not required for the diagnosis, but some elevations in opening pressure will always be present.2,5 With the exception of a high opening pressure, the patient’s CSF analysis is normal.
Differential Diagnosis
Idiopathic intracranial hypertension is essentially a diagnosis of exclusion, one that is made after exclusion of all other potential causes of increased ICP (Table). Since contrast CT and MRI can identify subtle anatomical deformities and small lesions, their absence on these studies can help establish a diagnosis of IIH.
Venous Sinus Thrombosis. Venous sinus thrombosis is a rare but devastating condition that also cannot be diagnosed from a noncontrast CT but must always be considered in the differential diagnosis of IIH.8-10 Venous sinus thrombosis is characterized by a clot in one of the large venous sinuses that drain blood from the brain; the clot causes pressure to back up into the smaller cerebral vasculature, eventually inducing either a hemorrhagic stroke from a stressed vessel rupturing, or an ischemic stroke from lack of blood flow to the affected area of the brain. This condition is even more rare than IIH (0.5 cases per 100,000 population), but it can be devastating if missed, carrying a mortality rate as high as 15% in some studies.11
Risk Factors
Risk factors known to cause cerebral venous clots include genetic thrombophilias, pregnancy or recent pregnancy, oral contraceptive use, inflammatory bowel disease, severe dehydration, local infection/trauma, and substance abuse. Regardless of risk factors, the most recent guidelines of the American Heart Association/American Stroke Association recommend imaging studies of the cerebral venous sinuses for any patient presenting with new-onset symptoms suggestive of IIH (Class 1, Level of Evidence C).11 The two imaging options for evaluation of the cerebral venous sinuses are CT venography or MR venography. Since the 2013 American College of Radiology Appropriateness Criteria do not indicate a preference of one modality over the other, the choice of can be left to your radiologist.12
Patient Disposition
Patients with IIH typically do not require inpatient admission. Only about 3% of IIH patients will have a fulminant course of rapid-onset of vision loss, but even the most severe and acute cases will deteriorate over weeks, not hours or days.13 Nevertheless, close neurology follow-up is essential. If rapid and thorough outpatient neurological care is unavailable, admission is required.
Management
Not every patient with IIH experiences amelioration or resolution of symptoms following an LP; moreover, there is no clear way to differentiate patients who will experience therapeutic effects from LP from those who will not. Serial LPs as treatment for IIH have been discussed in the literature, but a ventriculoperitoneal shunt is a more practical approach in patients who do not respond to an initial LP.2,14
CSF Volume. The volume of CSF that can be removed safely may be 15 to 25 mL or more. A 1974 paper by Johnston and Paterson15 described five pseudotumor patients whose CSF was drained until their pressure had normalized; the amount removed varied from 15 to 25 mL, without adverse effects. A 1975 case series by Weisberg6 described safe removal of up to 30 mL of CSF in pseudotumor patients—the precise amount removed was determined by that which was necessary to lower the CSF pressure into the normal range. In 2007, a case report by Aly and Lawther16 of a pregnant woman with IIH describes twice weekly LP drainage of 30 mL.
There is nothing in the current literature to suggest that removing 10 to 30 mL of CSF instead of the 4 to 8 mL typically drawn in a diagnostic LP is going to pose any risk to the patient. The main complication associated with therapeutic LP is post-LP headache.5,17,18 There are currently no studies documenting outcomes after specific amounts of CSF removal.
Lifestyle Modifications: Weight Loss. No prospective, randomized controlled trials have proven weight loss to be effective in ameliorating the symptoms of IIH; however, several studies have found that rapid weight loss—whether through aggressive dieting or gastric bypass surgery—can improve symptoms dramatically within several months.19,20 One small study by Johnson et al has suggested that a 6% weight reduction is associated with marked improvement in papilledema.21Pharmacotherapy. The accepted first-line medication to alleviate symptoms of IIH is acetazolamide, and its use is supported by a recent randomized controlled trial conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).22 Most neurologists will administer a starting dose of acetazolamide 500 mg twice a day, and then increase the dose until symptoms are controlled or adverse effects appear (eg, fatigue, nausea/vomiting/diarrhea, electrolyte abnormalities, kidney stones) that contraindicate further dosage increases. In the NORDIC trial, patients were given up to 4 g of acetazolamide daily.22
Other medications, including loop diuretics and corticosteroids, should not be used except under the direct supervision of a neurologist.2,14
Refractory Cases
A patient who fails conservative treatment should be referred to a neurosurgeon for placement of a CSF shunt, optic nerve sheath fenestration, or placement of a venous sinus stent.23
Case Conclusion
After a noncontrast CT of the head was interpreted as completely normal, an LP was performed with the patient in the left lateral recumbent position. The opening CSF pressure exceeded 55 cm H2O (the upper limit of the manometer). The CSF was clear, and opening pressure was rechecked after each 5 mL draw. After 15 mL had been removed, the patient reported a sudden, dramatic disappearance of her headache and clearing of her vision. After 19 mL of CSF had been removed, the CSF pressure had dropped into the normal range (<20 cm H2O), and the procedure was ended.
To definitively rule out venous sinus thrombosis, a CT venogram was performed in the ED, and interpreted as normal. All other CSF results (cell count, protein, glucose, and gram stain) were normal. After complete resolution of the patient’s symptoms, she was discharged home with a prescription for acetazolamide 500 mg twice daily and instructions to follow-up with a neurologist within 48 hours. At discharge, the patient also received weight-loss counseling and was instructed to return immediately to the ED if her headache recurred or if she experienced any new neurological symptoms.
Summary
Idiopathic intracranial hypertension, also referred to as pseudotumor cerebri, is a rare but potentially vision-threatening cause of headache. Patients with signs and symptoms of IIH often initially present to the ED for evaluation and management. While the etiology of IIH is poorly understood, its clinical picture is unique: elevated ICP (sometimes markedly so) with no other significant findings on noncontrast head CT or CSF analysis. Venous sinus thrombosis, a life-threatening mimic of IIH, must always be included in the differential diagnosis.
Idiopathic intracranial hypertension is initially treated with rapid weight loss and acetazolamide. Many patients experience instant, though sometimes only transient, symptom relief from LP. No definitive studies to support any specific approach, including “therapeutic lumbar punctures.” The condition is rarely fulminant, and hospital admission is not typically required as long as urgent outpatient neurology follow-up is available.
Case
A 24-year-old woman presented to the ED for evaluation of a 3-week history of worsening headache and a 5-day history of increasingly blurry vision. The patient stated that she had initially contacted her primary care physician, but instead presented to the ED because he had no open appointments until the following week and recommended that she go to the ED.
The patient described her headache as a pulsating and throbbing pain over her entire head, which only mildly improved after taking over-the-counter (OTC) ibuprofen. She further noted that her headache was somewhat worse when lying down, and reported the sensation of hearing her own pulsating heartbeat in her ears.
The patient had no personal or family history of migraines, tension headaches, aneurysms, clotting disorders, bleeding disorders, or renal disease, and stated that she had never experienced this type of headache before. She denied photophobia, phonophobia, neck stiffness, fever, vomiting, cough, numbness or weakness in her extremities, or pain anywhere else in her body.
Over the past 5 days, the patient noticed her vision had become increasingly blurry. She was not on any prescription medications, stating the only medication she used was occasional OTC ibuprofen. She had no known allergy to medications and denied smoking or recreational drug use; she admitted to occasional alcohol consumption.
The patient resided with her husband, who had no similar symptoms. Physical examination showed an obese woman (height, 5 ft 6 in; weight, 195 lb; body mass index, 32 kg/m2), lying supine in apparent discomfort. Vital signs at presentation were all normal, and oxygen saturation was normal on room air.
A bedside ocular examination showed 20/100 in both eyes while using glasses; no visual field cuts or obvious central scotoma was present. The patient was alert and oriented to time and place. The neurological examination showed intact cranial nerves, 5/5 strength in all extremities, intact sensation in all extremities, no pronator drift, negative Romberg test, and a normal gait. Fundoscopic examination revealed mildly blurred medial optic discs bilaterally. The rest of the physical examination was normal.
Discussion
Pseudotumor cerebri, more commonly referred to as idiopathic intracranial hypertension (IIH), is characterized by increased intracranial pressure (ICP) with no explanatory findings on imaging studies or in cerebrospinal fluid (CSF) analysis, and may be accompanied by symptoms of chronic headache, tinnitus, papilledema and progressive vision loss caused by optic nerve damage.1 Though historically IIH was referred to by several other names, including “benign intracranial hypertension,” the condition is not benign—when untreated, IIH can cause chronic disabling headaches and permanent vision loss.1
Clinical Course
The clinical course of IIH is unpredictable: In some patients, vision loss occurs gradually over the course of several weeks, while in others, loss occurs over a several month period. There are also patients with IIH who do not experience any alteration or loss of vision. Furthermore, some patients will experience permanent resolution of symptoms after a single lumbar puncture (LP); others have symptom recurrence after less than 24 hours; and some patients spontaneously remit on their own with no treatment whatsoever.1-4
Etiology
In the United States, IIH is a rare cause of headache, occurring in just 1 person per 100,000 annually.1 Though 90% of IIH cases occur in obese women of childbearing age, the etiology of IIH is unknown. Lumbar puncture usually alleviates the patient’s headache, but the CSF pressure typically returns to its pre-tap levels after a few hours.4,5 Neither CSF overproduction nor insufficient CSF resorption is responsible for causing IIH. One theory on the etiology of IIH proposes its cause to be due to a congenital malformation of the venous sinuses. This theory would explain why the symptoms so closely mimic those of venous sinus thrombosis, and why some IIH patients experience relief of symptoms after placement of a venous sinus stent.2
Symptoms
As noted previously, the most common symptom of IIH is headache, which patients usually describe as pressure-like and throbbing, and often involving retro-ocular pain. One feature in over half of patients is pulse-synchronous tinnitus (ie, hearing their own heartbeat in their ears). Eye pain, photophobia, blurry vision, and nausea/vomiting are all common symptoms in IIH, but these symptoms are also present in other causes of headache. The IIH headache might be relapsing and remitting, and can last from a few hours to weeks.2-4,6
Diagnosis
Imaging Studies. Noncontrast computed tomography (CT) imaging studies do not typically demonstrate any abnormal findings.1 Magnetic resonance imaging (MRI) studies show some inconsistent and subtle findings, such as flattening of the backs of the eyeballs, empty sella, or tortuous optic nerves.1
Lumbar Puncture. On LP, a very high opening pressure is a hallmark of IIH. An opening pressure <20 cm H2O is generally considered normal, 20 cm to 25 cm H2O is “equivocal,” and >25 cm H2O is abnormal.7 Patients presenting with IIH commonly have an opening pressure that exceeds 200 cm H2O.1-3 Extremely high pressures, however, are not required for the diagnosis, but some elevations in opening pressure will always be present.2,5 With the exception of a high opening pressure, the patient’s CSF analysis is normal.
