User login
Apply for These 2017 Research Grants Before They Close
Round out 2016 by preparing for a 2017 AGA Research Foundation grant. Eight grants are currently open for both established and young investigators, but all will close in January 2017. Make sure you’re prepared to submit your application before the deadline.
Complete information about these and other research awards is available on gastro.org. All recipients will be acknowledged at the Research Recognition Celebration at Digestive Disease Week® 2017 (www.ddw.org) in Chicago, IL.
Jan 6. 2017
- The AGA-Elsevier Gut Microbiome Pilot Research Award will provide $25,000 to support pilot research projects pertaining to the gut microbiome.
- The AGA-Elsevier Pilot Research Award is a 1-year grant providing young investigators, instructors, research associates, or equivalents $25,000 to support pilot research projects in gastroenterology- or hepatology-related areas.
- The AGA-Medtronic Research & Development Pilot Award in Technology grants $31,000 for 1 year to investigators to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Jan. 13, 2017
- The AGA-Rome Foundation Functional GI and Motility Disorders Pilot Research Award offers $50,000 for 1 year to early-stage investigators, established investigators, postdoctoral research fellows and combined research and clinical fellows to support pilot research projects pertaining to functional GI and motility disorders.
- The AGA Microbiome Junior Investigator Research Award is a 2-year award of $60,000 for junior investigators engaged in basic, translational, clinical, or health services research related to the gut microbiome.
- The AGA-Pfizer Pilot Research Award in Inflammatory Bowel Disease is a 1-year, $30,000 award offered to established and young investigators to support pilot research projects related to IBD.
Jan. 20, 2017
- The AGA-June & Donald O. Castell, MD, Esophageal Clinical Research Award is a 1-year, $25,000 grant that provides research or salary support for junior faculty involved in clinical research in esophageal diseases.
- The AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer is a 1-year, $40,000 award designed to support a young investigator, instructor, research associate or equivalent who currently holds a federal or nonfederal career development award devoted to conducting research related to digestive cancer.
Round out 2016 by preparing for a 2017 AGA Research Foundation grant. Eight grants are currently open for both established and young investigators, but all will close in January 2017. Make sure you’re prepared to submit your application before the deadline.
Complete information about these and other research awards is available on gastro.org. All recipients will be acknowledged at the Research Recognition Celebration at Digestive Disease Week® 2017 (www.ddw.org) in Chicago, IL.
Jan 6. 2017
- The AGA-Elsevier Gut Microbiome Pilot Research Award will provide $25,000 to support pilot research projects pertaining to the gut microbiome.
- The AGA-Elsevier Pilot Research Award is a 1-year grant providing young investigators, instructors, research associates, or equivalents $25,000 to support pilot research projects in gastroenterology- or hepatology-related areas.
- The AGA-Medtronic Research & Development Pilot Award in Technology grants $31,000 for 1 year to investigators to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Jan. 13, 2017
- The AGA-Rome Foundation Functional GI and Motility Disorders Pilot Research Award offers $50,000 for 1 year to early-stage investigators, established investigators, postdoctoral research fellows and combined research and clinical fellows to support pilot research projects pertaining to functional GI and motility disorders.
- The AGA Microbiome Junior Investigator Research Award is a 2-year award of $60,000 for junior investigators engaged in basic, translational, clinical, or health services research related to the gut microbiome.
- The AGA-Pfizer Pilot Research Award in Inflammatory Bowel Disease is a 1-year, $30,000 award offered to established and young investigators to support pilot research projects related to IBD.
Jan. 20, 2017
- The AGA-June & Donald O. Castell, MD, Esophageal Clinical Research Award is a 1-year, $25,000 grant that provides research or salary support for junior faculty involved in clinical research in esophageal diseases.
- The AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer is a 1-year, $40,000 award designed to support a young investigator, instructor, research associate or equivalent who currently holds a federal or nonfederal career development award devoted to conducting research related to digestive cancer.
Round out 2016 by preparing for a 2017 AGA Research Foundation grant. Eight grants are currently open for both established and young investigators, but all will close in January 2017. Make sure you’re prepared to submit your application before the deadline.
Complete information about these and other research awards is available on gastro.org. All recipients will be acknowledged at the Research Recognition Celebration at Digestive Disease Week® 2017 (www.ddw.org) in Chicago, IL.
Jan 6. 2017
- The AGA-Elsevier Gut Microbiome Pilot Research Award will provide $25,000 to support pilot research projects pertaining to the gut microbiome.
- The AGA-Elsevier Pilot Research Award is a 1-year grant providing young investigators, instructors, research associates, or equivalents $25,000 to support pilot research projects in gastroenterology- or hepatology-related areas.
- The AGA-Medtronic Research & Development Pilot Award in Technology grants $31,000 for 1 year to investigators to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Jan. 13, 2017
- The AGA-Rome Foundation Functional GI and Motility Disorders Pilot Research Award offers $50,000 for 1 year to early-stage investigators, established investigators, postdoctoral research fellows and combined research and clinical fellows to support pilot research projects pertaining to functional GI and motility disorders.
- The AGA Microbiome Junior Investigator Research Award is a 2-year award of $60,000 for junior investigators engaged in basic, translational, clinical, or health services research related to the gut microbiome.
- The AGA-Pfizer Pilot Research Award in Inflammatory Bowel Disease is a 1-year, $30,000 award offered to established and young investigators to support pilot research projects related to IBD.
Jan. 20, 2017
- The AGA-June & Donald O. Castell, MD, Esophageal Clinical Research Award is a 1-year, $25,000 grant that provides research or salary support for junior faculty involved in clinical research in esophageal diseases.
- The AGA-Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer is a 1-year, $40,000 award designed to support a young investigator, instructor, research associate or equivalent who currently holds a federal or nonfederal career development award devoted to conducting research related to digestive cancer.
Memorial and honorary gifts: A special tribute
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research or celebrate a special occasion such as a birthday while supporting the work of our mission through a gift to the AGA Research Foundation? Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit:
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research, which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax. A cash gift of $25,000 or more qualifies for membership in the AGA Legacy Society, which recognizes the foundation’s most generous individual donors.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one. A gift of $50,000 or more in your will qualifies for membership in the AGA Legacy Society.
- Named DDW Sessions. A named DDW session is established with a minimum gift of $125,000 over the course of 5 years or through an irrevocable planned gift. Gifts of cash, appreciated securities, life insurance, or property are gift vehicles that may be used to establish a named session. Donors will be eligible for naming recognition of a DDW AGA Institute Council session for 10 years. A gift at that level will qualifies for membership in the AGA Legacy Society.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.gastro.org/contribute or contact Harmony Excellent at 301-272-1602 or [email protected].
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research or celebrate a special occasion such as a birthday while supporting the work of our mission through a gift to the AGA Research Foundation? Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit:
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research, which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax. A cash gift of $25,000 or more qualifies for membership in the AGA Legacy Society, which recognizes the foundation’s most generous individual donors.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one. A gift of $50,000 or more in your will qualifies for membership in the AGA Legacy Society.
- Named DDW Sessions. A named DDW session is established with a minimum gift of $125,000 over the course of 5 years or through an irrevocable planned gift. Gifts of cash, appreciated securities, life insurance, or property are gift vehicles that may be used to establish a named session. Donors will be eligible for naming recognition of a DDW AGA Institute Council session for 10 years. A gift at that level will qualifies for membership in the AGA Legacy Society.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.gastro.org/contribute or contact Harmony Excellent at 301-272-1602 or [email protected].
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research or celebrate a special occasion such as a birthday while supporting the work of our mission through a gift to the AGA Research Foundation? Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit:
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research, which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax. A cash gift of $25,000 or more qualifies for membership in the AGA Legacy Society, which recognizes the foundation’s most generous individual donors.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one. A gift of $50,000 or more in your will qualifies for membership in the AGA Legacy Society.
- Named DDW Sessions. A named DDW session is established with a minimum gift of $125,000 over the course of 5 years or through an irrevocable planned gift. Gifts of cash, appreciated securities, life insurance, or property are gift vehicles that may be used to establish a named session. Donors will be eligible for naming recognition of a DDW AGA Institute Council session for 10 years. A gift at that level will qualifies for membership in the AGA Legacy Society.
Your next step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.gastro.org/contribute or contact Harmony Excellent at 301-272-1602 or [email protected].
Elizabeth A. Thiele, MD, PhD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Child, adolescent autism patients visiting EDs in higher numbers
NEW YORK – Emergency departments are seeing more pediatric and adolescent patients with autism spectrum disorder, and are struggling to meet their needs, experts say.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry, researchers presented results from studies attempting to quantify and better understand the uptick in ED visits, while clinicians shared strategies aimed at improving care in a setting that, nearly all agreed, presents unique obstacles for treating children with ASD.
Bright lights, excess noise, frequently changing care staff, and a lack of training in nonverbal communication strategies were among the problems the clinicians highlighted.
“There’s been a huge increase in recent years in the number of children with ASD that are coming into the ED because of either behavioral crises or general pediatric medical concerns that require us to intervene,” said Eron Y. Friedlaender, MD, MPH, of Children’s Hospital of Philadelphia (CHOP). “Yet, we struggle to offer kids with challenging behaviors or communication vulnerabilities the same standard of care that we’re used to offering.”
John J. McGonigle, PhD, head of the autism center at the University of Pittsburgh, noted that incidents tied to safety issues, such as disruptive behavior, aggression, and self-injury, were occurring among young ASD patients in the ED. In 2015, he said, Pennsylvania’s statewide patient safety data reporting system reviewed hospital records from 2004 to 2014 and recorded 138 events in the ED involving patients with ASD, 86 of them involving children and adolescents.
Dr. McGonigle said that such incidents, often accompanied by use of restraints, can be reduced through better training, and that ED practitioners and staff can be shown how to help calm patients and to provide the kind of simple, clear communication required to diagnose and treat them effectively. He showed excerpts from a training video produced at his institution to illustrate those strategies.
Patients with ASD should be moved away from bright fluorescent lights, and excess medical equipment and noise – ideally to a sensory room, Dr. McGonigle said – and given toys or other comforting activities appropriate to their interests. The number of people in and out of a patient’s room should be limited, and providers always should knock first on a door and wait for an answer, and introduce themselves by name, whether or not the child is able to respond.
Clinicians should recruit caregivers to help question patients, keep questions to a yes-no format, and not insist on eye contact. A “first-then” approach should be used to explain any intervention, describing the intervention and then an age-appropriate reward to follow. Interventions, even noninvasive ones, can be modeled or demonstrated first on caregivers.
Psychiatric crises are an important driver of ED visits among ASD patients, but crisis behavior should not be assumed to have a psychiatric cause, Dr. McGonigle stressed. Behavior mimicking a psychiatric episode “could be triggered by stomachache, ear infection, bowel obstruction, [urinary tract infection], hyper- or hypoglycemia.”
Communicating about pain is particularly challenging in patients with ASD, Dr. McGonigle said. The usual pain scales used in the pediatric ED rely on representations of facial expressions. These should be replaced by demonstrations using toys, tablet computers, or drawings to identify sources of pain, with a caregiver present to help.
Finding barriers to care
Dr. Friedlaender described a pilot study she and her colleagues conducted in her institution’s sedation unit that was designed to help them understand the barriers to optimal care for ASD patients, and to find ways around them. Many of the studies the investigators consulted “came from the dental literature, where there is a significant number of special-needs kids who need support during procedural care. [Dentists] were among the first to publish on how to make this a reasonable experience.”
