User login
Wanted: Better evidence on fast-track lung resection
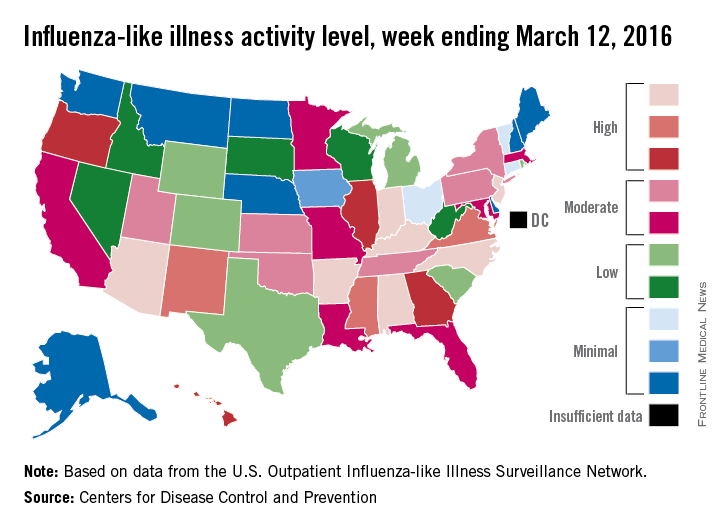
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”

|
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”

|
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”

|
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
Key clinical point: Well-designed clinical trials are needed to determine the effectiveness of fast-track recovery pathways in lung resection.
Major finding: Fast-track lung resection patients showed no differences in readmissions, overall complication and death rates compared to patients subjected to a traditional recovery course.
Data source: Systematic review of six studies published from 1997 to 2012 that involved 1,612 individuals who had lung resection.
Disclosures: The study authors had no financial relationships to disclose.
Antisclerostin osteoporosis drugs might worsen or unmask rheumatoid arthritis
Antisclerostin monoclonal antibodies have shown their ability to increase bone density in phase II and III trials of men and women with osteoporosis but could potentially have the opposite effect in patients with rheumatoid arthritis or other chronic inflammatory diseases in which tumor necrosis factor–alpha (TNF-alpha) plays an important role, according to new research.
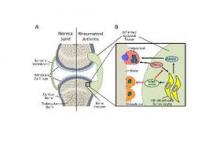
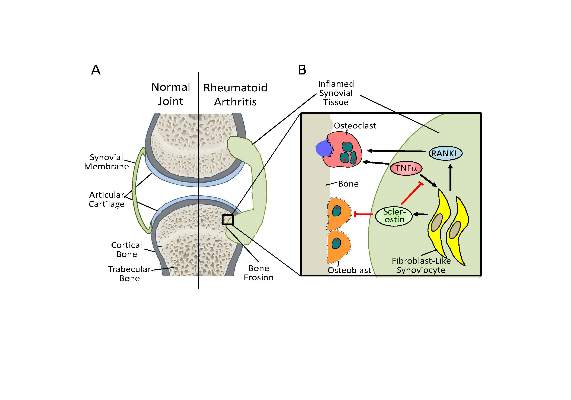
The new work, conducted by Corinna Wehmeyer, Ph.D., of the Institute of Experimental Musculoskeletal Medicine at University Hospital Muenster (Germany) and her colleagues, shows that the bone formation–inhibiting protein sclerostin is not expressed in bone only, as was previously thought, but is also expressed on the synovial cells of patients with rheumatoid arthritis (RA).
Dr. Wehmeyer and her associates were surprised to find that inhibiting sclerostin in a human TNF-alpha transgenic mouse model of RA actually accelerated joint damage rather than prevented it, suggesting that sclerostin actually had a protective role in the presence of chronic TNF-alpha–mediated inflammation. They confirmed this by demonstrating that sclerostin inhibited TNF-alpha signaling in fibroblast-like synoviocytes and showing that blocking sclerostin caused less or little worsening of bone erosions in mouse models of RA that are more dependent on a robust T and B cell response accompanied by high cytokine expression within the joint, rather than damage driven by TNF-alpha.
“These findings strongly suggest that in chronic TNF-alpha–mediated inflammation, sclerostin expression is upregulated as part of an attempt to reestablish bone homeostasis, where it exerts protective functions,” the authors wrote (Sci Transl Med. 2016 Mar 16;8:330ra34. doi: 10.1126/scitranslmed.aac4351).
The research needs confirmation in humans with RA and potentially in other chronic inflammatory diseases in which TNF-alpha plays an important role. “Nevertheless, the preliminary data in three different models indicate that sclerostin antibody therapy could be contraindicated in patients with chronic TNF-alpha–dependent inflammatory conditions. The possibility of adverse pathological effects means that caution should be taken both when considering such treatment in RA or in patients with chronic TNF-alpha–dependent comorbidities. Thus, to translate these findings to patients, first strategies to use sclerostin inhibition should exclude inflammatory comorbidities and very thoroughly monitor inflammatory events in patients to which such therapies are applied,” the researchers advised.
In an editorial, Dr. Frank Rauch of McGill University, Montreal, and Dr. Rick Adachi of the department of rheumatology at McMaster University, Hamilton, Ont., wrote that antisclerostin “treatment might accelerate joint destruction, at least when the inflammatory process is not quelled first. Patients with established RA usually undergo anti-inflammatory treatment, and it is unclear whether sclerostin inactivation would be detrimental in this context. Mouse data suggest that antisclerostin treatment might bring about regression of bone erosions when combined with TNF-alpha inhibition. The new work mirrors the situation of patients who have unrecognized RA while on antisclerostin therapy or who develop RA while receiving this treatment” (Sci Transl Med. 2016 Mar 16;8:330fs7. doi: 10.1126/scitranslmed.aaf4628).
Antisclerostin antibodies in trials
Trials of the antisclerostin monoclonal antibodies romosozumab and blosozumab have been successful in treating postmenopausal women and men with osteoporosis.
Romosozumab codevelopers UCB and Amgen reported that the biologic agent significantly reduced the rate of new vertebral fractures by 73% versus placebo at 12 months in the randomized, double-blind phase III FRAME (Fracture Study in Postmenopausal Women With Osteoporosis) study. In the 7,180-patient trial, the reduction was 75% versus placebo at 24 months after both treatment groups had been transitioned to denosumab given every 6 months in the second year of treatment. Romosozumab also significantly lowered the relative risk of clinical fractures (composite of vertebral and nonvertebral fractures) by 36% at 12 months, but the difference was not statistically significant at 24 months.
In the initial 12-month treatment period, the most commonly reported adverse events in both arms (greater than 10%) were arthralgia, nasopharyngitis, and back pain. There were no differences in the proportions of patients who reported hearing loss or worsening of knee osteoarthritis. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after 3 months of romosozumab treatment.
Phase III results from the 244-patient BRIDGE (Placebo-Controlled Study Evaluating the Efficacy and Safety of Romosozumab in Treating Men With Osteoporosis) trial found a significant increase in bone mineral density (BMD) at the lumbar spine at 12 months, which was the study’s primary endpoint. Other significant increases in femoral neck and total hip BMD were detected at 12 months. Cardiovascular severe adverse events occurred in 4.9% of men on romosozumab and 2.5% on placebo, including death in 0.6% and 1.2%, respectively. At least 5% or more of patients who received romosozumab reported nasopharyngitis, back pain, hypertension, headache, and constipation. About 5% of patients who received romosozumab in each trial had injection-site reactions, most of which were mild.
A phase II trial of blosozumab in 120 postmenopausal women with low bone mineral density (mean lumbar spine T-score –2.8) showed that the drug increased BMD in the lumbar spine by 17.7% above baseline at 52 weeks, femoral neck by 8.4%, and total hip by 6.2%, compared with decreases of 1.6%, 0.6%, and 0.7%, respectively, with placebo (J Bone Miner Res. 2015 Feb;30[2]:216-24). However, mild injection-site reactions were reported by up to 40% of women taking blosozumab, and 35% developed antidrug antibodies after exposure to blosozumab. Eli Lilly, its developer, is looking at possible ways to reformulate the drug before it moves to phase III.
The study in Science Translational Medicine was supported by the German Research Foundation. The authors had no competing interests to disclose.
Antisclerostin monoclonal antibodies have shown their ability to increase bone density in phase II and III trials of men and women with osteoporosis but could potentially have the opposite effect in patients with rheumatoid arthritis or other chronic inflammatory diseases in which tumor necrosis factor–alpha (TNF-alpha) plays an important role, according to new research.
The new work, conducted by Corinna Wehmeyer, Ph.D., of the Institute of Experimental Musculoskeletal Medicine at University Hospital Muenster (Germany) and her colleagues, shows that the bone formation–inhibiting protein sclerostin is not expressed in bone only, as was previously thought, but is also expressed on the synovial cells of patients with rheumatoid arthritis (RA).
Dr. Wehmeyer and her associates were surprised to find that inhibiting sclerostin in a human TNF-alpha transgenic mouse model of RA actually accelerated joint damage rather than prevented it, suggesting that sclerostin actually had a protective role in the presence of chronic TNF-alpha–mediated inflammation. They confirmed this by demonstrating that sclerostin inhibited TNF-alpha signaling in fibroblast-like synoviocytes and showing that blocking sclerostin caused less or little worsening of bone erosions in mouse models of RA that are more dependent on a robust T and B cell response accompanied by high cytokine expression within the joint, rather than damage driven by TNF-alpha.
“These findings strongly suggest that in chronic TNF-alpha–mediated inflammation, sclerostin expression is upregulated as part of an attempt to reestablish bone homeostasis, where it exerts protective functions,” the authors wrote (Sci Transl Med. 2016 Mar 16;8:330ra34. doi: 10.1126/scitranslmed.aac4351).