Differential Diagnosis
Idiopathic intracranial hypertension is essentially a diagnosis of exclusion, one that is made after exclusion of all other potential causes of increased ICP (Table). Since contrast CT and MRI can identify subtle anatomical deformities and small lesions, their absence on these studies can help establish a diagnosis of IIH.
Venous Sinus Thrombosis. Venous sinus thrombosis is a rare but devastating condition that also cannot be diagnosed from a noncontrast CT but must always be considered in the differential diagnosis of IIH.8-10 Venous sinus thrombosis is characterized by a clot in one of the large venous sinuses that drain blood from the brain; the clot causes pressure to back up into the smaller cerebral vasculature, eventually inducing either a hemorrhagic stroke from a stressed vessel rupturing, or an ischemic stroke from lack of blood flow to the affected area of the brain. This condition is even more rare than IIH (0.5 cases per 100,000 population), but it can be devastating if missed, carrying a mortality rate as high as 15% in some studies.11
Risk Factors
Risk factors known to cause cerebral venous clots include genetic thrombophilias, pregnancy or recent pregnancy, oral contraceptive use, inflammatory bowel disease, severe dehydration, local infection/trauma, and substance abuse. Regardless of risk factors, the most recent guidelines of the American Heart Association/American Stroke Association recommend imaging studies of the cerebral venous sinuses for any patient presenting with new-onset symptoms suggestive of IIH (Class 1, Level of Evidence C).11 The two imaging options for evaluation of the cerebral venous sinuses are CT venography or MR venography. Since the 2013 American College of Radiology Appropriateness Criteria do not indicate a preference of one modality over the other, the choice of can be left to your radiologist.12
Patient Disposition
Patients with IIH typically do not require inpatient admission. Only about 3% of IIH patients will have a fulminant course of rapid-onset of vision loss, but even the most severe and acute cases will deteriorate over weeks, not hours or days.13 Nevertheless, close neurology follow-up is essential. If rapid and thorough outpatient neurological care is unavailable, admission is required.
Management
Not every patient with IIH experiences amelioration or resolution of symptoms following an LP; moreover, there is no clear way to differentiate patients who will experience therapeutic effects from LP from those who will not. Serial LPs as treatment for IIH have been discussed in the literature, but a ventriculoperitoneal shunt is a more practical approach in patients who do not respond to an initial LP.2,14
CSF Volume. The volume of CSF that can be removed safely may be 15 to 25 mL or more. A 1974 paper by Johnston and Paterson15 described five pseudotumor patients whose CSF was drained until their pressure had normalized; the amount removed varied from 15 to 25 mL, without adverse effects. A 1975 case series by Weisberg6 described safe removal of up to 30 mL of CSF in pseudotumor patients—the precise amount removed was determined by that which was necessary to lower the CSF pressure into the normal range. In 2007, a case report by Aly and Lawther16 of a pregnant woman with IIH describes twice weekly LP drainage of 30 mL.
There is nothing in the current literature to suggest that removing 10 to 30 mL of CSF instead of the 4 to 8 mL typically drawn in a diagnostic LP is going to pose any risk to the patient. The main complication associated with therapeutic LP is post-LP headache.5,17,18 There are currently no studies documenting outcomes after specific amounts of CSF removal.
Lifestyle Modifications: Weight Loss. No prospective, randomized controlled trials have proven weight loss to be effective in ameliorating the symptoms of IIH; however, several studies have found that rapid weight loss—whether through aggressive dieting or gastric bypass surgery—can improve symptoms dramatically within several months.19,20 One small study by Johnson et al has suggested that a 6% weight reduction is associated with marked improvement in papilledema.21Pharmacotherapy. The accepted first-line medication to alleviate symptoms of IIH is acetazolamide, and its use is supported by a recent randomized controlled trial conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).22 Most neurologists will administer a starting dose of acetazolamide 500 mg twice a day, and then increase the dose until symptoms are controlled or adverse effects appear (eg, fatigue, nausea/vomiting/diarrhea, electrolyte abnormalities, kidney stones) that contraindicate further dosage increases. In the NORDIC trial, patients were given up to 4 g of acetazolamide daily.22
Other medications, including loop diuretics and corticosteroids, should not be used except under the direct supervision of a neurologist.2,14
Refractory Cases
A patient who fails conservative treatment should be referred to a neurosurgeon for placement of a CSF shunt, optic nerve sheath fenestration, or placement of a venous sinus stent.23
Case Conclusion
After a noncontrast CT of the head was interpreted as completely normal, an LP was performed with the patient in the left lateral recumbent position. The opening CSF pressure exceeded 55 cm H2O (the upper limit of the manometer). The CSF was clear, and opening pressure was rechecked after each 5 mL draw. After 15 mL had been removed, the patient reported a sudden, dramatic disappearance of her headache and clearing of her vision. After 19 mL of CSF had been removed, the CSF pressure had dropped into the normal range (<20 cm H2O), and the procedure was ended.
To definitively rule out venous sinus thrombosis, a CT venogram was performed in the ED, and interpreted as normal. All other CSF results (cell count, protein, glucose, and gram stain) were normal. After complete resolution of the patient’s symptoms, she was discharged home with a prescription for acetazolamide 500 mg twice daily and instructions to follow-up with a neurologist within 48 hours. At discharge, the patient also received weight-loss counseling and was instructed to return immediately to the ED if her headache recurred or if she experienced any new neurological symptoms.
Summary
Idiopathic intracranial hypertension, also referred to as pseudotumor cerebri, is a rare but potentially vision-threatening cause of headache. Patients with signs and symptoms of IIH often initially present to the ED for evaluation and management. While the etiology of IIH is poorly understood, its clinical picture is unique: elevated ICP (sometimes markedly so) with no other significant findings on noncontrast head CT or CSF analysis. Venous sinus thrombosis, a life-threatening mimic of IIH, must always be included in the differential diagnosis.
Idiopathic intracranial hypertension is initially treated with rapid weight loss and acetazolamide. Many patients experience instant, though sometimes only transient, symptom relief from LP. No definitive studies to support any specific approach, including “therapeutic lumbar punctures.” The condition is rarely fulminant, and hospital admission is not typically required as long as urgent outpatient neurology follow-up is available.
1. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986-1893. doi:10.3174/ajnr.A2404.
2. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488-494. doi:10.1136/jnnp-2011-302029. 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114(Pt 1A):155-180.
4. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617. doi:10.1016/j.ncl.2010.03.003.
5. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58(10):1551-1553.
6. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
7. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690-1691.
8. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537-1542.
9. Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6(5):601-604.
10. Sylaja PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S. Differential diagnosis of patients with intracranial sinus venous thrombosis related isolated intracranial hypertension from those with idiopathic intracranial hypertension. J Neurol Sci. 2003;215(1-2):9-12.
11. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi:10.1161/STR.0b013e31820a8364.
12. American College of Radiology ACR Appropriateness Criteria: Headache. https://acsearch.acr.org/docs/69482/Narrative/. Updated 2013. Accessed January 19, 2017.
13. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229-232.
14. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380-390. doi:10.1136/practneurol-2014-000821.
15. Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97(2):301-312.
16. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anesthesia. 2007;62(2):178-181.
17. Panikkath R, Welker J, Johnston R, Lado-Abeal J. Intracranial hypertension and intracranial hypotension causing headache in the same patient. Proc (Bayl Univ Med Cent). 2014;27(3):217-218.
18. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778-781. doi:10.1111/j.1460-9592.2004.01529.x.
19. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi:10.1136/bmj.c2701.
20. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-1098.
21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105(12):2313-2317. doi:10.1016/S0161-6420(98)91234-9.
22. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee; Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-1651. doi:10.1001/jama.2014.3312.
23. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol. 2014;98(10):1360-1363. doi:10.1136/bjophthalmol-2014-304953.
1. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986-1893. doi:10.3174/ajnr.A2404.
2. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488-494. doi:10.1136/jnnp-2011-302029. 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114(Pt 1A):155-180.
4. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617. doi:10.1016/j.ncl.2010.03.003.
5. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58(10):1551-1553.
6. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
7. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690-1691.
8. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537-1542.
9. Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6(5):601-604.
10. Sylaja PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S. Differential diagnosis of patients with intracranial sinus venous thrombosis related isolated intracranial hypertension from those with idiopathic intracranial hypertension. J Neurol Sci. 2003;215(1-2):9-12.
11. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi:10.1161/STR.0b013e31820a8364.
12. American College of Radiology ACR Appropriateness Criteria: Headache. https://acsearch.acr.org/docs/69482/Narrative/. Updated 2013. Accessed January 19, 2017.
13. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229-232.
14. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380-390. doi:10.1136/practneurol-2014-000821.
15. Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97(2):301-312.
16. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anesthesia. 2007;62(2):178-181.
17. Panikkath R, Welker J, Johnston R, Lado-Abeal J. Intracranial hypertension and intracranial hypotension causing headache in the same patient. Proc (Bayl Univ Med Cent). 2014;27(3):217-218.
18. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778-781. doi:10.1111/j.1460-9592.2004.01529.x.
19. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi:10.1136/bmj.c2701.
20. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-1098.
21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105(12):2313-2317. doi:10.1016/S0161-6420(98)91234-9.
22. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee; Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-1651. doi:10.1001/jama.2014.3312.
23. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol. 2014;98(10):1360-1363. doi:10.1136/bjophthalmol-2014-304953.
