User login
Minocycline-induced hyperpigmentation
A 64-year-old woman had a remote history of generalized fatigue, tightness of the hands, tingling and numbness of the face, joint stiffness, and bluish discoloration of the fingers that worsened with cold weather. Laboratory testing at that time had revealed an antinuclear antibody titer over 1:320 (reference range < 1:10), anti-Scl-70 antibody 100 U/mL (< 32 U/mL), and thyroid-stimulating hormone 10.78 mIU/L (0.4–5.5). Pulmonary function testing showed a pattern of restrictive lung disease. She was diagnosed with hypothyroidism, Raynaud phenomenon, and scleroderma. She was referred to a rheumatologist, who prescribed levothyroxine and penicillamine.
Despite treatment, she continued to feel fatigued, and she requested the addition of minocycline to the scleroderma treatment after seeing a report on television. Minocycline 100 mg twice daily was prescribed. She reported improvement of her symptoms for the next 2 years but was then lost to follow-up with the rheumatologist. She continued to take penicillamine and minocycline as prescribed by her primary care physician.
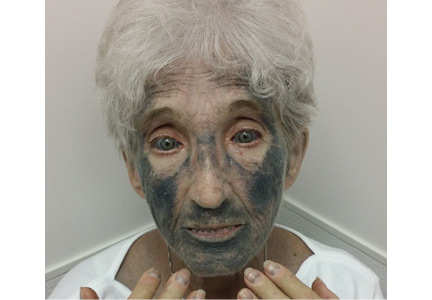
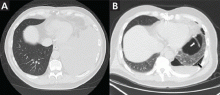
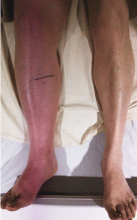
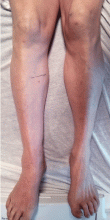
She presented to our clinic with bluish discoloration (Figure 1) that had started 1 year before as a small area but had spread to involve the entire face, fingers, gums, teeth, and sclera, and included a dark discoloration of the neck and upper chest. She had been taking minocycline for nearly 9 years. We referred her to a dermatologist, who diagnosed minocycline-induced hyperpigmentation. Her minocycline was stopped. Skin biopsy was not done, as the dermatologist was confident making the diagnosis without biopsy. At 1 year later, she continued to have the widespread skin pigmentation with no improvement at all.
DIFFERENTIAL DIAGNOSIS
Hyperpigmentation is the darkening in the natural color of the skin, usually from increased deposition of melanin in the epidermis or dermis, or both. It can occur in different degrees of blue, brown, and black (from lightest to darkest). Less frequently, it may be caused by the deposition in the dermis of an endogenous or exogenous pigment, such as hemosiderin, iron, or heavy metal.1 The hyperpigmentation can be circumscribed or more diffuse.
The differential diagnosis of diffuse skin pigmentation includes Addison disease, hyperthyroidism, hemochromatosis, erythema dyschromicum perstans, cutaneous malignancies, sunburn, and drug-induced hyperpigmentation.1,2 Medications commonly cited as causing hyperpigmentation include minocycline, amiodarone, bleomycin, prostaglandins, oral contraceptives, phenothiazine, and antimalarial drugs.1,3 In Addison disease, the pigmentation is typically diffuse, with accentuation in sun-exposed areas, flexures, palmar and plantar creases, and areas of pressure or friction.2 The bronze discoloration of hemochromatosis is from a combination of hemosiderin deposition and increased melanin production.1 Erythema dyschromicum perstans presents with brownish oval-shaped macules and patches. Early lesions may have thin, raised, erythematous borders that typically involve the trunk, but they may spread to the neck, upper extremities, and face.4
The role of minocycline in the treatment of scleroderma is controversial. Early reports involving a small number of patients showed a benefit of minocycline in decreasing symptoms,5,6 but these findings were not achieved in a larger multicenter trial.7
Types of minocycline-induced hyperpigmentation
Three types of minocycline-induced hyperpigmentation occur3,8:
- Type 1—blue-grey coloration on the face in areas of inflammation
- Type 2—blue-grey coloration on normal skin on the skin of the shins and forearms
- Type 3—the least common, characterized by diffuse muddy brown or blue-grey discoloration in sun-exposed areas, as in our patient.
The prevalence of minocycline-induced hyperpigmentation varies between 2.4% and 41% and is highest in patients with rheumatoid arthritis.3,9 Type 1 pigmentation is not correlated with treatment duration or cumulative dose, while type 2 and 3 are associated with long-term therapy.8 In type 3, changes are nonspecific, consisting of increased melanin in basal keratinocytes and melanin-only staining dermal melanophages. Types 1 and 2 may resolve slowly, whereas type 3 can persist indefinitely.3,8,10
TREATMENT
Treatment involves early recognition, discontinuation of the drug, and avoidance of sun exposure. Treatment with pigment-specific lasers has shown promise.8,10
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Thiboutot DM. Clinical review 74: dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab 1995; 80:3082–3087.
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat 2009; 17:123–126.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of Cinderella or ashy dermatosis. Int J Dermatol 2004; 43:230–232.
- Le CH, Morales A, Trentham DE. Minocycline in early diffuse scleroderma. Lancet 1998; 352:1755–1756.
- Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62:267–269.
- Mayes MD, O’Donnell D, Rothfield NF, Csuka ME. Minocycline is not effective in systemic sclerosis: results of an open-label multicenter trial. Arthritis Rheum 2004; 50:553–557.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. London, UK: Saunders/Elsevier; 2011:125–126.
- Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–1257.
- Vangipuram RK, DeLozier WL, Geddes E, Friedman PM. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med 2016; 48:234–237.
A 64-year-old woman had a remote history of generalized fatigue, tightness of the hands, tingling and numbness of the face, joint stiffness, and bluish discoloration of the fingers that worsened with cold weather. Laboratory testing at that time had revealed an antinuclear antibody titer over 1:320 (reference range < 1:10), anti-Scl-70 antibody 100 U/mL (< 32 U/mL), and thyroid-stimulating hormone 10.78 mIU/L (0.4–5.5). Pulmonary function testing showed a pattern of restrictive lung disease. She was diagnosed with hypothyroidism, Raynaud phenomenon, and scleroderma. She was referred to a rheumatologist, who prescribed levothyroxine and penicillamine.
Despite treatment, she continued to feel fatigued, and she requested the addition of minocycline to the scleroderma treatment after seeing a report on television. Minocycline 100 mg twice daily was prescribed. She reported improvement of her symptoms for the next 2 years but was then lost to follow-up with the rheumatologist. She continued to take penicillamine and minocycline as prescribed by her primary care physician.
She presented to our clinic with bluish discoloration (Figure 1) that had started 1 year before as a small area but had spread to involve the entire face, fingers, gums, teeth, and sclera, and included a dark discoloration of the neck and upper chest. She had been taking minocycline for nearly 9 years. We referred her to a dermatologist, who diagnosed minocycline-induced hyperpigmentation. Her minocycline was stopped. Skin biopsy was not done, as the dermatologist was confident making the diagnosis without biopsy. At 1 year later, she continued to have the widespread skin pigmentation with no improvement at all.
DIFFERENTIAL DIAGNOSIS
Hyperpigmentation is the darkening in the natural color of the skin, usually from increased deposition of melanin in the epidermis or dermis, or both. It can occur in different degrees of blue, brown, and black (from lightest to darkest). Less frequently, it may be caused by the deposition in the dermis of an endogenous or exogenous pigment, such as hemosiderin, iron, or heavy metal.1 The hyperpigmentation can be circumscribed or more diffuse.
The differential diagnosis of diffuse skin pigmentation includes Addison disease, hyperthyroidism, hemochromatosis, erythema dyschromicum perstans, cutaneous malignancies, sunburn, and drug-induced hyperpigmentation.1,2 Medications commonly cited as causing hyperpigmentation include minocycline, amiodarone, bleomycin, prostaglandins, oral contraceptives, phenothiazine, and antimalarial drugs.1,3 In Addison disease, the pigmentation is typically diffuse, with accentuation in sun-exposed areas, flexures, palmar and plantar creases, and areas of pressure or friction.2 The bronze discoloration of hemochromatosis is from a combination of hemosiderin deposition and increased melanin production.1 Erythema dyschromicum perstans presents with brownish oval-shaped macules and patches. Early lesions may have thin, raised, erythematous borders that typically involve the trunk, but they may spread to the neck, upper extremities, and face.4
The role of minocycline in the treatment of scleroderma is controversial. Early reports involving a small number of patients showed a benefit of minocycline in decreasing symptoms,5,6 but these findings were not achieved in a larger multicenter trial.7
Types of minocycline-induced hyperpigmentation
Three types of minocycline-induced hyperpigmentation occur3,8:
- Type 1—blue-grey coloration on the face in areas of inflammation
- Type 2—blue-grey coloration on normal skin on the skin of the shins and forearms
- Type 3—the least common, characterized by diffuse muddy brown or blue-grey discoloration in sun-exposed areas, as in our patient.
The prevalence of minocycline-induced hyperpigmentation varies between 2.4% and 41% and is highest in patients with rheumatoid arthritis.3,9 Type 1 pigmentation is not correlated with treatment duration or cumulative dose, while type 2 and 3 are associated with long-term therapy.8 In type 3, changes are nonspecific, consisting of increased melanin in basal keratinocytes and melanin-only staining dermal melanophages. Types 1 and 2 may resolve slowly, whereas type 3 can persist indefinitely.3,8,10
TREATMENT
Treatment involves early recognition, discontinuation of the drug, and avoidance of sun exposure. Treatment with pigment-specific lasers has shown promise.8,10
A 64-year-old woman had a remote history of generalized fatigue, tightness of the hands, tingling and numbness of the face, joint stiffness, and bluish discoloration of the fingers that worsened with cold weather. Laboratory testing at that time had revealed an antinuclear antibody titer over 1:320 (reference range < 1:10), anti-Scl-70 antibody 100 U/mL (< 32 U/mL), and thyroid-stimulating hormone 10.78 mIU/L (0.4–5.5). Pulmonary function testing showed a pattern of restrictive lung disease. She was diagnosed with hypothyroidism, Raynaud phenomenon, and scleroderma. She was referred to a rheumatologist, who prescribed levothyroxine and penicillamine.
Despite treatment, she continued to feel fatigued, and she requested the addition of minocycline to the scleroderma treatment after seeing a report on television. Minocycline 100 mg twice daily was prescribed. She reported improvement of her symptoms for the next 2 years but was then lost to follow-up with the rheumatologist. She continued to take penicillamine and minocycline as prescribed by her primary care physician.
She presented to our clinic with bluish discoloration (Figure 1) that had started 1 year before as a small area but had spread to involve the entire face, fingers, gums, teeth, and sclera, and included a dark discoloration of the neck and upper chest. She had been taking minocycline for nearly 9 years. We referred her to a dermatologist, who diagnosed minocycline-induced hyperpigmentation. Her minocycline was stopped. Skin biopsy was not done, as the dermatologist was confident making the diagnosis without biopsy. At 1 year later, she continued to have the widespread skin pigmentation with no improvement at all.
DIFFERENTIAL DIAGNOSIS
Hyperpigmentation is the darkening in the natural color of the skin, usually from increased deposition of melanin in the epidermis or dermis, or both. It can occur in different degrees of blue, brown, and black (from lightest to darkest). Less frequently, it may be caused by the deposition in the dermis of an endogenous or exogenous pigment, such as hemosiderin, iron, or heavy metal.1 The hyperpigmentation can be circumscribed or more diffuse.
The differential diagnosis of diffuse skin pigmentation includes Addison disease, hyperthyroidism, hemochromatosis, erythema dyschromicum perstans, cutaneous malignancies, sunburn, and drug-induced hyperpigmentation.1,2 Medications commonly cited as causing hyperpigmentation include minocycline, amiodarone, bleomycin, prostaglandins, oral contraceptives, phenothiazine, and antimalarial drugs.1,3 In Addison disease, the pigmentation is typically diffuse, with accentuation in sun-exposed areas, flexures, palmar and plantar creases, and areas of pressure or friction.2 The bronze discoloration of hemochromatosis is from a combination of hemosiderin deposition and increased melanin production.1 Erythema dyschromicum perstans presents with brownish oval-shaped macules and patches. Early lesions may have thin, raised, erythematous borders that typically involve the trunk, but they may spread to the neck, upper extremities, and face.4
The role of minocycline in the treatment of scleroderma is controversial. Early reports involving a small number of patients showed a benefit of minocycline in decreasing symptoms,5,6 but these findings were not achieved in a larger multicenter trial.7
Types of minocycline-induced hyperpigmentation
Three types of minocycline-induced hyperpigmentation occur3,8:
- Type 1—blue-grey coloration on the face in areas of inflammation
- Type 2—blue-grey coloration on normal skin on the skin of the shins and forearms
- Type 3—the least common, characterized by diffuse muddy brown or blue-grey discoloration in sun-exposed areas, as in our patient.
The prevalence of minocycline-induced hyperpigmentation varies between 2.4% and 41% and is highest in patients with rheumatoid arthritis.3,9 Type 1 pigmentation is not correlated with treatment duration or cumulative dose, while type 2 and 3 are associated with long-term therapy.8 In type 3, changes are nonspecific, consisting of increased melanin in basal keratinocytes and melanin-only staining dermal melanophages. Types 1 and 2 may resolve slowly, whereas type 3 can persist indefinitely.3,8,10
TREATMENT
Treatment involves early recognition, discontinuation of the drug, and avoidance of sun exposure. Treatment with pigment-specific lasers has shown promise.8,10
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Thiboutot DM. Clinical review 74: dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab 1995; 80:3082–3087.
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat 2009; 17:123–126.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of Cinderella or ashy dermatosis. Int J Dermatol 2004; 43:230–232.
- Le CH, Morales A, Trentham DE. Minocycline in early diffuse scleroderma. Lancet 1998; 352:1755–1756.
- Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62:267–269.
- Mayes MD, O’Donnell D, Rothfield NF, Csuka ME. Minocycline is not effective in systemic sclerosis: results of an open-label multicenter trial. Arthritis Rheum 2004; 50:553–557.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. London, UK: Saunders/Elsevier; 2011:125–126.
- Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–1257.
- Vangipuram RK, DeLozier WL, Geddes E, Friedman PM. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med 2016; 48:234–237.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Thiboutot DM. Clinical review 74: dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab 1995; 80:3082–3087.
- Geria AN, Tajirian AL, Kihiczak G, Schwartz RA. Minocycline-induced skin pigmentation: an update. Acta Dermatovenerol Croat 2009; 17:123–126.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of Cinderella or ashy dermatosis. Int J Dermatol 2004; 43:230–232.
- Le CH, Morales A, Trentham DE. Minocycline in early diffuse scleroderma. Lancet 1998; 352:1755–1756.
- Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62:267–269.
- Mayes MD, O’Donnell D, Rothfield NF, Csuka ME. Minocycline is not effective in systemic sclerosis: results of an open-label multicenter trial. Arthritis Rheum 2004; 50:553–557.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. London, UK: Saunders/Elsevier; 2011:125–126.
- Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–1257.
- Vangipuram RK, DeLozier WL, Geddes E, Friedman PM. Complete resolution of minocycline pigmentation following a single treatment with non-ablative 1550-nm fractional resurfacing in combination with the 755-nm Q-switched alexandrite laser. Lasers Surg Med 2016; 48:234–237.
When should an indwelling pleural catheter be considered for malignant pleural effusion?
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
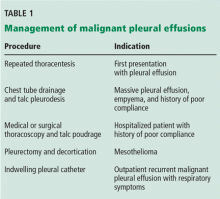
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
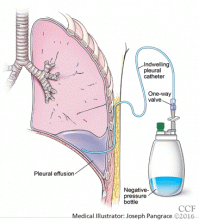
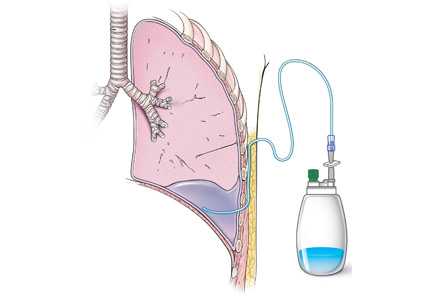
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
Women’s health 2016: An update for internists
Women's health encompasses a variety of topics relevant to the daily practice of internists. Staying up to date with the evidence in this wide field is a challenge.
This article reviews important studies published in 2015 and early 2016 on treatment of urinary tract infections, the optimal duration of bisphosphonate use, ovarian cancer screening, the impact of oral contraceptives and lactation on mortality rates, and the risks and benefits of intrauterine contraception. We critically appraised the studies and judged that their methodology was strong and appropriate for inclusion in this review.
IBUPROFEN FOR URINARY TRACT INFECTIONS
A 36-year-old woman reports 4 days of mild to moderate dysuria, frequency, and urgency. She denies fever, nausea, or back pain. Her last urinary tract infection was 2 years ago. Office urinalysis reveals leukocyte esterase and nitrites. She has read an article about antibiotic resistance and Clostridium difficile infection and asks you if antibiotics are truly necessary. What do you recommend?
Urinary tract infections are often self-limited
Uncomplicated urinary tract infections account for 25% of antibiotic prescriptions in primary care.1
Several small studies have suggested that many of these infections are self-limited, resolving within 3 to 14 days without antibiotics (Table 1).2–6 A potential disadvantage of withholding treatment is slower bacterial clearance and resolution of symptoms, but reducing the number of antibiotic prescriptions may help slow antibiotic resistance.7,8 Surveys and qualitative studies have suggested that women are concerned about the harms of antibiotic treatment and so may be willing to avoid or postpone antibiotic use.9–11
Ibuprofen vs fosfomycin
Gágyor et al6 conducted a double-blind, randomized multicenter trial in 42 general practices in Germany to assess whether treating the symptoms of uncomplicated urinary tract infection with ibuprofen would reduce antibiotic use without worsening outcomes.
Of the 779 eligible women with suspected urinary tract infection, 281 declined to participate in the study, 4 did not participate for reasons not specified, 246 received a single dose of fosfomycin 3 g, and 248 were treated with ibuprofen 400 mg three times a day for 3 days. Participants scored their daily symptoms and activity impairment, and safety data were collected for adverse events and relapses up to day 28 and within 6 and 12 months. In both groups, if symptoms worsened or persisted, antibiotic therapy was initiated at the discretion of the treating physician.
Exclusion criteria included fever, “loin” (back) tenderness, pregnancy, renal disease, a previous urinary tract infection within 2 weeks, urinary catheterization, and a contraindication to nonsteroidal anti-inflammatory medications.
Results. Within 28 days of symptom onset, women in the ibuprofen group had received 81 courses of antibiotics for symptoms of urinary tract infection (plus another 13 courses for other reasons), compared with 277 courses for urinary tract infection in the fosfomycin group (plus 6 courses for other reasons), for a relative rate reduction in antibiotic use of 66.5% (95% confidence interval [CI] 58.8%–74.4%, P < .001). The women who received ibuprofen were more likely to need antibiotics after initial treatment because of refractory symptoms but were still less likely to receive antibiotics overall (Table 1).
The mean duration of symptoms was slightly shorter in the fosfomycin group (4.6 vs 5.6 days, P < .001). However, the percentage of patients who had a recurrent urinary tract infection within 2 to 4 weeks was higher in the fosfomycin-treated patients (11% vs 6% P = .049).
Although the study was not powered to show significant differences in pyelonephritis, five patients in the ibuprofen group developed pyelonephritis compared with one in the antibiotic-treated group (P = .12).
An important limitation of the study was that nonparticipants had higher symptom scores, which may mean that the results are not generalizable to women who have recurrent urinary tract infections, longer duration of symptoms, or symptoms that are more severe. The strengths of the study were that more than half of all potentially eligible women were enrolled, and baseline data were collected from nonparticipants.
Can our patient avoid antibiotics?
Given the mild nature of her symptoms, the clinician should discuss with her the risks vs benefits of delaying antibiotics, once it has been determined that she has no risk factors for severe urinary tract infection. Her symptoms are likely to resolve within 1 week even if she declines antibiotic treatment, though they may last a day longer with ibuprofen alone than if she had received antibiotics. She should watch for symptoms of pyelonephritis (eg, flank pain, fever, chills, vomiting) and should seek prompt medical care if such symptoms occur.
DISCONTINUING BISPHOSPHONATES
A 64-year-old woman has taken alendronate for her osteoporosis for 5 years. She has no history of fractures. Her original bone density scans showed a T-score of –2.6 at the spine and –1.5 at the hip. Since she started to take alendronate, there has been no further loss in bone mineral density. She is tolerating the drug well and does not take any other medications. Should she continue the bisphosphonate?
Optimal duration of therapy unknown
The risks and benefits of long-term bisphosphonate use are debated.
In the Fracture Intervention Trial (FIT),12 women with low bone mineral density of the femoral neck were randomized to receive alendronate or placebo and were followed for 36 months. The alendronate group had significantly fewer vertebral fractures and clinical fractures overall. Then, in the FIT Long-term Extension (FLEX) study,13 1,009 alendronate-treated women in the FIT study were rerandomized to receive 5 years of additional treatment or to stop treatment. Bone density in the untreated women decreased, although not to the level it was before treatment. At the end of the study, there was no difference in hip fracture rate between the two groups (3% of each group had had a hip fracture), although women in the treated group had a lower rate of clinical vertebral fracture (2% vs 5%, relative risk 0.5, 95% CI 0.2–0.8).
In addition, rare but serious risks have been associated with bisphosphonate use, specifically atypical femoral fracture and osteonecrosis of the jaw. A US Food and Drug Administration (FDA) evaluation of long-term bisphosphonate use concluded that there was evidence of an increased risk of osteonecrosis of the jaw with longer duration of use, but causality was not established. The evaluation also noted conflicting results about the association with atypical femoral fracture.14
Based on this report and focusing on the absence of nonspine benefit after 5 years, the FDA suggested that bisphosphonates may be safely discontinued in some patients without compromising therapeutic gains, but no adequate clinical trial has yet delineated how long the benefits of treatment are maintained after cessation. A periodic reevaluation of continued need was recommended.14
New recommendations from the American Society for Bone and Mineral Research
Age is the greatest risk factor for fracture.15 Therefore, deciding whether to discontinue a bisphosphonate when a woman is older, and hence at higher risk, is a challenge.
A task force of the American Society for Bone and Mineral Research (ASBMR) has developed an evidence-based guideline on managing osteoporosis in patients on long-term bisphosphonate treatment.16 The goal was to provide guidance on the duration of bisphosphonate therapy from the perspective of risk vs benefit. The authors conducted a systematic review focusing on two randomized controlled trials (FLEX13 and the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Pivotal Fracture Trial17) that provided data on long-term bisphosphonate use.
The task force recommended16 that after 5 years of oral bisphosphonates or 3 years of intravenous bisphosphonates, risk should be reassessed. In women at high fracture risk, they recommended continuing the oral bisphosphonate for 10 years or the intravenous bisphosphonate for 6 years. Factors that favored continuation of bisphosphonate therapy were as follows:
- An osteoporotic fracture before or during therapy
- A hip bone mineral density T-score ≤ –2.5
- High risk of fracture, defined as age older than 70 or 75, other strong risk factors for fracture, or a FRAX fracture risk score18 above a country-specific threshold.
(The FRAX score is based on age, sex, weight, height, previous fracture, hip fracture in a parent, current smoking, use of glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol use, and bone mineral density in the femoral neck. It gives an estimate of the 10-year risk of major osteoporotic fracture and hip fracture. High risk would be a 10-year risk of major osteoporotic fracture greater than 20% or a 10-year risk of hip fracture greater than 3%.)
For women at high risk, the risks of atypical femoral fracture and osteonecrosis of the jaw are outweighed by the benefit of a reduction in vertebral fracture risk. For women not at high risk of fracture, a drug holiday of 2 to 3 years can be considered after 3 to 5 years of treatment.
Although the task force recommended reassessment after 2 to 3 years of drug holiday, how best to do this is not clear. The task force did not recommend a specific approach to reassessment, so decisions about when to restart therapy after a drug holiday could potentially be informed by subsequent bone mineral density testing if it were to show persistent bone loss. Another option could be to restart bisphosphonates after a defined amount of time (eg, 3–5 years) for women who have previously experienced benefit.
The task force recommendations are in line with those of other societies, the FDA, and expert opinion.19–23
The American Association of Clinical Endocrinologists recommends considering a drug holiday in low-risk patients after 4 to 5 years of treatment. For high-risk patients, they recommend 1 to 2 years of drug holiday after 10 years of treatment. They encourage restarting treatment if bone mineral density decreases, bone turnover markers rise, or fracture occurs.19 This is a grade C recommendation, meaning the advice is based on descriptive studies and expert opinion.
Although some clinicians restart bisphosphonates when markers of bone turnover such as NTX (N-telopeptide of type 1 collagen) rise to premenopausal levels, there is no evidence to support this strategy.24
The task force recommendations are based on limited evidence that primarily comes from white postmenopausal women. Another important limitation is that the outcomes are primarily vertebral fractures. However, until additional evidence is available, these guidelines can be useful in guiding decision-making.
Should our patient continue therapy?
Our patient is relatively young and does not have any of the high-risk features noted within the task force recommendations. She has responded well to bisphosphonate treatment and so can consider a drug holiday at this time.
OVARIAN CANCER SCREENING
A 50-year-old woman requests screening for ovarian cancer. She is postmenopausal and has no personal or family history of cancer. She is concerned because a friend forwarded an e-mail stating, “Please tell all your female friends and relatives to insist on a cancer antigen (CA) 125 blood test every year as part of their annual exam. This is an inexpensive and simple blood test. Don’t take no for an answer. If I had known then what I know now, we would have caught my cancer much earlier, before it was stage III!” What should you tell the patient?
Ovarian cancer is the most deadly of female reproductive cancers, largely because in most patients the cancer has already spread beyond the ovary by the time of clinical detection. Death rates from ovarian cancer have decreased only slightly in the past 30 years.
Little benefit and considerable harm of screening
In 2011, the Prostate Lung Colorectal Ovarian (PLCO) Cancer Screening trial25 randomized more than 68,000 women ages 55 to 74 from the general US population to annual screening with CA 125 testing and transvaginal ultrasonography compared with usual care. They were followed for a median of 12.4 years.
Screening did not affect stage at diagnosis (77%–78% were in stage III or IV in both the screening and usual care groups), nor did it reduce the rate of death from ovarian cancer. In addition, false-positive findings led to some harm: nearly one in three women who had a positive screening test underwent surgery. Of 3,285 women with false-positive results, 1,080 underwent surgery, and 15% of these had at least one serious complication. The trial was stopped early due to evidence of futility.
A new UK study also found no benefit from screening
In the PLCO study, a CA 125 result of 35 U/mL or greater was classified as abnormal. However, researchers in the United Kingdom postulated that instead of using a single cutoff for a normal or abnormal CA 125 level, it would be better to interpret the CA 125 result according to a somewhat complicated (and proprietary) algorithm called the Risk of Ovarian Cancer Algorithm (ROCA).26,27 The ROCA takes into account a woman’s age, menopausal status, known genetic mutations (BRCA 1 or 2 or Lynch syndrome), Ashkenazi Jewish descent, and family history of ovarian or breast cancer, as well as any change in CA 125 level over time.
In a 2016 UK study,26 202,638 postmenopausal women ages 50 to 74 were randomized to no screening, annual screening with transvaginal ultrasonography, or multimodal screening with an annual CA 125 blood test interpreted with the ROCA algorithm, adding transvaginal ultrasonography as a second-line test when needed if the CA 125 level was abnormal based on the ROCA. Women with abnormal findings on multimodal screening or ultrasonography had repeat tests, and women with persistent abnormalities underwent clinical evaluation and, when appropriate, surgery.
Participants were at average risk of ovarian cancer; those with suspected familial ovarian cancer syndrome were excluded, as were those with a personal history of ovarian cancer or other active cancer.
Results. At a median follow-up of 11.1 years, the percentage of women who were diagnosed with ovarian cancer was 0.7% in the multimodal screening group, 0.6% in the screening ultrasonography group, and 0.6% in the no-screening group. Comparing either multimodal or screening ultrasonography with no screening, there was no statistically significant reduction in mortality rate over 14 years of follow-up.
Screening had significant costs and potential harms. For every ovarian or peritoneal cancer detected by screening, an additional 2 women in the multimodal screening group and 10 women in the ultrasonography group underwent needless surgery.
Strengths of this trial included its large size, allowing adequate power to detect differences in outcomes, its multicenter setting, its high compliance rate, and the low crossover rate in the no-screening group. However, the design of the study makes it difficult to anticipate the late effects of screening. Also, the patient must purchase ROCA testing online and must also pay a consultation fee. Insurance providers do not cover this test.
Should our patient proceed with ovarian cancer screening?
No. Current evidence shows no clear benefit to ovarian cancer screening for average-risk women, and we should not recommend yearly ultrasonography and CA 125 level testing, as they are likely to cause harm without providing benefit. The US Preventive Services Task Force recommends against screening for ovarian cancer.28 For premenopausal women, pregnancy, hormonal contraception, and breastfeeding all significantly decrease ovarian cancer risk by suppressing ovulation.29–31
REPRODUCTIVE FACTORS AND THE RISK OF DEATH
A 26-year-old woman comes in to discuss her contraceptive options. She has been breastfeeding since the birth of her first baby 6 months ago, and wonders how lactation and contraception may affect her long-term health.
Questions about the safety of contraceptive options are common, especially in breastfeeding mothers.
In 2010, the long-term Royal College of General Practitioners’ Oral Contraceptive Study reported that the all-cause mortality rate was actually lower in women who used oral contraceptives.32 Similarly, in 2013, an Oxford study that followed 17,032 women for over 30 years reported no association between oral contraceptives and breast cancer.33
However, in 2014, results from the Nurses’ Health Study indicated that breast cancer rates were higher in oral contraceptive users, although reassuringly, the study found no difference in all-cause mortality rates in women who had used oral contraception.34
The European Prospective Investigation Into Cancer and Nutrition
To further characterize relationships between reproductive characteristics and mortality rates, investigators analyzed data from the European Prospective Investigation Into Cancer and Nutrition,35 which recruited 322,972 women from 10 countries between 1992 and 2000. Analyses were stratified by study center and participant age and were adjusted for body mass index, physical activity, education level, smoking, and menopausal status; alcohol intake was examined as a potential confounder but was excluded from final models.
Findings. Over an average 13 years of follow-up, the rate of all-cause mortality was 20% lower in parous than in nulliparous women. In parous women, the all-cause mortality rate was additionally 18% lower in those who had breastfed vs those who had never breastfed, although breastfeeding duration was not associated with mortality. Use of oral contraceptives lowered all-cause mortality by 10% among nonsmokers; in smokers, no association with all-cause mortality was seen for oral contraceptive use, as smoking is such a powerful risk factor for mortality. The primary contributor to all-cause mortality appeared to be ischemic heart disease, the incidence of which was significantly lower in parous women (by 14%) and those who breastfed (by 20%) and was not related to oral contraceptive use.35
Strengths of this study included the large sample size recruited from countries across Europe, with varying rates of breastfeeding and contraceptive use. However, as with all observational studies, it remains subject to the possibility of residual confounding.
What should we tell this patient?
After congratulating her for breastfeeding, we can reassure her about the safety of all available contraceptives. According to the US Centers for Disease Control and Prevention (CDC),36 after 42 days postpartum most women can use combined hormonal contraception. All other methods can be used immediately postpartum, including progestin-only pills.
As lactational amenorrhea is only effective while mothers are exclusively breastfeeding, and short interpregnancy intervals have been associated with higher rates of adverse pregnancy outcomes,37 this patient will likely benefit from promptly starting a prescription contraceptive.
HIGHLY EFFECTIVE REVERSIBLE CONTRACEPTION
This same 26-year-old patient is concerned that she will not remember to take an oral contraceptive every day, and expresses interest in a more convenient method of contraception. However, she is concerned about the potential risks.
Although intrauterine contraceptives (IUCs) are typically 20 times more effective than oral contraceptives38 and have been used by millions of women worldwide, rates of use in the United States have been lower than in many other countries.39
A study of intrauterine contraception
To clarify the safety of IUCs, researchers followed 61,448 women who underwent IUC placement in six European countries between 2006 and 2013.40 Most participants received an IUC containing levonorgestrel, while 30% received a copper IUC.
Findings. Overall, rates of uterine perforation were low (approximately 1 per 1,000 insertions). The most significant risk factors for perforation were breastfeeding at the time of insertion and insertion less than 36 weeks after the last delivery. None of the perforations in the study led to serious illness or injury of intra-abdominal or pelvic structures. Interestingly, women using a levonorgestrel IUC were considerably less likely to experience a contraceptive failure than those using a copper IUC.41
Strengths of this study included the prospective data collection and power to examine rare clinical outcomes. However, it was industry-funded.
The risk of pelvic infection with an IUC is so low that the CDC does not recommend prophylactic antibiotics with the insertion procedure. If women have other indications for testing for sexually transmitted disease, an IUC can be placed the same day as testing, and before results are available.42 If a woman is found to have a sexually transmitted disease while she has an IUC in place, she should be treated with antibiotics, and there is no need to remove the IUC.43
Subdermal implants
Another highly effective contraceptive option for this patient is the progestin-only subdermal contraceptive implant (marketed in the United States as Nexplanon). Implants have been well-studied and found to have no adverse effect on lactation.44
Learning to place a subdermal contraceptive is far easier than learning to place an IUC, but it requires a few hours of FDA-mandated in-person training. Unfortunately, relatively few clinicians have obtained this training.45 As placing a subdermal contraceptive is like placing an intravenous line without needing to hit the vein, this procedure can easily be incorporated into a primary care practice. Training from the manufacturer is available to providers who request it.
What should we tell this patient?
An IUC is a great option for many women. When pregnancy is desired, the device is easily removed. Of the three IUCs now available in the United States, those containing 52 mg of levonorgestrel (marketed in the United States as Mirena and Liletta) are the most effective.
The only option more effective than these IUCs is subdermal contraception.46 These reversible contraceptives are typically more effective than permanent contraceptives (ie, tubal ligation)47 and can be removed at any time if a patient wishes to switch to another method or to become pregnant.
Pregnancy rates following attempts at “sterilization” are higher than many realize. There are a variety of approaches to “tying tubes,” some of which may not result in complete tubal occlusion. The failure rate of the laparoscopic approach, according to the US Collaborative Review of Sterilization, ranges from 7.5 per 1,000 procedures for unipolar coagulation to a high of 36.5 per 1,000 for the spring clip.48 The relatively commonly used Filshie clip was not included in this study, but its failure rate is reported to be between 1% and 2%.
- Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012; 366:1028–1037.
- Christiaens TC, De Meyere M, Verschraegen G, et al. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract 2002; 52:729–734.
- Bleidorn J, Gágyor I, Kochen MM, Wegscheider K, Hummers-Pradier E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?—results of a randomized controlled pilot trial. BMC Med 2010; 8:30. doi: 10.1186/1741-7015-8-30.
- Little P, Moore MV, Turner S, et al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ 2010; 340:c199.
- Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis 2004; 36:296–301.
- Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 2015; 351:h6544. doi: 10.1136/bmj.h6544.
- Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract 2007; 57:785–792.
- Gottesman BS, Carmeli Y, Shitrit P, Chowers M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin Infect Dis 2009; 49:869–875.
- Knottnerus BJ, Geerlings SE, Moll van Charante EP, ter Riet G. Women with symptoms of uncomplicated urinary tract infection are often willing to delay antibiotic treatment: a prospective cohort study. BMC Fam Pract 2013; 14:71. doi: 10.1186/1471-2296-14-71.
- Leydon GM, Turner S, Smith H, Little P; UTIS team. Women’s views about management and cause of urinary tract infection: qualitative interview study. BMJ 2010; 340:c279. doi: 10.1136/bmj.c279.
- Willems CS, van den Broek D’Obrenan J, Numans ME, Verheij TJ, van der Velden AW. Cystitis: antibiotic prescribing, consultation, attitudes and opinions. Fam Pract 2014; 31:149–155.
- Black DM, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- US Food and Drug Administration. Background document for meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM270958.pdf. Accessed November 3, 2016.
- Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005; 16:581–589.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31:16–35.
- Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012; 27:243–254.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases. FRAX WHO fracture risk assessment tool. www.shef.ac.uk/FRAX/. Accessed October 7, 2016.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010; 16(suppl 3):1–37.
- Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med 2012; 366:2048–2051.
- Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med 2012; 366:2051–2053.
- Brown JP, Morin S, Leslie W, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician 2014; 60:324–333.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95:1555–1565.
- Bauer DC, Schwartz A, Palermo L, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med 2014; 174:1126–1134.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016; 387:945–956.
- Abcodia Inc. The ROCA test. www.therocatest.co.uk/for-clinicians/about-roca. Accessed November 3, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2012; 157:900–904.
- Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer 2001; 84:714–721.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer, Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008; 371:303–314.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr 2015; 104:96–113.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927. doi: 10.1136/bmj.c927.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association contraceptive study. Contraception 2013; 88:678–683.
- Charlton BM, Rich-Edwards JW, Colditz GA, et al. Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: prospective cohort study. BMJ 2014; 349:g6356. doi: 10.1136/bmj.g6356.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252. doi: 10.1186/s12916-015-0484-3.
- Centers for Disease Control and Prevention (CDC). Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. MMWR Morb Mortal Wkly Rep 2011; 60:878–883.
- Bigelow CA, Bryant AS. Short interpregnancy intervals: an evidence-based guide for clinicians. Obstet Gynecol Surv 2015; 70:458–464.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Buhling KJ, Zite NB, Lotke P, Black K; INTRA Writing Group. Worldwide use of intrauterine contraception: a review. Contraception 2014; 89:162–173.
- Heinemann K, Reed S, Moehner S, Minh TD. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception 2015; 91:274–279.
- Heinemann K, Reed S, Moehner S, Minh TD. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception 2015; 91:280–283.
- Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement. Am J Obstet Gynecol 2016 May 12. pii: S0002-9378(16)30212-5. doi: 10.1016/j.ajog.2016.05.017. [Epub ahead of print]
- Division of Reproductive health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013; 62(RR-05):1–60.
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol 2011; 117:1114–1121.
- Nisen MB, Peterson LE, Cochrane A, Rubin SE. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception 2016; 93:432–437.
- Polis CB, Bradley SE, Bankole A, Onda T, Croft T, Singh S. Typical-use contraceptive failure rates in 43 countries with Demographic and Health Survey data: summary of a detailed report. Contraception 2016; 94:11–17.
- Gariepy AM, Creinin MD, Smith KJ, Xu X. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception 2014; 90:174–181.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussel J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Rerview of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
Women's health encompasses a variety of topics relevant to the daily practice of internists. Staying up to date with the evidence in this wide field is a challenge.
This article reviews important studies published in 2015 and early 2016 on treatment of urinary tract infections, the optimal duration of bisphosphonate use, ovarian cancer screening, the impact of oral contraceptives and lactation on mortality rates, and the risks and benefits of intrauterine contraception. We critically appraised the studies and judged that their methodology was strong and appropriate for inclusion in this review.
IBUPROFEN FOR URINARY TRACT INFECTIONS
A 36-year-old woman reports 4 days of mild to moderate dysuria, frequency, and urgency. She denies fever, nausea, or back pain. Her last urinary tract infection was 2 years ago. Office urinalysis reveals leukocyte esterase and nitrites. She has read an article about antibiotic resistance and Clostridium difficile infection and asks you if antibiotics are truly necessary. What do you recommend?
Urinary tract infections are often self-limited
Uncomplicated urinary tract infections account for 25% of antibiotic prescriptions in primary care.1
Several small studies have suggested that many of these infections are self-limited, resolving within 3 to 14 days without antibiotics (Table 1).2–6 A potential disadvantage of withholding treatment is slower bacterial clearance and resolution of symptoms, but reducing the number of antibiotic prescriptions may help slow antibiotic resistance.7,8 Surveys and qualitative studies have suggested that women are concerned about the harms of antibiotic treatment and so may be willing to avoid or postpone antibiotic use.9–11
Ibuprofen vs fosfomycin
Gágyor et al6 conducted a double-blind, randomized multicenter trial in 42 general practices in Germany to assess whether treating the symptoms of uncomplicated urinary tract infection with ibuprofen would reduce antibiotic use without worsening outcomes.
Of the 779 eligible women with suspected urinary tract infection, 281 declined to participate in the study, 4 did not participate for reasons not specified, 246 received a single dose of fosfomycin 3 g, and 248 were treated with ibuprofen 400 mg three times a day for 3 days. Participants scored their daily symptoms and activity impairment, and safety data were collected for adverse events and relapses up to day 28 and within 6 and 12 months. In both groups, if symptoms worsened or persisted, antibiotic therapy was initiated at the discretion of the treating physician.
Exclusion criteria included fever, “loin” (back) tenderness, pregnancy, renal disease, a previous urinary tract infection within 2 weeks, urinary catheterization, and a contraindication to nonsteroidal anti-inflammatory medications.
Results. Within 28 days of symptom onset, women in the ibuprofen group had received 81 courses of antibiotics for symptoms of urinary tract infection (plus another 13 courses for other reasons), compared with 277 courses for urinary tract infection in the fosfomycin group (plus 6 courses for other reasons), for a relative rate reduction in antibiotic use of 66.5% (95% confidence interval [CI] 58.8%–74.4%, P < .001). The women who received ibuprofen were more likely to need antibiotics after initial treatment because of refractory symptoms but were still less likely to receive antibiotics overall (Table 1).
The mean duration of symptoms was slightly shorter in the fosfomycin group (4.6 vs 5.6 days, P < .001). However, the percentage of patients who had a recurrent urinary tract infection within 2 to 4 weeks was higher in the fosfomycin-treated patients (11% vs 6% P = .049).
Although the study was not powered to show significant differences in pyelonephritis, five patients in the ibuprofen group developed pyelonephritis compared with one in the antibiotic-treated group (P = .12).
An important limitation of the study was that nonparticipants had higher symptom scores, which may mean that the results are not generalizable to women who have recurrent urinary tract infections, longer duration of symptoms, or symptoms that are more severe. The strengths of the study were that more than half of all potentially eligible women were enrolled, and baseline data were collected from nonparticipants.
Can our patient avoid antibiotics?
Given the mild nature of her symptoms, the clinician should discuss with her the risks vs benefits of delaying antibiotics, once it has been determined that she has no risk factors for severe urinary tract infection. Her symptoms are likely to resolve within 1 week even if she declines antibiotic treatment, though they may last a day longer with ibuprofen alone than if she had received antibiotics. She should watch for symptoms of pyelonephritis (eg, flank pain, fever, chills, vomiting) and should seek prompt medical care if such symptoms occur.
DISCONTINUING BISPHOSPHONATES
A 64-year-old woman has taken alendronate for her osteoporosis for 5 years. She has no history of fractures. Her original bone density scans showed a T-score of –2.6 at the spine and –1.5 at the hip. Since she started to take alendronate, there has been no further loss in bone mineral density. She is tolerating the drug well and does not take any other medications. Should she continue the bisphosphonate?
Optimal duration of therapy unknown
The risks and benefits of long-term bisphosphonate use are debated.
In the Fracture Intervention Trial (FIT),12 women with low bone mineral density of the femoral neck were randomized to receive alendronate or placebo and were followed for 36 months. The alendronate group had significantly fewer vertebral fractures and clinical fractures overall. Then, in the FIT Long-term Extension (FLEX) study,13 1,009 alendronate-treated women in the FIT study were rerandomized to receive 5 years of additional treatment or to stop treatment. Bone density in the untreated women decreased, although not to the level it was before treatment. At the end of the study, there was no difference in hip fracture rate between the two groups (3% of each group had had a hip fracture), although women in the treated group had a lower rate of clinical vertebral fracture (2% vs 5%, relative risk 0.5, 95% CI 0.2–0.8).
In addition, rare but serious risks have been associated with bisphosphonate use, specifically atypical femoral fracture and osteonecrosis of the jaw. A US Food and Drug Administration (FDA) evaluation of long-term bisphosphonate use concluded that there was evidence of an increased risk of osteonecrosis of the jaw with longer duration of use, but causality was not established. The evaluation also noted conflicting results about the association with atypical femoral fracture.14
Based on this report and focusing on the absence of nonspine benefit after 5 years, the FDA suggested that bisphosphonates may be safely discontinued in some patients without compromising therapeutic gains, but no adequate clinical trial has yet delineated how long the benefits of treatment are maintained after cessation. A periodic reevaluation of continued need was recommended.14
New recommendations from the American Society for Bone and Mineral Research
Age is the greatest risk factor for fracture.15 Therefore, deciding whether to discontinue a bisphosphonate when a woman is older, and hence at higher risk, is a challenge.
A task force of the American Society for Bone and Mineral Research (ASBMR) has developed an evidence-based guideline on managing osteoporosis in patients on long-term bisphosphonate treatment.16 The goal was to provide guidance on the duration of bisphosphonate therapy from the perspective of risk vs benefit. The authors conducted a systematic review focusing on two randomized controlled trials (FLEX13 and the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Pivotal Fracture Trial17) that provided data on long-term bisphosphonate use.
The task force recommended16 that after 5 years of oral bisphosphonates or 3 years of intravenous bisphosphonates, risk should be reassessed. In women at high fracture risk, they recommended continuing the oral bisphosphonate for 10 years or the intravenous bisphosphonate for 6 years. Factors that favored continuation of bisphosphonate therapy were as follows:
- An osteoporotic fracture before or during therapy
- A hip bone mineral density T-score ≤ –2.5
- High risk of fracture, defined as age older than 70 or 75, other strong risk factors for fracture, or a FRAX fracture risk score18 above a country-specific threshold.
(The FRAX score is based on age, sex, weight, height, previous fracture, hip fracture in a parent, current smoking, use of glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol use, and bone mineral density in the femoral neck. It gives an estimate of the 10-year risk of major osteoporotic fracture and hip fracture. High risk would be a 10-year risk of major osteoporotic fracture greater than 20% or a 10-year risk of hip fracture greater than 3%.)
For women at high risk, the risks of atypical femoral fracture and osteonecrosis of the jaw are outweighed by the benefit of a reduction in vertebral fracture risk. For women not at high risk of fracture, a drug holiday of 2 to 3 years can be considered after 3 to 5 years of treatment.
Although the task force recommended reassessment after 2 to 3 years of drug holiday, how best to do this is not clear. The task force did not recommend a specific approach to reassessment, so decisions about when to restart therapy after a drug holiday could potentially be informed by subsequent bone mineral density testing if it were to show persistent bone loss. Another option could be to restart bisphosphonates after a defined amount of time (eg, 3–5 years) for women who have previously experienced benefit.
The task force recommendations are in line with those of other societies, the FDA, and expert opinion.19–23
The American Association of Clinical Endocrinologists recommends considering a drug holiday in low-risk patients after 4 to 5 years of treatment. For high-risk patients, they recommend 1 to 2 years of drug holiday after 10 years of treatment. They encourage restarting treatment if bone mineral density decreases, bone turnover markers rise, or fracture occurs.19 This is a grade C recommendation, meaning the advice is based on descriptive studies and expert opinion.
Although some clinicians restart bisphosphonates when markers of bone turnover such as NTX (N-telopeptide of type 1 collagen) rise to premenopausal levels, there is no evidence to support this strategy.24
The task force recommendations are based on limited evidence that primarily comes from white postmenopausal women. Another important limitation is that the outcomes are primarily vertebral fractures. However, until additional evidence is available, these guidelines can be useful in guiding decision-making.
Should our patient continue therapy?
Our patient is relatively young and does not have any of the high-risk features noted within the task force recommendations. She has responded well to bisphosphonate treatment and so can consider a drug holiday at this time.
OVARIAN CANCER SCREENING
A 50-year-old woman requests screening for ovarian cancer. She is postmenopausal and has no personal or family history of cancer. She is concerned because a friend forwarded an e-mail stating, “Please tell all your female friends and relatives to insist on a cancer antigen (CA) 125 blood test every year as part of their annual exam. This is an inexpensive and simple blood test. Don’t take no for an answer. If I had known then what I know now, we would have caught my cancer much earlier, before it was stage III!” What should you tell the patient?
Ovarian cancer is the most deadly of female reproductive cancers, largely because in most patients the cancer has already spread beyond the ovary by the time of clinical detection. Death rates from ovarian cancer have decreased only slightly in the past 30 years.
Little benefit and considerable harm of screening
In 2011, the Prostate Lung Colorectal Ovarian (PLCO) Cancer Screening trial25 randomized more than 68,000 women ages 55 to 74 from the general US population to annual screening with CA 125 testing and transvaginal ultrasonography compared with usual care. They were followed for a median of 12.4 years.
Screening did not affect stage at diagnosis (77%–78% were in stage III or IV in both the screening and usual care groups), nor did it reduce the rate of death from ovarian cancer. In addition, false-positive findings led to some harm: nearly one in three women who had a positive screening test underwent surgery. Of 3,285 women with false-positive results, 1,080 underwent surgery, and 15% of these had at least one serious complication. The trial was stopped early due to evidence of futility.
A new UK study also found no benefit from screening
In the PLCO study, a CA 125 result of 35 U/mL or greater was classified as abnormal. However, researchers in the United Kingdom postulated that instead of using a single cutoff for a normal or abnormal CA 125 level, it would be better to interpret the CA 125 result according to a somewhat complicated (and proprietary) algorithm called the Risk of Ovarian Cancer Algorithm (ROCA).26,27 The ROCA takes into account a woman’s age, menopausal status, known genetic mutations (BRCA 1 or 2 or Lynch syndrome), Ashkenazi Jewish descent, and family history of ovarian or breast cancer, as well as any change in CA 125 level over time.
In a 2016 UK study,26 202,638 postmenopausal women ages 50 to 74 were randomized to no screening, annual screening with transvaginal ultrasonography, or multimodal screening with an annual CA 125 blood test interpreted with the ROCA algorithm, adding transvaginal ultrasonography as a second-line test when needed if the CA 125 level was abnormal based on the ROCA. Women with abnormal findings on multimodal screening or ultrasonography had repeat tests, and women with persistent abnormalities underwent clinical evaluation and, when appropriate, surgery.
Participants were at average risk of ovarian cancer; those with suspected familial ovarian cancer syndrome were excluded, as were those with a personal history of ovarian cancer or other active cancer.
Results. At a median follow-up of 11.1 years, the percentage of women who were diagnosed with ovarian cancer was 0.7% in the multimodal screening group, 0.6% in the screening ultrasonography group, and 0.6% in the no-screening group. Comparing either multimodal or screening ultrasonography with no screening, there was no statistically significant reduction in mortality rate over 14 years of follow-up.
Screening had significant costs and potential harms. For every ovarian or peritoneal cancer detected by screening, an additional 2 women in the multimodal screening group and 10 women in the ultrasonography group underwent needless surgery.
Strengths of this trial included its large size, allowing adequate power to detect differences in outcomes, its multicenter setting, its high compliance rate, and the low crossover rate in the no-screening group. However, the design of the study makes it difficult to anticipate the late effects of screening. Also, the patient must purchase ROCA testing online and must also pay a consultation fee. Insurance providers do not cover this test.
Should our patient proceed with ovarian cancer screening?
No. Current evidence shows no clear benefit to ovarian cancer screening for average-risk women, and we should not recommend yearly ultrasonography and CA 125 level testing, as they are likely to cause harm without providing benefit. The US Preventive Services Task Force recommends against screening for ovarian cancer.28 For premenopausal women, pregnancy, hormonal contraception, and breastfeeding all significantly decrease ovarian cancer risk by suppressing ovulation.29–31
REPRODUCTIVE FACTORS AND THE RISK OF DEATH
A 26-year-old woman comes in to discuss her contraceptive options. She has been breastfeeding since the birth of her first baby 6 months ago, and wonders how lactation and contraception may affect her long-term health.
Questions about the safety of contraceptive options are common, especially in breastfeeding mothers.
In 2010, the long-term Royal College of General Practitioners’ Oral Contraceptive Study reported that the all-cause mortality rate was actually lower in women who used oral contraceptives.32 Similarly, in 2013, an Oxford study that followed 17,032 women for over 30 years reported no association between oral contraceptives and breast cancer.33
However, in 2014, results from the Nurses’ Health Study indicated that breast cancer rates were higher in oral contraceptive users, although reassuringly, the study found no difference in all-cause mortality rates in women who had used oral contraception.34
The European Prospective Investigation Into Cancer and Nutrition
To further characterize relationships between reproductive characteristics and mortality rates, investigators analyzed data from the European Prospective Investigation Into Cancer and Nutrition,35 which recruited 322,972 women from 10 countries between 1992 and 2000. Analyses were stratified by study center and participant age and were adjusted for body mass index, physical activity, education level, smoking, and menopausal status; alcohol intake was examined as a potential confounder but was excluded from final models.
Findings. Over an average 13 years of follow-up, the rate of all-cause mortality was 20% lower in parous than in nulliparous women. In parous women, the all-cause mortality rate was additionally 18% lower in those who had breastfed vs those who had never breastfed, although breastfeeding duration was not associated with mortality. Use of oral contraceptives lowered all-cause mortality by 10% among nonsmokers; in smokers, no association with all-cause mortality was seen for oral contraceptive use, as smoking is such a powerful risk factor for mortality. The primary contributor to all-cause mortality appeared to be ischemic heart disease, the incidence of which was significantly lower in parous women (by 14%) and those who breastfed (by 20%) and was not related to oral contraceptive use.35
Strengths of this study included the large sample size recruited from countries across Europe, with varying rates of breastfeeding and contraceptive use. However, as with all observational studies, it remains subject to the possibility of residual confounding.
What should we tell this patient?
After congratulating her for breastfeeding, we can reassure her about the safety of all available contraceptives. According to the US Centers for Disease Control and Prevention (CDC),36 after 42 days postpartum most women can use combined hormonal contraception. All other methods can be used immediately postpartum, including progestin-only pills.
As lactational amenorrhea is only effective while mothers are exclusively breastfeeding, and short interpregnancy intervals have been associated with higher rates of adverse pregnancy outcomes,37 this patient will likely benefit from promptly starting a prescription contraceptive.
HIGHLY EFFECTIVE REVERSIBLE CONTRACEPTION
This same 26-year-old patient is concerned that she will not remember to take an oral contraceptive every day, and expresses interest in a more convenient method of contraception. However, she is concerned about the potential risks.
Although intrauterine contraceptives (IUCs) are typically 20 times more effective than oral contraceptives38 and have been used by millions of women worldwide, rates of use in the United States have been lower than in many other countries.39
A study of intrauterine contraception
To clarify the safety of IUCs, researchers followed 61,448 women who underwent IUC placement in six European countries between 2006 and 2013.40 Most participants received an IUC containing levonorgestrel, while 30% received a copper IUC.
Findings. Overall, rates of uterine perforation were low (approximately 1 per 1,000 insertions). The most significant risk factors for perforation were breastfeeding at the time of insertion and insertion less than 36 weeks after the last delivery. None of the perforations in the study led to serious illness or injury of intra-abdominal or pelvic structures. Interestingly, women using a levonorgestrel IUC were considerably less likely to experience a contraceptive failure than those using a copper IUC.41
Strengths of this study included the prospective data collection and power to examine rare clinical outcomes. However, it was industry-funded.
The risk of pelvic infection with an IUC is so low that the CDC does not recommend prophylactic antibiotics with the insertion procedure. If women have other indications for testing for sexually transmitted disease, an IUC can be placed the same day as testing, and before results are available.42 If a woman is found to have a sexually transmitted disease while she has an IUC in place, she should be treated with antibiotics, and there is no need to remove the IUC.43
Subdermal implants
Another highly effective contraceptive option for this patient is the progestin-only subdermal contraceptive implant (marketed in the United States as Nexplanon). Implants have been well-studied and found to have no adverse effect on lactation.44
Learning to place a subdermal contraceptive is far easier than learning to place an IUC, but it requires a few hours of FDA-mandated in-person training. Unfortunately, relatively few clinicians have obtained this training.45 As placing a subdermal contraceptive is like placing an intravenous line without needing to hit the vein, this procedure can easily be incorporated into a primary care practice. Training from the manufacturer is available to providers who request it.
What should we tell this patient?
An IUC is a great option for many women. When pregnancy is desired, the device is easily removed. Of the three IUCs now available in the United States, those containing 52 mg of levonorgestrel (marketed in the United States as Mirena and Liletta) are the most effective.
The only option more effective than these IUCs is subdermal contraception.46 These reversible contraceptives are typically more effective than permanent contraceptives (ie, tubal ligation)47 and can be removed at any time if a patient wishes to switch to another method or to become pregnant.
Pregnancy rates following attempts at “sterilization” are higher than many realize. There are a variety of approaches to “tying tubes,” some of which may not result in complete tubal occlusion. The failure rate of the laparoscopic approach, according to the US Collaborative Review of Sterilization, ranges from 7.5 per 1,000 procedures for unipolar coagulation to a high of 36.5 per 1,000 for the spring clip.48 The relatively commonly used Filshie clip was not included in this study, but its failure rate is reported to be between 1% and 2%.
Women's health encompasses a variety of topics relevant to the daily practice of internists. Staying up to date with the evidence in this wide field is a challenge.
This article reviews important studies published in 2015 and early 2016 on treatment of urinary tract infections, the optimal duration of bisphosphonate use, ovarian cancer screening, the impact of oral contraceptives and lactation on mortality rates, and the risks and benefits of intrauterine contraception. We critically appraised the studies and judged that their methodology was strong and appropriate for inclusion in this review.
IBUPROFEN FOR URINARY TRACT INFECTIONS
A 36-year-old woman reports 4 days of mild to moderate dysuria, frequency, and urgency. She denies fever, nausea, or back pain. Her last urinary tract infection was 2 years ago. Office urinalysis reveals leukocyte esterase and nitrites. She has read an article about antibiotic resistance and Clostridium difficile infection and asks you if antibiotics are truly necessary. What do you recommend?
Urinary tract infections are often self-limited
Uncomplicated urinary tract infections account for 25% of antibiotic prescriptions in primary care.1
Several small studies have suggested that many of these infections are self-limited, resolving within 3 to 14 days without antibiotics (Table 1).2–6 A potential disadvantage of withholding treatment is slower bacterial clearance and resolution of symptoms, but reducing the number of antibiotic prescriptions may help slow antibiotic resistance.7,8 Surveys and qualitative studies have suggested that women are concerned about the harms of antibiotic treatment and so may be willing to avoid or postpone antibiotic use.9–11
Ibuprofen vs fosfomycin
Gágyor et al6 conducted a double-blind, randomized multicenter trial in 42 general practices in Germany to assess whether treating the symptoms of uncomplicated urinary tract infection with ibuprofen would reduce antibiotic use without worsening outcomes.
Of the 779 eligible women with suspected urinary tract infection, 281 declined to participate in the study, 4 did not participate for reasons not specified, 246 received a single dose of fosfomycin 3 g, and 248 were treated with ibuprofen 400 mg three times a day for 3 days. Participants scored their daily symptoms and activity impairment, and safety data were collected for adverse events and relapses up to day 28 and within 6 and 12 months. In both groups, if symptoms worsened or persisted, antibiotic therapy was initiated at the discretion of the treating physician.
Exclusion criteria included fever, “loin” (back) tenderness, pregnancy, renal disease, a previous urinary tract infection within 2 weeks, urinary catheterization, and a contraindication to nonsteroidal anti-inflammatory medications.
Results. Within 28 days of symptom onset, women in the ibuprofen group had received 81 courses of antibiotics for symptoms of urinary tract infection (plus another 13 courses for other reasons), compared with 277 courses for urinary tract infection in the fosfomycin group (plus 6 courses for other reasons), for a relative rate reduction in antibiotic use of 66.5% (95% confidence interval [CI] 58.8%–74.4%, P < .001). The women who received ibuprofen were more likely to need antibiotics after initial treatment because of refractory symptoms but were still less likely to receive antibiotics overall (Table 1).
The mean duration of symptoms was slightly shorter in the fosfomycin group (4.6 vs 5.6 days, P < .001). However, the percentage of patients who had a recurrent urinary tract infection within 2 to 4 weeks was higher in the fosfomycin-treated patients (11% vs 6% P = .049).
Although the study was not powered to show significant differences in pyelonephritis, five patients in the ibuprofen group developed pyelonephritis compared with one in the antibiotic-treated group (P = .12).
An important limitation of the study was that nonparticipants had higher symptom scores, which may mean that the results are not generalizable to women who have recurrent urinary tract infections, longer duration of symptoms, or symptoms that are more severe. The strengths of the study were that more than half of all potentially eligible women were enrolled, and baseline data were collected from nonparticipants.
Can our patient avoid antibiotics?
Given the mild nature of her symptoms, the clinician should discuss with her the risks vs benefits of delaying antibiotics, once it has been determined that she has no risk factors for severe urinary tract infection. Her symptoms are likely to resolve within 1 week even if she declines antibiotic treatment, though they may last a day longer with ibuprofen alone than if she had received antibiotics. She should watch for symptoms of pyelonephritis (eg, flank pain, fever, chills, vomiting) and should seek prompt medical care if such symptoms occur.
DISCONTINUING BISPHOSPHONATES
A 64-year-old woman has taken alendronate for her osteoporosis for 5 years. She has no history of fractures. Her original bone density scans showed a T-score of –2.6 at the spine and –1.5 at the hip. Since she started to take alendronate, there has been no further loss in bone mineral density. She is tolerating the drug well and does not take any other medications. Should she continue the bisphosphonate?
Optimal duration of therapy unknown
The risks and benefits of long-term bisphosphonate use are debated.
In the Fracture Intervention Trial (FIT),12 women with low bone mineral density of the femoral neck were randomized to receive alendronate or placebo and were followed for 36 months. The alendronate group had significantly fewer vertebral fractures and clinical fractures overall. Then, in the FIT Long-term Extension (FLEX) study,13 1,009 alendronate-treated women in the FIT study were rerandomized to receive 5 years of additional treatment or to stop treatment. Bone density in the untreated women decreased, although not to the level it was before treatment. At the end of the study, there was no difference in hip fracture rate between the two groups (3% of each group had had a hip fracture), although women in the treated group had a lower rate of clinical vertebral fracture (2% vs 5%, relative risk 0.5, 95% CI 0.2–0.8).
In addition, rare but serious risks have been associated with bisphosphonate use, specifically atypical femoral fracture and osteonecrosis of the jaw. A US Food and Drug Administration (FDA) evaluation of long-term bisphosphonate use concluded that there was evidence of an increased risk of osteonecrosis of the jaw with longer duration of use, but causality was not established. The evaluation also noted conflicting results about the association with atypical femoral fracture.14
Based on this report and focusing on the absence of nonspine benefit after 5 years, the FDA suggested that bisphosphonates may be safely discontinued in some patients without compromising therapeutic gains, but no adequate clinical trial has yet delineated how long the benefits of treatment are maintained after cessation. A periodic reevaluation of continued need was recommended.14
New recommendations from the American Society for Bone and Mineral Research
Age is the greatest risk factor for fracture.15 Therefore, deciding whether to discontinue a bisphosphonate when a woman is older, and hence at higher risk, is a challenge.
A task force of the American Society for Bone and Mineral Research (ASBMR) has developed an evidence-based guideline on managing osteoporosis in patients on long-term bisphosphonate treatment.16 The goal was to provide guidance on the duration of bisphosphonate therapy from the perspective of risk vs benefit. The authors conducted a systematic review focusing on two randomized controlled trials (FLEX13 and the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Pivotal Fracture Trial17) that provided data on long-term bisphosphonate use.
The task force recommended16 that after 5 years of oral bisphosphonates or 3 years of intravenous bisphosphonates, risk should be reassessed. In women at high fracture risk, they recommended continuing the oral bisphosphonate for 10 years or the intravenous bisphosphonate for 6 years. Factors that favored continuation of bisphosphonate therapy were as follows:
- An osteoporotic fracture before or during therapy
- A hip bone mineral density T-score ≤ –2.5
- High risk of fracture, defined as age older than 70 or 75, other strong risk factors for fracture, or a FRAX fracture risk score18 above a country-specific threshold.
(The FRAX score is based on age, sex, weight, height, previous fracture, hip fracture in a parent, current smoking, use of glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol use, and bone mineral density in the femoral neck. It gives an estimate of the 10-year risk of major osteoporotic fracture and hip fracture. High risk would be a 10-year risk of major osteoporotic fracture greater than 20% or a 10-year risk of hip fracture greater than 3%.)
For women at high risk, the risks of atypical femoral fracture and osteonecrosis of the jaw are outweighed by the benefit of a reduction in vertebral fracture risk. For women not at high risk of fracture, a drug holiday of 2 to 3 years can be considered after 3 to 5 years of treatment.
Although the task force recommended reassessment after 2 to 3 years of drug holiday, how best to do this is not clear. The task force did not recommend a specific approach to reassessment, so decisions about when to restart therapy after a drug holiday could potentially be informed by subsequent bone mineral density testing if it were to show persistent bone loss. Another option could be to restart bisphosphonates after a defined amount of time (eg, 3–5 years) for women who have previously experienced benefit.
The task force recommendations are in line with those of other societies, the FDA, and expert opinion.19–23
The American Association of Clinical Endocrinologists recommends considering a drug holiday in low-risk patients after 4 to 5 years of treatment. For high-risk patients, they recommend 1 to 2 years of drug holiday after 10 years of treatment. They encourage restarting treatment if bone mineral density decreases, bone turnover markers rise, or fracture occurs.19 This is a grade C recommendation, meaning the advice is based on descriptive studies and expert opinion.
Although some clinicians restart bisphosphonates when markers of bone turnover such as NTX (N-telopeptide of type 1 collagen) rise to premenopausal levels, there is no evidence to support this strategy.24
The task force recommendations are based on limited evidence that primarily comes from white postmenopausal women. Another important limitation is that the outcomes are primarily vertebral fractures. However, until additional evidence is available, these guidelines can be useful in guiding decision-making.
Should our patient continue therapy?
Our patient is relatively young and does not have any of the high-risk features noted within the task force recommendations. She has responded well to bisphosphonate treatment and so can consider a drug holiday at this time.
OVARIAN CANCER SCREENING
A 50-year-old woman requests screening for ovarian cancer. She is postmenopausal and has no personal or family history of cancer. She is concerned because a friend forwarded an e-mail stating, “Please tell all your female friends and relatives to insist on a cancer antigen (CA) 125 blood test every year as part of their annual exam. This is an inexpensive and simple blood test. Don’t take no for an answer. If I had known then what I know now, we would have caught my cancer much earlier, before it was stage III!” What should you tell the patient?
Ovarian cancer is the most deadly of female reproductive cancers, largely because in most patients the cancer has already spread beyond the ovary by the time of clinical detection. Death rates from ovarian cancer have decreased only slightly in the past 30 years.
Little benefit and considerable harm of screening
In 2011, the Prostate Lung Colorectal Ovarian (PLCO) Cancer Screening trial25 randomized more than 68,000 women ages 55 to 74 from the general US population to annual screening with CA 125 testing and transvaginal ultrasonography compared with usual care. They were followed for a median of 12.4 years.
Screening did not affect stage at diagnosis (77%–78% were in stage III or IV in both the screening and usual care groups), nor did it reduce the rate of death from ovarian cancer. In addition, false-positive findings led to some harm: nearly one in three women who had a positive screening test underwent surgery. Of 3,285 women with false-positive results, 1,080 underwent surgery, and 15% of these had at least one serious complication. The trial was stopped early due to evidence of futility.
A new UK study also found no benefit from screening
In the PLCO study, a CA 125 result of 35 U/mL or greater was classified as abnormal. However, researchers in the United Kingdom postulated that instead of using a single cutoff for a normal or abnormal CA 125 level, it would be better to interpret the CA 125 result according to a somewhat complicated (and proprietary) algorithm called the Risk of Ovarian Cancer Algorithm (ROCA).26,27 The ROCA takes into account a woman’s age, menopausal status, known genetic mutations (BRCA 1 or 2 or Lynch syndrome), Ashkenazi Jewish descent, and family history of ovarian or breast cancer, as well as any change in CA 125 level over time.
In a 2016 UK study,26 202,638 postmenopausal women ages 50 to 74 were randomized to no screening, annual screening with transvaginal ultrasonography, or multimodal screening with an annual CA 125 blood test interpreted with the ROCA algorithm, adding transvaginal ultrasonography as a second-line test when needed if the CA 125 level was abnormal based on the ROCA. Women with abnormal findings on multimodal screening or ultrasonography had repeat tests, and women with persistent abnormalities underwent clinical evaluation and, when appropriate, surgery.
Participants were at average risk of ovarian cancer; those with suspected familial ovarian cancer syndrome were excluded, as were those with a personal history of ovarian cancer or other active cancer.
Results. At a median follow-up of 11.1 years, the percentage of women who were diagnosed with ovarian cancer was 0.7% in the multimodal screening group, 0.6% in the screening ultrasonography group, and 0.6% in the no-screening group. Comparing either multimodal or screening ultrasonography with no screening, there was no statistically significant reduction in mortality rate over 14 years of follow-up.
