User login
Where do you network?
According to a CDC study, 59% of physicians use social media networks.
Do you use social media networking? If so, share your favorite places to network in the poll below, or leave a note for your colleagues in the comments field.
According to a CDC study, 59% of physicians use social media networks.
Do you use social media networking? If so, share your favorite places to network in the poll below, or leave a note for your colleagues in the comments field.
According to a CDC study, 59% of physicians use social media networks.
Do you use social media networking? If so, share your favorite places to network in the poll below, or leave a note for your colleagues in the comments field.
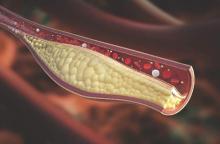
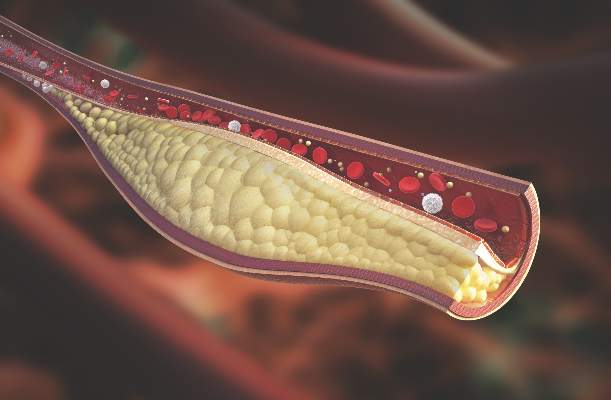
Psoriatic arthritis patients have elevated risk for coronary artery plaque
MIAMI – Patients with psoriatic arthritis had a higher prevalence and greater extent of coronary artery plaque in a pilot study comparison with healthy control patients that may point to increased risk independent of traditional cardiovascular risk factors.
In the study, coronary artery plaque as assessed by cardiac computed tomography angiography (CCTA) occurred in 39 (78%) of 50 patients with psoriatic arthritis, a significantly higher rate than that observed for healthy controls (11 of 25, 44%).
Investigators not only measured plaque volume, but also assessed the type of plaque: calcified, noncalcified, or mixed. Mixed plaque predominated. This could be important because “noncalcified and mixed carry higher risk for rupture and later cardiovascular events,” Agnes Szentpetery, MD, a research fellow at St. Vincent’s University Hospital in Dublin, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
She and her colleagues also found more clinically significant stenosis among the 50 participants with psoriatic arthritis, compared with 25 healthy controls matched for age, sex, smoking status, and presence of metabolic syndrome. “This pilot study is the first to assess coronary plaques in asymptomatic patients with psoriatic arthritis with CCTA,” Dr. Szentpetery said.
Total plaque volume was higher in the psoriatic arthritis group versus controls, and higher in the left main artery for psoriatic arthritis patients, both with and without metabolic syndrome.
The study points to increased risk independent of traditional cardiovascular risk factors. For example, CCTA revealed no difference in plaque volume between patients with and without metabolic disease. In addition, a previous study suggests “the burden of carotid artery plaques is higher in patients with psoriatic arthritis compared to those with psoriasis alone,” Dr. Szentpetery said, citing a cross-sectional study comparing 125 people with psoriasis to 114 others with psoriatic arthritis (Ann Rheum Dis. 2013 May;72[5]:715-20).
Perhaps not surprisingly, inflammation could be driving the association between psoriatic and cardiovascular disease risk. Other investigators suggest chronic, low-grade inflammation leads to atherosclerosis through a maladaptive immune response and altered lipid metabolism, for example (Nat Med. 2011 Nov;17[11]:1410-22).
In the current study, the patients with psoriatic arthritis had well-established disease, occurring for a mean duration of 19 years. Mean age was 58 years, and 54% were men. Approximately 60% were taking disease-modifying antirheumatic drugs, two-thirds were taking biologics, and about one-third were on combination treatment. Controls were similar demographically with a mean age of 57 years, and 52% were men.
Interestingly, Psoriasis Area and Severity Index (PASI) scores did not correlate with increased risk. During discussion after the presentation of the study, a researcher unaffiliated with the study offered an answer. “It could be their skin disease was controlled by the biologics. You had 67% on biologics,” said Nehal Mehta, MD, Clinical Research Scholar in the section of inflammation and cardiometabolic disease at the National Heart, Lung, and Blood Institute. “We at the NIH see a strong correlation between PASI and coronary artery disease risk.”
“We know methotrexate and anti-TNF agents can have a protective effect on atherosclerosis, but we did not look at this specifically,” Dr. Szentpetery said. Overall, PASI scores were relatively low in the study population, she added, which “may explain why we did not see the correlation with PASI scores.”
Dr. Szentpetery and Dr. Mehta had no relevant financial disclosures.
MIAMI – Patients with psoriatic arthritis had a higher prevalence and greater extent of coronary artery plaque in a pilot study comparison with healthy control patients that may point to increased risk independent of traditional cardiovascular risk factors.
In the study, coronary artery plaque as assessed by cardiac computed tomography angiography (CCTA) occurred in 39 (78%) of 50 patients with psoriatic arthritis, a significantly higher rate than that observed for healthy controls (11 of 25, 44%).
Investigators not only measured plaque volume, but also assessed the type of plaque: calcified, noncalcified, or mixed. Mixed plaque predominated. This could be important because “noncalcified and mixed carry higher risk for rupture and later cardiovascular events,” Agnes Szentpetery, MD, a research fellow at St. Vincent’s University Hospital in Dublin, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
She and her colleagues also found more clinically significant stenosis among the 50 participants with psoriatic arthritis, compared with 25 healthy controls matched for age, sex, smoking status, and presence of metabolic syndrome. “This pilot study is the first to assess coronary plaques in asymptomatic patients with psoriatic arthritis with CCTA,” Dr. Szentpetery said.
Total plaque volume was higher in the psoriatic arthritis group versus controls, and higher in the left main artery for psoriatic arthritis patients, both with and without metabolic syndrome.
The study points to increased risk independent of traditional cardiovascular risk factors. For example, CCTA revealed no difference in plaque volume between patients with and without metabolic disease. In addition, a previous study suggests “the burden of carotid artery plaques is higher in patients with psoriatic arthritis compared to those with psoriasis alone,” Dr. Szentpetery said, citing a cross-sectional study comparing 125 people with psoriasis to 114 others with psoriatic arthritis (Ann Rheum Dis. 2013 May;72[5]:715-20).
Perhaps not surprisingly, inflammation could be driving the association between psoriatic and cardiovascular disease risk. Other investigators suggest chronic, low-grade inflammation leads to atherosclerosis through a maladaptive immune response and altered lipid metabolism, for example (Nat Med. 2011 Nov;17[11]:1410-22).
In the current study, the patients with psoriatic arthritis had well-established disease, occurring for a mean duration of 19 years. Mean age was 58 years, and 54% were men. Approximately 60% were taking disease-modifying antirheumatic drugs, two-thirds were taking biologics, and about one-third were on combination treatment. Controls were similar demographically with a mean age of 57 years, and 52% were men.
Interestingly, Psoriasis Area and Severity Index (PASI) scores did not correlate with increased risk. During discussion after the presentation of the study, a researcher unaffiliated with the study offered an answer. “It could be their skin disease was controlled by the biologics. You had 67% on biologics,” said Nehal Mehta, MD, Clinical Research Scholar in the section of inflammation and cardiometabolic disease at the National Heart, Lung, and Blood Institute. “We at the NIH see a strong correlation between PASI and coronary artery disease risk.”
“We know methotrexate and anti-TNF agents can have a protective effect on atherosclerosis, but we did not look at this specifically,” Dr. Szentpetery said. Overall, PASI scores were relatively low in the study population, she added, which “may explain why we did not see the correlation with PASI scores.”
Dr. Szentpetery and Dr. Mehta had no relevant financial disclosures.
MIAMI – Patients with psoriatic arthritis had a higher prevalence and greater extent of coronary artery plaque in a pilot study comparison with healthy control patients that may point to increased risk independent of traditional cardiovascular risk factors.
In the study, coronary artery plaque as assessed by cardiac computed tomography angiography (CCTA) occurred in 39 (78%) of 50 patients with psoriatic arthritis, a significantly higher rate than that observed for healthy controls (11 of 25, 44%).
Investigators not only measured plaque volume, but also assessed the type of plaque: calcified, noncalcified, or mixed. Mixed plaque predominated. This could be important because “noncalcified and mixed carry higher risk for rupture and later cardiovascular events,” Agnes Szentpetery, MD, a research fellow at St. Vincent’s University Hospital in Dublin, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
She and her colleagues also found more clinically significant stenosis among the 50 participants with psoriatic arthritis, compared with 25 healthy controls matched for age, sex, smoking status, and presence of metabolic syndrome. “This pilot study is the first to assess coronary plaques in asymptomatic patients with psoriatic arthritis with CCTA,” Dr. Szentpetery said.
Total plaque volume was higher in the psoriatic arthritis group versus controls, and higher in the left main artery for psoriatic arthritis patients, both with and without metabolic syndrome.
The study points to increased risk independent of traditional cardiovascular risk factors. For example, CCTA revealed no difference in plaque volume between patients with and without metabolic disease. In addition, a previous study suggests “the burden of carotid artery plaques is higher in patients with psoriatic arthritis compared to those with psoriasis alone,” Dr. Szentpetery said, citing a cross-sectional study comparing 125 people with psoriasis to 114 others with psoriatic arthritis (Ann Rheum Dis. 2013 May;72[5]:715-20).
Perhaps not surprisingly, inflammation could be driving the association between psoriatic and cardiovascular disease risk. Other investigators suggest chronic, low-grade inflammation leads to atherosclerosis through a maladaptive immune response and altered lipid metabolism, for example (Nat Med. 2011 Nov;17[11]:1410-22).
In the current study, the patients with psoriatic arthritis had well-established disease, occurring for a mean duration of 19 years. Mean age was 58 years, and 54% were men. Approximately 60% were taking disease-modifying antirheumatic drugs, two-thirds were taking biologics, and about one-third were on combination treatment. Controls were similar demographically with a mean age of 57 years, and 52% were men.
Interestingly, Psoriasis Area and Severity Index (PASI) scores did not correlate with increased risk. During discussion after the presentation of the study, a researcher unaffiliated with the study offered an answer. “It could be their skin disease was controlled by the biologics. You had 67% on biologics,” said Nehal Mehta, MD, Clinical Research Scholar in the section of inflammation and cardiometabolic disease at the National Heart, Lung, and Blood Institute. “We at the NIH see a strong correlation between PASI and coronary artery disease risk.”
“We know methotrexate and anti-TNF agents can have a protective effect on atherosclerosis, but we did not look at this specifically,” Dr. Szentpetery said. Overall, PASI scores were relatively low in the study population, she added, which “may explain why we did not see the correlation with PASI scores.”
Dr. Szentpetery and Dr. Mehta had no relevant financial disclosures.
AT 2016 GRAPPA ANNUAL MEETING
Key clinical point:Imaging reveals a higher rate and greater extent of coronary plaque in people with psoriatic arthritis versus healthy controls.
Major finding: 78% of people with PsA had coronary artery plaque versus 44% of controls, a significant difference.
Data source: Comparison of 50 people with PsA versus 25 healthy controls undergoing CCTA.
Disclosures: Dr. Szentpetery and Dr. Mehta had no relevant disclosures.
Study may explain how LSCs evade treatment

Image by Robert Paulson
New research suggests leukemia stem cells (LSCs) can “hide” in gonadal adipose tissue (GAT) and transform the tissue so they can survive treatment.
Experiments in a mouse model of chronic myeloid leukemia (CML) showed that LSCs are enriched in GAT.
While there, the LSCs create a microenvironment that supports leukemic growth and resistance to treatment, and expression of the fatty acid transporter CD36 makes LSCs particularly resistant.
Craig Jordan, PhD, of University of Colorado in Aurora, and his colleagues conducted this research and detailed their findings in Cell Stem Cell.
The researchers began by examining cancer cells found in GAT from mice with blast crisis CML. Rather than containing the expected mix of regular leukemia cells and LSCs, the tissue was enriched for LSCs.
And these GAT-resident LSCs used a different energy source than LSCs in the bone marrow microenvironment. The GAT-resident LSCs powered their survival and growth with fatty acids, manufacturing energy by the process of fatty acid oxidization.
In fact, the GAT-resident LSCs actively signaled fat to undergo lipolysis, which released fatty acids into the microenvironment.
“The basic biology was fascinating,” Dr Jordan said. “The tumor adapted the local environment to suit itself.”
Dr Jordan and his colleagues also found that CD36 played a role. CD36+ LSCs were enriched in GAT, were more likely to migrate to GAT than to bone marrow, and were protected from treatment by GAT.
The researchers tested the effects of several drugs (cytarabine, doxorubicin, etoposide, SN-38, irinotecan, and dasatinib) on CD36+ LSCs, CD36- LSCs, and bulk leukemia cells ex vivo.
Both CD36+ and CD36- LSCs were more resistant to treatment than bulk leukemia cells, but CD36+ LSCs were preferentially drug-resistant.
The researchers observed similar results in leukemic mice and found evidence to suggest that CD36 plays a similar role in patients with blast crisis CML and those with acute myeloid leukemia. ![]()

Image by Robert Paulson
New research suggests leukemia stem cells (LSCs) can “hide” in gonadal adipose tissue (GAT) and transform the tissue so they can survive treatment.
Experiments in a mouse model of chronic myeloid leukemia (CML) showed that LSCs are enriched in GAT.
While there, the LSCs create a microenvironment that supports leukemic growth and resistance to treatment, and expression of the fatty acid transporter CD36 makes LSCs particularly resistant.
Craig Jordan, PhD, of University of Colorado in Aurora, and his colleagues conducted this research and detailed their findings in Cell Stem Cell.
The researchers began by examining cancer cells found in GAT from mice with blast crisis CML. Rather than containing the expected mix of regular leukemia cells and LSCs, the tissue was enriched for LSCs.
And these GAT-resident LSCs used a different energy source than LSCs in the bone marrow microenvironment. The GAT-resident LSCs powered their survival and growth with fatty acids, manufacturing energy by the process of fatty acid oxidization.
In fact, the GAT-resident LSCs actively signaled fat to undergo lipolysis, which released fatty acids into the microenvironment.
“The basic biology was fascinating,” Dr Jordan said. “The tumor adapted the local environment to suit itself.”
Dr Jordan and his colleagues also found that CD36 played a role. CD36+ LSCs were enriched in GAT, were more likely to migrate to GAT than to bone marrow, and were protected from treatment by GAT.
The researchers tested the effects of several drugs (cytarabine, doxorubicin, etoposide, SN-38, irinotecan, and dasatinib) on CD36+ LSCs, CD36- LSCs, and bulk leukemia cells ex vivo.
Both CD36+ and CD36- LSCs were more resistant to treatment than bulk leukemia cells, but CD36+ LSCs were preferentially drug-resistant.
The researchers observed similar results in leukemic mice and found evidence to suggest that CD36 plays a similar role in patients with blast crisis CML and those with acute myeloid leukemia. ![]()

Image by Robert Paulson
New research suggests leukemia stem cells (LSCs) can “hide” in gonadal adipose tissue (GAT) and transform the tissue so they can survive treatment.
Experiments in a mouse model of chronic myeloid leukemia (CML) showed that LSCs are enriched in GAT.
While there, the LSCs create a microenvironment that supports leukemic growth and resistance to treatment, and expression of the fatty acid transporter CD36 makes LSCs particularly resistant.
Craig Jordan, PhD, of University of Colorado in Aurora, and his colleagues conducted this research and detailed their findings in Cell Stem Cell.
The researchers began by examining cancer cells found in GAT from mice with blast crisis CML. Rather than containing the expected mix of regular leukemia cells and LSCs, the tissue was enriched for LSCs.
And these GAT-resident LSCs used a different energy source than LSCs in the bone marrow microenvironment. The GAT-resident LSCs powered their survival and growth with fatty acids, manufacturing energy by the process of fatty acid oxidization.
In fact, the GAT-resident LSCs actively signaled fat to undergo lipolysis, which released fatty acids into the microenvironment.
“The basic biology was fascinating,” Dr Jordan said. “The tumor adapted the local environment to suit itself.”
Dr Jordan and his colleagues also found that CD36 played a role. CD36+ LSCs were enriched in GAT, were more likely to migrate to GAT than to bone marrow, and were protected from treatment by GAT.
The researchers tested the effects of several drugs (cytarabine, doxorubicin, etoposide, SN-38, irinotecan, and dasatinib) on CD36+ LSCs, CD36- LSCs, and bulk leukemia cells ex vivo.
Both CD36+ and CD36- LSCs were more resistant to treatment than bulk leukemia cells, but CD36+ LSCs were preferentially drug-resistant.
The researchers observed similar results in leukemic mice and found evidence to suggest that CD36 plays a similar role in patients with blast crisis CML and those with acute myeloid leukemia. ![]()
Ibrutinib approved for first-line treatment of CLL

Photo courtesy of Janssen
Health Canada has approved the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica®) as a first-line treatment for patients with active chronic lymphocytic leukemia (CLL).
This is the fourth approval for ibrutinib in Canada. The drug is now approved for use in all CLL patients, patients with Waldenström’s macroglobulinemia, and patients with relapsed or refractory mantle cell lymphoma (conditional approval).
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The latest approval of ibrutinib is based on results from the phase 3 RESONATE-2 trial
(PCYC-1115), which were presented at the 2015 ASH Annual Meeting and
simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five
patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()

Photo courtesy of Janssen
Health Canada has approved the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica®) as a first-line treatment for patients with active chronic lymphocytic leukemia (CLL).
This is the fourth approval for ibrutinib in Canada. The drug is now approved for use in all CLL patients, patients with Waldenström’s macroglobulinemia, and patients with relapsed or refractory mantle cell lymphoma (conditional approval).
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The latest approval of ibrutinib is based on results from the phase 3 RESONATE-2 trial
(PCYC-1115), which were presented at the 2015 ASH Annual Meeting and
simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five
patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()

Photo courtesy of Janssen
Health Canada has approved the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica®) as a first-line treatment for patients with active chronic lymphocytic leukemia (CLL).
This is the fourth approval for ibrutinib in Canada. The drug is now approved for use in all CLL patients, patients with Waldenström’s macroglobulinemia, and patients with relapsed or refractory mantle cell lymphoma (conditional approval).
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The latest approval of ibrutinib is based on results from the phase 3 RESONATE-2 trial
(PCYC-1115), which were presented at the 2015 ASH Annual Meeting and
simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five
patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()
Radiologists no longer have higher risk of cancer-related death

Photo by Rhoda Baer
Radiologists who graduated from medical school after 1940 do not have an increased risk of dying from radiation-related causes such as cancers, according to a study published in Radiology.
However, the study suggested that male radiologists who graduated before 1940 had a higher risk of death from certain cancers, including acute myeloid leukemia and non-Hodgkin lymphoma.
Researchers said these findings point to the success of efforts to reduce occupational radiation doses over the past several decades.
The team noted that female radiologists did not have an increased risk of all-cause mortality or cancer-related mortality, regardless of when they graduated from medical school.
However, the small number of women in this study prevented the researchers from studying the subjects’ mortality rates in detail. And very few female radiologists worked during the early period of the study, when radiation exposures were likely highest.