Malpractice Counsel: Pain in the Back, Pain in the Butt(ocks)
A Pain in the Back
A 57-year-old man presented to the ED with a chief complaint of severe low back pain that radiated into his left buttock. The patient stated the pain started immediately after he had bent over to pick up a small refrigerator. He denied any abdominal pain, lower extremity numbness, weakness, or bowel or bladder dysfunction. The patient’s medical history was significant for hypertension, for which he was taking amlodipine, and sleep apnea. The patient stated that he had a continuous positive airway pressure (CPAP) machine for his sleep apnea, but did not use it regularly. Regarding his social history, the patient denied tobacco use, but did admit to daily alcohol consumption.
The patient’s vital signs were all normal. Physical examination was remarkable only for bilateral lumbar paraspinal muscle tenderness, which was greater on the left side. There was no midline tenderness. Straight leg-raise testing was negative bilaterally, and the patient had normal strength and deep tendon reflexes in the lower extremities. The abdomen was soft and nontender.
The emergency physician (EP) diagnosed the patient with muscle strain, and discharged him home with a prescription for hydrocodone, along with instructions to follow-up with his primary care physician (PCP) within the next few days.
Three days later, the patient presented again to the same ED complaining of increased lower back pain. He denied any new injury or overuse, and continued to deny any lower extremity numbness or weakness or bowel/bladder dysfunction. Similarly, the physical examination was unchanged. The patient was given an intramuscular (IM) injection of hydromorphone with promethazine, as well as oral diazepam, and discharged home with instructions to continue to take the hydrocodone as needed for pain.
According to his wife, the patient retired to bed shortly after arriving home from the ED. Approximately 90 minutes later, she discovered the patient unresponsive in bed and called emergency medical services (EMS). He was brought back to the same hospital ED via EMS and was emergently intubated upon arrival. Unfortunately, the patient had suffered an anoxic brain injury and never regained consciousness; he died 1 week later.
The patient’s wife sued the EP, claiming the anoxic brain injury was related to the drugs ordered by the EP in combination with the patient’s alcohol use. She alleged that if the EP had observed the patient in the ED for signs of respiratory distress, his condition would have been treated and the anoxic brain injury would have been prevented. The plaintiff also faulted the EP for not informing the patient of the risks of drinking alcohol while taking the prescription pain medication.
The EP asserted his care of the patient was appropriate, and that there was no reason to keep the patient for observation. Regarding counseling the patient about the risks associated with concomitant alcohol consumption and pain medication, the EP stated that he had relied on the nurse who administered the medications to provide such counsel. The EP further maintained the plaintiff’s death was due to the patient not using his CPAP machine as prescribed for sleep apnea, along with his alcohol consumption the evening of the event. At trial, a defense verdict was returned.
Discussion
Unfortunately, there are several unknowns in this case. Did the patient drink any alcohol after returning home from the second ED visit, prior to going to sleep? If so, how much did he consume? Did he take any of the narcotic pills prescribed from the first ED visit and, if so, how many did he take and in what time frame?
The combination of narcotics, benzodiazepines, and alcohol has long been known to be a potentially lethal combination, resulting in respiratory depression, respiratory arrest, anoxic brain injury and even death. As EPs, we are confronted with patients complaining of pain during every shift. Complicating matters, in national patient surveys concerning the care received in the ED, patients are specifically asked if their pain was adequately treated. At the same time, there is a national effort across all specialties to reduce the amount of opioids prescribed to patients. The EP should therefore attempt to select the least potent medication that will adequately control the patient’s pain.
The WHO Pain Ladder
In 1986, the World Health Organization (WHO) developed a three-step analgesic ladder to guide the management of cancer pain.1 This guide has since been expanded to include pain of noncancer etiology. Mild pain, defined on the numerical rating scale (NRS) as 1 to 3, is considered step 1.1,2 Moderate pain (NRS of 4-6) is considered step 2, and severe pain (NRS of 7-10) is step 3. For step 1 pain, acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) is recommended. For step 2 pain, a weak narcotic (eg, codeine, hydrocodone), with or without acetaminophen or an NSAID, is recommended. For step 3 pain, a strong narcotic agent such as morphine or hydromorphone is advised.1
The WHO’s ladder is not meant to serve as a strict protocol, but rather as a tool to guide the clinician in determining a reasonable starting point in pain management. Although the EP in this case did not ask the patient to rate his pain, from all indications it appeared to be severe (step 3) and as such, the choice of prescribing hydromorphone was a reasonable one. However, most experts agree that it is best to titrate an analgesic to the desired effect. In patients with severe pain, this means employing the intravenous (IV) route, not the IM route, which was used in this case. This is because the IM route can result in variable absorption and an unpredictable time of onset and duration of action.
Concomitant Antiemetic Therapy
It is common practice to administer an antiemetic simultaneously with a narcotic to prevent or lessen associated nausea and vomiting. The clinician must be aware, however, that all antiemetics act as central nervous system (CNS) depressants to some degree. The addition of diazepam in this case is problematic because all benzodiazepines cause sedation and anxiolysis. The combination of benzodiazepines with other CNS depressants, such as opioid analgesics, can lead to excessive sedation, resulting in partial airway obstruction, respiratory depression, and hypoxia.3 The risk of an adverse outcome significantly increases with concomitant alcohol consumption.
The EP must carefully consider the risks and benefits any time opioids and benzodiazepines are administered in combination. In addition, the underlying health of the patient must be considered. Risk factors for opioid-induced respiratory depression includes age older than 50 years, a history of sleep apnea, preexisting pulmonary disease (eg, chronic obstructive pulmonary disease), anatomic oral or airway abnormalities, and renal or hepatic impairment.3 Finally, patients should be informed of the dangers of mixing alcohol with opioids and benzodiazepines—whether such counsel is given by a physician, nurse, or pharmacist.
References
1. World Health Organization. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: World Health Organization; 1996:1-69.
2. Todd KH. Pain assessment instruments for us in the emergency department. Emerg Med Clin North Am. 2005;23(2):285-295.
3. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing. 2011;12(3):118-145.
A Pain in the Buttock(s)
A 33-year-old Hispanic man, whose only spoken language was Spanish, presented to the ED for evaluation of pain in his right buttock. A coworker who accompanied the patient to the ED and served as his translator stated the patient’s pain began the previous day, immediately following a work-related injury in which the patient had slipped and fallen backward, landing on his buttocks. The patient denied any head injury, loss of consciousness, or neck pain. He further stated that he was otherwise in good health and was not taking any medications. Regarding social history, the patient denied any alcohol or drug use.
The patient’s vital signs were normal, as was his physical examination. The EP ordered an anteroposterior X-ray of the pelvis, which radiology services interpreted as normal. The EP diagnosed a buttock contusion secondary to fall, and discharged the patient home with instructions to take over-the-counter (OTC) ibuprofen for pain as needed.
Four days later, the patient presented to the same ED, complaining of low back pain radiating down his right leg. He denied any new injuries or falls, any lower extremity weakness, or bowel or bladder discomfort. Through a translator, the patient further noted that although he had been taking the OTC ibuprofen as prescribed, it had not alleviated his pain.
On physical examination, the patient’s vital signs were: blood pressure (BP), 112/62 mm Hg; heart rate (HR), 96 beats/min; respiratory rate (RR), 20 breaths/min; and temperature (T), 101.8˚F. Oxygen saturation was 98% on room air. The lung, heart, and abdominal examinations were normal. The patient was noted to be tender to palpation over the sacral and coccygeal region. There was no documentation of a lower extremity or neurological examination.
The EP ordered a computed tomography (CT) scan of the pelvis without contrast. This was interpreted by radiology services as demonstrating moderate facet arthropathy at L4/L5 on the left side, with a facet joint cyst extending in the central canal; no fracture was identified. The radiologist suggested that these findings could be better evaluated with a magnetic resonance imaging (MRI) study of the lumbar spine, if clinically indicated. The EP decided against ordering the MRI, diagnosed the patient with a contusion of the coccyx, and discharged him home with instructions to continue taking OTC ibuprofen; he also prescribed combination acetaminophen/oxycodone for severe pain, and instructed the patient to follow-up with his PCP in 2 days.
Forty eight hours later, the patient returned to the same ED via EMS, now with the complaint of inability to walk or urinate. He continued to have a fever and was tachycardic with an HR of 110 beats/min; BP, RR, and T were normal, as was his oxygen saturation on room air. The patient was noted to have significant lower extremity weakness. A Foley catheter was placed, and 1,200 cc of urine was obtained. An emergent MRI of the lumbar spine revealed an extensive lumbar epidural abscess along the lumbar spine. The patient was started on IV antibiotics and taken to the operating room for evacuation of the epidural abscess. Unfortunately, he had a prolonged inpatient stay due to persistent bilateral lower extremity weakness and neurogenic bowel and bladder dysfunction; he eventually was discharged to a skilled nursing facility.
The patient sued the EP and hospital for their failure to appreciate the significance of his presentation at the second ED visit. The attorneys for the plaintiff argued the defendants failed to order laboratory tests in the presence of fever, and failed to consult neurosurgery services. The plaintiff’s neurosurgeon expert stated the extensive lumbar epidural abscess seen on the MRI during the third ED visit would have been appreciable on the second ED visit if an MRI had been obtained at that time. The defendants settled the case for $1 million.