The research needs confirmation in humans with RA and potentially in other chronic inflammatory diseases in which TNF-alpha plays an important role. “Nevertheless, the preliminary data in three different models indicate that sclerostin antibody therapy could be contraindicated in patients with chronic TNF-alpha–dependent inflammatory conditions. The possibility of adverse pathological effects means that caution should be taken both when considering such treatment in RA or in patients with chronic TNF-alpha–dependent comorbidities. Thus, to translate these findings to patients, first strategies to use sclerostin inhibition should exclude inflammatory comorbidities and very thoroughly monitor inflammatory events in patients to which such therapies are applied,” the researchers advised.
In an editorial, Dr. Frank Rauch of McGill University, Montreal, and Dr. Rick Adachi of the department of rheumatology at McMaster University, Hamilton, Ont., wrote that antisclerostin “treatment might accelerate joint destruction, at least when the inflammatory process is not quelled first. Patients with established RA usually undergo anti-inflammatory treatment, and it is unclear whether sclerostin inactivation would be detrimental in this context. Mouse data suggest that antisclerostin treatment might bring about regression of bone erosions when combined with TNF-alpha inhibition. The new work mirrors the situation of patients who have unrecognized RA while on antisclerostin therapy or who develop RA while receiving this treatment” (Sci Transl Med. 2016 Mar 16;8:330fs7. doi: 10.1126/scitranslmed.aaf4628).
Antisclerostin antibodies in trials
Trials of the antisclerostin monoclonal antibodies romosozumab and blosozumab have been successful in treating postmenopausal women and men with osteoporosis.
Romosozumab codevelopers UCB and Amgen reported that the biologic agent significantly reduced the rate of new vertebral fractures by 73% versus placebo at 12 months in the randomized, double-blind phase III FRAME (Fracture Study in Postmenopausal Women With Osteoporosis) study. In the 7,180-patient trial, the reduction was 75% versus placebo at 24 months after both treatment groups had been transitioned to denosumab given every 6 months in the second year of treatment. Romosozumab also significantly lowered the relative risk of clinical fractures (composite of vertebral and nonvertebral fractures) by 36% at 12 months, but the difference was not statistically significant at 24 months.
In the initial 12-month treatment period, the most commonly reported adverse events in both arms (greater than 10%) were arthralgia, nasopharyngitis, and back pain. There were no differences in the proportions of patients who reported hearing loss or worsening of knee osteoarthritis. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after 3 months of romosozumab treatment.
Phase III results from the 244-patient BRIDGE (Placebo-Controlled Study Evaluating the Efficacy and Safety of Romosozumab in Treating Men With Osteoporosis) trial found a significant increase in bone mineral density (BMD) at the lumbar spine at 12 months, which was the study’s primary endpoint. Other significant increases in femoral neck and total hip BMD were detected at 12 months. Cardiovascular severe adverse events occurred in 4.9% of men on romosozumab and 2.5% on placebo, including death in 0.6% and 1.2%, respectively. At least 5% or more of patients who received romosozumab reported nasopharyngitis, back pain, hypertension, headache, and constipation. About 5% of patients who received romosozumab in each trial had injection-site reactions, most of which were mild.
A phase II trial of blosozumab in 120 postmenopausal women with low bone mineral density (mean lumbar spine T-score –2.8) showed that the drug increased BMD in the lumbar spine by 17.7% above baseline at 52 weeks, femoral neck by 8.4%, and total hip by 6.2%, compared with decreases of 1.6%, 0.6%, and 0.7%, respectively, with placebo (J Bone Miner Res. 2015 Feb;30[2]:216-24). However, mild injection-site reactions were reported by up to 40% of women taking blosozumab, and 35% developed antidrug antibodies after exposure to blosozumab. Eli Lilly, its developer, is looking at possible ways to reformulate the drug before it moves to phase III.
The study in Science Translational Medicine was supported by the German Research Foundation. The authors had no competing interests to disclose.
Antisclerostin monoclonal antibodies have shown their ability to increase bone density in phase II and III trials of men and women with osteoporosis but could potentially have the opposite effect in patients with rheumatoid arthritis or other chronic inflammatory diseases in which tumor necrosis factor–alpha (TNF-alpha) plays an important role, according to new research.
The new work, conducted by Corinna Wehmeyer, Ph.D., of the Institute of Experimental Musculoskeletal Medicine at University Hospital Muenster (Germany) and her colleagues, shows that the bone formation–inhibiting protein sclerostin is not expressed in bone only, as was previously thought, but is also expressed on the synovial cells of patients with rheumatoid arthritis (RA).
Dr. Wehmeyer and her associates were surprised to find that inhibiting sclerostin in a human TNF-alpha transgenic mouse model of RA actually accelerated joint damage rather than prevented it, suggesting that sclerostin actually had a protective role in the presence of chronic TNF-alpha–mediated inflammation. They confirmed this by demonstrating that sclerostin inhibited TNF-alpha signaling in fibroblast-like synoviocytes and showing that blocking sclerostin caused less or little worsening of bone erosions in mouse models of RA that are more dependent on a robust T and B cell response accompanied by high cytokine expression within the joint, rather than damage driven by TNF-alpha.
“These findings strongly suggest that in chronic TNF-alpha–mediated inflammation, sclerostin expression is upregulated as part of an attempt to reestablish bone homeostasis, where it exerts protective functions,” the authors wrote (Sci Transl Med. 2016 Mar 16;8:330ra34. doi: 10.1126/scitranslmed.aac4351).
The research needs confirmation in humans with RA and potentially in other chronic inflammatory diseases in which TNF-alpha plays an important role. “Nevertheless, the preliminary data in three different models indicate that sclerostin antibody therapy could be contraindicated in patients with chronic TNF-alpha–dependent inflammatory conditions. The possibility of adverse pathological effects means that caution should be taken both when considering such treatment in RA or in patients with chronic TNF-alpha–dependent comorbidities. Thus, to translate these findings to patients, first strategies to use sclerostin inhibition should exclude inflammatory comorbidities and very thoroughly monitor inflammatory events in patients to which such therapies are applied,” the researchers advised.
In an editorial, Dr. Frank Rauch of McGill University, Montreal, and Dr. Rick Adachi of the department of rheumatology at McMaster University, Hamilton, Ont., wrote that antisclerostin “treatment might accelerate joint destruction, at least when the inflammatory process is not quelled first. Patients with established RA usually undergo anti-inflammatory treatment, and it is unclear whether sclerostin inactivation would be detrimental in this context. Mouse data suggest that antisclerostin treatment might bring about regression of bone erosions when combined with TNF-alpha inhibition. The new work mirrors the situation of patients who have unrecognized RA while on antisclerostin therapy or who develop RA while receiving this treatment” (Sci Transl Med. 2016 Mar 16;8:330fs7. doi: 10.1126/scitranslmed.aaf4628).
Antisclerostin antibodies in trials
Trials of the antisclerostin monoclonal antibodies romosozumab and blosozumab have been successful in treating postmenopausal women and men with osteoporosis.
Romosozumab codevelopers UCB and Amgen reported that the biologic agent significantly reduced the rate of new vertebral fractures by 73% versus placebo at 12 months in the randomized, double-blind phase III FRAME (Fracture Study in Postmenopausal Women With Osteoporosis) study. In the 7,180-patient trial, the reduction was 75% versus placebo at 24 months after both treatment groups had been transitioned to denosumab given every 6 months in the second year of treatment. Romosozumab also significantly lowered the relative risk of clinical fractures (composite of vertebral and nonvertebral fractures) by 36% at 12 months, but the difference was not statistically significant at 24 months.
In the initial 12-month treatment period, the most commonly reported adverse events in both arms (greater than 10%) were arthralgia, nasopharyngitis, and back pain. There were no differences in the proportions of patients who reported hearing loss or worsening of knee osteoarthritis. There were two positively adjudicated events of osteonecrosis of the jaw in the romosozumab treatment group, one after completing romosozumab dosing and the other after completing romosozumab treatment and receiving the initial dose of denosumab. There was one positively adjudicated event of atypical femoral fracture after 3 months of romosozumab treatment.
Phase III results from the 244-patient BRIDGE (Placebo-Controlled Study Evaluating the Efficacy and Safety of Romosozumab in Treating Men With Osteoporosis) trial found a significant increase in bone mineral density (BMD) at the lumbar spine at 12 months, which was the study’s primary endpoint. Other significant increases in femoral neck and total hip BMD were detected at 12 months. Cardiovascular severe adverse events occurred in 4.9% of men on romosozumab and 2.5% on placebo, including death in 0.6% and 1.2%, respectively. At least 5% or more of patients who received romosozumab reported nasopharyngitis, back pain, hypertension, headache, and constipation. About 5% of patients who received romosozumab in each trial had injection-site reactions, most of which were mild.
A phase II trial of blosozumab in 120 postmenopausal women with low bone mineral density (mean lumbar spine T-score –2.8) showed that the drug increased BMD in the lumbar spine by 17.7% above baseline at 52 weeks, femoral neck by 8.4%, and total hip by 6.2%, compared with decreases of 1.6%, 0.6%, and 0.7%, respectively, with placebo (J Bone Miner Res. 2015 Feb;30[2]:216-24). However, mild injection-site reactions were reported by up to 40% of women taking blosozumab, and 35% developed antidrug antibodies after exposure to blosozumab. Eli Lilly, its developer, is looking at possible ways to reformulate the drug before it moves to phase III.
The study in Science Translational Medicine was supported by the German Research Foundation. The authors had no competing interests to disclose.