To conduct this study, the researchers analyzed records from the American Medical Association Physician Masterfile, a database established in 1906 that has grown to include current and historical data for more than 1.4 million physicians, residents, and medical students in the US.
The team compared cancer incidence and mortality rates between 43,763 radiologists and 64,990 psychiatrists who graduated from medical school between 1916 and 2006. Psychiatrists were chosen as a comparison group because they are unlikely to have had occupational radiation exposure.
“Our most important finding is that radiologists have lower death rates from all causes of death combined, compared to psychiatrists, and had similar risks of cancer deaths overall,” said study author Martha Linet, MD, of the National Cancer Institute in Bethesda, Maryland.
Results in males
The researchers found that, among male subjects who graduated after 1940, the risk of all-cause mortality was lower for the radiologists than the psychiatrists (relative risk [RR]=0.94; 95% CI: 0.90, 0.97), and the risk of death from cancer was similar (RR=1.00; 95% CI: 0.93, 1.07).
In contrast, male radiologists who graduated before 1940 had higher mortality rates from certain cancers.
They had a higher risk of skin cancer mortality (RR=6.38; 95% CI: 1.75, 23.20) that was driven by an excess of melanoma (RR=8.75; 95% CI: 1.89, 40.53).
They had an increased risk of death from all myeloid leukemias (RR=1.43; 95% CI: 1.00, 2.05) that was driven by acute myeloid leukemia and/or myelodysplastic syndromes (RR=4.68; 95% CI: 0.91, 24.18).
And they had an increased risk of death from lymphomas (RR=2.24; 95% CI: 1.31, 3.86) that was driven by non-Hodgkin lymphoma (RR=2.69; 95% CI: 1.33, 5.45).
The researchers also found an increased risk of cerebrovascular deaths in the male radiologists who graduated before 1940 (RR=1.49; 95% CI: 1.11, 2.01).
The team said the reduced health risks for more recent radiology graduates are likely due to developments and improvements in radiation protection and monitoring, along with improvements in equipment safety.
“Most of the findings of increased risk were in the earlier radiologists,” Dr Linet noted. “We do feel there is evidence that decreases in dose in the United States and other countries seem to have paid off, reducing risks in recent graduates.”
Results in females
The researchers said there were no clear increases in mortality in the female radiologists compared with the female psychiatrists.
The risk of all-cause mortality was lower in the radiologists, as was the risk of death from circulatory diseases, but the risk of cancer-related mortality was similar between the radiologists and the psychiatrists.
However, the researchers said the relatively small number of female deaths in this study prevented detailed investigation. Only 2% of female radiologists (208/8851) and 3% of female psychiatrists (524/17,493) died, compared to 12% of male radiologists (4260/43,763) and 16% of male psychiatrists (7815/47,443). ![]()

Photo by Rhoda Baer
Radiologists who graduated from medical school after 1940 do not have an increased risk of dying from radiation-related causes such as cancers, according to a study published in Radiology.
However, the study suggested that male radiologists who graduated before 1940 had a higher risk of death from certain cancers, including acute myeloid leukemia and non-Hodgkin lymphoma.
Researchers said these findings point to the success of efforts to reduce occupational radiation doses over the past several decades.
The team noted that female radiologists did not have an increased risk of all-cause mortality or cancer-related mortality, regardless of when they graduated from medical school.
However, the small number of women in this study prevented the researchers from studying the subjects’ mortality rates in detail. And very few female radiologists worked during the early period of the study, when radiation exposures were likely highest.
To conduct this study, the researchers analyzed records from the American Medical Association Physician Masterfile, a database established in 1906 that has grown to include current and historical data for more than 1.4 million physicians, residents, and medical students in the US.
The team compared cancer incidence and mortality rates between 43,763 radiologists and 64,990 psychiatrists who graduated from medical school between 1916 and 2006. Psychiatrists were chosen as a comparison group because they are unlikely to have had occupational radiation exposure.
“Our most important finding is that radiologists have lower death rates from all causes of death combined, compared to psychiatrists, and had similar risks of cancer deaths overall,” said study author Martha Linet, MD, of the National Cancer Institute in Bethesda, Maryland.
Results in males
The researchers found that, among male subjects who graduated after 1940, the risk of all-cause mortality was lower for the radiologists than the psychiatrists (relative risk [RR]=0.94; 95% CI: 0.90, 0.97), and the risk of death from cancer was similar (RR=1.00; 95% CI: 0.93, 1.07).
In contrast, male radiologists who graduated before 1940 had higher mortality rates from certain cancers.
They had a higher risk of skin cancer mortality (RR=6.38; 95% CI: 1.75, 23.20) that was driven by an excess of melanoma (RR=8.75; 95% CI: 1.89, 40.53).
They had an increased risk of death from all myeloid leukemias (RR=1.43; 95% CI: 1.00, 2.05) that was driven by acute myeloid leukemia and/or myelodysplastic syndromes (RR=4.68; 95% CI: 0.91, 24.18).
And they had an increased risk of death from lymphomas (RR=2.24; 95% CI: 1.31, 3.86) that was driven by non-Hodgkin lymphoma (RR=2.69; 95% CI: 1.33, 5.45).
The researchers also found an increased risk of cerebrovascular deaths in the male radiologists who graduated before 1940 (RR=1.49; 95% CI: 1.11, 2.01).
The team said the reduced health risks for more recent radiology graduates are likely due to developments and improvements in radiation protection and monitoring, along with improvements in equipment safety.
“Most of the findings of increased risk were in the earlier radiologists,” Dr Linet noted. “We do feel there is evidence that decreases in dose in the United States and other countries seem to have paid off, reducing risks in recent graduates.”
Results in females
The researchers said there were no clear increases in mortality in the female radiologists compared with the female psychiatrists.
The risk of all-cause mortality was lower in the radiologists, as was the risk of death from circulatory diseases, but the risk of cancer-related mortality was similar between the radiologists and the psychiatrists.
However, the researchers said the relatively small number of female deaths in this study prevented detailed investigation. Only 2% of female radiologists (208/8851) and 3% of female psychiatrists (524/17,493) died, compared to 12% of male radiologists (4260/43,763) and 16% of male psychiatrists (7815/47,443). ![]()

Photo by Rhoda Baer
Radiologists who graduated from medical school after 1940 do not have an increased risk of dying from radiation-related causes such as cancers, according to a study published in Radiology.
However, the study suggested that male radiologists who graduated before 1940 had a higher risk of death from certain cancers, including acute myeloid leukemia and non-Hodgkin lymphoma.
Researchers said these findings point to the success of efforts to reduce occupational radiation doses over the past several decades.
The team noted that female radiologists did not have an increased risk of all-cause mortality or cancer-related mortality, regardless of when they graduated from medical school.
However, the small number of women in this study prevented the researchers from studying the subjects’ mortality rates in detail. And very few female radiologists worked during the early period of the study, when radiation exposures were likely highest.
To conduct this study, the researchers analyzed records from the American Medical Association Physician Masterfile, a database established in 1906 that has grown to include current and historical data for more than 1.4 million physicians, residents, and medical students in the US.
The team compared cancer incidence and mortality rates between 43,763 radiologists and 64,990 psychiatrists who graduated from medical school between 1916 and 2006. Psychiatrists were chosen as a comparison group because they are unlikely to have had occupational radiation exposure.
“Our most important finding is that radiologists have lower death rates from all causes of death combined, compared to psychiatrists, and had similar risks of cancer deaths overall,” said study author Martha Linet, MD, of the National Cancer Institute in Bethesda, Maryland.
Results in males
The researchers found that, among male subjects who graduated after 1940, the risk of all-cause mortality was lower for the radiologists than the psychiatrists (relative risk [RR]=0.94; 95% CI: 0.90, 0.97), and the risk of death from cancer was similar (RR=1.00; 95% CI: 0.93, 1.07).
In contrast, male radiologists who graduated before 1940 had higher mortality rates from certain cancers.
They had a higher risk of skin cancer mortality (RR=6.38; 95% CI: 1.75, 23.20) that was driven by an excess of melanoma (RR=8.75; 95% CI: 1.89, 40.53).
They had an increased risk of death from all myeloid leukemias (RR=1.43; 95% CI: 1.00, 2.05) that was driven by acute myeloid leukemia and/or myelodysplastic syndromes (RR=4.68; 95% CI: 0.91, 24.18).
And they had an increased risk of death from lymphomas (RR=2.24; 95% CI: 1.31, 3.86) that was driven by non-Hodgkin lymphoma (RR=2.69; 95% CI: 1.33, 5.45).
The researchers also found an increased risk of cerebrovascular deaths in the male radiologists who graduated before 1940 (RR=1.49; 95% CI: 1.11, 2.01).
The team said the reduced health risks for more recent radiology graduates are likely due to developments and improvements in radiation protection and monitoring, along with improvements in equipment safety.
“Most of the findings of increased risk were in the earlier radiologists,” Dr Linet noted. “We do feel there is evidence that decreases in dose in the United States and other countries seem to have paid off, reducing risks in recent graduates.”
Results in females
The researchers said there were no clear increases in mortality in the female radiologists compared with the female psychiatrists.
The risk of all-cause mortality was lower in the radiologists, as was the risk of death from circulatory diseases, but the risk of cancer-related mortality was similar between the radiologists and the psychiatrists.
However, the researchers said the relatively small number of female deaths in this study prevented detailed investigation. Only 2% of female radiologists (208/8851) and 3% of female psychiatrists (524/17,493) died, compared to 12% of male radiologists (4260/43,763) and 16% of male psychiatrists (7815/47,443). ![]()
Drug can prevent nausea, vomiting caused by chemo

Photo by Bill Branson
Results of a phase 3 study suggest the antipsychotic agent olanzapine can also be used to reduce nausea and vomiting caused by chemotherapy.
In this study, cancer patients receiving highly emetogenic chemotherapy also received combination anti-emetic therapy including olanzapine or placebo.
Those patients who received olanzapine were significantly less likely to experience nausea and vomiting in the 120 hours after starting chemotherapy.
These results were published in NEJM.
“We’ve long known the nausea and vomiting that come along with chemotherapy are a major problem and affect the quality of life of our patients,” said study author Steven Powell, MD, of Sanford Cancer Center in Sioux Falls, South Dakota.
“The findings of this study, fortunately, provide physicians with a tool to better address the needs of those they are treating for cancer.”
Dr Powell and his colleagues evaluated cancer patients who had received no previous chemotherapy but were receiving cisplatin or cyclophosphamide and doxorubicin during the study period.
To prevent nausea and vomiting, all of the patients received a 5-HT3–receptor antagonist, dexamethasone, and an NK1-receptor antagonist. Roughly half also received olanzapine, and the other half received placebo.
Overall, 380 patients were evaluable—192 assigned to olanzapine and 188 to placebo.
In the first 24 hours after starting chemotherapy, the proportion of patients who did not have chemotherapy-induced nausea was significantly greater in the olanzapine arm than the placebo arm—74% and 45%, respectively (P=0.002).
The same was true at 25 hours to 120 hours after the start of chemotherapy—42% and 25%, respectively (P=0.002)—and for the overall 120-hour period—37% and 22%, respectively (P=0.002).
The complete response rate—defined as no vomiting and no rescue therapy—was significantly higher in the olanzapine arm than the placebo arm in the first 24 hours—86% and 65% (P<0.001)—at 25 hours to 120 hours—67% and 52%, respectively (P=0.007)—and overall—64% and 41%, respectively (P<0.001).
There were two grade 3 adverse events and three grade 4 adverse events in the olanzapine arm, but none of these were attributed to olanzapine.
Patients in the olanzapine arm had significantly increased sedation on day 2 compared with baseline, but this resolved on days 3, 4, and 5, although the patients were still receiving olanzapine on days 3 and 4. ![]()

Photo by Bill Branson
Results of a phase 3 study suggest the antipsychotic agent olanzapine can also be used to reduce nausea and vomiting caused by chemotherapy.
In this study, cancer patients receiving highly emetogenic chemotherapy also received combination anti-emetic therapy including olanzapine or placebo.
Those patients who received olanzapine were significantly less likely to experience nausea and vomiting in the 120 hours after starting chemotherapy.
These results were published in NEJM.
“We’ve long known the nausea and vomiting that come along with chemotherapy are a major problem and affect the quality of life of our patients,” said study author Steven Powell, MD, of Sanford Cancer Center in Sioux Falls, South Dakota.
“The findings of this study, fortunately, provide physicians with a tool to better address the needs of those they are treating for cancer.”
Dr Powell and his colleagues evaluated cancer patients who had received no previous chemotherapy but were receiving cisplatin or cyclophosphamide and doxorubicin during the study period.
To prevent nausea and vomiting, all of the patients received a 5-HT3–receptor antagonist, dexamethasone, and an NK1-receptor antagonist. Roughly half also received olanzapine, and the other half received placebo.
Overall, 380 patients were evaluable—192 assigned to olanzapine and 188 to placebo.
In the first 24 hours after starting chemotherapy, the proportion of patients who did not have chemotherapy-induced nausea was significantly greater in the olanzapine arm than the placebo arm—74% and 45%, respectively (P=0.002).
The same was true at 25 hours to 120 hours after the start of chemotherapy—42% and 25%, respectively (P=0.002)—and for the overall 120-hour period—37% and 22%, respectively (P=0.002).
The complete response rate—defined as no vomiting and no rescue therapy—was significantly higher in the olanzapine arm than the placebo arm in the first 24 hours—86% and 65% (P<0.001)—at 25 hours to 120 hours—67% and 52%, respectively (P=0.007)—and overall—64% and 41%, respectively (P<0.001).
There were two grade 3 adverse events and three grade 4 adverse events in the olanzapine arm, but none of these were attributed to olanzapine.
Patients in the olanzapine arm had significantly increased sedation on day 2 compared with baseline, but this resolved on days 3, 4, and 5, although the patients were still receiving olanzapine on days 3 and 4. ![]()

Photo by Bill Branson
Results of a phase 3 study suggest the antipsychotic agent olanzapine can also be used to reduce nausea and vomiting caused by chemotherapy.
In this study, cancer patients receiving highly emetogenic chemotherapy also received combination anti-emetic therapy including olanzapine or placebo.
Those patients who received olanzapine were significantly less likely to experience nausea and vomiting in the 120 hours after starting chemotherapy.
These results were published in NEJM.
“We’ve long known the nausea and vomiting that come along with chemotherapy are a major problem and affect the quality of life of our patients,” said study author Steven Powell, MD, of Sanford Cancer Center in Sioux Falls, South Dakota.
“The findings of this study, fortunately, provide physicians with a tool to better address the needs of those they are treating for cancer.”
Dr Powell and his colleagues evaluated cancer patients who had received no previous chemotherapy but were receiving cisplatin or cyclophosphamide and doxorubicin during the study period.
To prevent nausea and vomiting, all of the patients received a 5-HT3–receptor antagonist, dexamethasone, and an NK1-receptor antagonist. Roughly half also received olanzapine, and the other half received placebo.
Overall, 380 patients were evaluable—192 assigned to olanzapine and 188 to placebo.
In the first 24 hours after starting chemotherapy, the proportion of patients who did not have chemotherapy-induced nausea was significantly greater in the olanzapine arm than the placebo arm—74% and 45%, respectively (P=0.002).
The same was true at 25 hours to 120 hours after the start of chemotherapy—42% and 25%, respectively (P=0.002)—and for the overall 120-hour period—37% and 22%, respectively (P=0.002).
The complete response rate—defined as no vomiting and no rescue therapy—was significantly higher in the olanzapine arm than the placebo arm in the first 24 hours—86% and 65% (P<0.001)—at 25 hours to 120 hours—67% and 52%, respectively (P=0.007)—and overall—64% and 41%, respectively (P<0.001).
There were two grade 3 adverse events and three grade 4 adverse events in the olanzapine arm, but none of these were attributed to olanzapine.
Patients in the olanzapine arm had significantly increased sedation on day 2 compared with baseline, but this resolved on days 3, 4, and 5, although the patients were still receiving olanzapine on days 3 and 4. ![]()
The Goals of Goals
In their study of goals of care (GOC) discussions and documentation, Wong et al. add to already robust evidence that communication, in this case from physicians caring for hospitalized patients back to long‐term care facilities, has room for improvement. They highlight that 37.5% of patients had documented discussions, and for cases in which these discussions resulted in changes to a patient's advance directive, only 1 in 4 were relayed in the discharge summary.[1]
As physicians caring for hospitalized patients and concerned with improving care quality and efficiency, many of us are familiar with potential systems solutions to augmenting communication: reminders in the electronic health record, checklists, multidisciplinary teams, scripts, and posthospitalization follow‐up phone calls. However, important as they are, these solutions often elide the underlying cognitive elements related to how we, as physicians, think about and engage in the diversity of cases presented to us, and to how we prioritize communication work.
Wong et al. looked at patient characteristics associated with performance of GOC discussions to understand when and why physicians might engage in GOC conversations in the hospital and to generate insights into potential targets for improvement. They found that characteristics of patients prior to hospital admission were not associated with GOC discussions; signs of acuity of illness were.[1] In other words, physicians in the hospital are pretty good at recognizing patients in extremis, and prioritize GOC discussions with these patients. What we are not good at, or might not be considering, is assessing the broader context of a patient's health.
Whether we interpret these results as appropriate prioritization, or as a sign that we are waiting too long to broach the subject of care goals, depends on how we conceptualize the hospital stay in the context of a patient's health story, and, by extension, the role of the hospitalist in this story. For some patients, an acute illness requiring hospitalization is unexpected and readily treated, and the patient rapidly returns to a prior level of health and function. The need for hospitalization represents an outlier state.
For other patients, often older, more debilitated, or with multiple and chronic medical conditions, minor changes in health or declines in mental, social, or physical function precipitate the need for hospitalization. Likewise, iatrogenic harms of hospitalizationsleeplessness, fasting, delirium, immobilitycan contribute to enduring decline.[2, 3] For these patients, the need for hospitalization is not so far from, or may be, their norm.[4]
I suspect that Wong et al.'s findings reflect a collective response to the uncertainties of prognostication, and the resultant discomfort in raising questions that are difficult to answer. How do we know it is time to start talking about the right amount of care? Some might answer, I think rightly, that it is rarely if ever too early, yet robust discussions are challenging if we are not sure of the relevance or the immediate goal. In the case of the patient who is ill, declining, yet not in extremis, many of us might conclude that raising the question would not produce actionable information; it would not change immediate in‐hospital management.
This common conclusion leads to a significant missed opportunity, both on an individual level for physicians and patients, and for hospital medicine as a specialty. Health, and the losses that come with declining health, are wrapped up with fundamental aspects of our identities, and take time and consideration to change and evolve. Decisions about our healthcare are statements about who we have been, who we are, and who we will no longer be. Especially for the second group of patients described above, each hospital stay affords a chance to assess, counsel, educate, support, and empower patients to move in the direction of their values, and to ready them for that eventuality when they or their loved ones are faced with decisions about how, and where, they will die. As specialists in hospital‐based healthcare, hospitalists have the privilege and professional duty to facilitate this journey.
