User login
NETWORKS
Interventional Chest/Diagnostic Procedures: Evolving approaches to manage central airway obstruction
Central airway obstruction (CAO) is a major cause of morbidity and mortality in patients with malignant and nonmalignant pulmonary disorders (Ernst et al. Am J Respir Crit Care Med. 2004;169:1278). It is associated with postobstructive pneumonia, respiratory compromise, and even respiratory failure. It often precludes the patients with malignancy from getting definitive treatment, such as surgical resection or chemotherapy. Therapeutic bronchoscopy using a rigid bronchoscope plays a central role in managing these patients.
Different modalities used during therapeutic bronchoscopy include debridement, airway dilation, and different heat therapies, such as laser, electrocautery, and argon plasma coagulation (Bolliger et al. Eur Respir J. 2006;27:1258). Airway stents are often placed to achieve durable airway patency. Endobronchial therapies with delayed effect include brachytherapy, photodynamic therapy, and cryotherapy (Vergnon et al. Eur Respir J. 2006;28:200). There is improvement in symptom control, quality of life, and spirometry with successful bronchoscopic intervention (Mahmood et al. Respiration. 2015;89:404). Patients with respiratory failure secondary to CAO can be weaned from mechanical ventilation (Murgu et al. Respiration. 2012;84:55).
It is often difficult to predict which patients will have a successful bronchoscopic intervention. Endobronchial disease and stent placement have been associated with successful outcome (Ost et al. Chest. 2015;147:1282). Patients with unsuccessful bronchoscopic intervention often have a poor prognosis, despite concurrent chemotherapy and radiation (Mahmood et al. Respiration. 2015;89:404).
As more fellowship programs are offering training in rigid bronchoscopy, there is a need to standardize the training and use validated tools to assess competency. RIGID-TASC (Rigid bronchoscopy Tool for Assessment of Skills and Competence) is one such tool, which can be utilized for this purpose to provide objective feedback to the trainee (Mahmood et al. Ann Am Thor Soc. 2016. doi: 10.1513/ Epub ahead of print).
Kamran Mahmood, MD, MPH, FCCP
Steering Committee Member
Pediatric Chest Medicine: CHEST Foundation campaign to fight difficult-to-control asthma
The CHEST Foundation and the Asthma and Allergy Network have joined forces to combat difficult-to-control asthma with the campaign “Asthma: Take Action. Take Control.” Affecting approximately 235 million people worldwide, asthma morbidity continues to have a significant impact on quality of life for both children and adults with asthma. In the United States alone, it accounts for health-care costs of approximately 60 billion dollars.
The campaign educates patients, caregivers, families, and health-care providers about current treatment options for asthma, highlights the importance of specialist referrals, and encourages patients to participate with their health-care provider to achieve asthma control. Because asthma may fall into this difficult-to-control category for many reasons, including poor adherence, unresponsiveness to conventional therapies, failure to recognize and manage triggers, and co-morbidities, this campaign developed materials to improve health literacy so that patients can take an active and informed role in asthma self-management. Written in an easy to understand format and language, the “Take Control” campaign highlights four key steps:
• Tell your doctor when it’s hard to breathe.
• Ask your doctor for an asthma action plan.
• Practice your asthma action plan.
• Know that asthma shouldn’t hold you back.
Newly developed materials include tips and resources for children and adults to learn about asthma and raise awareness about difficult-to-control asthma. These materials can be found at asthma.chestnet.org.
Mary Cataletto MD, FCCP
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation: Current clinical usefulness of the PETCO2 during exercise testing
Dynamic measurement of the PETCO2 in cardiopulmonary exercise testing may demonstrate unique changes throughout exercise in specific diseases and is often underutilized during interpretation. Though it can be affected by hyperventilation and the VD/VT relationship, normally it rises from rest to lactate threshold (LT), then declines from peak exercise through recovery (Ramos RP, et al. Pulm Med. 2013;2013:359021. doi: 10.1155/2013/359021.) In severe pulmonary hypertension and shunts, the reverse occurs, declining in early exercise and then rising during recovery (Sun XG, et al. Circulation. 2002;105[1]:54). Blunting or reversal of this exercise decline in PETCO2 has been correlated with clinical improvement in therapeutic trials (Oudiz RJ, et al. Eur J Heart Fail. 2007;9[9]:917). Studies in severe CHF have correlated prognosis with lower values at rest and greater decline from rest to peak exercise, the latter being affected by adequacy of effort and assessed by RQ. They, however, do not take into account the normal rise and fall before and after LT (Arena R, et al. Am Heart J. 2008;156[5]:982) (Hoshimoto-Iwamoto M, et al. J Physiol Sci. 2009;59[1]:49). In pulmonary hypertension, as the disease progresses, the unique reversal of the normal slopes of the PETCO2 that occurs, negative in early exercise and positive during recovery in association with an excessive alveolar ventilator response, needs further clinical investigation and correlation (Yasunobu Y, et al. Chest. 2005;127[5]:1637). The dynamic changes that occur in the PETCO2 throughout exercise may be an additional tool to use in selective conditions to more accurately assess prognosis and monitor response to therapy.
Said Chaaban, MD; and Zachary Morris, MD, FCCP
Steering Committee Members
Pulmonary Vascular Disease: Estrogen in PAH: Is it good or bad?
The role of sex hormones in the development and perpetuation of pulmonary arterial hypertension (PAH) continues to be an open field of active research. Epidemiology reveals that PAH is more prevalent in women in both idiopathic and heritable cases.1 On the other hand, data demonstrate that prognosis of PAH in men is worse than in women and, in animal research, estrogens provide a protective effect. This constitutes the “estrogen paradox.” Estrogen plays a protective role in the vasculature, modulating proliferative and vasoactive signaling by direct and receptor-mediated mechanisms.2,3 In animal models of PAH, estrogen increases nitric oxide and prostacyclin production and decreases endothelin-1, resulting in beneficial vascular effects.4 However, the Women’s Health Initiative revealed that hormone replacement therapy increases the risk for adverse cardiovascular events.5 In familial PAH, estrogen is a potent mitogen of pulmonary vascular smooth muscle cells.6 A recently published study, first in humans, by Ventetuolo et al.7 showed higher levels of estrogen (E2) and lower level of dehydroepiandrosterone-sulfate (DHEAS) in men with PAH, compared with normal men without cardiovascular disease (MESA study), supporting the role of the estrogen pathway in the development of PAH. Experimental data implicate estrogens as promoters of vascular proliferation and cell damage but also as inhibitors of pulmonary vasoreactivity. In vitro, estrogen is mitogenic and promotes proliferation of pulmonary vascular smooth muscle cells.6 Despite advances, the role of sex and estrogen in PAH is not fully understood. More preclinical and clinical data are necessary to establish a potential role for estrogen-based therapies in this disease.
Sandeep Sahay, MD; and Hector R Cajigas, MD
Steering Committee Members
References
1. Frost AE, et al. Chest. 2011;139:128.
2. Brouchet L, et al. Circulation. 2001;103:423.
3. Pendaries C, et al. Proc Natl Acad Sci USA. 2002;99:2205.
4. Lahm T, et al. Shock. 2008;30:660.
5. Manson JE, et al. N Engl J Med. 2003;349:523.
6. Farhat MY, et al. Br J Pharmacol. 1992;107:679.
7. Ventetuolo CE, et al. Am J Respir Crit Care Med. 2016;193:1168.
Thoracic Oncology: The “new” lung cancer staging system
Definition of lung cancer stage is an essential part of defining prognosis, developing treatment plans, and conducting and reporting on clinical research studies. The stage classification system is determined by the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC). The 7th edition of the lung cancer staging system, published in 2009, was a landmark effort based on a large multicenter international database created by the Staging and Prognostic Factors (SPFC) of the International Association for the Study of Lung Cancer (IASLC) and backed by careful validation and statistical analysis.
The IASLC Lung Cancer Staging Committee has been working on the 8th edition of the TNM classification for lung cancer. The database used for analysis consists of 94,708 patients diagnosed between 1999 and 2010, and included cases from 35 sources and 16 countries. Multiple analyses were performed to assess the ability of T, N, and M descriptors to predict prognosis and to identify new cutpoints for inclusion in the eight edition.1-3 The proposed changes include new cutpoints for the T component based on 1-cm increments, new categories for the N component, a new M category to specifically identify patients with oligometastatic disease, and multiple updates to the overall TNM stage groupings.4 In addition, the proposal includes recommendations for coding T stage for subsolid nodules and assessment of tumor size in part-solid nodules.5 These proposed changes will be submitted to the UICC and AJCC for inclusion in the eighth edition and will be enacted in January 2017.
Anil Vachani, MD, FCCP
NetWork Vice-Chair
References
1. Rami-Porta R, et al. J Thorac Oncol. 2015;10:990.
2. Eberhardt WEE, et al. J Thorac Oncol. 2015;10:1515.
3. Asamura H, et al. J Thorac Oncol. 2015;10:1675.
4. Goldstraw P, et al. J Thorac Oncol. 2016;11:39.
5. Travis WD, et al. J Thorac Oncol. 2016;11:1204.
Interventional Chest/Diagnostic Procedures: Evolving approaches to manage central airway obstruction
Central airway obstruction (CAO) is a major cause of morbidity and mortality in patients with malignant and nonmalignant pulmonary disorders (Ernst et al. Am J Respir Crit Care Med. 2004;169:1278). It is associated with postobstructive pneumonia, respiratory compromise, and even respiratory failure. It often precludes the patients with malignancy from getting definitive treatment, such as surgical resection or chemotherapy. Therapeutic bronchoscopy using a rigid bronchoscope plays a central role in managing these patients.
Different modalities used during therapeutic bronchoscopy include debridement, airway dilation, and different heat therapies, such as laser, electrocautery, and argon plasma coagulation (Bolliger et al. Eur Respir J. 2006;27:1258). Airway stents are often placed to achieve durable airway patency. Endobronchial therapies with delayed effect include brachytherapy, photodynamic therapy, and cryotherapy (Vergnon et al. Eur Respir J. 2006;28:200). There is improvement in symptom control, quality of life, and spirometry with successful bronchoscopic intervention (Mahmood et al. Respiration. 2015;89:404). Patients with respiratory failure secondary to CAO can be weaned from mechanical ventilation (Murgu et al. Respiration. 2012;84:55).
It is often difficult to predict which patients will have a successful bronchoscopic intervention. Endobronchial disease and stent placement have been associated with successful outcome (Ost et al. Chest. 2015;147:1282). Patients with unsuccessful bronchoscopic intervention often have a poor prognosis, despite concurrent chemotherapy and radiation (Mahmood et al. Respiration. 2015;89:404).
As more fellowship programs are offering training in rigid bronchoscopy, there is a need to standardize the training and use validated tools to assess competency. RIGID-TASC (Rigid bronchoscopy Tool for Assessment of Skills and Competence) is one such tool, which can be utilized for this purpose to provide objective feedback to the trainee (Mahmood et al. Ann Am Thor Soc. 2016. doi: 10.1513/ Epub ahead of print).
Kamran Mahmood, MD, MPH, FCCP
Steering Committee Member
Pediatric Chest Medicine: CHEST Foundation campaign to fight difficult-to-control asthma
The CHEST Foundation and the Asthma and Allergy Network have joined forces to combat difficult-to-control asthma with the campaign “Asthma: Take Action. Take Control.” Affecting approximately 235 million people worldwide, asthma morbidity continues to have a significant impact on quality of life for both children and adults with asthma. In the United States alone, it accounts for health-care costs of approximately 60 billion dollars.
The campaign educates patients, caregivers, families, and health-care providers about current treatment options for asthma, highlights the importance of specialist referrals, and encourages patients to participate with their health-care provider to achieve asthma control. Because asthma may fall into this difficult-to-control category for many reasons, including poor adherence, unresponsiveness to conventional therapies, failure to recognize and manage triggers, and co-morbidities, this campaign developed materials to improve health literacy so that patients can take an active and informed role in asthma self-management. Written in an easy to understand format and language, the “Take Control” campaign highlights four key steps:
• Tell your doctor when it’s hard to breathe.
• Ask your doctor for an asthma action plan.
• Practice your asthma action plan.
• Know that asthma shouldn’t hold you back.
Newly developed materials include tips and resources for children and adults to learn about asthma and raise awareness about difficult-to-control asthma. These materials can be found at asthma.chestnet.org.
Mary Cataletto MD, FCCP
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation: Current clinical usefulness of the PETCO2 during exercise testing
Dynamic measurement of the PETCO2 in cardiopulmonary exercise testing may demonstrate unique changes throughout exercise in specific diseases and is often underutilized during interpretation. Though it can be affected by hyperventilation and the VD/VT relationship, normally it rises from rest to lactate threshold (LT), then declines from peak exercise through recovery (Ramos RP, et al. Pulm Med. 2013;2013:359021. doi: 10.1155/2013/359021.) In severe pulmonary hypertension and shunts, the reverse occurs, declining in early exercise and then rising during recovery (Sun XG, et al. Circulation. 2002;105[1]:54). Blunting or reversal of this exercise decline in PETCO2 has been correlated with clinical improvement in therapeutic trials (Oudiz RJ, et al. Eur J Heart Fail. 2007;9[9]:917). Studies in severe CHF have correlated prognosis with lower values at rest and greater decline from rest to peak exercise, the latter being affected by adequacy of effort and assessed by RQ. They, however, do not take into account the normal rise and fall before and after LT (Arena R, et al. Am Heart J. 2008;156[5]:982) (Hoshimoto-Iwamoto M, et al. J Physiol Sci. 2009;59[1]:49). In pulmonary hypertension, as the disease progresses, the unique reversal of the normal slopes of the PETCO2 that occurs, negative in early exercise and positive during recovery in association with an excessive alveolar ventilator response, needs further clinical investigation and correlation (Yasunobu Y, et al. Chest. 2005;127[5]:1637). The dynamic changes that occur in the PETCO2 throughout exercise may be an additional tool to use in selective conditions to more accurately assess prognosis and monitor response to therapy.