FROM SCIENCE TRANSLATIONAL MEDICINE
Confluent Erythematous Plaques on the Palm
The Diagnosis: Palmoplantar Lichen Planus
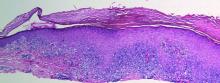
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
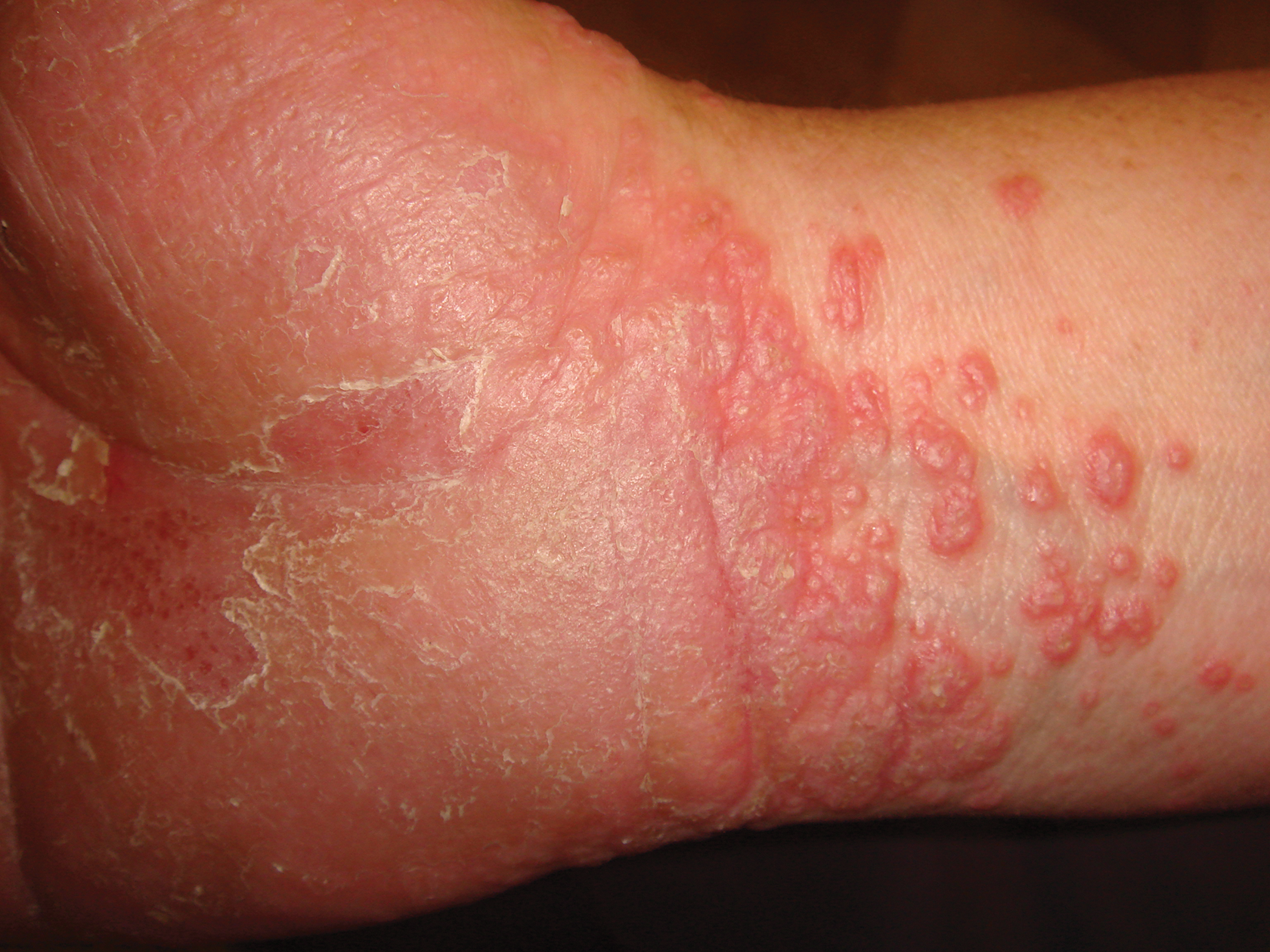
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
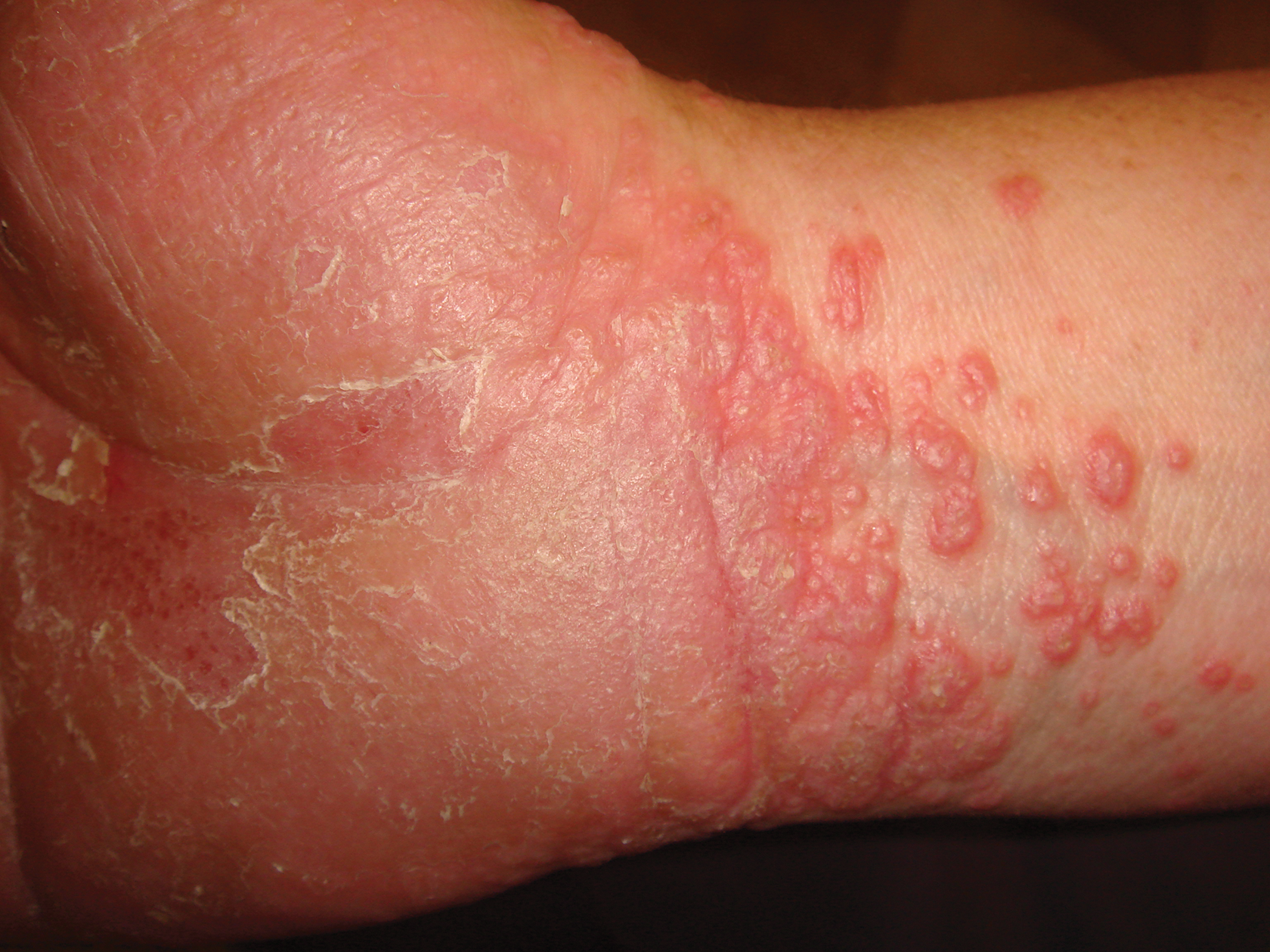
A 45-year-old woman was referred to dermatology by her general internist for the management of a pruritic rash on the hands and feet that was unresponsive to topical steroid creams. The pruritus also was unresponsive to hydroxyzine and aspirin. Erythematous plaques were present on the palms and soles. Physical examination revealed thickened volar skin with a yellowish surface. There were individual papules with atrophic tops at the edge of the plaques on the inner wrists. The patient’s medical history was otherwise unremarkable. Blood tests for glucose and liver function did not reveal any abnormalities.
Condom use low among female teens using LARC
WASHINGTON – While use of long-acting reversible contraception (LARC) has steadily been increasing over recent years, a new study shows that adolescent females who use LARC might be neglecting to wear condoms when engaging in sexual intercourse, regardless of their number of partners, thus predisposing them to a high risk of contracting sexually transmitted infections (STIs).
“Like moderately effective methods of contraception, [LARC] does not protect against STIs, and so use of a condom in conjunction with [LARC] is recommended for STI prevention,” lead author Riley J. Steiner of the Centers for Disease Control and Prevention in Atlanta, explained at the annual meeting of the Society for Adolescent Health and Medicine. The study also was published in JAMA Pediatrics (2016 Mar 14. doi:10.1001/jamapediatrics.2016.0007)
She added that, “We really think that establishing a link between LARC and condom use early on, prior to widespread adolescent uptake of LARC, can help provide a useful reference point for future monitoring and, ultimately, inform STI prevention efforts as LARC is brought to scale.”
Ms. Steiner and her coinvestigators used data from the 2013 national Youth Risk Behavior Survey, a self-administered “paper and pencil” questionnaire conducted every 2 years for students in grades 9-12 in public and private high schools across the United States. Analysis of the data – which looked for age, race, and type of contraceptive used – was conducted in July and August of 2015.