However, as hospitalists, we are often meeting patients for the first time; how do we assimilate an understanding of that point in time within the context of a patient's life with enough confidence to engage discussions? As Wong et al. show, it appears that in regard to very ill patients, respiratory rate and Glasgow Coma Scale inform action.[1] What signs or observations help inform action earlier in the trajectory of decline, to allow for anticipatory guidance and discussion? Increasingly, we see evidence that measures of frailty and functional status, applied in the hospital, are associated with hospital outcomes including readmission and death.[5, 6, 7] Future work might explore if training physicians to systematically assess frailty and functional status leads to greater frequency of, and comfort with, initiating GOC discussions during hospitalization.
Moreover, an emphasis on evaluating frailty and function, and explicitly including this assessment in our clinical decision‐making might help shift our thinking toward valuing each hospitalization as an opportunity to both intervene to improve function[8, 9] and to support, educate, and prepare patients under our care for the journey aheadin other words, to fully engage with our role as specialists in the comprehensive and coordinated treatment of patients who require hospitalization.
- , , , . Goals of care discussions among hospitalized long‐term care residents: predictors and associated outcomes of care. J Hosp Med. 2016;11(12):824–831.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30(11):1688–1695.
- , , , et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30‐day risk of readmission or death [published online May 17, 2016]. J Hosp Med. doi: 10.1002/jhm.2607.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
- , , , et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial [published online May 31, 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1870.
- . Activating hospitalized older patients to confront the epidemic of low mobility [published online May 31 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1874.
In their study of goals of care (GOC) discussions and documentation, Wong et al. add to already robust evidence that communication, in this case from physicians caring for hospitalized patients back to long‐term care facilities, has room for improvement. They highlight that 37.5% of patients had documented discussions, and for cases in which these discussions resulted in changes to a patient's advance directive, only 1 in 4 were relayed in the discharge summary.[1]
As physicians caring for hospitalized patients and concerned with improving care quality and efficiency, many of us are familiar with potential systems solutions to augmenting communication: reminders in the electronic health record, checklists, multidisciplinary teams, scripts, and posthospitalization follow‐up phone calls. However, important as they are, these solutions often elide the underlying cognitive elements related to how we, as physicians, think about and engage in the diversity of cases presented to us, and to how we prioritize communication work.
Wong et al. looked at patient characteristics associated with performance of GOC discussions to understand when and why physicians might engage in GOC conversations in the hospital and to generate insights into potential targets for improvement. They found that characteristics of patients prior to hospital admission were not associated with GOC discussions; signs of acuity of illness were.[1] In other words, physicians in the hospital are pretty good at recognizing patients in extremis, and prioritize GOC discussions with these patients. What we are not good at, or might not be considering, is assessing the broader context of a patient's health.
Whether we interpret these results as appropriate prioritization, or as a sign that we are waiting too long to broach the subject of care goals, depends on how we conceptualize the hospital stay in the context of a patient's health story, and, by extension, the role of the hospitalist in this story. For some patients, an acute illness requiring hospitalization is unexpected and readily treated, and the patient rapidly returns to a prior level of health and function. The need for hospitalization represents an outlier state.
For other patients, often older, more debilitated, or with multiple and chronic medical conditions, minor changes in health or declines in mental, social, or physical function precipitate the need for hospitalization. Likewise, iatrogenic harms of hospitalizationsleeplessness, fasting, delirium, immobilitycan contribute to enduring decline.[2, 3] For these patients, the need for hospitalization is not so far from, or may be, their norm.[4]
I suspect that Wong et al.'s findings reflect a collective response to the uncertainties of prognostication, and the resultant discomfort in raising questions that are difficult to answer. How do we know it is time to start talking about the right amount of care? Some might answer, I think rightly, that it is rarely if ever too early, yet robust discussions are challenging if we are not sure of the relevance or the immediate goal. In the case of the patient who is ill, declining, yet not in extremis, many of us might conclude that raising the question would not produce actionable information; it would not change immediate in‐hospital management.
This common conclusion leads to a significant missed opportunity, both on an individual level for physicians and patients, and for hospital medicine as a specialty. Health, and the losses that come with declining health, are wrapped up with fundamental aspects of our identities, and take time and consideration to change and evolve. Decisions about our healthcare are statements about who we have been, who we are, and who we will no longer be. Especially for the second group of patients described above, each hospital stay affords a chance to assess, counsel, educate, support, and empower patients to move in the direction of their values, and to ready them for that eventuality when they or their loved ones are faced with decisions about how, and where, they will die. As specialists in hospital‐based healthcare, hospitalists have the privilege and professional duty to facilitate this journey.
However, as hospitalists, we are often meeting patients for the first time; how do we assimilate an understanding of that point in time within the context of a patient's life with enough confidence to engage discussions? As Wong et al. show, it appears that in regard to very ill patients, respiratory rate and Glasgow Coma Scale inform action.[1] What signs or observations help inform action earlier in the trajectory of decline, to allow for anticipatory guidance and discussion? Increasingly, we see evidence that measures of frailty and functional status, applied in the hospital, are associated with hospital outcomes including readmission and death.[5, 6, 7] Future work might explore if training physicians to systematically assess frailty and functional status leads to greater frequency of, and comfort with, initiating GOC discussions during hospitalization.
Moreover, an emphasis on evaluating frailty and function, and explicitly including this assessment in our clinical decision‐making might help shift our thinking toward valuing each hospitalization as an opportunity to both intervene to improve function[8, 9] and to support, educate, and prepare patients under our care for the journey aheadin other words, to fully engage with our role as specialists in the comprehensive and coordinated treatment of patients who require hospitalization.
In their study of goals of care (GOC) discussions and documentation, Wong et al. add to already robust evidence that communication, in this case from physicians caring for hospitalized patients back to long‐term care facilities, has room for improvement. They highlight that 37.5% of patients had documented discussions, and for cases in which these discussions resulted in changes to a patient's advance directive, only 1 in 4 were relayed in the discharge summary.[1]
As physicians caring for hospitalized patients and concerned with improving care quality and efficiency, many of us are familiar with potential systems solutions to augmenting communication: reminders in the electronic health record, checklists, multidisciplinary teams, scripts, and posthospitalization follow‐up phone calls. However, important as they are, these solutions often elide the underlying cognitive elements related to how we, as physicians, think about and engage in the diversity of cases presented to us, and to how we prioritize communication work.
Wong et al. looked at patient characteristics associated with performance of GOC discussions to understand when and why physicians might engage in GOC conversations in the hospital and to generate insights into potential targets for improvement. They found that characteristics of patients prior to hospital admission were not associated with GOC discussions; signs of acuity of illness were.[1] In other words, physicians in the hospital are pretty good at recognizing patients in extremis, and prioritize GOC discussions with these patients. What we are not good at, or might not be considering, is assessing the broader context of a patient's health.
Whether we interpret these results as appropriate prioritization, or as a sign that we are waiting too long to broach the subject of care goals, depends on how we conceptualize the hospital stay in the context of a patient's health story, and, by extension, the role of the hospitalist in this story. For some patients, an acute illness requiring hospitalization is unexpected and readily treated, and the patient rapidly returns to a prior level of health and function. The need for hospitalization represents an outlier state.
For other patients, often older, more debilitated, or with multiple and chronic medical conditions, minor changes in health or declines in mental, social, or physical function precipitate the need for hospitalization. Likewise, iatrogenic harms of hospitalizationsleeplessness, fasting, delirium, immobilitycan contribute to enduring decline.[2, 3] For these patients, the need for hospitalization is not so far from, or may be, their norm.[4]
I suspect that Wong et al.'s findings reflect a collective response to the uncertainties of prognostication, and the resultant discomfort in raising questions that are difficult to answer. How do we know it is time to start talking about the right amount of care? Some might answer, I think rightly, that it is rarely if ever too early, yet robust discussions are challenging if we are not sure of the relevance or the immediate goal. In the case of the patient who is ill, declining, yet not in extremis, many of us might conclude that raising the question would not produce actionable information; it would not change immediate in‐hospital management.
This common conclusion leads to a significant missed opportunity, both on an individual level for physicians and patients, and for hospital medicine as a specialty. Health, and the losses that come with declining health, are wrapped up with fundamental aspects of our identities, and take time and consideration to change and evolve. Decisions about our healthcare are statements about who we have been, who we are, and who we will no longer be. Especially for the second group of patients described above, each hospital stay affords a chance to assess, counsel, educate, support, and empower patients to move in the direction of their values, and to ready them for that eventuality when they or their loved ones are faced with decisions about how, and where, they will die. As specialists in hospital‐based healthcare, hospitalists have the privilege and professional duty to facilitate this journey.
However, as hospitalists, we are often meeting patients for the first time; how do we assimilate an understanding of that point in time within the context of a patient's life with enough confidence to engage discussions? As Wong et al. show, it appears that in regard to very ill patients, respiratory rate and Glasgow Coma Scale inform action.[1] What signs or observations help inform action earlier in the trajectory of decline, to allow for anticipatory guidance and discussion? Increasingly, we see evidence that measures of frailty and functional status, applied in the hospital, are associated with hospital outcomes including readmission and death.[5, 6, 7] Future work might explore if training physicians to systematically assess frailty and functional status leads to greater frequency of, and comfort with, initiating GOC discussions during hospitalization.
Moreover, an emphasis on evaluating frailty and function, and explicitly including this assessment in our clinical decision‐making might help shift our thinking toward valuing each hospitalization as an opportunity to both intervene to improve function[8, 9] and to support, educate, and prepare patients under our care for the journey aheadin other words, to fully engage with our role as specialists in the comprehensive and coordinated treatment of patients who require hospitalization.
- , , , . Goals of care discussions among hospitalized long‐term care residents: predictors and associated outcomes of care. J Hosp Med. 2016;11(12):824–831.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30(11):1688–1695.
- , , , et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30‐day risk of readmission or death [published online May 17, 2016]. J Hosp Med. doi: 10.1002/jhm.2607.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
- , , , et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial [published online May 31, 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1870.
- . Activating hospitalized older patients to confront the epidemic of low mobility [published online May 31 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1874.
- , , , . Goals of care discussions among hospitalized long‐term care residents: predictors and associated outcomes of care. J Hosp Med. 2016;11(12):824–831.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223.
- , , . Hospitalization‐associated disability: “she was probably able to ambulate, but I'm not sure.” JAMA. 2011;306(16):1782–1793.
- , . The hospital‐dependent patient. N Engl J Med. 2014;370(8):694–697.
- , , , et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30(11):1688–1695.
- , , , et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30‐day risk of readmission or death [published online May 17, 2016]. J Hosp Med. doi: 10.1002/jhm.2607.
- , , , et al. Association between frailty and 30‐day outcomes after discharge from hospital. CMAJ. 2015;187(11):799–804.
- , , , et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial [published online May 31, 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1870.
- . Activating hospitalized older patients to confront the epidemic of low mobility [published online May 31 2016]. JAMA Intern Med. doi: 10.1001/jamainternmed.2016.1874.
GOC Discussions Among LTC Residents
Hospitalizations of long‐term care (LTC) residents are known to be frequent, costly, often preventable,[1, 2, 3] and potentially associated with negative health outcomes.[4] Often, an advance directive (AD) is made at LTC admission and updated annually when residents are in relatively stable health. An AD is a document that helps to inform a substitute decision maker (SDM) about the consent process for life‐sustaining treatments and is a resource that supports advance care planning (ACP). ACP is a process that allows individuals to consider, express, and plan for future healthcare in the event that they lack capacity to make their own decisions. When an LTC resident's health deteriorates and hospitalization is required, there is an opportunity to update prognosis, discuss risks and benefits of previously held treatment preferences, as well as reassess goals of care (GOC).
Engaging in ACP discussions during relatively stable health can help ensure patient preferences are followed.[5, 6] These discussions, however, are often insufficient, as they involve decision making for hypothetical situations that may not cover all potential scenarios, and may not reflect a patient's reality at the time of health status decline. Discussions held in the moment more authentically reflect the decisions of patients and/or SDM based on the specific needs and clinical realities particular to the patient at that time.[7] GOC discussions, defined in this context as ACP discussions occurring during hospitalization, have the potential to better align patient wishes with care received,[6] improve quality of life and satisfaction,[8, 9, 10] and reduce unwanted extra care.[11, 12] Although in‐the‐moment GOC discussions are recommended for all hospitalized patients who are seriously ill with a high risk of dying,[13] research suggests that this occurs infrequently for elderly patients. A recent multicenter survey of seriously ill hospitalized elderly patients found that only 25% of patients and 32% of family members reported that they had been asked about prior ACP or AD.[14] Another study of hospitalized LTC residents found that resuscitation status and family discussion was documented in only 55% and 42% of admissions, respectively.[15]
Further investigation is required to determine how often LTC patients have GOC discussions, what prompts these discussions, and what are the outcomes. Previous studies have focused on barriers to performing GOC discussions, rather than the factors that are associated with them.[16] By understanding why these discussions currently happen, we can potentially improve how often they occur and the quality of their outcomes.
The objectives of this study were to determine the rate of documented GOC discussions among hospitalized LTC residents, identify factors that were associated with documentation, and examine the association between documentation and outcomes of care.
METHODS
Study Population
We conducted a retrospective chart review of a random convenience sample of hospitalized patients admitted via the emergency department (ED) to the general internal medicine (GIM) service from January 1, 2012 through December 31, 2012, at 2 academic teaching hospitals in Toronto, Canada. Patients were identified through a search of each hospitals' electronic patient record (EPR). Patients were eligible for inclusion if they were (1) a LTC resident and (2) at least 65 years of age. For patients with multiple admissions to the GIM service during the specified 12‐month period, we only included data from the first hospitalization (index hospitalization). The hospital's research ethics board approved this study.
Our primary variable of interest was documentation in the hospital medical record of a discussion between physicians and the patient/family/SDM regarding GOC. A GOC discussion was considered to have taken place if there was documentation of (1) understanding/expectation of treatment options or (2) patient's preferences for life‐sustaining measures. Examples illustrating each criterion are provided in the Supporting Information, Appendix 1, in the online version of this article.
Factors Associated With GOC Documentation
From the EPR, we obtained visit‐level data including age, gender, Canadian Emergency Department Triage and Acuity Scale, vital signs at ED admission including temperature, respiratory rate, oxygen saturation, Glasgow Coma Scale (GCS) and shock index (defined as heart rate divided by systolic blood pressure), admission and discharge dates/times, discharge diagnosis, transfer to intensive care unit (ICU), and hospital use (number of ED visits and hospitalizations to the 2 study hospitals in the 1‐year period prior to index hospitalization).
Trained study personnel (J.W.) used a structured abstraction form to collect data from the hospital medical record that were not available through the EPR, including years living in LTC, contents of LTC AD forms, presence of SDM (identified as immediate family or surrogate with whom the care team communicated), dementia diagnosis (defined as documentation of dementia in the patient's past medical history and/or history of present illness), and measures of functional status. When available, we extracted the AD from LTC; they consisted of 4 levels (level 1: comfort careno transfer to hospital, no cardiopulmonary resuscitation [CPR]; level 2: supportive careadministration of antibiotics and/or other procedures that can be provided within LTC, no transfer to the hospital, no CPR; level 3: transfer to the hospitalno CPR; level 4: aggressive interventiontransfer to hospital for aggressive treatment, CPR).
GOC Documentation in the Discharge Summary
For the subset of patients who survived hospitalization and were discharged back to LTC, we examined whether the ADs ordered during hospitalization were communicated back to LTC via the discharge summary. We additionally assessed if the ADs determined during hospitalization differed from preferences documented prior to hospitalization. Physician orders for ADs were categorized as level 1: comfort measures only, level 3: no CPR, or level 4: full code. LTC level 2 was considered equivalent to physician‐ordered level 3 at admission; a patient with an LTC level 2 with no CPR (level 3) documented during hospitalized would be considered to have no change in the AD. An increase or decrease in the AD was determined by comparing LTC levels 1, 3, and 4 to physician‐ordered level 1, 3, and 4.
Outcomes of GOC Documentation
From the EPR, we obtained visit‐level outcome data including length of stay (LOS), resource intensity weight (RIW) (calculated based on patient case‐mix, severity, age, and procedures performed), visit disposition, number of ED visits and hospitalizations to the 2 study hospitals in the year following index hospitalization, in‐hospital death, and 1‐year mortality. We determined 1‐year mortality by following up with the LTC homes to determine whether the resident had died within the year following index hospitalization; only patients from LTC homes that responded to our request for data were included in 1‐year mortality analyses. We collected physician orders for the AD from chart review.
Statistical Analysis
Patients with and without documented GOC discussions were compared. Descriptive statistics including frequencies and percentages were used to characterize study variables. Differences between the study groups were assessed using Pearson 2/Fisher exact test. Multivariate logistic regression, which included variables that were significant in the bivariate analysis, was used to identify independent predictors of GOC discussion. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were presented for the logistic model. Patients with missing predictor data were excluded.
We also examined whether there was a correlation between GOC discussion and outcomes of care using Pearson 2/Fisher exact test. Outcomes included orders for the AD, LOS in days (stratified into quartiles), RIW (stratified into quartiles), visit disposition, hospital use in the year following index hospitalization, and 1‐year mortality following discharge back to LTC.
Lastly, to better understand the independent predictors of in‐hospital and 1‐year mortality, we used Pearson 2/Fisher exact test followed by logistic regression that included significant variables from the bivariate analyses.
All analyses were 2‐sided, and a P value of 0.05 was considered statistically significant. We used SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
We identified a total of 7084 hospitalizations to GIM between January 1, 2012 and December 31, 2012, of which 665 (9.4%) met inclusion criteria of residence in LTC and age 65 years. Of these 665 hospitalizations, 512 were unique patients. We randomly selected a convenience sample of 200 index hospitalizations of the 512 eligible hospitalizations (39%) to perform the chart review.
Predictors of GOC Documentation
Of the 200 randomly sampled charts that were reviewed, 75 (37.5%) had a documented GOC discussion.