One key insight gleaned from this literature, Dr. Friedlaender said, was that a simple screening question – whether the child could sit still for a haircut – proved sensitive in indicating a need for accommodation.
The CHOP researchers created a three-question universal screening tool that schedulers asked of all caregivers when a child presented to the ED. In addition to asking whether the child could sit still, schedulers asked whether he or she had a behavioral diagnosis or special communication needs. Of 458 families who completed the screening, 96 answered positively to at least one of the questions, and 79, or 17% of the cohort, indicated a behavioral diagnosis.
Such information previously had been missed, Dr. Friedlaender said, because “many families didn’t consider autism part of a medical history – if we didn’t ask about it, they didn’t share it.”
Her group also conducted a study on the effectiveness of self-reported pain scales in 43 verbal ASD children aged 6-17 who had undergone surgical procedures. Dr. Friedlaender said she suspected that it was impractical to ask children with ASD to use only pictures of facial expressions to indicate their pain.
The subjects were asked to circle images of faces with expressions corresponding to their pain. They also were asked to locate their pain by drawing it on tablet computers, and given poker chips to represent their degree of pain, with one chip the least and four the most. Caregivers were recruited to assist with questioning and interpreting responses.
All children in the study were able to describe and locate their pain. “We learned that there isn’t one universal pain tool that works for all kids,” Dr. Friedlaender said, “but that facial expressions and body language don’t often match pain scores” in ASD children. The study also revealed that parent or caregiver mediation is helpful in discerning the location and intensity of pain.
Why ED use is high
Other research presented at AACAP sought to grasp the scope of, and reasons behind, the increase in ASD youth seen in hospital emergency departments.
Michael J. Murray, MD, of Pennsylvania State University in Hershey, found using commercial insurance data from large employers showing that ED visits increased from 3% in 2005 to nearly 16% in 2013 among youth diagnosed with ASD, while a non-ASD comparison cohort saw a far more consistent rate of ED visits across the same time period, of about 3%. Adolescents with ASD were nearly five times more likely to have had an ED visit than were non-ASD adolescents (95% confidence interval, 4.678-4.875).
Dr. Murray said in an interview that the ASD cohort identified in his study “was smaller than it should have been,” compared with Centers for Disease Control and Prevention prevalence data. One likely reason is that not all the insurers had to cover ASD in the first years of the study period. Dr. Murray said he thinks a new study using public insurance data might provide a fuller picture.
Dr. Murray and colleagues’ study, which looked at youth aged 12-21, revealed that being older increased the likelihood of an ED visit. “We think it may have to do with the whole transition out of school,” he said. “This is the first generation with ASD that’s accustomed to having good school-based supports.” The transition to adulthood “is a really important time, and that’s when we’re pulling away from them.”
Sarah Lytle, MD, of University Hospitals Cleveland Medical Center, presented a literature review of ASD youth in the ED from 2006 through 2015. Dr. Lytle found that children with ASD were more likely to visit the ED than were those without ASD. In addition, the review showed a higher proportion of ED visits for psychiatric problems (13% of visits vs. 2% for non-ASD youth). Youth with ASD were more likely to be admitted to a psychiatric unit or medically boarded in the ED, she found. They also were more likely to have public insurance.
Dr. Lytle’s study drew from a dozen published studies in different age groups (subjects ranged from 0 to 24 years across studies). Though it was difficult to draw conclusions related to which saw the highest ED use, one study found the risk of ED use higher in adolescents, compared with younger children, she said. “One thing I see clinically is that when kids hit the age of 12, pediatric psychiatric units often won’t take them,” she said, as children are physically bigger and may be harder to manage. “And then they’re cycled into the ED,” she said.
Creating the ‘ASD care pathway’
Clinicians from New York shared their experiences designing and implementing an autism care pathway within the state’s only pediatric psychiatric emergency department.
At NYU Health and Hospitals/Bellevue, a public hospital, clinicians found themselves struggling to manage ASD patients, who comprise between 10% and 20% of children seen. “Most of our staff, and even our child psychiatrists, had previously had very little experience working with kids with autism, and that was true for most of our child psychiatrists as well,” said Ruth S. Gerson, MD, who oversees the hospital’s Children’s Comprehensive Psychiatric Emergency Program.
The ASD patients “were in crisis all the time, and having constant behavioral outbursts,” said Beryl J. Filton, PhD. The team responded by developing an autism-specific care pathway for the ED and inpatient units, with a 4-hour training course for all staff members.
The pathway begins with a tip sheet for providers conducting the initial evaluation in the ED. Providers “ask questions specific to symptoms of autism: Does the child have words? How much do they understand? Do they communicate in other ways that are nonverbal? Then we talk about the child’s warning signs, triggers, preferred activities and rewards,” Dr. Filton said. This allows providers to gather information up front that can be used during the ED stay.
Picture books and visual communication boards are used to create a visual schedule for patients, so that they know what to expect, and staff have been trained to communicate through gesturing, modeling, and physical guidance, she said. “First-then” verbal and visual prompts are used before any intervention, including noninvasive interventions, and patients are put on a schedule of rewards as regular as every 15 minutes. They also are engaged in scheduled “motor breaks,” or brief periods of physical activity.
Dr. Filton, like the other providers, emphasized the importance of decreasing excess stimulation around patients with ASD and communicating coping options to them nonverbally. “We talk a lot with staff when patients are getting agitated about giving space and waiting,” she said. “One important thing to recognize is that these patients can take longer after an episode of agitation to return to baseline. So we talk with staff about being on high alert for even a couple hours after an agitated episode to keep demands low and rewards high.”
Many of the strategies and principles that have worked at Bellevue can be generalized to other settings, Dr. Filton said. “Using more than verbal communication, gesturing, visual supports cuing patients, and having reward systems” are effective anywhere for managing patients with autism, she said. The main challenge, she added, is achieving consistency, “making sure all the staff know the same information about the patient.”
Dr. Gerson said some of her team’s challenges come from being part of a public institution serving a low-income community with fragmented health care delivery. “A number of families that are coming in in crisis may not have known that their child had autism,” she said. “We see many who have never been formally diagnosed – even teenagers. Or the child has the diagnosis, but no one helped the family get the services they’re legally eligible for,” she said. “And then the family comes in to the ED and says: ‘We need you to fix all this.’ ”
What ED providers can do, she said, is use the improved assessment tools, and communication and coping strategies outlined in the pathway to “focus on determining the immediate crisis – whether there is change from the child’s usual behaviors, and what’s the pattern of that change.” While youth with ASD have higher rates of comorbid psychiatric disorders, “statistically that’s less likely to be the case in the ED than the stuff that plagues all of us: stomachaches, toothaches, constipation, or psychosocial stressors, such as changes at home or at school.”
One of the goals in creating the ASD care pathway, Dr. Gerson said, was to avoid unnecessary hospitalizations. “We’ve changed our assessment, and really drilled down to determine what hospitalization can and cannot accomplish,” so that only the children likely to benefit stay.
“At the same time, we have to make sure that when we discharge, we’re not leaving families with nothing, that we’re setting them up to receive services and resources to stabilize and support them in the community.”
NEW YORK – Emergency departments are seeing more pediatric and adolescent patients with autism spectrum disorder, and are struggling to meet their needs, experts say.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry, researchers presented results from studies attempting to quantify and better understand the uptick in ED visits, while clinicians shared strategies aimed at improving care in a setting that, nearly all agreed, presents unique obstacles for treating children with ASD.
Bright lights, excess noise, frequently changing care staff, and a lack of training in nonverbal communication strategies were among the problems the clinicians highlighted.
“There’s been a huge increase in recent years in the number of children with ASD that are coming into the ED because of either behavioral crises or general pediatric medical concerns that require us to intervene,” said Eron Y. Friedlaender, MD, MPH, of Children’s Hospital of Philadelphia (CHOP). “Yet, we struggle to offer kids with challenging behaviors or communication vulnerabilities the same standard of care that we’re used to offering.”
John J. McGonigle, PhD, head of the autism center at the University of Pittsburgh, noted that incidents tied to safety issues, such as disruptive behavior, aggression, and self-injury, were occurring among young ASD patients in the ED. In 2015, he said, Pennsylvania’s statewide patient safety data reporting system reviewed hospital records from 2004 to 2014 and recorded 138 events in the ED involving patients with ASD, 86 of them involving children and adolescents.
Dr. McGonigle said that such incidents, often accompanied by use of restraints, can be reduced through better training, and that ED practitioners and staff can be shown how to help calm patients and to provide the kind of simple, clear communication required to diagnose and treat them effectively. He showed excerpts from a training video produced at his institution to illustrate those strategies.
Patients with ASD should be moved away from bright fluorescent lights, and excess medical equipment and noise – ideally to a sensory room, Dr. McGonigle said – and given toys or other comforting activities appropriate to their interests. The number of people in and out of a patient’s room should be limited, and providers always should knock first on a door and wait for an answer, and introduce themselves by name, whether or not the child is able to respond.
Clinicians should recruit caregivers to help question patients, keep questions to a yes-no format, and not insist on eye contact. A “first-then” approach should be used to explain any intervention, describing the intervention and then an age-appropriate reward to follow. Interventions, even noninvasive ones, can be modeled or demonstrated first on caregivers.
Psychiatric crises are an important driver of ED visits among ASD patients, but crisis behavior should not be assumed to have a psychiatric cause, Dr. McGonigle stressed. Behavior mimicking a psychiatric episode “could be triggered by stomachache, ear infection, bowel obstruction, [urinary tract infection], hyper- or hypoglycemia.”
Communicating about pain is particularly challenging in patients with ASD, Dr. McGonigle said. The usual pain scales used in the pediatric ED rely on representations of facial expressions. These should be replaced by demonstrations using toys, tablet computers, or drawings to identify sources of pain, with a caregiver present to help.
Finding barriers to care
Dr. Friedlaender described a pilot study she and her colleagues conducted in her institution’s sedation unit that was designed to help them understand the barriers to optimal care for ASD patients, and to find ways around them. Many of the studies the investigators consulted “came from the dental literature, where there is a significant number of special-needs kids who need support during procedural care. [Dentists] were among the first to publish on how to make this a reasonable experience.”
One key insight gleaned from this literature, Dr. Friedlaender said, was that a simple screening question – whether the child could sit still for a haircut – proved sensitive in indicating a need for accommodation.
The CHOP researchers created a three-question universal screening tool that schedulers asked of all caregivers when a child presented to the ED. In addition to asking whether the child could sit still, schedulers asked whether he or she had a behavioral diagnosis or special communication needs. Of 458 families who completed the screening, 96 answered positively to at least one of the questions, and 79, or 17% of the cohort, indicated a behavioral diagnosis.
Such information previously had been missed, Dr. Friedlaender said, because “many families didn’t consider autism part of a medical history – if we didn’t ask about it, they didn’t share it.”
Her group also conducted a study on the effectiveness of self-reported pain scales in 43 verbal ASD children aged 6-17 who had undergone surgical procedures. Dr. Friedlaender said she suspected that it was impractical to ask children with ASD to use only pictures of facial expressions to indicate their pain.