Discussion
Spinal epidural abscesses (SEAs) are an uncommon but serious infection that must be recognized and treated promptly to avoid permanent neurological complications. These abscesses occur most commonly in the thoracolumbar area, where the epidural space is larger. Since the epidural space is a vertical sheath, an abscess that begins at one level commonly extends to multiple levels; SEAs frequently range three to five spinal cord segments.1 The median age of onset for an SEA is approximately 50 years, and they are more common in men.1 Risk factors for the development of an SEA include epidural catheter placement, paraspinal injections of glucocorticoids or analgesics, IV drug abuse, human immunodeficiency virus infection, diabetes mellitus, alcohol abuse, trauma, tattoos, acupuncture, and hemodialysis.1,2 The most common pathogens causing an SEA are Staphylococcus aureus, gram-negative bacilli, and Streptococci.2 The percentage of S aureus that are methicillin-resistant (ie, MRSA) varies by geographic location, ranging from 40% to 68%.3,4
Signs and Symptoms
Typically, patients with an SEA initially present with fever, malaise, and nonspecific symptoms and, as seen in this case, generally present several times to a physician before the correct diagnosis is made.1 Unfortunately, the classic triad of fever, spinal pain, and neurological deficits is only infrequently observed. Fever is present in approximately two-thirds of patients, and spinal pain is present approximately 90% of the time.2
There are four stages of disease progression associated with SEAs. A typical scenario involves the initial complaint of back pain (stage I); followed by pain in the distribution of an affected nerve root (stage II); then motor weakness, sensory changes, and bladder or bowel dysfunction (stage III); and, finally, paralysis (stage IV).1,2
Diagnosis
Laboratory studies typically are not helpful in making the diagnosis. A complete blood count may show leukocytosis, but values can also be within the normal reference range. Acute phase reactants like erythrocyte sedimentation rate and C-reactive protein are commonly elevated with an SEA, but are neither sensitive nor specific.1
To make the diagnosis, the best test is a gadolinium-enhanced MRI of the spine.2 It may be prudent to image the entire spine because multiple skip lesions are common, and a patient may not have pain or tenderness in all affected areas. If MRI is not available, a CT scan of the spine with IV contrast is an acceptable alternative.1
Once an SEA is identified, it is important to determine the organism(s) responsible for the infection. The best culture source is from the abscess itself (90%) followed by blood cultures (62%) and cerebrospinal fluid, which are positive only 19% of the time.1
Treatment
Once an SEA is diagnosed, a multidisciplinary approach involving hospitalists, interventional radiology, neurosurgery, and/or orthopedics is best. The most effective management is to treat patients with a combination of surgical decompression and drainage with systemic antibiotic therapy, typically for a minimum of 4 weeks. A minority of select patients may be treated with antibiotics alone.
References
1. Sexton, DJ, Sampson JH. Spinal epidural abscess. UpToDate Web site. http://www.uptodate.com/contents/spinal-epidural-abscess. Updated June 23, 2016. Accessed January 9, 2017.
2. Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020. doi:10.1056/NEJMra055111.
3. Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes. J Microbiol Immunol Infect. 2008;41(3):215-221.
4. Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep. 2014;16(11):436. doi:10.1007/s11908-014-0436-7.
A Pain in the Back
A 57-year-old man presented to the ED with a chief complaint of severe low back pain that radiated into his left buttock. The patient stated the pain started immediately after he had bent over to pick up a small refrigerator. He denied any abdominal pain, lower extremity numbness, weakness, or bowel or bladder dysfunction. The patient’s medical history was significant for hypertension, for which he was taking amlodipine, and sleep apnea. The patient stated that he had a continuous positive airway pressure (CPAP) machine for his sleep apnea, but did not use it regularly. Regarding his social history, the patient denied tobacco use, but did admit to daily alcohol consumption.
The patient’s vital signs were all normal. Physical examination was remarkable only for bilateral lumbar paraspinal muscle tenderness, which was greater on the left side. There was no midline tenderness. Straight leg-raise testing was negative bilaterally, and the patient had normal strength and deep tendon reflexes in the lower extremities. The abdomen was soft and nontender.
The emergency physician (EP) diagnosed the patient with muscle strain, and discharged him home with a prescription for hydrocodone, along with instructions to follow-up with his primary care physician (PCP) within the next few days.
Three days later, the patient presented again to the same ED complaining of increased lower back pain. He denied any new injury or overuse, and continued to deny any lower extremity numbness or weakness or bowel/bladder dysfunction. Similarly, the physical examination was unchanged. The patient was given an intramuscular (IM) injection of hydromorphone with promethazine, as well as oral diazepam, and discharged home with instructions to continue to take the hydrocodone as needed for pain.
According to his wife, the patient retired to bed shortly after arriving home from the ED. Approximately 90 minutes later, she discovered the patient unresponsive in bed and called emergency medical services (EMS). He was brought back to the same hospital ED via EMS and was emergently intubated upon arrival. Unfortunately, the patient had suffered an anoxic brain injury and never regained consciousness; he died 1 week later.
The patient’s wife sued the EP, claiming the anoxic brain injury was related to the drugs ordered by the EP in combination with the patient’s alcohol use. She alleged that if the EP had observed the patient in the ED for signs of respiratory distress, his condition would have been treated and the anoxic brain injury would have been prevented. The plaintiff also faulted the EP for not informing the patient of the risks of drinking alcohol while taking the prescription pain medication.
The EP asserted his care of the patient was appropriate, and that there was no reason to keep the patient for observation. Regarding counseling the patient about the risks associated with concomitant alcohol consumption and pain medication, the EP stated that he had relied on the nurse who administered the medications to provide such counsel. The EP further maintained the plaintiff’s death was due to the patient not using his CPAP machine as prescribed for sleep apnea, along with his alcohol consumption the evening of the event. At trial, a defense verdict was returned.
Discussion
Unfortunately, there are several unknowns in this case. Did the patient drink any alcohol after returning home from the second ED visit, prior to going to sleep? If so, how much did he consume? Did he take any of the narcotic pills prescribed from the first ED visit and, if so, how many did he take and in what time frame?
The combination of narcotics, benzodiazepines, and alcohol has long been known to be a potentially lethal combination, resulting in respiratory depression, respiratory arrest, anoxic brain injury and even death. As EPs, we are confronted with patients complaining of pain during every shift. Complicating matters, in national patient surveys concerning the care received in the ED, patients are specifically asked if their pain was adequately treated. At the same time, there is a national effort across all specialties to reduce the amount of opioids prescribed to patients. The EP should therefore attempt to select the least potent medication that will adequately control the patient’s pain.
The WHO Pain Ladder
In 1986, the World Health Organization (WHO) developed a three-step analgesic ladder to guide the management of cancer pain.1 This guide has since been expanded to include pain of noncancer etiology. Mild pain, defined on the numerical rating scale (NRS) as 1 to 3, is considered step 1.1,2 Moderate pain (NRS of 4-6) is considered step 2, and severe pain (NRS of 7-10) is step 3. For step 1 pain, acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) is recommended. For step 2 pain, a weak narcotic (eg, codeine, hydrocodone), with or without acetaminophen or an NSAID, is recommended. For step 3 pain, a strong narcotic agent such as morphine or hydromorphone is advised.1
The WHO’s ladder is not meant to serve as a strict protocol, but rather as a tool to guide the clinician in determining a reasonable starting point in pain management. Although the EP in this case did not ask the patient to rate his pain, from all indications it appeared to be severe (step 3) and as such, the choice of prescribing hydromorphone was a reasonable one. However, most experts agree that it is best to titrate an analgesic to the desired effect. In patients with severe pain, this means employing the intravenous (IV) route, not the IM route, which was used in this case. This is because the IM route can result in variable absorption and an unpredictable time of onset and duration of action.
Concomitant Antiemetic Therapy
It is common practice to administer an antiemetic simultaneously with a narcotic to prevent or lessen associated nausea and vomiting. The clinician must be aware, however, that all antiemetics act as central nervous system (CNS) depressants to some degree. The addition of diazepam in this case is problematic because all benzodiazepines cause sedation and anxiolysis. The combination of benzodiazepines with other CNS depressants, such as opioid analgesics, can lead to excessive sedation, resulting in partial airway obstruction, respiratory depression, and hypoxia.3 The risk of an adverse outcome significantly increases with concomitant alcohol consumption.
The EP must carefully consider the risks and benefits any time opioids and benzodiazepines are administered in combination. In addition, the underlying health of the patient must be considered. Risk factors for opioid-induced respiratory depression includes age older than 50 years, a history of sleep apnea, preexisting pulmonary disease (eg, chronic obstructive pulmonary disease), anatomic oral or airway abnormalities, and renal or hepatic impairment.3 Finally, patients should be informed of the dangers of mixing alcohol with opioids and benzodiazepines—whether such counsel is given by a physician, nurse, or pharmacist.
References
1. World Health Organization. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: World Health Organization; 1996:1-69.
2. Todd KH. Pain assessment instruments for us in the emergency department. Emerg Med Clin North Am. 2005;23(2):285-295.
3. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing. 2011;12(3):118-145.
A Pain in the Buttock(s)
A 33-year-old Hispanic man, whose only spoken language was Spanish, presented to the ED for evaluation of pain in his right buttock. A coworker who accompanied the patient to the ED and served as his translator stated the patient’s pain began the previous day, immediately following a work-related injury in which the patient had slipped and fallen backward, landing on his buttocks. The patient denied any head injury, loss of consciousness, or neck pain. He further stated that he was otherwise in good health and was not taking any medications. Regarding social history, the patient denied any alcohol or drug use.
The patient’s vital signs were normal, as was his physical examination. The EP ordered an anteroposterior X-ray of the pelvis, which radiology services interpreted as normal. The EP diagnosed a buttock contusion secondary to fall, and discharged the patient home with instructions to take over-the-counter (OTC) ibuprofen for pain as needed.
Four days later, the patient presented to the same ED, complaining of low back pain radiating down his right leg. He denied any new injuries or falls, any lower extremity weakness, or bowel or bladder discomfort. Through a translator, the patient further noted that although he had been taking the OTC ibuprofen as prescribed, it had not alleviated his pain.
On physical examination, the patient’s vital signs were: blood pressure (BP), 112/62 mm Hg; heart rate (HR), 96 beats/min; respiratory rate (RR), 20 breaths/min; and temperature (T), 101.8˚F. Oxygen saturation was 98% on room air. The lung, heart, and abdominal examinations were normal. The patient was noted to be tender to palpation over the sacral and coccygeal region. There was no documentation of a lower extremity or neurological examination.
The EP ordered a computed tomography (CT) scan of the pelvis without contrast. This was interpreted by radiology services as demonstrating moderate facet arthropathy at L4/L5 on the left side, with a facet joint cyst extending in the central canal; no fracture was identified. The radiologist suggested that these findings could be better evaluated with a magnetic resonance imaging (MRI) study of the lumbar spine, if clinically indicated. The EP decided against ordering the MRI, diagnosed the patient with a contusion of the coccyx, and discharged him home with instructions to continue taking OTC ibuprofen; he also prescribed combination acetaminophen/oxycodone for severe pain, and instructed the patient to follow-up with his PCP in 2 days.
