User login
Drug granted conditional approval to treat CLL in Canada

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()
What Hospitalists Can Really Learn from Aviation
The aviation safety model is often discussed in a healthcare context but in a way that may miss the most important points, a new article in BMJ Quality & Safety suggests.
The article, “Learning from Near Misses in Aviation: So Much More to It Than You Thought” by Robert Wears, MD, PhD, MS, of University of Florida’s Department of Emergency Medicine, suggests healthcare still has important lessons to learn from aviation. The article focuses on a book called Close Calls: Managing Risk and Resilience in Airline Flight Safety by Carl Macrae.
“Although the book itself is about airlines, it has important lessons for improving safety in healthcare, especially with respect to management of incidents or ‘near misses,’” Dr. Wears writes. “Its rich descriptions and detailed explanation of the practical, everyday work of flight safety investigators should be required reading for anyone interested in patient safety. It will destroy many of the myths and misconceptions about reporting systems and learning from incidents that have caused us to expend so much effort for such meager results; it will also overturn the normative model of safety prevalent in healthcare.”
Dr. Wears says he wanted to write the article for two reasons.
“First, the patient safety orthodoxy has been obsessed with systems for reporting incidents, accidents, hazards, general ‘hiccups’ in clinical work for years, but almost nothing of value has come from this effort despite frequent badgering of physicians to report more,” he says. “Second, mainstream patient safety has also been enamored of the aviation safety model, but its ideas about how aviation safety is actually accomplished are naive and simplistic.”
He emphasizes that patient safety efforts to date have focused on the wrong things: too much on acquiring and storing reports and too little on analyzing them to develop an understanding of the systems in which hazards to patients arise.
“Making sense of incidents is far more important than classifying, counting, or trending them,” Dr. Wears says.
Hospitalists are on the front line of these issues, of course.
“Hospitalists regularly encounter hazards to patients in their daily work and, for the most part, successfully manage to mitigate or work around them, but the hazards remain in the system, only to pop up again sometime later. … A rich description of how a successful and effective safety reporting and analysis effort really works—not how we imagine it to work—could help us exchange our current wasteful and ineffective approach for something better,” he says.
Reference
- Wears R. Learning from near misses in aviation: so much more to it than you thought [published online ahead of print September 1, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2016-005990.
The aviation safety model is often discussed in a healthcare context but in a way that may miss the most important points, a new article in BMJ Quality & Safety suggests.
The article, “Learning from Near Misses in Aviation: So Much More to It Than You Thought” by Robert Wears, MD, PhD, MS, of University of Florida’s Department of Emergency Medicine, suggests healthcare still has important lessons to learn from aviation. The article focuses on a book called Close Calls: Managing Risk and Resilience in Airline Flight Safety by Carl Macrae.
“Although the book itself is about airlines, it has important lessons for improving safety in healthcare, especially with respect to management of incidents or ‘near misses,’” Dr. Wears writes. “Its rich descriptions and detailed explanation of the practical, everyday work of flight safety investigators should be required reading for anyone interested in patient safety. It will destroy many of the myths and misconceptions about reporting systems and learning from incidents that have caused us to expend so much effort for such meager results; it will also overturn the normative model of safety prevalent in healthcare.”
Dr. Wears says he wanted to write the article for two reasons.
“First, the patient safety orthodoxy has been obsessed with systems for reporting incidents, accidents, hazards, general ‘hiccups’ in clinical work for years, but almost nothing of value has come from this effort despite frequent badgering of physicians to report more,” he says. “Second, mainstream patient safety has also been enamored of the aviation safety model, but its ideas about how aviation safety is actually accomplished are naive and simplistic.”
He emphasizes that patient safety efforts to date have focused on the wrong things: too much on acquiring and storing reports and too little on analyzing them to develop an understanding of the systems in which hazards to patients arise.
“Making sense of incidents is far more important than classifying, counting, or trending them,” Dr. Wears says.
Hospitalists are on the front line of these issues, of course.
“Hospitalists regularly encounter hazards to patients in their daily work and, for the most part, successfully manage to mitigate or work around them, but the hazards remain in the system, only to pop up again sometime later. … A rich description of how a successful and effective safety reporting and analysis effort really works—not how we imagine it to work—could help us exchange our current wasteful and ineffective approach for something better,” he says.
Reference
- Wears R. Learning from near misses in aviation: so much more to it than you thought [published online ahead of print September 1, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2016-005990.
The aviation safety model is often discussed in a healthcare context but in a way that may miss the most important points, a new article in BMJ Quality & Safety suggests.
The article, “Learning from Near Misses in Aviation: So Much More to It Than You Thought” by Robert Wears, MD, PhD, MS, of University of Florida’s Department of Emergency Medicine, suggests healthcare still has important lessons to learn from aviation. The article focuses on a book called Close Calls: Managing Risk and Resilience in Airline Flight Safety by Carl Macrae.
“Although the book itself is about airlines, it has important lessons for improving safety in healthcare, especially with respect to management of incidents or ‘near misses,’” Dr. Wears writes. “Its rich descriptions and detailed explanation of the practical, everyday work of flight safety investigators should be required reading for anyone interested in patient safety. It will destroy many of the myths and misconceptions about reporting systems and learning from incidents that have caused us to expend so much effort for such meager results; it will also overturn the normative model of safety prevalent in healthcare.”
Dr. Wears says he wanted to write the article for two reasons.
“First, the patient safety orthodoxy has been obsessed with systems for reporting incidents, accidents, hazards, general ‘hiccups’ in clinical work for years, but almost nothing of value has come from this effort despite frequent badgering of physicians to report more,” he says. “Second, mainstream patient safety has also been enamored of the aviation safety model, but its ideas about how aviation safety is actually accomplished are naive and simplistic.”
He emphasizes that patient safety efforts to date have focused on the wrong things: too much on acquiring and storing reports and too little on analyzing them to develop an understanding of the systems in which hazards to patients arise.
“Making sense of incidents is far more important than classifying, counting, or trending them,” Dr. Wears says.
Hospitalists are on the front line of these issues, of course.
“Hospitalists regularly encounter hazards to patients in their daily work and, for the most part, successfully manage to mitigate or work around them, but the hazards remain in the system, only to pop up again sometime later. … A rich description of how a successful and effective safety reporting and analysis effort really works—not how we imagine it to work—could help us exchange our current wasteful and ineffective approach for something better,” he says.
Reference
- Wears R. Learning from near misses in aviation: so much more to it than you thought [published online ahead of print September 1, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2016-005990.
Educating Patients about Sleep Tools
One of the biggest complaints of hospital patients today is poor sleep, which is not conducive to healing or good health in general.
“The reason I’m interested, as a cardiologist, is that sleep disorders are associated with an increased risk of cardiovascular mortality,” says Peter M. Farrehi, MD, assistant professor of internal medicine at the University of Michigan and lead author of a recent sleep study published in The American Journal of Medicine.
Most information about sleeping in the hospital comes from ICU studies, he says.
Dr. Farrehi wanted to actually test an intervention rather than simply survey patients. All patients received an eye mask, ear plugs, and a white-noise machine, then were randomized to receive an education-based script on the importance of using these sleep-enhancing tools or a discussion about the general benefits of sleep.
“To avoid bias in the study both from the research staff and also hospital staff, I didn't want only the intervention to have the tools,” he says. “This was a double-blind, randomized control trial in the hospital, which is really unusual.”
Patients in the group that was taught about the sleep-enhancing tools had a statistically significant difference in their perceptions of fatigue and a trend toward improving their sleep and wake disturbances.
Dr. Farrehi suggests hospitalists talk to their patients complaining of poor sleep about these sleep tools. If they are not available in their hospital, hospitalists might refer their medical director to this paper to see if there is any interest in purchasing these sleep tools.
Reference
- 1. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of sleep tool education during hospitalization: a randomized controlled trial [published online ahead of print August 23, 2016]. Am J Med. doi:10.1016/j.amjmed.2016.08.001.
One of the biggest complaints of hospital patients today is poor sleep, which is not conducive to healing or good health in general.
“The reason I’m interested, as a cardiologist, is that sleep disorders are associated with an increased risk of cardiovascular mortality,” says Peter M. Farrehi, MD, assistant professor of internal medicine at the University of Michigan and lead author of a recent sleep study published in The American Journal of Medicine.