The subjects were asked to circle images of faces with expressions corresponding to their pain. They also were asked to locate their pain by drawing it on tablet computers, and given poker chips to represent their degree of pain, with one chip the least and four the most. Caregivers were recruited to assist with questioning and interpreting responses.
All children in the study were able to describe and locate their pain. “We learned that there isn’t one universal pain tool that works for all kids,” Dr. Friedlaender said, “but that facial expressions and body language don’t often match pain scores” in ASD children. The study also revealed that parent or caregiver mediation is helpful in discerning the location and intensity of pain.
Why ED use is high
Other research presented at AACAP sought to grasp the scope of, and reasons behind, the increase in ASD youth seen in hospital emergency departments.
Michael J. Murray, MD, of Pennsylvania State University in Hershey, found using commercial insurance data from large employers showing that ED visits increased from 3% in 2005 to nearly 16% in 2013 among youth diagnosed with ASD, while a non-ASD comparison cohort saw a far more consistent rate of ED visits across the same time period, of about 3%. Adolescents with ASD were nearly five times more likely to have had an ED visit than were non-ASD adolescents (95% confidence interval, 4.678-4.875).
Dr. Murray said in an interview that the ASD cohort identified in his study “was smaller than it should have been,” compared with Centers for Disease Control and Prevention prevalence data. One likely reason is that not all the insurers had to cover ASD in the first years of the study period. Dr. Murray said he thinks a new study using public insurance data might provide a fuller picture.
Dr. Murray and colleagues’ study, which looked at youth aged 12-21, revealed that being older increased the likelihood of an ED visit. “We think it may have to do with the whole transition out of school,” he said. “This is the first generation with ASD that’s accustomed to having good school-based supports.” The transition to adulthood “is a really important time, and that’s when we’re pulling away from them.”
Sarah Lytle, MD, of University Hospitals Cleveland Medical Center, presented a literature review of ASD youth in the ED from 2006 through 2015. Dr. Lytle found that children with ASD were more likely to visit the ED than were those without ASD. In addition, the review showed a higher proportion of ED visits for psychiatric problems (13% of visits vs. 2% for non-ASD youth). Youth with ASD were more likely to be admitted to a psychiatric unit or medically boarded in the ED, she found. They also were more likely to have public insurance.
Dr. Lytle’s study drew from a dozen published studies in different age groups (subjects ranged from 0 to 24 years across studies). Though it was difficult to draw conclusions related to which saw the highest ED use, one study found the risk of ED use higher in adolescents, compared with younger children, she said. “One thing I see clinically is that when kids hit the age of 12, pediatric psychiatric units often won’t take them,” she said, as children are physically bigger and may be harder to manage. “And then they’re cycled into the ED,” she said.
Creating the ‘ASD care pathway’
Clinicians from New York shared their experiences designing and implementing an autism care pathway within the state’s only pediatric psychiatric emergency department.
At NYU Health and Hospitals/Bellevue, a public hospital, clinicians found themselves struggling to manage ASD patients, who comprise between 10% and 20% of children seen. “Most of our staff, and even our child psychiatrists, had previously had very little experience working with kids with autism, and that was true for most of our child psychiatrists as well,” said Ruth S. Gerson, MD, who oversees the hospital’s Children’s Comprehensive Psychiatric Emergency Program.
The ASD patients “were in crisis all the time, and having constant behavioral outbursts,” said Beryl J. Filton, PhD. The team responded by developing an autism-specific care pathway for the ED and inpatient units, with a 4-hour training course for all staff members.
The pathway begins with a tip sheet for providers conducting the initial evaluation in the ED. Providers “ask questions specific to symptoms of autism: Does the child have words? How much do they understand? Do they communicate in other ways that are nonverbal? Then we talk about the child’s warning signs, triggers, preferred activities and rewards,” Dr. Filton said. This allows providers to gather information up front that can be used during the ED stay.
Picture books and visual communication boards are used to create a visual schedule for patients, so that they know what to expect, and staff have been trained to communicate through gesturing, modeling, and physical guidance, she said. “First-then” verbal and visual prompts are used before any intervention, including noninvasive interventions, and patients are put on a schedule of rewards as regular as every 15 minutes. They also are engaged in scheduled “motor breaks,” or brief periods of physical activity.
Dr. Filton, like the other providers, emphasized the importance of decreasing excess stimulation around patients with ASD and communicating coping options to them nonverbally. “We talk a lot with staff when patients are getting agitated about giving space and waiting,” she said. “One important thing to recognize is that these patients can take longer after an episode of agitation to return to baseline. So we talk with staff about being on high alert for even a couple hours after an agitated episode to keep demands low and rewards high.”
Many of the strategies and principles that have worked at Bellevue can be generalized to other settings, Dr. Filton said. “Using more than verbal communication, gesturing, visual supports cuing patients, and having reward systems” are effective anywhere for managing patients with autism, she said. The main challenge, she added, is achieving consistency, “making sure all the staff know the same information about the patient.”
Dr. Gerson said some of her team’s challenges come from being part of a public institution serving a low-income community with fragmented health care delivery. “A number of families that are coming in in crisis may not have known that their child had autism,” she said. “We see many who have never been formally diagnosed – even teenagers. Or the child has the diagnosis, but no one helped the family get the services they’re legally eligible for,” she said. “And then the family comes in to the ED and says: ‘We need you to fix all this.’ ”
What ED providers can do, she said, is use the improved assessment tools, and communication and coping strategies outlined in the pathway to “focus on determining the immediate crisis – whether there is change from the child’s usual behaviors, and what’s the pattern of that change.” While youth with ASD have higher rates of comorbid psychiatric disorders, “statistically that’s less likely to be the case in the ED than the stuff that plagues all of us: stomachaches, toothaches, constipation, or psychosocial stressors, such as changes at home or at school.”
One of the goals in creating the ASD care pathway, Dr. Gerson said, was to avoid unnecessary hospitalizations. “We’ve changed our assessment, and really drilled down to determine what hospitalization can and cannot accomplish,” so that only the children likely to benefit stay.
“At the same time, we have to make sure that when we discharge, we’re not leaving families with nothing, that we’re setting them up to receive services and resources to stabilize and support them in the community.”
NEW YORK – Emergency departments are seeing more pediatric and adolescent patients with autism spectrum disorder, and are struggling to meet their needs, experts say.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry, researchers presented results from studies attempting to quantify and better understand the uptick in ED visits, while clinicians shared strategies aimed at improving care in a setting that, nearly all agreed, presents unique obstacles for treating children with ASD.
Bright lights, excess noise, frequently changing care staff, and a lack of training in nonverbal communication strategies were among the problems the clinicians highlighted.
“There’s been a huge increase in recent years in the number of children with ASD that are coming into the ED because of either behavioral crises or general pediatric medical concerns that require us to intervene,” said Eron Y. Friedlaender, MD, MPH, of Children’s Hospital of Philadelphia (CHOP). “Yet, we struggle to offer kids with challenging behaviors or communication vulnerabilities the same standard of care that we’re used to offering.”
John J. McGonigle, PhD, head of the autism center at the University of Pittsburgh, noted that incidents tied to safety issues, such as disruptive behavior, aggression, and self-injury, were occurring among young ASD patients in the ED. In 2015, he said, Pennsylvania’s statewide patient safety data reporting system reviewed hospital records from 2004 to 2014 and recorded 138 events in the ED involving patients with ASD, 86 of them involving children and adolescents.
Dr. McGonigle said that such incidents, often accompanied by use of restraints, can be reduced through better training, and that ED practitioners and staff can be shown how to help calm patients and to provide the kind of simple, clear communication required to diagnose and treat them effectively. He showed excerpts from a training video produced at his institution to illustrate those strategies.
Patients with ASD should be moved away from bright fluorescent lights, and excess medical equipment and noise – ideally to a sensory room, Dr. McGonigle said – and given toys or other comforting activities appropriate to their interests. The number of people in and out of a patient’s room should be limited, and providers always should knock first on a door and wait for an answer, and introduce themselves by name, whether or not the child is able to respond.
Clinicians should recruit caregivers to help question patients, keep questions to a yes-no format, and not insist on eye contact. A “first-then” approach should be used to explain any intervention, describing the intervention and then an age-appropriate reward to follow. Interventions, even noninvasive ones, can be modeled or demonstrated first on caregivers.
Psychiatric crises are an important driver of ED visits among ASD patients, but crisis behavior should not be assumed to have a psychiatric cause, Dr. McGonigle stressed. Behavior mimicking a psychiatric episode “could be triggered by stomachache, ear infection, bowel obstruction, [urinary tract infection], hyper- or hypoglycemia.”
Communicating about pain is particularly challenging in patients with ASD, Dr. McGonigle said. The usual pain scales used in the pediatric ED rely on representations of facial expressions. These should be replaced by demonstrations using toys, tablet computers, or drawings to identify sources of pain, with a caregiver present to help.
Finding barriers to care
Dr. Friedlaender described a pilot study she and her colleagues conducted in her institution’s sedation unit that was designed to help them understand the barriers to optimal care for ASD patients, and to find ways around them. Many of the studies the investigators consulted “came from the dental literature, where there is a significant number of special-needs kids who need support during procedural care. [Dentists] were among the first to publish on how to make this a reasonable experience.”
One key insight gleaned from this literature, Dr. Friedlaender said, was that a simple screening question – whether the child could sit still for a haircut – proved sensitive in indicating a need for accommodation.
The CHOP researchers created a three-question universal screening tool that schedulers asked of all caregivers when a child presented to the ED. In addition to asking whether the child could sit still, schedulers asked whether he or she had a behavioral diagnosis or special communication needs. Of 458 families who completed the screening, 96 answered positively to at least one of the questions, and 79, or 17% of the cohort, indicated a behavioral diagnosis.
Such information previously had been missed, Dr. Friedlaender said, because “many families didn’t consider autism part of a medical history – if we didn’t ask about it, they didn’t share it.”
Her group also conducted a study on the effectiveness of self-reported pain scales in 43 verbal ASD children aged 6-17 who had undergone surgical procedures. Dr. Friedlaender said she suspected that it was impractical to ask children with ASD to use only pictures of facial expressions to indicate their pain.
The subjects were asked to circle images of faces with expressions corresponding to their pain. They also were asked to locate their pain by drawing it on tablet computers, and given poker chips to represent their degree of pain, with one chip the least and four the most. Caregivers were recruited to assist with questioning and interpreting responses.
All children in the study were able to describe and locate their pain. “We learned that there isn’t one universal pain tool that works for all kids,” Dr. Friedlaender said, “but that facial expressions and body language don’t often match pain scores” in ASD children. The study also revealed that parent or caregiver mediation is helpful in discerning the location and intensity of pain.
Why ED use is high
Other research presented at AACAP sought to grasp the scope of, and reasons behind, the increase in ASD youth seen in hospital emergency departments.
Michael J. Murray, MD, of Pennsylvania State University in Hershey, found using commercial insurance data from large employers showing that ED visits increased from 3% in 2005 to nearly 16% in 2013 among youth diagnosed with ASD, while a non-ASD comparison cohort saw a far more consistent rate of ED visits across the same time period, of about 3%. Adolescents with ASD were nearly five times more likely to have had an ED visit than were non-ASD adolescents (95% confidence interval, 4.678-4.875).