Characteristics of the study patients and results of bivariate analysis of the association between patient characteristics and GOC discussion are summarized in Table 1. No significant differences in demographic and baseline characteristics were seen between patients with and without discussion. However, a number of visit characteristics were found to be significantly associated with discussion. Forty percent of patients in the GOC discussion group had GCS scores 11 compared to 15.2% in the no‐discussion group. Higher respiratory rate, lower oxygen saturation, and ICU transfer were also significantly associated with discussions.
| Goals of Care Discussion Documented in Medical Chart | |||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Baseline characteristics | |||||
| Gender, n (%) | 0.88 | ||||
| Male | 48 | (38.4) | 30 | (40.0) | |
| Female | 77 | (61.6) | 45 | (60.0) | |
| Age, y, n (%) | 0.85 | ||||
| 6579 | 36 | (28.8) | 19 | (25.3) | |
| 8084 | 30 | (24.0) | 19 | (25.3) | |
| 8589 | 30 | (24.0) | 16 | (21.3) | |
| 90101 | 29 | (23.2) | 21 | (28.0) | |
| Years living in long‐term care, n (%)* | 0.65 | ||||
| [0, 1) | 28 | (22.4) | 12 | (16.0) | |
| [1, 3) | 31 | (24.8) | 22 | (29.3) | |
| [3, 6) | 33 | (26.4) | 22 | (29.3) | |
| [6, 22) | 25 | (20.0) | 13 | (17.3) | |
| Unknown | 8 | (6.4) | 6 | (8.0) | |
| AD from long‐term care, n (%) | 0.14 | ||||
| Comfort measures only | 2 | (1.6) | 1 | (1.3) | |
| Supportive care with no transfer to hospital | 0 | (0.0) | 3 | (4.0) | |
| Supportive care with transfer to hospital | 70 | (56.0) | 44 | (58.7) | |
| Aggressive care | 53 | (42.4) | 27 | (36.0) | |
| Years since most recent AD signed, n (%)* | 0.12 | ||||
| [0, 1) | 79 | (63.2) | 48 | (64.0) | |
| [1, 2) | 21 | (16.8) | 6 | (8.0) | |
| [2, 6) | 9 | (7.2) | 10 | (13.3) | |
| Unknown | 16 | (12.8) | 11 | (14.7) | |
| Substitute decision maker, n (%) | 0.06 | ||||
| Child | 81 | (64.8) | 44 | (58.7) | |
| Spouse | 9 | (7.2) | 15 | (20.0) | |
| Other | 26 | (20.8) | 13 | (17.3) | |
| Public guardian trustee | 6 | (4.8) | 2 | (2.7) | |
| Unknown | 3 | (2.4) | 1 | (1.3) | |
| Dementia, n (%) | 1.00 | ||||
| No | 47 | (37.6) | 28 | (37.3) | |
| Yes | 78 | (62.4) | 47 | (62.7) | |
| Mobility, n (%) | 0.26 | ||||
| Walk without assistance | 5 | (4.0) | 3 | (4.0) | |
| Walker | 16 | (12.8) | 3 | (4.0) | |
| Wheelchair | 43 | (34.4) | 29 | (38.7) | |
| Bedridden | 7 | (5.6) | 4 | (5.3) | |
| Unknown | 54 | (43.2) | 36 | (48.0) | |
| Continence, n (%) | 0.05 | ||||
| Mostly continent | 16 | (12.8) | 3 | (4.0) | |
| Incontinent | 49 | (39.2) | 34 | (45.3) | |
| Catheter/stoma | 7 | (5.6) | 1 | (1.3) | |
| Unknown | 53 | (42.4) | 37 | (49.3) | |
| Feeding, n (%) | 0.17 | ||||
| Mostly feeds self | 38 | (30.4) | 13 | (17.3) | |
| Needs to be fed | 17 | (13.6) | 14 | (18.7) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 62 | (49.6) | 43 | (57.3) | |
| Diet, n (%) | 0.68 | ||||
| Normal | 43 | (34.4) | 16 | (21.3) | |
| Dysphagic | 32 | (25.6) | 15 | (20.0) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 42 | (33.6) | 39 | (52.0) | |
| Previous ED visits in last year, n (%) | 0.43 | ||||
| 0 | 70 | (56.0) | 41 | (54.7) | |
| 1 | 35 | (28.0) | 17 | (22.7) | |
| 2+ | 20 | (16.0) | 17 | (22.7) | |
| Previous hospitalizations in last year, n (%) | 0.19 | ||||
| 0 | 98 | (78.4) | 54 | (72.0) | |
| 1 | 23 | (18.4) | 14 | (18.7) | |
| 2+ | 4 | (3.2) | 7 | (9.3) | |
| Visit characteristics | |||||
| Glasgow Coma Scale, n (%) | 0.001 | ||||
| 7 | 4 | (3.2) | 4 | (5.3) | |
| 711 | 15 | (12.0) | 26 | (34.7) | |
| 1213 | 7 | (5.6) | 8 | (10.7) | |
| 1415 | 85 | (68.0) | 32 | (42.7) | |
| Unknown | 14 | (11.2) | 5 | (6.7) | |
| Shock index, n (%) | 0.13 | ||||
| 1 | 105 | (84.0) | 54 | (72.0) | |
| >1 | 19 | (15.2) | 18 | (24.0) | |
| Unknown | 1 | (0.8) | 3 | (4.0) | |
| Respiratory rate, n (%) | 0.02 | ||||
| 20 | 59 | (47.2) | 21 | (28.0) | |
| 20 | 66 | (52.8) | 52 | (69.3) | |
| Unknown | 0 | (0.0) | 2 | (2.7) | |
| Oxygen saturation, n (%) | 0.03 | ||||
| 88 | 2 | (1.6) | 6 | (8.0) | |
| 88 | 122 | (97.6) | 65 | (86.7) | |
| Unknown | 1 | (0.8) | 4 | (5.3) | |
| Temperature, n (%) | 0.09 | ||||
| 38.0 | 100 | (80.0) | 51 | (68.0) | |
| 38.0 | 25 | (20.0) | 23 | (30.7) | |
| Unknown | 0 | (0.0) | 1 | (1.3) | |
| Canadian Triage and Acuity Scale, n (%) | 0.13 | ||||
| Resuscitation | 1 | (0.8) | 3 | (4.0) | |
| Emergent | 70 | (56.0) | 49 | (65.3) | |
| Urgent | 52 | (41.6) | 22 | (29.3) | |
| Less urgent and nonurgent | 2 | (1.6) | 1 | (1.3) | |
| Discharge diagnosis, n (%) | 0.29 | ||||
| Aspiration pneumonia | 12 | (9.6) | 12 | (16.0) | |
| Chronic obstructive pulmonary disease | 15 | (12.0) | 3 | (4.0) | |
| Dehydration/disorders fluid/electrolytes | 9 | (7.2) | 5 | (6.7) | |
| Gastrointestinal hemorrhage | 4 | (3.2) | 3 | (4.0) | |
| Heart failure | 11 | (8.8) | 2 | (2.7) | |
| Infection (other or not identified) | 9 | (7.2) | 9 | (12.0) | |
| Influenza/pneumonia | 14 | (11.2) | 11 | (14.7) | |
| Lower urinary tract infection | 11 | (8.8) | 6 | (8.0) | |
| Other | 40 | (32.0) | 24 | (32.0) | |
| Hospitalization included ICU stay, n (%) | 0.01 | ||||
| No | 124 | (99.2) | 69 | (92.0) | |
| Yes | 1 | (0.8) | 6 | (8.0) | |
When these 4 significant clinical and visit characteristics were tested together in a logistic regression analysis, 2 remained statistically significant (Table 2). Patients with lower GCS scores (GCS 1213 and 711) were more likely to have discussions (AOR: 4.4 [95% CI: 1.4‐13.9] and AOR: 5.9 [95% CI: 2.6‐13.2], respectively) and patients with higher respiratory rates were also more likely to have discussions (AOR: 2.3 [95% CI: 1.1‐4.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Glasgow Coma Scale | 0.001 | ||
| 7 | 1.77 | 0.33‐9.58 | 0.51 |
| 711 | 5.90 | 2.64‐13.22 | 0.001 |
| 1213 | 4.43 | 1.41‐13.91 | 0.01 |
| 1415 | Reference | ||
| Respiration | |||
| 20 | Reference | ||
| 20 | 2.32 | 1.12‐4.78 | 0.02 |
| Oxygen saturation | |||
| 88 | 3.35 | 0.55‐20.56 | 0.19 |
| 88 | Reference | 0.05‐1.83 | |
| Hospitalization included ICU stay | |||
| No | Reference | ||
| Yes | 7.87 | 0.83‐74.73 | 0.07 |
GOC Documentation in the Discharge Summary
For the subset of patients who survived index hospitalization and were discharged back to LTC (176 patients or 88%), we also investigated whether the ADs were documented in the discharge summary back to LTC (data not shown). Of the 42 patients (23.9%) who had a change in the AD (18 patients had an AD increase in care intensity due to hospitalization; 24 had a decrease), only 11 (26%) had this AD change documented in the discharge summary.
Outcomes of GOC Documentation
A number of outcomes differed significantly between patients with and without GOC discussions in unadjusted comparisons (Table 3). Patients with discussions had higher rates of orders for no CPR (80% vs 55%) and orders for comfort measures only (7% vs 0%). They also had higher rates of in‐hospital death (29% vs 1%), 1‐year mortality (63% vs 28%), and longer LOS. However, RIW and subsequent hospital use were not found to be significant.
| Variable | Goals of Care Discussion Documented in Medical Chart | ||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Physician orders, n (%) | 0.001 | ||||
| Comfort measures only | 0 | (0.0) | 5 | (6.7) | |
| No cardiopulmonary resuscitation | 69 | (55.2) | 60 | (80.0) | |
| Full code | 56 | (44.8) | 10 | (13.3) | |
| Visit disposition, n (%) | 0.001 | ||||
| Long‐term care home | 124 | (99.2) | 52 | (69.3) | |
| Died | 1 | (0.8) | 22 | (29.3) | |
| Transfer to palliative care facility | 0 | (0.0) | 1 | (1.3) | |
| Resource intensity weight, n (%) | 0.43 | ||||
| 0.250.75 | 35 | (28.0) | 19 | (25.3) | |
| 0.761.14 | 29 | (23.2) | 16 | (21.3) | |
| 1.151.60 | 34 | (27.2) | 16 | (21.3) | |
| 1.6125.5 | 27 | (21.6) | 24 | (32.0) | |
| Length of stay, d, n (%) | 0.01 | ||||
| 0.672.97 | 30 | (24.0) | 20 | (26.7) | |
| 2.984.60 | 40 | (32.0) | 10 | (13.3) | |
| 4.618.65 | 30 | (24.0) | 20 | (26.7) | |
| 8.66+ | 25 | (20.0) | 25 | (33.3) | |
| Subsequent emergency department visits in next year, n (% of applicable) | 0.38 | ||||
| 0 | 66 | (53.2) | 32 | (61.5) | |
| 1 | 30 | (24.2) | 13 | (25.0) | |
| 2+ | 28 | (22.6) | 7 | (13.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Subsequent hospitalizations in next year, n (% of applicable) | 0.87 | ||||
| 0 | 87 | (70.2) | 38 | (73.1) | |
| 1 | 24 | (19.4) | 10 | (19.2) | |
| 2+ | 13 | (10.5) | 4 | (7.7) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| 1‐year mortality, n (% of applicable) | 0.001 | ||||
| Alive | 82 | (71.9) | 15 | (37.5) | |
| Dead | 32 | (28.1) | 25 | (62.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Not applicable (unsuccessful follow‐up with long‐term care home) | 10 | 12 | |||
Predictors of In‐hospital Death and 1‐Year Mortality
Given the significant positive associations between discussions and in‐hospital death and 1‐year mortality, we performed separate logistic regression analyses to test whether discussions independently predicted in‐hospital death and 1‐year mortality (Table 4). After adjusting for variables significant in their respective bivariate analyses, patients with discussions continued to have higher odds of in‐hospital death (AOR: 52.0 [95% CI: 6.2‐440.4]) and 1‐year mortality (AOR: 4.1 [95% CI: 1.7‐9.6]). Of note, the presence of dementia had significantly lower adjusted odds of in‐hospital death compared to the reference group of no dementia (AOR: 0.3 [95% CI: 0.1‐0.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| In‐hospital death odds ratios | |||
| Advance directives from long‐term care | 0.91 | ||
| Comfort measures only | Reference | ||
| Supportive care no transfer | 3.43E +18 | 0‐. | 1.00 |
| Transfer to hospital | 3.10E +8 | 0‐. | 1.00 |
| Aggressive care | 4.85E +8 | 0‐. | 1.00 |
| Dementia | |||
| No | Reference | ||
| Yes | .25 | 0.08‐0.79 | 0.02 |
| Previous hospitalizations in last year | 0.05 | ||
| 0 | Reference | ||
| 1 | 0.43 | 0.08‐2.38 | 0.34 |
| 2+ | 6.30 | 1.10‐36.06 | 0.04 |
| Respiration | |||
| 20 | Reference | ||
| 20 | 3.64 | 0.82‐16.24 | 0.09 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 52.04 | 6.15‐440.40 | 0.001 |
| 1‐year mortality odds ratios | |||
| Oxygen saturation, n (%) | |||
| 88 | 12.15 | 1.18‐124.97 | 0.04 |
| 88 | Reference | ||
| Previous ED visits in last year | 0.06 | ||
| 0 | Reference | ||
| 1 | 3.07 | 1.15‐8.17 | 0.03 |
| 2+ | 3.21 | 0.87‐11.81 | 0.08 |
| Previous hospitalizations in last year | 0.55 | ||
| 0 | Reference | ||
| 1 | 1.66 | 0.57‐4.86 | 0.36 |
| 2+ | 2.52 | 0.30‐20.89 | 0.39 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 4.07 | 1.73‐9.56 | 0.001 |
DISCUSSION
Our retrospective study of LTC residents admitted to the GIM service showed that these admissions comprised 9.4% of all admissions and that GOC discussions occurred infrequently (37.5%). Our study revealed no differences in baseline patient characteristics associated with discussions, whereas patient acuity at hospital presentation independently contributed to the likelihood of discussions. We found strong associations between documentation and certain outcomes of care, including orders for AD, LOS, in‐hospital death, and 1‐year mortality. No significant associations were found between documentation and subsequent hospital use. Lastly, we found that consistent communication back to the LTC home when there was a change in AD was very poor; only 26% of discharge summaries included this documentation.
Our finding of infrequent GOC discussions during hospitalization aligns with prior studies. A study that identified code status discussions in transcripts of audio‐recorded admission encounters found that code status was discussed in only 24% of seriously ill patient admissions.[17] Furthermore, in a study specific to LTC residents, only 42% of admissions longer than 48 hours had a documented GOC discussion.[15]
We found visit‐level, but not baseline, characteristics were associated with discussions. These findings are supported by a recent study that found that whether GOC discussions took place largely depended on the acute condition presented on admission.[15] Although these results suggest that clinicians are appropriately prioritizing sicker patients who might have the most pressing need for GOC discussions, they also highlight the gap in care for less‐sick patients and the need to broaden clinical practice and consider underlying conditions and functional status. Of note, although the GCS score was found to be significantly associated with discussions, patients in the lowest GCS range did not have significantly different odds of discussions compared to the reference level (highest GCS range). A recent study by You et al. may offer some insight into this finding. They found that patients lacking capacity to make GOC decisions was ranked fifth, whereas lack of SDM availability was eighth among 21 barriers to GOC discussions, as perceived by hospital‐based clinicians.[16]
A major finding of this study was that both in‐hospital and 1‐year mortality were strongly associated with having a GOC discussion, suggesting that patients at higher risk of dying are more likely to have discussions. This is reflected by illness severity measured at initial assessment and by persistence of the association between discussions and mortality after discharge back to LTC. To the best of our knowledge, no previous studies have reported these findings. There are likely some unmeasured clinical factors such as clinical deterioration during hospitalization that contributed to this strong association. Interestingly, in our logistic regression analysis for independent predictors of in‐hospital death, we found that having dementia was associated with lower odds of in‐hospital death. One interpretation of this finding is that perhaps only patients with mild dementia were hospitalized, and those with more advanced dementia had an AD established in LTC that allowed them to remain in their LTC home. This possibility is supported by a systematic review of factors associated with LTC home hospitalization, which found that dementia was shown to be associated with less hospitalization.[18]
For patients who survived hospitalization, we did not find an association between GOC discussions and hospital use in the year following index hospitalization. In both groups, nearly 30% of patients had 1 or more subsequent hospitalizations. This is relevant especially in light of the finding that among patients where GOC discussions resulted in an AD change, only 26% of discharge summaries back to LTC included this documentation. We can only speculate that had these discussions been properly documented, subsequent hospitalizations would have decreased in the GOC group. Previous research has found that omissions of critical information in discharge summaries were common. In a study of hip fracture and stroke patients discharged from a large Midwestern academic medical center in the United States, code status was included in the discharge summary only 7% of the time.[19] The discharge summary is the primary means of sharing patient information between the hospital and LTC home. If GOC discussions are not included in the discharge summary, it is very unlikely that this information will be subsequently updated in the LTC medical record and impact the care the patient receives. A key recommendation for hospital‐based providers is ensuring that GOC discussions are clearly, consistently, and completely documented in the discharge summary so that the care provided is based on the patients' wishes.
Our study has several limitations. Our analysis was based on chart review, and although our analyses take into account a number of patient characteristics, we did not capture other characteristics that might influence GOC discussions such as culture/religion, language barriers, SDM availability, or whether patients clinically deteriorated during the index admission. Additionally, provider‐level predictors, including seniority, previous GOC training, and time available to conduct these discussions, were not captured. We also did not capture the timing or number of occasions that GOC discussions took place during hospitalization. Due to the retrospective nature of our study, we were able to only look at documented GOC discussions. GOC discussions may have happened but were never documented. However, the standard of care is to document these discussions as part of the medical record, and if they are not documented, it can be considered not to have happened and indicates a lower quality of practice. A recent survey of Canadian hospital‐based healthcare providers identified standardized GOC documentation as an effective practice to improve GOC communication.[20] Finally, because our study was conducted in 2 academic hospitals, our results may be less generalizable to other community hospitals. However, our hospitals' catchment areas capture a diverse population, both culturally and in terms of their socioeconomic status.
CONCLUSION
GOC discussions occurred infrequently, appeared to be triggered by illness severity, and were poorly communicated back to LTC. Important outcomes of care, including in‐hospital death and 1‐year mortality, were associated with discussions. This study serves to identify gaps in who might benefit from GOC discussions and illustrates opportunities for improvement including implementing standardized documentation practices.
Disclosures
Hannah J. Wong, PhD, and Robert C. Wu, MD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Robert C. Wu, MD, Hannah J. Wong, PhD, and Michelle Grinman, MD, were responsible for the conception and design of the study. Robert C. Wu, MD, Hannah J. Wong, PhD, and Jamie Wang were responsible for the acquisition of the data. All of the authors were responsible for the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. Hannah J. Wong, PhD obtained the funding. Hannah J. Wong, PhD, and Robert C. Wu, MD, supervised the study. The authors report no conflicts of interest.
- , , , , . Trends in emergency department visits for ambulatory care sensitive conditions by elderly nursing home residents, 2001 to 2010. JAMA Intern Med. 2014;174(1):156–158.
- , , , . Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909.
- , , , , . Potentially avoidable hospitalizations for elderly long‐stay residents in nursing homes. Med Care. 2013;51(8):673–681.
- , . Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , , , . The consistency between treatments provided to nursing facility residents and orders on the physician orders for life‐sustaining treatment form. J Am Geriatr Soc. 2011;59(11):2091–2099.
- , , . What should be the goal of advance care planning? JAMA Intern Med. 2014;174(7):1093–1094.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444.
- , . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , . Predictors of nursing home residents' time to hospitalization. Health Serv Res. 2011;46(1 pt 1):82–104.
- , , , . Regional variation in the association between advance directives and end‐of‐life Medicare expenditures. JAMA. 2011;306(13):1447–1453.
- , , . Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014;186(6):425–432.
- , , , et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778–787.
- , , , . Hospitalisation of high‐care residents of aged care facilities: are goals of care discussed? Intern Med J. 2013;43(2):144–149.
- , , , et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549–556.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , . Strategies for effective goals of care discussions and decision‐making: perspectives from a multi‐centre survey of Canadian hospital‐based healthcare providers. BMC Palliat Care. 2015;14:38.