Said Chaaban, MD; and Zachary Morris, MD, FCCP
Steering Committee Members
Pulmonary Vascular Disease: Estrogen in PAH: Is it good or bad?
The role of sex hormones in the development and perpetuation of pulmonary arterial hypertension (PAH) continues to be an open field of active research. Epidemiology reveals that PAH is more prevalent in women in both idiopathic and heritable cases.1 On the other hand, data demonstrate that prognosis of PAH in men is worse than in women and, in animal research, estrogens provide a protective effect. This constitutes the “estrogen paradox.” Estrogen plays a protective role in the vasculature, modulating proliferative and vasoactive signaling by direct and receptor-mediated mechanisms.2,3 In animal models of PAH, estrogen increases nitric oxide and prostacyclin production and decreases endothelin-1, resulting in beneficial vascular effects.4 However, the Women’s Health Initiative revealed that hormone replacement therapy increases the risk for adverse cardiovascular events.5 In familial PAH, estrogen is a potent mitogen of pulmonary vascular smooth muscle cells.6 A recently published study, first in humans, by Ventetuolo et al.7 showed higher levels of estrogen (E2) and lower level of dehydroepiandrosterone-sulfate (DHEAS) in men with PAH, compared with normal men without cardiovascular disease (MESA study), supporting the role of the estrogen pathway in the development of PAH. Experimental data implicate estrogens as promoters of vascular proliferation and cell damage but also as inhibitors of pulmonary vasoreactivity. In vitro, estrogen is mitogenic and promotes proliferation of pulmonary vascular smooth muscle cells.6 Despite advances, the role of sex and estrogen in PAH is not fully understood. More preclinical and clinical data are necessary to establish a potential role for estrogen-based therapies in this disease.
Sandeep Sahay, MD; and Hector R Cajigas, MD
Steering Committee Members
References
1. Frost AE, et al. Chest. 2011;139:128.
2. Brouchet L, et al. Circulation. 2001;103:423.
3. Pendaries C, et al. Proc Natl Acad Sci USA. 2002;99:2205.
4. Lahm T, et al. Shock. 2008;30:660.
5. Manson JE, et al. N Engl J Med. 2003;349:523.
6. Farhat MY, et al. Br J Pharmacol. 1992;107:679.
7. Ventetuolo CE, et al. Am J Respir Crit Care Med. 2016;193:1168.
Thoracic Oncology: The “new” lung cancer staging system
Definition of lung cancer stage is an essential part of defining prognosis, developing treatment plans, and conducting and reporting on clinical research studies. The stage classification system is determined by the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC). The 7th edition of the lung cancer staging system, published in 2009, was a landmark effort based on a large multicenter international database created by the Staging and Prognostic Factors (SPFC) of the International Association for the Study of Lung Cancer (IASLC) and backed by careful validation and statistical analysis.
The IASLC Lung Cancer Staging Committee has been working on the 8th edition of the TNM classification for lung cancer. The database used for analysis consists of 94,708 patients diagnosed between 1999 and 2010, and included cases from 35 sources and 16 countries. Multiple analyses were performed to assess the ability of T, N, and M descriptors to predict prognosis and to identify new cutpoints for inclusion in the eight edition.1-3 The proposed changes include new cutpoints for the T component based on 1-cm increments, new categories for the N component, a new M category to specifically identify patients with oligometastatic disease, and multiple updates to the overall TNM stage groupings.4 In addition, the proposal includes recommendations for coding T stage for subsolid nodules and assessment of tumor size in part-solid nodules.5 These proposed changes will be submitted to the UICC and AJCC for inclusion in the eighth edition and will be enacted in January 2017.
Anil Vachani, MD, FCCP
NetWork Vice-Chair
References
1. Rami-Porta R, et al. J Thorac Oncol. 2015;10:990.
2. Eberhardt WEE, et al. J Thorac Oncol. 2015;10:1515.
3. Asamura H, et al. J Thorac Oncol. 2015;10:1675.
4. Goldstraw P, et al. J Thorac Oncol. 2016;11:39.
5. Travis WD, et al. J Thorac Oncol. 2016;11:1204.
Interventional Chest/Diagnostic Procedures: Evolving approaches to manage central airway obstruction
Central airway obstruction (CAO) is a major cause of morbidity and mortality in patients with malignant and nonmalignant pulmonary disorders (Ernst et al. Am J Respir Crit Care Med. 2004;169:1278). It is associated with postobstructive pneumonia, respiratory compromise, and even respiratory failure. It often precludes the patients with malignancy from getting definitive treatment, such as surgical resection or chemotherapy. Therapeutic bronchoscopy using a rigid bronchoscope plays a central role in managing these patients.
Different modalities used during therapeutic bronchoscopy include debridement, airway dilation, and different heat therapies, such as laser, electrocautery, and argon plasma coagulation (Bolliger et al. Eur Respir J. 2006;27:1258). Airway stents are often placed to achieve durable airway patency. Endobronchial therapies with delayed effect include brachytherapy, photodynamic therapy, and cryotherapy (Vergnon et al. Eur Respir J. 2006;28:200). There is improvement in symptom control, quality of life, and spirometry with successful bronchoscopic intervention (Mahmood et al. Respiration. 2015;89:404). Patients with respiratory failure secondary to CAO can be weaned from mechanical ventilation (Murgu et al. Respiration. 2012;84:55).
It is often difficult to predict which patients will have a successful bronchoscopic intervention. Endobronchial disease and stent placement have been associated with successful outcome (Ost et al. Chest. 2015;147:1282). Patients with unsuccessful bronchoscopic intervention often have a poor prognosis, despite concurrent chemotherapy and radiation (Mahmood et al. Respiration. 2015;89:404).
As more fellowship programs are offering training in rigid bronchoscopy, there is a need to standardize the training and use validated tools to assess competency. RIGID-TASC (Rigid bronchoscopy Tool for Assessment of Skills and Competence) is one such tool, which can be utilized for this purpose to provide objective feedback to the trainee (Mahmood et al. Ann Am Thor Soc. 2016. doi: 10.1513/ Epub ahead of print).
Kamran Mahmood, MD, MPH, FCCP
Steering Committee Member
Pediatric Chest Medicine: CHEST Foundation campaign to fight difficult-to-control asthma
The CHEST Foundation and the Asthma and Allergy Network have joined forces to combat difficult-to-control asthma with the campaign “Asthma: Take Action. Take Control.” Affecting approximately 235 million people worldwide, asthma morbidity continues to have a significant impact on quality of life for both children and adults with asthma. In the United States alone, it accounts for health-care costs of approximately 60 billion dollars.
The campaign educates patients, caregivers, families, and health-care providers about current treatment options for asthma, highlights the importance of specialist referrals, and encourages patients to participate with their health-care provider to achieve asthma control. Because asthma may fall into this difficult-to-control category for many reasons, including poor adherence, unresponsiveness to conventional therapies, failure to recognize and manage triggers, and co-morbidities, this campaign developed materials to improve health literacy so that patients can take an active and informed role in asthma self-management. Written in an easy to understand format and language, the “Take Control” campaign highlights four key steps:
• Tell your doctor when it’s hard to breathe.
• Ask your doctor for an asthma action plan.
• Practice your asthma action plan.
• Know that asthma shouldn’t hold you back.
Newly developed materials include tips and resources for children and adults to learn about asthma and raise awareness about difficult-to-control asthma. These materials can be found at asthma.chestnet.org.
Mary Cataletto MD, FCCP
Steering Committee Member
Pulmonary Physiology, Function, and Rehabilitation: Current clinical usefulness of the PETCO2 during exercise testing
Dynamic measurement of the PETCO2 in cardiopulmonary exercise testing may demonstrate unique changes throughout exercise in specific diseases and is often underutilized during interpretation. Though it can be affected by hyperventilation and the VD/VT relationship, normally it rises from rest to lactate threshold (LT), then declines from peak exercise through recovery (Ramos RP, et al. Pulm Med. 2013;2013:359021. doi: 10.1155/2013/359021.) In severe pulmonary hypertension and shunts, the reverse occurs, declining in early exercise and then rising during recovery (Sun XG, et al. Circulation. 2002;105[1]:54). Blunting or reversal of this exercise decline in PETCO2 has been correlated with clinical improvement in therapeutic trials (Oudiz RJ, et al. Eur J Heart Fail. 2007;9[9]:917). Studies in severe CHF have correlated prognosis with lower values at rest and greater decline from rest to peak exercise, the latter being affected by adequacy of effort and assessed by RQ. They, however, do not take into account the normal rise and fall before and after LT (Arena R, et al. Am Heart J. 2008;156[5]:982) (Hoshimoto-Iwamoto M, et al. J Physiol Sci. 2009;59[1]:49). In pulmonary hypertension, as the disease progresses, the unique reversal of the normal slopes of the PETCO2 that occurs, negative in early exercise and positive during recovery in association with an excessive alveolar ventilator response, needs further clinical investigation and correlation (Yasunobu Y, et al. Chest. 2005;127[5]:1637). The dynamic changes that occur in the PETCO2 throughout exercise may be an additional tool to use in selective conditions to more accurately assess prognosis and monitor response to therapy.
Said Chaaban, MD; and Zachary Morris, MD, FCCP
Steering Committee Members
Pulmonary Vascular Disease: Estrogen in PAH: Is it good or bad?
The role of sex hormones in the development and perpetuation of pulmonary arterial hypertension (PAH) continues to be an open field of active research. Epidemiology reveals that PAH is more prevalent in women in both idiopathic and heritable cases.1 On the other hand, data demonstrate that prognosis of PAH in men is worse than in women and, in animal research, estrogens provide a protective effect. This constitutes the “estrogen paradox.” Estrogen plays a protective role in the vasculature, modulating proliferative and vasoactive signaling by direct and receptor-mediated mechanisms.2,3 In animal models of PAH, estrogen increases nitric oxide and prostacyclin production and decreases endothelin-1, resulting in beneficial vascular effects.4 However, the Women’s Health Initiative revealed that hormone replacement therapy increases the risk for adverse cardiovascular events.5 In familial PAH, estrogen is a potent mitogen of pulmonary vascular smooth muscle cells.6 A recently published study, first in humans, by Ventetuolo et al.7 showed higher levels of estrogen (E2) and lower level of dehydroepiandrosterone-sulfate (DHEAS) in men with PAH, compared with normal men without cardiovascular disease (MESA study), supporting the role of the estrogen pathway in the development of PAH. Experimental data implicate estrogens as promoters of vascular proliferation and cell damage but also as inhibitors of pulmonary vasoreactivity. In vitro, estrogen is mitogenic and promotes proliferation of pulmonary vascular smooth muscle cells.6 Despite advances, the role of sex and estrogen in PAH is not fully understood. More preclinical and clinical data are necessary to establish a potential role for estrogen-based therapies in this disease.
Sandeep Sahay, MD; and Hector R Cajigas, MD
Steering Committee Members
References
1. Frost AE, et al. Chest. 2011;139:128.
2. Brouchet L, et al. Circulation. 2001;103:423.
3. Pendaries C, et al. Proc Natl Acad Sci USA. 2002;99:2205.
4. Lahm T, et al. Shock. 2008;30:660.
5. Manson JE, et al. N Engl J Med. 2003;349:523.
6. Farhat MY, et al. Br J Pharmacol. 1992;107:679.
7. Ventetuolo CE, et al. Am J Respir Crit Care Med. 2016;193:1168.
Thoracic Oncology: The “new” lung cancer staging system
Definition of lung cancer stage is an essential part of defining prognosis, developing treatment plans, and conducting and reporting on clinical research studies. The stage classification system is determined by the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC). The 7th edition of the lung cancer staging system, published in 2009, was a landmark effort based on a large multicenter international database created by the Staging and Prognostic Factors (SPFC) of the International Association for the Study of Lung Cancer (IASLC) and backed by careful validation and statistical analysis.
The IASLC Lung Cancer Staging Committee has been working on the 8th edition of the TNM classification for lung cancer. The database used for analysis consists of 94,708 patients diagnosed between 1999 and 2010, and included cases from 35 sources and 16 countries. Multiple analyses were performed to assess the ability of T, N, and M descriptors to predict prognosis and to identify new cutpoints for inclusion in the eight edition.1-3 The proposed changes include new cutpoints for the T component based on 1-cm increments, new categories for the N component, a new M category to specifically identify patients with oligometastatic disease, and multiple updates to the overall TNM stage groupings.4 In addition, the proposal includes recommendations for coding T stage for subsolid nodules and assessment of tumor size in part-solid nodules.5 These proposed changes will be submitted to the UICC and AJCC for inclusion in the eighth edition and will be enacted in January 2017.
Anil Vachani, MD, FCCP
NetWork Vice-Chair
References
1. Rami-Porta R, et al. J Thorac Oncol. 2015;10:990.
2. Eberhardt WEE, et al. J Thorac Oncol. 2015;10:1515.
3. Asamura H, et al. J Thorac Oncol. 2015;10:1675.
4. Goldstraw P, et al. J Thorac Oncol. 2016;11:39.
5. Travis WD, et al. J Thorac Oncol. 2016;11:1204.
Common Canister Policy: The devil is in the details
Metered-dose inhalers (MDIs) have been available for more than 50 years and are routinely used to deliver inhalation therapy to patients with asthma and chronic obstructive pulmonary disease. Given the ever-escalating costs of health care, various measures have been targeted by hospitals or health systems to eke out savings. Given the ubiquity of MDIs in the ICU, collaborative efforts by administrators and clinicians have focused on MDIs. These efforts, intended to curb rising costs and waste associated with MDI use, have resulted in a variety of protocols generically referred to as common canister policies (CCPs). While the concept of CCPs came into existence in the mid-1990s, casual observation suggests they are gaining momentum at hospitals and long-term care facilities. Most data regarding CCPs come from abstracts or posters; few studies have been published in peer-reviewed journals. Data on the efficacy and safety of CCPs in the ICU are particularly limited. Although most reports on CCPs have originated in community-based hospitals, some academic medical centers have also explored this concept.