Screening had significant costs and potential harms. For every ovarian or peritoneal cancer detected by screening, an additional 2 women in the multimodal screening group and 10 women in the ultrasonography group underwent needless surgery.
Strengths of this trial included its large size, allowing adequate power to detect differences in outcomes, its multicenter setting, its high compliance rate, and the low crossover rate in the no-screening group. However, the design of the study makes it difficult to anticipate the late effects of screening. Also, the patient must purchase ROCA testing online and must also pay a consultation fee. Insurance providers do not cover this test.
Should our patient proceed with ovarian cancer screening?
No. Current evidence shows no clear benefit to ovarian cancer screening for average-risk women, and we should not recommend yearly ultrasonography and CA 125 level testing, as they are likely to cause harm without providing benefit. The US Preventive Services Task Force recommends against screening for ovarian cancer.28 For premenopausal women, pregnancy, hormonal contraception, and breastfeeding all significantly decrease ovarian cancer risk by suppressing ovulation.29–31
REPRODUCTIVE FACTORS AND THE RISK OF DEATH
A 26-year-old woman comes in to discuss her contraceptive options. She has been breastfeeding since the birth of her first baby 6 months ago, and wonders how lactation and contraception may affect her long-term health.
Questions about the safety of contraceptive options are common, especially in breastfeeding mothers.
In 2010, the long-term Royal College of General Practitioners’ Oral Contraceptive Study reported that the all-cause mortality rate was actually lower in women who used oral contraceptives.32 Similarly, in 2013, an Oxford study that followed 17,032 women for over 30 years reported no association between oral contraceptives and breast cancer.33
However, in 2014, results from the Nurses’ Health Study indicated that breast cancer rates were higher in oral contraceptive users, although reassuringly, the study found no difference in all-cause mortality rates in women who had used oral contraception.34
The European Prospective Investigation Into Cancer and Nutrition
To further characterize relationships between reproductive characteristics and mortality rates, investigators analyzed data from the European Prospective Investigation Into Cancer and Nutrition,35 which recruited 322,972 women from 10 countries between 1992 and 2000. Analyses were stratified by study center and participant age and were adjusted for body mass index, physical activity, education level, smoking, and menopausal status; alcohol intake was examined as a potential confounder but was excluded from final models.
Findings. Over an average 13 years of follow-up, the rate of all-cause mortality was 20% lower in parous than in nulliparous women. In parous women, the all-cause mortality rate was additionally 18% lower in those who had breastfed vs those who had never breastfed, although breastfeeding duration was not associated with mortality. Use of oral contraceptives lowered all-cause mortality by 10% among nonsmokers; in smokers, no association with all-cause mortality was seen for oral contraceptive use, as smoking is such a powerful risk factor for mortality. The primary contributor to all-cause mortality appeared to be ischemic heart disease, the incidence of which was significantly lower in parous women (by 14%) and those who breastfed (by 20%) and was not related to oral contraceptive use.35
Strengths of this study included the large sample size recruited from countries across Europe, with varying rates of breastfeeding and contraceptive use. However, as with all observational studies, it remains subject to the possibility of residual confounding.
What should we tell this patient?
After congratulating her for breastfeeding, we can reassure her about the safety of all available contraceptives. According to the US Centers for Disease Control and Prevention (CDC),36 after 42 days postpartum most women can use combined hormonal contraception. All other methods can be used immediately postpartum, including progestin-only pills.
As lactational amenorrhea is only effective while mothers are exclusively breastfeeding, and short interpregnancy intervals have been associated with higher rates of adverse pregnancy outcomes,37 this patient will likely benefit from promptly starting a prescription contraceptive.
HIGHLY EFFECTIVE REVERSIBLE CONTRACEPTION
This same 26-year-old patient is concerned that she will not remember to take an oral contraceptive every day, and expresses interest in a more convenient method of contraception. However, she is concerned about the potential risks.
Although intrauterine contraceptives (IUCs) are typically 20 times more effective than oral contraceptives38 and have been used by millions of women worldwide, rates of use in the United States have been lower than in many other countries.39
A study of intrauterine contraception
To clarify the safety of IUCs, researchers followed 61,448 women who underwent IUC placement in six European countries between 2006 and 2013.40 Most participants received an IUC containing levonorgestrel, while 30% received a copper IUC.
Findings. Overall, rates of uterine perforation were low (approximately 1 per 1,000 insertions). The most significant risk factors for perforation were breastfeeding at the time of insertion and insertion less than 36 weeks after the last delivery. None of the perforations in the study led to serious illness or injury of intra-abdominal or pelvic structures. Interestingly, women using a levonorgestrel IUC were considerably less likely to experience a contraceptive failure than those using a copper IUC.41
Strengths of this study included the prospective data collection and power to examine rare clinical outcomes. However, it was industry-funded.
The risk of pelvic infection with an IUC is so low that the CDC does not recommend prophylactic antibiotics with the insertion procedure. If women have other indications for testing for sexually transmitted disease, an IUC can be placed the same day as testing, and before results are available.42 If a woman is found to have a sexually transmitted disease while she has an IUC in place, she should be treated with antibiotics, and there is no need to remove the IUC.43
Subdermal implants
Another highly effective contraceptive option for this patient is the progestin-only subdermal contraceptive implant (marketed in the United States as Nexplanon). Implants have been well-studied and found to have no adverse effect on lactation.44
Learning to place a subdermal contraceptive is far easier than learning to place an IUC, but it requires a few hours of FDA-mandated in-person training. Unfortunately, relatively few clinicians have obtained this training.45 As placing a subdermal contraceptive is like placing an intravenous line without needing to hit the vein, this procedure can easily be incorporated into a primary care practice. Training from the manufacturer is available to providers who request it.
What should we tell this patient?
An IUC is a great option for many women. When pregnancy is desired, the device is easily removed. Of the three IUCs now available in the United States, those containing 52 mg of levonorgestrel (marketed in the United States as Mirena and Liletta) are the most effective.
The only option more effective than these IUCs is subdermal contraception.46 These reversible contraceptives are typically more effective than permanent contraceptives (ie, tubal ligation)47 and can be removed at any time if a patient wishes to switch to another method or to become pregnant.
Pregnancy rates following attempts at “sterilization” are higher than many realize. There are a variety of approaches to “tying tubes,” some of which may not result in complete tubal occlusion. The failure rate of the laparoscopic approach, according to the US Collaborative Review of Sterilization, ranges from 7.5 per 1,000 procedures for unipolar coagulation to a high of 36.5 per 1,000 for the spring clip.48 The relatively commonly used Filshie clip was not included in this study, but its failure rate is reported to be between 1% and 2%.
- Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012; 366:1028–1037.
- Christiaens TC, De Meyere M, Verschraegen G, et al. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract 2002; 52:729–734.
- Bleidorn J, Gágyor I, Kochen MM, Wegscheider K, Hummers-Pradier E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?—results of a randomized controlled pilot trial. BMC Med 2010; 8:30. doi: 10.1186/1741-7015-8-30.
- Little P, Moore MV, Turner S, et al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ 2010; 340:c199.
- Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis 2004; 36:296–301.
- Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 2015; 351:h6544. doi: 10.1136/bmj.h6544.
- Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract 2007; 57:785–792.
- Gottesman BS, Carmeli Y, Shitrit P, Chowers M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin Infect Dis 2009; 49:869–875.
- Knottnerus BJ, Geerlings SE, Moll van Charante EP, ter Riet G. Women with symptoms of uncomplicated urinary tract infection are often willing to delay antibiotic treatment: a prospective cohort study. BMC Fam Pract 2013; 14:71. doi: 10.1186/1471-2296-14-71.
- Leydon GM, Turner S, Smith H, Little P; UTIS team. Women’s views about management and cause of urinary tract infection: qualitative interview study. BMJ 2010; 340:c279. doi: 10.1136/bmj.c279.
- Willems CS, van den Broek D’Obrenan J, Numans ME, Verheij TJ, van der Velden AW. Cystitis: antibiotic prescribing, consultation, attitudes and opinions. Fam Pract 2014; 31:149–155.
- Black DM, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- US Food and Drug Administration. Background document for meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM270958.pdf. Accessed November 3, 2016.
- Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005; 16:581–589.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31:16–35.
- Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012; 27:243–254.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases. FRAX WHO fracture risk assessment tool. www.shef.ac.uk/FRAX/. Accessed October 7, 2016.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010; 16(suppl 3):1–37.
- Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med 2012; 366:2048–2051.
- Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med 2012; 366:2051–2053.
- Brown JP, Morin S, Leslie W, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician 2014; 60:324–333.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95:1555–1565.
- Bauer DC, Schwartz A, Palermo L, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med 2014; 174:1126–1134.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016; 387:945–956.
- Abcodia Inc. The ROCA test. www.therocatest.co.uk/for-clinicians/about-roca. Accessed November 3, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2012; 157:900–904.
- Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer 2001; 84:714–721.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer, Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008; 371:303–314.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr 2015; 104:96–113.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927. doi: 10.1136/bmj.c927.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association contraceptive study. Contraception 2013; 88:678–683.
- Charlton BM, Rich-Edwards JW, Colditz GA, et al. Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: prospective cohort study. BMJ 2014; 349:g6356. doi: 10.1136/bmj.g6356.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252. doi: 10.1186/s12916-015-0484-3.
- Centers for Disease Control and Prevention (CDC). Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. MMWR Morb Mortal Wkly Rep 2011; 60:878–883.
- Bigelow CA, Bryant AS. Short interpregnancy intervals: an evidence-based guide for clinicians. Obstet Gynecol Surv 2015; 70:458–464.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Buhling KJ, Zite NB, Lotke P, Black K; INTRA Writing Group. Worldwide use of intrauterine contraception: a review. Contraception 2014; 89:162–173.
- Heinemann K, Reed S, Moehner S, Minh TD. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception 2015; 91:274–279.
- Heinemann K, Reed S, Moehner S, Minh TD. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception 2015; 91:280–283.
- Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement. Am J Obstet Gynecol 2016 May 12. pii: S0002-9378(16)30212-5. doi: 10.1016/j.ajog.2016.05.017. [Epub ahead of print]
- Division of Reproductive health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013; 62(RR-05):1–60.
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol 2011; 117:1114–1121.
- Nisen MB, Peterson LE, Cochrane A, Rubin SE. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception 2016; 93:432–437.
- Polis CB, Bradley SE, Bankole A, Onda T, Croft T, Singh S. Typical-use contraceptive failure rates in 43 countries with Demographic and Health Survey data: summary of a detailed report. Contraception 2016; 94:11–17.
- Gariepy AM, Creinin MD, Smith KJ, Xu X. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception 2014; 90:174–181.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussel J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Rerview of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
- Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012; 366:1028–1037.
- Christiaens TC, De Meyere M, Verschraegen G, et al. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract 2002; 52:729–734.
- Bleidorn J, Gágyor I, Kochen MM, Wegscheider K, Hummers-Pradier E. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?—results of a randomized controlled pilot trial. BMC Med 2010; 8:30. doi: 10.1186/1741-7015-8-30.
- Little P, Moore MV, Turner S, et al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ 2010; 340:c199.
- Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis 2004; 36:296–301.
- Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 2015; 351:h6544. doi: 10.1136/bmj.h6544.
- Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract 2007; 57:785–792.
- Gottesman BS, Carmeli Y, Shitrit P, Chowers M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin Infect Dis 2009; 49:869–875.
- Knottnerus BJ, Geerlings SE, Moll van Charante EP, ter Riet G. Women with symptoms of uncomplicated urinary tract infection are often willing to delay antibiotic treatment: a prospective cohort study. BMC Fam Pract 2013; 14:71. doi: 10.1186/1471-2296-14-71.
- Leydon GM, Turner S, Smith H, Little P; UTIS team. Women’s views about management and cause of urinary tract infection: qualitative interview study. BMJ 2010; 340:c279. doi: 10.1136/bmj.c279.
- Willems CS, van den Broek D’Obrenan J, Numans ME, Verheij TJ, van der Velden AW. Cystitis: antibiotic prescribing, consultation, attitudes and opinions. Fam Pract 2014; 31:149–155.
- Black DM, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- US Food and Drug Administration. Background document for meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM270958.pdf. Accessed November 3, 2016.
- Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005; 16:581–589.
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31:16–35.
- Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012; 27:243–254.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases. FRAX WHO fracture risk assessment tool. www.shef.ac.uk/FRAX/. Accessed October 7, 2016.
- Watts NB, Bilezikian JP, Camacho PM, et al; AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 2010; 16(suppl 3):1–37.
- Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med 2012; 366:2048–2051.
- Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med 2012; 366:2051–2053.
- Brown JP, Morin S, Leslie W, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician 2014; 60:324–333.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95:1555–1565.
- Bauer DC, Schwartz A, Palermo L, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern Med 2014; 174:1126–1134.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016; 387:945–956.
- Abcodia Inc. The ROCA test. www.therocatest.co.uk/for-clinicians/about-roca. Accessed November 3, 2016.
- Moyer VA; US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2012; 157:900–904.
- Titus-Ernstoff L, Perez K, Cramer DW, Harlow BL, Baron JA, Greenberg ER. Menstrual and reproductive factors in relation to ovarian cancer risk. Br J Cancer 2001; 84:714–721.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer, Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008; 371:303–314.
- Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr 2015; 104:96–113.
- Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from Royal College of General Practitioners’ Oral Contraception Study. BMJ 2010; 340:c927. doi: 10.1136/bmj.c927.
- Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-Family Planning Association contraceptive study. Contraception 2013; 88:678–683.
- Charlton BM, Rich-Edwards JW, Colditz GA, et al. Oral contraceptive use and mortality after 36 years of follow-up in the Nurses’ Health Study: prospective cohort study. BMJ 2014; 349:g6356. doi: 10.1136/bmj.g6356.
- Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Med 2015; 13:252. doi: 10.1186/s12916-015-0484-3.
- Centers for Disease Control and Prevention (CDC). Update to CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. MMWR Morb Mortal Wkly Rep 2011; 60:878–883.
- Bigelow CA, Bryant AS. Short interpregnancy intervals: an evidence-based guide for clinicians. Obstet Gynecol Surv 2015; 70:458–464.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012; 366:1998–2007.
- Buhling KJ, Zite NB, Lotke P, Black K; INTRA Writing Group. Worldwide use of intrauterine contraception: a review. Contraception 2014; 89:162–173.
- Heinemann K, Reed S, Moehner S, Minh TD. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception 2015; 91:274–279.
- Heinemann K, Reed S, Moehner S, Minh TD. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception 2015; 91:280–283.
- Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement. Am J Obstet Gynecol 2016 May 12. pii: S0002-9378(16)30212-5. doi: 10.1016/j.ajog.2016.05.017. [Epub ahead of print]
- Division of Reproductive health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013; 62(RR-05):1–60.
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol 2011; 117:1114–1121.
- Nisen MB, Peterson LE, Cochrane A, Rubin SE. US family physicians’ intrauterine and implantable contraception provision: results from a national survey. Contraception 2016; 93:432–437.
- Polis CB, Bradley SE, Bankole A, Onda T, Croft T, Singh S. Typical-use contraceptive failure rates in 43 countries with Demographic and Health Survey data: summary of a detailed report. Contraception 2016; 94:11–17.
- Gariepy AM, Creinin MD, Smith KJ, Xu X. Probability of pregnancy after sterilization: a comparison of hysteroscopic versus laparoscopic sterilization. Contraception 2014; 90:174–181.
- Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussel J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Rerview of Sterilization. Am J Obstet Gynecol 1996; 174:1161–1168.
KEY POINTS
- Many women with mild uncomplicated urinary tract infections can avoid taking antibiotics and instead receive treatment for symptoms alone.
- The American Society for Bone and Mineral Research now recommends reassessing the risk of osteoporotic fracture after 3 to 5 years of bisphosphonate therapy. Women at high risk may benefit from extending bisphosphonate therapy to 10 years.
- Current evidence shows no clear benefit of ovarian cancer screening for women at average risk, and we should not recommend yearly ultrasonography or cancer antigen 125 level testing, either of which is likely to cause harm without providing benefit.
- A large observational study found death rates were lower in parous than in nulliparous women, in women who had breastfed than in those who had never breastfed, and in nonsmokers who had used oral contraceptives.
- Intrauterine contraception and subdermal implants are safe and are the most effective contraceptive options.
What stool testing is appropriate when diarrhea develops in a hospitalized patient?
A 72-year-old woman is admitted with fever and shortness of breath. Chest radiography demonstrates a consolidation in the right lower lobe, and ceftriaxone and azithromycin are given to treat community-acquired pneumonia. After initial improvement she develops abdominal discomfort and profuse diarrhea on day 5 of hospitalization. What stool testing should be ordered?
Most cases of diarrhea in hospitalized patients are not due to infection, but the most common infectious cause is Clostridium difficile. In the absence of unusual circumstances such as a norovirus outbreak or diarrhea in an immunocompromised patient, testing for C difficile is the only recommended assay. A multistep algorithm with a combination of antigen detection and nucleic acid amplification techniques provides the best sensitivity and specificity. Repeated testing after an initially negative test and performing a test of cure are of limited utility and incur added costs, and thus are not recommended.
CAUSES OF DIARRHEA IN THE HOSPITAL
Diarrhea is defined as at least 1 day with three or more unformed stools or a significant increase in stool frequency above baseline.
Nosocomial diarrhea is an acute episode of diarrhea in a hospitalized patient that was not present on admission and that arises after 3 days of hospitalization. It is fairly common, developing in 12% to 32% of patients at some point during their hospitalization.1
Most cases of nosocomial diarrhea are not due to infection, but rather secondary to enteral feeding, medications, and underlying illness. C difficile is the most common infectious cause and accounts for 10% to 20% of all cases of nosocomial diarrhea.2 Other pathogens associated with nosocomial diarrhea are unusual, although outbreaks of norovirus in healthcare facilities have occurred,3 and isolated cases of Klebsiella oxytoca causing acute abdominal pain, bloody diarrhea, and leukocytosis after exposure to antibiotics have been reported.1
RECOMMENDED TESTING
The evaluation of a hospitalized patient in whom diarrhea develops should initially focus on the clinical presentation, with attention to signs of sepsis. Stable patients with mild symptoms may respond to withdrawal of the offending agent (if any), while patients with moderate or severe symptoms (including those with fever, hypotension, leukocytosis, acute kidney injury, or a decreased serum bicarbonate level) should be tested for C difficile infection (Figure 1).
In general, stool testing should adhere to the “3-day rule”—ie, fecal specimens from patients with diarrhea that develops after 3 days of hospitalization have a very low yield when cultured for standard bacteria or examined for ova and parasites. Thus, only testing for C difficile infection should be ordered.4
In an outbreak of norovirus, especially if vomiting is present, norovirus testing by reverse transcriptase polymerase chain reaction (PCR) could be considered.
Fecal white blood cell testing should not be ordered, as it neither sensitive nor specific.5
Immunocompromised patients (such as those with organ transplants or late-stage human immunodeficiency virus infection) occasionally contract diarrhea due to causes other than C difficile, and consultation with a gastroenterologist or an infectious diseases physician could be considered if diarrhea persists and no cause is apparent.
In the rare situation when a patient is hospitalized after very recent overseas travel and then contracts diarrhea, causes of traveler’s diarrhea should be considered.
TESTING FOR C DIFFICILE INFECTION
A number of diagnostic tests for C difficile infection are available.
Toxigenic culture (culture followed by detection of a toxigenic isolate) and C difficile cytotoxin neutralization assay are considered the reference standards, having high sensitivity and specificity. However, both are time- and labor-intensive, with turnaround times of at least 2 to 3 days and up to 9 days, limiting their clinical utility and resulting in delay in both diagnosis and implementation of infection control measures.2,6
Enzyme immunoassays (EIAs) are faster. EIAs are available to detect glutamate dehydrogenase (GDH) and toxins A and B, all produced by C difficile. The GDH EIA is 92% sensitive and 93% specific but should not be used alone as it does not distinguish between toxigenic and nontoxigenic strains of C difficile.2,6 The toxin A/B EIA is 97% specific, but since its sensitivity may be as low as 73%, it too should not be used alone.6
Nucleic acid amplification tests such as PCR and loop-mediated isothermal amplification (LAMP) identify toxigenic C difficile by detecting tcdA, tcdB, or tcdC genes, which regulate toxin production. These tests have sensitivities and specificities well over 90%.6
Since molecular tests (ie, nucleic acid amplification tests) for C difficile infection became available in 2009, they have been widely adopted and are commercially available.7 Facilities that use them have reported a 50% to 100% increase in C difficile infection rates,7 but the increase may not be real. Rather, it may reflect increased detection of colonization by the more-sensitive tests.
In a prospective, observational, cohort study,7 1,416 hospitalized patients with diarrhea that developed 72 hours after hospitalization were tested for C difficile infection by both toxin EIA and PCR. Those with positive results on both tests had a longer duration of diarrhea, more C difficile infection-related complications, more C difficile infection-related deaths, and greater risk of diarrhea during follow-up. For those who had negative results on toxin EIA testing, the results of PCR testing made no difference, and neither did treatment for C difficile infection, suggesting that most patients with negative toxin test results do not need treatment for C difficile even if PCR testing is positive.
In light of the limited sensitivity of some toxin EIAs and the increased identification of asymptomatic colonization with nucleic acid amplification testing, the optimal approach may be to combine rapid testing methods. Algorithms that include nucleic acid amplification testing have the best sensitivity (68% to 100%) and specificity (92% to 100%).7 Clinical guidelines suggest using a GDH EIA as the initial step, and then confirming positive results with either nucleic acid amplification testing alone or toxin EIA followed by nucleic acid amplification testing if the toxin EIA is negative.8 However, the best diagnostic approach remains controversial, and multistep algorithms may be impractical in some laboratories.
Knowledge of the laboratory test used can help clinicians appreciate the limitations of specimen testing. Table 1 outlines some of the performance characteristics of the available assays.9–11
The preferred approach at our institution is a multistep algorithm using both the GDH and toxin EIAs in the initial step, followed by a LAMP assay for the C difficile toxin genes in cases of discordant EIA results.
Repeat testing after an initial negative test may be positive in fewer than 5% of cases, can increase the chance of false-positive results, does not improve sensitivity and negative predictive values, and is therefore not recommended.2,8 Similarly, a test of cure after symptoms resolve is not recommended, as the toxin EIA can be positive for up to 30 days after resolution of symptoms, and a positive nucleic acid amplification test may only reflect colonization.2,8
RETURNING TO OUR PATIENT
Returning to the patient hospitalized with community-acquired pneumonia, C difficile infection is the most likely cause of her diarrhea. If her respiratory symptoms have improved, then cessation of ceftriaxone and azithromycin should be considered because she has completed 5 days of therapy. In addition, given her profuse diarrhea, testing for C difficile is recommended with a multistep approach.
- Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridum difficile. Clin Infect Dis 2012; 55:982–989.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Greig JD, Lee MB. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect 2012; 140:1151–1160.
- Guerrant RL, Van Gilder T, Steiner TS, et al; Infectious Diseases Society of America. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001; 32:331–351.
- Savola KL, Baron EJ, Tompkins LS, Passaro DJ. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. Clin Microbiol 2001; 39:266–269.
- Bagdasarian N, Rao, K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015; 313:398–408.
- Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–1801.
- Surawica CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–498.
- Staneck JL, Weckbah LS, Allen SD, et al. Multicenter evaluation of four methods for Clostridium difficile detection: immunocard C. difficile, cytotoxin assay, culture, and latex agglutination. J Clin Microbiol 1996; 34:2718–2721.
- Novak-Weekley SM, Marlow EM, Miller JM, et al. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol 2010; 48:889–893.
- Schroeder LF, Robilotti E, Peterson LR, Banaei N, Dowdy DW. Economic evaluation of laboratory testing strategies for hospital-associated Clostridium difficle infection. J Clin Microbiol 2014; 52:489–496.
A 72-year-old woman is admitted with fever and shortness of breath. Chest radiography demonstrates a consolidation in the right lower lobe, and ceftriaxone and azithromycin are given to treat community-acquired pneumonia. After initial improvement she develops abdominal discomfort and profuse diarrhea on day 5 of hospitalization. What stool testing should be ordered?
Most cases of diarrhea in hospitalized patients are not due to infection, but the most common infectious cause is Clostridium difficile. In the absence of unusual circumstances such as a norovirus outbreak or diarrhea in an immunocompromised patient, testing for C difficile is the only recommended assay. A multistep algorithm with a combination of antigen detection and nucleic acid amplification techniques provides the best sensitivity and specificity. Repeated testing after an initially negative test and performing a test of cure are of limited utility and incur added costs, and thus are not recommended.
CAUSES OF DIARRHEA IN THE HOSPITAL
Diarrhea is defined as at least 1 day with three or more unformed stools or a significant increase in stool frequency above baseline.
Nosocomial diarrhea is an acute episode of diarrhea in a hospitalized patient that was not present on admission and that arises after 3 days of hospitalization. It is fairly common, developing in 12% to 32% of patients at some point during their hospitalization.1
Most cases of nosocomial diarrhea are not due to infection, but rather secondary to enteral feeding, medications, and underlying illness. C difficile is the most common infectious cause and accounts for 10% to 20% of all cases of nosocomial diarrhea.2 Other pathogens associated with nosocomial diarrhea are unusual, although outbreaks of norovirus in healthcare facilities have occurred,3 and isolated cases of Klebsiella oxytoca causing acute abdominal pain, bloody diarrhea, and leukocytosis after exposure to antibiotics have been reported.1
RECOMMENDED TESTING
The evaluation of a hospitalized patient in whom diarrhea develops should initially focus on the clinical presentation, with attention to signs of sepsis. Stable patients with mild symptoms may respond to withdrawal of the offending agent (if any), while patients with moderate or severe symptoms (including those with fever, hypotension, leukocytosis, acute kidney injury, or a decreased serum bicarbonate level) should be tested for C difficile infection (Figure 1).
In general, stool testing should adhere to the “3-day rule”—ie, fecal specimens from patients with diarrhea that develops after 3 days of hospitalization have a very low yield when cultured for standard bacteria or examined for ova and parasites. Thus, only testing for C difficile infection should be ordered.4
In an outbreak of norovirus, especially if vomiting is present, norovirus testing by reverse transcriptase polymerase chain reaction (PCR) could be considered.
Fecal white blood cell testing should not be ordered, as it neither sensitive nor specific.5
Immunocompromised patients (such as those with organ transplants or late-stage human immunodeficiency virus infection) occasionally contract diarrhea due to causes other than C difficile, and consultation with a gastroenterologist or an infectious diseases physician could be considered if diarrhea persists and no cause is apparent.
In the rare situation when a patient is hospitalized after very recent overseas travel and then contracts diarrhea, causes of traveler’s diarrhea should be considered.
TESTING FOR C DIFFICILE INFECTION
A number of diagnostic tests for C difficile infection are available.
Toxigenic culture (culture followed by detection of a toxigenic isolate) and C difficile cytotoxin neutralization assay are considered the reference standards, having high sensitivity and specificity. However, both are time- and labor-intensive, with turnaround times of at least 2 to 3 days and up to 9 days, limiting their clinical utility and resulting in delay in both diagnosis and implementation of infection control measures.2,6
Enzyme immunoassays (EIAs) are faster. EIAs are available to detect glutamate dehydrogenase (GDH) and toxins A and B, all produced by C difficile. The GDH EIA is 92% sensitive and 93% specific but should not be used alone as it does not distinguish between toxigenic and nontoxigenic strains of C difficile.2,6 The toxin A/B EIA is 97% specific, but since its sensitivity may be as low as 73%, it too should not be used alone.6
Nucleic acid amplification tests such as PCR and loop-mediated isothermal amplification (LAMP) identify toxigenic C difficile by detecting tcdA, tcdB, or tcdC genes, which regulate toxin production. These tests have sensitivities and specificities well over 90%.6
Since molecular tests (ie, nucleic acid amplification tests) for C difficile infection became available in 2009, they have been widely adopted and are commercially available.7 Facilities that use them have reported a 50% to 100% increase in C difficile infection rates,7 but the increase may not be real. Rather, it may reflect increased detection of colonization by the more-sensitive tests.
In a prospective, observational, cohort study,7 1,416 hospitalized patients with diarrhea that developed 72 hours after hospitalization were tested for C difficile infection by both toxin EIA and PCR. Those with positive results on both tests had a longer duration of diarrhea, more C difficile infection-related complications, more C difficile infection-related deaths, and greater risk of diarrhea during follow-up. For those who had negative results on toxin EIA testing, the results of PCR testing made no difference, and neither did treatment for C difficile infection, suggesting that most patients with negative toxin test results do not need treatment for C difficile even if PCR testing is positive.
In light of the limited sensitivity of some toxin EIAs and the increased identification of asymptomatic colonization with nucleic acid amplification testing, the optimal approach may be to combine rapid testing methods. Algorithms that include nucleic acid amplification testing have the best sensitivity (68% to 100%) and specificity (92% to 100%).7 Clinical guidelines suggest using a GDH EIA as the initial step, and then confirming positive results with either nucleic acid amplification testing alone or toxin EIA followed by nucleic acid amplification testing if the toxin EIA is negative.8 However, the best diagnostic approach remains controversial, and multistep algorithms may be impractical in some laboratories.
Knowledge of the laboratory test used can help clinicians appreciate the limitations of specimen testing. Table 1 outlines some of the performance characteristics of the available assays.9–11
The preferred approach at our institution is a multistep algorithm using both the GDH and toxin EIAs in the initial step, followed by a LAMP assay for the C difficile toxin genes in cases of discordant EIA results.
Repeat testing after an initial negative test may be positive in fewer than 5% of cases, can increase the chance of false-positive results, does not improve sensitivity and negative predictive values, and is therefore not recommended.2,8 Similarly, a test of cure after symptoms resolve is not recommended, as the toxin EIA can be positive for up to 30 days after resolution of symptoms, and a positive nucleic acid amplification test may only reflect colonization.2,8
RETURNING TO OUR PATIENT
Returning to the patient hospitalized with community-acquired pneumonia, C difficile infection is the most likely cause of her diarrhea. If her respiratory symptoms have improved, then cessation of ceftriaxone and azithromycin should be considered because she has completed 5 days of therapy. In addition, given her profuse diarrhea, testing for C difficile is recommended with a multistep approach.
A 72-year-old woman is admitted with fever and shortness of breath. Chest radiography demonstrates a consolidation in the right lower lobe, and ceftriaxone and azithromycin are given to treat community-acquired pneumonia. After initial improvement she develops abdominal discomfort and profuse diarrhea on day 5 of hospitalization. What stool testing should be ordered?
Most cases of diarrhea in hospitalized patients are not due to infection, but the most common infectious cause is Clostridium difficile. In the absence of unusual circumstances such as a norovirus outbreak or diarrhea in an immunocompromised patient, testing for C difficile is the only recommended assay. A multistep algorithm with a combination of antigen detection and nucleic acid amplification techniques provides the best sensitivity and specificity. Repeated testing after an initially negative test and performing a test of cure are of limited utility and incur added costs, and thus are not recommended.
CAUSES OF DIARRHEA IN THE HOSPITAL
Diarrhea is defined as at least 1 day with three or more unformed stools or a significant increase in stool frequency above baseline.
Nosocomial diarrhea is an acute episode of diarrhea in a hospitalized patient that was not present on admission and that arises after 3 days of hospitalization. It is fairly common, developing in 12% to 32% of patients at some point during their hospitalization.1
Most cases of nosocomial diarrhea are not due to infection, but rather secondary to enteral feeding, medications, and underlying illness. C difficile is the most common infectious cause and accounts for 10% to 20% of all cases of nosocomial diarrhea.2 Other pathogens associated with nosocomial diarrhea are unusual, although outbreaks of norovirus in healthcare facilities have occurred,3 and isolated cases of Klebsiella oxytoca causing acute abdominal pain, bloody diarrhea, and leukocytosis after exposure to antibiotics have been reported.1
RECOMMENDED TESTING
The evaluation of a hospitalized patient in whom diarrhea develops should initially focus on the clinical presentation, with attention to signs of sepsis. Stable patients with mild symptoms may respond to withdrawal of the offending agent (if any), while patients with moderate or severe symptoms (including those with fever, hypotension, leukocytosis, acute kidney injury, or a decreased serum bicarbonate level) should be tested for C difficile infection (Figure 1).
In general, stool testing should adhere to the “3-day rule”—ie, fecal specimens from patients with diarrhea that develops after 3 days of hospitalization have a very low yield when cultured for standard bacteria or examined for ova and parasites. Thus, only testing for C difficile infection should be ordered.4
In an outbreak of norovirus, especially if vomiting is present, norovirus testing by reverse transcriptase polymerase chain reaction (PCR) could be considered.
Fecal white blood cell testing should not be ordered, as it neither sensitive nor specific.5
Immunocompromised patients (such as those with organ transplants or late-stage human immunodeficiency virus infection) occasionally contract diarrhea due to causes other than C difficile, and consultation with a gastroenterologist or an infectious diseases physician could be considered if diarrhea persists and no cause is apparent.
In the rare situation when a patient is hospitalized after very recent overseas travel and then contracts diarrhea, causes of traveler’s diarrhea should be considered.
TESTING FOR C DIFFICILE INFECTION
A number of diagnostic tests for C difficile infection are available.
Toxigenic culture (culture followed by detection of a toxigenic isolate) and C difficile cytotoxin neutralization assay are considered the reference standards, having high sensitivity and specificity. However, both are time- and labor-intensive, with turnaround times of at least 2 to 3 days and up to 9 days, limiting their clinical utility and resulting in delay in both diagnosis and implementation of infection control measures.2,6
Enzyme immunoassays (EIAs) are faster. EIAs are available to detect glutamate dehydrogenase (GDH) and toxins A and B, all produced by C difficile. The GDH EIA is 92% sensitive and 93% specific but should not be used alone as it does not distinguish between toxigenic and nontoxigenic strains of C difficile.2,6 The toxin A/B EIA is 97% specific, but since its sensitivity may be as low as 73%, it too should not be used alone.6
Nucleic acid amplification tests such as PCR and loop-mediated isothermal amplification (LAMP) identify toxigenic C difficile by detecting tcdA, tcdB, or tcdC genes, which regulate toxin production. These tests have sensitivities and specificities well over 90%.6
Since molecular tests (ie, nucleic acid amplification tests) for C difficile infection became available in 2009, they have been widely adopted and are commercially available.7 Facilities that use them have reported a 50% to 100% increase in C difficile infection rates,7 but the increase may not be real. Rather, it may reflect increased detection of colonization by the more-sensitive tests.