Most information about sleeping in the hospital comes from ICU studies, he says.
Dr. Farrehi wanted to actually test an intervention rather than simply survey patients. All patients received an eye mask, ear plugs, and a white-noise machine, then were randomized to receive an education-based script on the importance of using these sleep-enhancing tools or a discussion about the general benefits of sleep.
“To avoid bias in the study both from the research staff and also hospital staff, I didn't want only the intervention to have the tools,” he says. “This was a double-blind, randomized control trial in the hospital, which is really unusual.”
Patients in the group that was taught about the sleep-enhancing tools had a statistically significant difference in their perceptions of fatigue and a trend toward improving their sleep and wake disturbances.
Dr. Farrehi suggests hospitalists talk to their patients complaining of poor sleep about these sleep tools. If they are not available in their hospital, hospitalists might refer their medical director to this paper to see if there is any interest in purchasing these sleep tools.
Reference
- 1. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of sleep tool education during hospitalization: a randomized controlled trial [published online ahead of print August 23, 2016]. Am J Med. doi:10.1016/j.amjmed.2016.08.001.
One of the biggest complaints of hospital patients today is poor sleep, which is not conducive to healing or good health in general.
“The reason I’m interested, as a cardiologist, is that sleep disorders are associated with an increased risk of cardiovascular mortality,” says Peter M. Farrehi, MD, assistant professor of internal medicine at the University of Michigan and lead author of a recent sleep study published in The American Journal of Medicine.
Most information about sleeping in the hospital comes from ICU studies, he says.
Dr. Farrehi wanted to actually test an intervention rather than simply survey patients. All patients received an eye mask, ear plugs, and a white-noise machine, then were randomized to receive an education-based script on the importance of using these sleep-enhancing tools or a discussion about the general benefits of sleep.
“To avoid bias in the study both from the research staff and also hospital staff, I didn't want only the intervention to have the tools,” he says. “This was a double-blind, randomized control trial in the hospital, which is really unusual.”
Patients in the group that was taught about the sleep-enhancing tools had a statistically significant difference in their perceptions of fatigue and a trend toward improving their sleep and wake disturbances.
Dr. Farrehi suggests hospitalists talk to their patients complaining of poor sleep about these sleep tools. If they are not available in their hospital, hospitalists might refer their medical director to this paper to see if there is any interest in purchasing these sleep tools.
Reference
- 1. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of sleep tool education during hospitalization: a randomized controlled trial [published online ahead of print August 23, 2016]. Am J Med. doi:10.1016/j.amjmed.2016.08.001.
Doc offers advice on choosing a frontline TKI

Photo by D. Meyer
NEW YORK—Evaluating treatment goals is essential when choosing which tyrosine kinase inhibitor (TKI) to prescribe for a patient with newly diagnosed chronic myeloid leukemia (CML), according to a speaker at the NCCN 11th Annual Congress: Hematologic Malignancies.
“Deciding what TKI to start people on really depends on what your goals are for that patient,” said the speaker, Jerald Radich, MD, of the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance in Seattle, Washington.
Because the 3 TKIs approved for frontline treatment of CML—imatinib, dasatinib, and nilotinib—produce “amazingly similar” responses, treatment compliance becomes an important factor in patient outcomes, he noted.
“If you take 90% of your imatinib, your MMR [major molecular response] is 90%,” he said. “Your CMR [complete molecular response] is 40%. So taking drug obviously trumps the decision of what drug to take.”
Dr Radich added that the major goal of treatment is to keep patients out of accelerated-phase blast crisis. Once people progress to blast crisis on a TKI, the median survival is less than 1 year.
“So that’s why you treat people aggressively, that’s why you monitor them molecularly, to prevent that from happening,” he said.
Treatment goals
Aside from preventing patients from progressing to blast crisis, treatment goals vary.
Achieving early molecular response (MR) impacts progression and survival, as does achieving a complete cytogenetic response (CCyR).
A major molecular response (MMR) is considered a “safe haven,” Dr Radich said, because once people achieve it, they almost never progress if they stay on drug.
And with a deep/complete molecular response (CMR), patients may potentially discontinue the drug.
So how your response goals line up determines how you use the agents for your treatment course, Dr Radich said.
In all response categories—patients with CCyR, MMR, MR, CMR—survival is virtually within 95% of survival for the general population.
“This is absolutely astonishing,” Dr Radich said.
He emphasized the importance of molecular testing at 3 months and achieving a BCR-ABL level of less than 10%.
Patients who have more than 10% blasts at 3 months have an 88% chance of achieving MMR at 4 years, while those who still have more than 10% blasts at 6 months have a 3.3% chance of achieving MMR at 4 years.
Toxicity
Side effects common to the 3 frontline TKIs are myelosuppression, transaminase elevation, and change in electrolytes. Dr Radich noted that imatinib doesn’t cause much myelosuppression.
“You can give imatinib on day 28 after allogeneic transplant, and it doesn’t affect the counts, which I think is pretty darn good proof that it doesn’t have any primary hematopoietic toxicity,” he said. “You can’t try that trick with the others.”
Venous and arterial cardiovascular events with TKIs are more recently coming to light.
Cardiovascular events with imatinib are about the same as the general population, Dr Radich said.
“[In] fact, some people think it might be protective,” he noted.
Discontinuation
“When we first started treating people with these drugs, we figured that they would be on them for life . . . ,” Dr Radich said. “[Y]ou’d always have a reservoir of CML cells because you can’t extinguish all the stem cells.”
A mathematical model predicted it would take 30 to 40 years to wipe out all CML cells with a TKI. The cumulative cure rate after 15 years of treatment would be 14%. After 30 years, it would be 31%.
Conducting a discontinuation trial would have been out of the question based on these predictions.
“Fortunately, some of the people who did the next trials hadn’t read that literature,” Dr Radich said.
One discontinuation trial (EURO-SKI) included patients who had been on drug for at least 3 years and had CMR for at least 1 year. About half stayed in PCR negativity, now up to 4 years.
A number of trials are now underway evaluating the possibility of TKI discontinuation, and they are showing that between 40% and 50% of patients can remain off drug for years.
Using generic imatinib
While generic imatinib is good for cost-effective, long-term use, second-generation TKIs are better at preventing accelerated-phase blast crisis, Dr Radich said.
The second generation is also better at producing deep remissions, and discontinuation could bring with it a cost savings.
Dr Radich calculated that it cost about $2.5 million for every patient who achieves treatment-free remission using a TKI, while transplant cost $1.31 million per patient who achieves treatment-free remission.
So generic imatinib is good for low- and intermediate-risk patients, as well as for older, sicker patients.
Second-generation TKIs are appropriate for higher-risk patients until they achieve a CCyR or MMR, then they can switch to generic imatinib.
And second-generation TKIs should be used for younger patients in whom drug discontinuation is important.
Frontline treatment observations
In summary, Dr Radich made the following observations about frontline treatment in CML.
- For overall survival, imatinib is equivalent to second-generation TKIs.
- To achieve a deep MR, a second-generation TKI is better than imatinib.
- Discontinuation is equally successful with all TKIs.
- For lower-risk CML, imatinib is equivalent to second-generation TKIs.
- When it comes to progression and possibly high-risk CML, second-generation TKIs are better than imatinib.
- Second-generation TKIs produce more long-term toxicities than imatinib.
- There is substantial cost savings with generics.


Photo by D. Meyer
NEW YORK—Evaluating treatment goals is essential when choosing which tyrosine kinase inhibitor (TKI) to prescribe for a patient with newly diagnosed chronic myeloid leukemia (CML), according to a speaker at the NCCN 11th Annual Congress: Hematologic Malignancies.
“Deciding what TKI to start people on really depends on what your goals are for that patient,” said the speaker, Jerald Radich, MD, of the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance in Seattle, Washington.
Because the 3 TKIs approved for frontline treatment of CML—imatinib, dasatinib, and nilotinib—produce “amazingly similar” responses, treatment compliance becomes an important factor in patient outcomes, he noted.
“If you take 90% of your imatinib, your MMR [major molecular response] is 90%,” he said. “Your CMR [complete molecular response] is 40%. So taking drug obviously trumps the decision of what drug to take.”