What is common canister policy?
CCPs allow a single MDI canister to be shared among patients in a designated care area (typically a ward or ICU), with each individual having his/her own one-way valve holding chamber or spacer (Larson T, et al. Curr Med Res Opin. 2015;31[4]:853). Each patient care unit or respiratory therapist has a set of inhalers to use until actuations run out, at which point new inhalers are delivered from the pharmacy. Because the holding chamber or spacer is not shared, the risk of patient-to-patient spread of disease is minimized. In addition, the provider involved in administration of the inhaler must follow a standardized cleaning protocol to ensure the common canister is sterilized after each use.
This policy is designed to be used with inhaled therapies delivered by MDI (albuterol, ipratropium, albuterol/ipratropium, fluticasone, budesonide/formoterol, fluticasone/salmeterol). CCP does not apply to other types of inhalers, such as dry powder or mist inhalers, because the use of a separate holding chamber or spacer is not feasible with these devices. CCP savings are realized through a reduction in the number of MDIs purchased and the ability of patients to be charged per inhalation of medication delivered. An alternative CCP practice is to issue an MDI to a single patient and, upon his/her discharge, to clean and reissue the patient’s partially used MDIs to subsequent patients until the medication is exhausted (Liou J, et al. Hosp Pharm. 2014;49:437).
What are the risks and benefits of CCP?
CCP was implemented to minimize costs associated with drug wasting, since patients would not need individual inhalers. Some analysts believe dispensing individual inhalers creates an inherent financial burden as the average length of stay for an acute respiratory hospitalization is 4-5 days (Larson T, et al). This concern appears valid as two studies of MDI and dry powder inhaler use in real-world practice found that 11%-13% of the total amount of drug was utilized, leaving 87%-89% of each device wasted at a cost of approximately $87,000 annually (Larson T, et al; Sakaan S, et al. Hosp Pharm. 2015;50[5]:386).
In addition to cost reductions, one study showed CCP reduced delays in delivery of MDI therapy to patients because the lag time between order entry and delivery of the MDI to the floor was eliminated (Filippelli A, et al. Abstract, ASHP Midyear Clinical Meeting, Dec 1997). In this study, CCP allowed respiratory care practitioners immediate access to the common MDI for their entire shift, creating more efficient delivery of MDI treatments. On a par with findings in prior studies, these investigators observed a 55% reduction in hospital purchase costs for MDIs. Patient-level costs were similarly reduced, as each patient was billed only for the number of doses administered from an MDI, rather than for an entire canister.
While CCP appears to reduce inhaler-related costs, it is still unclear whether CCP increases the risk of iatrogenic infection. There is a particular paucity of information on the use of CCP in high-risk patients – those with cystic fibrosis, those in isolation, patients receiving mechanical ventilation, and those who are post transplant or otherwise immunocompromised (Larson T, et al). These patients have an inherently increased risk of developing nosocomial infections including ventilator-acquired pneumonia. A recent prospective study compared MDI CCP with single-patient MDI use in 353 patients supported by mechanical ventilation. Although CCP was associated with cost savings and similar rates of ventilator-acquired pneumonia, hospital mortality, and length of stay, there was a greater frequency of ventilator-associated events among patients in the CCP arm of the study (Gowan M, et al. Respir Care. 2016 May 3. pii: respcare.04550. [Epub ahead of print]).
The safety of CCP hinges on proper cleaning of the MDI between users. Typical cleaning protocols include: 1. spraying the MDI mouthpiece with compressed air; 2. cleaning the entire MDI with 70% isopropyl alcohol spray, immersion in isopropyl alcohol for 2 minutes, or cleaning with a bleach swab; and 3. allowing the MDI to air dry before returning it to the shared stock for reissue (Larson T, et al). Although cleaning protocols minimize potential patient harm, they may not always be followed properly. Human errors that put patients at risk for nosocomial infection while utilizing CCP have been reported. In two such instances, patients isolated for methicillin-resistant Staphylococcus aureus infection had their individual MDIs put back into the common canister stock and utilized by other patients for approximately 24 hours (Larson T, et al). Once this was noticed, the patients who received inhalations from the “at-risk” MDI were monitored in isolation. No cross-infection occurred, but the mistake paradoxically increased hospital costs. In another reported instance, a bone marrow transplant patient received MDI therapy from the common canister stock (Larson T, et al). Although no harm occurred, this broke protocol as these patients were excluded from the program because of their increased risk of infection from cross-contamination. Other reports describe protocol breaches such as clinicians not returning MDIs to stock in a timely manner or keeping MDIs in their coat pockets. These events highlight the need for health care professionals associated with CCP to adhere to protocols.
Cross-contamination has been studied at institutions utilizing CCPs. While the majority of reports show no growth in postuse MDI cultures, one study reported growth of group D streptococci when alcohol disinfection did not occur and Staphylococcus epidermidis in 5% of the cultures taken after disinfection per protocol (Grissinger M. PT. 2013;38[8]:434). Although the bacteria that grew in these studies could be considered environmental contaminants, these findings reinforce the need for concern regarding iatrogenic infection.
The legal landscape
The decision to enact CCP requires careful analysis, planning, and communication by all key decision makers. State laws must be reviewed for formal statements or regulations regarding CCP. Protocol standards should also be evaluated against Joint Commission and Centers for Medicare & Medicaid Services standards for medication administration and storage. Before initiating CCP, communication should occur among risk managers, the pharmacy and therapeutics committee, pulmonologists, respiratory therapists, the medical executive committee, infection control personnel, and the professional liability insurance provider. A contingency plan should be put in place should cross-contamination occur. Note that while the goal of CCP is cost savings, no economic analysis to date has considered the incremental costs of cross-contamination and iatrogenic infection.
What alternative strategies to CCP exist?
CCP aims to turn a single-user multidose inhaler into one that is a unit-dose inhaler shared by multiple patients. One alternative strategy of unit-dose inhalations is nebulization as each treatment consists of a single-use ampule of medication. Another strategy is the use of institutional dose packages that allow hospitals to purchase single-user inhalers limited to five or seven doses of therapy. The prices for nebulized treatments and institutional dose packages may offer cost savings similar to CCP while obviating the increased risk of nosocomial infection.
Dr. Malesker is professor of pharmacy practice and medicine, department of pharmacy practice, School of Pharmacy and Health Professions, Creighton University, Omaha, Neb.
Metered-dose inhalers (MDIs) have been available for more than 50 years and are routinely used to deliver inhalation therapy to patients with asthma and chronic obstructive pulmonary disease. Given the ever-escalating costs of health care, various measures have been targeted by hospitals or health systems to eke out savings. Given the ubiquity of MDIs in the ICU, collaborative efforts by administrators and clinicians have focused on MDIs. These efforts, intended to curb rising costs and waste associated with MDI use, have resulted in a variety of protocols generically referred to as common canister policies (CCPs). While the concept of CCPs came into existence in the mid-1990s, casual observation suggests they are gaining momentum at hospitals and long-term care facilities. Most data regarding CCPs come from abstracts or posters; few studies have been published in peer-reviewed journals. Data on the efficacy and safety of CCPs in the ICU are particularly limited. Although most reports on CCPs have originated in community-based hospitals, some academic medical centers have also explored this concept.
What is common canister policy?
CCPs allow a single MDI canister to be shared among patients in a designated care area (typically a ward or ICU), with each individual having his/her own one-way valve holding chamber or spacer (Larson T, et al. Curr Med Res Opin. 2015;31[4]:853). Each patient care unit or respiratory therapist has a set of inhalers to use until actuations run out, at which point new inhalers are delivered from the pharmacy. Because the holding chamber or spacer is not shared, the risk of patient-to-patient spread of disease is minimized. In addition, the provider involved in administration of the inhaler must follow a standardized cleaning protocol to ensure the common canister is sterilized after each use.
This policy is designed to be used with inhaled therapies delivered by MDI (albuterol, ipratropium, albuterol/ipratropium, fluticasone, budesonide/formoterol, fluticasone/salmeterol). CCP does not apply to other types of inhalers, such as dry powder or mist inhalers, because the use of a separate holding chamber or spacer is not feasible with these devices. CCP savings are realized through a reduction in the number of MDIs purchased and the ability of patients to be charged per inhalation of medication delivered. An alternative CCP practice is to issue an MDI to a single patient and, upon his/her discharge, to clean and reissue the patient’s partially used MDIs to subsequent patients until the medication is exhausted (Liou J, et al. Hosp Pharm. 2014;49:437).
What are the risks and benefits of CCP?
CCP was implemented to minimize costs associated with drug wasting, since patients would not need individual inhalers. Some analysts believe dispensing individual inhalers creates an inherent financial burden as the average length of stay for an acute respiratory hospitalization is 4-5 days (Larson T, et al). This concern appears valid as two studies of MDI and dry powder inhaler use in real-world practice found that 11%-13% of the total amount of drug was utilized, leaving 87%-89% of each device wasted at a cost of approximately $87,000 annually (Larson T, et al; Sakaan S, et al. Hosp Pharm. 2015;50[5]:386).
In addition to cost reductions, one study showed CCP reduced delays in delivery of MDI therapy to patients because the lag time between order entry and delivery of the MDI to the floor was eliminated (Filippelli A, et al. Abstract, ASHP Midyear Clinical Meeting, Dec 1997). In this study, CCP allowed respiratory care practitioners immediate access to the common MDI for their entire shift, creating more efficient delivery of MDI treatments. On a par with findings in prior studies, these investigators observed a 55% reduction in hospital purchase costs for MDIs. Patient-level costs were similarly reduced, as each patient was billed only for the number of doses administered from an MDI, rather than for an entire canister.
While CCP appears to reduce inhaler-related costs, it is still unclear whether CCP increases the risk of iatrogenic infection. There is a particular paucity of information on the use of CCP in high-risk patients – those with cystic fibrosis, those in isolation, patients receiving mechanical ventilation, and those who are post transplant or otherwise immunocompromised (Larson T, et al). These patients have an inherently increased risk of developing nosocomial infections including ventilator-acquired pneumonia. A recent prospective study compared MDI CCP with single-patient MDI use in 353 patients supported by mechanical ventilation. Although CCP was associated with cost savings and similar rates of ventilator-acquired pneumonia, hospital mortality, and length of stay, there was a greater frequency of ventilator-associated events among patients in the CCP arm of the study (Gowan M, et al. Respir Care. 2016 May 3. pii: respcare.04550. [Epub ahead of print]).
The safety of CCP hinges on proper cleaning of the MDI between users. Typical cleaning protocols include: 1. spraying the MDI mouthpiece with compressed air; 2. cleaning the entire MDI with 70% isopropyl alcohol spray, immersion in isopropyl alcohol for 2 minutes, or cleaning with a bleach swab; and 3. allowing the MDI to air dry before returning it to the shared stock for reissue (Larson T, et al). Although cleaning protocols minimize potential patient harm, they may not always be followed properly. Human errors that put patients at risk for nosocomial infection while utilizing CCP have been reported. In two such instances, patients isolated for methicillin-resistant Staphylococcus aureus infection had their individual MDIs put back into the common canister stock and utilized by other patients for approximately 24 hours (Larson T, et al). Once this was noticed, the patients who received inhalations from the “at-risk” MDI were monitored in isolation. No cross-infection occurred, but the mistake paradoxically increased hospital costs. In another reported instance, a bone marrow transplant patient received MDI therapy from the common canister stock (Larson T, et al). Although no harm occurred, this broke protocol as these patients were excluded from the program because of their increased risk of infection from cross-contamination. Other reports describe protocol breaches such as clinicians not returning MDIs to stock in a timely manner or keeping MDIs in their coat pockets. These events highlight the need for health care professionals associated with CCP to adhere to protocols.
Cross-contamination has been studied at institutions utilizing CCPs. While the majority of reports show no growth in postuse MDI cultures, one study reported growth of group D streptococci when alcohol disinfection did not occur and Staphylococcus epidermidis in 5% of the cultures taken after disinfection per protocol (Grissinger M. PT. 2013;38[8]:434). Although the bacteria that grew in these studies could be considered environmental contaminants, these findings reinforce the need for concern regarding iatrogenic infection.
The legal landscape
The decision to enact CCP requires careful analysis, planning, and communication by all key decision makers. State laws must be reviewed for formal statements or regulations regarding CCP. Protocol standards should also be evaluated against Joint Commission and Centers for Medicare & Medicaid Services standards for medication administration and storage. Before initiating CCP, communication should occur among risk managers, the pharmacy and therapeutics committee, pulmonologists, respiratory therapists, the medical executive committee, infection control personnel, and the professional liability insurance provider. A contingency plan should be put in place should cross-contamination occur. Note that while the goal of CCP is cost savings, no economic analysis to date has considered the incremental costs of cross-contamination and iatrogenic infection.
What alternative strategies to CCP exist?
CCP aims to turn a single-user multidose inhaler into one that is a unit-dose inhaler shared by multiple patients. One alternative strategy of unit-dose inhalations is nebulization as each treatment consists of a single-use ampule of medication. Another strategy is the use of institutional dose packages that allow hospitals to purchase single-user inhalers limited to five or seven doses of therapy. The prices for nebulized treatments and institutional dose packages may offer cost savings similar to CCP while obviating the increased risk of nosocomial infection.
Dr. Malesker is professor of pharmacy practice and medicine, department of pharmacy practice, School of Pharmacy and Health Professions, Creighton University, Omaha, Neb.
Metered-dose inhalers (MDIs) have been available for more than 50 years and are routinely used to deliver inhalation therapy to patients with asthma and chronic obstructive pulmonary disease. Given the ever-escalating costs of health care, various measures have been targeted by hospitals or health systems to eke out savings. Given the ubiquity of MDIs in the ICU, collaborative efforts by administrators and clinicians have focused on MDIs. These efforts, intended to curb rising costs and waste associated with MDI use, have resulted in a variety of protocols generically referred to as common canister policies (CCPs). While the concept of CCPs came into existence in the mid-1990s, casual observation suggests they are gaining momentum at hospitals and long-term care facilities. Most data regarding CCPs come from abstracts or posters; few studies have been published in peer-reviewed journals. Data on the efficacy and safety of CCPs in the ICU are particularly limited. Although most reports on CCPs have originated in community-based hospitals, some academic medical centers have also explored this concept.