In a prospective, observational, cohort study,7 1,416 hospitalized patients with diarrhea that developed 72 hours after hospitalization were tested for C difficile infection by both toxin EIA and PCR. Those with positive results on both tests had a longer duration of diarrhea, more C difficile infection-related complications, more C difficile infection-related deaths, and greater risk of diarrhea during follow-up. For those who had negative results on toxin EIA testing, the results of PCR testing made no difference, and neither did treatment for C difficile infection, suggesting that most patients with negative toxin test results do not need treatment for C difficile even if PCR testing is positive.
In light of the limited sensitivity of some toxin EIAs and the increased identification of asymptomatic colonization with nucleic acid amplification testing, the optimal approach may be to combine rapid testing methods. Algorithms that include nucleic acid amplification testing have the best sensitivity (68% to 100%) and specificity (92% to 100%).7 Clinical guidelines suggest using a GDH EIA as the initial step, and then confirming positive results with either nucleic acid amplification testing alone or toxin EIA followed by nucleic acid amplification testing if the toxin EIA is negative.8 However, the best diagnostic approach remains controversial, and multistep algorithms may be impractical in some laboratories.
Knowledge of the laboratory test used can help clinicians appreciate the limitations of specimen testing. Table 1 outlines some of the performance characteristics of the available assays.9–11
The preferred approach at our institution is a multistep algorithm using both the GDH and toxin EIAs in the initial step, followed by a LAMP assay for the C difficile toxin genes in cases of discordant EIA results.
Repeat testing after an initial negative test may be positive in fewer than 5% of cases, can increase the chance of false-positive results, does not improve sensitivity and negative predictive values, and is therefore not recommended.2,8 Similarly, a test of cure after symptoms resolve is not recommended, as the toxin EIA can be positive for up to 30 days after resolution of symptoms, and a positive nucleic acid amplification test may only reflect colonization.2,8
RETURNING TO OUR PATIENT
Returning to the patient hospitalized with community-acquired pneumonia, C difficile infection is the most likely cause of her diarrhea. If her respiratory symptoms have improved, then cessation of ceftriaxone and azithromycin should be considered because she has completed 5 days of therapy. In addition, given her profuse diarrhea, testing for C difficile is recommended with a multistep approach.
- Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridum difficile. Clin Infect Dis 2012; 55:982–989.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Greig JD, Lee MB. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect 2012; 140:1151–1160.
- Guerrant RL, Van Gilder T, Steiner TS, et al; Infectious Diseases Society of America. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001; 32:331–351.
- Savola KL, Baron EJ, Tompkins LS, Passaro DJ. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. Clin Microbiol 2001; 39:266–269.
- Bagdasarian N, Rao, K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015; 313:398–408.
- Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–1801.
- Surawica CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–498.
- Staneck JL, Weckbah LS, Allen SD, et al. Multicenter evaluation of four methods for Clostridium difficile detection: immunocard C. difficile, cytotoxin assay, culture, and latex agglutination. J Clin Microbiol 1996; 34:2718–2721.
- Novak-Weekley SM, Marlow EM, Miller JM, et al. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol 2010; 48:889–893.
- Schroeder LF, Robilotti E, Peterson LR, Banaei N, Dowdy DW. Economic evaluation of laboratory testing strategies for hospital-associated Clostridium difficle infection. J Clin Microbiol 2014; 52:489–496.
- Polage CR, Solnick JV, Cohen SH. Nosocomial diarrhea: evaluation and treatment of causes other than Clostridum difficile. Clin Infect Dis 2012; 55:982–989.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Greig JD, Lee MB. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect 2012; 140:1151–1160.
- Guerrant RL, Van Gilder T, Steiner TS, et al; Infectious Diseases Society of America. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001; 32:331–351.
- Savola KL, Baron EJ, Tompkins LS, Passaro DJ. Fecal leukocyte stain has diagnostic value for outpatients but not inpatients. Clin Microbiol 2001; 39:266–269.
- Bagdasarian N, Rao, K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 2015; 313:398–408.
- Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–1801.
- Surawica CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–498.
- Staneck JL, Weckbah LS, Allen SD, et al. Multicenter evaluation of four methods for Clostridium difficile detection: immunocard C. difficile, cytotoxin assay, culture, and latex agglutination. J Clin Microbiol 1996; 34:2718–2721.
- Novak-Weekley SM, Marlow EM, Miller JM, et al. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol 2010; 48:889–893.
- Schroeder LF, Robilotti E, Peterson LR, Banaei N, Dowdy DW. Economic evaluation of laboratory testing strategies for hospital-associated Clostridium difficle infection. J Clin Microbiol 2014; 52:489–496.
Hiding in clear sight: Complications of immunosuppressive therapies
In this issue of the Journal, Ota et al provide a clinical image and vignette of a woman with emphysematous cystitis and a psoas abscess. Genitourinary infections with Escherichia coli are well known to occasionally produce gas—especially, it seems, in people with diabetes. But I thought it valuable to publish these images to provide a reminder of this infectious complication, as well as to highlight the cloaking effect of pharmacologic immunosuppression.
The patient they describe was receiving corticosteroids for brain metastases and was thus likely receiving a high dose. She had experienced abdominal pain for 3 days before seeking medical attention, but the infectious process undoubtedly predated that. Despite receiving appropriate antibiotics for more than 3 weeks, she harbored an expanding psoas abscess that was heralded by fever after the antibiotics were discontinued. This scenario is of little surprise in an ill 69-year-old diabetic woman with metastatic cancer who was receiving high-dose corticosteroids. We have all been taught about and likely have witnessed the devastating effect of delayed diagnosis of abdominal infections in patients on high-dose steroids.
With the current explosion of new targeted therapies for systemic and organ-specific inflammatory diseases, it is hard to keep up with their names, not to mention their mechanisms of action and potential complications. Much attention has been given, highlighted by the requisite warnings in direct-to-consumer advertising, to the reactivation of tuberculosis, the occurrence of fungal infections, and the risk of malignancy in patients taking many of these drugs. The risk of cancer seems to have been overstated, at least for the anti-tumor necrosis factor (anti-TNF) agents, but there is no question that some new biologics carry a real risk of reactivating latent tuberculosis and even some viral infections. But I have seen a more common problem, one that is inadequately emphasized: the delayed diagnosis of deep-tissue infection due to a blunting of the signs of inflammation that would normally accompany the infection.
Anti-TNF therapies (eg, infliximab, etanercept, adalimumab) are being increasingly prescribed for the gamut of systemic and organ-specific inflammatory diseases, from sarcoidosis and rheumatoid arthritis to uveitis. I have no doubt that these agents suppress the inflammatory response in ways that can delay diagnosis. I have seen it happen in patients with diverticular abscess, bacterial pneumonia, epidural abscess, and bacterial septic arthritis, and I believe it is a more concerning clinical issue than any actual increase in the number of opportunistic infections.
The anti-interleukin 6 biologic agent tocilizumab, like the anti-TNF agents, not only blunts the inflammatory response and masks infection, but also seems to contribute to an increased occurrence of lower intestinal perforation.1 This is potentially important, as it seems likely that indications for this agent will be expanded to diseases other than rheumatoid arthritis.
Equal concern is likely warranted at the present time in patients receiving newer drugs such as Janus kinase (JAK) inhibitors and blockers of the interleukin 17 and 23 pathways, at least until more “real-life” patient experience is accumulated. Not all anti-inflammatory and immunosuppressive drugs have this dramatic blunting effect on findings of infection-associated inflammation (methotrexate seems not to), but we need to be wary and should perform extra-fastidious physical examinations followed by imaging studies when our patients complain of any localizing symptoms that are not readily and completely explained.
At the end of a tumultuous and divisive year, we at the Journal send to you, our readers, our heartfelt wishes for personal tranquility and for a universally peaceful and harmonious 2017.
- Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 2016 Jul 12. pii: annrheumdis-2016-209773. doi: 10.1136/annrheumdis-2016-209773. [Epub ahead of print]
In this issue of the Journal, Ota et al provide a clinical image and vignette of a woman with emphysematous cystitis and a psoas abscess. Genitourinary infections with Escherichia coli are well known to occasionally produce gas—especially, it seems, in people with diabetes. But I thought it valuable to publish these images to provide a reminder of this infectious complication, as well as to highlight the cloaking effect of pharmacologic immunosuppression.
The patient they describe was receiving corticosteroids for brain metastases and was thus likely receiving a high dose. She had experienced abdominal pain for 3 days before seeking medical attention, but the infectious process undoubtedly predated that. Despite receiving appropriate antibiotics for more than 3 weeks, she harbored an expanding psoas abscess that was heralded by fever after the antibiotics were discontinued. This scenario is of little surprise in an ill 69-year-old diabetic woman with metastatic cancer who was receiving high-dose corticosteroids. We have all been taught about and likely have witnessed the devastating effect of delayed diagnosis of abdominal infections in patients on high-dose steroids.
With the current explosion of new targeted therapies for systemic and organ-specific inflammatory diseases, it is hard to keep up with their names, not to mention their mechanisms of action and potential complications. Much attention has been given, highlighted by the requisite warnings in direct-to-consumer advertising, to the reactivation of tuberculosis, the occurrence of fungal infections, and the risk of malignancy in patients taking many of these drugs. The risk of cancer seems to have been overstated, at least for the anti-tumor necrosis factor (anti-TNF) agents, but there is no question that some new biologics carry a real risk of reactivating latent tuberculosis and even some viral infections. But I have seen a more common problem, one that is inadequately emphasized: the delayed diagnosis of deep-tissue infection due to a blunting of the signs of inflammation that would normally accompany the infection.
Anti-TNF therapies (eg, infliximab, etanercept, adalimumab) are being increasingly prescribed for the gamut of systemic and organ-specific inflammatory diseases, from sarcoidosis and rheumatoid arthritis to uveitis. I have no doubt that these agents suppress the inflammatory response in ways that can delay diagnosis. I have seen it happen in patients with diverticular abscess, bacterial pneumonia, epidural abscess, and bacterial septic arthritis, and I believe it is a more concerning clinical issue than any actual increase in the number of opportunistic infections.
The anti-interleukin 6 biologic agent tocilizumab, like the anti-TNF agents, not only blunts the inflammatory response and masks infection, but also seems to contribute to an increased occurrence of lower intestinal perforation.1 This is potentially important, as it seems likely that indications for this agent will be expanded to diseases other than rheumatoid arthritis.
Equal concern is likely warranted at the present time in patients receiving newer drugs such as Janus kinase (JAK) inhibitors and blockers of the interleukin 17 and 23 pathways, at least until more “real-life” patient experience is accumulated. Not all anti-inflammatory and immunosuppressive drugs have this dramatic blunting effect on findings of infection-associated inflammation (methotrexate seems not to), but we need to be wary and should perform extra-fastidious physical examinations followed by imaging studies when our patients complain of any localizing symptoms that are not readily and completely explained.
At the end of a tumultuous and divisive year, we at the Journal send to you, our readers, our heartfelt wishes for personal tranquility and for a universally peaceful and harmonious 2017.
In this issue of the Journal, Ota et al provide a clinical image and vignette of a woman with emphysematous cystitis and a psoas abscess. Genitourinary infections with Escherichia coli are well known to occasionally produce gas—especially, it seems, in people with diabetes. But I thought it valuable to publish these images to provide a reminder of this infectious complication, as well as to highlight the cloaking effect of pharmacologic immunosuppression.
The patient they describe was receiving corticosteroids for brain metastases and was thus likely receiving a high dose. She had experienced abdominal pain for 3 days before seeking medical attention, but the infectious process undoubtedly predated that. Despite receiving appropriate antibiotics for more than 3 weeks, she harbored an expanding psoas abscess that was heralded by fever after the antibiotics were discontinued. This scenario is of little surprise in an ill 69-year-old diabetic woman with metastatic cancer who was receiving high-dose corticosteroids. We have all been taught about and likely have witnessed the devastating effect of delayed diagnosis of abdominal infections in patients on high-dose steroids.
With the current explosion of new targeted therapies for systemic and organ-specific inflammatory diseases, it is hard to keep up with their names, not to mention their mechanisms of action and potential complications. Much attention has been given, highlighted by the requisite warnings in direct-to-consumer advertising, to the reactivation of tuberculosis, the occurrence of fungal infections, and the risk of malignancy in patients taking many of these drugs. The risk of cancer seems to have been overstated, at least for the anti-tumor necrosis factor (anti-TNF) agents, but there is no question that some new biologics carry a real risk of reactivating latent tuberculosis and even some viral infections. But I have seen a more common problem, one that is inadequately emphasized: the delayed diagnosis of deep-tissue infection due to a blunting of the signs of inflammation that would normally accompany the infection.
Anti-TNF therapies (eg, infliximab, etanercept, adalimumab) are being increasingly prescribed for the gamut of systemic and organ-specific inflammatory diseases, from sarcoidosis and rheumatoid arthritis to uveitis. I have no doubt that these agents suppress the inflammatory response in ways that can delay diagnosis. I have seen it happen in patients with diverticular abscess, bacterial pneumonia, epidural abscess, and bacterial septic arthritis, and I believe it is a more concerning clinical issue than any actual increase in the number of opportunistic infections.
The anti-interleukin 6 biologic agent tocilizumab, like the anti-TNF agents, not only blunts the inflammatory response and masks infection, but also seems to contribute to an increased occurrence of lower intestinal perforation.1 This is potentially important, as it seems likely that indications for this agent will be expanded to diseases other than rheumatoid arthritis.
Equal concern is likely warranted at the present time in patients receiving newer drugs such as Janus kinase (JAK) inhibitors and blockers of the interleukin 17 and 23 pathways, at least until more “real-life” patient experience is accumulated. Not all anti-inflammatory and immunosuppressive drugs have this dramatic blunting effect on findings of infection-associated inflammation (methotrexate seems not to), but we need to be wary and should perform extra-fastidious physical examinations followed by imaging studies when our patients complain of any localizing symptoms that are not readily and completely explained.
At the end of a tumultuous and divisive year, we at the Journal send to you, our readers, our heartfelt wishes for personal tranquility and for a universally peaceful and harmonious 2017.
- Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 2016 Jul 12. pii: annrheumdis-2016-209773. doi: 10.1136/annrheumdis-2016-209773. [Epub ahead of print]
- Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 2016 Jul 12. pii: annrheumdis-2016-209773. doi: 10.1136/annrheumdis-2016-209773. [Epub ahead of print]
Phlegmasia cerulea dolens from radiation-induced venous stenosis
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
A 77-year-old man presented with a 5-day history of painful swelling of his right leg. He reported no trauma, no recent surgery, no history of thrombophilic disorder, and no prolonged immobilization. However, he had a history of prostate cancer, treated 10 years earlier with pelvic radiation.
Examination revealed massive right leg swelling extending from the thigh to the ankle, along with bluish-red skin discoloration (Figure 1). Doppler ultrasonography demonstrated acute thrombosis involving the right iliofemoral veins. These findings were consistent with phlegmasia cerulea dolens.
Urgent percutaneous catheter-directed thrombolysis was performed. Venography revealed extensive thrombosis of the femoral vein (Figure 2A) extending into the right external iliac vein. This was treated with catheter-directed pharmacomechanical thrombectomy.
Venography after this procedure showed significant improvement in venous blood flow (Figure 2B). However, stenosis of the right external iliac vein was also noted (Figure 2C) and was treated with balloon angioplasty (Figure 2D) followed by placement of a stent (14 × 40 mm).
In the immediate postprocedural period, there was marked reduction in swelling and normalization of skin color (Figure 3). The patient did not experience significant bleeding during or after the procedure. Treatment with intravenous unfractionated heparin was continued during the hospital stay, and he was discharged on warfarin with a therapeutic international normalized ratio. At a follow-up visit 3 months later, he was asymptomatic.
A RARE BUT SEVERE TYPE OF ACUTE DEEP VEIN THROMBOSIS
Phlegmasia cerulea dolens (painful cyanotic swollen leg) is a rare and severe form of acute deep vein thrombosis (DVT) characterized by marked limb pain, swelling, and blue discoloration.1 DVT is the most common cause of acute-onset unilateral leg pain, swelling, and skin discoloration.2
The differential diagnosis
The differential diagnosis includes infection (cellulitis, necrotizing fasciitis), compartment syndrome from limb injury, musculoskeletal conditions such as ruptured Baker cyst, venous stasis due to external compression (May-Thurner syndrome, iliac vein compression syndrome, pelvic tumor), acute limb ischemia from arterial obstruction, and complex regional pain syndrome (reflex sympathetic dystrophy).
Management recommendations
As in most cases of DVT, initial treatment of phlegmasia cerulea dolens involves systemic anticoagulation with heparin, elevation of the affected extremity, and fluid resuscitation if the patient is hypotensive. However, phlegmasia cerulea dolens is a major indication for catheter-directed thrombolysis,3,4 so an urgent vascular surgery or interventional cardiology consultation is also required. The American College of Chest Physicians recommends catheter-directed thrombolysis for acute DVT of the iliofemoral veins in patients with symptoms for less than 14 days, good functional capacity, and a life expectancy beyond 1 year.5 This intervention results in reduced incidence of postthrombotic syndrome and improved quality of life5,6 compared with anticoagulation therapy alone.
Who is at risk?
Risk factors for phlegmasia cerulea dolens include a history of malignancy, inherited or acquired thrombophilia, surgery, radiation therapy, trauma, placement of an inferior vena cava filter, and pregnancy. In our patient, the iliac vein stenosis most likely was the result of the radiation therapy he had undergone for prostate cancer.
Arterial stenosis is a well-known complication of radiation therapy and is associated with an increased risk of cardiovascular events.7,8 Radiation induces endothelial damage followed by proliferation of smooth muscle cells, resulting in luminal stenosis and thrombosis. At the cellular level, radiation leads to an acute increase in pro-inflammatory cytokines and endothelial adhesion molecules, causing the recruitment of inflammatory cells to radiation-exposed vessels and chronic activation of transcription factor NF-kappa B, leading to long-term inflammation and angiogenesis.9
Carotid, coronary, and iliac artery stenosis are known to occur around 10 years after radiation therapy to the head, neck, breast, and pelvis. Radiation-induced iliac vein stenosis is rare and can manifest as acute proximal DVT.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Ely JW, Osheroff JA, Chambliss ML, Ebell MH. Approach to leg edema of unclear etiology. J Am Board Fam Med 2006; 19:148–160.
- Casey ET, Murad MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55:1463–1473.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):454S–545S.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375.
- Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol 2010; 55:1237–1239.
- Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 2010; 55:1227–1236.
Abdominal pain under immunosuppressive conditions
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.
Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.
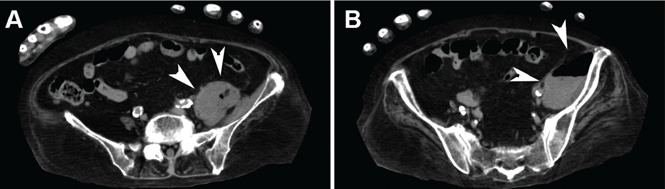
EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.

Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.

EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
A 69-year-old diabetic woman with stage 4 non–small-cell lung cancer presented with a 3-day history of abdominal pain and loss of appetite. She was being treated with corticosteroids for a brain metastasis.
Computed tomography (CT) (Figure 1) revealed air within the bladder wall and lumen; diffuse air in the intraperitoneum and retroperitoneum; air distributed from the left iliopsoas muscle to the left femur that spread around the obturator muscle; air in the left ureter; and an abscess in the psoas major muscle extending to the ala of the ilium. A diagnosis of emphysematous cystitis complicated by extensive abdominal emphysema and abscess was made.

Blood cultures were negative, but urine cultures grew extended-spectrum beta-lactamase-producing Escherichia coli, which was sensitive to meropenem. Meropenem was given intravenously for 24 days and was stopped when levels of inflammatory markers improved and urine cultures were negative. However, on day 29, the patient developed a fever. Follow-up CT showed that the abscess in the psoas muscle had enlarged (Figure 2). We chose not to surgically drain the abscess because the patient had terminal lung cancer. The patient expired 6 days later, 35 days after her hospital admission.

EMPHYSEMATOUS CYSTITIS ASSOCIATED WITH A PSOAS MUSCLE ABSCESS
Emphysematous cystitis is an uncommon urinary tract infection characterized by air within the bladder wall and lumen that is caused by gas-producing pathogens.1,2 The disease is often found in elderly diabetic women. Treatment of emphysematous cystitis typically includes intravenous antibiotics, adequate bladder drainage, and, for diabetic patients, appropriate glycemic control.
Psoas muscle abscess is a collection of pus in the retroperitoneal space.3 It can be primary, caused by hematogenous spread from the site of an occult infection, or secondary, caused by contiguous spread from adjacent infected organs, including those of the urinary tract. Psoas muscle abscess associated with emphysematous cystitis, as in our patient, is rare. We have seen only one other report in the medical literature.4
TREATMENT
The treatment of psoas muscle abscess involves the use of broad-spectrum antibiotics and drainage.5 Small abscesses (less than 3.5 cm) can be controlled with antibiotics alone. Image-guided percutaneous drainage is a safe, minimally invasive option. Surgery is indicated for unsuccessful percutaneous drainage, loculated abscesses, and abscesses difficult to approach percutaneously, or when the underlying disease requires definitive surgical management.
As in our patient, the presence of additional comorbid immunosuppressive conditions2 such as lung cancer and treatment with corticosteroids can allow the infection to become widespread and life-threatening.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100:17–20.
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86:47–53.
- Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004; 80:459–462.
- Choi JK, Kwon JC. Bilateral psoas muscle abscess associated with emphysematous cystitis. Case Rep Med 2015; 2015:285652.
- Tabrizian P, Nguyen SQ, Greenstein A, Rajhbeharrysingh U, Divino CM. Management and treatment of iliopsoas abscess. Arch Surg 2009; 144:946–949.
Taurine, energy drinks, and neuroendocrine effects
Taurine—an amino acid found in abundance in the human brain, retina, heart, and reproductive organs, as well as in meat and seafood—is also a major ingredient in “energy drinks” (Table 1).1,2 Given the tremendous popularity of these drinks in the United States, it would seem important to know and to recognize taurine’s neuroendocrine effects. Unfortunately, little is known about the effects of taurine supplementation in humans.
This paper reviews the sparse data to provide clinicians some background on the structure, synthesis, distribution, metabolism, mechanisms, effects, safety, and proposed therapeutic targets of taurine.
TAURINE’S THERAPEUTIC POTENTIAL
Taurine has been reported to have widespread anti-inflammatory actions.3,4 Taurine supplementation has been proposed to have beneficial effects in the treatment of epilepsy,5 heart failure,6,7 cystic fibrosis,8 and diabetes9 and has been shown in animal studies to protect against neurotoxic insults from alcohol, ammonia, lead, and other substances.10–16
In addition, taurine analogues such as homotaurine and N-acetyl-homotaurine (acamprosate) have been probed for possible therapeutic applications. Homotaurine has been shown to have antiamyloid activity that could in theory protect against the progression of Alzheimer disease,17 and acamprosate is approved by the US Food and Drug Administration (FDA) for the treatment of alcohol use disorders.18
TAURINE CONSUMPTION
Energy drinks are widely consumed in the United States, with an estimated 354 million gallons sold in 2009, or approximately 5.25 L/year per person over age 10.1 In 2012, US sales of energy drinks exceeded $12 billion,19 with young men, particularly those in the military deployed in war zones, being the biggest consumers.20–22 Analyses have found that of 49 nonalcoholic energy drinks tested, the average concentration of taurine was 3,180 mg/L, or approximately 750 mg per 8-oz serving.23,24 Popular brands include Red Bull, Monster, Rockstar (Table 1), NOS, Amp, and Full Throttle.
Taurine is plentiful in the human body, which contains up to 1 g of taurine per kg.25 Foods such as poultry, beef, pork, seafood, and processed meats have a high taurine content (Table 2).26–29 People who eat meat and seafood have plentiful taurine intake, whereas vegetarians and vegans consume much less and have significantly lower circulating levels30 because plants do not contain taurine in appreciable amounts.26,29
The typical American diet provides between 123 and 178 mg of taurine daily.26 Consumption of one 8-oz energy drink can increase the average intake 6 to 16 times. A lacto-ovo vegetarian diet provides only about 17 mg of taurine daily, and an 8-oz energy drink can increase the average intake by 44 to 117 mg.26 And since a vegan diet provides essentially no taurine,30 energy drink intake in any amount would constitute a major relative increase in taurine consumption.
ATTEMPTS TO STUDY TAURINE'S EFFECTS
Since most clinical trials to date have looked at the effects of taurine in combination with other ingredients such as caffeine, creatine, and glucose31–35 in drinks such as Red Bull, these studies cannot be used to determine the effects of taurine alone. In the few clinical trials that have tested isolated taurine consumption, data are not sufficient to make a conclusion on direct effects on energy metabolism.
Rutherford et al36 tested the effect of oral taurine supplementation (1,660 mg) on endurance in trained male cyclists 1 hour before exercise, but observed no effect on fluid intake, heart rate, subjective exertion, or time-trial performance. A small increase (16%) in total fat oxidation was observed during the 90-minute exercise period. Since mitochondria are the main location of fatty acid degradation, this effect may be attributed to taurine supplementation, with subsequent improvement in mitochondrial function.
Zhang et al37 found a 30-second increase in cycling energy capacity after 7 days of 6 g oral taurine supplementation, but the study was neither blinded nor placebo-controlled.
Kammerer et al38 tested the effect of 1 g of taurine supplementation on physical and mental performance in young adult soldiers 45 minutes before physical fitness and cognitive testing. This double-blind, placebo-controlled randomized trial found no effect of taurine on cardiorespiratory fitness indices, concentration, or immediate memory, nor did it find any effect of an 80-mg dose of caffeine.
In sum, the available data are far from sufficient to determine the direct effect of taurine consumption on energy metabolism in healthy people.
PHARMACOLOGY OF TAURINE
Chemical structure
Taurine, or 2-aminoethane sulfonic acid, is a conditionally essential amino acid, ie, we can usually make enough in our own bodies. It was first prepared on a large scale for physiologic investigation almost 90 years ago, through the purification of ox bile.39 It can be obtained either exogenously through dietary sources or endogenously through biosynthesis from methionine and cysteine precursors, both essential sulfur-containing alpha-amino acids.40 Both sources are important to maintain physiologic levels of taurine, and either can help compensate for the other in cases of deficiency.41
The structure of taurine has two main differences from the essential amino acids. First, taurine’s amino group is attached to the beta-carbon rather than the alpha-carbon, making it a beta-amino acid instead of an alpha-amino acid.42 Second, the acid group in taurine is sulfonic acid, whereas the essential amino acids have a carboxylic acid.43 Because of its distinctive structure, taurine is not used as a structural unit in proteins,43 existing mostly as a free amino acid within cells, readily positioned to perform several unique functions.
Synthesis
De novo synthesis of taurine involves several enzymes and at least five pathways,44 mostly differing by the order in which sulfur is oxidized and decarboxylated.45
The rate-limiting enzyme of the predominant pathway is thought to be cysteine sulfinate decarboxylase (CSD), and its presence within an organ indicates involvement in taurine production.44 CSD has been found in the liver,46 the primary site of taurine biosynthesis, as well as in the retina,47 brain,48 kidney,49 mammary glands,50,51 and reproductive organs.52
Distribution
Taurine levels are highest in electrically excitable tissues such as the central nervous system, retina, and heart; in secretory structures such as the pineal gland and the pituitary gland (including the posterior lobe or neurohypophysis); and in platelets25 and neutrophils.53
In the fetal brain, the taurine concentration is higher than that of any other amino acid,54 but the concentration in the brain decreases with advancing age, whereas glutamate levels increase over time to make it the predominant amino acid in the adult brain.54 Regardless, taurine is still the second most prevalent amino acid in the adult brain, its levels comparable to those of gamma-aminobutyric acid (GABA).55
Taurine has also been found in variable amounts in the liver, muscle, kidney, pancreas, spleen, small intestine, and lungs,56 as well as in several other locations.45,57
Taurine is also present in the male and female reproductive organs. In male rats, taurine and taurine biosynthesis have been localized to Leydig cells of the testes, the cellular source of testosterone in males, as well as the cremaster muscle, efferent ducts, and peritubular myoid cells surrounding seminiferous tubules.58 More recently, taurine has been detected in the testes of humans59 and is also found in sperm and seminal fluid.60 Levels of taurine in spermatozoa are correlated with sperm quality, presumably by protecting against lipid peroxidation through taurine’s antioxidant effects,61,62 as well as through contribution to the spermatozoa maturation process by facilitating the capacitation, motility, and acrosomal reaction of sperm.63
In female rats, taurine has been found in uterine tissue,64 oviducts,65 uterine fluid (where it is the predominant amino acid),66 and thecal cells of developing follicles of ovaries, cells responsible for the synthesis of androgens such as testosterone and androstenedione.65 Taurine is also a major component of human breast milk67 and is important for proper neonatal nutrition.68
Metabolism and excretion
Ninety-five percent of taurine is excreted in urine, about 70% as taurine itself, and the rest as sulfate. Most of the sulfate derived from taurine is produced by bacterial metabolism in the gut and then absorbed.69 However, taurine can also be conjugated with bile acids to act as a detergent in lipid emulsification.70 In this form, it may be subjected to the enterohepatic circulation, which gives bacteria another chance to convert it into inorganic sulfate for excretion in urine.69
MECHANISMS AND NEUROENDOCRINE EFFECTS
As a free amino acid, taurine has widespread distribution and unique biochemical and physiologic properties and exhibits several organ-specific functions; however, indisputable evidence of a taurine-specific receptor is lacking, and its putative existence71 is controversial.72 Nonetheless, taurine is a neuromodulator with a variety of actions.
Neurotransmission
Taurine is known to be an inhibitory neurotransmitter and neuromodulator.73 It is structurally analogous to GABA, the main inhibitory neurotransmitter in the brain.45 Accordingly, it binds to GABA receptors to serve as an agonist,74,75 causing neuronal hyperpolarization and inhibition. Taurine has an even higher affinity for glycine receptors75 where it has long been known to act as an agonist.76 GABA and glycine receptors both belong to the Cys-loop receptor superfamily,77 with conservation of subunits that allows taurine to bind each receptor, albeit at different affinities. The binding effects of taurine on GABA and glycine receptors have not been well documented quantitatively; however, it is known that taurine has a substantially lower affinity than GABA and glycine for their respective receptors.76
Catecholamines and the sympathetic nervous system
Surprisingly little is known about the effects of taurine on norepinephrine, dopamine, and the human sympathetic nervous system.78 Humans with borderline hypertension given 6 g of taurine orally for 7 days79 experienced decreases in epinephrine secretion and blood pressure, but normotensive study participants did not experience similar results, possibly because of a better ability to regulate sympathetic tone. Mizushima et al80 showed that a longer period of taurine intake (6 g orally for 3 weeks) could elicit a decrease in norepinephrine in healthy men with normal blood pressure. Other similar studies81–83 also suggested interplay between taurine and catecholamines, but the extent is still undetermined.
Growth hormone, prolactin, sex hormones, and cortisol
Taurine appears to have a complex relationship with several hormones, although its direct effects on hormone secretion remain obscure. Clinical studies of the acute and chronic neuroendocrine effects of taurine loading in humans are needed.
In female rats, secretion of prolactin is increased by the intraventricular injection of 5 μL of 2.0 μmol taurine over a 10-minute period.84 Ikuyama et al85 found an increase in prolactin and growth hormone secretion in adult male rats given 10 μL of 0.25 μmol and 1.0 μmol taurine intraventricularly, yet a higher dose of 4.0 μmol had no effect on either hormone. Furthermore, prolactin receptor deficiency is seen in CSD knockout mice, but the receptor is restored with taurine supplementation.86
Mantovani and DeVivo87 reported that 375 to 8,000 mg/day of taurine given orally for 4 to 6 months to epileptic patients stimulated the secretion of growth hormone. However, in another study, a single 75-mg/kg dose of oral taurine did not trigger an acute increase in levels of growth hormone or prolactin in humans.88 Energy drinks may contain up to 1,000 mg of taurine per 8-oz serving, but the effects of larger doses on growth hormone, which is banned as a supplement by major athletic organizations because of its anabolic and possible performance-enhancing effects, remain to be determined.
Taurine may have effects on human sex hormones, based on the limited observations in rodents.89–94
Although human salivary cortisol concentrations were purportedly assessed in response to 2,000 mg of oral taurine,95 the methods and reported data are not adequate to draw any conclusions.
Energy metabolism
Mammals are unable to directly use taurine in energy production because they cannot directly reduce it.25 Instead, bacteria in the gut use it as a source of energy, carbon, nitrogen, and sulfur.96 However, taurine deficiency appears to impair the cellular respiratory chain, resulting in diminished production of adenosine triphosphate and diminished uptake of long-chain fatty acids by mitochondria, at least in the heart.97
Taurine is present in human mitochondria and regulates mitochondrial function. For example, taurine in mitochondria assists in conjugation of transfer RNA for leucine, lysine, glutamate, and glutamine.98 In TauT knockout mice, deficiency of taurine causes mitochondrial dysfunction, triggering a greater than 80% decrease in exercise capacity.99 Several studies in rodents have shown increased exercise capacity after taurine supplementation.100–102 In addition, taurine is critical for the growth of blastocytes, skeletal muscle, and myocardium; it is necessary for mitochondrial development and is also important for muscular endurance.103,104
Antioxidation, anti-inflammation, and other functions
Taurine is a major antioxidant, scavenging reactive oxygen and protecting against oxidative stress to organs including the brain,97,105,106 where it increasingly appears to have neuroprotective effects.107,108
Cellular taurine also has anti-inflammatory actions.3 One of the proposed mechanisms is taurine inhibition of NF-kappa B, an important transcription factor for the synthesis of pro-inflammatory cytokines.4 This function may be important in protecting polyunsaturated fatty acids from oxidative stress—helping to maintain and stabilize the structure and function of plasma membranes within the lungs,109 heart,110 brain,111 liver,112 and spermatozoa.61,62
Taurine is also conjugated to bile acids synthesized in the liver, forming bile salts70 that act as detergents to help emulsify and digest lipids in the body. In addition, taurine facilitates xenobiotic detoxification in the liver by conjugating with several drugs to aid in their excretion.25 Taurine is also implicated in calcium modulation113 and homeostasis.114 Through inhibition of several types of calcium channels, taurine has been shown to decrease calcium influx into cells, effectively serving a cytoprotective role against calcium overload.115,116
TAURINE DEFICIENCY
Fetal and neonatal deficiency
Though taurine is considered nonessential in adults because it can be readily synthesized endogenously, it is thought to be conditionally essential in neonatal nutrition.68 It is the second most abundant free amino acid in human breast milk117 and the most abundant free amino acid in fetal brain.118 In cases of long-term parenteral nutrition, neonates can become drastically taurine deficient119 due to suboptimal CSD activity,118 leading to retinal dysfunction.41 Taurine deficiencies can lead to functional and structural brain damage.118 Moreover, maternal taurine deficiency results in neurologic abnormalities in offspring120 and may lead to oxidative stress throughout life.121
In 1984, the FDA approved the inclusion of taurine in infant formulas based on research showing that taurine-deficient infants had impaired fat absorption, bile acid secretion, retinal function, and hepatic function.122 But still under debate are the amount and duration of taurine supplementation required by preterm and low-birth-weight infants, as several randomized controlled trials failed to show statistically significant effects on growth.123 Nonetheless, given the alleged detrimental ramifications of a lack of taurine supplementation, as well as the ethical dilemma of performing additional research trials on infants, it is presumed that infant formulas and parenteral nutrition for preterm and low-birth-weight infants will continue to contain taurine.