Forty eight hours later, the patient returned to the same ED via EMS, now with the complaint of inability to walk or urinate. He continued to have a fever and was tachycardic with an HR of 110 beats/min; BP, RR, and T were normal, as was his oxygen saturation on room air. The patient was noted to have significant lower extremity weakness. A Foley catheter was placed, and 1,200 cc of urine was obtained. An emergent MRI of the lumbar spine revealed an extensive lumbar epidural abscess along the lumbar spine. The patient was started on IV antibiotics and taken to the operating room for evacuation of the epidural abscess. Unfortunately, he had a prolonged inpatient stay due to persistent bilateral lower extremity weakness and neurogenic bowel and bladder dysfunction; he eventually was discharged to a skilled nursing facility.
The patient sued the EP and hospital for their failure to appreciate the significance of his presentation at the second ED visit. The attorneys for the plaintiff argued the defendants failed to order laboratory tests in the presence of fever, and failed to consult neurosurgery services. The plaintiff’s neurosurgeon expert stated the extensive lumbar epidural abscess seen on the MRI during the third ED visit would have been appreciable on the second ED visit if an MRI had been obtained at that time. The defendants settled the case for $1 million.
Discussion
Spinal epidural abscesses (SEAs) are an uncommon but serious infection that must be recognized and treated promptly to avoid permanent neurological complications. These abscesses occur most commonly in the thoracolumbar area, where the epidural space is larger. Since the epidural space is a vertical sheath, an abscess that begins at one level commonly extends to multiple levels; SEAs frequently range three to five spinal cord segments.1 The median age of onset for an SEA is approximately 50 years, and they are more common in men.1 Risk factors for the development of an SEA include epidural catheter placement, paraspinal injections of glucocorticoids or analgesics, IV drug abuse, human immunodeficiency virus infection, diabetes mellitus, alcohol abuse, trauma, tattoos, acupuncture, and hemodialysis.1,2 The most common pathogens causing an SEA are Staphylococcus aureus, gram-negative bacilli, and Streptococci.2 The percentage of S aureus that are methicillin-resistant (ie, MRSA) varies by geographic location, ranging from 40% to 68%.3,4
Signs and Symptoms
Typically, patients with an SEA initially present with fever, malaise, and nonspecific symptoms and, as seen in this case, generally present several times to a physician before the correct diagnosis is made.1 Unfortunately, the classic triad of fever, spinal pain, and neurological deficits is only infrequently observed. Fever is present in approximately two-thirds of patients, and spinal pain is present approximately 90% of the time.2
There are four stages of disease progression associated with SEAs. A typical scenario involves the initial complaint of back pain (stage I); followed by pain in the distribution of an affected nerve root (stage II); then motor weakness, sensory changes, and bladder or bowel dysfunction (stage III); and, finally, paralysis (stage IV).1,2
Diagnosis
Laboratory studies typically are not helpful in making the diagnosis. A complete blood count may show leukocytosis, but values can also be within the normal reference range. Acute phase reactants like erythrocyte sedimentation rate and C-reactive protein are commonly elevated with an SEA, but are neither sensitive nor specific.1
To make the diagnosis, the best test is a gadolinium-enhanced MRI of the spine.2 It may be prudent to image the entire spine because multiple skip lesions are common, and a patient may not have pain or tenderness in all affected areas. If MRI is not available, a CT scan of the spine with IV contrast is an acceptable alternative.1
Once an SEA is identified, it is important to determine the organism(s) responsible for the infection. The best culture source is from the abscess itself (90%) followed by blood cultures (62%) and cerebrospinal fluid, which are positive only 19% of the time.1
Treatment
Once an SEA is diagnosed, a multidisciplinary approach involving hospitalists, interventional radiology, neurosurgery, and/or orthopedics is best. The most effective management is to treat patients with a combination of surgical decompression and drainage with systemic antibiotic therapy, typically for a minimum of 4 weeks. A minority of select patients may be treated with antibiotics alone.
References
1. Sexton, DJ, Sampson JH. Spinal epidural abscess. UpToDate Web site. http://www.uptodate.com/contents/spinal-epidural-abscess. Updated June 23, 2016. Accessed January 9, 2017.
2. Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020. doi:10.1056/NEJMra055111.
3. Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes. J Microbiol Immunol Infect. 2008;41(3):215-221.
4. Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep. 2014;16(11):436. doi:10.1007/s11908-014-0436-7.
A Pain in the Back
A 57-year-old man presented to the ED with a chief complaint of severe low back pain that radiated into his left buttock. The patient stated the pain started immediately after he had bent over to pick up a small refrigerator. He denied any abdominal pain, lower extremity numbness, weakness, or bowel or bladder dysfunction. The patient’s medical history was significant for hypertension, for which he was taking amlodipine, and sleep apnea. The patient stated that he had a continuous positive airway pressure (CPAP) machine for his sleep apnea, but did not use it regularly. Regarding his social history, the patient denied tobacco use, but did admit to daily alcohol consumption.
The patient’s vital signs were all normal. Physical examination was remarkable only for bilateral lumbar paraspinal muscle tenderness, which was greater on the left side. There was no midline tenderness. Straight leg-raise testing was negative bilaterally, and the patient had normal strength and deep tendon reflexes in the lower extremities. The abdomen was soft and nontender.
The emergency physician (EP) diagnosed the patient with muscle strain, and discharged him home with a prescription for hydrocodone, along with instructions to follow-up with his primary care physician (PCP) within the next few days.
Three days later, the patient presented again to the same ED complaining of increased lower back pain. He denied any new injury or overuse, and continued to deny any lower extremity numbness or weakness or bowel/bladder dysfunction. Similarly, the physical examination was unchanged. The patient was given an intramuscular (IM) injection of hydromorphone with promethazine, as well as oral diazepam, and discharged home with instructions to continue to take the hydrocodone as needed for pain.
According to his wife, the patient retired to bed shortly after arriving home from the ED. Approximately 90 minutes later, she discovered the patient unresponsive in bed and called emergency medical services (EMS). He was brought back to the same hospital ED via EMS and was emergently intubated upon arrival. Unfortunately, the patient had suffered an anoxic brain injury and never regained consciousness; he died 1 week later.
The patient’s wife sued the EP, claiming the anoxic brain injury was related to the drugs ordered by the EP in combination with the patient’s alcohol use. She alleged that if the EP had observed the patient in the ED for signs of respiratory distress, his condition would have been treated and the anoxic brain injury would have been prevented. The plaintiff also faulted the EP for not informing the patient of the risks of drinking alcohol while taking the prescription pain medication.
The EP asserted his care of the patient was appropriate, and that there was no reason to keep the patient for observation. Regarding counseling the patient about the risks associated with concomitant alcohol consumption and pain medication, the EP stated that he had relied on the nurse who administered the medications to provide such counsel. The EP further maintained the plaintiff’s death was due to the patient not using his CPAP machine as prescribed for sleep apnea, along with his alcohol consumption the evening of the event. At trial, a defense verdict was returned.
Discussion
Unfortunately, there are several unknowns in this case. Did the patient drink any alcohol after returning home from the second ED visit, prior to going to sleep? If so, how much did he consume? Did he take any of the narcotic pills prescribed from the first ED visit and, if so, how many did he take and in what time frame?
The combination of narcotics, benzodiazepines, and alcohol has long been known to be a potentially lethal combination, resulting in respiratory depression, respiratory arrest, anoxic brain injury and even death. As EPs, we are confronted with patients complaining of pain during every shift. Complicating matters, in national patient surveys concerning the care received in the ED, patients are specifically asked if their pain was adequately treated. At the same time, there is a national effort across all specialties to reduce the amount of opioids prescribed to patients. The EP should therefore attempt to select the least potent medication that will adequately control the patient’s pain.
The WHO Pain Ladder
In 1986, the World Health Organization (WHO) developed a three-step analgesic ladder to guide the management of cancer pain.1 This guide has since been expanded to include pain of noncancer etiology. Mild pain, defined on the numerical rating scale (NRS) as 1 to 3, is considered step 1.1,2 Moderate pain (NRS of 4-6) is considered step 2, and severe pain (NRS of 7-10) is step 3. For step 1 pain, acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID) is recommended. For step 2 pain, a weak narcotic (eg, codeine, hydrocodone), with or without acetaminophen or an NSAID, is recommended. For step 3 pain, a strong narcotic agent such as morphine or hydromorphone is advised.1
The WHO’s ladder is not meant to serve as a strict protocol, but rather as a tool to guide the clinician in determining a reasonable starting point in pain management. Although the EP in this case did not ask the patient to rate his pain, from all indications it appeared to be severe (step 3) and as such, the choice of prescribing hydromorphone was a reasonable one. However, most experts agree that it is best to titrate an analgesic to the desired effect. In patients with severe pain, this means employing the intravenous (IV) route, not the IM route, which was used in this case. This is because the IM route can result in variable absorption and an unpredictable time of onset and duration of action.
Concomitant Antiemetic Therapy
It is common practice to administer an antiemetic simultaneously with a narcotic to prevent or lessen associated nausea and vomiting. The clinician must be aware, however, that all antiemetics act as central nervous system (CNS) depressants to some degree. The addition of diazepam in this case is problematic because all benzodiazepines cause sedation and anxiolysis. The combination of benzodiazepines with other CNS depressants, such as opioid analgesics, can lead to excessive sedation, resulting in partial airway obstruction, respiratory depression, and hypoxia.3 The risk of an adverse outcome significantly increases with concomitant alcohol consumption.
The EP must carefully consider the risks and benefits any time opioids and benzodiazepines are administered in combination. In addition, the underlying health of the patient must be considered. Risk factors for opioid-induced respiratory depression includes age older than 50 years, a history of sleep apnea, preexisting pulmonary disease (eg, chronic obstructive pulmonary disease), anatomic oral or airway abnormalities, and renal or hepatic impairment.3 Finally, patients should be informed of the dangers of mixing alcohol with opioids and benzodiazepines—whether such counsel is given by a physician, nurse, or pharmacist.
References
1. World Health Organization. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: World Health Organization; 1996:1-69.