What is common canister policy?
CCPs allow a single MDI canister to be shared among patients in a designated care area (typically a ward or ICU), with each individual having his/her own one-way valve holding chamber or spacer (Larson T, et al. Curr Med Res Opin. 2015;31[4]:853). Each patient care unit or respiratory therapist has a set of inhalers to use until actuations run out, at which point new inhalers are delivered from the pharmacy. Because the holding chamber or spacer is not shared, the risk of patient-to-patient spread of disease is minimized. In addition, the provider involved in administration of the inhaler must follow a standardized cleaning protocol to ensure the common canister is sterilized after each use.
This policy is designed to be used with inhaled therapies delivered by MDI (albuterol, ipratropium, albuterol/ipratropium, fluticasone, budesonide/formoterol, fluticasone/salmeterol). CCP does not apply to other types of inhalers, such as dry powder or mist inhalers, because the use of a separate holding chamber or spacer is not feasible with these devices. CCP savings are realized through a reduction in the number of MDIs purchased and the ability of patients to be charged per inhalation of medication delivered. An alternative CCP practice is to issue an MDI to a single patient and, upon his/her discharge, to clean and reissue the patient’s partially used MDIs to subsequent patients until the medication is exhausted (Liou J, et al. Hosp Pharm. 2014;49:437).
What are the risks and benefits of CCP?
CCP was implemented to minimize costs associated with drug wasting, since patients would not need individual inhalers. Some analysts believe dispensing individual inhalers creates an inherent financial burden as the average length of stay for an acute respiratory hospitalization is 4-5 days (Larson T, et al). This concern appears valid as two studies of MDI and dry powder inhaler use in real-world practice found that 11%-13% of the total amount of drug was utilized, leaving 87%-89% of each device wasted at a cost of approximately $87,000 annually (Larson T, et al; Sakaan S, et al. Hosp Pharm. 2015;50[5]:386).
In addition to cost reductions, one study showed CCP reduced delays in delivery of MDI therapy to patients because the lag time between order entry and delivery of the MDI to the floor was eliminated (Filippelli A, et al. Abstract, ASHP Midyear Clinical Meeting, Dec 1997). In this study, CCP allowed respiratory care practitioners immediate access to the common MDI for their entire shift, creating more efficient delivery of MDI treatments. On a par with findings in prior studies, these investigators observed a 55% reduction in hospital purchase costs for MDIs. Patient-level costs were similarly reduced, as each patient was billed only for the number of doses administered from an MDI, rather than for an entire canister.
While CCP appears to reduce inhaler-related costs, it is still unclear whether CCP increases the risk of iatrogenic infection. There is a particular paucity of information on the use of CCP in high-risk patients – those with cystic fibrosis, those in isolation, patients receiving mechanical ventilation, and those who are post transplant or otherwise immunocompromised (Larson T, et al). These patients have an inherently increased risk of developing nosocomial infections including ventilator-acquired pneumonia. A recent prospective study compared MDI CCP with single-patient MDI use in 353 patients supported by mechanical ventilation. Although CCP was associated with cost savings and similar rates of ventilator-acquired pneumonia, hospital mortality, and length of stay, there was a greater frequency of ventilator-associated events among patients in the CCP arm of the study (Gowan M, et al. Respir Care. 2016 May 3. pii: respcare.04550. [Epub ahead of print]).
The safety of CCP hinges on proper cleaning of the MDI between users. Typical cleaning protocols include: 1. spraying the MDI mouthpiece with compressed air; 2. cleaning the entire MDI with 70% isopropyl alcohol spray, immersion in isopropyl alcohol for 2 minutes, or cleaning with a bleach swab; and 3. allowing the MDI to air dry before returning it to the shared stock for reissue (Larson T, et al). Although cleaning protocols minimize potential patient harm, they may not always be followed properly. Human errors that put patients at risk for nosocomial infection while utilizing CCP have been reported. In two such instances, patients isolated for methicillin-resistant Staphylococcus aureus infection had their individual MDIs put back into the common canister stock and utilized by other patients for approximately 24 hours (Larson T, et al). Once this was noticed, the patients who received inhalations from the “at-risk” MDI were monitored in isolation. No cross-infection occurred, but the mistake paradoxically increased hospital costs. In another reported instance, a bone marrow transplant patient received MDI therapy from the common canister stock (Larson T, et al). Although no harm occurred, this broke protocol as these patients were excluded from the program because of their increased risk of infection from cross-contamination. Other reports describe protocol breaches such as clinicians not returning MDIs to stock in a timely manner or keeping MDIs in their coat pockets. These events highlight the need for health care professionals associated with CCP to adhere to protocols.
Cross-contamination has been studied at institutions utilizing CCPs. While the majority of reports show no growth in postuse MDI cultures, one study reported growth of group D streptococci when alcohol disinfection did not occur and Staphylococcus epidermidis in 5% of the cultures taken after disinfection per protocol (Grissinger M. PT. 2013;38[8]:434). Although the bacteria that grew in these studies could be considered environmental contaminants, these findings reinforce the need for concern regarding iatrogenic infection.
The legal landscape
The decision to enact CCP requires careful analysis, planning, and communication by all key decision makers. State laws must be reviewed for formal statements or regulations regarding CCP. Protocol standards should also be evaluated against Joint Commission and Centers for Medicare & Medicaid Services standards for medication administration and storage. Before initiating CCP, communication should occur among risk managers, the pharmacy and therapeutics committee, pulmonologists, respiratory therapists, the medical executive committee, infection control personnel, and the professional liability insurance provider. A contingency plan should be put in place should cross-contamination occur. Note that while the goal of CCP is cost savings, no economic analysis to date has considered the incremental costs of cross-contamination and iatrogenic infection.
What alternative strategies to CCP exist?
CCP aims to turn a single-user multidose inhaler into one that is a unit-dose inhaler shared by multiple patients. One alternative strategy of unit-dose inhalations is nebulization as each treatment consists of a single-use ampule of medication. Another strategy is the use of institutional dose packages that allow hospitals to purchase single-user inhalers limited to five or seven doses of therapy. The prices for nebulized treatments and institutional dose packages may offer cost savings similar to CCP while obviating the increased risk of nosocomial infection.
Dr. Malesker is professor of pharmacy practice and medicine, department of pharmacy practice, School of Pharmacy and Health Professions, Creighton University, Omaha, Neb.
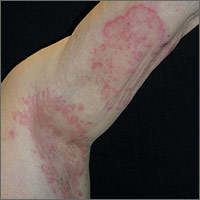
Pruritic rash in armpit
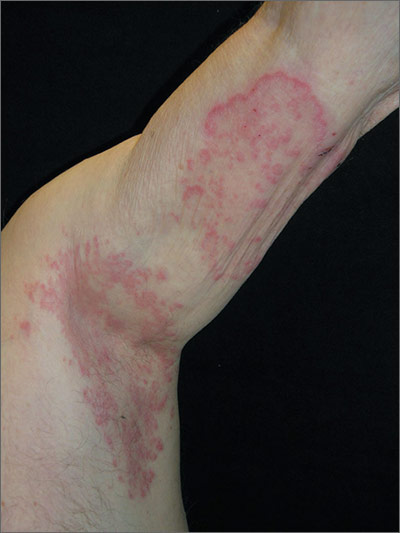
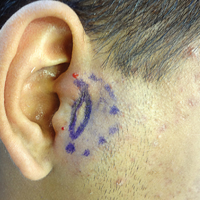
The FP suspected that this was tinea corporis, and after looking for other signs of tinea, found that the patient had some interdigital tinea pedis with thickened dysmorphic toenails. A potassium hydroxide (KOH) preparation was performed on the rash and toenails (See video on how to perform a KOH preparation here). Both were clearly positive for branching hyphae, confirming the diagnoses of tinea corporis and onychomycosis. While KOH preparation is the definitive test to confirm a suspected fungal infection, it is often helpful to look for other sites of fungus (such as the feet) when tinea is suspected.
The diagnosis of tinea corporis was unrelated to the patient’s deodorant, even though contact dermatitis can occur in the axilla as a result of a new deodorant containing a contact allergen. In most cases, however, the contact dermatitis will be bilateral.
The FP discussed the treatment options with the patient, including oral or topical antifungal medicines, and the patient chose to go straight to oral therapy. He stated that he had tried an over-the-counter antifungal medicine recently and did not find it very helpful. The FP explained that it would take a minimum of 3 months of oral therapy to also treat the patient’s toenails. The patient denied any history of hepatitis or heavy alcohol abuse and had normal liver function tests (LFTs) in the previous year.
The FP prescribed one month of oral terbinafine 250 mg/d to eradicate the skin infection and to begin treating the toenails. Two to 3 weeks of oral terbinafine is often adequate for tinea corporis if there is only one site involved. A follow-up appointment was set for one month later at which time the FP planned to order repeat LFTs and discuss the risks and benefits of another 2 months of oral terbinafine to eradicate the onychomycosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Jimenez A. Tinea corporis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:788-794.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP suspected that this was tinea corporis, and after looking for other signs of tinea, found that the patient had some interdigital tinea pedis with thickened dysmorphic toenails. A potassium hydroxide (KOH) preparation was performed on the rash and toenails (See video on how to perform a KOH preparation here). Both were clearly positive for branching hyphae, confirming the diagnoses of tinea corporis and onychomycosis. While KOH preparation is the definitive test to confirm a suspected fungal infection, it is often helpful to look for other sites of fungus (such as the feet) when tinea is suspected.
The diagnosis of tinea corporis was unrelated to the patient’s deodorant, even though contact dermatitis can occur in the axilla as a result of a new deodorant containing a contact allergen. In most cases, however, the contact dermatitis will be bilateral.
The FP discussed the treatment options with the patient, including oral or topical antifungal medicines, and the patient chose to go straight to oral therapy. He stated that he had tried an over-the-counter antifungal medicine recently and did not find it very helpful. The FP explained that it would take a minimum of 3 months of oral therapy to also treat the patient’s toenails. The patient denied any history of hepatitis or heavy alcohol abuse and had normal liver function tests (LFTs) in the previous year.
The FP prescribed one month of oral terbinafine 250 mg/d to eradicate the skin infection and to begin treating the toenails. Two to 3 weeks of oral terbinafine is often adequate for tinea corporis if there is only one site involved. A follow-up appointment was set for one month later at which time the FP planned to order repeat LFTs and discuss the risks and benefits of another 2 months of oral terbinafine to eradicate the onychomycosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Jimenez A. Tinea corporis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:788-794.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP suspected that this was tinea corporis, and after looking for other signs of tinea, found that the patient had some interdigital tinea pedis with thickened dysmorphic toenails. A potassium hydroxide (KOH) preparation was performed on the rash and toenails (See video on how to perform a KOH preparation here). Both were clearly positive for branching hyphae, confirming the diagnoses of tinea corporis and onychomycosis. While KOH preparation is the definitive test to confirm a suspected fungal infection, it is often helpful to look for other sites of fungus (such as the feet) when tinea is suspected.
The diagnosis of tinea corporis was unrelated to the patient’s deodorant, even though contact dermatitis can occur in the axilla as a result of a new deodorant containing a contact allergen. In most cases, however, the contact dermatitis will be bilateral.
The FP discussed the treatment options with the patient, including oral or topical antifungal medicines, and the patient chose to go straight to oral therapy. He stated that he had tried an over-the-counter antifungal medicine recently and did not find it very helpful. The FP explained that it would take a minimum of 3 months of oral therapy to also treat the patient’s toenails. The patient denied any history of hepatitis or heavy alcohol abuse and had normal liver function tests (LFTs) in the previous year.
The FP prescribed one month of oral terbinafine 250 mg/d to eradicate the skin infection and to begin treating the toenails. Two to 3 weeks of oral terbinafine is often adequate for tinea corporis if there is only one site involved. A follow-up appointment was set for one month later at which time the FP planned to order repeat LFTs and discuss the risks and benefits of another 2 months of oral terbinafine to eradicate the onychomycosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Jimenez A. Tinea corporis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:788-794.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Strongly recommend HPV vaccine to increase uptake
To convince parents to get their kids vaccinated against human papillomavirus (HPV), tell them “I strongly believe in the importance of this cancer-preventing vaccine for [their child’s name],” researchers concluded after an online survey of 1,504 parents of children aged 11-17 years.
Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice, also the highest physician endorsement for the various arguments (Cancer Epidemiol Biomarkers Prev. 2016 Sep 30. doi: 10.1158/1055-9965.EPI-16-0224).
In general, parents disinclined to vaccinate were most receptive to messages about HPV infection being common, cancers caused by HPV, and HPV vaccine effectiveness.
Other persuasive arguments included “[child’s name] can get anal/cervical cancer as an adult, but you can stop that right now;” and “there will be many things in [child’s name]’s life that you can’t control. But you can control whether [he/she] gets some dangerous kinds of HPV.”
Both “placed the onus of protection on the parent and emphasized the control they possess over their child’s health. This finding is aligned with the tenets of various theories of fear appeals, which posit that fear messages inspire action if the receiver believes he or she has some control over the situation. Parents without prior intentions to vaccinate their child may be particularly receptive to messages that arouse fear while fostering a sense of efficacy,” said Teri Malo, PhD, of the University of North Carolina, Chapel Hill, and associates.
Although almost 60% of parents said they’d pay attention if told they could control if their child gets HPV cancer, only 37% of physicians said they’d use the argument. It’s not clear why, but the team said the finding was “concerning ... given that it was one of the top messages that would persuade parents without prior intentions to vaccinate their child.”