Primary outcome of the analysis was to determine the contraceptive method used the most recent time a female had sexual intercourse: either LARC – via an intrauterine device or an implant – oral contraceptives, Depo-Provera, a patch, or a ring. In total, 2,288 females were included in the study; 41% used condoms, 22% used oral contraceptives, 16% used no contraceptive methods whatsoever, 12% used “withdrawal or other method,” 6% used either Depo-Provera, a patch, or a ring, 2% said they were unsure of what contraceptive, if any, they used, and only 2% of females used LARC.
However, of the 2% that used LARC, adjusted odds ratios revealed that they were significantly more likely not to use condoms (adjusted prevalence ratio [aPR], 0.42; 95% confidence interval, 0.21-0.84) as opposed to females on oral contraceptives. There was no significant difference found in condom use between females on LARC versus those on Depo-Provera, a patch, or a ring (aPR = 0.57; 95% CI, 0.26-1.25).
“Health care professionals may be more likely to offer LARC to adolescents who report not using condoms or using them infrequently, as LARC methods are particularly well suited for adolescents who have difficulty adhering to coitally dependent methods,” Ms. Steiner and her associates said, adding that it is currently unknown “whether the association varies by partnership type; it is possible that the observed differences occur largely among adolescents who consider themselves to be in committed partnerships and thus are less concerned about STIs.”
Females included in the study were 57% white, with just over a third of all 2,288 subjects being in the 12th grade. Condom use was most prevalent among 9th graders (47%), while non-Hispanic blacks and Hispanic females tended to use condoms the most (47% and 46%, respectively). LARC use, though low overall, was highest among 12th graders (3%) and non-Hispanic whites (2%).
The study was funded partly by grants from the National Institute of Allergy and Infectious Diseases, and the Centers for Disease Control and Prevention. Ms. Steiner did not report any relevant financial disclosures.
WASHINGTON – While use of long-acting reversible contraception (LARC) has steadily been increasing over recent years, a new study shows that adolescent females who use LARC might be neglecting to wear condoms when engaging in sexual intercourse, regardless of their number of partners, thus predisposing them to a high risk of contracting sexually transmitted infections (STIs).
“Like moderately effective methods of contraception, [LARC] does not protect against STIs, and so use of a condom in conjunction with [LARC] is recommended for STI prevention,” lead author Riley J. Steiner of the Centers for Disease Control and Prevention in Atlanta, explained at the annual meeting of the Society for Adolescent Health and Medicine. The study also was published in JAMA Pediatrics (2016 Mar 14. doi:10.1001/jamapediatrics.2016.0007)
She added that, “We really think that establishing a link between LARC and condom use early on, prior to widespread adolescent uptake of LARC, can help provide a useful reference point for future monitoring and, ultimately, inform STI prevention efforts as LARC is brought to scale.”
Ms. Steiner and her coinvestigators used data from the 2013 national Youth Risk Behavior Survey, a self-administered “paper and pencil” questionnaire conducted every 2 years for students in grades 9-12 in public and private high schools across the United States. Analysis of the data – which looked for age, race, and type of contraceptive used – was conducted in July and August of 2015.
Primary outcome of the analysis was to determine the contraceptive method used the most recent time a female had sexual intercourse: either LARC – via an intrauterine device or an implant – oral contraceptives, Depo-Provera, a patch, or a ring. In total, 2,288 females were included in the study; 41% used condoms, 22% used oral contraceptives, 16% used no contraceptive methods whatsoever, 12% used “withdrawal or other method,” 6% used either Depo-Provera, a patch, or a ring, 2% said they were unsure of what contraceptive, if any, they used, and only 2% of females used LARC.
However, of the 2% that used LARC, adjusted odds ratios revealed that they were significantly more likely not to use condoms (adjusted prevalence ratio [aPR], 0.42; 95% confidence interval, 0.21-0.84) as opposed to females on oral contraceptives. There was no significant difference found in condom use between females on LARC versus those on Depo-Provera, a patch, or a ring (aPR = 0.57; 95% CI, 0.26-1.25).
“Health care professionals may be more likely to offer LARC to adolescents who report not using condoms or using them infrequently, as LARC methods are particularly well suited for adolescents who have difficulty adhering to coitally dependent methods,” Ms. Steiner and her associates said, adding that it is currently unknown “whether the association varies by partnership type; it is possible that the observed differences occur largely among adolescents who consider themselves to be in committed partnerships and thus are less concerned about STIs.”
Females included in the study were 57% white, with just over a third of all 2,288 subjects being in the 12th grade. Condom use was most prevalent among 9th graders (47%), while non-Hispanic blacks and Hispanic females tended to use condoms the most (47% and 46%, respectively). LARC use, though low overall, was highest among 12th graders (3%) and non-Hispanic whites (2%).
The study was funded partly by grants from the National Institute of Allergy and Infectious Diseases, and the Centers for Disease Control and Prevention. Ms. Steiner did not report any relevant financial disclosures.
WASHINGTON – While use of long-acting reversible contraception (LARC) has steadily been increasing over recent years, a new study shows that adolescent females who use LARC might be neglecting to wear condoms when engaging in sexual intercourse, regardless of their number of partners, thus predisposing them to a high risk of contracting sexually transmitted infections (STIs).
“Like moderately effective methods of contraception, [LARC] does not protect against STIs, and so use of a condom in conjunction with [LARC] is recommended for STI prevention,” lead author Riley J. Steiner of the Centers for Disease Control and Prevention in Atlanta, explained at the annual meeting of the Society for Adolescent Health and Medicine. The study also was published in JAMA Pediatrics (2016 Mar 14. doi:10.1001/jamapediatrics.2016.0007)
She added that, “We really think that establishing a link between LARC and condom use early on, prior to widespread adolescent uptake of LARC, can help provide a useful reference point for future monitoring and, ultimately, inform STI prevention efforts as LARC is brought to scale.”
Ms. Steiner and her coinvestigators used data from the 2013 national Youth Risk Behavior Survey, a self-administered “paper and pencil” questionnaire conducted every 2 years for students in grades 9-12 in public and private high schools across the United States. Analysis of the data – which looked for age, race, and type of contraceptive used – was conducted in July and August of 2015.
Primary outcome of the analysis was to determine the contraceptive method used the most recent time a female had sexual intercourse: either LARC – via an intrauterine device or an implant – oral contraceptives, Depo-Provera, a patch, or a ring. In total, 2,288 females were included in the study; 41% used condoms, 22% used oral contraceptives, 16% used no contraceptive methods whatsoever, 12% used “withdrawal or other method,” 6% used either Depo-Provera, a patch, or a ring, 2% said they were unsure of what contraceptive, if any, they used, and only 2% of females used LARC.
However, of the 2% that used LARC, adjusted odds ratios revealed that they were significantly more likely not to use condoms (adjusted prevalence ratio [aPR], 0.42; 95% confidence interval, 0.21-0.84) as opposed to females on oral contraceptives. There was no significant difference found in condom use between females on LARC versus those on Depo-Provera, a patch, or a ring (aPR = 0.57; 95% CI, 0.26-1.25).
“Health care professionals may be more likely to offer LARC to adolescents who report not using condoms or using them infrequently, as LARC methods are particularly well suited for adolescents who have difficulty adhering to coitally dependent methods,” Ms. Steiner and her associates said, adding that it is currently unknown “whether the association varies by partnership type; it is possible that the observed differences occur largely among adolescents who consider themselves to be in committed partnerships and thus are less concerned about STIs.”
Females included in the study were 57% white, with just over a third of all 2,288 subjects being in the 12th grade. Condom use was most prevalent among 9th graders (47%), while non-Hispanic blacks and Hispanic females tended to use condoms the most (47% and 46%, respectively). LARC use, though low overall, was highest among 12th graders (3%) and non-Hispanic whites (2%).
The study was funded partly by grants from the National Institute of Allergy and Infectious Diseases, and the Centers for Disease Control and Prevention. Ms. Steiner did not report any relevant financial disclosures.
AT THE SAHM ANNUAL MEETING
Key clinical point: Adolescent females using LARC to prevent pregnancy often don’t use condoms, even if they have more than one sexual partner, leading to a high risk of contracting and transmitting STIs.
Major finding: 1.8% of sexually active females included in the study used LARC; however, these females were 60% less likely to use condoms, compared with females using oral contraceptives.
Data source: Cross-sectional analysis of data on 2,288 sexually active females from the 2013 national Youth Risk Behavior Survey of U.S. students in grades 9-12.
Disclosures: The study was funded partly by grants from the National Institute of Allergy and Infectious Diseases, and the CDC. Ms. Steiner did not report any relevant financial disclosures.
Sharpening the Saw
Few movies have universal appeal these days; but one that comes close is Bill Murray’s 1993 classic Groundhog Day in which Murray’s character is trapped in a time loop, living the same day over and over until he finally “gets it right.”
One reason that this film resonates with so many, I think, is that we are all, in essence, similarly trapped. Not in a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years, because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or take a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lesson; or get away a long weekend away with my wife. And we take longer vacations, without fail, each year.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle, you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. And trust me, your practice will still be there when you return.
Six weeks ago, my wife and I packed our carry-ons, bought rail passes, and took off for Japan. As we whisked around the archipelago on those incredibly punctual Shinkansen bullet trains, I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out.
When our whistle-stop trip was over, I returned ready to take on the world, and my practice, anew.
More than once I’ve recounted the story of K. Alexander Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But, in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few movies have universal appeal these days; but one that comes close is Bill Murray’s 1993 classic Groundhog Day in which Murray’s character is trapped in a time loop, living the same day over and over until he finally “gets it right.”
One reason that this film resonates with so many, I think, is that we are all, in essence, similarly trapped. Not in a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years, because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or take a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lesson; or get away a long weekend away with my wife. And we take longer vacations, without fail, each year.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle, you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. And trust me, your practice will still be there when you return.