Dr. Murray said in an interview that the ASD cohort identified in his study “was smaller than it should have been,” compared with Centers for Disease Control and Prevention prevalence data. One likely reason is that not all the insurers had to cover ASD in the first years of the study period. Dr. Murray said he thinks a new study using public insurance data might provide a fuller picture.
Dr. Murray and colleagues’ study, which looked at youth aged 12-21, revealed that being older increased the likelihood of an ED visit. “We think it may have to do with the whole transition out of school,” he said. “This is the first generation with ASD that’s accustomed to having good school-based supports.” The transition to adulthood “is a really important time, and that’s when we’re pulling away from them.”
Sarah Lytle, MD, of University Hospitals Cleveland Medical Center, presented a literature review of ASD youth in the ED from 2006 through 2015. Dr. Lytle found that children with ASD were more likely to visit the ED than were those without ASD. In addition, the review showed a higher proportion of ED visits for psychiatric problems (13% of visits vs. 2% for non-ASD youth). Youth with ASD were more likely to be admitted to a psychiatric unit or medically boarded in the ED, she found. They also were more likely to have public insurance.
Dr. Lytle’s study drew from a dozen published studies in different age groups (subjects ranged from 0 to 24 years across studies). Though it was difficult to draw conclusions related to which saw the highest ED use, one study found the risk of ED use higher in adolescents, compared with younger children, she said. “One thing I see clinically is that when kids hit the age of 12, pediatric psychiatric units often won’t take them,” she said, as children are physically bigger and may be harder to manage. “And then they’re cycled into the ED,” she said.
Creating the ‘ASD care pathway’
Clinicians from New York shared their experiences designing and implementing an autism care pathway within the state’s only pediatric psychiatric emergency department.
At NYU Health and Hospitals/Bellevue, a public hospital, clinicians found themselves struggling to manage ASD patients, who comprise between 10% and 20% of children seen. “Most of our staff, and even our child psychiatrists, had previously had very little experience working with kids with autism, and that was true for most of our child psychiatrists as well,” said Ruth S. Gerson, MD, who oversees the hospital’s Children’s Comprehensive Psychiatric Emergency Program.
The ASD patients “were in crisis all the time, and having constant behavioral outbursts,” said Beryl J. Filton, PhD. The team responded by developing an autism-specific care pathway for the ED and inpatient units, with a 4-hour training course for all staff members.
The pathway begins with a tip sheet for providers conducting the initial evaluation in the ED. Providers “ask questions specific to symptoms of autism: Does the child have words? How much do they understand? Do they communicate in other ways that are nonverbal? Then we talk about the child’s warning signs, triggers, preferred activities and rewards,” Dr. Filton said. This allows providers to gather information up front that can be used during the ED stay.
Picture books and visual communication boards are used to create a visual schedule for patients, so that they know what to expect, and staff have been trained to communicate through gesturing, modeling, and physical guidance, she said. “First-then” verbal and visual prompts are used before any intervention, including noninvasive interventions, and patients are put on a schedule of rewards as regular as every 15 minutes. They also are engaged in scheduled “motor breaks,” or brief periods of physical activity.
Dr. Filton, like the other providers, emphasized the importance of decreasing excess stimulation around patients with ASD and communicating coping options to them nonverbally. “We talk a lot with staff when patients are getting agitated about giving space and waiting,” she said. “One important thing to recognize is that these patients can take longer after an episode of agitation to return to baseline. So we talk with staff about being on high alert for even a couple hours after an agitated episode to keep demands low and rewards high.”
Many of the strategies and principles that have worked at Bellevue can be generalized to other settings, Dr. Filton said. “Using more than verbal communication, gesturing, visual supports cuing patients, and having reward systems” are effective anywhere for managing patients with autism, she said. The main challenge, she added, is achieving consistency, “making sure all the staff know the same information about the patient.”
Dr. Gerson said some of her team’s challenges come from being part of a public institution serving a low-income community with fragmented health care delivery. “A number of families that are coming in in crisis may not have known that their child had autism,” she said. “We see many who have never been formally diagnosed – even teenagers. Or the child has the diagnosis, but no one helped the family get the services they’re legally eligible for,” she said. “And then the family comes in to the ED and says: ‘We need you to fix all this.’ ”
What ED providers can do, she said, is use the improved assessment tools, and communication and coping strategies outlined in the pathway to “focus on determining the immediate crisis – whether there is change from the child’s usual behaviors, and what’s the pattern of that change.” While youth with ASD have higher rates of comorbid psychiatric disorders, “statistically that’s less likely to be the case in the ED than the stuff that plagues all of us: stomachaches, toothaches, constipation, or psychosocial stressors, such as changes at home or at school.”
One of the goals in creating the ASD care pathway, Dr. Gerson said, was to avoid unnecessary hospitalizations. “We’ve changed our assessment, and really drilled down to determine what hospitalization can and cannot accomplish,” so that only the children likely to benefit stay.
“At the same time, we have to make sure that when we discharge, we’re not leaving families with nothing, that we’re setting them up to receive services and resources to stabilize and support them in the community.”
EXPERT ANALYSIS FROM AACAP 2016
Potential Operating Room Fire Hazard of Bone Cement
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
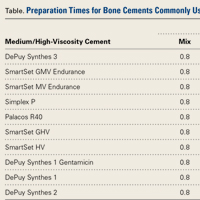
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Approximately 600 cases of operating room (OR) fires are reported annually.1 The incidence of OR fires in the United States equals that of wrong-site surgeries, and 20% of cases have associated morbidity.1,2 The estimated mortality rate is 1 to 2 cases per year.3-5 The most commonly involved anatomical regions are the airway (33%) and the face (28%).4 Most surgical fires are reported in anesthetized patients with open oxygen delivery systems during head, neck, and upper chest surgeries; electrosurgical instruments are the ignition source in 90% of these cases.6 Despite extensive fire safety education and training, complete elimination of OR fires still has not been achieved.
Each fire requires an ignition source, a fuel source, and an oxidizer.7 In the OR, the 2 most common oxidizers are oxygen and nitrous oxide. Head and neck surgeries have a high concentration of these gases near the working field and therefore a higher risk and incidence of fires. Furthermore, surgical drapes and equipment (eg, closed or semi-closed breathing systems, masks) may potentiate this risk by reducing ventilation in areas where gases can accumulate and ignite. Ignition sources provide the energy that starts fires; common sources are electrocautery, lasers, fiber-optic light cords, drills/burrs, and defibrillator paddles. Fires are propagated by fuel sources, which encompass any flammable material, including tracheal tubes, sponges, alcohol-based solutions, hair, gastrointestinal tract gases, gloves, and packaging materials.8 Of note, alcohol-based skin-preparation agents emit flammable vapors that can ignite.9-14 Before draping or exposure to an ignition source, chlorhexidine gluconate-based preparations must be allowed to dry for at least 3 minutes after application to hairless skin and up to 1 hour after application to hair.15 Inadequate drying poses a risk of fire.10We present the case of an OR fire ignited by electrocautery near freshly applied bone cement. No patient information is disclosed in this report.
Case Report
Our patient was evaluated in clinic and scheduled for total knee arthroplasty (TKA). All preoperative safety checklists and time-out procedures were followed and documented at the start of surgery. The TKA was performed with a standard medial patellar arthrotomy. Tourniquet control was used after Esmarch exsanguination. The surgery proceeded uneventfully until just after the bone cement was applied to the tibial surface. The surgeon was using a Bovie to resect residual lateral meniscus tissue when a fire instantaneously erupted within the joint space. Fortunately, the surgeon quickly suffocated the fire with a dry towel. The ignited bone cement was removed, and the patient was examined. There was no injury to surrounding tissue or joint space. Surgery was resumed with application of new bone cement to the tibial surface. The artificial joint was then successfully implanted and the case completed without further incident. The patient was discharged from the hospital and followed up as an outpatient without any postoperative complications.
Discussion
Bone cement, which is commonly used in artificial joint anchoring, craniofacial reconstruction, and vertebroplasty, has liquid and powder components. The liquid monomer methyl methacrylate (MMA) is colorless and flammable and has a distinct odor.16 Exposure to heat or light can prematurely polymerize MMA, requiring the addition of hydroquinone to inhibit the reaction.16 The powder polymethylmethacrylate affords excellent structural support, radiopacity, and facility of use.17 Dibenzoyl peroxide and N,N-dimethyl-p-toluidine are added to the powder to facilitate the polymerization reaction at room temperature (ie, cold curing of cement). Premature application of unpolymerized cement increases the risk of fire from the volatile liquid component.
In the OR, bone cement is prepared by mixing together its powder and liquid components.18 The reaction is exothermic polymerization. The liquid is highly volatile and flammable in both liquid and vapor states.16,19 The vapors are denser than air and can concentrate in poorly ventilated areas. The OR and the application site must be adequately ventilated to eliminate any pockets of vapor accumulation.16 A vacuum mixer can be used to minimize fume exposure, enhance cement strength, and reduce fire risk while combining the 2 components.
MMA’s flash point, the temperature at which the fumes could ignite in the presence of an ignition source, is 10.5ºC. The auto-ignition point, the temperature at which MMA spontaneously combusts, is 421ºC.20 The OR is usually warmer than the flash point temperature, but the electrocautery tip can generate up to 1200ºC of heat.21 Therefore, bone cement is a potential fire hazard, and use of Bovies or other ignition sources in its vicinity must be avoided.
The Table lists the recommended times for preparing various bone cement products.22,23Mix time is the time needed to combine the liquid and powder into a homogenous putty.
For OR fires, the standard guidelines for rapid containment and safety apply. These guidelines are detailed by the American Society of Anesthesiologists.8 Briefly, delivery of all airway gases to the patient is discontinued. Any burning material is removed and extinguished by the OR staff.1 Carbon dioxide fire extinguishers are used to put out any patient fires and minimize the risk of thermal injury. (Water-mist fire extinguishers can contaminate surgical wounds and present an electric shock hazard with surgical devices and should be avoided.24) If a fire occurs in a patient’s airway, the tracheal tube is removed, and airway patency is maintained with use of other invasive or noninvasive techniques. Often, noninvasive positive pressure ventilation without supplemental oxygen is used until the fire is controlled and the patient is safe. Once the patient fire is controlled, ventilation is restarted, and the patient is evacuated from the OR and away from any other hazards, as required. Last, the patient is physically examined for any injuries and treated.24 Specific to TKA, the procedure is resumed after removal of all bone cement, inspection of the operative site, and treatment of any fire-related injuries.
We have reported the case of an OR fire during TKA. Appropriate selection and use of bone cement products, proper assessment of set time, and avoidance of electrocautery near cement application sites may dramatically reduce associated fire risks.
Am J Orthop. 2016;45(7):E512-E514. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
1. Hart SR, Yajnik A, Ashford J, Springer R, Harvey S. Operating room fire safety. Ochsner J. 2011;11(1):37-42.
2. American Society of Anesthesiologists Task Force on Operating Room Fires; Caplan RA, Barker SJ, Connis RT, et al. Practice advisory for the prevention and management of operating room fires. Anesthesiology. 2008;108(5):786-801.
3. Bruley M. Surgical fires: perioperative communication is essential to prevent this rare but devastating complication. Qual Saf HealthCare. 2004;13(6):467-471.