Hospitalizations of long‐term care (LTC) residents are known to be frequent, costly, often preventable,[1, 2, 3] and potentially associated with negative health outcomes.[4] Often, an advance directive (AD) is made at LTC admission and updated annually when residents are in relatively stable health. An AD is a document that helps to inform a substitute decision maker (SDM) about the consent process for life‐sustaining treatments and is a resource that supports advance care planning (ACP). ACP is a process that allows individuals to consider, express, and plan for future healthcare in the event that they lack capacity to make their own decisions. When an LTC resident's health deteriorates and hospitalization is required, there is an opportunity to update prognosis, discuss risks and benefits of previously held treatment preferences, as well as reassess goals of care (GOC).
Engaging in ACP discussions during relatively stable health can help ensure patient preferences are followed.[5, 6] These discussions, however, are often insufficient, as they involve decision making for hypothetical situations that may not cover all potential scenarios, and may not reflect a patient's reality at the time of health status decline. Discussions held in the moment more authentically reflect the decisions of patients and/or SDM based on the specific needs and clinical realities particular to the patient at that time.[7] GOC discussions, defined in this context as ACP discussions occurring during hospitalization, have the potential to better align patient wishes with care received,[6] improve quality of life and satisfaction,[8, 9, 10] and reduce unwanted extra care.[11, 12] Although in‐the‐moment GOC discussions are recommended for all hospitalized patients who are seriously ill with a high risk of dying,[13] research suggests that this occurs infrequently for elderly patients. A recent multicenter survey of seriously ill hospitalized elderly patients found that only 25% of patients and 32% of family members reported that they had been asked about prior ACP or AD.[14] Another study of hospitalized LTC residents found that resuscitation status and family discussion was documented in only 55% and 42% of admissions, respectively.[15]
Further investigation is required to determine how often LTC patients have GOC discussions, what prompts these discussions, and what are the outcomes. Previous studies have focused on barriers to performing GOC discussions, rather than the factors that are associated with them.[16] By understanding why these discussions currently happen, we can potentially improve how often they occur and the quality of their outcomes.
The objectives of this study were to determine the rate of documented GOC discussions among hospitalized LTC residents, identify factors that were associated with documentation, and examine the association between documentation and outcomes of care.
METHODS
Study Population
We conducted a retrospective chart review of a random convenience sample of hospitalized patients admitted via the emergency department (ED) to the general internal medicine (GIM) service from January 1, 2012 through December 31, 2012, at 2 academic teaching hospitals in Toronto, Canada. Patients were identified through a search of each hospitals' electronic patient record (EPR). Patients were eligible for inclusion if they were (1) a LTC resident and (2) at least 65 years of age. For patients with multiple admissions to the GIM service during the specified 12‐month period, we only included data from the first hospitalization (index hospitalization). The hospital's research ethics board approved this study.
Our primary variable of interest was documentation in the hospital medical record of a discussion between physicians and the patient/family/SDM regarding GOC. A GOC discussion was considered to have taken place if there was documentation of (1) understanding/expectation of treatment options or (2) patient's preferences for life‐sustaining measures. Examples illustrating each criterion are provided in the Supporting Information, Appendix 1, in the online version of this article.
Factors Associated With GOC Documentation
From the EPR, we obtained visit‐level data including age, gender, Canadian Emergency Department Triage and Acuity Scale, vital signs at ED admission including temperature, respiratory rate, oxygen saturation, Glasgow Coma Scale (GCS) and shock index (defined as heart rate divided by systolic blood pressure), admission and discharge dates/times, discharge diagnosis, transfer to intensive care unit (ICU), and hospital use (number of ED visits and hospitalizations to the 2 study hospitals in the 1‐year period prior to index hospitalization).
Trained study personnel (J.W.) used a structured abstraction form to collect data from the hospital medical record that were not available through the EPR, including years living in LTC, contents of LTC AD forms, presence of SDM (identified as immediate family or surrogate with whom the care team communicated), dementia diagnosis (defined as documentation of dementia in the patient's past medical history and/or history of present illness), and measures of functional status. When available, we extracted the AD from LTC; they consisted of 4 levels (level 1: comfort careno transfer to hospital, no cardiopulmonary resuscitation [CPR]; level 2: supportive careadministration of antibiotics and/or other procedures that can be provided within LTC, no transfer to the hospital, no CPR; level 3: transfer to the hospitalno CPR; level 4: aggressive interventiontransfer to hospital for aggressive treatment, CPR).
GOC Documentation in the Discharge Summary
For the subset of patients who survived hospitalization and were discharged back to LTC, we examined whether the ADs ordered during hospitalization were communicated back to LTC via the discharge summary. We additionally assessed if the ADs determined during hospitalization differed from preferences documented prior to hospitalization. Physician orders for ADs were categorized as level 1: comfort measures only, level 3: no CPR, or level 4: full code. LTC level 2 was considered equivalent to physician‐ordered level 3 at admission; a patient with an LTC level 2 with no CPR (level 3) documented during hospitalized would be considered to have no change in the AD. An increase or decrease in the AD was determined by comparing LTC levels 1, 3, and 4 to physician‐ordered level 1, 3, and 4.
Outcomes of GOC Documentation
From the EPR, we obtained visit‐level outcome data including length of stay (LOS), resource intensity weight (RIW) (calculated based on patient case‐mix, severity, age, and procedures performed), visit disposition, number of ED visits and hospitalizations to the 2 study hospitals in the year following index hospitalization, in‐hospital death, and 1‐year mortality. We determined 1‐year mortality by following up with the LTC homes to determine whether the resident had died within the year following index hospitalization; only patients from LTC homes that responded to our request for data were included in 1‐year mortality analyses. We collected physician orders for the AD from chart review.
Statistical Analysis
Patients with and without documented GOC discussions were compared. Descriptive statistics including frequencies and percentages were used to characterize study variables. Differences between the study groups were assessed using Pearson 2/Fisher exact test. Multivariate logistic regression, which included variables that were significant in the bivariate analysis, was used to identify independent predictors of GOC discussion. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were presented for the logistic model. Patients with missing predictor data were excluded.
We also examined whether there was a correlation between GOC discussion and outcomes of care using Pearson 2/Fisher exact test. Outcomes included orders for the AD, LOS in days (stratified into quartiles), RIW (stratified into quartiles), visit disposition, hospital use in the year following index hospitalization, and 1‐year mortality following discharge back to LTC.
Lastly, to better understand the independent predictors of in‐hospital and 1‐year mortality, we used Pearson 2/Fisher exact test followed by logistic regression that included significant variables from the bivariate analyses.
All analyses were 2‐sided, and a P value of 0.05 was considered statistically significant. We used SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
We identified a total of 7084 hospitalizations to GIM between January 1, 2012 and December 31, 2012, of which 665 (9.4%) met inclusion criteria of residence in LTC and age 65 years. Of these 665 hospitalizations, 512 were unique patients. We randomly selected a convenience sample of 200 index hospitalizations of the 512 eligible hospitalizations (39%) to perform the chart review.
Predictors of GOC Documentation
Of the 200 randomly sampled charts that were reviewed, 75 (37.5%) had a documented GOC discussion.
Characteristics of the study patients and results of bivariate analysis of the association between patient characteristics and GOC discussion are summarized in Table 1. No significant differences in demographic and baseline characteristics were seen between patients with and without discussion. However, a number of visit characteristics were found to be significantly associated with discussion. Forty percent of patients in the GOC discussion group had GCS scores 11 compared to 15.2% in the no‐discussion group. Higher respiratory rate, lower oxygen saturation, and ICU transfer were also significantly associated with discussions.
| Goals of Care Discussion Documented in Medical Chart | |||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Baseline characteristics | |||||
| Gender, n (%) | 0.88 | ||||
| Male | 48 | (38.4) | 30 | (40.0) | |
| Female | 77 | (61.6) | 45 | (60.0) | |
| Age, y, n (%) | 0.85 | ||||
| 6579 | 36 | (28.8) | 19 | (25.3) | |
| 8084 | 30 | (24.0) | 19 | (25.3) | |
| 8589 | 30 | (24.0) | 16 | (21.3) | |
| 90101 | 29 | (23.2) | 21 | (28.0) | |
| Years living in long‐term care, n (%)* | 0.65 | ||||
| [0, 1) | 28 | (22.4) | 12 | (16.0) | |
| [1, 3) | 31 | (24.8) | 22 | (29.3) | |
| [3, 6) | 33 | (26.4) | 22 | (29.3) | |
| [6, 22) | 25 | (20.0) | 13 | (17.3) | |
| Unknown | 8 | (6.4) | 6 | (8.0) | |
| AD from long‐term care, n (%) | 0.14 | ||||
| Comfort measures only | 2 | (1.6) | 1 | (1.3) | |
| Supportive care with no transfer to hospital | 0 | (0.0) | 3 | (4.0) | |
| Supportive care with transfer to hospital | 70 | (56.0) | 44 | (58.7) | |
| Aggressive care | 53 | (42.4) | 27 | (36.0) | |
| Years since most recent AD signed, n (%)* | 0.12 | ||||
| [0, 1) | 79 | (63.2) | 48 | (64.0) | |
| [1, 2) | 21 | (16.8) | 6 | (8.0) | |
| [2, 6) | 9 | (7.2) | 10 | (13.3) | |
| Unknown | 16 | (12.8) | 11 | (14.7) | |
| Substitute decision maker, n (%) | 0.06 | ||||
| Child | 81 | (64.8) | 44 | (58.7) | |
| Spouse | 9 | (7.2) | 15 | (20.0) | |
| Other | 26 | (20.8) | 13 | (17.3) | |
| Public guardian trustee | 6 | (4.8) | 2 | (2.7) | |
| Unknown | 3 | (2.4) | 1 | (1.3) | |
| Dementia, n (%) | 1.00 | ||||
| No | 47 | (37.6) | 28 | (37.3) | |
| Yes | 78 | (62.4) | 47 | (62.7) | |
| Mobility, n (%) | 0.26 | ||||
| Walk without assistance | 5 | (4.0) | 3 | (4.0) | |
| Walker | 16 | (12.8) | 3 | (4.0) | |
| Wheelchair | 43 | (34.4) | 29 | (38.7) | |
| Bedridden | 7 | (5.6) | 4 | (5.3) | |
| Unknown | 54 | (43.2) | 36 | (48.0) | |
| Continence, n (%) | 0.05 | ||||
| Mostly continent | 16 | (12.8) | 3 | (4.0) | |
| Incontinent | 49 | (39.2) | 34 | (45.3) | |
| Catheter/stoma | 7 | (5.6) | 1 | (1.3) | |
| Unknown | 53 | (42.4) | 37 | (49.3) | |
| Feeding, n (%) | 0.17 | ||||
| Mostly feeds self | 38 | (30.4) | 13 | (17.3) | |
| Needs to be fed | 17 | (13.6) | 14 | (18.7) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 62 | (49.6) | 43 | (57.3) | |
| Diet, n (%) | 0.68 | ||||
| Normal | 43 | (34.4) | 16 | (21.3) | |
| Dysphagic | 32 | (25.6) | 15 | (20.0) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 42 | (33.6) | 39 | (52.0) | |
| Previous ED visits in last year, n (%) | 0.43 | ||||
| 0 | 70 | (56.0) | 41 | (54.7) | |
| 1 | 35 | (28.0) | 17 | (22.7) | |
| 2+ | 20 | (16.0) | 17 | (22.7) | |
| Previous hospitalizations in last year, n (%) | 0.19 | ||||
| 0 | 98 | (78.4) | 54 | (72.0) | |
| 1 | 23 | (18.4) | 14 | (18.7) | |
| 2+ | 4 | (3.2) | 7 | (9.3) | |
| Visit characteristics | |||||
| Glasgow Coma Scale, n (%) | 0.001 | ||||
| 7 | 4 | (3.2) | 4 | (5.3) | |
| 711 | 15 | (12.0) | 26 | (34.7) | |
| 1213 | 7 | (5.6) | 8 | (10.7) | |
| 1415 | 85 | (68.0) | 32 | (42.7) | |
| Unknown | 14 | (11.2) | 5 | (6.7) | |
| Shock index, n (%) | 0.13 | ||||
| 1 | 105 | (84.0) | 54 | (72.0) | |
| >1 | 19 | (15.2) | 18 | (24.0) | |
| Unknown | 1 | (0.8) | 3 | (4.0) | |
| Respiratory rate, n (%) | 0.02 | ||||
| 20 | 59 | (47.2) | 21 | (28.0) | |
| 20 | 66 | (52.8) | 52 | (69.3) | |
| Unknown | 0 | (0.0) | 2 | (2.7) | |
| Oxygen saturation, n (%) | 0.03 | ||||
| 88 | 2 | (1.6) | 6 | (8.0) | |
| 88 | 122 | (97.6) | 65 | (86.7) | |
| Unknown | 1 | (0.8) | 4 | (5.3) | |
| Temperature, n (%) | 0.09 | ||||
| 38.0 | 100 | (80.0) | 51 | (68.0) | |
| 38.0 | 25 | (20.0) | 23 | (30.7) | |
| Unknown | 0 | (0.0) | 1 | (1.3) | |
| Canadian Triage and Acuity Scale, n (%) | 0.13 | ||||
| Resuscitation | 1 | (0.8) | 3 | (4.0) | |
| Emergent | 70 | (56.0) | 49 | (65.3) | |
| Urgent | 52 | (41.6) | 22 | (29.3) | |
| Less urgent and nonurgent | 2 | (1.6) | 1 | (1.3) | |
| Discharge diagnosis, n (%) | 0.29 | ||||
| Aspiration pneumonia | 12 | (9.6) | 12 | (16.0) | |
| Chronic obstructive pulmonary disease | 15 | (12.0) | 3 | (4.0) | |
| Dehydration/disorders fluid/electrolytes | 9 | (7.2) | 5 | (6.7) | |
| Gastrointestinal hemorrhage | 4 | (3.2) | 3 | (4.0) | |
| Heart failure | 11 | (8.8) | 2 | (2.7) | |
| Infection (other or not identified) | 9 | (7.2) | 9 | (12.0) | |
| Influenza/pneumonia | 14 | (11.2) | 11 | (14.7) | |
| Lower urinary tract infection | 11 | (8.8) | 6 | (8.0) | |
| Other | 40 | (32.0) | 24 | (32.0) | |
| Hospitalization included ICU stay, n (%) | 0.01 | ||||
| No | 124 | (99.2) | 69 | (92.0) | |
| Yes | 1 | (0.8) | 6 | (8.0) | |
When these 4 significant clinical and visit characteristics were tested together in a logistic regression analysis, 2 remained statistically significant (Table 2). Patients with lower GCS scores (GCS 1213 and 711) were more likely to have discussions (AOR: 4.4 [95% CI: 1.4‐13.9] and AOR: 5.9 [95% CI: 2.6‐13.2], respectively) and patients with higher respiratory rates were also more likely to have discussions (AOR: 2.3 [95% CI: 1.1‐4.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Glasgow Coma Scale | 0.001 | ||
| 7 | 1.77 | 0.33‐9.58 | 0.51 |
| 711 | 5.90 | 2.64‐13.22 | 0.001 |
| 1213 | 4.43 | 1.41‐13.91 | 0.01 |
| 1415 | Reference | ||
| Respiration | |||
| 20 | Reference | ||
| 20 | 2.32 | 1.12‐4.78 | 0.02 |
| Oxygen saturation | |||
| 88 | 3.35 | 0.55‐20.56 | 0.19 |
| 88 | Reference | 0.05‐1.83 | |
| Hospitalization included ICU stay | |||
| No | Reference | ||
| Yes | 7.87 | 0.83‐74.73 | 0.07 |
GOC Documentation in the Discharge Summary
For the subset of patients who survived index hospitalization and were discharged back to LTC (176 patients or 88%), we also investigated whether the ADs were documented in the discharge summary back to LTC (data not shown). Of the 42 patients (23.9%) who had a change in the AD (18 patients had an AD increase in care intensity due to hospitalization; 24 had a decrease), only 11 (26%) had this AD change documented in the discharge summary.
Outcomes of GOC Documentation
A number of outcomes differed significantly between patients with and without GOC discussions in unadjusted comparisons (Table 3). Patients with discussions had higher rates of orders for no CPR (80% vs 55%) and orders for comfort measures only (7% vs 0%). They also had higher rates of in‐hospital death (29% vs 1%), 1‐year mortality (63% vs 28%), and longer LOS. However, RIW and subsequent hospital use were not found to be significant.
| Variable | Goals of Care Discussion Documented in Medical Chart | ||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Physician orders, n (%) | 0.001 | ||||
| Comfort measures only | 0 | (0.0) | 5 | (6.7) | |
| No cardiopulmonary resuscitation | 69 | (55.2) | 60 | (80.0) | |
| Full code | 56 | (44.8) | 10 | (13.3) | |
| Visit disposition, n (%) | 0.001 | ||||
| Long‐term care home | 124 | (99.2) | 52 | (69.3) | |
| Died | 1 | (0.8) | 22 | (29.3) | |
| Transfer to palliative care facility | 0 | (0.0) | 1 | (1.3) | |
| Resource intensity weight, n (%) | 0.43 | ||||
| 0.250.75 | 35 | (28.0) | 19 | (25.3) | |
| 0.761.14 | 29 | (23.2) | 16 | (21.3) | |
| 1.151.60 | 34 | (27.2) | 16 | (21.3) | |
| 1.6125.5 | 27 | (21.6) | 24 | (32.0) | |
| Length of stay, d, n (%) | 0.01 | ||||
| 0.672.97 | 30 | (24.0) | 20 | (26.7) | |
| 2.984.60 | 40 | (32.0) | 10 | (13.3) | |
| 4.618.65 | 30 | (24.0) | 20 | (26.7) | |
| 8.66+ | 25 | (20.0) | 25 | (33.3) | |
| Subsequent emergency department visits in next year, n (% of applicable) | 0.38 | ||||
| 0 | 66 | (53.2) | 32 | (61.5) | |
| 1 | 30 | (24.2) | 13 | (25.0) | |
| 2+ | 28 | (22.6) | 7 | (13.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Subsequent hospitalizations in next year, n (% of applicable) | 0.87 | ||||
| 0 | 87 | (70.2) | 38 | (73.1) | |
| 1 | 24 | (19.4) | 10 | (19.2) | |
| 2+ | 13 | (10.5) | 4 | (7.7) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| 1‐year mortality, n (% of applicable) | 0.001 | ||||
| Alive | 82 | (71.9) | 15 | (37.5) | |
| Dead | 32 | (28.1) | 25 | (62.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Not applicable (unsuccessful follow‐up with long‐term care home) | 10 | 12 | |||
Predictors of In‐hospital Death and 1‐Year Mortality
Given the significant positive associations between discussions and in‐hospital death and 1‐year mortality, we performed separate logistic regression analyses to test whether discussions independently predicted in‐hospital death and 1‐year mortality (Table 4). After adjusting for variables significant in their respective bivariate analyses, patients with discussions continued to have higher odds of in‐hospital death (AOR: 52.0 [95% CI: 6.2‐440.4]) and 1‐year mortality (AOR: 4.1 [95% CI: 1.7‐9.6]). Of note, the presence of dementia had significantly lower adjusted odds of in‐hospital death compared to the reference group of no dementia (AOR: 0.3 [95% CI: 0.1‐0.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| In‐hospital death odds ratios | |||
| Advance directives from long‐term care | 0.91 | ||
| Comfort measures only | Reference | ||
| Supportive care no transfer | 3.43E +18 | 0‐. | 1.00 |
| Transfer to hospital | 3.10E +8 | 0‐. | 1.00 |
| Aggressive care | 4.85E +8 | 0‐. | 1.00 |
| Dementia | |||
| No | Reference | ||
| Yes | .25 | 0.08‐0.79 | 0.02 |
| Previous hospitalizations in last year | 0.05 | ||
| 0 | Reference | ||
| 1 | 0.43 | 0.08‐2.38 | 0.34 |
| 2+ | 6.30 | 1.10‐36.06 | 0.04 |
| Respiration | |||
| 20 | Reference | ||
| 20 | 3.64 | 0.82‐16.24 | 0.09 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 52.04 | 6.15‐440.40 | 0.001 |
| 1‐year mortality odds ratios | |||
| Oxygen saturation, n (%) | |||
| 88 | 12.15 | 1.18‐124.97 | 0.04 |
| 88 | Reference | ||
| Previous ED visits in last year | 0.06 | ||
| 0 | Reference | ||
| 1 | 3.07 | 1.15‐8.17 | 0.03 |
| 2+ | 3.21 | 0.87‐11.81 | 0.08 |
| Previous hospitalizations in last year | 0.55 | ||
| 0 | Reference | ||
| 1 | 1.66 | 0.57‐4.86 | 0.36 |
| 2+ | 2.52 | 0.30‐20.89 | 0.39 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 4.07 | 1.73‐9.56 | 0.001 |
DISCUSSION
Our retrospective study of LTC residents admitted to the GIM service showed that these admissions comprised 9.4% of all admissions and that GOC discussions occurred infrequently (37.5%). Our study revealed no differences in baseline patient characteristics associated with discussions, whereas patient acuity at hospital presentation independently contributed to the likelihood of discussions. We found strong associations between documentation and certain outcomes of care, including orders for AD, LOS, in‐hospital death, and 1‐year mortality. No significant associations were found between documentation and subsequent hospital use. Lastly, we found that consistent communication back to the LTC home when there was a change in AD was very poor; only 26% of discharge summaries included this documentation.