At present, 40% of girls and 22% of boys aged 13-17 years receive all three HPV shots. The goal of the study was to help doctors increase those numbers with persuasive arguments. “Physician communication about human papillomavirus vaccine is a key determinant of uptake,” but not all physicians strongly recommend the vaccine, especially if they anticipate parent resistance. “We sought to identify messages that would motivate HPV vaccination,” the researchers said.
Argument by analogy was the weakest approach. Only 5% of parents found “would you wait until [child’s name] is in a car accident before you tell [him/her] to wear a seatbelt?” persuasive, and only 9% of physicians said they’d use it, which made it the least endorsed statement in the study.
The same arguments seemed to work regardless of parents’ race, education, or income, or their child’s age or sex, which led the team to suggest the messages will work across demographic subgroups.
Slightly more moms than dads filled out the survey, and most were white, with about 9% of parents black and 14% Hispanic. Most parents lived in metropolitan areas, nearly two-thirds had at least some college, and almost half reported an annual household income of at least $75,000. For simplicity, parents limited their responses to their child with the most recent birthday. About half of children were boys, and just over half hadn’t been vaccinated against HPV.
About two-thirds of the physicians were men. Over half had practiced for at least 20 years, just over half were pediatricians, and the rest were family practitioners.
The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
To convince parents to get their kids vaccinated against human papillomavirus (HPV), tell them “I strongly believe in the importance of this cancer-preventing vaccine for [their child’s name],” researchers concluded after an online survey of 1,504 parents of children aged 11-17 years.
Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice, also the highest physician endorsement for the various arguments (Cancer Epidemiol Biomarkers Prev. 2016 Sep 30. doi: 10.1158/1055-9965.EPI-16-0224).
In general, parents disinclined to vaccinate were most receptive to messages about HPV infection being common, cancers caused by HPV, and HPV vaccine effectiveness.
Other persuasive arguments included “[child’s name] can get anal/cervical cancer as an adult, but you can stop that right now;” and “there will be many things in [child’s name]’s life that you can’t control. But you can control whether [he/she] gets some dangerous kinds of HPV.”
Both “placed the onus of protection on the parent and emphasized the control they possess over their child’s health. This finding is aligned with the tenets of various theories of fear appeals, which posit that fear messages inspire action if the receiver believes he or she has some control over the situation. Parents without prior intentions to vaccinate their child may be particularly receptive to messages that arouse fear while fostering a sense of efficacy,” said Teri Malo, PhD, of the University of North Carolina, Chapel Hill, and associates.
Although almost 60% of parents said they’d pay attention if told they could control if their child gets HPV cancer, only 37% of physicians said they’d use the argument. It’s not clear why, but the team said the finding was “concerning ... given that it was one of the top messages that would persuade parents without prior intentions to vaccinate their child.”
At present, 40% of girls and 22% of boys aged 13-17 years receive all three HPV shots. The goal of the study was to help doctors increase those numbers with persuasive arguments. “Physician communication about human papillomavirus vaccine is a key determinant of uptake,” but not all physicians strongly recommend the vaccine, especially if they anticipate parent resistance. “We sought to identify messages that would motivate HPV vaccination,” the researchers said.
Argument by analogy was the weakest approach. Only 5% of parents found “would you wait until [child’s name] is in a car accident before you tell [him/her] to wear a seatbelt?” persuasive, and only 9% of physicians said they’d use it, which made it the least endorsed statement in the study.
The same arguments seemed to work regardless of parents’ race, education, or income, or their child’s age or sex, which led the team to suggest the messages will work across demographic subgroups.
Slightly more moms than dads filled out the survey, and most were white, with about 9% of parents black and 14% Hispanic. Most parents lived in metropolitan areas, nearly two-thirds had at least some college, and almost half reported an annual household income of at least $75,000. For simplicity, parents limited their responses to their child with the most recent birthday. About half of children were boys, and just over half hadn’t been vaccinated against HPV.
About two-thirds of the physicians were men. Over half had practiced for at least 20 years, just over half were pediatricians, and the rest were family practitioners.
The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
To convince parents to get their kids vaccinated against human papillomavirus (HPV), tell them “I strongly believe in the importance of this cancer-preventing vaccine for [their child’s name],” researchers concluded after an online survey of 1,504 parents of children aged 11-17 years.
Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice, also the highest physician endorsement for the various arguments (Cancer Epidemiol Biomarkers Prev. 2016 Sep 30. doi: 10.1158/1055-9965.EPI-16-0224).
In general, parents disinclined to vaccinate were most receptive to messages about HPV infection being common, cancers caused by HPV, and HPV vaccine effectiveness.
Other persuasive arguments included “[child’s name] can get anal/cervical cancer as an adult, but you can stop that right now;” and “there will be many things in [child’s name]’s life that you can’t control. But you can control whether [he/she] gets some dangerous kinds of HPV.”
Both “placed the onus of protection on the parent and emphasized the control they possess over their child’s health. This finding is aligned with the tenets of various theories of fear appeals, which posit that fear messages inspire action if the receiver believes he or she has some control over the situation. Parents without prior intentions to vaccinate their child may be particularly receptive to messages that arouse fear while fostering a sense of efficacy,” said Teri Malo, PhD, of the University of North Carolina, Chapel Hill, and associates.
Although almost 60% of parents said they’d pay attention if told they could control if their child gets HPV cancer, only 37% of physicians said they’d use the argument. It’s not clear why, but the team said the finding was “concerning ... given that it was one of the top messages that would persuade parents without prior intentions to vaccinate their child.”
At present, 40% of girls and 22% of boys aged 13-17 years receive all three HPV shots. The goal of the study was to help doctors increase those numbers with persuasive arguments. “Physician communication about human papillomavirus vaccine is a key determinant of uptake,” but not all physicians strongly recommend the vaccine, especially if they anticipate parent resistance. “We sought to identify messages that would motivate HPV vaccination,” the researchers said.
Argument by analogy was the weakest approach. Only 5% of parents found “would you wait until [child’s name] is in a car accident before you tell [him/her] to wear a seatbelt?” persuasive, and only 9% of physicians said they’d use it, which made it the least endorsed statement in the study.
The same arguments seemed to work regardless of parents’ race, education, or income, or their child’s age or sex, which led the team to suggest the messages will work across demographic subgroups.
Slightly more moms than dads filled out the survey, and most were white, with about 9% of parents black and 14% Hispanic. Most parents lived in metropolitan areas, nearly two-thirds had at least some college, and almost half reported an annual household income of at least $75,000. For simplicity, parents limited their responses to their child with the most recent birthday. About half of children were boys, and just over half hadn’t been vaccinated against HPV.
About two-thirds of the physicians were men. Over half had practiced for at least 20 years, just over half were pediatricians, and the rest were family practitioners.
The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
Key clinical point:
Major finding: Sixty-five percent of parents agreed it was a persuasive argument, the highest percentage among 15 statements in favor of vaccination; 69% of 776 surveyed physicians said they’d use it in practice.
Data source: Online survey of 1,504 parents of children aged 11-17 years old, and 776 physicians.
Disclosures: The work was funded by an unrestricted educational grant from Pfizer to senior investigator Noel Brewer, PhD. Dr. Brewer receives commercial research grants from Merck, maker of the HPV vaccine Gardasil, and Pfizer. He is also a Merck consultant and advisory board member. Coauthor Melissa B. Gilkey, PhD, and Dr. Malo were supported by other grants. The report noted that “the costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with” U.S. law.
Almost half of health providers will see PQRS pay cut
About half of all doctors who participate in the Physician Quality Reporting System (PQRS) soon will learn that their Medicare pay will be cut by up to 2% in 2017.
The Centers for Medicare & Medicaid Services has completed its assessments for reporting year 2015 and has begun notifying physicians that a 2.0% negative payment adjustment is forthcoming for those who did not satisfactorily report PQRS quality measures or who failed to satisfactorily participate in a qualified clinical data registry.
Doctors have just 2 months to challenge findings that they believe were made in error to spare themselves the 2017 cut, according to a CMS announcement.
If doctors believe their 2017 PQRS pay cut is erroneous, they can submit an informal review request by 11:59 p.m. EST on Nov. 30. CMS will investigate the merits of all review requests and issue a decision within 90 days. All requests for informal review must be submitted via a Web-based tool on the quality reporting communication support page. There are no hardship exemptions for the PQRS pay cuts.
In addition, some 2015 PQRS performance scores will be publicly reported on the Physician Compare website. CMS is hosting two sessions in October to provide further information about such public reporting.
In 2015, approximately 1.15 million professionals were eligible and able to participate in PQRS; just over half (624,077 or 54%) of eligible professionals successfully submitted data. The rest – about 528,000, or 46% – will see a pay cut in 2017, according to CMS.
Last year, slightly more health providers – 558,885 eligible professionals – were subject to the 2016 PQRS pay cut based on their 2014 reporting experience.
Walter J. Gorski, director of regulatory affairs for the American College of Physicians, said the college will be monitoring the notifications as they go out to physicians to assess impacts at the individual practice level and overall.
“In past years, the PQRS experience reports have shown a low level of participation in the program and, thus, a large number of physicians being subject to these negative adjustments,” Mr. Gorski said in an interview. “This low level of overall participation has long been a concern of ours and something we have recommended that CMS work to mitigate by simplifying reporting – something that we are pushing strongly for as the program is rolled into the new MIPS pathway [Merit-Based Incentive Payment System] within the [Quality Payment Program].”
The new Quality Payment Program will replace both PQRS and the Value Modifier program, as well as the separate payment adjustments under the Medicare EHR Incentive Program. The streamlined program will have reduced quality reporting requirements and a flexible design that allows eligible clinicians to pick the pace of participation during the first year, according to CMS.
“The newly announced ‘pick your pace’ approach for the first year of MIPS reporting will give physicians an opportunity to get their feet wet with quality reporting in a way that will protect them from future negative adjustments,” Mr. Gorski said.
[email protected]
On Twitter @legal_med
About half of all doctors who participate in the Physician Quality Reporting System (PQRS) soon will learn that their Medicare pay will be cut by up to 2% in 2017.
The Centers for Medicare & Medicaid Services has completed its assessments for reporting year 2015 and has begun notifying physicians that a 2.0% negative payment adjustment is forthcoming for those who did not satisfactorily report PQRS quality measures or who failed to satisfactorily participate in a qualified clinical data registry.
Doctors have just 2 months to challenge findings that they believe were made in error to spare themselves the 2017 cut, according to a CMS announcement.
If doctors believe their 2017 PQRS pay cut is erroneous, they can submit an informal review request by 11:59 p.m. EST on Nov. 30. CMS will investigate the merits of all review requests and issue a decision within 90 days. All requests for informal review must be submitted via a Web-based tool on the quality reporting communication support page. There are no hardship exemptions for the PQRS pay cuts.
In addition, some 2015 PQRS performance scores will be publicly reported on the Physician Compare website. CMS is hosting two sessions in October to provide further information about such public reporting.
In 2015, approximately 1.15 million professionals were eligible and able to participate in PQRS; just over half (624,077 or 54%) of eligible professionals successfully submitted data. The rest – about 528,000, or 46% – will see a pay cut in 2017, according to CMS.
Last year, slightly more health providers – 558,885 eligible professionals – were subject to the 2016 PQRS pay cut based on their 2014 reporting experience.
Walter J. Gorski, director of regulatory affairs for the American College of Physicians, said the college will be monitoring the notifications as they go out to physicians to assess impacts at the individual practice level and overall.
“In past years, the PQRS experience reports have shown a low level of participation in the program and, thus, a large number of physicians being subject to these negative adjustments,” Mr. Gorski said in an interview. “This low level of overall participation has long been a concern of ours and something we have recommended that CMS work to mitigate by simplifying reporting – something that we are pushing strongly for as the program is rolled into the new MIPS pathway [Merit-Based Incentive Payment System] within the [Quality Payment Program].”
The new Quality Payment Program will replace both PQRS and the Value Modifier program, as well as the separate payment adjustments under the Medicare EHR Incentive Program. The streamlined program will have reduced quality reporting requirements and a flexible design that allows eligible clinicians to pick the pace of participation during the first year, according to CMS.
“The newly announced ‘pick your pace’ approach for the first year of MIPS reporting will give physicians an opportunity to get their feet wet with quality reporting in a way that will protect them from future negative adjustments,” Mr. Gorski said.
[email protected]
On Twitter @legal_med
About half of all doctors who participate in the Physician Quality Reporting System (PQRS) soon will learn that their Medicare pay will be cut by up to 2% in 2017.
The Centers for Medicare & Medicaid Services has completed its assessments for reporting year 2015 and has begun notifying physicians that a 2.0% negative payment adjustment is forthcoming for those who did not satisfactorily report PQRS quality measures or who failed to satisfactorily participate in a qualified clinical data registry.
Doctors have just 2 months to challenge findings that they believe were made in error to spare themselves the 2017 cut, according to a CMS announcement.
If doctors believe their 2017 PQRS pay cut is erroneous, they can submit an informal review request by 11:59 p.m. EST on Nov. 30. CMS will investigate the merits of all review requests and issue a decision within 90 days. All requests for informal review must be submitted via a Web-based tool on the quality reporting communication support page. There are no hardship exemptions for the PQRS pay cuts.
In addition, some 2015 PQRS performance scores will be publicly reported on the Physician Compare website. CMS is hosting two sessions in October to provide further information about such public reporting.
In 2015, approximately 1.15 million professionals were eligible and able to participate in PQRS; just over half (624,077 or 54%) of eligible professionals successfully submitted data. The rest – about 528,000, or 46% – will see a pay cut in 2017, according to CMS.
Last year, slightly more health providers – 558,885 eligible professionals – were subject to the 2016 PQRS pay cut based on their 2014 reporting experience.
Walter J. Gorski, director of regulatory affairs for the American College of Physicians, said the college will be monitoring the notifications as they go out to physicians to assess impacts at the individual practice level and overall.
“In past years, the PQRS experience reports have shown a low level of participation in the program and, thus, a large number of physicians being subject to these negative adjustments,” Mr. Gorski said in an interview. “This low level of overall participation has long been a concern of ours and something we have recommended that CMS work to mitigate by simplifying reporting – something that we are pushing strongly for as the program is rolled into the new MIPS pathway [Merit-Based Incentive Payment System] within the [Quality Payment Program].”