2. Todd KH. Pain assessment instruments for us in the emergency department. Emerg Med Clin North Am. 2005;23(2):285-295.
3. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Management Nursing. 2011;12(3):118-145.
A Pain in the Buttock(s)
A 33-year-old Hispanic man, whose only spoken language was Spanish, presented to the ED for evaluation of pain in his right buttock. A coworker who accompanied the patient to the ED and served as his translator stated the patient’s pain began the previous day, immediately following a work-related injury in which the patient had slipped and fallen backward, landing on his buttocks. The patient denied any head injury, loss of consciousness, or neck pain. He further stated that he was otherwise in good health and was not taking any medications. Regarding social history, the patient denied any alcohol or drug use.
The patient’s vital signs were normal, as was his physical examination. The EP ordered an anteroposterior X-ray of the pelvis, which radiology services interpreted as normal. The EP diagnosed a buttock contusion secondary to fall, and discharged the patient home with instructions to take over-the-counter (OTC) ibuprofen for pain as needed.
Four days later, the patient presented to the same ED, complaining of low back pain radiating down his right leg. He denied any new injuries or falls, any lower extremity weakness, or bowel or bladder discomfort. Through a translator, the patient further noted that although he had been taking the OTC ibuprofen as prescribed, it had not alleviated his pain.
On physical examination, the patient’s vital signs were: blood pressure (BP), 112/62 mm Hg; heart rate (HR), 96 beats/min; respiratory rate (RR), 20 breaths/min; and temperature (T), 101.8˚F. Oxygen saturation was 98% on room air. The lung, heart, and abdominal examinations were normal. The patient was noted to be tender to palpation over the sacral and coccygeal region. There was no documentation of a lower extremity or neurological examination.
The EP ordered a computed tomography (CT) scan of the pelvis without contrast. This was interpreted by radiology services as demonstrating moderate facet arthropathy at L4/L5 on the left side, with a facet joint cyst extending in the central canal; no fracture was identified. The radiologist suggested that these findings could be better evaluated with a magnetic resonance imaging (MRI) study of the lumbar spine, if clinically indicated. The EP decided against ordering the MRI, diagnosed the patient with a contusion of the coccyx, and discharged him home with instructions to continue taking OTC ibuprofen; he also prescribed combination acetaminophen/oxycodone for severe pain, and instructed the patient to follow-up with his PCP in 2 days.
Forty eight hours later, the patient returned to the same ED via EMS, now with the complaint of inability to walk or urinate. He continued to have a fever and was tachycardic with an HR of 110 beats/min; BP, RR, and T were normal, as was his oxygen saturation on room air. The patient was noted to have significant lower extremity weakness. A Foley catheter was placed, and 1,200 cc of urine was obtained. An emergent MRI of the lumbar spine revealed an extensive lumbar epidural abscess along the lumbar spine. The patient was started on IV antibiotics and taken to the operating room for evacuation of the epidural abscess. Unfortunately, he had a prolonged inpatient stay due to persistent bilateral lower extremity weakness and neurogenic bowel and bladder dysfunction; he eventually was discharged to a skilled nursing facility.
The patient sued the EP and hospital for their failure to appreciate the significance of his presentation at the second ED visit. The attorneys for the plaintiff argued the defendants failed to order laboratory tests in the presence of fever, and failed to consult neurosurgery services. The plaintiff’s neurosurgeon expert stated the extensive lumbar epidural abscess seen on the MRI during the third ED visit would have been appreciable on the second ED visit if an MRI had been obtained at that time. The defendants settled the case for $1 million.
Discussion
Spinal epidural abscesses (SEAs) are an uncommon but serious infection that must be recognized and treated promptly to avoid permanent neurological complications. These abscesses occur most commonly in the thoracolumbar area, where the epidural space is larger. Since the epidural space is a vertical sheath, an abscess that begins at one level commonly extends to multiple levels; SEAs frequently range three to five spinal cord segments.1 The median age of onset for an SEA is approximately 50 years, and they are more common in men.1 Risk factors for the development of an SEA include epidural catheter placement, paraspinal injections of glucocorticoids or analgesics, IV drug abuse, human immunodeficiency virus infection, diabetes mellitus, alcohol abuse, trauma, tattoos, acupuncture, and hemodialysis.1,2 The most common pathogens causing an SEA are Staphylococcus aureus, gram-negative bacilli, and Streptococci.2 The percentage of S aureus that are methicillin-resistant (ie, MRSA) varies by geographic location, ranging from 40% to 68%.3,4
Signs and Symptoms
Typically, patients with an SEA initially present with fever, malaise, and nonspecific symptoms and, as seen in this case, generally present several times to a physician before the correct diagnosis is made.1 Unfortunately, the classic triad of fever, spinal pain, and neurological deficits is only infrequently observed. Fever is present in approximately two-thirds of patients, and spinal pain is present approximately 90% of the time.2
There are four stages of disease progression associated with SEAs. A typical scenario involves the initial complaint of back pain (stage I); followed by pain in the distribution of an affected nerve root (stage II); then motor weakness, sensory changes, and bladder or bowel dysfunction (stage III); and, finally, paralysis (stage IV).1,2
Diagnosis
Laboratory studies typically are not helpful in making the diagnosis. A complete blood count may show leukocytosis, but values can also be within the normal reference range. Acute phase reactants like erythrocyte sedimentation rate and C-reactive protein are commonly elevated with an SEA, but are neither sensitive nor specific.1
To make the diagnosis, the best test is a gadolinium-enhanced MRI of the spine.2 It may be prudent to image the entire spine because multiple skip lesions are common, and a patient may not have pain or tenderness in all affected areas. If MRI is not available, a CT scan of the spine with IV contrast is an acceptable alternative.1
Once an SEA is identified, it is important to determine the organism(s) responsible for the infection. The best culture source is from the abscess itself (90%) followed by blood cultures (62%) and cerebrospinal fluid, which are positive only 19% of the time.1
Treatment
Once an SEA is diagnosed, a multidisciplinary approach involving hospitalists, interventional radiology, neurosurgery, and/or orthopedics is best. The most effective management is to treat patients with a combination of surgical decompression and drainage with systemic antibiotic therapy, typically for a minimum of 4 weeks. A minority of select patients may be treated with antibiotics alone.
References
1. Sexton, DJ, Sampson JH. Spinal epidural abscess. UpToDate Web site. http://www.uptodate.com/contents/spinal-epidural-abscess. Updated June 23, 2016. Accessed January 9, 2017.
2. Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012-2020. doi:10.1056/NEJMra055111.
3. Chen WC, Wang JL, Wang JT, Chen YC, Chang SC. Spinal epidural abscess due to Staphylococcus aureus: clinical manifestations and outcomes. J Microbiol Immunol Infect. 2008;41(3):215-221.
4. Krishnamohan P, Berger JR. Spinal epidural abscess. Curr Infect Dis Rep. 2014;16(11):436. doi:10.1007/s11908-014-0436-7.
Emergency Imaging: Abdominal Pain 6 Months After Cesarean Delivery
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).
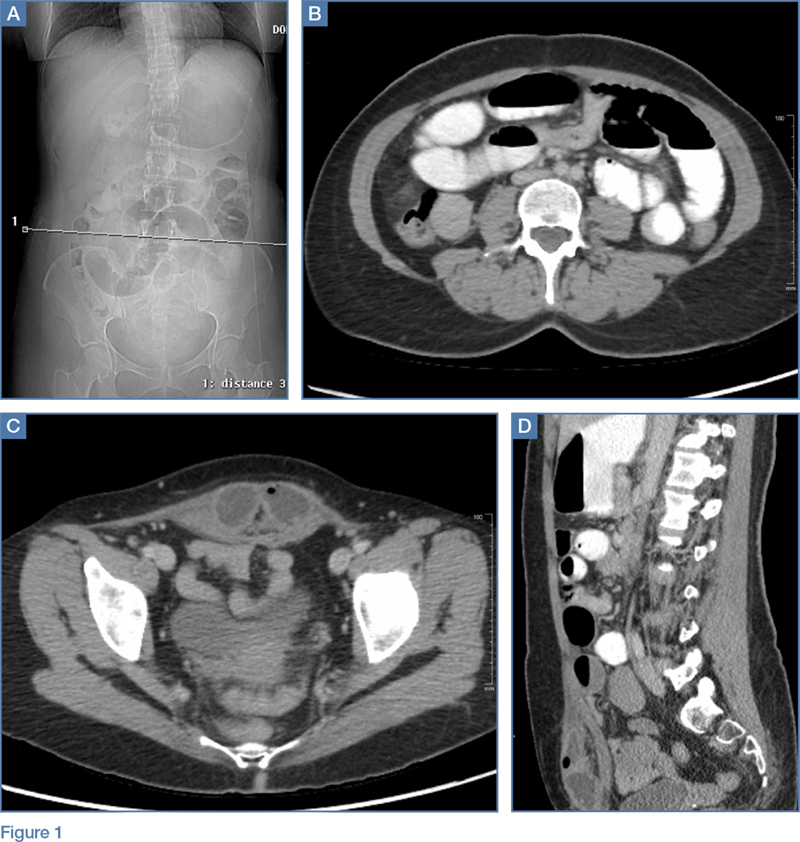
What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.