4. Daane SP, Toth BA. Fire in the operating room: principles and prevention. Plast Reconstr Surg. 2005;115(5):73e-75e.
5. Rinder CS. Fire safety in the operating room. Curr Opin Anaesthesiol. 2008;21(6):790-795.
6. Mathias JM. Fast action, team coordination critical when surgical fires occur. OR Manager. 2013;29(11):9-10.
7. Culp WC Jr, Kimbrough BA, Luna S. Flammability of surgical drapes and materials in varying concentrations of oxygen. Anesthesiology. 2013;119(4):770-776.
8. Apfelbaum JL, Caplan RA, Barker SJ, et al; American Society of Anesthesiologists Task Force on Operating Room Fires. Practice advisory for the prevention and management of operating room fires: an updated report by the American Society of Anesthesiologists Task Force on Operating Room Fires. Anesthesiology. 2013;118(2):271-290.
9. Barker SJ, Polson JS. Fire in the operating room: a case report and laboratory study. Anesth Analg. 2001;93(4):960-965.
10. Fire hazard created by the misuse of DuraPrep solution. Health Devices. 1998;27(11):400-402.
11. Hurt TL, Schweich PJ. Do not get burned: preventing iatrogenic fires and burns in the emergency department. Pediatr Emerg Care. 2003;19(4):255-259.
12. Prasad R, Quezado Z, St Andre A, O’Grady NP. Fires in the operating room and intensive care unit: awareness is the key to prevention. Anesth Analg. 2006;102(1):172-174.
13. Shah SC. Correspondence: operating room flash fire. Anesth Analg. 1974;53(2):288.
14. Tooher R, Maddern GJ, Simpson J. Surgical fires and alcohol-based skin preparations. ANZ J Surg. 2004;74(5):382-385.
15. Using ChloraPrep™ products and the skin prep portfolio. http://www.carefusion.com/medical-products/infection-prevention/skin-preparation/using-chloraprep.aspx. Accessed October 7, 2016.16. DePuy CMW. DePuy Orthopaedic Gentamicin Bone Cements. Blackpool, United Kingdom: DePuy International Ltd; 2008.
17. Dall’Oca C, Maluta T, Cavani F, et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem. 2014;58(2):2255.
18. Zimmer Biomet. Bone Cement: Biomet Cement and Cementing Systems. http://www.biomet.com/wps/portal/internet/Biomet/Healthcare-Professionals/products/orthopedics. 2014. Accessed October 7, 2016.
19. Sigma-Aldrich. Methyl methacrylate. http://www.sigmaaldrich.com/catalog/product/aldrich/w400201?lang=en®ion=US. Accessed October 7, 2016.
20. DePuy Synthes. Unmedicated bone cements MSDS. Blackpool, United Kingdom: DePuy International Ltd. http://msdsdigital.com/unmedicated-bone-cements-msds. Accessed October 7, 2016.
21. Mir MR, Sun GS, Wang CM. Electrocautery. http://emedicine.medscape.com/article/2111163-overview#showall. Accessed October 7, 2016.
22. DePuy Synthes. Bone cement time setting.
23. Berry DJ, Lieberman JR, eds. Surgery of the Hip. New York, NY: Elsevier; 2011.
24. ECRI Institute. Surgical Fire Prevention. https://www.ecri.org/Accident_Investigation/Pages/Surgical-Fire-Prevention.aspx. 2014. Accessed October 7, 2016.
Anxiety, poverty contribute to depression in at-risk children
Fear and anxiety, economic disadvantage, and recent psychosocial adversity contribute significantly to the onset of major depressive disorder in children and adolescents with a strong family history of the condition.
A 4-year longitudinal study followed the offspring of 279 families in which one parent had experienced at least two episodes of major depressive disorder (MDD) and in which there was a biologically related child living with that index parent.
Fear and anxiety showed a strong and significant association with new-onset major depressive disorder, as did irritability. Furthermore, the association between the two symptoms was low but significant, suggesting that they do not often co-occur (JAMA Psychiatry. 2016 Dec 7. doi: 10.1001/jamapsychiatry.2016.3140).
“The results suggested that generalized anxiety symptoms were driving the predictive effect of fear/anxiety on new-onset MDD and that fear/anxiety (and not irritability) predicted an especially early MDD onset,” wrote Frances Rice, PhD, of the division of psychological medicine and clinical neurosciences at Cardiff University, Wales, and coauthors.
Recent psychosocial adversity – stressful events such as the death of a friend, illness, bullying, or parents fighting – also showed a strong association with new-onset major depressive disorder, while economic disadvantage had a lesser but still significant contribution. Both of these also were associated with fear and anxiety, and irritability.
Greater family history and more severe parental depression also contributed significantly to the emergence of depression in the offspring, although those factors were not associated with the clinical antecedents such as fear and anxiety.
“Therefore, the indicators of social risk predicted MDD independent of correlated familial risk, parental depression severity, and clinical antecedents in the child,” the authors wrote. “This result has important implications for treatment and prevention and highlights the need to resolve not only clinical phenomena in the child but also wider contextual difficulties.”
The study also suggested that neither disruptive behavior nor low mood were significantly associated with new-onset MDD in children and adolescents.
The children and adolescents in the study had a mean of 1.85 DSM-IV major depressive disorder symptoms at follow-up, and 20 of them – six males and 14 females – had new-onset MDD, with a mean age of onset of 14.4 years.
“Our findings suggest that primary prevention methods for depression in groups with high familial risk will need to include effective treatment of parental depression, irritability, and fear/anxiety in the child and consider social risk factors,” Dr. Rice and her coauthors wrote.
The research and researchers were supported by the Sir Jules Thorn Charitable Trust, the Medical Research Council, the Economic and Social Research Council, the British Academy, and the British Medical Association. No conflicts of interest were declared.
Fear and anxiety, economic disadvantage, and recent psychosocial adversity contribute significantly to the onset of major depressive disorder in children and adolescents with a strong family history of the condition.
A 4-year longitudinal study followed the offspring of 279 families in which one parent had experienced at least two episodes of major depressive disorder (MDD) and in which there was a biologically related child living with that index parent.
Fear and anxiety showed a strong and significant association with new-onset major depressive disorder, as did irritability. Furthermore, the association between the two symptoms was low but significant, suggesting that they do not often co-occur (JAMA Psychiatry. 2016 Dec 7. doi: 10.1001/jamapsychiatry.2016.3140).
“The results suggested that generalized anxiety symptoms were driving the predictive effect of fear/anxiety on new-onset MDD and that fear/anxiety (and not irritability) predicted an especially early MDD onset,” wrote Frances Rice, PhD, of the division of psychological medicine and clinical neurosciences at Cardiff University, Wales, and coauthors.
Recent psychosocial adversity – stressful events such as the death of a friend, illness, bullying, or parents fighting – also showed a strong association with new-onset major depressive disorder, while economic disadvantage had a lesser but still significant contribution. Both of these also were associated with fear and anxiety, and irritability.
Greater family history and more severe parental depression also contributed significantly to the emergence of depression in the offspring, although those factors were not associated with the clinical antecedents such as fear and anxiety.
“Therefore, the indicators of social risk predicted MDD independent of correlated familial risk, parental depression severity, and clinical antecedents in the child,” the authors wrote. “This result has important implications for treatment and prevention and highlights the need to resolve not only clinical phenomena in the child but also wider contextual difficulties.”
The study also suggested that neither disruptive behavior nor low mood were significantly associated with new-onset MDD in children and adolescents.
The children and adolescents in the study had a mean of 1.85 DSM-IV major depressive disorder symptoms at follow-up, and 20 of them – six males and 14 females – had new-onset MDD, with a mean age of onset of 14.4 years.
“Our findings suggest that primary prevention methods for depression in groups with high familial risk will need to include effective treatment of parental depression, irritability, and fear/anxiety in the child and consider social risk factors,” Dr. Rice and her coauthors wrote.
The research and researchers were supported by the Sir Jules Thorn Charitable Trust, the Medical Research Council, the Economic and Social Research Council, the British Academy, and the British Medical Association. No conflicts of interest were declared.
Fear and anxiety, economic disadvantage, and recent psychosocial adversity contribute significantly to the onset of major depressive disorder in children and adolescents with a strong family history of the condition.
A 4-year longitudinal study followed the offspring of 279 families in which one parent had experienced at least two episodes of major depressive disorder (MDD) and in which there was a biologically related child living with that index parent.
Fear and anxiety showed a strong and significant association with new-onset major depressive disorder, as did irritability. Furthermore, the association between the two symptoms was low but significant, suggesting that they do not often co-occur (JAMA Psychiatry. 2016 Dec 7. doi: 10.1001/jamapsychiatry.2016.3140).
“The results suggested that generalized anxiety symptoms were driving the predictive effect of fear/anxiety on new-onset MDD and that fear/anxiety (and not irritability) predicted an especially early MDD onset,” wrote Frances Rice, PhD, of the division of psychological medicine and clinical neurosciences at Cardiff University, Wales, and coauthors.
Recent psychosocial adversity – stressful events such as the death of a friend, illness, bullying, or parents fighting – also showed a strong association with new-onset major depressive disorder, while economic disadvantage had a lesser but still significant contribution. Both of these also were associated with fear and anxiety, and irritability.
Greater family history and more severe parental depression also contributed significantly to the emergence of depression in the offspring, although those factors were not associated with the clinical antecedents such as fear and anxiety.
“Therefore, the indicators of social risk predicted MDD independent of correlated familial risk, parental depression severity, and clinical antecedents in the child,” the authors wrote. “This result has important implications for treatment and prevention and highlights the need to resolve not only clinical phenomena in the child but also wider contextual difficulties.”
The study also suggested that neither disruptive behavior nor low mood were significantly associated with new-onset MDD in children and adolescents.
The children and adolescents in the study had a mean of 1.85 DSM-IV major depressive disorder symptoms at follow-up, and 20 of them – six males and 14 females – had new-onset MDD, with a mean age of onset of 14.4 years.
“Our findings suggest that primary prevention methods for depression in groups with high familial risk will need to include effective treatment of parental depression, irritability, and fear/anxiety in the child and consider social risk factors,” Dr. Rice and her coauthors wrote.
The research and researchers were supported by the Sir Jules Thorn Charitable Trust, the Medical Research Council, the Economic and Social Research Council, the British Academy, and the British Medical Association. No conflicts of interest were declared.
FROM JAMA PSYCHIATRY
Key clinical point:
Major finding: Fear and anxiety, psychosocial adversity, economic disadvantage, and stronger family history showed a strong and significant association with new-onset major depressive disorder..
Data source: Longitudinal cohort study of the offspring of 279 families in which one parent had experienced at least two episodes of major depressive disorder.
Disclosures: The research and researchers were supported by the Sir Jules Thorn Charitable Trust, the Medical Research Council, the Economic and Social Research Council, the British Academy, and the British Medical Association. No conflicts of interest were declared.
Noncancerous disease has a significant impact on lung cancer surgery survival
After older patients undergo lung resection for stage I non–small-cell lung cancer, they are actually at greater risk of death from something other than lung cancer for up to 2.5 years, according to researchers at Memorial Sloan Kettering Cancer Center, New York. The findings were published online in the Journal of Clinical Oncology (2016;34: doi: 10.1200/JCO.2016.69.0834).
“As age increases, the risk of competing events increases, such as death from noncancer diseases,” wrote Takashi Eguchi, MD, and coauthors. “In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors – specifically, comorbidities associated with increasing age – contribute to the risk of death.”