Six weeks ago, my wife and I packed our carry-ons, bought rail passes, and took off for Japan. As we whisked around the archipelago on those incredibly punctual Shinkansen bullet trains, I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out.
When our whistle-stop trip was over, I returned ready to take on the world, and my practice, anew.
More than once I’ve recounted the story of K. Alexander Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But, in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few movies have universal appeal these days; but one that comes close is Bill Murray’s 1993 classic Groundhog Day in which Murray’s character is trapped in a time loop, living the same day over and over until he finally “gets it right.”
One reason that this film resonates with so many, I think, is that we are all, in essence, similarly trapped. Not in a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years, because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. Sooner or later, no matter how dedicated we are, the grind gets to all of us, leading to fatigue, irritability, and a progressive decline in motivation. And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well-being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of 1 a month) to catch up on journals or take a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lesson; or get away a long weekend away with my wife. And we take longer vacations, without fail, each year.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle, you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money. This is breaking the routine, clearing the cobwebs, living your life. And trust me, your practice will still be there when you return.
Six weeks ago, my wife and I packed our carry-ons, bought rail passes, and took off for Japan. As we whisked around the archipelago on those incredibly punctual Shinkansen bullet trains, I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out.
When our whistle-stop trip was over, I returned ready to take on the world, and my practice, anew.
More than once I’ve recounted the story of K. Alexander Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But, in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
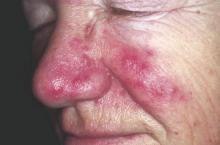
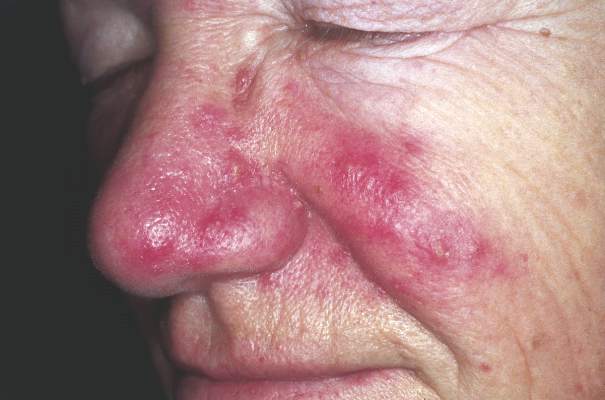
Rosacea linked to increased Parkinson’s disease risk
Individuals with rosacea were nearly twice as likely to develop Parkinson’s disease as those without rosacea, based on an analysis of results of a nationwide cohort study of 5.4 million people conducted in Denmark. The findings were published online March 21 in JAMA Neurology.
Data from previous studies suggest a link between rosacea and Parkinson’s disease (PD), as both may stem from elevated activity of matrix metalloproteinases, wrote Dr. Alexander Egeberg of the University of Copenhagen, and his associates.
To explore the possible link between rosacea and Parkinson’s disease, the researchers reviewed data on about 5.4 million adults in a Danish database of all citizens aged 18 and older, over a 15-year period from January 1, 1997, to December 31, 2011. A total of 22,387 individuals were diagnosed with Parkinson’s disease and 68,053 were diagnosed with rosacea (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0022).
The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among rosacea patients vs. 3.54 per 10,000 person-years in the general population, the researchers noted.
Overall, the Parkinson’s disease risk was significantly higher among rosacea patients, with an adjusted incidence rate ratio (IRR) of 1.71 for rosacea patients compared with the general population. The IRR was even higher in patients with ocular rosacea (adjusted IRR, 2.03).
However, treatment with tetracycline appeared to reduce the Parkinson’s risk in the rosacea patients: After controlling for multiple variables, having filled a prescription for tetracycline was associated with a 2% drop in the risk of PD (adjusted IRR, 0.98).
A sensitivity analysis showed no dose-dependent relationship with disease severity; the IRRs for Parkinson’s disease in patients with mild rosacea and moderate to severe rosacea were 1.82 and 1.84, respectively. However, moderate to severe rosacea was defined as cases where tetracyclines were used, and the neuroprotective effect of tetracyclines might impact the relationship between rosacea and Parkinson’s disease, the researchers noted.
“It is tempting to speculate that, in patients with coexistent rosacea, Parkinson’s disease may display other phenotypic characteristics, and it is possible that rosacea-associated features, such as facial flushing, may contribute to support a Parkinson’s disease diagnosis at an early phase of the disease,” the researchers noted.
Limitations of the study include its observational design, which does not allow the establishment of causation, the researchers noted, and additional research is needed to confirm the observations and clinical consequences.
Lead author Dr. Egeberg was employed by Pfizer at the time of the study; one of the coauthors was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
An intriguing aspect of the study was the lack of disease severity impact on the incidence rate ratio (IRR) on Parkinson’s disease, Dr. Thomas S. Wingo wrote in an accompanying editorial. He added: “In other words, people with moderate to severe rosacea have the same IRR for PD as do those who have moderate disease. To explain this result, the authors hypothesized that a disease-severity effect might be blunted by the treatment of moderate to severe rosacea with tetracycline. The reason for this possible effect is that tetracycline is chemically similar to minocycline, which has shown evidence for exerting a protective effect in animal models of PD, although this effect has not been consistently seen.”
Dr. Wingo noted that the “intriguing finding that increased tetracycline use is associated with a small but appreciable reduction in the risk of PD should be further explored. Of particular interest would be to understand the temporal association between the use of tetracycline and effect on PD risk” (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0291).
Dr. Wingo is a member of the departments of neurology and human genetics at Emory University, Atlanta. He had no financial conflicts to disclose.
An intriguing aspect of the study was the lack of disease severity impact on the incidence rate ratio (IRR) on Parkinson’s disease, Dr. Thomas S. Wingo wrote in an accompanying editorial. He added: “In other words, people with moderate to severe rosacea have the same IRR for PD as do those who have moderate disease. To explain this result, the authors hypothesized that a disease-severity effect might be blunted by the treatment of moderate to severe rosacea with tetracycline. The reason for this possible effect is that tetracycline is chemically similar to minocycline, which has shown evidence for exerting a protective effect in animal models of PD, although this effect has not been consistently seen.”
Dr. Wingo noted that the “intriguing finding that increased tetracycline use is associated with a small but appreciable reduction in the risk of PD should be further explored. Of particular interest would be to understand the temporal association between the use of tetracycline and effect on PD risk” (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0291).
Dr. Wingo is a member of the departments of neurology and human genetics at Emory University, Atlanta. He had no financial conflicts to disclose.
An intriguing aspect of the study was the lack of disease severity impact on the incidence rate ratio (IRR) on Parkinson’s disease, Dr. Thomas S. Wingo wrote in an accompanying editorial. He added: “In other words, people with moderate to severe rosacea have the same IRR for PD as do those who have moderate disease. To explain this result, the authors hypothesized that a disease-severity effect might be blunted by the treatment of moderate to severe rosacea with tetracycline. The reason for this possible effect is that tetracycline is chemically similar to minocycline, which has shown evidence for exerting a protective effect in animal models of PD, although this effect has not been consistently seen.”
Dr. Wingo noted that the “intriguing finding that increased tetracycline use is associated with a small but appreciable reduction in the risk of PD should be further explored. Of particular interest would be to understand the temporal association between the use of tetracycline and effect on PD risk” (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0291).
Dr. Wingo is a member of the departments of neurology and human genetics at Emory University, Atlanta. He had no financial conflicts to disclose.
Individuals with rosacea were nearly twice as likely to develop Parkinson’s disease as those without rosacea, based on an analysis of results of a nationwide cohort study of 5.4 million people conducted in Denmark. The findings were published online March 21 in JAMA Neurology.
Data from previous studies suggest a link between rosacea and Parkinson’s disease (PD), as both may stem from elevated activity of matrix metalloproteinases, wrote Dr. Alexander Egeberg of the University of Copenhagen, and his associates.
To explore the possible link between rosacea and Parkinson’s disease, the researchers reviewed data on about 5.4 million adults in a Danish database of all citizens aged 18 and older, over a 15-year period from January 1, 1997, to December 31, 2011. A total of 22,387 individuals were diagnosed with Parkinson’s disease and 68,053 were diagnosed with rosacea (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0022).
The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among rosacea patients vs. 3.54 per 10,000 person-years in the general population, the researchers noted.
Overall, the Parkinson’s disease risk was significantly higher among rosacea patients, with an adjusted incidence rate ratio (IRR) of 1.71 for rosacea patients compared with the general population. The IRR was even higher in patients with ocular rosacea (adjusted IRR, 2.03).
However, treatment with tetracycline appeared to reduce the Parkinson’s risk in the rosacea patients: After controlling for multiple variables, having filled a prescription for tetracycline was associated with a 2% drop in the risk of PD (adjusted IRR, 0.98).
A sensitivity analysis showed no dose-dependent relationship with disease severity; the IRRs for Parkinson’s disease in patients with mild rosacea and moderate to severe rosacea were 1.82 and 1.84, respectively. However, moderate to severe rosacea was defined as cases where tetracyclines were used, and the neuroprotective effect of tetracyclines might impact the relationship between rosacea and Parkinson’s disease, the researchers noted.
“It is tempting to speculate that, in patients with coexistent rosacea, Parkinson’s disease may display other phenotypic characteristics, and it is possible that rosacea-associated features, such as facial flushing, may contribute to support a Parkinson’s disease diagnosis at an early phase of the disease,” the researchers noted.
Limitations of the study include its observational design, which does not allow the establishment of causation, the researchers noted, and additional research is needed to confirm the observations and clinical consequences.