Age- and disease-related deficiency
Although taurine deficiency is rare in neonates, it is perhaps inevitable with advancing age. Healthy elderly patients ages 61 to 81 have up to a 49% decrease in plasma taurine concentration compared with healthy individuals ages 27 to 57.124 While reduced renal retention125 and taurine intake126 can account for depressed taurine levels, Eppler and Dawson127 found that tissue and circulating taurine concentrations decrease over the human life span primarily due to an age-dependent depletion of CSD activity in the liver. This effectively impairs the biosynthesis of endogenous taurine from cysteine or methionine or both, forcing a greater reliance on exogenous sources.
While specific mechanisms have not been fully elucidated, taurine deficiency has also been identified in patients suffering from diseases including but not limited to disorders of bone (osteogenesis imperfecta, osteoporosis),128 blood (acute myelogenous leukemia),129 central nervous system (schizophrenia, Friedreich ataxia-spinocerebellar degeneration),130,131 retina (retinitis pigmentosa),132 circulatory system and heart (essential hypertension, atherosclerosis),133 digestion (Gaucher disease),134 absorption (short-bowel syndrome),135 cellular proliferation (cancer),136 and membrane channels (cystic fibrosis),137 as well as in patients restricted to long-term parenteral nutrition.138 However, the apparent correlation between taurine deficiency and these conditions does not necessarily mean causation; more study is needed to elucidate a direct connection.
SAFETY AND TOXICITY OF TAURINE SUPPLEMENTATION
An upper safe level of intake for taurine has not been established. To date, several studies have involved heavy taurine supplementation without serious adverse effects. While the largest dosage of taurine tested in humans appears to be 10 g/day for 6 months,139 a number of studies have used 1 to 6 g/day for periods of 1 week to 1 year.140 However, the assessment of potential acute, subacute, and chronic adverse effects has not been comprehensive. The Scientific Committee on Food of the European Commission141 reviewed several toxicologic studies on taurine through 2003 and were unable to expose any carcinogenic or teratogenic potential. Nevertheless, based on the available data from trials in humans and lower animals, Shao and Hathcock140 suggested an observed safe level of taurine of 3 g/day, a conservatively smaller dose that carries a higher level of confidence. Because there is no “observed adverse effect level” for daily taurine intake,141 more research must be done to ensure safety of higher amounts of taurine administration and to define a tolerable upper limit of intake.
Interactions with medications
To date, the literature is scarce regarding potential interactions between taurine and commonly used medications.
Although no evidence specifically links taurine with adverse effects when used concurrently with other medications, there may be a link between taurine supplementation and various cytochrome P450 systems responsible for hepatic drug metabolism. Specifically, taurine inhibits cytochrome P450 2E1, a highly conserved xenobiotic-metabolizing P450 responsible for the breakdown of more than 70 substrates, including several commonly used anesthetics, analgesics, antidepressants, antibacterials, and antiepileptics.142 Of note, taurine may contribute to the attenuation of oxidative stress in the liver in the presence of alcohol143 and acetaminophen,144 two substances frequently used and abused. Since the P450 2E1 system catalyzes comparable reactions in rodents and humans,142 rodents should plausibly serve as a model for further testing of the effects of taurine on various substrates.
POTENTIAL THERAPEUTIC APPLICATIONS
More analysis is needed to fully unlock and understand taurine’s potential value in healthcare.
Correction of late-life taurine decline in humans could be beneficial for cognitive performance, energy metabolism, sexual function, and vision, but clinical studies remain to be performed. While a decline in taurine with age may intensify the stress caused by reactive oxygen species, taurine supplementation has been shown to decrease the presence of oxidative markers127 and to serve a neuroprotective role in rodents.145,146 Taurine levels increase in the hippocampus after experimentally induced gliosis147 and are neuroprotective against glutamate excitotoxicity.148,149 Furthermore, data in Alzheimer disease, Huntington disease, and brain ischemia experimental models show that taurine inhibits neuronal death (apoptosis).13,150,151 Taurine has even been proposed as a potential preventive treatment for Alzheimer dementia, as it stabilizes protein conformations to prevent their aggregation and subsequent dysfunction.152 Although improvement in memory and cognitive performance has been linked to taurine supplementation in old mice,145,153 similar results have not been found in adult mice whose taurine levels are within normal limits. Taurine also has transient anticonvulsant effects in some epileptic humans.154
Within the male reproductive organs, the age-related decline in taurine may or may not have implications regarding sexuality, as only very limited rat data are available.89–91
In cats, taurine supplementation has been found to prevent the progressive degeneration of retinal photoreceptors seen in retinitis pigmentosa, a genetic disease that causes the loss of vision.155
While several energy drink companies have advertised that taurine plays a role in improving cognitive and physical performance, there are few human studies that examine this contention in the absence of confounding factors such as caffeine or glucose.36,37,95 Taurine supplementation in patients with heart failure has been shown to increase exercise capacity vs placebo.156 This supports the idea that in cases of taurine deficiency, such as those seen in cardiomyopathy,157 taurine supplementation could have restorative effects. However, we are not aware of any double-blind, placebo-controlled clinical trial of taurine alone in healthy patients that measured energy parameters as clinical outcomes.
Although it remains possible that acute supraphysiologic taurine levels achieved by supplementation could transiently trigger various psychoneuroendocrine responses in healthy people, clinical research is needed in which taurine is the sole intervention. At present, the most compelling clinical reason to prescribe or recommend taurine supplementation is taurine deficiency.
- US Food and Drug Administration (FDA). Caffeine intake by the US population. www.fda.gov/downloads/AboutFDA/ CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/UCM333191.pdf. Accessed October 4, 2016.
- McLellan TM, Lieberman HR. Do energy drinks contain active components other than caffeine? Nutr Rev 2012; 70:730–744.
- Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol 1993; 54:119–124.
- Kontny E, Szczepanska K, Kowalczewski J, et al. The mechanism of taurine chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheum 2000; 43:2169–2177.
- Barbeau A, Inoue N, Tsukada Y, Butterworth RF. The neuropharmacology of taurine. Life Sci 1975; 17:669–677.
- Kramer JH, Chovan JP, Schaffer SW. Effect of taurine on calcium paradox and ischemic heart failure. Am J Physiol 1981; 240:H238–H246.
- Azuma J, Sawamura A, Awata N, et al. Therapeutic effect of taurine in congestive heart failure: a double-blind crossover trial. Clin Cardiol 1985; 8:276–282.
- Darling PB, Lepage G, Leroy C, Masson P, Roy CC. Effect of taurine supplements on fat absorption in cystic fibrosis. Pediatr Res 1985; 19:578–582.
- Franconi F, Di Leo MA, Bennardini F, Ghirlanda G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res 2004; 29:143–150.
- Malcangio M, Bartolini A, Ghelardini C, et al. Effect of ICV taurine on the impairment of learning, convulsions and death caused by hypoxia. Psychopharmacology (Berl) 1989; 98:316–320.
- Rivas-Arancibia S, Dorado-Martínez C, Borgonio-Pérez G, et al. Effects of taurine on ozone-induced memory deficits and lipid peroxidation levels in brains of young, mature, and old rats. Environ Res 2000; 82:7–17.
- Vohra BP, Hui X. Improvement of impaired memory in mice by taurine. Neural Plast 2000; 7:245–259.
- Tadros MG, Khalifa AE, Abdel-Naim AB, Arafa HM. Neuroprotective effect of taurine in 3-nitropropionic acid-induced experimental animal model of Huntington’s disease phenotype. Pharmacol Biochem Behav 2005; 82:574–582.
- Zhu DM, Wang M, She JQ, Yu K, Ruan DY. Protection by a taurine supplemented diet from lead-induced deficits of long-term potentiation/depotentiation in dentate gyrus of rats in vivo. Neuroscience 2005; 134:215–224.
- Chepkova AN, Sergeeva OA, Haas HL. Taurine rescues hippocampal long-term potentiation from ammonia-induced impairment. Neurobiol Dis 2006; 23:512–521.
- Wang GH, Jiang ZL, Fan XJ, Zhang L, Li X, Ke KF. Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 2007; 52:1199–1209.
- Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, Trabucchi M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: a review. Aging Clin Exp Res 2012; 24:580–587.
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014; 311:1889–1900.
- Sorkin BC, Camp KM, Haggans CJ, et al. Executive summary of NIH workshop on the use and biology of energy drinks: current knowledge and critical gaps. Nutr Rev 2014; 72(suppl 1):1–8.
- Centers for Disease Control and Prevention (CDC). Energy drink consumption and its association with sleep problems among US service members on a combat deployment—Afghanistan, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:895–898.
- Bailey RL, Saldanha LG, Dwyer JT. Estimating caffeine intake from energy drinks and dietary supplements in the United States. Nutr Rev 2014; 72(suppl 1):9–13.
- Stephens MB, Attipoe S, Jones D, Ledford CJ, Deuster PA. Energy drink and energy shot use in the military. Nutr Rev 2014; 72(suppl 1):72–77.
- Triebel S, Sproll C, Reusch H, Godelmann R, Lachenmeier DW. Rapid analysis of taurine in energy drinks using amino acid analyzer and Fourier transform infrared (FTIR) spectroscopy as basis for toxicological evaluation. Amino Acids 2007; 33:451–457.
- Beverage Industry. 2013 state of the industry: energy drinks & shots. www.bevindustry.com/articles/86552-state-of-the-industry-energy-drinks-shots. Accessed October 4, 2016.
- Huxtable RJ. Physiological actions of taurine. Physiol Rev 1992; 72:101–163.
- Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. JPEN J Parenter Enteral Nutr 1990; 14:183–188.
- Manzi P, Pizzoferrato L. Taurine in milk and yoghurt marketed in Italy. Int J Food Sci Nutr 2013; 64:112–116.
- Dragnes BT, Larsen R, Ernstsen MH, Mæhre H, Elvevoll EO. Impact of processing on the taurine content in processed seafood and their corresponding unprocessed raw materials. Int J Food Sci Nutr 2009; 60:143–152.
- Laidlaw SA, Shultz TD, Cecchino JT, Kopple JD. Plasma and urine taurine levels in vegans. Am J Clin Nutr 1988; 47:660–663.
- Rana SK, Sanders TA. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br J Nutr 1986; 56:17–27.
- Alford C, Cox H, Wescott R. The effects of Red Bull energy drink on human performance and mood. Amino Acids 2001; 21:139–150.
- Hoffman JR, Ratamess NA, Ross R, Shanklin M, Kang J, Faigenbaum AD. Effect of a pre-exercise energy supplement on the acute hormonal response to resistance exercise. J Strength Cond Res 2008; 22:874–882.
- Ivy JL, Kammer L, Ding Z, et al. Improved cycling time-trial performance after ingestion of a caffeine energy drink. Int J Sport Nutr Exerc Metab 2009; 19:61–78.
- Gonzalez AM, Walsh AL, Ratamess NA, Kang J, Hoffman JR. Effect of a pre-workout energy supplement on acute multi-joint resistance exercise. J Sports Sci Med 2011; 10:261–266.
- Yeh TS, Chan KH, Hsu MC, Liu JF. Supplementation with soybean peptides, taurine, pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J Med Food 2011; 14:219–225.
- Rutherford JA, Spriet LL, Stellingwerff T. The effect of acute taurine ingestion on endurance performance and metabolism in well-trained cyclists. Int J Sport Nutr Exerc Metab 2010; 20:322–329.
- Zhang M, Izumi I, Kagamimori S, et al. Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids 2004; 26:203–207.
- Kammerer M, Jaramillo JA, García A, Calderón JC, Valbuena LH. Effects of energy drink major bioactive compounds on the performance of young adults in fitness and cognitive tests: a randomized controlled trial. J Int Soc Sports Nutr 2014; 11:44.
- Kermack WO, Slater RH. The preparation of taurine in considerable quantity. Biochem J 1927; 21:1065–1067.
- Huxtable RJ, Lippincott SE. Diet and biosynthesis as sources of taurine in the mouse. J Nutr 1982; 112:1003–1010.
- Ohkuma S, Tamura J, Kuriyama K. Roles of endogenous and exogenous taurine and glycine in the formation of conjugated bile acids: analyses using freshly isolated and primary cultured rat hepatocytes. Jpn J Pharmacol 1984; 35:347–358.
- Chapman GE, Greenwood CE. Taurine in nutrition and brain development. Nutrition Research 1988; 8:955–968.
- Andrews S, Schmidt CLA. Titration curves of taurine and cysteic acid. J Biol Chem 1927; 73:651–654.
- Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol 1989; 32:471–533.
- Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 1968; 48:424–511.
- Hope DB. Pyridoxal phosphate as the coenzyme of the mammalian decarboxylase for L-cysteine sulphinic and L-cysteic acids. Biochem J 1955; 59:497–500.
- Pasantes-Morales H, Lopez-Colome AM, Salceda R, Mandel P. Cysteine sulphinate decarboxylase in chick and rat retina during development. J Neurochem 1976; 27:1103–1106.
- Oertel WH, Schmechel DE, Weise VK, et al. Comparison of cysteine sulphinic acid decarboxylase isoenzymes and glutamic acid decarboxylase in rat liver and brain. Neuroscience 1981; 6:2701–2714.
- Reymond I, Bitoun M, Levillain O, Tappaz M. Regional expression and histological localization of cysteine sulfinate decarboxylase mRNA in the rat kidney. J Histochem Cytochem 2000; 48:1461–1468.
- Hu JM, Ikemura R, Chang KT, Suzuki M, Nishihara M, Takahashi M. Expression of cysteine sulfinate decarboxylase mRNA in rat mammary gland. J Vet Med Sci 2000; 62:829–834.
- Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 2007; 137:1887–1894.
- Li JH, Ling YQ, Fan JJ, Zhang XP, Cui S. Expression of cysteine sulfinate decarboxylase (CSD) in male reproductive organs of mice. Histochem Cell Biol 2006; 125:607–613.
- Green TR, Fellman JH, Eicher AL, Pratt KL. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta 1991; 1073:91–97.
- Lefauconnier JM, Portemer C, Chatagner F. Free amino acids and related substances in human glial tumours and in fetal brain: comparison with normal adult brain. Brain Res 1976; 117:105–113.
- Sturman JA, Rassin DK, Gaull GE. Taurine in development. Life Sci 1977; 21:1–22.
- Sturman JA. Taurine pool sizes in the rat: effects of vitamin B-6 deficiency and high taurine diet. J Nutr 1973; 103:1566–1580.
- Terauchi A, Nakazaw A, Johkura K, Yan L, Usuda N. Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids 1998; 15:151–160.
- Lobo MV, Alonso FJ, del Río RM. Immunohistochemical localization of taurine in the male reproductive organs of the rat. J Histochem Cytochem 2000; 48:313–320.
- Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod 2010; 25:847–852.
- Holmes RP, Goodman HO, Shihabi ZK, Jarow JP. The taurine and hypotaurine content of human semen. J Androl 1992; 13:289–292.
- Alvarez JG, Storey BT. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol Reprod 1983; 29:548–555.
- Das J, Ghosh J, Manna P, Sinha M, Sil PC. Taurine protects rat testes against NaAsO(2)-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 2009; 187:201–210.
- Mrsny RJ, Meizel S. Inhibition of hamster sperm Na+, K+-ATPase activity by taurine and hypotaurine. Life Sci 1985; 36:271–275.
- Phoenix J, Wray S. Changes in human and rat uterine phosphoethanolamine and taurine with pregnancy and parturition. Exp Physiol 1994; 79:601–604.
- Lobo MV, Alonso FJ, Latorre A, del Río RM. Immunohistochemical localization of taurine in the rat ovary, oviduct, and uterus. J Histochem Cytochem 2001; 49:1133–1142.
- Casslén BG. Free amino acids in human uterine fluid. Possible role of high taurine concentration. J Reprod Med 1987; 32:181–184.
- Pamblanco M, Portolés M, Paredes C, Ten A, Comín J. Free amino acids in preterm and term milk from mothers delivering appropriate- or small-for-gestational-age infants. Am J Clin Nutr 1989; 50:778–781.
- Rigo J, Senterre J. Is taurine essential for the neonates? Biol Neonate 1977; 32:73–76.
- Sturman JA, Hepner GW, Hofmann AF, Thomas PJ. Metabolism of [35S]taurine in man. J Nutr 1975; 105:1206–1214.
- Bremer J. Species differences in the conjugation of free bile acids with taurine and glycine. Biochem J 1956; 63:507–513.
- Wu JY, Tang XW, Tsai WH. Taurine receptor: kinetic analysis and pharmacological studies. Adv Exp Med Biol 1992; 315:263–268.
- Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis 2012; 18:2673–2686.
- Haas HL, Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res 1973; 52:399–402.
- Bureau MH, Olsen RW. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Eur J Pharmacol 1991; 207:9–16.
- Bhattarai JP, Park SJ, Chun SW, Cho DH, Han SK. Activation of synaptic and extrasynaptic glycine receptors by taurine in preoptic hypothalamic neurons. Neurosci Lett 2015; 608:51–56.
- Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S. Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Res 1988; 464:97–105.
- Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci 2010; 31:161–174.
- Fujita T, Sato Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J Clin Invest 1988; 82:993–997.
- Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987; 75:525–532.
- Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol 1996; 403:615–622.
- Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl 1984; 2:S563–S565.
- Fujita T, Sato Y, Ando K. Changes in cardiac and hypothalamic noradrenergic activity with taurine in DOCA-salt rats. Am J Physiol 1986; 251:H926–H933.
- Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension 1987; 9:81–87.
- Scheibel J, Elsasser T, Ondo JG. A neuromodulatory role for taurine in controlling prolactin secretion in female rats. Psychoneuroendocrinology 1981; 6:139–144.
- Ikuyama S, Okajima T, Kato K, Ibayashi H. Effect of taurine on growth hormone and prolactin secretion in rats: possible interaction with opioid peptidergic system. Life Sci 1988; 43:807–812.
- Park E, Park SY, Dobkin C, Schuller-Levis G. Development of a novel cysteine sulfinic acid decarboxylase knockout mouse: dietary taurine reduces neonatal mortality. J Amino Acids 2014; 2014:346809.
- Mantovani J, DeVivo DC. Effects of taurine on seizures and growth hormone release in epileptic patients. Arch Neurol 1979; 36:672–674.
- Carlson HE, Miglietta JT, Roginsky MS, Stegink LD. Stimulation of pituitary hormone secretion by neurotransmitter amino acids in humans. Metabolism 1989; 38:1179–1182.
- Yang J, Wu G, Feng Y, Lv Q, Lin S, Hu J. Effects of taurine on male reproduction in rats of different ages. J Biomed Sci 2010; 17(suppl 1):S9.
- Yang J, Lin S, Feng Y, Wu G, Hu J. Taurine enhances the sexual response and mating ability in aged male rats. Adv Exp Med Biol 2013; 776:347–355.
- Yang J, Zong X, Wu G, Lin S, Feng Y, Hu J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids 2015; 47:1549–1558.
- Scheibel J, Elsasser T, Ondo JG. Stimulation of LH and FSH secretion following intraventricular injection of cysteic acid but not taurine. Brain Res 1980; 201:99–106.
- Price MT, Olney JW, Mitchell MV, Fuller T, Cicero TJ. Luteinizing hormone releasing action of N-methyl aspartate is blocked by GABA or taurine but not by dopamine antagonists. Brain Res 1978; 158:461–465.
- Ma Q, Zhao J, Cao W, Liu J, Cui S. Estradiol decreases taurine level by reducing cysteine sulfinic acid decarboxylase via the estrogen receptor-a in female mice liver. Am J Physiol Gastrointest Liver Physiol 2015; 308:G277–G286.
- Giles GE, Mahoney CR, Brunyé TT, Gardony AL, Taylor HA, Kanarek RB. Differential cognitive effects of energy drink ingredients: caffeine, taurine, and glucose. Pharmacol Biochem Behav 2012; 102:569–577.
- Giehl TJ, Qoronfleh MW, Wilkinson BJ. Transport, nutritional and metabolic studies of taurine in staphylococci. J Gen Microbiol 1987; 133:849–856.
- Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids 2015 Oct 16. Epub ahead of print.
- Schaffer SW, Jong CJ, Ito T, Azuma J. Role of taurine in the pathologies of MELAS and MERRF. Amino Acids 2014; 46:47–56.
- Warskulat U, Heller-Stilb B, Oermann E, et al. Phenotype of the taurine transporter knockout mouse. Methods Enzymol 2007; 428:439–458.
- Yatabe Y, Miyakawa S, Miyazaki T, Matsuzaki Y, Ochiai N. Effects of taurine administration in rat skeletal muscles on exercise. J Orthop Sci 2003; 8:415–419.
- Miyazaki T, Matsuzaki Y, Ikegami T, et al. Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acids 2004; 27:291–298.
- Goodman CA, Horvath D, Stathis C, et al. Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. J Appl Physiol (1985) 2009; 107:144–154.
- Van Winkle LJ, Patel M, Wasserlauf HG, Dickinson HR, Campione AL. Osmotic regulation of taurine transport via system beta and novel processes in mouse preimplantation conceptuses. Biochim Biophys Acta 1994; 1191:244–255.
- Ito T, Kimura Y, Uozumi Y, et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 2008; 44:927–937.
- Aydın AF, Çoban J, Dogan-Ekici I, Betül-Kalaz E, Dogru-Abbasoglu S, Uysal M. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab Brain Dis 2016; 31:337–345.
- Nagai K, Fukuno S, Oda A, Konishi H. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs 2016; 27:17–23.
- Caletti G, Almeida FB, Agnes G, Nin MS, Barros HM, Gomez R. Antidepressant dose of taurine increases mRNA expression of GABAA receptor a2 subunit and BDNF in the hippocampus of diabetic rats. Behav Brain Res 2015; 283:11–55.
- Gu Y, Zhao Y, Qian K, Sun M. Taurine attenuates hippocampal and corpus callosum damage, and enhances neurological recovery after closed head injury in rats. Neuroscience 2015; 291:331–340.
- Banks MA, Porter DW, Pailes WH, Schwegler-Berry D, Martin WG, Castranova V. Taurine content of isolated rat alveolar type I cells. Comp Biochem Physiol B 1991; 100:795–799.
- Raschke P, Massoudy P, Becker BF. Taurine protects the heart from neutrophil-induced reperfusion injury. Free Radic Biol Med 1995; 19:461–471.
- Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids 2004; 27:91–96.
- Refik Mas M, Comert B, Oncu K, et al. The effect of taurine treatment on oxidative stress in experimental liver fibrosis. Hepatol Res 2004; 28:207–215.
- Sebring LA, Huxtable RJ. Taurine modulation of calcium binding to cardiac sarcolemma. J Pharmacol Exp Ther 1985; 232:445–451.
- Schaffer SW, Kramer J, Chovan JP. Regulation of calcium homeostasis in the heart by taurine. Fed Proc 1980; 39:2691–2694.
- Foos TM, Wu JY. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 2002; 27:21–26.
- Ito T, Schaffer S, Azuma J. The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids 2014; 46:111–119.
- Gaull GE. Taurine in pediatric nutrition: review and update. Pediatrics 1989; 83:433–442.
- Sturman JA, Rassin DK, Gaull GE. Taurine in developing rat brain: transfer of [35S] taurine to pups via the milk. Pediatr Res 1977; 11:28–33.
- Geggel HS, Ament ME, Heckenlively JR, Martin DA, Kopple JD. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N Engl J Med 1985; 312:142–146.
- Sturman JA, Gargano AD, Messing JM, Imaki H. Feline maternal taurine deficiency: effect on mother and offspring. J Nutr 1986; 116:655–667.
- Lerdweeraphon W, Wyss JM, Boonmars T, Roysommuti S. Perinatal taurine exposure affects adult oxidative stress. Am J Physiol Regul Integr Comp Physiol 2013; 305:R95–R97.
- Chesney RW, Helms RA, Christensen M, Budreau AM, Han X, Sturman JA. The role of taurine in infant nutrition. Adv Exp Med Biol 1998; 442:463–476.
- Verner A, Craig S, McGuire W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev 2007; 4:CD006072.
- Jeevanandam M, Young DH, Ramias L, Schiller WR. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am J Clin Nutr 1990; 51:1040–1045.
- Dawson R Jr, Eppler B, Patterson TA, Shih D, Liu S. The effects of taurine in a rodent model of aging. Adv Exp Med Biol 1996; 403:37–50.
- Han X, Budreau AM, Chesney RW. Molecular cloning and functional expression of an LLC-PK1 cell taurine transporter that is adaptively regulated by taurine. Adv Exp Med Biol 1998; 442:261–268.
- Eppler B, Dawson R Jr. Dietary taurine manipulations in aged male Fischer 344 rat tissue: taurine concentration, taurine biosynthesis, and oxidative markers. Biochem Pharmacol 2001; 62:29–39.
- D’Eufemia P, Finocchiaro R, Celli M, et al. Taurine deficiency in thalassemia major-induced osteoporosis treated with neridronate. Biomed Pharmacother 2010; 64:271–274.
- Muscaritoli M, Conversano L, Petti MC, et al. Plasma amino acid concentrations in patients with acute myelogenous leukemia. Nutrition 1999; 15:195–199.
- Do KQ, Lauer CJ, Schreiber W, et al. Gamma-glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem 1995; 65:2652–2662.
- Lemieux B, Giguère R, Shapcott D. Studies on the role of taurine in Friedreich’s ataxia. Can J Neurol Sci 1984; 11(suppl 4):610–615.
- Airaksinen EM, Oja SS, Marnela KM, Sihvola P. Taurine and other amino acids of platelets and plasma in retinitis pigmentosa. Ann Clin Res 1980; 12:52–54.
- Kohashi N, Katori R. Decrease of urinary taurine in essential hypertension. Jpn Heart J 1983; 24:91–102.
- vom Dahl S, Mönnighoff I, Häussinger D. Decrease of plasma taurine in Gaucher disease and its sustained correction during enzyme replacement therapy. Amino Acids 2000; 19:585–592.
- Schneider SM, Joly F, Gehrardt MF, et al. Taurine status and response to intravenous taurine supplementation in adults with short-bowel syndrome undergoing long-term parenteral nutrition: a pilot study. Br J Nutr 2006; 96:365–370.
- Gray GE, Landel AM, Meguid MM. Taurine-supplemented total parenteral nutrition and taurine status of malnourished cancer patients. Nutrition 1994; 10:11–15.
- Thompson GN. Assessment of taurine deficiency in cystic fibrosis. Clin Chim Acta 1988; 171:233–237.
- Cho KH, Kim ES, Chen JD. Taurine intake and excretion in patients undergoing long term enteral nutrition. Adv Exp Med Biol 2000; 483:605–612.
- Durelli L, Mutani R, Fassio F. The treatment of myotonia: evaluation of chronic oral taurine therapy. Neurology 1983; 33:599–603.
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol 2008; 50:376–399.
- European Commission; Scientific Committee on Food. Opinion on additional information on energy drinks. http://ec.europa.eu/food/fs/sc/scf/out169_en.pdf. Accessed October 4, 2016.
- Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther 2000; 25:165–175.
- Kerai MD, Waterfield CJ, Kenyon SH, Asker DS, Timbrell JA. Reversal of ethanol-induced hepatic steatosis and lipid peroxidation by taurine: a study in rats. Alcohol Alcohol 1999; 34:529–541.
- Das J, Ghosh J, Manna P, Sil PC. Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res 2010; 44:340–355.
- El Idrissi A, Shen CH, L’amoreaux WJ. Neuroprotective role of taurine during aging. Amino Acids 2013; 45:735–750.
- Gharibani P, Modi J, Menzie J, et al. Comparison between single and combined post-treatment with S-methyl-N,N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience 2015; 300:460–473.
- Junyent F, De Lemos L, Utrera J, et al. Content and traffic of taurine in hippocampal reactive astrocytes. Hippocampus 2011; 21:185–197.
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 1999; 19:9459–9468.
- Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res 2005; 1038:123–131.
- Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 2005; 49:1140–1148.
- Takatani T, Takahashi K, Uozumi Y, et al. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol 2004; 287:C949–C953.
- Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis 2010; 20(suppl 2):S439–S452.
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett 2008; 436:19–22.
- Oja SS, Saransaari P. Taurine and epilepsy. Epilepsy Res 2013; 104:187–194.
- Berson EL, Hayes KC, Rabin AR, Schmidt SY, Watson G. Retinal degeneration in cats fed casein. II. Supplementation with methionine, cysteine, or taurine. Invest Ophthalmol 1976; 15:52–58.
- Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 2011; 57:333–337.
- Eby G, Halcomb WW. Elimination of cardiac arrhythmias using oral taurine with l-arginine with case histories: hypothesis for nitric oxide stabilization of the sinus node. Med Hypotheses 2006; 67:1200–1204.
Taurine—an amino acid found in abundance in the human brain, retina, heart, and reproductive organs, as well as in meat and seafood—is also a major ingredient in “energy drinks” (Table 1).1,2 Given the tremendous popularity of these drinks in the United States, it would seem important to know and to recognize taurine’s neuroendocrine effects. Unfortunately, little is known about the effects of taurine supplementation in humans.
This paper reviews the sparse data to provide clinicians some background on the structure, synthesis, distribution, metabolism, mechanisms, effects, safety, and proposed therapeutic targets of taurine.
TAURINE’S THERAPEUTIC POTENTIAL
Taurine has been reported to have widespread anti-inflammatory actions.3,4 Taurine supplementation has been proposed to have beneficial effects in the treatment of epilepsy,5 heart failure,6,7 cystic fibrosis,8 and diabetes9 and has been shown in animal studies to protect against neurotoxic insults from alcohol, ammonia, lead, and other substances.10–16
In addition, taurine analogues such as homotaurine and N-acetyl-homotaurine (acamprosate) have been probed for possible therapeutic applications. Homotaurine has been shown to have antiamyloid activity that could in theory protect against the progression of Alzheimer disease,17 and acamprosate is approved by the US Food and Drug Administration (FDA) for the treatment of alcohol use disorders.18
TAURINE CONSUMPTION
Energy drinks are widely consumed in the United States, with an estimated 354 million gallons sold in 2009, or approximately 5.25 L/year per person over age 10.1 In 2012, US sales of energy drinks exceeded $12 billion,19 with young men, particularly those in the military deployed in war zones, being the biggest consumers.20–22 Analyses have found that of 49 nonalcoholic energy drinks tested, the average concentration of taurine was 3,180 mg/L, or approximately 750 mg per 8-oz serving.23,24 Popular brands include Red Bull, Monster, Rockstar (Table 1), NOS, Amp, and Full Throttle.
Taurine is plentiful in the human body, which contains up to 1 g of taurine per kg.25 Foods such as poultry, beef, pork, seafood, and processed meats have a high taurine content (Table 2).26–29 People who eat meat and seafood have plentiful taurine intake, whereas vegetarians and vegans consume much less and have significantly lower circulating levels30 because plants do not contain taurine in appreciable amounts.26,29
The typical American diet provides between 123 and 178 mg of taurine daily.26 Consumption of one 8-oz energy drink can increase the average intake 6 to 16 times. A lacto-ovo vegetarian diet provides only about 17 mg of taurine daily, and an 8-oz energy drink can increase the average intake by 44 to 117 mg.26 And since a vegan diet provides essentially no taurine,30 energy drink intake in any amount would constitute a major relative increase in taurine consumption.
ATTEMPTS TO STUDY TAURINE'S EFFECTS
Since most clinical trials to date have looked at the effects of taurine in combination with other ingredients such as caffeine, creatine, and glucose31–35 in drinks such as Red Bull, these studies cannot be used to determine the effects of taurine alone. In the few clinical trials that have tested isolated taurine consumption, data are not sufficient to make a conclusion on direct effects on energy metabolism.
Rutherford et al36 tested the effect of oral taurine supplementation (1,660 mg) on endurance in trained male cyclists 1 hour before exercise, but observed no effect on fluid intake, heart rate, subjective exertion, or time-trial performance. A small increase (16%) in total fat oxidation was observed during the 90-minute exercise period. Since mitochondria are the main location of fatty acid degradation, this effect may be attributed to taurine supplementation, with subsequent improvement in mitochondrial function.
Zhang et al37 found a 30-second increase in cycling energy capacity after 7 days of 6 g oral taurine supplementation, but the study was neither blinded nor placebo-controlled.
Kammerer et al38 tested the effect of 1 g of taurine supplementation on physical and mental performance in young adult soldiers 45 minutes before physical fitness and cognitive testing. This double-blind, placebo-controlled randomized trial found no effect of taurine on cardiorespiratory fitness indices, concentration, or immediate memory, nor did it find any effect of an 80-mg dose of caffeine.
In sum, the available data are far from sufficient to determine the direct effect of taurine consumption on energy metabolism in healthy people.
PHARMACOLOGY OF TAURINE
Chemical structure
Taurine, or 2-aminoethane sulfonic acid, is a conditionally essential amino acid, ie, we can usually make enough in our own bodies. It was first prepared on a large scale for physiologic investigation almost 90 years ago, through the purification of ox bile.39 It can be obtained either exogenously through dietary sources or endogenously through biosynthesis from methionine and cysteine precursors, both essential sulfur-containing alpha-amino acids.40 Both sources are important to maintain physiologic levels of taurine, and either can help compensate for the other in cases of deficiency.41
The structure of taurine has two main differences from the essential amino acids. First, taurine’s amino group is attached to the beta-carbon rather than the alpha-carbon, making it a beta-amino acid instead of an alpha-amino acid.42 Second, the acid group in taurine is sulfonic acid, whereas the essential amino acids have a carboxylic acid.43 Because of its distinctive structure, taurine is not used as a structural unit in proteins,43 existing mostly as a free amino acid within cells, readily positioned to perform several unique functions.