Dr Radich added that the major goal of treatment is to keep patients out of accelerated-phase blast crisis. Once people progress to blast crisis on a TKI, the median survival is less than 1 year.
“So that’s why you treat people aggressively, that’s why you monitor them molecularly, to prevent that from happening,” he said.
Treatment goals
Aside from preventing patients from progressing to blast crisis, treatment goals vary.
Achieving early molecular response (MR) impacts progression and survival, as does achieving a complete cytogenetic response (CCyR).
A major molecular response (MMR) is considered a “safe haven,” Dr Radich said, because once people achieve it, they almost never progress if they stay on drug.
And with a deep/complete molecular response (CMR), patients may potentially discontinue the drug.
So how your response goals line up determines how you use the agents for your treatment course, Dr Radich said.
In all response categories—patients with CCyR, MMR, MR, CMR—survival is virtually within 95% of survival for the general population.
“This is absolutely astonishing,” Dr Radich said.
He emphasized the importance of molecular testing at 3 months and achieving a BCR-ABL level of less than 10%.
Patients who have more than 10% blasts at 3 months have an 88% chance of achieving MMR at 4 years, while those who still have more than 10% blasts at 6 months have a 3.3% chance of achieving MMR at 4 years.
Toxicity
Side effects common to the 3 frontline TKIs are myelosuppression, transaminase elevation, and change in electrolytes. Dr Radich noted that imatinib doesn’t cause much myelosuppression.
“You can give imatinib on day 28 after allogeneic transplant, and it doesn’t affect the counts, which I think is pretty darn good proof that it doesn’t have any primary hematopoietic toxicity,” he said. “You can’t try that trick with the others.”
Venous and arterial cardiovascular events with TKIs are more recently coming to light.
Cardiovascular events with imatinib are about the same as the general population, Dr Radich said.
“[In] fact, some people think it might be protective,” he noted.
Discontinuation
“When we first started treating people with these drugs, we figured that they would be on them for life . . . ,” Dr Radich said. “[Y]ou’d always have a reservoir of CML cells because you can’t extinguish all the stem cells.”
A mathematical model predicted it would take 30 to 40 years to wipe out all CML cells with a TKI. The cumulative cure rate after 15 years of treatment would be 14%. After 30 years, it would be 31%.
Conducting a discontinuation trial would have been out of the question based on these predictions.
“Fortunately, some of the people who did the next trials hadn’t read that literature,” Dr Radich said.
One discontinuation trial (EURO-SKI) included patients who had been on drug for at least 3 years and had CMR for at least 1 year. About half stayed in PCR negativity, now up to 4 years.
A number of trials are now underway evaluating the possibility of TKI discontinuation, and they are showing that between 40% and 50% of patients can remain off drug for years.
Using generic imatinib
While generic imatinib is good for cost-effective, long-term use, second-generation TKIs are better at preventing accelerated-phase blast crisis, Dr Radich said.
The second generation is also better at producing deep remissions, and discontinuation could bring with it a cost savings.
Dr Radich calculated that it cost about $2.5 million for every patient who achieves treatment-free remission using a TKI, while transplant cost $1.31 million per patient who achieves treatment-free remission.
So generic imatinib is good for low- and intermediate-risk patients, as well as for older, sicker patients.
Second-generation TKIs are appropriate for higher-risk patients until they achieve a CCyR or MMR, then they can switch to generic imatinib.
And second-generation TKIs should be used for younger patients in whom drug discontinuation is important.
Frontline treatment observations
In summary, Dr Radich made the following observations about frontline treatment in CML.
- For overall survival, imatinib is equivalent to second-generation TKIs.
- To achieve a deep MR, a second-generation TKI is better than imatinib.
- Discontinuation is equally successful with all TKIs.
- For lower-risk CML, imatinib is equivalent to second-generation TKIs.
- When it comes to progression and possibly high-risk CML, second-generation TKIs are better than imatinib.
- Second-generation TKIs produce more long-term toxicities than imatinib.
- There is substantial cost savings with generics.


Photo by D. Meyer
NEW YORK—Evaluating treatment goals is essential when choosing which tyrosine kinase inhibitor (TKI) to prescribe for a patient with newly diagnosed chronic myeloid leukemia (CML), according to a speaker at the NCCN 11th Annual Congress: Hematologic Malignancies.
“Deciding what TKI to start people on really depends on what your goals are for that patient,” said the speaker, Jerald Radich, MD, of the Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance in Seattle, Washington.
Because the 3 TKIs approved for frontline treatment of CML—imatinib, dasatinib, and nilotinib—produce “amazingly similar” responses, treatment compliance becomes an important factor in patient outcomes, he noted.
“If you take 90% of your imatinib, your MMR [major molecular response] is 90%,” he said. “Your CMR [complete molecular response] is 40%. So taking drug obviously trumps the decision of what drug to take.”
Dr Radich added that the major goal of treatment is to keep patients out of accelerated-phase blast crisis. Once people progress to blast crisis on a TKI, the median survival is less than 1 year.
“So that’s why you treat people aggressively, that’s why you monitor them molecularly, to prevent that from happening,” he said.
Treatment goals
Aside from preventing patients from progressing to blast crisis, treatment goals vary.
Achieving early molecular response (MR) impacts progression and survival, as does achieving a complete cytogenetic response (CCyR).
A major molecular response (MMR) is considered a “safe haven,” Dr Radich said, because once people achieve it, they almost never progress if they stay on drug.
And with a deep/complete molecular response (CMR), patients may potentially discontinue the drug.
So how your response goals line up determines how you use the agents for your treatment course, Dr Radich said.
In all response categories—patients with CCyR, MMR, MR, CMR—survival is virtually within 95% of survival for the general population.
“This is absolutely astonishing,” Dr Radich said.
He emphasized the importance of molecular testing at 3 months and achieving a BCR-ABL level of less than 10%.
Patients who have more than 10% blasts at 3 months have an 88% chance of achieving MMR at 4 years, while those who still have more than 10% blasts at 6 months have a 3.3% chance of achieving MMR at 4 years.
Toxicity
Side effects common to the 3 frontline TKIs are myelosuppression, transaminase elevation, and change in electrolytes. Dr Radich noted that imatinib doesn’t cause much myelosuppression.
“You can give imatinib on day 28 after allogeneic transplant, and it doesn’t affect the counts, which I think is pretty darn good proof that it doesn’t have any primary hematopoietic toxicity,” he said. “You can’t try that trick with the others.”
Venous and arterial cardiovascular events with TKIs are more recently coming to light.
Cardiovascular events with imatinib are about the same as the general population, Dr Radich said.
“[In] fact, some people think it might be protective,” he noted.
Discontinuation
“When we first started treating people with these drugs, we figured that they would be on them for life . . . ,” Dr Radich said. “[Y]ou’d always have a reservoir of CML cells because you can’t extinguish all the stem cells.”
A mathematical model predicted it would take 30 to 40 years to wipe out all CML cells with a TKI. The cumulative cure rate after 15 years of treatment would be 14%. After 30 years, it would be 31%.
Conducting a discontinuation trial would have been out of the question based on these predictions.
“Fortunately, some of the people who did the next trials hadn’t read that literature,” Dr Radich said.
One discontinuation trial (EURO-SKI) included patients who had been on drug for at least 3 years and had CMR for at least 1 year. About half stayed in PCR negativity, now up to 4 years.
A number of trials are now underway evaluating the possibility of TKI discontinuation, and they are showing that between 40% and 50% of patients can remain off drug for years.
Using generic imatinib
While generic imatinib is good for cost-effective, long-term use, second-generation TKIs are better at preventing accelerated-phase blast crisis, Dr Radich said.
The second generation is also better at producing deep remissions, and discontinuation could bring with it a cost savings.
Dr Radich calculated that it cost about $2.5 million for every patient who achieves treatment-free remission using a TKI, while transplant cost $1.31 million per patient who achieves treatment-free remission.
So generic imatinib is good for low- and intermediate-risk patients, as well as for older, sicker patients.
Second-generation TKIs are appropriate for higher-risk patients until they achieve a CCyR or MMR, then they can switch to generic imatinib.
And second-generation TKIs should be used for younger patients in whom drug discontinuation is important.
Frontline treatment observations
In summary, Dr Radich made the following observations about frontline treatment in CML.