Lead author Dr. Egeberg was employed by Pfizer at the time of the study; one of the coauthors was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
Individuals with rosacea were nearly twice as likely to develop Parkinson’s disease as those without rosacea, based on an analysis of results of a nationwide cohort study of 5.4 million people conducted in Denmark. The findings were published online March 21 in JAMA Neurology.
Data from previous studies suggest a link between rosacea and Parkinson’s disease (PD), as both may stem from elevated activity of matrix metalloproteinases, wrote Dr. Alexander Egeberg of the University of Copenhagen, and his associates.
To explore the possible link between rosacea and Parkinson’s disease, the researchers reviewed data on about 5.4 million adults in a Danish database of all citizens aged 18 and older, over a 15-year period from January 1, 1997, to December 31, 2011. A total of 22,387 individuals were diagnosed with Parkinson’s disease and 68,053 were diagnosed with rosacea (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0022).
The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among rosacea patients vs. 3.54 per 10,000 person-years in the general population, the researchers noted.
Overall, the Parkinson’s disease risk was significantly higher among rosacea patients, with an adjusted incidence rate ratio (IRR) of 1.71 for rosacea patients compared with the general population. The IRR was even higher in patients with ocular rosacea (adjusted IRR, 2.03).
However, treatment with tetracycline appeared to reduce the Parkinson’s risk in the rosacea patients: After controlling for multiple variables, having filled a prescription for tetracycline was associated with a 2% drop in the risk of PD (adjusted IRR, 0.98).
A sensitivity analysis showed no dose-dependent relationship with disease severity; the IRRs for Parkinson’s disease in patients with mild rosacea and moderate to severe rosacea were 1.82 and 1.84, respectively. However, moderate to severe rosacea was defined as cases where tetracyclines were used, and the neuroprotective effect of tetracyclines might impact the relationship between rosacea and Parkinson’s disease, the researchers noted.
“It is tempting to speculate that, in patients with coexistent rosacea, Parkinson’s disease may display other phenotypic characteristics, and it is possible that rosacea-associated features, such as facial flushing, may contribute to support a Parkinson’s disease diagnosis at an early phase of the disease,” the researchers noted.
Limitations of the study include its observational design, which does not allow the establishment of causation, the researchers noted, and additional research is needed to confirm the observations and clinical consequences.
Lead author Dr. Egeberg was employed by Pfizer at the time of the study; one of the coauthors was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
FROM JAMA NEUROLOGY
Key clinical point: Rosacea was found to be an independent risk factor for Parkinson’s disease, an effect reduced by the use of tetracycline.
Major finding: The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among people with rosacea vs. 3.54 per 10,000 person-years in the general population over a 15-year follow-up period.
Data source: The data come from a nationwide cohort study conducted in Denmark that included 5.4 million adults aged 18 years and older.
Disclosures: Lead author Dr. Alexander Egeberg was employed by Pfizer at the time of the study; a study coauthor was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
U.S. flu activity: Another week, another increase
Influenza-like illness (ILI) activity in the U.S. showed no signs of slowing down during the week ending March 12, 2016, as the number of states at the highest level increased to seven, compared with four the previous week, according to the Centers for Disease Control and Prevention.
The seven states at level 10 on the CDC’s 1-10 scale of ILI activity were Alabama, Arizona, Arkansas, Indiana, Kentucky, New Jersey, and North Carolina. Other states in the “high” range for the week were Mississippi, New Mexico, and Virginia at level 9 and Georgia, Hawaii, Illinois, and Oregon at level 8, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.
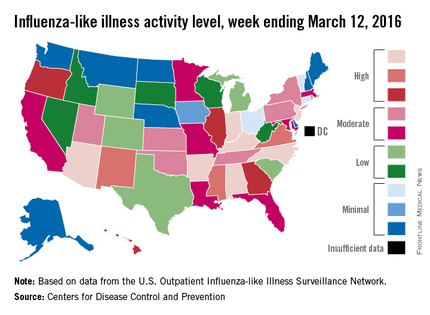
The proportion of outpatient visits for ILI was 3.7% for the week, up from 3.5% the previous week and another new high for the season. The national baseline is 2.1%. The geographic spread of influenza in 40 states and Puerto Rico was reported as widespread, the CDC said.
There were eight flu-related pediatric deaths reported to the CDC, of which only one occurred during the week ending March 12. For the season so far, a total of 28 flu-related pediatric deaths have been reported in 14 states and Puerto Rico.
Since Oct. 1, 2015, 4,006 laboratory-confirmed flu-associated hospitalizations have been reported to the CDC’s Influenza Hospitalization Surveillance Network, which covers more than 70 counties in a group of 10 Emerging Infections Program states plus four additional states. The overall hospitalization rate for the season is 14.5 per 100,000 population, with the highest rate occurring in adults aged 65 years and older (37.2 per 100,000), followed by adults aged 50-64 (21.3) and children aged 0-4 years (20.9), the CDC report noted.
Influenza-like illness (ILI) activity in the U.S. showed no signs of slowing down during the week ending March 12, 2016, as the number of states at the highest level increased to seven, compared with four the previous week, according to the Centers for Disease Control and Prevention.
The seven states at level 10 on the CDC’s 1-10 scale of ILI activity were Alabama, Arizona, Arkansas, Indiana, Kentucky, New Jersey, and North Carolina. Other states in the “high” range for the week were Mississippi, New Mexico, and Virginia at level 9 and Georgia, Hawaii, Illinois, and Oregon at level 8, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.

The proportion of outpatient visits for ILI was 3.7% for the week, up from 3.5% the previous week and another new high for the season. The national baseline is 2.1%. The geographic spread of influenza in 40 states and Puerto Rico was reported as widespread, the CDC said.
There were eight flu-related pediatric deaths reported to the CDC, of which only one occurred during the week ending March 12. For the season so far, a total of 28 flu-related pediatric deaths have been reported in 14 states and Puerto Rico.
Since Oct. 1, 2015, 4,006 laboratory-confirmed flu-associated hospitalizations have been reported to the CDC’s Influenza Hospitalization Surveillance Network, which covers more than 70 counties in a group of 10 Emerging Infections Program states plus four additional states. The overall hospitalization rate for the season is 14.5 per 100,000 population, with the highest rate occurring in adults aged 65 years and older (37.2 per 100,000), followed by adults aged 50-64 (21.3) and children aged 0-4 years (20.9), the CDC report noted.
Influenza-like illness (ILI) activity in the U.S. showed no signs of slowing down during the week ending March 12, 2016, as the number of states at the highest level increased to seven, compared with four the previous week, according to the Centers for Disease Control and Prevention.
The seven states at level 10 on the CDC’s 1-10 scale of ILI activity were Alabama, Arizona, Arkansas, Indiana, Kentucky, New Jersey, and North Carolina. Other states in the “high” range for the week were Mississippi, New Mexico, and Virginia at level 9 and Georgia, Hawaii, Illinois, and Oregon at level 8, the CDC’s Influenza-like Illness Surveillance Network (ILINet) reported.

The proportion of outpatient visits for ILI was 3.7% for the week, up from 3.5% the previous week and another new high for the season. The national baseline is 2.1%. The geographic spread of influenza in 40 states and Puerto Rico was reported as widespread, the CDC said.
There were eight flu-related pediatric deaths reported to the CDC, of which only one occurred during the week ending March 12. For the season so far, a total of 28 flu-related pediatric deaths have been reported in 14 states and Puerto Rico.
Since Oct. 1, 2015, 4,006 laboratory-confirmed flu-associated hospitalizations have been reported to the CDC’s Influenza Hospitalization Surveillance Network, which covers more than 70 counties in a group of 10 Emerging Infections Program states plus four additional states. The overall hospitalization rate for the season is 14.5 per 100,000 population, with the highest rate occurring in adults aged 65 years and older (37.2 per 100,000), followed by adults aged 50-64 (21.3) and children aged 0-4 years (20.9), the CDC report noted.
VTEP Guidelines Can Be Systematically Implemented in Pediatric Inpatients
Clinical question: Can venous thromboembolism prophylaxis (VTEP) guidelines be systematically implemented in a pediatric inpatient population?
Background: VTEP for hospitalized adult medical patients has been characterized in the literature as being safe and efficacious, although mortality benefits are unclear.1 Systematic risk stratification based on electronic medical records (EMRs) with resultant implementation of pharmacologic and mechanical thromboprophylaxis has been shown to improve appropriate VTEP ordering in the adult inpatient population.2
Although the incidence of VTE is known to be increasing in the pediatric population, systematic VTEP implementation in hospitalized children is not well-described. Prior studies have shown the safety of systematic VTEP implementation through a protocol identifying high-risk pediatric inpatients with resultant initiation of appropriate VTEP. 3,4
Risk stratification in prior studies has taken into consideration risk factors such as altered mobility, presence of a central venous catheter (CVC), spinal cord injury (SCI), major lower-extremity orthopedic surgery, major trauma, active malignancy, acute infection, obesity, estrogen use, inflammatory bowel disease (IBD), prior VTE, and family history of VTE.4
Study design: Prospective cohort study using QI methodology.
Setting: A 455-bed, tertiary, freestanding children’s hospital.
Synopsis: After reviewing current literature for VTEP in adults and children and existing institutional pathways for VTEP in adults, traumatic brain injury, and SCI, a multidisciplinary committee formulated VTEP guidelines for 12- to 17-year-old patients. Pharmacologic prophylaxis was considered appropriate in the absence of contraindications and only if CVC and altered mobility were present as risk factors. Using a previously published logistic regression model evaluating VTE risk factors, patients were further categorized as high, moderate, or low risk.