Synthesis
De novo synthesis of taurine involves several enzymes and at least five pathways,44 mostly differing by the order in which sulfur is oxidized and decarboxylated.45
The rate-limiting enzyme of the predominant pathway is thought to be cysteine sulfinate decarboxylase (CSD), and its presence within an organ indicates involvement in taurine production.44 CSD has been found in the liver,46 the primary site of taurine biosynthesis, as well as in the retina,47 brain,48 kidney,49 mammary glands,50,51 and reproductive organs.52
Distribution
Taurine levels are highest in electrically excitable tissues such as the central nervous system, retina, and heart; in secretory structures such as the pineal gland and the pituitary gland (including the posterior lobe or neurohypophysis); and in platelets25 and neutrophils.53
In the fetal brain, the taurine concentration is higher than that of any other amino acid,54 but the concentration in the brain decreases with advancing age, whereas glutamate levels increase over time to make it the predominant amino acid in the adult brain.54 Regardless, taurine is still the second most prevalent amino acid in the adult brain, its levels comparable to those of gamma-aminobutyric acid (GABA).55
Taurine has also been found in variable amounts in the liver, muscle, kidney, pancreas, spleen, small intestine, and lungs,56 as well as in several other locations.45,57
Taurine is also present in the male and female reproductive organs. In male rats, taurine and taurine biosynthesis have been localized to Leydig cells of the testes, the cellular source of testosterone in males, as well as the cremaster muscle, efferent ducts, and peritubular myoid cells surrounding seminiferous tubules.58 More recently, taurine has been detected in the testes of humans59 and is also found in sperm and seminal fluid.60 Levels of taurine in spermatozoa are correlated with sperm quality, presumably by protecting against lipid peroxidation through taurine’s antioxidant effects,61,62 as well as through contribution to the spermatozoa maturation process by facilitating the capacitation, motility, and acrosomal reaction of sperm.63
In female rats, taurine has been found in uterine tissue,64 oviducts,65 uterine fluid (where it is the predominant amino acid),66 and thecal cells of developing follicles of ovaries, cells responsible for the synthesis of androgens such as testosterone and androstenedione.65 Taurine is also a major component of human breast milk67 and is important for proper neonatal nutrition.68
Metabolism and excretion
Ninety-five percent of taurine is excreted in urine, about 70% as taurine itself, and the rest as sulfate. Most of the sulfate derived from taurine is produced by bacterial metabolism in the gut and then absorbed.69 However, taurine can also be conjugated with bile acids to act as a detergent in lipid emulsification.70 In this form, it may be subjected to the enterohepatic circulation, which gives bacteria another chance to convert it into inorganic sulfate for excretion in urine.69
MECHANISMS AND NEUROENDOCRINE EFFECTS
As a free amino acid, taurine has widespread distribution and unique biochemical and physiologic properties and exhibits several organ-specific functions; however, indisputable evidence of a taurine-specific receptor is lacking, and its putative existence71 is controversial.72 Nonetheless, taurine is a neuromodulator with a variety of actions.
Neurotransmission
Taurine is known to be an inhibitory neurotransmitter and neuromodulator.73 It is structurally analogous to GABA, the main inhibitory neurotransmitter in the brain.45 Accordingly, it binds to GABA receptors to serve as an agonist,74,75 causing neuronal hyperpolarization and inhibition. Taurine has an even higher affinity for glycine receptors75 where it has long been known to act as an agonist.76 GABA and glycine receptors both belong to the Cys-loop receptor superfamily,77 with conservation of subunits that allows taurine to bind each receptor, albeit at different affinities. The binding effects of taurine on GABA and glycine receptors have not been well documented quantitatively; however, it is known that taurine has a substantially lower affinity than GABA and glycine for their respective receptors.76
Catecholamines and the sympathetic nervous system
Surprisingly little is known about the effects of taurine on norepinephrine, dopamine, and the human sympathetic nervous system.78 Humans with borderline hypertension given 6 g of taurine orally for 7 days79 experienced decreases in epinephrine secretion and blood pressure, but normotensive study participants did not experience similar results, possibly because of a better ability to regulate sympathetic tone. Mizushima et al80 showed that a longer period of taurine intake (6 g orally for 3 weeks) could elicit a decrease in norepinephrine in healthy men with normal blood pressure. Other similar studies81–83 also suggested interplay between taurine and catecholamines, but the extent is still undetermined.
Growth hormone, prolactin, sex hormones, and cortisol
Taurine appears to have a complex relationship with several hormones, although its direct effects on hormone secretion remain obscure. Clinical studies of the acute and chronic neuroendocrine effects of taurine loading in humans are needed.
In female rats, secretion of prolactin is increased by the intraventricular injection of 5 μL of 2.0 μmol taurine over a 10-minute period.84 Ikuyama et al85 found an increase in prolactin and growth hormone secretion in adult male rats given 10 μL of 0.25 μmol and 1.0 μmol taurine intraventricularly, yet a higher dose of 4.0 μmol had no effect on either hormone. Furthermore, prolactin receptor deficiency is seen in CSD knockout mice, but the receptor is restored with taurine supplementation.86
Mantovani and DeVivo87 reported that 375 to 8,000 mg/day of taurine given orally for 4 to 6 months to epileptic patients stimulated the secretion of growth hormone. However, in another study, a single 75-mg/kg dose of oral taurine did not trigger an acute increase in levels of growth hormone or prolactin in humans.88 Energy drinks may contain up to 1,000 mg of taurine per 8-oz serving, but the effects of larger doses on growth hormone, which is banned as a supplement by major athletic organizations because of its anabolic and possible performance-enhancing effects, remain to be determined.
Taurine may have effects on human sex hormones, based on the limited observations in rodents.89–94
Although human salivary cortisol concentrations were purportedly assessed in response to 2,000 mg of oral taurine,95 the methods and reported data are not adequate to draw any conclusions.
Energy metabolism
Mammals are unable to directly use taurine in energy production because they cannot directly reduce it.25 Instead, bacteria in the gut use it as a source of energy, carbon, nitrogen, and sulfur.96 However, taurine deficiency appears to impair the cellular respiratory chain, resulting in diminished production of adenosine triphosphate and diminished uptake of long-chain fatty acids by mitochondria, at least in the heart.97
Taurine is present in human mitochondria and regulates mitochondrial function. For example, taurine in mitochondria assists in conjugation of transfer RNA for leucine, lysine, glutamate, and glutamine.98 In TauT knockout mice, deficiency of taurine causes mitochondrial dysfunction, triggering a greater than 80% decrease in exercise capacity.99 Several studies in rodents have shown increased exercise capacity after taurine supplementation.100–102 In addition, taurine is critical for the growth of blastocytes, skeletal muscle, and myocardium; it is necessary for mitochondrial development and is also important for muscular endurance.103,104
Antioxidation, anti-inflammation, and other functions
Taurine is a major antioxidant, scavenging reactive oxygen and protecting against oxidative stress to organs including the brain,97,105,106 where it increasingly appears to have neuroprotective effects.107,108
Cellular taurine also has anti-inflammatory actions.3 One of the proposed mechanisms is taurine inhibition of NF-kappa B, an important transcription factor for the synthesis of pro-inflammatory cytokines.4 This function may be important in protecting polyunsaturated fatty acids from oxidative stress—helping to maintain and stabilize the structure and function of plasma membranes within the lungs,109 heart,110 brain,111 liver,112 and spermatozoa.61,62
Taurine is also conjugated to bile acids synthesized in the liver, forming bile salts70 that act as detergents to help emulsify and digest lipids in the body. In addition, taurine facilitates xenobiotic detoxification in the liver by conjugating with several drugs to aid in their excretion.25 Taurine is also implicated in calcium modulation113 and homeostasis.114 Through inhibition of several types of calcium channels, taurine has been shown to decrease calcium influx into cells, effectively serving a cytoprotective role against calcium overload.115,116
TAURINE DEFICIENCY
Fetal and neonatal deficiency
Though taurine is considered nonessential in adults because it can be readily synthesized endogenously, it is thought to be conditionally essential in neonatal nutrition.68 It is the second most abundant free amino acid in human breast milk117 and the most abundant free amino acid in fetal brain.118 In cases of long-term parenteral nutrition, neonates can become drastically taurine deficient119 due to suboptimal CSD activity,118 leading to retinal dysfunction.41 Taurine deficiencies can lead to functional and structural brain damage.118 Moreover, maternal taurine deficiency results in neurologic abnormalities in offspring120 and may lead to oxidative stress throughout life.121
In 1984, the FDA approved the inclusion of taurine in infant formulas based on research showing that taurine-deficient infants had impaired fat absorption, bile acid secretion, retinal function, and hepatic function.122 But still under debate are the amount and duration of taurine supplementation required by preterm and low-birth-weight infants, as several randomized controlled trials failed to show statistically significant effects on growth.123 Nonetheless, given the alleged detrimental ramifications of a lack of taurine supplementation, as well as the ethical dilemma of performing additional research trials on infants, it is presumed that infant formulas and parenteral nutrition for preterm and low-birth-weight infants will continue to contain taurine.
Age- and disease-related deficiency
Although taurine deficiency is rare in neonates, it is perhaps inevitable with advancing age. Healthy elderly patients ages 61 to 81 have up to a 49% decrease in plasma taurine concentration compared with healthy individuals ages 27 to 57.124 While reduced renal retention125 and taurine intake126 can account for depressed taurine levels, Eppler and Dawson127 found that tissue and circulating taurine concentrations decrease over the human life span primarily due to an age-dependent depletion of CSD activity in the liver. This effectively impairs the biosynthesis of endogenous taurine from cysteine or methionine or both, forcing a greater reliance on exogenous sources.
While specific mechanisms have not been fully elucidated, taurine deficiency has also been identified in patients suffering from diseases including but not limited to disorders of bone (osteogenesis imperfecta, osteoporosis),128 blood (acute myelogenous leukemia),129 central nervous system (schizophrenia, Friedreich ataxia-spinocerebellar degeneration),130,131 retina (retinitis pigmentosa),132 circulatory system and heart (essential hypertension, atherosclerosis),133 digestion (Gaucher disease),134 absorption (short-bowel syndrome),135 cellular proliferation (cancer),136 and membrane channels (cystic fibrosis),137 as well as in patients restricted to long-term parenteral nutrition.138 However, the apparent correlation between taurine deficiency and these conditions does not necessarily mean causation; more study is needed to elucidate a direct connection.
SAFETY AND TOXICITY OF TAURINE SUPPLEMENTATION
An upper safe level of intake for taurine has not been established. To date, several studies have involved heavy taurine supplementation without serious adverse effects. While the largest dosage of taurine tested in humans appears to be 10 g/day for 6 months,139 a number of studies have used 1 to 6 g/day for periods of 1 week to 1 year.140 However, the assessment of potential acute, subacute, and chronic adverse effects has not been comprehensive. The Scientific Committee on Food of the European Commission141 reviewed several toxicologic studies on taurine through 2003 and were unable to expose any carcinogenic or teratogenic potential. Nevertheless, based on the available data from trials in humans and lower animals, Shao and Hathcock140 suggested an observed safe level of taurine of 3 g/day, a conservatively smaller dose that carries a higher level of confidence. Because there is no “observed adverse effect level” for daily taurine intake,141 more research must be done to ensure safety of higher amounts of taurine administration and to define a tolerable upper limit of intake.
Interactions with medications
To date, the literature is scarce regarding potential interactions between taurine and commonly used medications.
Although no evidence specifically links taurine with adverse effects when used concurrently with other medications, there may be a link between taurine supplementation and various cytochrome P450 systems responsible for hepatic drug metabolism. Specifically, taurine inhibits cytochrome P450 2E1, a highly conserved xenobiotic-metabolizing P450 responsible for the breakdown of more than 70 substrates, including several commonly used anesthetics, analgesics, antidepressants, antibacterials, and antiepileptics.142 Of note, taurine may contribute to the attenuation of oxidative stress in the liver in the presence of alcohol143 and acetaminophen,144 two substances frequently used and abused. Since the P450 2E1 system catalyzes comparable reactions in rodents and humans,142 rodents should plausibly serve as a model for further testing of the effects of taurine on various substrates.
POTENTIAL THERAPEUTIC APPLICATIONS
More analysis is needed to fully unlock and understand taurine’s potential value in healthcare.
Correction of late-life taurine decline in humans could be beneficial for cognitive performance, energy metabolism, sexual function, and vision, but clinical studies remain to be performed. While a decline in taurine with age may intensify the stress caused by reactive oxygen species, taurine supplementation has been shown to decrease the presence of oxidative markers127 and to serve a neuroprotective role in rodents.145,146 Taurine levels increase in the hippocampus after experimentally induced gliosis147 and are neuroprotective against glutamate excitotoxicity.148,149 Furthermore, data in Alzheimer disease, Huntington disease, and brain ischemia experimental models show that taurine inhibits neuronal death (apoptosis).13,150,151 Taurine has even been proposed as a potential preventive treatment for Alzheimer dementia, as it stabilizes protein conformations to prevent their aggregation and subsequent dysfunction.152 Although improvement in memory and cognitive performance has been linked to taurine supplementation in old mice,145,153 similar results have not been found in adult mice whose taurine levels are within normal limits. Taurine also has transient anticonvulsant effects in some epileptic humans.154
Within the male reproductive organs, the age-related decline in taurine may or may not have implications regarding sexuality, as only very limited rat data are available.89–91
In cats, taurine supplementation has been found to prevent the progressive degeneration of retinal photoreceptors seen in retinitis pigmentosa, a genetic disease that causes the loss of vision.155
While several energy drink companies have advertised that taurine plays a role in improving cognitive and physical performance, there are few human studies that examine this contention in the absence of confounding factors such as caffeine or glucose.36,37,95 Taurine supplementation in patients with heart failure has been shown to increase exercise capacity vs placebo.156 This supports the idea that in cases of taurine deficiency, such as those seen in cardiomyopathy,157 taurine supplementation could have restorative effects. However, we are not aware of any double-blind, placebo-controlled clinical trial of taurine alone in healthy patients that measured energy parameters as clinical outcomes.
Although it remains possible that acute supraphysiologic taurine levels achieved by supplementation could transiently trigger various psychoneuroendocrine responses in healthy people, clinical research is needed in which taurine is the sole intervention. At present, the most compelling clinical reason to prescribe or recommend taurine supplementation is taurine deficiency.
Taurine—an amino acid found in abundance in the human brain, retina, heart, and reproductive organs, as well as in meat and seafood—is also a major ingredient in “energy drinks” (Table 1).1,2 Given the tremendous popularity of these drinks in the United States, it would seem important to know and to recognize taurine’s neuroendocrine effects. Unfortunately, little is known about the effects of taurine supplementation in humans.
This paper reviews the sparse data to provide clinicians some background on the structure, synthesis, distribution, metabolism, mechanisms, effects, safety, and proposed therapeutic targets of taurine.
TAURINE’S THERAPEUTIC POTENTIAL
Taurine has been reported to have widespread anti-inflammatory actions.3,4 Taurine supplementation has been proposed to have beneficial effects in the treatment of epilepsy,5 heart failure,6,7 cystic fibrosis,8 and diabetes9 and has been shown in animal studies to protect against neurotoxic insults from alcohol, ammonia, lead, and other substances.10–16
In addition, taurine analogues such as homotaurine and N-acetyl-homotaurine (acamprosate) have been probed for possible therapeutic applications. Homotaurine has been shown to have antiamyloid activity that could in theory protect against the progression of Alzheimer disease,17 and acamprosate is approved by the US Food and Drug Administration (FDA) for the treatment of alcohol use disorders.18
TAURINE CONSUMPTION
Energy drinks are widely consumed in the United States, with an estimated 354 million gallons sold in 2009, or approximately 5.25 L/year per person over age 10.1 In 2012, US sales of energy drinks exceeded $12 billion,19 with young men, particularly those in the military deployed in war zones, being the biggest consumers.20–22 Analyses have found that of 49 nonalcoholic energy drinks tested, the average concentration of taurine was 3,180 mg/L, or approximately 750 mg per 8-oz serving.23,24 Popular brands include Red Bull, Monster, Rockstar (Table 1), NOS, Amp, and Full Throttle.
Taurine is plentiful in the human body, which contains up to 1 g of taurine per kg.25 Foods such as poultry, beef, pork, seafood, and processed meats have a high taurine content (Table 2).26–29 People who eat meat and seafood have plentiful taurine intake, whereas vegetarians and vegans consume much less and have significantly lower circulating levels30 because plants do not contain taurine in appreciable amounts.26,29
The typical American diet provides between 123 and 178 mg of taurine daily.26 Consumption of one 8-oz energy drink can increase the average intake 6 to 16 times. A lacto-ovo vegetarian diet provides only about 17 mg of taurine daily, and an 8-oz energy drink can increase the average intake by 44 to 117 mg.26 And since a vegan diet provides essentially no taurine,30 energy drink intake in any amount would constitute a major relative increase in taurine consumption.
ATTEMPTS TO STUDY TAURINE'S EFFECTS
Since most clinical trials to date have looked at the effects of taurine in combination with other ingredients such as caffeine, creatine, and glucose31–35 in drinks such as Red Bull, these studies cannot be used to determine the effects of taurine alone. In the few clinical trials that have tested isolated taurine consumption, data are not sufficient to make a conclusion on direct effects on energy metabolism.
Rutherford et al36 tested the effect of oral taurine supplementation (1,660 mg) on endurance in trained male cyclists 1 hour before exercise, but observed no effect on fluid intake, heart rate, subjective exertion, or time-trial performance. A small increase (16%) in total fat oxidation was observed during the 90-minute exercise period. Since mitochondria are the main location of fatty acid degradation, this effect may be attributed to taurine supplementation, with subsequent improvement in mitochondrial function.
Zhang et al37 found a 30-second increase in cycling energy capacity after 7 days of 6 g oral taurine supplementation, but the study was neither blinded nor placebo-controlled.
Kammerer et al38 tested the effect of 1 g of taurine supplementation on physical and mental performance in young adult soldiers 45 minutes before physical fitness and cognitive testing. This double-blind, placebo-controlled randomized trial found no effect of taurine on cardiorespiratory fitness indices, concentration, or immediate memory, nor did it find any effect of an 80-mg dose of caffeine.
In sum, the available data are far from sufficient to determine the direct effect of taurine consumption on energy metabolism in healthy people.
PHARMACOLOGY OF TAURINE
Chemical structure
Taurine, or 2-aminoethane sulfonic acid, is a conditionally essential amino acid, ie, we can usually make enough in our own bodies. It was first prepared on a large scale for physiologic investigation almost 90 years ago, through the purification of ox bile.39 It can be obtained either exogenously through dietary sources or endogenously through biosynthesis from methionine and cysteine precursors, both essential sulfur-containing alpha-amino acids.40 Both sources are important to maintain physiologic levels of taurine, and either can help compensate for the other in cases of deficiency.41
The structure of taurine has two main differences from the essential amino acids. First, taurine’s amino group is attached to the beta-carbon rather than the alpha-carbon, making it a beta-amino acid instead of an alpha-amino acid.42 Second, the acid group in taurine is sulfonic acid, whereas the essential amino acids have a carboxylic acid.43 Because of its distinctive structure, taurine is not used as a structural unit in proteins,43 existing mostly as a free amino acid within cells, readily positioned to perform several unique functions.
Synthesis
De novo synthesis of taurine involves several enzymes and at least five pathways,44 mostly differing by the order in which sulfur is oxidized and decarboxylated.45
The rate-limiting enzyme of the predominant pathway is thought to be cysteine sulfinate decarboxylase (CSD), and its presence within an organ indicates involvement in taurine production.44 CSD has been found in the liver,46 the primary site of taurine biosynthesis, as well as in the retina,47 brain,48 kidney,49 mammary glands,50,51 and reproductive organs.52
Distribution
Taurine levels are highest in electrically excitable tissues such as the central nervous system, retina, and heart; in secretory structures such as the pineal gland and the pituitary gland (including the posterior lobe or neurohypophysis); and in platelets25 and neutrophils.53
In the fetal brain, the taurine concentration is higher than that of any other amino acid,54 but the concentration in the brain decreases with advancing age, whereas glutamate levels increase over time to make it the predominant amino acid in the adult brain.54 Regardless, taurine is still the second most prevalent amino acid in the adult brain, its levels comparable to those of gamma-aminobutyric acid (GABA).55
Taurine has also been found in variable amounts in the liver, muscle, kidney, pancreas, spleen, small intestine, and lungs,56 as well as in several other locations.45,57
Taurine is also present in the male and female reproductive organs. In male rats, taurine and taurine biosynthesis have been localized to Leydig cells of the testes, the cellular source of testosterone in males, as well as the cremaster muscle, efferent ducts, and peritubular myoid cells surrounding seminiferous tubules.58 More recently, taurine has been detected in the testes of humans59 and is also found in sperm and seminal fluid.60 Levels of taurine in spermatozoa are correlated with sperm quality, presumably by protecting against lipid peroxidation through taurine’s antioxidant effects,61,62 as well as through contribution to the spermatozoa maturation process by facilitating the capacitation, motility, and acrosomal reaction of sperm.63
In female rats, taurine has been found in uterine tissue,64 oviducts,65 uterine fluid (where it is the predominant amino acid),66 and thecal cells of developing follicles of ovaries, cells responsible for the synthesis of androgens such as testosterone and androstenedione.65 Taurine is also a major component of human breast milk67 and is important for proper neonatal nutrition.68
Metabolism and excretion
Ninety-five percent of taurine is excreted in urine, about 70% as taurine itself, and the rest as sulfate. Most of the sulfate derived from taurine is produced by bacterial metabolism in the gut and then absorbed.69 However, taurine can also be conjugated with bile acids to act as a detergent in lipid emulsification.70 In this form, it may be subjected to the enterohepatic circulation, which gives bacteria another chance to convert it into inorganic sulfate for excretion in urine.69
MECHANISMS AND NEUROENDOCRINE EFFECTS
As a free amino acid, taurine has widespread distribution and unique biochemical and physiologic properties and exhibits several organ-specific functions; however, indisputable evidence of a taurine-specific receptor is lacking, and its putative existence71 is controversial.72 Nonetheless, taurine is a neuromodulator with a variety of actions.
Neurotransmission
Taurine is known to be an inhibitory neurotransmitter and neuromodulator.73 It is structurally analogous to GABA, the main inhibitory neurotransmitter in the brain.45 Accordingly, it binds to GABA receptors to serve as an agonist,74,75 causing neuronal hyperpolarization and inhibition. Taurine has an even higher affinity for glycine receptors75 where it has long been known to act as an agonist.76 GABA and glycine receptors both belong to the Cys-loop receptor superfamily,77 with conservation of subunits that allows taurine to bind each receptor, albeit at different affinities. The binding effects of taurine on GABA and glycine receptors have not been well documented quantitatively; however, it is known that taurine has a substantially lower affinity than GABA and glycine for their respective receptors.76
Catecholamines and the sympathetic nervous system
Surprisingly little is known about the effects of taurine on norepinephrine, dopamine, and the human sympathetic nervous system.78 Humans with borderline hypertension given 6 g of taurine orally for 7 days79 experienced decreases in epinephrine secretion and blood pressure, but normotensive study participants did not experience similar results, possibly because of a better ability to regulate sympathetic tone. Mizushima et al80 showed that a longer period of taurine intake (6 g orally for 3 weeks) could elicit a decrease in norepinephrine in healthy men with normal blood pressure. Other similar studies81–83 also suggested interplay between taurine and catecholamines, but the extent is still undetermined.
Growth hormone, prolactin, sex hormones, and cortisol
Taurine appears to have a complex relationship with several hormones, although its direct effects on hormone secretion remain obscure. Clinical studies of the acute and chronic neuroendocrine effects of taurine loading in humans are needed.
In female rats, secretion of prolactin is increased by the intraventricular injection of 5 μL of 2.0 μmol taurine over a 10-minute period.84 Ikuyama et al85 found an increase in prolactin and growth hormone secretion in adult male rats given 10 μL of 0.25 μmol and 1.0 μmol taurine intraventricularly, yet a higher dose of 4.0 μmol had no effect on either hormone. Furthermore, prolactin receptor deficiency is seen in CSD knockout mice, but the receptor is restored with taurine supplementation.86
Mantovani and DeVivo87 reported that 375 to 8,000 mg/day of taurine given orally for 4 to 6 months to epileptic patients stimulated the secretion of growth hormone. However, in another study, a single 75-mg/kg dose of oral taurine did not trigger an acute increase in levels of growth hormone or prolactin in humans.88 Energy drinks may contain up to 1,000 mg of taurine per 8-oz serving, but the effects of larger doses on growth hormone, which is banned as a supplement by major athletic organizations because of its anabolic and possible performance-enhancing effects, remain to be determined.
Taurine may have effects on human sex hormones, based on the limited observations in rodents.89–94
Although human salivary cortisol concentrations were purportedly assessed in response to 2,000 mg of oral taurine,95 the methods and reported data are not adequate to draw any conclusions.
Energy metabolism
Mammals are unable to directly use taurine in energy production because they cannot directly reduce it.25 Instead, bacteria in the gut use it as a source of energy, carbon, nitrogen, and sulfur.96 However, taurine deficiency appears to impair the cellular respiratory chain, resulting in diminished production of adenosine triphosphate and diminished uptake of long-chain fatty acids by mitochondria, at least in the heart.97
Taurine is present in human mitochondria and regulates mitochondrial function. For example, taurine in mitochondria assists in conjugation of transfer RNA for leucine, lysine, glutamate, and glutamine.98 In TauT knockout mice, deficiency of taurine causes mitochondrial dysfunction, triggering a greater than 80% decrease in exercise capacity.99 Several studies in rodents have shown increased exercise capacity after taurine supplementation.100–102 In addition, taurine is critical for the growth of blastocytes, skeletal muscle, and myocardium; it is necessary for mitochondrial development and is also important for muscular endurance.103,104
Antioxidation, anti-inflammation, and other functions
Taurine is a major antioxidant, scavenging reactive oxygen and protecting against oxidative stress to organs including the brain,97,105,106 where it increasingly appears to have neuroprotective effects.107,108
Cellular taurine also has anti-inflammatory actions.3 One of the proposed mechanisms is taurine inhibition of NF-kappa B, an important transcription factor for the synthesis of pro-inflammatory cytokines.4 This function may be important in protecting polyunsaturated fatty acids from oxidative stress—helping to maintain and stabilize the structure and function of plasma membranes within the lungs,109 heart,110 brain,111 liver,112 and spermatozoa.61,62
Taurine is also conjugated to bile acids synthesized in the liver, forming bile salts70 that act as detergents to help emulsify and digest lipids in the body. In addition, taurine facilitates xenobiotic detoxification in the liver by conjugating with several drugs to aid in their excretion.25 Taurine is also implicated in calcium modulation113 and homeostasis.114 Through inhibition of several types of calcium channels, taurine has been shown to decrease calcium influx into cells, effectively serving a cytoprotective role against calcium overload.115,116
TAURINE DEFICIENCY
Fetal and neonatal deficiency
Though taurine is considered nonessential in adults because it can be readily synthesized endogenously, it is thought to be conditionally essential in neonatal nutrition.68 It is the second most abundant free amino acid in human breast milk117 and the most abundant free amino acid in fetal brain.118 In cases of long-term parenteral nutrition, neonates can become drastically taurine deficient119 due to suboptimal CSD activity,118 leading to retinal dysfunction.41 Taurine deficiencies can lead to functional and structural brain damage.118 Moreover, maternal taurine deficiency results in neurologic abnormalities in offspring120 and may lead to oxidative stress throughout life.121
In 1984, the FDA approved the inclusion of taurine in infant formulas based on research showing that taurine-deficient infants had impaired fat absorption, bile acid secretion, retinal function, and hepatic function.122 But still under debate are the amount and duration of taurine supplementation required by preterm and low-birth-weight infants, as several randomized controlled trials failed to show statistically significant effects on growth.123 Nonetheless, given the alleged detrimental ramifications of a lack of taurine supplementation, as well as the ethical dilemma of performing additional research trials on infants, it is presumed that infant formulas and parenteral nutrition for preterm and low-birth-weight infants will continue to contain taurine.
Age- and disease-related deficiency
Although taurine deficiency is rare in neonates, it is perhaps inevitable with advancing age. Healthy elderly patients ages 61 to 81 have up to a 49% decrease in plasma taurine concentration compared with healthy individuals ages 27 to 57.124 While reduced renal retention125 and taurine intake126 can account for depressed taurine levels, Eppler and Dawson127 found that tissue and circulating taurine concentrations decrease over the human life span primarily due to an age-dependent depletion of CSD activity in the liver. This effectively impairs the biosynthesis of endogenous taurine from cysteine or methionine or both, forcing a greater reliance on exogenous sources.
While specific mechanisms have not been fully elucidated, taurine deficiency has also been identified in patients suffering from diseases including but not limited to disorders of bone (osteogenesis imperfecta, osteoporosis),128 blood (acute myelogenous leukemia),129 central nervous system (schizophrenia, Friedreich ataxia-spinocerebellar degeneration),130,131 retina (retinitis pigmentosa),132 circulatory system and heart (essential hypertension, atherosclerosis),133 digestion (Gaucher disease),134 absorption (short-bowel syndrome),135 cellular proliferation (cancer),136 and membrane channels (cystic fibrosis),137 as well as in patients restricted to long-term parenteral nutrition.138 However, the apparent correlation between taurine deficiency and these conditions does not necessarily mean causation; more study is needed to elucidate a direct connection.
SAFETY AND TOXICITY OF TAURINE SUPPLEMENTATION
An upper safe level of intake for taurine has not been established. To date, several studies have involved heavy taurine supplementation without serious adverse effects. While the largest dosage of taurine tested in humans appears to be 10 g/day for 6 months,139 a number of studies have used 1 to 6 g/day for periods of 1 week to 1 year.140 However, the assessment of potential acute, subacute, and chronic adverse effects has not been comprehensive. The Scientific Committee on Food of the European Commission141 reviewed several toxicologic studies on taurine through 2003 and were unable to expose any carcinogenic or teratogenic potential. Nevertheless, based on the available data from trials in humans and lower animals, Shao and Hathcock140 suggested an observed safe level of taurine of 3 g/day, a conservatively smaller dose that carries a higher level of confidence. Because there is no “observed adverse effect level” for daily taurine intake,141 more research must be done to ensure safety of higher amounts of taurine administration and to define a tolerable upper limit of intake.
Interactions with medications
To date, the literature is scarce regarding potential interactions between taurine and commonly used medications.
Although no evidence specifically links taurine with adverse effects when used concurrently with other medications, there may be a link between taurine supplementation and various cytochrome P450 systems responsible for hepatic drug metabolism. Specifically, taurine inhibits cytochrome P450 2E1, a highly conserved xenobiotic-metabolizing P450 responsible for the breakdown of more than 70 substrates, including several commonly used anesthetics, analgesics, antidepressants, antibacterials, and antiepileptics.142 Of note, taurine may contribute to the attenuation of oxidative stress in the liver in the presence of alcohol143 and acetaminophen,144 two substances frequently used and abused. Since the P450 2E1 system catalyzes comparable reactions in rodents and humans,142 rodents should plausibly serve as a model for further testing of the effects of taurine on various substrates.
POTENTIAL THERAPEUTIC APPLICATIONS
More analysis is needed to fully unlock and understand taurine’s potential value in healthcare.
Correction of late-life taurine decline in humans could be beneficial for cognitive performance, energy metabolism, sexual function, and vision, but clinical studies remain to be performed. While a decline in taurine with age may intensify the stress caused by reactive oxygen species, taurine supplementation has been shown to decrease the presence of oxidative markers127 and to serve a neuroprotective role in rodents.145,146 Taurine levels increase in the hippocampus after experimentally induced gliosis147 and are neuroprotective against glutamate excitotoxicity.148,149 Furthermore, data in Alzheimer disease, Huntington disease, and brain ischemia experimental models show that taurine inhibits neuronal death (apoptosis).13,150,151 Taurine has even been proposed as a potential preventive treatment for Alzheimer dementia, as it stabilizes protein conformations to prevent their aggregation and subsequent dysfunction.152 Although improvement in memory and cognitive performance has been linked to taurine supplementation in old mice,145,153 similar results have not been found in adult mice whose taurine levels are within normal limits. Taurine also has transient anticonvulsant effects in some epileptic humans.154
Within the male reproductive organs, the age-related decline in taurine may or may not have implications regarding sexuality, as only very limited rat data are available.89–91
In cats, taurine supplementation has been found to prevent the progressive degeneration of retinal photoreceptors seen in retinitis pigmentosa, a genetic disease that causes the loss of vision.155
While several energy drink companies have advertised that taurine plays a role in improving cognitive and physical performance, there are few human studies that examine this contention in the absence of confounding factors such as caffeine or glucose.36,37,95 Taurine supplementation in patients with heart failure has been shown to increase exercise capacity vs placebo.156 This supports the idea that in cases of taurine deficiency, such as those seen in cardiomyopathy,157 taurine supplementation could have restorative effects. However, we are not aware of any double-blind, placebo-controlled clinical trial of taurine alone in healthy patients that measured energy parameters as clinical outcomes.
Although it remains possible that acute supraphysiologic taurine levels achieved by supplementation could transiently trigger various psychoneuroendocrine responses in healthy people, clinical research is needed in which taurine is the sole intervention. At present, the most compelling clinical reason to prescribe or recommend taurine supplementation is taurine deficiency.
- US Food and Drug Administration (FDA). Caffeine intake by the US population. www.fda.gov/downloads/AboutFDA/ CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/UCM333191.pdf. Accessed October 4, 2016.
- McLellan TM, Lieberman HR. Do energy drinks contain active components other than caffeine? Nutr Rev 2012; 70:730–744.
- Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol 1993; 54:119–124.
- Kontny E, Szczepanska K, Kowalczewski J, et al. The mechanism of taurine chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheum 2000; 43:2169–2177.
- Barbeau A, Inoue N, Tsukada Y, Butterworth RF. The neuropharmacology of taurine. Life Sci 1975; 17:669–677.
- Kramer JH, Chovan JP, Schaffer SW. Effect of taurine on calcium paradox and ischemic heart failure. Am J Physiol 1981; 240:H238–H246.
- Azuma J, Sawamura A, Awata N, et al. Therapeutic effect of taurine in congestive heart failure: a double-blind crossover trial. Clin Cardiol 1985; 8:276–282.
- Darling PB, Lepage G, Leroy C, Masson P, Roy CC. Effect of taurine supplements on fat absorption in cystic fibrosis. Pediatr Res 1985; 19:578–582.
- Franconi F, Di Leo MA, Bennardini F, Ghirlanda G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res 2004; 29:143–150.