The new Quality Payment Program will replace both PQRS and the Value Modifier program, as well as the separate payment adjustments under the Medicare EHR Incentive Program. The streamlined program will have reduced quality reporting requirements and a flexible design that allows eligible clinicians to pick the pace of participation during the first year, according to CMS.
“The newly announced ‘pick your pace’ approach for the first year of MIPS reporting will give physicians an opportunity to get their feet wet with quality reporting in a way that will protect them from future negative adjustments,” Mr. Gorski said.
[email protected]
On Twitter @legal_med
Lessons from largest measles outbreak in 20 years
The largest measles outbreak in the United States in more than 2 decades, with an attack rate several orders of magnitude larger than the usual annual nationwide incidence of the disease, could have been better controlled if clinicians had recognized the index cases sooner, according to a report published online Oct. 6 in the New England Journal of Medicine.
At 4 months’ duration, the 2014 measles outbreak in undervaccinated Amish communities across Ohio was also the longest-lasting one on record since the disease was eliminated in the United States, said Paul A. Gastañaduy, MD, of the division of viral diseases, Centers for Disease Control and Prevention, and his associates.
Two index cases developed in young Amish men (aged 22 and 23 years) who had just returned from a church-sponsored trip to the Philippines to perform charitable work among victims of a typhoon. They had not received any guidance about contagious diseases before their trip. Both developed prodromal symptoms (cough, coryza, or conjunctivitis) the day after their return, then a generalized red maculopapular rash 2 days later. They were then admitted to a local hospital and found to have thrombocytopenia and were diagnosed as having dengue fever. Only after a febrile illness with rash developed in 2 more returning relief workers, then in 12 additional contacts within the Amish community, was measles recognized and reported to the health department a full 30 days later. “Health care providers should maintain a high awareness of measles when returning unvaccinated travelers present with a fever and rash,” Dr. Gastañaduy and his associates said (N Engl J Med. 2016 Oct 6. doi: 10.1056/NEJMoa1602295).
A total of 383 people developed measles in this outbreak; 380 were Amish, and the other 3 were epidemiologically linked to the Amish community. The Amish sect doesn’t specifically prohibit vaccination, but immunization rates are low because the people’s personal and cultural beliefs limit participation in preventive health care. The fact that this outbreak was confined almost exclusively to the Amish indicates that high vaccine coverage in the general Ohio population probably prevented further spread of measles, the investigators noted. A total of 52% of the cases occurred among children and adolescents aged 5-17 years and 25% among persons aged 18-39 years.
After the outbreak was recognized, “engagement of local leaders, isolation of infectious persons, quarantine of those exposed, and vaccination of susceptible persons” allowed it to be contained. Local health departments implemented enhanced surveillance and met with community leaders to encourage case reporting, emphasize the importance of vaccination, inform residents about testing, and alert health care providers statewide about the outbreak. A total of 120 free vaccination clinics were held and 10,644 people were immunized.
This study was supported by the Ohio Department of Health and the CDC. Dr. Gastañaduy and his associates reported having no relevant financial disclosures.
The largest measles outbreak in the United States in more than 2 decades, with an attack rate several orders of magnitude larger than the usual annual nationwide incidence of the disease, could have been better controlled if clinicians had recognized the index cases sooner, according to a report published online Oct. 6 in the New England Journal of Medicine.
At 4 months’ duration, the 2014 measles outbreak in undervaccinated Amish communities across Ohio was also the longest-lasting one on record since the disease was eliminated in the United States, said Paul A. Gastañaduy, MD, of the division of viral diseases, Centers for Disease Control and Prevention, and his associates.
Two index cases developed in young Amish men (aged 22 and 23 years) who had just returned from a church-sponsored trip to the Philippines to perform charitable work among victims of a typhoon. They had not received any guidance about contagious diseases before their trip. Both developed prodromal symptoms (cough, coryza, or conjunctivitis) the day after their return, then a generalized red maculopapular rash 2 days later. They were then admitted to a local hospital and found to have thrombocytopenia and were diagnosed as having dengue fever. Only after a febrile illness with rash developed in 2 more returning relief workers, then in 12 additional contacts within the Amish community, was measles recognized and reported to the health department a full 30 days later. “Health care providers should maintain a high awareness of measles when returning unvaccinated travelers present with a fever and rash,” Dr. Gastañaduy and his associates said (N Engl J Med. 2016 Oct 6. doi: 10.1056/NEJMoa1602295).
A total of 383 people developed measles in this outbreak; 380 were Amish, and the other 3 were epidemiologically linked to the Amish community. The Amish sect doesn’t specifically prohibit vaccination, but immunization rates are low because the people’s personal and cultural beliefs limit participation in preventive health care. The fact that this outbreak was confined almost exclusively to the Amish indicates that high vaccine coverage in the general Ohio population probably prevented further spread of measles, the investigators noted. A total of 52% of the cases occurred among children and adolescents aged 5-17 years and 25% among persons aged 18-39 years.
After the outbreak was recognized, “engagement of local leaders, isolation of infectious persons, quarantine of those exposed, and vaccination of susceptible persons” allowed it to be contained. Local health departments implemented enhanced surveillance and met with community leaders to encourage case reporting, emphasize the importance of vaccination, inform residents about testing, and alert health care providers statewide about the outbreak. A total of 120 free vaccination clinics were held and 10,644 people were immunized.
This study was supported by the Ohio Department of Health and the CDC. Dr. Gastañaduy and his associates reported having no relevant financial disclosures.
The largest measles outbreak in the United States in more than 2 decades, with an attack rate several orders of magnitude larger than the usual annual nationwide incidence of the disease, could have been better controlled if clinicians had recognized the index cases sooner, according to a report published online Oct. 6 in the New England Journal of Medicine.
At 4 months’ duration, the 2014 measles outbreak in undervaccinated Amish communities across Ohio was also the longest-lasting one on record since the disease was eliminated in the United States, said Paul A. Gastañaduy, MD, of the division of viral diseases, Centers for Disease Control and Prevention, and his associates.
Two index cases developed in young Amish men (aged 22 and 23 years) who had just returned from a church-sponsored trip to the Philippines to perform charitable work among victims of a typhoon. They had not received any guidance about contagious diseases before their trip. Both developed prodromal symptoms (cough, coryza, or conjunctivitis) the day after their return, then a generalized red maculopapular rash 2 days later. They were then admitted to a local hospital and found to have thrombocytopenia and were diagnosed as having dengue fever. Only after a febrile illness with rash developed in 2 more returning relief workers, then in 12 additional contacts within the Amish community, was measles recognized and reported to the health department a full 30 days later. “Health care providers should maintain a high awareness of measles when returning unvaccinated travelers present with a fever and rash,” Dr. Gastañaduy and his associates said (N Engl J Med. 2016 Oct 6. doi: 10.1056/NEJMoa1602295).
A total of 383 people developed measles in this outbreak; 380 were Amish, and the other 3 were epidemiologically linked to the Amish community. The Amish sect doesn’t specifically prohibit vaccination, but immunization rates are low because the people’s personal and cultural beliefs limit participation in preventive health care. The fact that this outbreak was confined almost exclusively to the Amish indicates that high vaccine coverage in the general Ohio population probably prevented further spread of measles, the investigators noted. A total of 52% of the cases occurred among children and adolescents aged 5-17 years and 25% among persons aged 18-39 years.
After the outbreak was recognized, “engagement of local leaders, isolation of infectious persons, quarantine of those exposed, and vaccination of susceptible persons” allowed it to be contained. Local health departments implemented enhanced surveillance and met with community leaders to encourage case reporting, emphasize the importance of vaccination, inform residents about testing, and alert health care providers statewide about the outbreak. A total of 120 free vaccination clinics were held and 10,644 people were immunized.
This study was supported by the Ohio Department of Health and the CDC. Dr. Gastañaduy and his associates reported having no relevant financial disclosures.
Key clinical point: The largest measles outbreak in the U.S. in more than 2 decades could have been better controlled if clinicians had recognized the index cases earlier.
Major finding: Only after a febrile illness with rash developed in 2 more returning relief workers, then in 12 additional contacts within the Amish community, was measles recognized and reported to the health department – a full 30 days after the 2 index patients presented.
Data source: A descriptive analysis of the epidemiologic features of a measles outbreak affecting 383 patients in nine counties in Ohio.
Disclosures: This study was supported by the Ohio Department of Health and the CDC. Dr. Gastañaduy and his associates reported having no relevant financial disclosures.
High-performing hospitals yield longer life expectancy after MI
Patients treated for acute MI at high-performing hospitals – those with the best performance on 30-day mortality quality measures assessed by the Centers for Medicare & Medicaid Services – had longer life expectancies than did patients treated at low-performing hospitals, according to a report published online Oct. 6 in the New England Journal of Medicine.
This survival benefit persisted through 17 years of follow-up, said Emily Bucholz, MD, of Boston Children’s Hospital, and her associates.
Until now, researchers didn’t know whether the short-term survival benefit at high-performing hospitals would wane over time, which “would lend support to the theory that these hospitals discharge more patients alive but with higher subsequent mortality.” The results of this secondary analysis of data from a nationally representative cohort study indicate that, instead, the superior quality of care delivered at high-performing hospitals appears to “produce an early benefit that endures over time,” Dr. Bucholz and her associates noted. The investigators assessed long-term life expectancy using data from the Cooperative Cardiovascular Project, which enrolled Medicare beneficiaries aged 65 years and older treated for acute MI in 1994-1996. They focused on 119,735 patients admitted to 1,824 hospitals. They grouped the hospitals according to their case mixes (a measure of patients’ severity of illness) and calculated risk-standardized mortality rates according to CMS criteria.
“We found that patients treated at high-performing hospitals ... lived, on average, between 0.74 and 1.14 years longer after acute MI than [did] those treated at low-performing hospitals. These findings were consistent across case-mix strata, which indicates that the relationship between hospital performance and long-term patient outcomes is independent of hospital case mix,” Dr. Bucholz and her associates said (N Engl J Med. 2016 Oct 6. doi: 10.1056/NEJMoa1513223).
These findings show that the early survival advantage achieved by high-performing hospitals is durable, and that “investing in initiatives to improve short-term hospital performance may also improve patient outcomes over the long term,” they added.
Patients treated for acute MI at high-performing hospitals – those with the best performance on 30-day mortality quality measures assessed by the Centers for Medicare & Medicaid Services – had longer life expectancies than did patients treated at low-performing hospitals, according to a report published online Oct. 6 in the New England Journal of Medicine.
This survival benefit persisted through 17 years of follow-up, said Emily Bucholz, MD, of Boston Children’s Hospital, and her associates.
Until now, researchers didn’t know whether the short-term survival benefit at high-performing hospitals would wane over time, which “would lend support to the theory that these hospitals discharge more patients alive but with higher subsequent mortality.” The results of this secondary analysis of data from a nationally representative cohort study indicate that, instead, the superior quality of care delivered at high-performing hospitals appears to “produce an early benefit that endures over time,” Dr. Bucholz and her associates noted. The investigators assessed long-term life expectancy using data from the Cooperative Cardiovascular Project, which enrolled Medicare beneficiaries aged 65 years and older treated for acute MI in 1994-1996. They focused on 119,735 patients admitted to 1,824 hospitals. They grouped the hospitals according to their case mixes (a measure of patients’ severity of illness) and calculated risk-standardized mortality rates according to CMS criteria.
“We found that patients treated at high-performing hospitals ... lived, on average, between 0.74 and 1.14 years longer after acute MI than [did] those treated at low-performing hospitals. These findings were consistent across case-mix strata, which indicates that the relationship between hospital performance and long-term patient outcomes is independent of hospital case mix,” Dr. Bucholz and her associates said (N Engl J Med. 2016 Oct 6. doi: 10.1056/NEJMoa1513223).
These findings show that the early survival advantage achieved by high-performing hospitals is durable, and that “investing in initiatives to improve short-term hospital performance may also improve patient outcomes over the long term,” they added.
Patients treated for acute MI at high-performing hospitals – those with the best performance on 30-day mortality quality measures assessed by the Centers for Medicare & Medicaid Services – had longer life expectancies than did patients treated at low-performing hospitals, according to a report published online Oct. 6 in the New England Journal of Medicine.
This survival benefit persisted through 17 years of follow-up, said Emily Bucholz, MD, of Boston Children’s Hospital, and her associates.
Until now, researchers didn’t know whether the short-term survival benefit at high-performing hospitals would wane over time, which “would lend support to the theory that these hospitals discharge more patients alive but with higher subsequent mortality.” The results of this secondary analysis of data from a nationally representative cohort study indicate that, instead, the superior quality of care delivered at high-performing hospitals appears to “produce an early benefit that endures over time,” Dr. Bucholz and her associates noted. The investigators assessed long-term life expectancy using data from the Cooperative Cardiovascular Project, which enrolled Medicare beneficiaries aged 65 years and older treated for acute MI in 1994-1996. They focused on 119,735 patients admitted to 1,824 hospitals. They grouped the hospitals according to their case mixes (a measure of patients’ severity of illness) and calculated risk-standardized mortality rates according to CMS criteria.
“We found that patients treated at high-performing hospitals ... lived, on average, between 0.74 and 1.14 years longer after acute MI than [did] those treated at low-performing hospitals. These findings were consistent across case-mix strata, which indicates that the relationship between hospital performance and long-term patient outcomes is independent of hospital case mix,” Dr. Bucholz and her associates said (N Engl J Med. 2016 Oct 6. doi: 10.1056/NEJMoa1513223).
These findings show that the early survival advantage achieved by high-performing hospitals is durable, and that “investing in initiatives to improve short-term hospital performance may also improve patient outcomes over the long term,” they added.
Key clinical point: Patients treated for MI at high-performing hospitals have longer life expectancies than those treated at low-performing hospitals.