Initial risk-factor categorization was via EMR-based order set, where risk factors were displayed, but subsequently was performed by an integrated tool based on an initial screening form completed by providers upon admission. Logic rules applied by the EMR led to specific VTEP recommendations, which were then selectable by the provider.
Over the first 17 months of EMR tool use, 148 patients on average were admitted each month. VTEP screening rates via the EMR tool increased from 48% in the first month to 81% in the final month. Despite EMR tool usage, VTEP orders did not always correlate with recommendations. Although not a stated objective of the study, none of the screened patients developed a VTE (compared to three cases of VTE in patients between 12 and 17 years of age the year prior).
Bottom line: VTEP guidelines can be systematically implemented via an EMR-based tool in a pediatric inpatient population.
Citation: Mahajerin A, Webber E, Morris J, Taylor K, Saysana M. Development and implementation results of a venous thromboembolism prophylaxis guideline in a tertiary care pediatric hospital. Hosp Pediatr. 2015;5(12):630-636.
References
- Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: controversies and perspectives. Am J Med. 2009;122(12):1077-1084.
- Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- Takemoto CM, Sohi S, Desai K, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332-338.
- Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326-1332.

Clinical question: Can venous thromboembolism prophylaxis (VTEP) guidelines be systematically implemented in a pediatric inpatient population?
Background: VTEP for hospitalized adult medical patients has been characterized in the literature as being safe and efficacious, although mortality benefits are unclear.1 Systematic risk stratification based on electronic medical records (EMRs) with resultant implementation of pharmacologic and mechanical thromboprophylaxis has been shown to improve appropriate VTEP ordering in the adult inpatient population.2
Although the incidence of VTE is known to be increasing in the pediatric population, systematic VTEP implementation in hospitalized children is not well-described. Prior studies have shown the safety of systematic VTEP implementation through a protocol identifying high-risk pediatric inpatients with resultant initiation of appropriate VTEP. 3,4
Risk stratification in prior studies has taken into consideration risk factors such as altered mobility, presence of a central venous catheter (CVC), spinal cord injury (SCI), major lower-extremity orthopedic surgery, major trauma, active malignancy, acute infection, obesity, estrogen use, inflammatory bowel disease (IBD), prior VTE, and family history of VTE.4
Study design: Prospective cohort study using QI methodology.
Setting: A 455-bed, tertiary, freestanding children’s hospital.
Synopsis: After reviewing current literature for VTEP in adults and children and existing institutional pathways for VTEP in adults, traumatic brain injury, and SCI, a multidisciplinary committee formulated VTEP guidelines for 12- to 17-year-old patients. Pharmacologic prophylaxis was considered appropriate in the absence of contraindications and only if CVC and altered mobility were present as risk factors. Using a previously published logistic regression model evaluating VTE risk factors, patients were further categorized as high, moderate, or low risk.
Initial risk-factor categorization was via EMR-based order set, where risk factors were displayed, but subsequently was performed by an integrated tool based on an initial screening form completed by providers upon admission. Logic rules applied by the EMR led to specific VTEP recommendations, which were then selectable by the provider.
Over the first 17 months of EMR tool use, 148 patients on average were admitted each month. VTEP screening rates via the EMR tool increased from 48% in the first month to 81% in the final month. Despite EMR tool usage, VTEP orders did not always correlate with recommendations. Although not a stated objective of the study, none of the screened patients developed a VTE (compared to three cases of VTE in patients between 12 and 17 years of age the year prior).
Bottom line: VTEP guidelines can be systematically implemented via an EMR-based tool in a pediatric inpatient population.
Citation: Mahajerin A, Webber E, Morris J, Taylor K, Saysana M. Development and implementation results of a venous thromboembolism prophylaxis guideline in a tertiary care pediatric hospital. Hosp Pediatr. 2015;5(12):630-636.
References
- Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: controversies and perspectives. Am J Med. 2009;122(12):1077-1084.
- Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- Takemoto CM, Sohi S, Desai K, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332-338.
- Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326-1332.

Clinical question: Can venous thromboembolism prophylaxis (VTEP) guidelines be systematically implemented in a pediatric inpatient population?
Background: VTEP for hospitalized adult medical patients has been characterized in the literature as being safe and efficacious, although mortality benefits are unclear.1 Systematic risk stratification based on electronic medical records (EMRs) with resultant implementation of pharmacologic and mechanical thromboprophylaxis has been shown to improve appropriate VTEP ordering in the adult inpatient population.2
Although the incidence of VTE is known to be increasing in the pediatric population, systematic VTEP implementation in hospitalized children is not well-described. Prior studies have shown the safety of systematic VTEP implementation through a protocol identifying high-risk pediatric inpatients with resultant initiation of appropriate VTEP. 3,4
Risk stratification in prior studies has taken into consideration risk factors such as altered mobility, presence of a central venous catheter (CVC), spinal cord injury (SCI), major lower-extremity orthopedic surgery, major trauma, active malignancy, acute infection, obesity, estrogen use, inflammatory bowel disease (IBD), prior VTE, and family history of VTE.4
Study design: Prospective cohort study using QI methodology.
Setting: A 455-bed, tertiary, freestanding children’s hospital.
Synopsis: After reviewing current literature for VTEP in adults and children and existing institutional pathways for VTEP in adults, traumatic brain injury, and SCI, a multidisciplinary committee formulated VTEP guidelines for 12- to 17-year-old patients. Pharmacologic prophylaxis was considered appropriate in the absence of contraindications and only if CVC and altered mobility were present as risk factors. Using a previously published logistic regression model evaluating VTE risk factors, patients were further categorized as high, moderate, or low risk.
Initial risk-factor categorization was via EMR-based order set, where risk factors were displayed, but subsequently was performed by an integrated tool based on an initial screening form completed by providers upon admission. Logic rules applied by the EMR led to specific VTEP recommendations, which were then selectable by the provider.
Over the first 17 months of EMR tool use, 148 patients on average were admitted each month. VTEP screening rates via the EMR tool increased from 48% in the first month to 81% in the final month. Despite EMR tool usage, VTEP orders did not always correlate with recommendations. Although not a stated objective of the study, none of the screened patients developed a VTE (compared to three cases of VTE in patients between 12 and 17 years of age the year prior).
Bottom line: VTEP guidelines can be systematically implemented via an EMR-based tool in a pediatric inpatient population.
Citation: Mahajerin A, Webber E, Morris J, Taylor K, Saysana M. Development and implementation results of a venous thromboembolism prophylaxis guideline in a tertiary care pediatric hospital. Hosp Pediatr. 2015;5(12):630-636.
References
- Spyropoulos AC, Mahan C. Venous thromboembolism prophylaxis in the medical patient: controversies and perspectives. Am J Med. 2009;122(12):1077-1084.
- Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
- Takemoto CM, Sohi S, Desai K, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr. 2014;164(2):332-338.
- Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326-1332.

Questions Have Been Raised about Potential Risks from Using Abbott Laboratories' Novel Coronary Stent
(Reuters) - U.S. government scientists have raised questions about the potential risks to patients of heart attacks and blood clots from Abbott Laboratories' novel coronary stent that dissolves after it is implanted.
Abbott is seeking U.S. approval to sell the stent, called Absorb, as an alternative to metal stents currently used in percutaneous coronary intervention. Unlike traditional stents that remain in place after implantation, Absorb is designed to disappear within three years of the procedure.
U.S. Food and Drug Administration staff, in documents released before an advisory panel meets on Tuesday to consider whether to recommend approval of the device, said they would ask the outside experts about data showing more heart attack and stent-related blood clots compared with Abbott's drug-coated metal Xience stent.
FDA said it also will ask the panel to address risks associated with the device when used in smaller arteries.
A large clinical trial released in October concluded that the Absorb stent, which is made of a plastic similar to dissolving sutures, was comparable to Xience in overall safety and effectiveness. Although Xience appeared to be numerically better than Absorb at one year on a variety of secondary measures, the differences were not considered statistically significant.
"These results are from physicians using a new therapy for the first time. Consequently, we expect them to improve with time and experience," said Abbott spokesman Jonathon Hamilton.
In the Absorb III clinical study, patients with small vessels comprised less than 20 percent of the total and experienced relatively low rates of adverse events even though many had known risk factors, including diabetes, Hamilton said.
More than 125,000 patients already have been treated with Absorb in more than 100 countries where it is commercially available.
If approved in the United States, Absorb would compete with Xience, the market-leading stent, and with Medtronic Plc's Resolute stent and Boston Scientific's Synergy and Promus stents. Synergy's polymer coating used to deliver a drug disappears over time, leaving a bare metal stent in place.
Wells Fargo analyst Larry Biegelsen said he expects Absorb to get a positive recommendation from the advisory panel, followed by FDA approval later this year.
"In the U.S., we estimate Absorb will capture about 5 percent of the total drug-eluting stent market, although our estimate may prove conservative if the post-approval data and experience with Absorb improves," Biegelsen wrote in a note to clients.
(Reuters) - U.S. government scientists have raised questions about the potential risks to patients of heart attacks and blood clots from Abbott Laboratories' novel coronary stent that dissolves after it is implanted.
Abbott is seeking U.S. approval to sell the stent, called Absorb, as an alternative to metal stents currently used in percutaneous coronary intervention. Unlike traditional stents that remain in place after implantation, Absorb is designed to disappear within three years of the procedure.
U.S. Food and Drug Administration staff, in documents released before an advisory panel meets on Tuesday to consider whether to recommend approval of the device, said they would ask the outside experts about data showing more heart attack and stent-related blood clots compared with Abbott's drug-coated metal Xience stent.
FDA said it also will ask the panel to address risks associated with the device when used in smaller arteries.
A large clinical trial released in October concluded that the Absorb stent, which is made of a plastic similar to dissolving sutures, was comparable to Xience in overall safety and effectiveness. Although Xience appeared to be numerically better than Absorb at one year on a variety of secondary measures, the differences were not considered statistically significant.