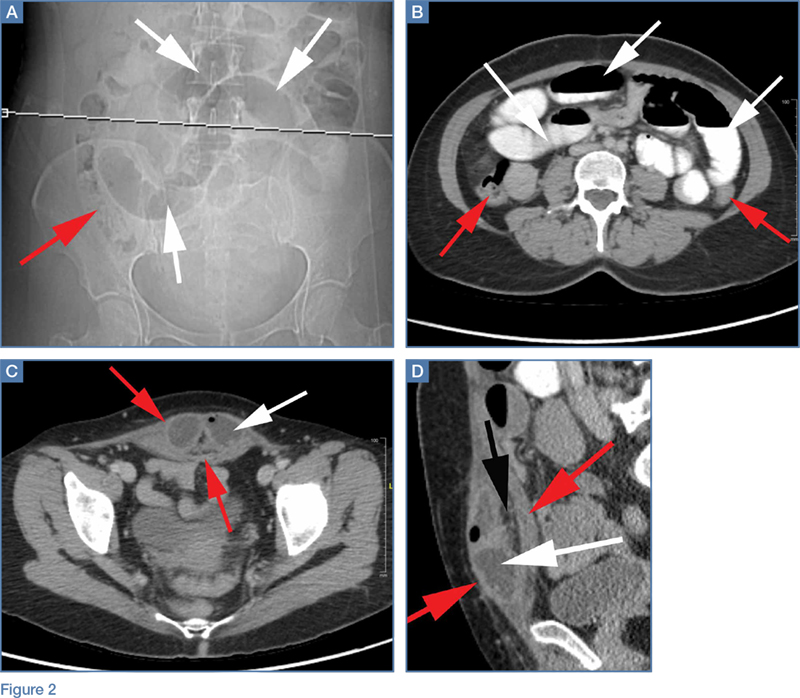
An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
First EDition: A-Fib Management Pathway in the ED, more
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Atrial Fibrillation Management Pathway in the ED May Lower Hospital Admissions
TED BOSWORTH
FRONTLINE MEDICAL NEWS
An atrial fibrillation (AF) treatment pathway designed specifically to reduce the proportion of patients with this complaint who are admitted to the hospital from the ED was remarkably effective, according to a pilot study presented at the annual International AF Symposium.
“In this single-center observational study, a multidisciplinary AF pathway was associated with 5-fold reduction in admission rate and 2.5-fold reduction in length-of-stay [LOS] for those who were admitted,” reported Jeremy N. Ruskin, MD.
Relative to many other countries, admission rates for AF in the United States are “extremely high,” according to Dr Ruskin, director of the cardiac arrhythmia service at Massachusetts General Hospital, Boston. Citing 2013 figures from the Nationwide Emergency Department Sample (NEDS) database, rates ranged between 60% and 80% by geographic region, with an average of about 66%. In contrast, and as an example of lower rates elsewhere, fewer than 40% of AF patients with similar characteristics presenting at EDs in Ontario, Canada were admitted. Similarly low admission rates have been reported in Europe.
The AF pathway tested in the study at Massachusetts General was developed through collaboration between electrophysiologists and emergency physicians (EPs). It is suitable for patients presenting with a primary complaint of AF without concomitant diseases, such as sepsis or myocardial infarction. Patients were entered into this study after it was shown that AF was the chief complaint. The first step was to determine whether participants were best suited to a rhythm-control or rate-control strategy.
“The rhythm-control group was anticoagulated and then underwent expedited cardioversion with TEE [transesophageal echocardiogram] if necessary. The rate-control group was anticoagulated and then given appropriate pharmacologic therapy,” Dr Ruskin explained. Once patients were on treatment, an electrophysiologist and an EP evaluated the patients’ response. For both groups, stable patients were discharged and unstable patients were admitted.
In this nonrandomized observational study conducted over a 1-year period, 94 patients were managed with the AF pathway. Admissions and outcomes in this group were compared with 265 patients who received usual care.
Only 16% of those managed through the AF pathway were admitted versus 80% (P < .001) in the usual care group. Among those admitted, LOS was shorter in patients managed along the AF pathway relative to usual care (32 vs 85 hours; P = .002). Dr Ruskin reported that both the cardioversion rate and the proportion of patients discharged on novel oral anticoagulation drugs were higher in the AF pathway group.
The reductions in hospital admissions would be expected to translate into large reductions in costs, particularly as follow-up showed no difference in return visits to the hospital between those entered into the AF pathway relative to those who received routine care, according to Dr Ruskin. Emphasizing the cost burden of AF admissions, he noted that the estimated charges for the more than 300,000 AF admissions in US hospitals in 2013 exceeded $7 billion.
Currently, there are no uniform guidelines for managing AF in the ED, and there is wide variation in practice among centers, according to Dr Ruskin. He provided data from the NEDS database demonstrating highly significant variations in rates of admission by geographic region (eg, rates were >10% higher in the northeast vs the west) and hospital type (eg, rates were twice as high in metropolitan than nonmetropolitan hospitals).
In the NEDS database, various patient characteristics were associated with increased odds ratios (ORs) for admission. These included hypertension (OR, 2.3), valvular disease (OR, 3.6), and congestive heart failure (OR, 3.7). However, Dr Ruskin indicated that patients with these or other characteristics associated with increased likelihood of admission, such as older age, have better outcomes with hospitalization.
The data from this initial observational study were recently published, and a larger prospective study of this AF pathway is already underway at both Massachusetts General and at Brigham and Women’s Hospital, Boston. If the data confirm that AF admissions can be safely reduced through this pathway, Dr Ruskin anticipates that implementation will be adopted at other hospitals in the Harvard system.
Ptaszek LM, White B, Lubitz SA, et al. Effect of a multidisciplinary approach for the management of patients with atrial fibrillation in the emergency department on hospital admission rate and length of stay. Am J Cardiol. 2016;118(1):64-71. doi:10.1016/j.amjcard.2016.04.014.
Understanding SSTI Admission, Treatment Crucial to Reducing Disease Burden
DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
Decreasing the burden of treating skin and soft tissue infections (SSTIs) is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Connecticut) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous antibiotic therapy or treated as outpatients.”
Dr Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average LOS and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion.
“Patients were categorized into two groups based on disposition of care, inpatient or outpatient, on index presentation,” the authors explained. “Economic data were collected using reports from hospital finance databases and included reports of total billed costs.”
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall (22.6% for inpatients and 28.3% for outpatients; P > .05) or for SSTI-related re-presentation (10.4% for inpatients and 15.1% for outpatients; P > .05). For those patients who were admitted, the mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of 0 were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of 1, and 60.9% of those with a CCI score of 2 or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S aureus (MRSA) was the most common (66.7%). According to the investigators, gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high-frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
Linder KE, Nicolau DP, Nailor MD. Epidemiology, treatment, and economics of patients presenting to the emergency department for skin and soft tissue infections. Hosp Pract (1995). 2017;16:1-7. doi:10.1080/21548331.2017.1279519.
Adolescents, Boys, Black Children Most Likely To Be Hospitalized for SJS and TEN
WHITNEY MCKNIGHT
FRONTLINE MEDICAL NEWS
Annual hospitalization rates in the United States for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were shown to be higher in adolescents, boys, and black children, in a cross-sectional analysis of discharge records from more than 4,100 hospitals.
Using relevant ICD-9 codes, researchers at Harvard University identified 1,571 patients hospitalized for SJS, TEN, or both in 2009 and 2012, as listed in the Kids Inpatient Database from the Agency for Healthcare Research and Quality. The highest hospitalization rates per 100,000 in each year were for adolescents between ages 15 and 19 years (P = .01), boys (P = .03), and black children (P = .82). The overall risk of death from these conditions was 1.5% in 2009 and 0.3% in 2012. The data were published online in a brief report.
Although the difference in the number of hospitalizations for black children was not significant when compared with other ethnic and racial groups, at 1.03 hospitalizations per 100,000 children (95% confidence interval [CI], 0.80-1.31) in 2009 and 1.06 hospitalizations per 100,000 children (95% CI, 0.86-1.30) in 2012, the rate was greatest in this group. The next highest ratio was in white children at 0.82 hospitalizations per 100,000 (95% CI, 0.74-0.91) in 2009, and 0.95 hospitalizations per 100,000 (95% CI, 0.86-1.05) in 2012.
With the number of SJS- and TEN-related hospitalizations between 0.1 and 1.0 per 100,000, lead author Yusuke Okubo, MD, MPH, and colleagues wrote that their data aligned with previous studies; however, regarding the emphasis on demographic differences, theirs was, to the best of their knowledge, “the first study to reveal these disparities.” Compared with adults, they added, mortality was “remarkably lower” in children.
Okubo Y, Nochioka K, Testa MA. Nationwide survey of Stevens-Johnson syndrome and toxic epidermal necrolysis in children in the United States. Pediatr Dermatol. 2016 Dec 19. doi:10.1111/pde.13050. [Epub ahead of print]
Guidelines Released for Diagnosing TB in Adults, Children
MARY ANN MOON
FRONTLINE MEDICAL NEWS
A clinical practice guideline for diagnosing pulmonary, extrapulmonary, and latent tuberculosis (TB) in adults and children has been released jointly by the American Thoracic Society, the Centers for Disease Control and Prevention, and the Infectious Diseases Society of America.
The American Academy of Pediatrics also provided input to the guideline, which includes 23 evidence-based recommendations. The document is intended to assist clinicians in high-resource countries with a low incidence of TB disease and latent TB infection, such as the United States, said David M. Lewinsohn, MD, PhD, and his associates on the joint task force that wrote the guideline.
There were 9,412 cases of TB disease reported in the United States in 2014, the most recent year for which data are available. This translates to a rate of 3.0 cases per 100,000 persons. Two-thirds of the cases in the United States developed in foreign-born persons. “The rate of disease was 13.4 times higher in foreign-born persons than in US-born individuals [15.3 vs 1.1 per 100,000, respectively],” wrote Dr Lewinsohn of pulmonary and critical care medicine, Oregon Health & Science University, Portland, and colleagues.
Even though the case rate is relatively low in the United States and has declined in recent years, “an estimated 11 million persons are infected with Mycobacterium tuberculosis. Thus…there remains a large reservoir of individuals who are infected. Without the application of improved diagnosis and effective treatment for latent [disease], new cases of TB will develop from within this group,” they noted.
Among the guideline’s strongest recommendations are the following:
- Acid-fast bacilli smear microscopy should be performed in all patients suspected of having pulmonary TB, using at least three sputum samples. A sputum volume of at least 3 mL is needed, but 5 to 10 mL would be better.
- Both liquid and solid mycobacterial cultures should be performed on every specimen from patients suspected of having TB disease, rather than either type alone.
- A diagnostic nucleic acid amplification test should be performed on the initial specimen from patients suspected of having pulmonary TB.
- Rapid molecular drug-susceptibility testing of respiratory specimens is advised for certain patients, with a focus on testing for rifampin susceptibility with or without isoniazid.
- Patients suspected of having extrapulmonary TB also should have mycobacterial cultures performed on all specimens.
- For all mycobacterial cultures that are positive for TB, a culture isolate should be submitted for genotyping to a regional genotyping laboratory.