Major finding: Patients treated at high-performing hospitals lived, on average, 0.74-1.14 years longer after acute MI than those treated at low-performing hospitals.
Data source: A secondary analysis of data in a nationally representative cohort study involving 119,735 MI patients treated at 1,824 hospitals and followed for 17 years.
Disclosures: This study was supported by the National Heart, Lung, and Blood Institute and the National Institute of General Medical Sciences. Dr. Bucholz reported having no relevant financial disclosures; one of her associates reported ties to Medtronic, Johnson & Johnson, and UnitedHealth.
Epidermodysplasia Verruciformis and the Risk for Malignancy
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.
The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.

The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.

The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
Practice Points
- Epidermodysplasia verruciformis (EV) is a rare genodermatosis that usually presents in early childhood and presents as verrucous papules and plaques most commonly on the skin of the head, neck, and upper extremities. It often is misdiagnosed at pityriasis versicolor.
- Mutations of the EVER1 and EVER2 genes have been identified as a source for developing EV.
- Epidermodysplasia verruciformis produces wartlike lesions in individuals who have a unique susceptibility to acquiring the human papillomavirus and early onset of nonmelanoma skin cancers, most commonly squamous cell carcinomas related to viral oncogenesis.
- Avoidance and protection from UV exposure is a critical component of treatment plans for patients with EV.
Diagnosis of a Rapidly Growing Preauricular Nodule: Chondroid Syringoma or Pleomorphic Adenoma?
To the Editor:
Chondroid syringoma is a rare benign mixed tumor that originates from the sweat glands, typically presenting with both epithelial and mesenchymal components.1 It differs from pleomorphic adenoma, which arises from salivary glands. The surgical approach for complete excision is different for the 2 tumors; therefore, definitive diagnosis is important. For chondroid syringoma, total excision is recommended,2 while for pleomorphic adenoma, extracapsular dissection or superficial parotidectomy is commonly indicated. We report a case of a preauricular nodule at presentation and highlight the importance of differentiating a chondroid syringoma from a pleomorphic adenoma. This case is unique because of the anatomic location of the nodule, making diagnosis difficult because the tumor was abutting the parotid gland and a biopsy included normal salivary gland cells.
A 19-year-old man with a history of moderate acne on the shoulders, back, and face presented with a rapidly growing, painless nodule on right preauricular region of 6 months’ duration. The nodule was originally thought to be acne related and monitored, as the patient was asymptomatic. On examination the patient was found to have a firm, fixed, nontender, subcutaneous nodule overlying the right temporomandibular joint just anterior to the right tragus (Figure 1). Laboratory results were unremarkable. Computed tomography showed a subcutaneous nonaggressive-appearing soft-tissue mass measuring 16×17×12 mm just anterior and inferior to the external auditory canal cartilaginous segment with no bony abnormalities. The patient was initially treated with incomplete excision of the area for a presumed sebaceous cyst; 2 months later, an abnormal biopsy prompted a complete excisional biopsy.
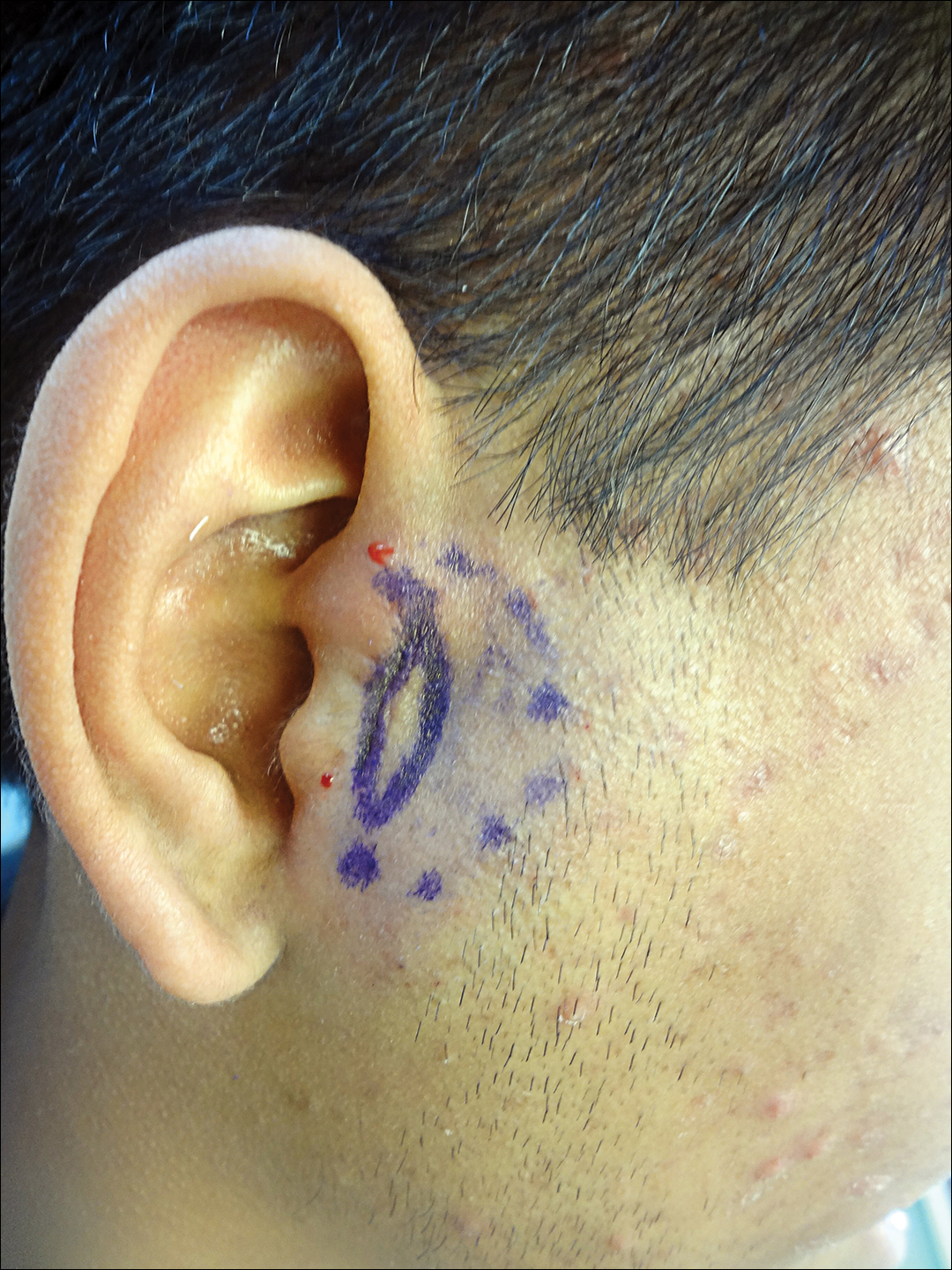
Histologically, the initial incomplete excision biopsy revealed myxoid and chondroid tissue with glandular elements and adjacent lymph node with strong positivity for S-100 protein and moderate positivity for glial fibrillary acid protein, consistent with chondroid syringoma (Figure 2). Histological findings on second excision biopsy performed 2 months later showed tumor cells surrounded by normal salivary gland cells; therefore, it was difficult to define the origin of this tumor. Subsequent magnetic resonance imaging showed no evidence of the tumor and normal parotid gland borders.
Chondroid syringoma is a rare nonmelanoma skin tumor of the head and neck, mostly benign in nature but with malignant potential. Predominantly, it presents in males as an asymptomatic, slow-growing, nontender nodule.2 Malignant chondroid syringomas are more rare, typically appear on the trunk and legs of females, and present as rapidly growing hard nodules. They can arise de novo or from a preexisting chondroid syringoma and can metastasize.3,4
Clinically and histologically, chondroid syringoma resembles a pleomorphic adenoma. Its diagnosis is dependent on the clinical location to exclude origin in a salivary gland.5 Folliculosebaceous and myoepithelial differentiation within the tumor has been reported.6 Immunocytochemistry is the same in both types and is used to identify 2 prominent components—epithelial and mesenchymal—found in both chondroid syringoma and pleomorphic adenoma. Immunocytochemistry differentiates the epithelial component, which expresses cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. In contrast, the mesenchymal component expresses S-100, vimentin, and neuron‐specific enolase, and less often glial fibrillary acidic protein, smooth muscle actin, calponin, or p63.5,7,8 Identification of both layers is a distinctive trait of both tumors, rendering it apart from other conditions in the differential diagnosis.5
Typical treatment options include excision, electrodesiccation, dermabrasion, and argon or CO2 laser. Total excision is recommended if there is a benign tumor and complete excision is a cure.2 One case of recurrent benign chondroid syringoma was treated by Mohs micrographic surgery on the eyebrow9; however, Mohs surgery was not recommended in our case due to concerns of spread if malignant as well as an unknown tumor depth, as these tumors have a tendency for deep infiltration.
Due to its anatomical location and presentation as an anterior preauricular mass, it was difficult to differentiate between chondroid syringoma from sweat gland origin and pleomorphic adenoma from the salivary gland. As seen in our case, it is important for physicians to be aware of the differential diagnosis for mixed tumors because it can have a notable effect on the type of surgical therapy and follow-up management.
- Hirsch P, Helwig EB. Chondroid syringoma. Arch Dermatol. 1961;84:835-847.
- Turhan-Haktanir N, Sahin O, Bukulmez A, et al. Chondroid syringoma in a child. Pediatr Dermatol. 2007;24:505-507.
- Mathiasen RA, Rasgon BM, Rumore G. Malignant chondroid syringoma of the face: a first reported case. Otolaryngol Head Neck Surg. 2005;133:305-307.
- Barnett MD, Wallack MK, Zuretti A, et al. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol. 2000;23:227-232.
- Dubb M, Michelow P. Cytologic features of chondroid syringoma in fine needle aspiration biopsies a report of 3 cases. Acta Cytol. 2010;54:183-186.
- Rauso R, Santagata M, Tartaro G, et al. Chondroid syringoma: rare tumor of orofacial region. Minerva Stomatol. 2009;58:383-388.
- Metzler G, Schaumburg-Lever G, Hornstein O, et al. Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol. 1996;18:83-89.
- Argenyi ZB, Balogh K, Goeken JA. Immunohistochemical characterization of chondroid syringomas. Am J Clin Pathol. 1988;90:662-669.
- Walls AC, Deng A, Geist DE. Mohs micrographic surgery for recurrent chondroid syringoma of the eyebrow. Dermatol Surg. 2012;38:800-802.
To the Editor:
Chondroid syringoma is a rare benign mixed tumor that originates from the sweat glands, typically presenting with both epithelial and mesenchymal components.1 It differs from pleomorphic adenoma, which arises from salivary glands. The surgical approach for complete excision is different for the 2 tumors; therefore, definitive diagnosis is important. For chondroid syringoma, total excision is recommended,2 while for pleomorphic adenoma, extracapsular dissection or superficial parotidectomy is commonly indicated. We report a case of a preauricular nodule at presentation and highlight the importance of differentiating a chondroid syringoma from a pleomorphic adenoma. This case is unique because of the anatomic location of the nodule, making diagnosis difficult because the tumor was abutting the parotid gland and a biopsy included normal salivary gland cells.
A 19-year-old man with a history of moderate acne on the shoulders, back, and face presented with a rapidly growing, painless nodule on right preauricular region of 6 months’ duration. The nodule was originally thought to be acne related and monitored, as the patient was asymptomatic. On examination the patient was found to have a firm, fixed, nontender, subcutaneous nodule overlying the right temporomandibular joint just anterior to the right tragus (Figure 1). Laboratory results were unremarkable. Computed tomography showed a subcutaneous nonaggressive-appearing soft-tissue mass measuring 16×17×12 mm just anterior and inferior to the external auditory canal cartilaginous segment with no bony abnormalities. The patient was initially treated with incomplete excision of the area for a presumed sebaceous cyst; 2 months later, an abnormal biopsy prompted a complete excisional biopsy.

Histologically, the initial incomplete excision biopsy revealed myxoid and chondroid tissue with glandular elements and adjacent lymph node with strong positivity for S-100 protein and moderate positivity for glial fibrillary acid protein, consistent with chondroid syringoma (Figure 2). Histological findings on second excision biopsy performed 2 months later showed tumor cells surrounded by normal salivary gland cells; therefore, it was difficult to define the origin of this tumor. Subsequent magnetic resonance imaging showed no evidence of the tumor and normal parotid gland borders.

Chondroid syringoma is a rare nonmelanoma skin tumor of the head and neck, mostly benign in nature but with malignant potential. Predominantly, it presents in males as an asymptomatic, slow-growing, nontender nodule.2 Malignant chondroid syringomas are more rare, typically appear on the trunk and legs of females, and present as rapidly growing hard nodules. They can arise de novo or from a preexisting chondroid syringoma and can metastasize.3,4
Clinically and histologically, chondroid syringoma resembles a pleomorphic adenoma. Its diagnosis is dependent on the clinical location to exclude origin in a salivary gland.5 Folliculosebaceous and myoepithelial differentiation within the tumor has been reported.6 Immunocytochemistry is the same in both types and is used to identify 2 prominent components—epithelial and mesenchymal—found in both chondroid syringoma and pleomorphic adenoma. Immunocytochemistry differentiates the epithelial component, which expresses cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. In contrast, the mesenchymal component expresses S-100, vimentin, and neuron‐specific enolase, and less often glial fibrillary acidic protein, smooth muscle actin, calponin, or p63.5,7,8 Identification of both layers is a distinctive trait of both tumors, rendering it apart from other conditions in the differential diagnosis.5
Typical treatment options include excision, electrodesiccation, dermabrasion, and argon or CO2 laser. Total excision is recommended if there is a benign tumor and complete excision is a cure.2 One case of recurrent benign chondroid syringoma was treated by Mohs micrographic surgery on the eyebrow9; however, Mohs surgery was not recommended in our case due to concerns of spread if malignant as well as an unknown tumor depth, as these tumors have a tendency for deep infiltration.