"These results are from physicians using a new therapy for the first time. Consequently, we expect them to improve with time and experience," said Abbott spokesman Jonathon Hamilton.
In the Absorb III clinical study, patients with small vessels comprised less than 20 percent of the total and experienced relatively low rates of adverse events even though many had known risk factors, including diabetes, Hamilton said.
More than 125,000 patients already have been treated with Absorb in more than 100 countries where it is commercially available.
If approved in the United States, Absorb would compete with Xience, the market-leading stent, and with Medtronic Plc's Resolute stent and Boston Scientific's Synergy and Promus stents. Synergy's polymer coating used to deliver a drug disappears over time, leaving a bare metal stent in place.
Wells Fargo analyst Larry Biegelsen said he expects Absorb to get a positive recommendation from the advisory panel, followed by FDA approval later this year.
"In the U.S., we estimate Absorb will capture about 5 percent of the total drug-eluting stent market, although our estimate may prove conservative if the post-approval data and experience with Absorb improves," Biegelsen wrote in a note to clients.
(Reuters) - U.S. government scientists have raised questions about the potential risks to patients of heart attacks and blood clots from Abbott Laboratories' novel coronary stent that dissolves after it is implanted.
Abbott is seeking U.S. approval to sell the stent, called Absorb, as an alternative to metal stents currently used in percutaneous coronary intervention. Unlike traditional stents that remain in place after implantation, Absorb is designed to disappear within three years of the procedure.
U.S. Food and Drug Administration staff, in documents released before an advisory panel meets on Tuesday to consider whether to recommend approval of the device, said they would ask the outside experts about data showing more heart attack and stent-related blood clots compared with Abbott's drug-coated metal Xience stent.
FDA said it also will ask the panel to address risks associated with the device when used in smaller arteries.
A large clinical trial released in October concluded that the Absorb stent, which is made of a plastic similar to dissolving sutures, was comparable to Xience in overall safety and effectiveness. Although Xience appeared to be numerically better than Absorb at one year on a variety of secondary measures, the differences were not considered statistically significant.
"These results are from physicians using a new therapy for the first time. Consequently, we expect them to improve with time and experience," said Abbott spokesman Jonathon Hamilton.
In the Absorb III clinical study, patients with small vessels comprised less than 20 percent of the total and experienced relatively low rates of adverse events even though many had known risk factors, including diabetes, Hamilton said.
More than 125,000 patients already have been treated with Absorb in more than 100 countries where it is commercially available.
If approved in the United States, Absorb would compete with Xience, the market-leading stent, and with Medtronic Plc's Resolute stent and Boston Scientific's Synergy and Promus stents. Synergy's polymer coating used to deliver a drug disappears over time, leaving a bare metal stent in place.
Wells Fargo analyst Larry Biegelsen said he expects Absorb to get a positive recommendation from the advisory panel, followed by FDA approval later this year.
"In the U.S., we estimate Absorb will capture about 5 percent of the total drug-eluting stent market, although our estimate may prove conservative if the post-approval data and experience with Absorb improves," Biegelsen wrote in a note to clients.
Computer models simulate HSCT recovery

Photo by Darren Baker
New research indicates that computer models can simulate the recovery of the immune system in patients undergoing hematopoietic stem cell transplant (HSCT).
The study suggests the possibility of using DNA sequencing and computer modeling to predict which HSCT recipients might suffer complications such as graft-versus-host-disease.
The research was published in Biology of Blood and Marrow Transplantation.
The study builds upon prior research, which showed that the immune system may be modeled as a dynamical system. Dynamical systems are mathematical objects used to model physical phenomena that change over time. These systems can be used to predict future states via observations of past and present states.
Researchers say the ability to predict immune system recovery after HSCT could potentially allow doctors to refine donor selection and better personalize post-transplant care to improve outcomes.
With this in mind, the team sequenced the DNA of 34 HSCT donor-recipient pairs and used the resulting information in a computer model to simulate how the recipient’s T-cell repertoire will recover following transplant.
“This study is the first to simulate the growth of the T-cell repertoire following transplantation using variables that aren’t accounted for in typical HLA donor-recipient matching,” said study author Amir Ahmed Toor, MD, of Virginia Commonwealth University in Richmond.
“Using a larger cohort of patients than in previous studies, we were able to mathematically predict the interactions of these variables, which led to simulations that appear to be very similar to clinically observed post-transplantation T-cell repertoire development.”
Previous research by Dr Toor and his colleagues revealed large variations between donor-recipient minor histocompatibility antigens that could potentially contribute to transplant complications not accounted for by HLA testing.
The models used in the computer simulations were driven by population growth formulas developed from past studies by Dr Toor and his colleagues that revealed distinct patterns of lymphocyte recovery in HSCT recipients.
Using matrix mathematics to develop the simulations, the researchers observed competition among T cells as the T-cell repertoire develops.
This competition leads to certain families of T cells becoming dominant and more numerous, which crowds out weaker T-cell families, causing them to develop later and in fewer numbers.
“We are attempting to account for the many variables that could impact T-cell repertoire development and, in turn, patient outcomes,” Dr Toor said.
“In future studies, we hope to explore the impact of organ-specific antigen expression. The knowledge gained from this research could potentially allow more accurate predictions of which organs could be most affected by graft-versus-host-disease.” ![]()

Photo by Darren Baker
New research indicates that computer models can simulate the recovery of the immune system in patients undergoing hematopoietic stem cell transplant (HSCT).
The study suggests the possibility of using DNA sequencing and computer modeling to predict which HSCT recipients might suffer complications such as graft-versus-host-disease.
The research was published in Biology of Blood and Marrow Transplantation.
The study builds upon prior research, which showed that the immune system may be modeled as a dynamical system. Dynamical systems are mathematical objects used to model physical phenomena that change over time. These systems can be used to predict future states via observations of past and present states.
Researchers say the ability to predict immune system recovery after HSCT could potentially allow doctors to refine donor selection and better personalize post-transplant care to improve outcomes.
With this in mind, the team sequenced the DNA of 34 HSCT donor-recipient pairs and used the resulting information in a computer model to simulate how the recipient’s T-cell repertoire will recover following transplant.
“This study is the first to simulate the growth of the T-cell repertoire following transplantation using variables that aren’t accounted for in typical HLA donor-recipient matching,” said study author Amir Ahmed Toor, MD, of Virginia Commonwealth University in Richmond.
“Using a larger cohort of patients than in previous studies, we were able to mathematically predict the interactions of these variables, which led to simulations that appear to be very similar to clinically observed post-transplantation T-cell repertoire development.”
Previous research by Dr Toor and his colleagues revealed large variations between donor-recipient minor histocompatibility antigens that could potentially contribute to transplant complications not accounted for by HLA testing.
The models used in the computer simulations were driven by population growth formulas developed from past studies by Dr Toor and his colleagues that revealed distinct patterns of lymphocyte recovery in HSCT recipients.
Using matrix mathematics to develop the simulations, the researchers observed competition among T cells as the T-cell repertoire develops.
This competition leads to certain families of T cells becoming dominant and more numerous, which crowds out weaker T-cell families, causing them to develop later and in fewer numbers.
“We are attempting to account for the many variables that could impact T-cell repertoire development and, in turn, patient outcomes,” Dr Toor said.
“In future studies, we hope to explore the impact of organ-specific antigen expression. The knowledge gained from this research could potentially allow more accurate predictions of which organs could be most affected by graft-versus-host-disease.” ![]()

Photo by Darren Baker
New research indicates that computer models can simulate the recovery of the immune system in patients undergoing hematopoietic stem cell transplant (HSCT).
The study suggests the possibility of using DNA sequencing and computer modeling to predict which HSCT recipients might suffer complications such as graft-versus-host-disease.
The research was published in Biology of Blood and Marrow Transplantation.
The study builds upon prior research, which showed that the immune system may be modeled as a dynamical system. Dynamical systems are mathematical objects used to model physical phenomena that change over time. These systems can be used to predict future states via observations of past and present states.
Researchers say the ability to predict immune system recovery after HSCT could potentially allow doctors to refine donor selection and better personalize post-transplant care to improve outcomes.
With this in mind, the team sequenced the DNA of 34 HSCT donor-recipient pairs and used the resulting information in a computer model to simulate how the recipient’s T-cell repertoire will recover following transplant.
“This study is the first to simulate the growth of the T-cell repertoire following transplantation using variables that aren’t accounted for in typical HLA donor-recipient matching,” said study author Amir Ahmed Toor, MD, of Virginia Commonwealth University in Richmond.
“Using a larger cohort of patients than in previous studies, we were able to mathematically predict the interactions of these variables, which led to simulations that appear to be very similar to clinically observed post-transplantation T-cell repertoire development.”
Previous research by Dr Toor and his colleagues revealed large variations between donor-recipient minor histocompatibility antigens that could potentially contribute to transplant complications not accounted for by HLA testing.
The models used in the computer simulations were driven by population growth formulas developed from past studies by Dr Toor and his colleagues that revealed distinct patterns of lymphocyte recovery in HSCT recipients.
Using matrix mathematics to develop the simulations, the researchers observed competition among T cells as the T-cell repertoire develops.
This competition leads to certain families of T cells becoming dominant and more numerous, which crowds out weaker T-cell families, causing them to develop later and in fewer numbers.
“We are attempting to account for the many variables that could impact T-cell repertoire development and, in turn, patient outcomes,” Dr Toor said.
“In future studies, we hope to explore the impact of organ-specific antigen expression. The knowledge gained from this research could potentially allow more accurate predictions of which organs could be most affected by graft-versus-host-disease.” ![]()