The researchers examined outcomes in three different age groups: younger than 65, 65-74, and 75 and older. The study focused on 2,186 patients with pathologic stage I non–small-cell lung cancer (NSCLC) among a population of 5,371 consecutive patients who had resection for primary lung cancer from 2000 to 2011. Seventy percent of patients in the study group were 65 and older, and 29.2% were 75 and older.
In all age groups, the calculated 5-year cumulative incidence of death (CID) for lung cancer–specific causes exceeded that for noncancer causes, but at significant intervals the 65-and-over groups were more likely to die from the latter. For the overall study group, noncancer-specific causes accounted for a higher CID through 18 months after surgery, when the CID for both cancer and noncancer causes crossed at around 2.9. At 5 years, the overall lung cancer–specific CID was 10.4 vs. 5.3 for noncancer specific causes.
However, in the older age groups, those trends were more pronounced. In those aged 65-74, CID for both causes met at around 3.15 at 18 months (10.7 for lung cancer–specific and 4.9 for noncancer specific at 5 years), whereas for those 75 and older, CID for noncancer causes exceeded that for lung cancer–related causes for 2.5 years, when both were around 6; reaching 13.2 for lung cancer–specific and 9 for noncancer-specific at 5 years.
In the 65-and-younger group, lung cancer– and noncancer-specific CIDs were equal for about 3 months after surgery, when the lung cancer deaths tracked upward and the trends diverged (at 5 years, CID was 7.5 for lung cancer–specific and 1 for noncancer specific).
“We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases,” Dr. Eguchi and colleagues said.
“We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases.” The findings underscore the importance of screening older patients for noncancer-specific diseases that could alter outcomes, the researchers said.
Of the 2,186 stage I NSCLC patients in the study, 167 developed severe morbidities after surgery; 68.3% developed respiratory problems and 18.6% went on to develop cardiovascular problems. Patients who had lobectomy were more likely to develop respiratory problems than were those who had sublobar resection, Dr. Eguchi and coauthors said.
Respiratory and cardiovascular diseases were the most frequent causes of death early after surgery. At 30 days, respiratory disease accounted for 5 deaths and cardiovascular disease 7 of 15 total deaths at 30 days; and at 90 days, 11 and 7, respectively, of 27 overall deaths. Even at 1 year, noncancer issues were the leading cause of death (50%), followed by lung cancer–specific causes (27.8%) and other cancer specific disease (13.3%).
“Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Dr. Eguchi and coauthors said. “These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.”
The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial disclosures.
Every surgeon performing lung resection comes across elderly patients who are at a higher risk than usual for a formal lung resection. In this era of screening and the abundant use of CT scans, this is increasingly common. Selection of the optimal treatment approach is often done intuitively, balancing the increased risk of surgery vs. the improved cancer-specific survival and the baseline life expectancy of the patient. This manuscript provides more quantitative estimates of this balance and draws attention, through a competing risks analysis, to the importance of non–cancer-related mortality in elderly patients.
The authors point out that non–cancer-related mortality is more common than cancer-related mortality for up to 2.5 years after surgery in patients greater than 75 years of age. This way of examining a situation is different from the usual emphasis on 30-day (and more recently the 90-day) perioperative mortality. The manuscript significantly adds to the decision-making framework of this increasingly important population and is a useful read for all lung cancer surgeons.
Sai Yendamuri, MD, is an attending surgeon in the department of thoracic surgery, the director, Thoracic Surgery Research Laboratory, and associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is associate medical editor for Thoracic Surgery News.
Every surgeon performing lung resection comes across elderly patients who are at a higher risk than usual for a formal lung resection. In this era of screening and the abundant use of CT scans, this is increasingly common. Selection of the optimal treatment approach is often done intuitively, balancing the increased risk of surgery vs. the improved cancer-specific survival and the baseline life expectancy of the patient. This manuscript provides more quantitative estimates of this balance and draws attention, through a competing risks analysis, to the importance of non–cancer-related mortality in elderly patients.
The authors point out that non–cancer-related mortality is more common than cancer-related mortality for up to 2.5 years after surgery in patients greater than 75 years of age. This way of examining a situation is different from the usual emphasis on 30-day (and more recently the 90-day) perioperative mortality. The manuscript significantly adds to the decision-making framework of this increasingly important population and is a useful read for all lung cancer surgeons.
Sai Yendamuri, MD, is an attending surgeon in the department of thoracic surgery, the director, Thoracic Surgery Research Laboratory, and associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is associate medical editor for Thoracic Surgery News.
Every surgeon performing lung resection comes across elderly patients who are at a higher risk than usual for a formal lung resection. In this era of screening and the abundant use of CT scans, this is increasingly common. Selection of the optimal treatment approach is often done intuitively, balancing the increased risk of surgery vs. the improved cancer-specific survival and the baseline life expectancy of the patient. This manuscript provides more quantitative estimates of this balance and draws attention, through a competing risks analysis, to the importance of non–cancer-related mortality in elderly patients.
The authors point out that non–cancer-related mortality is more common than cancer-related mortality for up to 2.5 years after surgery in patients greater than 75 years of age. This way of examining a situation is different from the usual emphasis on 30-day (and more recently the 90-day) perioperative mortality. The manuscript significantly adds to the decision-making framework of this increasingly important population and is a useful read for all lung cancer surgeons.
Sai Yendamuri, MD, is an attending surgeon in the department of thoracic surgery, the director, Thoracic Surgery Research Laboratory, and associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is associate medical editor for Thoracic Surgery News.
After older patients undergo lung resection for stage I non–small-cell lung cancer, they are actually at greater risk of death from something other than lung cancer for up to 2.5 years, according to researchers at Memorial Sloan Kettering Cancer Center, New York. The findings were published online in the Journal of Clinical Oncology (2016;34: doi: 10.1200/JCO.2016.69.0834).
“As age increases, the risk of competing events increases, such as death from noncancer diseases,” wrote Takashi Eguchi, MD, and coauthors. “In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors – specifically, comorbidities associated with increasing age – contribute to the risk of death.”
The researchers examined outcomes in three different age groups: younger than 65, 65-74, and 75 and older. The study focused on 2,186 patients with pathologic stage I non–small-cell lung cancer (NSCLC) among a population of 5,371 consecutive patients who had resection for primary lung cancer from 2000 to 2011. Seventy percent of patients in the study group were 65 and older, and 29.2% were 75 and older.
In all age groups, the calculated 5-year cumulative incidence of death (CID) for lung cancer–specific causes exceeded that for noncancer causes, but at significant intervals the 65-and-over groups were more likely to die from the latter. For the overall study group, noncancer-specific causes accounted for a higher CID through 18 months after surgery, when the CID for both cancer and noncancer causes crossed at around 2.9. At 5 years, the overall lung cancer–specific CID was 10.4 vs. 5.3 for noncancer specific causes.
However, in the older age groups, those trends were more pronounced. In those aged 65-74, CID for both causes met at around 3.15 at 18 months (10.7 for lung cancer–specific and 4.9 for noncancer specific at 5 years), whereas for those 75 and older, CID for noncancer causes exceeded that for lung cancer–related causes for 2.5 years, when both were around 6; reaching 13.2 for lung cancer–specific and 9 for noncancer-specific at 5 years.
In the 65-and-younger group, lung cancer– and noncancer-specific CIDs were equal for about 3 months after surgery, when the lung cancer deaths tracked upward and the trends diverged (at 5 years, CID was 7.5 for lung cancer–specific and 1 for noncancer specific).
“We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases,” Dr. Eguchi and colleagues said.
“We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases.” The findings underscore the importance of screening older patients for noncancer-specific diseases that could alter outcomes, the researchers said.
Of the 2,186 stage I NSCLC patients in the study, 167 developed severe morbidities after surgery; 68.3% developed respiratory problems and 18.6% went on to develop cardiovascular problems. Patients who had lobectomy were more likely to develop respiratory problems than were those who had sublobar resection, Dr. Eguchi and coauthors said.
Respiratory and cardiovascular diseases were the most frequent causes of death early after surgery. At 30 days, respiratory disease accounted for 5 deaths and cardiovascular disease 7 of 15 total deaths at 30 days; and at 90 days, 11 and 7, respectively, of 27 overall deaths. Even at 1 year, noncancer issues were the leading cause of death (50%), followed by lung cancer–specific causes (27.8%) and other cancer specific disease (13.3%).
“Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Dr. Eguchi and coauthors said. “These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.”
The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial disclosures.
After older patients undergo lung resection for stage I non–small-cell lung cancer, they are actually at greater risk of death from something other than lung cancer for up to 2.5 years, according to researchers at Memorial Sloan Kettering Cancer Center, New York. The findings were published online in the Journal of Clinical Oncology (2016;34: doi: 10.1200/JCO.2016.69.0834).
“As age increases, the risk of competing events increases, such as death from noncancer diseases,” wrote Takashi Eguchi, MD, and coauthors. “In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors – specifically, comorbidities associated with increasing age – contribute to the risk of death.”
The researchers examined outcomes in three different age groups: younger than 65, 65-74, and 75 and older. The study focused on 2,186 patients with pathologic stage I non–small-cell lung cancer (NSCLC) among a population of 5,371 consecutive patients who had resection for primary lung cancer from 2000 to 2011. Seventy percent of patients in the study group were 65 and older, and 29.2% were 75 and older.
In all age groups, the calculated 5-year cumulative incidence of death (CID) for lung cancer–specific causes exceeded that for noncancer causes, but at significant intervals the 65-and-over groups were more likely to die from the latter. For the overall study group, noncancer-specific causes accounted for a higher CID through 18 months after surgery, when the CID for both cancer and noncancer causes crossed at around 2.9. At 5 years, the overall lung cancer–specific CID was 10.4 vs. 5.3 for noncancer specific causes.
However, in the older age groups, those trends were more pronounced. In those aged 65-74, CID for both causes met at around 3.15 at 18 months (10.7 for lung cancer–specific and 4.9 for noncancer specific at 5 years), whereas for those 75 and older, CID for noncancer causes exceeded that for lung cancer–related causes for 2.5 years, when both were around 6; reaching 13.2 for lung cancer–specific and 9 for noncancer-specific at 5 years.
In the 65-and-younger group, lung cancer– and noncancer-specific CIDs were equal for about 3 months after surgery, when the lung cancer deaths tracked upward and the trends diverged (at 5 years, CID was 7.5 for lung cancer–specific and 1 for noncancer specific).
“We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases,” Dr. Eguchi and colleagues said.
“We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases.” The findings underscore the importance of screening older patients for noncancer-specific diseases that could alter outcomes, the researchers said.
Of the 2,186 stage I NSCLC patients in the study, 167 developed severe morbidities after surgery; 68.3% developed respiratory problems and 18.6% went on to develop cardiovascular problems. Patients who had lobectomy were more likely to develop respiratory problems than were those who had sublobar resection, Dr. Eguchi and coauthors said.
Respiratory and cardiovascular diseases were the most frequent causes of death early after surgery. At 30 days, respiratory disease accounted for 5 deaths and cardiovascular disease 7 of 15 total deaths at 30 days; and at 90 days, 11 and 7, respectively, of 27 overall deaths. Even at 1 year, noncancer issues were the leading cause of death (50%), followed by lung cancer–specific causes (27.8%) and other cancer specific disease (13.3%).
“Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Dr. Eguchi and coauthors said. “These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.”
The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Risk of non-cancer death after curative resection of stage 1 non–small-call lung cancer (NSCLC) exceeded that of lung-cancer deaths 1.5 to 2.5 years after surgery in older patients.
Major finding: In patients aged 75 and older the risk of non–lung-cancer–related death exceeded the risk of death from lung cancer for 2.5 years after surgery, whereas in patients 65 and younger the risk of non–lung cancer death exceeded that of lung-cancer death for 3 months after surgery.
Data Source: Single-center analysis of 5,371 consecutive patients who had curative lung cancer resection from 2000 to 2011, 2,186 of whom had stage 1 NSCLC.
Disclosures: The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial relationships to disclose.
Expandable cardiac valve in children found feasible
Young children and infants who require cardiac valve replacement are limited to fixed-diameter prostheses that cannot accommodate their growth, but researchers at Boston Children’s Hospital have reinforced an expandable bovine jugular vein graft using an external stent and implanted it in 42 patients with acceptable short-term results, according to a report in the Journal of Thoracic and Cardiovascular Surgery.
In 4 years, the modified Melody valve (Medtronic) has proved amenable to enlargement via catheterization as the child grows, Sitaram M. Emani, MD, and coauthors said. “The valve was competent with low gradient acutely postoperatively in all patients,” Dr. Emani and his coauthors said (J Thorac Cardiovasc Surg. 2016 Dec;152[6]:1514-23).
The Melody valve is approved for transcatheter implantation into the RVOT and can be modified for the semilunar or AV positions, Dr. Emani and his coauthors said. The valve has achieved competence within a size range of 10-22 mm.
The researchers implanted the valve in four positions: RVOT (14), mitral (24), aortic (one), or tricuspid (three). The median age at implantation was 10 months, with a range of 3 weeks to 5.8 years. The patients had an average of one previous valve replacement or repair procedure.
Six deaths occurred in the study population; three before discharge. Those three patients had replacement to salvage a moribund circulation in the setting of mechanical circulatory support and severely depressed ventricular function. The other three patients who died were able to demonstrate adequate valve function, and autopsy did not show any signs of valvular thrombosis or deterioration, the researchers said.
Using Kaplan-Meier analysis, Dr. Emani and his coauthors estimated the freedom from death or transplantation was 83% at 12 months and 77% at 24 months.
The average time between catheter-based dilations was around 10 months, they wrote, “but this might be increased by more aggressive dilation.” Early in the study, the researchers were not aggressive with dilations because of concerns about valve injury, but then they found that patients tolerated increases in valve diameter by as much as 4 mm.
“Further investigation is needed to determine whether the device retains expandability over the long term after multiple dilations,” Dr. Emani and his coauthors said.
Further refinements in valve design and implantation techniques may lead to prevention of reoperation and perivalvular complications, they suggested.
The researchers did note a couple limitations of their study: the heterogeneous cohort prohibits any generalization of the outcomes, particularly mortality, and concomitant procedures performed during the valve replacement would affect mortality.
Dr. Emani and his colleagues had no financial relationships to disclose.
In his invited commentary, Carl L. Backer, MD, of Northwestern University, Chicago, noted three advantages of the use of the stent-expandable bovine valve for infants and children: It can be used when no prosthetic is small enough to fit in the annulus, it does not require anticoagulation with warfarin after placement, and it can be dilated as the child grows (J Thorac Cardiovasc Surg. 2016 Dec:152[6];1524-5).
But, Dr. Backer added, “one note of caution that I would raise relates to the late complications noted in previous trials with tissue valves on the left side of the heart in children.” He noted such experience had been reported with the Mitroflow bovine pericardial bioprosthesis (LivaNova) (Circulation. 2014;130[1];51-60) and the Perimount Magna (Edwards Lifesciences) (Ann Thorac Surg. 2016;102[1];308-11). “The follow-up of Emani and colleagues at two years is good; however, there is always the possibility that premature calcification and stenosis of these valves, particularly those placed on the left side of the heart, could lead to the need for early explantation,” Dr. Backer said.
Nonetheless, the findings of Dr. Emani and his colleagues showed that the concept of implanting a stented jugular vein graft valve in infants and young children “is proving to be safe and efficacious,” Dr. Backer said. “In these children for whom there are limited options, this appears to be an important addition to our surgical strategies,” he concluded.
Dr. Backer had no financial relationships to disclose.
In his invited commentary, Carl L. Backer, MD, of Northwestern University, Chicago, noted three advantages of the use of the stent-expandable bovine valve for infants and children: It can be used when no prosthetic is small enough to fit in the annulus, it does not require anticoagulation with warfarin after placement, and it can be dilated as the child grows (J Thorac Cardiovasc Surg. 2016 Dec:152[6];1524-5).
But, Dr. Backer added, “one note of caution that I would raise relates to the late complications noted in previous trials with tissue valves on the left side of the heart in children.” He noted such experience had been reported with the Mitroflow bovine pericardial bioprosthesis (LivaNova) (Circulation. 2014;130[1];51-60) and the Perimount Magna (Edwards Lifesciences) (Ann Thorac Surg. 2016;102[1];308-11). “The follow-up of Emani and colleagues at two years is good; however, there is always the possibility that premature calcification and stenosis of these valves, particularly those placed on the left side of the heart, could lead to the need for early explantation,” Dr. Backer said.
Nonetheless, the findings of Dr. Emani and his colleagues showed that the concept of implanting a stented jugular vein graft valve in infants and young children “is proving to be safe and efficacious,” Dr. Backer said. “In these children for whom there are limited options, this appears to be an important addition to our surgical strategies,” he concluded.
Dr. Backer had no financial relationships to disclose.
In his invited commentary, Carl L. Backer, MD, of Northwestern University, Chicago, noted three advantages of the use of the stent-expandable bovine valve for infants and children: It can be used when no prosthetic is small enough to fit in the annulus, it does not require anticoagulation with warfarin after placement, and it can be dilated as the child grows (J Thorac Cardiovasc Surg. 2016 Dec:152[6];1524-5).
But, Dr. Backer added, “one note of caution that I would raise relates to the late complications noted in previous trials with tissue valves on the left side of the heart in children.” He noted such experience had been reported with the Mitroflow bovine pericardial bioprosthesis (LivaNova) (Circulation. 2014;130[1];51-60) and the Perimount Magna (Edwards Lifesciences) (Ann Thorac Surg. 2016;102[1];308-11). “The follow-up of Emani and colleagues at two years is good; however, there is always the possibility that premature calcification and stenosis of these valves, particularly those placed on the left side of the heart, could lead to the need for early explantation,” Dr. Backer said.
Nonetheless, the findings of Dr. Emani and his colleagues showed that the concept of implanting a stented jugular vein graft valve in infants and young children “is proving to be safe and efficacious,” Dr. Backer said. “In these children for whom there are limited options, this appears to be an important addition to our surgical strategies,” he concluded.
Dr. Backer had no financial relationships to disclose.
Young children and infants who require cardiac valve replacement are limited to fixed-diameter prostheses that cannot accommodate their growth, but researchers at Boston Children’s Hospital have reinforced an expandable bovine jugular vein graft using an external stent and implanted it in 42 patients with acceptable short-term results, according to a report in the Journal of Thoracic and Cardiovascular Surgery.
In 4 years, the modified Melody valve (Medtronic) has proved amenable to enlargement via catheterization as the child grows, Sitaram M. Emani, MD, and coauthors said. “The valve was competent with low gradient acutely postoperatively in all patients,” Dr. Emani and his coauthors said (J Thorac Cardiovasc Surg. 2016 Dec;152[6]:1514-23).
The Melody valve is approved for transcatheter implantation into the RVOT and can be modified for the semilunar or AV positions, Dr. Emani and his coauthors said. The valve has achieved competence within a size range of 10-22 mm.
The researchers implanted the valve in four positions: RVOT (14), mitral (24), aortic (one), or tricuspid (three). The median age at implantation was 10 months, with a range of 3 weeks to 5.8 years. The patients had an average of one previous valve replacement or repair procedure.
Six deaths occurred in the study population; three before discharge. Those three patients had replacement to salvage a moribund circulation in the setting of mechanical circulatory support and severely depressed ventricular function. The other three patients who died were able to demonstrate adequate valve function, and autopsy did not show any signs of valvular thrombosis or deterioration, the researchers said.
Using Kaplan-Meier analysis, Dr. Emani and his coauthors estimated the freedom from death or transplantation was 83% at 12 months and 77% at 24 months.
The average time between catheter-based dilations was around 10 months, they wrote, “but this might be increased by more aggressive dilation.” Early in the study, the researchers were not aggressive with dilations because of concerns about valve injury, but then they found that patients tolerated increases in valve diameter by as much as 4 mm.
“Further investigation is needed to determine whether the device retains expandability over the long term after multiple dilations,” Dr. Emani and his coauthors said.
Further refinements in valve design and implantation techniques may lead to prevention of reoperation and perivalvular complications, they suggested.
The researchers did note a couple limitations of their study: the heterogeneous cohort prohibits any generalization of the outcomes, particularly mortality, and concomitant procedures performed during the valve replacement would affect mortality.
Dr. Emani and his colleagues had no financial relationships to disclose.
Young children and infants who require cardiac valve replacement are limited to fixed-diameter prostheses that cannot accommodate their growth, but researchers at Boston Children’s Hospital have reinforced an expandable bovine jugular vein graft using an external stent and implanted it in 42 patients with acceptable short-term results, according to a report in the Journal of Thoracic and Cardiovascular Surgery.
In 4 years, the modified Melody valve (Medtronic) has proved amenable to enlargement via catheterization as the child grows, Sitaram M. Emani, MD, and coauthors said. “The valve was competent with low gradient acutely postoperatively in all patients,” Dr. Emani and his coauthors said (J Thorac Cardiovasc Surg. 2016 Dec;152[6]:1514-23).
The Melody valve is approved for transcatheter implantation into the RVOT and can be modified for the semilunar or AV positions, Dr. Emani and his coauthors said. The valve has achieved competence within a size range of 10-22 mm.
The researchers implanted the valve in four positions: RVOT (14), mitral (24), aortic (one), or tricuspid (three). The median age at implantation was 10 months, with a range of 3 weeks to 5.8 years. The patients had an average of one previous valve replacement or repair procedure.
Six deaths occurred in the study population; three before discharge. Those three patients had replacement to salvage a moribund circulation in the setting of mechanical circulatory support and severely depressed ventricular function. The other three patients who died were able to demonstrate adequate valve function, and autopsy did not show any signs of valvular thrombosis or deterioration, the researchers said.
Using Kaplan-Meier analysis, Dr. Emani and his coauthors estimated the freedom from death or transplantation was 83% at 12 months and 77% at 24 months.
The average time between catheter-based dilations was around 10 months, they wrote, “but this might be increased by more aggressive dilation.” Early in the study, the researchers were not aggressive with dilations because of concerns about valve injury, but then they found that patients tolerated increases in valve diameter by as much as 4 mm.
“Further investigation is needed to determine whether the device retains expandability over the long term after multiple dilations,” Dr. Emani and his coauthors said.
Further refinements in valve design and implantation techniques may lead to prevention of reoperation and perivalvular complications, they suggested.