Our finding of infrequent GOC discussions during hospitalization aligns with prior studies. A study that identified code status discussions in transcripts of audio‐recorded admission encounters found that code status was discussed in only 24% of seriously ill patient admissions.[17] Furthermore, in a study specific to LTC residents, only 42% of admissions longer than 48 hours had a documented GOC discussion.[15]
We found visit‐level, but not baseline, characteristics were associated with discussions. These findings are supported by a recent study that found that whether GOC discussions took place largely depended on the acute condition presented on admission.[15] Although these results suggest that clinicians are appropriately prioritizing sicker patients who might have the most pressing need for GOC discussions, they also highlight the gap in care for less‐sick patients and the need to broaden clinical practice and consider underlying conditions and functional status. Of note, although the GCS score was found to be significantly associated with discussions, patients in the lowest GCS range did not have significantly different odds of discussions compared to the reference level (highest GCS range). A recent study by You et al. may offer some insight into this finding. They found that patients lacking capacity to make GOC decisions was ranked fifth, whereas lack of SDM availability was eighth among 21 barriers to GOC discussions, as perceived by hospital‐based clinicians.[16]
A major finding of this study was that both in‐hospital and 1‐year mortality were strongly associated with having a GOC discussion, suggesting that patients at higher risk of dying are more likely to have discussions. This is reflected by illness severity measured at initial assessment and by persistence of the association between discussions and mortality after discharge back to LTC. To the best of our knowledge, no previous studies have reported these findings. There are likely some unmeasured clinical factors such as clinical deterioration during hospitalization that contributed to this strong association. Interestingly, in our logistic regression analysis for independent predictors of in‐hospital death, we found that having dementia was associated with lower odds of in‐hospital death. One interpretation of this finding is that perhaps only patients with mild dementia were hospitalized, and those with more advanced dementia had an AD established in LTC that allowed them to remain in their LTC home. This possibility is supported by a systematic review of factors associated with LTC home hospitalization, which found that dementia was shown to be associated with less hospitalization.[18]
For patients who survived hospitalization, we did not find an association between GOC discussions and hospital use in the year following index hospitalization. In both groups, nearly 30% of patients had 1 or more subsequent hospitalizations. This is relevant especially in light of the finding that among patients where GOC discussions resulted in an AD change, only 26% of discharge summaries back to LTC included this documentation. We can only speculate that had these discussions been properly documented, subsequent hospitalizations would have decreased in the GOC group. Previous research has found that omissions of critical information in discharge summaries were common. In a study of hip fracture and stroke patients discharged from a large Midwestern academic medical center in the United States, code status was included in the discharge summary only 7% of the time.[19] The discharge summary is the primary means of sharing patient information between the hospital and LTC home. If GOC discussions are not included in the discharge summary, it is very unlikely that this information will be subsequently updated in the LTC medical record and impact the care the patient receives. A key recommendation for hospital‐based providers is ensuring that GOC discussions are clearly, consistently, and completely documented in the discharge summary so that the care provided is based on the patients' wishes.
Our study has several limitations. Our analysis was based on chart review, and although our analyses take into account a number of patient characteristics, we did not capture other characteristics that might influence GOC discussions such as culture/religion, language barriers, SDM availability, or whether patients clinically deteriorated during the index admission. Additionally, provider‐level predictors, including seniority, previous GOC training, and time available to conduct these discussions, were not captured. We also did not capture the timing or number of occasions that GOC discussions took place during hospitalization. Due to the retrospective nature of our study, we were able to only look at documented GOC discussions. GOC discussions may have happened but were never documented. However, the standard of care is to document these discussions as part of the medical record, and if they are not documented, it can be considered not to have happened and indicates a lower quality of practice. A recent survey of Canadian hospital‐based healthcare providers identified standardized GOC documentation as an effective practice to improve GOC communication.[20] Finally, because our study was conducted in 2 academic hospitals, our results may be less generalizable to other community hospitals. However, our hospitals' catchment areas capture a diverse population, both culturally and in terms of their socioeconomic status.
CONCLUSION
GOC discussions occurred infrequently, appeared to be triggered by illness severity, and were poorly communicated back to LTC. Important outcomes of care, including in‐hospital death and 1‐year mortality, were associated with discussions. This study serves to identify gaps in who might benefit from GOC discussions and illustrates opportunities for improvement including implementing standardized documentation practices.
Disclosures
Hannah J. Wong, PhD, and Robert C. Wu, MD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Robert C. Wu, MD, Hannah J. Wong, PhD, and Michelle Grinman, MD, were responsible for the conception and design of the study. Robert C. Wu, MD, Hannah J. Wong, PhD, and Jamie Wang were responsible for the acquisition of the data. All of the authors were responsible for the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. Hannah J. Wong, PhD obtained the funding. Hannah J. Wong, PhD, and Robert C. Wu, MD, supervised the study. The authors report no conflicts of interest.
Hospitalizations of long‐term care (LTC) residents are known to be frequent, costly, often preventable,[1, 2, 3] and potentially associated with negative health outcomes.[4] Often, an advance directive (AD) is made at LTC admission and updated annually when residents are in relatively stable health. An AD is a document that helps to inform a substitute decision maker (SDM) about the consent process for life‐sustaining treatments and is a resource that supports advance care planning (ACP). ACP is a process that allows individuals to consider, express, and plan for future healthcare in the event that they lack capacity to make their own decisions. When an LTC resident's health deteriorates and hospitalization is required, there is an opportunity to update prognosis, discuss risks and benefits of previously held treatment preferences, as well as reassess goals of care (GOC).
Engaging in ACP discussions during relatively stable health can help ensure patient preferences are followed.[5, 6] These discussions, however, are often insufficient, as they involve decision making for hypothetical situations that may not cover all potential scenarios, and may not reflect a patient's reality at the time of health status decline. Discussions held in the moment more authentically reflect the decisions of patients and/or SDM based on the specific needs and clinical realities particular to the patient at that time.[7] GOC discussions, defined in this context as ACP discussions occurring during hospitalization, have the potential to better align patient wishes with care received,[6] improve quality of life and satisfaction,[8, 9, 10] and reduce unwanted extra care.[11, 12] Although in‐the‐moment GOC discussions are recommended for all hospitalized patients who are seriously ill with a high risk of dying,[13] research suggests that this occurs infrequently for elderly patients. A recent multicenter survey of seriously ill hospitalized elderly patients found that only 25% of patients and 32% of family members reported that they had been asked about prior ACP or AD.[14] Another study of hospitalized LTC residents found that resuscitation status and family discussion was documented in only 55% and 42% of admissions, respectively.[15]
Further investigation is required to determine how often LTC patients have GOC discussions, what prompts these discussions, and what are the outcomes. Previous studies have focused on barriers to performing GOC discussions, rather than the factors that are associated with them.[16] By understanding why these discussions currently happen, we can potentially improve how often they occur and the quality of their outcomes.
The objectives of this study were to determine the rate of documented GOC discussions among hospitalized LTC residents, identify factors that were associated with documentation, and examine the association between documentation and outcomes of care.
METHODS
Study Population
We conducted a retrospective chart review of a random convenience sample of hospitalized patients admitted via the emergency department (ED) to the general internal medicine (GIM) service from January 1, 2012 through December 31, 2012, at 2 academic teaching hospitals in Toronto, Canada. Patients were identified through a search of each hospitals' electronic patient record (EPR). Patients were eligible for inclusion if they were (1) a LTC resident and (2) at least 65 years of age. For patients with multiple admissions to the GIM service during the specified 12‐month period, we only included data from the first hospitalization (index hospitalization). The hospital's research ethics board approved this study.
Our primary variable of interest was documentation in the hospital medical record of a discussion between physicians and the patient/family/SDM regarding GOC. A GOC discussion was considered to have taken place if there was documentation of (1) understanding/expectation of treatment options or (2) patient's preferences for life‐sustaining measures. Examples illustrating each criterion are provided in the Supporting Information, Appendix 1, in the online version of this article.
Factors Associated With GOC Documentation
From the EPR, we obtained visit‐level data including age, gender, Canadian Emergency Department Triage and Acuity Scale, vital signs at ED admission including temperature, respiratory rate, oxygen saturation, Glasgow Coma Scale (GCS) and shock index (defined as heart rate divided by systolic blood pressure), admission and discharge dates/times, discharge diagnosis, transfer to intensive care unit (ICU), and hospital use (number of ED visits and hospitalizations to the 2 study hospitals in the 1‐year period prior to index hospitalization).
Trained study personnel (J.W.) used a structured abstraction form to collect data from the hospital medical record that were not available through the EPR, including years living in LTC, contents of LTC AD forms, presence of SDM (identified as immediate family or surrogate with whom the care team communicated), dementia diagnosis (defined as documentation of dementia in the patient's past medical history and/or history of present illness), and measures of functional status. When available, we extracted the AD from LTC; they consisted of 4 levels (level 1: comfort careno transfer to hospital, no cardiopulmonary resuscitation [CPR]; level 2: supportive careadministration of antibiotics and/or other procedures that can be provided within LTC, no transfer to the hospital, no CPR; level 3: transfer to the hospitalno CPR; level 4: aggressive interventiontransfer to hospital for aggressive treatment, CPR).
GOC Documentation in the Discharge Summary
For the subset of patients who survived hospitalization and were discharged back to LTC, we examined whether the ADs ordered during hospitalization were communicated back to LTC via the discharge summary. We additionally assessed if the ADs determined during hospitalization differed from preferences documented prior to hospitalization. Physician orders for ADs were categorized as level 1: comfort measures only, level 3: no CPR, or level 4: full code. LTC level 2 was considered equivalent to physician‐ordered level 3 at admission; a patient with an LTC level 2 with no CPR (level 3) documented during hospitalized would be considered to have no change in the AD. An increase or decrease in the AD was determined by comparing LTC levels 1, 3, and 4 to physician‐ordered level 1, 3, and 4.
Outcomes of GOC Documentation
From the EPR, we obtained visit‐level outcome data including length of stay (LOS), resource intensity weight (RIW) (calculated based on patient case‐mix, severity, age, and procedures performed), visit disposition, number of ED visits and hospitalizations to the 2 study hospitals in the year following index hospitalization, in‐hospital death, and 1‐year mortality. We determined 1‐year mortality by following up with the LTC homes to determine whether the resident had died within the year following index hospitalization; only patients from LTC homes that responded to our request for data were included in 1‐year mortality analyses. We collected physician orders for the AD from chart review.
Statistical Analysis
Patients with and without documented GOC discussions were compared. Descriptive statistics including frequencies and percentages were used to characterize study variables. Differences between the study groups were assessed using Pearson 2/Fisher exact test. Multivariate logistic regression, which included variables that were significant in the bivariate analysis, was used to identify independent predictors of GOC discussion. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were presented for the logistic model. Patients with missing predictor data were excluded.
We also examined whether there was a correlation between GOC discussion and outcomes of care using Pearson 2/Fisher exact test. Outcomes included orders for the AD, LOS in days (stratified into quartiles), RIW (stratified into quartiles), visit disposition, hospital use in the year following index hospitalization, and 1‐year mortality following discharge back to LTC.
Lastly, to better understand the independent predictors of in‐hospital and 1‐year mortality, we used Pearson 2/Fisher exact test followed by logistic regression that included significant variables from the bivariate analyses.
All analyses were 2‐sided, and a P value of 0.05 was considered statistically significant. We used SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
We identified a total of 7084 hospitalizations to GIM between January 1, 2012 and December 31, 2012, of which 665 (9.4%) met inclusion criteria of residence in LTC and age 65 years. Of these 665 hospitalizations, 512 were unique patients. We randomly selected a convenience sample of 200 index hospitalizations of the 512 eligible hospitalizations (39%) to perform the chart review.
Predictors of GOC Documentation
Of the 200 randomly sampled charts that were reviewed, 75 (37.5%) had a documented GOC discussion.
Characteristics of the study patients and results of bivariate analysis of the association between patient characteristics and GOC discussion are summarized in Table 1. No significant differences in demographic and baseline characteristics were seen between patients with and without discussion. However, a number of visit characteristics were found to be significantly associated with discussion. Forty percent of patients in the GOC discussion group had GCS scores 11 compared to 15.2% in the no‐discussion group. Higher respiratory rate, lower oxygen saturation, and ICU transfer were also significantly associated with discussions.
| Goals of Care Discussion Documented in Medical Chart | |||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Baseline characteristics | |||||
| Gender, n (%) | 0.88 | ||||
| Male | 48 | (38.4) | 30 | (40.0) | |
| Female | 77 | (61.6) | 45 | (60.0) | |
| Age, y, n (%) | 0.85 | ||||
| 6579 | 36 | (28.8) | 19 | (25.3) | |
| 8084 | 30 | (24.0) | 19 | (25.3) | |
| 8589 | 30 | (24.0) | 16 | (21.3) | |
| 90101 | 29 | (23.2) | 21 | (28.0) | |
| Years living in long‐term care, n (%)* | 0.65 | ||||
| [0, 1) | 28 | (22.4) | 12 | (16.0) | |
| [1, 3) | 31 | (24.8) | 22 | (29.3) | |
| [3, 6) | 33 | (26.4) | 22 | (29.3) | |
| [6, 22) | 25 | (20.0) | 13 | (17.3) | |
| Unknown | 8 | (6.4) | 6 | (8.0) | |
| AD from long‐term care, n (%) | 0.14 | ||||
| Comfort measures only | 2 | (1.6) | 1 | (1.3) | |
| Supportive care with no transfer to hospital | 0 | (0.0) | 3 | (4.0) | |
| Supportive care with transfer to hospital | 70 | (56.0) | 44 | (58.7) | |
| Aggressive care | 53 | (42.4) | 27 | (36.0) | |
| Years since most recent AD signed, n (%)* | 0.12 | ||||
| [0, 1) | 79 | (63.2) | 48 | (64.0) | |
| [1, 2) | 21 | (16.8) | 6 | (8.0) | |
| [2, 6) | 9 | (7.2) | 10 | (13.3) | |
| Unknown | 16 | (12.8) | 11 | (14.7) | |
| Substitute decision maker, n (%) | 0.06 | ||||
| Child | 81 | (64.8) | 44 | (58.7) | |
| Spouse | 9 | (7.2) | 15 | (20.0) | |
| Other | 26 | (20.8) | 13 | (17.3) | |
| Public guardian trustee | 6 | (4.8) | 2 | (2.7) | |
| Unknown | 3 | (2.4) | 1 | (1.3) | |
| Dementia, n (%) | 1.00 | ||||
| No | 47 | (37.6) | 28 | (37.3) | |
| Yes | 78 | (62.4) | 47 | (62.7) | |
| Mobility, n (%) | 0.26 | ||||
| Walk without assistance | 5 | (4.0) | 3 | (4.0) | |
| Walker | 16 | (12.8) | 3 | (4.0) | |
| Wheelchair | 43 | (34.4) | 29 | (38.7) | |
| Bedridden | 7 | (5.6) | 4 | (5.3) | |
| Unknown | 54 | (43.2) | 36 | (48.0) | |
| Continence, n (%) | 0.05 | ||||
| Mostly continent | 16 | (12.8) | 3 | (4.0) | |
| Incontinent | 49 | (39.2) | 34 | (45.3) | |
| Catheter/stoma | 7 | (5.6) | 1 | (1.3) | |
| Unknown | 53 | (42.4) | 37 | (49.3) | |
| Feeding, n (%) | 0.17 | ||||
| Mostly feeds self | 38 | (30.4) | 13 | (17.3) | |
| Needs to be fed | 17 | (13.6) | 14 | (18.7) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 62 | (49.6) | 43 | (57.3) | |
| Diet, n (%) | 0.68 | ||||
| Normal | 43 | (34.4) | 16 | (21.3) | |
| Dysphagic | 32 | (25.6) | 15 | (20.0) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 42 | (33.6) | 39 | (52.0) | |
| Previous ED visits in last year, n (%) | 0.43 | ||||
| 0 | 70 | (56.0) | 41 | (54.7) | |
| 1 | 35 | (28.0) | 17 | (22.7) | |
| 2+ | 20 | (16.0) | 17 | (22.7) | |
| Previous hospitalizations in last year, n (%) | 0.19 | ||||
| 0 | 98 | (78.4) | 54 | (72.0) | |
| 1 | 23 | (18.4) | 14 | (18.7) | |
| 2+ | 4 | (3.2) | 7 | (9.3) | |
| Visit characteristics | |||||
| Glasgow Coma Scale, n (%) | 0.001 | ||||
| 7 | 4 | (3.2) | 4 | (5.3) | |
| 711 | 15 | (12.0) | 26 | (34.7) | |
| 1213 | 7 | (5.6) | 8 | (10.7) | |
| 1415 | 85 | (68.0) | 32 | (42.7) | |
| Unknown | 14 | (11.2) | 5 | (6.7) | |
| Shock index, n (%) | 0.13 | ||||
| 1 | 105 | (84.0) | 54 | (72.0) | |
| >1 | 19 | (15.2) | 18 | (24.0) | |
| Unknown | 1 | (0.8) | 3 | (4.0) | |
| Respiratory rate, n (%) | 0.02 | ||||
| 20 | 59 | (47.2) | 21 | (28.0) | |
| 20 | 66 | (52.8) | 52 | (69.3) | |
| Unknown | 0 | (0.0) | 2 | (2.7) | |
| Oxygen saturation, n (%) | 0.03 | ||||
| 88 | 2 | (1.6) | 6 | (8.0) | |
| 88 | 122 | (97.6) | 65 | (86.7) | |
| Unknown | 1 | (0.8) | 4 | (5.3) | |
| Temperature, n (%) | 0.09 | ||||
| 38.0 | 100 | (80.0) | 51 | (68.0) | |
| 38.0 | 25 | (20.0) | 23 | (30.7) | |
| Unknown | 0 | (0.0) | 1 | (1.3) | |
| Canadian Triage and Acuity Scale, n (%) | 0.13 | ||||
| Resuscitation | 1 | (0.8) | 3 | (4.0) | |
| Emergent | 70 | (56.0) | 49 | (65.3) | |
| Urgent | 52 | (41.6) | 22 | (29.3) | |
| Less urgent and nonurgent | 2 | (1.6) | 1 | (1.3) | |
| Discharge diagnosis, n (%) | 0.29 | ||||
| Aspiration pneumonia | 12 | (9.6) | 12 | (16.0) | |
| Chronic obstructive pulmonary disease | 15 | (12.0) | 3 | (4.0) | |
| Dehydration/disorders fluid/electrolytes | 9 | (7.2) | 5 | (6.7) | |
| Gastrointestinal hemorrhage | 4 | (3.2) | 3 | (4.0) | |
| Heart failure | 11 | (8.8) | 2 | (2.7) | |
| Infection (other or not identified) | 9 | (7.2) | 9 | (12.0) | |
| Influenza/pneumonia | 14 | (11.2) | 11 | (14.7) | |
| Lower urinary tract infection | 11 | (8.8) | 6 | (8.0) | |
| Other | 40 | (32.0) | 24 | (32.0) | |
| Hospitalization included ICU stay, n (%) | 0.01 | ||||
| No | 124 | (99.2) | 69 | (92.0) | |
| Yes | 1 | (0.8) | 6 | (8.0) | |
When these 4 significant clinical and visit characteristics were tested together in a logistic regression analysis, 2 remained statistically significant (Table 2). Patients with lower GCS scores (GCS 1213 and 711) were more likely to have discussions (AOR: 4.4 [95% CI: 1.4‐13.9] and AOR: 5.9 [95% CI: 2.6‐13.2], respectively) and patients with higher respiratory rates were also more likely to have discussions (AOR: 2.3 [95% CI: 1.1‐4.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Glasgow Coma Scale | 0.001 | ||
| 7 | 1.77 | 0.33‐9.58 | 0.51 |
| 711 | 5.90 | 2.64‐13.22 | 0.001 |
| 1213 | 4.43 | 1.41‐13.91 | 0.01 |
| 1415 | Reference | ||
| Respiration | |||
| 20 | Reference | ||
| 20 | 2.32 | 1.12‐4.78 | 0.02 |
| Oxygen saturation | |||
| 88 | 3.35 | 0.55‐20.56 | 0.19 |
| 88 | Reference | 0.05‐1.83 | |
| Hospitalization included ICU stay | |||
| No | Reference | ||
| Yes | 7.87 | 0.83‐74.73 | 0.07 |
GOC Documentation in the Discharge Summary
For the subset of patients who survived index hospitalization and were discharged back to LTC (176 patients or 88%), we also investigated whether the ADs were documented in the discharge summary back to LTC (data not shown). Of the 42 patients (23.9%) who had a change in the AD (18 patients had an AD increase in care intensity due to hospitalization; 24 had a decrease), only 11 (26%) had this AD change documented in the discharge summary.