- For patients aged 5 and older who are suspected of having latent TB infection, an interferon-gamma release assay (IGRA) is advised rather than a tuberculin skin test, especially if the patient is not likely to return to have the test result read. A tuberculin skin test is an acceptable alternative if IGRA is not available, is too expensive, or is too burdensome.
The guideline also addresses bronchoscopic sampling, cell counts and chemistries from fluid specimens collected from sites suspected of harboring extrapulmonary TB (such as pleural, cerebrospinal, ascetic, or joint fluids), and measurement of adenosine deaminase levels.
Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):e1-e33. doi:10.1093/cid/ciw694.
Estate planning
The latest anniversary of my birth recently passed; I am now a provider and a beneficiary in the Medicare system. Fortunately, I have learned to celebrate these annual events, and the changes they bring, rather than dread them. I now appreciate that life gets better as we get older, on all levels – except, perhaps, the physical.
But I have also learned that birthdays are a good time to pause and consider the various financial arrangements that I’ve set up over the years, and to determine whether any of them need updating.
Estate plans, in particular, need regular review and revision. Nothing important has changed in your life since you drafted your will, you say? Well, chances are the laws have changed, or other factors may have rendered your plan obsolete without your even realizing it.
I am assuming, of course, that you have in fact drafted a will. If not – regardless of your age – do it as soon as possible. Stuff happens; if you die without a will (“intestate,” in lawyer lingo), your heirs will be at the mercy of attorneys, bureaucrats, state and federal laws, and greed. Quarrels will ensue; decisions will be made that are almost certainly at variance with what you would have wanted; and a substantial chunk of your estate that could have gone to loved ones, or to charity, will be lost to taxes and legal fees.
In other words, if you don’t write a will, others will write one for you – one your heirs probably won’t like. Don’t let that happen. That said, let’s consider some variables that mandate your constant vigilance:
Laws change. Trust laws, in particular, have changed a great deal in recent years, and trust strategies have changed with them. New instruments such as perpetual trusts, trust protectors, directed trusts, and total return trusts may or may not work to your advantage, but you won’t know without asking. State laws change too.
Once a year, my wife and I meet with our estate lawyer to learn about any new legislation that may have affected our plan. A few years ago, for example, I learned that my irrevocable trust was no longer irrevocable; new laws now permit certain provisions to be modified.
Laws that don’t directly regulate wills and trusts can impact your plan as well. For instance, the ever-popular Health Insurance Portability and Accountability Act (HIPAA) applies to your estate as well as your practice; under its provisions, your family cannot access your medical information or make treatment and life-support decisions without your specific permission. So if a Health Care Power of Attorney is not already part of your will, add it. And remember to modify it if your medical status, or your philosophy of life, changes.
Financial markets change. It’s not exactly a secret that asset values and interest rates are considerably different than they were even a few years ago. Real estate or securities bequests could now be significantly larger or smaller. Your accountant and estate lawyer should take a look at your assets periodically, and their apportionment in your will, to be sure all arrangements remain as you intend. And be sure to notify them whenever the composition of your assets changes, even if their value doesn’t. Say, for example, you sell a business or property, and reinvest the proceeds in something completely different; a different set of tax laws will apply, and your will must reflect that.
Fiduciaries change. Keep track of the executor of your estate and the trustee(s) of your trust(s), and be prepared to make changes if needed. If your brother-in-law is your executor, and your sister divorces him, you may want to name a new executor. A once-vigorous trustee who is now old or sick should be replaced. Trustees are often financial institutions; if a corporate trustee goes belly up, or the employee you were working with leaves or retires, you’ll need a replacement.
Personal circumstances change. Some changes – marriage, divorce, the death of an heir, or the birth of a new one – obviously require modifications to wills and trusts. But any significant alteration of your personal or financial circumstances probably merits a phone call to your financial planners. The need for changes, and your options, are not always obvious.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The latest anniversary of my birth recently passed; I am now a provider and a beneficiary in the Medicare system. Fortunately, I have learned to celebrate these annual events, and the changes they bring, rather than dread them. I now appreciate that life gets better as we get older, on all levels – except, perhaps, the physical.
But I have also learned that birthdays are a good time to pause and consider the various financial arrangements that I’ve set up over the years, and to determine whether any of them need updating.
Estate plans, in particular, need regular review and revision. Nothing important has changed in your life since you drafted your will, you say? Well, chances are the laws have changed, or other factors may have rendered your plan obsolete without your even realizing it.
I am assuming, of course, that you have in fact drafted a will. If not – regardless of your age – do it as soon as possible. Stuff happens; if you die without a will (“intestate,” in lawyer lingo), your heirs will be at the mercy of attorneys, bureaucrats, state and federal laws, and greed. Quarrels will ensue; decisions will be made that are almost certainly at variance with what you would have wanted; and a substantial chunk of your estate that could have gone to loved ones, or to charity, will be lost to taxes and legal fees.
In other words, if you don’t write a will, others will write one for you – one your heirs probably won’t like. Don’t let that happen. That said, let’s consider some variables that mandate your constant vigilance:
Laws change. Trust laws, in particular, have changed a great deal in recent years, and trust strategies have changed with them. New instruments such as perpetual trusts, trust protectors, directed trusts, and total return trusts may or may not work to your advantage, but you won’t know without asking. State laws change too.
Once a year, my wife and I meet with our estate lawyer to learn about any new legislation that may have affected our plan. A few years ago, for example, I learned that my irrevocable trust was no longer irrevocable; new laws now permit certain provisions to be modified.
Laws that don’t directly regulate wills and trusts can impact your plan as well. For instance, the ever-popular Health Insurance Portability and Accountability Act (HIPAA) applies to your estate as well as your practice; under its provisions, your family cannot access your medical information or make treatment and life-support decisions without your specific permission. So if a Health Care Power of Attorney is not already part of your will, add it. And remember to modify it if your medical status, or your philosophy of life, changes.
Financial markets change. It’s not exactly a secret that asset values and interest rates are considerably different than they were even a few years ago. Real estate or securities bequests could now be significantly larger or smaller. Your accountant and estate lawyer should take a look at your assets periodically, and their apportionment in your will, to be sure all arrangements remain as you intend. And be sure to notify them whenever the composition of your assets changes, even if their value doesn’t. Say, for example, you sell a business or property, and reinvest the proceeds in something completely different; a different set of tax laws will apply, and your will must reflect that.
Fiduciaries change. Keep track of the executor of your estate and the trustee(s) of your trust(s), and be prepared to make changes if needed. If your brother-in-law is your executor, and your sister divorces him, you may want to name a new executor. A once-vigorous trustee who is now old or sick should be replaced. Trustees are often financial institutions; if a corporate trustee goes belly up, or the employee you were working with leaves or retires, you’ll need a replacement.
Personal circumstances change. Some changes – marriage, divorce, the death of an heir, or the birth of a new one – obviously require modifications to wills and trusts. But any significant alteration of your personal or financial circumstances probably merits a phone call to your financial planners. The need for changes, and your options, are not always obvious.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The latest anniversary of my birth recently passed; I am now a provider and a beneficiary in the Medicare system. Fortunately, I have learned to celebrate these annual events, and the changes they bring, rather than dread them. I now appreciate that life gets better as we get older, on all levels – except, perhaps, the physical.
But I have also learned that birthdays are a good time to pause and consider the various financial arrangements that I’ve set up over the years, and to determine whether any of them need updating.
Estate plans, in particular, need regular review and revision. Nothing important has changed in your life since you drafted your will, you say? Well, chances are the laws have changed, or other factors may have rendered your plan obsolete without your even realizing it.
I am assuming, of course, that you have in fact drafted a will. If not – regardless of your age – do it as soon as possible. Stuff happens; if you die without a will (“intestate,” in lawyer lingo), your heirs will be at the mercy of attorneys, bureaucrats, state and federal laws, and greed. Quarrels will ensue; decisions will be made that are almost certainly at variance with what you would have wanted; and a substantial chunk of your estate that could have gone to loved ones, or to charity, will be lost to taxes and legal fees.
In other words, if you don’t write a will, others will write one for you – one your heirs probably won’t like. Don’t let that happen. That said, let’s consider some variables that mandate your constant vigilance:
Laws change. Trust laws, in particular, have changed a great deal in recent years, and trust strategies have changed with them. New instruments such as perpetual trusts, trust protectors, directed trusts, and total return trusts may or may not work to your advantage, but you won’t know without asking. State laws change too.
Once a year, my wife and I meet with our estate lawyer to learn about any new legislation that may have affected our plan. A few years ago, for example, I learned that my irrevocable trust was no longer irrevocable; new laws now permit certain provisions to be modified.
Laws that don’t directly regulate wills and trusts can impact your plan as well. For instance, the ever-popular Health Insurance Portability and Accountability Act (HIPAA) applies to your estate as well as your practice; under its provisions, your family cannot access your medical information or make treatment and life-support decisions without your specific permission. So if a Health Care Power of Attorney is not already part of your will, add it. And remember to modify it if your medical status, or your philosophy of life, changes.
Financial markets change. It’s not exactly a secret that asset values and interest rates are considerably different than they were even a few years ago. Real estate or securities bequests could now be significantly larger or smaller. Your accountant and estate lawyer should take a look at your assets periodically, and their apportionment in your will, to be sure all arrangements remain as you intend. And be sure to notify them whenever the composition of your assets changes, even if their value doesn’t. Say, for example, you sell a business or property, and reinvest the proceeds in something completely different; a different set of tax laws will apply, and your will must reflect that.
Fiduciaries change. Keep track of the executor of your estate and the trustee(s) of your trust(s), and be prepared to make changes if needed. If your brother-in-law is your executor, and your sister divorces him, you may want to name a new executor. A once-vigorous trustee who is now old or sick should be replaced. Trustees are often financial institutions; if a corporate trustee goes belly up, or the employee you were working with leaves or retires, you’ll need a replacement.
Personal circumstances change. Some changes – marriage, divorce, the death of an heir, or the birth of a new one – obviously require modifications to wills and trusts. But any significant alteration of your personal or financial circumstances probably merits a phone call to your financial planners. The need for changes, and your options, are not always obvious.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].