- Malcangio M, Bartolini A, Ghelardini C, et al. Effect of ICV taurine on the impairment of learning, convulsions and death caused by hypoxia. Psychopharmacology (Berl) 1989; 98:316–320.
- Rivas-Arancibia S, Dorado-Martínez C, Borgonio-Pérez G, et al. Effects of taurine on ozone-induced memory deficits and lipid peroxidation levels in brains of young, mature, and old rats. Environ Res 2000; 82:7–17.
- Vohra BP, Hui X. Improvement of impaired memory in mice by taurine. Neural Plast 2000; 7:245–259.
- Tadros MG, Khalifa AE, Abdel-Naim AB, Arafa HM. Neuroprotective effect of taurine in 3-nitropropionic acid-induced experimental animal model of Huntington’s disease phenotype. Pharmacol Biochem Behav 2005; 82:574–582.
- Zhu DM, Wang M, She JQ, Yu K, Ruan DY. Protection by a taurine supplemented diet from lead-induced deficits of long-term potentiation/depotentiation in dentate gyrus of rats in vivo. Neuroscience 2005; 134:215–224.
- Chepkova AN, Sergeeva OA, Haas HL. Taurine rescues hippocampal long-term potentiation from ammonia-induced impairment. Neurobiol Dis 2006; 23:512–521.
- Wang GH, Jiang ZL, Fan XJ, Zhang L, Li X, Ke KF. Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 2007; 52:1199–1209.
- Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, Trabucchi M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: a review. Aging Clin Exp Res 2012; 24:580–587.
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014; 311:1889–1900.
- Sorkin BC, Camp KM, Haggans CJ, et al. Executive summary of NIH workshop on the use and biology of energy drinks: current knowledge and critical gaps. Nutr Rev 2014; 72(suppl 1):1–8.
- Centers for Disease Control and Prevention (CDC). Energy drink consumption and its association with sleep problems among US service members on a combat deployment—Afghanistan, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:895–898.
- Bailey RL, Saldanha LG, Dwyer JT. Estimating caffeine intake from energy drinks and dietary supplements in the United States. Nutr Rev 2014; 72(suppl 1):9–13.
- Stephens MB, Attipoe S, Jones D, Ledford CJ, Deuster PA. Energy drink and energy shot use in the military. Nutr Rev 2014; 72(suppl 1):72–77.
- Triebel S, Sproll C, Reusch H, Godelmann R, Lachenmeier DW. Rapid analysis of taurine in energy drinks using amino acid analyzer and Fourier transform infrared (FTIR) spectroscopy as basis for toxicological evaluation. Amino Acids 2007; 33:451–457.
- Beverage Industry. 2013 state of the industry: energy drinks & shots. www.bevindustry.com/articles/86552-state-of-the-industry-energy-drinks-shots. Accessed October 4, 2016.
- Huxtable RJ. Physiological actions of taurine. Physiol Rev 1992; 72:101–163.
- Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. JPEN J Parenter Enteral Nutr 1990; 14:183–188.
- Manzi P, Pizzoferrato L. Taurine in milk and yoghurt marketed in Italy. Int J Food Sci Nutr 2013; 64:112–116.
- Dragnes BT, Larsen R, Ernstsen MH, Mæhre H, Elvevoll EO. Impact of processing on the taurine content in processed seafood and their corresponding unprocessed raw materials. Int J Food Sci Nutr 2009; 60:143–152.
- Laidlaw SA, Shultz TD, Cecchino JT, Kopple JD. Plasma and urine taurine levels in vegans. Am J Clin Nutr 1988; 47:660–663.
- Rana SK, Sanders TA. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br J Nutr 1986; 56:17–27.
- Alford C, Cox H, Wescott R. The effects of Red Bull energy drink on human performance and mood. Amino Acids 2001; 21:139–150.
- Hoffman JR, Ratamess NA, Ross R, Shanklin M, Kang J, Faigenbaum AD. Effect of a pre-exercise energy supplement on the acute hormonal response to resistance exercise. J Strength Cond Res 2008; 22:874–882.
- Ivy JL, Kammer L, Ding Z, et al. Improved cycling time-trial performance after ingestion of a caffeine energy drink. Int J Sport Nutr Exerc Metab 2009; 19:61–78.
- Gonzalez AM, Walsh AL, Ratamess NA, Kang J, Hoffman JR. Effect of a pre-workout energy supplement on acute multi-joint resistance exercise. J Sports Sci Med 2011; 10:261–266.
- Yeh TS, Chan KH, Hsu MC, Liu JF. Supplementation with soybean peptides, taurine, pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J Med Food 2011; 14:219–225.
- Rutherford JA, Spriet LL, Stellingwerff T. The effect of acute taurine ingestion on endurance performance and metabolism in well-trained cyclists. Int J Sport Nutr Exerc Metab 2010; 20:322–329.
- Zhang M, Izumi I, Kagamimori S, et al. Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids 2004; 26:203–207.
- Kammerer M, Jaramillo JA, García A, Calderón JC, Valbuena LH. Effects of energy drink major bioactive compounds on the performance of young adults in fitness and cognitive tests: a randomized controlled trial. J Int Soc Sports Nutr 2014; 11:44.
- Kermack WO, Slater RH. The preparation of taurine in considerable quantity. Biochem J 1927; 21:1065–1067.
- Huxtable RJ, Lippincott SE. Diet and biosynthesis as sources of taurine in the mouse. J Nutr 1982; 112:1003–1010.
- Ohkuma S, Tamura J, Kuriyama K. Roles of endogenous and exogenous taurine and glycine in the formation of conjugated bile acids: analyses using freshly isolated and primary cultured rat hepatocytes. Jpn J Pharmacol 1984; 35:347–358.
- Chapman GE, Greenwood CE. Taurine in nutrition and brain development. Nutrition Research 1988; 8:955–968.
- Andrews S, Schmidt CLA. Titration curves of taurine and cysteic acid. J Biol Chem 1927; 73:651–654.
- Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol 1989; 32:471–533.
- Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 1968; 48:424–511.
- Hope DB. Pyridoxal phosphate as the coenzyme of the mammalian decarboxylase for L-cysteine sulphinic and L-cysteic acids. Biochem J 1955; 59:497–500.
- Pasantes-Morales H, Lopez-Colome AM, Salceda R, Mandel P. Cysteine sulphinate decarboxylase in chick and rat retina during development. J Neurochem 1976; 27:1103–1106.
- Oertel WH, Schmechel DE, Weise VK, et al. Comparison of cysteine sulphinic acid decarboxylase isoenzymes and glutamic acid decarboxylase in rat liver and brain. Neuroscience 1981; 6:2701–2714.
- Reymond I, Bitoun M, Levillain O, Tappaz M. Regional expression and histological localization of cysteine sulfinate decarboxylase mRNA in the rat kidney. J Histochem Cytochem 2000; 48:1461–1468.
- Hu JM, Ikemura R, Chang KT, Suzuki M, Nishihara M, Takahashi M. Expression of cysteine sulfinate decarboxylase mRNA in rat mammary gland. J Vet Med Sci 2000; 62:829–834.
- Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 2007; 137:1887–1894.
- Li JH, Ling YQ, Fan JJ, Zhang XP, Cui S. Expression of cysteine sulfinate decarboxylase (CSD) in male reproductive organs of mice. Histochem Cell Biol 2006; 125:607–613.
- Green TR, Fellman JH, Eicher AL, Pratt KL. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta 1991; 1073:91–97.
- Lefauconnier JM, Portemer C, Chatagner F. Free amino acids and related substances in human glial tumours and in fetal brain: comparison with normal adult brain. Brain Res 1976; 117:105–113.
- Sturman JA, Rassin DK, Gaull GE. Taurine in development. Life Sci 1977; 21:1–22.
- Sturman JA. Taurine pool sizes in the rat: effects of vitamin B-6 deficiency and high taurine diet. J Nutr 1973; 103:1566–1580.
- Terauchi A, Nakazaw A, Johkura K, Yan L, Usuda N. Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids 1998; 15:151–160.
- Lobo MV, Alonso FJ, del Río RM. Immunohistochemical localization of taurine in the male reproductive organs of the rat. J Histochem Cytochem 2000; 48:313–320.
- Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod 2010; 25:847–852.
- Holmes RP, Goodman HO, Shihabi ZK, Jarow JP. The taurine and hypotaurine content of human semen. J Androl 1992; 13:289–292.
- Alvarez JG, Storey BT. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol Reprod 1983; 29:548–555.
- Das J, Ghosh J, Manna P, Sinha M, Sil PC. Taurine protects rat testes against NaAsO(2)-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 2009; 187:201–210.
- Mrsny RJ, Meizel S. Inhibition of hamster sperm Na+, K+-ATPase activity by taurine and hypotaurine. Life Sci 1985; 36:271–275.
- Phoenix J, Wray S. Changes in human and rat uterine phosphoethanolamine and taurine with pregnancy and parturition. Exp Physiol 1994; 79:601–604.
- Lobo MV, Alonso FJ, Latorre A, del Río RM. Immunohistochemical localization of taurine in the rat ovary, oviduct, and uterus. J Histochem Cytochem 2001; 49:1133–1142.
- Casslén BG. Free amino acids in human uterine fluid. Possible role of high taurine concentration. J Reprod Med 1987; 32:181–184.
- Pamblanco M, Portolés M, Paredes C, Ten A, Comín J. Free amino acids in preterm and term milk from mothers delivering appropriate- or small-for-gestational-age infants. Am J Clin Nutr 1989; 50:778–781.
- Rigo J, Senterre J. Is taurine essential for the neonates? Biol Neonate 1977; 32:73–76.
- Sturman JA, Hepner GW, Hofmann AF, Thomas PJ. Metabolism of [35S]taurine in man. J Nutr 1975; 105:1206–1214.
- Bremer J. Species differences in the conjugation of free bile acids with taurine and glycine. Biochem J 1956; 63:507–513.
- Wu JY, Tang XW, Tsai WH. Taurine receptor: kinetic analysis and pharmacological studies. Adv Exp Med Biol 1992; 315:263–268.
- Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis 2012; 18:2673–2686.
- Haas HL, Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res 1973; 52:399–402.
- Bureau MH, Olsen RW. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Eur J Pharmacol 1991; 207:9–16.
- Bhattarai JP, Park SJ, Chun SW, Cho DH, Han SK. Activation of synaptic and extrasynaptic glycine receptors by taurine in preoptic hypothalamic neurons. Neurosci Lett 2015; 608:51–56.
- Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S. Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Res 1988; 464:97–105.
- Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci 2010; 31:161–174.
- Fujita T, Sato Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J Clin Invest 1988; 82:993–997.
- Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987; 75:525–532.
- Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol 1996; 403:615–622.
- Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl 1984; 2:S563–S565.
- Fujita T, Sato Y, Ando K. Changes in cardiac and hypothalamic noradrenergic activity with taurine in DOCA-salt rats. Am J Physiol 1986; 251:H926–H933.
- Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension 1987; 9:81–87.
- Scheibel J, Elsasser T, Ondo JG. A neuromodulatory role for taurine in controlling prolactin secretion in female rats. Psychoneuroendocrinology 1981; 6:139–144.
- Ikuyama S, Okajima T, Kato K, Ibayashi H. Effect of taurine on growth hormone and prolactin secretion in rats: possible interaction with opioid peptidergic system. Life Sci 1988; 43:807–812.
- Park E, Park SY, Dobkin C, Schuller-Levis G. Development of a novel cysteine sulfinic acid decarboxylase knockout mouse: dietary taurine reduces neonatal mortality. J Amino Acids 2014; 2014:346809.
- Mantovani J, DeVivo DC. Effects of taurine on seizures and growth hormone release in epileptic patients. Arch Neurol 1979; 36:672–674.
- Carlson HE, Miglietta JT, Roginsky MS, Stegink LD. Stimulation of pituitary hormone secretion by neurotransmitter amino acids in humans. Metabolism 1989; 38:1179–1182.
- Yang J, Wu G, Feng Y, Lv Q, Lin S, Hu J. Effects of taurine on male reproduction in rats of different ages. J Biomed Sci 2010; 17(suppl 1):S9.
- Yang J, Lin S, Feng Y, Wu G, Hu J. Taurine enhances the sexual response and mating ability in aged male rats. Adv Exp Med Biol 2013; 776:347–355.
- Yang J, Zong X, Wu G, Lin S, Feng Y, Hu J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids 2015; 47:1549–1558.
- Scheibel J, Elsasser T, Ondo JG. Stimulation of LH and FSH secretion following intraventricular injection of cysteic acid but not taurine. Brain Res 1980; 201:99–106.
- Price MT, Olney JW, Mitchell MV, Fuller T, Cicero TJ. Luteinizing hormone releasing action of N-methyl aspartate is blocked by GABA or taurine but not by dopamine antagonists. Brain Res 1978; 158:461–465.
- Ma Q, Zhao J, Cao W, Liu J, Cui S. Estradiol decreases taurine level by reducing cysteine sulfinic acid decarboxylase via the estrogen receptor-a in female mice liver. Am J Physiol Gastrointest Liver Physiol 2015; 308:G277–G286.
- Giles GE, Mahoney CR, Brunyé TT, Gardony AL, Taylor HA, Kanarek RB. Differential cognitive effects of energy drink ingredients: caffeine, taurine, and glucose. Pharmacol Biochem Behav 2012; 102:569–577.
- Giehl TJ, Qoronfleh MW, Wilkinson BJ. Transport, nutritional and metabolic studies of taurine in staphylococci. J Gen Microbiol 1987; 133:849–856.
- Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids 2015 Oct 16. Epub ahead of print.
- Schaffer SW, Jong CJ, Ito T, Azuma J. Role of taurine in the pathologies of MELAS and MERRF. Amino Acids 2014; 46:47–56.
- Warskulat U, Heller-Stilb B, Oermann E, et al. Phenotype of the taurine transporter knockout mouse. Methods Enzymol 2007; 428:439–458.
- Yatabe Y, Miyakawa S, Miyazaki T, Matsuzaki Y, Ochiai N. Effects of taurine administration in rat skeletal muscles on exercise. J Orthop Sci 2003; 8:415–419.
- Miyazaki T, Matsuzaki Y, Ikegami T, et al. Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acids 2004; 27:291–298.
- Goodman CA, Horvath D, Stathis C, et al. Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. J Appl Physiol (1985) 2009; 107:144–154.
- Van Winkle LJ, Patel M, Wasserlauf HG, Dickinson HR, Campione AL. Osmotic regulation of taurine transport via system beta and novel processes in mouse preimplantation conceptuses. Biochim Biophys Acta 1994; 1191:244–255.
- Ito T, Kimura Y, Uozumi Y, et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 2008; 44:927–937.
- Aydın AF, Çoban J, Dogan-Ekici I, Betül-Kalaz E, Dogru-Abbasoglu S, Uysal M. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab Brain Dis 2016; 31:337–345.
- Nagai K, Fukuno S, Oda A, Konishi H. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs 2016; 27:17–23.
- Caletti G, Almeida FB, Agnes G, Nin MS, Barros HM, Gomez R. Antidepressant dose of taurine increases mRNA expression of GABAA receptor a2 subunit and BDNF in the hippocampus of diabetic rats. Behav Brain Res 2015; 283:11–55.
- Gu Y, Zhao Y, Qian K, Sun M. Taurine attenuates hippocampal and corpus callosum damage, and enhances neurological recovery after closed head injury in rats. Neuroscience 2015; 291:331–340.
- Banks MA, Porter DW, Pailes WH, Schwegler-Berry D, Martin WG, Castranova V. Taurine content of isolated rat alveolar type I cells. Comp Biochem Physiol B 1991; 100:795–799.
- Raschke P, Massoudy P, Becker BF. Taurine protects the heart from neutrophil-induced reperfusion injury. Free Radic Biol Med 1995; 19:461–471.
- Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids 2004; 27:91–96.
- Refik Mas M, Comert B, Oncu K, et al. The effect of taurine treatment on oxidative stress in experimental liver fibrosis. Hepatol Res 2004; 28:207–215.
- Sebring LA, Huxtable RJ. Taurine modulation of calcium binding to cardiac sarcolemma. J Pharmacol Exp Ther 1985; 232:445–451.
- Schaffer SW, Kramer J, Chovan JP. Regulation of calcium homeostasis in the heart by taurine. Fed Proc 1980; 39:2691–2694.
- Foos TM, Wu JY. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 2002; 27:21–26.
- Ito T, Schaffer S, Azuma J. The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids 2014; 46:111–119.
- Gaull GE. Taurine in pediatric nutrition: review and update. Pediatrics 1989; 83:433–442.
- Sturman JA, Rassin DK, Gaull GE. Taurine in developing rat brain: transfer of [35S] taurine to pups via the milk. Pediatr Res 1977; 11:28–33.
- Geggel HS, Ament ME, Heckenlively JR, Martin DA, Kopple JD. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N Engl J Med 1985; 312:142–146.
- Sturman JA, Gargano AD, Messing JM, Imaki H. Feline maternal taurine deficiency: effect on mother and offspring. J Nutr 1986; 116:655–667.
- Lerdweeraphon W, Wyss JM, Boonmars T, Roysommuti S. Perinatal taurine exposure affects adult oxidative stress. Am J Physiol Regul Integr Comp Physiol 2013; 305:R95–R97.
- Chesney RW, Helms RA, Christensen M, Budreau AM, Han X, Sturman JA. The role of taurine in infant nutrition. Adv Exp Med Biol 1998; 442:463–476.
- Verner A, Craig S, McGuire W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev 2007; 4:CD006072.
- Jeevanandam M, Young DH, Ramias L, Schiller WR. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am J Clin Nutr 1990; 51:1040–1045.
- Dawson R Jr, Eppler B, Patterson TA, Shih D, Liu S. The effects of taurine in a rodent model of aging. Adv Exp Med Biol 1996; 403:37–50.
- Han X, Budreau AM, Chesney RW. Molecular cloning and functional expression of an LLC-PK1 cell taurine transporter that is adaptively regulated by taurine. Adv Exp Med Biol 1998; 442:261–268.
- Eppler B, Dawson R Jr. Dietary taurine manipulations in aged male Fischer 344 rat tissue: taurine concentration, taurine biosynthesis, and oxidative markers. Biochem Pharmacol 2001; 62:29–39.
- D’Eufemia P, Finocchiaro R, Celli M, et al. Taurine deficiency in thalassemia major-induced osteoporosis treated with neridronate. Biomed Pharmacother 2010; 64:271–274.
- Muscaritoli M, Conversano L, Petti MC, et al. Plasma amino acid concentrations in patients with acute myelogenous leukemia. Nutrition 1999; 15:195–199.
- Do KQ, Lauer CJ, Schreiber W, et al. Gamma-glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem 1995; 65:2652–2662.
- Lemieux B, Giguère R, Shapcott D. Studies on the role of taurine in Friedreich’s ataxia. Can J Neurol Sci 1984; 11(suppl 4):610–615.
- Airaksinen EM, Oja SS, Marnela KM, Sihvola P. Taurine and other amino acids of platelets and plasma in retinitis pigmentosa. Ann Clin Res 1980; 12:52–54.
- Kohashi N, Katori R. Decrease of urinary taurine in essential hypertension. Jpn Heart J 1983; 24:91–102.
- vom Dahl S, Mönnighoff I, Häussinger D. Decrease of plasma taurine in Gaucher disease and its sustained correction during enzyme replacement therapy. Amino Acids 2000; 19:585–592.
- Schneider SM, Joly F, Gehrardt MF, et al. Taurine status and response to intravenous taurine supplementation in adults with short-bowel syndrome undergoing long-term parenteral nutrition: a pilot study. Br J Nutr 2006; 96:365–370.
- Gray GE, Landel AM, Meguid MM. Taurine-supplemented total parenteral nutrition and taurine status of malnourished cancer patients. Nutrition 1994; 10:11–15.
- Thompson GN. Assessment of taurine deficiency in cystic fibrosis. Clin Chim Acta 1988; 171:233–237.
- Cho KH, Kim ES, Chen JD. Taurine intake and excretion in patients undergoing long term enteral nutrition. Adv Exp Med Biol 2000; 483:605–612.
- Durelli L, Mutani R, Fassio F. The treatment of myotonia: evaluation of chronic oral taurine therapy. Neurology 1983; 33:599–603.
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol 2008; 50:376–399.
- European Commission; Scientific Committee on Food. Opinion on additional information on energy drinks. http://ec.europa.eu/food/fs/sc/scf/out169_en.pdf. Accessed October 4, 2016.
- Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther 2000; 25:165–175.
- Kerai MD, Waterfield CJ, Kenyon SH, Asker DS, Timbrell JA. Reversal of ethanol-induced hepatic steatosis and lipid peroxidation by taurine: a study in rats. Alcohol Alcohol 1999; 34:529–541.
- Das J, Ghosh J, Manna P, Sil PC. Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res 2010; 44:340–355.
- El Idrissi A, Shen CH, L’amoreaux WJ. Neuroprotective role of taurine during aging. Amino Acids 2013; 45:735–750.
- Gharibani P, Modi J, Menzie J, et al. Comparison between single and combined post-treatment with S-methyl-N,N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience 2015; 300:460–473.
- Junyent F, De Lemos L, Utrera J, et al. Content and traffic of taurine in hippocampal reactive astrocytes. Hippocampus 2011; 21:185–197.
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 1999; 19:9459–9468.
- Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res 2005; 1038:123–131.
- Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 2005; 49:1140–1148.
- Takatani T, Takahashi K, Uozumi Y, et al. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol 2004; 287:C949–C953.
- Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis 2010; 20(suppl 2):S439–S452.
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett 2008; 436:19–22.
- Oja SS, Saransaari P. Taurine and epilepsy. Epilepsy Res 2013; 104:187–194.
- Berson EL, Hayes KC, Rabin AR, Schmidt SY, Watson G. Retinal degeneration in cats fed casein. II. Supplementation with methionine, cysteine, or taurine. Invest Ophthalmol 1976; 15:52–58.
- Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 2011; 57:333–337.
- Eby G, Halcomb WW. Elimination of cardiac arrhythmias using oral taurine with l-arginine with case histories: hypothesis for nitric oxide stabilization of the sinus node. Med Hypotheses 2006; 67:1200–1204.
- US Food and Drug Administration (FDA). Caffeine intake by the US population. www.fda.gov/downloads/AboutFDA/ CentersOffices/OfficeofFoods/CFSAN/CFSANFOIAElectronicReadingRoom/UCM333191.pdf. Accessed October 4, 2016.
- McLellan TM, Lieberman HR. Do energy drinks contain active components other than caffeine? Nutr Rev 2012; 70:730–744.
- Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol 1993; 54:119–124.
- Kontny E, Szczepanska K, Kowalczewski J, et al. The mechanism of taurine chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheum 2000; 43:2169–2177.
- Barbeau A, Inoue N, Tsukada Y, Butterworth RF. The neuropharmacology of taurine. Life Sci 1975; 17:669–677.
- Kramer JH, Chovan JP, Schaffer SW. Effect of taurine on calcium paradox and ischemic heart failure. Am J Physiol 1981; 240:H238–H246.
- Azuma J, Sawamura A, Awata N, et al. Therapeutic effect of taurine in congestive heart failure: a double-blind crossover trial. Clin Cardiol 1985; 8:276–282.
- Darling PB, Lepage G, Leroy C, Masson P, Roy CC. Effect of taurine supplements on fat absorption in cystic fibrosis. Pediatr Res 1985; 19:578–582.
- Franconi F, Di Leo MA, Bennardini F, Ghirlanda G. Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res 2004; 29:143–150.
- Malcangio M, Bartolini A, Ghelardini C, et al. Effect of ICV taurine on the impairment of learning, convulsions and death caused by hypoxia. Psychopharmacology (Berl) 1989; 98:316–320.
- Rivas-Arancibia S, Dorado-Martínez C, Borgonio-Pérez G, et al. Effects of taurine on ozone-induced memory deficits and lipid peroxidation levels in brains of young, mature, and old rats. Environ Res 2000; 82:7–17.
- Vohra BP, Hui X. Improvement of impaired memory in mice by taurine. Neural Plast 2000; 7:245–259.
- Tadros MG, Khalifa AE, Abdel-Naim AB, Arafa HM. Neuroprotective effect of taurine in 3-nitropropionic acid-induced experimental animal model of Huntington’s disease phenotype. Pharmacol Biochem Behav 2005; 82:574–582.
- Zhu DM, Wang M, She JQ, Yu K, Ruan DY. Protection by a taurine supplemented diet from lead-induced deficits of long-term potentiation/depotentiation in dentate gyrus of rats in vivo. Neuroscience 2005; 134:215–224.
- Chepkova AN, Sergeeva OA, Haas HL. Taurine rescues hippocampal long-term potentiation from ammonia-induced impairment. Neurobiol Dis 2006; 23:512–521.
- Wang GH, Jiang ZL, Fan XJ, Zhang L, Li X, Ke KF. Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 2007; 52:1199–1209.
- Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, Trabucchi M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: a review. Aging Clin Exp Res 2012; 24:580–587.
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014; 311:1889–1900.
- Sorkin BC, Camp KM, Haggans CJ, et al. Executive summary of NIH workshop on the use and biology of energy drinks: current knowledge and critical gaps. Nutr Rev 2014; 72(suppl 1):1–8.
- Centers for Disease Control and Prevention (CDC). Energy drink consumption and its association with sleep problems among US service members on a combat deployment—Afghanistan, 2010. MMWR Morb Mortal Wkly Rep 2012; 61:895–898.
- Bailey RL, Saldanha LG, Dwyer JT. Estimating caffeine intake from energy drinks and dietary supplements in the United States. Nutr Rev 2014; 72(suppl 1):9–13.
- Stephens MB, Attipoe S, Jones D, Ledford CJ, Deuster PA. Energy drink and energy shot use in the military. Nutr Rev 2014; 72(suppl 1):72–77.
- Triebel S, Sproll C, Reusch H, Godelmann R, Lachenmeier DW. Rapid analysis of taurine in energy drinks using amino acid analyzer and Fourier transform infrared (FTIR) spectroscopy as basis for toxicological evaluation. Amino Acids 2007; 33:451–457.
- Beverage Industry. 2013 state of the industry: energy drinks & shots. www.bevindustry.com/articles/86552-state-of-the-industry-energy-drinks-shots. Accessed October 4, 2016.
- Huxtable RJ. Physiological actions of taurine. Physiol Rev 1992; 72:101–163.
- Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. JPEN J Parenter Enteral Nutr 1990; 14:183–188.
- Manzi P, Pizzoferrato L. Taurine in milk and yoghurt marketed in Italy. Int J Food Sci Nutr 2013; 64:112–116.
- Dragnes BT, Larsen R, Ernstsen MH, Mæhre H, Elvevoll EO. Impact of processing on the taurine content in processed seafood and their corresponding unprocessed raw materials. Int J Food Sci Nutr 2009; 60:143–152.
- Laidlaw SA, Shultz TD, Cecchino JT, Kopple JD. Plasma and urine taurine levels in vegans. Am J Clin Nutr 1988; 47:660–663.
- Rana SK, Sanders TA. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br J Nutr 1986; 56:17–27.
- Alford C, Cox H, Wescott R. The effects of Red Bull energy drink on human performance and mood. Amino Acids 2001; 21:139–150.
- Hoffman JR, Ratamess NA, Ross R, Shanklin M, Kang J, Faigenbaum AD. Effect of a pre-exercise energy supplement on the acute hormonal response to resistance exercise. J Strength Cond Res 2008; 22:874–882.
- Ivy JL, Kammer L, Ding Z, et al. Improved cycling time-trial performance after ingestion of a caffeine energy drink. Int J Sport Nutr Exerc Metab 2009; 19:61–78.
- Gonzalez AM, Walsh AL, Ratamess NA, Kang J, Hoffman JR. Effect of a pre-workout energy supplement on acute multi-joint resistance exercise. J Sports Sci Med 2011; 10:261–266.
- Yeh TS, Chan KH, Hsu MC, Liu JF. Supplementation with soybean peptides, taurine, pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J Med Food 2011; 14:219–225.
- Rutherford JA, Spriet LL, Stellingwerff T. The effect of acute taurine ingestion on endurance performance and metabolism in well-trained cyclists. Int J Sport Nutr Exerc Metab 2010; 20:322–329.
- Zhang M, Izumi I, Kagamimori S, et al. Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids 2004; 26:203–207.
- Kammerer M, Jaramillo JA, García A, Calderón JC, Valbuena LH. Effects of energy drink major bioactive compounds on the performance of young adults in fitness and cognitive tests: a randomized controlled trial. J Int Soc Sports Nutr 2014; 11:44.
- Kermack WO, Slater RH. The preparation of taurine in considerable quantity. Biochem J 1927; 21:1065–1067.
- Huxtable RJ, Lippincott SE. Diet and biosynthesis as sources of taurine in the mouse. J Nutr 1982; 112:1003–1010.
- Ohkuma S, Tamura J, Kuriyama K. Roles of endogenous and exogenous taurine and glycine in the formation of conjugated bile acids: analyses using freshly isolated and primary cultured rat hepatocytes. Jpn J Pharmacol 1984; 35:347–358.
- Chapman GE, Greenwood CE. Taurine in nutrition and brain development. Nutrition Research 1988; 8:955–968.
- Andrews S, Schmidt CLA. Titration curves of taurine and cysteic acid. J Biol Chem 1927; 73:651–654.
- Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol 1989; 32:471–533.
- Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 1968; 48:424–511.
- Hope DB. Pyridoxal phosphate as the coenzyme of the mammalian decarboxylase for L-cysteine sulphinic and L-cysteic acids. Biochem J 1955; 59:497–500.
- Pasantes-Morales H, Lopez-Colome AM, Salceda R, Mandel P. Cysteine sulphinate decarboxylase in chick and rat retina during development. J Neurochem 1976; 27:1103–1106.
- Oertel WH, Schmechel DE, Weise VK, et al. Comparison of cysteine sulphinic acid decarboxylase isoenzymes and glutamic acid decarboxylase in rat liver and brain. Neuroscience 1981; 6:2701–2714.
- Reymond I, Bitoun M, Levillain O, Tappaz M. Regional expression and histological localization of cysteine sulfinate decarboxylase mRNA in the rat kidney. J Histochem Cytochem 2000; 48:1461–1468.
- Hu JM, Ikemura R, Chang KT, Suzuki M, Nishihara M, Takahashi M. Expression of cysteine sulfinate decarboxylase mRNA in rat mammary gland. J Vet Med Sci 2000; 62:829–834.
- Ueki I, Stipanuk MH. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J Nutr 2007; 137:1887–1894.
- Li JH, Ling YQ, Fan JJ, Zhang XP, Cui S. Expression of cysteine sulfinate decarboxylase (CSD) in male reproductive organs of mice. Histochem Cell Biol 2006; 125:607–613.
- Green TR, Fellman JH, Eicher AL, Pratt KL. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta 1991; 1073:91–97.
- Lefauconnier JM, Portemer C, Chatagner F. Free amino acids and related substances in human glial tumours and in fetal brain: comparison with normal adult brain. Brain Res 1976; 117:105–113.
- Sturman JA, Rassin DK, Gaull GE. Taurine in development. Life Sci 1977; 21:1–22.
- Sturman JA. Taurine pool sizes in the rat: effects of vitamin B-6 deficiency and high taurine diet. J Nutr 1973; 103:1566–1580.
- Terauchi A, Nakazaw A, Johkura K, Yan L, Usuda N. Immunohistochemical localization of taurine in various tissues of the mouse. Amino Acids 1998; 15:151–160.
- Lobo MV, Alonso FJ, del Río RM. Immunohistochemical localization of taurine in the male reproductive organs of the rat. J Histochem Cytochem 2000; 48:313–320.
- Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ. A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod 2010; 25:847–852.
- Holmes RP, Goodman HO, Shihabi ZK, Jarow JP. The taurine and hypotaurine content of human semen. J Androl 1992; 13:289–292.
- Alvarez JG, Storey BT. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol Reprod 1983; 29:548–555.
- Das J, Ghosh J, Manna P, Sinha M, Sil PC. Taurine protects rat testes against NaAsO(2)-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 2009; 187:201–210.
- Mrsny RJ, Meizel S. Inhibition of hamster sperm Na+, K+-ATPase activity by taurine and hypotaurine. Life Sci 1985; 36:271–275.
- Phoenix J, Wray S. Changes in human and rat uterine phosphoethanolamine and taurine with pregnancy and parturition. Exp Physiol 1994; 79:601–604.
- Lobo MV, Alonso FJ, Latorre A, del Río RM. Immunohistochemical localization of taurine in the rat ovary, oviduct, and uterus. J Histochem Cytochem 2001; 49:1133–1142.
- Casslén BG. Free amino acids in human uterine fluid. Possible role of high taurine concentration. J Reprod Med 1987; 32:181–184.
- Pamblanco M, Portolés M, Paredes C, Ten A, Comín J. Free amino acids in preterm and term milk from mothers delivering appropriate- or small-for-gestational-age infants. Am J Clin Nutr 1989; 50:778–781.
- Rigo J, Senterre J. Is taurine essential for the neonates? Biol Neonate 1977; 32:73–76.
- Sturman JA, Hepner GW, Hofmann AF, Thomas PJ. Metabolism of [35S]taurine in man. J Nutr 1975; 105:1206–1214.
- Bremer J. Species differences in the conjugation of free bile acids with taurine and glycine. Biochem J 1956; 63:507–513.
- Wu JY, Tang XW, Tsai WH. Taurine receptor: kinetic analysis and pharmacological studies. Adv Exp Med Biol 1992; 315:263–268.
- Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis 2012; 18:2673–2686.
- Haas HL, Hösli L. The depression of brain stem neurones by taurine and its interaction with strychnine and bicuculline. Brain Res 1973; 52:399–402.
- Bureau MH, Olsen RW. Taurine acts on a subclass of GABAA receptors in mammalian brain in vitro. Eur J Pharmacol 1991; 207:9–16.
- Bhattarai JP, Park SJ, Chun SW, Cho DH, Han SK. Activation of synaptic and extrasynaptic glycine receptors by taurine in preoptic hypothalamic neurons. Neurosci Lett 2015; 608:51–56.
- Horikoshi T, Asanuma A, Yanagisawa K, Anzai K, Goto S. Taurine and beta-alanine act on both GABA and glycine receptors in Xenopus oocyte injected with mouse brain messenger RNA. Brain Res 1988; 464:97–105.
- Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci 2010; 31:161–174.
- Fujita T, Sato Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J Clin Invest 1988; 82:993–997.
- Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation 1987; 75:525–532.
- Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol 1996; 403:615–622.
- Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl 1984; 2:S563–S565.