- For overall survival, imatinib is equivalent to second-generation TKIs.
- To achieve a deep MR, a second-generation TKI is better than imatinib.
- Discontinuation is equally successful with all TKIs.
- For lower-risk CML, imatinib is equivalent to second-generation TKIs.
- When it comes to progression and possibly high-risk CML, second-generation TKIs are better than imatinib.
- Second-generation TKIs produce more long-term toxicities than imatinib.
- There is substantial cost savings with generics.

Findings may aid drug delivery, bioimaging

to illuminate microfluidic device
simulating a blood vessel.
Photo from Anson Ma/UConn
A study published in Biophysical Journal has revealed new information about how particles behave in the bloodstream, and investigators believe the findings have implications for bioimaging and targeted drug delivery in cancer.
The investigators used a microfluidic channel device to observe, track, and measure how individual particles behave in a simulated blood vessel.
Their goal was to learn more about the physics influencing a particle’s behavior as it travels in the blood and to determine which particle size might be the most effective for delivering drugs to their targets.
“Even before particles reach a target site, you have to worry about what is going to happen with them after they get injected into the bloodstream,” said study author Anson Ma, PhD, of the University of Connecticut in Storrs, Connecticut.
“Are they going to clump together? How are they going to move around? Are they going to get swept away and flushed out of our bodies?”
Using a high-powered fluorescence microscope, Dr Ma and his colleagues were able to observe particles being carried along in the simulated blood vessel in what could be described as a vascular “Running of the Bulls.”
Red blood cells raced through the middle of the channel, and the particles were carried along in the rush, bumping and bouncing off the blood cells until they were pushed to open spaces—called the cell-free layer—along the vessel’s walls.
The investigators found that larger particles—the optimum size appeared to be about 2 microns—were most likely to get pushed to the cell-free layer, where their chances of carrying a drug to a targeted site are greatest.
The team also determined that 2 microns was the largest size that should be used if particles are going to have any chance of going through the leaky blood vessel walls to the site.
“When it comes to using particles for the delivery of cancer drugs, size matters,” Dr Ma said. “When you have a bigger particle, the chance of it bumping into blood cells is much higher, there are a lot more collisions, and they tend to get pushed to the blood vessel walls.”
These results were somewhat surprising. The investigators had theorized that smaller particles would probably be the most effective since they would move the most in collisions with blood cells.
But the opposite proved true. The smaller particles appeared to skirt through the mass of moving blood cells and were less likely to get bounced to the cell-free layer.
Knowing how particles behave in the circulatory system should help improve targeted drug delivery, Dr Ma said. And this should further reduce the side effects caused by potent cancer drugs missing their target.
Measuring how different sized particles move in the bloodstream may also be beneficial in bioimaging, where the goal is to keep particles circulating in the bloodstream long enough for imaging to occur. In that case, smaller particles would be better, Dr Ma said.
Moving forward, Dr Ma would like to explore other aspects of particle flow in the circulatory system, such as how particles behave when they pass through a constricted area, like from a blood vessel to a capillary.
Capillaries are only about 7 microns in diameter. Dr Ma said he would like to know how that constricted space might impact particle flow or the ability of particles to accumulate near the vessel walls.
“We have all of this complex geometry in our bodies,” Dr Ma said. “Most people just assume there is no impact when a particle moves from a bigger channel to a smaller channel because they haven’t quantified it. Our plan is to do some experiments to look at this more carefully, building on the work that we just published.” ![]()

to illuminate microfluidic device
simulating a blood vessel.
Photo from Anson Ma/UConn
A study published in Biophysical Journal has revealed new information about how particles behave in the bloodstream, and investigators believe the findings have implications for bioimaging and targeted drug delivery in cancer.
The investigators used a microfluidic channel device to observe, track, and measure how individual particles behave in a simulated blood vessel.
Their goal was to learn more about the physics influencing a particle’s behavior as it travels in the blood and to determine which particle size might be the most effective for delivering drugs to their targets.
“Even before particles reach a target site, you have to worry about what is going to happen with them after they get injected into the bloodstream,” said study author Anson Ma, PhD, of the University of Connecticut in Storrs, Connecticut.
“Are they going to clump together? How are they going to move around? Are they going to get swept away and flushed out of our bodies?”
Using a high-powered fluorescence microscope, Dr Ma and his colleagues were able to observe particles being carried along in the simulated blood vessel in what could be described as a vascular “Running of the Bulls.”
Red blood cells raced through the middle of the channel, and the particles were carried along in the rush, bumping and bouncing off the blood cells until they were pushed to open spaces—called the cell-free layer—along the vessel’s walls.
The investigators found that larger particles—the optimum size appeared to be about 2 microns—were most likely to get pushed to the cell-free layer, where their chances of carrying a drug to a targeted site are greatest.
The team also determined that 2 microns was the largest size that should be used if particles are going to have any chance of going through the leaky blood vessel walls to the site.
“When it comes to using particles for the delivery of cancer drugs, size matters,” Dr Ma said. “When you have a bigger particle, the chance of it bumping into blood cells is much higher, there are a lot more collisions, and they tend to get pushed to the blood vessel walls.”
These results were somewhat surprising. The investigators had theorized that smaller particles would probably be the most effective since they would move the most in collisions with blood cells.
But the opposite proved true. The smaller particles appeared to skirt through the mass of moving blood cells and were less likely to get bounced to the cell-free layer.
Knowing how particles behave in the circulatory system should help improve targeted drug delivery, Dr Ma said. And this should further reduce the side effects caused by potent cancer drugs missing their target.
Measuring how different sized particles move in the bloodstream may also be beneficial in bioimaging, where the goal is to keep particles circulating in the bloodstream long enough for imaging to occur. In that case, smaller particles would be better, Dr Ma said.
Moving forward, Dr Ma would like to explore other aspects of particle flow in the circulatory system, such as how particles behave when they pass through a constricted area, like from a blood vessel to a capillary.
Capillaries are only about 7 microns in diameter. Dr Ma said he would like to know how that constricted space might impact particle flow or the ability of particles to accumulate near the vessel walls.
“We have all of this complex geometry in our bodies,” Dr Ma said. “Most people just assume there is no impact when a particle moves from a bigger channel to a smaller channel because they haven’t quantified it. Our plan is to do some experiments to look at this more carefully, building on the work that we just published.” ![]()

to illuminate microfluidic device
simulating a blood vessel.
Photo from Anson Ma/UConn
A study published in Biophysical Journal has revealed new information about how particles behave in the bloodstream, and investigators believe the findings have implications for bioimaging and targeted drug delivery in cancer.
The investigators used a microfluidic channel device to observe, track, and measure how individual particles behave in a simulated blood vessel.
Their goal was to learn more about the physics influencing a particle’s behavior as it travels in the blood and to determine which particle size might be the most effective for delivering drugs to their targets.
“Even before particles reach a target site, you have to worry about what is going to happen with them after they get injected into the bloodstream,” said study author Anson Ma, PhD, of the University of Connecticut in Storrs, Connecticut.
“Are they going to clump together? How are they going to move around? Are they going to get swept away and flushed out of our bodies?”
Using a high-powered fluorescence microscope, Dr Ma and his colleagues were able to observe particles being carried along in the simulated blood vessel in what could be described as a vascular “Running of the Bulls.”
Red blood cells raced through the middle of the channel, and the particles were carried along in the rush, bumping and bouncing off the blood cells until they were pushed to open spaces—called the cell-free layer—along the vessel’s walls.
The investigators found that larger particles—the optimum size appeared to be about 2 microns—were most likely to get pushed to the cell-free layer, where their chances of carrying a drug to a targeted site are greatest.
The team also determined that 2 microns was the largest size that should be used if particles are going to have any chance of going through the leaky blood vessel walls to the site.
“When it comes to using particles for the delivery of cancer drugs, size matters,” Dr Ma said. “When you have a bigger particle, the chance of it bumping into blood cells is much higher, there are a lot more collisions, and they tend to get pushed to the blood vessel walls.”
These results were somewhat surprising. The investigators had theorized that smaller particles would probably be the most effective since they would move the most in collisions with blood cells.
But the opposite proved true. The smaller particles appeared to skirt through the mass of moving blood cells and were less likely to get bounced to the cell-free layer.