The researchers did note a couple limitations of their study: the heterogeneous cohort prohibits any generalization of the outcomes, particularly mortality, and concomitant procedures performed during the valve replacement would affect mortality.
Dr. Emani and his colleagues had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: An expandable cardiac valve adapted for infants and children demonstrates acceptable function and can accommodate the child’s growth.
Major finding: At 12 months after implantation of a bovine jugular vein graft reinforced with an external stent modified for surgical valve replacement in pediatric patients, Kaplan-Meier analysis indicated that 83% of those surviving would be free from reoperation at 12 months.
Data source: Single-center study of 42 patients who underwent implantation between 2010 and 2014.
Disclosures: Dr. Emani and his coauthors had no financial relationships to disclose.
Shulkin Addresses APRN Rule, Health Care Vacancies, and Access
At the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016, Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD. The below video that discusses ongoing efforts to improve coordination of care with community providers, the VA’s commitment to expanding the scope of practice for advanced practice registered nurses (APRNs), and the recruitment challenges for filling more than 46,000 health care vacancies. Dr. Shulkin also discussed VA progress over the past 18 months.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016, Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD. The below video that discusses ongoing efforts to improve coordination of care with community providers, the VA’s commitment to expanding the scope of practice for advanced practice registered nurses (APRNs), and the recruitment challenges for filling more than 46,000 health care vacancies. Dr. Shulkin also discussed VA progress over the past 18 months.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016, Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD. The below video that discusses ongoing efforts to improve coordination of care with community providers, the VA’s commitment to expanding the scope of practice for advanced practice registered nurses (APRNs), and the recruitment challenges for filling more than 46,000 health care vacancies. Dr. Shulkin also discussed VA progress over the past 18 months.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Cancer Prevention and Gastrointestinal Risk
Low-dose aspirin is recommended to help prevent colorectal cancer and vascular disease as well as to reduce mortality. Research suggests that low-dose aspirin also can be useful as an adjunct treatment in established cancers. The potential, however, is offset by concerns about bleeding.
Are the concerns valid? When gauging the risks vs benefits of aspirin for patients at risk for bleeding, considering not only the severity of events, but also the frequency is important, say researchers from Cardiff University, Hywel Dda University, University of Cambridge, Harvard, University of Zaragoza, University of South Wales, Swansea University, National University of Singapore, and University of Leicester.
Related: A Better Way to Predict Colorectal Cancer Relapse?
They point out that most estimates of harm from aspirin are based on the frequency of “major” gastrointestinal (GI) bleeding together with cerebral bleeding. But they also note that although GI bleeds can be severe, they’re “acute events usually followed by recovery without sequelae.” Strokes, on the other hand, can have lasting effects in survivors; and people who survive heart attacks or cancer may require complex and lifelong interventions.
The researchers suggest weighing the risk of fatal bleeds attributable to aspirin with the severity of the disease events prevented. And the distinction between GI bleeding and fatal bleeding isn’t trivial, they contend. Studies have already shown that the risk of death from aspirin-related GI bleeds is lower than that of spontaneous GI bleeding.
To put the discussion on a more even footing, the research team looked at 11 long-term, randomized controlled trials of aspirin prophylaxis with data on fatal adverse effects (AEs). They also incorporated new data from direct e-mail contact with the authors of some of the studies.
They found that although aspirin increases the risk of GI bleeding by about 60%, low-dose aspirin is associated with a lower risk of fatality among the patients who develop GI bleeding. The researchers note that no report mentioned the use of gastric protection by proton pump inhibitor or other treatment.
Most important, the researchers say, is the finding that among all the subjects who were taking aspirin, there was no increase in death from GI bleeding compared with those randomized to take no aspirin.
Related: Colorectal Screening: Available but Underused
Also important, they add, is to bear in mind the duration of aspirin taking. Almost all studies of cancer prevention, they note, show a 3- to 5-year delay before the benefits of aspirin are clinically apparent (because the effects on cells take a while to show up, that is, as the absence of a tumor).
However, the incidence of GI bleeding attributable to aspirin seems to drop over time: Within the first month of aspirin taking, the risk increases more than 4-fold, but then declines rapidly. After about 3 to 5 years of taking aspirin, there seems to be no excess in GI bleeds. Similarly, most deaths from bleeding happen with the first month, implying underlying untreated gastric pathology, the researchers say.
GI bleeds are still the majority of the AEs caused by aspirin, but thanks to better treatments, GI bleeding and fatal bleeding have declined as much as 20% to 50% in some countries, such as Scotland, Spain, U.S., and Wales. In the risk-benefit analysis, however, the researchers say, the “undesirable effect” of prophylactic aspirin as serious as a vascular event or cancer is rare: Low-dose aspirin is associated with 1 death and 1 disabling hemorrhagic stroke per year in every 10,000 people, and that number could be lower if hypertension is treated adequately.
Source:
Elwood PC, Morgan G, Galante J, et al. PLoS One. 2016;11(11): e0166166.
doi: 10.1371/journal.pone.0166166.
Low-dose aspirin is recommended to help prevent colorectal cancer and vascular disease as well as to reduce mortality. Research suggests that low-dose aspirin also can be useful as an adjunct treatment in established cancers. The potential, however, is offset by concerns about bleeding.
Are the concerns valid? When gauging the risks vs benefits of aspirin for patients at risk for bleeding, considering not only the severity of events, but also the frequency is important, say researchers from Cardiff University, Hywel Dda University, University of Cambridge, Harvard, University of Zaragoza, University of South Wales, Swansea University, National University of Singapore, and University of Leicester.
Related: A Better Way to Predict Colorectal Cancer Relapse?
They point out that most estimates of harm from aspirin are based on the frequency of “major” gastrointestinal (GI) bleeding together with cerebral bleeding. But they also note that although GI bleeds can be severe, they’re “acute events usually followed by recovery without sequelae.” Strokes, on the other hand, can have lasting effects in survivors; and people who survive heart attacks or cancer may require complex and lifelong interventions.
The researchers suggest weighing the risk of fatal bleeds attributable to aspirin with the severity of the disease events prevented. And the distinction between GI bleeding and fatal bleeding isn’t trivial, they contend. Studies have already shown that the risk of death from aspirin-related GI bleeds is lower than that of spontaneous GI bleeding.
To put the discussion on a more even footing, the research team looked at 11 long-term, randomized controlled trials of aspirin prophylaxis with data on fatal adverse effects (AEs). They also incorporated new data from direct e-mail contact with the authors of some of the studies.
They found that although aspirin increases the risk of GI bleeding by about 60%, low-dose aspirin is associated with a lower risk of fatality among the patients who develop GI bleeding. The researchers note that no report mentioned the use of gastric protection by proton pump inhibitor or other treatment.
Most important, the researchers say, is the finding that among all the subjects who were taking aspirin, there was no increase in death from GI bleeding compared with those randomized to take no aspirin.
Related: Colorectal Screening: Available but Underused
Also important, they add, is to bear in mind the duration of aspirin taking. Almost all studies of cancer prevention, they note, show a 3- to 5-year delay before the benefits of aspirin are clinically apparent (because the effects on cells take a while to show up, that is, as the absence of a tumor).
However, the incidence of GI bleeding attributable to aspirin seems to drop over time: Within the first month of aspirin taking, the risk increases more than 4-fold, but then declines rapidly. After about 3 to 5 years of taking aspirin, there seems to be no excess in GI bleeds. Similarly, most deaths from bleeding happen with the first month, implying underlying untreated gastric pathology, the researchers say.
GI bleeds are still the majority of the AEs caused by aspirin, but thanks to better treatments, GI bleeding and fatal bleeding have declined as much as 20% to 50% in some countries, such as Scotland, Spain, U.S., and Wales. In the risk-benefit analysis, however, the researchers say, the “undesirable effect” of prophylactic aspirin as serious as a vascular event or cancer is rare: Low-dose aspirin is associated with 1 death and 1 disabling hemorrhagic stroke per year in every 10,000 people, and that number could be lower if hypertension is treated adequately.
Source:
Elwood PC, Morgan G, Galante J, et al. PLoS One. 2016;11(11): e0166166.
doi: 10.1371/journal.pone.0166166.
Low-dose aspirin is recommended to help prevent colorectal cancer and vascular disease as well as to reduce mortality. Research suggests that low-dose aspirin also can be useful as an adjunct treatment in established cancers. The potential, however, is offset by concerns about bleeding.
Are the concerns valid? When gauging the risks vs benefits of aspirin for patients at risk for bleeding, considering not only the severity of events, but also the frequency is important, say researchers from Cardiff University, Hywel Dda University, University of Cambridge, Harvard, University of Zaragoza, University of South Wales, Swansea University, National University of Singapore, and University of Leicester.
Related: A Better Way to Predict Colorectal Cancer Relapse?
They point out that most estimates of harm from aspirin are based on the frequency of “major” gastrointestinal (GI) bleeding together with cerebral bleeding. But they also note that although GI bleeds can be severe, they’re “acute events usually followed by recovery without sequelae.” Strokes, on the other hand, can have lasting effects in survivors; and people who survive heart attacks or cancer may require complex and lifelong interventions.
The researchers suggest weighing the risk of fatal bleeds attributable to aspirin with the severity of the disease events prevented. And the distinction between GI bleeding and fatal bleeding isn’t trivial, they contend. Studies have already shown that the risk of death from aspirin-related GI bleeds is lower than that of spontaneous GI bleeding.
To put the discussion on a more even footing, the research team looked at 11 long-term, randomized controlled trials of aspirin prophylaxis with data on fatal adverse effects (AEs). They also incorporated new data from direct e-mail contact with the authors of some of the studies.
They found that although aspirin increases the risk of GI bleeding by about 60%, low-dose aspirin is associated with a lower risk of fatality among the patients who develop GI bleeding. The researchers note that no report mentioned the use of gastric protection by proton pump inhibitor or other treatment.
Most important, the researchers say, is the finding that among all the subjects who were taking aspirin, there was no increase in death from GI bleeding compared with those randomized to take no aspirin.
Related: Colorectal Screening: Available but Underused
Also important, they add, is to bear in mind the duration of aspirin taking. Almost all studies of cancer prevention, they note, show a 3- to 5-year delay before the benefits of aspirin are clinically apparent (because the effects on cells take a while to show up, that is, as the absence of a tumor).
However, the incidence of GI bleeding attributable to aspirin seems to drop over time: Within the first month of aspirin taking, the risk increases more than 4-fold, but then declines rapidly. After about 3 to 5 years of taking aspirin, there seems to be no excess in GI bleeds. Similarly, most deaths from bleeding happen with the first month, implying underlying untreated gastric pathology, the researchers say.
GI bleeds are still the majority of the AEs caused by aspirin, but thanks to better treatments, GI bleeding and fatal bleeding have declined as much as 20% to 50% in some countries, such as Scotland, Spain, U.S., and Wales. In the risk-benefit analysis, however, the researchers say, the “undesirable effect” of prophylactic aspirin as serious as a vascular event or cancer is rare: Low-dose aspirin is associated with 1 death and 1 disabling hemorrhagic stroke per year in every 10,000 people, and that number could be lower if hypertension is treated adequately.
Source:
Elwood PC, Morgan G, Galante J, et al. PLoS One. 2016;11(11): e0166166.
doi: 10.1371/journal.pone.0166166.