- Fujita T, Sato Y, Ando K. Changes in cardiac and hypothalamic noradrenergic activity with taurine in DOCA-salt rats. Am J Physiol 1986; 251:H926–H933.
- Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension 1987; 9:81–87.
- Scheibel J, Elsasser T, Ondo JG. A neuromodulatory role for taurine in controlling prolactin secretion in female rats. Psychoneuroendocrinology 1981; 6:139–144.
- Ikuyama S, Okajima T, Kato K, Ibayashi H. Effect of taurine on growth hormone and prolactin secretion in rats: possible interaction with opioid peptidergic system. Life Sci 1988; 43:807–812.
- Park E, Park SY, Dobkin C, Schuller-Levis G. Development of a novel cysteine sulfinic acid decarboxylase knockout mouse: dietary taurine reduces neonatal mortality. J Amino Acids 2014; 2014:346809.
- Mantovani J, DeVivo DC. Effects of taurine on seizures and growth hormone release in epileptic patients. Arch Neurol 1979; 36:672–674.
- Carlson HE, Miglietta JT, Roginsky MS, Stegink LD. Stimulation of pituitary hormone secretion by neurotransmitter amino acids in humans. Metabolism 1989; 38:1179–1182.
- Yang J, Wu G, Feng Y, Lv Q, Lin S, Hu J. Effects of taurine on male reproduction in rats of different ages. J Biomed Sci 2010; 17(suppl 1):S9.
- Yang J, Lin S, Feng Y, Wu G, Hu J. Taurine enhances the sexual response and mating ability in aged male rats. Adv Exp Med Biol 2013; 776:347–355.
- Yang J, Zong X, Wu G, Lin S, Feng Y, Hu J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids 2015; 47:1549–1558.
- Scheibel J, Elsasser T, Ondo JG. Stimulation of LH and FSH secretion following intraventricular injection of cysteic acid but not taurine. Brain Res 1980; 201:99–106.
- Price MT, Olney JW, Mitchell MV, Fuller T, Cicero TJ. Luteinizing hormone releasing action of N-methyl aspartate is blocked by GABA or taurine but not by dopamine antagonists. Brain Res 1978; 158:461–465.
- Ma Q, Zhao J, Cao W, Liu J, Cui S. Estradiol decreases taurine level by reducing cysteine sulfinic acid decarboxylase via the estrogen receptor-a in female mice liver. Am J Physiol Gastrointest Liver Physiol 2015; 308:G277–G286.
- Giles GE, Mahoney CR, Brunyé TT, Gardony AL, Taylor HA, Kanarek RB. Differential cognitive effects of energy drink ingredients: caffeine, taurine, and glucose. Pharmacol Biochem Behav 2012; 102:569–577.
- Giehl TJ, Qoronfleh MW, Wilkinson BJ. Transport, nutritional and metabolic studies of taurine in staphylococci. J Gen Microbiol 1987; 133:849–856.
- Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids 2015 Oct 16. Epub ahead of print.
- Schaffer SW, Jong CJ, Ito T, Azuma J. Role of taurine in the pathologies of MELAS and MERRF. Amino Acids 2014; 46:47–56.
- Warskulat U, Heller-Stilb B, Oermann E, et al. Phenotype of the taurine transporter knockout mouse. Methods Enzymol 2007; 428:439–458.
- Yatabe Y, Miyakawa S, Miyazaki T, Matsuzaki Y, Ochiai N. Effects of taurine administration in rat skeletal muscles on exercise. J Orthop Sci 2003; 8:415–419.
- Miyazaki T, Matsuzaki Y, Ikegami T, et al. Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acids 2004; 27:291–298.
- Goodman CA, Horvath D, Stathis C, et al. Taurine supplementation increases skeletal muscle force production and protects muscle function during and after high-frequency in vitro stimulation. J Appl Physiol (1985) 2009; 107:144–154.
- Van Winkle LJ, Patel M, Wasserlauf HG, Dickinson HR, Campione AL. Osmotic regulation of taurine transport via system beta and novel processes in mouse preimplantation conceptuses. Biochim Biophys Acta 1994; 1191:244–255.
- Ito T, Kimura Y, Uozumi Y, et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 2008; 44:927–937.
- Aydın AF, Çoban J, Dogan-Ekici I, Betül-Kalaz E, Dogru-Abbasoglu S, Uysal M. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab Brain Dis 2016; 31:337–345.
- Nagai K, Fukuno S, Oda A, Konishi H. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs 2016; 27:17–23.
- Caletti G, Almeida FB, Agnes G, Nin MS, Barros HM, Gomez R. Antidepressant dose of taurine increases mRNA expression of GABAA receptor a2 subunit and BDNF in the hippocampus of diabetic rats. Behav Brain Res 2015; 283:11–55.
- Gu Y, Zhao Y, Qian K, Sun M. Taurine attenuates hippocampal and corpus callosum damage, and enhances neurological recovery after closed head injury in rats. Neuroscience 2015; 291:331–340.
- Banks MA, Porter DW, Pailes WH, Schwegler-Berry D, Martin WG, Castranova V. Taurine content of isolated rat alveolar type I cells. Comp Biochem Physiol B 1991; 100:795–799.
- Raschke P, Massoudy P, Becker BF. Taurine protects the heart from neutrophil-induced reperfusion injury. Free Radic Biol Med 1995; 19:461–471.
- Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids 2004; 27:91–96.
- Refik Mas M, Comert B, Oncu K, et al. The effect of taurine treatment on oxidative stress in experimental liver fibrosis. Hepatol Res 2004; 28:207–215.
- Sebring LA, Huxtable RJ. Taurine modulation of calcium binding to cardiac sarcolemma. J Pharmacol Exp Ther 1985; 232:445–451.
- Schaffer SW, Kramer J, Chovan JP. Regulation of calcium homeostasis in the heart by taurine. Fed Proc 1980; 39:2691–2694.
- Foos TM, Wu JY. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 2002; 27:21–26.
- Ito T, Schaffer S, Azuma J. The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids 2014; 46:111–119.
- Gaull GE. Taurine in pediatric nutrition: review and update. Pediatrics 1989; 83:433–442.
- Sturman JA, Rassin DK, Gaull GE. Taurine in developing rat brain: transfer of [35S] taurine to pups via the milk. Pediatr Res 1977; 11:28–33.
- Geggel HS, Ament ME, Heckenlively JR, Martin DA, Kopple JD. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N Engl J Med 1985; 312:142–146.
- Sturman JA, Gargano AD, Messing JM, Imaki H. Feline maternal taurine deficiency: effect on mother and offspring. J Nutr 1986; 116:655–667.
- Lerdweeraphon W, Wyss JM, Boonmars T, Roysommuti S. Perinatal taurine exposure affects adult oxidative stress. Am J Physiol Regul Integr Comp Physiol 2013; 305:R95–R97.
- Chesney RW, Helms RA, Christensen M, Budreau AM, Han X, Sturman JA. The role of taurine in infant nutrition. Adv Exp Med Biol 1998; 442:463–476.
- Verner A, Craig S, McGuire W. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev 2007; 4:CD006072.
- Jeevanandam M, Young DH, Ramias L, Schiller WR. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am J Clin Nutr 1990; 51:1040–1045.
- Dawson R Jr, Eppler B, Patterson TA, Shih D, Liu S. The effects of taurine in a rodent model of aging. Adv Exp Med Biol 1996; 403:37–50.
- Han X, Budreau AM, Chesney RW. Molecular cloning and functional expression of an LLC-PK1 cell taurine transporter that is adaptively regulated by taurine. Adv Exp Med Biol 1998; 442:261–268.
- Eppler B, Dawson R Jr. Dietary taurine manipulations in aged male Fischer 344 rat tissue: taurine concentration, taurine biosynthesis, and oxidative markers. Biochem Pharmacol 2001; 62:29–39.
- D’Eufemia P, Finocchiaro R, Celli M, et al. Taurine deficiency in thalassemia major-induced osteoporosis treated with neridronate. Biomed Pharmacother 2010; 64:271–274.
- Muscaritoli M, Conversano L, Petti MC, et al. Plasma amino acid concentrations in patients with acute myelogenous leukemia. Nutrition 1999; 15:195–199.
- Do KQ, Lauer CJ, Schreiber W, et al. Gamma-glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem 1995; 65:2652–2662.
- Lemieux B, Giguère R, Shapcott D. Studies on the role of taurine in Friedreich’s ataxia. Can J Neurol Sci 1984; 11(suppl 4):610–615.
- Airaksinen EM, Oja SS, Marnela KM, Sihvola P. Taurine and other amino acids of platelets and plasma in retinitis pigmentosa. Ann Clin Res 1980; 12:52–54.
- Kohashi N, Katori R. Decrease of urinary taurine in essential hypertension. Jpn Heart J 1983; 24:91–102.
- vom Dahl S, Mönnighoff I, Häussinger D. Decrease of plasma taurine in Gaucher disease and its sustained correction during enzyme replacement therapy. Amino Acids 2000; 19:585–592.
- Schneider SM, Joly F, Gehrardt MF, et al. Taurine status and response to intravenous taurine supplementation in adults with short-bowel syndrome undergoing long-term parenteral nutrition: a pilot study. Br J Nutr 2006; 96:365–370.
- Gray GE, Landel AM, Meguid MM. Taurine-supplemented total parenteral nutrition and taurine status of malnourished cancer patients. Nutrition 1994; 10:11–15.
- Thompson GN. Assessment of taurine deficiency in cystic fibrosis. Clin Chim Acta 1988; 171:233–237.
- Cho KH, Kim ES, Chen JD. Taurine intake and excretion in patients undergoing long term enteral nutrition. Adv Exp Med Biol 2000; 483:605–612.
- Durelli L, Mutani R, Fassio F. The treatment of myotonia: evaluation of chronic oral taurine therapy. Neurology 1983; 33:599–603.
- Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol 2008; 50:376–399.
- European Commission; Scientific Committee on Food. Opinion on additional information on energy drinks. http://ec.europa.eu/food/fs/sc/scf/out169_en.pdf. Accessed October 4, 2016.
- Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. J Clin Pharm Ther 2000; 25:165–175.
- Kerai MD, Waterfield CJ, Kenyon SH, Asker DS, Timbrell JA. Reversal of ethanol-induced hepatic steatosis and lipid peroxidation by taurine: a study in rats. Alcohol Alcohol 1999; 34:529–541.
- Das J, Ghosh J, Manna P, Sil PC. Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res 2010; 44:340–355.
- El Idrissi A, Shen CH, L’amoreaux WJ. Neuroprotective role of taurine during aging. Amino Acids 2013; 45:735–750.
- Gharibani P, Modi J, Menzie J, et al. Comparison between single and combined post-treatment with S-methyl-N,N-diethylthiolcarbamate sulfoxide and taurine following transient focal cerebral ischemia in rat brain. Neuroscience 2015; 300:460–473.
- Junyent F, De Lemos L, Utrera J, et al. Content and traffic of taurine in hippocampal reactive astrocytes. Hippocampus 2011; 21:185–197.
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 1999; 19:9459–9468.
- Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res 2005; 1038:123–131.
- Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 2005; 49:1140–1148.
- Takatani T, Takahashi K, Uozumi Y, et al. Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Cell Physiol 2004; 287:C949–C953.
- Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis 2010; 20(suppl 2):S439–S452.
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett 2008; 436:19–22.
- Oja SS, Saransaari P. Taurine and epilepsy. Epilepsy Res 2013; 104:187–194.
- Berson EL, Hayes KC, Rabin AR, Schmidt SY, Watson G. Retinal degeneration in cats fed casein. II. Supplementation with methionine, cysteine, or taurine. Invest Ophthalmol 1976; 15:52–58.
- Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 2011; 57:333–337.
- Eby G, Halcomb WW. Elimination of cardiac arrhythmias using oral taurine with l-arginine with case histories: hypothesis for nitric oxide stabilization of the sinus node. Med Hypotheses 2006; 67:1200–1204.
KEY POINTS
- Energy drinks are widely consumed in the United States, with an estimated 354 million gallons sold in 2009, or approximately 5.25 L/year per person over age 10.
- Taurine has been reported to have anti-inflammatory action. Supplementation has been proposed to have beneficial effects in epilepsy, heart failure, cystic fibrosis, and diabetes, and has been shown in animal studies to protect against neurotoxic insults from alcohol, ammonia, lead, and other substances.
- Taurine is an inhibitory neurotransmitter and neuromodulator. It is structurally analogous to gamma-aminobutyric acid, the main inhibitory neurotransmitter in the brain.
Thrombolysis in submassive pulmonary embolism: Finding the balance
In this issue of the Journal, Ataya et al1 provide a comprehensive review of thrombolysis in submassive pulmonary embolism, a subject of much debate. In massive pulmonary embolism, thrombolytic therapy is usually indicated2; in submassive pulmonary embolism, the decision is not so clear. Which patients with submassive embolism would benefit from thrombolysis, and which patients require only anticoagulant therapy? The answer lies in finding the balance between the potential benefit of thrombolytic therapy—preventing death or hemodynamic collapse—and the numerically low but potentially catastrophic risk of intracranial bleeding.
In general, submassive pulmonary embolism refers to an acute pulmonary embolus serious enough to cause evidence of right ventricular dysfunction or necrosis but not hemodynamic instability (ie, with systolic blood pressure > 90 mm Hg) on presentation.3 It accounts for about 25% of cases of pulmonary embolism,4,5 and perhaps 0.5 to 1% of patients admitted to intensive care units across the country.6 The 30-day mortality rate can be as high as 30%, making it a condition that requires prompt identification and appropriate management.
But clinical trials have failed to demonstrate a substantial improvement in mortality rates with thrombolytic therapy in patients with submassive pulmonary embolism, and have shown improvement only in other clinical end points.7 Part of the problem is that this is a heterogeneous condition, posing a challenge for the optimal design and interpretation of studies.
WHO IS AT RISK OF DEATH OR DETERIORATION?
If clinicians could ascertain in each patient whether the risk-benefit ratio is favorable for thrombolytic therapy, it would be easier to provide optimal care. This is not a straightforward task, and it requires integration of clinical judgment, high index of suspicion for deterioration, and clinical tools.
One of the challenges is that it is difficult to identify normotensive patients at the highest risk of poor outcomes. Several factors are associated with a higher risk of death within 30 days (Table 1). While each of these has a negative predictive value of about 95% or even higher (meaning that it is very good at predicting who will not die), they all have very low positive predictive values (meaning that none of them, by itself, is very good at predicting who will die).
For this reason, a multimodal approach to risk stratification has emerged. For example, Jiménez et al8 showed that normotensive patients with acute pulmonary embolism and a combination of abnormal Simplified Pulmonary Embolism Severity Index, elevated B-type natriuretic peptide level, elevated troponin level, and lower-extremity deep vein thrombosis had a 26% rate of complications (death, hemodynamic collapse, or recurrent pulmonary embolism) within 30 days.
Bova et al9 showed that the combination of borderline low systolic blood pressure (90–100 mm Hg), tachycardia (heart rate ≥ 110 beats per minute), elevated troponin, and right ventricular dysfunction by echocardiography or computed tomography allowed for the separation of three groups with significantly different rates of poor outcomes.
WHO IS AT RISK OF BLEEDING?
Estimation of the risk of bleeding is currently less sophisticated, and we need a bleeding score to use in the setting of acute pulmonary embolism. A few studies have shed some light on this issue beyond the known absolute and relative contraindications to thrombolysis.
Ataya et al1 note a meta-analysis10 showing that systemic thrombolytic therapy was not associated with an increased risk of major bleeding in patients age 65 or younger. Similarly, a large observational study showed a strong association between the risk of intracerebral hemorrhage and increasing age11 and also identified comorbidities such as kidney disease as risk factors. While the frequently cited Pulmonary Embolism Thrombolysis trial12 showed a significantly higher risk of stroke with tenecteplase, careful review of its data reveals that all 10 of the 506 patients in the tenecteplase group who sustained a hemorrhagic stroke were age 65 or older.12
A TEAM APPROACH
Thus, in patients with acute pulmonary embolism, clinicians face the difficult task of assessing the patient’s risk of death and clinical worsening and balancing that risk against the risk of bleeding, to identify those who may benefit from early reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis, mechanical treatment, and surgical embolectomy.
Given the absence of high-quality evidence to guide these decisions, several institutions have developed multidisciplinary pulmonary embolism response teams to provide rapid evaluation and risk stratification and to recommend and implement advanced therapies, as appropriate. This is a novel concept that is still evolving but holds promise, as it integrates the experience and expertise of physicians in multiple specialties, such as pulmonary and critical care medicine, vascular medicine, interventional radiology, interventional cardiology, emergency medicine, and cardiothoracic surgery, who can then fill the currently existing knowledge gaps for clinical care and, possibly, research.13
Early published experience has documented the feasibility of this multidisciplinary approach.14 The first 95 patients treated at Cleveland Clinic had a 30-day mortality rate of 3.2%, which was lower than the expected 9% rate predicted by the Pulmonary Embolism Severity Index score (unpublished observation).
Figure 1 shows the algorithm currently used by Cleveland Clinic’s pulmonary embolism response team, with the caveat that no algorithm can fully capture the extent of the complexities and discussions that each case triggers within the team.
TOWARD BETTER UNDERSTANDING
As Ataya et al point out,1 the current state of the evidence does not allow a clear, simplistic, one-size-fits-all approach. A question that arises from this controversial topic is whether we should look for markers of right ventricular dysfunction in every patient admitted with a diagnosis of pulmonary embolism, or only in those with a significant anatomic burden of clot on imaging. Would testing everyone be an appropriate way to identify patients at risk of further deterioration early and therefore prevent adverse outcomes in a timely manner? Or would it not be cost-effective and translate into ordering more diagnostic testing, as well as an increase in downstream workup with higher healthcare costs?
Once we better understand this condition and the factors that predict a higher risk of deterioration, we should be able to design prospective studies that can help elucidate the most appropriate diagnostic and therapeutic approach for such challenging cases. In the meantime, it is important to appraise the evidence in a critical way, as Ataya et al have done in their review.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. The role of thrombolytic therapy in patients with submassive pulmonary embolism. Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28–e32.
- Busse LW, Vourlekis JS. Submassive pulmonary embolism. Crit Care Clin 2014; 30:447–473.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5:97–103.
- Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med 2010; 15:419–428.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718–726.
- Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J 2014; 44:694–703.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med 2012; 125:50–56.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133:98–103.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150:384–393.
In this issue of the Journal, Ataya et al1 provide a comprehensive review of thrombolysis in submassive pulmonary embolism, a subject of much debate. In massive pulmonary embolism, thrombolytic therapy is usually indicated2; in submassive pulmonary embolism, the decision is not so clear. Which patients with submassive embolism would benefit from thrombolysis, and which patients require only anticoagulant therapy? The answer lies in finding the balance between the potential benefit of thrombolytic therapy—preventing death or hemodynamic collapse—and the numerically low but potentially catastrophic risk of intracranial bleeding.
In general, submassive pulmonary embolism refers to an acute pulmonary embolus serious enough to cause evidence of right ventricular dysfunction or necrosis but not hemodynamic instability (ie, with systolic blood pressure > 90 mm Hg) on presentation.3 It accounts for about 25% of cases of pulmonary embolism,4,5 and perhaps 0.5 to 1% of patients admitted to intensive care units across the country.6 The 30-day mortality rate can be as high as 30%, making it a condition that requires prompt identification and appropriate management.
But clinical trials have failed to demonstrate a substantial improvement in mortality rates with thrombolytic therapy in patients with submassive pulmonary embolism, and have shown improvement only in other clinical end points.7 Part of the problem is that this is a heterogeneous condition, posing a challenge for the optimal design and interpretation of studies.
WHO IS AT RISK OF DEATH OR DETERIORATION?
If clinicians could ascertain in each patient whether the risk-benefit ratio is favorable for thrombolytic therapy, it would be easier to provide optimal care. This is not a straightforward task, and it requires integration of clinical judgment, high index of suspicion for deterioration, and clinical tools.
One of the challenges is that it is difficult to identify normotensive patients at the highest risk of poor outcomes. Several factors are associated with a higher risk of death within 30 days (Table 1). While each of these has a negative predictive value of about 95% or even higher (meaning that it is very good at predicting who will not die), they all have very low positive predictive values (meaning that none of them, by itself, is very good at predicting who will die).
For this reason, a multimodal approach to risk stratification has emerged. For example, Jiménez et al8 showed that normotensive patients with acute pulmonary embolism and a combination of abnormal Simplified Pulmonary Embolism Severity Index, elevated B-type natriuretic peptide level, elevated troponin level, and lower-extremity deep vein thrombosis had a 26% rate of complications (death, hemodynamic collapse, or recurrent pulmonary embolism) within 30 days.
Bova et al9 showed that the combination of borderline low systolic blood pressure (90–100 mm Hg), tachycardia (heart rate ≥ 110 beats per minute), elevated troponin, and right ventricular dysfunction by echocardiography or computed tomography allowed for the separation of three groups with significantly different rates of poor outcomes.
WHO IS AT RISK OF BLEEDING?
Estimation of the risk of bleeding is currently less sophisticated, and we need a bleeding score to use in the setting of acute pulmonary embolism. A few studies have shed some light on this issue beyond the known absolute and relative contraindications to thrombolysis.
Ataya et al1 note a meta-analysis10 showing that systemic thrombolytic therapy was not associated with an increased risk of major bleeding in patients age 65 or younger. Similarly, a large observational study showed a strong association between the risk of intracerebral hemorrhage and increasing age11 and also identified comorbidities such as kidney disease as risk factors. While the frequently cited Pulmonary Embolism Thrombolysis trial12 showed a significantly higher risk of stroke with tenecteplase, careful review of its data reveals that all 10 of the 506 patients in the tenecteplase group who sustained a hemorrhagic stroke were age 65 or older.12
A TEAM APPROACH
Thus, in patients with acute pulmonary embolism, clinicians face the difficult task of assessing the patient’s risk of death and clinical worsening and balancing that risk against the risk of bleeding, to identify those who may benefit from early reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis, mechanical treatment, and surgical embolectomy.
Given the absence of high-quality evidence to guide these decisions, several institutions have developed multidisciplinary pulmonary embolism response teams to provide rapid evaluation and risk stratification and to recommend and implement advanced therapies, as appropriate. This is a novel concept that is still evolving but holds promise, as it integrates the experience and expertise of physicians in multiple specialties, such as pulmonary and critical care medicine, vascular medicine, interventional radiology, interventional cardiology, emergency medicine, and cardiothoracic surgery, who can then fill the currently existing knowledge gaps for clinical care and, possibly, research.13
Early published experience has documented the feasibility of this multidisciplinary approach.14 The first 95 patients treated at Cleveland Clinic had a 30-day mortality rate of 3.2%, which was lower than the expected 9% rate predicted by the Pulmonary Embolism Severity Index score (unpublished observation).
Figure 1 shows the algorithm currently used by Cleveland Clinic’s pulmonary embolism response team, with the caveat that no algorithm can fully capture the extent of the complexities and discussions that each case triggers within the team.
TOWARD BETTER UNDERSTANDING
As Ataya et al point out,1 the current state of the evidence does not allow a clear, simplistic, one-size-fits-all approach. A question that arises from this controversial topic is whether we should look for markers of right ventricular dysfunction in every patient admitted with a diagnosis of pulmonary embolism, or only in those with a significant anatomic burden of clot on imaging. Would testing everyone be an appropriate way to identify patients at risk of further deterioration early and therefore prevent adverse outcomes in a timely manner? Or would it not be cost-effective and translate into ordering more diagnostic testing, as well as an increase in downstream workup with higher healthcare costs?
Once we better understand this condition and the factors that predict a higher risk of deterioration, we should be able to design prospective studies that can help elucidate the most appropriate diagnostic and therapeutic approach for such challenging cases. In the meantime, it is important to appraise the evidence in a critical way, as Ataya et al have done in their review.
In this issue of the Journal, Ataya et al1 provide a comprehensive review of thrombolysis in submassive pulmonary embolism, a subject of much debate. In massive pulmonary embolism, thrombolytic therapy is usually indicated2; in submassive pulmonary embolism, the decision is not so clear. Which patients with submassive embolism would benefit from thrombolysis, and which patients require only anticoagulant therapy? The answer lies in finding the balance between the potential benefit of thrombolytic therapy—preventing death or hemodynamic collapse—and the numerically low but potentially catastrophic risk of intracranial bleeding.
In general, submassive pulmonary embolism refers to an acute pulmonary embolus serious enough to cause evidence of right ventricular dysfunction or necrosis but not hemodynamic instability (ie, with systolic blood pressure > 90 mm Hg) on presentation.3 It accounts for about 25% of cases of pulmonary embolism,4,5 and perhaps 0.5 to 1% of patients admitted to intensive care units across the country.6 The 30-day mortality rate can be as high as 30%, making it a condition that requires prompt identification and appropriate management.
But clinical trials have failed to demonstrate a substantial improvement in mortality rates with thrombolytic therapy in patients with submassive pulmonary embolism, and have shown improvement only in other clinical end points.7 Part of the problem is that this is a heterogeneous condition, posing a challenge for the optimal design and interpretation of studies.
WHO IS AT RISK OF DEATH OR DETERIORATION?
If clinicians could ascertain in each patient whether the risk-benefit ratio is favorable for thrombolytic therapy, it would be easier to provide optimal care. This is not a straightforward task, and it requires integration of clinical judgment, high index of suspicion for deterioration, and clinical tools.
One of the challenges is that it is difficult to identify normotensive patients at the highest risk of poor outcomes. Several factors are associated with a higher risk of death within 30 days (Table 1). While each of these has a negative predictive value of about 95% or even higher (meaning that it is very good at predicting who will not die), they all have very low positive predictive values (meaning that none of them, by itself, is very good at predicting who will die).
For this reason, a multimodal approach to risk stratification has emerged. For example, Jiménez et al8 showed that normotensive patients with acute pulmonary embolism and a combination of abnormal Simplified Pulmonary Embolism Severity Index, elevated B-type natriuretic peptide level, elevated troponin level, and lower-extremity deep vein thrombosis had a 26% rate of complications (death, hemodynamic collapse, or recurrent pulmonary embolism) within 30 days.
Bova et al9 showed that the combination of borderline low systolic blood pressure (90–100 mm Hg), tachycardia (heart rate ≥ 110 beats per minute), elevated troponin, and right ventricular dysfunction by echocardiography or computed tomography allowed for the separation of three groups with significantly different rates of poor outcomes.
WHO IS AT RISK OF BLEEDING?
Estimation of the risk of bleeding is currently less sophisticated, and we need a bleeding score to use in the setting of acute pulmonary embolism. A few studies have shed some light on this issue beyond the known absolute and relative contraindications to thrombolysis.
Ataya et al1 note a meta-analysis10 showing that systemic thrombolytic therapy was not associated with an increased risk of major bleeding in patients age 65 or younger. Similarly, a large observational study showed a strong association between the risk of intracerebral hemorrhage and increasing age11 and also identified comorbidities such as kidney disease as risk factors. While the frequently cited Pulmonary Embolism Thrombolysis trial12 showed a significantly higher risk of stroke with tenecteplase, careful review of its data reveals that all 10 of the 506 patients in the tenecteplase group who sustained a hemorrhagic stroke were age 65 or older.12
A TEAM APPROACH
Thus, in patients with acute pulmonary embolism, clinicians face the difficult task of assessing the patient’s risk of death and clinical worsening and balancing that risk against the risk of bleeding, to identify those who may benefit from early reperfusion therapies, including systemic thrombolysis, catheter-directed thrombolysis, mechanical treatment, and surgical embolectomy.
Given the absence of high-quality evidence to guide these decisions, several institutions have developed multidisciplinary pulmonary embolism response teams to provide rapid evaluation and risk stratification and to recommend and implement advanced therapies, as appropriate. This is a novel concept that is still evolving but holds promise, as it integrates the experience and expertise of physicians in multiple specialties, such as pulmonary and critical care medicine, vascular medicine, interventional radiology, interventional cardiology, emergency medicine, and cardiothoracic surgery, who can then fill the currently existing knowledge gaps for clinical care and, possibly, research.13
Early published experience has documented the feasibility of this multidisciplinary approach.14 The first 95 patients treated at Cleveland Clinic had a 30-day mortality rate of 3.2%, which was lower than the expected 9% rate predicted by the Pulmonary Embolism Severity Index score (unpublished observation).
Figure 1 shows the algorithm currently used by Cleveland Clinic’s pulmonary embolism response team, with the caveat that no algorithm can fully capture the extent of the complexities and discussions that each case triggers within the team.
TOWARD BETTER UNDERSTANDING
As Ataya et al point out,1 the current state of the evidence does not allow a clear, simplistic, one-size-fits-all approach. A question that arises from this controversial topic is whether we should look for markers of right ventricular dysfunction in every patient admitted with a diagnosis of pulmonary embolism, or only in those with a significant anatomic burden of clot on imaging. Would testing everyone be an appropriate way to identify patients at risk of further deterioration early and therefore prevent adverse outcomes in a timely manner? Or would it not be cost-effective and translate into ordering more diagnostic testing, as well as an increase in downstream workup with higher healthcare costs?
Once we better understand this condition and the factors that predict a higher risk of deterioration, we should be able to design prospective studies that can help elucidate the most appropriate diagnostic and therapeutic approach for such challenging cases. In the meantime, it is important to appraise the evidence in a critical way, as Ataya et al have done in their review.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. The role of thrombolytic therapy in patients with submassive pulmonary embolism. Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28–e32.
- Busse LW, Vourlekis JS. Submassive pulmonary embolism. Crit Care Clin 2014; 30:447–473.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5:97–103.
- Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med 2010; 15:419–428.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718–726.
- Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J 2014; 44:694–703.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med 2012; 125:50–56.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133:98–103.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150:384–393.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. The role of thrombolytic therapy in patients with submassive pulmonary embolism. Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005; 112:e28–e32.
- Busse LW, Vourlekis JS. Submassive pulmonary embolism. Crit Care Clin 2014; 30:447–473.
- Tapson VF. Acute pulmonary embolism. N Engl J Med 2008; 358:1037–1052.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Bahloul M, Chaari A, Kallel H, et al. Pulmonary embolism in intensive care unit: predictive factors, clinical manifestations and outcome. Ann Thorac Med 2010; 5:97–103.
- Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med 2010; 15:419–428.
- Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med 2014; 189:718–726.
- Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J 2014; 44:694–703.
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311:2414–2421.
- Stein PD, Matta F, Steinberger DS, Keyes DC. Intracerebral hemorrhage with thrombolytic therapy for acute pulmonary embolism. Am J Med 2012; 125:50–56.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation 2016; 133:98–103.
- Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest 2016; 150:384–393.
Minerals used in dermatology part of NIH-Smithsonian exhibit
A geologic display currently on exhibit at the National Institutes of Health Clinical Center in Bethesda, Md., includes several samples of minerals that can be utilized in dermatology.
The “Minerals in Medicine” exhibit, put on by the Clinical Center in partnership with the Smithsonian National Museum of Natural History, includes “more than 40 minerals that are crucial to human health and biomedicine,” according to an NIH statement.
Edward W. Cowen, MD, senior clinician and head of the dermatology consultation service in the National Cancer Institute’s Center for Cancer Research, noted that copper ions have been used for centuries as a disinfecting agent. “In modern medicine, copper-impregnated wound care dressings have been proposed as a mechanism to decrease bacterial colonization with less bacterial resistance than is seen with conventional antibiotic therapy,” he said in an interview.
Sulphur with calcite also is displayed. “Sulfur is found in oral antibiotics used every day, such as penicillin and sulfamethoxazole/trimethoprim, and many topical preparations, ranging from soap to creams to shampoos, where it is effective for the treatment of acne, rosacea, seborrheic dermatitis, and scabies infestation,” Dr. Cowen added. “One common adverse effect of topical medications containing elemental sulfur is the unpleasant smell – sulfur compounds are responsible for the unique fragrance of skunks, among other odors. Interestingly, researchers have found that the exquisite human sensitivity of our olfactory receptors to detect the smell of sulfur is due to another element – copper,” he said.
A geologic display currently on exhibit at the National Institutes of Health Clinical Center in Bethesda, Md., includes several samples of minerals that can be utilized in dermatology.
The “Minerals in Medicine” exhibit, put on by the Clinical Center in partnership with the Smithsonian National Museum of Natural History, includes “more than 40 minerals that are crucial to human health and biomedicine,” according to an NIH statement.
Edward W. Cowen, MD, senior clinician and head of the dermatology consultation service in the National Cancer Institute’s Center for Cancer Research, noted that copper ions have been used for centuries as a disinfecting agent. “In modern medicine, copper-impregnated wound care dressings have been proposed as a mechanism to decrease bacterial colonization with less bacterial resistance than is seen with conventional antibiotic therapy,” he said in an interview.
Sulphur with calcite also is displayed. “Sulfur is found in oral antibiotics used every day, such as penicillin and sulfamethoxazole/trimethoprim, and many topical preparations, ranging from soap to creams to shampoos, where it is effective for the treatment of acne, rosacea, seborrheic dermatitis, and scabies infestation,” Dr. Cowen added. “One common adverse effect of topical medications containing elemental sulfur is the unpleasant smell – sulfur compounds are responsible for the unique fragrance of skunks, among other odors. Interestingly, researchers have found that the exquisite human sensitivity of our olfactory receptors to detect the smell of sulfur is due to another element – copper,” he said.
A geologic display currently on exhibit at the National Institutes of Health Clinical Center in Bethesda, Md., includes several samples of minerals that can be utilized in dermatology.
The “Minerals in Medicine” exhibit, put on by the Clinical Center in partnership with the Smithsonian National Museum of Natural History, includes “more than 40 minerals that are crucial to human health and biomedicine,” according to an NIH statement.
Edward W. Cowen, MD, senior clinician and head of the dermatology consultation service in the National Cancer Institute’s Center for Cancer Research, noted that copper ions have been used for centuries as a disinfecting agent. “In modern medicine, copper-impregnated wound care dressings have been proposed as a mechanism to decrease bacterial colonization with less bacterial resistance than is seen with conventional antibiotic therapy,” he said in an interview.
Sulphur with calcite also is displayed. “Sulfur is found in oral antibiotics used every day, such as penicillin and sulfamethoxazole/trimethoprim, and many topical preparations, ranging from soap to creams to shampoos, where it is effective for the treatment of acne, rosacea, seborrheic dermatitis, and scabies infestation,” Dr. Cowen added. “One common adverse effect of topical medications containing elemental sulfur is the unpleasant smell – sulfur compounds are responsible for the unique fragrance of skunks, among other odors. Interestingly, researchers have found that the exquisite human sensitivity of our olfactory receptors to detect the smell of sulfur is due to another element – copper,” he said.