Knowing how particles behave in the circulatory system should help improve targeted drug delivery, Dr Ma said. And this should further reduce the side effects caused by potent cancer drugs missing their target.
Measuring how different sized particles move in the bloodstream may also be beneficial in bioimaging, where the goal is to keep particles circulating in the bloodstream long enough for imaging to occur. In that case, smaller particles would be better, Dr Ma said.
Moving forward, Dr Ma would like to explore other aspects of particle flow in the circulatory system, such as how particles behave when they pass through a constricted area, like from a blood vessel to a capillary.
Capillaries are only about 7 microns in diameter. Dr Ma said he would like to know how that constricted space might impact particle flow or the ability of particles to accumulate near the vessel walls.
“We have all of this complex geometry in our bodies,” Dr Ma said. “Most people just assume there is no impact when a particle moves from a bigger channel to a smaller channel because they haven’t quantified it. Our plan is to do some experiments to look at this more carefully, building on the work that we just published.” ![]()
Treating Chronic Disease in Disadvantaged Populations
In 2015, 7 of the top 10 causes of death were chronic diseases, according to the CDC, and many disproportionately affect “health disparity populations”: minorities, underserved rural populations, and other disadvantaged groups who generally have lower detection rates, leading to late-stage diagnosis and treatment and worse outcomes.
In response to a need for “more robust, ecological approaches to address chronic diseases” among those groups, the National Institute on Minority Health and Health Disparities (NIMHD) is launching the Transdisciplinary Collaborative Centers (TCC) for Health Disparities Research on Chronic Disease Prevention program.
The program comprises 2 centers of community organizations, academic institutions, clinicians and health care systems, and state and local public health agencies. The 2 centers will share about $20 million to conduct research into community-based, multilevel interventions to combat heart disease, cancer, diabetes, and other chronic diseases. The emphasis will be on prevention, early detection, and early treatment.
The research programs will “translate community needs into practice” at local clinics, churches, and community centers, says NIMHD. Projects include developing interventions to control hypertension among American Indians, Alaska Natives, and Native Hawaiians and other Pacific Islanders. In another project researchers will apply community-engaged research in Flint, Michigan, investigating the effectiveness of interventions aimed at improving physical activity and healthy food consumption.
The new program “looks beyond individual behavioral risk factors,” NIMHD says, to engage the family, community, health care systems, and policy impacts that affect health. NIMHD Director Dr. Eliseo Pérez-Stable says, “Multilevel interventions that take into account complex interactions between individuals and their environments can better address determinants of health and enhance chronic disease prevention and health promotion for local communities.”
In 2015, 7 of the top 10 causes of death were chronic diseases, according to the CDC, and many disproportionately affect “health disparity populations”: minorities, underserved rural populations, and other disadvantaged groups who generally have lower detection rates, leading to late-stage diagnosis and treatment and worse outcomes.
In response to a need for “more robust, ecological approaches to address chronic diseases” among those groups, the National Institute on Minority Health and Health Disparities (NIMHD) is launching the Transdisciplinary Collaborative Centers (TCC) for Health Disparities Research on Chronic Disease Prevention program.
The program comprises 2 centers of community organizations, academic institutions, clinicians and health care systems, and state and local public health agencies. The 2 centers will share about $20 million to conduct research into community-based, multilevel interventions to combat heart disease, cancer, diabetes, and other chronic diseases. The emphasis will be on prevention, early detection, and early treatment.
The research programs will “translate community needs into practice” at local clinics, churches, and community centers, says NIMHD. Projects include developing interventions to control hypertension among American Indians, Alaska Natives, and Native Hawaiians and other Pacific Islanders. In another project researchers will apply community-engaged research in Flint, Michigan, investigating the effectiveness of interventions aimed at improving physical activity and healthy food consumption.
The new program “looks beyond individual behavioral risk factors,” NIMHD says, to engage the family, community, health care systems, and policy impacts that affect health. NIMHD Director Dr. Eliseo Pérez-Stable says, “Multilevel interventions that take into account complex interactions between individuals and their environments can better address determinants of health and enhance chronic disease prevention and health promotion for local communities.”
In 2015, 7 of the top 10 causes of death were chronic diseases, according to the CDC, and many disproportionately affect “health disparity populations”: minorities, underserved rural populations, and other disadvantaged groups who generally have lower detection rates, leading to late-stage diagnosis and treatment and worse outcomes.
In response to a need for “more robust, ecological approaches to address chronic diseases” among those groups, the National Institute on Minority Health and Health Disparities (NIMHD) is launching the Transdisciplinary Collaborative Centers (TCC) for Health Disparities Research on Chronic Disease Prevention program.
The program comprises 2 centers of community organizations, academic institutions, clinicians and health care systems, and state and local public health agencies. The 2 centers will share about $20 million to conduct research into community-based, multilevel interventions to combat heart disease, cancer, diabetes, and other chronic diseases. The emphasis will be on prevention, early detection, and early treatment.
The research programs will “translate community needs into practice” at local clinics, churches, and community centers, says NIMHD. Projects include developing interventions to control hypertension among American Indians, Alaska Natives, and Native Hawaiians and other Pacific Islanders. In another project researchers will apply community-engaged research in Flint, Michigan, investigating the effectiveness of interventions aimed at improving physical activity and healthy food consumption.
The new program “looks beyond individual behavioral risk factors,” NIMHD says, to engage the family, community, health care systems, and policy impacts that affect health. NIMHD Director Dr. Eliseo Pérez-Stable says, “Multilevel interventions that take into account complex interactions between individuals and their environments can better address determinants of health and enhance chronic disease prevention and health promotion for local communities.”
"I Got Blisters On My Fingers!"
The stubborn focal eruption on this 6-year-old boy’s left fourth finger remains unaffected after a one-week course of oral cephalexin, so his pediatrician refers him to dermatology. The problem initially manifested as a cluster of tiny blisters, which tingled a bit but didn’t hurt. When redness developed around it, the patient’s mother brought him for evaluation.
The boy experienced the same problem in the same location almost exactly a year ago. It was treated the same way and within a week or two had resolved.
The child is otherwise reasonably healthy, despite being allergy-prone. He has a history of seasonal allergies and is susceptible to skin infections, such as staph.
EXAMINATION
On the dorsal aspect of his finger are grouped vesicles on an erythematous base, measuring about 1 cm altogether. Several of these demonstrate central umbilication, and several are filled with pus. The erythema is minimal, and there is no tenderness on palpation. Palpation of the epitrochlear area reveals a tiny, nontender node.
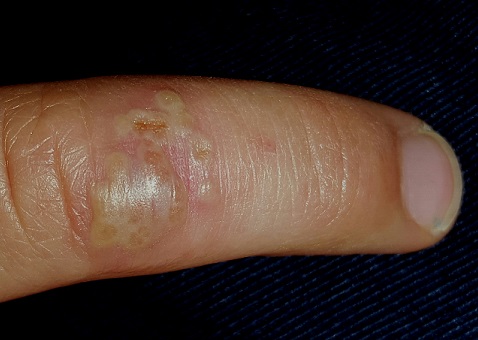
What is the diagnosis?
DISCUSSION
Herpetic whitlow (HW), while uncommon, is far from rare. Essentially a cold sore occurring away from the usual labial/facial location, HW presents with premonitory symptoms of itch, tingle, and slight pain, followed by the appearance of grouped vesicles on an erythematous base. The vesicles are often umbillicated, as with most herpetic conditions. And, like most herpes simplex–related outbreaks, HW tends to recur in the same location—an extremely useful piece of information.
Its appearance on unusual areas can cause confusion, but herpes simplex can manifest almost anywhere: toes, legs, nipples, the tips of ears, and even the eye. On the thicker skin of fingers, these vesicles may be a bit harder to recognize than they would be on a penis or lip.
Because it is often assumed that pus designates a bacterial infection, its presence in this situation can be puzzling. But pus is really just a collection of dead white cells, often seen in conditions unrelated to bacteria (eg, psoriasis).
One additional factor played a role in the diagnosis of this condition. Atopic children (with seasonal allergies, asthma, eczema, and other related phenomena) are exceptionally susceptible to skin infections of all kinds, including bacterial, viral, and fungal. Further evaluation revealed that this child had a history of molluscum contagiosum and impetigo.