Outcomes of GOC Documentation
A number of outcomes differed significantly between patients with and without GOC discussions in unadjusted comparisons (Table 3). Patients with discussions had higher rates of orders for no CPR (80% vs 55%) and orders for comfort measures only (7% vs 0%). They also had higher rates of in‐hospital death (29% vs 1%), 1‐year mortality (63% vs 28%), and longer LOS. However, RIW and subsequent hospital use were not found to be significant.
| Variable | Goals of Care Discussion Documented in Medical Chart | ||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Physician orders, n (%) | 0.001 | ||||
| Comfort measures only | 0 | (0.0) | 5 | (6.7) | |
| No cardiopulmonary resuscitation | 69 | (55.2) | 60 | (80.0) | |
| Full code | 56 | (44.8) | 10 | (13.3) | |
| Visit disposition, n (%) | 0.001 | ||||
| Long‐term care home | 124 | (99.2) | 52 | (69.3) | |
| Died | 1 | (0.8) | 22 | (29.3) | |
| Transfer to palliative care facility | 0 | (0.0) | 1 | (1.3) | |
| Resource intensity weight, n (%) | 0.43 | ||||
| 0.250.75 | 35 | (28.0) | 19 | (25.3) | |
| 0.761.14 | 29 | (23.2) | 16 | (21.3) | |
| 1.151.60 | 34 | (27.2) | 16 | (21.3) | |
| 1.6125.5 | 27 | (21.6) | 24 | (32.0) | |
| Length of stay, d, n (%) | 0.01 | ||||
| 0.672.97 | 30 | (24.0) | 20 | (26.7) | |
| 2.984.60 | 40 | (32.0) | 10 | (13.3) | |
| 4.618.65 | 30 | (24.0) | 20 | (26.7) | |
| 8.66+ | 25 | (20.0) | 25 | (33.3) | |
| Subsequent emergency department visits in next year, n (% of applicable) | 0.38 | ||||
| 0 | 66 | (53.2) | 32 | (61.5) | |
| 1 | 30 | (24.2) | 13 | (25.0) | |
| 2+ | 28 | (22.6) | 7 | (13.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Subsequent hospitalizations in next year, n (% of applicable) | 0.87 | ||||
| 0 | 87 | (70.2) | 38 | (73.1) | |
| 1 | 24 | (19.4) | 10 | (19.2) | |
| 2+ | 13 | (10.5) | 4 | (7.7) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| 1‐year mortality, n (% of applicable) | 0.001 | ||||
| Alive | 82 | (71.9) | 15 | (37.5) | |
| Dead | 32 | (28.1) | 25 | (62.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Not applicable (unsuccessful follow‐up with long‐term care home) | 10 | 12 | |||
Predictors of In‐hospital Death and 1‐Year Mortality
Given the significant positive associations between discussions and in‐hospital death and 1‐year mortality, we performed separate logistic regression analyses to test whether discussions independently predicted in‐hospital death and 1‐year mortality (Table 4). After adjusting for variables significant in their respective bivariate analyses, patients with discussions continued to have higher odds of in‐hospital death (AOR: 52.0 [95% CI: 6.2‐440.4]) and 1‐year mortality (AOR: 4.1 [95% CI: 1.7‐9.6]). Of note, the presence of dementia had significantly lower adjusted odds of in‐hospital death compared to the reference group of no dementia (AOR: 0.3 [95% CI: 0.1‐0.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| In‐hospital death odds ratios | |||
| Advance directives from long‐term care | 0.91 | ||
| Comfort measures only | Reference | ||
| Supportive care no transfer | 3.43E +18 | 0‐. | 1.00 |
| Transfer to hospital | 3.10E +8 | 0‐. | 1.00 |
| Aggressive care | 4.85E +8 | 0‐. | 1.00 |
| Dementia | |||
| No | Reference | ||
| Yes | .25 | 0.08‐0.79 | 0.02 |
| Previous hospitalizations in last year | 0.05 | ||
| 0 | Reference | ||
| 1 | 0.43 | 0.08‐2.38 | 0.34 |
| 2+ | 6.30 | 1.10‐36.06 | 0.04 |
| Respiration | |||
| 20 | Reference | ||
| 20 | 3.64 | 0.82‐16.24 | 0.09 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 52.04 | 6.15‐440.40 | 0.001 |
| 1‐year mortality odds ratios | |||
| Oxygen saturation, n (%) | |||
| 88 | 12.15 | 1.18‐124.97 | 0.04 |
| 88 | Reference | ||
| Previous ED visits in last year | 0.06 | ||
| 0 | Reference | ||
| 1 | 3.07 | 1.15‐8.17 | 0.03 |
| 2+ | 3.21 | 0.87‐11.81 | 0.08 |
| Previous hospitalizations in last year | 0.55 | ||
| 0 | Reference | ||
| 1 | 1.66 | 0.57‐4.86 | 0.36 |
| 2+ | 2.52 | 0.30‐20.89 | 0.39 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 4.07 | 1.73‐9.56 | 0.001 |
DISCUSSION
Our retrospective study of LTC residents admitted to the GIM service showed that these admissions comprised 9.4% of all admissions and that GOC discussions occurred infrequently (37.5%). Our study revealed no differences in baseline patient characteristics associated with discussions, whereas patient acuity at hospital presentation independently contributed to the likelihood of discussions. We found strong associations between documentation and certain outcomes of care, including orders for AD, LOS, in‐hospital death, and 1‐year mortality. No significant associations were found between documentation and subsequent hospital use. Lastly, we found that consistent communication back to the LTC home when there was a change in AD was very poor; only 26% of discharge summaries included this documentation.
Our finding of infrequent GOC discussions during hospitalization aligns with prior studies. A study that identified code status discussions in transcripts of audio‐recorded admission encounters found that code status was discussed in only 24% of seriously ill patient admissions.[17] Furthermore, in a study specific to LTC residents, only 42% of admissions longer than 48 hours had a documented GOC discussion.[15]
We found visit‐level, but not baseline, characteristics were associated with discussions. These findings are supported by a recent study that found that whether GOC discussions took place largely depended on the acute condition presented on admission.[15] Although these results suggest that clinicians are appropriately prioritizing sicker patients who might have the most pressing need for GOC discussions, they also highlight the gap in care for less‐sick patients and the need to broaden clinical practice and consider underlying conditions and functional status. Of note, although the GCS score was found to be significantly associated with discussions, patients in the lowest GCS range did not have significantly different odds of discussions compared to the reference level (highest GCS range). A recent study by You et al. may offer some insight into this finding. They found that patients lacking capacity to make GOC decisions was ranked fifth, whereas lack of SDM availability was eighth among 21 barriers to GOC discussions, as perceived by hospital‐based clinicians.[16]
A major finding of this study was that both in‐hospital and 1‐year mortality were strongly associated with having a GOC discussion, suggesting that patients at higher risk of dying are more likely to have discussions. This is reflected by illness severity measured at initial assessment and by persistence of the association between discussions and mortality after discharge back to LTC. To the best of our knowledge, no previous studies have reported these findings. There are likely some unmeasured clinical factors such as clinical deterioration during hospitalization that contributed to this strong association. Interestingly, in our logistic regression analysis for independent predictors of in‐hospital death, we found that having dementia was associated with lower odds of in‐hospital death. One interpretation of this finding is that perhaps only patients with mild dementia were hospitalized, and those with more advanced dementia had an AD established in LTC that allowed them to remain in their LTC home. This possibility is supported by a systematic review of factors associated with LTC home hospitalization, which found that dementia was shown to be associated with less hospitalization.[18]
For patients who survived hospitalization, we did not find an association between GOC discussions and hospital use in the year following index hospitalization. In both groups, nearly 30% of patients had 1 or more subsequent hospitalizations. This is relevant especially in light of the finding that among patients where GOC discussions resulted in an AD change, only 26% of discharge summaries back to LTC included this documentation. We can only speculate that had these discussions been properly documented, subsequent hospitalizations would have decreased in the GOC group. Previous research has found that omissions of critical information in discharge summaries were common. In a study of hip fracture and stroke patients discharged from a large Midwestern academic medical center in the United States, code status was included in the discharge summary only 7% of the time.[19] The discharge summary is the primary means of sharing patient information between the hospital and LTC home. If GOC discussions are not included in the discharge summary, it is very unlikely that this information will be subsequently updated in the LTC medical record and impact the care the patient receives. A key recommendation for hospital‐based providers is ensuring that GOC discussions are clearly, consistently, and completely documented in the discharge summary so that the care provided is based on the patients' wishes.
Our study has several limitations. Our analysis was based on chart review, and although our analyses take into account a number of patient characteristics, we did not capture other characteristics that might influence GOC discussions such as culture/religion, language barriers, SDM availability, or whether patients clinically deteriorated during the index admission. Additionally, provider‐level predictors, including seniority, previous GOC training, and time available to conduct these discussions, were not captured. We also did not capture the timing or number of occasions that GOC discussions took place during hospitalization. Due to the retrospective nature of our study, we were able to only look at documented GOC discussions. GOC discussions may have happened but were never documented. However, the standard of care is to document these discussions as part of the medical record, and if they are not documented, it can be considered not to have happened and indicates a lower quality of practice. A recent survey of Canadian hospital‐based healthcare providers identified standardized GOC documentation as an effective practice to improve GOC communication.[20] Finally, because our study was conducted in 2 academic hospitals, our results may be less generalizable to other community hospitals. However, our hospitals' catchment areas capture a diverse population, both culturally and in terms of their socioeconomic status.
CONCLUSION
GOC discussions occurred infrequently, appeared to be triggered by illness severity, and were poorly communicated back to LTC. Important outcomes of care, including in‐hospital death and 1‐year mortality, were associated with discussions. This study serves to identify gaps in who might benefit from GOC discussions and illustrates opportunities for improvement including implementing standardized documentation practices.
Disclosures
Hannah J. Wong, PhD, and Robert C. Wu, MD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Robert C. Wu, MD, Hannah J. Wong, PhD, and Michelle Grinman, MD, were responsible for the conception and design of the study. Robert C. Wu, MD, Hannah J. Wong, PhD, and Jamie Wang were responsible for the acquisition of the data. All of the authors were responsible for the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. Hannah J. Wong, PhD obtained the funding. Hannah J. Wong, PhD, and Robert C. Wu, MD, supervised the study. The authors report no conflicts of interest.
- , , , , . Trends in emergency department visits for ambulatory care sensitive conditions by elderly nursing home residents, 2001 to 2010. JAMA Intern Med. 2014;174(1):156–158.
- , , , . Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909.
- , , , , . Potentially avoidable hospitalizations for elderly long‐stay residents in nursing homes. Med Care. 2013;51(8):673–681.
- , . Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , , , . The consistency between treatments provided to nursing facility residents and orders on the physician orders for life‐sustaining treatment form. J Am Geriatr Soc. 2011;59(11):2091–2099.
- , , . What should be the goal of advance care planning? JAMA Intern Med. 2014;174(7):1093–1094.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444.
- , . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , . Predictors of nursing home residents' time to hospitalization. Health Serv Res. 2011;46(1 pt 1):82–104.
- , , , . Regional variation in the association between advance directives and end‐of‐life Medicare expenditures. JAMA. 2011;306(13):1447–1453.
- , , . Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014;186(6):425–432.
- , , , et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778–787.
- , , , . Hospitalisation of high‐care residents of aged care facilities: are goals of care discussed? Intern Med J. 2013;43(2):144–149.
- , , , et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549–556.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , . Strategies for effective goals of care discussions and decision‐making: perspectives from a multi‐centre survey of Canadian hospital‐based healthcare providers. BMC Palliat Care. 2015;14:38.
- , , , , . Trends in emergency department visits for ambulatory care sensitive conditions by elderly nursing home residents, 2001 to 2010. JAMA Intern Med. 2014;174(1):156–158.
- , , , . Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909.
- , , , , . Potentially avoidable hospitalizations for elderly long‐stay residents in nursing homes. Med Care. 2013;51(8):673–681.
- , . Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , , , . The consistency between treatments provided to nursing facility residents and orders on the physician orders for life‐sustaining treatment form. J Am Geriatr Soc. 2011;59(11):2091–2099.
- , , . What should be the goal of advance care planning? JAMA Intern Med. 2014;174(7):1093–1094.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444.
- , . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , . Predictors of nursing home residents' time to hospitalization. Health Serv Res. 2011;46(1 pt 1):82–104.
- , , , . Regional variation in the association between advance directives and end‐of‐life Medicare expenditures. JAMA. 2011;306(13):1447–1453.
- , , . Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014;186(6):425–432.
- , , , et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778–787.
- , , , . Hospitalisation of high‐care residents of aged care facilities: are goals of care discussed? Intern Med J. 2013;43(2):144–149.
- , , , et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549–556.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , . Strategies for effective goals of care discussions and decision‐making: perspectives from a multi‐centre survey of Canadian hospital‐based healthcare providers. BMC Palliat Care. 2015;14:38.
New data shed light on impact of resecting the primary tumor in stage IV breast cancer
CHICAGO – The survival impact of resecting the primary tumor in women with de novo stage IV breast cancer depends on receipt of and response to prior systemic therapy, suggested a pair of studies reported at the annual meeting of the American Society of Clinical Oncology.
A randomized trial conducted in Turkey found that, relative to peers who received initial systemic therapy, women who underwent initial resection of the primary tumor had a one-third lower risk of death at 5 years. But a prospective registry study conducted in the United States found that elective resection after a response to first-line therapy did not significantly improve overall survival, with patients living roughly 6 years regardless of whether they had the surgery or not.
Findings in context
“I think these studies have just confirmed what we know, and that is that tumor biology is critical,” said invited discussant Elizabeth A. Mittendorf, MD, PhD, of University of Texas MD Anderson Cancer Center, Houston. “Patients who do not respond to systemic therapy will do poorly, so I don’t think it’s unwise to consider a biologic ‘stress test’ with initiation of first-line therapy, knowing that patients who do not respond will not benefit from surgery.”
Those with hormone receptor–positive or HER2-positive disease are most likely to benefit from targeted therapy and may see even higher response rates as novel targeted agents are introduced. “But despite the increase in response to therapy, we really have no data at this time to suggest any benefit from surgery,” she added. “There may be some utility in continuing to enroll these patients in a clinical trial. I would suggest that it would need to be a subtype-specific trial and would question whether we have the appetite to conduct such a study.”
More information on managing de novo stage IV breast cancer is expected from ongoing trials such as the Eastern Cooperative Oncology Group’s 2108 trial, which is randomizing patients having a response or stable disease with first-line therapy to either early local therapy or delayed local therapy only at the time of progression, according to Dr. Mittendorf.
Poor accrual necessitated redesign of the trial. “As part of that redesign, there was a decrease in the target enrollment, which causes me concern that the trial will not be powered to inform its primary endpoint of overall survival,” she commented. However, “it’s interesting to note that in early 2014, shortly after the report of the trials from India and Turkey at the San Antonio Breast Cancer Symposium, there was a significant increase in enrollment, suggesting that this is a clinically important question.”
Turkish study: MF07-01
The first study – trial MF07-01 of the Turkish Federation of Breast Diseases Societies – was presented by Atilla Soran, MD, of Magee-Womens Hospital of University of Pittsburgh Medical Center.
He and his colleagues enrolled in the trial women with de novo stage IV breast cancer whose primary tumor was amenable to complete surgical resection and who were healthy enough to be treated.
The women were randomized evenly either to initial systemic therapy followed by local therapy only if local progression occurred, or to initial local therapy, consisting of surgery with or without radiation therapy of the breast and axilla, followed by systemic therapy.
Among the 274 evaluable women having a median follow-up of about 40 months, the 3-year rate of overall survival did not differ significantly between the two groups, Dr. Soran reported.
However, the 5-year rate of overall survival was 41.6% in the initial surgery group and 24.4% in the initial systemic therapy group, a difference translating to a significant reduction in the risk of death (hazard ratio, 0.66; P = .005). Median survival was 46 months and 37 months, respectively.