Due to its anatomical location and presentation as an anterior preauricular mass, it was difficult to differentiate between chondroid syringoma from sweat gland origin and pleomorphic adenoma from the salivary gland. As seen in our case, it is important for physicians to be aware of the differential diagnosis for mixed tumors because it can have a notable effect on the type of surgical therapy and follow-up management.
To the Editor:
Chondroid syringoma is a rare benign mixed tumor that originates from the sweat glands, typically presenting with both epithelial and mesenchymal components.1 It differs from pleomorphic adenoma, which arises from salivary glands. The surgical approach for complete excision is different for the 2 tumors; therefore, definitive diagnosis is important. For chondroid syringoma, total excision is recommended,2 while for pleomorphic adenoma, extracapsular dissection or superficial parotidectomy is commonly indicated. We report a case of a preauricular nodule at presentation and highlight the importance of differentiating a chondroid syringoma from a pleomorphic adenoma. This case is unique because of the anatomic location of the nodule, making diagnosis difficult because the tumor was abutting the parotid gland and a biopsy included normal salivary gland cells.
A 19-year-old man with a history of moderate acne on the shoulders, back, and face presented with a rapidly growing, painless nodule on right preauricular region of 6 months’ duration. The nodule was originally thought to be acne related and monitored, as the patient was asymptomatic. On examination the patient was found to have a firm, fixed, nontender, subcutaneous nodule overlying the right temporomandibular joint just anterior to the right tragus (Figure 1). Laboratory results were unremarkable. Computed tomography showed a subcutaneous nonaggressive-appearing soft-tissue mass measuring 16×17×12 mm just anterior and inferior to the external auditory canal cartilaginous segment with no bony abnormalities. The patient was initially treated with incomplete excision of the area for a presumed sebaceous cyst; 2 months later, an abnormal biopsy prompted a complete excisional biopsy.

Histologically, the initial incomplete excision biopsy revealed myxoid and chondroid tissue with glandular elements and adjacent lymph node with strong positivity for S-100 protein and moderate positivity for glial fibrillary acid protein, consistent with chondroid syringoma (Figure 2). Histological findings on second excision biopsy performed 2 months later showed tumor cells surrounded by normal salivary gland cells; therefore, it was difficult to define the origin of this tumor. Subsequent magnetic resonance imaging showed no evidence of the tumor and normal parotid gland borders.

Chondroid syringoma is a rare nonmelanoma skin tumor of the head and neck, mostly benign in nature but with malignant potential. Predominantly, it presents in males as an asymptomatic, slow-growing, nontender nodule.2 Malignant chondroid syringomas are more rare, typically appear on the trunk and legs of females, and present as rapidly growing hard nodules. They can arise de novo or from a preexisting chondroid syringoma and can metastasize.3,4
Clinically and histologically, chondroid syringoma resembles a pleomorphic adenoma. Its diagnosis is dependent on the clinical location to exclude origin in a salivary gland.5 Folliculosebaceous and myoepithelial differentiation within the tumor has been reported.6 Immunocytochemistry is the same in both types and is used to identify 2 prominent components—epithelial and mesenchymal—found in both chondroid syringoma and pleomorphic adenoma. Immunocytochemistry differentiates the epithelial component, which expresses cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. In contrast, the mesenchymal component expresses S-100, vimentin, and neuron‐specific enolase, and less often glial fibrillary acidic protein, smooth muscle actin, calponin, or p63.5,7,8 Identification of both layers is a distinctive trait of both tumors, rendering it apart from other conditions in the differential diagnosis.5
Typical treatment options include excision, electrodesiccation, dermabrasion, and argon or CO2 laser. Total excision is recommended if there is a benign tumor and complete excision is a cure.2 One case of recurrent benign chondroid syringoma was treated by Mohs micrographic surgery on the eyebrow9; however, Mohs surgery was not recommended in our case due to concerns of spread if malignant as well as an unknown tumor depth, as these tumors have a tendency for deep infiltration.
Due to its anatomical location and presentation as an anterior preauricular mass, it was difficult to differentiate between chondroid syringoma from sweat gland origin and pleomorphic adenoma from the salivary gland. As seen in our case, it is important for physicians to be aware of the differential diagnosis for mixed tumors because it can have a notable effect on the type of surgical therapy and follow-up management.
- Hirsch P, Helwig EB. Chondroid syringoma. Arch Dermatol. 1961;84:835-847.
- Turhan-Haktanir N, Sahin O, Bukulmez A, et al. Chondroid syringoma in a child. Pediatr Dermatol. 2007;24:505-507.
- Mathiasen RA, Rasgon BM, Rumore G. Malignant chondroid syringoma of the face: a first reported case. Otolaryngol Head Neck Surg. 2005;133:305-307.
- Barnett MD, Wallack MK, Zuretti A, et al. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol. 2000;23:227-232.
- Dubb M, Michelow P. Cytologic features of chondroid syringoma in fine needle aspiration biopsies a report of 3 cases. Acta Cytol. 2010;54:183-186.
- Rauso R, Santagata M, Tartaro G, et al. Chondroid syringoma: rare tumor of orofacial region. Minerva Stomatol. 2009;58:383-388.
- Metzler G, Schaumburg-Lever G, Hornstein O, et al. Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol. 1996;18:83-89.
- Argenyi ZB, Balogh K, Goeken JA. Immunohistochemical characterization of chondroid syringomas. Am J Clin Pathol. 1988;90:662-669.
- Walls AC, Deng A, Geist DE. Mohs micrographic surgery for recurrent chondroid syringoma of the eyebrow. Dermatol Surg. 2012;38:800-802.
- Hirsch P, Helwig EB. Chondroid syringoma. Arch Dermatol. 1961;84:835-847.
- Turhan-Haktanir N, Sahin O, Bukulmez A, et al. Chondroid syringoma in a child. Pediatr Dermatol. 2007;24:505-507.
- Mathiasen RA, Rasgon BM, Rumore G. Malignant chondroid syringoma of the face: a first reported case. Otolaryngol Head Neck Surg. 2005;133:305-307.
- Barnett MD, Wallack MK, Zuretti A, et al. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol. 2000;23:227-232.
- Dubb M, Michelow P. Cytologic features of chondroid syringoma in fine needle aspiration biopsies a report of 3 cases. Acta Cytol. 2010;54:183-186.
- Rauso R, Santagata M, Tartaro G, et al. Chondroid syringoma: rare tumor of orofacial region. Minerva Stomatol. 2009;58:383-388.
- Metzler G, Schaumburg-Lever G, Hornstein O, et al. Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol. 1996;18:83-89.
- Argenyi ZB, Balogh K, Goeken JA. Immunohistochemical characterization of chondroid syringomas. Am J Clin Pathol. 1988;90:662-669.
- Walls AC, Deng A, Geist DE. Mohs micrographic surgery for recurrent chondroid syringoma of the eyebrow. Dermatol Surg. 2012;38:800-802.
Practice Points
- Clinically and histologically, pleomorphic adenomas and chondroid syringoma both have identical presentations. Differentiation can be determined by knowing where the mixed tumor originated.
- Both lesions warrant different surgical management techniques. Pleomorphic adenoma requires extracapsular dissection or superficial parotidectomy, while complete excision is recommended for chondroid syringoma.
Diagnostic error most common claim against internists
The majority of lawsuits against internists stem from alleged diagnostics errors, results of a new study show.
Of 1,180 legal claims against internists, 39% were related to failed, delayed, or wrong diagnosis allegations, according to an analysis published by The Doctors Company, a nationwide medical malpractice insurer. Negligence associated with medical treatment accounted for 32% of the claims, while 19% were related to alleged medication errors.
The Doctors Company evaluated 1,180 claims from its database against internal medicine physicians that closed from 2007 to 2014. Of diagnostic-related cases, 56% alleged inadequate patient assessments, such as failure to order or delay in ordering diagnostic tests. The final diagnoses most commonly related to these allegations were myocardial infarction (6%), lung cancer (5%), and colorectal cancer (5%). More than 200 other diagnoses were seen in fewer than 2% of the claims, according to the study.
In cases that involved injury, a top contributing factor was patient assessment issues, such as failure to establish a differential diagnosis or inadequate assessment. Patient factors, such as noncompliance, were also a primary contributer to injuries. The third most common factor for injury was poor communication among providers, family, and patients, such as inadequate education about the risks of medications.
The findings hopefully will assist internists and risk managers in understanding common allegations and factors behind lawsuits so that improvements can be made, study coauthor David B. Troxel, MD, medical director for The Doctors Company said in a statement.
“With the data on patient allegations and the actual factors that led to injuries included in this study, physicians and risk managers can identify system weaknesses and reduce risk of harm to patients,” he said.
“Physicians, internists, would benefit from something we’re doing in my group where we talk about why it is that there are cognitive errors,” he said. “Is there a system problem here, rather than someone who just made a mistake?”
Dr. Marcus advised physicians to read a recent report by the National Academies of Sciences, Engineering, and Medicine, which calls for more emphasis on identifying and learning from diagnostic errors and near misses in clinical practice, a payment and care delivery environment that supports the diagnostic process, and a dedicated focus on new research.
Ensuring that patients understand the treatment plan and follow-up care management are also key risk mitigation steps, adds Dr. Troxel.
“Patient compliance is a major problem,” he said in an interview. “It’s not that patients are not listening to the doctor. Sometimes they don’t understand what the doctor is explaining. We encourage physicians to really make sure the patient understands by asking them to repeat back what you’ve told them. Make sure they got the information.”
[email protected]
On Twitter @legal_med
The majority of lawsuits against internists stem from alleged diagnostics errors, results of a new study show.
Of 1,180 legal claims against internists, 39% were related to failed, delayed, or wrong diagnosis allegations, according to an analysis published by The Doctors Company, a nationwide medical malpractice insurer. Negligence associated with medical treatment accounted for 32% of the claims, while 19% were related to alleged medication errors.
The Doctors Company evaluated 1,180 claims from its database against internal medicine physicians that closed from 2007 to 2014. Of diagnostic-related cases, 56% alleged inadequate patient assessments, such as failure to order or delay in ordering diagnostic tests. The final diagnoses most commonly related to these allegations were myocardial infarction (6%), lung cancer (5%), and colorectal cancer (5%). More than 200 other diagnoses were seen in fewer than 2% of the claims, according to the study.
In cases that involved injury, a top contributing factor was patient assessment issues, such as failure to establish a differential diagnosis or inadequate assessment. Patient factors, such as noncompliance, were also a primary contributer to injuries. The third most common factor for injury was poor communication among providers, family, and patients, such as inadequate education about the risks of medications.
The findings hopefully will assist internists and risk managers in understanding common allegations and factors behind lawsuits so that improvements can be made, study coauthor David B. Troxel, MD, medical director for The Doctors Company said in a statement.
“With the data on patient allegations and the actual factors that led to injuries included in this study, physicians and risk managers can identify system weaknesses and reduce risk of harm to patients,” he said.
“Physicians, internists, would benefit from something we’re doing in my group where we talk about why it is that there are cognitive errors,” he said. “Is there a system problem here, rather than someone who just made a mistake?”
Dr. Marcus advised physicians to read a recent report by the National Academies of Sciences, Engineering, and Medicine, which calls for more emphasis on identifying and learning from diagnostic errors and near misses in clinical practice, a payment and care delivery environment that supports the diagnostic process, and a dedicated focus on new research.
Ensuring that patients understand the treatment plan and follow-up care management are also key risk mitigation steps, adds Dr. Troxel.
“Patient compliance is a major problem,” he said in an interview. “It’s not that patients are not listening to the doctor. Sometimes they don’t understand what the doctor is explaining. We encourage physicians to really make sure the patient understands by asking them to repeat back what you’ve told them. Make sure they got the information.”
[email protected]
On Twitter @legal_med
The majority of lawsuits against internists stem from alleged diagnostics errors, results of a new study show.
Of 1,180 legal claims against internists, 39% were related to failed, delayed, or wrong diagnosis allegations, according to an analysis published by The Doctors Company, a nationwide medical malpractice insurer. Negligence associated with medical treatment accounted for 32% of the claims, while 19% were related to alleged medication errors.
The Doctors Company evaluated 1,180 claims from its database against internal medicine physicians that closed from 2007 to 2014. Of diagnostic-related cases, 56% alleged inadequate patient assessments, such as failure to order or delay in ordering diagnostic tests. The final diagnoses most commonly related to these allegations were myocardial infarction (6%), lung cancer (5%), and colorectal cancer (5%). More than 200 other diagnoses were seen in fewer than 2% of the claims, according to the study.
In cases that involved injury, a top contributing factor was patient assessment issues, such as failure to establish a differential diagnosis or inadequate assessment. Patient factors, such as noncompliance, were also a primary contributer to injuries. The third most common factor for injury was poor communication among providers, family, and patients, such as inadequate education about the risks of medications.
The findings hopefully will assist internists and risk managers in understanding common allegations and factors behind lawsuits so that improvements can be made, study coauthor David B. Troxel, MD, medical director for The Doctors Company said in a statement.
“With the data on patient allegations and the actual factors that led to injuries included in this study, physicians and risk managers can identify system weaknesses and reduce risk of harm to patients,” he said.
“Physicians, internists, would benefit from something we’re doing in my group where we talk about why it is that there are cognitive errors,” he said. “Is there a system problem here, rather than someone who just made a mistake?”
Dr. Marcus advised physicians to read a recent report by the National Academies of Sciences, Engineering, and Medicine, which calls for more emphasis on identifying and learning from diagnostic errors and near misses in clinical practice, a payment and care delivery environment that supports the diagnostic process, and a dedicated focus on new research.
Ensuring that patients understand the treatment plan and follow-up care management are also key risk mitigation steps, adds Dr. Troxel.
“Patient compliance is a major problem,” he said in an interview. “It’s not that patients are not listening to the doctor. Sometimes they don’t understand what the doctor is explaining. We encourage physicians to really make sure the patient understands by asking them to repeat back what you’ve told them. Make sure they got the information.”
[email protected]
On Twitter @legal_med