If the nature of this outbreak had been unclear, it could have been cultured (though it could take a week or two to get results) or even biopsied. The latter option would have shown the cytopathic effects of the virus.
No treatment was possible, nor was it necessary. HW has to be treated within the first day or two of the outbreak to be effective. Since this patient’s outbreaks only occur once a year, he does not need prophylactic treatment. Over time, as he and his immune system mature, he can be expected to have fewer attacks.
In terms of contagion, he represents no more threat to others than a child with a cold sore or a wart.
TAKE-HOME LEARNING POINTS
• Herpetic whitlow (HW), although commonly found on fingers, can occur almost anywhere.
• The causative organism is herpes simplex; each episode begins with premonitory symptoms, followed within a few days by the appearance of grouped vesicles on an erythematous base. After a week or two, the episode resolves.
• Herpes infections of all kinds in immunocompetent patients tend to recur in the same places repeatedly.
• Atopy predisposes to skin infections of all kinds.
• Pus is not necessarily an indication of bacterial infection; it is often seen in viral infections.
The stubborn focal eruption on this 6-year-old boy’s left fourth finger remains unaffected after a one-week course of oral cephalexin, so his pediatrician refers him to dermatology. The problem initially manifested as a cluster of tiny blisters, which tingled a bit but didn’t hurt. When redness developed around it, the patient’s mother brought him for evaluation.
The boy experienced the same problem in the same location almost exactly a year ago. It was treated the same way and within a week or two had resolved.
The child is otherwise reasonably healthy, despite being allergy-prone. He has a history of seasonal allergies and is susceptible to skin infections, such as staph.
EXAMINATION
On the dorsal aspect of his finger are grouped vesicles on an erythematous base, measuring about 1 cm altogether. Several of these demonstrate central umbilication, and several are filled with pus. The erythema is minimal, and there is no tenderness on palpation. Palpation of the epitrochlear area reveals a tiny, nontender node.

What is the diagnosis?
DISCUSSION
Herpetic whitlow (HW), while uncommon, is far from rare. Essentially a cold sore occurring away from the usual labial/facial location, HW presents with premonitory symptoms of itch, tingle, and slight pain, followed by the appearance of grouped vesicles on an erythematous base. The vesicles are often umbillicated, as with most herpetic conditions. And, like most herpes simplex–related outbreaks, HW tends to recur in the same location—an extremely useful piece of information.
Its appearance on unusual areas can cause confusion, but herpes simplex can manifest almost anywhere: toes, legs, nipples, the tips of ears, and even the eye. On the thicker skin of fingers, these vesicles may be a bit harder to recognize than they would be on a penis or lip.
Because it is often assumed that pus designates a bacterial infection, its presence in this situation can be puzzling. But pus is really just a collection of dead white cells, often seen in conditions unrelated to bacteria (eg, psoriasis).
One additional factor played a role in the diagnosis of this condition. Atopic children (with seasonal allergies, asthma, eczema, and other related phenomena) are exceptionally susceptible to skin infections of all kinds, including bacterial, viral, and fungal. Further evaluation revealed that this child had a history of molluscum contagiosum and impetigo.
If the nature of this outbreak had been unclear, it could have been cultured (though it could take a week or two to get results) or even biopsied. The latter option would have shown the cytopathic effects of the virus.
No treatment was possible, nor was it necessary. HW has to be treated within the first day or two of the outbreak to be effective. Since this patient’s outbreaks only occur once a year, he does not need prophylactic treatment. Over time, as he and his immune system mature, he can be expected to have fewer attacks.
In terms of contagion, he represents no more threat to others than a child with a cold sore or a wart.
TAKE-HOME LEARNING POINTS
• Herpetic whitlow (HW), although commonly found on fingers, can occur almost anywhere.
• The causative organism is herpes simplex; each episode begins with premonitory symptoms, followed within a few days by the appearance of grouped vesicles on an erythematous base. After a week or two, the episode resolves.
• Herpes infections of all kinds in immunocompetent patients tend to recur in the same places repeatedly.
• Atopy predisposes to skin infections of all kinds.
• Pus is not necessarily an indication of bacterial infection; it is often seen in viral infections.
The stubborn focal eruption on this 6-year-old boy’s left fourth finger remains unaffected after a one-week course of oral cephalexin, so his pediatrician refers him to dermatology. The problem initially manifested as a cluster of tiny blisters, which tingled a bit but didn’t hurt. When redness developed around it, the patient’s mother brought him for evaluation.
The boy experienced the same problem in the same location almost exactly a year ago. It was treated the same way and within a week or two had resolved.
The child is otherwise reasonably healthy, despite being allergy-prone. He has a history of seasonal allergies and is susceptible to skin infections, such as staph.
EXAMINATION
On the dorsal aspect of his finger are grouped vesicles on an erythematous base, measuring about 1 cm altogether. Several of these demonstrate central umbilication, and several are filled with pus. The erythema is minimal, and there is no tenderness on palpation. Palpation of the epitrochlear area reveals a tiny, nontender node.

What is the diagnosis?
DISCUSSION
Herpetic whitlow (HW), while uncommon, is far from rare. Essentially a cold sore occurring away from the usual labial/facial location, HW presents with premonitory symptoms of itch, tingle, and slight pain, followed by the appearance of grouped vesicles on an erythematous base. The vesicles are often umbillicated, as with most herpetic conditions. And, like most herpes simplex–related outbreaks, HW tends to recur in the same location—an extremely useful piece of information.
Its appearance on unusual areas can cause confusion, but herpes simplex can manifest almost anywhere: toes, legs, nipples, the tips of ears, and even the eye. On the thicker skin of fingers, these vesicles may be a bit harder to recognize than they would be on a penis or lip.
Because it is often assumed that pus designates a bacterial infection, its presence in this situation can be puzzling. But pus is really just a collection of dead white cells, often seen in conditions unrelated to bacteria (eg, psoriasis).
One additional factor played a role in the diagnosis of this condition. Atopic children (with seasonal allergies, asthma, eczema, and other related phenomena) are exceptionally susceptible to skin infections of all kinds, including bacterial, viral, and fungal. Further evaluation revealed that this child had a history of molluscum contagiosum and impetigo.
If the nature of this outbreak had been unclear, it could have been cultured (though it could take a week or two to get results) or even biopsied. The latter option would have shown the cytopathic effects of the virus.
No treatment was possible, nor was it necessary. HW has to be treated within the first day or two of the outbreak to be effective. Since this patient’s outbreaks only occur once a year, he does not need prophylactic treatment. Over time, as he and his immune system mature, he can be expected to have fewer attacks.
In terms of contagion, he represents no more threat to others than a child with a cold sore or a wart.
TAKE-HOME LEARNING POINTS
• Herpetic whitlow (HW), although commonly found on fingers, can occur almost anywhere.
• The causative organism is herpes simplex; each episode begins with premonitory symptoms, followed within a few days by the appearance of grouped vesicles on an erythematous base. After a week or two, the episode resolves.
• Herpes infections of all kinds in immunocompetent patients tend to recur in the same places repeatedly.
• Atopy predisposes to skin infections of all kinds.
• Pus is not necessarily an indication of bacterial infection; it is often seen in viral infections.
Subscribe to the new CHEST SEEK Library
The CHEST SEEK Library includes nearly 1,500 pulmonary, critical care, and sleep medicine questions in a 1-year subscription. Whether preparing for board exams or just wanting a solid review, sharpen your skills with this comprehensive collection for an introductory price of $149 ($199 for nonmembers). This is the best deal ever offered for SEEK, which can now be accessed via Apple, Android, and Web browser. Do not wait. The introductory price will only be offered for a limited time. Learn more at https://www.chestnet.org/Store.
The CHEST SEEK Library includes nearly 1,500 pulmonary, critical care, and sleep medicine questions in a 1-year subscription. Whether preparing for board exams or just wanting a solid review, sharpen your skills with this comprehensive collection for an introductory price of $149 ($199 for nonmembers). This is the best deal ever offered for SEEK, which can now be accessed via Apple, Android, and Web browser. Do not wait. The introductory price will only be offered for a limited time. Learn more at https://www.chestnet.org/Store.