The benefit was similar in women whose tumors had hormone receptors, whose tumors were negative for HER2, and who were younger than age 55. There was no significant benefit of up-front surgery for women with bone-only metastases, “but we believe that when we follow these patients longer, the difference is going to be statistically significant,” he said.
On the other hand, there was a trend among women who had multiple pulmonary and/or liver metastases whereby they were more likely to die if they initially had surgery instead of systemic therapy.
Locoregional progression/relapse occurred in 1% of the initial surgery group but 11% of the initial systemic therapy group. Among women who did not have locoregional progression/relapse, surgery still had a survival benefit (HR, 0.61; P = .001).
“We know that with systemic therapy, immunotherapy, radiation therapy, and imaging as developments, patients are living longer when you compare to a decade ago or 20 years ago,” said Dr. Soran. “But we also believe that there is a role for surgery of the primary tumor in those patients.”
“Performance status, age, and comorbidities must be taken into account, and the burden of metastatic disease needs to be considered,” he maintained. “The benefit of surgery at presentation is dependent on the completeness of resection, and axillary surgery and locoregional radiation therapy should be considered regardless of the metastasis.”
U.S. study: TBCRC 013
The second study – the Translational Breast Cancer Research Consortium’s study 013 – was presented by Tari A. King, MD, chief of breast surgery at the Dana-Farber Cancer Institute, associate division chief for breast surgery at Brigham and Women’s Hospital, and associate professor of surgery at Harvard Medical School, all in Boston.
The investigators analyzed data from the study’s cohort A, consisting of 112 patients with de novo stage IV breast cancer who had an intact primary tumor. All patients were given first-line systemic therapy; those who had a response were additionally offered elective resection of their primary tumor.
The median duration of follow-up was 54 months. Overall, 85% of the women had a response to their first-line therapy, Dr. King reported.
Some 43% of responders opted to undergo elective surgery to resect their primary tumor, defined as surgery performed in the absence of local symptoms or the need for local control, with specific type and extent left up to the treating physician.
In a multivariate analysis among responders surviving at least 6 months, median survival was 71 months with elective surgery and 65 months without it, a nonsignificant difference.
Findings were similar among subsets of women having estrogen receptor–positive tumors or HER2-positive tumors, and various combinations of these features.
In recursive partitioning analysis, response to first-line therapy, HER2 status, and age were the major determinants of survival.
“Importantly, although we were not able to demonstrate a survival benefit with the use of surgery, surgery also did not impact progression-free survival,” noted Dr. King.
Ultimately, 4% of responders who did not have elective surgery and 18% of nonresponders went on to have palliative resection of their primary.
“As this was a registry study, patients selected for surgery were more likely to have single-organ metastatic disease and to have received first-line chemotherapy, yet despite this selection bias, surgery did not impact survival in any tumor subtype,” Dr. King summarized. “Among patients who responded to therapy, HER2 status and patient age remained strong prognostic factors. Further investigation is needed to determine if subsets of patients will ultimately benefit from surgery.”
“In the absence of additional prospective data, our findings do not support surgery for the primary tumor outside of a clinical trial,” she concluded.
CHICAGO – The survival impact of resecting the primary tumor in women with de novo stage IV breast cancer depends on receipt of and response to prior systemic therapy, suggested a pair of studies reported at the annual meeting of the American Society of Clinical Oncology.
A randomized trial conducted in Turkey found that, relative to peers who received initial systemic therapy, women who underwent initial resection of the primary tumor had a one-third lower risk of death at 5 years. But a prospective registry study conducted in the United States found that elective resection after a response to first-line therapy did not significantly improve overall survival, with patients living roughly 6 years regardless of whether they had the surgery or not.
Findings in context
“I think these studies have just confirmed what we know, and that is that tumor biology is critical,” said invited discussant Elizabeth A. Mittendorf, MD, PhD, of University of Texas MD Anderson Cancer Center, Houston. “Patients who do not respond to systemic therapy will do poorly, so I don’t think it’s unwise to consider a biologic ‘stress test’ with initiation of first-line therapy, knowing that patients who do not respond will not benefit from surgery.”
Those with hormone receptor–positive or HER2-positive disease are most likely to benefit from targeted therapy and may see even higher response rates as novel targeted agents are introduced. “But despite the increase in response to therapy, we really have no data at this time to suggest any benefit from surgery,” she added. “There may be some utility in continuing to enroll these patients in a clinical trial. I would suggest that it would need to be a subtype-specific trial and would question whether we have the appetite to conduct such a study.”
More information on managing de novo stage IV breast cancer is expected from ongoing trials such as the Eastern Cooperative Oncology Group’s 2108 trial, which is randomizing patients having a response or stable disease with first-line therapy to either early local therapy or delayed local therapy only at the time of progression, according to Dr. Mittendorf.
Poor accrual necessitated redesign of the trial. “As part of that redesign, there was a decrease in the target enrollment, which causes me concern that the trial will not be powered to inform its primary endpoint of overall survival,” she commented. However, “it’s interesting to note that in early 2014, shortly after the report of the trials from India and Turkey at the San Antonio Breast Cancer Symposium, there was a significant increase in enrollment, suggesting that this is a clinically important question.”
Turkish study: MF07-01
The first study – trial MF07-01 of the Turkish Federation of Breast Diseases Societies – was presented by Atilla Soran, MD, of Magee-Womens Hospital of University of Pittsburgh Medical Center.
He and his colleagues enrolled in the trial women with de novo stage IV breast cancer whose primary tumor was amenable to complete surgical resection and who were healthy enough to be treated.
The women were randomized evenly either to initial systemic therapy followed by local therapy only if local progression occurred, or to initial local therapy, consisting of surgery with or without radiation therapy of the breast and axilla, followed by systemic therapy.
Among the 274 evaluable women having a median follow-up of about 40 months, the 3-year rate of overall survival did not differ significantly between the two groups, Dr. Soran reported.
However, the 5-year rate of overall survival was 41.6% in the initial surgery group and 24.4% in the initial systemic therapy group, a difference translating to a significant reduction in the risk of death (hazard ratio, 0.66; P = .005). Median survival was 46 months and 37 months, respectively.
The benefit was similar in women whose tumors had hormone receptors, whose tumors were negative for HER2, and who were younger than age 55. There was no significant benefit of up-front surgery for women with bone-only metastases, “but we believe that when we follow these patients longer, the difference is going to be statistically significant,” he said.
On the other hand, there was a trend among women who had multiple pulmonary and/or liver metastases whereby they were more likely to die if they initially had surgery instead of systemic therapy.
Locoregional progression/relapse occurred in 1% of the initial surgery group but 11% of the initial systemic therapy group. Among women who did not have locoregional progression/relapse, surgery still had a survival benefit (HR, 0.61; P = .001).
“We know that with systemic therapy, immunotherapy, radiation therapy, and imaging as developments, patients are living longer when you compare to a decade ago or 20 years ago,” said Dr. Soran. “But we also believe that there is a role for surgery of the primary tumor in those patients.”
“Performance status, age, and comorbidities must be taken into account, and the burden of metastatic disease needs to be considered,” he maintained. “The benefit of surgery at presentation is dependent on the completeness of resection, and axillary surgery and locoregional radiation therapy should be considered regardless of the metastasis.”
U.S. study: TBCRC 013
The second study – the Translational Breast Cancer Research Consortium’s study 013 – was presented by Tari A. King, MD, chief of breast surgery at the Dana-Farber Cancer Institute, associate division chief for breast surgery at Brigham and Women’s Hospital, and associate professor of surgery at Harvard Medical School, all in Boston.
The investigators analyzed data from the study’s cohort A, consisting of 112 patients with de novo stage IV breast cancer who had an intact primary tumor. All patients were given first-line systemic therapy; those who had a response were additionally offered elective resection of their primary tumor.
The median duration of follow-up was 54 months. Overall, 85% of the women had a response to their first-line therapy, Dr. King reported.
Some 43% of responders opted to undergo elective surgery to resect their primary tumor, defined as surgery performed in the absence of local symptoms or the need for local control, with specific type and extent left up to the treating physician.
In a multivariate analysis among responders surviving at least 6 months, median survival was 71 months with elective surgery and 65 months without it, a nonsignificant difference.
Findings were similar among subsets of women having estrogen receptor–positive tumors or HER2-positive tumors, and various combinations of these features.
In recursive partitioning analysis, response to first-line therapy, HER2 status, and age were the major determinants of survival.
“Importantly, although we were not able to demonstrate a survival benefit with the use of surgery, surgery also did not impact progression-free survival,” noted Dr. King.
Ultimately, 4% of responders who did not have elective surgery and 18% of nonresponders went on to have palliative resection of their primary.
“As this was a registry study, patients selected for surgery were more likely to have single-organ metastatic disease and to have received first-line chemotherapy, yet despite this selection bias, surgery did not impact survival in any tumor subtype,” Dr. King summarized. “Among patients who responded to therapy, HER2 status and patient age remained strong prognostic factors. Further investigation is needed to determine if subsets of patients will ultimately benefit from surgery.”
“In the absence of additional prospective data, our findings do not support surgery for the primary tumor outside of a clinical trial,” she concluded.
CHICAGO – The survival impact of resecting the primary tumor in women with de novo stage IV breast cancer depends on receipt of and response to prior systemic therapy, suggested a pair of studies reported at the annual meeting of the American Society of Clinical Oncology.
A randomized trial conducted in Turkey found that, relative to peers who received initial systemic therapy, women who underwent initial resection of the primary tumor had a one-third lower risk of death at 5 years. But a prospective registry study conducted in the United States found that elective resection after a response to first-line therapy did not significantly improve overall survival, with patients living roughly 6 years regardless of whether they had the surgery or not.
Findings in context
“I think these studies have just confirmed what we know, and that is that tumor biology is critical,” said invited discussant Elizabeth A. Mittendorf, MD, PhD, of University of Texas MD Anderson Cancer Center, Houston. “Patients who do not respond to systemic therapy will do poorly, so I don’t think it’s unwise to consider a biologic ‘stress test’ with initiation of first-line therapy, knowing that patients who do not respond will not benefit from surgery.”
Those with hormone receptor–positive or HER2-positive disease are most likely to benefit from targeted therapy and may see even higher response rates as novel targeted agents are introduced. “But despite the increase in response to therapy, we really have no data at this time to suggest any benefit from surgery,” she added. “There may be some utility in continuing to enroll these patients in a clinical trial. I would suggest that it would need to be a subtype-specific trial and would question whether we have the appetite to conduct such a study.”
More information on managing de novo stage IV breast cancer is expected from ongoing trials such as the Eastern Cooperative Oncology Group’s 2108 trial, which is randomizing patients having a response or stable disease with first-line therapy to either early local therapy or delayed local therapy only at the time of progression, according to Dr. Mittendorf.
Poor accrual necessitated redesign of the trial. “As part of that redesign, there was a decrease in the target enrollment, which causes me concern that the trial will not be powered to inform its primary endpoint of overall survival,” she commented. However, “it’s interesting to note that in early 2014, shortly after the report of the trials from India and Turkey at the San Antonio Breast Cancer Symposium, there was a significant increase in enrollment, suggesting that this is a clinically important question.”
Turkish study: MF07-01
The first study – trial MF07-01 of the Turkish Federation of Breast Diseases Societies – was presented by Atilla Soran, MD, of Magee-Womens Hospital of University of Pittsburgh Medical Center.
He and his colleagues enrolled in the trial women with de novo stage IV breast cancer whose primary tumor was amenable to complete surgical resection and who were healthy enough to be treated.
The women were randomized evenly either to initial systemic therapy followed by local therapy only if local progression occurred, or to initial local therapy, consisting of surgery with or without radiation therapy of the breast and axilla, followed by systemic therapy.
Among the 274 evaluable women having a median follow-up of about 40 months, the 3-year rate of overall survival did not differ significantly between the two groups, Dr. Soran reported.
However, the 5-year rate of overall survival was 41.6% in the initial surgery group and 24.4% in the initial systemic therapy group, a difference translating to a significant reduction in the risk of death (hazard ratio, 0.66; P = .005). Median survival was 46 months and 37 months, respectively.
The benefit was similar in women whose tumors had hormone receptors, whose tumors were negative for HER2, and who were younger than age 55. There was no significant benefit of up-front surgery for women with bone-only metastases, “but we believe that when we follow these patients longer, the difference is going to be statistically significant,” he said.
On the other hand, there was a trend among women who had multiple pulmonary and/or liver metastases whereby they were more likely to die if they initially had surgery instead of systemic therapy.
Locoregional progression/relapse occurred in 1% of the initial surgery group but 11% of the initial systemic therapy group. Among women who did not have locoregional progression/relapse, surgery still had a survival benefit (HR, 0.61; P = .001).
“We know that with systemic therapy, immunotherapy, radiation therapy, and imaging as developments, patients are living longer when you compare to a decade ago or 20 years ago,” said Dr. Soran. “But we also believe that there is a role for surgery of the primary tumor in those patients.”
“Performance status, age, and comorbidities must be taken into account, and the burden of metastatic disease needs to be considered,” he maintained. “The benefit of surgery at presentation is dependent on the completeness of resection, and axillary surgery and locoregional radiation therapy should be considered regardless of the metastasis.”
U.S. study: TBCRC 013
The second study – the Translational Breast Cancer Research Consortium’s study 013 – was presented by Tari A. King, MD, chief of breast surgery at the Dana-Farber Cancer Institute, associate division chief for breast surgery at Brigham and Women’s Hospital, and associate professor of surgery at Harvard Medical School, all in Boston.
The investigators analyzed data from the study’s cohort A, consisting of 112 patients with de novo stage IV breast cancer who had an intact primary tumor. All patients were given first-line systemic therapy; those who had a response were additionally offered elective resection of their primary tumor.
The median duration of follow-up was 54 months. Overall, 85% of the women had a response to their first-line therapy, Dr. King reported.
Some 43% of responders opted to undergo elective surgery to resect their primary tumor, defined as surgery performed in the absence of local symptoms or the need for local control, with specific type and extent left up to the treating physician.
In a multivariate analysis among responders surviving at least 6 months, median survival was 71 months with elective surgery and 65 months without it, a nonsignificant difference.
Findings were similar among subsets of women having estrogen receptor–positive tumors or HER2-positive tumors, and various combinations of these features.
In recursive partitioning analysis, response to first-line therapy, HER2 status, and age were the major determinants of survival.
“Importantly, although we were not able to demonstrate a survival benefit with the use of surgery, surgery also did not impact progression-free survival,” noted Dr. King.
Ultimately, 4% of responders who did not have elective surgery and 18% of nonresponders went on to have palliative resection of their primary.
“As this was a registry study, patients selected for surgery were more likely to have single-organ metastatic disease and to have received first-line chemotherapy, yet despite this selection bias, surgery did not impact survival in any tumor subtype,” Dr. King summarized. “Among patients who responded to therapy, HER2 status and patient age remained strong prognostic factors. Further investigation is needed to determine if subsets of patients will ultimately benefit from surgery.”
“In the absence of additional prospective data, our findings do not support surgery for the primary tumor outside of a clinical trial,” she concluded.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Resecting the primary tumor up front had a survival benefit, whereas resecting it after a response to systemic therapy did not.
Major finding: Compared with initial systemic therapy, initial resection of the primary tumor reduced the risk of death (HR, 0.66). But after a response to first-line therapy, median survival with elective resection was not significantly superior to that without it (71 vs. 65 months).
Data source: A randomized, controlled trial among 274 women with de novo stage IV breast cancer (MF07-01 trial) and a prospective registry study among 112 women with de novo stage IV breast cancer (TBCRC 013 study).
Disclosures: Dr. Soran disclosed that he has a consulting or advisory role with NanoVision. Dr. King disclosed that she had no relevant conflicts of interest.
New IDSA aspergillosis guidelines endorse galactomannan for diagnosis
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
New aspergillosis guidelines from the Infectious Diseases Society of America recommend serum and bronchoalveolar lavage galactomannan as a marker for the diagnosis of invasive Aspergillus in adult and pediatric patients who have hematologic malignancies or have undergone hematopoietic stem cell transplants.
Serial monitoring of serum galactomannan (GM) is also useful to monitor disease progression, therapeutic response, and prognosis in hematologic malignancy and hematopoietic stem cell transplant (HSCT) patients who have elevated baseline GM (Clin Infect Dis. 2016 Jun 29. doi: 10.1093/cid/ciw326).
Serum beta-D-glucan assays also are recommended for diagnosing invasive Aspergillus (IA) in high-risk hematologic malignancy and allogeneic HSCT patients, although these tests are not very specific for the infection.
The advice illustrates the Society’s emphasis on early diagnosis in its new guidelines, which supplant the group’s 2008 guidance. There are almost 100 recommendations covering – in depth – the management of invasive, allergic, and chronic Aspergillus infections in all their manifestations. It’s a step-by-step, how-to manual for handling the problem.
“Aspergillosis mortality rates have decreased significantly in recent years, but there is still significant mortality from the infection, and we have a ways to go. We felt that early diagnosis was key, which is why it’s such an important part of these guidelines,” said lead author Thomas Patterson, MD, chief of the Division of Infectious Diseases at the University of Texas Health Science Center, San Antonio. He highlighted the most important developments in a recent interview.
“We know a lot more since 2008 about the benefits of using biomarkers like GM in bronchoalveolar lavage samples, which could be highly useful for diagnosis. However, biomarkers have not been as well validated for biologic response and are not recommended” in most cases for monitoring how well patients are doing. Also, “biomarkers are not as useful in solid organ transplants; we discuss that” in the guidelines, Dr. Patterson said.
The society came out against routine polymerase chain reaction (PCR) testing of blood samples for diagnosis. Although there has been a lot of work on the technique, the evidence isn’t strong enough yet to establish overall clinical benefit, but there is emerging evidence for the diagnostic use of PCR in conjunction with radiologic findings.
For treatment, voriconazole remains the go-to drug, but the guidelines make room for more recently approved therapies. “We now have isavuconazole, which may be better tolerated,” but it’s recommended only as an alternative to voriconazole because evidence comes mostly from a single clinical trial, he said.
Posaconazole extended-release tablets are strongly recommended as prophylaxis based on high-quality evidence from studies in neutropenic patients. Posaconazole extended-release tablets result in significantly higher antifungal blood levels than those seen with voriconazole, and “it certainly has been useful in some patients”; however, posaconazole is not approved for primary therapy in the United States, Dr. Patterson said.
A large clinical trial that tested voriconazole plus an echinocandin against voriconazole alone found that in patients diagnosed using serum galactomannan – especially those with hematologic malignancies – outcomes were better with the combination. “The panel felt combinations could be considered in some patients” but didn’t recommend them for routine use because [again,] there’s not strong evidence,” he said.
For now, it seems that higher-risk patients with hematologic malignancies and those with more widespread disease might be the ones who benefit most from combination therapy.
“We also discussed allergic and saprophytic diseases. We know that some patients with allergic bronchopulmonary aspergillosis will respond to antifungal therapy, and perhaps reduce their need for steroids, so that’s now part of the suggestions, as well,” he said.
The IDSA funded the work. Dr. Patterson receives research funding from Astellas, Merck, and Revolution Medicines, and has been an adviser to numerous drug companies.
FROM CLINICAL INFECTIOUS DISEASES