The CHEST SEEK Library includes nearly 1,500 pulmonary, critical care, and sleep medicine questions in a 1-year subscription. Whether preparing for board exams or just wanting a solid review, sharpen your skills with this comprehensive collection for an introductory price of $149 ($199 for nonmembers). This is the best deal ever offered for SEEK, which can now be accessed via Apple, Android, and Web browser. Do not wait. The introductory price will only be offered for a limited time. Learn more at https://www.chestnet.org/Store.
PRESIDENT’S REPORT The Six F’s for Our Most Important Resource: Faculty Volunteers
This has been an extraordinary year for CHEST, particularly in the core area of clinical education. In the past fiscal year, we exceeded our educational goals. We set out to reach 10,000 learners through educational programming including live courses and conferences, and online activities; in the end, we served 15,547.
Other goals accomplished include demonstrating a significant increase in average learner knowledge acquisition and procedural skills improvement; identifying top priorities for online offerings and delivering five stand-alone online modules that can serve as a point of entry to wider audiences; recording professional attendance at CHEST 2015 of 5,149 people; offering online training for guideline development and the panelists engaged in CHEST guidelines; achieving an attendance at CHEST World Congress in Shanghai of 2,089; and working with leading Chinese medical societies to see the China-CHEST Pulmonary and Critical Care Medicine Fellowship Program formally adopted by the government in China as one of the four first-ever subspecialty training programs to be implemented nationwide.
This is a lot!
These accomplishments depend on intense work and collaboration between our incredibly talented faculty and volunteers from among CHEST membership and CHEST’s amazing professional staff of 105 employees, of which 28 are dedicated full time to the development and delivery of education and best practices. Through this partnership, we continue to meet CHEST’s mission: To champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.
Without these dedicated women and men, CHEST would be utterly unable to complete its mission. Our faculty work in a vast array of opportunities, including writing questions for SEEK, serving as a content expert for guidelines, proposing and delivering sessions at CHEST, running Board Review courses, recording videos, facilitating hands-on simulation sessions, and more. While intrinsically gratifying, there are many difficult elements to such work, requiring commitment that begins long before the delivery of an event or the launch of a new activity. Reviewing existing literature and knowledge on a topic to determine whether an activity will meet the needs of our membership; coming up with valid learning objectives; generating just the right multiple choice questions and other assessments to measure our success at helping learners reach those objectives; peer reviewing content to ensure we’re teaching to the latest science and established best practices; and then measuring learner outcomes – these elements put the “state of the art” into CHEST’s internationally recognized state-of-the-art educational program.
To achieve our mission, we have been asking CHEST’s valiant and dedicated volunteers to do more than ever before, and some of what we have asked them to do has been frustrating, tedious, and less than rewarding. The reasons for this are many, including the imperfect technology platforms we’ve asked our volunteers to use; the disconnect between the educational goals we have set and the implementation of the clear processes, communication, and on-boarding of staff required to support them; and the lag of recognition proportionate to the nature of these new asks.
So, how do we show our member-faculty that they are our most important resource? Recently, we have had internal discussions about how to acknowledge the priceless contributions made by our faculty volunteers. To that end, CHEST staff and volunteer leadership have developed a Faculty & Volunteer Treatment Action Plan, recently approved by the CHEST Board of Regents. This is part of our comprehensive “six F’s” plan:
Formal recognition and rewards. Recognition and rewards are different – but both important. Recognition is expressing gratitude for an expected job that was well done and includes a formal thank-you. Rewards are additional, tangible benefits for exceptional services. We now have enhanced guidelines for travel, honoraria, and amenities for our volunteer faculty. Also of note, two new awards will be bestowed annually beginning at CHEST 2017 Los Angeles – the Early Career Clinician Educator Award and the Master Clinician Educator Award. These are some additional ways we will more appropriately highlight the people who have helped make us CHEST, the leader in clinical education in chest medicine.
Feedback. In addition to learner satisfaction data, CHEST provides an unprecedented level of learner outcomes data to our faculty. We are even introducing a new peer-review of teaching (PRT) program so faculty can get even more feedback from expert colleagues.
Faculty Development. As an education-focused organization, training and development plays a foundational role. We are working to develop a comprehensive clinician educator program that will grow our bench of faculty. A newly launched database will more proactively track and match interested members with teaching opportunities within the organization.
Face Time. Easy access to leadership and staff is important. We are implementing staff training that will better position all CHEST employees to more effectively facilitate and support the work we ask of our faculty. On another front, we are engaged in identifying new, user-friendly systems for session submission, conflict of interest disclosure, and review, as well as developing content.
Food. It is a simple but well-established fact that having a stocked lounge area for busy faculty on the run between teaching sessions enhances efficiency, communication, camaraderie, and overall morale.
Fun. The fun of discovering better ways to take care of our patients, be it from the teacher or learner perspective, in an engaging, effective learning environment is and always has been at the center of what we do.
CHEST’s volunteer leaders, in service to their peers, the field, and the organization, have risen to many challenges over and over again. We realize we need to do a better job of rewarding and recognizing their irreplaceable contributions. The above initiatives, and others, we hope, will help demonstrate to our most precious resource, our member-faculty, that we truly value and appreciate their invaluable contributions on behalf of CHEST. Stay tuned and stay with us.
This has been an extraordinary year for CHEST, particularly in the core area of clinical education. In the past fiscal year, we exceeded our educational goals. We set out to reach 10,000 learners through educational programming including live courses and conferences, and online activities; in the end, we served 15,547.
Other goals accomplished include demonstrating a significant increase in average learner knowledge acquisition and procedural skills improvement; identifying top priorities for online offerings and delivering five stand-alone online modules that can serve as a point of entry to wider audiences; recording professional attendance at CHEST 2015 of 5,149 people; offering online training for guideline development and the panelists engaged in CHEST guidelines; achieving an attendance at CHEST World Congress in Shanghai of 2,089; and working with leading Chinese medical societies to see the China-CHEST Pulmonary and Critical Care Medicine Fellowship Program formally adopted by the government in China as one of the four first-ever subspecialty training programs to be implemented nationwide.
This is a lot!
These accomplishments depend on intense work and collaboration between our incredibly talented faculty and volunteers from among CHEST membership and CHEST’s amazing professional staff of 105 employees, of which 28 are dedicated full time to the development and delivery of education and best practices. Through this partnership, we continue to meet CHEST’s mission: To champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.
Without these dedicated women and men, CHEST would be utterly unable to complete its mission. Our faculty work in a vast array of opportunities, including writing questions for SEEK, serving as a content expert for guidelines, proposing and delivering sessions at CHEST, running Board Review courses, recording videos, facilitating hands-on simulation sessions, and more. While intrinsically gratifying, there are many difficult elements to such work, requiring commitment that begins long before the delivery of an event or the launch of a new activity. Reviewing existing literature and knowledge on a topic to determine whether an activity will meet the needs of our membership; coming up with valid learning objectives; generating just the right multiple choice questions and other assessments to measure our success at helping learners reach those objectives; peer reviewing content to ensure we’re teaching to the latest science and established best practices; and then measuring learner outcomes – these elements put the “state of the art” into CHEST’s internationally recognized state-of-the-art educational program.
To achieve our mission, we have been asking CHEST’s valiant and dedicated volunteers to do more than ever before, and some of what we have asked them to do has been frustrating, tedious, and less than rewarding. The reasons for this are many, including the imperfect technology platforms we’ve asked our volunteers to use; the disconnect between the educational goals we have set and the implementation of the clear processes, communication, and on-boarding of staff required to support them; and the lag of recognition proportionate to the nature of these new asks.
So, how do we show our member-faculty that they are our most important resource? Recently, we have had internal discussions about how to acknowledge the priceless contributions made by our faculty volunteers. To that end, CHEST staff and volunteer leadership have developed a Faculty & Volunteer Treatment Action Plan, recently approved by the CHEST Board of Regents. This is part of our comprehensive “six F’s” plan:
Formal recognition and rewards. Recognition and rewards are different – but both important. Recognition is expressing gratitude for an expected job that was well done and includes a formal thank-you. Rewards are additional, tangible benefits for exceptional services. We now have enhanced guidelines for travel, honoraria, and amenities for our volunteer faculty. Also of note, two new awards will be bestowed annually beginning at CHEST 2017 Los Angeles – the Early Career Clinician Educator Award and the Master Clinician Educator Award. These are some additional ways we will more appropriately highlight the people who have helped make us CHEST, the leader in clinical education in chest medicine.
Feedback. In addition to learner satisfaction data, CHEST provides an unprecedented level of learner outcomes data to our faculty. We are even introducing a new peer-review of teaching (PRT) program so faculty can get even more feedback from expert colleagues.
Faculty Development. As an education-focused organization, training and development plays a foundational role. We are working to develop a comprehensive clinician educator program that will grow our bench of faculty. A newly launched database will more proactively track and match interested members with teaching opportunities within the organization.
Face Time. Easy access to leadership and staff is important. We are implementing staff training that will better position all CHEST employees to more effectively facilitate and support the work we ask of our faculty. On another front, we are engaged in identifying new, user-friendly systems for session submission, conflict of interest disclosure, and review, as well as developing content.
Food. It is a simple but well-established fact that having a stocked lounge area for busy faculty on the run between teaching sessions enhances efficiency, communication, camaraderie, and overall morale.
Fun. The fun of discovering better ways to take care of our patients, be it from the teacher or learner perspective, in an engaging, effective learning environment is and always has been at the center of what we do.
CHEST’s volunteer leaders, in service to their peers, the field, and the organization, have risen to many challenges over and over again. We realize we need to do a better job of rewarding and recognizing their irreplaceable contributions. The above initiatives, and others, we hope, will help demonstrate to our most precious resource, our member-faculty, that we truly value and appreciate their invaluable contributions on behalf of CHEST. Stay tuned and stay with us.
This has been an extraordinary year for CHEST, particularly in the core area of clinical education. In the past fiscal year, we exceeded our educational goals. We set out to reach 10,000 learners through educational programming including live courses and conferences, and online activities; in the end, we served 15,547.
Other goals accomplished include demonstrating a significant increase in average learner knowledge acquisition and procedural skills improvement; identifying top priorities for online offerings and delivering five stand-alone online modules that can serve as a point of entry to wider audiences; recording professional attendance at CHEST 2015 of 5,149 people; offering online training for guideline development and the panelists engaged in CHEST guidelines; achieving an attendance at CHEST World Congress in Shanghai of 2,089; and working with leading Chinese medical societies to see the China-CHEST Pulmonary and Critical Care Medicine Fellowship Program formally adopted by the government in China as one of the four first-ever subspecialty training programs to be implemented nationwide.
This is a lot!
These accomplishments depend on intense work and collaboration between our incredibly talented faculty and volunteers from among CHEST membership and CHEST’s amazing professional staff of 105 employees, of which 28 are dedicated full time to the development and delivery of education and best practices. Through this partnership, we continue to meet CHEST’s mission: To champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.
Without these dedicated women and men, CHEST would be utterly unable to complete its mission. Our faculty work in a vast array of opportunities, including writing questions for SEEK, serving as a content expert for guidelines, proposing and delivering sessions at CHEST, running Board Review courses, recording videos, facilitating hands-on simulation sessions, and more. While intrinsically gratifying, there are many difficult elements to such work, requiring commitment that begins long before the delivery of an event or the launch of a new activity. Reviewing existing literature and knowledge on a topic to determine whether an activity will meet the needs of our membership; coming up with valid learning objectives; generating just the right multiple choice questions and other assessments to measure our success at helping learners reach those objectives; peer reviewing content to ensure we’re teaching to the latest science and established best practices; and then measuring learner outcomes – these elements put the “state of the art” into CHEST’s internationally recognized state-of-the-art educational program.
To achieve our mission, we have been asking CHEST’s valiant and dedicated volunteers to do more than ever before, and some of what we have asked them to do has been frustrating, tedious, and less than rewarding. The reasons for this are many, including the imperfect technology platforms we’ve asked our volunteers to use; the disconnect between the educational goals we have set and the implementation of the clear processes, communication, and on-boarding of staff required to support them; and the lag of recognition proportionate to the nature of these new asks.
So, how do we show our member-faculty that they are our most important resource? Recently, we have had internal discussions about how to acknowledge the priceless contributions made by our faculty volunteers. To that end, CHEST staff and volunteer leadership have developed a Faculty & Volunteer Treatment Action Plan, recently approved by the CHEST Board of Regents. This is part of our comprehensive “six F’s” plan:
Formal recognition and rewards. Recognition and rewards are different – but both important. Recognition is expressing gratitude for an expected job that was well done and includes a formal thank-you. Rewards are additional, tangible benefits for exceptional services. We now have enhanced guidelines for travel, honoraria, and amenities for our volunteer faculty. Also of note, two new awards will be bestowed annually beginning at CHEST 2017 Los Angeles – the Early Career Clinician Educator Award and the Master Clinician Educator Award. These are some additional ways we will more appropriately highlight the people who have helped make us CHEST, the leader in clinical education in chest medicine.
Feedback. In addition to learner satisfaction data, CHEST provides an unprecedented level of learner outcomes data to our faculty. We are even introducing a new peer-review of teaching (PRT) program so faculty can get even more feedback from expert colleagues.
Faculty Development. As an education-focused organization, training and development plays a foundational role. We are working to develop a comprehensive clinician educator program that will grow our bench of faculty. A newly launched database will more proactively track and match interested members with teaching opportunities within the organization.
Face Time. Easy access to leadership and staff is important. We are implementing staff training that will better position all CHEST employees to more effectively facilitate and support the work we ask of our faculty. On another front, we are engaged in identifying new, user-friendly systems for session submission, conflict of interest disclosure, and review, as well as developing content.
Food. It is a simple but well-established fact that having a stocked lounge area for busy faculty on the run between teaching sessions enhances efficiency, communication, camaraderie, and overall morale.
Fun. The fun of discovering better ways to take care of our patients, be it from the teacher or learner perspective, in an engaging, effective learning environment is and always has been at the center of what we do.
CHEST’s volunteer leaders, in service to their peers, the field, and the organization, have risen to many challenges over and over again. We realize we need to do a better job of rewarding and recognizing their irreplaceable contributions. The above initiatives, and others, we hope, will help demonstrate to our most precious resource, our member-faculty, that we truly value and appreciate their invaluable contributions on behalf of CHEST. Stay tuned and stay with us.
This Month in CHEST: Editor’s Picks
Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. By Dr. J. Corren, et al.
Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase III Study. By Dr. L. Bjermar, et al.
A Critical Review of the Quality of Cough Clinical Practice Guidelines. By Dr. M. Jiang, et al.
Procalcitonin as an Early Marker of the Need for Invasive Respiratory or Vasopressor Support in Adults With Community-Acquired Pneumonia. By Dr. W. H. Self, et al.
Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. By Dr. C. Bai, et al.
Occupational and Environmental Contributions to Chronic Cough in Adults: Chest Expert Panel Report. By Dr. S. M. Tarlo, et al, on behalf of the CHEST Expert Cough Panel.
Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. By Dr. J. Corren, et al.
Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase III Study. By Dr. L. Bjermar, et al.
A Critical Review of the Quality of Cough Clinical Practice Guidelines. By Dr. M. Jiang, et al.
Procalcitonin as an Early Marker of the Need for Invasive Respiratory or Vasopressor Support in Adults With Community-Acquired Pneumonia. By Dr. W. H. Self, et al.
Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. By Dr. C. Bai, et al.
Occupational and Environmental Contributions to Chronic Cough in Adults: Chest Expert Panel Report. By Dr. S. M. Tarlo, et al, on behalf of the CHEST Expert Cough Panel.
Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. By Dr. J. Corren, et al.
Reslizumab for Inadequately Controlled Asthma With Elevated Blood Eosinophil Levels: A Randomized Phase III Study. By Dr. L. Bjermar, et al.
A Critical Review of the Quality of Cough Clinical Practice Guidelines. By Dr. M. Jiang, et al.
Procalcitonin as an Early Marker of the Need for Invasive Respiratory or Vasopressor Support in Adults With Community-Acquired Pneumonia. By Dr. W. H. Self, et al.
Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. By Dr. C. Bai, et al.
Occupational and Environmental Contributions to Chronic Cough in Adults: Chest Expert Panel Report. By Dr. S. M. Tarlo, et al, on behalf of the CHEST Expert Cough Panel.





